
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
WORLD HEALTH ORGANIZATION
SAFETY EVALUATION OF CERTAIN FOOD
ADDITIVES AND CONTAMINANTS
WHO FOOD ADDITIVES SERIES: 44
Prepared by the Fifty-third meeting of the Joint FAO/WHO
Expert Committee on Food Additives (JECFA)
World Health Organization, Geneva, 2000
IPCS - International Programme on Chemical Safety
ALIPHATIC PRIMARY ALCOHOLS, ALDEHYDES, CARBOXYLIC ACIDS,
ACETALS, AND ESTERS CONTAINING ADDITIONAL OXYGENATED FUNCTIONAL
GROUPS
First draft prepared by Dr P.J. Abbott
Australia New Zealand Food Authority, Canberra, Australia
Evaluation
Introduction
Estimated daily per capita intake
Metabolism
Application of the Procedure for the Safety Evaluation of
Flavouring Agents
Consideration of combined intakes
Conclusions
Relevant background information
Explanation
Additional considerations on intake
Biological data
Absorption, distribution, metabolism, and excretion
Esters and diesters
alpha-Keto-and alpha-hydroxy acids and their esters
Acetals
beta-Keto and beta-hydroxy acids and their esters
gamma-Keto or gamma-hydroxy acids and their esters
omega-Substituted derivatives
Aliphatic di-and tricarboxylic acids and their esters
Toxicological studies
Acute toxicity
Short-term and long-term studies of toxicity
Genotoxicity
Other relevant studies
References
1. EVALUATION
1.1 Introduction
The Committee evaluated a group of 47 flavouring agents that
includes aliphatic primary alcohols, aldehydes, carboxylic acids,
acetals, and esters containing additional oxygenated functional groups
(see Table 1) using the Procedure for the Safety Evaluation of
Flavouring Agents (Figure 1, p. 122).
The Committee previously evaluated eight members of this group
for other functional uses. Fumaric acid (No. 618) was first considered
by the Committee at its tenth meeting (Annex 1, reference 13), and at
its thirty-fifth meeting (Annex 1, reference 88) the Committee
established a group ADI of 'not specified'1 for fumaric acid and its
salts. Triethyl citrate (No. 629) was first considered by the
Committee at its twenty-third meeting (Annex 1, reference 50), and at
its twenty-eighth meeting (Annex 1, reference 66) the Committee
established an ADI of 0-20 mg/kg bw. Diethyl tartrate (No. 622) was
first considered by the Committee at its twenty-third meeting (Annex
1, reference 50), but an evaluation was not possible on the basis of
the data available at that time. As no additional data were available
to the Committee at its twenty-fifth meeting (Annex 1, reference 56),
no ADI was allocated. The Committee also evaluated related terpenoid
flavouring agents, including linalool, linalyl acetate, citronellol,
citral, and geranyl acetate, and established a group ADI of 0-0.5
mg/kg bw at its twenty-third meeting (Annex 1, reference 50).
1.2 Estimated daily per capita intake
The estimated per capita intake of these agents, modified to
calculate intake of flavouring agents (see p. 121), was derived from
surveys in Europe and the United States. The total annual production
of the 47 substances in this group is 200 tonnes in Europe and 1700
tonnes in the United States, which is equivalent to a total estimated
daily per capita intake of 28 mg in Europe and 300 mg in the United
States.
Fumaric acid (No. 618) and (-)-malic acid (No. 619) account for
approximately 59% of the total daily per capita intake of these 47
substances in Europe and 88% in the United States. The estimated total
daily consumption of fumaric acid resulting from its use as a
flavouring agent is approximately 0.9 mg/person in Europe and 219
mg/person in the United States. The total daily consumption of
(-)-malic acid is estimated to be 16 mg/person in Europe and 58
mg/person in the United States.
Of the 47 substances evaluated, 25 have been detected as natural
components of traditional foods (Maarse et al., 1994).
1.3 Metabolism
Studies on the absorption, metabolism, and elimination of
aliphatic primary alcohols, aldehydes, carboxylic acids, acetals, and
esters with additional oxygenated functional groups show that these
substances are readily hydroly-sed and absorbed and are completely
metabolized. Many of these substances or their metabolites are
endogenous in humans.
1 ADI 'not specified' is a term applicable to a food component of very
low toxicity which, on the basis of the available chemical,
biological, toxicological, and other data, the total dietary intake of
the substance arising from its use at the levels necessary to achieve
the desired effect and from its acceptable background in food, does
not, in the opinion of the Committee, represent a hazard to health.
For this reason and for those stated in the evaluation, the
establishment of an ADI expressed in numerical form is deemed
unnecessary.
Many of the substances in this group are esters or diesters and
are expected to undergo hydrolysis to their corresponding alcohol
(saturated linear or branched-chain aliphatic primary alcohols or
branched-chain hydroxy or keto alcohols). The presence of a second
oxygenated functional group has little if any effect on the hydrolysis
of these esters. ß-Keto acids and derivatives such as acetoacetic acid
easily undergo decarboxylation and, with alpha-keto and
alpha-hydroxyacids, yield breakdown products which are incorporated
into normal biochemical pathways. The gamma-keto acids and related
substances may undergo complete or partial ß-oxidation to yield
metabolites, which are eliminated in the urine. The omega-substituted
derivatives are readily oxidized and/or excreted in the urine. The
simple aliphatic di-and tricarboxylic acids either occur endogenously
in humans or are structurally related to endogenous substances. These
substances are metabolized through the fatty acid ß-oxidation pathway
or the tricarboxylic acid cycle.
1.4 Application of the Procedure for the Safety Evaluation of
Flavouring Agents
Step 1. In applying the Procedure for the Safety Evaluation of
Flavouring Agents (Figure 1, p. 122) to the above-mentioned
aliphatic primary alcohols, aldehydes, carboxylic acids,
acetals, and esters containing additional oxygenated
functional groups, the Committee assigned all 47 substances
to structural class I (Cramer et al., 1978).
Step 2. Metabolic data on individual members of the group are
limited, but the common structural features and common
pathways of metabolism allow some general conclusions to be
drawn on the likely metabolic fate of these agents. Fourteen
substances are found normally in human metabolism, and 28
substances in the group are esters or diesters that would be
expected to be metabolized to innocuous products. There was
evidence that the other substances in the group, including
acetals, derivatives of beta-keto and beta-hydroxy acids,
gamma-keto and gamma-hydroxy acids, and aliphatic di-and
tricarboxylic acids, are also metabolized to innocuous
products. For all substances in this group, therefore, the
evaluation should proceed via the left-hand side of the
decision-tree.
Table 1. Summary of results of the safety evaluation of 47 aliphatic primary alcohols, aldehydes,
carboxylic acids, acetals, and esters containing additional oxygenated functional groups
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
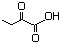
2-Oxobutyric acid 589 600-18-0 No N/R N/R No safety concern
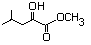
 Methyl 2-hydroxy-4-methylpentanoate 590 40348-72-9 No N/R N/R No safety concern
Methyl 2-hydroxy-4-methylpentanoate 590 40348-72-9 No N/R N/R No safety concern
 Methyl 2-oxo-3-methyl-pentanoate 591 3682-42-6 No N/R N/R No safety concern
Methyl 2-oxo-3-methyl-pentanoate 591 3682-42-6 No N/R N/R No safety concern
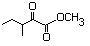 Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
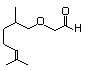
Citronelloxyacetaldehyde 592 7492-67-3 No N/R N/R No safety concern
Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
Citronelloxyacetaldehyde 592 7492-67-3 No N/R N/R No safety concern
 3-Oxobutanal dimethyl acetal 593 5436-21-5 No N/R N/R No safety concern
3-Oxobutanal dimethyl acetal 593 5436-21-5 No N/R N/R No safety concern
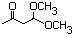 Ethyl 3-hydroxybutyrate 594 5405-41-4 No N/R N/R No safety concern
Ethyl 3-hydroxybutyrate 594 5405-41-4 No N/R N/R No safety concern
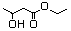 Ethyl acetoacetate 595 141-97-9 Yes Yesb N/R No safety concern
Ethyl acetoacetate 595 141-97-9 Yes Yesb N/R No safety concern
 Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
Butyl acetoacetate 596 591-60-6 No N/R N/R No safety concern
Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
Butyl acetoacetate 596 591-60-6 No N/R N/R No safety concern
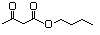 Isobutyl acetoacetate 597 7779-75-1 No N/R N/R No safety concern
Isobutyl acetoacetate 597 7779-75-1 No N/R N/R No safety concern
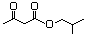 Isoamyl acetoacetate 598 2308-18-1 No N/R N/R No safety concern
Isoamyl acetoacetate 598 2308-18-1 No N/R N/R No safety concern
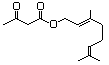
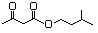 Geranyl acetoacetate 599 10032-00-5 No N/R N/R No safety concern
Geranyl acetoacetate 599 10032-00-5 No N/R N/R No safety concern
 Methyl 3-hydroxyhexanoate 600 21188-58-9 No N/R N/R No safety concern
Methyl 3-hydroxyhexanoate 600 21188-58-9 No N/R N/R No safety concern
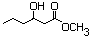 Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
Ethyl 3-hydroxyhexanoate 601 2305-25-1 No N/R N/R No safety concern
Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
Ethyl 3-hydroxyhexanoate 601 2305-25-1 No N/R N/R No safety concern
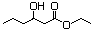 Ethyl 3-oxohexanoate 602 3249-68-1 No N/R N/R No safety concern
Ethyl 3-oxohexanoate 602 3249-68-1 No N/R N/R No safety concern
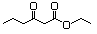 Ethyl 2,4-dioxohexanoate 603 13246-52-1 No N/R N/R No safety concern
Ethyl 2,4-dioxohexanoate 603 13246-52-1 No N/R N/R No safety concern
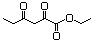 3-(Hydroxymethyl)-2-heptanone 604 65405-68-7 No NR N/R No safety concern
3-(Hydroxymethyl)-2-heptanone 604 65405-68-7 No NR N/R No safety concern
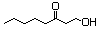 Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
1,3-Nonanediol acetate 605 1322-17-4 No N/R N/R No safety concern
(mixed esters)
Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
1,3-Nonanediol acetate 605 1322-17-4 No N/R N/R No safety concern
(mixed esters)
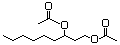 Laevulinic acid 606 123-76-2 No N/R N/R No safety concern
Laevulinic acid 606 123-76-2 No N/R N/R No safety concern
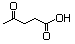 Ethyl laevulinate 607 539-88-8 No N/R N/R No safety concern
Ethyl laevulinate 607 539-88-8 No N/R N/R No safety concern
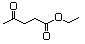 Butyl laevulinate 608 2052-15-5 No N/R N/R No safety concern
Butyl laevulinate 608 2052-15-5 No N/R N/R No safety concern
 Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
1,4-Nonanediol diacetate 609 67715-81-5 No N/R N/R No safety concern
Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
1,4-Nonanediol diacetate 609 67715-81-5 No N/R N/R No safety concern
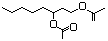 Hydroxycitronellol 610 107-74-4 No N/R N/R No safety concern
Hydroxycitronellol 610 107-74-4 No N/R N/R No safety concern
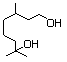 Hydroxycitronellal 611 107-75-5 No N/R N/R No safety concern
Hydroxycitronellal 611 107-75-5 No N/R N/R No safety concern
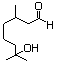 Hydroxycitronellal dimethyl 612 141-92-4 No N/R N/R No safety concern
acetal
Hydroxycitronellal dimethyl 612 141-92-4 No N/R N/R No safety concern
acetal
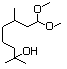 Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
Hydroxycitronellal diethyl 613 7779-94-4 No N/R N/R No safety concern
acetal
Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
Hydroxycitronellal diethyl 613 7779-94-4 No N/R N/R No safety concern
acetal
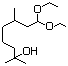 Diethyl malonate 614 105-53-3 No N/R N/R No safety concern
Diethyl malonate 614 105-53-3 No N/R N/R No safety concern
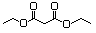 Butyl ethyl malonate 615 17373-84-1 No N/R N/R No safety concern
Butyl ethyl malonate 615 17373-84-1 No N/R N/R No safety concern
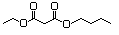 Dimethyl succinate 616 106-65-0 No N/R N/R No safety concern
Dimethyl succinate 616 106-65-0 No N/R N/R No safety concern
 Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
Diethyl succinate 617 123-25-1 No N/R N/R No safety concern
Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
Diethyl succinate 617 123-25-1 No N/R N/R No safety concern
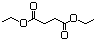 Fumaric acidc 618 110-17-8 Yes Yesd N/R No safety concern
Fumaric acidc 618 110-17-8 Yes Yesd N/R No safety concern
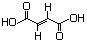 (-)-Malic acid 619 97-67-6 Yes Yesd N/R No safety concern
(-)-Malic acid 619 97-67-6 Yes Yesd N/R No safety concern
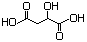 Diethyl malate 620 7554-12-3 No N/R N/R No safety concern
Diethyl malate 620 7554-12-3 No N/R N/R No safety concern
 Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
Tartaric acid (+-, --, ±-, meso-) 621 87-69-4 Yes No Yes. NOEL No safety concern
was 1200
mg/kg bw
per day in
a two-year
study in rats
Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
Tartaric acid (+-, --, ±-, meso-) 621 87-69-4 Yes No Yes. NOEL No safety concern
was 1200
mg/kg bw
per day in
a two-year
study in rats
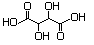 Diethyl tartrate 622 87-91-2 No N/R N/R No safety concern
Diethyl tartrate 622 87-91-2 No N/R N/R No safety concern
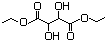 Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
Adipic acid 623 124-04-9 Yes No Yes. The NOEL
for the
structurally
related
compound,
dibutyl
sebacate, was
6200 mg/kg bw
per day in a
two-year
study in rats
Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
Adipic acid 623 124-04-9 Yes No Yes. The NOEL
for the
structurally
related
compound,
dibutyl
sebacate, was
6200 mg/kg bw
per day in a
two-year
study in rats
 Diethyl sebacate 624 110-40-7 No N/R N/R No safety concern
Diethyl sebacate 624 110-40-7 No N/R N/R No safety concern
 Dibutyl sebacate 625 109-43-3 No N/R N/R No safety concern
Dibutyl sebacate 625 109-43-3 No N/R N/R No safety concern
 Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
Ethylene brassylate 626 105-95-3 No N/R N/R No safety concern
Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
Ethylene brassylate 626 105-95-3 No N/R N/R No safety concern
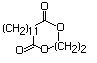 Aconitic acid 627 499-12-7 No N/R N/R No safety concern
Aconitic acid 627 499-12-7 No N/R N/R No safety concern
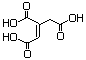 Ethyl aconitate (mixed esters) 628 - No N/R N/R No safety concern
Ethyl aconitate (mixed esters) 628 - No N/R N/R No safety concern
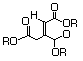 Triethyl citratec 629 77-93-0 Yes Yesd N/R No safety concern
Triethyl citratec 629 77-93-0 Yes Yesd N/R No safety concern
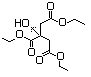 Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
Tributyl acetylcitrate 630 77-90-7 No N/R N/R No safety concern
Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
Tributyl acetylcitrate 630 77-90-7 No N/R N/R No safety concern
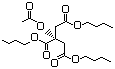 3-Methyl-2-oxobutanoic acid 631 759-05-7 No N/R N/R No safety concern
and sodium salt 3715-29-6
3-Methyl-2-oxobutanoic acid 631 759-05-7 No N/R N/R No safety concern
and sodium salt 3715-29-6
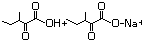
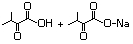 3-Methyl-2-oxopentanoic acid 632 1460-34-0 No N/R N/R No safety concern
and sodium salt 3715-31-9
3-Methyl-2-oxopentanoic acid 632 1460-34-0 No N/R N/R No safety concern
and sodium salt 3715-31-9
 4-Methyl-2-oxopentanoic acid 633 816-66-0 No N/R N/R No safety concern
and sodium salt 4502-00-5
4-Methyl-2-oxopentanoic acid 633 816-66-0 No N/R N/R No safety concern
and sodium salt 4502-00-5
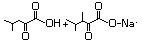 Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
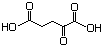
2-Oxopentandioic acid 634 328-50-7 No N/R N/R No safety concern
Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
2-Oxopentandioic acid 634 328-50-7 No N/R N/R No safety concern
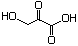
 3-Hydroxy-2-oxopropionic acid 635 1113-60-6 No N/R N/R No safety concern
3-Hydroxy-2-oxopropionic acid 635 1113-60-6 No N/R N/R No safety concern
 All of the substances in the group are in structural class I, the human intake threshold of which is 1800 µg per person
per day, and all of the substances in the group are metabolized to innocuous products.
a The threshold for human inatke of substances in class I is 1800 µg per day.
b Ethyl acetoacetate is expected to be hydrolysed to acetoacetic acid, which is endogenous in humans.
c The ADI for this substance was maintained.
d Fumaric acid, (-)-malic acid, and triethyl citrate are components of the tricarboxylic acid cycle.
Step A3. The estimated daily per capita intakes in Europe and the
United States of 41 of the substances in this group are
below the threshold of concern for substances in class I
(1800 µg), indicating that they would not raise concern for
safety. The intakes of six substances, namely, ethyl
acetoacetate (No. 595; 1900 µg/person per day in Europe and
3900 µg/person per day in the United States), fumaric acid
(No. 618; 220 000 µg/person per day in the United States);
(-)-malic acid (No. 619; 16 000 µg/person per day in Europe
and 58 000 µg/person per day in the United States), tartaric
acid (No. 621; 4400 µg/person per day in Europe and 14 000
µg/person per day in the United States), adipic acid (No.
623; 18 000 µg/person per day in the United States), and
triethyl citrate (No. 629; 3400 µg/person per day in Europe
and 2400 µg/person per day in the United States), are
greater than the threshold for human intake for class I
(1800 µg). The evaluation of the safety of these six
substances therefore proceeds to step A4.
Step A4. Four of the six substances for which the intake exceeds the
threshold of concern for class I are endogenous in humans.
Three of these four substances, namely, fumaric acid (No.
618), (-)-malic acid (No. 619), and triethyl citrate (No.
629), are components of the tricarboxylic acid cycle. The
fourth substance, ethyl acetoacetate (No. 595), is expected
to be hydrolysed to acetoacetic acid, which is endogenous in
humans and is formed from the condensation of two acetyl
coenzyme A units in the fatty acid pathway. For tartaric
acid and adipic acid, the evaluation should proceed to
step A5.
Step A5. The NOEL for tartaric acid in a two-year study of toxicity
in rats was 1200 mg/kg bw per day, the highest dose tested,
which provides adequate margins of safety (> 10 000 and
> 1000) for the known levels of intake (74 and 230 µg/kg bw
per day in Europe and the United States, respectively). No
NOEL was available for adipic acid, but the NOEL for the
structurally related material, dibutyl sebacate, in a
two-year study in rats was 6200 mg/kg bw per day, which
provides adequate margins of safety (> 100 000 000 and >
10 000 times) for the known levels of intake of adipic acid
(0.2 and 300 µg/kg bw per day in Europe and the United
States, respectively). These substances would not therefore
be expected to raise concern.
Table 1 summarizes the stepwise evaluation of the 47 aliphatic
primary alcohols, aldehydes, carboxylic acids, acetals, and esters
containing additional oxygenated functional groups used as flavouring
agents.
1.5 Consideration of combined intake
All of the 47 aliphatic primary alcohols, aldehydes, carboxylic
acids, acetals, and esters containing additional oxygenated functional
groups that were evaluated would be efficiently metabolized by common
biochemical pathways to innocuous substances.
In the unlikely event that foods containing all 47 substances
were consumed simultaneously on a daily basis, the total estimated
daily per capita intake of these substances in Europe and the United
States would exceed the threshold for human intake of substances in
class I. The Committee considered that such intake would not give rise
to perturbations outside the physiological range.
1.6 Conclusions
The Committee concluded that the safety of flavouring agents in
this group would not raise concern when they were used at he current
levels of estimated intake.
No data on toxicity were available for application of the
Procedure to 45 of the 47 substances in this group. For the remaining
two substances, tartaric acid (No. 621) and adipic acid (No. 623), the
data on toxicity were consistent with the results of the safety
evaluation made with the Procedure.
The ADIs for fumaric acid and its salts and for triethyl citrate
were maintained at the present meeting.
2. RELEVANT BACKGROUND INFORMATION
2.1 Explanation
Forty-seven aliphatic primary alcohols, aldehydes, carboxylic
acids, acetals, and esters containing additional oxygenated functional
groups are included in this group of flavouring agents (see Table 1).
The substances were selected on the basis of the criteria that all
members of the group are simple aliphatic primary alcohols, aldehydes,
carboxylic acids, acetals, and esters and contain additional
oxygenated functional groups. Eight substances in this group (Nos 589,
591, 603, 631-635) are alpha-keto acids, esters, or related
substances; five substances (Nos 590, 619-622) are alpha-hydroxy
acids, esters, or related substances; 12 substances (Nos 593-602, 614,
615) are beta-keto or beta-hydroxy alcohols, aldehydes, carboxylic
acids, and related acetals and esters; five substances (Nos 605-609)
are gamma-keto acids, esters, or related substances; four substances
(Nos 610-613) are omega-substituted alcohols, aldehydes, or acetals;
and 22 substances (Nos 614-631) are simple, aliphatic di-and
tricarboxylic acids or their esters.
2.2 Additonal considerations on intake
The total annual production of each of the 47 substances in this
group is shown in Table 2.
2.3 Biological data
2.3.1 Absorption, metabolism, and elimination
2.3.1.1 Ester and diesters
Twenty-eight substances in this group (Nos 590, 591, 594-603,
605, 607-609, 614-617, 620, 622, 624-626, and 628-630) are esters or
diesters, including one cyclic diester, which are expected to undergo
hydrolysis to their corresponding alcohol (saturated linear or
branched-chain aliphatic primary alcohols or branched-chain hydroxy or
keto alcohols) and acid components (alpha, beta-, or gamma-keto or
hydroxy acids or simple aliphatic acids, diacids, or triacids), which
would be further metabolized. Hydrolysis occurs in the intestinal
tract, blood, and liver and in most tissues and is catalysed by
carboxylesterases or esterases, the most important of which are the
B-esterases (Anders, 1989; Heymann, 1980). Acetyl esters are the
preferred substrates of C-esterases (Heymann, 1980). The presence of a
second oxygenated functional group has little if any effect on
hydrolysis of these esters.
Evidence for hydrolysis of these esters has come from various
experiments. Incubation of aqueous methyl 2-oxo-3-methylpentanoate
(No. 591) with a 2% pancreatin solution (pH 7.5) resulted in virtually
complete hydrolysis (> 98%) within 80 min (Leegwater & Van Straten,
1979). Dibutyl sebacate (No. 625) in 10% acacia solution was also
hydrolysed in vitro in a 10% crude pancreatic lipase solution
(Smith, 1953). 14C-Tributylacetyl citrate (No. 630) administered to
male Sprague-Dawley rats by gavage at a dose of 70 mg/kg bw was
rapidly absorbed (half-life, 1 h) and partially hydrolysed. More than
87% of the radiolabel was eliminated within 24 h of dosing. At least
nine urinary metabolites representing 59-70% of the dose were
detected. Five were identified as the partially hydrolysed mono-, di-,
and trialkylesters of citric acid. Three metabolites representing
25-26% of the dose were identified in the faeces. Approximately 2% was
eliminated as 14CO2 (Hiser et al., 1992). Hydrolysis of the cyclic
diester ethylene brassylate (No. 626) would be expected to occur on
the basis of the hydrolysis of structurally related lactones like
omega-6-hexadecenlactone. In simulated intestinal fluid,
omega-6-hexadecenlactone underwent nearly complete hydrolysis (92%) to
its open-chain form within 15 min (Morgareidge, 1962a).
The alcohol, aldehyde, and acid components of these esters,
diesters, and cyclic diester are completely metabolized. At higher
concentrations, they may be conjugated with glucuronic acid and
excreted.
2.3.1.2 alpha-Keto-and alpha-hydroxy acids and their esters
alpha-Keto-and alpha-hydroxyacids and their esters (Nos 589-591,
603, 631-635) would be expected to be metabolized in the same way as
endogenous alpha-ketoacids formed from oxidative deamination of amino
acids, such as isoleucine, methionine, and valine, in vivo.
2-Oxobutyric acid (alpha-ketobutyric acid, No. 589) is produced
endogenously in humans as a product of methionine degradation and
undergoes alpha-decarboxylation to yield propionyl-coenzyme A, which
ultimately enters the tricarboxylic acid cycle as succinyl-coenzyme A.
Nos 631-635 are intermediates formed endogenously from the oxidative
deamination of valine, isoleucine, leucine, glutamic acid, and serine,
respectively (Voet & Voet, 1990).
2.3.1.3 Acetals
Three substances in this group are acetals (Nos 593, 612, and
613), which are likely to undergo uncatalysed hydrolysis in vivo to
yield their component aldehydes and alcohols. 3-Oxobutanal dimethyl
acetal (No. 593) would be expected to undergo hydrolysis to yield
methanol and acetoacetaldehyde, which may be oxidized to acetoacetic
acid. More than 99% of hydroxycitronellal dimethyl acetal (No. 612)
was hydrolysed to the terpenoid hydroxycitronellal and methanol in
simulated gastric juice (pH 2.1) after 1 h, and > 6% was hydrolysed
in intestinal fluid (pH 7.5) after 2 h (Morgareidge, 1962b).
Hydroxy-citronellal diethyl acetal (No. 613) would be expected to
undergo similar metabolism.
