
FAO Nutrition Meetings
Resort Series No. 44A
WHO/Food Add./68.33
TOXICOLOGICAL EVALUATION OF SOME
FLAVOURING SUBSTANCES AND
NON-NUTRITIVE SWEETENING AGENTS
Geneva, 21-28 August 1967
The Eleventh Report of the Joint FAO/WHO Expert Committee on Food
Additives is published as FAO Nutrition Meetings Report Series,
1967, No. 44; Wld Hlth Org. techn. Rep. Ser., 1968, 383. This
Report contains general considerations, including the principles
adopted for the evaluation, and a summary of the results of the
evaluations of a number of food additives. Additional information,
such as biological data and a toxicological evaluation, considered at
that meeting, is to be found in this document.
Food and Agriculture Organization of the United Nations
World Health Organization
1967
CITRAL
Chemical name 3,7-Dimethyl-2,6-octadienal
Empirical formula C10H16O
Structural formula
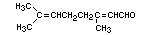 Molecular weight 152.24
Definition Citral contains not less than 95 per
cent. C10H16O.
Description Citral is the principal constituent of
lemongrass oil and Backhousia
citriodora oil. It is usually isolated
from citral-containing oils by chemical
means. It may be prepared synthetically.
The product of commerce is a mixture of
two geometric isomers, a- and ß-citrals.
Citral is a pale yellow liquid having a
strong lemon like odour.
Biological Data
Biochemical aspects
This aldehyde is probably metabolized to
1,5-dimethyl-1,5-hexadien-1,6-dicarboxylic acid and
7-carboxy-3-methylocta-6-enoic acid (Williams, 1959). There is some
conflicting evidence that this compound may interfere through its
CH=CH-CHO group with SH groups in the cell.
Acute toxicity
Animal Route LD50 Reference
(mg/kg
body-wegiht)
Rat oral 4960 Jenner et al, 1964
There is an extensive literature on local reactions due to this
compound, and various pharmacological effects which are not shown
under normal circumstances.
Short-term studies
Rat. In a 12-week feeding study in 15 males and 15 females,
using mixed citral conpounds there was no adverse effect noticeable at
50 mg/kg body-weight/day (Oser, 1967). In another study lasting 13
weeks groups of 10 male and 10 female rats were fed diets containing
0, 0.1, 0.25 and 1.0 per cent. of citral without any adverse effects
(Hagen et al., 1967). On the other hand, young rats fed 0.15 mg citral
daily for 26 days showed reduced body-weight gain in both sexes
(Shillinger, 1950).
Rabbit. Oral administration of 2.8 mg/kg body-weight daily for
3 months reduced body-weight gain, raised fasting blood sugar level
and prolonged the hyperglycaemic period, produced urobilinuria,
proteinuria and histological evidence of chronic nephritis
(Shillinger, 1950).
Long-term studies
None available.
Comment
The biochemical studies are not conclusive, particularly in
regard to the local activity of this substance and its pharmacological
effects under certain circumstances. However, it is possible to
evaluate this compound on the basis of short-term studies. Because of
the local action of the compound, it is prudent to employ a safety
factor of 500.
EVALUATION
Level causing no toxicological effect
Rat: 1 per cent. (= 10 000 ppm) in the diet, equivalent to 500
mg/kg body-weight/day.
Estimate of acceptable daily intake for man
mg/kg body-weight
Conditional acceptance 0-1
Further work required
Biochemical and metabolic studies and long-term studies,1 with
special attention to possible systemic effects on the eyes.
REFERENCES
Hagan, E. C., Hansen, W. H., Fitzhugh, O. C., Jenner, F. M., Jones, W.
I., Taylor, J. M., Long, E. L., Nelson, A. A. & Brouwer, J. B. (1967)
Fd Cosmet. Toxicol., 5(2), 141
Jenner, P. M., Hagan, E. C., Taylor, J. M., Cook, E. L. & Fitzhugh, O.
