
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
WORLD HEALTH ORGANIZATION
SAFETY EVALUATION OF CERTAIN FOOD
ADDITIVES AND CONTAMINANTS
WHO FOOD ADDITIVES SERIES: 44
Prepared by the Fifty-third meeting of the Joint FAO/WHO
Expert Committee on Food Additives (JECFA)
World Health Organization, Geneva, 2000
IPCS - International Programme on Chemical Safety
SIMPLE ALIPHATIC AND AROMATIC SULFIDES AND THIOLS
First draft prepared by Dr A. Mattia1 and Professor A.G. Renwick2
1Division of Product Policy, Office of Premarket Approval, Center for
Food Safety and Applied Nutrition, US Food and Drug Administration,
Washington DC, United States; 2Clinical Pharmacology Group,
University of Southampton, Southampton, United Kingdom
Evaluation
Introduction
Estimated daily intake
Metabolic considerations
Application of the Procedure for the Safety Evaluation of
Flavouring Agents
Consideration of combined intakes
Conclusions
Relevant background information
Explanation
Additional considerations on intake
Biological data
Absorption, distribution, metabolism, and excretion
Simple sulfides (thioethers)
Acyclic sulfides with oxidized side-chains
Cyclic sulfides
Simple thiols
Thiols with oxidized side-chains
Dithiols
Simple disulfides
Disulfides with oxidized side-chains
Trisulfides
Heterocyclic disulfides
Thioesters
Sulfoxides
Toxicological studies
Acute toxicity
Short-term studies of toxicity
Ninety-day studies of toxicity
Long-term studies of toxicity and carcinogenicity
Genotoxicity
Developmental toxicity
Chemoprevention
Observations in humans
References
1. EVALUATION
1.1 Introduction
The Committee evaluated a group of 137 flavouring agents that
includes aliphatic and aromatic sulfides and thiols, with and without
an additional oxygenated functional group (see Table 1), using the
Procedure for the Safety Assessment of Flavouring Agents (Figure 1, p.
122). The Committee has not previously evaluated any member of the
group.
1.2 Estimated daily intake
The total annual volume of the 137 sulfides and thiols in this
group is approximately 6000 kg in Europe and 5300 kg in the United
States. Approximately 51% and 52%, respectively, of the total annual
volume in Europe and in the United States is accounted for by methyl
sulfide (No. 452). The estimated daily intake, modified for use to
calculate the intake of flavouring agents and for 'eaters only' (see
introduction to this section), of methyl sulfide is 10 µg/kg bw in
Europe and 9 µg/kg bw in the United States; the intake of the
remaining substances in this group is lower. The estimated modified
daily intake of methyl 3-methylthiopropionate (No. 472) in Europe and
of bis(methylthio)methane (No. 533) in the United States is 2 µg/kg
bw. The estimated modified intake of four substances in Europe,
3(methylthio)propional-dehyde (No. 466), ethyl 3-methyl-thiopropionate
(No. 476), methyl mercaptan (No. 508), and allyl disulfide (No. 572),
and two substances in the United States, benzenethiol (No. 525) and
3-methyl-1,2,4-trithiane (No. 574), is estimated to be 0.0003-0.2
µg/kg bw per day. The values for the intake of each substance in the
group in Europe and in the United States are reported in Table 1.
Sulfides and thiols have been detected in a variety of foods,
including onion, garlic, beer, cabbage, tea, and coffee. Of the
substances in this group, 106 have been reported to occur naturally in
foods. Quantitative data on the natural occurrence of 19 substances in
the group demonstrate that they are consumed predominantly from
traditional foods, with the exception of methyl 3-methylthiopropionate
(No. 472) and ethyl 3-methylthiopropionate (No. 476).
One-hundred-and-six of the 137 sulfur-containing substances in
this group of flavours have been detected as natural components of
traditional foods (Maarse et al., 1994; see Table 2). Quantitative
data on the natural occurrence of 19 of these substances have been
reported which indicate that the intake of 17 of them is predominantly
from food (i.e. a consumption ratio >1; Stofberg & Kirschman, 1985;
Stofberg & Grundschober, 1987). The exceptions are methyl
3-methylthiopropionate (No. 472) (consumption ratio = 0.0006 in Europe
and 0.1 in the United States) and ethyl 3-methylthiopropionate (No.
476) (consumption ratio = 0.05 in Europe and 1 in the United States)
suggesting that the intake of these two substances is greater from
their use as flavours than from their consumption in food.
1.3 Metabolic considerations
The large group of 137 flavouring agents considered at this
meeting was divided into 12 subgroups on the basis of the position of
the sulfur atom, to facilitate the assessment of their metabolism and
the data on toxicity. The subgroups are:
(i) simple sulfides (thioethers), in which the sulfur is located
between two unoxidized alkyl or aryl side-chains (Nos
452-455, 457-460, and 533);
(ii) acyclic sulfides with oxidized side-chains, in which an
alcohol, aldehyde, ketone, ester, carboxylic acid, or phenol
group is present (Nos 461-463, 465-481, 495-497, 500-503,
and 505);
(iii) cyclic sulfides (Nos 456, 464, 498, 499, 534, 543, 550, and
562);
(iv) simple thiols with unoxidized aliphatic or aromatic
side-chains (Nos 508-531);
(v) thiols with oxidized side chains, in which an alcohol,
aldehyde, ketone, ester, or carboxylic acid group is present
(Nos 544-549, 551-561, and 563);
(vi) dithiols (Nos 532 and 535-542);
(vii) simple disulfides (Nos 564-572 and 575-579);
(viii) disulfides with oxidized side chains (Nos 580 and 581);
(ix) trisulfides and polysulfides (Nos 582-588);
(x) heterocyclic disulfides (Nos 573 and 574);
(xi) thioesters (Nos 482-494, 504, and 506a and b); and
(xii) sulfoxides (No. 507).
All of the sulfur substances considered are of low relative
molecular mass and are sufficiently lipophilic to be absorbed from the
intestine. These flavouring agents would be metabolized through many
different pathways. As metabolism would usually result in increased
polarity and greater likelihood of excretion, they would not be
expected to accumulate in the body. Many substances, such as thiols
and disulfides, would be able to form disulfide bonds with endogenous
thiols. Disulfides formed with cysteine could be excreted in the urine
as the xenobiotic cysteine disulfide, whereas formation of disulfides
with endogenous macromolecules would delay elimination and could
result in effects such as enzyme inhibition.
Table 1. Summary of results of safety evaluations of aliphatic and aromatic sulfides and thiols
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Subgroup (i): Simple sulfides (thioethers)
Structural class I
Methyl sulfide 452 75-18-3 No Yes. A NOEL of 250 N/R No safety
mg/kg bw per day was concern
reported in a 14-week
study in rats treated
with methyl sulfide at
multiple doses.
 Methyl ethyl 453 624-89-5 No Yes. A NOEL of 250 mg/kg bw N/R No safety
sulfide per day was reported in concern
a 14-week study in rats
treated with methyl sulfide
at multiple doses.
Methyl ethyl 453 624-89-5 No Yes. A NOEL of 250 mg/kg bw N/R No safety
sulfide per day was reported in concern
a 14-week study in rats
treated with methyl sulfide
at multiple doses.
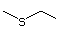 Diethyl sulfide 454 352-93-2 No Yes. A NOEL of 250 mg/kg bw N/R No safety
per day was reported in a concern
14-week study in rats treated
with methyl sulfide at multiple
doses.
Diethyl sulfide 454 352-93-2 No Yes. A NOEL of 250 mg/kg bw N/R No safety
per day was reported in a concern
14-week study in rats treated
with methyl sulfide at multiple
doses.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Butyl sulfide 455 544-40-1 No Yes. A NOEL of 250 mg/kg bw per N/R No safety
day was reported in a 14-week concern
study in rats treated with
methyl sulfide at multiple doses.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Butyl sulfide 455 544-40-1 No Yes. A NOEL of 250 mg/kg bw per N/R No safety
day was reported in a 14-week concern
study in rats treated with
methyl sulfide at multiple doses.
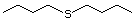 (1-Butenyl-1) 457 32951-19-2 No Yes. A NOEL of 250 mg/kg bw per N/R No safety
methyl sulfide day was reported in a 14-week concern
study in rats treated with methyl
sulfide at multiple doses.
(1-Butenyl-1) 457 32951-19-2 No Yes. A NOEL of 250 mg/kg bw per N/R No safety
methyl sulfide day was reported in a 14-week concern
study in rats treated with methyl
sulfide at multiple doses.
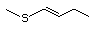 Bis(methylthio)methane 533 1618-26-4 No Yes. A NOEL of 250 mg/kg bw per N/R No safety
day was reported in a 14-week concern
study in rats treated with methyl
sulfide (No. 452) at multiple
doses
Bis(methylthio)methane 533 1618-26-4 No Yes. A NOEL of 250 mg/kg bw per N/R No safety
day was reported in a 14-week concern
study in rats treated with methyl
sulfide (No. 452) at multiple
doses
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Structural class II
Allyl sulfide 458 592-88-1 No No. Allyl mercaptan is not predicted Yes No safety
to be a metabolite of allyl sufide. concern
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Structural class II
Allyl sulfide 458 592-88-1 No No. Allyl mercaptan is not predicted Yes No safety
to be a metabolite of allyl sufide. concern
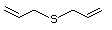 Methyl phenyl sulfide 459 100-68-5 No No Yes No safety
concern
Methyl phenyl sulfide 459 100-68-5 No No Yes No safety
concern
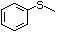 Benzyl methyl sulfide 460 766-92-7 No No Yes No safety
concern
Benzyl methyl sulfide 460 766-92-7 No No Yes No safety
concern
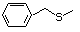 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Subgroup (ii): Acyclic sulfides with oxidized side-chains
Structural class I
3-(Methylthio)propanol 461 505-10-2 No Yes. A NOEL of 1.4 mg/kg bw per N/R No safety
day was reported in a 90-day syudy concern
in rats given
2-(methylthiomethyl)-3-phenyl-propenal
(No. 505) only at that dose. Data
for methyl sulfide (No. 452) are
relevant to compounds with a simple
oxidized side-chain.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Subgroup (ii): Acyclic sulfides with oxidized side-chains
Structural class I
3-(Methylthio)propanol 461 505-10-2 No Yes. A NOEL of 1.4 mg/kg bw per N/R No safety
day was reported in a 90-day syudy concern
in rats given
2-(methylthiomethyl)-3-phenyl-propenal
(No. 505) only at that dose. Data
for methyl sulfide (No. 452) are
relevant to compounds with a simple
oxidized side-chain.
 4-(Methylthio)butanol 462 999999-26-9 No Yes. A NOEL of 1.4 mg/kg bw was N/R No safety
in a reported 90-day syudy concern
in rats given
2-(methylthiomethyl)-3-phenyl-propenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
4-(Methylthio)butanol 462 999999-26-9 No Yes. A NOEL of 1.4 mg/kg bw was N/R No safety
in a reported 90-day syudy concern
in rats given
2-(methylthiomethyl)-3-phenyl-propenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
3-(Methylthio)-1-hexanol 463 51755-66-9 No Yes. A NOEL of 1.4 mg/kg bw was reported N/R No safety
in a 90-day syudy in rats given concern
2-(methylthiomethyl)-3-phenyl-propenal
(No. 505) only at that dose.
Data for methyl sulfide (No. 452) are
relevant to compounds with a simple
oxidized side-chain.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
3-(Methylthio)-1-hexanol 463 51755-66-9 No Yes. A NOEL of 1.4 mg/kg bw was reported N/R No safety
in a 90-day syudy in rats given concern
2-(methylthiomethyl)-3-phenyl-propenal
(No. 505) only at that dose.
Data for methyl sulfide (No. 452) are
relevant to compounds with a simple
oxidized side-chain.
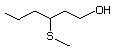 2-Methylthioacetaldehyde 465 23328-62-3 No Yes. A NOEL of 1.4 mg/kg bw was reported N/R No safety
in a 90-day syudy in rats given concern
2-(methylthiomethyl)-3-phenyl-propenal
(No. 505) only at that dose.
Data for methyl sulfide (No. 452) are
relevant to compounds with a simple
oxidized side-chain.
2-Methylthioacetaldehyde 465 23328-62-3 No Yes. A NOEL of 1.4 mg/kg bw was reported N/R No safety
in a 90-day syudy in rats given concern
2-(methylthiomethyl)-3-phenyl-propenal
(No. 505) only at that dose.
Data for methyl sulfide (No. 452) are
relevant to compounds with a simple
oxidized side-chain.
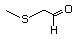 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
3-(Methylthio) 466 3268-49-3 No Yes. A NOEL of 1.4 mg/kg bw per day was N/R No safety
propionaldehyde reported in a 90-day study in rats concern
treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
3-(Methylthio) 466 3268-49-3 No Yes. A NOEL of 1.4 mg/kg bw per day was N/R No safety
propionaldehyde reported in a 90-day study in rats concern
treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
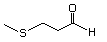 3-(Methylthio)butanal 467 16630-52-7 No Yes. A NOEL of 1.4 mg/kg bw per day was N/R No safety
reported in a 90-day study in rats concern
treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
3-(Methylthio)butanal 467 16630-52-7 No Yes. A NOEL of 1.4 mg/kg bw per day was N/R No safety
reported in a 90-day study in rats concern
treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
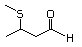 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
4-(Methylthio)butanal 468 42919-64-2 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
4-(Methylthio)butanal 468 42919-64-2 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
 3-Methylthiohexanal 469 38433-74-8 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
3-Methylthiohexanal 469 38433-74-8 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
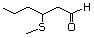 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
2-(Methylthio) 470 40878-72-6 No No No No safety
methyl-2-butenal concern
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
2-(Methylthio) 470 40878-72-6 No No No No safety
methyl-2-butenal concern
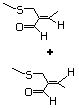 2,8-Dithianon-4-ene-4-carbox- 471 59902-01-1 No No No No safety
aldehyde concern
2,8-Dithianon-4-ene-4-carbox- 471 59902-01-1 No No No No safety
aldehyde concern
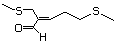 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Methyl 3-methylthiopropionate 472 13532-18-8 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain. The simple side-chain acid
and ester would be predicted to be of
low toxicity.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Methyl 3-methylthiopropionate 472 13532-18-8 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain. The simple side-chain acid
and ester would be predicted to be of
low toxicity.
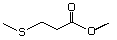 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
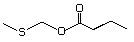
Methylthiomethyl butyrate 473 74758-93-3 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Methylthiomethyl butyrate 473 74758-93-3 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
 Methyl 4-(methylthio)butyrate 474 53053-51-3 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
Methyl 4-(methylthio)butyrate 474 53053-51-3 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
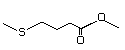 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Ethyl 2-(methylthio)acetate 475 4455-13-4 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Ethyl 2-(methylthio)acetate 475 4455-13-4 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
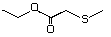 Ethyl 3-methylthiopropionate 476 133327-56-5 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
Ethyl 3-methylthiopropionate 476 133327-56-5 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
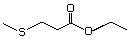 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Ethyl 4-(methylthio)butyrate 477 22014-48-8 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Ethyl 4-(methylthio)butyrate 477 22014-48-8 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
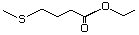 3-(Methylthio)propyl acetate 478 16630-55-0 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data
for methyl sulfide (No. 452) are
relevant to compounds with a simple
oxidized side-chain.
3-(Methylthio)propyl acetate 478 16630-55-0 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data
for methyl sulfide (No. 452) are
relevant to compounds with a simple
oxidized side-chain.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Methylthiomethyl hexanoate 479 74758-91-1 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Methylthiomethyl hexanoate 479 74758-91-1 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
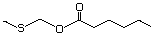 Ethyl 3-(methylthio)butyrate 480 Pending No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day syudy in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
Ethyl 3-(methylthio)butyrate 480 Pending No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day syudy in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
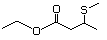 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
3-(Methylthio)hexyl acetate 481 51755-85-2 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
3-(Methylthio)hexyl acetate 481 51755-85-2 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
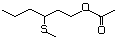 1-Methylthio-2-propanone 495 14109-72-9 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study concern
in rats given
2-(methylthiomethyl)-3-phenyl-propenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
1-Methylthio-2-propanone 495 14109-72-9 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study concern
in rats given
2-(methylthiomethyl)-3-phenyl-propenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
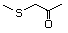 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
1-(Methylthio)-2-butanone 496 13678-58-5 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
1-(Methylthio)-2-butanone 496 13678-58-5 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
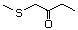 4-(Methylthio)-2-butanone 497 34047-39-7 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
4-(Methylthio)-2-butanone 497 34047-39-7 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
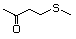 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
4-(Methylthio)-4-methyl-2- 500 23550-40-5 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
pentanone was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
4-(Methylthio)-4-methyl-2- 500 23550-40-5 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
pentanone was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
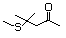 Di(butan-3-one-1-yl) sulfide 502 40790-04-3 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
Di(butan-3-one-1-yl) sulfide 502 40790-04-3 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
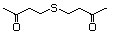 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Structural class II
ortho-(Methylthio)phenol 503 1073-29-6 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Structural class II
ortho-(Methylthio)phenol 503 1073-29-6 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
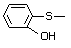 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Structural class III
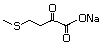
4-(Methylthio)-2-oxobutanoic 501 51828-97-8 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
acid, sodium salt was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Structural class III
4-(Methylthio)-2-oxobutanoic 501 51828-97-8 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
acid, sodium salt was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
 2-(Methylthiomethyl)-3-phenyl 505 65887-08-3 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
- propenal was reported in a 90-day study in concern
rats treated with this substance only
at that dose. Data for methyl sulfide
(No. 452) are relevant to compounds
with a simple oxidized side-chain.
2-(Methylthiomethyl)-3-phenyl 505 65887-08-3 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
- propenal was reported in a 90-day study in concern
rats treated with this substance only
at that dose. Data for methyl sulfide
(No. 452) are relevant to compounds
with a simple oxidized side-chain.
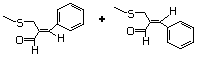 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Subgroup (iii): Cyclic sulfides
Structural class I
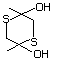
2,5-Dimethyl-2,5-dihydroxy-1,4- 562 55704-78-4 No Yes. A NOEL of 3.1 mg/kg bw per day N/R No safety
dithiane was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Subgroup (iii): Cyclic sulfides
Structural class I
2,5-Dimethyl-2,5-dihydroxy-1,4- 562 55704-78-4 No Yes. A NOEL of 3.1 mg/kg bw per day N/R No safety
dithiane was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
 2,5-Dihydroxy-1,4-dithiane 550 40018-26-6 No Yes. A NOEL of 3.1 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2,5-dimethyl-2,5-dihydroxy-1,4-dithiane
(No. 562) only at that dose.
2,5-Dihydroxy-1,4-dithiane 550 40018-26-6 No Yes. A NOEL of 3.1 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2,5-dimethyl-2,5-dihydroxy-1,4-dithiane
(No. 562) only at that dose.
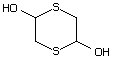 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Structural class II
2-Methyl-4-propyl-1,3-oxathiane 464 67715-80-4 No Yes. A NOEL of 0.44 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Structural class II
2-Methyl-4-propyl-1,3-oxathiane 464 67715-80-4 No Yes. A NOEL of 0.44 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
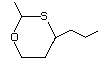 4,5-Dihydro-3-(2H)-thiophenone 498 1003-04-9 No Yes. A NOEL of 9.2 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
4,5-Dihydro-3-(2H)-thiophenone 498 1003-04-9 No Yes. A NOEL of 9.2 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
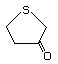 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
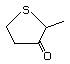
2-Methyltetrahydrothiophen-3-one 499 13679-85-1 No Yes. A NOEL of 9.2 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
4,5-dihydro-3-(2H)-thiophenone (No. 498)
only at that dose.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
2-Methyltetrahydrothiophen-3-one 499 13679-85-1 No Yes. A NOEL of 9.2 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
4,5-dihydro-3-(2H)-thiophenone (No. 498)
only at that dose.
 1,4-Dithiane 456 505-29-3 No Yes. NOELs of 0.44, 7, and 0.2 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-methyl-4-propyl-1,3-oxathiane
(No. 464), 2-methyl-1,3-dithiolane
(No. 534), and tri-thioacetone
(No. 543), respectively, only at those
doses.
1,4-Dithiane 456 505-29-3 No Yes. NOELs of 0.44, 7, and 0.2 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-methyl-4-propyl-1,3-oxathiane
(No. 464), 2-methyl-1,3-dithiolane
(No. 534), and tri-thioacetone
(No. 543), respectively, only at those
doses.
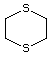 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
2-Methyl-1,3-dithiolane 534 5616-51-3 No Yes. A NOEL of 7 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
2-Methyl-1,3-dithiolane 534 5616-51-3 No Yes. A NOEL of 7 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
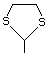 Trithioacetone 543 828-26-2 No Yes. A NOEL of 0.2 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
Trithioacetone 543 828-26-2 No Yes. A NOEL of 0.2 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
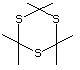 Subgroup (iv): Thiols
Structural class I
Methyl mercaptan 508 74-93-1 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
Subgroup (iv): Thiols
Structural class I
Methyl mercaptan 508 74-93-1 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
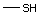 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Propanethiol 509 107-03-9 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Propanethiol 509 107-03-9 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
 2-Propanethiol 510 75-33-2 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
2-Propanethiol 510 75-33-2 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
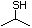 1-Butanethiol 511 109-79-5 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
1-Butanethiol 511 109-79-5 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
2-Methyl-1-propanethiol 512 513-44-0 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
2-Methyl-1-propanethiol 512 513-44-0 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
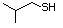 3-Methylbutanethiol 513 541-31-1 No Yes. A NOEL of 0.56 mg/kg bw per day NR No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
3-Methylbutanethiol 513 541-31-1 No Yes. A NOEL of 0.56 mg/kg bw per day NR No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
 2-Pentanethiol 514 2084-19-7 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
2-Pentanethiol 514 2084-19-7 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
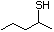 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
2-Methyl-1-butanethiol 515 1878-18-8 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
2-Methyl-1-butanethiol 515 1878-18-8 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
 3-Methyl-2-butanethiol 517 2084-18-6 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
3-Methyl-2-butanethiol 517 2084-18-6 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
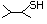 1-Hexanethiol 518 111-31-9 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
1-Hexanethiol 518 111-31-9 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
32-Ethylhexanethiol 519 7341-17-5 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
32-Ethylhexanethiol 519 7341-17-5 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
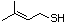 Prenythiol 522 5287-45-6 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose; see diallyl
trisulfide (No. 587, subgroup ix), which
is predicted to be metabolized to allyl
disulfide and allyl thiol.
Prenythiol 522 5287-45-6 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose; see diallyl
trisulfide (No. 587, subgroup ix), which
is predicted to be metabolized to allyl
disulfide and allyl thiol.
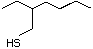 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
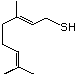
Thiogeraniol 524 39067-80-6 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose; see
diallyl trisulfide (No. 587, subgroup
ix), which is predicted to be
metabolized to allyl disulfide and
allyl thiol.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Thiogeraniol 524 39067-80-6 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose; see
diallyl trisulfide (No. 587, subgroup
ix), which is predicted to be
metabolized to allyl disulfide and
allyl thiol.
 Structural class II
Cyclopentanethiol 516 1679-07-8 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
Structural class II
Cyclopentanethiol 516 1679-07-8 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
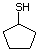 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
2,3- or 10-Mercaptopinane 520 23832-18-0 No Yes. A NOEL of 0.06 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose. NOELs of 0.56, 0.52, 0.43,
and 3.4 mg/kg bw per day were reported
in 90-day studies in rats treated with
cyclo-pentanethiol (No. 516),
ortho-toluenethiol (No. 528),
2,6-dimethylthiophenol (No. 530), and
2-naphthalenethiol (No. 531),
respectively, only at those doses.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
2,3- or 10-Mercaptopinane 520 23832-18-0 No Yes. A NOEL of 0.06 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose. NOELs of 0.56, 0.52, 0.43,
and 3.4 mg/kg bw per day were reported
in 90-day studies in rats treated with
cyclo-pentanethiol (No. 516),
ortho-toluenethiol (No. 528),
2,6-dimethylthiophenol (No. 530), and
2-naphthalenethiol (No. 531),
respectively, only at those doses.
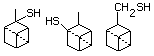 Allyl mercaptan 521 870-23-5 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
Allyl mercaptan 521 870-23-5 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
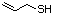 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
1-para-Menthene-8-thiol 523 71159-90-5 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
1-para-Menthene-8-thiol 523 71159-90-5 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
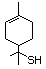 Benzenethiol 525 108-98-5 No Yes. NOELs of 0.52, 0.43, and 3.4 N/R No safety
mg/kg bw per day were reported in concern
90-day studies in rats treated with
ortho-toluenethiol (No. 528),
2,6-dimethyl-thiophenol (No. 530), and
2-naphthal-enethiol (No. 531),
respectively, only at those doses.
Benzenethiol 525 108-98-5 No Yes. NOELs of 0.52, 0.43, and 3.4 N/R No safety
mg/kg bw per day were reported in concern
90-day studies in rats treated with
ortho-toluenethiol (No. 528),
2,6-dimethyl-thiophenol (No. 530), and
2-naphthal-enethiol (No. 531),
respectively, only at those doses.
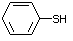 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Benzyl mercaptan 526 100-53-8 No Yes. NOELs of 0.52, 0.43, and 3.4 N/R No safety
mg/kg bw per day were reported in concern
90-day studies in rats treated with
ortho-toluenethiol (No. 528),
2,6-dimethyl-thiophenol (No. 530), and
2-naphthal-enethiol (No. 531),
respectively, only at those doses.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Benzyl mercaptan 526 100-53-8 No Yes. NOELs of 0.52, 0.43, and 3.4 N/R No safety
mg/kg bw per day were reported in concern
90-day studies in rats treated with
ortho-toluenethiol (No. 528),
2,6-dimethyl-thiophenol (No. 530), and
2-naphthal-enethiol (No. 531),
respectively, only at those doses.
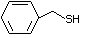 Phenethyl mercaptan 527 4410-99-5 No Yes. NOELs of 0.52, 0.43, and 3.4 N/R No safety
mg/kg bw per day were reported in concern
90-day studies in rats treated with
ortho-toluenethiol (No. 528),
2,6-dimethyl-thiophenol (No. 530), and
2-naphthal-enethiol (No. 531),
respectively, only at those doses.
Phenethyl mercaptan 527 4410-99-5 No Yes. NOELs of 0.52, 0.43, and 3.4 N/R No safety
mg/kg bw per day were reported in concern
90-day studies in rats treated with
ortho-toluenethiol (No. 528),
2,6-dimethyl-thiophenol (No. 530), and
2-naphthal-enethiol (No. 531),
respectively, only at those doses.
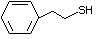 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
ortho-Toluenethiol 528 137-06-4 No Yes. A NOEL of 0.52 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
ortho-Toluenethiol 528 137-06-4 No Yes. A NOEL of 0.52 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
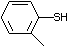 2,6-Dimethylthiophenol 530 118-72-9 No Yes. A NOEL of 0.43 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
2,6-Dimethylthiophenol 530 118-72-9 No Yes. A NOEL of 0.43 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
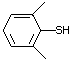 2-Naphthalenethiol 531 91-60-1 No Yes. A NOEL of 3.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with this substance
only at that dose.
2-Naphthalenethiol 531 91-60-1 No Yes. A NOEL of 3.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with this substance
only at that dose.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Structural class III
2-Ethylthiophenol 529 4500-58-7 No Yes. NOELs of 0.52, 0.43, and 3.4 N/R No safety
mg/kg bw per day were reported in concern
90-day studies in rats treated with
ortho-toluenethiol (No. 528),
2,6-dimethyl-thiophenol (No. 530),
and 2-naphthal-enethiol (No. 531),
respectively, only at those doses.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Structural class III
2-Ethylthiophenol 529 4500-58-7 No Yes. NOELs of 0.52, 0.43, and 3.4 N/R No safety
mg/kg bw per day were reported in concern
90-day studies in rats treated with
ortho-toluenethiol (No. 528),
2,6-dimethyl-thiophenol (No. 530),
and 2-naphthal-enethiol (No. 531),
respectively, only at those doses.
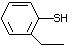 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Subgroup (v): Thiols with oxidized side-chains
Structural class I
2-Mercaptopropionic acid 551 79-42-5 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Subgroup (v): Thiols with oxidized side-chains
Structural class I
2-Mercaptopropionic acid 551 79-42-5 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
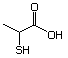 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
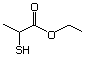
Ethyl 2-mercaptopropionate 552 19788-49-9 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Ethyl 2-mercaptopropionate 552 19788-49-9 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
 Ethyl 3-mercaptopropionate 553 5466-06-8 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
Ethyl 3-mercaptopropionate 553 5466-06-8 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
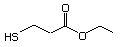 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
3-Mercaptohexyl acetate 554 136954-20-6 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
3-Mercaptohexyl acetate 554 136954-20-6 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
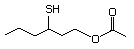 3-Mercaptohexyl butyrate 555 136954-21-7 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
3-Mercaptohexyl butyrate 555 136954-21-7 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
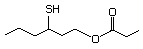 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
3-Mercaptohexyl hexanoate 556 136954-22-8 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
3-Mercaptohexyl hexanoate 556 136954-22-8 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
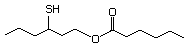 1-Mercapto-2-propanone 557 24653-75-6 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
1-Mercapto-2-propanone 557 24653-75-6 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
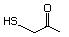 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
3-Mercapto-2-butanone 558 40789-98-8 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
3-Mercapto-2-butanone 558 40789-98-8 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
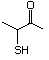 2-Keto-4-butanethiol 559 34619-12-0 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
2-Keto-4-butanethiol 559 34619-12-0 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
3-Mercapto-2-pentanone 560 67633-97-0 No Yes. A NOEL of 1.9 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
given this substance only at that dose.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
3-Mercapto-2-pentanone 560 67633-97-0 No Yes. A NOEL of 1.9 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
given this substance only at that dose.
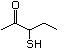 3-Mercapto-3-methyl-1-butanol 544 34300-94-2 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
3-Mercapto-3-methyl-1-butanol 544 34300-94-2 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
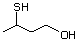 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
3-Mercaptohexanol 545 51755-83-0 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
3-Mercaptohexanol 545 51755-83-0 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
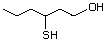 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
2-Mercapto-3-butanol 546 37887-04-0 No Yes. A NOEL of 1.9 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
given this substance only at that dose.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
2-Mercapto-3-butanol 546 37887-04-0 No Yes. A NOEL of 1.9 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
given this substance only at that dose.
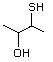 alpha-methyl-beta-hydroxypropyl 547 54957-02-7 No Yes. A NOEL of 2.8 mg/kg bw per day N/R No safety
alpha-methyl-beta-mercaptopropyl was reported in a 90-day study in rats concern
sulfide given this substance only at that dose.
alpha-methyl-beta-hydroxypropyl 547 54957-02-7 No Yes. A NOEL of 2.8 mg/kg bw per day N/R No safety
alpha-methyl-beta-mercaptopropyl was reported in a 90-day study in rats concern
sulfide given this substance only at that dose.
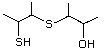 4-Methyoxy-2-methyl-2-butane- 548 94087-83-9 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
4-Methyoxy-2-methyl-2-butane- 548 94087-83-9 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
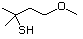 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
3-Methyl-3-mercaptobutyl formate 549 50746-10-6 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
3-Methyl-3-mercaptobutyl formate 549 50746-10-6 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
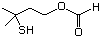 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Structural class II
para-Mentha-8-thiol-3-one 561 38462-22-5 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those doses.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Structural class II
para-Mentha-8-thiol-3-one 561 38462-22-5 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those doses.
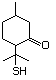 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Structural class III
Sodium 3-mercapto-oxo-propionate 563 10255-67-1 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses. See subgroup (i), No. 452 for the
sulfur moiety; the oxopropionate moiety
would be predicted to be of low
toxicity.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Structural class III
Sodium 3-mercapto-oxo-propionate 563 10255-67-1 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses. See subgroup (i), No. 452 for the
sulfur moiety; the oxopropionate moiety
would be predicted to be of low
toxicity.
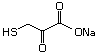 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Subgroup (vi): Dithiols
Structural class I
1,2-Ethanedithiol 532 540-63-6 No Yes. NOELs of 0.7 mg/kg bw per day N/R No safety
were reported in 90-day studies in rats concern
treated with 2,3-butanedithiol
(No. 539) and 1,8-octanedithiol
(No. 541) only at that dose.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Subgroup (vi): Dithiols
Structural class I
1,2-Ethanedithiol 532 540-63-6 No Yes. NOELs of 0.7 mg/kg bw per day N/R No safety
were reported in 90-day studies in rats concern
treated with 2,3-butanedithiol
(No. 539) and 1,8-octanedithiol
(No. 541) only at that dose.
 1,3-Propanedithiol 535 109-80-8 No Yes. NOELs of 0.7 mg/kg bw per day N/R No safety
were reported in 90-day studies in rats concern
treated with 2,3-butanedithiol (No. 539)
and 1,8-octanedithiol (No. 541) only at
that dose.
1,3-Propanedithiol 535 109-80-8 No Yes. NOELs of 0.7 mg/kg bw per day N/R No safety
were reported in 90-day studies in rats concern
treated with 2,3-butanedithiol (No. 539)
and 1,8-octanedithiol (No. 541) only at
that dose.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
1,2-Propanedithiol 536 814-67-5 No Yes. NOELs of 0.7 mg/kg bw per day N/R No safety
were reported in 90-day studies in rats concern
treated with 2,3-butanedithiol (No. 539)
and 1,8-octanedithiol (No. 541) only at
that dose.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
1,2-Propanedithiol 536 814-67-5 No Yes. NOELs of 0.7 mg/kg bw per day N/R No safety
were reported in 90-day studies in rats concern
treated with 2,3-butanedithiol (No. 539)
and 1,8-octanedithiol (No. 541) only at
that dose.
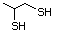 1,2-Butanedithiol 537 16128-68-0 No Yes. NOELs of 0.7 mg/kg bw per day N/R No safety
were reported in 90-day studies in rats concern
treated with 2,3-butanedithiol (No. 539)
and 1,8-octanedithiol (No. 541) only at
that dose.
1,2-Butanedithiol 537 16128-68-0 No Yes. NOELs of 0.7 mg/kg bw per day N/R No safety
were reported in 90-day studies in rats concern
treated with 2,3-butanedithiol (No. 539)
and 1,8-octanedithiol (No. 541) only at
that dose.
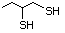 1,3-Butanedithiol 538 24330-52-7 No Yes. NOELs of 0.7 mg/kg bw per day N/R No safety
were reported in 90-day studies in rats concern
treated with 2,3-butanedithiol (No. 539)
and 1,8-octanedithiol (No. 541) only at
that dose.
1,3-Butanedithiol 538 24330-52-7 No Yes. NOELs of 0.7 mg/kg bw per day N/R No safety
were reported in 90-day studies in rats concern
treated with 2,3-butanedithiol (No. 539)
and 1,8-octanedithiol (No. 541) only at
that dose.
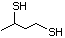 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
2,3-Butanedithiol 539 4532-64-3 No Yes. A NOEL of 0.7 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
given this substance only at that dose.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
2,3-Butanedithiol 539 4532-64-3 No Yes. A NOEL of 0.7 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
given this substance only at that dose.
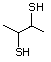 1,6-Hexanedithiol 540 1191-43-1 No Yes. NOELs of 0.7 mg/kg bw per day N/R No safety
were reported in 90-day studies in rats concern
treated with 2,3-butanedithiol (No. 539)
and 1,8-octanedithiol (No. 541) only at
that dose.
1,6-Hexanedithiol 540 1191-43-1 No Yes. NOELs of 0.7 mg/kg bw per day N/R No safety
were reported in 90-day studies in rats concern
treated with 2,3-butanedithiol (No. 539)
and 1,8-octanedithiol (No. 541) only at
that dose.
 1,8-Octanedithiol 541 1191-62-4 No Yes. A NOEL of 0.7 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
given this substance only at that dose.
1,8-Octanedithiol 541 1191-62-4 No Yes. A NOEL of 0.7 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
given this substance only at that dose.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
1,9-Nonanedithiol 542 3489-28-9 No Yes. NOELs of 0.7 mg/kg bw per day N/R No safety
were reported in 90-day studies in rats concern
treated with 2,3-butanedithiol (No. 539)
and 1,8-octanedithiol (No. 541) only at
that dose.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
1,9-Nonanedithiol 542 3489-28-9 No Yes. NOELs of 0.7 mg/kg bw per day N/R No safety
were reported in 90-day studies in rats concern
treated with 2,3-butanedithiol (No. 539)
and 1,8-octanedithiol (No. 541) only at
that dose.
 Subgroup (vii): Simple disulfides
Structural class I
Dimethyl disulfide 564 624-92-0 No Yes. A NOEL of 7.3 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with propyl disulfide (No. 566)
at two doses.
Subgroup (vii): Simple disulfides
Structural class I
Dimethyl disulfide 564 624-92-0 No Yes. A NOEL of 7.3 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with propyl disulfide (No. 566)
at two doses.
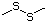 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Methyl propyl disulfide 565 2179-60-4 No Yes. A NOEL of 7.3 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with propyl disulfide (No. 566)
at two doses.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Methyl propyl disulfide 565 2179-60-4 No Yes. A NOEL of 7.3 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with propyl disulfide (No. 566)
at two doses.
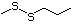 Propyl disulfide 566 629-19-6 No Yes. A NOEL of 7.3 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance at two
doses.
Propyl disulfide 566 629-19-6 No Yes. A NOEL of 7.3 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance at two
doses.
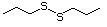 Diisopropyl disulfide 567 4253-89-8 No Yes. NOELs of 7.3 and 0.23 mg/kg bw N/R No safety
per day were reported in 90-day studies concern
in rats treated with propyl disulfide
and dicyclohexyl disulfide at two and
single doses, respectively.
Diisopropyl disulfide 567 4253-89-8 No Yes. NOELs of 7.3 and 0.23 mg/kg bw N/R No safety
per day were reported in 90-day studies concern
in rats treated with propyl disulfide
and dicyclohexyl disulfide at two and
single doses, respectively.
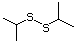 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Methyl 1-propenyl disulfide 569 5905-47-5 No Yes. A NOEL of 7.3 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with propyl disulfide (No. 566)
at two doses.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Methyl 1-propenyl disulfide 569 5905-47-5 No Yes. A NOEL of 7.3 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with propyl disulfide (No. 566)
at two doses.
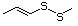 Propenyl propyl disulfide 570 5905-46-4 - Yes. A NOEL of 7.3 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with propyl disulfide (No. 566)
at two doses.
Propenyl propyl disulfide 570 5905-46-4 - Yes. A NOEL of 7.3 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with propyl disulfide (No. 566)
at two doses.
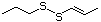 Methyl 3-methyl-1-butenyl 571 Pending - Yes. A NOEL of 7.3 mg/kg bw per day N/R No safety
disulfide was reported in a 90-day study in rats concern
treated with propyl disulfide (No. 566)
at two doses.
Methyl 3-methyl-1-butenyl 571 Pending - Yes. A NOEL of 7.3 mg/kg bw per day N/R No safety
disulfide was reported in a 90-day study in rats concern
treated with propyl disulfide (No. 566)
at two doses.
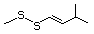 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Structural class II
Allyl methyl disulfide 568 2179-58-0 No Yes. A NOEL of 4.6 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with diallyl trisulfide
(No. 587, group ix) at a single dose for
90 days; this substance is predicted to
be metabolized to allyl mercaptan.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Structural class II
Allyl methyl disulfide 568 2179-58-0 No Yes. A NOEL of 4.6 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with diallyl trisulfide
(No. 587, group ix) at a single dose for
90 days; this substance is predicted to
be metabolized to allyl mercaptan.
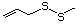 Allyl disulfide 572 2179-57-9 No Yes. A NOEL of 4.6 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with diallyl trisulfide
(No. 587, group ix) at a single dose for
90 days; this substance is predicted to
be metabolized to allyl mercaptan.
Allyl disulfide 572 2179-57-9 No Yes. A NOEL of 4.6 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with diallyl trisulfide
(No. 587, group ix) at a single dose for
90 days; this substance is predicted to
be metabolized to allyl mercaptan.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Dicyclohexyl disulfide 575 2550-40-5 No Yes. A NOEL of 0.23 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Dicyclohexyl disulfide 575 2550-40-5 No Yes. A NOEL of 0.23 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
 Methyl phenyl disulfide 576 14173-25-2 No Yes. A NOEL of 3.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with 2-naphthalenethiol
(No. 531, group iv) at a single dose for
90 days; this substance is predicted to
be reduced rapidly to thiophenol.
Methyl phenyl disulfide 576 14173-25-2 No Yes. A NOEL of 3.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with 2-naphthalenethiol
(No. 531, group iv) at a single dose for
90 days; this substance is predicted to
be reduced rapidly to thiophenol.
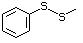 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Methyl benzyl disulfide 577 699-10-5 No Yes. A NOEL of 1.2 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Methyl benzyl disulfide 577 699-10-5 No Yes. A NOEL of 1.2 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
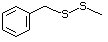 Benzyl disulfide 579 150-60-7 No Yes. A NOEL of 1.2 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with methyl benzyl disulfide
(No. 579) only at that dose
Benzyl disulfide 579 150-60-7 No Yes. A NOEL of 1.2 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with methyl benzyl disulfide
(No. 579) only at that dose
 Structural class III
Phenyl disulfide 578 882-33-7 No Yes. A NOEL of 3.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with 2-naphthalenethiol
(No. 531, group iv) at a single dose for
90 days; this substance is predicted to
be reduced rapidly to thiophenol.
Structural class III
Phenyl disulfide 578 882-33-7 No Yes. A NOEL of 3.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with 2-naphthalenethiol
(No. 531, group iv) at a single dose for
90 days; this substance is predicted to
be reduced rapidly to thiophenol.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Subgroup (viii): Disulfides with oxidized side-chains
Structural class I
2-Methyl-2-(methyldithio) 580 67952-60-7 No Yes. NOELs of 7.3, 0.23, 1.2,and 1.9 N/R No safety
-propanal mg/kg bw per day were reported in concern
90-day studies in rats treated with
propyl disulfide (No. 566), dicyclohexyl
disulfide (No. 575), methyl benzyl
disulfide (No. 577), and
3-mercapto-2-pentanone (No. 560),
respectively, only at those doses.
See cyclopentanethiol (No. 516,
sub-group iv) for the thiol products
of reduction.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Subgroup (viii): Disulfides with oxidized side-chains
Structural class I
2-Methyl-2-(methyldithio) 580 67952-60-7 No Yes. NOELs of 7.3, 0.23, 1.2,and 1.9 N/R No safety
-propanal mg/kg bw per day were reported in concern
90-day studies in rats treated with
propyl disulfide (No. 566), dicyclohexyl
disulfide (No. 575), methyl benzyl
disulfide (No. 577), and
3-mercapto-2-pentanone (No. 560),
respectively, only at those doses.
See cyclopentanethiol (No. 516,
sub-group iv) for the thiol products
of reduction.
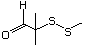 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Ethyl 2-(methyldithio)propionate 581 23747-43-5 No Yes. NOELs of 7.3, 0.23, 1.2,and 1.9 N/R No safety
mg/kg bw per day were reported in concern
90-day studies in rats treated with
propyl disulfide (No. 566), dicyclohexyl
disulfide (No. 575), methyl benzyl
disulfide (No. 577), and
3-mercapto-2-pentanone (No. 560),
respectively, only at those doses.
See cyclopentanethiol (No. 516,
sub-group iv) for the thiol products of
reduction.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Ethyl 2-(methyldithio)propionate 581 23747-43-5 No Yes. NOELs of 7.3, 0.23, 1.2,and 1.9 N/R No safety
mg/kg bw per day were reported in concern
90-day studies in rats treated with
propyl disulfide (No. 566), dicyclohexyl
disulfide (No. 575), methyl benzyl
disulfide (No. 577), and
3-mercapto-2-pentanone (No. 560),
respectively, only at those doses.
See cyclopentanethiol (No. 516,
sub-group iv) for the thiol products of
reduction.
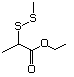 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Subgroup (ix): Trisulfides
Structural class I
Dimethyl trisulfide 582 3658-80-8 No Yes. A NOEL of 4.8 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with dipropyl trisulfide
(No. 585) only at that dose
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Subgroup (ix): Trisulfides
Structural class I
Dimethyl trisulfide 582 3658-80-8 No Yes. A NOEL of 4.8 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with dipropyl trisulfide
(No. 585) only at that dose
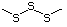 Methyl ethyl trisulfide 583 31499-71-5 No Yes. A NOEL of 4.8 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with dipropyl trisulfide
(No. 585) only at that dose
Methyl ethyl trisulfide 583 31499-71-5 No Yes. A NOEL of 4.8 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with dipropyl trisulfide
(No. 585) only at that dose
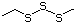 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Methyl propyl trisulfide 584 17619-36-2 No Yes. A NOEL of 4.8 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with dipropyl trisulfide
(No. 585) only at that dose
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Methyl propyl trisulfide 584 17619-36-2 No Yes. A NOEL of 4.8 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with dipropyl trisulfide
(No. 585) only at that dose
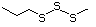 Dipropyl trisulfide 585 6028-61-1 No Yes. A NOEL of 4.8 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
Dipropyl trisulfide 585 6028-61-1 No Yes. A NOEL of 4.8 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
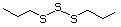 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Structural class II
Allyl methyl trisulfide 586 34135-85-8 No Yes. A NOEL of 4.6 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with diallyl trisulfide
(No. 587) only at that dose.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Structural class II
Allyl methyl trisulfide 586 34135-85-8 No Yes. A NOEL of 4.6 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with diallyl trisulfide
(No. 587) only at that dose.
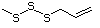 Diallyl trisulfide 587 2050-87-5 No Yes. A NOEL of 4.6 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
Diallyl trisulfide 587 2050-87-5 No Yes. A NOEL of 4.6 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
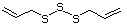 Diallyl polysulfide 588 72869-75-1 No Yes. A NOEL of 4.6 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with diallyl trisulfide
(No. 587) only at that dose.
Diallyl polysulfide 588 72869-75-1 No Yes. A NOEL of 4.6 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with diallyl trisulfide
(No. 587) only at that dose.
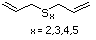 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Subgroup (x): Heterocyclic disulfides
Structural class II
3,5-Dimethyl-1,2,4-trithiolane 573 23654-92-4 No Yes. A NOEL of 1.9 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Subgroup (x): Heterocyclic disulfides
Structural class II
3,5-Dimethyl-1,2,4-trithiolane 573 23654-92-4 No Yes. A NOEL of 1.9 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
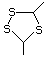 3-Methyl-1,2,4-trithiane 574 43040-01-3 No Yes. NOELs of 1.9 and 0.3 mg/kg bw N/R No safety
per day were reported in 90-day studies concern
in rats treated with
3,5-dimethyl-1,2,4-tri-thiolane
(No. 573) and this substance,
respectively, only at those doses.
3-Methyl-1,2,4-trithiane 574 43040-01-3 No Yes. NOELs of 1.9 and 0.3 mg/kg bw N/R No safety
per day were reported in 90-day studies concern
in rats treated with
3,5-dimethyl-1,2,4-tri-thiolane
(No. 573) and this substance,
respectively, only at those doses.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Subgroup (xi): Thioesters
Structural class I
S-Methyl thioacetate 482 1534-08-3 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate
(No. 483) only at that dose, and a NOEL
of 1000 mg/kg bw per day was reported
in a 90-day study in rats treated with
methyl thio-butyrate (No. 484) at
multiple doses.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Subgroup (xi): Thioesters
Structural class I
S-Methyl thioacetate 482 1534-08-3 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate
(No. 483) only at that dose, and a NOEL
of 1000 mg/kg bw per day was reported
in a 90-day study in rats treated with
methyl thio-butyrate (No. 484) at
multiple doses.
 Ethyl thioacetate 483 625-60-5 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
Ethyl thioacetate 483 625-60-5 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
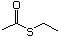 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Methyl thiobutyrate 484 2432-51-1 No Yes. A NOEL of 1000 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Methyl thiobutyrate 484 2432-51-1 No Yes. A NOEL of 1000 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
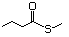 Propyl thioacetate 485 2307-10-0 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate (No. 483)
only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses.
Propyl thioacetate 485 2307-10-0 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate (No. 483)
only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses.
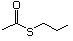 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Methyl 2-methylthiobutyrate 486 42075-45-6 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate (No. 483)
only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Methyl 2-methylthiobutyrate 486 42075-45-6 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate (No. 483)
only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses.
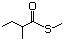 SS-Methyl 3-methylbutanethioate 487 23747-45-7 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate (No. 483)
only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses.
SS-Methyl 3-methylbutanethioate 487 23747-45-7 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate (No. 483)
only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses.
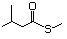 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
S-Methyl 4-methylpentanethioate 488 61122-71-2 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate (No. 483)
only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
S-Methyl 4-methylpentanethioate 488 61122-71-2 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate (No. 483)
only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses.
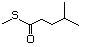 S-Methyl hexanethioate 489 20756-86-9 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate (No. 483)
only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses.
S-Methyl hexanethioate 489 20756-86-9 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate (No. 483)
only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses.
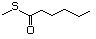 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Allyl thiopropionate 490 41820-22-8 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate (No. 483)
only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses. See diallyl trisulfide
(No. 587, subgroup ix), which is
predicted to be metabolized to allyl
thiol (allyl mercaptan) via diallyl
disulfide.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Allyl thiopropionate 490 41820-22-8 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate (No. 483)
only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses. See diallyl trisulfide
(No. 587, subgroup ix), which is
predicted to be metabolized to allyl
thiol (allyl mercaptan) via diallyl
disulfide.
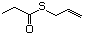 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Prenyl thioacetate 491 33049-93-3 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate (No. 483)
only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses. See diallyl trisulfide (No. 587,
subgroup ix), which is predicted to be
metabolized to allyl thiol (allyl
mercaptan) via diallyl disulfide.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Prenyl thioacetate 491 33049-93-3 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate (No. 483)
only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses. See diallyl trisulfide (No. 587,
subgroup ix), which is predicted to be
metabolized to allyl thiol (allyl
mercaptan) via diallyl disulfide.
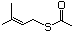 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Methylthio 2-(acetyloxy) 492 74586-09-7 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate (No. 483)
only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Methylthio 2-(acetyloxy) 492 74586-09-7 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate (No. 483)
only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses.
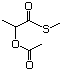 Methylthio 2-(propionyloxy) 493 999999-90-9 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate (No. 483)
only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses.
Methylthio 2-(propionyloxy) 493 999999-90-9 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate (No. 483)
only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses.
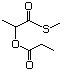 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
3-Acetylmercaptohexyl acetate 494 136954-25-1 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate (No. 483)
only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
3-Acetylmercaptohexyl acetate 494 136954-25-1 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate (No. 483)
only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses.
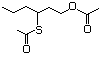 Subgroup (xi), Structural class II
S-Methyl benzothioate 504 5925-68-8 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate (No. 483)
only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses.
Subgroup (xi), Structural class II
S-Methyl benzothioate 504 5925-68-8 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate (No. 483)
only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
s- and 506a cis, No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
trans-Menthone-8-thioacetate and 57129-12-1 was reported in a 90-day study in rats concern
506b trans, treated with ethyl thioacetate (No. 483)
57074-34-7 only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
s- and 506a cis, No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
trans-Menthone-8-thioacetate and 57129-12-1 was reported in a 90-day study in rats concern
506b trans, treated with ethyl thioacetate (No. 483)
57074-34-7 only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Subgroup (xii), Structural class III: Sulfoxides
Methylsulfinylmethane (DMSO) 507 67-68-5 No Yes. A NOEL of 3000 mg/kg bw per day N/R No safety
was reported in monkeys given this concern
substance at multiple doses for 74-87
weeks; similar results in rats, dogs,
and humans.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Subgroup (xii), Structural class III: Sulfoxides
Methylsulfinylmethane (DMSO) 507 67-68-5 No Yes. A NOEL of 3000 mg/kg bw per day N/R No safety
was reported in monkeys given this concern
substance at multiple doses for 74-87
weeks; similar results in rats, dogs,
and humans.
 N/R, not relevant to the evaluation; ND, no data reported; bw, body weight
a None of the substances in this group are predicted to be metabolized to innocuous products. They were placed in subgroups
(i)-(xii) on the basis of the position of the sulfur atom.
b The thresholds for human intake are 1800 µg/day for class I, 540 µg/day for class II, and 90 µg/day for class III.
Table 2. Annual volume and intake of aliphatic and aromatic sulfides and thiols used as
flavouring substances in Europe and the United States
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
Methyl sulfide (452)
Europe 3100 590 10
United States 2800 530 9 57 000
Methyl ethyl sulfide (453)
Europe NR NA NA
United States (A) 9 2 0.03 +
Diethyl sulfide (454)
Europe NR NA NA
United States (A) 68 13 0.22 +
Butyl sulfide (455)
Europe 19 4 0.1
United States 0.5 0.1 0.002 +
1,4-Dithiane (456)
Europe NR NA NA
United States (A) 0.45 0.1 0.001 +
(1-Butenyl-1) methyl sulfide (457)
Europe NR NA NA
United States (A) 0.45 0.1 0.001 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
Allyl sulfide (458)
Europe NR NA NA
United States 2 0.4 0.01 +
Methyl phenyl sulfide (459)
Europe NR NA NA
United States (A) 2 0.4 0.01 +
Benzyl methyl sulfide (460)
Europe 1.1 0.2 0.003
United States (1982) 0.1 0.02 0.0003 +
3-(Methylthio)propanol (461)
Europe 23 4 0.1
United States 4 1 0.01 +
4-(Methylthio)butanol (462)
Europe 0.1 0.02 0.0003
United States 0.5 0.1 0.002 +
3-(Methylthio)-1-hexanol (463)
Europe 26 5 0.1
United States 0.5 0.1 0.002 +
2-Methyl-4-propyl-1,3-oxathiane (464)
Europe 11 2 0.03
United States (A) 5 1 0.02 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
2-Methylthioacetaldehyde (465)
Europe NR NA NA
United States (1970) 3 1 0.01 +
3-(Methylthio)propionaldehyde (466)
Europe 230 45 1
United States 130 25 0.4 637
3-(Methylthio)butanal (467)
Europe 0.7 0.1 0.002
United States 0.5 0.1 0.002 +
4-(Methylthio)butanal (468)
Europe NR NA NA
United States 0.1 0.02 0.0003 -
3-Methylthiohexanal (469)
Europe NR NA NA
United States (A) 4.5 1 0.01 +
2-(Methylthio)methyl-2-butenal (470)
Europe 0.2 0.04 0.001
United States (1982) 0.5 0.1 0.002 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
2,8-Dithianon-4-en-4-carboxaldehyde (471)
Europe 0.1 0.01 0.0002
United States 0.5 0.0: 0.002 +
Methyl 3-methylthiopropionate (472)
Europe 770 146 2
United States 46 9 0.1 5
Methylthiomethyl butyrate (473)
Europe NR NA NA
United States (A) 0.45 0.1 0.001 +
Methyl 4-(methylthio)butyrate (474)
Europe 0.5 0.1 0.002
United States 0.5 0.1 0.002 -
Ethyl 2-(methylthio)acetate (475)
Europe NR NA NA
United States (A) 0.45 0.1 0.001 +
Ethyl 3-methylthiopropionate (476)
Europe 200 37 1
United States 9 2 0.03 9
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
Ethyl 4-(methylthio)butyrate (477)
Europe NR NA NA
United States (A) 11 2 0.03 -
3-(Methylthio)propyl acetate (478)
Europe NR NA NA
United States (A) 59 11 0.2 +
Methylthiomethyl hexanoate (479)
Europe NR NA NA
United States (A) 0.45 0.1 0.001 +
Ethyl 3-(methylthio)butyrate (480)
Europe NR NA NA
United States (A) 0.45 0.1 0.001 +
3-(Methylthio)hexyl acetate (481)
Europe 0.4 0.1 0.001
United States (A) 45 9 0.1 +
S-Methyl thioacetate (482)
Europe NR NA NA
United States (A) 0.01 0.002 0.00003 2
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
Ethyl thioacetate (483)
Europe 0.1 0.02 0.0003
United States 0.1 0.02 0.0003 56
Methyl thiobutyrate (484)
Europe 24 5 0.1
United States 27 5 0.1 +
Propyl thioacetate (485)
Europe 2.2 0.4 0.01
United States 0.1 0.02 0.0003 +
Methyl 2-methylthiobutyrate (486)
Europe 0.8 0.2 0.003
United States 0.5 0.01 0.002 +
S-Methyl 3-methylbutanethioate (487)
Europe NR NA NA
United States (A) 1 0.19 0.003 +
S-Methyl 4-methylpentanethioate (488)
Europe NR NA NA
United States (A) 0.0045 0.001 0.00001 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
S-Methyl hexanethioate (489)
Europe NR NA NA
United States (A) 0.45 0.1 0.001 3
Allyl thiopropionate (490)
Europe NR NA NA
United States 0.5 0.1 0.002 -
Prenyl thioacetate (491)
Europe NR NA NA
United States (A) 1 0.19 0.003 +
Methyl 2-(acetyloxy) propionate (492)
Europe NR NA NA
United States (A) 45 9 0.1 -
Methylthio 2-(propionyloxy) propionate (493)
Europe NR NA NA
United States (A) 45 9 0.1 -
3-Acetylmercaptohexyl acetate (494)
Europe NR NA NA
United States (A) 2.2 0.4 0.01 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
1-Methylthio-2-propanone (495)
Europe NR NA NA
United States (A) 1 0.2 0.003 -
1-(Methylthio)-2-butanone (496)
Europe 0.03 0.01 0.0001
United States (1982) 0.1 0.02 0.0003 +
4-(Methylthio)-2-butanone (497)
Europe 0.1 0.02 0.0003
United States 2 0.4 0.01 +
4,5-Dihydro-3(2H)-thiophenone (498)
Europe 3.6 1 0.01
United States 13 2 0.04 +
2-Methyltetrahydrothiophen-3-one (499)
Europe 100 19 0.3
United States (A) 0.5 0.1 0.002 5036
4-(Methylthio)-4-methyl-2-pentanone (500)
Europe 0.2 0.04 0.0006
United States 0.5 0.1 0.002 -
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
4-(Methylthio)-2-oxobutanoic acid, sodium salt (501)
Europe NR NA NA
United States (A) 1 0.2 0.003 +
Di(butan-3-one-1-yl) sulfide (502)
Europe NR NA NA
United States (1982) 0.1 0.02 0.0003 -
ortho-(Methylthio)phenol (503)
Europe 5 1 0.02
United States (1970) 5 1 0.02 +
S-Methyl benzothioate (504)
Europe NR NA NA
United States (A) 0.0045 0.001 0.00001 +
2-(Methylthiomethyl)-3-phenylpropenal (505)
Europe NR NA NA
United States 10 2 0.03 -
cis- and trans-Menthone-8-thioacetate (506)
Europe NR NA NA
United States (A) 2.3 0.4 0.01 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
Methylsulfinylmethane (507)
Europe NR NA NA
United States (A) 0.0045 0.001 0.00001 45 000
Methyl mercaptan (508)
Europe 440 83 1
United States 0.9 0.2 0.003 +
Propanethiol (509)
Europe 18 3 0.1
United States 38 7 0.1 360
2-Propanethiol (510)
Europe NR NA NA
United States (A) 0.022 0.004 0.0001 +
1-Butanethiol (511)
Europe 3.2 0.5 0.01
United States 0.2 0.04 0.001 +
2-Methyl-1-propanethiol (512)
Europe NR NA NA
United States (A) 6.8 1.3 0.021 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
3-Methylbutanethiol (513)
Europe NR NA NA
United States (A) 0.045 0.01 0.0001 23
2-Pentanethiol (514)
Europe 12 2 0.04
United States (A) 11 2 0.03 +
2-Methyl-1-butanethiol (515)
Europe 2.5 0.5 0.01
United States (1982) 0.1 0.02 0.0003 +
Cyclopentanethiol (516)
Europe NR NA NA
United States (A) 4.5 1 0.01 -
3-Methyl-2-butanethiol (517)
Europe 0.1 0.02 0.0003
United States 0.1 0.02 0.0003 +
1-Hexanethiol (518)
Europe NR NA NA
United States (A) 0.045 0.01 0.0001 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
2-Ethylhexanethiol (519)
Europe NR NA NA
United States (A) 0.045 0.01 0.0001 +
2,3, or 10-Mercatopinane (520)
Europe 0.3 0.1 0.001
United States 50 10 0.2 -
Allyl mercaptan (521)
Europe 1.3 0.2 0.003
United States 8 2 0.03 480
Prenythiol (522)
Europe NR NA NA
United States (A) 1 0.2 0.003 +
1-para-Menthene-8-thiol (523)
Europe 2.8 1 0.01
United States (A) 4.5 1 0.01 +
Thiogeraniol (524)
Europe 9 2 0.03
United States 0.1 0.02 0.0003 -
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
Benzenethiol (525)
Europe 6 1 0.02
United States 160 30 1 +
Benzyl mercaptan (526)
Europe 10 2 0.03
United States 2 0.4 0.01 +
Phenethyl mercaptan (527)
Europe NR NA NA
United States (A) 1 0.2 0.003 +
ortho-Toluenethiol (528)
Europe 140 27 0.4
United States 0.9 0.2 0.003 +
2-Ethylthiophenol (529)
Europe 0.001 0.0002 0.0
United States 0.5 0.10 0.002 -
2,6-Dimethylthiophenol (530)
Europe 11 2 0.03
United States 0.1 0.02 0.0003 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
2-Naphthalenethiol (531)
Europe NR NA NA
United States (A) 0.34 0.1 0.001 +
1,2-Ethanedithiol (532)
Europe 0.01 0.002 0.00003
United States (A) 4.5 0.9 0.01 +
Bis(methylthio)methane (533)
Europe NR NA NA
United States (A) 500 94 2 +
2-Methyl-1,3-dithiolane (534)
Europe 0.5 0.1 0.002
United States (A) 23 4 0.1 +
1,3-Propanedithiol (535)
Europe 7 1 0.02
United States (A) 4.5 0.9 0.01 +
1,2-Propanedithiol (536)
Europe NR NA NA
United States (A) 4.5 0.9 0.01 -
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
1,2-Butanedithiol (537)
Europe NR NA NA
United States 0.9 0.2 0.003 -
1,3-Butanedithiol (538)
Europe NR NA NA
United States (A) 4.5 0.9 0.01 -
2,3-Butanedithiol (539)
Europe 0.4 0.1 0.001
United States 0.9 0.2 0.003 -
1,6-Hexanedithiol (540)
Europe 13 2.5 0.04
United States 0.5 0.1 0.002 +
1,8-Octanedithiol (541)
Europe 17 3 0.1
United States (A) 4.5 0.9 0.01 -
1,9-Nonanedithiol (542)
Europe 0.01 0.002 0.00003
United States (A) 4.5 0.9 0.01 -
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
Trithioacetone (543)
Europe 12 2 0.04
United States 2 0.4 0.01 +
3-Mercapto-3-methyl-1-butanol (544)
Europe NR NA NA
United States (A) 10 1.9 0.03 +
3-Mercaptohexanol (545)
Europe NR NA NA
United States (A) 4.5 1 0.01 +
2-Mercapto-3-butanol (546)
Europe 33 6 0.1
United States 0.5 0.1 0.002 -
alpha-Methyl-ß-hydroxypropyl alpha-methyl-ß-mercaptopropyl sulfide (547)
Europe NR NA NA
United States 4 1 0.01 -
4-Methyoxy-2-methyl-2-butanethiol (548)
Europe NR NA NA
United States (A) 4 0.8 0.01 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
3-Mercapto-3-methylbutyl formate (549)
Europe NR NA NA
United States (A) 0.5 0.1 0.002 +
2,5-Dihydroxy-1,4-dithiane (550)
Europe NR NA NA
United States (A) 0.45 0.1 0.001 -
2-Mercaptopropionic acid (551)
Europe 17 3 0.1
United States 440 84 1 -
Ethyl 2-mercaptopropionate (552)
Europe 3.2 0.5 0.01
United States 2 0.4 0.01 +
Ethyl 3-mercaptopropionate (553)
Europe 0.6 0.1 0.002
United States (A) 230 43 1 +
3-Mercaptohexyl acetate (554)
Europe NR NA NA
United States (A) 1 0.2 0.003 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
3-Mercaptohexyl butyrate (555)
Europe NR NA NA
United States (A) 1 0.2 0.003 +
3-Mercaptohexyl hexanoate (556)
Europe NR NA NA
United States (A) 1 0.2 0.003 +
1-Mercapto-2-propanone (557)
Europe NR NA NA
United States (A) 0.45 0.09 0.001 +
3-Mercapto-2-butanone (558)
Europe 26 5 0.1
United States (1982) 0.2 0.04 0.001 +
2-Keto-4-butanethiol (559)
Europe NR NA NA
United States 0.5 0.1 0.002 -
3-Mercapto-2-pentanone (560)
Europe NR NA NA
United States 0.5 0.1 0.002 -
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
para-Mentha-8-thiol-3-one (561)
Europe 86 16 0.3
United States 9 2 0.03 +
2,5-Dimethyl-2,5-dihydroxy-1,4-dithiane (562)
Europe 1.2 0.2 0.004
United States 0.9 0.2 0.003 -
Sodium 3-mercaptooxopropionate (563)
Europe NR NA NA
United States (A) 1 0.2 0.003 -
Dimethyl disulfide (564)
Europe 57 11 0.2
United States 13 2 0.04 1 400
Methyl propyl disulfide (565)
Europe 32 6 0.10
United States 0.1 0.02 0.0003 13 000
Propyl disulfide (566)
Europe 28 5 0.1
United States 0.5 0.1 0.002 14 000
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
Diisopropyl disulfide (567)
Europe NR NA NA
United States (A) 41 8 0.1 +
Allyl methyl disulfide (568)
Europe 0.01 0.002 0.00003
United States (1982) 0.1 0.02 0.0003 +
Methyl 1-propenyl disulfide (569)
Europe NR NA NA
United States (1982) 4 1 0.01 1
Propenyl propyl disulfide (570)
Europe NR NA NA
United States (1970) 40 8 0.1 +
Methyl 3-methyl-1-butenyl disulfide (571)
Europe NR NA NA
United States (A) 0.45 0.1 0.001 +
Allyl disulfide (572)
Europe 480 92 2
United States 44 8 0.1 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
3,5-Dimethyl-1,2,4-trithiolane (573)
Europe 0.2 0.04 0.001
United States (1982) 0.5 0.0: 0.002 +
3-Methyl-1,2,4-trithiane (574)
Europe 0.6 0.1 0.002
United States (A) 230 43 1 +
Dicyclohexyl disulfide (575)
Europe 0.1 0.02 0.0003
United States 0.9 0.2 0.003 -
Methyl phenyl disulfide (576)
Europe NR NA NA
United States (A) 1 0.2 0.003 +
Methyl benzyl disulfide (577)
Europe 0.1 0.02 0.0003
United States 0.5 0.1 0.002 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
Phenyl disulfide (578)
Europe NR NA NA
United States (1982) 0.2 0.04 0.001 -
Benzyl disulfide (579)
Europe 0.1 0.01 0.0002
United States (1982) 0.5 0.1 0.002 +
2-Methyl-2-(methyldithio) propanal (580)
Europe NR NA NA
United States (A) 9 2 0.03 +
Ethyl 2-(methyldithio)propionate (581)
Europe NR NA NA
United States (A) 0.45 0.1 0.001 +
Dimethyl trisulfide (582)
Europe 9 2 0.03
United States 0.1 0.02 0.0003 3 000
Methyl ethyl trisulfide (583)
Europe NR NA NA
United States (A) 3 1 0.01 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
Methyl propyl trisulfide (584)
Europe 1.7 0.3 0.01
United States 0.4 0.1 0.001 13 000
Dipropyl trisulfide (585)
Europe 60 11 0.2
United States 5 1 0.02 16 000
Allyl methyl trisulfide (586)
Europe NR NA NA
United States (A) 4.5 0.9 0.01 +
Diallyl trisulfide (587)
Europe 29 6 0.1
United States (1970) 0.1 0.02 0.0003 +
Diallyl polysulfide (588)
Europe 10 2 0.03
United States 0.1 0.02 0.0003 -
TOTAL
Europe 6 100
United States 5 300
A, volume anticipated on the basis of recent estimates (communication from the Flavor and Extract
Manufacturers' Association to the Committee); NR, not reported; NA, not applicable; +, reported
to occur naturally in foods (CIVO-TNO, 1994), but quantitative data were not available; -, not
reported to occur naturally in foods
a Volumes reported in a survey in Europe (International Organization of the Flavor
Industry, 1995) and in a survey in the United States (National Academy of Sciences, 1989).
Most of the data for the United States are for 1987 (and there is no entry); when data
for 1987 were not available, data for 1982 or 1970 were used, as indicated.
b Per capita intake (mg/day) calculated as follows:
[(annual usage, kg) × (1 × 109 mg/kg) / (population × 0.6 × 365 days)], where population
(10% 'eaters only') = 32 × 106 for Europe and 24 × 106 for the USA; 0.6 represents the
assumption that only 60% of the annual usage of the flavour was reported in the survey.
Intake (mg/kg bw per day) calculated as follows: [(mg/day)/body weight], where body weight = 60 kg.
c From Stofberg & Kirschman (1985); Stofberg & Grundschober (1987)
Potential toxicity can be deduced by comparison with structural
analogues on the basis of metabolic similarities. In the absence of
information on the toxicity of structural analogues, however, it is
not possible to conclude a priori that the substances are
metabolized to innocuous products.
(i) Simple sulfides (thioethers)
Once alkyl and aromatic thioethers, commonly called 'sulfides',
enter the systemic circulation, they are rapidly oxidized to
sulfoxides and, depending on the structure of the thioether, may be
further oxidized to sulfones. Sulfoxides and sulfones are major
urinary metabolites of simple sulfides. Aliphatic thioethers (Nos
452-455, 457, 458, and 533) and thioethers containing an aromatic
nucleus (Nos 459 and 460) yield mixtures of sulfoxide and sulfone
metabolites. Enzymes of the cytochrome P450 superfamily and
flavin-containing monooxygenases catalyse the oxidation of thioethers
to sulfoxides. Oxidation of sulfoxides to the corresponding sulfones
occurs both in tissues and in aerobic microorganisms and is an
irreversible metabolic reaction in mammals. Sulfoxides can also be
metabolized back to the thioether by aldehyde oxidase, thioredoxin and
its reductase, and the gut microflora in the anaerobic environment of
the lower bowel.
The methyl aromatic thioethers (Nos 459 and 460) are predicted to
be major metabolites of the corresponding aromatic thiols (Nos 525 and
526, subgroup iv) and would be oxidized to sulfoxides and sulfones,
which would be excreted.
(ii) Acyclic sulfides with oxidized side-chains
The presence of other functional groups, such as alcohol (Nos
461-463), aldehyde (Nos 465-471 and 505), ester (Nos 472-481), acid
(No. 501), ß-ketone (Nos 495-497, 500, and 502), and phenol (No. 503),
provides centres of greater polarity and additional sites for the
biotransformation of thioethers. The presence of these polar groups
would also result in increased renal excretion. The biotransformations
of such oxygenated, carbon-containing, functional groups are well
characterized and have been described for groups of flavouring agents
previously evaluated by the Committee. Concurrent metabolism of
various substrates at both sulfur and oxygenated functional groups has
been reported, and sulfoxide formation usually predominates as the
major metabolic pathway of detoxification. Experiments in vitro
suggest that hydrolysis of carboxyl esters occurs in the presence of
thioether (sulfide) groups. In consequence, thioethers with oxidized
side-chains would be expected to be eliminated more rapidly than
simple sulfides.
(iii) Cyclic sulfides
Oxidation of unsubstituted and methyl-substituted cyclic
thioethers by the cytochrome P450 superfamily produces the
corresponding sulfoxides. The mono-sulfoxides are predicted to be the
main urinary metabolites of simple cyclic sulfides (Nos 456, 534, and
543). The metabolism of cyclic sulfides containing oxidized carbon
atoms (Nos 464, 498, 499, 550, and 562) has not been studied but would
be predicted to involve extensive S-oxidation and possibly oxidation
or conjugation of alcohol groups. The polarity of the hydroxy
thioethers (Nos 550 and 562) may allow their elimination unchanged.
(iv) Simple thiols
The simple thiol flavouring agents considered are alkyl and
alicyclic thiols (Nos 508-524, 526, and 527) and aromatic thiols
(thiophenols; Nos 525 and 528-531). These substances may be
metabolized along several pathways. Simple aliphatic and aromatic
thiols undergo S-methylation in mammals to produce the corresponding
methyl thioether or sulfide. Methylation is catalysed by thiopurine
methyltransferase in the cytoplasm and thiol methyltransferase in
microsomes, and both reactions require S-adenosyl-l-methionine as a
methyl group donor. Thiopurine methyltransferase is present in human
liver, kidney, and erythrocytes; preferential substrates for this
enzyme include aromatic and heterocyclic thiols. S-Methylation of
aliphatic thiols is catalysed by microsomal thiol methyltransferase,
and the resulting methyl thioether (sulfide) metabolite would undergo
S-oxidation to give the methyl sulfoxide and methyl sulfone
analogues as urinary products.
Thiols may react with glutathione and other endogenous thiol
substances to form mixed disulfides. Both microsomal and cytoplasmlic
thioltransferases have been reported to catalyse the formation of
mixed disulfides. The resulting mixed disulfides can undergo reduction
back to thiols, oxidative desulfuration, or oxidation to a sulfonic
acid via the intermediate thiosulfinate and sulfinic acids. The
principal form in the circulation would probably be a mixed disulfide
formed with albumin.
S-Glucuronidation of aromatic thiols has been reported, and
this may be a pathway for the metabolism of aromatic thiols
(thiophenols) (Nos 525 and 528-531) and simple aromatic disulfides
(Nos 576 and 578; subgroup vii) after their reduction (see below).
Glucuronyl transferases behave similarly towards hydroxyl and
sulfydryl groups, and the two activities have the same subcellular
location and optimal pH.
Thiols may be oxidized to form sulfenic acids (RSOH), which are
unstable and readily undergo further oxidation to sulfinic (RSO2H)
and sulfonic (RSO3H) acids or combine with nucleophiles. The sulfonic
acid group is a highly polar centre and makes molelcules highly
soluble in water. In general, sulfonic acids are stable to metabolism.
Alkyl thiols of low relative molecular mass undergo oxidative
desulfuration in vivo to yield CO2 and SO4=. This reaction has
been shown, for example, for methanethiol (methyl mercaptan; No. 508).
Whereas the carbon atom from thiols may be used in the biosynthesis of
amino acids, the sulfur atom is not used significantly in the
synthesis of sulfur-containing amino acids.
(v) Thiols with oxidized side chains
Although alkyl thiols with oxidized side-chains (Nos 544-549,
551-561, and 563) comprise a significant proportion of the flavouring
agents evaluated, their metabolic fate has not been studied. Their
metabolism is predicted to involve a combination of the pathways
described above for simple thiols and further oxidation or conjugation
of the oxidized side-chain. The class III compound, sodium
3-mercapto-oxopropionate (No. 563), would be expected to be eliminated
very rapidly by metabolism at both the thiol and keto-acid groups.
(vi) Dithiols
The metabolism of the simple aliphatic dithiols evaluated (Nos
532 and 535-542) is predicted to involve the pathways described above
for simple thiols. Urinary metabolites could result from methylation,
S-oxidation of one S atom to yield a polar sulfonate, and the
formation of mixed disulfides of low relative molecular mass such as
cysteine, an endogenous thiol. The longer, linear dithiols (Nos 535
and 540-542) could form intramolecular disulfide bonds, with
interconversion between dithiol and cyclic disulfide forms.
(vii) Simple disulfides
The reduction of xenobiotic disulfides is believed to be
extensive, and the reaction may be catalysed enzymatically by
thioltransferases and chemically by exchange with glutathione,
thioredoxin, cysteine, and other endogenous thiols. Reduction of the
non-cyclic disulfides considered in the group of flavours (Nos 564-572
and 575-579) would result in the formation of thiols of low relative
molecular mass, which would then be metabolized by the various
pathways described above for simple thiols.
(viii) Disulfides with oxidized side-chains
As discussed above for subgroups (ii) and (iii), additional sites
of carbon oxidation would result in greater polarity and further
oxidation or conjugation of the flavouring agents evaluated (Nos 580
and 581). However, as disulfide bonds in substances in subgroup (v)
are susceptible to reductive cleavage, this would be expected to be
the initial metabolic reaction, and the polarity of the side-chains
would primarily affect elimination of the thiol fragments.
(ix) Trisulfides and polysulfides
The trisulfide of glutathione is labile and readily converted to
the disulfide, the sulfur being released as hydrogen sulfide.
Trisulfides and polysulfides are predicted to be converted rapidly to
the corresponding disulfides, which would then be reduced to thiols,
which themselves would follow the pathways described above for thiols.
The potential toxicity of trisulfides (Nos 582-587) and polysulfides
(No. 588) is probably related to their metabolic lability and to the
nature of the eventual thiol (e.g. allyl thiol).
(x) Heterocyclic disulfides: The heterocyclic disulfides (Nos 573 and
574) are five-and six-carbon rings which also contain a cyclic
thioether bond. Lipoic acid, an endogenous substance, is a
five-membered cyclic disulfide which undergoes rapid redox cycling
between ring disulfide and open dithiol forms. The principal metabolic
pathways are predicted to be disulfide reduction with ring opening to
produce a dithiol, and S-oxidation of the cyclic thioether.
(xi) Thioesters
Thioester groups (-S-CO-) are present in a number of the
flavouring agents evaluated (Nos 482-494, 504, and 506). Hydrolysis of
esters has been considered previously by the Committee, but that of
thioesters has not. Thioesters are hydrolysed by lipase and esterases,
and the rate of hydrolysis increases as the length of the C-chain of
the carboxylic acid fragment increases, and decreases as oxygenation
of the carbon chain in the thiol moiety increases.
Hydrolysis could result in a thioic acid and an alcohol or a
carboxylic acid and a thiol (the metabolic fates of which have been
outlined above). Data for dithioic acids and esters indicate that the
esters of monothioic acids would be poor substrates for oxidation, but
the monothioic acid released by hydrolysis would be oxidized to the
dioxo acid. Other possibilities for elimination in vivo include renal
excretion of thiocarboxylic acid. The substances evaluated (with the
exceptions of prenyl thioacetate (No. 491) and allyl thiopropionate
(No. 490)) are simple linear alkyl compounds, branched-chain alkyl
compounds, or their side-chain hydroxyester analogues, so that
comparison of the toxicity of the different substances is reasonable.
(xii) Sulfoxides
The metabolism of sulfoxides by oxidation and reduction is
described above under 'Thioethers'. The only sulfoxide flavouring
agent evaluated was methylsulfinylmethane (dimethyl sulfoxide, DMSO;
No. 507), for which data were available on both metabolism and
toxicity in experimental animals and humans. DMSO is readily absorbed
and excreted in urine as the parent sulfoxide and dimethyl sulfone.
1.4 Application of the Procedure for the Safety
Evaluation of Flavouring Agents
Step 1. In applying the Procedure for the Safety Evaluation of
Flavouring Agents to the above-mentioned aliphatic and
aromatic sulfides and thiols, the Committee assigned 97 of
the 137 substances (Nos 452-455, 457, 461-463, 465-497, 500,
502, 508-515, 517-519, 522, 524, 532, 533, 535-542, 544-560,
562, 564-567, 569-571, and 580-585), which may or may not
contain an additional oxygenated functional group, to
structural class I. The Committee assigned 34 of the 137
substances to structural class II because they are aromatic
sulfides or thiols (Nos 459, 460, 503, 504, 525-528, 530,
531, 576, 577, and 579), alicyclic (Nos 506, 516, 520, 523,
561, and 575) or heterocyclic substances (Nos 456, 464, 498,
499, 534, 543, 573, and 574), or allyl mercaptan or sulfides
(Nos 458, 521, 568, 572, and 586-588), which are common
components of food. The aromatic thiols or thioethers that
are not common compo-nents of food (Nos 505, 529, and 578)
were assigned to structural class III. The remaining three
substances were also assigned to structural class III by
virtue of the fact that they are aliphatic thiols or
thioethers containing more than three functional groups (Nos
501 and 563) or do not contain divalent sulfur (No. 507).
Step 2. None of the substances in this group can be predicted to be
metabolized to innocuous products. The evaluation of these
substan-ces therefore proceeded via the right-hand side of
the decision tree.
Step B3. The estimated daily per capita intake in Europe and in the
United States for each of the substances in the overall
group of 137 flavouring agents is below the threshold for
human intake for the structural class in which the substance
falls. The thresholds are 1800 µg/person per day for class
I, 540 µg/person per day for class II, and 90 µg/person per
day for class III.
Step B4. The Committee considered the results of 90-day studies of
toxicity in rodents for 27 substances in this group of
flavouring agents. The Committee noted that the single or
multiple doses of flavouring agents tested in a number of
studies had no effect in rats and that the NOELs were
consequently derived from studies that did not show toxic
effects. The results of long-term studies in multiple
species were considered for one substance, DMSO (No. 507).
To facilitate comparisons of the toxicity of structurally
related substances, the agents were considered in subgroups
(i)- (xii) on the basis of the position of the sulfur atom
(see Tables 1 and 3). Toxicity was compared within and
across subgroups with no restriction on the basis of
structural class assignment (Step 1).
(i) Simple sulfides (thioethers): This subgroup comprises
nine flavouring agents that are simple thioethers. The NOEL
for methyl sulfide (No. 452) in a 14-week study in rats
treated with multiple doses by gavage was 250 mg/kg bw per
day. This NOEL provides an adequate basis for evaluation of
five structurally and metabolically related substances (Nos
453, 454, 455, 457, and 533); however, the NOEL for methyl
sulfide was considered inappropriate for evaluation of allyl
sulfide (No. 458) and two aromatic thioethers, methyl phenyl
sulfide and benzyl methyl sulfide (Nos 459 and 460,
respectively), and the evaluation of these three substances
therefore proceeded to step B5.
(ii) Acyclic sulfides with oxidized side-chains: This
subgroup comprises 28 flavouring agents that are acyclic
thioethers with oxidized side-chains. The NOEL in a 90-day
study in rats treated with a single dose of
2-(methylthiomethyl)-3-phenylpropenal (No. 505) was 1.4
mg/kg bw per day. This substance is an aromatic compound
with a sulfide group in an unsaturated side-chain; it was
assigned to structural class III, because it is not a common
component of food. Data for methyl sulfide (No. 452) were
also considered relevant for assessing the toxicity of
sulfide substances with oxidized side-chains (Nos 461-463,
465-469, 472-481, 495-497, and 500-503). Although
2-(methylthiomethyl)-3-phenylpropenal (No. 505) is not an
aryl thioether, the safety margin between its NOEL and the
intake of ortho-(methylthio)phenol pentanone (No. 503) was
considered to be adequate. The NOEL for
2-(methylthiomethyl)-3-phenylpropenal (No. 505) does not
provide a high margin of safety (i.e. > 1000) for methyl
3-methylthiopropionate (No. 472) at the current estimated
level of intake; however, the simple side-chain acid and
ester would have little toxic potential, and the NOEL for
methyl sulfide (No. 452) provides an adequate margin of
safety. The NOELs for 2-(methylthio-methyl)-3-phenylpropenal
(No. 505) and methyl sulfide (No. 452, subgroup (i)) were
considered inappropriate for evaluation of the toxicity of
two alpha,beta-unsaturated carbonyls (Nos 470 and 471)
because they are potentially more reactive and toxic, and
the evaluation of these substances therefore proceeded to
step B5.
(iii) Cyclic sulfides: This subgroup comprises eight
substances that are cyclic thioethers. NOELs of 0.44 mg/kg
bw per day for 2-methyl-4-propyl-1,3-oxathiane (No. 464),
9.2 mg/kg bw per day for 4,5-dihydro-3(2 H)-thiophenone
(No. 498), 7 mg/kg bw per day for 2-methyl-1,3-dithiolane
(No. 534), 0.2 mg/kg bw per day for trithioace-tone (No.
543), and 3.1 mg/kg bw per day for
2,5-dimethyl-2,5-dihydroxy-1,4-dithiane (No. 562) were
reported. These values provide an adequate margin of safety
for evaluation of the toxicity of substances No. 456, 499,
and 550.
Table 3. Comparison of the toxicity and intake data used in the safety evaluations of
137 simple aliphatic and aromatic sulfides and thiols, by subgroupa
Subgroup Adequate NOEL Adequate NOEL No adequate NOEL
for substanceb for structurally for substance or
related substanceb related substance,
but intake
< 1.5 µg/dayc
(i) Simple sulfides No. 452 Nos 453-455, Nos 458-460
(thioethers) 457, 533
(ii) Acyclic thioethers No. 505 Nos 461-463, Nos 470, 471
with oxidized 465-469, 472-481,
side-chains 495-497, 500-503
(iii) Cyclic sulfides Nos 464, 498, Nos 456, 499, N/R
534, 543, 562 550
(iv) Thiols Nos 516, 520, Nos 508-515, N/R
528, 530, 531 517-519, 521-527,
529
(v) Thiols with Nos 546, 547, Nos 544, 545, 548, N/R
oxidized 560 549, 551-559, 561,
side-chains 563
(vi) Dithiols Nos 539, 541 Nos 532, 535-538, N/R
540, 542
(vii) Simple disulfides Nos 566, 575, Nos 564, 565, N/R
577 567-572, 576, 578,
579
(viii) Disulfides with None Nos 580, 581 N/R
oxidized
side-chains
(ix) Trisulfides and Nos 585, 587 Nos 582-584, 586, N/R
polysulfides 588
(x) Heterocyclic No. 573 No. 574 N/R
disulfides
Table 3. (continued)
Subgroup Adequate NOEL Adequate NOEL No adequate NOEL
for substanceb for structurally for substance or
related substanceb related substance,
but intake
< 1.5 µg/dayc
(xi) Thioesters Nos 483, 484 Nos 482, 485-494, N/R
504, 506
(xii) Sulfoxides No. 507 N/R N/R
N/R, not relevant to the evaluation
a See Table 1 for further details of the evaluations.
b See Figure 1, step B4 and pp. 121-123 for further information.
c See Figure 1, step B5 and pp. 121-123 for further information.
(iv) Simple thiols: This subgroup comprises 24 flavouring
agents that are simple thiols. NOELs of 0.56 mg/kg bw per
day for cyclopentanethiol (No. 516), 0.06 mg/kg bw per day
for 2,3-and 10-mercaptopinane (No. 520), 0.52 mg/kg bw per
day for ortho-toluenethiol (No. 528), 0.43 mg/kg bw per
day for 2,6-dimethyl-thiophenol (No. 530), and 3.4 mg/kg bw
per day for 2-naphthalenethiol (No. 531) were reported.
These values provide adequate margins of safety for the
individual substances and for the other structurally related
thiols in the group in relation to current estimates of
intake, with the exception of 2,3 and 10-mercaptopinane (No.
520). A margin of safety of about 300 (based on an estimated
modified per capita intake of 0.2 µg/kg bw per day in the
United States) was obtained from the results of a 90-day
study in which only a single dose of 0.06 mg/kg bw per day
was tested. This substance was therefore also evaluated by
comparison with other substances in this subgroup (Nos 516,
528, 530, and 531) for which there was an adequate margin of
safety. The Committee noted that Nos 521-524 are unsaturated
thiols. Of these, allyl mercaptan (allyl thiol; No. 521)
would be expected to be more toxic than others that have
double bonds in different positions (by analogy to their
oxygenated analogues). Although data were not available on
allyl mercaptan (No. 521), the NOEL of diallyl trisulfide
(No. 587), which would be converted to allyl mercaptan after
reduction to allyl disulfide, was 4.6 mg/kg bw per day in a
90-day study in rats. This NOEL was considered to provide an
adequate safety margin for Nos 521-524.
(v) Thiols with oxidized side-chains: This subgroup
comprises thiols with oxygenated side-chains. The NOELs
available for three of the substances in class I (1.9 mg/kg
bw per day for 2-mercapto-3-butanol (No. 546), 2.8 mg/kg bw
per day for alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide (No. 547), and 1.9
mg/kg bw per day for 3-mercapto-2-pentanone (No. 560)) were
considered to provide adequate safety margins for the
flavouring agents in this subgroup, including the one
substance in structural class III, 3-mercapto-oxopropionate
(No. 563). Although the last compound has more than three
functional groups, the oxopropionate moiety would have
little toxic potential, and the NOELs for Nos 546, 547, and
560 were considered to provide an adequate margin of safety.
(vi) Dithiols: This subgroup comprises flavouring agents
that are dithiols. The NOEL for 2,3-butanedithiol (No. 539)
and 1,6-hexane-dithiol (No. 540) was 0.7 mg/kg bw per day,
which provides adequate margins of safety for all of the
substances in the subgroup.
(vii) Simple disulfides: This subgroup comprises 14
flavouring agents that are disulfides. The major metabolites
of these unsaturated disulfides would be thiols. The NOELs
were 7.7 mg/kg bw per day for propyl disulfide (No. 566),
0.23 mg/kg bw per day for dicyclohexyl disulfide (No. 575),
and 1.2 mg/kg bw per day for methyl benzyl disulfide (No.
577). These values provide adequate margins of safety for
each substance tested and for four structurally related
substances, Nos 564, 565, 567, and 579, at currently
estimated levels of intake. The substances in this subgroup
for which NOELs are available do not include unsaturated or
aryl disulfides. The aryl disulfides, methyl phenyl
disulfide and phenyl disulfide (Nos 576 and 578,
respectively), would be rapidly reduced to thiophenol, and
the NOEL of 3.4 mg/kg bw per day for 2-naphtha-lenethiol
(No. 531, subgroup iv) was considered to provide an adequate
safety margin for these agents. The Committee was aware that
propenyl disulfides can cause haemolytic anaemia in certain
species after short-term exposure. This effect would be of
concern to susceptible individuals. Substances Nos 568 and
572 would be metabolized to allyl mercaptan (No. 521;
subgroup iv). The Committee noted that the NOEL for diallyl
trisulfide (No. 587; subgroup ix) in a 90-day study in rats
given a single dose was 4.6 mg/kg bw per day, and that this
would provide an adequate safety margin for the allyl thiol
produced on reduction of substances Nos 568 and 572.
Di(1-propenyl) disulfide was about four times more potent
than allyl disulfide (No. 572) and about 20 times more
potent than propyl disulfide (No. 566). The intakes of the
related propenyl and butenyl flavours Nos 569, 570, and 571
gave safety margins of > 50 000 in comparison with the NOEL
for propyl disulfide (No. 566), and this was considered to
be adequate to allow for the differences in potency.
(viii) Disulfides with oxidized side-chains: This subgroup
consists of two disulfides with oxidized side-chains,
2-methyl-2-(methyldithio)-propanal (No. 580) and ethyl
2-(methyldithio)propionate (No. 581). The toxicity of these
agents has not been studied, but the Committee considered
these substances by analogy to disulfides (subgroup vii) and
concluded that adequate margins of safety were available,
given their greater polarity and the presence of thiols with
and without oxidized side-chains, i.e. subgroups (iv) and
(v).
(ix) Trisulfides and polysulfides: This subgroup consists
of six trisulfides and one polysulfide. NOELs of 4.8 mg/kg
bw per day for dipropyl trisulfide (No. 585) and 4.6 g/kg bw
per day for diallyl trisulfide (No. 587) were reported,
which gave adequate margins of safety for all substances in
this subgroup.
(x) Heterocyclic disulfides: This subgroup comprises two
flavouring agents that are heterocyclic disulfides. The NOEL
of 1.9 mg/kg bw per day for 3,5-dimethyl-1,2,4-trithiolane
(No. 573) provides an adequate margin of safety for this
substance at current levels of use. 3-Methyl-1,2,4-trithiane
(No. 574) was reported to have no effect at the single dose
of 0.3 mg/kg bw per day tested in a 90-day study; this dose
provides a margin of safety of only 100 at the estimated
modified per capita intake level of 1 µg/kg bw per day in
the United States. However, the NOEL for the closely related
compound 3,5-dimethyl-1,2,4-trithiolane provides an adequate
margin of safety (> 1000).
(xi) Thioesters: This subgroup consists of 15 thioesters.
The NOELs of 6.5 mg/kg bw per day for ethylthioacetate (No.
483) and 1000 mg/kg bw per day for methylthiobutyrate (No.
484) give an adequate margin of safety for all other esters
in this group. The conclusion that the current intake levels
of allyl thiopropionate and prenyl thioacetate are safe is
supported by the NOEL of 4.6 mg/kg bw per day for the
unsaturated diallyl trisulfide (No. 587), which would be
reduced to allyl disulfide and then allyl sulfide.
(xii) Sulfoxides: This subgroup consists of only one
substance, DMSO (No. 507). The NOEL in monkeys given DMSO by
gavage for 74-87 weeks was 3000 mg/kg bw per day. This NOEL
and other data provide an adequate margin of safety for the
use of DMSO as a flavouring agent at the estimated modified
daily per capita intake of 0.001 µg/day in the United
States.
Step B5. Five substances, allyl sulfide (No. 458), methyl phenyl
sulfide (No. 459), benzyl methyl sulfide (No. 460),
2-(methylthio)methyl-2-butenal (No. 470), and
2,8-dithianon-4-ene-carboxaldehyde (No. 471), were evaluated
at this step of the Procedure. The modified daily per
capita intake of all five substances is less than 1.5 µg
in Europe and in the United States. Applying the criteria
for Step B5 outlined in Annex 5 of the evaluations published
after its forty-ninth meeting Annex 1, reference 132), the
Committee concluded that use of these substances at their
current levels of intake poses no safety concern.
In summary, for 100 agents in subgroups (iii) to (xii), a NOEL
was available for the substance, a closely related substance, or a
predicted major metabolite that provided an adequate margin of safety
(> 1000). For 6/9 of the agents in subgroup (i) and 26/28 in subgroup
(ii), a NOEL for the substance or a closely related substance was
available that provided an adequate margin of safety (i.e. > 1000).
Therefore, the Committee determined at Step B4 of the Procedure that
the safety of these 132 substances would be expected to be of no
concern when they are used at their currently estimated level of daily
intake. The evaluation of the remaining five substances (Nos 458, 459,
and 460 in subgroup (i) and Nos 470 and 471 in subgroup (ii))
proceeded to Step B5 of the Procedure. The comparisons of toxicity and
the intake considerations for each subgroup that were used to apply
Steps B4 and B5 of the Procedure to the evaluation of individual
substances in this group of flavouring agents are summarized in Table
3 and given in more detail in Table 1.
1.5 Consideration of combined intakes
In the unlikely event that all 137 aliphatic and aromatic
sulfides and thiols in the overall group or in subgroups were to be
consumed simultaneously on a daily basis, the estimated combined
intake would not exceed the human intake threshold for class I
substances. The powerful aroma of these substances limits the level of
their use in foods.
1.6 Conclusions
The Committee concluded that the 137 flavouring agents comprising
aromatic sulfides and thiols evaluated at the present meeting could
not be predicted to be metabolized to innocuous products. According to
the Procedure, data on toxicity were needed to evaluate the safety of
this group of flavouring agents. The primary data that were used in
the evaluations consisted of 27 90-day studies in rodents with 25 of
the substances and long-term studies in multiple species for one
substance (DMSO). Most of these studies were conducted at a single
dose or multiple doses, which had no effects. The Committee noted that
the NOELs were thus derived from studies in which no toxic effects
were seen.
On the basis of the available data on the toxicity of
representative substances in each subgroup and on their metabolism,
the Committee determined that the safety of 132 of the flavouring
agents poses no concern when they are consumed at their current levels
of intake. The safety of the remaining five substances was considered
to pose no concern when they are consumed at levels of intake < 1.5
µg/day.
Other data on toxicity, including the results of short-term tests
for toxicity and studies of teratogenicity and genotoxicity, were
consistent with the results of the safety evaluation.
2. RELEVANT BACKGROUND INFORMATION
2.1 Explanation
The 137 compounds evaluated have a diverse array of chemical
structures, each of which contains a sulfur atom attached to a carbon,
hydrogen, oxygen, or second sulfur atom. The substances were separated
into 12 subgroups on the basis of the position of the sulfur atom, as
indicated in section 1.3 above. The key data on metabolism and
toxicity relevant to the safety evaluation are presented below by
subgroup. Information on intake, special studies, and observations in
humans are also discussed.
2.2 Additional considerations on intake
Thiols (i.e. mercaptans), thioethers, and their oxygenated
derivatives are known for their powerful aromas. They provide cooked,
browned, and roasted flavours when added as flavouring substances.
Their strong organoleptic properties make a significant contribution
to the taste and smell of various foods even when they are added at
low concentrations. The thresholds of odour detection for aromatic and
aliphatic thiols and thioethers range from 0.08 µg/m3 for amyl
mercaptan (not in any subgroup) to 0.03 mg/m3 for benzyl mercaptan
(No. 526); the odour threshold for methyl sulfide (No. 452), the
thioether with the highest annual volume of use as a flavouring
substance, is about 1 µg/m3, and that for the corresponding thiol,
methyl mercaptan (No. 508), is 2 µg/m3 (Farr & Kirwin, 1994). In
tests for sensory tolerance, most of the panelists reported that
atmospheres containing thiols, thioesters, or thioethers at
concentrations > 1 mg/m3 are intolerable (Flavor and Extract
Manufacturers' Association, 1996).
A direct result of the extremely low odour threshold of thiols
and thioethers is that their use in food is self-limiting, because
high concentrations would result in foods that are repulsive. The
levels of use in the vast majority of food categories are much less
than 1 mg/m3. In foods in which substantial evaporation of the sulfur
derivative occurs during processing, such as hard candies and baked
goods, the concentration is usually < 10 mg/m3. The low levels of
use in food are reflected in the low reported annual volumes of use of
thiols and thioethers as flavouring substances.
2.3 Biological data
2.3.1 Absorption, distribution, metabolism, and excretion
Simple, non-polar thioether, disulfide, and thiol flavouring
substances would be absorbed rapidly from the gastrointestinal tract
intact and excreted in urine, probably as metabolites, and in some
cases in the expired air as the parent compound. Essentially complete
absorption has been reported for methyl sulfide (No. 452) (Williams et
al., 1966) and dipropyl sulfide (Nickson & Mitchell, 1994). The
presence of other functional groups, such as an alcohol (e.g. Nos
461-463), aldehyde (e.g. Nos 465-471, 505), ester (e.g. Nos 472-481
and 492-494), acid (e.g. No. 501), alpha-ketone (or thioester) (e.g.
Nos 482-494, 504, and 506), beta-ketone (e.g. Nos 495-500, and 502),
or phenol (e.g. No. 503), provides a centre of greater polarity which
could slow the rate of absorption and would provide other sites for
oxidative metabolism (see below). The metabolism of sulfur centres is
shown in Figure 1. All the sulfur compounds considered at this meeting
are of low relative molecular mass and of sufficient lipophilicity to
be absorbed from the intestine. Some functional groups such as
aldehydes, esters, and thioesters probably undergo presystemic
metabolism in the gut lumen, gut wall, or liver.
(i) Simple sulfides (thioethers)
Thioethers are commonly called 'sulfides', as if the hydrogen
atoms of hydrogen sulfide were replaced by alkyl or aryl substituents.
Flavours are usually given such trivial names, which are simplified
even further when the two substituents are the same; for instance,
dimethyl thioether is called 'methyl sulfide'. The presence of a lone
pair of electrons on a divalent sulfur allows rapid oxidation of the
sulfide to a sulfoxide, which occurs both as part of metabolism and
also before ingestion or absorption under aerobic conditions. For
example, allyl sulfide (No. 458), a volatile constituent of onions and
garlic, is oxidized rapidly in air to the corresponding sulfoxide,
which is partly respon-sible for the lachrymatory effects of these
foods (Brodnitz & Pascale, 1971).
In many natural and pharmaceutical compounds, one or more oxygen
atoms is linked to the sulfur centre of an organic molecule, and the
chemical and biological properties of the compound depend on the
nature of the substituents on the sulfur and oxygen atoms. The
oxidation state of sulfur is of major importance in determining the
polarity of the compound and hence its interaction with biological
systems. Once alkyl and aromatic sulfides enter the systemic
circulation, they are oxidized rapidly to sulfoxides and, depending on
the structure of the sulfide, may be further oxidized to the sulfone.
Aliphatic sulfides generally yield mixtures of sulfone and sulfoxide
metabolites; the relative amounts excreted depend on the polarity of
the sulfide (Damani, 1987). Methyl sulfide (No. 452) administered to
rabbits is excreted in the urine as DMSO and dimethyl sulfone
(Williams et al., 1966), whereas the sulfone is the main urinary
metabolite of diethyl sulfide (No. 454) (Hoodi & Damani, 1984).