2.3.1.5 beta-Keto-and beta-hydroxy acids and their esters
Esters of beta-keto or beta-hydroxy acids (Nos 594-603, 605) are
hydrolysed to acetoacetic acid or its beta-hydroxy or aldehyde
precursor. The last two can be oxidized in vivo to acetoacetic acid,
which is endogenous in humans and is formed from the condensation of
two acetyl coenzyme A units in the fatty acid pathway. It is released
from the liver into the bloodstream and transported to peripheral
tissues, where it is converted to acetyl coenzyme A and is completely
metabolized. When the endogenous levels are high, beta-ketoacids may
undergo non-enzymatic decarboxylation, which for acetoacetic acid
yields acetone and carbon ioxide (Voet & Voet, 1990).
2.3.1.6 gamma-Keto and gamma-hydroxy acids and their esters
Small amounts of gamma-hydroxy and gamma-keto acids and related
substances (Nos 606-609) are expected to be completely metabolized to
carbon dioxide. With greater exposure, the ketone function may be
reduced to the corresponding secondary alcohol (Bosron & Ting-Kai,
1980) and excreted as the glucuronic acid conjugate (Williams, 1959).
Products of partial beta-oxidation or glucuronic acid conjugation have
been identified in the urine. For example, a 1-g dose of the
structurally related substance gamma-hydroxybutyrate was excreted in
human urine unchanged and as S-3,4-dihydroxybutyrate and glycolate
(Lee, 1977).
2.3.1.7 omega-Substituted derivatives
omega-Substituted derivatives (Nos 610-613) may undergo complete
oxidation or conjugation with glucuronic acid and are then excreted
primarily in the urine. Products of incomplete oxidation and reduction
have also been observed. In rabbits, orally administered
hydroxycitronellal (No. 611) is reduced to hydroxy-citronellol (No.
610) and oxidized to hydroxycitronellic acid, both of which are
excreted in the urine (Ishida et al., 1989).
2.3.1.8 Aliphatic di- and tricarboxylic acids and their esters
The simple aliphatic di- and tricarboxylic acids either occur
endogenously in humans (Nos 618, 619, 627, and 634) or are
structurally related to endogenous substances (Nos 621-626, and 630).
The esters of these acids (616, 617, 620, 628, and 629) are
hydrolysed, as discussed above. Succinic acid, derived from the esters
(Nos 616 and 617), fumaric acid (No. 618), (-)-malic acid (No. 619),
aconitic acid (No. 627), citric acid derived from triethyl citrate
(No. 629), and 2-oxopentandioic acid (No. 634) are components of the
tricarboxylic acid cycle (Voet & Voet, 1990). Fumaric acid is present
in the blood, brain, liver, muscle, and kidney of normal rats
(Marshall et al., 1949), and citric, tartaric, malic, aconitic,
fumaric, and adipic acids are present in adult human urine (Osteux &
Laturaze, 1954). alpha-Ketoglutaric acid is an intermediate metabolite
of citric acid, fumaric acid, and succinic acid and is formed by
alpha-oxidation (Krebs et al., 1938; Simola & Krusius, 1938).
Simple aliphatic di-and tricarboxylic acids and their esters (Nos
614-635) are metabolized (after hydrolysis in the case of esters) in
the fatty acid beta-oxidation pathway or tricarboxylic acid cycle.
When 14C-labelled (-)-malic acid (No. 619) was administered to male
albino Wistar rats by gavage at a dose of 2.5 mg/kg bw, 93% of the
radiolabel was recovered in expired air, urine, and faeces (Daniel,
1969). Radiolabelled adipic acid fed to rats by stomach tube at a dose
of 200-300 mg/kg bw was partially or completely metabolized, and the
radiolabelled products identified in the urine included glutamic acid,
lactic acid, beta-ketoadipic acid, and citric acid. The presence of
the beta-oxidation metabolite beta-ketoadipic acid indicates that
adipic acid participates in beta-oxidation in the fatty acid pathway
(Rusoff et al., 1960).
The linear and branched-chain aliphatic primary alcohol
components would be oxidized in the presence of alcohol dehydrogenase
to their corresponding aldehydes which, in turn, would be oxidized to
their corresponding carboxylic acids (Bosron & Ting-Kai, 1980; Levi &
Hodgson, 1989; Feldman & Weiner, 1972). The resulting carboxylic acids
would be metabolized in the fatty acid pathway and tricarboxylic acid
cycle (Voet & Voet, 1990). Branched-chain diols or keto alcohols may
undergo oxidation to their corresponding aldehydes and carboxylic
acid, which would be further metabolized or excreted.
2.4 Toxicological studies
2.4.1 Acute toxicity
The available data on this group of aliphatic primary alcohols,
aldehydes, carboxylic acids, acetals, and esters which contain
additional oxygenated functional groups demonstrate that they have
little acute toxicity when given orally. Oral LD50 values have been
reported for 29 of the 47 substances in the group; these range from
1628 to > 34 000 mg/kg bw in male and female rats and from 1900 to >
31 000 mg/kg bw in male and female mice (Smyth et al., 1949, 1951;
Smith, 1953; Smyth et al., 1954; Horn et al., 1957; Finkelstein &
Gold, 1959; Wolven & Leverstein, 1962; Jenner et al., 1964;
Levenstein, 1969; Smyth et al., 1969; Hart & Wong, 1971; Levenstein,
1973; Moreno, 1973; Pellmont, 1973; Shelanski & Moldovan, 1973;
Lawrence et al., 1974; Moreno, 1976, 1977; Vernot et al., 1977;
Moreno, 1978; Pellmont, 1978; Moreno et al., 1979; Moreno, 1980;
Levenstein, 1981; Hoechst, 1995).
2.4.2 Short-term and long-term studies of toxicity
The results of short-term and long-term studies of the toxicity
of the substances in this group are shown in Table 3. Details of the
studies which were critical to the evaluation of the safety of
tartaric acid and adipic acid are given below.
2.4.2.1 Tartaric acid (No. 621)
Rats
The toxicity of fumaric, tartaric, oxalic, and maleic acids was
compared in groups of 12 weanling Osborne-Mendel rats of each sex,
with 24 of each sex in the control group. The animals were given diets
containing tartaric or fumaric acid at concentrations of 0, 0.1, 0.5,
0.8, or 1.2%, equivalent to 100, 500, 800, or 1200 mg/kg bw per day.
The mortality rates in treated groups were not different from those of
controls, and there was no statistically significant difference in
body-weight gain or weekly food consumption. Necropsy performed on
most animals at two years did not reveal any macroscopic changes.
Histopathological examination of a wide range of tissues revealed no
treatment-related changes. The NOEL was 1200 mg/kg bw per day
(Fitzhugh & Nelson, 1947).
Rabbits
In a study of the toxicity of citric, fumaric, and tartaric
acids, 15 New Zealand rabbits (sex not specified) weighing 1-3 kg were
given the sodium salt of tartaric acid in the diet at a concentration
of 7.7% for 150 days, equivalent to 2300 mg/kg bw per day. A control
group was fed ground diet alone. Each animal was examined daily, and
food intake and body weights were determined weekly. Haematological
and urinary analyses were performed after 60 days of treatment on five
treated and six control rabbits. Two animals were examined grossly 30
days after treatment, and one animal was examined after 60 days. The
testis was examined histologically. At 100 days, half of the surviving
rabbits were examined grossly, and the liver, kidney, and testis were
examined microscopically. At the end of the study at 150 days, all
animals were killed and examined grossly and histologically.
Haematological and urinary analyses showed no changes. No significant
gross or histopathological changes attributable to tartaric acid were
observed (Packman et al., 1963).
Table 3. Results of short-term and long-term studies of the toxicity of aliphatic
primary alcohols, aldehydes, carboxylic acids, acetals, and esters with additional
oxygenated functional groups
No. Substance Species Sex No. test Route Duration NOEL Reference
groupsa/no. (mg/kg bw
per test per day)
groupb
595 Ethyl acetoacetate Rat M/F 3/32 Diet 28-29 days 300 Cook et al. (1992)
606 Laevulinic acid Rat NR 2/3 Diet 16 days 1000 Tischer et al. (1942)
611 Hydroxycitronellal Rat M/F 2/20, 2/60 Diet 2 years 250 Bar & Griepentrog
(1967)
614 Diethyl malonate Rat M/F 2/20 Diet 13 weeks < 500c,d Posternak (1964)
614 Diethyl malonate Rat M/F 2/20-32 Diet 90 days 406 Posternak et al.
(1969)
618 Fumaric acid Rat M/F 8/12 Diet 2 years 1200 Fitzhugh & Nelson
(1947)
618 Fumaric acid Rat NR 2/14, 14/20 Diet 2 years 1380 Levey et al. (1946)
618 Fumaric acid Guinea-pig M/F NR Diet 1 year 400 Levey et al. (1946)
618 Fumaric acide Rabbit NR 3/15 Diet 150 days 2070 Packman et al. (1963)
621 Tartaric acid Rat M/F 8/12 Diet 2 years 1200 Fitzhugh & Nelson
(1947)
621 Tartaric acide Rabbit NR 3/15 Diet 150 days 2300c Packman et al.
(1963)
621 Tartaric acid Dog NR 1/4 Oral 90-114 days < 990c Krop et al. (1945)
Table 3. (continued)
No. Substance Species Sex No. test Route Duration NOEL Reference
groupsa/no. (mg/kg bw
per test per day)
groupb
624 Diethyl sebacate Rat M/F 2/10 Diet 17-18 or 1000 Hagan et al. (1967)
27-28
weeks
625 Dibutyl sebacate Rat M 4/10 Diet 1 year 1250 Smith (1953)
625 Dibutyl sebacate Rat M 5/16 Diet 2 years 6250 Smith (1953)
629 Triethyl citrate Rat M/F 3/7 Diet 2 months 4000 Finkelstein
& Gold (1959)
629 Triethyl citrate Cat NR 1/6 Gavage 2 months < 285 Finkelstein
& Gold (1959)
630 Tributyl Rat M/F 2/4 Diet 2 months 5000 Finkelstein
acetylcitrate & Gold (1959)
630 Tributyl Cat NR 1/2 Gavage 2 months < 5700c Finkelstein
acetylcitrate & Gold (1959)
M, male; F, female; NR, not reported
a Number of test groups does not include controls.
b Number per test group comprises male and female animals.
c Only one dose tested
d Changes in relative liver weight and glomerular and renal tubular histological appearance observed
e Administered as the sodium salt
Dogs
As part of a comparison of the toxicity of hydroxyacetic acid,
citric acid, and tartaric acid, four dogs (sex not specified) received
tartaric acid daily in a gelatin capsule at a dose of 990 mg/kg bw per
day for periods of 90 to 114 days. The changes in body weight varied
from a 30% gain to a 32% loss. Haematological and urinary parameters
were examined. Urinary casts (gelled protein) were observed in all
dogs and were graded as hyaline (clear) in three dogs. Blood chemical
parameters remained normal except in one dog which showed azotaemia
(increased concentrations of urea in the blood) and died at 90 days,
according to the authors due to nephrotoxicity. There was no NOEL
(Krop et al., 1945).
2.4.2.2 Diethyl sebacate (No. 624)
Rats
In a study of the toxicity of about 50 flavouring agents, groups
of five weanling Osborne-Mendel rats of each sex were fed diethyl
sebacate (referred to in the paper as ethyl sebacate) at a dietary
concentration of 1000 mg/kg for 27-28 weeks or 10 000 mg/kg for 17-18
weeks, equivalent to 100 and 1000 mg/kg bw per day. A group of 10
males and 10 females served as controls. Body weights, food intake,
and general condition were recorded weekly, and haematological
examinations were performed at the end of the study. All tissues were
examined grossly at necropsy. The livers, kidneys, spleens, hearts,
and testes from six controls and eight animals at the high dose,
evenly divided by sex, were weighed and examined microscopically.
There was no difference in growth rate or food consumption between
test and control animals, and haematological examination revealed
normal values. No macroscopic or microscopic changes were observed in
the tissues. The NOEL was 1000 mg/kg bw per day (Hagan et al., 1967).
2.4.2.3 Dibutyl sebacate (No. 625)
Rats
Groups of 10 male Sprague-Dawley rats, five weeks old, were fed
dibutyl sebacate at dietary concentrations of 0, 0.01, 0.05, 0.25, or
1.25%, equivalent to 0, 10, 50, 250, and 1250 mg/kg bw per day, for
one year. Body weight and food intake were measured periodically
throughout the study. Measurement of haematological parameters and
microscopic examination at necropsy revealed no adverse effects
(Smith, 1953).
Groups of 16 five-to six-week-old male Sprague-Dawley rats were
given dibutyl sebacate in the diet at concentrations of 0 (two control
groups), 0.01, 0.05, 0.25, 1.25, or 6.25%, equivalent to 0, 10, 50,
250, 1250, and 6250 mg/kg bw per day, for two years. Administration of
dibutyl sebacate did not adversely affect the growth or survival of
the animals. Body weight and food intake were measured periodically
throughout the study. Measurement of haematological parameters and
microscopic examination at necropsy revealed no adverse effects. The
lesions observed in older control and treated rats at necropsy
included inflammatory changes in the lungs, enlarged and discoloured
kidneys, and fatty changes in the liver. The incidence of these gross
lesions was not considered to be associated with the administration of
dibutyl sebacate. The NOEL was 6250 mg/kg bw per day (Smith, 1953).
2.4.4 Genotoxicity
The results of tests for the genotoxicity of substances in this
group are shown in Table 4.
2.4.5 Other relevant studies
2.4.5.1 Adipic acid (No. 623)
In a study of teratogenicity, groups of 20-24 pregnant rats were
given adipic acid by oral intubation on days 6-15 of gestation at
doses of 0, 3, 13, 62, or 288 mg/kg bw per day. A sixth group of 24
pregnant females was given aspirin at a dose of 250 mg/kg bw per day
as a positive control. The maternal parameters evaluated included
clinical signs of toxicity, body weight, and food consumption. The
fetuses were removed surgically from all dams on day 20. The numbers
of implantation sites, resorption sites, and live births were counted,
and the body weights of live pups and external, visceral, and skeletal
abnormalities were evaluated. Administration of adipic acid had no
adverse effect on the maternal parameters evaluated, nor did it
adversely affect fetal survival or the number of abnormalities in soft
or skeletal tissues (Morgareidge, 1973).
In a study of potential peroxisome proliferation, male Fischer
344 rats were fed adipic acid at a dietary concentration of 2%,
equivalent to about 2000 mg/kg bw per day, for three weeks. Control
animals received powdered Purina rat chow alone. No effect on hepatic
peroxisomes or their associated enzymes was observed in treated
animals (Moody & Reddy, 1978).
2.4.5.2 Tartaric acid (No. 621)
The potential immunotoxicity of tartaric acid was evaluated in a
rapid screening protocol in which groups of 10-20 female CD1 or
B6C3F1 mice were given the material orally at doses up to 3000 mg/kg
bw per day (doses not specified) for five days. A group of control
animals was also evaluated. The animals received an infectious
challenge on day 3 of dosing and immunization on day 5, and the
antibody plaque-forming cell response was measured four days later.
Deaths and survival were monitored for 10 days after infection. There
were no statistically significant differences in spleen weight, thymus
weight, spleen cellularity, anti-sheep red blood cell or
plaque-forming cell response, or death due to Listeria infection
between test and control animals (Vollmuth et al., 1989).
Table 4. Results of studies of the genotoxicity of aliphatic primary alcohols, aldehydes, carboxylic acids,
acetals, and esters with additional oxygenated functional groups
No. Substance End-point Test system Concentration Results Reference
595 Ethyl acetoacetate Gene mutation B. subtilis 20 mg/disc Negative Oda et al.
H17, M45 rec+/- (1978)
595 Ethyl acetoacetate Gene mutation B. subtilis 20 ml/disc Positive Yoo (1986)
H17, M45 rec+/-
595 Ethyl acetoacetate Gene mutation E. coli WP2 uvrA 25-320 mg/plate Positive Yoo (1986)
595 Ethyl acetoacetate Gene mutation B. subtilis 10-20 ml/ml Weakly Kuroda et al.
H17, M45 rec+/- positive (1984)
(test tube)
595 Ethyl acetoacetate Chromosomal Chinese hamster 2 mg/ml Negative Ishidate et al.
aberration cells (1984)
595 Ethyl acetoacetate Gene mutation S. typhimurium 25 mg/plate Negativea Ishidate et al.
TA92, TA1535, TA100, (1984)
TA1537, TA94, TA98
(preincubation
protocol)
595 Ethyl acetoacetate Gene mutation S. typhimurium TA97, 0.01-10 mg/plate Negativea Fujita & Sasaki
TA102 (preincubation (1987)
protocol)
610 Hydroxycitronellol Gene mutation S. typhimurium TA1535, 3.6 mg/plate Negativea Wild et al.
TA100, TA1537, TA1538, (1983)
TA98
610 Hydroxycitronellol Micronucleus Mouse 1204 mg/kg bw Negative Wild et al.
formation (1983)
610 Hydroxycitronellol Gene mutation D. melanogaster 10 mmol/L Negative Wild et al.
(1983)
611 Hydroxycitronellal Gene mutation S. typhimurium TA1535, 3.6 mg/plate Negativea Wild et al.
TA100, TA1537, TA1538, (1983)
TA98
Table 4. (continued)
No. Substance End-point Test system Concentration Results Reference
611 Hydoxycitronellal Micronucleus Mouse 861 mg/kg bw Negative Wild et al.
formation (1983)
611 Hydoxycitronellal Gene mutation D. melanogaster 37 mmol/L Negative Wild et al.
(1983)
612 Hydroxycitronellal Gene mutation S. typhimurium TA1535, 3.6 mg/plate Negativea Wild et al.
dimethyl acetal TA100, TA1537, TA1538, (1983)
TA98
612 Hydroxycitronellal Micronucleus Mouse 763 mg/kg bw Negative Wild et al.
dimethyl acetal formation (1983)
612 Hydroxycitronellal Gene mutation D. melanogaster 25 mmol/L Negative Wild et al.
dimethyl acetal (1983)
614 Diethyl malonate Gene mutation S. typhimurium TA98, 3 mmol/plate Negativea Florin et al.
TA100, TA1535, TA1537 (480 mg/plate)b (1980)
616 Dimethyl succinate Gene mutation S. typhimurium TA100, 20 000 mg/plate Negativea Andersen & Jensen
TA1535, TA1537, TA98 (1984)
616 Dimethyl succinate Gene mutation S. typhimurium TA97, 10 mg/plate Negativea Zeiger et al.
TA98, TA102, TA104, (1992)
TA1535, TA1538
618 Fumaric acid Gene mutation S. typhimurium TA100 1000 mg/plate Negativea Rapson et al.
(1980)
618 Fumaric acid Gene mutation S. typhimurium TA98, 2000 mg/plate Negative Zeiger et al.
TA100, TA1535, TA97 (1988)
(preincubation
protocol)
619 (-)-Malic acid Gene mutation S. typhimurium TA97, 2000 mg/plate Negativea Al-Ani & Al-Lami
TA98, TA100, TA104 (1988)
Table 4. (continued)
No. Substance End-point Test system Concentration Results Reference
623 Adipic acid Gene mutation E. coli WP2 uvrA 5000 mg/plate Negativea Shimizu et al.
(1985)
623 Adipic acid Gene mutation S. typhimurium TA100, 5000 mg/plate Negativea Shimizu et al.
TA98, (1985)
623 Adipic acid Gene mutation D. melanogaster 4000 ppm Negative Ramel & Magnusson
(1979)
625 Dibutyl sebacate Gene mutation S. typhimurium TA1535, 3.6 mg/plate Negativea Wild et al.
TA100, TA1537, TA1538, (1983)
TA98
625 Dibutyl sebacate Micronucleus Mouse 2829 mg/kg bw Negative Wild et al.
formation (1983)
625 Dibutyl sebacate Gene mutation D. melanogaster 19 mmol/L Negative Wild et al.
(1983)
626 Ethylene brassylate Gene mutation S. typhimurium TA1535, 3.6 mg/plate Negativea Wild et al.
TA100, TA1537, TA1538, (1983)
TA98
627 Aconitic acid Gene mutation S. typhimurium TA100, 20 000 mg/plate Negativea Andersen & Jensen
TA1535, TA1537, TA98 (1984)
a With and without metabolic activation
b Calculation based on relative molecular mass of 160.17
3. REFERENCES
Al-An, F.Y. & Al-Lami, S.K. (1988) Absence of mutagenic activity of
acidity regulators in the Ames Salmonella/microsome test. Mutat.
Res., 206, 467-470.
Anders, M.W. (1989) Biotransformation and bioactivation of xenobiotics
by the kidney. In: Hutson, D.H., Caldwell, J., & Paulson, G.D., eds,
Intermediary Xenobiotic Metabolism in Animals, New York: Taylor &
Francis, pp. 81-97.
Andersen, P.H. & Jensen, N.J. (1984) Mutagenic investigation of
flavourings: Dimethyl succinate, ethyl pyruvate and aconitic acid are
negative in the Salmonella/mammalian-microsome test. Food Addit.
Contam., 1, 283-288.
Bar, V.F. & Griepentrog, F. (1967) Where we stand concerning the
evaluation of flavouring substances from the viewpoint of health.
Med. Ernahr., 8, 244-251.
Bosron, W.F. & Ting-Kai, L. (1980) Alcohol dehydrogenase. In: Jacoby,
W.B., ed., Enzymatic Basis of Detoxification, Vol. 1, New York:
Academic Press, pp. 231-248.
Cook, W.M., Purchase, R., Ford, G.P., Creasy, D.M., Brantom, P.G. &
Gangolli, S.D. (1992) A 28-day feeding study with ethyl acetoacetate
in rats. Food Chem. Toxicol., 30, 567-573.
Cramer, G.M., Ford, R.A. & Hall, R.L. (1978) Estimation of toxic
hazard: A decision tree approach. Food Cosmet. Toxicol., 16,
255-276.
Daniel, J.W. (1969) The metabolism of l-and dl-malic acids by rats.
Food Cosmet. Toxicol., 7, 103-106.
Feldman, R.I. & Weiner, H. (1972) Horse liver aldehyde dehydrogenase.
I. Purification and characterization. J. Biol. Chem., 247, 260-266.
Finkelstein, M. & Gold, H. (1959) Toxicology of the citric acid
esters: Tributyl citrate, acetyltributyl citrate, triethyl citrate,
and acetyltriethyl citrate. Toxicol. Appl. Pharmacol., 1, 283-298.
Fitzhugh, O.G. & Nelson, A. (1947) The comparative chronic toxicities
of fumaric, tartaric, oxalic, and maleic acids. J. Am. Pharm.
Assoc., 36, 217-219.
Florin, I., Rutberg, L., Curvall, M. & Enzell, C.R. (1980) Screening
of tobacco smoke constituents for mutagenicity using the Ames' test.
Toxicologist, 15, 219-232.
Fujita, H. & Sasaki, M. (1987) Mutagenicity test of food additives
with Salmonella typhimurium TA97 and TA102. II. Ann. Rep. Tokyo
Metr. Res. Lab. Public Health, 38, 423-430.
Hagan, E.C., Hansen, W.H., Fitzhugh, O.G., Jenner, P.M., Jones, W.I.,
Taylor, J.M., Long, E.L., Nelson, A.A. & Brouwer, J.B. (1967) Food
flavourings and compounds of related structure. II. Subacute and
chronic toxicity. Food Cosmet. Toxicol., 5, 141-157.
Hart, E.R. & Wong, L.C.K. (1971) Acute oral toxicity studies in rats,
acute dermal toxicity and primary skin irritation studies in rabbits
of fragrance materials. Unpublished report by Bionetics Research
Laboratories. Submitted to WHO by the Flavor and Extract
Manufacturers' Association.
Heymann, E. (1980) Carboxylesterases and amidases. In: Jacoby, W.B.,
ed., Enzymatic Basis of Detoxication, 2nd Ed., New York, Academic
Press, pp. 291-323.
Hise,r M.F., Markley, B.J., Reitz, R.H. & Nieusma, J.L. (1992)
Metabolism and disposition of acetyl tributyl citrate in male
Sprague-Dawley rats. Toxicologist, 12, 161.
Hoechst (1995) Material safety data sheet for 3-hydroxy-2-oxopropionic
acid. Unpublished document submitted to WHO by the Flavor and Extract
Manufacturers' Association.
Horn, H.J., Holland, E.G. & Hazleton, L.W. (1957) Safety of adipic
acid as compared with citric and tartaric acid. J. Agric. Food
Chem., 5, 759-762.
International Organization of the Flavour Industry (1975) European
inquiry on volume of use. Unpublished report submitted to WHO by the
Flavor and Extract Manufacturers' Association.
Ishida, R., Toyota, M. & Asakawa, Y. (1989) Terpenoid
biotransformation in mammals. V. Metabolism of (+)-citronellal,
(+/-)-7-hydroxycitronellal, citral, (-)-perillaldehyde, (-)-myrtenal,
cuminaldehyde, thujone, and (+/-)-carvone in rabbits. Xenobiotica,
19, 843-855.
Ishidate, M., Jr, Sofuni, T., Yoshikawa, K., Hayashi, M., Nohmi, T.,
Sawada, M. & Matsu, A. (1984) Primary mutagenicity screening of food
additives currently used in Japan. Food Chem. Toxicol., 22, 623-636.
Jenner, P.M., Hagan, E.C., Taylor, J.M., Cook, E.L. & Fitzhugh, O.G.
(1964) Food flavourings and compounds of related structure. I. Acute
oral toxicity. Food Cosmet. Toxicol., 2, 327-343.
Krebs, H.A., Salvin, E. & Johnson, W.A. (1938) The formation of citric
acid and alpha-ketoglutaric acids in the mammalian body. Biochem. J.,
32, 113-117.
Krop, S., Gold, H. & Paterno, C.A. (1945) On the toxicity of
hydroxyacetic acid after prolonged administration: Comparison with its
sodium salt and citric and tartaric acids. J. Am. Pharm. Assoc., 24,
86-89.
Kuroda, K., Tanaka, S., Yu, Y.S. & Ishibashi, T. (1984) Rec-assay of
food additives. Nippon Kosnu Eisei Zasshi, 31, 277-281.
