
FAO Nutrition Meetings
Resort Series No. 44A
WHO/Food Add./68.33
TOXICOLOGICAL EVALUATION OF SOME
FLAVOURING SUBSTANCES AND
NON-NUTRITIVE SWEETENING AGENTS
Geneva, 21-28 August 1967
The Eleventh Report of the Joint FAO/WHO Expert Committee on Food
Additives is published as FAO Nutrition Meetings Report Series,
1967, No. 44; Wld Hlth Org. techn. Rep. Ser., 1968, 383. This
Report contains general considerations, including the principles
adopted for the evaluation, and a summary of the results of the
evaluations of a number of food additives. Additional information,
such as biological data and a toxicological evaluation, considered at
that meeting, is to be found in this document.
Food and Agriculture Organization of the United Nations
World Health Organization
1967
d,1-MENTHOL and 1-MENTHOL
Chemical name d,1-3-p-Menthanol
Empirical formula C10H20O
Structural formula
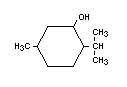 Molecular weight 156.27
Description Menthol is an alcohol obtained from
various mint oils or prepared
synthetically. It may be either
levorotatory (1-menthol) from natural
or synthetic sources, or racemic
(d1-menthol) produced synthetically.
It occurs as colourless, hexagonal
crystals, usually needle-like, or in
fused masses, or as a crystalline
powder. It has a pleasant,
peppermint-like odour.
Biological Data
Biochemical aspects
In the dog, 5 per cent. of orally administered menthol
metabolizes to 1-menthyl-5-glucuronide (Williams, 1959). For other
animals, the reported proportions vary. In the rabbit, the larger the
ingested dose, the less conjugation (Quick, 1924). Other workers
reported 31-34 per cent. glucuronide excretion in rats after oral or
continuous i.v. dosing (Harken, 1961). Rabbits are said to eliminate
48 per cent. of 1-menthol and 59 per cent. of d1-menthol as
glucuronide (Williams, 1938). Menthol is absorbed percutaneously, and
extracts a local anaesthetic action in mice (Macht, 1939).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
(a) 1-menthol
Mouse s.c. 5000-6000 Flury, 1920
i.p. 2000(LD) Macht, 1939
Rat oral 3300 Herken, 1961
s.c. 1000-2500 Flury, 1920
i.p. 710 Herken, 1961
1500(LD) Macht, 1939
Guinea-pig i.p. 4000(LD) Macht, 1939
Cat oral 800-1000 Flury, 1920
i.p. 800-1000 Flury, 1920
i.v. 34(LD) Macht, 1939
Rabbit i.p. approx. 2000 Herken, 1961
(b) d1-menthol
Mouse s.c. 1400-1600 Flury & Seel, 1926
Rat oral 2900 Herken, 1961
3180 Jenner et al., 1964
i.p. 750 Herken, 1961
Cat oral 1500-1600 Flury & Seel, 1926
i.p. 1500-1600 Flury & Seel, 1926
Rabbit i.p. approx. 2000 Herken, 1961
Short-term studies
Rat. Groups of 40 male and 40 female rats received 0, 100 and
200 mg/kg body-weight of either 1- or d1-menthol in their diet for
5-1/2 weeks. There was no adverse effect on weight gain, excretion of
glucuronide, water and electrolytes, nor interference with CNS
reactions to cardrazol or electric shock, or on i.v. hexobarbital
sleeping time as compared with controls (Herken, 1961).
Long-term studies
None available.
Observations in man
Smoking 80 mentholated cigarettes resulted in irritability,
gastrointestinal upsets, tremors, ataxia, bradycardia and toxic
psychosis. Taking 64 mg of menthol three times a day produced
tiredness and apathy within 3 days and nausea, exhaustion and
bradycardia in 7 days (Luke, 1962). Chronic urticaria with basophil
leucopenia on challenge has been reported after contact with menthol
in toothpaste, mentholated cigarettes, peppermint sweets, etc. (Papa &
Shelley, 1964; McCowan, 1966). The usual human oral dose is 60-120 mg.
Comments
Metabolic studies are not very revealing. There is much human
experience from long-established therapeutic use. Further metabolic
studies and adequate long-term studies are desirable.
EVALUATION
Level causing no toxicological effect
Rat. 200 mg/kg body-weight of d1- pr 1-menthol/day.
Estimate of acceptable daily intake for man
mg/kg body-weight
Unconditional acceptance 0-0.2
Conditional acceptance 0.2-2
REFERENCES
Flury, F. (1920) Abderhalden's Handbuch der Biologischen
Arbeitsmethoden,39, 1365
Flury, F. & Seel, H. (1926) Münch. Med. Wschr., 48, 2011
Herken, H. (1961) Report to Schering, AG
Jenner, P. M., Hagan, E. C., Taylor, J. M., Cook, E. L. & Fitzhugh, O.
