
FAO Nutrition Meetings
Resort Series No. 44A
WHO/Food Add./68.33
TOXICOLOGICAL EVALUATION OF SOME
FLAVOURING SUBSTANCES AND
NON-NUTRITIVE SWEETENING AGENTS
Geneva, 21-28 August 1967
The Eleventh Report of the Joint FAO/WHO Expert Committee on Food
Additives is published as FAO Nutrition Meetings Report Series,
1967, No. 44; Wld Hlth Org. techn. Rep. Ser., 1968, 383. This
Report contains general considerations, including the principles
adopted for the evaluation, and a summary of the results of the
evaluations of a number of food additives. Additional information,
such as biological data and a toxicological evaluation, considered at
that meeting, is to be found in this document.
Food and Agriculture Organization of the United Nations
World Health Organization
1967
CALCIUM CYCLAMATE
Chemical name Calcium cyclohexanesulfamate; Calcium
cyclohexylsulfamate
Empirical formula C12H24CaN2O6S2.2H2O
Structural formula
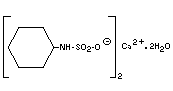 Molecular weight 432.57
Definition Calcium cyclamate contains not less than
98 per cent. and not more than the
equivalent of 101 per cent.
C12H24CaN2O6S2.
Description White, odourless crystals or crystalline
powder.
Use Non-nutritive sweetener.
Biological Data
Biochemical aspects
The metabolic fate was studied in rats using single oral doses of
0.46, 0.93 or 1.85 g/kg body-weight. 17-30 per cent. was excreted in
the urine and 70-80 per cent. in the faeces over 72 hours, absorption
being slightly less than for the sodium salt (Hwang, 1966). In another
experiment calcium cyclamate excretion was followed using gas-liquid
chromatography. At 1 per cent. of the diet 17 per cent. was recovered
in the urine and 70 per cent. in the faeces; at 5 per cent. of the
diet, 14 per cent. and 46 per cent.; and at 10 per cent. in the diet,
15 per cent. and 30 per cent. (Derse & Daun, 1966).
In the rat, 99 per cent. of the radioactivity excreted in the
urine following single oral doses (714 and 909 mg/kg) was in the form
of unchanged cyclamate (Sonders & Miller, 1966).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Mouse oral 7200 Abbott Laboratories, 1966
i.v. 570 Abbott Laboratories, 1966
Rat s.c. 100 Golberg, 1965
Rabbit i.v. 125 Abbott Laboratories, 1966
Special studies
The laxative and faecal softening effects were studied in rat,
mouse, dog and monkey, using 35S-labelled calcium cyclamate. Over 70
per cent. of the compound remained unabsorbed in the gut, no
significant systemic effects were noted, and laxative action was due
to the increased bulk secondary to osmosis as shown by the motility of
isolated gut. No gross intestinal mucosal changes were noted. The
laxative dose (ED50) was 2.8 g/kg in the rat (Hwang, 1966). Similar
results were noted in a repeat study on rats, mice and dogs, these
being ascribed entirely to osmotic hypertonicity (Taylor et al.,
1967).
Rat. Groups of 10 weanling rats were fed 0, 5 and 10 per cent.
calcium cyclamate for 22 weeks; 3-day balance studies were done in
the eighth, fourteenth, twentieth and twenty-second weeks, in which
chromatographic recoveries from urine and faeces were compared with
consumption. These recoveries were simultaneously compared with
isotope recoveries from additional labelled doses of the sodium salt
administered orally at the beginning of each balance period. Average
chromatographic recoveries ranged from 76.3 to over 100 per cent.
Gross, histological and radiological examination of the survivors
showed no changes attributable to cyclamate (Sonders et al., 1967).
In a test designed to study the influence of cyclamate on
caloric utilization, groups of 6 male rats were fed 5 g/day basal
diets supplemented with 0, 1 and 2 g/day of sucrose with and without
1, 2 and 4 per cent. calcium cyclamate, and 2.33 per cent. sodium
sulfate (osmotically equivalent to the 4 per cent. cyclamate level)
for 10 days. A smaller weight gain and mild diarrhoea were seen in the
4 per cent. cyclamate group. No catharsis was seen in the sodium
sulfate group. Mild diarrhoea, but without a clear-cut effect on
weight gain, was again found at the 4 per cent. cyclamate level in a
further test in which the same animals, redistributed to groups, were
tested in the same manner with starch instead of sucrose (Frost &
Main, 1967).
Short-term studies
Rat. Calcium cyclamate was fed to groups of rats at 0, 5 and 10
per cent. of the diet for 8 weeks. Growth rates were reduced despite
greater food and water intake at both levels, with possible
histological changes in adrenal and other organs. All faeces were soft
and moist. Urinalysis and haematology were normal. After 8 weeks half
the animals were placed on restricted food intake (60 per cent. of
ad lib. feeding). Females and males from both series were mated
twice to produce an F1a and F1b generation. Both filial generations
showed a dose-related reduction in pup weight. Animals on restricted
food intake conceived and gestated but could not raise their young
(Nees & Derse, 1965; 1967).
