
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
WORLD HEALTH ORGANIZATION
Toxicological evaluation of certain veterinary drug
residues in food
WHO FOOD ADDITIVES SERIES 39
Prepared by:
The forty-eighth meeting of the Joint FAO/WHO Expert
Committee on Food Additives (JECFA)
World Health Organization, Geneva 1997
FLUAZURON
First draft prepared by
M.E.J. Pronk and G.J. Schefferlie
Centre for Substances and Risk Assessment
National Institute of Public Health and the Environment
Bilthoven, The Netherlands
1. Explanation
2. Biological data
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
2.1.2 Biotransformation
2.2 Toxicological studies
2.2.1 Acute toxicity
2.2.2 Short-term toxicity
2.2.3 Long-term toxicity and carcinogenicity
2.2.4 Genotoxicity
2.2.5 Reproductive toxicity
2.2.5.1 Multigeneration reproductive toxicity
2.2.5.2 Developmental toxicity
2.2.6 Special studies on target animals
2.2.7 Special studies on heat degradation
3. Comments
4. Evaluation
5. References
1. EXPLANATION
Fluazuron is an ectoparasiticide used for topical tick control in
beef cattle. It has not been evaluated previously by the Committee.
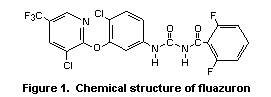
The chemical name of fluazuron is 3-[3-(3-chloro-5-
trifluoromethyl-2-pyridinyloxy)-4-chlorophenyl]-1-(2,6-
difluorobenzoyl)urea. The structure is shown in Figure 1. The purity
of technical-grade fluazuron that was used in the studies of toxicity
was > 98% and 99.2% in most studies.
 Fluazuron belongs to the class of benzoylphenyl urea derivatives,
which inhibit the growth and development of arthropod insects and
members of the order Acarina by acting on chitin formation. These
'insect growth regulators' do not act on the insect nervous system.
Fluazuron specifically affects ticks. Chitin synthesis in ticks occurs
during engorgement, moulting, and embryogenesis. Female ticks that
have absorbed a minimal amount of fluazuron show signs of disturbed
engorgement and lay eggs from which either no or non-viable larvae
hatch. Male ticks that take up minimal amounts of plasma are not
visibly affected by the compound. Fluazuron interrupts the life cycle
of immature ticks by interfering with cuticle formation during
engorgement and moulting. The affected ticks die.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
Rats
Fluazuron uniformly radiolabelled with 14C in the
4-chlorophenyl moiety ([U-14C-Cl]phenyl-labelled fluazuron; specific
activity, 48.1 µCi/mg) was administered orally in
N-methylpyrrolidone and PEG 200 by stomach tube to Tif:RAIf (SPF)
rats at a dose of 0.5 mg/kg bw per day for one week. Three rats of
each sex were killed 24 h and 2, 4, 8, and 12 weeks after the final
dose. Urine and faeces were collected daily during treatment and for
one week thereafter. After sacrifice, subcutaneous, renal, and
abdominal fat, blood, brain, kidneys, skeletal muscle, liver, and the
remaining carcass were sampled for radiolabel with a liquid
scintillation counter. The study was certified for compliance with GLP
and quality assurance.
By 24 h after the last dose, about 60% had been absorbed from the
gastrointestinal tract into the general circulation, as calculated
from the amount of radiolabel in urine, tissues, and carcass. During
the administration and observation periods, a total of 62% of the
administered dose was excreted, with 59% via the faeces and only 3%
via the urine. The extent of absorption and the route and rate of
excretion were independent of sex. At 24 h, the highest residue levels
were found in the adipose tissues (12-18 mg/kg fluazuron equivalents)
and significantly lower levels in other tissues: liver, 1.3; kidney,
0.84; lungs, 0.53; muscle, 0.39; and brain, 0.20 mg/kg. The same
distribution was seen at all time points. By 12 weeks after the last
dose, the amount of residual radiolabel had declined to 0.15-0.26
mg/kg fluazuron equivalents in adipose tissues and to < 0.03 mg/kg in
all other tissues. The depletion of radiolabel from all tissues
followed first-order kinetics, with a half-time of about 13 days for
all tissues in animals of each sex. The ratio in fat:blood (201 ± 28)
was relatively constant over the experimental period (Schulze-Aurich,
1992a).
Cattle
Male cross-bred beef cattle received single topical applications
of [U-14C-Cl]phenyl-labelled fluazuron (as the commercial pour-on
formulation Acatak; specific activity, 4.86 µCi/mg) at a dose of 1.5
mg/kg bw, administered on both sides of the spine between the shoulder
and the rump. The animals were slaughtered in groups of three during
weeks 2, 4, 6, and 16 after treatment. Blood, urine, and faeces were
collected from the animals slaughtered at the end of the study at
several times after treatment. At slaughter, samples were taken of
ventral and dorsal subcutaneous, renal, and omental fat; blood; brain;
kidney; hindquarter, forequarter, and tenderloin muscle; liver; bile;
and skin at the site of administration. All samples were analysed for
radiolabel with a liquid scintillation counter. The study was
certified for compliance with GLP and quality assurance.
The total intake was at least 60% of the administered dose, as
indicated by urinary and faecal excretion. It was not clear, however,
whether intake was via the dermal and/or the oral route (licking); the
portion absorbed into the systemic circulation was also unknown.
Fluazuron was slowly absorbed and distributed to tissues. Radiolabel
was initially observed in plasma 16 h after treatment. The mean plasma
levels remained fairly constant between 9 and 35 days after treatment,
ranging from 0.035 to 0.041 mg/litre fluazuron equivalents. The levels
declined thereafter, with a mean elimination half-time of about 73
days, to a mean level of 0.007 mg/litre 16 weeks after treatment. The
main route of elimination was the faeces (40% of the administered dose
within the first four weeks, increasing gradually to 62% by 16 weeks),
whereas renal excretion was of minor importance (1% of the dose after
16 weeks). There was some indication of biliary excretion. Maximum
residue levels were found two weeks after administration in almost all
tissues. The levels were highest in renal fat (4.8 mg/kg fluazuron
equivalents), omental fat (4.3 mg/kg), subcutaneous fat (ventral: 3.9
mg/kg; dorsal: 2.8 mg/kg), and skin (3 mg/kg). Lower levels were found
in liver (0.5 mg/kg), kidney (0.4 mg/kg), muscle (0.1 mg/kg), and
brain (0.08 mg/kg). The levels decreased slowly up to 16 weeks after
treatment, when they were 0.5-0.6 mg/kg in fat, 0.05-0.06 mg/kg in
liver and kidney, and 0.01-0.02 mg/kg in muscle and brain. The
depletion half-times for the different tissues varied from 4.5 to 5.5
weeks, but that in skin was 1.5 weeks (McLean & Dunsire, 1996).
Castrated Hereford steers received single subcutaneous injections
of [U-14C-Cl]phenyl-labelled fluazuron in a vehicle consisting of
PEG 200 dilaurate, Cremophor EL, citric acid, and
N-methyl-2-pyrrolidone (specific activity, 3.95 µCi/mg) behind the
left shoulder at a dose of 1.5 mg/kg bw. The animals were slaughtered
in groups of three, two days and 2, 6, and 16 weeks after treatment.
Blood, urine, and faeces were collected at several times after
treatment. At slaughter, samples were taken of subcutaneous, renal,
and omental fat; blood; brain; kidney; hindquarter and forequarter
muscle; liver; bile; and skin at the site of administration. All
samples were analysed for radiolabel with a liquid scintillation
counter. The study was certified for compliance with GLP and quality
assurance.
The compound was absorbed slowly from the injection site, the
mean maximum plasma level of 0.1 mg/litre fluazuron equivalents being
reached after 48 h. The plasma levels declined with a mean elimination
half-time of about 78 days; by 16 weeks after treatment, the mean
plasma level was still 0.01 mg/litre. The main route of elimination
was the faeces (23% of the administered dose after 16 weeks), whereas
renal excretion was of minor importance (1% of the dose after 16
weeks). There was some indication of biliary excretion. In all tissues
except subcutaneous fat, maximum residue levels were found 48 h after
administration. The levels were highest in renal fat (4.6 mg/kg
fluazuron equivalents) and omental fat (3.3 mg/kg), with lower levels
in liver (0.8 mg/kg), kidney (0.5 mg/kg), brain (0.2 mg/kg), and
muscle (0.1 mg/kg). In subcutaneous fat, a maximum residue level of
2.7 mg/kg was found two weeks after dosing. The residue levels were
comparable in all tissues after two and six weeks; after 16 weeks, the
levels had declined to 0.9-1 mg/kg in fat and 0.1 mg/kg in both liver
and kidney. The radiolabel collected at the injection site accounted
for 52% of the administered dose after 48 h (643 mg/kg fluazuron
equivalents), 26% after six weeks (396 mg/kg), and 5% after 16 weeks
(52 mg/kg) (Cameron et al., 1992).
2.1.2 Biotransformation
Rats
In the study of Schulze-Aurich (1992a) described above,
metabolites were identified in all tissue, faecal, and urine samples
by thin-layer chromatography and in fat and faeces also by
high-performance liquid chromatography and mass spectrometry-nuclear
magnetic resonance. In all tissues taken at all times of sacrifice,
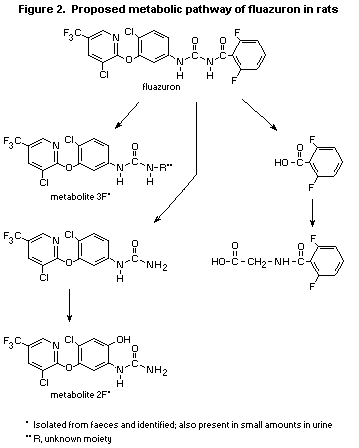
the residues consisted only of unchanged fluazuron. In faeces, six
metabolic fractions were present and two metabolites were identified:
2F (3.2% of the dose) and 3F (5.8% of the dose) (see Figure 2), but
the major compound was unchanged fluazuron (26% of the dose). In
urine, eight metabolic fractions were found, including metabolites 2F
(0.6% of the dose) and 3F (0.45%); no unchanged fluazuron was present.
Hence, fluazuron is metabolized slowly but to a significant extent;
the pattern of metabolites in faeces indicates that about two-thirds
of the dose is metabolized and one-third is eliminated unchanged.
Metabolism proceeds by cleavage of the urea moiety, followed by
hydroxylation in position 6 of the phenyl ring, leading to 3-[3-(3-
chloro-5-trifluoromethyl-2-pyridinyloxy)-4-chloro-6-
hydroxyphenyl]urea. The main cleavage product, 2,6-difluorobenzoic
acid, is partly conjugated with glycine to form 2,6-difluorohippuric
acid.
Cattle
The nature of the residues in tissues, faeces, and bile of cattle
after pour-on administration of fluazuron at 1.5 mg/kg bw was
investigated by means of thin-layer chromatography and, in fat, also
by mass spectrometry. The study was certified for compliance with GLP
and quality assurance. Fluazuron is not extensively metabolized after
pour-on administration, as unchanged fluazuron was the only
radiolabelled component detected in almost all tissues taken at all
slaughter times, generally accounting for > 90% of the total
residues. Additional metabolites were detected at low levels only in
muscle (3% of the total residues) and skin (24%) samples taken at 16
weeks. Two metabolic fractions were found in faeces. The major
compound was unchanged fluazuron (about 92% of the radiolabel), while
the other, more polar fraction accounted for 3% of faecal radiolabel.
In bile, unchanged fluazuron was the major compound (76% of
radiolabel), and the other 24% remained at the origin of the
chromatography system (Johnson et al., 1996).
Fluazuron belongs to the class of benzoylphenyl urea derivatives,
which inhibit the growth and development of arthropod insects and
members of the order Acarina by acting on chitin formation. These
'insect growth regulators' do not act on the insect nervous system.
Fluazuron specifically affects ticks. Chitin synthesis in ticks occurs
during engorgement, moulting, and embryogenesis. Female ticks that
have absorbed a minimal amount of fluazuron show signs of disturbed
engorgement and lay eggs from which either no or non-viable larvae
hatch. Male ticks that take up minimal amounts of plasma are not
visibly affected by the compound. Fluazuron interrupts the life cycle
of immature ticks by interfering with cuticle formation during
engorgement and moulting. The affected ticks die.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
Rats
Fluazuron uniformly radiolabelled with 14C in the
4-chlorophenyl moiety ([U-14C-Cl]phenyl-labelled fluazuron; specific
activity, 48.1 µCi/mg) was administered orally in
N-methylpyrrolidone and PEG 200 by stomach tube to Tif:RAIf (SPF)
rats at a dose of 0.5 mg/kg bw per day for one week. Three rats of
each sex were killed 24 h and 2, 4, 8, and 12 weeks after the final
dose. Urine and faeces were collected daily during treatment and for
one week thereafter. After sacrifice, subcutaneous, renal, and
abdominal fat, blood, brain, kidneys, skeletal muscle, liver, and the
remaining carcass were sampled for radiolabel with a liquid
scintillation counter. The study was certified for compliance with GLP
and quality assurance.
By 24 h after the last dose, about 60% had been absorbed from the
gastrointestinal tract into the general circulation, as calculated
from the amount of radiolabel in urine, tissues, and carcass. During
the administration and observation periods, a total of 62% of the
administered dose was excreted, with 59% via the faeces and only 3%
via the urine. The extent of absorption and the route and rate of
excretion were independent of sex. At 24 h, the highest residue levels
were found in the adipose tissues (12-18 mg/kg fluazuron equivalents)
and significantly lower levels in other tissues: liver, 1.3; kidney,
0.84; lungs, 0.53; muscle, 0.39; and brain, 0.20 mg/kg. The same
distribution was seen at all time points. By 12 weeks after the last
dose, the amount of residual radiolabel had declined to 0.15-0.26
mg/kg fluazuron equivalents in adipose tissues and to < 0.03 mg/kg in
all other tissues. The depletion of radiolabel from all tissues
followed first-order kinetics, with a half-time of about 13 days for
all tissues in animals of each sex. The ratio in fat:blood (201 ± 28)
was relatively constant over the experimental period (Schulze-Aurich,
1992a).
Cattle
Male cross-bred beef cattle received single topical applications
of [U-14C-Cl]phenyl-labelled fluazuron (as the commercial pour-on
formulation Acatak; specific activity, 4.86 µCi/mg) at a dose of 1.5
mg/kg bw, administered on both sides of the spine between the shoulder
and the rump. The animals were slaughtered in groups of three during
weeks 2, 4, 6, and 16 after treatment. Blood, urine, and faeces were
collected from the animals slaughtered at the end of the study at
several times after treatment. At slaughter, samples were taken of
ventral and dorsal subcutaneous, renal, and omental fat; blood; brain;
kidney; hindquarter, forequarter, and tenderloin muscle; liver; bile;
and skin at the site of administration. All samples were analysed for
radiolabel with a liquid scintillation counter. The study was
certified for compliance with GLP and quality assurance.
The total intake was at least 60% of the administered dose, as
indicated by urinary and faecal excretion. It was not clear, however,
whether intake was via the dermal and/or the oral route (licking); the
portion absorbed into the systemic circulation was also unknown.
Fluazuron was slowly absorbed and distributed to tissues. Radiolabel
was initially observed in plasma 16 h after treatment. The mean plasma
levels remained fairly constant between 9 and 35 days after treatment,
ranging from 0.035 to 0.041 mg/litre fluazuron equivalents. The levels
declined thereafter, with a mean elimination half-time of about 73
days, to a mean level of 0.007 mg/litre 16 weeks after treatment. The
main route of elimination was the faeces (40% of the administered dose
within the first four weeks, increasing gradually to 62% by 16 weeks),
whereas renal excretion was of minor importance (1% of the dose after
16 weeks). There was some indication of biliary excretion. Maximum
residue levels were found two weeks after administration in almost all
tissues. The levels were highest in renal fat (4.8 mg/kg fluazuron
equivalents), omental fat (4.3 mg/kg), subcutaneous fat (ventral: 3.9
mg/kg; dorsal: 2.8 mg/kg), and skin (3 mg/kg). Lower levels were found
in liver (0.5 mg/kg), kidney (0.4 mg/kg), muscle (0.1 mg/kg), and
brain (0.08 mg/kg). The levels decreased slowly up to 16 weeks after
treatment, when they were 0.5-0.6 mg/kg in fat, 0.05-0.06 mg/kg in
liver and kidney, and 0.01-0.02 mg/kg in muscle and brain. The
depletion half-times for the different tissues varied from 4.5 to 5.5
weeks, but that in skin was 1.5 weeks (McLean & Dunsire, 1996).
Castrated Hereford steers received single subcutaneous injections
of [U-14C-Cl]phenyl-labelled fluazuron in a vehicle consisting of
PEG 200 dilaurate, Cremophor EL, citric acid, and
N-methyl-2-pyrrolidone (specific activity, 3.95 µCi/mg) behind the
left shoulder at a dose of 1.5 mg/kg bw. The animals were slaughtered
in groups of three, two days and 2, 6, and 16 weeks after treatment.
Blood, urine, and faeces were collected at several times after
treatment. At slaughter, samples were taken of subcutaneous, renal,
and omental fat; blood; brain; kidney; hindquarter and forequarter
muscle; liver; bile; and skin at the site of administration. All
samples were analysed for radiolabel with a liquid scintillation
counter. The study was certified for compliance with GLP and quality
assurance.
The compound was absorbed slowly from the injection site, the
mean maximum plasma level of 0.1 mg/litre fluazuron equivalents being
reached after 48 h. The plasma levels declined with a mean elimination
half-time of about 78 days; by 16 weeks after treatment, the mean
plasma level was still 0.01 mg/litre. The main route of elimination
was the faeces (23% of the administered dose after 16 weeks), whereas
renal excretion was of minor importance (1% of the dose after 16
weeks). There was some indication of biliary excretion. In all tissues
except subcutaneous fat, maximum residue levels were found 48 h after
administration. The levels were highest in renal fat (4.6 mg/kg
fluazuron equivalents) and omental fat (3.3 mg/kg), with lower levels
in liver (0.8 mg/kg), kidney (0.5 mg/kg), brain (0.2 mg/kg), and
muscle (0.1 mg/kg). In subcutaneous fat, a maximum residue level of
2.7 mg/kg was found two weeks after dosing. The residue levels were
comparable in all tissues after two and six weeks; after 16 weeks, the
levels had declined to 0.9-1 mg/kg in fat and 0.1 mg/kg in both liver
and kidney. The radiolabel collected at the injection site accounted
for 52% of the administered dose after 48 h (643 mg/kg fluazuron
equivalents), 26% after six weeks (396 mg/kg), and 5% after 16 weeks
(52 mg/kg) (Cameron et al., 1992).
2.1.2 Biotransformation
Rats
In the study of Schulze-Aurich (1992a) described above,
metabolites were identified in all tissue, faecal, and urine samples
by thin-layer chromatography and in fat and faeces also by
high-performance liquid chromatography and mass spectrometry-nuclear
magnetic resonance. In all tissues taken at all times of sacrifice,
the residues consisted only of unchanged fluazuron. In faeces, six
metabolic fractions were present and two metabolites were identified:
2F (3.2% of the dose) and 3F (5.8% of the dose) (see Figure 2), but
the major compound was unchanged fluazuron (26% of the dose). In
urine, eight metabolic fractions were found, including metabolites 2F
(0.6% of the dose) and 3F (0.45%); no unchanged fluazuron was present.
Hence, fluazuron is metabolized slowly but to a significant extent;
the pattern of metabolites in faeces indicates that about two-thirds
of the dose is metabolized and one-third is eliminated unchanged.
Metabolism proceeds by cleavage of the urea moiety, followed by
hydroxylation in position 6 of the phenyl ring, leading to 3-[3-(3-
chloro-5-trifluoromethyl-2-pyridinyloxy)-4-chloro-6-
hydroxyphenyl]urea. The main cleavage product, 2,6-difluorobenzoic
acid, is partly conjugated with glycine to form 2,6-difluorohippuric
acid.
Cattle
The nature of the residues in tissues, faeces, and bile of cattle
after pour-on administration of fluazuron at 1.5 mg/kg bw was
investigated by means of thin-layer chromatography and, in fat, also
by mass spectrometry. The study was certified for compliance with GLP
and quality assurance. Fluazuron is not extensively metabolized after
pour-on administration, as unchanged fluazuron was the only
radiolabelled component detected in almost all tissues taken at all
slaughter times, generally accounting for > 90% of the total
residues. Additional metabolites were detected at low levels only in
muscle (3% of the total residues) and skin (24%) samples taken at 16
weeks. Two metabolic fractions were found in faeces. The major
compound was unchanged fluazuron (about 92% of the radiolabel), while
the other, more polar fraction accounted for 3% of faecal radiolabel.
In bile, unchanged fluazuron was the major compound (76% of
radiolabel), and the other 24% remained at the origin of the
chromatography system (Johnson et al., 1996).
 The nature of the residues in tissues and excreta of steers after
subcutaneous administration of fluazuron at 1.5 mg/kg bw was
investigated by thin-layer chromatography. The study was certified for
compliance with GLP and quality assurance. In all tissues taken at all
slaughter times, unchanged fluazuron was the only detectable fraction,
accounting for more than 90% of the total residues. In faeces, eight
metabolic fractions were found. The major compound was unchanged
fluazuron (about 70% of radiolabel), while the other, unidentified
fractions were more polar. Urine contained only unidentified
metabolized products, which were more polar than unchanged fluazuron.
Within 16 weeks of treatment, about 24% of the administered dose was
eliminated in faeces and urine, 16% as unchanged fluazuron and 8% as
its degradation products. Hence, fluazuron is metabolized to a lesser
extent in cattle than in rats (Schulze-Aurich, 1992b).
2.2 Toxicological studies
2.2.1 Acute toxicity
In studies of technical-grade fluazuron in distilled water
containing 0.5% carboxymethylcellulose and 0.1% polysorbate 80 in male
and female Tif:RAIf (SPF) rats, one study that followed OECD test
guideline 401 with quality assurance certification showed an oral
LD50 value of > 5000 mg/kg bw (Schoch, 1986a); in a study in the
same strain that followed OECD test guideline 402 with quality
assurance certification, the dermal LD50 was > 2000 mg/kg bw
(Schoch, 1986b). In a study that followed OECD test guideline 403, the
LC50 after exposure by inhalation was > 5994 mg/m3 (Hartmann &
Schoch, 1987). The toxic symptoms observed were sedation, dyspnoea,
exophthalmos, ruffled fur, and abnormal body positions. The animals
recovered within 6-11 days.
Three male New Zealand white rabbits received an instillation of
0.1 ml (56 mg) technical-grade fluazuron in a study that followed OECD
test guideline 405 with GLP and quality assurance certification. The
animals developed slight redness and chemosis of the cornea and
conjunctivae up to 24 h. No irritation of the iris was observed
(Schoch, 1986c).
In a study that followed OECD test guideline 404 with GLP and
quality assurance certification, three male New Zealand white rabbits
that received a semi-occlusive topical application of 0.5 g
technical-grade fluazuron developed no dermal reactions (Schoch,
1986d).
In 10 male and 10 female Pirbright white guinea-pigs submitted to
an optimization test, in a study that followed OECD test guideline 404
with GLP and quality assurance certification, no skin sensitization
was observed after challenge with technical-grade fluazuron
intradermally in 20% propylene glycol or epidermally in vaseline
(Schoch & Gfeller, 1987).
2.2.2 Short-term toxicity
Rats
In a study of dermal toxicity that followed OECD test guideline
410 with GLP and quality assurance certification, gauze patches
moistened with distilled water and technical-grade fluazuron at doses
of 0, 10, 100, or 1000 mg/kg bw were applied to the shaven skin of
groups of five male and five female Tif:RAIf (SPF) rats for 6 h/day,
five days per week for three weeks. The only effect observed was a
slight but significant prolongation of prothrombin time in male rats
at the two higher doses. The NOEL was 10 mg/kg bw per day (Schoch
et al., 1988).
Groups of 10 male and 10 female Tif:RAIf (SPF) rats received
technical-grade fluazuron by gavage for four weeks at intended doses
of 0, 10, 100, or 1000 mg/kg bw per day. The vehicle was distilled
water containing 0.5% carboxymethylcellulose and 0.1% Tween 80. Since
no clinical signs of toxicity were observed, the upper dose was
increased to 2000 mg/kg bw per day from day 10 onwards. The final
actual doses were 0, 3.2-5.6, 35-60, and 1660-1920 mg/kg bw per day.
The study was certified for compliance with GLP and quality assurance.
