
FAO Nutrition Meetings
Resort Series No. 44A
WHO/Food Add./68.33
TOXICOLOGICAL EVALUATION OF SOME
FLAVOURING SUBSTANCES AND
NON-NUTRITIVE SWEETENING AGENTS
Geneva, 21-28 August 1967
The Eleventh Report of the Joint FAO/WHO Expert Committee on Food
Additives is published as FAO Nutrition Meetings Report Series,
1967, No. 44; Wld Hlth Org. techn. Rep. Ser., 1968, 383. This
Report contains general considerations, including the principles
adopted for the evaluation, and a summary of the results of the
evaluations of a number of food additives. Additional information,
such as biological data and a toxicological evaluation, considered at
that meeting, is to be found in this document.
Food and Agriculture Organization of the United Nations
World Health Organization
1967
SODIUM CYCLAMATE
Chemical name Sodium cyclohexanesulfamate; Sodium
cyclohexylsulfamate
Empirical formula C6H12NNaO3S
Structural formula
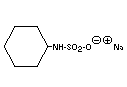 Molecular weight 201.22
Definition Sodium cyclamate contains not less than
98 per cent. and not more than the
equivalent of 101 per cent.
C6H12NNaO3S.
Description White, odourless crystals or
crystalline powder which in dilute
solution is about 30 times as sweet as
sucrose.
Use Non-nutritive sweetener
Biological Data
Biochemical aspects
Absorption of sodium cyclamate from rat stomach perfused in
situ amounted to 4 per cent. per hour at pH 1 and nil at pH 3;
absorption from the small gut occurred at a similar rate of about 4
per cent. per hour (Kojima et al., 1966b).
Whole-body autoradiography of the female rat 5 minutes after i.v.
injection of 100 mg/kg of sodium 14C-cyclamate showed relatively
uniform tissue distribution, with some concentration in the lungs,
kidneys, salivary gland and bloodstream; at 7 hours, the radioactivity
was concentrated in the intestinal contents, the salivary gland and an
unidentified area of the brain; at 48 hours some activity remained in
the gastrointestinal contents. In the pregnant rat, little penetration
into foetal tissue was found at 5 minutes, although the placentas
showed high concentrations; at 7 hours, maternal tissues except the
renal medulla and placenta were relatively free of isotope, with most
of the activity concentrated in the gastrointestinal contents, but the
foetuses showed high activity, with concentrations in the
gastrointestinal contents and kidneys. Forty-eight hours after
injection of the dam, radioactivity in the day-old rat was confined to
the lower gastrointestinal tract, the kidneys, and perhaps the skin
(Schechter & Roth, 1967).
35S-labelled sodium cyclamate was shown to cross the placental
barrier within 2 hours after i.v. administration in the rat.
Concentration in foetal tissues declined at a slower rate than that in
maternal blood (Abbott Laboratories, 1966).
Seventy-two per cent. of intraperitoneally administered
35S-labelled sodium cyclamate was excreted unchanged in the urine of
rats after 5 hours. Single i.p. doses at up to 3.2 g/kg were excreted
rapidly in 24 hours, while 1.4 g/kg was excreted equally rapidly by
normal and unilaterally nephrectomized rats (Taylor et al., 1951).
Repeated oral doses of 80-120 mg/kg for 5 days led to 85 per
cent. excretion in 5 days and another 3 per cent. in the 4 days
following the last dose. About 2/3 was excreted in the faeces and the
remainder in the urine (Taylor et al., 1951). Single oral doses of
0.5, 1 or 2 g/kg body-weight were excreted to the extent of 30 per
cent. in urine and 70 per cent. in faeces in 3 days (substantially in
the first 24 hours). Using 35S-labelled sodium cyclamate in groups of
4 rats given 2 or 4 g/kg body-weight, after 8 hours 75-80 per cent.
remained unabsorbed in the gut, 18-25 per cent. was absorbed and
mostly excreted in the urine and 3-8 per cent. remained in the
carcass. Feeding rats 1 per cent. sodium cyclamate for 54 days prior
to an i.p. injection of the substance showed 77 per cent. of the
injected dose to be excreted in 24 hours, hence no cumulation had
occurred (Hwang, 1966). When 14C-labelled sodium cyclamate was
administered orally to rats, 22 per cent. of the activity was excreted
in the urine, 72 per cent. in the faeces and 0.2 per cent. remained in
the carcass; 98-99 per cent. of the activity was excreted as cyclamate
(Miller et al., 1966). However, when sodium cyclamate excretion was
followed using a gas-liquid chromatography method, at 5 per cent.
dietary intake only 5 per cent. as recovered in the urine and 19 per
cent. in the faeces, while at 10 per cent. dietary intakes some 7 per
cent. appeared in the urine and 18 per cent. in the faeces. No
explanation for these low recoveries was offered (Derse & Daun, 1966).
Two rats given a total of 6.67 g/kg of sodium 14C-cyclamate
orally over an 8-hour period had total carcass activity levels of 0.33
per cent. and 0.24 per cent. of this dose after 5 days; recoveries
from excreta and cage washings were calculated to be 103.25 per cent.
and 102.59 per cent. of the doses given (Miller et al., 1966).
About 80 per cent. of a 1000 mg/kg dose of orally administered
14C-cyclamate was found to be eliminated by the rat in the first 24
hours, approximately equal amounts appearing in urine and faeces. Only
unchanged cyclamate was chromatographically identified in the urine.
0.12 per cent. of the dose was transmitted to litter animals,
apparently via the milk (Hood et al., 1966).
Oral sodium cyclamate at 1 per cent. in the diet for one month
did not alter the BMR of rats (Richards et al., 1951).
In the rabbit, sodium cyclamate was excreted unchanged in the
urine to the extent of 80-90 per cent. in 12 hours after i.v., i.p. or
oral administration (Audrieth & Sveda, 1944).
Of 600 mg orally administered sodium cyclamate in the dog, 70 per
cent. was excreted in the urine within 8 hours and 85 per cent. within
24 hours, while of 100 mg/kg body-weight administered i.v. 92 per
cent. was excreted. One dog receiving 35S-labelled sodium cyclamate
i.v. excreted only 0.5 per cent. in the bile and 67 per cent. in the
urine after 5 hours (Taylor et al., 1951). Three dogs receiving
0.75-1 g/kg body-weight sodium cyclamate i.g. excreted 21-64 per cent.
in the urine within 11-17 hours. Cyclamate blood levels were low
(Hwang, 1966).
In two dogs given single 14C-labelled doses of 200 and 1000
mg/kg, severe diarrhoea was seen 9-10 hours later. Total recovery of
radioactivity from the excreta by the fourth day amounted to about
97-98 per cent. In the animal given 1000 mg/kg, urinary excretion
accounted for 27 per cent. of the dose; in this animal, peak
whole-blood activity levels were found in the first hour after
administration, with a steady decline to less than 1 per cent. of peak
by the forty-eighth hour. In the other, the peak whole-blood activity
level probably occurred during the eighth hour or later, and by 72
hours was down to 7 per cent. of the highest level measured.
Ninety-two hours after administration, no activity was detected in the
skin, eyes, testes, brain, thyroid or skeletal muscle of the low-dose
dog; total activity in the spleen, liver, lungs, pancreas, heart and
kidneys was calculated as 0.027 per cent. of the dose administered. In
a dog given 200 mg/kg 3 times a day for 8 days no activity was
detected in whole blood 44 hours after the last dose. During the test
and in the 9 days following, about 40 per cent. of the dose
administered was recovered from the urine and 54 per cent. from the
faeces. Nine days after the last dose the activity in the spleen,
liver, lungs and pancreas totalled 0.005 per cent. of the doses given,
and activity in a sample of skeletal muscle was about 10 per cent. of
that found in a biopsy sample taken during the last day of
administration (Miller et al., 1966; 1967).
About 80 per cent. of a dose of 1000 mg/kg of 14C-labelled
cyclamate administered orally in the dog was excreted in the urine in
24 hours; two apparent metabolites plus unchanged cyclamate were found
by chromatography of urine samples up to 10 hours after ingestion.
Only cyclamate and possibly a very similar metabolite were found after
24-48 hours. No activity was detectable in the bloodstream after 48
hours. Two dogs were given 200 mg/kg of labelled cyclamate three times
a day for one week. Between 65 and 97 per cent. of the total dose had
been excreted by the eighth day after cessation of administration.
Whole-blood activity levels had dropped to approximately 40 per cent.
of the dose-cessation levels and tissue levels had dropped to 25 per
cent. or less of those found in two other dogs given the same regime
and sacrificed at the end of one week (Hood et al., 1966).
Only unchanged sodium cyclamate was identified
chromatographically in a dog urine sample taken 17 hours after oral
administration of 200 mg/kg (Sonders, 1967). 35S-labelled cyclamate
appeared in the milk of lactating dogs partly from blood and partly
from active secretion but disappeared after 24 hours (Abbott
Laboratories, 1966).
In the lactating dog, 4-24 hours after i.v. injection of a 20 mg
14C-labelled dose, concentrations of cyclamate were found to be
higher in the milk than in simultaneous blood samples; cyclamate
appeared in the milk at levels of about 2 µg/ml or less, and
disappeared from the milk at about the same rate as from the
bloodstream (Wiegand, 1967).
Intravenous injection of 35S-labelled sodium cyclamate into
rats, rabbits and dogs showed that, with the exception of the kidney
and perhaps the liver, gastrointestinal tract and spleen, there was no
significant concentration in the various organs examined. Sodium
cyclamate penetrated into the brain with difficulty but crossed the
placenta very easily in the rat and was found in the foetus (Taylor et
al., 1951).
Cyclohexylamine excretion
Humans, dogs but not rabbits excrete cyclohexylamine after oral
doses of 1-3 g of sodium cyclamate. No glucuronates were detected.
Some 0.7 per cent. of the oral dose is excreted as cyclohexylamine in
man while in dogs only trace amounts are detected (Kojima &
Ichibagase, 1966). Rats receiving sodium cyclamate in their diet
excreted cyclohexylamine in their urine (Leahy et al., 1966). Oral
administration of 3 g sodium cyclamate on each of 3 days to 5 human
volunteers resulted in excretion of cyclohexylamine in man,
representing 0.8 per cent. of the dose administered (Leahy et al.,
1967). Further extension of the work to 40 volunteers showed 5
excretors again at 0.8 per cent. of the administered dose while one
subject excreted 7.5 per cent. of the administered dose as
cyclohexylamine (Huntingdon Laboratories, 1967).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Mouse oral 10 000-12 000 Richards et al., 1951
11 000 Abbott Laboratories, 1966
17 000 Taylor et al., 1967
1 525 Mollet, 1966
i.v. 4 000 Richards et al., 1951
4 800 Taylor et al., 1967
i.p. 10 000-12 000 Fitzhugh et al., 1951
7 100 Taylor et al., 1967
Rat oral 12 000 Richards et al., 1951
17 500 Taylor et al., 1967
i.v. 3 000-4 000 Richards et al., 1951
3 500 Taylor et al., 1967
i.p. 6 000 Taylor et al., 1967
Pretreatment of mice dams with 0.5 per cent. sodium cyclamate
raised the acute i.p. toxicity of the substance in their pups.
Changing the lipid content of the diet from 6.5 per cent. to 40 per
cent. increased the i.p. acute toxicity of sodium cyclamate in mice
(Taylor et al., 1967), Rats consuming up to 8 g/kg body-weight/day of
sodium cyclamate for 5 days showed no abnormal findings regarding
haematology, tissue chemistry and histopathology apart from a slight
decrease in hepatic triglycerides at the 8 g level (Stein et al.,
1967).
Diffuse, mild vesiculation of the endoplasmic reticulum
associated with vacuolization was observed in the liver and kidney of
monkeys given 4 or 8 g/kg of sodium cyclamate orally and sacrificed 1
or 48 hours later. No changes in various biochemical values were
associated with these early morphologic alterations (Stein et al.,
1967). No ultrastructural changes attributed to cyclamate were found
in the hepatic cells of two monkeys 48 hours after single doses of
4000 mg/kg or of one animal 1 hour after an oral dose of 7170 mg/kg
(Richter et al., 1967).
Special studies
Laxative and faecal softening effects were studied in rats, mice,
dogs and monkeys using 35S-labelled compounds. Approximately70 per
cent. of cyclamate remained unabsorbed in the gut and no significant
systemic effects were noted. Laxative action as shown by motility of
isolated gut was ascribed to the increased bulk secondary to osmosis.
Mice were slightly more susceptible. The laxative dose (ED50) in the
rat was about 1.9 g/kg body-weight. No gross changes in rat or mouse
intestinal mucosa were noted (Hwang, 1966). Similar results were noted
in a repeat study on rats, mice and dogs. Intravenous sodium cyclamate
in mice did not affect intestinal motility, stool frequency and
appearance (Taylor et al, 1967). Sodium cyclamate had no effect on
the water balance of hypertensive rats with renal disease (Fregley &
Kier, 1961).
Short-term studies
Rat. Groups of 10 weanling rats were fed 0, 1, 5 and 10 per
cent. sodium cyclamate for 22 weeks. Three-day balance studies were
done in the eighth, fourteenth, twentieth and twenty-second weeks, in
which chromatographic recoveries from urine and faeces were compared
with consumption. These recoveries were simultaneously compared with
isotope recoveries from additional doses of the labelled sodium salt
administered orally at the beginning of each balance period. Average
chromatographic recoveries ranged from 77.7 to over 100 per cent. and
isotope recoveries from 91.5 to over 100 per cent. Weight gain was
adversely affected in the 10 per cent. group. Gross, histological and
radiological examination of survivors showed no changes (Sonders et
al., 1967).
