
FAO Nutrition Meetings
Resort Series No. 44A
WHO/Food Add./68.33
TOXICOLOGICAL EVALUATION OF SOME
FLAVOURING SUBSTANCES AND
NON-NUTRITIVE SWEETENING AGENTS
Geneva, 21-28 August 1967
The Eleventh Report of the Joint FAO/WHO Expert Committee on Food
Additives is published as FAO Nutrition Meetings Report Series,
1967, No. 44; Wld Hlth Org. techn. Rep. Ser., 1968, 383. This
Report contains general considerations, including the principles
adopted for the evaluation, and a summary of the results of the
evaluations of a number of food additives. Additional information,
such as biological data and a toxicological evaluation, considered at
that meeting, is to be found in this document.
Food and Agriculture Organization of the United Nations
World Health Organization
1967
PIPERONAL
Synonyms Heliotropine; Piperonyl aldehyde
Chemical name Piperonal
Empirical formula C8H6O3
Structural formula
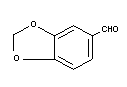 Molecular weight 150.14
Definition Piperonal contains not less than 99 per
cent. C8H6O3.
Description Piperonal is found in oils of Spirea
ulmaria L., Doriphora sassafras
Endl., and other oils. It is prepared by
oxidation of isosafrole. It is a white
crystalline substance with a sweet
floral odour resembling heliotrope and
free from safrole by-odour.
Biological Data
Biochemical aspects
This is probably metabolized to piperonylic acid. The glycine
conjugate of piperonylic acid has been isolated from human urine
(Heffter, 1895).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Mouse i.p. >500 Patty, 1963
Rat oral 2700 Jenner et al., 1964
i.p. 1500-1700 Binet, 1896
Intragastric administration of 900 mg/kg body-weight per day to 3
male and 3 female rats for 4 days produced slight gross liver damage
in some animals (Taylor et. al., 1964). When rats were given 13-115 mg
Piperonal perorally for 5-9 days, fatty infiltration of the liver
parenchymal cells was noted (Shillinger, 1950). Ten grams have been
ingested by man without any adverse effect (Von Oettingen, 1949).
Short-term studies
Rat. A 12-week feeding study on 15 males and 15-females, using
mixed aldehydes, produced no adverse effect at 14.2 mg/kg
body-weight/day (Oser, 1967). In another study, groups of 5 male and 5
female rats were fed diets containing 0 and 1.0 per cent. piperonal
for 15 weeks, and 0.1 per cent. for 28 weeks. No adverse effects were
noted on body-weight gain, haematology, organ weights of major organs
and histology of major tissues (Hagan et al., 1965; Hagan et al.,
1967).
Long-term studies
Rat. Groups of 20 male and 20 female rats were fed on diets
containing 0, 0.1 and 0.5 per cent. piperonal for 2 years without any
specific adverse effect (Baer & Griepentrog, 1967).
Comments
The long-term study in rats compensates for the scanty
information on the metabolic fate of this compound, and the evaluation
is based on this. Further metabolic studies are desirable.
EVALUATION
Level causing no toxicological effect
Rat. 0.5 per cent. (= 5000 ppm) in the diet, equivalent to 250
mg/kg body-weight/day.
Estimate of acceptable daily intake for man
mg/kg body-weight
Unconditional acceptance 0-2.5
REFERENCES
Baer, F. & Griepentrog, F. (1967) Unpublished report
Binet, P. (1896) Rev. Med. Suisse, 16, 449
Hagan, E. C., Jenner, P. M., Jones, W. I., Fitzhugh, O. G., Long, E.
C., Brouwer, J. G. & Webb, W. K. (1965) Toxic. appl. Pharm., 7, 18
Hagan, E. C., Hanse, W. H., Fitzhugh, O. G., Jenner, P. M., Jones, W.
