
FAO Nutrition Meetings
Resort Series No. 44A
WHO/Food Add./68.33
TOXICOLOGICAL EVALUATION OF SOME
FLAVOURING SUBSTANCES AND
NON-NUTRITIVE SWEETENING AGENTS
Geneva, 21-28 August 1967
The Eleventh Report of the Joint FAO/WHO Expert Committee on Food
Additives is published as FAO Nutrition Meetings Report Series,
1967, No. 44; Wld Hlth Org. techn. Rep. Ser., 1968, 383. This
Report contains general considerations, including the principles
adopted for the evaluation, and a summary of the results of the
evaluations of a number of food additives. Additional information,
such as biological data and a toxicological evaluation, considered at
that meeting, is to be found in this document.
Food and Agriculture Organization of the United Nations
World Health Organization
1967
trans-ANETHOLE
Chemical name trans-p-Propenylanisole
Empirical formula C10H12O
Structural formula
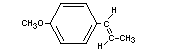 Molecular weight 148.21
Description Anethole is prepared from anise oil and
other sources, or it is prepared
synthetically. It is a colourless or faintly
yellow liquid at or above 23°. It has a
sweet taste and a characteristic anise-like
odour. It is affected by light.
Limit of cis-isomer Not more than 1 per cent. (by gas-liquid
chromatography).
Biological Data
Biochemical Aspects
This ether is probably metabolized to anisic acid (Kühling &
Giacosa, 1890).
Acute toxicity
Animal Route LD50 Reference
(mg/kg body-weight)
trans-isomer cis-isomer
Mouse oral 5000 - Boissier et al.,1967
Mouse oral 3050 - Jenner et al., 1964
Mouse i.p. 1410 95 Caujolle & Meynier, 1958
Mouse i.p. 650 135 Boissier et al., 1967
Rat oral 3200 - Boissier et al., 1967
Rat oral 2940 150 Shelansky & Gabriel, 1958
Rat oral 2090 - Jenner et al., 1964
Rat i.p. - 67 Caujolle & Meynier, 1958
Rat i.p. 900 93 Boissier et al., 1967
Guinea-pig oral 2167 - Jenner et al., 1964
Feeding 3 male and 3 female rats 700 mg/kg body-weight/day
intragastrically for 4 days produced slight gross changes in the liver
(Taylor et al., 1964).
Short-term studies
Trans-isomer
Rat. Groups of 5 males and 5 females were fed dietary levels of
0, 1000, 3000, 10 000 and 30 000 ppm of trans-isomer for 90 days.
There was no survival past 20 days at the highest level, and survival
was affected at the 10 000 ppm level. Depressed food consumption and
body-weight gain, in proportion to the level fed, were found at 3000
ppm and above. No examination was made of animals dying during the
study, but post-study gross and microscopic examination of major
organs of survivors disclosed hepatocellular oedema, degeneration and
regeneration, in proportion to the level fed, at, 3000 ppm and above.
No effect was seen at 1000 ppm. There were no differences between the
groups in periodic examinations of blood and urine (Kay & Calandra,
1959).
Groups of 5 male and 5 female rats were fed diets containing 0,
0.25 and 1.0 per cent. of anethole. The group in the highest level was
sacrificed after 15 weeks and showed slight hydropic changes of
hepatic cells in males only, while the group on the lowest level was
kept for 1 year without any evidence of adverse effects (Hagan et al.,
1967).
Cis-isomer
Rat. Groups of 5 males and 5 females were fed dietary levels of
0, 10, 30, 100, 300, 1000 and 3000 ppm of cis-isomer for 90 days.
Survival was affected and food consumption and body-weight gain were
depressed at the highest level. No examination was made of animals
dying during the study, but post-study gross and microscopic
examination of major organs of survivors disclosed hepatocellular
oedema, degeneration and regeneration, in proportion to the level fed,
at 300 ppm and above. No effect was seen at 100 ppm. There were no
differences between the groups in periodic examination of blood and
urine (Kay & Calandbra, 1959).
