
First draft prepared by
Richard A. Canady1, Raymond D. Coker2, S. Kathleen Egan1, Rudolf Krska3, Monica Olsen4, Silvia Resnik5, and Josef Schlatter6
1Food and Drug Administration, Washington DC, USA;
2
University of Greenwich, Kent, United Kingdom;3
Tulln Institute for Agrobiotechnology, Centre for Analytical Chemistry, Tulln, Austria;4
National Food Administration, Uppsala, Sweden;5
Commission for Scientific Research, University of Buenos Aires, Argentina;6
Swiss Federal Office of Public Health, Zurich, SwitzerlandT-2 and HT-2 toxins are type-A trichothecene mycotoxins, which are closely-related epoxy sesquiterpenoids. Surveys have revealed the presence of T-2 and HT-2 toxins in grains such as wheat, maize, oats, barley, rice, beans, and soya beans as well as in some cereal-based products. T-2 and HT-2 toxins have been reported to be produced by Fusarium sporotrichioides, F. poae, F. equiseti, and F. acuminatum. The most important producer is F. sporotrichioides, a saprophyte (i.e. not pathogenic to plants) which grows at –2 to 35 °C and only at high water activities (above 0.88). In consequence, T-2 and HT-2 toxins are not normally found in grain at harvest but result from water damage to the grain such as may occur when it remains for extended periods in the field at or after harvest, especially in cold weather, or in grain that becomes wet during storage.
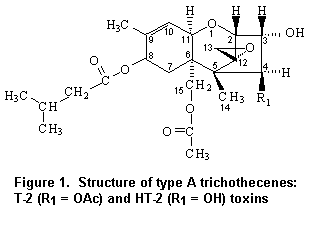
T-2 toxin is the trivial name for 4beta,15-diacetoxy-3alpha,dihydroxy-8alpha-[3-methylbutyryl-oxy]-12,13-epoxytrichothec-9-ene, which is shown in Figure 1. Corresponding to the molecular formula C24H34O9, its relative molecular mass is 466.5 g/mol. 15-Acetoxy-3alpha,4beta-dihydroxy-8alpha-[3-methylbutyryloxy]-12,13-epoxytrichothec-9-ene is the systematic name of HT-2 toxin. The molecular formula is C22H32O8, and the relative molecular mass is 424.5 g/mol. The structures of T-2 and HT-2 toxins differ only in the functional group at the C-4 position. As T-2 toxin is readily metabolized to HT-2 toxin, these two mycotoxins were evaluated together.

Neither T-2 nor HT-2 toxin has been evaluated previously by the Committee.
(a) Gastrointestinal metabolism
T-2 toxin is metabolized extensively in the small intestine. [3H]T-2 toxin at a dose of 5 nmol/L (2.3 µg) or 500 nmol/L (230 µg T-2 toxin plus 2.3 µg [3H]T-2 toxin), both in 0.5 ml of an ethanol:saline solution, was injected into semi-intact isolated jejunal loops from female Sprague-Dawley rats. Blood draining from the loops was collected and analysed by gas–liquid chromatography–mass spectroscopy at various times. Both T-2 and HT-2 toxin were found in the blood within 1 min after injection of T-2 toxin into the lumen of the intestinal loop. Only 2% of the dose of 5 nmol/L was recovered in plasma as unchanged T-2 toxin after 50 min, while 25% was recovered as HT-2 toxin, 4–7% as 3’-OH-HT-2 toxin, and smaller amounts as 3’-OH-T-2 toxin, T-2 tetraol, and 4-deacetylneosolaniol. No glucuronide or sulfate conjugates were detected. Similarly, less than 1% of the dose was recovered as T-2 toxin in the intestinal lumen, while 15% was recovered in the intestinal lumen as HT-2 toxin and 5% as 3’-OH-HT-2 toxin. The dose of 500 nmol/L caused extensive tissue damage, but the ability to metabolize T-2 toxin was retained. Intestinal fluid extracted from the jejunal loops was incubated with T-2 toxin at either 5 or 500 nmol/L for 50 min. Less metabolism was seen than in intact preparations: after 50 min of incubation with intestinal fluid alone, 14% of the administered dose was recovered as HT-2 toxin at the 5 nmol/L concentration and 12% at 500 nmol/L. The rest of the dose was recovered as T-2 toxin, and no other metabolites were observed. Filtration (0.45-µm pore) to remove bacteria from the intestinal fluid did not appreciably reduce the amount of T-2 toxin that was deacetylated to HT-2 toxin in 50 min (Conrady-Lorck et al., 1988).
T-2 toxin was also deacetylated to HT-2 toxin in sheep rumen. When T-2 toxin at a dose of 0.02 mg/mL was incubated with sheep rumenal fluid for 0.5, 1, 2, or 3 h, it was deacetylated at a rate of 1.7 mg/L-h. The proportion of a 100-µg dose of T-2 toxin recovered as HT-2 toxin increased over 0.5, 1, 2, and 3 h of incubation with sheep rumenal fluid, to approximately 50% HT-2 toxin by 3 h. Gas chromatography (with a flame ionization detector) was used to detect T-2 toxin and HT-2 toxin. HT-2 toxin was the only T-2 toxin metabolite analysed in the culture medium (Kiessling et al., 1984).
Intestinal de-epoxidation of T-2 toxin also occurs, with deacetylation to HT-2 toxin in rats. De-epoxy HT-2 toxin was the predominant metabolite observed when T-2 toxin (at 0.1 mg/mL) was incubated with rat caecal microorganisms for 4 days. T-2 tetraol, 4-deacetylneosolaniol, T-2 toxin, HT-2 toxin, and T-2 triol were not found in the culture medium after 4 days (Swanson et al., 1987).
(b) Bioavailability
No studies of the systemic bioavailability of T-2 or HT-2 toxin were available. As T-2 toxin is extensively metabolized in the small intestine, however, the bioavailability of unchanged T-2 toxin may be quite low and the predominant absorbed chemical species may be HT-2 toxin (Conrady-Lorck et al., 1988; Kiessling et al., 1984; Swanson et al., 1987).
(c) Distribution
Placental transfer of T-2 toxin to fetal tissues was observed after intravenous injection of [14C]T-2 toxin to dams. Preferential distribution of T-2 toxin to thymus and spleen rather than liver was observed in fetuses after intraperitoneal administration of T-2 toxin to dams (Lafarge-Frayssinet et al., 1990).
In 6-week-old broiler chicks fed a diet containing T-2 toxin at 2 mg/kg for 5 weeks and then intubated with a single dose of [3H]T-2 toxin at 0.5 mg/kg bw, the radiolabel reached a maximum concentration in most tissues 4 h after dosing; the exceptions were muscle, skin, and bile, in which the maximum level was reached after 12 h. After 48 h, the chicks contained the equivalent of 39 µgkg of T-2 toxin and/or its metabolites in the muscle and 40 µg/kg in liver, as calculated on the basis of the specific activity of the radiolabelled T-2 toxin administered (Chi et al., 1978a).
In a weanling cross-bred pig (weighing 7.5 kg bw) intubated with [3H]T-2 toxin at a dose of 0.1 mg/kg bw, the percentage of administered radiolabel 18 h after dosing was 0.7% in muscle, 0.43% in liver, 0.08% in kidney, 0.06% in bile, 22% in urine, and 25% in faeces. The percentage of administered radiolabel 18 h after dosing in another pig intubated with T-2 toxin at 0.4 mg/kg bw was 0.7% in muscle, 0.29% in liver, 0.08% in kidney, 0.14% in bile, 18% in urine, and 0.86% in faeces (Robison et al., 1979a).
Four hours after intravenous administration of [3H]T-2 toxin to pigs, the largest amount of radiolabel was located in the gastrointestinal tract (15–24% of the dose), and 4.7–5.2% of the dose was found in the remaining tissues; muscle accounted for 2.9–3.2% of the dose and liver for 0.7–1.7% (Corley et al., 1986).
The fate and distribution of an intramuscular dose of 1.04 mg/kg bw of [3H]T-2 toxin was studied in guinea-pigs. Except in the large intestine and bile, the amount of radiolabel had peaked by 30 min and rapidly declined, with no measurable long-term accumulation. The tissue distribution was expressed in intervals over a 30-min to 672-h period, with six animals per time, as picomoles per milligram of tissue. Three hours after dosing, the distribution of T-2 toxin-derived radiolabel corresponded to 850 pmol/mg of tissue (wet weight) in kidney, 970 pmol/mg in liver, 490 pmol/mg in lung, 570 pmol/mg in spleen, 280 pmol/mg in adrenals, 630 pmol/mg in fat, 230 pmol/mg in heart, 430 pmol/mg in muscle, 440 pmol/mg in testis, and 160 pmol/mg in brain (equal to 400, 450, 230, 260, 130, 290, 110, 200, 200, and 77 µg/g of tissue). At 3 h, the distribution of T-2 toxin-derived radiolabel corresponded to 290 pmol/ml of plasma and 2700 pmol/ml of bile. At 72 h after dosing, the distribution of T-2 toxin-derived radiolabel corresponded to 90 pmol/mg in kidney, 100 pmol/mg in liver, 61 pmol/mg in lung, 59 pmol/mg in spleen, 49 pmol/mg in adrenals, 39 pmol/mg in fat, 36 pmol/mg in heart, 51 pmol/mg in muscle, 41 pmol/mg in testis, and 24 pmol/mg in brain (equal to 43, 47, 28, 28, 23, 18, 17, 24, 19, and 11 µg/g of tissue). At this time, the distribution of radiolabel corresponded to 88 pmol/ml of plasma and 39 000 pmol/ml of bile (equal to 41 and 18 000 µg/L) (Pace et al., 1985).
In pigs given T-2 toxin at 1.2 mg/kg bw by intra-aortal injection, the plasma and tissue concentrations of T-2 toxin decreased rapidly. By 4 h after dosing, no T-2 toxin was detected in any of the tissues examined (analysis by gas–liquid chromatography; limit of quantification, 40 ng/g). However, at 1, 2, and 3 h after injection, larger amounts of T-2 toxin were seen in spleen than in either kidney or muscle. T-2 toxin was not detected in liver at any time (Beasley et al., 1986).
A lactating Jersey cow weighing 375 kg was given 180 mg of T-2 toxin orally, equal to 0.48 mg/kg bw per day, by capsule for 3 days and was then given 160 mg of [3H]T-2 toxin, equal to 0.42 mg/kg bw. Although almost all the administered dose was eliminated within 72 h, measurable tritium remained in the bile, liver, and kidney (equivalent, respectively, to 27, 18, and 14 µg/kg of tissue) 3 days after dosing. These concentrations are higher than those in whole blood (13 µg/kg), plasma (10 µg/kg), and other tissues, including the spleen (9.4 µg/kg), heart (10 µg/kg), mammary gland (11 µg/kg), ovaries (11 µg/kg), muscle (8.8 µg/kg), and fat (4.7 µg/kg) (Yoshizawa et al., 1981).
(d) Excretion
Excretion was measured in faeces and urine collected for 6 days after oral, intravenous, and dermal administration of [3H]T-2 toxin to rats at a dose of 0.15 or 0.6 mg/kg bw. After oral administration, > 95% of the administered radiolabel was excreted within 72 h. After intravenous administration, similarly rapid elimination of the dose of 0.15 mg/kg bw was seen, but < 80% of the dose of 0.6 mg/kg bw was eliminated within 72 h. Extensive vascular damage was seen at the higher dose in these animals. After dermal application, < 60% of either dose was excreted within 72 h. More radiolabel was excreted in faeces than in urine with all routes and both doses. After oral administration, faecal excretion accounted for approximately 80% of the administered dose. A similar ratio (80:20, faeces:urine) was seen with the dose of 0.15 mg/kg given intravenously (Pfeiffer et al., 1988).
In 6-week-old broiler chicks given [3H]T-2 toxin by crop intubation at a dose of 0.5 mg/kg bw, the radiolabel peaked at 4 h in most tissues but at 12 h in muscle, skin, and bile. Bile contained the highest concentration of radiolabel of the tissues examined over the 48-h observation period. The pattern of distribution and excretion indicated that biliary excretion dominated (Chi et al., 1978a).
Biliary excretion was also found after intramuscular injection of [3H]T-2 toxin at a dose of 1 mg/kg bw to guinea-pigs. The concentration in bile at 12 h was 540 000 pmol/ml (equal to 250 000 µg/L), whereas that in plasma was 290 pmol/ml (equal to 140 µg/L). Over the first 5 days 75% of the administered radiolabel was excreted in urine and faeces at a ratio of 4:1. The amount peaked in urine at 24 h; 99% of the administered dose was eliminated by 28 days (Pace et al., 1985).
(e) Transmission (excretion into eggs and milk)
Radiolabel was transmitted into the eggs of laying hens that had been intubated gastrically with a single or several doses of [3H]T-2 toxin. In birds given a single dose of 0.25 mg/kg bw, the maximum residues were found in eggs 24 h later; the yolk contained 0.04% of the total dose and the white contained 0.13%. In birds given 0.1 mg/kg bw per day for 8 days, radiolabel accumulated in eggs until the fifth day of dosing, remained unchanged until the last day of dosing, and rapidly decreased thereafter. The maximum percentage of administered radiolabel recovered in egg white and yolk after the repeated doses was 0.41% and 0.28%, respectively. Assuming that birds weighing 1.6 kg consumed 100 g of the diet containing T-2 toxin at 1.6 mg/kg each day, the concentration of residues (T-2 toxin and/or its metabolites) in the contaminated eggs would be about 0.9 µg/egg (Chi et al., 1978b).
A pregnant Holstein cow was given 182 mg of T-2 toxin, equal to 0.5 mg/kg bw per day, for 15 days, and milk was sampled on days 2, 4, 5, 8, 10, and 12 after initiation of treatment. T-2 toxin was detected at concentrations of 10–160 ng/kg of milk. A sow was given T-2 toxin in feed at a concentration of 12 mg/kg, equivalent to 0.48 mg/kg bw per day, for 220 days, and its milk was analysed on day 190 of treatment. T-2 toxin was found at a concentration of 76 ng/g. No analysis for metabolites was reported (Robison et al., 1979b). The Committee noted that the purity and source of the T-2 toxin were not reported.
T-2 toxin was found at a concentration of 2 ng/ml in cows’ milk after administration of 160–180 mg/day for 4 days (equivalent to 0.42–0.48 mg/kg bw per day) (Yoshizawa et al., 1981).
Metabolic transformations of T-2 toxin and HT-2 toxin that have been demonstrated in animals are shown in Table 1.
Table 1. Transformation of T-2 toxin
|
Animal |
Transformation reaction |
References |
|
Rat |
De-epoxidation, hydrolysis, 3’-hydroxylation, glucuronide conjugation |
Pace (1986); Pfeiffer et al. (1988); Yoshizawa et al. (1985a,b); Gareis et al. (1986) |
|
Guinea-pig |
Hydrolysis, 3’-hydroxylation |
Pace et al. (1985) |
|
Chicken |
Hydrolysis, 3’-hydroxylation |
Yoshizawa et al. (1980); Visconti & Mirocha (1985) |
|
Dog |
Hydrolysis, glucuronide conjugation |
Sintov et al. (1986, 1987, 1988) |
|
Pig |
Hydrolysis, 3’-hydroxylation, de-epoxidation, glucuronide conjugation |
Corley et al. (1985, 1986) |
|
Cow |
Hydrolysis, 3’-hydroxylation, 7’-hydroxylation, de-epoxidation |
Yoshizawa et al. (1981, 1982a,b); Pawlosky & Mirocha (1984); Chatterjee et al. (1986) |
|
Monkey |
Hydrolysis, 3’-hydroxylation |
Yoshizawa et al. (1984) |
Male Sprague-Dawley rats received [3H]T-2 toxin at a dose of 0.15 or 0.6 mg/kg bw by oral, intravenous, or dermal administration, and 16 peaks in urine and faecal extracts were compared with known metabolite standards by high-performance liquid chromatography (HPLC) to identify radiolabel associated with T-2 toxin, HT-2 toxin, 3’-OH-HT-2, 3’-OH-T-2, neosolaniol, T-2 tetraol, and 4-deacetylneosolaniol. Averaged over all doses and routes, 68% of the recovered radiolabel in urine was associated with T-2 toxin (5.6%), HT-2 toxin (8.9%), 3’-OH-HT-2 (29%), 3’-OH-T-2 (3.2%), and T-2 tetraol (21%). On the basis of retention times, de-epoxy 3’-OH-T-2 triol, de-epoxy 3’-OH-HT-2, and 3’-OH-T-2 triol were also tentatively identified in excreta. The relative conversion rates to the identified metabolites were not affected by dose, but higher relative rates of conversion to T-2 tetraol, HT-2 toxin, and de-epoxy tetraol and lower rates of conversion to 3’-OH-HT-2 were seen after intravenous than after oral administration. More de-epoxy 3’-OH-HT-2 was found in orally dosed animals than those treated intravenously. In general, de-epoxidation appeared to be an important route of detoxication. T-2 toxin represented a greater percentage of the recovered radiolabel in urine over time, suggesting slow release from cutaneous fat stores. An increase over time in the percentage of recovered radioactivity as the de-epoxy metabolites was also observed (Pfeiffer et al., 1988).
C-4 deacetylation of T-2 toxin to HT-2 toxin was demonstrated in microsomal preparations of liver, kidney, and spleen from various species. The reaction rates of hepatic microsomes from various species, expressed in nmol/mg protein per 10 min, were: rabbit, 3000; human, 330; mouse, 75; chicken, 55; rat, 36; and guinea-pig, 14 (equal to 1400, 150, 35, 26, 17, and 6 µg/mg of protein) (Ohta et al., 1977; Johnsen et al., 1986).
Metabolism of T-2 toxin to hydrolysates at the C-4, C-8, and C-15 positions and hydroxylation at the C-3´ position by liver homogenates from rats, mice, and monkeys were demonstrated in vitro. Hydrolytic transformation was also observed in hepatic homogenates from rabbits, pigs, and cows (Yoshizawa et al., 1984, 1985b).
Enzymic conversion of T-2 toxin to HT-2 toxin was examined in liver homogenates from male Wistar rats, in studies in which 0.5 µmol (230 µg) of T-2 toxin were added to 2 ml of a cytosol/microsomal fraction. Complete conversion to HT-2 toxin was demonstrated within 60 min, and no other metabolites were reported (analysis by gas chromatography (GC)–mass spectrometry (MS)). There was much less conversion to HT-2 toxin in liver cytosol, and no metabolism was reported in plasma. Co-incubation of T-2 toxin in a liver homogenate with paraoxon showed that inhibition of serine esterases completely blocked conversion of T-2 toxin to HT-2 toxin. Further analysis with serine esterases showed that carboxylesterase but not cholinesterase was effective in converting T-2 toxin to HT-2 toxin (Johnsen et al., 1986)
Inhibition of carboxylesterase with tri-ortho-cresyl phosphate (TOCP) in groups of 10 mice (strain and sex unspecificed) given T-2 toxin at 0.5, 1, 2, 3, or 4 mg/kg bw intravenously led to the death of all animals at 2, 3, and 4 mg/kg bw, but not at 0.5 or 1 mg/kg bw (Johnsen et al., 1986).
Glucuronide-conjugated HT-2 toxin was the main metabolite recovered from bile collected from isolated, perfused male Wistar rat livers. Only trace amounts of T-2 toxin were recovered as the glucuronide conjugate. Analysis was performed with gas–liquid chromatography–mass spectroscopy. beta-Glucuronidase was used to determine glucuronide conjugates (Gareis et al., 1986).
Glucuronide conjugates of T-2 toxin and metabolites were identified in the urine and bile of two sows after intravenous administration of [3H]T-2 toxin at 0.15 mg/kg bw. Urine was collected hourly for 4 h through a catheter, and bile was collected after the animals were killed at 4 h. High-performance thin-layer chromatography with comparison to standards was used to identify and quantify the metabolites. Glucuronide conjugates were determined with beta-glucuronidase for T-2 toxin, 3’-OH-T-2, neosolaniol HT-2, 3’-OH-HT-2, T-2 triol, 4-deacetylneosolaniol, and T-2 tetraol. De-epoxy derivatives were not determined. Less than 1% of the recovered T-2 toxin and < 10% of the recovered HT-2 toxin was unconjugated in either bile or urine. The main metabolite recovered in bile was conjugated T-2 toxin, followed by conjugated HT-2 toxin. Conjugated metabolites represented an average of 77% of the recovered radiolabel in bile, and all metabolites accounted for an average of 92% of the radiolabel. In urine, conjugated metabolites represented an average of 63% of the recovered radiolabel, with an average of 90% of the recovered radiolabel accounted for by metabolites (Corley et al., 1985).
The effect of T-2 toxin on intestinal absorption of monosaccharides was studied in rats. The absorption of 3-O-methylglucose was reduced one- to threefold after injection of T-2 toxin into the jejunal lumen or after intravenous injection. Jejunal function was impaired by specific damage to the active transport and diffusional movement of monosaccharides (Kumagai & Shimizu, 1988).
Three groups of 1-day-old chicks (10 chicks per group) were given diets containing T-2 toxin at a concentration of 0.5–15 mg/kg, equivalent to 0.06–1.9 mg/kg bw, for 3 weeks. Plasma vitamin E activity and hepatic vitamin A content were measured. A dose-dependent decrease in plasma vitamin E activity was observed, with a 65% decrease from that of controls in chicks fed the diet containing 15 mg/kg. This decrease was considered to be due to a reduction in the plasma concentration of lipoproteins, which are required for the transport of vitamin E (Coffin & Combs, 1981).
Several authors reported changes in the serum concentrations of glucose and essential elements in animals given T-2 toxin in the feed (for example, Rafai et al., 1995a; Weaver et al., 1978). The Committee noted that, as feed refusal was observed at all doses tested and decreased feed intake would result in changes in metabolism and energy use, observations such as these are difficult to interpret. Experimental designs that control for feed intake are needed before changes in metabolism and energy use can be assessed.
(b) Inhibition of protein synthesis
The effects of T-2 toxin on protein synthesis were studied in Swiss mice and hepatoma cell cultures. T-2 toxin was given as an intraperitoneal dose of 0.75 mg/kg bw per day for 3 or 7 days. Protein synthesis was inhibited in the animals and in cells obtained from bone marrow, spleen, and thymus. Protein synthesis was also inhibited in vitro in hepatoma cell cultures and phytohaemagglutinin-stimulated lymphocytes (Rosenstein & Lafarge-Frayssinet, 1983).
Chinese hamster ovary and African green monkey kidney (Vero) cell cultures were exposed to T-2 toxin at 0.01 or 1 ng/ml for 1 or 12 h. The cells showed morphological changes indicative of inhibition of protein synthesis, including disassociation of polysomes and matrix density, ballooning of the intracristal space, and malalignment of cristae in mitochondria. The Chinese hamster ovary cells had bleb formations of the plasma membrane, which indicates inhibition of protein synthesis (Trusal, 1985).
Protein inhibition was also observed in muscle, heart, liver, and spleen of rats that received intraperitoneal injections of T-2 toxin at 0.3, 0.75, or 2 mg/kg bw (Thompson & Wannemacher, 1990).
The effects of T-2 toxin on rat hepatocytes in culture were studied by adding it at 0.01 or 1 ng/ml for 1 or 12 h. The lower concentration caused 75% inhibition of protein synthesis within 1 h. At the higher concentration, the hepatocytes recovered from a 1-h but not a 12-h exposure. Cell damage (release of lactate dehydrogenase) lagged behind inhibition of protein synthesis, which was 90% at 1 ng/ml. Ultra-structural alterations were present in the endoplasmic reticulum and mitochondria. The rough endoplasmic reticulum showed degranulation, and mitochondria had translucent foci and electron-dense cores (Trusal & O’Brien, 1986).
T-2 toxin induced disaggregation of polysomes in HeLa cells (Liao et al., 1976). It interacted with the peptidyl transferase centre on the 60S ribosomal subunit and inhibited transpeptidation of peptide-bond formation. These results were consistent with the effects of an inhibitor of prolongation or termination of protein synthesis (Stafford & McLaughlin, 1973).
The ribosomal subunits of Myrothecium verrucaria, a producer of macrocyclic trichothecenes, were resistant to T-2 toxin. The authors concluded that the 60S subunits of eukaryotes are responsible for the sensitivity to T-2 toxin (Hobden & Cundliffe, 1980).
(c) Inhibition of nucleic acid synthesis
The incorporation of [3H]thymidine into DNA in cell lines from thymus was strongly inhibited by T-2 toxin at 10 ng/ml and slightly inhibited at 0.1–10 ng/ml. Concentrations of 0.1–1 ng/ml caused a transient increase in DNA polymerase and alpha- and beta-terminal deoxynucleotidyl transferase activity, whereas concentrations > 1 ng/ml caused strong inhibition (Munsch & Mueller, 1980)
A single dose, three daily doses, or seven daily doses of T-2 toxin at 0.75 mg/kg inhibited DNA synthesis ex vivo in cell cultures from the spleen, thymus, and bone marrow of treated mice. T-2 toxin also inhibited DNA synthesis in vitro in cultures of hepatoma cells and in phytohaemagglutinin-stimulated lymphocytes (Rosenstein & Lafarge-Frayssinet, 1983).
The effects of T-2 toxin on DNA synthesis in phytohemagglutinin-stimulated human peripheral blood lymphocytes was assayed by incorporation of [3H]thymidine. Total inhibition was obtained with 8 ng /ml and 80% inhibition with 1.5 ng/ml (Cooray, 1984).
In synchronously dividing Tetrahymena cells, incorporation of radiolabelled thymidine and uracil into DNA and RNA, respectively, was inhibited. Neither DNA nor RNA synthesis nor RNA hybridase activity was altered by T-2 toxin in vitro in isolated nuclei from normal cells or from cells pretreated with T-2 toxin (Iwahashi et al., 1982).
Thymidine uptake and incorporation into DNA was biphasic, with increased uptake at 0.4 pg/ml reduced uptake at 4 pg/ml, and reduced incorporation at 40 pg/ml (Bunner & Morris, 1988).
(d) Alterations of cellular membranes
At a concentration of T-2 toxin of 20 µg/ml, no entry of [14C]sucrose or [3H]inulin was observed in bovine erythrocytes in vitro. Very little radiolabel was bound to bovine erythrocytes, and the binding was independent of the T-2 toxin concentration. The toxin had no effect on the entrapment of sucrose or inulin. Carrier erythrocytes retained 85% of [14C]sucrose and only 18% of [3H]T-2 toxin. Thus, T-2 toxin diffused from carrier cells more rapidly than sucrose. The authors concluded that the interaction of T-2 toxin with bovine erythrocytes was minimal and intercalation with the inner bilayer was unlikely, because the increase in cell volume that would have resulted did not occur (DeLoach et al., 1987).
The effects of T-2 toxin on membrane function were studied in L-6 myoblasts. The minimal effective concentration of T-2 toxin for reducing uptake of calcium and glucose and for reducing uptake of leucine and tyrosine and their incorporation into protein was 4 pg/ml. The uptake of rubidium was increased at 0.4 pg/ml and reduced at 4 pg/ml or more. Calcium efflux was reduced after 1, 5, and 15 min of exposure to T-2 toxin at a concentration of 40 pg/ml. The authors concluded that that T-2 toxin has multiple effects on membrane function at low concentrations and that these effects are independent of inhibition of protein synthesis (Bunner & Morris, 1988).