Lawrence, W.H., Malik, M., & Autian, J. (1974) Development of a
toxicity evaluation program for dental materials. II. Screening for
systemic toxicity. J. Biomed. Mater. Res., 8, 11-34.
Lee, C.R. (1977) Evidence for the beta-oxidation of orally
administered 4-hydroxybutyrate in humans. Biochem. Med., 17, 284-291.
Leegwater, D.C. & Van Straten, S. (1979) in vitro digestion test on
methyl-2-keto-3-methyl valerate. Unpublished report from Central
Institute for Nutrition and Food Research. Submitted to WHO by the
Flavor and Extract Manufacturers' Association.
Levenstein, I. (1969) Acute oral toxicity reports on rats. Unpublished
report from Leberco Laboratories. Submitted to WHO by the Flavor and
Extract Manufacturers' Association.
Levenstein, I. (1973) Acute oral toxicity reports on rats. Unpublished
report from Leberco Laboratories. Submtted to WHO by the Flavor and
Extract Manufacturers' Association.
Levenstein, I. (1981) Acute oral toxicity reports on rats. Unpublished
report from Leberco Laboratories. Submtted to WHO by the Flavor and
Extract Manufacturers' Association.
Levey, S., Lasichak, A.G., Brimi, R., Orten, J.M., Smyth, C.J. &
Smith, A.H. (1946) A study to determine the toxicity of fumaric acid.
J. Am. Pharm. Assoc., 35, 298-304.
Levi, E. & Hodgson, E. (1989) Metabolites resulting from oxidative and
reductive processes. In: Hutson, D.H., Caldwell, J. & Paulson, G.D.,
eds, Intermediary Xenobiotic Metabolism in Animals, London: Taylor &
Francis, pp. 119-138.
Maarse, C.A. Visscher, L.C., Willemsens, L.M., Nijssen, M.H. &
Boelens, M.H., eds (1994) Volatile Components in Food, 6th Ed.,
Suppl. 5, Zeist: TNO Nutrition and Food Research.
Marshall, L.M., Orten, J.M. & Smith, A.H. (1949) The determination of
fumaric acid in animal tissues by partition chromatography. J. Biol.
Chem., 179, 1127-1139.
Moody, D.E. & Reddy, J.K. (1978) Hepatic peroxisome (microbody)
proliferation in rats fed plasticizers and related compounds.
Toxicol. Appl. Pharmacol., 45, 497-504.
Moreno, O.M. (1973) Acute toxicity studies on rats and rabbits.
Unpublished report from MB Research Laboratories. Submtted to WHO by
the Flavor and Extract Manufacturers' Association.
Moreno, O.M. (1976) Acute toxicity studies in rats, mice, rabbits and
guinea pigs. Unpublished report from MB Research Laboratories.
Submtted to WHO by the Flavor and Extract Manufacturers' Association.
Moreno, O.M. (1977) Acute toxicity study in rats, rabbits and guinea
pigs. Unpublished report from MB Research Laboratories. Submtted to
WHO by the Flavor and Extract Manufacturers' Association.
Moreno, O.M. (1978) Acute, toxicity studies in rats, mice, rabbits and
guinea pigs. Unpublished report from MB Research Laboratories.
Submtted to WHO by the Flavor and Extract Manufacturers' Association.
Moreno, O.M. (1980) Acute toxicity studies. Unpublished report from MB
Research Laboratories. Submtted to WHO by the Flavor and Extract
Manufacturers' Association.
Moreno, O.M., Moreno, M.T. & Altenbach, E.J. (1979) Acute oral
toxicity study in rats with methyl-2-oxo-3-methylpentanoate.
Unpublished report from MB Research Laboratories. Submtted to WHO by
the Flavor and Extract Manufacturers' Association.
Morgareidge, K. (1962a) in vitro digestion of four lactones.
Unpublished report from the Food and Drug Research Laboratories.
Submitted to WHO by the Flavor and Extract Manufacturers' Association.
Morgareidge, K. (1962b) in vitro digestion of four acetals.
Unpublished report from the Food and Drug Research Laboratories.
Submitted to WHO by the Flavor and Extract Manufacturers' Association.
Morgareidge, K. (1973) Teratologic evaluation of adipic acid in rats.
Unpublished report from the Food and Drug Research Laboratories.
Submitted to WHO by the Flavor and Extract Manufacturers' Association.
National Academy of Sciences (1989) 1987 Poundage and technical
effects update of substances added to food. Washington DC: Committee
on Food Additive Survey Data.
Oda, Y., Hamono, Y., Inoue, K., Yamamoto, H., Niihara, T. & Kunita, N.
(1978) Mutagenicity of food flavours in bacteria. Shokuhin Eisei
Hen, 9, 177-181.
Osteux, R. & Laturaze, J. (1954) Paper chromatography of the organic
acids found in urine. C.R. Acad. Sci. (Paris), 239, 512-513.
Packman, E.W., Abbott, D.D. & Harrisson, W.E. (1963) Comparative
subacute toxicity for rabbits of citric, fumaric, and tartaric acids.
Toxicol. Appl. Pharmacol., 5, 163-167.
Pellmont, B. (1973) Acute oral toxicity of ethyl-3-oxohexanoate.
Unpublished report. Submitted to WHO by the Flavor and Extract
Manufacturers' Association.
Pellmont, B. (1978) Acute oral toxicity in mice with
methyl-2-hydroxy-4-methyl-pentanoate. Unpublished report. Submtted to
WHO by the Flavor and Extract Manufacturers' Association.
Posternak, J.M. (1964) Diethyl malonate. Unpublished report from
Firmenich & Co. Submtted to WHO by the Flavor and Extract
Manufacturers' Association.
Posternak, J.M., Linder, A. & Vodoz, C.A. (1969) Summaries of
toxicological data. Toxicological tests on flavouring matters. Food
Cosmet. Toxicol., 7, 405-407.
Ramel, C. & Magnusson, J. (1979) Chemical induction of nondisjunction
in Drosophila. Environ. Health Perspectives, 31, 59-66.
Rapson, W.H., Nazar, M.A. & Butsky, V.V. (1980) Mutagenicity produced
by aqueous chlorination of organic compounds. Bull. Environ. Contam.
Toxicol., 24, 590-596.
Rusoff, I.I., Balldwin, R.R., Dominues, F.J., Monder, C., Ohan, W.J. &
Thiessen, R., Jr (1960) Intermediary metabolism of adipic acid.
Toxicol. Appl. Pharmacol., 2, 316-330.
Shelanski, M.V. & Moldovan, M. (1973) Acute oral and dermal toxicity
studies. Unpublished report from Food and Drug Research Laboratories.
Submtted to WHO by the Flavor and Extract Manufacturers' Association.
Shimizu, H., Suzuki, Y., Takemura, N., Goto, S. & Matsushita, H.
(1985) The results of microbial mutation test for forty-three
industrial chemicals. Jpn. J. Ind. Health, 27, 400-419.
Simola, P.E. & Krusius, F.E. (1938) The formation of ketoglutaric acid
in animal metabolism. Suomen Kemistilehti, 11, B-9.
Smith, C.C. (1953) Toxicity of butyl stearate, dibutyl sebacate,
dibutyl phthalate, and methoxyethyl oleate. Arch. Ind. Hyg. Occup.
Med., 7, 310-318.
Smyth, H.F., Carpenter, C.P. & Weil, C.S. (1949) Range-finding
toxicity data. List III. J. Ind. Hyg. Toxicol., 31, 60-62.
Smyth, H.F., Carpenter, C.P. & Weil, C.S. (1951) Range-finding
toxicity data. List IV. Arch. Ind. Hyg. Occup. Med., 4, 119-122.
Smyth, H.F., Carpenter, C.P., Weil, C.S. & Pozzani, U.C. (1954)
Range-finding toxicity data. List V. Arch. Ind. Hyg., 10, 61-68.
Smyth, H.F., Carpenter, C.P., Weil, C.S., Pozzani, V.C., Striegel,
J.A. & Nycum, J.S. (1969) Range-finding toxicity data. List VII. Am.
Ind. Hyg. Ass. J., 30, 470-476.
Tischer, R.G., Fellers, C.R. & Doyle, B.J. (1942) The non-toxicity of
levulinic acid. J. Am. Pharm. Assoc., 31, 217-220.
Vernot, E.H., MacEwen, J.D., Huan, C.C. & Kinkead, E.R. (1977) Acute
toxicity and skin corrosion data for some organic and inorganic
compounds and aqueous solutions. Toxicol. Appl. Pharmacol., 42,
417-423.
Voet, D. & Voet, J.G., eds (1990) Biochemistry, New York: John Wiley
& Sons, pp. 506-527, 632-633, 690.
Vollmuth, T.A., Heck, J.D., Ratajczak, H.V. & Thomas, P.T. (1989)
Immunotoxicity assessment of flavouring ingredients using a rapid and
economical screen. Toxicologist, 9, 206.
Wild, D., King, M.T., Gocke, E. & Eckhardt, K. (1983) Study of
artificial flavouring substances for mutagenicity in the
Salmonella/microsome, basc and micronucleus test. Food Chem.
Toxicol., 21, 707-719.
Williams, R.T., ed. (1959) Detoxication Mechanisms. The Metabolism
and Detoxication of Drugs, Toxic Substances, and Other Organic
Compounds, 2nd Ed., London: Chapman & Hall, pp. 119-120.
Wolven, A. & Leverstein, I (1962) Acute oral toxicity study of diethyl
malonate in mice. Unpublished report from Givaudan Corporation.
Submitted to WHO by the Flavor and Extract Manufacturers' Association.
Yoo, Y.S. (1986) Mutagenic and antimutagenic activities of flavouring
agents used in foodstuffs. J. Osaka City Med. Center, 34, 267-288.
Zeiger, E., Anderson, B., Haworth, S., Lawlor, T. & Mortelmans, K.
(1988) Salmonella mutagenicity tests: IV. Results from the testing of
300 chemicals. Environ. Mol. Mutag., 11 (Suppl. 12), 1-158.
Zeiger, E., Anderson, B., Haworth, S, Lawlor, T. & Mortelmans, K.
(1992) Salmonella mutagenicity tests: V. Results from the testing of
311 chemicals. Environ. Mol. Mutag., 19 (Suppl. 21), 2-141.
All of the substances in the group are in structural class I, the human intake threshold of which is 1800 µg per person
per day, and all of the substances in the group are metabolized to innocuous products.
a The threshold for human inatke of substances in class I is 1800 µg per day.
b Ethyl acetoacetate is expected to be hydrolysed to acetoacetic acid, which is endogenous in humans.
c The ADI for this substance was maintained.
d Fumaric acid, (-)-malic acid, and triethyl citrate are components of the tricarboxylic acid cycle.
Step A3. The estimated daily per capita intakes in Europe and the
United States of 41 of the substances in this group are
below the threshold of concern for substances in class I
(1800 µg), indicating that they would not raise concern for
safety. The intakes of six substances, namely, ethyl
acetoacetate (No. 595; 1900 µg/person per day in Europe and
3900 µg/person per day in the United States), fumaric acid
(No. 618; 220 000 µg/person per day in the United States);
(-)-malic acid (No. 619; 16 000 µg/person per day in Europe
and 58 000 µg/person per day in the United States), tartaric
acid (No. 621; 4400 µg/person per day in Europe and 14 000
µg/person per day in the United States), adipic acid (No.
623; 18 000 µg/person per day in the United States), and
triethyl citrate (No. 629; 3400 µg/person per day in Europe
and 2400 µg/person per day in the United States), are
greater than the threshold for human intake for class I
(1800 µg). The evaluation of the safety of these six
substances therefore proceeds to step A4.
Step A4. Four of the six substances for which the intake exceeds the
threshold of concern for class I are endogenous in humans.
Three of these four substances, namely, fumaric acid (No.
618), (-)-malic acid (No. 619), and triethyl citrate (No.
629), are components of the tricarboxylic acid cycle. The
fourth substance, ethyl acetoacetate (No. 595), is expected
to be hydrolysed to acetoacetic acid, which is endogenous in
humans and is formed from the condensation of two acetyl
coenzyme A units in the fatty acid pathway. For tartaric
acid and adipic acid, the evaluation should proceed to
step A5.
Step A5. The NOEL for tartaric acid in a two-year study of toxicity
in rats was 1200 mg/kg bw per day, the highest dose tested,
which provides adequate margins of safety (> 10 000 and
> 1000) for the known levels of intake (74 and 230 µg/kg bw
per day in Europe and the United States, respectively). No
NOEL was available for adipic acid, but the NOEL for the
structurally related material, dibutyl sebacate, in a
two-year study in rats was 6200 mg/kg bw per day, which
provides adequate margins of safety (> 100 000 000 and >
10 000 times) for the known levels of intake of adipic acid
(0.2 and 300 µg/kg bw per day in Europe and the United
States, respectively). These substances would not therefore
be expected to raise concern.
Table 1 summarizes the stepwise evaluation of the 47 aliphatic
primary alcohols, aldehydes, carboxylic acids, acetals, and esters
containing additional oxygenated functional groups used as flavouring
agents.
1.5 Consideration of combined intake
All of the 47 aliphatic primary alcohols, aldehydes, carboxylic
acids, acetals, and esters containing additional oxygenated functional
groups that were evaluated would be efficiently metabolized by common
biochemical pathways to innocuous substances.
In the unlikely event that foods containing all 47 substances
were consumed simultaneously on a daily basis, the total estimated
daily per capita intake of these substances in Europe and the United
States would exceed the threshold for human intake of substances in
class I. The Committee considered that such intake would not give rise
to perturbations outside the physiological range.
1.6 Conclusions
The Committee concluded that the safety of flavouring agents in
this group would not raise concern when they were used at he current
levels of estimated intake.
No data on toxicity were available for application of the
Procedure to 45 of the 47 substances in this group. For the remaining
two substances, tartaric acid (No. 621) and adipic acid (No. 623), the
data on toxicity were consistent with the results of the safety
evaluation made with the Procedure.
The ADIs for fumaric acid and its salts and for triethyl citrate
were maintained at the present meeting.
2. RELEVANT BACKGROUND INFORMATION
2.1 Explanation
Forty-seven aliphatic primary alcohols, aldehydes, carboxylic
acids, acetals, and esters containing additional oxygenated functional
groups are included in this group of flavouring agents (see Table 1).
The substances were selected on the basis of the criteria that all
members of the group are simple aliphatic primary alcohols, aldehydes,
carboxylic acids, acetals, and esters and contain additional
oxygenated functional groups. Eight substances in this group (Nos 589,
591, 603, 631-635) are alpha-keto acids, esters, or related
substances; five substances (Nos 590, 619-622) are alpha-hydroxy
acids, esters, or related substances; 12 substances (Nos 593-602, 614,
615) are beta-keto or beta-hydroxy alcohols, aldehydes, carboxylic
acids, and related acetals and esters; five substances (Nos 605-609)
are gamma-keto acids, esters, or related substances; four substances
(Nos 610-613) are omega-substituted alcohols, aldehydes, or acetals;
and 22 substances (Nos 614-631) are simple, aliphatic di-and
tricarboxylic acids or their esters.
2.2 Additonal considerations on intake
The total annual production of each of the 47 substances in this
group is shown in Table 2.
2.3 Biological data
2.3.1 Absorption, metabolism, and elimination
2.3.1.1 Ester and diesters
Twenty-eight substances in this group (Nos 590, 591, 594-603,
605, 607-609, 614-617, 620, 622, 624-626, and 628-630) are esters or
diesters, including one cyclic diester, which are expected to undergo
hydrolysis to their corresponding alcohol (saturated linear or
branched-chain aliphatic primary alcohols or branched-chain hydroxy or
keto alcohols) and acid components (alpha, beta-, or gamma-keto or
hydroxy acids or simple aliphatic acids, diacids, or triacids), which
would be further metabolized. Hydrolysis occurs in the intestinal
tract, blood, and liver and in most tissues and is catalysed by
carboxylesterases or esterases, the most important of which are the
B-esterases (Anders, 1989; Heymann, 1980). Acetyl esters are the
preferred substrates of C-esterases (Heymann, 1980). The presence of a
second oxygenated functional group has little if any effect on
hydrolysis of these esters.
Evidence for hydrolysis of these esters has come from various
experiments. Incubation of aqueous methyl 2-oxo-3-methylpentanoate
(No. 591) with a 2% pancreatin solution (pH 7.5) resulted in virtually
complete hydrolysis (> 98%) within 80 min (Leegwater & Van Straten,
1979). Dibutyl sebacate (No. 625) in 10% acacia solution was also
hydrolysed in vitro in a 10% crude pancreatic lipase solution
(Smith, 1953). 14C-Tributylacetyl citrate (No. 630) administered to
male Sprague-Dawley rats by gavage at a dose of 70 mg/kg bw was
rapidly absorbed (half-life, 1 h) and partially hydrolysed. More than
87% of the radiolabel was eliminated within 24 h of dosing. At least
nine urinary metabolites representing 59-70% of the dose were
detected. Five were identified as the partially hydrolysed mono-, di-,
and trialkylesters of citric acid. Three metabolites representing
25-26% of the dose were identified in the faeces. Approximately 2% was
eliminated as 14CO2 (Hiser et al., 1992). Hydrolysis of the cyclic
diester ethylene brassylate (No. 626) would be expected to occur on
the basis of the hydrolysis of structurally related lactones like
omega-6-hexadecenlactone. In simulated intestinal fluid,
omega-6-hexadecenlactone underwent nearly complete hydrolysis (92%) to
its open-chain form within 15 min (Morgareidge, 1962a).
The alcohol, aldehyde, and acid components of these esters,
diesters, and cyclic diester are completely metabolized. At higher
concentrations, they may be conjugated with glucuronic acid and
excreted.
2.3.1.2 alpha-Keto-and alpha-hydroxy acids and their esters
alpha-Keto-and alpha-hydroxyacids and their esters (Nos 589-591,
603, 631-635) would be expected to be metabolized in the same way as
endogenous alpha-ketoacids formed from oxidative deamination of amino
acids, such as isoleucine, methionine, and valine, in vivo.
2-Oxobutyric acid (alpha-ketobutyric acid, No. 589) is produced
endogenously in humans as a product of methionine degradation and
undergoes alpha-decarboxylation to yield propionyl-coenzyme A, which
ultimately enters the tricarboxylic acid cycle as succinyl-coenzyme A.
Nos 631-635 are intermediates formed endogenously from the oxidative
deamination of valine, isoleucine, leucine, glutamic acid, and serine,
respectively (Voet & Voet, 1990).
2.3.1.3 Acetals
Three substances in this group are acetals (Nos 593, 612, and
613), which are likely to undergo uncatalysed hydrolysis in vivo to
yield their component aldehydes and alcohols. 3-Oxobutanal dimethyl
acetal (No. 593) would be expected to undergo hydrolysis to yield
methanol and acetoacetaldehyde, which may be oxidized to acetoacetic
acid. More than 99% of hydroxycitronellal dimethyl acetal (No. 612)
was hydrolysed to the terpenoid hydroxycitronellal and methanol in
simulated gastric juice (pH 2.1) after 1 h, and > 6% was hydrolysed
in intestinal fluid (pH 7.5) after 2 h (Morgareidge, 1962b).
Hydroxy-citronellal diethyl acetal (No. 613) would be expected to
undergo similar metabolism.
2.3.1.5 beta-Keto-and beta-hydroxy acids and their esters
Esters of beta-keto or beta-hydroxy acids (Nos 594-603, 605) are
hydrolysed to acetoacetic acid or its beta-hydroxy or aldehyde
precursor. The last two can be oxidized in vivo to acetoacetic acid,
which is endogenous in humans and is formed from the condensation of
two acetyl coenzyme A units in the fatty acid pathway. It is released
from the liver into the bloodstream and transported to peripheral
tissues, where it is converted to acetyl coenzyme A and is completely
metabolized. When the endogenous levels are high, beta-ketoacids may
undergo non-enzymatic decarboxylation, which for acetoacetic acid
yields acetone and carbon ioxide (Voet & Voet, 1990).
2.3.1.6 gamma-Keto and gamma-hydroxy acids and their esters
Small amounts of gamma-hydroxy and gamma-keto acids and related
substances (Nos 606-609) are expected to be completely metabolized to
carbon dioxide. With greater exposure, the ketone function may be
reduced to the corresponding secondary alcohol (Bosron & Ting-Kai,
1980) and excreted as the glucuronic acid conjugate (Williams, 1959).
Products of partial beta-oxidation or glucuronic acid conjugation have
been identified in the urine. For example, a 1-g dose of the
structurally related substance gamma-hydroxybutyrate was excreted in
human urine unchanged and as S-3,4-dihydroxybutyrate and glycolate
(Lee, 1977).
2.3.1.7 omega-Substituted derivatives
omega-Substituted derivatives (Nos 610-613) may undergo complete
oxidation or conjugation with glucuronic acid and are then excreted
primarily in the urine. Products of incomplete oxidation and reduction
have also been observed. In rabbits, orally administered
hydroxycitronellal (No. 611) is reduced to hydroxy-citronellol (No.
610) and oxidized to hydroxycitronellic acid, both of which are
excreted in the urine (Ishida et al., 1989).
2.3.1.8 Aliphatic di- and tricarboxylic acids and their esters
The simple aliphatic di- and tricarboxylic acids either occur
endogenously in humans (Nos 618, 619, 627, and 634) or are
structurally related to endogenous substances (Nos 621-626, and 630).
The esters of these acids (616, 617, 620, 628, and 629) are
hydrolysed, as discussed above. Succinic acid, derived from the esters
(Nos 616 and 617), fumaric acid (No. 618), (-)-malic acid (No. 619),
aconitic acid (No. 627), citric acid derived from triethyl citrate
(No. 629), and 2-oxopentandioic acid (No. 634) are components of the
tricarboxylic acid cycle (Voet & Voet, 1990). Fumaric acid is present
in the blood, brain, liver, muscle, and kidney of normal rats
(Marshall et al., 1949), and citric, tartaric, malic, aconitic,
fumaric, and adipic acids are present in adult human urine (Osteux &
Laturaze, 1954). alpha-Ketoglutaric acid is an intermediate metabolite
of citric acid, fumaric acid, and succinic acid and is formed by
alpha-oxidation (Krebs et al., 1938; Simola & Krusius, 1938).
Simple aliphatic di-and tricarboxylic acids and their esters (Nos
614-635) are metabolized (after hydrolysis in the case of esters) in
the fatty acid beta-oxidation pathway or tricarboxylic acid cycle.
When 14C-labelled (-)-malic acid (No. 619) was administered to male
albino Wistar rats by gavage at a dose of 2.5 mg/kg bw, 93% of the
radiolabel was recovered in expired air, urine, and faeces (Daniel,
1969). Radiolabelled adipic acid fed to rats by stomach tube at a dose
of 200-300 mg/kg bw was partially or completely metabolized, and the
radiolabelled products identified in the urine included glutamic acid,
lactic acid, beta-ketoadipic acid, and citric acid. The presence of
the beta-oxidation metabolite beta-ketoadipic acid indicates that
adipic acid participates in beta-oxidation in the fatty acid pathway
(Rusoff et al., 1960).
The linear and branched-chain aliphatic primary alcohol
components would be oxidized in the presence of alcohol dehydrogenase
to their corresponding aldehydes which, in turn, would be oxidized to
their corresponding carboxylic acids (Bosron & Ting-Kai, 1980; Levi &
Hodgson, 1989; Feldman & Weiner, 1972). The resulting carboxylic acids
would be metabolized in the fatty acid pathway and tricarboxylic acid
cycle (Voet & Voet, 1990). Branched-chain diols or keto alcohols may
undergo oxidation to their corresponding aldehydes and carboxylic
acid, which would be further metabolized or excreted.
2.4 Toxicological studies
2.4.1 Acute toxicity
The available data on this group of aliphatic primary alcohols,
aldehydes, carboxylic acids, acetals, and esters which contain
additional oxygenated functional groups demonstrate that they have
little acute toxicity when given orally. Oral LD50 values have been
reported for 29 of the 47 substances in the group; these range from
1628 to > 34 000 mg/kg bw in male and female rats and from 1900 to >
31 000 mg/kg bw in male and female mice (Smyth et al., 1949, 1951;
Smith, 1953; Smyth et al., 1954; Horn et al., 1957; Finkelstein &
Gold, 1959; Wolven & Leverstein, 1962; Jenner et al., 1964;
Levenstein, 1969; Smyth et al., 1969; Hart & Wong, 1971; Levenstein,
1973; Moreno, 1973; Pellmont, 1973; Shelanski & Moldovan, 1973;
Lawrence et al., 1974; Moreno, 1976, 1977; Vernot et al., 1977;
Moreno, 1978; Pellmont, 1978; Moreno et al., 1979; Moreno, 1980;
Levenstein, 1981; Hoechst, 1995).
2.4.2 Short-term and long-term studies of toxicity
The results of short-term and long-term studies of the toxicity
of the substances in this group are shown in Table 3. Details of the
studies which were critical to the evaluation of the safety of
tartaric acid and adipic acid are given below.
2.4.2.1 Tartaric acid (No. 621)
Rats
The toxicity of fumaric, tartaric, oxalic, and maleic acids was
compared in groups of 12 weanling Osborne-Mendel rats of each sex,
with 24 of each sex in the control group. The animals were given diets
containing tartaric or fumaric acid at concentrations of 0, 0.1, 0.5,
0.8, or 1.2%, equivalent to 100, 500, 800, or 1200 mg/kg bw per day.
The mortality rates in treated groups were not different from those of
controls, and there was no statistically significant difference in
body-weight gain or weekly food consumption. Necropsy performed on
most animals at two years did not reveal any macroscopic changes.
Histopathological examination of a wide range of tissues revealed no
treatment-related changes. The NOEL was 1200 mg/kg bw per day
(Fitzhugh & Nelson, 1947).
