
First draft prepared by
D. Bellinger1, M. Bolger2, R. Goyer3,L. Barraj4, and J. Baines5
1 Harvard Medical School, Boston, Massachusetts, USA
2 Food and Drug Administration, Washington DC, USA
3 University of Western Ontario, Canada
4Novigen Sciences Inc., Washington DC, USA
5Australia New Zealand Food Authority, Canberra, Australia
|
Estimates of the relationship of dietary intake of cadmium to tubular dysfunction |
Cadmium was evaluated by the Committee at its sixteenth, thirty-third, and forty-first meetings (Annex 1, references 30, 83, and 107). At its sixteenth meeting, the Committee allocated a provisional tolerable weekly intake (PTWI) of 400–500 µg of cadmium per person. At its thirty-third meeting, the Committee retained this PTWI but expressed it in terms of intake per kilogram of body weight (7 µg/kg bw). In 1992, the International Programme on Chemical Safety published a monograph on cadmium which provided a detailed review of the available information on the health effects of cadmium and a description of the models on which the PTWI was based (WHO, 1992). At its forty-first meeting, the Committee used the monograph as the basis for its evaluation and retained the PTWI of 7 µg/kg bw.
At its forty-first meeting, the Committee identified a number of unresolved issues related to the intake and health effects of cadmium in the general populations of various countries. These issues were the dose–response relationship between the daily or accumulative intake of cadmium and renal dysfunction (beta2-microglobulinuria) in the general population; re-examination of the existing epidemiological information correlating cadmium intake and beta2-microglobulinuria among inhabitants of a cadmium-polluted region; examination of data on cadmium intake and its health effects in the general populations of various countries, including data on cadmium concentrations in foods; evaluation of the critical concentration of cadmium in the renal cortex in two groups exposed to high and low concentrations of cadmium; studies on the chemical identity and bioavailability of cadmium compounds in food; re-examination of mathematical models for estimating the biological half-life of cadmium; and studies on the involvement of the renal glomeruli in long-term intoxication with cadmium. At that meeting, the Committee also reaffirmed that ‘there is only a relatively small safety margin between exposure in the normal diet and exposure that produces deleterious effects’.
At its present meeting, the Committee based its evaluation on the IPCS monograph (WHO, 1992) and on updated information on intake, bioavailability, and health effects.
In mammals, cadmium is virtually absent at birth but accumulates with time, especially in the liver and kidneys. Rapid renal concentration occurs mainly during the early years of life (Henke et al., 1970), and 50–75% of the total body burden is found in these two organs.
(a) Absorption
The factors that affect the absorption of ingested cadmium include the animal species, type of cadmium compound, dose, frequency of administration, age or stage of development, pregnancy and lactation, presence or absence of drugs, nutritional status, and interactions with various nutrients. Studies in experimental animals have shown that 0.5–8% of cadmium nitrate or chloride is absorbed after a single exposure (Friberg et al., 1974). In humans given radioactive cadmium, the average amount absorbed is 5% (Flanagan et al., 1978).
Metallothioneins are metal-binding proteins of low relative molecular mass with a high content of cysteine residues which have a particular affinity for cadmium and can affect its toxicity. The regulation, degradation, and biological significance of mammalian metallothioneins has been reviewed in detail (Nordberg, 1998; Ono & Cherian, 1998; Miles et al., 2000). Those aspects relevant to the absorption, distribution, and metabolism of cadmium are summarized here and in the sections on nephropathy and effects on the nervous system.
Several functions have been assigned to metallothionein. Its synthesis may be induced by the essential metals zinc and copper, and it is involved in the storage of these metals. Zinc–metallothionein can detoxify free radicals. Administration of zinc and induction of metallothionein can inhibit the toxicity of agents such as carbon tetrachloride, ethanol, and ionizing radiation, which act in part through oxidative injury. Metallothionein is also induced by cadmium, and intracellular binding of cadmium to metallothionein protects against the toxicity of cadmium. Cadmium is transported in the plasma as a complex with metallothionein and may be toxic to the kidney when excreted in the glomerular filtrate. Most of the cadmium in urine is bound to metallothionein.
Metallothionein in the gastrointestinal mucosa might play a role in the gastrointestinal transport of cadmium. Its presence in cells of the placenta impairs the transport of cadmium from maternal to fetal blood and across the blood–brain barrier, but only when the concentration of cadmium in low. Newborns are virtually cadmium-free, whereas zinc and copper are readily supplied to the fetus. The concentrations of cadmium–metallothionein in blood are higher in pregnant rats exposed to cadmium than in unexposed rats, suggesting that absorption of cadmium, zinc, and copper from the gastrointestinal tract during pregnancy is increased.
Metallothionein occurs as at least four genetic variants or isomers in humans, I, II, III, and IV. The two major forms, I and II, are ubiquitous in most organs, particularly in liver and kidney but also in brain. The two forms have different isoelectric points but identical arrangements of cystinyl residues. Metallothionein isolated from adult or fetal human livers contained mainly zinc and copper, whereas that from human kidney contained zinc, copper, and cadmium. The metals are bound to the peptide by mercaptide bonds and arranged in two distinct clusters: a four-metal cluster called the alpha domain and a three-metal cluster called cluster beta at the C terminal of the protein. The alpha cluster is an obligate zinc cluster, whereas the zinc in cluster beta may be replaced by the essential metal copper or by the toxic metal cadmium. Interaction with metallothionein is the basis for metabolic interactions between these metals.
Metallothionein-III is found in human brain and differs from I and II by having six glutamic acid residues near the terminal part of the protein. Metallothionein-III is thought to be a growth inhibitory factor, and its expression is not regulated by metals; however, it does bind zinc. Its expression is down-regulated in Alzheimer disease. Another proposed role for metallothionein-III is participation in the utilization of zinc as a neuromodulator, since metallothionein-III is present in the neurons that store zinc in their terminal vesicles. Metallothionein-IV occurs during differentiation of stratified squamous epithelium, but it is not known to have a role in the absorption or toxicity of cadmium.
Cadmium bound to metallothionein in food does not appear to be absorbed or distributed in the same way as inorganic cadmium compounds (see WHO, 1992). Mice exposed to cadmium–metallothionein had lower concentrations of cadmium in blood and liver but a higher concentration in kidney than mice exposed to the same amount of cadmium chloride (Cherian et al., 1978). Although fishermen with an extremely high intake of cadmium from eating oysters had raised blood and urine cadmium concentrations, the increase was not as great as would have been expected from the amount of cadmium ingested (Sharma et al., 1983).
In mice, the degree of absorption depended on the age of the animal: young mice retained 10%, while adult mice retained only 1% (Matsusaka et al., 1972). A similar difference in absorption with age was found in neonatal mice, which absorbed cadmium to a much greater extent than adult mice (Engstrom & Nordberg, 1979).
Low dietary concentrations of calcium and protein promote absorption of cadmium from the intestinal tract of experimental animals (Friberg et al., 1975). A low iron status in laboratory animals and humans has also been shown to result in greater absorption of cadmium (Hamilton & Valberg, 1974; Flanagan et al., 1978). In particular, women with low body iron stores, as reflected by low serum ferritin concentrations, had an average gastrointestinal absorption rate twice as high as that of a control group of women, of the order of 10%.
Studies in rats showed that iron status is an important predictor of cadmium bioavailability. A high iron status resulted in decreased total and fractional cadmium accumulation from wheat endosperm and bran diets. In rats with reduced iron status, the inclusion of wheat bran in the diet increased cadmium uptake (Wing at al., 1992). Similar effects have been found in other studies of marginal iron status. The bioavailability of cadmium in rats can be increased significantly even in the absence of overt deficiency (Reeves, 2000).
Concomitant administration of zinc (20 mg/kg of diet as zinc sulfate) for 5 days and cadmium (10 mg/L as cadmium chloride in drinking-water) for 12 weeks to male rats did not mitigate the toxicity of cadmium (Flora et al., 1998).
Substitution of a mixed diet with a vegetarian diet for 3 months resulted in a tendency to increased elimination of cadmium in the faeces of 16 persons (Vahter et al., 1992). Cadmium intake and uptake from a vegetarian diet, a high-fibre diet and a normal mixed diet were measured over 4 days in two groups of nonsmoking women aged 20–50. No difference in the intake of nutrients was found, except that the high-fibre diet obviously had a higher fibre content and also a higher concentration of cadmium. No differences in blood or urinary cadmium concentrations were found between the two groups, which suggests that fibre had an inhibitory effect on cadmium absorption. The serum ferritin concentrations of 67% of the study population were below 30 µg/L, indicating reduced body iron stores, which were highly correlated with blood cadmium concentrations. This suggests that cadmium absorption is enhanced when the body iron stores are suboptimal. The blood cadmium concentrations mainly reflected long, light exposure, as the variation in blood cadmium concentration was accounted for by urinary cadmium, serum ferritin, age and fibre intake (Berglund et al. 1994). In a similar study, less cadmium was absorbed from a shellfish diet than from a mixed diet, even though the former containing twice as much cadmium as the latter. In persons on the mixed diet, serum ferritin was negatively correlated with blood cadmium when the serum ferritin concentrations were < 20 µg/L, indicating enhanced absorption when the iron status is suboptimal (Vahter et al., 1996).
The relative bioavailability of cadmium was investigated in female Sprague-Dawley rats which received diets in which rice was polluted with cadmium at a concentration of 0.02, 0.04, 0.12, or 1.1 mg/kg of diet or with cadmium chloride at 5.1, 20, or 40 mg of cadmium per kg of diet for up to 8 months. No cadmium-related toxic effects were observed. The concentrations of cadmium in the liver and kidney and of metallothionein in the liver, kidney, serum, and urine increased in a dose-dependent manner at 4 and 8 months, but the intestinal metallothionein concentrations remained constant during the same period. The rates of distribution of cadmium in the liver increased with dose, while those in the kidney decreased with dose. This finding probably reflects dose-dependent differences in the form of absorbed cadmium and tissue metallothionein storage capacity (Hiratsuka et al., 1990).
The gastrointestinal absorption and organ distribution of cadmium in mice was determined after 9 weeks of exposure to three fibre-rich foods: wheat bran, sugar-beet fibre, and carrots. The group given wheat bran had significantly less cadmium accumulation in the liver and kidneys than the other groups. The higher concentrations of inositol hexates and phytates and a higher zinc concentration were considered to be responsible for the differential absorption (Lind et al., 1998).
In rats fed sunflower kernels with concentrations of cadmium of 0.2–1.0 mg/kg, those fed marginal concentrations of dietary calcium absorbed 50% more cadmium than rats that were fed adequate concentrations of calcium. Rats given marginal dietary iron absorbed 160% more cadmium than those fed adequate iron. In contrast, the natural concentration of zinc in sunflower seeds which provided 90% of the rats’ nutritional requirement, was enough to deter excessive absorption of cadmium (Reeves, 2000).
The pattern of accumulation of cadmium in the liver and kidneys was investigated in female Sprague-Dawley rats fed a diet containing cadmium chloride at 8, 40, 200, or 800 mg/kg for 2 or 4 months. As expected, the cadmium concentrations in the two organs increased with dose; however, the renal concentrations plateaued between the two highest doses. In addition, the ratio of cadmium concentrations in the kidney and liver decreased with dose, suggesting preferential, dose-dependent distribution to the two organs. The parallel shift in the curves illustrating total cadmium intake to renal cadmium concentrations over time indicated that renal accumulation is fairly constant and small (Ando et al., 1998).
The intestinal absorption of cadmium was investigated in groups of 14 female rats which received diets containing 28% purified rice or 72% ordinary rice containing cadmium at 0.02, 0.04, 0.12, or 1.0 mg/kg of diet. At 1, 4, and 8 months, seven animals per group were killed for determination of the cadmium and metallothionein concentrations in the liver and kidneys and of metallothionein in the intestinal mucosa, serum, and urine. The remaining animals were given cadmium-109. No cadmium-related toxic effects were seen. The cadmium concentrations were dose-related, as were those of metallothionein, except in the intestinal mucosa. The rates of distribution to the liver increased with dose, whereas the renal concentrations decreased with dose. From 0.2 to 1% of cadmium-109 was found to have been absorbed (Ando, 2000).
Six groups of 50 Sprague-Dawley rats were given diets containing cadmium-polluted rice or cadmium chloride at concentrations up to 40 mg/kg of diet from 5 weeks of age until 2 years. The only toxic effects seen during the study were changes in mean corpuscular volume and mean corpuscular haemoglobin. As has been observed in other studies, dose-dependent increases in hepatic and renal accumulation of cadmium and metallothionein were noted. The maximum cadmium concentrations were 130 mg/kg in liver and 120 mg/kg in kidneys. The urinary cadmium concentrations became dose-dependent with time (Ando, 2000; Mitsumori & Ando, 2000).
An analysis of the results of studies of bioavailability in rodents indicated that: (1) the bioavailability of cadmium from drinking-water and food is similar; (2) since the presence of cadmium decreases both food and water consumption, its availability must be assessed from the actual doses rather than the concentrations administered; (3) the composition of the diet and gastrointestinal tract status are major determinants of the bioavailability of cadmium; and (4) studies of the effect of the composition of the total diet on bioavailability are more relevant than studies of the medium of exposure (Ruoff et al., 1994).
The bioavailability of cadmium was assessed in weanling male rats on two diets: one consisting of a purified rodent diet to which 20% fresh sunflower kernels containing cadmium at 330 or 780 µg/kg was added and the other consisting of a purified diet containing cadmium chloride. After 10 weeks, a test meal containing cadmium-109 was fed in order to assess absorption by whole-body counting techniques. The absorption of cadmium varied from 0.39 to 0.55%, and that from the kernels was 30% less than from the diet containing cadmium chloride. In addition, the concentrations of cadmum in tissues tended to be lower in the animals on the kernel diet (Reeves et al., 1994). In subsequent studies, the protocol was modified to include a sunflower-seed diet that contained a low concentration of cadmium and other diets containing a concentration of 195 mg/kg. The body burden of cadmium was increased twofold in those animals given the diet containing sunflower seeds. Labelling of the sunflowers had no effect on the amount of label detected in the liver and kidneys (Reeves & Vanderpool, 1998).
On the basis of a 7-day food diary, 66 men and women who ate sunflower seeds were divided into two groups: those who ate more and those who ate less than 1 oz (28 g) per week. The dietary intake of cadmium was assessed from dietary surveys; the body burden of cadmium from the concentrations in whole blood, erythrocytes, and urine; and renal function from the presence of N-acetyl-beta-glucosaminidase and beta2-microglobulin. The whole blood and erythrocyte concentrations of cadmium and urinary cadmium excretion were not affected by increased cadmium intake. The amounts of N-acetyl-beta-glucosaminidase and beta2-microglobulin in the urine were elevated with increased dietary intake of cadmium when the values were expressed on the basis of volume but not when expressed on the basis of creatinine (Reeves et al., 1997). In subsequent studies, healthy men and women aged 23–59 were divided into three groups of eight or nine persons. One group ate a sunflower-seed diet, the second a sunflower and peanut diet, and the third a peanut diet. The sunflower seed diet, which contained cadmium at 0.52 mg/kg, increased the dietary concentration of cadmium from 100 to 240 µg/week. The peanuts contained cadmium at 0.11 mg/kg. There was no evidence that increased cadmium intake resulted in increased cadmium body burdens or affected renal function, as measured by changes in N-acetyl-beta-glucosaminidase activity. Faecal cadmium excretion increased as a function of increased sunflower-seed consumption. The concentrations of cadmium in hair did not change with diet (Reeves & Vanderpool, 1997; Reeves, 2000).
Administration of the stable isotope, cadmium-113, to 14 female and four male volunteers aged 30–70 years, given a typical western diet low in cadmium, indicated that the bioavailability of cadmium from sunflower seeds is no different from that from other types of food. Faecal excretion was 15 µg/day. The apparent absorption of cadmium-113 was 11 ± 4.4% for women and 2.1 ± 4.3% for men. No significant correlation was found between cadmium absorption and serum ferritin concentration (Vanderpool & Reeves, 2000).
Dietary uptake of cadmium was assessed in 17 nonsmoking women, 20–50 years of age, who ate shellfish at least once a week. A control group of 34 women who ate a mixed diet with little shellfish was used. The shellfish diets, containing a median of 22 µg of cadmium per day, had twice as much cadmium as the control diets. Duplicate diet studies indicated that faecal excretion of cadmium represented 100% and 99% of the intake from the two diets, respectively. The concentrations of cadmium in blood were not statistically significantly different in the two groups, suggesting less absorption of cadmium by persons on the shellfish diet. However, greater absorption could have occurred, because they had a low iron status, as indicated by reduced serum ferritin concentrations. When the iron status was accounted for, the difference in the bioavailability of cadmium between the two groups diminished (Vahter et al., 1996).
(b) Distribution
Once absorbed, cadmium is transported in the blood, mainly in erythrocytes, and is bound intracellularly to protein fractions of low and high relative molecular mass (Nordberg, 1972). The fraction with a low relative molecular mass is similar to metallothionein. Plasma metallothionein has an important role in the transport of cadmium. It can contain up to 11% of cadmium by weight, bound to sulfhydryl groups (Elinder & Nordberg, 1985), and occurs in large quantities in the liver, particularly after exposure to cadmium. Metallothionein occurs in varying amounts in other tissues, such as the kidneys, and its concentration correlates with that of cadmium in these tissues. The low relative molecular mass of free metallothionein in plasma allows it to filter through the glomeruli and subsequently be reabsorbed in the proximal tubules, which in turn results in selective accumulation of cadmium in the renal cortex (Nordberg, 1972). Transport of cadmium bound to metallothionein from blood to renal tubular cells is rapid and virtually complete, while free cadmium is not taken up by the kidneys to a similar extent (Johnson & Foulkes, 1980).
While cadmium can reach the embryo or fetus early in gestation, little transfer occurs across the fully developed placenta (Ahokas & Dilts, 1979). Cadmium can induce metallothionein in the placenta, and the placenta retains cadmium after exposure to low concentrations. The essential elements zinc and copper are also bound to metallothionein in the placenta but, for reasons not understood, cadmium is retained and the essential metals are transported to the fetus (Goyer, 1995). The concentrations of cadmium in the organs of embryos, fetuses and neonates are three orders of magnitude lower than the corresponding concentrations in adult women (Chaube et al., 1973).
Long-term exposure to cadmium leads to selective accumulation in the liver and renal cortex, such that up to 75% of the total body burden is found in these organs (Friberg et al., 1985). The accumulation of cadmium in the liver and its subsequent redistribution to the kidney is due to efficient metallothionein synthesis in the liver; cadmium–metallothionein is slowly released into the plasma, filtered through the glomeruli and reabsorbed in the proximal tubules. After exposure to normal dietary concentrations of cadmium (10–30 µg/day), about 50% of the body burden is found in kidneys, about 15% in the liver, and about 20% in muscle (Kjellstrom, 1979). Lower concentrations are found in brain, bone, and fat (Sumino et al., 1975; Cherry, 1981). Accumulation in the kidneys continues up to 50–60 years of age and falls thereafter, possibly due to age-related changes in kidney function. The hepatic and renal concentrations may fall subsequent to renal damage and increased leakage of bound cadmium into the urine (Nomiyama et al., 1982). Differences in the population mean concentrations of cadmium in the renal cortex in various countries have been attributed to differences in daily dietary intake (Friberg et al., 1986).
(c) Excretion
Little cadmium is normally excreted in the urine. The rate of excretion increases slowly with increasing body burden, but, as renal dysfunction develops, it increases sharply and the hepatic and renal cadmium concentrations fall (Nordberg & Piscator, 1972; Nomiyama & Nomiyama, 1976). In the general population, the mean urinary cadmium concentration, mainly bound to metallothionein, ranges from < 0.5 to 2.0 pg/L, representing about 0.01% of the body burden, and increases with age (Nordberg & Strangert, 1976; Kowal et al., 1979). Estimates of biliary and gastrointestinal excretion after oral administration of cadmium are somewhat uncertain in that most faecal cadmium is unabsorbed material. The mechanism of faecal excretion may involve both sloughed mucosal cells and excretion in the bile. After an initial rapid phase, biliary excretion represents 0.02–0.04% of the body burden, and most is associated with a fraction of low relative molecular mass (Elinder & Pannone, 1979). After low or moderate doses, the amount excreted in the faeces is about the same as that excreted in urine. Minor routes of excretion include hair, breast milk, and pancreatic fluid, but collectively these routes make little contribution to the total excretion or biological half-time of cadmium. The slow excretion of cadmium results in extremely long biological half-times in animals, lasting from 200 days to 22 years (Friberg et al., 1985). The retention functions are multi-phasic, involving several compartments with different half-times. The half-time of the slowest compartment is usually greater than 20% of the life span of the animal.
The toxic effects of cadmium in food are largely related to long-term exposure to low doses. Acute effects may occur after ingestion of very high concentrations. Such effects are reviewed briefly to illustrate the differences in the solubility and absorption of various forms of cadmium, but the major emphasis is on effects of long-term oral intake. The effects of cadmium as documented in Environmental Health Criteria No. 134 (WHO, 1992) and more recent information are summarized below.
The LD50 for rats and mice treated orally ranges from about 100 to 300 mg/kg bw after a single gavage dose of cadmium chloride (Table 1, Agency for Toxic Substances and Disease Registry, 1999). One study (Kostial et al., 1978) suggested that immature mice may be more sensitive than mature mice or rats. Some deaths occurred at doses as low as 15 mg/kg bw administered continually in drinking-water for 10 days. Some of the differences between studies may due to differences in species.