Dipropyl sulfide, a volatile component of onions and garlic, is
metabolized mainly to the corresponding sulfoxide with small amounts
of the sulfone and trace amounts of inorganic sulfate. Individual
studies on the sulfoxide and sulfone indicate that these metabolites
are relatively stable under physiological conditions (Nickson &
Mitchell, 1994; Nickson et al., 1995). Male Wistar rats eliminated 93%
of a single oral dose of [35S]-dipropyl sulfide over three days, with
66% in the urine and lesser amounts in exhaled air (18%), faeces
(4.6%), and the carcass (1.5%). The sulfoxide was the only compound
detected in plasma. About 25% of the radiolabel was recovered in urine
on day 1 and 39% on day 2, and approximately 25% of the radiolabel was
eliminated in bile over 48 h as the sulfoxide (20%) and sulfone (5%).
Enterohepatic recirculation was probably responsible for the delayed
urinary excretion and the plasma profiles, which showed a slow
increase with peaks at 12-15 h. Urinary metabolites collected during
the first 24 h included the sulfoxide (92%), sulfone (5%), and sulfate
(3%); on days 2 and 3, the sulfoxide accounted for > 98% of daily
urinary metabolites (Nickson & Mitchell, 1994). Methyl phenyl sulfide
administered per se orally to rats or produced by methylation of
benzenethiol (No. 525) was eliminated in the urine as methyl phenyl
sulfone and hydroxylated sulfones (McBain & Menn, 1969).
The major enzyme systems involved in xenobiotic oxidation are the
cytochrome P450 superfamily (CYP-450) (Souhaili-El Amri et al., 1987)
and the flavin-containing monooxygenases (FMO) (Levi & Hodgson, 1988;
Ziegler, 1989; Hodgson & Levi, 1992). The oxidation of thioethers to
sulfoxides is catalysed by these two enzyme systems (Renwick, 1989).
Simple aliphatic (e.g. diethyl sulfide), alicyclic (e.g. thiolane),
and aromatic (e.g. ethyl para-tolyl sulfide) sulfides are oxidized
primarily by FMO and to a lesser extent, by CYP-450. The oxidation of
diethyl sulfide and thiolane in rats is catalysed almost exclusively
by FMO (Hoodi & Damani, 1984; Damani, 1987). 4-Chlorophenyl methyl
sulfide was oxidized by FMO and CYP-450 to the sulfoxide and sulfone
derivatives in vitro (Nnane & Damani, 1995), and diphenyl sulfoxide
was oxidized to the corresponding sulfone in perfused guinea-pig liver
(Yoshihara & Tatsumi, 1990).
Enzyme-catalysed thioether oxidation may be stereoselective, FMO
and CYP-450 selecting different enantiomers (Cashman & Williams,
1990). The stereochemistry of sulfoxidation by the isoenzymes of FMO
has been studied in humans (Rettie et al., 1994). When methyl
para-tolyl sulfide was incubated with human fetal liver and human
kidney microsomes in which CYP-450 activity had been eliminated, the
resulting sulfoxide showed an enantiomeric excess (> 86%) of the
N/R, not relevant to the evaluation; ND, no data reported; bw, body weight
a None of the substances in this group are predicted to be metabolized to innocuous products. They were placed in subgroups
(i)-(xii) on the basis of the position of the sulfur atom.
b The thresholds for human intake are 1800 µg/day for class I, 540 µg/day for class II, and 90 µg/day for class III.
Table 2. Annual volume and intake of aliphatic and aromatic sulfides and thiols used as
flavouring substances in Europe and the United States
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
Methyl sulfide (452)
Europe 3100 590 10
United States 2800 530 9 57 000
Methyl ethyl sulfide (453)
Europe NR NA NA
United States (A) 9 2 0.03 +
Diethyl sulfide (454)
Europe NR NA NA
United States (A) 68 13 0.22 +
Butyl sulfide (455)
Europe 19 4 0.1
United States 0.5 0.1 0.002 +
1,4-Dithiane (456)
Europe NR NA NA
United States (A) 0.45 0.1 0.001 +
(1-Butenyl-1) methyl sulfide (457)
Europe NR NA NA
United States (A) 0.45 0.1 0.001 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
Allyl sulfide (458)
Europe NR NA NA
United States 2 0.4 0.01 +
Methyl phenyl sulfide (459)
Europe NR NA NA
United States (A) 2 0.4 0.01 +
Benzyl methyl sulfide (460)
Europe 1.1 0.2 0.003
United States (1982) 0.1 0.02 0.0003 +
3-(Methylthio)propanol (461)
Europe 23 4 0.1
United States 4 1 0.01 +
4-(Methylthio)butanol (462)
Europe 0.1 0.02 0.0003
United States 0.5 0.1 0.002 +
3-(Methylthio)-1-hexanol (463)
Europe 26 5 0.1
United States 0.5 0.1 0.002 +
2-Methyl-4-propyl-1,3-oxathiane (464)
Europe 11 2 0.03
United States (A) 5 1 0.02 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
2-Methylthioacetaldehyde (465)
Europe NR NA NA
United States (1970) 3 1 0.01 +
3-(Methylthio)propionaldehyde (466)
Europe 230 45 1
United States 130 25 0.4 637
3-(Methylthio)butanal (467)
Europe 0.7 0.1 0.002
United States 0.5 0.1 0.002 +
4-(Methylthio)butanal (468)
Europe NR NA NA
United States 0.1 0.02 0.0003 -
3-Methylthiohexanal (469)
Europe NR NA NA
United States (A) 4.5 1 0.01 +
2-(Methylthio)methyl-2-butenal (470)
Europe 0.2 0.04 0.001
United States (1982) 0.5 0.1 0.002 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
2,8-Dithianon-4-en-4-carboxaldehyde (471)
Europe 0.1 0.01 0.0002
United States 0.5 0.0: 0.002 +
Methyl 3-methylthiopropionate (472)
Europe 770 146 2
United States 46 9 0.1 5
Methylthiomethyl butyrate (473)
Europe NR NA NA
United States (A) 0.45 0.1 0.001 +
Methyl 4-(methylthio)butyrate (474)
Europe 0.5 0.1 0.002
United States 0.5 0.1 0.002 -
Ethyl 2-(methylthio)acetate (475)
Europe NR NA NA
United States (A) 0.45 0.1 0.001 +
Ethyl 3-methylthiopropionate (476)
Europe 200 37 1
United States 9 2 0.03 9
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
Ethyl 4-(methylthio)butyrate (477)
Europe NR NA NA
United States (A) 11 2 0.03 -
3-(Methylthio)propyl acetate (478)
Europe NR NA NA
United States (A) 59 11 0.2 +
Methylthiomethyl hexanoate (479)
Europe NR NA NA
United States (A) 0.45 0.1 0.001 +
Ethyl 3-(methylthio)butyrate (480)
Europe NR NA NA
United States (A) 0.45 0.1 0.001 +
3-(Methylthio)hexyl acetate (481)
Europe 0.4 0.1 0.001
United States (A) 45 9 0.1 +
S-Methyl thioacetate (482)
Europe NR NA NA
United States (A) 0.01 0.002 0.00003 2
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
Ethyl thioacetate (483)
Europe 0.1 0.02 0.0003
United States 0.1 0.02 0.0003 56
Methyl thiobutyrate (484)
Europe 24 5 0.1
United States 27 5 0.1 +
Propyl thioacetate (485)
Europe 2.2 0.4 0.01
United States 0.1 0.02 0.0003 +
Methyl 2-methylthiobutyrate (486)
Europe 0.8 0.2 0.003
United States 0.5 0.01 0.002 +
S-Methyl 3-methylbutanethioate (487)
Europe NR NA NA
United States (A) 1 0.19 0.003 +
S-Methyl 4-methylpentanethioate (488)
Europe NR NA NA
United States (A) 0.0045 0.001 0.00001 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
S-Methyl hexanethioate (489)
Europe NR NA NA
United States (A) 0.45 0.1 0.001 3
Allyl thiopropionate (490)
Europe NR NA NA
United States 0.5 0.1 0.002 -
Prenyl thioacetate (491)
Europe NR NA NA
United States (A) 1 0.19 0.003 +
Methyl 2-(acetyloxy) propionate (492)
Europe NR NA NA
United States (A) 45 9 0.1 -
Methylthio 2-(propionyloxy) propionate (493)
Europe NR NA NA
United States (A) 45 9 0.1 -
3-Acetylmercaptohexyl acetate (494)
Europe NR NA NA
United States (A) 2.2 0.4 0.01 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
1-Methylthio-2-propanone (495)
Europe NR NA NA
United States (A) 1 0.2 0.003 -
1-(Methylthio)-2-butanone (496)
Europe 0.03 0.01 0.0001
United States (1982) 0.1 0.02 0.0003 +
4-(Methylthio)-2-butanone (497)
Europe 0.1 0.02 0.0003
United States 2 0.4 0.01 +
4,5-Dihydro-3(2H)-thiophenone (498)
Europe 3.6 1 0.01
United States 13 2 0.04 +
2-Methyltetrahydrothiophen-3-one (499)
Europe 100 19 0.3
United States (A) 0.5 0.1 0.002 5036
4-(Methylthio)-4-methyl-2-pentanone (500)
Europe 0.2 0.04 0.0006
United States 0.5 0.1 0.002 -
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
4-(Methylthio)-2-oxobutanoic acid, sodium salt (501)
Europe NR NA NA
United States (A) 1 0.2 0.003 +
Di(butan-3-one-1-yl) sulfide (502)
Europe NR NA NA
United States (1982) 0.1 0.02 0.0003 -
ortho-(Methylthio)phenol (503)
Europe 5 1 0.02
United States (1970) 5 1 0.02 +
S-Methyl benzothioate (504)
Europe NR NA NA
United States (A) 0.0045 0.001 0.00001 +
2-(Methylthiomethyl)-3-phenylpropenal (505)
Europe NR NA NA
United States 10 2 0.03 -
cis- and trans-Menthone-8-thioacetate (506)
Europe NR NA NA
United States (A) 2.3 0.4 0.01 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
Methylsulfinylmethane (507)
Europe NR NA NA
United States (A) 0.0045 0.001 0.00001 45 000
Methyl mercaptan (508)
Europe 440 83 1
United States 0.9 0.2 0.003 +
Propanethiol (509)
Europe 18 3 0.1
United States 38 7 0.1 360
2-Propanethiol (510)
Europe NR NA NA
United States (A) 0.022 0.004 0.0001 +
1-Butanethiol (511)
Europe 3.2 0.5 0.01
United States 0.2 0.04 0.001 +
2-Methyl-1-propanethiol (512)
Europe NR NA NA
United States (A) 6.8 1.3 0.021 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
3-Methylbutanethiol (513)
Europe NR NA NA
United States (A) 0.045 0.01 0.0001 23
2-Pentanethiol (514)
Europe 12 2 0.04
United States (A) 11 2 0.03 +
2-Methyl-1-butanethiol (515)
Europe 2.5 0.5 0.01
United States (1982) 0.1 0.02 0.0003 +
Cyclopentanethiol (516)
Europe NR NA NA
United States (A) 4.5 1 0.01 -
3-Methyl-2-butanethiol (517)
Europe 0.1 0.02 0.0003
United States 0.1 0.02 0.0003 +
1-Hexanethiol (518)
Europe NR NA NA
United States (A) 0.045 0.01 0.0001 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
2-Ethylhexanethiol (519)
Europe NR NA NA
United States (A) 0.045 0.01 0.0001 +
2,3, or 10-Mercatopinane (520)
Europe 0.3 0.1 0.001
United States 50 10 0.2 -
Allyl mercaptan (521)
Europe 1.3 0.2 0.003
United States 8 2 0.03 480
Prenythiol (522)
Europe NR NA NA
United States (A) 1 0.2 0.003 +
1-para-Menthene-8-thiol (523)
Europe 2.8 1 0.01
United States (A) 4.5 1 0.01 +
Thiogeraniol (524)
Europe 9 2 0.03
United States 0.1 0.02 0.0003 -
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
Benzenethiol (525)
Europe 6 1 0.02
United States 160 30 1 +
Benzyl mercaptan (526)
Europe 10 2 0.03
United States 2 0.4 0.01 +
Phenethyl mercaptan (527)
Europe NR NA NA
United States (A) 1 0.2 0.003 +
ortho-Toluenethiol (528)
Europe 140 27 0.4
United States 0.9 0.2 0.003 +
2-Ethylthiophenol (529)
Europe 0.001 0.0002 0.0
United States 0.5 0.10 0.002 -
2,6-Dimethylthiophenol (530)
Europe 11 2 0.03
United States 0.1 0.02 0.0003 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
2-Naphthalenethiol (531)
Europe NR NA NA
United States (A) 0.34 0.1 0.001 +
1,2-Ethanedithiol (532)
Europe 0.01 0.002 0.00003
United States (A) 4.5 0.9 0.01 +
Bis(methylthio)methane (533)
Europe NR NA NA
United States (A) 500 94 2 +
2-Methyl-1,3-dithiolane (534)
Europe 0.5 0.1 0.002
United States (A) 23 4 0.1 +
1,3-Propanedithiol (535)
Europe 7 1 0.02
United States (A) 4.5 0.9 0.01 +
1,2-Propanedithiol (536)
Europe NR NA NA
United States (A) 4.5 0.9 0.01 -
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
1,2-Butanedithiol (537)
Europe NR NA NA
United States 0.9 0.2 0.003 -
1,3-Butanedithiol (538)
Europe NR NA NA
United States (A) 4.5 0.9 0.01 -
2,3-Butanedithiol (539)
Europe 0.4 0.1 0.001
United States 0.9 0.2 0.003 -
1,6-Hexanedithiol (540)
Europe 13 2.5 0.04
United States 0.5 0.1 0.002 +
1,8-Octanedithiol (541)
Europe 17 3 0.1
United States (A) 4.5 0.9 0.01 -
1,9-Nonanedithiol (542)
Europe 0.01 0.002 0.00003
United States (A) 4.5 0.9 0.01 -
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
Trithioacetone (543)
Europe 12 2 0.04
United States 2 0.4 0.01 +
3-Mercapto-3-methyl-1-butanol (544)
Europe NR NA NA
United States (A) 10 1.9 0.03 +
3-Mercaptohexanol (545)
Europe NR NA NA
United States (A) 4.5 1 0.01 +
2-Mercapto-3-butanol (546)
Europe 33 6 0.1
United States 0.5 0.1 0.002 -
alpha-Methyl-ß-hydroxypropyl alpha-methyl-ß-mercaptopropyl sulfide (547)
Europe NR NA NA
United States 4 1 0.01 -
4-Methyoxy-2-methyl-2-butanethiol (548)
Europe NR NA NA
United States (A) 4 0.8 0.01 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
3-Mercapto-3-methylbutyl formate (549)
Europe NR NA NA
United States (A) 0.5 0.1 0.002 +
2,5-Dihydroxy-1,4-dithiane (550)
Europe NR NA NA
United States (A) 0.45 0.1 0.001 -
2-Mercaptopropionic acid (551)
Europe 17 3 0.1
United States 440 84 1 -
Ethyl 2-mercaptopropionate (552)
Europe 3.2 0.5 0.01
United States 2 0.4 0.01 +
Ethyl 3-mercaptopropionate (553)
Europe 0.6 0.1 0.002
United States (A) 230 43 1 +
3-Mercaptohexyl acetate (554)
Europe NR NA NA
United States (A) 1 0.2 0.003 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
3-Mercaptohexyl butyrate (555)
Europe NR NA NA
United States (A) 1 0.2 0.003 +
3-Mercaptohexyl hexanoate (556)
Europe NR NA NA
United States (A) 1 0.2 0.003 +
1-Mercapto-2-propanone (557)
Europe NR NA NA
United States (A) 0.45 0.09 0.001 +
3-Mercapto-2-butanone (558)
Europe 26 5 0.1
United States (1982) 0.2 0.04 0.001 +
2-Keto-4-butanethiol (559)
Europe NR NA NA
United States 0.5 0.1 0.002 -
3-Mercapto-2-pentanone (560)
Europe NR NA NA
United States 0.5 0.1 0.002 -
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
para-Mentha-8-thiol-3-one (561)
Europe 86 16 0.3
United States 9 2 0.03 +
2,5-Dimethyl-2,5-dihydroxy-1,4-dithiane (562)
Europe 1.2 0.2 0.004
United States 0.9 0.2 0.003 -
Sodium 3-mercaptooxopropionate (563)
Europe NR NA NA
United States (A) 1 0.2 0.003 -
Dimethyl disulfide (564)
Europe 57 11 0.2
United States 13 2 0.04 1 400
Methyl propyl disulfide (565)
Europe 32 6 0.10
United States 0.1 0.02 0.0003 13 000
Propyl disulfide (566)
Europe 28 5 0.1
United States 0.5 0.1 0.002 14 000
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
Diisopropyl disulfide (567)
Europe NR NA NA
United States (A) 41 8 0.1 +
Allyl methyl disulfide (568)
Europe 0.01 0.002 0.00003
United States (1982) 0.1 0.02 0.0003 +
Methyl 1-propenyl disulfide (569)
Europe NR NA NA
United States (1982) 4 1 0.01 1
Propenyl propyl disulfide (570)
Europe NR NA NA
United States (1970) 40 8 0.1 +
Methyl 3-methyl-1-butenyl disulfide (571)
Europe NR NA NA
United States (A) 0.45 0.1 0.001 +
Allyl disulfide (572)
Europe 480 92 2
United States 44 8 0.1 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
3,5-Dimethyl-1,2,4-trithiolane (573)
Europe 0.2 0.04 0.001
United States (1982) 0.5 0.0: 0.002 +
3-Methyl-1,2,4-trithiane (574)
Europe 0.6 0.1 0.002
United States (A) 230 43 1 +
Dicyclohexyl disulfide (575)
Europe 0.1 0.02 0.0003
United States 0.9 0.2 0.003 -
Methyl phenyl disulfide (576)
Europe NR NA NA
United States (A) 1 0.2 0.003 +
Methyl benzyl disulfide (577)
Europe 0.1 0.02 0.0003
United States 0.5 0.1 0.002 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
Phenyl disulfide (578)
Europe NR NA NA
United States (1982) 0.2 0.04 0.001 -
Benzyl disulfide (579)
Europe 0.1 0.01 0.0002
United States (1982) 0.5 0.1 0.002 +
2-Methyl-2-(methyldithio) propanal (580)
Europe NR NA NA
United States (A) 9 2 0.03 +
Ethyl 2-(methyldithio)propionate (581)
Europe NR NA NA
United States (A) 0.45 0.1 0.001 +
Dimethyl trisulfide (582)
Europe 9 2 0.03
United States 0.1 0.02 0.0003 3 000
Methyl ethyl trisulfide (583)
Europe NR NA NA
United States (A) 3 1 0.01 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
Methyl propyl trisulfide (584)
Europe 1.7 0.3 0.01
United States 0.4 0.1 0.001 13 000
Dipropyl trisulfide (585)
Europe 60 11 0.2
United States 5 1 0.02 16 000
Allyl methyl trisulfide (586)
Europe NR NA NA
United States (A) 4.5 0.9 0.01 +
Diallyl trisulfide (587)
Europe 29 6 0.1
United States (1970) 0.1 0.02 0.0003 +
Diallyl polysulfide (588)
Europe 10 2 0.03
United States 0.1 0.02 0.0003 -
TOTAL
Europe 6 100
United States 5 300
A, volume anticipated on the basis of recent estimates (communication from the Flavor and Extract
Manufacturers' Association to the Committee); NR, not reported; NA, not applicable; +, reported
to occur naturally in foods (CIVO-TNO, 1994), but quantitative data were not available; -, not
reported to occur naturally in foods
a Volumes reported in a survey in Europe (International Organization of the Flavor
Industry, 1995) and in a survey in the United States (National Academy of Sciences, 1989).
Most of the data for the United States are for 1987 (and there is no entry); when data
for 1987 were not available, data for 1982 or 1970 were used, as indicated.
b Per capita intake (mg/day) calculated as follows:
[(annual usage, kg) × (1 × 109 mg/kg) / (population × 0.6 × 365 days)], where population
(10% 'eaters only') = 32 × 106 for Europe and 24 × 106 for the USA; 0.6 represents the
assumption that only 60% of the annual usage of the flavour was reported in the survey.
Intake (mg/kg bw per day) calculated as follows: [(mg/day)/body weight], where body weight = 60 kg.
c From Stofberg & Kirschman (1985); Stofberg & Grundschober (1987)
Potential toxicity can be deduced by comparison with structural
analogues on the basis of metabolic similarities. In the absence of
information on the toxicity of structural analogues, however, it is
not possible to conclude a priori that the substances are
metabolized to innocuous products.
(i) Simple sulfides (thioethers)
Once alkyl and aromatic thioethers, commonly called 'sulfides',
enter the systemic circulation, they are rapidly oxidized to
sulfoxides and, depending on the structure of the thioether, may be
further oxidized to sulfones. Sulfoxides and sulfones are major
urinary metabolites of simple sulfides. Aliphatic thioethers (Nos
452-455, 457, 458, and 533) and thioethers containing an aromatic
nucleus (Nos 459 and 460) yield mixtures of sulfoxide and sulfone
metabolites. Enzymes of the cytochrome P450 superfamily and
flavin-containing monooxygenases catalyse the oxidation of thioethers
to sulfoxides. Oxidation of sulfoxides to the corresponding sulfones
occurs both in tissues and in aerobic microorganisms and is an
irreversible metabolic reaction in mammals. Sulfoxides can also be
metabolized back to the thioether by aldehyde oxidase, thioredoxin and
its reductase, and the gut microflora in the anaerobic environment of
the lower bowel.
The methyl aromatic thioethers (Nos 459 and 460) are predicted to
be major metabolites of the corresponding aromatic thiols (Nos 525 and
526, subgroup iv) and would be oxidized to sulfoxides and sulfones,
which would be excreted.
(ii) Acyclic sulfides with oxidized side-chains
The presence of other functional groups, such as alcohol (Nos
461-463), aldehyde (Nos 465-471 and 505), ester (Nos 472-481), acid
(No. 501), ß-ketone (Nos 495-497, 500, and 502), and phenol (No. 503),
provides centres of greater polarity and additional sites for the
biotransformation of thioethers. The presence of these polar groups
would also result in increased renal excretion. The biotransformations
of such oxygenated, carbon-containing, functional groups are well
characterized and have been described for groups of flavouring agents
previously evaluated by the Committee. Concurrent metabolism of
various substrates at both sulfur and oxygenated functional groups has
been reported, and sulfoxide formation usually predominates as the
major metabolic pathway of detoxification. Experiments in vitro
suggest that hydrolysis of carboxyl esters occurs in the presence of
thioether (sulfide) groups. In consequence, thioethers with oxidized
side-chains would be expected to be eliminated more rapidly than
simple sulfides.
(iii) Cyclic sulfides
Oxidation of unsubstituted and methyl-substituted cyclic
thioethers by the cytochrome P450 superfamily produces the
corresponding sulfoxides. The mono-sulfoxides are predicted to be the
main urinary metabolites of simple cyclic sulfides (Nos 456, 534, and
543). The metabolism of cyclic sulfides containing oxidized carbon
atoms (Nos 464, 498, 499, 550, and 562) has not been studied but would
be predicted to involve extensive S-oxidation and possibly oxidation
or conjugation of alcohol groups. The polarity of the hydroxy
thioethers (Nos 550 and 562) may allow their elimination unchanged.
(iv) Simple thiols
The simple thiol flavouring agents considered are alkyl and
alicyclic thiols (Nos 508-524, 526, and 527) and aromatic thiols
(thiophenols; Nos 525 and 528-531). These substances may be
metabolized along several pathways. Simple aliphatic and aromatic
thiols undergo S-methylation in mammals to produce the corresponding
methyl thioether or sulfide. Methylation is catalysed by thiopurine
methyltransferase in the cytoplasm and thiol methyltransferase in
microsomes, and both reactions require S-adenosyl-l-methionine as a
methyl group donor. Thiopurine methyltransferase is present in human
liver, kidney, and erythrocytes; preferential substrates for this
enzyme include aromatic and heterocyclic thiols. S-Methylation of
aliphatic thiols is catalysed by microsomal thiol methyltransferase,
and the resulting methyl thioether (sulfide) metabolite would undergo
S-oxidation to give the methyl sulfoxide and methyl sulfone
analogues as urinary products.
Thiols may react with glutathione and other endogenous thiol
substances to form mixed disulfides. Both microsomal and cytoplasmlic
thioltransferases have been reported to catalyse the formation of
mixed disulfides. The resulting mixed disulfides can undergo reduction
back to thiols, oxidative desulfuration, or oxidation to a sulfonic
acid via the intermediate thiosulfinate and sulfinic acids. The
principal form in the circulation would probably be a mixed disulfide
formed with albumin.
S-Glucuronidation of aromatic thiols has been reported, and
this may be a pathway for the metabolism of aromatic thiols
(thiophenols) (Nos 525 and 528-531) and simple aromatic disulfides
(Nos 576 and 578; subgroup vii) after their reduction (see below).
Glucuronyl transferases behave similarly towards hydroxyl and
sulfydryl groups, and the two activities have the same subcellular
location and optimal pH.
Thiols may be oxidized to form sulfenic acids (RSOH), which are
unstable and readily undergo further oxidation to sulfinic (RSO2H)
and sulfonic (RSO3H) acids or combine with nucleophiles. The sulfonic
acid group is a highly polar centre and makes molelcules highly
soluble in water. In general, sulfonic acids are stable to metabolism.
Alkyl thiols of low relative molecular mass undergo oxidative
desulfuration in vivo to yield CO2 and SO4=. This reaction has
been shown, for example, for methanethiol (methyl mercaptan; No. 508).
Whereas the carbon atom from thiols may be used in the biosynthesis of
amino acids, the sulfur atom is not used significantly in the
synthesis of sulfur-containing amino acids.
(v) Thiols with oxidized side chains
Although alkyl thiols with oxidized side-chains (Nos 544-549,
551-561, and 563) comprise a significant proportion of the flavouring
agents evaluated, their metabolic fate has not been studied. Their
metabolism is predicted to involve a combination of the pathways
described above for simple thiols and further oxidation or conjugation
of the oxidized side-chain. The class III compound, sodium
3-mercapto-oxopropionate (No. 563), would be expected to be eliminated
very rapidly by metabolism at both the thiol and keto-acid groups.
(vi) Dithiols
The metabolism of the simple aliphatic dithiols evaluated (Nos
532 and 535-542) is predicted to involve the pathways described above
for simple thiols. Urinary metabolites could result from methylation,
S-oxidation of one S atom to yield a polar sulfonate, and the
formation of mixed disulfides of low relative molecular mass such as
cysteine, an endogenous thiol. The longer, linear dithiols (Nos 535
and 540-542) could form intramolecular disulfide bonds, with
interconversion between dithiol and cyclic disulfide forms.
(vii) Simple disulfides
The reduction of xenobiotic disulfides is believed to be
extensive, and the reaction may be catalysed enzymatically by
thioltransferases and chemically by exchange with glutathione,
thioredoxin, cysteine, and other endogenous thiols. Reduction of the
non-cyclic disulfides considered in the group of flavours (Nos 564-572
and 575-579) would result in the formation of thiols of low relative
molecular mass, which would then be metabolized by the various
pathways described above for simple thiols.
(viii) Disulfides with oxidized side-chains
As discussed above for subgroups (ii) and (iii), additional sites
of carbon oxidation would result in greater polarity and further
oxidation or conjugation of the flavouring agents evaluated (Nos 580
and 581). However, as disulfide bonds in substances in subgroup (v)
are susceptible to reductive cleavage, this would be expected to be
the initial metabolic reaction, and the polarity of the side-chains
would primarily affect elimination of the thiol fragments.
(ix) Trisulfides and polysulfides
The trisulfide of glutathione is labile and readily converted to
the disulfide, the sulfur being released as hydrogen sulfide.
Trisulfides and polysulfides are predicted to be converted rapidly to
the corresponding disulfides, which would then be reduced to thiols,
which themselves would follow the pathways described above for thiols.
The potential toxicity of trisulfides (Nos 582-587) and polysulfides
(No. 588) is probably related to their metabolic lability and to the
nature of the eventual thiol (e.g. allyl thiol).
(x) Heterocyclic disulfides: The heterocyclic disulfides (Nos 573 and
574) are five-and six-carbon rings which also contain a cyclic
thioether bond. Lipoic acid, an endogenous substance, is a
five-membered cyclic disulfide which undergoes rapid redox cycling
between ring disulfide and open dithiol forms. The principal metabolic
pathways are predicted to be disulfide reduction with ring opening to
produce a dithiol, and S-oxidation of the cyclic thioether.
(xi) Thioesters
Thioester groups (-S-CO-) are present in a number of the
flavouring agents evaluated (Nos 482-494, 504, and 506). Hydrolysis of
esters has been considered previously by the Committee, but that of
thioesters has not. Thioesters are hydrolysed by lipase and esterases,
and the rate of hydrolysis increases as the length of the C-chain of
the carboxylic acid fragment increases, and decreases as oxygenation
of the carbon chain in the thiol moiety increases.
Hydrolysis could result in a thioic acid and an alcohol or a
carboxylic acid and a thiol (the metabolic fates of which have been
outlined above). Data for dithioic acids and esters indicate that the
esters of monothioic acids would be poor substrates for oxidation, but
the monothioic acid released by hydrolysis would be oxidized to the
dioxo acid. Other possibilities for elimination in vivo include renal
excretion of thiocarboxylic acid. The substances evaluated (with the
exceptions of prenyl thioacetate (No. 491) and allyl thiopropionate
(No. 490)) are simple linear alkyl compounds, branched-chain alkyl
compounds, or their side-chain hydroxyester analogues, so that
comparison of the toxicity of the different substances is reasonable.
(xii) Sulfoxides
The metabolism of sulfoxides by oxidation and reduction is
described above under 'Thioethers'. The only sulfoxide flavouring
agent evaluated was methylsulfinylmethane (dimethyl sulfoxide, DMSO;
No. 507), for which data were available on both metabolism and
toxicity in experimental animals and humans. DMSO is readily absorbed
and excreted in urine as the parent sulfoxide and dimethyl sulfone.
1.4 Application of the Procedure for the Safety
Evaluation of Flavouring Agents
Step 1. In applying the Procedure for the Safety Evaluation of
Flavouring Agents to the above-mentioned aliphatic and
aromatic sulfides and thiols, the Committee assigned 97 of
the 137 substances (Nos 452-455, 457, 461-463, 465-497, 500,
502, 508-515, 517-519, 522, 524, 532, 533, 535-542, 544-560,
562, 564-567, 569-571, and 580-585), which may or may not
contain an additional oxygenated functional group, to
structural class I. The Committee assigned 34 of the 137
substances to structural class II because they are aromatic
sulfides or thiols (Nos 459, 460, 503, 504, 525-528, 530,
531, 576, 577, and 579), alicyclic (Nos 506, 516, 520, 523,
561, and 575) or heterocyclic substances (Nos 456, 464, 498,
499, 534, 543, 573, and 574), or allyl mercaptan or sulfides
(Nos 458, 521, 568, 572, and 586-588), which are common
components of food. The aromatic thiols or thioethers that
are not common compo-nents of food (Nos 505, 529, and 578)
were assigned to structural class III. The remaining three
substances were also assigned to structural class III by
virtue of the fact that they are aliphatic thiols or
thioethers containing more than three functional groups (Nos
501 and 563) or do not contain divalent sulfur (No. 507).
Step 2. None of the substances in this group can be predicted to be
metabolized to innocuous products. The evaluation of these
substan-ces therefore proceeded via the right-hand side of
the decision tree.
Step B3. The estimated daily per capita intake in Europe and in the
United States for each of the substances in the overall
group of 137 flavouring agents is below the threshold for
human intake for the structural class in which the substance
falls. The thresholds are 1800 µg/person per day for class
I, 540 µg/person per day for class II, and 90 µg/person per
day for class III.
Step B4. The Committee considered the results of 90-day studies of
toxicity in rodents for 27 substances in this group of
flavouring agents. The Committee noted that the single or
multiple doses of flavouring agents tested in a number of
studies had no effect in rats and that the NOELs were
consequently derived from studies that did not show toxic
effects. The results of long-term studies in multiple
species were considered for one substance, DMSO (No. 507).
To facilitate comparisons of the toxicity of structurally
related substances, the agents were considered in subgroups
(i)- (xii) on the basis of the position of the sulfur atom
(see Tables 1 and 3). Toxicity was compared within and
across subgroups with no restriction on the basis of
structural class assignment (Step 1).
(i) Simple sulfides (thioethers): This subgroup comprises
nine flavouring agents that are simple thioethers. The NOEL
for methyl sulfide (No. 452) in a 14-week study in rats
treated with multiple doses by gavage was 250 mg/kg bw per
day. This NOEL provides an adequate basis for evaluation of
five structurally and metabolically related substances (Nos
453, 454, 455, 457, and 533); however, the NOEL for methyl
sulfide was considered inappropriate for evaluation of allyl
sulfide (No. 458) and two aromatic thioethers, methyl phenyl
sulfide and benzyl methyl sulfide (Nos 459 and 460,
respectively), and the evaluation of these three substances
therefore proceeded to step B5.
(ii) Acyclic sulfides with oxidized side-chains: This
subgroup comprises 28 flavouring agents that are acyclic
thioethers with oxidized side-chains. The NOEL in a 90-day
study in rats treated with a single dose of
2-(methylthiomethyl)-3-phenylpropenal (No. 505) was 1.4
mg/kg bw per day. This substance is an aromatic compound
with a sulfide group in an unsaturated side-chain; it was
assigned to structural class III, because it is not a common
component of food. Data for methyl sulfide (No. 452) were
also considered relevant for assessing the toxicity of
sulfide substances with oxidized side-chains (Nos 461-463,
465-469, 472-481, 495-497, and 500-503). Although
2-(methylthiomethyl)-3-phenylpropenal (No. 505) is not an
aryl thioether, the safety margin between its NOEL and the
intake of ortho-(methylthio)phenol pentanone (No. 503) was
considered to be adequate. The NOEL for
2-(methylthiomethyl)-3-phenylpropenal (No. 505) does not
provide a high margin of safety (i.e. > 1000) for methyl
3-methylthiopropionate (No. 472) at the current estimated
level of intake; however, the simple side-chain acid and
ester would have little toxic potential, and the NOEL for
methyl sulfide (No. 452) provides an adequate margin of
safety. The NOELs for 2-(methylthio-methyl)-3-phenylpropenal
(No. 505) and methyl sulfide (No. 452, subgroup (i)) were
considered inappropriate for evaluation of the toxicity of
two alpha,beta-unsaturated carbonyls (Nos 470 and 471)
because they are potentially more reactive and toxic, and
the evaluation of these substances therefore proceeded to
step B5.
(iii) Cyclic sulfides: This subgroup comprises eight
substances that are cyclic thioethers. NOELs of 0.44 mg/kg
bw per day for 2-methyl-4-propyl-1,3-oxathiane (No. 464),
9.2 mg/kg bw per day for 4,5-dihydro-3(2 H)-thiophenone
(No. 498), 7 mg/kg bw per day for 2-methyl-1,3-dithiolane
(No. 534), 0.2 mg/kg bw per day for trithioace-tone (No.
543), and 3.1 mg/kg bw per day for
2,5-dimethyl-2,5-dihydroxy-1,4-dithiane (No. 562) were
reported. These values provide an adequate margin of safety
for evaluation of the toxicity of substances No. 456, 499,
and 550.
Table 3. Comparison of the toxicity and intake data used in the safety evaluations of
137 simple aliphatic and aromatic sulfides and thiols, by subgroupa
Subgroup Adequate NOEL Adequate NOEL No adequate NOEL
for substanceb for structurally for substance or
related substanceb related substance,
but intake
< 1.5 µg/dayc
(i) Simple sulfides No. 452 Nos 453-455, Nos 458-460
(thioethers) 457, 533
(ii) Acyclic thioethers No. 505 Nos 461-463, Nos 470, 471
with oxidized 465-469, 472-481,
side-chains 495-497, 500-503
(iii) Cyclic sulfides Nos 464, 498, Nos 456, 499, N/R
534, 543, 562 550
(iv) Thiols Nos 516, 520, Nos 508-515, N/R
528, 530, 531 517-519, 521-527,
529
(v) Thiols with Nos 546, 547, Nos 544, 545, 548, N/R
oxidized 560 549, 551-559, 561,
side-chains 563
(vi) Dithiols Nos 539, 541 Nos 532, 535-538, N/R
540, 542
(vii) Simple disulfides Nos 566, 575, Nos 564, 565, N/R
577 567-572, 576, 578,
579
(viii) Disulfides with None Nos 580, 581 N/R
oxidized
side-chains
(ix) Trisulfides and Nos 585, 587 Nos 582-584, 586, N/R
polysulfides 588
(x) Heterocyclic No. 573 No. 574 N/R
disulfides
Table 3. (continued)
Subgroup Adequate NOEL Adequate NOEL No adequate NOEL
for substanceb for structurally for substance or
related substanceb related substance,
but intake
< 1.5 µg/dayc
(xi) Thioesters Nos 483, 484 Nos 482, 485-494, N/R
504, 506
(xii) Sulfoxides No. 507 N/R N/R
N/R, not relevant to the evaluation
a See Table 1 for further details of the evaluations.
b See Figure 1, step B4 and pp. 121-123 for further information.
c See Figure 1, step B5 and pp. 121-123 for further information.
(iv) Simple thiols: This subgroup comprises 24 flavouring
agents that are simple thiols. NOELs of 0.56 mg/kg bw per
day for cyclopentanethiol (No. 516), 0.06 mg/kg bw per day
for 2,3-and 10-mercaptopinane (No. 520), 0.52 mg/kg bw per
day for ortho-toluenethiol (No. 528), 0.43 mg/kg bw per
day for 2,6-dimethyl-thiophenol (No. 530), and 3.4 mg/kg bw
per day for 2-naphthalenethiol (No. 531) were reported.
These values provide adequate margins of safety for the
individual substances and for the other structurally related
thiols in the group in relation to current estimates of
intake, with the exception of 2,3 and 10-mercaptopinane (No.
520). A margin of safety of about 300 (based on an estimated
modified per capita intake of 0.2 µg/kg bw per day in the
United States) was obtained from the results of a 90-day
study in which only a single dose of 0.06 mg/kg bw per day
was tested. This substance was therefore also evaluated by
comparison with other substances in this subgroup (Nos 516,
528, 530, and 531) for which there was an adequate margin of
safety. The Committee noted that Nos 521-524 are unsaturated
thiols. Of these, allyl mercaptan (allyl thiol; No. 521)
would be expected to be more toxic than others that have
double bonds in different positions (by analogy to their
oxygenated analogues). Although data were not available on
allyl mercaptan (No. 521), the NOEL of diallyl trisulfide
(No. 587), which would be converted to allyl mercaptan after
reduction to allyl disulfide, was 4.6 mg/kg bw per day in a
90-day study in rats. This NOEL was considered to provide an
adequate safety margin for Nos 521-524.
(v) Thiols with oxidized side-chains: This subgroup
comprises thiols with oxygenated side-chains. The NOELs
available for three of the substances in class I (1.9 mg/kg
bw per day for 2-mercapto-3-butanol (No. 546), 2.8 mg/kg bw
per day for alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide (No. 547), and 1.9
mg/kg bw per day for 3-mercapto-2-pentanone (No. 560)) were
considered to provide adequate safety margins for the
flavouring agents in this subgroup, including the one
substance in structural class III, 3-mercapto-oxopropionate
(No. 563). Although the last compound has more than three
functional groups, the oxopropionate moiety would have
little toxic potential, and the NOELs for Nos 546, 547, and
560 were considered to provide an adequate margin of safety.
(vi) Dithiols: This subgroup comprises flavouring agents
that are dithiols. The NOEL for 2,3-butanedithiol (No. 539)
and 1,6-hexane-dithiol (No. 540) was 0.7 mg/kg bw per day,
which provides adequate margins of safety for all of the
substances in the subgroup.
(vii) Simple disulfides: This subgroup comprises 14
flavouring agents that are disulfides. The major metabolites
of these unsaturated disulfides would be thiols. The NOELs
were 7.7 mg/kg bw per day for propyl disulfide (No. 566),
0.23 mg/kg bw per day for dicyclohexyl disulfide (No. 575),
and 1.2 mg/kg bw per day for methyl benzyl disulfide (No.
577). These values provide adequate margins of safety for
each substance tested and for four structurally related
substances, Nos 564, 565, 567, and 579, at currently
estimated levels of intake. The substances in this subgroup
for which NOELs are available do not include unsaturated or
aryl disulfides. The aryl disulfides, methyl phenyl
disulfide and phenyl disulfide (Nos 576 and 578,
respectively), would be rapidly reduced to thiophenol, and
the NOEL of 3.4 mg/kg bw per day for 2-naphtha-lenethiol
(No. 531, subgroup iv) was considered to provide an adequate
safety margin for these agents. The Committee was aware that
propenyl disulfides can cause haemolytic anaemia in certain
species after short-term exposure. This effect would be of
concern to susceptible individuals. Substances Nos 568 and
572 would be metabolized to allyl mercaptan (No. 521;
subgroup iv). The Committee noted that the NOEL for diallyl
trisulfide (No. 587; subgroup ix) in a 90-day study in rats
given a single dose was 4.6 mg/kg bw per day, and that this
would provide an adequate safety margin for the allyl thiol
produced on reduction of substances Nos 568 and 572.
Di(1-propenyl) disulfide was about four times more potent
than allyl disulfide (No. 572) and about 20 times more
potent than propyl disulfide (No. 566). The intakes of the
related propenyl and butenyl flavours Nos 569, 570, and 571
gave safety margins of > 50 000 in comparison with the NOEL
for propyl disulfide (No. 566), and this was considered to
be adequate to allow for the differences in potency.
(viii) Disulfides with oxidized side-chains: This subgroup
consists of two disulfides with oxidized side-chains,
2-methyl-2-(methyldithio)-propanal (No. 580) and ethyl
2-(methyldithio)propionate (No. 581). The toxicity of these
agents has not been studied, but the Committee considered
these substances by analogy to disulfides (subgroup vii) and
concluded that adequate margins of safety were available,
given their greater polarity and the presence of thiols with
and without oxidized side-chains, i.e. subgroups (iv) and
(v).
(ix) Trisulfides and polysulfides: This subgroup consists
of six trisulfides and one polysulfide. NOELs of 4.8 mg/kg
bw per day for dipropyl trisulfide (No. 585) and 4.6 g/kg bw
per day for diallyl trisulfide (No. 587) were reported,
which gave adequate margins of safety for all substances in
this subgroup.
(x) Heterocyclic disulfides: This subgroup comprises two
flavouring agents that are heterocyclic disulfides. The NOEL
of 1.9 mg/kg bw per day for 3,5-dimethyl-1,2,4-trithiolane
(No. 573) provides an adequate margin of safety for this
substance at current levels of use. 3-Methyl-1,2,4-trithiane
(No. 574) was reported to have no effect at the single dose
of 0.3 mg/kg bw per day tested in a 90-day study; this dose
provides a margin of safety of only 100 at the estimated
modified per capita intake level of 1 µg/kg bw per day in
the United States. However, the NOEL for the closely related
compound 3,5-dimethyl-1,2,4-trithiolane provides an adequate
margin of safety (> 1000).
(xi) Thioesters: This subgroup consists of 15 thioesters.
The NOELs of 6.5 mg/kg bw per day for ethylthioacetate (No.
483) and 1000 mg/kg bw per day for methylthiobutyrate (No.
484) give an adequate margin of safety for all other esters
in this group. The conclusion that the current intake levels
of allyl thiopropionate and prenyl thioacetate are safe is
supported by the NOEL of 4.6 mg/kg bw per day for the
unsaturated diallyl trisulfide (No. 587), which would be
reduced to allyl disulfide and then allyl sulfide.
(xii) Sulfoxides: This subgroup consists of only one
substance, DMSO (No. 507). The NOEL in monkeys given DMSO by
gavage for 74-87 weeks was 3000 mg/kg bw per day. This NOEL
and other data provide an adequate margin of safety for the
use of DMSO as a flavouring agent at the estimated modified
daily per capita intake of 0.001 µg/day in the United
States.
Step B5. Five substances, allyl sulfide (No. 458), methyl phenyl
sulfide (No. 459), benzyl methyl sulfide (No. 460),
2-(methylthio)methyl-2-butenal (No. 470), and
2,8-dithianon-4-ene-carboxaldehyde (No. 471), were evaluated
at this step of the Procedure. The modified daily per
capita intake of all five substances is less than 1.5 µg
in Europe and in the United States. Applying the criteria
for Step B5 outlined in Annex 5 of the evaluations published
after its forty-ninth meeting Annex 1, reference 132), the
Committee concluded that use of these substances at their
current levels of intake poses no safety concern.
In summary, for 100 agents in subgroups (iii) to (xii), a NOEL
was available for the substance, a closely related substance, or a
predicted major metabolite that provided an adequate margin of safety
(> 1000). For 6/9 of the agents in subgroup (i) and 26/28 in subgroup
(ii), a NOEL for the substance or a closely related substance was
available that provided an adequate margin of safety (i.e. > 1000).
Therefore, the Committee determined at Step B4 of the Procedure that
the safety of these 132 substances would be expected to be of no
concern when they are used at their currently estimated level of daily
intake. The evaluation of the remaining five substances (Nos 458, 459,
and 460 in subgroup (i) and Nos 470 and 471 in subgroup (ii))
proceeded to Step B5 of the Procedure. The comparisons of toxicity and
the intake considerations for each subgroup that were used to apply
Steps B4 and B5 of the Procedure to the evaluation of individual
substances in this group of flavouring agents are summarized in Table
3 and given in more detail in Table 1.
1.5 Consideration of combined intakes
In the unlikely event that all 137 aliphatic and aromatic
sulfides and thiols in the overall group or in subgroups were to be
consumed simultaneously on a daily basis, the estimated combined
intake would not exceed the human intake threshold for class I
substances. The powerful aroma of these substances limits the level of
their use in foods.
1.6 Conclusions
The Committee concluded that the 137 flavouring agents comprising
aromatic sulfides and thiols evaluated at the present meeting could
not be predicted to be metabolized to innocuous products. According to
the Procedure, data on toxicity were needed to evaluate the safety of
this group of flavouring agents. The primary data that were used in
the evaluations consisted of 27 90-day studies in rodents with 25 of
the substances and long-term studies in multiple species for one
substance (DMSO). Most of these studies were conducted at a single
dose or multiple doses, which had no effects. The Committee noted that
the NOELs were thus derived from studies in which no toxic effects
were seen.
On the basis of the available data on the toxicity of
representative substances in each subgroup and on their metabolism,
the Committee determined that the safety of 132 of the flavouring
agents poses no concern when they are consumed at their current levels
of intake. The safety of the remaining five substances was considered
to pose no concern when they are consumed at levels of intake < 1.5
µg/day.
Other data on toxicity, including the results of short-term tests
for toxicity and studies of teratogenicity and genotoxicity, were
consistent with the results of the safety evaluation.
2. RELEVANT BACKGROUND INFORMATION
2.1 Explanation
The 137 compounds evaluated have a diverse array of chemical
structures, each of which contains a sulfur atom attached to a carbon,
hydrogen, oxygen, or second sulfur atom. The substances were separated
into 12 subgroups on the basis of the position of the sulfur atom, as
indicated in section 1.3 above. The key data on metabolism and
toxicity relevant to the safety evaluation are presented below by
subgroup. Information on intake, special studies, and observations in
humans are also discussed.
2.2 Additional considerations on intake
Thiols (i.e. mercaptans), thioethers, and their oxygenated
derivatives are known for their powerful aromas. They provide cooked,
browned, and roasted flavours when added as flavouring substances.
Their strong organoleptic properties make a significant contribution
to the taste and smell of various foods even when they are added at
low concentrations. The thresholds of odour detection for aromatic and
aliphatic thiols and thioethers range from 0.08 µg/m3 for amyl
mercaptan (not in any subgroup) to 0.03 mg/m3 for benzyl mercaptan
(No. 526); the odour threshold for methyl sulfide (No. 452), the
thioether with the highest annual volume of use as a flavouring
substance, is about 1 µg/m3, and that for the corresponding thiol,
methyl mercaptan (No. 508), is 2 µg/m3 (Farr & Kirwin, 1994). In
tests for sensory tolerance, most of the panelists reported that
atmospheres containing thiols, thioesters, or thioethers at
concentrations > 1 mg/m3 are intolerable (Flavor and Extract
Manufacturers' Association, 1996).
A direct result of the extremely low odour threshold of thiols
and thioethers is that their use in food is self-limiting, because
high concentrations would result in foods that are repulsive. The
levels of use in the vast majority of food categories are much less
than 1 mg/m3. In foods in which substantial evaporation of the sulfur
derivative occurs during processing, such as hard candies and baked
goods, the concentration is usually < 10 mg/m3. The low levels of
use in food are reflected in the low reported annual volumes of use of
thiols and thioethers as flavouring substances.
2.3 Biological data
2.3.1 Absorption, distribution, metabolism, and excretion
Simple, non-polar thioether, disulfide, and thiol flavouring
substances would be absorbed rapidly from the gastrointestinal tract
intact and excreted in urine, probably as metabolites, and in some
cases in the expired air as the parent compound. Essentially complete
absorption has been reported for methyl sulfide (No. 452) (Williams et
al., 1966) and dipropyl sulfide (Nickson & Mitchell, 1994). The
presence of other functional groups, such as an alcohol (e.g. Nos
461-463), aldehyde (e.g. Nos 465-471, 505), ester (e.g. Nos 472-481
and 492-494), acid (e.g. No. 501), alpha-ketone (or thioester) (e.g.
Nos 482-494, 504, and 506), beta-ketone (e.g. Nos 495-500, and 502),
or phenol (e.g. No. 503), provides a centre of greater polarity which
could slow the rate of absorption and would provide other sites for
oxidative metabolism (see below). The metabolism of sulfur centres is
shown in Figure 1. All the sulfur compounds considered at this meeting
are of low relative molecular mass and of sufficient lipophilicity to
be absorbed from the intestine. Some functional groups such as
aldehydes, esters, and thioesters probably undergo presystemic
metabolism in the gut lumen, gut wall, or liver.
(i) Simple sulfides (thioethers)
Thioethers are commonly called 'sulfides', as if the hydrogen
atoms of hydrogen sulfide were replaced by alkyl or aryl substituents.
Flavours are usually given such trivial names, which are simplified
even further when the two substituents are the same; for instance,
dimethyl thioether is called 'methyl sulfide'. The presence of a lone
pair of electrons on a divalent sulfur allows rapid oxidation of the
sulfide to a sulfoxide, which occurs both as part of metabolism and
also before ingestion or absorption under aerobic conditions. For
example, allyl sulfide (No. 458), a volatile constituent of onions and
garlic, is oxidized rapidly in air to the corresponding sulfoxide,
which is partly respon-sible for the lachrymatory effects of these
foods (Brodnitz & Pascale, 1971).
In many natural and pharmaceutical compounds, one or more oxygen
atoms is linked to the sulfur centre of an organic molecule, and the
chemical and biological properties of the compound depend on the
nature of the substituents on the sulfur and oxygen atoms. The
oxidation state of sulfur is of major importance in determining the
polarity of the compound and hence its interaction with biological
systems. Once alkyl and aromatic sulfides enter the systemic
circulation, they are oxidized rapidly to sulfoxides and, depending on
the structure of the sulfide, may be further oxidized to the sulfone.
Aliphatic sulfides generally yield mixtures of sulfone and sulfoxide
metabolites; the relative amounts excreted depend on the polarity of
the sulfide (Damani, 1987). Methyl sulfide (No. 452) administered to
rabbits is excreted in the urine as DMSO and dimethyl sulfone
(Williams et al., 1966), whereas the sulfone is the main urinary
metabolite of diethyl sulfide (No. 454) (Hoodi & Damani, 1984).
Dipropyl sulfide, a volatile component of onions and garlic, is
metabolized mainly to the corresponding sulfoxide with small amounts
of the sulfone and trace amounts of inorganic sulfate. Individual
studies on the sulfoxide and sulfone indicate that these metabolites
are relatively stable under physiological conditions (Nickson &
Mitchell, 1994; Nickson et al., 1995). Male Wistar rats eliminated 93%
of a single oral dose of [35S]-dipropyl sulfide over three days, with
66% in the urine and lesser amounts in exhaled air (18%), faeces
(4.6%), and the carcass (1.5%). The sulfoxide was the only compound
detected in plasma. About 25% of the radiolabel was recovered in urine
on day 1 and 39% on day 2, and approximately 25% of the radiolabel was
eliminated in bile over 48 h as the sulfoxide (20%) and sulfone (5%).
Enterohepatic recirculation was probably responsible for the delayed
urinary excretion and the plasma profiles, which showed a slow
increase with peaks at 12-15 h. Urinary metabolites collected during
the first 24 h included the sulfoxide (92%), sulfone (5%), and sulfate
(3%); on days 2 and 3, the sulfoxide accounted for > 98% of daily
urinary metabolites (Nickson & Mitchell, 1994). Methyl phenyl sulfide
administered per se orally to rats or produced by methylation of
benzenethiol (No. 525) was eliminated in the urine as methyl phenyl
sulfone and hydroxylated sulfones (McBain & Menn, 1969).
The major enzyme systems involved in xenobiotic oxidation are the
cytochrome P450 superfamily (CYP-450) (Souhaili-El Amri et al., 1987)
and the flavin-containing monooxygenases (FMO) (Levi & Hodgson, 1988;
Ziegler, 1989; Hodgson & Levi, 1992). The oxidation of thioethers to
sulfoxides is catalysed by these two enzyme systems (Renwick, 1989).
Simple aliphatic (e.g. diethyl sulfide), alicyclic (e.g. thiolane),
and aromatic (e.g. ethyl para-tolyl sulfide) sulfides are oxidized
primarily by FMO and to a lesser extent, by CYP-450. The oxidation of
diethyl sulfide and thiolane in rats is catalysed almost exclusively
by FMO (Hoodi & Damani, 1984; Damani, 1987). 4-Chlorophenyl methyl
sulfide was oxidized by FMO and CYP-450 to the sulfoxide and sulfone
derivatives in vitro (Nnane & Damani, 1995), and diphenyl sulfoxide
was oxidized to the corresponding sulfone in perfused guinea-pig liver
(Yoshihara & Tatsumi, 1990).
Enzyme-catalysed thioether oxidation may be stereoselective, FMO
and CYP-450 selecting different enantiomers (Cashman & Williams,
1990). The stereochemistry of sulfoxidation by the isoenzymes of FMO
has been studied in humans (Rettie et al., 1994). When methyl
para-tolyl sulfide was incubated with human fetal liver and human
kidney microsomes in which CYP-450 activity had been eliminated, the
resulting sulfoxide showed an enantiomeric excess (> 86%) of the
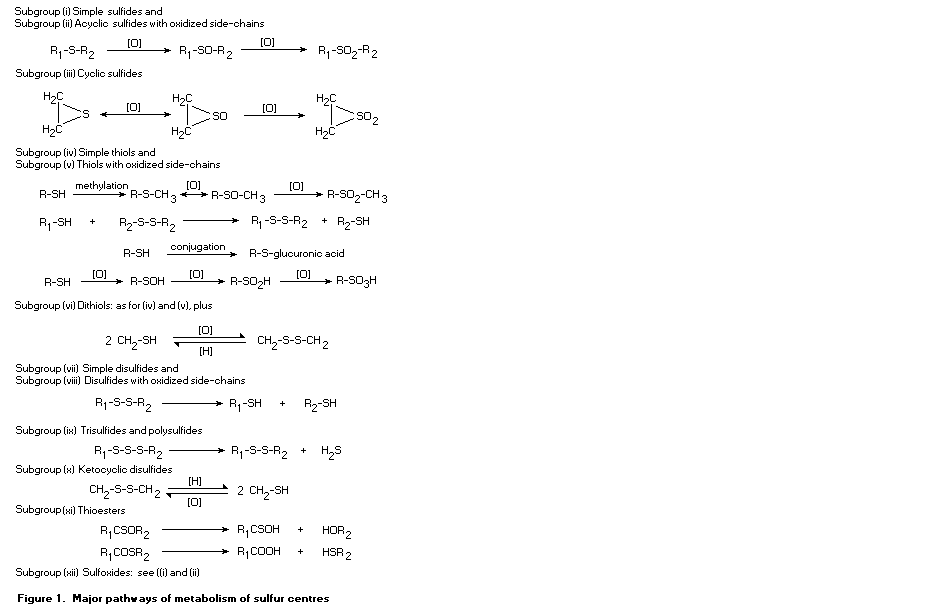 R-isomer. Decreasing stereoselectivity was seen as the size of the
alkyl group (ethyl, propyl, or isopropyl) (Sadeque et al., 1992) or
the pH (8.5-10) increased (Rettie et al., 1990). Oxidation of propyl
and butyl para-tolyl sulfide by the predominant human liver FMO
isozyme, FMO3, resulted in greater formation of the R-enantiomer
(73-88%), whereas oxidation of the methyl or ethyl derivative by human
FMO5 resulted in a > 90% preference for formation of the
S-stereoisomer of the sulfoxide (Sadeque et al., 1995). The influence
of the stereochemistry of sulfoxides on the biological and
toxicological properties of sulfides has not been evaluated.
On the basis of numerous examples of successive oxidation of
sulfides to sulfoxides and sulfones by FMO and CYP-450 enzymes in a
variety of test systems (Cashman & Williams, 1990; Cashman et al.,
1990; Rettie et al., 1990; Yoshihara & Tatsumi, 1990; Sadeque et al.,
1992; Cashman et al., 1995a,b; Elfarra et al., 1995; Nnane & Damani,
1995; Sadeque et al., 1995), the oxidation pathway appears to be a
major route for the metabolism of thioethers in humans (Ziegler, 1980;
Nickson & Mitchell, 1994).
The sulfoxide group is part of an interconvertible redox series
involving the sulfide (or thioether), sulfoxide, and sulfone. The
sulfoxide group is present in numerous molecules, which show a
diversity of biological activity; examples include pharmaceuticals
such as sulindac and flosequinan, veterinary drugs such as
albendazole, and numerous pesticides. Oxidation of a sulfoxide yields
a sulfone, which is commonly found as a metabolite of sulfoxides but
is less important as an active chemical entity. Oxidation of
sulfoxides to the corresponding sulfones occurs in both tissues and
aerobic microorganisms. The oxidation of the sulfoxide drug sulindac
to its inactive sulfone is a major metabolic pathway in humans (Strong
et al., 1985). The enzymes involved have not been studied extensively
in mammalian tissues (Levi & Hodgson, 1988) or microbes (Davis &
Guenthner, 1985). Oxidation of the sulfoxide to the sulfone is an
irreversible reaction which is probably catalysed by CYP-450 (Damani,
1987). The relative amounts of sulfoxide and sulfone excreted depend
on the stability and hydrophilicity of the sulfoxide (Damani, 1987).
After administration of [35S]-dipropyl sulfoxide to rats,
essentially all of the administered radiolabel was recovered over the
following three days, in the urine (80%), exhaled air (1.4%), faeces
(5.0%), and the carcass (13.0%) (Nickson & Mitchell, 1994). The
profile of urinary metabolites was the same after administration of
the sulfoxide or the sulfide (see above); the principal quantitative
difference was that more sulfone was excreted in urine and bile after
sulfoxide administration. Dipropyl sulfone is physiologically stable
in rats in vitro and is excreted unchanged in urine (83%) and bile
(Nickson et al., 1995). More than 98% was excreted unchanged in the
urine with trace amounts of inorganic sulfate. No reduction of the
sulfone group or oxidation of the hydrocarbon chain was observed.
As the sulfoxide may also be reduced back to the thioether, it is not
always possible to determine if the activity of a chemical in vivo
resides in the parent compound or in one of the inter-convertible
redox forms.
The three important sites of sulfoxide reduction in vivo are
liver, kidney, and gut microflora. The tissue enzymes that can reduce
sulfoxides in vitro are primarily aldehyde oxidase (Tatsumi et al.,
1982, 1983; Yoshihara & Tatsumi, 1985a,b) and thioredoxin and its
reductase (Anders et al., 1980, 1981). Studies with tissue homogenates
containing various cofactors and inhibitors have shown that the main
enzyme involved in the liver is aldehyde oxidase, whereas thioredoxin
is important in the kidney (Lee & Renwick, 1995a). The former enzyme
was active mainly under anaerobic conditions, while sulfoxide
reduction by thioredoxin occurred under both aerobic and anaerobic
conditions. The possible role of aldehyde oxidase under normal and
hypoxic conditions was shown in isolated perfused guinea-pig liver;
CYP-P450-catalysed oxidation of both diphenylsulfide and
diphenylsulfoxide to the sulfone predominated under aerobic conditions
(Yoshihara & Tatsumi, 1990), whereas oxidation of diphenylsulfoxide to
the sulfone was decreased under hypoxic conditions and trace amounts
of diphenylsulfide were formed. Infusion of the sulfoxide with
2-hydroxypyrimidine and benzaldehyde, which are electron donors for
aldehyde oxidase, resulted in increased reduction. The importance of
the kidney and thioredoxin in vivo under normal oxygen conditions is
indicated by the fact that patients with end-stage renal disease show
decreased reduction of sulindac in comparison with controls (Gibson et
al., 1987).
The gut microflora in the anaerobic environment of the lower
bowel can be an important and sometimes the sole site of reduction of
sulfoxides. Studies in germ-free animals or animals treated with
antibiotics to suppress the gut microflora and in patients indicated
that the gut bacteria were the main, or possibly the sole, site of
sulfoxide reduction of the drug sulfinpyrazone in vivo (Renwick et
al., 1982; Strong et al., 1984a,b, 1986). The intestinal bacteria can
also reduce sulindac in vitro (Strong et al., 1985), and the
thioether is a major circulating metabolite in vivo. The
contribution of the intestinal microflora to the reduction of sulindac
in humans was demonstrated by a study in normal subjects and patients
who had undergone an ileostomy. The tissues produced an initial peak
0-12 h after dosing, corresponding to 45% of the total thioether, and
the intestinal bacteria produced a second peak at 12-72 h,
corresponding to 55% of the total thioether (Strong et al., 1985).
Studies with over 200 isolated strains of bacteria from human faeces
(Strong et al., 1987) showed significant sulfoxide reduction by many
aerobic organisms such as Escherichia coli under anaerobic
conditions. Few strict anaerobes showed high reducing activity in
vitro, but they are probably essential in vivo in providing the
anaerobic environment in which the aerobes can express their sulfoxide
reductase activity (Strong et al., 1987). Recent studies with
xenobiotics as substrates have indicated the presence of a number of
soluble enzymes in E. coli that are capable of sulfoxide reduction
(Lee & Renwick, 1995b).
The enzymes involved in the reduction of the sulfoxide group
present in DMSO or of the sulfoxide metabolites of the thioethers are
likely to be the hepatic and renal systems discussed above. In order
for the gut flora to be involved in the metabolism of the flavouring
substances, the sulfur derivatives must either be incompletely
absorbed or reach the lower gut as biliary metabolites (Renwick &
George, 1989). The significant biliary excretion of the sulfoxide
metabolite after administration of dipropyl sulfide (see above) raises
the possibility of reduction by the intestinal microflora, followed by
reabsorption and subsequent reoxidation.
(ii) Acyclic sulfides with oxidized side-chains
Oxidized side-chains provide additional sites for the
biotransformation of sulfides, and, as they are polar groups, they
would increase renal excretion. When the thioether contains an
additional oxygenated functional group on a carbon atom (i.e. alcohol,
aldehyde, acid, or ketone), C-oxidation competes with sulfoxidation.
The biotransformation of such oxygenated carbon-containing functional
groups is well characterized and may have been considered in groups of
flavouring substances previously evaluated by the Committee.
Concurrent metabolism via both sulfur and oxygenated functional groups
has been reported for various substrates (El Fatih et al., 1988;
Gachon et al., 1988; Feng & Solsten, 1991; Wilson et al., 1991; Black
et al., 1993). In the presence of oxygenated functional groups,
sulfoxide formation is usually the major metabolic detoxication
pathway. The main urinary metabolites seen after intraperitoneal
administration of thiodiglycol [(HOCH2CH2)2S] to male rats were the
corresponding sulfoxide (90%) and acid thioether
S-(2-hydroxyethylthio)acetic acid (10%). The corresponding sulfone and
combined C-and S-oxidation product S-(2-hydroxyethylsulfinyl)acetic
acid were minor metabolites (Black et al., 1993). Phenacyl phenyl
sulfide is oxidized to the sulfoxide in vitro by FMO but split by
CYP-450 to give thiophenol (Oae et al., 1985). The corresponding
sulfoxides are the principal urinary metabolites of aliphatic
methylthiocarboxylic acids (Alterman et al., 1995), of the mucolytic
drug S-carboxymethyl-L-cysteine ( S-containing amino acid), of the
histamine antagonist cimetidine ( S-containing amidine) in humans
(Mitchell et al., 1982), and of other thioether drugs (Renwick, 1996).
Experiments in vitro suggest that carboxyl ester hydrolysis
occurs in the presence of thioether groups. The thioether
3-(methylthio)hexyl acetate (No. 481) was partially (9%) hydrolysed in
simulated gastric juice containing pepsin (pH = 1.1; 37-38 °C) after 4
h and completely hydrolysed in simulated intestinal fluid containing
pancreatin (Flavor & Extract Manufacturers' Association, 1991).
Thioethers with a polar anionic group such as carboxylic acid one
or more carbon atoms away for the sulfur are inhibitors of rather than
substrates for FMO (Taylor & Ziegler, 1987) and would probably be
eliminated without S-oxidation.
(iii) Cyclic sulfides
There are few published data on the fate of simple cyclic
thioethers. S-Oxidation is likely to be a major pathway in vivo,
and oxidation in vitro results in a chiral sulfoxide when the ring
is asymmetric. Oxidation of unsubstituted and methyl-substituted
cyclic sulfides by a phenobarbital-type CYP-450 yielded the
corresponding sulfoxides, but further oxidation of sulfoxides to
sulfones was not detected (Takata et al., 1983).
The metabolism of the cyclic sulfides with oxidized carbon atoms
(Nos 464, 498, 499, 550, and 562) has not been studied but would be
predicted to comprise extensive S-oxidation and possibly oxidation
or conjugation of alcohol groups.
Substituted 1,3-dithiolane derivatives undergo stereoselective
S-oxidation to the sulfoxide as a major metabolic pathway (Grosa et
al., 1991) with slower oxidation to the cyclic sulfone ( S,S-dioxide)
(Cashman & Olsen, 1990). The reactions are catalysed by both FMO and
CYP-450 (CYP2B); oxidation at both sulfur atoms was not reported. The
cyclic mono-sulfoxides of substances Nos 456, 534, and 543 are
predicted to be the main urinary metabolites, while steric hindrance
and the higher polarity of the hydroxy thioethers (Nos 550 and 562)
may allow their elimination unchanged.
(iv) Simple thiols
A large number of the flavouring agents in this group are either
alkyl or alicyclic thiols (Nos 511-524, 526, 527, and 530), arylthiols
(thiophenols) (Nos 525, 528, 529, and 531-534), dithiols (Nos
535-542), or alkylthiols with oxidized side-chains (Nos 544-547, 549,
551-561, and 563). The thiol group is weakly acidic, and endogenous
thiols have important nucleophilic functions in vivo.
Whereas alcohols are oxidized to give carbonyl compounds
(aldehydes, ketones, and carboxylic acids), thiols are not readily
oxidized to the corres-ponding thiocarbonyls. The availability of
sulfur d-orbitals allows valencies of 4 and 6, so that oxidation of
the thiol group can lead to sulfenic acids (RSOH), sulfinic acids
(RSO2H), or sulfonic acids (RSO3H), as well as disulfides (RSSR').
The metabolic pathways of thiols include oxidation to unstable
sulfenic acids (RSOH), which may be oxidized to the corresponding
sulfinic (RSO2H) and sulfonic acids (RSO3H); methylation to yield
methyl sulfides, which can be oxidized to methyl sulfoxides and
sulfones; reaction with endogenous thiols such as glutathione to form
mixed disulfides; conjugation with glucuronic acid; and oxidation of
the alpha-carbon resulting in desulfuration and formation of an
aldehyde intermediate (McBain & Menn, 1969; Dutton & Illing, 1972;
Maiorino et al., 1988; Richardson et al., 1991; Renwick, 1996).
Sulfenic acids are formed by mild oxidation of thiols but are
unstable and readily undergo further oxidation to sulfinic and
sulfonic acids or combine with nucleophiles. Reactions of sulfenic
acids include dimerization with elimination of water to produce
R-SO-S-R and oxidation to the corresponding polar sulfinic (RSO2H)
and sulfonic (RSO3H) acids. Oxidation of the thiol group of cysteine
in proteins can give stabilized sulfenic acid groups which are
critical to the active site of enzymes; redox cycling between thiol
and sulfenic acid in the active site may be essential for the
catalytic activity of a number of bacterial redox enzymes. The
sulfinic acid group is an intermediate redox state between sulfenic
and sulfonic acids. Endogenous sulfinic acids include cysteine
sulfinic acid, which is released during neuronal stimulation (Klancnik
et al., 1992) and may be involved in synaptic transmission in the
hippocampus. Cysteine and homocysteine sulfinic acids are excitatory
and cytotoxic acidic amino acids (Frandsen et al., 1993). The sulfonic
acid group is a highly polar centre and makes the molecule very
soluble in water. In general, sulfonic acids are stable to metabolism,
and desulfonation was not found in vivo with simple alkyl
sulfo-nates (Taylor et al., 1978) or with branched-chain sulfonates
(Michael, 1968).
Simple aliphatic and aromatic thiols undergo S-methylation in
mammals to produce the corresponding methyl thioether or sulfide,
which may be successively oxidized to the corresponding sulfoxides and
sulfones. Methylation is catalysed by thiopurine methyltransferase in
the cytoplasm and by thiol methyltransferase in microsomes, both of
which require S-adenosyl-L-methionine as a methyl group donor.
Thiopurine methyltransferase is present in human liver, kidney, and
erythrocytes (Woodson et al., 1982; Szumlanski et al., 1988), and
preferential substrates for this enzyme include aromatic and
heterocyclic thiols (Woodson & Weinshilboum, 1983; Woodson et al.,
1983). The apparent Km values of thiophenols (Ames et al., 1986)
are at least two orders of magnitude lower than those of aliphatic
substrates (Woodson & Weinshilboum, 1983). The activity of thiopurine
methyltransferase in humans shows genetic polymorphism, 89% of
subjects showing high activity, 11% showing intermediate activity, and
0.3% showing no activity (Weinshilboum & Sladek, 1980). Studies of
families indicate that the polymorphism is due to a single genetic
locus with two alleles, TPMTH for high activity and TPMTL for low
activity (Keith et al., 1983). The frequencies of the TPMTH and
TPMTL genes are 94% and 6%, respectively, resulting in the trimodal
frequency distribution of thiopurine methyltransferase activity in the
general population. The impact of such differences in 'methylator
status' on the metabolism of thiol-containing flavouring substances is
unknown but is likely to be small because of the presence of
alternative S-oxidation and conjugation pathways. S-Methylation of
aliphatic thiols is catalysed by microsomal thiol methyltrans-ferase,
and thiol substances such as 2-mercaptoethanol, methylmercaptan, and
2-mercaptopropionic acid are substrates for this enzyme (Bremer &
Greenberg, 1961), but the endogenous sulfur amino acid derivatives
homocysteine and glutathione are not. Microsomal thiol
methyltransferase and cytoplasmic thiopurine methyltransferase
activities are regulated independently in humans (Keith et al., 1983),
and therefore S-methylation by thiol methyltrans-ferase may occur in
individuals with low or negligible thiopurine methyltrans-ferase
activity.
Examples of S-methylation in vivo include both aliphatic and
aromatic substrates. Methyl ethyl sulfide (No. 453), which was
detected in the urine of guinea-pigs and mice given an oral dose of
diethyl disulfide (Snow, 1957), was presumably formed by reductive
cleavage to ethanethiol and subsequent methylation (Snow, 1957); minor
urinary metabolites were the sulfoxide and sulfone (Parkinson, 1996).
The sulfoxide and sulfone derivative of benzyl methyl sulfide (No.
460) were excreted in the urine of rats given an oral dose of
S-benzyl- N-malonyl-L-cysteine, presumably via methylation and
oxidation of the intermediate benzyl mercaptan (No. 525) (Richardson
et al., 1991). The urine of rats given an oral dose of [35S]phenyl
mercaptan contained S-methylated metabolites such as phenyl methyl
sulfone and ortho-and para-hydroxylated phenyl methyl sulfone
(McBain & Menn, 1969).
Thiols may react with glutathione and other endogenous thiol
compounds to form mixed disulfides, and both membrane-bound and
cytosolic thioltrans-ferases have been reported to catalyse their
formation. The resulting mixed disulfides can undergo reduction back
to thiols, oxidative desulfuration, or oxidation to a sulfonic acid
via the intermediate thiosulfinate and sulfinic acids (see above).
Thus, the disulfide represents a reversible metabolic 'reservoir' of
the xenobiotic thiol compound. The principal form in the circulation
would probably be a mixed disulfide with albumin. The mixed disulfides
formed from conjugation of a thiol with glutathione or cysteine are
not substrates for cysteine conjugate C-S lyase. C-S lyase is found in
the liver, kidney, and intestinal microflora and catalyses the
reduction of the C-S bonds of cysteine conjugates, which are formed
between organic compounds and glutathione and then undergo a series of
hydrolysis steps. The thiol product is usually methylated to form a
thioether, which may then be oxidized to sulfoxide and sulfone
analogues; compounds that undergo this metabolic pathway include
bromo-benzene, paracetamol (acetominophen), propachlor (a herbicide),
and chlorinated biphenyls. With some organic compounds, conjugation
with glutathione followed by hydrolysis and C-S lyase results in a
novel, unstable thiol; for example, some halogenated substrates and
S-phenyl-L-cysteine formed from conjugation of bromobenzene yield
unstable thiols which are toxic to the kidney (Tateishi et al., 1978;
Shaw & Blagbrough, 1989; Tateishi & Tomisawa, 1989).
S-Glucuronidation of aromatic thiols has been reported, and
this is a possible pathway for the aromatic thiols (thio-phenols) (Nos
525 and 528-531) and aromatic disulfides (Nos 576-578) after their
reduction. This reaction produced thiophenol glucuronide via
conjugation to glutathione, with subse-quent C-S lyase action on the
intermediate cysteine conjugate to yield thiophenol. The metabolism of
benzothiazole-2-sulfonamide was the first of a S-glucuronide to be
fully elucidated. Three metabolites were detected in the urine of
treated rats, benzothiazole-2-thioglucuronide, benzothiazole-2-thiol,
and benzothiazole-1-mercapturate (see Buckberry & Teesdale-Spittle,
1996); the thiol group is introduced into position 2 by displacement
of the sulfonamide by glutathione. Glucuronyl transferases behave in a
similar way towards hydroxyls and sulfydryls, and the two activities
have the same subcellular location and optimal pH (Dutton & Illing,
1972).
Thiols of low relative molecular mass undergo oxidative
desulfuration in vivo to yield CO2 and SO4=. For example, 40% of
14C-labelled methyl mercaptan (No. 508) administered to rats was
eliminated as CO2, 6.4% was exhaled as unchanged compound within 6 h,
and only 2.3% was excreted in the urine. 14C also was detected in
the beta-carbon of serine and in the methyl groups of methionine,
choline, and creatine (Canellakis & Tarver, 1953), and formalde-hyde
has been shown to be an intermediate in the oxidation of methanethiol
in vitro (Mazel et al., 1964). Whereas the carbon atom from a thiol
may be used in the biosynthesis of amino acids, the sulfur atom is not
used significantly in the synthesis of sulfur-containing amino acids:
31% of 35S-labelled methyl mercaptan was excreted in the urine as
sulfate ion (Mazel et al., 1964).
(v) Thiols with oxidized side-chains
Although these compounds comprise a significant proportion of the
flavouring agents in this group, their metabolite fate has not been
studied specifically. The metabolism is predicted to be a combination
of the pathways described above for simple thiols, combined with
further oxidation or conjugation of the oxidized side-chain.
(vi) Dithiols
The metabolism of the simple aliphatic dithiols in this group is
predicted to involve the pathways described above for thiols,
particularly oxidation of sulfur and alpha-carbon atoms and formation
of disulfides by reaction with endogenous thiols such as glutathione.
S-Oxidation of one S-atom could yield a polar thiol-sulfonate. Data
for 2,3-dimercapto-1-propanesulfonate (Maiorino et al., 1996) indicate
that a thiol-sulfonate would be excreted in the urine as such and also
as disulfides formed with endogenous thiols of low relative molecular
mass such as cysteine. The longer, linear dithiols (Nos 535, 538, and
540-542) may form an intramolecular disulfide bond and exist in
equilibrium between dithiol and cyclic disulfide.
(vii) Simple disulfides
Disulfide bonds are important elements in protein folding and
structure (Waring, 1996), and redox interconversion between disulfide
and dithiol is important in the regulation of the activity of some
enzymes. Interconversion is catalysed by a number of enzymes; for
example, glutathione reductase reduces the dimeric disulfide to two
molecules of the monomeric thiol glutathione. The reduction of
xenobiotic disulfides is believed to be extensive and may be catalysed
enzymatically by glutathione reductase (Waring, 1996) or
thioltrans-ferases (Wells et al., 1993) as well as chemically by
exchange with glutathione, thioredoxin, cysteine, and other endogenous
thiols. The non-cyclic disulfides in this group of flavours would be
metabolized to form low-relative-molecular-mass thiols, which would
then follow the pathways described above for thiols.
(viii) Disulfides with oxidized side-chains
As discussed above for groups (ii) and (iii), the additional
sites of carbon oxidation would result in greater polarity and the
potential for further oxidation or conjugation. Because of the
instability of disulfide bonds to reductive cleavage, this would be
expected to be the initial metabolic reaction, and the polarity of the
side-chains would mainly affect the elimination of thiol fragments.
(ix) Trisulfides
The trisulfide of glutathione is labile and readily converted to
the disulfide, the sulfur being released as hydrogen sulfide (Moutiez
et al., 1994). The metabolic lability of trisulfides is associated
with biological activity, such as inhibition of CYP-450 (Ogasawara et
al., 1998) and enhancement by diallyl trisulfide (No. 587) of
unscheduled DNA synthesis produced in rat hepatocytes by known
mutagens (Deng et al., 1994). The toxicity of trisulfides (Nos
582-587) and polysulfides (No. 588) is probably related to their
metabolic lability and the nature of the eventual thiol, such as allyl
thiol.
(x) Heterocyclic disulfides
The heterocyclic disulfides (Nos 573 and 574) are five-and
six-membered rings which also contain a cyclic thioether bond. Lipoic
acid is a five-membered cyclic disulfide, and rapid redox cycling
between ring disulfide and open dithiol is important in its metabolic
role in vivo. The five-membered disulfide (No. 573) would be
predicted to undergo more rapid reductive ring opening to a dithiol
than the six-membered compound (No. 574). The principal metabolic
pathways are predicted to be disulfide reduction to produce a dithiol
and S-oxidation of the thioether.
(xi) Thioesters
The thioester group (-S-CO-) is present in a number of the
flavouring substances in this group (Nos 482-494, 504, and 506). The
hydrolysis of other esters has been considered previously by the
Committee. Esters are hydrolysed by lipase and esterases (Kurooka et
al., 1976), and the rate of hydrolysis increases as the length of the
C-chain of the carboxylic acid fragment increases (Greenzaid & Jencks,
1971) and decreases as oxygenation of the carbon chain in the thiol
moiety increases (Kurooka et al., 1976).
Dithionic acids are oxidized by FMO to the dioxo analogue with
the monothioc acid as an intermediate (Taylor & Ziegler, 1987), but
simple esters of dithioic acids are poor substrates for oxidation. The
esters of monothioic acids are also probably poor substrates for
oxidation, but the monothioic acid released by hydrolysis would be
oxidized to the dioxo-acid. Another possibility for elimination in
vivo is renal excretion of the thiocarboxylic acid. With the
exceptions of prenyl thioacetate (No. 491) and methylthio
2-(acetyloxy)propio-nate (No. 492), the compounds evaluated are simple
linear alkyl compounds, branched alkyl compounds, or their side-chain
hydroxy-ester analogues, so that their toxicity can reasonably be
compared.
(xii) Sulfoxides
The metabolism of sulfoxides by oxidation and reduction is
described above for thioethers. The only sulfoxide flavouring agent is
DMSO (No. 507). The absorption, metabolism, and excretion of DMSO were
studied in rhesus monkeys given a daily oral dose of 3 g/kg bw for 14
days. DMSO was absorbed rapidly, reached a peak serum concentration
after about 4 h, and was cleared from the blood within 72 h of the end
of treatment. Dimethyl sulfone was detected in the blood 2 h after
treatment, reached a steady-state concentration after four days, and
was cleared from the blood 120 h after the end of treatment. Urinary
excretion of DMSO and dimethyl sulfone accounted for approximately 60%
and 16%, respectively, of the total ingested dose. Neither DMSO nor
dimethyl sulfone was detected in the faeces (Layman & Jacob, 1985).
The fate of DMSO in humans is similar to that in monkeys, but the
elimination is slower. When an oral dose of 1 g/kg bw was given to six
subjects, peak serum concentrations were observed approximately 4 h
after administration. The peak concentration of dimethyl sulfone was
slightly higher than that of DMSO and occurred after 72-96 h.
Approximately 51% of the dose was excreted in the urine unchanged over
the first 120 h, and up to 22% was excreted as dimethyl sulfone.
Repeated daily oral administration of DMSO at 0.5 g/kg bw per day for
14 days to one adult resulted in peak serum concentrations of DMSO
within eight days (Hucker et al., 1967).
2.3.2 Toxicological studies
The available data on the toxicity of sulfides, thiols, and
oxygenated functional derivatives in the group of 137
sulfur-containing flavouring substances are presented below. Although
the studies of acute and short-term toxicity were of limited use for
evaluating the safety of these substances, because of their short
duration, they are included for completeness. Studies related to
chemoprevention are also described.
2.3.2.1 Acute toxicity
Oral LD50 values have been reported for 52 of the 137 sulfur
compounds. The values ranged from 11 to 28 000 mg/kg bw in male and
female rats (Fairchild & Stokinger, 1958; Brown et al., 1963; Elf
Atochem, 1963; Harper & Ginn, 1964; Sommer & Tauberger, 1964; Willson
et al., 1965; Koptyaev, 1967; Fishman et al., 1969; McBain & Menn,
1969; Fogelman et al., 1970; Oser, 1970; deGroot et al., 1974;
Christias, 1975; Moreno, 1975; British Industrial Biological Research
Association, 1976; Griffiths et al., 1979; Mondino & Peano, 1979;
Opdyke, 1979; Moran et al., 1980; Moreno, 1980; Panasevich et al.,
1980; Butterworth & Mason, 1981; Mondino et al., 1981; Peano et al.,
1981; Mondino & Peano, 1982; Piccirillo & Lunchicki, 1982; Elf
Atochem, 1985; Schafer & Bowles, 1985; Collinson, 1989; Watanabe &
Kinosaki, 1989a,b; Phillips Petroleum Co., 1990a,b,c; Rush, 1991; Elf
Atochem, 1992; Farr & Kirwin, 1994; Sanders, 1997). In male and female
mice, the values ranged from 61 to > 5000 mg/kg bw (Koptyaev, 1967;
Oser, 1970; Shellen-berger, 1971a,b; Selyuzhitskii, 1972; Fogelman &
DeProspo, 1973a,b; Fogelman & Suppers, 1973; Fogelman & DeProspo,
1974; Fogelman & Suppers, 1974a,b; Bailey, 1976a,b,c; Gallo et al.,
1976; Griffiths & Babish, 1978; Moran et al., 1980; Butterworth &
Mason, 1981; Schafer & Bowles, 1985).
2.3.2.2 Short-term studies of toxicity
2,8-Dithianon-4-ene-4-carboxaldehyde (No. 471)
Five male and five female rats (strain not specified) received
0.33 or 3.3 mg/kg bw per day of 2,8-dithianon-4-ene-4-carboxaldehyde
(No. 471) by gavage on six days a week for two weeks. Individual body
weights and feed and water consumption were recorded at one and two
weeks. Haemoglobin levels determined on day 14 were normal. At
necropsy on day 14, the liver and kidney were weighed and examined
macro-and microscopically. No treat-ment-related effects were reported
(de Groot et al., 1974).
Methylthio 2-(acetyloxy)propionate (No. 492) and
methylthio 2-(propionyloxy)propionate (No. 493)
Groups of five Fischer 344 rats of each sex were given either
methylthio 2-(acetyloxy)propionate (No. 492) or methylthio
2-(propionyloxy)propionate (No. 493) in the diet for 14 days at a dose
of approximately 500 mg/kg bw per day. Control animals received a
basal diet. The animals were observed and tested daily for clinical
signs of toxicity. Body weights were measured on days 1, 6, and 14,
and feed consumption was measured on days 7 and 14. At necropsy,
organs were weighed and the liver and kidneys were examined
histologically.
Neither test material produced any abnormal clinical or
microscopic changes. The potential treatment-related effects included
depressed feed consumption, decreased body-weight gains of treated
males, and depressed relative kidney weights in treated females. The
depressed feed consumption and decreased body-weight gains observed in
treated males were probably due to the strong odour of the test
compounds, which decreased the palatability of the diets. The
biological significance, if any, of the decreased relative kidney
weights in the females (7 and 6%, respectively, with methylthio
2-(acetyloxy)-propionate and methylthio 2-(propionyloxy)propionate) is
questionable because the change was small and there were no
histological changes (Hermansky & Weaver, 1990).
Prenyl thioacetate (No. 491), prenylthiol (No. 522),
3-mercaptohexanol (No. 545), 3-methyl-3-mercaptobutyl formate
(No. 549), 3-mercaptohexyl acetate (No. 554), 3-mercaptohexyl
butyrate (No. 555), and 3-mercaptohexyl hexanoate (No. 556)
A series of 14-day studies were performed with prenyl thioacetate
(No. 491), prenylthiol (No. 522), 3-mercaptohexanol (No. 545),
3-methyl-3-mercaptobutyl formate (No. 549), 3-mercaptohexyl acetate
(No. 554), 3-mercaptohexyl butyrate (No. 555), and 3-mercaptohexyl
hexanoate (No. 556). Each substance was provided in the diets of 10
Sprague-Dawley rats of each sex at a target dose of 10 mg/kg bw per
day. Weekly measurements of body weight and feed consumption indicated
that the average daily intakes were 10-13 mg/kg bw per day. Control
animals received chow alone for the same period. The animals were
observed for gross signs of toxicity and deaths at least once daily
for the duration of the study. Body weights and feed consumption were
measured on days 8 and 15. Gross necropsies were performed on all
rats, and the kidneys and liver were removed for histopathological
examination at the time of autopsy. Body-weight gain, organ-to-body
weight ratios, and the weights of the kidney and liver of test and
control animals were not significantly different in any of the seven
studies. Gross necropsy and histopathological examination of kidney
and liver tissues revealed no lesions related to administration of the
test substances (Wnorowski, 1996a-e, 1997a,b].
Prenyl thioacetate (No. 491) and prenylthiol (No. 522),
respectively, are in the thioester and simple thiol subgroups of the
larger group of sulfur-containing flavouring substances; the other
five substances (Nos 545, 549, and 554-556) are thiols with oxidized
side-chains.
Allyl disulfide (No. 572), propyl disulfide (No. 566), and
phenyl disulfide (No. 578)
In two separate, but similarly conducted studies, allyl disulfide
(No. 572) was administered at a concentration of 250 or 1000 µmol/kg
(36 or 150 mg/kg bw per day); propyl disulfide (No. 566) at 250, 1000,
or 5000 µmol/kg (38, 150, or 750 mg/kg bw per day); and phenyl
disulfide (No. 578) at 500 µmol/kg (110 mg/kg bw per day), as
solutions in peanut oil or Tween 80, to groups of six or seven female
Sprague-Dawley rats. The doses were given by oral intubation for six
consecutive days. Control groups of 12 or 14 rats received the vehicle
alone. The animals were killed on day 7 of the experiment, and blood
was collected from the posterior vena cava. At necropsy, splenic
weights were recorded and expressed as a percentage of body weight,
and splenic darkening was assessed visually on an arbitrary scale of
0-5. Blood was examined for various parameters. Sections of splenic
sinusoidal engorgement, focal hepatic erythropoiesis, and iron
deposition in the spleen, liver, and kidneys were also scored on a
scale of 0-5. The number and size of Heinz bodies were measured to
estimate any decrease in the optical density of red cell lysates.
The results for animals given the lower dose of allyl disulfide
and the two lower doses of propyl disulfide were similar to those of
controls, whereas those given the higher doses of allyl and propyl
disulfide had enlargement and darkening of the spleen, increased iron
concentrations in the spleen, liver, and kidneys, and decreased packed
cell volume and haemoglobin concentra-tions associated with Heinz body
formation. No degenerative changes were seen in the spleen, liver, or
kidneys, but destruction of erthrocytes was indicated by deposition of
iron in these organs. Similar findings were made in rats given phenyl
disulfide.
The haemolysis observed was attributed to oxidative stress due to
'active oxygen' species formed by intra-erythrocytic redox cycling of
disulfides. Thiol-disulfide exchange with glutathione results in
reduction of disulfides to their corresponding thiols. Although
generally recognized as antioxidants, thiols act as pro-oxidants after
one-electron oxidation to a thyil radical. In erythrocytes, such
oxidation is mediated by oxyhaemoglobin and results in the formation
of methaemoglobin, hydrogen peroxide, and a thiolate anion. The
hydrogen peroxide and the more active oxygen species formed from the
thyil radical are responsible for initiating the cellular damage that
leads to haemolysis. Although human erythrocytes are usually resistant
to oxidative damage, certain individuals are susceptible because of
hereditary deficiencies (Munday et al., 1990; Munday & Manns, 1994).
2.3.2.3 Ninety-day studies of toxicity
Ninety-day studies were available for 26 of the flavouring
substances (Table 4). To facilitate comparisons of the toxicity of
structurally related substances, the studies are grouped according to
the 12 subgroups. A 90-day study was available for one or more
substance in each subgroup except that of disulfides with oxidized
side-chains. Most of the studies were performed at only one dose that
produced no adverse effects.
(i) Simple sulfides (thioethers)
Methyl sulfide (No. 452)
Four groups of 15 male and 15 female weanling SPF rats of the
Wistar strain were given methyl sulfide by oral intubation at
concentrations of 0 (control), 2.5, 25, or 250 mg/kg bw per day for 14
weeks. Two further groups of five animals of each sex were given 0.25
or 250 mg/kg bw per day for two or six weeks, respectively. The
animals were weighed on day 0 and then weekly throughout the study.
Feed and water consumption were measured for 24 h before the day of
weighing. Urine was collected during weeks 2, 6, and 14 and examined
for appearance, microscopic constituents, and content of glucose,
ketones, bile salts, and blood. The specific gravity and volume of
urine produced over 6 h were also noted. At the end of the appropriate
period of dosing, the rats were killed and blood was taken for
haematological examination. Gross abnormalities were noted and organs
weighed, and histological examinations were performed. No adverse
effect occurred with any treatment (Butterworth et al., 1975). The
dose of 250 mg/kg bw per day that had no effect is at least 10 000
times the per capita intakes of 10 and 9 mg/kg bw per day due to use
of methyl sulfide as a flavouring substance in Europe and the United
States, respectively.
In a study designed to investigate the mechanism of lenticular
changes caused by DMSO (No. 507), 10 eight-week-old New Zealand
rabbits were given 2000 mg/kg bw per day methyl sulfide (No. 452) in
drinking-water for 13 weeks. Control animals received drinking-water
only. Photographs were taken each week of the animals' lens to
evaluate the occurrence of changes. At termination, the kidney, liver,
spleen, heart, lung, adrenals, and lens were weighed and examined for
gross physical abnormalities. Treated animals had a 50% increase in
lung weight, with pulmonary congestion and some haemorrhagic spots.
The kidneys showed signs of pyelonephritis. There was no evidence of
lenticular changes (Wood et al., 1971).
(ii) Acyclic sulfides with oxidized side-chains
2-(Methylthiomethyl)-3-phenylpropenal (No. 505)
In a standardized protocol (referred to below as such for other
compounds), groups of 15 male and 15 female weanling Sprague-Dawley
rats were given a single dose of 0 or 1.4 mg/kg bw per day of
2-(methylthiomethyl)-3-phenylpropenal (No. 505) dissolved in acetone
and blended into a basal laboratory diet for 92 days. The rats were
housed individually and given feed and tap water ad libitum. The
acetone was fully evaporated before presentation of the diet to the
animals. Samples of each diet were taken weekly to assess the
stability and concentration of the test material. Appearance,
behaviour, appetite, elimination, gross signs of toxic effects, and
deaths were recorded daily, and body weights and feed consumption were
measured weekly. Haematological examinations, blood chemical
determinations, and urine analyses were performed during weeks 6 and
12 on eight males and eight females from each group. At necropsy, the
weights of the liver, kidneys, thyroid, spleen, and adrenal glands
were recorded. At termination, the weights of the thyroid, liver,
kidneys, spleen, and adrenal glands were recorded. Tissues from 26
sites and all grossly observable abnormal sites were preserved in
formalin for histopathological examination.
The body weights of females were reduced throughout the study,
and both males and females had decreased feed consumption, but these
findings did not appear to be biologically significant. No significant
differences were seen between treated and control animals with respect
to haematological, blood chemical, or urinary parameters. The absolute
and relative organ weights of the treated and control animals were
similar and there was no evidence of gross or histological changes
(Cox et al., 1979).
The dose of 2-(methylthiomethyl)-3-phenylpropenal that showed no
effects (i.e. 1.4 mg/kg bw per day) is more than 10 000 times the
estimated per capita intake of 0.03 µg/kg bw per day of this
flavouring substance in the United States.
(iii) Cyclic sulfides
2-Methyl-4-propyl-1,3-oxathiane (No. 464)
Groups of 16 male and 16 female weanling Wistar rats were given
2-methyl-4-propyl-1,3-oxathiane (No. 464) by oral intubation at a
daily dose of 0.44 mg/kg bw for 90 days or corn oil alone. The rats
were weighed on the first day of treatment and thereafter at regular
intervals throughout the study. Feed intake was recorded at three-or
four-day intervals. Blood was collected from half the rats in the
study at six weeks and from all rats at 12 weeks and was examined for
haemoglobin concentration, packed cell volume, and erythrocyte and
leukocyte counts. Urea concentration was measured. At necropsy, organs
were weighed and gross and histological examinations performed.
A slight increase in body-weight gain was seen in treated males,
which was associated with increased feed intake thought to result from
alteration of feeding behaviour due to intubation of highly flavoured
solutions. Isolated differences from controls were seen in
haematological parameters, organ weights, and histological appearance,
but none was statistically significant and were considered not to
Table 4. Results of short-term studies of toxicity and long-term studies of toxicity and carcinogenicity on aliphatic and aromatic sulfides
and thiols
No. Substance Species Sex No. test Route Duration NOEL Reference
groupsa/no. (mg/kg bw)
per test
groupb
452 Methyl sulfide Rat MF 3/30 Gavage 14 weeks 250c Butterworth
et al. (1975)
Rabbit MF 1/10 Oral 13 weeks < 2000 Wood et al. (1971)
464 2-Methyl-4-propyl-1,3-oxathiane Rat MF 1/32 Gavage 13 weeks 0.44c British Industrial
Biological Research
Association (1976)
471 2,8-Dithianon-4-en-4-carboxaldehyde Rat MF 2/10 Gavage 2 weeks ND de Groot
et al. (1974)
483 Ethyl thioacetate Rat MF 1/46 Oral 13 weeks 6.5c Shellenberger (1970)
484 Methyl thiobutyrate Rat M 3/10 Oral 13 weeks 1000c,d Wheldon et al. (1970)
491 Prenyl thioacetate Rat MF 1/20 Diet 2 weeks ND Wnorowski (1997a)
498 4,5-Dihydro-3(2H)-thiophenone Rat MF 1/30 Oral 92 days 9.2c Morgareidge (1970)
505 2-(Methylthiomethyl)-3-phenylpropenal Rat MF 1/30 Oral 92 days 1.4c Cox et al. (1979)
507 Methylsulfinylmethane Rat 4/20 Oral 13 weeks 2200 Sommer & Tauberger
(1964)
Rat MF 3/100 Gavage 18 months < 1100 Noel et al. (1975)
Dog MF 3/10 Gavage 18 weeks 1100
or 2 years
Monkey MF 3/4 Gavage 74-87 weeks 3000 Vogin et al. (1970)
516 Cyclopentanethiol Rat MF 1/30 Oral 92 days 0.56c Morgareidge &
Oser (1970a)
520 2,3- or 10-Mercaptopinane Rat MF 1/30 Oral 92 days 0.06c Oser (1966)
522 Prenylthiol Rat MF 1/20 Diet 2 weeks ND Wnorowski (1997b)
528 ortho-Toluenethiol Rat MF 1/20-32 Diet 90 days 0.52c Posternak
et al. (1969)
Table 4. (contd)
No. Substance Species Sex No. test Route Duration NOEL Reference
groupsa/no. (mg/kg bw)
per test
groupb
530 2,6-Dimethylthiophenol Rat MF 1/64 Gavage 13 weeks 0.43c Peano et al. (1981)
531 2-Naphthalenethiol Rat MF 1/30 Oral 92 days 3.4c Morgareidge (1971a)
534 2-Methyl-1,3-dithiolane Rat MF 1/32 Gavage 91 days 7.0c Griffiths
et al. (1979)
539 2,3-Butanedithiol Rat MF 1/30 Oral 92 days 0.7c Morgareidge
et al. (1974a)
541 1,8-Octanedithiol Rat MF 1/30 Oral 92 days 0.7c Morgareidge
et al. (1974c)
543 Trithioacetone Rat MF 1/30 Oral 92 days 0.2c Cox et al. (1973a)
545 3-Mercaptohexanol Rat MF 1/20 Diet 2 weeks ND Wnorowski (1996a)
546 2-Mercapto-3-butanol Rat MF 1/30 Oral 92 days 1.9c Cox et al. (1974a)
547 a-Methyl-b-hydroxypropyl Rat MF 1/30 Oral 92 days 2.8c Morgareidge
a-methyl-b-mercaptopropyl et al. (1974c)
sulfide
549 3-Mercapto-3-methylbutyl Rat MF 1/20 Diet 2 weeks ND Wnorowski (1996a)
formate
554 3-Mercaptohexyl acetate Rat MF 1/20 Diet 2 weeks ND Wnorowski (1996b)
555 3-Mercaptohexyl butyrate Rat MF 1/20 Diet 2 weeks ND Wnorowski (1996c)
556 3-Mercaptohexyl hexanoate Rat MF 1/20 Diet 2 weeks ND Wnorowski (1996d)
560 3-Mercapto-2-pentanone Rat MF 1/30 Oral 92 days 1.9c Morgareidge (1971b)
562 2,5-Dimethyl-2,5-dihydroxy Rat MF 1/30 Diet 92 days 3.1c Cox et al. (1973b)
-1,4-dithiane
566 Propyl disulfide Rat F 1/6 Gavage 6 days ND Munday & Manns (1994)
Rat M 1/10-16 Diet 90 days 7.3c Posternak
et al. (1969)
F 1/10-16 Diet 90 days 8.2c
572 Allyl disulfide Rat F 2/6 Gavage 6 days 36c Munday & Manns (1994)
Table 4. (contd)
No. Substance Species Sex No. test Route Duration NOEL Reference
groupsa/no. (mg/kg bw)
per test
groupb
573 3,5-Dimethyl-1,2,4-trithiolane Rat, MF 1/32 Gavage 90 days 1.9c British Industrial
Biological Research
Association (1976)
574 3-Methyl-1,2,4-trithiane Rat MF 1/32 Oral 13 weeks 0.3c Mondino (1981)
575 Dicyclohexyl disulfide Rat MF 1/30 Oral 92 days 0.23c Cox et al. (1974b)
577 Methyl benzyl disulfide Rat MF 1/30 Oral 92 days 1.2c Gallo et al. (1976)
578 Phenyl disulfide Rat F 1/6 Gavage 6 days ND Munday et al. (1990)
585 Dipropyl trisulfide Rat MF 1/30 Oral 92 days 4.8c Morgareidge
& Oser (1970b)
587 Diallyl trisulfide Rat MF 1/30 Oral 92 days 4.6c Morgareidge
& Oser (1970c)
M, male; F, female; ND, not determined
a No. of test groups does not include controls
b No. per test group comprises males and females
c Study performed with either a single dose or multiple doses that had no effect; the value is therefore the highest dose tested.
d Dose increased from 500 to 1000 mg after six weeks
represent adverse effects (British Industrial Biological Research
Association, 1976). The dose of 0.44 mg/kg bw per day of
2-methyl-4-propyl-1,3-oxathiane that showed no effects is more than 10
000 times the estimated per capita intakes of 0.03 mg/kg bw per day in
Europe and 0.02 mg/kg bw per day in the United States.
