
FAO Nutrition Meetings
Resort Series No. 44A
WHO/Food Add./68.33
TOXICOLOGICAL EVALUATION OF SOME
FLAVOURING SUBSTANCES AND
NON-NUTRITIVE SWEETENING AGENTS
Geneva, 21-28 August 1967
The Eleventh Report of the Joint FAO/WHO Expert Committee on Food
Additives is published as FAO Nutrition Meetings Report Series,
1967, No. 44; Wld Hlth Org. techn. Rep. Ser., 1968, 383. This
Report contains general considerations, including the principles
adopted for the evaluation, and a summary of the results of the
evaluations of a number of food additives. Additional information,
such as biological data and a toxicological evaluation, considered at
that meeting, is to be found in this document.
Food and Agriculture Organization of the United Nations
World Health Organization
1967
SACCHARIN
Chemical name o-Benzosulfimide;
2,3-dihydro-3-oxobenzisosulfonazole
Empirical formula C7H5NO3S
Structural formula
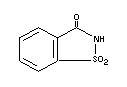 Molecular weight 183.19
Definition Saccharin contains not less than 98 per
cent C7H5NO3S after drying.
Description White crystals or a white, crystalline
powder, odourless, or with a faint,
aromatic odour
Use Non-nutritive sweetener
CALCIUM SACCHARIN
Chemical name Calcium o-Benzosulfimide; Calcium
salt of 2,3-dihydro-
3-oxobenzisosulfonazole
Empirical formula C14H8CaN2O6S2.3-1/2H2O
Structural formula
Molecular weight 183.19
Definition Saccharin contains not less than 98 per
cent C7H5NO3S after drying.
Description White crystals or a white, crystalline
powder, odourless, or with a faint,
aromatic odour
Use Non-nutritive sweetener
CALCIUM SACCHARIN
Chemical name Calcium o-Benzosulfimide; Calcium
salt of 2,3-dihydro-
3-oxobenzisosulfonazole
Empirical formula C14H8CaN2O6S2.3-1/2H2O
Structural formula
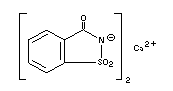 Molecular weight 467.49
Definition Calcium saccharin contains not less
than 95 per cent. C14H8CaN2O6S2
calculated on anhydrous basis.
Description White crystals or a white, crystalline
powder, which is odourless or has a
faint, aromatic, odour and an intensely
sweet taste even in dilute solutions.
Use Non-nutritive sweetener
SODIUM SACCHARIN
Chemical name Sodium o-Benzosulfamide; Sodium salt
of 2,3-dihydro-3-oxobenzisosulfonazole
Empirical formula C7H4NNaO3S.2H2O
Structural formula
Molecular weight 467.49
Definition Calcium saccharin contains not less
than 95 per cent. C14H8CaN2O6S2
calculated on anhydrous basis.
Description White crystals or a white, crystalline
powder, which is odourless or has a
faint, aromatic, odour and an intensely
sweet taste even in dilute solutions.
Use Non-nutritive sweetener
SODIUM SACCHARIN
Chemical name Sodium o-Benzosulfamide; Sodium salt
of 2,3-dihydro-3-oxobenzisosulfonazole
Empirical formula C7H4NNaO3S.2H2O
Structural formula
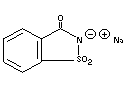 Molecular weight 241.20
Definition Sodium saccharin contains not less than
98 per cent. and not more than the
equivalent of 101 per cent.
C7H4NNaO3S (205.17) after drying.
Description White crystals or a white, crystalline,
efflorescent powder which is odourless
or has a faint, aromatic odour and an
intensely sweet taste, even in very
dilute solutions.
Use Non-nutritive sweetener
Biological Data
Biochemical aspects
Saccharin and saccharin sodium have been in use since the late
nineteenth century, the sodium salt being more soluble but of the same
sweetening power as the acid form.
Orally administered saccharin appears in the urine of man within
a half hour and is completely eliminated unchanged in 16-48 hours
(Staub & Staehelin, 1933), some 90 per cent. being excreted in the
urine in 24 hours (Folin & Herter, 1912). Intravenous saccharin sodium
in doses of 2.5 g has been used without adverse effect in sick and
well people to determine circulation time (Fisher et al., 1933). No
effect has been noted on human nitrogen balance or protein utilization
in man by doses up to 4 g/day. Daily doses of 5 g reduced albumin
absorption and utilization by 0.94 per cent. (Neunann, 1926). No
abnormal effects on total urinary nitrogen excretion or uric acid
output was noted by other workers after giving oral saccharin (Folin &
Herter, 1912). No deleterious effects on blood sugar, kidney function,
vitamin utilization, blood coagulation or enzyme activity has been
detected in man (NAS-NRC, 1955). Early observations of hypoglycaemia
induced by saccharin were confirmed in obese-hyperglycaemic mice by
feeding 125-175 mg/animal daily for 4 weeks. No effect was shown on
lean mice and adult rats. Intraperitoneal administration of 100 mg to
fed rats and 10 mg to fed mice caused hypoglycaemia, but no effect was
seen in fasted animals even after administration of 1 g (Thompson &
Mayer, 1959).
Intravenously administered saccharin sodium appears in the
thoracic and cervical lymph within a few minutes, is distributed
throughout all body fluids and appears in saliva and tears.
One gram of saccharin given in water to goats appeared in the
milk after 9 hours (Carlson et al., 1923). In vivo perfusion of rat
stomach and small intestine demonstrated considerable absorption from
the stomach at pH 1.0 and slow absorption from the small intestine,
i.e. <9 per cent., in two hours (Kojima et al., 1966).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Mouse i.p. 17 500 Tanaka, 1964
Rabbit oral 5000-8000(LD) Folin & Herter, 1912
Dog i.v. 2500(LD) Becht, 1920
Short-term studies
Dog. One male and one female dog received 150 mg/day of
saccharin in their food for 18 months without any adverse effects on
weight, fertility or other bodily functions. Their pups developed
normally (Bonjean, 1922).
If given doses of 175 mg to 350 mg/day for 100 days, dogs
developed hyperaemia of lungs, liver, myocardium and kidney as well as
cloudy swelling of renal glomeruli and convoluted tubules (De Nito,
1936).
