
FAO Nutrition Meetings
Resort Series No. 44A
WHO/Food Add./68.33
TOXICOLOGICAL EVALUATION OF SOME
FLAVOURING SUBSTANCES AND
NON-NUTRITIVE SWEETENING AGENTS
Geneva, 21-28 August 1967
The Eleventh Report of the Joint FAO/WHO Expert Committee on Food
Additives is published as FAO Nutrition Meetings Report Series,
1967, No. 44; Wld Hlth Org. techn. Rep. Ser., 1968, 383. This
Report contains general considerations, including the principles
adopted for the evaluation, and a summary of the results of the
evaluations of a number of food additives. Additional information,
such as biological data and a toxicological evaluation, considered at
that meeting, is to be found in this document.
Food and Agriculture Organization of the United Nations
World Health Organization
1967
BENZALDEHYDE
Chemical name Benzoic aldehyde
Empirical formula C7H6O
Structural Formula
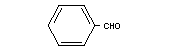 Molecular weight 106.12
Definition Benzaldehyde contains not less than 97
per cent. C7H6O.
Description Benzaldehyde occurs as a constituent of
oils of bitter almond, peach and apricot
kernel. It is usually prepared
synthetically. It is a colourless liquid
having an odour resembling that of bitter
almond oil, and a burning taste. It is
affected by light and it oxidizes in air
to benzoic acid.
Biological Data
Biochemical aspects
Benzaldehyde is hydrogenated in the rabbit to benzoyl alcohol,
which is further oxidized to benzoic acid and excreted as hippuric
acid (Bray et al., 1951).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Rat oral 1300 Taylor et al, 1964
Rat s.c. 5000 (LD) Macht, 1922
Guinea-pig oral 1000 Jenner et al, 1914
Groups of 3 male and 3 female rats were given one-third of the
LD50 intragastrically daily for 4 days. Animals were then killed and
the livers examined for gross changes. None were detected (Taylor et
al., 1964). The fatal human dose in an acute poisoning case was
estimated at 50-60 ml (Dadlez, 1928).
Short-term studies
Rat. Groups of 5 male and 5 female rats were given 0 and 0.1
per cent. in their diet for 27-28 weeks and 1 per cent. for 16 weeks
without tissue damage as determined by gross and histological
examination (Hagan et al., 1964).
Long-term studies
None available.
Comments
The evaluation is based on information concerning the metabolic
pathway and on the short-term studies. It is expressed in terms of the
final metabolite, benzoic acid.
EVALUATION
Level causing no toxicological effect
Rat: 1 per cent. (= 10 000 ppm) in the diet, equivalent to 500
mg/kg body-weight/day.
Estimate of acceptable daily intake for man
mg/kg body-weight1
Unconditional acceptance 0-5
REFERENCES
Bray, H. G., Thorpe, W. V. & White, K. (1951) Biochem. J., 48, 88
Dadlez, J. (1928) Compt. Rend. Hebd. Seance Acad. Sci. Paris, 99,
1038
Hagan. E. C., Hansen, W. H., Fitzhugh, O. G., Jenner, P. M., Jones, W.
I., Taylor, J. M., Long, E. L., Nelson, A. A. & Brouwer, J. B. (1967)
Fd Cosmet. Toxicol., 5.(2), 141
Jenner, P. M., Hagan, E. C., Taylor, J. M., Cook, E. L. & Fitzhugh, O.
G. (1964) Fd Cosmet. Toxicol., 2, 327
Macht, (1922) Arch. int. pharmacod., 27, 163
Taylor, J. M., Jenner, P. M. & Jones, W. I. (1964) Toxicol. appl.
Pharmacol., 6, 378
1 Calculated as total benzoic acid from all food additive sources.
Molecular weight 106.12
Definition Benzaldehyde contains not less than 97
per cent. C7H6O.
Description Benzaldehyde occurs as a constituent of
oils of bitter almond, peach and apricot
kernel. It is usually prepared
synthetically. It is a colourless liquid
having an odour resembling that of bitter
almond oil, and a burning taste. It is
affected by light and it oxidizes in air
to benzoic acid.