Rabbits
In a study of the toxicity of citric, fumaric, and tartaric
acids, 15 New Zealand rabbits (sex not specified) weighing 1-3 kg were
given the sodium salt of tartaric acid in the diet at a concentration
of 7.7% for 150 days, equivalent to 2300 mg/kg bw per day. A control
group was fed ground diet alone. Each animal was examined daily, and
food intake and body weights were determined weekly. Haematological
and urinary analyses were performed after 60 days of treatment on five
treated and six control rabbits. Two animals were examined grossly 30
days after treatment, and one animal was examined after 60 days. The
testis was examined histologically. At 100 days, half of the surviving
rabbits were examined grossly, and the liver, kidney, and testis were
examined microscopically. At the end of the study at 150 days, all
animals were killed and examined grossly and histologically.
Haematological and urinary analyses showed no changes. No significant
gross or histopathological changes attributable to tartaric acid were
observed (Packman et al., 1963).
Table 3. Results of short-term and long-term studies of the toxicity of aliphatic
primary alcohols, aldehydes, carboxylic acids, acetals, and esters with additional
oxygenated functional groups
No. Substance Species Sex No. test Route Duration NOEL Reference
groupsa/no. (mg/kg bw
per test per day)
groupb
595 Ethyl acetoacetate Rat M/F 3/32 Diet 28-29 days 300 Cook et al. (1992)
606 Laevulinic acid Rat NR 2/3 Diet 16 days 1000 Tischer et al. (1942)
611 Hydroxycitronellal Rat M/F 2/20, 2/60 Diet 2 years 250 Bar & Griepentrog
(1967)
614 Diethyl malonate Rat M/F 2/20 Diet 13 weeks < 500c,d Posternak (1964)
614 Diethyl malonate Rat M/F 2/20-32 Diet 90 days 406 Posternak et al.
(1969)
618 Fumaric acid Rat M/F 8/12 Diet 2 years 1200 Fitzhugh & Nelson
(1947)
618 Fumaric acid Rat NR 2/14, 14/20 Diet 2 years 1380 Levey et al. (1946)
618 Fumaric acid Guinea-pig M/F NR Diet 1 year 400 Levey et al. (1946)
618 Fumaric acide Rabbit NR 3/15 Diet 150 days 2070 Packman et al. (1963)
621 Tartaric acid Rat M/F 8/12 Diet 2 years 1200 Fitzhugh & Nelson
(1947)
621 Tartaric acide Rabbit NR 3/15 Diet 150 days 2300c Packman et al.
(1963)
621 Tartaric acid Dog NR 1/4 Oral 90-114 days < 990c Krop et al. (1945)
Table 3. (continued)
No. Substance Species Sex No. test Route Duration NOEL Reference
groupsa/no. (mg/kg bw
per test per day)
groupb
624 Diethyl sebacate Rat M/F 2/10 Diet 17-18 or 1000 Hagan et al. (1967)
27-28
weeks
625 Dibutyl sebacate Rat M 4/10 Diet 1 year 1250 Smith (1953)
625 Dibutyl sebacate Rat M 5/16 Diet 2 years 6250 Smith (1953)
629 Triethyl citrate Rat M/F 3/7 Diet 2 months 4000 Finkelstein
& Gold (1959)
629 Triethyl citrate Cat NR 1/6 Gavage 2 months < 285 Finkelstein
& Gold (1959)
630 Tributyl Rat M/F 2/4 Diet 2 months 5000 Finkelstein
acetylcitrate & Gold (1959)
630 Tributyl Cat NR 1/2 Gavage 2 months < 5700c Finkelstein
acetylcitrate & Gold (1959)
M, male; F, female; NR, not reported
a Number of test groups does not include controls.
b Number per test group comprises male and female animals.
c Only one dose tested
d Changes in relative liver weight and glomerular and renal tubular histological appearance observed
e Administered as the sodium salt
Dogs
As part of a comparison of the toxicity of hydroxyacetic acid,
citric acid, and tartaric acid, four dogs (sex not specified) received
tartaric acid daily in a gelatin capsule at a dose of 990 mg/kg bw per
day for periods of 90 to 114 days. The changes in body weight varied
from a 30% gain to a 32% loss. Haematological and urinary parameters
were examined. Urinary casts (gelled protein) were observed in all
dogs and were graded as hyaline (clear) in three dogs. Blood chemical
parameters remained normal except in one dog which showed azotaemia
(increased concentrations of urea in the blood) and died at 90 days,
according to the authors due to nephrotoxicity. There was no NOEL
(Krop et al., 1945).
2.4.2.2 Diethyl sebacate (No. 624)
Rats
In a study of the toxicity of about 50 flavouring agents, groups
of five weanling Osborne-Mendel rats of each sex were fed diethyl
sebacate (referred to in the paper as ethyl sebacate) at a dietary
concentration of 1000 mg/kg for 27-28 weeks or 10 000 mg/kg for 17-18
weeks, equivalent to 100 and 1000 mg/kg bw per day. A group of 10
males and 10 females served as controls. Body weights, food intake,
and general condition were recorded weekly, and haematological
examinations were performed at the end of the study. All tissues were
examined grossly at necropsy. The livers, kidneys, spleens, hearts,
and testes from six controls and eight animals at the high dose,
evenly divided by sex, were weighed and examined microscopically.
There was no difference in growth rate or food consumption between
test and control animals, and haematological examination revealed
normal values. No macroscopic or microscopic changes were observed in
the tissues. The NOEL was 1000 mg/kg bw per day (Hagan et al., 1967).
2.4.2.3 Dibutyl sebacate (No. 625)
Rats
Groups of 10 male Sprague-Dawley rats, five weeks old, were fed
dibutyl sebacate at dietary concentrations of 0, 0.01, 0.05, 0.25, or
1.25%, equivalent to 0, 10, 50, 250, and 1250 mg/kg bw per day, for
one year. Body weight and food intake were measured periodically
throughout the study. Measurement of haematological parameters and
microscopic examination at necropsy revealed no adverse effects
(Smith, 1953).
Groups of 16 five-to six-week-old male Sprague-Dawley rats were
given dibutyl sebacate in the diet at concentrations of 0 (two control
groups), 0.01, 0.05, 0.25, 1.25, or 6.25%, equivalent to 0, 10, 50,
250, 1250, and 6250 mg/kg bw per day, for two years. Administration of
dibutyl sebacate did not adversely affect the growth or survival of
the animals. Body weight and food intake were measured periodically
throughout the study. Measurement of haematological parameters and
microscopic examination at necropsy revealed no adverse effects. The
lesions observed in older control and treated rats at necropsy
included inflammatory changes in the lungs, enlarged and discoloured
kidneys, and fatty changes in the liver. The incidence of these gross
lesions was not considered to be associated with the administration of
dibutyl sebacate. The NOEL was 6250 mg/kg bw per day (Smith, 1953).
2.4.4 Genotoxicity
The results of tests for the genotoxicity of substances in this
group are shown in Table 4.
2.4.5 Other relevant studies
2.4.5.1 Adipic acid (No. 623)
In a study of teratogenicity, groups of 20-24 pregnant rats were
given adipic acid by oral intubation on days 6-15 of gestation at
doses of 0, 3, 13, 62, or 288 mg/kg bw per day. A sixth group of 24
pregnant females was given aspirin at a dose of 250 mg/kg bw per day
as a positive control. The maternal parameters evaluated included
clinical signs of toxicity, body weight, and food consumption. The
fetuses were removed surgically from all dams on day 20. The numbers
of implantation sites, resorption sites, and live births were counted,
and the body weights of live pups and external, visceral, and skeletal
abnormalities were evaluated. Administration of adipic acid had no
adverse effect on the maternal parameters evaluated, nor did it
adversely affect fetal survival or the number of abnormalities in soft
or skeletal tissues (Morgareidge, 1973).
In a study of potential peroxisome proliferation, male Fischer
344 rats were fed adipic acid at a dietary concentration of 2%,
equivalent to about 2000 mg/kg bw per day, for three weeks. Control
animals received powdered Purina rat chow alone. No effect on hepatic
peroxisomes or their associated enzymes was observed in treated
animals (Moody & Reddy, 1978).
2.4.5.2 Tartaric acid (No. 621)
The potential immunotoxicity of tartaric acid was evaluated in a
rapid screening protocol in which groups of 10-20 female CD1 or
B6C3F1 mice were given the material orally at doses up to 3000 mg/kg
bw per day (doses not specified) for five days. A group of control
animals was also evaluated. The animals received an infectious
challenge on day 3 of dosing and immunization on day 5, and the
antibody plaque-forming cell response was measured four days later.
Deaths and survival were monitored for 10 days after infection. There
were no statistically significant differences in spleen weight, thymus
weight, spleen cellularity, anti-sheep red blood cell or
plaque-forming cell response, or death due to Listeria infection
between test and control animals (Vollmuth et al., 1989).
Table 4. Results of studies of the genotoxicity of aliphatic primary alcohols, aldehydes, carboxylic acids,
acetals, and esters with additional oxygenated functional groups
No. Substance End-point Test system Concentration Results Reference
595 Ethyl acetoacetate Gene mutation B. subtilis 20 mg/disc Negative Oda et al.
H17, M45 rec+/- (1978)
595 Ethyl acetoacetate Gene mutation B. subtilis 20 ml/disc Positive Yoo (1986)
H17, M45 rec+/-
595 Ethyl acetoacetate Gene mutation E. coli WP2 uvrA 25-320 mg/plate Positive Yoo (1986)
595 Ethyl acetoacetate Gene mutation B. subtilis 10-20 ml/ml Weakly Kuroda et al.
H17, M45 rec+/- positive (1984)
(test tube)
595 Ethyl acetoacetate Chromosomal Chinese hamster 2 mg/ml Negative Ishidate et al.
aberration cells (1984)
595 Ethyl acetoacetate Gene mutation S. typhimurium 25 mg/plate Negativea Ishidate et al.
TA92, TA1535, TA100, (1984)
TA1537, TA94, TA98
(preincubation
protocol)
595 Ethyl acetoacetate Gene mutation S. typhimurium TA97, 0.01-10 mg/plate Negativea Fujita & Sasaki
TA102 (preincubation (1987)
protocol)
610 Hydroxycitronellol Gene mutation S. typhimurium TA1535, 3.6 mg/plate Negativea Wild et al.
TA100, TA1537, TA1538, (1983)
TA98
610 Hydroxycitronellol Micronucleus Mouse 1204 mg/kg bw Negative Wild et al.
formation (1983)
610 Hydroxycitronellol Gene mutation D. melanogaster 10 mmol/L Negative Wild et al.
(1983)
611 Hydroxycitronellal Gene mutation S. typhimurium TA1535, 3.6 mg/plate Negativea Wild et al.
TA100, TA1537, TA1538, (1983)
TA98
Table 4. (continued)
No. Substance End-point Test system Concentration Results Reference
611 Hydoxycitronellal Micronucleus Mouse 861 mg/kg bw Negative Wild et al.
formation (1983)
611 Hydoxycitronellal Gene mutation D. melanogaster 37 mmol/L Negative Wild et al.
(1983)
612 Hydroxycitronellal Gene mutation S. typhimurium TA1535, 3.6 mg/plate Negativea Wild et al.
dimethyl acetal TA100, TA1537, TA1538, (1983)
TA98
612 Hydroxycitronellal Micronucleus Mouse 763 mg/kg bw Negative Wild et al.
dimethyl acetal formation (1983)
612 Hydroxycitronellal Gene mutation D. melanogaster 25 mmol/L Negative Wild et al.
dimethyl acetal (1983)
614 Diethyl malonate Gene mutation S. typhimurium TA98, 3 mmol/plate Negativea Florin et al.
TA100, TA1535, TA1537 (480 mg/plate)b (1980)
616 Dimethyl succinate Gene mutation S. typhimurium TA100, 20 000 mg/plate Negativea Andersen & Jensen
TA1535, TA1537, TA98 (1984)
616 Dimethyl succinate Gene mutation S. typhimurium TA97, 10 mg/plate Negativea Zeiger et al.
TA98, TA102, TA104, (1992)
TA1535, TA1538
618 Fumaric acid Gene mutation S. typhimurium TA100 1000 mg/plate Negativea Rapson et al.
(1980)
618 Fumaric acid Gene mutation S. typhimurium TA98, 2000 mg/plate Negative Zeiger et al.
TA100, TA1535, TA97 (1988)
(preincubation
protocol)
619 (-)-Malic acid Gene mutation S. typhimurium TA97, 2000 mg/plate Negativea Al-Ani & Al-Lami
TA98, TA100, TA104 (1988)
Table 4. (continued)
No. Substance End-point Test system Concentration Results Reference
623 Adipic acid Gene mutation E. coli WP2 uvrA 5000 mg/plate Negativea Shimizu et al.
(1985)
623 Adipic acid Gene mutation S. typhimurium TA100, 5000 mg/plate Negativea Shimizu et al.
TA98, (1985)
623 Adipic acid Gene mutation D. melanogaster 4000 ppm Negative Ramel & Magnusson
(1979)
625 Dibutyl sebacate Gene mutation S. typhimurium TA1535, 3.6 mg/plate Negativea Wild et al.
TA100, TA1537, TA1538, (1983)
TA98
625 Dibutyl sebacate Micronucleus Mouse 2829 mg/kg bw Negative Wild et al.
formation (1983)
625 Dibutyl sebacate Gene mutation D. melanogaster 19 mmol/L Negative Wild et al.
(1983)
626 Ethylene brassylate Gene mutation S. typhimurium TA1535, 3.6 mg/plate Negativea Wild et al.
TA100, TA1537, TA1538, (1983)
TA98
627 Aconitic acid Gene mutation S. typhimurium TA100, 20 000 mg/plate Negativea Andersen & Jensen
TA1535, TA1537, TA98 (1984)
a With and without metabolic activation
b Calculation based on relative molecular mass of 160.17
3. REFERENCES
Al-An, F.Y. & Al-Lami, S.K. (1988) Absence of mutagenic activity of
acidity regulators in the Ames Salmonella/microsome test. Mutat.
Res., 206, 467-470.
Anders, M.W. (1989) Biotransformation and bioactivation of xenobiotics
by the kidney. In: Hutson, D.H., Caldwell, J., & Paulson, G.D., eds,
Intermediary Xenobiotic Metabolism in Animals, New York: Taylor &
Francis, pp. 81-97.
Andersen, P.H. & Jensen, N.J. (1984) Mutagenic investigation of
flavourings: Dimethyl succinate, ethyl pyruvate and aconitic acid are
negative in the Salmonella/mammalian-microsome test. Food Addit.
Contam., 1, 283-288.
Bar, V.F. & Griepentrog, F. (1967) Where we stand concerning the
evaluation of flavouring substances from the viewpoint of health.
Med. Ernahr., 8, 244-251.
Bosron, W.F. & Ting-Kai, L. (1980) Alcohol dehydrogenase. In: Jacoby,
W.B., ed., Enzymatic Basis of Detoxification, Vol. 1, New York:
Academic Press, pp. 231-248.
Cook, W.M., Purchase, R., Ford, G.P., Creasy, D.M., Brantom, P.G. &
Gangolli, S.D. (1992) A 28-day feeding study with ethyl acetoacetate
in rats. Food Chem. Toxicol., 30, 567-573.
Cramer, G.M., Ford, R.A. & Hall, R.L. (1978) Estimation of toxic
hazard: A decision tree approach. Food Cosmet. Toxicol., 16,
255-276.
Daniel, J.W. (1969) The metabolism of l-and dl-malic acids by rats.
Food Cosmet. Toxicol., 7, 103-106.
Feldman, R.I. & Weiner, H. (1972) Horse liver aldehyde dehydrogenase.
I. Purification and characterization. J. Biol. Chem., 247, 260-266.
Finkelstein, M. & Gold, H. (1959) Toxicology of the citric acid
esters: Tributyl citrate, acetyltributyl citrate, triethyl citrate,
and acetyltriethyl citrate. Toxicol. Appl. Pharmacol., 1, 283-298.
Fitzhugh, O.G. & Nelson, A. (1947) The comparative chronic toxicities
of fumaric, tartaric, oxalic, and maleic acids. J. Am. Pharm.
Assoc., 36, 217-219.
Florin, I., Rutberg, L., Curvall, M. & Enzell, C.R. (1980) Screening
of tobacco smoke constituents for mutagenicity using the Ames' test.
Toxicologist, 15, 219-232.
Fujita, H. & Sasaki, M. (1987) Mutagenicity test of food additives
with Salmonella typhimurium TA97 and TA102. II. Ann. Rep. Tokyo
Metr. Res. Lab. Public Health, 38, 423-430.
Hagan, E.C., Hansen, W.H., Fitzhugh, O.G., Jenner, P.M., Jones, W.I.,
Taylor, J.M., Long, E.L., Nelson, A.A. & Brouwer, J.B. (1967) Food
flavourings and compounds of related structure. II. Subacute and
chronic toxicity. Food Cosmet. Toxicol., 5, 141-157.
Hart, E.R. & Wong, L.C.K. (1971) Acute oral toxicity studies in rats,
acute dermal toxicity and primary skin irritation studies in rabbits
of fragrance materials. Unpublished report by Bionetics Research
Laboratories. Submitted to WHO by the Flavor and Extract
Manufacturers' Association.
Heymann, E. (1980) Carboxylesterases and amidases. In: Jacoby, W.B.,
ed., Enzymatic Basis of Detoxication, 2nd Ed., New York, Academic
Press, pp. 291-323.
Hise,r M.F., Markley, B.J., Reitz, R.H. & Nieusma, J.L. (1992)
Metabolism and disposition of acetyl tributyl citrate in male
Sprague-Dawley rats. Toxicologist, 12, 161.
Hoechst (1995) Material safety data sheet for 3-hydroxy-2-oxopropionic
acid. Unpublished document submitted to WHO by the Flavor and Extract
Manufacturers' Association.
Horn, H.J., Holland, E.G. & Hazleton, L.W. (1957) Safety of adipic
acid as compared with citric and tartaric acid. J. Agric. Food
Chem., 5, 759-762.
International Organization of the Flavour Industry (1975) European
inquiry on volume of use. Unpublished report submitted to WHO by the
Flavor and Extract Manufacturers' Association.
Ishida, R., Toyota, M. & Asakawa, Y. (1989) Terpenoid
biotransformation in mammals. V. Metabolism of (+)-citronellal,
(+/-)-7-hydroxycitronellal, citral, (-)-perillaldehyde, (-)-myrtenal,
cuminaldehyde, thujone, and (+/-)-carvone in rabbits. Xenobiotica,
19, 843-855.
Ishidate, M., Jr, Sofuni, T., Yoshikawa, K., Hayashi, M., Nohmi, T.,
Sawada, M. & Matsu, A. (1984) Primary mutagenicity screening of food
additives currently used in Japan. Food Chem. Toxicol., 22, 623-636.
Jenner, P.M., Hagan, E.C., Taylor, J.M., Cook, E.L. & Fitzhugh, O.G.
(1964) Food flavourings and compounds of related structure. I. Acute
oral toxicity. Food Cosmet. Toxicol., 2, 327-343.
Krebs, H.A., Salvin, E. & Johnson, W.A. (1938) The formation of citric
acid and alpha-ketoglutaric acids in the mammalian body. Biochem. J.,
32, 113-117.
Krop, S., Gold, H. & Paterno, C.A. (1945) On the toxicity of
hydroxyacetic acid after prolonged administration: Comparison with its
sodium salt and citric and tartaric acids. J. Am. Pharm. Assoc., 24,
86-89.
Kuroda, K., Tanaka, S., Yu, Y.S. & Ishibashi, T. (1984) Rec-assay of
food additives. Nippon Kosnu Eisei Zasshi, 31, 277-281.
Lawrence, W.H., Malik, M., & Autian, J. (1974) Development of a
toxicity evaluation program for dental materials. II. Screening for
systemic toxicity. J. Biomed. Mater. Res., 8, 11-34.
Lee, C.R. (1977) Evidence for the beta-oxidation of orally
administered 4-hydroxybutyrate in humans. Biochem. Med., 17, 284-291.
Leegwater, D.C. & Van Straten, S. (1979) in vitro digestion test on
methyl-2-keto-3-methyl valerate. Unpublished report from Central
Institute for Nutrition and Food Research. Submitted to WHO by the
Flavor and Extract Manufacturers' Association.
Levenstein, I. (1969) Acute oral toxicity reports on rats. Unpublished
report from Leberco Laboratories. Submitted to WHO by the Flavor and
Extract Manufacturers' Association.
Levenstein, I. (1973) Acute oral toxicity reports on rats. Unpublished
report from Leberco Laboratories. Submtted to WHO by the Flavor and
Extract Manufacturers' Association.
Levenstein, I. (1981) Acute oral toxicity reports on rats. Unpublished
report from Leberco Laboratories. Submtted to WHO by the Flavor and
Extract Manufacturers' Association.
Levey, S., Lasichak, A.G., Brimi, R., Orten, J.M., Smyth, C.J. &
Smith, A.H. (1946) A study to determine the toxicity of fumaric acid.
J. Am. Pharm. Assoc., 35, 298-304.
Levi, E. & Hodgson, E. (1989) Metabolites resulting from oxidative and
reductive processes. In: Hutson, D.H., Caldwell, J. & Paulson, G.D.,
eds, Intermediary Xenobiotic Metabolism in Animals, London: Taylor &
Francis, pp. 119-138.
Maarse, C.A. Visscher, L.C., Willemsens, L.M., Nijssen, M.H. &
Boelens, M.H., eds (1994) Volatile Components in Food, 6th Ed.,
Suppl. 5, Zeist: TNO Nutrition and Food Research.
Marshall, L.M., Orten, J.M. & Smith, A.H. (1949) The determination of
fumaric acid in animal tissues by partition chromatography. J. Biol.
Chem., 179, 1127-1139.
Moody, D.E. & Reddy, J.K. (1978) Hepatic peroxisome (microbody)
proliferation in rats fed plasticizers and related compounds.
Toxicol. Appl. Pharmacol., 45, 497-504.
Moreno, O.M. (1973) Acute toxicity studies on rats and rabbits.
Unpublished report from MB Research Laboratories. Submtted to WHO by
the Flavor and Extract Manufacturers' Association.
Moreno, O.M. (1976) Acute toxicity studies in rats, mice, rabbits and
guinea pigs. Unpublished report from MB Research Laboratories.
Submtted to WHO by the Flavor and Extract Manufacturers' Association.
Moreno, O.M. (1977) Acute toxicity study in rats, rabbits and guinea
pigs. Unpublished report from MB Research Laboratories. Submtted to
WHO by the Flavor and Extract Manufacturers' Association.
Moreno, O.M. (1978) Acute, toxicity studies in rats, mice, rabbits and
guinea pigs. Unpublished report from MB Research Laboratories.
Submtted to WHO by the Flavor and Extract Manufacturers' Association.
Moreno, O.M. (1980) Acute toxicity studies. Unpublished report from MB
Research Laboratories. Submtted to WHO by the Flavor and Extract
Manufacturers' Association.
Moreno, O.M., Moreno, M.T. & Altenbach, E.J. (1979) Acute oral
toxicity study in rats with methyl-2-oxo-3-methylpentanoate.
Unpublished report from MB Research Laboratories. Submtted to WHO by
the Flavor and Extract Manufacturers' Association.
Morgareidge, K. (1962a) in vitro digestion of four lactones.
Unpublished report from the Food and Drug Research Laboratories.
Submitted to WHO by the Flavor and Extract Manufacturers' Association.
Morgareidge, K. (1962b) in vitro digestion of four acetals.
Unpublished report from the Food and Drug Research Laboratories.
Submitted to WHO by the Flavor and Extract Manufacturers' Association.
Morgareidge, K. (1973) Teratologic evaluation of adipic acid in rats.
Unpublished report from the Food and Drug Research Laboratories.
Submitted to WHO by the Flavor and Extract Manufacturers' Association.
National Academy of Sciences (1989) 1987 Poundage and technical
effects update of substances added to food. Washington DC: Committee
on Food Additive Survey Data.
Oda, Y., Hamono, Y., Inoue, K., Yamamoto, H., Niihara, T. & Kunita, N.
(1978) Mutagenicity of food flavours in bacteria. Shokuhin Eisei
Hen, 9, 177-181.
Osteux, R. & Laturaze, J. (1954) Paper chromatography of the organic
acids found in urine. C.R. Acad. Sci. (Paris), 239, 512-513.
Packman, E.W., Abbott, D.D. & Harrisson, W.E. (1963) Comparative
subacute toxicity for rabbits of citric, fumaric, and tartaric acids.
Toxicol. Appl. Pharmacol., 5, 163-167.
Pellmont, B. (1973) Acute oral toxicity of ethyl-3-oxohexanoate.
Unpublished report. Submitted to WHO by the Flavor and Extract
Manufacturers' Association.
Pellmont, B. (1978) Acute oral toxicity in mice with
methyl-2-hydroxy-4-methyl-pentanoate. Unpublished report. Submtted to
WHO by the Flavor and Extract Manufacturers' Association.
Posternak, J.M. (1964) Diethyl malonate. Unpublished report from
Firmenich & Co. Submtted to WHO by the Flavor and Extract
Manufacturers' Association.
Posternak, J.M., Linder, A. & Vodoz, C.A. (1969) Summaries of
toxicological data. Toxicological tests on flavouring matters. Food
Cosmet. Toxicol., 7, 405-407.
Ramel, C. & Magnusson, J. (1979) Chemical induction of nondisjunction
in Drosophila. Environ. Health Perspectives, 31, 59-66.
Rapson, W.H., Nazar, M.A. & Butsky, V.V. (1980) Mutagenicity produced
by aqueous chlorination of organic compounds. Bull. Environ. Contam.
Toxicol., 24, 590-596.
Rusoff, I.I., Balldwin, R.R., Dominues, F.J., Monder, C., Ohan, W.J. &
Thiessen, R., Jr (1960) Intermediary metabolism of adipic acid.
Toxicol. Appl. Pharmacol., 2, 316-330.
Shelanski, M.V. & Moldovan, M. (1973) Acute oral and dermal toxicity
studies. Unpublished report from Food and Drug Research Laboratories.
Submtted to WHO by the Flavor and Extract Manufacturers' Association.