Table 1. LD50 values for rats and mice given cadmium chloride orally
|
Strain, sex |
Exposure |
Dose |
Effect |
Reference |
|
Sprague-Dawley rats, male |
Gavage, once/day |
220 |
LD50 at 14 days |
Kotsonis & Klaassen (1977) |
|
Sprague-Dawley rats, male |
Water, twice/week |
42 |
7/9 died |
Kotsonis & Klaassen (1978) |
|
Rats (strain unspecified), 2-wk-old |
Gavage, once |
29 |
LD50 at 8 days |
Kostial et al. (1978) |
|
Sprague-Dawley rats |
Gavage, once |
15 |
1/10 males, 1/10 females died |
Borzelleca et al. (1989) |
|
|
Gavage, once; water, 10 days |
15 |
2/10 males, 1/10 females died |
|
|
Sprague-Dawley rats |
Gavage, once |
|
LD50 at 24 h |
Shimizu & Morita (1990) |
|
CBA/Bom mice, male |
Gavage, once |
30 |
2/54 died within 10 days |
Anderson et al. (1988) |
|
Swiss-Webster mice, male |
Gavage, once |
96 |
LD50 at 96 h |
Baer & Benson (1987) |
|
ICR mice |
Gavage, once |
110 |
5/10 died within 8 days |
Basinger et al. (1988) |
Modified from Agency for Toxic Substances and Disease Registry (1999)
Table 2 shows the LD50 values for different cadmium compounds administered by gavage or in drinking-water. Because the toxicity of cadmium compounds is believed to be attributable to the cadmium ion per se, the differences in LD50 values are considered to be due to differences in solubility or rate of dissolution and the availability of the cadmium ion.
Table 2. LD50 values for cadmium compounds in mice and rats treated by intragastric administration
|
Compound |
Relative molecular mass |
LD50 (confidence interval) (mg/kg bw) |
LD50 for cadmium ion (mg/kg bw) |
|
Mice |
|||
|
Cadmium |
112.4 |
890 (640–1200) |
890 |
|
Cadmium oxide |
128.4 |
72 (41–110) |
63 |
|
Cadmium sulfate |
208.5 |
88 (70–100) |
47 |
|
Cadmium chloride |
183.3 |
94 (76–110) |
57 |
|
Cadmium nitrate |
236.4 |
100 (79–120) |
48 |
|
Cadmium iodide |
366.2 |
170 (140–190) |
51 |
|
Cadmium caprylate |
394.8 |
300 (200–460) |
85 |
|
Cadmium carbonate |
172.4 |
310 (220–400) |
200 |
|
Cadmium stearate |
679.4 |
590 (560–620) |
98 |
|
Cadmium sulfide |
144.5 |
1200 (1100–1200) |
910 |
|
Cadmium sulfoselenide |
335.8 |
2400 (2400–2500) |
1600 |
|
Barium–cadmium stearate |
1383.7 |
3200 (2800–3600) |
260 |
|
Rats |
|||
|
Cadmium caprylate |
394.8 |
950 (610–1500) |
270 |
|
Cadmium stearate |
679.4 |
1200 (880–1600) |
200 |
|
Barium–cadmium stearate |
1383.7 |
2000 (1700–2200) |
160 |
Modified from WHO (1992)
Pathological effects resulting from a single, high, parenteral dose or a single exposure by inhalation are summarized here only briefly. WHO (1992) pointed out that the most marked effect in experimental animals given a single parenteral dose of cadmium at 2–4 mg/kg bw is testicular necrosis. This effect is probably the result of endothelial damage to small blood vessels followed by increased capillary permeability and haemorrhage. A similar effect occurred in the ovaries of prepubertal rats and in the ovaries of adult rats in persistent estrus.
Acute effects are also seen in other organs after a single high dose. Exposure to cadmium by inhalation at concentrations of 5–20 mg/m3 for 50–120 min caused pulmonary oedema in rats and rabbits. Parenteral administration at doses close to the LD50 had pronounced effects on small blood vessels, including those of the nervous system. Morphological effects were found in the livers of rats given a single parenteral dose of 3.9 mg/kg bw. The systemic effects may be fatal, and oral administration of a high dose of cadmium caused desquamation and necrosis of gastric and intestinal mucosa.
(a) Renal effects
The kidney is the critical organ in humans and other mammals exposed for long periods to the relatively small amounts of cadmium that might occur in foods. Many studies in experimental animals have demonstrated an association between morphological and/or functional changes in the kidney and the renal concentration of cadmium. In most of these studies, cadmium was given parenterally rather than in food or water. The many studies of this type performed before 1990 were reported in detail and tabulated by WHO (1992) and are only summarized here.
Long-term exposure to cadmium leads to pathological changes in the kidneys. The morphological changes are initially limited to proximal tubular epithelial cell degeneration, but this is followed by cellular atrophy, interstitial fibrosis, and glomerular sclerosis. The morphological changes are associated with biochemical evidence of renal tubular dysfunction, including the presence of proteins of low relative molecular mass in the urine (beta2-microglobulin and retinol-binding protein), glucosuria, aminoaciduria, hypercalciuria, enzymuria (N-acetyl-beta-glucosaminidase), and urinary concentration of cadmium and metallothionein (WHO, 1992; Agency for Toxic Substances and Disease Registry, 1999). The ultrastructural changes include increased numbers of lysosomes and swelling of mitochondria. In addition to the tubular changes, there have been reports of pathological changes in mesangial cells and increased thickening of the glomerular basement membrane.
Impaired glomerular filtration, seen as increases in serum creatinine and blood urea nitrogen concentrations, is a less sensitive indicator of cadmium-induced nephropathy than tubular dysfunction or injury. Uriu et al. (1998) found a decline in the glomerular filtration rate in rats treated parenterally with cadmium chloride, and the reduced rate was associated with a lowered filtration fraction, although this may be a functional effect of cadmium rather than a morphological effect. Nevertheless, continued exposure to cadmium results in progressive glomerular sclerosis with impairment of glomerular filtration.
Various hypotheses have been proposed to explain the nephrotoxicity of cadmium and particularly the role of the metal-binding protein metallothionein. It has been shown in experimental animals and in vitro that metallothionein protects against the toxicity of cadmium by binding to it to form a cadmium–metallothionein complex that is non-toxic when stored within cells. Renal-cell injury occurs when the critical concentration of cadmium is exceeded. The mechanism of this toxicity is unknown, but it has been suggested that an unidentified form of cadmium that is not bound to metallothionein becomes available (Goyer et al., 1989). Parenterally administered cadmium–metallothionein is very toxic to renal epithelial cells (Cherian et al., 1976; Goyer et al., 1984), and it has been suggested that cadmium–metallothionein in the glomerular filtrate injures renal cells as it excreted by the nephron. Hepatic metallothionein may be an important source of plasma metallothionein, and the critical factor in cadmium nephropathy may be the release of cadmium–metallothionein from the liver. Jin et al. (1987) reported that hypercalciuria developed in rats injected with cadmium–metallothionein.
Repeated injections of small amounts of cadmium–metallothionein to rats that had not been exposed to cadmium produced nephrotoxicity similar to that which occurs after long-term ingestion of cadmium (Wang et al., 1993). When the livers of rats exposed to cadmium were transplanted into normal rats, the plasma concentration of cadmium–metallothionein and nephrotoxicity increased in spite of low concentrations of renal cadmium (Chan et al., 1992). The irreversibility of proteinuria after cessation of exposure to cadmium, despite declining renal concentrations, may be due to continued exposure of the kidney to cadmium–metallothionein as it is slowly released from the liver. Inflammatory foci were found in the interstitium of the kidneys of mice with no metallothionein that were exposed to cadmium (Liu et al., 1999), suggesting that cadmium that is not bound to metallothionein might also play a role in cadmium-induced nephropathy.
As mentioned above, the intestinal absorption and potential toxicity of cadmium may be influenced by metabolically related metal ions (WHO, 1992). Diets with low concentrations of calcium and protein enhance the gastrointestinal absorption of cadmium, and iron-deficient animals absorb more cadmium than animals with nutritionally adequate iron. The renal toxicity of cadmium is reduced or abolished by increased intakes of zinc, copper, and selenium (Nordberg, 1978). The preventive effects of pretreatment with zinc and copper have been suggested to be the result of increased production of metallothionein in the liver and renal cortex (Liu et al., 1994).
Studies summarized by WHO (1992) indicate that a concentration of cadmium in the renal cortex of 100–200 µg/g is likely to be the critical concentration for 50% of the population. Measurements of cadmium in human renal cortex in vivo suggest that renal tubular damage would be experienced by about 50% of people with a concentration of 200 µg/g in the renal cortex and about 10% of people with a concentration of 300 µg/g. A one-compartment metabolic model (Friberg et al., 1974; Task Group on Metal Toxicity, 1976) has been used to estimate the daily intake of cadmium by a nonsmoker that would be required to attain a renal cortical concentration of 200 µg/g at the age of 50 years.
Studies in animal models are, in general, supportive of the proposed concept of a critical concentration of cadmium in the renal cortex, although the LOAEL may be lower than 200 µg/g. Morphological changes in the renal tubules were reported by Kawai et al. (1976) in rats given cadmium at 10–200 mg/L of drinking-water for 8.5 months. The average renal concentration of cadmium was about 38 µg/g wet weight of kidney, which corresponds to about 50 µg/g in the renal cortex. Aughey et al. (1984) administered cadmium at 50 mg/L of drinking-water to rats for 6 months and also found histological evidence of renal toxicity with a renal cortical cadmium concentration of 50 µg/g. Bernard et al. (1981) produced proteinuria in rats by exposing them to cadmium in drinking-water at a dose of 200 µg/L for up to 11 months. After 8–9 months, there was a 25% prevalence of increased proteinuria, corresponding to a renal cortical concentration of about 200 µg/g. However, evidence of renal tubular injury was seen in some animals with a renal cortical cadmium concentration as low as 40 µg/g, indicating that the critical concentration of cadmium in the renal cortex may be about 50 µg/g for about 10% of the population.
Thus, cadmium produces renal tubular dysfunction in non-human mammalian species that is analogous to the effect in humans of exposure to low concentrations of cadmium. Renal tubular dysfunction induced in animals by low concentrations may progress to interstitial nephropathy with longer exposure. The presence of proteins of low relative molecular mass in the urine is a sensitive biomarker of renal tubular dysfunction, although hypercalciuria may be an equally sensitive biomarker. The critical concentration of cadmium for the induction of nephropathy is 50–200 µg/g of renal cortex.
Limited information was available about the dose–response relationships for renal tubular dysfunction in experimental models. The following selected studies addressed dose–response relationships after oral exposure to cadmium.
Ten male Flemish giant and 10 male New Zealand rabbits were fed commercial lab chow containing cadmium chloride at 160 mg/kg of diet for 200 days, resulting in an average cadmium intake of 15 mg/kg bw per day. The weight of the kidneys and the incidences of glomerular sclerosis, tubular-cell degeneration, and interstitial fibrosis were increased (Stowe et al., 1972).
In male and female rats given cadmium in the drinking-water at a dose of 0, 5, 13, 32, or 50 mg/L to for 92 and 84 weeks, respectively, the kidney weights and the frequency of histological changes in the renal tubules were found to increase with dose (Fingerle et al., 1982).
Wistar rats were given cadmium at 5 or 50 mg/L in drinking-water for up to 2 years. The concentration of metallothionein in urine, measured by radioimmuno-assay, corresponded to the concentration of cadmium in the drinking-water and increased linearly over time. The authors therefore concluded that the urinary metallothionein concentration is a sensitive indicator of oral intake of cadmium. The maximum concentration of cadmium in the renal cortex was 90 mg/kg. No morphological or functional changes were observed in the kidneys (Shaikh et al., 1989).
Female Sprague-Dawley rats were given cadmium in the drinking-water for up to 18 months. From the second month, the animals showed increased albuminuria, which preceded the onset of tubular proteinuria (beta2-microglobulin) by about 6 months. The results suggest that cadmium enhances glomerular filtration by impairing glomerular anion depletion, mainly by loss of sialic acid (Bernard et al., 1992a).
Renal tubular dysfunction was detected in rhesus monkeys exposed to cadmium at 1.2 mg/kg bw per day for 9 years, but not in monkeys exposed to 0.4 mg/kg bw per day (Masaoka et al., 1994).
In a study in which rats received a diet containing cadmium chloride at a concentration of 0, 8, 200, or 600 mg/kg of diet for 2, 4, and 8 months from 5 weeks of age, the rats receiving 600 mg/kg of diet were were found to have anaemia, reduction of the femoral bone, periportal liver-cell necrosis, and renal tubular-cell necrosis at 4 months. Rats receiving 200 mg/kg of diet had toxic renal lesions after 2 months, when the renal concentration of cadmium was 104–244 µg/g. No renal lesions were found after 2 months in rats receiving 200 mg/kg of diet for 8 months, when the renal concentration was 91–183 µg/g. The authors suggested that a concentration of cadmium of 40 mg/kg of diet was the NOEL. Also in this study, the hepatic concentration of cadmium increased linearly throughout treatment, whereas the renal concentration in rats at 600 mg/kg of diet plateaued at about 250 µg/g within the first 2 months (Ando et al., 1998; Mitsumori et al., 1998). The authors suggested that the difference in the pattern of distribution of cadmium in the liver and kidneys was due to a difference in the form of absobed cadmium, i.e. free ion or cadmium–metallothionein complex (Hiratsuba et al., 1999). An alternative explanation is that the lower concentrations in the kidneys at higher doses of cadmium reflect hypercadmiumuria due to renal tubular toxicity.
NOEL and LOEL values from selected studies of animals given cadmium orally are shown in Table 3. Stowe et al. (1972) and Bernard et al. (1992a), studying rabbits and rats, respectively, and using different end-points and sexes, determined the LOEL to be 13–15 mg/kg bw per day. The LOEL for tubular dysfunction in rhesus monkeys was lower, 4.0 mg/kg bw per day (Masoaka et al., 1994), but greater than the concentration reported by Shaikh et al. (1989) for renal tubular dysfunction, 2.6 mg/kg bw per day. An increased concentration of metallothionein in urine may be interpreted as a biomarker of exposure to cadmium and is not a pathological effect. Cloudy swelling of the renal tubular epithelium (Fingerle et al., 1982) is a mild change and its diagnosis is somewhat subjective. These studies suggest an LOEL of 13–15 mg/kg bw per day and a NOEL of 0.8 mg/kg bw per day. Direct extrapolation to humans would require a number of assumptions that are difficult to support, but it is interesting that this NOEL is well above the value for the provisional tolerable weekly intake of 400–500 µg, i.e. 0.007 mg/kg bw per week or 0.001 mg/kg bw per day.
Table 3. NOEL and LOEL values for the renal effects of cadmium chloride in drinking-water
|
Species, sex |
Effect |
NOEL |
LOEL |
Reference |
|
Rabbits, male |
Tubular necrosis Interstital necrosis at 200 days |
ND |
15 |
Stowe et al. (1972) |
|
Sprague-Dawley rats |
Cloudy swelling of tubular epithelium at 92 weeks (males), 84 weeks (females) |
0.8 |
1.5 |
Fingerle et al. (1982) |
|
Wistar rats, male |
Increased urinary metallothionein; no tubular dysfunction |
2.6 |
ND |
Shaikh et al. (1989) |
|
Sprague-Dawley rats, female |
Albuminuria |
ND |
13 |
Bernard et al. (1992a) |
|
Rhesus monkeys, male |
Tubular dysfunction |
0.4 |
4.0 |
Masaoka et al. (1994) |
ND, not determined
(b) Effects on bone and calcium metabolism
One of the major health effects of excess cadmium in food is a severe bone disease known as itai-itai disease. The populations known to have been affected were exposed to excess cadmium in rice. The disease has characteristics of osteoporosis and osteomalacia and is associated with severe cadmium nephropathy. Women in affected populations may be severely debilitated. Populations with low dietary exposure to cadmium and minimal renal tubular proteinuria have been shown to have hypercalciuria. A potential continuum between the hypercalciuria and itai-itai disease and other more common disorders of the bone such as osteoporosis in the general population is conceptually possible but not established clinically (Goyer et al., 1994).
There is debate about whether the skeletal effects of cadmium are due only to its toxicity or to its toxicity in conjunction with renal disease and nutritional deficiencies, particularly of calcium (Nogawa etNishijo, 1999). Oral administration of cadmium to rats on a low-protein, low-calcium diet resulted in a negative calcium balance and reduced calcium and zinc contents in bone.
Cadmium in bone may interfere with calcification, decalcification, and bone remodelling. Administration of cadmium to beagle dogs at 25 mg/L in drinking-water for 6 months reduced the bone turnover rate, consistent with calcium deficiency or osteomalacia (Anderson & Danylchuk, 1979). A study in vitro suggested that cadmium may have a direct effect on bone and calcium metabolism (Wang & Bhattacharyya, 1993).
A proposed mechanism for the decreased calcium absorption and negative calcium balance seen in rats exposed to cadmium is that this metal inhibits activation of vitamin D in the renal cortex (Feldman & Cousins, 1974). Cadmium inhibits vitamin D-stimulated intestinal calcium transport in rats (Ando et al., 1981; Pleasants, 1993). Renal conversion of 25-hydroxycholecalceferol to 1,25-dihydroxycholecalceferol in rats is inhibited by a high dietary concentration of cadmium when the dietary concentration of calcium is low, but the effect was not seen in rats given a low-calcium diet (Lorentzon & Larsson, 1977). Nevertheless, there is considerable evidence that a low dietary calcium concentration enhances the effects of cadmium on bone. In a series of studies in Japan, described in detail in WHO (1992), on the effects of dietary cadmium in monkeys, a dose of 30 mg/kg bw was found to worsen osteomalacic changes in the bones of animals fed diets with low concentrations of protein, calcium, and vitamin D.
The effects of cadmium at a dose of 0, 0.25, 5, or 50 mg/kg of diet were studied in female mice bred for six consecutive 42-day cycles of pregnancy and lactation and in non-pregnant controls. The multiparous mice exposed to the highest dose showed significant losses in body weight (3–11%), femur calcium content (15–27%), and the femur calcium to dry weight ratio (5–7%). The authors concluded that the combination of cadmium, calcium loss, and multiparity has a synergistic effect on bone metabolism (Bhattacharyya et al., 1988). Multiparity and ovariectory further increased the cadmium-related reductions in bone calcium and density, and the effect was further enhanced by dietary calcium deficiency (Whelton et al., 1997).
Several studies on the effects of oral administration of cadmium on bone and calcium metabolism showed a decreased calcium content in bone and increased urinary excretion of cadmium. Administration of cadmium at 10 mg/L of drinking-water for 9 months to rats fed a normal diet resulted in decalcification and cortical atrophy in the skeleton, associated with renal cortical concentrations in the order of 50 mg/kg (Kawai et al., 1976).
Rats given cadmium in the drinking-water at 50 mg/L for about 9 months showed reduced calcium and phosphorus absorption from the intestine (Sugawara & Sugawara, 1974).
Cadmium chloride administered in drinking-water to rats at a dose of 1 or 4 mg/kg bw daily for 6 months caused changes in calcium metabolism and bone structure characteristic of osteomalacia (WHO, 1992). In contrast, no significant change in bone calcification was reported after a 24-week exposure to cadmium at 8 mg/kg bw in drinking-water (Kotsonis & Klaassen, 1978), and no significant change in the amount of stable or radiolabelled calcium was found in the tissues of rats given cadmium at 3.8 mg/kg bw per day in drinking-water for 22 days of gestation (Kelman et al., 1978).
(c) Carcinogenicity
Studies in experimental animals treated by injection or inhalation have provided considerable evidence that cadmium is carcinogenic. In rats, cadmium produces a variety of tumours, including malignant tumours at the site of injection and in the lungs after inhalation. Cadmium chloride, oxide, sulfate, and sulfide produced local sarcomas in rats after subcutaneous injection, and cadmium powder and cadmium sulfide produced local sarcomas in rats after intramuscular administration (Environmental Protection Agency, 1984). Cadmium chloride caused a dose-dependent increase in the incidence of lung carcinomas in rats exposed by inhalation and a low incidence (5/100) of prostatic carcinomas after injection into the ventral prostate (Takenaka et al., 1983). Exposure to cadmium chloride by inhalation produced a dose-dependent increase in the incidence of lung carcinomas in rats (Takenaka et al., 1983; Oldiges, 1989).
Tumours can be induced in the ventral lobe of the prostate of rats by oral or parenteral administration and by direct injection. The frequency of proliferative lesions in the prostate was increased in rats fed diets deficient in zinc as compared with that in rats fed diets with adequate zinc; the incidence was lower in rats fed zinc-deficient diets, but with no dose–response relationship. The incidence of benign testicular interstitial tumours was also increased at high doses. The authors concluded that cadmium given orally caused tumours of the prostate, testis, and haematopoeitic system in rats and that this effect was inhibited by dietary deficiency of zinc (Waalkes & Rehm, 1994). As the lobular structure of the prostate is absent in humans and the ventral prostate in rats is considered to have no human analogue, tumours of the dorsolateral lobe are more closely comparable to the human disease. Nevertheless, subcutaneous injection of cadmium to a particularly sensitive strain of rat (Noble/Cr) produced proliferative lesions of the dorsolateral prostate, tumours of the testis and pituitary adenomas, and sarcomas at the injection site (Waalkes et al., 1999).