Long-term studies
No data available.
Comments
This ether is probably metabolized to anisic acid. The available
information is scanty and toxicity appears to be dependent on
stereoisomeric configuration. The short-term studies can be used for
evaluation. Further metabolic investigation and long-term studies are
essential.
EVALUATION
Level causing no toxicological effect
Rat: 2500 ppm in the diet, equivalent to 125 mg/kg
body-weight/day.
Estimate of acceptable daily intake for man
mg/kg body-weight
Conditional acceptance 0-1.25
Further work required
Biochemical, metabolic and long-term studies on the
trans-stereoisomer with emphasis on the effect on the liver.
REFERENCES
Boissier, J. R., Simon, P. & LaBourhis, B. (1967) Thérapie, 22,
309
Canjolle, F. & Meynier, D. (1958) Compt. Rend., 246, 1465
Hagan, E. C., Hansen, W. H., Fitzhugh, O. G., Jenner, P. M., Jones, W.
I., Taylor, J. M., Long, E. L., Nelson, A. A. & Brouwer, J. B. (1967)
Fd Cosmet. Toxicol., 5(2), 141
Jenner, P. M., Hagan, E. C., Taylor, J. M., Cook, E. L. & Fitzhugh, O.
G. (1964) Fd Cosmet. Toxicol., 2, 327
Kay, J. H. & Calandra, J. C. (1959) Unpublished report submitted by
Hercules Powder Company, Inc.
Kühling, O. & Giacosa, P. (1890) Ann. chim. Farm., 11, 304
Taylor, J. M., Jenner, P. M. & Jones, W. I. (1964) Toxicol. appl.
Pharmacol.,6, 378
Molecular weight 148.21
Description Anethole is prepared from anise oil and
other sources, or it is prepared
synthetically. It is a colourless or faintly
yellow liquid at or above 23°. It has a
sweet taste and a characteristic anise-like
odour. It is affected by light.
Limit of cis-isomer Not more than 1 per cent. (by gas-liquid
chromatography).
Biological Data
Biochemical Aspects
This ether is probably metabolized to anisic acid (Kühling &
Giacosa, 1890).
Acute toxicity
Animal Route LD50 Reference
(mg/kg body-weight)
trans-isomer cis-isomer
Mouse oral 5000 - Boissier et al.,1967
Mouse oral 3050 - Jenner et al., 1964
Mouse i.p. 1410 95 Caujolle & Meynier, 1958
Mouse i.p. 650 135 Boissier et al., 1967
Rat oral 3200 - Boissier et al., 1967
Rat oral 2940 150 Shelansky & Gabriel, 1958
Rat oral 2090 - Jenner et al., 1964
Rat i.p. - 67 Caujolle & Meynier, 1958
Rat i.p. 900 93 Boissier et al., 1967
Guinea-pig oral 2167 - Jenner et al., 1964
Feeding 3 male and 3 female rats 700 mg/kg body-weight/day
intragastrically for 4 days produced slight gross changes in the liver
(Taylor et al., 1964).
Short-term studies
Trans-isomer
Rat. Groups of 5 males and 5 females were fed dietary levels of
0, 1000, 3000, 10 000 and 30 000 ppm of trans-isomer for 90 days.
There was no survival past 20 days at the highest level, and survival
was affected at the 10 000 ppm level. Depressed food consumption and
body-weight gain, in proportion to the level fed, were found at 3000
ppm and above. No examination was made of animals dying during the
study, but post-study gross and microscopic examination of major
organs of survivors disclosed hepatocellular oedema, degeneration and
regeneration, in proportion to the level fed, at, 3000 ppm and above.
No effect was seen at 1000 ppm. There were no differences between the
groups in periodic examinations of blood and urine (Kay & Calandra,
1959).