Groups of 10 male and 10 female rats received diets containing 0,
1, 2 or 3 per cent. of sodium cyclamate for 11 months. No adverse
effects (apart from occasional lax stools at the 2 per cent. and 3 per
cent. levels) were noted on growth rate, liver and kidney weight,
haematology and urinalysis. When rats of corresponding test groups
were mated, there was no adverse effect on fertility or litter
characteristics. Of the F1 generation, groups of 3-8 mice and 8
females were kept on 1, 2 and 3 per cent. sodium cyclamate in their
diet and again correspondingly mated to give the F2 generation. Pairs
of corresponding groups continued to receive 0, 1, 2 and 3 per cent.
in their diet and were remated to produce an F3 generation. No
adverse effects were seen on conception rate, foetal development and
litter characteristics (Taylor et al., 1967).
Guinea-pig. Groups of 7 males received 0.5 or 2 per cent.
sodium cyclamate in their drinking water for 5 months and controls
were given 0.3 per cent. saline. Fluid intake was restricted to 30 ml
per day per animal. There was an apparent increase in mortality at the
2 per cent. level. There was also a 20 per cent. decrease in
body-weight at the 2 per cent. level compared with controls. Histology
of the liver of test animals showed "reduced protoplasm" and
vacuolization at both dietary levels though less at 0.5 per cent.
Histology of controls was not reported. Serum lactic dehydrogenase and
SGOT levels were raised at 2 per cent., but not significantly
(Hellauer, 1967).
Dog. Three groups of 2, 4 and 4 dogs received 0, 2 and 4 g/kg
body-weight of sodium cyclamate i.g. daily for 30 days without
deleterious effect on body-weight, appearance, marrow picture, hepatic
function tests, gross autopsy and histology of liver, kidney and
gastrointestinal tract (Taylor et al., 1967). In another experiment
dogs were given 0.5 g/kg body-weight sodium cyclamate daily for 11
months without adverse effect on weight, blood, liver and kidney
function or urine (Hwang & Richards, 1955). Four groups of 3 dogs
received 0, or 0.5 g/kg body-weight sodium cyclamate 6 days per week
i.g. for 11 months. At the tenth month soft stools were seen. No
adverse effects were seen regarding body-weight, haemoglobin,
urinalysis and tests of hepatic and renal function, and on gross
autopsy (Taylor et al., 1967). Groups of 2 dogs were kept on diets
containing 0, 0.2 or 0.4 per cent. sodium cyclamate daily for 15
months. All gained weight; haematology and liver and kidney function
tests were normal; urinalysis did not differ from controls. Gross and
microscopic examination of the major organs was normal (Richards et
al., 1951).
Monkey. Monkeys were given 4 g/kg body-weight of sodium
cyclamate daily for 9 months without significant changes in
haematology, serum enzymes, urine or gross autopsy findings. Hepatic
and renal demethylase and desulfurase activities, lysosomal activity
and mitochondrial oxygen utilization were not affected, but slight
enlargement of pancreatic islands of Langerhans was noted.
Electron microscopy revealed some subcellular alterations (Stein et
al., 1967).
Teratogenicity studies
Mouse. Groups of 2-4 mice with control groups of 3 mice were
given single i.g. doses of sodium cyclamate at various stages of
pregnancy as follows: 62.5 mg on day 4-5, 125 mg on day 4-5 and 6-7,
250 mg on day 6-7 and 8-10 and 500 mg on day 6-7 and 8-10. The uteri
were removed on the eighteenth day, opened and the foetuses assessed
and absorption sites counted. No abnormalities were detected following
any administrations on days 8-10 but in all other instances the
majority of embryos had either been resorbed or appeared dead or
showed delayed development compared with controls. The foetal LD50
was calculated to be 180 mg/kg body-weight (Tanaka, 1964a; 1964b).
In embryotoxicity studies still in progress, groups of 10-14
female mice, mated at a little over 2 months of age, were given
intragastric, aqueous doses of 0, 5 and 10 g/kg of sodium cyclamate on
the eighth day of gestation. On examination of the uteri and foetuses
on the eighteenth day of gestation, no effect was found (Lorke, 1967).
Rat. Groups of 20-24 pregnant females were given 50, 100 and
150 mg/kg body-weight of sodium cyclamate daily from the sixth to the
fifteenth day of gestation. Three hundred and sixty-three pregnant
animals served as controls. The dams were autopsied on the
twenty-first day and the foetuses examined grossly. The number of
dead, malformed or reported foetuses did not differ from controls
(Bein et al., 1967).
Rabbit. Four females were given 1 g/kg body-weight sodium
cyclamate orally from day 1-18 of gestation. All offspring were normal
and survival and litter size corresponded with controls (Abbott
Laboratories, 1966).
Dog. Groups of 6 dogs (2 male and 4 female) were given 0, 0.45,
0.9 and 1.35 g/kg of sodium cyclamate for about 6 months. The dogs
were bred and the offspring after weaning at 8 weeks were placed on
the same regimen that their respective parents were receiving. After
20 months there was no adverse effect on the parents. All progeny were
born normal and have shown no effect of treatment (Abbott
Laboratories, 1966).
Long-term studies
Rat. Groups of 8-20 male and female rats were fed diets
containing 0, 0.5, 0.1 and 1.0 per cent. sodium cyclamate for 18, 24
or 30 months. Mating was permitted and the F1 generation kept on the
same dietary regime. The experiment was carried into the third
generation at the 0.05 per cent. level. Weight gains, haematology and
urinalysis were normal. Clinical and pathological studies showed no
difference from controls as regards major organs. Mortality and tumour
incidence were similar to those of controls. Normal litters were
produced (Richards et al., 1951).
In another experiment groups of 18-24 rats received 0, 0.01, 0.1,
0.5, 1 and 5 per cent. sodium cyclamate in their diet for 24 months.
Slight growth depression was noted at 5 per cent. associated with
marked diarrhoea. Mortality was similar in all groups. Organ weights,
histopathology of major organs and tumour incidence were no different
from controls (Fitzhugh et al., 1951).
Observations in man
After i.v. administration to healthy men of 1 g sodium cyclamate,
7O-90 per cent. was excreted in the urine within 3 hours and 84-99 per
cent. within 24 hours. Using 35S-labelled sodium cyclamate i.v. in 3
patients, the urinary clearance indicated excretion by glomerular
filtration and tubular secretion. No general metabolic effects were
noted (Schoenberger et al., 1953). One healthy man was given 300 mg
sodium cyclamate orally and excreted 79.5 per cent. in the urine over
7 days, 26 per cent. being excreted in the first 24 hours. Of an oral
dose of 200 mg sodium cyclamate 77 per cent. was recovered in 3 days
(Richards et al., 1951). Doses of 1 or 5 g sodium cyclamate were given
to 3 subjects and 40-48 per cent. was recovered in the urine in 24
hours (Kojima et al., 1966a). The amounts of cyclamate remaining in
the body during 2, 5 or 10 g daily administration can be calculated
for daily excretion rates ranging from 10-90 per cent. For 5 g and 75
per cent. excretion, the cyclamate residue in the body is 1.67 g
(Taylor, 1954). Phototoxicity has been reported as an immediate
reaction to UV radiation (Kennedy et al., 1961). Photo-allergy as a
delayed reaction to UV radiation has also been noted (Tatsuji &
Tosbie, 1963).
Two human subjects were given 14C-labelled doses of 4D mg/kg and
88 mg/kg. Urine recoveries in 4 days were 51.8 and 73.5 per cent.
respectively and faecal recoveries were 50.5 and 24.5 per cent.
Urinary cyclohexylamine accounted for 0.156 and 0.123 per cent. of the
isotopic material present. Reverse isotope dilution analysis indicated
that all of the urinary isotope was unchanged cyclamate. Peak plasma
activity levels were found in the eighth hour and the first hour
respectively and peak whole-blood levels in the first and sixth hours
(Sonders & Weigand, 1967).
Groups of 32 and 30 patients with chronic hepatic or renal
disease were given 5 and 2 g/day of sodium cyclamate for 6-6.5 months.
On the basis of clinical chemical studies, cyclamate did not affect
the patient's progress (Zöllner & Schneller, 1967).
Comments
Although this substance has been studied extensively in animals
and man, there are certain lacunae such as the exact mechanism of the
laxative effect, bearing in mind the changes observed in cellular and
subcellular structures in histoenzymatic studies, the influence on the
foetus in view of the easy transplacental passage and the significance
of the appearance of the metabolite cyclohexylamine in the urine of
man. The teratogenic effects which have only been seen in some studies
in mice may well be related to physical and environmental factors
rather than inherent teratogenic potential of sodium cyclamate.
Long-term experiments have only been conducted in one species and
further feeding studies in a second species are needed. The possible
nutritional aspect of prolonged unrestricted use of cyclamate may
require consideration.
EVALUATION
In view of the extensive studies in animal and man and widespread
use in some countries, on the one hand, and the gaps in information
about certain biological effects on the other, a temporary acceptable
daily intake is allocated with some further work specified below.
Level causing no significant toxicological effect
Man: 12 000 mg per day, equivalent to 200 mg/kg/day (soft
stools and more frequent bowel movements were observed in some
subjects).
Estimate of acceptable daily intake for man
mg/kg body-weight
Temporary acceptance 0-50
Further work required within 3 years
(1) Investigation into the metabolism to cyclohexylamine and other
possible metabolites and full toxicological evaluation of these
metabolites including any possible human genetic aspects.
(2) Investigation into the exact mechanism of the laxative effect
including any possible enteropathic cellular and subcellular changes.
(3) Lifespan studies in a second species including observations on
reproduction and development of several filial generations.
REFERENCES
Abbott Laboratories (1966) Review of cyclamates
Audrieth, I. F. &, Sveda, M. (1944) J. Org. Chem., 9, 89
Batterman, R. C. (1966) Unpublished report submitted by Abbott
Laboratories
Bein, H. J,, Fritz, H., Hess, R. & Loustalot, P. (1967) Unpublished
report submitted by CIBA
Berndt, C. W. & Calandra, J. C. (1965) Unpublished report submitted by
Abbots Laboratories
Bernier, J. J. (1967) Unpublished report
Dedmon, R. E., Ryan, W. G. & Kark, R. M. (1961) Unpublished report
submitted by Abbott Laboratories
Derse, P. H. & Daun R. J. (1966) J. Ass. Off. Agr. Chem., 49, 1090
Fitzhugh, O. G, Nelson, A. A. & Frawley, J. P. (1951) J. Amer.
Pharm. Ass., Sci Ed., 40, 583
Freese, H. B., Jenike, T. S. & Rubenkoenig, H. L. (1967) Unpublished
report submitted by Abbott Laboratories
Fregley, M. J. & Kier, L. B. (1966) Tox. Appl. Pharm., 9, 124
Frost, D. V. & Main, B. T. Unpublished report submitted by Abbott
Laboratories 1967
GoLberg, L. (1965) Unpublished report
Hellauer, H. (1967) Unpublished report
Hood, S. L., Somaini, S. M., Zink, V. R. & Bogner, R. L. (1966)
Unpublished report submitted by Abbott Laboratories
Huntingdon Research Centre (1967) Unpublished report
Hwang, K. (1966) Arch. int. Pharmacodyn., 163, 302
Hwang, K. & Richards, R. K. (1955) Unpublished report
Kark, R. M. Unpublished report submitted by Abbott Laboratories 1967
Kennedy, B., O'Quinn, S., Perret, W. J., Tilley, J. C. & Hennington,
V. M. (1961) J. Louis. State Med. 113, 365
Kojima, S. & Ichibagase, H. (1966) Chem. Pharm. Bull., 14, 971
Kojima, S., Ichibagase, H. & Iguchi, S. (1966a) Chem. Pharm. Bull.,
14, 959
Kojima S., Ichibagase, H. & Iguchi, S. (1966b) Chem. Pharm. Bull.,
14, 965
Leahy, J. S., Wakefield, M. & Taylor, T. (1967) Fd Cosmet. Toxicol.,
5, 447
Lorke, D. (1967) Unpublished report submitted by Farbenfabriken Bayer
A. G.
Miller, J. P., Michael Crawford, L. E., Sonders, R. C. & Cardinal, E.
V. (1966) Biochem, biophys. Res. Comm., 25, 153
Miller, J. P., Michael Crawford, L. E. & Netwal, J. C. (1967)
Unpublished report submitted by Abbott Laboratories
Mollet, M. (1966) Unpublished report submitted by Abbott Laboratories
National Academy of Sciences - National Research Council (1955)
The Safety of Artificial Sweeteners for Use in Foods, Publication
386
Nees, P. O. & Derse, P. H. (1965) Nature (Lond.), 208, 81
Nees, P. O. & Derse, P. H. (1967) Nature (Lond.), 211, 1191
Radding, R. S. Unpublished report submitted by Abbott Laboratories
1967
Richards, R. K., Taylor, J. D., O'Brien, J. L. & Duescher, H. O.
(1951) J. Amer. Pharm. Ass., Sci. Ed., 40, 1
Richter, W. R., Stein, R. J., Rdzok, E. J. & Moize, S. M. (1967)
Unpublished report submitted by Abbott Laboratories
Schoenberger,. J. A., Rix, D. M., Sakamoto, A., Taylor, J. D. & Kark,
R. M. (1953) Amer. J. Med. Sci., 225, 551
Schechter, P. & Roth, L. J. (1967) Unpublished report submitted by
Abbott Laboratories
Sonders, R. C. & Miller, J. P. (1966) Unpublished report submitted by
Abbott Laboratories
Sonders, R. C. (1967) Unpublished report submitted by Abbott
Laboratories
Sonders, R. C. & Wiegand, R. G. (1967) Unpublished report submitted by
Abbott Laboratories
Sonders, R. C., Wiegand, R. G. & Netwal, J. C. (1967) Unpublished
report submitted by Abbott Laboratories
Stein, A. A., Serrone, D. M. & Coulston, F. (1967) Tox. Appl.