G. (1964) Fd Cosmet. Toxicol., 2, 327
Oser, B. L. (1967) Unpublished Report
Shillinger, Y. I. (1950) Gig. i. San., 3, 37
Williams, R. T. (1959) Detoxication Mechanisms, Second Edition,
Chapman & Hall, London
1 When considering the group of flavouring substances citral,
citronellol, linalol, linalyl acetate and geranyl acetate, the
Committee stressed the urgent need to elucidate the metabolic pathways
which may be common to these widely distributed substances. They found
it reasonable to require that one or more of these substances should
be made the subject of long-term studies. Whether this limitation can
be made and which substances should be chosen may follow from a
consideration of the biochemical evidence when this becomes available.
Molecular weight 152.24
Definition Citral contains not less than 95 per
cent. C10H16O.
Description Citral is the principal constituent of
lemongrass oil and Backhousia
citriodora oil. It is usually isolated
from citral-containing oils by chemical
means. It may be prepared synthetically.
The product of commerce is a mixture of
two geometric isomers, a- and ß-citrals.
Citral is a pale yellow liquid having a
strong lemon like odour.
Biological Data
Biochemical aspects
This aldehyde is probably metabolized to
1,5-dimethyl-1,5-hexadien-1,6-dicarboxylic acid and
7-carboxy-3-methylocta-6-enoic acid (Williams, 1959). There is some
conflicting evidence that this compound may interfere through its
CH=CH-CHO group with SH groups in the cell.
Acute toxicity
Animal Route LD50 Reference
(mg/kg
body-wegiht)
Rat oral 4960 Jenner et al, 1964
There is an extensive literature on local reactions due to this
compound, and various pharmacological effects which are not shown
under normal circumstances.
Short-term studies
Rat. In a 12-week feeding study in 15 males and 15 females,
using mixed citral conpounds there was no adverse effect noticeable at
50 mg/kg body-weight/day (Oser, 1967). In another study lasting 13
weeks groups of 10 male and 10 female rats were fed diets containing
0, 0.1, 0.25 and 1.0 per cent. of citral without any adverse effects
(Hagen et al., 1967). On the other hand, young rats fed 0.15 mg citral
daily for 26 days showed reduced body-weight gain in both sexes
(Shillinger, 1950).
Rabbit. Oral administration of 2.8 mg/kg body-weight daily for
3 months reduced body-weight gain, raised fasting blood sugar level
and prolonged the hyperglycaemic period, produced urobilinuria,
proteinuria and histological evidence of chronic nephritis
(Shillinger, 1950).
Long-term studies
None available.
Comment
The biochemical studies are not conclusive, particularly in
regard to the local activity of this substance and its pharmacological
effects under certain circumstances. However, it is possible to
evaluate this compound on the basis of short-term studies. Because of
the local action of the compound, it is prudent to employ a safety
factor of 500.
EVALUATION
Level causing no toxicological effect
Rat: 1 per cent. (= 10 000 ppm) in the diet, equivalent to 500
mg/kg body-weight/day.
Estimate of acceptable daily intake for man
mg/kg body-weight
Conditional acceptance 0-1
Further work required
Biochemical and metabolic studies and long-term studies,1 with
special attention to possible systemic effects on the eyes.
REFERENCES
Hagan, E. C., Hansen, W. H., Fitzhugh, O. C., Jenner, F. M., Jones, W.
I., Taylor, J. M., Long, E. L., Nelson, A. A. & Brouwer, J. B. (1967)
Fd Cosmet. Toxicol., 5(2), 141
Jenner, P. M., Hagan, E. C., Taylor, J. M., Cook, E. L. & Fitzhugh, O.