Teratogenicity and fertility studies
Mouse. Groups of 21 pregnant mice received 42-168 mg/kg
body-weight/day of saccharin through the production of 3 successive
litters without deleterious effect on growth, litter number and pups
per litter when compared with controls fed sugar. No histological
studies were performed (Lehmann, 1929). Groups of 4 pregnant mice
received 125 mg/kg saccharin intragastrically on days 4-5 and 6-7 of
pregnancy, 250 mg/kg on days 4-5 and 6-7 of pregnancy and 500 mg/kg or
days 8-10 of pregnancy. Controls received saline. Abnormalities of the
foetus in terms of resorption or retarded development were seen at 125
mg/kg and 250 mg/kg but not at 500 mg/kg. The mean foetal LD50 was
calculated at 155 mg/kg (Tanaka, 1964).
Rat. Eighteen pregnant controls and 15 pregnant test rats
received either 0 or 6000 mg/kg/day of saccharin sodium from day 1 to
20 of pregnancy. Six of the controls and 6 test animals were killed on
the twenty-first day and no foetal abnormalities were noted. Litter
size, foetal mortality and foetal weight were unaffected. All test
animals showed some reduced weight gain during the first week and
gastric ulceration. The remaining 9 test and 12 control animals were
allowed to go on to parturition and to suckle their young for 21
days. Again there was no difference between controls and test animals
as regards litter size, percentage weaned and weaning weights (Bough
et al., 1967). In another experiment 2 groups of 10 males and 20
females were given 0 or 10 000 ppm of saccharin in the diet for 60
premating days. No effect was seen on libido or fertility. Litter size
at birth and number of pups weaned were comparable for both groups.
After 6 weeks without treatment the animals were remated, again
without difference in litter size and number of pups per litter (Bough
et al., 1967).
Rabbit. Oral doses of 0 and 600 mg/kg/day saccharin sodium were
given to Californian rabbits (8 test and 7 controls) from day 1-29 of
pregnancy, and the foetuses examined at day 30. The test mothers
showed reduced body-weight gain in the first week, and gastric
ulceration. There was no adverse effect on litter size, foetal
mortality or foetal weight (Bough et al., 1967).
Carcinogenicity studies
Saccharin and croton oil together were tested for dermal
cocarcinogenicity in mice. Although the treated group showed a greater
incidence of skin papillomata compared with controls, the difference
was not statistically significant (Salaman & Roe, 1956).
Paraffin wax pellets containing saccharin when implanted in the
mouse bladder induce a significant incidence of bladder tumours and
this is interpreted as demonstrating a cocarcinogenic effect (Allen et
al., 1957).
Long-term studies
Rat. Groups of 25 rats (5 males and 20 females) were fed diets
containing 1.0, or 10 per cent. saccharin for 36 weeks. Similar
groups of rats were fed diets containing 0, 0.1, or 1.0 per cent.
saccharin for a life time. One female from each group in the second
experiment was bred and 4 progeny from each litter were fed a diet
containing the same amount of saccharin as their parents for a life
time. Growth was retarded at the 10 per cent. level but no adverse
effects were seen at the lower levels on histological examination of
major organs (Fantus & Hektoen, 1923).
In another study, groups of 7-10 male and 9-10 male rats were fed
0, 0.01, 0.1, 0.5, 1.0 and 5.0 per cent. of saccharin in their diet
for 2 years. There was slight retardation of growth at the 5 per cent.
level. All other levels showed no deleterious effect on rate of weight
gain, mortality, haematology, organ weights or histology (Fitzhugh et
al., 1951). A further study on groups of 20 male and 20 female rats
fed 0, 0.005, 0.05, 0.5 and 5.0 per cent. saccharin in their diet for
2 years included a similar group given 1 ml of an aqueous 1 per cent.
solution of trypan blue s.c. every 2 weeks for 1 year as a positive
control. In the 5 per cent. group and the trypan blue group, mortality
was higher than in the controls. Mortality was lower than for the
controls in the 0.005 per cent. group. Retardation of growth was
observed in the males and females of the 5 per cent. group despite
greater food consumption. There was no difference in the incidence of
tumours between test and control groups (Lessel, 1967).
Observations in man
Doses of 1.5-3.0 g/day in man cause a persistent sweet metallic
taste (Carlson et al., 1923). Single doses of 5-10 g have been
tolerated and even 100 g orally is said to have caused no harm. A few
non-fatal cases of acute poisoning and allergic response have been
reported (NAS-NRC, 1955).
During high-intake balance studies, 3 male volunteers received
0.3 g/day of saccharin sodium for a maximum of 4 months and 1-1.5
g/day of saccharin sodium for a maximum of 2 months. All saccharin
administered was fully accounted for. Seven volunteers received from
0.15-0.3 g per day of saccharin for 1.3 months without adverse effects
except for increased urine output (Folin & Herter, 1912). 90-180
mg/day was well tolerated by children aged 10-12 years for 3 months
(Jessen, 1890). Diabetic patients have received as much as 4.8 g daily
for 5 months without adverse effect (Neumann, 1926), and 0.4-0.5 g/day
for 15-24 years without any adverse effects (NRC, 1955).
Comments
The extensive biochemical studies with saccharin and sodium
saccharin show the inertness of these substances. Following an oral
dose saccharin appears unchanged in the urine of man within a
half-hour and is completely excreted within 48 hours. The
long-recorded use by man without any apparent deleterious effects in
normal individuals and diabetic patients indicates the safety of the
normal intakes of saccharin. Although long-term animal studies are
limited to rats, two reports show no effects at dosage levels as high
as 1 per cent. and only slight growth retardation at 5 per cent. These
studies are adequate to rule out carcinogenicity. The cocarcinogenic
studies are limited to skin application and bladder implantation in
mice and lack significance in the oral use of saccharin for man.
Reports on studies in mice, rats and rabbits are adequate to show the
lack of any effect on fertility and progeny.
EVALUATION
Level causing no significant toxicological effects
Rat: 1 per cent. (10 000 ppm) in the diet, equivalent to 500
mg/kg body-weight/day.
Acceptable daily intake for man
mg/kg body-weight
Unconditional acceptance 0-5
Conditional acceptance1 5-15
1 For dietetic foods only
REFERENCES
Allen, M. J., Boyland, E., Dukes, C. E., Horning, E. S. & Watson, J.
G. (1957) Brit. J. Cancer, 11, 212
Becht, F. C. (1920) J. Pharmacol. exp. Therap., 16, 155
Bonjean, E. (1922) Rev. Hyg., 44, 50
Bough, R. G., Lessel, B., Sutton, M. M. & Williams, G. A. H.