The effect of T-2 toxin on lipid peroxidation and the antioxidant status of liver and blood was examined in three species of fowl. As measured by malondialdehyde concentrations in liver homogenate and blood plasma, lipid peroxidation was increased in ducks, chickens, and geese given feed containing T-2 toxin at concentrations of 0.2–0.6 mg/kg (Mezes et al., 1999).
The LD50 values for T-2 toxin and HT-2 toxin in several animal species are summarized in Tables 2 and 3. Strain and sex differences in death rates were seen.
Table 2. Results of studies of the acute toxicity of T-2 toxin
|
Species |
Sex |
Route |
LD50 |
Reference |
|
Mice |
Male |
Oral |
10 |
Ueno (1984) |
|
Mice |
Male |
intraperitoneal |
5.2 |
Ueno (1984) |
|
Mice |
Female |
Intraperitoneal |
14 |
Fairhurst et al. (1987) |
|
Mice |
Male |
Intraperitoneal |
5.3 |
Yoshizawa et al. (1982b) |
|
Mice |
Male |
Intraperitoneal |
9.1 |
Thompson & Wannemacher (1986) |
|
Mice |
Female |
Intraperitoneal |
0.4 |
Lindenfelser et al. (1974) |
|
Mice |
Male |
Subcutaneous |
2.1 |
Ueno (1984) |
|
Mice |
Female |
Subcutaneous |
6.4–8.0 |
Fairhurst et al. (1987) |
|
Mice |
Male |
Subcutaneous |
3.3 |
Thompson & Wannemacher (1986) |
|
Mice |
Male |
Intravenous |
4.2 |
Ueno (1984) |
|
Mice |
Female |
Intravenous |
11 |
Fairhurst et al. (1987) |
|
Rats |
Male |
Intraperitoneal |
1.5 |
Creasia et al. (1990) |
|
Rats |
Male |
Intraperitoneal |
0.9 |
Martin et al. (1986) |
|
Rats |
Female |
Intraperitoneal |
1.3 |
Fairhurst et al. (1987) |
|
Rats |
NS |
Intraperitoneal |
2.2 |
Brennecke & Neufeld (1982) |
|
Rats |
Male |
Subcutaneous |
1 |
Bergmann et al. (1985) |
|
Rats |
Female |
Subcutaneous |
2 |
Fairhurst et al. (1987) |
|
Rats |
NS |
Subcutaneous |
0.56 |
Brennecke & Neufeld (1982) |
|
Rats |
Male |
Intramuscular |
0.85 |
Chan & Gentry (1984) |
|
Rats |
NS |
Intramuscular |
0.47 |
Brennecke & Neufeld (1982) |
|
Rats |
Female |
Intravenous |
0.9 |
Fairhurst et al. (1987) |
|
Rats |
Male |
Intravenous |
0.74 |
Feuerstein et al. (1985) |
|
Rats |
Male |
Inhalation |
0.05 |
Creasia et al. (1990) |
|
Guinea-pigs |
Male |
Intraperitoneal |
1.2 |
Creasia et al. (1990) |
|
Guinea-pigs |
Female |
Subcutaneous |
1-2 |
Marrs et al. (1985) |
|
Guinea-pigs |
Male |
Intravenous |
1-2 |
Fairhurst et al. (1987) |
|
Guinea-pigs |
Male |
Intravenous |
1.3 |
Feuerstein et al. (1985) |
|
Guinea-pigs |
Male |
Inhalation |
0.4 |
Creasia et al. (1990) |
|
Rabbits |
Male/ Female |
Intramuscular |
1.1 |
Chan & Gentry (1984) |
|
7-day-old broiler chicks |
Male |
Oral |
4 |
Hoerr et al. (1981) |
|
Laying hens |
Female |
Oral |
6.3 |
Chi et al. (1977a) |
|
Day-old cockerels |
Male |
Oral |
1.84 |
Lansden et al. (1978) |
|
Day-old broiler chicks |
NS |
Oral |
5 |
Chi et al. (1978a) |
|
Pigs |
|
Intravenous |
1.21 |
Weaver et al. (1978) |
NS, not specified
Table 3. Results of studies of the acute toxicity of HT-2 toxin
|
Species |
Sex |
Route |
LD50 |
Reference |
|
Mice |
Male |
Intraperitoneal |
10 |
Thompson & Wannemacher (1986) |
|
Mice |
Male |
Intraperitoneal |
6.5 |
Yoshizawa et al. (1982b) |
|
Mice |
Male |
Subcutaneous |
6.7 |
Thompson & Wannemacher (1986) |
|
Rats |
Male |
Subcutaneous |
1 |
Bergmann et al. (1988) |
|
1-day-old broiler chicks |
Not specified |
Oral |
7.2 |
Chi et al. (1978) |
Groups of five to seven male and female mice of strains ICR:CD1, BALB/c, C57Bl/6, and DBA/2 were given single doses of T-2 toxin at 2.5, 5, or 10 mg/kg bw by oral gavage. T-2 toxin was more lethal in C57Bl/6 and BALB/c strains and more lethal in the females of those strains. Five of six C57Bl/6 females and three of seven BALB/c females at 10 mg/kg bw but none of the males at this dose had died by 24 h, whereas one of six male and female DBA/2 mice at 10 mg/kg bw, one of six female BALB/c mice at 5 mg/kg bw, and none of the ICR:CD1 mice had died by 24 h. At 48 h, none of the male DBA/2 mice given the same doses of T-2 toxin had died. The mitotic index of intestinal crypt epithelial cells was depressed at all doses in all strains, with no difference by sex, strain, or dose. The apoptotic index in these cells varied by dose, was higher in C57Bl/6 mice than in the other three strains, and was reportedly generally greater in females (Li et al., 1997; Shinozuka et al., 1997a,b).
Sex differences were also observed after exposure by inhalation. When 9-week-old Swiss ICR mice weighing 15–20 g were exposed to T-2 toxin at a concentration of 120 µg/L of air for 10 min (dose per kg bw not estimated), adrenal lesions in the zona fasciculata were observed in 11 of 11 females and in none of 10 males. Extensive necrosis of cortical lymphocytes and necrosis of lymphoid cells in the follicles of the spleen were observed in both males and females (Thurman et al., 1986).
Adaptation to the local and systemic acute effects of T-2 toxin was observed in groups of eight CD-1 mice after dermal application to shaved areas on the back of 10 or 25 µg per animal weekly for 22 weeks. Initially, T-2 toxin caused necrosis, scarring, and sloughing of skin. Application of 10 µg caused reactions that healed within 2–3 weeks, and application 4 or 7 days later and weekly thereafter caused minor or no irritation. Furthermore, a lower acute mortality rate than expected was observed in the group given the higher dermal dose: at 25 µg, only 2/20 mice died after an intraperitoneal LD50 dose (0.4 mg/kg bw), while 10/16 mice given the same intraperitoneal LD50 dose died after having received 10 µg of T-2 toxin (Lindenfelser et al., 1974).
Newborn mice appeared to be more sensitive than adults to a single subcuta-neous dose of T-2 toxin (Ueno et al., 1973a); however, the LD50 after oral adminis-tration did not differ with age in poultry. The birds died within 48 h of receiving T-2 toxin. Within 4 h, they showed asthenia, inappetence, diarrhoea, and panting. The abdominal cavities of birds given lethal doses contained a white chalk-like material that covered much of the viscera (Chi et al., 1977b).
Inhaled T-2 toxin was at least 10 times more toxic than orally administered material (Creasia et al., 1987). Surprisingly, no epithelial necrosis was reported after exposure to T-2 toxin by inhalation, although inflammation and necrosis were seen after dermal application or ingestion of high doses. The LC50 of an aerosolized saline solution of T-2 toxin was 0.035 mg/L of air in mice (Creasia & Thurman, 1993).
Intraperitoneal injection of T-2 toxin caused myocardial damage in rats. A single dose of 2 mg/kg bw or four doses of 0.3 mg/kg bw per day caused focal necrosis of endothelial cells and other histological signs of cell damage (Yarom et al., 1987) Two months after the last of 10 daily intraperitoneal injections of T-2 toxin at 0.3 mg/kg bw, hypertrophy, focal fibrosis, and abundant cellularity were seen (Yarom et al., 1983).
The LD50 of T-2 toxin dissolved in ethanol and administered intravenously was 1.2 mg/kg bw in normal, healthy, cross-bred pigs weighing 3–50 kg. Soon after administration, emesis was followed by eager consumption of feed, moderate posterior paresis, staggering gait, extreme listlessness, and frequent defaecation of normal stools. Between 1 and 6 h, severe posterior paresis, knuckling-over of the rear feet, and extreme lethargy were observed. These signs were followed by severe posterior paresis, frequent falling because of hind-quarter weakness, and dragging of both rear legs. Twenty-four hours after administration, the surviving pigs appeared normal (Weaver et al., 1978; Coppock et al., 1985).
Similar clinical signs were observed in pigs exposed to T-2 toxin by inhalation. Necrosis was present in the epithelial cells of the mucosa and in the crypt cells of the jejunum and ileum, the Peyer’s patches of the ileum, the lymphoid elements of the caecum, the lymphoid follicles in the spleen, and the germinal centre of the mesenteric lymph node (Weaver et al., 1978; Pang et al., 1988).
Eighteen white, cross-bred female pigs weighing 40–60 kg, immunized against Erysipelothrix rhusiopathiae, were given purified T-2 toxin dissolved in 70% ethanol intravenously at a dose of 0 (five pigs), 0.6 (five pigs), 1.2 (one pig), 4.8 (five pigs), or 5.4 (two pigs) mg/kg bw. The animals at 4.8 or 5.4 mg/kg bw died 5–10.5 h later, and the other animals were killed 12–24 h after treatment. Gross lesions were seen in pigs given doses > 1.2 mg/kg bw, consisting of oedema, congestion, and haemorrhage of the lymph nodes and pancreas and congestion and haemorrhage of the gastrointestinal mucosa, subendocardium, adrenal glands, and meninges. The histological alterations parallelled the gross lesions. Other lesions were widespread degeneration and necrosis of lymphoid tissue and the surface and crypt epithelium of the intestines. Scattered foci of necrosis were present in the pancreas, myocardium, bone marrow, adrenal cortex, and the tubular epithelium of the renal medulla. Most of the lesions were dose-dependent. The T-2 toxin-induced lesions in the lymphoid and gastrointestinal tract of pigs were similar to those described in other species. The heart and pancreas were additional target organs in pigs (Pang et al., 1987a).
Groups of 17 castrated male, cross-bred, specific pathogen-free pigs, 9–11 weeks of age, were used to characterize pulmonary and systemic responses to nebulized T-2 toxin (mixed with 100–200 µCi of technetium) at a dose of 9 mg/kg bw given by endotracheal intubation. The animals were exposed to aerosols in pairs, one animal receiving T-2 toxin and the other acting as a control. The pigs retained 20–30% of the T-2 toxin. Five pairs of animals were killed 1, 3, and 7 days after dosing; two pairs in which one treated pig died and the other was killed in a moribund state 0–10 h after dosing were designated 0.33-day groups. The treated pigs vomited after exposure and showed cyanosis, anorexia, and lethargy; they then became laterally recumbent. Alveolar macrophages showed reduced phagocytosis, and the blastogenic responses of pulmonary lymphocytes, but not peripheral blood lymphocytes, to mitogen were reduced. The lesions in the pigs that died included multifocal interstitial pneumonia, necrosis of lymphoid tissue, necrohaemorrhagic gastroenteritis, oedema of gall-bladder mucosa, and multifocal areas of necrosis in the heart and pancreas. Inhalation of T-2 toxin produced a clinical and morphological syndrome resembling that caused by intravenously administered T-2 toxin at doses > 1.2 mg/kg bw (approximate LD50) or death. The lesions produced after inhalation were more severe than those seen after intravenous administration (Pang et al., 1987a).
Rats
Wistar rats fed T-2 toxin (purity not specified) at a concentration of 5, 10, or 15 mg/kg of feed, equivalent to 0.25, 0.5, and 0.75 mg/kg bw per day, for 4 weeks showed gastric lesions, which were diffuse and severe in the rats at the highest dose, focal but definite in those at 0.5 mg/kg bw per day, and negligible in rats at the lowest dose (Ohtsubo & Saito, 1977). The Committee noted that few experimental details were provided, e.g. on the purity of the T-2 toxin used, the way in which it was incorporated into the diet, and the number of rats per dose.
Twenty-four male Wistar rats received subcutaneous injections of T-2 toxin at 0.05 mg/kg bw per day for 28 days, and groups of six were examined at 7, 14, 21, and 28 days. Changes ranging from dystrophia or necrosis to hyperplasia were observed in the liver, kidney, and heart, with progression of severity with duration of exposure. Necrotic changes were observed from the third week. The changes in the kidney appeared to be more severe and occurred earlier (Sinovec & Jovanovic, 1993).
Groups of five to six female Holtzman albino rats were given diets containing T-2 toxin (purity not specified) for 3 weeks to 8 months at a concentration of 5 or 15 mg/kg (equivalent to 0.25 and 0.75 mg/kg bw per day) for up to 3 weeks and 10 mg/kg (equivalent to 0.5 mg/kg bw per day) during alternate 4-week periods for 8 months (4 weeks on the T-2 toxin-containing diet alternating with 4 weeks on control diet). The body weight of rats fed a diet containing 15 mg/kg feed for 19 days was markedly reduced. Slight growth depression was reported in rats fed a diet containing T-2 toxin at 5 mg/kg for 3 weeks. No gastric lesions were observed in any of the treated animals. Focal changes and cytoplasmic degradation (but no macroscopic abnormalities) were seen in the livers of four rats fed diets containing 5 mg/kg feed for 3 weeks followed by 15 mg/kg feed for 3 weeks. Severe inflammation around the nose and mouth were also seen at this time. After 8 months of alternating 4-week exposures to 10 mg/kg of diet, histopathological examination of the livers showed no evidence of toxic hepatitis, cirrhosis, neoplasia, or hyperplasia of either hepatocytes or cholangioles (Marasas et al., 1969).
Poultry
Chickens were fed a diet containing T-2 toxin at a concentration of 1–16 mg/kg for 3 weeks. The T-2 toxin was extracted from a F. tricinctum culture and purified by the method of Burmeister (1971) to yield a crystalline product melting at 148–150 °C. Birds at 4, 8, and 16 mg/kg of diet showed reduced growth and developed yellow–white lesions in the mouth consisting of a fibrinous surface layer and a heavy infiltration of the underlying tissues by granular leukocytes. Escherichia coli and Staphylococcus epidermis were isolated from the lesions (Wyatt et al., 1972).
Groups of 36 broiler chicks aged 1 day to 9 weeks received a diet containing T-2 toxin (purity not specified) at a concentration of 0.2, 0.4, 2, or 4 mg/kg, equivalent to 0.025, 0.05, 0.25, and 0.5 mg/kg bw per day. Those at the highest concentration had reduced body-weight gain and feed consumption and developed oral lesions characterized by circumscribed, proliferating, yellow caseous plaques at the margin of the beak, the mucosa of the hard palate, and the tongue and the angle of the mouth. No lesions were observed in the bone marrow or in peripheral blood (Chi et al., 1977a).
In 1-day-old broiler chicks fed a diet containing T-2 toxin at 1, 2, 4, 8, or 16 mg/kg (equivalent to 0.125, 0.25, 0.5, 1, and 2 mg/kg bw per day) for 3 weeks, the growth rate, the weight of the pancreas, and the weight of the spleen were decreased at concentrations > 4 mg/kg. The T-2 toxin was extracted from F. tricinctum culture and purified by the method of Burmeister (1971) to yield a crystalline product melting at 148–150 °C. Oral lesions were seen at concentrations > 1 mg/kg (Wyatt et al., 1973).
T-2 toxin was administered to 10 laying hens at a concentration of 20 mg/kg of feed for 3 weeks, equivalent to 0.86–0.91 mg/kg bw on the basis of the reported weekly feed consumption during the study. The T-2 toxin was extracted from F. tricinctum culture and purified by the method of Burmeister (1971) to yield a crystalline product melting at 148–150 °C. In comparison with untreated controls, the T-2 toxin-treated hens had reduced feed consumption, body weight, and egg production and thinner egg shells (Wyatt et al., 1975).
Single-comb white Leghorn hens were fed a diet containing T-2 toxin (purity not specified) at 0.5, 1, 2, 4, or 8 mg/kg for 8 weeks. Feed consumption, egg production, and shell thickness were significantly decreased in hens fed the highest concentration. Furthermore, the hatchability of fertile eggs of hens fed 2 or 8 mg/kg of diet was lower than that of hens fed the control diet (Chi et al., 1977b).
Feed consumption and egg production were also reduced in groups of 10 laying hens, 33 weeks old, fed a diet containing T-2 toxin (purity not specified) at 2 mg/kg for 24 days. The total amount of T-2 toxin consumed per chicken per day was 0.2 mg on the basis of feed intake rates and the concentration of T-2 toxin in the feed. Body weights were not reported; however, the authors stated that there was no reduction in body weight (Diaz et al., 1994).
Cats
T-2 toxin was administered to four groups of four to six cats orally in gelatin capsules on alternate days at a dose of 0.06, 0.08, or 0.1 mg/kg bw per day, until death. The animals survived for 6–40 days. Emesis, anorexia, bloody diarrhoea, and ataxia were observed. The cats lost weight and became emaciated. The gross lesions observed included multiple petechiae to ecchymotic haemorrhages of the intestinal tract, lymph nodes, and heart. The lumen of the gut contained copious amounts of dark-red material. The microscopic lesions included haemorrhages in the gut, lymph nodes, heart, and meninges, necrosis of the gastrointestinal epithelium, and decreased cellularity of the bone marrow, lymph nodes, and spleen. The mean survival time was inversely related to the dose of T-2 toxin (Lutsky et al., 1978). The Committee noted that, given the substantial detoxication of T-2 toxin by glucuronide conjugation in rats, dogs, and pigs (Corley et al., 1985; Gareis et al., 1986; Sintov et al., 1986, 1987), the severe effects in cats at this low dose were likely to be a result of deficient glucuronide conjugation in this species. For example, as stated in Annex 1, reference 123, p. 40: "The urinary metabolites of benzoic acid were determined after oral administration of 14C-benzoic acid, mostly at a dose of 50 mg/kg bw, to a large variety of species, including primates, rodents, carnivores, reptiles, and birds.… Substantial amounts of the glucuronide of benzoic acid, in addition to hippuric acid, were detected in the urine of carnivores, except in cats (Bridges et al., 1970)." and "An outbreak of poisoning affected 28 cats that had eaten meat containing 2.39% benzoic acid. The effects were nervousness, excitability, and loss of balance and vision. Convulsions occurred, and 17 cats died or were killed. Autopsies showed damage to the intestinal mucosa and liver. The sensitivity of cats may be due to their failure to form benzoyl glucuronide, … (Bedford & Clarke, 1971)."
Pigs
Groups of 10 pigs were fed a diet containing T-2 toxin at 0.0, 0.5, 1, 2, 3, 4, 5, 10, or 15 mg/kg for 3 weeks. On the basis of feed intake and weekly measurement of the T-2 toxin concentration in the feed, the 3-week average daily intakes were equal to 0.029, 0.062, 0.10, 0.13, 0.1, 0.08, 0.09, and 0.23 mg/kg bw per day, respectively. The T-2 toxin was extracted from a culture of F. tricinctum and determined to be > 90% pure by gas and liquid chromatography. Feed was refused at all concentrations tested. Decreased weight gain was observed at concentrations > 1.0 mg/kg of feed. Hyper- and parakeratosis, acanthosis, erosion, and inflammatory-cell infiltration were observed on histological examination of the skin near the mouth and snout of pigs at all doses. Clinical haematological parameters, such as serum enzyme activities and urea and glucose concentrations, were affected at 0.029 and 0.062 mg/kg bw per day (Rafai et al., 1995a). The Committee noted that the changes in clinical haematological parameters were not clearly dose-related and may have been due in part to reduced feed intake.
Nine young male pigs were fed a diet containing T-2 toxin (purity, 99%) at a concentration of 8 mg/kg for 30 days, equivalent to 0.64 mg/kg bw per day. Feed intake, weight gain, and haemoglobin concentrations were reduced in treated pigs. Serum alkaline phosphatase activity was also significantly decreased (Harvey et al., 1994).
Ruminants
Groups of five male Suffolk-Finn-Columbian lambs were fed diets containing T-2 toxin (purity, 99%) to give a dose of 0.0, 0.3, or 0.6 mg/kg bw per day for 21 days. All treated lambs developed focal hyperaemia and dermatitis at the mucocutaneous junction of the commissure of the lips, diarrhoea, leukopenia, lymphopenia, and lymphoid depletion of the mesenteric lymph nodes and spleen (Friend et al., 1983a).
Four calves were given T-2 toxin at a dose of 0.08, 0.16, 0.32, or 0.6 mg/kg bw per day orally in capsules for 30 days. The calf at the highest dose developed a hunched stance and died on day 20. Some evidence of mild enteritis was seen at all doses. Bloody faeces were observed at doses > 0.32 mg/kg bw per day. At necropsy, abomasal ulcers were present in the calf at 0.16 mg/kg bw per day and rumenal ulcers in the two calves given the two higher doses. Prothrombin times and the activity of serum aspartate aminotransferase were increased in calves at the two higher doses (Pier et al., 1976).
No standardized long-term studies of HT-2 toxin were available.
Groups of 50 male and 50 female weanling CD-1 mice were fed a semi-synthetic diet containing T-2 toxin (purity, 99%) at 1.5 or 3 mg/kg, equivalent to 0.22 and 0.45 mg/kg bw per day, for 71 weeks. The survival rate (reported in a graph) was consistently lower in the control groups of both males and females, beginning from about week 35; the survival rate of treated males at 71 weeks was 75% or more, whereas that of the controls was about 62%. No statistically significant differences among the groups were found in feed consumption or body-weight gain. A dose-related increase in heart weight was seen in males receiving T-2 toxin, being statistically different from controls at the high dose. No changes were seen in the heart weights of females, and no treatment-related weight changes were reported for other organs in either males or females. No treatment-related changes were reported in haematological parameters or in the response to sheep red blood cell challenge. A dose-related increase in the frequency of squamous mucosa hyperplasia was found in the forestomach of both male and female mice, with an increased frequency of hyperkeratosis. The incidence of pulmonary adenoma increased in a dose-related manner in males (10, 15, and 23% for controls and at the low and high dose, respectively) but not in females. The incidences of pulmonary adenomas and hepatic adenomas in males at the high dose were statistically significantly higher than in controls. The incidence of pulmonary adenocarcinoma was 5% in control males and 6% in males at the high dose (Schiefer et al., 1987). The Committee noted the lower survival rate of control rats.
Dermal application of 100 µg of 7,12-dimethylbenz[a]anthracene (DMBA) to 45 BALB/c mice, followed 1 week later by T-2 toxin at 0.5 µg, three times a week for 26 weeks, resulted in papillomas in eight mice and a carcinoma in one, with one papilloma in 15 mice that received DMBA alone. In the same study, 35 BALB/c mice were given dermal applications of 5 µg of T-2 toxin for 6 days, followed 1 week later by application of 12-O-tetradecanoylphorbol 13-acetate (TPA) or acetone three times a week for 26 weeks. No tumours were observed in either group. In 21 BALB/c mice, no papillomas were seen after DMBA or T-2 toxin alone [dosing regimen not translated from Chinese], but papillomas occurred in two of 22 mice treated with DMBA followed by T-2 toxin and four of 21 mice treated with T-2 toxin followed by TPA. One skin carcinoma was observed in mice treated with DMBA followed by T-2 toxin (Yang & Xia, 1988a,b).
Dermal application of 5, 10, or 20 µg of T-2 toxin to the backs of 20 mice (strain not specified) followed after 2 weeks by croton oil twice weekly for 10 weeks did not produce papillomas. Two of 20 mice developed papillomas after dermal treatment with DMBA followed 2 weeks later by 10 µg of T-2 toxin twice weekly for 10 weeks; however, the papilloma rate was not statistically different from that in DMBA-treated controls (Marasas et al., 1969). The Committee noted that the statistical test and the results for DMBA-treated mice were not reported.
In a promotion–initiation study in groups of eight CD-1 mice, an initial dose of DMBA, aflatoxin B1, or T-2 toxin (25 µg) was applied to shaved areas on the back and was followed 4 days later by weekly applications of 10 or 25 µg of T-2 toxin to the same area for 22 weeks. One of eight mice treated with DMBA once and 25 µg of T-2 toxin for 22 weeks developed papillomas. No papillomas were observed in any other T-2 toxin-treated groups. Adaptation to the dermal effects of T-2 toxin was observed.: the initial T-2 toxin treatment caused severe skin reactions (necrosis and sloughing), while applications 4 or 7 days later and weekly thereafter caused only minor or no irritation. The authors concluded that T-2 toxin was not initiating but was a weak promoter (Lindenfelser et al., 1974)
Fifty male Kunming mice received T-2 toxin in ethanol:saline at a dose of 0.1 mg/kg bw per day three times per week by oral gavage for up to 25 weeks. Forestomach papillomas occurred in five of 35 treated animals, with one each at weeks 6 and 20 and three at week 25 of exposure. No papillomas of the forestomach were observed in 30 control mice (Yang & Xia, 1988a). The Committee noted that few experimental details were provided.
Groups of 16–22 female DDD mice were given feed containing T-2 toxin at a concentration of 0, 10, or 15 mg/kg, equivalent to 0, 1.5, and 2.2 mg/kg bw per day, for 12 months. Two mice from each group were killed at 3, 6, and 9 months. At 12 months, all controls were killed, and five mice in each treated group were fed a T-2 toxin-free diet for 3 months. Lesions were observed in the oesophageal region of the stomach of mice fed T-2 toxin, which included hyperkeratosis, acanthosis, and papillomatosis with inflammatory-cell infiltration of the squamous epithelium. These changes were found 13 weeks after the start of treatment and persisted throughout the 12-month feeding; however, most had subsided by 3 months after cessation of treatment. One adenocarcinoma of the glandular stomach and two hepatocellular carcinomas were observed in mice at the high dose. No papillomas of the forestomach were found (Ohtsubo & Saito, 1977). The Committee noted that few experimental details were provided, including the purity of the T-2 toxin used and the way in which it was incorporated into the diet.
A working group convened by IARC (1993) evaluated the experimental data on the carcinogenicity of T-2 toxin and concluded that it was not classifiable as to its carcinogenicity to humans (Group 3).
The results of studies on the genotoxicity of T-2 toxin are summarized in Table 4.
