
FAO Nutrition Meetings
Resort Series No. 44A
WHO/Food Add./68.33
TOXICOLOGICAL EVALUATION OF SOME
FLAVOURING SUBSTANCES AND
NON-NUTRITIVE SWEETENING AGENTS
Geneva, 21-28 August 1967
The Eleventh Report of the Joint FAO/WHO Expert Committee on Food
Additives is published as FAO Nutrition Meetings Report Series,
1967, No. 44; Wld Hlth Org. techn. Rep. Ser., 1968, 383. This
Report contains general considerations, including the principles
adopted for the evaluation, and a summary of the results of the
evaluations of a number of food additives. Additional information,
such as biological data and a toxicological evaluation, considered at
that meeting, is to be found in this document.
Food and Agriculture Organization of the United Nations
World Health Organization
1967
METHYL ANTHRANILATE
Chemical name Methyl 2-aminobenzoate
Empirical formula C8H9NO2
Structural formula
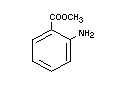 Molecular weight 151.17
Definition Methyl anthranilate contains not less than 98
per cent. C8H9NO2.
Description Methyl anthranilate is found in neroli oil
and in citrus and other oils. It is prepared
synthetically by esterification of
anthranilic acid. It is a colourless to pale
yellow liquid with a bluish fluorescence. It
has a grape-like odour.
Biological Data
Biochemical aspects
This ester is probably hydrolysed and the anthranilate excreted
mostly as o-aminobenzoyl glucuronide (Charconnet-Harding et al., 1953)
Some anthranilic acid is excreted unchanged, while other reported
metabolites include o-aminohippuric acid, 5-hydroxyanthranilic acid
and anthranoylanthranilic acid (Williams, 1959).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Mouse oral 3900 Jenner et al., 1964
Rat oral 2910 Jenner et al., 1964
Guinea-pig oral 2780 Jenner et al., 1964,
Rat oral 3000 Dow Chemical Company, 1967
Guinea-pig oral 4000 Dow Chemical Company, 1967
Short-term studies
Rat. Groups of 10 male and 10 female rats were fed diets
containing 0, 0.1 and 1.0 per cent. ester for 13 weeks without adverse
effect on weight gain, organ weights or histology of major organs
(Hagan et al., 1967). In another study on groups of 10 males and 10
females fed 0, 0.3 and 1.0. per cent., no adverse effect was noted at
0.3 per cent., but at 1.0 per cent. there were statistically
significant increases in liver and kidney weights of male rats, with
renal histologic changes (Dow Chemical Company, 1967).
Long-term studies
None available.
Comments
Evaluation is based on the short-term studies and the probable
metabolic fate.
EVALUATION
Level Causing No Toxicological Effect
Rat. 3000 ppm in the diet, equivalent to 150 mg/kg/day.
Estimate of acceptable daily intake for man
mg/kg body-weight
Conditional acceptance 0-1.5
Further work required
Biochemical, metabolic and long-term tests, including
carcinogenicity are required.
REFERENCES
Charconnet-Harding, F., Dalgliesh, C.E. & Neuberger, A. (1953)
Biochem. J., 53, 513
Dow Chemical Company (1967) Unpublished report
Hagan, E. C., Hansen, W. H., Fitzhugh, O. G., Jenner, P. M., Jones, W.
I., Taylor, J. M., Long, E. L. Nelson, A. A. & Brouwer, J. B. (1967)
Fd Cosmet. Toxicol., 5(2) 141
Jenner, P. M. Hagan, E. C., Taylor, J. M., Cook, E. L. & Fitzhugh, O.
G. (1964) Fd Cosmet. Toxicol., 3, 327
Williams, R. T. (1959) Detoxication Mechanisms, Second Edition,
Chapman & Hall, London
Molecular weight 151.17
Definition Methyl anthranilate contains not less than 98
per cent. C8H9NO2.
Description Methyl anthranilate is found in neroli oil
and in citrus and other oils. It is prepared
synthetically by esterification of
anthranilic acid. It is a colourless to pale
yellow liquid with a bluish fluorescence. It
has a grape-like odour.