Cadmium has therefore been shown to be carcinogenic in animals after administration orally, by inhalation, or by injection. A dose-dependent increase in the incidence of lung tumours was found in mice and rats after inhalation of cadmium chloride. Oral intake is associated with proliferative lesions of the ventral lobe of the prostate in rats fed diets adequate in zinc, whereas dietary deficiency of zinc appears to inhibit the tumorigenic effect of cadmium. The relevance of these studies to prostate carcinogenesis in humans is questionable because of anatomical differences between the human and rodent prostate.
(a) Access of cadmium to the brain
The blood–brain barrier and circumventricular epithelial cells with tight junctions limit the entrance of cadmium into the central nervous system. In addition, the choroid plexus epithelium accumulates highly toxic metals from the blood or cerebrospinal fluid (Murphy, 1997). Injected cadmium cannot easily enter the brain as it is blocked not only by the blood–brain barrier and blood cerebrospinal sufaces but also by the ependymal and pial surfaces (Takeda et al., 1999). Studies with radiotracers have shown the greatest concentration of cadmium in circumventricular areas, including the choroid plexus, hypophysis, meninges, pineal gland, and olfactory bulb in rats (Arivison, 1987). In a study of 7–14-day-old mice, the tracer moved from the blood vessels to the brain parenchyma and particularly the granular layer of the cerebellum (Agency for Toxic Substances and Disease Registry, 1999). In other studies, no radiolabel was found in brain parenchyma or spinal cord, but some was found in sensory ganglia and spinal roots. Therefore, the peripheral nervous system may be particularly sensitive to cadmium (Murphy, 1997).
Metallothionein in glial cells and ependymal cells near circumventricular organs also minimizes the diffusion of cadmium into other parts of the brain. Use of immunohistochemical staining techniques showed different concentrations of metallothionein in rat and mouse brains, but as the concentration appeared to be higher in adult mouse brain than in young or adult rat brain it may be difficult to compare the results of studies in the two species (Nishimura et al., 1992). Cadmium accumulation and expression of mRNA of metallothionein-I, -II, and -III were observed in the brains of adult mice and also during different stages of development. There appeared to be an inverse relationship between expression of metallothionein and cadmium accumulation in the brain (Choudhuri et al., 1996). The effect of metallothionein on the neurotoxicity of cadmium is thus not clear.
(b) Effects
A variety of neurobehavioural and biochemical effects are produced on the nervous system of rodents given repeated doses of cadmium (Murphy, 1997). As cadmium and zinc are metabolically competitive, cadmium may replace zinc in a number of metalloenzymes, proteins, and ion channels. Exposure to cadmium generally increases the concentrations of noradrenaline and dopamine and impairs enzymes involved in the synthesis of neurotransmitters. Serotonin production may be altered, depending on age, brain region, and duration of exposure.
Behavioural effects that are not apparent clinically can be detected in the offspring of animals exposed to small repeated doses of cadmium during pregnancy (Murphy, 1997). The offspring of pregnant rats given 50 mg/L in drinking-water, corresponding to 4–5 mg/kg bw per day, throughout gestation had the same body weight as controls but lower brain weights after 7 and 14 days but not 21 days (Gupta & Chandra, 1991). Brain protein, DNA, and RNA contents were the similar in control and treated rats, although there was a twofold increase in the concentration of cadmium in the tissues. The activities of several enzymes were decreased at various times after birth: succinate dehydrogenase at 7 days, cyclic nucleotide phosphorylase at 14 days, and acetylcholine at 21 days.
When cadmium was given during gestation and lactation at 5–6 mg/kg bw in drinking-water, the brain zinc and DNA contents and DNA synthetase and thymidine kinase activities were reduced. The brains weighed less at 7, 14, and 21 days after birth (Gupta et al., 1993).
Cadmium chloride administered by gavage to female rats on 5 days a week for 5 weeks and then during mating and gestation at a dose of cadmium of 0.04, 0.4, or 4 mg/kg bw per day significantly reduced locomotor activity in offspring tested at 2 months of age (Baranski et al., 1983). Administration of 60 mg/L (corresponding to 5 mg/kg bw per day) in drinking-water during days 1–20 of gestation caused alterations in locomotor activity and behaviour in the open field in adult offspring of each sex. Adult female offspring showed decreased acquisition of avoidance behaviour. The copper content of the brains of 2-week old offspring of each sex and of 16-week-old females was decreased, but the concentration of zinc in brain was decreased only in 16-week-old animals (Baranski, 1986). These result suggest that changes in the concentrations of essential metals may also play a role in the apparent effects of cadmium during development.
The offspring of rats given cadmium at a dose of 0.20, 0.62, or 2.0 mg/kg bw per day on days 7 and 15 of gestation showed decreased horizontal motor activity, increased immobility after treatment with amphetamine during a 5-min stress swim (at all doses), increased social interactions (at 0.62 and 2.0 mg/kg bw per day), and extended latency of reacquisition (at 0.62 and 2.0 mg/kg bw per day). The weights of these animals at birth and weaning were not different from those of controls, and the dams showed no toxic effects (Agency for Toxic Substances and Disease Registry, 1999).
Administration of cadmium orally to weanling rats for 30 days caused increased secretion of dopamine, noradrenaline, and serotonin in certain regions of the brain at 1.0 mg/kg bw per day but not at 0.1 mg/kg bw per day; however, locomotor activity was increased in both groups (Babitch, 1988). Neonatal animals given repeated doses of cadmium showed decreased exploration in open fields and inner squares and improved performance in T-mazes.
In male rats given cadmium chloride at 10 mg/L of drinking-water, corresponding to a dose of cadmium of 2 mg/kg bw per day, ad libitum for 90 days, the concentration of cadmium in the brain was increased by 76% after 30 days and by 165% after 90 days. The permeability of the blood–brain barrier to fluorescein dye and the concentration of malondialdehyde in brain microvessels were increased at the end of exposure, whereas the activity of superoxide dismutase was decreased. The authors concluded that cadmium-induced dysfunction of the blood–brain barrier may be related to depletion of microvessel antioxidants and increased lipid peroxidation (Shukla et al., 1996).
Cadmium is thus a potential neurotoxin, but some level of protection is provided by metallothionein in the brain. The presence of metallothionein in the placenta protects the fetus when exposure is low. No consistent dose–response relationships were seen in these studies because of differences in species and design.
High doses of cadmium compounds administered to rodents induced severe placental damage and fetal death when given at a late stage of pregnancy. The teratogenic effects induced when cadmium was given in the early stages of pregnancy included exencephaly, hydrocephaly, cleft lip and palate, microphthalmia, micrognathia, club foot, and dysplastic tail. No teratogenic effects were reported after oral intake of low doses (WHO, 1992).
Much of the information that has become available since publication of Environmental Health Criteria 134 (WHO, 1992) on the effects of long-term exposure to low doses of cadmium on human health is from a cross-sectional, population-based epidemiological study conducted between 1985 and 1989 in Belgium, known as the Cadmibel, or Cadmium in Belgium, Study (Lauwerys et al., 1990). As this study provides data on a variety of health end-points, it is described in detail. The health effects of cadmium, by organ system, are then described on the basis of the findings of the Cadmibel Study and those of other, recent studies.
(a) Design
The goals of the Cadmibel Study were twofold: (i) to assess the acceptable internal dose of cadmium for the general population from concentrations in blood and urine and (ii) to establish whether environmental exposure induces renal changes or affects blood pressure.
Residents of four areas of Belgium were selected for study. These comprised two areas in which exposure to cadmium was known to be high owing to past industrial activities: Liege (an urban area and the site of a cadmium-producing plant that was closed in 1981) and N-Kempen (a rural area near two non-ferrous smelters), and two less polluted or control areas matched with regard to urbanization and socioeconomic status: Charleroi (an urban area with iron foundries in operation) and Hechtel-Eksel (a rural area).
A target sample size of at least 300 persons in each of the four areas was selected in order to provide sufficient statistical power (alpha = 0.05, beta = 0.1) to detect a 10% difference in protein excretion, a 5% difference in creatinine clearance, a difference of 5 mm Hg in systolic blood pressure, and a difference of 3 mm Hg in diastolic blood pressure. The study subjects within each area were chosen to include equal numbers of men and women and equal numbers of individuals in the age groups 20–39, 40–59, and 60–79 years. Persons who were occupationally exposed to heavy metals (cadmium, zinc, lead, mercury) were excluded.
The household was selected as the sampling unit. In each area, at least 200 households headed by a Belgian citizen were selected randomly. All family members 20–80 years of age were eligible for inclusion in the study if they had lived in the area for at least 8 years, as long as the number of individuals to be included in each sex and age group (50) had not been reached. Of 4532 persons who met the criteria for eligibility, 2327 were enrolled, comprising 78% in the rural districts and 39% in the urban districts.
The field staff consisted of 10 trained nurses and social workers. One team operated in the rural areas and the other in the urban areas. Data were collected during two home visits. At the first visit, five blood pressure readings were taken from the person in a sitting position, and pulse rate (over 1 min), height, and weight were recorded. A container was provided for collection of a 24-h urine sample, and a questionnaire covering medical history, occupational history, smoking, alcohol, diet, water, and drug intake was left to be completed. At the second home visit, the same staff member repeated the set of measurements and collected the urine sample and completed questionnaire. At one of the home visits (preferably the first), a spot urine sample was collected. Within a week of the urine collection, a physician collected a 20-mL venous blood sample.
A series of biomarkers were analysed in the samples. Blood was tested for cadmium, lead, zinc protoporphyrin, and selenium. Serum was analysed for creatinine, alkaline phosphatase, gamma-glutamyltranspeptidase, total cholesterol, high-density lipoprotein cholesterol, calcium, magnesium, zinc, ferritin, and beta2-microglobulin. The 24-h urine sample was tested for creatinine, cadmium, calcium, sodium, potassium, copper, total amino acids, total protein, beta2-microglobulin, retinol-binding protein, albumin, and N-acetyl-beta-glucosaminidase, and the spot urine sample was tested for creatinine, cadmium, and beta2-microglobulin. Reagent strip analyses were performed on both urine samples.
For the purposes of a follow-up study conducted in 1991–94, a random sample of 1419 members of the original study population was selected, of whom 1104 were enrolled.
(b) Evaluation
The Cadmibel Study has several noteworthy features that make it a valuable source of data on the health effects of exposure of populations to cadmium. First, in contrast to studies in which the exposure of individuals was classified only on the basis of the area of residence, data on exposure to cadmium were available for each individual from the measurements of biological markers in urine and blood. Second, early biological effects were selected as health indicators, rather than the clinical entities that are the late stages in the expression of pathological processes, the expression of which may be affected by many factors. The effects of such factors can be reduced by studying biological effects that occur closer in time to exposure and thus provide a stronger basis for estimating the association between exposure and effect.
The use of early indicators also reduces the likelihood of selection bias due to migration out of the area by ill people who attribute their illness to local pollution. One important limitation of studying early biological effects is uncertainty about their significance. For instance, a decline in tubular reabsorptive capacity from 99.9% to 99.0% will result a 10-fold increase in the amount of beta2-microglobulin excreted in the urine (Jarup et al., 1998a). Some other markers of tubular damage are equally or more sensitive than beta2-microglobulin (e.g. retinol-binding protein, alpha1-microglobulin, N-acetyl-beta-glucosaminidase, Clara cell protein).
Use of the household as the unit of sampling has several strengths. First, it reduces the cost and logistics of field visits by allowing the home visitor to collect data on several people at once. Second, the likelihood that an eligible individual will participate in the study may be increased if other family members have agreed to participate. Third, blood pressure readings may be more accurate when performed in the familiar setting of the home. Fourth, it allows familial aggregation of certain measures (e.g. blood pressure) and environmental factors related to exposure to cadmium (e.g. consumption of well water). This potential does not appear to have been fully exploited, however, and studying multiple family members introduces a possible problem in terms of lack of statistical independence of the observations.
Another noteworthy feature of the Cadmibel Study was the use of detailed protocols for training home visitors and for maintaining the quality of blood pressure measurements (Staessen et al., 1991) and the analysis of the biological samples (Claeys et al., 1992). In addition, detailed information was collected on potential confounding exposures, including other chemicals (e.g. lead, arsenic) and those related to life style (e.g. smoking, alcohol use).
The low rate of participation of eligible individuals in the two regions with low exposure (39%) is a potential weakness, particularly in light of the 78% participation rate of persons in the regions of high exposure. This would reduce the relevance of the findings if the participants in the control areas were not representative of the total population of eligible controls, and would have produced a selection bias if the likelihood of participation was systematically related to either exposure or disease status. There is little evidence that such a bias was present, however.
(c) Major findings with regard to exposure to cadmium
The main findings of the Cadmibel Study with regard to exposure are: (1) the body burden of cadmium increases with age, at least until 60 years; (2) higher alcohol consumption is associated with less excretion of cadmium in urine; (3) smokers have a higher burden of cadmium than do nonsmokers; (4) premenopausal women have a higher burden of cadmium than do similarly aged men (at least among nonsmokers); (5) urinary excretion of cadmium increases in women after the menopause; and (6) residence in areas where there is heavy environmental pollution with cadmium is associated with higher body burdens, including a 30% greater urinary excretion (Lauwerys et al., 1991; Sartor et al., 1992a,b).
In 1699 persons in the Cadmibel Study, the urinary concentration of cadmium was significantly associated with five biomarkers of renal function: urinary excretion of retinol-binding protein, N-acetyl-beta-glucosaminidase activity, beta2-microglobulin, amino acids, and calcium (Buchet et al., 1990). In multivariate models in which adjustments were made for blood lead, zinc protoporphyrin, age, sex, smoking habits, diuresis, presence of diabetes or urinary-tract disorders, consumption of analgesics, place of residence, and first-order interaction terms involving cadmium concentration in blood or urine, all of the variables except beta2-microglobulin were also associated with the concentration of cadmium in the 24-h urine sample. Dose–response relationships were found. A positive association seen between urinary excretion of cadmium and calcium suggests that increased exposure to cadmium may be associated with increased renal wasting of calcium. For two biomarkers, N-acetyl-beta-glucosaminidase and beta2-microglobulin, the interaction between a diagnosis of diabetes and urinary excretion of cadmium in the 24-h sample was statistically significant, suggesting that individuals with diabetes may be particularly vulnerable to the adverse renal effects of cadmium. The blood cadmium concentration was not significantly associated with any of the five biomarkers of renal function.
Additional logistic regression analyses were carried out to estimate the rates of excretion of cadmium associated with a 10% prevalence of abnormal values for the five biomarkers. The rates were 2.9 µg/24 h for retinol-binding protein, 2.7 µg/24 h for N-acetyl-beta-glucosaminidase, 3.0 µg/24 h for beta2-microglobulin, 4.3 µg/24 h for amino acids, and 1.9 µg/24 h for calcium. Jung et al. (1993) reported similar findings, a mean urinary cadmium excretion of 5.4 µg/g creatinine being associated with a 11–22% prevalence of abnormalities in renal markers such as alpha1-microglobulin, retinol-binding protein, and N-acetyl-beta-glucosaminidase in urine.
These findings suggest that an increased body burden of cadmium, estimated from 24-h urinary excretion, is associated with changes in proximal tubular function but not with changes in glomerular function. Blood cadmium concentration, which reflects more recent exposures than does urinary cadmium excretion, was not associated with changes in renal function. The dose–response analyses suggested that these changes occurred largely when urinary excretion exceeded 2 µg/24 h (or 0.5–2 µg/g of creatinine), a rate that is considered to reflect a renal cortical concentration of 10–40 mg/kg (Jarup et al., 1998a). It was estimated that 10% of the general population of Belgium has a body burden of cadmium that would be associated with an excretion rate of 2 µg/24 h and are thus at increased risk for the observed renal changes. Specifically, at a urinary cadmium concentration of 2–4 µg/24 h, the prevalence of tubular proteinuria would be 10%, whereas the background prevalence is 5%. In a comprehensive review of the literature on this topic, Jarup et al. (1998b) related renal cortical and urinary concentrations of cadmium with the percentage of the general population that can be expected to have renal dysfunction (Table 4). The findings are somewhat at variance with those obtained in studies of adult male workers, in whom the NOEL is considerably higher, the rate of excretion of cadmium being about 10 µg/g of creatinine. This value is likely to reflect a ‘healthy worker effect’, i.e. a reduced representation among workers of individuals susceptible to nephrotoxicity (Lauwerys et al., 1995).
Table 4. Prevalence of tubular effects in the general population in relation to concentration of cadmium in the renal cortex and urine, on the basis of all data published up to mid-1997
|
Cadmium in renal |
Cadmium in urine |
Prevalence |
|
< 50 |
< 2.5 |
0 |
|
51–60 |
2.75 |
1 |
|
61–70 |
3.25 |
2 |
|
71–80 |
3.75 |
3 |
|
81–90 |
4.25 |
4 |
|
91–100 |
4.75 |
5 |
|
101–110 |
5.25 |
6 |
|
111–120 |
5.75 |
8 |
|
121–130 |
6.25 |
10 |
|
131–140 |
6.75 |
12 |
|
141–150 |
7.25 |
14 |
|
151–160 |
7.75 |
17 |
|
161–170 |
8.25 |
20 |
|
171–180 |
8.75 |
23 |
|
181–190 |
9.25 |
26 |
|
191–200 |
9.75 |
30 |
|
> 200 |
> 10.25 |
> 35 |
A follow-up investigation of 703 of 1107 randomly selected Cadmibel participants (Staessen et al., 1994) confirmed the association between the body burden of cadmium and changes in biomarkers of proximal tubular function. In this study, however, more direct indicators of glomerular function were also measured, which were serum creatinine, beta2-microglobulin, and creatinine clearance. These markers tended to be altered adversely in more heavily cadmium-polluted regions, although the associations were much weaker when tested in relation to 24-h urinary excretion of cadmium, which is a biomarker of individual exposure. This indicates that the ecological association might reflect confounding by unmeasured differences between regions.
In order to evaluate the possibility that increased urinary excretion of cadmium is the result, rather than the cause, of tubular dysfunction, Bernard et al. (1992b) studied 114 pregnant women, some of whom had reversible tubular proteinuria unrelated to cadmium. Urinary excretion of cadmium was not related to excretion of the two renal markers measured, retinol-binding protein and beta2-microglobulin, suggesting that tubular dysfunction does not necessarily increase urinary cadmium excretion. The tubular dysfunction identified in the Cadmibel Study population is therefore likely to be attributable to increased exposure to cadmium.
Additional evidence that tubular dysfunction is associated with environmental exposure to cadmium was provided by Jarup et al. (1995a) in a study of 72 adults living near a nickel–cadmium battery plant that had been shut down. In this study, the urinary cadmium concentrations were greater than those of controls and decreased with distance of the residence from the plant. The urinary concentrations of cadmium (0.12–3.2 nmol/mmol of creatinine) were positively correlated with the activity of N-acetyl-beta-glucosaminidase in a dose–response manner, with no evidence of a threshold.
The health significance of the changes in renal function is uncertain. Cadmium-induced proteinuria is considered to be irreversible, but the level of exposure to cadmium that is required to place an individual at increased risk for progressive alterations in renal function is not known. Roels et al. (1989) studied a group of 23 workers who had been removed from jobs involving exposure to cadmium because of the finding of increased urinary excretion of beta2-microglobulin (> 300 µg/L) and/or retinol-binding protein (> 300 µ/L) and a mean urinary concentration of cadmium at baseline of 22.2 µg/L. At follow-up 5 years later, none of the renal parameters had returned to normal. Furthermore, those of creatinine and beta2-microglobulin in serum had increased significantly over this period even though exposure had been discontinued, suggesting progressive impairment of glomerular function independent of the tubular effects. The estimated decline in glomerular filtration rate among these workers was approximately five times that expected as the result of normal ageing. These findings clearly suggest that the increased urinary excretion of plasma proteins associated with occupational exposure to cadmium should be considered an adverse effect. A study of 179 men confirmed the observation that exposure to cadmium reduces the transient increase in glomerular filtration rate that occurs in response to a protein load (reserve filtration capacity) and accelerates the age-related decline in glomerular filtration rate, but only in the presence of cadmium-induced microproteinuria (Roels et al., 1991). Additional evidence for this conclusion has been reported (Jarup et al., 1993, 1995b, 1997). Jarup et al. (1995b) reported that 7/13 workers with blood concentrations of cadmium exceeding 50 nmol/L showed evidence of glomerular lesions (glomerular filtration rates < 80% of the reference value). Jarup et al. (1997) concluded that alpha1-microglobulin is a more sensitive biomarker of early tubular dysfunction than is beta2-microglobulin. An increased risk for kidney stones has also been reported among workers exposed to cadmium (Jarup & Elinder, 1993; Jarup et al., 1997), the incidence rate ratio increasing substantially in the presence of tubular proteinuria.
The prognosis of the renal effects seen at normal levels of exposure to cadmium appears to be good. In a 5-year follow-up study of 593 participants in the Cadmibel Study, Hotz et al. (1999) found no evidence of progressive renal damage. In men, the mean urinary excretion of cadmium at follow-up was 7.5 nmol/24 h (a decline of 16% from baseline), while in women, the mean value was 7.6 nmol/24 h (a decline of 14% from baseline). The investigators concluded that the renal effects due to cadmium in this population were weak, stable, or even reversible after the introduction of measures to reduce exposure. They also concluded that the tubular effects were not necessarily associated with a subsequent deterioration in glomerular function. In studies of workers in cadmium smelters (Bernard et al., 1995; Nortier et al., 1997), however, urinary excretion of the B isozyme of the lysosomal enzyme N-acetylgluco-saminidase was dose-related even among individuals with a rate of urinary excretion of cadmium of 2 µg/g of creatinine. Thus, excretion of the lesional form of this enzyme was related to the concentration of cadmium, without clear evidence of a threshold. The investigators speculated that cadmium-induced apoptosis might account for this association.