4,5-Dihydro-3(2H)-thiophenone (No. 498)
4,5-Dihydro-3(2H)-thiophenone (No. 498) was tested in a
standardized study (see above) at a single dose of 9.16 mg/kg bw per
day. No effects were observed (Morgareidge, 1970). This dose is 100
000 times greater than the estimated per capita intakes of 0.01 µg/kg
bw per day in Europe and 0.04 µg/kg bw per day in the United States.
2-Methyl-1,3-dithiolane (No. 534)
A group of 16 male and 16 female Sprague-Dawley rats received an
aqueous propylglycol solution (0.2% w/w) containing 7 mg/kg bw of
2-methyl-1,3-dithiolane (No. 534) daily by oral intubation for 91
days. Control animals received 0.02% propylene glycol only. The rats
were observed routinely for body weight and feed consumption.
Haematological and blood chemical parameters were determined in weeks
4 and 13. No difference was seen between control and treated groups in
body-weight gain or feed consumption. A slight, nonsignificant
reduction in haemoglobin concentration was seen in treated females.
Gross and histological examinations at termination showed no
dose-related effects, and organ weights were normal (Griffiths et al.,
1979). The dose of 7 mg/kg bw per day is greater than 1 000 000 times
the estimated per capita intake of 0.002 mg/kg bw per day of
2-methyl-1,3-dithiolane in Europe and 10 000 times that of 0.1 mg/kg
bw per day in the United States.
Trithioacetone (No. 543)
Trithioacetone (No. 543) was tested in a standardized study (see
above) at a single dose of 0.205 mg/kg bw per day. No effects were
observed (Cox et al., 1973a). This dose is 10 000 greater than the
estimated intakes of 0.04 µg/kg bw per day in Europe and 0.01 µg/kg bw
per day in the United States.
2,5-Dimethyl-2,5-dihydroxy-1,4-dithiane (No. 562)
2,5-Dimethyl-2,5-dihydroxy-1,4-dithiane (No. 562) was tested in a
standar-dized study (see above) at a single dose of 3.1 mg/kg bw per
day. No effects were observed (Cox et al., 1973b). This dose is 1 000
000 greater than the estimated per capita intakes of 0.004 µg/kg bw
per day in Europe and 0.003 µg/kg bw per day in the United States.
(iv) Simple thiols
Cyclopentanethiol (No. 516)
Cyclopentanethiol (No. 516) was tested in a standardized study
(see above) at a single dose of 0.56 mg/kg bw per day. No effects were
observed (Morgareidge & Oser, 1970a). This dose is 10 000 times
greater than the estimated per capita intake of 0.01 µg/kg bw per day
in the United States.
2,3-or 10-Mercaptopinane (No. 520)
2,3-or 10-Mercaptopinane (No. 520) was tested in a standardized
study (see above) at a single dose of 0.06 mg/kg bw per day, No
effects were observed (Oser, 1966). This dose is 10 000 times greater
than the estimated per capita intake of 0.001 µg/kg bw per day in
Europe and 100 times that of 0.2 µg/kg bw per day in the United
States.
ortho- Toluenethiol (No. 528)
Groups of 10-16 male and 10-16 female rats were given 0.52 mg/kg
bw per day of ortho-toluenethiol (No. 528) in the diet for 90 days.
Control animals received untreated diet. Feed consumption and body
weights were recorded weekly. Haematological examinations and blood
urea determinations were conducted on half the animals at seven weeks
and on all animals at 13 weeks. At necropsy, organs were weighed and
examined grossly and histologically. No adverse effects were observed
(Posternak et al., 1969). The dose that had no effect is greater than
1000 times the estimated per capita intake of 0.4 mg/kg bw per day in
Europe and 100 000 times that of 0.003 mg/kg bw per day in the United
States.
2,6-Dimethylthiophenol (No. 530)
2,6-Dimethylthiophenol (No. 530) was administered in corn oil by
gavage to 32 male and 32 female Sprague-Dawley rats at a concentration
calculated to result in an average intake of 0.43 mg/kg bw per day for
13 weeks. Control animals received only corn oil. Body weights and
feed intake were measured weekly, and haematological and blood
chemical parameters were measured at weeks 4 and 13. Organs were
weighted and gross and histological examinations were performed at the
time of autopsy. No significant difference was seen between treated
and control animals (Peano et al., 1981). The dose that had no effect
is greater then 10 000 times the estimated per capita intake of 0.03
mg/kg bw per day in Europe and greater than 1 000 000 times that of
0.0003 mg/kg bw per day in the United States.
2-Naphthalenethiol (No. 531)
2-Naphthalenethiol (No. 531) was tested in a standardized study
(see above) at a single dose of 3.4 mg/kg bw per day. No effects were
observed (Morgareidge, 1971a). The dose that had no effect is 1 000
000 times greater than the estimated per capita intake of 0.001 µg/kg
bw per day in the United States.
(v) Thiols with oxidized side-chains
2-Mercapto-3-butanol (No. 546)
2-Mercapto-3-butanol (No. 546) was tested in a standardized study
(see above). No effects were observed (Cox et al., 1974a). The dose of
1.9 mg/kg bw per day that had no effect is 1000 times greater than the
estimated per capita intake of 0.1 µg/kg bw per day in Europe and
10 000 times that of 0.002 µg/kg bw per day in the United States.
alpha-Methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl
sulfide (No. 547)
alpha-Methyl-beta-hydroxypropyl alpha-methyl-beta-mercaptopropyl
sulfide (No. 547) was tested in a standardized study (see above) at a
single dose of 2.815 mg/kg bw per day. No effects were observed
(Morgareidge et al., 1974b). This dose is 100 000 times greater than
the estimated per capita intake of 0.01 µg/kg bw in the United States.
3-Mercapto-2-pentanone (No. 560)
3-Mercapto-2-pentanone (No. 560) was tested in a standardized
study (see above) at a single dose of 1.89 mg/kg bw per day. No
effects were observed (Morgareidge, 1971b). This dose is 100 000 times
greater than the estimated per capita intake of 0.002 µg/kg bw per day
in the United States.
(vi) Dithiols
2,3-Butanedithiol (No. 539) and 1,8-octanedithiol (No. 541)
2,3-Butanedithiol (No. 539) and 1,8-octanedithiol (No. 541) were
tested in standardized studies (see above) at single doses of 0.703
and 0.705 mg/kg bw per day, respectively. No effects were observed
(Morgareidge et al., 1974a,c). The dose of 2,3-butanedithiol that had
no effect is 100 000 times greater than the estimated per capita
intakes of 0.001 µg/kg bw per day in Europe and 0.003 µg/kg bw per day
in the United States. The dose of 1,8-octanedithiol that had no effect
is 1000 times greater than the estimated per capita intake of 0.1
µg/kg bw per day in Europe and 10 000 times that of 0.01 µg/kg bw per
day in the United States.
(vii) Simple disulfides
Propyl disulfide (No. 566)
Three groups of 10-16 male and 10-16 female rats were given 7.3
or 8.2 mg/kg bw per day of propyl disulfide (No. 566) in the diet for
90 days. Control animals received untreated diet. feed consumption and
body weights were recorded weekly, and haematological examinations and
blood urea determinations were conducted on half the animals at seven
weeks and on all animals at 13 weeks. At necropsy, organs were weighed
and examined grossly and histologically. No adverse effects were
observed (Posternak et al., 1969). The doses that had no effects are
10 000 times greater than the estimated per capita intake of 0.1 mg/kg
bw per day in Europe and 1 000 000 times that of 0.002 mg/kg bw per
day in the United States.
Dicyclohexyl disulfide (No. 575)
Dicyclohexyl disulfide (No. 575) was tested in a standardized
study (see above) at a single dose of 0.23 mg/kg bw per day. No
effects were observed (Cox et al., 1974b). The dose that had no effect
is 100 000 times greater than the estimated per capita intake of
0.0003 µg/kg bw per day in Europe and 10 000 that of 0.003 µg/kg bw
per day in the United States.
Methyl benzyl disulfide (No. 577)
Methyl benzyl disulfide (No. 577) was tested in a standardized
study (see above) at a single dose of 1.2 mg/kg bw per day, No effects
were observed (Gallo et al., 1976). The dose that had no effect is 1
000 000 times greater than the estimated per capita intake of 0.0003
µg/kg bw per day in Europe and 100 000 that of 0.002 µg/kg bw per day
in the United States.
(viii) Disulfides with oxidized side-chains
No studies on the two substances in this group were available,
but their toxicity can be compared by analogy to that of the
disulfides.
(ix) Trisulfides
Dipropyl trisulfide (No. 585)
Dipropyl trisulfide (No. 585) was tested in a standardized study
(see above) at a single dose of 4.8 mg/kg bw per day, No effects were
observed (Morgareidge & Oser, 1970b). The dose that had no effect is
10 000 times greater than the estimated per capita intake of 0.2 µg/kg
bw per day in Europe and 100 000 that of 0.02 µg/kg bw per day in the
United States.
Diallyl trisulfide (No. 587)
Diallyl trisulfide (No. 587) was tested in a standardized study
(see above) at a single dose of 4.6 mg/kg bw per day, No effects were
observed (Morgareidge & Oser, 1970c). The dose that had no effect is
10 000 times greater than the estimated per capita intake of 0.1 µg/kg
bw per day in Europe and 100 000 that of 0.0003 µg/kg bw per day in
the United States.
(x) Heterocyclic disulfides
3,5-Dimethyl-1,2,4-trithiolane (No. 573)
Groups of 16 male and 16 female weanling Wistar rats were given
3,5-dimethyl-1,2,4-trithiolane (No. 573) by oral intubation at a daily
dose of 1.9 mg/kg bw for 90 days or corn oil alone. The rats were
weighed on the first day of treatment and thereafter at regular
intervals throughout the study. Feed intake was recorded at three- or
four-day intervals. Blood was collected from half the rats at six
weeks and from all rats at 12 weeks and was examined for haemoglobin
concentration, packed cell volume, and numbers of erythrocyte and
leukocytes. The urea concentration was measured. At necropsy, organs
were weighed and gross and histological examinations were performed.
A slight increase in body-weight gain was seen in treated males,
which was associated with increased feed intake thought to result from
alteration of feeding behaviour due to intubation of highly flavoured
solutions. Isolated differences from controls were seen in
haematological parameters, organ weights, and histological appearance,
but none was statistically significant and they were considered not to
represent adverse effects (British Industrial Biological Research
Association, 1976). The dose that had no effect is 100 000 times
greater than the estimated per capita intakes of 0.001 mg/kg bw per
day in Europe and 0.002 mg/kg bw per day in the United States.
3-Methyl-1,2,4-trithiane (No. 574)
3-Methyl-1,2,4-trithiane (No. 574) was administered orally to 16
male and 16 female rats (strain not specified) at a dose of 0.3 mg/kg
bw per day for 13 weeks. Body weights and feed intake were measured
weekly. Haematological parameters and blood urea were determined at
weeks 4 and 13. At necropsy, organs were weighed and examined
histologically. No adverse effects were observed (Mondino, 1981). The
dose that had no effect is 100 000 and 100 times greater than the
estimated per capita intakes of 0.002 mg/kg bw per day in Europe and
1 mg/kg bw per day in the United States.
(xi) Thioesters
Ethyl thioacetate (No. 483)
Groups of 23 male and 23 female rats were given ethyl thioacetate
(No. 483) at a dose of 6.5 mg/kg bw per day in the diet, while a
control group received basal diet alone. The animals were observed
daily for appearance, physiological responses, behaviour, any
pharmacological or toxicological responses, and deaths. Body weights
and feed consumption were recorded weekly. During weeks 6 and 13,
urine was obtained from eight males and eight females for estimation
of pH and specific gravity, for microscopic examination of sediment,
and for qualitative estimates of albumin, glucose, occult blood,
ketones, and bilirubin. Eight animals of each sex were killed after
six weeks, and blood was taken for haematological testing. The
remaining animals were necropsied at 13 weeks and their tissues
examined for gross pathological changes. Organs were weighed and
tissues retained for histological evaluations. The growth, feed
consumption, and feed utilization of the test animals were similar to
those of controls throughout the study, and blood biochemical and
haematological indices were normal. Likewise, there were no
significant differences in the average absolute or relative organ
weights. All rats appeared normal throughout the study, and no gross
pathological lesions were seen in any tissue (Shellenberger, 1970).
The dose that had no effect is 1 000 000 times greater than the
estimated per capita intake of 0.0003 mg/kg bw per day in both Europe
and the United States.
Methyl thiobutyrate (No. 484)
Three groups of 10 male rats were fed 20, 200, or 500 mg/kg bw
per day of methyl thiobutyrate (No. 484) for 13 weeks, the dose given
to the third group being increased from 500 to 1000 mg/kg bw per day
after six weeks. A control group received only the basal diet. Feed
consumption and body weights were recorded weekly. A slight decrease
in weight gain was observed in treated rats as compared with controls,
which was attributed to decreased feed intake associated with
unpalatability. At necropsy, haematological examinations showed normal
values, and no differences in relative or absolute organ weights or
gross or histological appearance was seen between test and control
animals (Wheldon et al., 1970). The dose of 1000 mg/kg bw per day
which had no effect is 1 000 000 times greater than the estimated per
capita intake of 0.1 mg/kg bw per day in both Europe and the United
States.
(xii) Sulfoxides
No 90-day study was available for this group of compounds, but
methylsulfinylmethane (DMSO; No. 507) has been tested in long-term
studies of toxicity and carcinogenicity in rats, dogs, and monkeys
(see below) and has also been given to humans (see below).
2.3.2.4 Long-term studies of toxicity and carcinogenicity
Long-term studies of toxicity and carcinogenicity in rats, dogs,
and monkeys were available only for DMSO (No. 507) (Table 4).
Groups of 50 male and 50 female Sprague-Dawley rats and groups of
five male and five female pure-bred Pembrokeshire Corgi dogs were
given a 50% aqueous solution containing undiluted DMSO at 1100, 3300,
or 9900 mg/kg bw per day by gavage on five days per week. A control
group received 1 L/kg bw per day of distilled water. The rats were
treated for 18 months and the dogs for either 18 weeks or two years.
All animals were observed daily for clinical signs, and body weight
and feed consumption were measured weekly. Haematological assessments,
biochemical measurements, and urinary analyses were performed after 4,
12, 20, 32, 51, 60, and 72 weeks for the rats and after 1, 3, 4.5, 6,
9, 12, 18, and 24 months for the dogs. Routine ophthalmo-scopic
examinations were also performed. At termination, detailed macroscopic
and histopathological examinations were performed.
A dose-related decrease in weight gain was observed in rats at
all doses, except in males receiving the lowest dose. The decrease in
weight gain was not accompanied by a decrease in feed intake. Male
rats at the high dose also showed a slight reduction in haemoglobin
and packed-cell volume. Examination of the eyes revealed no changes in
the retina or vitreous humour. No adverse effects on body weight or
feed intake were seen in the dogs. Persistent diuresis was observed in
those receiving the intermediate and high doses, but there was no
renal damage. Increased packed-cell volume and haemoglobin levels were
observed at the highest dose, although the erthrocytes had normal
haemoglobin concentrations and were of normal size. The most
significant observation in dogs was lenticular changes, comprising
changes in the refractive index of the central portion of the lens,
transitory, dense, white equatorial lens opacity, the appearance of
persistent opalescence in the central region of the lens, and changes
in the vitreous humour. Biochemical analyses of the lenses of affected
animals revealed an increase in insoluble protein and reductions in
soluble protein, glutathione, and water. These changes were observed
during the fifth month in dogs receiving the highest dose and during
the ninth to tenth months in those at the intermediate dose. Nuclear
refractive changes were observed after six months in dogs at the low
dose, but none of these animals had opalescence. Those animals that
were withdrawn from the study at 18 weeks showed partial regression of
the lenticular changes at the end of the two-year period, and the
lenses of dogs that received the intermediate dose had apparently
reverted to a normal state. No abnormalities other than those in the
eye were detected on macroscopic or histopathological examination
(Noel et al., 1975).
Four groups of rhesus monkeys received doses equivalent to 0,
990, 3000, or 9000 mg/kg bw per day of a 90% solution of DMSO by
gastric intubation or dermal application for 74-87 weeks. Three
females and one male received only water by oral intubation, and two
males and one female received only water dermally and served as
controls. Two animals of each sex were treated with 990 and 2970 mg/kg
bw per day, and three animals of each sex received 8910 mg/kg bw per
day by each route of administration. The topical administration
consisted of direct application to the entire abdominal skin. Physical
signs, behaviour, and survival were recorded daily. Body weights,
water consumption, blood pressure, heart rate, respiratory rate,
neurological reflexes, and complete haematological ophthalmological
and urinary analyses were performed during weeks 1, 4, 7, 12, 24, 37,
51, and 73. At termination, gross and histomorphological examinations
were made.
All animals treated topically showed scaling and flaking on the
area of application but no other adverse behavioural or physical
signs. Animals given the highest dose orally died between weeks 15 and
53 of the study. No other treatment-related changes were found in the
monkeys treated orally, in physical examinations, in haematological,
ophthalmological, urinary, or biochemical parameters, or in absolute
or relative organ weights. Histologically, atelectasis and emphysema
were the only pathological changes observed, and were seen only at the
highest dose. Some regurgitation and/or tracheal inspiration of DMSO,
which is a highly volatile, irritating substance, may have occurred
(Vogin et al., 1970).
2.3.2.5 Genotoxicity
Studies of mutagenicity and genotoxicity were available for 14
mono-, di-, and trisulfides, thiols, and related oxygenated
derivatives in this group and three structurally related substances
(Table 5). The compounds were tested for mutagenicity in vitro at
concentrations up to 300 000 mg/plate. No effect was found in
Salmonella typhimurium strains TA97, TA98, TA100, TA102, TA1535,
TA1537, TA1538, or TA2637 with or without metabolic activation (Lavoie
et al., 1979; Eder et al., 1980, 1982; Wild et al., 1983; Aeschbacher
et al., 1989; Watanabe & Morimoto, 1989a,b; Phillips Petroleum Co.,
1990a; Zeiger et al., 1992; Hakura et al., 1993; Wang et al., 1994;
Karekar et al., 1996; Brams et al., 1997). The only positive results
found were with 1,2-ethanedithiol (No. 532) and DMSO (No. 507). The
latter is widely used as a solvent in assays for genotoxicity in
vitro.
In a test for reverse mutation in nine strains of S.
typhimurium and Escherichia coli strains WP2 and WP2uvrA, modified
by the use of preincubation, DMSO was mutagenic in S. typhimurium
TA1537 and TA2637 and in E. coli WP2uvrA at concentrations of
0.2-0.4 ml/plate, with and without metabolic activation (Hakura et
al., 1993). These are relatively high concentrations, some being
cytolethal, and no mutagenic activity was observed at concentrations
used routinely in this assay.
The ability of 1,2-ethanedithiol (No. 532) to induce specific
locus mutations at the Tk locus in cultured L5178Y Tk+/- mouse
lymphoma cells was evaluated in the presence and absence of a
metabolic activation system prepared from Aroclor-induced rat liver.
When unactivated cultures were treated with concentrations of 13-150
µg/ml, total cell survival was only 0.9-32%. A dose-related increase
in mutant frequency was seen relative to control cultures in the
solvent, DMSO. In cultures with metabolic activation, no evidence of
mutagenicity was seen at concentrations of 0.07-1 µg/ml. The survival
rate in these cultures (3-49%) was greater than that in cultures
without activation. Concentrations of 16 and 50 µg/ml of
1,2-ethanedithiol were reported to induce a significant
( p < 0.002), concentration-related increase in the frequency of
sister chromatid exchange in Chinese hamster ovary cells in the
absence of metabolic activation, and concentrations of 1.6 and 5 µg/ml
induced a significant ( p < 0.004) increase in the frequency of
sister chromatid exchange in the presence of metabolic activation. No
cytotoxicity was observed at concentra-tions up to 5 µg/ml (Phillips
Petroleum Co., 1990a).
The validity of the L5178YTk+/- mouse lymphoma cell assay has
been evaluated in relation to potentially acidifying substances of low
relative molecular mass by Brusick (1986) and Heck et al. (1989).
Those authors concluded that the assay was not valid, since positive
results can be induced by changes in the pH and osmolality of the
culture medium. The results of assays for sister chromatid exchange
may also be artefacts resulting from lysosome breakdown secondary to
cytotoxicity. Cytotoxic concentrations of substances may also cause
release of DNase which induces chromosomal aberrations and DNA
double-strand breaks (Zajac-Kaye & Ts'o, 1984; Bradley et al., 1987).
Cells with double-strand breaks that survive could be subject to a
variety of genotoxic consequences, including chromosomal breaks and
rearrangements. As cytotoxicity and lysosomal breakdown were not
evaluated in the study of sister chromatid exchange with
1,2-ethandithiol, the results are difficult to interpret.
Groups of male ICR mice were given two doses 48 h apart of a
mixture containing allyl sulfide (No. 458), allyl disulfide (No. 572),
or diallyl trisulfide (No. 587) in corn oil at doses of 10 or 20 mg/ml
by gavage. The doses were estimated to provide 0.33 or 0.67 mmol/kg bw
or 50 or 100 mg/kg bw on the basis of the composition of the mixture.
No increase in the frequency of mirconucleated polychromatic
erythrocytes was seen in bone-marrow cells (Marks et al., 1992).
The results of these assays in vivo and in vitro indicate
that aliphatic and aromatic thiols and sulfides are not genotoxic.
2.3.2.6 Developmental toxicity
Studies on teratogenicity were available only for 1-butanethiol
(No. 511) and benzenethiol (No. 525).
Table 5. Studies of genotoxicity with aliphatic and aromatic sulfides and thiols
No. Substance End-point Test system Concentration Results Reference
458 Allyl sulfide Reverse mutation S. typhimurium TA100 0.004-0.44 µg/ml Negativea Eder et al. (1982)
458 Allyl sulfide Micronucleus formation Mouse 38-77 mg/kg bw Negative Marks et al. (1992)
492 Methylthio 2-(acetyloxy) Reverse mutation S. typhimurium TA100, TA98, 0.156-5 mg/plate Negativea Watanabe &
propionate TA1535, TA1537 Morimoto (1989a)
493 Methylthio 2-(propionyloxy) Reverse mutation S. typhimurium TA98, TA100, 0.156-5 mg/plate Negativea Watanabe &
propionate TA1535, TA1537 Morimoto (1989b)
507 DMSOc Reverse mutation S. typhimurium TA97, TA98, 0.005-10 µmol/ Negativea Karekar et al.
TA100, TA102 plate (1996)
507 DMSO Reverse mutation S. typhimurium TA98, TA100 12.5-200 µg/plate Negativea Wang et al. (1994)
507 DMSO Reverse mutation S. typhimurium TA97, TA98, 100 000-300 000 Negativea Brams et al. (1987)
TA100 µg/plate
507 DMSO Reverse mutation S. typhimurium TA97, TA98, 100-10 000 µg/ Negativea Zeiger et al. (1992)
TA100, TA1535, TA1537 plate
507 DMSO Reverse mutation S. typhimurium TA97, TA98, 0.1-0.4 ml/plate Negativea Hakura et al. (1993)
TA100, TA102, TA104,
TA1535, TA1538
507 DMSO Reverse mutation S. typhimurium TA1537, 0.1-0.4 ml/plate Positived Hakura et al. (1993)
TA2637
521 Allyl mercaptan Reverse mutation S. typhimurium TA100 0.005-1.4 µg/ml Negativea Eder et al. (1980)
525 Benzenethiol Reverse mutation S. typhimurium TA98, TA100 25-500 µg/plate Negative Lavoie et al. (1979)
526 Benzyl mercaptan Reverse mutation S. typhimurium TA98, TA100, < 3.6 mg/plate Negativea Wild et al. (1983)
TA1535, TA1537, TA1538
532 1,2-Ethanedithiol Reverse mutation S. typhimurium TA98, TA100, 5000 µg/plate Negativea Phillips Petroleum
TA1535, TA1537, TA1538 Co. (1990a)
532 1,2-Ethanedithiol Sister chromatid Chinese hamster ovary cells 16 µg/mle Positivef Phillips Petroleum
exchange 1.6 µg/mlg Positive Co. (1990a)
532 1,2-Ethanedithiol Forward mutation L5178Y mouse lymphoma 150 µg/mle Positivef Phillips Petroleum
cells, Tk locus 1 µg/mlg Negative Co. (1990a)
551 2-Mercaptopropionic Reverse mutation S. typhimurium TA98, TA100, < 3.6 mg/plate Negativea Wild et al. (1983)
acid TA1535, TA1537, TA1538
Table 5 (contd)
No. Substance End-point Test system Concentration Results Reference
564 Dimethyl disulfide Reverse mutation S. typhimurium TA98, TA100, 0.011-1.1 mmol Negativea Aeschbacher et al.
TA102 (1989)
572 Allyl disulfide Reverse mutation S. typhimurium TA100 0.0015-0.15 µg/ml Negativea Eder et al. (1980)
572 Allyl disulfide Micronucleus formation Mouse 48-98 mg/kg bw Negativeb Marks et al. (1992)
578 Phenyl disulfide Reverse mutation S. typhimurium TA98, TA100, < 3.6 mg/plate Negativea Wild et al. (1983)
TA1535, TA1537, TA1538
579 Benzyl disulfide Reverse mutation S. typhimurium TA98, TA100, < 3.6 mg/plate Negativea Wild et al. (1983)
TA1535, TA1537, TA1538
587 Diallyl trisulfide Micronucleus formation Mouse 59-120 mg/kg bw Negativeb Marks et al. (1992)
Related substances
tert-Dodecyl Reverse mutation S. typhimurium TA98, TA100, 0.018-10 000 µg/ Negativea Zeiger et al. (1987)
mercaptan TA1535, TA1537 plate
Dimethyl sulfone Reverse mutation S. typhimurium TA98, TA100, 0.003-0.3 mmol Negativea Aeschbacher et al.
TA102 (1989)
n-Butyl sulfone Disc method E. coli Sd-4-73 Not specified Negative Szybalski, (1958)
100 2-Mercaptopropionic Basc mutation Drosophila 10 mmol/L Negative Wild et al. (1983)
acid
a With and without metabolic activation
b Mixture of allyl sulfide, allyl disulfide, and allyl trisulfide in a ratio of 68:20:12
c Tested as solvent control
d At doses that caused lethality; negative results at doses used routinely in this test
e Without metabolic activation
f Positive results in tests for sister chromatid exchange may be a result of lysosome breakdown, and positive results in mouse lymphoma
cells may be due to pH and osmolality of culture medium
g With metabolic activation
1-Butanethiol (No. 511)
Pregnant COBS CD rats were exposed to 1-butanethiol by whole-body
inhalation for 6 h per day on days 6-19 of gestation at doses of 10,
68, or 152 mg/kg of diet. The control group was exposed to filtered
air only. The fetuses were removed from all rats on gestation day 20.
The animals were examined for gross physical changes, viable fetuses,
fetal body weights, maternal body-weight changes, and
post-implantation losses or total implantations. 1-Butanethiol had no
effect at the lowest dose, but significant differences in fetal body
weights were seen at 68 and 152 mg/kg of diet (Thomas et al., 1987).
Benzenethiol (No. 525)
Groups of 15-26 New Zealand white rabbits were given benzenethiol
in corn oil orally at doses of 10, 30, or 40 mg/kg bw per day on days
6-19 of gestation. Maternal body weight and feed and water consumption
were recorded. On gestation day 30, the animals were killed and
maternal organs and fetuses were weighed. The fetuses were also
examined for external, visceral, and skeletal malformations. There was
no effect at 10 mg/kg bw per day, but significant differences in
maternal body weights were seen at 30 and 40 mg/kg bw per day.
In the same study, groups of 25 rats were given 20, 35, or 50
mg/kg bw per day orally on days 6-15 of gestation and were killed on
gestation day 20. They were examined for the same parameters as the
rabbits. Decreased mater-nal body weight was seen at all doses, and
fetal body weights were decreased at 35 and 50 mg/kg bw per day. At 50
mg/kg bw, the relative maternal liver weights were increased and
increased post-implantation losses occurred, with a slight but
nonsignificant decrease in the live litter size (George et al., 1995).
2.3.2.7 Chemoprevention
Several organosulfur compounds inhibit carcinogenesis by
increasing the metabolism, detoxification, and elimination of
carcinogens by phase I and II enzymes. For example, the effect of
oltipraz in inhibiting colon tumorigenicity is associated with
increased activity of glutathione S-transferase, NAD(P)H quinone
reductase, and UDP-glucuronosyl transferase in the colon (Reddy et
al., 1993). This effect may partly explain the cancer-inhibiting
effect of foods like garlic that contain organosulfur compounds.
Allyl sulfide (No. 458)
Seven-week-old female A/J mice were given allyl sulfide at a dose
of 200 mg/kg bw per day in the diet for three days. Two hours after
treatment, the animals were given either a single dose of 100 mg/kg bw
of the carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and
kept for 16 weeks for determination of lung tumour production or
killed immediately for preparation of lung and liver microsomes.
Administration of the test material before carcinogen treatment
significantly decreased the lung tumour incidence (by 38%) and tumour
multiplicity (by 0.6) (Hong et al., 1994).
Allyl mercaptan (No. 521)
Groups of male weanling rats were given free access to a purified
diet for two weeks and then given the same diet containing 0.2% allyl
mercaptan dissolved in corn oil, corresponding to a calculated (Food
and Drug Administration, 1993) daily intake of allyl mercaptan of 200
mg/kg bw. After two weeks, the rats were injected intraperitoneally
with a single dose of 2 mg/kg bw aflatoxin B1, 910 mg/kg bw
N-nitrosodimethylamine, or 50 mg/kg bw N-methyl- N-nitrosourea
and killed 4 h later. Hepatic DNA damage was evaluated as
single-strand breaks by the alkaline elution technique, and the amount
of DNA eluted was determined by a microfluorimetric procedure. Allyl
mercaptan significantly reduced (by 64%) the incidence of DNA
single-strand breaks induced by aflatoxin B1 and reduced that induced
by N-nitrosodimethylamine by 39%. It had no effect on the incidence
of breaks induced by N-methyl- N-nitrosourea (LeBon et al., 1997).
Propyl disulfide (No. 566)
Cultures of the adult human tumour cell lines HCT-15 (colon),
A549 (lung), and SKMEL-2 (skin) were plated for 24 h and then propyl
disulfide was added at a dose of 100 mmol for up to 48 h. The cells
were harvested and intracellular free calcium and apoptotic cells were
determined. There was no effect on apoptosis, but a 12% increase in
the intracellular concentration of calcium was observed (Sundaram &
Milner, 1996).
Allyl disulfide (No. 572)
Cultures of the adult human tumour cell lines HCT-15 (colon),
A549 (lung), and SKMEL-2 (skin) were plated for 24 h and then allyl
disulfide was added at a dose of 100 or 500 mmol for up to 48 h. The
cells were harvested and intracellular free calcium, apoptotic cells,
and DNA fragmentation were determined. The proliferation of the HCT-15
and SKMEL-2 cell lines was depressed by 90% and that of the A549 line
by 75%, and the intracellular calcium concentration was increased by
41%. Morphological changes characteristic of apoptosis were observed
in treated cells, and a fivefold increase in DNA fragmentation was
seen (Sundaram & Milner, 1996).
Groups of 12 male Fischer 344 rats received diallyl disulfide at
a concentra-tion of 0, 100, or 200 mg/kg of diet. After two weeks, the
animals received azoxymethane, a known carcinogen, subcutaneously once
a week for two weeks at a dose of 15 mg/kg bw per week. Treatment
continued for 52 weeks, at which time the animals were killed. Body
weights were recorded every two weeks for the first 10 weeks and then
every four to six weeks. At autopsy, the animals were dissected in
order to detect microscopic tumours, and their location, number, and
size were recorded. Colon tumors with a diameter > 0.5 cm were cut
into two halves: one portion was used to measure enzyme activity and
the other for histopathology. Animals fed 200 mg/kg of diet showed a
slight but significant decrease in body weight. Neither the incidence
nor the multiplicity of colon adenomas differed between the control
and experimental groups, but both doses significantly reduced the
incidence and multiplicity of invasive colon adenocarcinomas. The
activities of glutathione S-transferase, NAD(P)H quinone reductase,
and UDP-glucuronosyl transferase in the liver, colonic mucosa, and
tumours were also significantly increased in the treated animals
(Reddy et al., 1993).
2.3.3 Observations in humans
Methylsulfinylmethane (DMSO; No. 507)
In clinical trials to determine the therapeutic effects of DMSO,
8900 mg/kg bw of 90% DMSO were applied to the entire trunk of 20 men
either twice daily for three weeks or once daily for 26 weeks.
Haematological examinations, blood chemical determinations, and urine
analyses performed at the beginning and end of the three-week study
revealed no significant changes. In the 26-week study, similar
examinations were performed at 0, 2, 4, 8, 12, 16, and 24 weeks, in
addition to thymol turbidity testing and sodium sulfobromophthalein
determinations. Except for the appearance of cutaneous signs such as
erythema, scaling, contact urticaria, and stinging or burning
sensations, DMSO was tolerated well by all but two individuals, who
developed systemic symptoms including a scaling rash, severe abdominal
pain, slight nausea, and chest pains during the second week of
treatment. One of these men did not continue the study; the other
continued treatment, and the symptoms eventually abated. Clinical and
laboratory investigations showed no evidence of adverse effects
(Kligman, 1965).
Two drops of aqueous solutions of increasing concentrations of
DMSO were instilled into the lower conjunctival sac of groups of 10
adult men, each eye being used only once without washing. No adverse
effects were seen until a concentration of 50% was reached, when the
men complained of transient burning. With 90% DMSO, all the men noted
temporary stinging and burning. The external eye was completely normal
24 h after treatment. The author concluded that DMSO is absorbed so
rapidly through the conjunctival mucosa that any irritation would not
be manifested (Kligman, 1965).
While studies by oral administration are most appropriate for
evaluating the safety of flavouring substances, the results of studies
of dermal absorption may be considered useful in their absence for
substances such as DMSO, which are readily absorbed into the systemic
circulation. The lack of treatment-related adverse effects (other than
irritation) in rhesus monkeys after both oral and dermal treatment
with DMSO (Vogin et al., 1970; see above) demonstrates that the
toxicity of DMSO is independent of route of administration. Therefore,
the lack of toxic effects other than irritation in studies in humans
treated dermally or in the eye (Kligman, 1965) suggests that oral oral
intake of DMSO used as a flavouring substance would not be toxic.
3. REFERENCES
Aeschbacher, H.U., Wolleb, U., Loliger, J., Spadone, J.C. & Liardon R.
(1989) Contribution of coffee aroma constituents to the mutagenicity
of coffee. Food Chem. Toxicol., 27, 227-232.
Alterman, M.A., Chaurassia, C.S., Lu, P. & Hanzlik R.P. (1995)
Metabolism of 10-methoxydecanoic acid (1) and 10-(methylthio)decanoic
acid(2), (omega-1)-oxa and -thia analogs of lauric acid. Int. Soc.
Study Xenobiotics, 8, 118.
Ames, M.M., Selassie, C.D., Woodson, L.C., Van Loon, J.A., Hansch, C.
& Weinshilboum, R.M. (1986) Thiopurine methyltransferase:
Structure-activity relationships for benzoic acid inhibitors and
thiophenol substrates. J. Med. Chem., 29, 345-358.
Anders, M.W., Ratnayake, J.H., Hanna, P.E. & Fucus, J.A. (1980)
Involvement of thioredoxin in sulfoxide reduction by mammalian
tissues. Biochem. Biophys. Res. Commun., 97, 846-851.
Anders, M.W., Ratnayake, J.H., Hanna, P.E. & Fucus, J.A. (1981)
Thioredoxin-dependent sulfoxide reduction by rat renal cytosol.
Biochem. Drug Metab. Disposition, 9, 307-310.
Bailey, D.E. (1976a) Acute oral toxicity (LD50) studies in mice with
1,8-octanedithiol. Unpublished study submitted to the Flavor and
Extract Manufacturers' Association by Food and Drug Research
Laboratories, Inc.
Bailey, D.E. (1976b) Acute oral toxicity of 1,2-propanedithiol in
mice. Unpublished study submitted to the Flavor and Extract
Manufacturers' Association by Food and Drug Research Laboratories,
Inc.
Bailey, D.E. (1976c) Acute oral toxicity (LD50) studies in mice with
compound 75-31065 (methyl benzyl disulfide). Unpublished study
submitted to the Flavor and Extract Manufacturers' Association by Food
and Drug Research Laboratories, Inc.
Black, R.M., Brewster, K., Clarke, R.J., Hambrook, J.L., Harrison,
J.M. & Howells, D.J. (1993) Metabolism of thiodiglycol
(2,2'-thiobis-ethanol): Isolation and identification of urinary
metabolites following intraperitoneal administration to rat.
Xenobiotica, 23, 473-481.
Bradley, M.O., Taylor, V.L., Armstrong, M.J. & Galloway, S.M. (1987)
Relationship among cytotoxicity, lysosomal breakdown, chromosome
aberrations, and DNA double-strand breaks. Mutat. Res., 189, 69-79.
Brams, A., Buchet, J.P., Crutzen-Fayt, M.C., DeMeester, C., Lauwerys,
R. & Leonard, A. (1987) A comparative study, with 40 chemicals, of the
efficiency of the Salmonella assay and the SOS Chromotest (kit
procedure). Toxicol. Lett., 38, 123-133.
Bremer, J. & Greenberg, D.M. (1961) Enzymic methylation of foreign
sulfhydryl compounds. Biochim. Biophys. Acta, 46, 217-224.
British Industrial Biological Research Association (1976) Short-term
toxicity of samples TT171, TT172, TT173, and TT174 in rats.
Unpublished study submitted to the Flavor and Extract Manufacturers'
Association.
Brodnitz, M.H. & Pascale, J.V. (1971) Thiopropanol S-oxide: A
lachrymatory factor in onions. J. Agric. Food Chem., 19, 269.
Brown, V.K., Robinson, J. & Stevenson, D.E. (1963) A note on the
toxicity and solvent properties of dimethyl sulfoxide. J. Pharm.
Pharmacol., 15, 688-692.
Brusick, D. (1986) Genotoxic effects in cultured mammalian cells
produced by low pH treatment conditions and increased ion
concentrations. Environ. Mutag., 8, 879-886.
Buckberry, L.D. & Teesdale-Spittle, P.H. (1996) Sulfur-hydrogen
compounds. In: Mitchell, S., ed., Biological Interactions of Sulfur
Compounds, London: Taylor & Francis Ltd, pp. 113-144-76.
Butterworth, K.R. & Mason, P.L. (1981) Acute toxicity of thioguaiacol
and of versalide in rodents. Food Chem. Toxicol., 19, 753-755.
Butterworth, K.R., Carpanini, F.M.B., Gaunt, I.F., Hardy, J., Kiss,
I.S. & Gangolli, S.D. (1975) Short-term toxicity of dimethyl sulfide
in rat. Food Cosmet. Toxicol., 13, 15-22.
Canellakis, E.S. & Tarver, H. (1953) The metabolism of methyl
mercaptan in the intact animal. Arch. Biochem. Biophys., 42,
446-455.
Cashman, J.R. & Olsen, L.D. (1990) Stereoselective S-oxygenation of
2-aryl-1,3-dithiolanes by the flavin-containing and cytocrome P-450
monooxygenases. Mol. Pharmacol., 38, 573-585.
Cashman, J.R. & Williams, D.E. (1990) Enantioselective S-oxygenation
of 2-aryl-1,3-dithiolanes by rabbit lung enzyme preparations.
Mol. Pharmacol., 37, 333.
Cashman, J.R., Olsen, L.D. & Bornheim, L.M. (1990) Enantioselective
S-oxygenation by flavin containing cytochrome P-450 mono-oxygenases.
Chem. Res. Toxicol., 3, 344.
Cashman, J.R., Yang, Z.C., Yang, L. & Wrighton, S.A. (1995a)
Stereo-and regioselective N-and S-oxygenation of tertiary amines and
sulfides in adult human liver microsomes. Int. Soc. Study
Xenobiotics, 8, 34.
Cashman, J.R., Park, S.B., Yang, Z.C., Washington, C.B., Gomez, D.Y.,
Giacomini, K. & Brett, C.M. (1995b) Chemical, enzymatic and human
enantioselective S-oxygenation of cimetidine. Int. Soc. Study
Xenobiotics, 8, 133.
Christias, C. (1975) Specific inhibition of sclerotium formation by
2-mercaptoethanol and related sulfhydryl compounds in Sclerotium
rolfsii. Can. J. Microbiol., 21, 1541-1547.
Collinson, V.A. (1989) ST 15 C 88 Acute oral toxicity study in the
rat. Unpublished study No. A/0/13008 from Toxicol Laboratories Ltd,
United Kingdom.
Cox, G.E., Bailey, D.E. & Morgareidge, K. (1973a) 90-Day feeding
studies in rats with trithioacetone. Unpublished report submitted to
the Flavor and Extract Manufacturers' Association by Food and Drug
Research Laboratories, Inc.
Cox, G.E., Bailey, D.E. & Morgareidge, K. (1973b) 90-Day feeding
studies in rats with compound ES-307/308 (14705). Unpublished report
submitted to the Flavor and Extract Manufacturers' Association by Food
and Drug Research Laboratories, Inc.
Cox, G.E., Bailey, D.E. & Morgareidge, K. (1974a) 90-Day feeding study
in rats with compound 14935 (2-mercapto-3-butanol). Unpublished report
submitted to the Flavor and Extract Manufacturers' Association by Food
and Drug Research Laboratories, Inc.
Cox, G.E., Bailey, D.E. & Morgareidge, K. (1974b) 90-Day feeding
studies in rats with compound 31098. Unpublished report submitted to
the Flavor and Extract Manufacturers' Association by Food and Drug
Research Laboratories, Inc.
Cox, G.E., Rucci, G. & Babish, J.G. (1979) 90-Day subacute dietary
toxicity study of IFF code No. 78-002-2 in Sprague-Dawley rats.
Unpublished report submitted to the Flavor and Extract Manufacturers'
Association by Food and Drug Research Laboratories, Inc.
Damani, L.A. (1987) Metabolism of sulphur-containing drugs. In:
Benford, D.J., Bridges, J.W. & Gibson, G.G., eds,
Drug Metabolism--From Molecules to Man, London: Taylor & Francis,
pp. 581-603.
Davis, P.J. & Guenthner, L.E. (1985) Sulindac oxidation/reduction by
microbial cultures; microbial models for mammalian metabolism.
Xenobiotica, 15, 845-857.
Deng, D.J., Mueller, K., Kasper, P. & Mueller, L. (1994) Effect of
diallyl trisulfide on induction of UDS by mutagenic drugs in primary
rat hepatocytes. Biomed. Environ. Sci., 7, 85-90.
Dutton, G.J. & Illing, H.P.A. (1972) Mechanism of biosynthesis of
thio-beta-D-glucosides. Biochem. J., 129, 539-550.
Eder, E., Neudecker, T., Lutz, D. & Henschler, D. (1980) Mutagenic
potential of allyl and allylic compounds. Structure-activity
relationship as determined by alkylating and direct in vitro mutagenic
properties. Biochem. Pharmacol., 29, 993-998.
Eder, E., Henschler, D. & Neudecker, T. (1982) Mutagenic properties of
allylic and alpha, beta-unsaturated compounds: Consideration of
alkylating mechanisms. Xenobiotica, 12, 831-848.
Elfarra, A.A., Duescher, R.J., Sausen, P.J., Lawton, M.P. & Philpot,
R.M. (1995) Potential role of the flavin-containing monooxygenases in
the metabolism of endogenous compounds. Int. Soc. Study Xenobiotics,
8, 9.
El Fatih, Karim, I.A., Millership, J.S., Temple, D.J. & Woolfson, A.D.
(1988) An investigation of the metabolism of
S-carboxymethyl-L-cysteine in man using a novel HPLC-ECD method.
Eur. J. Drug. Metab. Pharmacokinet., 13, 253.
Elf Atochem (1963) Unpublished study from Pharmacology Research Inc.
Elf Atochem (1985) Unpublished study from Product Safety Labs.
Elf Atochem (1992) Unpublished study from Centre International de
Toxicologie.
Fairchild, E.J. & Stokinger, H.E. (1958) Toxicologic studies on
organic sulfur compounds. 1. Acute toxicity of some aliphatic and
aromatic thiols (mercaptans). Am. Ind. Hyg. Assoc. J., 19, 171-189.
Farr, C.H. & Kirwin, C.J. (1994) Organic sulfur compounds. In:
Clayton, D.G. & Clayton, F.E., eds, Patty's Industrial Hygiene
and Toxicology, 4th Ed., Vol. 2, New York: John Wiley & Sons, pp.
4311-4372.
Feng, P.C.C. & Solsten, R.T. (1991) In vitro transformation of
dithiopyr by rat liver enzymes: Conversion of methylthioesters to
acids by oxygenases. Xenobiotica, 21, 1265.
Fenwick, G.R. & Hanley, A.B. (1985) The genus Allium, part 2. CRC
Crit. Rev. Food Sci. Nutr., 22, 273-377.
Fishman, F.G., Jenkins, L.J., Jr, Coon, R.A. & Jones, R.A. (1969)
Effects of acute and repeated inhalation of dimethyl sulfoxide in
rats. Toxicol. Appl. Pharmacol., 15, 74-82.
Flavor and Extract Manufacturers' Association (1991) In vitro
hydrolysis of 3-methylthiohexy acetate. Unpublished report No. 183/91.
Flavor and Extract Manufacturers' Association (1996) Sensory tolerance
test protocol. Unpublished report.
Fogelman, R.W. & DeProspo, J. (1973a) Acute oral toxicity in mice with
ES-307/308. Unpublished report submitted to the Flavor and Extract
Manufacturers' Association by Affiliated Medical Research Institute.
Fogelman, R.W. & DeProspo, J. (1973b) Acute oral toxicity in mice with
SB-11-2876 (trithioacetate). Unpublished report submitted to the
Flavor and Extract Manufacturers' Association by Affiliated Medical
Research Institute.
Fogelman, R.W. & DeProspo, J. (1974) Acute oral toxicity in mice with
1,2-ethanedithiol. Unpublished report submitted to the Flavor and
Extract Manufacturers' Association by Affiliated Medical Research
Institute.
Fogelman, R.W. & Suppers, L. (1973) Acute oral toxicity in mice with
1,8-octanedithiol. Unpublished report submitted to the Flavor and
Extract Manufacturers' Association by Affiliated Medical Research
Institute.
Fogelman, R.W. & Suppers, L. (1974a) Acute oral toxicity in mice with
1,8-octanedithiol. Unpublished report submitted to the Flavor and
Extract Manufacturers' Association by Affiliated Medical Research
Institute.
Fogelman, R.W. & Suppers, L. (1974b) Acute oral toxicity in mice with
1,2-propanedithiol. Unpublished report submitted to the Flavor and
Extract Manufacturers' Association by Affiliated Medical Research
Institute.
Fogelman, R.W., Bukva, N.F. & Margolin, S. (1970) Acute oral toxicity
of 2-4252. Unpublished study submitted by Affiliated Medical
Enterprises, Inc. Princeton, NJ, USA.
Food and Drug Administration (1993) Priority-based Assessment of
Food Additives (PAFA) Database, Waskington DC: Center for Food
Safety and Applied Nutrition, p. 58.
Frandsen, A., Schousboe, A. & Griffiths, R. (1993) Cytotoxic actions
and effects on intracellular Ca2+ and cGMP concentrations of
sulphur-containing excitatory amino acids in cultured cerebral
cortical neurons. J. Neurosci. Res., 34, 331-339.
Gachon, F., Nicolas, C., Maurizis, C., Verny, M., Chabard, J.L.,
Faurie, M. & Gaillard, G. (1988) Disposition and metabolism of
letosteine in rats. Drug Metab. Disposition, 16, 853.
Gallo, M.A., Cox, G.E. & Babish, J.G. (1976) 90-Day feeding studies in
rats with compound 75-31065 (methyl benzyl disulfide). Unpublished
report to the Flavor and Extract Manufacturers' Association by Food
and Drug Research Laboratories, Inc..
George, J.D., Price, C.J., Navarro, H.A., Barr, M.C., Myers, C.B.,
Hunter, E.S., Schwetz, B.A. & Shelby, M.D. (1995) Developmental
toxicity of thiophenol (thio) in rats and rabbits. Toxicologist, 15,
160.
Gibson, T.P., Dobrinska, M.R., Lin, J.H., Entwistle, L.A. & Davies,
R.O. (1987) Biotransformation of sulindac in end-stage renal disease.
Clin. Pharmacol. Ther., 42, 82-88.
Greenzaid, P. & Jencks, W.P. (1971) Pig liver esterase. Reactions with
alcohols, structure-reactivity correlations, and the acyl-enzyme
intermediate. Biochemistry, 10, 1210.
Griffiths, P.J. & Babish, J.G. (1978) Acute oral toxicity (LD50) of
DSE 3303S (15277) in albino mice (BLU: Ha(ICR)). Unpublished report
No. B-3 to the Flavor and Extract Manufacturers' Association from Food
and Drug Research Laboratories, Inc.
Griffiths, P.J., Giessinger, M. & Fouillet, X. (1979) Report on acute
oral toxicity (LD/50) and three-month toxicity of TT 184. Unpublished
report to the Flavor and Extract Manufacturers' Association from
Battelle Geneva Research Centres.
de Groot, A.P., Spanjers, M.T. & van der Heijden, C.A. (1974) Acute
and sub-acute oral toxicity studies in rats with five flavour
compounds. Unpublished report submitted to the Flavor and Extract
Manufacturers' Association.
Grosa, G., Caputo, O., Cerutti, M., Biglino, G., Franzone, J.S.%
Cravanzola, C. (1991) Metabolism of
7-(1,3-dithiolan-2-ylmethyl)1,3-dimethylxanthine by rat liver
microsomes: Diastereoselective metabolism of the 1,3-dithiolane ring.
Drug Metab. Disposition, 19, 454-457.
Hakura, A., Mochida, H. & Yamatsu, K. (1993) Dimethyl sulfoxide (DMSO)
is mutagenic for bacterial mutagenicity tester strains. Mutat. Res.,
303, 127-133.
Harper, K.H. & Gin, H.B. (1964) The acute oral toxicity to rats of
T59. Unpublished report.
Heck, J.D., Vollmuth, T.A., Cifone, M.A., Jagannath, D.R., Myhr, B. &
Curren, R.D. (1989) An evaluation of food flavoring ingredients in a
genetic toxicity screening battery. Toxicologist, 9, 257.
Hermansky, S.J. & Weaver, E.C. (1990) Fourteen-day dietary minimum
toxicity screen (MTS) with acetyl lactic, thiomethyl ester and
propiodyl lactic, thiomethyl ester in Fischer 344 rats. Unpublished
report 53-553 to the Flavor and Extract Manufacturers' Association
from Bushy Run Research Center, Pennsylvania, USA.
Hodgson, E. & Levi, P.A. (1992) The role of the flavin-containing
monooxygenases (EC 1.14.13.8) in the metabolism and mode of action of
agricultural chemicals. Xenobiotica, 22, 1175-1183.
Hong, J.Y., Lin, M.C., Wang, Z.Y., Wang, E.J. & Yang, C.S. (1994)
Inhibition of chemical toxicity and carcinogenesis by diallyl sulfide
and diallyl sulfone. In: Huang, M.-T., Osawa, T., Ho, C.-T. & Rosen,
R.T., eds, Food Phytochemicals for Cancer prevention I, Fruits and
Vegetables (ACS Symposium Series 546), Washington DC: American
Chemical Society, pp. 97-101.
Hoodi, A.A. & Damani, L.A. (1984) Cytochrome P-450 and non-P-450
sulphoxidations. J. Pharm. Pharmacol., 36, 62P.
Hucker, H.B., Miller, J.K., Hochberg, A., Brobyn, R.D., Riordan, F.H.
& Calesnick, B. (1967) Studies on the absorption, excretion, and
metabolism of dimethyl sulfoxide (DMSO) in man. J. Pharmacol. Exp.
Ther., 155, 309-317.
International Organization of the Flavor Industry (1995) European
inquiry on volume of use. Private communication to the Flavor and
Extract Manufacturers' Association.
Karekar, V.R., Mujumdar, A.M., Joshi, S.S., Dhuley, J., Shinde, S.L. &
Ghaskadbi, S. (1996) Assessment of genotoxic effect of piperine using
Salmonella typhimurium and somatic and germ cells of Swiss albino
mice. Arzneimittel-Forsch., 46, 972-975.
Keith, R.A., Abraham, R.T., Pazmino, P. & Weinshilboum, R.M. (1983)
Correlation of low and high affinity thiol methyltransferase and
phenol methyltransferase activities in human erythrocyte membranes.
Clin. Chim. Acta, 131, 257-272.
Klancnik, J.M., Cuenod, M., Gahwiler, B.H., Jiang, Z.P. & Do, K.Q.
(1992) Release of endogenous amino acids, including homocysteic acid
and cysteine sulphinic acid, from rat hippocampal slices evoked by
electrical stimulation of Schaffer collateral-commissural fibres.
Neuroscience, 49, 557-570.
Kligman, A.M. (1965) Dimethyl sulfoxide--Part 2. J. Am. Med. Assoc.,
193, 151-156.
Koptyaev, V.G. (1967) Experimental determination of maximum
permissible concentration of dimethyl sulfide in water bodies. Hyg.
Sanit., 32, 315-320.
Kurooka, S., Hashimoto, M., Tomita, M., Maki, A. & Yoshimura, Y.
(1976) Relationship between the structure of S-acyl thiol compounds
and their rates of hydrolysis by pancreatic lipase and hepatic
carboxylic esterase. J. Biochem., 79, 533-541.
Lavoie, E., Tulley, L., Fow, E. & Hoffmann, D. (1979) Mutagenicity of
aminophenyl and nitrophenyl ethers, sulfides, and disulfides. Mutat.
Res., 67, 123-131.
Layman, D.L. & Jacob, S.W. (1985) The absorption, metabolism, and
excretion of dimethyl sulfoxide by rhesus monkeys. Life Sci., 37,
2431-2437.
LeBon, A.M., Roy, C., Dupont, C. & Suschete, M. (1997) In vivo
antigenotoxic effects of dietary allyl sulfides in the rat. Cancer
Lett., 114, 131-134.
Lee, S.C. & Renwick, A.G. (1995a) Sulphoxide reduction by rat
intestinal flora and by Escherichia coli in vitro. Biochem.
Pharmacol., 49, 1567-1576.
Lee, S.C. & Renwick, A.G. (1995b) Sulphoxide reduction by rat and
rabbit tissues in vitro. Biochem. Pharmacol., 49, 1557-1565.
Levi, P.E. & Hodgson, E. (1988) Stereospecificity in the oxidation of
phorate and phorate sulphoxide by purified FAD-containing
mono-oxygenase and cytochrome P-450 isozymes. Xenobiotica, 18,
29-39.
Maarse, H., Visscher, C.A., Willemsens, L.C., Nijssen, L.M. & Boelens,
M.H., eds (1994) Volatile Components in Food, 6th Ed., Suppl. 5,
Zeist, TNO Nutrition and Food Research.
Maiorino, R.M., Bruce, D.C. & Aposhian, H.V. (1988) Determination and
metabolism of dithiol chelating agents. VI. Isolation and
identification of the mixed disulfides of meso-2,3-dimercaptosuccinic
acid with L-cysteine in human urine. Toxicol. Appl. Pharmacol., 97,
338.
Maiorino, R.M., Xu, Z.F. & Aposhian, H.V. (1996) Determination and
metabolism of dithiol chelating agents. XXII. In humans, sodium
1,3-dimercapto-1-propanesulfonate is bound to plasma albumin via mixed
disulfide formation and is found in the urine as cyclic polymeric
disulfides. J. Pharmacol. Exp. Ther., 277, 375-384.
Marks, H.S., Anderson, J.L. & Stoewsand, G.S. (1992) Inhibition of
benzo[a]pyrene-induced bone marrow micronuclei formation by diallyl
thioethers in mice. J. Toxicol. Environ. Health, 37, 1-9
Mazel, P., Henderson, J.F. & Axelrod, J. (1964) S-Demethylation by
microsomal enzymes. J. Pharmacol. Exp. Ther., 143, 1-6.
McBain, D.A. & Menn, J.J. (1969) S-Methylation, oxidation,
hydroxylation, and conjugation of thiophenol in the rat. Biochem.
Pharmacol., 18, 2282-2285.
Michael, W.R. 1968) Metabolism of linear alkylate sulfonate and alkyl
benzene sulfonate in albino rats. Toxicol. Appl. Pharmacol., 12,
473-485.
Mitchell, S.C., Idle, J.R. & Smith, R.L. (1982) The metabolism of
[14C] cimetidine in man. Xenobiotica, 12, 283.
Mondino, A. (1981) Thirteen week repeated dose study of the test
article TT 191 (3-methyl-1,2,4-trithiane) orally administered to
Sprague Dawley Charles River CD (SD) BR rats. Unpublished study from
Instituto di richierche biomedicine 'Antoine Marxer' SpA.
Mondino, A. & Peano, S. (1979) Acute oral toxicity of
2,6-dimethylthiophenol. Unpublished report to the Flavor and Extract
Manufacturers' Association.
Mondino, A. & Peano, S. (1982) Acute toxicity study, TT 201 and TT202.
Unpublished report from Instituto di richierche biomedicine 'Antoine
Marxer' SpA.
Mondino, A., Peano, S. & Berruto, P. (1981) Acute toxicity study,
TT191. Unpublished report from Instituto di richierche biomedicine
'Antoine Marxer' SpA.
Moran, E.J., Easterday, O.D. & Oser, B.L. (1980) Acute oral toxicity
of selected flavor chemicals. Drug Chem. Toxicol., 3, 249-258.
Moreno, O.M. (1975) Acute toxicity studies on rats, rabbits, and
guinea pigs. Unpublished report to RIFM.
Moreno, O.M. (1980) Acute toxicity studies. Unpublished report to
RIFM.
Morgareidge, K. (1970) 90-Day feeding study in rats with
tetrahydrothiophene-3-one (31015). Unpublished study submitted to the
Flavor and Extract Manufacturers' Association.
Morgareidge K. (1971a) 90-Day feeding study with 2-naphthalenethiol in
rats. Unpublished study submitted to the Flavor and Extract
Manufacturers' Association.
Morgareidge, K. (1971b) 90-Day feeding study with
2-keto-3-pentanethiol in rats. Unpubli-shed study submitted to the
Flavor and Extract Manufacturers' Association.
Morgareidge, K. & Oser, B.L. (1970a) 90-Day feeding studies in rats
with cyclopentane-thiol. Unpublished study submitted to the Flavor and
Extract Manufacturers' Association.
Morgareidge, K. & Oser, B.L. (1970b) 90-Day feeding studies in rats
with dipropyltrisulfide (30204). Unpublished study submitted to the
Flavor and Extract Manufacturers' Association.
Morgareidge, K. & Oser, B.L. (1970c) 90-Day feeding studies in rats
with diallyltrisulfide. Unpublished study submitted by Food and Drug
Research Laboratories, Inc. to the Flavor and Extract Manufacturers'
Association.
Morgareidge, K., Bailey, D.E. & Cox, G.E. (1974a) 90-Day feeding study
on 2,3-butanedithiol in rats. Unpublished study submitted to the
Flavor and Extract Manufac-turers' Association.
Morgareidge, K., Bailey, D.E. & Cox, G.E. (1974b) 90-Day feeding study
in rats with compound 14966. Unpublished study submitted to the Flavor
and Extract Manufac-turers' Association.
Morgareidge, K., Bailey, D.E. & Cox, G.E. (1974c) 90-Day feeding study
of 1,8-octane-dithiol in rats. Unpublished study submitted to the
Flavor and Extract Manufacturers' Association.
Moutiez, M., Aumercier, M., Tessier, E., Parmentier, B., Tartar, A. &
Sergheraert, C. (1994) Reduction of a trisulfide derivative of
glutathione by glutathione reductases. Biochem. Biophys. Res.
Commun., 202, 1380-1386.
Munday, R. & Manns, E. (1994) Comparative toxicity of prop(en)yl
disulfides derived from Alliaceae. Possible involvement of 1-propenyl
disulfides in onion-induced hemolytic anemia. J. Agric. Food Chem.,
42, 959-962.
Munday, R., Manns, E. & Fowke, E.A. (1990) Steric effects on the
haemolytic activity of aromatic disulphides in rats. Food Chem.
Toxicol., 28, 561-566.
National Academy of Sciences (1970) Evaluating the Safety of Food
Chemicals, Washington DC.
National Academy of Sciences (1982) Evaluating the Safety of Food
Chemicals, Washington DC.
National Academy of Sciences (1987) Evaluating the Safety of Food
Chemicals, Washington DC.
Nickson, R.M. & Mitchell, S.C. (1994) Fate of dipropyl sulfide and
dipropyl sulfoxide in rat. Xenobiotica, 24, 157-168.
Nickson, R.M., Mitchell, S.C. & Zhang, A.Q. (1995) Fate of dipropyl
sulfone in rat. Xenobiotica, 25, 1391-1398.
Nnane, P. & Damani, L.A. (1995) The involvement of rat liver CYP2B1
and CYP2D1 in the microsomal sulphoxidation of 4-chlorophenyl methyl
sulphide. Int. Soc. Study Xenobiotics, 8, 110.
Noel, P.R.B., Barnett, K.C., Davies, R.E., Jolly, D.W., Leahy, J.S.,
Mawdesley-Thomas, L.E., Shillam, K.W.G., Squires, P.F., Street, A.E.,
Tucker, W.C. & Worden, A.N. (1975) The toxicity of dimethyl sulphoxide
(DMSO) for the dog, pig, rat, and rabbit. Toxicologist, 3, 143-169.
Oae, S., Mikami, A., Matsura, T., Ogawa-Asada, K., Watanabe, Y.,
Fujimori, K. & Iyanagi, T. (1985) Comparison of sulfite oxygenation
mechanism for liver microsomal FAD-containing monooxygenase with that
for cytochrome P-450. Biochem Biophys. Res. Commun., 131, 567-573.
Ogasawara, Y., Isoda, S. & Tanabe, S. (1998) A labile sulfur in
trisulfide affects cytochrome P-450 dependent lipid peroxidation in
rat liver microsomes. Toxicol. Lett., 99, 191-198.
Opdyke, D.L.J. (1979) Monographs on fragrance raw materials. Food
Cosmet. Toxicol., 17, 769.
Oser, B.L. (1966) Feeding studies with pinanyl mercaptan (PK) in rats.
Unpublished study submitted to the Flavor and Extract Manufacturers'
Association.
Oser, B.L. (1970) The acute oral toxicity to mice of ten compounds.
Unpublished study submitted to the Flavor and Extract Manufacturers'
Association.
Panasevich, R.E., Mallory, V.T. & Matthews, R.J. (1980) Dose-related
finding and single dose oral study of ethyl 4-(methylthio)butyrate.
Unpublished study submitted to the Flavor and Extract Manufacturers'
Association.
Parkinson, A. (1996) Biotransformation of xenobiotics. In: Klaassen,
C.D., ed., Casarret and Doull's Toxicology: The Basic Science of
Poisons, 5th Ed., New York, McGraw-Hill Co., pp. 113-118.
Peano, S., Roffino, A., Milone, M.F., Orlando, L. & Berruto, G. (1981)
Thirteen week repeated dose study with 2,6-dimethylthiophenol orally
administered to Sprague-Dawley Charles River CD (SD) BR rats.
Unpublished study submitted to the Flavor and Extract Manufacturers'
Association by Instituto di richierche biomedicine 'Antoine Marxer'
SpA.
Phillips Petroleum Co. (1990a) Toxicity study
summary--1,2-Ethanedithiol (TOX071).
Phillips Petroleum Co. (1990b) Toxicity study summary--Isopropyl
mercaptan (TOX083).
Phillips Petroleum Co. (1990c) Toxicity study summary--Tertiary butyl
mercaptan (TOX1-6).
Piccirillo, V.J. & Lunchicki, C. (1982) Acute oral toxicity (LD50)
study in the rat with methyl-2-methylthiobutyrate. Unpublished report
submitted to the Flavor and Extract Manufacturers' Association.
Posternak, J.M., Linder, A. & Vodoz, C.A. (1969) Summaries of
toxicological data. Toxicological tests on flavoring matters. Food
Cosmet. Toxicol., 7, 405-407.
Reddy, B.S., Rao, C.V., Rivenson, A. & Kelloff, G. (1993)
Chemoprevention of colon carcinogenesis by organosulfur compounds.
Cancer Res., 53, 3493-3498.
Renwick, A.G. (1989) Sulphoxides and sulphones. In: Damani, L.A., ed.,
Sulphur-containing Drugs and Related Organic Compounds. Chemistry,
Biochemistry and Toxicology (Ellis Horwood Series in Biochemical
Pharmacology), Vol. 1, Part B, New York: John Wiley & Sons, pp.
133-154.
Renwick, A.G. (1996) Sulfur-oxygen compounds. In: Mitchell, S., ed.,
Biological Interactions of Sulfur Compounds, London: Taylor &
Francis, pp. 42-76.
Renwick, A.G. & George, C.F. (1989) Metabolism of xenobiotics in the
gastro-intestinal tract. In: Hudson, D.H., Caldwell, J. & Paulson,
G.D., eds, Intermediary Xenobiotic Metabolism in Animals:
Methodology, Mechanisms and Significance, London, Taylor & Francis,
pp. 13-40.
Renwick, A.G., Evans, S.P., Sweatman, T.W., Cumberland, J. & George,
C.F. (1982) The role of the gut flora in the reduction of
sulphinpyrazone in the rat. Biochem. Pharmacol., 31, 2649.
Rettie, A.E., Bogucki, B.D., Lim, I. & Meier, G.P. (1990)
Stereoselective sulfoxidation of a series of alkyl p-tolyl sulfides by
microsomal and purified flavin-containing monooxygenases.
Mol. Pharmacol., 37, 643.
Rettie, A.E., Lawton, M.P., Jaffer, A., Sadeque, M. & Meier, G.P.
(1994) Prochiral sulfoxidation as a probe for multiple forms of the
microsomal flavin-containing monooxygenase: Studies with rabbit FMO1,
FMO2, FMO3, and FMO5 expressed in Escherichia coli. Arch. Biochem.
Biophys., 311, 369.
Richardson, K.A., Edward, V.T., Jones, B.C. & Hutson, D.H. (1991)
Metabolism in the rat of a model xenobiotic plant metabolite
S-benzyl-N-malonyl-L-cysteine. Xenobiotica, 21, 371.
Rush, R.E. (1991) 14-Day dietary toxicity study in rats with
3-(methylthio)hexyl acetate (MTHA). Unpublished report No. 3141.5 from
Springborn Laboratories, Inc. to the Flavor and Extract Manufacturers'
Association.
Sadeque, A.J.M., Eddy, A.C., Meierand, G.P. & Rettie, A.E. (1992)
Stereoselective sulfoxidation by human flavin-containing
monooxygenase. Drug Metab. Disposition, 20, 832.
Sadeque, A.J.M., Philpot, R.M. & Rettie, A.E. (1995) Chiral
sulfoxidation by human liver FMO3 and FMO5. Int. Soc. Study
Xenobiotics, 8.
Sanders, A. (1997) Acute oral toxicity (limit test) in the rat.
Unpublished SPL project No. 012/234 submitted to the Flavor and
Extract Manufacturers' Association.
Schafer, E.W. & Bowles, W.A., Jr (1985) Acute oral toxicity and
repellency of 933 chemicals to house and deer mice. Arch. Environ.
Contam. Toxicol., 14, 111-129.
Selyuzhitskii, G.V. (1972) Experimental data on substantiation of the
maximum allowable concentraion of methyl mercaptan, dimethyl sulfide
and dimethyl disulfide in the air of the working area of pulp paper
plants. Gig. Tr. Prof. Zabol., 16, 46-47.
Shaw, P.N. & Blagbrough, I.S. (1989) Cysteine conjugate beta-lyase,
II: Isolation, properties and structure-activity relationships. In:
Damani, L.A., ed., Sulphur-containing Drugs and Related Organic
Compounds, Vol. 2, Part B, Chichester: Ellis Horwood Ltd, pp.
135-156.
Shellenberger, T.E. (1970) Subacute toxicity evaluation of ethyl
thioacetate in rats. Final report. Unpublished report GSRI Project
NC-373 from Gulf South Research Institute submitted to the Flavor and
Extract Manufacturers' Association.
Shellenberger, T.E. (1971a) Acute toxicological evaluations of
2-naphthalenethiol in mice. Unpublished report GSRI Project NC-398
from Gulf South Research Institute submitted to the Flavor and Extract
Manufacturers' Association.
Shellenberger, T.E. (1971b) Acute toxicological evaluations of
chemicals with mice--2-Keto-3-pentanethiol. Unpublished study from
Gulf South Research Institute.
Snow, G.A. (1957) The metabolism of compounds related to ethanethiol.
J. Biochem., 65, 77-82.
Sommer, S. & Tauberger, G. (1964) Toxicologic investigations of
dimethyl sulfoxide. Arnzeimittel. Forsch., 14, 1050-1053.
Souhaili-El Amri, H., Fargetton, X., Delatour, P. & Batt, A.M. (1987)
Sulphoxidation of albendazole by the FAD-containing and cytochrome
P-450 dependent mono-oxygenase from pig liver microsomes.
Xenobiotica, 17, 1159-1168.
Stofberg, J. & Grundschober, F. (1987) Consumption ratio and food
predominance of flavoring materials. Perfumer Flavorist, 12, 27.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of
flavouring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23, 857-860.
Strong, H.A., Renwick, A.G. & George, C.F. (1984a) The site of
reduction of sulphinpyrazone in the rabbit. Xenobiotica, 14,
815-826.
Strong, H.A., Oates, J., Sembi, J., Renwick, A.G. & George, C.F.
(1984b) Role of the gut flora in the reduction of sulphinpyrazone in
humans. J. Pharmacol. Exp. Ther., 230, 726-732.
Strong, H.A., Warner, J., Renwick, A.G. & George, C.F. (1985) Sulindac
metabolism: The importance of an intact colon. Clin. Pharmacol.
Ther., 38, 387-393.
Strong, H.A., Angus, R., Oates, J., Sembi, J., Howarth, P., Renwick,
A.G. & George, C.F. (1986) Effects of ischaemic heart disease, Crohn's
disease and anti-microbial therapy on the pharmacokinetics of
sulphinpyrazone. Clin. Pharmacokinet., 11, 402-411.
Strong, H.A., Renwick, A.G., George, C.F., Liu, Y.F. & Hill, M.J.
(1987) The reduction of sulphinpyrazone and sulindac by intestinal
bacteria. Xenobiotica, 17, 685-696.
Sundaram, S.G. & Milner, J.A. (1996) Diallyl disulfide induces
apoptosis of human colon tumor cells. Carcinogenesis, 17, 669-673.
Szumlanski, C.L., Scott, M.C. & Weinshilboum, R.M. (1988) Thiopurine
methyltransferase pharmacogenetics: Human liver enzyme activity.
Clin. Pharmacol. Ther., 43, 134.
Szybalski, W. (1958) Special microbiological systems. II. Observations
on chemical mutagenesis in microorganisms. Ann. N.Y. Acad. Sci., 76,
475-489.
Takata, T., Yamazaki, M., Fujimori, K., Kim, H.Y., Iyanagi, T. & Oae,
S. (1983) Enzymatic oxygenation of sulfides with cytochrome P-450 from
rabbit liver: Stereochemistry of sulfoxide formation. Bull. Chem.
Soc. Jpn., 55, 2300-2310.
Tateishi, M. & Tomisawa, H. (1989) Cysteine conjugate beta-lyase. I:
Toxic thiol production. In: Damani, L.A., ed., Sulphur-containing
Drugs and Related Organic Compounds, Vol. 2, Part B, Chichester:
Ellis Horwood Ltd, pp. 121-134.
Tateishi, M., Suzuki, S. & Shimizu, H. (1978) Cysteine conjugate
beta-lyase in rat liver. J. Biol. Chem., 253, 8854.
Tatsumi, K., Kitamura, S. & Yamada, H. (1982) Involvement of liver
aldehyde oxidase in sulfoxide reduction. Chem. Pharm. Bull., 30,
4585-4588.
Tatsumi, K., Kitamura, S. & Yamada, H. (1983) Sulfoxide reductase
activity of liver aldehyde oxidase. Biochim. Biophys. Acta, 747,
86-92.
Taylor, K.L. & Ziegler, D.M. (1987) Studies on substrate specificity
of the hog liver flavin-containing monooxygenase. Anionic organic
sulfur compounds. Biochem. Pharmacol., 36, 141-146.
Taylor, A.J., Olavesen, A.H., Black, J.G. & Howes, D. (1978) The
metabolism of the surfactants dodecylsulfonate and hexadecylsulfonate
in the rat. Toxicol. Appl. Pharmacol., 45, 105-117.
Thomas, W.C., Seckar, J.A., Johnson, J.T., Ulrich, C.E, Klonne, D.R.,
Schardein, J.L. & Kirwin, C.J. (1987) Inhalation teratology studies of
n-butyl mercaptan in rats and mice. Fundam. Appl. Toxicol., 8,
170-178.
Vogin, E.E., Carson, S., Cannon, G., Linegar, C.R. & Rubin, L.F.
(1970) Chronic toxicity of DMSO in primates. Toxicol. Appl.
Pharmacol., 16, 606-612.
Wang, C.J., Lin, Y.L. & Lin, J.K. (1994) Mutagenicity and cytotoxicity
of nitropyrrole compounds derived from the reaction of 2-acetyl
pyrrole with nitrate. Food Chem. Toxicol., 32, 839-844.
Waring, R.H. (1996) Sulfur-sulfur compounds. In: Mitchell, S., ed.,
Biological Interactions of Sulfur Compounds, London: Taylor &
Francis, pp. 145-173.
Watanabe, S. & Kinosaki, A. (1989a) Acute oral toxicity in the rat
with acetyllactic acid thiomethyl ester. Unpublished report submitted
to the Flavor and Extract Manufacturers' Association.
Watanabe, S. & Kinosaki, A. (1989b) Acute oral toxicity in the rat
with propionyl lactic acid thiomethyl ester. Unpublished report
submitted to the Flavor and Extract Manufacturers' Association.
Watanabe, S. & Morimoto, Y. (1989a) Mutagenicity study of acetyl
lactic acid thiomethyl ester in Salmonella typhimurium and
Escherichia coli. Unpublished study submitted to the Flavor and
Extract Manufacturers' Association.
Watanabe, S. & Morimoto, Y. (1989b) Mutagenicity study of propionyl
lactic acid thiomethyl ester in Salmonella typhimurium and
Escherichia coli. Unpublished study submitted to the Flavor and
Extract Manufacturers' Association.
Weinshilboum, R.M. & Sladek, S.L. (1980) Mercaptopurine
pharmacogenetics: Monogenic inheritance of erythrocyte thiopurine
methyltransferase activity. Am. J. Hum. Genet., 32, 651-662.
Wells, W.W., Yang, Y., Diets, T.L. & Gan, Z.R. (1993)
Thioltransferases. Adv. Enzymol. Relat. Areas Mol. Biol., 66,
149-201.
Wheldon, G.H., Amyes, S.J., Street, A.E., Hague, P.H. &
Mawdesley-Thomas, L.E. (1970) Toxicity of Wa 4295, Sa 927, Stl 3048,
and Wa 3328 in dietary administration to rats over a period of 13
weeks. Unpublished report from Huntington Research Centre to the
Flavor and Extract Manufacturers' Association.
Wild, D., King, M.T., Gocke, E. & Eckhardt, K. (1983) Study of
artificial flavouring substances for mutagenicity in the
Salmonella/microsome, basc and micronucleus tests. Food Chem.
Toxicol., 21, 707-719.
Williams, K.I.H., Burstein, S.H. & Layne, D.S. (1966) Metabolism of
dimethyl sulfide, dimethyl sulfoxide, and dimethyl sulfone in the
rabbit. Arch. Biochem. Biophys., 117, 84-87.
Willson, J.E., Brown, D.E. & Timmens, E.K. (1965) A toxicologic study
of dimethyl sulfoxide. Toxicol. Appl. Pharmacol., 7, 104-122.
Wilson, K., Chissick, H., Fowler, A.M., Frearson, F.J., Gittins, M. &
Swinbourne, F.J. (1991) Metabolism of benzothiazole I. Identification
of ring-cleavage products. Xenobiotica, 21, 1179.
Wnorowski, G. (1996a) 14-Day dietary toxicity study: Rats. Unpublished
study No. 4442 from Product Safety Laboratories to the Flavor and
Extract Manufacturers' Association.
Wnorowski, G. (1996b) 14-Day dietary toxicity study: Rats. Unpublished
study No. 4453 from Product Safety Laboratories to the Flavor and
Extract Manufacturers' Association.
Wnorowski, G. (1996c) 14-Day dietary toxicity study: Rats. Unpublished
study No. 4456 from Product Safety Laboratories to the Flavor and
Extract Manufacturers' Association.
Wnorowski, G. (1996d) 14-Day dietary toxicity study: Rats. Unpublished
study No. 4454 from Product Safety Laboratories to the Flavor and
Extract Manufacturers' Association.
Wnorowski, G. (1996e) 14-Day dietary toxicity study: Rats. Unpublished
study No. 4457 from Product Safety Laboratories to the Flavor and
Extract Manufacturers' Association.
Wnorowski, G. (1997a) 14-Day repeat dose oral toxicity study: Rats.
Unpublished study No. 5211 from Product Safety Laboratories to the
Flavor and Extract Manufacturers' Association.
Wnorowski, G. (1997b) 14-Day dietary toxicity study: Rats. Unpublished
study No. 5210 from Product Safety Laboratories to the Flavor and
Extract Manufacturers' Association.
Wood, D.C., Wirth, N.V., Weber, F.S. & Palmquist, M.A. (1971)
Mechanism considerations of dimethyl sulfoxide (DMSO)-lenticular
changes in rabbits. J. Pharmacol. Exp. Ther., 177, 528-535.
Woodson, L.C. & Weinshilboum, R.M. (1983) Human kidney thiopurine
methyltransferase: Purification and biochemical properties. Biochem.
Pharmacol., 32, 819-826.
Woodson, L.C., Dunnette, J.H. & Weinshilboum, R.M. (1982)
Pharmacogenetics of human thiopurine methyltransferase:
Kidney-erythrocyte correlation and immunotitra-tion studies.
J. Pharmacol. Exp. Ther., 222, 174-181.
Woodson, L.C., Ames, M.M, Selassie, C.D., Hansch, C. & Weinshilboum,
R.M. (1983) Thiopurine methyltransferase: Aromatic thiol substrates
and inhibition by benzoic acid derivatives. Mol. Pharmacol., 24,
471-478.
Yoshihara, S. & Tatsumi, K. (1985a) Sulfoxide reduction catalysed by
guinea pig liver aldehyde oxidase in combination with one electron
reducing flavoenzymes. J. Pharmacobio-Dyn., 8, 996-1005.
Yoshihara, S. & Tatsumi, K. (1985b) Guinea pig liver aldehyde oxidase
as a sulfoxide reductase: Its purification and characterisation.
Arch. Biochem. Biophys., 242, 213-224.
Yoshihara, S. & Tatsumi, K. (1990) Metabolism of diphenyl sulphoxide
in perfused guinea pig liver. Drug Metab. Disposition, 18, 876.
Zajac-Kaye, M. & Ts'o, P.O.P. (1984) DNAase I encapsulation in
liposomes can induce neoplastic transformation of Syrian hamster
embryo cells in culture. Cell, 39, 427-437.
Zeiger, E., Anderson, B., Haworth, S., Lawlor, T., Mortelmans, K. &
Speck, W. (1987) Salmonella mutagenicity test: III. Results from the
testing of 255 chemicals. Environ. Mutag., 9 (Suppl. 9), 1-110.
Zeiger, E., Anderson, B., Haworth, S., Lawlor, T. & Mortelmans, K.
(1992) Salmonella mutagenicity tests. V. Results from the testing of
311 chemicals. Environ. Mol. Mutag., 19 (Suppl.21), 2-141.
Ziegler, D.M. (1980) Microsomal flavin-coenzyme monooxygenase:
Oxygenation of nucleophilic nitrogen and sulfur compounds. In: Jakoby,
W.B., ed., Enzymatic Basis of Detoxification, Vol. 2, Chichester:
Ellis Horwood, pp. 201-227.
Ziegler, D.M. (1989) S-Oxygenases. I. Chemistry and biochemistry. In:
Damani, L.A., ed., Sulphur-containing Drugs and Related Organic
Compounds, Vol. 2, Part A, Chichester: Ellis Horwood, pp. 53-66.
R-isomer. Decreasing stereoselectivity was seen as the size of the
alkyl group (ethyl, propyl, or isopropyl) (Sadeque et al., 1992) or
the pH (8.5-10) increased (Rettie et al., 1990). Oxidation of propyl
and butyl para-tolyl sulfide by the predominant human liver FMO
isozyme, FMO3, resulted in greater formation of the R-enantiomer
(73-88%), whereas oxidation of the methyl or ethyl derivative by human
FMO5 resulted in a > 90% preference for formation of the
S-stereoisomer of the sulfoxide (Sadeque et al., 1995). The influence
of the stereochemistry of sulfoxides on the biological and
toxicological properties of sulfides has not been evaluated.
On the basis of numerous examples of successive oxidation of
sulfides to sulfoxides and sulfones by FMO and CYP-450 enzymes in a
variety of test systems (Cashman & Williams, 1990; Cashman et al.,
1990; Rettie et al., 1990; Yoshihara & Tatsumi, 1990; Sadeque et al.,
1992; Cashman et al., 1995a,b; Elfarra et al., 1995; Nnane & Damani,
1995; Sadeque et al., 1995), the oxidation pathway appears to be a
major route for the metabolism of thioethers in humans (Ziegler, 1980;
Nickson & Mitchell, 1994).
The sulfoxide group is part of an interconvertible redox series
involving the sulfide (or thioether), sulfoxide, and sulfone. The
sulfoxide group is present in numerous molecules, which show a
diversity of biological activity; examples include pharmaceuticals
such as sulindac and flosequinan, veterinary drugs such as
albendazole, and numerous pesticides. Oxidation of a sulfoxide yields
a sulfone, which is commonly found as a metabolite of sulfoxides but
is less important as an active chemical entity. Oxidation of
sulfoxides to the corresponding sulfones occurs in both tissues and
aerobic microorganisms. The oxidation of the sulfoxide drug sulindac
to its inactive sulfone is a major metabolic pathway in humans (Strong
et al., 1985). The enzymes involved have not been studied extensively
in mammalian tissues (Levi & Hodgson, 1988) or microbes (Davis &
Guenthner, 1985). Oxidation of the sulfoxide to the sulfone is an
irreversible reaction which is probably catalysed by CYP-450 (Damani,
1987). The relative amounts of sulfoxide and sulfone excreted depend
on the stability and hydrophilicity of the sulfoxide (Damani, 1987).
After administration of [35S]-dipropyl sulfoxide to rats,
essentially all of the administered radiolabel was recovered over the
following three days, in the urine (80%), exhaled air (1.4%), faeces
(5.0%), and the carcass (13.0%) (Nickson & Mitchell, 1994). The
profile of urinary metabolites was the same after administration of
the sulfoxide or the sulfide (see above); the principal quantitative
difference was that more sulfone was excreted in urine and bile after
sulfoxide administration. Dipropyl sulfone is physiologically stable
in rats in vitro and is excreted unchanged in urine (83%) and bile
(Nickson et al., 1995). More than 98% was excreted unchanged in the
urine with trace amounts of inorganic sulfate. No reduction of the
sulfone group or oxidation of the hydrocarbon chain was observed.
As the sulfoxide may also be reduced back to the thioether, it is not
always possible to determine if the activity of a chemical in vivo
resides in the parent compound or in one of the inter-convertible
redox forms.
The three important sites of sulfoxide reduction in vivo are
liver, kidney, and gut microflora. The tissue enzymes that can reduce
sulfoxides in vitro are primarily aldehyde oxidase (Tatsumi et al.,
1982, 1983; Yoshihara & Tatsumi, 1985a,b) and thioredoxin and its
reductase (Anders et al., 1980, 1981). Studies with tissue homogenates
containing various cofactors and inhibitors have shown that the main
enzyme involved in the liver is aldehyde oxidase, whereas thioredoxin
is important in the kidney (Lee & Renwick, 1995a). The former enzyme
was active mainly under anaerobic conditions, while sulfoxide
reduction by thioredoxin occurred under both aerobic and anaerobic
conditions. The possible role of aldehyde oxidase under normal and
hypoxic conditions was shown in isolated perfused guinea-pig liver;
CYP-P450-catalysed oxidation of both diphenylsulfide and
diphenylsulfoxide to the sulfone predominated under aerobic conditions
(Yoshihara & Tatsumi, 1990), whereas oxidation of diphenylsulfoxide to
the sulfone was decreased under hypoxic conditions and trace amounts
of diphenylsulfide were formed. Infusion of the sulfoxide with
2-hydroxypyrimidine and benzaldehyde, which are electron donors for
aldehyde oxidase, resulted in increased reduction. The importance of
the kidney and thioredoxin in vivo under normal oxygen conditions is
indicated by the fact that patients with end-stage renal disease show
decreased reduction of sulindac in comparison with controls (Gibson et
al., 1987).
The gut microflora in the anaerobic environment of the lower
bowel can be an important and sometimes the sole site of reduction of
sulfoxides. Studies in germ-free animals or animals treated with
antibiotics to suppress the gut microflora and in patients indicated
that the gut bacteria were the main, or possibly the sole, site of
sulfoxide reduction of the drug sulfinpyrazone in vivo (Renwick et
al., 1982; Strong et al., 1984a,b, 1986). The intestinal bacteria can
also reduce sulindac in vitro (Strong et al., 1985), and the
thioether is a major circulating metabolite in vivo. The
contribution of the intestinal microflora to the reduction of sulindac
in humans was demonstrated by a study in normal subjects and patients
who had undergone an ileostomy. The tissues produced an initial peak
0-12 h after dosing, corresponding to 45% of the total thioether, and
the intestinal bacteria produced a second peak at 12-72 h,
corresponding to 55% of the total thioether (Strong et al., 1985).
Studies with over 200 isolated strains of bacteria from human faeces
(Strong et al., 1987) showed significant sulfoxide reduction by many
aerobic organisms such as Escherichia coli under anaerobic
conditions. Few strict anaerobes showed high reducing activity in
vitro, but they are probably essential in vivo in providing the
anaerobic environment in which the aerobes can express their sulfoxide
reductase activity (Strong et al., 1987). Recent studies with
xenobiotics as substrates have indicated the presence of a number of
soluble enzymes in E. coli that are capable of sulfoxide reduction
(Lee & Renwick, 1995b).
The enzymes involved in the reduction of the sulfoxide group
present in DMSO or of the sulfoxide metabolites of the thioethers are
likely to be the hepatic and renal systems discussed above. In order
for the gut flora to be involved in the metabolism of the flavouring
substances, the sulfur derivatives must either be incompletely
absorbed or reach the lower gut as biliary metabolites (Renwick &
George, 1989). The significant biliary excretion of the sulfoxide
metabolite after administration of dipropyl sulfide (see above) raises
the possibility of reduction by the intestinal microflora, followed by
reabsorption and subsequent reoxidation.
(ii) Acyclic sulfides with oxidized side-chains
Oxidized side-chains provide additional sites for the
biotransformation of sulfides, and, as they are polar groups, they
would increase renal excretion. When the thioether contains an
additional oxygenated functional group on a carbon atom (i.e. alcohol,
aldehyde, acid, or ketone), C-oxidation competes with sulfoxidation.
The biotransformation of such oxygenated carbon-containing functional
groups is well characterized and may have been considered in groups of
flavouring substances previously evaluated by the Committee.
Concurrent metabolism via both sulfur and oxygenated functional groups
has been reported for various substrates (El Fatih et al., 1988;
Gachon et al., 1988; Feng & Solsten, 1991; Wilson et al., 1991; Black
et al., 1993). In the presence of oxygenated functional groups,
sulfoxide formation is usually the major metabolic detoxication
pathway. The main urinary metabolites seen after intraperitoneal
administration of thiodiglycol [(HOCH2CH2)2S] to male rats were the
corresponding sulfoxide (90%) and acid thioether
S-(2-hydroxyethylthio)acetic acid (10%). The corresponding sulfone and
combined C-and S-oxidation product S-(2-hydroxyethylsulfinyl)acetic
acid were minor metabolites (Black et al., 1993). Phenacyl phenyl
sulfide is oxidized to the sulfoxide in vitro by FMO but split by
CYP-450 to give thiophenol (Oae et al., 1985). The corresponding
sulfoxides are the principal urinary metabolites of aliphatic
methylthiocarboxylic acids (Alterman et al., 1995), of the mucolytic
drug S-carboxymethyl-L-cysteine ( S-containing amino acid), of the
histamine antagonist cimetidine ( S-containing amidine) in humans
(Mitchell et al., 1982), and of other thioether drugs (Renwick, 1996).
Experiments in vitro suggest that carboxyl ester hydrolysis
occurs in the presence of thioether groups. The thioether
3-(methylthio)hexyl acetate (No. 481) was partially (9%) hydrolysed in
simulated gastric juice containing pepsin (pH = 1.1; 37-38 °C) after 4
h and completely hydrolysed in simulated intestinal fluid containing
pancreatin (Flavor & Extract Manufacturers' Association, 1991).
Thioethers with a polar anionic group such as carboxylic acid one
or more carbon atoms away for the sulfur are inhibitors of rather than
substrates for FMO (Taylor & Ziegler, 1987) and would probably be
eliminated without S-oxidation.
(iii) Cyclic sulfides
There are few published data on the fate of simple cyclic
thioethers. S-Oxidation is likely to be a major pathway in vivo,
and oxidation in vitro results in a chiral sulfoxide when the ring
is asymmetric. Oxidation of unsubstituted and methyl-substituted
cyclic sulfides by a phenobarbital-type CYP-450 yielded the
corresponding sulfoxides, but further oxidation of sulfoxides to
sulfones was not detected (Takata et al., 1983).
The metabolism of the cyclic sulfides with oxidized carbon atoms
(Nos 464, 498, 499, 550, and 562) has not been studied but would be
predicted to comprise extensive S-oxidation and possibly oxidation
or conjugation of alcohol groups.
Substituted 1,3-dithiolane derivatives undergo stereoselective
S-oxidation to the sulfoxide as a major metabolic pathway (Grosa et
al., 1991) with slower oxidation to the cyclic sulfone ( S,S-dioxide)
(Cashman & Olsen, 1990). The reactions are catalysed by both FMO and
CYP-450 (CYP2B); oxidation at both sulfur atoms was not reported. The
cyclic mono-sulfoxides of substances Nos 456, 534, and 543 are
predicted to be the main urinary metabolites, while steric hindrance
and the higher polarity of the hydroxy thioethers (Nos 550 and 562)
may allow their elimination unchanged.
(iv) Simple thiols
A large number of the flavouring agents in this group are either
alkyl or alicyclic thiols (Nos 511-524, 526, 527, and 530), arylthiols
(thiophenols) (Nos 525, 528, 529, and 531-534), dithiols (Nos
535-542), or alkylthiols with oxidized side-chains (Nos 544-547, 549,
551-561, and 563). The thiol group is weakly acidic, and endogenous
thiols have important nucleophilic functions in vivo.
Whereas alcohols are oxidized to give carbonyl compounds
(aldehydes, ketones, and carboxylic acids), thiols are not readily
oxidized to the corres-ponding thiocarbonyls. The availability of
sulfur d-orbitals allows valencies of 4 and 6, so that oxidation of
the thiol group can lead to sulfenic acids (RSOH), sulfinic acids
(RSO2H), or sulfonic acids (RSO3H), as well as disulfides (RSSR').
The metabolic pathways of thiols include oxidation to unstable
sulfenic acids (RSOH), which may be oxidized to the corresponding
sulfinic (RSO2H) and sulfonic acids (RSO3H); methylation to yield
methyl sulfides, which can be oxidized to methyl sulfoxides and
sulfones; reaction with endogenous thiols such as glutathione to form
mixed disulfides; conjugation with glucuronic acid; and oxidation of
the alpha-carbon resulting in desulfuration and formation of an
aldehyde intermediate (McBain & Menn, 1969; Dutton & Illing, 1972;
Maiorino et al., 1988; Richardson et al., 1991; Renwick, 1996).
Sulfenic acids are formed by mild oxidation of thiols but are
unstable and readily undergo further oxidation to sulfinic and
sulfonic acids or combine with nucleophiles. Reactions of sulfenic
acids include dimerization with elimination of water to produce
R-SO-S-R and oxidation to the corresponding polar sulfinic (RSO2H)
and sulfonic (RSO3H) acids. Oxidation of the thiol group of cysteine
in proteins can give stabilized sulfenic acid groups which are
critical to the active site of enzymes; redox cycling between thiol
and sulfenic acid in the active site may be essential for the
catalytic activity of a number of bacterial redox enzymes. The
sulfinic acid group is an intermediate redox state between sulfenic
and sulfonic acids. Endogenous sulfinic acids include cysteine
sulfinic acid, which is released during neuronal stimulation (Klancnik
et al., 1992) and may be involved in synaptic transmission in the
hippocampus. Cysteine and homocysteine sulfinic acids are excitatory
and cytotoxic acidic amino acids (Frandsen et al., 1993). The sulfonic
acid group is a highly polar centre and makes the molecule very
soluble in water. In general, sulfonic acids are stable to metabolism,
and desulfonation was not found in vivo with simple alkyl
sulfo-nates (Taylor et al., 1978) or with branched-chain sulfonates
(Michael, 1968).
Simple aliphatic and aromatic thiols undergo S-methylation in
mammals to produce the corresponding methyl thioether or sulfide,
which may be successively oxidized to the corresponding sulfoxides and
sulfones. Methylation is catalysed by thiopurine methyltransferase in
the cytoplasm and by thiol methyltransferase in microsomes, both of
which require S-adenosyl-L-methionine as a methyl group donor.
Thiopurine methyltransferase is present in human liver, kidney, and
erythrocytes (Woodson et al., 1982; Szumlanski et al., 1988), and
preferential substrates for this enzyme include aromatic and
heterocyclic thiols (Woodson & Weinshilboum, 1983; Woodson et al.,
1983). The apparent Km values of thiophenols (Ames et al., 1986)
are at least two orders of magnitude lower than those of aliphatic
substrates (Woodson & Weinshilboum, 1983). The activity of thiopurine
methyltransferase in humans shows genetic polymorphism, 89% of
subjects showing high activity, 11% showing intermediate activity, and
0.3% showing no activity (Weinshilboum & Sladek, 1980). Studies of
families indicate that the polymorphism is due to a single genetic
locus with two alleles, TPMTH for high activity and TPMTL for low
activity (Keith et al., 1983). The frequencies of the TPMTH and
TPMTL genes are 94% and 6%, respectively, resulting in the trimodal
frequency distribution of thiopurine methyltransferase activity in the
general population. The impact of such differences in 'methylator
status' on the metabolism of thiol-containing flavouring substances is
unknown but is likely to be small because of the presence of
alternative S-oxidation and conjugation pathways. S-Methylation of
aliphatic thiols is catalysed by microsomal thiol methyltrans-ferase,
and thiol substances such as 2-mercaptoethanol, methylmercaptan, and
2-mercaptopropionic acid are substrates for this enzyme (Bremer &
Greenberg, 1961), but the endogenous sulfur amino acid derivatives
homocysteine and glutathione are not. Microsomal thiol
methyltransferase and cytoplasmic thiopurine methyltransferase
activities are regulated independently in humans (Keith et al., 1983),
and therefore S-methylation by thiol methyltrans-ferase may occur in
individuals with low or negligible thiopurine methyltrans-ferase
activity.
Examples of S-methylation in vivo include both aliphatic and
aromatic substrates. Methyl ethyl sulfide (No. 453), which was
detected in the urine of guinea-pigs and mice given an oral dose of
diethyl disulfide (Snow, 1957), was presumably formed by reductive
cleavage to ethanethiol and subsequent methylation (Snow, 1957); minor
urinary metabolites were the sulfoxide and sulfone (Parkinson, 1996).
The sulfoxide and sulfone derivative of benzyl methyl sulfide (No.
460) were excreted in the urine of rats given an oral dose of
S-benzyl- N-malonyl-L-cysteine, presumably via methylation and
oxidation of the intermediate benzyl mercaptan (No. 525) (Richardson
et al., 1991). The urine of rats given an oral dose of [35S]phenyl
mercaptan contained S-methylated metabolites such as phenyl methyl
sulfone and ortho-and para-hydroxylated phenyl methyl sulfone
(McBain & Menn, 1969).
Thiols may react with glutathione and other endogenous thiol
compounds to form mixed disulfides, and both membrane-bound and
cytosolic thioltrans-ferases have been reported to catalyse their
formation. The resulting mixed disulfides can undergo reduction back
to thiols, oxidative desulfuration, or oxidation to a sulfonic acid
via the intermediate thiosulfinate and sulfinic acids (see above).
Thus, the disulfide represents a reversible metabolic 'reservoir' of
the xenobiotic thiol compound. The principal form in the circulation
would probably be a mixed disulfide with albumin. The mixed disulfides
formed from conjugation of a thiol with glutathione or cysteine are
not substrates for cysteine conjugate C-S lyase. C-S lyase is found in
the liver, kidney, and intestinal microflora and catalyses the
reduction of the C-S bonds of cysteine conjugates, which are formed
between organic compounds and glutathione and then undergo a series of
hydrolysis steps. The thiol product is usually methylated to form a
thioether, which may then be oxidized to sulfoxide and sulfone
analogues; compounds that undergo this metabolic pathway include
bromo-benzene, paracetamol (acetominophen), propachlor (a herbicide),
and chlorinated biphenyls. With some organic compounds, conjugation
with glutathione followed by hydrolysis and C-S lyase results in a
novel, unstable thiol; for example, some halogenated substrates and
S-phenyl-L-cysteine formed from conjugation of bromobenzene yield
unstable thiols which are toxic to the kidney (Tateishi et al., 1978;
Shaw & Blagbrough, 1989; Tateishi & Tomisawa, 1989).
S-Glucuronidation of aromatic thiols has been reported, and
this is a possible pathway for the aromatic thiols (thio-phenols) (Nos
525 and 528-531) and aromatic disulfides (Nos 576-578) after their
reduction. This reaction produced thiophenol glucuronide via
conjugation to glutathione, with subse-quent C-S lyase action on the
intermediate cysteine conjugate to yield thiophenol. The metabolism of
benzothiazole-2-sulfonamide was the first of a S-glucuronide to be
fully elucidated. Three metabolites were detected in the urine of
treated rats, benzothiazole-2-thioglucuronide, benzothiazole-2-thiol,
and benzothiazole-1-mercapturate (see Buckberry & Teesdale-Spittle,
1996); the thiol group is introduced into position 2 by displacement
of the sulfonamide by glutathione. Glucuronyl transferases behave in a
similar way towards hydroxyls and sulfydryls, and the two activities
have the same subcellular location and optimal pH (Dutton & Illing,
1972).
Thiols of low relative molecular mass undergo oxidative
desulfuration in vivo to yield CO2 and SO4=. For example, 40% of
14C-labelled methyl mercaptan (No. 508) administered to rats was
eliminated as CO2, 6.4% was exhaled as unchanged compound within 6 h,
and only 2.3% was excreted in the urine. 14C also was detected in
the beta-carbon of serine and in the methyl groups of methionine,
choline, and creatine (Canellakis & Tarver, 1953), and formalde-hyde
has been shown to be an intermediate in the oxidation of methanethiol
in vitro (Mazel et al., 1964). Whereas the carbon atom from a thiol
may be used in the biosynthesis of amino acids, the sulfur atom is not
used significantly in the synthesis of sulfur-containing amino acids:
31% of 35S-labelled methyl mercaptan was excreted in the urine as
sulfate ion (Mazel et al., 1964).
(v) Thiols with oxidized side-chains
Although these compounds comprise a significant proportion of the
flavouring agents in this group, their metabolite fate has not been
studied specifically. The metabolism is predicted to be a combination
of the pathways described above for simple thiols, combined with
further oxidation or conjugation of the oxidized side-chain.
(vi) Dithiols
The metabolism of the simple aliphatic dithiols in this group is
predicted to involve the pathways described above for thiols,
particularly oxidation of sulfur and alpha-carbon atoms and formation
of disulfides by reaction with endogenous thiols such as glutathione.
S-Oxidation of one S-atom could yield a polar thiol-sulfonate. Data
for 2,3-dimercapto-1-propanesulfonate (Maiorino et al., 1996) indicate
that a thiol-sulfonate would be excreted in the urine as such and also
as disulfides formed with endogenous thiols of low relative molecular
mass such as cysteine. The longer, linear dithiols (Nos 535, 538, and
540-542) may form an intramolecular disulfide bond and exist in
equilibrium between dithiol and cyclic disulfide.
(vii) Simple disulfides
Disulfide bonds are important elements in protein folding and
structure (Waring, 1996), and redox interconversion between disulfide
and dithiol is important in the regulation of the activity of some
enzymes. Interconversion is catalysed by a number of enzymes; for
example, glutathione reductase reduces the dimeric disulfide to two
molecules of the monomeric thiol glutathione. The reduction of
xenobiotic disulfides is believed to be extensive and may be catalysed
enzymatically by glutathione reductase (Waring, 1996) or
thioltrans-ferases (Wells et al., 1993) as well as chemically by
exchange with glutathione, thioredoxin, cysteine, and other endogenous
thiols. The non-cyclic disulfides in this group of flavours would be
metabolized to form low-relative-molecular-mass thiols, which would
then follow the pathways described above for thiols.
