
4-ETHOXYPHENYLUREA
Synonyms Dulcin; Sucrol; Valzin
Chemical name 4-Ethoxyphenylurea
Empirical formula C9H12O2N2
Structural formula
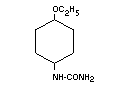 Molecular weight 180.21
Description Colourless or white crystals, or a
white crystalline powder, which is
odourless and has a very sweet taste
which is appreciable even after a
3000-fold dilution.
Biological Data
Biochemical aspects
Early experiments indicated p-aminophenol as a metabolite in
human urine after oral intakes at 1 g of 4-ethoxyphenylurea (Rost &
Braun, 1926). More recent studies in rabbits and rats, given oral or
intragastric: 4-ethoxyphenylurea at the rate of 500 mg/kg body-weight,
showed rapid absorption into the blood within 3 hours and slow
disappearance from the body. Three per cent. was excreted in the urine
in 48 hours, none appeared in the faeces, the rest being metabolized
slowly. Most tissues except fat contain 4-ethoxyphenylurea which
disappears slowly (Akagi & Aoki, 1962; Akagi et al., 1965).
Twenty-three per cent. 4-ethoxyphenylurea is absorbed from rat stomach
within 1 hour end over 80 per cent. absorbed from rat small intestine
within 2 hours, probably by simple diffusion (Kojima et al., 1966).
In the rabbit 4D per cent. of orally administered
4-ethoxyphenylurea is de-ethylated to p-hydroxyphenylurea as the
principal metabolite. This appears in the urine either as free
compound (7 per cent.) conjugated as O-sulfate (23 per cent.) or as
O-glucuronide (11 per cent.). Three per cent. of 4-ethoxyphenylurea ix
excreted unchanged as p-ethoxyphenylurea 27 per cent. as
N-glucuronide or p-ethoxyphenylurea, which appears to be the major
common pathway for disposal of arylureas, and a trace as
p-ethoxyphenylurea sulfamate. Small amounts of p-phenetidine,
p-aminophenol and urea have also been isolated (Akagi & Aoki, 1962;
Akagi et al., 1966).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Mouse oral 700-1000 Tanaka, 1964
Rat (young) oral 4900 ) Bekemeier et al., 1958
(adult) oral 3200 )
Dog oral 1000 (LD) Flury
Doses of 0.4-0.6 g/day caused ataxia, vomiting and weight loss in
dogs but O.1 g/day for 30 days had no adverse effects. Similar effects
were seen in monkeys, cats and guinea-pigs (Rost & Braun, 1926).
Teratogenicity studies
Mouse. Groups of 3 or 4 female mice were mated and given 50
mg/kg body-weight 4-ethoxyphenylurea intragastrically on days 8-10 and
6-7 post-conception, 30 mg/kg body-weight on days 6-7 post-conception
and 10 mg/kg body-weight on days 4-5 post-conception. Foetuses were
examined on the eighteenth day of pregnancy. 50 mg/kg had no
deleterious effect on development or foetal survival on days 8-10, but
retarded development and caused foetal death on days 6-7. Similar
effects occurred with 30 mg/kg and 10 mg/kg, including subnormal, as
well as retarded development in almost all foetuses (Tanaka, 1964).
Long-term studies
Rat. Groups of 7-10 male and 7-9 female rats were fed 0, 0.01,
0.1, 0.25, 0.5 and 1.0 per cent. 4-ethoxyphenylurea in their diet for
up to 2 years. Survival and growth rate were adversely and
significantly affected in the 0.5 and 1.0 per cent. groups. The
weights of liver, kidney and spleen were significantly raised at the
0.5 and 1.0 per cent. levels. Histopathology showed liver tumours,
some benign some malignant, at the 0.1 per cent. and higher levels.