G. (1964) Fd Cosmet. Toxicol., 2, 327
Oser, B. L. (1967) Unpublished Report
Shillinger, Y. I. (1950) Gig. i. San., 3, 37
Williams, R. T. (1959) Detoxication Mechanisms, Second Edition,
Chapman & Hall, London
1 When considering the group of flavouring substances citral,
citronellol, linalol, linalyl acetate and geranyl acetate, the
Committee stressed the urgent need to elucidate the metabolic pathways
which may be common to these widely distributed substances. They found
it reasonable to require that one or more of these substances should
be made the subject of long-term studies. Whether this limitation can
be made and which substances should be chosen may follow from a
consideration of the biochemical evidence when this becomes available.
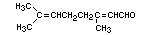 Molecular weight 152.24
Definition Citral contains not less than 95 per
cent. C10H16O.
Description Citral is the principal constituent of
lemongrass oil and Backhousia
citriodora oil. It is usually isolated
from citral-containing oils by chemical
means. It may be prepared synthetically.
The product of commerce is a mixture of
two geometric isomers, a- and ß-citrals.
Citral is a pale yellow liquid having a
strong lemon like odour.
Biological Data
Biochemical aspects
This aldehyde is probably metabolized to
1,5-dimethyl-1,5-hexadien-1,6-dicarboxylic acid and
7-carboxy-3-methylocta-6-enoic acid (Williams, 1959). There is some
conflicting evidence that this compound may interfere through its
CH=CH-CHO group with SH groups in the cell.
Acute toxicity
Animal Route LD50 Reference
(mg/kg
body-wegiht)
Rat oral 4960 Jenner et al, 1964
There is an extensive literature on local reactions due to this
compound, and various pharmacological effects which are not shown
under normal circumstances.
Short-term studies
Rat. In a 12-week feeding study in 15 males and 15 females,
using mixed citral conpounds there was no adverse effect noticeable at
50 mg/kg body-weight/day (Oser, 1967). In another study lasting 13
weeks groups of 10 male and 10 female rats were fed diets containing
0, 0.1, 0.25 and 1.0 per cent. of citral without any adverse effects
(Hagen et al., 1967). On the other hand, young rats fed 0.15 mg citral
daily for 26 days showed reduced body-weight gain in both sexes
(Shillinger, 1950).
Rabbit. Oral administration of 2.8 mg/kg body-weight daily for
3 months reduced body-weight gain, raised fasting blood sugar level
and prolonged the hyperglycaemic period, produced urobilinuria,
proteinuria and histological evidence of chronic nephritis
(Shillinger, 1950).
Long-term studies
None available.
Comment
The biochemical studies are not conclusive, particularly in
regard to the local activity of this substance and its pharmacological
effects under certain circumstances. However, it is possible to
evaluate this compound on the basis of short-term studies. Because of
the local action of the compound, it is prudent to employ a safety
factor of 500.
EVALUATION
Level causing no toxicological effect
Rat: 1 per cent. (= 10 000 ppm) in the diet, equivalent to 500
mg/kg body-weight/day.
Estimate of acceptable daily intake for man
mg/kg body-weight
Conditional acceptance 0-1
Further work required
Biochemical and metabolic studies and long-term studies,1 with
special attention to possible systemic effects on the eyes.
REFERENCES
Hagan, E. C., Hansen, W. H., Fitzhugh, O. C., Jenner, F. M., Jones, W.
I., Taylor, J. M., Long, E. L., Nelson, A. A. & Brouwer, J. B. (1967)
Fd Cosmet. Toxicol., 5(2), 141
Jenner, P. M., Hagan, E. C., Taylor, J. M., Cook, E. L. & Fitzhugh, O.
G. (1964) Fd Cosmet. Toxicol., 2, 327
Oser, B. L. (1967) Unpublished Report
Shillinger, Y. I. (1950) Gig. i. San., 3, 37
Williams, R. T. (1959) Detoxication Mechanisms, Second Edition,
Chapman & Hall, London
1 When considering the group of flavouring substances citral,
citronellol, linalol, linalyl acetate and geranyl acetate, the
Committee stressed the urgent need to elucidate the metabolic pathways
which may be common to these widely distributed substances. They found
it reasonable to require that one or more of these substances should
be made the subject of long-term studies. Whether this limitation can
be made and which substances should be chosen may follow from a
consideration of the biochemical evidence when this becomes available.