Groups of 40 rats were fed 0 and 5 per cent. calcium cyclamate
for 4 months. Soft faeces without frank diarrhoea, depressed rate of
weight gain and ceca grossly dilated with fluid were seen in the test
group. However, appetite was unaffected. Discrete moderate
histological abnormalities unrelated to length of treatment were seen
The mucosal architecture was unimpaired except for a rise in crypt
height; 50 per cent. of test animals showed ballooning of the tips of
mucosal cells with cytoplasmic vacuolization, while 80 per cent.
showed oedema of the ileum and enlarged villi without congestion or
inflammation. The mitotic index did not differ from that of the
controls, but there was hyperplasia of myenteric ganglion cells and
other evidence of accelerated cellular replacement.
Histochemically there was non-specific goblet cell hyperplasia,
abnormal RNA change but normal fat absorption. Histoenzymatic studies
showed various qualitative anomalies and alteration in distribution
particularly depression of ATPase, variable depression of acid and
alkaline phosphatase, reduced Krebs cycle activity but little
disturbance of Embden-Meyerhoff pathways. Pentose cycle enzymes were
increased, indicating a metabolic change induced by enhanced nucleic
acid synthesis. No definite changes were noted in enzymes involved in
protein and lipid metabolism (Bernier, 1967).
Observations in man
Intravenous doses of 1 g calcium cyclamate were tolerated
without ill effects (Schoenberger et al., 1953). Oral doses of 5 to 12
g for 14-21 days had no other effect except soft stools without
significant effect in frequency of bowel movement (Wisconsin A. R. F.,
1965).
Following administration of a single oral dose of 2 g calcium
cyclamate (containing 35S-labelled material) to a man, 31.2 per cent.
was excreted in the urine and 65.5 per cent. in the faeces over 3-4
days. In another study, 2 volunteers ingested 5 g calcium cyclamate
daily for 18 days without any adverse effect on metabolism of
nitrogen, calcium, phosphorus, sodium and potassium. Some 37 per cent.
of the daily dose was excreted in the urine and 50-61 per cent. in the
faeces (Schoenberger et al., 1953).
Urinary excretion was studied by intravenous infusion of a single
dose of 1 g, followed by oral administration of 5 g/day for 2 weeks
and a concluding intravenous administration of a 1 g dose. In 2 normal
subjects, 80 per cent. of the i.v. dose was excreted, whether
preceding or following the oral course. In 7 nephritics, a mean of 87
per cent. of the i.v. dose was excreted before the oral course, and 93
per cent. after. Of the oral doses, mean daily excretion in the normal
subjects was 31 per cent., and in 3 nephritics it was 13 per cent.
(Dedmon et al., 1961).
A study of cyclamate ingestion was carried out in 164 children,
aged 3-16 years, in four weight classes of 15-45 lbs., 45-75 lbs.,
75-105 lbs. and 105-135 lbs., with arbitrarily assigned body weights
of 30, 60, 90 and 120 lbs. for individuals within these classes, for
the purpose of administration of daily doses. These classes were
further subdivided into three groups of 15-16 children each. These
groups received 0, 1 and 1.5 g/30 lbs. body-weight/ day for 12 weeks.
After a rest period of two weeks the groups were redistributed to the
same dosages for another 12 weeks.
The compound was administered as artificially sweetened
carbonated soft drinks, and, in the higher weight classes, in capsule
supplements, whereas the 0 g/30 lbs. group received sugar-sweetened
soft drinks only. Soft stools, but no diarrhoea, were seen in some of
the children. No effect was noted in physical examinations, nor in
peripheral blood picture, examination of urine, and laboratory
parameters of liver function (Freese et al., 1967).
Ten healthy males were given 5 g/day in aqueous solution for 5
days, removed from the regimen for 7 days, then returned to 5 g/day
for another 5 days. Slightly increased water excretion in urine and
faeces but no change in serum sodium and potassium, BUN and serum
alkaline phosphatase activity were found. Blood cell counts were
unchanged (Berndt & Calandra, 1965).
Groups of 28 male volunteers took 5-7 g/day of calcium cyclamate
for 21 days, or 7-12 g/day for 14 days, without significant change in
blood cell counts or composition of the urine. One man experienced a
significant increase in the frequency of bowel movements at 7 g/day;
12 g/day produced questionably significant increases in 4 of 28. Bulk
and softness of stools was increased at 10-12 g/day but the group mean
of frequency of bowel movements was not affected at this level
(NAS-NRC, 1955).
Six male volunteers received 5 g calcium cyclamate daily in
divided doses for 7-1/2 months. No unusual symptoms were reported
except increased bulk and mushiness of stools without increase in
number of bowel movements. No adverse effects were noted in
haemopoietic, cardiovascular, renal or hepatic systems (Schoenberger
et al., 1953).
Thirty adult males ingested 1.8-6.42 g/day in dietetic foods, by
capsule and in soft drinks, for 48 weeks without apparent effect on
renal, hepatic or thyroid functions or on peripheral blood picture
(Radding, 1967).
Forty-two diabetic outpatients kept daily records of their
voluntary cyclamate intake for 6-9 months: the cumulative daily
averages of intake for the period ranged from 209 to 3107 mg/day. No
changes in bowel habits or gastrointestinal signs or symptoms were
noted. At six months, the group average results of blood cell counts,
examination of urine, determination of blood urea nitrogen and liver
function tests, except serum alkaline phosphatase, were not different
from base-line determinations. Mean serum alkaline phosphatase
activity was lowered, and mean fasting blood sugar was slightly
increased (Stern, 1967).