Splenic enlargement also occurred at the 0.1 per cent. level with
congestion, hyperplasia and haemosiderosis. Pigmentation of the renal
proximal convoluted tubules and anaemia were noted at the 0.5 and 1.0
per cent. levels (Fitzhugh et al., 1951). In another experiment,
groups of 15 young and adult rats were given 0.2 g 4-ethoxyphenylurea
per animal daily for up to 22 months. No malignant tumours were noted
(Lettre & Wrba, 1955). In another experiment, 20 male and 30 female
rats were given 0.2 g 4-ethoxyphenylurea/kg body-weight
intragastrically daily for 13 months without any adverse effects being
noted on organ weight or tumour incidence; but 60 per cent. of the
animals died during this time. No deleterious effects were noted on
fertility, litter size and development of pups. Thirty-nine males of
the F1 generation were kept on 0.2 g 4-ethoxyphenylurea/kg daily for
22 months without any noticeable effect on weight gain, incidence of
malignant tumours or mortality (Bekemeier et al., 1958). In another
experiment groups of 30 rats were fed 0 or 1.0 per cent.
4-ethoxyphenylurea in the diet for 21-24 months. Over 75 per cent. of
test animals developed tumours (1/3 malignant) of the urinary tract,
66 per cent. had also stones of the renal pelvis and bladder.
Splenomegaly and haemosiderosis of the spleen were also noted
(Griepentrog, 1959).
Observations
Up to 0.6 g daily in 4 male and 7 female volunteers and 0.1 g
daily for 14 days in 30 volunteers produced no adverse effects and no
evidence of p-aminophenol in the urine (Rost & Braun, 1926). Two
deaths, accompanied by abdominal pain, vomiting, coma and fits, have
been reported in children after an intake of 8-10 g. Doses of 20-35 g
taken by adults produced dizziness, nausea, methaemoglobinuria with
cyanosis, hypotension, dyspnoea, paraesthesiae, and coronary
disturbance in one case (Buhr, 1948).
Five volunteers received approximately 0.11 9 4-ethoxyphenylurea
daily for 41 weeks without any apparent ill effects; 1.5 g/day for 3
weeks is harmless to man, apart from slight temperature lowering.
Diabetics have received 4-ethoxyphenylurea for 1 year without
deleterious effects (Roest & Braun, 1926).
Comments
Biochemical studies show that 4-ethoxyphenylurea is rapidly
absorbed and slowly excreted. In the rabbit the greater amount is
excreted in the urine as metabolites, of which the principal one is
the de-ethylated 4-ethoxyphenylurea, p-hydroxyphenylurea. Early
human studies indicated that approximately 0.1 g daily had no
deleterious effects; however, long-term studies in rats revealed
tumour production at relatively low dosage levels. In one study
dietary levels of 0.1-1.0 per cent. 4-ethoxyphenylurea produced liver
tumours, and in another a level of 1.0 per cent. produced malignant
bladder lesions and urinary stones. Other studies at 0.2 and 0.3 per
cent. levels did not confirm either observation. Therefore long-term
animal studies are inconclusive.
EVALUATION
The data are not satisfactory for the evaluation of
4-ethoxyphenylurea as an artificial sweetener in foods. Although the
substance has been used by humans without any observed deleterious
effect, animal studies indicate that 4-ethoxyphenylurea may possess
tumourigenic properties. Until satisfactory studies are completed to
show that 4-ethoxyphenylurea is safe this substance should not be used
as a food additive.
REFERENCES
Akigi, M. & Aoki, I. (1962) Chem. Pharm. Bull., 10, 200
Akagi, M., Oketani, Y. & Vematsu, T. (1965) Chem. Pharm. Bull.,
13, 1200
Akagi, M., Aoki, I. & Vematsu, T. (1966) Chem. Pharm. Bull., 14, 1
Bekemeier, H., Hannig, E. & Pfennigsdorf, G. (1958) Arzneim.-
Forsch., 8, 150
Buhr, G. (1948) Med. Klinik, 43, 105
Fitzhugh, O. G., Nelson, A. A. & Frawley, J. P. (1951) J. Amer.
Pharm. Assoc., 60, 583
Griepentrog, F. (1959) Arzneim. Forseh., 9, 123
Kojima, S., Ichibagase, H. & Iguchi, S. (1966) Chem. Pharm. Bull.,
14, 965
Lettre, H. & Wrba, H. (1955) Naturwiss., 42, 217
Rost, R. & Braun, A. (1926) Arb. Reichsg. Amter, 57, 212
Tanaka, R. (1964) Jap. J. Public Hlth, 11/14, 909
Flury, F. Abderhaldens Handbuch 4, 7b, 1345
Molecular weight 180.21
Description Colourless or white crystals, or a
white crystalline powder, which is
odourless and has a very sweet taste
which is appreciable even after a
3000-fold dilution.