Groups of 5 male and 5 female rats were fed diets containing 0,
0.25 and 1.0 per cent. of anethole. The group in the highest level was
sacrificed after 15 weeks and showed slight hydropic changes of
hepatic cells in males only, while the group on the lowest level was
kept for 1 year without any evidence of adverse effects (Hagan et al.,
1967).
Cis-isomer
Rat. Groups of 5 males and 5 females were fed dietary levels of
0, 10, 30, 100, 300, 1000 and 3000 ppm of cis-isomer for 90 days.
Survival was affected and food consumption and body-weight gain were
depressed at the highest level. No examination was made of animals
dying during the study, but post-study gross and microscopic
examination of major organs of survivors disclosed hepatocellular
oedema, degeneration and regeneration, in proportion to the level fed,
at 300 ppm and above. No effect was seen at 100 ppm. There were no
differences between the groups in periodic examination of blood and
urine (Kay & Calandbra, 1959).
Long-term studies
No data available.
Comments
This ether is probably metabolized to anisic acid. The available
information is scanty and toxicity appears to be dependent on
stereoisomeric configuration. The short-term studies can be used for
evaluation. Further metabolic investigation and long-term studies are
essential.
EVALUATION
Level causing no toxicological effect
Rat: 2500 ppm in the diet, equivalent to 125 mg/kg
body-weight/day.
Estimate of acceptable daily intake for man
mg/kg body-weight
Conditional acceptance 0-1.25
Further work required
Biochemical, metabolic and long-term studies on the
trans-stereoisomer with emphasis on the effect on the liver.
REFERENCES
Boissier, J. R., Simon, P. & LaBourhis, B. (1967) Thérapie, 22,
309
Canjolle, F. & Meynier, D. (1958) Compt. Rend., 246, 1465
Hagan, E. C., Hansen, W. H., Fitzhugh, O. G., Jenner, P. M., Jones, W.
I., Taylor, J. M., Long, E. L., Nelson, A. A. & Brouwer, J. B. (1967)
Fd Cosmet. Toxicol., 5(2), 141
Jenner, P. M., Hagan, E. C., Taylor, J. M., Cook, E. L. & Fitzhugh, O.
G. (1964) Fd Cosmet. Toxicol., 2, 327
Kay, J. H. & Calandra, J. C. (1959) Unpublished report submitted by
Hercules Powder Company, Inc.
Kühling, O. & Giacosa, P. (1890) Ann. chim. Farm., 11, 304
Taylor, J. M., Jenner, P. M. & Jones, W. I. (1964) Toxicol. appl.
Pharmacol.,6, 378
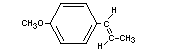 Molecular weight 148.21
Description Anethole is prepared from anise oil and
other sources, or it is prepared
synthetically. It is a colourless or faintly
yellow liquid at or above 23°. It has a
sweet taste and a characteristic anise-like
odour. It is affected by light.
Limit of cis-isomer Not more than 1 per cent. (by gas-liquid
chromatography).
Biological Data
Biochemical Aspects
This ether is probably metabolized to anisic acid (Kühling &
Giacosa, 1890).
Acute toxicity
Animal Route LD50 Reference
(mg/kg body-weight)
trans-isomer cis-isomer
Mouse oral 5000 - Boissier et al.,1967
Mouse oral 3050 - Jenner et al., 1964
Mouse i.p. 1410 95 Caujolle & Meynier, 1958
Mouse i.p. 650 135 Boissier et al., 1967
Rat oral 3200 - Boissier et al., 1967
Rat oral 2940 150 Shelansky & Gabriel, 1958
Rat oral 2090 - Jenner et al., 1964
Rat i.p. - 67 Caujolle & Meynier, 1958
Rat i.p. 900 93 Boissier et al., 1967
Guinea-pig oral 2167 - Jenner et al., 1964
Feeding 3 male and 3 female rats 700 mg/kg body-weight/day
intragastrically for 4 days produced slight gross changes in the liver
(Taylor et al., 1964).
Short-term studies
Trans-isomer
Rat. Groups of 5 males and 5 females were fed dietary levels of
0, 1000, 3000, 10 000 and 30 000 ppm of trans-isomer for 90 days.