Table 4. Results of assays for genotoxicity with T-2 toxin
|
Test system |
Test object |
Concentration |
Results |
Reference |
|
In vitro |
||||
|
Reverse mutationa |
S. typhimurium , TA100, TA1535, TA1537, TA98 |
50 µg/ml |
Negativea |
Wehner et al. (1978) |
|
S. typhimurium , TA1535, TA1537, TA1538 |
50 000 ng/ml |
Negativea |
Kuczuk et al. (1978) |
|
|
S. typhimurium , TA100 |
100 µg/plate |
Negativea |
Takahashi et al. (1992) |
|
|
SOS DNA repair |
E. coli PQ37 (spot test) |
Not specified |
Negativea |
Auffray & Boutibonnes (1986) |
|
SOS DNA repair |
E. coli PQ37 |
1000 ng/ml |
Negativea |
Krivobok et al. (1987) |
|
Differential toxicity |
B. subtilis rec strains |
100 µg/plate |
Negative |
Ueno & Kubota (1976) |
|
Mitotic crossing-over |
Saccharomyces cerevisiae ade2 locus |
100 µg/plate |
Negativea |
Kuczuk et al. (1978) |
|
Petite forward mutation |
Saccharomyces cerevisiae |
50 000 ng/ml |
Negative |
Schappert & Khachatourians (1986) |
|
DNA single-strand breaks |
BALB/c mouse primary hepatocytes |
5 ng/ml |
Weakly positive |
Lafarge- Frayssinet et al. (1981) |
|
BALB/c mouse spleen lymphocytes |
5 ng/ml |
Positive |
||
|
BALB/c mouse thymic lymphocytes |
5 ng/ml |
Positive |
||
|
Gene mutation |
Chinese hamster V79 fibroblasts, thioguanine |
100 ng/ml |
Positiveb |
Zhu et al. (1987) |
|
Sister chromatid exchange |
Chinese hamster V79 fibroblasts |
2300 ng/ml |
Weakly positivea |
Thust et al. (1983) |
|
Chinese hamster V79 fibroblasts |
100 ng/ml |
Weakly positiveb |
Zhu et al. (1987) |
|
|
Human lymphocytes |
3 ng/ml |
Negativea |
Cooray (1984) |
|
|
Chromosomal aberrations |
Chinese hamster V79 fibroblasts |
500 ng/ml |
Positivea |
Thust et al. (1983) |
|
Chinese hamster V79 fibroblasts |
5 ng/ml |
Positive |
Hsia et al. (1986) |
|
|
Chinese hamster V79 fibroblasts |
50 ng/ml |
Weakly positivea |
Zhu et al. (1987) |
|
|
Chinese hamster V79 fibroblasts |
1 ng/ml |
Positive |
Hsia et al. (1988) |
|
|
Human lymphocytes |
0.1 ng/ml |
Positive |
Hsia et al. (1986) |
|
|
Micronucleus formation |
Chinese hamster V79 fibroblasts |
50 ng/ml |
Positivea |
Zhu et al. (1987) |
|
Unscheduled DNA synthesis |
Human fibroblasts |
5 ng/ml |
Positive |
Oldham et al. (1980) |
|
Inhibition of intercellular communication |
Chinese hamster V79 cells |
3 ng/ml |
Positive |
Jone et al. (1987) |
|
In vivo |
||||
|
Polyploidy induction |
Allium cepa |
20 000 ng/ml |
Positive |
Linnainmaa et al. (1979) |
|
Sex-linked recessive lethal mutations |
Drosophila melanogaster |
63 000 ng/ml |
Weakly positive |
Sorsa et al. (1980) |
|
Drosophila melanogaster |
100–1000 mg/kg in feed, 2–3 days |
Negative |
||
|
Sex-linked chromosomal loss |
Adult Drosophila melanogaster |
20 mg/kg in feed, 48 h |
Positive |
Sorsa et al. (1980) |
|
DNA single-strand breaks |
BALB/c mouse liver |
3 mg/kg bw, intraperitoneally |
Negative |
Lafarge-Frayssinet et al. (1981) |
|
BALB/c mouse spleen |
3 mg/kg bw, intraperitoneally |
Positive |
||
|
BALB/c mouse thymus |
3 mg/kg bw, intraperitoneally |
Weakly positive |
||
|
Micronucleus induction |
Chinese hamster bone marrow |
3 mg/kg bw, intraperitoneally |
Negative |
Norppa et al. (1980) |
|
Chromosomal aberrations |
Chinese hamster bone marrow |
1.7 mg/kg bw, intraperitoneally |
Weakly positive |
Norppa et al. (1980) |
|
Mice |
0.1 mg/kg of feed |
Positive |
Bilgrami et al. (1995) |
|
Adapted from IARC (1993)
a With and without metabolic activation
b With metabolic activation
Hydroxyurea did not alter unscheduled DNA synthesis in cells treated with T-2 toxin or HT-2 toxin at 6 ng/ml; however, the combination of rat liver microsomes and hydroxyurea increased the rate of unscheduled DNA synthesis in cells exposed to HT-2 toxin at 100 µg/L. The authors concluded that the microsomal drug-metabolizing enzyme system participates in the induction of DNA damage by T-2 toxin (Agrelo & Schoental, 1980).
No data were available on the reproductive toxicity of HT-2 toxin.
A two-generation study of reproductive and developmental toxicity was conducted in which 90 female CD-1 mice were fed a semi-synthetic diet containing T-2 toxin (purity, 99%) at a concentration of 1.5 or 3 mg/kg, equivalent to 0.22 and 0.45 mg/kg bw per day. The body-weight gain of dams was similar in all groups. T-2 toxin had minimal, if any, effects on female reproduction and fetal development and was not teratogenic or fetotoxic. Offspring of dams at the higher concentration (through milk) had an initial depression of weight gain, but the weights by 6 weeks of age were reported to be within the normal range for Swiss outbred mice. The weights of the spleen of male offspring of treated dams were greater than that of male offspring of control dams (Rousseaux et al., 1986).
Groups of five female B6C3F1 mice received T-2 toxin by gavage at a dose of 1.2 or 1.5 mg/kg bw per day on days 14–17 of gestation. On day 18, fetal liver cells were collected and pooled per dam for staining and flow cytometry. Depletion of cells expressing CD44 and CD45 antigens was observed. Subsequent analysis of a prolymphocyte-enriched culture of fetal liver cells exposed to T-2 toxin also showed selective elimination of a subpopulation of lymphocytes suggested to be of the CD45+ B-lineage. Similar reductions were found in CD44lo and CD45R+ bone-marrow cells of adult mice exposed to T-2 toxin by gavage at 1.8 mg/kg bw per day, suggesting that B-cell precursors are a sensitive target for T-2 toxin (Holladay et al., 1995).
T-2 toxin was injected intraperitoneally at a dose of 0.5, 1, or 1.5 mg/kg bw into pregnant mice on day 7, 8, 9, 10, or 11 of gestation (number of mice per dose and per gestation day not reported). The two higher doses caused significant maternal mortality, fetal deaths, and fetal body-weight loss. Gross malformations were seen in 37% of the fetuses of dams given 1 mg/kg bw (eight litters) or 1.5 mg/kg bw (four litters) on day 10. The most frequent anomalies were bent, shortened, or missing tails and limb malformations including oligodactyly and syndactyly. Exencephaly, open eyes, retarded jaw, and skeletal malformations of the rib or vertebrae were also found (Stanford et al., 1975). The Committee noted that intraperitoneal injection of T-2 toxin might have resulted in high concentrations in the conceptus and that the malformations occurred at doses that were maternally toxic.
Pregnant CD-1 mice (18 litters per treatment group) were given T-2 toxin dissolved in propylene glycol intraperitoneally at a dose of 0.5 mg/kg bw on day 8 or 10 of gestation. Treatment induced grossly malformed fetuses, principally with tail and limb anomalies. A higher incidence of malformations was observed when T-2 toxin was combined with ochratoxin A at 4 mg/kg bw (Hood et al., 1978). The Committee noted that intraperitoneal injection of T-2 toxin might have resulted in high concentrations in the conceptus.
T-2 toxin dissolved in a 1:1 mixture of propylene glycol and 0.1 N sodium bicarbonate was administered intraperitoneally at a dose of 0.5 mg/kg bw alone or in combination with rubratoxin B at 0.4 mg/kg bw to pregnant CD-1 mice on day 1 of gestation. Only T-2 toxin caused gross malformations. The combination of toxins increased the adverse effects on fetal body weight and mortality rate but not the incidence or severity of the gross malformations (Hood, 1986). The Committee noted that intraperitoneal injection of T-2 toxin might have resulted in high concentrations to the conceptus.
Groups of 10–29 CD-1 mice received crystalline T-2 toxin dissolved in propylene glycol orally by gavage at a dose of 0, 0.5, 1, 2, 3, 3.5, or 4 mg/kg bw on day 9 of gestation. One control group was fed normally and another was starved for 36 h after injection of the vehicle. Fetal morphology and implantation loss were examined on day 18 of gestation. Three of 20 starved controls and 0/19, 1/10, 0/20, 3/20, 9/25, 10/16, and 21/29 of mice given T-2 toxin at 0.0, 0.5, 1, 2, 3, 3.5, and 4 mg/kg bw, respectively, died. T-2 toxin at doses of 3.5 and 4 mg/kg caused more maternal deaths than in the starved controls. Over the 9-day period, reduced feed consumption and weight gain were seen at 4 mg/kg bw, and reduced weight gain alone was seen at 3.5 mg/kg bw. The rate of fetal resorption was 6% in the starved control group and 100%, 73%, and 4% at 4, 3.5, and 0 mg/kg bw, respectively. More skeletal abnormalities were seen in the starved controls and T-2 toxin-treated mice at 3 mg/kg bw.
In a second experiment, a single dose of T-2 toxin at 3 mg/kg bw was given by oral gavage on day 6, 7, 8, 9, 10, 11, or 12 of gestation to CD-1 mice. Fetal morphology and implantation loss were examined on day 18 of gestation. Control groups for each treated group were starved for 36 h after administration of the vehicle. The fetuses of 279 dams were examined, but the number of dams per treated and control group and the number of animals that died before examination were not reported. The treated females lost more fetuses than controls, and there were more dead fetuses among litters treated on day 9 of gestation than on other days. Major skeletal defects (not defined) were reported to be more numerous in mice treated on day 7 of gestation than in those treated on other days or in starved controls. The authors concluded that a single oral dose of T-2 toxin in propylene glycol was primarily maternally toxic and embryolethal; the defective development was possibly secondary to the maternal toxicity (Rousseaux & Schiefer, 1987).
Wag rat dams were treated on days 14–20 of gestation with T-2 toxin at 0.1, 0.2, or 0.4 mg/kg bw per day by intraperitoneal injection or at 0.1 or 0.4 mg/kg bw per day in the diet. Feed intake, weight gain, and the numbers of treated and control dams were not reported. Four to 29 rat pups per dose and time of sacrifice were examined; no mention was made of efforts to control for litter effects. Differences in thymus weight were seen in all treated groups. In rat pups examined 1 day after birth, the thymus weights, expressed as a percentage of total body weight, were 30–39% lower in treated rats, with the exception of those treated at 0.1 mg/kg bw per day intraperitoneally, which had thymus weights 11% lower than those of controls. Liver weight as a percentage bw was reported to be higher in 1-day-old pups of dams dosed with 0.4 mg/kg bw per day intraperitoneally. No differences were seen in liver or spleen weights at other doses and times. One week after parturition, thymic atrophy was less marked, suggesting that the effect was transient. The lymphoblastic response of spleen T and B cells (but not thymus T cells) to phytohaemagglutinin, concanavalin A, and lipopolysaccharide was reduced in 4- and 6-day-old offspring of dams that received T-2 toxin at 0.2 mg/kg bw per day intraperitoneally on days 18–20 of gestation (Lafarge-Frayssinet et al., 1990). The Committee noted the lack of detail in reporting and the lack of statistical analysis of the any of the reported differences. In general, the differences were difficult to interpret.
(c) Reproductive endocrine effects
Groups of 10 New Zealand white rabbits were fed a diet consisting of ‘naturally infected’ wheat containing T-2 toxin at a concentration of 0.19 mg/kg, equivalent to 0.008 mg/kg bw per day, for 32 days. A control group was fed uncontaminated wheat. The treated animals were then given gonadotropin-releasing hormone to induce false gestation plus T-2 toxin for a total duration of T-2 toxin treatment of 50 days; progesterone levels were monitored during this time. The concentrations of T-2 toxin and zearalenone were determined by HPLC; no other toxins were assayed. Two animals died during the 32-day treatment period, and one animal died during the subsequent treatment, each of Staphylococcus aureus infection. No control animals died. No gross morphological differences were found in three treated and three control animals killed after 32 days. Three of the five animals given T-2 toxin and gonadotropin-releasing hormone showed abnormal progression of progesterone concentrations, and one of these animals died about 1 week after initiation of hormone treatment. Serum creatinine and alanine aminotransferase activity were higher in treated than in control animals, and the serum cholinesterase concentration was decreased. No differences in feed intake were seen. Body weights were not reported (Fekete & Huszenicza, 1993). The Committee noted the poor description of the experimental design, that naturally infected feed was used, and that the process of infection and the organism used were not described.
Perturbation of progesterone progression after stimulation of ovarian activity was reported in groups of four to five ewes given T-2 toxin (purity, 95%) at 0.005 or 0.015 mg/kg bw per day for 21 days orally by intubation. The authors concluded that progesterone cycling was affected at the higher dose (Huszenicza et al., 2000). The Committee noted that the report did not permit evaluation of the results. Examples of progesterone profiles were given, but no statistical analysis was reported.
Four heifers fed a diet that stimulated ruminal acidosis were given T-2 toxin (purity, 95%) at a dose of 0.025 mg/kg bw per day by oral intubation for 20 days after initiation of ovulation. Rumenal acidosis was induced in order to investigate an increased rate of abortions that had been observed in a dairy herd on a similar diet contaminated with T-2 toxin. Three heifers were fed the acidosis-stimulating diet without T-2 toxin; no heifers were fed diets that did not stimulate rumenal acidosis. The ovarian activity of the heifers was synchronized by administering 25 mg of prostaglandin F2 intramuscularly 23 and 12 days before T-2 toxin and on the day of initiation of T-2 toxin treatment. Plasma progesterone was sampled on alternate days starting 12 days before T-2 toxin treatment and continuing 10 days afterwards. The authors reported that ultrasonography showed no difference in the number of antral follicles or the size of the dominant follicle. All the treated heifers ovulated, but the mean time to ovulation was significantly greater in T-2 toxin-treated animals when compared with their pre-treatment ovulation time and with the ovulation time of controls that did not receive T-2 toxin. The progesterone concentrations remained low for about 2 days longer after the last prostaglandin F2 injection in the T-2 toxin-treated heifers, suggesting that, under acidotic conditions, a low dose of T-2 toxin can retard follicle maturation and ovulation (Huszenicza et al., 2000).
T-2 toxin inhibited testosterone secretion in gerbil testicular interstitial cells in vitro. The median inhibitory dose was 0.042 nmol/L, equivalent to 0.02 ng/mL (Fenske & Fink-Gremmels, 1990).
(a) Cell proliferation and apoptosis in dermal tissues
Hairless WBN/ILA-Ht rats were given single applications of 10 µL of a 0.5 mg/mL solution of T-2 toxin on each of four dorsal skin areas. Control animals were treated with the vehicle, a 20% ethanol solution. Biopsies of treated skin were taken 3, 6, 12, and 24 h after application, and the number of cells staining for proliferating cell nuclear antigen (PCNA) was used as a measure of proliferation. Less epidermal basal-cell proliferation was seen in treated than control animals 3 h after treatment, and the number continued to decrease during the observation period. No degranulation of mast cells was seen, but the number of mast cells increased during the observation period, especially around the small vessels of the dermis. Basal cells showing acidophilic degeneration were noted 12 h after treatment. DNA fragmentation was analysed by tritiated thymidine-mediated dUTP-biotin nick end-labelling (TUNEL). Basal cells showing acidophilic degeneration were generally positive, indicating apoptosis. The number of these cells increased at the 12- and 24-h observation times (Albarenque et al., 1999).
Groups of five male CD-1 mice were given T-2 toxin orally by gavage at a dose of 0.1, 0.5, or 2.5 mg/kg bw per day for 2 weeks. Increased formation of macrophage colonies from cultured bone marrow was observed, but no change was seen in the number of granulocyte or granulocyte–macrophage (GM) colonies. At 0.5 mg/kg bw per day, no change in macrophage colonies was observed in bone-marrow culture, but an increased number of granulocyte colonies and a decreased number of GM colonies were observed. At 2.5 mg/kg bw per day, there was no change in the number of granulocyte colonies, but an increased number of macrophages and a decreased number of GM colonies were seen. Increased spleen weight and decreased thymus weight (both relative to body weight) and a decreased red blood cell count were observed at the highest dose. No change was seen in the leukocyte count at any dose. Exposure of gGM progenitor cells from unexposed mice to T-2 toxin inhibited their proliferation into granulocyte or GM colonies at concentrations > 1 nmol/L (0.47 ng/ml) and inhibited their proliferation to macrophage colonies at concentrations > 3 nmol/L (1.4 ng/ml) (Dugyala et al., 1994).
Groups of 10 male BALB/c mice received T-2 toxin by subcutaneous injection at a dose of 0.17, 0.3, 0.44, 0.66, 1, or 1.5 mg/kg bw 1 h before an intraperitoneal injection of 59Fe. Blood was drawn 24 and 72 h after 59Fe injection. Dose-dependent inhibition of 59Fe uptake into erythrocytes was seen at doses of T-2 toxin > 0.3 mg/kg bw at both 24 and 72 h. The mean response to the lowest dose was also lower than that of controls but was not statistically significant. The leukocyte count was affected in a dose- and time-dependent manner. In a separate experiment, groups of four to six male BALB/c mice received T-2 toxin at the same doses, followed 3, 24, or 72 h later by withdrawal of blood for leukocytre counting. Dose-dependent leukocytosis was observed 3 h after injection of T-2 toxin, with increases of several hundred per cent in the count at doses > 0.66 mg/kg bw. Conversely, 24 h after injection, the leukocyte count was less than 50% that of controls at the same doses. By 72 h after T-2 toxin injection at doses > 0.3 mg/kg bw, the leukocyte count was numerically lower but was not significantly lower than that of controls. The erythrocyte count was not affected at the doses tested, nor was the serum Fe concentration changed by treatment, suggesting that the effect on Fe incorporation occurred at the level of erythrocyte formation. The lowest statistically significant level of effect on 59Fe uptake in circulating erythrocytes was 0.3 mg/kg bw (Faifer & Godoy, 1991).
The proliferation of colony-forming units (CFU) GM colonies was assessed in the bone marrow of groups of eight to ten male BALB/c mice that received a single subcutaneous injection of T-2 toxin at 0.17, 0.3, 0.6, or 1 mg/kg bw. All doses caused a decrease in the number of colonies 1 h after dosing. The effect was transient, with a slight increase 72 h after exposure. Significant inhibition of 59Fe uptake in circulating erythrocytes was seen at doses > 0.3 mg/kg bw 1 h after dosing, with non-significant inhibition at 0.17 mg/kg bw. A significant rebound to levels of 59Fe uptake above that of controls was seen even at the lowest dose 72 h after treatment (Faifer et al., 1992).
Recovery after T-2 toxin treatment occurred more readily in spleen than in bone marrow in male BALB/c mice. A single subcutaneous dose of 2 mg/kg bw reduced 59Fe uptake into erythrocyte precursors in bone marrow and spleen. The uptake into spleen returned to control levels by 3 days, but that into bone marrow was depressed for 15–21 days. The cellularity of spleen and femur marrow had decreased 1 day after T-2 toxin injection, but that of the femur returned to normal and that of the spleen was increased to 200% of the control value by day 6. By day 35, the cellularity of the spleen had returned to normal (Velazco et al., 1996).
Repeated doses of T-2 toxin at 2 mg/kg bw per day for 3 days to groups of five or more male BALB/c mice did not cause additional damage to their blood-forming capacity over that seen with single doses. The repeated doses caused similar cellular depletion in the spleen and femur marrow, with a similar recovery period (Velazco et al., 1996). Furthermore, repeated doses did not inhibit the haematopoietic response to bleeding, consisting in removal of approximately one-third of the total blood volume on each of two successive days, the volume being replaced by saline. Rather, there was an increased response, measured as total nucleated cellularity and total erythroid cellularity in spleen up to 35 days after T-2 toxin treatment (Godoy et al., 1997).
Twenty male weanling outbred Swiss mice were fed a dry diet containing purified crystalline T-2 toxin at a concentration of 20 mg/kg for 41 days, and another group of four animals received the same diet for 21 days, followed by control diet for 7 days. One control group of 20 animals was maintained on restricted diet; a second control group consisting of eight animals was given the diet ad libitum; and 12 animals were killed at day 0. Haematological examinations were made weekly of treated and control animals. During the first 3 weeks of treatment, lymphoid tissues, bone marrow, and splenic red pulp became hypoplastic, resulting in anaemia, lymphopenia, and eosinopenia. Subsequently, during continued treatment, regeneration occurred, leading to hyperplasia of the haematopoietic cells by 6 weeks. All treated animals also developed perioral dermatitis and ulceration of the gastric mucosa. The authors concluded that T-2 toxin is irritating and suppresses haematopoiesis; however, the haematopoietic effects were transient at the dose tested and did not lead to haematopoietic failure (Hayes et al., 1980).
Incubation of CFU-GM from the bone marrow of rats with T-2 toxin and HT-2 toxin at 1 nmol/L (equivalent to 0.47 ng/ml) for 7, 10, or 14 days inhibited the growth of the cells (Parent-Massin & Thouvenot, 1995).
Hartley guinea-pigs (number not stated) given T-2 toxin dissolved in ethanol by intramuscular injection at the 24-h LD50 dose of 1 mg/kg bw showed decreased activities of all coagulation factors except fibrinogen. Platelet aggregation in the whole blood response to ADP and collagen was depressed. The animals also showed an initial rise, followed by a fall in erythrocyte volume fraction, leukocytosis, and a fall in the platelet count. These changes, which were found within a few hours of administration, reached a maximum at 24 h and returned to normal over the next 2 days. Pretreatment with vitamin K1 did not prevent the effects of T-2 toxin on coagulation. Addition of the toxin to plasma and blood of untreated guinea-pigs at a concentration of 1 mg/mL did not affect clotting times or platelet aggregation, indicating that T-2 toxin itself did not have a direct effect on the activity of coagulation factors (Cosgriff et al., 1984).
Eight New Zealand white rabbits were given T-2 toxin dissolved in dimethyl sulfoxide by intravenous injection at 0.5 mg/kg bw; and five rabbits were given a single oral dose of 2 mg/kg bw by gavage. The rabbits treated intravenously showed reductions in both packed cell volume and total leukocyte count, but no significant alterations in haematological parameters were seen in rabbits given T-2 toxin orally. In another study, nine New Zealand white rabbits were given a single intravenous injection of T-2 toxin dissolved in dimethyl sulfoxide at a dose of 0.5 mg/kg bw; five animals received daily subcutaneous injections of vitamin K at a dose of 0.5 mg/kg bw per day for 5 days before administration of the same dose of T-2 toxin for the subsequent 4 days. Two groups of eight rabbits served as controls. Blood samples were taken from each animal before treatment with dimethyl sulfoxide and 6–96 h later. The concentrations of coagulation factors VII, VIII, IX, X, and XI were decreased by about 40% within 6 h of administration of T-2 toxin, and the fibrinogen content was elevated at 24 h. The reduction in coagulation factors did not induce clinical haemorrhage, however, and administration of vitamin K did not alter the effects of T-2 toxin, indicating that the effect of the toxin on coagulation was not due to antagonism of vitamin K (Gentry & Cooper, 1981).
Cats given T-2 toxin subcutaneously or in the feed showed leukocytosis followed by leukopenia. The frequency of dosing and the dose were different for individual cats, and there were few cats per dose, so that the dose–response relationship could not be analysed. When three cats were given T-2 toxin subcutaneously at 0.05 mg/kg bw per day for 12 days, a transient increase in leukocyte count was seen in two cats within the first few days. This transient increase was followed by a decrease in all cats, to < 20% of the initial values by the last day of dosing. Of the three cats, two died within 35 days after the last injection. Deaths occurred at all doses tested (Sato et al., 1975).
Extracted, purified T-2 toxin was administered to cats in gelatin capsules every 2 days at a dose of 0.08 mg/kg bw per day (six cats) or 0.1 mg/kg bw per day (four cats). Pancytopenia and death occurred within 6–24 days for all cats (Lutsky et al., 1978).
Ten male cats were anaesthetized with ketamine and given T-2 toxin orally by capsule at a dose of 0.08 mg/kg bw every 48 h until death; two cats received the vehicle only. Blood was drawn at each administration. All 10 treated cats but neither of the controls died within 32 days of initiation of treatment, with an average survival time of 21 days. The clinical signs included vomiting, bloody faeces, weakness, lassitude, ataxia, dyspnoea, dehydration, loss of weight, and pre-terminal anorexia. Progressive decreases were observed in total leukocyte count, erythrocte volume fraction, haemoglobin, and thrombocyte count during treatment. Initial leukocytosis was observed in most cats. On gross examination, subcutaneous petaechiae, haemorrhagic lymph nodes, and multiple haemorrhagic erosions were observed in the gastric and intestinal mucosa. Petaechial haemorrhages and ecchymoses were observed on the myocardium (Lutsky & Mor, 1981).
The effects of T-2 toxin on blood coagulation were studied in groups of 40-day-old chickens fed diets containing T-2 toxin at a concentration of 1, 2, 4, 8, or 16 mg/kg, equivalent to 0.12, 0.25, 0.5, 1, and 2 mg/kg bw. Forty birds served as controls. The activities of factor X, prothrombin, and fibrinogen were reduced only at the highest concentration. The activity of factor VII was reduced at the three higher concentrations (Doerr et al., 1981).
In a study of the effects of ochratoxin and T-2 toxin, nine young pigs were given feed containing T-2 toxin (purity, 99%) at 8 mg/kg, equivalent to 0.64 mg/kg bw per day, for 30 days. Treatment reduced the haemoglobin concentration and serum alkaline phosphatase activity. No effect was reported on the erythrocte volume fraction or mean cell volume (Harvey et al., 1994).
The erythrocyte volume fraction was reduced in young pigs fed a diet containing T-2 toxin extracted from a culture of F. tricinctum (> 90% pure by gas and liquid chromatography) at a concentration of 3 mg/kg of diet, equivalent to 0.13 mg/kg bw per day. Groups of two 7-week-old pigs (sex unspecified) received diets containing T-2 toxin at concentrations equivalent to doses of 0, 0.029, 0.062, 0.10, or 0.13 mg/kg bw per day for 21 days. A dose-related reduction in leukocyte count was observed at all doses. The haemoglobin concentration was also decreased in a dose-related manner, at doses ž 0.062 mg/kg bw per day. A reduction in the erythrocyte count was observed at 0.10 and 0.13 mg/kg bw per day (Rafai et al., 1995b). The Committee noted that the changes observed might have been due to reduced feed intake.
Two calves were given T-2 toxin by stomach tube at a dose of 0.2 mg/kg bw per day for 11 days. There were no controls. The treated animals developed clinical signs of weakness and inappetence, and one died. Prothrombin time was prolonged in both animals, and one had marked neutrophilia. No haemorrhagic syndrome was found (Patterson et al., 1979).
Nine male cynomolgus monkeys received a single intramuscular injection of T-2 toxin at a dose of 0.65 mg/kg bw (LD20 dose). Three monkeys served as controls. Haematological parameters were measured before injection and 0, 6, 12, and 24 h and 2, 3, and 7 days after treatment. Samples of 8 ml of blood were drawn through a heparinized catheter implanted surgically 7 days before treatment. The monkeys wore leather restraints for the duration of surgery, recovery, and the observation period; they were observed for signs of toxicity and particularly for evidence of haemorrhage. Three of the treated monkeys died < 24 h after treatment, and two more died during the observation period; none of the controls died. The animals that died were necropsied, and necrosis of lymphoid tissues and petaechial haemorrhage of the colon and heart were observed. A parallel decline in erythrocyte volume fraction (10–25% over 3 days) was observed in both the surviving treated and control monkeys. Transient leukocytosis was seen, the neutrophil and lymphocyte counts in treated animals being four to five times those before treatment and in controls. The lymphocyte counts returned to normal within 1 day and those of neutrophils within 3 days. Slight lymphopenia was seen at days 2 and 3. Prolongation of prothrombin and activated thromboplastin times and decreased activities of multiple coagulation factors were also observed within hours of administration, which reached a maximum at 24 h and returned to normal over the next 3 days. Fibrin–fibrinogen degradation products were not detected at any time. The platelet counts of treated animals varied nonsignificantly over the observation period but were significantly increased in controls. Despite the changes in haematological parameters, none of the surviving animals had clinical signs of haemorrhage (Cosgriff et al., 1986).