Biological Data
Biochemical aspects
Early experiments indicated p-aminophenol as a metabolite in
human urine after oral intakes at 1 g of 4-ethoxyphenylurea (Rost &
Braun, 1926). More recent studies in rabbits and rats, given oral or
intragastric: 4-ethoxyphenylurea at the rate of 500 mg/kg body-weight,
showed rapid absorption into the blood within 3 hours and slow
disappearance from the body. Three per cent. was excreted in the urine
in 48 hours, none appeared in the faeces, the rest being metabolized
slowly. Most tissues except fat contain 4-ethoxyphenylurea which
disappears slowly (Akagi & Aoki, 1962; Akagi et al., 1965).
Twenty-three per cent. 4-ethoxyphenylurea is absorbed from rat stomach
within 1 hour end over 80 per cent. absorbed from rat small intestine
within 2 hours, probably by simple diffusion (Kojima et al., 1966).
In the rabbit 4D per cent. of orally administered
4-ethoxyphenylurea is de-ethylated to p-hydroxyphenylurea as the
principal metabolite. This appears in the urine either as free
compound (7 per cent.) conjugated as O-sulfate (23 per cent.) or as
O-glucuronide (11 per cent.). Three per cent. of 4-ethoxyphenylurea ix
excreted unchanged as p-ethoxyphenylurea 27 per cent. as
N-glucuronide or p-ethoxyphenylurea, which appears to be the major
common pathway for disposal of arylureas, and a trace as
p-ethoxyphenylurea sulfamate. Small amounts of p-phenetidine,
p-aminophenol and urea have also been isolated (Akagi & Aoki, 1962;
Akagi et al., 1966).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Mouse oral 700-1000 Tanaka, 1964
Rat (young) oral 4900 ) Bekemeier et al., 1958
(adult) oral 3200 )
Dog oral 1000 (LD) Flury
Doses of 0.4-0.6 g/day caused ataxia, vomiting and weight loss in
dogs but O.1 g/day for 30 days had no adverse effects. Similar effects
were seen in monkeys, cats and guinea-pigs (Rost & Braun, 1926).
Teratogenicity studies
Mouse. Groups of 3 or 4 female mice were mated and given 50
mg/kg body-weight 4-ethoxyphenylurea intragastrically on days 8-10 and
6-7 post-conception, 30 mg/kg body-weight on days 6-7 post-conception
and 10 mg/kg body-weight on days 4-5 post-conception. Foetuses were
examined on the eighteenth day of pregnancy. 50 mg/kg had no
deleterious effect on development or foetal survival on days 8-10, but
retarded development and caused foetal death on days 6-7. Similar
effects occurred with 30 mg/kg and 10 mg/kg, including subnormal, as
well as retarded development in almost all foetuses (Tanaka, 1964).
Long-term studies
Rat. Groups of 7-10 male and 7-9 female rats were fed 0, 0.01,
0.1, 0.25, 0.5 and 1.0 per cent. 4-ethoxyphenylurea in their diet for
up to 2 years. Survival and growth rate were adversely and
significantly affected in the 0.5 and 1.0 per cent. groups. The
weights of liver, kidney and spleen were significantly raised at the
0.5 and 1.0 per cent. levels. Histopathology showed liver tumours,
some benign some malignant, at the 0.1 per cent. and higher levels.