Biological Data
Biochemical aspects
This ester is probably hydrolysed and the anthranilate excreted
mostly as o-aminobenzoyl glucuronide (Charconnet-Harding et al., 1953)
Some anthranilic acid is excreted unchanged, while other reported
metabolites include o-aminohippuric acid, 5-hydroxyanthranilic acid
and anthranoylanthranilic acid (Williams, 1959).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Mouse oral 3900 Jenner et al., 1964
Rat oral 2910 Jenner et al., 1964
Guinea-pig oral 2780 Jenner et al., 1964,
Rat oral 3000 Dow Chemical Company, 1967
Guinea-pig oral 4000 Dow Chemical Company, 1967
Short-term studies
Rat. Groups of 10 male and 10 female rats were fed diets
containing 0, 0.1 and 1.0 per cent. ester for 13 weeks without adverse
effect on weight gain, organ weights or histology of major organs
(Hagan et al., 1967). In another study on groups of 10 males and 10
females fed 0, 0.3 and 1.0. per cent., no adverse effect was noted at
0.3 per cent., but at 1.0 per cent. there were statistically
significant increases in liver and kidney weights of male rats, with
renal histologic changes (Dow Chemical Company, 1967).
Long-term studies
None available.
Comments
Evaluation is based on the short-term studies and the probable
metabolic fate.
EVALUATION
Level Causing No Toxicological Effect
Rat. 3000 ppm in the diet, equivalent to 150 mg/kg/day.
Estimate of acceptable daily intake for man
mg/kg body-weight
Conditional acceptance 0-1.5
Further work required
Biochemical, metabolic and long-term tests, including
carcinogenicity are required.
REFERENCES
Charconnet-Harding, F., Dalgliesh, C.E. & Neuberger, A. (1953)
Biochem. J., 53, 513
Dow Chemical Company (1967) Unpublished report
Hagan, E. C., Hansen, W. H., Fitzhugh, O. G., Jenner, P. M., Jones, W.
I., Taylor, J. M., Long, E. L. Nelson, A. A. & Brouwer, J. B. (1967)
Fd Cosmet. Toxicol., 5(2) 141
Jenner, P. M. Hagan, E. C., Taylor, J. M., Cook, E. L. & Fitzhugh, O.
G. (1964) Fd Cosmet. Toxicol., 3, 327
Williams, R. T. (1959) Detoxication Mechanisms, Second Edition,
Chapman & Hall, London
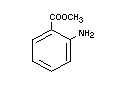 Molecular weight 151.17
Definition Methyl anthranilate contains not less than 98
per cent. C8H9NO2.
Description Methyl anthranilate is found in neroli oil
and in citrus and other oils. It is prepared
synthetically by esterification of
anthranilic acid. It is a colourless to pale
yellow liquid with a bluish fluorescence. It
has a grape-like odour.
Biological Data
Biochemical aspects
This ester is probably hydrolysed and the anthranilate excreted
mostly as o-aminobenzoyl glucuronide (Charconnet-Harding et al., 1953)
Some anthranilic acid is excreted unchanged, while other reported
metabolites include o-aminohippuric acid, 5-hydroxyanthranilic acid
and anthranoylanthranilic acid (Williams, 1959).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Mouse oral 3900 Jenner et al., 1964
Rat oral 2910 Jenner et al., 1964
Guinea-pig oral 2780 Jenner et al., 1964,
Rat oral 3000 Dow Chemical Company, 1967
Guinea-pig oral 4000 Dow Chemical Company, 1967
Short-term studies
Rat. Groups of 10 male and 10 female rats were fed diets
containing 0, 0.1 and 1.0 per cent. ester for 13 weeks without adverse
effect on weight gain, organ weights or histology of major organs
(Hagan et al., 1967). In another study on groups of 10 males and 10
females fed 0, 0.3 and 1.0. per cent., no adverse effect was noted at
0.3 per cent., but at 1.0 per cent. there were statistically
significant increases in liver and kidney weights of male rats, with
renal histologic changes (Dow Chemical Company, 1967).