G. (1964) Fd Cosmet. Toxicol., 2, 327
Luke, E. (1962), Lancet, i, 110
Macht, D. I. (1939) Arch. Int. Pharmacodyn., 63, 43
McGowan, E. M. (1966) Arch. Derm.,94, 62
Papa, C. M. & Shelley, W. B. (1964) J. Amer. med. Ass., 189, 546
Quick, A.J. (1924) J. biol. Chem., 61, 681
Williams, R. T. (1938) Biochem. J., 32, 1849
Williams, R. T. (1959) Detoxication Mechanisms, Second edition,
Chapman & Hall, London
Molecular weight 156.27
Description Menthol is an alcohol obtained from
various mint oils or prepared
synthetically. It may be either
levorotatory (1-menthol) from natural
or synthetic sources, or racemic
(d1-menthol) produced synthetically.
It occurs as colourless, hexagonal
crystals, usually needle-like, or in
fused masses, or as a crystalline
powder. It has a pleasant,
peppermint-like odour.
Biological Data
Biochemical aspects
In the dog, 5 per cent. of orally administered menthol
metabolizes to 1-menthyl-5-glucuronide (Williams, 1959). For other
animals, the reported proportions vary. In the rabbit, the larger the
ingested dose, the less conjugation (Quick, 1924). Other workers
reported 31-34 per cent. glucuronide excretion in rats after oral or
continuous i.v. dosing (Harken, 1961). Rabbits are said to eliminate
48 per cent. of 1-menthol and 59 per cent. of d1-menthol as
glucuronide (Williams, 1938). Menthol is absorbed percutaneously, and
extracts a local anaesthetic action in mice (Macht, 1939).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
(a) 1-menthol
Mouse s.c. 5000-6000 Flury, 1920
i.p. 2000(LD) Macht, 1939
Rat oral 3300 Herken, 1961
s.c. 1000-2500 Flury, 1920
i.p. 710 Herken, 1961
1500(LD) Macht, 1939
Guinea-pig i.p. 4000(LD) Macht, 1939
Cat oral 800-1000 Flury, 1920
i.p. 800-1000 Flury, 1920
i.v. 34(LD) Macht, 1939
Rabbit i.p. approx. 2000 Herken, 1961
(b) d1-menthol
Mouse s.c. 1400-1600 Flury & Seel, 1926
Rat oral 2900 Herken, 1961
3180 Jenner et al., 1964
i.p. 750 Herken, 1961
Cat oral 1500-1600 Flury & Seel, 1926
i.p. 1500-1600 Flury & Seel, 1926
Rabbit i.p. approx. 2000 Herken, 1961
Short-term studies
Rat. Groups of 40 male and 40 female rats received 0, 100 and
200 mg/kg body-weight of either 1- or d1-menthol in their diet for
5-1/2 weeks. There was no adverse effect on weight gain, excretion of
glucuronide, water and electrolytes, nor interference with CNS
reactions to cardrazol or electric shock, or on i.v. hexobarbital
sleeping time as compared with controls (Herken, 1961).
Long-term studies
None available.
Observations in man
Smoking 80 mentholated cigarettes resulted in irritability,
gastrointestinal upsets, tremors, ataxia, bradycardia and toxic
psychosis. Taking 64 mg of menthol three times a day produced
tiredness and apathy within 3 days and nausea, exhaustion and
bradycardia in 7 days (Luke, 1962). Chronic urticaria with basophil
leucopenia on challenge has been reported after contact with menthol
in toothpaste, mentholated cigarettes, peppermint sweets, etc. (Papa &
Shelley, 1964; McCowan, 1966). The usual human oral dose is 60-120 mg.
Comments
Metabolic studies are not very revealing. There is much human
experience from long-established therapeutic use. Further metabolic
studies and adequate long-term studies are desirable.
EVALUATION
Level causing no toxicological effect
Rat. 200 mg/kg body-weight of d1- pr 1-menthol/day.
Estimate of acceptable daily intake for man
mg/kg body-weight
Unconditional acceptance 0-0.2
Conditional acceptance 0.2-2
REFERENCES
Flury, F. (1920) Abderhalden's Handbuch der Biologischen
Arbeitsmethoden,39, 1365
Flury, F. & Seel, H. (1926) Münch. Med. Wschr., 48, 2011
Herken, H. (1961) Report to Schering, AG
Jenner, P. M., Hagan, E. C., Taylor, J. M., Cook, E. L. & Fitzhugh, O.