Twenty-one patients with chronic renal disease took up to 5.3
g/cay of calcium cyclamate by capsule for 6 months without any
alteration in their clinical courses attributable to cyclamate. Of the
original 29 persons started on the regimen, one stopped taking
cyclamate after the first month because of diarrhoea (Kark, 1967).
Seventeen patients with functional gastrointestinal disease took
4-5 g/day by capsule for 6-10 weeks. Of the original group of 20, one
stopped the regimen after 3 weeks because of exacerbation of a
pre-existing dermatitis, and another stopped after 5 days because of
diarrhoea. No other adverse effects were noted (Batterman, 1966).
Comments
See Sodium Cyclamate
EVALUATION
See Sodium Cyclamate
REFERENCES
See Sodium Cyclamate
Molecular weight 432.57
Definition Calcium cyclamate contains not less than
98 per cent. and not more than the
equivalent of 101 per cent.
C12H24CaN2O6S2.
Description White, odourless crystals or crystalline
powder.
Use Non-nutritive sweetener.
Biological Data
Biochemical aspects
The metabolic fate was studied in rats using single oral doses of
0.46, 0.93 or 1.85 g/kg body-weight. 17-30 per cent. was excreted in
the urine and 70-80 per cent. in the faeces over 72 hours, absorption
being slightly less than for the sodium salt (Hwang, 1966). In another
experiment calcium cyclamate excretion was followed using gas-liquid
chromatography. At 1 per cent. of the diet 17 per cent. was recovered
in the urine and 70 per cent. in the faeces; at 5 per cent. of the
diet, 14 per cent. and 46 per cent.; and at 10 per cent. in the diet,
15 per cent. and 30 per cent. (Derse & Daun, 1966).
In the rat, 99 per cent. of the radioactivity excreted in the
urine following single oral doses (714 and 909 mg/kg) was in the form
of unchanged cyclamate (Sonders & Miller, 1966).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Mouse oral 7200 Abbott Laboratories, 1966
i.v. 570 Abbott Laboratories, 1966
Rat s.c. 100 Golberg, 1965
Rabbit i.v. 125 Abbott Laboratories, 1966
Special studies
The laxative and faecal softening effects were studied in rat,
mouse, dog and monkey, using 35S-labelled calcium cyclamate. Over 70
per cent. of the compound remained unabsorbed in the gut, no
significant systemic effects were noted, and laxative action was due
to the increased bulk secondary to osmosis as shown by the motility of
isolated gut. No gross intestinal mucosal changes were noted. The
laxative dose (ED50) was 2.8 g/kg in the rat (Hwang, 1966). Similar
results were noted in a repeat study on rats, mice and dogs, these
being ascribed entirely to osmotic hypertonicity (Taylor et al.,
1967).
Rat. Groups of 10 weanling rats were fed 0, 5 and 10 per cent.
calcium cyclamate for 22 weeks; 3-day balance studies were done in
the eighth, fourteenth, twentieth and twenty-second weeks, in which
chromatographic recoveries from urine and faeces were compared with
consumption. These recoveries were simultaneously compared with
isotope recoveries from additional labelled doses of the sodium salt
administered orally at the beginning of each balance period. Average
chromatographic recoveries ranged from 76.3 to over 100 per cent.
Gross, histological and radiological examination of the survivors
showed no changes attributable to cyclamate (Sonders et al., 1967).
In a test designed to study the influence of cyclamate on
caloric utilization, groups of 6 male rats were fed 5 g/day basal
diets supplemented with 0, 1 and 2 g/day of sucrose with and without
1, 2 and 4 per cent. calcium cyclamate, and 2.33 per cent. sodium
sulfate (osmotically equivalent to the 4 per cent. cyclamate level)
for 10 days. A smaller weight gain and mild diarrhoea were seen in the
4 per cent. cyclamate group. No catharsis was seen in the sodium
sulfate group. Mild diarrhoea, but without a clear-cut effect on
weight gain, was again found at the 4 per cent. cyclamate level in a
further test in which the same animals, redistributed to groups, were
tested in the same manner with starch instead of sucrose (Frost &
Main, 1967).
Short-term studies
Rat. Calcium cyclamate was fed to groups of rats at 0, 5 and 10
per cent. of the diet for 8 weeks. Growth rates were reduced despite
greater food and water intake at both levels, with possible
histological changes in adrenal and other organs. All faeces were soft
and moist. Urinalysis and haematology were normal. After 8 weeks half
the animals were placed on restricted food intake (60 per cent. of
ad lib. feeding). Females and males from both series were mated
twice to produce an F1a and F1b generation. Both filial generations
showed a dose-related reduction in pup weight. Animals on restricted
food intake conceived and gestated but could not raise their young
(Nees & Derse, 1965; 1967).
Groups of 40 rats were fed 0 and 5 per cent. calcium cyclamate
for 4 months. Soft faeces without frank diarrhoea, depressed rate of
weight gain and ceca grossly dilated with fluid were seen in the test
group. However, appetite was unaffected. Discrete moderate
histological abnormalities unrelated to length of treatment were seen
The mucosal architecture was unimpaired except for a rise in crypt
height; 50 per cent. of test animals showed ballooning of the tips of
mucosal cells with cytoplasmic vacuolization, while 80 per cent.
showed oedema of the ileum and enlarged villi without congestion or
inflammation. The mitotic index did not differ from that of the
controls, but there was hyperplasia of myenteric ganglion cells and
other evidence of accelerated cellular replacement.