Splenic enlargement also occurred at the 0.1 per cent. level with
congestion, hyperplasia and haemosiderosis. Pigmentation of the renal
proximal convoluted tubules and anaemia were noted at the 0.5 and 1.0
per cent. levels (Fitzhugh et al., 1951). In another experiment,
groups of 15 young and adult rats were given 0.2 g 4-ethoxyphenylurea
per animal daily for up to 22 months. No malignant tumours were noted
(Lettre & Wrba, 1955). In another experiment, 20 male and 30 female
rats were given 0.2 g 4-ethoxyphenylurea/kg body-weight
intragastrically daily for 13 months without any adverse effects being
noted on organ weight or tumour incidence; but 60 per cent. of the
animals died during this time. No deleterious effects were noted on
fertility, litter size and development of pups. Thirty-nine males of
the F1 generation were kept on 0.2 g 4-ethoxyphenylurea/kg daily for
22 months without any noticeable effect on weight gain, incidence of
malignant tumours or mortality (Bekemeier et al., 1958). In another
experiment groups of 30 rats were fed 0 or 1.0 per cent.
4-ethoxyphenylurea in the diet for 21-24 months. Over 75 per cent. of
test animals developed tumours (1/3 malignant) of the urinary tract,
66 per cent. had also stones of the renal pelvis and bladder.
Splenomegaly and haemosiderosis of the spleen were also noted
(Griepentrog, 1959).
Observations
Up to 0.6 g daily in 4 male and 7 female volunteers and 0.1 g
daily for 14 days in 30 volunteers produced no adverse effects and no
evidence of p-aminophenol in the urine (Rost & Braun, 1926). Two
deaths, accompanied by abdominal pain, vomiting, coma and fits, have
been reported in children after an intake of 8-10 g. Doses of 20-35 g
taken by adults produced dizziness, nausea, methaemoglobinuria with
cyanosis, hypotension, dyspnoea, paraesthesiae, and coronary
disturbance in one case (Buhr, 1948).
Five volunteers received approximately 0.11 9 4-ethoxyphenylurea
daily for 41 weeks without any apparent ill effects; 1.5 g/day for 3
weeks is harmless to man, apart from slight temperature lowering.
Diabetics have received 4-ethoxyphenylurea for 1 year without
deleterious effects (Roest & Braun, 1926).
Comments
Biochemical studies show that 4-ethoxyphenylurea is rapidly
absorbed and slowly excreted. In the rabbit the greater amount is
excreted in the urine as metabolites, of which the principal one is
the de-ethylated 4-ethoxyphenylurea, p-hydroxyphenylurea. Early
human studies indicated that approximately 0.1 g daily had no
deleterious effects; however, long-term studies in rats revealed
tumour production at relatively low dosage levels. In one study
dietary levels of 0.1-1.0 per cent. 4-ethoxyphenylurea produced liver
tumours, and in another a level of 1.0 per cent. produced malignant
bladder lesions and urinary stones. Other studies at 0.2 and 0.3 per
cent. levels did not confirm either observation. Therefore long-term
animal studies are inconclusive.
EVALUATION
The data are not satisfactory for the evaluation of
4-ethoxyphenylurea as an artificial sweetener in foods. Although the
substance has been used by humans without any observed deleterious
effect, animal studies indicate that 4-ethoxyphenylurea may possess
tumourigenic properties. Until satisfactory studies are completed to
show that 4-ethoxyphenylurea is safe this substance should not be used
as a food additive.
REFERENCES
Akigi, M. & Aoki, I. (1962) Chem. Pharm. Bull., 10, 200
Akagi, M., Oketani, Y. & Vematsu, T. (1965) Chem. Pharm. Bull.,
13, 1200
Akagi, M., Aoki, I. & Vematsu, T. (1966) Chem. Pharm. Bull., 14, 1
Bekemeier, H., Hannig, E. & Pfennigsdorf, G. (1958) Arzneim.-
Forsch., 8, 150
Buhr, G. (1948) Med. Klinik, 43, 105
Fitzhugh, O. G., Nelson, A. A. & Frawley, J. P. (1951) J. Amer.
Pharm. Assoc., 60, 583
Griepentrog, F. (1959) Arzneim. Forseh., 9, 123
Kojima, S., Ichibagase, H. & Iguchi, S. (1966) Chem. Pharm. Bull.,
14, 965
Lettre, H. & Wrba, H. (1955) Naturwiss., 42, 217
Rost, R. & Braun, A. (1926) Arb. Reichsg. Amter, 57, 212
Tanaka, R. (1964) Jap. J. Public Hlth, 11/14, 909
Flury, F. Abderhaldens Handbuch 4, 7b, 1345
 Molecular weight 180.21
Description Colourless or white crystals, or a
white crystalline powder, which is
odourless and has a very sweet taste
which is appreciable even after a
3000-fold dilution.