In reviewing the literature on exposure to cadmium and renal dysfunction, Jarup et al. (1998b) concluded that in order to prevent renal tubular damage that can proceed to clinical disease and perhaps contribute to early death, the concentration of cadmium in the kidneys should be maintained below 50 mg/kg and that in the urine below 2.5 µg/g of creatinine.
In the Cadmibel study population, the association between exposure to cadmium and cardiovascular disease was evaluated in a subsample of 2086 persons of a mean age of 48 years (range, 20–88 years). The mean concentration of cadmium in blood was 11 nmol/L in the areas of heavy exposure and 8.5 nmol/L in the control areas, and the concentrations in the 24-h urine samples were 8.7 nmol and 7.2 nmol, respectively. Systolic blood pressure was significantly higher (5 mm Hg) in the urban area of heavy exposure than in the urban control area (Staessen et al., 1991a, 2000), but the difference remained when adjustments were made for cadmium concentrations in blood, suggesting that cadmium was not causal in this association. Other factors that were controlled in the analyses were age, body mass index, smoking, and alcohol intake. Diastolic blood pressure was negatively correlated with both blood and urinary cadmium concentrations. No differences in blood pressure were found in the two rural areas. The prevalences of hypertension (systolic blood pressure > 140 or diastolic blood pressure > 90 mm Hg or both, or being on anti-hypertensive treatment) and self-reported ischaemic heart disease did not differ between the areas of heavy and low exposure or with the concentration of cadmium in blood.
Whittemore et al. (1991) used data on 951 adult men and women with no occupational exposure to cadmium who were participating in a cross-sectional health survey in the United States. Although significant positive associations were found between the urinary concentration of cadmium and both systolic and diastolic blood pressure after adjustment for age, sex, race, body weight, smoking, and use of hypertensive medication, the associations were highly unstable when examined in subgroups defined on the basis of sex and smoking status. Among current smokers, the positive association between urinary cadmium and systolic and diastolic blood pressure remained among women and that with diastolic blood pressure among men. No association was found in former smokers or lifelong nonsmokers of either sex. When persons being treated for hypertension were excluded from the analyses, no significant associations were found. The associations between urinary cadmium concentration and blood pressure among current smokers may therefore be the result of the increased urinary excretion of cadmium associated with anti-hypertensive medications.
Among individuals residing in Shipham, England, an area in which the soil is contaminated by cadmium from old zinc mines, blood pressure was not significantly associated with urinary or blood concentrations of cadmium (Morgan & Simms, 1988). A volunteer bias may have distorted these results insofar as the participation rate was much lower among long-time residents, who presumably had had longer exposure to cadmium. Given the similarity of these results to those obtained in the Cadmibel and United States studies, however, the effect of this bias is likely to have been small.
In the Cadmibel Study, 24-h urinary excretion of cadmium was associated with three biomarkers of calcium metabolism. Serum alkaline phosphatase activity increased with increasing excretion of cadmium after adjustment for age, body mass index, alcohol, use of diuretics, use of contraceptives, and menopausal status. A doubling in the excretion of cadmium was associated with a rise of 3–4% in serum alkaline phosphatase activity (Staessen et al., 1991b). Urinary calcium excretion (calciuria) was also positively related to urinary cadmium excretion. The total calcium concentration in serum was negatively associated with urinary cadmium excretion, but this association was significant only for men. Adjustment for alcohol intake and gamma-glutamyltranspeptidase activity enabled the investigators to eliminate liver dysfunction as an explanation for these associations. Although the changes were statistically significant, the amount of variation accounted for by cadmium excretion was quite small (1–2%), and the clinical significance of the changes is uncertain. Nevertheless, no threshold was apparent for the associations, contradicting the view that cadmium affects calcium metabolism only at very high concentrations. The inferences are limited, moreover, by the fact that neither calcium-regulating hormones nor direct indices of bone metabolism were measured.
Very heavy exposure to cadmium is known to be associated with osteoporosis and osteomalacia (itai-itai disease). The risk for clinically significant effects on bone at lower levels of exposure has been clarified in recent studies. Jarup et al. (1998b) and Alfven et al. (2000) measured mineral density in the bones of the forearm, lumbar spine, and hip by dual-energy X-ray absorptiometry in 43 persons with occupational exposure to cadmium. For all three bones, the covariate-adjusted mineral density was inversely and significantly associated with cadmium concentrations in both blood and urine. A dose–effect relationship was seen only for the forearm, however. A dose–response relationship was suggested for osteoporosis (z score for bone mineral density, less than –2.0). These data suggest that cadmium causes adverse effects on bone in persons with urinary cadmium concentrations up to about 8 nmol/mmol of creatinine and blood cadmium concentrations up to about 89 nmol/L.
In the follow-up to the Cadmibel Study, 506 participants underwent measurements of forearm bone density by single photon absorptiometry (Staessen et al., 1999), providing data on the association at environmental levels of exposure. The participants also gave information on fractures, which was verified from medical records. In post-menopausal women, proximal and distal bone densities decreased by nearly 0.01 g/cm2 with a doubling of 24-h urinary cadmium excretion over that at baseline (median, 6.6 years earlier). Similarly, a doubling of cadmium excretion at baseline was associated with a 73% increase in the risk for fractures in women, corresponding to a population attributable risk of 29% for women with rates of excretion of cadmium above the age-adjusted median value in the cohort. In men, bone density decreased with age and with increased calcium excretion at baseline, but was not associated with the urinary cadmium concentration. It is noteworthy that the mean urinary cadmium excretion in the study sample was approximately 1 µg/g of creatinine. These findings suggest that environmental exposure to cadmium is associated with decreased bone density and an increased risk for fractures in women, possibly due to increasing calcium loss and bone resorption. The reasons for the apparent increase in the vulnerability of women might be related to depleted iron stores, resulting in increased gastrointestinal cadmium absorption, and to the reduction in estrogen concentrations associated with the menopause.
The mechanism by which cadmium induces bone damage is not clear. It may be mediated by renal tubular damage and specifically by reduced activation of vitamin D3 to 1,25-dihydroxycalcitriol, resulting in reduced calcium absorption (Aoshima & Kasuya, 1991). Tsuritani et al. (1992) reported that the serum concentration of 1,25-dihydroxyvitamin D was positively associated with the rate of clearance of creatinine and tubular resorption of phosphate, and inversely related to serum concentrations of creatinine, beta2-microglobulin, and parathyroid hormone, although only in women.
A follow-up study of 3178 residents of a cadmium-polluted area of Japan suggested higher rates of premature death (Nishijo et al., 1994, 1995, 1999). The age-adjusted standardized mortality ratios were higher among persons with high urinary concentrations of beta2-microglobulin, protein, and amino acids than among individuals without such increases or among the general Japanese population. Urinary protein was the biomarker most strongly related to risk for death. In a study conducted in another cadmium-exposed population in Japan, Iwata et al. (1992) found that people with signs of tubular dysfunction (increased urinary concentrations of beta2-microglobulin or total amino nitrogen) were at significantly increased risk of dying during the follow-up period. For example, in a Cox proportional hazard model, the hazard ratio in women associated with a 10-fold increase in urinary beta2-microglobulin was 1.4 (95% CI, 1.0–2.0). In neither of these studies were cause-specific mortality rates examined, but such analyses were carried out as part of a long-term follow-up study of the mortality rates of residents of Shipham, England (Elliott et al., 2000). Although the overall mortality rate was lower than expected, the standardized incidence ratio for cancer was elevated (1.7; 95% CI, 1.1–2.5). The standardized mortality ratios for cancers of the genitourinary tract, and particularly of the prostate (2.6; 95% CI, 1.5–4.5) and ovary (2.6; 95% CI, 1.1–6.2), were elevated. The ratio for hypertension, cerebrovascular disease, and nephritis and nephrosis was also elevated, but the lower confidence bound fell just below 1.0 (1.3; 95% CI, 0.99–1.6). Both these studies were ecological in design, and exposure status was inferred from area of residence rather than from measurements of the body burden of cadmium.
Although associations have been reported between exposure to cadmium and increased rates of cancers of the lung, kidney, breast, and prostate, other studies did not confirm these associations. The studies that did have been faulted on methodological grounds, including failure to adjust for confounding by known risk factors.
The nervous system has not received as much attention as a potential target organ for cadmium as have the kidney and the skeletal system, possibly because cadmium does not cross the blood–brain barrier, at least in adults, and any neurotoxic effect must be secondary to its other effects, such as interference with zinc metabolism and the expression of metallothionein-III (Jin et al., 1998). Several studies of workers exposed to cadmium showed increased frequencies of subjective symptoms such as fatigue, headache, sleep disturbances, disturbances of sensory and motor functions, anorexia, and anosmia (Murphy, 1997). A case report of two individuals exposed to cadmium during a fire suggested that it had caused persistent neurophysiological deficits (e.g. slowed reaction times, abnormal balance, constricted visual fields, decreased vibration sensitivity) and persistent neuropsychological deficits (e.g. delayed recall, dexterity, coordination, decision-making) (Kilburn & McKinley, 1996). No controls were included in this investigation, nor could the deficits be ascribed solely to cadmium, as exposure to other potentially neurotoxic agents was involved.
The association between exposure to cadmium and peripheral neuropathy was investigated in 13 retired workers who had been exposed to cadmium (and lead) for 12–36 years, ending 1–24 years prior to the study. They were matched on age to unexposed controls. At the time of the examination, the mean urinary cadmium concentration of the exposed men was 8.8 µg/g of creatinine, and the mean blood concentration was 0.6 µg/dL. Peripheral neuropathy was diagnosed if two of four criteria were met: (i) self-reported complaint of polyneuropathy; (ii) neurophysiological findings including sympathetic skin response and changes in motor nerve conduction velocity, sensory nerve conduction velocity, and a needle electromyograph; (iii) distal symmetrical areflexia; and (4) distal symmetrical anaesthesia for vibration sense, temperature, or blunt–sharp discrimination. The prevalence of peripheral neuropathy was significantly higher in the exposed (53%) than in the unexposed group (11%), with an odds ratio of 9.9 (95% CI, 1.6–62). Multiple logistic regression analyses suggested a dose–response relationship between the risk for peripheral neuropathy and both urinary and blood cadmium concentrations. The investigators speculated that these findings might reflect a combination of cadmium-induced demyelinization and axonal damage. No differences were found between the two groups in a standard clinical neurological examination.
Hart et al. (1993) investigated the potential neurobehavioural effects of occupational exposure to cadmium in 31 workers with a mean duration of exposure of 14 years. The workers were classified by the concentration of cadmium in a 24-h urine sample as low (mean, 6.0; range, 1–11 [units not stated]) and high (mean, 43; range, 25–110). No group of unexposed persons was included. Efforts were made to assess the potential confounding effects of exposure to known neurotoxicants. The group with high cadmium concentrations achieved significantly lower scores for attention, psychomotor speed (symbol–digit modalities, digit symbol) and memory (paired-associate learning, digit symbol incidental recall) but not in measures of general intelligence, vigilance, conceptual reasoning, mental flexibility, motor speed, or mood state. The three persons with high beta2-microglobulin concentrations tended to perform less well in the neurobehavioural tests than those with normal concentrations.
The only well-controlled epidemiological study of adult exposure to cadmium and neurobehavioural effects was reported by Viaene et al. (2000). A sample of 42 workers with a mean of 12.6 years of exposure to cadmium and 47 controls were given a questionnaire (the neurotoxicity symptom checklist-60), a standard neurological examination, and computer-administered neurobehavioural tests of visuomotor performance, memory, and concentration. The statistical analyses were adjusted for a variety of potential confounders, including age, reported alcohol consumption, smoking habits, exposures to other neurotoxicants such as lead and organic solvents, education, and use of hypnotic medications. The exposure indices were the maximum concentration of cadmium measured in urine during each person’s work years (mean, 13 µg/g of creatinine) and the urinary concentration at the time of the examination (mean, 4.6 µg/g of creatinine). The exposed workers reported significantly more signs and symptoms of peripheral neuropathy, particularly in the categories of sensorimotor ability, equilibrium, and concentration. The only finding in the neurological examination that was related to exposure to cadmium was a higher frequency of a positive forehead reflex. In the neurobehavioral tests, the exposed workers showed significant slowing of simple reaction time and significantly worse scores in the symbol–digit substitution test. The scores in the two tests were also significantly related to the maximum urinary concentration of cadmium. These neurological effects were seen in workers without microproteinuria, suggesting that the effects on the nervous system are unlikely to be secondary to the effects of cadmium on renal function.
The neurotoxicity of cadmium in children was investigated in several studies in the 1970s and 1980s, but has received little attention since. In most of these studies, the biomarker of exposure was the concentration of cadmium in hair. In case–control studies in which the hair concentration of cadmium of a clinically defined group was compared with that of a reference group, higher concentrations were reported in mentally retarded children (Marlowe et al., 1983; Jiang, et al., 1990) and in children with learning difficulties or dyslexia (Pihl & Parkes, 1977; Capel et al., 1981) but not in children with autism (Shearer et al., 1982; Wecker et al., 1985) or with any of several neuropsychiatric diagnoses (motor, perceptual, speech, or attention disorders) (Gillberg et al., 1982). In cohort studies, Thatcher et al. (1982, 1984) reported that the concentration of cadmium in hair was significantly inversely related to covariate-adjusted scores in the revised Wechsler intelligence scale for children, particularly verbal intelligence quotient, and to scores for visual evoked potentials. Bonithon-Kopp et al. (1986) and Marlowe and colleagues (Stellern et al., 1983; Marlowe et al., 1985a) reported associations between increased hair concentrations of cadmium and children’s performance in visual–motor tasks. Marlowe et al. (1985b) also reported that lead and cadmium acted synergistically to impair children’s classroom behaviour. Lewis et al. (1992) combined the concentrations of seven metals (including cadmium) in amniotic fluid into a ‘prenatal toxic risk’ factor and reported that children with higher factor scores had significantly lower adjusted scores in the McCarthy scales of children’s abilities. The strong correlations among the concentrations of the various metals precluded an evaluation of their individual contributions to the children’s outcomes. No population-based studies of the neurotoxicity of cadmium have been conducted in children with validated biomarkers of exposure.
The kidney is the critical organ for the long-term effects of cadmium, and the critical effect is renal tubular dysfunction, which is most often manifested as the presence of proteins of low relative molecular mass in the urine. Studies in experimental animals indicate that histological changes occur in the renal tubules at a dose lower than that required to produce this proteinuria. In experimental animals, tubular lesions are usually seen histologically when the renal cortical concentration of cadmium is 200–300 mg/kg of wet weight. There is some evidence that the average critical concentration is as high as 300–400 mg/kg for more severe signs of renal damage.
The critical renal cortical concentrations at which a small but significant proportion of an exposed human population will have effects can be estimated. Data from human autopsies or biopsies are usually cross-sectional, i.e. the renal cadmium concentration and the effects were measured more or less simultaneously. They are difficult to interpret with regard to critical concentrations, as the cases with the most severe cadmium-induced renal dysfunction had the lowest renal concentrations of cadmium, since cadmium is lost from the kidney as the damage progresses.
Renal cadmium concentrations are disproportionately low when those in the liver are high and renal effects have developed. The average critical renal concentration in groups of exposed workers varied from 200 to 320 µg/g of tissue. The dose required to achieve a critical renal cortical concentration can be estimated on the basis of certain assumptions (e.g. one-quarter to one-third of the body burden of cadmium is in the kidney) on bioavailability, a specific critical renal cortical concentration, and a toxicokinetics model (e.g. one compartment). On the basis of a single-compartment model, the daily intake that would give rise to a concentration of 200 µg/g of wet weight in the renal cortex in a person aged 50 would be 260–480 µg/day. This prediction is based on an assumed gastrointestinal absorption of 5%, various biological half-times, and various proportions of the body burden in the kidneys. If a 10% absorption rate is assumed, the corresponding intake would be 140–260 µg/day (WHO, 1992).
The dose–response relationship can be estiumated from epidemiological data derived from population studies of the associations between exposure and response. Analysis of new population-based data indicates that the early renal effects of cadmium are prevalent after lower intakes than those indicated by the model used to establish the PTWI. That model is based on the assumption that at a renal cortical concentration of cadmium of about 200 µg/g, about 10% of the population would have renal tubular dysfunction.
In the recent meta-analysis of data from several studies of workers and environmentally exposed populations, an estimate was made of the prevalence of cadmium-induced tubular proteinuria that would be expected to occur in individuals with specific urinary concentrations of cadmium. This estimate suggests that the risk for tubular dysfunction begins to increase when urinary excretion of cadmium exceeds 2.5 µg/g of creatinine.
Information in the literature indicates an empirical relationship between the concentrations of cadmium in the diet and and in the urine. This relationship is a function of age, as both variables increase with advancing years. Furthermore, diets are made up of a mixture of foods with various cadmium contents, and some foods with high concentrations of cadmium, such as shellfish and some grains, have low bioavailability. The estimates of dietary intake were derived from data for Japan, Sweden, and the USA. The mean dietary intake of cadmium by nonsmoking women in many areas of Japan was 26 µg/day (range, 19–51 µg/day), and the mean urinary excretion of cadmium was 4.4 µg/g of creatinine (range, 3.6–7.0). These results indicate that the ratio of dietary cadmium intake to urinary cadmium excretion is 6 (range, 3–15). Among Swedish nonsmoking women, the rate of cadmium excretion in the urine was 0.15 µg/g of creatinine and the median dietary intake was 10 µg/day (range, 5.7–26). The estimated ratio of dietary cadmium intake to urinary cadmium excretion ranged from 40 to 175 (Jarup et al., 1998b).
The relationship between urinary excretion of cadmium and predicted dietary intake can be derived from a theoretical model. The dietary intakes can be predicted by assuming values for creatinine excretion, bioavailability, and the amount of absorbed cadmium excreted in the urine. It is also assumed that there are no significant changes in dietary intake of cadmium over time. By choosing a set of assumptions, a table can be constructed relating dietary intake to urinary excretion (Table 5). The three scenarios shown are reasonable, as they are based on the toxicokinetics of cadmium. This table can be used to predict a range of dietary intakes for various urinary concentrations of cadmium, which depend on the assumed values for bioavailability and for the percentage of absorbed cadmium that is excreted in urine. Table 5 also shows the links between predicted cadmium intakes and the prevalence rates of renal tubular dysfunction. For population groups for whom it is reasonable to assume that 10% of their dietary cadmium is bioavailable and that 100% of the absorbed cadmium is excreted in urine, the model predicts that dietary intakes of cadmium greater than 0.5 µg/kg bw per day would result in an increased prevalence of renal tubular dysfunction.
Table 5. Relationship between predicted cadmium intake, urinary cadmium excretion at steady state, and predicted excess prevalence of renal tubular dysfunction
|
Scenario |
Urinary excretion of cadmium |
Predicted intake of cadmiumb |
Predicted excess prevalence of renal tubular dysfunction in population (%) |
|
|
µg/day |
µg/ kg bw per dayc |
|||
|
Ratio of dietary cadmium intake to urinary excretion, 12 |
2.5 |
30 |
0.5 |
0 |
|
|
4.2 |
50 |
0.8 |
4 |
|
|
8.2 |
100 |
1.7 |
20 |
|
Ratio of dietary cadmium intake to urinary excretion, 24 |
2.5 |
60 |
1.0 |
0 |
|
|
4.2 |
100 |
1.7 |
4 |
|
|
8.2 |
200 |
3.3 |
20 |
|
Ratio of dietary cadmium intake to urinary excretion, 48 |
2.5 |
120 |
2.0 |
0 |
|
|
4.2 |
200 |
3.3 |
4 |
|
|
8.2 |
400 |
6.7 |
20 |
Assuming no significant change in cadmium intake over time
a
Derived mainly from studies of occupational exposure to cadmium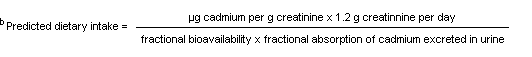
c Body weight of 60 kg assumed. The PTWI corresponds to a daily intake of 1 µg/kg bw.
The estimates of excess prevalence (Table 5) are based on studies of large, heterogeneous populations. The confidence intervals for the point estimates used are unknown and may be wide. Therefore, the estimates themselves may be conservative, in that they may be overestimates of the risks associated with dietary intake of cadmium, especially at lower concentrations. In addition, the variation in the ratios of intake to excretion in these studies may be due in part to differences in the ages of individuals in each study population. In the data set used for the United States population (Paschal et al., 2000), dietary intake of cadmium was found to be highest in early life and to decrease gradually with age, whereas urinary excretion is relatively low early in life and increases gradually with age, approaching critical concentrations at over 40 to 50 years of age.