(viii) Disulfides with oxidized side-chains
As discussed above for groups (ii) and (iii), the additional
sites of carbon oxidation would result in greater polarity and the
potential for further oxidation or conjugation. Because of the
instability of disulfide bonds to reductive cleavage, this would be
expected to be the initial metabolic reaction, and the polarity of the
side-chains would mainly affect the elimination of thiol fragments.
(ix) Trisulfides
The trisulfide of glutathione is labile and readily converted to
the disulfide, the sulfur being released as hydrogen sulfide (Moutiez
et al., 1994). The metabolic lability of trisulfides is associated
with biological activity, such as inhibition of CYP-450 (Ogasawara et
al., 1998) and enhancement by diallyl trisulfide (No. 587) of
unscheduled DNA synthesis produced in rat hepatocytes by known
mutagens (Deng et al., 1994). The toxicity of trisulfides (Nos
582-587) and polysulfides (No. 588) is probably related to their
metabolic lability and the nature of the eventual thiol, such as allyl
thiol.
(x) Heterocyclic disulfides
The heterocyclic disulfides (Nos 573 and 574) are five-and
six-membered rings which also contain a cyclic thioether bond. Lipoic
acid is a five-membered cyclic disulfide, and rapid redox cycling
between ring disulfide and open dithiol is important in its metabolic
role in vivo. The five-membered disulfide (No. 573) would be
predicted to undergo more rapid reductive ring opening to a dithiol
than the six-membered compound (No. 574). The principal metabolic
pathways are predicted to be disulfide reduction to produce a dithiol
and S-oxidation of the thioether.
(xi) Thioesters
The thioester group (-S-CO-) is present in a number of the
flavouring substances in this group (Nos 482-494, 504, and 506). The
hydrolysis of other esters has been considered previously by the
Committee. Esters are hydrolysed by lipase and esterases (Kurooka et
al., 1976), and the rate of hydrolysis increases as the length of the
C-chain of the carboxylic acid fragment increases (Greenzaid & Jencks,
1971) and decreases as oxygenation of the carbon chain in the thiol
moiety increases (Kurooka et al., 1976).
Dithionic acids are oxidized by FMO to the dioxo analogue with
the monothioc acid as an intermediate (Taylor & Ziegler, 1987), but
simple esters of dithioic acids are poor substrates for oxidation. The
esters of monothioic acids are also probably poor substrates for
oxidation, but the monothioic acid released by hydrolysis would be
oxidized to the dioxo-acid. Another possibility for elimination in
vivo is renal excretion of the thiocarboxylic acid. With the
exceptions of prenyl thioacetate (No. 491) and methylthio
2-(acetyloxy)propio-nate (No. 492), the compounds evaluated are simple
linear alkyl compounds, branched alkyl compounds, or their side-chain
hydroxy-ester analogues, so that their toxicity can reasonably be
compared.
(xii) Sulfoxides
The metabolism of sulfoxides by oxidation and reduction is
described above for thioethers. The only sulfoxide flavouring agent is
DMSO (No. 507). The absorption, metabolism, and excretion of DMSO were
studied in rhesus monkeys given a daily oral dose of 3 g/kg bw for 14
days. DMSO was absorbed rapidly, reached a peak serum concentration
after about 4 h, and was cleared from the blood within 72 h of the end
of treatment. Dimethyl sulfone was detected in the blood 2 h after
treatment, reached a steady-state concentration after four days, and
was cleared from the blood 120 h after the end of treatment. Urinary
excretion of DMSO and dimethyl sulfone accounted for approximately 60%
and 16%, respectively, of the total ingested dose. Neither DMSO nor
dimethyl sulfone was detected in the faeces (Layman & Jacob, 1985).
The fate of DMSO in humans is similar to that in monkeys, but the
elimination is slower. When an oral dose of 1 g/kg bw was given to six
subjects, peak serum concentrations were observed approximately 4 h
after administration. The peak concentration of dimethyl sulfone was
slightly higher than that of DMSO and occurred after 72-96 h.
Approximately 51% of the dose was excreted in the urine unchanged over
the first 120 h, and up to 22% was excreted as dimethyl sulfone.
Repeated daily oral administration of DMSO at 0.5 g/kg bw per day for
14 days to one adult resulted in peak serum concentrations of DMSO
within eight days (Hucker et al., 1967).
2.3.2 Toxicological studies
The available data on the toxicity of sulfides, thiols, and
oxygenated functional derivatives in the group of 137
sulfur-containing flavouring substances are presented below. Although
the studies of acute and short-term toxicity were of limited use for
evaluating the safety of these substances, because of their short
duration, they are included for completeness. Studies related to
chemoprevention are also described.
2.3.2.1 Acute toxicity
Oral LD50 values have been reported for 52 of the 137 sulfur
compounds. The values ranged from 11 to 28 000 mg/kg bw in male and
female rats (Fairchild & Stokinger, 1958; Brown et al., 1963; Elf
Atochem, 1963; Harper & Ginn, 1964; Sommer & Tauberger, 1964; Willson
et al., 1965; Koptyaev, 1967; Fishman et al., 1969; McBain & Menn,
1969; Fogelman et al., 1970; Oser, 1970; deGroot et al., 1974;
Christias, 1975; Moreno, 1975; British Industrial Biological Research
Association, 1976; Griffiths et al., 1979; Mondino & Peano, 1979;
Opdyke, 1979; Moran et al., 1980; Moreno, 1980; Panasevich et al.,
1980; Butterworth & Mason, 1981; Mondino et al., 1981; Peano et al.,
1981; Mondino & Peano, 1982; Piccirillo & Lunchicki, 1982; Elf
Atochem, 1985; Schafer & Bowles, 1985; Collinson, 1989; Watanabe &
Kinosaki, 1989a,b; Phillips Petroleum Co., 1990a,b,c; Rush, 1991; Elf
Atochem, 1992; Farr & Kirwin, 1994; Sanders, 1997). In male and female
mice, the values ranged from 61 to > 5000 mg/kg bw (Koptyaev, 1967;
Oser, 1970; Shellen-berger, 1971a,b; Selyuzhitskii, 1972; Fogelman &
DeProspo, 1973a,b; Fogelman & Suppers, 1973; Fogelman & DeProspo,
1974; Fogelman & Suppers, 1974a,b; Bailey, 1976a,b,c; Gallo et al.,
1976; Griffiths & Babish, 1978; Moran et al., 1980; Butterworth &
Mason, 1981; Schafer & Bowles, 1985).
2.3.2.2 Short-term studies of toxicity
2,8-Dithianon-4-ene-4-carboxaldehyde (No. 471)
Five male and five female rats (strain not specified) received
0.33 or 3.3 mg/kg bw per day of 2,8-dithianon-4-ene-4-carboxaldehyde
(No. 471) by gavage on six days a week for two weeks. Individual body
weights and feed and water consumption were recorded at one and two
weeks. Haemoglobin levels determined on day 14 were normal. At
necropsy on day 14, the liver and kidney were weighed and examined
macro-and microscopically. No treat-ment-related effects were reported
(de Groot et al., 1974).
Methylthio 2-(acetyloxy)propionate (No. 492) and
methylthio 2-(propionyloxy)propionate (No. 493)
Groups of five Fischer 344 rats of each sex were given either
methylthio 2-(acetyloxy)propionate (No. 492) or methylthio
2-(propionyloxy)propionate (No. 493) in the diet for 14 days at a dose
of approximately 500 mg/kg bw per day. Control animals received a
basal diet. The animals were observed and tested daily for clinical
signs of toxicity. Body weights were measured on days 1, 6, and 14,
and feed consumption was measured on days 7 and 14. At necropsy,
organs were weighed and the liver and kidneys were examined
histologically.
Neither test material produced any abnormal clinical or
microscopic changes. The potential treatment-related effects included
depressed feed consumption, decreased body-weight gains of treated
males, and depressed relative kidney weights in treated females. The
depressed feed consumption and decreased body-weight gains observed in
treated males were probably due to the strong odour of the test
compounds, which decreased the palatability of the diets. The
biological significance, if any, of the decreased relative kidney
weights in the females (7 and 6%, respectively, with methylthio
2-(acetyloxy)-propionate and methylthio 2-(propionyloxy)propionate) is
questionable because the change was small and there were no
histological changes (Hermansky & Weaver, 1990).
Prenyl thioacetate (No. 491), prenylthiol (No. 522),
3-mercaptohexanol (No. 545), 3-methyl-3-mercaptobutyl formate
(No. 549), 3-mercaptohexyl acetate (No. 554), 3-mercaptohexyl
butyrate (No. 555), and 3-mercaptohexyl hexanoate (No. 556)
A series of 14-day studies were performed with prenyl thioacetate
(No. 491), prenylthiol (No. 522), 3-mercaptohexanol (No. 545),
3-methyl-3-mercaptobutyl formate (No. 549), 3-mercaptohexyl acetate
(No. 554), 3-mercaptohexyl butyrate (No. 555), and 3-mercaptohexyl
hexanoate (No. 556). Each substance was provided in the diets of 10
Sprague-Dawley rats of each sex at a target dose of 10 mg/kg bw per
day. Weekly measurements of body weight and feed consumption indicated
that the average daily intakes were 10-13 mg/kg bw per day. Control
animals received chow alone for the same period. The animals were
observed for gross signs of toxicity and deaths at least once daily
for the duration of the study. Body weights and feed consumption were
measured on days 8 and 15. Gross necropsies were performed on all
rats, and the kidneys and liver were removed for histopathological
examination at the time of autopsy. Body-weight gain, organ-to-body
weight ratios, and the weights of the kidney and liver of test and
control animals were not significantly different in any of the seven
studies. Gross necropsy and histopathological examination of kidney
and liver tissues revealed no lesions related to administration of the
test substances (Wnorowski, 1996a-e, 1997a,b].
Prenyl thioacetate (No. 491) and prenylthiol (No. 522),
respectively, are in the thioester and simple thiol subgroups of the
larger group of sulfur-containing flavouring substances; the other
five substances (Nos 545, 549, and 554-556) are thiols with oxidized
side-chains.
Allyl disulfide (No. 572), propyl disulfide (No. 566), and
phenyl disulfide (No. 578)
In two separate, but similarly conducted studies, allyl disulfide
(No. 572) was administered at a concentration of 250 or 1000 µmol/kg
(36 or 150 mg/kg bw per day); propyl disulfide (No. 566) at 250, 1000,
or 5000 µmol/kg (38, 150, or 750 mg/kg bw per day); and phenyl
disulfide (No. 578) at 500 µmol/kg (110 mg/kg bw per day), as
solutions in peanut oil or Tween 80, to groups of six or seven female
Sprague-Dawley rats. The doses were given by oral intubation for six
consecutive days. Control groups of 12 or 14 rats received the vehicle
alone. The animals were killed on day 7 of the experiment, and blood
was collected from the posterior vena cava. At necropsy, splenic
weights were recorded and expressed as a percentage of body weight,
and splenic darkening was assessed visually on an arbitrary scale of
0-5. Blood was examined for various parameters. Sections of splenic
sinusoidal engorgement, focal hepatic erythropoiesis, and iron
deposition in the spleen, liver, and kidneys were also scored on a
scale of 0-5. The number and size of Heinz bodies were measured to
estimate any decrease in the optical density of red cell lysates.
The results for animals given the lower dose of allyl disulfide
and the two lower doses of propyl disulfide were similar to those of
controls, whereas those given the higher doses of allyl and propyl
disulfide had enlargement and darkening of the spleen, increased iron
concentrations in the spleen, liver, and kidneys, and decreased packed
cell volume and haemoglobin concentra-tions associated with Heinz body
formation. No degenerative changes were seen in the spleen, liver, or
kidneys, but destruction of erthrocytes was indicated by deposition of
iron in these organs. Similar findings were made in rats given phenyl
disulfide.
The haemolysis observed was attributed to oxidative stress due to
'active oxygen' species formed by intra-erythrocytic redox cycling of
disulfides. Thiol-disulfide exchange with glutathione results in
reduction of disulfides to their corresponding thiols. Although
generally recognized as antioxidants, thiols act as pro-oxidants after
one-electron oxidation to a thyil radical. In erythrocytes, such
oxidation is mediated by oxyhaemoglobin and results in the formation
of methaemoglobin, hydrogen peroxide, and a thiolate anion. The
hydrogen peroxide and the more active oxygen species formed from the
thyil radical are responsible for initiating the cellular damage that
leads to haemolysis. Although human erythrocytes are usually resistant
to oxidative damage, certain individuals are susceptible because of
hereditary deficiencies (Munday et al., 1990; Munday & Manns, 1994).
2.3.2.3 Ninety-day studies of toxicity
Ninety-day studies were available for 26 of the flavouring
substances (Table 4). To facilitate comparisons of the toxicity of
structurally related substances, the studies are grouped according to
the 12 subgroups. A 90-day study was available for one or more
substance in each subgroup except that of disulfides with oxidized
side-chains. Most of the studies were performed at only one dose that
produced no adverse effects.
(i) Simple sulfides (thioethers)
Methyl sulfide (No. 452)
Four groups of 15 male and 15 female weanling SPF rats of the
Wistar strain were given methyl sulfide by oral intubation at
concentrations of 0 (control), 2.5, 25, or 250 mg/kg bw per day for 14
weeks. Two further groups of five animals of each sex were given 0.25
or 250 mg/kg bw per day for two or six weeks, respectively. The
animals were weighed on day 0 and then weekly throughout the study.
Feed and water consumption were measured for 24 h before the day of
weighing. Urine was collected during weeks 2, 6, and 14 and examined
for appearance, microscopic constituents, and content of glucose,
ketones, bile salts, and blood. The specific gravity and volume of
urine produced over 6 h were also noted. At the end of the appropriate
period of dosing, the rats were killed and blood was taken for
haematological examination. Gross abnormalities were noted and organs
weighed, and histological examinations were performed. No adverse
effect occurred with any treatment (Butterworth et al., 1975). The
dose of 250 mg/kg bw per day that had no effect is at least 10 000
times the per capita intakes of 10 and 9 mg/kg bw per day due to use
of methyl sulfide as a flavouring substance in Europe and the United
States, respectively.
In a study designed to investigate the mechanism of lenticular
changes caused by DMSO (No. 507), 10 eight-week-old New Zealand
rabbits were given 2000 mg/kg bw per day methyl sulfide (No. 452) in
drinking-water for 13 weeks. Control animals received drinking-water
only. Photographs were taken each week of the animals' lens to
evaluate the occurrence of changes. At termination, the kidney, liver,
spleen, heart, lung, adrenals, and lens were weighed and examined for
gross physical abnormalities. Treated animals had a 50% increase in
lung weight, with pulmonary congestion and some haemorrhagic spots.
The kidneys showed signs of pyelonephritis. There was no evidence of
lenticular changes (Wood et al., 1971).
(ii) Acyclic sulfides with oxidized side-chains
2-(Methylthiomethyl)-3-phenylpropenal (No. 505)
In a standardized protocol (referred to below as such for other
compounds), groups of 15 male and 15 female weanling Sprague-Dawley
rats were given a single dose of 0 or 1.4 mg/kg bw per day of
2-(methylthiomethyl)-3-phenylpropenal (No. 505) dissolved in acetone
and blended into a basal laboratory diet for 92 days. The rats were
housed individually and given feed and tap water ad libitum. The
acetone was fully evaporated before presentation of the diet to the
animals. Samples of each diet were taken weekly to assess the
stability and concentration of the test material. Appearance,
behaviour, appetite, elimination, gross signs of toxic effects, and
deaths were recorded daily, and body weights and feed consumption were
measured weekly. Haematological examinations, blood chemical
determinations, and urine analyses were performed during weeks 6 and
12 on eight males and eight females from each group. At necropsy, the
weights of the liver, kidneys, thyroid, spleen, and adrenal glands
were recorded. At termination, the weights of the thyroid, liver,
kidneys, spleen, and adrenal glands were recorded. Tissues from 26
sites and all grossly observable abnormal sites were preserved in
formalin for histopathological examination.
The body weights of females were reduced throughout the study,
and both males and females had decreased feed consumption, but these
findings did not appear to be biologically significant. No significant
differences were seen between treated and control animals with respect
to haematological, blood chemical, or urinary parameters. The absolute
and relative organ weights of the treated and control animals were
similar and there was no evidence of gross or histological changes
(Cox et al., 1979).
The dose of 2-(methylthiomethyl)-3-phenylpropenal that showed no
effects (i.e. 1.4 mg/kg bw per day) is more than 10 000 times the
estimated per capita intake of 0.03 µg/kg bw per day of this
flavouring substance in the United States.
(iii) Cyclic sulfides
2-Methyl-4-propyl-1,3-oxathiane (No. 464)
Groups of 16 male and 16 female weanling Wistar rats were given
2-methyl-4-propyl-1,3-oxathiane (No. 464) by oral intubation at a
daily dose of 0.44 mg/kg bw for 90 days or corn oil alone. The rats
were weighed on the first day of treatment and thereafter at regular
intervals throughout the study. Feed intake was recorded at three-or
four-day intervals. Blood was collected from half the rats in the
study at six weeks and from all rats at 12 weeks and was examined for
haemoglobin concentration, packed cell volume, and erythrocyte and
leukocyte counts. Urea concentration was measured. At necropsy, organs
were weighed and gross and histological examinations performed.
A slight increase in body-weight gain was seen in treated males,
which was associated with increased feed intake thought to result from
alteration of feeding behaviour due to intubation of highly flavoured
solutions. Isolated differences from controls were seen in
haematological parameters, organ weights, and histological appearance,
but none was statistically significant and were considered not to
Table 4. Results of short-term studies of toxicity and long-term studies of toxicity and carcinogenicity on aliphatic and aromatic sulfides
and thiols
No. Substance Species Sex No. test Route Duration NOEL Reference
groupsa/no. (mg/kg bw)
per test
groupb
452 Methyl sulfide Rat MF 3/30 Gavage 14 weeks 250c Butterworth
et al. (1975)
Rabbit MF 1/10 Oral 13 weeks < 2000 Wood et al. (1971)
464 2-Methyl-4-propyl-1,3-oxathiane Rat MF 1/32 Gavage 13 weeks 0.44c British Industrial
Biological Research
Association (1976)
471 2,8-Dithianon-4-en-4-carboxaldehyde Rat MF 2/10 Gavage 2 weeks ND de Groot
et al. (1974)
483 Ethyl thioacetate Rat MF 1/46 Oral 13 weeks 6.5c Shellenberger (1970)
484 Methyl thiobutyrate Rat M 3/10 Oral 13 weeks 1000c,d Wheldon et al. (1970)
491 Prenyl thioacetate Rat MF 1/20 Diet 2 weeks ND Wnorowski (1997a)
498 4,5-Dihydro-3(2H)-thiophenone Rat MF 1/30 Oral 92 days 9.2c Morgareidge (1970)
505 2-(Methylthiomethyl)-3-phenylpropenal Rat MF 1/30 Oral 92 days 1.4c Cox et al. (1979)
507 Methylsulfinylmethane Rat 4/20 Oral 13 weeks 2200 Sommer & Tauberger
(1964)
Rat MF 3/100 Gavage 18 months < 1100 Noel et al. (1975)
Dog MF 3/10 Gavage 18 weeks 1100
or 2 years
Monkey MF 3/4 Gavage 74-87 weeks 3000 Vogin et al. (1970)
516 Cyclopentanethiol Rat MF 1/30 Oral 92 days 0.56c Morgareidge &
Oser (1970a)
520 2,3- or 10-Mercaptopinane Rat MF 1/30 Oral 92 days 0.06c Oser (1966)
522 Prenylthiol Rat MF 1/20 Diet 2 weeks ND Wnorowski (1997b)
528 ortho-Toluenethiol Rat MF 1/20-32 Diet 90 days 0.52c Posternak
et al. (1969)
Table 4. (contd)
No. Substance Species Sex No. test Route Duration NOEL Reference
groupsa/no. (mg/kg bw)
per test
groupb
530 2,6-Dimethylthiophenol Rat MF 1/64 Gavage 13 weeks 0.43c Peano et al. (1981)
531 2-Naphthalenethiol Rat MF 1/30 Oral 92 days 3.4c Morgareidge (1971a)
534 2-Methyl-1,3-dithiolane Rat MF 1/32 Gavage 91 days 7.0c Griffiths
et al. (1979)
539 2,3-Butanedithiol Rat MF 1/30 Oral 92 days 0.7c Morgareidge
et al. (1974a)
541 1,8-Octanedithiol Rat MF 1/30 Oral 92 days 0.7c Morgareidge
et al. (1974c)
543 Trithioacetone Rat MF 1/30 Oral 92 days 0.2c Cox et al. (1973a)
545 3-Mercaptohexanol Rat MF 1/20 Diet 2 weeks ND Wnorowski (1996a)
546 2-Mercapto-3-butanol Rat MF 1/30 Oral 92 days 1.9c Cox et al. (1974a)
547 a-Methyl-b-hydroxypropyl Rat MF 1/30 Oral 92 days 2.8c Morgareidge
a-methyl-b-mercaptopropyl et al. (1974c)
sulfide
549 3-Mercapto-3-methylbutyl Rat MF 1/20 Diet 2 weeks ND Wnorowski (1996a)
formate
554 3-Mercaptohexyl acetate Rat MF 1/20 Diet 2 weeks ND Wnorowski (1996b)
555 3-Mercaptohexyl butyrate Rat MF 1/20 Diet 2 weeks ND Wnorowski (1996c)
556 3-Mercaptohexyl hexanoate Rat MF 1/20 Diet 2 weeks ND Wnorowski (1996d)
560 3-Mercapto-2-pentanone Rat MF 1/30 Oral 92 days 1.9c Morgareidge (1971b)
562 2,5-Dimethyl-2,5-dihydroxy Rat MF 1/30 Diet 92 days 3.1c Cox et al. (1973b)
-1,4-dithiane
566 Propyl disulfide Rat F 1/6 Gavage 6 days ND Munday & Manns (1994)
Rat M 1/10-16 Diet 90 days 7.3c Posternak
et al. (1969)
F 1/10-16 Diet 90 days 8.2c
572 Allyl disulfide Rat F 2/6 Gavage 6 days 36c Munday & Manns (1994)
Table 4. (contd)
No. Substance Species Sex No. test Route Duration NOEL Reference
groupsa/no. (mg/kg bw)
per test
groupb
573 3,5-Dimethyl-1,2,4-trithiolane Rat, MF 1/32 Gavage 90 days 1.9c British Industrial
Biological Research
Association (1976)
574 3-Methyl-1,2,4-trithiane Rat MF 1/32 Oral 13 weeks 0.3c Mondino (1981)
575 Dicyclohexyl disulfide Rat MF 1/30 Oral 92 days 0.23c Cox et al. (1974b)
577 Methyl benzyl disulfide Rat MF 1/30 Oral 92 days 1.2c Gallo et al. (1976)
578 Phenyl disulfide Rat F 1/6 Gavage 6 days ND Munday et al. (1990)
585 Dipropyl trisulfide Rat MF 1/30 Oral 92 days 4.8c Morgareidge
& Oser (1970b)
587 Diallyl trisulfide Rat MF 1/30 Oral 92 days 4.6c Morgareidge
& Oser (1970c)
M, male; F, female; ND, not determined
a No. of test groups does not include controls
b No. per test group comprises males and females
c Study performed with either a single dose or multiple doses that had no effect; the value is therefore the highest dose tested.
d Dose increased from 500 to 1000 mg after six weeks
represent adverse effects (British Industrial Biological Research
Association, 1976). The dose of 0.44 mg/kg bw per day of
2-methyl-4-propyl-1,3-oxathiane that showed no effects is more than 10
000 times the estimated per capita intakes of 0.03 mg/kg bw per day in
Europe and 0.02 mg/kg bw per day in the United States.
4,5-Dihydro-3(2H)-thiophenone (No. 498)
4,5-Dihydro-3(2H)-thiophenone (No. 498) was tested in a
standardized study (see above) at a single dose of 9.16 mg/kg bw per
day. No effects were observed (Morgareidge, 1970). This dose is 100
000 times greater than the estimated per capita intakes of 0.01 µg/kg
bw per day in Europe and 0.04 µg/kg bw per day in the United States.
2-Methyl-1,3-dithiolane (No. 534)
A group of 16 male and 16 female Sprague-Dawley rats received an
aqueous propylglycol solution (0.2% w/w) containing 7 mg/kg bw of
2-methyl-1,3-dithiolane (No. 534) daily by oral intubation for 91
days. Control animals received 0.02% propylene glycol only. The rats
were observed routinely for body weight and feed consumption.
Haematological and blood chemical parameters were determined in weeks
4 and 13. No difference was seen between control and treated groups in
body-weight gain or feed consumption. A slight, nonsignificant
reduction in haemoglobin concentration was seen in treated females.
Gross and histological examinations at termination showed no
dose-related effects, and organ weights were normal (Griffiths et al.,
1979). The dose of 7 mg/kg bw per day is greater than 1 000 000 times
the estimated per capita intake of 0.002 mg/kg bw per day of
2-methyl-1,3-dithiolane in Europe and 10 000 times that of 0.1 mg/kg
bw per day in the United States.
Trithioacetone (No. 543)
Trithioacetone (No. 543) was tested in a standardized study (see
above) at a single dose of 0.205 mg/kg bw per day. No effects were
observed (Cox et al., 1973a). This dose is 10 000 greater than the
estimated intakes of 0.04 µg/kg bw per day in Europe and 0.01 µg/kg bw
per day in the United States.
2,5-Dimethyl-2,5-dihydroxy-1,4-dithiane (No. 562)
2,5-Dimethyl-2,5-dihydroxy-1,4-dithiane (No. 562) was tested in a
standar-dized study (see above) at a single dose of 3.1 mg/kg bw per
day. No effects were observed (Cox et al., 1973b). This dose is 1 000
000 greater than the estimated per capita intakes of 0.004 µg/kg bw
per day in Europe and 0.003 µg/kg bw per day in the United States.
(iv) Simple thiols
Cyclopentanethiol (No. 516)
Cyclopentanethiol (No. 516) was tested in a standardized study
(see above) at a single dose of 0.56 mg/kg bw per day. No effects were
observed (Morgareidge & Oser, 1970a). This dose is 10 000 times
greater than the estimated per capita intake of 0.01 µg/kg bw per day
in the United States.
2,3-or 10-Mercaptopinane (No. 520)
2,3-or 10-Mercaptopinane (No. 520) was tested in a standardized
study (see above) at a single dose of 0.06 mg/kg bw per day, No
effects were observed (Oser, 1966). This dose is 10 000 times greater
than the estimated per capita intake of 0.001 µg/kg bw per day in
Europe and 100 times that of 0.2 µg/kg bw per day in the United
States.
ortho- Toluenethiol (No. 528)
Groups of 10-16 male and 10-16 female rats were given 0.52 mg/kg
bw per day of ortho-toluenethiol (No. 528) in the diet for 90 days.
Control animals received untreated diet. Feed consumption and body
weights were recorded weekly. Haematological examinations and blood
urea determinations were conducted on half the animals at seven weeks
and on all animals at 13 weeks. At necropsy, organs were weighed and
examined grossly and histologically. No adverse effects were observed
(Posternak et al., 1969). The dose that had no effect is greater than
1000 times the estimated per capita intake of 0.4 mg/kg bw per day in
Europe and 100 000 times that of 0.003 mg/kg bw per day in the United
States.
2,6-Dimethylthiophenol (No. 530)
2,6-Dimethylthiophenol (No. 530) was administered in corn oil by
gavage to 32 male and 32 female Sprague-Dawley rats at a concentration
calculated to result in an average intake of 0.43 mg/kg bw per day for
13 weeks. Control animals received only corn oil. Body weights and
feed intake were measured weekly, and haematological and blood
chemical parameters were measured at weeks 4 and 13. Organs were
weighted and gross and histological examinations were performed at the
time of autopsy. No significant difference was seen between treated
and control animals (Peano et al., 1981). The dose that had no effect
is greater then 10 000 times the estimated per capita intake of 0.03
mg/kg bw per day in Europe and greater than 1 000 000 times that of
0.0003 mg/kg bw per day in the United States.
2-Naphthalenethiol (No. 531)
2-Naphthalenethiol (No. 531) was tested in a standardized study
(see above) at a single dose of 3.4 mg/kg bw per day. No effects were
observed (Morgareidge, 1971a). The dose that had no effect is 1 000
000 times greater than the estimated per capita intake of 0.001 µg/kg
bw per day in the United States.
(v) Thiols with oxidized side-chains
2-Mercapto-3-butanol (No. 546)
2-Mercapto-3-butanol (No. 546) was tested in a standardized study
(see above). No effects were observed (Cox et al., 1974a). The dose of
1.9 mg/kg bw per day that had no effect is 1000 times greater than the
estimated per capita intake of 0.1 µg/kg bw per day in Europe and
10 000 times that of 0.002 µg/kg bw per day in the United States.
alpha-Methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl
sulfide (No. 547)
alpha-Methyl-beta-hydroxypropyl alpha-methyl-beta-mercaptopropyl
sulfide (No. 547) was tested in a standardized study (see above) at a
single dose of 2.815 mg/kg bw per day. No effects were observed
(Morgareidge et al., 1974b). This dose is 100 000 times greater than
the estimated per capita intake of 0.01 µg/kg bw in the United States.
3-Mercapto-2-pentanone (No. 560)
3-Mercapto-2-pentanone (No. 560) was tested in a standardized
study (see above) at a single dose of 1.89 mg/kg bw per day. No
effects were observed (Morgareidge, 1971b). This dose is 100 000 times
greater than the estimated per capita intake of 0.002 µg/kg bw per day
in the United States.
(vi) Dithiols
2,3-Butanedithiol (No. 539) and 1,8-octanedithiol (No. 541)
2,3-Butanedithiol (No. 539) and 1,8-octanedithiol (No. 541) were
tested in standardized studies (see above) at single doses of 0.703
and 0.705 mg/kg bw per day, respectively. No effects were observed
(Morgareidge et al., 1974a,c). The dose of 2,3-butanedithiol that had
no effect is 100 000 times greater than the estimated per capita
intakes of 0.001 µg/kg bw per day in Europe and 0.003 µg/kg bw per day
in the United States. The dose of 1,8-octanedithiol that had no effect
is 1000 times greater than the estimated per capita intake of 0.1
µg/kg bw per day in Europe and 10 000 times that of 0.01 µg/kg bw per
day in the United States.
(vii) Simple disulfides
Propyl disulfide (No. 566)
Three groups of 10-16 male and 10-16 female rats were given 7.3
or 8.2 mg/kg bw per day of propyl disulfide (No. 566) in the diet for
90 days. Control animals received untreated diet. feed consumption and
body weights were recorded weekly, and haematological examinations and
blood urea determinations were conducted on half the animals at seven
weeks and on all animals at 13 weeks. At necropsy, organs were weighed
and examined grossly and histologically. No adverse effects were
observed (Posternak et al., 1969). The doses that had no effects are
10 000 times greater than the estimated per capita intake of 0.1 mg/kg
bw per day in Europe and 1 000 000 times that of 0.002 mg/kg bw per
day in the United States.
Dicyclohexyl disulfide (No. 575)
Dicyclohexyl disulfide (No. 575) was tested in a standardized
study (see above) at a single dose of 0.23 mg/kg bw per day. No
effects were observed (Cox et al., 1974b). The dose that had no effect
is 100 000 times greater than the estimated per capita intake of
0.0003 µg/kg bw per day in Europe and 10 000 that of 0.003 µg/kg bw
per day in the United States.
Methyl benzyl disulfide (No. 577)
Methyl benzyl disulfide (No. 577) was tested in a standardized
study (see above) at a single dose of 1.2 mg/kg bw per day, No effects
were observed (Gallo et al., 1976). The dose that had no effect is 1
000 000 times greater than the estimated per capita intake of 0.0003
µg/kg bw per day in Europe and 100 000 that of 0.002 µg/kg bw per day
in the United States.
(viii) Disulfides with oxidized side-chains
No studies on the two substances in this group were available,
but their toxicity can be compared by analogy to that of the
disulfides.
(ix) Trisulfides
Dipropyl trisulfide (No. 585)
Dipropyl trisulfide (No. 585) was tested in a standardized study
(see above) at a single dose of 4.8 mg/kg bw per day, No effects were
observed (Morgareidge & Oser, 1970b). The dose that had no effect is
10 000 times greater than the estimated per capita intake of 0.2 µg/kg
bw per day in Europe and 100 000 that of 0.02 µg/kg bw per day in the
United States.
Diallyl trisulfide (No. 587)
Diallyl trisulfide (No. 587) was tested in a standardized study
(see above) at a single dose of 4.6 mg/kg bw per day, No effects were
observed (Morgareidge & Oser, 1970c). The dose that had no effect is
10 000 times greater than the estimated per capita intake of 0.1 µg/kg
bw per day in Europe and 100 000 that of 0.0003 µg/kg bw per day in
the United States.
(x) Heterocyclic disulfides
3,5-Dimethyl-1,2,4-trithiolane (No. 573)
Groups of 16 male and 16 female weanling Wistar rats were given
3,5-dimethyl-1,2,4-trithiolane (No. 573) by oral intubation at a daily
dose of 1.9 mg/kg bw for 90 days or corn oil alone. The rats were
weighed on the first day of treatment and thereafter at regular
intervals throughout the study. Feed intake was recorded at three- or
four-day intervals. Blood was collected from half the rats at six
weeks and from all rats at 12 weeks and was examined for haemoglobin
concentration, packed cell volume, and numbers of erythrocyte and
leukocytes. The urea concentration was measured. At necropsy, organs
were weighed and gross and histological examinations were performed.
A slight increase in body-weight gain was seen in treated males,
which was associated with increased feed intake thought to result from
alteration of feeding behaviour due to intubation of highly flavoured
solutions. Isolated differences from controls were seen in
haematological parameters, organ weights, and histological appearance,
but none was statistically significant and they were considered not to
represent adverse effects (British Industrial Biological Research
Association, 1976). The dose that had no effect is 100 000 times
greater than the estimated per capita intakes of 0.001 mg/kg bw per
day in Europe and 0.002 mg/kg bw per day in the United States.
3-Methyl-1,2,4-trithiane (No. 574)
3-Methyl-1,2,4-trithiane (No. 574) was administered orally to 16
male and 16 female rats (strain not specified) at a dose of 0.3 mg/kg
bw per day for 13 weeks. Body weights and feed intake were measured
weekly. Haematological parameters and blood urea were determined at
weeks 4 and 13. At necropsy, organs were weighed and examined
histologically. No adverse effects were observed (Mondino, 1981). The
dose that had no effect is 100 000 and 100 times greater than the
estimated per capita intakes of 0.002 mg/kg bw per day in Europe and
1 mg/kg bw per day in the United States.
(xi) Thioesters
Ethyl thioacetate (No. 483)
Groups of 23 male and 23 female rats were given ethyl thioacetate
(No. 483) at a dose of 6.5 mg/kg bw per day in the diet, while a
control group received basal diet alone. The animals were observed
daily for appearance, physiological responses, behaviour, any
pharmacological or toxicological responses, and deaths. Body weights
and feed consumption were recorded weekly. During weeks 6 and 13,
urine was obtained from eight males and eight females for estimation
of pH and specific gravity, for microscopic examination of sediment,
and for qualitative estimates of albumin, glucose, occult blood,
ketones, and bilirubin. Eight animals of each sex were killed after
six weeks, and blood was taken for haematological testing. The
remaining animals were necropsied at 13 weeks and their tissues
examined for gross pathological changes. Organs were weighed and
tissues retained for histological evaluations. The growth, feed
consumption, and feed utilization of the test animals were similar to
those of controls throughout the study, and blood biochemical and
haematological indices were normal. Likewise, there were no
significant differences in the average absolute or relative organ
weights. All rats appeared normal throughout the study, and no gross
pathological lesions were seen in any tissue (Shellenberger, 1970).
The dose that had no effect is 1 000 000 times greater than the
estimated per capita intake of 0.0003 mg/kg bw per day in both Europe
and the United States.
Methyl thiobutyrate (No. 484)
Three groups of 10 male rats were fed 20, 200, or 500 mg/kg bw
per day of methyl thiobutyrate (No. 484) for 13 weeks, the dose given
to the third group being increased from 500 to 1000 mg/kg bw per day
after six weeks. A control group received only the basal diet. Feed
consumption and body weights were recorded weekly. A slight decrease
in weight gain was observed in treated rats as compared with controls,
which was attributed to decreased feed intake associated with
unpalatability. At necropsy, haematological examinations showed normal
values, and no differences in relative or absolute organ weights or
gross or histological appearance was seen between test and control
animals (Wheldon et al., 1970). The dose of 1000 mg/kg bw per day
which had no effect is 1 000 000 times greater than the estimated per
capita intake of 0.1 mg/kg bw per day in both Europe and the United
States.
(xii) Sulfoxides
No 90-day study was available for this group of compounds, but
methylsulfinylmethane (DMSO; No. 507) has been tested in long-term
studies of toxicity and carcinogenicity in rats, dogs, and monkeys
(see below) and has also been given to humans (see below).
2.3.2.4 Long-term studies of toxicity and carcinogenicity
Long-term studies of toxicity and carcinogenicity in rats, dogs,
and monkeys were available only for DMSO (No. 507) (Table 4).
Groups of 50 male and 50 female Sprague-Dawley rats and groups of
five male and five female pure-bred Pembrokeshire Corgi dogs were
given a 50% aqueous solution containing undiluted DMSO at 1100, 3300,
or 9900 mg/kg bw per day by gavage on five days per week. A control
group received 1 L/kg bw per day of distilled water. The rats were
treated for 18 months and the dogs for either 18 weeks or two years.
All animals were observed daily for clinical signs, and body weight
and feed consumption were measured weekly. Haematological assessments,
biochemical measurements, and urinary analyses were performed after 4,
12, 20, 32, 51, 60, and 72 weeks for the rats and after 1, 3, 4.5, 6,
9, 12, 18, and 24 months for the dogs. Routine ophthalmo-scopic
examinations were also performed. At termination, detailed macroscopic
and histopathological examinations were performed.
A dose-related decrease in weight gain was observed in rats at
all doses, except in males receiving the lowest dose. The decrease in
weight gain was not accompanied by a decrease in feed intake. Male
rats at the high dose also showed a slight reduction in haemoglobin
and packed-cell volume. Examination of the eyes revealed no changes in
the retina or vitreous humour. No adverse effects on body weight or
feed intake were seen in the dogs. Persistent diuresis was observed in
those receiving the intermediate and high doses, but there was no
renal damage. Increased packed-cell volume and haemoglobin levels were
observed at the highest dose, although the erthrocytes had normal
haemoglobin concentrations and were of normal size. The most
significant observation in dogs was lenticular changes, comprising
changes in the refractive index of the central portion of the lens,
transitory, dense, white equatorial lens opacity, the appearance of
persistent opalescence in the central region of the lens, and changes
in the vitreous humour. Biochemical analyses of the lenses of affected
animals revealed an increase in insoluble protein and reductions in
soluble protein, glutathione, and water. These changes were observed
during the fifth month in dogs receiving the highest dose and during
the ninth to tenth months in those at the intermediate dose. Nuclear
refractive changes were observed after six months in dogs at the low
dose, but none of these animals had opalescence. Those animals that
were withdrawn from the study at 18 weeks showed partial regression of
the lenticular changes at the end of the two-year period, and the
lenses of dogs that received the intermediate dose had apparently
reverted to a normal state. No abnormalities other than those in the
eye were detected on macroscopic or histopathological examination
(Noel et al., 1975).
Four groups of rhesus monkeys received doses equivalent to 0,
990, 3000, or 9000 mg/kg bw per day of a 90% solution of DMSO by
gastric intubation or dermal application for 74-87 weeks. Three
females and one male received only water by oral intubation, and two
males and one female received only water dermally and served as
controls. Two animals of each sex were treated with 990 and 2970 mg/kg
bw per day, and three animals of each sex received 8910 mg/kg bw per
day by each route of administration. The topical administration
consisted of direct application to the entire abdominal skin. Physical
signs, behaviour, and survival were recorded daily. Body weights,
water consumption, blood pressure, heart rate, respiratory rate,
neurological reflexes, and complete haematological ophthalmological
and urinary analyses were performed during weeks 1, 4, 7, 12, 24, 37,
51, and 73. At termination, gross and histomorphological examinations
were made.
All animals treated topically showed scaling and flaking on the
area of application but no other adverse behavioural or physical
signs. Animals given the highest dose orally died between weeks 15 and
53 of the study. No other treatment-related changes were found in the
monkeys treated orally, in physical examinations, in haematological,
ophthalmological, urinary, or biochemical parameters, or in absolute
or relative organ weights. Histologically, atelectasis and emphysema
were the only pathological changes observed, and were seen only at the
highest dose. Some regurgitation and/or tracheal inspiration of DMSO,
which is a highly volatile, irritating substance, may have occurred
(Vogin et al., 1970).
2.3.2.5 Genotoxicity
Studies of mutagenicity and genotoxicity were available for 14
mono-, di-, and trisulfides, thiols, and related oxygenated
derivatives in this group and three structurally related substances
(Table 5). The compounds were tested for mutagenicity in vitro at
concentrations up to 300 000 mg/plate. No effect was found in
Salmonella typhimurium strains TA97, TA98, TA100, TA102, TA1535,
TA1537, TA1538, or TA2637 with or without metabolic activation (Lavoie
et al., 1979; Eder et al., 1980, 1982; Wild et al., 1983; Aeschbacher
et al., 1989; Watanabe & Morimoto, 1989a,b; Phillips Petroleum Co.,
1990a; Zeiger et al., 1992; Hakura et al., 1993; Wang et al., 1994;
Karekar et al., 1996; Brams et al., 1997). The only positive results
found were with 1,2-ethanedithiol (No. 532) and DMSO (No. 507). The
latter is widely used as a solvent in assays for genotoxicity in
vitro.
In a test for reverse mutation in nine strains of S.
typhimurium and Escherichia coli strains WP2 and WP2uvrA, modified
by the use of preincubation, DMSO was mutagenic in S. typhimurium
TA1537 and TA2637 and in E. coli WP2uvrA at concentrations of
0.2-0.4 ml/plate, with and without metabolic activation (Hakura et
al., 1993). These are relatively high concentrations, some being
cytolethal, and no mutagenic activity was observed at concentrations
used routinely in this assay.
The ability of 1,2-ethanedithiol (No. 532) to induce specific
locus mutations at the Tk locus in cultured L5178Y Tk+/- mouse
lymphoma cells was evaluated in the presence and absence of a
metabolic activation system prepared from Aroclor-induced rat liver.
When unactivated cultures were treated with concentrations of 13-150
µg/ml, total cell survival was only 0.9-32%. A dose-related increase
in mutant frequency was seen relative to control cultures in the
solvent, DMSO. In cultures with metabolic activation, no evidence of
mutagenicity was seen at concentrations of 0.07-1 µg/ml. The survival
rate in these cultures (3-49%) was greater than that in cultures
without activation. Concentrations of 16 and 50 µg/ml of
1,2-ethanedithiol were reported to induce a significant
( p < 0.002), concentration-related increase in the frequency of
sister chromatid exchange in Chinese hamster ovary cells in the
absence of metabolic activation, and concentrations of 1.6 and 5 µg/ml
induced a significant ( p < 0.004) increase in the frequency of
sister chromatid exchange in the presence of metabolic activation. No
cytotoxicity was observed at concentra-tions up to 5 µg/ml (Phillips
Petroleum Co., 1990a).
The validity of the L5178YTk+/- mouse lymphoma cell assay has
been evaluated in relation to potentially acidifying substances of low
relative molecular mass by Brusick (1986) and Heck et al. (1989).
Those authors concluded that the assay was not valid, since positive
results can be induced by changes in the pH and osmolality of the
culture medium. The results of assays for sister chromatid exchange
may also be artefacts resulting from lysosome breakdown secondary to
cytotoxicity. Cytotoxic concentrations of substances may also cause
release of DNase which induces chromosomal aberrations and DNA
double-strand breaks (Zajac-Kaye & Ts'o, 1984; Bradley et al., 1987).
Cells with double-strand breaks that survive could be subject to a
variety of genotoxic consequences, including chromosomal breaks and
rearrangements. As cytotoxicity and lysosomal breakdown were not
evaluated in the study of sister chromatid exchange with
1,2-ethandithiol, the results are difficult to interpret.
Groups of male ICR mice were given two doses 48 h apart of a
mixture containing allyl sulfide (No. 458), allyl disulfide (No. 572),
or diallyl trisulfide (No. 587) in corn oil at doses of 10 or 20 mg/ml
by gavage. The doses were estimated to provide 0.33 or 0.67 mmol/kg bw
or 50 or 100 mg/kg bw on the basis of the composition of the mixture.
No increase in the frequency of mirconucleated polychromatic
erythrocytes was seen in bone-marrow cells (Marks et al., 1992).
The results of these assays in vivo and in vitro indicate
that aliphatic and aromatic thiols and sulfides are not genotoxic.
2.3.2.6 Developmental toxicity
Studies on teratogenicity were available only for 1-butanethiol
(No. 511) and benzenethiol (No. 525).
Table 5. Studies of genotoxicity with aliphatic and aromatic sulfides and thiols
No. Substance End-point Test system Concentration Results Reference
458 Allyl sulfide Reverse mutation S. typhimurium TA100 0.004-0.44 µg/ml Negativea Eder et al. (1982)
458 Allyl sulfide Micronucleus formation Mouse 38-77 mg/kg bw Negative Marks et al. (1992)
492 Methylthio 2-(acetyloxy) Reverse mutation S. typhimurium TA100, TA98, 0.156-5 mg/plate Negativea Watanabe &
propionate TA1535, TA1537 Morimoto (1989a)
493 Methylthio 2-(propionyloxy) Reverse mutation S. typhimurium TA98, TA100, 0.156-5 mg/plate Negativea Watanabe &
propionate TA1535, TA1537 Morimoto (1989b)
507 DMSOc Reverse mutation S. typhimurium TA97, TA98, 0.005-10 µmol/ Negativea Karekar et al.
TA100, TA102 plate (1996)
507 DMSO Reverse mutation S. typhimurium TA98, TA100 12.5-200 µg/plate Negativea Wang et al. (1994)
507 DMSO Reverse mutation S. typhimurium TA97, TA98, 100 000-300 000 Negativea Brams et al. (1987)
TA100 µg/plate
507 DMSO Reverse mutation S. typhimurium TA97, TA98, 100-10 000 µg/ Negativea Zeiger et al. (1992)
TA100, TA1535, TA1537 plate
507 DMSO Reverse mutation S. typhimurium TA97, TA98, 0.1-0.4 ml/plate Negativea Hakura et al. (1993)
TA100, TA102, TA104,
TA1535, TA1538
507 DMSO Reverse mutation S. typhimurium TA1537, 0.1-0.4 ml/plate Positived Hakura et al. (1993)
TA2637
521 Allyl mercaptan Reverse mutation S. typhimurium TA100 0.005-1.4 µg/ml Negativea Eder et al. (1980)
525 Benzenethiol Reverse mutation S. typhimurium TA98, TA100 25-500 µg/plate Negative Lavoie et al. (1979)
526 Benzyl mercaptan Reverse mutation S. typhimurium TA98, TA100, < 3.6 mg/plate Negativea Wild et al. (1983)
TA1535, TA1537, TA1538
532 1,2-Ethanedithiol Reverse mutation S. typhimurium TA98, TA100, 5000 µg/plate Negativea Phillips Petroleum
TA1535, TA1537, TA1538 Co. (1990a)
532 1,2-Ethanedithiol Sister chromatid Chinese hamster ovary cells 16 µg/mle Positivef Phillips Petroleum
exchange 1.6 µg/mlg Positive Co. (1990a)
532 1,2-Ethanedithiol Forward mutation L5178Y mouse lymphoma 150 µg/mle Positivef Phillips Petroleum
cells, Tk locus 1 µg/mlg Negative Co. (1990a)
551 2-Mercaptopropionic Reverse mutation S. typhimurium TA98, TA100, < 3.6 mg/plate Negativea Wild et al. (1983)
acid TA1535, TA1537, TA1538
Table 5 (contd)
No. Substance End-point Test system Concentration Results Reference
564 Dimethyl disulfide Reverse mutation S. typhimurium TA98, TA100, 0.011-1.1 mmol Negativea Aeschbacher et al.
TA102 (1989)
572 Allyl disulfide Reverse mutation S. typhimurium TA100 0.0015-0.15 µg/ml Negativea Eder et al. (1980)
572 Allyl disulfide Micronucleus formation Mouse 48-98 mg/kg bw Negativeb Marks et al. (1992)
578 Phenyl disulfide Reverse mutation S. typhimurium TA98, TA100, < 3.6 mg/plate Negativea Wild et al. (1983)
TA1535, TA1537, TA1538
579 Benzyl disulfide Reverse mutation S. typhimurium TA98, TA100, < 3.6 mg/plate Negativea Wild et al. (1983)
TA1535, TA1537, TA1538
587 Diallyl trisulfide Micronucleus formation Mouse 59-120 mg/kg bw Negativeb Marks et al. (1992)
Related substances
tert-Dodecyl Reverse mutation S. typhimurium TA98, TA100, 0.018-10 000 µg/ Negativea Zeiger et al. (1987)
mercaptan TA1535, TA1537 plate
Dimethyl sulfone Reverse mutation S. typhimurium TA98, TA100, 0.003-0.3 mmol Negativea Aeschbacher et al.
TA102 (1989)
n-Butyl sulfone Disc method E. coli Sd-4-73 Not specified Negative Szybalski, (1958)
100 2-Mercaptopropionic Basc mutation Drosophila 10 mmol/L Negative Wild et al. (1983)
acid
a With and without metabolic activation
b Mixture of allyl sulfide, allyl disulfide, and allyl trisulfide in a ratio of 68:20:12
c Tested as solvent control
d At doses that caused lethality; negative results at doses used routinely in this test
e Without metabolic activation
f Positive results in tests for sister chromatid exchange may be a result of lysosome breakdown, and positive results in mouse lymphoma
cells may be due to pH and osmolality of culture medium
g With metabolic activation
1-Butanethiol (No. 511)
Pregnant COBS CD rats were exposed to 1-butanethiol by whole-body
inhalation for 6 h per day on days 6-19 of gestation at doses of 10,
68, or 152 mg/kg of diet. The control group was exposed to filtered
air only. The fetuses were removed from all rats on gestation day 20.
The animals were examined for gross physical changes, viable fetuses,
fetal body weights, maternal body-weight changes, and
post-implantation losses or total implantations. 1-Butanethiol had no
effect at the lowest dose, but significant differences in fetal body
weights were seen at 68 and 152 mg/kg of diet (Thomas et al., 1987).
Benzenethiol (No. 525)
Groups of 15-26 New Zealand white rabbits were given benzenethiol
in corn oil orally at doses of 10, 30, or 40 mg/kg bw per day on days
6-19 of gestation. Maternal body weight and feed and water consumption
were recorded. On gestation day 30, the animals were killed and
maternal organs and fetuses were weighed. The fetuses were also
examined for external, visceral, and skeletal malformations. There was
no effect at 10 mg/kg bw per day, but significant differences in
maternal body weights were seen at 30 and 40 mg/kg bw per day.
In the same study, groups of 25 rats were given 20, 35, or 50
mg/kg bw per day orally on days 6-15 of gestation and were killed on
gestation day 20. They were examined for the same parameters as the
rabbits. Decreased mater-nal body weight was seen at all doses, and
fetal body weights were decreased at 35 and 50 mg/kg bw per day. At 50
mg/kg bw, the relative maternal liver weights were increased and
increased post-implantation losses occurred, with a slight but
nonsignificant decrease in the live litter size (George et al., 1995).
2.3.2.7 Chemoprevention
Several organosulfur compounds inhibit carcinogenesis by
increasing the metabolism, detoxification, and elimination of
carcinogens by phase I and II enzymes. For example, the effect of
oltipraz in inhibiting colon tumorigenicity is associated with
increased activity of glutathione S-transferase, NAD(P)H quinone
reductase, and UDP-glucuronosyl transferase in the colon (Reddy et
al., 1993). This effect may partly explain the cancer-inhibiting
effect of foods like garlic that contain organosulfur compounds.
Allyl sulfide (No. 458)
Seven-week-old female A/J mice were given allyl sulfide at a dose
of 200 mg/kg bw per day in the diet for three days. Two hours after
treatment, the animals were given either a single dose of 100 mg/kg bw
of the carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and
kept for 16 weeks for determination of lung tumour production or
killed immediately for preparation of lung and liver microsomes.
Administration of the test material before carcinogen treatment
significantly decreased the lung tumour incidence (by 38%) and tumour
multiplicity (by 0.6) (Hong et al., 1994).
Allyl mercaptan (No. 521)
Groups of male weanling rats were given free access to a purified
diet for two weeks and then given the same diet containing 0.2% allyl
mercaptan dissolved in corn oil, corresponding to a calculated (Food
and Drug Administration, 1993) daily intake of allyl mercaptan of 200
mg/kg bw. After two weeks, the rats were injected intraperitoneally
with a single dose of 2 mg/kg bw aflatoxin B1, 910 mg/kg bw
N-nitrosodimethylamine, or 50 mg/kg bw N-methyl- N-nitrosourea
and killed 4 h later. Hepatic DNA damage was evaluated as
single-strand breaks by the alkaline elution technique, and the amount
of DNA eluted was determined by a microfluorimetric procedure. Allyl
mercaptan significantly reduced (by 64%) the incidence of DNA
single-strand breaks induced by aflatoxin B1 and reduced that induced
by N-nitrosodimethylamine by 39%. It had no effect on the incidence
of breaks induced by N-methyl- N-nitrosourea (LeBon et al., 1997).
Propyl disulfide (No. 566)
Cultures of the adult human tumour cell lines HCT-15 (colon),
A549 (lung), and SKMEL-2 (skin) were plated for 24 h and then propyl
disulfide was added at a dose of 100 mmol for up to 48 h. The cells
were harvested and intracellular free calcium and apoptotic cells were
determined. There was no effect on apoptosis, but a 12% increase in
the intracellular concentration of calcium was observed (Sundaram &
Milner, 1996).
Allyl disulfide (No. 572)
Cultures of the adult human tumour cell lines HCT-15 (colon),
A549 (lung), and SKMEL-2 (skin) were plated for 24 h and then allyl
disulfide was added at a dose of 100 or 500 mmol for up to 48 h. The
cells were harvested and intracellular free calcium, apoptotic cells,
and DNA fragmentation were determined. The proliferation of the HCT-15
and SKMEL-2 cell lines was depressed by 90% and that of the A549 line
by 75%, and the intracellular calcium concentration was increased by
41%. Morphological changes characteristic of apoptosis were observed
in treated cells, and a fivefold increase in DNA fragmentation was
seen (Sundaram & Milner, 1996).
Groups of 12 male Fischer 344 rats received diallyl disulfide at
a concentra-tion of 0, 100, or 200 mg/kg of diet. After two weeks, the
animals received azoxymethane, a known carcinogen, subcutaneously once
a week for two weeks at a dose of 15 mg/kg bw per week. Treatment
continued for 52 weeks, at which time the animals were killed. Body
weights were recorded every two weeks for the first 10 weeks and then
every four to six weeks. At autopsy, the animals were dissected in
order to detect microscopic tumours, and their location, number, and
size were recorded. Colon tumors with a diameter > 0.5 cm were cut
into two halves: one portion was used to measure enzyme activity and
the other for histopathology. Animals fed 200 mg/kg of diet showed a
slight but significant decrease in body weight. Neither the incidence
nor the multiplicity of colon adenomas differed between the control
and experimental groups, but both doses significantly reduced the
incidence and multiplicity of invasive colon adenocarcinomas. The
activities of glutathione S-transferase, NAD(P)H quinone reductase,
and UDP-glucuronosyl transferase in the liver, colonic mucosa, and
tumours were also significantly increased in the treated animals
(Reddy et al., 1993).
2.3.3 Observations in humans
Methylsulfinylmethane (DMSO; No. 507)
In clinical trials to determine the therapeutic effects of DMSO,
8900 mg/kg bw of 90% DMSO were applied to the entire trunk of 20 men
either twice daily for three weeks or once daily for 26 weeks.
Haematological examinations, blood chemical determinations, and urine
analyses performed at the beginning and end of the three-week study
revealed no significant changes. In the 26-week study, similar
examinations were performed at 0, 2, 4, 8, 12, 16, and 24 weeks, in
addition to thymol turbidity testing and sodium sulfobromophthalein
determinations. Except for the appearance of cutaneous signs such as
erythema, scaling, contact urticaria, and stinging or burning
sensations, DMSO was tolerated well by all but two individuals, who
developed systemic symptoms including a scaling rash, severe abdominal
pain, slight nausea, and chest pains during the second week of
treatment. One of these men did not continue the study; the other
continued treatment, and the symptoms eventually abated. Clinical and
laboratory investigations showed no evidence of adverse effects
(Kligman, 1965).
Two drops of aqueous solutions of increasing concentrations of
DMSO were instilled into the lower conjunctival sac of groups of 10
adult men, each eye being used only once without washing. No adverse
effects were seen until a concentration of 50% was reached, when the
men complained of transient burning. With 90% DMSO, all the men noted
temporary stinging and burning. The external eye was completely normal
24 h after treatment. The author concluded that DMSO is absorbed so
rapidly through the conjunctival mucosa that any irritation would not
be manifested (Kligman, 1965).
While studies by oral administration are most appropriate for
evaluating the safety of flavouring substances, the results of studies
of dermal absorption may be considered useful in their absence for
substances such as DMSO, which are readily absorbed into the systemic
circulation. The lack of treatment-related adverse effects (other than
irritation) in rhesus monkeys after both oral and dermal treatment
with DMSO (Vogin et al., 1970; see above) demonstrates that the
toxicity of DMSO is independent of route of administration. Therefore,
the lack of toxic effects other than irritation in studies in humans
treated dermally or in the eye (Kligman, 1965) suggests that oral oral
intake of DMSO used as a flavouring substance would not be toxic.
3. REFERENCES
Aeschbacher, H.U., Wolleb, U., Loliger, J., Spadone, J.C. & Liardon R.
(1989) Contribution of coffee aroma constituents to the mutagenicity
of coffee. Food Chem. Toxicol., 27, 227-232.
Alterman, M.A., Chaurassia, C.S., Lu, P. & Hanzlik R.P. (1995)
Metabolism of 10-methoxydecanoic acid (1) and 10-(methylthio)decanoic
acid(2), (omega-1)-oxa and -thia analogs of lauric acid. Int. Soc.
Study Xenobiotics, 8, 118.
Ames, M.M., Selassie, C.D., Woodson, L.C., Van Loon, J.A., Hansch, C.
& Weinshilboum, R.M. (1986) Thiopurine methyltransferase:
Structure-activity relationships for benzoic acid inhibitors and
thiophenol substrates. J. Med. Chem., 29, 345-358.
Anders, M.W., Ratnayake, J.H., Hanna, P.E. & Fucus, J.A. (1980)
Involvement of thioredoxin in sulfoxide reduction by mammalian
tissues. Biochem. Biophys. Res. Commun., 97, 846-851.
Anders, M.W., Ratnayake, J.H., Hanna, P.E. & Fucus, J.A. (1981)
Thioredoxin-dependent sulfoxide reduction by rat renal cytosol.
Biochem. Drug Metab. Disposition, 9, 307-310.
Bailey, D.E. (1976a) Acute oral toxicity (LD50) studies in mice with
1,8-octanedithiol. Unpublished study submitted to the Flavor and
Extract Manufacturers' Association by Food and Drug Research
Laboratories, Inc.
Bailey, D.E. (1976b) Acute oral toxicity of 1,2-propanedithiol in
mice. Unpublished study submitted to the Flavor and Extract
Manufacturers' Association by Food and Drug Research Laboratories,
Inc.
Bailey, D.E. (1976c) Acute oral toxicity (LD50) studies in mice with
compound 75-31065 (methyl benzyl disulfide). Unpublished study
submitted to the Flavor and Extract Manufacturers' Association by Food
and Drug Research Laboratories, Inc.
Black, R.M., Brewster, K., Clarke, R.J., Hambrook, J.L., Harrison,
J.M. & Howells, D.J. (1993) Metabolism of thiodiglycol
(2,2'-thiobis-ethanol): Isolation and identification of urinary
metabolites following intraperitoneal administration to rat.
Xenobiotica, 23, 473-481.
Bradley, M.O., Taylor, V.L., Armstrong, M.J. & Galloway, S.M. (1987)
Relationship among cytotoxicity, lysosomal breakdown, chromosome
aberrations, and DNA double-strand breaks. Mutat. Res., 189, 69-79.
Brams, A., Buchet, J.P., Crutzen-Fayt, M.C., DeMeester, C., Lauwerys,
R. & Leonard, A. (1987) A comparative study, with 40 chemicals, of the
efficiency of the Salmonella assay and the SOS Chromotest (kit
procedure). Toxicol. Lett., 38, 123-133.
Bremer, J. & Greenberg, D.M. (1961) Enzymic methylation of foreign
sulfhydryl compounds. Biochim. Biophys. Acta, 46, 217-224.
British Industrial Biological Research Association (1976) Short-term
toxicity of samples TT171, TT172, TT173, and TT174 in rats.
Unpublished study submitted to the Flavor and Extract Manufacturers'
Association.
Brodnitz, M.H. & Pascale, J.V. (1971) Thiopropanol S-oxide: A
lachrymatory factor in onions. J. Agric. Food Chem., 19, 269.
Brown, V.K., Robinson, J. & Stevenson, D.E. (1963) A note on the
toxicity and solvent properties of dimethyl sulfoxide. J. Pharm.
Pharmacol., 15, 688-692.
Brusick, D. (1986) Genotoxic effects in cultured mammalian cells
produced by low pH treatment conditions and increased ion
concentrations. Environ. Mutag., 8, 879-886.
Buckberry, L.D. & Teesdale-Spittle, P.H. (1996) Sulfur-hydrogen
compounds. In: Mitchell, S., ed., Biological Interactions of Sulfur
Compounds, London: Taylor & Francis Ltd, pp. 113-144-76.
Butterworth, K.R. & Mason, P.L. (1981) Acute toxicity of thioguaiacol
and of versalide in rodents. Food Chem. Toxicol., 19, 753-755.
Butterworth, K.R., Carpanini, F.M.B., Gaunt, I.F., Hardy, J., Kiss,
I.S. & Gangolli, S.D. (1975) Short-term toxicity of dimethyl sulfide
in rat. Food Cosmet. Toxicol., 13, 15-22.
Canellakis, E.S. & Tarver, H. (1953) The metabolism of methyl
mercaptan in the intact animal. Arch. Biochem. Biophys., 42,
446-455.
Cashman, J.R. & Olsen, L.D. (1990) Stereoselective S-oxygenation of
2-aryl-1,3-dithiolanes by the flavin-containing and cytocrome P-450
monooxygenases. Mol. Pharmacol., 38, 573-585.
Cashman, J.R. & Williams, D.E. (1990) Enantioselective S-oxygenation
of 2-aryl-1,3-dithiolanes by rabbit lung enzyme preparations.
Mol. Pharmacol., 37, 333.
Cashman, J.R., Olsen, L.D. & Bornheim, L.M. (1990) Enantioselective
S-oxygenation by flavin containing cytochrome P-450 mono-oxygenases.
Chem. Res. Toxicol., 3, 344.
Cashman, J.R., Yang, Z.C., Yang, L. & Wrighton, S.A. (1995a)
Stereo-and regioselective N-and S-oxygenation of tertiary amines and
sulfides in adult human liver microsomes. Int. Soc. Study
Xenobiotics, 8, 34.
Cashman, J.R., Park, S.B., Yang, Z.C., Washington, C.B., Gomez, D.Y.,
Giacomini, K. & Brett, C.M. (1995b) Chemical, enzymatic and human
enantioselective S-oxygenation of cimetidine. Int. Soc. Study
Xenobiotics, 8, 133.
Christias, C. (1975) Specific inhibition of sclerotium formation by
2-mercaptoethanol and related sulfhydryl compounds in Sclerotium
rolfsii. Can. J. Microbiol., 21, 1541-1547.
Collinson, V.A. (1989) ST 15 C 88 Acute oral toxicity study in the
rat. Unpublished study No. A/0/13008 from Toxicol Laboratories Ltd,
United Kingdom.
Cox, G.E., Bailey, D.E. & Morgareidge, K. (1973a) 90-Day feeding
studies in rats with trithioacetone. Unpublished report submitted to
the Flavor and Extract Manufacturers' Association by Food and Drug
Research Laboratories, Inc.
Cox, G.E., Bailey, D.E. & Morgareidge, K. (1973b) 90-Day feeding
studies in rats with compound ES-307/308 (14705). Unpublished report
submitted to the Flavor and Extract Manufacturers' Association by Food
and Drug Research Laboratories, Inc.
Cox, G.E., Bailey, D.E. & Morgareidge, K. (1974a) 90-Day feeding study
in rats with compound 14935 (2-mercapto-3-butanol). Unpublished report
submitted to the Flavor and Extract Manufacturers' Association by Food
and Drug Research Laboratories, Inc.
Cox, G.E., Bailey, D.E. & Morgareidge, K. (1974b) 90-Day feeding
studies in rats with compound 31098. Unpublished report submitted to
the Flavor and Extract Manufacturers' Association by Food and Drug
Research Laboratories, Inc.
Cox, G.E., Rucci, G. & Babish, J.G. (1979) 90-Day subacute dietary
toxicity study of IFF code No. 78-002-2 in Sprague-Dawley rats.
Unpublished report submitted to the Flavor and Extract Manufacturers'
Association by Food and Drug Research Laboratories, Inc.
Damani, L.A. (1987) Metabolism of sulphur-containing drugs. In:
Benford, D.J., Bridges, J.W. & Gibson, G.G., eds,
Drug Metabolism--From Molecules to Man, London: Taylor & Francis,
pp. 581-603.
Davis, P.J. & Guenthner, L.E. (1985) Sulindac oxidation/reduction by
microbial cultures; microbial models for mammalian metabolism.
Xenobiotica, 15, 845-857.
Deng, D.J., Mueller, K., Kasper, P. & Mueller, L. (1994) Effect of
diallyl trisulfide on induction of UDS by mutagenic drugs in primary
rat hepatocytes. Biomed. Environ. Sci., 7, 85-90.
Dutton, G.J. & Illing, H.P.A. (1972) Mechanism of biosynthesis of
thio-beta-D-glucosides. Biochem. J., 129, 539-550.
Eder, E., Neudecker, T., Lutz, D. & Henschler, D. (1980) Mutagenic
potential of allyl and allylic compounds. Structure-activity
relationship as determined by alkylating and direct in vitro mutagenic
properties. Biochem. Pharmacol., 29, 993-998.
Eder, E., Henschler, D. & Neudecker, T. (1982) Mutagenic properties of
allylic and alpha, beta-unsaturated compounds: Consideration of
alkylating mechanisms. Xenobiotica, 12, 831-848.
Elfarra, A.A., Duescher, R.J., Sausen, P.J., Lawton, M.P. & Philpot,
R.M. (1995) Potential role of the flavin-containing monooxygenases in
the metabolism of endogenous compounds. Int. Soc. Study Xenobiotics,
8, 9.
El Fatih, Karim, I.A., Millership, J.S., Temple, D.J. & Woolfson, A.D.
(1988) An investigation of the metabolism of
S-carboxymethyl-L-cysteine in man using a novel HPLC-ECD method.
Eur. J. Drug. Metab. Pharmacokinet., 13, 253.
Elf Atochem (1963) Unpublished study from Pharmacology Research Inc.
Elf Atochem (1985) Unpublished study from Product Safety Labs.
Elf Atochem (1992) Unpublished study from Centre International de
Toxicologie.
Fairchild, E.J. & Stokinger, H.E. (1958) Toxicologic studies on
organic sulfur compounds. 1. Acute toxicity of some aliphatic and
aromatic thiols (mercaptans). Am. Ind. Hyg. Assoc. J., 19, 171-189.
Farr, C.H. & Kirwin, C.J. (1994) Organic sulfur compounds. In:
Clayton, D.G. & Clayton, F.E., eds, Patty's Industrial Hygiene
and Toxicology, 4th Ed., Vol. 2, New York: John Wiley & Sons, pp.
4311-4372.
Feng, P.C.C. & Solsten, R.T. (1991) In vitro transformation of
dithiopyr by rat liver enzymes: Conversion of methylthioesters to
acids by oxygenases. Xenobiotica, 21, 1265.
Fenwick, G.R. & Hanley, A.B. (1985) The genus Allium, part 2. CRC
Crit. Rev. Food Sci. Nutr., 22, 273-377.
Fishman, F.G., Jenkins, L.J., Jr, Coon, R.A. & Jones, R.A. (1969)
Effects of acute and repeated inhalation of dimethyl sulfoxide in
rats. Toxicol. Appl. Pharmacol., 15, 74-82.
Flavor and Extract Manufacturers' Association (1991) In vitro
hydrolysis of 3-methylthiohexy acetate. Unpublished report No. 183/91.
Flavor and Extract Manufacturers' Association (1996) Sensory tolerance
test protocol. Unpublished report.
Fogelman, R.W. & DeProspo, J. (1973a) Acute oral toxicity in mice with
ES-307/308. Unpublished report submitted to the Flavor and Extract
Manufacturers' Association by Affiliated Medical Research Institute.
Fogelman, R.W. & DeProspo, J. (1973b) Acute oral toxicity in mice with
SB-11-2876 (trithioacetate). Unpublished report submitted to the
Flavor and Extract Manufacturers' Association by Affiliated Medical
Research Institute.
Fogelman, R.W. & DeProspo, J. (1974) Acute oral toxicity in mice with
1,2-ethanedithiol. Unpublished report submitted to the Flavor and
Extract Manufacturers' Association by Affiliated Medical Research
Institute.
Fogelman, R.W. & Suppers, L. (1973) Acute oral toxicity in mice with
1,8-octanedithiol. Unpublished report submitted to the Flavor and
Extract Manufacturers' Association by Affiliated Medical Research
Institute.
Fogelman, R.W. & Suppers, L. (1974a) Acute oral toxicity in mice with
1,8-octanedithiol. Unpublished report submitted to the Flavor and
Extract Manufacturers' Association by Affiliated Medical Research
Institute.
Fogelman, R.W. & Suppers, L. (1974b) Acute oral toxicity in mice with
1,2-propanedithiol. Unpublished report submitted to the Flavor and
Extract Manufacturers' Association by Affiliated Medical Research
Institute.
Fogelman, R.W., Bukva, N.F. & Margolin, S. (1970) Acute oral toxicity
of 2-4252. Unpublished study submitted by Affiliated Medical
Enterprises, Inc. Princeton, NJ, USA.
Food and Drug Administration (1993) Priority-based Assessment of
Food Additives (PAFA) Database, Waskington DC: Center for Food
Safety and Applied Nutrition, p. 58.
Frandsen, A., Schousboe, A. & Griffiths, R. (1993) Cytotoxic actions
and effects on intracellular Ca2+ and cGMP concentrations of
sulphur-containing excitatory amino acids in cultured cerebral
cortical neurons. J. Neurosci. Res., 34, 331-339.
Gachon, F., Nicolas, C., Maurizis, C., Verny, M., Chabard, J.L.,
Faurie, M. & Gaillard, G. (1988) Disposition and metabolism of
letosteine in rats. Drug Metab. Disposition, 16, 853.
Gallo, M.A., Cox, G.E. & Babish, J.G. (1976) 90-Day feeding studies in
rats with compound 75-31065 (methyl benzyl disulfide). Unpublished
report to the Flavor and Extract Manufacturers' Association by Food
and Drug Research Laboratories, Inc..
George, J.D., Price, C.J., Navarro, H.A., Barr, M.C., Myers, C.B.,
Hunter, E.S., Schwetz, B.A. & Shelby, M.D. (1995) Developmental
toxicity of thiophenol (thio) in rats and rabbits. Toxicologist, 15,
160.
Gibson, T.P., Dobrinska, M.R., Lin, J.H., Entwistle, L.A. & Davies,
R.O. (1987) Biotransformation of sulindac in end-stage renal disease.
Clin. Pharmacol. Ther., 42, 82-88.
Greenzaid, P. & Jencks, W.P. (1971) Pig liver esterase. Reactions with
alcohols, structure-reactivity correlations, and the acyl-enzyme
intermediate. Biochemistry, 10, 1210.
Griffiths, P.J. & Babish, J.G. (1978) Acute oral toxicity (LD50) of
DSE 3303S (15277) in albino mice (BLU: Ha(ICR)). Unpublished report
No. B-3 to the Flavor and Extract Manufacturers' Association from Food
and Drug Research Laboratories, Inc.
Griffiths, P.J., Giessinger, M. & Fouillet, X. (1979) Report on acute
oral toxicity (LD/50) and three-month toxicity of TT 184. Unpublished
report to the Flavor and Extract Manufacturers' Association from
Battelle Geneva Research Centres.
de Groot, A.P., Spanjers, M.T. & van der Heijden, C.A. (1974) Acute
and sub-acute oral toxicity studies in rats with five flavour
compounds. Unpublished report submitted to the Flavor and Extract
Manufacturers' Association.
Grosa, G., Caputo, O., Cerutti, M., Biglino, G., Franzone, J.S.%
Cravanzola, C. (1991) Metabolism of
7-(1,3-dithiolan-2-ylmethyl)1,3-dimethylxanthine by rat liver
microsomes: Diastereoselective metabolism of the 1,3-dithiolane ring.
Drug Metab. Disposition, 19, 454-457.
Hakura, A., Mochida, H. & Yamatsu, K. (1993) Dimethyl sulfoxide (DMSO)
is mutagenic for bacterial mutagenicity tester strains. Mutat. Res.,
303, 127-133.
Harper, K.H. & Gin, H.B. (1964) The acute oral toxicity to rats of
T59. Unpublished report.
Heck, J.D., Vollmuth, T.A., Cifone, M.A., Jagannath, D.R., Myhr, B. &
Curren, R.D. (1989) An evaluation of food flavoring ingredients in a
genetic toxicity screening battery. Toxicologist, 9, 257.
Hermansky, S.J. & Weaver, E.C. (1990) Fourteen-day dietary minimum
toxicity screen (MTS) with acetyl lactic, thiomethyl ester and
propiodyl lactic, thiomethyl ester in Fischer 344 rats. Unpublished
report 53-553 to the Flavor and Extract Manufacturers' Association
from Bushy Run Research Center, Pennsylvania, USA.
Hodgson, E. & Levi, P.A. (1992) The role of the flavin-containing
monooxygenases (EC 1.14.13.8) in the metabolism and mode of action of
agricultural chemicals. Xenobiotica, 22, 1175-1183.
Hong, J.Y., Lin, M.C., Wang, Z.Y., Wang, E.J. & Yang, C.S. (1994)
Inhibition of chemical toxicity and carcinogenesis by diallyl sulfide
and diallyl sulfone. In: Huang, M.-T., Osawa, T., Ho, C.-T. & Rosen,
R.T., eds, Food Phytochemicals for Cancer prevention I, Fruits and
Vegetables (ACS Symposium Series 546), Washington DC: American
Chemical Society, pp. 97-101.
Hoodi, A.A. & Damani, L.A. (1984) Cytochrome P-450 and non-P-450
sulphoxidations. J. Pharm. Pharmacol., 36, 62P.
Hucker, H.B., Miller, J.K., Hochberg, A., Brobyn, R.D., Riordan, F.H.
& Calesnick, B. (1967) Studies on the absorption, excretion, and
metabolism of dimethyl sulfoxide (DMSO) in man. J. Pharmacol. Exp.
Ther., 155, 309-317.
International Organization of the Flavor Industry (1995) European
inquiry on volume of use. Private communication to the Flavor and
Extract Manufacturers' Association.
Karekar, V.R., Mujumdar, A.M., Joshi, S.S., Dhuley, J., Shinde, S.L. &
Ghaskadbi, S. (1996) Assessment of genotoxic effect of piperine using
Salmonella typhimurium and somatic and germ cells of Swiss albino
mice. Arzneimittel-Forsch., 46, 972-975.
Keith, R.A., Abraham, R.T., Pazmino, P. & Weinshilboum, R.M. (1983)
Correlation of low and high affinity thiol methyltransferase and
phenol methyltransferase activities in human erythrocyte membranes.
Clin. Chim. Acta, 131, 257-272.
Klancnik, J.M., Cuenod, M., Gahwiler, B.H., Jiang, Z.P. & Do, K.Q.
(1992) Release of endogenous amino acids, including homocysteic acid
and cysteine sulphinic acid, from rat hippocampal slices evoked by
electrical stimulation of Schaffer collateral-commissural fibres.
Neuroscience, 49, 557-570.
Kligman, A.M. (1965) Dimethyl sulfoxide--Part 2. J. Am. Med. Assoc.,
193, 151-156.
Koptyaev, V.G. (1967) Experimental determination of maximum
permissible concentration of dimethyl sulfide in water bodies. Hyg.
Sanit., 32, 315-320.
Kurooka, S., Hashimoto, M., Tomita, M., Maki, A. & Yoshimura, Y.
(1976) Relationship between the structure of S-acyl thiol compounds
and their rates of hydrolysis by pancreatic lipase and hepatic
carboxylic esterase. J. Biochem., 79, 533-541.
Lavoie, E., Tulley, L., Fow, E. & Hoffmann, D. (1979) Mutagenicity of
aminophenyl and nitrophenyl ethers, sulfides, and disulfides. Mutat.
Res., 67, 123-131.
Layman, D.L. & Jacob, S.W. (1985) The absorption, metabolism, and
excretion of dimethyl sulfoxide by rhesus monkeys. Life Sci., 37,
2431-2437.
LeBon, A.M., Roy, C., Dupont, C. & Suschete, M. (1997) In vivo
antigenotoxic effects of dietary allyl sulfides in the rat. Cancer
Lett., 114, 131-134.
Lee, S.C. & Renwick, A.G. (1995a) Sulphoxide reduction by rat
intestinal flora and by Escherichia coli in vitro. Biochem.
Pharmacol., 49, 1567-1576.
Lee, S.C. & Renwick, A.G. (1995b) Sulphoxide reduction by rat and
rabbit tissues in vitro. Biochem. Pharmacol., 49, 1557-1565.
Levi, P.E. & Hodgson, E. (1988) Stereospecificity in the oxidation of
phorate and phorate sulphoxide by purified FAD-containing
mono-oxygenase and cytochrome P-450 isozymes. Xenobiotica, 18,
29-39.
Maarse, H., Visscher, C.A., Willemsens, L.C., Nijssen, L.M. & Boelens,
M.H., eds (1994) Volatile Components in Food, 6th Ed., Suppl. 5,
Zeist, TNO Nutrition and Food Research.
Maiorino, R.M., Bruce, D.C. & Aposhian, H.V. (1988) Determination and
metabolism of dithiol chelating agents. VI. Isolation and
identification of the mixed disulfides of meso-2,3-dimercaptosuccinic
acid with L-cysteine in human urine. Toxicol. Appl. Pharmacol., 97,
338.
Maiorino, R.M., Xu, Z.F. & Aposhian, H.V. (1996) Determination and
metabolism of dithiol chelating agents. XXII. In humans, sodium
1,3-dimercapto-1-propanesulfonate is bound to plasma albumin via mixed
disulfide formation and is found in the urine as cyclic polymeric
disulfides. J. Pharmacol. Exp. Ther., 277, 375-384.
Marks, H.S., Anderson, J.L. & Stoewsand, G.S. (1992) Inhibition of
benzo[a]pyrene-induced bone marrow micronuclei formation by diallyl
thioethers in mice. J. Toxicol. Environ. Health, 37, 1-9
Mazel, P., Henderson, J.F. & Axelrod, J. (1964) S-Demethylation by
microsomal enzymes. J. Pharmacol. Exp. Ther., 143, 1-6.
McBain, D.A. & Menn, J.J. (1969) S-Methylation, oxidation,
hydroxylation, and conjugation of thiophenol in the rat. Biochem.
Pharmacol., 18, 2282-2285.
Michael, W.R. 1968) Metabolism of linear alkylate sulfonate and alkyl
benzene sulfonate in albino rats. Toxicol. Appl. Pharmacol., 12,
473-485.
Mitchell, S.C., Idle, J.R. & Smith, R.L. (1982) The metabolism of
[14C] cimetidine in man. Xenobiotica, 12, 283.
Mondino, A. (1981) Thirteen week repeated dose study of the test
article TT 191 (3-methyl-1,2,4-trithiane) orally administered to
Sprague Dawley Charles River CD (SD) BR rats. Unpublished study from
Instituto di richierche biomedicine 'Antoine Marxer' SpA.
Mondino, A. & Peano, S. (1979) Acute oral toxicity of
2,6-dimethylthiophenol. Unpublished report to the Flavor and Extract
Manufacturers' Association.
Mondino, A. & Peano, S. (1982) Acute toxicity study, TT 201 and TT202.
Unpublished report from Instituto di richierche biomedicine 'Antoine
Marxer' SpA.
Mondino, A., Peano, S. & Berruto, P. (1981) Acute toxicity study,
TT191. Unpublished report from Instituto di richierche biomedicine
'Antoine Marxer' SpA.
Moran, E.J., Easterday, O.D. & Oser, B.L. (1980) Acute oral toxicity
of selected flavor chemicals. Drug Chem. Toxicol., 3, 249-258.
Moreno, O.M. (1975) Acute toxicity studies on rats, rabbits, and
guinea pigs. Unpublished report to RIFM.
Moreno, O.M. (1980) Acute toxicity studies. Unpublished report to
RIFM.
Morgareidge, K. (1970) 90-Day feeding study in rats with
tetrahydrothiophene-3-one (31015). Unpublished study submitted to the
Flavor and Extract Manufacturers' Association.
Morgareidge K. (1971a) 90-Day feeding study with 2-naphthalenethiol in
rats. Unpublished study submitted to the Flavor and Extract
Manufacturers' Association.
Morgareidge, K. (1971b) 90-Day feeding study with
2-keto-3-pentanethiol in rats. Unpubli-shed study submitted to the
Flavor and Extract Manufacturers' Association.
Morgareidge, K. & Oser, B.L. (1970a) 90-Day feeding studies in rats
with cyclopentane-thiol. Unpublished study submitted to the Flavor and
Extract Manufacturers' Association.
Morgareidge, K. & Oser, B.L. (1970b) 90-Day feeding studies in rats
with dipropyltrisulfide (30204). Unpublished study submitted to the
Flavor and Extract Manufacturers' Association.
Morgareidge, K. & Oser, B.L. (1970c) 90-Day feeding studies in rats
with diallyltrisulfide. Unpublished study submitted by Food and Drug
Research Laboratories, Inc. to the Flavor and Extract Manufacturers'
Association.
Morgareidge, K., Bailey, D.E. & Cox, G.E. (1974a) 90-Day feeding study
on 2,3-butanedithiol in rats. Unpublished study submitted to the
Flavor and Extract Manufac-turers' Association.
Morgareidge, K., Bailey, D.E. & Cox, G.E. (1974b) 90-Day feeding study
in rats with compound 14966. Unpublished study submitted to the Flavor
and Extract Manufac-turers' Association.
Morgareidge, K., Bailey, D.E. & Cox, G.E. (1974c) 90-Day feeding study
of 1,8-octane-dithiol in rats. Unpublished study submitted to the
Flavor and Extract Manufacturers' Association.
Moutiez, M., Aumercier, M., Tessier, E., Parmentier, B., Tartar, A. &
Sergheraert, C. (1994) Reduction of a trisulfide derivative of
glutathione by glutathione reductases. Biochem. Biophys. Res.
Commun., 202, 1380-1386.
Munday, R. & Manns, E. (1994) Comparative toxicity of prop(en)yl
disulfides derived from Alliaceae. Possible involvement of 1-propenyl
disulfides in onion-induced hemolytic anemia. J. Agric. Food Chem.,
42, 959-962.
Munday, R., Manns, E. & Fowke, E.A. (1990) Steric effects on the
haemolytic activity of aromatic disulphides in rats. Food Chem.
Toxicol., 28, 561-566.
National Academy of Sciences (1970) Evaluating the Safety of Food
Chemicals, Washington DC.
National Academy of Sciences (1982) Evaluating the Safety of Food
Chemicals, Washington DC.
National Academy of Sciences (1987) Evaluating the Safety of Food
Chemicals, Washington DC.
Nickson, R.M. & Mitchell, S.C. (1994) Fate of dipropyl sulfide and
dipropyl sulfoxide in rat. Xenobiotica, 24, 157-168.
Nickson, R.M., Mitchell, S.C. & Zhang, A.Q. (1995) Fate of dipropyl
sulfone in rat. Xenobiotica, 25, 1391-1398.
Nnane, P. & Damani, L.A. (1995) The involvement of rat liver CYP2B1
and CYP2D1 in the microsomal sulphoxidation of 4-chlorophenyl methyl
sulphide. Int. Soc. Study Xenobiotics, 8, 110.
Noel, P.R.B., Barnett, K.C., Davies, R.E., Jolly, D.W., Leahy, J.S.,
Mawdesley-Thomas, L.E., Shillam, K.W.G., Squires, P.F., Street, A.E.,
Tucker, W.C. & Worden, A.N. (1975) The toxicity of dimethyl sulphoxide
(DMSO) for the dog, pig, rat, and rabbit. Toxicologist, 3, 143-169.
Oae, S., Mikami, A., Matsura, T., Ogawa-Asada, K., Watanabe, Y.,
Fujimori, K. & Iyanagi, T. (1985) Comparison of sulfite oxygenation
mechanism for liver microsomal FAD-containing monooxygenase with that
for cytochrome P-450. Biochem Biophys. Res. Commun., 131, 567-573.
Ogasawara, Y., Isoda, S. & Tanabe, S. (1998) A labile sulfur in
trisulfide affects cytochrome P-450 dependent lipid peroxidation in
rat liver microsomes. Toxicol. Lett., 99, 191-198.
Opdyke, D.L.J. (1979) Monographs on fragrance raw materials. Food
Cosmet. Toxicol., 17, 769.
Oser, B.L. (1966) Feeding studies with pinanyl mercaptan (PK) in rats.
Unpublished study submitted to the Flavor and Extract Manufacturers'
Association.
Oser, B.L. (1970) The acute oral toxicity to mice of ten compounds.
Unpublished study submitted to the Flavor and Extract Manufacturers'
Association.
Panasevich, R.E., Mallory, V.T. & Matthews, R.J. (1980) Dose-related
finding and single dose oral study of ethyl 4-(methylthio)butyrate.
Unpublished study submitted to the Flavor and Extract Manufacturers'
Association.
Parkinson, A. (1996) Biotransformation of xenobiotics. In: Klaassen,
C.D., ed., Casarret and Doull's Toxicology: The Basic Science of
Poisons, 5th Ed., New York, McGraw-Hill Co., pp. 113-118.
Peano, S., Roffino, A., Milone, M.F., Orlando, L. & Berruto, G. (1981)
Thirteen week repeated dose study with 2,6-dimethylthiophenol orally
administered to Sprague-Dawley Charles River CD (SD) BR rats.
Unpublished study submitted to the Flavor and Extract Manufacturers'
Association by Instituto di richierche biomedicine 'Antoine Marxer'
SpA.
Phillips Petroleum Co. (1990a) Toxicity study
summary--1,2-Ethanedithiol (TOX071).
Phillips Petroleum Co. (1990b) Toxicity study summary--Isopropyl
mercaptan (TOX083).
Phillips Petroleum Co. (1990c) Toxicity study summary--Tertiary butyl
mercaptan (TOX1-6).
Piccirillo, V.J. & Lunchicki, C. (1982) Acute oral toxicity (LD50)
study in the rat with methyl-2-methylthiobutyrate. Unpublished report
submitted to the Flavor and Extract Manufacturers' Association.
Posternak, J.M., Linder, A. & Vodoz, C.A. (1969) Summaries of
toxicological data. Toxicological tests on flavoring matters. Food
Cosmet. Toxicol., 7, 405-407.
Reddy, B.S., Rao, C.V., Rivenson, A. & Kelloff, G. (1993)
Chemoprevention of colon carcinogenesis by organosulfur compounds.
Cancer Res., 53, 3493-3498.
Renwick, A.G. (1989) Sulphoxides and sulphones. In: Damani, L.A., ed.,
Sulphur-containing Drugs and Related Organic Compounds. Chemistry,
Biochemistry and Toxicology (Ellis Horwood Series in Biochemical
Pharmacology), Vol. 1, Part B, New York: John Wiley & Sons, pp.
133-154.
Renwick, A.G. (1996) Sulfur-oxygen compounds. In: Mitchell, S., ed.,
Biological Interactions of Sulfur Compounds, London: Taylor &
Francis, pp. 42-76.
Renwick, A.G. & George, C.F. (1989) Metabolism of xenobiotics in the
gastro-intestinal tract. In: Hudson, D.H., Caldwell, J. & Paulson,
G.D., eds, Intermediary Xenobiotic Metabolism in Animals:
Methodology, Mechanisms and Significance, London, Taylor & Francis,
pp. 13-40.
Renwick, A.G., Evans, S.P., Sweatman, T.W., Cumberland, J. & George,
C.F. (1982) The role of the gut flora in the reduction of
sulphinpyrazone in the rat. Biochem. Pharmacol., 31, 2649.
Rettie, A.E., Bogucki, B.D., Lim, I. & Meier, G.P. (1990)
Stereoselective sulfoxidation of a series of alkyl p-tolyl sulfides by
microsomal and purified flavin-containing monooxygenases.
Mol. Pharmacol., 37, 643.
Rettie, A.E., Lawton, M.P., Jaffer, A., Sadeque, M. & Meier, G.P.
(1994) Prochiral sulfoxidation as a probe for multiple forms of the
microsomal flavin-containing monooxygenase: Studies with rabbit FMO1,
FMO2, FMO3, and FMO5 expressed in Escherichia coli. Arch. Biochem.
Biophys., 311, 369.
Richardson, K.A., Edward, V.T., Jones, B.C. & Hutson, D.H. (1991)
Metabolism in the rat of a model xenobiotic plant metabolite
S-benzyl-N-malonyl-L-cysteine. Xenobiotica, 21, 371.
Rush, R.E. (1991) 14-Day dietary toxicity study in rats with
3-(methylthio)hexyl acetate (MTHA). Unpublished report No. 3141.5 from
Springborn Laboratories, Inc. to the Flavor and Extract Manufacturers'
Association.
Sadeque, A.J.M., Eddy, A.C., Meierand, G.P. & Rettie, A.E. (1992)
Stereoselective sulfoxidation by human flavin-containing
monooxygenase. Drug Metab. Disposition, 20, 832.
Sadeque, A.J.M., Philpot, R.M. & Rettie, A.E. (1995) Chiral
sulfoxidation by human liver FMO3 and FMO5. Int. Soc. Study
Xenobiotics, 8.
Sanders, A. (1997) Acute oral toxicity (limit test) in the rat.
Unpublished SPL project No. 012/234 submitted to the Flavor and
Extract Manufacturers' Association.
Schafer, E.W. & Bowles, W.A., Jr (1985) Acute oral toxicity and
repellency of 933 chemicals to house and deer mice. Arch. Environ.
Contam. Toxicol., 14, 111-129.
Selyuzhitskii, G.V. (1972) Experimental data on substantiation of the
maximum allowable concentraion of methyl mercaptan, dimethyl sulfide
and dimethyl disulfide in the air of the working area of pulp paper
plants. Gig. Tr. Prof. Zabol., 16, 46-47.
Shaw, P.N. & Blagbrough, I.S. (1989) Cysteine conjugate beta-lyase,
II: Isolation, properties and structure-activity relationships. In:
Damani, L.A., ed., Sulphur-containing Drugs and Related Organic
Compounds, Vol. 2, Part B, Chichester: Ellis Horwood Ltd, pp.
135-156.
Shellenberger, T.E. (1970) Subacute toxicity evaluation of ethyl
thioacetate in rats. Final report. Unpublished report GSRI Project
NC-373 from Gulf South Research Institute submitted to the Flavor and
Extract Manufacturers' Association.
Shellenberger, T.E. (1971a) Acute toxicological evaluations of
2-naphthalenethiol in mice. Unpublished report GSRI Project NC-398
from Gulf South Research Institute submitted to the Flavor and Extract
Manufacturers' Association.
Shellenberger, T.E. (1971b) Acute toxicological evaluations of
chemicals with mice--2-Keto-3-pentanethiol. Unpublished study from
Gulf South Research Institute.
Snow, G.A. (1957) The metabolism of compounds related to ethanethiol.
J. Biochem., 65, 77-82.
Sommer, S. & Tauberger, G. (1964) Toxicologic investigations of
dimethyl sulfoxide. Arnzeimittel. Forsch., 14, 1050-1053.
Souhaili-El Amri, H., Fargetton, X., Delatour, P. & Batt, A.M. (1987)
Sulphoxidation of albendazole by the FAD-containing and cytochrome
P-450 dependent mono-oxygenase from pig liver microsomes.
Xenobiotica, 17, 1159-1168.
Stofberg, J. & Grundschober, F. (1987) Consumption ratio and food
predominance of flavoring materials. Perfumer Flavorist, 12, 27.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of
flavouring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23, 857-860.
Strong, H.A., Renwick, A.G. & George, C.F. (1984a) The site of
reduction of sulphinpyrazone in the rabbit. Xenobiotica, 14,
815-826.
Strong, H.A., Oates, J., Sembi, J., Renwick, A.G. & George, C.F.
(1984b) Role of the gut flora in the reduction of sulphinpyrazone in
humans. J. Pharmacol. Exp. Ther., 230, 726-732.
Strong, H.A., Warner, J., Renwick, A.G. & George, C.F. (1985) Sulindac
metabolism: The importance of an intact colon. Clin. Pharmacol.
Ther., 38, 387-393.
Strong, H.A., Angus, R., Oates, J., Sembi, J., Howarth, P., Renwick,
A.G. & George, C.F. (1986) Effects of ischaemic heart disease, Crohn's
disease and anti-microbial therapy on the pharmacokinetics of
sulphinpyrazone. Clin. Pharmacokinet., 11, 402-411.
Strong, H.A., Renwick, A.G., George, C.F., Liu, Y.F. & Hill, M.J.
(1987) The reduction of sulphinpyrazone and sulindac by intestinal
bacteria. Xenobiotica, 17, 685-696.
Sundaram, S.G. & Milner, J.A. (1996) Diallyl disulfide induces
apoptosis of human colon tumor cells. Carcinogenesis, 17, 669-673.
Szumlanski, C.L., Scott, M.C. & Weinshilboum, R.M. (1988) Thiopurine
methyltransferase pharmacogenetics: Human liver enzyme activity.
Clin. Pharmacol. Ther., 43, 134.
Szybalski, W. (1958) Special microbiological systems. II. Observations
on chemical mutagenesis in microorganisms. Ann. N.Y. Acad. Sci., 76,
475-489.
Takata, T., Yamazaki, M., Fujimori, K., Kim, H.Y., Iyanagi, T. & Oae,
S. (1983) Enzymatic oxygenation of sulfides with cytochrome P-450 from
rabbit liver: Stereochemistry of sulfoxide formation. Bull. Chem.
Soc. Jpn., 55, 2300-2310.
Tateishi, M. & Tomisawa, H. (1989) Cysteine conjugate beta-lyase. I:
Toxic thiol production. In: Damani, L.A., ed., Sulphur-containing
Drugs and Related Organic Compounds, Vol. 2, Part B, Chichester:
Ellis Horwood Ltd, pp. 121-134.
Tateishi, M., Suzuki, S. & Shimizu, H. (1978) Cysteine conjugate
beta-lyase in rat liver. J. Biol. Chem., 253, 8854.
Tatsumi, K., Kitamura, S. & Yamada, H. (1982) Involvement of liver
aldehyde oxidase in sulfoxide reduction. Chem. Pharm. Bull., 30,
4585-4588.
Tatsumi, K., Kitamura, S. & Yamada, H. (1983) Sulfoxide reductase
activity of liver aldehyde oxidase. Biochim. Biophys. Acta, 747,
86-92.
Taylor, K.L. & Ziegler, D.M. (1987) Studies on substrate specificity
of the hog liver flavin-containing monooxygenase. Anionic organic
sulfur compounds. Biochem. Pharmacol., 36, 141-146.
Taylor, A.J., Olavesen, A.H., Black, J.G. & Howes, D. (1978) The
metabolism of the surfactants dodecylsulfonate and hexadecylsulfonate
in the rat. Toxicol. Appl. Pharmacol., 45, 105-117.
Thomas, W.C., Seckar, J.A., Johnson, J.T., Ulrich, C.E, Klonne, D.R.,
Schardein, J.L. & Kirwin, C.J. (1987) Inhalation teratology studies of
n-butyl mercaptan in rats and mice. Fundam. Appl. Toxicol., 8,
170-178.
Vogin, E.E., Carson, S., Cannon, G., Linegar, C.R. & Rubin, L.F.
(1970) Chronic toxicity of DMSO in primates. Toxicol. Appl.
Pharmacol., 16, 606-612.
Wang, C.J., Lin, Y.L. & Lin, J.K. (1994) Mutagenicity and cytotoxicity
of nitropyrrole compounds derived from the reaction of 2-acetyl
pyrrole with nitrate. Food Chem. Toxicol., 32, 839-844.
Waring, R.H. (1996) Sulfur-sulfur compounds. In: Mitchell, S., ed.,
Biological Interactions of Sulfur Compounds, London: Taylor &
Francis, pp. 145-173.
Watanabe, S. & Kinosaki, A. (1989a) Acute oral toxicity in the rat
with acetyllactic acid thiomethyl ester. Unpublished report submitted
to the Flavor and Extract Manufacturers' Association.
Watanabe, S. & Kinosaki, A. (1989b) Acute oral toxicity in the rat
with propionyl lactic acid thiomethyl ester. Unpublished report
submitted to the Flavor and Extract Manufacturers' Association.
Watanabe, S. & Morimoto, Y. (1989a) Mutagenicity study of acetyl
lactic acid thiomethyl ester in Salmonella typhimurium and
Escherichia coli. Unpublished study submitted to the Flavor and
Extract Manufacturers' Association.
Watanabe, S. & Morimoto, Y. (1989b) Mutagenicity study of propionyl
lactic acid thiomethyl ester in Salmonella typhimurium and
Escherichia coli. Unpublished study submitted to the Flavor and
Extract Manufacturers' Association.
Weinshilboum, R.M. & Sladek, S.L. (1980) Mercaptopurine
pharmacogenetics: Monogenic inheritance of erythrocyte thiopurine
methyltransferase activity. Am. J. Hum. Genet., 32, 651-662.
Wells, W.W., Yang, Y., Diets, T.L. & Gan, Z.R. (1993)
Thioltransferases. Adv. Enzymol. Relat. Areas Mol. Biol., 66,
149-201.
Wheldon, G.H., Amyes, S.J., Street, A.E., Hague, P.H. &
Mawdesley-Thomas, L.E. (1970) Toxicity of Wa 4295, Sa 927, Stl 3048,
and Wa 3328 in dietary administration to rats over a period of 13
weeks. Unpublished report from Huntington Research Centre to the
Flavor and Extract Manufacturers' Association.
Wild, D., King, M.T., Gocke, E. & Eckhardt, K. (1983) Study of
artificial flavouring substances for mutagenicity in the
Salmonella/microsome, basc and micronucleus tests. Food Chem.
Toxicol., 21, 707-719.
Williams, K.I.H., Burstein, S.H. & Layne, D.S. (1966) Metabolism of
dimethyl sulfide, dimethyl sulfoxide, and dimethyl sulfone in the
rabbit. Arch. Biochem. Biophys., 117, 84-87.
Willson, J.E., Brown, D.E. & Timmens, E.K. (1965) A toxicologic study
of dimethyl sulfoxide. Toxicol. Appl. Pharmacol., 7, 104-122.
Wilson, K., Chissick, H., Fowler, A.M., Frearson, F.J., Gittins, M. &
Swinbourne, F.J. (1991) Metabolism of benzothiazole I. Identification
of ring-cleavage products. Xenobiotica, 21, 1179.
Wnorowski, G. (1996a) 14-Day dietary toxicity study: Rats. Unpublished
study No. 4442 from Product Safety Laboratories to the Flavor and
Extract Manufacturers' Association.
Wnorowski, G. (1996b) 14-Day dietary toxicity study: Rats. Unpublished
study No. 4453 from Product Safety Laboratories to the Flavor and
Extract Manufacturers' Association.
Wnorowski, G. (1996c) 14-Day dietary toxicity study: Rats. Unpublished
study No. 4456 from Product Safety Laboratories to the Flavor and
Extract Manufacturers' Association.
Wnorowski, G. (1996d) 14-Day dietary toxicity study: Rats. Unpublished
study No. 4454 from Product Safety Laboratories to the Flavor and
Extract Manufacturers' Association.
Wnorowski, G. (1996e) 14-Day dietary toxicity study: Rats. Unpublished
study No. 4457 from Product Safety Laboratories to the Flavor and
Extract Manufacturers' Association.
Wnorowski, G. (1997a) 14-Day repeat dose oral toxicity study: Rats.
