
FAO Nutrition Meetings
Resort Series No. 44A
WHO/Food Add./68.33
TOXICOLOGICAL EVALUATION OF SOME
FLAVOURING SUBSTANCES AND
NON-NUTRITIVE SWEETENING AGENTS
Geneva, 21-28 August 1967
The Eleventh Report of the Joint FAO/WHO Expert Committee on Food
Additives is published as FAO Nutrition Meetings Report Series,
1967, No. 44; Wld Hlth Org. techn. Rep. Ser., 1968, 383. This
Report contains general considerations, including the principles
adopted for the evaluation, and a summary of the results of the
evaluations of a number of food additives. Additional information,
such as biological data and a toxicological evaluation, considered at
that meeting, is to be found in this document.
Food and Agriculture Organization of the United Nations
World Health Organization
1967
d-CARVONE and 1-CARVONE
Chemical names d-2-Methyl-5-isopropenyl-2-cyclohexenone;
and 1-2-Methyl-5-isopropenyl-2-cyclohexenone
Empirical formula C10H14O
Structural formula
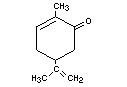 Molecular weight 150.22
Definition d-Carvone and 1-Carvone contain not less
than 95 per cent. C10H14O.
Descriptions d-Carvone is usually prepared by fractional
distillation from caraway oil. It may be
prepared in a similar manner from either
dillseed oil or dillweed oil, but this type
differs in odour and flavour from that
dervied from caraway oil. It is a colourless
to light yellow liquid having an odour of
caraway.
1-Carvone occurs in several essential oils.
it may be isolated from spearmint oil or
synthesized commercially from D-limonene.
It is a colourless to pale straw-coloured
liquid having an odour of spearmint.
Biological Data
Biochemical aspects
The rabbit metabolized this ketone to
1,5-dimethyl-1,5-hexadien-1,6-dicarboxylic acid and a carbinol in
which one ethylenic linkage is saturated and the keto group reduced
(Fischer & Bielig, 1940).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Rat oral 1640 Jenner et al., 1964
Guinea-pig oral 766 Jenner et al., 1964
Short-term studies
Rat. Groups of 5 male and 5 female rats were kept on diets
containing 0, 0.1 and 1.0 per cent. of carvone for 28 weeks. The group
at the highest level was sacrificed after 16 weeks, when growth
retardation and testicular atrophy were noted. No adverse effects were
seen at the 0.1 per cent. level (Hagan et al., 1967).
A one-year study was performed or groups of 5 male and 5 female
rats fed a diet containing 0 or 0.25 per cent. carvone. No adverse
effects were noted on body-weight gain, organ weight of major organs
or in the histology of the main organs and tissues, including testes
(Hagan et al., 1967).
Long-term studies
None available.
Comments
Depite the scanty metabolic data the evaluation is based on
short-term studies. Further biochemical studies are required.
EVALUATION
Level causing no toxicological effect
Rat: 0.25 per cent. (= 2500 ppm) in the diet, equivalent to 125
mg/kg body-weight/day.
Estimate of acceptable daily intake for man
mg/kg body-weight
Conditional acceptance 0-1.25
Further work required
Biochemical and metabolic studies in animals and man.
REFERENCES
Fischer, F. G. & Bielig, H. J. (1940) Hoppe-Seylers Z., 266, 73
Hagan E. C., Hansen, W. H., Fitzhugh, O. G., Jenner, P. M., Jones, W.
I., Taylor, J. M., Long, E.L., Nelson, A. A. & Brouwer, J. B. (1967)
Fd Cosmet. Toxicol., 5, (2), 141
Jenner, P. M., Hagan, E. C., Taylor, J. M., Cook, E. L. & Fitzhugh, O.
G., (1964) Fd Cosmet Toxicol., 2, 372
Molecular weight 150.22
Definition d-Carvone and 1-Carvone contain not less
than 95 per cent. C10H14O.