Exposure to cadmium can occur from food, water, smoking, or at work. The assessment reported here is limited to food. Cadmium is found in food as a result of contamination of soil with cadmium. Estimation of the intake of cadmium, like most contaminants, is complicated by the skewed distribution of residues, as it does not reach foods through controlled or predictable agricultural or manufacturing processes. In addition, crops vary widely with respect to the degree of absorption from soil, depending on soil type and salinity, and to the bioavailability of the cadmium. Estimation of the potential risks associated with intake of cadmium is further complicated by the fact that its absorption by the intestines is increased under conditions of iron, zinc, and/or calcium deficiency (Chaney et al., 1996).
Australia, China, and the USA submitted data for evaluation, and additional data were available from a review prepared in Denmark for the Codex Committee on Food Additives and Contaminants (CCFAC, 1998, based, in part, on national data) and a review prepared by the European Commission. Information was also obtained from government publications and the general literature. The data covered 22 countries in Asia, Europe, North America, and Oceania.
A discussion paper prepared in Denmark(Codex Alimentarius Commission, 1998) identified leafy vegetables and cereals as the most significant sources of cadmium in the diet. Other sources include offal, since cadmium accumulates in the kidney and liver of animals. Oilseeds, crustaceans, and molluscs may also absorb large amounts of cadmium. In view of the relatively low consumption of these foods, however, their contribution to total cadmium intake is generally negligible.
Five regional diets were designed within the WHO Global Environment Monitoring System—Food Contamination Monitoring and Assessment Programme (GEMS/Food) to describe food consumption patterns in a wide variety of the world’s populations on the basis of data from food balance sheets. These diets were used with the available data on cadmium residues to estimate typical cadmium intakes. In addition, the assessments of individual countries based on national consumption and residue data were summarized when available.
The Australia New Zealand Food Authority (2000) submitted estimates of cadmium intake and detailed data on residues, the latter from the Australian market basket survey, in which composite samples are collected randomly throughout Australia, and other surveys. Food is sampled according to categories of staple, national, and regional foods. Each sample represents a composite of three to four samples. The number of samples analysed varies from six to 28, depending on the food. The study is repeated every 2 years. All foods are analysed in the state in which they would be consumed. Samples are taken of foods in 14 major groups: cereal and cereal products, meat and poultry, seafood, eggs, fats and oils, dairy products, vegetables, fruit, nuts and seeds, beverages, snack foods, sugar and confectionery, condiments, and foods for infants. Cadmium concentrations were available for samples collected between 1992 and 1998.
Data were also submitted to the Australia New Zealand Food Authority from surveillance surveys, typically in response to calls for data. Targeted surveys or surveys in which fewer than five samples were taken were not used. Data from studies conducted from 1985 onwards were used, but most were carried out after 1990.
The highest median concentrations of cadmium were found in shellfish and other seafood, organ meats, cocoa beans, potato products, peanuts, and various oilseeds.
Foods bought at the retail level in five Canadian cities during the period 1986–88 as part of a total diet survey were prepared for consumption and combined into 113 composite samples. The concentrations of residues of cadmium in these foods were summarized by Dabeka and McKenzie (1995). The median concentrations were highest in bakery goods, cereals, and vegetables.
The 1990 total diet study in China consisted of four market basket surveys covering 12 provinces. Each market basket contained 13 composite samples. Summary data from this study and on the concentrations of cadmium in 634 raw food samples collected at local markets in Beijing, Shanghai, Jiangsu, Sichuan, and Guandong in 1992 were available (Yang et al., 1994). The highest mean cadmium concentrations were observed in rice, wheat, vegetables, and aquatic foods.
In the 1995 total diet study in the Czech Republic, 12 ‘consumer baskets of foods’, each consisting of 160 foodstuffs, were collected five times during the year and prepared according to standard recipes (National Institute of Public Health, 1996). The cadmium concentrations were not given in the summary report, but the levels of food consumption and cadmium intakes were used to calculate the median cadmium concentration in each food. The highest median concentrations were calculated for bread and rolls, leafy vegetables, and potatoes.
The cadmium contents of 117 foods typical of the German diet and bought between 1988 and 1991 were analysed for cadmium in 1506 samples (Muller et al., 1996). The mean concentrations of most foods were generally low, few foods having average residue concentrations greater than 5 µg/kg. Potatoes, carrots, lettuce, herbs, pulses, organ meats, flour and farinaceous products, and cocoa and products had the highest values.
Tsombaris and Tsoukali-Papadopoulou (1994a) reported the cadmium concentrations in food samples collected in Thessaloniki over 3 years. The highest mean values were reported for lamb and beef liver, vegetables, and pasta.
The 1997–98 total diet study in New Zealand (Vanoort et al., 2000) was carried out by the Institute of Environmental Science and Research Ltd, the New Zealand Institute for Crop and Food Research Ltd, and health protection officers. Samples were collected of 114 foods, comprising 66 national and 48 regional foods. The samples were collected at two dates to provide a measure of seasonal variation and were combined to provide 532 food samples for analysis. Data on cadmium concentrations were available in summary form. The highest mean concentrations were found in oysters and mussels, organ meats, potato crisps, and peanut butter.
Jorhem and Sundstrom (1993) presented summary data on the cadmium contents of foods collected in Swedish markets between 1983 and 1990. Between one to 893 samples were collected and analysed. The highest mean concentrations were reported in beef and pork kidney, various seeds, wheat bran, and dark chocolate.
The concentrations of cadmium and other metals in foods collected for the 1997 total diet study were reported by the Ministry for Agriculture, Fisheries and Food (1999a). Samples of foods in 20 groups were obtained from 20 towns in the United Kingdom. The mean concentrations were highest in offal, nuts, bread and cereals, potatoes, and green vegetables.
A total diet study is conducted yearly in the USA by the Food and Drug Administra-tion to provide information on the concentrations of pesticides, contaminants, and nutrients in the food supply and in the diets of several subgroups defined by age and sex. More than 200 foods are collected and analysed for contaminants and nutrients. The samples are collected in supermarkets in various areas three or four times a year. Foods that require cooking are prepared according to specified instructions or recipes. Foods from each collection that are ready for the table are combined and homogenized to produce a composite representing a specified region. Aliquots are analysed for organic contaminants, elements (including cadmium), and moisture (Pennington et al., 1996; Food & Drug Administration, 2000). Data on cadmium concentrations were available for samples collected between 1991 and 1999. The highest average concentrations were found in spinach, beef liver, potato products, and peanuts. Additional data on residues of cadmium in poultry and livestock are available in the published literature (Coleman et al., 1992).
Data on residues of cadmium in countries of the European Union were available in a report prepared by the European Commission (1996). The objective of the report was to provide a scientific basis for evaluating and managing risks to public health due to dietary exposure to cadmium. The report provided the concentrations of cadmium in 15 countries (Austria, Belgium, Denmark, Finland, France, Germany, Greece, Ireland, Italy, the Netherlands, Norway, Portugal, Spain, Sweden, and the United Kingdom). The data on 16 main food groups (milk, cheese, milk products, fats and oils, fruits, leafy vegetables, other vegetables, cereals and products, meat and products, organs and offal, equine products, fish and products, molluscs and crustaceans, eggs and products, alcoholic beverages, and non-alcoholic beverages) were grouped. The data on the concentrations in other foods groups (including breast milk) were combined into 13 additional categories. Both the occurrence and the concentrations of cadmium were provided, when applicable. The largest numbers of samples were of fruit, vegetables, cereals, meat, organ meat, and molluscs and crustaceans. The highest mean cadmium concentrations were found in organ meats and offal, meat and meat by-products, molluscs and crustaceans, seeds, and mushrooms.
Summary data compiled in Denmark on the proposed maximum limits on cadmium in foods and discussed in CCFAC were also available. These data will form part of a paper that Denmark has been asked to prepare for the Thirty-third Session of CCFAC in 2001, as a contribution for the risk management of cadmium. Data from various European countries, Australia, Canada, China, Japan, Taiwan, and the USA were compiled either from written comments submitted by the various countries or from the published literature.
Data on cadmium residues in selected foods were available from the published literature for several other countries including Finland (Tahvonen & Kumpulainen, 1994a,b), Japan (Watanabe et al., 1992), the Netherlands (Kreis et al., 1992), Pakistan (Ahmad et al., 1994), and Slovakia (Ursinyova & Hladikova, 1997). Although information on residues of cadmium are available in other publications, it was decided, in the interests of time, to limit the assessment to the data listed above.
Estimates of the intake of cadmium were available for the populations of 14 countries and are summarized below and in Table 6. Most of the estimates were based on total diet studies. The Australian and Dutch estimates were based on data on consumption from national nutrition surveys, while the Japanese estimates and one set of Swedish estimates were based on studies of duplicate diets. Differences in cadmium intakes between these populations reflect differences in cadmium concentrations and food intakes and in the sources of data on consumption.
Table 6. Estimates of national cadmium intake
|
Country |
Type of data on consumption |
Estimated average Intake |
|
Austriaa |
Disappearance |
0.15 |
|
Australia |
National nutrition survey |
0.1 |
|
Belgiuma |
Household purchases, 24-h records, and FAO food balance sheets |
0.39 |
|
Canada |
Total diet study |
0.22 |
|
China |
Total diet studies |
Adult males: 0.21–0.51 |
|
Czech Republic |
Total diet study |
0.26 |
|
Denmarka |
National consumption survey |
0.28 |
|
Finlanda |
Not specified |
0.16 |
|
Francea |
Household consumption survey |
0.22 |
|
Germany |
Total diet study |
0.18 |
|
|
Food consumption surveya |
Males: 0.19 |
|
Greece |
Total diet study |
0.74, 0.94a |
|
Italya |
National consumption survey |
0.33 |
|
Japan |
Duplicate diet study |
Adult males: 0.36 |
|
Netherlands |
Food consumption survey |
Males 16–70 years: 0.33–0.40 |
|
New Zealand |
Total diet study |
Young males: 0.40/0.24b |
|
Norwaya |
|
0.14 |
|
Portugala |
National consumption survey and household budget data |
0.26 |
|
Spaina |
Not specified |
0.30 |
|
Swedena |
Not specified |
Males: 0.12 |
|
United Kingdom |
Total diet study |
0.17 |
|
|
National nutrition survey |
0.20 |
|
United States |
Total diet study |
Adult males: 0.14–0.15 |
a
From European Commissionb
Intake from all foods / intake when the consumption of oysters is excludedThe estimates of intake of cadmium by the Australian population are based on the median concentrations in food samples collected in the Australian market basket survey, on the maximum concentrations specified in the Australian Food Standards Code, and on data on consumption from the 1995 national nutrition survey. Estimates based on maximum concentrations were also presented in the summary report prepared by the Australia New Zealand Food Authority. The estimated average intake based on the maximum concentrations was 0.6 µg/kg bw per day, while the 95th percentile of the intake distribution derived from the maximum concentrations was 1 µg/kg bw per day. More realistic estimates were derived from survey data, in which the estimated average intake of cadmium was 0.1 µg/kg bw per day and the 95th percentile was 0.4 µg/kg bw per day. Six foods (potatoes, milk, wheat flour, carrots, cocoa beans, and meat) contributed more than 50% of the total cadmium intake.
The estimated average intake of cadmium in 1986–88 by the Canadian population was 0.22 µg/kg bw per day. Bakery goods and cereals and vegetables contributed more than 75% of the total intake (Dabeka & McKenzie, 1995).
Estimates of the average intake of cadmium by Chinese men ranged from 0.21 to 0.51 µg/kg bw per day (assuming 60 kg body weight), cereals and vegetables contributing more than 70% of the total cadmium intake. Average estimates were also derived for other population subgroups.
The estimated average intake of cadmium, derived from median cadmium concentrations, was 0.26 µg/kg bw per day. Potatoes, bread, and rolls contributed more than 60% of the total cadmium intake. The estimate based on maximum concentrations was 0.51 µg/kg bw per day.
The average intakes of cadmium by men and women in Germany were derived for 1988 and 1991 (Muller et al., 1998). The total cadmium intake was similar in both periods (0.14 µg/day for men and 0.10 and 0.11 µg/day for women in the two periods, respectively), but the contributions of various foods differed, reflecting changes in dietary habits such as decreased consumption of breads, cakes, and pastries. They also reflected a change in cadmium concentrations, including an increase in that in vegetables. The intake of cadmium ranged from 0.16 µg/kg bw per day for women in 1988 to 0.19 µg/kg bw per day for men in the same year. The value for both men and women in 1991 was 0.18 µg/kg bw per day.
The daily intakes of cadmium from common foods were estimated by Tsoumbaris and Tsoukali-Papadopoulou (1994b). The concentrations in samples collected in Thessaloniki and data on consumption were derived from three sources: answers to questionnaires, data from the Agricultural Bank of Greece, and WHO. The estimated average intakes derived with each of these sources were 0.74 µg/kg bw per day, 0.94 µg/kg bw per day, and 0.80 µg/kg bw per day, respectively.
Estimates of the intake of cadmium by Japanese men and women aged 30–69 were derived from analysis of duplicate diets collected twice in the same village in north-eastern Japan, once in 1976–77 and once in 1989 (Watanabe et al., 1992). The daily intake of cadmium was assumed to follow a log-normal distribution. The estimated intake decreased from 0.47 µg/kg bw per day for men and 0.46 µg/kg bw per day for women in 1976–77 to 0.36 µg/kg bw per day for men and 0.31 µg/kg bw per day for women in 1989. The apparent decline in intake was attributed in part to a decline in cadmium concentrations in cooked rice.
Estimates of lifetime accumulated cadmium intake from foods and of average daily cadmium intake were derived for three populations who (a) do not grow their own vegetables and (b) grow their own vegetables in soils not necessarily contaminated with cadmium and (c) persons living in an area in which the soil was known to be heavily contaminated with cadmium and who grow and eat their own vegetables (Kreis et al., 1992). The estimated cadmium intake of adults ranged from 0.31 to 0.40 µg/kg bw per day.
The intake of cadmium was calculated for six population subgroups in the total diet study. The values were 0.40 µg/kg bw per day for men aged 19–24, 0.33 µg/kg bw per day for men aged 25 or more, and 0.33 µg/kg bw per day for women aged 25 or more. High concentrations of cadmium residues in oysters contributed 39%, 43%, and 52%, respectively, of the total intake of cadmium of these three subgroups.
Vahter et al. (1990), Berglund et al. (1991), and Jorhem et al. (1998) reported estimates of the intakes of cadmium by 15 nonsmoking women. The estimates were based on analyses of duplicate diets collected over 7 days. The cadmium intakes ranged from 0.10 to 0.22 µg/kg bw per day, with an average of 0.12 µg/kg bw per day for males and 0.13 µg/kg bw for females.
Jorhem and Sundstrom (1993) presented summary data on the cadmium contents of foods and the associated estimated cadmium intakes of the Swedish population. Total cadmium intake was estimated to be 0.20 µg/kg bw per day. Wheat flour and potatoes contributed most to the total cadmium intake (0.11 µg/kg bw per day).
Estimates of the cadmium intake of the population of the United Kingdom and of adult consumers were derived from data on residues of cadmium from the total diet study of the Ministry of Agriculture, Fisheries and Food and data on consumption from the national food survey and the dietary and nutritional survey of British adults. The cadmium concentrations were assumed to be equal to the upper bound of the mean concentrations in the 20 groups of foods included in the total diet survey. The estimated cadmium intake of the total population was 0.20 µg/kg bw per day, and that of the 97.5th percentile of the distribution of intake by adult consumers was 0.23 µg/kg bw per day.
Estimates of the cadmium intake of the population of the USA and 14 subpopulations were derived from the sources described earlier. The values for groups subdivided by age and sex ranged from 0.13 to 0.20 µg/kg bw per day.
The intakes of 15 major food groups (cheese and milk products were combined) were estimated for each country that submitted data on consumption and cadmium concentrations. When the data were available, estimates of both high and average intakes were provided. The most important sources of cadmium were cereals, vegetables, fruits, and molluscs. While cereals, vegetables, and fruits have lower concentrations of cadmium than molluscs, they are eaten much more frequently. Estimates of total intake were also derived for each country and varied from 0.12 µg/kg bw per day for the female population of Sweden to 0.94 µg/kg bw per day in Greece.
The intake of cadmium from wheat flour in Pakistan was 0.89 µg/kg bw per day. The concentrations were measured in samples of wheat and wheat by-products collected from local markets in three cities (Ahmad et al., 1994).
In Slovakia, 35 samples of breast milk, eight samples of infant formula, and 12 samples of pasteurized cows’ milk were analysed. The median daily intakes of cadmium from these sources by a 5-kg infant, assuming a daily intake of 800 g of milk, were 0.08 µg/kg bw from breast milk, 0.1 µg/kg bw from infant formula, and 0.29 µg/kg bw from cows’ milk (Ursinyova & Hladikova, 1997).
The Ministry of Agriculture, Fisheries and Food (1999b) in the United Kingdom estimated the average daily intakes of cadmium from infant foods to be 0.1 µg/kg bw for 0–6-month-old infants and 0.16 µg/kg bw for 6–12-month-old infants. The maximum intake for infants aged 0–6 months was 0.43 µg/kg bw per day, and the 97.5th percentile intake for those aged 6–12 months was 0.41 µg/kg bw per day. In another study, the Ministry of Agriculture, Fisheries and Food estimated the intakes of cadmium from 101 samples of vegetarian diets, each representing a 7-day diet, to be 5–25 µg/day, with an average of 15 µg/day.
The data on cadmium residues summarized above were used to assess the cadmium intakes in the five GEMS/Food regional diets. To simplify the assessment, the data on residues were grouped for the major food groups in the regional diets. Within each food group, and for each country for which data were available, the median residue concentrations were derived for foods with similar patterns of cadmium residues, where possible (otherwise, mean residues were used), and these values were combined with the total consumption of these foods within each diet. Thus, for instance, since cadmium residues have been shown to occur at higher concentrations in leafy vegetables than in other vegetables, the foods in the ‘Vegetables’ category were combined into three groups: leafy vegetables, mushrooms, and all other vegetables. If no data were available on some foods in a region, surrogate data on similar food groups or other regions were used. Since no data were available for the African and Latin American regions, the European data on residues were used. Note that the ‘European’ regional diet does not represent only that of Europe, since it also includes those of Australia, Canada, New Zealand, and the United States. The concentrations of cadmium used in the assessment are summarized in Table 7, and the resulting estimates of cadmium intake from the five regional diets are summarized in Table 8.