No treatment-related effects were seen on mortality rate,
clinical or ophthalmoscopic signs, body weight, or food or water
consumption, macroscopically or microscopically. Although treated
animals showed some statistically significant changes in blood
chemistry, these were not considered to be treatment-related (i.e. not
dose-related or of no biological relevance). All males at the medium
and high doses had a slight, statistically significant, dose-related
increase in prothrombin time and a slight, statistically significant,
dose-related decrease in platelet count. The mean liver weight was
increased in all treated animals, reaching statistical significance in
those at the two higher doses. Males at these doses also had a
decrease in mean thymus weights, which was statistically significant
only at the highest dose. These weight changes were not accompanied by
histopathological findings. The NOEL was 3.2-5.6 mg/kg bw per day, but
the validity of the study is limited due to the fact that the doses
intended to be the low and medium doses were not reached (Thevenaz
et al., 1987).
Groups of 20 male and 20 female Tif:RAIf (SPF) rats were fed
technical-grade fluazuron in the diet at concentrations of 0, 100,
600, 3500, or 20 000 mg/kg feed for three months, to give calculated
mean daily intakes of 0, 6.4, 39, 220, or 1300 mg/kg bw for males and
0, 6.6, 41, 240, or 1400 mg/kg bw for females. The study followed OECD
test guideline 408 with GLP and quality assurance certification.
No treatment-related effects were seen on mortality, clinical or
ophthalmoscopic signs, or macroscopically. Although body weight and
food consumption were marginally lower in treated males than in
controls, food conversion was not influenced. Treated males and
females showed some statistically significant changes in blood
chemistry; however, as these findings did not reflect any consistent
alteration (i.e. not dose-related or of no biological relevance), they
were not considered to be treatment-related. All treated males had
slightly increased prothrombin time (dose-related, statistically
significant in rats at 3500 and 20 000 mg/kg feed), in platelet count
(not dose-related, significant at 3500 mg/kg feed), and in lymphocyte
count (dose-related, significant at 20 000 mg/kg feed). The absolute
and relative weights of the liver and glycogen deposition were
significantly increased in all treated males, due, at least at 100
mg/kg feed, to poor study design, as sections were not made at random.
Changes in the absolute and relative weights of the heart (males),
kidney, ovaries, and adrenals (females) were not accompanied by
abnormal histopathological findings. Fluazuron treatment resulted in
slight to moderate hepatocellular hypertrophy in males and females at
3500 and 20 000 mg/kg feed and a slight increase in the incidence and
intensity of thyroid follicular hypertrophy and pituitary cell
hypertrophy in males at these doses. The authors considered the NOEL
to be 600 mg/kg feed (equal to 39-41 mg/kg bw per day); however,
effects on liver weight were observed in males at this dose. The
Committee concluded that the NOEL was 100 mg/kg feed, equal to 6.4
mg/kg bw per day, on the basis of effects on the liver. It should be
noted that similar findings were not observed in a long-term study of
toxicity and carcinogenicity in rats (see below) (Basler et al.,
1987).
Dogs
In a one-month range-finding study, groups of two male and two
female pure-bred beagle dogs were given technical-grade fluazuron at
doses of 200 or 50 000 mg/kg feed, equal to mean daily intakes of 8.5
or 2200 mg/kg bw per day, respectively. The study was certified for
compliance with GLP and quality assurance. No deaths occurred.
Clinical symptoms, food consumption, body-weight gain,
ophthalmoscopic, haematological, clinical, and urinary parameters, and
macroscopic and histopathological findings indicated no change that
could be considered to be related to treatment (Bloch et al., 1987).
In a three-month study, groups of four to six pedigree beagle
dogs of each sex were fed diets containing technical-grade fluazuron
at 0, 500, 5000, or 50 000 mg/kg feed. The study followed OECD test
guideline 409 with GLP and quality assurance certification. As several
animals in each group showed alterations indicative of an infection
(possibly leptospirosis) during the experiment, the study was not
considered useful for evaluating the safety of fluazuron. The only
findings of note that could not be attributed to the infectious
disease were degenerative, inflammatory, or hypertrophic changes in
the major arteries of females at 5000 and 50 000 mg/kg; however, this
effect was not confirmed in a 52-week feeding study with an interim
kill at 13 weeks (see below) (Gretener et al., 1988).
Groups of six male and six female beagle dogs received diets
containing technical-grade fluazuron at 0, 200, 3000, or 50 000 mg/kg
feed for one year. Two dogs of each sex per group were killed for
interim examinations after 13 weeks. The observations included
mortality, clinical signs, body weight, food consumption,
ophthalmoscopy, haematology, blood chemistry, urinalysis, organ
weights, and macroscopic and microscopic examinations. The calculated
mean intakes were 0, 7.5, 110, or 1900 mg/kg bw per day for males and
0, 7.1, 120, or 2000 mg/kg bw per day for females. The study followed
OECD test guideline 452 with GLP and quality assurance certification.
No deaths occurred. Treatment mainly affected two males at the high
dose (one killed at 13 weeks and the other at 52 weeks), which had
decreased food consumption, transient body-weight loss, and increased
activities of alkaline phosphatase and aspartate and alanine
aminotransferases from week 13 onwards; histopathological examination
revealed minimal multifocal areas of haemorrhage with slight
multifocal chronic inflammation of the liver. A slight increase in
alkaline phosphatase activity was also found in males at the middle
dose from week 13 onwards and in females at the high dose from week 39
onwards. Females at the high dose also had a slightly decreased
phosphorus concentration. The NOEL was 200 mg/kg feed, equal to 7.5
mg/kg bw per day, on the basis of changes in hepatic enzyme activities
(Briffaux, 1988a, 1990).
2.2.3 Long-term toxicity and carcinogenicity
Mice
Groups of 60 male and 60 female Tif: MAGf (SPF) mice were fed
diets containing technical-grade fluazuron at doses of 0, 40, 400,
4000, or 9000 mg/kg feed for two years, equal to average achieved
intakes of 0, 4.5, 45, 450, or 990 mg/kg bw per day for males and 0,
4.3, 43, 430, or 970 mg/kg bw per day for females. The observations
included clinical signs, mortality, body weight, food and water
consumption, haematological changes (10 animals of each sex per
group), and organ weights; detailed macroscopic and histopathological
examinations were perfomed. The study followed OECD test guideline 451
with GLP and quality assurance certification.
Clinical signs, body weight, and food consumption were unaffected
by treatment, as was survival (40-45% for controls, 43-58% for treated
animals). Water consumption was increased consistently in females at
9000 mg/kg feed and in females at 400 and 4000 mg/kg feed in the
second year of the study, but in females at 40 mg/kg feed and in all
treated males it was comparable to that of controls. Haematological
examination revealed no treatment-related changes, and the organ
weights and macroscopic findings at necropsy were comparable in
control and treated animals. The treatment-related non-neoplastic
changes included an increased incidence of cataract of the crystalline
lens characterized by mild necrosis and calcification of subcapsular
lens fibres in animals of each sex at 4000 and 9000 mg/kg feed and a
trend to increased diffuse hyperplasia of prostatic glandular tissue
in males at 4000 and 9000 mg/kg feed. In addition, the following
uterine changes, which were not dose-related, were noted: increased
incidences of inflammatory polyps at 400, 4000, and 9000 mg/kg feed,
increased dilatation of the lumen at 4000 and 9000 mg/kg feed, and
increased incidences of haematomas and dilatation of blood vessels
associated with thrombosis at 9000 mg/kg feed. Tumour incidences were
not increased, although a marginally increased incidence of systemic
infiltration with malignant lymphoma was observed in some organs of
some animals at the high dose. The total number of lymphomas per
animal and the total number of animals bearing lymphomas were not
significantly different from those among controls. On the basis of the
pathological changes in the uterus, the NOEL was 40 mg/kg feed, equal
to 4.3 mg/kg bw per day (Bachmann et al., 1991a).
In the same study, the total amount of fluazuron in the body
appeared to reach a maximum at approximately 400 mg/kg feed: the
fluazuron level was 4.9-8.7 µg/ml in blood and 880-970 mg/kg in fat in
animals of each sex at 4000 and 9000 mg/kg feed and only slightly
lower in those at 400 mg/kg feed. At 40 mg/kg feed, a steady state was
not reached. The blood:fat ratio was approximately 1:200 in all groups
(Maier, 1991a).
Rats
Groups of 80 male and 80 female Tif:RAIf (SPF) rats received
diets containing technical-grade fluazuron at 0, 50, 500, 10 000, or
20 000 mg/kg feed for two years, equal to average achieved intakes of
0, 1.9, 18, 380, or 780 mg/kg bw per day for males and 0, 2.1, 21,
440, or 920 mg/kg bw per day for females. Ten rats of each sex were
killed after one year for interim necropsy. The observations included
clinical signs, mortality, body weight, food consumption,
ophthalmoscopy, haematology (20 rats of each sex per group), blood
chemistry (10 of each sex per group), urinalysis (10 of each sex per
group), and organ weights; detailed macroscopic and histopathological
examinations were performed. The study followed OECD test guideline
453 with GLP and quality assurance certification.
The survival of the animals (56-67% for controls, 54-73% for
treated animals) was not affected by treatment. Minor effects were
observed in animals at the highest dose at interim sacrifice but not
at terminal sacrifice. In females, the relative liver and kidney
weights were significantly decreased, with no associated morphological
changes; in males, minimal hypertrophy of hepatocytes was seen.
Females at the highest dose also had decreased body-weight gain during
the second year of treatment, but this failed to reach statistical
significance. The findings were considered not to be of toxicological
significance. The incidence of tumours was not increased. The total
amount of fluazuron in the body appeared to reach a maximum at 500
mg/kg feed: the level was 1.3-2.3 µg/ml in blood and 290-440 mg/kg in
fat in animals of each sex at 500 mg/kg feed and above. At 50 mg/kg
feed, a steady state was not reached. The blood:fat ratio was
approximately 1:200 in all groups (Bachmann et al., 1991b; Maier,
1991b).
2.2.4 Genotoxicity
The results of tests for genotoxicity carried out with fluazuron
are summarized in Table 1.
2.2.5 Reproductive toxicity
2.2.5.1 Multigeneration reproductive toxicity
In a preliminary study of reproductive toxicity, groups of seven
male and 14 female Ico:OFA Sprague-Dawley rats received
technical-grade fluazuron at dietary concentrations of 0, 200, 1000,
7000, or 20 000 mg/kg feed. Males were dosed for two weeks before
mating and during the mating period and were sacrificed thereafter.
Females were dosed from two weeks before mating up to 21 days post
partum, when both the females and their litters were sacrificed. The
study was certified for quality assurance.
There were no deaths or treatment-related clinical signs in
animals of either sex during the study. The food consumption and
body-weight gain of F0 animals and the precoital period and duration
of the gestation period were not affected by treatment, and no effects
were seen on insemination, fecundity, fertility, resorption indices,
stillbirths, nursing behaviour, the numbers of live F1 pups, their
physical development, or neonatal or postnatal mortality. The mean
weight at birth of F1 pups was not affected by treatment, but a
slight retardation in growth rate was seen towards the end of the
lactation period in animals at 7000 and 20 000 mg/kg feed. No
treatment-related effects were seen in any animal at necropsy
(Briffaux, 1988b).
In a two-generation study of reproductive toxicity, groups of 30
male and 30 female Ico:OFA Sprague-Dawley rats received diets
containing technical-grade fluazuron at 0, 100, 1500, or 20 000 mg/kg
feed for at least 100 days before mating and then throughout gestation
and lactation for two successive generations. Two litters were bred
per generation. Males and females of the first F1 litter (F1a)
were selected to breed the next generation. After weaning of the F1b
and F2b pups, the parental animals were killed and necropsied.
Unselected F1a pups and F1b, F2a, and F2b pups were killed and
necropsied after 21 days of lactation. The study followed OECD test
guideline 416 with GLP and quality assurance certification.
The only effects found were on food consumption and body weight,
not on reproductive function. F1 females at the high dose showed a
slight reduction in body weight in the first gestation period, and the
food consumption of those at the middle and high doses tended to be
slightly reduced at the end of both lactation periods. Neonatal
viability of both generations of rats at the high dose was slightly
reduced after the first matings, but the litters from subsequent
matings were not affected. Pup viability from day 4 post partum to
weaning was not affected by treatment during any phase of the study.
The body weights at birth of pups in all litters were comparable to
those of controls, but the body-weight gain of those at the high dose
was reduced for all generations (F1a, F1b, F2a, F2b) and of
those at the middle dose for the F1a, F2a, and F2b generations.
The NOEL was 100 mg/kg feed, equivalent to 5 mg/kg bw per day, on the
basis of postnatal toxicity (Barrow & Briffaux, 1991).
2.2.5.2 Developmental toxicity
Rats
Technical-grade fluazuron was administered by gavage in a 0.1%
aqueous solution of polysorbate 80 at doses of 0, 10, 100, or 1000
mg/kg bw per day to groups of 24 mated female Tif:RAIf (SPF) rats on
days 6-15 of gestation. On day 21 of gestation, the dams were killed
and necropsied, and the fetuses were weighed, sexed, and examined for
external, visceral, and skeletal abnormalities. The study followed
OECD test guideline 414 with GLP and quality assurance certification.
One dam at the middle dose aborted its entire litter. No
treatment-related effects were observed in dams, and the numbers of
implantations, live young, and resorptions and fetal and litter
weights were unaffected by treatment. There was no indication of
teratogenicity. Hence, fluazuron at doses up to 1000 mg/kg bw per day
had no maternal, embryo- or fetal toxicity or teratogenicity (Thouin,
1988).
Rabbits
Groups of 20 mated female Chinchilla-type rabbits received
technical-grade fluazuron suspended in 3% (w/w) aqueous corn starch by
gavage at doses of 0, 10, 100, or 1000 mg/kg bw per day on days 7-19
of pregnancy. The dams were killed and necropsied on day 29 of
pregnancy, and various parameters examined. Fetuses were subjected to
gross necropsy and visceral and skeletal examination. The study
followed OECD test guideline 414 with GLP and quality assurance
certification. One female at the high dose died due to faulty
intubation, and another resorbed all of its implants. The incidence of
post-implantation loss was slightly increased in all treated groups,
mainly due to a slightly higher rate of early resorptions, but was
still within the historical control range and was considered not to be
related to treatment. The number of live fetuses and the litter weight
were unaffected by treatment. The body weights of the fetuses of all
treated dams were slightly increased; the increases in group means
were statistically significant but the litter means were not. No other
effects were observed. Hence, at doses up to 1000 mg/kg bw per day
fluazuron induced no maternal, embryo or fetal toxicity and was not
teratogenic (Thomann, 1988).
Table 1. Results of assays for genotoxicity with fluazuron
End-point Test object Concentration Result S9 QA Reference
In vitro
Reverse S. typhimurium 1.14-278 µg/mla Negative + No Deparade &
mutation TA98, TA100, Negative - Arni (1985)
TA1535, TA1537
Gene V79 Chinese 12.5-500 ng/ml Negative + Yes Dollenmeier &
mutation hamster cells 0.625-25 µg/ml Negative - Puri (1987)
Chromosomal Human lymphocytes 14.1-225 µg/ml Negative + Yes Strasser &
aberration 7.5-120.0 µg/ml Negative - Muller (1987)
DNA repair Rat hepatocytes 0.4-300 µg/ml Negative Yes Puri & Muller (1987)
DNA repair Human fibroblasts 0.2-50 µg/ml Negative Yes Meyer & Puri (1987)
In vivo
Nuclear Chinese hamster 1.25-5.0 g/kg bwb Negativec Yes Strasser & Arni
anomalies bone marrow (1987)
S9, 9000 × g fraction of rat liver; QA, quality assurance
a No cytotoxicity, but precipitation occurred at the highest dose in the presence of S9
b Daily doses given by gavage on each of two consecutive days; cyclophosphamide used as a positive control
c The authors considered 5.0 g/kg bw to be the highest applicable dose, but as cytotoxicity was not
determined it was not clear whether the bone marrow was exposed.
2.2.6 Special studies on target animals
In a study of tolerability, groups of three male and three female
Hereford cattle received the pour-on formulation Acatak at 0
(control), once, three times, or five times the recommended dose of 2
mg/kg bw fluazuron, along the back from the neck to the rump. The
animals were observed for the following eight weeks and monitored for
subclinical side-effects. The study was certified for quality
assurance. Apart from the formation of a crusty layer on the skin and
coat, which caused no irritation or discomfort to the animals, the
formulation was well tolerated at all doses. No drug-related changes
were observed in body weight, feed consumption, body temperature, or
haematological or blood biochemical parameters (Bowen & Strong, 1993).
When the label instructions for application of the pour-on formulation
Acatak were followed, a crusty layer formed on the coats of the
cattle, but the animals displayed no clinical signs of irritation.
Histopathological examination showed only minor pathological changes
in the skin, which had no effect on the hide quality (Strong, 1993;
Strong & Bowen, 1993).
Subcutaneous injection of Acatik, a commercial formulation
containing fluazuron as the active ingredient, induced no side-effects
or local reactions at the injection site in cattle receiving single
doses of 1 mg/kg bw fluazuron or dogs receiving double doses of 2.5
and then 5 mg/kg bw (Suarez, 1988; Genchi, 1990).
2.2.7 Special studies on heat degradation
The degradation of fluazuron residues present in cattle meat and
fat was investigated after normal dry cooking, cooking in water or
oil, or microwave treatment. The meat and fat samples were derived
from steers treated with fluazuron at 1.5 mg/kg bw by subcutaneous
injection, which is not the recommended route of administration. The
study was certified for compliance with GLP and quality assurance.
Residues of fluazuron were partly (in meat) or completely (in fat)
degraded to [3-(3-chloro-5-trifluoro-methyl-2-pyridinyloxy)-4-
chlorophenyl]-1-amine. This degradation product did not show mutagenic
potential in an assay for reverse mutation in Salmonella typhimurium
strains TA98, TA100, TA1535, and TA1537 or in Escherichia coli
strain WP2uvrA, when the known mutagen found in cooked meat, MeIQx
(2-amino-3,8- dimethylimidaz[4,5-f]quinoxaline), was used as the
reference substance (Schulze-Aurich, 1992c; Maier, 1993).
3. COMMENTS
The Committee considered the results of studies on the
pharmacokinetics, metabolism, acute, short-term and long-term
toxicity, carcinogenicity, genotoxicity, and reproductive toxicity of
fluazuron. All of the studies critical for the evaluation were carried
out according to appropriate standards for study protocol and conduct.
Analysis 24 h after oral administration of radiolabelled
fluazuron to rats showed that an average of 60% was absorbed through
the gut. Most of the absorbed radiolabel was taken up into adipose
tissues, while significantly lower levels were found in liver, kidney,
lung, muscle, and brain. Ninety percent of the radiolabel in tissues
was attached to unchanged fluazuron. Fluazuron was eliminated slowly
from adipose tissues, following first-order kinetics, with a half-life
of about 13 days. Elimination occurred primarily in the faeces. One
week after administration, 59% had been excreted in faeces and 3% in
urine. About one-third of the dose was eliminated as unchanged
fluazuron in the faeces; the remaining two-thirds was metabolized by
cleavage of the urea moiety between the benzoyl carbon and the urea
nitrogen, followed by hydroxylation in the phenyl ring.
When radiolabelled fluazuron was administered topically to
cattle, radiolabel was slowly absorbed, either percutaneously, orally
(by licking), or by both routes. A steady state between absorption and
elimination was observed for three to four weeks after treatment. The
absorbed radiolabel was taken up mainly by adipose tissues and to a
lesser extent by other tissues. Depletion of fluazuron from plasma and
tissues was slow, with half-lives of elimination of 10.5 and 4.5-5.5
weeks, respectively. The major route of elimination was the faeces
(62% of the dose within 16 weeks), while renal excretion was of minor
importance (1% of the dose within 16 weeks). There was some indication
of biliary excretion. Fluazuron was not extensively metabolized, as
unchanged fluazuron accounted for more than 90% of the total residues
in tissues and faeces. A similar slow absorption, distribution, and
elimination pattern was observed after subcutaneous administration of
radiolabelled fluazuron to steers. The pattern of metabolites excreted
in faeces was somewhat more complex, however, indicating that about
one-third of the fluazuron was metabolized into more polar
metabolites. Although the fate of fluazuron in rats was similar to
that in cattle, they metabolized fluazuron to a greater extent than
cattle.
Orally administered fluazuron was found to have low acute
toxicity, with an LD50 value of > 5000 mg/kg bw in rats.
The short-term toxicity of fluazuron was evaluated by oral
administration to rats and dogs. In a 28-day study, rats received
fluazuron by gavage at doses of 0, 10, 100, or 2000 mg/kg bw per day.
Increased prothrombin time and liver weight and decreased platelet
counts and thymus weight were observed, mainly in animals at the two
higher doses, and particularly in males. Male rats were also more
sensitive than females to the effects of fluazuron in a 13-week
feeding study at dietary concentrations of 0, 100, 600, 3500, or
20 000 mg/kg feed (equal to 6.4-1400 mg/kg bw per day). Male rats at
the two higher doses had increased prothrombin time, platelet and
lymphocyte counts, and absolute and relative liver weights, as well as
hypertrophy of thyroid follicular cells, pituitary cells, and
hepatocytes. In males, increases in absolute and relative liver
weights were also observed at 600 mg/kg feed. In females,
hepatocellular hypertrophy occurred at 3500 and 20 000 mg/kg feed. The
NOEL was 100 mg/kg feed, equal to 6.4 mg/kg bw per day, on the basis
of effects on the liver.
In a one-year feeding study, dogs received fluazuron at dietary
concentrations of 0, 200, 3000, or 50 000 mg/kg feed. Effects were
observed primarily in males at the highest dose, consisting of
decreased food consumption, transient body-weight loss, increased
serum activities of alkaline phosphatase and aspartate and alanine
aminotransferases, and minimal multifocal haemorrhage with slight
multifocal chronic inflammation in the liver. A slight increase in
alkaline phosphatase activity was also observed in males at 3000 mg/kg
feed and in females at 50 000 mg/kg feed. The NOEL was 200 mg/kg feed,
equal to 7.5 mg/kg bw per day, on the basis of changes in hepatic
enzyme activities.
In a study of carcinogenicity, mice received diets containing 0,
40, 400, 4000, or 9000 mg/kg feed for two years (equal to 4.3-990
mg/kg bw per day). Females at the two higher levels showed increased
water consumption, cataracts, and uterine changes (inflammatory
polyps, luminal dilatation and, at 9000 mg/kg feed only, haematomas
and dilatation of blood vessels associated with thrombosis). Increased
water consumption and inflammatory uterine polyps were also observed
in females at 400 mg/kg feed. Male mice at the two higher levels had
cataracts, and a trend to an increasing frequency of diffuse
hyperplasia of prostatic glandular tissue was observed. The NOEL was
40 mg/kg feed, equal to 4.3 mg/kg bw per day, on the basis of
pathological changes in the uterus.
In a long-term study of toxicity and carcinogenicity, rats were
given fluazuron at 0, 50, 500, 10 000, or 20 000 mg/kg feed for two
years (equal to 1.9-920 mg/kg bw per day), and a proportion of the
animals were killed at one year. No toxicologically significant
effects were observed at any dose. The toxic effects on the liver
observed in the 13-week study in rats were not observed after either
one or two years.
In the long-term studies of toxicity, the total amount of
fluazuron in the body appeared to reach a maximum at relatively low
levels (approximately 400 and 500 mg/kg feed, equal to 43 and 18 mg/kg
bw per day, in mice and rats, respectively), as the concentration of
the compound in blood and fat did not increase at higher levels. The
reason is not clear. It is not known whether a similar effect occurred
in the short-term studies, because blood and fat levels were not
measured.
Fluazuron has been tested in vitro for its ability to induce
reverse mutations in Salmonella typhimurium, gene mutations in Chinese
hamster cells, chromosomal aberration in human lymphocytes, and DNA
repair in rat hepatocytes and human fibroblasts. It has also been
tested in vivo for its ability to induce nuclear anomalies in
Chinese hamster bone marrow. All the results were negative. On the
basis of these data and the results of the bioassays in rodents, the
Committee concluded that fluazuron has no genotoxic or carcinogenic
potential.
In a two-generation study of reproductive toxicity in rats at
dietary concentrations of 0, 100, 1500, or 20 000 mg/kg feed
(equivalent to 5-1000 mg/kg bw per day), fluazuron had no adverse
effects on reproductive function. The only effects observed were
slightly retarded growth of pups at 1500 and 20 000 mg/kg feed and a
slight increase in neonatal mortality at 20 000 mg/kg feed. The NOEL
was 100 mg/kg feed, equivalent to 5 mg/kg bw per day, on the basis of
postnatal toxicity.