Unpublished report submitted by Boots Pure Drug Co., 1967
Carlson, A. J., Eldridge, C. J., Martin, H. P. & Foran, F. L. (1923)
J Metab. Res., 3, 45
De Nito, G. (1936) Boll. Soc. ital. Biol. sper., 11, 934
Fantus, B. & Hektoen, L. (1923) J. Amer. Pharm Ass., Sci. Ed., 12,
318
Fishberg, A. M., Hitzig, W. M. & King, F. H. (1933) Proc. Soc. exp
Biol. Med., 30, 651
Fitzhugh, O. G., Nelson, A. A. & Frawley, J. P. (1951) J. Amer.
Pharm. Ass., Sci. Ed., 60, 583
Folin, O. & Herter, C. (1912) US Dept. Agric. Rep. No. 94
Jessen, F. (1890) Arch. f. Hyg., 10, 64
Kojima, S., Ichibagase, H. & Igudin, S. (1966) Chem. Pharm. Bull.,
14, 965
Kusaka, -. (1926) Folia Japon. Pharmakol., 2, 370
Lehmann, K. B. (1929) Arch. f. Hyg., 101, 39
Lessel, B. Unpublished report submitted by Boots Pure Drug Co., 1967
National Academy of Sciences - National Research Council (1955)
The safety of artificial sweeteners for use in food, Publication No.
386
Neumann, R. O. (1926) Arch. f. Hyg., 263; 97, 275
Salaman, M. H. & Roe, F. J. C. (1950) Brit. J. Cancer, 10, 363
Staub, H. & Staehelin, R. (1936) Med. Press Circ., 193, 2
Tanaka, R. (1964) J. Iwate med. Ass., 16, 330
Thompson, M. M. & Mayer, J. (1959) Amer. J. Clin. Nutr., 7, 80
Molecular weight 241.20
Definition Sodium saccharin contains not less than
98 per cent. and not more than the
equivalent of 101 per cent.
C7H4NNaO3S (205.17) after drying.
Description White crystals or a white, crystalline,
efflorescent powder which is odourless
or has a faint, aromatic odour and an
intensely sweet taste, even in very
dilute solutions.
Use Non-nutritive sweetener
Biological Data
Biochemical aspects
Saccharin and saccharin sodium have been in use since the late
nineteenth century, the sodium salt being more soluble but of the same
sweetening power as the acid form.
Orally administered saccharin appears in the urine of man within
a half hour and is completely eliminated unchanged in 16-48 hours
(Staub & Staehelin, 1933), some 90 per cent. being excreted in the
urine in 24 hours (Folin & Herter, 1912). Intravenous saccharin sodium
in doses of 2.5 g has been used without adverse effect in sick and
well people to determine circulation time (Fisher et al., 1933). No
effect has been noted on human nitrogen balance or protein utilization
in man by doses up to 4 g/day. Daily doses of 5 g reduced albumin
absorption and utilization by 0.94 per cent. (Neunann, 1926). No
abnormal effects on total urinary nitrogen excretion or uric acid
output was noted by other workers after giving oral saccharin (Folin &
Herter, 1912). No deleterious effects on blood sugar, kidney function,
vitamin utilization, blood coagulation or enzyme activity has been
detected in man (NAS-NRC, 1955). Early observations of hypoglycaemia
induced by saccharin were confirmed in obese-hyperglycaemic mice by
feeding 125-175 mg/animal daily for 4 weeks. No effect was shown on
lean mice and adult rats. Intraperitoneal administration of 100 mg to
fed rats and 10 mg to fed mice caused hypoglycaemia, but no effect was
seen in fasted animals even after administration of 1 g (Thompson &
Mayer, 1959).
Intravenously administered saccharin sodium appears in the
thoracic and cervical lymph within a few minutes, is distributed
throughout all body fluids and appears in saliva and tears.
One gram of saccharin given in water to goats appeared in the
milk after 9 hours (Carlson et al., 1923). In vivo perfusion of rat
stomach and small intestine demonstrated considerable absorption from
the stomach at pH 1.0 and slow absorption from the small intestine,
i.e. <9 per cent., in two hours (Kojima et al., 1966).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Mouse i.p. 17 500 Tanaka, 1964
Rabbit oral 5000-8000(LD) Folin & Herter, 1912
Dog i.v. 2500(LD) Becht, 1920
Short-term studies
Dog. One male and one female dog received 150 mg/day of
saccharin in their food for 18 months without any adverse effects on
weight, fertility or other bodily functions. Their pups developed
normally (Bonjean, 1922).
If given doses of 175 mg to 350 mg/day for 100 days, dogs
developed hyperaemia of lungs, liver, myocardium and kidney as well as
cloudy swelling of renal glomeruli and convoluted tubules (De Nito,
1936).
Teratogenicity and fertility studies
Mouse. Groups of 21 pregnant mice received 42-168 mg/kg
body-weight/day of saccharin through the production of 3 successive
litters without deleterious effect on growth, litter number and pups
per litter when compared with controls fed sugar. No histological
studies were performed (Lehmann, 1929). Groups of 4 pregnant mice
received 125 mg/kg saccharin intragastrically on days 4-5 and 6-7 of
pregnancy, 250 mg/kg on days 4-5 and 6-7 of pregnancy and 500 mg/kg or
days 8-10 of pregnancy. Controls received saline. Abnormalities of the
foetus in terms of resorption or retarded development were seen at 125
mg/kg and 250 mg/kg but not at 500 mg/kg. The mean foetal LD50 was
calculated at 155 mg/kg (Tanaka, 1964).
Rat. Eighteen pregnant controls and 15 pregnant test rats
received either 0 or 6000 mg/kg/day of saccharin sodium from day 1 to
20 of pregnancy. Six of the controls and 6 test animals were killed on
the twenty-first day and no foetal abnormalities were noted. Litter
size, foetal mortality and foetal weight were unaffected. All test
animals showed some reduced weight gain during the first week and
gastric ulceration. The remaining 9 test and 12 control animals were
allowed to go on to parturition and to suckle their young for 21
days. Again there was no difference between controls and test animals
as regards litter size, percentage weaned and weaning weights (Bough
et al., 1967). In another experiment 2 groups of 10 males and 20
females were given 0 or 10 000 ppm of saccharin in the diet for 60
premating days. No effect was seen on libido or fertility. Litter size
at birth and number of pups weaned were comparable for both groups.