Biological Data
Biochemical aspects
Benzaldehyde is hydrogenated in the rabbit to benzoyl alcohol,
which is further oxidized to benzoic acid and excreted as hippuric
acid (Bray et al., 1951).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Rat oral 1300 Taylor et al, 1964
Rat s.c. 5000 (LD) Macht, 1922
Guinea-pig oral 1000 Jenner et al, 1914
Groups of 3 male and 3 female rats were given one-third of the
LD50 intragastrically daily for 4 days. Animals were then killed and
the livers examined for gross changes. None were detected (Taylor et
al., 1964). The fatal human dose in an acute poisoning case was
estimated at 50-60 ml (Dadlez, 1928).
Short-term studies
Rat. Groups of 5 male and 5 female rats were given 0 and 0.1
per cent. in their diet for 27-28 weeks and 1 per cent. for 16 weeks
without tissue damage as determined by gross and histological
examination (Hagan et al., 1964).
Long-term studies
None available.
Comments
The evaluation is based on information concerning the metabolic
pathway and on the short-term studies. It is expressed in terms of the
final metabolite, benzoic acid.
EVALUATION
Level causing no toxicological effect
Rat: 1 per cent. (= 10 000 ppm) in the diet, equivalent to 500
mg/kg body-weight/day.
Estimate of acceptable daily intake for man
mg/kg body-weight1
Unconditional acceptance 0-5
REFERENCES
Bray, H. G., Thorpe, W. V. & White, K. (1951) Biochem. J., 48, 88
Dadlez, J. (1928) Compt. Rend. Hebd. Seance Acad. Sci. Paris, 99,
1038
Hagan. E. C., Hansen, W. H., Fitzhugh, O. G., Jenner, P. M., Jones, W.
I., Taylor, J. M., Long, E. L., Nelson, A. A. & Brouwer, J. B. (1967)
Fd Cosmet. Toxicol., 5.(2), 141
Jenner, P. M., Hagan, E. C., Taylor, J. M., Cook, E. L. & Fitzhugh, O.
G. (1964) Fd Cosmet. Toxicol., 2, 327
Macht, (1922) Arch. int. pharmacod., 27, 163
Taylor, J. M., Jenner, P. M. & Jones, W. I. (1964) Toxicol. appl.
Pharmacol., 6, 378
1 Calculated as total benzoic acid from all food additive sources.
 Molecular weight 106.12
Definition Benzaldehyde contains not less than 97
per cent. C7H6O.
Description Benzaldehyde occurs as a constituent of
oils of bitter almond, peach and apricot
kernel. It is usually prepared
synthetically. It is a colourless liquid
having an odour resembling that of bitter
almond oil, and a burning taste. It is
affected by light and it oxidizes in air
to benzoic acid.
Biological Data
Biochemical aspects
Benzaldehyde is hydrogenated in the rabbit to benzoyl alcohol,
which is further oxidized to benzoic acid and excreted as hippuric
acid (Bray et al., 1951).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Rat oral 1300 Taylor et al, 1964
Rat s.c. 5000 (LD) Macht, 1922
Guinea-pig oral 1000 Jenner et al, 1914
Groups of 3 male and 3 female rats were given one-third of the
LD50 intragastrically daily for 4 days. Animals were then killed and
the livers examined for gross changes. None were detected (Taylor et
al., 1964). The fatal human dose in an acute poisoning case was
estimated at 50-60 ml (Dadlez, 1928).
Short-term studies
Rat. Groups of 5 male and 5 female rats were given 0 and 0.1
per cent. in their diet for 27-28 weeks and 1 per cent. for 16 weeks
without tissue damage as determined by gross and histological
examination (Hagan et al., 1964).
Long-term studies
None available.
Comments
The evaluation is based on information concerning the metabolic
pathway and on the short-term studies. It is expressed in terms of the
final metabolite, benzoic acid.