Three male and two female adult rhesus monkeys were given T-2 toxin in 20 ml of milk, initially at 1 mg/kg bw per day for 4 days and at 0.5 mg/kg bw per day on days 5–15. Three males and three females served as controls. All three treated males died of respiratory failure between days 0 and 15. After 30 days’ recovery, the two treated females and two additional males received T-2 toxin at 0.1 mg/kg bw per day for 15 days. All monkeys given 1 mg/kg per day showed signs of toxicity similar to those of alimentary toxic aleukia in humans, i.e. vomiting, apathy, and weakness of the lower limbs. The signs were more severe in males, which also developed petaecheal haemorrhages on the face. All males developed severe leukocytopenia, follicular atrophy of the spleen and lymph nodes, and pneumonia, suggesting involvement of the immune system. Bone-marrow changes were not found at necropsy. All animals at 0.1 mg/kg per day developed leukocytopenia and mild anaemia after 15 days of treatment (Rukmini et al., 1980).
Development of burst-forming unit–erythroid (BFU-E) colonies was scored in cells harvested from human umbilical cord after addition of T-2 toxin or HT-2 toxin to the culture medium for 14 days. The size of the colonies was measured microscopically, and the degree of cell differentiation was measured from the total porphyrin and haemoglobin content. The concentrations tested were 0.1, 0.5, 2.2, and 10 nmol/L (equivalent to 0.047, 0.23, 1.1, and 4.7 ng/ml) for T-2 toxin and 0.1, 0.5, 2.5, and 100 nmol/L (equivalent to 0.042, 0.21, 1.1, and 42 ng/ml) for HT-2 toxin. Significant differences in colony growth were observed at 0.1–2.5 nmol/L of HT-2 toxin, with no difference in porphyrin or haemoglobin concentrations, even though growth was inhibited. No colonies survived at the highest dose of HT-2 toxin (100 nmol/L). No differences in growth were observed with T-2 toxin up to a concentration of 2.2 nmol/L, but the highest concentration caused complete loss of BFU-E colonies. Intermediate doses did change the porphyrin or haemoglobin concentrations, although no dose–response relationship was evident. At the lowest dose of T-2 toxin (0.1 nmol/L), the haemoglobin concentration was greater than that of controls, and at 0.5 nmol/L a decrease in porphyrin concentration was observed (Rio et al., 1997). The Committee noted that the number of individuals on which the mean values were based was not reported.
In cells harvested from human umbilical cord, inhibition of CFU-GM colony growth was observed after 7 days of culture with T-2 toxin at a concentration of 0.1 nmol/L (equivalent to 0.047 ng/ml); however, no inhibition was observed after 10 and 14 days. At 2 nmol/L, T-2 toxin inhibited colony growth at 7, 10, and 14 days. With HT-2 toxin, a concentration of 1 nmol/L (equivalent to 0.42 ng/ml) inhibited growth at 7 days of culture, with no inhibition at day 10 or 14. At 10 nmol/L (equivalent to 4.2 ng/ml), HT-2 toxin inhibited growth at 7, 10, and 14 days (Parent-Massin et al., 1994).
Similar results were obtained for growth of rat and human CFU-GM colonies. GM progenitors were collected from the marrow of rats and from the umbilical cord of humans and were cultured and incubated with T-2 toxin and HT-2 toxin for 14 days. The median inhibitory concentrations (IC50) for rat CFU-GM at 14 days were 2.6 nmol/L for T-2 toxin (equivalent to 1.2 ng/ml) and 2.2 nmol/L for HT-2 toxin (equivalent to 0.93 ng/ml), and the values for human CFU-GM were 1.4 nmol/L (equivalent to 0.65 ng/ml) for T-2 toxin and 1.8 nmol/L (equivalent to 0.76 ng/ml) for HT-2 toxin (Lautraite et al., 1995, 1996).
When platelets isolated from 12 healthy volunteers were incubated with T-2 toxin at doses of 5–500 µg/109 platelets for 20 min, a concentration-related inhibition of platelet aggregation was seen with various activators, including adrenaline, arachidonic acid, and collagen, with release of dense bodies consisting mainly of serotonin-containing granules. There was also a change in membrane permeability but no change in shape. No parallel inhibition of thromboxane synthesis or significant alterations in platelet calcium content were observed. The microtubular system was unaffected (Yarom et al., 1984a).
(c) Effect on vascular parameters
Intravenous administration of T-2 toxin to pigs at a dose of 4 or 8 mg/kg bw resulted in a shock syndrome characterized by reductions in cardiac output and blood pressure and increased plasma concentrations of adrenaline, noradrenaline, thromboxane B2, 6-keto-prostaglandin F1a, and lactate. The pigs given the higher dose showed signs including persistent vomiting, watery diarrhoea, abdominal straining, cold extremities, coma, and death (Lorenzana et al., 1985).
In the bovine ear perfusion system in vitro, T-2 toxin caused dose-dependent vasoconstriction of the peripheral vasculature but was a less potent vasoconstrictor than histamine or noradrenaline. The presence of known histamine or noradrenergic antagonists did not affect the response to T-2 toxin (Wilson & Gentry, 1985). T-2 toxin administered systemically markedly increased peripheral vascular resistance in conscious rats. The cardiac output gradually decreased, eventually resulting in cardiovascular collapse and death (Feuerstein et al., 1985).
Lethal intravenous doses of T-2 toxin to male Sprague-Dawley rats reduced blood flow and increased vascular resistance in hindquarter, mesenteric, and renal vascular beds. The mean arterial pressure and heart rate were not significantly altered after an intravenous dose of 1 mg/kg bw. Two of five rats died within 5 h, and only one animal survived to 24 h after treatment. The maximum fall in blood flow in mesenteric and renal vascular beds occurred 4 h after injection of T-2 toxin (Siren & Feuerstein, 1986).
Mice
(i) Effects of T-2 toxin on spleen and thymus cellularity and lymphoproliferative response
A single dose of T-2 toxin at 10 mg/kg bw given to female ICR:CD1 mice by oral gavage induced apoptosis in thymus and spleen, as measured by TUNEL. The spleen and thymus weights were decreased, as were the lymphocyte and platelet counts in blood. Hypocellularity was observed in bone marrow and spleen, with specific depletion of myelocytes in bone marrow due to loss of immature granulocytes, erythroblasts, and lymphocytes (Shinozuka et al., 1997a,b, 1998, 1999). TUNEL, indicating apoptosis, was also seen after treatment of female ICR:CD1mice with T-2 toxin at 2.5 mg/kg bw by oral gavage (Shinozuka et al., 1997a,b) or intraperitoneal injection of female BALB/c mice at 2.5 or 5 mg/kg bw (Ihara et al., 1997; Sugamata et al., 1998).
Thymic atrophy and apoptosis were observed in groups of female BALB/c mice after a single intraperitoneal dose of 1.8 or 3.5 mg/kg bw. A dose of 0.35 mg/kg bw per day did not affect thymus weight or cellularity. The percentage of DNA fragmentation, as a measure of apoptosis, was increased with T-2 toxin treatment at 1.8 mg/kg bw per day but was reduced in animals that received a protein synthesis inhibitor (cycloheximide) 5 min after T-2 toxin, suggesting that protein synthesis may be necessary for the toxicity of T-2 toxin to thymus cells (Islam et al., 1998a).
Depletion of fetal liver B lymphocytes was observed in the offspring of female B6C3F1 mice exposed on days 14–17 of gestation to T-2 toxin by oral gavage at a dose of 1.2 or 1.5 mg/kg bw per day for 4 days. Fetal livers were collected on day 18 of gestation for cell flow cytometric analysis. A specific subpopulation of CD44+ lymphocytes (CD44lo) was reduced in fetal livers of dams at 1.5 mg/kg bw per day. The number of CD45R+ lymphocytes was decreased at both 1.2 and 1.5 mg/kg bw per day. Subsequent analysis of a prolymphocyte-enriched culture of fetal liver cells exposed to T-2 toxin in vitro suggested selective elimination of a subpopulation of B-lineage lymphocytes, CD45R+. Similar reductions were seen in CD44lo and CD45R+ bone-marrow cells in adult mice exposed to T-2 toxin by gavage at 1.8 mg/kg bw per day, suggesting that B-cell precursors represent a sensitive target for T-2 toxin (Holladay et al., 1993).
The proliferative response of lymphocytes to phytohaemagglutinin and lipopoly-saccharide was examined in male French IC mice treated with T-2 toxin (purity unspecified) at doses representing one-half or one-quarter of the LD50 for 2 days or one-twelfth of the LD50 for 15 days. No other information on doses was reported. Stimulation of both T and B cells was inhibited reversibly, and the ability to synthesize antibodies to sheep red blood cells was suppressed. The effects on lymphocytes and fibrosarcoma cells in culture included a direct cytostatic action at high concentrations and stimulation at low concentrations. The histological observations included severe lymphoid damage in the thymus and spleen. The immune system therefore appears to be sensitive to the trichothecenes and is impaired at doses that do not inhibit other organs (Lafarge-Frayssinet et al., 1979).
The proliferative response of spleen lymphocytes of female BALB/c mice to concanavalin A, phytohaemagglutinin, pokeweed mitogen, and lipopolysaccharide was examined in vitro and in vivo. Spleen lymphocytes from five mice were incubated with T-2 toxin at a concentration of 0, 0.25, 0.5, 0.75, 1, or 2.5 nmol/L (equivalent to 0.12, 0.23, 0.35, 0.47, and 1.2 ng/ml) and the mitogens. The lymphoproliferative response in vitro depended on the concentration of T-2 toxin and the mitogen; the response was increased with T-2 toxin at 0.5 nmol/L and concanavalin A and decreased with T-2 toxin at 2.5 nmol/L alone or with concanavalin A; decreased with T-2 toxin at concentrations > 0.75 nmol/L T-2 toxin and phytohaemagglutinin, pokeweed mitogen, or lipopolysaccharide. For exposure in vivo, groups of three mice were given intraperitoneal injections of T-2 toxin at 0, 0.8, or 1.6 mg/kg bw per day on days 0, 7, and 14; the spleens were harvested on day 15, and lymphocytes cultured with mitogen. In T-2 toxin treated groups, more lymphoproliferation was seen at 0.08 mg/kg bw per day (a 1000% increase) and less at 1.6 mg/kg bw per day, when compared with controls. When lymphocytes were cultured with mitogen, the lymphoproliferative response was decreased with T-2 toxin at 1.6 mg/kg bw per day plus phytohaemagglutinin, pokeweed mitogen, or lipopolysaccharide. In a separate experiment in which groups of three mice were immunized with keyhole limpet haemocyanin and Bordetella pertusis antigen by subcutaneous injection on days 1 and 8 and treated as in the initial experiment, the lymphoproliferative response was increased with T-2 toxin at 1.6 mg/kg bw per day and concanavalin A or phytohaemagglutinin (Paucod et al., 1990).
Male Swiss mice were fed a diet containing T-2 toxin (purity, 99%) at 5, 10, or 20 mg/kg, equivalent to 0.75, 1.5, and 3.0 mg/kg bw per day, for up to 48 days. A control group fed the same amount of food as that consumed by the mice at 20 mg/kg of diet was used to determine the effects of dietary restriction caused by feed refusal in the treated groups; an additional control group was fed ad libitum. Eight mice from each control and treated group were killed and examined after 7, 14, 21, 28, and 48 days of treatment. After 7 days, the spleen cell count was significantly lower in the pair-fed control group than in the group fed T-2 toxin at 20 mg/kg of diet, and was lower in all T-2 toxin-treated groups than in controls fed ad libitum. At subsequent times, the mean count in pair-fed controls was no different from that of the group given T-2 toxin at the highest concentration, and both were lower than in controls fed ad libitum. The lymphoproliferative response of the spleen to concanavalin A and lipopolysaccharide at the same times was significantly lower at 20 mg/kg of diet and in pair-fed controls than in the group fed ad libitum. The mean proliferative response to both mitogens was lower in the group given T-2 toxin at 20 mg/kg of diet than in the pair-fed control group, but the difference was not significant (Friend et al., 1983b).
The T-2 toxin metabolites 3’-OH-T-2 , HT-2 toxin, 3’-OH-HT-2, neosolaniol, and T-2 tetraol were tested for their ability to induce apoptosis in the thymus, assessed by DNA fragmentation analysis after an intravenous dose of 1.6 mg/kg bw to female BALB/c mice. 3’-OH-T-2, T-2 toxin, HT-2 toxin, and 3’-OH-HT-2 caused a significant increase in DNA fragmentation. On this basis, 3’-OH-T-2 was as effective as T-2 toxin in causing thymic apoptosis, while HT-2 toxin and 3’-OH-HT-2 were less effective. Neosolaniol and T-2 tetraol did not cause thymic cell apoptosis (Islam et al., 1998b).
(ii) Antibody formation
Four male Swiss IC mice received T-2 toxin by intraperitoneal injection at a dose of 0.75 mg/kg bw per day for 7 days. On day 3 of treatment, the mice were immunized with sheep erythrocytes, and all animals were killed 5 days later. T-2 toxin decreased the antibody titres, as measured by haemagglutination stimulation, and reduced the thymic weight. In a second study, seven groups of five mice received T-2 toxin intraperitoneally at a dose of 0.5, 0.6, 0.75, 1, or 2 mg/kg bw per day for 7 days. The mice were immunized on day 3 and killed 5 days later. Antibody-producing cells from the spleen were counted as the number of plaque-forming cells on sheep erythrocytes. A dose-dependent inhibition of plaque-forming cells was observed in T-2 toxin-treated mice, with total suppression of the immune response at 2 mg/kg bw per day. A dose-dependent reduction in thymus weight and antibody titre to sheep red blood cells was seen at 0.5–1 mg/kg bw per day. The immune suppressive effect disappeared within 6 days after cessation of treatment.
In another experiment, 36 Swiss IC mice were treated intraperitoneally with T-2 toxin at 0.75 mg/kg bw per day for 7 days, and groups of four mice were killed at nine times over the subsequent 83 days. The mice were immunized with sheep red blood cells 5 days before being killed. The thymus weight and antibody titre to sheep red blood cells titre returned to the range seen in controls as early as 6 days after cessation of T-2 toxin treatment (Rosenstein et al., 1979).
(iii) Graft rejection
Inhibition of cellular immunity by trichothecenes included effects on grafting. The mean length of survival of skin grafted from C57Bl/6 mice onto Swiss mice was 8.7 days in control recipients and 12 days in recipients treated with T-2 toxin at 0.75 mg/kg bw per day for 7 days before skin grafting and then three times a week for 20 days, indicating that T-2 toxin suppressed immunity, resulting in allograft rejection. The areas of the graft in T-2 toxin-treated mice lacked the typical cellular infiltrates of a cell-mediated immune response of macrophages and lymphocytes (Rosenstein et al., 1979).
(iv) Delayed hypersensitivity
Delayed hypersensitivity is an immune response mediated by sensitized T lymphocytes. The effect of T-2 toxin on T lymphocyte responses was studied in female BDF1 mice sensitized by subcutaneous injection of sheep red blood cells followed by measurement of foot-pad swelling. No appreciable effect on delayed hypersensitivity was observed when mice received T-2 toxin at 3 mg/kg bw before or on the day of sensitization. However, when T-2 toxin was administered 2 or 3 days after sensitization, marked enhancement of the response was seen. Since the lifetime of the effective T-2 toxin dose in vivo was short and the optimal timing of injection corresponded to the time of appearance of suppresser cells, it was presumed that trichothecenes interfere with the proliferation of suppresser T cells that appear in mice tolerant to delayed hypersensitivity (Masuko et al., 1977; Otokawa et al., 1979).
(v) Resistance to infection
Mice
C3H/HeN mice resistant to Salmonella were used to examine interactions between T-2 toxin and Salmonella infection. Groups of 30 female mice were given T-2 toxin at 1 mg/kg bw by oral gavage every 2 days for 3 weeks, for 11 doses over 21 days. S. typhimurium was inoculated at a dose of 1 x 107, 2.5 x 106, or 5 x 106 CFU on day 2 of the 3-week exposure. Two control groups were used: one given the vehicle only and one given the vehicle by gavage plus S. typhimurium inoculation. The body-weight gains of the groups receiving T-2 toxin or the vehicle only were equivalent, but that of the group receiving T-2 toxin plus S. typhimurium was significantly lower than that of the other groups by day 5. Survival was monitored for up to 30 days (9 days after the end of T-2 toxin treatment). No deaths occurred with T-2 toxin alone, with the vehicle, or at the lowest dose of S. typhimurium alone; the mortality rate was significantly greater in all groups receiving T-2 toxin plus S. typhimurium than in that given S. typhimurium plus vehicle.
In the same study, the mortality rate of groups of 10 animals given T-2 toxin at a dose of 0.1, 0.25, 0.5, or 1 mg/kg bw per day plus S. typhimurium at 3 x 105 CFU was also examined over a 30-day period. Three deaths occurred in the group given vehicle plus S. typhimurium, and a statistically significant increase in the mortality rate (7/10) was observed with T-2 toxin at 0.5 mg/kg bw per day. At 0.1 and 0.25 mg/kg bw per day, the mortality rate was 3/10 and 6/10, respectively. In mice inoculated with 3 x 105 CFU S. typhimurium and given the vehicle or T-2 toxin at 1 mg/kg bw per day (11 treatments over 21 days), the spleen weight and accumulation of S. typhimurium in the spleen were affected by T-2 toxin treatment. Five or more mice given T-2 toxin or the vehicle were killed and their spleens examined on days 2, 5, 7, 12, 15, 18, and 23. The spleen weight was lower by day 12, and remained lower, in T-2 toxin-treated mice than in controls given S. typhimurium plus vehicle. The S. typhimurium count in the spleen was higher in T-2 toxin-treated mice on day 2, no different from that in vehicle controls on days 5–12, and again higher in T-2 toxin-treated mice on days 15–23. In mice treated with S. typhimurium at doses of 3 x 102 to 3 × 106 CFU per mouse and examined on day 10 after inoculation, those treated with T-2 toxin at 1 mg/kg bw per day on alternate days had significantly higher S. typhimurium counts in the spleen. However, the spleen weight in mice treated with T-2 toxin plus S. typhimurium was higher than that of mice treated with S. typhimurium at 3 × 102 CFU per mouse plus vehicle, and no different from that of mice treated with S. typhimurium at 3 × 103 to 3 × 106 CFU plus vehicle (Tai & Pestka, 1988).
Groups of 10 male BALB/c mice, 7 weeks of age, were given T-2 toxin in their drinking-water at a concentration of 0, 0.2, 1, or 6 mg/L for 4 weeks (equivalent to 0, 0.024, 0.12, and 0.72 mg/kg bw per day), and were given S. enteritidis (1 x 107 cells in 0.2 ml) by gastric intubation on day 14. The concentrations of T-2 toxin were insufficient to cause water or food refusal. The survival ratio of T-2 toxin treated to control animals after exposure to S. enteritidis was expressed as < 0.5, < 1.2, or > 2. The ratio was < 1.2 with T-2 toxin at concentrations of 1 and 6 mg/L (i.e. no effect) but was > 2 at 0.2 mg/L (improved survival). (Sugita-Konishi et al., 1998). The Committee noted that the data were presented as ranges of ratios and a statistical analysis was not reported.
Groups of 10–14 ddY male mice weighing 18–20 g were inoculated intravenously with mycobacteria into the tail vein. In a first study, the mice received 0.01 mg of tubercle bacteria (species not indicated) and 0.1 mg of T-2 toxin orally 12 times, starting the day before inoculation, seven times at 1-day intervals, and then five times daily. For comparison, a group of mice was given 5 mg of cortisone acetate intraperitoneally on a similar schedule. A third group was inoculated with tubercle bacteria only. At the end of the 20-day observation period, the mice in the first group had a lower spleen weight and a higher tubercle bacteria count in the spleen than those in the other two groups, indicating greater depression of resistance with T-2 toxin than with cortisone.
In a second study, two groups of animals were inoculated with 0.25 mg of a culture of Mycobacterium bovis. One group was given 0.1 mg of T-2 toxin daily for 6 days, starting 8 days after injection, and there were two groups of controls. The average length of survival of the T-2 toxin-treated group was 19 days, whereas that of the untreated group was 35 days, indicating decreased resistance (Kanai & Kondo, 1984).
Single or 7 days of administration of T-2 toxin by gavage to female Han:NMRI mice reduced the virulence of Staphylococcus hyicus and Mycobacterium avium in mastitis infections. In the single-dose experiment, mice received T-2 toxin at 2.6 mg/kg bw, followed 6 h later by surgical removal of the mammary gland tips and inoculation of the glands with either 5 × 109 CFU of S. hyicus or 6 × 107 CFU of M. avium. In the 7-day experiment, mice received T-2 toxin at 0.75 mg/kg bw per day by gavage, followed by infection with 5 × 109 CFU of S. hyicus. The severity of the mastitis resulting from the infection was scored 48 h after inoculation with S. hyicus and 17 days after inoculation with M. avium. The infection was generally less severe in animals treated with T-2 toxin than in controls; this difference was more readily apparent in the S. hyicus-treated mice. The mice were killed after assessment of the severity of mastitis and serum immunoglobulin (Ig)M, IgG, and IgA were determined. With both protocols of exposure to T-2 toxin, the serum IgA level was significantly increased in infected T-2 toxin-treated animals over that in controls infected with either S. hyicus or M. avium. The IgM and IgG levels were significantly increased only in S. hyicus-infected mice that had received 7 days’ treatment with T-2 toxin at 0.75 mg/kg bw per day (Atroshi et al., 1994).
The effects of T-2 toxin on cell-mediated resistance were studied in female ICR mice infected with Listeria monocytogenes. Groups of 17 animals (10 in the control group) were inoculated intraperitoneally with 4 × 105 (LD50) or 4 x 104 CFU of L. monocytogenes, given a single oral dose of T-2 toxin at 4 mg/kg bw, and observed for 15 days. The bacteria multiplied rapidly in the spleen after T-2 toxin treatment, and the mortality rate was increased in both treated groups. Necrosis and depletion of lymphoid tissue were observed in the thymus, the periarteriolar lymphoid sheaths, and the lymphoid follicles of the spleen. The cellular response to L. monocytogenes in the spleen and liver was decreased by treatment with T-2 toxin, and the lesions were sparsely populated with mononuclear cells. The foci of necrosis were larger, with numerous colonies of bacteria. The influx and number of lymphocytes and macrophages were greater in Listeria-elicited peritoneal exudates. The immunotoxic effects of T-2 toxin were comparable with those produced by cyclophosphamide and were attributed to depletion of T lymphocytes and subsequent failure of T cell-dependent macrophages to clear the host of bacteria (Corrier & Ziprin, 1986a).
In a continuation of this study, groups of 17 female ICR mice were inoculated intraperitoneally on day 1 with 4 × 105 (LD50) or 4 × 104 CFU of bacteria, treated orally on days 0, 1, 2, and 3 with T-2 toxin at 0, 1, or 2 mg/kg bw per day, and observed for 15 days. Suppression of resistance was indicated by rapid multiplication of L. monocytogenes in the spleen and an increased mortality rate of mice in both groups treated with 2 mg/kg bw per day. The thymuses and spleens of T-2 toxin-treated mice showed necrosis and depletion of lymphoid cells. Foci of necrosis induced by Listeria infection in the spleen and liver were larger in treated mice, and the inflammatory reaction was sparse (Corrier & Ziprin, 1986a,b).
Increased resistance to L. monocytogenes infection was, however, observed by the same group in mice given T-2 toxin several days before inoculation of bacteria. Groups of 16 female ICR mice were given T-2 toxin by stomach tube at a dose of 0, 0.5, 1, or 2 mg/kg bw on days –5, –4, –3, –2, –1, +1, and +3. On day 0, half of each treated group was inoculated intraperitoneally with 106 (LD100) and half with 105 (LD50) L. monocytogenes, respectively. An additional 20 mice were given T-2 toxin alone at 2 mg/kg bw per day on the same days. Although the cytotoxic effect of T-2 toxin on lymphoid tissue was marked, enhanced resistance to Listeria infection was seen by a decrease in the number of deaths due to listeriosis (in both bacteria-exposed groups) in a T-2 toxin dose-dependent manner. No specific cause for the increased resistance seen with T-2 toxin treatment prior to bacterial infection was identified (Corrier & Ziprin, 1986b).
Young male white Swiss mice received diets containing T-2 toxin (purity, 99%) at a concentration of 10 or 20 mg/kg, equivalent to 1.5 and 3.0 mg/kg bw per day, for 2–3 weeks. The mice were then inoculated intraperitoneally with Herpes simplex virus (HSV-1). Mice fed the high concentration of T-2 toxin were highly susceptible to HSV-1 infection, and about 75% died with extensive hepatic and adrenal necrosis and little or no inflammatory cellular reaction in the affected tissues or the central nervous system. No necrotizing encephalitis was found in treated mice. Mice fed the lower concentration of T-2 toxin had lesions of intermediate severity between those seen at the high dose and in the virus-infected controls (Friend et al., 1983c). Feeding of diets containing T-2 toxin at 5, 10, or 20 mg/kg, equivalent to 0.75, 1.5, and 3 mg/kg bw per day, for 3–6 weeks did not reactivate the virus in mice latently infected with HSV-1 (Friend et al., 1983b).
A study of the effect of T-2 toxin and aflatoxin B1 on activation of toxoplasmosis in mice suggested that T-2 toxin precipitates Toxoplasma gondii cyst rupture. A 3 x 3 study design was used to test aflatoxin B1, T-2 toxin, and T. gondii infection in 30 female CF-1 mice. Groups of five mice were infected with T. gondii and 1 month later were given T-2 toxin as an intragastric dose of 0.5 mg/kg bw per day for 50 days. When compared with a control group of five mice that received T. gondii alone, T-2 toxin-treated mice had more severe brain lesions and focal proliferation of glial cells and an increased degree of perivascular cuffing and necrotic and degenerate changes in neurons. The number and percentage of both ruptured and unruptured T. gondii cysts was greater in T-2 toxin-treated animals. Furthermore, the body-weight gain of the T-2 toxin-treated, T. gondii-infected mice was lower than that of untreated and T. gondii-infected control mice; no difference in body-weight gain was observed between T-2 toxin-treated and control mice (Venturini et al., 1996).
Rats
Groups of four male Sabra rats received T-2 toxin as a single intraperitoneal injection of 1 mg/kg bw or as 0.5 mg/kg bw per day for 5 days. Twenty-four hours after the last T-2 toxin injection, the rats were inoculated intramuscularly with 0.1 ml of a medium containing Staphylococcus aureus at 109/ml. Rats given multiple intraperitoneal injections of T-2 toxin showed more oedema and myofibril necrosis at the site of injection of the bacteria than vehicle-injected controls, and the cellular infiltrate in T-2 toxin-treated rats was sparse and bacteria were abundant.