Fluazuron was not maternally toxic and did not cause
embryotoxicity, fetotoxicity, or teratogenicity in rats or rabbits at
oral doses up to 1000 mg/kg bw per day.
Because fluazuron belongs to a class of insect growth regulators
that do not act on the nervous system and because the central nervous
system was not a target organ in the short-term or long-term studies
of toxicity in rats, mice, or dogs, special studies of neurotoxicity
have not been performed. The Committee concluded that such studies
were unnecessary.
4. EVALUATION
The Committee established an ADI of 0-40 µg/kg bw for fluazuronâ
on the basis of the NOEL of 4.3 mg/kg bw per day for pathological
changes in the uterus in the two-year study in mice and a 100-fold
safety factor. The ADI was rounded to one significant figure, as is
the usual practice (Annex 1, reference 91, section 2.7).
5. REFERENCES
Bachmann, M., Hunter, B., Gretener, P., Krinke, G., Landes, C.,
Christen, P., Seewald, W., Heinemann, G.W. & Basler, W. (1991a) CGA
157419 tech: Lifetime carcinogenicity study in mice. Unpublished
report No. 860086 from Ciba-Geigy Ltd, Stein, Switzerland. Submitted
to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Bachmann, M., Hunter, B., Gretener, P., Froehlich, E., Faccini, J.M.,
Krinke, G., Christen, P., Seewald, W., Heinemann, G.W. & Basler, W.
(1991b) CGA 157419 tech: Carcinogenicity and chronic toxicity study in
rats. Unpublished report No. 860087 from Ciba-Geigy Ltd, Stein,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Barrow, P. & Briffaux, J.P. (1991) CGA 157419 tech: Two generation
oral (dietary administration) reproduction study in the rat.
Unpublished report No. 860089 from Hazleton France, L'Arbresle, France
(Hazleton report No. 710779). Submitted to WHO by Ciba-Geigy Ltd,
Basel, Switzerland.
Basler, W., Fankhauser, H., Gretener, P., Froehlich, E., Heider, K. &
Gfeller, W. (1987) CGA 157419 tech: 3-month oral toxicity study in
rats (administration in food). Unpublished report No. 860080 from
Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd, Basel, Switzerland.
Bloch, I., Frei, T., Luetkemeier, H. & Wilson, J. (1987) CGA 157419:
4-week oral range-finding toxicity (feeding) study in beagle dogs.
Unpublished report No. 860083 from Research & Consulting Company AG,
Itingen, Switzerland (RCC report No. 072977). Submitted to WHO by
Ciba-Geigy Ltd, Basel, Switzerland.
Bowen, F.L. & Strong, M.B. (1993) The tolerability of cattle to
exposure to the acarine growth regulator Acatak. Unpublished report
No. 93/3/1398 from Ciba-Geigy Ltd, Kemps Creek, Australia. Submitted
to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Briffaux, J.P. (1988a) CGA 157419 tech: 52 week oral (dietary
administration) toxicity study in the beagle dog. Interim report for
the first 13 weeks of the study. Unpublished report No. 860085 from
Hazleton France, L'Arbresle, France (Hazleton report No. 712829).
Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Briffaux, J.P. (1988b) CGA 157419: Preliminary study for a
2-generation oral (dietary administration) reproduction study in the
rat. Unpublished report No. 860400 from Hazleton France, L'Arbresle,
France (Hazleton report No. 703623). Submitted to WHO by Ciba-Geigy
Ltd, Basel, Switzerland.
Briffaux, J.P. (1990) CGA 157419 tech: 52 week oral (dietary
administration) toxicity study in the beagle dog. Unpublished report
No. 860085 from Hazleton France, L'Arbresle, France (Hazleton report
No. 094089). Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Cameron, B.D., Somers, K. & Speirs, G.C. (1992) The distribution and
excretion of [U-14C]Cl-phenyl CGA 157419 after subcutaneous
injection to cattle. Unpublished report No. 141232 from Inveresk
Research International, Tranent, Scotland, United Kingdom (Inveresk
report No. 6189). Submitted to WHO by Ciba-Geigy Ltd, Basel,
Switzerland.
Deparade, E. & Arni, P. (1985) Salmonella Cobas. Bact. pilot test with
CGA 157419 techn. (test for mutagenic effects on bacteria).
Unpublished report No. 850235 from Ciba-Geigy Ltd, Basel, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Dollenmeier, P. & Puri, E. (1987) CGA 157419 tech: V79 Chinese hamster
point mutation test (with and without microsomal activation).
Unpublished report No. 860071 from Ciba-Geigy Ltd, Basel, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Genchi, C. (1990) Tolerability of Acatik formulation A-8560 A in dogs.
Unpublished report from Facolta di Medicina Veterinaria, Universita
degli studi di Milano, Milan, Italy. Submitted to WHO by Ciba-Geigy
Ltd, Basel, Switzerland.
Gretener, P., Froehlich, E., Heider, K., Christen, P. & Gfeller, W.
(1988) CGA 157419 tech: 3-month subchronic oral toxicity study in
beagle dogs. Unpublished report No. 860084 from Ciba-Geigy Ltd, Stein,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Hartmann, H.R. & Schoch, M. (1987) CGA 157419 tech: Acute aerosol
inhalation toxicity in the rat. Unpublished report No. 860078 from
Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd, Basel, Switzerland.
Johnson, S., Johnston, A.M. & Prout, M.S. (1996)
[Chlor-phenyl-(U)-14C]-CGA 157419: Nature of metabolites in excreta
and tissues in ruminant cattle following single topical
administration. Unpublished report No. 156940 from Inveresk Research,
Tranent, Scotland, United Kingdom (Inveresk report No. 13026).
Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Maier, W. (1991a) CGA 157419: Determination of residues in whole blood
and fat of mice after subchronic and chronic exposure. Summary report
and assessment. Unpublished report from Ciba-Geigy Ltd, Basel,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Maier, W. (1991b) CGA 157419: Determination of residues in whole blood
and fat of rats after subchronic and chronic exposure. Summary report
and assessment. Unpublished report from Ciba-Geigy Ltd, Basel,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Maier, W. (1993) CGA 157419: Toxicological assessment of heat
degradation products. Unpublished report from Ciba-Geigy Ltd, Basel,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
McLean, C.L. & Dunsire, J.P. (1996) The absorption, distribution,
excretion, and residue depletion of [chlor-phenyl-(U)-14C]-CGA
157419 in ruminant cattle following topical administration.
Unpublished report No. 155601 from Inveresk Research, Tranent,
Scotland, United Kingdom (Inveresk report No. 12446). Submitted to WHO
by Ciba-Geigy Ltd, Basel, Switzerland.
Meyer, A. & Puri, E. (1987) CGA 157419 tech: Autoradiographic
DNA-repair test on human fibroblasts (OECD-conform). Unpublished
report No. 861231 from Ciba-Geigy Ltd, Basel, Switzerland. Submitted
to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Puri, E. & Muller, D. (1987) CGA 157419 tech: Autoradiographic
DNA-repair test on rat hepatocytes (OECD-conform). Unpublished report
No. 861230 from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO
by Ciba-Geigy Ltd, Basel, Switzerland.
Schoch, M. (1986a) CGA 157419 tech: Acute oral toxicity in the rat.
Unpublished report No. 860073 from Ciba-Geigy Ltd, Basel, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Schoch, M. (1986b) CGA 157419 tech: Acute dermal toxicity in the rat.
Unpublished report No. 860076 from Ciba-Geigy Ltd, Basel, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Schoch, M. (1986c) CGA 157419 tech: Acute eye irritation/corrosion
study in the rabbit. Unpublished report No. 860074 from Ciba-Geigy
Ltd, Basel, Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basel,
Switzerland.
Schoch, M. (1986d) CGA 157419 tech: Acute dermal irritation/corrosion
study in the rabbit. Unpublished report No. 860075 from Ciba-Geigy
Ltd, Basel, Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basel,
Switzerland.
Schoch, M. & Gfeller, W. (1987) CGA 157419 tech: Skin sensitization
test in the guinea pig (optimization test). Unpublished report No.
860066 from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd, Basel, Switzerland.
Schoch, M., Gretener, P., Froehlich, E., Germer, M., Krinke, G. &
Gfeller, W. (1988) CGA 157419 tech: 21-day repeated dose dermal
toxicity in the rat. Unpublished report No. 860077 from Ciba-Geigy
Ltd, Stein, Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basel,
Switzerland.
Schulze-Aurich, J. (1992a) Absorption, distribution, depletion of
residues and metabolic pathways of [U-14C]Cl-phenyl CGA 157419 in
the rat. Unpublished report No. 8/92 from Ciba-Geigy Ltd, Basel,
Switzerland. Submitted to WHO by Ciba- Geigy Ltd, Basel, Switzerland.
Schulze-Aurich, J. (1992b) The nature of the metabolites in tissues
and excreta of male cattle after single subcutaneous administration of
[U-14C]Cl-phenyl CGA 157419. Unpublished report No. 9/92 from
Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd, Basel, Switzerland.
Schulze-Aurich, J. (1992c) The fate of residues of [U-14C]Cl-phenyl
CGA 157419 in cattle tissues upon cooking. Unpublished report No.
10/92 from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd, Basel, Switzerland.
Strasser, F. & Arni, P. (1987) CGA 157419 tech: Nucleus anomaly test
in somatic interphase nuclei of Chinese hamster. Unpublished report
No. 860068 from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO
by Ciba-Geigy Ltd, Basel, Switzerland.
Strasser, F. & Muller, D. (1987) CGA 157419 tech: Chromosome studies
on human lymphocytes in vitro. Unpublished report No. 860070 from
Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd, Basel, Switzerland.
Strong, M.B. (1993) Effect of Acatak treatment on hide quality.
Unpublished report No. 93M/MBS/46 from Ciba-Geigy Ltd, Kemps Creek,
Australia. Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Strong, M.B. & Bowen, F.L. (1993) Reaction of cattle skin to Acatak.
Unpublished report No. 93/6/1410 from Ciba-Geigy Ltd, Kemps Creek,
Australia. Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Suarez, M. (1988) Acatik field trial. Unpublished report No. NPP 84.04
from Ciba-Geigy Ltd, Argentina. Submitted to WHO by Ciba-Geigy Ltd,
Basel, Switzerland.
Thevenaz, P., Gretener, P., Zak, F., Malinowski, W., Landes, C.,
Basler, W. & Gfeller, W. (1987) CGA 157419 tech: 28-day oral
cumulative toxicity study in rats (gavage). Unpublished report No.
860081 from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd, Basel, Switzerland.
Thomann, P. (1988) CGA 157419 tech: Developmental toxicity
(teratogenicity) study in rabbits. Unpublished report No. 860090 from
Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd, Basel, Switzerland.
Thouin, M.H. (1988) CGA 157419 tech: Teratogenicity study in rats.
Unpublished report No. 860088 from Ciba-Geigy Ltd, Basel, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
The nature of the residues in tissues and excreta of steers after
subcutaneous administration of fluazuron at 1.5 mg/kg bw was
investigated by thin-layer chromatography. The study was certified for
compliance with GLP and quality assurance. In all tissues taken at all
slaughter times, unchanged fluazuron was the only detectable fraction,
accounting for more than 90% of the total residues. In faeces, eight
metabolic fractions were found. The major compound was unchanged
fluazuron (about 70% of radiolabel), while the other, unidentified
fractions were more polar. Urine contained only unidentified
metabolized products, which were more polar than unchanged fluazuron.
Within 16 weeks of treatment, about 24% of the administered dose was
eliminated in faeces and urine, 16% as unchanged fluazuron and 8% as
its degradation products. Hence, fluazuron is metabolized to a lesser
extent in cattle than in rats (Schulze-Aurich, 1992b).
2.2 Toxicological studies
2.2.1 Acute toxicity
In studies of technical-grade fluazuron in distilled water
containing 0.5% carboxymethylcellulose and 0.1% polysorbate 80 in male
and female Tif:RAIf (SPF) rats, one study that followed OECD test
guideline 401 with quality assurance certification showed an oral
LD50 value of > 5000 mg/kg bw (Schoch, 1986a); in a study in the
same strain that followed OECD test guideline 402 with quality
assurance certification, the dermal LD50 was > 2000 mg/kg bw
(Schoch, 1986b). In a study that followed OECD test guideline 403, the
LC50 after exposure by inhalation was > 5994 mg/m3 (Hartmann &
Schoch, 1987). The toxic symptoms observed were sedation, dyspnoea,
exophthalmos, ruffled fur, and abnormal body positions. The animals
recovered within 6-11 days.
Three male New Zealand white rabbits received an instillation of
0.1 ml (56 mg) technical-grade fluazuron in a study that followed OECD
test guideline 405 with GLP and quality assurance certification. The
animals developed slight redness and chemosis of the cornea and
conjunctivae up to 24 h. No irritation of the iris was observed
(Schoch, 1986c).
In a study that followed OECD test guideline 404 with GLP and
quality assurance certification, three male New Zealand white rabbits
that received a semi-occlusive topical application of 0.5 g
technical-grade fluazuron developed no dermal reactions (Schoch,
1986d).
In 10 male and 10 female Pirbright white guinea-pigs submitted to
an optimization test, in a study that followed OECD test guideline 404
with GLP and quality assurance certification, no skin sensitization
was observed after challenge with technical-grade fluazuron
intradermally in 20% propylene glycol or epidermally in vaseline
(Schoch & Gfeller, 1987).
2.2.2 Short-term toxicity
Rats
In a study of dermal toxicity that followed OECD test guideline
410 with GLP and quality assurance certification, gauze patches
moistened with distilled water and technical-grade fluazuron at doses
of 0, 10, 100, or 1000 mg/kg bw were applied to the shaven skin of
groups of five male and five female Tif:RAIf (SPF) rats for 6 h/day,
five days per week for three weeks. The only effect observed was a
slight but significant prolongation of prothrombin time in male rats
at the two higher doses. The NOEL was 10 mg/kg bw per day (Schoch
et al., 1988).
Groups of 10 male and 10 female Tif:RAIf (SPF) rats received
technical-grade fluazuron by gavage for four weeks at intended doses
of 0, 10, 100, or 1000 mg/kg bw per day. The vehicle was distilled
water containing 0.5% carboxymethylcellulose and 0.1% Tween 80. Since
no clinical signs of toxicity were observed, the upper dose was
increased to 2000 mg/kg bw per day from day 10 onwards. The final
actual doses were 0, 3.2-5.6, 35-60, and 1660-1920 mg/kg bw per day.
The study was certified for compliance with GLP and quality assurance.
No treatment-related effects were seen on mortality rate,
clinical or ophthalmoscopic signs, body weight, or food or water
consumption, macroscopically or microscopically. Although treated
animals showed some statistically significant changes in blood
chemistry, these were not considered to be treatment-related (i.e. not
dose-related or of no biological relevance). All males at the medium
and high doses had a slight, statistically significant, dose-related
increase in prothrombin time and a slight, statistically significant,
dose-related decrease in platelet count. The mean liver weight was
increased in all treated animals, reaching statistical significance in
those at the two higher doses. Males at these doses also had a
decrease in mean thymus weights, which was statistically significant
only at the highest dose. These weight changes were not accompanied by
histopathological findings. The NOEL was 3.2-5.6 mg/kg bw per day, but
the validity of the study is limited due to the fact that the doses
intended to be the low and medium doses were not reached (Thevenaz
et al., 1987).
Groups of 20 male and 20 female Tif:RAIf (SPF) rats were fed
technical-grade fluazuron in the diet at concentrations of 0, 100,
600, 3500, or 20 000 mg/kg feed for three months, to give calculated
mean daily intakes of 0, 6.4, 39, 220, or 1300 mg/kg bw for males and
0, 6.6, 41, 240, or 1400 mg/kg bw for females. The study followed OECD
test guideline 408 with GLP and quality assurance certification.
No treatment-related effects were seen on mortality, clinical or
ophthalmoscopic signs, or macroscopically. Although body weight and
food consumption were marginally lower in treated males than in
controls, food conversion was not influenced. Treated males and
females showed some statistically significant changes in blood
chemistry; however, as these findings did not reflect any consistent
alteration (i.e. not dose-related or of no biological relevance), they
were not considered to be treatment-related. All treated males had
slightly increased prothrombin time (dose-related, statistically
significant in rats at 3500 and 20 000 mg/kg feed), in platelet count
(not dose-related, significant at 3500 mg/kg feed), and in lymphocyte
count (dose-related, significant at 20 000 mg/kg feed). The absolute
and relative weights of the liver and glycogen deposition were
significantly increased in all treated males, due, at least at 100
mg/kg feed, to poor study design, as sections were not made at random.
Changes in the absolute and relative weights of the heart (males),
kidney, ovaries, and adrenals (females) were not accompanied by
abnormal histopathological findings. Fluazuron treatment resulted in
slight to moderate hepatocellular hypertrophy in males and females at
3500 and 20 000 mg/kg feed and a slight increase in the incidence and
intensity of thyroid follicular hypertrophy and pituitary cell
hypertrophy in males at these doses. The authors considered the NOEL
to be 600 mg/kg feed (equal to 39-41 mg/kg bw per day); however,
effects on liver weight were observed in males at this dose. The
Committee concluded that the NOEL was 100 mg/kg feed, equal to 6.4
mg/kg bw per day, on the basis of effects on the liver. It should be
noted that similar findings were not observed in a long-term study of
toxicity and carcinogenicity in rats (see below) (Basler et al.,
1987).
Dogs
In a one-month range-finding study, groups of two male and two
female pure-bred beagle dogs were given technical-grade fluazuron at
doses of 200 or 50 000 mg/kg feed, equal to mean daily intakes of 8.5
or 2200 mg/kg bw per day, respectively. The study was certified for
compliance with GLP and quality assurance. No deaths occurred.
Clinical symptoms, food consumption, body-weight gain,
ophthalmoscopic, haematological, clinical, and urinary parameters, and
macroscopic and histopathological findings indicated no change that
could be considered to be related to treatment (Bloch et al., 1987).
In a three-month study, groups of four to six pedigree beagle
dogs of each sex were fed diets containing technical-grade fluazuron
at 0, 500, 5000, or 50 000 mg/kg feed. The study followed OECD test
guideline 409 with GLP and quality assurance certification. As several
animals in each group showed alterations indicative of an infection
(possibly leptospirosis) during the experiment, the study was not
considered useful for evaluating the safety of fluazuron. The only
findings of note that could not be attributed to the infectious
disease were degenerative, inflammatory, or hypertrophic changes in
the major arteries of females at 5000 and 50 000 mg/kg; however, this
effect was not confirmed in a 52-week feeding study with an interim
kill at 13 weeks (see below) (Gretener et al., 1988).
Groups of six male and six female beagle dogs received diets
containing technical-grade fluazuron at 0, 200, 3000, or 50 000 mg/kg
feed for one year. Two dogs of each sex per group were killed for
interim examinations after 13 weeks. The observations included
mortality, clinical signs, body weight, food consumption,
ophthalmoscopy, haematology, blood chemistry, urinalysis, organ
weights, and macroscopic and microscopic examinations. The calculated
mean intakes were 0, 7.5, 110, or 1900 mg/kg bw per day for males and
0, 7.1, 120, or 2000 mg/kg bw per day for females. The study followed
OECD test guideline 452 with GLP and quality assurance certification.
No deaths occurred. Treatment mainly affected two males at the high
dose (one killed at 13 weeks and the other at 52 weeks), which had
decreased food consumption, transient body-weight loss, and increased
activities of alkaline phosphatase and aspartate and alanine
aminotransferases from week 13 onwards; histopathological examination
revealed minimal multifocal areas of haemorrhage with slight
multifocal chronic inflammation of the liver. A slight increase in
alkaline phosphatase activity was also found in males at the middle
dose from week 13 onwards and in females at the high dose from week 39
onwards. Females at the high dose also had a slightly decreased
phosphorus concentration. The NOEL was 200 mg/kg feed, equal to 7.5
mg/kg bw per day, on the basis of changes in hepatic enzyme activities
(Briffaux, 1988a, 1990).
2.2.3 Long-term toxicity and carcinogenicity
Mice
Groups of 60 male and 60 female Tif: MAGf (SPF) mice were fed
diets containing technical-grade fluazuron at doses of 0, 40, 400,
4000, or 9000 mg/kg feed for two years, equal to average achieved
intakes of 0, 4.5, 45, 450, or 990 mg/kg bw per day for males and 0,
4.3, 43, 430, or 970 mg/kg bw per day for females. The observations
included clinical signs, mortality, body weight, food and water
consumption, haematological changes (10 animals of each sex per
group), and organ weights; detailed macroscopic and histopathological
examinations were perfomed. The study followed OECD test guideline 451
with GLP and quality assurance certification.
Clinical signs, body weight, and food consumption were unaffected
by treatment, as was survival (40-45% for controls, 43-58% for treated
animals). Water consumption was increased consistently in females at
9000 mg/kg feed and in females at 400 and 4000 mg/kg feed in the
second year of the study, but in females at 40 mg/kg feed and in all
treated males it was comparable to that of controls. Haematological
examination revealed no treatment-related changes, and the organ
weights and macroscopic findings at necropsy were comparable in
control and treated animals. The treatment-related non-neoplastic
changes included an increased incidence of cataract of the crystalline
lens characterized by mild necrosis and calcification of subcapsular
lens fibres in animals of each sex at 4000 and 9000 mg/kg feed and a
trend to increased diffuse hyperplasia of prostatic glandular tissue
in males at 4000 and 9000 mg/kg feed. In addition, the following
uterine changes, which were not dose-related, were noted: increased
incidences of inflammatory polyps at 400, 4000, and 9000 mg/kg feed,
increased dilatation of the lumen at 4000 and 9000 mg/kg feed, and
increased incidences of haematomas and dilatation of blood vessels
associated with thrombosis at 9000 mg/kg feed. Tumour incidences were
not increased, although a marginally increased incidence of systemic
infiltration with malignant lymphoma was observed in some organs of
some animals at the high dose. The total number of lymphomas per
animal and the total number of animals bearing lymphomas were not
significantly different from those among controls. On the basis of the
pathological changes in the uterus, the NOEL was 40 mg/kg feed, equal
to 4.3 mg/kg bw per day (Bachmann et al., 1991a).
In the same study, the total amount of fluazuron in the body
appeared to reach a maximum at approximately 400 mg/kg feed: the
fluazuron level was 4.9-8.7 µg/ml in blood and 880-970 mg/kg in fat in
animals of each sex at 4000 and 9000 mg/kg feed and only slightly
lower in those at 400 mg/kg feed. At 40 mg/kg feed, a steady state was
not reached. The blood:fat ratio was approximately 1:200 in all groups
(Maier, 1991a).
Rats
Groups of 80 male and 80 female Tif:RAIf (SPF) rats received
diets containing technical-grade fluazuron at 0, 50, 500, 10 000, or
20 000 mg/kg feed for two years, equal to average achieved intakes of
0, 1.9, 18, 380, or 780 mg/kg bw per day for males and 0, 2.1, 21,
440, or 920 mg/kg bw per day for females. Ten rats of each sex were
killed after one year for interim necropsy. The observations included
clinical signs, mortality, body weight, food consumption,
ophthalmoscopy, haematology (20 rats of each sex per group), blood
chemistry (10 of each sex per group), urinalysis (10 of each sex per
group), and organ weights; detailed macroscopic and histopathological
examinations were performed. The study followed OECD test guideline
453 with GLP and quality assurance certification.
The survival of the animals (56-67% for controls, 54-73% for
treated animals) was not affected by treatment. Minor effects were
observed in animals at the highest dose at interim sacrifice but not
at terminal sacrifice. In females, the relative liver and kidney
weights were significantly decreased, with no associated morphological
changes; in males, minimal hypertrophy of hepatocytes was seen.
Females at the highest dose also had decreased body-weight gain during
the second year of treatment, but this failed to reach statistical
significance. The findings were considered not to be of toxicological
significance. The incidence of tumours was not increased. The total
amount of fluazuron in the body appeared to reach a maximum at 500
mg/kg feed: the level was 1.3-2.3 µg/ml in blood and 290-440 mg/kg in
fat in animals of each sex at 500 mg/kg feed and above. At 50 mg/kg
feed, a steady state was not reached. The blood:fat ratio was
approximately 1:200 in all groups (Bachmann et al., 1991b; Maier,
1991b).