G. (1964) Fd Cosmet. Toxicol., 2, 327
Luke, E. (1962), Lancet, i, 110
Macht, D. I. (1939) Arch. Int. Pharmacodyn., 63, 43
McGowan, E. M. (1966) Arch. Derm.,94, 62
Papa, C. M. & Shelley, W. B. (1964) J. Amer. med. Ass., 189, 546
Quick, A.J. (1924) J. biol. Chem., 61, 681
Williams, R. T. (1938) Biochem. J., 32, 1849
Williams, R. T. (1959) Detoxication Mechanisms, Second edition,
Chapman & Hall, London
 Molecular weight 156.27
Description Menthol is an alcohol obtained from
various mint oils or prepared
synthetically. It may be either
levorotatory (1-menthol) from natural
or synthetic sources, or racemic
(d1-menthol) produced synthetically.
It occurs as colourless, hexagonal
crystals, usually needle-like, or in
fused masses, or as a crystalline
powder. It has a pleasant,
peppermint-like odour.
Biological Data
Biochemical aspects
In the dog, 5 per cent. of orally administered menthol
metabolizes to 1-menthyl-5-glucuronide (Williams, 1959). For other
animals, the reported proportions vary. In the rabbit, the larger the
ingested dose, the less conjugation (Quick, 1924). Other workers
reported 31-34 per cent. glucuronide excretion in rats after oral or
continuous i.v. dosing (Harken, 1961). Rabbits are said to eliminate
48 per cent. of 1-menthol and 59 per cent. of d1-menthol as
glucuronide (Williams, 1938). Menthol is absorbed percutaneously, and
extracts a local anaesthetic action in mice (Macht, 1939).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
(a) 1-menthol
Mouse s.c. 5000-6000 Flury, 1920
i.p. 2000(LD) Macht, 1939
Rat oral 3300 Herken, 1961
s.c. 1000-2500 Flury, 1920
i.p. 710 Herken, 1961
1500(LD) Macht, 1939
Guinea-pig i.p. 4000(LD) Macht, 1939
Cat oral 800-1000 Flury, 1920
i.p. 800-1000 Flury, 1920
i.v. 34(LD) Macht, 1939
Rabbit i.p. approx. 2000 Herken, 1961
(b) d1-menthol
Mouse s.c. 1400-1600 Flury & Seel, 1926
Rat oral 2900 Herken, 1961
3180 Jenner et al., 1964
i.p. 750 Herken, 1961
Cat oral 1500-1600 Flury & Seel, 1926
i.p. 1500-1600 Flury & Seel, 1926
Rabbit i.p. approx. 2000 Herken, 1961
Short-term studies
Rat. Groups of 40 male and 40 female rats received 0, 100 and
200 mg/kg body-weight of either 1- or d1-menthol in their diet for
5-1/2 weeks. There was no adverse effect on weight gain, excretion of
glucuronide, water and electrolytes, nor interference with CNS
reactions to cardrazol or electric shock, or on i.v. hexobarbital
sleeping time as compared with controls (Herken, 1961).
Long-term studies
None available.
Observations in man
Smoking 80 mentholated cigarettes resulted in irritability,
gastrointestinal upsets, tremors, ataxia, bradycardia and toxic
psychosis. Taking 64 mg of menthol three times a day produced
tiredness and apathy within 3 days and nausea, exhaustion and
bradycardia in 7 days (Luke, 1962). Chronic urticaria with basophil
leucopenia on challenge has been reported after contact with menthol
in toothpaste, mentholated cigarettes, peppermint sweets, etc. (Papa &
Shelley, 1964; McCowan, 1966). The usual human oral dose is 60-120 mg.
Comments
Metabolic studies are not very revealing. There is much human
experience from long-established therapeutic use. Further metabolic
studies and adequate long-term studies are desirable.
EVALUATION
Level causing no toxicological effect
Rat. 200 mg/kg body-weight of d1- pr 1-menthol/day.
Estimate of acceptable daily intake for man
mg/kg body-weight
Unconditional acceptance 0-0.2
Conditional acceptance 0.2-2
REFERENCES
Flury, F. (1920) Abderhalden's Handbuch der Biologischen
Arbeitsmethoden,39, 1365
Flury, F. & Seel, H. (1926) Münch. Med. Wschr., 48, 2011
Herken, H. (1961) Report to Schering, AG
Jenner, P. M., Hagan, E. C., Taylor, J. M., Cook, E. L. & Fitzhugh, O.