Unpublished study No. 5211 from Product Safety Laboratories to the
Flavor and Extract Manufacturers' Association.
Wnorowski, G. (1997b) 14-Day dietary toxicity study: Rats. Unpublished
study No. 5210 from Product Safety Laboratories to the Flavor and
Extract Manufacturers' Association.
Wood, D.C., Wirth, N.V., Weber, F.S. & Palmquist, M.A. (1971)
Mechanism considerations of dimethyl sulfoxide (DMSO)-lenticular
changes in rabbits. J. Pharmacol. Exp. Ther., 177, 528-535.
Woodson, L.C. & Weinshilboum, R.M. (1983) Human kidney thiopurine
methyltransferase: Purification and biochemical properties. Biochem.
Pharmacol., 32, 819-826.
Woodson, L.C., Dunnette, J.H. & Weinshilboum, R.M. (1982)
Pharmacogenetics of human thiopurine methyltransferase:
Kidney-erythrocyte correlation and immunotitra-tion studies.
J. Pharmacol. Exp. Ther., 222, 174-181.
Woodson, L.C., Ames, M.M, Selassie, C.D., Hansch, C. & Weinshilboum,
R.M. (1983) Thiopurine methyltransferase: Aromatic thiol substrates
and inhibition by benzoic acid derivatives. Mol. Pharmacol., 24,
471-478.
Yoshihara, S. & Tatsumi, K. (1985a) Sulfoxide reduction catalysed by
guinea pig liver aldehyde oxidase in combination with one electron
reducing flavoenzymes. J. Pharmacobio-Dyn., 8, 996-1005.
Yoshihara, S. & Tatsumi, K. (1985b) Guinea pig liver aldehyde oxidase
as a sulfoxide reductase: Its purification and characterisation.
Arch. Biochem. Biophys., 242, 213-224.
Yoshihara, S. & Tatsumi, K. (1990) Metabolism of diphenyl sulphoxide
in perfused guinea pig liver. Drug Metab. Disposition, 18, 876.
Zajac-Kaye, M. & Ts'o, P.O.P. (1984) DNAase I encapsulation in
liposomes can induce neoplastic transformation of Syrian hamster
embryo cells in culture. Cell, 39, 427-437.
Zeiger, E., Anderson, B., Haworth, S., Lawlor, T., Mortelmans, K. &
Speck, W. (1987) Salmonella mutagenicity test: III. Results from the
testing of 255 chemicals. Environ. Mutag., 9 (Suppl. 9), 1-110.
Zeiger, E., Anderson, B., Haworth, S., Lawlor, T. & Mortelmans, K.
(1992) Salmonella mutagenicity tests. V. Results from the testing of
311 chemicals. Environ. Mol. Mutag., 19 (Suppl.21), 2-141.
Ziegler, D.M. (1980) Microsomal flavin-coenzyme monooxygenase:
Oxygenation of nucleophilic nitrogen and sulfur compounds. In: Jakoby,
W.B., ed., Enzymatic Basis of Detoxification, Vol. 2, Chichester:
Ellis Horwood, pp. 201-227.
Ziegler, D.M. (1989) S-Oxygenases. I. Chemistry and biochemistry. In:
Damani, L.A., ed., Sulphur-containing Drugs and Related Organic
Compounds, Vol. 2, Part A, Chichester: Ellis Horwood, pp. 53-66.
 Methyl ethyl 453 624-89-5 No Yes. A NOEL of 250 mg/kg bw N/R No safety
sulfide per day was reported in concern
a 14-week study in rats
treated with methyl sulfide
at multiple doses.
Methyl ethyl 453 624-89-5 No Yes. A NOEL of 250 mg/kg bw N/R No safety
sulfide per day was reported in concern
a 14-week study in rats
treated with methyl sulfide
at multiple doses.
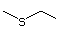 Diethyl sulfide 454 352-93-2 No Yes. A NOEL of 250 mg/kg bw N/R No safety
per day was reported in a concern
14-week study in rats treated
with methyl sulfide at multiple
doses.
Diethyl sulfide 454 352-93-2 No Yes. A NOEL of 250 mg/kg bw N/R No safety
per day was reported in a concern
14-week study in rats treated
with methyl sulfide at multiple
doses.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Butyl sulfide 455 544-40-1 No Yes. A NOEL of 250 mg/kg bw per N/R No safety
day was reported in a 14-week concern
study in rats treated with
methyl sulfide at multiple doses.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Butyl sulfide 455 544-40-1 No Yes. A NOEL of 250 mg/kg bw per N/R No safety
day was reported in a 14-week concern
study in rats treated with
methyl sulfide at multiple doses.
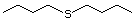 (1-Butenyl-1) 457 32951-19-2 No Yes. A NOEL of 250 mg/kg bw per N/R No safety
methyl sulfide day was reported in a 14-week concern
study in rats treated with methyl
sulfide at multiple doses.
(1-Butenyl-1) 457 32951-19-2 No Yes. A NOEL of 250 mg/kg bw per N/R No safety
methyl sulfide day was reported in a 14-week concern
study in rats treated with methyl
sulfide at multiple doses.
 Bis(methylthio)methane 533 1618-26-4 No Yes. A NOEL of 250 mg/kg bw per N/R No safety
day was reported in a 14-week concern
study in rats treated with methyl
sulfide (No. 452) at multiple
doses
Bis(methylthio)methane 533 1618-26-4 No Yes. A NOEL of 250 mg/kg bw per N/R No safety
day was reported in a 14-week concern
study in rats treated with methyl
sulfide (No. 452) at multiple
doses
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Structural class II
Allyl sulfide 458 592-88-1 No No. Allyl mercaptan is not predicted Yes No safety
to be a metabolite of allyl sufide. concern
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Structural class II
Allyl sulfide 458 592-88-1 No No. Allyl mercaptan is not predicted Yes No safety
to be a metabolite of allyl sufide. concern
 Methyl phenyl sulfide 459 100-68-5 No No Yes No safety
concern
Methyl phenyl sulfide 459 100-68-5 No No Yes No safety
concern
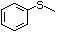 Benzyl methyl sulfide 460 766-92-7 No No Yes No safety
concern
Benzyl methyl sulfide 460 766-92-7 No No Yes No safety
concern
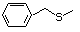 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Subgroup (ii): Acyclic sulfides with oxidized side-chains
Structural class I
3-(Methylthio)propanol 461 505-10-2 No Yes. A NOEL of 1.4 mg/kg bw per N/R No safety
day was reported in a 90-day syudy concern
in rats given
2-(methylthiomethyl)-3-phenyl-propenal
(No. 505) only at that dose. Data
for methyl sulfide (No. 452) are
relevant to compounds with a simple
oxidized side-chain.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Subgroup (ii): Acyclic sulfides with oxidized side-chains
Structural class I
3-(Methylthio)propanol 461 505-10-2 No Yes. A NOEL of 1.4 mg/kg bw per N/R No safety
day was reported in a 90-day syudy concern
in rats given
2-(methylthiomethyl)-3-phenyl-propenal
(No. 505) only at that dose. Data
for methyl sulfide (No. 452) are
relevant to compounds with a simple
oxidized side-chain.
 4-(Methylthio)butanol 462 999999-26-9 No Yes. A NOEL of 1.4 mg/kg bw was N/R No safety
in a reported 90-day syudy concern
in rats given
2-(methylthiomethyl)-3-phenyl-propenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
4-(Methylthio)butanol 462 999999-26-9 No Yes. A NOEL of 1.4 mg/kg bw was N/R No safety
in a reported 90-day syudy concern
in rats given
2-(methylthiomethyl)-3-phenyl-propenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
3-(Methylthio)-1-hexanol 463 51755-66-9 No Yes. A NOEL of 1.4 mg/kg bw was reported N/R No safety
in a 90-day syudy in rats given concern
2-(methylthiomethyl)-3-phenyl-propenal
(No. 505) only at that dose.
Data for methyl sulfide (No. 452) are
relevant to compounds with a simple
oxidized side-chain.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
3-(Methylthio)-1-hexanol 463 51755-66-9 No Yes. A NOEL of 1.4 mg/kg bw was reported N/R No safety
in a 90-day syudy in rats given concern
2-(methylthiomethyl)-3-phenyl-propenal
(No. 505) only at that dose.
Data for methyl sulfide (No. 452) are
relevant to compounds with a simple
oxidized side-chain.
 2-Methylthioacetaldehyde 465 23328-62-3 No Yes. A NOEL of 1.4 mg/kg bw was reported N/R No safety
in a 90-day syudy in rats given concern
2-(methylthiomethyl)-3-phenyl-propenal
(No. 505) only at that dose.
Data for methyl sulfide (No. 452) are
relevant to compounds with a simple
oxidized side-chain.
2-Methylthioacetaldehyde 465 23328-62-3 No Yes. A NOEL of 1.4 mg/kg bw was reported N/R No safety
in a 90-day syudy in rats given concern
2-(methylthiomethyl)-3-phenyl-propenal
(No. 505) only at that dose.
Data for methyl sulfide (No. 452) are
relevant to compounds with a simple
oxidized side-chain.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
3-(Methylthio) 466 3268-49-3 No Yes. A NOEL of 1.4 mg/kg bw per day was N/R No safety
propionaldehyde reported in a 90-day study in rats concern
treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
3-(Methylthio) 466 3268-49-3 No Yes. A NOEL of 1.4 mg/kg bw per day was N/R No safety
propionaldehyde reported in a 90-day study in rats concern
treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
 3-(Methylthio)butanal 467 16630-52-7 No Yes. A NOEL of 1.4 mg/kg bw per day was N/R No safety
reported in a 90-day study in rats concern
treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
3-(Methylthio)butanal 467 16630-52-7 No Yes. A NOEL of 1.4 mg/kg bw per day was N/R No safety
reported in a 90-day study in rats concern
treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
4-(Methylthio)butanal 468 42919-64-2 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
4-(Methylthio)butanal 468 42919-64-2 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
 3-Methylthiohexanal 469 38433-74-8 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
3-Methylthiohexanal 469 38433-74-8 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
2-(Methylthio) 470 40878-72-6 No No No No safety
methyl-2-butenal concern
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
2-(Methylthio) 470 40878-72-6 No No No No safety
methyl-2-butenal concern
 2,8-Dithianon-4-ene-4-carbox- 471 59902-01-1 No No No No safety
aldehyde concern
2,8-Dithianon-4-ene-4-carbox- 471 59902-01-1 No No No No safety
aldehyde concern
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Methyl 3-methylthiopropionate 472 13532-18-8 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain. The simple side-chain acid
and ester would be predicted to be of
low toxicity.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Methyl 3-methylthiopropionate 472 13532-18-8 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain. The simple side-chain acid
and ester would be predicted to be of
low toxicity.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Methylthiomethyl butyrate 473 74758-93-3 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Methylthiomethyl butyrate 473 74758-93-3 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
 Methyl 4-(methylthio)butyrate 474 53053-51-3 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
Methyl 4-(methylthio)butyrate 474 53053-51-3 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Ethyl 2-(methylthio)acetate 475 4455-13-4 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Ethyl 2-(methylthio)acetate 475 4455-13-4 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
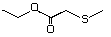 Ethyl 3-methylthiopropionate 476 133327-56-5 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
Ethyl 3-methylthiopropionate 476 133327-56-5 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Ethyl 4-(methylthio)butyrate 477 22014-48-8 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Ethyl 4-(methylthio)butyrate 477 22014-48-8 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
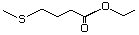 3-(Methylthio)propyl acetate 478 16630-55-0 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data
for methyl sulfide (No. 452) are
relevant to compounds with a simple
oxidized side-chain.
3-(Methylthio)propyl acetate 478 16630-55-0 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data
for methyl sulfide (No. 452) are
relevant to compounds with a simple
oxidized side-chain.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Methylthiomethyl hexanoate 479 74758-91-1 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Methylthiomethyl hexanoate 479 74758-91-1 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
 Ethyl 3-(methylthio)butyrate 480 Pending No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day syudy in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
Ethyl 3-(methylthio)butyrate 480 Pending No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day syudy in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
3-(Methylthio)hexyl acetate 481 51755-85-2 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
3-(Methylthio)hexyl acetate 481 51755-85-2 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
 1-Methylthio-2-propanone 495 14109-72-9 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study concern
in rats given
2-(methylthiomethyl)-3-phenyl-propenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
1-Methylthio-2-propanone 495 14109-72-9 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study concern
in rats given
2-(methylthiomethyl)-3-phenyl-propenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
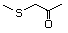 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
1-(Methylthio)-2-butanone 496 13678-58-5 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
1-(Methylthio)-2-butanone 496 13678-58-5 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
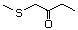 4-(Methylthio)-2-butanone 497 34047-39-7 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
4-(Methylthio)-2-butanone 497 34047-39-7 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
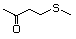 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
4-(Methylthio)-4-methyl-2- 500 23550-40-5 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
pentanone was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
4-(Methylthio)-4-methyl-2- 500 23550-40-5 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
pentanone was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
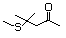 Di(butan-3-one-1-yl) sulfide 502 40790-04-3 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
Di(butan-3-one-1-yl) sulfide 502 40790-04-3 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
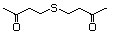 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Structural class II
ortho-(Methylthio)phenol 503 1073-29-6 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Structural class II
ortho-(Methylthio)phenol 503 1073-29-6 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
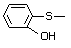 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Structural class III
4-(Methylthio)-2-oxobutanoic 501 51828-97-8 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
acid, sodium salt was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Structural class III
4-(Methylthio)-2-oxobutanoic 501 51828-97-8 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
acid, sodium salt was reported in a 90-day study in concern
rats treated with
2-(methylthiomethyl)-3-phenylpropenal
(No. 505) only at that dose. Data for
methyl sulfide (No. 452) are relevant
to compounds with a simple oxidized
side-chain.
 2-(Methylthiomethyl)-3-phenyl 505 65887-08-3 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
- propenal was reported in a 90-day study in concern
rats treated with this substance only
at that dose. Data for methyl sulfide
(No. 452) are relevant to compounds
with a simple oxidized side-chain.
2-(Methylthiomethyl)-3-phenyl 505 65887-08-3 No Yes. A NOEL of 1.4 mg/kg bw per day N/R No safety
- propenal was reported in a 90-day study in concern
rats treated with this substance only
at that dose. Data for methyl sulfide
(No. 452) are relevant to compounds
with a simple oxidized side-chain.
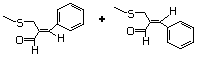 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Subgroup (iii): Cyclic sulfides
Structural class I
2,5-Dimethyl-2,5-dihydroxy-1,4- 562 55704-78-4 No Yes. A NOEL of 3.1 mg/kg bw per day N/R No safety
dithiane was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Subgroup (iii): Cyclic sulfides
Structural class I
2,5-Dimethyl-2,5-dihydroxy-1,4- 562 55704-78-4 No Yes. A NOEL of 3.1 mg/kg bw per day N/R No safety
dithiane was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
 2,5-Dihydroxy-1,4-dithiane 550 40018-26-6 No Yes. A NOEL of 3.1 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2,5-dimethyl-2,5-dihydroxy-1,4-dithiane
(No. 562) only at that dose.
2,5-Dihydroxy-1,4-dithiane 550 40018-26-6 No Yes. A NOEL of 3.1 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
2,5-dimethyl-2,5-dihydroxy-1,4-dithiane
(No. 562) only at that dose.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Structural class II
2-Methyl-4-propyl-1,3-oxathiane 464 67715-80-4 No Yes. A NOEL of 0.44 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Structural class II
2-Methyl-4-propyl-1,3-oxathiane 464 67715-80-4 No Yes. A NOEL of 0.44 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
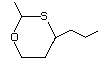 4,5-Dihydro-3-(2H)-thiophenone 498 1003-04-9 No Yes. A NOEL of 9.2 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
4,5-Dihydro-3-(2H)-thiophenone 498 1003-04-9 No Yes. A NOEL of 9.2 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
2-Methyltetrahydrothiophen-3-one 499 13679-85-1 No Yes. A NOEL of 9.2 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
4,5-dihydro-3-(2H)-thiophenone (No. 498)
only at that dose.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
2-Methyltetrahydrothiophen-3-one 499 13679-85-1 No Yes. A NOEL of 9.2 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with
4,5-dihydro-3-(2H)-thiophenone (No. 498)
only at that dose.
 1,4-Dithiane 456 505-29-3 No Yes. NOELs of 0.44, 7, and 0.2 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-methyl-4-propyl-1,3-oxathiane
(No. 464), 2-methyl-1,3-dithiolane
(No. 534), and tri-thioacetone
(No. 543), respectively, only at those
doses.
1,4-Dithiane 456 505-29-3 No Yes. NOELs of 0.44, 7, and 0.2 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-methyl-4-propyl-1,3-oxathiane
(No. 464), 2-methyl-1,3-dithiolane
(No. 534), and tri-thioacetone
(No. 543), respectively, only at those
doses.
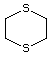 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
2-Methyl-1,3-dithiolane 534 5616-51-3 No Yes. A NOEL of 7 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
2-Methyl-1,3-dithiolane 534 5616-51-3 No Yes. A NOEL of 7 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
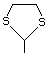 Trithioacetone 543 828-26-2 No Yes. A NOEL of 0.2 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
Trithioacetone 543 828-26-2 No Yes. A NOEL of 0.2 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
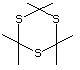 Subgroup (iv): Thiols
Structural class I
Methyl mercaptan 508 74-93-1 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
Subgroup (iv): Thiols
Structural class I
Methyl mercaptan 508 74-93-1 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Propanethiol 509 107-03-9 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Propanethiol 509 107-03-9 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
 2-Propanethiol 510 75-33-2 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
2-Propanethiol 510 75-33-2 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
 1-Butanethiol 511 109-79-5 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
1-Butanethiol 511 109-79-5 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
2-Methyl-1-propanethiol 512 513-44-0 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
2-Methyl-1-propanethiol 512 513-44-0 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
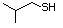 3-Methylbutanethiol 513 541-31-1 No Yes. A NOEL of 0.56 mg/kg bw per day NR No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
3-Methylbutanethiol 513 541-31-1 No Yes. A NOEL of 0.56 mg/kg bw per day NR No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
 2-Pentanethiol 514 2084-19-7 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
2-Pentanethiol 514 2084-19-7 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
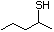 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
2-Methyl-1-butanethiol 515 1878-18-8 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
2-Methyl-1-butanethiol 515 1878-18-8 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
 3-Methyl-2-butanethiol 517 2084-18-6 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
3-Methyl-2-butanethiol 517 2084-18-6 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
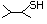 1-Hexanethiol 518 111-31-9 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
1-Hexanethiol 518 111-31-9 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
32-Ethylhexanethiol 519 7341-17-5 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
32-Ethylhexanethiol 519 7341-17-5 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
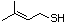 Prenythiol 522 5287-45-6 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose; see diallyl
trisulfide (No. 587, subgroup ix), which
is predicted to be metabolized to allyl
disulfide and allyl thiol.
Prenythiol 522 5287-45-6 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose; see diallyl
trisulfide (No. 587, subgroup ix), which
is predicted to be metabolized to allyl
disulfide and allyl thiol.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Thiogeraniol 524 39067-80-6 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose; see
diallyl trisulfide (No. 587, subgroup
ix), which is predicted to be
metabolized to allyl disulfide and
allyl thiol.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Thiogeraniol 524 39067-80-6 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose; see
diallyl trisulfide (No. 587, subgroup
ix), which is predicted to be
metabolized to allyl disulfide and
allyl thiol.
 Structural class II
Cyclopentanethiol 516 1679-07-8 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
Structural class II
Cyclopentanethiol 516 1679-07-8 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
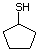 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
2,3- or 10-Mercaptopinane 520 23832-18-0 No Yes. A NOEL of 0.06 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose. NOELs of 0.56, 0.52, 0.43,
and 3.4 mg/kg bw per day were reported
in 90-day studies in rats treated with
cyclo-pentanethiol (No. 516),
ortho-toluenethiol (No. 528),
2,6-dimethylthiophenol (No. 530), and
2-naphthalenethiol (No. 531),
respectively, only at those doses.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
2,3- or 10-Mercaptopinane 520 23832-18-0 No Yes. A NOEL of 0.06 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose. NOELs of 0.56, 0.52, 0.43,
and 3.4 mg/kg bw per day were reported
in 90-day studies in rats treated with
cyclo-pentanethiol (No. 516),
ortho-toluenethiol (No. 528),
2,6-dimethylthiophenol (No. 530), and
2-naphthalenethiol (No. 531),
respectively, only at those doses.
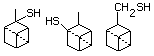 Allyl mercaptan 521 870-23-5 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
Allyl mercaptan 521 870-23-5 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
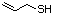 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
1-para-Menthene-8-thiol 523 71159-90-5 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
1-para-Menthene-8-thiol 523 71159-90-5 No Yes. A NOEL of 0.56 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with cyclopentanethiol
(No. 516) only at that dose.
 Benzenethiol 525 108-98-5 No Yes. NOELs of 0.52, 0.43, and 3.4 N/R No safety
mg/kg bw per day were reported in concern
90-day studies in rats treated with
ortho-toluenethiol (No. 528),
2,6-dimethyl-thiophenol (No. 530), and
2-naphthal-enethiol (No. 531),
respectively, only at those doses.
Benzenethiol 525 108-98-5 No Yes. NOELs of 0.52, 0.43, and 3.4 N/R No safety
mg/kg bw per day were reported in concern
90-day studies in rats treated with
ortho-toluenethiol (No. 528),
2,6-dimethyl-thiophenol (No. 530), and
2-naphthal-enethiol (No. 531),
respectively, only at those doses.
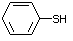 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Benzyl mercaptan 526 100-53-8 No Yes. NOELs of 0.52, 0.43, and 3.4 N/R No safety
mg/kg bw per day were reported in concern
90-day studies in rats treated with
ortho-toluenethiol (No. 528),
2,6-dimethyl-thiophenol (No. 530), and
2-naphthal-enethiol (No. 531),
respectively, only at those doses.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Benzyl mercaptan 526 100-53-8 No Yes. NOELs of 0.52, 0.43, and 3.4 N/R No safety
mg/kg bw per day were reported in concern
90-day studies in rats treated with
ortho-toluenethiol (No. 528),
2,6-dimethyl-thiophenol (No. 530), and
2-naphthal-enethiol (No. 531),
respectively, only at those doses.
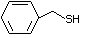 Phenethyl mercaptan 527 4410-99-5 No Yes. NOELs of 0.52, 0.43, and 3.4 N/R No safety
mg/kg bw per day were reported in concern
90-day studies in rats treated with
ortho-toluenethiol (No. 528),
2,6-dimethyl-thiophenol (No. 530), and
2-naphthal-enethiol (No. 531),
respectively, only at those doses.
Phenethyl mercaptan 527 4410-99-5 No Yes. NOELs of 0.52, 0.43, and 3.4 N/R No safety
mg/kg bw per day were reported in concern
90-day studies in rats treated with
ortho-toluenethiol (No. 528),
2,6-dimethyl-thiophenol (No. 530), and
2-naphthal-enethiol (No. 531),
respectively, only at those doses.
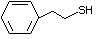 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
ortho-Toluenethiol 528 137-06-4 No Yes. A NOEL of 0.52 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
ortho-Toluenethiol 528 137-06-4 No Yes. A NOEL of 0.52 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
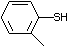 2,6-Dimethylthiophenol 530 118-72-9 No Yes. A NOEL of 0.43 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
2,6-Dimethylthiophenol 530 118-72-9 No Yes. A NOEL of 0.43 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
 2-Naphthalenethiol 531 91-60-1 No Yes. A NOEL of 3.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with this substance
only at that dose.
2-Naphthalenethiol 531 91-60-1 No Yes. A NOEL of 3.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in concern
rats treated with this substance
only at that dose.
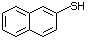 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Structural class III
2-Ethylthiophenol 529 4500-58-7 No Yes. NOELs of 0.52, 0.43, and 3.4 N/R No safety
mg/kg bw per day were reported in concern
90-day studies in rats treated with
ortho-toluenethiol (No. 528),
2,6-dimethyl-thiophenol (No. 530),
and 2-naphthal-enethiol (No. 531),
respectively, only at those doses.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Structural class III
2-Ethylthiophenol 529 4500-58-7 No Yes. NOELs of 0.52, 0.43, and 3.4 N/R No safety
mg/kg bw per day were reported in concern
90-day studies in rats treated with
ortho-toluenethiol (No. 528),
2,6-dimethyl-thiophenol (No. 530),
and 2-naphthal-enethiol (No. 531),
respectively, only at those doses.
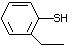 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Subgroup (v): Thiols with oxidized side-chains
Structural class I
2-Mercaptopropionic acid 551 79-42-5 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Subgroup (v): Thiols with oxidized side-chains
Structural class I
2-Mercaptopropionic acid 551 79-42-5 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Ethyl 2-mercaptopropionate 552 19788-49-9 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Ethyl 2-mercaptopropionate 552 19788-49-9 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
 Ethyl 3-mercaptopropionate 553 5466-06-8 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
Ethyl 3-mercaptopropionate 553 5466-06-8 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
3-Mercaptohexyl acetate 554 136954-20-6 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
3-Mercaptohexyl acetate 554 136954-20-6 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
 3-Mercaptohexyl butyrate 555 136954-21-7 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
3-Mercaptohexyl butyrate 555 136954-21-7 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
3-Mercaptohexyl hexanoate 556 136954-22-8 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
3-Mercaptohexyl hexanoate 556 136954-22-8 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
 1-Mercapto-2-propanone 557 24653-75-6 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
1-Mercapto-2-propanone 557 24653-75-6 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
3-Mercapto-2-butanone 558 40789-98-8 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
3-Mercapto-2-butanone 558 40789-98-8 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
 2-Keto-4-butanethiol 559 34619-12-0 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
2-Keto-4-butanethiol 559 34619-12-0 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
3-Mercapto-2-pentanone 560 67633-97-0 No Yes. A NOEL of 1.9 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
given this substance only at that dose.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
3-Mercapto-2-pentanone 560 67633-97-0 No Yes. A NOEL of 1.9 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
given this substance only at that dose.
 3-Mercapto-3-methyl-1-butanol 544 34300-94-2 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
3-Mercapto-3-methyl-1-butanol 544 34300-94-2 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
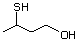 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
3-Mercaptohexanol 545 51755-83-0 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
3-Mercaptohexanol 545 51755-83-0 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
2-Mercapto-3-butanol 546 37887-04-0 No Yes. A NOEL of 1.9 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
given this substance only at that dose.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
2-Mercapto-3-butanol 546 37887-04-0 No Yes. A NOEL of 1.9 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
given this substance only at that dose.
 alpha-methyl-beta-hydroxypropyl 547 54957-02-7 No Yes. A NOEL of 2.8 mg/kg bw per day N/R No safety
alpha-methyl-beta-mercaptopropyl was reported in a 90-day study in rats concern
sulfide given this substance only at that dose.
alpha-methyl-beta-hydroxypropyl 547 54957-02-7 No Yes. A NOEL of 2.8 mg/kg bw per day N/R No safety
alpha-methyl-beta-mercaptopropyl was reported in a 90-day study in rats concern
sulfide given this substance only at that dose.
 4-Methyoxy-2-methyl-2-butane- 548 94087-83-9 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
4-Methyoxy-2-methyl-2-butane- 548 94087-83-9 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
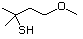 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
3-Methyl-3-mercaptobutyl formate 549 50746-10-6 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
3-Methyl-3-mercaptobutyl formate 549 50746-10-6 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Structural class II
para-Mentha-8-thiol-3-one 561 38462-22-5 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those doses.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Structural class II
para-Mentha-8-thiol-3-one 561 38462-22-5 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those doses.
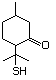 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Structural class III
Sodium 3-mercapto-oxo-propionate 563 10255-67-1 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses. See subgroup (i), No. 452 for the
sulfur moiety; the oxopropionate moiety
would be predicted to be of low
toxicity.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Structural class III
Sodium 3-mercapto-oxo-propionate 563 10255-67-1 No Yes. NOELs of 1,9, 2.8, and 1.9 mg/kg N/R No safety
bw per day were reported in 90-day concern
studies in rats treated with
2-mercapto-3-butanol (No. 546),
alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide
(No. 547), and 3-mercapto-2-pentanone
(No. 560), respectively, only at those
doses. See subgroup (i), No. 452 for the
sulfur moiety; the oxopropionate moiety
would be predicted to be of low
toxicity.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Subgroup (vi): Dithiols
Structural class I
1,2-Ethanedithiol 532 540-63-6 No Yes. NOELs of 0.7 mg/kg bw per day N/R No safety
were reported in 90-day studies in rats concern
treated with 2,3-butanedithiol
(No. 539) and 1,8-octanedithiol
(No. 541) only at that dose.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Subgroup (vi): Dithiols
Structural class I
1,2-Ethanedithiol 532 540-63-6 No Yes. NOELs of 0.7 mg/kg bw per day N/R No safety
were reported in 90-day studies in rats concern
treated with 2,3-butanedithiol
(No. 539) and 1,8-octanedithiol
(No. 541) only at that dose.
 1,3-Propanedithiol 535 109-80-8 No Yes. NOELs of 0.7 mg/kg bw per day N/R No safety
were reported in 90-day studies in rats concern
treated with 2,3-butanedithiol (No. 539)
and 1,8-octanedithiol (No. 541) only at
that dose.
1,3-Propanedithiol 535 109-80-8 No Yes. NOELs of 0.7 mg/kg bw per day N/R No safety
were reported in 90-day studies in rats concern
treated with 2,3-butanedithiol (No. 539)
and 1,8-octanedithiol (No. 541) only at
that dose.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
1,2-Propanedithiol 536 814-67-5 No Yes. NOELs of 0.7 mg/kg bw per day N/R No safety
were reported in 90-day studies in rats concern
treated with 2,3-butanedithiol (No. 539)
and 1,8-octanedithiol (No. 541) only at
that dose.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
1,2-Propanedithiol 536 814-67-5 No Yes. NOELs of 0.7 mg/kg bw per day N/R No safety
were reported in 90-day studies in rats concern
treated with 2,3-butanedithiol (No. 539)
and 1,8-octanedithiol (No. 541) only at
that dose.
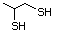 1,2-Butanedithiol 537 16128-68-0 No Yes. NOELs of 0.7 mg/kg bw per day N/R No safety
were reported in 90-day studies in rats concern
treated with 2,3-butanedithiol (No. 539)
and 1,8-octanedithiol (No. 541) only at
that dose.
1,2-Butanedithiol 537 16128-68-0 No Yes. NOELs of 0.7 mg/kg bw per day N/R No safety
were reported in 90-day studies in rats concern
treated with 2,3-butanedithiol (No. 539)
and 1,8-octanedithiol (No. 541) only at
that dose.
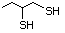 1,3-Butanedithiol 538 24330-52-7 No Yes. NOELs of 0.7 mg/kg bw per day N/R No safety
were reported in 90-day studies in rats concern
treated with 2,3-butanedithiol (No. 539)
and 1,8-octanedithiol (No. 541) only at
that dose.
1,3-Butanedithiol 538 24330-52-7 No Yes. NOELs of 0.7 mg/kg bw per day N/R No safety
were reported in 90-day studies in rats concern
treated with 2,3-butanedithiol (No. 539)
and 1,8-octanedithiol (No. 541) only at
that dose.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
2,3-Butanedithiol 539 4532-64-3 No Yes. A NOEL of 0.7 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
given this substance only at that dose.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
2,3-Butanedithiol 539 4532-64-3 No Yes. A NOEL of 0.7 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
given this substance only at that dose.
 1,6-Hexanedithiol 540 1191-43-1 No Yes. NOELs of 0.7 mg/kg bw per day N/R No safety
were reported in 90-day studies in rats concern
treated with 2,3-butanedithiol (No. 539)
and 1,8-octanedithiol (No. 541) only at
that dose.
1,6-Hexanedithiol 540 1191-43-1 No Yes. NOELs of 0.7 mg/kg bw per day N/R No safety
were reported in 90-day studies in rats concern
treated with 2,3-butanedithiol (No. 539)
and 1,8-octanedithiol (No. 541) only at
that dose.
 1,8-Octanedithiol 541 1191-62-4 No Yes. A NOEL of 0.7 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
given this substance only at that dose.
1,8-Octanedithiol 541 1191-62-4 No Yes. A NOEL of 0.7 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
given this substance only at that dose.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
1,9-Nonanedithiol 542 3489-28-9 No Yes. NOELs of 0.7 mg/kg bw per day N/R No safety
were reported in 90-day studies in rats concern
treated with 2,3-butanedithiol (No. 539)
and 1,8-octanedithiol (No. 541) only at
that dose.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
1,9-Nonanedithiol 542 3489-28-9 No Yes. NOELs of 0.7 mg/kg bw per day N/R No safety
were reported in 90-day studies in rats concern
treated with 2,3-butanedithiol (No. 539)
and 1,8-octanedithiol (No. 541) only at
that dose.
 Subgroup (vii): Simple disulfides
Structural class I
Dimethyl disulfide 564 624-92-0 No Yes. A NOEL of 7.3 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with propyl disulfide (No. 566)
at two doses.
Subgroup (vii): Simple disulfides
Structural class I
Dimethyl disulfide 564 624-92-0 No Yes. A NOEL of 7.3 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with propyl disulfide (No. 566)
at two doses.
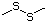 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Methyl propyl disulfide 565 2179-60-4 No Yes. A NOEL of 7.3 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with propyl disulfide (No. 566)
at two doses.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Methyl propyl disulfide 565 2179-60-4 No Yes. A NOEL of 7.3 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with propyl disulfide (No. 566)
at two doses.
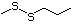 Propyl disulfide 566 629-19-6 No Yes. A NOEL of 7.3 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance at two
doses.
Propyl disulfide 566 629-19-6 No Yes. A NOEL of 7.3 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance at two
doses.
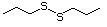 Diisopropyl disulfide 567 4253-89-8 No Yes. NOELs of 7.3 and 0.23 mg/kg bw N/R No safety
per day were reported in 90-day studies concern
in rats treated with propyl disulfide
and dicyclohexyl disulfide at two and
single doses, respectively.
Diisopropyl disulfide 567 4253-89-8 No Yes. NOELs of 7.3 and 0.23 mg/kg bw N/R No safety
per day were reported in 90-day studies concern
in rats treated with propyl disulfide
and dicyclohexyl disulfide at two and
single doses, respectively.
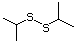 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Methyl 1-propenyl disulfide 569 5905-47-5 No Yes. A NOEL of 7.3 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with propyl disulfide (No. 566)
at two doses.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Methyl 1-propenyl disulfide 569 5905-47-5 No Yes. A NOEL of 7.3 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with propyl disulfide (No. 566)
at two doses.
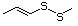 Propenyl propyl disulfide 570 5905-46-4 - Yes. A NOEL of 7.3 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with propyl disulfide (No. 566)
at two doses.
Propenyl propyl disulfide 570 5905-46-4 - Yes. A NOEL of 7.3 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with propyl disulfide (No. 566)
at two doses.
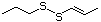 Methyl 3-methyl-1-butenyl 571 Pending - Yes. A NOEL of 7.3 mg/kg bw per day N/R No safety
disulfide was reported in a 90-day study in rats concern
treated with propyl disulfide (No. 566)
at two doses.
Methyl 3-methyl-1-butenyl 571 Pending - Yes. A NOEL of 7.3 mg/kg bw per day N/R No safety
disulfide was reported in a 90-day study in rats concern
treated with propyl disulfide (No. 566)
at two doses.
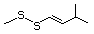 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Structural class II
Allyl methyl disulfide 568 2179-58-0 No Yes. A NOEL of 4.6 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with diallyl trisulfide
(No. 587, group ix) at a single dose for
90 days; this substance is predicted to
be metabolized to allyl mercaptan.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Structural class II
Allyl methyl disulfide 568 2179-58-0 No Yes. A NOEL of 4.6 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with diallyl trisulfide
(No. 587, group ix) at a single dose for
90 days; this substance is predicted to
be metabolized to allyl mercaptan.
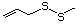 Allyl disulfide 572 2179-57-9 No Yes. A NOEL of 4.6 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with diallyl trisulfide
(No. 587, group ix) at a single dose for
90 days; this substance is predicted to
be metabolized to allyl mercaptan.
Allyl disulfide 572 2179-57-9 No Yes. A NOEL of 4.6 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with diallyl trisulfide
(No. 587, group ix) at a single dose for
90 days; this substance is predicted to
be metabolized to allyl mercaptan.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Dicyclohexyl disulfide 575 2550-40-5 No Yes. A NOEL of 0.23 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Dicyclohexyl disulfide 575 2550-40-5 No Yes. A NOEL of 0.23 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
 Methyl phenyl disulfide 576 14173-25-2 No Yes. A NOEL of 3.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with 2-naphthalenethiol
(No. 531, group iv) at a single dose for
90 days; this substance is predicted to
be reduced rapidly to thiophenol.
Methyl phenyl disulfide 576 14173-25-2 No Yes. A NOEL of 3.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with 2-naphthalenethiol
(No. 531, group iv) at a single dose for
90 days; this substance is predicted to
be reduced rapidly to thiophenol.
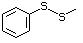 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Methyl benzyl disulfide 577 699-10-5 No Yes. A NOEL of 1.2 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Methyl benzyl disulfide 577 699-10-5 No Yes. A NOEL of 1.2 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
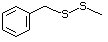 Benzyl disulfide 579 150-60-7 No Yes. A NOEL of 1.2 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with methyl benzyl disulfide
(No. 579) only at that dose
Benzyl disulfide 579 150-60-7 No Yes. A NOEL of 1.2 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with methyl benzyl disulfide
(No. 579) only at that dose
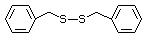 Structural class III
Phenyl disulfide 578 882-33-7 No Yes. A NOEL of 3.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with 2-naphthalenethiol
(No. 531, group iv) at a single dose for
90 days; this substance is predicted to
be reduced rapidly to thiophenol.
Structural class III
Phenyl disulfide 578 882-33-7 No Yes. A NOEL of 3.4 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with 2-naphthalenethiol
(No. 531, group iv) at a single dose for
90 days; this substance is predicted to
be reduced rapidly to thiophenol.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Subgroup (viii): Disulfides with oxidized side-chains
Structural class I
2-Methyl-2-(methyldithio) 580 67952-60-7 No Yes. NOELs of 7.3, 0.23, 1.2,and 1.9 N/R No safety
-propanal mg/kg bw per day were reported in concern
90-day studies in rats treated with
propyl disulfide (No. 566), dicyclohexyl
disulfide (No. 575), methyl benzyl
disulfide (No. 577), and
3-mercapto-2-pentanone (No. 560),
respectively, only at those doses.
See cyclopentanethiol (No. 516,
sub-group iv) for the thiol products
of reduction.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Subgroup (viii): Disulfides with oxidized side-chains
Structural class I
2-Methyl-2-(methyldithio) 580 67952-60-7 No Yes. NOELs of 7.3, 0.23, 1.2,and 1.9 N/R No safety
-propanal mg/kg bw per day were reported in concern
90-day studies in rats treated with
propyl disulfide (No. 566), dicyclohexyl
disulfide (No. 575), methyl benzyl
disulfide (No. 577), and
3-mercapto-2-pentanone (No. 560),
respectively, only at those doses.
See cyclopentanethiol (No. 516,
sub-group iv) for the thiol products
of reduction.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Ethyl 2-(methyldithio)propionate 581 23747-43-5 No Yes. NOELs of 7.3, 0.23, 1.2,and 1.9 N/R No safety
mg/kg bw per day were reported in concern
90-day studies in rats treated with
propyl disulfide (No. 566), dicyclohexyl
disulfide (No. 575), methyl benzyl
disulfide (No. 577), and
3-mercapto-2-pentanone (No. 560),
respectively, only at those doses.
See cyclopentanethiol (No. 516,
sub-group iv) for the thiol products of
reduction.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Ethyl 2-(methyldithio)propionate 581 23747-43-5 No Yes. NOELs of 7.3, 0.23, 1.2,and 1.9 N/R No safety
mg/kg bw per day were reported in concern
90-day studies in rats treated with
propyl disulfide (No. 566), dicyclohexyl
disulfide (No. 575), methyl benzyl
disulfide (No. 577), and
3-mercapto-2-pentanone (No. 560),
respectively, only at those doses.
See cyclopentanethiol (No. 516,
sub-group iv) for the thiol products of
reduction.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Subgroup (ix): Trisulfides
Structural class I
Dimethyl trisulfide 582 3658-80-8 No Yes. A NOEL of 4.8 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with dipropyl trisulfide
(No. 585) only at that dose
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Subgroup (ix): Trisulfides
Structural class I
Dimethyl trisulfide 582 3658-80-8 No Yes. A NOEL of 4.8 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with dipropyl trisulfide
(No. 585) only at that dose
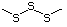 Methyl ethyl trisulfide 583 31499-71-5 No Yes. A NOEL of 4.8 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with dipropyl trisulfide
(No. 585) only at that dose
Methyl ethyl trisulfide 583 31499-71-5 No Yes. A NOEL of 4.8 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with dipropyl trisulfide
(No. 585) only at that dose
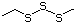 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Methyl propyl trisulfide 584 17619-36-2 No Yes. A NOEL of 4.8 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with dipropyl trisulfide
(No. 585) only at that dose
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Methyl propyl trisulfide 584 17619-36-2 No Yes. A NOEL of 4.8 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with dipropyl trisulfide
(No. 585) only at that dose
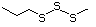 Dipropyl trisulfide 585 6028-61-1 No Yes. A NOEL of 4.8 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
Dipropyl trisulfide 585 6028-61-1 No Yes. A NOEL of 4.8 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
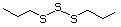 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Structural class II
Allyl methyl trisulfide 586 34135-85-8 No Yes. A NOEL of 4.6 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with diallyl trisulfide
(No. 587) only at that dose.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Structural class II
Allyl methyl trisulfide 586 34135-85-8 No Yes. A NOEL of 4.6 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with diallyl trisulfide
(No. 587) only at that dose.
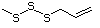 Diallyl trisulfide 587 2050-87-5 No Yes. A NOEL of 4.6 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
Diallyl trisulfide 587 2050-87-5 No Yes. A NOEL of 4.6 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
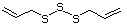 Diallyl polysulfide 588 72869-75-1 No Yes. A NOEL of 4.6 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with diallyl trisulfide
(No. 587) only at that dose.
Diallyl polysulfide 588 72869-75-1 No Yes. A NOEL of 4.6 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with diallyl trisulfide
(No. 587) only at that dose.
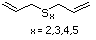 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Subgroup (x): Heterocyclic disulfides
Structural class II
3,5-Dimethyl-1,2,4-trithiolane 573 23654-92-4 No Yes. A NOEL of 1.9 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Subgroup (x): Heterocyclic disulfides
Structural class II
3,5-Dimethyl-1,2,4-trithiolane 573 23654-92-4 No Yes. A NOEL of 1.9 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
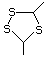 3-Methyl-1,2,4-trithiane 574 43040-01-3 No Yes. NOELs of 1.9 and 0.3 mg/kg bw N/R No safety
per day were reported in 90-day studies concern
in rats treated with
3,5-dimethyl-1,2,4-tri-thiolane
(No. 573) and this substance,
respectively, only at those doses.
3-Methyl-1,2,4-trithiane 574 43040-01-3 No Yes. NOELs of 1.9 and 0.3 mg/kg bw N/R No safety
per day were reported in 90-day studies concern
in rats treated with
3,5-dimethyl-1,2,4-tri-thiolane
(No. 573) and this substance,
respectively, only at those doses.
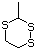 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Subgroup (xi): Thioesters
Structural class I
S-Methyl thioacetate 482 1534-08-3 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate
(No. 483) only at that dose, and a NOEL
of 1000 mg/kg bw per day was reported
in a 90-day study in rats treated with
methyl thio-butyrate (No. 484) at
multiple doses.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Subgroup (xi): Thioesters
Structural class I
S-Methyl thioacetate 482 1534-08-3 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate
(No. 483) only at that dose, and a NOEL
of 1000 mg/kg bw per day was reported
in a 90-day study in rats treated with
methyl thio-butyrate (No. 484) at
multiple doses.
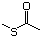 Ethyl thioacetate 483 625-60-5 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
Ethyl thioacetate 483 625-60-5 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Methyl thiobutyrate 484 2432-51-1 No Yes. A NOEL of 1000 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Methyl thiobutyrate 484 2432-51-1 No Yes. A NOEL of 1000 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with this substance only at
that dose.
 Propyl thioacetate 485 2307-10-0 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate (No. 483)
only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses.
Propyl thioacetate 485 2307-10-0 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate (No. 483)
only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Methyl 2-methylthiobutyrate 486 42075-45-6 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate (No. 483)
only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Methyl 2-methylthiobutyrate 486 42075-45-6 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate (No. 483)
only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses.
 SS-Methyl 3-methylbutanethioate 487 23747-45-7 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate (No. 483)
only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses.
SS-Methyl 3-methylbutanethioate 487 23747-45-7 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate (No. 483)
only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses.
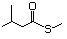 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
S-Methyl 4-methylpentanethioate 488 61122-71-2 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate (No. 483)
only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
S-Methyl 4-methylpentanethioate 488 61122-71-2 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate (No. 483)
only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses.
 S-Methyl hexanethioate 489 20756-86-9 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate (No. 483)
only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses.
S-Methyl hexanethioate 489 20756-86-9 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate (No. 483)
only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses.
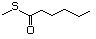 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Allyl thiopropionate 490 41820-22-8 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate (No. 483)
only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses. See diallyl trisulfide
(No. 587, subgroup ix), which is
predicted to be metabolized to allyl
thiol (allyl mercaptan) via diallyl
disulfide.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Allyl thiopropionate 490 41820-22-8 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate (No. 483)
only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses. See diallyl trisulfide
(No. 587, subgroup ix), which is
predicted to be metabolized to allyl
thiol (allyl mercaptan) via diallyl
disulfide.
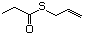 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Prenyl thioacetate 491 33049-93-3 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate (No. 483)
only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses. See diallyl trisulfide (No. 587,
subgroup ix), which is predicted to be
metabolized to allyl thiol (allyl
mercaptan) via diallyl disulfide.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Prenyl thioacetate 491 33049-93-3 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate (No. 483)
only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses. See diallyl trisulfide (No. 587,
subgroup ix), which is predicted to be
metabolized to allyl thiol (allyl
mercaptan) via diallyl disulfide.
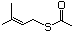 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Methylthio 2-(acetyloxy) 492 74586-09-7 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate (No. 483)
only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Methylthio 2-(acetyloxy) 492 74586-09-7 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate (No. 483)
only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses.
 Methylthio 2-(propionyloxy) 493 999999-90-9 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate (No. 483)
only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses.
Methylthio 2-(propionyloxy) 493 999999-90-9 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate (No. 483)
only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
3-Acetylmercaptohexyl acetate 494 136954-25-1 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate (No. 483)
only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
3-Acetylmercaptohexyl acetate 494 136954-25-1 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate (No. 483)
only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses.
 Subgroup (xi), Structural class II
S-Methyl benzothioate 504 5925-68-8 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate (No. 483)
only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses.
Subgroup (xi), Structural class II
S-Methyl benzothioate 504 5925-68-8 No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
was reported in a 90-day study in rats concern
treated with ethyl thioacetate (No. 483)
only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
s- and 506a cis, No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
trans-Menthone-8-thioacetate and 57129-12-1 was reported in a 90-day study in rats concern
506b trans, treated with ethyl thioacetate (No. 483)
57074-34-7 only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
s- and 506a cis, No Yes. A NOEL of 6.5 mg/kg bw per day N/R No safety
trans-Menthone-8-thioacetate and 57129-12-1 was reported in a 90-day study in rats concern
506b trans, treated with ethyl thioacetate (No. 483)
57074-34-7 only at that dose, and a NOEL of 1000
mg/kg bw per day was reported in a
90-day study in rats treated with methyl
thio-butyrate (No. 484) at multiple
doses.
 Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Subgroup (xii), Structural class III: Sulfoxides
Methylsulfinylmethane (DMSO) 507 67-68-5 No Yes. A NOEL of 3000 mg/kg bw per day N/R No safety
was reported in monkeys given this concern
substance at multiple doses for 74-87
weeks; similar results in rats, dogs,
and humans.
Table 1. (continued)
Substance JECFA CAS No. Step B3 Step B4. Adequate Step B5 Conclusion
and structure No. Does margin of safety Does intake based on
intake for substance or exceed current
exceed related substance? 1.5 µg/day? intake
threshold
for human
intake?
Subgroup (xii), Structural class III: Sulfoxides
Methylsulfinylmethane (DMSO) 507 67-68-5 No Yes. A NOEL of 3000 mg/kg bw per day N/R No safety
was reported in monkeys given this concern
substance at multiple doses for 74-87
weeks; similar results in rats, dogs,
and humans.
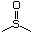 N/R, not relevant to the evaluation; ND, no data reported; bw, body weight
a None of the substances in this group are predicted to be metabolized to innocuous products. They were placed in subgroups
(i)-(xii) on the basis of the position of the sulfur atom.
b The thresholds for human intake are 1800 µg/day for class I, 540 µg/day for class II, and 90 µg/day for class III.
Table 2. Annual volume and intake of aliphatic and aromatic sulfides and thiols used as
flavouring substances in Europe and the United States
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
Methyl sulfide (452)
Europe 3100 590 10
United States 2800 530 9 57 000
Methyl ethyl sulfide (453)
Europe NR NA NA
United States (A) 9 2 0.03 +
Diethyl sulfide (454)
Europe NR NA NA
United States (A) 68 13 0.22 +
Butyl sulfide (455)
Europe 19 4 0.1
United States 0.5 0.1 0.002 +
1,4-Dithiane (456)
Europe NR NA NA
United States (A) 0.45 0.1 0.001 +
(1-Butenyl-1) methyl sulfide (457)
Europe NR NA NA
United States (A) 0.45 0.1 0.001 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
Allyl sulfide (458)
Europe NR NA NA
United States 2 0.4 0.01 +
Methyl phenyl sulfide (459)
Europe NR NA NA
United States (A) 2 0.4 0.01 +
Benzyl methyl sulfide (460)
Europe 1.1 0.2 0.003
United States (1982) 0.1 0.02 0.0003 +
3-(Methylthio)propanol (461)
Europe 23 4 0.1
United States 4 1 0.01 +
4-(Methylthio)butanol (462)
Europe 0.1 0.02 0.0003
United States 0.5 0.1 0.002 +
3-(Methylthio)-1-hexanol (463)
Europe 26 5 0.1
United States 0.5 0.1 0.002 +
2-Methyl-4-propyl-1,3-oxathiane (464)
Europe 11 2 0.03
United States (A) 5 1 0.02 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
2-Methylthioacetaldehyde (465)
Europe NR NA NA
United States (1970) 3 1 0.01 +
3-(Methylthio)propionaldehyde (466)
Europe 230 45 1
United States 130 25 0.4 637
3-(Methylthio)butanal (467)
Europe 0.7 0.1 0.002
United States 0.5 0.1 0.002 +
4-(Methylthio)butanal (468)
Europe NR NA NA
United States 0.1 0.02 0.0003 -
3-Methylthiohexanal (469)
Europe NR NA NA
United States (A) 4.5 1 0.01 +
2-(Methylthio)methyl-2-butenal (470)
Europe 0.2 0.04 0.001
United States (1982) 0.5 0.1 0.002 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
2,8-Dithianon-4-en-4-carboxaldehyde (471)
Europe 0.1 0.01 0.0002
United States 0.5 0.0: 0.002 +
Methyl 3-methylthiopropionate (472)
Europe 770 146 2
United States 46 9 0.1 5
Methylthiomethyl butyrate (473)
Europe NR NA NA
United States (A) 0.45 0.1 0.001 +
Methyl 4-(methylthio)butyrate (474)
Europe 0.5 0.1 0.002
United States 0.5 0.1 0.002 -
Ethyl 2-(methylthio)acetate (475)
Europe NR NA NA
United States (A) 0.45 0.1 0.001 +
Ethyl 3-methylthiopropionate (476)
Europe 200 37 1
United States 9 2 0.03 9
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
Ethyl 4-(methylthio)butyrate (477)
Europe NR NA NA
United States (A) 11 2 0.03 -
3-(Methylthio)propyl acetate (478)
Europe NR NA NA
United States (A) 59 11 0.2 +
Methylthiomethyl hexanoate (479)
Europe NR NA NA
United States (A) 0.45 0.1 0.001 +
Ethyl 3-(methylthio)butyrate (480)
Europe NR NA NA
United States (A) 0.45 0.1 0.001 +
3-(Methylthio)hexyl acetate (481)
Europe 0.4 0.1 0.001
United States (A) 45 9 0.1 +
S-Methyl thioacetate (482)
Europe NR NA NA
United States (A) 0.01 0.002 0.00003 2
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
Ethyl thioacetate (483)
Europe 0.1 0.02 0.0003
United States 0.1 0.02 0.0003 56
Methyl thiobutyrate (484)
Europe 24 5 0.1
United States 27 5 0.1 +
Propyl thioacetate (485)
Europe 2.2 0.4 0.01
United States 0.1 0.02 0.0003 +
Methyl 2-methylthiobutyrate (486)
Europe 0.8 0.2 0.003
United States 0.5 0.01 0.002 +
S-Methyl 3-methylbutanethioate (487)
Europe NR NA NA
United States (A) 1 0.19 0.003 +
S-Methyl 4-methylpentanethioate (488)
Europe NR NA NA
United States (A) 0.0045 0.001 0.00001 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
S-Methyl hexanethioate (489)
Europe NR NA NA
United States (A) 0.45 0.1 0.001 3
Allyl thiopropionate (490)
Europe NR NA NA
United States 0.5 0.1 0.002 -
Prenyl thioacetate (491)
Europe NR NA NA
United States (A) 1 0.19 0.003 +
Methyl 2-(acetyloxy) propionate (492)
Europe NR NA NA
United States (A) 45 9 0.1 -
Methylthio 2-(propionyloxy) propionate (493)
Europe NR NA NA
United States (A) 45 9 0.1 -
3-Acetylmercaptohexyl acetate (494)
Europe NR NA NA
United States (A) 2.2 0.4 0.01 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
1-Methylthio-2-propanone (495)
Europe NR NA NA
United States (A) 1 0.2 0.003 -
1-(Methylthio)-2-butanone (496)
Europe 0.03 0.01 0.0001
United States (1982) 0.1 0.02 0.0003 +
4-(Methylthio)-2-butanone (497)
Europe 0.1 0.02 0.0003
United States 2 0.4 0.01 +
4,5-Dihydro-3(2H)-thiophenone (498)
Europe 3.6 1 0.01
United States 13 2 0.04 +
2-Methyltetrahydrothiophen-3-one (499)
Europe 100 19 0.3
United States (A) 0.5 0.1 0.002 5036
4-(Methylthio)-4-methyl-2-pentanone (500)
Europe 0.2 0.04 0.0006
United States 0.5 0.1 0.002 -
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
4-(Methylthio)-2-oxobutanoic acid, sodium salt (501)
Europe NR NA NA
United States (A) 1 0.2 0.003 +
Di(butan-3-one-1-yl) sulfide (502)
Europe NR NA NA
United States (1982) 0.1 0.02 0.0003 -
ortho-(Methylthio)phenol (503)
Europe 5 1 0.02
United States (1970) 5 1 0.02 +
S-Methyl benzothioate (504)
Europe NR NA NA
United States (A) 0.0045 0.001 0.00001 +
2-(Methylthiomethyl)-3-phenylpropenal (505)
Europe NR NA NA
United States 10 2 0.03 -
cis- and trans-Menthone-8-thioacetate (506)
Europe NR NA NA
United States (A) 2.3 0.4 0.01 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
Methylsulfinylmethane (507)
Europe NR NA NA
United States (A) 0.0045 0.001 0.00001 45 000
Methyl mercaptan (508)
Europe 440 83 1
United States 0.9 0.2 0.003 +
Propanethiol (509)
Europe 18 3 0.1
United States 38 7 0.1 360
2-Propanethiol (510)
Europe NR NA NA
United States (A) 0.022 0.004 0.0001 +
1-Butanethiol (511)
Europe 3.2 0.5 0.01
United States 0.2 0.04 0.001 +
2-Methyl-1-propanethiol (512)
Europe NR NA NA
United States (A) 6.8 1.3 0.021 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
3-Methylbutanethiol (513)
Europe NR NA NA
United States (A) 0.045 0.01 0.0001 23
2-Pentanethiol (514)
Europe 12 2 0.04
United States (A) 11 2 0.03 +
2-Methyl-1-butanethiol (515)
Europe 2.5 0.5 0.01
United States (1982) 0.1 0.02 0.0003 +
Cyclopentanethiol (516)
Europe NR NA NA
United States (A) 4.5 1 0.01 -
3-Methyl-2-butanethiol (517)
Europe 0.1 0.02 0.0003
United States 0.1 0.02 0.0003 +
1-Hexanethiol (518)
Europe NR NA NA
United States (A) 0.045 0.01 0.0001 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
2-Ethylhexanethiol (519)
Europe NR NA NA
United States (A) 0.045 0.01 0.0001 +
2,3, or 10-Mercatopinane (520)
Europe 0.3 0.1 0.001
United States 50 10 0.2 -
Allyl mercaptan (521)
Europe 1.3 0.2 0.003
United States 8 2 0.03 480
Prenythiol (522)
Europe NR NA NA
United States (A) 1 0.2 0.003 +
1-para-Menthene-8-thiol (523)
Europe 2.8 1 0.01
United States (A) 4.5 1 0.01 +
Thiogeraniol (524)
Europe 9 2 0.03
United States 0.1 0.02 0.0003 -
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
Benzenethiol (525)
Europe 6 1 0.02
United States 160 30 1 +
Benzyl mercaptan (526)
Europe 10 2 0.03
United States 2 0.4 0.01 +
Phenethyl mercaptan (527)
Europe NR NA NA
United States (A) 1 0.2 0.003 +
ortho-Toluenethiol (528)
Europe 140 27 0.4
United States 0.9 0.2 0.003 +
2-Ethylthiophenol (529)
Europe 0.001 0.0002 0.0
United States 0.5 0.10 0.002 -
2,6-Dimethylthiophenol (530)
Europe 11 2 0.03
United States 0.1 0.02 0.0003 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
2-Naphthalenethiol (531)
Europe NR NA NA
United States (A) 0.34 0.1 0.001 +
1,2-Ethanedithiol (532)
Europe 0.01 0.002 0.00003
United States (A) 4.5 0.9 0.01 +
Bis(methylthio)methane (533)
Europe NR NA NA
United States (A) 500 94 2 +
2-Methyl-1,3-dithiolane (534)
Europe 0.5 0.1 0.002
United States (A) 23 4 0.1 +
1,3-Propanedithiol (535)
Europe 7 1 0.02
United States (A) 4.5 0.9 0.01 +
1,2-Propanedithiol (536)
Europe NR NA NA
United States (A) 4.5 0.9 0.01 -
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
1,2-Butanedithiol (537)
Europe NR NA NA
United States 0.9 0.2 0.003 -
1,3-Butanedithiol (538)
Europe NR NA NA
United States (A) 4.5 0.9 0.01 -
2,3-Butanedithiol (539)
Europe 0.4 0.1 0.001
United States 0.9 0.2 0.003 -
1,6-Hexanedithiol (540)
Europe 13 2.5 0.04
United States 0.5 0.1 0.002 +
1,8-Octanedithiol (541)
Europe 17 3 0.1
United States (A) 4.5 0.9 0.01 -
1,9-Nonanedithiol (542)
Europe 0.01 0.002 0.00003
United States (A) 4.5 0.9 0.01 -
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
Trithioacetone (543)
Europe 12 2 0.04
United States 2 0.4 0.01 +
3-Mercapto-3-methyl-1-butanol (544)
Europe NR NA NA
United States (A) 10 1.9 0.03 +
3-Mercaptohexanol (545)
Europe NR NA NA
United States (A) 4.5 1 0.01 +
2-Mercapto-3-butanol (546)
Europe 33 6 0.1
United States 0.5 0.1 0.002 -
alpha-Methyl-ß-hydroxypropyl alpha-methyl-ß-mercaptopropyl sulfide (547)
Europe NR NA NA
United States 4 1 0.01 -
4-Methyoxy-2-methyl-2-butanethiol (548)
Europe NR NA NA
United States (A) 4 0.8 0.01 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
3-Mercapto-3-methylbutyl formate (549)
Europe NR NA NA
United States (A) 0.5 0.1 0.002 +
2,5-Dihydroxy-1,4-dithiane (550)
Europe NR NA NA
United States (A) 0.45 0.1 0.001 -
2-Mercaptopropionic acid (551)
Europe 17 3 0.1
United States 440 84 1 -
Ethyl 2-mercaptopropionate (552)
Europe 3.2 0.5 0.01
United States 2 0.4 0.01 +
Ethyl 3-mercaptopropionate (553)
Europe 0.6 0.1 0.002
United States (A) 230 43 1 +
3-Mercaptohexyl acetate (554)
Europe NR NA NA
United States (A) 1 0.2 0.003 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
3-Mercaptohexyl butyrate (555)
Europe NR NA NA
United States (A) 1 0.2 0.003 +
3-Mercaptohexyl hexanoate (556)
Europe NR NA NA
United States (A) 1 0.2 0.003 +
1-Mercapto-2-propanone (557)
Europe NR NA NA
United States (A) 0.45 0.09 0.001 +
3-Mercapto-2-butanone (558)
Europe 26 5 0.1
United States (1982) 0.2 0.04 0.001 +
2-Keto-4-butanethiol (559)
Europe NR NA NA
United States 0.5 0.1 0.002 -
3-Mercapto-2-pentanone (560)
Europe NR NA NA
United States 0.5 0.1 0.002 -
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
para-Mentha-8-thiol-3-one (561)
Europe 86 16 0.3
United States 9 2 0.03 +
2,5-Dimethyl-2,5-dihydroxy-1,4-dithiane (562)
Europe 1.2 0.2 0.004
United States 0.9 0.2 0.003 -
Sodium 3-mercaptooxopropionate (563)
Europe NR NA NA
United States (A) 1 0.2 0.003 -
Dimethyl disulfide (564)
Europe 57 11 0.2
United States 13 2 0.04 1 400
Methyl propyl disulfide (565)
Europe 32 6 0.10
United States 0.1 0.02 0.0003 13 000
Propyl disulfide (566)
Europe 28 5 0.1
United States 0.5 0.1 0.002 14 000
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
Diisopropyl disulfide (567)
Europe NR NA NA
United States (A) 41 8 0.1 +
Allyl methyl disulfide (568)
Europe 0.01 0.002 0.00003
United States (1982) 0.1 0.02 0.0003 +
Methyl 1-propenyl disulfide (569)
Europe NR NA NA
United States (1982) 4 1 0.01 1
Propenyl propyl disulfide (570)
Europe NR NA NA
United States (1970) 40 8 0.1 +
Methyl 3-methyl-1-butenyl disulfide (571)
Europe NR NA NA
United States (A) 0.45 0.1 0.001 +
Allyl disulfide (572)
Europe 480 92 2
United States 44 8 0.1 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
3,5-Dimethyl-1,2,4-trithiolane (573)
Europe 0.2 0.04 0.001
United States (1982) 0.5 0.0: 0.002 +
3-Methyl-1,2,4-trithiane (574)
Europe 0.6 0.1 0.002
United States (A) 230 43 1 +
Dicyclohexyl disulfide (575)
Europe 0.1 0.02 0.0003
United States 0.9 0.2 0.003 -
Methyl phenyl disulfide (576)
Europe NR NA NA
United States (A) 1 0.2 0.003 +
Methyl benzyl disulfide (577)
Europe 0.1 0.02 0.0003
United States 0.5 0.1 0.002 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
Phenyl disulfide (578)
Europe NR NA NA
United States (1982) 0.2 0.04 0.001 -
Benzyl disulfide (579)
Europe 0.1 0.01 0.0002
United States (1982) 0.5 0.1 0.002 +
2-Methyl-2-(methyldithio) propanal (580)
Europe NR NA NA
United States (A) 9 2 0.03 +
Ethyl 2-(methyldithio)propionate (581)
Europe NR NA NA
United States (A) 0.45 0.1 0.001 +
Dimethyl trisulfide (582)
Europe 9 2 0.03
United States 0.1 0.02 0.0003 3 000
Methyl ethyl trisulfide (583)
Europe NR NA NA
United States (A) 3 1 0.01 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
Methyl propyl trisulfide (584)
Europe 1.7 0.3 0.01
United States 0.4 0.1 0.001 13 000
Dipropyl trisulfide (585)
Europe 60 11 0.2
United States 5 1 0.02 16 000
Allyl methyl trisulfide (586)
Europe NR NA NA
United States (A) 4.5 0.9 0.01 +
Diallyl trisulfide (587)
Europe 29 6 0.1
United States (1970) 0.1 0.02 0.0003 +
Diallyl polysulfide (588)
Europe 10 2 0.03
United States 0.1 0.02 0.0003 -
TOTAL
Europe 6 100
United States 5 300
A, volume anticipated on the basis of recent estimates (communication from the Flavor and Extract
Manufacturers' Association to the Committee); NR, not reported; NA, not applicable; +, reported
to occur naturally in foods (CIVO-TNO, 1994), but quantitative data were not available; -, not
reported to occur naturally in foods
a Volumes reported in a survey in Europe (International Organization of the Flavor
Industry, 1995) and in a survey in the United States (National Academy of Sciences, 1989).
Most of the data for the United States are for 1987 (and there is no entry); when data
for 1987 were not available, data for 1982 or 1970 were used, as indicated.
b Per capita intake (mg/day) calculated as follows:
[(annual usage, kg) × (1 × 109 mg/kg) / (population × 0.6 × 365 days)], where population
(10% 'eaters only') = 32 × 106 for Europe and 24 × 106 for the USA; 0.6 represents the
assumption that only 60% of the annual usage of the flavour was reported in the survey.
Intake (mg/kg bw per day) calculated as follows: [(mg/day)/body weight], where body weight = 60 kg.
c From Stofberg & Kirschman (1985); Stofberg & Grundschober (1987)
Potential toxicity can be deduced by comparison with structural
analogues on the basis of metabolic similarities. In the absence of
information on the toxicity of structural analogues, however, it is
not possible to conclude a priori that the substances are
metabolized to innocuous products.
(i) Simple sulfides (thioethers)
Once alkyl and aromatic thioethers, commonly called 'sulfides',
enter the systemic circulation, they are rapidly oxidized to
sulfoxides and, depending on the structure of the thioether, may be
further oxidized to sulfones. Sulfoxides and sulfones are major
urinary metabolites of simple sulfides. Aliphatic thioethers (Nos
452-455, 457, 458, and 533) and thioethers containing an aromatic
nucleus (Nos 459 and 460) yield mixtures of sulfoxide and sulfone
metabolites. Enzymes of the cytochrome P450 superfamily and
flavin-containing monooxygenases catalyse the oxidation of thioethers
to sulfoxides. Oxidation of sulfoxides to the corresponding sulfones
occurs both in tissues and in aerobic microorganisms and is an
irreversible metabolic reaction in mammals. Sulfoxides can also be
metabolized back to the thioether by aldehyde oxidase, thioredoxin and
its reductase, and the gut microflora in the anaerobic environment of
the lower bowel.
The methyl aromatic thioethers (Nos 459 and 460) are predicted to
be major metabolites of the corresponding aromatic thiols (Nos 525 and
526, subgroup iv) and would be oxidized to sulfoxides and sulfones,
which would be excreted.
(ii) Acyclic sulfides with oxidized side-chains
The presence of other functional groups, such as alcohol (Nos
461-463), aldehyde (Nos 465-471 and 505), ester (Nos 472-481), acid
(No. 501), ß-ketone (Nos 495-497, 500, and 502), and phenol (No. 503),
provides centres of greater polarity and additional sites for the
biotransformation of thioethers. The presence of these polar groups
would also result in increased renal excretion. The biotransformations
of such oxygenated, carbon-containing, functional groups are well
characterized and have been described for groups of flavouring agents
previously evaluated by the Committee. Concurrent metabolism of
various substrates at both sulfur and oxygenated functional groups has
been reported, and sulfoxide formation usually predominates as the
major metabolic pathway of detoxification. Experiments in vitro
suggest that hydrolysis of carboxyl esters occurs in the presence of
thioether (sulfide) groups. In consequence, thioethers with oxidized
side-chains would be expected to be eliminated more rapidly than
simple sulfides.
(iii) Cyclic sulfides
Oxidation of unsubstituted and methyl-substituted cyclic
thioethers by the cytochrome P450 superfamily produces the
corresponding sulfoxides. The mono-sulfoxides are predicted to be the
main urinary metabolites of simple cyclic sulfides (Nos 456, 534, and
543). The metabolism of cyclic sulfides containing oxidized carbon
atoms (Nos 464, 498, 499, 550, and 562) has not been studied but would
be predicted to involve extensive S-oxidation and possibly oxidation
or conjugation of alcohol groups. The polarity of the hydroxy
thioethers (Nos 550 and 562) may allow their elimination unchanged.
(iv) Simple thiols
The simple thiol flavouring agents considered are alkyl and
alicyclic thiols (Nos 508-524, 526, and 527) and aromatic thiols
(thiophenols; Nos 525 and 528-531). These substances may be
metabolized along several pathways. Simple aliphatic and aromatic
thiols undergo S-methylation in mammals to produce the corresponding
methyl thioether or sulfide. Methylation is catalysed by thiopurine
methyltransferase in the cytoplasm and thiol methyltransferase in
microsomes, and both reactions require S-adenosyl-l-methionine as a
methyl group donor. Thiopurine methyltransferase is present in human
liver, kidney, and erythrocytes; preferential substrates for this
enzyme include aromatic and heterocyclic thiols. S-Methylation of
aliphatic thiols is catalysed by microsomal thiol methyltransferase,
and the resulting methyl thioether (sulfide) metabolite would undergo
S-oxidation to give the methyl sulfoxide and methyl sulfone
analogues as urinary products.
Thiols may react with glutathione and other endogenous thiol
substances to form mixed disulfides. Both microsomal and cytoplasmlic
thioltransferases have been reported to catalyse the formation of
mixed disulfides. The resulting mixed disulfides can undergo reduction
back to thiols, oxidative desulfuration, or oxidation to a sulfonic
acid via the intermediate thiosulfinate and sulfinic acids. The
principal form in the circulation would probably be a mixed disulfide
formed with albumin.
S-Glucuronidation of aromatic thiols has been reported, and
this may be a pathway for the metabolism of aromatic thiols
(thiophenols) (Nos 525 and 528-531) and simple aromatic disulfides
(Nos 576 and 578; subgroup vii) after their reduction (see below).
Glucuronyl transferases behave similarly towards hydroxyl and
sulfydryl groups, and the two activities have the same subcellular
location and optimal pH.
Thiols may be oxidized to form sulfenic acids (RSOH), which are
unstable and readily undergo further oxidation to sulfinic (RSO2H)
and sulfonic (RSO3H) acids or combine with nucleophiles. The sulfonic
acid group is a highly polar centre and makes molelcules highly
soluble in water. In general, sulfonic acids are stable to metabolism.
Alkyl thiols of low relative molecular mass undergo oxidative
desulfuration in vivo to yield CO2 and SO4=. This reaction has
been shown, for example, for methanethiol (methyl mercaptan; No. 508).
Whereas the carbon atom from thiols may be used in the biosynthesis of
amino acids, the sulfur atom is not used significantly in the
synthesis of sulfur-containing amino acids.
(v) Thiols with oxidized side chains
Although alkyl thiols with oxidized side-chains (Nos 544-549,
551-561, and 563) comprise a significant proportion of the flavouring
agents evaluated, their metabolic fate has not been studied. Their
metabolism is predicted to involve a combination of the pathways
described above for simple thiols and further oxidation or conjugation
of the oxidized side-chain. The class III compound, sodium
3-mercapto-oxopropionate (No. 563), would be expected to be eliminated
very rapidly by metabolism at both the thiol and keto-acid groups.
(vi) Dithiols
The metabolism of the simple aliphatic dithiols evaluated (Nos
532 and 535-542) is predicted to involve the pathways described above
for simple thiols. Urinary metabolites could result from methylation,
S-oxidation of one S atom to yield a polar sulfonate, and the
formation of mixed disulfides of low relative molecular mass such as
cysteine, an endogenous thiol. The longer, linear dithiols (Nos 535
and 540-542) could form intramolecular disulfide bonds, with
interconversion between dithiol and cyclic disulfide forms.
(vii) Simple disulfides
The reduction of xenobiotic disulfides is believed to be
extensive, and the reaction may be catalysed enzymatically by
thioltransferases and chemically by exchange with glutathione,
thioredoxin, cysteine, and other endogenous thiols. Reduction of the
non-cyclic disulfides considered in the group of flavours (Nos 564-572
and 575-579) would result in the formation of thiols of low relative
molecular mass, which would then be metabolized by the various
pathways described above for simple thiols.
(viii) Disulfides with oxidized side-chains
As discussed above for subgroups (ii) and (iii), additional sites
of carbon oxidation would result in greater polarity and further
oxidation or conjugation of the flavouring agents evaluated (Nos 580
and 581). However, as disulfide bonds in substances in subgroup (v)
are susceptible to reductive cleavage, this would be expected to be
the initial metabolic reaction, and the polarity of the side-chains
would primarily affect elimination of the thiol fragments.
(ix) Trisulfides and polysulfides
The trisulfide of glutathione is labile and readily converted to
the disulfide, the sulfur being released as hydrogen sulfide.
Trisulfides and polysulfides are predicted to be converted rapidly to
the corresponding disulfides, which would then be reduced to thiols,
which themselves would follow the pathways described above for thiols.
The potential toxicity of trisulfides (Nos 582-587) and polysulfides
(No. 588) is probably related to their metabolic lability and to the
nature of the eventual thiol (e.g. allyl thiol).
(x) Heterocyclic disulfides: The heterocyclic disulfides (Nos 573 and
574) are five-and six-carbon rings which also contain a cyclic
thioether bond. Lipoic acid, an endogenous substance, is a
five-membered cyclic disulfide which undergoes rapid redox cycling
between ring disulfide and open dithiol forms. The principal metabolic
pathways are predicted to be disulfide reduction with ring opening to
produce a dithiol, and S-oxidation of the cyclic thioether.
(xi) Thioesters
Thioester groups (-S-CO-) are present in a number of the
flavouring agents evaluated (Nos 482-494, 504, and 506). Hydrolysis of
esters has been considered previously by the Committee, but that of
thioesters has not. Thioesters are hydrolysed by lipase and esterases,
and the rate of hydrolysis increases as the length of the C-chain of
the carboxylic acid fragment increases, and decreases as oxygenation
of the carbon chain in the thiol moiety increases.
Hydrolysis could result in a thioic acid and an alcohol or a
carboxylic acid and a thiol (the metabolic fates of which have been
outlined above). Data for dithioic acids and esters indicate that the
esters of monothioic acids would be poor substrates for oxidation, but
the monothioic acid released by hydrolysis would be oxidized to the
dioxo acid. Other possibilities for elimination in vivo include renal
excretion of thiocarboxylic acid. The substances evaluated (with the
exceptions of prenyl thioacetate (No. 491) and allyl thiopropionate
(No. 490)) are simple linear alkyl compounds, branched-chain alkyl
compounds, or their side-chain hydroxyester analogues, so that
comparison of the toxicity of the different substances is reasonable.
(xii) Sulfoxides
The metabolism of sulfoxides by oxidation and reduction is
described above under 'Thioethers'. The only sulfoxide flavouring
agent evaluated was methylsulfinylmethane (dimethyl sulfoxide, DMSO;
No. 507), for which data were available on both metabolism and
toxicity in experimental animals and humans. DMSO is readily absorbed
and excreted in urine as the parent sulfoxide and dimethyl sulfone.
1.4 Application of the Procedure for the Safety
Evaluation of Flavouring Agents
Step 1. In applying the Procedure for the Safety Evaluation of
Flavouring Agents to the above-mentioned aliphatic and
aromatic sulfides and thiols, the Committee assigned 97 of
the 137 substances (Nos 452-455, 457, 461-463, 465-497, 500,
502, 508-515, 517-519, 522, 524, 532, 533, 535-542, 544-560,
562, 564-567, 569-571, and 580-585), which may or may not
contain an additional oxygenated functional group, to
structural class I. The Committee assigned 34 of the 137
substances to structural class II because they are aromatic
sulfides or thiols (Nos 459, 460, 503, 504, 525-528, 530,
531, 576, 577, and 579), alicyclic (Nos 506, 516, 520, 523,
561, and 575) or heterocyclic substances (Nos 456, 464, 498,
499, 534, 543, 573, and 574), or allyl mercaptan or sulfides
(Nos 458, 521, 568, 572, and 586-588), which are common
components of food. The aromatic thiols or thioethers that
are not common compo-nents of food (Nos 505, 529, and 578)
were assigned to structural class III. The remaining three
substances were also assigned to structural class III by
virtue of the fact that they are aliphatic thiols or
thioethers containing more than three functional groups (Nos
501 and 563) or do not contain divalent sulfur (No. 507).
Step 2. None of the substances in this group can be predicted to be
metabolized to innocuous products. The evaluation of these
substan-ces therefore proceeded via the right-hand side of
the decision tree.
Step B3. The estimated daily per capita intake in Europe and in the
United States for each of the substances in the overall
group of 137 flavouring agents is below the threshold for
human intake for the structural class in which the substance
falls. The thresholds are 1800 µg/person per day for class
I, 540 µg/person per day for class II, and 90 µg/person per
day for class III.
Step B4. The Committee considered the results of 90-day studies of
toxicity in rodents for 27 substances in this group of
flavouring agents. The Committee noted that the single or
multiple doses of flavouring agents tested in a number of
studies had no effect in rats and that the NOELs were
consequently derived from studies that did not show toxic
effects. The results of long-term studies in multiple
species were considered for one substance, DMSO (No. 507).
To facilitate comparisons of the toxicity of structurally
related substances, the agents were considered in subgroups
(i)- (xii) on the basis of the position of the sulfur atom
(see Tables 1 and 3). Toxicity was compared within and
across subgroups with no restriction on the basis of
structural class assignment (Step 1).
(i) Simple sulfides (thioethers): This subgroup comprises
nine flavouring agents that are simple thioethers. The NOEL
for methyl sulfide (No. 452) in a 14-week study in rats
treated with multiple doses by gavage was 250 mg/kg bw per
day. This NOEL provides an adequate basis for evaluation of
five structurally and metabolically related substances (Nos
453, 454, 455, 457, and 533); however, the NOEL for methyl
sulfide was considered inappropriate for evaluation of allyl
sulfide (No. 458) and two aromatic thioethers, methyl phenyl
sulfide and benzyl methyl sulfide (Nos 459 and 460,
respectively), and the evaluation of these three substances
therefore proceeded to step B5.
(ii) Acyclic sulfides with oxidized side-chains: This
subgroup comprises 28 flavouring agents that are acyclic
thioethers with oxidized side-chains. The NOEL in a 90-day
study in rats treated with a single dose of
2-(methylthiomethyl)-3-phenylpropenal (No. 505) was 1.4
mg/kg bw per day. This substance is an aromatic compound
with a sulfide group in an unsaturated side-chain; it was
assigned to structural class III, because it is not a common
component of food. Data for methyl sulfide (No. 452) were
also considered relevant for assessing the toxicity of
sulfide substances with oxidized side-chains (Nos 461-463,
465-469, 472-481, 495-497, and 500-503). Although
2-(methylthiomethyl)-3-phenylpropenal (No. 505) is not an
aryl thioether, the safety margin between its NOEL and the
intake of ortho-(methylthio)phenol pentanone (No. 503) was
considered to be adequate. The NOEL for
2-(methylthiomethyl)-3-phenylpropenal (No. 505) does not
provide a high margin of safety (i.e. > 1000) for methyl
3-methylthiopropionate (No. 472) at the current estimated
level of intake; however, the simple side-chain acid and
ester would have little toxic potential, and the NOEL for
methyl sulfide (No. 452) provides an adequate margin of
safety. The NOELs for 2-(methylthio-methyl)-3-phenylpropenal
(No. 505) and methyl sulfide (No. 452, subgroup (i)) were
considered inappropriate for evaluation of the toxicity of
two alpha,beta-unsaturated carbonyls (Nos 470 and 471)
because they are potentially more reactive and toxic, and
the evaluation of these substances therefore proceeded to
step B5.
(iii) Cyclic sulfides: This subgroup comprises eight
substances that are cyclic thioethers. NOELs of 0.44 mg/kg
bw per day for 2-methyl-4-propyl-1,3-oxathiane (No. 464),
9.2 mg/kg bw per day for 4,5-dihydro-3(2 H)-thiophenone
(No. 498), 7 mg/kg bw per day for 2-methyl-1,3-dithiolane
(No. 534), 0.2 mg/kg bw per day for trithioace-tone (No.
543), and 3.1 mg/kg bw per day for
2,5-dimethyl-2,5-dihydroxy-1,4-dithiane (No. 562) were
reported. These values provide an adequate margin of safety
for evaluation of the toxicity of substances No. 456, 499,
and 550.
Table 3. Comparison of the toxicity and intake data used in the safety evaluations of
137 simple aliphatic and aromatic sulfides and thiols, by subgroupa
Subgroup Adequate NOEL Adequate NOEL No adequate NOEL
for substanceb for structurally for substance or
related substanceb related substance,
but intake
< 1.5 µg/dayc
(i) Simple sulfides No. 452 Nos 453-455, Nos 458-460
(thioethers) 457, 533
(ii) Acyclic thioethers No. 505 Nos 461-463, Nos 470, 471
with oxidized 465-469, 472-481,
side-chains 495-497, 500-503
(iii) Cyclic sulfides Nos 464, 498, Nos 456, 499, N/R
534, 543, 562 550
(iv) Thiols Nos 516, 520, Nos 508-515, N/R
528, 530, 531 517-519, 521-527,
529
(v) Thiols with Nos 546, 547, Nos 544, 545, 548, N/R
oxidized 560 549, 551-559, 561,
side-chains 563
(vi) Dithiols Nos 539, 541 Nos 532, 535-538, N/R
540, 542
(vii) Simple disulfides Nos 566, 575, Nos 564, 565, N/R
577 567-572, 576, 578,
579
(viii) Disulfides with None Nos 580, 581 N/R
oxidized
side-chains
(ix) Trisulfides and Nos 585, 587 Nos 582-584, 586, N/R
polysulfides 588
(x) Heterocyclic No. 573 No. 574 N/R
disulfides
Table 3. (continued)
Subgroup Adequate NOEL Adequate NOEL No adequate NOEL
for substanceb for structurally for substance or
related substanceb related substance,
but intake
< 1.5 µg/dayc
(xi) Thioesters Nos 483, 484 Nos 482, 485-494, N/R
504, 506
(xii) Sulfoxides No. 507 N/R N/R
N/R, not relevant to the evaluation
a See Table 1 for further details of the evaluations.
b See Figure 1, step B4 and pp. 121-123 for further information.
c See Figure 1, step B5 and pp. 121-123 for further information.
(iv) Simple thiols: This subgroup comprises 24 flavouring
agents that are simple thiols. NOELs of 0.56 mg/kg bw per
day for cyclopentanethiol (No. 516), 0.06 mg/kg bw per day
for 2,3-and 10-mercaptopinane (No. 520), 0.52 mg/kg bw per
day for ortho-toluenethiol (No. 528), 0.43 mg/kg bw per
day for 2,6-dimethyl-thiophenol (No. 530), and 3.4 mg/kg bw
per day for 2-naphthalenethiol (No. 531) were reported.
These values provide adequate margins of safety for the
individual substances and for the other structurally related
thiols in the group in relation to current estimates of
intake, with the exception of 2,3 and 10-mercaptopinane (No.
520). A margin of safety of about 300 (based on an estimated
modified per capita intake of 0.2 µg/kg bw per day in the
United States) was obtained from the results of a 90-day
study in which only a single dose of 0.06 mg/kg bw per day
was tested. This substance was therefore also evaluated by
comparison with other substances in this subgroup (Nos 516,
528, 530, and 531) for which there was an adequate margin of
safety. The Committee noted that Nos 521-524 are unsaturated
thiols. Of these, allyl mercaptan (allyl thiol; No. 521)
would be expected to be more toxic than others that have
double bonds in different positions (by analogy to their
oxygenated analogues). Although data were not available on
allyl mercaptan (No. 521), the NOEL of diallyl trisulfide
(No. 587), which would be converted to allyl mercaptan after
reduction to allyl disulfide, was 4.6 mg/kg bw per day in a
90-day study in rats. This NOEL was considered to provide an
adequate safety margin for Nos 521-524.
(v) Thiols with oxidized side-chains: This subgroup
comprises thiols with oxygenated side-chains. The NOELs
available for three of the substances in class I (1.9 mg/kg
bw per day for 2-mercapto-3-butanol (No. 546), 2.8 mg/kg bw
per day for alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide (No. 547), and 1.9
mg/kg bw per day for 3-mercapto-2-pentanone (No. 560)) were
considered to provide adequate safety margins for the
flavouring agents in this subgroup, including the one
substance in structural class III, 3-mercapto-oxopropionate
(No. 563). Although the last compound has more than three
functional groups, the oxopropionate moiety would have
little toxic potential, and the NOELs for Nos 546, 547, and
560 were considered to provide an adequate margin of safety.
(vi) Dithiols: This subgroup comprises flavouring agents
that are dithiols. The NOEL for 2,3-butanedithiol (No. 539)
and 1,6-hexane-dithiol (No. 540) was 0.7 mg/kg bw per day,
which provides adequate margins of safety for all of the
substances in the subgroup.
(vii) Simple disulfides: This subgroup comprises 14
flavouring agents that are disulfides. The major metabolites
of these unsaturated disulfides would be thiols. The NOELs
were 7.7 mg/kg bw per day for propyl disulfide (No. 566),
0.23 mg/kg bw per day for dicyclohexyl disulfide (No. 575),
and 1.2 mg/kg bw per day for methyl benzyl disulfide (No.
577). These values provide adequate margins of safety for
each substance tested and for four structurally related
substances, Nos 564, 565, 567, and 579, at currently
estimated levels of intake. The substances in this subgroup
for which NOELs are available do not include unsaturated or
aryl disulfides. The aryl disulfides, methyl phenyl
disulfide and phenyl disulfide (Nos 576 and 578,
respectively), would be rapidly reduced to thiophenol, and
the NOEL of 3.4 mg/kg bw per day for 2-naphtha-lenethiol
(No. 531, subgroup iv) was considered to provide an adequate
safety margin for these agents. The Committee was aware that
propenyl disulfides can cause haemolytic anaemia in certain
species after short-term exposure. This effect would be of
concern to susceptible individuals. Substances Nos 568 and
572 would be metabolized to allyl mercaptan (No. 521;
subgroup iv). The Committee noted that the NOEL for diallyl
trisulfide (No. 587; subgroup ix) in a 90-day study in rats
given a single dose was 4.6 mg/kg bw per day, and that this
would provide an adequate safety margin for the allyl thiol
produced on reduction of substances Nos 568 and 572.
Di(1-propenyl) disulfide was about four times more potent
than allyl disulfide (No. 572) and about 20 times more
potent than propyl disulfide (No. 566). The intakes of the
related propenyl and butenyl flavours Nos 569, 570, and 571
gave safety margins of > 50 000 in comparison with the NOEL
for propyl disulfide (No. 566), and this was considered to
be adequate to allow for the differences in potency.
(viii) Disulfides with oxidized side-chains: This subgroup
consists of two disulfides with oxidized side-chains,
2-methyl-2-(methyldithio)-propanal (No. 580) and ethyl
2-(methyldithio)propionate (No. 581). The toxicity of these
agents has not been studied, but the Committee considered
these substances by analogy to disulfides (subgroup vii) and
concluded that adequate margins of safety were available,
given their greater polarity and the presence of thiols with
and without oxidized side-chains, i.e. subgroups (iv) and
(v).
(ix) Trisulfides and polysulfides: This subgroup consists
of six trisulfides and one polysulfide. NOELs of 4.8 mg/kg
bw per day for dipropyl trisulfide (No. 585) and 4.6 g/kg bw
per day for diallyl trisulfide (No. 587) were reported,
which gave adequate margins of safety for all substances in
this subgroup.
(x) Heterocyclic disulfides: This subgroup comprises two
flavouring agents that are heterocyclic disulfides. The NOEL
of 1.9 mg/kg bw per day for 3,5-dimethyl-1,2,4-trithiolane
(No. 573) provides an adequate margin of safety for this
substance at current levels of use. 3-Methyl-1,2,4-trithiane
(No. 574) was reported to have no effect at the single dose
of 0.3 mg/kg bw per day tested in a 90-day study; this dose
provides a margin of safety of only 100 at the estimated
modified per capita intake level of 1 µg/kg bw per day in
the United States. However, the NOEL for the closely related
compound 3,5-dimethyl-1,2,4-trithiolane provides an adequate
margin of safety (> 1000).
(xi) Thioesters: This subgroup consists of 15 thioesters.
The NOELs of 6.5 mg/kg bw per day for ethylthioacetate (No.
483) and 1000 mg/kg bw per day for methylthiobutyrate (No.
484) give an adequate margin of safety for all other esters
in this group. The conclusion that the current intake levels
of allyl thiopropionate and prenyl thioacetate are safe is
supported by the NOEL of 4.6 mg/kg bw per day for the
unsaturated diallyl trisulfide (No. 587), which would be
reduced to allyl disulfide and then allyl sulfide.
(xii) Sulfoxides: This subgroup consists of only one
substance, DMSO (No. 507). The NOEL in monkeys given DMSO by
gavage for 74-87 weeks was 3000 mg/kg bw per day. This NOEL
and other data provide an adequate margin of safety for the
use of DMSO as a flavouring agent at the estimated modified
daily per capita intake of 0.001 µg/day in the United
States.
Step B5. Five substances, allyl sulfide (No. 458), methyl phenyl
sulfide (No. 459), benzyl methyl sulfide (No. 460),
2-(methylthio)methyl-2-butenal (No. 470), and
2,8-dithianon-4-ene-carboxaldehyde (No. 471), were evaluated
at this step of the Procedure. The modified daily per
capita intake of all five substances is less than 1.5 µg
in Europe and in the United States. Applying the criteria
for Step B5 outlined in Annex 5 of the evaluations published
after its forty-ninth meeting Annex 1, reference 132), the
Committee concluded that use of these substances at their
current levels of intake poses no safety concern.
In summary, for 100 agents in subgroups (iii) to (xii), a NOEL
was available for the substance, a closely related substance, or a
predicted major metabolite that provided an adequate margin of safety
(> 1000). For 6/9 of the agents in subgroup (i) and 26/28 in subgroup
(ii), a NOEL for the substance or a closely related substance was
available that provided an adequate margin of safety (i.e. > 1000).
Therefore, the Committee determined at Step B4 of the Procedure that
the safety of these 132 substances would be expected to be of no
concern when they are used at their currently estimated level of daily
intake. The evaluation of the remaining five substances (Nos 458, 459,
and 460 in subgroup (i) and Nos 470 and 471 in subgroup (ii))
proceeded to Step B5 of the Procedure. The comparisons of toxicity and
the intake considerations for each subgroup that were used to apply
Steps B4 and B5 of the Procedure to the evaluation of individual
substances in this group of flavouring agents are summarized in Table
3 and given in more detail in Table 1.
1.5 Consideration of combined intakes
In the unlikely event that all 137 aliphatic and aromatic
sulfides and thiols in the overall group or in subgroups were to be
consumed simultaneously on a daily basis, the estimated combined
intake would not exceed the human intake threshold for class I
substances. The powerful aroma of these substances limits the level of
their use in foods.
1.6 Conclusions
The Committee concluded that the 137 flavouring agents comprising
aromatic sulfides and thiols evaluated at the present meeting could
not be predicted to be metabolized to innocuous products. According to
the Procedure, data on toxicity were needed to evaluate the safety of
this group of flavouring agents. The primary data that were used in
the evaluations consisted of 27 90-day studies in rodents with 25 of
the substances and long-term studies in multiple species for one
substance (DMSO). Most of these studies were conducted at a single
dose or multiple doses, which had no effects. The Committee noted that
the NOELs were thus derived from studies in which no toxic effects
were seen.
On the basis of the available data on the toxicity of
representative substances in each subgroup and on their metabolism,
the Committee determined that the safety of 132 of the flavouring
agents poses no concern when they are consumed at their current levels
of intake. The safety of the remaining five substances was considered
to pose no concern when they are consumed at levels of intake < 1.5
µg/day.
Other data on toxicity, including the results of short-term tests
for toxicity and studies of teratogenicity and genotoxicity, were
consistent with the results of the safety evaluation.
2. RELEVANT BACKGROUND INFORMATION
2.1 Explanation
The 137 compounds evaluated have a diverse array of chemical
structures, each of which contains a sulfur atom attached to a carbon,
hydrogen, oxygen, or second sulfur atom. The substances were separated
into 12 subgroups on the basis of the position of the sulfur atom, as
indicated in section 1.3 above. The key data on metabolism and
toxicity relevant to the safety evaluation are presented below by
subgroup. Information on intake, special studies, and observations in
humans are also discussed.
2.2 Additional considerations on intake
Thiols (i.e. mercaptans), thioethers, and their oxygenated
derivatives are known for their powerful aromas. They provide cooked,
browned, and roasted flavours when added as flavouring substances.
Their strong organoleptic properties make a significant contribution
to the taste and smell of various foods even when they are added at
low concentrations. The thresholds of odour detection for aromatic and
aliphatic thiols and thioethers range from 0.08 µg/m3 for amyl
mercaptan (not in any subgroup) to 0.03 mg/m3 for benzyl mercaptan
(No. 526); the odour threshold for methyl sulfide (No. 452), the
thioether with the highest annual volume of use as a flavouring
substance, is about 1 µg/m3, and that for the corresponding thiol,
methyl mercaptan (No. 508), is 2 µg/m3 (Farr & Kirwin, 1994). In
tests for sensory tolerance, most of the panelists reported that
atmospheres containing thiols, thioesters, or thioethers at
concentrations > 1 mg/m3 are intolerable (Flavor and Extract
Manufacturers' Association, 1996).
A direct result of the extremely low odour threshold of thiols
and thioethers is that their use in food is self-limiting, because
high concentrations would result in foods that are repulsive. The
levels of use in the vast majority of food categories are much less
than 1 mg/m3. In foods in which substantial evaporation of the sulfur
derivative occurs during processing, such as hard candies and baked
goods, the concentration is usually < 10 mg/m3. The low levels of
use in food are reflected in the low reported annual volumes of use of
thiols and thioethers as flavouring substances.
2.3 Biological data
2.3.1 Absorption, distribution, metabolism, and excretion
Simple, non-polar thioether, disulfide, and thiol flavouring
substances would be absorbed rapidly from the gastrointestinal tract
intact and excreted in urine, probably as metabolites, and in some
cases in the expired air as the parent compound. Essentially complete
absorption has been reported for methyl sulfide (No. 452) (Williams et
al., 1966) and dipropyl sulfide (Nickson & Mitchell, 1994). The
presence of other functional groups, such as an alcohol (e.g. Nos
461-463), aldehyde (e.g. Nos 465-471, 505), ester (e.g. Nos 472-481
and 492-494), acid (e.g. No. 501), alpha-ketone (or thioester) (e.g.
Nos 482-494, 504, and 506), beta-ketone (e.g. Nos 495-500, and 502),
or phenol (e.g. No. 503), provides a centre of greater polarity which
could slow the rate of absorption and would provide other sites for
oxidative metabolism (see below). The metabolism of sulfur centres is
shown in Figure 1. All the sulfur compounds considered at this meeting
are of low relative molecular mass and of sufficient lipophilicity to
be absorbed from the intestine. Some functional groups such as
aldehydes, esters, and thioesters probably undergo presystemic
metabolism in the gut lumen, gut wall, or liver.
(i) Simple sulfides (thioethers)
Thioethers are commonly called 'sulfides', as if the hydrogen
atoms of hydrogen sulfide were replaced by alkyl or aryl substituents.
Flavours are usually given such trivial names, which are simplified
even further when the two substituents are the same; for instance,
dimethyl thioether is called 'methyl sulfide'. The presence of a lone
pair of electrons on a divalent sulfur allows rapid oxidation of the
sulfide to a sulfoxide, which occurs both as part of metabolism and
also before ingestion or absorption under aerobic conditions. For
example, allyl sulfide (No. 458), a volatile constituent of onions and
garlic, is oxidized rapidly in air to the corresponding sulfoxide,
which is partly respon-sible for the lachrymatory effects of these
foods (Brodnitz & Pascale, 1971).
In many natural and pharmaceutical compounds, one or more oxygen
atoms is linked to the sulfur centre of an organic molecule, and the
chemical and biological properties of the compound depend on the
nature of the substituents on the sulfur and oxygen atoms. The
oxidation state of sulfur is of major importance in determining the
polarity of the compound and hence its interaction with biological
systems. Once alkyl and aromatic sulfides enter the systemic
circulation, they are oxidized rapidly to sulfoxides and, depending on
the structure of the sulfide, may be further oxidized to the sulfone.
Aliphatic sulfides generally yield mixtures of sulfone and sulfoxide
metabolites; the relative amounts excreted depend on the polarity of
the sulfide (Damani, 1987). Methyl sulfide (No. 452) administered to
rabbits is excreted in the urine as DMSO and dimethyl sulfone
(Williams et al., 1966), whereas the sulfone is the main urinary
metabolite of diethyl sulfide (No. 454) (Hoodi & Damani, 1984).
Dipropyl sulfide, a volatile component of onions and garlic, is
metabolized mainly to the corresponding sulfoxide with small amounts
of the sulfone and trace amounts of inorganic sulfate. Individual
studies on the sulfoxide and sulfone indicate that these metabolites
are relatively stable under physiological conditions (Nickson &
Mitchell, 1994; Nickson et al., 1995). Male Wistar rats eliminated 93%
of a single oral dose of [35S]-dipropyl sulfide over three days, with
66% in the urine and lesser amounts in exhaled air (18%), faeces
(4.6%), and the carcass (1.5%). The sulfoxide was the only compound
detected in plasma. About 25% of the radiolabel was recovered in urine
on day 1 and 39% on day 2, and approximately 25% of the radiolabel was
eliminated in bile over 48 h as the sulfoxide (20%) and sulfone (5%).
Enterohepatic recirculation was probably responsible for the delayed
urinary excretion and the plasma profiles, which showed a slow
increase with peaks at 12-15 h. Urinary metabolites collected during
the first 24 h included the sulfoxide (92%), sulfone (5%), and sulfate
(3%); on days 2 and 3, the sulfoxide accounted for > 98% of daily
urinary metabolites (Nickson & Mitchell, 1994). Methyl phenyl sulfide
administered per se orally to rats or produced by methylation of
benzenethiol (No. 525) was eliminated in the urine as methyl phenyl
sulfone and hydroxylated sulfones (McBain & Menn, 1969).
The major enzyme systems involved in xenobiotic oxidation are the
cytochrome P450 superfamily (CYP-450) (Souhaili-El Amri et al., 1987)
and the flavin-containing monooxygenases (FMO) (Levi & Hodgson, 1988;
Ziegler, 1989; Hodgson & Levi, 1992). The oxidation of thioethers to
sulfoxides is catalysed by these two enzyme systems (Renwick, 1989).
Simple aliphatic (e.g. diethyl sulfide), alicyclic (e.g. thiolane),
and aromatic (e.g. ethyl para-tolyl sulfide) sulfides are oxidized
primarily by FMO and to a lesser extent, by CYP-450. The oxidation of
diethyl sulfide and thiolane in rats is catalysed almost exclusively
by FMO (Hoodi & Damani, 1984; Damani, 1987). 4-Chlorophenyl methyl
sulfide was oxidized by FMO and CYP-450 to the sulfoxide and sulfone
derivatives in vitro (Nnane & Damani, 1995), and diphenyl sulfoxide
was oxidized to the corresponding sulfone in perfused guinea-pig liver
(Yoshihara & Tatsumi, 1990).
Enzyme-catalysed thioether oxidation may be stereoselective, FMO
and CYP-450 selecting different enantiomers (Cashman & Williams,
1990). The stereochemistry of sulfoxidation by the isoenzymes of FMO
has been studied in humans (Rettie et al., 1994). When methyl
para-tolyl sulfide was incubated with human fetal liver and human
kidney microsomes in which CYP-450 activity had been eliminated, the
resulting sulfoxide showed an enantiomeric excess (> 86%) of the
N/R, not relevant to the evaluation; ND, no data reported; bw, body weight
a None of the substances in this group are predicted to be metabolized to innocuous products. They were placed in subgroups
(i)-(xii) on the basis of the position of the sulfur atom.
b The thresholds for human intake are 1800 µg/day for class I, 540 µg/day for class II, and 90 µg/day for class III.