2.2.4 Genotoxicity
The results of tests for genotoxicity carried out with fluazuron
are summarized in Table 1.
2.2.5 Reproductive toxicity
2.2.5.1 Multigeneration reproductive toxicity
In a preliminary study of reproductive toxicity, groups of seven
male and 14 female Ico:OFA Sprague-Dawley rats received
technical-grade fluazuron at dietary concentrations of 0, 200, 1000,
7000, or 20 000 mg/kg feed. Males were dosed for two weeks before
mating and during the mating period and were sacrificed thereafter.
Females were dosed from two weeks before mating up to 21 days post
partum, when both the females and their litters were sacrificed. The
study was certified for quality assurance.
There were no deaths or treatment-related clinical signs in
animals of either sex during the study. The food consumption and
body-weight gain of F0 animals and the precoital period and duration
of the gestation period were not affected by treatment, and no effects
were seen on insemination, fecundity, fertility, resorption indices,
stillbirths, nursing behaviour, the numbers of live F1 pups, their
physical development, or neonatal or postnatal mortality. The mean
weight at birth of F1 pups was not affected by treatment, but a
slight retardation in growth rate was seen towards the end of the
lactation period in animals at 7000 and 20 000 mg/kg feed. No
treatment-related effects were seen in any animal at necropsy
(Briffaux, 1988b).
In a two-generation study of reproductive toxicity, groups of 30
male and 30 female Ico:OFA Sprague-Dawley rats received diets
containing technical-grade fluazuron at 0, 100, 1500, or 20 000 mg/kg
feed for at least 100 days before mating and then throughout gestation
and lactation for two successive generations. Two litters were bred
per generation. Males and females of the first F1 litter (F1a)
were selected to breed the next generation. After weaning of the F1b
and F2b pups, the parental animals were killed and necropsied.
Unselected F1a pups and F1b, F2a, and F2b pups were killed and
necropsied after 21 days of lactation. The study followed OECD test
guideline 416 with GLP and quality assurance certification.
The only effects found were on food consumption and body weight,
not on reproductive function. F1 females at the high dose showed a
slight reduction in body weight in the first gestation period, and the
food consumption of those at the middle and high doses tended to be
slightly reduced at the end of both lactation periods. Neonatal
viability of both generations of rats at the high dose was slightly
reduced after the first matings, but the litters from subsequent
matings were not affected. Pup viability from day 4 post partum to
weaning was not affected by treatment during any phase of the study.
The body weights at birth of pups in all litters were comparable to
those of controls, but the body-weight gain of those at the high dose
was reduced for all generations (F1a, F1b, F2a, F2b) and of
those at the middle dose for the F1a, F2a, and F2b generations.
The NOEL was 100 mg/kg feed, equivalent to 5 mg/kg bw per day, on the
basis of postnatal toxicity (Barrow & Briffaux, 1991).
2.2.5.2 Developmental toxicity
Rats
Technical-grade fluazuron was administered by gavage in a 0.1%
aqueous solution of polysorbate 80 at doses of 0, 10, 100, or 1000
mg/kg bw per day to groups of 24 mated female Tif:RAIf (SPF) rats on
days 6-15 of gestation. On day 21 of gestation, the dams were killed
and necropsied, and the fetuses were weighed, sexed, and examined for
external, visceral, and skeletal abnormalities. The study followed
OECD test guideline 414 with GLP and quality assurance certification.
One dam at the middle dose aborted its entire litter. No
treatment-related effects were observed in dams, and the numbers of
implantations, live young, and resorptions and fetal and litter
weights were unaffected by treatment. There was no indication of
teratogenicity. Hence, fluazuron at doses up to 1000 mg/kg bw per day
had no maternal, embryo- or fetal toxicity or teratogenicity (Thouin,
1988).
Rabbits
Groups of 20 mated female Chinchilla-type rabbits received
technical-grade fluazuron suspended in 3% (w/w) aqueous corn starch by
gavage at doses of 0, 10, 100, or 1000 mg/kg bw per day on days 7-19
of pregnancy. The dams were killed and necropsied on day 29 of
pregnancy, and various parameters examined. Fetuses were subjected to
gross necropsy and visceral and skeletal examination. The study
followed OECD test guideline 414 with GLP and quality assurance
certification. One female at the high dose died due to faulty
intubation, and another resorbed all of its implants. The incidence of
post-implantation loss was slightly increased in all treated groups,
mainly due to a slightly higher rate of early resorptions, but was
still within the historical control range and was considered not to be
related to treatment. The number of live fetuses and the litter weight
were unaffected by treatment. The body weights of the fetuses of all
treated dams were slightly increased; the increases in group means
were statistically significant but the litter means were not. No other
effects were observed. Hence, at doses up to 1000 mg/kg bw per day
fluazuron induced no maternal, embryo or fetal toxicity and was not
teratogenic (Thomann, 1988).
Table 1. Results of assays for genotoxicity with fluazuron
End-point Test object Concentration Result S9 QA Reference
In vitro
Reverse S. typhimurium 1.14-278 µg/mla Negative + No Deparade &
mutation TA98, TA100, Negative - Arni (1985)
TA1535, TA1537
Gene V79 Chinese 12.5-500 ng/ml Negative + Yes Dollenmeier &
mutation hamster cells 0.625-25 µg/ml Negative - Puri (1987)
Chromosomal Human lymphocytes 14.1-225 µg/ml Negative + Yes Strasser &
aberration 7.5-120.0 µg/ml Negative - Muller (1987)
DNA repair Rat hepatocytes 0.4-300 µg/ml Negative Yes Puri & Muller (1987)
DNA repair Human fibroblasts 0.2-50 µg/ml Negative Yes Meyer & Puri (1987)
In vivo
Nuclear Chinese hamster 1.25-5.0 g/kg bwb Negativec Yes Strasser & Arni
anomalies bone marrow (1987)
S9, 9000 × g fraction of rat liver; QA, quality assurance
a No cytotoxicity, but precipitation occurred at the highest dose in the presence of S9
b Daily doses given by gavage on each of two consecutive days; cyclophosphamide used as a positive control
c The authors considered 5.0 g/kg bw to be the highest applicable dose, but as cytotoxicity was not
determined it was not clear whether the bone marrow was exposed.
2.2.6 Special studies on target animals
In a study of tolerability, groups of three male and three female
Hereford cattle received the pour-on formulation Acatak at 0
(control), once, three times, or five times the recommended dose of 2
mg/kg bw fluazuron, along the back from the neck to the rump. The
animals were observed for the following eight weeks and monitored for
subclinical side-effects. The study was certified for quality
assurance. Apart from the formation of a crusty layer on the skin and
coat, which caused no irritation or discomfort to the animals, the
formulation was well tolerated at all doses. No drug-related changes
were observed in body weight, feed consumption, body temperature, or
haematological or blood biochemical parameters (Bowen & Strong, 1993).
When the label instructions for application of the pour-on formulation
Acatak were followed, a crusty layer formed on the coats of the
cattle, but the animals displayed no clinical signs of irritation.
Histopathological examination showed only minor pathological changes
in the skin, which had no effect on the hide quality (Strong, 1993;
Strong & Bowen, 1993).
Subcutaneous injection of Acatik, a commercial formulation
containing fluazuron as the active ingredient, induced no side-effects
or local reactions at the injection site in cattle receiving single
doses of 1 mg/kg bw fluazuron or dogs receiving double doses of 2.5
and then 5 mg/kg bw (Suarez, 1988; Genchi, 1990).
2.2.7 Special studies on heat degradation
The degradation of fluazuron residues present in cattle meat and
fat was investigated after normal dry cooking, cooking in water or
oil, or microwave treatment. The meat and fat samples were derived
from steers treated with fluazuron at 1.5 mg/kg bw by subcutaneous
injection, which is not the recommended route of administration. The
study was certified for compliance with GLP and quality assurance.
Residues of fluazuron were partly (in meat) or completely (in fat)
degraded to [3-(3-chloro-5-trifluoro-methyl-2-pyridinyloxy)-4-
chlorophenyl]-1-amine. This degradation product did not show mutagenic
potential in an assay for reverse mutation in Salmonella typhimurium
strains TA98, TA100, TA1535, and TA1537 or in Escherichia coli
strain WP2uvrA, when the known mutagen found in cooked meat, MeIQx
(2-amino-3,8- dimethylimidaz[4,5-f]quinoxaline), was used as the
reference substance (Schulze-Aurich, 1992c; Maier, 1993).
3. COMMENTS
The Committee considered the results of studies on the
pharmacokinetics, metabolism, acute, short-term and long-term
toxicity, carcinogenicity, genotoxicity, and reproductive toxicity of
fluazuron. All of the studies critical for the evaluation were carried
out according to appropriate standards for study protocol and conduct.
Analysis 24 h after oral administration of radiolabelled
fluazuron to rats showed that an average of 60% was absorbed through
the gut. Most of the absorbed radiolabel was taken up into adipose
tissues, while significantly lower levels were found in liver, kidney,
lung, muscle, and brain. Ninety percent of the radiolabel in tissues
was attached to unchanged fluazuron. Fluazuron was eliminated slowly
from adipose tissues, following first-order kinetics, with a half-life
of about 13 days. Elimination occurred primarily in the faeces. One
week after administration, 59% had been excreted in faeces and 3% in
urine. About one-third of the dose was eliminated as unchanged
fluazuron in the faeces; the remaining two-thirds was metabolized by
cleavage of the urea moiety between the benzoyl carbon and the urea
nitrogen, followed by hydroxylation in the phenyl ring.
When radiolabelled fluazuron was administered topically to
cattle, radiolabel was slowly absorbed, either percutaneously, orally
(by licking), or by both routes. A steady state between absorption and
elimination was observed for three to four weeks after treatment. The
absorbed radiolabel was taken up mainly by adipose tissues and to a
lesser extent by other tissues. Depletion of fluazuron from plasma and
tissues was slow, with half-lives of elimination of 10.5 and 4.5-5.5
weeks, respectively. The major route of elimination was the faeces
(62% of the dose within 16 weeks), while renal excretion was of minor
importance (1% of the dose within 16 weeks). There was some indication
of biliary excretion. Fluazuron was not extensively metabolized, as
unchanged fluazuron accounted for more than 90% of the total residues
in tissues and faeces. A similar slow absorption, distribution, and
elimination pattern was observed after subcutaneous administration of
radiolabelled fluazuron to steers. The pattern of metabolites excreted
in faeces was somewhat more complex, however, indicating that about
one-third of the fluazuron was metabolized into more polar
metabolites. Although the fate of fluazuron in rats was similar to
that in cattle, they metabolized fluazuron to a greater extent than
cattle.
Orally administered fluazuron was found to have low acute
toxicity, with an LD50 value of > 5000 mg/kg bw in rats.
The short-term toxicity of fluazuron was evaluated by oral
administration to rats and dogs. In a 28-day study, rats received
fluazuron by gavage at doses of 0, 10, 100, or 2000 mg/kg bw per day.
Increased prothrombin time and liver weight and decreased platelet
counts and thymus weight were observed, mainly in animals at the two
higher doses, and particularly in males. Male rats were also more
sensitive than females to the effects of fluazuron in a 13-week
feeding study at dietary concentrations of 0, 100, 600, 3500, or
20 000 mg/kg feed (equal to 6.4-1400 mg/kg bw per day). Male rats at
the two higher doses had increased prothrombin time, platelet and
lymphocyte counts, and absolute and relative liver weights, as well as
hypertrophy of thyroid follicular cells, pituitary cells, and
hepatocytes. In males, increases in absolute and relative liver
weights were also observed at 600 mg/kg feed. In females,
hepatocellular hypertrophy occurred at 3500 and 20 000 mg/kg feed. The
NOEL was 100 mg/kg feed, equal to 6.4 mg/kg bw per day, on the basis
of effects on the liver.
In a one-year feeding study, dogs received fluazuron at dietary
concentrations of 0, 200, 3000, or 50 000 mg/kg feed. Effects were
observed primarily in males at the highest dose, consisting of
decreased food consumption, transient body-weight loss, increased
serum activities of alkaline phosphatase and aspartate and alanine
aminotransferases, and minimal multifocal haemorrhage with slight
multifocal chronic inflammation in the liver. A slight increase in
alkaline phosphatase activity was also observed in males at 3000 mg/kg
feed and in females at 50 000 mg/kg feed. The NOEL was 200 mg/kg feed,
equal to 7.5 mg/kg bw per day, on the basis of changes in hepatic
enzyme activities.
In a study of carcinogenicity, mice received diets containing 0,
40, 400, 4000, or 9000 mg/kg feed for two years (equal to 4.3-990
mg/kg bw per day). Females at the two higher levels showed increased
water consumption, cataracts, and uterine changes (inflammatory
polyps, luminal dilatation and, at 9000 mg/kg feed only, haematomas
and dilatation of blood vessels associated with thrombosis). Increased
water consumption and inflammatory uterine polyps were also observed
in females at 400 mg/kg feed. Male mice at the two higher levels had
cataracts, and a trend to an increasing frequency of diffuse
hyperplasia of prostatic glandular tissue was observed. The NOEL was
40 mg/kg feed, equal to 4.3 mg/kg bw per day, on the basis of
pathological changes in the uterus.
In a long-term study of toxicity and carcinogenicity, rats were
given fluazuron at 0, 50, 500, 10 000, or 20 000 mg/kg feed for two
years (equal to 1.9-920 mg/kg bw per day), and a proportion of the
animals were killed at one year. No toxicologically significant
effects were observed at any dose. The toxic effects on the liver
observed in the 13-week study in rats were not observed after either
one or two years.
In the long-term studies of toxicity, the total amount of
fluazuron in the body appeared to reach a maximum at relatively low
levels (approximately 400 and 500 mg/kg feed, equal to 43 and 18 mg/kg
bw per day, in mice and rats, respectively), as the concentration of
the compound in blood and fat did not increase at higher levels. The
reason is not clear. It is not known whether a similar effect occurred
in the short-term studies, because blood and fat levels were not
measured.
Fluazuron has been tested in vitro for its ability to induce
reverse mutations in Salmonella typhimurium, gene mutations in Chinese
hamster cells, chromosomal aberration in human lymphocytes, and DNA
repair in rat hepatocytes and human fibroblasts. It has also been
tested in vivo for its ability to induce nuclear anomalies in
Chinese hamster bone marrow. All the results were negative. On the
basis of these data and the results of the bioassays in rodents, the
Committee concluded that fluazuron has no genotoxic or carcinogenic
potential.
In a two-generation study of reproductive toxicity in rats at
dietary concentrations of 0, 100, 1500, or 20 000 mg/kg feed
(equivalent to 5-1000 mg/kg bw per day), fluazuron had no adverse
effects on reproductive function. The only effects observed were
slightly retarded growth of pups at 1500 and 20 000 mg/kg feed and a
slight increase in neonatal mortality at 20 000 mg/kg feed. The NOEL
was 100 mg/kg feed, equivalent to 5 mg/kg bw per day, on the basis of
postnatal toxicity.
Fluazuron was not maternally toxic and did not cause
embryotoxicity, fetotoxicity, or teratogenicity in rats or rabbits at
oral doses up to 1000 mg/kg bw per day.
Because fluazuron belongs to a class of insect growth regulators
that do not act on the nervous system and because the central nervous
system was not a target organ in the short-term or long-term studies
of toxicity in rats, mice, or dogs, special studies of neurotoxicity
have not been performed. The Committee concluded that such studies
were unnecessary.
4. EVALUATION
The Committee established an ADI of 0-40 µg/kg bw for fluazuronâ
on the basis of the NOEL of 4.3 mg/kg bw per day for pathological
changes in the uterus in the two-year study in mice and a 100-fold
safety factor. The ADI was rounded to one significant figure, as is
the usual practice (Annex 1, reference 91, section 2.7).
5. REFERENCES
Bachmann, M., Hunter, B., Gretener, P., Krinke, G., Landes, C.,
Christen, P., Seewald, W., Heinemann, G.W. & Basler, W. (1991a) CGA
157419 tech: Lifetime carcinogenicity study in mice. Unpublished
report No. 860086 from Ciba-Geigy Ltd, Stein, Switzerland. Submitted
to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Bachmann, M., Hunter, B., Gretener, P., Froehlich, E., Faccini, J.M.,
Krinke, G., Christen, P., Seewald, W., Heinemann, G.W. & Basler, W.
(1991b) CGA 157419 tech: Carcinogenicity and chronic toxicity study in
rats. Unpublished report No. 860087 from Ciba-Geigy Ltd, Stein,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Barrow, P. & Briffaux, J.P. (1991) CGA 157419 tech: Two generation
oral (dietary administration) reproduction study in the rat.
Unpublished report No. 860089 from Hazleton France, L'Arbresle, France
(Hazleton report No. 710779). Submitted to WHO by Ciba-Geigy Ltd,
Basel, Switzerland.
Basler, W., Fankhauser, H., Gretener, P., Froehlich, E., Heider, K. &
Gfeller, W. (1987) CGA 157419 tech: 3-month oral toxicity study in
rats (administration in food). Unpublished report No. 860080 from
Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd, Basel, Switzerland.
Bloch, I., Frei, T., Luetkemeier, H. & Wilson, J. (1987) CGA 157419:
4-week oral range-finding toxicity (feeding) study in beagle dogs.
Unpublished report No. 860083 from Research & Consulting Company AG,
Itingen, Switzerland (RCC report No. 072977). Submitted to WHO by
Ciba-Geigy Ltd, Basel, Switzerland.
Bowen, F.L. & Strong, M.B. (1993) The tolerability of cattle to
exposure to the acarine growth regulator Acatak. Unpublished report
No. 93/3/1398 from Ciba-Geigy Ltd, Kemps Creek, Australia. Submitted
to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Briffaux, J.P. (1988a) CGA 157419 tech: 52 week oral (dietary
administration) toxicity study in the beagle dog. Interim report for
the first 13 weeks of the study. Unpublished report No. 860085 from
Hazleton France, L'Arbresle, France (Hazleton report No. 712829).
Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Briffaux, J.P. (1988b) CGA 157419: Preliminary study for a
2-generation oral (dietary administration) reproduction study in the
rat. Unpublished report No. 860400 from Hazleton France, L'Arbresle,
France (Hazleton report No. 703623). Submitted to WHO by Ciba-Geigy
Ltd, Basel, Switzerland.
Briffaux, J.P. (1990) CGA 157419 tech: 52 week oral (dietary
administration) toxicity study in the beagle dog. Unpublished report
No. 860085 from Hazleton France, L'Arbresle, France (Hazleton report
No. 094089). Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Cameron, B.D., Somers, K. & Speirs, G.C. (1992) The distribution and
excretion of [U-14C]Cl-phenyl CGA 157419 after subcutaneous
injection to cattle. Unpublished report No. 141232 from Inveresk
Research International, Tranent, Scotland, United Kingdom (Inveresk
report No. 6189). Submitted to WHO by Ciba-Geigy Ltd, Basel,
Switzerland.
Deparade, E. & Arni, P. (1985) Salmonella Cobas. Bact. pilot test with
CGA 157419 techn. (test for mutagenic effects on bacteria).
Unpublished report No. 850235 from Ciba-Geigy Ltd, Basel, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Dollenmeier, P. & Puri, E. (1987) CGA 157419 tech: V79 Chinese hamster
point mutation test (with and without microsomal activation).
Unpublished report No. 860071 from Ciba-Geigy Ltd, Basel, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Genchi, C. (1990) Tolerability of Acatik formulation A-8560 A in dogs.
Unpublished report from Facolta di Medicina Veterinaria, Universita
degli studi di Milano, Milan, Italy. Submitted to WHO by Ciba-Geigy
Ltd, Basel, Switzerland.
Gretener, P., Froehlich, E., Heider, K., Christen, P. & Gfeller, W.
(1988) CGA 157419 tech: 3-month subchronic oral toxicity study in
beagle dogs. Unpublished report No. 860084 from Ciba-Geigy Ltd, Stein,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Hartmann, H.R. & Schoch, M. (1987) CGA 157419 tech: Acute aerosol
inhalation toxicity in the rat. Unpublished report No. 860078 from
Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd, Basel, Switzerland.
Johnson, S., Johnston, A.M. & Prout, M.S. (1996)
[Chlor-phenyl-(U)-14C]-CGA 157419: Nature of metabolites in excreta
and tissues in ruminant cattle following single topical
administration. Unpublished report No. 156940 from Inveresk Research,
Tranent, Scotland, United Kingdom (Inveresk report No. 13026).
Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Maier, W. (1991a) CGA 157419: Determination of residues in whole blood
and fat of mice after subchronic and chronic exposure. Summary report
and assessment. Unpublished report from Ciba-Geigy Ltd, Basel,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Maier, W. (1991b) CGA 157419: Determination of residues in whole blood
and fat of rats after subchronic and chronic exposure. Summary report
and assessment. Unpublished report from Ciba-Geigy Ltd, Basel,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Maier, W. (1993) CGA 157419: Toxicological assessment of heat
degradation products. Unpublished report from Ciba-Geigy Ltd, Basel,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
McLean, C.L. & Dunsire, J.P. (1996) The absorption, distribution,
excretion, and residue depletion of [chlor-phenyl-(U)-14C]-CGA
157419 in ruminant cattle following topical administration.
Unpublished report No. 155601 from Inveresk Research, Tranent,
Scotland, United Kingdom (Inveresk report No. 12446). Submitted to WHO
by Ciba-Geigy Ltd, Basel, Switzerland.
Meyer, A. & Puri, E. (1987) CGA 157419 tech: Autoradiographic
DNA-repair test on human fibroblasts (OECD-conform). Unpublished
report No. 861231 from Ciba-Geigy Ltd, Basel, Switzerland. Submitted
to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Puri, E. & Muller, D. (1987) CGA 157419 tech: Autoradiographic
DNA-repair test on rat hepatocytes (OECD-conform). Unpublished report
No. 861230 from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO
by Ciba-Geigy Ltd, Basel, Switzerland.
Schoch, M. (1986a) CGA 157419 tech: Acute oral toxicity in the rat.
Unpublished report No. 860073 from Ciba-Geigy Ltd, Basel, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Schoch, M. (1986b) CGA 157419 tech: Acute dermal toxicity in the rat.
Unpublished report No. 860076 from Ciba-Geigy Ltd, Basel, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Schoch, M. (1986c) CGA 157419 tech: Acute eye irritation/corrosion
study in the rabbit. Unpublished report No. 860074 from Ciba-Geigy
Ltd, Basel, Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basel,
Switzerland.
Schoch, M. (1986d) CGA 157419 tech: Acute dermal irritation/corrosion
study in the rabbit. Unpublished report No. 860075 from Ciba-Geigy
Ltd, Basel, Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basel,
Switzerland.
Schoch, M. & Gfeller, W. (1987) CGA 157419 tech: Skin sensitization
test in the guinea pig (optimization test). Unpublished report No.
860066 from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd, Basel, Switzerland.
Schoch, M., Gretener, P., Froehlich, E., Germer, M., Krinke, G. &
Gfeller, W. (1988) CGA 157419 tech: 21-day repeated dose dermal
toxicity in the rat. Unpublished report No. 860077 from Ciba-Geigy
Ltd, Stein, Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basel,
Switzerland.
Schulze-Aurich, J. (1992a) Absorption, distribution, depletion of
residues and metabolic pathways of [U-14C]Cl-phenyl CGA 157419 in
the rat. Unpublished report No. 8/92 from Ciba-Geigy Ltd, Basel,
Switzerland. Submitted to WHO by Ciba- Geigy Ltd, Basel, Switzerland.
Schulze-Aurich, J. (1992b) The nature of the metabolites in tissues
and excreta of male cattle after single subcutaneous administration of
[U-14C]Cl-phenyl CGA 157419. Unpublished report No. 9/92 from
Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd, Basel, Switzerland.
Schulze-Aurich, J. (1992c) The fate of residues of [U-14C]Cl-phenyl
CGA 157419 in cattle tissues upon cooking. Unpublished report No.
10/92 from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd, Basel, Switzerland.
Strasser, F. & Arni, P. (1987) CGA 157419 tech: Nucleus anomaly test
in somatic interphase nuclei of Chinese hamster. Unpublished report
No. 860068 from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO
by Ciba-Geigy Ltd, Basel, Switzerland.
Strasser, F. & Muller, D. (1987) CGA 157419 tech: Chromosome studies
on human lymphocytes in vitro. Unpublished report No. 860070 from
Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd, Basel, Switzerland.
Strong, M.B. (1993) Effect of Acatak treatment on hide quality.
Unpublished report No. 93M/MBS/46 from Ciba-Geigy Ltd, Kemps Creek,
Australia. Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Strong, M.B. & Bowen, F.L. (1993) Reaction of cattle skin to Acatak.