Histochemically there was non-specific goblet cell hyperplasia,
abnormal RNA change but normal fat absorption. Histoenzymatic studies
showed various qualitative anomalies and alteration in distribution
particularly depression of ATPase, variable depression of acid and
alkaline phosphatase, reduced Krebs cycle activity but little
disturbance of Embden-Meyerhoff pathways. Pentose cycle enzymes were
increased, indicating a metabolic change induced by enhanced nucleic
acid synthesis. No definite changes were noted in enzymes involved in
protein and lipid metabolism (Bernier, 1967).
Observations in man
Intravenous doses of 1 g calcium cyclamate were tolerated
without ill effects (Schoenberger et al., 1953). Oral doses of 5 to 12
g for 14-21 days had no other effect except soft stools without
significant effect in frequency of bowel movement (Wisconsin A. R. F.,
1965).
Following administration of a single oral dose of 2 g calcium
cyclamate (containing 35S-labelled material) to a man, 31.2 per cent.
was excreted in the urine and 65.5 per cent. in the faeces over 3-4
days. In another study, 2 volunteers ingested 5 g calcium cyclamate
daily for 18 days without any adverse effect on metabolism of
nitrogen, calcium, phosphorus, sodium and potassium. Some 37 per cent.
of the daily dose was excreted in the urine and 50-61 per cent. in the
faeces (Schoenberger et al., 1953).
Urinary excretion was studied by intravenous infusion of a single
dose of 1 g, followed by oral administration of 5 g/day for 2 weeks
and a concluding intravenous administration of a 1 g dose. In 2 normal
subjects, 80 per cent. of the i.v. dose was excreted, whether
preceding or following the oral course. In 7 nephritics, a mean of 87
per cent. of the i.v. dose was excreted before the oral course, and 93
per cent. after. Of the oral doses, mean daily excretion in the normal
subjects was 31 per cent., and in 3 nephritics it was 13 per cent.
(Dedmon et al., 1961).
A study of cyclamate ingestion was carried out in 164 children,
aged 3-16 years, in four weight classes of 15-45 lbs., 45-75 lbs.,
75-105 lbs. and 105-135 lbs., with arbitrarily assigned body weights
of 30, 60, 90 and 120 lbs. for individuals within these classes, for
the purpose of administration of daily doses. These classes were
further subdivided into three groups of 15-16 children each. These
groups received 0, 1 and 1.5 g/30 lbs. body-weight/ day for 12 weeks.
After a rest period of two weeks the groups were redistributed to the
same dosages for another 12 weeks.
The compound was administered as artificially sweetened
carbonated soft drinks, and, in the higher weight classes, in capsule
supplements, whereas the 0 g/30 lbs. group received sugar-sweetened
soft drinks only. Soft stools, but no diarrhoea, were seen in some of
the children. No effect was noted in physical examinations, nor in
peripheral blood picture, examination of urine, and laboratory
parameters of liver function (Freese et al., 1967).
Ten healthy males were given 5 g/day in aqueous solution for 5
days, removed from the regimen for 7 days, then returned to 5 g/day
for another 5 days. Slightly increased water excretion in urine and
faeces but no change in serum sodium and potassium, BUN and serum
alkaline phosphatase activity were found. Blood cell counts were
unchanged (Berndt & Calandra, 1965).
Groups of 28 male volunteers took 5-7 g/day of calcium cyclamate
for 21 days, or 7-12 g/day for 14 days, without significant change in
blood cell counts or composition of the urine. One man experienced a
significant increase in the frequency of bowel movements at 7 g/day;
12 g/day produced questionably significant increases in 4 of 28. Bulk
and softness of stools was increased at 10-12 g/day but the group mean
of frequency of bowel movements was not affected at this level
(NAS-NRC, 1955).
Six male volunteers received 5 g calcium cyclamate daily in
divided doses for 7-1/2 months. No unusual symptoms were reported
except increased bulk and mushiness of stools without increase in
number of bowel movements. No adverse effects were noted in
haemopoietic, cardiovascular, renal or hepatic systems (Schoenberger
et al., 1953).
Thirty adult males ingested 1.8-6.42 g/day in dietetic foods, by
capsule and in soft drinks, for 48 weeks without apparent effect on
renal, hepatic or thyroid functions or on peripheral blood picture
(Radding, 1967).
Forty-two diabetic outpatients kept daily records of their
voluntary cyclamate intake for 6-9 months: the cumulative daily
averages of intake for the period ranged from 209 to 3107 mg/day. No
changes in bowel habits or gastrointestinal signs or symptoms were
noted. At six months, the group average results of blood cell counts,
examination of urine, determination of blood urea nitrogen and liver
function tests, except serum alkaline phosphatase, were not different
from base-line determinations. Mean serum alkaline phosphatase
activity was lowered, and mean fasting blood sugar was slightly
increased (Stern, 1967).
Twenty-one patients with chronic renal disease took up to 5.3
g/cay of calcium cyclamate by capsule for 6 months without any
alteration in their clinical courses attributable to cyclamate. Of the
original 29 persons started on the regimen, one stopped taking
cyclamate after the first month because of diarrhoea (Kark, 1967).