Pharm., 10, 381
Stern, S. B. (1967) Unpublished report submitted by Abbott
Laboratories
Tanaka, R. (1964a) Jap. J. Publ. Health, 11, 909
Tanaka, R. (1964b) J. Iwate Med. Ass., 16, 330
Tatsuji, K. & Tosbie, A. (1963) Med. Cult, 5, 796
Taylor, J. D. (1954) Unpublished report
Taylor, J. D., Richards, R. K. & Davis, J. C. (1951) Proc Soc. Exp.
Biol. Med., 78, 530
Taylor, J. D., Richards, R. K., Wiegand, R. G. & Weinberg, M. S.
(1967) Fd Cosmet. Toxicol.,
Tremolieres, J., Krebs, M. & Pedron, A. (1967) Unpublished report
submitted by Abbott Laboratories
Wisconsin Alumni Research Foundation (1965) Unpublished report
Zöllner, N. & Schnelle, K. (1967) Arzneim.-Forsch. (Drug Res.) 17,
1568
Molecular weight 201.22
Definition Sodium cyclamate contains not less than
98 per cent. and not more than the
equivalent of 101 per cent.
C6H12NNaO3S.
Description White, odourless crystals or
crystalline powder which in dilute
solution is about 30 times as sweet as
sucrose.
Use Non-nutritive sweetener
Biological Data
Biochemical aspects
Absorption of sodium cyclamate from rat stomach perfused in
situ amounted to 4 per cent. per hour at pH 1 and nil at pH 3;
absorption from the small gut occurred at a similar rate of about 4
per cent. per hour (Kojima et al., 1966b).
Whole-body autoradiography of the female rat 5 minutes after i.v.
injection of 100 mg/kg of sodium 14C-cyclamate showed relatively
uniform tissue distribution, with some concentration in the lungs,
kidneys, salivary gland and bloodstream; at 7 hours, the radioactivity
was concentrated in the intestinal contents, the salivary gland and an
unidentified area of the brain; at 48 hours some activity remained in
the gastrointestinal contents. In the pregnant rat, little penetration
into foetal tissue was found at 5 minutes, although the placentas
showed high concentrations; at 7 hours, maternal tissues except the
renal medulla and placenta were relatively free of isotope, with most
of the activity concentrated in the gastrointestinal contents, but the
foetuses showed high activity, with concentrations in the
gastrointestinal contents and kidneys. Forty-eight hours after
injection of the dam, radioactivity in the day-old rat was confined to
the lower gastrointestinal tract, the kidneys, and perhaps the skin
(Schechter & Roth, 1967).
35S-labelled sodium cyclamate was shown to cross the placental
barrier within 2 hours after i.v. administration in the rat.
Concentration in foetal tissues declined at a slower rate than that in
maternal blood (Abbott Laboratories, 1966).
Seventy-two per cent. of intraperitoneally administered
35S-labelled sodium cyclamate was excreted unchanged in the urine of
rats after 5 hours. Single i.p. doses at up to 3.2 g/kg were excreted
rapidly in 24 hours, while 1.4 g/kg was excreted equally rapidly by
normal and unilaterally nephrectomized rats (Taylor et al., 1951).
Repeated oral doses of 80-120 mg/kg for 5 days led to 85 per
cent. excretion in 5 days and another 3 per cent. in the 4 days
following the last dose. About 2/3 was excreted in the faeces and the
remainder in the urine (Taylor et al., 1951). Single oral doses of
0.5, 1 or 2 g/kg body-weight were excreted to the extent of 30 per
cent. in urine and 70 per cent. in faeces in 3 days (substantially in
the first 24 hours). Using 35S-labelled sodium cyclamate in groups of
4 rats given 2 or 4 g/kg body-weight, after 8 hours 75-80 per cent.
remained unabsorbed in the gut, 18-25 per cent. was absorbed and
mostly excreted in the urine and 3-8 per cent. remained in the
carcass. Feeding rats 1 per cent. sodium cyclamate for 54 days prior
to an i.p. injection of the substance showed 77 per cent. of the
injected dose to be excreted in 24 hours, hence no cumulation had
occurred (Hwang, 1966). When 14C-labelled sodium cyclamate was
administered orally to rats, 22 per cent. of the activity was excreted
in the urine, 72 per cent. in the faeces and 0.2 per cent. remained in
the carcass; 98-99 per cent. of the activity was excreted as cyclamate
(Miller et al., 1966). However, when sodium cyclamate excretion was
followed using a gas-liquid chromatography method, at 5 per cent.
dietary intake only 5 per cent. as recovered in the urine and 19 per
cent. in the faeces, while at 10 per cent. dietary intakes some 7 per
cent. appeared in the urine and 18 per cent. in the faeces. No
explanation for these low recoveries was offered (Derse & Daun, 1966).
Two rats given a total of 6.67 g/kg of sodium 14C-cyclamate
orally over an 8-hour period had total carcass activity levels of 0.33
per cent. and 0.24 per cent. of this dose after 5 days; recoveries
from excreta and cage washings were calculated to be 103.25 per cent.
and 102.59 per cent. of the doses given (Miller et al., 1966).
About 80 per cent. of a 1000 mg/kg dose of orally administered
14C-cyclamate was found to be eliminated by the rat in the first 24
hours, approximately equal amounts appearing in urine and faeces. Only
unchanged cyclamate was chromatographically identified in the urine.
0.12 per cent. of the dose was transmitted to litter animals,
apparently via the milk (Hood et al., 1966).
Oral sodium cyclamate at 1 per cent. in the diet for one month
did not alter the BMR of rats (Richards et al., 1951).
In the rabbit, sodium cyclamate was excreted unchanged in the
urine to the extent of 80-90 per cent. in 12 hours after i.v., i.p. or
oral administration (Audrieth & Sveda, 1944).
Of 600 mg orally administered sodium cyclamate in the dog, 70 per
cent. was excreted in the urine within 8 hours and 85 per cent. within
24 hours, while of 100 mg/kg body-weight administered i.v. 92 per
cent. was excreted. One dog receiving 35S-labelled sodium cyclamate
i.v. excreted only 0.5 per cent. in the bile and 67 per cent. in the
urine after 5 hours (Taylor et al., 1951). Three dogs receiving
0.75-1 g/kg body-weight sodium cyclamate i.g. excreted 21-64 per cent.
in the urine within 11-17 hours. Cyclamate blood levels were low
(Hwang, 1966).
In two dogs given single 14C-labelled doses of 200 and 1000
mg/kg, severe diarrhoea was seen 9-10 hours later. Total recovery of
radioactivity from the excreta by the fourth day amounted to about
97-98 per cent. In the animal given 1000 mg/kg, urinary excretion
accounted for 27 per cent. of the dose; in this animal, peak
whole-blood activity levels were found in the first hour after
administration, with a steady decline to less than 1 per cent. of peak
by the forty-eighth hour. In the other, the peak whole-blood activity
level probably occurred during the eighth hour or later, and by 72
hours was down to 7 per cent. of the highest level measured.
Ninety-two hours after administration, no activity was detected in the
skin, eyes, testes, brain, thyroid or skeletal muscle of the low-dose
dog; total activity in the spleen, liver, lungs, pancreas, heart and
kidneys was calculated as 0.027 per cent. of the dose administered. In
a dog given 200 mg/kg 3 times a day for 8 days no activity was
detected in whole blood 44 hours after the last dose. During the test
and in the 9 days following, about 40 per cent. of the dose
administered was recovered from the urine and 54 per cent. from the
faeces. Nine days after the last dose the activity in the spleen,
liver, lungs and pancreas totalled 0.005 per cent. of the doses given,
and activity in a sample of skeletal muscle was about 10 per cent. of
that found in a biopsy sample taken during the last day of
administration (Miller et al., 1966; 1967).
About 80 per cent. of a dose of 1000 mg/kg of 14C-labelled
cyclamate administered orally in the dog was excreted in the urine in
24 hours; two apparent metabolites plus unchanged cyclamate were found
by chromatography of urine samples up to 10 hours after ingestion.
Only cyclamate and possibly a very similar metabolite were found after
24-48 hours. No activity was detectable in the bloodstream after 48
hours. Two dogs were given 200 mg/kg of labelled cyclamate three times
a day for one week. Between 65 and 97 per cent. of the total dose had
been excreted by the eighth day after cessation of administration.
Whole-blood activity levels had dropped to approximately 40 per cent.
of the dose-cessation levels and tissue levels had dropped to 25 per
cent. or less of those found in two other dogs given the same regime
and sacrificed at the end of one week (Hood et al., 1966).
Only unchanged sodium cyclamate was identified
chromatographically in a dog urine sample taken 17 hours after oral
administration of 200 mg/kg (Sonders, 1967). 35S-labelled cyclamate
appeared in the milk of lactating dogs partly from blood and partly
from active secretion but disappeared after 24 hours (Abbott
Laboratories, 1966).
In the lactating dog, 4-24 hours after i.v. injection of a 20 mg
14C-labelled dose, concentrations of cyclamate were found to be
higher in the milk than in simultaneous blood samples; cyclamate
appeared in the milk at levels of about 2 µg/ml or less, and
disappeared from the milk at about the same rate as from the
bloodstream (Wiegand, 1967).
Intravenous injection of 35S-labelled sodium cyclamate into
rats, rabbits and dogs showed that, with the exception of the kidney
and perhaps the liver, gastrointestinal tract and spleen, there was no
significant concentration in the various organs examined. Sodium
cyclamate penetrated into the brain with difficulty but crossed the
placenta very easily in the rat and was found in the foetus (Taylor et
al., 1951).
Cyclohexylamine excretion
Humans, dogs but not rabbits excrete cyclohexylamine after oral
doses of 1-3 g of sodium cyclamate. No glucuronates were detected.
Some 0.7 per cent. of the oral dose is excreted as cyclohexylamine in
man while in dogs only trace amounts are detected (Kojima &
Ichibagase, 1966). Rats receiving sodium cyclamate in their diet
excreted cyclohexylamine in their urine (Leahy et al., 1966). Oral
administration of 3 g sodium cyclamate on each of 3 days to 5 human
volunteers resulted in excretion of cyclohexylamine in man,
representing 0.8 per cent. of the dose administered (Leahy et al.,
1967). Further extension of the work to 40 volunteers showed 5
excretors again at 0.8 per cent. of the administered dose while one
subject excreted 7.5 per cent. of the administered dose as
cyclohexylamine (Huntingdon Laboratories, 1967).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Mouse oral 10 000-12 000 Richards et al., 1951
11 000 Abbott Laboratories, 1966
17 000 Taylor et al., 1967
1 525 Mollet, 1966
i.v. 4 000 Richards et al., 1951
4 800 Taylor et al., 1967
i.p. 10 000-12 000 Fitzhugh et al., 1951
7 100 Taylor et al., 1967
Rat oral 12 000 Richards et al., 1951
17 500 Taylor et al., 1967
i.v. 3 000-4 000 Richards et al., 1951
3 500 Taylor et al., 1967
i.p. 6 000 Taylor et al., 1967
Pretreatment of mice dams with 0.5 per cent. sodium cyclamate
raised the acute i.p. toxicity of the substance in their pups.
Changing the lipid content of the diet from 6.5 per cent. to 40 per
cent. increased the i.p. acute toxicity of sodium cyclamate in mice
(Taylor et al., 1967), Rats consuming up to 8 g/kg body-weight/day of
sodium cyclamate for 5 days showed no abnormal findings regarding
haematology, tissue chemistry and histopathology apart from a slight
decrease in hepatic triglycerides at the 8 g level (Stein et al.,
1967).
Diffuse, mild vesiculation of the endoplasmic reticulum
associated with vacuolization was observed in the liver and kidney of
monkeys given 4 or 8 g/kg of sodium cyclamate orally and sacrificed 1
or 48 hours later. No changes in various biochemical values were
associated with these early morphologic alterations (Stein et al.,
1967). No ultrastructural changes attributed to cyclamate were found
in the hepatic cells of two monkeys 48 hours after single doses of
4000 mg/kg or of one animal 1 hour after an oral dose of 7170 mg/kg
(Richter et al., 1967).
Special studies
Laxative and faecal softening effects were studied in rats, mice,
dogs and monkeys using 35S-labelled compounds. Approximately70 per
cent. of cyclamate remained unabsorbed in the gut and no significant
systemic effects were noted. Laxative action as shown by motility of
isolated gut was ascribed to the increased bulk secondary to osmosis.
Mice were slightly more susceptible. The laxative dose (ED50) in the
rat was about 1.9 g/kg body-weight. No gross changes in rat or mouse
intestinal mucosa were noted (Hwang, 1966). Similar results were noted
in a repeat study on rats, mice and dogs. Intravenous sodium cyclamate
in mice did not affect intestinal motility, stool frequency and
appearance (Taylor et al, 1967). Sodium cyclamate had no effect on
the water balance of hypertensive rats with renal disease (Fregley &
Kier, 1961).
Short-term studies
Rat. Groups of 10 weanling rats were fed 0, 1, 5 and 10 per
cent. sodium cyclamate for 22 weeks. Three-day balance studies were
done in the eighth, fourteenth, twentieth and twenty-second weeks, in
which chromatographic recoveries from urine and faeces were compared
with consumption. These recoveries were simultaneously compared with
isotope recoveries from additional doses of the labelled sodium salt
administered orally at the beginning of each balance period. Average
chromatographic recoveries ranged from 77.7 to over 100 per cent. and
isotope recoveries from 91.5 to over 100 per cent. Weight gain was
adversely affected in the 10 per cent. group. Gross, histological and
radiological examination of survivors showed no changes (Sonders et
al., 1967).
Groups of 10 male and 10 female rats received diets containing 0,
1, 2 or 3 per cent. of sodium cyclamate for 11 months. No adverse
effects (apart from occasional lax stools at the 2 per cent. and 3 per
cent. levels) were noted on growth rate, liver and kidney weight,
haematology and urinalysis. When rats of corresponding test groups
were mated, there was no adverse effect on fertility or litter
characteristics. Of the F1 generation, groups of 3-8 mice and 8
females were kept on 1, 2 and 3 per cent. sodium cyclamate in their
diet and again correspondingly mated to give the F2 generation. Pairs
of corresponding groups continued to receive 0, 1, 2 and 3 per cent.
in their diet and were remated to produce an F3 generation. No
adverse effects were seen on conception rate, foetal development and
litter characteristics (Taylor et al., 1967).