In separate experiments, reduced leukocyte counts were observed 24 h after intraperitoneal injection of T-2 toxin at 0.5 mg/kg bw as a single dose or after up to five daily doses. The number of myeloid cells in the femur marrow was also markedly decreased by single or up to three daily intraperitoneal injections of T-2 toxin at 0.5 mg/kg bw (Yarom et al., 1984b).
Chickens
Groups of 200 broiler chicks were fed a diet containing T-2 toxin at 16 mg/kg, equivalent to 2 mg/kg bw per day, for 3 weeks or control diet. The T-2 toxin was extracted from F. tricinctum culture and purified by the method of Burmeister (1971) to yield a crystalline product melting at 148–150 °C. After 1 week, groups of 40 chicks in each group were inoculated orally with either Salmonella worthington, S. thompson, S. derby, or S. typhimurium. Two to seven chicks exposed to T-2 toxin and Salmonella died, whereas no deaths were observed after exposure to Salmonella or T-2 toxin alone. The weight gain of T-2 toxin-treated chicks was markedly reduced: for example, 420 g for controls and 260 g for treated chicks at 3 weeks. The weight gain was not affected by Salmonella. The T-2 toxin-treated chicks also had lower relative weights of the spleen and bursa of Fabricius. Salmonella increased the relative spleen weight of both T-2 toxin-treated and control birds but did not affect the relative weight of the bursa (Boonchuvit et al., 1975).
Pigs
Six weaned pigs received diets containing T-2 toxin at a concentration of 5 mg/kg, equivalent to 0.2 mg/kg bw per day, for 25 days, and their immune response was evaluated in vitro by testing for blast transformation, immune-rosette formation, and IgG-positive cell counts. T-2 toxin caused a 40–50% reduction in immune responsiveness and a decreased total leukocyte count but an increase in adrenocortical activity. The neutralizing antibody titres to enteritic B vaccine were lower in the treated pigs. It was concluded that T-2 toxin had a distinct immunosuppressive effect during the early phase of immune induction by altering the function of both T and B lymphocytes (Rafai & Tuboly, 1982).
Groups of 10 pigs (sex not specified), 7 weeks of age, were fed a diet containing T-2 toxin at a concentration of 0, 0.5, 1, 2, or 3 mg/kg for 3 weeks, equal on the basis of feed intake and weekly measurements of T-2 toxin in feed to 0.029, 0.062, 0.10, and 0.13 mg/kg bw per day. The T-2 toxin was extracted from a culture of F. tricinctum and determined to be > 90% pure by gas and liquid chromatography. On the first and fourth days of exposure to T-2 toxin, the pigs were immunized by an intramuscular injection of 5 mL of horse globulin. Blood was drawn before the first immunization and on days 7, 14, and 21 and used to determine the antibody titre, the lymphoproliferative response to mitogens in vitro, and the immune complex, cytotoxic reaction, and phagocytic activity of circulating granulocytes. Blood drawn on day 21 was also used to assess the erythrocyte count, erythrocyte volume fraction, mean cell volume, haemoglobin concentration, leukocyte count, and proportion of T lymphocytes. A T-2 toxin dose-related decrease in feed intake was observed, with average daily intakes over the 3-week period of 820, 710, 770, 660, and 480 g/day at 0, 0.5, 1, 2, and 3 mg/kg of diet, respectively. The titre of antibodies to horse globulin was significantly lower in T-2 toxin-treated than control pigs at all doses tested at 14 and 21 days. The leukocyte count and the proportion of T lymphocytes were lower in all treated groups, and a dose-related decrease in the size of the thymus lobules and spleen follicles was noted, although no statistical analysis of trend or differences from control was presented. A decreased proliferative response of lymphocytes to phytohaemagglutinin and concanavalin A was observed at all concentrations of T-2 toxin at 21 days but not at earlier times (Rafai et al., 1995b). The Committee noted that, as pair-fed animals were not used, the potential confounding effects of differences in feed intake and weight gain on the end-points cannot be evaluated.
Cows
Alterations in the levels of several serum proteins and immunoglobulins were reported in calves given T-2 toxin orally at 0.6 mg/kg per day for 43 days. The total protein, albumin, and immunoglobulin fractions, including the alpha-beta1- and beta2-globulin fractions and IgA and IgM and complement protein values, were decreased in T-2 toxin-treated calves (Mann et al., 1983).
Lymphocytes from calves given a diet containing T-2 toxin at 0.6 mg/kg for up to 43 days had a decreased proliferative response to phytohaemagglutinin on days 1, 8, and 29 after administration and decreased proliferative responses to concanavalin A and pokeweed mitogen on day 29 after dosing (Buening et al., 1982).
In five calves treated orally with T-2 toxin at 0.3 mg/kg bw per day for 56 days, neutrophil function and the cutaneous reaction to injected phytohaemagglutinin were reduced. In a second study, six calves were given T-2 toxin at a dose of 0.5 mg/kg bw per day for 28 days. The number of B lymphocytes and the response of the B-cell-enriched fraction to phytohaemagglutinin were increased by treatment. Exposure of mononuclear cells, B-cell-enriched or T-cell enriched fraction, to T-2 toxin in vitro at 1.4 ng/ml reduced the lymphoblastic response to mitogens by 50% (Mann et al., 1984).
Monkeys
Seven male rhesus monkeys (Macaca mulatta) were given T-2 toxin by stomach tube at a dose of 0.1 mg/kg bw per day for 4–5 weeks; no vehicle controls were used. Three animals died. Although the cause of death was not reported, acute fibrous pericarditis and pneumonitis were observed in the one animal that was autopsied. The surviving monkeys showed a reduction in leukocyte count, a reduction in the bactericidal activity of neutrophils (phagocytosis of E. coli), a reduction in the transformation of lymphocytes by mitogens, and a reduction in the numbers of C-cell and T-cell lymphocytes (Jagadeesan et al., 1982). The Committee noted that no statistical analysis of the results was reported and the results were reported only as mean plus or minus standard error.
Human lymphocytes in vitro
Apoptosis of human peripheral lymphocytes was observed after exposure in vitro to T-2 toxin at a concentration of 0.1, 1, 10, or 100 ng/mL of culture medium. Apoptosis was measured by flow cytometry with propidium iodide staining of peripheral lymphocytes after culture for various times with T-2 toxin. A concentration- and time-dependent apoptotic response was observed that was inhibited by chelating intracellular calcium with BAPTA-AM, a chelator activated by cytosolic esterases. No response was seen with T-2 toxin at 0.01 or 0.1 ng/mL after up to 5 days of incubation. Increased apoptotic cell counts were observed after 3 or 5 days of incubation with concentrations > 1 ng/mL. Apoptosis was observed in all lymphocyte types (Yoshino et al., 1997).
Addition of T-2 toxin at 1.6 ng/ml to culture medium inhibited the proliferative response of human peripheral lymphocytes to concanavalin A, and 2.4 ng/ml inhibited the responses to phytohaemagglutinin and pokeweed mitogen. Addition of T-2 toxin at 2 ng/ml to human peripheral lymphocytes at the same time as any of the three mitogens caused maximal inhibition of the proliferative response, but inhibition was still observed when T-2 toxin was added as long as 66 h after addition of mitogen. Conversely, T-2 toxin stimulated lymphocyte proliferation when added > 16 h after initiation of control cultures without addition of mitogen (Tomar et al., 1988).
Inter-individual differences and the effects of combined exposure to four trichothecenes on the mitogen-stimulated lymphocyte proliferation response were studied. The mean IC50 for inhibition of proliferation of lymphocytes from 10 women was 1.3 nmol/L for phytohaemagglutinin and 1.2 nmol/L for pokeweed mitogen (equivalent to 0.61 and 0.56 ng/ml). The mean IC50 for inhibition of proliferation of lymphocytes from five men was 1.4 nmol/L for phytohaemagglutinin and 0.9 nmol/L for pokeweed mitogen (equivalent to 0.65 and 0.42 ng/ml). The difference in the IC50 for phytohaemagglutinin and pokeweed mitogen between men was significant. No significant differences were reported between men and women for any measure. A three- to fourfold difference in the IC50 for inhibition of proliferation was observed in lymphocytes from six male and 16 female subjects. Combinations of T-2 toxin, diacetoxyscirpenol, deoxynivalenol, and nivalenol had either additive or marginally antagonistic interactions. The IC50 values for inhibition by T-2 toxin of IgA, IgG, and IgM production were slightly higher than those for inhibition of mitogen-stimulated proliferation. Increased immunoglobulin production was observed at the lower doses tested (a U-shaped dose–response curve). At 0.2 nmol/L (equivalent to 0.093 ng/ml), T-2 toxin caused an increase in IgA and IgM (but not IgG) production in vitro, while 2 nmol/L (equivalent to 0.93 ng/ml) decreased the IgM and IgA levels (Thuvander et al., 1999).
Human lymphocyte proliferation after mitogen stimulation was examined in cell cultures by flow cytometry and measurement of bromodeoxyuridine (BrdU) incorporation. T-2 toxin at 1–2 nmol/L (equivalent to 0.47–0.93 ng/ml) in the culture medium inhibited cell proliferation and altered the proportions of cells (increases and decreases at different times) expressing CD69, CD25, and CD71 markers in CD4+ and CD8+ cells. The authors concluded that T-2 toxin and deoxynivalenol inhibited the cell cycle in a similar way and that proliferation was affected early in the cell cycle, before CD25 expression (Johannisson et al., 1999)
The initial hydrolysis of T-2 toxin to HT-2 toxin and hydroxylation to 3’-OH T-2 slightly decreased inhibition of the lymphoproliferative response, as measured by tritiated thymidine uptake in human lymphocyte cultures; however, metabolism to 3’OH HT-2, T-2 triol, and T-2 tetraol affected the response more dramatically. The IC50 values for inhibition of mitogen-induced proliferation of cultured human lymphocytes by T-2 toxin, HT-2 toxin, 3’-OH T-2, 3’-OH HT-2, T-2 triol, and T-2 tetraol were 1.5, 3.5, 4.0, 50, 150, and 150 ng/ml, respectively (Forsell et al., 1985). The IC50 values for inhibition of tritiated thymidine uptake in mitogen-stimulated human lymphocytes were higher for the trichothecenes fusarenon-X, nivalenol, deoxy-nivalenol, and 15-acetyldeoxynivalenol, at 18, 72, 140, and 240 ng/ml, respectively. These results suggest that the lymphotoxicity of trichothecenes is related to the C-4 substituent (Forsell & Pestka, 1985).
Four-week-old male broiler chickens were intubated with a single dose of T-2 toxin at 2.5 mg/kg bw, and the brain concentrations of dopamine, noradrenaline, serotonin, and selected blood components were determined 4–48 h after administration. A significant increase in the dopamine concentration and a reduction in the noradrenaline concentration in the brain were found. The brain serotonin content did not change (Chi et al., 1981).
Weanling male Wistar rats were given T-2 toxin orally at 2 mg/kg bw, and the concentrations of neurotransmitters were determined. T-2 toxin increased the concentrations of tryptophan, serotonin, and dopamine in the brain but decreased that of 3,4-dihydroxyphenylacetic acid (MacDonald et al., 1988).
In male Sprague-Dawley rats given T-2 toxin orally at 22 mg/kg bw, the concentrations of serotonin and 5-hydroxy-3-indoleacetic acid were significantly increased in all regions of the brain examined, whereas those of noradrenaline and dopamine were unaltered (Fitzpatrick et al., 1988). In male Sprague-Dawley rats that received T-2 toxin at 1 mg/kg bw by intravenous injection, the concentrations of vasopressin, oxytocin, and leucine encephalin decreased in the posterior pituitary, and the concentrations of methionine enkephalin increased (Zamir et al., 1985).
Changes in monoamine neurotransmitter levels were also observed in groups of 10 male Sprague-Dawley rats that received feed containing T-2 toxin (purity unspecified) at a concentration of 2.5 or 10 mg/kg, equal to 0.26 and 0.63 mg/kg bw per day for 7 days and 0.24 and 0.71 mg/kg bw per day for 14 days on the basis of reported feed consumption and body weights. Feed consumption, feed use efficiency, and weight gain were decreased at both doses. When specific brain areas were assessed in a slice–micropunch procedure, decreased noradrenaline in the substantia nigra and decreased 3,4-dihydroxyphenylacetic acid in the paraventricular nucleus and medial forebrain bundle were observed. Increased concentrations of serotonin, 5-hydroxy-3-indoleacetic acid, dopamine, and noradrenaline were found in the nucleus raphe magnus and increased adrenaline in the substantia nigra (Wang et al., 1993a).
Neurotransmitters were also assessed by a slice–micropunch procedure in discrete brain regions of groups of 10 male Sprague-Dawley rats 2–10 h after administration of a single dose of T-2 toxin at 0.1, 1, or 2.5 mg/kg bw by gavage. The serotonin concentration was increased in the nucleus raphe magnus, the medial forebrain bundle, and the paraventricular nucleus, that of 3,4-dihydroxyphenylacetic acid in the medial forebrain bundle and paraventricular nucleus, and that of noradrenaline in the locus coeruleus at all doses. The concentration of noradrenaline was decreased in the substantia nigra at 0.1 mg/kg bw (Wang et al., 1998a).
The permeability of the blood–brain barrier was assessed in 10 male Sprague-Dawley rats by measuring the intracerebral recovery of labelled, systemically administered mannitol or dextran 2 h after intraperitoneal injection of T-2 toxin (purity unspecified) at 0.2 or 1 mg/kg bw or after 7 days on a diet containing T-2 toxin at a concentration of 10 mg/kg, equal to 0.93 mg/kg bw per day on the basis of reported feed consumption and weight gain. Monoamine oxidase activity and protein synthesis (labelled leucine incorporation) were also measured, except in the group given the low intraperitoneal dose. A dose-related increase in mannitol uptake was seen after intraperitoneal injections of T-2 toxin, but a statistically significant difference from control was seen only at the highest dose; mannitol uptake was also increased in some areas of the brain by administration of 10 mg/kg of diet. Dextran uptake was not affected. Protein synthesis in brain tissue was increased by the intraperitoneal dose of 1 mg/kg bw but not by intake of T-2 toxin in the diet. Monoamine oxidase activity was not affected by T-2 toxin given intraperitoneally but was decreased by administration of T-2 toxin in the feed at a concentration of 2.5 or 10 mg/kg, equal to 0.32 and 0.88 mg/kg bw per day on the basis of the reported feed consumption and weight gain. The Committee noted that the reduction in monoamine oxidase activity was the same at both concentrations. Effects on feed consumption and weight gain were seen with the 7-day dietary exposure. The authors noted that addition of a pair-fed control group would have allowed control for the possible effects of reduced feed intake on brain protein synthesis and enzyme activity in the rats treated in the diet (Wang et al., 1998b).
The effects of T-2 toxin were assessed in male Wistar rats given a single dose of 0.4 or 2 mg/kg bw by oral gavage on motor performance, nociceptor measures, open field behaviour, passive avoidance 4 and 8 h after dosing, sequential performance on an elevated plus-maze, rotarod, horizontal bridges, and passive avoidance or reaction to a hot plate 4–8.5 h after dosing. No effects on behaviour were observed at 0.4 mg/kg bw. At 2 mg/kg bw, body-weight gain was decreased 7 days after dosing, recumbancy was more prevalent, and sniffing was decreased. Passive avoidance was impaired, suggesting an effect on learning; and step-through latency was shortened. The latency of the response to a hot plate was not affected (Sirkka et al., 1992).
Effects of crude extracts of fungal cultures or solutions containing T-2 toxin on the skin have been reported (Bamburg & Strong, 1971; Saito & Ohtsubo, 1974). All of the incidents were accidental and involved few persons, who developed severe irritation, loss of sensitivity, and desquamation. Despite the presence of T-2 toxin in the contact material, the involvement of other compounds could not be ruled out.
Alimentary toxic aleukia was reported in the former USSR during the period 1931–47, which was attributed to the presence of toxic Fusarium species in mouldy over-wintered grain. An association was established with the ingestion of grain invaded by some moulds, in particular F. poae and F. sporotrichioides. The main pathological changes were necrotic lesions of the oral cavity, oesophagus, and stomach and, in particular, pronounced leukopenia. The primary lesions were bone-marrow hypoplasia and aplasia. The disease was lethal in a high proportion of cases (Sarkisov et al., 1944; Bilai, 1977; Leonov, 1977; Joffe, 1986; Beardall & Miller, 1994).
The clinical symptoms reported in alimentary toxic aleukia and the identification of Fusarium in foods suggested that it was associated with mycotoxins, identified years later in fungal cultures of Fusarium species under laboratory conditions, including T-2 toxin (Mirocha & Pathre, 1973) and wortmannin (Mirocha & Abbas, 1989).
Scabby grain toxicosis, a disease of both humans and farm animals, was reported from Japan and Korea during 1946–63. The commonest clinical symptoms were nausea, vomiting, diarrhoea, and abdominal pain. All the cases were acute, with recovery within a few days; none was lethal. Fusarium fungi, F. graminearum in particular, were isolated from suspected cereals (Tochinai, 1933; Hirayama & Yamamoto, 1948, 1950; Nakamura et al., 1951; Tsunoda et al., 1957; Cho, 1964; Ogasawara, 1965; Chung, 1975).
Three investigations of outbreaks of trichothecene-related disease involving either T-2 toxin or deoxynivalenol have been reported, two in China involving maize, wheat, or rice (Luo, 1988; Wang et al., 1993b) and one in India involving wheat (Bhat et al., 1989). Each involved 100 or more cases.
During the first incident, outbreaks of poisoning with mouldy maize and scabby wheat were reported. Among approximately 600 persons who ate mouldy cereals, there were 463 cases of poisoning (77%). The latency for the onset of symptoms was 5–30 min. These included nausea, vomiting, abdominal pain, diarrhoea, dizziness, and headache. No deaths occurred. Pigs and chicks fed the same mouldy cereals were also affected. Analysis of five samples of mouldy maize by GC–MS and radioimmunoassay revealed the presence of deoxynivalenol at 0.34–93 mg/kg and zearalenone at 0.004–0.59 mg/kg; neither T-2 toxin nor nivalenol was found. Analysis by TLC of 19 samples of scabby wheat collected from affected and unaffected families showed a deoxynivalenol content of 1–40 mg/kg, which was significantly higher than that in the non-scabby wheat samples. Zearalenone was detected in two samples at 0.25 and 0.5 mg/kg. No T-2 toxin was found (Luo, 1988).
A similar outbreak was reported in Kashmir, India, in 1987, which was ascribed to the consumption of bread made from flour that had become mouldy during storage following unseasonal rains in the wheat-harvesting season. Fusarium spp. were grown from the wheat and found to contain mycotoxins. Of the 224 persons investigated randomly, 97 had symptoms including abdominal pain (100%), throat irritation (63%), diarrhoea (39%), blood in stools (5%), and vomiting (7%). Symptoms developed 15 min to 1 h after consumption of locally baked bread. The following concentrations of mycotoxins were found in 12 of 24 samples of refined wheat flour used in the preparation of bread: deoxynivalenol at 0.35–8.4 mg/kg), acetyl-deoxynivalenol at 0.64–2.5 mg/kg (no details of the analysis of this derivative were provided), nivalenol at 0.03–0.1 mg/kg, and T-2 toxin at 0.55–0.8 mg/kg. Deoxynivalenol, its acetyl derivative, and nivealenol were measured quantitatively by HPLC and T-2 toxin by TLC, but no rigorous confirmation of identity was undertaken (Bhat et al., 1987).
The most recent report of a trichothecene-related outbreak involved 97 persons with symptoms out of an estimated 165 persons who had eaten polished rice from which F. heterosporum and F. graminearum were isolated. Of 29 persons examined, 28 reported nausea with lesser incidences of dizziness, vomiting, chills, abdominal pain and distention, thoracic constriction, and diarrhoea. The latent period for symptoms was 10–30 min after ingestion of the rice. Fungal culture of suspected rice demonstrated the presence of F. heterosporum and F. graminearum, and ELISA was used to show the presence of T-2 toxin. No analysis was conducted to determine whether other mycotoxins were present. T-2 toxin was present in three samples of rice at 0.18–0.42 mg/kg (Wang et al., 1993b).
T-2 toxin forms white needles, with a melting-point of 151–152 °C, and its specific rotation has been determined as [alpha]26D = +15 (c = 2.58, ethanol). All trichothecenes are stable under the usual conditions of storage for long times, and the epoxide group at the C-12,13 position is extremely stable to nucleophilic attack (Shepherd & Gilbert, 1988). Biochemically, all trichothecenes are derived from isoprenoid pathways, as are many secondary metabolites. Trichodiene is an intermediate compound derived from farnesol.
Immunoassay and TLC are the only screening tests for T-2 toxin- and HT-2 toxin that are applicable for routine analysis of cereals. Extraction is usually performed with acetonitrile and water, methanol, or chloroform and ethanol. Immunoassays for the determination of T-2 toxin and HT-2 toxin are based mainly on monoclonal antibodies.
Tricothecenes Appendix 3 gives the performance characteristics of the screening tests that have been developed for the detection of T-2 toxin and HT-2 toxin. The detection limits of the assays for T-2 toxin range from 0.2 to 50 ng/g. TLC methods allow detection of T-2 toxin down to 100 ng/g.
Various combinations of solvents, usually acetonitrile and water and methanol and water, have been used to extract type-A trichothecenes from grain, food, and feeds. Extraction is performed mainly by high-speed blending or mechanical shaking. Subsequent clean-up is done on prepacked silica gel, Florisil, or cyano and C18 solid-phase extraction cartridges. Multifunctional MycoSep™ columns, which contain activated charcoal, alumina, and Celite are also being used for the determination of type-A trichothecenes.
Generally, type-A trichothecenes are less polar than type-B toxins, as they have fewer free hydroxyl groups and lack a keto group at C-8 position. Therefore, different methods are used to extract these two types. For example, HPLC with detection of ultra-violet absorbance is not applicable to type-A trichothecenes owing to the lack of a conjugated keto group at the C-8 position. HPLC methods for T-2 toxin and HT-2 toxin toxins are being developed in which a variety of derivatization reagents are used to allow detection by fluorescence (Lawrence & Scott, 1993).
Although TLC is also used for quantitative determination of T-2 toxin and HT-2 toxin, GC analysis is the method of choice for the determination of type-A tricho-thecenes. Most GC methods are based on derivatization of hydroxyl groups in order to increase volatility and sensitivity. Both trimethylsilylation and fluoroacylation are currently used for derivatization. MS detection and use of ECD or flame ionization detection, after derivatization with a fluoroacylation reagent are recommended for reliable detection of type-A trichothecenes. The formation of fluoroacyl derivatives by trifluoroacetic anhydride, pentafluoropropionyl, or heptafluorobutyryl derivatives is also commonly used in order to increase the sensitivity of type-A trichothecenes to ECD (Langseth & Rundberget, 1998). LC with atmospheric pressure chemical ionization and MS has also been used for the determination of T-2 and HT-2 toxins (Berger et al., 1999).
Tricothecenes Appendix 4 lists the performance characteristics of the quantitative methods that have been developed for the determination of T-2 toxin and HT-2 toxin. Figure 2 shows a typical chromatogram obtained after separation and detection by GC with ECD of a mixture of HT-2 and T-2 in a standard solution of 400 ng/ml overlaid with a wheat sample spiked with HT-2 and T-2 at 500 ng/ml. The trace shows the advantage of this method for sensitive, simultaneous quantification of both trichothecenes. The typical LODs of quantitative methods for the determination of T-2 toxin and HT-2 toxin in cereals are 3 ng/g for T-2 toxin and 1 ng/g for HT-2 toxin by LC with MS; 10 ng/g for T-2 toxin and HT-2 toxin by GC with MS; and 10 ng/g for T-2 toxin and HT-2 toxin with GC and ECD. The typical recovery is 70–120%.
There are no published sampling plans for the determination of T-2 toxin and HT-2 toxin in foods, and no details of the sampling variability of these toxins have been reported. The generation of meaningful data requires the collection of representative samples from carefully selected batches of food which, in turn, are representative of clearly defined locations (e.g. country, region within a country).
In wheat contaminated at low concentrations (up to 1 mg/kg), the mycotoxins are typically found near the surface of the kernel, whereas at high levels of contamination the mycotoxins may be more evenly distributed (Charmley & Prelusky, 1994). Many infected kernels can be removed in gravity separators, which separate particles on the basis of differences in specific gravity, size, shape, and surface texture.
Natural degradation of T-2 toxin and HT-2 toxin has been observed in cereal grain both in the field and during storage. It is difficult to understand how the concentrations of mycotoxin are decreased in a natural system, although several explanations have been put forward. The concentration of free mycotoxin in a natural ecosystem may be the result of concurrent synthesis, transport, conjugation, release from bound forms, and degradation by the plant or by other microbes (Karlovski, 1999).
The effectiveness of milling practices in reducing trichothecene concentrations in the flour fractions from that of the whole grain were reviewed by Patey & Gilbert (1989), Scott (1991), and Charmley & Prelusky (1994). Most of the studies concern deoxynivalenol, and few are available on T-2 toxin and HT-2 toxin. In a laboratory-scale experiment, wet milling of maize containing T-2 toxin resulted in the loss of two-thirds in the steep or process water and a high concentration in the germ (Patey & Gilbert, 1989).
The trichothecenes are stable at 120 °C, moderately stable at 180 °C, and decompose within 30–40 min at 210 °C (Kamimura, 1989). T-2 toxin and HT-2 toxin were reported to be relatively stable during baking processes (Patey & Gilbert, 1989).
During cooking of noodles and spaghetti, considerable amounts of trichothecenes may leach into the boiling water (Scott, 1991).
T-2 toxin was deacetylated to HT-2 toxin by rumen microbes (Kiessling et al., 1984). In another study, rumen microorganisms transformed T-2 toxin to HT-2 toxin, T-2 triol, de-epoxy HT-2, and de-epoxy T-2 triol (Swanson et al., 1987).
Bacterial communities isolated from soil and water samples readily detoxified T-2 toxin (Beeton & Bull, 1989). The main degradation pathway in most isolates involved side-chain cleavage of acetyl moieties to produce HT-2 toxin and T-2 triol. In all cases, the complete communities were more active against T-2 toxin in terms of rates of degradation than any single bacterial component. Figure 3 shows the microbial transformation of trichothecenes to their de-epoxylated forms.
Data on contamination of grains and food products with T-2 toxin and HT-2 toxin were submitted to the Committee by Brazil, China, Finland, Germany, Norway, Sweden, and the United Kingdom. Other data on contamination of food with these toxins were taken from literature published between 1990 and early 2000. Previous data were reviewed in Environmental Health Criteria 105 (WHO, 1990). As the information available was not complete in many cases, many authors were contacted and asked to send details of their sampling and analytical methods and additional data; for example, the 90th percentile was generally not included in the papers.
Surveys for T-2 toxin and HT-2 toxin revealed their presence in grains such as wheat, maize, oats, barley, rice, beans, and soya beans as well as in some cereal-based products. There have been occasional reports of the presence of T-2 toxin and HT-2 toxin in human foods. Bread, breakfast cereals, and other cereal foods were found to be contaminated with T-2 toxin, and infant foods, bread, noodles, and cereal foods were found to contain HT-2 toxin (Patel et al., 1996; Schollenberger et al., 1999, 2000a,b).