Seventeen patients with functional gastrointestinal disease took
4-5 g/day by capsule for 6-10 weeks. Of the original group of 20, one
stopped the regimen after 3 weeks because of exacerbation of a
pre-existing dermatitis, and another stopped after 5 days because of
diarrhoea. No other adverse effects were noted (Batterman, 1966).
Comments
See Sodium Cyclamate
EVALUATION
See Sodium Cyclamate
REFERENCES
See Sodium Cyclamate
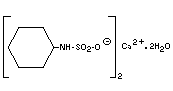 Molecular weight 432.57
Definition Calcium cyclamate contains not less than
98 per cent. and not more than the
equivalent of 101 per cent.
C12H24CaN2O6S2.
Description White, odourless crystals or crystalline
powder.
Use Non-nutritive sweetener.
Biological Data
Biochemical aspects
The metabolic fate was studied in rats using single oral doses of
0.46, 0.93 or 1.85 g/kg body-weight. 17-30 per cent. was excreted in
the urine and 70-80 per cent. in the faeces over 72 hours, absorption
being slightly less than for the sodium salt (Hwang, 1966). In another
experiment calcium cyclamate excretion was followed using gas-liquid
chromatography. At 1 per cent. of the diet 17 per cent. was recovered
in the urine and 70 per cent. in the faeces; at 5 per cent. of the
diet, 14 per cent. and 46 per cent.; and at 10 per cent. in the diet,
15 per cent. and 30 per cent. (Derse & Daun, 1966).
In the rat, 99 per cent. of the radioactivity excreted in the
urine following single oral doses (714 and 909 mg/kg) was in the form
of unchanged cyclamate (Sonders & Miller, 1966).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Mouse oral 7200 Abbott Laboratories, 1966
i.v. 570 Abbott Laboratories, 1966
Rat s.c. 100 Golberg, 1965
Rabbit i.v. 125 Abbott Laboratories, 1966
Special studies
The laxative and faecal softening effects were studied in rat,
mouse, dog and monkey, using 35S-labelled calcium cyclamate. Over 70
per cent. of the compound remained unabsorbed in the gut, no
significant systemic effects were noted, and laxative action was due
to the increased bulk secondary to osmosis as shown by the motility of
isolated gut. No gross intestinal mucosal changes were noted. The
laxative dose (ED50) was 2.8 g/kg in the rat (Hwang, 1966). Similar
results were noted in a repeat study on rats, mice and dogs, these
being ascribed entirely to osmotic hypertonicity (Taylor et al.,
1967).
Rat. Groups of 10 weanling rats were fed 0, 5 and 10 per cent.
calcium cyclamate for 22 weeks; 3-day balance studies were done in
the eighth, fourteenth, twentieth and twenty-second weeks, in which
chromatographic recoveries from urine and faeces were compared with
consumption. These recoveries were simultaneously compared with
isotope recoveries from additional labelled doses of the sodium salt
administered orally at the beginning of each balance period. Average
chromatographic recoveries ranged from 76.3 to over 100 per cent.
Gross, histological and radiological examination of the survivors
showed no changes attributable to cyclamate (Sonders et al., 1967).
In a test designed to study the influence of cyclamate on
caloric utilization, groups of 6 male rats were fed 5 g/day basal
diets supplemented with 0, 1 and 2 g/day of sucrose with and without
1, 2 and 4 per cent. calcium cyclamate, and 2.33 per cent. sodium
sulfate (osmotically equivalent to the 4 per cent. cyclamate level)
for 10 days. A smaller weight gain and mild diarrhoea were seen in the
4 per cent. cyclamate group. No catharsis was seen in the sodium
sulfate group. Mild diarrhoea, but without a clear-cut effect on
weight gain, was again found at the 4 per cent. cyclamate level in a
further test in which the same animals, redistributed to groups, were
tested in the same manner with starch instead of sucrose (Frost &
Main, 1967).
Short-term studies
Rat. Calcium cyclamate was fed to groups of rats at 0, 5 and 10
per cent. of the diet for 8 weeks. Growth rates were reduced despite
greater food and water intake at both levels, with possible
histological changes in adrenal and other organs. All faeces were soft
and moist. Urinalysis and haematology were normal. After 8 weeks half
the animals were placed on restricted food intake (60 per cent. of
ad lib. feeding). Females and males from both series were mated
twice to produce an F1a and F1b generation. Both filial generations
showed a dose-related reduction in pup weight. Animals on restricted
food intake conceived and gestated but could not raise their young
(Nees & Derse, 1965; 1967).
Groups of 40 rats were fed 0 and 5 per cent. calcium cyclamate
for 4 months. Soft faeces without frank diarrhoea, depressed rate of
weight gain and ceca grossly dilated with fluid were seen in the test
group. However, appetite was unaffected. Discrete moderate
histological abnormalities unrelated to length of treatment were seen
The mucosal architecture was unimpaired except for a rise in crypt
height; 50 per cent. of test animals showed ballooning of the tips of
mucosal cells with cytoplasmic vacuolization, while 80 per cent.
showed oedema of the ileum and enlarged villi without congestion or
inflammation. The mitotic index did not differ from that of the
controls, but there was hyperplasia of myenteric ganglion cells and
other evidence of accelerated cellular replacement.