Table 7. Concentrations of cadmium deriveda from various surveysb (mg/kg)
|
Code |
Commodity |
Middle Eastern |
Far Eastern |
European |
|
|
Cereals |
|||
|
GC 649 |
Rice |
0.010 |
0.048 |
0.010 |
|
GC 654 |
Wheat |
0.048 |
0.023 |
0.028 |
|
|
Othere |
0.016 |
0.016 |
0.016 |
|
VR 75 |
All roots and tubers |
0.027 |
0.011 |
0.027 |
|
|
Pulses |
|||
|
VD 541 |
Soya beans |
0.012 |
0.015 |
0.012 |
|
|
Other |
0.008 |
0.015 |
0.008 |
|
|
All sugar and honey |
0.003 |
0.000 |
0.003 |
|
|
Nuts and oilseeds |
|||
|
SO 697 |
Groundnuts, shelled |
0.049 |
0.049 |
0.049 |
|
SO 702 |
Sunflower seeds |
0.220 |
0.220 |
0.220 |
|
|
Other oilseeds |
0.148 |
0.148 |
0.148 |
|
|
Vegetable oils and fats |
|||
|
OR 541 |
Soya bean oil |
0.003 |
0.003 |
0.003 |
|
OR 702 |
Sunflower seed oil |
0.003 |
0.003 |
0.003 |
|
OR 172 |
Other vegetable oils |
0.002 |
0.002 |
0.002 |
|
|
Stimulants |
|||
|
SB 715 |
Cocoa beans |
0.120 |
0.120 |
0.120 |
|
|
Other |
0.002 |
0.002 |
0.002 |
|
HS 93 |
All spices |
0.000 |
0.000 |
0.000 |
|
|
Vegetables |
|||
|
VL 53 |
Leafy vegetables |
0.074 |
0.023 |
0.016 |
|
VO 450 |
Mushrooms |
0.009 |
0.023 |
0.009 |
|
|
Other |
0.038 |
0.023 |
0.009 |
|
|
Fish and seafood |
|||
|
IM 151 |
Molluscs, except cephalopods, fresh |
1.360 |
0.124 |
1.360 |
|
IM 150 |
Molluscs, canned |
1.360 |
0.124 |
1.360 |
|
|
Other |
0.048 |
0.012 |
0.010 |
|
PE 112 |
All eggs |
0.001 |
0.006 |
0.001 |
|
|
All fruit |
0.016 |
0.011 |
0.003 |
|
|
Milk and milk products |
|||
|
ML 106 |
Milk |
0.001 |
0.004 |
0.001 |
|
|
Milk products |
0.005 |
0.004 |
0.001 |
|
|
Meat and offal |
|||
|
MO 1280 |
Cattle kidney |
0.146 |
0.156 |
0.154 |
|
MO 1281 |
Cattle liver |
0.146 |
0.378 |
0.077 |
|
MM 816 |
Horsemeat |
0.270 |
0.626 |
0.270 |
|
MO 97 |
Edible offal of cattle, pigs, sheep |
0.146 |
0.012 |
0.041 |
|
PM 110 |
Poultry meat |
0.025 |
0.009 |
0.002 |
|
PO 111 |
Edible offal of poultry |
0.025 |
0.019 |
0.005 |
|
PF 111 |
Poultry fat |
0.025 |
0.009 |
0.003 |
|
PO 113 |
Poultry skin |
0.025 |
0.009 |
0.003 |
|
|
Other meat |
0.053 |
0.009 |
0.003 |
|
|
All animals oils and fats |
0.001 |
0.001 |
0.001 |
a
Arithmetic average of median concentrations (when possible, otherwise mean residue concentration) for each countryb
When concentrations were not available for a particular food group, the European average was used.Table 8. Estimates of cadmium intake (µg/kg bw per day, assuming body weight of 60 kg) in the WHO GEMS/Food regional diets
|
Code |
Commodity |
Middle Eastern |
Far Eastern |
African |
Latin American |
European |
|
|
Cereals |
|||||
|
GC 649 |
Rice |
0.008 |
0.224 |
0.017 |
0.014 |
0.002 |
|
GC 654 |
Wheat |
0.264 |
0.044 |
0.013 |
0.055 |
0.084 |
|
|
Other |
0.015 |
0.016 |
0.050 |
0.013 |
0.010 |
|
GC 80 |
All |
0.287 |
0.283 |
0.080 |
0.082 |
0.095 |
|
VR 75 |
All roots and tubers |
0.028 |
0.020 |
0.146 |
0.072 |
0.110 |
|
|
Pulses |
|||||
|
VD 541 |
Soya beans |
0.001 |
0.001 |
0.000 |
0.000 |
0.000 |
|
|
Other |
0.003 |
0.004 |
0.002 |
0.003 |
0.002 |
|
VD 70 |
All |
0.004 |
0.005 |
0.002 |
0.003 |
0.002 |
|
|
All sugar and honey |
0.004 |
0.000 |
0.002 |
0.005 |
0.005 |
|
|
Nuts and oilseeds |
|||||
|
SO 697 |
Groundnuts, shelled |
0.000 |
0.000 |
0.002 |
0.000 |
0.002 |
|
SO 702 |
Sunflower seeds |
0.004 |
0.000 |
0.002 |
0.000 |
0.000 |
|
|
Other oilseeds |
0.028 |
0.123 |
0.077 |
0.141 |
0.067 |
|
|
All |
0.032 |
0.123 |
0.081 |
0.142 |
0.069 |
|
|
Vegetable oils and fats |
|||||
|
OR 541 |
Soya bean oil |
0.000 |
0.000 |
0.000 |
0.001 |
0.000 |
|
OR 702 |
Sunflower seed oil |
0.000 |
0.000 |
0.000 |
0.000 |
0.000 |
|
OR 172 |
Other vegetable oils |
0.001 |
0.000 |
0.001 |
0.000 |
0.001 |
|
|
All |
0.001 |
0.000 |
0.001 |
0.001 |
0.001 |
|
|
Stimulants |
|||||
|
SB 715 |
Cocoa beans |
0.001 |
0.000 |
0.000 |
0.003 |
0.006 |
|
|
Other |
0.000 |
0.000 |
0.000 |
0.000 |
0.000 |
|
|
All |
0.001 |
0.000 |
0.000 |
0.003 |
0.007 |
|
HS 93 |
All spices |
0.000 |
0.000 |
0.000 |
0.000 |
0.000 |
|
|
Vegetables |
|||||
|
VL 53 |
Leafy vegetables |
0.010 |
0.004 |
0.000 |
0.005 |
0.014 |
|
VO 450 |
Mushrooms |
0.000 |
0.000 |
0.000 |
0.000 |
0.001 |
|
|
Other |
0.142 |
0.065 |
0.012 |
0.020 |
0.048 |
|
|
All |
0.152 |
0.069 |
0.012 |
0.025 |
0.063 |
|
|
Fish and seafood |
|||||
|
IM 151 |
Molluscs, excluding cephalopods, fresh |
0.000 |
0.008 |
0.011 |
0.018 |
0.188 |
|
IM 150 |
Molluscs, canned |
0.000 |
0.000 |
0.000 |
0.000 |
0.018 |
|
|
Other |
0.010 |
0.006 |
0.006 |
0.007 |
0.006 |
|
|
All |
0.010 |
0.014 |
0.017 |
0.026 |
0.212 |
|
PE 112 |
All eggs |
0.000 |
0.001 |
0.000 |
0.000 |
0.001 |
|
|
All fruit |
0.055 |
0.016 |
0.005 |
0.014 |
0.011 |
|
|
Milk and milk products |
|||||
|
ML 106 |
Milk |
0.002 |
0.002 |
0.001 |
0.003 |
0.006 |
|
|
Milk products |
0.001 |
0.000 |
0.000 |
0.000 |
0.001 |
|
AO 31 |
All |
0.004 |
0.002 |
0.001 |
0.003 |
0.007 |
|
|
Meat and offal |
|||||
|
MO 1280 |
Cattle kidney |
0.000 |
0.000 |
0.000 |
0.001 |
0.001 |
|
MO 1281 |
Cattle liver |
0.000 |
0.000 |
0.000 |
0.000 |
0.001 |
|
MM 816 |
Horsemeat |
0.000 |
0.000 |
0.001 |
0.000 |
0.004 |
|
MO 97 |
Edible offal of cattle, pigs, and sheep |
0.009 |
0.000 |
0.002 |
0.004 |
0.008 |
|
PM 110 |
Poultry meat |
0.013 |
0.002 |
0.000 |
0.001 |
0.002 |
|
PO 111 |
Edible offal of poultry |
0.000 |
0.000 |
0.000 |
0.000 |
0.000 |
|
PF 111 |
Poultry fat |
0.001 |
0.000 |
0.000 |
0.000 |
0.000 |
|
PO 113 |
Poultry skin |
0.000 |
0.000 |
0.000 |
0.000 |
0.000 |
|
|
Other meat |
0.029 |
0.004 |
0.001 |
0.002 |
0.006 |
|
|
All |
0.053 |
0.007 |
0.004 |
0.008 |
0.021 |
|
|
Animals oils and fats |
0.000 |
0.000 |
0.000 |
0.000 |
0.000 |
|
|
Total diet (µg/kg bw per day) |
0.632 |
0.541 |
0.352 |
0.383 |
0.603 |
The mean intake for a 60-kg person varied from 0.35 to 0.63 µg/kg bw per day. It should be noted that these might be overestimates, since the GEMS/Food diets represent conservative estimates of consumption. Furthermore, the estimates derived for the African and Latin American diets might not be representative of exposure in these two regions.
Cadmium concentrations vary from food to food, higher values being observed in organ meats, molluscs, wheat, rice, leafy vegetables, potatoes, carrots, and oilseeds. In addition, the concentrations in specific foods show some variation between countries and within countries by region or season. The relative contribution of the various foods to the total intake of cadmium depends only partly on the cadmium concentration in these foods, as for most contaminants and food additives. The amounts consumed also play a significant role in the intake of cadmium. Consumption patterns differ among countries and among subpopulations within a country. Nevertheless, the same general patterns of cadmium intake were observed for most of the populations considered.
In a recent national health survey in the United States, the urinary concentration of cadmium was measured in 22 162 participants. Both the uncorrected concentration and that corrected for creatinine increased with age and with amount of smoking. The arithmetic mean urinary cadmium concentration was 0.57 µg/L or 0.48 µg/g of creatinine. About 2.3% of the subjects had concentrations greater than 2 µg/g of creatinine, and only 0.2% had concentrations that exceeded those consistent with the PTWI (Paschal et al., 2000).
In another analysis in which the urinary concentrations from the above study were used to verify those predicted, it was demonstrated that women excrete twice as much cadmium as males. The predictions were derived with the ‘cadmium dietary exposure model’ in which data on cadmium concentrations in food are derived from national surveys (the total diet study) and food consumption patterns are used to estimate dietary intake. The model was linked to a modification of the cadmium biokinetics model of Kjellstrom and Nordberg (WHO, 1992) to derive estimates of renal cortical concentrations and urinary cadmium concentrations. The peak renal cortical concentration in males was 15 µg/g of wet cortex, while that in females was 29 µg/g of wet cortex (Diamond et al., 2000).
Watanabe et al. (2000) also studied the dietary intake of cadmium by the Japanese population and at the same time measured biomarkers. Peripheral blood, urine, and duplicate food samples over 24 h were collected from 399 nonsmoking women in 1977–81 and from 588 nonsmoking women in 1991–97 at 27 survey sites throughout Japan. The average cadmium concentration in Japanese diets was 38 ± 2.0 µg/day in 1977–81 and 26 ± 2.1 µg/day in 1991–97. The changes in cadmium intake were attributed mainly to reduced consumption of rice. The current concentration is still high, however, when compared with international values.
Ikeda et al. (2000) reported the results of a nationwide survey in which cadmium was measured in 24-h duplicate food samples, blood, and urine. Analyses for 607 nonsmoking women showed that the concentrations of alpha1-microglobulin and possibly beta2-microglobulin increased as a function of the cadmium concentrations in these three media. The geometric mean concentrations were 25 µg/L in food, 1.8 µg/L in blood, and 3.9 µg/g of creatinine.The evidence for renal dysfunction was of borderline significance.
The bioavailability of cadmium can be affected markedly by nutritional factors. Low iron status, as determined from serum ferritin levels, which is prevalent among women, increases the uptake of cadmium from the gastrointestinal tract. Furthermore, the bioavailability of cadmium from some grains or seeds and foods in which it is bound to phytates, metallothionein, and other proteins may be reduced. The Committee examined the information on bioavailability that had become available since its forty-first meeting and concluded that it did not significantly differ from that considered previously. While information suggesting altered bioavailability due to dietary and nutritional factors exists, the bulk of the evidence indicates that the overall point estimate of 5% for bioavailability that was used in previous models of the relationship between cadmium intake and critical effects is appropriate. For specific populations, such as people with iron deficiency, the bioavailability of cadmium may range from 5 to 10%. Studies in which experimental animals were given parenteral injections of cadmium do not address the question of the bioavailability of ingested cadmium.
Cadmium has an extremely long biological half-life in mammals (estimated to be 17 years or longer in humans) and accumulates in the liver and kidney. The presence of cadmium in food can result in long-term intake of low concentrations. The toxic effects of this metal on the kidneys (e.g. tubular dysfunction) are the most sensitive for use in evaluating its health effects. While cadmium can affect organs other than the kidneys, it generally does so at doses higher than those associated with renal effects. Acute effects can occur after ingestion of very high concentrations, and these may be fatal, owing to widespread systemic distribution. Such outcomes do not arise from typical dietary concentrations.
Non-renal effects
Neurodevelopmental and neurobehavioural effects have been demonstrated in experimental animals given repeated doses of cadmium by gavage. Studies in which cadmium was administered by injection showed that it cannot easily enter the brain, its entry being blocked by the blood–brain barrier. Cadmium can replace zinc in a number of metallo-enzymes, proteins, and ion channels, generally increases the brain concentrations of noradrenaline and dopamine, and impairs enzymes required for the production of neurotransmitters. The peripheral nervous system may also be susceptible to long-term exposure to cadmium, but the investigations of these effects were limited. Studies of occupationally exposed adults have shown increased prevalences of peripheral neuropathies and neurobehavioural deficits in specific domains (e.g. attention, psychomotor speed, memory). No population-based studies of neurotoxicity after environmental exposure of adults or children to cadmium were available in which validated biomarkers of exposure were used.
Experimental studies have shown that cadmium can induce metallothionein synthesis in the placenta and that cadmium is retained in the placenta after exposure to low concentrations. Large doses of cadmium compounds administered parenterally to several rodent species at a late stage of gestation induced severe placental damage and fetal deaths, whereas similar doses given parenterally in the early stages of gestation had teratogenic effects. Teratogenic effects have not been reported after oral intake of cadmium by animals or humans.
Cadmium is carcinogenic in experimental animals when given by injection or inhalation, and exposure of workers by inhalation has been shown to result in pulmonary cancer. There was no evidence that cadmium is carcinogenic to humans exposed by the oral route.
Large, population-based studies provided little evidence that changes in blood pressure or in the prevalence of ischaemic heart disease are related to blood or urinary cadmium concentrations.
Excretion of cadmium in the urine is weakly but significantly associated with elevated urinary calcium concentrations and increased serum alkaline phosphatase activity. Studies of occupationally exposed persons suggest that high urinary and blood cadmium concentrations are associated with low bone mineral density. In environmentally exposed post-menopausal women, higher urinary cadmium excretion was associated with hypercalciuria, osteoporosis, reduced bone density, and an increased risk for fractures. The relationship between the effect of cadmium on calcium metabolism and osteoporosis should be investigated further in so far as the effects on bone might be a more sensitive indicator of the toxicity of cadmium than the renal effects.
Renal effects
The kidney is the critical target organ in mammals, including humans, exposed for long periods to small amounts of cadmium. Cadmium produces renal tubular dysfunction characterized by hypercalciuria and increased excretion of several low-molecular-mass proteins. In particular, beta2-microglobulin has served as a biomarker of toxicity and may complement urinary cadmium as a biomarker of intake from various foods.
The renal tubular dysfunction seen in non-human mammalian species exposed to low dietary concentrations of cadmium is analogous to that produced in humans; in animals, this may progress to interstitial nephropathy and glomerulopathy with longer exposure. The critical renal concentration of cadmium that is associated with cadmium-induced nephropathy in animals is 50–200 µg/g of renal cortex, which is consistent with the results of studies of humans. Recent studies in various species indicate that when the concentration of cadmium in the renal cortex exceeds 250 µg/g, continued exposure results in further increases in the concentration in the liver but no further increase in that in the renal cortex. This effect has been suggested to reflect increased loss of cadmium in the urine owing to tubular dysfunction. Experimental studies have shown that impaired glomerular filtration (i.e. increased serum creatinine and blood urea nitrogen concentrations) is a less sensitive indicator of cadmium-induced nephropathy than are indicators of tubular dysfunction or injury.
Many reports from Japan and a large population-based study of environmental exposure to cadmium in Belgium confirm that the major risk factors for cadmium-induced renal effects in humans exposed other than occupationally include increasing age, higher alcohol consumption, cigarette smoking, and residence in a cadmium-contaminated region. In these studies, renal effects were investigated by using urinary biomarkers of renal dysfunction. Several markers of proximal tubular function, including N-acetyl-beta-glucosaminidase activity and the concentrations of retinol-binding protein, beta2-microglobulin, amino acids, and calcium, are related to the urinary cadmium concentrations of environmentally exposed individuals. No threshold was found for the relationship between N-acetyl-beta-glucosaminidase activity and urinary cadmium concentration. A 10% prevalence of abnormal values for these markers was found when urinary cadmium concentrations exceeded 2–4 µg/24 h or the estimated concentration in the renal cortex was > 50 µg/g. Estimates of the relation-ship between urinary cadmium concentration and abnormal values for these markers were considerably lower in studies of environmental exposure than in studies of occupational exposure; however, past exposure would be underestimated in areas where cadmium concentrations in the environment had been reduced prior to the time the study was conducted. Certain individuals, such as patients with diabetes or pre-existing renal disorders, appear to be at increased risk for cadmium-related renal dysfunction.
Follow-up studies of workers with cadmium-related renal dysfunction suggest that many of the changes are irreversible, with continued declines in glomerular function for decades after cessation of heavy exposure. The low-molecular-mass proteinuria associated with long-term exposure to cadmium is deemed to be irreversible. This assumption is borne out by the observation in studies of occupational and environmental exposure that renal tubular function in people who excrete > 1000 µg of beta2-microglobulin in the urine per 24 h does not improve or even worsens within 5 years of reduction of exposure to cadmium. The prognosis appears to be more favourable for individuals with lower body burdens. In the Belgian study, some of the subtle tubular effects seen at the time the participants entered the study were no longer apparent at follow-up or, at least, were not associated with a decline in glomerular function in the interim.
A comprehensive meta-analysis of the relevant epidemiological studies and a risk assessment suggested that the risk for renal dysfunction and progression to clinical disease could be lowered if exposure to cadmium were reduced such that the concentrations of cadmium in the kidney and urine were maintained below 50 µg/g of renal cortex and 2.5 µg/g of creatinine, respectively.
The diet is the major route of human exposure to cadmium. Contamination of foods with cadmium results from its presence in soil and water. Estimation of the intake of cadmium, like that of most contaminants, is complicated by the skewed distributions of residues, since cadmium does not reach foods through controlled or predictable agricultural or manufacturing processes. In addition, crops differ widely with respect to their absorption of cadmium from soil, depending on soil type and salinity, and in the bioavailability of cadmium. The cadmium concentrations in food samples vary widely, but the highest average concentrations are detected in molluscs, kidney, liver, cereals, cocoa, and leafy vegetables. Estimates of mean cadmium intake from national food surveys and total diet studies generally ranged from 0.1 to 0.5 µg/kg bw per day. The estimates derived from the WHO regional diets, based on food balance sheets, ranged from 0.35 to 0.63 µg/kg bw per day.
Analysis of new data from population-based studies indicates that the early renal effects of cadmium are prevalent at lower intakes than those indicated by the model used by the Committee to confirm the PTWI at its forty-first meeting. That model was based on the assumption that about 10% of a population with a concentration of cadmium in the renal cortex of about 200 µg/g would experience renal tubular dysfunction.
In a recent meta-analysis of data from several studies of workers and general populations exposed to cadmium, the prevalence of cadmium-induced tubular proteinuria that would be expected to occur in individuals with specific urinary concentrations of cadmium was estimated. This analysis suggests that the risk for tubular dysfunction begins to increase when the urinary excretion of cadmium exceeds 2.5 µg/g of creatinine. The Committee considered this value to represent no excess prevalence of renal tubular dysfunction.
The Committee used data in the literature to investigate the empirical relationship between the concentration of cadmium in the diet and urinary concentrations. This relationship is a function of increasing cadmium concentration in the renal cortex and increasing urinary excretion with age. Furthermore, diets are made up of a mixture of foods with different cadmium contents, and its bioavailability from some of the foods that contain high concentrations, such as shellfish and some grains, is low. The estimates were derived from data from Japan, Sweden, and the USA. The mean dietary intake of cadmium by nonsmoking women in many areas of Japan was 26 µg/day (range, 19–51 µg/day), and the mean urinary excretion of cadmium was 4.4 µg/g of creatinine (range, 3.6–7.0 µg/g of creatinine). These data indicate a ratio of dietary cadmium to urinary cadmium excretion of 6 (range, 3–15). The data from Sweden indicate that female nonsmokers have a urinary cadmium excretion of 0.15 µg/g of creatinine and a median dietary intake of 10 µg/day (range, 5.7–26 µg/day). The estimated ratio of dietary cadmium to urinary cadmium excretion ranged from 40 to 175. The mean dietary intake of cadmium in a total diet study in the USA was 5.5 µg/day, and the mean value for urinary excretion of cadmium obtained independently in a national survey of health and nutrition in the USA was 0.5 µg/g of creatinine, resulting in a ratio of dietary cadmium to urinary cadmium excretion of 11.
The relationship between urinary cadmium excretion and dietary intake of cadmium can be predicted from a theoretical model. Possible dietary intakes can be predicted from the amount of cadmium excreted in the urine if it is assumed that there are no significant changes in the dietary intake of cadmium over time. Once a set of assumptions has been chosen, a table can be constructed, relating dietary intake to urinary cadmium excretion (Table 5). The three selected scenarios are reasonable, as they are based on data for the toxicokinetics of cadmium. This table can be used to predict a range of dietary intakes for different urinary cadmium concentrations, which depend on the assumed values for bioavailability and for the percentage of absorbed cadmium that is excreted in urine. Table 5 also links the predicted cadmium intakes to prevalence rates of renal tubular dysfunction. For population groups in which it is reasonable to assume that 10% of dietary cadmium is bioavailable and that 100% of the absorbed cadmium is excreted in urine, the model predicts that dietary intakes of cadmium > 0.5 µg/kg bw per day would result in an increased prevalence of renal tubular dysfunction.
The estimates of excess prevalence (Table 5) were derived from studies of large, heterogeneous populations. As the confidence intervals for the point estimates are unknown, and may be wide, the estimates may be conservative in that they may be overestimates of the risks associated with dietary intake of cadmium, especially at lower levels. The Committee concluded that the incidences of renal tubular dysfunction in populations with various dietary intakes of cadmium can serve as a reasonable basis for risk assessment if the assumptions made when applying the predictive model are scientifically based and clearly described.
Even though new information indicates that a proportion of the general population may be at increased risk for tubular dysfunction when exposed at the current PTWI of 7 µg/kg bw, the Committee maintained this value because the risk estimates that can be made at present are imprecise. The range of predicted dietary intakes that may be associated with an excess prevalence of renal tubular dysfunction (Table 5) can be used to indicate risk at various levels of intake by potentially sensitive groups within a population.
The Committee recommended that seven areas be investigated in order to increase confidence in the estimates of excess prevalence of renal tubular dysfunction.
1. The toxicokinetics of cadmium should be investigated in controlled experimental studies in humans of the relationship between dietary intake and urinary excretion of cadmium by the general population and by groups at high risk, such as people with iron deficiency, renal disease, or diabetes mellitus.
2. Surveys should be conducted in which individual records of the food consumption of specific population subgroups are kept.
3. Studies should be conducted of the bioavailability of cadmium from specific foods and of the factors that affect bioavailability, such as age, health status, and dietary nutrients.
4. The relationship between biomarkers of renal tubular dysfunction and biomarkers of exposure should be elucidated.
5. The relationship between renal tubular dysfunction (as determined by specific biomarkers), clinical disease, and mortality should be studied.