I., Taylor J.M., Long, E.L. Nelson, A.A. & Brouwer, J. P. (1967)
Fd Cosmet. Toxicol., 5(2), 141
Heffter, A. (1895) Arch. exp. Pathol. Pharmacol., 35, 342
Jenner, P. M., Hagan, E. C., Taylor, J. M., Cook, E. L. & Fitzhugh, O.
G. (1964) Fd Cosmet. Toxicol., 2, 327
Oser, B. L. (1967) Unpublished report
Patty, F. A. (1963) Industrial Hygiene and Toxicology Vol. II.
Interscience
Shillinger, J. I. (1950) Gig. i. San., 3, 37
Taylor, J. M., Jenner, P. M. & Jones, W. I. (1964)Toxic. appl.
Pharm., 6, 378
Von Oettingen, W. F. (1949) Nat. Inst. Health Bull., No. 190, 342
Molecular weight 150.14
Definition Piperonal contains not less than 99 per
cent. C8H6O3.
Description Piperonal is found in oils of Spirea
ulmaria L., Doriphora sassafras
Endl., and other oils. It is prepared by
oxidation of isosafrole. It is a white
crystalline substance with a sweet
floral odour resembling heliotrope and
free from safrole by-odour.
Biological Data
Biochemical aspects
This is probably metabolized to piperonylic acid. The glycine
conjugate of piperonylic acid has been isolated from human urine
(Heffter, 1895).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Mouse i.p. >500 Patty, 1963
Rat oral 2700 Jenner et al., 1964
i.p. 1500-1700 Binet, 1896
Intragastric administration of 900 mg/kg body-weight per day to 3
male and 3 female rats for 4 days produced slight gross liver damage
in some animals (Taylor et. al., 1964). When rats were given 13-115 mg
Piperonal perorally for 5-9 days, fatty infiltration of the liver
parenchymal cells was noted (Shillinger, 1950). Ten grams have been
ingested by man without any adverse effect (Von Oettingen, 1949).
Short-term studies
Rat. A 12-week feeding study on 15 males and 15-females, using
mixed aldehydes, produced no adverse effect at 14.2 mg/kg
body-weight/day (Oser, 1967). In another study, groups of 5 male and 5
female rats were fed diets containing 0 and 1.0 per cent. piperonal
for 15 weeks, and 0.1 per cent. for 28 weeks. No adverse effects were
noted on body-weight gain, haematology, organ weights of major organs
and histology of major tissues (Hagan et al., 1965; Hagan et al.,
1967).
Long-term studies
Rat. Groups of 20 male and 20 female rats were fed on diets
containing 0, 0.1 and 0.5 per cent. piperonal for 2 years without any
specific adverse effect (Baer & Griepentrog, 1967).
Comments
The long-term study in rats compensates for the scanty
information on the metabolic fate of this compound, and the evaluation
is based on this. Further metabolic studies are desirable.
EVALUATION
Level causing no toxicological effect
Rat. 0.5 per cent. (= 5000 ppm) in the diet, equivalent to 250
mg/kg body-weight/day.
Estimate of acceptable daily intake for man
mg/kg body-weight
Unconditional acceptance 0-2.5
REFERENCES
Baer, F. & Griepentrog, F. (1967) Unpublished report
Binet, P. (1896) Rev. Med. Suisse, 16, 449
Hagan, E. C., Jenner, P. M., Jones, W. I., Fitzhugh, O. G., Long, E.
C., Brouwer, J. G. & Webb, W. K. (1965) Toxic. appl. Pharm., 7, 18
Hagan, E. C., Hanse, W. H., Fitzhugh, O. G., Jenner, P. M., Jones, W.
I., Taylor J.M., Long, E.L. Nelson, A.A. & Brouwer, J. P. (1967)
Fd Cosmet. Toxicol., 5(2), 141
Heffter, A. (1895) Arch. exp. Pathol. Pharmacol., 35, 342
Jenner, P. M., Hagan, E. C., Taylor, J. M., Cook, E. L. & Fitzhugh, O.