Biological Data
Biochemical aspects
Early experiments indicated p-aminophenol as a metabolite in
human urine after oral intakes at 1 g of 4-ethoxyphenylurea (Rost &
Braun, 1926). More recent studies in rabbits and rats, given oral or
intragastric: 4-ethoxyphenylurea at the rate of 500 mg/kg body-weight,
showed rapid absorption into the blood within 3 hours and slow
disappearance from the body. Three per cent. was excreted in the urine
in 48 hours, none appeared in the faeces, the rest being metabolized
slowly. Most tissues except fat contain 4-ethoxyphenylurea which
disappears slowly (Akagi & Aoki, 1962; Akagi et al., 1965).
Twenty-three per cent. 4-ethoxyphenylurea is absorbed from rat stomach
within 1 hour end over 80 per cent. absorbed from rat small intestine
within 2 hours, probably by simple diffusion (Kojima et al., 1966).
In the rabbit 4D per cent. of orally administered
4-ethoxyphenylurea is de-ethylated to p-hydroxyphenylurea as the
principal metabolite. This appears in the urine either as free
compound (7 per cent.) conjugated as O-sulfate (23 per cent.) or as
O-glucuronide (11 per cent.). Three per cent. of 4-ethoxyphenylurea ix
excreted unchanged as p-ethoxyphenylurea 27 per cent. as
N-glucuronide or p-ethoxyphenylurea, which appears to be the major
common pathway for disposal of arylureas, and a trace as
p-ethoxyphenylurea sulfamate. Small amounts of p-phenetidine,
p-aminophenol and urea have also been isolated (Akagi & Aoki, 1962;
Akagi et al., 1966).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Mouse oral 700-1000 Tanaka, 1964
Rat (young) oral 4900 ) Bekemeier et al., 1958
(adult) oral 3200 )
Dog oral 1000 (LD) Flury
Doses of 0.4-0.6 g/day caused ataxia, vomiting and weight loss in
dogs but O.1 g/day for 30 days had no adverse effects. Similar effects
were seen in monkeys, cats and guinea-pigs (Rost & Braun, 1926).
Teratogenicity studies
Mouse. Groups of 3 or 4 female mice were mated and given 50
mg/kg body-weight 4-ethoxyphenylurea intragastrically on days 8-10 and
6-7 post-conception, 30 mg/kg body-weight on days 6-7 post-conception
and 10 mg/kg body-weight on days 4-5 post-conception. Foetuses were
examined on the eighteenth day of pregnancy. 50 mg/kg had no
deleterious effect on development or foetal survival on days 8-10, but
retarded development and caused foetal death on days 6-7. Similar
effects occurred with 30 mg/kg and 10 mg/kg, including subnormal, as
well as retarded development in almost all foetuses (Tanaka, 1964).
Long-term studies
Rat. Groups of 7-10 male and 7-9 female rats were fed 0, 0.01,
0.1, 0.25, 0.5 and 1.0 per cent. 4-ethoxyphenylurea in their diet for
up to 2 years. Survival and growth rate were adversely and
significantly affected in the 0.5 and 1.0 per cent. groups. The
weights of liver, kidney and spleen were significantly raised at the
0.5 and 1.0 per cent. levels. Histopathology showed liver tumours,
some benign some malignant, at the 0.1 per cent. and higher levels.