Unpublished report No. 93/6/1410 from Ciba-Geigy Ltd, Kemps Creek,
Australia. Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Suarez, M. (1988) Acatik field trial. Unpublished report No. NPP 84.04
from Ciba-Geigy Ltd, Argentina. Submitted to WHO by Ciba-Geigy Ltd,
Basel, Switzerland.
Thevenaz, P., Gretener, P., Zak, F., Malinowski, W., Landes, C.,
Basler, W. & Gfeller, W. (1987) CGA 157419 tech: 28-day oral
cumulative toxicity study in rats (gavage). Unpublished report No.
860081 from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd, Basel, Switzerland.
Thomann, P. (1988) CGA 157419 tech: Developmental toxicity
(teratogenicity) study in rabbits. Unpublished report No. 860090 from
Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd, Basel, Switzerland.
Thouin, M.H. (1988) CGA 157419 tech: Teratogenicity study in rats.
Unpublished report No. 860088 from Ciba-Geigy Ltd, Basel, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
 Fluazuron belongs to the class of benzoylphenyl urea derivatives,
which inhibit the growth and development of arthropod insects and
members of the order Acarina by acting on chitin formation. These
'insect growth regulators' do not act on the insect nervous system.
Fluazuron specifically affects ticks. Chitin synthesis in ticks occurs
during engorgement, moulting, and embryogenesis. Female ticks that
have absorbed a minimal amount of fluazuron show signs of disturbed
engorgement and lay eggs from which either no or non-viable larvae
hatch. Male ticks that take up minimal amounts of plasma are not
visibly affected by the compound. Fluazuron interrupts the life cycle
of immature ticks by interfering with cuticle formation during
engorgement and moulting. The affected ticks die.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
Rats
Fluazuron uniformly radiolabelled with 14C in the
4-chlorophenyl moiety ([U-14C-Cl]phenyl-labelled fluazuron; specific
activity, 48.1 µCi/mg) was administered orally in
N-methylpyrrolidone and PEG 200 by stomach tube to Tif:RAIf (SPF)
rats at a dose of 0.5 mg/kg bw per day for one week. Three rats of
each sex were killed 24 h and 2, 4, 8, and 12 weeks after the final
dose. Urine and faeces were collected daily during treatment and for
one week thereafter. After sacrifice, subcutaneous, renal, and
abdominal fat, blood, brain, kidneys, skeletal muscle, liver, and the
remaining carcass were sampled for radiolabel with a liquid
scintillation counter. The study was certified for compliance with GLP
and quality assurance.
By 24 h after the last dose, about 60% had been absorbed from the
gastrointestinal tract into the general circulation, as calculated
from the amount of radiolabel in urine, tissues, and carcass. During
the administration and observation periods, a total of 62% of the
administered dose was excreted, with 59% via the faeces and only 3%
via the urine. The extent of absorption and the route and rate of
excretion were independent of sex. At 24 h, the highest residue levels
were found in the adipose tissues (12-18 mg/kg fluazuron equivalents)
and significantly lower levels in other tissues: liver, 1.3; kidney,
0.84; lungs, 0.53; muscle, 0.39; and brain, 0.20 mg/kg. The same
distribution was seen at all time points. By 12 weeks after the last
dose, the amount of residual radiolabel had declined to 0.15-0.26
mg/kg fluazuron equivalents in adipose tissues and to < 0.03 mg/kg in
all other tissues. The depletion of radiolabel from all tissues
followed first-order kinetics, with a half-time of about 13 days for
all tissues in animals of each sex. The ratio in fat:blood (201 ± 28)
was relatively constant over the experimental period (Schulze-Aurich,
1992a).
Cattle
Male cross-bred beef cattle received single topical applications
of [U-14C-Cl]phenyl-labelled fluazuron (as the commercial pour-on
formulation Acatak; specific activity, 4.86 µCi/mg) at a dose of 1.5
mg/kg bw, administered on both sides of the spine between the shoulder
and the rump. The animals were slaughtered in groups of three during
weeks 2, 4, 6, and 16 after treatment. Blood, urine, and faeces were
collected from the animals slaughtered at the end of the study at
several times after treatment. At slaughter, samples were taken of
ventral and dorsal subcutaneous, renal, and omental fat; blood; brain;
kidney; hindquarter, forequarter, and tenderloin muscle; liver; bile;
and skin at the site of administration. All samples were analysed for
radiolabel with a liquid scintillation counter. The study was
certified for compliance with GLP and quality assurance.
The total intake was at least 60% of the administered dose, as
indicated by urinary and faecal excretion. It was not clear, however,
whether intake was via the dermal and/or the oral route (licking); the
portion absorbed into the systemic circulation was also unknown.
Fluazuron was slowly absorbed and distributed to tissues. Radiolabel
was initially observed in plasma 16 h after treatment. The mean plasma
levels remained fairly constant between 9 and 35 days after treatment,
ranging from 0.035 to 0.041 mg/litre fluazuron equivalents. The levels
declined thereafter, with a mean elimination half-time of about 73
days, to a mean level of 0.007 mg/litre 16 weeks after treatment. The
main route of elimination was the faeces (40% of the administered dose
within the first four weeks, increasing gradually to 62% by 16 weeks),
whereas renal excretion was of minor importance (1% of the dose after
16 weeks). There was some indication of biliary excretion. Maximum
residue levels were found two weeks after administration in almost all
tissues. The levels were highest in renal fat (4.8 mg/kg fluazuron
equivalents), omental fat (4.3 mg/kg), subcutaneous fat (ventral: 3.9
mg/kg; dorsal: 2.8 mg/kg), and skin (3 mg/kg). Lower levels were found
in liver (0.5 mg/kg), kidney (0.4 mg/kg), muscle (0.1 mg/kg), and
brain (0.08 mg/kg). The levels decreased slowly up to 16 weeks after
treatment, when they were 0.5-0.6 mg/kg in fat, 0.05-0.06 mg/kg in
liver and kidney, and 0.01-0.02 mg/kg in muscle and brain. The
depletion half-times for the different tissues varied from 4.5 to 5.5
weeks, but that in skin was 1.5 weeks (McLean & Dunsire, 1996).
Castrated Hereford steers received single subcutaneous injections
of [U-14C-Cl]phenyl-labelled fluazuron in a vehicle consisting of
PEG 200 dilaurate, Cremophor EL, citric acid, and
N-methyl-2-pyrrolidone (specific activity, 3.95 µCi/mg) behind the
left shoulder at a dose of 1.5 mg/kg bw. The animals were slaughtered
in groups of three, two days and 2, 6, and 16 weeks after treatment.
Blood, urine, and faeces were collected at several times after
treatment. At slaughter, samples were taken of subcutaneous, renal,
and omental fat; blood; brain; kidney; hindquarter and forequarter
muscle; liver; bile; and skin at the site of administration. All
samples were analysed for radiolabel with a liquid scintillation
counter. The study was certified for compliance with GLP and quality
assurance.
The compound was absorbed slowly from the injection site, the
mean maximum plasma level of 0.1 mg/litre fluazuron equivalents being
reached after 48 h. The plasma levels declined with a mean elimination
half-time of about 78 days; by 16 weeks after treatment, the mean
plasma level was still 0.01 mg/litre. The main route of elimination
was the faeces (23% of the administered dose after 16 weeks), whereas
renal excretion was of minor importance (1% of the dose after 16
weeks). There was some indication of biliary excretion. In all tissues
except subcutaneous fat, maximum residue levels were found 48 h after
administration. The levels were highest in renal fat (4.6 mg/kg
fluazuron equivalents) and omental fat (3.3 mg/kg), with lower levels
in liver (0.8 mg/kg), kidney (0.5 mg/kg), brain (0.2 mg/kg), and
muscle (0.1 mg/kg). In subcutaneous fat, a maximum residue level of
2.7 mg/kg was found two weeks after dosing. The residue levels were
comparable in all tissues after two and six weeks; after 16 weeks, the
levels had declined to 0.9-1 mg/kg in fat and 0.1 mg/kg in both liver
and kidney. The radiolabel collected at the injection site accounted
for 52% of the administered dose after 48 h (643 mg/kg fluazuron
equivalents), 26% after six weeks (396 mg/kg), and 5% after 16 weeks
(52 mg/kg) (Cameron et al., 1992).
2.1.2 Biotransformation
Rats
In the study of Schulze-Aurich (1992a) described above,
metabolites were identified in all tissue, faecal, and urine samples
by thin-layer chromatography and in fat and faeces also by
high-performance liquid chromatography and mass spectrometry-nuclear
magnetic resonance. In all tissues taken at all times of sacrifice,
the residues consisted only of unchanged fluazuron. In faeces, six
metabolic fractions were present and two metabolites were identified:
2F (3.2% of the dose) and 3F (5.8% of the dose) (see Figure 2), but
the major compound was unchanged fluazuron (26% of the dose). In
urine, eight metabolic fractions were found, including metabolites 2F
(0.6% of the dose) and 3F (0.45%); no unchanged fluazuron was present.
Hence, fluazuron is metabolized slowly but to a significant extent;
the pattern of metabolites in faeces indicates that about two-thirds
of the dose is metabolized and one-third is eliminated unchanged.
Metabolism proceeds by cleavage of the urea moiety, followed by
hydroxylation in position 6 of the phenyl ring, leading to 3-[3-(3-
chloro-5-trifluoromethyl-2-pyridinyloxy)-4-chloro-6-
hydroxyphenyl]urea. The main cleavage product, 2,6-difluorobenzoic
acid, is partly conjugated with glycine to form 2,6-difluorohippuric
acid.
Cattle
The nature of the residues in tissues, faeces, and bile of cattle
after pour-on administration of fluazuron at 1.5 mg/kg bw was
investigated by means of thin-layer chromatography and, in fat, also
by mass spectrometry. The study was certified for compliance with GLP
and quality assurance. Fluazuron is not extensively metabolized after
pour-on administration, as unchanged fluazuron was the only
radiolabelled component detected in almost all tissues taken at all
slaughter times, generally accounting for > 90% of the total
residues. Additional metabolites were detected at low levels only in
muscle (3% of the total residues) and skin (24%) samples taken at 16
weeks. Two metabolic fractions were found in faeces. The major
compound was unchanged fluazuron (about 92% of the radiolabel), while
the other, more polar fraction accounted for 3% of faecal radiolabel.
In bile, unchanged fluazuron was the major compound (76% of
radiolabel), and the other 24% remained at the origin of the
chromatography system (Johnson et al., 1996).
Fluazuron belongs to the class of benzoylphenyl urea derivatives,
which inhibit the growth and development of arthropod insects and
members of the order Acarina by acting on chitin formation. These
'insect growth regulators' do not act on the insect nervous system.
Fluazuron specifically affects ticks. Chitin synthesis in ticks occurs
during engorgement, moulting, and embryogenesis. Female ticks that
have absorbed a minimal amount of fluazuron show signs of disturbed
engorgement and lay eggs from which either no or non-viable larvae
hatch. Male ticks that take up minimal amounts of plasma are not
visibly affected by the compound. Fluazuron interrupts the life cycle
of immature ticks by interfering with cuticle formation during
engorgement and moulting. The affected ticks die.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
Rats
Fluazuron uniformly radiolabelled with 14C in the
4-chlorophenyl moiety ([U-14C-Cl]phenyl-labelled fluazuron; specific
activity, 48.1 µCi/mg) was administered orally in
N-methylpyrrolidone and PEG 200 by stomach tube to Tif:RAIf (SPF)
rats at a dose of 0.5 mg/kg bw per day for one week. Three rats of
each sex were killed 24 h and 2, 4, 8, and 12 weeks after the final
dose. Urine and faeces were collected daily during treatment and for
one week thereafter. After sacrifice, subcutaneous, renal, and
abdominal fat, blood, brain, kidneys, skeletal muscle, liver, and the
remaining carcass were sampled for radiolabel with a liquid
scintillation counter. The study was certified for compliance with GLP
and quality assurance.
By 24 h after the last dose, about 60% had been absorbed from the
gastrointestinal tract into the general circulation, as calculated
from the amount of radiolabel in urine, tissues, and carcass. During
the administration and observation periods, a total of 62% of the
administered dose was excreted, with 59% via the faeces and only 3%
via the urine. The extent of absorption and the route and rate of
excretion were independent of sex. At 24 h, the highest residue levels
were found in the adipose tissues (12-18 mg/kg fluazuron equivalents)
and significantly lower levels in other tissues: liver, 1.3; kidney,
0.84; lungs, 0.53; muscle, 0.39; and brain, 0.20 mg/kg. The same
distribution was seen at all time points. By 12 weeks after the last
dose, the amount of residual radiolabel had declined to 0.15-0.26
mg/kg fluazuron equivalents in adipose tissues and to < 0.03 mg/kg in
all other tissues. The depletion of radiolabel from all tissues
followed first-order kinetics, with a half-time of about 13 days for
all tissues in animals of each sex. The ratio in fat:blood (201 ± 28)
was relatively constant over the experimental period (Schulze-Aurich,
1992a).
Cattle
Male cross-bred beef cattle received single topical applications
of [U-14C-Cl]phenyl-labelled fluazuron (as the commercial pour-on
formulation Acatak; specific activity, 4.86 µCi/mg) at a dose of 1.5
mg/kg bw, administered on both sides of the spine between the shoulder
and the rump. The animals were slaughtered in groups of three during
weeks 2, 4, 6, and 16 after treatment. Blood, urine, and faeces were
collected from the animals slaughtered at the end of the study at
several times after treatment. At slaughter, samples were taken of
ventral and dorsal subcutaneous, renal, and omental fat; blood; brain;
kidney; hindquarter, forequarter, and tenderloin muscle; liver; bile;
and skin at the site of administration. All samples were analysed for
radiolabel with a liquid scintillation counter. The study was
certified for compliance with GLP and quality assurance.
The total intake was at least 60% of the administered dose, as
indicated by urinary and faecal excretion. It was not clear, however,
whether intake was via the dermal and/or the oral route (licking); the
portion absorbed into the systemic circulation was also unknown.
Fluazuron was slowly absorbed and distributed to tissues. Radiolabel
was initially observed in plasma 16 h after treatment. The mean plasma
levels remained fairly constant between 9 and 35 days after treatment,
ranging from 0.035 to 0.041 mg/litre fluazuron equivalents. The levels
declined thereafter, with a mean elimination half-time of about 73
days, to a mean level of 0.007 mg/litre 16 weeks after treatment. The
main route of elimination was the faeces (40% of the administered dose
within the first four weeks, increasing gradually to 62% by 16 weeks),
whereas renal excretion was of minor importance (1% of the dose after
16 weeks). There was some indication of biliary excretion. Maximum
residue levels were found two weeks after administration in almost all
tissues. The levels were highest in renal fat (4.8 mg/kg fluazuron
equivalents), omental fat (4.3 mg/kg), subcutaneous fat (ventral: 3.9
mg/kg; dorsal: 2.8 mg/kg), and skin (3 mg/kg). Lower levels were found
in liver (0.5 mg/kg), kidney (0.4 mg/kg), muscle (0.1 mg/kg), and
brain (0.08 mg/kg). The levels decreased slowly up to 16 weeks after
treatment, when they were 0.5-0.6 mg/kg in fat, 0.05-0.06 mg/kg in
liver and kidney, and 0.01-0.02 mg/kg in muscle and brain. The
depletion half-times for the different tissues varied from 4.5 to 5.5
weeks, but that in skin was 1.5 weeks (McLean & Dunsire, 1996).
Castrated Hereford steers received single subcutaneous injections
of [U-14C-Cl]phenyl-labelled fluazuron in a vehicle consisting of
PEG 200 dilaurate, Cremophor EL, citric acid, and
N-methyl-2-pyrrolidone (specific activity, 3.95 µCi/mg) behind the
left shoulder at a dose of 1.5 mg/kg bw. The animals were slaughtered
in groups of three, two days and 2, 6, and 16 weeks after treatment.
Blood, urine, and faeces were collected at several times after
treatment. At slaughter, samples were taken of subcutaneous, renal,
and omental fat; blood; brain; kidney; hindquarter and forequarter
muscle; liver; bile; and skin at the site of administration. All
samples were analysed for radiolabel with a liquid scintillation
counter. The study was certified for compliance with GLP and quality
assurance.
The compound was absorbed slowly from the injection site, the
mean maximum plasma level of 0.1 mg/litre fluazuron equivalents being
reached after 48 h. The plasma levels declined with a mean elimination
half-time of about 78 days; by 16 weeks after treatment, the mean
plasma level was still 0.01 mg/litre. The main route of elimination
was the faeces (23% of the administered dose after 16 weeks), whereas
renal excretion was of minor importance (1% of the dose after 16
weeks). There was some indication of biliary excretion. In all tissues
except subcutaneous fat, maximum residue levels were found 48 h after
administration. The levels were highest in renal fat (4.6 mg/kg
fluazuron equivalents) and omental fat (3.3 mg/kg), with lower levels
in liver (0.8 mg/kg), kidney (0.5 mg/kg), brain (0.2 mg/kg), and
muscle (0.1 mg/kg). In subcutaneous fat, a maximum residue level of
2.7 mg/kg was found two weeks after dosing. The residue levels were
comparable in all tissues after two and six weeks; after 16 weeks, the
levels had declined to 0.9-1 mg/kg in fat and 0.1 mg/kg in both liver
and kidney. The radiolabel collected at the injection site accounted
for 52% of the administered dose after 48 h (643 mg/kg fluazuron
equivalents), 26% after six weeks (396 mg/kg), and 5% after 16 weeks
(52 mg/kg) (Cameron et al., 1992).
2.1.2 Biotransformation
Rats
In the study of Schulze-Aurich (1992a) described above,
metabolites were identified in all tissue, faecal, and urine samples
by thin-layer chromatography and in fat and faeces also by
high-performance liquid chromatography and mass spectrometry-nuclear
magnetic resonance. In all tissues taken at all times of sacrifice,
the residues consisted only of unchanged fluazuron. In faeces, six
metabolic fractions were present and two metabolites were identified:
2F (3.2% of the dose) and 3F (5.8% of the dose) (see Figure 2), but
the major compound was unchanged fluazuron (26% of the dose). In
urine, eight metabolic fractions were found, including metabolites 2F
(0.6% of the dose) and 3F (0.45%); no unchanged fluazuron was present.
Hence, fluazuron is metabolized slowly but to a significant extent;
the pattern of metabolites in faeces indicates that about two-thirds
of the dose is metabolized and one-third is eliminated unchanged.
Metabolism proceeds by cleavage of the urea moiety, followed by
hydroxylation in position 6 of the phenyl ring, leading to 3-[3-(3-
chloro-5-trifluoromethyl-2-pyridinyloxy)-4-chloro-6-
hydroxyphenyl]urea. The main cleavage product, 2,6-difluorobenzoic
acid, is partly conjugated with glycine to form 2,6-difluorohippuric
acid.
Cattle
The nature of the residues in tissues, faeces, and bile of cattle
after pour-on administration of fluazuron at 1.5 mg/kg bw was
investigated by means of thin-layer chromatography and, in fat, also
by mass spectrometry. The study was certified for compliance with GLP
and quality assurance. Fluazuron is not extensively metabolized after
pour-on administration, as unchanged fluazuron was the only
radiolabelled component detected in almost all tissues taken at all
slaughter times, generally accounting for > 90% of the total
residues. Additional metabolites were detected at low levels only in
muscle (3% of the total residues) and skin (24%) samples taken at 16
weeks. Two metabolic fractions were found in faeces. The major
compound was unchanged fluazuron (about 92% of the radiolabel), while
the other, more polar fraction accounted for 3% of faecal radiolabel.
In bile, unchanged fluazuron was the major compound (76% of
radiolabel), and the other 24% remained at the origin of the
chromatography system (Johnson et al., 1996).
 The nature of the residues in tissues and excreta of steers after
subcutaneous administration of fluazuron at 1.5 mg/kg bw was
investigated by thin-layer chromatography. The study was certified for
compliance with GLP and quality assurance. In all tissues taken at all
slaughter times, unchanged fluazuron was the only detectable fraction,
accounting for more than 90% of the total residues. In faeces, eight
metabolic fractions were found. The major compound was unchanged
fluazuron (about 70% of radiolabel), while the other, unidentified
fractions were more polar. Urine contained only unidentified
metabolized products, which were more polar than unchanged fluazuron.
Within 16 weeks of treatment, about 24% of the administered dose was
eliminated in faeces and urine, 16% as unchanged fluazuron and 8% as
its degradation products. Hence, fluazuron is metabolized to a lesser
extent in cattle than in rats (Schulze-Aurich, 1992b).
2.2 Toxicological studies
2.2.1 Acute toxicity
In studies of technical-grade fluazuron in distilled water
containing 0.5% carboxymethylcellulose and 0.1% polysorbate 80 in male
and female Tif:RAIf (SPF) rats, one study that followed OECD test
guideline 401 with quality assurance certification showed an oral
LD50 value of > 5000 mg/kg bw (Schoch, 1986a); in a study in the
same strain that followed OECD test guideline 402 with quality
assurance certification, the dermal LD50 was > 2000 mg/kg bw
(Schoch, 1986b). In a study that followed OECD test guideline 403, the
LC50 after exposure by inhalation was > 5994 mg/m3 (Hartmann &
Schoch, 1987). The toxic symptoms observed were sedation, dyspnoea,
exophthalmos, ruffled fur, and abnormal body positions. The animals
recovered within 6-11 days.
Three male New Zealand white rabbits received an instillation of
0.1 ml (56 mg) technical-grade fluazuron in a study that followed OECD
test guideline 405 with GLP and quality assurance certification. The
animals developed slight redness and chemosis of the cornea and
conjunctivae up to 24 h. No irritation of the iris was observed
(Schoch, 1986c).
In a study that followed OECD test guideline 404 with GLP and
quality assurance certification, three male New Zealand white rabbits
that received a semi-occlusive topical application of 0.5 g
technical-grade fluazuron developed no dermal reactions (Schoch,
1986d).
In 10 male and 10 female Pirbright white guinea-pigs submitted to
an optimization test, in a study that followed OECD test guideline 404
with GLP and quality assurance certification, no skin sensitization
was observed after challenge with technical-grade fluazuron
intradermally in 20% propylene glycol or epidermally in vaseline
(Schoch & Gfeller, 1987).
2.2.2 Short-term toxicity
Rats
In a study of dermal toxicity that followed OECD test guideline
410 with GLP and quality assurance certification, gauze patches
moistened with distilled water and technical-grade fluazuron at doses
of 0, 10, 100, or 1000 mg/kg bw were applied to the shaven skin of
groups of five male and five female Tif:RAIf (SPF) rats for 6 h/day,
five days per week for three weeks. The only effect observed was a
slight but significant prolongation of prothrombin time in male rats
at the two higher doses. The NOEL was 10 mg/kg bw per day (Schoch
et al., 1988).
Groups of 10 male and 10 female Tif:RAIf (SPF) rats received
technical-grade fluazuron by gavage for four weeks at intended doses
of 0, 10, 100, or 1000 mg/kg bw per day. The vehicle was distilled
water containing 0.5% carboxymethylcellulose and 0.1% Tween 80. Since
no clinical signs of toxicity were observed, the upper dose was
increased to 2000 mg/kg bw per day from day 10 onwards. The final
actual doses were 0, 3.2-5.6, 35-60, and 1660-1920 mg/kg bw per day.
The study was certified for compliance with GLP and quality assurance.
No treatment-related effects were seen on mortality rate,
clinical or ophthalmoscopic signs, body weight, or food or water
consumption, macroscopically or microscopically. Although treated
animals showed some statistically significant changes in blood
chemistry, these were not considered to be treatment-related (i.e. not
dose-related or of no biological relevance). All males at the medium
and high doses had a slight, statistically significant, dose-related
increase in prothrombin time and a slight, statistically significant,
dose-related decrease in platelet count. The mean liver weight was
increased in all treated animals, reaching statistical significance in
those at the two higher doses. Males at these doses also had a
decrease in mean thymus weights, which was statistically significant
only at the highest dose. These weight changes were not accompanied by
histopathological findings. The NOEL was 3.2-5.6 mg/kg bw per day, but
the validity of the study is limited due to the fact that the doses
intended to be the low and medium doses were not reached (Thevenaz
et al., 1987).