Guinea-pig. Groups of 7 males received 0.5 or 2 per cent.
sodium cyclamate in their drinking water for 5 months and controls
were given 0.3 per cent. saline. Fluid intake was restricted to 30 ml
per day per animal. There was an apparent increase in mortality at the
2 per cent. level. There was also a 20 per cent. decrease in
body-weight at the 2 per cent. level compared with controls. Histology
of the liver of test animals showed "reduced protoplasm" and
vacuolization at both dietary levels though less at 0.5 per cent.
Histology of controls was not reported. Serum lactic dehydrogenase and
SGOT levels were raised at 2 per cent., but not significantly
(Hellauer, 1967).
Dog. Three groups of 2, 4 and 4 dogs received 0, 2 and 4 g/kg
body-weight of sodium cyclamate i.g. daily for 30 days without
deleterious effect on body-weight, appearance, marrow picture, hepatic
function tests, gross autopsy and histology of liver, kidney and
gastrointestinal tract (Taylor et al., 1967). In another experiment
dogs were given 0.5 g/kg body-weight sodium cyclamate daily for 11
months without adverse effect on weight, blood, liver and kidney
function or urine (Hwang & Richards, 1955). Four groups of 3 dogs
received 0, or 0.5 g/kg body-weight sodium cyclamate 6 days per week
i.g. for 11 months. At the tenth month soft stools were seen. No
adverse effects were seen regarding body-weight, haemoglobin,
urinalysis and tests of hepatic and renal function, and on gross
autopsy (Taylor et al., 1967). Groups of 2 dogs were kept on diets
containing 0, 0.2 or 0.4 per cent. sodium cyclamate daily for 15
months. All gained weight; haematology and liver and kidney function
tests were normal; urinalysis did not differ from controls. Gross and
microscopic examination of the major organs was normal (Richards et
al., 1951).
Monkey. Monkeys were given 4 g/kg body-weight of sodium
cyclamate daily for 9 months without significant changes in
haematology, serum enzymes, urine or gross autopsy findings. Hepatic
and renal demethylase and desulfurase activities, lysosomal activity
and mitochondrial oxygen utilization were not affected, but slight
enlargement of pancreatic islands of Langerhans was noted.
Electron microscopy revealed some subcellular alterations (Stein et
al., 1967).
Teratogenicity studies
Mouse. Groups of 2-4 mice with control groups of 3 mice were
given single i.g. doses of sodium cyclamate at various stages of
pregnancy as follows: 62.5 mg on day 4-5, 125 mg on day 4-5 and 6-7,
250 mg on day 6-7 and 8-10 and 500 mg on day 6-7 and 8-10. The uteri
were removed on the eighteenth day, opened and the foetuses assessed
and absorption sites counted. No abnormalities were detected following
any administrations on days 8-10 but in all other instances the
majority of embryos had either been resorbed or appeared dead or
showed delayed development compared with controls. The foetal LD50
was calculated to be 180 mg/kg body-weight (Tanaka, 1964a; 1964b).
In embryotoxicity studies still in progress, groups of 10-14
female mice, mated at a little over 2 months of age, were given
intragastric, aqueous doses of 0, 5 and 10 g/kg of sodium cyclamate on
the eighth day of gestation. On examination of the uteri and foetuses
on the eighteenth day of gestation, no effect was found (Lorke, 1967).
Rat. Groups of 20-24 pregnant females were given 50, 100 and
150 mg/kg body-weight of sodium cyclamate daily from the sixth to the
fifteenth day of gestation. Three hundred and sixty-three pregnant
animals served as controls. The dams were autopsied on the
twenty-first day and the foetuses examined grossly. The number of
dead, malformed or reported foetuses did not differ from controls
(Bein et al., 1967).
Rabbit. Four females were given 1 g/kg body-weight sodium
cyclamate orally from day 1-18 of gestation. All offspring were normal
and survival and litter size corresponded with controls (Abbott
Laboratories, 1966).
Dog. Groups of 6 dogs (2 male and 4 female) were given 0, 0.45,
0.9 and 1.35 g/kg of sodium cyclamate for about 6 months. The dogs
were bred and the offspring after weaning at 8 weeks were placed on
the same regimen that their respective parents were receiving. After
20 months there was no adverse effect on the parents. All progeny were
born normal and have shown no effect of treatment (Abbott
Laboratories, 1966).
Long-term studies
Rat. Groups of 8-20 male and female rats were fed diets
containing 0, 0.5, 0.1 and 1.0 per cent. sodium cyclamate for 18, 24
or 30 months. Mating was permitted and the F1 generation kept on the
same dietary regime. The experiment was carried into the third
generation at the 0.05 per cent. level. Weight gains, haematology and
urinalysis were normal. Clinical and pathological studies showed no
difference from controls as regards major organs. Mortality and tumour
incidence were similar to those of controls. Normal litters were
produced (Richards et al., 1951).
In another experiment groups of 18-24 rats received 0, 0.01, 0.1,
0.5, 1 and 5 per cent. sodium cyclamate in their diet for 24 months.
Slight growth depression was noted at 5 per cent. associated with
marked diarrhoea. Mortality was similar in all groups. Organ weights,
histopathology of major organs and tumour incidence were no different
from controls (Fitzhugh et al., 1951).
Observations in man
After i.v. administration to healthy men of 1 g sodium cyclamate,
7O-90 per cent. was excreted in the urine within 3 hours and 84-99 per
cent. within 24 hours. Using 35S-labelled sodium cyclamate i.v. in 3
patients, the urinary clearance indicated excretion by glomerular
filtration and tubular secretion. No general metabolic effects were
noted (Schoenberger et al., 1953). One healthy man was given 300 mg
sodium cyclamate orally and excreted 79.5 per cent. in the urine over
7 days, 26 per cent. being excreted in the first 24 hours. Of an oral
dose of 200 mg sodium cyclamate 77 per cent. was recovered in 3 days
(Richards et al., 1951). Doses of 1 or 5 g sodium cyclamate were given
to 3 subjects and 40-48 per cent. was recovered in the urine in 24
hours (Kojima et al., 1966a). The amounts of cyclamate remaining in
the body during 2, 5 or 10 g daily administration can be calculated
for daily excretion rates ranging from 10-90 per cent. For 5 g and 75
per cent. excretion, the cyclamate residue in the body is 1.67 g
(Taylor, 1954). Phototoxicity has been reported as an immediate
reaction to UV radiation (Kennedy et al., 1961). Photo-allergy as a
delayed reaction to UV radiation has also been noted (Tatsuji &
Tosbie, 1963).
Two human subjects were given 14C-labelled doses of 4D mg/kg and
88 mg/kg. Urine recoveries in 4 days were 51.8 and 73.5 per cent.
respectively and faecal recoveries were 50.5 and 24.5 per cent.
Urinary cyclohexylamine accounted for 0.156 and 0.123 per cent. of the
isotopic material present. Reverse isotope dilution analysis indicated
that all of the urinary isotope was unchanged cyclamate. Peak plasma
activity levels were found in the eighth hour and the first hour
respectively and peak whole-blood levels in the first and sixth hours
(Sonders & Weigand, 1967).
Groups of 32 and 30 patients with chronic hepatic or renal
disease were given 5 and 2 g/day of sodium cyclamate for 6-6.5 months.
On the basis of clinical chemical studies, cyclamate did not affect
the patient's progress (Zöllner & Schneller, 1967).
Comments
Although this substance has been studied extensively in animals
and man, there are certain lacunae such as the exact mechanism of the
laxative effect, bearing in mind the changes observed in cellular and
subcellular structures in histoenzymatic studies, the influence on the
foetus in view of the easy transplacental passage and the significance
of the appearance of the metabolite cyclohexylamine in the urine of
man. The teratogenic effects which have only been seen in some studies
in mice may well be related to physical and environmental factors
rather than inherent teratogenic potential of sodium cyclamate.
Long-term experiments have only been conducted in one species and
further feeding studies in a second species are needed. The possible
nutritional aspect of prolonged unrestricted use of cyclamate may
require consideration.
EVALUATION
In view of the extensive studies in animal and man and widespread
use in some countries, on the one hand, and the gaps in information
about certain biological effects on the other, a temporary acceptable
daily intake is allocated with some further work specified below.
Level causing no significant toxicological effect
Man: 12 000 mg per day, equivalent to 200 mg/kg/day (soft
stools and more frequent bowel movements were observed in some
subjects).
Estimate of acceptable daily intake for man
mg/kg body-weight
Temporary acceptance 0-50
Further work required within 3 years
(1) Investigation into the metabolism to cyclohexylamine and other
possible metabolites and full toxicological evaluation of these
metabolites including any possible human genetic aspects.
(2) Investigation into the exact mechanism of the laxative effect
including any possible enteropathic cellular and subcellular changes.
(3) Lifespan studies in a second species including observations on
reproduction and development of several filial generations.
REFERENCES
Abbott Laboratories (1966) Review of cyclamates
Audrieth, I. F. &, Sveda, M. (1944) J. Org. Chem., 9, 89
Batterman, R. C. (1966) Unpublished report submitted by Abbott
Laboratories
Bein, H. J,, Fritz, H., Hess, R. & Loustalot, P. (1967) Unpublished
report submitted by CIBA
Berndt, C. W. & Calandra, J. C. (1965) Unpublished report submitted by
Abbots Laboratories
Bernier, J. J. (1967) Unpublished report
Dedmon, R. E., Ryan, W. G. & Kark, R. M. (1961) Unpublished report
submitted by Abbott Laboratories
Derse, P. H. & Daun R. J. (1966) J. Ass. Off. Agr. Chem., 49, 1090
Fitzhugh, O. G, Nelson, A. A. & Frawley, J. P. (1951) J. Amer.
Pharm. Ass., Sci Ed., 40, 583
Freese, H. B., Jenike, T. S. & Rubenkoenig, H. L. (1967) Unpublished
report submitted by Abbott Laboratories
Fregley, M. J. & Kier, L. B. (1966) Tox. Appl. Pharm., 9, 124
Frost, D. V. & Main, B. T. Unpublished report submitted by Abbott
Laboratories 1967
GoLberg, L. (1965) Unpublished report
Hellauer, H. (1967) Unpublished report
Hood, S. L., Somaini, S. M., Zink, V. R. & Bogner, R. L. (1966)
Unpublished report submitted by Abbott Laboratories
Huntingdon Research Centre (1967) Unpublished report
Hwang, K. (1966) Arch. int. Pharmacodyn., 163, 302
Hwang, K. & Richards, R. K. (1955) Unpublished report
Kark, R. M. Unpublished report submitted by Abbott Laboratories 1967
Kennedy, B., O'Quinn, S., Perret, W. J., Tilley, J. C. & Hennington,
V. M. (1961) J. Louis. State Med. 113, 365
Kojima, S. & Ichibagase, H. (1966) Chem. Pharm. Bull., 14, 971
Kojima, S., Ichibagase, H. & Iguchi, S. (1966a) Chem. Pharm. Bull.,
14, 959
Kojima S., Ichibagase, H. & Iguchi, S. (1966b) Chem. Pharm. Bull.,
14, 965
Leahy, J. S., Wakefield, M. & Taylor, T. (1967) Fd Cosmet. Toxicol.,
5, 447
Lorke, D. (1967) Unpublished report submitted by Farbenfabriken Bayer
A. G.
Miller, J. P., Michael Crawford, L. E., Sonders, R. C. & Cardinal, E.
V. (1966) Biochem, biophys. Res. Comm., 25, 153
Miller, J. P., Michael Crawford, L. E. & Netwal, J. C. (1967)
Unpublished report submitted by Abbott Laboratories
Mollet, M. (1966) Unpublished report submitted by Abbott Laboratories
National Academy of Sciences - National Research Council (1955)
The Safety of Artificial Sweeteners for Use in Foods, Publication
386
Nees, P. O. & Derse, P. H. (1965) Nature (Lond.), 208, 81
Nees, P. O. & Derse, P. H. (1967) Nature (Lond.), 211, 1191
Radding, R. S. Unpublished report submitted by Abbott Laboratories
1967
Richards, R. K., Taylor, J. D., O'Brien, J. L. & Duescher, H. O.
(1951) J. Amer. Pharm. Ass., Sci. Ed., 40, 1
Richter, W. R., Stein, R. J., Rdzok, E. J. & Moize, S. M. (1967)
Unpublished report submitted by Abbott Laboratories
Schoenberger,. J. A., Rix, D. M., Sakamoto, A., Taylor, J. D. & Kark,
R. M. (1953) Amer. J. Med. Sci., 225, 551
Schechter, P. & Roth, L. J. (1967) Unpublished report submitted by
Abbott Laboratories
Sonders, R. C. & Miller, J. P. (1966) Unpublished report submitted by
Abbott Laboratories
Sonders, R. C. (1967) Unpublished report submitted by Abbott
Laboratories
Sonders, R. C. & Wiegand, R. G. (1967) Unpublished report submitted by
Abbott Laboratories
Sonders, R. C., Wiegand, R. G. & Netwal, J. C. (1967) Unpublished
report submitted by Abbott Laboratories
Stein, A. A., Serrone, D. M. & Coulston, F. (1967) Tox. Appl.
Pharm., 10, 381
Stern, S. B. (1967) Unpublished report submitted by Abbott
Laboratories
Tanaka, R. (1964a) Jap. J. Publ. Health, 11, 909
Tanaka, R. (1964b) J. Iwate Med. Ass., 16, 330
Tatsuji, K. & Tosbie, A. (1963) Med. Cult, 5, 796
Taylor, J. D. (1954) Unpublished report
Taylor, J. D., Richards, R. K. & Davis, J. C. (1951) Proc Soc. Exp.