Splenic enlargement also occurred at the 0.1 per cent. level with
congestion, hyperplasia and haemosiderosis. Pigmentation of the renal
proximal convoluted tubules and anaemia were noted at the 0.5 and 1.0
per cent. levels (Fitzhugh et al., 1951). In another experiment,
groups of 15 young and adult rats were given 0.2 g 4-ethoxyphenylurea
per animal daily for up to 22 months. No malignant tumours were noted
(Lettre & Wrba, 1955). In another experiment, 20 male and 30 female
rats were given 0.2 g 4-ethoxyphenylurea/kg body-weight
intragastrically daily for 13 months without any adverse effects being
noted on organ weight or tumour incidence; but 60 per cent. of the
animals died during this time. No deleterious effects were noted on
fertility, litter size and development of pups. Thirty-nine males of
the F1 generation were kept on 0.2 g 4-ethoxyphenylurea/kg daily for
22 months without any noticeable effect on weight gain, incidence of
malignant tumours or mortality (Bekemeier et al., 1958). In another
experiment groups of 30 rats were fed 0 or 1.0 per cent.
4-ethoxyphenylurea in the diet for 21-24 months. Over 75 per cent. of
test animals developed tumours (1/3 malignant) of the urinary tract,
66 per cent. had also stones of the renal pelvis and bladder.
Splenomegaly and haemosiderosis of the spleen were also noted
(Griepentrog, 1959).
Observations
Up to 0.6 g daily in 4 male and 7 female volunteers and 0.1 g
daily for 14 days in 30 volunteers produced no adverse effects and no
evidence of p-aminophenol in the urine (Rost & Braun, 1926). Two
deaths, accompanied by abdominal pain, vomiting, coma and fits, have
been reported in children after an intake of 8-10 g. Doses of 20-35 g
taken by adults produced dizziness, nausea, methaemoglobinuria with
cyanosis, hypotension, dyspnoea, paraesthesiae, and coronary
disturbance in one case (Buhr, 1948).
Five volunteers received approximately 0.11 9 4-ethoxyphenylurea
daily for 41 weeks without any apparent ill effects; 1.5 g/day for 3
weeks is harmless to man, apart from slight temperature lowering.
Diabetics have received 4-ethoxyphenylurea for 1 year without
deleterious effects (Roest & Braun, 1926).
Comments
Biochemical studies show that 4-ethoxyphenylurea is rapidly
absorbed and slowly excreted. In the rabbit the greater amount is
excreted in the urine as metabolites, of which the principal one is
the de-ethylated 4-ethoxyphenylurea, p-hydroxyphenylurea. Early
human studies indicated that approximately 0.1 g daily had no
deleterious effects; however, long-term studies in rats revealed
tumour production at relatively low dosage levels. In one study
dietary levels of 0.1-1.0 per cent. 4-ethoxyphenylurea produced liver
tumours, and in another a level of 1.0 per cent. produced malignant
bladder lesions and urinary stones. Other studies at 0.2 and 0.3 per
cent. levels did not confirm either observation. Therefore long-term
animal studies are inconclusive.
EVALUATION
The data are not satisfactory for the evaluation of
4-ethoxyphenylurea as an artificial sweetener in foods. Although the
substance has been used by humans without any observed deleterious
effect, animal studies indicate that 4-ethoxyphenylurea may possess
tumourigenic properties. Until satisfactory studies are completed to
show that 4-ethoxyphenylurea is safe this substance should not be used
as a food additive.
REFERENCES
Akigi, M. & Aoki, I. (1962) Chem. Pharm. Bull., 10, 200
Akagi, M., Oketani, Y. & Vematsu, T. (1965) Chem. Pharm. Bull.,
13, 1200
Akagi, M., Aoki, I. & Vematsu, T. (1966) Chem. Pharm. Bull., 14, 1
Bekemeier, H., Hannig, E. & Pfennigsdorf, G. (1958) Arzneim.-
Forsch., 8, 150
Buhr, G. (1948) Med. Klinik, 43, 105
Fitzhugh, O. G., Nelson, A. A. & Frawley, J. P. (1951) J. Amer.
Pharm. Assoc., 60, 583
Griepentrog, F. (1959) Arzneim. Forseh., 9, 123
Kojima, S., Ichibagase, H. & Iguchi, S. (1966) Chem. Pharm. Bull.,
14, 965
Lettre, H. & Wrba, H. (1955) Naturwiss., 42, 217
Rost, R. & Braun, A. (1926) Arb. Reichsg. Amter, 57, 212
Tanaka, R. (1964) Jap. J. Public Hlth, 11/14, 909
Flury, F. Abderhaldens Handbuch 4, 7b, 1345
Molecular weight 180.21
Description Colourless or white crystals, or a
white crystalline powder, which is
odourless and has a very sweet taste
which is appreciable even after a
3000-fold dilution.