6. The influence of cadmium on calcium metabolism and osteoporosis requires clarification.
7. Studies should be conducted to determine the effect of exposure to cadmium (integrated over a lifetime) on the development of osteoporosis later in life.
Agency for Toxic Substances and Disease Registry (1999) Draft Txoicological Profile for Arsenic, Washington DC, US Public Health Service, US Department of Health and Human Services.
Ahmad, S., Waheed, S., Mannan, A., Fatima, I. & Qureishi, I.H. (1994) Evaluation of trace elements in wheat and wheat by-products. J. AOAC Int., 77, 11–18.
Ahokas, R. & Dilts, P. (1979) Cadmium uptake by the rat embryo as a function of gestational age. Am. J. Obstet. Gynecol., 135, 219–222.
Alfven, T., Elinder, C.-G., Carlsson, M., Grubb, A., Hellstrom, L., Persson, B., Pettersson, C., Spang, G., Schutz, A. & Jarup, L. (2000) Low level cadmium exposure and osteoporosis. J. Bone Min. Res. (in press).
Anderson, C. & Danylchuk, K.D. (1979) Plasma levels of immunoreactive parathyroid hormone in dogs chronically exposed to low levels of cadmium chloride. J. Environ. Pathol. Toxicol., 2, 1151–1159.
Anderson, O., Nielsen, J.B. & Svendsen, P. (1988) Oral cadmium chloride intoxication in mice: Effects of dose on tissue damage, intestinal absorption and relative organ distribution. Toxicology, 48, 225–236.
Ando, M. (2000) Study on the intestinal absorption of cadmium in rats given minimum amounts of cadmium chloride or cadmium-polluted rice for 8 months.
Ando, M., Shimizu, M., Sayato, Y., Tanimura, A. & Tobe, M. (1981) The inhibition of vitamin D-stimulated intestinal calcium transport in rats after continuous oral administration of cadmium. Toxicol. Appl. Pharmacol., 61, 297–301.
Ando, M., Hiratsuka, H., Nakagawa, J., Sato, S., Hayashi, Y. & Mitsumori, K. (1998) Cadmium accumulation in rats treated orally with cadmium chloride for 8 months. J. Toxicol. Sci., 23, 243–248.
Aoshima, K. & Kasuya, M. (1991) Preliminary study on serum levels of 1,25-dihydroxyvitamin D and 25-hydroxyvitamin D in cadmium-induced renal tubular dysfunction. Toxicol. Lett., 57, 91–99.
Arivison, B. (1980) Regional differences in the severity of cadmium-induced lesions in the peripheral nervous system in mice. Acta Neuropathol., 49, 213–224.
Aughey, E., Fell, G., Scott, R. & Black, M. (1984) Histopathology of early effects of oral cadmium in the rat kidney. Environ. Health Perspectives, 54, 153–161.
Australia/New Zealand Food Authority (2000) Dietary exposure assessment for cadmium. Submitted to WHO.
Baer, K.N. & Benson, W.H. (1987) Influence of chemical and environmetal stressors on acute cadmium toxicity. J. Toxicol. Environ. Health, 22, 35–44.
Baranski, B. (1986) Effect of maternal cadmium exposure on postnatal development and tissue cadmium, copper and zinc concentrations in rats. Toxicology, 58, 255–260.
Baranski, B., Stetkiewicz, I., Sitarek, K. & Szymczak, W. (1983) Effects of oral, subchronic cadmium administration on fertility, prenatal and postnatal progeny development in rats. Arch. Toxicol., 54, 297–302.
Basinger, M., Jones, M. & Holscher, M. (1988) Antagonists for acute oral cadmium chloride intoxification. J. Toxicol. Environ. Health, 23, 77–89.
Bergland, M., Akesson, A., Nermell, B. & Vahter, M. (1994) Intestinal absorption of dietary cadmium in women depends on body iron stores and fiber intake. Environ. Health Perspectives, 102, 1058–1066.
Berglund, M., Vahter, M. & Slorach, S. (1991) Determination of human exposure to lead and cadmium: A WHO/UNEP pilot study. Chem. Spec. Bioavailability, 3, 69–72.
Bernard, A., Lauwerys, R. & Gengoux, P. (1981) Characterization of the proteinuria induce by prolonged oral administration of cadmium in female rats. Toxicology, 20, 345–357.
Bernard, A., Lauwerys, R. & Gengoux, P. (1981) Characterization of the proteinuria induced by prolonged oral administration of cadmium in female rats. Toxicology, 20, 345–357.
Bernard, A., Lauwerys. R. & Amor, A.O. (1992a) Loss of glomerular polyanion correlated with albuminuria in experimental cadmium nephropathy. Arch. Pathol., 66, 272–278.
Bernard, A., Roels, H., Thielemans, N., Van Lierde, M. & Lauwerys, R. (1992b) Assessment of the causality of the cadmium–protein relationships in the urine of the general population with reference to the Cadmibel Study. In: Nordberg. G.F., Herber, R.F.M. & Alessio, L., eds, Cadmium in the Human Environment: Toxicity and Carcinogenicity, Lyon: IARCPress, pp. 341–346.
Bernard, A., Thielemans, N., Roels, H. & Lauwerys, R. (1995) Association between NAG-B and cadmium in urine with no evidence of a threshold. Occup. Environ. Med., 52, 177–180.
Bhattacharyya, M., Whelton, B., Peterson, D., Carnes, B., Moretti, E., Toomey, J. & Williams, L. (1988) Skeletal changes in multiparous mice fed nutrient-sufficient diet containing cadmium. Toxicology, 50, 193–204.
Bonithon-Kopp, C., Huel, G., Moreau, T. & Wendling, R. (1986) Prenatal exposure to lead and cadmium and psychomotor development of the child at 6 years. Neurobehav. Toxicol. Teratol., 8, 307–310.
Borzelleca, J.F., Clarke, E.C. & Condcie, L.W., Jr (1989) Short-term toxicity (1 and 10 days) of cadmium chloride in male and female rats: Gavage and drinking water. J. Am. Coll. Toxicol., 8, 377–404.
Buchet, J.P., Lauwerys, R., Roels, H., Bernard, A., Bruaux, P., Claeys, F., Duciffre, G., De Plaen, P., Staessen, J., Amery, A., Lijnen, P., Phijs, L., Rondia, D., Sartor, F., Saint Remy, A. & Nick, L. (1990) Renal effects of cadmium body burden of the general population. Lancet, 336, 699–702.
Capel, I.D., Pinnock, M.H., Dorrell, H.M., Williams, D.C. & Grant, E.C.G. (1981) Comparison of concentrations of some trace, bulk, and toxic metals in the hair of normal and dyslexic children. Clin. Chem., 27, 879–891.
Chan, H.M., Zhu, L., Zhong, R., Grant, D., Goyer, R.A. & Cherian, M.G. (1993) Nephrotoxicity in rats following liver transplantation from cadmium-exposed rats. Toxicol. Appl. Pharmacol., 123, 89–96.
Chaney, R.L., Ryan, J.A., Li, Y.M., Welch, R.M., Reeves, P.G., Brown, S.L. & Green, C.E. (1996) Sources of Cadmium in the Environment, Paris: Organisation for Economic Co-operation and Development.
Chaube, S., Nishimura, H. & Swinyard, C. (1973) Zinc and cadmium in normal human embryos and fetuses. Arch. Environ. Health, 26, 237–240.
Cherian, M.G., Goyer, R.A. & Delaquerriere-Richardson, L. (1976) Cadmium metallothionein induced nephropathy. Toxicol. Appl. Pharmacol., 38, 399–408.
Cherian, M.G., Goyer, R.A. & Valberg, L. (1978) Gastrointestinal absorptiion and organ distribution of oral cadmium chloride and cadmium metallothionein in mice. J. Toxicol. Environ. Health, 4, 861–868.
Cherry, W. (1981) Distribution of cadmium in human tissues. In: Nrigu, J., ed., Cadmium in the Environment, Vol. II, Chichester, John Wiley, pp. 111–122.
Choudhuri, S., Liu, W.L., Berman, N.E. & Klaassen, C.D. (1996) Cadmium accumulation and metallothionein expression in brain of mice at different stages of development. Toxicol. Lett., 84, 127–133.
Claeys, F., Ducoffre, G., Sartor, F. & Roels, H. (1992) Analytical quality control of cadmium and lead in blood and cadmium in urine: Results of its implementation during a five-year epidemiological study. In: Nordberg. G.F., Herber, R.F.M. & Alessio, L., eds, Cadmium in the Human Environment: Toxicity and Carcinogenicity, Lyon: IARCPress, pp. 83–92.
Codex Alimentarius Commission (1999) Discussion paper on cadmium prepared by Denmark (CX/FAC 99/21), Joint FAO/WHO Foods Standards Programme, Codex Committee on Food Additives and Contaminants.
Coleman, M.E., Elder, R.S. & Basu, P. (1992) Trace metals in edible tissues of livestock and poultry. J. AOAC Int., 75, 615–625.
Dabeka, R.W. & McKenzie, A.D. (1995) Survey of lead, cadmium, fluoride, nickel, and cobalt in food composites and estimation of dietary intakes of these elements by Canadians in 1986–88. J. AOAC Int., 78, 897–909.
Diamond, G., Thayer, W., Choudry, H., Lockwood, T. Stitele, W., Goodrum, P. & Hassett, J. (2000) Development of the cadmium dietary exposure model. Presentation at the annual meeting of the Society of Toxicology.
Elinder, C.-G. & Nordberg, M. (1985) In: Friberg, L., Elinder, C.-G., Kjellstrom, T. & Nordberg, G.F. eds, Cadmium and Health: A Toxicological and Epidemiological Appraisal, Boca Raton, FL: CRC Press.
Elinder, C.-G. & Pannone, M. (1979) Biliary excretion of cadmium. Environ. Health Perspectives, 28, 123–126.
Elliott, P., Arnold, R., Cockins, S., Eaton, N., Jarup, L., Jones, J., Quinn, M., Rosato, M., Thornton, I., Toledano, M., Tristan, E. & Wakefield, J. (2000) Risk of mortality, cancer incidence, and stroke in a population potentially exposed to cadmium. Occup. Environ. Med., 57, 94–97.
Engstrom, B. & Nordberg, G. (1979) Factors influencing absorption and retention of oral Cd in mice: Age, pretreatment, and subsequent pretreatment with non-radioactive cadmium. Acta pharmacol. Toxicology, 45, 315–324.
Environmental Protection Agency (1984) Updated mutagenicity and cacinogenicity assessment of cadmium (addendum to the health assessment for cadmium May, 1981; EPA-600/8-81-023), Washington DC.
European Commission (1996) Dietary Exposure to Cadmium, Report on Tasks for Scientific Cooperation (Report of experts participating in Task 3.2.4), Brussels: Food Science and Technology.
Feldman, S. & Cousins, R. (1973) Influence of cadmium on the metabolism of 25-hydroxycholecalciferol in chicks. Nutr. Rep. Int., 8, 251–259
Fingerle, H., Fischer, G. & Classen, H.G. (1982) Failure to produce hypertension in rats by chronic exposure to cadmium. Food Chem. Toxicol., 20, 301–306.
Flanagan, P., McLellan, J., Haist, J., Cherian, M., Chamberlaijn, M.& Valberg, L. (1978) Increased dietary cadsmium absorption in mice and human subjects with iron deficiency. Gastroenterology, 74, 841–846.
Flora, S., Gubrelay, U., Kannan, M. & Ramesh, M. (1998) Effects of zinc supplementation during chelating agent administration in cadmium intoxication in rats. J. Appl. Toxicol., 18, 357–362.
Food and Drug Administration (2000) Total Diet Study. http://vm.cfsan.fda.gov/~comm/tds-toc.html.
Friberg, L., Piscator, M., Nordberg, G. & Kjellstrom, T. (1974) Camium in the Environment, 2nd Ed., Cleveland, Ohio, CRC Press.
Friberg, L., Kjellstrom, T., Nordberg, G. & Piscator, M. (1975) Cadmium in the Environment, Vol. II, A Toxicological and Epidemiological Appraisal, Washington DC, US Environmental Protection Agency.
Friberg, L., Elinder, C., Kjellstrom, T. & Nordberg, G. (1985) Cadmium and Health, A Toxicological and Epidemiological Appraisal, Vol. I, Exposure, Dose and Metabolism, Cleveland, OH, CRC Press.
Gillberg, C., Noren, J.G., Wahlstrom, J. & Rasmussen, P. (1982) Heavy metals and neuropsychiatric disorders in six-year-old children. Acta Paedopsychiatr., 48, 253–263.
Goyer, R.A. (1995) Transplacental transfer of lead and cadmium. In: Goyer, R.A. & Cherian, M.G., eds, Toxicology of Metals: Biochemical Aspects, Heidelberg: Springer-Verlag, pp. 1–13.
Goyer, R.A., Cherian, M.G. & Delaquerriere-Richardson, L. (1984) Correlation of parameters of cadmium exposure with onset of cadmium-induced nephropathy in rat. J. Environ. Pathol. Toxicol. Oncol., 5, 89–100.
Goyer, R.A., Miller, C.R., Zhu, S. & Victery, W. (1989) Non-metallothionein-bound cadmium in the pathogenesis of cadmium nephrotoxicity in the rat. Toxicol. Appl. Pharmacol., 101, 232–244.
Goyer, R.A., Epstein, S., Bhattacharyya, M. & Pounds, J. (1994) Environmental risk factors for osteoporosis. Environ. Health Perspectives, 102, 390–394.
Gupta, A., Murthy, R., Thakur, S., Dubey, M. & Chandra, S. (1993) Neurochemical changes in developing rat brain after pre- and postnatal cadmium exposure. Bull. Environ. Contam. Toxicol., 51, 12–17.
Hamilton, D. & Valberg, L. (1974) Relationship between cadmium and iron absorption. Am. J. Physiol., 227, 1033–1037
Hart, R.P., Rose, C.S. & Hamer, R.M. (1989) Neuropsychological effects of occupational exposure to cadmium. J. Clin. Exp. Neuropsychol., 11, 933–943.
Henke, G., Sachs, H. & Bohn, G. (1970) Cadmium determination by neutron activation analysis of liver and kidneys from children and young people. Arch. Toxikol., 26, 8–16.(in German).
Hiratsuka, H., Satoh, S., Satoh, M., Nishjima, M., Katsuki, Y., Suzuki, J., Nakagawa, J., Sumiyoshi, M., Shibutani, M., Mitsumori, K., Tanaka-Kagawa, T. & Ando, M. (1999) Tissue distribution of cadmium in rats given minimum amounts of cadmium-polluted rice or cadmium chloride for 8 months. Toxicol. Appl. Pharmacol., 160, 183–191.
Hotz, P., Buchet, J.P., Bernard, A., Lison, D. & Lauwerys, R. (1989) Renal effects of low-level environmental cadmium exposure: 5-year follow-up of a subcohort from the Cadmibel Study. Lancet, 354, 1508–
Ikeda, M., Zang, Z.-W., Moon, C.-S., Shimbo, S., Watanabe, T., Nakatsuka, H., Matsuda-Inoguchi, N. & Higashikawa, K. (2000) Possible effects of environmental cadmium exposure on kidney function in the Japanese general population. Int. Arch. Occup. Environ. Health, 73, 15–25.
Iwata, K., Saito, H., Moryiama, M. & Nakano, A. (1992) Follow up study of renal tubular dysfunction and mortality in residents of an area polluted with cadmium. Br. J. Ind. Med., 49, 736–737.
Jarup, L. & Elinder, C.G. (1993) Incidence of renal stones among cadmium exposed battery workers. Br. J. Ind. Med., 50, 598–602.
Jarup, L., Persson, B., Edling, C. & Elinder, C.G. (1993) Renal function impairment in workers previously exposed to cadmium. Nephron, 64, 75–81.
Jarup, L., Carlsson, M.D., Elinder, C.G., Hellstrom, L., Persson, B. & Schutz, A. (1995a) Enzymuria in a population living near a cadmium battery plant. Occup. Environ. Med., 52, 770–772.
Jarup, L., Persson, B. & Elinder, C.G. (1995b) Decreaed glomerular filtration rate in solderers exposed to cadmium. Occup. Environ. Med., 52, 818–822.
Jarup, L., Persson, B. & Elinder, C.G. (1997) Blood cadmium as an indicator of dose in a long-term follow-up of workers previously exposed to cadmium. Scand. J. Work Environ. Health, 23, 31–36.
Jarup, L., Alfven, T., Persson, B., Toss, G. & Elinder, C.G. (1998a) Cadmium may be a risk factor for osteoporosis. Occup. Environ. Med., 55, 435–439.
Jarup, K., Berglund, M., Elinder, C., Nordberg, G. & Vahter, M. (1998b) Health effects of cadmium exposure—A review of the literature and a risk estimate. Scand. J. Work Environ. Health, 24 (Suppl. 1).
Jiang, H.M., Guo, H. & Zhu, H. (1990) Clinical significance of hair cadmium content in the diagnosis of mental retardation of children. Chin. Med. J., 103, 331–334.
Jin, T., Nordberg, G.F. & Nordberg, M. (1987) Resistance to acute nephrotoxicity induced by cadmium–metallothionein dependence on pretreatment with cadmium chloride. Pharmacol. Toxicol., 61, 89–93.
Jin, T., Lu, J. & Nordberg, M. (1998) Toxicokinetics and biochemistry of cadmium with special emphasis on the role of metallothionein. Neurotoxicology, 19, 529–535.
Johnson, D. & Foulkes, E. (1980) On the proposed role of metallothionein in the transport of cadmium. Environ. Res., 21, 360–365.
Jorhem, L. & Sundstrom, B. (1993) Levels of lead, cadmium, zinc, copper, nickel, chromium, manganese, and cobalt in foods on the Swedish market, 1983–1990. J. Food Compos. Anal., 6, 223–241.
Jorhem, L., Becker, W. & Slorach, S. (1998) Intake of 17 elements by Swedish women, determined by a 234-h duplicate portion study. J. Food Compos. Anal., 11, 32–46.
Jung, K., Pergrande, M., Graubaum, H.-C., Fels, L.M., Endl, U. & Stolte, H. (1993) Urinary proteins and enzymes as early indicators of renal dysfunction in chronic exposure to cadmium. Clin. Chem., 39, 757–765.
Kawai, K., Fukuda, K. & Kimura, M. (1976) Morphological alterations in experimental cadmium exposure with special reference to the onset of renal lesion. In: Nordberg, G.F., ed., Effects and Dose–Response Relationships of Toxic Metals, Amsterdam: Elsevier Scientific, pp. 359–370.
Kelman, B.J., Walter, B.K. & Jarboe, G.E. (1978) Effect of dietary cadmium on calcium metabolism in the rat during gestation. Proc. Soc. Exp. Biol. Med., 158, 614–617.
Kilburn, K.H. & McKinley, K.L. (1996) Persistent neurotoxicity from a battery fire: Is cadmium the culprit? South. Med. J., 89, 693–698.
Kostial, K., Kello, D., Jugo, S., Rabar, I. & Maljkovic, T. (1978) Influence of age on metal metabolism and toxicity. Environ. Health Perspectives, 25, 81–86.
Kotsonis, F.N. & Klaassen, C.D. (1977) Toxicity and distribution of cadmium administered to rats at sublethal doses. Toxicol. Appl. Pharmacol., 41, 667–680.
Kotsonis, F.N. & Klaassen, C.D. (1978) The relationship of metallothionein to the toxcitiy of cadmium after prolonged adminstration to rats. Toxicol. Appl. Pharmacol., 46, 39–54.
Kowal, N., Johnson, D., Kraemer, D. & Pahren, H. (1979) Normal levels of cadmium in diet, urine, blood and tissues of inhabitants of the Unites States. J. Toxicol. Environ. Health, 5, 995–1014.
Kreis, I.A., Wijga, A. & van Wijnen, J.H. (1992). Assessment of the lifetime accumulated cadmium intake from food in Kempenland. Sci. Total Environ., 127, 281–292.
Lauwerys, R., Amery, A., Bernard, A., Bruax, P., Buchet, J.-P., Claeys, F., De Plaen, P., Ducoffre, G., Fagard, R., Lijnen, P., Nick, L., Roels, H., Rondia, D., Saint-Remy, A., Sartor, F. & Staessen, J. (1990) Health effects of environmental exposure to cadmium: Objectives, design and organization of the Cadmibel Study: A cross-sectional morbidity study carried out in Belgium from 1985 to 1989. Environ. Health Perspectives, 87, 283–289.
Lauwerys, R., Bernard, A., Buchet, J.P., et al. (1991) Does environmental exposure to cadmium represent a health risk? Conclusions from the Cadmibel Study. Acta Clin. Belg., 46, 219–225.
Lauwerys, R.R., Bernard, A., Roels, H. & Buchet, J.-P. (1995) Health risk assessment of long-term exposure to non-genotoxic chemicals: Application of biological indices. Toxicol. Lett., 77, 39–44.
Lehotzky, K., Ungvary, G. & Polinak, D. (1990) Behavorial deficits due to prenatal exposure to cadmium chloride in CFY rat pups. Neurotox. Teratol., 12, 169–172.
Lewis, M., Worobey, J., Ramsey, D.S. & McCormack, M.K. (1992) Prenatal exposure to heavy metals: Effect on childhood cognitive skills and health status. Pediatrics, 89, 1010–1015.
Lind, X., Jin, T., Nirdberg, G., /sjostrom, M. & Zhou, Y. (1994) Influence of zinc and copper administration on metal disposition in rats with cadmium–metallothione induced nephrotoxicity. Toxicol. Appl. Pharmacol., 126, 84–90.