Groups of 20 male and 20 female Tif:RAIf (SPF) rats were fed
technical-grade fluazuron in the diet at concentrations of 0, 100,
600, 3500, or 20 000 mg/kg feed for three months, to give calculated
mean daily intakes of 0, 6.4, 39, 220, or 1300 mg/kg bw for males and
0, 6.6, 41, 240, or 1400 mg/kg bw for females. The study followed OECD
test guideline 408 with GLP and quality assurance certification.
No treatment-related effects were seen on mortality, clinical or
ophthalmoscopic signs, or macroscopically. Although body weight and
food consumption were marginally lower in treated males than in
controls, food conversion was not influenced. Treated males and
females showed some statistically significant changes in blood
chemistry; however, as these findings did not reflect any consistent
alteration (i.e. not dose-related or of no biological relevance), they
were not considered to be treatment-related. All treated males had
slightly increased prothrombin time (dose-related, statistically
significant in rats at 3500 and 20 000 mg/kg feed), in platelet count
(not dose-related, significant at 3500 mg/kg feed), and in lymphocyte
count (dose-related, significant at 20 000 mg/kg feed). The absolute
and relative weights of the liver and glycogen deposition were
significantly increased in all treated males, due, at least at 100
mg/kg feed, to poor study design, as sections were not made at random.
Changes in the absolute and relative weights of the heart (males),
kidney, ovaries, and adrenals (females) were not accompanied by
abnormal histopathological findings. Fluazuron treatment resulted in
slight to moderate hepatocellular hypertrophy in males and females at
3500 and 20 000 mg/kg feed and a slight increase in the incidence and
intensity of thyroid follicular hypertrophy and pituitary cell
hypertrophy in males at these doses. The authors considered the NOEL
to be 600 mg/kg feed (equal to 39-41 mg/kg bw per day); however,
effects on liver weight were observed in males at this dose. The
Committee concluded that the NOEL was 100 mg/kg feed, equal to 6.4
mg/kg bw per day, on the basis of effects on the liver. It should be
noted that similar findings were not observed in a long-term study of
toxicity and carcinogenicity in rats (see below) (Basler et al.,
1987).
Dogs
In a one-month range-finding study, groups of two male and two
female pure-bred beagle dogs were given technical-grade fluazuron at
doses of 200 or 50 000 mg/kg feed, equal to mean daily intakes of 8.5
or 2200 mg/kg bw per day, respectively. The study was certified for
compliance with GLP and quality assurance. No deaths occurred.
Clinical symptoms, food consumption, body-weight gain,
ophthalmoscopic, haematological, clinical, and urinary parameters, and
macroscopic and histopathological findings indicated no change that
could be considered to be related to treatment (Bloch et al., 1987).
In a three-month study, groups of four to six pedigree beagle
dogs of each sex were fed diets containing technical-grade fluazuron
at 0, 500, 5000, or 50 000 mg/kg feed. The study followed OECD test
guideline 409 with GLP and quality assurance certification. As several
animals in each group showed alterations indicative of an infection
(possibly leptospirosis) during the experiment, the study was not
considered useful for evaluating the safety of fluazuron. The only
findings of note that could not be attributed to the infectious
disease were degenerative, inflammatory, or hypertrophic changes in
the major arteries of females at 5000 and 50 000 mg/kg; however, this
effect was not confirmed in a 52-week feeding study with an interim
kill at 13 weeks (see below) (Gretener et al., 1988).
Groups of six male and six female beagle dogs received diets
containing technical-grade fluazuron at 0, 200, 3000, or 50 000 mg/kg
feed for one year. Two dogs of each sex per group were killed for
interim examinations after 13 weeks. The observations included
mortality, clinical signs, body weight, food consumption,
ophthalmoscopy, haematology, blood chemistry, urinalysis, organ
weights, and macroscopic and microscopic examinations. The calculated
mean intakes were 0, 7.5, 110, or 1900 mg/kg bw per day for males and
0, 7.1, 120, or 2000 mg/kg bw per day for females. The study followed
OECD test guideline 452 with GLP and quality assurance certification.
No deaths occurred. Treatment mainly affected two males at the high
dose (one killed at 13 weeks and the other at 52 weeks), which had
decreased food consumption, transient body-weight loss, and increased
activities of alkaline phosphatase and aspartate and alanine
aminotransferases from week 13 onwards; histopathological examination
revealed minimal multifocal areas of haemorrhage with slight
multifocal chronic inflammation of the liver. A slight increase in
alkaline phosphatase activity was also found in males at the middle
dose from week 13 onwards and in females at the high dose from week 39
onwards. Females at the high dose also had a slightly decreased
phosphorus concentration. The NOEL was 200 mg/kg feed, equal to 7.5
mg/kg bw per day, on the basis of changes in hepatic enzyme activities
(Briffaux, 1988a, 1990).
2.2.3 Long-term toxicity and carcinogenicity
Mice
Groups of 60 male and 60 female Tif: MAGf (SPF) mice were fed
diets containing technical-grade fluazuron at doses of 0, 40, 400,
4000, or 9000 mg/kg feed for two years, equal to average achieved
intakes of 0, 4.5, 45, 450, or 990 mg/kg bw per day for males and 0,
4.3, 43, 430, or 970 mg/kg bw per day for females. The observations
included clinical signs, mortality, body weight, food and water
consumption, haematological changes (10 animals of each sex per
group), and organ weights; detailed macroscopic and histopathological
examinations were perfomed. The study followed OECD test guideline 451
with GLP and quality assurance certification.
Clinical signs, body weight, and food consumption were unaffected
by treatment, as was survival (40-45% for controls, 43-58% for treated
animals). Water consumption was increased consistently in females at
9000 mg/kg feed and in females at 400 and 4000 mg/kg feed in the
second year of the study, but in females at 40 mg/kg feed and in all
treated males it was comparable to that of controls. Haematological
examination revealed no treatment-related changes, and the organ
weights and macroscopic findings at necropsy were comparable in
control and treated animals. The treatment-related non-neoplastic
changes included an increased incidence of cataract of the crystalline
lens characterized by mild necrosis and calcification of subcapsular
lens fibres in animals of each sex at 4000 and 9000 mg/kg feed and a
trend to increased diffuse hyperplasia of prostatic glandular tissue
in males at 4000 and 9000 mg/kg feed. In addition, the following
uterine changes, which were not dose-related, were noted: increased
incidences of inflammatory polyps at 400, 4000, and 9000 mg/kg feed,
increased dilatation of the lumen at 4000 and 9000 mg/kg feed, and
increased incidences of haematomas and dilatation of blood vessels
associated with thrombosis at 9000 mg/kg feed. Tumour incidences were
not increased, although a marginally increased incidence of systemic
infiltration with malignant lymphoma was observed in some organs of
some animals at the high dose. The total number of lymphomas per
animal and the total number of animals bearing lymphomas were not
significantly different from those among controls. On the basis of the
pathological changes in the uterus, the NOEL was 40 mg/kg feed, equal
to 4.3 mg/kg bw per day (Bachmann et al., 1991a).
In the same study, the total amount of fluazuron in the body
appeared to reach a maximum at approximately 400 mg/kg feed: the
fluazuron level was 4.9-8.7 µg/ml in blood and 880-970 mg/kg in fat in
animals of each sex at 4000 and 9000 mg/kg feed and only slightly
lower in those at 400 mg/kg feed. At 40 mg/kg feed, a steady state was
not reached. The blood:fat ratio was approximately 1:200 in all groups
(Maier, 1991a).
Rats
Groups of 80 male and 80 female Tif:RAIf (SPF) rats received
diets containing technical-grade fluazuron at 0, 50, 500, 10 000, or
20 000 mg/kg feed for two years, equal to average achieved intakes of
0, 1.9, 18, 380, or 780 mg/kg bw per day for males and 0, 2.1, 21,
440, or 920 mg/kg bw per day for females. Ten rats of each sex were
killed after one year for interim necropsy. The observations included
clinical signs, mortality, body weight, food consumption,
ophthalmoscopy, haematology (20 rats of each sex per group), blood
chemistry (10 of each sex per group), urinalysis (10 of each sex per
group), and organ weights; detailed macroscopic and histopathological
examinations were performed. The study followed OECD test guideline
453 with GLP and quality assurance certification.
The survival of the animals (56-67% for controls, 54-73% for
treated animals) was not affected by treatment. Minor effects were
observed in animals at the highest dose at interim sacrifice but not
at terminal sacrifice. In females, the relative liver and kidney
weights were significantly decreased, with no associated morphological
changes; in males, minimal hypertrophy of hepatocytes was seen.
Females at the highest dose also had decreased body-weight gain during
the second year of treatment, but this failed to reach statistical
significance. The findings were considered not to be of toxicological
significance. The incidence of tumours was not increased. The total
amount of fluazuron in the body appeared to reach a maximum at 500
mg/kg feed: the level was 1.3-2.3 µg/ml in blood and 290-440 mg/kg in
fat in animals of each sex at 500 mg/kg feed and above. At 50 mg/kg
feed, a steady state was not reached. The blood:fat ratio was
approximately 1:200 in all groups (Bachmann et al., 1991b; Maier,
1991b).
2.2.4 Genotoxicity
The results of tests for genotoxicity carried out with fluazuron
are summarized in Table 1.
2.2.5 Reproductive toxicity
2.2.5.1 Multigeneration reproductive toxicity
In a preliminary study of reproductive toxicity, groups of seven
male and 14 female Ico:OFA Sprague-Dawley rats received
technical-grade fluazuron at dietary concentrations of 0, 200, 1000,
7000, or 20 000 mg/kg feed. Males were dosed for two weeks before
mating and during the mating period and were sacrificed thereafter.
Females were dosed from two weeks before mating up to 21 days post
partum, when both the females and their litters were sacrificed. The
study was certified for quality assurance.
There were no deaths or treatment-related clinical signs in
animals of either sex during the study. The food consumption and
body-weight gain of F0 animals and the precoital period and duration
of the gestation period were not affected by treatment, and no effects
were seen on insemination, fecundity, fertility, resorption indices,
stillbirths, nursing behaviour, the numbers of live F1 pups, their
physical development, or neonatal or postnatal mortality. The mean
weight at birth of F1 pups was not affected by treatment, but a
slight retardation in growth rate was seen towards the end of the
lactation period in animals at 7000 and 20 000 mg/kg feed. No
treatment-related effects were seen in any animal at necropsy
(Briffaux, 1988b).
In a two-generation study of reproductive toxicity, groups of 30
male and 30 female Ico:OFA Sprague-Dawley rats received diets
containing technical-grade fluazuron at 0, 100, 1500, or 20 000 mg/kg
feed for at least 100 days before mating and then throughout gestation
and lactation for two successive generations. Two litters were bred
per generation. Males and females of the first F1 litter (F1a)
were selected to breed the next generation. After weaning of the F1b
and F2b pups, the parental animals were killed and necropsied.
Unselected F1a pups and F1b, F2a, and F2b pups were killed and
necropsied after 21 days of lactation. The study followed OECD test
guideline 416 with GLP and quality assurance certification.
The only effects found were on food consumption and body weight,
not on reproductive function. F1 females at the high dose showed a
slight reduction in body weight in the first gestation period, and the
food consumption of those at the middle and high doses tended to be
slightly reduced at the end of both lactation periods. Neonatal
viability of both generations of rats at the high dose was slightly
reduced after the first matings, but the litters from subsequent
matings were not affected. Pup viability from day 4 post partum to
weaning was not affected by treatment during any phase of the study.
The body weights at birth of pups in all litters were comparable to
those of controls, but the body-weight gain of those at the high dose
was reduced for all generations (F1a, F1b, F2a, F2b) and of
those at the middle dose for the F1a, F2a, and F2b generations.
The NOEL was 100 mg/kg feed, equivalent to 5 mg/kg bw per day, on the
basis of postnatal toxicity (Barrow & Briffaux, 1991).
2.2.5.2 Developmental toxicity
Rats
Technical-grade fluazuron was administered by gavage in a 0.1%
aqueous solution of polysorbate 80 at doses of 0, 10, 100, or 1000
mg/kg bw per day to groups of 24 mated female Tif:RAIf (SPF) rats on
days 6-15 of gestation. On day 21 of gestation, the dams were killed
and necropsied, and the fetuses were weighed, sexed, and examined for
external, visceral, and skeletal abnormalities. The study followed
OECD test guideline 414 with GLP and quality assurance certification.
One dam at the middle dose aborted its entire litter. No
treatment-related effects were observed in dams, and the numbers of
implantations, live young, and resorptions and fetal and litter
weights were unaffected by treatment. There was no indication of
teratogenicity. Hence, fluazuron at doses up to 1000 mg/kg bw per day
had no maternal, embryo- or fetal toxicity or teratogenicity (Thouin,
1988).
Rabbits
Groups of 20 mated female Chinchilla-type rabbits received
technical-grade fluazuron suspended in 3% (w/w) aqueous corn starch by
gavage at doses of 0, 10, 100, or 1000 mg/kg bw per day on days 7-19
of pregnancy. The dams were killed and necropsied on day 29 of
pregnancy, and various parameters examined. Fetuses were subjected to
gross necropsy and visceral and skeletal examination. The study
followed OECD test guideline 414 with GLP and quality assurance
certification. One female at the high dose died due to faulty
intubation, and another resorbed all of its implants. The incidence of
post-implantation loss was slightly increased in all treated groups,
mainly due to a slightly higher rate of early resorptions, but was
still within the historical control range and was considered not to be
related to treatment. The number of live fetuses and the litter weight
were unaffected by treatment. The body weights of the fetuses of all
treated dams were slightly increased; the increases in group means
were statistically significant but the litter means were not. No other
effects were observed. Hence, at doses up to 1000 mg/kg bw per day
fluazuron induced no maternal, embryo or fetal toxicity and was not
teratogenic (Thomann, 1988).
Table 1. Results of assays for genotoxicity with fluazuron
End-point Test object Concentration Result S9 QA Reference
In vitro
Reverse S. typhimurium 1.14-278 µg/mla Negative + No Deparade &
mutation TA98, TA100, Negative - Arni (1985)
TA1535, TA1537
Gene V79 Chinese 12.5-500 ng/ml Negative + Yes Dollenmeier &
mutation hamster cells 0.625-25 µg/ml Negative - Puri (1987)
Chromosomal Human lymphocytes 14.1-225 µg/ml Negative + Yes Strasser &
aberration 7.5-120.0 µg/ml Negative - Muller (1987)
DNA repair Rat hepatocytes 0.4-300 µg/ml Negative Yes Puri & Muller (1987)
DNA repair Human fibroblasts 0.2-50 µg/ml Negative Yes Meyer & Puri (1987)
In vivo
Nuclear Chinese hamster 1.25-5.0 g/kg bwb Negativec Yes Strasser & Arni
anomalies bone marrow (1987)
S9, 9000 × g fraction of rat liver; QA, quality assurance
a No cytotoxicity, but precipitation occurred at the highest dose in the presence of S9
b Daily doses given by gavage on each of two consecutive days; cyclophosphamide used as a positive control
c The authors considered 5.0 g/kg bw to be the highest applicable dose, but as cytotoxicity was not
determined it was not clear whether the bone marrow was exposed.
2.2.6 Special studies on target animals
In a study of tolerability, groups of three male and three female
Hereford cattle received the pour-on formulation Acatak at 0
(control), once, three times, or five times the recommended dose of 2
mg/kg bw fluazuron, along the back from the neck to the rump. The
animals were observed for the following eight weeks and monitored for
subclinical side-effects. The study was certified for quality
assurance. Apart from the formation of a crusty layer on the skin and
coat, which caused no irritation or discomfort to the animals, the
formulation was well tolerated at all doses. No drug-related changes
were observed in body weight, feed consumption, body temperature, or
haematological or blood biochemical parameters (Bowen & Strong, 1993).
When the label instructions for application of the pour-on formulation
Acatak were followed, a crusty layer formed on the coats of the
cattle, but the animals displayed no clinical signs of irritation.
Histopathological examination showed only minor pathological changes
in the skin, which had no effect on the hide quality (Strong, 1993;
Strong & Bowen, 1993).
Subcutaneous injection of Acatik, a commercial formulation
containing fluazuron as the active ingredient, induced no side-effects
or local reactions at the injection site in cattle receiving single
doses of 1 mg/kg bw fluazuron or dogs receiving double doses of 2.5
and then 5 mg/kg bw (Suarez, 1988; Genchi, 1990).
2.2.7 Special studies on heat degradation
The degradation of fluazuron residues present in cattle meat and
fat was investigated after normal dry cooking, cooking in water or
oil, or microwave treatment. The meat and fat samples were derived
from steers treated with fluazuron at 1.5 mg/kg bw by subcutaneous
injection, which is not the recommended route of administration. The
study was certified for compliance with GLP and quality assurance.
Residues of fluazuron were partly (in meat) or completely (in fat)
degraded to [3-(3-chloro-5-trifluoro-methyl-2-pyridinyloxy)-4-
chlorophenyl]-1-amine. This degradation product did not show mutagenic
potential in an assay for reverse mutation in Salmonella typhimurium
strains TA98, TA100, TA1535, and TA1537 or in Escherichia coli
strain WP2uvrA, when the known mutagen found in cooked meat, MeIQx
(2-amino-3,8- dimethylimidaz[4,5-f]quinoxaline), was used as the
reference substance (Schulze-Aurich, 1992c; Maier, 1993).
3. COMMENTS
The Committee considered the results of studies on the
pharmacokinetics, metabolism, acute, short-term and long-term
toxicity, carcinogenicity, genotoxicity, and reproductive toxicity of
fluazuron. All of the studies critical for the evaluation were carried
out according to appropriate standards for study protocol and conduct.
Analysis 24 h after oral administration of radiolabelled
fluazuron to rats showed that an average of 60% was absorbed through
the gut. Most of the absorbed radiolabel was taken up into adipose
tissues, while significantly lower levels were found in liver, kidney,
lung, muscle, and brain. Ninety percent of the radiolabel in tissues
was attached to unchanged fluazuron. Fluazuron was eliminated slowly
from adipose tissues, following first-order kinetics, with a half-life
of about 13 days. Elimination occurred primarily in the faeces. One
week after administration, 59% had been excreted in faeces and 3% in
urine. About one-third of the dose was eliminated as unchanged
fluazuron in the faeces; the remaining two-thirds was metabolized by
cleavage of the urea moiety between the benzoyl carbon and the urea
nitrogen, followed by hydroxylation in the phenyl ring.
When radiolabelled fluazuron was administered topically to
cattle, radiolabel was slowly absorbed, either percutaneously, orally
(by licking), or by both routes. A steady state between absorption and
elimination was observed for three to four weeks after treatment. The
absorbed radiolabel was taken up mainly by adipose tissues and to a
lesser extent by other tissues. Depletion of fluazuron from plasma and
tissues was slow, with half-lives of elimination of 10.5 and 4.5-5.5
weeks, respectively. The major route of elimination was the faeces
(62% of the dose within 16 weeks), while renal excretion was of minor
importance (1% of the dose within 16 weeks). There was some indication
of biliary excretion. Fluazuron was not extensively metabolized, as
unchanged fluazuron accounted for more than 90% of the total residues
in tissues and faeces. A similar slow absorption, distribution, and
elimination pattern was observed after subcutaneous administration of
radiolabelled fluazuron to steers. The pattern of metabolites excreted
in faeces was somewhat more complex, however, indicating that about
one-third of the fluazuron was metabolized into more polar
metabolites. Although the fate of fluazuron in rats was similar to
that in cattle, they metabolized fluazuron to a greater extent than
cattle.
Orally administered fluazuron was found to have low acute
toxicity, with an LD50 value of > 5000 mg/kg bw in rats.
The short-term toxicity of fluazuron was evaluated by oral
administration to rats and dogs. In a 28-day study, rats received
fluazuron by gavage at doses of 0, 10, 100, or 2000 mg/kg bw per day.
Increased prothrombin time and liver weight and decreased platelet
counts and thymus weight were observed, mainly in animals at the two
higher doses, and particularly in males. Male rats were also more
sensitive than females to the effects of fluazuron in a 13-week
feeding study at dietary concentrations of 0, 100, 600, 3500, or
20 000 mg/kg feed (equal to 6.4-1400 mg/kg bw per day). Male rats at
the two higher doses had increased prothrombin time, platelet and
lymphocyte counts, and absolute and relative liver weights, as well as
hypertrophy of thyroid follicular cells, pituitary cells, and
hepatocytes. In males, increases in absolute and relative liver
weights were also observed at 600 mg/kg feed. In females,
hepatocellular hypertrophy occurred at 3500 and 20 000 mg/kg feed. The
NOEL was 100 mg/kg feed, equal to 6.4 mg/kg bw per day, on the basis
of effects on the liver.
In a one-year feeding study, dogs received fluazuron at dietary
concentrations of 0, 200, 3000, or 50 000 mg/kg feed. Effects were
observed primarily in males at the highest dose, consisting of
decreased food consumption, transient body-weight loss, increased
serum activities of alkaline phosphatase and aspartate and alanine
aminotransferases, and minimal multifocal haemorrhage with slight
multifocal chronic inflammation in the liver. A slight increase in
alkaline phosphatase activity was also observed in males at 3000 mg/kg
feed and in females at 50 000 mg/kg feed. The NOEL was 200 mg/kg feed,
equal to 7.5 mg/kg bw per day, on the basis of changes in hepatic
enzyme activities.
In a study of carcinogenicity, mice received diets containing 0,
40, 400, 4000, or 9000 mg/kg feed for two years (equal to 4.3-990
mg/kg bw per day). Females at the two higher levels showed increased
water consumption, cataracts, and uterine changes (inflammatory
polyps, luminal dilatation and, at 9000 mg/kg feed only, haematomas
and dilatation of blood vessels associated with thrombosis). Increased
water consumption and inflammatory uterine polyps were also observed
in females at 400 mg/kg feed. Male mice at the two higher levels had
cataracts, and a trend to an increasing frequency of diffuse
hyperplasia of prostatic glandular tissue was observed. The NOEL was
40 mg/kg feed, equal to 4.3 mg/kg bw per day, on the basis of
pathological changes in the uterus.
In a long-term study of toxicity and carcinogenicity, rats were
given fluazuron at 0, 50, 500, 10 000, or 20 000 mg/kg feed for two
years (equal to 1.9-920 mg/kg bw per day), and a proportion of the
animals were killed at one year. No toxicologically significant
effects were observed at any dose. The toxic effects on the liver
observed in the 13-week study in rats were not observed after either
one or two years.
In the long-term studies of toxicity, the total amount of
fluazuron in the body appeared to reach a maximum at relatively low
levels (approximately 400 and 500 mg/kg feed, equal to 43 and 18 mg/kg
bw per day, in mice and rats, respectively), as the concentration of
the compound in blood and fat did not increase at higher levels. The
reason is not clear. It is not known whether a similar effect occurred
in the short-term studies, because blood and fat levels were not
measured.
Fluazuron has been tested in vitro for its ability to induce
reverse mutations in Salmonella typhimurium, gene mutations in Chinese
hamster cells, chromosomal aberration in human lymphocytes, and DNA
repair in rat hepatocytes and human fibroblasts. It has also been
tested in vivo for its ability to induce nuclear anomalies in
Chinese hamster bone marrow. All the results were negative. On the
basis of these data and the results of the bioassays in rodents, the
Committee concluded that fluazuron has no genotoxic or carcinogenic
potential.
In a two-generation study of reproductive toxicity in rats at
dietary concentrations of 0, 100, 1500, or 20 000 mg/kg feed
(equivalent to 5-1000 mg/kg bw per day), fluazuron had no adverse
effects on reproductive function. The only effects observed were
slightly retarded growth of pups at 1500 and 20 000 mg/kg feed and a
slight increase in neonatal mortality at 20 000 mg/kg feed. The NOEL
was 100 mg/kg feed, equivalent to 5 mg/kg bw per day, on the basis of
postnatal toxicity.
Fluazuron was not maternally toxic and did not cause
embryotoxicity, fetotoxicity, or teratogenicity in rats or rabbits at
oral doses up to 1000 mg/kg bw per day.
Because fluazuron belongs to a class of insect growth regulators
that do not act on the nervous system and because the central nervous
system was not a target organ in the short-term or long-term studies
of toxicity in rats, mice, or dogs, special studies of neurotoxicity
have not been performed. The Committee concluded that such studies
were unnecessary.
4. EVALUATION
The Committee established an ADI of 0-40 µg/kg bw for fluazuronâ
on the basis of the NOEL of 4.3 mg/kg bw per day for pathological
changes in the uterus in the two-year study in mice and a 100-fold
safety factor. The ADI was rounded to one significant figure, as is
the usual practice (Annex 1, reference 91, section 2.7).