EVALUATION
Level causing no toxicological effect
Rat: 1 per cent. (= 10 000 ppm) in the diet, equivalent to 500
mg/kg body-weight/day.
Estimate of acceptable daily intake for man
mg/kg body-weight1
Unconditional acceptance 0-5
REFERENCES
Bray, H. G., Thorpe, W. V. & White, K. (1951) Biochem. J., 48, 88
Dadlez, J. (1928) Compt. Rend. Hebd. Seance Acad. Sci. Paris, 99,
1038
Hagan. E. C., Hansen, W. H., Fitzhugh, O. G., Jenner, P. M., Jones, W.
I., Taylor, J. M., Long, E. L., Nelson, A. A. & Brouwer, J. B. (1967)
Fd Cosmet. Toxicol., 5.(2), 141
Jenner, P. M., Hagan, E. C., Taylor, J. M., Cook, E. L. & Fitzhugh, O.
G. (1964) Fd Cosmet. Toxicol., 2, 327
Macht, (1922) Arch. int. pharmacod., 27, 163
Taylor, J. M., Jenner, P. M. & Jones, W. I. (1964) Toxicol. appl.
Pharmacol., 6, 378
1 Calculated as total benzoic acid from all food additive sources.
Molecular weight 106.12
Definition Benzaldehyde contains not less than 97
per cent. C7H6O.
Description Benzaldehyde occurs as a constituent of
oils of bitter almond, peach and apricot
kernel. It is usually prepared
synthetically. It is a colourless liquid
having an odour resembling that of bitter
almond oil, and a burning taste. It is
affected by light and it oxidizes in air
to benzoic acid.
Biological Data
Biochemical aspects
Benzaldehyde is hydrogenated in the rabbit to benzoyl alcohol,
which is further oxidized to benzoic acid and excreted as hippuric
acid (Bray et al., 1951).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Rat oral 1300 Taylor et al, 1964
Rat s.c. 5000 (LD) Macht, 1922
Guinea-pig oral 1000 Jenner et al, 1914
Groups of 3 male and 3 female rats were given one-third of the
LD50 intragastrically daily for 4 days. Animals were then killed and
the livers examined for gross changes. None were detected (Taylor et
al., 1964). The fatal human dose in an acute poisoning case was
estimated at 50-60 ml (Dadlez, 1928).
Short-term studies
Rat. Groups of 5 male and 5 female rats were given 0 and 0.1
per cent. in their diet for 27-28 weeks and 1 per cent. for 16 weeks
without tissue damage as determined by gross and histological
examination (Hagan et al., 1964).
Long-term studies
None available.
Comments
The evaluation is based on information concerning the metabolic
pathway and on the short-term studies. It is expressed in terms of the
final metabolite, benzoic acid.
EVALUATION
Level causing no toxicological effect
Rat: 1 per cent. (= 10 000 ppm) in the diet, equivalent to 500
mg/kg body-weight/day.
Estimate of acceptable daily intake for man
mg/kg body-weight1
Unconditional acceptance 0-5
REFERENCES
Bray, H. G., Thorpe, W. V. & White, K. (1951) Biochem. J., 48, 88
Dadlez, J. (1928) Compt. Rend. Hebd. Seance Acad. Sci. Paris, 99,
1038
Hagan. E. C., Hansen, W. H., Fitzhugh, O. G., Jenner, P. M., Jones, W.
I., Taylor, J. M., Long, E. L., Nelson, A. A. & Brouwer, J. B. (1967)
Fd Cosmet. Toxicol., 5.(2), 141
Jenner, P. M., Hagan, E. C., Taylor, J. M., Cook, E. L. & Fitzhugh, O.
G. (1964) Fd Cosmet. Toxicol., 2, 327
Macht, (1922) Arch. int. pharmacod., 27, 163
Taylor, J. M., Jenner, P. M. & Jones, W. I. (1964) Toxicol. appl.
Pharmacol., 6, 378
1 Calculated as total benzoic acid from all food additive sources.