Biol. Med., 78, 530
Taylor, J. D., Richards, R. K., Wiegand, R. G. & Weinberg, M. S.
(1967) Fd Cosmet. Toxicol.,
Tremolieres, J., Krebs, M. & Pedron, A. (1967) Unpublished report
submitted by Abbott Laboratories
Wisconsin Alumni Research Foundation (1965) Unpublished report
Zöllner, N. & Schnelle, K. (1967) Arzneim.-Forsch. (Drug Res.) 17,
1568
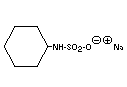 Molecular weight 201.22
Definition Sodium cyclamate contains not less than
98 per cent. and not more than the
equivalent of 101 per cent.
C6H12NNaO3S.
Description White, odourless crystals or
crystalline powder which in dilute
solution is about 30 times as sweet as
sucrose.
Use Non-nutritive sweetener
Biological Data
Biochemical aspects
Absorption of sodium cyclamate from rat stomach perfused in
situ amounted to 4 per cent. per hour at pH 1 and nil at pH 3;
absorption from the small gut occurred at a similar rate of about 4
per cent. per hour (Kojima et al., 1966b).
Whole-body autoradiography of the female rat 5 minutes after i.v.
injection of 100 mg/kg of sodium 14C-cyclamate showed relatively
uniform tissue distribution, with some concentration in the lungs,
kidneys, salivary gland and bloodstream; at 7 hours, the radioactivity
was concentrated in the intestinal contents, the salivary gland and an
unidentified area of the brain; at 48 hours some activity remained in
the gastrointestinal contents. In the pregnant rat, little penetration
into foetal tissue was found at 5 minutes, although the placentas
showed high concentrations; at 7 hours, maternal tissues except the
renal medulla and placenta were relatively free of isotope, with most
of the activity concentrated in the gastrointestinal contents, but the
foetuses showed high activity, with concentrations in the
gastrointestinal contents and kidneys. Forty-eight hours after
injection of the dam, radioactivity in the day-old rat was confined to
the lower gastrointestinal tract, the kidneys, and perhaps the skin
(Schechter & Roth, 1967).
35S-labelled sodium cyclamate was shown to cross the placental
barrier within 2 hours after i.v. administration in the rat.
Concentration in foetal tissues declined at a slower rate than that in
maternal blood (Abbott Laboratories, 1966).
Seventy-two per cent. of intraperitoneally administered
35S-labelled sodium cyclamate was excreted unchanged in the urine of
rats after 5 hours. Single i.p. doses at up to 3.2 g/kg were excreted
rapidly in 24 hours, while 1.4 g/kg was excreted equally rapidly by
normal and unilaterally nephrectomized rats (Taylor et al., 1951).
Repeated oral doses of 80-120 mg/kg for 5 days led to 85 per
cent. excretion in 5 days and another 3 per cent. in the 4 days
following the last dose. About 2/3 was excreted in the faeces and the
remainder in the urine (Taylor et al., 1951). Single oral doses of
0.5, 1 or 2 g/kg body-weight were excreted to the extent of 30 per
cent. in urine and 70 per cent. in faeces in 3 days (substantially in
the first 24 hours). Using 35S-labelled sodium cyclamate in groups of
4 rats given 2 or 4 g/kg body-weight, after 8 hours 75-80 per cent.
remained unabsorbed in the gut, 18-25 per cent. was absorbed and
mostly excreted in the urine and 3-8 per cent. remained in the
carcass. Feeding rats 1 per cent. sodium cyclamate for 54 days prior
to an i.p. injection of the substance showed 77 per cent. of the
injected dose to be excreted in 24 hours, hence no cumulation had
occurred (Hwang, 1966). When 14C-labelled sodium cyclamate was
administered orally to rats, 22 per cent. of the activity was excreted
in the urine, 72 per cent. in the faeces and 0.2 per cent. remained in
the carcass; 98-99 per cent. of the activity was excreted as cyclamate
(Miller et al., 1966). However, when sodium cyclamate excretion was
followed using a gas-liquid chromatography method, at 5 per cent.
dietary intake only 5 per cent. as recovered in the urine and 19 per
cent. in the faeces, while at 10 per cent. dietary intakes some 7 per
cent. appeared in the urine and 18 per cent. in the faeces. No
explanation for these low recoveries was offered (Derse & Daun, 1966).
Two rats given a total of 6.67 g/kg of sodium 14C-cyclamate
orally over an 8-hour period had total carcass activity levels of 0.33
per cent. and 0.24 per cent. of this dose after 5 days; recoveries
from excreta and cage washings were calculated to be 103.25 per cent.
and 102.59 per cent. of the doses given (Miller et al., 1966).
About 80 per cent. of a 1000 mg/kg dose of orally administered
14C-cyclamate was found to be eliminated by the rat in the first 24
hours, approximately equal amounts appearing in urine and faeces. Only
unchanged cyclamate was chromatographically identified in the urine.
0.12 per cent. of the dose was transmitted to litter animals,
apparently via the milk (Hood et al., 1966).
Oral sodium cyclamate at 1 per cent. in the diet for one month
did not alter the BMR of rats (Richards et al., 1951).
In the rabbit, sodium cyclamate was excreted unchanged in the
urine to the extent of 80-90 per cent. in 12 hours after i.v., i.p. or
oral administration (Audrieth & Sveda, 1944).
Of 600 mg orally administered sodium cyclamate in the dog, 70 per
cent. was excreted in the urine within 8 hours and 85 per cent. within
24 hours, while of 100 mg/kg body-weight administered i.v. 92 per
cent. was excreted. One dog receiving 35S-labelled sodium cyclamate
i.v. excreted only 0.5 per cent. in the bile and 67 per cent. in the
urine after 5 hours (Taylor et al., 1951). Three dogs receiving
0.75-1 g/kg body-weight sodium cyclamate i.g. excreted 21-64 per cent.
in the urine within 11-17 hours. Cyclamate blood levels were low
(Hwang, 1966).
In two dogs given single 14C-labelled doses of 200 and 1000
mg/kg, severe diarrhoea was seen 9-10 hours later. Total recovery of
radioactivity from the excreta by the fourth day amounted to about
97-98 per cent. In the animal given 1000 mg/kg, urinary excretion
accounted for 27 per cent. of the dose; in this animal, peak
whole-blood activity levels were found in the first hour after
administration, with a steady decline to less than 1 per cent. of peak
by the forty-eighth hour. In the other, the peak whole-blood activity
level probably occurred during the eighth hour or later, and by 72
hours was down to 7 per cent. of the highest level measured.
Ninety-two hours after administration, no activity was detected in the
skin, eyes, testes, brain, thyroid or skeletal muscle of the low-dose
dog; total activity in the spleen, liver, lungs, pancreas, heart and
kidneys was calculated as 0.027 per cent. of the dose administered. In
a dog given 200 mg/kg 3 times a day for 8 days no activity was
detected in whole blood 44 hours after the last dose. During the test
and in the 9 days following, about 40 per cent. of the dose
administered was recovered from the urine and 54 per cent. from the
faeces. Nine days after the last dose the activity in the spleen,
liver, lungs and pancreas totalled 0.005 per cent. of the doses given,
and activity in a sample of skeletal muscle was about 10 per cent. of
that found in a biopsy sample taken during the last day of
administration (Miller et al., 1966; 1967).
About 80 per cent. of a dose of 1000 mg/kg of 14C-labelled
cyclamate administered orally in the dog was excreted in the urine in
24 hours; two apparent metabolites plus unchanged cyclamate were found
by chromatography of urine samples up to 10 hours after ingestion.
Only cyclamate and possibly a very similar metabolite were found after
24-48 hours. No activity was detectable in the bloodstream after 48
hours. Two dogs were given 200 mg/kg of labelled cyclamate three times
a day for one week. Between 65 and 97 per cent. of the total dose had
been excreted by the eighth day after cessation of administration.
Whole-blood activity levels had dropped to approximately 40 per cent.
of the dose-cessation levels and tissue levels had dropped to 25 per
cent. or less of those found in two other dogs given the same regime
and sacrificed at the end of one week (Hood et al., 1966).
Only unchanged sodium cyclamate was identified
chromatographically in a dog urine sample taken 17 hours after oral
administration of 200 mg/kg (Sonders, 1967). 35S-labelled cyclamate
appeared in the milk of lactating dogs partly from blood and partly
from active secretion but disappeared after 24 hours (Abbott
Laboratories, 1966).
In the lactating dog, 4-24 hours after i.v. injection of a 20 mg
14C-labelled dose, concentrations of cyclamate were found to be
higher in the milk than in simultaneous blood samples; cyclamate
appeared in the milk at levels of about 2 µg/ml or less, and
disappeared from the milk at about the same rate as from the
bloodstream (Wiegand, 1967).
Intravenous injection of 35S-labelled sodium cyclamate into
rats, rabbits and dogs showed that, with the exception of the kidney
and perhaps the liver, gastrointestinal tract and spleen, there was no
significant concentration in the various organs examined. Sodium
cyclamate penetrated into the brain with difficulty but crossed the
placenta very easily in the rat and was found in the foetus (Taylor et
al., 1951).
Cyclohexylamine excretion
Humans, dogs but not rabbits excrete cyclohexylamine after oral
doses of 1-3 g of sodium cyclamate. No glucuronates were detected.
Some 0.7 per cent. of the oral dose is excreted as cyclohexylamine in
man while in dogs only trace amounts are detected (Kojima &
Ichibagase, 1966). Rats receiving sodium cyclamate in their diet
excreted cyclohexylamine in their urine (Leahy et al., 1966). Oral
administration of 3 g sodium cyclamate on each of 3 days to 5 human
volunteers resulted in excretion of cyclohexylamine in man,
representing 0.8 per cent. of the dose administered (Leahy et al.,
1967). Further extension of the work to 40 volunteers showed 5
excretors again at 0.8 per cent. of the administered dose while one
subject excreted 7.5 per cent. of the administered dose as
cyclohexylamine (Huntingdon Laboratories, 1967).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Mouse oral 10 000-12 000 Richards et al., 1951
11 000 Abbott Laboratories, 1966
17 000 Taylor et al., 1967
1 525 Mollet, 1966
i.v. 4 000 Richards et al., 1951
4 800 Taylor et al., 1967
i.p. 10 000-12 000 Fitzhugh et al., 1951
7 100 Taylor et al., 1967
Rat oral 12 000 Richards et al., 1951
17 500 Taylor et al., 1967
i.v. 3 000-4 000 Richards et al., 1951
3 500 Taylor et al., 1967
i.p. 6 000 Taylor et al., 1967
Pretreatment of mice dams with 0.5 per cent. sodium cyclamate
raised the acute i.p. toxicity of the substance in their pups.
Changing the lipid content of the diet from 6.5 per cent. to 40 per
cent. increased the i.p. acute toxicity of sodium cyclamate in mice
(Taylor et al., 1967), Rats consuming up to 8 g/kg body-weight/day of
sodium cyclamate for 5 days showed no abnormal findings regarding
haematology, tissue chemistry and histopathology apart from a slight
decrease in hepatic triglycerides at the 8 g level (Stein et al.,
1967).
Diffuse, mild vesiculation of the endoplasmic reticulum
associated with vacuolization was observed in the liver and kidney of
monkeys given 4 or 8 g/kg of sodium cyclamate orally and sacrificed 1
or 48 hours later. No changes in various biochemical values were
associated with these early morphologic alterations (Stein et al.,
1967). No ultrastructural changes attributed to cyclamate were found
in the hepatic cells of two monkeys 48 hours after single doses of
4000 mg/kg or of one animal 1 hour after an oral dose of 7170 mg/kg
(Richter et al., 1967).
Special studies
Laxative and faecal softening effects were studied in rats, mice,
dogs and monkeys using 35S-labelled compounds. Approximately70 per
cent. of cyclamate remained unabsorbed in the gut and no significant
systemic effects were noted. Laxative action as shown by motility of
isolated gut was ascribed to the increased bulk secondary to osmosis.
Mice were slightly more susceptible. The laxative dose (ED50) in the
rat was about 1.9 g/kg body-weight. No gross changes in rat or mouse
intestinal mucosa were noted (Hwang, 1966). Similar results were noted
in a repeat study on rats, mice and dogs. Intravenous sodium cyclamate
in mice did not affect intestinal motility, stool frequency and
appearance (Taylor et al, 1967). Sodium cyclamate had no effect on
the water balance of hypertensive rats with renal disease (Fregley &
Kier, 1961).
Short-term studies
Rat. Groups of 10 weanling rats were fed 0, 1, 5 and 10 per
cent. sodium cyclamate for 22 weeks. Three-day balance studies were
done in the eighth, fourteenth, twentieth and twenty-second weeks, in
which chromatographic recoveries from urine and faeces were compared
with consumption. These recoveries were simultaneously compared with
isotope recoveries from additional doses of the labelled sodium salt
administered orally at the beginning of each balance period. Average
chromatographic recoveries ranged from 77.7 to over 100 per cent. and
isotope recoveries from 91.5 to over 100 per cent. Weight gain was
adversely affected in the 10 per cent. group. Gross, histological and
radiological examination of survivors showed no changes (Sonders et
al., 1967).
Groups of 10 male and 10 female rats received diets containing 0,
1, 2 or 3 per cent. of sodium cyclamate for 11 months. No adverse
effects (apart from occasional lax stools at the 2 per cent. and 3 per
cent. levels) were noted on growth rate, liver and kidney weight,
haematology and urinalysis. When rats of corresponding test groups
were mated, there was no adverse effect on fertility or litter
characteristics. Of the F1 generation, groups of 3-8 mice and 8
females were kept on 1, 2 and 3 per cent. sodium cyclamate in their
diet and again correspondingly mated to give the F2 generation. Pairs
of corresponding groups continued to receive 0, 1, 2 and 3 per cent.
in their diet and were remated to produce an F3 generation. No
adverse effects were seen on conception rate, foetal development and
litter characteristics (Taylor et al., 1967).