Long-term studies
None available.
Comments
Evaluation is based on the short-term studies and the probable
metabolic fate.
EVALUATION
Level Causing No Toxicological Effect
Rat. 3000 ppm in the diet, equivalent to 150 mg/kg/day.
Estimate of acceptable daily intake for man
mg/kg body-weight
Conditional acceptance 0-1.5
Further work required
Biochemical, metabolic and long-term tests, including
carcinogenicity are required.
REFERENCES
Charconnet-Harding, F., Dalgliesh, C.E. & Neuberger, A. (1953)
Biochem. J., 53, 513
Dow Chemical Company (1967) Unpublished report
Hagan, E. C., Hansen, W. H., Fitzhugh, O. G., Jenner, P. M., Jones, W.
I., Taylor, J. M., Long, E. L. Nelson, A. A. & Brouwer, J. B. (1967)
Fd Cosmet. Toxicol., 5(2) 141
Jenner, P. M. Hagan, E. C., Taylor, J. M., Cook, E. L. & Fitzhugh, O.
G. (1964) Fd Cosmet. Toxicol., 3, 327
Williams, R. T. (1959) Detoxication Mechanisms, Second Edition,
Chapman & Hall, London
Molecular weight 151.17
Definition Methyl anthranilate contains not less than 98
per cent. C8H9NO2.
Description Methyl anthranilate is found in neroli oil
and in citrus and other oils. It is prepared
synthetically by esterification of
anthranilic acid. It is a colourless to pale
yellow liquid with a bluish fluorescence. It
has a grape-like odour.
Biological Data
Biochemical aspects
This ester is probably hydrolysed and the anthranilate excreted
mostly as o-aminobenzoyl glucuronide (Charconnet-Harding et al., 1953)
Some anthranilic acid is excreted unchanged, while other reported
metabolites include o-aminohippuric acid, 5-hydroxyanthranilic acid
and anthranoylanthranilic acid (Williams, 1959).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Mouse oral 3900 Jenner et al., 1964
Rat oral 2910 Jenner et al., 1964
Guinea-pig oral 2780 Jenner et al., 1964,
Rat oral 3000 Dow Chemical Company, 1967
Guinea-pig oral 4000 Dow Chemical Company, 1967
Short-term studies
Rat. Groups of 10 male and 10 female rats were fed diets
containing 0, 0.1 and 1.0 per cent. ester for 13 weeks without adverse
effect on weight gain, organ weights or histology of major organs
(Hagan et al., 1967). In another study on groups of 10 males and 10
females fed 0, 0.3 and 1.0. per cent., no adverse effect was noted at
0.3 per cent., but at 1.0 per cent. there were statistically
significant increases in liver and kidney weights of male rats, with
renal histologic changes (Dow Chemical Company, 1967).
Long-term studies
None available.
Comments
Evaluation is based on the short-term studies and the probable
metabolic fate.
EVALUATION
Level Causing No Toxicological Effect
Rat. 3000 ppm in the diet, equivalent to 150 mg/kg/day.
Estimate of acceptable daily intake for man
mg/kg body-weight
Conditional acceptance 0-1.5
Further work required
Biochemical, metabolic and long-term tests, including
carcinogenicity are required.
REFERENCES
Charconnet-Harding, F., Dalgliesh, C.E. & Neuberger, A. (1953)
Biochem. J., 53, 513
Dow Chemical Company (1967) Unpublished report
Hagan, E. C., Hansen, W. H., Fitzhugh, O. G., Jenner, P. M., Jones, W.
I., Taylor, J. M., Long, E. L. Nelson, A. A. & Brouwer, J. B. (1967)
Fd Cosmet. Toxicol., 5(2) 141
Jenner, P. M. Hagan, E. C., Taylor, J. M., Cook, E. L. & Fitzhugh, O.
G. (1964) Fd Cosmet. Toxicol., 3, 327
Williams, R. T. (1959) Detoxication Mechanisms, Second Edition,
Chapman & Hall, London