Descriptions d-Carvone is usually prepared by fractional
distillation from caraway oil. It may be
prepared in a similar manner from either
dillseed oil or dillweed oil, but this type
differs in odour and flavour from that
dervied from caraway oil. It is a colourless
to light yellow liquid having an odour of
caraway.
1-Carvone occurs in several essential oils.
it may be isolated from spearmint oil or
synthesized commercially from D-limonene.
It is a colourless to pale straw-coloured
liquid having an odour of spearmint.
Biological Data
Biochemical aspects
The rabbit metabolized this ketone to
1,5-dimethyl-1,5-hexadien-1,6-dicarboxylic acid and a carbinol in
which one ethylenic linkage is saturated and the keto group reduced
(Fischer & Bielig, 1940).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Rat oral 1640 Jenner et al., 1964
Guinea-pig oral 766 Jenner et al., 1964
Short-term studies
Rat. Groups of 5 male and 5 female rats were kept on diets
containing 0, 0.1 and 1.0 per cent. of carvone for 28 weeks. The group
at the highest level was sacrificed after 16 weeks, when growth
retardation and testicular atrophy were noted. No adverse effects were
seen at the 0.1 per cent. level (Hagan et al., 1967).
A one-year study was performed or groups of 5 male and 5 female
rats fed a diet containing 0 or 0.25 per cent. carvone. No adverse
effects were noted on body-weight gain, organ weight of major organs
or in the histology of the main organs and tissues, including testes
(Hagan et al., 1967).
Long-term studies
None available.
Comments
Depite the scanty metabolic data the evaluation is based on
short-term studies. Further biochemical studies are required.
EVALUATION
Level causing no toxicological effect
Rat: 0.25 per cent. (= 2500 ppm) in the diet, equivalent to 125
mg/kg body-weight/day.
Estimate of acceptable daily intake for man
mg/kg body-weight
Conditional acceptance 0-1.25
Further work required
Biochemical and metabolic studies in animals and man.
REFERENCES
Fischer, F. G. & Bielig, H. J. (1940) Hoppe-Seylers Z., 266, 73
Hagan E. C., Hansen, W. H., Fitzhugh, O. G., Jenner, P. M., Jones, W.
I., Taylor, J. M., Long, E.L., Nelson, A. A. & Brouwer, J. B. (1967)
Fd Cosmet. Toxicol., 5, (2), 141
Jenner, P. M., Hagan, E. C., Taylor, J. M., Cook, E. L. & Fitzhugh, O.
G., (1964) Fd Cosmet Toxicol., 2, 372
 Molecular weight 150.22
Definition d-Carvone and 1-Carvone contain not less
than 95 per cent. C10H14O.
Descriptions d-Carvone is usually prepared by fractional
distillation from caraway oil. It may be
prepared in a similar manner from either
dillseed oil or dillweed oil, but this type
differs in odour and flavour from that
dervied from caraway oil. It is a colourless
to light yellow liquid having an odour of
caraway.
1-Carvone occurs in several essential oils.
it may be isolated from spearmint oil or
synthesized commercially from D-limonene.
It is a colourless to pale straw-coloured
liquid having an odour of spearmint.
Biological Data
Biochemical aspects
The rabbit metabolized this ketone to
1,5-dimethyl-1,5-hexadien-1,6-dicarboxylic acid and a carbinol in
which one ethylenic linkage is saturated and the keto group reduced
(Fischer & Bielig, 1940).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Rat oral 1640 Jenner et al., 1964
Guinea-pig oral 766 Jenner et al., 1964
Short-term studies
Rat. Groups of 5 male and 5 female rats were kept on diets
containing 0, 0.1 and 1.0 per cent. of carvone for 28 weeks. The group
at the highest level was sacrificed after 16 weeks, when growth
retardation and testicular atrophy were noted. No adverse effects were
seen at the 0.1 per cent. level (Hagan et al., 1967).