G. (1964) Fd Cosmet. Toxicol., 2, 327
Luke, E. (1962), Lancet, i, 110
Macht, D. I. (1939) Arch. Int. Pharmacodyn., 63, 43
McGowan, E. M. (1966) Arch. Derm.,94, 62
Papa, C. M. & Shelley, W. B. (1964) J. Amer. med. Ass., 189, 546
Quick, A.J. (1924) J. biol. Chem., 61, 681
Williams, R. T. (1938) Biochem. J., 32, 1849
Williams, R. T. (1959) Detoxication Mechanisms, Second edition,
Chapman & Hall, London
Molecular weight 156.27
Description Menthol is an alcohol obtained from
various mint oils or prepared
synthetically. It may be either
levorotatory (1-menthol) from natural
or synthetic sources, or racemic
(d1-menthol) produced synthetically.
It occurs as colourless, hexagonal
crystals, usually needle-like, or in
fused masses, or as a crystalline
powder. It has a pleasant,
peppermint-like odour.
Biological Data
Biochemical aspects
In the dog, 5 per cent. of orally administered menthol
metabolizes to 1-menthyl-5-glucuronide (Williams, 1959). For other
animals, the reported proportions vary. In the rabbit, the larger the
ingested dose, the less conjugation (Quick, 1924). Other workers
reported 31-34 per cent. glucuronide excretion in rats after oral or
continuous i.v. dosing (Harken, 1961). Rabbits are said to eliminate
48 per cent. of 1-menthol and 59 per cent. of d1-menthol as
glucuronide (Williams, 1938). Menthol is absorbed percutaneously, and
extracts a local anaesthetic action in mice (Macht, 1939).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
(a) 1-menthol
Mouse s.c. 5000-6000 Flury, 1920
i.p. 2000(LD) Macht, 1939
Rat oral 3300 Herken, 1961
s.c. 1000-2500 Flury, 1920
i.p. 710 Herken, 1961
1500(LD) Macht, 1939
Guinea-pig i.p. 4000(LD) Macht, 1939
Cat oral 800-1000 Flury, 1920
i.p. 800-1000 Flury, 1920
i.v. 34(LD) Macht, 1939
Rabbit i.p. approx. 2000 Herken, 1961
(b) d1-menthol
Mouse s.c. 1400-1600 Flury & Seel, 1926
Rat oral 2900 Herken, 1961
3180 Jenner et al., 1964
i.p. 750 Herken, 1961
Cat oral 1500-1600 Flury & Seel, 1926
i.p. 1500-1600 Flury & Seel, 1926
Rabbit i.p. approx. 2000 Herken, 1961
Short-term studies
Rat. Groups of 40 male and 40 female rats received 0, 100 and
200 mg/kg body-weight of either 1- or d1-menthol in their diet for
5-1/2 weeks. There was no adverse effect on weight gain, excretion of
glucuronide, water and electrolytes, nor interference with CNS
reactions to cardrazol or electric shock, or on i.v. hexobarbital
sleeping time as compared with controls (Herken, 1961).
Long-term studies
None available.
Observations in man
Smoking 80 mentholated cigarettes resulted in irritability,
gastrointestinal upsets, tremors, ataxia, bradycardia and toxic
psychosis. Taking 64 mg of menthol three times a day produced
tiredness and apathy within 3 days and nausea, exhaustion and
bradycardia in 7 days (Luke, 1962). Chronic urticaria with basophil
leucopenia on challenge has been reported after contact with menthol
in toothpaste, mentholated cigarettes, peppermint sweets, etc. (Papa &
Shelley, 1964; McCowan, 1966). The usual human oral dose is 60-120 mg.
Comments
Metabolic studies are not very revealing. There is much human
experience from long-established therapeutic use. Further metabolic
studies and adequate long-term studies are desirable.
EVALUATION
Level causing no toxicological effect
Rat. 200 mg/kg body-weight of d1- pr 1-menthol/day.
Estimate of acceptable daily intake for man
mg/kg body-weight
Unconditional acceptance 0-0.2
Conditional acceptance 0.2-2
REFERENCES
Flury, F. (1920) Abderhalden's Handbuch der Biologischen
Arbeitsmethoden,39, 1365
Flury, F. & Seel, H. (1926) Münch. Med. Wschr., 48, 2011
Herken, H. (1961) Report to Schering, AG
Jenner, P. M., Hagan, E. C., Taylor, J. M., Cook, E. L. & Fitzhugh, O.
G. (1964) Fd Cosmet. Toxicol., 2, 327
Luke, E. (1962), Lancet, i, 110
Macht, D. I. (1939) Arch. Int. Pharmacodyn., 63, 43
McGowan, E. M. (1966) Arch. Derm.,94, 62
Papa, C. M. & Shelley, W. B. (1964) J. Amer. med. Ass., 189, 546
Quick, A.J. (1924) J. biol. Chem., 61, 681
Williams, R. T. (1938) Biochem. J., 32, 1849
Williams, R. T. (1959) Detoxication Mechanisms, Second edition,
Chapman & Hall, London