G. (1964) Fd Cosmet. Toxicol., 2, 327
Oser, B. L. (1967) Unpublished report
Patty, F. A. (1963) Industrial Hygiene and Toxicology Vol. II.
Interscience
Shillinger, J. I. (1950) Gig. i. San., 3, 37
Taylor, J. M., Jenner, P. M. & Jones, W. I. (1964)Toxic. appl.
Pharm., 6, 378
Von Oettingen, W. F. (1949) Nat. Inst. Health Bull., No. 190, 342
 Molecular weight 150.14
Definition Piperonal contains not less than 99 per
cent. C8H6O3.
Description Piperonal is found in oils of Spirea
ulmaria L., Doriphora sassafras
Endl., and other oils. It is prepared by
oxidation of isosafrole. It is a white
crystalline substance with a sweet
floral odour resembling heliotrope and
free from safrole by-odour.
Biological Data
Biochemical aspects
This is probably metabolized to piperonylic acid. The glycine
conjugate of piperonylic acid has been isolated from human urine
(Heffter, 1895).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Mouse i.p. >500 Patty, 1963
Rat oral 2700 Jenner et al., 1964
i.p. 1500-1700 Binet, 1896
Intragastric administration of 900 mg/kg body-weight per day to 3
male and 3 female rats for 4 days produced slight gross liver damage
in some animals (Taylor et. al., 1964). When rats were given 13-115 mg
Piperonal perorally for 5-9 days, fatty infiltration of the liver
parenchymal cells was noted (Shillinger, 1950). Ten grams have been
ingested by man without any adverse effect (Von Oettingen, 1949).
Short-term studies
Rat. A 12-week feeding study on 15 males and 15-females, using
mixed aldehydes, produced no adverse effect at 14.2 mg/kg
body-weight/day (Oser, 1967). In another study, groups of 5 male and 5
female rats were fed diets containing 0 and 1.0 per cent. piperonal
for 15 weeks, and 0.1 per cent. for 28 weeks. No adverse effects were
noted on body-weight gain, haematology, organ weights of major organs
and histology of major tissues (Hagan et al., 1965; Hagan et al.,
1967).
Long-term studies
Rat. Groups of 20 male and 20 female rats were fed on diets
containing 0, 0.1 and 0.5 per cent. piperonal for 2 years without any
specific adverse effect (Baer & Griepentrog, 1967).
Comments
The long-term study in rats compensates for the scanty
information on the metabolic fate of this compound, and the evaluation
is based on this. Further metabolic studies are desirable.
EVALUATION
Level causing no toxicological effect
Rat. 0.5 per cent. (= 5000 ppm) in the diet, equivalent to 250
mg/kg body-weight/day.
Estimate of acceptable daily intake for man
mg/kg body-weight
Unconditional acceptance 0-2.5
REFERENCES
Baer, F. & Griepentrog, F. (1967) Unpublished report
Binet, P. (1896) Rev. Med. Suisse, 16, 449
Hagan, E. C., Jenner, P. M., Jones, W. I., Fitzhugh, O. G., Long, E.
C., Brouwer, J. G. & Webb, W. K. (1965) Toxic. appl. Pharm., 7, 18
Hagan, E. C., Hanse, W. H., Fitzhugh, O. G., Jenner, P. M., Jones, W.
I., Taylor J.M., Long, E.L. Nelson, A.A. & Brouwer, J. P. (1967)
Fd Cosmet. Toxicol., 5(2), 141
Heffter, A. (1895) Arch. exp. Pathol. Pharmacol., 35, 342
Jenner, P. M., Hagan, E. C., Taylor, J. M., Cook, E. L. & Fitzhugh, O.
G. (1964) Fd Cosmet. Toxicol., 2, 327
Oser, B. L. (1967) Unpublished report
Patty, F. A. (1963) Industrial Hygiene and Toxicology Vol. II.