There was no survival past 20 days at the highest level, and survival
was affected at the 10 000 ppm level. Depressed food consumption and
body-weight gain, in proportion to the level fed, were found at 3000
ppm and above. No examination was made of animals dying during the
study, but post-study gross and microscopic examination of major
organs of survivors disclosed hepatocellular oedema, degeneration and
regeneration, in proportion to the level fed, at, 3000 ppm and above.
No effect was seen at 1000 ppm. There were no differences between the
groups in periodic examinations of blood and urine (Kay & Calandra,
1959).
Groups of 5 male and 5 female rats were fed diets containing 0,
0.25 and 1.0 per cent. of anethole. The group in the highest level was
sacrificed after 15 weeks and showed slight hydropic changes of
hepatic cells in males only, while the group on the lowest level was
kept for 1 year without any evidence of adverse effects (Hagan et al.,
1967).
Cis-isomer
Rat. Groups of 5 males and 5 females were fed dietary levels of
0, 10, 30, 100, 300, 1000 and 3000 ppm of cis-isomer for 90 days.
Survival was affected and food consumption and body-weight gain were
depressed at the highest level. No examination was made of animals
dying during the study, but post-study gross and microscopic
examination of major organs of survivors disclosed hepatocellular
oedema, degeneration and regeneration, in proportion to the level fed,
at 300 ppm and above. No effect was seen at 100 ppm. There were no
differences between the groups in periodic examination of blood and
urine (Kay & Calandbra, 1959).
Long-term studies
No data available.
Comments
This ether is probably metabolized to anisic acid. The available
information is scanty and toxicity appears to be dependent on
stereoisomeric configuration. The short-term studies can be used for
evaluation. Further metabolic investigation and long-term studies are
essential.
EVALUATION
Level causing no toxicological effect
Rat: 2500 ppm in the diet, equivalent to 125 mg/kg
body-weight/day.
Estimate of acceptable daily intake for man
mg/kg body-weight
Conditional acceptance 0-1.25
Further work required
Biochemical, metabolic and long-term studies on the
trans-stereoisomer with emphasis on the effect on the liver.
REFERENCES
Boissier, J. R., Simon, P. & LaBourhis, B. (1967) Thérapie, 22,
309
Canjolle, F. & Meynier, D. (1958) Compt. Rend., 246, 1465
Hagan, E. C., Hansen, W. H., Fitzhugh, O. G., Jenner, P. M., Jones, W.
I., Taylor, J. M., Long, E. L., Nelson, A. A. & Brouwer, J. B. (1967)
Fd Cosmet. Toxicol., 5(2), 141
Jenner, P. M., Hagan, E. C., Taylor, J. M., Cook, E. L. & Fitzhugh, O.
G. (1964) Fd Cosmet. Toxicol., 2, 327
Kay, J. H. & Calandra, J. C. (1959) Unpublished report submitted by
Hercules Powder Company, Inc.
Kühling, O. & Giacosa, P. (1890) Ann. chim. Farm., 11, 304
Taylor, J. M., Jenner, P. M. & Jones, W. I. (1964) Toxicol. appl.
Pharmacol.,6, 378
Molecular weight 148.21
Description Anethole is prepared from anise oil and
other sources, or it is prepared
synthetically. It is a colourless or faintly
yellow liquid at or above 23°. It has a
sweet taste and a characteristic anise-like
odour. It is affected by light.
Limit of cis-isomer Not more than 1 per cent. (by gas-liquid
chromatography).
Biological Data
Biochemical Aspects
This ether is probably metabolized to anisic acid (Kühling &
Giacosa, 1890).