After 6 weeks without treatment the animals were remated, again
without difference in litter size and number of pups per litter (Bough
et al., 1967).
Rabbit. Oral doses of 0 and 600 mg/kg/day saccharin sodium were
given to Californian rabbits (8 test and 7 controls) from day 1-29 of
pregnancy, and the foetuses examined at day 30. The test mothers
showed reduced body-weight gain in the first week, and gastric
ulceration. There was no adverse effect on litter size, foetal
mortality or foetal weight (Bough et al., 1967).
Carcinogenicity studies
Saccharin and croton oil together were tested for dermal
cocarcinogenicity in mice. Although the treated group showed a greater
incidence of skin papillomata compared with controls, the difference
was not statistically significant (Salaman & Roe, 1956).
Paraffin wax pellets containing saccharin when implanted in the
mouse bladder induce a significant incidence of bladder tumours and
this is interpreted as demonstrating a cocarcinogenic effect (Allen et
al., 1957).
Long-term studies
Rat. Groups of 25 rats (5 males and 20 females) were fed diets
containing 1.0, or 10 per cent. saccharin for 36 weeks. Similar
groups of rats were fed diets containing 0, 0.1, or 1.0 per cent.
saccharin for a life time. One female from each group in the second
experiment was bred and 4 progeny from each litter were fed a diet
containing the same amount of saccharin as their parents for a life
time. Growth was retarded at the 10 per cent. level but no adverse
effects were seen at the lower levels on histological examination of
major organs (Fantus & Hektoen, 1923).
In another study, groups of 7-10 male and 9-10 male rats were fed
0, 0.01, 0.1, 0.5, 1.0 and 5.0 per cent. of saccharin in their diet
for 2 years. There was slight retardation of growth at the 5 per cent.
level. All other levels showed no deleterious effect on rate of weight
gain, mortality, haematology, organ weights or histology (Fitzhugh et
al., 1951). A further study on groups of 20 male and 20 female rats
fed 0, 0.005, 0.05, 0.5 and 5.0 per cent. saccharin in their diet for
2 years included a similar group given 1 ml of an aqueous 1 per cent.
solution of trypan blue s.c. every 2 weeks for 1 year as a positive
control. In the 5 per cent. group and the trypan blue group, mortality
was higher than in the controls. Mortality was lower than for the
controls in the 0.005 per cent. group. Retardation of growth was
observed in the males and females of the 5 per cent. group despite
greater food consumption. There was no difference in the incidence of
tumours between test and control groups (Lessel, 1967).
Observations in man
Doses of 1.5-3.0 g/day in man cause a persistent sweet metallic
taste (Carlson et al., 1923). Single doses of 5-10 g have been
tolerated and even 100 g orally is said to have caused no harm. A few
non-fatal cases of acute poisoning and allergic response have been
reported (NAS-NRC, 1955).
During high-intake balance studies, 3 male volunteers received
0.3 g/day of saccharin sodium for a maximum of 4 months and 1-1.5
g/day of saccharin sodium for a maximum of 2 months. All saccharin
administered was fully accounted for. Seven volunteers received from
0.15-0.3 g per day of saccharin for 1.3 months without adverse effects
except for increased urine output (Folin & Herter, 1912). 90-180
mg/day was well tolerated by children aged 10-12 years for 3 months
(Jessen, 1890). Diabetic patients have received as much as 4.8 g daily
for 5 months without adverse effect (Neumann, 1926), and 0.4-0.5 g/day
for 15-24 years without any adverse effects (NRC, 1955).
Comments
The extensive biochemical studies with saccharin and sodium
saccharin show the inertness of these substances. Following an oral
dose saccharin appears unchanged in the urine of man within a
half-hour and is completely excreted within 48 hours. The
long-recorded use by man without any apparent deleterious effects in
normal individuals and diabetic patients indicates the safety of the
normal intakes of saccharin. Although long-term animal studies are
limited to rats, two reports show no effects at dosage levels as high
as 1 per cent. and only slight growth retardation at 5 per cent. These
studies are adequate to rule out carcinogenicity. The cocarcinogenic
studies are limited to skin application and bladder implantation in
mice and lack significance in the oral use of saccharin for man.
Reports on studies in mice, rats and rabbits are adequate to show the
lack of any effect on fertility and progeny.
EVALUATION
Level causing no significant toxicological effects
Rat: 1 per cent. (10 000 ppm) in the diet, equivalent to 500
mg/kg body-weight/day.
Acceptable daily intake for man
mg/kg body-weight
Unconditional acceptance 0-5
Conditional acceptance1 5-15
1 For dietetic foods only
REFERENCES
Allen, M. J., Boyland, E., Dukes, C. E., Horning, E. S. & Watson, J.
G. (1957) Brit. J. Cancer, 11, 212
Becht, F. C. (1920) J. Pharmacol. exp. Therap., 16, 155
Bonjean, E. (1922) Rev. Hyg., 44, 50
Bough, R. G., Lessel, B., Sutton, M. M. & Williams, G. A. H.
Unpublished report submitted by Boots Pure Drug Co., 1967
Carlson, A. J., Eldridge, C. J., Martin, H. P. & Foran, F. L. (1923)
J Metab. Res., 3, 45
De Nito, G. (1936) Boll. Soc. ital. Biol. sper., 11, 934
Fantus, B. & Hektoen, L. (1923) J. Amer. Pharm Ass., Sci. Ed., 12,
318
Fishberg, A. M., Hitzig, W. M. & King, F. H. (1933) Proc. Soc. exp
Biol. Med., 30, 651
Fitzhugh, O. G., Nelson, A. A. & Frawley, J. P. (1951) J. Amer.
Pharm. Ass., Sci. Ed., 60, 583
Folin, O. & Herter, C. (1912) US Dept. Agric. Rep. No. 94
Jessen, F. (1890) Arch. f. Hyg., 10, 64
Kojima, S., Ichibagase, H. & Igudin, S. (1966) Chem. Pharm. Bull.,
14, 965
Kusaka, -. (1926) Folia Japon. Pharmakol., 2, 370
Lehmann, K. B. (1929) Arch. f. Hyg., 101, 39
Lessel, B. Unpublished report submitted by Boots Pure Drug Co., 1967
National Academy of Sciences - National Research Council (1955)
The safety of artificial sweeteners for use in food, Publication No.