Shimizu, H., Suzuki, Y., Takemura, N., Goto, S. & Matsushita, H.
(1985) The results of microbial mutation test for forty-three
industrial chemicals. Jpn. J. Ind. Health, 27, 400-419.
Simola, P.E. & Krusius, F.E. (1938) The formation of ketoglutaric acid
in animal metabolism. Suomen Kemistilehti, 11, B-9.
Smith, C.C. (1953) Toxicity of butyl stearate, dibutyl sebacate,
dibutyl phthalate, and methoxyethyl oleate. Arch. Ind. Hyg. Occup.
Med., 7, 310-318.
Smyth, H.F., Carpenter, C.P. & Weil, C.S. (1949) Range-finding
toxicity data. List III. J. Ind. Hyg. Toxicol., 31, 60-62.
Smyth, H.F., Carpenter, C.P. & Weil, C.S. (1951) Range-finding
toxicity data. List IV. Arch. Ind. Hyg. Occup. Med., 4, 119-122.
Smyth, H.F., Carpenter, C.P., Weil, C.S. & Pozzani, U.C. (1954)
Range-finding toxicity data. List V. Arch. Ind. Hyg., 10, 61-68.
Smyth, H.F., Carpenter, C.P., Weil, C.S., Pozzani, V.C., Striegel,
J.A. & Nycum, J.S. (1969) Range-finding toxicity data. List VII. Am.
Ind. Hyg. Ass. J., 30, 470-476.
Tischer, R.G., Fellers, C.R. & Doyle, B.J. (1942) The non-toxicity of
levulinic acid. J. Am. Pharm. Assoc., 31, 217-220.
Vernot, E.H., MacEwen, J.D., Huan, C.C. & Kinkead, E.R. (1977) Acute
toxicity and skin corrosion data for some organic and inorganic
compounds and aqueous solutions. Toxicol. Appl. Pharmacol., 42,
417-423.
Voet, D. & Voet, J.G., eds (1990) Biochemistry, New York: John Wiley
& Sons, pp. 506-527, 632-633, 690.
Vollmuth, T.A., Heck, J.D., Ratajczak, H.V. & Thomas, P.T. (1989)
Immunotoxicity assessment of flavouring ingredients using a rapid and
economical screen. Toxicologist, 9, 206.
Wild, D., King, M.T., Gocke, E. & Eckhardt, K. (1983) Study of
artificial flavouring substances for mutagenicity in the
Salmonella/microsome, basc and micronucleus test. Food Chem.
Toxicol., 21, 707-719.
Williams, R.T., ed. (1959) Detoxication Mechanisms. The Metabolism
and Detoxication of Drugs, Toxic Substances, and Other Organic
Compounds, 2nd Ed., London: Chapman & Hall, pp. 119-120.
Wolven, A. & Leverstein, I (1962) Acute oral toxicity study of diethyl
malonate in mice. Unpublished report from Givaudan Corporation.
Submitted to WHO by the Flavor and Extract Manufacturers' Association.
Yoo, Y.S. (1986) Mutagenic and antimutagenic activities of flavouring
agents used in foodstuffs. J. Osaka City Med. Center, 34, 267-288.
Zeiger, E., Anderson, B., Haworth, S., Lawlor, T. & Mortelmans, K.
(1988) Salmonella mutagenicity tests: IV. Results from the testing of
300 chemicals. Environ. Mol. Mutag., 11 (Suppl. 12), 1-158.
Zeiger, E., Anderson, B., Haworth, S, Lawlor, T. & Mortelmans, K.
(1992) Salmonella mutagenicity tests: V. Results from the testing of
311 chemicals. Environ. Mol. Mutag., 19 (Suppl. 21), 2-141.
 Methyl 2-hydroxy-4-methylpentanoate 590 40348-72-9 No N/R N/R No safety concern
Methyl 2-hydroxy-4-methylpentanoate 590 40348-72-9 No N/R N/R No safety concern
 Methyl 2-oxo-3-methyl-pentanoate 591 3682-42-6 No N/R N/R No safety concern
Methyl 2-oxo-3-methyl-pentanoate 591 3682-42-6 No N/R N/R No safety concern
 Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
Citronelloxyacetaldehyde 592 7492-67-3 No N/R N/R No safety concern
Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
Citronelloxyacetaldehyde 592 7492-67-3 No N/R N/R No safety concern
 3-Oxobutanal dimethyl acetal 593 5436-21-5 No N/R N/R No safety concern
3-Oxobutanal dimethyl acetal 593 5436-21-5 No N/R N/R No safety concern
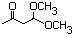 Ethyl 3-hydroxybutyrate 594 5405-41-4 No N/R N/R No safety concern
Ethyl 3-hydroxybutyrate 594 5405-41-4 No N/R N/R No safety concern
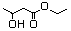 Ethyl acetoacetate 595 141-97-9 Yes Yesb N/R No safety concern
Ethyl acetoacetate 595 141-97-9 Yes Yesb N/R No safety concern
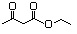 Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
Butyl acetoacetate 596 591-60-6 No N/R N/R No safety concern
Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
Butyl acetoacetate 596 591-60-6 No N/R N/R No safety concern
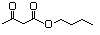 Isobutyl acetoacetate 597 7779-75-1 No N/R N/R No safety concern
Isobutyl acetoacetate 597 7779-75-1 No N/R N/R No safety concern
 Isoamyl acetoacetate 598 2308-18-1 No N/R N/R No safety concern
Isoamyl acetoacetate 598 2308-18-1 No N/R N/R No safety concern
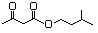 Geranyl acetoacetate 599 10032-00-5 No N/R N/R No safety concern
Geranyl acetoacetate 599 10032-00-5 No N/R N/R No safety concern
 Methyl 3-hydroxyhexanoate 600 21188-58-9 No N/R N/R No safety concern
Methyl 3-hydroxyhexanoate 600 21188-58-9 No N/R N/R No safety concern
 Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
Ethyl 3-hydroxyhexanoate 601 2305-25-1 No N/R N/R No safety concern
Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
Ethyl 3-hydroxyhexanoate 601 2305-25-1 No N/R N/R No safety concern
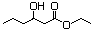 Ethyl 3-oxohexanoate 602 3249-68-1 No N/R N/R No safety concern
Ethyl 3-oxohexanoate 602 3249-68-1 No N/R N/R No safety concern
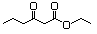 Ethyl 2,4-dioxohexanoate 603 13246-52-1 No N/R N/R No safety concern
Ethyl 2,4-dioxohexanoate 603 13246-52-1 No N/R N/R No safety concern
 3-(Hydroxymethyl)-2-heptanone 604 65405-68-7 No NR N/R No safety concern
3-(Hydroxymethyl)-2-heptanone 604 65405-68-7 No NR N/R No safety concern
 Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
1,3-Nonanediol acetate 605 1322-17-4 No N/R N/R No safety concern
(mixed esters)
Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
1,3-Nonanediol acetate 605 1322-17-4 No N/R N/R No safety concern
(mixed esters)
 Laevulinic acid 606 123-76-2 No N/R N/R No safety concern
Laevulinic acid 606 123-76-2 No N/R N/R No safety concern
 Ethyl laevulinate 607 539-88-8 No N/R N/R No safety concern
Ethyl laevulinate 607 539-88-8 No N/R N/R No safety concern
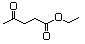 Butyl laevulinate 608 2052-15-5 No N/R N/R No safety concern
Butyl laevulinate 608 2052-15-5 No N/R N/R No safety concern
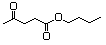 Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
1,4-Nonanediol diacetate 609 67715-81-5 No N/R N/R No safety concern
Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
1,4-Nonanediol diacetate 609 67715-81-5 No N/R N/R No safety concern
 Hydroxycitronellol 610 107-74-4 No N/R N/R No safety concern
Hydroxycitronellol 610 107-74-4 No N/R N/R No safety concern
 Hydroxycitronellal 611 107-75-5 No N/R N/R No safety concern
Hydroxycitronellal 611 107-75-5 No N/R N/R No safety concern
 Hydroxycitronellal dimethyl 612 141-92-4 No N/R N/R No safety concern
acetal
Hydroxycitronellal dimethyl 612 141-92-4 No N/R N/R No safety concern
acetal
 Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
Hydroxycitronellal diethyl 613 7779-94-4 No N/R N/R No safety concern
acetal
Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
Hydroxycitronellal diethyl 613 7779-94-4 No N/R N/R No safety concern
acetal
 Diethyl malonate 614 105-53-3 No N/R N/R No safety concern
Diethyl malonate 614 105-53-3 No N/R N/R No safety concern
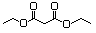 Butyl ethyl malonate 615 17373-84-1 No N/R N/R No safety concern
Butyl ethyl malonate 615 17373-84-1 No N/R N/R No safety concern
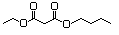 Dimethyl succinate 616 106-65-0 No N/R N/R No safety concern
Dimethyl succinate 616 106-65-0 No N/R N/R No safety concern
 Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
Diethyl succinate 617 123-25-1 No N/R N/R No safety concern
Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
Diethyl succinate 617 123-25-1 No N/R N/R No safety concern
 Fumaric acidc 618 110-17-8 Yes Yesd N/R No safety concern
Fumaric acidc 618 110-17-8 Yes Yesd N/R No safety concern
 (-)-Malic acid 619 97-67-6 Yes Yesd N/R No safety concern
(-)-Malic acid 619 97-67-6 Yes Yesd N/R No safety concern
 Diethyl malate 620 7554-12-3 No N/R N/R No safety concern
Diethyl malate 620 7554-12-3 No N/R N/R No safety concern
 Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
Tartaric acid (+-, --, ±-, meso-) 621 87-69-4 Yes No Yes. NOEL No safety concern
was 1200
mg/kg bw
per day in
a two-year
study in rats
Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
Tartaric acid (+-, --, ±-, meso-) 621 87-69-4 Yes No Yes. NOEL No safety concern
was 1200
mg/kg bw
per day in
a two-year
study in rats
 Diethyl tartrate 622 87-91-2 No N/R N/R No safety concern
Diethyl tartrate 622 87-91-2 No N/R N/R No safety concern
 Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
Adipic acid 623 124-04-9 Yes No Yes. The NOEL
for the
structurally
related
compound,
dibutyl
sebacate, was
6200 mg/kg bw
per day in a
two-year
study in rats
Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
Adipic acid 623 124-04-9 Yes No Yes. The NOEL
for the
structurally
related
compound,
dibutyl
sebacate, was
6200 mg/kg bw
per day in a
two-year
study in rats
 Diethyl sebacate 624 110-40-7 No N/R N/R No safety concern
Diethyl sebacate 624 110-40-7 No N/R N/R No safety concern
 Dibutyl sebacate 625 109-43-3 No N/R N/R No safety concern
Dibutyl sebacate 625 109-43-3 No N/R N/R No safety concern
 Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
Ethylene brassylate 626 105-95-3 No N/R N/R No safety concern
Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
Ethylene brassylate 626 105-95-3 No N/R N/R No safety concern
 Aconitic acid 627 499-12-7 No N/R N/R No safety concern
Aconitic acid 627 499-12-7 No N/R N/R No safety concern
 Ethyl aconitate (mixed esters) 628 - No N/R N/R No safety concern
Ethyl aconitate (mixed esters) 628 - No N/R N/R No safety concern
 Triethyl citratec 629 77-93-0 Yes Yesd N/R No safety concern
Triethyl citratec 629 77-93-0 Yes Yesd N/R No safety concern
 Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
Tributyl acetylcitrate 630 77-90-7 No N/R N/R No safety concern
Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
Tributyl acetylcitrate 630 77-90-7 No N/R N/R No safety concern
 3-Methyl-2-oxobutanoic acid 631 759-05-7 No N/R N/R No safety concern
and sodium salt 3715-29-6
3-Methyl-2-oxobutanoic acid 631 759-05-7 No N/R N/R No safety concern
and sodium salt 3715-29-6
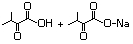 3-Methyl-2-oxopentanoic acid 632 1460-34-0 No N/R N/R No safety concern
and sodium salt 3715-31-9
3-Methyl-2-oxopentanoic acid 632 1460-34-0 No N/R N/R No safety concern
and sodium salt 3715-31-9
 4-Methyl-2-oxopentanoic acid 633 816-66-0 No N/R N/R No safety concern
and sodium salt 4502-00-5
4-Methyl-2-oxopentanoic acid 633 816-66-0 No N/R N/R No safety concern
and sodium salt 4502-00-5
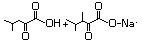 Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
2-Oxopentandioic acid 634 328-50-7 No N/R N/R No safety concern
Table 1. (continued)
Substance JECFA CAS No. Step A3a Step A4 Step A5 Conclusion based
and structure No. Does intake Is the Adequate NOEL on current intake
exceed the substance or for substance
threshold for are its or related
human intake? metabolites substance?
endogenous?
2-Oxopentandioic acid 634 328-50-7 No N/R N/R No safety concern
 3-Hydroxy-2-oxopropionic acid 635 1113-60-6 No N/R N/R No safety concern
3-Hydroxy-2-oxopropionic acid 635 1113-60-6 No N/R N/R No safety concern
 All of the substances in the group are in structural class I, the human intake threshold of which is 1800 µg per person
per day, and all of the substances in the group are metabolized to innocuous products.
a The threshold for human inatke of substances in class I is 1800 µg per day.
b Ethyl acetoacetate is expected to be hydrolysed to acetoacetic acid, which is endogenous in humans.
c The ADI for this substance was maintained.
d Fumaric acid, (-)-malic acid, and triethyl citrate are components of the tricarboxylic acid cycle.
Step A3. The estimated daily per capita intakes in Europe and the
United States of 41 of the substances in this group are
below the threshold of concern for substances in class I
(1800 µg), indicating that they would not raise concern for
safety. The intakes of six substances, namely, ethyl
acetoacetate (No. 595; 1900 µg/person per day in Europe and
3900 µg/person per day in the United States), fumaric acid
(No. 618; 220 000 µg/person per day in the United States);
(-)-malic acid (No. 619; 16 000 µg/person per day in Europe
and 58 000 µg/person per day in the United States), tartaric
acid (No. 621; 4400 µg/person per day in Europe and 14 000
µg/person per day in the United States), adipic acid (No.
623; 18 000 µg/person per day in the United States), and
triethyl citrate (No. 629; 3400 µg/person per day in Europe
and 2400 µg/person per day in the United States), are
greater than the threshold for human intake for class I
(1800 µg). The evaluation of the safety of these six
substances therefore proceeds to step A4.
Step A4. Four of the six substances for which the intake exceeds the
threshold of concern for class I are endogenous in humans.
Three of these four substances, namely, fumaric acid (No.
618), (-)-malic acid (No. 619), and triethyl citrate (No.
629), are components of the tricarboxylic acid cycle. The
fourth substance, ethyl acetoacetate (No. 595), is expected
to be hydrolysed to acetoacetic acid, which is endogenous in
humans and is formed from the condensation of two acetyl
coenzyme A units in the fatty acid pathway. For tartaric
acid and adipic acid, the evaluation should proceed to
step A5.
Step A5. The NOEL for tartaric acid in a two-year study of toxicity
in rats was 1200 mg/kg bw per day, the highest dose tested,
which provides adequate margins of safety (> 10 000 and
> 1000) for the known levels of intake (74 and 230 µg/kg bw
per day in Europe and the United States, respectively). No
NOEL was available for adipic acid, but the NOEL for the
structurally related material, dibutyl sebacate, in a
two-year study in rats was 6200 mg/kg bw per day, which
provides adequate margins of safety (> 100 000 000 and >
10 000 times) for the known levels of intake of adipic acid
(0.2 and 300 µg/kg bw per day in Europe and the United
States, respectively). These substances would not therefore
be expected to raise concern.
Table 1 summarizes the stepwise evaluation of the 47 aliphatic
primary alcohols, aldehydes, carboxylic acids, acetals, and esters
containing additional oxygenated functional groups used as flavouring
agents.
1.5 Consideration of combined intake
All of the 47 aliphatic primary alcohols, aldehydes, carboxylic
acids, acetals, and esters containing additional oxygenated functional
groups that were evaluated would be efficiently metabolized by common
biochemical pathways to innocuous substances.
In the unlikely event that foods containing all 47 substances
were consumed simultaneously on a daily basis, the total estimated
daily per capita intake of these substances in Europe and the United
States would exceed the threshold for human intake of substances in
class I. The Committee considered that such intake would not give rise
to perturbations outside the physiological range.
1.6 Conclusions
The Committee concluded that the safety of flavouring agents in
this group would not raise concern when they were used at he current
levels of estimated intake.
No data on toxicity were available for application of the
Procedure to 45 of the 47 substances in this group. For the remaining
two substances, tartaric acid (No. 621) and adipic acid (No. 623), the
data on toxicity were consistent with the results of the safety
evaluation made with the Procedure.
The ADIs for fumaric acid and its salts and for triethyl citrate
were maintained at the present meeting.
2. RELEVANT BACKGROUND INFORMATION
2.1 Explanation
Forty-seven aliphatic primary alcohols, aldehydes, carboxylic
acids, acetals, and esters containing additional oxygenated functional
groups are included in this group of flavouring agents (see Table 1).
The substances were selected on the basis of the criteria that all
members of the group are simple aliphatic primary alcohols, aldehydes,
carboxylic acids, acetals, and esters and contain additional
oxygenated functional groups. Eight substances in this group (Nos 589,
591, 603, 631-635) are alpha-keto acids, esters, or related
substances; five substances (Nos 590, 619-622) are alpha-hydroxy
acids, esters, or related substances; 12 substances (Nos 593-602, 614,
615) are beta-keto or beta-hydroxy alcohols, aldehydes, carboxylic
acids, and related acetals and esters; five substances (Nos 605-609)
are gamma-keto acids, esters, or related substances; four substances
(Nos 610-613) are omega-substituted alcohols, aldehydes, or acetals;
and 22 substances (Nos 614-631) are simple, aliphatic di-and
tricarboxylic acids or their esters.
2.2 Additonal considerations on intake
The total annual production of each of the 47 substances in this
group is shown in Table 2.
2.3 Biological data
2.3.1 Absorption, metabolism, and elimination
2.3.1.1 Ester and diesters
Twenty-eight substances in this group (Nos 590, 591, 594-603,
605, 607-609, 614-617, 620, 622, 624-626, and 628-630) are esters or
diesters, including one cyclic diester, which are expected to undergo
hydrolysis to their corresponding alcohol (saturated linear or
branched-chain aliphatic primary alcohols or branched-chain hydroxy or
keto alcohols) and acid components (alpha, beta-, or gamma-keto or
hydroxy acids or simple aliphatic acids, diacids, or triacids), which
would be further metabolized. Hydrolysis occurs in the intestinal
tract, blood, and liver and in most tissues and is catalysed by
carboxylesterases or esterases, the most important of which are the
B-esterases (Anders, 1989; Heymann, 1980). Acetyl esters are the
preferred substrates of C-esterases (Heymann, 1980). The presence of a
second oxygenated functional group has little if any effect on
hydrolysis of these esters.
Evidence for hydrolysis of these esters has come from various
experiments. Incubation of aqueous methyl 2-oxo-3-methylpentanoate
(No. 591) with a 2% pancreatin solution (pH 7.5) resulted in virtually
complete hydrolysis (> 98%) within 80 min (Leegwater & Van Straten,
1979). Dibutyl sebacate (No. 625) in 10% acacia solution was also
hydrolysed in vitro in a 10% crude pancreatic lipase solution
(Smith, 1953). 14C-Tributylacetyl citrate (No. 630) administered to
male Sprague-Dawley rats by gavage at a dose of 70 mg/kg bw was
rapidly absorbed (half-life, 1 h) and partially hydrolysed. More than
87% of the radiolabel was eliminated within 24 h of dosing. At least
nine urinary metabolites representing 59-70% of the dose were
detected. Five were identified as the partially hydrolysed mono-, di-,
and trialkylesters of citric acid. Three metabolites representing
25-26% of the dose were identified in the faeces. Approximately 2% was
eliminated as 14CO2 (Hiser et al., 1992). Hydrolysis of the cyclic
diester ethylene brassylate (No. 626) would be expected to occur on
the basis of the hydrolysis of structurally related lactones like
omega-6-hexadecenlactone. In simulated intestinal fluid,
omega-6-hexadecenlactone underwent nearly complete hydrolysis (92%) to
its open-chain form within 15 min (Morgareidge, 1962a).
The alcohol, aldehyde, and acid components of these esters,
diesters, and cyclic diester are completely metabolized. At higher
concentrations, they may be conjugated with glucuronic acid and
excreted.
2.3.1.2 alpha-Keto-and alpha-hydroxy acids and their esters
alpha-Keto-and alpha-hydroxyacids and their esters (Nos 589-591,
603, 631-635) would be expected to be metabolized in the same way as
endogenous alpha-ketoacids formed from oxidative deamination of amino
acids, such as isoleucine, methionine, and valine, in vivo.
2-Oxobutyric acid (alpha-ketobutyric acid, No. 589) is produced
endogenously in humans as a product of methionine degradation and
undergoes alpha-decarboxylation to yield propionyl-coenzyme A, which
ultimately enters the tricarboxylic acid cycle as succinyl-coenzyme A.
Nos 631-635 are intermediates formed endogenously from the oxidative
deamination of valine, isoleucine, leucine, glutamic acid, and serine,
respectively (Voet & Voet, 1990).
2.3.1.3 Acetals
Three substances in this group are acetals (Nos 593, 612, and
613), which are likely to undergo uncatalysed hydrolysis in vivo to
yield their component aldehydes and alcohols. 3-Oxobutanal dimethyl
acetal (No. 593) would be expected to undergo hydrolysis to yield
methanol and acetoacetaldehyde, which may be oxidized to acetoacetic
acid. More than 99% of hydroxycitronellal dimethyl acetal (No. 612)
was hydrolysed to the terpenoid hydroxycitronellal and methanol in
simulated gastric juice (pH 2.1) after 1 h, and > 6% was hydrolysed
in intestinal fluid (pH 7.5) after 2 h (Morgareidge, 1962b).
Hydroxy-citronellal diethyl acetal (No. 613) would be expected to
undergo similar metabolism.
2.3.1.5 beta-Keto-and beta-hydroxy acids and their esters
Esters of beta-keto or beta-hydroxy acids (Nos 594-603, 605) are
hydrolysed to acetoacetic acid or its beta-hydroxy or aldehyde
precursor. The last two can be oxidized in vivo to acetoacetic acid,
which is endogenous in humans and is formed from the condensation of
two acetyl coenzyme A units in the fatty acid pathway. It is released
from the liver into the bloodstream and transported to peripheral
tissues, where it is converted to acetyl coenzyme A and is completely
metabolized. When the endogenous levels are high, beta-ketoacids may
undergo non-enzymatic decarboxylation, which for acetoacetic acid
yields acetone and carbon ioxide (Voet & Voet, 1990).
2.3.1.6 gamma-Keto and gamma-hydroxy acids and their esters
Small amounts of gamma-hydroxy and gamma-keto acids and related
substances (Nos 606-609) are expected to be completely metabolized to
carbon dioxide. With greater exposure, the ketone function may be
reduced to the corresponding secondary alcohol (Bosron & Ting-Kai,
1980) and excreted as the glucuronic acid conjugate (Williams, 1959).
Products of partial beta-oxidation or glucuronic acid conjugation have
been identified in the urine. For example, a 1-g dose of the
structurally related substance gamma-hydroxybutyrate was excreted in
human urine unchanged and as S-3,4-dihydroxybutyrate and glycolate
(Lee, 1977).
2.3.1.7 omega-Substituted derivatives
omega-Substituted derivatives (Nos 610-613) may undergo complete
oxidation or conjugation with glucuronic acid and are then excreted
primarily in the urine. Products of incomplete oxidation and reduction
have also been observed. In rabbits, orally administered
hydroxycitronellal (No. 611) is reduced to hydroxy-citronellol (No.
610) and oxidized to hydroxycitronellic acid, both of which are
excreted in the urine (Ishida et al., 1989).
2.3.1.8 Aliphatic di- and tricarboxylic acids and their esters
The simple aliphatic di- and tricarboxylic acids either occur
endogenously in humans (Nos 618, 619, 627, and 634) or are
structurally related to endogenous substances (Nos 621-626, and 630).
The esters of these acids (616, 617, 620, 628, and 629) are
hydrolysed, as discussed above. Succinic acid, derived from the esters
(Nos 616 and 617), fumaric acid (No. 618), (-)-malic acid (No. 619),
aconitic acid (No. 627), citric acid derived from triethyl citrate
(No. 629), and 2-oxopentandioic acid (No. 634) are components of the
tricarboxylic acid cycle (Voet & Voet, 1990). Fumaric acid is present
in the blood, brain, liver, muscle, and kidney of normal rats
(Marshall et al., 1949), and citric, tartaric, malic, aconitic,
fumaric, and adipic acids are present in adult human urine (Osteux &
Laturaze, 1954). alpha-Ketoglutaric acid is an intermediate metabolite
of citric acid, fumaric acid, and succinic acid and is formed by
alpha-oxidation (Krebs et al., 1938; Simola & Krusius, 1938).
Simple aliphatic di-and tricarboxylic acids and their esters (Nos
614-635) are metabolized (after hydrolysis in the case of esters) in
the fatty acid beta-oxidation pathway or tricarboxylic acid cycle.
When 14C-labelled (-)-malic acid (No. 619) was administered to male
albino Wistar rats by gavage at a dose of 2.5 mg/kg bw, 93% of the
radiolabel was recovered in expired air, urine, and faeces (Daniel,
1969). Radiolabelled adipic acid fed to rats by stomach tube at a dose
of 200-300 mg/kg bw was partially or completely metabolized, and the
radiolabelled products identified in the urine included glutamic acid,
lactic acid, beta-ketoadipic acid, and citric acid. The presence of
the beta-oxidation metabolite beta-ketoadipic acid indicates that
adipic acid participates in beta-oxidation in the fatty acid pathway
(Rusoff et al., 1960).