Histochemically there was non-specific goblet cell hyperplasia,
abnormal RNA change but normal fat absorption. Histoenzymatic studies
showed various qualitative anomalies and alteration in distribution
particularly depression of ATPase, variable depression of acid and
alkaline phosphatase, reduced Krebs cycle activity but little
disturbance of Embden-Meyerhoff pathways. Pentose cycle enzymes were
increased, indicating a metabolic change induced by enhanced nucleic
acid synthesis. No definite changes were noted in enzymes involved in
protein and lipid metabolism (Bernier, 1967).
Observations in man
Intravenous doses of 1 g calcium cyclamate were tolerated
without ill effects (Schoenberger et al., 1953). Oral doses of 5 to 12
g for 14-21 days had no other effect except soft stools without
significant effect in frequency of bowel movement (Wisconsin A. R. F.,
1965).
Following administration of a single oral dose of 2 g calcium
cyclamate (containing 35S-labelled material) to a man, 31.2 per cent.
was excreted in the urine and 65.5 per cent. in the faeces over 3-4
days. In another study, 2 volunteers ingested 5 g calcium cyclamate
daily for 18 days without any adverse effect on metabolism of
nitrogen, calcium, phosphorus, sodium and potassium. Some 37 per cent.
of the daily dose was excreted in the urine and 50-61 per cent. in the
faeces (Schoenberger et al., 1953).
Urinary excretion was studied by intravenous infusion of a single
dose of 1 g, followed by oral administration of 5 g/day for 2 weeks
and a concluding intravenous administration of a 1 g dose. In 2 normal
subjects, 80 per cent. of the i.v. dose was excreted, whether
preceding or following the oral course. In 7 nephritics, a mean of 87
per cent. of the i.v. dose was excreted before the oral course, and 93
per cent. after. Of the oral doses, mean daily excretion in the normal
subjects was 31 per cent., and in 3 nephritics it was 13 per cent.
(Dedmon et al., 1961).
A study of cyclamate ingestion was carried out in 164 children,
aged 3-16 years, in four weight classes of 15-45 lbs., 45-75 lbs.,
75-105 lbs. and 105-135 lbs., with arbitrarily assigned body weights
of 30, 60, 90 and 120 lbs. for individuals within these classes, for
the purpose of administration of daily doses. These classes were
further subdivided into three groups of 15-16 children each. These
groups received 0, 1 and 1.5 g/30 lbs. body-weight/ day for 12 weeks.
After a rest period of two weeks the groups were redistributed to the
same dosages for another 12 weeks.
The compound was administered as artificially sweetened
carbonated soft drinks, and, in the higher weight classes, in capsule
supplements, whereas the 0 g/30 lbs. group received sugar-sweetened
soft drinks only. Soft stools, but no diarrhoea, were seen in some of
the children. No effect was noted in physical examinations, nor in
peripheral blood picture, examination of urine, and laboratory
parameters of liver function (Freese et al., 1967).
Ten healthy males were given 5 g/day in aqueous solution for 5
days, removed from the regimen for 7 days, then returned to 5 g/day
for another 5 days. Slightly increased water excretion in urine and
faeces but no change in serum sodium and potassium, BUN and serum
alkaline phosphatase activity were found. Blood cell counts were
unchanged (Berndt & Calandra, 1965).
Groups of 28 male volunteers took 5-7 g/day of calcium cyclamate
for 21 days, or 7-12 g/day for 14 days, without significant change in
blood cell counts or composition of the urine. One man experienced a
significant increase in the frequency of bowel movements at 7 g/day;
12 g/day produced questionably significant increases in 4 of 28. Bulk
and softness of stools was increased at 10-12 g/day but the group mean
of frequency of bowel movements was not affected at this level
(NAS-NRC, 1955).
Six male volunteers received 5 g calcium cyclamate daily in
divided doses for 7-1/2 months. No unusual symptoms were reported
except increased bulk and mushiness of stools without increase in
number of bowel movements. No adverse effects were noted in
haemopoietic, cardiovascular, renal or hepatic systems (Schoenberger
et al., 1953).
Thirty adult males ingested 1.8-6.42 g/day in dietetic foods, by
capsule and in soft drinks, for 48 weeks without apparent effect on
renal, hepatic or thyroid functions or on peripheral blood picture
(Radding, 1967).
Forty-two diabetic outpatients kept daily records of their
voluntary cyclamate intake for 6-9 months: the cumulative daily
averages of intake for the period ranged from 209 to 3107 mg/day. No
changes in bowel habits or gastrointestinal signs or symptoms were
noted. At six months, the group average results of blood cell counts,
examination of urine, determination of blood urea nitrogen and liver
function tests, except serum alkaline phosphatase, were not different
from base-line determinations. Mean serum alkaline phosphatase
activity was lowered, and mean fasting blood sugar was slightly
increased (Stern, 1967).
Twenty-one patients with chronic renal disease took up to 5.3
g/cay of calcium cyclamate by capsule for 6 months without any
alteration in their clinical courses attributable to cyclamate. Of the
original 29 persons started on the regimen, one stopped taking
cyclamate after the first month because of diarrhoea (Kark, 1967).
Seventeen patients with functional gastrointestinal disease took
4-5 g/day by capsule for 6-10 weeks. Of the original group of 20, one
stopped the regimen after 3 weeks because of exacerbation of a
pre-existing dermatitis, and another stopped after 5 days because of
diarrhoea. No other adverse effects were noted (Batterman, 1966).