386
Neumann, R. O. (1926) Arch. f. Hyg., 263; 97, 275
Salaman, M. H. & Roe, F. J. C. (1950) Brit. J. Cancer, 10, 363
Staub, H. & Staehelin, R. (1936) Med. Press Circ., 193, 2
Tanaka, R. (1964) J. Iwate med. Ass., 16, 330
Thompson, M. M. & Mayer, J. (1959) Amer. J. Clin. Nutr., 7, 80
 Molecular weight 183.19
Definition Saccharin contains not less than 98 per
cent C7H5NO3S after drying.
Description White crystals or a white, crystalline
powder, odourless, or with a faint,
aromatic odour
Use Non-nutritive sweetener
CALCIUM SACCHARIN
Chemical name Calcium o-Benzosulfimide; Calcium
salt of 2,3-dihydro-
3-oxobenzisosulfonazole
Empirical formula C14H8CaN2O6S2.3-1/2H2O
Structural formula
Molecular weight 183.19
Definition Saccharin contains not less than 98 per
cent C7H5NO3S after drying.
Description White crystals or a white, crystalline
powder, odourless, or with a faint,
aromatic odour
Use Non-nutritive sweetener
CALCIUM SACCHARIN
Chemical name Calcium o-Benzosulfimide; Calcium
salt of 2,3-dihydro-
3-oxobenzisosulfonazole
Empirical formula C14H8CaN2O6S2.3-1/2H2O
Structural formula
 Molecular weight 467.49
Definition Calcium saccharin contains not less
than 95 per cent. C14H8CaN2O6S2
calculated on anhydrous basis.
Description White crystals or a white, crystalline
powder, which is odourless or has a
faint, aromatic, odour and an intensely
sweet taste even in dilute solutions.
Use Non-nutritive sweetener
SODIUM SACCHARIN
Chemical name Sodium o-Benzosulfamide; Sodium salt
of 2,3-dihydro-3-oxobenzisosulfonazole
Empirical formula C7H4NNaO3S.2H2O
Structural formula
Molecular weight 467.49
Definition Calcium saccharin contains not less
than 95 per cent. C14H8CaN2O6S2
calculated on anhydrous basis.
Description White crystals or a white, crystalline
powder, which is odourless or has a
faint, aromatic, odour and an intensely
sweet taste even in dilute solutions.
Use Non-nutritive sweetener
SODIUM SACCHARIN
Chemical name Sodium o-Benzosulfamide; Sodium salt
of 2,3-dihydro-3-oxobenzisosulfonazole
Empirical formula C7H4NNaO3S.2H2O
Structural formula
 Molecular weight 241.20
Definition Sodium saccharin contains not less than
98 per cent. and not more than the
equivalent of 101 per cent.
C7H4NNaO3S (205.17) after drying.
Description White crystals or a white, crystalline,
efflorescent powder which is odourless
or has a faint, aromatic odour and an
intensely sweet taste, even in very
dilute solutions.
Use Non-nutritive sweetener
Biological Data
Biochemical aspects
Saccharin and saccharin sodium have been in use since the late
nineteenth century, the sodium salt being more soluble but of the same
sweetening power as the acid form.
Orally administered saccharin appears in the urine of man within
a half hour and is completely eliminated unchanged in 16-48 hours
(Staub & Staehelin, 1933), some 90 per cent. being excreted in the
urine in 24 hours (Folin & Herter, 1912). Intravenous saccharin sodium
in doses of 2.5 g has been used without adverse effect in sick and
well people to determine circulation time (Fisher et al., 1933). No
effect has been noted on human nitrogen balance or protein utilization
in man by doses up to 4 g/day. Daily doses of 5 g reduced albumin
absorption and utilization by 0.94 per cent. (Neunann, 1926). No
abnormal effects on total urinary nitrogen excretion or uric acid
output was noted by other workers after giving oral saccharin (Folin &
Herter, 1912). No deleterious effects on blood sugar, kidney function,
vitamin utilization, blood coagulation or enzyme activity has been
detected in man (NAS-NRC, 1955). Early observations of hypoglycaemia
induced by saccharin were confirmed in obese-hyperglycaemic mice by
feeding 125-175 mg/animal daily for 4 weeks. No effect was shown on
lean mice and adult rats. Intraperitoneal administration of 100 mg to
fed rats and 10 mg to fed mice caused hypoglycaemia, but no effect was
seen in fasted animals even after administration of 1 g (Thompson &
Mayer, 1959).
Intravenously administered saccharin sodium appears in the
thoracic and cervical lymph within a few minutes, is distributed
throughout all body fluids and appears in saliva and tears.
One gram of saccharin given in water to goats appeared in the
milk after 9 hours (Carlson et al., 1923). In vivo perfusion of rat
stomach and small intestine demonstrated considerable absorption from
the stomach at pH 1.0 and slow absorption from the small intestine,
i.e. <9 per cent., in two hours (Kojima et al., 1966).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Mouse i.p. 17 500 Tanaka, 1964
Rabbit oral 5000-8000(LD) Folin & Herter, 1912
Dog i.v. 2500(LD) Becht, 1920
Short-term studies
Dog. One male and one female dog received 150 mg/day of
saccharin in their food for 18 months without any adverse effects on
weight, fertility or other bodily functions. Their pups developed
normally (Bonjean, 1922).
If given doses of 175 mg to 350 mg/day for 100 days, dogs
developed hyperaemia of lungs, liver, myocardium and kidney as well as
cloudy swelling of renal glomeruli and convoluted tubules (De Nito,
1936).
Teratogenicity and fertility studies
Mouse. Groups of 21 pregnant mice received 42-168 mg/kg
body-weight/day of saccharin through the production of 3 successive
litters without deleterious effect on growth, litter number and pups
per litter when compared with controls fed sugar. No histological
studies were performed (Lehmann, 1929). Groups of 4 pregnant mice
received 125 mg/kg saccharin intragastrically on days 4-5 and 6-7 of
pregnancy, 250 mg/kg on days 4-5 and 6-7 of pregnancy and 500 mg/kg or
days 8-10 of pregnancy. Controls received saline. Abnormalities of the
foetus in terms of resorption or retarded development were seen at 125
mg/kg and 250 mg/kg but not at 500 mg/kg. The mean foetal LD50 was
calculated at 155 mg/kg (Tanaka, 1964).