Interscience
Shillinger, J. I. (1950) Gig. i. San., 3, 37
Taylor, J. M., Jenner, P. M. & Jones, W. I. (1964)Toxic. appl.
Pharm., 6, 378
Von Oettingen, W. F. (1949) Nat. Inst. Health Bull., No. 190, 342
Molecular weight 150.14
Definition Piperonal contains not less than 99 per
cent. C8H6O3.
Description Piperonal is found in oils of Spirea
ulmaria L., Doriphora sassafras
Endl., and other oils. It is prepared by
oxidation of isosafrole. It is a white
crystalline substance with a sweet
floral odour resembling heliotrope and
free from safrole by-odour.
Biological Data
Biochemical aspects
This is probably metabolized to piperonylic acid. The glycine
conjugate of piperonylic acid has been isolated from human urine
(Heffter, 1895).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Mouse i.p. >500 Patty, 1963
Rat oral 2700 Jenner et al., 1964
i.p. 1500-1700 Binet, 1896
Intragastric administration of 900 mg/kg body-weight per day to 3
male and 3 female rats for 4 days produced slight gross liver damage
in some animals (Taylor et. al., 1964). When rats were given 13-115 mg
Piperonal perorally for 5-9 days, fatty infiltration of the liver
parenchymal cells was noted (Shillinger, 1950). Ten grams have been
ingested by man without any adverse effect (Von Oettingen, 1949).
Short-term studies
Rat. A 12-week feeding study on 15 males and 15-females, using
mixed aldehydes, produced no adverse effect at 14.2 mg/kg
body-weight/day (Oser, 1967). In another study, groups of 5 male and 5
female rats were fed diets containing 0 and 1.0 per cent. piperonal
for 15 weeks, and 0.1 per cent. for 28 weeks. No adverse effects were
noted on body-weight gain, haematology, organ weights of major organs
and histology of major tissues (Hagan et al., 1965; Hagan et al.,
1967).
Long-term studies
Rat. Groups of 20 male and 20 female rats were fed on diets
containing 0, 0.1 and 0.5 per cent. piperonal for 2 years without any
specific adverse effect (Baer & Griepentrog, 1967).
Comments
The long-term study in rats compensates for the scanty
information on the metabolic fate of this compound, and the evaluation
is based on this. Further metabolic studies are desirable.
EVALUATION
Level causing no toxicological effect
Rat. 0.5 per cent. (= 5000 ppm) in the diet, equivalent to 250
mg/kg body-weight/day.
Estimate of acceptable daily intake for man
mg/kg body-weight
Unconditional acceptance 0-2.5
REFERENCES
Baer, F. & Griepentrog, F. (1967) Unpublished report
Binet, P. (1896) Rev. Med. Suisse, 16, 449
Hagan, E. C., Jenner, P. M., Jones, W. I., Fitzhugh, O. G., Long, E.
C., Brouwer, J. G. & Webb, W. K. (1965) Toxic. appl. Pharm., 7, 18
Hagan, E. C., Hanse, W. H., Fitzhugh, O. G., Jenner, P. M., Jones, W.
I., Taylor J.M., Long, E.L. Nelson, A.A. & Brouwer, J. P. (1967)
Fd Cosmet. Toxicol., 5(2), 141
Heffter, A. (1895) Arch. exp. Pathol. Pharmacol., 35, 342
Jenner, P. M., Hagan, E. C., Taylor, J. M., Cook, E. L. & Fitzhugh, O.
G. (1964) Fd Cosmet. Toxicol., 2, 327
Oser, B. L. (1967) Unpublished report
Patty, F. A. (1963) Industrial Hygiene and Toxicology Vol. II.
Interscience
Shillinger, J. I. (1950) Gig. i. San., 3, 37
Taylor, J. M., Jenner, P. M. & Jones, W. I. (1964)Toxic. appl.
Pharm., 6, 378
Von Oettingen, W. F. (1949) Nat. Inst. Health Bull., No. 190, 342