Table 2. Annual volume and intake of aliphatic and aromatic sulfides and thiols used as
flavouring substances in Europe and the United States
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
Methyl sulfide (452)
Europe 3100 590 10
United States 2800 530 9 57 000
Methyl ethyl sulfide (453)
Europe NR NA NA
United States (A) 9 2 0.03 +
Diethyl sulfide (454)
Europe NR NA NA
United States (A) 68 13 0.22 +
Butyl sulfide (455)
Europe 19 4 0.1
United States 0.5 0.1 0.002 +
1,4-Dithiane (456)
Europe NR NA NA
United States (A) 0.45 0.1 0.001 +
(1-Butenyl-1) methyl sulfide (457)
Europe NR NA NA
United States (A) 0.45 0.1 0.001 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
Allyl sulfide (458)
Europe NR NA NA
United States 2 0.4 0.01 +
Methyl phenyl sulfide (459)
Europe NR NA NA
United States (A) 2 0.4 0.01 +
Benzyl methyl sulfide (460)
Europe 1.1 0.2 0.003
United States (1982) 0.1 0.02 0.0003 +
3-(Methylthio)propanol (461)
Europe 23 4 0.1
United States 4 1 0.01 +
4-(Methylthio)butanol (462)
Europe 0.1 0.02 0.0003
United States 0.5 0.1 0.002 +
3-(Methylthio)-1-hexanol (463)
Europe 26 5 0.1
United States 0.5 0.1 0.002 +
2-Methyl-4-propyl-1,3-oxathiane (464)
Europe 11 2 0.03
United States (A) 5 1 0.02 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
2-Methylthioacetaldehyde (465)
Europe NR NA NA
United States (1970) 3 1 0.01 +
3-(Methylthio)propionaldehyde (466)
Europe 230 45 1
United States 130 25 0.4 637
3-(Methylthio)butanal (467)
Europe 0.7 0.1 0.002
United States 0.5 0.1 0.002 +
4-(Methylthio)butanal (468)
Europe NR NA NA
United States 0.1 0.02 0.0003 -
3-Methylthiohexanal (469)
Europe NR NA NA
United States (A) 4.5 1 0.01 +
2-(Methylthio)methyl-2-butenal (470)
Europe 0.2 0.04 0.001
United States (1982) 0.5 0.1 0.002 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
2,8-Dithianon-4-en-4-carboxaldehyde (471)
Europe 0.1 0.01 0.0002
United States 0.5 0.0: 0.002 +
Methyl 3-methylthiopropionate (472)
Europe 770 146 2
United States 46 9 0.1 5
Methylthiomethyl butyrate (473)
Europe NR NA NA
United States (A) 0.45 0.1 0.001 +
Methyl 4-(methylthio)butyrate (474)
Europe 0.5 0.1 0.002
United States 0.5 0.1 0.002 -
Ethyl 2-(methylthio)acetate (475)
Europe NR NA NA
United States (A) 0.45 0.1 0.001 +
Ethyl 3-methylthiopropionate (476)
Europe 200 37 1
United States 9 2 0.03 9
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
Ethyl 4-(methylthio)butyrate (477)
Europe NR NA NA
United States (A) 11 2 0.03 -
3-(Methylthio)propyl acetate (478)
Europe NR NA NA
United States (A) 59 11 0.2 +
Methylthiomethyl hexanoate (479)
Europe NR NA NA
United States (A) 0.45 0.1 0.001 +
Ethyl 3-(methylthio)butyrate (480)
Europe NR NA NA
United States (A) 0.45 0.1 0.001 +
3-(Methylthio)hexyl acetate (481)
Europe 0.4 0.1 0.001
United States (A) 45 9 0.1 +
S-Methyl thioacetate (482)
Europe NR NA NA
United States (A) 0.01 0.002 0.00003 2
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
Ethyl thioacetate (483)
Europe 0.1 0.02 0.0003
United States 0.1 0.02 0.0003 56
Methyl thiobutyrate (484)
Europe 24 5 0.1
United States 27 5 0.1 +
Propyl thioacetate (485)
Europe 2.2 0.4 0.01
United States 0.1 0.02 0.0003 +
Methyl 2-methylthiobutyrate (486)
Europe 0.8 0.2 0.003
United States 0.5 0.01 0.002 +
S-Methyl 3-methylbutanethioate (487)
Europe NR NA NA
United States (A) 1 0.19 0.003 +
S-Methyl 4-methylpentanethioate (488)
Europe NR NA NA
United States (A) 0.0045 0.001 0.00001 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
S-Methyl hexanethioate (489)
Europe NR NA NA
United States (A) 0.45 0.1 0.001 3
Allyl thiopropionate (490)
Europe NR NA NA
United States 0.5 0.1 0.002 -
Prenyl thioacetate (491)
Europe NR NA NA
United States (A) 1 0.19 0.003 +
Methyl 2-(acetyloxy) propionate (492)
Europe NR NA NA
United States (A) 45 9 0.1 -
Methylthio 2-(propionyloxy) propionate (493)
Europe NR NA NA
United States (A) 45 9 0.1 -
3-Acetylmercaptohexyl acetate (494)
Europe NR NA NA
United States (A) 2.2 0.4 0.01 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
1-Methylthio-2-propanone (495)
Europe NR NA NA
United States (A) 1 0.2 0.003 -
1-(Methylthio)-2-butanone (496)
Europe 0.03 0.01 0.0001
United States (1982) 0.1 0.02 0.0003 +
4-(Methylthio)-2-butanone (497)
Europe 0.1 0.02 0.0003
United States 2 0.4 0.01 +
4,5-Dihydro-3(2H)-thiophenone (498)
Europe 3.6 1 0.01
United States 13 2 0.04 +
2-Methyltetrahydrothiophen-3-one (499)
Europe 100 19 0.3
United States (A) 0.5 0.1 0.002 5036
4-(Methylthio)-4-methyl-2-pentanone (500)
Europe 0.2 0.04 0.0006
United States 0.5 0.1 0.002 -
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
4-(Methylthio)-2-oxobutanoic acid, sodium salt (501)
Europe NR NA NA
United States (A) 1 0.2 0.003 +
Di(butan-3-one-1-yl) sulfide (502)
Europe NR NA NA
United States (1982) 0.1 0.02 0.0003 -
ortho-(Methylthio)phenol (503)
Europe 5 1 0.02
United States (1970) 5 1 0.02 +
S-Methyl benzothioate (504)
Europe NR NA NA
United States (A) 0.0045 0.001 0.00001 +
2-(Methylthiomethyl)-3-phenylpropenal (505)
Europe NR NA NA
United States 10 2 0.03 -
cis- and trans-Menthone-8-thioacetate (506)
Europe NR NA NA
United States (A) 2.3 0.4 0.01 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
Methylsulfinylmethane (507)
Europe NR NA NA
United States (A) 0.0045 0.001 0.00001 45 000
Methyl mercaptan (508)
Europe 440 83 1
United States 0.9 0.2 0.003 +
Propanethiol (509)
Europe 18 3 0.1
United States 38 7 0.1 360
2-Propanethiol (510)
Europe NR NA NA
United States (A) 0.022 0.004 0.0001 +
1-Butanethiol (511)
Europe 3.2 0.5 0.01
United States 0.2 0.04 0.001 +
2-Methyl-1-propanethiol (512)
Europe NR NA NA
United States (A) 6.8 1.3 0.021 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
3-Methylbutanethiol (513)
Europe NR NA NA
United States (A) 0.045 0.01 0.0001 23
2-Pentanethiol (514)
Europe 12 2 0.04
United States (A) 11 2 0.03 +
2-Methyl-1-butanethiol (515)
Europe 2.5 0.5 0.01
United States (1982) 0.1 0.02 0.0003 +
Cyclopentanethiol (516)
Europe NR NA NA
United States (A) 4.5 1 0.01 -
3-Methyl-2-butanethiol (517)
Europe 0.1 0.02 0.0003
United States 0.1 0.02 0.0003 +
1-Hexanethiol (518)
Europe NR NA NA
United States (A) 0.045 0.01 0.0001 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
2-Ethylhexanethiol (519)
Europe NR NA NA
United States (A) 0.045 0.01 0.0001 +
2,3, or 10-Mercatopinane (520)
Europe 0.3 0.1 0.001
United States 50 10 0.2 -
Allyl mercaptan (521)
Europe 1.3 0.2 0.003
United States 8 2 0.03 480
Prenythiol (522)
Europe NR NA NA
United States (A) 1 0.2 0.003 +
1-para-Menthene-8-thiol (523)
Europe 2.8 1 0.01
United States (A) 4.5 1 0.01 +
Thiogeraniol (524)
Europe 9 2 0.03
United States 0.1 0.02 0.0003 -
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
Benzenethiol (525)
Europe 6 1 0.02
United States 160 30 1 +
Benzyl mercaptan (526)
Europe 10 2 0.03
United States 2 0.4 0.01 +
Phenethyl mercaptan (527)
Europe NR NA NA
United States (A) 1 0.2 0.003 +
ortho-Toluenethiol (528)
Europe 140 27 0.4
United States 0.9 0.2 0.003 +
2-Ethylthiophenol (529)
Europe 0.001 0.0002 0.0
United States 0.5 0.10 0.002 -
2,6-Dimethylthiophenol (530)
Europe 11 2 0.03
United States 0.1 0.02 0.0003 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
2-Naphthalenethiol (531)
Europe NR NA NA
United States (A) 0.34 0.1 0.001 +
1,2-Ethanedithiol (532)
Europe 0.01 0.002 0.00003
United States (A) 4.5 0.9 0.01 +
Bis(methylthio)methane (533)
Europe NR NA NA
United States (A) 500 94 2 +
2-Methyl-1,3-dithiolane (534)
Europe 0.5 0.1 0.002
United States (A) 23 4 0.1 +
1,3-Propanedithiol (535)
Europe 7 1 0.02
United States (A) 4.5 0.9 0.01 +
1,2-Propanedithiol (536)
Europe NR NA NA
United States (A) 4.5 0.9 0.01 -
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
1,2-Butanedithiol (537)
Europe NR NA NA
United States 0.9 0.2 0.003 -
1,3-Butanedithiol (538)
Europe NR NA NA
United States (A) 4.5 0.9 0.01 -
2,3-Butanedithiol (539)
Europe 0.4 0.1 0.001
United States 0.9 0.2 0.003 -
1,6-Hexanedithiol (540)
Europe 13 2.5 0.04
United States 0.5 0.1 0.002 +
1,8-Octanedithiol (541)
Europe 17 3 0.1
United States (A) 4.5 0.9 0.01 -
1,9-Nonanedithiol (542)
Europe 0.01 0.002 0.00003
United States (A) 4.5 0.9 0.01 -
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
Trithioacetone (543)
Europe 12 2 0.04
United States 2 0.4 0.01 +
3-Mercapto-3-methyl-1-butanol (544)
Europe NR NA NA
United States (A) 10 1.9 0.03 +
3-Mercaptohexanol (545)
Europe NR NA NA
United States (A) 4.5 1 0.01 +
2-Mercapto-3-butanol (546)
Europe 33 6 0.1
United States 0.5 0.1 0.002 -
alpha-Methyl-ß-hydroxypropyl alpha-methyl-ß-mercaptopropyl sulfide (547)
Europe NR NA NA
United States 4 1 0.01 -
4-Methyoxy-2-methyl-2-butanethiol (548)
Europe NR NA NA
United States (A) 4 0.8 0.01 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
3-Mercapto-3-methylbutyl formate (549)
Europe NR NA NA
United States (A) 0.5 0.1 0.002 +
2,5-Dihydroxy-1,4-dithiane (550)
Europe NR NA NA
United States (A) 0.45 0.1 0.001 -
2-Mercaptopropionic acid (551)
Europe 17 3 0.1
United States 440 84 1 -
Ethyl 2-mercaptopropionate (552)
Europe 3.2 0.5 0.01
United States 2 0.4 0.01 +
Ethyl 3-mercaptopropionate (553)
Europe 0.6 0.1 0.002
United States (A) 230 43 1 +
3-Mercaptohexyl acetate (554)
Europe NR NA NA
United States (A) 1 0.2 0.003 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
3-Mercaptohexyl butyrate (555)
Europe NR NA NA
United States (A) 1 0.2 0.003 +
3-Mercaptohexyl hexanoate (556)
Europe NR NA NA
United States (A) 1 0.2 0.003 +
1-Mercapto-2-propanone (557)
Europe NR NA NA
United States (A) 0.45 0.09 0.001 +
3-Mercapto-2-butanone (558)
Europe 26 5 0.1
United States (1982) 0.2 0.04 0.001 +
2-Keto-4-butanethiol (559)
Europe NR NA NA
United States 0.5 0.1 0.002 -
3-Mercapto-2-pentanone (560)
Europe NR NA NA
United States 0.5 0.1 0.002 -
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
para-Mentha-8-thiol-3-one (561)
Europe 86 16 0.3
United States 9 2 0.03 +
2,5-Dimethyl-2,5-dihydroxy-1,4-dithiane (562)
Europe 1.2 0.2 0.004
United States 0.9 0.2 0.003 -
Sodium 3-mercaptooxopropionate (563)
Europe NR NA NA
United States (A) 1 0.2 0.003 -
Dimethyl disulfide (564)
Europe 57 11 0.2
United States 13 2 0.04 1 400
Methyl propyl disulfide (565)
Europe 32 6 0.10
United States 0.1 0.02 0.0003 13 000
Propyl disulfide (566)
Europe 28 5 0.1
United States 0.5 0.1 0.002 14 000
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
Diisopropyl disulfide (567)
Europe NR NA NA
United States (A) 41 8 0.1 +
Allyl methyl disulfide (568)
Europe 0.01 0.002 0.00003
United States (1982) 0.1 0.02 0.0003 +
Methyl 1-propenyl disulfide (569)
Europe NR NA NA
United States (1982) 4 1 0.01 1
Propenyl propyl disulfide (570)
Europe NR NA NA
United States (1970) 40 8 0.1 +
Methyl 3-methyl-1-butenyl disulfide (571)
Europe NR NA NA
United States (A) 0.45 0.1 0.001 +
Allyl disulfide (572)
Europe 480 92 2
United States 44 8 0.1 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
3,5-Dimethyl-1,2,4-trithiolane (573)
Europe 0.2 0.04 0.001
United States (1982) 0.5 0.0: 0.002 +
3-Methyl-1,2,4-trithiane (574)
Europe 0.6 0.1 0.002
United States (A) 230 43 1 +
Dicyclohexyl disulfide (575)
Europe 0.1 0.02 0.0003
United States 0.9 0.2 0.003 -
Methyl phenyl disulfide (576)
Europe NR NA NA
United States (A) 1 0.2 0.003 +
Methyl benzyl disulfide (577)
Europe 0.1 0.02 0.0003
United States 0.5 0.1 0.002 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
Phenyl disulfide (578)
Europe NR NA NA
United States (1982) 0.2 0.04 0.001 -
Benzyl disulfide (579)
Europe 0.1 0.01 0.0002
United States (1982) 0.5 0.1 0.002 +
2-Methyl-2-(methyldithio) propanal (580)
Europe NR NA NA
United States (A) 9 2 0.03 +
Ethyl 2-(methyldithio)propionate (581)
Europe NR NA NA
United States (A) 0.45 0.1 0.001 +
Dimethyl trisulfide (582)
Europe 9 2 0.03
United States 0.1 0.02 0.0003 3 000
Methyl ethyl trisulfide (583)
Europe NR NA NA
United States (A) 3 1 0.01 +
Table 2. (cont'd)
Substance (No.) Most recent Intakeb Annual volume in
annual volume naturally
(kg)a µg/day µg/kg bw occurring
per day foods (kg)c
Methyl propyl trisulfide (584)
Europe 1.7 0.3 0.01
United States 0.4 0.1 0.001 13 000
Dipropyl trisulfide (585)
Europe 60 11 0.2
United States 5 1 0.02 16 000
Allyl methyl trisulfide (586)
Europe NR NA NA
United States (A) 4.5 0.9 0.01 +
Diallyl trisulfide (587)
Europe 29 6 0.1
United States (1970) 0.1 0.02 0.0003 +
Diallyl polysulfide (588)
Europe 10 2 0.03
United States 0.1 0.02 0.0003 -
TOTAL
Europe 6 100
United States 5 300
A, volume anticipated on the basis of recent estimates (communication from the Flavor and Extract
Manufacturers' Association to the Committee); NR, not reported; NA, not applicable; +, reported
to occur naturally in foods (CIVO-TNO, 1994), but quantitative data were not available; -, not
reported to occur naturally in foods
a Volumes reported in a survey in Europe (International Organization of the Flavor
Industry, 1995) and in a survey in the United States (National Academy of Sciences, 1989).
Most of the data for the United States are for 1987 (and there is no entry); when data
for 1987 were not available, data for 1982 or 1970 were used, as indicated.
b Per capita intake (mg/day) calculated as follows:
[(annual usage, kg) × (1 × 109 mg/kg) / (population × 0.6 × 365 days)], where population
(10% 'eaters only') = 32 × 106 for Europe and 24 × 106 for the USA; 0.6 represents the
assumption that only 60% of the annual usage of the flavour was reported in the survey.
Intake (mg/kg bw per day) calculated as follows: [(mg/day)/body weight], where body weight = 60 kg.
c From Stofberg & Kirschman (1985); Stofberg & Grundschober (1987)
Potential toxicity can be deduced by comparison with structural
analogues on the basis of metabolic similarities. In the absence of
information on the toxicity of structural analogues, however, it is
not possible to conclude a priori that the substances are
metabolized to innocuous products.
(i) Simple sulfides (thioethers)
Once alkyl and aromatic thioethers, commonly called 'sulfides',
enter the systemic circulation, they are rapidly oxidized to
sulfoxides and, depending on the structure of the thioether, may be
further oxidized to sulfones. Sulfoxides and sulfones are major
urinary metabolites of simple sulfides. Aliphatic thioethers (Nos
452-455, 457, 458, and 533) and thioethers containing an aromatic
nucleus (Nos 459 and 460) yield mixtures of sulfoxide and sulfone
metabolites. Enzymes of the cytochrome P450 superfamily and
flavin-containing monooxygenases catalyse the oxidation of thioethers
to sulfoxides. Oxidation of sulfoxides to the corresponding sulfones
occurs both in tissues and in aerobic microorganisms and is an
irreversible metabolic reaction in mammals. Sulfoxides can also be
metabolized back to the thioether by aldehyde oxidase, thioredoxin and
its reductase, and the gut microflora in the anaerobic environment of
the lower bowel.
The methyl aromatic thioethers (Nos 459 and 460) are predicted to
be major metabolites of the corresponding aromatic thiols (Nos 525 and
526, subgroup iv) and would be oxidized to sulfoxides and sulfones,
which would be excreted.
(ii) Acyclic sulfides with oxidized side-chains
The presence of other functional groups, such as alcohol (Nos
461-463), aldehyde (Nos 465-471 and 505), ester (Nos 472-481), acid
(No. 501), ß-ketone (Nos 495-497, 500, and 502), and phenol (No. 503),
provides centres of greater polarity and additional sites for the
biotransformation of thioethers. The presence of these polar groups
would also result in increased renal excretion. The biotransformations
of such oxygenated, carbon-containing, functional groups are well
characterized and have been described for groups of flavouring agents
previously evaluated by the Committee. Concurrent metabolism of
various substrates at both sulfur and oxygenated functional groups has
been reported, and sulfoxide formation usually predominates as the
major metabolic pathway of detoxification. Experiments in vitro
suggest that hydrolysis of carboxyl esters occurs in the presence of
thioether (sulfide) groups. In consequence, thioethers with oxidized
side-chains would be expected to be eliminated more rapidly than
simple sulfides.
(iii) Cyclic sulfides
Oxidation of unsubstituted and methyl-substituted cyclic
thioethers by the cytochrome P450 superfamily produces the
corresponding sulfoxides. The mono-sulfoxides are predicted to be the
main urinary metabolites of simple cyclic sulfides (Nos 456, 534, and
543). The metabolism of cyclic sulfides containing oxidized carbon
atoms (Nos 464, 498, 499, 550, and 562) has not been studied but would
be predicted to involve extensive S-oxidation and possibly oxidation
or conjugation of alcohol groups. The polarity of the hydroxy
thioethers (Nos 550 and 562) may allow their elimination unchanged.
(iv) Simple thiols
The simple thiol flavouring agents considered are alkyl and
alicyclic thiols (Nos 508-524, 526, and 527) and aromatic thiols
(thiophenols; Nos 525 and 528-531). These substances may be
metabolized along several pathways. Simple aliphatic and aromatic
thiols undergo S-methylation in mammals to produce the corresponding
methyl thioether or sulfide. Methylation is catalysed by thiopurine
methyltransferase in the cytoplasm and thiol methyltransferase in
microsomes, and both reactions require S-adenosyl-l-methionine as a
methyl group donor. Thiopurine methyltransferase is present in human
liver, kidney, and erythrocytes; preferential substrates for this
enzyme include aromatic and heterocyclic thiols. S-Methylation of
aliphatic thiols is catalysed by microsomal thiol methyltransferase,
and the resulting methyl thioether (sulfide) metabolite would undergo
S-oxidation to give the methyl sulfoxide and methyl sulfone
analogues as urinary products.
Thiols may react with glutathione and other endogenous thiol
substances to form mixed disulfides. Both microsomal and cytoplasmlic
thioltransferases have been reported to catalyse the formation of
mixed disulfides. The resulting mixed disulfides can undergo reduction
back to thiols, oxidative desulfuration, or oxidation to a sulfonic
acid via the intermediate thiosulfinate and sulfinic acids. The
principal form in the circulation would probably be a mixed disulfide
formed with albumin.
S-Glucuronidation of aromatic thiols has been reported, and
this may be a pathway for the metabolism of aromatic thiols
(thiophenols) (Nos 525 and 528-531) and simple aromatic disulfides
(Nos 576 and 578; subgroup vii) after their reduction (see below).
Glucuronyl transferases behave similarly towards hydroxyl and
sulfydryl groups, and the two activities have the same subcellular
location and optimal pH.
Thiols may be oxidized to form sulfenic acids (RSOH), which are
unstable and readily undergo further oxidation to sulfinic (RSO2H)
and sulfonic (RSO3H) acids or combine with nucleophiles. The sulfonic
acid group is a highly polar centre and makes molelcules highly
soluble in water. In general, sulfonic acids are stable to metabolism.
Alkyl thiols of low relative molecular mass undergo oxidative
desulfuration in vivo to yield CO2 and SO4=. This reaction has
been shown, for example, for methanethiol (methyl mercaptan; No. 508).
Whereas the carbon atom from thiols may be used in the biosynthesis of
amino acids, the sulfur atom is not used significantly in the
synthesis of sulfur-containing amino acids.
(v) Thiols with oxidized side chains
Although alkyl thiols with oxidized side-chains (Nos 544-549,
551-561, and 563) comprise a significant proportion of the flavouring
agents evaluated, their metabolic fate has not been studied. Their
metabolism is predicted to involve a combination of the pathways
described above for simple thiols and further oxidation or conjugation
of the oxidized side-chain. The class III compound, sodium
3-mercapto-oxopropionate (No. 563), would be expected to be eliminated
very rapidly by metabolism at both the thiol and keto-acid groups.
(vi) Dithiols
The metabolism of the simple aliphatic dithiols evaluated (Nos
532 and 535-542) is predicted to involve the pathways described above
for simple thiols. Urinary metabolites could result from methylation,
S-oxidation of one S atom to yield a polar sulfonate, and the
formation of mixed disulfides of low relative molecular mass such as
cysteine, an endogenous thiol. The longer, linear dithiols (Nos 535
and 540-542) could form intramolecular disulfide bonds, with
interconversion between dithiol and cyclic disulfide forms.
(vii) Simple disulfides
The reduction of xenobiotic disulfides is believed to be
extensive, and the reaction may be catalysed enzymatically by
thioltransferases and chemically by exchange with glutathione,
thioredoxin, cysteine, and other endogenous thiols. Reduction of the
non-cyclic disulfides considered in the group of flavours (Nos 564-572
and 575-579) would result in the formation of thiols of low relative
molecular mass, which would then be metabolized by the various
pathways described above for simple thiols.
(viii) Disulfides with oxidized side-chains
As discussed above for subgroups (ii) and (iii), additional sites
of carbon oxidation would result in greater polarity and further
oxidation or conjugation of the flavouring agents evaluated (Nos 580
and 581). However, as disulfide bonds in substances in subgroup (v)
are susceptible to reductive cleavage, this would be expected to be
the initial metabolic reaction, and the polarity of the side-chains
would primarily affect elimination of the thiol fragments.
(ix) Trisulfides and polysulfides
The trisulfide of glutathione is labile and readily converted to
the disulfide, the sulfur being released as hydrogen sulfide.
Trisulfides and polysulfides are predicted to be converted rapidly to
the corresponding disulfides, which would then be reduced to thiols,
which themselves would follow the pathways described above for thiols.
The potential toxicity of trisulfides (Nos 582-587) and polysulfides
(No. 588) is probably related to their metabolic lability and to the
nature of the eventual thiol (e.g. allyl thiol).
(x) Heterocyclic disulfides: The heterocyclic disulfides (Nos 573 and
574) are five-and six-carbon rings which also contain a cyclic
thioether bond. Lipoic acid, an endogenous substance, is a
five-membered cyclic disulfide which undergoes rapid redox cycling
between ring disulfide and open dithiol forms. The principal metabolic
pathways are predicted to be disulfide reduction with ring opening to
produce a dithiol, and S-oxidation of the cyclic thioether.
(xi) Thioesters
Thioester groups (-S-CO-) are present in a number of the
flavouring agents evaluated (Nos 482-494, 504, and 506). Hydrolysis of
esters has been considered previously by the Committee, but that of
thioesters has not. Thioesters are hydrolysed by lipase and esterases,
and the rate of hydrolysis increases as the length of the C-chain of
the carboxylic acid fragment increases, and decreases as oxygenation
of the carbon chain in the thiol moiety increases.
Hydrolysis could result in a thioic acid and an alcohol or a
carboxylic acid and a thiol (the metabolic fates of which have been
outlined above). Data for dithioic acids and esters indicate that the
esters of monothioic acids would be poor substrates for oxidation, but
the monothioic acid released by hydrolysis would be oxidized to the
dioxo acid. Other possibilities for elimination in vivo include renal
excretion of thiocarboxylic acid. The substances evaluated (with the
exceptions of prenyl thioacetate (No. 491) and allyl thiopropionate
(No. 490)) are simple linear alkyl compounds, branched-chain alkyl
compounds, or their side-chain hydroxyester analogues, so that
comparison of the toxicity of the different substances is reasonable.
(xii) Sulfoxides
The metabolism of sulfoxides by oxidation and reduction is
described above under 'Thioethers'. The only sulfoxide flavouring
agent evaluated was methylsulfinylmethane (dimethyl sulfoxide, DMSO;
No. 507), for which data were available on both metabolism and
toxicity in experimental animals and humans. DMSO is readily absorbed
and excreted in urine as the parent sulfoxide and dimethyl sulfone.
1.4 Application of the Procedure for the Safety
Evaluation of Flavouring Agents
Step 1. In applying the Procedure for the Safety Evaluation of
Flavouring Agents to the above-mentioned aliphatic and
aromatic sulfides and thiols, the Committee assigned 97 of
the 137 substances (Nos 452-455, 457, 461-463, 465-497, 500,
502, 508-515, 517-519, 522, 524, 532, 533, 535-542, 544-560,
562, 564-567, 569-571, and 580-585), which may or may not
contain an additional oxygenated functional group, to
structural class I. The Committee assigned 34 of the 137
substances to structural class II because they are aromatic
sulfides or thiols (Nos 459, 460, 503, 504, 525-528, 530,
531, 576, 577, and 579), alicyclic (Nos 506, 516, 520, 523,
561, and 575) or heterocyclic substances (Nos 456, 464, 498,
499, 534, 543, 573, and 574), or allyl mercaptan or sulfides
(Nos 458, 521, 568, 572, and 586-588), which are common
components of food. The aromatic thiols or thioethers that
are not common compo-nents of food (Nos 505, 529, and 578)
were assigned to structural class III. The remaining three
substances were also assigned to structural class III by
virtue of the fact that they are aliphatic thiols or
thioethers containing more than three functional groups (Nos
501 and 563) or do not contain divalent sulfur (No. 507).
Step 2. None of the substances in this group can be predicted to be
metabolized to innocuous products. The evaluation of these
substan-ces therefore proceeded via the right-hand side of
the decision tree.
Step B3. The estimated daily per capita intake in Europe and in the
United States for each of the substances in the overall
group of 137 flavouring agents is below the threshold for
human intake for the structural class in which the substance
falls. The thresholds are 1800 µg/person per day for class
I, 540 µg/person per day for class II, and 90 µg/person per
day for class III.
Step B4. The Committee considered the results of 90-day studies of
toxicity in rodents for 27 substances in this group of
flavouring agents. The Committee noted that the single or
multiple doses of flavouring agents tested in a number of
studies had no effect in rats and that the NOELs were
consequently derived from studies that did not show toxic
effects. The results of long-term studies in multiple
species were considered for one substance, DMSO (No. 507).
To facilitate comparisons of the toxicity of structurally
related substances, the agents were considered in subgroups
(i)- (xii) on the basis of the position of the sulfur atom
(see Tables 1 and 3). Toxicity was compared within and
across subgroups with no restriction on the basis of
structural class assignment (Step 1).
(i) Simple sulfides (thioethers): This subgroup comprises
nine flavouring agents that are simple thioethers. The NOEL
for methyl sulfide (No. 452) in a 14-week study in rats
treated with multiple doses by gavage was 250 mg/kg bw per
day. This NOEL provides an adequate basis for evaluation of
five structurally and metabolically related substances (Nos
453, 454, 455, 457, and 533); however, the NOEL for methyl
sulfide was considered inappropriate for evaluation of allyl
sulfide (No. 458) and two aromatic thioethers, methyl phenyl
sulfide and benzyl methyl sulfide (Nos 459 and 460,
respectively), and the evaluation of these three substances
therefore proceeded to step B5.
(ii) Acyclic sulfides with oxidized side-chains: This
subgroup comprises 28 flavouring agents that are acyclic
thioethers with oxidized side-chains. The NOEL in a 90-day
study in rats treated with a single dose of
2-(methylthiomethyl)-3-phenylpropenal (No. 505) was 1.4
mg/kg bw per day. This substance is an aromatic compound
with a sulfide group in an unsaturated side-chain; it was
assigned to structural class III, because it is not a common
component of food. Data for methyl sulfide (No. 452) were
also considered relevant for assessing the toxicity of
sulfide substances with oxidized side-chains (Nos 461-463,
465-469, 472-481, 495-497, and 500-503). Although
2-(methylthiomethyl)-3-phenylpropenal (No. 505) is not an
aryl thioether, the safety margin between its NOEL and the
intake of ortho-(methylthio)phenol pentanone (No. 503) was
considered to be adequate. The NOEL for
2-(methylthiomethyl)-3-phenylpropenal (No. 505) does not
provide a high margin of safety (i.e. > 1000) for methyl
3-methylthiopropionate (No. 472) at the current estimated
level of intake; however, the simple side-chain acid and
ester would have little toxic potential, and the NOEL for
methyl sulfide (No. 452) provides an adequate margin of
safety. The NOELs for 2-(methylthio-methyl)-3-phenylpropenal
(No. 505) and methyl sulfide (No. 452, subgroup (i)) were
considered inappropriate for evaluation of the toxicity of
two alpha,beta-unsaturated carbonyls (Nos 470 and 471)
because they are potentially more reactive and toxic, and
the evaluation of these substances therefore proceeded to
step B5.
(iii) Cyclic sulfides: This subgroup comprises eight
substances that are cyclic thioethers. NOELs of 0.44 mg/kg
bw per day for 2-methyl-4-propyl-1,3-oxathiane (No. 464),
9.2 mg/kg bw per day for 4,5-dihydro-3(2 H)-thiophenone
(No. 498), 7 mg/kg bw per day for 2-methyl-1,3-dithiolane
(No. 534), 0.2 mg/kg bw per day for trithioace-tone (No.
543), and 3.1 mg/kg bw per day for
2,5-dimethyl-2,5-dihydroxy-1,4-dithiane (No. 562) were
reported. These values provide an adequate margin of safety
for evaluation of the toxicity of substances No. 456, 499,
and 550.
Table 3. Comparison of the toxicity and intake data used in the safety evaluations of
137 simple aliphatic and aromatic sulfides and thiols, by subgroupa
Subgroup Adequate NOEL Adequate NOEL No adequate NOEL
for substanceb for structurally for substance or
related substanceb related substance,
but intake
< 1.5 µg/dayc
(i) Simple sulfides No. 452 Nos 453-455, Nos 458-460
(thioethers) 457, 533
(ii) Acyclic thioethers No. 505 Nos 461-463, Nos 470, 471
with oxidized 465-469, 472-481,
side-chains 495-497, 500-503
(iii) Cyclic sulfides Nos 464, 498, Nos 456, 499, N/R
534, 543, 562 550
(iv) Thiols Nos 516, 520, Nos 508-515, N/R
528, 530, 531 517-519, 521-527,
529
(v) Thiols with Nos 546, 547, Nos 544, 545, 548, N/R
oxidized 560 549, 551-559, 561,
side-chains 563
(vi) Dithiols Nos 539, 541 Nos 532, 535-538, N/R
540, 542
(vii) Simple disulfides Nos 566, 575, Nos 564, 565, N/R
577 567-572, 576, 578,
579
(viii) Disulfides with None Nos 580, 581 N/R
oxidized
side-chains
(ix) Trisulfides and Nos 585, 587 Nos 582-584, 586, N/R
polysulfides 588
(x) Heterocyclic No. 573 No. 574 N/R
disulfides
Table 3. (continued)
Subgroup Adequate NOEL Adequate NOEL No adequate NOEL
for substanceb for structurally for substance or
related substanceb related substance,
but intake
< 1.5 µg/dayc
(xi) Thioesters Nos 483, 484 Nos 482, 485-494, N/R
504, 506
(xii) Sulfoxides No. 507 N/R N/R
N/R, not relevant to the evaluation
a See Table 1 for further details of the evaluations.
b See Figure 1, step B4 and pp. 121-123 for further information.
c See Figure 1, step B5 and pp. 121-123 for further information.
(iv) Simple thiols: This subgroup comprises 24 flavouring
agents that are simple thiols. NOELs of 0.56 mg/kg bw per
day for cyclopentanethiol (No. 516), 0.06 mg/kg bw per day
for 2,3-and 10-mercaptopinane (No. 520), 0.52 mg/kg bw per
day for ortho-toluenethiol (No. 528), 0.43 mg/kg bw per
day for 2,6-dimethyl-thiophenol (No. 530), and 3.4 mg/kg bw
per day for 2-naphthalenethiol (No. 531) were reported.
These values provide adequate margins of safety for the
individual substances and for the other structurally related
thiols in the group in relation to current estimates of
intake, with the exception of 2,3 and 10-mercaptopinane (No.
520). A margin of safety of about 300 (based on an estimated
modified per capita intake of 0.2 µg/kg bw per day in the
United States) was obtained from the results of a 90-day
study in which only a single dose of 0.06 mg/kg bw per day
was tested. This substance was therefore also evaluated by
comparison with other substances in this subgroup (Nos 516,
528, 530, and 531) for which there was an adequate margin of
safety. The Committee noted that Nos 521-524 are unsaturated
thiols. Of these, allyl mercaptan (allyl thiol; No. 521)
would be expected to be more toxic than others that have
double bonds in different positions (by analogy to their
oxygenated analogues). Although data were not available on
allyl mercaptan (No. 521), the NOEL of diallyl trisulfide
(No. 587), which would be converted to allyl mercaptan after
reduction to allyl disulfide, was 4.6 mg/kg bw per day in a
90-day study in rats. This NOEL was considered to provide an
adequate safety margin for Nos 521-524.
(v) Thiols with oxidized side-chains: This subgroup
comprises thiols with oxygenated side-chains. The NOELs
available for three of the substances in class I (1.9 mg/kg
bw per day for 2-mercapto-3-butanol (No. 546), 2.8 mg/kg bw
per day for alpha-methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl sulfide (No. 547), and 1.9
mg/kg bw per day for 3-mercapto-2-pentanone (No. 560)) were
considered to provide adequate safety margins for the
flavouring agents in this subgroup, including the one
substance in structural class III, 3-mercapto-oxopropionate
(No. 563). Although the last compound has more than three
functional groups, the oxopropionate moiety would have
little toxic potential, and the NOELs for Nos 546, 547, and
560 were considered to provide an adequate margin of safety.
(vi) Dithiols: This subgroup comprises flavouring agents
that are dithiols. The NOEL for 2,3-butanedithiol (No. 539)
and 1,6-hexane-dithiol (No. 540) was 0.7 mg/kg bw per day,
which provides adequate margins of safety for all of the
substances in the subgroup.
(vii) Simple disulfides: This subgroup comprises 14
flavouring agents that are disulfides. The major metabolites
of these unsaturated disulfides would be thiols. The NOELs
were 7.7 mg/kg bw per day for propyl disulfide (No. 566),
0.23 mg/kg bw per day for dicyclohexyl disulfide (No. 575),
and 1.2 mg/kg bw per day for methyl benzyl disulfide (No.
577). These values provide adequate margins of safety for
each substance tested and for four structurally related
substances, Nos 564, 565, 567, and 579, at currently
estimated levels of intake. The substances in this subgroup
for which NOELs are available do not include unsaturated or
aryl disulfides. The aryl disulfides, methyl phenyl
disulfide and phenyl disulfide (Nos 576 and 578,
respectively), would be rapidly reduced to thiophenol, and
the NOEL of 3.4 mg/kg bw per day for 2-naphtha-lenethiol
(No. 531, subgroup iv) was considered to provide an adequate
safety margin for these agents. The Committee was aware that
propenyl disulfides can cause haemolytic anaemia in certain
species after short-term exposure. This effect would be of
concern to susceptible individuals. Substances Nos 568 and
572 would be metabolized to allyl mercaptan (No. 521;
subgroup iv). The Committee noted that the NOEL for diallyl
trisulfide (No. 587; subgroup ix) in a 90-day study in rats
given a single dose was 4.6 mg/kg bw per day, and that this
would provide an adequate safety margin for the allyl thiol
produced on reduction of substances Nos 568 and 572.
Di(1-propenyl) disulfide was about four times more potent
than allyl disulfide (No. 572) and about 20 times more
potent than propyl disulfide (No. 566). The intakes of the
related propenyl and butenyl flavours Nos 569, 570, and 571
gave safety margins of > 50 000 in comparison with the NOEL
for propyl disulfide (No. 566), and this was considered to
be adequate to allow for the differences in potency.
(viii) Disulfides with oxidized side-chains: This subgroup
consists of two disulfides with oxidized side-chains,
2-methyl-2-(methyldithio)-propanal (No. 580) and ethyl
2-(methyldithio)propionate (No. 581). The toxicity of these
agents has not been studied, but the Committee considered
these substances by analogy to disulfides (subgroup vii) and
concluded that adequate margins of safety were available,
given their greater polarity and the presence of thiols with
and without oxidized side-chains, i.e. subgroups (iv) and
(v).
(ix) Trisulfides and polysulfides: This subgroup consists
of six trisulfides and one polysulfide. NOELs of 4.8 mg/kg
bw per day for dipropyl trisulfide (No. 585) and 4.6 g/kg bw
per day for diallyl trisulfide (No. 587) were reported,
which gave adequate margins of safety for all substances in
this subgroup.
(x) Heterocyclic disulfides: This subgroup comprises two
flavouring agents that are heterocyclic disulfides. The NOEL
of 1.9 mg/kg bw per day for 3,5-dimethyl-1,2,4-trithiolane
(No. 573) provides an adequate margin of safety for this
substance at current levels of use. 3-Methyl-1,2,4-trithiane
(No. 574) was reported to have no effect at the single dose
of 0.3 mg/kg bw per day tested in a 90-day study; this dose
provides a margin of safety of only 100 at the estimated
modified per capita intake level of 1 µg/kg bw per day in
the United States. However, the NOEL for the closely related
compound 3,5-dimethyl-1,2,4-trithiolane provides an adequate
margin of safety (> 1000).
(xi) Thioesters: This subgroup consists of 15 thioesters.
The NOELs of 6.5 mg/kg bw per day for ethylthioacetate (No.
483) and 1000 mg/kg bw per day for methylthiobutyrate (No.
484) give an adequate margin of safety for all other esters
in this group. The conclusion that the current intake levels
of allyl thiopropionate and prenyl thioacetate are safe is
supported by the NOEL of 4.6 mg/kg bw per day for the
unsaturated diallyl trisulfide (No. 587), which would be
reduced to allyl disulfide and then allyl sulfide.
(xii) Sulfoxides: This subgroup consists of only one
substance, DMSO (No. 507). The NOEL in monkeys given DMSO by
gavage for 74-87 weeks was 3000 mg/kg bw per day. This NOEL
and other data provide an adequate margin of safety for the
use of DMSO as a flavouring agent at the estimated modified
daily per capita intake of 0.001 µg/day in the United
States.
Step B5. Five substances, allyl sulfide (No. 458), methyl phenyl
sulfide (No. 459), benzyl methyl sulfide (No. 460),
2-(methylthio)methyl-2-butenal (No. 470), and
2,8-dithianon-4-ene-carboxaldehyde (No. 471), were evaluated
at this step of the Procedure. The modified daily per
capita intake of all five substances is less than 1.5 µg
in Europe and in the United States. Applying the criteria
for Step B5 outlined in Annex 5 of the evaluations published
after its forty-ninth meeting Annex 1, reference 132), the
Committee concluded that use of these substances at their
current levels of intake poses no safety concern.
In summary, for 100 agents in subgroups (iii) to (xii), a NOEL
was available for the substance, a closely related substance, or a
predicted major metabolite that provided an adequate margin of safety
(> 1000). For 6/9 of the agents in subgroup (i) and 26/28 in subgroup
(ii), a NOEL for the substance or a closely related substance was
available that provided an adequate margin of safety (i.e. > 1000).
Therefore, the Committee determined at Step B4 of the Procedure that
the safety of these 132 substances would be expected to be of no
concern when they are used at their currently estimated level of daily
intake. The evaluation of the remaining five substances (Nos 458, 459,
and 460 in subgroup (i) and Nos 470 and 471 in subgroup (ii))
proceeded to Step B5 of the Procedure. The comparisons of toxicity and
the intake considerations for each subgroup that were used to apply
Steps B4 and B5 of the Procedure to the evaluation of individual
substances in this group of flavouring agents are summarized in Table
3 and given in more detail in Table 1.
1.5 Consideration of combined intakes
In the unlikely event that all 137 aliphatic and aromatic
sulfides and thiols in the overall group or in subgroups were to be
consumed simultaneously on a daily basis, the estimated combined
intake would not exceed the human intake threshold for class I
substances. The powerful aroma of these substances limits the level of
their use in foods.
1.6 Conclusions
The Committee concluded that the 137 flavouring agents comprising
aromatic sulfides and thiols evaluated at the present meeting could
not be predicted to be metabolized to innocuous products. According to
the Procedure, data on toxicity were needed to evaluate the safety of
this group of flavouring agents. The primary data that were used in
the evaluations consisted of 27 90-day studies in rodents with 25 of
the substances and long-term studies in multiple species for one
substance (DMSO). Most of these studies were conducted at a single
dose or multiple doses, which had no effects. The Committee noted that
the NOELs were thus derived from studies in which no toxic effects
were seen.
On the basis of the available data on the toxicity of
representative substances in each subgroup and on their metabolism,
the Committee determined that the safety of 132 of the flavouring
agents poses no concern when they are consumed at their current levels
of intake. The safety of the remaining five substances was considered
to pose no concern when they are consumed at levels of intake < 1.5
µg/day.
Other data on toxicity, including the results of short-term tests
for toxicity and studies of teratogenicity and genotoxicity, were
consistent with the results of the safety evaluation.
2. RELEVANT BACKGROUND INFORMATION
2.1 Explanation
The 137 compounds evaluated have a diverse array of chemical
structures, each of which contains a sulfur atom attached to a carbon,
hydrogen, oxygen, or second sulfur atom. The substances were separated
into 12 subgroups on the basis of the position of the sulfur atom, as
indicated in section 1.3 above. The key data on metabolism and
toxicity relevant to the safety evaluation are presented below by
subgroup. Information on intake, special studies, and observations in
humans are also discussed.
2.2 Additional considerations on intake
Thiols (i.e. mercaptans), thioethers, and their oxygenated
derivatives are known for their powerful aromas. They provide cooked,
browned, and roasted flavours when added as flavouring substances.
Their strong organoleptic properties make a significant contribution
to the taste and smell of various foods even when they are added at
low concentrations. The thresholds of odour detection for aromatic and
aliphatic thiols and thioethers range from 0.08 µg/m3 for amyl
mercaptan (not in any subgroup) to 0.03 mg/m3 for benzyl mercaptan
(No. 526); the odour threshold for methyl sulfide (No. 452), the
thioether with the highest annual volume of use as a flavouring
substance, is about 1 µg/m3, and that for the corresponding thiol,
methyl mercaptan (No. 508), is 2 µg/m3 (Farr & Kirwin, 1994). In
tests for sensory tolerance, most of the panelists reported that
atmospheres containing thiols, thioesters, or thioethers at
concentrations > 1 mg/m3 are intolerable (Flavor and Extract
Manufacturers' Association, 1996).
A direct result of the extremely low odour threshold of thiols
and thioethers is that their use in food is self-limiting, because
high concentrations would result in foods that are repulsive. The
levels of use in the vast majority of food categories are much less
than 1 mg/m3. In foods in which substantial evaporation of the sulfur
derivative occurs during processing, such as hard candies and baked
goods, the concentration is usually < 10 mg/m3. The low levels of
use in food are reflected in the low reported annual volumes of use of
thiols and thioethers as flavouring substances.
2.3 Biological data
2.3.1 Absorption, distribution, metabolism, and excretion
Simple, non-polar thioether, disulfide, and thiol flavouring
substances would be absorbed rapidly from the gastrointestinal tract
intact and excreted in urine, probably as metabolites, and in some
cases in the expired air as the parent compound. Essentially complete
absorption has been reported for methyl sulfide (No. 452) (Williams et
al., 1966) and dipropyl sulfide (Nickson & Mitchell, 1994). The
presence of other functional groups, such as an alcohol (e.g. Nos
461-463), aldehyde (e.g. Nos 465-471, 505), ester (e.g. Nos 472-481
and 492-494), acid (e.g. No. 501), alpha-ketone (or thioester) (e.g.
Nos 482-494, 504, and 506), beta-ketone (e.g. Nos 495-500, and 502),
or phenol (e.g. No. 503), provides a centre of greater polarity which
could slow the rate of absorption and would provide other sites for
oxidative metabolism (see below). The metabolism of sulfur centres is
shown in Figure 1. All the sulfur compounds considered at this meeting
are of low relative molecular mass and of sufficient lipophilicity to
be absorbed from the intestine. Some functional groups such as
aldehydes, esters, and thioesters probably undergo presystemic
metabolism in the gut lumen, gut wall, or liver.
(i) Simple sulfides (thioethers)
Thioethers are commonly called 'sulfides', as if the hydrogen
atoms of hydrogen sulfide were replaced by alkyl or aryl substituents.
Flavours are usually given such trivial names, which are simplified
even further when the two substituents are the same; for instance,
dimethyl thioether is called 'methyl sulfide'. The presence of a lone
pair of electrons on a divalent sulfur allows rapid oxidation of the
sulfide to a sulfoxide, which occurs both as part of metabolism and
also before ingestion or absorption under aerobic conditions. For
example, allyl sulfide (No. 458), a volatile constituent of onions and
garlic, is oxidized rapidly in air to the corresponding sulfoxide,
which is partly respon-sible for the lachrymatory effects of these
foods (Brodnitz & Pascale, 1971).
In many natural and pharmaceutical compounds, one or more oxygen
atoms is linked to the sulfur centre of an organic molecule, and the
chemical and biological properties of the compound depend on the
nature of the substituents on the sulfur and oxygen atoms. The
oxidation state of sulfur is of major importance in determining the
polarity of the compound and hence its interaction with biological
systems. Once alkyl and aromatic sulfides enter the systemic
circulation, they are oxidized rapidly to sulfoxides and, depending on
the structure of the sulfide, may be further oxidized to the sulfone.
Aliphatic sulfides generally yield mixtures of sulfone and sulfoxide
metabolites; the relative amounts excreted depend on the polarity of
the sulfide (Damani, 1987). Methyl sulfide (No. 452) administered to
rabbits is excreted in the urine as DMSO and dimethyl sulfone
(Williams et al., 1966), whereas the sulfone is the main urinary
metabolite of diethyl sulfide (No. 454) (Hoodi & Damani, 1984).
Dipropyl sulfide, a volatile component of onions and garlic, is
metabolized mainly to the corresponding sulfoxide with small amounts
of the sulfone and trace amounts of inorganic sulfate. Individual
studies on the sulfoxide and sulfone indicate that these metabolites
are relatively stable under physiological conditions (Nickson &
Mitchell, 1994; Nickson et al., 1995). Male Wistar rats eliminated 93%
of a single oral dose of [35S]-dipropyl sulfide over three days, with
66% in the urine and lesser amounts in exhaled air (18%), faeces
(4.6%), and the carcass (1.5%). The sulfoxide was the only compound
detected in plasma. About 25% of the radiolabel was recovered in urine
on day 1 and 39% on day 2, and approximately 25% of the radiolabel was
eliminated in bile over 48 h as the sulfoxide (20%) and sulfone (5%).
Enterohepatic recirculation was probably responsible for the delayed
urinary excretion and the plasma profiles, which showed a slow
increase with peaks at 12-15 h. Urinary metabolites collected during
the first 24 h included the sulfoxide (92%), sulfone (5%), and sulfate
(3%); on days 2 and 3, the sulfoxide accounted for > 98% of daily
urinary metabolites (Nickson & Mitchell, 1994). Methyl phenyl sulfide
administered per se orally to rats or produced by methylation of
benzenethiol (No. 525) was eliminated in the urine as methyl phenyl
sulfone and hydroxylated sulfones (McBain & Menn, 1969).
The major enzyme systems involved in xenobiotic oxidation are the
cytochrome P450 superfamily (CYP-450) (Souhaili-El Amri et al., 1987)
and the flavin-containing monooxygenases (FMO) (Levi & Hodgson, 1988;
Ziegler, 1989; Hodgson & Levi, 1992). The oxidation of thioethers to
sulfoxides is catalysed by these two enzyme systems (Renwick, 1989).
Simple aliphatic (e.g. diethyl sulfide), alicyclic (e.g. thiolane),
and aromatic (e.g. ethyl para-tolyl sulfide) sulfides are oxidized
primarily by FMO and to a lesser extent, by CYP-450. The oxidation of
diethyl sulfide and thiolane in rats is catalysed almost exclusively
by FMO (Hoodi & Damani, 1984; Damani, 1987). 4-Chlorophenyl methyl
sulfide was oxidized by FMO and CYP-450 to the sulfoxide and sulfone
derivatives in vitro (Nnane & Damani, 1995), and diphenyl sulfoxide
was oxidized to the corresponding sulfone in perfused guinea-pig liver
(Yoshihara & Tatsumi, 1990).
Enzyme-catalysed thioether oxidation may be stereoselective, FMO
and CYP-450 selecting different enantiomers (Cashman & Williams,
1990). The stereochemistry of sulfoxidation by the isoenzymes of FMO
has been studied in humans (Rettie et al., 1994). When methyl
para-tolyl sulfide was incubated with human fetal liver and human
kidney microsomes in which CYP-450 activity had been eliminated, the
resulting sulfoxide showed an enantiomeric excess (> 86%) of the
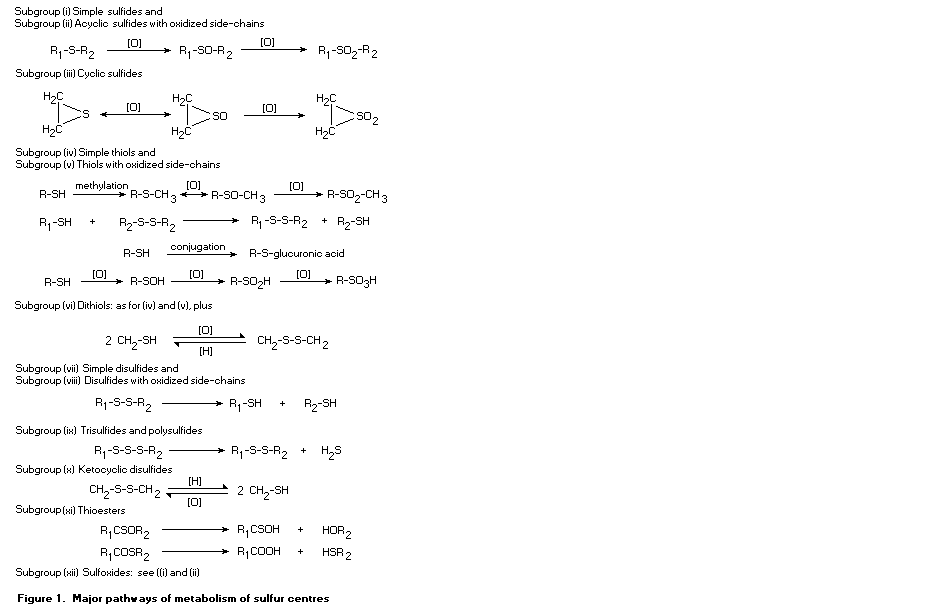 R-isomer. Decreasing stereoselectivity was seen as the size of the
alkyl group (ethyl, propyl, or isopropyl) (Sadeque et al., 1992) or
the pH (8.5-10) increased (Rettie et al., 1990). Oxidation of propyl
and butyl para-tolyl sulfide by the predominant human liver FMO
isozyme, FMO3, resulted in greater formation of the R-enantiomer
(73-88%), whereas oxidation of the methyl or ethyl derivative by human
FMO5 resulted in a > 90% preference for formation of the
S-stereoisomer of the sulfoxide (Sadeque et al., 1995). The influence
of the stereochemistry of sulfoxides on the biological and
toxicological properties of sulfides has not been evaluated.
On the basis of numerous examples of successive oxidation of
sulfides to sulfoxides and sulfones by FMO and CYP-450 enzymes in a
variety of test systems (Cashman & Williams, 1990; Cashman et al.,
1990; Rettie et al., 1990; Yoshihara & Tatsumi, 1990; Sadeque et al.,
1992; Cashman et al., 1995a,b; Elfarra et al., 1995; Nnane & Damani,
1995; Sadeque et al., 1995), the oxidation pathway appears to be a
major route for the metabolism of thioethers in humans (Ziegler, 1980;
Nickson & Mitchell, 1994).
The sulfoxide group is part of an interconvertible redox series
involving the sulfide (or thioether), sulfoxide, and sulfone. The
sulfoxide group is present in numerous molecules, which show a
diversity of biological activity; examples include pharmaceuticals
such as sulindac and flosequinan, veterinary drugs such as
albendazole, and numerous pesticides. Oxidation of a sulfoxide yields
a sulfone, which is commonly found as a metabolite of sulfoxides but
is less important as an active chemical entity. Oxidation of
sulfoxides to the corresponding sulfones occurs in both tissues and
aerobic microorganisms. The oxidation of the sulfoxide drug sulindac
to its inactive sulfone is a major metabolic pathway in humans (Strong
et al., 1985). The enzymes involved have not been studied extensively
in mammalian tissues (Levi & Hodgson, 1988) or microbes (Davis &
Guenthner, 1985). Oxidation of the sulfoxide to the sulfone is an
irreversible reaction which is probably catalysed by CYP-450 (Damani,
1987). The relative amounts of sulfoxide and sulfone excreted depend
on the stability and hydrophilicity of the sulfoxide (Damani, 1987).
After administration of [35S]-dipropyl sulfoxide to rats,
essentially all of the administered radiolabel was recovered over the
following three days, in the urine (80%), exhaled air (1.4%), faeces
(5.0%), and the carcass (13.0%) (Nickson & Mitchell, 1994). The
profile of urinary metabolites was the same after administration of
the sulfoxide or the sulfide (see above); the principal quantitative
difference was that more sulfone was excreted in urine and bile after
sulfoxide administration. Dipropyl sulfone is physiologically stable
in rats in vitro and is excreted unchanged in urine (83%) and bile
(Nickson et al., 1995). More than 98% was excreted unchanged in the
urine with trace amounts of inorganic sulfate. No reduction of the
sulfone group or oxidation of the hydrocarbon chain was observed.
As the sulfoxide may also be reduced back to the thioether, it is not
always possible to determine if the activity of a chemical in vivo
resides in the parent compound or in one of the inter-convertible
redox forms.
The three important sites of sulfoxide reduction in vivo are
liver, kidney, and gut microflora. The tissue enzymes that can reduce
sulfoxides in vitro are primarily aldehyde oxidase (Tatsumi et al.,
1982, 1983; Yoshihara & Tatsumi, 1985a,b) and thioredoxin and its
reductase (Anders et al., 1980, 1981). Studies with tissue homogenates
containing various cofactors and inhibitors have shown that the main
enzyme involved in the liver is aldehyde oxidase, whereas thioredoxin
is important in the kidney (Lee & Renwick, 1995a). The former enzyme
was active mainly under anaerobic conditions, while sulfoxide
reduction by thioredoxin occurred under both aerobic and anaerobic
conditions. The possible role of aldehyde oxidase under normal and
hypoxic conditions was shown in isolated perfused guinea-pig liver;
CYP-P450-catalysed oxidation of both diphenylsulfide and
diphenylsulfoxide to the sulfone predominated under aerobic conditions
(Yoshihara & Tatsumi, 1990), whereas oxidation of diphenylsulfoxide to
the sulfone was decreased under hypoxic conditions and trace amounts
of diphenylsulfide were formed. Infusion of the sulfoxide with
2-hydroxypyrimidine and benzaldehyde, which are electron donors for
aldehyde oxidase, resulted in increased reduction. The importance of
the kidney and thioredoxin in vivo under normal oxygen conditions is
indicated by the fact that patients with end-stage renal disease show
decreased reduction of sulindac in comparison with controls (Gibson et
al., 1987).
The gut microflora in the anaerobic environment of the lower
bowel can be an important and sometimes the sole site of reduction of
sulfoxides. Studies in germ-free animals or animals treated with
antibiotics to suppress the gut microflora and in patients indicated
that the gut bacteria were the main, or possibly the sole, site of
sulfoxide reduction of the drug sulfinpyrazone in vivo (Renwick et
al., 1982; Strong et al., 1984a,b, 1986). The intestinal bacteria can
also reduce sulindac in vitro (Strong et al., 1985), and the
thioether is a major circulating metabolite in vivo. The
contribution of the intestinal microflora to the reduction of sulindac
in humans was demonstrated by a study in normal subjects and patients
who had undergone an ileostomy. The tissues produced an initial peak
0-12 h after dosing, corresponding to 45% of the total thioether, and
the intestinal bacteria produced a second peak at 12-72 h,
corresponding to 55% of the total thioether (Strong et al., 1985).
Studies with over 200 isolated strains of bacteria from human faeces
(Strong et al., 1987) showed significant sulfoxide reduction by many
aerobic organisms such as Escherichia coli under anaerobic
conditions. Few strict anaerobes showed high reducing activity in
vitro, but they are probably essential in vivo in providing the
anaerobic environment in which the aerobes can express their sulfoxide
reductase activity (Strong et al., 1987). Recent studies with
xenobiotics as substrates have indicated the presence of a number of
soluble enzymes in E. coli that are capable of sulfoxide reduction
(Lee & Renwick, 1995b).
The enzymes involved in the reduction of the sulfoxide group
present in DMSO or of the sulfoxide metabolites of the thioethers are
likely to be the hepatic and renal systems discussed above. In order
for the gut flora to be involved in the metabolism of the flavouring
substances, the sulfur derivatives must either be incompletely
absorbed or reach the lower gut as biliary metabolites (Renwick &
George, 1989). The significant biliary excretion of the sulfoxide
metabolite after administration of dipropyl sulfide (see above) raises
the possibility of reduction by the intestinal microflora, followed by
reabsorption and subsequent reoxidation.
(ii) Acyclic sulfides with oxidized side-chains
Oxidized side-chains provide additional sites for the
biotransformation of sulfides, and, as they are polar groups, they
would increase renal excretion. When the thioether contains an
additional oxygenated functional group on a carbon atom (i.e. alcohol,
aldehyde, acid, or ketone), C-oxidation competes with sulfoxidation.
The biotransformation of such oxygenated carbon-containing functional
groups is well characterized and may have been considered in groups of
flavouring substances previously evaluated by the Committee.
Concurrent metabolism via both sulfur and oxygenated functional groups
has been reported for various substrates (El Fatih et al., 1988;
Gachon et al., 1988; Feng & Solsten, 1991; Wilson et al., 1991; Black
et al., 1993). In the presence of oxygenated functional groups,
sulfoxide formation is usually the major metabolic detoxication
pathway. The main urinary metabolites seen after intraperitoneal
administration of thiodiglycol [(HOCH2CH2)2S] to male rats were the
corresponding sulfoxide (90%) and acid thioether
S-(2-hydroxyethylthio)acetic acid (10%). The corresponding sulfone and
combined C-and S-oxidation product S-(2-hydroxyethylsulfinyl)acetic
acid were minor metabolites (Black et al., 1993). Phenacyl phenyl
sulfide is oxidized to the sulfoxide in vitro by FMO but split by
CYP-450 to give thiophenol (Oae et al., 1985). The corresponding
sulfoxides are the principal urinary metabolites of aliphatic
methylthiocarboxylic acids (Alterman et al., 1995), of the mucolytic
drug S-carboxymethyl-L-cysteine ( S-containing amino acid), of the
histamine antagonist cimetidine ( S-containing amidine) in humans
(Mitchell et al., 1982), and of other thioether drugs (Renwick, 1996).
Experiments in vitro suggest that carboxyl ester hydrolysis
occurs in the presence of thioether groups. The thioether
3-(methylthio)hexyl acetate (No. 481) was partially (9%) hydrolysed in
simulated gastric juice containing pepsin (pH = 1.1; 37-38 °C) after 4
h and completely hydrolysed in simulated intestinal fluid containing
pancreatin (Flavor & Extract Manufacturers' Association, 1991).
Thioethers with a polar anionic group such as carboxylic acid one
or more carbon atoms away for the sulfur are inhibitors of rather than
substrates for FMO (Taylor & Ziegler, 1987) and would probably be
eliminated without S-oxidation.
(iii) Cyclic sulfides
There are few published data on the fate of simple cyclic
thioethers. S-Oxidation is likely to be a major pathway in vivo,
and oxidation in vitro results in a chiral sulfoxide when the ring
is asymmetric. Oxidation of unsubstituted and methyl-substituted
cyclic sulfides by a phenobarbital-type CYP-450 yielded the
corresponding sulfoxides, but further oxidation of sulfoxides to
sulfones was not detected (Takata et al., 1983).
The metabolism of the cyclic sulfides with oxidized carbon atoms
(Nos 464, 498, 499, 550, and 562) has not been studied but would be
predicted to comprise extensive S-oxidation and possibly oxidation
or conjugation of alcohol groups.
Substituted 1,3-dithiolane derivatives undergo stereoselective
S-oxidation to the sulfoxide as a major metabolic pathway (Grosa et
al., 1991) with slower oxidation to the cyclic sulfone ( S,S-dioxide)
(Cashman & Olsen, 1990). The reactions are catalysed by both FMO and
CYP-450 (CYP2B); oxidation at both sulfur atoms was not reported. The
cyclic mono-sulfoxides of substances Nos 456, 534, and 543 are
predicted to be the main urinary metabolites, while steric hindrance
and the higher polarity of the hydroxy thioethers (Nos 550 and 562)
may allow their elimination unchanged.
(iv) Simple thiols
A large number of the flavouring agents in this group are either
alkyl or alicyclic thiols (Nos 511-524, 526, 527, and 530), arylthiols
(thiophenols) (Nos 525, 528, 529, and 531-534), dithiols (Nos
535-542), or alkylthiols with oxidized side-chains (Nos 544-547, 549,
551-561, and 563). The thiol group is weakly acidic, and endogenous
thiols have important nucleophilic functions in vivo.
Whereas alcohols are oxidized to give carbonyl compounds
(aldehydes, ketones, and carboxylic acids), thiols are not readily
oxidized to the corres-ponding thiocarbonyls. The availability of
sulfur d-orbitals allows valencies of 4 and 6, so that oxidation of
the thiol group can lead to sulfenic acids (RSOH), sulfinic acids
(RSO2H), or sulfonic acids (RSO3H), as well as disulfides (RSSR').
The metabolic pathways of thiols include oxidation to unstable
sulfenic acids (RSOH), which may be oxidized to the corresponding
sulfinic (RSO2H) and sulfonic acids (RSO3H); methylation to yield
methyl sulfides, which can be oxidized to methyl sulfoxides and
sulfones; reaction with endogenous thiols such as glutathione to form
mixed disulfides; conjugation with glucuronic acid; and oxidation of
the alpha-carbon resulting in desulfuration and formation of an
aldehyde intermediate (McBain & Menn, 1969; Dutton & Illing, 1972;
Maiorino et al., 1988; Richardson et al., 1991; Renwick, 1996).
Sulfenic acids are formed by mild oxidation of thiols but are
unstable and readily undergo further oxidation to sulfinic and
sulfonic acids or combine with nucleophiles. Reactions of sulfenic
acids include dimerization with elimination of water to produce
R-SO-S-R and oxidation to the corresponding polar sulfinic (RSO2H)
and sulfonic (RSO3H) acids. Oxidation of the thiol group of cysteine
in proteins can give stabilized sulfenic acid groups which are
critical to the active site of enzymes; redox cycling between thiol
and sulfenic acid in the active site may be essential for the
catalytic activity of a number of bacterial redox enzymes. The
sulfinic acid group is an intermediate redox state between sulfenic
and sulfonic acids. Endogenous sulfinic acids include cysteine
sulfinic acid, which is released during neuronal stimulation (Klancnik
et al., 1992) and may be involved in synaptic transmission in the
hippocampus. Cysteine and homocysteine sulfinic acids are excitatory
and cytotoxic acidic amino acids (Frandsen et al., 1993). The sulfonic
acid group is a highly polar centre and makes the molecule very
soluble in water. In general, sulfonic acids are stable to metabolism,
and desulfonation was not found in vivo with simple alkyl
sulfo-nates (Taylor et al., 1978) or with branched-chain sulfonates
(Michael, 1968).
Simple aliphatic and aromatic thiols undergo S-methylation in
mammals to produce the corresponding methyl thioether or sulfide,
which may be successively oxidized to the corresponding sulfoxides and
sulfones. Methylation is catalysed by thiopurine methyltransferase in
the cytoplasm and by thiol methyltransferase in microsomes, both of
which require S-adenosyl-L-methionine as a methyl group donor.
Thiopurine methyltransferase is present in human liver, kidney, and
erythrocytes (Woodson et al., 1982; Szumlanski et al., 1988), and
preferential substrates for this enzyme include aromatic and
heterocyclic thiols (Woodson & Weinshilboum, 1983; Woodson et al.,
1983). The apparent Km values of thiophenols (Ames et al., 1986)
are at least two orders of magnitude lower than those of aliphatic
substrates (Woodson & Weinshilboum, 1983). The activity of thiopurine
methyltransferase in humans shows genetic polymorphism, 89% of
subjects showing high activity, 11% showing intermediate activity, and
0.3% showing no activity (Weinshilboum & Sladek, 1980). Studies of
families indicate that the polymorphism is due to a single genetic
locus with two alleles, TPMTH for high activity and TPMTL for low
activity (Keith et al., 1983). The frequencies of the TPMTH and
TPMTL genes are 94% and 6%, respectively, resulting in the trimodal
frequency distribution of thiopurine methyltransferase activity in the
general population. The impact of such differences in 'methylator
status' on the metabolism of thiol-containing flavouring substances is
unknown but is likely to be small because of the presence of
alternative S-oxidation and conjugation pathways. S-Methylation of
aliphatic thiols is catalysed by microsomal thiol methyltrans-ferase,
and thiol substances such as 2-mercaptoethanol, methylmercaptan, and
2-mercaptopropionic acid are substrates for this enzyme (Bremer &
Greenberg, 1961), but the endogenous sulfur amino acid derivatives
homocysteine and glutathione are not. Microsomal thiol
methyltransferase and cytoplasmic thiopurine methyltransferase
activities are regulated independently in humans (Keith et al., 1983),
and therefore S-methylation by thiol methyltrans-ferase may occur in
individuals with low or negligible thiopurine methyltrans-ferase
activity.
Examples of S-methylation in vivo include both aliphatic and
aromatic substrates. Methyl ethyl sulfide (No. 453), which was
detected in the urine of guinea-pigs and mice given an oral dose of
diethyl disulfide (Snow, 1957), was presumably formed by reductive
cleavage to ethanethiol and subsequent methylation (Snow, 1957); minor
urinary metabolites were the sulfoxide and sulfone (Parkinson, 1996).
The sulfoxide and sulfone derivative of benzyl methyl sulfide (No.
460) were excreted in the urine of rats given an oral dose of
S-benzyl- N-malonyl-L-cysteine, presumably via methylation and
oxidation of the intermediate benzyl mercaptan (No. 525) (Richardson
et al., 1991). The urine of rats given an oral dose of [35S]phenyl
mercaptan contained S-methylated metabolites such as phenyl methyl
sulfone and ortho-and para-hydroxylated phenyl methyl sulfone
(McBain & Menn, 1969).
Thiols may react with glutathione and other endogenous thiol
compounds to form mixed disulfides, and both membrane-bound and
cytosolic thioltrans-ferases have been reported to catalyse their
formation. The resulting mixed disulfides can undergo reduction back
to thiols, oxidative desulfuration, or oxidation to a sulfonic acid
via the intermediate thiosulfinate and sulfinic acids (see above).
Thus, the disulfide represents a reversible metabolic 'reservoir' of
the xenobiotic thiol compound. The principal form in the circulation
would probably be a mixed disulfide with albumin. The mixed disulfides
formed from conjugation of a thiol with glutathione or cysteine are
not substrates for cysteine conjugate C-S lyase. C-S lyase is found in
the liver, kidney, and intestinal microflora and catalyses the
reduction of the C-S bonds of cysteine conjugates, which are formed
between organic compounds and glutathione and then undergo a series of
hydrolysis steps. The thiol product is usually methylated to form a
thioether, which may then be oxidized to sulfoxide and sulfone
analogues; compounds that undergo this metabolic pathway include
bromo-benzene, paracetamol (acetominophen), propachlor (a herbicide),
and chlorinated biphenyls. With some organic compounds, conjugation
with glutathione followed by hydrolysis and C-S lyase results in a
novel, unstable thiol; for example, some halogenated substrates and
S-phenyl-L-cysteine formed from conjugation of bromobenzene yield
unstable thiols which are toxic to the kidney (Tateishi et al., 1978;
Shaw & Blagbrough, 1989; Tateishi & Tomisawa, 1989).
S-Glucuronidation of aromatic thiols has been reported, and
this is a possible pathway for the aromatic thiols (thio-phenols) (Nos
525 and 528-531) and aromatic disulfides (Nos 576-578) after their
reduction. This reaction produced thiophenol glucuronide via
conjugation to glutathione, with subse-quent C-S lyase action on the
intermediate cysteine conjugate to yield thiophenol. The metabolism of
benzothiazole-2-sulfonamide was the first of a S-glucuronide to be
fully elucidated. Three metabolites were detected in the urine of
treated rats, benzothiazole-2-thioglucuronide, benzothiazole-2-thiol,
and benzothiazole-1-mercapturate (see Buckberry & Teesdale-Spittle,
1996); the thiol group is introduced into position 2 by displacement
of the sulfonamide by glutathione. Glucuronyl transferases behave in a
similar way towards hydroxyls and sulfydryls, and the two activities
have the same subcellular location and optimal pH (Dutton & Illing,
1972).
Thiols of low relative molecular mass undergo oxidative
desulfuration in vivo to yield CO2 and SO4=. For example, 40% of
14C-labelled methyl mercaptan (No. 508) administered to rats was
eliminated as CO2, 6.4% was exhaled as unchanged compound within 6 h,
and only 2.3% was excreted in the urine. 14C also was detected in
the beta-carbon of serine and in the methyl groups of methionine,
choline, and creatine (Canellakis & Tarver, 1953), and formalde-hyde
has been shown to be an intermediate in the oxidation of methanethiol
in vitro (Mazel et al., 1964). Whereas the carbon atom from a thiol
may be used in the biosynthesis of amino acids, the sulfur atom is not
used significantly in the synthesis of sulfur-containing amino acids:
31% of 35S-labelled methyl mercaptan was excreted in the urine as
sulfate ion (Mazel et al., 1964).
(v) Thiols with oxidized side-chains
Although these compounds comprise a significant proportion of the
flavouring agents in this group, their metabolite fate has not been
studied specifically. The metabolism is predicted to be a combination
of the pathways described above for simple thiols, combined with
further oxidation or conjugation of the oxidized side-chain.
(vi) Dithiols
The metabolism of the simple aliphatic dithiols in this group is
predicted to involve the pathways described above for thiols,
particularly oxidation of sulfur and alpha-carbon atoms and formation
of disulfides by reaction with endogenous thiols such as glutathione.
S-Oxidation of one S-atom could yield a polar thiol-sulfonate. Data
for 2,3-dimercapto-1-propanesulfonate (Maiorino et al., 1996) indicate
that a thiol-sulfonate would be excreted in the urine as such and also
as disulfides formed with endogenous thiols of low relative molecular
mass such as cysteine. The longer, linear dithiols (Nos 535, 538, and
540-542) may form an intramolecular disulfide bond and exist in
equilibrium between dithiol and cyclic disulfide.
(vii) Simple disulfides
Disulfide bonds are important elements in protein folding and
structure (Waring, 1996), and redox interconversion between disulfide
and dithiol is important in the regulation of the activity of some
enzymes. Interconversion is catalysed by a number of enzymes; for
example, glutathione reductase reduces the dimeric disulfide to two
molecules of the monomeric thiol glutathione. The reduction of
xenobiotic disulfides is believed to be extensive and may be catalysed
enzymatically by glutathione reductase (Waring, 1996) or
thioltrans-ferases (Wells et al., 1993) as well as chemically by
exchange with glutathione, thioredoxin, cysteine, and other endogenous
thiols. The non-cyclic disulfides in this group of flavours would be
metabolized to form low-relative-molecular-mass thiols, which would
then follow the pathways described above for thiols.
(viii) Disulfides with oxidized side-chains
As discussed above for groups (ii) and (iii), the additional
sites of carbon oxidation would result in greater polarity and the
potential for further oxidation or conjugation. Because of the
instability of disulfide bonds to reductive cleavage, this would be
expected to be the initial metabolic reaction, and the polarity of the
side-chains would mainly affect the elimination of thiol fragments.
(ix) Trisulfides
The trisulfide of glutathione is labile and readily converted to
the disulfide, the sulfur being released as hydrogen sulfide (Moutiez
et al., 1994). The metabolic lability of trisulfides is associated
with biological activity, such as inhibition of CYP-450 (Ogasawara et
al., 1998) and enhancement by diallyl trisulfide (No. 587) of
unscheduled DNA synthesis produced in rat hepatocytes by known
mutagens (Deng et al., 1994). The toxicity of trisulfides (Nos
582-587) and polysulfides (No. 588) is probably related to their
metabolic lability and the nature of the eventual thiol, such as allyl
thiol.
(x) Heterocyclic disulfides
The heterocyclic disulfides (Nos 573 and 574) are five-and
six-membered rings which also contain a cyclic thioether bond. Lipoic
acid is a five-membered cyclic disulfide, and rapid redox cycling
between ring disulfide and open dithiol is important in its metabolic
role in vivo. The five-membered disulfide (No. 573) would be
predicted to undergo more rapid reductive ring opening to a dithiol
than the six-membered compound (No. 574). The principal metabolic
pathways are predicted to be disulfide reduction to produce a dithiol
and S-oxidation of the thioether.
(xi) Thioesters
The thioester group (-S-CO-) is present in a number of the
flavouring substances in this group (Nos 482-494, 504, and 506). The
hydrolysis of other esters has been considered previously by the
Committee. Esters are hydrolysed by lipase and esterases (Kurooka et
al., 1976), and the rate of hydrolysis increases as the length of the
C-chain of the carboxylic acid fragment increases (Greenzaid & Jencks,
1971) and decreases as oxygenation of the carbon chain in the thiol
moiety increases (Kurooka et al., 1976).
Dithionic acids are oxidized by FMO to the dioxo analogue with
the monothioc acid as an intermediate (Taylor & Ziegler, 1987), but
simple esters of dithioic acids are poor substrates for oxidation. The
esters of monothioic acids are also probably poor substrates for
oxidation, but the monothioic acid released by hydrolysis would be
oxidized to the dioxo-acid. Another possibility for elimination in
vivo is renal excretion of the thiocarboxylic acid. With the
exceptions of prenyl thioacetate (No. 491) and methylthio
2-(acetyloxy)propio-nate (No. 492), the compounds evaluated are simple
linear alkyl compounds, branched alkyl compounds, or their side-chain
hydroxy-ester analogues, so that their toxicity can reasonably be
compared.
(xii) Sulfoxides
The metabolism of sulfoxides by oxidation and reduction is
described above for thioethers. The only sulfoxide flavouring agent is
DMSO (No. 507). The absorption, metabolism, and excretion of DMSO were
studied in rhesus monkeys given a daily oral dose of 3 g/kg bw for 14
days. DMSO was absorbed rapidly, reached a peak serum concentration
after about 4 h, and was cleared from the blood within 72 h of the end
of treatment. Dimethyl sulfone was detected in the blood 2 h after
treatment, reached a steady-state concentration after four days, and
was cleared from the blood 120 h after the end of treatment. Urinary
excretion of DMSO and dimethyl sulfone accounted for approximately 60%
and 16%, respectively, of the total ingested dose. Neither DMSO nor
dimethyl sulfone was detected in the faeces (Layman & Jacob, 1985).
The fate of DMSO in humans is similar to that in monkeys, but the
elimination is slower. When an oral dose of 1 g/kg bw was given to six
subjects, peak serum concentrations were observed approximately 4 h
after administration. The peak concentration of dimethyl sulfone was
slightly higher than that of DMSO and occurred after 72-96 h.
Approximately 51% of the dose was excreted in the urine unchanged over
the first 120 h, and up to 22% was excreted as dimethyl sulfone.
Repeated daily oral administration of DMSO at 0.5 g/kg bw per day for
14 days to one adult resulted in peak serum concentrations of DMSO
within eight days (Hucker et al., 1967).
2.3.2 Toxicological studies
The available data on the toxicity of sulfides, thiols, and
oxygenated functional derivatives in the group of 137
sulfur-containing flavouring substances are presented below. Although
the studies of acute and short-term toxicity were of limited use for
evaluating the safety of these substances, because of their short
duration, they are included for completeness. Studies related to
chemoprevention are also described.
2.3.2.1 Acute toxicity
Oral LD50 values have been reported for 52 of the 137 sulfur
compounds. The values ranged from 11 to 28 000 mg/kg bw in male and
female rats (Fairchild & Stokinger, 1958; Brown et al., 1963; Elf
Atochem, 1963; Harper & Ginn, 1964; Sommer & Tauberger, 1964; Willson
et al., 1965; Koptyaev, 1967; Fishman et al., 1969; McBain & Menn,
1969; Fogelman et al., 1970; Oser, 1970; deGroot et al., 1974;
Christias, 1975; Moreno, 1975; British Industrial Biological Research
Association, 1976; Griffiths et al., 1979; Mondino & Peano, 1979;
Opdyke, 1979; Moran et al., 1980; Moreno, 1980; Panasevich et al.,
1980; Butterworth & Mason, 1981; Mondino et al., 1981; Peano et al.,
1981; Mondino & Peano, 1982; Piccirillo & Lunchicki, 1982; Elf
Atochem, 1985; Schafer & Bowles, 1985; Collinson, 1989; Watanabe &
Kinosaki, 1989a,b; Phillips Petroleum Co., 1990a,b,c; Rush, 1991; Elf
Atochem, 1992; Farr & Kirwin, 1994; Sanders, 1997). In male and female
mice, the values ranged from 61 to > 5000 mg/kg bw (Koptyaev, 1967;
Oser, 1970; Shellen-berger, 1971a,b; Selyuzhitskii, 1972; Fogelman &
DeProspo, 1973a,b; Fogelman & Suppers, 1973; Fogelman & DeProspo,
1974; Fogelman & Suppers, 1974a,b; Bailey, 1976a,b,c; Gallo et al.,
1976; Griffiths & Babish, 1978; Moran et al., 1980; Butterworth &
Mason, 1981; Schafer & Bowles, 1985).
2.3.2.2 Short-term studies of toxicity
2,8-Dithianon-4-ene-4-carboxaldehyde (No. 471)
Five male and five female rats (strain not specified) received
0.33 or 3.3 mg/kg bw per day of 2,8-dithianon-4-ene-4-carboxaldehyde
(No. 471) by gavage on six days a week for two weeks. Individual body
weights and feed and water consumption were recorded at one and two
weeks. Haemoglobin levels determined on day 14 were normal. At
necropsy on day 14, the liver and kidney were weighed and examined
macro-and microscopically. No treat-ment-related effects were reported
(de Groot et al., 1974).
Methylthio 2-(acetyloxy)propionate (No. 492) and
methylthio 2-(propionyloxy)propionate (No. 493)
Groups of five Fischer 344 rats of each sex were given either
methylthio 2-(acetyloxy)propionate (No. 492) or methylthio
2-(propionyloxy)propionate (No. 493) in the diet for 14 days at a dose
of approximately 500 mg/kg bw per day. Control animals received a
basal diet. The animals were observed and tested daily for clinical
signs of toxicity. Body weights were measured on days 1, 6, and 14,
and feed consumption was measured on days 7 and 14. At necropsy,
organs were weighed and the liver and kidneys were examined
histologically.
Neither test material produced any abnormal clinical or
microscopic changes. The potential treatment-related effects included
depressed feed consumption, decreased body-weight gains of treated
males, and depressed relative kidney weights in treated females. The
depressed feed consumption and decreased body-weight gains observed in
treated males were probably due to the strong odour of the test
compounds, which decreased the palatability of the diets. The
biological significance, if any, of the decreased relative kidney
weights in the females (7 and 6%, respectively, with methylthio
2-(acetyloxy)-propionate and methylthio 2-(propionyloxy)propionate) is
questionable because the change was small and there were no
histological changes (Hermansky & Weaver, 1990).
Prenyl thioacetate (No. 491), prenylthiol (No. 522),
3-mercaptohexanol (No. 545), 3-methyl-3-mercaptobutyl formate
(No. 549), 3-mercaptohexyl acetate (No. 554), 3-mercaptohexyl
butyrate (No. 555), and 3-mercaptohexyl hexanoate (No. 556)
A series of 14-day studies were performed with prenyl thioacetate
(No. 491), prenylthiol (No. 522), 3-mercaptohexanol (No. 545),
3-methyl-3-mercaptobutyl formate (No. 549), 3-mercaptohexyl acetate
(No. 554), 3-mercaptohexyl butyrate (No. 555), and 3-mercaptohexyl
hexanoate (No. 556). Each substance was provided in the diets of 10
Sprague-Dawley rats of each sex at a target dose of 10 mg/kg bw per
day. Weekly measurements of body weight and feed consumption indicated
that the average daily intakes were 10-13 mg/kg bw per day. Control
animals received chow alone for the same period. The animals were
observed for gross signs of toxicity and deaths at least once daily
for the duration of the study. Body weights and feed consumption were
measured on days 8 and 15. Gross necropsies were performed on all
rats, and the kidneys and liver were removed for histopathological
examination at the time of autopsy. Body-weight gain, organ-to-body
weight ratios, and the weights of the kidney and liver of test and
control animals were not significantly different in any of the seven
studies. Gross necropsy and histopathological examination of kidney
and liver tissues revealed no lesions related to administration of the
test substances (Wnorowski, 1996a-e, 1997a,b].
Prenyl thioacetate (No. 491) and prenylthiol (No. 522),
respectively, are in the thioester and simple thiol subgroups of the
larger group of sulfur-containing flavouring substances; the other
five substances (Nos 545, 549, and 554-556) are thiols with oxidized
side-chains.
Allyl disulfide (No. 572), propyl disulfide (No. 566), and
phenyl disulfide (No. 578)
In two separate, but similarly conducted studies, allyl disulfide
(No. 572) was administered at a concentration of 250 or 1000 µmol/kg
(36 or 150 mg/kg bw per day); propyl disulfide (No. 566) at 250, 1000,
or 5000 µmol/kg (38, 150, or 750 mg/kg bw per day); and phenyl
disulfide (No. 578) at 500 µmol/kg (110 mg/kg bw per day), as
solutions in peanut oil or Tween 80, to groups of six or seven female
Sprague-Dawley rats. The doses were given by oral intubation for six
consecutive days. Control groups of 12 or 14 rats received the vehicle
alone. The animals were killed on day 7 of the experiment, and blood
was collected from the posterior vena cava. At necropsy, splenic
weights were recorded and expressed as a percentage of body weight,
and splenic darkening was assessed visually on an arbitrary scale of
0-5. Blood was examined for various parameters. Sections of splenic
sinusoidal engorgement, focal hepatic erythropoiesis, and iron
deposition in the spleen, liver, and kidneys were also scored on a
scale of 0-5. The number and size of Heinz bodies were measured to
estimate any decrease in the optical density of red cell lysates.
The results for animals given the lower dose of allyl disulfide
and the two lower doses of propyl disulfide were similar to those of
controls, whereas those given the higher doses of allyl and propyl
disulfide had enlargement and darkening of the spleen, increased iron
concentrations in the spleen, liver, and kidneys, and decreased packed
cell volume and haemoglobin concentra-tions associated with Heinz body
formation. No degenerative changes were seen in the spleen, liver, or
kidneys, but destruction of erthrocytes was indicated by deposition of
iron in these organs. Similar findings were made in rats given phenyl
disulfide.
The haemolysis observed was attributed to oxidative stress due to
'active oxygen' species formed by intra-erythrocytic redox cycling of
disulfides. Thiol-disulfide exchange with glutathione results in
reduction of disulfides to their corresponding thiols. Although
generally recognized as antioxidants, thiols act as pro-oxidants after
one-electron oxidation to a thyil radical. In erythrocytes, such
oxidation is mediated by oxyhaemoglobin and results in the formation
of methaemoglobin, hydrogen peroxide, and a thiolate anion. The
hydrogen peroxide and the more active oxygen species formed from the
thyil radical are responsible for initiating the cellular damage that
leads to haemolysis. Although human erythrocytes are usually resistant
to oxidative damage, certain individuals are susceptible because of
hereditary deficiencies (Munday et al., 1990; Munday & Manns, 1994).
2.3.2.3 Ninety-day studies of toxicity
Ninety-day studies were available for 26 of the flavouring
substances (Table 4). To facilitate comparisons of the toxicity of
structurally related substances, the studies are grouped according to
the 12 subgroups. A 90-day study was available for one or more
substance in each subgroup except that of disulfides with oxidized
side-chains. Most of the studies were performed at only one dose that
produced no adverse effects.
(i) Simple sulfides (thioethers)
Methyl sulfide (No. 452)
Four groups of 15 male and 15 female weanling SPF rats of the
Wistar strain were given methyl sulfide by oral intubation at
concentrations of 0 (control), 2.5, 25, or 250 mg/kg bw per day for 14
weeks. Two further groups of five animals of each sex were given 0.25
or 250 mg/kg bw per day for two or six weeks, respectively. The
animals were weighed on day 0 and then weekly throughout the study.
Feed and water consumption were measured for 24 h before the day of
weighing. Urine was collected during weeks 2, 6, and 14 and examined
for appearance, microscopic constituents, and content of glucose,
ketones, bile salts, and blood. The specific gravity and volume of
urine produced over 6 h were also noted. At the end of the appropriate
period of dosing, the rats were killed and blood was taken for
haematological examination. Gross abnormalities were noted and organs
weighed, and histological examinations were performed. No adverse
effect occurred with any treatment (Butterworth et al., 1975). The
dose of 250 mg/kg bw per day that had no effect is at least 10 000
times the per capita intakes of 10 and 9 mg/kg bw per day due to use
of methyl sulfide as a flavouring substance in Europe and the United
States, respectively.
In a study designed to investigate the mechanism of lenticular
changes caused by DMSO (No. 507), 10 eight-week-old New Zealand
rabbits were given 2000 mg/kg bw per day methyl sulfide (No. 452) in
drinking-water for 13 weeks. Control animals received drinking-water
only. Photographs were taken each week of the animals' lens to
evaluate the occurrence of changes. At termination, the kidney, liver,
spleen, heart, lung, adrenals, and lens were weighed and examined for
gross physical abnormalities. Treated animals had a 50% increase in
lung weight, with pulmonary congestion and some haemorrhagic spots.
The kidneys showed signs of pyelonephritis. There was no evidence of
lenticular changes (Wood et al., 1971).
(ii) Acyclic sulfides with oxidized side-chains
2-(Methylthiomethyl)-3-phenylpropenal (No. 505)
In a standardized protocol (referred to below as such for other
compounds), groups of 15 male and 15 female weanling Sprague-Dawley
rats were given a single dose of 0 or 1.4 mg/kg bw per day of
2-(methylthiomethyl)-3-phenylpropenal (No. 505) dissolved in acetone
and blended into a basal laboratory diet for 92 days. The rats were
housed individually and given feed and tap water ad libitum. The
acetone was fully evaporated before presentation of the diet to the
animals. Samples of each diet were taken weekly to assess the
stability and concentration of the test material. Appearance,
behaviour, appetite, elimination, gross signs of toxic effects, and
deaths were recorded daily, and body weights and feed consumption were
measured weekly. Haematological examinations, blood chemical
determinations, and urine analyses were performed during weeks 6 and
12 on eight males and eight females from each group. At necropsy, the
weights of the liver, kidneys, thyroid, spleen, and adrenal glands
were recorded. At termination, the weights of the thyroid, liver,
kidneys, spleen, and adrenal glands were recorded. Tissues from 26
sites and all grossly observable abnormal sites were preserved in
formalin for histopathological examination.
The body weights of females were reduced throughout the study,
and both males and females had decreased feed consumption, but these
findings did not appear to be biologically significant. No significant
differences were seen between treated and control animals with respect
to haematological, blood chemical, or urinary parameters. The absolute
and relative organ weights of the treated and control animals were
similar and there was no evidence of gross or histological changes
(Cox et al., 1979).
The dose of 2-(methylthiomethyl)-3-phenylpropenal that showed no
effects (i.e. 1.4 mg/kg bw per day) is more than 10 000 times the
estimated per capita intake of 0.03 µg/kg bw per day of this
flavouring substance in the United States.
(iii) Cyclic sulfides
2-Methyl-4-propyl-1,3-oxathiane (No. 464)
Groups of 16 male and 16 female weanling Wistar rats were given
2-methyl-4-propyl-1,3-oxathiane (No. 464) by oral intubation at a
daily dose of 0.44 mg/kg bw for 90 days or corn oil alone. The rats
were weighed on the first day of treatment and thereafter at regular
intervals throughout the study. Feed intake was recorded at three-or
four-day intervals. Blood was collected from half the rats in the
study at six weeks and from all rats at 12 weeks and was examined for
haemoglobin concentration, packed cell volume, and erythrocyte and
leukocyte counts. Urea concentration was measured. At necropsy, organs
were weighed and gross and histological examinations performed.
A slight increase in body-weight gain was seen in treated males,
which was associated with increased feed intake thought to result from
alteration of feeding behaviour due to intubation of highly flavoured
solutions. Isolated differences from controls were seen in
haematological parameters, organ weights, and histological appearance,
but none was statistically significant and were considered not to
Table 4. Results of short-term studies of toxicity and long-term studies of toxicity and carcinogenicity on aliphatic and aromatic sulfides
and thiols
No. Substance Species Sex No. test Route Duration NOEL Reference
groupsa/no. (mg/kg bw)
per test
groupb
452 Methyl sulfide Rat MF 3/30 Gavage 14 weeks 250c Butterworth
et al. (1975)
Rabbit MF 1/10 Oral 13 weeks < 2000 Wood et al. (1971)
464 2-Methyl-4-propyl-1,3-oxathiane Rat MF 1/32 Gavage 13 weeks 0.44c British Industrial
Biological Research
Association (1976)
471 2,8-Dithianon-4-en-4-carboxaldehyde Rat MF 2/10 Gavage 2 weeks ND de Groot
et al. (1974)
483 Ethyl thioacetate Rat MF 1/46 Oral 13 weeks 6.5c Shellenberger (1970)
484 Methyl thiobutyrate Rat M 3/10 Oral 13 weeks 1000c,d Wheldon et al. (1970)
491 Prenyl thioacetate Rat MF 1/20 Diet 2 weeks ND Wnorowski (1997a)
498 4,5-Dihydro-3(2H)-thiophenone Rat MF 1/30 Oral 92 days 9.2c Morgareidge (1970)
505 2-(Methylthiomethyl)-3-phenylpropenal Rat MF 1/30 Oral 92 days 1.4c Cox et al. (1979)
507 Methylsulfinylmethane Rat 4/20 Oral 13 weeks 2200 Sommer & Tauberger
(1964)
Rat MF 3/100 Gavage 18 months < 1100 Noel et al. (1975)
Dog MF 3/10 Gavage 18 weeks 1100
or 2 years
Monkey MF 3/4 Gavage 74-87 weeks 3000 Vogin et al. (1970)
516 Cyclopentanethiol Rat MF 1/30 Oral 92 days 0.56c Morgareidge &
Oser (1970a)
520 2,3- or 10-Mercaptopinane Rat MF 1/30 Oral 92 days 0.06c Oser (1966)
522 Prenylthiol Rat MF 1/20 Diet 2 weeks ND Wnorowski (1997b)
528 ortho-Toluenethiol Rat MF 1/20-32 Diet 90 days 0.52c Posternak
et al. (1969)
Table 4. (contd)
No. Substance Species Sex No. test Route Duration NOEL Reference
groupsa/no. (mg/kg bw)
per test
groupb
530 2,6-Dimethylthiophenol Rat MF 1/64 Gavage 13 weeks 0.43c Peano et al. (1981)
531 2-Naphthalenethiol Rat MF 1/30 Oral 92 days 3.4c Morgareidge (1971a)
534 2-Methyl-1,3-dithiolane Rat MF 1/32 Gavage 91 days 7.0c Griffiths
et al. (1979)
539 2,3-Butanedithiol Rat MF 1/30 Oral 92 days 0.7c Morgareidge
et al. (1974a)
541 1,8-Octanedithiol Rat MF 1/30 Oral 92 days 0.7c Morgareidge
et al. (1974c)
543 Trithioacetone Rat MF 1/30 Oral 92 days 0.2c Cox et al. (1973a)
545 3-Mercaptohexanol Rat MF 1/20 Diet 2 weeks ND Wnorowski (1996a)
546 2-Mercapto-3-butanol Rat MF 1/30 Oral 92 days 1.9c Cox et al. (1974a)
547 a-Methyl-b-hydroxypropyl Rat MF 1/30 Oral 92 days 2.8c Morgareidge
a-methyl-b-mercaptopropyl et al. (1974c)
sulfide
549 3-Mercapto-3-methylbutyl Rat MF 1/20 Diet 2 weeks ND Wnorowski (1996a)
formate
554 3-Mercaptohexyl acetate Rat MF 1/20 Diet 2 weeks ND Wnorowski (1996b)
555 3-Mercaptohexyl butyrate Rat MF 1/20 Diet 2 weeks ND Wnorowski (1996c)
556 3-Mercaptohexyl hexanoate Rat MF 1/20 Diet 2 weeks ND Wnorowski (1996d)
560 3-Mercapto-2-pentanone Rat MF 1/30 Oral 92 days 1.9c Morgareidge (1971b)
562 2,5-Dimethyl-2,5-dihydroxy Rat MF 1/30 Diet 92 days 3.1c Cox et al. (1973b)
-1,4-dithiane
566 Propyl disulfide Rat F 1/6 Gavage 6 days ND Munday & Manns (1994)
Rat M 1/10-16 Diet 90 days 7.3c Posternak
et al. (1969)
F 1/10-16 Diet 90 days 8.2c
572 Allyl disulfide Rat F 2/6 Gavage 6 days 36c Munday & Manns (1994)
Table 4. (contd)
No. Substance Species Sex No. test Route Duration NOEL Reference
groupsa/no. (mg/kg bw)
per test
groupb
573 3,5-Dimethyl-1,2,4-trithiolane Rat, MF 1/32 Gavage 90 days 1.9c British Industrial
Biological Research
Association (1976)
574 3-Methyl-1,2,4-trithiane Rat MF 1/32 Oral 13 weeks 0.3c Mondino (1981)
575 Dicyclohexyl disulfide Rat MF 1/30 Oral 92 days 0.23c Cox et al. (1974b)
577 Methyl benzyl disulfide Rat MF 1/30 Oral 92 days 1.2c Gallo et al. (1976)
578 Phenyl disulfide Rat F 1/6 Gavage 6 days ND Munday et al. (1990)
585 Dipropyl trisulfide Rat MF 1/30 Oral 92 days 4.8c Morgareidge
& Oser (1970b)
587 Diallyl trisulfide Rat MF 1/30 Oral 92 days 4.6c Morgareidge
& Oser (1970c)
M, male; F, female; ND, not determined
a No. of test groups does not include controls
b No. per test group comprises males and females
c Study performed with either a single dose or multiple doses that had no effect; the value is therefore the highest dose tested.
d Dose increased from 500 to 1000 mg after six weeks
represent adverse effects (British Industrial Biological Research
Association, 1976). The dose of 0.44 mg/kg bw per day of
2-methyl-4-propyl-1,3-oxathiane that showed no effects is more than 10
000 times the estimated per capita intakes of 0.03 mg/kg bw per day in
Europe and 0.02 mg/kg bw per day in the United States.
4,5-Dihydro-3(2H)-thiophenone (No. 498)
4,5-Dihydro-3(2H)-thiophenone (No. 498) was tested in a
standardized study (see above) at a single dose of 9.16 mg/kg bw per
day. No effects were observed (Morgareidge, 1970). This dose is 100
000 times greater than the estimated per capita intakes of 0.01 µg/kg
bw per day in Europe and 0.04 µg/kg bw per day in the United States.
2-Methyl-1,3-dithiolane (No. 534)
A group of 16 male and 16 female Sprague-Dawley rats received an
aqueous propylglycol solution (0.2% w/w) containing 7 mg/kg bw of
2-methyl-1,3-dithiolane (No. 534) daily by oral intubation for 91
days. Control animals received 0.02% propylene glycol only. The rats
were observed routinely for body weight and feed consumption.
Haematological and blood chemical parameters were determined in weeks
4 and 13. No difference was seen between control and treated groups in
body-weight gain or feed consumption. A slight, nonsignificant
reduction in haemoglobin concentration was seen in treated females.
Gross and histological examinations at termination showed no
dose-related effects, and organ weights were normal (Griffiths et al.,
1979). The dose of 7 mg/kg bw per day is greater than 1 000 000 times
the estimated per capita intake of 0.002 mg/kg bw per day of
2-methyl-1,3-dithiolane in Europe and 10 000 times that of 0.1 mg/kg
bw per day in the United States.
Trithioacetone (No. 543)
Trithioacetone (No. 543) was tested in a standardized study (see
above) at a single dose of 0.205 mg/kg bw per day. No effects were
observed (Cox et al., 1973a). This dose is 10 000 greater than the
estimated intakes of 0.04 µg/kg bw per day in Europe and 0.01 µg/kg bw
per day in the United States.
2,5-Dimethyl-2,5-dihydroxy-1,4-dithiane (No. 562)
2,5-Dimethyl-2,5-dihydroxy-1,4-dithiane (No. 562) was tested in a
standar-dized study (see above) at a single dose of 3.1 mg/kg bw per
day. No effects were observed (Cox et al., 1973b). This dose is 1 000
000 greater than the estimated per capita intakes of 0.004 µg/kg bw
per day in Europe and 0.003 µg/kg bw per day in the United States.
(iv) Simple thiols
Cyclopentanethiol (No. 516)
Cyclopentanethiol (No. 516) was tested in a standardized study
(see above) at a single dose of 0.56 mg/kg bw per day. No effects were
observed (Morgareidge & Oser, 1970a). This dose is 10 000 times
greater than the estimated per capita intake of 0.01 µg/kg bw per day
in the United States.
2,3-or 10-Mercaptopinane (No. 520)
2,3-or 10-Mercaptopinane (No. 520) was tested in a standardized
study (see above) at a single dose of 0.06 mg/kg bw per day, No
effects were observed (Oser, 1966). This dose is 10 000 times greater
than the estimated per capita intake of 0.001 µg/kg bw per day in
Europe and 100 times that of 0.2 µg/kg bw per day in the United
States.
ortho- Toluenethiol (No. 528)
Groups of 10-16 male and 10-16 female rats were given 0.52 mg/kg
bw per day of ortho-toluenethiol (No. 528) in the diet for 90 days.
Control animals received untreated diet. Feed consumption and body
weights were recorded weekly. Haematological examinations and blood
urea determinations were conducted on half the animals at seven weeks
and on all animals at 13 weeks. At necropsy, organs were weighed and
examined grossly and histologically. No adverse effects were observed
(Posternak et al., 1969). The dose that had no effect is greater than
1000 times the estimated per capita intake of 0.4 mg/kg bw per day in
Europe and 100 000 times that of 0.003 mg/kg bw per day in the United
States.