5. REFERENCES
Bachmann, M., Hunter, B., Gretener, P., Krinke, G., Landes, C.,
Christen, P., Seewald, W., Heinemann, G.W. & Basler, W. (1991a) CGA
157419 tech: Lifetime carcinogenicity study in mice. Unpublished
report No. 860086 from Ciba-Geigy Ltd, Stein, Switzerland. Submitted
to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Bachmann, M., Hunter, B., Gretener, P., Froehlich, E., Faccini, J.M.,
Krinke, G., Christen, P., Seewald, W., Heinemann, G.W. & Basler, W.
(1991b) CGA 157419 tech: Carcinogenicity and chronic toxicity study in
rats. Unpublished report No. 860087 from Ciba-Geigy Ltd, Stein,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Barrow, P. & Briffaux, J.P. (1991) CGA 157419 tech: Two generation
oral (dietary administration) reproduction study in the rat.
Unpublished report No. 860089 from Hazleton France, L'Arbresle, France
(Hazleton report No. 710779). Submitted to WHO by Ciba-Geigy Ltd,
Basel, Switzerland.
Basler, W., Fankhauser, H., Gretener, P., Froehlich, E., Heider, K. &
Gfeller, W. (1987) CGA 157419 tech: 3-month oral toxicity study in
rats (administration in food). Unpublished report No. 860080 from
Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd, Basel, Switzerland.
Bloch, I., Frei, T., Luetkemeier, H. & Wilson, J. (1987) CGA 157419:
4-week oral range-finding toxicity (feeding) study in beagle dogs.
Unpublished report No. 860083 from Research & Consulting Company AG,
Itingen, Switzerland (RCC report No. 072977). Submitted to WHO by
Ciba-Geigy Ltd, Basel, Switzerland.
Bowen, F.L. & Strong, M.B. (1993) The tolerability of cattle to
exposure to the acarine growth regulator Acatak. Unpublished report
No. 93/3/1398 from Ciba-Geigy Ltd, Kemps Creek, Australia. Submitted
to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Briffaux, J.P. (1988a) CGA 157419 tech: 52 week oral (dietary
administration) toxicity study in the beagle dog. Interim report for
the first 13 weeks of the study. Unpublished report No. 860085 from
Hazleton France, L'Arbresle, France (Hazleton report No. 712829).
Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Briffaux, J.P. (1988b) CGA 157419: Preliminary study for a
2-generation oral (dietary administration) reproduction study in the
rat. Unpublished report No. 860400 from Hazleton France, L'Arbresle,
France (Hazleton report No. 703623). Submitted to WHO by Ciba-Geigy
Ltd, Basel, Switzerland.
Briffaux, J.P. (1990) CGA 157419 tech: 52 week oral (dietary
administration) toxicity study in the beagle dog. Unpublished report
No. 860085 from Hazleton France, L'Arbresle, France (Hazleton report
No. 094089). Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Cameron, B.D., Somers, K. & Speirs, G.C. (1992) The distribution and
excretion of [U-14C]Cl-phenyl CGA 157419 after subcutaneous
injection to cattle. Unpublished report No. 141232 from Inveresk
Research International, Tranent, Scotland, United Kingdom (Inveresk
report No. 6189). Submitted to WHO by Ciba-Geigy Ltd, Basel,
Switzerland.
Deparade, E. & Arni, P. (1985) Salmonella Cobas. Bact. pilot test with
CGA 157419 techn. (test for mutagenic effects on bacteria).
Unpublished report No. 850235 from Ciba-Geigy Ltd, Basel, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Dollenmeier, P. & Puri, E. (1987) CGA 157419 tech: V79 Chinese hamster
point mutation test (with and without microsomal activation).
Unpublished report No. 860071 from Ciba-Geigy Ltd, Basel, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Genchi, C. (1990) Tolerability of Acatik formulation A-8560 A in dogs.
Unpublished report from Facolta di Medicina Veterinaria, Universita
degli studi di Milano, Milan, Italy. Submitted to WHO by Ciba-Geigy
Ltd, Basel, Switzerland.
Gretener, P., Froehlich, E., Heider, K., Christen, P. & Gfeller, W.
(1988) CGA 157419 tech: 3-month subchronic oral toxicity study in
beagle dogs. Unpublished report No. 860084 from Ciba-Geigy Ltd, Stein,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Hartmann, H.R. & Schoch, M. (1987) CGA 157419 tech: Acute aerosol
inhalation toxicity in the rat. Unpublished report No. 860078 from
Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd, Basel, Switzerland.
Johnson, S., Johnston, A.M. & Prout, M.S. (1996)
[Chlor-phenyl-(U)-14C]-CGA 157419: Nature of metabolites in excreta
and tissues in ruminant cattle following single topical
administration. Unpublished report No. 156940 from Inveresk Research,
Tranent, Scotland, United Kingdom (Inveresk report No. 13026).
Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Maier, W. (1991a) CGA 157419: Determination of residues in whole blood
and fat of mice after subchronic and chronic exposure. Summary report
and assessment. Unpublished report from Ciba-Geigy Ltd, Basel,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Maier, W. (1991b) CGA 157419: Determination of residues in whole blood
and fat of rats after subchronic and chronic exposure. Summary report
and assessment. Unpublished report from Ciba-Geigy Ltd, Basel,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Maier, W. (1993) CGA 157419: Toxicological assessment of heat
degradation products. Unpublished report from Ciba-Geigy Ltd, Basel,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
McLean, C.L. & Dunsire, J.P. (1996) The absorption, distribution,
excretion, and residue depletion of [chlor-phenyl-(U)-14C]-CGA
157419 in ruminant cattle following topical administration.
Unpublished report No. 155601 from Inveresk Research, Tranent,
Scotland, United Kingdom (Inveresk report No. 12446). Submitted to WHO
by Ciba-Geigy Ltd, Basel, Switzerland.
Meyer, A. & Puri, E. (1987) CGA 157419 tech: Autoradiographic
DNA-repair test on human fibroblasts (OECD-conform). Unpublished
report No. 861231 from Ciba-Geigy Ltd, Basel, Switzerland. Submitted
to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Puri, E. & Muller, D. (1987) CGA 157419 tech: Autoradiographic
DNA-repair test on rat hepatocytes (OECD-conform). Unpublished report
No. 861230 from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO
by Ciba-Geigy Ltd, Basel, Switzerland.
Schoch, M. (1986a) CGA 157419 tech: Acute oral toxicity in the rat.
Unpublished report No. 860073 from Ciba-Geigy Ltd, Basel, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Schoch, M. (1986b) CGA 157419 tech: Acute dermal toxicity in the rat.
Unpublished report No. 860076 from Ciba-Geigy Ltd, Basel, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Schoch, M. (1986c) CGA 157419 tech: Acute eye irritation/corrosion
study in the rabbit. Unpublished report No. 860074 from Ciba-Geigy
Ltd, Basel, Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basel,
Switzerland.
Schoch, M. (1986d) CGA 157419 tech: Acute dermal irritation/corrosion
study in the rabbit. Unpublished report No. 860075 from Ciba-Geigy
Ltd, Basel, Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basel,
Switzerland.
Schoch, M. & Gfeller, W. (1987) CGA 157419 tech: Skin sensitization
test in the guinea pig (optimization test). Unpublished report No.
860066 from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd, Basel, Switzerland.
Schoch, M., Gretener, P., Froehlich, E., Germer, M., Krinke, G. &
Gfeller, W. (1988) CGA 157419 tech: 21-day repeated dose dermal
toxicity in the rat. Unpublished report No. 860077 from Ciba-Geigy
Ltd, Stein, Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basel,
Switzerland.
Schulze-Aurich, J. (1992a) Absorption, distribution, depletion of
residues and metabolic pathways of [U-14C]Cl-phenyl CGA 157419 in
the rat. Unpublished report No. 8/92 from Ciba-Geigy Ltd, Basel,
Switzerland. Submitted to WHO by Ciba- Geigy Ltd, Basel, Switzerland.
Schulze-Aurich, J. (1992b) The nature of the metabolites in tissues
and excreta of male cattle after single subcutaneous administration of
[U-14C]Cl-phenyl CGA 157419. Unpublished report No. 9/92 from
Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd, Basel, Switzerland.
Schulze-Aurich, J. (1992c) The fate of residues of [U-14C]Cl-phenyl
CGA 157419 in cattle tissues upon cooking. Unpublished report No.
10/92 from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd, Basel, Switzerland.
Strasser, F. & Arni, P. (1987) CGA 157419 tech: Nucleus anomaly test
in somatic interphase nuclei of Chinese hamster. Unpublished report
No. 860068 from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO
by Ciba-Geigy Ltd, Basel, Switzerland.
Strasser, F. & Muller, D. (1987) CGA 157419 tech: Chromosome studies
on human lymphocytes in vitro. Unpublished report No. 860070 from
Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd, Basel, Switzerland.
Strong, M.B. (1993) Effect of Acatak treatment on hide quality.
Unpublished report No. 93M/MBS/46 from Ciba-Geigy Ltd, Kemps Creek,
Australia. Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Strong, M.B. & Bowen, F.L. (1993) Reaction of cattle skin to Acatak.
Unpublished report No. 93/6/1410 from Ciba-Geigy Ltd, Kemps Creek,
Australia. Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Suarez, M. (1988) Acatik field trial. Unpublished report No. NPP 84.04
from Ciba-Geigy Ltd, Argentina. Submitted to WHO by Ciba-Geigy Ltd,
Basel, Switzerland.
Thevenaz, P., Gretener, P., Zak, F., Malinowski, W., Landes, C.,
Basler, W. & Gfeller, W. (1987) CGA 157419 tech: 28-day oral
cumulative toxicity study in rats (gavage). Unpublished report No.
860081 from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd, Basel, Switzerland.
Thomann, P. (1988) CGA 157419 tech: Developmental toxicity
(teratogenicity) study in rabbits. Unpublished report No. 860090 from
Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd, Basel, Switzerland.
Thouin, M.H. (1988) CGA 157419 tech: Teratogenicity study in rats.
Unpublished report No. 860088 from Ciba-Geigy Ltd, Basel, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
The nature of the residues in tissues and excreta of steers after
subcutaneous administration of fluazuron at 1.5 mg/kg bw was
investigated by thin-layer chromatography. The study was certified for
compliance with GLP and quality assurance. In all tissues taken at all
slaughter times, unchanged fluazuron was the only detectable fraction,
accounting for more than 90% of the total residues. In faeces, eight
metabolic fractions were found. The major compound was unchanged
fluazuron (about 70% of radiolabel), while the other, unidentified
fractions were more polar. Urine contained only unidentified
metabolized products, which were more polar than unchanged fluazuron.
Within 16 weeks of treatment, about 24% of the administered dose was
eliminated in faeces and urine, 16% as unchanged fluazuron and 8% as
its degradation products. Hence, fluazuron is metabolized to a lesser
extent in cattle than in rats (Schulze-Aurich, 1992b).
2.2 Toxicological studies
2.2.1 Acute toxicity
In studies of technical-grade fluazuron in distilled water
containing 0.5% carboxymethylcellulose and 0.1% polysorbate 80 in male
and female Tif:RAIf (SPF) rats, one study that followed OECD test
guideline 401 with quality assurance certification showed an oral
LD50 value of > 5000 mg/kg bw (Schoch, 1986a); in a study in the
same strain that followed OECD test guideline 402 with quality
assurance certification, the dermal LD50 was > 2000 mg/kg bw
(Schoch, 1986b). In a study that followed OECD test guideline 403, the
LC50 after exposure by inhalation was > 5994 mg/m3 (Hartmann &
Schoch, 1987). The toxic symptoms observed were sedation, dyspnoea,
exophthalmos, ruffled fur, and abnormal body positions. The animals
recovered within 6-11 days.
Three male New Zealand white rabbits received an instillation of
0.1 ml (56 mg) technical-grade fluazuron in a study that followed OECD
test guideline 405 with GLP and quality assurance certification. The
animals developed slight redness and chemosis of the cornea and
conjunctivae up to 24 h. No irritation of the iris was observed
(Schoch, 1986c).
In a study that followed OECD test guideline 404 with GLP and
quality assurance certification, three male New Zealand white rabbits
that received a semi-occlusive topical application of 0.5 g
technical-grade fluazuron developed no dermal reactions (Schoch,
1986d).
In 10 male and 10 female Pirbright white guinea-pigs submitted to
an optimization test, in a study that followed OECD test guideline 404
with GLP and quality assurance certification, no skin sensitization
was observed after challenge with technical-grade fluazuron
intradermally in 20% propylene glycol or epidermally in vaseline
(Schoch & Gfeller, 1987).
2.2.2 Short-term toxicity
Rats
In a study of dermal toxicity that followed OECD test guideline
410 with GLP and quality assurance certification, gauze patches
moistened with distilled water and technical-grade fluazuron at doses
of 0, 10, 100, or 1000 mg/kg bw were applied to the shaven skin of
groups of five male and five female Tif:RAIf (SPF) rats for 6 h/day,
five days per week for three weeks. The only effect observed was a
slight but significant prolongation of prothrombin time in male rats
at the two higher doses. The NOEL was 10 mg/kg bw per day (Schoch
et al., 1988).
Groups of 10 male and 10 female Tif:RAIf (SPF) rats received
technical-grade fluazuron by gavage for four weeks at intended doses
of 0, 10, 100, or 1000 mg/kg bw per day. The vehicle was distilled
water containing 0.5% carboxymethylcellulose and 0.1% Tween 80. Since
no clinical signs of toxicity were observed, the upper dose was
increased to 2000 mg/kg bw per day from day 10 onwards. The final
actual doses were 0, 3.2-5.6, 35-60, and 1660-1920 mg/kg bw per day.
The study was certified for compliance with GLP and quality assurance.
No treatment-related effects were seen on mortality rate,
clinical or ophthalmoscopic signs, body weight, or food or water
consumption, macroscopically or microscopically. Although treated
animals showed some statistically significant changes in blood
chemistry, these were not considered to be treatment-related (i.e. not
dose-related or of no biological relevance). All males at the medium
and high doses had a slight, statistically significant, dose-related
increase in prothrombin time and a slight, statistically significant,
dose-related decrease in platelet count. The mean liver weight was
increased in all treated animals, reaching statistical significance in
those at the two higher doses. Males at these doses also had a
decrease in mean thymus weights, which was statistically significant
only at the highest dose. These weight changes were not accompanied by
histopathological findings. The NOEL was 3.2-5.6 mg/kg bw per day, but
the validity of the study is limited due to the fact that the doses
intended to be the low and medium doses were not reached (Thevenaz
et al., 1987).
Groups of 20 male and 20 female Tif:RAIf (SPF) rats were fed
technical-grade fluazuron in the diet at concentrations of 0, 100,
600, 3500, or 20 000 mg/kg feed for three months, to give calculated
mean daily intakes of 0, 6.4, 39, 220, or 1300 mg/kg bw for males and
0, 6.6, 41, 240, or 1400 mg/kg bw for females. The study followed OECD
test guideline 408 with GLP and quality assurance certification.
No treatment-related effects were seen on mortality, clinical or
ophthalmoscopic signs, or macroscopically. Although body weight and
food consumption were marginally lower in treated males than in
controls, food conversion was not influenced. Treated males and
females showed some statistically significant changes in blood
chemistry; however, as these findings did not reflect any consistent
alteration (i.e. not dose-related or of no biological relevance), they
were not considered to be treatment-related. All treated males had
slightly increased prothrombin time (dose-related, statistically
significant in rats at 3500 and 20 000 mg/kg feed), in platelet count
(not dose-related, significant at 3500 mg/kg feed), and in lymphocyte
count (dose-related, significant at 20 000 mg/kg feed). The absolute
and relative weights of the liver and glycogen deposition were
significantly increased in all treated males, due, at least at 100
mg/kg feed, to poor study design, as sections were not made at random.
Changes in the absolute and relative weights of the heart (males),
kidney, ovaries, and adrenals (females) were not accompanied by
abnormal histopathological findings. Fluazuron treatment resulted in
slight to moderate hepatocellular hypertrophy in males and females at
3500 and 20 000 mg/kg feed and a slight increase in the incidence and
intensity of thyroid follicular hypertrophy and pituitary cell
hypertrophy in males at these doses. The authors considered the NOEL
to be 600 mg/kg feed (equal to 39-41 mg/kg bw per day); however,
effects on liver weight were observed in males at this dose. The
Committee concluded that the NOEL was 100 mg/kg feed, equal to 6.4
mg/kg bw per day, on the basis of effects on the liver. It should be
noted that similar findings were not observed in a long-term study of
toxicity and carcinogenicity in rats (see below) (Basler et al.,
1987).
Dogs
In a one-month range-finding study, groups of two male and two
female pure-bred beagle dogs were given technical-grade fluazuron at
doses of 200 or 50 000 mg/kg feed, equal to mean daily intakes of 8.5
or 2200 mg/kg bw per day, respectively. The study was certified for
compliance with GLP and quality assurance. No deaths occurred.
Clinical symptoms, food consumption, body-weight gain,
ophthalmoscopic, haematological, clinical, and urinary parameters, and
macroscopic and histopathological findings indicated no change that
could be considered to be related to treatment (Bloch et al., 1987).
In a three-month study, groups of four to six pedigree beagle
dogs of each sex were fed diets containing technical-grade fluazuron
at 0, 500, 5000, or 50 000 mg/kg feed. The study followed OECD test
guideline 409 with GLP and quality assurance certification. As several
animals in each group showed alterations indicative of an infection
(possibly leptospirosis) during the experiment, the study was not
considered useful for evaluating the safety of fluazuron. The only
findings of note that could not be attributed to the infectious
disease were degenerative, inflammatory, or hypertrophic changes in
the major arteries of females at 5000 and 50 000 mg/kg; however, this
effect was not confirmed in a 52-week feeding study with an interim
kill at 13 weeks (see below) (Gretener et al., 1988).
Groups of six male and six female beagle dogs received diets
containing technical-grade fluazuron at 0, 200, 3000, or 50 000 mg/kg
feed for one year. Two dogs of each sex per group were killed for
interim examinations after 13 weeks. The observations included
mortality, clinical signs, body weight, food consumption,
ophthalmoscopy, haematology, blood chemistry, urinalysis, organ
weights, and macroscopic and microscopic examinations. The calculated
mean intakes were 0, 7.5, 110, or 1900 mg/kg bw per day for males and
0, 7.1, 120, or 2000 mg/kg bw per day for females. The study followed
OECD test guideline 452 with GLP and quality assurance certification.
No deaths occurred. Treatment mainly affected two males at the high
dose (one killed at 13 weeks and the other at 52 weeks), which had
decreased food consumption, transient body-weight loss, and increased
activities of alkaline phosphatase and aspartate and alanine
aminotransferases from week 13 onwards; histopathological examination
revealed minimal multifocal areas of haemorrhage with slight
multifocal chronic inflammation of the liver. A slight increase in
alkaline phosphatase activity was also found in males at the middle
dose from week 13 onwards and in females at the high dose from week 39
onwards. Females at the high dose also had a slightly decreased
phosphorus concentration. The NOEL was 200 mg/kg feed, equal to 7.5
mg/kg bw per day, on the basis of changes in hepatic enzyme activities
(Briffaux, 1988a, 1990).
2.2.3 Long-term toxicity and carcinogenicity
Mice
Groups of 60 male and 60 female Tif: MAGf (SPF) mice were fed
diets containing technical-grade fluazuron at doses of 0, 40, 400,
4000, or 9000 mg/kg feed for two years, equal to average achieved
intakes of 0, 4.5, 45, 450, or 990 mg/kg bw per day for males and 0,
4.3, 43, 430, or 970 mg/kg bw per day for females. The observations
included clinical signs, mortality, body weight, food and water
consumption, haematological changes (10 animals of each sex per
group), and organ weights; detailed macroscopic and histopathological
examinations were perfomed. The study followed OECD test guideline 451
with GLP and quality assurance certification.
Clinical signs, body weight, and food consumption were unaffected
by treatment, as was survival (40-45% for controls, 43-58% for treated
animals). Water consumption was increased consistently in females at
9000 mg/kg feed and in females at 400 and 4000 mg/kg feed in the
second year of the study, but in females at 40 mg/kg feed and in all
treated males it was comparable to that of controls. Haematological
examination revealed no treatment-related changes, and the organ
weights and macroscopic findings at necropsy were comparable in
control and treated animals. The treatment-related non-neoplastic
changes included an increased incidence of cataract of the crystalline
lens characterized by mild necrosis and calcification of subcapsular
lens fibres in animals of each sex at 4000 and 9000 mg/kg feed and a
trend to increased diffuse hyperplasia of prostatic glandular tissue
in males at 4000 and 9000 mg/kg feed. In addition, the following
uterine changes, which were not dose-related, were noted: increased
incidences of inflammatory polyps at 400, 4000, and 9000 mg/kg feed,
increased dilatation of the lumen at 4000 and 9000 mg/kg feed, and
increased incidences of haematomas and dilatation of blood vessels
associated with thrombosis at 9000 mg/kg feed. Tumour incidences were
not increased, although a marginally increased incidence of systemic
infiltration with malignant lymphoma was observed in some organs of
some animals at the high dose. The total number of lymphomas per
animal and the total number of animals bearing lymphomas were not
significantly different from those among controls. On the basis of the
pathological changes in the uterus, the NOEL was 40 mg/kg feed, equal
to 4.3 mg/kg bw per day (Bachmann et al., 1991a).
In the same study, the total amount of fluazuron in the body
appeared to reach a maximum at approximately 400 mg/kg feed: the
fluazuron level was 4.9-8.7 µg/ml in blood and 880-970 mg/kg in fat in
animals of each sex at 4000 and 9000 mg/kg feed and only slightly
lower in those at 400 mg/kg feed. At 40 mg/kg feed, a steady state was
not reached. The blood:fat ratio was approximately 1:200 in all groups
(Maier, 1991a).
Rats
Groups of 80 male and 80 female Tif:RAIf (SPF) rats received
diets containing technical-grade fluazuron at 0, 50, 500, 10 000, or
20 000 mg/kg feed for two years, equal to average achieved intakes of
0, 1.9, 18, 380, or 780 mg/kg bw per day for males and 0, 2.1, 21,
440, or 920 mg/kg bw per day for females. Ten rats of each sex were
killed after one year for interim necropsy. The observations included
clinical signs, mortality, body weight, food consumption,
ophthalmoscopy, haematology (20 rats of each sex per group), blood
chemistry (10 of each sex per group), urinalysis (10 of each sex per
group), and organ weights; detailed macroscopic and histopathological
examinations were performed. The study followed OECD test guideline
453 with GLP and quality assurance certification.
The survival of the animals (56-67% for controls, 54-73% for
treated animals) was not affected by treatment. Minor effects were
observed in animals at the highest dose at interim sacrifice but not
at terminal sacrifice. In females, the relative liver and kidney
weights were significantly decreased, with no associated morphological
changes; in males, minimal hypertrophy of hepatocytes was seen.
Females at the highest dose also had decreased body-weight gain during
the second year of treatment, but this failed to reach statistical
significance. The findings were considered not to be of toxicological
significance. The incidence of tumours was not increased. The total
amount of fluazuron in the body appeared to reach a maximum at 500
mg/kg feed: the level was 1.3-2.3 µg/ml in blood and 290-440 mg/kg in
fat in animals of each sex at 500 mg/kg feed and above. At 50 mg/kg
feed, a steady state was not reached. The blood:fat ratio was
approximately 1:200 in all groups (Bachmann et al., 1991b; Maier,
1991b).
2.2.4 Genotoxicity
The results of tests for genotoxicity carried out with fluazuron
are summarized in Table 1.
2.2.5 Reproductive toxicity
2.2.5.1 Multigeneration reproductive toxicity
In a preliminary study of reproductive toxicity, groups of seven
male and 14 female Ico:OFA Sprague-Dawley rats received
technical-grade fluazuron at dietary concentrations of 0, 200, 1000,
7000, or 20 000 mg/kg feed. Males were dosed for two weeks before
mating and during the mating period and were sacrificed thereafter.
Females were dosed from two weeks before mating up to 21 days post
partum, when both the females and their litters were sacrificed. The
study was certified for quality assurance.
There were no deaths or treatment-related clinical signs in
animals of either sex during the study. The food consumption and
body-weight gain of F0 animals and the precoital period and duration
of the gestation period were not affected by treatment, and no effects
were seen on insemination, fecundity, fertility, resorption indices,
stillbirths, nursing behaviour, the numbers of live F1 pups, their
physical development, or neonatal or postnatal mortality. The mean
weight at birth of F1 pups was not affected by treatment, but a
slight retardation in growth rate was seen towards the end of the
lactation period in animals at 7000 and 20 000 mg/kg feed. No
treatment-related effects were seen in any animal at necropsy
(Briffaux, 1988b).