Rat. Eighteen pregnant controls and 15 pregnant test rats
received either 0 or 6000 mg/kg/day of saccharin sodium from day 1 to
20 of pregnancy. Six of the controls and 6 test animals were killed on
the twenty-first day and no foetal abnormalities were noted. Litter
size, foetal mortality and foetal weight were unaffected. All test
animals showed some reduced weight gain during the first week and
gastric ulceration. The remaining 9 test and 12 control animals were
allowed to go on to parturition and to suckle their young for 21
days. Again there was no difference between controls and test animals
as regards litter size, percentage weaned and weaning weights (Bough
et al., 1967). In another experiment 2 groups of 10 males and 20
females were given 0 or 10 000 ppm of saccharin in the diet for 60
premating days. No effect was seen on libido or fertility. Litter size
at birth and number of pups weaned were comparable for both groups.
After 6 weeks without treatment the animals were remated, again
without difference in litter size and number of pups per litter (Bough
et al., 1967).
Rabbit. Oral doses of 0 and 600 mg/kg/day saccharin sodium were
given to Californian rabbits (8 test and 7 controls) from day 1-29 of
pregnancy, and the foetuses examined at day 30. The test mothers
showed reduced body-weight gain in the first week, and gastric
ulceration. There was no adverse effect on litter size, foetal
mortality or foetal weight (Bough et al., 1967).
Carcinogenicity studies
Saccharin and croton oil together were tested for dermal
cocarcinogenicity in mice. Although the treated group showed a greater
incidence of skin papillomata compared with controls, the difference
was not statistically significant (Salaman & Roe, 1956).
Paraffin wax pellets containing saccharin when implanted in the
mouse bladder induce a significant incidence of bladder tumours and
this is interpreted as demonstrating a cocarcinogenic effect (Allen et
al., 1957).
Long-term studies
Rat. Groups of 25 rats (5 males and 20 females) were fed diets
containing 1.0, or 10 per cent. saccharin for 36 weeks. Similar
groups of rats were fed diets containing 0, 0.1, or 1.0 per cent.
saccharin for a life time. One female from each group in the second
experiment was bred and 4 progeny from each litter were fed a diet
containing the same amount of saccharin as their parents for a life
time. Growth was retarded at the 10 per cent. level but no adverse
effects were seen at the lower levels on histological examination of
major organs (Fantus & Hektoen, 1923).
In another study, groups of 7-10 male and 9-10 male rats were fed
0, 0.01, 0.1, 0.5, 1.0 and 5.0 per cent. of saccharin in their diet
for 2 years. There was slight retardation of growth at the 5 per cent.
level. All other levels showed no deleterious effect on rate of weight
gain, mortality, haematology, organ weights or histology (Fitzhugh et
al., 1951). A further study on groups of 20 male and 20 female rats
fed 0, 0.005, 0.05, 0.5 and 5.0 per cent. saccharin in their diet for
2 years included a similar group given 1 ml of an aqueous 1 per cent.
solution of trypan blue s.c. every 2 weeks for 1 year as a positive
control. In the 5 per cent. group and the trypan blue group, mortality
was higher than in the controls. Mortality was lower than for the
controls in the 0.005 per cent. group. Retardation of growth was
observed in the males and females of the 5 per cent. group despite
greater food consumption. There was no difference in the incidence of
tumours between test and control groups (Lessel, 1967).
Observations in man
Doses of 1.5-3.0 g/day in man cause a persistent sweet metallic
taste (Carlson et al., 1923). Single doses of 5-10 g have been
tolerated and even 100 g orally is said to have caused no harm. A few
non-fatal cases of acute poisoning and allergic response have been
reported (NAS-NRC, 1955).
During high-intake balance studies, 3 male volunteers received
0.3 g/day of saccharin sodium for a maximum of 4 months and 1-1.5
g/day of saccharin sodium for a maximum of 2 months. All saccharin
administered was fully accounted for. Seven volunteers received from
0.15-0.3 g per day of saccharin for 1.3 months without adverse effects
except for increased urine output (Folin & Herter, 1912). 90-180
mg/day was well tolerated by children aged 10-12 years for 3 months
(Jessen, 1890). Diabetic patients have received as much as 4.8 g daily
for 5 months without adverse effect (Neumann, 1926), and 0.4-0.5 g/day
for 15-24 years without any adverse effects (NRC, 1955).
Comments
The extensive biochemical studies with saccharin and sodium
saccharin show the inertness of these substances. Following an oral
dose saccharin appears unchanged in the urine of man within a
half-hour and is completely excreted within 48 hours. The
long-recorded use by man without any apparent deleterious effects in
normal individuals and diabetic patients indicates the safety of the
normal intakes of saccharin. Although long-term animal studies are
limited to rats, two reports show no effects at dosage levels as high
as 1 per cent. and only slight growth retardation at 5 per cent. These
studies are adequate to rule out carcinogenicity. The cocarcinogenic
studies are limited to skin application and bladder implantation in
mice and lack significance in the oral use of saccharin for man.
Reports on studies in mice, rats and rabbits are adequate to show the
lack of any effect on fertility and progeny.
EVALUATION
Level causing no significant toxicological effects
Rat: 1 per cent. (10 000 ppm) in the diet, equivalent to 500
mg/kg body-weight/day.
Acceptable daily intake for man
mg/kg body-weight
Unconditional acceptance 0-5
Conditional acceptance1 5-15
1 For dietetic foods only
REFERENCES
Allen, M. J., Boyland, E., Dukes, C. E., Horning, E. S. & Watson, J.
G. (1957) Brit. J. Cancer, 11, 212
Becht, F. C. (1920) J. Pharmacol. exp. Therap., 16, 155
Bonjean, E. (1922) Rev. Hyg., 44, 50
Bough, R. G., Lessel, B., Sutton, M. M. & Williams, G. A. H.
Unpublished report submitted by Boots Pure Drug Co., 1967
Carlson, A. J., Eldridge, C. J., Martin, H. P. & Foran, F. L. (1923)
J Metab. Res., 3, 45
De Nito, G. (1936) Boll. Soc. ital. Biol. sper., 11, 934
Fantus, B. & Hektoen, L. (1923) J. Amer. Pharm Ass., Sci. Ed., 12,
318
Fishberg, A. M., Hitzig, W. M. & King, F. H. (1933) Proc. Soc. exp
Biol. Med., 30, 651
Fitzhugh, O. G., Nelson, A. A. & Frawley, J. P. (1951) J. Amer.