The results of surveys for T-2 toxin and HT-2 toxin are presented in Appendices A and B, respectively. The references in these tables include the primary reference (P), the reference for the sampling method (S), and the reference for the analytical method (A) given by the authors. The analytical methods are described in Tricothecenes Appendix 5, and the sampling methods are described in Trichothecenes Appendix 6. The data were included on a case-by-case basis: when there was no information about sampling or analytical method, the data were not included. Mean values were recalculated when they were given as the mean for positive samples or when ‘undetected’ was considered to be half the LOD. The mean values shown are therefore the means of all samples, with those below the LOD taken as zero.
Although soya bean samples were contaminated at concentrations ranging from not detectable to 1100 µg/g (Jacobsen et al., 1995), these data were not included in the Appendices because they represented soya beans that had been refused or heavily discounted by local grain merchants, and the concentration of toxins was analysed after hydrolysis and reported as T-2 toxin equivalents.
WHO (1990) reported that T-2 toxin was present at concentrations > 100 µg/kg in only a few of 999 samples. This value was therefore chosen to represent contamination with T-2 toxin and HT-2 toxin for the decade 1990–2000. In a total of 8918 samples, T-2 toxin was found in 64% and HT-2 toxin in 36%. Concentrations > 100 µg/kg were found for T-2 toxin in only 37 and HT-2 toxin in 77 samples. Occasionally, high levels of both toxins were found; for T-2 toxin, for example, 820 µg/kg in wheat in Asia (Chen et al., 1995), 1700 µg/kg in oats in Europe (Müller et al., 1998), and 2400 µg/kg in maize in America (Saubois et al., 1992); and for HT-2 toxin, 2000 µg/kg in oats in Europe (Müller et al., 1998). When the LOD or LOQ was > 100 µg/kg, therefore, the chance of detecting the toxins was quite low. These data were included in the Appendix in order to illustrate this point. As the LOQ diminishes, there is an incremental increase in the frequency of contamination and an incremental increase in the average values. For example, when the LOQ is between 1 and 50 µg/kg, the mean concentration in oats is up to 200 µg/kg for HT-2 toxin (Langseth, 2000) and 76 µg/kg for T-2 toxin (Müller et al., 1998). In other studies, the LOQ was > 100 µg/kg, and the toxins were not detected or the average concentration was low. For example, a mean concentration of 13 µg/kg was reported in heavily contaminated wheat in 1986 (LOQ = 500 µg/kg; Quiroga et al., 1995).
The analytical method used most commonly for these trichothecenes was GC–MS or GC–ECD followed by ELISA, with an LOD of 1–1000 µg/kg. Different authors defined the LOD and LOQ in different ways. The LOD was sometimes expressed as two or three times the background and sometimes as the smallest quantity of the standard that could be detected; the LOQ was sometimes defined as five to six times the ‘noise’ in standard solutions, without taking into account recovery in the matrix under study. Some investigators included recovery values in their estimate of the LOQ. It would be advisable if investigators reported the method by which the LOD and LOQ were calculated, in order to standardize this procedure.
Intake is best estimated by considering results in which the recovery at the LOQ is > 60%. Analytical procedures must be improved to allow better quantification at lower cost and to improve the recovery of both mycotoxins in most matrices.
During 1990–2000 (WHO, 1990), 7% of 999 samples were reported to be contami-nated with T-2 toxin, but variable methods were used to derive the data. The vast majority of studies did not provide evidence that the methods used had been tested rigorously and that the confirmation of identity was adequate. Few of the reported data have been corroborated. In the analyses in Europe, 52% of samples containing T-2 toxin and 88% of those containing HT-2 toxin were detected at LOQ or LOD values 100 µg/kg, and the frequency of contamination was 11% and 14%, respectively. HT-2 toxin appears to be a more frequent contaminant, but the relationship varies among studies. Although there are inadequate data for tropical and subtropical zones, where, for example, maize, beans, and rice are grown. the few samples from those regions that have been analysed show contamination.
Confirmed information on the distribution of these toxins has been reported in the form of a frequency distribution table for all type A trichothecenes (Park et al., 1996). As dried extracts were hydrolysed before analysis, the individual distribution cannot be ascertained.
Few of the data submitted to the Committee could be used to make a distribution analysis, and the high percentage of ‘undetected’ entries obviated estimation of a distribution curve. Although the number of positive samples was high in some sets of data, the figures were rounded in such a way that any of the distribution functions recommended in the literature for other mycotoxins provided a suitable adjustment.
The literature contains few data for an analysis of annual variation in contamina-tion with T-2 toxin and HT-2 toxin, because studies with a LOQ < 10 µg/kg are required for this purpose. Nevertheless, annual variations can be seen in Appendices A and B. For example, a survey in Sweden showed annual variations in the average contamination with T-2 toxin and HT-2 toxin in oats (Pettersson, 2000).
Figure 5 shows the annual variation in T-2 toxin contamination in Germany in three substrates: wheat, oats, and barley. All samples were analysed by the same persons by a method with a LOQ of < 5 µg/kg. Although the samples were of feed, the results clearly illustrate not only the annual variations that can occur in contamination but also differences with respect to substrates. Oats appeared to be more sensitive to contamination with T-2 toxin than wheat or barley, which is consistent with results (Appendix A) from Finland (Hietaniemi & Kumpulainen, 1991), Norway (Langseth, 2000), and Sweden (Thuvander et al., 2000).
The influence of climatic conditions on the amount and frequency of T-2 toxin and HT-2 toxin contamination is difficult to understand (Müller et al., 1998). A larger number of samples would be necessary to determine the effect of such conditions on the behaviour of Fusarium species and accumulation of the toxins.
The dietary intake of T-2 and HT-2 toxins was assessed according to the recommendations of a FAO/WHO workshop on methods for assessing exposure to contaminants and toxins, which was held in Geneva in June 2000 (WHO, 2000). The workshop recommended that the median concentration be given when data on individual samples were available, whereas a mean should be given when only pooled or aggregated data were available. In the case of commodities that contribute significantly to intake, distribution curves should be generated to allow risk managers to determine the effects on dietary intake of different maximum levels.
The workshop further recommended that international estimates of dietary intake should be calculated by multiplying the mean or median concentration by the values for consumption of the commodity in the five GEMS/Food regional diets (WHO, 1998). The diets (African, European (which includes Australia, Canada, New Zealand, and the USA), Far Eastern, Latin American, and Middle Eastern) were established on the basis of information on food balance sheets compiled by FAO. Since such information is available for most countries, the data are comparable across countries and regions of the world. The regional diets represent the average availability of food commodities per capita rather than actual food consumption.
The report of the workshop noted that national intake estimates should also be reported when available, as they may provide information about intake by specific population subgroups or heavy consumers, which cannot be derived from GEMS/Food regional diets.
For this assessment, concentrations of T-2 and HT-2 toxins in food commodities and in some processed foods were submitted to the Committee or were obtained from the literature. The quality and reporting of the data are discussed in the previous section. As the dietary intakes are based on the GEMS/Food regional diets, which include information on consumption of raw or minimally processed foods, concentra-tions of T-2 and HT-2 toxins in processed foods were not used to estimate dietary intake.
Information was available on the concentrations of six commodities: barley, maize, oats, rice, rye, and wheat, and other cereals. Data on T-2 toxin were received from 16 countries, representing four of the five GEMS/Food regional diets, while data on HT-2 toxin were received from 11 countries (Table 5); no data were reported for the Middle Eastern diet.
Table 5. Countries for which data on concentrations of T-2 and HT-2 toxins were available, by geographical regional diet
|
Toxin |
Far Eastern |
African |
Latin American |
European |
|
T-2 |
China |
South Africa |
Argentina |
Austria |
|
|
India |
|
Brazil |
Bulgaria |
|
|
Republic of Korea |
|
Chile |
Canada |
|
|
|
|
Ecuador |
Finland |
|
|
|
|
|
Germany |
|
|
|
|
|
Norway |
|
|
|
|
|
Sweden |
|
|
|
|
|
United Kingdom |
|
HT-2 |
China |
|
Brazil |
Austria |
|
|
Republic of Korea |
|
Chile |
Canada |
|
|
|
|
|
Finland |
|
|
|
|
|
Germany |
|
|
|
|
|
Norway |
|
|
|
|
|
Sweden |
|
|
|
|
|
United Kingdom |
Most of the data available for this evaluation were pooled; that is, each data point represented the mean concentration in a number of individual samples. In calculating the mean values, samples in which the concentration was below the LOQ or LOD were assumed to have a value of zero. The maximum analytical value and the number of samples with concentrations below the LOD or LOQ were also reported for each data point. For T-2 and HT-2 toxins, a total of 175 data points (mean values) representing 8410 individual samples were included in the intake assessment (Table 6). Of those 175 data points, 147 were reported from countries represented by the GEMS/Food European diet. The remaining 28 data points represented intake of the six commodities in the other regional diets.
Table 6. Numbers of countries, data points, and samples from which information on concentrations of T-2 and HT-2 was available
|
Toxin |
Commodity |
No. of countries |
Data used in intake estimates |
|
|
No. of data points (means) |
No. of individual samples |
|||
|
T-2 |
Barley |
6 |
10 |
372 |
|
Maize |
9 |
16 |
1239 |
|
|
Oats |
4 |
21 |
758 |
|
|
Rice |
2 |
2 |
125 |
|
|
Rye |
3 |
4 |
83 |
|
|
Wheat |
10 |
38 |
2564 |
|
|
HT-2 |
Barley |
4 |
10 |
364 |
|
Maize |
5 |
9 |
292 |
|
|
Oats |
4 |
21 |
758 |
|
|
Rice |
1 |
1 |
26 |
|
|
Rye |
3 |
5 |
87 |
|
|
Wheat |
3 |
35 |
1740 |
|
For each commodity, the data were sorted according to the country groupings of the GEMS/Food regional diets. The number of data points reported, the number of individual samples represented, the highest maximum analytical value reported, the proportion of samples with concentrations below the LOD or LOQ, and the average of all mean values are summarized in Table 7.
Table 7. Summary of data on concentrations of T-2 and HT-2 toxins in grains in GEMS/Food regional diets
|
Commodity |
Far Eastern |
African |
Latin American |
European |
|
T-2 |
||||
|
Barley |
|
|
|
|
|
No. of data points |
1 |
|
|
9 |
|
No. of individual samples |
30 |
|
|
342 |
|
Unweighted mean (µg/kg) |
0 |
|
|
3.1 |
|
Maximum value (µg/kg) |
0 |
|
|
310 |
|
% < LOD or LOQ |
100 |
|
|
90 |
|
Weighted mean, all samples: 4.6 µg/kg |
|
|
|
|
|
Maize |
|
|
|
|
|
No. of data points |
3 |
4 |
5 |
4 |
|
No. of individual samples |
40 |
685 |
346 |
168 |
|
Unweighted mean (µg/kg) |
0 |
0 |
39 |
3 |
|
Maximum value (µg/kg) |
0 |
0 |
2400 |
260 |
|
% < LOD or LOQ |
100 |
100 |
92 |
98 |
|
Weighted mean, all samples: 3.2 µg/kg |
|
|
|
|
|
Oats |
|
|
|
|
|
No. of data points |
|
|
|
21 |
|
No. of individual samples |
|
|
|
758 |
|
Unweighted mean (µg/kg) |
|
|
|
18 |
|
Maximum value (µg/kg) |
|
|
|
530 |
|
% < LOD or LOQ |
|
|
|
71 |
|
Weighted mean, all samples: 21 µg/kg |
|
|
|
|
|
Rice |
|
|
|
|
|
No. of data points |
|
|
1 |
1 |
|
No. of individual samples |
|
|
99 |
26 |
|
Unweighted mean (µg/kg) |
|
|
27 |
0.7 |
|
Maximum value (µg/kg) |
|
|
NA |
19 |
|
% < LOD or LOQ |
|
|
94 |
96 |
|
Weighted mean, all samples: 0.7 µg/kg |
|
|
|
|
|
Rye |
|
|
|
|
|
No. of data points |
|
|
|
4 |
|
No. of individual samples |
|
|
|
83 |
|
Unweighted mean (µg/kg) |
|
|
|
0.1 |
|
Maximum value (µg/kg) |
|
|
|
17 |
|
% < LOD or LOQ |
|
|
|
99 |
|
Weighted mean, all samples: 0.2 µg/kg |
|
|
|
|
|
Wheat |
|
|
|
|
|
No. of data points |
2 |
|
3 |
33 |
|
No. of individual samples |
512 |
|
319 |
1 733 |
|
Unweighted mean (µg/kg) |
22 |
|
24 |
1.1 |
|
Maximum value (µg/kg) |
122 |
|
800 |
249 |
|
% < LOD or LOQ |
4 |
|
93 |
96 |
|
Weighted mean, all samples: 1.6 µg/kg |
|
|
|
|
|
HT-2 |
||||
|
Barley |
|
|
|
|
|
No. of data points |
1 |
|
|
9 |
|
No. of individual samples |
30 |
|
|
334 |
|
Unweighted mean (µg/kg) |
0 |
|
|
3.8 |
|
Maximum value (µg/kg) |
0 |
|
|
290 |
|
% < LOD or LOQ |
100 |
|
|
91 |
|
Weighted mean, all samples: 4.4 µg/kg |
|
|
|
|
|
Maize |
|
|
|
|
|
No. of data points |
3 |
|
1 |
5 |
|
No. of individual samples |
40 |
|
68 |
184 |
|
Unweighted mean (µg/kg) |
0 |
|
0 |
7 |
|
Maximum value (µg/kg) |
0 |
|
0 |
230 |
|
% < LOD or LOQ |
100 |
|
100 |
97 |
|
Weighted mean, all samples: 2.9 µg/kg |
|
|
|
|
|
Oats |
|
|
|
|
|
No. of data points |
|
|
|
21 |
|
No. of individual samples |
|
|
|
758 |
|
Unweighted mean (µg/kg) |
|
|
|
44 |
|
Maximum value (µg/kg) |
|
|
|
2 000 |
|
% < LOD or LOQ |
|
|
|
67 |
|
Weighted mean, all samples: 35 µg/kg |
|
|
|
|
|
Rice |
|
|
|
|
|
No. of data points |
|
|
|
1 |
|
No. of individual samples |
|
|
|
26 |
|
Unweighted mean (µg/kg) |
|
|
|
0 |
|
Maximum value (µg/kg) |
|
|
|
0 |
|
% < LOD or LOQ |
|
|
|
100 |
|
Weighted mean, all samples: 0 µg/kg |
|
|
|
|
|
Rye |
|
|
|
|
|
No. of data points |
|
|
|
5 |
|
No. of individual samples |
|
|
|
87 |
|
Unweighted mean (µg/kg) |
|
|
|
0.01 |
|
Maximum value (µg/kg) |
|
|
|
23 |
|
% < LOD or LOQ |
|
|
|
99 |
|
Weighted mean, all samples: 0.01 µg/kg |
|
|
|
|
|
Wheat |
|
|
|
|
|
No. of data points |
2 |
|
2 |
31 |
|
No. of individual samples |
39 |
|
58 |
1 643 |
|
Unweighted mean (µg/kg) |
0 |
|
0 |
1.6 |
|
Maximum value (µg/kg) |
0 |
|
0 |
310 |
|
% < LOD or LOQ |
100 |
100 |
90 |
|
Weighted mean, all samples: 1.9 µg/kg
The concentrations of T-2 toxin and HT-2 toxin available for this assessment are summarized by commodity and regional diet in Table 7. In view of the limited amount of data on these toxins in diets other than the European diet, only the latter were used in the dietary intake estimates. The mean of all the data for each commodity in the European diet, weighted by sample size, was used as the basis for the intake estimates.
Barley: Information on the concentrations of T-2 toxin in barley was received from six countries with two regional diets (the Far Eastern and European). A total of 372 samples were analysed, of which 342 were for the European diet. Of the samples for the European diet, the results for 90% were below the LOD or LOQ. The weighted mean value was 4.6 µg/kg.
Information on the concentrations of HT-2 toxin in barley were received from four countries with the same two diets. Of the 364 samples analysed, 334 were for the European diet. The maximum value reported was 290 µg/kg, and the weighted mean for the European diet was 4.4 µg/kg.
Maize: Nine countries representing four regional diets reported information on the concentration of T-2 toxin in maize, in a total of 1239 samples. Of these, 97% had results below the LOD or LOQ. Of the 168 samples for the European diet, the results for 98% were below the LOD or LOQ. The weighted mean in samples for the European diet was 3.2 µg/kg.
Five countries representing three regional diets reported information on the concentrations of HT-2 toxin in maize. Of the 292 samples, 184 were for the European diet. The results for all samples from the Far Eastern and Latin American diets were below the LOD or LOQ. The weighted mean of values for the European diet was 2.9 µg/kg. The maximum individual value reported was 230 µg/kg.
Oats: Four countries representing the European diet provided data on the concentration of T-2 toxin in a total of 758 samples of oats. The values for 71% of all samples were below the LOD or LOQ. The maximum individual value reported was 530 µg/kg, and the weighted mean was 21 µg/kg.
Four countries representing the European diet submitted data on the concentration of HT-2 toxin in 758 samples of oats. The results for 67% were below the LOD or LOQ. The maximum individual value reported was 2000 µg/kg, and the weighted mean was 35 µg/kg.
Rice: Only two countries (Ecuador and Germany) reported data on T-2 toxin in rice. Of the 26 samples representing the European diet, the results for 96% were below the LOD or LOQ. The maximum analytical value reported was 19 µg/kg, and the weighted mean for the European diet was 0.7 µg/kg.
Data on HT-2 toxin in rice were submitted by one country with the European diet, for only 26 samples. None of the samples contained quantifiable concentrations.
Rye: Three countries, all with the European diet, submitted data on the concentra-tion of T-2 toxin in rye. The results for 99% of the 83 samples were < LOD/LOQ. The maximum individual value reported was 17 µg/kg, and the weighted mean was 0.2 µg/kg.
Three countries with the European diet submitted data on the concentrations of HT-2 toxin in 87 samples of rye. The results for 99% of the samples were < LOD/LOQ. The maximum individual value reported was 23 µg/kg, and the weighted mean of all samples was 0.01 µg/kg.
Wheat: Ten countries with three regional diets reported data on the T-2 toxin concentrations in 2564 samples of wheat. Of those representing the European diet, 96% had values below the LOD or LOQ; the weighted mean was 1.6 µg/kg.
Three countries with three regional diets provided data on HT-2 toxin concentra-tions in wheat. Of 1740 samples, 1643 represented the European diet. For 90% of these, the results were below the LOD or LOQ. A maximum value of 310 µg/kg was reported for this diet, and the weighted mean of all samples was 1.9 µg/kg.
As noted above, dietary intakes were estimated only for the European diet, as limited information was available on T-2 toxin and HT-2 toxin in other regional diets. The average intakes of the two toxins were calculated by multiplying the weighted mean concentration of each commodity times the corresponding amount consumed in the GEMS/Food European diet. Intakes were calculated per person per day and converted to intake per kilogram of body weight per day, assuming a body weight of 60 kg, as recommended for international intake assessments (WHO, 1985).
The average intake of T-2 toxin in the European diet was estimated to be 7.6 ng/kg bw per day, while that of HT-2 toxin was estimated to be 8.7 ng/kg bw per day (Table 8). These estimates are based on the assumption that consumers choose foods randomly with respect to the distribution of concentrations of contaminants and are, therefore, exposed to an approximation of the mean of that distribution over time.
Table 8. Estimated intakes of T-2 and HT-2 toxins in the European diet
|
Toxin |
Commodity |
Weighted mean |
Consumption |
Intake |
% total intake |
||
|
ng/person per day |
µg/person per day |
ng/kg bw per day |
|||||
|
T-2 |
Barley |
4.6 |
20 |
91 |
0.09 |
1.5 |
20 |
|
Maize |
3.2 |
8.8 |
28 |
0.03 |
0.5 |
6 |
|
|
Oats |
21 |
2.0 |
42 |
0.04 |
0.7 |
2 |
|
|
Rice |
0.7 |
12 |
8 |
0.01 |
0.1 |
2 |
|
|
Rye |
0.2 |
1.5 |
0 |
0 |
0 |
0 |
|
|
Wheat |
1.6 |
180 |
280 |
0.28 |
4.7 |
63 |
|
|
Total intake |
|
|
450 |
0.45 |
7.6 |
100 |
|
|
HT-2 |
Barley |
4.4 |
20 |
87 |
0.09 |
1.5 |
17 |
|
Maize |
2.9 |
8.8 |
26 |
0.03 |
0.4 |
5 |
|
|
Oats |
35 |
2.0 |
70 |
0.07 |
1.2 |
13 |
|
|
Rice |
0 |
12 |
0 |
0 |
0 |
0 |
|
|
Rye |
0.01 |
1.5 |
0 |
0 |
0 |
0 |
|
|
Wheat |
1.9 |
180 |
340 |
0.34 |
5.6 |
65 |
|
|
Total intake |
|
|
520 |
0.52 |
8.7 |
100 |
|
The distributions of dietary intakes could not be constructed from the available data. Nonetheless, intakes at high levels can be approximated by multiplying the average intake by a factor of two for a single food commodity and three for the total diet (WHO, 1985).
Information on food consumption patterns at the national level was submitted by Norway and the United Kingdom. The intakes of T-2 toxin and HT-2 toxin were calculated by multiplying the national food consumption values by the weighted mean concentrations of T-2 toxin in samples from the European diet. Norway submitted information on the median and 95th percentile consumption of oats, rye, and wheat by eight population subgroups. Males aged 16–29 had the highest consumption of these foods and the highest intakes of the toxins on the basis of kilograms of body weight (Table 9). Children 6 years of age had the lowest intakes per kilogram of body weight.
Table 9. Intake of T-2 and HT-2 toxins from grains in Norway
|
Population group |
Grain |
Concentration of toxin (µg/kg) |
Body weight |
Median consumption |
95th percentile consumption |
||||
|
Grain (g/person per day) |
Toxin intake |
Grain (g/person per day) |
Toxin intake |
||||||
|
µg/person per day |
µg/kg bw per day |
µg/person per day |
µg/kg bw per day |
||||||
|
T-2 |
|||||||||
|
Males, females |
Oats |
21 |
23 |
6.2 |
0.13 |
0.006 |
26 |
0.54 |
0.02 |
|
Rye |
0.2 |
23 |
13 |
< 0.01 |
< 0.001 |
25 |
0.01 |
< 0.001 |
|
|
Wheat |
1.6 |
23 |
180 |
0.28 |
0.012 |
380 |
0.60 |
0.026 |
|
|
Males, females |
Oats |
21 |
35 |
8.2 |
0.17 |
0.0005 |
34 |
0.71 |
0.020 |
|
Rye |
0.2 |
35 |
16 |
< 0.01 |
< 0.001 |
32 |
0.01 |
< 0.001 |
|
|
Wheat |
1.6 |
35 |
230 |
0.37 |
0.010 |
490 |
0.79 |
0.022 |
|
|
Males |
Oats |
21 |
75 |
7.5 |
0.16 |
0.002 |
76 |
1.6 |
0.021 |
|
Rye |
0.2 |
75 |
15 |
< 0.01 |
< 0.001 |
31 |
0.01 |
< 0.001 |
|
|
Wheat |
1.6 |
75 |
280 |
0.44 |
0.006 |
700 |
1.1 |
0.015 |
|
|
Males |
Oats |
21 |
83 |
7.7 |
0.16 |
0.002 |
63 |
1.3 |
0.016 |
|
Rye |
0.2 |
83 |
14 |
< 0.01 |
< 0.001 |
28 |
0.01 |
< 0.001 |
|
|
Wheat |
1.6 |
83 |
240 |
0.38 |
0.005 |
570 |
0.91 |
0.011 |
|
|
Males |
Oats |
21 |
79 |
6.5 |
0.14 |
0.002 |
67 |
1.4 |
0.018 |
|
Rye |
0.2 |
79 |
13 |
< 0.01 |
< 0.001 |
25 |
0.01 |
< 0.001 |
|
|
Wheat |
1.6 |
79 |
190 |
0.31 |
0.004 |
720 |
1.2 |
0.015 |
|
|
Females |
Oats |
21 |
63 |
6.3 |
0.13 |
0.002 |
45 |
0.94 |
0.015 |
|
Rye |
0.2 |
63 |
11 |
< 0.01 |
< 0.001 |
19 |
< 0.01 |
< 0.001 |
|
|
Wheat |
1.6 |
63 |
190 |
0.31 |
0.005 |
440 |
0.71 |
0.011 |
|
|
Females |
Oats |
21 |
65 |
5.8 |
0.12 |
0.002 |
46 |
0.96 |
0.015 |
|
Rye |
0.2 |
65 |
10 |
< 0.01 |
< 0.001 |
18 |
< 0.01 |
< 0.001 |
|
|
Wheat |
1.6 |
65 |
170 |
0.28 |
0.004 |
390 |
0.62 |
0.010 |
|
|
Females |
Oats |
21 |
69 |
5.1 |
0.11 |
0.002 |
56 |
1.2 |
0.017 |
|
Rye |
0.2 |
69 |
10.0 |
< 0.01 |
< 0.001 |
17 |
< 0.01 |
< 0.001 |
|
|
Wheat |
1.6 |
69 |
160 |
0.25 |
0.004 |
360 |
0.58 |
0.008 |
|
|
HT-2 |
|||||||||
|
Males, females |
Oats |
35 |
23 |
6.2 |
0.22 |
0.015 |
26 |
0.91 |
0.039 |
|
Rye |
0.01 |
23 |
12.6 |
< 0.01 |
< 0.001 |
25 |
< 0.01 |
< 0.001 |
|
|
Wheat |
1.9 |
23 |
180 |
0.34 |
0.015 |
380 |
0.72 |
0.031 |
|
|
Males, females |
Oats |
35 |
35 |
8.2 |
0.29 |
0.008 |
34 |
1.2 |
0.034 |
|
Rye |
0.01 |
35 |
16 |
< 0.01 |
< 0.001 |
32 |
< 0.01 |
< 0.001 |
|
|
Wheat |
1.9 |
35 |
230 |
0.44 |
0.012 |
490 |
0.93 |
0.027 |
|
|
Males |
Oats |
35 |
75 |
7.5 |
0.26 |
0.003 |
76 |
2.6 |
0.035 |
|
Rye |
0.01 |
75 |
15.4 |
< 0.01 |
< 0.001 |
31 |
< 0.01 |
< 0.001 |
|
|
Wheat |
1.9 |
75 |
280 |
0.52 |
0.007 |
700 |
1.32 |
0.018 |
|
|
Males |
Oats |
35 |
83 |
7.7 |
0.27 |
0.003 |
63 |
2.2 |
0.026 |
|
Rye |
0.01 |
83 |
14 |
< 0.01 |
< 0.001 |
28 |
< 0.01 |
< 0.001 |
|
|
Wheat |
1.9 |
83 |
240 |
0.45 |
0.005 |
570 |
1.1 |
0.013 |
|
|
Males |
Oats |
35 |
79 |
6.5 |
0.23 |
0.003 |
67 |
2.3 |
0.030 |
|
Rye |
0.01 |
79 |
13 |
< 0.01 |
< 0.001 |
25 |
< 0.01 |
< 0.001 |
|
|
Wheat |
1.9 |
79 |
190 |
0.37 |
0.005 |
720 |
1.4 |
0.017 |
|
|
Females |
Oats |
35 |
63 |
6.3 |
0.22 |
0.003 |
45 |
1.6 |
0.025 |
|
Rye |
0.01 |
63 |
11 |
< 0.01 |
< 0.001 |
19 |
< 0.01 |
< 0.001 |
|
|
Wheat |
1.9 |
63 |
190 |
0.37 |
0.006 |
440 |
0.84 |
0.013 |
|
|
Females |
Oats |
35 |
65 |
5.8 |
0.20 |
0.003 |
46 |
1.6 |
0.025 |
|
Rye |
0.01 |
65 |
10 |
< 0.01 |
< 0.001 |
18 |
< 0.01 |
< 0.001 |
|
|
Wheat |
1.9 |
65 |
170 |
0.33 |
0.005 |
390 |
0.74 |
0.011 |
|
|
Females |
Oats |
35 |
69 |
5.1 |
0.18 |
0.003 |
56 |
2.0 |
0.028 |
|
Rye |
0.01 |
69 |
10 |
< 0.01 |
< 0.001 |
17 |
< 0.01 |
< 0.001 |
|
|
Wheat |
1.9 |
69 |
160 |
0.30 |
0.004 |
360 |
0.69 |
0.010 |
|
Sources of information on food consumption and body weight: Children 6–10 years, Norkost (1997); males and females > 16 years,
Langseth (2000). Toxin concentrations, weighted mean of European data
The United Kingdom provided information on the mean, median, and 97.5th percentile consumption of grains by two population subgroups, children aged 1.5–4.5 years and persons aged 16–64 years. The estimated intakes are reported in Table 10.