2,6-Dimethylthiophenol (No. 530)
2,6-Dimethylthiophenol (No. 530) was administered in corn oil by
gavage to 32 male and 32 female Sprague-Dawley rats at a concentration
calculated to result in an average intake of 0.43 mg/kg bw per day for
13 weeks. Control animals received only corn oil. Body weights and
feed intake were measured weekly, and haematological and blood
chemical parameters were measured at weeks 4 and 13. Organs were
weighted and gross and histological examinations were performed at the
time of autopsy. No significant difference was seen between treated
and control animals (Peano et al., 1981). The dose that had no effect
is greater then 10 000 times the estimated per capita intake of 0.03
mg/kg bw per day in Europe and greater than 1 000 000 times that of
0.0003 mg/kg bw per day in the United States.
2-Naphthalenethiol (No. 531)
2-Naphthalenethiol (No. 531) was tested in a standardized study
(see above) at a single dose of 3.4 mg/kg bw per day. No effects were
observed (Morgareidge, 1971a). The dose that had no effect is 1 000
000 times greater than the estimated per capita intake of 0.001 µg/kg
bw per day in the United States.
(v) Thiols with oxidized side-chains
2-Mercapto-3-butanol (No. 546)
2-Mercapto-3-butanol (No. 546) was tested in a standardized study
(see above). No effects were observed (Cox et al., 1974a). The dose of
1.9 mg/kg bw per day that had no effect is 1000 times greater than the
estimated per capita intake of 0.1 µg/kg bw per day in Europe and
10 000 times that of 0.002 µg/kg bw per day in the United States.
alpha-Methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl
sulfide (No. 547)
alpha-Methyl-beta-hydroxypropyl alpha-methyl-beta-mercaptopropyl
sulfide (No. 547) was tested in a standardized study (see above) at a
single dose of 2.815 mg/kg bw per day. No effects were observed
(Morgareidge et al., 1974b). This dose is 100 000 times greater than
the estimated per capita intake of 0.01 µg/kg bw in the United States.
3-Mercapto-2-pentanone (No. 560)
3-Mercapto-2-pentanone (No. 560) was tested in a standardized
study (see above) at a single dose of 1.89 mg/kg bw per day. No
effects were observed (Morgareidge, 1971b). This dose is 100 000 times
greater than the estimated per capita intake of 0.002 µg/kg bw per day
in the United States.
(vi) Dithiols
2,3-Butanedithiol (No. 539) and 1,8-octanedithiol (No. 541)
2,3-Butanedithiol (No. 539) and 1,8-octanedithiol (No. 541) were
tested in standardized studies (see above) at single doses of 0.703
and 0.705 mg/kg bw per day, respectively. No effects were observed
(Morgareidge et al., 1974a,c). The dose of 2,3-butanedithiol that had
no effect is 100 000 times greater than the estimated per capita
intakes of 0.001 µg/kg bw per day in Europe and 0.003 µg/kg bw per day
in the United States. The dose of 1,8-octanedithiol that had no effect
is 1000 times greater than the estimated per capita intake of 0.1
µg/kg bw per day in Europe and 10 000 times that of 0.01 µg/kg bw per
day in the United States.
(vii) Simple disulfides
Propyl disulfide (No. 566)
Three groups of 10-16 male and 10-16 female rats were given 7.3
or 8.2 mg/kg bw per day of propyl disulfide (No. 566) in the diet for
90 days. Control animals received untreated diet. feed consumption and
body weights were recorded weekly, and haematological examinations and
blood urea determinations were conducted on half the animals at seven
weeks and on all animals at 13 weeks. At necropsy, organs were weighed
and examined grossly and histologically. No adverse effects were
observed (Posternak et al., 1969). The doses that had no effects are
10 000 times greater than the estimated per capita intake of 0.1 mg/kg
bw per day in Europe and 1 000 000 times that of 0.002 mg/kg bw per
day in the United States.
Dicyclohexyl disulfide (No. 575)
Dicyclohexyl disulfide (No. 575) was tested in a standardized
study (see above) at a single dose of 0.23 mg/kg bw per day. No
effects were observed (Cox et al., 1974b). The dose that had no effect
is 100 000 times greater than the estimated per capita intake of
0.0003 µg/kg bw per day in Europe and 10 000 that of 0.003 µg/kg bw
per day in the United States.
Methyl benzyl disulfide (No. 577)
Methyl benzyl disulfide (No. 577) was tested in a standardized
study (see above) at a single dose of 1.2 mg/kg bw per day, No effects
were observed (Gallo et al., 1976). The dose that had no effect is 1
000 000 times greater than the estimated per capita intake of 0.0003
µg/kg bw per day in Europe and 100 000 that of 0.002 µg/kg bw per day
in the United States.
(viii) Disulfides with oxidized side-chains
No studies on the two substances in this group were available,
but their toxicity can be compared by analogy to that of the
disulfides.
(ix) Trisulfides
Dipropyl trisulfide (No. 585)
Dipropyl trisulfide (No. 585) was tested in a standardized study
(see above) at a single dose of 4.8 mg/kg bw per day, No effects were
observed (Morgareidge & Oser, 1970b). The dose that had no effect is
10 000 times greater than the estimated per capita intake of 0.2 µg/kg
bw per day in Europe and 100 000 that of 0.02 µg/kg bw per day in the
United States.
Diallyl trisulfide (No. 587)
Diallyl trisulfide (No. 587) was tested in a standardized study
(see above) at a single dose of 4.6 mg/kg bw per day, No effects were
observed (Morgareidge & Oser, 1970c). The dose that had no effect is
10 000 times greater than the estimated per capita intake of 0.1 µg/kg
bw per day in Europe and 100 000 that of 0.0003 µg/kg bw per day in
the United States.
(x) Heterocyclic disulfides
3,5-Dimethyl-1,2,4-trithiolane (No. 573)
Groups of 16 male and 16 female weanling Wistar rats were given
3,5-dimethyl-1,2,4-trithiolane (No. 573) by oral intubation at a daily
dose of 1.9 mg/kg bw for 90 days or corn oil alone. The rats were
weighed on the first day of treatment and thereafter at regular
intervals throughout the study. Feed intake was recorded at three- or
four-day intervals. Blood was collected from half the rats at six
weeks and from all rats at 12 weeks and was examined for haemoglobin
concentration, packed cell volume, and numbers of erythrocyte and
leukocytes. The urea concentration was measured. At necropsy, organs
were weighed and gross and histological examinations were performed.
A slight increase in body-weight gain was seen in treated males,
which was associated with increased feed intake thought to result from
alteration of feeding behaviour due to intubation of highly flavoured
solutions. Isolated differences from controls were seen in
haematological parameters, organ weights, and histological appearance,
but none was statistically significant and they were considered not to
represent adverse effects (British Industrial Biological Research
Association, 1976). The dose that had no effect is 100 000 times
greater than the estimated per capita intakes of 0.001 mg/kg bw per
day in Europe and 0.002 mg/kg bw per day in the United States.
3-Methyl-1,2,4-trithiane (No. 574)
3-Methyl-1,2,4-trithiane (No. 574) was administered orally to 16
male and 16 female rats (strain not specified) at a dose of 0.3 mg/kg
bw per day for 13 weeks. Body weights and feed intake were measured
weekly. Haematological parameters and blood urea were determined at
weeks 4 and 13. At necropsy, organs were weighed and examined
histologically. No adverse effects were observed (Mondino, 1981). The
dose that had no effect is 100 000 and 100 times greater than the
estimated per capita intakes of 0.002 mg/kg bw per day in Europe and
1 mg/kg bw per day in the United States.
(xi) Thioesters
Ethyl thioacetate (No. 483)
Groups of 23 male and 23 female rats were given ethyl thioacetate
(No. 483) at a dose of 6.5 mg/kg bw per day in the diet, while a
control group received basal diet alone. The animals were observed
daily for appearance, physiological responses, behaviour, any
pharmacological or toxicological responses, and deaths. Body weights
and feed consumption were recorded weekly. During weeks 6 and 13,
urine was obtained from eight males and eight females for estimation
of pH and specific gravity, for microscopic examination of sediment,
and for qualitative estimates of albumin, glucose, occult blood,
ketones, and bilirubin. Eight animals of each sex were killed after
six weeks, and blood was taken for haematological testing. The
remaining animals were necropsied at 13 weeks and their tissues
examined for gross pathological changes. Organs were weighed and
tissues retained for histological evaluations. The growth, feed
consumption, and feed utilization of the test animals were similar to
those of controls throughout the study, and blood biochemical and
haematological indices were normal. Likewise, there were no
significant differences in the average absolute or relative organ
weights. All rats appeared normal throughout the study, and no gross
pathological lesions were seen in any tissue (Shellenberger, 1970).
The dose that had no effect is 1 000 000 times greater than the
estimated per capita intake of 0.0003 mg/kg bw per day in both Europe
and the United States.
Methyl thiobutyrate (No. 484)
Three groups of 10 male rats were fed 20, 200, or 500 mg/kg bw
per day of methyl thiobutyrate (No. 484) for 13 weeks, the dose given
to the third group being increased from 500 to 1000 mg/kg bw per day
after six weeks. A control group received only the basal diet. Feed
consumption and body weights were recorded weekly. A slight decrease
in weight gain was observed in treated rats as compared with controls,
which was attributed to decreased feed intake associated with
unpalatability. At necropsy, haematological examinations showed normal
values, and no differences in relative or absolute organ weights or
gross or histological appearance was seen between test and control
animals (Wheldon et al., 1970). The dose of 1000 mg/kg bw per day
which had no effect is 1 000 000 times greater than the estimated per
capita intake of 0.1 mg/kg bw per day in both Europe and the United
States.
(xii) Sulfoxides
No 90-day study was available for this group of compounds, but
methylsulfinylmethane (DMSO; No. 507) has been tested in long-term
studies of toxicity and carcinogenicity in rats, dogs, and monkeys
(see below) and has also been given to humans (see below).
2.3.2.4 Long-term studies of toxicity and carcinogenicity
Long-term studies of toxicity and carcinogenicity in rats, dogs,
and monkeys were available only for DMSO (No. 507) (Table 4).
Groups of 50 male and 50 female Sprague-Dawley rats and groups of
five male and five female pure-bred Pembrokeshire Corgi dogs were
given a 50% aqueous solution containing undiluted DMSO at 1100, 3300,
or 9900 mg/kg bw per day by gavage on five days per week. A control
group received 1 L/kg bw per day of distilled water. The rats were
treated for 18 months and the dogs for either 18 weeks or two years.
All animals were observed daily for clinical signs, and body weight
and feed consumption were measured weekly. Haematological assessments,
biochemical measurements, and urinary analyses were performed after 4,
12, 20, 32, 51, 60, and 72 weeks for the rats and after 1, 3, 4.5, 6,
9, 12, 18, and 24 months for the dogs. Routine ophthalmo-scopic
examinations were also performed. At termination, detailed macroscopic
and histopathological examinations were performed.
A dose-related decrease in weight gain was observed in rats at
all doses, except in males receiving the lowest dose. The decrease in
weight gain was not accompanied by a decrease in feed intake. Male
rats at the high dose also showed a slight reduction in haemoglobin
and packed-cell volume. Examination of the eyes revealed no changes in
the retina or vitreous humour. No adverse effects on body weight or
feed intake were seen in the dogs. Persistent diuresis was observed in
those receiving the intermediate and high doses, but there was no
renal damage. Increased packed-cell volume and haemoglobin levels were
observed at the highest dose, although the erthrocytes had normal
haemoglobin concentrations and were of normal size. The most
significant observation in dogs was lenticular changes, comprising
changes in the refractive index of the central portion of the lens,
transitory, dense, white equatorial lens opacity, the appearance of
persistent opalescence in the central region of the lens, and changes
in the vitreous humour. Biochemical analyses of the lenses of affected
animals revealed an increase in insoluble protein and reductions in
soluble protein, glutathione, and water. These changes were observed
during the fifth month in dogs receiving the highest dose and during
the ninth to tenth months in those at the intermediate dose. Nuclear
refractive changes were observed after six months in dogs at the low
dose, but none of these animals had opalescence. Those animals that
were withdrawn from the study at 18 weeks showed partial regression of
the lenticular changes at the end of the two-year period, and the
lenses of dogs that received the intermediate dose had apparently
reverted to a normal state. No abnormalities other than those in the
eye were detected on macroscopic or histopathological examination
(Noel et al., 1975).
Four groups of rhesus monkeys received doses equivalent to 0,
990, 3000, or 9000 mg/kg bw per day of a 90% solution of DMSO by
gastric intubation or dermal application for 74-87 weeks. Three
females and one male received only water by oral intubation, and two
males and one female received only water dermally and served as
controls. Two animals of each sex were treated with 990 and 2970 mg/kg
bw per day, and three animals of each sex received 8910 mg/kg bw per
day by each route of administration. The topical administration
consisted of direct application to the entire abdominal skin. Physical
signs, behaviour, and survival were recorded daily. Body weights,
water consumption, blood pressure, heart rate, respiratory rate,
neurological reflexes, and complete haematological ophthalmological
and urinary analyses were performed during weeks 1, 4, 7, 12, 24, 37,
51, and 73. At termination, gross and histomorphological examinations
were made.
All animals treated topically showed scaling and flaking on the
area of application but no other adverse behavioural or physical
signs. Animals given the highest dose orally died between weeks 15 and
53 of the study. No other treatment-related changes were found in the
monkeys treated orally, in physical examinations, in haematological,
ophthalmological, urinary, or biochemical parameters, or in absolute
or relative organ weights. Histologically, atelectasis and emphysema
were the only pathological changes observed, and were seen only at the
highest dose. Some regurgitation and/or tracheal inspiration of DMSO,
which is a highly volatile, irritating substance, may have occurred
(Vogin et al., 1970).
2.3.2.5 Genotoxicity
Studies of mutagenicity and genotoxicity were available for 14
mono-, di-, and trisulfides, thiols, and related oxygenated
derivatives in this group and three structurally related substances
(Table 5). The compounds were tested for mutagenicity in vitro at
concentrations up to 300 000 mg/plate. No effect was found in
Salmonella typhimurium strains TA97, TA98, TA100, TA102, TA1535,
TA1537, TA1538, or TA2637 with or without metabolic activation (Lavoie
et al., 1979; Eder et al., 1980, 1982; Wild et al., 1983; Aeschbacher
et al., 1989; Watanabe & Morimoto, 1989a,b; Phillips Petroleum Co.,
1990a; Zeiger et al., 1992; Hakura et al., 1993; Wang et al., 1994;
Karekar et al., 1996; Brams et al., 1997). The only positive results
found were with 1,2-ethanedithiol (No. 532) and DMSO (No. 507). The
latter is widely used as a solvent in assays for genotoxicity in
vitro.
In a test for reverse mutation in nine strains of S.
typhimurium and Escherichia coli strains WP2 and WP2uvrA, modified
by the use of preincubation, DMSO was mutagenic in S. typhimurium
TA1537 and TA2637 and in E. coli WP2uvrA at concentrations of
0.2-0.4 ml/plate, with and without metabolic activation (Hakura et
al., 1993). These are relatively high concentrations, some being
cytolethal, and no mutagenic activity was observed at concentrations
used routinely in this assay.
The ability of 1,2-ethanedithiol (No. 532) to induce specific
locus mutations at the Tk locus in cultured L5178Y Tk+/- mouse
lymphoma cells was evaluated in the presence and absence of a
metabolic activation system prepared from Aroclor-induced rat liver.
When unactivated cultures were treated with concentrations of 13-150
µg/ml, total cell survival was only 0.9-32%. A dose-related increase
in mutant frequency was seen relative to control cultures in the
solvent, DMSO. In cultures with metabolic activation, no evidence of
mutagenicity was seen at concentrations of 0.07-1 µg/ml. The survival
rate in these cultures (3-49%) was greater than that in cultures
without activation. Concentrations of 16 and 50 µg/ml of
1,2-ethanedithiol were reported to induce a significant
( p < 0.002), concentration-related increase in the frequency of
sister chromatid exchange in Chinese hamster ovary cells in the
absence of metabolic activation, and concentrations of 1.6 and 5 µg/ml
induced a significant ( p < 0.004) increase in the frequency of
sister chromatid exchange in the presence of metabolic activation. No
cytotoxicity was observed at concentra-tions up to 5 µg/ml (Phillips
Petroleum Co., 1990a).
The validity of the L5178YTk+/- mouse lymphoma cell assay has
been evaluated in relation to potentially acidifying substances of low
relative molecular mass by Brusick (1986) and Heck et al. (1989).
Those authors concluded that the assay was not valid, since positive
results can be induced by changes in the pH and osmolality of the
culture medium. The results of assays for sister chromatid exchange
may also be artefacts resulting from lysosome breakdown secondary to
cytotoxicity. Cytotoxic concentrations of substances may also cause
release of DNase which induces chromosomal aberrations and DNA
double-strand breaks (Zajac-Kaye & Ts'o, 1984; Bradley et al., 1987).
Cells with double-strand breaks that survive could be subject to a
variety of genotoxic consequences, including chromosomal breaks and
rearrangements. As cytotoxicity and lysosomal breakdown were not
evaluated in the study of sister chromatid exchange with
1,2-ethandithiol, the results are difficult to interpret.
Groups of male ICR mice were given two doses 48 h apart of a
mixture containing allyl sulfide (No. 458), allyl disulfide (No. 572),
or diallyl trisulfide (No. 587) in corn oil at doses of 10 or 20 mg/ml
by gavage. The doses were estimated to provide 0.33 or 0.67 mmol/kg bw
or 50 or 100 mg/kg bw on the basis of the composition of the mixture.
No increase in the frequency of mirconucleated polychromatic
erythrocytes was seen in bone-marrow cells (Marks et al., 1992).
The results of these assays in vivo and in vitro indicate
that aliphatic and aromatic thiols and sulfides are not genotoxic.
2.3.2.6 Developmental toxicity
Studies on teratogenicity were available only for 1-butanethiol
(No. 511) and benzenethiol (No. 525).
Table 5. Studies of genotoxicity with aliphatic and aromatic sulfides and thiols
No. Substance End-point Test system Concentration Results Reference
458 Allyl sulfide Reverse mutation S. typhimurium TA100 0.004-0.44 µg/ml Negativea Eder et al. (1982)
458 Allyl sulfide Micronucleus formation Mouse 38-77 mg/kg bw Negative Marks et al. (1992)
492 Methylthio 2-(acetyloxy) Reverse mutation S. typhimurium TA100, TA98, 0.156-5 mg/plate Negativea Watanabe &
propionate TA1535, TA1537 Morimoto (1989a)
493 Methylthio 2-(propionyloxy) Reverse mutation S. typhimurium TA98, TA100, 0.156-5 mg/plate Negativea Watanabe &
propionate TA1535, TA1537 Morimoto (1989b)
507 DMSOc Reverse mutation S. typhimurium TA97, TA98, 0.005-10 µmol/ Negativea Karekar et al.
TA100, TA102 plate (1996)
507 DMSO Reverse mutation S. typhimurium TA98, TA100 12.5-200 µg/plate Negativea Wang et al. (1994)
507 DMSO Reverse mutation S. typhimurium TA97, TA98, 100 000-300 000 Negativea Brams et al. (1987)
TA100 µg/plate
507 DMSO Reverse mutation S. typhimurium TA97, TA98, 100-10 000 µg/ Negativea Zeiger et al. (1992)
TA100, TA1535, TA1537 plate
507 DMSO Reverse mutation S. typhimurium TA97, TA98, 0.1-0.4 ml/plate Negativea Hakura et al. (1993)
TA100, TA102, TA104,
TA1535, TA1538
507 DMSO Reverse mutation S. typhimurium TA1537, 0.1-0.4 ml/plate Positived Hakura et al. (1993)
TA2637
521 Allyl mercaptan Reverse mutation S. typhimurium TA100 0.005-1.4 µg/ml Negativea Eder et al. (1980)
525 Benzenethiol Reverse mutation S. typhimurium TA98, TA100 25-500 µg/plate Negative Lavoie et al. (1979)
526 Benzyl mercaptan Reverse mutation S. typhimurium TA98, TA100, < 3.6 mg/plate Negativea Wild et al. (1983)
TA1535, TA1537, TA1538
532 1,2-Ethanedithiol Reverse mutation S. typhimurium TA98, TA100, 5000 µg/plate Negativea Phillips Petroleum
TA1535, TA1537, TA1538 Co. (1990a)
532 1,2-Ethanedithiol Sister chromatid Chinese hamster ovary cells 16 µg/mle Positivef Phillips Petroleum
exchange 1.6 µg/mlg Positive Co. (1990a)
532 1,2-Ethanedithiol Forward mutation L5178Y mouse lymphoma 150 µg/mle Positivef Phillips Petroleum
cells, Tk locus 1 µg/mlg Negative Co. (1990a)
551 2-Mercaptopropionic Reverse mutation S. typhimurium TA98, TA100, < 3.6 mg/plate Negativea Wild et al. (1983)
acid TA1535, TA1537, TA1538
Table 5 (contd)
No. Substance End-point Test system Concentration Results Reference
564 Dimethyl disulfide Reverse mutation S. typhimurium TA98, TA100, 0.011-1.1 mmol Negativea Aeschbacher et al.
TA102 (1989)
572 Allyl disulfide Reverse mutation S. typhimurium TA100 0.0015-0.15 µg/ml Negativea Eder et al. (1980)
572 Allyl disulfide Micronucleus formation Mouse 48-98 mg/kg bw Negativeb Marks et al. (1992)
578 Phenyl disulfide Reverse mutation S. typhimurium TA98, TA100, < 3.6 mg/plate Negativea Wild et al. (1983)
TA1535, TA1537, TA1538
579 Benzyl disulfide Reverse mutation S. typhimurium TA98, TA100, < 3.6 mg/plate Negativea Wild et al. (1983)
TA1535, TA1537, TA1538
587 Diallyl trisulfide Micronucleus formation Mouse 59-120 mg/kg bw Negativeb Marks et al. (1992)
Related substances
tert-Dodecyl Reverse mutation S. typhimurium TA98, TA100, 0.018-10 000 µg/ Negativea Zeiger et al. (1987)
mercaptan TA1535, TA1537 plate
Dimethyl sulfone Reverse mutation S. typhimurium TA98, TA100, 0.003-0.3 mmol Negativea Aeschbacher et al.
TA102 (1989)
n-Butyl sulfone Disc method E. coli Sd-4-73 Not specified Negative Szybalski, (1958)
100 2-Mercaptopropionic Basc mutation Drosophila 10 mmol/L Negative Wild et al. (1983)
acid
a With and without metabolic activation
b Mixture of allyl sulfide, allyl disulfide, and allyl trisulfide in a ratio of 68:20:12
c Tested as solvent control
d At doses that caused lethality; negative results at doses used routinely in this test
e Without metabolic activation
f Positive results in tests for sister chromatid exchange may be a result of lysosome breakdown, and positive results in mouse lymphoma
cells may be due to pH and osmolality of culture medium
g With metabolic activation
1-Butanethiol (No. 511)
Pregnant COBS CD rats were exposed to 1-butanethiol by whole-body
inhalation for 6 h per day on days 6-19 of gestation at doses of 10,
68, or 152 mg/kg of diet. The control group was exposed to filtered
air only. The fetuses were removed from all rats on gestation day 20.
The animals were examined for gross physical changes, viable fetuses,
fetal body weights, maternal body-weight changes, and
post-implantation losses or total implantations. 1-Butanethiol had no
effect at the lowest dose, but significant differences in fetal body
weights were seen at 68 and 152 mg/kg of diet (Thomas et al., 1987).
Benzenethiol (No. 525)
Groups of 15-26 New Zealand white rabbits were given benzenethiol
in corn oil orally at doses of 10, 30, or 40 mg/kg bw per day on days
6-19 of gestation. Maternal body weight and feed and water consumption
were recorded. On gestation day 30, the animals were killed and
maternal organs and fetuses were weighed. The fetuses were also
examined for external, visceral, and skeletal malformations. There was
no effect at 10 mg/kg bw per day, but significant differences in
maternal body weights were seen at 30 and 40 mg/kg bw per day.
In the same study, groups of 25 rats were given 20, 35, or 50
mg/kg bw per day orally on days 6-15 of gestation and were killed on
gestation day 20. They were examined for the same parameters as the
rabbits. Decreased mater-nal body weight was seen at all doses, and
fetal body weights were decreased at 35 and 50 mg/kg bw per day. At 50
mg/kg bw, the relative maternal liver weights were increased and
increased post-implantation losses occurred, with a slight but
nonsignificant decrease in the live litter size (George et al., 1995).
2.3.2.7 Chemoprevention
Several organosulfur compounds inhibit carcinogenesis by
increasing the metabolism, detoxification, and elimination of
carcinogens by phase I and II enzymes. For example, the effect of
oltipraz in inhibiting colon tumorigenicity is associated with
increased activity of glutathione S-transferase, NAD(P)H quinone
reductase, and UDP-glucuronosyl transferase in the colon (Reddy et
al., 1993). This effect may partly explain the cancer-inhibiting
effect of foods like garlic that contain organosulfur compounds.
Allyl sulfide (No. 458)
Seven-week-old female A/J mice were given allyl sulfide at a dose
of 200 mg/kg bw per day in the diet for three days. Two hours after
treatment, the animals were given either a single dose of 100 mg/kg bw
of the carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and
kept for 16 weeks for determination of lung tumour production or
killed immediately for preparation of lung and liver microsomes.
Administration of the test material before carcinogen treatment
significantly decreased the lung tumour incidence (by 38%) and tumour
multiplicity (by 0.6) (Hong et al., 1994).
Allyl mercaptan (No. 521)
Groups of male weanling rats were given free access to a purified
diet for two weeks and then given the same diet containing 0.2% allyl
mercaptan dissolved in corn oil, corresponding to a calculated (Food
and Drug Administration, 1993) daily intake of allyl mercaptan of 200
mg/kg bw. After two weeks, the rats were injected intraperitoneally
with a single dose of 2 mg/kg bw aflatoxin B1, 910 mg/kg bw
N-nitrosodimethylamine, or 50 mg/kg bw N-methyl- N-nitrosourea
and killed 4 h later. Hepatic DNA damage was evaluated as
single-strand breaks by the alkaline elution technique, and the amount
of DNA eluted was determined by a microfluorimetric procedure. Allyl
mercaptan significantly reduced (by 64%) the incidence of DNA
single-strand breaks induced by aflatoxin B1 and reduced that induced
by N-nitrosodimethylamine by 39%. It had no effect on the incidence
of breaks induced by N-methyl- N-nitrosourea (LeBon et al., 1997).
Propyl disulfide (No. 566)
Cultures of the adult human tumour cell lines HCT-15 (colon),
A549 (lung), and SKMEL-2 (skin) were plated for 24 h and then propyl
disulfide was added at a dose of 100 mmol for up to 48 h. The cells
were harvested and intracellular free calcium and apoptotic cells were
determined. There was no effect on apoptosis, but a 12% increase in
the intracellular concentration of calcium was observed (Sundaram &
Milner, 1996).
Allyl disulfide (No. 572)
Cultures of the adult human tumour cell lines HCT-15 (colon),
A549 (lung), and SKMEL-2 (skin) were plated for 24 h and then allyl
disulfide was added at a dose of 100 or 500 mmol for up to 48 h. The
cells were harvested and intracellular free calcium, apoptotic cells,
and DNA fragmentation were determined. The proliferation of the HCT-15
and SKMEL-2 cell lines was depressed by 90% and that of the A549 line
by 75%, and the intracellular calcium concentration was increased by
41%. Morphological changes characteristic of apoptosis were observed
in treated cells, and a fivefold increase in DNA fragmentation was
seen (Sundaram & Milner, 1996).
Groups of 12 male Fischer 344 rats received diallyl disulfide at
a concentra-tion of 0, 100, or 200 mg/kg of diet. After two weeks, the
animals received azoxymethane, a known carcinogen, subcutaneously once
a week for two weeks at a dose of 15 mg/kg bw per week. Treatment
continued for 52 weeks, at which time the animals were killed. Body
weights were recorded every two weeks for the first 10 weeks and then
every four to six weeks. At autopsy, the animals were dissected in
order to detect microscopic tumours, and their location, number, and
size were recorded. Colon tumors with a diameter > 0.5 cm were cut
into two halves: one portion was used to measure enzyme activity and
the other for histopathology. Animals fed 200 mg/kg of diet showed a
slight but significant decrease in body weight. Neither the incidence
nor the multiplicity of colon adenomas differed between the control
and experimental groups, but both doses significantly reduced the
incidence and multiplicity of invasive colon adenocarcinomas. The
activities of glutathione S-transferase, NAD(P)H quinone reductase,
and UDP-glucuronosyl transferase in the liver, colonic mucosa, and
tumours were also significantly increased in the treated animals
(Reddy et al., 1993).
2.3.3 Observations in humans
Methylsulfinylmethane (DMSO; No. 507)
In clinical trials to determine the therapeutic effects of DMSO,
8900 mg/kg bw of 90% DMSO were applied to the entire trunk of 20 men
either twice daily for three weeks or once daily for 26 weeks.
Haematological examinations, blood chemical determinations, and urine
analyses performed at the beginning and end of the three-week study
revealed no significant changes. In the 26-week study, similar
examinations were performed at 0, 2, 4, 8, 12, 16, and 24 weeks, in
addition to thymol turbidity testing and sodium sulfobromophthalein
determinations. Except for the appearance of cutaneous signs such as
erythema, scaling, contact urticaria, and stinging or burning
sensations, DMSO was tolerated well by all but two individuals, who
developed systemic symptoms including a scaling rash, severe abdominal
pain, slight nausea, and chest pains during the second week of
treatment. One of these men did not continue the study; the other
continued treatment, and the symptoms eventually abated. Clinical and
laboratory investigations showed no evidence of adverse effects
(Kligman, 1965).
Two drops of aqueous solutions of increasing concentrations of
DMSO were instilled into the lower conjunctival sac of groups of 10
adult men, each eye being used only once without washing. No adverse
effects were seen until a concentration of 50% was reached, when the
men complained of transient burning. With 90% DMSO, all the men noted
temporary stinging and burning. The external eye was completely normal
24 h after treatment. The author concluded that DMSO is absorbed so
rapidly through the conjunctival mucosa that any irritation would not
be manifested (Kligman, 1965).
While studies by oral administration are most appropriate for
evaluating the safety of flavouring substances, the results of studies
of dermal absorption may be considered useful in their absence for
substances such as DMSO, which are readily absorbed into the systemic
circulation. The lack of treatment-related adverse effects (other than
irritation) in rhesus monkeys after both oral and dermal treatment
with DMSO (Vogin et al., 1970; see above) demonstrates that the
toxicity of DMSO is independent of route of administration. Therefore,
the lack of toxic effects other than irritation in studies in humans
treated dermally or in the eye (Kligman, 1965) suggests that oral oral
intake of DMSO used as a flavouring substance would not be toxic.
3. REFERENCES
Aeschbacher, H.U., Wolleb, U., Loliger, J., Spadone, J.C. & Liardon R.
(1989) Contribution of coffee aroma constituents to the mutagenicity
of coffee. Food Chem. Toxicol., 27, 227-232.
Alterman, M.A., Chaurassia, C.S., Lu, P. & Hanzlik R.P. (1995)
Metabolism of 10-methoxydecanoic acid (1) and 10-(methylthio)decanoic
acid(2), (omega-1)-oxa and -thia analogs of lauric acid. Int. Soc.
Study Xenobiotics, 8, 118.
Ames, M.M., Selassie, C.D., Woodson, L.C., Van Loon, J.A., Hansch, C.
& Weinshilboum, R.M. (1986) Thiopurine methyltransferase:
Structure-activity relationships for benzoic acid inhibitors and
thiophenol substrates. J. Med. Chem., 29, 345-358.
Anders, M.W., Ratnayake, J.H., Hanna, P.E. & Fucus, J.A. (1980)
Involvement of thioredoxin in sulfoxide reduction by mammalian
tissues. Biochem. Biophys. Res. Commun., 97, 846-851.
Anders, M.W., Ratnayake, J.H., Hanna, P.E. & Fucus, J.A. (1981)
Thioredoxin-dependent sulfoxide reduction by rat renal cytosol.
Biochem. Drug Metab. Disposition, 9, 307-310.
Bailey, D.E. (1976a) Acute oral toxicity (LD50) studies in mice with
1,8-octanedithiol. Unpublished study submitted to the Flavor and
Extract Manufacturers' Association by Food and Drug Research
Laboratories, Inc.
Bailey, D.E. (1976b) Acute oral toxicity of 1,2-propanedithiol in
mice. Unpublished study submitted to the Flavor and Extract
Manufacturers' Association by Food and Drug Research Laboratories,
Inc.
Bailey, D.E. (1976c) Acute oral toxicity (LD50) studies in mice with
compound 75-31065 (methyl benzyl disulfide). Unpublished study
submitted to the Flavor and Extract Manufacturers' Association by Food
and Drug Research Laboratories, Inc.
Black, R.M., Brewster, K., Clarke, R.J., Hambrook, J.L., Harrison,
J.M. & Howells, D.J. (1993) Metabolism of thiodiglycol
(2,2'-thiobis-ethanol): Isolation and identification of urinary
metabolites following intraperitoneal administration to rat.
Xenobiotica, 23, 473-481.
Bradley, M.O., Taylor, V.L., Armstrong, M.J. & Galloway, S.M. (1987)
Relationship among cytotoxicity, lysosomal breakdown, chromosome
aberrations, and DNA double-strand breaks. Mutat. Res., 189, 69-79.
Brams, A., Buchet, J.P., Crutzen-Fayt, M.C., DeMeester, C., Lauwerys,
R. & Leonard, A. (1987) A comparative study, with 40 chemicals, of the
efficiency of the Salmonella assay and the SOS Chromotest (kit
procedure). Toxicol. Lett., 38, 123-133.
Bremer, J. & Greenberg, D.M. (1961) Enzymic methylation of foreign
sulfhydryl compounds. Biochim. Biophys. Acta, 46, 217-224.
British Industrial Biological Research Association (1976) Short-term
toxicity of samples TT171, TT172, TT173, and TT174 in rats.
Unpublished study submitted to the Flavor and Extract Manufacturers'
Association.
Brodnitz, M.H. & Pascale, J.V. (1971) Thiopropanol S-oxide: A
lachrymatory factor in onions. J. Agric. Food Chem., 19, 269.
Brown, V.K., Robinson, J. & Stevenson, D.E. (1963) A note on the
toxicity and solvent properties of dimethyl sulfoxide. J. Pharm.
Pharmacol., 15, 688-692.
Brusick, D. (1986) Genotoxic effects in cultured mammalian cells
produced by low pH treatment conditions and increased ion
concentrations. Environ. Mutag., 8, 879-886.
Buckberry, L.D. & Teesdale-Spittle, P.H. (1996) Sulfur-hydrogen
compounds. In: Mitchell, S., ed., Biological Interactions of Sulfur
Compounds, London: Taylor & Francis Ltd, pp. 113-144-76.
Butterworth, K.R. & Mason, P.L. (1981) Acute toxicity of thioguaiacol
and of versalide in rodents. Food Chem. Toxicol., 19, 753-755.
Butterworth, K.R., Carpanini, F.M.B., Gaunt, I.F., Hardy, J., Kiss,
I.S. & Gangolli, S.D. (1975) Short-term toxicity of dimethyl sulfide
in rat. Food Cosmet. Toxicol., 13, 15-22.
Canellakis, E.S. & Tarver, H. (1953) The metabolism of methyl
mercaptan in the intact animal. Arch. Biochem. Biophys., 42,
446-455.
Cashman, J.R. & Olsen, L.D. (1990) Stereoselective S-oxygenation of
2-aryl-1,3-dithiolanes by the flavin-containing and cytocrome P-450
monooxygenases. Mol. Pharmacol., 38, 573-585.
Cashman, J.R. & Williams, D.E. (1990) Enantioselective S-oxygenation
of 2-aryl-1,3-dithiolanes by rabbit lung enzyme preparations.
Mol. Pharmacol., 37, 333.
Cashman, J.R., Olsen, L.D. & Bornheim, L.M. (1990) Enantioselective
S-oxygenation by flavin containing cytochrome P-450 mono-oxygenases.
Chem. Res. Toxicol., 3, 344.
Cashman, J.R., Yang, Z.C., Yang, L. & Wrighton, S.A. (1995a)
Stereo-and regioselective N-and S-oxygenation of tertiary amines and
sulfides in adult human liver microsomes. Int. Soc. Study
Xenobiotics, 8, 34.
Cashman, J.R., Park, S.B., Yang, Z.C., Washington, C.B., Gomez, D.Y.,
Giacomini, K. & Brett, C.M. (1995b) Chemical, enzymatic and human
enantioselective S-oxygenation of cimetidine. Int. Soc. Study
Xenobiotics, 8, 133.
Christias, C. (1975) Specific inhibition of sclerotium formation by
2-mercaptoethanol and related sulfhydryl compounds in Sclerotium
rolfsii. Can. J. Microbiol., 21, 1541-1547.
Collinson, V.A. (1989) ST 15 C 88 Acute oral toxicity study in the
rat. Unpublished study No. A/0/13008 from Toxicol Laboratories Ltd,
United Kingdom.
Cox, G.E., Bailey, D.E. & Morgareidge, K. (1973a) 90-Day feeding
studies in rats with trithioacetone. Unpublished report submitted to
the Flavor and Extract Manufacturers' Association by Food and Drug
Research Laboratories, Inc.
Cox, G.E., Bailey, D.E. & Morgareidge, K. (1973b) 90-Day feeding
studies in rats with compound ES-307/308 (14705). Unpublished report
submitted to the Flavor and Extract Manufacturers' Association by Food
and Drug Research Laboratories, Inc.
Cox, G.E., Bailey, D.E. & Morgareidge, K. (1974a) 90-Day feeding study
in rats with compound 14935 (2-mercapto-3-butanol). Unpublished report
submitted to the Flavor and Extract Manufacturers' Association by Food
and Drug Research Laboratories, Inc.
Cox, G.E., Bailey, D.E. & Morgareidge, K. (1974b) 90-Day feeding
studies in rats with compound 31098. Unpublished report submitted to
the Flavor and Extract Manufacturers' Association by Food and Drug
Research Laboratories, Inc.
Cox, G.E., Rucci, G. & Babish, J.G. (1979) 90-Day subacute dietary
toxicity study of IFF code No. 78-002-2 in Sprague-Dawley rats.
Unpublished report submitted to the Flavor and Extract Manufacturers'
Association by Food and Drug Research Laboratories, Inc.
Damani, L.A. (1987) Metabolism of sulphur-containing drugs. In:
Benford, D.J., Bridges, J.W. & Gibson, G.G., eds,
Drug Metabolism--From Molecules to Man, London: Taylor & Francis,
pp. 581-603.
Davis, P.J. & Guenthner, L.E. (1985) Sulindac oxidation/reduction by
microbial cultures; microbial models for mammalian metabolism.
Xenobiotica, 15, 845-857.
Deng, D.J., Mueller, K., Kasper, P. & Mueller, L. (1994) Effect of
diallyl trisulfide on induction of UDS by mutagenic drugs in primary
rat hepatocytes. Biomed. Environ. Sci., 7, 85-90.
Dutton, G.J. & Illing, H.P.A. (1972) Mechanism of biosynthesis of
thio-beta-D-glucosides. Biochem. J., 129, 539-550.
Eder, E., Neudecker, T., Lutz, D. & Henschler, D. (1980) Mutagenic
potential of allyl and allylic compounds. Structure-activity
relationship as determined by alkylating and direct in vitro mutagenic
properties. Biochem. Pharmacol., 29, 993-998.
Eder, E., Henschler, D. & Neudecker, T. (1982) Mutagenic properties of
allylic and alpha, beta-unsaturated compounds: Consideration of
alkylating mechanisms. Xenobiotica, 12, 831-848.
Elfarra, A.A., Duescher, R.J., Sausen, P.J., Lawton, M.P. & Philpot,
R.M. (1995) Potential role of the flavin-containing monooxygenases in
the metabolism of endogenous compounds. Int. Soc. Study Xenobiotics,
8, 9.
El Fatih, Karim, I.A., Millership, J.S., Temple, D.J. & Woolfson, A.D.
(1988) An investigation of the metabolism of
S-carboxymethyl-L-cysteine in man using a novel HPLC-ECD method.
Eur. J. Drug. Metab. Pharmacokinet., 13, 253.
Elf Atochem (1963) Unpublished study from Pharmacology Research Inc.
Elf Atochem (1985) Unpublished study from Product Safety Labs.
Elf Atochem (1992) Unpublished study from Centre International de
Toxicologie.
Fairchild, E.J. & Stokinger, H.E. (1958) Toxicologic studies on
organic sulfur compounds. 1. Acute toxicity of some aliphatic and
aromatic thiols (mercaptans). Am. Ind. Hyg. Assoc. J., 19, 171-189.
Farr, C.H. & Kirwin, C.J. (1994) Organic sulfur compounds. In:
Clayton, D.G. & Clayton, F.E., eds, Patty's Industrial Hygiene
and Toxicology, 4th Ed., Vol. 2, New York: John Wiley & Sons, pp.
4311-4372.
Feng, P.C.C. & Solsten, R.T. (1991) In vitro transformation of
dithiopyr by rat liver enzymes: Conversion of methylthioesters to
acids by oxygenases. Xenobiotica, 21, 1265.
Fenwick, G.R. & Hanley, A.B. (1985) The genus Allium, part 2. CRC
Crit. Rev. Food Sci. Nutr., 22, 273-377.
Fishman, F.G., Jenkins, L.J., Jr, Coon, R.A. & Jones, R.A. (1969)
Effects of acute and repeated inhalation of dimethyl sulfoxide in
rats. Toxicol. Appl. Pharmacol., 15, 74-82.
Flavor and Extract Manufacturers' Association (1991) In vitro
hydrolysis of 3-methylthiohexy acetate. Unpublished report No. 183/91.
Flavor and Extract Manufacturers' Association (1996) Sensory tolerance
test protocol. Unpublished report.
Fogelman, R.W. & DeProspo, J. (1973a) Acute oral toxicity in mice with
ES-307/308. Unpublished report submitted to the Flavor and Extract
Manufacturers' Association by Affiliated Medical Research Institute.
Fogelman, R.W. & DeProspo, J. (1973b) Acute oral toxicity in mice with
SB-11-2876 (trithioacetate). Unpublished report submitted to the
Flavor and Extract Manufacturers' Association by Affiliated Medical
Research Institute.
Fogelman, R.W. & DeProspo, J. (1974) Acute oral toxicity in mice with
1,2-ethanedithiol. Unpublished report submitted to the Flavor and
Extract Manufacturers' Association by Affiliated Medical Research
Institute.
Fogelman, R.W. & Suppers, L. (1973) Acute oral toxicity in mice with
1,8-octanedithiol. Unpublished report submitted to the Flavor and
Extract Manufacturers' Association by Affiliated Medical Research
Institute.
Fogelman, R.W. & Suppers, L. (1974a) Acute oral toxicity in mice with
1,8-octanedithiol. Unpublished report submitted to the Flavor and
Extract Manufacturers' Association by Affiliated Medical Research
Institute.
Fogelman, R.W. & Suppers, L. (1974b) Acute oral toxicity in mice with
1,2-propanedithiol. Unpublished report submitted to the Flavor and
Extract Manufacturers' Association by Affiliated Medical Research
Institute.
Fogelman, R.W., Bukva, N.F. & Margolin, S. (1970) Acute oral toxicity
of 2-4252. Unpublished study submitted by Affiliated Medical
Enterprises, Inc. Princeton, NJ, USA.
Food and Drug Administration (1993) Priority-based Assessment of
Food Additives (PAFA) Database, Waskington DC: Center for Food
Safety and Applied Nutrition, p. 58.
Frandsen, A., Schousboe, A. & Griffiths, R. (1993) Cytotoxic actions
and effects on intracellular Ca2+ and cGMP concentrations of
sulphur-containing excitatory amino acids in cultured cerebral
cortical neurons. J. Neurosci. Res., 34, 331-339.
Gachon, F., Nicolas, C., Maurizis, C., Verny, M., Chabard, J.L.,
Faurie, M. & Gaillard, G. (1988) Disposition and metabolism of
letosteine in rats. Drug Metab. Disposition, 16, 853.
Gallo, M.A., Cox, G.E. & Babish, J.G. (1976) 90-Day feeding studies in
rats with compound 75-31065 (methyl benzyl disulfide). Unpublished
report to the Flavor and Extract Manufacturers' Association by Food
and Drug Research Laboratories, Inc..
George, J.D., Price, C.J., Navarro, H.A., Barr, M.C., Myers, C.B.,
Hunter, E.S., Schwetz, B.A. & Shelby, M.D. (1995) Developmental
toxicity of thiophenol (thio) in rats and rabbits. Toxicologist, 15,
160.
Gibson, T.P., Dobrinska, M.R., Lin, J.H., Entwistle, L.A. & Davies,
R.O. (1987) Biotransformation of sulindac in end-stage renal disease.
Clin. Pharmacol. Ther., 42, 82-88.
Greenzaid, P. & Jencks, W.P. (1971) Pig liver esterase. Reactions with
alcohols, structure-reactivity correlations, and the acyl-enzyme
intermediate. Biochemistry, 10, 1210.
Griffiths, P.J. & Babish, J.G. (1978) Acute oral toxicity (LD50) of
DSE 3303S (15277) in albino mice (BLU: Ha(ICR)). Unpublished report
No. B-3 to the Flavor and Extract Manufacturers' Association from Food
and Drug Research Laboratories, Inc.
Griffiths, P.J., Giessinger, M. & Fouillet, X. (1979) Report on acute
oral toxicity (LD/50) and three-month toxicity of TT 184. Unpublished
report to the Flavor and Extract Manufacturers' Association from
Battelle Geneva Research Centres.
de Groot, A.P., Spanjers, M.T. & van der Heijden, C.A. (1974) Acute
and sub-acute oral toxicity studies in rats with five flavour
compounds. Unpublished report submitted to the Flavor and Extract
Manufacturers' Association.
Grosa, G., Caputo, O., Cerutti, M., Biglino, G., Franzone, J.S.%
Cravanzola, C. (1991) Metabolism of
7-(1,3-dithiolan-2-ylmethyl)1,3-dimethylxanthine by rat liver
microsomes: Diastereoselective metabolism of the 1,3-dithiolane ring.
Drug Metab. Disposition, 19, 454-457.
Hakura, A., Mochida, H. & Yamatsu, K. (1993) Dimethyl sulfoxide (DMSO)
is mutagenic for bacterial mutagenicity tester strains. Mutat. Res.,
303, 127-133.
Harper, K.H. & Gin, H.B. (1964) The acute oral toxicity to rats of
T59. Unpublished report.
Heck, J.D., Vollmuth, T.A., Cifone, M.A., Jagannath, D.R., Myhr, B. &
Curren, R.D. (1989) An evaluation of food flavoring ingredients in a
genetic toxicity screening battery. Toxicologist, 9, 257.
Hermansky, S.J. & Weaver, E.C. (1990) Fourteen-day dietary minimum
toxicity screen (MTS) with acetyl lactic, thiomethyl ester and
propiodyl lactic, thiomethyl ester in Fischer 344 rats. Unpublished
report 53-553 to the Flavor and Extract Manufacturers' Association
from Bushy Run Research Center, Pennsylvania, USA.
Hodgson, E. & Levi, P.A. (1992) The role of the flavin-containing
monooxygenases (EC 1.14.13.8) in the metabolism and mode of action of
agricultural chemicals. Xenobiotica, 22, 1175-1183.
Hong, J.Y., Lin, M.C., Wang, Z.Y., Wang, E.J. & Yang, C.S. (1994)
Inhibition of chemical toxicity and carcinogenesis by diallyl sulfide
and diallyl sulfone. In: Huang, M.-T., Osawa, T., Ho, C.-T. & Rosen,
R.T., eds, Food Phytochemicals for Cancer prevention I, Fruits and
Vegetables (ACS Symposium Series 546), Washington DC: American
Chemical Society, pp. 97-101.
Hoodi, A.A. & Damani, L.A. (1984) Cytochrome P-450 and non-P-450
sulphoxidations. J. Pharm. Pharmacol., 36, 62P.
Hucker, H.B., Miller, J.K., Hochberg, A., Brobyn, R.D., Riordan, F.H.
& Calesnick, B. (1967) Studies on the absorption, excretion, and
metabolism of dimethyl sulfoxide (DMSO) in man. J. Pharmacol. Exp.
Ther., 155, 309-317.
International Organization of the Flavor Industry (1995) European
inquiry on volume of use. Private communication to the Flavor and
Extract Manufacturers' Association.
Karekar, V.R., Mujumdar, A.M., Joshi, S.S., Dhuley, J., Shinde, S.L. &
Ghaskadbi, S. (1996) Assessment of genotoxic effect of piperine using
Salmonella typhimurium and somatic and germ cells of Swiss albino
mice. Arzneimittel-Forsch., 46, 972-975.
Keith, R.A., Abraham, R.T., Pazmino, P. & Weinshilboum, R.M. (1983)
Correlation of low and high affinity thiol methyltransferase and
phenol methyltransferase activities in human erythrocyte membranes.
Clin. Chim. Acta, 131, 257-272.
Klancnik, J.M., Cuenod, M., Gahwiler, B.H., Jiang, Z.P. & Do, K.Q.
(1992) Release of endogenous amino acids, including homocysteic acid
and cysteine sulphinic acid, from rat hippocampal slices evoked by
electrical stimulation of Schaffer collateral-commissural fibres.
Neuroscience, 49, 557-570.
Kligman, A.M. (1965) Dimethyl sulfoxide--Part 2. J. Am. Med. Assoc.,
193, 151-156.
Koptyaev, V.G. (1967) Experimental determination of maximum
permissible concentration of dimethyl sulfide in water bodies. Hyg.
Sanit., 32, 315-320.
Kurooka, S., Hashimoto, M., Tomita, M., Maki, A. & Yoshimura, Y.
(1976) Relationship between the structure of S-acyl thiol compounds
and their rates of hydrolysis by pancreatic lipase and hepatic
carboxylic esterase. J. Biochem., 79, 533-541.
Lavoie, E., Tulley, L., Fow, E. & Hoffmann, D. (1979) Mutagenicity of
aminophenyl and nitrophenyl ethers, sulfides, and disulfides. Mutat.
Res., 67, 123-131.
Layman, D.L. & Jacob, S.W. (1985) The absorption, metabolism, and
excretion of dimethyl sulfoxide by rhesus monkeys. Life Sci., 37,
2431-2437.
LeBon, A.M., Roy, C., Dupont, C. & Suschete, M. (1997) In vivo
antigenotoxic effects of dietary allyl sulfides in the rat. Cancer
Lett., 114, 131-134.
Lee, S.C. & Renwick, A.G. (1995a) Sulphoxide reduction by rat
intestinal flora and by Escherichia coli in vitro. Biochem.
Pharmacol., 49, 1567-1576.
Lee, S.C. & Renwick, A.G. (1995b) Sulphoxide reduction by rat and
rabbit tissues in vitro. Biochem. Pharmacol., 49, 1557-1565.
Levi, P.E. & Hodgson, E. (1988) Stereospecificity in the oxidation of
phorate and phorate sulphoxide by purified FAD-containing
mono-oxygenase and cytochrome P-450 isozymes. Xenobiotica, 18,
29-39.
Maarse, H., Visscher, C.A., Willemsens, L.C., Nijssen, L.M. & Boelens,
M.H., eds (1994) Volatile Components in Food, 6th Ed., Suppl. 5,
Zeist, TNO Nutrition and Food Research.
Maiorino, R.M., Bruce, D.C. & Aposhian, H.V. (1988) Determination and
metabolism of dithiol chelating agents. VI. Isolation and
identification of the mixed disulfides of meso-2,3-dimercaptosuccinic
acid with L-cysteine in human urine. Toxicol. Appl. Pharmacol., 97,
338.
Maiorino, R.M., Xu, Z.F. & Aposhian, H.V. (1996) Determination and
metabolism of dithiol chelating agents. XXII. In humans, sodium
1,3-dimercapto-1-propanesulfonate is bound to plasma albumin via mixed
disulfide formation and is found in the urine as cyclic polymeric
disulfides. J. Pharmacol. Exp. Ther., 277, 375-384.
Marks, H.S., Anderson, J.L. & Stoewsand, G.S. (1992) Inhibition of
benzo[a]pyrene-induced bone marrow micronuclei formation by diallyl
thioethers in mice. J. Toxicol. Environ. Health, 37, 1-9
Mazel, P., Henderson, J.F. & Axelrod, J. (1964) S-Demethylation by
microsomal enzymes. J. Pharmacol. Exp. Ther., 143, 1-6.
McBain, D.A. & Menn, J.J. (1969) S-Methylation, oxidation,
hydroxylation, and conjugation of thiophenol in the rat. Biochem.
Pharmacol., 18, 2282-2285.
Michael, W.R. 1968) Metabolism of linear alkylate sulfonate and alkyl
benzene sulfonate in albino rats. Toxicol. Appl. Pharmacol., 12,
473-485.
Mitchell, S.C., Idle, J.R. & Smith, R.L. (1982) The metabolism of
[14C] cimetidine in man. Xenobiotica, 12, 283.
Mondino, A. (1981) Thirteen week repeated dose study of the test
article TT 191 (3-methyl-1,2,4-trithiane) orally administered to
Sprague Dawley Charles River CD (SD) BR rats. Unpublished study from
Instituto di richierche biomedicine 'Antoine Marxer' SpA.
Mondino, A. & Peano, S. (1979) Acute oral toxicity of
2,6-dimethylthiophenol. Unpublished report to the Flavor and Extract
Manufacturers' Association.
Mondino, A. & Peano, S. (1982) Acute toxicity study, TT 201 and TT202.
Unpublished report from Instituto di richierche biomedicine 'Antoine
Marxer' SpA.
Mondino, A., Peano, S. & Berruto, P. (1981) Acute toxicity study,
TT191. Unpublished report from Instituto di richierche biomedicine
'Antoine Marxer' SpA.
Moran, E.J., Easterday, O.D. & Oser, B.L. (1980) Acute oral toxicity
of selected flavor chemicals. Drug Chem. Toxicol., 3, 249-258.
Moreno, O.M. (1975) Acute toxicity studies on rats, rabbits, and
guinea pigs. Unpublished report to RIFM.
Moreno, O.M. (1980) Acute toxicity studies. Unpublished report to
RIFM.
Morgareidge, K. (1970) 90-Day feeding study in rats with
tetrahydrothiophene-3-one (31015). Unpublished study submitted to the
Flavor and Extract Manufacturers' Association.
Morgareidge K. (1971a) 90-Day feeding study with 2-naphthalenethiol in
rats. Unpublished study submitted to the Flavor and Extract
Manufacturers' Association.
Morgareidge, K. (1971b) 90-Day feeding study with
2-keto-3-pentanethiol in rats. Unpubli-shed study submitted to the
Flavor and Extract Manufacturers' Association.
Morgareidge, K. & Oser, B.L. (1970a) 90-Day feeding studies in rats
with cyclopentane-thiol. Unpublished study submitted to the Flavor and
Extract Manufacturers' Association.
Morgareidge, K. & Oser, B.L. (1970b) 90-Day feeding studies in rats
with dipropyltrisulfide (30204). Unpublished study submitted to the
Flavor and Extract Manufacturers' Association.
Morgareidge, K. & Oser, B.L. (1970c) 90-Day feeding studies in rats
with diallyltrisulfide. Unpublished study submitted by Food and Drug
Research Laboratories, Inc. to the Flavor and Extract Manufacturers'
Association.
Morgareidge, K., Bailey, D.E. & Cox, G.E. (1974a) 90-Day feeding study
on 2,3-butanedithiol in rats. Unpublished study submitted to the
Flavor and Extract Manufac-turers' Association.
Morgareidge, K., Bailey, D.E. & Cox, G.E. (1974b) 90-Day feeding study
in rats with compound 14966. Unpublished study submitted to the Flavor
and Extract Manufac-turers' Association.
Morgareidge, K., Bailey, D.E. & Cox, G.E. (1974c) 90-Day feeding study
of 1,8-octane-dithiol in rats. Unpublished study submitted to the
Flavor and Extract Manufacturers' Association.
Moutiez, M., Aumercier, M., Tessier, E., Parmentier, B., Tartar, A. &
Sergheraert, C. (1994) Reduction of a trisulfide derivative of
glutathione by glutathione reductases. Biochem. Biophys. Res.
Commun., 202, 1380-1386.
Munday, R. & Manns, E. (1994) Comparative toxicity of prop(en)yl
disulfides derived from Alliaceae. Possible involvement of 1-propenyl
disulfides in onion-induced hemolytic anemia. J. Agric. Food Chem.,
42, 959-962.
Munday, R., Manns, E. & Fowke, E.A. (1990) Steric effects on the
haemolytic activity of aromatic disulphides in rats. Food Chem.
Toxicol., 28, 561-566.
National Academy of Sciences (1970) Evaluating the Safety of Food
Chemicals, Washington DC.
National Academy of Sciences (1982) Evaluating the Safety of Food
Chemicals, Washington DC.
National Academy of Sciences (1987) Evaluating the Safety of Food
Chemicals, Washington DC.
Nickson, R.M. & Mitchell, S.C. (1994) Fate of dipropyl sulfide and
dipropyl sulfoxide in rat. Xenobiotica, 24, 157-168.
Nickson, R.M., Mitchell, S.C. & Zhang, A.Q. (1995) Fate of dipropyl
sulfone in rat. Xenobiotica, 25, 1391-1398.
Nnane, P. & Damani, L.A. (1995) The involvement of rat liver CYP2B1
and CYP2D1 in the microsomal sulphoxidation of 4-chlorophenyl methyl
sulphide. Int. Soc. Study Xenobiotics, 8, 110.
Noel, P.R.B., Barnett, K.C., Davies, R.E., Jolly, D.W., Leahy, J.S.,
Mawdesley-Thomas, L.E., Shillam, K.W.G., Squires, P.F., Street, A.E.,
Tucker, W.C. & Worden, A.N. (1975) The toxicity of dimethyl sulphoxide
(DMSO) for the dog, pig, rat, and rabbit. Toxicologist, 3, 143-169.
Oae, S., Mikami, A., Matsura, T., Ogawa-Asada, K., Watanabe, Y.,
Fujimori, K. & Iyanagi, T. (1985) Comparison of sulfite oxygenation
mechanism for liver microsomal FAD-containing monooxygenase with that
for cytochrome P-450. Biochem Biophys. Res. Commun., 131, 567-573.
Ogasawara, Y., Isoda, S. & Tanabe, S. (1998) A labile sulfur in
trisulfide affects cytochrome P-450 dependent lipid peroxidation in
rat liver microsomes. Toxicol. Lett., 99, 191-198.
Opdyke, D.L.J. (1979) Monographs on fragrance raw materials. Food
Cosmet. Toxicol., 17, 769.
Oser, B.L. (1966) Feeding studies with pinanyl mercaptan (PK) in rats.
Unpublished study submitted to the Flavor and Extract Manufacturers'
Association.
Oser, B.L. (1970) The acute oral toxicity to mice of ten compounds.
Unpublished study submitted to the Flavor and Extract Manufacturers'
Association.
Panasevich, R.E., Mallory, V.T. & Matthews, R.J. (1980) Dose-related
finding and single dose oral study of ethyl 4-(methylthio)butyrate.
Unpublished study submitted to the Flavor and Extract Manufacturers'
Association.
Parkinson, A. (1996) Biotransformation of xenobiotics. In: Klaassen,
C.D., ed., Casarret and Doull's Toxicology: The Basic Science of
Poisons, 5th Ed., New York, McGraw-Hill Co., pp. 113-118.
Peano, S., Roffino, A., Milone, M.F., Orlando, L. & Berruto, G. (1981)
Thirteen week repeated dose study with 2,6-dimethylthiophenol orally
administered to Sprague-Dawley Charles River CD (SD) BR rats.
Unpublished study submitted to the Flavor and Extract Manufacturers'
Association by Instituto di richierche biomedicine 'Antoine Marxer'
SpA.
Phillips Petroleum Co. (1990a) Toxicity study
summary--1,2-Ethanedithiol (TOX071).
Phillips Petroleum Co. (1990b) Toxicity study summary--Isopropyl
mercaptan (TOX083).
Phillips Petroleum Co. (1990c) Toxicity study summary--Tertiary butyl
mercaptan (TOX1-6).
Piccirillo, V.J. & Lunchicki, C. (1982) Acute oral toxicity (LD50)
study in the rat with methyl-2-methylthiobutyrate. Unpublished report
submitted to the Flavor and Extract Manufacturers' Association.
Posternak, J.M., Linder, A. & Vodoz, C.A. (1969) Summaries of
toxicological data. Toxicological tests on flavoring matters. Food
Cosmet. Toxicol., 7, 405-407.
Reddy, B.S., Rao, C.V., Rivenson, A. & Kelloff, G. (1993)
Chemoprevention of colon carcinogenesis by organosulfur compounds.
Cancer Res., 53, 3493-3498.
Renwick, A.G. (1989) Sulphoxides and sulphones. In: Damani, L.A., ed.,
Sulphur-containing Drugs and Related Organic Compounds. Chemistry,
Biochemistry and Toxicology (Ellis Horwood Series in Biochemical
Pharmacology), Vol. 1, Part B, New York: John Wiley & Sons, pp.
133-154.
Renwick, A.G. (1996) Sulfur-oxygen compounds. In: Mitchell, S., ed.,
Biological Interactions of Sulfur Compounds, London: Taylor &
Francis, pp. 42-76.
Renwick, A.G. & George, C.F. (1989) Metabolism of xenobiotics in the
gastro-intestinal tract. In: Hudson, D.H., Caldwell, J. & Paulson,
G.D., eds, Intermediary Xenobiotic Metabolism in Animals:
Methodology, Mechanisms and Significance, London, Taylor & Francis,
pp. 13-40.
Renwick, A.G., Evans, S.P., Sweatman, T.W., Cumberland, J. & George,
C.F. (1982) The role of the gut flora in the reduction of
sulphinpyrazone in the rat. Biochem. Pharmacol., 31, 2649.
Rettie, A.E., Bogucki, B.D., Lim, I. & Meier, G.P. (1990)
Stereoselective sulfoxidation of a series of alkyl p-tolyl sulfides by
microsomal and purified flavin-containing monooxygenases.
Mol. Pharmacol., 37, 643.
Rettie, A.E., Lawton, M.P., Jaffer, A., Sadeque, M. & Meier, G.P.
(1994) Prochiral sulfoxidation as a probe for multiple forms of the
microsomal flavin-containing monooxygenase: Studies with rabbit FMO1,
FMO2, FMO3, and FMO5 expressed in Escherichia coli. Arch. Biochem.
Biophys., 311, 369.
Richardson, K.A., Edward, V.T., Jones, B.C. & Hutson, D.H. (1991)
Metabolism in the rat of a model xenobiotic plant metabolite
S-benzyl-N-malonyl-L-cysteine. Xenobiotica, 21, 371.
Rush, R.E. (1991) 14-Day dietary toxicity study in rats with
3-(methylthio)hexyl acetate (MTHA). Unpublished report No. 3141.5 from
Springborn Laboratories, Inc. to the Flavor and Extract Manufacturers'
Association.
Sadeque, A.J.M., Eddy, A.C., Meierand, G.P. & Rettie, A.E. (1992)
Stereoselective sulfoxidation by human flavin-containing
monooxygenase. Drug Metab. Disposition, 20, 832.
Sadeque, A.J.M., Philpot, R.M. & Rettie, A.E. (1995) Chiral
sulfoxidation by human liver FMO3 and FMO5. Int. Soc. Study
Xenobiotics, 8.
Sanders, A. (1997) Acute oral toxicity (limit test) in the rat.
Unpublished SPL project No. 012/234 submitted to the Flavor and
Extract Manufacturers' Association.
Schafer, E.W. & Bowles, W.A., Jr (1985) Acute oral toxicity and
repellency of 933 chemicals to house and deer mice. Arch. Environ.
Contam. Toxicol., 14, 111-129.
Selyuzhitskii, G.V. (1972) Experimental data on substantiation of the
maximum allowable concentraion of methyl mercaptan, dimethyl sulfide
and dimethyl disulfide in the air of the working area of pulp paper
plants. Gig. Tr. Prof. Zabol., 16, 46-47.
Shaw, P.N. & Blagbrough, I.S. (1989) Cysteine conjugate beta-lyase,
II: Isolation, properties and structure-activity relationships. In:
Damani, L.A., ed., Sulphur-containing Drugs and Related Organic
Compounds, Vol. 2, Part B, Chichester: Ellis Horwood Ltd, pp.
135-156.
Shellenberger, T.E. (1970) Subacute toxicity evaluation of ethyl
thioacetate in rats. Final report. Unpublished report GSRI Project
NC-373 from Gulf South Research Institute submitted to the Flavor and
Extract Manufacturers' Association.
Shellenberger, T.E. (1971a) Acute toxicological evaluations of
2-naphthalenethiol in mice. Unpublished report GSRI Project NC-398
from Gulf South Research Institute submitted to the Flavor and Extract
Manufacturers' Association.
Shellenberger, T.E. (1971b) Acute toxicological evaluations of
chemicals with mice--2-Keto-3-pentanethiol. Unpublished study from
Gulf South Research Institute.
Snow, G.A. (1957) The metabolism of compounds related to ethanethiol.
J. Biochem., 65, 77-82.
Sommer, S. & Tauberger, G. (1964) Toxicologic investigations of
dimethyl sulfoxide. Arnzeimittel. Forsch., 14, 1050-1053.
Souhaili-El Amri, H., Fargetton, X., Delatour, P. & Batt, A.M. (1987)
Sulphoxidation of albendazole by the FAD-containing and cytochrome
P-450 dependent mono-oxygenase from pig liver microsomes.
Xenobiotica, 17, 1159-1168.
Stofberg, J. & Grundschober, F. (1987) Consumption ratio and food
predominance of flavoring materials. Perfumer Flavorist, 12, 27.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of
flavouring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23, 857-860.
Strong, H.A., Renwick, A.G. & George, C.F. (1984a) The site of
reduction of sulphinpyrazone in the rabbit. Xenobiotica, 14,
815-826.
Strong, H.A., Oates, J., Sembi, J., Renwick, A.G. & George, C.F.
(1984b) Role of the gut flora in the reduction of sulphinpyrazone in
humans. J. Pharmacol. Exp. Ther., 230, 726-732.
Strong, H.A., Warner, J., Renwick, A.G. & George, C.F. (1985) Sulindac
metabolism: The importance of an intact colon. Clin. Pharmacol.
Ther., 38, 387-393.
Strong, H.A., Angus, R., Oates, J., Sembi, J., Howarth, P., Renwick,
A.G. & George, C.F. (1986) Effects of ischaemic heart disease, Crohn's
disease and anti-microbial therapy on the pharmacokinetics of
sulphinpyrazone. Clin. Pharmacokinet., 11, 402-411.
Strong, H.A., Renwick, A.G., George, C.F., Liu, Y.F. & Hill, M.J.
(1987) The reduction of sulphinpyrazone and sulindac by intestinal
bacteria. Xenobiotica, 17, 685-696.
Sundaram, S.G. & Milner, J.A. (1996) Diallyl disulfide induces
apoptosis of human colon tumor cells. Carcinogenesis, 17, 669-673.
Szumlanski, C.L., Scott, M.C. & Weinshilboum, R.M. (1988) Thiopurine
methyltransferase pharmacogenetics: Human liver enzyme activity.
Clin. Pharmacol. Ther., 43, 134.
Szybalski, W. (1958) Special microbiological systems. II. Observations
on chemical mutagenesis in microorganisms. Ann. N.Y. Acad. Sci., 76,
475-489.
Takata, T., Yamazaki, M., Fujimori, K., Kim, H.Y., Iyanagi, T. & Oae,
S. (1983) Enzymatic oxygenation of sulfides with cytochrome P-450 from
rabbit liver: Stereochemistry of sulfoxide formation. Bull. Chem.
Soc. Jpn., 55, 2300-2310.
Tateishi, M. & Tomisawa, H. (1989) Cysteine conjugate beta-lyase. I:
Toxic thiol production. In: Damani, L.A., ed., Sulphur-containing
Drugs and Related Organic Compounds, Vol. 2, Part B, Chichester:
Ellis Horwood Ltd, pp. 121-134.
Tateishi, M., Suzuki, S. & Shimizu, H. (1978) Cysteine conjugate
beta-lyase in rat liver. J. Biol. Chem., 253, 8854.
Tatsumi, K., Kitamura, S. & Yamada, H. (1982) Involvement of liver
aldehyde oxidase in sulfoxide reduction. Chem. Pharm. Bull., 30,
4585-4588.
Tatsumi, K., Kitamura, S. & Yamada, H. (1983) Sulfoxide reductase
activity of liver aldehyde oxidase. Biochim. Biophys. Acta, 747,
86-92.
Taylor, K.L. & Ziegler, D.M. (1987) Studies on substrate specificity
of the hog liver flavin-containing monooxygenase. Anionic organic
sulfur compounds. Biochem. Pharmacol., 36, 141-146.
Taylor, A.J., Olavesen, A.H., Black, J.G. & Howes, D. (1978) The
metabolism of the surfactants dodecylsulfonate and hexadecylsulfonate
in the rat. Toxicol. Appl. Pharmacol., 45, 105-117.
Thomas, W.C., Seckar, J.A., Johnson, J.T., Ulrich, C.E, Klonne, D.R.,
Schardein, J.L. & Kirwin, C.J. (1987) Inhalation teratology studies of
n-butyl mercaptan in rats and mice. Fundam. Appl. Toxicol., 8,
170-178.
Vogin, E.E., Carson, S., Cannon, G., Linegar, C.R. & Rubin, L.F.
(1970) Chronic toxicity of DMSO in primates. Toxicol. Appl.
Pharmacol., 16, 606-612.
Wang, C.J., Lin, Y.L. & Lin, J.K. (1994) Mutagenicity and cytotoxicity
of nitropyrrole compounds derived from the reaction of 2-acetyl
pyrrole with nitrate. Food Chem. Toxicol., 32, 839-844.
Waring, R.H. (1996) Sulfur-sulfur compounds. In: Mitchell, S., ed.,
Biological Interactions of Sulfur Compounds, London: Taylor &
Francis, pp. 145-173.
Watanabe, S. & Kinosaki, A. (1989a) Acute oral toxicity in the rat
with acetyllactic acid thiomethyl ester. Unpublished report submitted
to the Flavor and Extract Manufacturers' Association.
Watanabe, S. & Kinosaki, A. (1989b) Acute oral toxicity in the rat
with propionyl lactic acid thiomethyl ester. Unpublished report
submitted to the Flavor and Extract Manufacturers' Association.
Watanabe, S. & Morimoto, Y. (1989a) Mutagenicity study of acetyl
lactic acid thiomethyl ester in Salmonella typhimurium and
Escherichia coli. Unpublished study submitted to the Flavor and
Extract Manufacturers' Association.
Watanabe, S. & Morimoto, Y. (1989b) Mutagenicity study of propionyl
lactic acid thiomethyl ester in Salmonella typhimurium and
Escherichia coli. Unpublished study submitted to the Flavor and
Extract Manufacturers' Association.
Weinshilboum, R.M. & Sladek, S.L. (1980) Mercaptopurine
pharmacogenetics: Monogenic inheritance of erythrocyte thiopurine
methyltransferase activity. Am. J. Hum. Genet., 32, 651-662.
Wells, W.W., Yang, Y., Diets, T.L. & Gan, Z.R. (1993)
Thioltransferases. Adv. Enzymol. Relat. Areas Mol. Biol., 66,
149-201.
Wheldon, G.H., Amyes, S.J., Street, A.E., Hague, P.H. &
Mawdesley-Thomas, L.E. (1970) Toxicity of Wa 4295, Sa 927, Stl 3048,
and Wa 3328 in dietary administration to rats over a period of 13
weeks. Unpublished report from Huntington Research Centre to the
Flavor and Extract Manufacturers' Association.
Wild, D., King, M.T., Gocke, E. & Eckhardt, K. (1983) Study of
artificial flavouring substances for mutagenicity in the
Salmonella/microsome, basc and micronucleus tests. Food Chem.
Toxicol., 21, 707-719.
Williams, K.I.H., Burstein, S.H. & Layne, D.S. (1966) Metabolism of
dimethyl sulfide, dimethyl sulfoxide, and dimethyl sulfone in the
rabbit. Arch. Biochem. Biophys., 117, 84-87.
Willson, J.E., Brown, D.E. & Timmens, E.K. (1965) A toxicologic study
of dimethyl sulfoxide. Toxicol. Appl. Pharmacol., 7, 104-122.
Wilson, K., Chissick, H., Fowler, A.M., Frearson, F.J., Gittins, M. &
Swinbourne, F.J. (1991) Metabolism of benzothiazole I. Identification
of ring-cleavage products. Xenobiotica, 21, 1179.
Wnorowski, G. (1996a) 14-Day dietary toxicity study: Rats. Unpublished
study No. 4442 from Product Safety Laboratories to the Flavor and
Extract Manufacturers' Association.
Wnorowski, G. (1996b) 14-Day dietary toxicity study: Rats. Unpublished
study No. 4453 from Product Safety Laboratories to the Flavor and
Extract Manufacturers' Association.
Wnorowski, G. (1996c) 14-Day dietary toxicity study: Rats. Unpublished
study No. 4456 from Product Safety Laboratories to the Flavor and
Extract Manufacturers' Association.
Wnorowski, G. (1996d) 14-Day dietary toxicity study: Rats. Unpublished
study No. 4454 from Product Safety Laboratories to the Flavor and
Extract Manufacturers' Association.
Wnorowski, G. (1996e) 14-Day dietary toxicity study: Rats. Unpublished
study No. 4457 from Product Safety Laboratories to the Flavor and
Extract Manufacturers' Association.
Wnorowski, G. (1997a) 14-Day repeat dose oral toxicity study: Rats.
Unpublished study No. 5211 from Product Safety Laboratories to the
Flavor and Extract Manufacturers' Association.
Wnorowski, G. (1997b) 14-Day dietary toxicity study: Rats. Unpublished
study No. 5210 from Product Safety Laboratories to the Flavor and
Extract Manufacturers' Association.
Wood, D.C., Wirth, N.V., Weber, F.S. & Palmquist, M.A. (1971)
Mechanism considerations of dimethyl sulfoxide (DMSO)-lenticular
changes in rabbits. J. Pharmacol. Exp. Ther., 177, 528-535.
Woodson, L.C. & Weinshilboum, R.M. (1983) Human kidney thiopurine
methyltransferase: Purification and biochemical properties. Biochem.
Pharmacol., 32, 819-826.
Woodson, L.C., Dunnette, J.H. & Weinshilboum, R.M. (1982)
Pharmacogenetics of human thiopurine methyltransferase:
Kidney-erythrocyte correlation and immunotitra-tion studies.
J. Pharmacol. Exp. Ther., 222, 174-181.
Woodson, L.C., Ames, M.M, Selassie, C.D., Hansch, C. & Weinshilboum,
R.M. (1983) Thiopurine methyltransferase: Aromatic thiol substrates
and inhibition by benzoic acid derivatives. Mol. Pharmacol., 24,
471-478.
Yoshihara, S. & Tatsumi, K. (1985a) Sulfoxide reduction catalysed by
guinea pig liver aldehyde oxidase in combination with one electron
reducing flavoenzymes. J. Pharmacobio-Dyn., 8, 996-1005.
Yoshihara, S. & Tatsumi, K. (1985b) Guinea pig liver aldehyde oxidase
as a sulfoxide reductase: Its purification and characterisation.
Arch. Biochem. Biophys., 242, 213-224.
Yoshihara, S. & Tatsumi, K. (1990) Metabolism of diphenyl sulphoxide
in perfused guinea pig liver. Drug Metab. Disposition, 18, 876.
Zajac-Kaye, M. & Ts'o, P.O.P. (1984) DNAase I encapsulation in
liposomes can induce neoplastic transformation of Syrian hamster
embryo cells in culture. Cell, 39, 427-437.
Zeiger, E., Anderson, B., Haworth, S., Lawlor, T., Mortelmans, K. &
Speck, W. (1987) Salmonella mutagenicity test: III. Results from the
testing of 255 chemicals. Environ. Mutag., 9 (Suppl. 9), 1-110.
Zeiger, E., Anderson, B., Haworth, S., Lawlor, T. & Mortelmans, K.
(1992) Salmonella mutagenicity tests. V. Results from the testing of
311 chemicals. Environ. Mol. Mutag., 19 (Suppl.21), 2-141.
Ziegler, D.M. (1980) Microsomal flavin-coenzyme monooxygenase:
Oxygenation of nucleophilic nitrogen and sulfur compounds. In: Jakoby,
W.B., ed., Enzymatic Basis of Detoxification, Vol. 2, Chichester:
Ellis Horwood, pp. 201-227.
Ziegler, D.M. (1989) S-Oxygenases. I. Chemistry and biochemistry. In:
Damani, L.A., ed., Sulphur-containing Drugs and Related Organic
Compounds, Vol. 2, Part A, Chichester: Ellis Horwood, pp. 53-66.
R-isomer. Decreasing stereoselectivity was seen as the size of the
alkyl group (ethyl, propyl, or isopropyl) (Sadeque et al., 1992) or
the pH (8.5-10) increased (Rettie et al., 1990). Oxidation of propyl
and butyl para-tolyl sulfide by the predominant human liver FMO
isozyme, FMO3, resulted in greater formation of the R-enantiomer
(73-88%), whereas oxidation of the methyl or ethyl derivative by human
FMO5 resulted in a > 90% preference for formation of the
S-stereoisomer of the sulfoxide (Sadeque et al., 1995). The influence
of the stereochemistry of sulfoxides on the biological and
toxicological properties of sulfides has not been evaluated.
On the basis of numerous examples of successive oxidation of
sulfides to sulfoxides and sulfones by FMO and CYP-450 enzymes in a
variety of test systems (Cashman & Williams, 1990; Cashman et al.,
1990; Rettie et al., 1990; Yoshihara & Tatsumi, 1990; Sadeque et al.,
1992; Cashman et al., 1995a,b; Elfarra et al., 1995; Nnane & Damani,
1995; Sadeque et al., 1995), the oxidation pathway appears to be a
major route for the metabolism of thioethers in humans (Ziegler, 1980;
Nickson & Mitchell, 1994).
The sulfoxide group is part of an interconvertible redox series
involving the sulfide (or thioether), sulfoxide, and sulfone. The
sulfoxide group is present in numerous molecules, which show a
diversity of biological activity; examples include pharmaceuticals
such as sulindac and flosequinan, veterinary drugs such as
albendazole, and numerous pesticides. Oxidation of a sulfoxide yields
a sulfone, which is commonly found as a metabolite of sulfoxides but
is less important as an active chemical entity. Oxidation of
sulfoxides to the corresponding sulfones occurs in both tissues and
aerobic microorganisms. The oxidation of the sulfoxide drug sulindac
to its inactive sulfone is a major metabolic pathway in humans (Strong
et al., 1985). The enzymes involved have not been studied extensively
in mammalian tissues (Levi & Hodgson, 1988) or microbes (Davis &
Guenthner, 1985). Oxidation of the sulfoxide to the sulfone is an
irreversible reaction which is probably catalysed by CYP-450 (Damani,
1987). The relative amounts of sulfoxide and sulfone excreted depend
on the stability and hydrophilicity of the sulfoxide (Damani, 1987).
After administration of [35S]-dipropyl sulfoxide to rats,
essentially all of the administered radiolabel was recovered over the
following three days, in the urine (80%), exhaled air (1.4%), faeces
(5.0%), and the carcass (13.0%) (Nickson & Mitchell, 1994). The
profile of urinary metabolites was the same after administration of
the sulfoxide or the sulfide (see above); the principal quantitative
difference was that more sulfone was excreted in urine and bile after
sulfoxide administration. Dipropyl sulfone is physiologically stable
in rats in vitro and is excreted unchanged in urine (83%) and bile
(Nickson et al., 1995). More than 98% was excreted unchanged in the
urine with trace amounts of inorganic sulfate. No reduction of the
sulfone group or oxidation of the hydrocarbon chain was observed.
As the sulfoxide may also be reduced back to the thioether, it is not
always possible to determine if the activity of a chemical in vivo
resides in the parent compound or in one of the inter-convertible
redox forms.
The three important sites of sulfoxide reduction in vivo are
liver, kidney, and gut microflora. The tissue enzymes that can reduce
sulfoxides in vitro are primarily aldehyde oxidase (Tatsumi et al.,
1982, 1983; Yoshihara & Tatsumi, 1985a,b) and thioredoxin and its
reductase (Anders et al., 1980, 1981). Studies with tissue homogenates
containing various cofactors and inhibitors have shown that the main
enzyme involved in the liver is aldehyde oxidase, whereas thioredoxin
is important in the kidney (Lee & Renwick, 1995a). The former enzyme
was active mainly under anaerobic conditions, while sulfoxide
reduction by thioredoxin occurred under both aerobic and anaerobic
conditions. The possible role of aldehyde oxidase under normal and
hypoxic conditions was shown in isolated perfused guinea-pig liver;
CYP-P450-catalysed oxidation of both diphenylsulfide and
diphenylsulfoxide to the sulfone predominated under aerobic conditions
(Yoshihara & Tatsumi, 1990), whereas oxidation of diphenylsulfoxide to
the sulfone was decreased under hypoxic conditions and trace amounts
of diphenylsulfide were formed. Infusion of the sulfoxide with
2-hydroxypyrimidine and benzaldehyde, which are electron donors for
aldehyde oxidase, resulted in increased reduction. The importance of
the kidney and thioredoxin in vivo under normal oxygen conditions is
indicated by the fact that patients with end-stage renal disease show
decreased reduction of sulindac in comparison with controls (Gibson et
al., 1987).
The gut microflora in the anaerobic environment of the lower
bowel can be an important and sometimes the sole site of reduction of
sulfoxides. Studies in germ-free animals or animals treated with
antibiotics to suppress the gut microflora and in patients indicated
that the gut bacteria were the main, or possibly the sole, site of
sulfoxide reduction of the drug sulfinpyrazone in vivo (Renwick et
al., 1982; Strong et al., 1984a,b, 1986). The intestinal bacteria can
also reduce sulindac in vitro (Strong et al., 1985), and the
thioether is a major circulating metabolite in vivo. The
contribution of the intestinal microflora to the reduction of sulindac
in humans was demonstrated by a study in normal subjects and patients
who had undergone an ileostomy. The tissues produced an initial peak
0-12 h after dosing, corresponding to 45% of the total thioether, and
the intestinal bacteria produced a second peak at 12-72 h,
corresponding to 55% of the total thioether (Strong et al., 1985).
Studies with over 200 isolated strains of bacteria from human faeces
(Strong et al., 1987) showed significant sulfoxide reduction by many
aerobic organisms such as Escherichia coli under anaerobic
conditions. Few strict anaerobes showed high reducing activity in
vitro, but they are probably essential in vivo in providing the
anaerobic environment in which the aerobes can express their sulfoxide
reductase activity (Strong et al., 1987). Recent studies with
xenobiotics as substrates have indicated the presence of a number of
soluble enzymes in E. coli that are capable of sulfoxide reduction
(Lee & Renwick, 1995b).
The enzymes involved in the reduction of the sulfoxide group
present in DMSO or of the sulfoxide metabolites of the thioethers are
likely to be the hepatic and renal systems discussed above. In order
for the gut flora to be involved in the metabolism of the flavouring
substances, the sulfur derivatives must either be incompletely
absorbed or reach the lower gut as biliary metabolites (Renwick &
George, 1989). The significant biliary excretion of the sulfoxide
metabolite after administration of dipropyl sulfide (see above) raises
the possibility of reduction by the intestinal microflora, followed by
reabsorption and subsequent reoxidation.
(ii) Acyclic sulfides with oxidized side-chains
Oxidized side-chains provide additional sites for the
biotransformation of sulfides, and, as they are polar groups, they
would increase renal excretion. When the thioether contains an
additional oxygenated functional group on a carbon atom (i.e. alcohol,
aldehyde, acid, or ketone), C-oxidation competes with sulfoxidation.
The biotransformation of such oxygenated carbon-containing functional
groups is well characterized and may have been considered in groups of
flavouring substances previously evaluated by the Committee.
Concurrent metabolism via both sulfur and oxygenated functional groups
has been reported for various substrates (El Fatih et al., 1988;
Gachon et al., 1988; Feng & Solsten, 1991; Wilson et al., 1991; Black
et al., 1993). In the presence of oxygenated functional groups,
sulfoxide formation is usually the major metabolic detoxication
pathway. The main urinary metabolites seen after intraperitoneal
administration of thiodiglycol [(HOCH2CH2)2S] to male rats were the
corresponding sulfoxide (90%) and acid thioether
S-(2-hydroxyethylthio)acetic acid (10%). The corresponding sulfone and
combined C-and S-oxidation product S-(2-hydroxyethylsulfinyl)acetic
acid were minor metabolites (Black et al., 1993). Phenacyl phenyl
sulfide is oxidized to the sulfoxide in vitro by FMO but split by
CYP-450 to give thiophenol (Oae et al., 1985). The corresponding
sulfoxides are the principal urinary metabolites of aliphatic
methylthiocarboxylic acids (Alterman et al., 1995), of the mucolytic
drug S-carboxymethyl-L-cysteine ( S-containing amino acid), of the
histamine antagonist cimetidine ( S-containing amidine) in humans
(Mitchell et al., 1982), and of other thioether drugs (Renwick, 1996).
Experiments in vitro suggest that carboxyl ester hydrolysis
occurs in the presence of thioether groups. The thioether
3-(methylthio)hexyl acetate (No. 481) was partially (9%) hydrolysed in
simulated gastric juice containing pepsin (pH = 1.1; 37-38 °C) after 4
h and completely hydrolysed in simulated intestinal fluid containing
pancreatin (Flavor & Extract Manufacturers' Association, 1991).
Thioethers with a polar anionic group such as carboxylic acid one
or more carbon atoms away for the sulfur are inhibitors of rather than
substrates for FMO (Taylor & Ziegler, 1987) and would probably be
eliminated without S-oxidation.
(iii) Cyclic sulfides
There are few published data on the fate of simple cyclic
thioethers. S-Oxidation is likely to be a major pathway in vivo,
and oxidation in vitro results in a chiral sulfoxide when the ring
is asymmetric. Oxidation of unsubstituted and methyl-substituted
cyclic sulfides by a phenobarbital-type CYP-450 yielded the
corresponding sulfoxides, but further oxidation of sulfoxides to
sulfones was not detected (Takata et al., 1983).
The metabolism of the cyclic sulfides with oxidized carbon atoms
(Nos 464, 498, 499, 550, and 562) has not been studied but would be
predicted to comprise extensive S-oxidation and possibly oxidation
or conjugation of alcohol groups.
Substituted 1,3-dithiolane derivatives undergo stereoselective
S-oxidation to the sulfoxide as a major metabolic pathway (Grosa et
al., 1991) with slower oxidation to the cyclic sulfone ( S,S-dioxide)
(Cashman & Olsen, 1990). The reactions are catalysed by both FMO and
CYP-450 (CYP2B); oxidation at both sulfur atoms was not reported. The
cyclic mono-sulfoxides of substances Nos 456, 534, and 543 are
predicted to be the main urinary metabolites, while steric hindrance
and the higher polarity of the hydroxy thioethers (Nos 550 and 562)
may allow their elimination unchanged.
(iv) Simple thiols
A large number of the flavouring agents in this group are either
alkyl or alicyclic thiols (Nos 511-524, 526, 527, and 530), arylthiols
(thiophenols) (Nos 525, 528, 529, and 531-534), dithiols (Nos
535-542), or alkylthiols with oxidized side-chains (Nos 544-547, 549,
551-561, and 563). The thiol group is weakly acidic, and endogenous
thiols have important nucleophilic functions in vivo.
Whereas alcohols are oxidized to give carbonyl compounds
(aldehydes, ketones, and carboxylic acids), thiols are not readily
oxidized to the corres-ponding thiocarbonyls. The availability of
sulfur d-orbitals allows valencies of 4 and 6, so that oxidation of
the thiol group can lead to sulfenic acids (RSOH), sulfinic acids
(RSO2H), or sulfonic acids (RSO3H), as well as disulfides (RSSR').
The metabolic pathways of thiols include oxidation to unstable
sulfenic acids (RSOH), which may be oxidized to the corresponding
sulfinic (RSO2H) and sulfonic acids (RSO3H); methylation to yield
methyl sulfides, which can be oxidized to methyl sulfoxides and
sulfones; reaction with endogenous thiols such as glutathione to form
mixed disulfides; conjugation with glucuronic acid; and oxidation of
the alpha-carbon resulting in desulfuration and formation of an
aldehyde intermediate (McBain & Menn, 1969; Dutton & Illing, 1972;
Maiorino et al., 1988; Richardson et al., 1991; Renwick, 1996).
Sulfenic acids are formed by mild oxidation of thiols but are
unstable and readily undergo further oxidation to sulfinic and
sulfonic acids or combine with nucleophiles. Reactions of sulfenic
acids include dimerization with elimination of water to produce
R-SO-S-R and oxidation to the corresponding polar sulfinic (RSO2H)
and sulfonic (RSO3H) acids. Oxidation of the thiol group of cysteine
in proteins can give stabilized sulfenic acid groups which are
critical to the active site of enzymes; redox cycling between thiol
and sulfenic acid in the active site may be essential for the
catalytic activity of a number of bacterial redox enzymes. The
sulfinic acid group is an intermediate redox state between sulfenic
and sulfonic acids. Endogenous sulfinic acids include cysteine
sulfinic acid, which is released during neuronal stimulation (Klancnik
et al., 1992) and may be involved in synaptic transmission in the
hippocampus. Cysteine and homocysteine sulfinic acids are excitatory
and cytotoxic acidic amino acids (Frandsen et al., 1993). The sulfonic
acid group is a highly polar centre and makes the molecule very
soluble in water. In general, sulfonic acids are stable to metabolism,
and desulfonation was not found in vivo with simple alkyl
sulfo-nates (Taylor et al., 1978) or with branched-chain sulfonates
(Michael, 1968).
Simple aliphatic and aromatic thiols undergo S-methylation in
mammals to produce the corresponding methyl thioether or sulfide,
which may be successively oxidized to the corresponding sulfoxides and
sulfones. Methylation is catalysed by thiopurine methyltransferase in
the cytoplasm and by thiol methyltransferase in microsomes, both of
which require S-adenosyl-L-methionine as a methyl group donor.
Thiopurine methyltransferase is present in human liver, kidney, and
erythrocytes (Woodson et al., 1982; Szumlanski et al., 1988), and
preferential substrates for this enzyme include aromatic and
heterocyclic thiols (Woodson & Weinshilboum, 1983; Woodson et al.,
1983). The apparent Km values of thiophenols (Ames et al., 1986)
are at least two orders of magnitude lower than those of aliphatic
substrates (Woodson & Weinshilboum, 1983). The activity of thiopurine
methyltransferase in humans shows genetic polymorphism, 89% of
subjects showing high activity, 11% showing intermediate activity, and
0.3% showing no activity (Weinshilboum & Sladek, 1980). Studies of
families indicate that the polymorphism is due to a single genetic
locus with two alleles, TPMTH for high activity and TPMTL for low
activity (Keith et al., 1983). The frequencies of the TPMTH and
TPMTL genes are 94% and 6%, respectively, resulting in the trimodal
frequency distribution of thiopurine methyltransferase activity in the
general population. The impact of such differences in 'methylator
status' on the metabolism of thiol-containing flavouring substances is
unknown but is likely to be small because of the presence of
alternative S-oxidation and conjugation pathways. S-Methylation of
aliphatic thiols is catalysed by microsomal thiol methyltrans-ferase,
and thiol substances such as 2-mercaptoethanol, methylmercaptan, and
2-mercaptopropionic acid are substrates for this enzyme (Bremer &
Greenberg, 1961), but the endogenous sulfur amino acid derivatives
homocysteine and glutathione are not. Microsomal thiol
methyltransferase and cytoplasmic thiopurine methyltransferase
activities are regulated independently in humans (Keith et al., 1983),
and therefore S-methylation by thiol methyltrans-ferase may occur in
individuals with low or negligible thiopurine methyltrans-ferase
activity.
Examples of S-methylation in vivo include both aliphatic and
aromatic substrates. Methyl ethyl sulfide (No. 453), which was
detected in the urine of guinea-pigs and mice given an oral dose of
diethyl disulfide (Snow, 1957), was presumably formed by reductive
cleavage to ethanethiol and subsequent methylation (Snow, 1957); minor
urinary metabolites were the sulfoxide and sulfone (Parkinson, 1996).
The sulfoxide and sulfone derivative of benzyl methyl sulfide (No.
460) were excreted in the urine of rats given an oral dose of
S-benzyl- N-malonyl-L-cysteine, presumably via methylation and
oxidation of the intermediate benzyl mercaptan (No. 525) (Richardson
et al., 1991). The urine of rats given an oral dose of [35S]phenyl
mercaptan contained S-methylated metabolites such as phenyl methyl
sulfone and ortho-and para-hydroxylated phenyl methyl sulfone
(McBain & Menn, 1969).
Thiols may react with glutathione and other endogenous thiol
compounds to form mixed disulfides, and both membrane-bound and
cytosolic thioltrans-ferases have been reported to catalyse their
formation. The resulting mixed disulfides can undergo reduction back
to thiols, oxidative desulfuration, or oxidation to a sulfonic acid
via the intermediate thiosulfinate and sulfinic acids (see above).
Thus, the disulfide represents a reversible metabolic 'reservoir' of
the xenobiotic thiol compound. The principal form in the circulation
would probably be a mixed disulfide with albumin. The mixed disulfides
formed from conjugation of a thiol with glutathione or cysteine are
not substrates for cysteine conjugate C-S lyase. C-S lyase is found in
the liver, kidney, and intestinal microflora and catalyses the
reduction of the C-S bonds of cysteine conjugates, which are formed
between organic compounds and glutathione and then undergo a series of
hydrolysis steps. The thiol product is usually methylated to form a
thioether, which may then be oxidized to sulfoxide and sulfone
analogues; compounds that undergo this metabolic pathway include
bromo-benzene, paracetamol (acetominophen), propachlor (a herbicide),
and chlorinated biphenyls. With some organic compounds, conjugation
with glutathione followed by hydrolysis and C-S lyase results in a
novel, unstable thiol; for example, some halogenated substrates and
S-phenyl-L-cysteine formed from conjugation of bromobenzene yield
unstable thiols which are toxic to the kidney (Tateishi et al., 1978;
Shaw & Blagbrough, 1989; Tateishi & Tomisawa, 1989).
S-Glucuronidation of aromatic thiols has been reported, and
this is a possible pathway for the aromatic thiols (thio-phenols) (Nos
525 and 528-531) and aromatic disulfides (Nos 576-578) after their
reduction. This reaction produced thiophenol glucuronide via
conjugation to glutathione, with subse-quent C-S lyase action on the
intermediate cysteine conjugate to yield thiophenol. The metabolism of
benzothiazole-2-sulfonamide was the first of a S-glucuronide to be
fully elucidated. Three metabolites were detected in the urine of
treated rats, benzothiazole-2-thioglucuronide, benzothiazole-2-thiol,
and benzothiazole-1-mercapturate (see Buckberry & Teesdale-Spittle,
1996); the thiol group is introduced into position 2 by displacement
of the sulfonamide by glutathione. Glucuronyl transferases behave in a
similar way towards hydroxyls and sulfydryls, and the two activities
have the same subcellular location and optimal pH (Dutton & Illing,
1972).
Thiols of low relative molecular mass undergo oxidative
desulfuration in vivo to yield CO2 and SO4=. For example, 40% of
14C-labelled methyl mercaptan (No. 508) administered to rats was
eliminated as CO2, 6.4% was exhaled as unchanged compound within 6 h,
and only 2.3% was excreted in the urine. 14C also was detected in
the beta-carbon of serine and in the methyl groups of methionine,
choline, and creatine (Canellakis & Tarver, 1953), and formalde-hyde
has been shown to be an intermediate in the oxidation of methanethiol
in vitro (Mazel et al., 1964). Whereas the carbon atom from a thiol
may be used in the biosynthesis of amino acids, the sulfur atom is not
used significantly in the synthesis of sulfur-containing amino acids:
31% of 35S-labelled methyl mercaptan was excreted in the urine as
sulfate ion (Mazel et al., 1964).
(v) Thiols with oxidized side-chains
Although these compounds comprise a significant proportion of the
flavouring agents in this group, their metabolite fate has not been
studied specifically. The metabolism is predicted to be a combination
of the pathways described above for simple thiols, combined with
further oxidation or conjugation of the oxidized side-chain.
(vi) Dithiols
The metabolism of the simple aliphatic dithiols in this group is
predicted to involve the pathways described above for thiols,
particularly oxidation of sulfur and alpha-carbon atoms and formation
of disulfides by reaction with endogenous thiols such as glutathione.
S-Oxidation of one S-atom could yield a polar thiol-sulfonate. Data
for 2,3-dimercapto-1-propanesulfonate (Maiorino et al., 1996) indicate
that a thiol-sulfonate would be excreted in the urine as such and also
as disulfides formed with endogenous thiols of low relative molecular
mass such as cysteine. The longer, linear dithiols (Nos 535, 538, and
540-542) may form an intramolecular disulfide bond and exist in
equilibrium between dithiol and cyclic disulfide.
(vii) Simple disulfides
Disulfide bonds are important elements in protein folding and
structure (Waring, 1996), and redox interconversion between disulfide
and dithiol is important in the regulation of the activity of some
enzymes. Interconversion is catalysed by a number of enzymes; for
example, glutathione reductase reduces the dimeric disulfide to two
molecules of the monomeric thiol glutathione. The reduction of
xenobiotic disulfides is believed to be extensive and may be catalysed
enzymatically by glutathione reductase (Waring, 1996) or
thioltrans-ferases (Wells et al., 1993) as well as chemically by
exchange with glutathione, thioredoxin, cysteine, and other endogenous
thiols. The non-cyclic disulfides in this group of flavours would be
metabolized to form low-relative-molecular-mass thiols, which would
then follow the pathways described above for thiols.
(viii) Disulfides with oxidized side-chains
As discussed above for groups (ii) and (iii), the additional
sites of carbon oxidation would result in greater polarity and the
potential for further oxidation or conjugation. Because of the
instability of disulfide bonds to reductive cleavage, this would be
expected to be the initial metabolic reaction, and the polarity of the
side-chains would mainly affect the elimination of thiol fragments.
(ix) Trisulfides
The trisulfide of glutathione is labile and readily converted to
the disulfide, the sulfur being released as hydrogen sulfide (Moutiez
et al., 1994). The metabolic lability of trisulfides is associated
with biological activity, such as inhibition of CYP-450 (Ogasawara et
al., 1998) and enhancement by diallyl trisulfide (No. 587) of
unscheduled DNA synthesis produced in rat hepatocytes by known
mutagens (Deng et al., 1994). The toxicity of trisulfides (Nos
582-587) and polysulfides (No. 588) is probably related to their
metabolic lability and the nature of the eventual thiol, such as allyl
thiol.
(x) Heterocyclic disulfides
The heterocyclic disulfides (Nos 573 and 574) are five-and
six-membered rings which also contain a cyclic thioether bond. Lipoic
acid is a five-membered cyclic disulfide, and rapid redox cycling
between ring disulfide and open dithiol is important in its metabolic
role in vivo. The five-membered disulfide (No. 573) would be
predicted to undergo more rapid reductive ring opening to a dithiol
than the six-membered compound (No. 574). The principal metabolic
pathways are predicted to be disulfide reduction to produce a dithiol
and S-oxidation of the thioether.
(xi) Thioesters
The thioester group (-S-CO-) is present in a number of the
flavouring substances in this group (Nos 482-494, 504, and 506). The
hydrolysis of other esters has been considered previously by the
Committee. Esters are hydrolysed by lipase and esterases (Kurooka et
al., 1976), and the rate of hydrolysis increases as the length of the
C-chain of the carboxylic acid fragment increases (Greenzaid & Jencks,
1971) and decreases as oxygenation of the carbon chain in the thiol
moiety increases (Kurooka et al., 1976).
Dithionic acids are oxidized by FMO to the dioxo analogue with
the monothioc acid as an intermediate (Taylor & Ziegler, 1987), but
simple esters of dithioic acids are poor substrates for oxidation. The
esters of monothioic acids are also probably poor substrates for
oxidation, but the monothioic acid released by hydrolysis would be
oxidized to the dioxo-acid. Another possibility for elimination in
vivo is renal excretion of the thiocarboxylic acid. With the
exceptions of prenyl thioacetate (No. 491) and methylthio
2-(acetyloxy)propio-nate (No. 492), the compounds evaluated are simple
linear alkyl compounds, branched alkyl compounds, or their side-chain
hydroxy-ester analogues, so that their toxicity can reasonably be
compared.