Guinea-pig. Groups of 7 males received 0.5 or 2 per cent.
sodium cyclamate in their drinking water for 5 months and controls
were given 0.3 per cent. saline. Fluid intake was restricted to 30 ml
per day per animal. There was an apparent increase in mortality at the
2 per cent. level. There was also a 20 per cent. decrease in
body-weight at the 2 per cent. level compared with controls. Histology
of the liver of test animals showed "reduced protoplasm" and
vacuolization at both dietary levels though less at 0.5 per cent.
Histology of controls was not reported. Serum lactic dehydrogenase and
SGOT levels were raised at 2 per cent., but not significantly
(Hellauer, 1967).
Dog. Three groups of 2, 4 and 4 dogs received 0, 2 and 4 g/kg
body-weight of sodium cyclamate i.g. daily for 30 days without
deleterious effect on body-weight, appearance, marrow picture, hepatic
function tests, gross autopsy and histology of liver, kidney and
gastrointestinal tract (Taylor et al., 1967). In another experiment
dogs were given 0.5 g/kg body-weight sodium cyclamate daily for 11
months without adverse effect on weight, blood, liver and kidney
function or urine (Hwang & Richards, 1955). Four groups of 3 dogs
received 0, or 0.5 g/kg body-weight sodium cyclamate 6 days per week
i.g. for 11 months. At the tenth month soft stools were seen. No
adverse effects were seen regarding body-weight, haemoglobin,
urinalysis and tests of hepatic and renal function, and on gross
autopsy (Taylor et al., 1967). Groups of 2 dogs were kept on diets
containing 0, 0.2 or 0.4 per cent. sodium cyclamate daily for 15
months. All gained weight; haematology and liver and kidney function
tests were normal; urinalysis did not differ from controls. Gross and
microscopic examination of the major organs was normal (Richards et
al., 1951).
Monkey. Monkeys were given 4 g/kg body-weight of sodium
cyclamate daily for 9 months without significant changes in
haematology, serum enzymes, urine or gross autopsy findings. Hepatic
and renal demethylase and desulfurase activities, lysosomal activity
and mitochondrial oxygen utilization were not affected, but slight
enlargement of pancreatic islands of Langerhans was noted.
Electron microscopy revealed some subcellular alterations (Stein et
al., 1967).
Teratogenicity studies
Mouse. Groups of 2-4 mice with control groups of 3 mice were
given single i.g. doses of sodium cyclamate at various stages of
pregnancy as follows: 62.5 mg on day 4-5, 125 mg on day 4-5 and 6-7,
250 mg on day 6-7 and 8-10 and 500 mg on day 6-7 and 8-10. The uteri
were removed on the eighteenth day, opened and the foetuses assessed
and absorption sites counted. No abnormalities were detected following
any administrations on days 8-10 but in all other instances the
majority of embryos had either been resorbed or appeared dead or
showed delayed development compared with controls. The foetal LD50
was calculated to be 180 mg/kg body-weight (Tanaka, 1964a; 1964b).
In embryotoxicity studies still in progress, groups of 10-14
female mice, mated at a little over 2 months of age, were given
intragastric, aqueous doses of 0, 5 and 10 g/kg of sodium cyclamate on
the eighth day of gestation. On examination of the uteri and foetuses
on the eighteenth day of gestation, no effect was found (Lorke, 1967).
Rat. Groups of 20-24 pregnant females were given 50, 100 and
150 mg/kg body-weight of sodium cyclamate daily from the sixth to the
fifteenth day of gestation. Three hundred and sixty-three pregnant
animals served as controls. The dams were autopsied on the
twenty-first day and the foetuses examined grossly. The number of
dead, malformed or reported foetuses did not differ from controls
(Bein et al., 1967).
Rabbit. Four females were given 1 g/kg body-weight sodium
cyclamate orally from day 1-18 of gestation. All offspring were normal
and survival and litter size corresponded with controls (Abbott
Laboratories, 1966).
Dog. Groups of 6 dogs (2 male and 4 female) were given 0, 0.45,
0.9 and 1.35 g/kg of sodium cyclamate for about 6 months. The dogs
were bred and the offspring after weaning at 8 weeks were placed on
the same regimen that their respective parents were receiving. After
20 months there was no adverse effect on the parents. All progeny were
born normal and have shown no effect of treatment (Abbott
Laboratories, 1966).
Long-term studies
Rat. Groups of 8-20 male and female rats were fed diets
containing 0, 0.5, 0.1 and 1.0 per cent. sodium cyclamate for 18, 24
or 30 months. Mating was permitted and the F1 generation kept on the
same dietary regime. The experiment was carried into the third
generation at the 0.05 per cent. level. Weight gains, haematology and
urinalysis were normal. Clinical and pathological studies showed no
difference from controls as regards major organs. Mortality and tumour
incidence were similar to those of controls. Normal litters were
produced (Richards et al., 1951).
In another experiment groups of 18-24 rats received 0, 0.01, 0.1,
0.5, 1 and 5 per cent. sodium cyclamate in their diet for 24 months.
Slight growth depression was noted at 5 per cent. associated with
marked diarrhoea. Mortality was similar in all groups. Organ weights,
histopathology of major organs and tumour incidence were no different
from controls (Fitzhugh et al., 1951).
Observations in man
After i.v. administration to healthy men of 1 g sodium cyclamate,
7O-90 per cent. was excreted in the urine within 3 hours and 84-99 per
cent. within 24 hours. Using 35S-labelled sodium cyclamate i.v. in 3
patients, the urinary clearance indicated excretion by glomerular
filtration and tubular secretion. No general metabolic effects were
noted (Schoenberger et al., 1953). One healthy man was given 300 mg
sodium cyclamate orally and excreted 79.5 per cent. in the urine over
7 days, 26 per cent. being excreted in the first 24 hours. Of an oral
dose of 200 mg sodium cyclamate 77 per cent. was recovered in 3 days
(Richards et al., 1951). Doses of 1 or 5 g sodium cyclamate were given
to 3 subjects and 40-48 per cent. was recovered in the urine in 24
hours (Kojima et al., 1966a). The amounts of cyclamate remaining in
the body during 2, 5 or 10 g daily administration can be calculated
for daily excretion rates ranging from 10-90 per cent. For 5 g and 75
per cent. excretion, the cyclamate residue in the body is 1.67 g
(Taylor, 1954). Phototoxicity has been reported as an immediate
reaction to UV radiation (Kennedy et al., 1961). Photo-allergy as a
delayed reaction to UV radiation has also been noted (Tatsuji &
Tosbie, 1963).
Two human subjects were given 14C-labelled doses of 4D mg/kg and
88 mg/kg. Urine recoveries in 4 days were 51.8 and 73.5 per cent.
respectively and faecal recoveries were 50.5 and 24.5 per cent.
Urinary cyclohexylamine accounted for 0.156 and 0.123 per cent. of the
isotopic material present. Reverse isotope dilution analysis indicated
that all of the urinary isotope was unchanged cyclamate. Peak plasma
activity levels were found in the eighth hour and the first hour
respectively and peak whole-blood levels in the first and sixth hours
(Sonders & Weigand, 1967).
Groups of 32 and 30 patients with chronic hepatic or renal
disease were given 5 and 2 g/day of sodium cyclamate for 6-6.5 months.
On the basis of clinical chemical studies, cyclamate did not affect
the patient's progress (Zöllner & Schneller, 1967).
Comments
Although this substance has been studied extensively in animals
and man, there are certain lacunae such as the exact mechanism of the
laxative effect, bearing in mind the changes observed in cellular and
subcellular structures in histoenzymatic studies, the influence on the
foetus in view of the easy transplacental passage and the significance
of the appearance of the metabolite cyclohexylamine in the urine of
man. The teratogenic effects which have only been seen in some studies
in mice may well be related to physical and environmental factors
rather than inherent teratogenic potential of sodium cyclamate.
Long-term experiments have only been conducted in one species and
further feeding studies in a second species are needed. The possible
nutritional aspect of prolonged unrestricted use of cyclamate may
require consideration.
EVALUATION
In view of the extensive studies in animal and man and widespread
use in some countries, on the one hand, and the gaps in information
about certain biological effects on the other, a temporary acceptable
daily intake is allocated with some further work specified below.
Level causing no significant toxicological effect
Man: 12 000 mg per day, equivalent to 200 mg/kg/day (soft
stools and more frequent bowel movements were observed in some
subjects).
Estimate of acceptable daily intake for man
mg/kg body-weight
Temporary acceptance 0-50
Further work required within 3 years
(1) Investigation into the metabolism to cyclohexylamine and other
possible metabolites and full toxicological evaluation of these
metabolites including any possible human genetic aspects.
(2) Investigation into the exact mechanism of the laxative effect
including any possible enteropathic cellular and subcellular changes.
(3) Lifespan studies in a second species including observations on
reproduction and development of several filial generations.
REFERENCES
Abbott Laboratories (1966) Review of cyclamates
Audrieth, I. F. &, Sveda, M. (1944) J. Org. Chem., 9, 89
Batterman, R. C. (1966) Unpublished report submitted by Abbott
Laboratories
Bein, H. J,, Fritz, H., Hess, R. & Loustalot, P. (1967) Unpublished
report submitted by CIBA
Berndt, C. W. & Calandra, J. C. (1965) Unpublished report submitted by
Abbots Laboratories
Bernier, J. J. (1967) Unpublished report
Dedmon, R. E., Ryan, W. G. & Kark, R. M. (1961) Unpublished report
submitted by Abbott Laboratories
Derse, P. H. & Daun R. J. (1966) J. Ass. Off. Agr. Chem., 49, 1090
Fitzhugh, O. G, Nelson, A. A. & Frawley, J. P. (1951) J. Amer.
Pharm. Ass., Sci Ed., 40, 583
Freese, H. B., Jenike, T. S. & Rubenkoenig, H. L. (1967) Unpublished
report submitted by Abbott Laboratories
Fregley, M. J. & Kier, L. B. (1966) Tox. Appl. Pharm., 9, 124
Frost, D. V. & Main, B. T. Unpublished report submitted by Abbott
Laboratories 1967
GoLberg, L. (1965) Unpublished report
Hellauer, H. (1967) Unpublished report
Hood, S. L., Somaini, S. M., Zink, V. R. & Bogner, R. L. (1966)
Unpublished report submitted by Abbott Laboratories
Huntingdon Research Centre (1967) Unpublished report
Hwang, K. (1966) Arch. int. Pharmacodyn., 163, 302
Hwang, K. & Richards, R. K. (1955) Unpublished report
Kark, R. M. Unpublished report submitted by Abbott Laboratories 1967
Kennedy, B., O'Quinn, S., Perret, W. J., Tilley, J. C. & Hennington,
V. M. (1961) J. Louis. State Med. 113, 365
Kojima, S. & Ichibagase, H. (1966) Chem. Pharm. Bull., 14, 971
Kojima, S., Ichibagase, H. & Iguchi, S. (1966a) Chem. Pharm. Bull.,
14, 959
Kojima S., Ichibagase, H. & Iguchi, S. (1966b) Chem. Pharm. Bull.,
14, 965
Leahy, J. S., Wakefield, M. & Taylor, T. (1967) Fd Cosmet. Toxicol.,
5, 447
Lorke, D. (1967) Unpublished report submitted by Farbenfabriken Bayer
A. G.
Miller, J. P., Michael Crawford, L. E., Sonders, R. C. & Cardinal, E.
V. (1966) Biochem, biophys. Res. Comm., 25, 153
Miller, J. P., Michael Crawford, L. E. & Netwal, J. C. (1967)
Unpublished report submitted by Abbott Laboratories
Mollet, M. (1966) Unpublished report submitted by Abbott Laboratories
National Academy of Sciences - National Research Council (1955)
The Safety of Artificial Sweeteners for Use in Foods, Publication
386
Nees, P. O. & Derse, P. H. (1965) Nature (Lond.), 208, 81
Nees, P. O. & Derse, P. H. (1967) Nature (Lond.), 211, 1191
Radding, R. S. Unpublished report submitted by Abbott Laboratories
1967
Richards, R. K., Taylor, J. D., O'Brien, J. L. & Duescher, H. O.
(1951) J. Amer. Pharm. Ass., Sci. Ed., 40, 1
Richter, W. R., Stein, R. J., Rdzok, E. J. & Moize, S. M. (1967)
Unpublished report submitted by Abbott Laboratories
Schoenberger,. J. A., Rix, D. M., Sakamoto, A., Taylor, J. D. & Kark,
R. M. (1953) Amer. J. Med. Sci., 225, 551
Schechter, P. & Roth, L. J. (1967) Unpublished report submitted by
Abbott Laboratories
Sonders, R. C. & Miller, J. P. (1966) Unpublished report submitted by
Abbott Laboratories
Sonders, R. C. (1967) Unpublished report submitted by Abbott
Laboratories
Sonders, R. C. & Wiegand, R. G. (1967) Unpublished report submitted by
Abbott Laboratories
Sonders, R. C., Wiegand, R. G. & Netwal, J. C. (1967) Unpublished
report submitted by Abbott Laboratories
Stein, A. A., Serrone, D. M. & Coulston, F. (1967) Tox. Appl.
Pharm., 10, 381
Stern, S. B. (1967) Unpublished report submitted by Abbott
Laboratories
Tanaka, R. (1964a) Jap. J. Publ. Health, 11, 909
Tanaka, R. (1964b) J. Iwate Med. Ass., 16, 330
Tatsuji, K. & Tosbie, A. (1963) Med. Cult, 5, 796
Taylor, J. D. (1954) Unpublished report
Taylor, J. D., Richards, R. K. & Davis, J. C. (1951) Proc Soc. Exp.
Biol. Med., 78, 530
Taylor, J. D., Richards, R. K., Wiegand, R. G. & Weinberg, M. S.