Table 10. Estimated intake of T-2 and HT-2 toxins by British children aged 1.5–4.5 years and British adults aged 16–64 years (eaters only)
|
Age group |
Grain |
Toxin |
% |
Consumption (g/person per day) |
Toxin intake (µg/person per day) |
||||
|
Mean |
Median |
97.5th %ile |
Mean |
Median |
97.5th %ile |
||||
|
T-2 |
|||||||||
|
1.5–4.5 |
Barley |
4.6 |
< 1 |
1.1 |
0.6 |
2.1 |
0.005 |
0.003 |
0.010 |
|
Maize |
3.2 |
66 |
10 |
6.6 |
37 |
0.032 |
0.021 |
0.12 |
|
|
Oats |
21 |
25 |
4.1 |
2.2 |
18 |
0.085 |
0.046 |
0.37 |
|
|
Rye |
0.2 |
1 |
2.0 |
1.7 |
4.9 |
< 0.001 |
< 0.001 |
0.001 |
|
|
Wheat |
1.6 |
99 |
47 |
45 |
100 |
0.076 |
0.072 |
0.16 |
|
|
16–64 |
Barley |
4.6 |
< 1 |
4.9 |
4.7 |
8.7 |
0.023 |
0.022 |
0.040 |
|
Maize |
3.2 |
50 |
12 |
7.6 |
50 |
0.040 |
0.024 |
0.16 |
|
|
Oats |
21 |
25 |
12 |
7.6 |
41 |
0.24 |
0.16 |
0.85 |
|
|
Rye |
0.2 |
9 |
7.4 |
3.7 |
39 |
0.001 |
0.001 |
0.008 |
|
|
Wheat |
1.6 |
99 |
130 |
120 |
250 |
0.20 |
0.19 |
0.40 |
|
|
HT-2 |
|||||||||
|
1.5–4.5 |
Barley |
4.4 |
< 1 |
1.1 |
0.6 |
2.1 |
0.005 |
0.003 |
0.009 |
|
Maize |
2.9 |
66 |
10 |
6.6 |
37 |
0.029 |
0.019 |
0.11 |
|
|
Oats |
35 |
25 |
4.1 |
2.2 |
18 |
0.14 |
0.077 |
0.62 |
|
|
Rye |
0.01 |
1 |
2.0 |
1.7 |
4.9 |
< 0.001 |
< 0.001 |
< 0.001 |
|
|
Wheat |
1.9 |
99 |
47 |
45 |
100 |
0.090 |
0.085 |
0.19 |
|
|
16–64 |
Barley |
4.4 |
< 1 |
4.9 |
4.7 |
8.7 |
0.022 |
0.021 |
0.038 |
|
Maize |
2.9 |
50 |
12 |
7.6 |
50 |
0.036 |
0.022 |
0.15 |
|
|
Oats |
35 |
25 |
12 |
7.6 |
41 |
0.40 |
0.26 |
1.4 |
|
|
Rye |
0.01 |
9 |
7.4 |
3.7 |
39 |
< 0.001 |
< 0.001 |
< 0.001 |
|
|
Wheat |
1.9 |
99 |
130 |
120 |
250 |
0.24 |
0.23 |
0.48 |
|
Sources of information on consumption: Gregory et al. (1990, 1992)
a Weighted mean of data for European diet
Fusarium species infect grain and produce mycotoxins when in the field. Measures taken to control or minimize Fusarium infection may also reduce the possibility of formation of T-2 toxin and HT-2 toxin. Such measures include culture control techniques, growing resistant cultivars, and the use of fungicides or biological antagonists. The measures, summarized by Parry et al. (1995), are described briefly below.
Culture control techniques include suitable crop rotation, appropriate use of fertilizers, irrigation, and weed control. Maize–wheat rotation increases the incidence of Fusarium head blight and should be avoided, whereas removal or ploughing in of crop debris reduces the incidence in wheat. Direct drilling or minimal cultivation increases the risk of infection when Fusarium-contaminated debris is present. High concentrations of nitrogen fertilizer may increase plant water stress, but the effect on Fusarium head blight is unclear. Effective weed control may be useful in reducing Fusarium inoculum, but the efficacy of weed control in reducing Fusarium head blight is debated. Irrigation may avoid water stress and reduce the severity of Fusarium foot rot in wheat, which may serve as an inoculum for the development of head blight. Overhead irrigation has been shown to increase the severity of the disease.
Differences between cultivars in susceptibility to Fusarium head blight has been recognized for more than 100 years. Most cultivars of wheat are susceptible, and only a few are moderately resistant. Few reports of immune species exist. Limited work has been done on breeding resistance into species other than wheat.
The effect of previous crop residues and tillage on Fusarium head blight in wheat were examined by Dill-Macky & Jones (2000), who confirmed that the incidence and severity of the disease were greatest when wheat followed maize and least when wheat followed soya beans. In addition, the incidence and severity were lower in molboard plow plots than in either chisel (reduced-till) or no-till plots. They suggested that changes in tillage practices, principally the move to conservation tillage and reduced-till systems, contributed to the recent epidemics of Fusarium head blight in midwestern USA.
Sub-lethal doses of certain fungicides have been shown to stimulate T-2 toxin production, while other fungicides may be highly effective inhibitors of T-2 toxin synthesis (D’Mello et al., 1998).
Parry et al. (1995) suggested that biological control measures could be a useful alternative to fungicide treatment, since the period during which the cereals are sensitive to the disease is short. There are few reports of such control of Fusarium head blight, and none seems to have been used in practice. Experimental use of biological control against diseases attributed to Fusarium spp. has been reported (Kempf & Wolf, 1989; Mao et al., 1998; Hoefnagels & Linderman, 1999).
Numerous chemicals have been tested for their ability to decontaminate trichothecene-contaminated grain or feed. Calcium hydroxide monomethylamine effectively decontaminated feeds containing T-2 toxin (Bauer et al. 1987), but the efficiacy was dependent on the moisture content of the feed and the processing temperature. A moisture content of 25% and a temperature of 100 °C also had to be maintained for 1 h in order to destroy the less toxic HT-2 toxin, which was formed during the detoxication process as a result of alkaline hydrolysis of T-2 toxin.
Bentonite and spent canola oil bleaching clays appear to exert beneficial effects by adsorbing T-2 toxin present in the diet and inhibiting its adsorption from the gastrointestinal tract (Carson & Smith, 1983). Bentonite fed at 10% was the most effective treatment in overcoming feed refusal and growth depression in rats.
A hydrated sodium calcium aluminosilicate protected some animals against the adverse effects of some Fusarium mycotoxins (Charmley & Prelusky, 1994); however, incorporation of this compound into contaminated diets had no effect on the toxicity of T-2 toxin in poultry (Kubena et al., 1990).
Superactivated charcoal had little effect in alleviating mycotoxicosis when T-2 toxin was fed to broiler chicks (Edrington et al., 1997).
Administration of monoclonal antibodies specific for T-2 toxin neutralized the inhibitory effects of the toxin on protein synthesis in vitro (Feuerstein et al., 1988).
Absorption, distribution, metabolism, and excretion
T-2 toxin is readily metabolized by mammalian gut microflora to several metabolites. HT-2 toxin is a primary metabolite in the gut and is absorbed into the blood after ingestion of T-2 toxin. Metabolism continues in the liver (with biliary excretion), resulting in a substantial, combined first-pass effect in the gut and liver. Metabolites of T-2 toxin include HT-2, 3’-hydroxy-HT-2, 3’-hydroxy-T-2, T-2 tetraol, de-epoxy 3’-hydroxy-T-2 triol, de-epoxy 3’-hydroxy-HT-2, and 3’-hydroxy-T-2 triol. Glucuronide conjugates are also formed extensively in most species (with the exception of cats). T-2 toxin and its metabolites are eliminated rapidly. In rats, more than 95% of a radioactively labelled oral dose of 0.15 mg/kg bw per day was excreted within 72 h. In the same study, a dose of 0.6 mg/kg bw per day was eliminated more slowly, suggesting potentially saturable metabolism or elimination pathways at doses that are relevant to those used in the studies of toxicity considered in this evaluation.
Toxicological studies
T-2 toxin is a potent inhibitor of protein synthesis both in vivo and in vitro. The effective concentration for protein inhibition in vitro is lower than the effective concentrations for all other effects that have been demonstrated.
The metabolites of T-2 toxin have not been studied in detail, but several primary metabolites were less toxic than the parent compound in vitro. Furthermore, T-2 toxin was 10 times more toxic when inhaled than after oral intake, suggesting that the first-pass effect reduces the toxicity, at least after acute exposure. The Committee noted that, although it is generally assumed that T-2 toxin is considerably (e.g. 10-fold) more toxic than deoxynivalenol, a comparison of the LOELs for similar species and end-points (see monograph on deoxynivalenol) suggests that these trichothecenes have roughly similar toxicity when they are ingested with food.
Strain and sex differences in susceptibility to the toxicity of T-2 toxin have been observed in mice given single oral doses by gavage in studies designed to evaluate this variation. A sex difference was also observed after administration by inhalation. The cause of the differences has not been identified. Differences in susceptibility to T-2 toxin among species were also suggested from a comparison of the results of short-term studies. Severe toxic effects, including haemorrhage in the intestinal tract, lymph nodes, and heart, that led to death within weeks were observed in cats that received a dose of T-2 toxin as low as 0.06 mg/kg bw per day in a gelatin capsule. In contrast, relatively mild effects were observed in 7-week-old pigs given T-2 toxin in the diet at doses up to 0.13 mg/kg bw per day for 3 weeks and in mice given 0.22 mg/kg bw per day in the diet in a 71-week study. The greater susceptibility of cats was to be expected in view of the demonstrated deficiencies in conjugation reactions in this species. Humans would not be expected to be similarly susceptible.
Little direct information was available on the toxicity of HT-2 toxin alone. The few comparative data available on T-2 and HT-2 toxins indicate that they induce adverse effects with similar potency. Furthermore, because T-2 toxin is rapidly converted to HT-2 toxin (and other metabolites common to T-2 and HT-2 toxins) in the gut, the toxicity of T-2 toxin in vivo can be considered to include that of HT-2 toxin. Hence, the results of studies of T-2 toxin can be used to approximate the effects of HT-2 toxin.
Information on the toxicity of T-2 toxin when ingested daily was limited largely to studies of less than 1 month. The immune system is a primary target of T-2 toxin, and the effects include changes in leukocyte count, delayed hypersensitivity, depletion of selective blood cell progenitors, depressed antibody formation, allograft rejection, and a blastogenic response to lectins. Either increased or decreased leukocyte counts were observed, depending at least in part on the dose and the time after administration of T-2 toxin when the leukocytes were counted. Similarly, both decreased and increased resistance to microbial infection has been observed in a number of studies. For example, in separate experiments in several laboratories, decreased resistance (leading in most cases to greater mortality rates) was observed in mice exposed to T-2 toxin at the time of infection with Salmonella typhimurium, Salmonella enteriditis, Mycobacterium bovis, Herpes simplex, Toxoplasma gondii, or Listeria monocytogenes. However, increased resistance leading to a reduced mortality rate was observed when mice were treated with T-2 toxin before infection with Listeria monocytogenes.
Feed refusal, reduced weight gain, and changes in organ weights, which are sensitive end-points, have been observed in most studies of feeding of T-2 toxin in which these parameters were recorded.
A 3-week study was conducted in which 7-week-old pigs were fed a diet providing an average T-2 toxin intake equal to 0.029, 0.062, 0.10, or 0.13 mg/kg bw per day. On the first and fourth days of administration, the pigs were immunized by an intramuscular injection of horse globulin. The titre of anitibodies to this antigen was significantly lower in T-2 toxin-treated than in control pigs at 14 and 21 days at all doses tested. The leukocyte count and the proportion of leukocytes made up by T-lymphocytes was lower in all treated groups. A decreased proliferative response to phytohaemagglutinin and concanavalin A was observed at all doses of T-2 toxin at 21 days. A dose-related decrease in feed intake was observed at all doses, and decreased weight gain was observed at doses of 0.062 mg/kg bw per day and above. The haemoglobin concentration was decreased in a dose-related manner at these doses. A reduction in erythrocyte count was observed at 0.10 and 0.13 mg/kg bw per day, and the erythrocyte volume fraction was reduced at 0.13 mg/kg bw per day. The Committee noted that, as pair-fed animals were not used as controls, the potential confounding effects of feed intake and differences in weight gain on the observed end-points could not be evaluated. A NOEL was not identified.
In a 71-week bioassay of the carcinogenicity of T-2 toxin administered in the feed of mice, the incidence of pulmonary adenomas and hepatic adenomas was statistically significantly increased at the end of the study in males at the highest dose, with no increase in tumour incidence in female mice. However, an increase in the incidence of benign tumours of the liver or lung in mice of one sex in a single study constitutes, at most, weak evidence of carcinogenicity. A dose-related increase in heart weight was seen in males (but not females) receiving T-2 toxin. No other treatment-related changes were reported. Studies of cancer initiation and promotion in mice suggest that T-2 toxin is not likely to be a potent carcinogen. A working group convened by IARC in 1993 evaluated the same experimental data and concluded that T-2 toxin is not classifiable with regard to its carcinogenicity to humans (Group 3).
Tests for genotoxicity with T-2 toxin in microorganisms gave uniformly negative results. In cultured mammalian cells, however, low concentrations of T-2 toxin induced DNA strand breaks, unscheduled DNA synthesis, gene mutation, chromosomal aberrations, and inhibition of gap-junctional intercellular communication. There was also evidence that DNA strand breaks and chromosomal aberrations are induced in vivo. It was unclear whether these effects are a consequence of interaction of T-2 toxin with genetic material or are secondary to inhibition of protein synthesis by T-2 toxin.
No embryotoxicity or gross fetal malformations were seen at intraperitoneal doses lower than 0.5 mg/kg bw per day. Continuous administration in the feed at concentrations of T-2 toxin equivalent to 0.22 and 0.45 mg/kg bw per day did not result in reproductive or gross developmental effects in CD-1 mice, although increased spleen weights were observed in male offspring of exposed dams at both doses.
The Committee noted that reduced feed intake is a potential confounder in studies of the toxicity of T-2 and HT-2 toxins. For example, in one study in mice in which pair-fed controls were used, changes in spleen weight, cell counts, and lymphoproliferative response were observed that paralleled those in mice given 3 mg/kg bw per day. The spleen weight, cell counts, and lymphoproliferative response were significantly lower in both the T-2 toxin-treated and pair-fed groups than in control mice fed ad libitum.
Observations in humans
The studies of adverse health effects in human populations were limited to a few investigations of outbreaks of acute poisoning, in which the effects reported included nausea, vomiting, pharyngeal irritation, abdominal pain and distension, diarrhoea, bloody stools, dizziness, and chills. In subsequent investigations, analyses of limited numbers of suspected food or grain samples indirectly linked the outbreaks to T-2 toxin. The concomitant occurrence of T-2 toxin with deoxynivalenol, acetyldeoxy-nivalenol, and nivalenol was reported in one of these outbreaks and the presence of these or other trichothecenes could not be ruled out in other studies. A series of food-related poisoning incidents referred to as alimentary toxic aleukia that occurred in 1931–47 in the former Soviet Union was associated with ingestion of grain infected with moulds, in particular F. poae and F. sporotrichioides. The dominant pathological changes were necrotic lesions of the oral cavity, oesophagus, and stomach and, in particular, pronounced leukopenia consisting primarily of bone-marrow hypoplasia and aplasia. The disease was lethal in a high proportion of cases. In investigations conducted three decades later, cultures implicated in the outbreak were shown to produce T-2 toxin.
Sampling protocols and analytical methods
No sampling plans for the determination of T-2 and HT-2 toxins in foods have been published, and details on variation in the sampling of these toxins have not been reported. Furthermore, no official methods for the determination of T-2 and HT-2 toxins have been published, although some methods that have been validated in collaborative studies have been reported.
The introduction of improved clean-up columns based on charcoal, alumina, and modified diatomaceous earth before chromatographic determination has simplified and accelerated the analysis of T-2 and HT-2 toxins. Use of these columns in combination with gas chromatography and electron capture detection or mass spectrometry after derivatization of T-2 and HT-2 toxins is the commonest technique for their quantification. These techniques allow determination of concentrations of a few nanograms per gram, even in complex food matrices. Effective clean-up and derivatization are required for quantification by liquid chromatography with ultra-violet or fluorescence detection. Liquid chromatography with mass spectrometric detection is a potentially useful technique for determining T-2 and HT-2 toxins directly and simultaneously; however, its high cost prohibits its use as a routine method. Thin-layer chromatography, particularly high-performance, can also be used for the determination of T-2 and HT-2 toxins. A few enzyme-linked immunosorbent assay methods have been developed for screening T-2 toxin.
Although a variety of analytical methods is available for quantifying T-2 and HT-2 toxins, inter-laboratory comparisons have clearly shown that appropriate screening methods and better analytical methods are needed for these mycotoxins, particularly with respect to the recovery, accuracy and precision of measurements. More widely available reference materials and regular international comparative studies are required for these mycotoxins in order to improve internal and external quality assurance.
Levels and patterns of contamination of food commodities
Data on the concentrations of T-2 and HT-2 toxins in food commodities were submitted by Brazil, China, Finland, Germany, Norway, Sweden, and the United Kingdom, and others were obtained from the literature. Gas chromatography with electron capture detection was the technique used most commonly for quantification of these toxins, followed in order by thin-layer chromatography and enzyme-linked immunosorbent assay.
Data from studies in which information on the sampling protocol or the analytical method was not provided were excluded from the evaluation. The remaining data were used only if the samples had been collected at random and if the analytical methods used were considered to be adequate.
Data were available on barley, oats, rice, rye, and wheat. Most of the 8918 samples were collected in Europe. The frequency of occurrence of contaminated samples was 11% for T-2 toxin and 14% for HT-2 toxin; high concentrations of the two toxins were occasionally found together. Annual variation was observed in the degree of contamination of oats, wheat, and barley. The mean concentrations in samples in which T-2 was found were 0.1–21 µg/kg in barley, 1.3–6.0 µg/kg in maize, 2.3–26 µg/kg in oats, 2.7–27 µg/kg in rice, 0.6 µg/kg in rye, and 0.1–60 µg/kg in wheat. The mean concentrations in samples in which HT-2 was found were 0.4–15 µg/kg in barley, 2.4–14 µg/kg in maize, 3.7–20 µg/kg in oats, 26–100 µg/kg in rice, 0.03 µg/kg in rye, and 0.2–20 µg/kg in wheat.
As T-2 and HT-2 toxins were not detected in a large proportion of samples from the United Kingdom, the distribution could not be derived. The use of distribution functions was also not practicable for the other data sets, mainly because of the way in which the data were reported.
Food consumption/dietary intake assessment
The average intakes of T-2 and HT-2 toxins can be estimated by multiplying the average concentrations in food commodities by estimates of the average food consumption. For the latter, the GEMS/Food regional diets were used. Most of the data on average concentrations of T-2 and HT-2 toxins that were available for the evaluation were pooled; that is, each data point represented the mean concentration of a number of individual samples. Data on processed food products were excluded from estimates of dietary intake. A total of 175 data points representing 8410 individual samples were used for the intake assessment. Of these, 147 were reported from countries in Europe; the remaining 28 data points represented only three commodities in the other four geographical regions. In view of the limited amount of information on these toxins in regions other than Europe, the dietary intakes of T-2 and HT-2 toxins were estimated from the GEMS/Food European-type diet only. The mean concentrations of T-2 and HT-2 toxins, weighted by sample size, were calculated for each commodity (barley, maize, oats, rice, rye, and wheat) from the data submitted, and the intakes of T-2 and HT-2 toxins were estimated by multiplying the weighted mean concentration in each commodity by the respective value for consumption in the GEMS/Food European diet.
The total intake of T-2 toxin was estimated to be 7.6 ng/kg bw per day, wheat and barley being the major dietary sources. The total intake of HT-2 toxin was estimated to be 8.7 ng/kg bw per day, wheat, barley, and oats being the most important dietary sources. These estimates were based on the assumption that consumers choose food randomly with respect to the distribution of concentrations of contaminants, which will approximate the mean over time.
In general, more data on the occurrence of T-2 and HT-2 toxins in food commodities, particularly from geographical regions other than Europe, are required to allow better estimates of intake. The Committee noted that the distribution of contamination in processed products could differ from that in raw cereals, as contamination tends to be more homogeneous after processing. Despite the limited amount of data on concentrations of T-2 and HT-2 toxins, the preliminary estimates of average contamination and dietary intake based on the GEMS/Food European diet proved to be useful. However, significant data gaps were identified in the assessment, with respect to both the quality and the geographical representativeness of the available data. Although it was not possible to estimate intakes at high consumption levels from the available data, such intakes may be approximated by multiplying the average intake by a factor of two for a single food commodity and three for the total diet.
Prevention and control
Preharvest measures taken to control or minimize Fusarium infection may also reduce the possibility of formation of T-2 and HT-2 toxins. Reducing the inoculum of Fusarium in host debris and other reservoirs in the field appears to be an effective control measure. Practices such as reduced tillage have been shown to increase the incidence of other trichothecenes and may also affect those of T-2 and HT-2 toxins. Good agricultural practice, such as immediate drying after harvesting and proper storage, prevents further contamination with T-2 and HT-2 toxins.
Physical, chemical, and biological methods have been used to decontaminate grain containing trichothecenes, but few studies were available on any reduction in the concentration of T-2 or HT-2 toxins. Thermal processing is usually ineffective.
The Committee concluded that there was substantial evidence for the immunotoxicity and haematotoxicity of T-2 toxin in several species, and that these are critical effects after short-term intake. Only one long-term study was available, and that study alone was not suitable for establishing a tolerable intake. Nonetheless, on the basis of the critical effects seen in several short-term studies, the Committee concluded that the safety of food contaminated with T-2 toxin could be evaluated from the LOEL of 0.029 mg/kg bw per day for changes in white and red blood cell counts identified in the 3-week dietary study in pigs. This LOEL was the lowest LOEL for adverse effects in the studies on T-2 toxin. It was considered to be close to a NOEL, as the effects on blood cell counts were subtle and reversible. Furthermore, other studies in pigs showed no effects at this dose.
The Committee used this LOEL and a safety factor of 500 to derive a provisional maximum tolerable daily intake (PMTDI) for T-2 of 60 ng/kg bw per day. The safety factor of 500 was used because there was no clear NOEL in the 3-week study in pigs and there were deficiencies in the database, including insufficient study of long-term administration of T-2 toxin and sex, species, and individual variations in sensitivity.
The Committee further concluded that the toxic effects of T-2 toxin and its metabolite HT-2 toxin could not be differentiated, and that the toxicity of T-2 toxin in vivo might be due at least partly to effects of HT-2 toxin. Hence, HT-2 toxin was included in the PMTDI, resulting in a group PMTDI of 60 ng/kg bw per day for T-2 and HT-2 toxins, alone or in combination.
Recommendations
|
• |
Further studies are needed to reduce the uncertainty in the evaluation of the carcinogenic potential of T-2 toxin. Standard bioassays in rats and mice, with pair-fed controls, would be preferred. Also, a longer-term study in pigs is needed in which a NOEL is identified, control groups are used to account for the potential effects of reduced feed consumption, and relevant, sensitive end-points of haematotoxicity and immunotoxicity are measured. In order to clarify differences among species, comparative studies of toxicity and toxicokinetics should be carried out in rodents, cats, and pigs. Studies are needed on the combined effects of other trichothecenes that contaminate foods consumed by humans. |
|
• |
As little information on the concentrations of T-2 and HT-2 toxins in food commodities was available from geographical regions other than Europe, dietary intake was estimated only on the basis of the GEMS/Food European regional diet. Dietary intake in other geographical regions should be evaluated when more data on the concentrations of T-2 and HT-2 toxin become available. The average intake of T-2 toxin was estimated to be 8 ng/kg bw per day, and that of HT-2 toxin was estimated to be 9 ng/kg bw per day. The total was therefore not expected to exceed the group PMTDI of 60 ng/kg bw per day. Nonetheless, more accurate information on human intake of T-2 toxin in various regions of the world and improved analytical methods and reference materials for the determination of both toxins are needed. |
Abouzied, M. (2000) A very sensitive rapid ELISA test for the detection and quantification of the trichothecene mycotoxins deoxynivalenol (DON). In: Proceedings of the X International IUPAC Symposium of Mycotoxins and Phytotoxins, 21–25 May 2000, São Paulo, Brazil.
Adler, A., Lew, H., Edinger, W., & Oberforster, M. (1992) Moniliformin in durum wheat. In: Proceedings of the 3rd European Seminar on Fusarium-Mycotoxins, Taxonomy, Pathogenicity and Host Resistance, 22–24 September 1992, Radzikow: Plant Breeding and Acclimatization Institute, pp. 22–27.
Agrelo, C.E. & Schoental, R. (1980) Synthesis of DNA in human fibroblasts treated with T-2 toxin and HT-2 toxin (the trichothecene metabolites of Fusarium species) and the effects of hydroxyurea. Toxicol. Lett., 5, 155–160.
Albarenque, S.M., Shinozuka, J., Iwamoto, S., Nakayama, H. & Doi, K. (1999) T-2 toxin-induced acute skin lesions in Wistar-derived hypotrichotic WBN/ILA-Ht rats. Histol. Histopathol., 14, 337–342.
Apro, N., Resnik, S. & Ferro Fontán, C. (1987) [Representative samples for mycotoxins analysis.] Anal. Asoc. Quím. Argentina, 75, 501–510 (in Spanish).
Atroshi, F., Rizzo, A.F., Veijalainen, P., Lindberg, L.A., Honkanen-Buzalski, T., Andersson, K., Hirvi, T. & Saloniemi, H. (1994) The effect of dietary exposure to DON and T-2 toxin on host resistance and serum immunoglobulins of normal and mastitic mice. J. Anim. Physiol. Anim. Nutr., 71, 223–233.
Auffray, Y. & Boutibonnes, P. (1986) Evaluation of the genotoxic activity of some mycotoxins using Escherichia coli in the SOS spot test. Mutat. Res., 171, 79–82.
Bamburg, J.R. & Strong, F.M. (1971) 12,13-Epoxytrichothecenes. In: Kadis, S., Ciegler, A. & Ajl, S.J., eds, Microbiological Toxins. VII. Algal and Fungal Toxins, New York: Academic Press, pp. 207–292.