Biological Data
Biochemical aspects
Early experiments indicated p-aminophenol as a metabolite in
human urine after oral intakes at 1 g of 4-ethoxyphenylurea (Rost &
Braun, 1926). More recent studies in rabbits and rats, given oral or
intragastric: 4-ethoxyphenylurea at the rate of 500 mg/kg body-weight,
showed rapid absorption into the blood within 3 hours and slow
disappearance from the body. Three per cent. was excreted in the urine
in 48 hours, none appeared in the faeces, the rest being metabolized
slowly. Most tissues except fat contain 4-ethoxyphenylurea which
disappears slowly (Akagi & Aoki, 1962; Akagi et al., 1965).
Twenty-three per cent. 4-ethoxyphenylurea is absorbed from rat stomach
within 1 hour end over 80 per cent. absorbed from rat small intestine
within 2 hours, probably by simple diffusion (Kojima et al., 1966).
In the rabbit 4D per cent. of orally administered
4-ethoxyphenylurea is de-ethylated to p-hydroxyphenylurea as the
principal metabolite. This appears in the urine either as free
compound (7 per cent.) conjugated as O-sulfate (23 per cent.) or as
O-glucuronide (11 per cent.). Three per cent. of 4-ethoxyphenylurea ix
excreted unchanged as p-ethoxyphenylurea 27 per cent. as
N-glucuronide or p-ethoxyphenylurea, which appears to be the major
common pathway for disposal of arylureas, and a trace as
p-ethoxyphenylurea sulfamate. Small amounts of p-phenetidine,
p-aminophenol and urea have also been isolated (Akagi & Aoki, 1962;
Akagi et al., 1966).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Mouse oral 700-1000 Tanaka, 1964
Rat (young) oral 4900 ) Bekemeier et al., 1958
(adult) oral 3200 )
Dog oral 1000 (LD) Flury
Doses of 0.4-0.6 g/day caused ataxia, vomiting and weight loss in
dogs but O.1 g/day for 30 days had no adverse effects. Similar effects
were seen in monkeys, cats and guinea-pigs (Rost & Braun, 1926).
Teratogenicity studies
Mouse. Groups of 3 or 4 female mice were mated and given 50
mg/kg body-weight 4-ethoxyphenylurea intragastrically on days 8-10 and
6-7 post-conception, 30 mg/kg body-weight on days 6-7 post-conception
and 10 mg/kg body-weight on days 4-5 post-conception. Foetuses were
examined on the eighteenth day of pregnancy. 50 mg/kg had no
deleterious effect on development or foetal survival on days 8-10, but
retarded development and caused foetal death on days 6-7. Similar
effects occurred with 30 mg/kg and 10 mg/kg, including subnormal, as
well as retarded development in almost all foetuses (Tanaka, 1964).
Long-term studies
Rat. Groups of 7-10 male and 7-9 female rats were fed 0, 0.01,
0.1, 0.25, 0.5 and 1.0 per cent. 4-ethoxyphenylurea in their diet for
up to 2 years. Survival and growth rate were adversely and
significantly affected in the 0.5 and 1.0 per cent. groups. The
weights of liver, kidney and spleen were significantly raised at the
0.5 and 1.0 per cent. levels. Histopathology showed liver tumours,
some benign some malignant, at the 0.1 per cent. and higher levels.
Splenic enlargement also occurred at the 0.1 per cent. level with
congestion, hyperplasia and haemosiderosis. Pigmentation of the renal
proximal convoluted tubules and anaemia were noted at the 0.5 and 1.0
per cent. levels (Fitzhugh et al., 1951). In another experiment,
groups of 15 young and adult rats were given 0.2 g 4-ethoxyphenylurea
per animal daily for up to 22 months. No malignant tumours were noted
(Lettre & Wrba, 1955). In another experiment, 20 male and 30 female
rats were given 0.2 g 4-ethoxyphenylurea/kg body-weight
intragastrically daily for 13 months without any adverse effects being
noted on organ weight or tumour incidence; but 60 per cent. of the
animals died during this time. No deleterious effects were noted on
fertility, litter size and development of pups. Thirty-nine males of
the F1 generation were kept on 0.2 g 4-ethoxyphenylurea/kg daily for
22 months without any noticeable effect on weight gain, incidence of
malignant tumours or mortality (Bekemeier et al., 1958). In another
experiment groups of 30 rats were fed 0 or 1.0 per cent.