(1967) Fd Cosmet. Toxicol.,
Tremolieres, J., Krebs, M. & Pedron, A. (1967) Unpublished report
submitted by Abbott Laboratories
Wisconsin Alumni Research Foundation (1965) Unpublished report
Zöllner, N. & Schnelle, K. (1967) Arzneim.-Forsch. (Drug Res.) 17,
1568
Molecular weight 201.22
Definition Sodium cyclamate contains not less than
98 per cent. and not more than the
equivalent of 101 per cent.
C6H12NNaO3S.
Description White, odourless crystals or
crystalline powder which in dilute
solution is about 30 times as sweet as
sucrose.
Use Non-nutritive sweetener
Biological Data
Biochemical aspects
Absorption of sodium cyclamate from rat stomach perfused in
situ amounted to 4 per cent. per hour at pH 1 and nil at pH 3;
absorption from the small gut occurred at a similar rate of about 4
per cent. per hour (Kojima et al., 1966b).
Whole-body autoradiography of the female rat 5 minutes after i.v.
injection of 100 mg/kg of sodium 14C-cyclamate showed relatively
uniform tissue distribution, with some concentration in the lungs,
kidneys, salivary gland and bloodstream; at 7 hours, the radioactivity
was concentrated in the intestinal contents, the salivary gland and an
unidentified area of the brain; at 48 hours some activity remained in
the gastrointestinal contents. In the pregnant rat, little penetration
into foetal tissue was found at 5 minutes, although the placentas
showed high concentrations; at 7 hours, maternal tissues except the
renal medulla and placenta were relatively free of isotope, with most
of the activity concentrated in the gastrointestinal contents, but the
foetuses showed high activity, with concentrations in the
gastrointestinal contents and kidneys. Forty-eight hours after
injection of the dam, radioactivity in the day-old rat was confined to
the lower gastrointestinal tract, the kidneys, and perhaps the skin
(Schechter & Roth, 1967).
35S-labelled sodium cyclamate was shown to cross the placental
barrier within 2 hours after i.v. administration in the rat.
Concentration in foetal tissues declined at a slower rate than that in
maternal blood (Abbott Laboratories, 1966).
Seventy-two per cent. of intraperitoneally administered
35S-labelled sodium cyclamate was excreted unchanged in the urine of
rats after 5 hours. Single i.p. doses at up to 3.2 g/kg were excreted
rapidly in 24 hours, while 1.4 g/kg was excreted equally rapidly by
normal and unilaterally nephrectomized rats (Taylor et al., 1951).
Repeated oral doses of 80-120 mg/kg for 5 days led to 85 per
cent. excretion in 5 days and another 3 per cent. in the 4 days
following the last dose. About 2/3 was excreted in the faeces and the
remainder in the urine (Taylor et al., 1951). Single oral doses of
0.5, 1 or 2 g/kg body-weight were excreted to the extent of 30 per
cent. in urine and 70 per cent. in faeces in 3 days (substantially in
the first 24 hours). Using 35S-labelled sodium cyclamate in groups of
4 rats given 2 or 4 g/kg body-weight, after 8 hours 75-80 per cent.
remained unabsorbed in the gut, 18-25 per cent. was absorbed and
mostly excreted in the urine and 3-8 per cent. remained in the
carcass. Feeding rats 1 per cent. sodium cyclamate for 54 days prior
to an i.p. injection of the substance showed 77 per cent. of the
injected dose to be excreted in 24 hours, hence no cumulation had
occurred (Hwang, 1966). When 14C-labelled sodium cyclamate was
administered orally to rats, 22 per cent. of the activity was excreted
in the urine, 72 per cent. in the faeces and 0.2 per cent. remained in
the carcass; 98-99 per cent. of the activity was excreted as cyclamate
(Miller et al., 1966). However, when sodium cyclamate excretion was
followed using a gas-liquid chromatography method, at 5 per cent.
dietary intake only 5 per cent. as recovered in the urine and 19 per
cent. in the faeces, while at 10 per cent. dietary intakes some 7 per
cent. appeared in the urine and 18 per cent. in the faeces. No
explanation for these low recoveries was offered (Derse & Daun, 1966).
Two rats given a total of 6.67 g/kg of sodium 14C-cyclamate
orally over an 8-hour period had total carcass activity levels of 0.33
per cent. and 0.24 per cent. of this dose after 5 days; recoveries
from excreta and cage washings were calculated to be 103.25 per cent.
and 102.59 per cent. of the doses given (Miller et al., 1966).
About 80 per cent. of a 1000 mg/kg dose of orally administered
14C-cyclamate was found to be eliminated by the rat in the first 24
hours, approximately equal amounts appearing in urine and faeces. Only
unchanged cyclamate was chromatographically identified in the urine.
0.12 per cent. of the dose was transmitted to litter animals,
apparently via the milk (Hood et al., 1966).
Oral sodium cyclamate at 1 per cent. in the diet for one month
did not alter the BMR of rats (Richards et al., 1951).
In the rabbit, sodium cyclamate was excreted unchanged in the
urine to the extent of 80-90 per cent. in 12 hours after i.v., i.p. or
oral administration (Audrieth & Sveda, 1944).
Of 600 mg orally administered sodium cyclamate in the dog, 70 per
cent. was excreted in the urine within 8 hours and 85 per cent. within
24 hours, while of 100 mg/kg body-weight administered i.v. 92 per
cent. was excreted. One dog receiving 35S-labelled sodium cyclamate
i.v. excreted only 0.5 per cent. in the bile and 67 per cent. in the
urine after 5 hours (Taylor et al., 1951). Three dogs receiving
0.75-1 g/kg body-weight sodium cyclamate i.g. excreted 21-64 per cent.
in the urine within 11-17 hours. Cyclamate blood levels were low
(Hwang, 1966).
In two dogs given single 14C-labelled doses of 200 and 1000
mg/kg, severe diarrhoea was seen 9-10 hours later. Total recovery of
radioactivity from the excreta by the fourth day amounted to about
97-98 per cent. In the animal given 1000 mg/kg, urinary excretion
accounted for 27 per cent. of the dose; in this animal, peak
whole-blood activity levels were found in the first hour after
administration, with a steady decline to less than 1 per cent. of peak
by the forty-eighth hour. In the other, the peak whole-blood activity
level probably occurred during the eighth hour or later, and by 72
hours was down to 7 per cent. of the highest level measured.
Ninety-two hours after administration, no activity was detected in the
skin, eyes, testes, brain, thyroid or skeletal muscle of the low-dose
dog; total activity in the spleen, liver, lungs, pancreas, heart and
kidneys was calculated as 0.027 per cent. of the dose administered. In
a dog given 200 mg/kg 3 times a day for 8 days no activity was
detected in whole blood 44 hours after the last dose. During the test
and in the 9 days following, about 40 per cent. of the dose
administered was recovered from the urine and 54 per cent. from the
faeces. Nine days after the last dose the activity in the spleen,
liver, lungs and pancreas totalled 0.005 per cent. of the doses given,
and activity in a sample of skeletal muscle was about 10 per cent. of
that found in a biopsy sample taken during the last day of
administration (Miller et al., 1966; 1967).
About 80 per cent. of a dose of 1000 mg/kg of 14C-labelled
cyclamate administered orally in the dog was excreted in the urine in
24 hours; two apparent metabolites plus unchanged cyclamate were found
by chromatography of urine samples up to 10 hours after ingestion.
Only cyclamate and possibly a very similar metabolite were found after
24-48 hours. No activity was detectable in the bloodstream after 48
hours. Two dogs were given 200 mg/kg of labelled cyclamate three times
a day for one week. Between 65 and 97 per cent. of the total dose had
been excreted by the eighth day after cessation of administration.
Whole-blood activity levels had dropped to approximately 40 per cent.
of the dose-cessation levels and tissue levels had dropped to 25 per
cent. or less of those found in two other dogs given the same regime
and sacrificed at the end of one week (Hood et al., 1966).
Only unchanged sodium cyclamate was identified
chromatographically in a dog urine sample taken 17 hours after oral
administration of 200 mg/kg (Sonders, 1967). 35S-labelled cyclamate
appeared in the milk of lactating dogs partly from blood and partly
from active secretion but disappeared after 24 hours (Abbott
Laboratories, 1966).
In the lactating dog, 4-24 hours after i.v. injection of a 20 mg
14C-labelled dose, concentrations of cyclamate were found to be
higher in the milk than in simultaneous blood samples; cyclamate
appeared in the milk at levels of about 2 µg/ml or less, and
disappeared from the milk at about the same rate as from the
bloodstream (Wiegand, 1967).
Intravenous injection of 35S-labelled sodium cyclamate into
rats, rabbits and dogs showed that, with the exception of the kidney
and perhaps the liver, gastrointestinal tract and spleen, there was no
significant concentration in the various organs examined. Sodium
cyclamate penetrated into the brain with difficulty but crossed the
placenta very easily in the rat and was found in the foetus (Taylor et
al., 1951).
Cyclohexylamine excretion
Humans, dogs but not rabbits excrete cyclohexylamine after oral
doses of 1-3 g of sodium cyclamate. No glucuronates were detected.
Some 0.7 per cent. of the oral dose is excreted as cyclohexylamine in
man while in dogs only trace amounts are detected (Kojima &
Ichibagase, 1966). Rats receiving sodium cyclamate in their diet
excreted cyclohexylamine in their urine (Leahy et al., 1966). Oral
administration of 3 g sodium cyclamate on each of 3 days to 5 human
volunteers resulted in excretion of cyclohexylamine in man,
representing 0.8 per cent. of the dose administered (Leahy et al.,
1967). Further extension of the work to 40 volunteers showed 5
excretors again at 0.8 per cent. of the administered dose while one
subject excreted 7.5 per cent. of the administered dose as
cyclohexylamine (Huntingdon Laboratories, 1967).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Mouse oral 10 000-12 000 Richards et al., 1951
11 000 Abbott Laboratories, 1966
17 000 Taylor et al., 1967
1 525 Mollet, 1966
i.v. 4 000 Richards et al., 1951
4 800 Taylor et al., 1967
i.p. 10 000-12 000 Fitzhugh et al., 1951
7 100 Taylor et al., 1967
Rat oral 12 000 Richards et al., 1951
17 500 Taylor et al., 1967
i.v. 3 000-4 000 Richards et al., 1951
3 500 Taylor et al., 1967
i.p. 6 000 Taylor et al., 1967
Pretreatment of mice dams with 0.5 per cent. sodium cyclamate
raised the acute i.p. toxicity of the substance in their pups.
Changing the lipid content of the diet from 6.5 per cent. to 40 per
cent. increased the i.p. acute toxicity of sodium cyclamate in mice
(Taylor et al., 1967), Rats consuming up to 8 g/kg body-weight/day of
sodium cyclamate for 5 days showed no abnormal findings regarding
haematology, tissue chemistry and histopathology apart from a slight
decrease in hepatic triglycerides at the 8 g level (Stein et al.,
1967).
Diffuse, mild vesiculation of the endoplasmic reticulum
associated with vacuolization was observed in the liver and kidney of
monkeys given 4 or 8 g/kg of sodium cyclamate orally and sacrificed 1
or 48 hours later. No changes in various biochemical values were
associated with these early morphologic alterations (Stein et al.,
1967). No ultrastructural changes attributed to cyclamate were found
in the hepatic cells of two monkeys 48 hours after single doses of
4000 mg/kg or of one animal 1 hour after an oral dose of 7170 mg/kg
(Richter et al., 1967).
Special studies
Laxative and faecal softening effects were studied in rats, mice,
dogs and monkeys using 35S-labelled compounds. Approximately70 per
cent. of cyclamate remained unabsorbed in the gut and no significant
systemic effects were noted. Laxative action as shown by motility of
isolated gut was ascribed to the increased bulk secondary to osmosis.
Mice were slightly more susceptible. The laxative dose (ED50) in the
rat was about 1.9 g/kg body-weight. No gross changes in rat or mouse
intestinal mucosa were noted (Hwang, 1966). Similar results were noted
in a repeat study on rats, mice and dogs. Intravenous sodium cyclamate
in mice did not affect intestinal motility, stool frequency and
appearance (Taylor et al, 1967). Sodium cyclamate had no effect on
the water balance of hypertensive rats with renal disease (Fregley &
Kier, 1961).
Short-term studies
Rat. Groups of 10 weanling rats were fed 0, 1, 5 and 10 per
cent. sodium cyclamate for 22 weeks. Three-day balance studies were
done in the eighth, fourteenth, twentieth and twenty-second weeks, in
which chromatographic recoveries from urine and faeces were compared
with consumption. These recoveries were simultaneously compared with
isotope recoveries from additional doses of the labelled sodium salt
administered orally at the beginning of each balance period. Average
chromatographic recoveries ranged from 77.7 to over 100 per cent. and
isotope recoveries from 91.5 to over 100 per cent. Weight gain was
adversely affected in the 10 per cent. group. Gross, histological and
radiological examination of survivors showed no changes (Sonders et
al., 1967).
Groups of 10 male and 10 female rats received diets containing 0,
1, 2 or 3 per cent. of sodium cyclamate for 11 months. No adverse
effects (apart from occasional lax stools at the 2 per cent. and 3 per
cent. levels) were noted on growth rate, liver and kidney weight,
haematology and urinalysis. When rats of corresponding test groups
were mated, there was no adverse effect on fertility or litter
characteristics. Of the F1 generation, groups of 3-8 mice and 8
females were kept on 1, 2 and 3 per cent. sodium cyclamate in their
diet and again correspondingly mated to give the F2 generation. Pairs
of corresponding groups continued to receive 0, 1, 2 and 3 per cent.
in their diet and were remated to produce an F3 generation. No
adverse effects were seen on conception rate, foetal development and
litter characteristics (Taylor et al., 1967).