Comments
See Sodium Cyclamate
EVALUATION
See Sodium Cyclamate
REFERENCES
See Sodium Cyclamate
Molecular weight 432.57
Definition Calcium cyclamate contains not less than
98 per cent. and not more than the
equivalent of 101 per cent.
C12H24CaN2O6S2.
Description White, odourless crystals or crystalline
powder.
Use Non-nutritive sweetener.
Biological Data
Biochemical aspects
The metabolic fate was studied in rats using single oral doses of
0.46, 0.93 or 1.85 g/kg body-weight. 17-30 per cent. was excreted in
the urine and 70-80 per cent. in the faeces over 72 hours, absorption
being slightly less than for the sodium salt (Hwang, 1966). In another
experiment calcium cyclamate excretion was followed using gas-liquid
chromatography. At 1 per cent. of the diet 17 per cent. was recovered
in the urine and 70 per cent. in the faeces; at 5 per cent. of the
diet, 14 per cent. and 46 per cent.; and at 10 per cent. in the diet,
15 per cent. and 30 per cent. (Derse & Daun, 1966).
In the rat, 99 per cent. of the radioactivity excreted in the
urine following single oral doses (714 and 909 mg/kg) was in the form
of unchanged cyclamate (Sonders & Miller, 1966).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Mouse oral 7200 Abbott Laboratories, 1966
i.v. 570 Abbott Laboratories, 1966
Rat s.c. 100 Golberg, 1965
Rabbit i.v. 125 Abbott Laboratories, 1966
Special studies
The laxative and faecal softening effects were studied in rat,
mouse, dog and monkey, using 35S-labelled calcium cyclamate. Over 70
per cent. of the compound remained unabsorbed in the gut, no
significant systemic effects were noted, and laxative action was due
to the increased bulk secondary to osmosis as shown by the motility of
isolated gut. No gross intestinal mucosal changes were noted. The
laxative dose (ED50) was 2.8 g/kg in the rat (Hwang, 1966). Similar
results were noted in a repeat study on rats, mice and dogs, these
being ascribed entirely to osmotic hypertonicity (Taylor et al.,
1967).
Rat. Groups of 10 weanling rats were fed 0, 5 and 10 per cent.
calcium cyclamate for 22 weeks; 3-day balance studies were done in
the eighth, fourteenth, twentieth and twenty-second weeks, in which
chromatographic recoveries from urine and faeces were compared with
consumption. These recoveries were simultaneously compared with
isotope recoveries from additional labelled doses of the sodium salt
administered orally at the beginning of each balance period. Average
chromatographic recoveries ranged from 76.3 to over 100 per cent.
Gross, histological and radiological examination of the survivors
showed no changes attributable to cyclamate (Sonders et al., 1967).
In a test designed to study the influence of cyclamate on
caloric utilization, groups of 6 male rats were fed 5 g/day basal
diets supplemented with 0, 1 and 2 g/day of sucrose with and without
1, 2 and 4 per cent. calcium cyclamate, and 2.33 per cent. sodium
sulfate (osmotically equivalent to the 4 per cent. cyclamate level)
for 10 days. A smaller weight gain and mild diarrhoea were seen in the
4 per cent. cyclamate group. No catharsis was seen in the sodium
sulfate group. Mild diarrhoea, but without a clear-cut effect on
weight gain, was again found at the 4 per cent. cyclamate level in a
further test in which the same animals, redistributed to groups, were
tested in the same manner with starch instead of sucrose (Frost &
Main, 1967).
Short-term studies
Rat. Calcium cyclamate was fed to groups of rats at 0, 5 and 10
per cent. of the diet for 8 weeks. Growth rates were reduced despite
greater food and water intake at both levels, with possible
histological changes in adrenal and other organs. All faeces were soft
and moist. Urinalysis and haematology were normal. After 8 weeks half
the animals were placed on restricted food intake (60 per cent. of
ad lib. feeding). Females and males from both series were mated
twice to produce an F1a and F1b generation. Both filial generations
showed a dose-related reduction in pup weight. Animals on restricted
food intake conceived and gestated but could not raise their young
(Nees & Derse, 1965; 1967).
Groups of 40 rats were fed 0 and 5 per cent. calcium cyclamate
for 4 months. Soft faeces without frank diarrhoea, depressed rate of
weight gain and ceca grossly dilated with fluid were seen in the test
group. However, appetite was unaffected. Discrete moderate
histological abnormalities unrelated to length of treatment were seen
The mucosal architecture was unimpaired except for a rise in crypt
height; 50 per cent. of test animals showed ballooning of the tips of
mucosal cells with cytoplasmic vacuolization, while 80 per cent.
showed oedema of the ileum and enlarged villi without congestion or
inflammation. The mitotic index did not differ from that of the
controls, but there was hyperplasia of myenteric ganglion cells and
other evidence of accelerated cellular replacement.
Histochemically there was non-specific goblet cell hyperplasia,
abnormal RNA change but normal fat absorption. Histoenzymatic studies
showed various qualitative anomalies and alteration in distribution
particularly depression of ATPase, variable depression of acid and
alkaline phosphatase, reduced Krebs cycle activity but little
disturbance of Embden-Meyerhoff pathways. Pentose cycle enzymes were
increased, indicating a metabolic change induced by enhanced nucleic
acid synthesis. No definite changes were noted in enzymes involved in
protein and lipid metabolism (Bernier, 1967).