(xii) Sulfoxides
The metabolism of sulfoxides by oxidation and reduction is
described above for thioethers. The only sulfoxide flavouring agent is
DMSO (No. 507). The absorption, metabolism, and excretion of DMSO were
studied in rhesus monkeys given a daily oral dose of 3 g/kg bw for 14
days. DMSO was absorbed rapidly, reached a peak serum concentration
after about 4 h, and was cleared from the blood within 72 h of the end
of treatment. Dimethyl sulfone was detected in the blood 2 h after
treatment, reached a steady-state concentration after four days, and
was cleared from the blood 120 h after the end of treatment. Urinary
excretion of DMSO and dimethyl sulfone accounted for approximately 60%
and 16%, respectively, of the total ingested dose. Neither DMSO nor
dimethyl sulfone was detected in the faeces (Layman & Jacob, 1985).
The fate of DMSO in humans is similar to that in monkeys, but the
elimination is slower. When an oral dose of 1 g/kg bw was given to six
subjects, peak serum concentrations were observed approximately 4 h
after administration. The peak concentration of dimethyl sulfone was
slightly higher than that of DMSO and occurred after 72-96 h.
Approximately 51% of the dose was excreted in the urine unchanged over
the first 120 h, and up to 22% was excreted as dimethyl sulfone.
Repeated daily oral administration of DMSO at 0.5 g/kg bw per day for
14 days to one adult resulted in peak serum concentrations of DMSO
within eight days (Hucker et al., 1967).
2.3.2 Toxicological studies
The available data on the toxicity of sulfides, thiols, and
oxygenated functional derivatives in the group of 137
sulfur-containing flavouring substances are presented below. Although
the studies of acute and short-term toxicity were of limited use for
evaluating the safety of these substances, because of their short
duration, they are included for completeness. Studies related to
chemoprevention are also described.
2.3.2.1 Acute toxicity
Oral LD50 values have been reported for 52 of the 137 sulfur
compounds. The values ranged from 11 to 28 000 mg/kg bw in male and
female rats (Fairchild & Stokinger, 1958; Brown et al., 1963; Elf
Atochem, 1963; Harper & Ginn, 1964; Sommer & Tauberger, 1964; Willson
et al., 1965; Koptyaev, 1967; Fishman et al., 1969; McBain & Menn,
1969; Fogelman et al., 1970; Oser, 1970; deGroot et al., 1974;
Christias, 1975; Moreno, 1975; British Industrial Biological Research
Association, 1976; Griffiths et al., 1979; Mondino & Peano, 1979;
Opdyke, 1979; Moran et al., 1980; Moreno, 1980; Panasevich et al.,
1980; Butterworth & Mason, 1981; Mondino et al., 1981; Peano et al.,
1981; Mondino & Peano, 1982; Piccirillo & Lunchicki, 1982; Elf
Atochem, 1985; Schafer & Bowles, 1985; Collinson, 1989; Watanabe &
Kinosaki, 1989a,b; Phillips Petroleum Co., 1990a,b,c; Rush, 1991; Elf
Atochem, 1992; Farr & Kirwin, 1994; Sanders, 1997). In male and female
mice, the values ranged from 61 to > 5000 mg/kg bw (Koptyaev, 1967;
Oser, 1970; Shellen-berger, 1971a,b; Selyuzhitskii, 1972; Fogelman &
DeProspo, 1973a,b; Fogelman & Suppers, 1973; Fogelman & DeProspo,
1974; Fogelman & Suppers, 1974a,b; Bailey, 1976a,b,c; Gallo et al.,
1976; Griffiths & Babish, 1978; Moran et al., 1980; Butterworth &
Mason, 1981; Schafer & Bowles, 1985).
2.3.2.2 Short-term studies of toxicity
2,8-Dithianon-4-ene-4-carboxaldehyde (No. 471)
Five male and five female rats (strain not specified) received
0.33 or 3.3 mg/kg bw per day of 2,8-dithianon-4-ene-4-carboxaldehyde
(No. 471) by gavage on six days a week for two weeks. Individual body
weights and feed and water consumption were recorded at one and two
weeks. Haemoglobin levels determined on day 14 were normal. At
necropsy on day 14, the liver and kidney were weighed and examined
macro-and microscopically. No treat-ment-related effects were reported
(de Groot et al., 1974).
Methylthio 2-(acetyloxy)propionate (No. 492) and
methylthio 2-(propionyloxy)propionate (No. 493)
Groups of five Fischer 344 rats of each sex were given either
methylthio 2-(acetyloxy)propionate (No. 492) or methylthio
2-(propionyloxy)propionate (No. 493) in the diet for 14 days at a dose
of approximately 500 mg/kg bw per day. Control animals received a
basal diet. The animals were observed and tested daily for clinical
signs of toxicity. Body weights were measured on days 1, 6, and 14,
and feed consumption was measured on days 7 and 14. At necropsy,
organs were weighed and the liver and kidneys were examined
histologically.
Neither test material produced any abnormal clinical or
microscopic changes. The potential treatment-related effects included
depressed feed consumption, decreased body-weight gains of treated
males, and depressed relative kidney weights in treated females. The
depressed feed consumption and decreased body-weight gains observed in
treated males were probably due to the strong odour of the test
compounds, which decreased the palatability of the diets. The
biological significance, if any, of the decreased relative kidney
weights in the females (7 and 6%, respectively, with methylthio
2-(acetyloxy)-propionate and methylthio 2-(propionyloxy)propionate) is
questionable because the change was small and there were no
histological changes (Hermansky & Weaver, 1990).
Prenyl thioacetate (No. 491), prenylthiol (No. 522),
3-mercaptohexanol (No. 545), 3-methyl-3-mercaptobutyl formate
(No. 549), 3-mercaptohexyl acetate (No. 554), 3-mercaptohexyl
butyrate (No. 555), and 3-mercaptohexyl hexanoate (No. 556)
A series of 14-day studies were performed with prenyl thioacetate
(No. 491), prenylthiol (No. 522), 3-mercaptohexanol (No. 545),
3-methyl-3-mercaptobutyl formate (No. 549), 3-mercaptohexyl acetate
(No. 554), 3-mercaptohexyl butyrate (No. 555), and 3-mercaptohexyl
hexanoate (No. 556). Each substance was provided in the diets of 10
Sprague-Dawley rats of each sex at a target dose of 10 mg/kg bw per
day. Weekly measurements of body weight and feed consumption indicated
that the average daily intakes were 10-13 mg/kg bw per day. Control
animals received chow alone for the same period. The animals were
observed for gross signs of toxicity and deaths at least once daily
for the duration of the study. Body weights and feed consumption were
measured on days 8 and 15. Gross necropsies were performed on all
rats, and the kidneys and liver were removed for histopathological
examination at the time of autopsy. Body-weight gain, organ-to-body
weight ratios, and the weights of the kidney and liver of test and
control animals were not significantly different in any of the seven
studies. Gross necropsy and histopathological examination of kidney
and liver tissues revealed no lesions related to administration of the
test substances (Wnorowski, 1996a-e, 1997a,b].
Prenyl thioacetate (No. 491) and prenylthiol (No. 522),
respectively, are in the thioester and simple thiol subgroups of the
larger group of sulfur-containing flavouring substances; the other
five substances (Nos 545, 549, and 554-556) are thiols with oxidized
side-chains.
Allyl disulfide (No. 572), propyl disulfide (No. 566), and
phenyl disulfide (No. 578)
In two separate, but similarly conducted studies, allyl disulfide
(No. 572) was administered at a concentration of 250 or 1000 µmol/kg
(36 or 150 mg/kg bw per day); propyl disulfide (No. 566) at 250, 1000,
or 5000 µmol/kg (38, 150, or 750 mg/kg bw per day); and phenyl
disulfide (No. 578) at 500 µmol/kg (110 mg/kg bw per day), as
solutions in peanut oil or Tween 80, to groups of six or seven female
Sprague-Dawley rats. The doses were given by oral intubation for six
consecutive days. Control groups of 12 or 14 rats received the vehicle
alone. The animals were killed on day 7 of the experiment, and blood
was collected from the posterior vena cava. At necropsy, splenic
weights were recorded and expressed as a percentage of body weight,
and splenic darkening was assessed visually on an arbitrary scale of
0-5. Blood was examined for various parameters. Sections of splenic
sinusoidal engorgement, focal hepatic erythropoiesis, and iron
deposition in the spleen, liver, and kidneys were also scored on a
scale of 0-5. The number and size of Heinz bodies were measured to
estimate any decrease in the optical density of red cell lysates.
The results for animals given the lower dose of allyl disulfide
and the two lower doses of propyl disulfide were similar to those of
controls, whereas those given the higher doses of allyl and propyl
disulfide had enlargement and darkening of the spleen, increased iron
concentrations in the spleen, liver, and kidneys, and decreased packed
cell volume and haemoglobin concentra-tions associated with Heinz body
formation. No degenerative changes were seen in the spleen, liver, or
kidneys, but destruction of erthrocytes was indicated by deposition of
iron in these organs. Similar findings were made in rats given phenyl
disulfide.
The haemolysis observed was attributed to oxidative stress due to
'active oxygen' species formed by intra-erythrocytic redox cycling of
disulfides. Thiol-disulfide exchange with glutathione results in
reduction of disulfides to their corresponding thiols. Although
generally recognized as antioxidants, thiols act as pro-oxidants after
one-electron oxidation to a thyil radical. In erythrocytes, such
oxidation is mediated by oxyhaemoglobin and results in the formation
of methaemoglobin, hydrogen peroxide, and a thiolate anion. The
hydrogen peroxide and the more active oxygen species formed from the
thyil radical are responsible for initiating the cellular damage that
leads to haemolysis. Although human erythrocytes are usually resistant
to oxidative damage, certain individuals are susceptible because of
hereditary deficiencies (Munday et al., 1990; Munday & Manns, 1994).
2.3.2.3 Ninety-day studies of toxicity
Ninety-day studies were available for 26 of the flavouring
substances (Table 4). To facilitate comparisons of the toxicity of
structurally related substances, the studies are grouped according to
the 12 subgroups. A 90-day study was available for one or more
substance in each subgroup except that of disulfides with oxidized
side-chains. Most of the studies were performed at only one dose that
produced no adverse effects.
(i) Simple sulfides (thioethers)
Methyl sulfide (No. 452)
Four groups of 15 male and 15 female weanling SPF rats of the
Wistar strain were given methyl sulfide by oral intubation at
concentrations of 0 (control), 2.5, 25, or 250 mg/kg bw per day for 14
weeks. Two further groups of five animals of each sex were given 0.25
or 250 mg/kg bw per day for two or six weeks, respectively. The
animals were weighed on day 0 and then weekly throughout the study.
Feed and water consumption were measured for 24 h before the day of
weighing. Urine was collected during weeks 2, 6, and 14 and examined
for appearance, microscopic constituents, and content of glucose,
ketones, bile salts, and blood. The specific gravity and volume of
urine produced over 6 h were also noted. At the end of the appropriate
period of dosing, the rats were killed and blood was taken for
haematological examination. Gross abnormalities were noted and organs
weighed, and histological examinations were performed. No adverse
effect occurred with any treatment (Butterworth et al., 1975). The
dose of 250 mg/kg bw per day that had no effect is at least 10 000
times the per capita intakes of 10 and 9 mg/kg bw per day due to use
of methyl sulfide as a flavouring substance in Europe and the United
States, respectively.
In a study designed to investigate the mechanism of lenticular
changes caused by DMSO (No. 507), 10 eight-week-old New Zealand
rabbits were given 2000 mg/kg bw per day methyl sulfide (No. 452) in
drinking-water for 13 weeks. Control animals received drinking-water
only. Photographs were taken each week of the animals' lens to
evaluate the occurrence of changes. At termination, the kidney, liver,
spleen, heart, lung, adrenals, and lens were weighed and examined for
gross physical abnormalities. Treated animals had a 50% increase in
lung weight, with pulmonary congestion and some haemorrhagic spots.
The kidneys showed signs of pyelonephritis. There was no evidence of
lenticular changes (Wood et al., 1971).
(ii) Acyclic sulfides with oxidized side-chains
2-(Methylthiomethyl)-3-phenylpropenal (No. 505)
In a standardized protocol (referred to below as such for other
compounds), groups of 15 male and 15 female weanling Sprague-Dawley
rats were given a single dose of 0 or 1.4 mg/kg bw per day of
2-(methylthiomethyl)-3-phenylpropenal (No. 505) dissolved in acetone
and blended into a basal laboratory diet for 92 days. The rats were
housed individually and given feed and tap water ad libitum. The
acetone was fully evaporated before presentation of the diet to the
animals. Samples of each diet were taken weekly to assess the
stability and concentration of the test material. Appearance,
behaviour, appetite, elimination, gross signs of toxic effects, and
deaths were recorded daily, and body weights and feed consumption were
measured weekly. Haematological examinations, blood chemical
determinations, and urine analyses were performed during weeks 6 and
12 on eight males and eight females from each group. At necropsy, the
weights of the liver, kidneys, thyroid, spleen, and adrenal glands
were recorded. At termination, the weights of the thyroid, liver,
kidneys, spleen, and adrenal glands were recorded. Tissues from 26
sites and all grossly observable abnormal sites were preserved in
formalin for histopathological examination.
The body weights of females were reduced throughout the study,
and both males and females had decreased feed consumption, but these
findings did not appear to be biologically significant. No significant
differences were seen between treated and control animals with respect
to haematological, blood chemical, or urinary parameters. The absolute
and relative organ weights of the treated and control animals were
similar and there was no evidence of gross or histological changes
(Cox et al., 1979).
The dose of 2-(methylthiomethyl)-3-phenylpropenal that showed no
effects (i.e. 1.4 mg/kg bw per day) is more than 10 000 times the
estimated per capita intake of 0.03 µg/kg bw per day of this
flavouring substance in the United States.
(iii) Cyclic sulfides
2-Methyl-4-propyl-1,3-oxathiane (No. 464)
Groups of 16 male and 16 female weanling Wistar rats were given
2-methyl-4-propyl-1,3-oxathiane (No. 464) by oral intubation at a
daily dose of 0.44 mg/kg bw for 90 days or corn oil alone. The rats
were weighed on the first day of treatment and thereafter at regular
intervals throughout the study. Feed intake was recorded at three-or
four-day intervals. Blood was collected from half the rats in the
study at six weeks and from all rats at 12 weeks and was examined for
haemoglobin concentration, packed cell volume, and erythrocyte and
leukocyte counts. Urea concentration was measured. At necropsy, organs
were weighed and gross and histological examinations performed.
A slight increase in body-weight gain was seen in treated males,
which was associated with increased feed intake thought to result from
alteration of feeding behaviour due to intubation of highly flavoured
solutions. Isolated differences from controls were seen in
haematological parameters, organ weights, and histological appearance,
but none was statistically significant and were considered not to
Table 4. Results of short-term studies of toxicity and long-term studies of toxicity and carcinogenicity on aliphatic and aromatic sulfides
and thiols
No. Substance Species Sex No. test Route Duration NOEL Reference
groupsa/no. (mg/kg bw)
per test
groupb
452 Methyl sulfide Rat MF 3/30 Gavage 14 weeks 250c Butterworth
et al. (1975)
Rabbit MF 1/10 Oral 13 weeks < 2000 Wood et al. (1971)
464 2-Methyl-4-propyl-1,3-oxathiane Rat MF 1/32 Gavage 13 weeks 0.44c British Industrial
Biological Research
Association (1976)
471 2,8-Dithianon-4-en-4-carboxaldehyde Rat MF 2/10 Gavage 2 weeks ND de Groot
et al. (1974)
483 Ethyl thioacetate Rat MF 1/46 Oral 13 weeks 6.5c Shellenberger (1970)
484 Methyl thiobutyrate Rat M 3/10 Oral 13 weeks 1000c,d Wheldon et al. (1970)
491 Prenyl thioacetate Rat MF 1/20 Diet 2 weeks ND Wnorowski (1997a)
498 4,5-Dihydro-3(2H)-thiophenone Rat MF 1/30 Oral 92 days 9.2c Morgareidge (1970)
505 2-(Methylthiomethyl)-3-phenylpropenal Rat MF 1/30 Oral 92 days 1.4c Cox et al. (1979)
507 Methylsulfinylmethane Rat 4/20 Oral 13 weeks 2200 Sommer & Tauberger
(1964)
Rat MF 3/100 Gavage 18 months < 1100 Noel et al. (1975)
Dog MF 3/10 Gavage 18 weeks 1100
or 2 years
Monkey MF 3/4 Gavage 74-87 weeks 3000 Vogin et al. (1970)
516 Cyclopentanethiol Rat MF 1/30 Oral 92 days 0.56c Morgareidge &
Oser (1970a)
520 2,3- or 10-Mercaptopinane Rat MF 1/30 Oral 92 days 0.06c Oser (1966)
522 Prenylthiol Rat MF 1/20 Diet 2 weeks ND Wnorowski (1997b)
528 ortho-Toluenethiol Rat MF 1/20-32 Diet 90 days 0.52c Posternak
et al. (1969)
Table 4. (contd)
No. Substance Species Sex No. test Route Duration NOEL Reference
groupsa/no. (mg/kg bw)
per test
groupb
530 2,6-Dimethylthiophenol Rat MF 1/64 Gavage 13 weeks 0.43c Peano et al. (1981)
531 2-Naphthalenethiol Rat MF 1/30 Oral 92 days 3.4c Morgareidge (1971a)
534 2-Methyl-1,3-dithiolane Rat MF 1/32 Gavage 91 days 7.0c Griffiths
et al. (1979)
539 2,3-Butanedithiol Rat MF 1/30 Oral 92 days 0.7c Morgareidge
et al. (1974a)
541 1,8-Octanedithiol Rat MF 1/30 Oral 92 days 0.7c Morgareidge
et al. (1974c)
543 Trithioacetone Rat MF 1/30 Oral 92 days 0.2c Cox et al. (1973a)
545 3-Mercaptohexanol Rat MF 1/20 Diet 2 weeks ND Wnorowski (1996a)
546 2-Mercapto-3-butanol Rat MF 1/30 Oral 92 days 1.9c Cox et al. (1974a)
547 a-Methyl-b-hydroxypropyl Rat MF 1/30 Oral 92 days 2.8c Morgareidge
a-methyl-b-mercaptopropyl et al. (1974c)
sulfide
549 3-Mercapto-3-methylbutyl Rat MF 1/20 Diet 2 weeks ND Wnorowski (1996a)
formate
554 3-Mercaptohexyl acetate Rat MF 1/20 Diet 2 weeks ND Wnorowski (1996b)
555 3-Mercaptohexyl butyrate Rat MF 1/20 Diet 2 weeks ND Wnorowski (1996c)
556 3-Mercaptohexyl hexanoate Rat MF 1/20 Diet 2 weeks ND Wnorowski (1996d)
560 3-Mercapto-2-pentanone Rat MF 1/30 Oral 92 days 1.9c Morgareidge (1971b)
562 2,5-Dimethyl-2,5-dihydroxy Rat MF 1/30 Diet 92 days 3.1c Cox et al. (1973b)
-1,4-dithiane
566 Propyl disulfide Rat F 1/6 Gavage 6 days ND Munday & Manns (1994)
Rat M 1/10-16 Diet 90 days 7.3c Posternak
et al. (1969)
F 1/10-16 Diet 90 days 8.2c
572 Allyl disulfide Rat F 2/6 Gavage 6 days 36c Munday & Manns (1994)
Table 4. (contd)
No. Substance Species Sex No. test Route Duration NOEL Reference
groupsa/no. (mg/kg bw)
per test
groupb
573 3,5-Dimethyl-1,2,4-trithiolane Rat, MF 1/32 Gavage 90 days 1.9c British Industrial
Biological Research
Association (1976)
574 3-Methyl-1,2,4-trithiane Rat MF 1/32 Oral 13 weeks 0.3c Mondino (1981)
575 Dicyclohexyl disulfide Rat MF 1/30 Oral 92 days 0.23c Cox et al. (1974b)
577 Methyl benzyl disulfide Rat MF 1/30 Oral 92 days 1.2c Gallo et al. (1976)
578 Phenyl disulfide Rat F 1/6 Gavage 6 days ND Munday et al. (1990)
585 Dipropyl trisulfide Rat MF 1/30 Oral 92 days 4.8c Morgareidge
& Oser (1970b)
587 Diallyl trisulfide Rat MF 1/30 Oral 92 days 4.6c Morgareidge
& Oser (1970c)
M, male; F, female; ND, not determined
a No. of test groups does not include controls
b No. per test group comprises males and females
c Study performed with either a single dose or multiple doses that had no effect; the value is therefore the highest dose tested.
d Dose increased from 500 to 1000 mg after six weeks
represent adverse effects (British Industrial Biological Research
Association, 1976). The dose of 0.44 mg/kg bw per day of
2-methyl-4-propyl-1,3-oxathiane that showed no effects is more than 10
000 times the estimated per capita intakes of 0.03 mg/kg bw per day in
Europe and 0.02 mg/kg bw per day in the United States.
4,5-Dihydro-3(2H)-thiophenone (No. 498)
4,5-Dihydro-3(2H)-thiophenone (No. 498) was tested in a
standardized study (see above) at a single dose of 9.16 mg/kg bw per
day. No effects were observed (Morgareidge, 1970). This dose is 100
000 times greater than the estimated per capita intakes of 0.01 µg/kg
bw per day in Europe and 0.04 µg/kg bw per day in the United States.
2-Methyl-1,3-dithiolane (No. 534)
A group of 16 male and 16 female Sprague-Dawley rats received an
aqueous propylglycol solution (0.2% w/w) containing 7 mg/kg bw of
2-methyl-1,3-dithiolane (No. 534) daily by oral intubation for 91
days. Control animals received 0.02% propylene glycol only. The rats
were observed routinely for body weight and feed consumption.
Haematological and blood chemical parameters were determined in weeks
4 and 13. No difference was seen between control and treated groups in
body-weight gain or feed consumption. A slight, nonsignificant
reduction in haemoglobin concentration was seen in treated females.
Gross and histological examinations at termination showed no
dose-related effects, and organ weights were normal (Griffiths et al.,
1979). The dose of 7 mg/kg bw per day is greater than 1 000 000 times
the estimated per capita intake of 0.002 mg/kg bw per day of
2-methyl-1,3-dithiolane in Europe and 10 000 times that of 0.1 mg/kg
bw per day in the United States.
Trithioacetone (No. 543)
Trithioacetone (No. 543) was tested in a standardized study (see
above) at a single dose of 0.205 mg/kg bw per day. No effects were
observed (Cox et al., 1973a). This dose is 10 000 greater than the
estimated intakes of 0.04 µg/kg bw per day in Europe and 0.01 µg/kg bw
per day in the United States.
2,5-Dimethyl-2,5-dihydroxy-1,4-dithiane (No. 562)
2,5-Dimethyl-2,5-dihydroxy-1,4-dithiane (No. 562) was tested in a
standar-dized study (see above) at a single dose of 3.1 mg/kg bw per
day. No effects were observed (Cox et al., 1973b). This dose is 1 000
000 greater than the estimated per capita intakes of 0.004 µg/kg bw
per day in Europe and 0.003 µg/kg bw per day in the United States.
(iv) Simple thiols
Cyclopentanethiol (No. 516)
Cyclopentanethiol (No. 516) was tested in a standardized study
(see above) at a single dose of 0.56 mg/kg bw per day. No effects were
observed (Morgareidge & Oser, 1970a). This dose is 10 000 times
greater than the estimated per capita intake of 0.01 µg/kg bw per day
in the United States.
2,3-or 10-Mercaptopinane (No. 520)
2,3-or 10-Mercaptopinane (No. 520) was tested in a standardized
study (see above) at a single dose of 0.06 mg/kg bw per day, No
effects were observed (Oser, 1966). This dose is 10 000 times greater
than the estimated per capita intake of 0.001 µg/kg bw per day in
Europe and 100 times that of 0.2 µg/kg bw per day in the United
States.
ortho- Toluenethiol (No. 528)
Groups of 10-16 male and 10-16 female rats were given 0.52 mg/kg
bw per day of ortho-toluenethiol (No. 528) in the diet for 90 days.
Control animals received untreated diet. Feed consumption and body
weights were recorded weekly. Haematological examinations and blood
urea determinations were conducted on half the animals at seven weeks
and on all animals at 13 weeks. At necropsy, organs were weighed and
examined grossly and histologically. No adverse effects were observed
(Posternak et al., 1969). The dose that had no effect is greater than
1000 times the estimated per capita intake of 0.4 mg/kg bw per day in
Europe and 100 000 times that of 0.003 mg/kg bw per day in the United
States.
2,6-Dimethylthiophenol (No. 530)
2,6-Dimethylthiophenol (No. 530) was administered in corn oil by
gavage to 32 male and 32 female Sprague-Dawley rats at a concentration
calculated to result in an average intake of 0.43 mg/kg bw per day for
13 weeks. Control animals received only corn oil. Body weights and
feed intake were measured weekly, and haematological and blood
chemical parameters were measured at weeks 4 and 13. Organs were
weighted and gross and histological examinations were performed at the
time of autopsy. No significant difference was seen between treated
and control animals (Peano et al., 1981). The dose that had no effect
is greater then 10 000 times the estimated per capita intake of 0.03
mg/kg bw per day in Europe and greater than 1 000 000 times that of
0.0003 mg/kg bw per day in the United States.
2-Naphthalenethiol (No. 531)
2-Naphthalenethiol (No. 531) was tested in a standardized study
(see above) at a single dose of 3.4 mg/kg bw per day. No effects were
observed (Morgareidge, 1971a). The dose that had no effect is 1 000
000 times greater than the estimated per capita intake of 0.001 µg/kg
bw per day in the United States.
(v) Thiols with oxidized side-chains
2-Mercapto-3-butanol (No. 546)
2-Mercapto-3-butanol (No. 546) was tested in a standardized study
(see above). No effects were observed (Cox et al., 1974a). The dose of
1.9 mg/kg bw per day that had no effect is 1000 times greater than the
estimated per capita intake of 0.1 µg/kg bw per day in Europe and
10 000 times that of 0.002 µg/kg bw per day in the United States.
alpha-Methyl-beta-hydroxypropyl
alpha-methyl-beta-mercaptopropyl
sulfide (No. 547)
alpha-Methyl-beta-hydroxypropyl alpha-methyl-beta-mercaptopropyl
sulfide (No. 547) was tested in a standardized study (see above) at a
single dose of 2.815 mg/kg bw per day. No effects were observed
(Morgareidge et al., 1974b). This dose is 100 000 times greater than
the estimated per capita intake of 0.01 µg/kg bw in the United States.
3-Mercapto-2-pentanone (No. 560)
3-Mercapto-2-pentanone (No. 560) was tested in a standardized
study (see above) at a single dose of 1.89 mg/kg bw per day. No
effects were observed (Morgareidge, 1971b). This dose is 100 000 times
greater than the estimated per capita intake of 0.002 µg/kg bw per day
in the United States.
(vi) Dithiols
2,3-Butanedithiol (No. 539) and 1,8-octanedithiol (No. 541)
2,3-Butanedithiol (No. 539) and 1,8-octanedithiol (No. 541) were
tested in standardized studies (see above) at single doses of 0.703
and 0.705 mg/kg bw per day, respectively. No effects were observed
(Morgareidge et al., 1974a,c). The dose of 2,3-butanedithiol that had
no effect is 100 000 times greater than the estimated per capita
intakes of 0.001 µg/kg bw per day in Europe and 0.003 µg/kg bw per day
in the United States. The dose of 1,8-octanedithiol that had no effect
is 1000 times greater than the estimated per capita intake of 0.1
µg/kg bw per day in Europe and 10 000 times that of 0.01 µg/kg bw per
day in the United States.
(vii) Simple disulfides
Propyl disulfide (No. 566)
Three groups of 10-16 male and 10-16 female rats were given 7.3
or 8.2 mg/kg bw per day of propyl disulfide (No. 566) in the diet for
90 days. Control animals received untreated diet. feed consumption and
body weights were recorded weekly, and haematological examinations and
blood urea determinations were conducted on half the animals at seven
weeks and on all animals at 13 weeks. At necropsy, organs were weighed
and examined grossly and histologically. No adverse effects were
observed (Posternak et al., 1969). The doses that had no effects are
10 000 times greater than the estimated per capita intake of 0.1 mg/kg
bw per day in Europe and 1 000 000 times that of 0.002 mg/kg bw per
day in the United States.
Dicyclohexyl disulfide (No. 575)
Dicyclohexyl disulfide (No. 575) was tested in a standardized
study (see above) at a single dose of 0.23 mg/kg bw per day. No
effects were observed (Cox et al., 1974b). The dose that had no effect
is 100 000 times greater than the estimated per capita intake of
0.0003 µg/kg bw per day in Europe and 10 000 that of 0.003 µg/kg bw
per day in the United States.
Methyl benzyl disulfide (No. 577)
Methyl benzyl disulfide (No. 577) was tested in a standardized
study (see above) at a single dose of 1.2 mg/kg bw per day, No effects
were observed (Gallo et al., 1976). The dose that had no effect is 1
000 000 times greater than the estimated per capita intake of 0.0003
µg/kg bw per day in Europe and 100 000 that of 0.002 µg/kg bw per day
in the United States.
(viii) Disulfides with oxidized side-chains
No studies on the two substances in this group were available,
but their toxicity can be compared by analogy to that of the
disulfides.
(ix) Trisulfides
Dipropyl trisulfide (No. 585)
Dipropyl trisulfide (No. 585) was tested in a standardized study
(see above) at a single dose of 4.8 mg/kg bw per day, No effects were
observed (Morgareidge & Oser, 1970b). The dose that had no effect is
10 000 times greater than the estimated per capita intake of 0.2 µg/kg
bw per day in Europe and 100 000 that of 0.02 µg/kg bw per day in the
United States.
Diallyl trisulfide (No. 587)
Diallyl trisulfide (No. 587) was tested in a standardized study
(see above) at a single dose of 4.6 mg/kg bw per day, No effects were
observed (Morgareidge & Oser, 1970c). The dose that had no effect is
10 000 times greater than the estimated per capita intake of 0.1 µg/kg
bw per day in Europe and 100 000 that of 0.0003 µg/kg bw per day in
the United States.
(x) Heterocyclic disulfides
3,5-Dimethyl-1,2,4-trithiolane (No. 573)
Groups of 16 male and 16 female weanling Wistar rats were given
3,5-dimethyl-1,2,4-trithiolane (No. 573) by oral intubation at a daily
dose of 1.9 mg/kg bw for 90 days or corn oil alone. The rats were
weighed on the first day of treatment and thereafter at regular
intervals throughout the study. Feed intake was recorded at three- or
four-day intervals. Blood was collected from half the rats at six
weeks and from all rats at 12 weeks and was examined for haemoglobin
concentration, packed cell volume, and numbers of erythrocyte and
leukocytes. The urea concentration was measured. At necropsy, organs
were weighed and gross and histological examinations were performed.
A slight increase in body-weight gain was seen in treated males,
which was associated with increased feed intake thought to result from
alteration of feeding behaviour due to intubation of highly flavoured
solutions. Isolated differences from controls were seen in
haematological parameters, organ weights, and histological appearance,
but none was statistically significant and they were considered not to
represent adverse effects (British Industrial Biological Research
Association, 1976). The dose that had no effect is 100 000 times
greater than the estimated per capita intakes of 0.001 mg/kg bw per
day in Europe and 0.002 mg/kg bw per day in the United States.
3-Methyl-1,2,4-trithiane (No. 574)
3-Methyl-1,2,4-trithiane (No. 574) was administered orally to 16
male and 16 female rats (strain not specified) at a dose of 0.3 mg/kg
bw per day for 13 weeks. Body weights and feed intake were measured
weekly. Haematological parameters and blood urea were determined at
weeks 4 and 13. At necropsy, organs were weighed and examined
histologically. No adverse effects were observed (Mondino, 1981). The
dose that had no effect is 100 000 and 100 times greater than the
estimated per capita intakes of 0.002 mg/kg bw per day in Europe and
1 mg/kg bw per day in the United States.
(xi) Thioesters
Ethyl thioacetate (No. 483)
Groups of 23 male and 23 female rats were given ethyl thioacetate
(No. 483) at a dose of 6.5 mg/kg bw per day in the diet, while a
control group received basal diet alone. The animals were observed
daily for appearance, physiological responses, behaviour, any
pharmacological or toxicological responses, and deaths. Body weights
and feed consumption were recorded weekly. During weeks 6 and 13,
urine was obtained from eight males and eight females for estimation
of pH and specific gravity, for microscopic examination of sediment,
and for qualitative estimates of albumin, glucose, occult blood,
ketones, and bilirubin. Eight animals of each sex were killed after
six weeks, and blood was taken for haematological testing. The
remaining animals were necropsied at 13 weeks and their tissues
examined for gross pathological changes. Organs were weighed and
tissues retained for histological evaluations. The growth, feed
consumption, and feed utilization of the test animals were similar to
those of controls throughout the study, and blood biochemical and
haematological indices were normal. Likewise, there were no
significant differences in the average absolute or relative organ
weights. All rats appeared normal throughout the study, and no gross
pathological lesions were seen in any tissue (Shellenberger, 1970).
The dose that had no effect is 1 000 000 times greater than the
estimated per capita intake of 0.0003 mg/kg bw per day in both Europe
and the United States.
Methyl thiobutyrate (No. 484)
Three groups of 10 male rats were fed 20, 200, or 500 mg/kg bw
per day of methyl thiobutyrate (No. 484) for 13 weeks, the dose given
to the third group being increased from 500 to 1000 mg/kg bw per day
after six weeks. A control group received only the basal diet. Feed
consumption and body weights were recorded weekly. A slight decrease
in weight gain was observed in treated rats as compared with controls,
which was attributed to decreased feed intake associated with
unpalatability. At necropsy, haematological examinations showed normal
values, and no differences in relative or absolute organ weights or
gross or histological appearance was seen between test and control
animals (Wheldon et al., 1970). The dose of 1000 mg/kg bw per day
which had no effect is 1 000 000 times greater than the estimated per
capita intake of 0.1 mg/kg bw per day in both Europe and the United
States.
(xii) Sulfoxides
No 90-day study was available for this group of compounds, but
methylsulfinylmethane (DMSO; No. 507) has been tested in long-term
studies of toxicity and carcinogenicity in rats, dogs, and monkeys
(see below) and has also been given to humans (see below).
2.3.2.4 Long-term studies of toxicity and carcinogenicity
Long-term studies of toxicity and carcinogenicity in rats, dogs,
and monkeys were available only for DMSO (No. 507) (Table 4).
Groups of 50 male and 50 female Sprague-Dawley rats and groups of
five male and five female pure-bred Pembrokeshire Corgi dogs were
given a 50% aqueous solution containing undiluted DMSO at 1100, 3300,
or 9900 mg/kg bw per day by gavage on five days per week. A control
group received 1 L/kg bw per day of distilled water. The rats were
treated for 18 months and the dogs for either 18 weeks or two years.
All animals were observed daily for clinical signs, and body weight
and feed consumption were measured weekly. Haematological assessments,
biochemical measurements, and urinary analyses were performed after 4,
12, 20, 32, 51, 60, and 72 weeks for the rats and after 1, 3, 4.5, 6,
9, 12, 18, and 24 months for the dogs. Routine ophthalmo-scopic
examinations were also performed. At termination, detailed macroscopic
and histopathological examinations were performed.
A dose-related decrease in weight gain was observed in rats at
all doses, except in males receiving the lowest dose. The decrease in
weight gain was not accompanied by a decrease in feed intake. Male
rats at the high dose also showed a slight reduction in haemoglobin
and packed-cell volume. Examination of the eyes revealed no changes in
the retina or vitreous humour. No adverse effects on body weight or
feed intake were seen in the dogs. Persistent diuresis was observed in
those receiving the intermediate and high doses, but there was no
renal damage. Increased packed-cell volume and haemoglobin levels were
observed at the highest dose, although the erthrocytes had normal
haemoglobin concentrations and were of normal size. The most
significant observation in dogs was lenticular changes, comprising
changes in the refractive index of the central portion of the lens,
transitory, dense, white equatorial lens opacity, the appearance of
persistent opalescence in the central region of the lens, and changes
in the vitreous humour. Biochemical analyses of the lenses of affected
animals revealed an increase in insoluble protein and reductions in
soluble protein, glutathione, and water. These changes were observed
during the fifth month in dogs receiving the highest dose and during
the ninth to tenth months in those at the intermediate dose. Nuclear
refractive changes were observed after six months in dogs at the low
dose, but none of these animals had opalescence. Those animals that
were withdrawn from the study at 18 weeks showed partial regression of
the lenticular changes at the end of the two-year period, and the
lenses of dogs that received the intermediate dose had apparently
reverted to a normal state. No abnormalities other than those in the
eye were detected on macroscopic or histopathological examination
(Noel et al., 1975).
Four groups of rhesus monkeys received doses equivalent to 0,
990, 3000, or 9000 mg/kg bw per day of a 90% solution of DMSO by
gastric intubation or dermal application for 74-87 weeks. Three
females and one male received only water by oral intubation, and two
males and one female received only water dermally and served as
controls. Two animals of each sex were treated with 990 and 2970 mg/kg
bw per day, and three animals of each sex received 8910 mg/kg bw per
day by each route of administration. The topical administration
consisted of direct application to the entire abdominal skin. Physical
signs, behaviour, and survival were recorded daily. Body weights,
water consumption, blood pressure, heart rate, respiratory rate,
neurological reflexes, and complete haematological ophthalmological
and urinary analyses were performed during weeks 1, 4, 7, 12, 24, 37,
51, and 73. At termination, gross and histomorphological examinations
were made.
All animals treated topically showed scaling and flaking on the
area of application but no other adverse behavioural or physical
signs. Animals given the highest dose orally died between weeks 15 and
53 of the study. No other treatment-related changes were found in the
monkeys treated orally, in physical examinations, in haematological,
ophthalmological, urinary, or biochemical parameters, or in absolute
or relative organ weights. Histologically, atelectasis and emphysema
were the only pathological changes observed, and were seen only at the
highest dose. Some regurgitation and/or tracheal inspiration of DMSO,
which is a highly volatile, irritating substance, may have occurred
(Vogin et al., 1970).
2.3.2.5 Genotoxicity
Studies of mutagenicity and genotoxicity were available for 14
mono-, di-, and trisulfides, thiols, and related oxygenated
derivatives in this group and three structurally related substances
(Table 5). The compounds were tested for mutagenicity in vitro at
concentrations up to 300 000 mg/plate. No effect was found in
Salmonella typhimurium strains TA97, TA98, TA100, TA102, TA1535,
TA1537, TA1538, or TA2637 with or without metabolic activation (Lavoie
et al., 1979; Eder et al., 1980, 1982; Wild et al., 1983; Aeschbacher
et al., 1989; Watanabe & Morimoto, 1989a,b; Phillips Petroleum Co.,
1990a; Zeiger et al., 1992; Hakura et al., 1993; Wang et al., 1994;
Karekar et al., 1996; Brams et al., 1997). The only positive results
found were with 1,2-ethanedithiol (No. 532) and DMSO (No. 507). The
latter is widely used as a solvent in assays for genotoxicity in
vitro.
In a test for reverse mutation in nine strains of S.
typhimurium and Escherichia coli strains WP2 and WP2uvrA, modified
by the use of preincubation, DMSO was mutagenic in S. typhimurium
TA1537 and TA2637 and in E. coli WP2uvrA at concentrations of
0.2-0.4 ml/plate, with and without metabolic activation (Hakura et
al., 1993). These are relatively high concentrations, some being
cytolethal, and no mutagenic activity was observed at concentrations
used routinely in this assay.
The ability of 1,2-ethanedithiol (No. 532) to induce specific
locus mutations at the Tk locus in cultured L5178Y Tk+/- mouse
lymphoma cells was evaluated in the presence and absence of a
metabolic activation system prepared from Aroclor-induced rat liver.
When unactivated cultures were treated with concentrations of 13-150
µg/ml, total cell survival was only 0.9-32%. A dose-related increase
in mutant frequency was seen relative to control cultures in the
solvent, DMSO. In cultures with metabolic activation, no evidence of
mutagenicity was seen at concentrations of 0.07-1 µg/ml. The survival
rate in these cultures (3-49%) was greater than that in cultures
without activation. Concentrations of 16 and 50 µg/ml of
1,2-ethanedithiol were reported to induce a significant
( p < 0.002), concentration-related increase in the frequency of
sister chromatid exchange in Chinese hamster ovary cells in the
absence of metabolic activation, and concentrations of 1.6 and 5 µg/ml
induced a significant ( p < 0.004) increase in the frequency of
sister chromatid exchange in the presence of metabolic activation. No
cytotoxicity was observed at concentra-tions up to 5 µg/ml (Phillips
Petroleum Co., 1990a).
The validity of the L5178YTk+/- mouse lymphoma cell assay has
been evaluated in relation to potentially acidifying substances of low
relative molecular mass by Brusick (1986) and Heck et al. (1989).
Those authors concluded that the assay was not valid, since positive
results can be induced by changes in the pH and osmolality of the
culture medium. The results of assays for sister chromatid exchange
may also be artefacts resulting from lysosome breakdown secondary to
cytotoxicity. Cytotoxic concentrations of substances may also cause
release of DNase which induces chromosomal aberrations and DNA
double-strand breaks (Zajac-Kaye & Ts'o, 1984; Bradley et al., 1987).
Cells with double-strand breaks that survive could be subject to a
variety of genotoxic consequences, including chromosomal breaks and
rearrangements. As cytotoxicity and lysosomal breakdown were not
evaluated in the study of sister chromatid exchange with
1,2-ethandithiol, the results are difficult to interpret.
Groups of male ICR mice were given two doses 48 h apart of a
mixture containing allyl sulfide (No. 458), allyl disulfide (No. 572),
or diallyl trisulfide (No. 587) in corn oil at doses of 10 or 20 mg/ml
by gavage. The doses were estimated to provide 0.33 or 0.67 mmol/kg bw
or 50 or 100 mg/kg bw on the basis of the composition of the mixture.
No increase in the frequency of mirconucleated polychromatic
erythrocytes was seen in bone-marrow cells (Marks et al., 1992).
The results of these assays in vivo and in vitro indicate
that aliphatic and aromatic thiols and sulfides are not genotoxic.
2.3.2.6 Developmental toxicity
Studies on teratogenicity were available only for 1-butanethiol
(No. 511) and benzenethiol (No. 525).
Table 5. Studies of genotoxicity with aliphatic and aromatic sulfides and thiols
No. Substance End-point Test system Concentration Results Reference
458 Allyl sulfide Reverse mutation S. typhimurium TA100 0.004-0.44 µg/ml Negativea Eder et al. (1982)
458 Allyl sulfide Micronucleus formation Mouse 38-77 mg/kg bw Negative Marks et al. (1992)
492 Methylthio 2-(acetyloxy) Reverse mutation S. typhimurium TA100, TA98, 0.156-5 mg/plate Negativea Watanabe &
propionate TA1535, TA1537 Morimoto (1989a)
493 Methylthio 2-(propionyloxy) Reverse mutation S. typhimurium TA98, TA100, 0.156-5 mg/plate Negativea Watanabe &
propionate TA1535, TA1537 Morimoto (1989b)
507 DMSOc Reverse mutation S. typhimurium TA97, TA98, 0.005-10 µmol/ Negativea Karekar et al.
TA100, TA102 plate (1996)
507 DMSO Reverse mutation S. typhimurium TA98, TA100 12.5-200 µg/plate Negativea Wang et al. (1994)
507 DMSO Reverse mutation S. typhimurium TA97, TA98, 100 000-300 000 Negativea Brams et al. (1987)
TA100 µg/plate
507 DMSO Reverse mutation S. typhimurium TA97, TA98, 100-10 000 µg/ Negativea Zeiger et al. (1992)
TA100, TA1535, TA1537 plate
507 DMSO Reverse mutation S. typhimurium TA97, TA98, 0.1-0.4 ml/plate Negativea Hakura et al. (1993)
TA100, TA102, TA104,
TA1535, TA1538
507 DMSO Reverse mutation S. typhimurium TA1537, 0.1-0.4 ml/plate Positived Hakura et al. (1993)
TA2637
521 Allyl mercaptan Reverse mutation S. typhimurium TA100 0.005-1.4 µg/ml Negativea Eder et al. (1980)
525 Benzenethiol Reverse mutation S. typhimurium TA98, TA100 25-500 µg/plate Negative Lavoie et al. (1979)
526 Benzyl mercaptan Reverse mutation S. typhimurium TA98, TA100, < 3.6 mg/plate Negativea Wild et al. (1983)
TA1535, TA1537, TA1538
532 1,2-Ethanedithiol Reverse mutation S. typhimurium TA98, TA100, 5000 µg/plate Negativea Phillips Petroleum
TA1535, TA1537, TA1538 Co. (1990a)
532 1,2-Ethanedithiol Sister chromatid Chinese hamster ovary cells 16 µg/mle Positivef Phillips Petroleum
exchange 1.6 µg/mlg Positive Co. (1990a)
532 1,2-Ethanedithiol Forward mutation L5178Y mouse lymphoma 150 µg/mle Positivef Phillips Petroleum
cells, Tk locus 1 µg/mlg Negative Co. (1990a)
551 2-Mercaptopropionic Reverse mutation S. typhimurium TA98, TA100, < 3.6 mg/plate Negativea Wild et al. (1983)
acid TA1535, TA1537, TA1538
Table 5 (contd)
No. Substance End-point Test system Concentration Results Reference
564 Dimethyl disulfide Reverse mutation S. typhimurium TA98, TA100, 0.011-1.1 mmol Negativea Aeschbacher et al.
TA102 (1989)
572 Allyl disulfide Reverse mutation S. typhimurium TA100 0.0015-0.15 µg/ml Negativea Eder et al. (1980)
572 Allyl disulfide Micronucleus formation Mouse 48-98 mg/kg bw Negativeb Marks et al. (1992)
578 Phenyl disulfide Reverse mutation S. typhimurium TA98, TA100, < 3.6 mg/plate Negativea Wild et al. (1983)
TA1535, TA1537, TA1538
579 Benzyl disulfide Reverse mutation S. typhimurium TA98, TA100, < 3.6 mg/plate Negativea Wild et al. (1983)
TA1535, TA1537, TA1538
587 Diallyl trisulfide Micronucleus formation Mouse 59-120 mg/kg bw Negativeb Marks et al. (1992)
Related substances
tert-Dodecyl Reverse mutation S. typhimurium TA98, TA100, 0.018-10 000 µg/ Negativea Zeiger et al. (1987)
mercaptan TA1535, TA1537 plate
Dimethyl sulfone Reverse mutation S. typhimurium TA98, TA100, 0.003-0.3 mmol Negativea Aeschbacher et al.
TA102 (1989)
n-Butyl sulfone Disc method E. coli Sd-4-73 Not specified Negative Szybalski, (1958)
100 2-Mercaptopropionic Basc mutation Drosophila 10 mmol/L Negative Wild et al. (1983)
acid
a With and without metabolic activation
b Mixture of allyl sulfide, allyl disulfide, and allyl trisulfide in a ratio of 68:20:12
c Tested as solvent control
d At doses that caused lethality; negative results at doses used routinely in this test
e Without metabolic activation
f Positive results in tests for sister chromatid exchange may be a result of lysosome breakdown, and positive results in mouse lymphoma
cells may be due to pH and osmolality of culture medium
g With metabolic activation
1-Butanethiol (No. 511)
Pregnant COBS CD rats were exposed to 1-butanethiol by whole-body
inhalation for 6 h per day on days 6-19 of gestation at doses of 10,
68, or 152 mg/kg of diet. The control group was exposed to filtered
air only. The fetuses were removed from all rats on gestation day 20.
The animals were examined for gross physical changes, viable fetuses,
fetal body weights, maternal body-weight changes, and
post-implantation losses or total implantations. 1-Butanethiol had no
effect at the lowest dose, but significant differences in fetal body
weights were seen at 68 and 152 mg/kg of diet (Thomas et al., 1987).
Benzenethiol (No. 525)
Groups of 15-26 New Zealand white rabbits were given benzenethiol
in corn oil orally at doses of 10, 30, or 40 mg/kg bw per day on days
6-19 of gestation. Maternal body weight and feed and water consumption
were recorded. On gestation day 30, the animals were killed and
maternal organs and fetuses were weighed. The fetuses were also
examined for external, visceral, and skeletal malformations. There was
no effect at 10 mg/kg bw per day, but significant differences in
maternal body weights were seen at 30 and 40 mg/kg bw per day.
In the same study, groups of 25 rats were given 20, 35, or 50
mg/kg bw per day orally on days 6-15 of gestation and were killed on
gestation day 20. They were examined for the same parameters as the
rabbits. Decreased mater-nal body weight was seen at all doses, and
fetal body weights were decreased at 35 and 50 mg/kg bw per day. At 50
mg/kg bw, the relative maternal liver weights were increased and
increased post-implantation losses occurred, with a slight but
nonsignificant decrease in the live litter size (George et al., 1995).
2.3.2.7 Chemoprevention
Several organosulfur compounds inhibit carcinogenesis by
increasing the metabolism, detoxification, and elimination of
carcinogens by phase I and II enzymes. For example, the effect of
oltipraz in inhibiting colon tumorigenicity is associated with
increased activity of glutathione S-transferase, NAD(P)H quinone
reductase, and UDP-glucuronosyl transferase in the colon (Reddy et
al., 1993). This effect may partly explain the cancer-inhibiting
effect of foods like garlic that contain organosulfur compounds.
Allyl sulfide (No. 458)
Seven-week-old female A/J mice were given allyl sulfide at a dose
of 200 mg/kg bw per day in the diet for three days. Two hours after
treatment, the animals were given either a single dose of 100 mg/kg bw
of the carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and
kept for 16 weeks for determination of lung tumour production or
killed immediately for preparation of lung and liver microsomes.
Administration of the test material before carcinogen treatment
significantly decreased the lung tumour incidence (by 38%) and tumour
multiplicity (by 0.6) (Hong et al., 1994).
Allyl mercaptan (No. 521)
Groups of male weanling rats were given free access to a purified
diet for two weeks and then given the same diet containing 0.2% allyl
mercaptan dissolved in corn oil, corresponding to a calculated (Food
and Drug Administration, 1993) daily intake of allyl mercaptan of 200
mg/kg bw. After two weeks, the rats were injected intraperitoneally
with a single dose of 2 mg/kg bw aflatoxin B1, 910 mg/kg bw
N-nitrosodimethylamine, or 50 mg/kg bw N-methyl- N-nitrosourea
and killed 4 h later. Hepatic DNA damage was evaluated as
single-strand breaks by the alkaline elution technique, and the amount
of DNA eluted was determined by a microfluorimetric procedure. Allyl
mercaptan significantly reduced (by 64%) the incidence of DNA
single-strand breaks induced by aflatoxin B1 and reduced that induced
by N-nitrosodimethylamine by 39%. It had no effect on the incidence
of breaks induced by N-methyl- N-nitrosourea (LeBon et al., 1997).
Propyl disulfide (No. 566)
Cultures of the adult human tumour cell lines HCT-15 (colon),
A549 (lung), and SKMEL-2 (skin) were plated for 24 h and then propyl
disulfide was added at a dose of 100 mmol for up to 48 h. The cells
were harvested and intracellular free calcium and apoptotic cells were
determined. There was no effect on apoptosis, but a 12% increase in
the intracellular concentration of calcium was observed (Sundaram &
Milner, 1996).
Allyl disulfide (No. 572)
Cultures of the adult human tumour cell lines HCT-15 (colon),
A549 (lung), and SKMEL-2 (skin) were plated for 24 h and then allyl
disulfide was added at a dose of 100 or 500 mmol for up to 48 h. The
cells were harvested and intracellular free calcium, apoptotic cells,
and DNA fragmentation were determined. The proliferation of the HCT-15
and SKMEL-2 cell lines was depressed by 90% and that of the A549 line
by 75%, and the intracellular calcium concentration was increased by
41%. Morphological changes characteristic of apoptosis were observed
in treated cells, and a fivefold increase in DNA fragmentation was
seen (Sundaram & Milner, 1996).
Groups of 12 male Fischer 344 rats received diallyl disulfide at
a concentra-tion of 0, 100, or 200 mg/kg of diet. After two weeks, the
animals received azoxymethane, a known carcinogen, subcutaneously once
a week for two weeks at a dose of 15 mg/kg bw per week. Treatment
continued for 52 weeks, at which time the animals were killed. Body
weights were recorded every two weeks for the first 10 weeks and then
every four to six weeks. At autopsy, the animals were dissected in
order to detect microscopic tumours, and their location, number, and
size were recorded. Colon tumors with a diameter > 0.5 cm were cut
into two halves: one portion was used to measure enzyme activity and
the other for histopathology. Animals fed 200 mg/kg of diet showed a
slight but significant decrease in body weight. Neither the incidence
nor the multiplicity of colon adenomas differed between the control
and experimental groups, but both doses significantly reduced the
incidence and multiplicity of invasive colon adenocarcinomas. The
activities of glutathione S-transferase, NAD(P)H quinone reductase,
and UDP-glucuronosyl transferase in the liver, colonic mucosa, and
tumours were also significantly increased in the treated animals
(Reddy et al., 1993).
2.3.3 Observations in humans
Methylsulfinylmethane (DMSO; No. 507)
In clinical trials to determine the therapeutic effects of DMSO,
8900 mg/kg bw of 90% DMSO were applied to the entire trunk of 20 men
either twice daily for three weeks or once daily for 26 weeks.
Haematological examinations, blood chemical determinations, and urine
analyses performed at the beginning and end of the three-week study
revealed no significant changes. In the 26-week study, similar
examinations were performed at 0, 2, 4, 8, 12, 16, and 24 weeks, in
addition to thymol turbidity testing and sodium sulfobromophthalein
determinations. Except for the appearance of cutaneous signs such as
erythema, scaling, contact urticaria, and stinging or burning
sensations, DMSO was tolerated well by all but two individuals, who
developed systemic symptoms including a scaling rash, severe abdominal
pain, slight nausea, and chest pains during the second week of
treatment. One of these men did not continue the study; the other
continued treatment, and the symptoms eventually abated. Clinical and
laboratory investigations showed no evidence of adverse effects
(Kligman, 1965).
Two drops of aqueous solutions of increasing concentrations of
DMSO were instilled into the lower conjunctival sac of groups of 10
adult men, each eye being used only once without washing. No adverse
effects were seen until a concentration of 50% was reached, when the
men complained of transient burning. With 90% DMSO, all the men noted
temporary stinging and burning. The external eye was completely normal
24 h after treatment. The author concluded that DMSO is absorbed so
rapidly through the conjunctival mucosa that any irritation would not
be manifested (Kligman, 1965).
While studies by oral administration are most appropriate for
evaluating the safety of flavouring substances, the results of studies
of dermal absorption may be considered useful in their absence for
substances such as DMSO, which are readily absorbed into the systemic
circulation. The lack of treatment-related adverse effects (other than
irritation) in rhesus monkeys after both oral and dermal treatment
with DMSO (Vogin et al., 1970; see above) demonstrates that the
toxicity of DMSO is independent of route of administration. Therefore,
the lack of toxic effects other than irritation in studies in humans
treated dermally or in the eye (Kligman, 1965) suggests that oral oral
intake of DMSO used as a flavouring substance would not be toxic.
3. REFERENCES
Aeschbacher, H.U., Wolleb, U., Loliger, J., Spadone, J.C. & Liardon R.
(1989) Contribution of coffee aroma constituents to the mutagenicity
of coffee. Food Chem. Toxicol., 27, 227-232.
Alterman, M.A., Chaurassia, C.S., Lu, P. & Hanzlik R.P. (1995)
Metabolism of 10-methoxydecanoic acid (1) and 10-(methylthio)decanoic
acid(2), (omega-1)-oxa and -thia analogs of lauric acid. Int. Soc.
Study Xenobiotics, 8, 118.
Ames, M.M., Selassie, C.D., Woodson, L.C., Van Loon, J.A., Hansch, C.
& Weinshilboum, R.M. (1986) Thiopurine methyltransferase:
Structure-activity relationships for benzoic acid inhibitors and
thiophenol substrates. J. Med. Chem., 29, 345-358.
Anders, M.W., Ratnayake, J.H., Hanna, P.E. & Fucus, J.A. (1980)
Involvement of thioredoxin in sulfoxide reduction by mammalian
tissues. Biochem. Biophys. Res. Commun., 97, 846-851.
Anders, M.W., Ratnayake, J.H., Hanna, P.E. & Fucus, J.A. (1981)
Thioredoxin-dependent sulfoxide reduction by rat renal cytosol.
Biochem. Drug Metab. Disposition, 9, 307-310.
Bailey, D.E. (1976a) Acute oral toxicity (LD50) studies in mice with
1,8-octanedithiol. Unpublished study submitted to the Flavor and
Extract Manufacturers' Association by Food and Drug Research
Laboratories, Inc.
Bailey, D.E. (1976b) Acute oral toxicity of 1,2-propanedithiol in
mice. Unpublished study submitted to the Flavor and Extract
Manufacturers' Association by Food and Drug Research Laboratories,
Inc.
Bailey, D.E. (1976c) Acute oral toxicity (LD50) studies in mice with
compound 75-31065 (methyl benzyl disulfide). Unpublished study
submitted to the Flavor and Extract Manufacturers' Association by Food
and Drug Research Laboratories, Inc.
Black, R.M., Brewster, K., Clarke, R.J., Hambrook, J.L., Harrison,
J.M. & Howells, D.J. (1993) Metabolism of thiodiglycol
(2,2'-thiobis-ethanol): Isolation and identification of urinary
metabolites following intraperitoneal administration to rat.
Xenobiotica, 23, 473-481.
Bradley, M.O., Taylor, V.L., Armstrong, M.J. & Galloway, S.M. (1987)
Relationship among cytotoxicity, lysosomal breakdown, chromosome
aberrations, and DNA double-strand breaks. Mutat. Res., 189, 69-79.
Brams, A., Buchet, J.P., Crutzen-Fayt, M.C., DeMeester, C., Lauwerys,
R. & Leonard, A. (1987) A comparative study, with 40 chemicals, of the
efficiency of the Salmonella assay and the SOS Chromotest (kit
procedure). Toxicol. Lett., 38, 123-133.
Bremer, J. & Greenberg, D.M. (1961) Enzymic methylation of foreign
sulfhydryl compounds. Biochim. Biophys. Acta, 46, 217-224.
British Industrial Biological Research Association (1976) Short-term
toxicity of samples TT171, TT172, TT173, and TT174 in rats.
Unpublished study submitted to the Flavor and Extract Manufacturers'
Association.
Brodnitz, M.H. & Pascale, J.V. (1971) Thiopropanol S-oxide: A
lachrymatory factor in onions. J. Agric. Food Chem., 19, 269.
Brown, V.K., Robinson, J. & Stevenson, D.E. (1963) A note on the
toxicity and solvent properties of dimethyl sulfoxide. J. Pharm.
Pharmacol., 15, 688-692.
Brusick, D. (1986) Genotoxic effects in cultured mammalian cells
produced by low pH treatment conditions and increased ion
concentrations. Environ. Mutag., 8, 879-886.
Buckberry, L.D. & Teesdale-Spittle, P.H. (1996) Sulfur-hydrogen
compounds. In: Mitchell, S., ed., Biological Interactions of Sulfur
Compounds, London: Taylor & Francis Ltd, pp. 113-144-76.
Butterworth, K.R. & Mason, P.L. (1981) Acute toxicity of thioguaiacol
and of versalide in rodents. Food Chem. Toxicol., 19, 753-755.
Butterworth, K.R., Carpanini, F.M.B., Gaunt, I.F., Hardy, J., Kiss,
I.S. & Gangolli, S.D. (1975) Short-term toxicity of dimethyl sulfide
in rat. Food Cosmet. Toxicol., 13, 15-22.
Canellakis, E.S. & Tarver, H. (1953) The metabolism of methyl
mercaptan in the intact animal. Arch. Biochem. Biophys., 42,
446-455.
Cashman, J.R. & Olsen, L.D. (1990) Stereoselective S-oxygenation of
2-aryl-1,3-dithiolanes by the flavin-containing and cytocrome P-450
monooxygenases. Mol. Pharmacol., 38, 573-585.
Cashman, J.R. & Williams, D.E. (1990) Enantioselective S-oxygenation
of 2-aryl-1,3-dithiolanes by rabbit lung enzyme preparations.
Mol. Pharmacol., 37, 333.
Cashman, J.R., Olsen, L.D. & Bornheim, L.M. (1990) Enantioselective
S-oxygenation by flavin containing cytochrome P-450 mono-oxygenases.
Chem. Res. Toxicol., 3, 344.
Cashman, J.R., Yang, Z.C., Yang, L. & Wrighton, S.A. (1995a)
Stereo-and regioselective N-and S-oxygenation of tertiary amines and
sulfides in adult human liver microsomes. Int. Soc. Study
Xenobiotics, 8, 34.
Cashman, J.R., Park, S.B., Yang, Z.C., Washington, C.B., Gomez, D.Y.,
Giacomini, K. & Brett, C.M. (1995b) Chemical, enzymatic and human
enantioselective S-oxygenation of cimetidine. Int. Soc. Study
Xenobiotics, 8, 133.
Christias, C. (1975) Specific inhibition of sclerotium formation by
2-mercaptoethanol and related sulfhydryl compounds in Sclerotium
rolfsii. Can. J. Microbiol., 21, 1541-1547.
Collinson, V.A. (1989) ST 15 C 88 Acute oral toxicity study in the
rat. Unpublished study No. A/0/13008 from Toxicol Laboratories Ltd,
United Kingdom.
Cox, G.E., Bailey, D.E. & Morgareidge, K. (1973a) 90-Day feeding
studies in rats with trithioacetone. Unpublished report submitted to
the Flavor and Extract Manufacturers' Association by Food and Drug
Research Laboratories, Inc.
Cox, G.E., Bailey, D.E. & Morgareidge, K. (1973b) 90-Day feeding
studies in rats with compound ES-307/308 (14705). Unpublished report
submitted to the Flavor and Extract Manufacturers' Association by Food
and Drug Research Laboratories, Inc.
Cox, G.E., Bailey, D.E. & Morgareidge, K. (1974a) 90-Day feeding study
in rats with compound 14935 (2-mercapto-3-butanol). Unpublished report
submitted to the Flavor and Extract Manufacturers' Association by Food
and Drug Research Laboratories, Inc.
Cox, G.E., Bailey, D.E. & Morgareidge, K. (1974b) 90-Day feeding
studies in rats with compound 31098. Unpublished report submitted to
the Flavor and Extract Manufacturers' Association by Food and Drug
Research Laboratories, Inc.
Cox, G.E., Rucci, G. & Babish, J.G. (1979) 90-Day subacute dietary
toxicity study of IFF code No. 78-002-2 in Sprague-Dawley rats.
Unpublished report submitted to the Flavor and Extract Manufacturers'
Association by Food and Drug Research Laboratories, Inc.
Damani, L.A. (1987) Metabolism of sulphur-containing drugs. In:
Benford, D.J., Bridges, J.W. & Gibson, G.G., eds,
Drug Metabolism--From Molecules to Man, London: Taylor & Francis,
pp. 581-603.
Davis, P.J. & Guenthner, L.E. (1985) Sulindac oxidation/reduction by
microbial cultures; microbial models for mammalian metabolism.
Xenobiotica, 15, 845-857.
Deng, D.J., Mueller, K., Kasper, P. & Mueller, L. (1994) Effect of
diallyl trisulfide on induction of UDS by mutagenic drugs in primary
rat hepatocytes. Biomed. Environ. Sci., 7, 85-90.
Dutton, G.J. & Illing, H.P.A. (1972) Mechanism of biosynthesis of
thio-beta-D-glucosides. Biochem. J., 129, 539-550.
Eder, E., Neudecker, T., Lutz, D. & Henschler, D. (1980) Mutagenic
potential of allyl and allylic compounds. Structure-activity
relationship as determined by alkylating and direct in vitro mutagenic
properties. Biochem. Pharmacol., 29, 993-998.
Eder, E., Henschler, D. & Neudecker, T. (1982) Mutagenic properties of
allylic and alpha, beta-unsaturated compounds: Consideration of
alkylating mechanisms. Xenobiotica, 12, 831-848.
Elfarra, A.A., Duescher, R.J., Sausen, P.J., Lawton, M.P. & Philpot,
R.M. (1995) Potential role of the flavin-containing monooxygenases in
the metabolism of endogenous compounds. Int. Soc. Study Xenobiotics,
8, 9.
El Fatih, Karim, I.A., Millership, J.S., Temple, D.J. & Woolfson, A.D.
(1988) An investigation of the metabolism of
S-carboxymethyl-L-cysteine in man using a novel HPLC-ECD method.
Eur. J. Drug. Metab. Pharmacokinet., 13, 253.
Elf Atochem (1963) Unpublished study from Pharmacology Research Inc.
Elf Atochem (1985) Unpublished study from Product Safety Labs.
Elf Atochem (1992) Unpublished study from Centre International de
Toxicologie.
Fairchild, E.J. & Stokinger, H.E. (1958) Toxicologic studies on
organic sulfur compounds. 1. Acute toxicity of some aliphatic and
aromatic thiols (mercaptans). Am. Ind. Hyg. Assoc. J., 19, 171-189.
Farr, C.H. & Kirwin, C.J. (1994) Organic sulfur compounds. In:
Clayton, D.G. & Clayton, F.E., eds, Patty's Industrial Hygiene
and Toxicology, 4th Ed., Vol. 2, New York: John Wiley & Sons, pp.
4311-4372.
Feng, P.C.C. & Solsten, R.T. (1991) In vitro transformation of
dithiopyr by rat liver enzymes: Conversion of methylthioesters to
acids by oxygenases. Xenobiotica, 21, 1265.
Fenwick, G.R. & Hanley, A.B. (1985) The genus Allium, part 2. CRC
Crit. Rev. Food Sci. Nutr., 22, 273-377.
Fishman, F.G., Jenkins, L.J., Jr, Coon, R.A. & Jones, R.A. (1969)
Effects of acute and repeated inhalation of dimethyl sulfoxide in
rats. Toxicol. Appl. Pharmacol., 15, 74-82.
Flavor and Extract Manufacturers' Association (1991) In vitro
hydrolysis of 3-methylthiohexy acetate. Unpublished report No. 183/91.
Flavor and Extract Manufacturers' Association (1996) Sensory tolerance
test protocol. Unpublished report.
Fogelman, R.W. & DeProspo, J. (1973a) Acute oral toxicity in mice with
ES-307/308. Unpublished report submitted to the Flavor and Extract
Manufacturers' Association by Affiliated Medical Research Institute.
Fogelman, R.W. & DeProspo, J. (1973b) Acute oral toxicity in mice with
SB-11-2876 (trithioacetate). Unpublished report submitted to the
Flavor and Extract Manufacturers' Association by Affiliated Medical
Research Institute.
Fogelman, R.W. & DeProspo, J. (1974) Acute oral toxicity in mice with
1,2-ethanedithiol. Unpublished report submitted to the Flavor and
Extract Manufacturers' Association by Affiliated Medical Research
Institute.
Fogelman, R.W. & Suppers, L. (1973) Acute oral toxicity in mice with
1,8-octanedithiol. Unpublished report submitted to the Flavor and
Extract Manufacturers' Association by Affiliated Medical Research
Institute.
Fogelman, R.W. & Suppers, L. (1974a) Acute oral toxicity in mice with
1,8-octanedithiol. Unpublished report submitted to the Flavor and
Extract Manufacturers' Association by Affiliated Medical Research
Institute.
Fogelman, R.W. & Suppers, L. (1974b) Acute oral toxicity in mice with
1,2-propanedithiol. Unpublished report submitted to the Flavor and
Extract Manufacturers' Association by Affiliated Medical Research
Institute.
Fogelman, R.W., Bukva, N.F. & Margolin, S. (1970) Acute oral toxicity
of 2-4252. Unpublished study submitted by Affiliated Medical
Enterprises, Inc. Princeton, NJ, USA.
Food and Drug Administration (1993) Priority-based Assessment of
Food Additives (PAFA) Database, Waskington DC: Center for Food
Safety and Applied Nutrition, p. 58.
Frandsen, A., Schousboe, A. & Griffiths, R. (1993) Cytotoxic actions
and effects on intracellular Ca2+ and cGMP concentrations of
sulphur-containing excitatory amino acids in cultured cerebral
cortical neurons. J. Neurosci. Res., 34, 331-339.
Gachon, F., Nicolas, C., Maurizis, C., Verny, M., Chabard, J.L.,
Faurie, M. & Gaillard, G. (1988) Disposition and metabolism of
letosteine in rats. Drug Metab. Disposition, 16, 853.
Gallo, M.A., Cox, G.E. & Babish, J.G. (1976) 90-Day feeding studies in
rats with compound 75-31065 (methyl benzyl disulfide). Unpublished
report to the Flavor and Extract Manufacturers' Association by Food
and Drug Research Laboratories, Inc..
George, J.D., Price, C.J., Navarro, H.A., Barr, M.C., Myers, C.B.,
Hunter, E.S., Schwetz, B.A. & Shelby, M.D. (1995) Developmental
toxicity of thiophenol (thio) in rats and rabbits. Toxicologist, 15,
160.
Gibson, T.P., Dobrinska, M.R., Lin, J.H., Entwistle, L.A. & Davies,
R.O. (1987) Biotransformation of sulindac in end-stage renal disease.
Clin. Pharmacol. Ther., 42, 82-88.
Greenzaid, P. & Jencks, W.P. (1971) Pig liver esterase. Reactions with
alcohols, structure-reactivity correlations, and the acyl-enzyme
intermediate. Biochemistry, 10, 1210.
Griffiths, P.J. & Babish, J.G. (1978) Acute oral toxicity (LD50) of
DSE 3303S (15277) in albino mice (BLU: Ha(ICR)). Unpublished report
No. B-3 to the Flavor and Extract Manufacturers' Association from Food
and Drug Research Laboratories, Inc.
Griffiths, P.J., Giessinger, M. & Fouillet, X. (1979) Report on acute
oral toxicity (LD/50) and three-month toxicity of TT 184. Unpublished
report to the Flavor and Extract Manufacturers' Association from
Battelle Geneva Research Centres.
de Groot, A.P., Spanjers, M.T. & van der Heijden, C.A. (1974) Acute
and sub-acute oral toxicity studies in rats with five flavour
compounds. Unpublished report submitted to the Flavor and Extract
Manufacturers' Association.
Grosa, G., Caputo, O., Cerutti, M., Biglino, G., Franzone, J.S.%
Cravanzola, C. (1991) Metabolism of
7-(1,3-dithiolan-2-ylmethyl)1,3-dimethylxanthine by rat liver
microsomes: Diastereoselective metabolism of the 1,3-dithiolane ring.
Drug Metab. Disposition, 19, 454-457.
Hakura, A., Mochida, H. & Yamatsu, K. (1993) Dimethyl sulfoxide (DMSO)
is mutagenic for bacterial mutagenicity tester strains. Mutat. Res.,
303, 127-133.
Harper, K.H. & Gin, H.B. (1964) The acute oral toxicity to rats of
T59. Unpublished report.
Heck, J.D., Vollmuth, T.A., Cifone, M.A., Jagannath, D.R., Myhr, B. &
Curren, R.D. (1989) An evaluation of food flavoring ingredients in a
genetic toxicity screening battery. Toxicologist, 9, 257.
Hermansky, S.J. & Weaver, E.C. (1990) Fourteen-day dietary minimum
toxicity screen (MTS) with acetyl lactic, thiomethyl ester and
propiodyl lactic, thiomethyl ester in Fischer 344 rats. Unpublished
report 53-553 to the Flavor and Extract Manufacturers' Association
from Bushy Run Research Center, Pennsylvania, USA.
Hodgson, E. & Levi, P.A. (1992) The role of the flavin-containing
monooxygenases (EC 1.14.13.8) in the metabolism and mode of action of
agricultural chemicals. Xenobiotica, 22, 1175-1183.
Hong, J.Y., Lin, M.C., Wang, Z.Y., Wang, E.J. & Yang, C.S. (1994)
Inhibition of chemical toxicity and carcinogenesis by diallyl sulfide
and diallyl sulfone. In: Huang, M.-T., Osawa, T., Ho, C.-T. & Rosen,
R.T., eds, Food Phytochemicals for Cancer prevention I, Fruits and
Vegetables (ACS Symposium Series 546), Washington DC: American
Chemical Society, pp. 97-101.
Hoodi, A.A. & Damani, L.A. (1984) Cytochrome P-450 and non-P-450
sulphoxidations. J. Pharm. Pharmacol., 36, 62P.
Hucker, H.B., Miller, J.K., Hochberg, A., Brobyn, R.D., Riordan, F.H.
& Calesnick, B. (1967) Studies on the absorption, excretion, and
metabolism of dimethyl sulfoxide (DMSO) in man. J. Pharmacol. Exp.
Ther., 155, 309-317.
International Organization of the Flavor Industry (1995) European
inquiry on volume of use. Private communication to the Flavor and
Extract Manufacturers' Association.
Karekar, V.R., Mujumdar, A.M., Joshi, S.S., Dhuley, J., Shinde, S.L. &
Ghaskadbi, S. (1996) Assessment of genotoxic effect of piperine using
Salmonella typhimurium and somatic and germ cells of Swiss albino
mice. Arzneimittel-Forsch., 46, 972-975.
Keith, R.A., Abraham, R.T., Pazmino, P. & Weinshilboum, R.M. (1983)
Correlation of low and high affinity thiol methyltransferase and
phenol methyltransferase activities in human erythrocyte membranes.
Clin. Chim. Acta, 131, 257-272.
Klancnik, J.M., Cuenod, M., Gahwiler, B.H., Jiang, Z.P. & Do, K.Q.
(1992) Release of endogenous amino acids, including homocysteic acid
and cysteine sulphinic acid, from rat hippocampal slices evoked by
electrical stimulation of Schaffer collateral-commissural fibres.
Neuroscience, 49, 557-570.
Kligman, A.M. (1965) Dimethyl sulfoxide--Part 2. J. Am. Med. Assoc.,
193, 151-156.
Koptyaev, V.G. (1967) Experimental determination of maximum
permissible concentration of dimethyl sulfide in water bodies. Hyg.
Sanit., 32, 315-320.
Kurooka, S., Hashimoto, M., Tomita, M., Maki, A. & Yoshimura, Y.
(1976) Relationship between the structure of S-acyl thiol compounds
and their rates of hydrolysis by pancreatic lipase and hepatic
carboxylic esterase. J. Biochem., 79, 533-541.
Lavoie, E., Tulley, L., Fow, E. & Hoffmann, D. (1979) Mutagenicity of
aminophenyl and nitrophenyl ethers, sulfides, and disulfides. Mutat.
Res., 67, 123-131.
Layman, D.L. & Jacob, S.W. (1985) The absorption, metabolism, and
excretion of dimethyl sulfoxide by rhesus monkeys. Life Sci., 37,
2431-2437.
LeBon, A.M., Roy, C., Dupont, C. & Suschete, M. (1997) In vivo
antigenotoxic effects of dietary allyl sulfides in the rat. Cancer
Lett., 114, 131-134.
Lee, S.C. & Renwick, A.G. (1995a) Sulphoxide reduction by rat
intestinal flora and by Escherichia coli in vitro. Biochem.
Pharmacol., 49, 1567-1576.
Lee, S.C. & Renwick, A.G. (1995b) Sulphoxide reduction by rat and
rabbit tissues in vitro. Biochem. Pharmacol., 49, 1557-1565.
Levi, P.E. & Hodgson, E. (1988) Stereospecificity in the oxidation of
phorate and phorate sulphoxide by purified FAD-containing
mono-oxygenase and cytochrome P-450 isozymes. Xenobiotica, 18,
29-39.
Maarse, H., Visscher, C.A., Willemsens, L.C., Nijssen, L.M. & Boelens,
M.H., eds (1994) Volatile Components in Food, 6th Ed., Suppl. 5,
Zeist, TNO Nutrition and Food Research.
Maiorino, R.M., Bruce, D.C. & Aposhian, H.V. (1988) Determination and
metabolism of dithiol chelating agents. VI. Isolation and
identification of the mixed disulfides of meso-2,3-dimercaptosuccinic
acid with L-cysteine in human urine. Toxicol. Appl. Pharmacol., 97,
338.
Maiorino, R.M., Xu, Z.F. & Aposhian, H.V. (1996) Determination and
metabolism of dithiol chelating agents. XXII. In humans, sodium
1,3-dimercapto-1-propanesulfonate is bound to plasma albumin via mixed
disulfide formation and is found in the urine as cyclic polymeric
disulfides. J. Pharmacol. Exp. Ther., 277, 375-384.
Marks, H.S., Anderson, J.L. & Stoewsand, G.S. (1992) Inhibition of
benzo[a]pyrene-induced bone marrow micronuclei formation by diallyl
thioethers in mice. J. Toxicol. Environ. Health, 37, 1-9
Mazel, P., Henderson, J.F. & Axelrod, J. (1964) S-Demethylation by
microsomal enzymes. J. Pharmacol. Exp. Ther., 143, 1-6.
McBain, D.A. & Menn, J.J. (1969) S-Methylation, oxidation,
hydroxylation, and conjugation of thiophenol in the rat. Biochem.
Pharmacol., 18, 2282-2285.
Michael, W.R. 1968) Metabolism of linear alkylate sulfonate and alkyl
benzene sulfonate in albino rats. Toxicol. Appl. Pharmacol., 12,
473-485.
Mitchell, S.C., Idle, J.R. & Smith, R.L. (1982) The metabolism of
[14C] cimetidine in man. Xenobiotica, 12, 283.
Mondino, A. (1981) Thirteen week repeated dose study of the test
article TT 191 (3-methyl-1,2,4-trithiane) orally administered to
Sprague Dawley Charles River CD (SD) BR rats. Unpublished study from
Instituto di richierche biomedicine 'Antoine Marxer' SpA.
Mondino, A. & Peano, S. (1979) Acute oral toxicity of
2,6-dimethylthiophenol. Unpublished report to the Flavor and Extract
Manufacturers' Association.
Mondino, A. & Peano, S. (1982) Acute toxicity study, TT 201 and TT202.
Unpublished report from Instituto di richierche biomedicine 'Antoine
Marxer' SpA.
Mondino, A., Peano, S. & Berruto, P. (1981) Acute toxicity study,
TT191. Unpublished report from Instituto di richierche biomedicine
'Antoine Marxer' SpA.
Moran, E.J., Easterday, O.D. & Oser, B.L. (1980) Acute oral toxicity
of selected flavor chemicals. Drug Chem. Toxicol., 3, 249-258.
Moreno, O.M. (1975) Acute toxicity studies on rats, rabbits, and
guinea pigs. Unpublished report to RIFM.
Moreno, O.M. (1980) Acute toxicity studies. Unpublished report to
RIFM.
Morgareidge, K. (1970) 90-Day feeding study in rats with
tetrahydrothiophene-3-one (31015). Unpublished study submitted to the
Flavor and Extract Manufacturers' Association.
Morgareidge K. (1971a) 90-Day feeding study with 2-naphthalenethiol in
rats. Unpublished study submitted to the Flavor and Extract
Manufacturers' Association.
Morgareidge, K. (1971b) 90-Day feeding study with
2-keto-3-pentanethiol in rats. Unpubli-shed study submitted to the
Flavor and Extract Manufacturers' Association.
Morgareidge, K. & Oser, B.L. (1970a) 90-Day feeding studies in rats
with cyclopentane-thiol. Unpublished study submitted to the Flavor and
Extract Manufacturers' Association.
Morgareidge, K. & Oser, B.L. (1970b) 90-Day feeding studies in rats
with dipropyltrisulfide (30204). Unpublished study submitted to the
Flavor and Extract Manufacturers' Association.
Morgareidge, K. & Oser, B.L. (1970c) 90-Day feeding studies in rats
with diallyltrisulfide. Unpublished study submitted by Food and Drug
Research Laboratories, Inc. to the Flavor and Extract Manufacturers'
Association.
Morgareidge, K., Bailey, D.E. & Cox, G.E. (1974a) 90-Day feeding study
on 2,3-butanedithiol in rats. Unpublished study submitted to the
Flavor and Extract Manufac-turers' Association.
Morgareidge, K., Bailey, D.E. & Cox, G.E. (1974b) 90-Day feeding study
in rats with compound 14966. Unpublished study submitted to the Flavor
and Extract Manufac-turers' Association.
Morgareidge, K., Bailey, D.E. & Cox, G.E. (1974c) 90-Day feeding study
of 1,8-octane-dithiol in rats. Unpublished study submitted to the
Flavor and Extract Manufacturers' Association.
Moutiez, M., Aumercier, M., Tessier, E., Parmentier, B., Tartar, A. &
Sergheraert, C. (1994) Reduction of a trisulfide derivative of
glutathione by glutathione reductases. Biochem. Biophys. Res.
Commun., 202, 1380-1386.
Munday, R. & Manns, E. (1994) Comparative toxicity of prop(en)yl
disulfides derived from Alliaceae. Possible involvement of 1-propenyl
disulfides in onion-induced hemolytic anemia. J. Agric. Food Chem.,
42, 959-962.
Munday, R., Manns, E. & Fowke, E.A. (1990) Steric effects on the
haemolytic activity of aromatic disulphides in rats. Food Chem.
Toxicol., 28, 561-566.
National Academy of Sciences (1970) Evaluating the Safety of Food
Chemicals, Washington DC.
National Academy of Sciences (1982) Evaluating the Safety of Food
Chemicals, Washington DC.
National Academy of Sciences (1987) Evaluating the Safety of Food
Chemicals, Washington DC.
Nickson, R.M. & Mitchell, S.C. (1994) Fate of dipropyl sulfide and
dipropyl sulfoxide in rat. Xenobiotica, 24, 157-168.
Nickson, R.M., Mitchell, S.C. & Zhang, A.Q. (1995) Fate of dipropyl
sulfone in rat. Xenobiotica, 25, 1391-1398.
Nnane, P. & Damani, L.A. (1995) The involvement of rat liver CYP2B1
and CYP2D1 in the microsomal sulphoxidation of 4-chlorophenyl methyl
sulphide. Int. Soc. Study Xenobiotics, 8, 110.
Noel, P.R.B., Barnett, K.C., Davies, R.E., Jolly, D.W., Leahy, J.S.,
Mawdesley-Thomas, L.E., Shillam, K.W.G., Squires, P.F., Street, A.E.,
Tucker, W.C. & Worden, A.N. (1975) The toxicity of dimethyl sulphoxide
(DMSO) for the dog, pig, rat, and rabbit. Toxicologist, 3, 143-169.
Oae, S., Mikami, A., Matsura, T., Ogawa-Asada, K., Watanabe, Y.,
Fujimori, K. & Iyanagi, T. (1985) Comparison of sulfite oxygenation
mechanism for liver microsomal FAD-containing monooxygenase with that
for cytochrome P-450. Biochem Biophys. Res. Commun., 131, 567-573.
Ogasawara, Y., Isoda, S. & Tanabe, S. (1998) A labile sulfur in
trisulfide affects cytochrome P-450 dependent lipid peroxidation in
rat liver microsomes. Toxicol. Lett., 99, 191-198.
Opdyke, D.L.J. (1979) Monographs on fragrance raw materials. Food
Cosmet. Toxicol., 17, 769.
Oser, B.L. (1966) Feeding studies with pinanyl mercaptan (PK) in rats.
Unpublished study submitted to the Flavor and Extract Manufacturers'
Association.
Oser, B.L. (1970) The acute oral toxicity to mice of ten compounds.
Unpublished study submitted to the Flavor and Extract Manufacturers'
Association.
Panasevich, R.E., Mallory, V.T. & Matthews, R.J. (1980) Dose-related
finding and single dose oral study of ethyl 4-(methylthio)butyrate.
Unpublished study submitted to the Flavor and Extract Manufacturers'
Association.
Parkinson, A. (1996) Biotransformation of xenobiotics. In: Klaassen,
C.D., ed., Casarret and Doull's Toxicology: The Basic Science of
Poisons, 5th Ed., New York, McGraw-Hill Co., pp. 113-118.
Peano, S., Roffino, A., Milone, M.F., Orlando, L. & Berruto, G. (1981)
Thirteen week repeated dose study with 2,6-dimethylthiophenol orally
administered to Sprague-Dawley Charles River CD (SD) BR rats.
Unpublished study submitted to the Flavor and Extract Manufacturers'
Association by Instituto di richierche biomedicine 'Antoine Marxer'
SpA.
Phillips Petroleum Co. (1990a) Toxicity study
summary--1,2-Ethanedithiol (TOX071).
Phillips Petroleum Co. (1990b) Toxicity study summary--Isopropyl
mercaptan (TOX083).
Phillips Petroleum Co. (1990c) Toxicity study summary--Tertiary butyl
mercaptan (TOX1-6).
Piccirillo, V.J. & Lunchicki, C. (1982) Acute oral toxicity (LD50)
study in the rat with methyl-2-methylthiobutyrate. Unpublished report
submitted to the Flavor and Extract Manufacturers' Association.
Posternak, J.M., Linder, A. & Vodoz, C.A. (1969) Summaries of
toxicological data. Toxicological tests on flavoring matters. Food
Cosmet. Toxicol., 7, 405-407.
Reddy, B.S., Rao, C.V., Rivenson, A. & Kelloff, G. (1993)
Chemoprevention of colon carcinogenesis by organosulfur compounds.
Cancer Res., 53, 3493-3498.
Renwick, A.G. (1989) Sulphoxides and sulphones. In: Damani, L.A., ed.,
Sulphur-containing Drugs and Related Organic Compounds. Chemistry,
Biochemistry and Toxicology (Ellis Horwood Series in Biochemical
Pharmacology), Vol. 1, Part B, New York: John Wiley & Sons, pp.
133-154.
Renwick, A.G. (1996) Sulfur-oxygen compounds. In: Mitchell, S., ed.,
Biological Interactions of Sulfur Compounds, London: Taylor &
Francis, pp. 42-76.
Renwick, A.G. & George, C.F. (1989) Metabolism of xenobiotics in the
gastro-intestinal tract. In: Hudson, D.H., Caldwell, J. & Paulson,
G.D., eds, Intermediary Xenobiotic Metabolism in Animals:
Methodology, Mechanisms and Significance, London, Taylor & Francis,
pp. 13-40.
Renwick, A.G., Evans, S.P., Sweatman, T.W., Cumberland, J. & George,
C.F. (1982) The role of the gut flora in the reduction of
sulphinpyrazone in the rat. Biochem. Pharmacol., 31, 2649.
Rettie, A.E., Bogucki, B.D., Lim, I. & Meier, G.P. (1990)
Stereoselective sulfoxidation of a series of alkyl p-tolyl sulfides by
microsomal and purified flavin-containing monooxygenases.
Mol. Pharmacol., 37, 643.
Rettie, A.E., Lawton, M.P., Jaffer, A., Sadeque, M. & Meier, G.P.
(1994) Prochiral sulfoxidation as a probe for multiple forms of the
microsomal flavin-containing monooxygenase: Studies with rabbit FMO1,
FMO2, FMO3, and FMO5 expressed in Escherichia coli. Arch. Biochem.
Biophys., 311, 369.
Richardson, K.A., Edward, V.T., Jones, B.C. & Hutson, D.H. (1991)
Metabolism in the rat of a model xenobiotic plant metabolite
S-benzyl-N-malonyl-L-cysteine. Xenobiotica, 21, 371.
Rush, R.E. (1991) 14-Day dietary toxicity study in rats with
3-(methylthio)hexyl acetate (MTHA). Unpublished report No. 3141.5 from
Springborn Laboratories, Inc. to the Flavor and Extract Manufacturers'
Association.
Sadeque, A.J.M., Eddy, A.C., Meierand, G.P. & Rettie, A.E. (1992)
Stereoselective sulfoxidation by human flavin-containing
monooxygenase. Drug Metab. Disposition, 20, 832.
Sadeque, A.J.M., Philpot, R.M. & Rettie, A.E. (1995) Chiral
sulfoxidation by human liver FMO3 and FMO5. Int. Soc. Study
Xenobiotics, 8.
Sanders, A. (1997) Acute oral toxicity (limit test) in the rat.
Unpublished SPL project No. 012/234 submitted to the Flavor and
Extract Manufacturers' Association.
Schafer, E.W. & Bowles, W.A., Jr (1985) Acute oral toxicity and
repellency of 933 chemicals to house and deer mice. Arch. Environ.
Contam. Toxicol., 14, 111-129.
Selyuzhitskii, G.V. (1972) Experimental data on substantiation of the
maximum allowable concentraion of methyl mercaptan, dimethyl sulfide
and dimethyl disulfide in the air of the working area of pulp paper
plants. Gig. Tr. Prof. Zabol., 16, 46-47.
Shaw, P.N. & Blagbrough, I.S. (1989) Cysteine conjugate beta-lyase,
II: Isolation, properties and structure-activity relationships. In:
Damani, L.A., ed., Sulphur-containing Drugs and Related Organic
Compounds, Vol. 2, Part B, Chichester: Ellis Horwood Ltd, pp.
135-156.
Shellenberger, T.E. (1970) Subacute toxicity evaluation of ethyl
thioacetate in rats. Final report. Unpublished report GSRI Project
NC-373 from Gulf South Research Institute submitted to the Flavor and
Extract Manufacturers' Association.
Shellenberger, T.E. (1971a) Acute toxicological evaluations of
2-naphthalenethiol in mice. Unpublished report GSRI Project NC-398
from Gulf South Research Institute submitted to the Flavor and Extract
Manufacturers' Association.
Shellenberger, T.E. (1971b) Acute toxicological evaluations of
chemicals with mice--2-Keto-3-pentanethiol. Unpublished study from
Gulf South Research Institute.
Snow, G.A. (1957) The metabolism of compounds related to ethanethiol.
J. Biochem., 65, 77-82.
Sommer, S. & Tauberger, G. (1964) Toxicologic investigations of
dimethyl sulfoxide. Arnzeimittel. Forsch., 14, 1050-1053.
Souhaili-El Amri, H., Fargetton, X., Delatour, P. & Batt, A.M. (1987)
Sulphoxidation of albendazole by the FAD-containing and cytochrome
P-450 dependent mono-oxygenase from pig liver microsomes.
Xenobiotica, 17, 1159-1168.
Stofberg, J. & Grundschober, F. (1987) Consumption ratio and food
predominance of flavoring materials. Perfumer Flavorist, 12, 27.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of
flavouring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23, 857-860.
Strong, H.A., Renwick, A.G. & George, C.F. (1984a) The site of
reduction of sulphinpyrazone in the rabbit. Xenobiotica, 14,
815-826.
Strong, H.A., Oates, J., Sembi, J., Renwick, A.G. & George, C.F.
(1984b) Role of the gut flora in the reduction of sulphinpyrazone in
humans. J. Pharmacol. Exp. Ther., 230, 726-732.
Strong, H.A., Warner, J., Renwick, A.G. & George, C.F. (1985) Sulindac
metabolism: The importance of an intact colon. Clin. Pharmacol.
Ther., 38, 387-393.
Strong, H.A., Angus, R., Oates, J., Sembi, J., Howarth, P., Renwick,
A.G. & George, C.F. (1986) Effects of ischaemic heart disease, Crohn's
disease and anti-microbial therapy on the pharmacokinetics of
sulphinpyrazone. Clin. Pharmacokinet., 11, 402-411.
Strong, H.A., Renwick, A.G., George, C.F., Liu, Y.F. & Hill, M.J.
(1987) The reduction of sulphinpyrazone and sulindac by intestinal
bacteria. Xenobiotica, 17, 685-696.
Sundaram, S.G. & Milner, J.A. (1996) Diallyl disulfide induces
apoptosis of human colon tumor cells. Carcinogenesis, 17, 669-673.
Szumlanski, C.L., Scott, M.C. & Weinshilboum, R.M. (1988) Thiopurine
methyltransferase pharmacogenetics: Human liver enzyme activity.
Clin. Pharmacol. Ther., 43, 134.
Szybalski, W. (1958) Special microbiological systems. II. Observations
on chemical mutagenesis in microorganisms. Ann. N.Y. Acad. Sci., 76,
475-489.
Takata, T., Yamazaki, M., Fujimori, K., Kim, H.Y., Iyanagi, T. & Oae,
S. (1983) Enzymatic oxygenation of sulfides with cytochrome P-450 from
rabbit liver: Stereochemistry of sulfoxide formation. Bull. Chem.
Soc. Jpn., 55, 2300-2310.
Tateishi, M. & Tomisawa, H. (1989) Cysteine conjugate beta-lyase. I:
Toxic thiol production. In: Damani, L.A., ed., Sulphur-containing
Drugs and Related Organic Compounds, Vol. 2, Part B, Chichester:
Ellis Horwood Ltd, pp. 121-134.
Tateishi, M., Suzuki, S. & Shimizu, H. (1978) Cysteine conjugate
beta-lyase in rat liver. J. Biol. Chem., 253, 8854.
Tatsumi, K., Kitamura, S. & Yamada, H. (1982) Involvement of liver
aldehyde oxidase in sulfoxide reduction. Chem. Pharm. Bull., 30,
4585-4588.
Tatsumi, K., Kitamura, S. & Yamada, H. (1983) Sulfoxide reductase
activity of liver aldehyde oxidase. Biochim. Biophys. Acta, 747,
86-92.
Taylor, K.L. & Ziegler, D.M. (1987) Studies on substrate specificity
of the hog liver flavin-containing monooxygenase. Anionic organic
sulfur compounds. Biochem. Pharmacol., 36, 141-146.
Taylor, A.J., Olavesen, A.H., Black, J.G. & Howes, D. (1978) The
metabolism of the surfactants dodecylsulfonate and hexadecylsulfonate
in the rat. Toxicol. Appl. Pharmacol., 45, 105-117.
Thomas, W.C., Seckar, J.A., Johnson, J.T., Ulrich, C.E, Klonne, D.R.,
Schardein, J.L. & Kirwin, C.J. (1987) Inhalation teratology studies of
n-butyl mercaptan in rats and mice. Fundam. Appl. Toxicol., 8,
170-178.
Vogin, E.E., Carson, S., Cannon, G., Linegar, C.R. & Rubin, L.F.
(1970) Chronic toxicity of DMSO in primates. Toxicol. Appl.
Pharmacol., 16, 606-612.
Wang, C.J., Lin, Y.L. & Lin, J.K. (1994) Mutagenicity and cytotoxicity
of nitropyrrole compounds derived from the reaction of 2-acetyl
pyrrole with nitrate. Food Chem. Toxicol., 32, 839-844.
Waring, R.H. (1996) Sulfur-sulfur compounds. In: Mitchell, S., ed.,
Biological Interactions of Sulfur Compounds, London: Taylor &
Francis, pp. 145-173.
Watanabe, S. & Kinosaki, A. (1989a) Acute oral toxicity in the rat
with acetyllactic acid thiomethyl ester. Unpublished report submitted
to the Flavor and Extract Manufacturers' Association.
Watanabe, S. & Kinosaki, A. (1989b) Acute oral toxicity in the rat
with propionyl lactic acid thiomethyl ester. Unpublished report
submitted to the Flavor and Extract Manufacturers' Association.
Watanabe, S. & Morimoto, Y. (1989a) Mutagenicity study of acetyl
lactic acid thiomethyl ester in Salmonella typhimurium and
Escherichia coli. Unpublished study submitted to the Flavor and
Extract Manufacturers' Association.
Watanabe, S. & Morimoto, Y. (1989b) Mutagenicity study of propionyl
lactic acid thiomethyl ester in Salmonella typhimurium and
Escherichia coli. Unpublished study submitted to the Flavor and
Extract Manufacturers' Association.
Weinshilboum, R.M. & Sladek, S.L. (1980) Mercaptopurine
pharmacogenetics: Monogenic inheritance of erythrocyte thiopurine
methyltransferase activity. Am. J. Hum. Genet., 32, 651-662.
Wells, W.W., Yang, Y., Diets, T.L. & Gan, Z.R. (1993)
Thioltransferases. Adv. Enzymol. Relat. Areas Mol. Biol., 66,
149-201.
Wheldon, G.H., Amyes, S.J., Street, A.E., Hague, P.H. &
Mawdesley-Thomas, L.E. (1970) Toxicity of Wa 4295, Sa 927, Stl 3048,
and Wa 3328 in dietary administration to rats over a period of 13
weeks. Unpublished report from Huntington Research Centre to the
Flavor and Extract Manufacturers' Association.
Wild, D., King, M.T., Gocke, E. & Eckhardt, K. (1983) Study of
artificial flavouring substances for mutagenicity in the
Salmonella/microsome, basc and micronucleus tests. Food Chem.
Toxicol., 21, 707-719.
Williams, K.I.H., Burstein, S.H. & Layne, D.S. (1966) Metabolism of
dimethyl sulfide, dimethyl sulfoxide, and dimethyl sulfone in the
rabbit. Arch. Biochem. Biophys., 117, 84-87.
Willson, J.E., Brown, D.E. & Timmens, E.K. (1965) A toxicologic study
of dimethyl sulfoxide. Toxicol. Appl. Pharmacol., 7, 104-122.
Wilson, K., Chissick, H., Fowler, A.M., Frearson, F.J., Gittins, M. &
Swinbourne, F.J. (1991) Metabolism of benzothiazole I. Identification
of ring-cleavage products. Xenobiotica, 21, 1179.
Wnorowski, G. (1996a) 14-Day dietary toxicity study: Rats. Unpublished
study No. 4442 from Product Safety Laboratories to the Flavor and
Extract Manufacturers' Association.
Wnorowski, G. (1996b) 14-Day dietary toxicity study: Rats. Unpublished
study No. 4453 from Product Safety Laboratories to the Flavor and
Extract Manufacturers' Association.
Wnorowski, G. (1996c) 14-Day dietary toxicity study: Rats. Unpublished
study No. 4456 from Product Safety Laboratories to the Flavor and
Extract Manufacturers' Association.
Wnorowski, G. (1996d) 14-Day dietary toxicity study: Rats. Unpublished
study No. 4454 from Product Safety Laboratories to the Flavor and
Extract Manufacturers' Association.
Wnorowski, G. (1996e) 14-Day dietary toxicity study: Rats. Unpublished
study No. 4457 from Product Safety Laboratories to the Flavor and
Extract Manufacturers' Association.
Wnorowski, G. (1997a) 14-Day repeat dose oral toxicity study: Rats.
Unpublished study No. 5211 from Product Safety Laboratories to the
Flavor and Extract Manufacturers' Association.
Wnorowski, G. (1997b) 14-Day dietary toxicity study: Rats. Unpublished
study No. 5210 from Product Safety Laboratories to the Flavor and
Extract Manufacturers' Association.
Wood, D.C., Wirth, N.V., Weber, F.S. & Palmquist, M.A. (1971)
Mechanism considerations of dimethyl sulfoxide (DMSO)-lenticular
changes in rabbits. J. Pharmacol. Exp. Ther., 177, 528-535.
Woodson, L.C. & Weinshilboum, R.M. (1983) Human kidney thiopurine
methyltransferase: Purification and biochemical properties. Biochem.
Pharmacol., 32, 819-826.
Woodson, L.C., Dunnette, J.H. & Weinshilboum, R.M. (1982)
Pharmacogenetics of human thiopurine methyltransferase:
Kidney-erythrocyte correlation and immunotitra-tion studies.
J. Pharmacol. Exp. Ther., 222, 174-181.
Woodson, L.C., Ames, M.M, Selassie, C.D., Hansch, C. & Weinshilboum,
R.M. (1983) Thiopurine methyltransferase: Aromatic thiol substrates
and inhibition by benzoic acid derivatives. Mol. Pharmacol., 24,
471-478.
Yoshihara, S. & Tatsumi, K. (1985a) Sulfoxide reduction catalysed by
guinea pig liver aldehyde oxidase in combination with one electron
reducing flavoenzymes. J. Pharmacobio-Dyn., 8, 996-1005.
Yoshihara, S. & Tatsumi, K. (1985b) Guinea pig liver aldehyde oxidase
as a sulfoxide reductase: Its purification and characterisation.
Arch. Biochem. Biophys., 242, 213-224.
Yoshihara, S. & Tatsumi, K. (1990) Metabolism of diphenyl sulphoxide
in perfused guinea pig liver. Drug Metab. Disposition, 18, 876.
Zajac-Kaye, M. & Ts'o, P.O.P. (1984) DNAase I encapsulation in
liposomes can induce neoplastic transformation of Syrian hamster
embryo cells in culture. Cell, 39, 427-437.
Zeiger, E., Anderson, B., Haworth, S., Lawlor, T., Mortelmans, K. &
Speck, W. (1987) Salmonella mutagenicity test: III. Results from the
testing of 255 chemicals. Environ. Mutag., 9 (Suppl. 9), 1-110.
Zeiger, E., Anderson, B., Haworth, S., Lawlor, T. & Mortelmans, K.
(1992) Salmonella mutagenicity tests. V. Results from the testing of
311 chemicals. Environ. Mol. Mutag., 19 (Suppl.21), 2-141.
Ziegler, D.M. (1980) Microsomal flavin-coenzyme monooxygenase:
Oxygenation of nucleophilic nitrogen and sulfur compounds. In: Jakoby,
W.B., ed., Enzymatic Basis of Detoxification, Vol. 2, Chichester:
Ellis Horwood, pp. 201-227.
Ziegler, D.M. (1989) S-Oxygenases. I. Chemistry and biochemistry. In:
Damani, L.A., ed., Sulphur-containing Drugs and Related Organic
Compounds, Vol. 2, Part A, Chichester: Ellis Horwood, pp. 53-66.