4-ethoxyphenylurea in the diet for 21-24 months. Over 75 per cent. of
test animals developed tumours (1/3 malignant) of the urinary tract,
66 per cent. had also stones of the renal pelvis and bladder.
Splenomegaly and haemosiderosis of the spleen were also noted
(Griepentrog, 1959).
Observations
Up to 0.6 g daily in 4 male and 7 female volunteers and 0.1 g
daily for 14 days in 30 volunteers produced no adverse effects and no
evidence of p-aminophenol in the urine (Rost & Braun, 1926). Two
deaths, accompanied by abdominal pain, vomiting, coma and fits, have
been reported in children after an intake of 8-10 g. Doses of 20-35 g
taken by adults produced dizziness, nausea, methaemoglobinuria with
cyanosis, hypotension, dyspnoea, paraesthesiae, and coronary
disturbance in one case (Buhr, 1948).
Five volunteers received approximately 0.11 9 4-ethoxyphenylurea
daily for 41 weeks without any apparent ill effects; 1.5 g/day for 3
weeks is harmless to man, apart from slight temperature lowering.
Diabetics have received 4-ethoxyphenylurea for 1 year without
deleterious effects (Roest & Braun, 1926).
Comments
Biochemical studies show that 4-ethoxyphenylurea is rapidly
absorbed and slowly excreted. In the rabbit the greater amount is
excreted in the urine as metabolites, of which the principal one is
the de-ethylated 4-ethoxyphenylurea, p-hydroxyphenylurea. Early
human studies indicated that approximately 0.1 g daily had no
deleterious effects; however, long-term studies in rats revealed
tumour production at relatively low dosage levels. In one study
dietary levels of 0.1-1.0 per cent. 4-ethoxyphenylurea produced liver
tumours, and in another a level of 1.0 per cent. produced malignant
bladder lesions and urinary stones. Other studies at 0.2 and 0.3 per
cent. levels did not confirm either observation. Therefore long-term
animal studies are inconclusive.
EVALUATION
The data are not satisfactory for the evaluation of
4-ethoxyphenylurea as an artificial sweetener in foods. Although the
substance has been used by humans without any observed deleterious
effect, animal studies indicate that 4-ethoxyphenylurea may possess
tumourigenic properties. Until satisfactory studies are completed to
show that 4-ethoxyphenylurea is safe this substance should not be used
as a food additive.
REFERENCES
Akigi, M. & Aoki, I. (1962) Chem. Pharm. Bull., 10, 200
Akagi, M., Oketani, Y. & Vematsu, T. (1965) Chem. Pharm. Bull.,
13, 1200
Akagi, M., Aoki, I. & Vematsu, T. (1966) Chem. Pharm. Bull., 14, 1
Bekemeier, H., Hannig, E. & Pfennigsdorf, G. (1958) Arzneim.-
Forsch., 8, 150
Buhr, G. (1948) Med. Klinik, 43, 105
Fitzhugh, O. G., Nelson, A. A. & Frawley, J. P. (1951) J. Amer.
Pharm. Assoc., 60, 583
Griepentrog, F. (1959) Arzneim. Forseh., 9, 123
Kojima, S., Ichibagase, H. & Iguchi, S. (1966) Chem. Pharm. Bull.,
14, 965
Lettre, H. & Wrba, H. (1955) Naturwiss., 42, 217
Rost, R. & Braun, A. (1926) Arb. Reichsg. Amter, 57, 212
Tanaka, R. (1964) Jap. J. Public Hlth, 11/14, 909
Flury, F. Abderhaldens Handbuch 4, 7b, 1345