Pharm. Ass., Sci. Ed., 60, 583
Folin, O. & Herter, C. (1912) US Dept. Agric. Rep. No. 94
Jessen, F. (1890) Arch. f. Hyg., 10, 64
Kojima, S., Ichibagase, H. & Igudin, S. (1966) Chem. Pharm. Bull.,
14, 965
Kusaka, -. (1926) Folia Japon. Pharmakol., 2, 370
Lehmann, K. B. (1929) Arch. f. Hyg., 101, 39
Lessel, B. Unpublished report submitted by Boots Pure Drug Co., 1967
National Academy of Sciences - National Research Council (1955)
The safety of artificial sweeteners for use in food, Publication No.
386
Neumann, R. O. (1926) Arch. f. Hyg., 263; 97, 275
Salaman, M. H. & Roe, F. J. C. (1950) Brit. J. Cancer, 10, 363
Staub, H. & Staehelin, R. (1936) Med. Press Circ., 193, 2
Tanaka, R. (1964) J. Iwate med. Ass., 16, 330
Thompson, M. M. & Mayer, J. (1959) Amer. J. Clin. Nutr., 7, 80
Molecular weight 241.20
Definition Sodium saccharin contains not less than
98 per cent. and not more than the
equivalent of 101 per cent.
C7H4NNaO3S (205.17) after drying.
Description White crystals or a white, crystalline,
efflorescent powder which is odourless
or has a faint, aromatic odour and an
intensely sweet taste, even in very
dilute solutions.
Use Non-nutritive sweetener
Biological Data
Biochemical aspects
Saccharin and saccharin sodium have been in use since the late
nineteenth century, the sodium salt being more soluble but of the same
sweetening power as the acid form.
Orally administered saccharin appears in the urine of man within
a half hour and is completely eliminated unchanged in 16-48 hours
(Staub & Staehelin, 1933), some 90 per cent. being excreted in the
urine in 24 hours (Folin & Herter, 1912). Intravenous saccharin sodium
in doses of 2.5 g has been used without adverse effect in sick and
well people to determine circulation time (Fisher et al., 1933). No
effect has been noted on human nitrogen balance or protein utilization
in man by doses up to 4 g/day. Daily doses of 5 g reduced albumin
absorption and utilization by 0.94 per cent. (Neunann, 1926). No
abnormal effects on total urinary nitrogen excretion or uric acid
output was noted by other workers after giving oral saccharin (Folin &
Herter, 1912). No deleterious effects on blood sugar, kidney function,
vitamin utilization, blood coagulation or enzyme activity has been
detected in man (NAS-NRC, 1955). Early observations of hypoglycaemia
induced by saccharin were confirmed in obese-hyperglycaemic mice by
feeding 125-175 mg/animal daily for 4 weeks. No effect was shown on
lean mice and adult rats. Intraperitoneal administration of 100 mg to
fed rats and 10 mg to fed mice caused hypoglycaemia, but no effect was
seen in fasted animals even after administration of 1 g (Thompson &
Mayer, 1959).
Intravenously administered saccharin sodium appears in the
thoracic and cervical lymph within a few minutes, is distributed
throughout all body fluids and appears in saliva and tears.
One gram of saccharin given in water to goats appeared in the
milk after 9 hours (Carlson et al., 1923). In vivo perfusion of rat
stomach and small intestine demonstrated considerable absorption from
the stomach at pH 1.0 and slow absorption from the small intestine,
i.e. <9 per cent., in two hours (Kojima et al., 1966).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Mouse i.p. 17 500 Tanaka, 1964
Rabbit oral 5000-8000(LD) Folin & Herter, 1912
Dog i.v. 2500(LD) Becht, 1920
Short-term studies
Dog. One male and one female dog received 150 mg/day of
saccharin in their food for 18 months without any adverse effects on
weight, fertility or other bodily functions. Their pups developed
normally (Bonjean, 1922).
If given doses of 175 mg to 350 mg/day for 100 days, dogs
developed hyperaemia of lungs, liver, myocardium and kidney as well as
cloudy swelling of renal glomeruli and convoluted tubules (De Nito,
1936).
Teratogenicity and fertility studies
Mouse. Groups of 21 pregnant mice received 42-168 mg/kg
body-weight/day of saccharin through the production of 3 successive
litters without deleterious effect on growth, litter number and pups
per litter when compared with controls fed sugar. No histological
studies were performed (Lehmann, 1929). Groups of 4 pregnant mice
received 125 mg/kg saccharin intragastrically on days 4-5 and 6-7 of
pregnancy, 250 mg/kg on days 4-5 and 6-7 of pregnancy and 500 mg/kg or
days 8-10 of pregnancy. Controls received saline. Abnormalities of the
foetus in terms of resorption or retarded development were seen at 125
mg/kg and 250 mg/kg but not at 500 mg/kg. The mean foetal LD50 was
calculated at 155 mg/kg (Tanaka, 1964).
Rat. Eighteen pregnant controls and 15 pregnant test rats
received either 0 or 6000 mg/kg/day of saccharin sodium from day 1 to
20 of pregnancy. Six of the controls and 6 test animals were killed on
the twenty-first day and no foetal abnormalities were noted. Litter
size, foetal mortality and foetal weight were unaffected. All test
animals showed some reduced weight gain during the first week and
gastric ulceration. The remaining 9 test and 12 control animals were
allowed to go on to parturition and to suckle their young for 21
days. Again there was no difference between controls and test animals
as regards litter size, percentage weaned and weaning weights (Bough
et al., 1967). In another experiment 2 groups of 10 males and 20
females were given 0 or 10 000 ppm of saccharin in the diet for 60
premating days. No effect was seen on libido or fertility. Litter size
at birth and number of pups weaned were comparable for both groups.
After 6 weeks without treatment the animals were remated, again
without difference in litter size and number of pups per litter (Bough
et al., 1967).
Rabbit. Oral doses of 0 and 600 mg/kg/day saccharin sodium were
given to Californian rabbits (8 test and 7 controls) from day 1-29 of
pregnancy, and the foetuses examined at day 30. The test mothers
showed reduced body-weight gain in the first week, and gastric
ulceration. There was no adverse effect on litter size, foetal
mortality or foetal weight (Bough et al., 1967).