Observations in man
Intravenous doses of 1 g calcium cyclamate were tolerated
without ill effects (Schoenberger et al., 1953). Oral doses of 5 to 12
g for 14-21 days had no other effect except soft stools without
significant effect in frequency of bowel movement (Wisconsin A. R. F.,
1965).
Following administration of a single oral dose of 2 g calcium
cyclamate (containing 35S-labelled material) to a man, 31.2 per cent.
was excreted in the urine and 65.5 per cent. in the faeces over 3-4
days. In another study, 2 volunteers ingested 5 g calcium cyclamate
daily for 18 days without any adverse effect on metabolism of
nitrogen, calcium, phosphorus, sodium and potassium. Some 37 per cent.
of the daily dose was excreted in the urine and 50-61 per cent. in the
faeces (Schoenberger et al., 1953).
Urinary excretion was studied by intravenous infusion of a single
dose of 1 g, followed by oral administration of 5 g/day for 2 weeks
and a concluding intravenous administration of a 1 g dose. In 2 normal
subjects, 80 per cent. of the i.v. dose was excreted, whether
preceding or following the oral course. In 7 nephritics, a mean of 87
per cent. of the i.v. dose was excreted before the oral course, and 93
per cent. after. Of the oral doses, mean daily excretion in the normal
subjects was 31 per cent., and in 3 nephritics it was 13 per cent.
(Dedmon et al., 1961).
A study of cyclamate ingestion was carried out in 164 children,
aged 3-16 years, in four weight classes of 15-45 lbs., 45-75 lbs.,
75-105 lbs. and 105-135 lbs., with arbitrarily assigned body weights
of 30, 60, 90 and 120 lbs. for individuals within these classes, for
the purpose of administration of daily doses. These classes were
further subdivided into three groups of 15-16 children each. These
groups received 0, 1 and 1.5 g/30 lbs. body-weight/ day for 12 weeks.
After a rest period of two weeks the groups were redistributed to the
same dosages for another 12 weeks.
The compound was administered as artificially sweetened
carbonated soft drinks, and, in the higher weight classes, in capsule
supplements, whereas the 0 g/30 lbs. group received sugar-sweetened
soft drinks only. Soft stools, but no diarrhoea, were seen in some of
the children. No effect was noted in physical examinations, nor in
peripheral blood picture, examination of urine, and laboratory
parameters of liver function (Freese et al., 1967).
Ten healthy males were given 5 g/day in aqueous solution for 5
days, removed from the regimen for 7 days, then returned to 5 g/day
for another 5 days. Slightly increased water excretion in urine and
faeces but no change in serum sodium and potassium, BUN and serum
alkaline phosphatase activity were found. Blood cell counts were
unchanged (Berndt & Calandra, 1965).
Groups of 28 male volunteers took 5-7 g/day of calcium cyclamate
for 21 days, or 7-12 g/day for 14 days, without significant change in
blood cell counts or composition of the urine. One man experienced a
significant increase in the frequency of bowel movements at 7 g/day;
12 g/day produced questionably significant increases in 4 of 28. Bulk
and softness of stools was increased at 10-12 g/day but the group mean
of frequency of bowel movements was not affected at this level
(NAS-NRC, 1955).
Six male volunteers received 5 g calcium cyclamate daily in
divided doses for 7-1/2 months. No unusual symptoms were reported
except increased bulk and mushiness of stools without increase in
number of bowel movements. No adverse effects were noted in
haemopoietic, cardiovascular, renal or hepatic systems (Schoenberger
et al., 1953).
Thirty adult males ingested 1.8-6.42 g/day in dietetic foods, by
capsule and in soft drinks, for 48 weeks without apparent effect on
renal, hepatic or thyroid functions or on peripheral blood picture
(Radding, 1967).
Forty-two diabetic outpatients kept daily records of their
voluntary cyclamate intake for 6-9 months: the cumulative daily
averages of intake for the period ranged from 209 to 3107 mg/day. No
changes in bowel habits or gastrointestinal signs or symptoms were
noted. At six months, the group average results of blood cell counts,
examination of urine, determination of blood urea nitrogen and liver
function tests, except serum alkaline phosphatase, were not different
from base-line determinations. Mean serum alkaline phosphatase
activity was lowered, and mean fasting blood sugar was slightly
increased (Stern, 1967).
Twenty-one patients with chronic renal disease took up to 5.3
g/cay of calcium cyclamate by capsule for 6 months without any
alteration in their clinical courses attributable to cyclamate. Of the
original 29 persons started on the regimen, one stopped taking
cyclamate after the first month because of diarrhoea (Kark, 1967).
Seventeen patients with functional gastrointestinal disease took
4-5 g/day by capsule for 6-10 weeks. Of the original group of 20, one
stopped the regimen after 3 weeks because of exacerbation of a
pre-existing dermatitis, and another stopped after 5 days because of
diarrhoea. No other adverse effects were noted (Batterman, 1966).
Comments
See Sodium Cyclamate
EVALUATION
See Sodium Cyclamate
REFERENCES
See Sodium Cyclamate