The linear and branched-chain aliphatic primary alcohol
components would be oxidized in the presence of alcohol dehydrogenase
to their corresponding aldehydes which, in turn, would be oxidized to
their corresponding carboxylic acids (Bosron & Ting-Kai, 1980; Levi &
Hodgson, 1989; Feldman & Weiner, 1972). The resulting carboxylic acids
would be metabolized in the fatty acid pathway and tricarboxylic acid
cycle (Voet & Voet, 1990). Branched-chain diols or keto alcohols may
undergo oxidation to their corresponding aldehydes and carboxylic
acid, which would be further metabolized or excreted.
2.4 Toxicological studies
2.4.1 Acute toxicity
The available data on this group of aliphatic primary alcohols,
aldehydes, carboxylic acids, acetals, and esters which contain
additional oxygenated functional groups demonstrate that they have
little acute toxicity when given orally. Oral LD50 values have been
reported for 29 of the 47 substances in the group; these range from
1628 to > 34 000 mg/kg bw in male and female rats and from 1900 to >
31 000 mg/kg bw in male and female mice (Smyth et al., 1949, 1951;
Smith, 1953; Smyth et al., 1954; Horn et al., 1957; Finkelstein &
Gold, 1959; Wolven & Leverstein, 1962; Jenner et al., 1964;
Levenstein, 1969; Smyth et al., 1969; Hart & Wong, 1971; Levenstein,
1973; Moreno, 1973; Pellmont, 1973; Shelanski & Moldovan, 1973;
Lawrence et al., 1974; Moreno, 1976, 1977; Vernot et al., 1977;
Moreno, 1978; Pellmont, 1978; Moreno et al., 1979; Moreno, 1980;
Levenstein, 1981; Hoechst, 1995).
2.4.2 Short-term and long-term studies of toxicity
The results of short-term and long-term studies of the toxicity
of the substances in this group are shown in Table 3. Details of the
studies which were critical to the evaluation of the safety of
tartaric acid and adipic acid are given below.
2.4.2.1 Tartaric acid (No. 621)
Rats
The toxicity of fumaric, tartaric, oxalic, and maleic acids was
compared in groups of 12 weanling Osborne-Mendel rats of each sex,
with 24 of each sex in the control group. The animals were given diets
containing tartaric or fumaric acid at concentrations of 0, 0.1, 0.5,
0.8, or 1.2%, equivalent to 100, 500, 800, or 1200 mg/kg bw per day.
The mortality rates in treated groups were not different from those of
controls, and there was no statistically significant difference in
body-weight gain or weekly food consumption. Necropsy performed on
most animals at two years did not reveal any macroscopic changes.
Histopathological examination of a wide range of tissues revealed no
treatment-related changes. The NOEL was 1200 mg/kg bw per day
(Fitzhugh & Nelson, 1947).
Rabbits
In a study of the toxicity of citric, fumaric, and tartaric
acids, 15 New Zealand rabbits (sex not specified) weighing 1-3 kg were
given the sodium salt of tartaric acid in the diet at a concentration
of 7.7% for 150 days, equivalent to 2300 mg/kg bw per day. A control
group was fed ground diet alone. Each animal was examined daily, and
food intake and body weights were determined weekly. Haematological
and urinary analyses were performed after 60 days of treatment on five
treated and six control rabbits. Two animals were examined grossly 30
days after treatment, and one animal was examined after 60 days. The
testis was examined histologically. At 100 days, half of the surviving
rabbits were examined grossly, and the liver, kidney, and testis were
examined microscopically. At the end of the study at 150 days, all
animals were killed and examined grossly and histologically.
Haematological and urinary analyses showed no changes. No significant
gross or histopathological changes attributable to tartaric acid were
observed (Packman et al., 1963).
Table 3. Results of short-term and long-term studies of the toxicity of aliphatic
primary alcohols, aldehydes, carboxylic acids, acetals, and esters with additional
oxygenated functional groups
No. Substance Species Sex No. test Route Duration NOEL Reference
groupsa/no. (mg/kg bw
per test per day)
groupb
595 Ethyl acetoacetate Rat M/F 3/32 Diet 28-29 days 300 Cook et al. (1992)
606 Laevulinic acid Rat NR 2/3 Diet 16 days 1000 Tischer et al. (1942)
611 Hydroxycitronellal Rat M/F 2/20, 2/60 Diet 2 years 250 Bar & Griepentrog
(1967)
614 Diethyl malonate Rat M/F 2/20 Diet 13 weeks < 500c,d Posternak (1964)
614 Diethyl malonate Rat M/F 2/20-32 Diet 90 days 406 Posternak et al.
(1969)
618 Fumaric acid Rat M/F 8/12 Diet 2 years 1200 Fitzhugh & Nelson
(1947)
618 Fumaric acid Rat NR 2/14, 14/20 Diet 2 years 1380 Levey et al. (1946)
618 Fumaric acid Guinea-pig M/F NR Diet 1 year 400 Levey et al. (1946)
618 Fumaric acide Rabbit NR 3/15 Diet 150 days 2070 Packman et al. (1963)
621 Tartaric acid Rat M/F 8/12 Diet 2 years 1200 Fitzhugh & Nelson
(1947)
621 Tartaric acide Rabbit NR 3/15 Diet 150 days 2300c Packman et al.
(1963)
621 Tartaric acid Dog NR 1/4 Oral 90-114 days < 990c Krop et al. (1945)
Table 3. (continued)
No. Substance Species Sex No. test Route Duration NOEL Reference
groupsa/no. (mg/kg bw
per test per day)
groupb
624 Diethyl sebacate Rat M/F 2/10 Diet 17-18 or 1000 Hagan et al. (1967)
27-28
weeks
625 Dibutyl sebacate Rat M 4/10 Diet 1 year 1250 Smith (1953)
625 Dibutyl sebacate Rat M 5/16 Diet 2 years 6250 Smith (1953)
629 Triethyl citrate Rat M/F 3/7 Diet 2 months 4000 Finkelstein
& Gold (1959)
629 Triethyl citrate Cat NR 1/6 Gavage 2 months < 285 Finkelstein
& Gold (1959)
630 Tributyl Rat M/F 2/4 Diet 2 months 5000 Finkelstein
acetylcitrate & Gold (1959)
630 Tributyl Cat NR 1/2 Gavage 2 months < 5700c Finkelstein
acetylcitrate & Gold (1959)
M, male; F, female; NR, not reported
a Number of test groups does not include controls.
b Number per test group comprises male and female animals.
c Only one dose tested
d Changes in relative liver weight and glomerular and renal tubular histological appearance observed
e Administered as the sodium salt
Dogs
As part of a comparison of the toxicity of hydroxyacetic acid,
citric acid, and tartaric acid, four dogs (sex not specified) received
tartaric acid daily in a gelatin capsule at a dose of 990 mg/kg bw per
day for periods of 90 to 114 days. The changes in body weight varied
from a 30% gain to a 32% loss. Haematological and urinary parameters
were examined. Urinary casts (gelled protein) were observed in all
dogs and were graded as hyaline (clear) in three dogs. Blood chemical
parameters remained normal except in one dog which showed azotaemia
(increased concentrations of urea in the blood) and died at 90 days,
according to the authors due to nephrotoxicity. There was no NOEL
(Krop et al., 1945).
2.4.2.2 Diethyl sebacate (No. 624)
Rats
In a study of the toxicity of about 50 flavouring agents, groups
of five weanling Osborne-Mendel rats of each sex were fed diethyl
sebacate (referred to in the paper as ethyl sebacate) at a dietary
concentration of 1000 mg/kg for 27-28 weeks or 10 000 mg/kg for 17-18
weeks, equivalent to 100 and 1000 mg/kg bw per day. A group of 10
males and 10 females served as controls. Body weights, food intake,
and general condition were recorded weekly, and haematological
examinations were performed at the end of the study. All tissues were
examined grossly at necropsy. The livers, kidneys, spleens, hearts,
and testes from six controls and eight animals at the high dose,
evenly divided by sex, were weighed and examined microscopically.
There was no difference in growth rate or food consumption between
test and control animals, and haematological examination revealed
normal values. No macroscopic or microscopic changes were observed in
the tissues. The NOEL was 1000 mg/kg bw per day (Hagan et al., 1967).
2.4.2.3 Dibutyl sebacate (No. 625)
Rats
Groups of 10 male Sprague-Dawley rats, five weeks old, were fed
dibutyl sebacate at dietary concentrations of 0, 0.01, 0.05, 0.25, or
1.25%, equivalent to 0, 10, 50, 250, and 1250 mg/kg bw per day, for
one year. Body weight and food intake were measured periodically
throughout the study. Measurement of haematological parameters and
microscopic examination at necropsy revealed no adverse effects
(Smith, 1953).
Groups of 16 five-to six-week-old male Sprague-Dawley rats were
given dibutyl sebacate in the diet at concentrations of 0 (two control
groups), 0.01, 0.05, 0.25, 1.25, or 6.25%, equivalent to 0, 10, 50,
250, 1250, and 6250 mg/kg bw per day, for two years. Administration of
dibutyl sebacate did not adversely affect the growth or survival of
the animals. Body weight and food intake were measured periodically
throughout the study. Measurement of haematological parameters and
microscopic examination at necropsy revealed no adverse effects. The
lesions observed in older control and treated rats at necropsy
included inflammatory changes in the lungs, enlarged and discoloured
kidneys, and fatty changes in the liver. The incidence of these gross
lesions was not considered to be associated with the administration of
dibutyl sebacate. The NOEL was 6250 mg/kg bw per day (Smith, 1953).
2.4.4 Genotoxicity
The results of tests for the genotoxicity of substances in this
group are shown in Table 4.
2.4.5 Other relevant studies
2.4.5.1 Adipic acid (No. 623)
In a study of teratogenicity, groups of 20-24 pregnant rats were
given adipic acid by oral intubation on days 6-15 of gestation at
doses of 0, 3, 13, 62, or 288 mg/kg bw per day. A sixth group of 24
pregnant females was given aspirin at a dose of 250 mg/kg bw per day
as a positive control. The maternal parameters evaluated included
clinical signs of toxicity, body weight, and food consumption. The
fetuses were removed surgically from all dams on day 20. The numbers
of implantation sites, resorption sites, and live births were counted,
and the body weights of live pups and external, visceral, and skeletal
abnormalities were evaluated. Administration of adipic acid had no
adverse effect on the maternal parameters evaluated, nor did it
adversely affect fetal survival or the number of abnormalities in soft
or skeletal tissues (Morgareidge, 1973).
In a study of potential peroxisome proliferation, male Fischer
344 rats were fed adipic acid at a dietary concentration of 2%,
equivalent to about 2000 mg/kg bw per day, for three weeks. Control
animals received powdered Purina rat chow alone. No effect on hepatic
peroxisomes or their associated enzymes was observed in treated
animals (Moody & Reddy, 1978).
2.4.5.2 Tartaric acid (No. 621)
The potential immunotoxicity of tartaric acid was evaluated in a
rapid screening protocol in which groups of 10-20 female CD1 or
B6C3F1 mice were given the material orally at doses up to 3000 mg/kg
bw per day (doses not specified) for five days. A group of control
animals was also evaluated. The animals received an infectious
challenge on day 3 of dosing and immunization on day 5, and the
antibody plaque-forming cell response was measured four days later.
Deaths and survival were monitored for 10 days after infection. There
were no statistically significant differences in spleen weight, thymus
weight, spleen cellularity, anti-sheep red blood cell or
plaque-forming cell response, or death due to Listeria infection
between test and control animals (Vollmuth et al., 1989).
Table 4. Results of studies of the genotoxicity of aliphatic primary alcohols, aldehydes, carboxylic acids,
acetals, and esters with additional oxygenated functional groups
No. Substance End-point Test system Concentration Results Reference
595 Ethyl acetoacetate Gene mutation B. subtilis 20 mg/disc Negative Oda et al.
H17, M45 rec+/- (1978)
595 Ethyl acetoacetate Gene mutation B. subtilis 20 ml/disc Positive Yoo (1986)
H17, M45 rec+/-
595 Ethyl acetoacetate Gene mutation E. coli WP2 uvrA 25-320 mg/plate Positive Yoo (1986)
595 Ethyl acetoacetate Gene mutation B. subtilis 10-20 ml/ml Weakly Kuroda et al.
H17, M45 rec+/- positive (1984)
(test tube)
595 Ethyl acetoacetate Chromosomal Chinese hamster 2 mg/ml Negative Ishidate et al.
aberration cells (1984)
595 Ethyl acetoacetate Gene mutation S. typhimurium 25 mg/plate Negativea Ishidate et al.
TA92, TA1535, TA100, (1984)
TA1537, TA94, TA98
(preincubation
protocol)
595 Ethyl acetoacetate Gene mutation S. typhimurium TA97, 0.01-10 mg/plate Negativea Fujita & Sasaki
TA102 (preincubation (1987)
protocol)
610 Hydroxycitronellol Gene mutation S. typhimurium TA1535, 3.6 mg/plate Negativea Wild et al.
TA100, TA1537, TA1538, (1983)
TA98
610 Hydroxycitronellol Micronucleus Mouse 1204 mg/kg bw Negative Wild et al.
formation (1983)
610 Hydroxycitronellol Gene mutation D. melanogaster 10 mmol/L Negative Wild et al.
(1983)
611 Hydroxycitronellal Gene mutation S. typhimurium TA1535, 3.6 mg/plate Negativea Wild et al.
TA100, TA1537, TA1538, (1983)
TA98
Table 4. (continued)
No. Substance End-point Test system Concentration Results Reference
611 Hydoxycitronellal Micronucleus Mouse 861 mg/kg bw Negative Wild et al.
formation (1983)
611 Hydoxycitronellal Gene mutation D. melanogaster 37 mmol/L Negative Wild et al.
(1983)
612 Hydroxycitronellal Gene mutation S. typhimurium TA1535, 3.6 mg/plate Negativea Wild et al.
dimethyl acetal TA100, TA1537, TA1538, (1983)
TA98
612 Hydroxycitronellal Micronucleus Mouse 763 mg/kg bw Negative Wild et al.
dimethyl acetal formation (1983)
612 Hydroxycitronellal Gene mutation D. melanogaster 25 mmol/L Negative Wild et al.
dimethyl acetal (1983)
614 Diethyl malonate Gene mutation S. typhimurium TA98, 3 mmol/plate Negativea Florin et al.
TA100, TA1535, TA1537 (480 mg/plate)b (1980)
616 Dimethyl succinate Gene mutation S. typhimurium TA100, 20 000 mg/plate Negativea Andersen & Jensen
TA1535, TA1537, TA98 (1984)
616 Dimethyl succinate Gene mutation S. typhimurium TA97, 10 mg/plate Negativea Zeiger et al.
TA98, TA102, TA104, (1992)
TA1535, TA1538
618 Fumaric acid Gene mutation S. typhimurium TA100 1000 mg/plate Negativea Rapson et al.
(1980)
618 Fumaric acid Gene mutation S. typhimurium TA98, 2000 mg/plate Negative Zeiger et al.
TA100, TA1535, TA97 (1988)
(preincubation
protocol)
619 (-)-Malic acid Gene mutation S. typhimurium TA97, 2000 mg/plate Negativea Al-Ani & Al-Lami
TA98, TA100, TA104 (1988)
Table 4. (continued)
No. Substance End-point Test system Concentration Results Reference
623 Adipic acid Gene mutation E. coli WP2 uvrA 5000 mg/plate Negativea Shimizu et al.
(1985)
623 Adipic acid Gene mutation S. typhimurium TA100, 5000 mg/plate Negativea Shimizu et al.
TA98, (1985)
623 Adipic acid Gene mutation D. melanogaster 4000 ppm Negative Ramel & Magnusson
(1979)
625 Dibutyl sebacate Gene mutation S. typhimurium TA1535, 3.6 mg/plate Negativea Wild et al.
TA100, TA1537, TA1538, (1983)
TA98
625 Dibutyl sebacate Micronucleus Mouse 2829 mg/kg bw Negative Wild et al.
formation (1983)
625 Dibutyl sebacate Gene mutation D. melanogaster 19 mmol/L Negative Wild et al.
(1983)
626 Ethylene brassylate Gene mutation S. typhimurium TA1535, 3.6 mg/plate Negativea Wild et al.
TA100, TA1537, TA1538, (1983)
TA98
627 Aconitic acid Gene mutation S. typhimurium TA100, 20 000 mg/plate Negativea Andersen & Jensen
TA1535, TA1537, TA98 (1984)
a With and without metabolic activation
b Calculation based on relative molecular mass of 160.17
3. REFERENCES
Al-An, F.Y. & Al-Lami, S.K. (1988) Absence of mutagenic activity of
acidity regulators in the Ames Salmonella/microsome test. Mutat.
Res., 206, 467-470.
Anders, M.W. (1989) Biotransformation and bioactivation of xenobiotics
by the kidney. In: Hutson, D.H., Caldwell, J., & Paulson, G.D., eds,
Intermediary Xenobiotic Metabolism in Animals, New York: Taylor &
Francis, pp. 81-97.
Andersen, P.H. & Jensen, N.J. (1984) Mutagenic investigation of
flavourings: Dimethyl succinate, ethyl pyruvate and aconitic acid are
negative in the Salmonella/mammalian-microsome test. Food Addit.
Contam., 1, 283-288.
Bar, V.F. & Griepentrog, F. (1967) Where we stand concerning the
evaluation of flavouring substances from the viewpoint of health.
Med. Ernahr., 8, 244-251.
Bosron, W.F. & Ting-Kai, L. (1980) Alcohol dehydrogenase. In: Jacoby,
W.B., ed., Enzymatic Basis of Detoxification, Vol. 1, New York:
Academic Press, pp. 231-248.
Cook, W.M., Purchase, R., Ford, G.P., Creasy, D.M., Brantom, P.G. &
Gangolli, S.D. (1992) A 28-day feeding study with ethyl acetoacetate
in rats. Food Chem. Toxicol., 30, 567-573.
Cramer, G.M., Ford, R.A. & Hall, R.L. (1978) Estimation of toxic
hazard: A decision tree approach. Food Cosmet. Toxicol., 16,
255-276.
Daniel, J.W. (1969) The metabolism of l-and dl-malic acids by rats.
Food Cosmet. Toxicol., 7, 103-106.
Feldman, R.I. & Weiner, H. (1972) Horse liver aldehyde dehydrogenase.
I. Purification and characterization. J. Biol. Chem., 247, 260-266.
Finkelstein, M. & Gold, H. (1959) Toxicology of the citric acid
esters: Tributyl citrate, acetyltributyl citrate, triethyl citrate,
and acetyltriethyl citrate. Toxicol. Appl. Pharmacol., 1, 283-298.
Fitzhugh, O.G. & Nelson, A. (1947) The comparative chronic toxicities
of fumaric, tartaric, oxalic, and maleic acids. J. Am. Pharm.
Assoc., 36, 217-219.
Florin, I., Rutberg, L., Curvall, M. & Enzell, C.R. (1980) Screening
of tobacco smoke constituents for mutagenicity using the Ames' test.
Toxicologist, 15, 219-232.
Fujita, H. & Sasaki, M. (1987) Mutagenicity test of food additives
with Salmonella typhimurium TA97 and TA102. II. Ann. Rep. Tokyo
Metr. Res. Lab. Public Health, 38, 423-430.
Hagan, E.C., Hansen, W.H., Fitzhugh, O.G., Jenner, P.M., Jones, W.I.,
Taylor, J.M., Long, E.L., Nelson, A.A. & Brouwer, J.B. (1967) Food
flavourings and compounds of related structure. II. Subacute and
chronic toxicity. Food Cosmet. Toxicol., 5, 141-157.
Hart, E.R. & Wong, L.C.K. (1971) Acute oral toxicity studies in rats,
acute dermal toxicity and primary skin irritation studies in rabbits
of fragrance materials. Unpublished report by Bionetics Research
Laboratories. Submitted to WHO by the Flavor and Extract
Manufacturers' Association.
Heymann, E. (1980) Carboxylesterases and amidases. In: Jacoby, W.B.,
ed., Enzymatic Basis of Detoxication, 2nd Ed., New York, Academic
Press, pp. 291-323.
Hise,r M.F., Markley, B.J., Reitz, R.H. & Nieusma, J.L. (1992)
Metabolism and disposition of acetyl tributyl citrate in male
Sprague-Dawley rats. Toxicologist, 12, 161.
Hoechst (1995) Material safety data sheet for 3-hydroxy-2-oxopropionic
acid. Unpublished document submitted to WHO by the Flavor and Extract
Manufacturers' Association.
Horn, H.J., Holland, E.G. & Hazleton, L.W. (1957) Safety of adipic
acid as compared with citric and tartaric acid. J. Agric. Food
Chem., 5, 759-762.
International Organization of the Flavour Industry (1975) European
inquiry on volume of use. Unpublished report submitted to WHO by the
Flavor and Extract Manufacturers' Association.
Ishida, R., Toyota, M. & Asakawa, Y. (1989) Terpenoid
biotransformation in mammals. V. Metabolism of (+)-citronellal,
(+/-)-7-hydroxycitronellal, citral, (-)-perillaldehyde, (-)-myrtenal,
cuminaldehyde, thujone, and (+/-)-carvone in rabbits. Xenobiotica,
19, 843-855.
Ishidate, M., Jr, Sofuni, T., Yoshikawa, K., Hayashi, M., Nohmi, T.,
Sawada, M. & Matsu, A. (1984) Primary mutagenicity screening of food
additives currently used in Japan. Food Chem. Toxicol., 22, 623-636.
Jenner, P.M., Hagan, E.C., Taylor, J.M., Cook, E.L. & Fitzhugh, O.G.
(1964) Food flavourings and compounds of related structure. I. Acute
oral toxicity. Food Cosmet. Toxicol., 2, 327-343.
Krebs, H.A., Salvin, E. & Johnson, W.A. (1938) The formation of citric
acid and alpha-ketoglutaric acids in the mammalian body. Biochem. J.,
32, 113-117.
Krop, S., Gold, H. & Paterno, C.A. (1945) On the toxicity of
hydroxyacetic acid after prolonged administration: Comparison with its
sodium salt and citric and tartaric acids. J. Am. Pharm. Assoc., 24,
86-89.
Kuroda, K., Tanaka, S., Yu, Y.S. & Ishibashi, T. (1984) Rec-assay of
food additives. Nippon Kosnu Eisei Zasshi, 31, 277-281.
Lawrence, W.H., Malik, M., & Autian, J. (1974) Development of a
toxicity evaluation program for dental materials. II. Screening for
systemic toxicity. J. Biomed. Mater. Res., 8, 11-34.
Lee, C.R. (1977) Evidence for the beta-oxidation of orally
administered 4-hydroxybutyrate in humans. Biochem. Med., 17, 284-291.
Leegwater, D.C. & Van Straten, S. (1979) in vitro digestion test on
methyl-2-keto-3-methyl valerate. Unpublished report from Central
Institute for Nutrition and Food Research. Submitted to WHO by the
Flavor and Extract Manufacturers' Association.
Levenstein, I. (1969) Acute oral toxicity reports on rats. Unpublished
report from Leberco Laboratories. Submitted to WHO by the Flavor and
Extract Manufacturers' Association.
Levenstein, I. (1973) Acute oral toxicity reports on rats. Unpublished
report from Leberco Laboratories. Submtted to WHO by the Flavor and
Extract Manufacturers' Association.
Levenstein, I. (1981) Acute oral toxicity reports on rats. Unpublished
report from Leberco Laboratories. Submtted to WHO by the Flavor and
Extract Manufacturers' Association.
Levey, S., Lasichak, A.G., Brimi, R., Orten, J.M., Smyth, C.J. &
Smith, A.H. (1946) A study to determine the toxicity of fumaric acid.
J. Am. Pharm. Assoc., 35, 298-304.
Levi, E. & Hodgson, E. (1989) Metabolites resulting from oxidative and
reductive processes. In: Hutson, D.H., Caldwell, J. & Paulson, G.D.,
eds, Intermediary Xenobiotic Metabolism in Animals, London: Taylor &
Francis, pp. 119-138.
Maarse, C.A. Visscher, L.C., Willemsens, L.M., Nijssen, M.H. &
Boelens, M.H., eds (1994) Volatile Components in Food, 6th Ed.,
Suppl. 5, Zeist: TNO Nutrition and Food Research.
Marshall, L.M., Orten, J.M. & Smith, A.H. (1949) The determination of
fumaric acid in animal tissues by partition chromatography. J. Biol.
Chem., 179, 1127-1139.
Moody, D.E. & Reddy, J.K. (1978) Hepatic peroxisome (microbody)
proliferation in rats fed plasticizers and related compounds.
Toxicol. Appl. Pharmacol., 45, 497-504.
Moreno, O.M. (1973) Acute toxicity studies on rats and rabbits.
Unpublished report from MB Research Laboratories. Submtted to WHO by
the Flavor and Extract Manufacturers' Association.
Moreno, O.M. (1976) Acute toxicity studies in rats, mice, rabbits and
guinea pigs. Unpublished report from MB Research Laboratories.
Submtted to WHO by the Flavor and Extract Manufacturers' Association.
Moreno, O.M. (1977) Acute toxicity study in rats, rabbits and guinea
pigs. Unpublished report from MB Research Laboratories. Submtted to
WHO by the Flavor and Extract Manufacturers' Association.
Moreno, O.M. (1978) Acute, toxicity studies in rats, mice, rabbits and
guinea pigs. Unpublished report from MB Research Laboratories.
Submtted to WHO by the Flavor and Extract Manufacturers' Association.
Moreno, O.M. (1980) Acute toxicity studies. Unpublished report from MB
Research Laboratories. Submtted to WHO by the Flavor and Extract
Manufacturers' Association.
Moreno, O.M., Moreno, M.T. & Altenbach, E.J. (1979) Acute oral
toxicity study in rats with methyl-2-oxo-3-methylpentanoate.
Unpublished report from MB Research Laboratories. Submtted to WHO by
the Flavor and Extract Manufacturers' Association.