In a two-generation study of reproductive toxicity, groups of 30
male and 30 female Ico:OFA Sprague-Dawley rats received diets
containing technical-grade fluazuron at 0, 100, 1500, or 20 000 mg/kg
feed for at least 100 days before mating and then throughout gestation
and lactation for two successive generations. Two litters were bred
per generation. Males and females of the first F1 litter (F1a)
were selected to breed the next generation. After weaning of the F1b
and F2b pups, the parental animals were killed and necropsied.
Unselected F1a pups and F1b, F2a, and F2b pups were killed and
necropsied after 21 days of lactation. The study followed OECD test
guideline 416 with GLP and quality assurance certification.
The only effects found were on food consumption and body weight,
not on reproductive function. F1 females at the high dose showed a
slight reduction in body weight in the first gestation period, and the
food consumption of those at the middle and high doses tended to be
slightly reduced at the end of both lactation periods. Neonatal
viability of both generations of rats at the high dose was slightly
reduced after the first matings, but the litters from subsequent
matings were not affected. Pup viability from day 4 post partum to
weaning was not affected by treatment during any phase of the study.
The body weights at birth of pups in all litters were comparable to
those of controls, but the body-weight gain of those at the high dose
was reduced for all generations (F1a, F1b, F2a, F2b) and of
those at the middle dose for the F1a, F2a, and F2b generations.
The NOEL was 100 mg/kg feed, equivalent to 5 mg/kg bw per day, on the
basis of postnatal toxicity (Barrow & Briffaux, 1991).
2.2.5.2 Developmental toxicity
Rats
Technical-grade fluazuron was administered by gavage in a 0.1%
aqueous solution of polysorbate 80 at doses of 0, 10, 100, or 1000
mg/kg bw per day to groups of 24 mated female Tif:RAIf (SPF) rats on
days 6-15 of gestation. On day 21 of gestation, the dams were killed
and necropsied, and the fetuses were weighed, sexed, and examined for
external, visceral, and skeletal abnormalities. The study followed
OECD test guideline 414 with GLP and quality assurance certification.
One dam at the middle dose aborted its entire litter. No
treatment-related effects were observed in dams, and the numbers of
implantations, live young, and resorptions and fetal and litter
weights were unaffected by treatment. There was no indication of
teratogenicity. Hence, fluazuron at doses up to 1000 mg/kg bw per day
had no maternal, embryo- or fetal toxicity or teratogenicity (Thouin,
1988).
Rabbits
Groups of 20 mated female Chinchilla-type rabbits received
technical-grade fluazuron suspended in 3% (w/w) aqueous corn starch by
gavage at doses of 0, 10, 100, or 1000 mg/kg bw per day on days 7-19
of pregnancy. The dams were killed and necropsied on day 29 of
pregnancy, and various parameters examined. Fetuses were subjected to
gross necropsy and visceral and skeletal examination. The study
followed OECD test guideline 414 with GLP and quality assurance
certification. One female at the high dose died due to faulty
intubation, and another resorbed all of its implants. The incidence of
post-implantation loss was slightly increased in all treated groups,
mainly due to a slightly higher rate of early resorptions, but was
still within the historical control range and was considered not to be
related to treatment. The number of live fetuses and the litter weight
were unaffected by treatment. The body weights of the fetuses of all
treated dams were slightly increased; the increases in group means
were statistically significant but the litter means were not. No other
effects were observed. Hence, at doses up to 1000 mg/kg bw per day
fluazuron induced no maternal, embryo or fetal toxicity and was not
teratogenic (Thomann, 1988).
Table 1. Results of assays for genotoxicity with fluazuron
End-point Test object Concentration Result S9 QA Reference
In vitro
Reverse S. typhimurium 1.14-278 µg/mla Negative + No Deparade &
mutation TA98, TA100, Negative - Arni (1985)
TA1535, TA1537
Gene V79 Chinese 12.5-500 ng/ml Negative + Yes Dollenmeier &
mutation hamster cells 0.625-25 µg/ml Negative - Puri (1987)
Chromosomal Human lymphocytes 14.1-225 µg/ml Negative + Yes Strasser &
aberration 7.5-120.0 µg/ml Negative - Muller (1987)
DNA repair Rat hepatocytes 0.4-300 µg/ml Negative Yes Puri & Muller (1987)
DNA repair Human fibroblasts 0.2-50 µg/ml Negative Yes Meyer & Puri (1987)
In vivo
Nuclear Chinese hamster 1.25-5.0 g/kg bwb Negativec Yes Strasser & Arni
anomalies bone marrow (1987)
S9, 9000 × g fraction of rat liver; QA, quality assurance
a No cytotoxicity, but precipitation occurred at the highest dose in the presence of S9
b Daily doses given by gavage on each of two consecutive days; cyclophosphamide used as a positive control
c The authors considered 5.0 g/kg bw to be the highest applicable dose, but as cytotoxicity was not
determined it was not clear whether the bone marrow was exposed.
2.2.6 Special studies on target animals
In a study of tolerability, groups of three male and three female
Hereford cattle received the pour-on formulation Acatak at 0
(control), once, three times, or five times the recommended dose of 2
mg/kg bw fluazuron, along the back from the neck to the rump. The
animals were observed for the following eight weeks and monitored for
subclinical side-effects. The study was certified for quality
assurance. Apart from the formation of a crusty layer on the skin and
coat, which caused no irritation or discomfort to the animals, the
formulation was well tolerated at all doses. No drug-related changes
were observed in body weight, feed consumption, body temperature, or
haematological or blood biochemical parameters (Bowen & Strong, 1993).
When the label instructions for application of the pour-on formulation
Acatak were followed, a crusty layer formed on the coats of the
cattle, but the animals displayed no clinical signs of irritation.
Histopathological examination showed only minor pathological changes
in the skin, which had no effect on the hide quality (Strong, 1993;
Strong & Bowen, 1993).
Subcutaneous injection of Acatik, a commercial formulation
containing fluazuron as the active ingredient, induced no side-effects
or local reactions at the injection site in cattle receiving single
doses of 1 mg/kg bw fluazuron or dogs receiving double doses of 2.5
and then 5 mg/kg bw (Suarez, 1988; Genchi, 1990).
2.2.7 Special studies on heat degradation
The degradation of fluazuron residues present in cattle meat and
fat was investigated after normal dry cooking, cooking in water or
oil, or microwave treatment. The meat and fat samples were derived
from steers treated with fluazuron at 1.5 mg/kg bw by subcutaneous
injection, which is not the recommended route of administration. The
study was certified for compliance with GLP and quality assurance.
Residues of fluazuron were partly (in meat) or completely (in fat)
degraded to [3-(3-chloro-5-trifluoro-methyl-2-pyridinyloxy)-4-
chlorophenyl]-1-amine. This degradation product did not show mutagenic
potential in an assay for reverse mutation in Salmonella typhimurium
strains TA98, TA100, TA1535, and TA1537 or in Escherichia coli
strain WP2uvrA, when the known mutagen found in cooked meat, MeIQx
(2-amino-3,8- dimethylimidaz[4,5-f]quinoxaline), was used as the
reference substance (Schulze-Aurich, 1992c; Maier, 1993).
3. COMMENTS
The Committee considered the results of studies on the
pharmacokinetics, metabolism, acute, short-term and long-term
toxicity, carcinogenicity, genotoxicity, and reproductive toxicity of
fluazuron. All of the studies critical for the evaluation were carried
out according to appropriate standards for study protocol and conduct.
Analysis 24 h after oral administration of radiolabelled
fluazuron to rats showed that an average of 60% was absorbed through
the gut. Most of the absorbed radiolabel was taken up into adipose
tissues, while significantly lower levels were found in liver, kidney,
lung, muscle, and brain. Ninety percent of the radiolabel in tissues
was attached to unchanged fluazuron. Fluazuron was eliminated slowly
from adipose tissues, following first-order kinetics, with a half-life
of about 13 days. Elimination occurred primarily in the faeces. One
week after administration, 59% had been excreted in faeces and 3% in
urine. About one-third of the dose was eliminated as unchanged
fluazuron in the faeces; the remaining two-thirds was metabolized by
cleavage of the urea moiety between the benzoyl carbon and the urea
nitrogen, followed by hydroxylation in the phenyl ring.
When radiolabelled fluazuron was administered topically to
cattle, radiolabel was slowly absorbed, either percutaneously, orally
(by licking), or by both routes. A steady state between absorption and
elimination was observed for three to four weeks after treatment. The
absorbed radiolabel was taken up mainly by adipose tissues and to a
lesser extent by other tissues. Depletion of fluazuron from plasma and
tissues was slow, with half-lives of elimination of 10.5 and 4.5-5.5
weeks, respectively. The major route of elimination was the faeces
(62% of the dose within 16 weeks), while renal excretion was of minor
importance (1% of the dose within 16 weeks). There was some indication
of biliary excretion. Fluazuron was not extensively metabolized, as
unchanged fluazuron accounted for more than 90% of the total residues
in tissues and faeces. A similar slow absorption, distribution, and
elimination pattern was observed after subcutaneous administration of
radiolabelled fluazuron to steers. The pattern of metabolites excreted
in faeces was somewhat more complex, however, indicating that about
one-third of the fluazuron was metabolized into more polar
metabolites. Although the fate of fluazuron in rats was similar to
that in cattle, they metabolized fluazuron to a greater extent than
cattle.
Orally administered fluazuron was found to have low acute
toxicity, with an LD50 value of > 5000 mg/kg bw in rats.
The short-term toxicity of fluazuron was evaluated by oral
administration to rats and dogs. In a 28-day study, rats received
fluazuron by gavage at doses of 0, 10, 100, or 2000 mg/kg bw per day.
Increased prothrombin time and liver weight and decreased platelet
counts and thymus weight were observed, mainly in animals at the two
higher doses, and particularly in males. Male rats were also more
sensitive than females to the effects of fluazuron in a 13-week
feeding study at dietary concentrations of 0, 100, 600, 3500, or
20 000 mg/kg feed (equal to 6.4-1400 mg/kg bw per day). Male rats at
the two higher doses had increased prothrombin time, platelet and
lymphocyte counts, and absolute and relative liver weights, as well as
hypertrophy of thyroid follicular cells, pituitary cells, and
hepatocytes. In males, increases in absolute and relative liver
weights were also observed at 600 mg/kg feed. In females,
hepatocellular hypertrophy occurred at 3500 and 20 000 mg/kg feed. The
NOEL was 100 mg/kg feed, equal to 6.4 mg/kg bw per day, on the basis
of effects on the liver.
In a one-year feeding study, dogs received fluazuron at dietary
concentrations of 0, 200, 3000, or 50 000 mg/kg feed. Effects were
observed primarily in males at the highest dose, consisting of
decreased food consumption, transient body-weight loss, increased
serum activities of alkaline phosphatase and aspartate and alanine
aminotransferases, and minimal multifocal haemorrhage with slight
multifocal chronic inflammation in the liver. A slight increase in
alkaline phosphatase activity was also observed in males at 3000 mg/kg
feed and in females at 50 000 mg/kg feed. The NOEL was 200 mg/kg feed,
equal to 7.5 mg/kg bw per day, on the basis of changes in hepatic
enzyme activities.
In a study of carcinogenicity, mice received diets containing 0,
40, 400, 4000, or 9000 mg/kg feed for two years (equal to 4.3-990
mg/kg bw per day). Females at the two higher levels showed increased
water consumption, cataracts, and uterine changes (inflammatory
polyps, luminal dilatation and, at 9000 mg/kg feed only, haematomas
and dilatation of blood vessels associated with thrombosis). Increased
water consumption and inflammatory uterine polyps were also observed
in females at 400 mg/kg feed. Male mice at the two higher levels had
cataracts, and a trend to an increasing frequency of diffuse
hyperplasia of prostatic glandular tissue was observed. The NOEL was
40 mg/kg feed, equal to 4.3 mg/kg bw per day, on the basis of
pathological changes in the uterus.
In a long-term study of toxicity and carcinogenicity, rats were
given fluazuron at 0, 50, 500, 10 000, or 20 000 mg/kg feed for two
years (equal to 1.9-920 mg/kg bw per day), and a proportion of the
animals were killed at one year. No toxicologically significant
effects were observed at any dose. The toxic effects on the liver
observed in the 13-week study in rats were not observed after either
one or two years.
In the long-term studies of toxicity, the total amount of
fluazuron in the body appeared to reach a maximum at relatively low
levels (approximately 400 and 500 mg/kg feed, equal to 43 and 18 mg/kg
bw per day, in mice and rats, respectively), as the concentration of
the compound in blood and fat did not increase at higher levels. The
reason is not clear. It is not known whether a similar effect occurred
in the short-term studies, because blood and fat levels were not
measured.
Fluazuron has been tested in vitro for its ability to induce
reverse mutations in Salmonella typhimurium, gene mutations in Chinese
hamster cells, chromosomal aberration in human lymphocytes, and DNA
repair in rat hepatocytes and human fibroblasts. It has also been
tested in vivo for its ability to induce nuclear anomalies in
Chinese hamster bone marrow. All the results were negative. On the
basis of these data and the results of the bioassays in rodents, the
Committee concluded that fluazuron has no genotoxic or carcinogenic
potential.
In a two-generation study of reproductive toxicity in rats at
dietary concentrations of 0, 100, 1500, or 20 000 mg/kg feed
(equivalent to 5-1000 mg/kg bw per day), fluazuron had no adverse
effects on reproductive function. The only effects observed were
slightly retarded growth of pups at 1500 and 20 000 mg/kg feed and a
slight increase in neonatal mortality at 20 000 mg/kg feed. The NOEL
was 100 mg/kg feed, equivalent to 5 mg/kg bw per day, on the basis of
postnatal toxicity.
Fluazuron was not maternally toxic and did not cause
embryotoxicity, fetotoxicity, or teratogenicity in rats or rabbits at
oral doses up to 1000 mg/kg bw per day.
Because fluazuron belongs to a class of insect growth regulators
that do not act on the nervous system and because the central nervous
system was not a target organ in the short-term or long-term studies
of toxicity in rats, mice, or dogs, special studies of neurotoxicity
have not been performed. The Committee concluded that such studies
were unnecessary.
4. EVALUATION
The Committee established an ADI of 0-40 µg/kg bw for fluazuronâ
on the basis of the NOEL of 4.3 mg/kg bw per day for pathological
changes in the uterus in the two-year study in mice and a 100-fold
safety factor. The ADI was rounded to one significant figure, as is
the usual practice (Annex 1, reference 91, section 2.7).
5. REFERENCES
Bachmann, M., Hunter, B., Gretener, P., Krinke, G., Landes, C.,
Christen, P., Seewald, W., Heinemann, G.W. & Basler, W. (1991a) CGA
157419 tech: Lifetime carcinogenicity study in mice. Unpublished
report No. 860086 from Ciba-Geigy Ltd, Stein, Switzerland. Submitted
to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Bachmann, M., Hunter, B., Gretener, P., Froehlich, E., Faccini, J.M.,
Krinke, G., Christen, P., Seewald, W., Heinemann, G.W. & Basler, W.
(1991b) CGA 157419 tech: Carcinogenicity and chronic toxicity study in
rats. Unpublished report No. 860087 from Ciba-Geigy Ltd, Stein,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Barrow, P. & Briffaux, J.P. (1991) CGA 157419 tech: Two generation
oral (dietary administration) reproduction study in the rat.
Unpublished report No. 860089 from Hazleton France, L'Arbresle, France
(Hazleton report No. 710779). Submitted to WHO by Ciba-Geigy Ltd,
Basel, Switzerland.
Basler, W., Fankhauser, H., Gretener, P., Froehlich, E., Heider, K. &
Gfeller, W. (1987) CGA 157419 tech: 3-month oral toxicity study in
rats (administration in food). Unpublished report No. 860080 from
Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd, Basel, Switzerland.
Bloch, I., Frei, T., Luetkemeier, H. & Wilson, J. (1987) CGA 157419:
4-week oral range-finding toxicity (feeding) study in beagle dogs.
Unpublished report No. 860083 from Research & Consulting Company AG,
Itingen, Switzerland (RCC report No. 072977). Submitted to WHO by
Ciba-Geigy Ltd, Basel, Switzerland.
Bowen, F.L. & Strong, M.B. (1993) The tolerability of cattle to
exposure to the acarine growth regulator Acatak. Unpublished report
No. 93/3/1398 from Ciba-Geigy Ltd, Kemps Creek, Australia. Submitted
to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Briffaux, J.P. (1988a) CGA 157419 tech: 52 week oral (dietary
administration) toxicity study in the beagle dog. Interim report for
the first 13 weeks of the study. Unpublished report No. 860085 from
Hazleton France, L'Arbresle, France (Hazleton report No. 712829).
Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Briffaux, J.P. (1988b) CGA 157419: Preliminary study for a
2-generation oral (dietary administration) reproduction study in the
rat. Unpublished report No. 860400 from Hazleton France, L'Arbresle,
France (Hazleton report No. 703623). Submitted to WHO by Ciba-Geigy
Ltd, Basel, Switzerland.
Briffaux, J.P. (1990) CGA 157419 tech: 52 week oral (dietary
administration) toxicity study in the beagle dog. Unpublished report
No. 860085 from Hazleton France, L'Arbresle, France (Hazleton report
No. 094089). Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Cameron, B.D., Somers, K. & Speirs, G.C. (1992) The distribution and
excretion of [U-14C]Cl-phenyl CGA 157419 after subcutaneous
injection to cattle. Unpublished report No. 141232 from Inveresk
Research International, Tranent, Scotland, United Kingdom (Inveresk
report No. 6189). Submitted to WHO by Ciba-Geigy Ltd, Basel,
Switzerland.
Deparade, E. & Arni, P. (1985) Salmonella Cobas. Bact. pilot test with
CGA 157419 techn. (test for mutagenic effects on bacteria).
Unpublished report No. 850235 from Ciba-Geigy Ltd, Basel, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Dollenmeier, P. & Puri, E. (1987) CGA 157419 tech: V79 Chinese hamster
point mutation test (with and without microsomal activation).
Unpublished report No. 860071 from Ciba-Geigy Ltd, Basel, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Genchi, C. (1990) Tolerability of Acatik formulation A-8560 A in dogs.
Unpublished report from Facolta di Medicina Veterinaria, Universita
degli studi di Milano, Milan, Italy. Submitted to WHO by Ciba-Geigy
Ltd, Basel, Switzerland.
Gretener, P., Froehlich, E., Heider, K., Christen, P. & Gfeller, W.
(1988) CGA 157419 tech: 3-month subchronic oral toxicity study in
beagle dogs. Unpublished report No. 860084 from Ciba-Geigy Ltd, Stein,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Hartmann, H.R. & Schoch, M. (1987) CGA 157419 tech: Acute aerosol
inhalation toxicity in the rat. Unpublished report No. 860078 from
Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd, Basel, Switzerland.
Johnson, S., Johnston, A.M. & Prout, M.S. (1996)
[Chlor-phenyl-(U)-14C]-CGA 157419: Nature of metabolites in excreta
and tissues in ruminant cattle following single topical
administration. Unpublished report No. 156940 from Inveresk Research,
Tranent, Scotland, United Kingdom (Inveresk report No. 13026).
Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Maier, W. (1991a) CGA 157419: Determination of residues in whole blood
and fat of mice after subchronic and chronic exposure. Summary report
and assessment. Unpublished report from Ciba-Geigy Ltd, Basel,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Maier, W. (1991b) CGA 157419: Determination of residues in whole blood
and fat of rats after subchronic and chronic exposure. Summary report
and assessment. Unpublished report from Ciba-Geigy Ltd, Basel,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Maier, W. (1993) CGA 157419: Toxicological assessment of heat
degradation products. Unpublished report from Ciba-Geigy Ltd, Basel,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
McLean, C.L. & Dunsire, J.P. (1996) The absorption, distribution,
excretion, and residue depletion of [chlor-phenyl-(U)-14C]-CGA
157419 in ruminant cattle following topical administration.
Unpublished report No. 155601 from Inveresk Research, Tranent,
Scotland, United Kingdom (Inveresk report No. 12446). Submitted to WHO
by Ciba-Geigy Ltd, Basel, Switzerland.
Meyer, A. & Puri, E. (1987) CGA 157419 tech: Autoradiographic
DNA-repair test on human fibroblasts (OECD-conform). Unpublished
report No. 861231 from Ciba-Geigy Ltd, Basel, Switzerland. Submitted
to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Puri, E. & Muller, D. (1987) CGA 157419 tech: Autoradiographic
DNA-repair test on rat hepatocytes (OECD-conform). Unpublished report
No. 861230 from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO
by Ciba-Geigy Ltd, Basel, Switzerland.
Schoch, M. (1986a) CGA 157419 tech: Acute oral toxicity in the rat.
Unpublished report No. 860073 from Ciba-Geigy Ltd, Basel, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Schoch, M. (1986b) CGA 157419 tech: Acute dermal toxicity in the rat.
Unpublished report No. 860076 from Ciba-Geigy Ltd, Basel, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Schoch, M. (1986c) CGA 157419 tech: Acute eye irritation/corrosion
study in the rabbit. Unpublished report No. 860074 from Ciba-Geigy
Ltd, Basel, Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basel,
Switzerland.
Schoch, M. (1986d) CGA 157419 tech: Acute dermal irritation/corrosion
study in the rabbit. Unpublished report No. 860075 from Ciba-Geigy
Ltd, Basel, Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basel,
Switzerland.
Schoch, M. & Gfeller, W. (1987) CGA 157419 tech: Skin sensitization
test in the guinea pig (optimization test). Unpublished report No.
860066 from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd, Basel, Switzerland.
Schoch, M., Gretener, P., Froehlich, E., Germer, M., Krinke, G. &
Gfeller, W. (1988) CGA 157419 tech: 21-day repeated dose dermal
toxicity in the rat. Unpublished report No. 860077 from Ciba-Geigy
Ltd, Stein, Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basel,
Switzerland.
Schulze-Aurich, J. (1992a) Absorption, distribution, depletion of
residues and metabolic pathways of [U-14C]Cl-phenyl CGA 157419 in
the rat. Unpublished report No. 8/92 from Ciba-Geigy Ltd, Basel,
Switzerland. Submitted to WHO by Ciba- Geigy Ltd, Basel, Switzerland.
Schulze-Aurich, J. (1992b) The nature of the metabolites in tissues
and excreta of male cattle after single subcutaneous administration of
[U-14C]Cl-phenyl CGA 157419. Unpublished report No. 9/92 from
Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd, Basel, Switzerland.
Schulze-Aurich, J. (1992c) The fate of residues of [U-14C]Cl-phenyl
CGA 157419 in cattle tissues upon cooking. Unpublished report No.
10/92 from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd, Basel, Switzerland.
Strasser, F. & Arni, P. (1987) CGA 157419 tech: Nucleus anomaly test
in somatic interphase nuclei of Chinese hamster. Unpublished report
No. 860068 from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO
by Ciba-Geigy Ltd, Basel, Switzerland.
Strasser, F. & Muller, D. (1987) CGA 157419 tech: Chromosome studies
on human lymphocytes in vitro. Unpublished report No. 860070 from
Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd, Basel, Switzerland.
Strong, M.B. (1993) Effect of Acatak treatment on hide quality.
Unpublished report No. 93M/MBS/46 from Ciba-Geigy Ltd, Kemps Creek,
Australia. Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Strong, M.B. & Bowen, F.L. (1993) Reaction of cattle skin to Acatak.
Unpublished report No. 93/6/1410 from Ciba-Geigy Ltd, Kemps Creek,
Australia. Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
Suarez, M. (1988) Acatik field trial. Unpublished report No. NPP 84.04
from Ciba-Geigy Ltd, Argentina. Submitted to WHO by Ciba-Geigy Ltd,
Basel, Switzerland.
Thevenaz, P., Gretener, P., Zak, F., Malinowski, W., Landes, C.,
Basler, W. & Gfeller, W. (1987) CGA 157419 tech: 28-day oral
cumulative toxicity study in rats (gavage). Unpublished report No.
860081 from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd, Basel, Switzerland.
Thomann, P. (1988) CGA 157419 tech: Developmental toxicity
(teratogenicity) study in rabbits. Unpublished report No. 860090 from
Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd, Basel, Switzerland.
Thouin, M.H. (1988) CGA 157419 tech: Teratogenicity study in rats.
Unpublished report No. 860088 from Ciba-Geigy Ltd, Basel, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd, Basel, Switzerland.