A one-year study was performed or groups of 5 male and 5 female
rats fed a diet containing 0 or 0.25 per cent. carvone. No adverse
effects were noted on body-weight gain, organ weight of major organs
or in the histology of the main organs and tissues, including testes
(Hagan et al., 1967).
Long-term studies
None available.
Comments
Depite the scanty metabolic data the evaluation is based on
short-term studies. Further biochemical studies are required.
EVALUATION
Level causing no toxicological effect
Rat: 0.25 per cent. (= 2500 ppm) in the diet, equivalent to 125
mg/kg body-weight/day.
Estimate of acceptable daily intake for man
mg/kg body-weight
Conditional acceptance 0-1.25
Further work required
Biochemical and metabolic studies in animals and man.
REFERENCES
Fischer, F. G. & Bielig, H. J. (1940) Hoppe-Seylers Z., 266, 73
Hagan E. C., Hansen, W. H., Fitzhugh, O. G., Jenner, P. M., Jones, W.
I., Taylor, J. M., Long, E.L., Nelson, A. A. & Brouwer, J. B. (1967)
Fd Cosmet. Toxicol., 5, (2), 141
Jenner, P. M., Hagan, E. C., Taylor, J. M., Cook, E. L. & Fitzhugh, O.
G., (1964) Fd Cosmet Toxicol., 2, 372
Molecular weight 150.22
Definition d-Carvone and 1-Carvone contain not less
than 95 per cent. C10H14O.
Descriptions d-Carvone is usually prepared by fractional
distillation from caraway oil. It may be
prepared in a similar manner from either
dillseed oil or dillweed oil, but this type
differs in odour and flavour from that
dervied from caraway oil. It is a colourless
to light yellow liquid having an odour of
caraway.
1-Carvone occurs in several essential oils.
it may be isolated from spearmint oil or
synthesized commercially from D-limonene.
It is a colourless to pale straw-coloured
liquid having an odour of spearmint.
Biological Data
Biochemical aspects
The rabbit metabolized this ketone to
1,5-dimethyl-1,5-hexadien-1,6-dicarboxylic acid and a carbinol in
which one ethylenic linkage is saturated and the keto group reduced
(Fischer & Bielig, 1940).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Rat oral 1640 Jenner et al., 1964
Guinea-pig oral 766 Jenner et al., 1964
Short-term studies
Rat. Groups of 5 male and 5 female rats were kept on diets
containing 0, 0.1 and 1.0 per cent. of carvone for 28 weeks. The group
at the highest level was sacrificed after 16 weeks, when growth
retardation and testicular atrophy were noted. No adverse effects were
seen at the 0.1 per cent. level (Hagan et al., 1967).
A one-year study was performed or groups of 5 male and 5 female
rats fed a diet containing 0 or 0.25 per cent. carvone. No adverse
effects were noted on body-weight gain, organ weight of major organs
or in the histology of the main organs and tissues, including testes
(Hagan et al., 1967).
Long-term studies
None available.
Comments
Depite the scanty metabolic data the evaluation is based on
short-term studies. Further biochemical studies are required.
EVALUATION
Level causing no toxicological effect
Rat: 0.25 per cent. (= 2500 ppm) in the diet, equivalent to 125
mg/kg body-weight/day.
Estimate of acceptable daily intake for man
mg/kg body-weight
Conditional acceptance 0-1.25
Further work required
Biochemical and metabolic studies in animals and man.
REFERENCES
Fischer, F. G. & Bielig, H. J. (1940) Hoppe-Seylers Z., 266, 73
Hagan E. C., Hansen, W. H., Fitzhugh, O. G., Jenner, P. M., Jones, W.
I., Taylor, J. M., Long, E.L., Nelson, A. A. & Brouwer, J. B. (1967)
Fd Cosmet. Toxicol., 5, (2), 141
Jenner, P. M., Hagan, E. C., Taylor, J. M., Cook, E. L. & Fitzhugh, O.
G., (1964) Fd Cosmet Toxicol., 2, 372