Morgareidge, K. (1962a) in vitro digestion of four lactones.
Unpublished report from the Food and Drug Research Laboratories.
Submitted to WHO by the Flavor and Extract Manufacturers' Association.
Morgareidge, K. (1962b) in vitro digestion of four acetals.
Unpublished report from the Food and Drug Research Laboratories.
Submitted to WHO by the Flavor and Extract Manufacturers' Association.
Morgareidge, K. (1973) Teratologic evaluation of adipic acid in rats.
Unpublished report from the Food and Drug Research Laboratories.
Submitted to WHO by the Flavor and Extract Manufacturers' Association.
National Academy of Sciences (1989) 1987 Poundage and technical
effects update of substances added to food. Washington DC: Committee
on Food Additive Survey Data.
Oda, Y., Hamono, Y., Inoue, K., Yamamoto, H., Niihara, T. & Kunita, N.
(1978) Mutagenicity of food flavours in bacteria. Shokuhin Eisei
Hen, 9, 177-181.
Osteux, R. & Laturaze, J. (1954) Paper chromatography of the organic
acids found in urine. C.R. Acad. Sci. (Paris), 239, 512-513.
Packman, E.W., Abbott, D.D. & Harrisson, W.E. (1963) Comparative
subacute toxicity for rabbits of citric, fumaric, and tartaric acids.
Toxicol. Appl. Pharmacol., 5, 163-167.
Pellmont, B. (1973) Acute oral toxicity of ethyl-3-oxohexanoate.
Unpublished report. Submitted to WHO by the Flavor and Extract
Manufacturers' Association.
Pellmont, B. (1978) Acute oral toxicity in mice with
methyl-2-hydroxy-4-methyl-pentanoate. Unpublished report. Submtted to
WHO by the Flavor and Extract Manufacturers' Association.
Posternak, J.M. (1964) Diethyl malonate. Unpublished report from
Firmenich & Co. Submtted to WHO by the Flavor and Extract
Manufacturers' Association.
Posternak, J.M., Linder, A. & Vodoz, C.A. (1969) Summaries of
toxicological data. Toxicological tests on flavouring matters. Food
Cosmet. Toxicol., 7, 405-407.
Ramel, C. & Magnusson, J. (1979) Chemical induction of nondisjunction
in Drosophila. Environ. Health Perspectives, 31, 59-66.
Rapson, W.H., Nazar, M.A. & Butsky, V.V. (1980) Mutagenicity produced
by aqueous chlorination of organic compounds. Bull. Environ. Contam.
Toxicol., 24, 590-596.
Rusoff, I.I., Balldwin, R.R., Dominues, F.J., Monder, C., Ohan, W.J. &
Thiessen, R., Jr (1960) Intermediary metabolism of adipic acid.
Toxicol. Appl. Pharmacol., 2, 316-330.
Shelanski, M.V. & Moldovan, M. (1973) Acute oral and dermal toxicity
studies. Unpublished report from Food and Drug Research Laboratories.
Submtted to WHO by the Flavor and Extract Manufacturers' Association.
Shimizu, H., Suzuki, Y., Takemura, N., Goto, S. & Matsushita, H.
(1985) The results of microbial mutation test for forty-three
industrial chemicals. Jpn. J. Ind. Health, 27, 400-419.
Simola, P.E. & Krusius, F.E. (1938) The formation of ketoglutaric acid
in animal metabolism. Suomen Kemistilehti, 11, B-9.
Smith, C.C. (1953) Toxicity of butyl stearate, dibutyl sebacate,
dibutyl phthalate, and methoxyethyl oleate. Arch. Ind. Hyg. Occup.
Med., 7, 310-318.
Smyth, H.F., Carpenter, C.P. & Weil, C.S. (1949) Range-finding
toxicity data. List III. J. Ind. Hyg. Toxicol., 31, 60-62.
Smyth, H.F., Carpenter, C.P. & Weil, C.S. (1951) Range-finding
toxicity data. List IV. Arch. Ind. Hyg. Occup. Med., 4, 119-122.
Smyth, H.F., Carpenter, C.P., Weil, C.S. & Pozzani, U.C. (1954)
Range-finding toxicity data. List V. Arch. Ind. Hyg., 10, 61-68.
Smyth, H.F., Carpenter, C.P., Weil, C.S., Pozzani, V.C., Striegel,
J.A. & Nycum, J.S. (1969) Range-finding toxicity data. List VII. Am.
Ind. Hyg. Ass. J., 30, 470-476.
Tischer, R.G., Fellers, C.R. & Doyle, B.J. (1942) The non-toxicity of
levulinic acid. J. Am. Pharm. Assoc., 31, 217-220.
Vernot, E.H., MacEwen, J.D., Huan, C.C. & Kinkead, E.R. (1977) Acute
toxicity and skin corrosion data for some organic and inorganic
compounds and aqueous solutions. Toxicol. Appl. Pharmacol., 42,
417-423.
Voet, D. & Voet, J.G., eds (1990) Biochemistry, New York: John Wiley
& Sons, pp. 506-527, 632-633, 690.
Vollmuth, T.A., Heck, J.D., Ratajczak, H.V. & Thomas, P.T. (1989)
Immunotoxicity assessment of flavouring ingredients using a rapid and
economical screen. Toxicologist, 9, 206.
Wild, D., King, M.T., Gocke, E. & Eckhardt, K. (1983) Study of
artificial flavouring substances for mutagenicity in the
Salmonella/microsome, basc and micronucleus test. Food Chem.
Toxicol., 21, 707-719.
Williams, R.T., ed. (1959) Detoxication Mechanisms. The Metabolism
and Detoxication of Drugs, Toxic Substances, and Other Organic
Compounds, 2nd Ed., London: Chapman & Hall, pp. 119-120.
Wolven, A. & Leverstein, I (1962) Acute oral toxicity study of diethyl
malonate in mice. Unpublished report from Givaudan Corporation.
Submitted to WHO by the Flavor and Extract Manufacturers' Association.
Yoo, Y.S. (1986) Mutagenic and antimutagenic activities of flavouring
agents used in foodstuffs. J. Osaka City Med. Center, 34, 267-288.
Zeiger, E., Anderson, B., Haworth, S., Lawlor, T. & Mortelmans, K.
(1988) Salmonella mutagenicity tests: IV. Results from the testing of
300 chemicals. Environ. Mol. Mutag., 11 (Suppl. 12), 1-158.
Zeiger, E., Anderson, B., Haworth, S, Lawlor, T. & Mortelmans, K.
(1992) Salmonella mutagenicity tests: V. Results from the testing of
311 chemicals. Environ. Mol. Mutag., 19 (Suppl. 21), 2-141.
All of the substances in the group are in structural class I, the human intake threshold of which is 1800 µg per person
per day, and all of the substances in the group are metabolized to innocuous products.
a The threshold for human inatke of substances in class I is 1800 µg per day.
b Ethyl acetoacetate is expected to be hydrolysed to acetoacetic acid, which is endogenous in humans.
c The ADI for this substance was maintained.
d Fumaric acid, (-)-malic acid, and triethyl citrate are components of the tricarboxylic acid cycle.
Step A3. The estimated daily per capita intakes in Europe and the
United States of 41 of the substances in this group are
below the threshold of concern for substances in class I
(1800 µg), indicating that they would not raise concern for
safety. The intakes of six substances, namely, ethyl
acetoacetate (No. 595; 1900 µg/person per day in Europe and
3900 µg/person per day in the United States), fumaric acid
(No. 618; 220 000 µg/person per day in the United States);
(-)-malic acid (No. 619; 16 000 µg/person per day in Europe
and 58 000 µg/person per day in the United States), tartaric
acid (No. 621; 4400 µg/person per day in Europe and 14 000
µg/person per day in the United States), adipic acid (No.
623; 18 000 µg/person per day in the United States), and
triethyl citrate (No. 629; 3400 µg/person per day in Europe
and 2400 µg/person per day in the United States), are
greater than the threshold for human intake for class I
(1800 µg). The evaluation of the safety of these six
substances therefore proceeds to step A4.
Step A4. Four of the six substances for which the intake exceeds the
threshold of concern for class I are endogenous in humans.
Three of these four substances, namely, fumaric acid (No.
618), (-)-malic acid (No. 619), and triethyl citrate (No.
629), are components of the tricarboxylic acid cycle. The
fourth substance, ethyl acetoacetate (No. 595), is expected
to be hydrolysed to acetoacetic acid, which is endogenous in
humans and is formed from the condensation of two acetyl
coenzyme A units in the fatty acid pathway. For tartaric
acid and adipic acid, the evaluation should proceed to
step A5.
Step A5. The NOEL for tartaric acid in a two-year study of toxicity
in rats was 1200 mg/kg bw per day, the highest dose tested,
which provides adequate margins of safety (> 10 000 and
> 1000) for the known levels of intake (74 and 230 µg/kg bw
per day in Europe and the United States, respectively). No
NOEL was available for adipic acid, but the NOEL for the
structurally related material, dibutyl sebacate, in a
two-year study in rats was 6200 mg/kg bw per day, which
provides adequate margins of safety (> 100 000 000 and >
10 000 times) for the known levels of intake of adipic acid
(0.2 and 300 µg/kg bw per day in Europe and the United
States, respectively). These substances would not therefore
be expected to raise concern.
Table 1 summarizes the stepwise evaluation of the 47 aliphatic
primary alcohols, aldehydes, carboxylic acids, acetals, and esters
containing additional oxygenated functional groups used as flavouring
agents.
1.5 Consideration of combined intake
All of the 47 aliphatic primary alcohols, aldehydes, carboxylic
acids, acetals, and esters containing additional oxygenated functional
groups that were evaluated would be efficiently metabolized by common
biochemical pathways to innocuous substances.
In the unlikely event that foods containing all 47 substances
were consumed simultaneously on a daily basis, the total estimated
daily per capita intake of these substances in Europe and the United
States would exceed the threshold for human intake of substances in
class I. The Committee considered that such intake would not give rise
to perturbations outside the physiological range.
1.6 Conclusions
The Committee concluded that the safety of flavouring agents in
this group would not raise concern when they were used at he current
levels of estimated intake.
No data on toxicity were available for application of the
Procedure to 45 of the 47 substances in this group. For the remaining
two substances, tartaric acid (No. 621) and adipic acid (No. 623), the
data on toxicity were consistent with the results of the safety
evaluation made with the Procedure.
The ADIs for fumaric acid and its salts and for triethyl citrate
were maintained at the present meeting.
2. RELEVANT BACKGROUND INFORMATION
2.1 Explanation
Forty-seven aliphatic primary alcohols, aldehydes, carboxylic
acids, acetals, and esters containing additional oxygenated functional
groups are included in this group of flavouring agents (see Table 1).
The substances were selected on the basis of the criteria that all
members of the group are simple aliphatic primary alcohols, aldehydes,
carboxylic acids, acetals, and esters and contain additional
oxygenated functional groups. Eight substances in this group (Nos 589,
591, 603, 631-635) are alpha-keto acids, esters, or related
substances; five substances (Nos 590, 619-622) are alpha-hydroxy
acids, esters, or related substances; 12 substances (Nos 593-602, 614,
615) are beta-keto or beta-hydroxy alcohols, aldehydes, carboxylic
acids, and related acetals and esters; five substances (Nos 605-609)
are gamma-keto acids, esters, or related substances; four substances
(Nos 610-613) are omega-substituted alcohols, aldehydes, or acetals;
and 22 substances (Nos 614-631) are simple, aliphatic di-and
tricarboxylic acids or their esters.
2.2 Additonal considerations on intake
The total annual production of each of the 47 substances in this
group is shown in Table 2.
2.3 Biological data
2.3.1 Absorption, metabolism, and elimination
2.3.1.1 Ester and diesters
Twenty-eight substances in this group (Nos 590, 591, 594-603,
605, 607-609, 614-617, 620, 622, 624-626, and 628-630) are esters or
diesters, including one cyclic diester, which are expected to undergo
hydrolysis to their corresponding alcohol (saturated linear or
branched-chain aliphatic primary alcohols or branched-chain hydroxy or
keto alcohols) and acid components (alpha, beta-, or gamma-keto or
hydroxy acids or simple aliphatic acids, diacids, or triacids), which
would be further metabolized. Hydrolysis occurs in the intestinal
tract, blood, and liver and in most tissues and is catalysed by
carboxylesterases or esterases, the most important of which are the
B-esterases (Anders, 1989; Heymann, 1980). Acetyl esters are the
preferred substrates of C-esterases (Heymann, 1980). The presence of a
second oxygenated functional group has little if any effect on
hydrolysis of these esters.
Evidence for hydrolysis of these esters has come from various
experiments. Incubation of aqueous methyl 2-oxo-3-methylpentanoate
(No. 591) with a 2% pancreatin solution (pH 7.5) resulted in virtually
complete hydrolysis (> 98%) within 80 min (Leegwater & Van Straten,
1979). Dibutyl sebacate (No. 625) in 10% acacia solution was also
hydrolysed in vitro in a 10% crude pancreatic lipase solution
(Smith, 1953). 14C-Tributylacetyl citrate (No. 630) administered to
male Sprague-Dawley rats by gavage at a dose of 70 mg/kg bw was
rapidly absorbed (half-life, 1 h) and partially hydrolysed. More than
87% of the radiolabel was eliminated within 24 h of dosing. At least
nine urinary metabolites representing 59-70% of the dose were
detected. Five were identified as the partially hydrolysed mono-, di-,
and trialkylesters of citric acid. Three metabolites representing
25-26% of the dose were identified in the faeces. Approximately 2% was
eliminated as 14CO2 (Hiser et al., 1992). Hydrolysis of the cyclic
diester ethylene brassylate (No. 626) would be expected to occur on
the basis of the hydrolysis of structurally related lactones like
omega-6-hexadecenlactone. In simulated intestinal fluid,
omega-6-hexadecenlactone underwent nearly complete hydrolysis (92%) to
its open-chain form within 15 min (Morgareidge, 1962a).
The alcohol, aldehyde, and acid components of these esters,
diesters, and cyclic diester are completely metabolized. At higher
concentrations, they may be conjugated with glucuronic acid and
excreted.
2.3.1.2 alpha-Keto-and alpha-hydroxy acids and their esters
alpha-Keto-and alpha-hydroxyacids and their esters (Nos 589-591,
603, 631-635) would be expected to be metabolized in the same way as
endogenous alpha-ketoacids formed from oxidative deamination of amino
acids, such as isoleucine, methionine, and valine, in vivo.
2-Oxobutyric acid (alpha-ketobutyric acid, No. 589) is produced
endogenously in humans as a product of methionine degradation and
undergoes alpha-decarboxylation to yield propionyl-coenzyme A, which
ultimately enters the tricarboxylic acid cycle as succinyl-coenzyme A.
Nos 631-635 are intermediates formed endogenously from the oxidative
deamination of valine, isoleucine, leucine, glutamic acid, and serine,
respectively (Voet & Voet, 1990).
2.3.1.3 Acetals
Three substances in this group are acetals (Nos 593, 612, and
613), which are likely to undergo uncatalysed hydrolysis in vivo to
yield their component aldehydes and alcohols. 3-Oxobutanal dimethyl
acetal (No. 593) would be expected to undergo hydrolysis to yield
methanol and acetoacetaldehyde, which may be oxidized to acetoacetic
acid. More than 99% of hydroxycitronellal dimethyl acetal (No. 612)
was hydrolysed to the terpenoid hydroxycitronellal and methanol in
simulated gastric juice (pH 2.1) after 1 h, and > 6% was hydrolysed
in intestinal fluid (pH 7.5) after 2 h (Morgareidge, 1962b).
Hydroxy-citronellal diethyl acetal (No. 613) would be expected to
undergo similar metabolism.
2.3.1.5 beta-Keto-and beta-hydroxy acids and their esters
Esters of beta-keto or beta-hydroxy acids (Nos 594-603, 605) are
hydrolysed to acetoacetic acid or its beta-hydroxy or aldehyde
precursor. The last two can be oxidized in vivo to acetoacetic acid,
which is endogenous in humans and is formed from the condensation of
two acetyl coenzyme A units in the fatty acid pathway. It is released
from the liver into the bloodstream and transported to peripheral
tissues, where it is converted to acetyl coenzyme A and is completely
metabolized. When the endogenous levels are high, beta-ketoacids may
undergo non-enzymatic decarboxylation, which for acetoacetic acid
yields acetone and carbon ioxide (Voet & Voet, 1990).
2.3.1.6 gamma-Keto and gamma-hydroxy acids and their esters
Small amounts of gamma-hydroxy and gamma-keto acids and related
substances (Nos 606-609) are expected to be completely metabolized to
carbon dioxide. With greater exposure, the ketone function may be
reduced to the corresponding secondary alcohol (Bosron & Ting-Kai,
1980) and excreted as the glucuronic acid conjugate (Williams, 1959).
Products of partial beta-oxidation or glucuronic acid conjugation have
been identified in the urine. For example, a 1-g dose of the
structurally related substance gamma-hydroxybutyrate was excreted in
human urine unchanged and as S-3,4-dihydroxybutyrate and glycolate
(Lee, 1977).
2.3.1.7 omega-Substituted derivatives
omega-Substituted derivatives (Nos 610-613) may undergo complete
oxidation or conjugation with glucuronic acid and are then excreted
primarily in the urine. Products of incomplete oxidation and reduction
have also been observed. In rabbits, orally administered
hydroxycitronellal (No. 611) is reduced to hydroxy-citronellol (No.
610) and oxidized to hydroxycitronellic acid, both of which are
excreted in the urine (Ishida et al., 1989).
2.3.1.8 Aliphatic di- and tricarboxylic acids and their esters
The simple aliphatic di- and tricarboxylic acids either occur
endogenously in humans (Nos 618, 619, 627, and 634) or are
structurally related to endogenous substances (Nos 621-626, and 630).
The esters of these acids (616, 617, 620, 628, and 629) are
hydrolysed, as discussed above. Succinic acid, derived from the esters
(Nos 616 and 617), fumaric acid (No. 618), (-)-malic acid (No. 619),
aconitic acid (No. 627), citric acid derived from triethyl citrate
(No. 629), and 2-oxopentandioic acid (No. 634) are components of the
tricarboxylic acid cycle (Voet & Voet, 1990). Fumaric acid is present
in the blood, brain, liver, muscle, and kidney of normal rats
(Marshall et al., 1949), and citric, tartaric, malic, aconitic,
fumaric, and adipic acids are present in adult human urine (Osteux &
Laturaze, 1954). alpha-Ketoglutaric acid is an intermediate metabolite
of citric acid, fumaric acid, and succinic acid and is formed by
alpha-oxidation (Krebs et al., 1938; Simola & Krusius, 1938).
Simple aliphatic di-and tricarboxylic acids and their esters (Nos
614-635) are metabolized (after hydrolysis in the case of esters) in
the fatty acid beta-oxidation pathway or tricarboxylic acid cycle.
When 14C-labelled (-)-malic acid (No. 619) was administered to male
albino Wistar rats by gavage at a dose of 2.5 mg/kg bw, 93% of the
radiolabel was recovered in expired air, urine, and faeces (Daniel,
1969). Radiolabelled adipic acid fed to rats by stomach tube at a dose
of 200-300 mg/kg bw was partially or completely metabolized, and the
radiolabelled products identified in the urine included glutamic acid,
lactic acid, beta-ketoadipic acid, and citric acid. The presence of
the beta-oxidation metabolite beta-ketoadipic acid indicates that
adipic acid participates in beta-oxidation in the fatty acid pathway
(Rusoff et al., 1960).
The linear and branched-chain aliphatic primary alcohol
components would be oxidized in the presence of alcohol dehydrogenase
to their corresponding aldehydes which, in turn, would be oxidized to
their corresponding carboxylic acids (Bosron & Ting-Kai, 1980; Levi &
Hodgson, 1989; Feldman & Weiner, 1972). The resulting carboxylic acids
would be metabolized in the fatty acid pathway and tricarboxylic acid
cycle (Voet & Voet, 1990). Branched-chain diols or keto alcohols may
undergo oxidation to their corresponding aldehydes and carboxylic
acid, which would be further metabolized or excreted.
2.4 Toxicological studies
2.4.1 Acute toxicity
The available data on this group of aliphatic primary alcohols,
aldehydes, carboxylic acids, acetals, and esters which contain
additional oxygenated functional groups demonstrate that they have
little acute toxicity when given orally. Oral LD50 values have been
reported for 29 of the 47 substances in the group; these range from
1628 to > 34 000 mg/kg bw in male and female rats and from 1900 to >
31 000 mg/kg bw in male and female mice (Smyth et al., 1949, 1951;
Smith, 1953; Smyth et al., 1954; Horn et al., 1957; Finkelstein &
Gold, 1959; Wolven & Leverstein, 1962; Jenner et al., 1964;
Levenstein, 1969; Smyth et al., 1969; Hart & Wong, 1971; Levenstein,
1973; Moreno, 1973; Pellmont, 1973; Shelanski & Moldovan, 1973;
Lawrence et al., 1974; Moreno, 1976, 1977; Vernot et al., 1977;
Moreno, 1978; Pellmont, 1978; Moreno et al., 1979; Moreno, 1980;
Levenstein, 1981; Hoechst, 1995).
2.4.2 Short-term and long-term studies of toxicity
The results of short-term and long-term studies of the toxicity
of the substances in this group are shown in Table 3. Details of the
studies which were critical to the evaluation of the safety of
tartaric acid and adipic acid are given below.
2.4.2.1 Tartaric acid (No. 621)
Rats
The toxicity of fumaric, tartaric, oxalic, and maleic acids was
compared in groups of 12 weanling Osborne-Mendel rats of each sex,
with 24 of each sex in the control group. The animals were given diets
containing tartaric or fumaric acid at concentrations of 0, 0.1, 0.5,
0.8, or 1.2%, equivalent to 100, 500, 800, or 1200 mg/kg bw per day.
The mortality rates in treated groups were not different from those of
controls, and there was no statistically significant difference in
body-weight gain or weekly food consumption. Necropsy performed on
most animals at two years did not reveal any macroscopic changes.
Histopathological examination of a wide range of tissues revealed no
treatment-related changes. The NOEL was 1200 mg/kg bw per day
(Fitzhugh & Nelson, 1947).
Rabbits
In a study of the toxicity of citric, fumaric, and tartaric
acids, 15 New Zealand rabbits (sex not specified) weighing 1-3 kg were
given the sodium salt of tartaric acid in the diet at a concentration
of 7.7% for 150 days, equivalent to 2300 mg/kg bw per day. A control
group was fed ground diet alone. Each animal was examined daily, and
food intake and body weights were determined weekly. Haematological
and urinary analyses were performed after 60 days of treatment on five
treated and six control rabbits. Two animals were examined grossly 30
days after treatment, and one animal was examined after 60 days. The
testis was examined histologically. At 100 days, half of the surviving
rabbits were examined grossly, and the liver, kidney, and testis were
examined microscopically. At the end of the study at 150 days, all
animals were killed and examined grossly and histologically.
Haematological and urinary analyses showed no changes. No significant
gross or histopathological changes attributable to tartaric acid were
observed (Packman et al., 1963).
Table 3. Results of short-term and long-term studies of the toxicity of aliphatic
primary alcohols, aldehydes, carboxylic acids, acetals, and esters with additional
oxygenated functional groups
No. Substance Species Sex No. test Route Duration NOEL Reference
groupsa/no. (mg/kg bw
per test per day)
groupb
595 Ethyl acetoacetate Rat M/F 3/32 Diet 28-29 days 300 Cook et al. (1992)
606 Laevulinic acid Rat NR 2/3 Diet 16 days 1000 Tischer et al. (1942)
611 Hydroxycitronellal Rat M/F 2/20, 2/60 Diet 2 years 250 Bar & Griepentrog
(1967)
614 Diethyl malonate Rat M/F 2/20 Diet 13 weeks < 500c,d Posternak (1964)
614 Diethyl malonate Rat M/F 2/20-32 Diet 90 days 406 Posternak et al.
(1969)
618 Fumaric acid Rat M/F 8/12 Diet 2 years 1200 Fitzhugh & Nelson
(1947)
618 Fumaric acid Rat NR 2/14, 14/20 Diet 2 years 1380 Levey et al. (1946)
618 Fumaric acid Guinea-pig M/F NR Diet 1 year 400 Levey et al. (1946)
618 Fumaric acide Rabbit NR 3/15 Diet 150 days 2070 Packman et al. (1963)
621 Tartaric acid Rat M/F 8/12 Diet 2 years 1200 Fitzhugh & Nelson
(1947)
621 Tartaric acide Rabbit NR 3/15 Diet 150 days 2300c Packman et al.
(1963)
621 Tartaric acid Dog NR 1/4 Oral 90-114 days < 990c Krop et al. (1945)
Table 3. (continued)
No. Substance Species Sex No. test Route Duration NOEL Reference
groupsa/no. (mg/kg bw
per test per day)
groupb
624 Diethyl sebacate Rat M/F 2/10 Diet 17-18 or 1000 Hagan et al. (1967)
27-28
weeks
625 Dibutyl sebacate Rat M 4/10 Diet 1 year 1250 Smith (1953)
625 Dibutyl sebacate Rat M 5/16 Diet 2 years 6250 Smith (1953)
629 Triethyl citrate Rat M/F 3/7 Diet 2 months 4000 Finkelstein
& Gold (1959)
629 Triethyl citrate Cat NR 1/6 Gavage 2 months < 285 Finkelstein
& Gold (1959)
630 Tributyl Rat M/F 2/4 Diet 2 months 5000 Finkelstein
acetylcitrate & Gold (1959)
630 Tributyl Cat NR 1/2 Gavage 2 months < 5700c Finkelstein
acetylcitrate & Gold (1959)
M, male; F, female; NR, not reported
a Number of test groups does not include controls.
b Number per test group comprises male and female animals.
c Only one dose tested
d Changes in relative liver weight and glomerular and renal tubular histological appearance observed
e Administered as the sodium salt
Dogs
As part of a comparison of the toxicity of hydroxyacetic acid,
citric acid, and tartaric acid, four dogs (sex not specified) received
tartaric acid daily in a gelatin capsule at a dose of 990 mg/kg bw per
day for periods of 90 to 114 days. The changes in body weight varied
from a 30% gain to a 32% loss. Haematological and urinary parameters
were examined. Urinary casts (gelled protein) were observed in all
dogs and were graded as hyaline (clear) in three dogs. Blood chemical
parameters remained normal except in one dog which showed azotaemia
(increased concentrations of urea in the blood) and died at 90 days,
according to the authors due to nephrotoxicity. There was no NOEL
(Krop et al., 1945).