Guinea-pig. Groups of 7 males received 0.5 or 2 per cent.
sodium cyclamate in their drinking water for 5 months and controls
were given 0.3 per cent. saline. Fluid intake was restricted to 30 ml
per day per animal. There was an apparent increase in mortality at the
2 per cent. level. There was also a 20 per cent. decrease in
body-weight at the 2 per cent. level compared with controls. Histology
of the liver of test animals showed "reduced protoplasm" and
vacuolization at both dietary levels though less at 0.5 per cent.
Histology of controls was not reported. Serum lactic dehydrogenase and
SGOT levels were raised at 2 per cent., but not significantly
(Hellauer, 1967).
Dog. Three groups of 2, 4 and 4 dogs received 0, 2 and 4 g/kg
body-weight of sodium cyclamate i.g. daily for 30 days without
deleterious effect on body-weight, appearance, marrow picture, hepatic
function tests, gross autopsy and histology of liver, kidney and
gastrointestinal tract (Taylor et al., 1967). In another experiment
dogs were given 0.5 g/kg body-weight sodium cyclamate daily for 11
months without adverse effect on weight, blood, liver and kidney
function or urine (Hwang & Richards, 1955). Four groups of 3 dogs
received 0, or 0.5 g/kg body-weight sodium cyclamate 6 days per week
i.g. for 11 months. At the tenth month soft stools were seen. No
adverse effects were seen regarding body-weight, haemoglobin,
urinalysis and tests of hepatic and renal function, and on gross
autopsy (Taylor et al., 1967). Groups of 2 dogs were kept on diets
containing 0, 0.2 or 0.4 per cent. sodium cyclamate daily for 15
months. All gained weight; haematology and liver and kidney function
tests were normal; urinalysis did not differ from controls. Gross and
microscopic examination of the major organs was normal (Richards et
al., 1951).
Monkey. Monkeys were given 4 g/kg body-weight of sodium
cyclamate daily for 9 months without significant changes in
haematology, serum enzymes, urine or gross autopsy findings. Hepatic
and renal demethylase and desulfurase activities, lysosomal activity
and mitochondrial oxygen utilization were not affected, but slight
enlargement of pancreatic islands of Langerhans was noted.
Electron microscopy revealed some subcellular alterations (Stein et
al., 1967).
Teratogenicity studies
Mouse. Groups of 2-4 mice with control groups of 3 mice were
given single i.g. doses of sodium cyclamate at various stages of
pregnancy as follows: 62.5 mg on day 4-5, 125 mg on day 4-5 and 6-7,
250 mg on day 6-7 and 8-10 and 500 mg on day 6-7 and 8-10. The uteri
were removed on the eighteenth day, opened and the foetuses assessed
and absorption sites counted. No abnormalities were detected following
any administrations on days 8-10 but in all other instances the
majority of embryos had either been resorbed or appeared dead or
showed delayed development compared with controls. The foetal LD50
was calculated to be 180 mg/kg body-weight (Tanaka, 1964a; 1964b).
In embryotoxicity studies still in progress, groups of 10-14
female mice, mated at a little over 2 months of age, were given
intragastric, aqueous doses of 0, 5 and 10 g/kg of sodium cyclamate on
the eighth day of gestation. On examination of the uteri and foetuses
on the eighteenth day of gestation, no effect was found (Lorke, 1967).
Rat. Groups of 20-24 pregnant females were given 50, 100 and
150 mg/kg body-weight of sodium cyclamate daily from the sixth to the
fifteenth day of gestation. Three hundred and sixty-three pregnant
animals served as controls. The dams were autopsied on the
twenty-first day and the foetuses examined grossly. The number of
dead, malformed or reported foetuses did not differ from controls
(Bein et al., 1967).
Rabbit. Four females were given 1 g/kg body-weight sodium
cyclamate orally from day 1-18 of gestation. All offspring were normal
and survival and litter size corresponded with controls (Abbott
Laboratories, 1966).
Dog. Groups of 6 dogs (2 male and 4 female) were given 0, 0.45,
0.9 and 1.35 g/kg of sodium cyclamate for about 6 months. The dogs
were bred and the offspring after weaning at 8 weeks were placed on
the same regimen that their respective parents were receiving. After
20 months there was no adverse effect on the parents. All progeny were
born normal and have shown no effect of treatment (Abbott
Laboratories, 1966).
Long-term studies
Rat. Groups of 8-20 male and female rats were fed diets
containing 0, 0.5, 0.1 and 1.0 per cent. sodium cyclamate for 18, 24
or 30 months. Mating was permitted and the F1 generation kept on the
same dietary regime. The experiment was carried into the third
generation at the 0.05 per cent. level. Weight gains, haematology and
urinalysis were normal. Clinical and pathological studies showed no
difference from controls as regards major organs. Mortality and tumour
incidence were similar to those of controls. Normal litters were
produced (Richards et al., 1951).
In another experiment groups of 18-24 rats received 0, 0.01, 0.1,
0.5, 1 and 5 per cent. sodium cyclamate in their diet for 24 months.
Slight growth depression was noted at 5 per cent. associated with
marked diarrhoea. Mortality was similar in all groups. Organ weights,
histopathology of major organs and tumour incidence were no different
from controls (Fitzhugh et al., 1951).
Observations in man
After i.v. administration to healthy men of 1 g sodium cyclamate,
7O-90 per cent. was excreted in the urine within 3 hours and 84-99 per
cent. within 24 hours. Using 35S-labelled sodium cyclamate i.v. in 3
patients, the urinary clearance indicated excretion by glomerular
filtration and tubular secretion. No general metabolic effects were
noted (Schoenberger et al., 1953). One healthy man was given 300 mg
sodium cyclamate orally and excreted 79.5 per cent. in the urine over
7 days, 26 per cent. being excreted in the first 24 hours. Of an oral
dose of 200 mg sodium cyclamate 77 per cent. was recovered in 3 days
(Richards et al., 1951). Doses of 1 or 5 g sodium cyclamate were given
to 3 subjects and 40-48 per cent. was recovered in the urine in 24
hours (Kojima et al., 1966a). The amounts of cyclamate remaining in
the body during 2, 5 or 10 g daily administration can be calculated
for daily excretion rates ranging from 10-90 per cent. For 5 g and 75
per cent. excretion, the cyclamate residue in the body is 1.67 g
(Taylor, 1954). Phototoxicity has been reported as an immediate
reaction to UV radiation (Kennedy et al., 1961). Photo-allergy as a
delayed reaction to UV radiation has also been noted (Tatsuji &
Tosbie, 1963).
Two human subjects were given 14C-labelled doses of 4D mg/kg and
88 mg/kg. Urine recoveries in 4 days were 51.8 and 73.5 per cent.
respectively and faecal recoveries were 50.5 and 24.5 per cent.
Urinary cyclohexylamine accounted for 0.156 and 0.123 per cent. of the
isotopic material present. Reverse isotope dilution analysis indicated
that all of the urinary isotope was unchanged cyclamate. Peak plasma
activity levels were found in the eighth hour and the first hour
respectively and peak whole-blood levels in the first and sixth hours
(Sonders & Weigand, 1967).
Groups of 32 and 30 patients with chronic hepatic or renal
disease were given 5 and 2 g/day of sodium cyclamate for 6-6.5 months.
On the basis of clinical chemical studies, cyclamate did not affect
the patient's progress (Zöllner & Schneller, 1967).
Comments
Although this substance has been studied extensively in animals
and man, there are certain lacunae such as the exact mechanism of the
laxative effect, bearing in mind the changes observed in cellular and
subcellular structures in histoenzymatic studies, the influence on the
foetus in view of the easy transplacental passage and the significance
of the appearance of the metabolite cyclohexylamine in the urine of
man. The teratogenic effects which have only been seen in some studies
in mice may well be related to physical and environmental factors
rather than inherent teratogenic potential of sodium cyclamate.
Long-term experiments have only been conducted in one species and
further feeding studies in a second species are needed. The possible
nutritional aspect of prolonged unrestricted use of cyclamate may
require consideration.
EVALUATION
In view of the extensive studies in animal and man and widespread
use in some countries, on the one hand, and the gaps in information
about certain biological effects on the other, a temporary acceptable
daily intake is allocated with some further work specified below.
Level causing no significant toxicological effect
Man: 12 000 mg per day, equivalent to 200 mg/kg/day (soft
stools and more frequent bowel movements were observed in some
subjects).
Estimate of acceptable daily intake for man
mg/kg body-weight
Temporary acceptance 0-50
Further work required within 3 years
(1) Investigation into the metabolism to cyclohexylamine and other
possible metabolites and full toxicological evaluation of these
metabolites including any possible human genetic aspects.
(2) Investigation into the exact mechanism of the laxative effect
including any possible enteropathic cellular and subcellular changes.
(3) Lifespan studies in a second species including observations on
reproduction and development of several filial generations.
REFERENCES
Abbott Laboratories (1966) Review of cyclamates
Audrieth, I. F. &, Sveda, M. (1944) J. Org. Chem., 9, 89
Batterman, R. C. (1966) Unpublished report submitted by Abbott
Laboratories
Bein, H. J,, Fritz, H., Hess, R. & Loustalot, P. (1967) Unpublished
report submitted by CIBA
Berndt, C. W. & Calandra, J. C. (1965) Unpublished report submitted by
Abbots Laboratories
Bernier, J. J. (1967) Unpublished report
Dedmon, R. E., Ryan, W. G. & Kark, R. M. (1961) Unpublished report
submitted by Abbott Laboratories
Derse, P. H. & Daun R. J. (1966) J. Ass. Off. Agr. Chem., 49, 1090
Fitzhugh, O. G, Nelson, A. A. & Frawley, J. P. (1951) J. Amer.
Pharm. Ass., Sci Ed., 40, 583
Freese, H. B., Jenike, T. S. & Rubenkoenig, H. L. (1967) Unpublished
report submitted by Abbott Laboratories
Fregley, M. J. & Kier, L. B. (1966) Tox. Appl. Pharm., 9, 124
Frost, D. V. & Main, B. T. Unpublished report submitted by Abbott
Laboratories 1967
GoLberg, L. (1965) Unpublished report
Hellauer, H. (1967) Unpublished report
Hood, S. L., Somaini, S. M., Zink, V. R. & Bogner, R. L. (1966)
Unpublished report submitted by Abbott Laboratories
Huntingdon Research Centre (1967) Unpublished report
Hwang, K. (1966) Arch. int. Pharmacodyn., 163, 302
Hwang, K. & Richards, R. K. (1955) Unpublished report
Kark, R. M. Unpublished report submitted by Abbott Laboratories 1967
Kennedy, B., O'Quinn, S., Perret, W. J., Tilley, J. C. & Hennington,
V. M. (1961) J. Louis. State Med. 113, 365
Kojima, S. & Ichibagase, H. (1966) Chem. Pharm. Bull., 14, 971
Kojima, S., Ichibagase, H. & Iguchi, S. (1966a) Chem. Pharm. Bull.,
14, 959
Kojima S., Ichibagase, H. & Iguchi, S. (1966b) Chem. Pharm. Bull.,
14, 965
Leahy, J. S., Wakefield, M. & Taylor, T. (1967) Fd Cosmet. Toxicol.,
5, 447
Lorke, D. (1967) Unpublished report submitted by Farbenfabriken Bayer
A. G.
Miller, J. P., Michael Crawford, L. E., Sonders, R. C. & Cardinal, E.
V. (1966) Biochem, biophys. Res. Comm., 25, 153
Miller, J. P., Michael Crawford, L. E. & Netwal, J. C. (1967)
Unpublished report submitted by Abbott Laboratories
Mollet, M. (1966) Unpublished report submitted by Abbott Laboratories
National Academy of Sciences - National Research Council (1955)
The Safety of Artificial Sweeteners for Use in Foods, Publication
386
Nees, P. O. & Derse, P. H. (1965) Nature (Lond.), 208, 81
Nees, P. O. & Derse, P. H. (1967) Nature (Lond.), 211, 1191
Radding, R. S. Unpublished report submitted by Abbott Laboratories
1967
Richards, R. K., Taylor, J. D., O'Brien, J. L. & Duescher, H. O.
(1951) J. Amer. Pharm. Ass., Sci. Ed., 40, 1
Richter, W. R., Stein, R. J., Rdzok, E. J. & Moize, S. M. (1967)
Unpublished report submitted by Abbott Laboratories
Schoenberger,. J. A., Rix, D. M., Sakamoto, A., Taylor, J. D. & Kark,
R. M. (1953) Amer. J. Med. Sci., 225, 551
Schechter, P. & Roth, L. J. (1967) Unpublished report submitted by
Abbott Laboratories
Sonders, R. C. & Miller, J. P. (1966) Unpublished report submitted by
Abbott Laboratories
Sonders, R. C. (1967) Unpublished report submitted by Abbott
Laboratories
Sonders, R. C. & Wiegand, R. G. (1967) Unpublished report submitted by
Abbott Laboratories
Sonders, R. C., Wiegand, R. G. & Netwal, J. C. (1967) Unpublished
report submitted by Abbott Laboratories
Stein, A. A., Serrone, D. M. & Coulston, F. (1967) Tox. Appl.
Pharm., 10, 381
Stern, S. B. (1967) Unpublished report submitted by Abbott
Laboratories
Tanaka, R. (1964a) Jap. J. Publ. Health, 11, 909
Tanaka, R. (1964b) J. Iwate Med. Ass., 16, 330
Tatsuji, K. & Tosbie, A. (1963) Med. Cult, 5, 796
Taylor, J. D. (1954) Unpublished report
Taylor, J. D., Richards, R. K. & Davis, J. C. (1951) Proc Soc. Exp.
Biol. Med., 78, 530
Taylor, J. D., Richards, R. K., Wiegand, R. G. & Weinberg, M. S.
(1967) Fd Cosmet. Toxicol.,
Tremolieres, J., Krebs, M. & Pedron, A. (1967) Unpublished report
submitted by Abbott Laboratories
Wisconsin Alumni Research Foundation (1965) Unpublished report
Zöllner, N. & Schnelle, K. (1967) Arzneim.-Forsch. (Drug Res.) 17,
1568
