INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
WORLD HEALTH ORGANIZATION
SAFETY EVALUATION OF CERTAIN FOOD
ADDITIVES AND CONTAMINANTS
WHO FOOD ADDITIVES SERIES: 44
Prepared by the Fifty-third meeting of the Joint FAO/WHO
Expert Committee on Food Additives (JECFA)
World Health Organization, Geneva, 2000
IPCS - International Programme on Chemical Safety
SAFETY EVAULATIONS OF GROUPS OF RELATED SUBSTANCES BY THE
PROCEDURE FOR THE SAFETY EVALUATION OF FLAVOURING AGENTS
INTRODUCTION
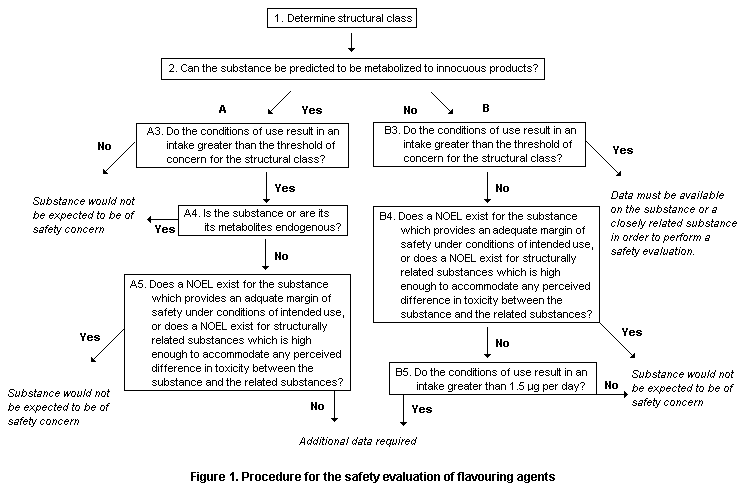
Two groups of flavouring agents were evaluated by the Procedure
for the Safety Evaluation of Flavouring Agents outlined in Figure 1
(Annex 1, references 116, 122, and 131).
The Committee noted that in applying the Procedure, the substance
is first assigned to one of the structural classes identified at the
forty-sixth meeting of the Committee (Annex 1, reference 122):
* Class I. Substances that have simple chemical structures and
efficient modes of metabolism that would suggest a low order of
toxicity when given by the oral route
* Class II. Substances that have structural features that are less
innocuous than those of substances in Class I but are not
suggestive of toxicity. Substances in this class may contain
reactive functional groups.
* Class III. Substances that have structural features that permit
no strong initial presumption of safety or may even suggest
significant toxicity.
A key element of the Procedure involves determining whether a
flavouring agent and the product(s) of its metabolism are innocuous
and/or endogenous. For the purposes of evaluation, the Committee used
the following definitions, adapted from the report of its forty-sixth
meeting:
Innocuous metabolic products are defined as products that are
known or readily predicted to be harmless to humans at the estimated
intake of the flavouring agent.
Endogenous substances are intermediary metabolites normally
present in human tissues and fluids, whether free or conjugated;
hormones and other substances with biochemical or physiological
regulatory functions are not included. The estimated intake of a
flavouring agent that is, or is metabolized to, an endogenous
substance should not give rise to perturbations outside the
physiological range.
Intake
Intakes were estimated from surveys carried out in Europe and the
United States. Estimates of the intake of flavouring agents by
populations typically involve the acquisition of data on the amounts
used in food. In Europe, a survey was conducted in 1995 by the
International Organization of the Flavor Industry, in which flavour
manufacturers reported the total amount of each flavouring agent
incorporated into food sold in the European Union during the previous
year. Manufacturers were requested to exclude use of flavouring agents
in pharmaceutical, tobacco, and cosmetic products. In the United
States, a series of surveys was conducted between 1970 and 1987 by the
National Academy of Sciences National Research Council (under contract
to the Food and Drug Administration) in which information was obtained
from ingredient manufacturers and food processers on the amount of
each substance destined for addition to the food supply and on the
usual and maximal levels at which each substance was added to a number
of broad food categories.
In using data from these surveys to estimate the intakes of
flavouring agents, it was assumed that only 60% of the total amount
used is reported and that the total amount used in food is consumed by
only 10% of the population.
Intake annual volume of production (kg) × 109 (µg/kg)
(µg/person =
per day) population of consumer × 0.6 × 365 days
The population of consumers was assumed to be 32 × 106 in Europe and
24 × 106 in the United States.