2.4.2.2 Diethyl sebacate (No. 624)
Rats
In a study of the toxicity of about 50 flavouring agents, groups
of five weanling Osborne-Mendel rats of each sex were fed diethyl
sebacate (referred to in the paper as ethyl sebacate) at a dietary
concentration of 1000 mg/kg for 27-28 weeks or 10 000 mg/kg for 17-18
weeks, equivalent to 100 and 1000 mg/kg bw per day. A group of 10
males and 10 females served as controls. Body weights, food intake,
and general condition were recorded weekly, and haematological
examinations were performed at the end of the study. All tissues were
examined grossly at necropsy. The livers, kidneys, spleens, hearts,
and testes from six controls and eight animals at the high dose,
evenly divided by sex, were weighed and examined microscopically.
There was no difference in growth rate or food consumption between
test and control animals, and haematological examination revealed
normal values. No macroscopic or microscopic changes were observed in
the tissues. The NOEL was 1000 mg/kg bw per day (Hagan et al., 1967).
2.4.2.3 Dibutyl sebacate (No. 625)
Rats
Groups of 10 male Sprague-Dawley rats, five weeks old, were fed
dibutyl sebacate at dietary concentrations of 0, 0.01, 0.05, 0.25, or
1.25%, equivalent to 0, 10, 50, 250, and 1250 mg/kg bw per day, for
one year. Body weight and food intake were measured periodically
throughout the study. Measurement of haematological parameters and
microscopic examination at necropsy revealed no adverse effects
(Smith, 1953).
Groups of 16 five-to six-week-old male Sprague-Dawley rats were
given dibutyl sebacate in the diet at concentrations of 0 (two control
groups), 0.01, 0.05, 0.25, 1.25, or 6.25%, equivalent to 0, 10, 50,
250, 1250, and 6250 mg/kg bw per day, for two years. Administration of
dibutyl sebacate did not adversely affect the growth or survival of
the animals. Body weight and food intake were measured periodically
throughout the study. Measurement of haematological parameters and
microscopic examination at necropsy revealed no adverse effects. The
lesions observed in older control and treated rats at necropsy
included inflammatory changes in the lungs, enlarged and discoloured
kidneys, and fatty changes in the liver. The incidence of these gross
lesions was not considered to be associated with the administration of
dibutyl sebacate. The NOEL was 6250 mg/kg bw per day (Smith, 1953).
2.4.4 Genotoxicity
The results of tests for the genotoxicity of substances in this
group are shown in Table 4.
2.4.5 Other relevant studies
2.4.5.1 Adipic acid (No. 623)
In a study of teratogenicity, groups of 20-24 pregnant rats were
given adipic acid by oral intubation on days 6-15 of gestation at
doses of 0, 3, 13, 62, or 288 mg/kg bw per day. A sixth group of 24
pregnant females was given aspirin at a dose of 250 mg/kg bw per day
as a positive control. The maternal parameters evaluated included
clinical signs of toxicity, body weight, and food consumption. The
fetuses were removed surgically from all dams on day 20. The numbers
of implantation sites, resorption sites, and live births were counted,
and the body weights of live pups and external, visceral, and skeletal
abnormalities were evaluated. Administration of adipic acid had no
adverse effect on the maternal parameters evaluated, nor did it
adversely affect fetal survival or the number of abnormalities in soft
or skeletal tissues (Morgareidge, 1973).
In a study of potential peroxisome proliferation, male Fischer
344 rats were fed adipic acid at a dietary concentration of 2%,
equivalent to about 2000 mg/kg bw per day, for three weeks. Control
animals received powdered Purina rat chow alone. No effect on hepatic
peroxisomes or their associated enzymes was observed in treated
animals (Moody & Reddy, 1978).
2.4.5.2 Tartaric acid (No. 621)
The potential immunotoxicity of tartaric acid was evaluated in a
rapid screening protocol in which groups of 10-20 female CD1 or
B6C3F1 mice were given the material orally at doses up to 3000 mg/kg
bw per day (doses not specified) for five days. A group of control
animals was also evaluated. The animals received an infectious
challenge on day 3 of dosing and immunization on day 5, and the
antibody plaque-forming cell response was measured four days later.
Deaths and survival were monitored for 10 days after infection. There
were no statistically significant differences in spleen weight, thymus
weight, spleen cellularity, anti-sheep red blood cell or
plaque-forming cell response, or death due to Listeria infection
between test and control animals (Vollmuth et al., 1989).
Table 4. Results of studies of the genotoxicity of aliphatic primary alcohols, aldehydes, carboxylic acids,
acetals, and esters with additional oxygenated functional groups
No. Substance End-point Test system Concentration Results Reference
595 Ethyl acetoacetate Gene mutation B. subtilis 20 mg/disc Negative Oda et al.
H17, M45 rec+/- (1978)
595 Ethyl acetoacetate Gene mutation B. subtilis 20 ml/disc Positive Yoo (1986)
H17, M45 rec+/-
595 Ethyl acetoacetate Gene mutation E. coli WP2 uvrA 25-320 mg/plate Positive Yoo (1986)
595 Ethyl acetoacetate Gene mutation B. subtilis 10-20 ml/ml Weakly Kuroda et al.
H17, M45 rec+/- positive (1984)
(test tube)
595 Ethyl acetoacetate Chromosomal Chinese hamster 2 mg/ml Negative Ishidate et al.
aberration cells (1984)
595 Ethyl acetoacetate Gene mutation S. typhimurium 25 mg/plate Negativea Ishidate et al.
TA92, TA1535, TA100, (1984)
TA1537, TA94, TA98
(preincubation
protocol)
595 Ethyl acetoacetate Gene mutation S. typhimurium TA97, 0.01-10 mg/plate Negativea Fujita & Sasaki
TA102 (preincubation (1987)
protocol)
610 Hydroxycitronellol Gene mutation S. typhimurium TA1535, 3.6 mg/plate Negativea Wild et al.
TA100, TA1537, TA1538, (1983)
TA98
610 Hydroxycitronellol Micronucleus Mouse 1204 mg/kg bw Negative Wild et al.
formation (1983)
610 Hydroxycitronellol Gene mutation D. melanogaster 10 mmol/L Negative Wild et al.
(1983)
611 Hydroxycitronellal Gene mutation S. typhimurium TA1535, 3.6 mg/plate Negativea Wild et al.
TA100, TA1537, TA1538, (1983)
TA98
Table 4. (continued)
No. Substance End-point Test system Concentration Results Reference
611 Hydoxycitronellal Micronucleus Mouse 861 mg/kg bw Negative Wild et al.
formation (1983)
611 Hydoxycitronellal Gene mutation D. melanogaster 37 mmol/L Negative Wild et al.
(1983)
612 Hydroxycitronellal Gene mutation S. typhimurium TA1535, 3.6 mg/plate Negativea Wild et al.
dimethyl acetal TA100, TA1537, TA1538, (1983)
TA98
612 Hydroxycitronellal Micronucleus Mouse 763 mg/kg bw Negative Wild et al.
dimethyl acetal formation (1983)
612 Hydroxycitronellal Gene mutation D. melanogaster 25 mmol/L Negative Wild et al.
dimethyl acetal (1983)
614 Diethyl malonate Gene mutation S. typhimurium TA98, 3 mmol/plate Negativea Florin et al.
TA100, TA1535, TA1537 (480 mg/plate)b (1980)
616 Dimethyl succinate Gene mutation S. typhimurium TA100, 20 000 mg/plate Negativea Andersen & Jensen
TA1535, TA1537, TA98 (1984)
616 Dimethyl succinate Gene mutation S. typhimurium TA97, 10 mg/plate Negativea Zeiger et al.
TA98, TA102, TA104, (1992)
TA1535, TA1538
618 Fumaric acid Gene mutation S. typhimurium TA100 1000 mg/plate Negativea Rapson et al.
(1980)
618 Fumaric acid Gene mutation S. typhimurium TA98, 2000 mg/plate Negative Zeiger et al.
TA100, TA1535, TA97 (1988)
(preincubation
protocol)
619 (-)-Malic acid Gene mutation S. typhimurium TA97, 2000 mg/plate Negativea Al-Ani & Al-Lami
TA98, TA100, TA104 (1988)
Table 4. (continued)
No. Substance End-point Test system Concentration Results Reference
623 Adipic acid Gene mutation E. coli WP2 uvrA 5000 mg/plate Negativea Shimizu et al.
(1985)
623 Adipic acid Gene mutation S. typhimurium TA100, 5000 mg/plate Negativea Shimizu et al.
TA98, (1985)
623 Adipic acid Gene mutation D. melanogaster 4000 ppm Negative Ramel & Magnusson
(1979)
625 Dibutyl sebacate Gene mutation S. typhimurium TA1535, 3.6 mg/plate Negativea Wild et al.
TA100, TA1537, TA1538, (1983)
TA98
625 Dibutyl sebacate Micronucleus Mouse 2829 mg/kg bw Negative Wild et al.
formation (1983)
625 Dibutyl sebacate Gene mutation D. melanogaster 19 mmol/L Negative Wild et al.
(1983)
626 Ethylene brassylate Gene mutation S. typhimurium TA1535, 3.6 mg/plate Negativea Wild et al.
TA100, TA1537, TA1538, (1983)
TA98
627 Aconitic acid Gene mutation S. typhimurium TA100, 20 000 mg/plate Negativea Andersen & Jensen
TA1535, TA1537, TA98 (1984)
a With and without metabolic activation
b Calculation based on relative molecular mass of 160.17
3. REFERENCES
Al-An, F.Y. & Al-Lami, S.K. (1988) Absence of mutagenic activity of
acidity regulators in the Ames Salmonella/microsome test. Mutat.
Res., 206, 467-470.
Anders, M.W. (1989) Biotransformation and bioactivation of xenobiotics
by the kidney. In: Hutson, D.H., Caldwell, J., & Paulson, G.D., eds,
Intermediary Xenobiotic Metabolism in Animals, New York: Taylor &
Francis, pp. 81-97.
Andersen, P.H. & Jensen, N.J. (1984) Mutagenic investigation of
flavourings: Dimethyl succinate, ethyl pyruvate and aconitic acid are
negative in the Salmonella/mammalian-microsome test. Food Addit.
Contam., 1, 283-288.
Bar, V.F. & Griepentrog, F. (1967) Where we stand concerning the
evaluation of flavouring substances from the viewpoint of health.
Med. Ernahr., 8, 244-251.
Bosron, W.F. & Ting-Kai, L. (1980) Alcohol dehydrogenase. In: Jacoby,
W.B., ed., Enzymatic Basis of Detoxification, Vol. 1, New York:
Academic Press, pp. 231-248.
Cook, W.M., Purchase, R., Ford, G.P., Creasy, D.M., Brantom, P.G. &
Gangolli, S.D. (1992) A 28-day feeding study with ethyl acetoacetate
in rats. Food Chem. Toxicol., 30, 567-573.
Cramer, G.M., Ford, R.A. & Hall, R.L. (1978) Estimation of toxic
hazard: A decision tree approach. Food Cosmet. Toxicol., 16,
255-276.
Daniel, J.W. (1969) The metabolism of l-and dl-malic acids by rats.
Food Cosmet. Toxicol., 7, 103-106.
Feldman, R.I. & Weiner, H. (1972) Horse liver aldehyde dehydrogenase.
I. Purification and characterization. J. Biol. Chem., 247, 260-266.
Finkelstein, M. & Gold, H. (1959) Toxicology of the citric acid
esters: Tributyl citrate, acetyltributyl citrate, triethyl citrate,
and acetyltriethyl citrate. Toxicol. Appl. Pharmacol., 1, 283-298.
Fitzhugh, O.G. & Nelson, A. (1947) The comparative chronic toxicities
of fumaric, tartaric, oxalic, and maleic acids. J. Am. Pharm.
Assoc., 36, 217-219.
Florin, I., Rutberg, L., Curvall, M. & Enzell, C.R. (1980) Screening
of tobacco smoke constituents for mutagenicity using the Ames' test.
Toxicologist, 15, 219-232.
Fujita, H. & Sasaki, M. (1987) Mutagenicity test of food additives
with Salmonella typhimurium TA97 and TA102. II. Ann. Rep. Tokyo
Metr. Res. Lab. Public Health, 38, 423-430.
Hagan, E.C., Hansen, W.H., Fitzhugh, O.G., Jenner, P.M., Jones, W.I.,
Taylor, J.M., Long, E.L., Nelson, A.A. & Brouwer, J.B. (1967) Food
flavourings and compounds of related structure. II. Subacute and
chronic toxicity. Food Cosmet. Toxicol., 5, 141-157.
Hart, E.R. & Wong, L.C.K. (1971) Acute oral toxicity studies in rats,
acute dermal toxicity and primary skin irritation studies in rabbits
of fragrance materials. Unpublished report by Bionetics Research
Laboratories. Submitted to WHO by the Flavor and Extract
Manufacturers' Association.
Heymann, E. (1980) Carboxylesterases and amidases. In: Jacoby, W.B.,
ed., Enzymatic Basis of Detoxication, 2nd Ed., New York, Academic
Press, pp. 291-323.
Hise,r M.F., Markley, B.J., Reitz, R.H. & Nieusma, J.L. (1992)
Metabolism and disposition of acetyl tributyl citrate in male
Sprague-Dawley rats. Toxicologist, 12, 161.
Hoechst (1995) Material safety data sheet for 3-hydroxy-2-oxopropionic
acid. Unpublished document submitted to WHO by the Flavor and Extract
Manufacturers' Association.
Horn, H.J., Holland, E.G. & Hazleton, L.W. (1957) Safety of adipic
acid as compared with citric and tartaric acid. J. Agric. Food
Chem., 5, 759-762.
International Organization of the Flavour Industry (1975) European
inquiry on volume of use. Unpublished report submitted to WHO by the
Flavor and Extract Manufacturers' Association.
Ishida, R., Toyota, M. & Asakawa, Y. (1989) Terpenoid
biotransformation in mammals. V. Metabolism of (+)-citronellal,
(+/-)-7-hydroxycitronellal, citral, (-)-perillaldehyde, (-)-myrtenal,
cuminaldehyde, thujone, and (+/-)-carvone in rabbits. Xenobiotica,
19, 843-855.
Ishidate, M., Jr, Sofuni, T., Yoshikawa, K., Hayashi, M., Nohmi, T.,
Sawada, M. & Matsu, A. (1984) Primary mutagenicity screening of food
additives currently used in Japan. Food Chem. Toxicol., 22, 623-636.
Jenner, P.M., Hagan, E.C., Taylor, J.M., Cook, E.L. & Fitzhugh, O.G.
(1964) Food flavourings and compounds of related structure. I. Acute
oral toxicity. Food Cosmet. Toxicol., 2, 327-343.
Krebs, H.A., Salvin, E. & Johnson, W.A. (1938) The formation of citric
acid and alpha-ketoglutaric acids in the mammalian body. Biochem. J.,
32, 113-117.
Krop, S., Gold, H. & Paterno, C.A. (1945) On the toxicity of
hydroxyacetic acid after prolonged administration: Comparison with its
sodium salt and citric and tartaric acids. J. Am. Pharm. Assoc., 24,
86-89.
Kuroda, K., Tanaka, S., Yu, Y.S. & Ishibashi, T. (1984) Rec-assay of
food additives. Nippon Kosnu Eisei Zasshi, 31, 277-281.
Lawrence, W.H., Malik, M., & Autian, J. (1974) Development of a
toxicity evaluation program for dental materials. II. Screening for
systemic toxicity. J. Biomed. Mater. Res., 8, 11-34.
Lee, C.R. (1977) Evidence for the beta-oxidation of orally
administered 4-hydroxybutyrate in humans. Biochem. Med., 17, 284-291.
Leegwater, D.C. & Van Straten, S. (1979) in vitro digestion test on
methyl-2-keto-3-methyl valerate. Unpublished report from Central
Institute for Nutrition and Food Research. Submitted to WHO by the
Flavor and Extract Manufacturers' Association.
Levenstein, I. (1969) Acute oral toxicity reports on rats. Unpublished
report from Leberco Laboratories. Submitted to WHO by the Flavor and
Extract Manufacturers' Association.
Levenstein, I. (1973) Acute oral toxicity reports on rats. Unpublished
report from Leberco Laboratories. Submtted to WHO by the Flavor and
Extract Manufacturers' Association.
Levenstein, I. (1981) Acute oral toxicity reports on rats. Unpublished
report from Leberco Laboratories. Submtted to WHO by the Flavor and
Extract Manufacturers' Association.
Levey, S., Lasichak, A.G., Brimi, R., Orten, J.M., Smyth, C.J. &
Smith, A.H. (1946) A study to determine the toxicity of fumaric acid.
J. Am. Pharm. Assoc., 35, 298-304.
Levi, E. & Hodgson, E. (1989) Metabolites resulting from oxidative and
reductive processes. In: Hutson, D.H., Caldwell, J. & Paulson, G.D.,
eds, Intermediary Xenobiotic Metabolism in Animals, London: Taylor &
Francis, pp. 119-138.
Maarse, C.A. Visscher, L.C., Willemsens, L.M., Nijssen, M.H. &
Boelens, M.H., eds (1994) Volatile Components in Food, 6th Ed.,
Suppl. 5, Zeist: TNO Nutrition and Food Research.
Marshall, L.M., Orten, J.M. & Smith, A.H. (1949) The determination of
fumaric acid in animal tissues by partition chromatography. J. Biol.
Chem., 179, 1127-1139.
Moody, D.E. & Reddy, J.K. (1978) Hepatic peroxisome (microbody)
proliferation in rats fed plasticizers and related compounds.
Toxicol. Appl. Pharmacol., 45, 497-504.
Moreno, O.M. (1973) Acute toxicity studies on rats and rabbits.
Unpublished report from MB Research Laboratories. Submtted to WHO by
the Flavor and Extract Manufacturers' Association.
Moreno, O.M. (1976) Acute toxicity studies in rats, mice, rabbits and
guinea pigs. Unpublished report from MB Research Laboratories.
Submtted to WHO by the Flavor and Extract Manufacturers' Association.
Moreno, O.M. (1977) Acute toxicity study in rats, rabbits and guinea
pigs. Unpublished report from MB Research Laboratories. Submtted to
WHO by the Flavor and Extract Manufacturers' Association.
Moreno, O.M. (1978) Acute, toxicity studies in rats, mice, rabbits and
guinea pigs. Unpublished report from MB Research Laboratories.
Submtted to WHO by the Flavor and Extract Manufacturers' Association.
Moreno, O.M. (1980) Acute toxicity studies. Unpublished report from MB
Research Laboratories. Submtted to WHO by the Flavor and Extract
Manufacturers' Association.
Moreno, O.M., Moreno, M.T. & Altenbach, E.J. (1979) Acute oral
toxicity study in rats with methyl-2-oxo-3-methylpentanoate.
Unpublished report from MB Research Laboratories. Submtted to WHO by
the Flavor and Extract Manufacturers' Association.
Morgareidge, K. (1962a) in vitro digestion of four lactones.
Unpublished report from the Food and Drug Research Laboratories.
Submitted to WHO by the Flavor and Extract Manufacturers' Association.
Morgareidge, K. (1962b) in vitro digestion of four acetals.
Unpublished report from the Food and Drug Research Laboratories.
Submitted to WHO by the Flavor and Extract Manufacturers' Association.
Morgareidge, K. (1973) Teratologic evaluation of adipic acid in rats.
Unpublished report from the Food and Drug Research Laboratories.
Submitted to WHO by the Flavor and Extract Manufacturers' Association.
National Academy of Sciences (1989) 1987 Poundage and technical
effects update of substances added to food. Washington DC: Committee
on Food Additive Survey Data.
Oda, Y., Hamono, Y., Inoue, K., Yamamoto, H., Niihara, T. & Kunita, N.
(1978) Mutagenicity of food flavours in bacteria. Shokuhin Eisei
Hen, 9, 177-181.
Osteux, R. & Laturaze, J. (1954) Paper chromatography of the organic
acids found in urine. C.R. Acad. Sci. (Paris), 239, 512-513.
Packman, E.W., Abbott, D.D. & Harrisson, W.E. (1963) Comparative
subacute toxicity for rabbits of citric, fumaric, and tartaric acids.
Toxicol. Appl. Pharmacol., 5, 163-167.
Pellmont, B. (1973) Acute oral toxicity of ethyl-3-oxohexanoate.
Unpublished report. Submitted to WHO by the Flavor and Extract
Manufacturers' Association.
Pellmont, B. (1978) Acute oral toxicity in mice with
methyl-2-hydroxy-4-methyl-pentanoate. Unpublished report. Submtted to
WHO by the Flavor and Extract Manufacturers' Association.
Posternak, J.M. (1964) Diethyl malonate. Unpublished report from
Firmenich & Co. Submtted to WHO by the Flavor and Extract
Manufacturers' Association.
Posternak, J.M., Linder, A. & Vodoz, C.A. (1969) Summaries of
toxicological data. Toxicological tests on flavouring matters. Food
Cosmet. Toxicol., 7, 405-407.
Ramel, C. & Magnusson, J. (1979) Chemical induction of nondisjunction
in Drosophila. Environ. Health Perspectives, 31, 59-66.
Rapson, W.H., Nazar, M.A. & Butsky, V.V. (1980) Mutagenicity produced
by aqueous chlorination of organic compounds. Bull. Environ. Contam.
Toxicol., 24, 590-596.
Rusoff, I.I., Balldwin, R.R., Dominues, F.J., Monder, C., Ohan, W.J. &
Thiessen, R., Jr (1960) Intermediary metabolism of adipic acid.
Toxicol. Appl. Pharmacol., 2, 316-330.
Shelanski, M.V. & Moldovan, M. (1973) Acute oral and dermal toxicity
studies. Unpublished report from Food and Drug Research Laboratories.
Submtted to WHO by the Flavor and Extract Manufacturers' Association.
Shimizu, H., Suzuki, Y., Takemura, N., Goto, S. & Matsushita, H.
(1985) The results of microbial mutation test for forty-three
industrial chemicals. Jpn. J. Ind. Health, 27, 400-419.
Simola, P.E. & Krusius, F.E. (1938) The formation of ketoglutaric acid
in animal metabolism. Suomen Kemistilehti, 11, B-9.
Smith, C.C. (1953) Toxicity of butyl stearate, dibutyl sebacate,
dibutyl phthalate, and methoxyethyl oleate. Arch. Ind. Hyg. Occup.
Med., 7, 310-318.
Smyth, H.F., Carpenter, C.P. & Weil, C.S. (1949) Range-finding
toxicity data. List III. J. Ind. Hyg. Toxicol., 31, 60-62.
Smyth, H.F., Carpenter, C.P. & Weil, C.S. (1951) Range-finding
toxicity data. List IV. Arch. Ind. Hyg. Occup. Med., 4, 119-122.
Smyth, H.F., Carpenter, C.P., Weil, C.S. & Pozzani, U.C. (1954)
Range-finding toxicity data. List V. Arch. Ind. Hyg., 10, 61-68.
Smyth, H.F., Carpenter, C.P., Weil, C.S., Pozzani, V.C., Striegel,
J.A. & Nycum, J.S. (1969) Range-finding toxicity data. List VII. Am.
Ind. Hyg. Ass. J., 30, 470-476.
Tischer, R.G., Fellers, C.R. & Doyle, B.J. (1942) The non-toxicity of
levulinic acid. J. Am. Pharm. Assoc., 31, 217-220.
Vernot, E.H., MacEwen, J.D., Huan, C.C. & Kinkead, E.R. (1977) Acute
toxicity and skin corrosion data for some organic and inorganic
compounds and aqueous solutions. Toxicol. Appl. Pharmacol., 42,
417-423.
Voet, D. & Voet, J.G., eds (1990) Biochemistry, New York: John Wiley
& Sons, pp. 506-527, 632-633, 690.
Vollmuth, T.A., Heck, J.D., Ratajczak, H.V. & Thomas, P.T. (1989)
Immunotoxicity assessment of flavouring ingredients using a rapid and
economical screen. Toxicologist, 9, 206.
Wild, D., King, M.T., Gocke, E. & Eckhardt, K. (1983) Study of
artificial flavouring substances for mutagenicity in the
Salmonella/microsome, basc and micronucleus test. Food Chem.
Toxicol., 21, 707-719.
Williams, R.T., ed. (1959) Detoxication Mechanisms. The Metabolism
and Detoxication of Drugs, Toxic Substances, and Other Organic
Compounds, 2nd Ed., London: Chapman & Hall, pp. 119-120.
Wolven, A. & Leverstein, I (1962) Acute oral toxicity study of diethyl
malonate in mice. Unpublished report from Givaudan Corporation.
Submitted to WHO by the Flavor and Extract Manufacturers' Association.
Yoo, Y.S. (1986) Mutagenic and antimutagenic activities of flavouring
agents used in foodstuffs. J. Osaka City Med. Center, 34, 267-288.
Zeiger, E., Anderson, B., Haworth, S., Lawlor, T. & Mortelmans, K.
(1988) Salmonella mutagenicity tests: IV. Results from the testing of
300 chemicals. Environ. Mol. Mutag., 11 (Suppl. 12), 1-158.
Zeiger, E., Anderson, B., Haworth, S, Lawlor, T. & Mortelmans, K.
(1992) Salmonella mutagenicity tests: V. Results from the testing of
311 chemicals. Environ. Mol. Mutag., 19 (Suppl. 21), 2-141.