Lind, Y., Engman, J., Jorhem, L. & Glynn, A. (1998) Accumulation of cadmium from wheat bran, sugar-beet fibre, carrots and cadmium in the liver and kidneys of mice. Br. J. Nutr., 80, 205–211.
Liu, J.L., & Klaassen, C.D. (1994) Nephrotoxicity of CdCl2 and Cd-metallothionein in cultured rat kidney proximal tubules and LLC-PK1 cells. Toxicol. Appl. Pharmacol., 128, 264–270.
Liu, Y., Liu, J., Habeebu, S. & Klaassen, C. (1999) Metallothionein protects against the nephrotoxicity produced by chronic CdMT exposure. Toxicol. Sci., 50, 221–227.
Lorentzon, R. & Larsson, S.E. (1977) Vitamin D metabolism in adult rats at low and normal calcium intake and the effect of cadmium exposure. Clin. Sci. Mol. Med., 53, 439–446.
Marlowe, M., Errera, J. & Jacobs, J. (1983) Increased lead and cadmium burdens among mentally retarded children and children with borderline intelligence. Am. J. Ment. Defic., 87, 477–483.
Marlowe, M., Stellern, J., Errera, J. & Moon, C. (1985a) Main and interaction effects of metal pollutants on visual–motor performance. Arch. Environ. Health, 40, 221–225.
Marlowe, M., Cossairt, A., Moon, C., Errera, J., MacNeel, A., Peak, R., Ray, J. & Schroeder, C. (1985b) Main and interaction effects of metallic toxins on classroom behavior. J. Abnorm. Child Psychol.,13, 185–198.
Masaoka, T., Akaori, F. & Arai, S. (1994) A nine year chronic toxicity study of cadmium ingestion in monkeys. I. Effects of dietary cadmium on the general health of monkeys. Vet. Hum. Toxicol., 36, 189–194.
Matsusaka, N., Tananka, M., Nishmura, Y., Yuyama, A. & Kobayashi, H. (1972) Whole body retention and intestinal absoprtion of Cd in young and adult mice. Med. Biol., 85, 275–279 (in Japanese).
Miles, A.T., Hawksworth, G.M., Beattie, J.H. & Rodilla, V. (2000) Induction, regulation, degradation and biological significance of mammalian metallothioneins (Abstract). Crit. Rev. Biochem. Mol. Biol., 35, 35–75.
Ministry of Agriculture, Fisheries and Food (1999a) Metals and Other Elements in Infant Foods (Food Surveillance Information Sheet Number 190), London.
Ministry of Agriculture, Fisheries and Food (1999b) 1997 Total Diet Study—Aluminium, Arsenic, Cadmium, Chromium, Copper, Lead, Mercury, Nickel, Selenium, Tin and Zinc (Food Surveillance Information Sheet Number 191), London.
Mitsumori, K. & Ando, M. (2000) Study of the relationship between the development of toxicities and cadmium accumulation in rats given minimum amounts of cadmium or cadmium-polluted rice for two years. (in press).
Mitsumori, K., Shibutani, M., Sato, S., Onodera, N., Nakagawa, J., Hayashi, Y. & Ando, M. (1998) Relationship between the development of hepato-renal toxicity and cadmium accumulation in rats given minimum to large amounts of cadmium chloride in the long-term: Preliminary study. Arch. Toxicol., 72, 545–552.
Morgan, H. & Simms, D. (1988) Discussion and conclusions. Sci. Total Environ., 7, 135–143.
Muller, M., Anke, M., Hartmann, E. & Illing-Gunther, H. (1996) Oral cadmium exposure of adults in Germany. 1: Cadmium content of foodstuffs and beverages. Food Addit. Contam., 13, 359–378.
Muller, M., Anke, M., Illing-Gunther, H. & Thiel, C. (1998) Oral cadmium exposure of adults in Germany. 2: Market basket calculations. Food Addit. Contam., 15, 135–141.
Murphy, V.A. (1997) Cadmium: Acute and chronic neurological disorders. In: Yasui M, Strong M, Ota K, Verity MA (Eds.) Mineral and Metal Neurotoxicology. Boca Raton, FL: CRC press, pp. 229–240.
Murphy, V. (2000) Cadmium: Acute and chronic neurological disorders. In: Yasui, M., Strong, M., Ota, K. & Verity, M.A., eds, Mineral and Metal Neurotoxicology, Boca Raton, FL: CRC Press, pp 229–240.
National Institute of Public Health (1996) The Health Risk Assessment of Dietary Exposure to Selected Chemical Substances, Prague: Division for Hygiene of Food Chains in Brno.
Nishijo, M., Nakagawa, H., Morikawa, Y., Tabata, M., Senma, M., Kitagawa, Y., Kawano, S., Ishizaki, M., Sugita, N., Nishi, M., Kido, T. & Nogawa, K. (1994) Prognostic factors of renal dysfunction induced by environmental cadmium pollution. Environ. Res., 64, 112–121.
Nishijo, M., Nakagawa, H., Morikawa, Y., Tabata, M., Senma, M., Miura, K., Takahara, H., Kawano, S., Nishi, M., Mizukoshi, K., Kido, T. & Nogawa, K. (1995) Mortality of inhabitants in an area polluted by cadmium: 15 year follow up. Occup. Environ. Med., 52, 181–184.
Nishijo, M., Nakagawa, H., Morikawa, Y., Tabata, M., Miura, T., Senma, M., Miura, K., Yoshita, K., Higashiguchi, K., Seto, T., Kido, T., Nogawa K., Mizukoshi, K. & Nishi, M. (1999) Relationship between urinary cadmium and mortality among inhabitants living in a cadmium polluted area in Japan. Toxicol. Lett., 108, 321–327.
Nishimura, N., Nishimura, H., Ghaffar, A. & Tohyama, C. (1992) Localization of metallothionein in the brain of rat and mouse. J. Histochem. Cytochem., 40, 309–315.
Nogawa, H. & Nishijo, M. (1999) Dose–response relationship and mortality in inhabitants of cadmium-polluted areas. In: Nogawa, K., Kurachi, M. & Kasuya, M., eds, Advances in the Prevention of Environmental Cadmium Pollution and Countermeasures, Kanazawa, Eiko Laboratory, pp. 110–115.
Nomiyama, K. & Nomiyama, H. (1976) Mechanisms of urinary excretion of cadmium: Experimental studies in rabbits. In: Noordberg, G., ed., Effects and Dose–Response Relationships of Toxic Metals, Amsterdam, Elsevier Science, pp. 371–379.
Nordberg, G. (1972) Cadmium metabolims and toxicity. Environ. Physiol. Biochem., 2, 7–36.
Nordberg, G. (1978) Studies on metallothionein and cadmium. Environ. Res., 15, 381–404.
Nordberg, M. (1998) Metallothioneins: Historical review and state of knowledge. Talanta, 46, 243–254.
Nordberg, G. & Piscator, M. (1972) Influence of long-term cadmium exposure on urinary excretion of protein and cadmium in mice. Environ. Physiol. Biochem., 2, 37–49.
Nordberg, G.F. & Strangert, P. (1976) In: Nordberg, G.F., ed., Effects and Dose–Response Relationships of Toxic Metals, Amsterdam: Elsevier, pp. 273–282. .
Nortier, J., Bernard, A., Roels, H., Deschodt-Lanckman, M., Gueuning, C. & Lauwerys, R. (1997) Urinary neutral endopeptidase in workers exposed to cadmiun: Interaction with cigarette smoking. Occup. Environ. Med., 54, 432–436.
Oldiges, H., Hochrainer, D. & Glaser, U. (1989) Long-term inhalation study with Wistar rats and four cadmium compounds. Toxicol. Environ. Chem., 19, 217–222.
Ono, S. & Cherian, M.G. (1998) Changes in brain metallothionein and zinc during development in transgenic mice. Biol. Trace Elem. Res., 61, 41–49.
Paschal, D., Burt, V., Caufdill, S., Gunter, E., Pirkle, J., Sampson, E., Miller, D. & Jackson, R. (2000) Expsoure of the US population aged 6 years and older to cadmium: 1988–1994. Arch. Environ. Contam. Toxicol., 38, 377–383.
Pennington, J.A.T., Capar, S.G. & Parfitt, C.H. (1996) History of the Food and Drug Administration’s Total Diet Study (Part II), 1987–1993. J. AOAC Int., 79, 163–170.
Pihl, R.O. & Parkes, M. (1977) Hair element content in learning disabled children. Science, 198, 204–206.
Pleasants, W., Waslien, C. & Naughton, B. (1993) Dietary modulation of the symptoms of cadmium toxicity in rats:effects of vitamins A, C, D, DD hormone and fluoride. Nutrit. Res., 13, 839–850.
Reeves, P. (2000) Mineral nutrient status affects the absorption of cadmium from sunflower kernals in rats. (in press).
Reeves, P. & Vanderpool, R. (1997) Cadmium burden of men and women who report regular consumption of confectionary sunflower kernals containing a natural abundance of cadmium. Environ. Health Perspectives, 105, 1098–1104.
Reeves, P. & Vanderpool, R. (1998) Organ content and fecal excretion of cadmium in male and female rats consuming variable amounts of naturally occuring sunflower kernals (Helianthus annus L.). J. Nutr. Biochem., 9, 636–644.
Reeves, P., Johnson, P. & Rossow, K. (1994) Absorption and organ content of cadmium from the kernals of confectionary sunflowers (Helianthus annus) fed to male rats. J. Agric. Food Chem., 42, 2836–2843.
Reeves, P., Nielsen, E., O’Brien-Nimens, C. & Vanderpool, R. (2000) Cadmium consumption and its availability from sunflower kernals: A study with human volunteers. (in press).
Roels, H.A., Lauwerys, R.R., Buchet, J.P., Bernard, A.M., Vos, A. & Oversteyns, M. (1989) Health significance of cadmium induced renal dysfunction: A five year follow up. Br. J. Ind. Med., 46, 755–764.
Roels, H.A., Lauwerys, R.R., Bernard, A.M., Buchet, J.P., Vos, A. & Oversteyns, M. (1991) Assessment of the filtration reserve capacity of the kidney in workers exposed to cadmium. Br. J. Ind. Med., 48, 365–374.
Ruoff, W., Diamond, G., Valazquez, S., Stitele, W. & Gefell, D. (1994) Bioavailability of cadmium in food and water: A case study on the derivation of relative bioavailability factors for inorganics anf their relevance to the reference dose. Regul. Toxicol. Pharmacol., 20, 139–160.
Sartor, F., Rondia, D., Claeys, F., Buchet, J.P., Ducoffre, G., Lauwerys, R., Staessen, J. & Amery, A. (1992a) Factors influencing the cadmium body burden in a population study. In: Nordberg, G.F., Herber, R.F.M. & Alessio, L., eds, Cadmium in the Human Environment: Toxicity and Carcinogenicity, Lyon: IARCPress, pp. 101–106.
Sartor, F.A., Rondia, D.J., Claeys, F.D., Staessen, J.A., Lauwerys, R.R., Bernard, A.M., Buchet, J.P., Roels, H.A., Bruaux, P.J., Ducoffre, G.M., Lijnen, P.J., Thijs, L.B. & Amery, A.K. (1992b) Impact of environmental cadmium pollution on cadmium exposure and body burden. Arch. Environ. Health, 47, 347–353.
Shaikh, Z.A., Harnett, K.M. & Perlin, S.A. (1989) Chronic cadmium intake results in dose-related excretion of metallothionein in urine. Experimentia, 45, 146–148.
Sharma, R., Kjellstrom, T. & McKenszie, J. (1983) Cadmium in blood and urine among smokers and non-smokers with high cadmium intake via food. Toxicology, 29, 163–171.
Shearer, T.R., Larson, K., Neuschwander, J. & Gedney, B. (1982) Minerals in the hair and nutrient intake of autistic children. J. Autism Dev. Disord., 12, 25–34.
Shimizo, M. & Morita, S. (1990) Effects of fasting on cadmium toxicity, glutathione metabolism and metallothionein synthesis in the rat. Toxicol. Appl. Pharmacol., 103, 28–39.
Shukla, A., Shukla, G.S. & Srimal, R.C. (1996) Cadmium-induced alterations in blood–brain barrier permeability and its possible correlation with decreased microvessel antioxidant potential in rat. Hum. Exp. Toxicol., 15, 400–405.
Staessen, J., Amery, A., Bernard, A., Bruax, P., Buchet, P., Bulpitt, C., Claeys, F., De Plaen, P., Ducoffre, G., Fagard, R., Lauwerys, R., Lijnen, P., Nick, L., Saint Remy, A., Roels, H., Rondia, D., Sartor, F. & Thijs, L. (1991a) Blood pressure, the prevalence of cardiovascular diseases and exposure to cadmium: A population study. Am. J. Epidemiol., 134, 257–267.
Staessen, J., Amery, A., Bernard, A., Bruax, P., Buchet, J.P., Claeys, F., De Plaen, P., Ducoffre, G., Fagard, R., Lauwerys, R.R., Lijnen, P., Nick, L., Saint Remy, A., Roels, H., Rondia, D., Sartor, F. & Thijs, L. (1991b) Effects of exposure to cadmium on calcium metabolism: A population study. Br. J. Ind. Med., 48, 710–714.
Staessen, J.A., Lauwerys, R.R., Ide, G., Roels, H., Vyncke, G. & Amery, A. (1994) Renal function and historical environmental cadmium pollution from zinc smelters. Lancet, 343, 1523–1527.
Staessen, J.A., Roels, H.A., Emelianov, D., Kuznetsova, T., Thijs, L., Vangronsveld, J. & Fagard, R. (1999) Environmental exposure to cadmium, forearm bone density, and risk of fractures: Prospective population study. Lancet, 353, 1140–1144.
Staessen, J., Kuzneetsova, T., Roels, H., Emelianov, D. & Fagard, R. (2000) Exposure to cadmium and conventional and ambulatory blood pressures in a prospective population. Am. J. Hypertension, 113, 146–156.
Stellern, J., Marlowe, M., Cossairt, A. & Errera, J. (1983) Low lead and cadmium levels and childhood visual-perception development. Percept. Motor Skills, 56, 539–544.
Stowe, H.D., Wilson, M. & Goyer, R.A. (1972) Clinical and morphological effects of oral cadmium toxicity in rabbits. Arch. Pathol., 94, 389–405.
Sugawara, C. & Sugawara, N. (1974) Cadmium toxicity for rat intestine, especially on the absorption of calcium and phosphorus. Jpn. J. Hyg., 28, 511–516.
Sumino, K., Hayakawa, K., Shibata, T. & Kitamura, S. (1975) Heavy metals in normal Japanese tissues. Arch. Environ. Health, 30, 487–494.
Tahvonen. R. & Kumpalainen, J. (1994a) Lead and cadmium contents in pork, beef and chicken, and in pig and cow liver in Finland during 1991. Food Addit. Contam., 11, 415–426.
Tahvonen. R. & Kumpalainen, J. (1994b) Lead and cadmium contents in Finnish breads. Food Addit. Contam., 11, 621–631.
Takeda, A., Takefuta, S., Ijiro, H., Okada, S. & Oku, N. (1999) 109-Cadmium transport in rat brain. Brain Res. Bull., 49, 453–457.
Takenaka, S., Oldiges, H., Konig, H., Hochrainer, D. & Oberdorster, G. (1983) Carcinogenicity of cadmium chloride aerosols in W rats. J. Natl Cancer Inst., 70, 367–373.
Task Group on Metal Toxicity (1976) Conceptual considerations: Critical organ, critical concentration in cells and organs, critical effect, subcritical effect, dose–response relationships. In: Nordberg, G., ed., Effects and Dose–Response of Toxic Metals, Amsterdam, Elsevier Science, pp. 10…13.
Thatcher, R.W., Lester, M.L., McAlaster, R. & Horst, R. (1982) Effects of low levels of cadmium and lead on cognitive functioning in children. Arch. Environ. Health, 37, 159–166.
Thatcher, R.W., McAlaster, R. & Lester, M.L. (1984) Evoked potentials related to hair cadmium and lead in children. Ann. NY Acad. Sci., 425, 384–390.
Tsoumbaris, P. & Tsoukali–Papadopoulou, H. (1994a) Heavy metals in common foodstuff: Quantitative analysis. Bull. Environ. Contam. Toxicol., 53, 61–66.
Tsoumbaris, P. & Tsoukali–Papadopoulou, H. (1994b) Heavy metals in common foodstuff: Daily intake. Bull. Environ. Contam. Toxicol., 53, 67–70.
Tsuritani, I., Honda, R., Ishizaki, M., Yamada, Y., Kido, T. & Nogawa, K. (1992) Impairment of vitamin D metabolism due to environmental cadmium exposure, and possible relevance to sex-related differences in vulnerability to the bone damage. J. Toxicol. Environ. Health, 37, 519–533.
Uriu, K., Kaizu, K., Komine, N., Ikeda, M., Qie, Y.L., Hashimoto, O., Matsuoka, A. & Eto, S. (1998) Renal hemodynamics in rats with cadmium-induced nephropathy. Toxicol. Appl. Pharmacol., 150, 76–85.
Ursinyova, M. & Hladikova, V. (1997) The intake of selected toxic elements from milk in infants. Fresenius Environ. Bull., 6, 627–632.
Vahter, M., Berglund, M., Friberg, L., Jorhem, L., Lind, B., Slorach, S. & Akesson, A. (1990) Dietary intake of lead and cadmium in Sweden. Var. Foda, 44 (Suppl. 2).
Vahter, M., Johansson, G., Akesson, A. & Rahnster, B. (1992) Faecal elimination of lead and cadmium in subjects on a mixed and lactovagetarian. Food Chem. Toxicol., 30, 281–287.
Vahter, M., Bergland, M., Nermell, B. & Akesson, A. (1996) Bioavailability of cadmium from shellfish and mixed diet in women. Toxicol. Appl. Pharmacol., 136, 332–341.
Vanderpool, R. & Reeves, P. (2000) Cadmium absorption in humans fed confectionary sunflower kernals (Helianthus annuus L.) labeled with a stable isotope of cadmium. (in press).
Vanoort, R., Cressey, P. & Silvers, K. (2000) 1997/98 New Zealand Total Diet Survey. Part 2: Elements. Report prepared for the Ministry of Health.
Viaene, M.K., Masschelein, R., Leenders, J., De Groof, M., Swerts, L.J.V.C. & Roels, H.A. (2000) Neurobehavioral effects of occupational exposure to cadmium: A cross sectional epidemiological study. Occup. Environ. Med., 57, 19–27.
Waalkes, M.P. & Rehm, S. (1994) Cadmium and prostate cancer. J. Toxicol. Environ. Health, 43, 251–269.
Waalkes, M.P., Anver, M. & Diwan, B.A. (1999) Carcinogenic effects of cadmium in the Noble (NBL/Cr) rat: Induction of pituitary, testicular, and injection site tumors and intraepithelial prolifertive lesions of the dorsolateral prostate. Toxicol. Sci., 52, 154–161.
Wang, C. & Bhattacharyya, M. (1993) Effect of cadmium on bone calcium and 45 Ca in onopregnant mice on a calcium-deficient diet: Evidence of direct effect of cadmium on bone. Toxicol. Appl. Pharmacol., 120, 228–239.
Wang, X., Chan, H.N., Goyer, R.A. & Cherian, M.G. (1993) Nephrotoxicity of repeated injections of cadmium–metallothionein in rats. Toxicol. Appl. Pharmacol., 119, 11–16.
Watanabe, T., Nakatsuka, H., Satoh, H., Yamamoto, R. & Ikeda, M. (1992). Reduced dietary cadmium intake in past 12 years in a rural area in Japan. Sci. Total Environ., 119, 43–50.
Watanabe, T., Zhang, Z.-W., Moon, C.-S., Shimbo, S., Nakatsuka, H., Matsuda-Inoguchi, N., Higashikawa, K. & Ikeda, M. (2000) Cadmium exposure of women in general populations in Japan during 1991–1997 compared with 1977–1981. Int. Arch. Occup. Environ. Health, 73, ???–???.
Wecker, L., Miller, S.B., Cochran, S.R., Dugger, D.L. & Johnson, W.D. (1985) Trace element concentrations in hair from autistic children. J. Ment. Defic. Res., 29, 15–22..
Whelton, B., Peterson, D., Moretti, E., Mauser, R. & Bhattacharyya, M.H. (1997) Hepatic levels of cadmium, zinc,and copper in multiparous, nulliparous and ovariectomized mice fed either a nutrient-sufficient or -deficient diet containing cadmium. Toxicology, 119, 141–153.
Whittemore, A.S., DiCiccio, Y. & Provenzano, G. (1991) Urinary cadmium and blood pressure: Results from the NHANES II survey. Environ. Health Perspectives, 91, 133–140.
WHO (1992) Cadmium (Environmental Health Criteria 134), Geneva.
Wing, A., Wing, K., Tidehag, P., Hallmans, G. & Sjostrom, R. (1992) Cadmium accumulation from diets in rats with different iron status. Nutr. Res., 12, 1205–1215.
Yang, H.F., Luo, X.Y., Shen, W., Zhou, Z.F., Jin, C.Y., Yu, F. & Liang, C.S. (1994) National food contamination monitoring programmes. Biomed. Environ. Sci., 7, 362–368.
See Also:
Toxicological Abbreviations