Acute toxicity
Animal Route LD50 Reference
(mg/kg body-weight)
trans-isomer cis-isomer
Mouse oral 5000 - Boissier et al.,1967
Mouse oral 3050 - Jenner et al., 1964
Mouse i.p. 1410 95 Caujolle & Meynier, 1958
Mouse i.p. 650 135 Boissier et al., 1967
Rat oral 3200 - Boissier et al., 1967
Rat oral 2940 150 Shelansky & Gabriel, 1958
Rat oral 2090 - Jenner et al., 1964
Rat i.p. - 67 Caujolle & Meynier, 1958
Rat i.p. 900 93 Boissier et al., 1967
Guinea-pig oral 2167 - Jenner et al., 1964
Feeding 3 male and 3 female rats 700 mg/kg body-weight/day
intragastrically for 4 days produced slight gross changes in the liver
(Taylor et al., 1964).
Short-term studies
Trans-isomer
Rat. Groups of 5 males and 5 females were fed dietary levels of
0, 1000, 3000, 10 000 and 30 000 ppm of trans-isomer for 90 days.
There was no survival past 20 days at the highest level, and survival
was affected at the 10 000 ppm level. Depressed food consumption and
body-weight gain, in proportion to the level fed, were found at 3000
ppm and above. No examination was made of animals dying during the
study, but post-study gross and microscopic examination of major
organs of survivors disclosed hepatocellular oedema, degeneration and
regeneration, in proportion to the level fed, at, 3000 ppm and above.
No effect was seen at 1000 ppm. There were no differences between the
groups in periodic examinations of blood and urine (Kay & Calandra,
1959).
Groups of 5 male and 5 female rats were fed diets containing 0,
0.25 and 1.0 per cent. of anethole. The group in the highest level was
sacrificed after 15 weeks and showed slight hydropic changes of
hepatic cells in males only, while the group on the lowest level was
kept for 1 year without any evidence of adverse effects (Hagan et al.,
1967).
Cis-isomer
Rat. Groups of 5 males and 5 females were fed dietary levels of
0, 10, 30, 100, 300, 1000 and 3000 ppm of cis-isomer for 90 days.
Survival was affected and food consumption and body-weight gain were
depressed at the highest level. No examination was made of animals
dying during the study, but post-study gross and microscopic
examination of major organs of survivors disclosed hepatocellular
oedema, degeneration and regeneration, in proportion to the level fed,
at 300 ppm and above. No effect was seen at 100 ppm. There were no
differences between the groups in periodic examination of blood and
urine (Kay & Calandbra, 1959).
Long-term studies
No data available.
Comments
This ether is probably metabolized to anisic acid. The available
information is scanty and toxicity appears to be dependent on
stereoisomeric configuration. The short-term studies can be used for
evaluation. Further metabolic investigation and long-term studies are
essential.
EVALUATION
Level causing no toxicological effect
Rat: 2500 ppm in the diet, equivalent to 125 mg/kg
body-weight/day.
Estimate of acceptable daily intake for man
mg/kg body-weight
Conditional acceptance 0-1.25
Further work required
Biochemical, metabolic and long-term studies on the
trans-stereoisomer with emphasis on the effect on the liver.
REFERENCES
Boissier, J. R., Simon, P. & LaBourhis, B. (1967) Thérapie, 22,
309
Canjolle, F. & Meynier, D. (1958) Compt. Rend., 246, 1465
Hagan, E. C., Hansen, W. H., Fitzhugh, O. G., Jenner, P. M., Jones, W.
I., Taylor, J. M., Long, E. L., Nelson, A. A. & Brouwer, J. B. (1967)
Fd Cosmet. Toxicol., 5(2), 141
Jenner, P. M., Hagan, E. C., Taylor, J. M., Cook, E. L. & Fitzhugh, O.
G. (1964) Fd Cosmet. Toxicol., 2, 327
Kay, J. H. & Calandra, J. C. (1959) Unpublished report submitted by
Hercules Powder Company, Inc.
Kühling, O. & Giacosa, P. (1890) Ann. chim. Farm., 11, 304
Taylor, J. M., Jenner, P. M. & Jones, W. I. (1964) Toxicol. appl.
Pharmacol.,6, 378