Molecular weight 152.24
Definition Citral contains not less than 95 per
cent. C10H16O.
Description Citral is the principal constituent of
lemongrass oil and Backhousia
citriodora oil. It is usually isolated
from citral-containing oils by chemical
means. It may be prepared synthetically.
The product of commerce is a mixture of
two geometric isomers, a- and ß-citrals.
Citral is a pale yellow liquid having a
strong lemon like odour.
Biological Data
Biochemical aspects
This aldehyde is probably metabolized to
1,5-dimethyl-1,5-hexadien-1,6-dicarboxylic acid and
7-carboxy-3-methylocta-6-enoic acid (Williams, 1959). There is some
conflicting evidence that this compound may interfere through its
CH=CH-CHO group with SH groups in the cell.
Acute toxicity
Animal Route LD50 Reference
(mg/kg
body-wegiht)
Rat oral 4960 Jenner et al, 1964
There is an extensive literature on local reactions due to this
compound, and various pharmacological effects which are not shown
under normal circumstances.
Short-term studies
Rat. In a 12-week feeding study in 15 males and 15 females,
using mixed citral conpounds there was no adverse effect noticeable at
50 mg/kg body-weight/day (Oser, 1967). In another study lasting 13
weeks groups of 10 male and 10 female rats were fed diets containing
0, 0.1, 0.25 and 1.0 per cent. of citral without any adverse effects
(Hagen et al., 1967). On the other hand, young rats fed 0.15 mg citral
daily for 26 days showed reduced body-weight gain in both sexes
(Shillinger, 1950).
Rabbit. Oral administration of 2.8 mg/kg body-weight daily for
3 months reduced body-weight gain, raised fasting blood sugar level
and prolonged the hyperglycaemic period, produced urobilinuria,
proteinuria and histological evidence of chronic nephritis
(Shillinger, 1950).
Long-term studies
None available.
Comment
The biochemical studies are not conclusive, particularly in
regard to the local activity of this substance and its pharmacological
effects under certain circumstances. However, it is possible to
evaluate this compound on the basis of short-term studies. Because of
the local action of the compound, it is prudent to employ a safety
factor of 500.
EVALUATION
Level causing no toxicological effect
Rat: 1 per cent. (= 10 000 ppm) in the diet, equivalent to 500
mg/kg body-weight/day.
Estimate of acceptable daily intake for man
mg/kg body-weight
Conditional acceptance 0-1
Further work required
Biochemical and metabolic studies and long-term studies,1 with
special attention to possible systemic effects on the eyes.
REFERENCES
Hagan, E. C., Hansen, W. H., Fitzhugh, O. C., Jenner, F. M., Jones, W.
I., Taylor, J. M., Long, E. L., Nelson, A. A. & Brouwer, J. B. (1967)
Fd Cosmet. Toxicol., 5(2), 141
Jenner, P. M., Hagan, E. C., Taylor, J. M., Cook, E. L. & Fitzhugh, O.
G. (1964) Fd Cosmet. Toxicol., 2, 327
Oser, B. L. (1967) Unpublished Report
Shillinger, Y. I. (1950) Gig. i. San., 3, 37
Williams, R. T. (1959) Detoxication Mechanisms, Second Edition,
Chapman & Hall, London
1 When considering the group of flavouring substances citral,
citronellol, linalol, linalyl acetate and geranyl acetate, the
Committee stressed the urgent need to elucidate the metabolic pathways
which may be common to these widely distributed substances. They found
it reasonable to require that one or more of these substances should
be made the subject of long-term studies. Whether this limitation can
be made and which substances should be chosen may follow from a
consideration of the biochemical evidence when this becomes available.