Carcinogenicity studies
Saccharin and croton oil together were tested for dermal
cocarcinogenicity in mice. Although the treated group showed a greater
incidence of skin papillomata compared with controls, the difference
was not statistically significant (Salaman & Roe, 1956).
Paraffin wax pellets containing saccharin when implanted in the
mouse bladder induce a significant incidence of bladder tumours and
this is interpreted as demonstrating a cocarcinogenic effect (Allen et
al., 1957).
Long-term studies
Rat. Groups of 25 rats (5 males and 20 females) were fed diets
containing 1.0, or 10 per cent. saccharin for 36 weeks. Similar
groups of rats were fed diets containing 0, 0.1, or 1.0 per cent.
saccharin for a life time. One female from each group in the second
experiment was bred and 4 progeny from each litter were fed a diet
containing the same amount of saccharin as their parents for a life
time. Growth was retarded at the 10 per cent. level but no adverse
effects were seen at the lower levels on histological examination of
major organs (Fantus & Hektoen, 1923).
In another study, groups of 7-10 male and 9-10 male rats were fed
0, 0.01, 0.1, 0.5, 1.0 and 5.0 per cent. of saccharin in their diet
for 2 years. There was slight retardation of growth at the 5 per cent.
level. All other levels showed no deleterious effect on rate of weight
gain, mortality, haematology, organ weights or histology (Fitzhugh et
al., 1951). A further study on groups of 20 male and 20 female rats
fed 0, 0.005, 0.05, 0.5 and 5.0 per cent. saccharin in their diet for
2 years included a similar group given 1 ml of an aqueous 1 per cent.
solution of trypan blue s.c. every 2 weeks for 1 year as a positive
control. In the 5 per cent. group and the trypan blue group, mortality
was higher than in the controls. Mortality was lower than for the
controls in the 0.005 per cent. group. Retardation of growth was
observed in the males and females of the 5 per cent. group despite
greater food consumption. There was no difference in the incidence of
tumours between test and control groups (Lessel, 1967).
Observations in man
Doses of 1.5-3.0 g/day in man cause a persistent sweet metallic
taste (Carlson et al., 1923). Single doses of 5-10 g have been
tolerated and even 100 g orally is said to have caused no harm. A few
non-fatal cases of acute poisoning and allergic response have been
reported (NAS-NRC, 1955).
During high-intake balance studies, 3 male volunteers received
0.3 g/day of saccharin sodium for a maximum of 4 months and 1-1.5
g/day of saccharin sodium for a maximum of 2 months. All saccharin
administered was fully accounted for. Seven volunteers received from
0.15-0.3 g per day of saccharin for 1.3 months without adverse effects
except for increased urine output (Folin & Herter, 1912). 90-180
mg/day was well tolerated by children aged 10-12 years for 3 months
(Jessen, 1890). Diabetic patients have received as much as 4.8 g daily
for 5 months without adverse effect (Neumann, 1926), and 0.4-0.5 g/day
for 15-24 years without any adverse effects (NRC, 1955).
Comments
The extensive biochemical studies with saccharin and sodium
saccharin show the inertness of these substances. Following an oral
dose saccharin appears unchanged in the urine of man within a
half-hour and is completely excreted within 48 hours. The
long-recorded use by man without any apparent deleterious effects in
normal individuals and diabetic patients indicates the safety of the
normal intakes of saccharin. Although long-term animal studies are
limited to rats, two reports show no effects at dosage levels as high
as 1 per cent. and only slight growth retardation at 5 per cent. These
studies are adequate to rule out carcinogenicity. The cocarcinogenic
studies are limited to skin application and bladder implantation in
mice and lack significance in the oral use of saccharin for man.
Reports on studies in mice, rats and rabbits are adequate to show the
lack of any effect on fertility and progeny.
EVALUATION
Level causing no significant toxicological effects
Rat: 1 per cent. (10 000 ppm) in the diet, equivalent to 500
mg/kg body-weight/day.
Acceptable daily intake for man
mg/kg body-weight
Unconditional acceptance 0-5
Conditional acceptance1 5-15
1 For dietetic foods only
REFERENCES
Allen, M. J., Boyland, E., Dukes, C. E., Horning, E. S. & Watson, J.
G. (1957) Brit. J. Cancer, 11, 212
Becht, F. C. (1920) J. Pharmacol. exp. Therap., 16, 155
Bonjean, E. (1922) Rev. Hyg., 44, 50
Bough, R. G., Lessel, B., Sutton, M. M. & Williams, G. A. H.
Unpublished report submitted by Boots Pure Drug Co., 1967
Carlson, A. J., Eldridge, C. J., Martin, H. P. & Foran, F. L. (1923)
J Metab. Res., 3, 45
De Nito, G. (1936) Boll. Soc. ital. Biol. sper., 11, 934
Fantus, B. & Hektoen, L. (1923) J. Amer. Pharm Ass., Sci. Ed., 12,
318
Fishberg, A. M., Hitzig, W. M. & King, F. H. (1933) Proc. Soc. exp
Biol. Med., 30, 651
Fitzhugh, O. G., Nelson, A. A. & Frawley, J. P. (1951) J. Amer.
Pharm. Ass., Sci. Ed., 60, 583
Folin, O. & Herter, C. (1912) US Dept. Agric. Rep. No. 94
Jessen, F. (1890) Arch. f. Hyg., 10, 64
Kojima, S., Ichibagase, H. & Igudin, S. (1966) Chem. Pharm. Bull.,
14, 965
Kusaka, -. (1926) Folia Japon. Pharmakol., 2, 370
Lehmann, K. B. (1929) Arch. f. Hyg., 101, 39
Lessel, B. Unpublished report submitted by Boots Pure Drug Co., 1967
National Academy of Sciences - National Research Council (1955)
The safety of artificial sweeteners for use in food, Publication No.
386
Neumann, R. O. (1926) Arch. f. Hyg., 263; 97, 275
Salaman, M. H. & Roe, F. J. C. (1950) Brit. J. Cancer, 10, 363
Staub, H. & Staehelin, R. (1936) Med. Press Circ., 193, 2
Tanaka, R. (1964) J. Iwate med. Ass., 16, 330
Thompson, M. M. & Mayer, J. (1959) Amer. J. Clin. Nutr., 7, 80