Barna-Vetro, I., Gyongyosi, A. & Solti, L. (1994) Monoclonal antibody-based enzyme-linked immunosorbent assay of fusarium T-2 and zearalenone toxins in cereals. Appl. Environ. Microbiol., 60, 729–731.
Barna-Vetró, I., Gyöngyösi, À. & Làszló, S. (1997) Immundiagnostics for mycotoxin determina-tion. Hung. Agric. Res., 2, 16–19.
Bauer, J., Gareis, M., Detzler, W., Gedek, B., Heinritzi, K. & Kabilka, G. (1987) Detoxification of mycotoxins in animal feeds. Tierarztl. Umsch., 42, 70–77.
Beardall, J.M. & Miller, J.D. (1994) Diseases in humans with mycotoxins as possible causes. In: Miller, J.D. & Trenholm, H.L., eds, Mycotoxins in Grain. Compounds Other than Aflatoxin, St Paul, Minnesota: Eagan Press, pp. 487–539.
Beasley, V.R., Swanson, S.P., Corley, R.A., Buck, W.B., Koritz, G.D. & Burmeister, H.R. (1986) Pharmacokinetics of the trichothecene mycotoxin, T-2 toxin, in swine and cattle. Toxicon, 21, 13–23.
Bedford, P.G.C. & Clarke, E.G.C. (1971) Suspected benzoic acid poisoning in the cat. Vet. Rec., 88, 599–601.
Beeton, S. & Bull, A.T. (1989) Biotransformation and detoxification of T-2 toxin by soil and freshwater bacteria. Appl. Environ. Microbiol., 55, 190–197.
Berger, U., Oehme, M. & Kuhn, F. (1999) Quantitative determination and structure elucidation of type A- and B-trichothecenes by HPLC/ion trap multiple mass spectrometry. J. Agric. Food Chem., 47, 4240–4245.
Bergmann, F., Yagen, B., & Soffer, D. (1985) Toxic and lethal effects of T-2 toxin upon intracerebral administration to rats. Arch. Toxicol., 58, 40-44.
Bergmann, F., Soffer, D. & Yagen, B. (1988) Cerebral toxicity of the trichothecene toxin T-2, of the products of its hydrolysis and of some related toxins. Toxicon, 26, 923–930.
Bhat, R.V., Ramakrishna, Y., Rao, B.S. & Nahdi, S. (1987) Trichothecene Mycotoxicosis, Hyderabad: Food and Drug Toxicology Research Centre, National Institute of Nutrition.
Bhat, R.V., Beedu, S.R., Ramakrishna, Y. & Munshi, K.L. (1989) Outbreak of trichothecene mycotoxicosis associated with consumption of mould-damaged wheat products in Kashmir Valley, India. Lancet, 7, 35–37.
Bilai, V.I. (1977) [Fusariums], Kiev: Naukova Dumka (in Russian).
Bilgrami, K.S., Masood, A. & Rahman, M.F. (1995) Cumulative effect of T-2 toxin and vitamin C on chromosomal abnormalities in the bone marrow cells of mice (Mus musculus). Cytobios, 81, 171–174.
Blaas, W., Kellert, M., Steinmeyer, S., Tiebach, R. & Weber, R. (1984) Determination of deoxynivalenol and nivalenol in cereals at trace levels. Z.Lebensm. Untersuch. Forsch., 179, 104–108,.
Boonchuvit, B., Hamilton, P.B. & Burmeister, H.R. (1975) Interaction of T-2 toxin with Salmonella infections of chickens. Poult. Sci., 54, 1693–1696.
Brennecke, L.H. & Neufeld, H.A. (1982) Pathologic effects and LD50 doses of T-2 toxin in rats by intramuscular, subcutaneous, and intraperitoneal routes of administration. Fed. Proc., 41, 924.
Bridges, J.W., French, M.R., Smith, R.L. & Williams, R.T. (1970) The fate of benzoic acid in various species. Biochem. J., 118, 47–51.
Buening, G.M., Mann, D.D., Hook, B. & Osweiler, G.D. (1982) The effect of T-2 toxin on the bovine immune system, cellular factors. Vet. Immunol. Immunopathol., 3, 411–417.
Bunner, D.L. & Morris, E.R. (1988) Alteration of multiple cell membrane functions in L-6 myoblasts by T-2 toxin, an important mechanism of action. Toxicol. Appl. Microbiol., 92, 113–121.
Burmeister, H.R. (1971) T-2 toxin production by Fusarium tricinctum on solid substrate. Appl. Microbiol.,21, 739–742.
Cahill, L.M., Kruger, S.C., McAlice, B.T., Ramsey C.S., Prioli, R. & Kohn, B. (1999) Quantification of deoxynivalenol in wheat using an immunoaffinity column and liquid chromatography. J. Chromatogr. A, 859, 23–28.
Carson, M.S. & Smith, T.K. (1983) Role of bentonite in prevention of T-2 toxicosis in rats. J. Anim. Sci., 57, 1498–1505.
Chan, P.K-C. & Gentry, P.A. (1984) LD50 values and serum biochemical changes induced by T-2 toxin in rats and rabbits. Toxicol. Appl. Pharmacol., 73, 402–410.
Charmley, L.L. & Prelusky, D.B. (1994) Decontamination of Fusarium mycotoxins. In: Miller, J.D. & Trenholm, H.L., eds, Mycotoxins in Grain—Compounds Other than Aflatoxin, St Paul, Minnesota: Eagan Press, pp. 421–435.
Chatterjee, K., Visconti, A. & Mirocha, C.J. (1986) Deepoxy T-2 tetraol, a metabolite of T-2 toxin found in cow urine. J. Agric. Food Chem., 34, 695–697.
Chen,Y.F., Zhao, P., Wei, G.L. & Wang, W. (1995) A survey of T-2 toxin in wheat in Guizhou province. Chin. J. Food Hyg., 7, 43–44.
Chi, M.S., Mirocha, C.J., Kurtz, H.J., Weaver, G., Bates, F., Shimoda, W. & Burmeister, H.R. (1977a) Acute toxicity of T-2 toxin in broiler chicks and laying hens. Poult. Sci., 56, 103–116.
Chi, M.S., Mirocha, C.J., Kurtz, H.J., Weaver, G., Bates, F. & Shimoda, W. (1977b) Effects of T-2 toxin on reproductive performance and health of laying hens. Poult. Sci., 56, 628–637.
Chi, M.S., Robison, T.S., Mirocha, C.J., Swanson, S.P. & Shimoda, W. (1978a) Excretion and tissue distribution of radioactivity from tritium labeled T-2 toxin in chicks. Toxicol. Appl. Pharmacol., 45, 391–402.
Chi, M.S., Robison, T.S., Mirocha, C.J., Behrens, J.C. & Shimoda, W. (1978b) Transmission of radioactivity into eggs from laying hens (Gallus domesticus) administered tritium labeled T-2 toxin. Poult. Sci., 57, 1234–1238.
Chi, M.S., El-Halawani, M.E., Waibel, P.E. & Mirocha, C.J. (1981) Effects of T-2 toxin on brain catecholamines and selected blood components in growing chickens. Poult. Sci., 60, 137–141.
Cho, B.R. (1964) Toxicity of water extracts of scabby barley to suckling mice. Am. J. Vet. Res., 25, 1267–1270.
Chu, F.S. (1991) Detection and determination of mycotoxins. In: Sharma, R.P. & Salunkhe, D.K., eds, Mycotoxins and Phytoalexins, Boca Raton, Florida: CRC Press, pp. 33–79.
Chung, H.S. (1975) Cereal scab causing mycotoxicoses in Korea and present status of mycotoxin researches. Kor. J. Mycol., 3, 31–36.
Coffin, J.L. & Combs, G.F., Jr (1981) Impaired vitamin E status of chicks fed T-2 toxin. Poult. Sci., 60, 385–392.
Cole, R.J. (1986) Modern Methods in the Analysis and Structural Elucidation of Mycotoxins, Orlando, Florida: Academic Press.
Conrady-Lorck, S., Gareis, M., Feng, X., Amselgruber, W., Forth, W. & Fichtl, B. (1988) Metabolism of T-2 toxin in vascularly autoperfused jejunal loops of rats. Toxicol. Appl. Pharmacol., 94, 23–33
Cooray, R. (1984) Effects of some mycotoxins on mitogen-induced blastogenesis and SCE frequency in human lymphocytes. Food Chem. Toxicol., 22, 529–534.
COPANT (1998) Norma Panamericana 989/78. Panamerican Committee of Technical Norms, Buenos Aires.
Coppock, R.W., Swanson, S.P., Gelberg, H.B., Koritz, G.D., Hoffman, W.E., Buck, W.B. & Vesonder, R.F. (1985) Preliminary study of the pharmacokinetics and toxicopathy of deoxynivalenol (vomitin) in swine. Am. J. Vet. Res., 46, 169–174.
Corley, R.A., Swanson, S.P. & Buck, W.B. (1985) Glucuronide conjugates of T-2 toxin and metabolites in swine bile and urine. J. Agric. Food Chem., 33, 1085–1089.
Corley, R.A., Swanson, S.P., Gullo, G.J., Johnson, L., Beasley, V.R. & Buck, W.B. (1986) Disposition of T-2 toxin, a trichothecene mycotoxin, in intravascularly dosed swine. J. Agric. Food Chem., 34, 868–875.
Corrier, D.E. & Ziprin, R.L. (1986a) Immunotoxic effects of T-2 toxin on cell-mediated immunity to listeriosis in mice, comparison with cyclophosphamide. Am. J. Vet. Res., 47, 1956–1960.
Corrier, D.E. & Ziprin, R.L. (1986b) Enhanced resistance to listeriosis induced in mice by preinoculation treatment with T-2 mycotoxin. Am. J. Vet. Res., 47, 856–859.
Cosgriff, T.M., Bunner, D.P., Wannemacher, R.W., Jr., Hodgson, L.A. & Dinterman R.E. (1984) The hemostatic derangement produced by T-2 toxin in guinea pigs. Toxicol. Appl. Pharmacol., 76, 454–463.
Cosgriff, T.M., Bunner, D.P., Wannemacher, R.W., Jr., Hodgson, L.A. & Dinterman, R.E. (1986) The hemostatic derangement produced by T-2 toxin in cynomolgus monkeys. Toxicol. Appl. Pharmacol., 82, 532–539.
Council for Agricultural Science and Technology (1989) Mycotoxins: Economic and Health Risks. Task Force Report No. 116 (November) Ames, Iowa, USA.
Creasia, D.A. & Thurman, J.D. (1993) Comparative acute inhalation toxicity of a saline suspension and an ethanol solution of T-2 mycotoxin in mice. Inhal. Toxicol., 5, 33–41.
Creasia, D.A., Thurman, J.D., Wannemacher, R.W., Jr & Bunner, D.L. (1987) Acute inhalation toxicity of T-2 mycotoxin in mice. Fundam. Appl. Toxicol., 8, 230–235.
Creasia, D.A., Thurman, J.D., Wannemacher, R.W., Jr & Bunner, D.L. (1990) Acute inhalation toxicity of T-2 mycotoxin in the rat and guinea pig. Fundam. Appl. Toxicol., 14, 54–59.
Croteau, St.M., Prelusky, D.B. & Trenholm, H.L. (1994) Analysis of trichothecene mycotoxins by gas chromatography with electron capture detection. J. Agric. Food Chem., 42, 928–933.
Dahlem, A.M., Swanson, S.P., Côte, L.-M., Yoshizawa, T. & Buck, W.B. (1986) Quantitation of deoxynivalenol and its metabolite in bovine urine and feces by gas chromatography with electron-capture detection. J. Chromatogr., 378, 226–233.
DeLoach, J.R., Andrews, K. & Nagi, A. (1987) Interaction of T-2 toxin with bovine erythrocyte, effects on cell lysis, permeability and entrapment. Toxicol. Appl. Pharmacol., 88, 123–131.
Diaz, G.J., Squires, E.J., Julian, R.J. & Boermans, H.J. (1994) Individual and combined effects of T-2 toxin and DAS in laying hens. Br. Poult. Sci., 35, 393–405.
Dill-Macky, R. & Jones, R.K. (2000) The effect of previous crop residues and tillage on Fusarium head blight of wheat. Plant Dis., 84, 71–76.
D’Mello, J.P.F., Porter, J.K., MacDonald, A.M.C. & Placinta, C.M. (1997) Fusarium mycotoxins. In: D’Mello, J.P.F., ed., Handbook of Plant and Fungal Toxicants, Boca Raton, Florida: CRC Press, p. 287.
D’Mello, J.P.F., MacDonald, A.M.C., Postel, D., Dijksma, T.P., Dujardin, A. & Placinta, C.M. (1998) Pesticide use and mycotoxin production in Fusarium and Aspergillus phytopathogens. Eur. J. Plant Pathol., 104, 741–751.
Doerr, J.A., Hamilton, P.B. & Burmeister, H.R. (1981) T-2 toxicosis and blood coagulation in young chickens. Toxicol. Appl. Pharmacol., 60, 157–162.
Dugyala, R.R., Kim, Y.-W. & Sharma, R.P. (1994) Effects of aflatoxin B1 and T-2 toxin on the granulocyte–macrophage progenitor cells in mouse bone marrow cultures. Immunopharmacology, 27, 57–65.
Edrington, T.S., Kubena, L.F., Harvey, R.B. & Rottinghaus, G.E. (1997) Influence of a superactivated charcoal on the toxic effects of aflatoxin or T-2 toxin in growing broilers. Poult. Sci., 76, 1205–1211.
Eppley, R.M., Trucksess, M.W., Nesheim, S., Thorpe, C.W., Wood, G.E. & Bohland, A.E. (1984) Deoxynivalenol in winter wheat: Thin layer chromatographic method and survey. J. Assoc. Off. Anal. Chem., 67, 43–45.
Eppley, R.M., Trucksess, M.W., Nesheim, S., Thorpe, C.W., & Pohland, A.E. (1986) Thin layer chromatographic method for determination of deoxynivalenol in wheat: Collaborative study. J. Assoc. Off. Anal. Chem., 69, 37–40.
Eskola, M., Parikka, P., & Rizzo, A. (2000a) Trichothecenes, ochratoxin A and zearalenone contamination and Fusarium infection in Finnish cereal samples in 1998. Food Addit. Contam. (in press).
Eskola, M., Rizzo, A. & Soupas, L. (2000b) Occurrence and amounts of some Fusarium toxins in Finnish cereal samples in 1998. Acta Agric. Scand. Sect. B (in press).
Faifer, G.C. & Godoy, H.M. (1991) Acute effects of T-2 toxin on radioactive ion incorporation into circulating erythrocytes in mice. Toxicology, 70, 133–140.
Faifer, G.C., Zabal, O. & Godoy, H.M. (1992) Further studies on the hematopoietic damage produced by a single dose of T-2 toxin in mice. Toxicology, 75, 169–174.
Fairhurst, S., Maxwell, S.A., Scawin, J.W. & Swanston, D.W. (1987) Skin effects of trichothecenes and their amelioration by decontamination. Toxicology, 46, 307–319.
Fekete, S. & Huszenicza, G. (1993) Effects of T-2 toxin on ovarian activity and some metabolic variables of rabbits. Lab. Anim. Sci., 43, 646–649.
Fenske, M. & Fink-Gremmels, J. (1990) Effects of fungal metabolites on testosterone secretion in vitro. Arch. Toxicol., 64, 72–75.
Fernandez, C., Stack, M.E. & Musser, St.M. (1994) Determination of deoxynivalenol in 1991 US winter and spring wheat by high-performance thin-layer chromatography. J. AOAC Int., 77, 628–630.
Feuerstein, G., Goldstein, D.S., Ramwell, P.W., Zerbe, R.L., Lux, W.E., Jr, Foden, A.I. & Bayorh, M.A. (1985) Cardiorespiratory sympathetic and biochemical response to T-2 toxin in the guinea pig and rat. J. Pharmacol. Exp. Ther., 232, 786–794.
Feuerstein, G., Powell, J.A., Knower, A.T. & Hunter, K.W. (1988) Monoclonal antibodies to T-2 toxin. In vitro neutralization of protein synthesis inhibition and protection of rats against lethal toxemia. J. Clin. Invest., 76, 2134–2138.
Fitzpatrick, D.W., Boyd, K.E. & Watts, B.M. (1988) Comparison of the trichothecenes deoxynivalenol and T-2 toxin for their effect on brain biogenic monoamines in the rat. Toxicol. Lett., 40, 241–245.
Fonseca, H. (1991) [Sampling system for aflatoxins analysis in grains.] Rev. Microbiol., 21, 66–70.
Forsell, J.H. & Pestka, J.J. (1985) Relation of 8-ketotrichothecene and zearalenone analog structure to inhibitions of mitogen-induced human lymphocyte blastogenesis. Appl. Environ. Microbiol., 50, 1304–1307.
Forsell, J.H., Kateby, J.R., Yoshizawa, T. & Pestka, J.J. (1985) Inhibition of mitogen-induced blastogenesis in human lymphocytes by T-2 toxin and its metabolites. Appl. Environ. Microbiol., 49, 1524–1526.
Friend, S.C.E., Hancock, D.S. & Schiefer, H.B. (1983a) Experimental T-2 toxicosis in sheep. Can. J. Comp. Med., 47, 291–297.
Friend, S.C.E., Babuik, L.A. & Schiefer, H.B. (1983b) The effects of dietary T-2 toxin on the immunological function and Herpes simplex reactivation in Swiss mice. Toxicol. Appl. Pharmacol., 69, 234–244.
Friend, S.C.E., Schiefer, H.B. & Babuik, L.A. (1983c) The effects of dietary T-2 toxin on acute Herpes simplex virus type 1 infection in mice. Vet. Pathol., 20, 737–760.
Furlong, E. & Valente Soares, L. (1995) Gas chromatographic method for quantitation and confirmation of trichothecenes in wheat. J. AOAC Int., 78, 386–390.
Furlong, E., Valente Soares, L., Lasca, C. & Kohara, E. (1995) Mycotoxins and fungi in wheat harvested during 1990 in test plots in the state of São Paulo, Brazil. Mycopathologia, 131, 185–190.
Gao, H.-P. & Yoshizawa, T. (1997) Further study on Fusarium mycotoxins in corn and wheat from a high-risk area for human esophageal cancer in China. Mycotoxins, 45, 51–55.
Gareis, M., Hashem, A., Bauer, J. & Gedek, B. (1986) Identification of glucuronide metabolites of T-2 toxin and diacetoxyscirpenol in the bile of isolated perfused rat lever. Toxicol. Appl. Pharmacol., 84, 168–172.
Gendloff, E.H., Pestka, J.J., Dixon, D.E. & Hart, L.P. (1987) Production of a monoclonal antibody to T-2 toxin with strong cross-reactivity to T-2 metabolites. Phytopatology, 77, 57–59.
Gentry, P.A. & Cooper, M.L. (1981) Effect of Fusarium T-2 toxin on hematological and biochemical parameters in the rabbit. Can. J. Comp. Med., 45, 400–405.
Godoy, H.M., Faifer, G.C. & Velazco, V. (1997) Effects of multiple doses of T-2 toxin on the erythroid response capacity of mice following an extensive experimental bleeding. Nat. Toxins, 5, 152–156.
Gregory, J.R., Foster, K., Tyler, H. & Wiseman, M. (1990) Dietary and Nutritional Survey of British Adults, London: Her Majesty’s Stationery Office.
Gregory, J.R., Collins, D.L, Davies, P.S.W., Hughes, J.M. & Clarke, P.C. (1992) National Diet and Nutrition Survey; Children Aged 11/2 to 41/2 Years. Volume 1: Report of the Diet and Nutrition Survey, London: Her Majesty’s Stationery Office.
Groves, F.D., Zhang, L., Chang, Y.S., Ross, P.F., Casper, H., Norred, W.P., You, W.C. & Fraumeni, J.F., Jr (1999) Fusarium mycotoxins in corn and corn products in a high-risk area for gastric cancer in Shandong Province, China. J. AOAC Int., 82, 657–662.
Hack, R., Maertlbauer, E. & Terplan, G. (1989) A monoclonal antibody-based enzyme immunoassay for the detection of T-2 tocin at picogram levels. Lett. Appl. Microbiol., 9, 133–135.
Harvey R.B., Kubena L.F., Elissalde M.H., Rottinghaus G.E. & Corrier D.E. (1994) Administration of ochratoxin A and T-2 toxin to growing swine. Am. J. Vet. Res., 55, 1757–1761.
Hayes, M.A., Bellamy, J.E.C. & Schiefer, H.B. (1980) Subacute toxicity of dietary T-2 toxin in mice, morphological and hematological effects. Can. J. Comp. Med., 44, 203–218.
He, Y.M., Zhang, Z. & Jia, Z.Z. (1998) A survey of natural occurrence of T-2 toxin in wheat in Beijing. Chin. J. Food Hyg., 10, 22–24.
Hietaniemi, V. & Kumpulainen, J. (1991) Contents of fusarium toxins in Finnish and imported grains and feeds. Food Addit. Contam., 8, 171–182.
Hirayama, S. & Yamamoto, M. (1948) [Biological studies on the poisonous wheat flour (I).] Eiseishikenjo Hokoku, 66, 85–98 (in Japanese).
Hirayama, S. & Yamamoto, M. (1950) [Biological studies on the poisonous wheat flour (II).] Eiseishikenjo Hokoku, 67, 117–121 (in Japanese).
Hobden, A.N. & Cundliffe, E. (1980) Ribosomal resistance to the 12,13-epoxy-trichothecene antibiotics in the producing organism Myrothecium verrucaria. Biochem. J., 190, 765–770.
Hoefnagels, M.H. & Linderman, R.G. (1999) Biological suppression of seedborne Fusarium spp. during cold stratification of Douglas fir seeds. Plant Dis., 83, 845–852.
Hoerr, F.J., Carlton, W.W. & Yagen, B. (1981) Mycotoxicosis caused by a single dose of T-2 toxin or diacetoxyscirpenol in broiler chickens. Vet. Pathol., 18, 653–664.
Holladay, S.D., Blaylock, B.L., Comment, C.E., Hendel, J.J. & Luster, M.L. (1993) Fetal thymic atrophy after exposure to T-2 toxin, selectivity for lymphoid progenitor cells, Toxicol. Appl. Pharmacol., 121, 8–14.
Holladay, S.D., Smith, B.J. & Luster, M.I. (1995) B lymphocyte precursor cells represent sensitive targets of T-2 mycotoxin exposure, Toxicol. Appl. Pharmacol., 131, 309–315.
Home Grown Cereals Authority (2000) Survey of mycotoxins in stored grain from the 1999 harvest in the UK, Project Report No. 230. Unpublished data supplied by the United Kingdom Home Grown Cereals Authority.
Hood, R.D. (1986) Effects of concurrent prenatal exposure to rubratoxin B and T-2 toxin in the mouse. Drug Chem. Toxicol., 9, 195–190.
Hood, R.D., Kuczuk, M.H. & Szczeck, G.M. (1978) Effects in mice of simultaneous prenatal exposure to ochratoxin A and T-2 toxin. Teratology, 17, 25–30.
Hsia, C.C., Gao, Y., Wu, J.L. & Tzian, B.L. (1986) Induction of chromosome aberrations by Fusarium T-2 toxin in cultured human peripheral blood lymphocytes and Chinese hamster fibroblasts. J. Cell. Physiol., Suppl. 4, 65–72.
Hsia, C.C., Wu, J.L., Lu, X.Q. & Li, Y.S. (1988) Natural occurrence and clastogenic effects of nivalenol, deoxynivalenol, 3-acetyldeoxynivalenol, 15-acetyldeoxynivalenol, and zearalenone in maize from a high risk area of esophageal cancer. Cancer Detect. Prev., 13, 79–86.
Hunter, K.W., Brimfield, A.A., Miller, M., Finkelman, F.D. & Chu, S.F. (1985) Preparation and characterization of monoclonal antibodies to the trichothecene mycotoxin T-2. Appl. Environ. Microbiol., 49, 168–172.
Huszenicza, G., Fekete, S., Szigeti, G., Kulcsar, M., Febel, H., Kellems, R.O., Nagy, P., Cseh, S., Veresegyhazy, T. & Hullar, I. (2000) Ovarian consequences of low dose peroral Fusarium (T-2) toxin in a ewe and heifer model. Theriogenology, 53, 1631–1639.
IARC (1993) IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, Vol. 56, Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Amines and Mycotoxins, Lyon: IARCPress, pp. 467–488.
Ihara, T., Sugamata, M., Sekijima, M., Okumura H., Yoshino, N. & Ueno, Y. (1997) Apoptotic cellular damage in mice after T-2 toxin-induced acute toxicosis. Nat. Toxins, 5, 141–145.
Islam, Z., Nagase, M., Yoshizawa, T., Yamauchi, K. & Sakato, N. (1998a) T-2 toxin induces thymic apoptosis in vivo in mice. Toxicol. Appl. Pharmacol., 148, 204–214.
Islam, Z., Nagase, M., Ota, A., Ueda, S., Yoshizawa, T. & Sakato, N. (1998b) Structure–function relationship of T-2 toxin and its metabolites in inducing thymic apoptosis in vivo in mice. Biosci. Biotechnol. Biochem., 62, 1492–1497.
Iwahashi, T., Tashiro, F. & Ueno, Y. (1982) Mechanism of cytotoxic effect of T-2 toxin on a protozoan, Tetrahymena pyriformis gl. Maikotokishin, 15, 31–33.
Jacobsen, B., Harlin, S., Swanson, R., Lambert, R., Beasley, V. & Sinclair, J. (1995) Occurrence of fungi and mycotoxins associated with field mold damaged soybeans in the midwest. Plant Dis., 79, 86–89.
Janardhana, G.R., Raveesha, K.A. & Shetty, H.S. (1999) Mycotoxin contamination of maize grains grown in Karnataka (India). Food Chem. Toxicol., 37, 863–868.
Jagadeesan, V., Rukmini, C., Vijayaraghovan, M. & Tulpule, P.G. (1982) Immune studies with T-2 toxin, effect of feeding and withdrawal in monkeys. Food Chem. Toxicol., 20, 83–87.
Jewers, K. (1987) [Related problems with sample collection for mycotoxins quantification and results interpretation.] In: Second International Conference on Mycotoxins FAO/WHO/UNEP (MYC 87/8.1), Thailand, 1987.
Joffe, A.Z. (1986) Fusarium Species, Their Biology and Toxicology, New York: J. Wiley & Sons.
Johannisson, A., Bjokhag, B., Hansson, W., Gadhasson, I.L., & Thuvander, A. (1999) Effects of four trichothecene mycotoxins on activation marker expression and cell proliferation of human lymphocytes in culture. Cell Biol. Toxicol., 15, 203–215.
Johnsen, H., Odden, E., Lie, O., Johnsen, B.A. & Fonnum, F. (1986) Metabolism of T-2 toxin by rat liver carboxylesterase. Biochem. Pharmacol., 35, 1469–1473.
Jone, C., Erickson, L., Trosko, J.E. & Chang, C.C. (1987) Effect of biological toxins on gap-junctional intercellular communication in Chinese hamster V79 cells. Cell Biol. Toxicol., 3, 1–15.
Josephs, R. (1999) Development, Application and Characterisation of Analytical Methods for the Determination of Agriculturally Important Fusarium Mycotoxins, Thesis, Technical University, Vienna.
Josephs, R.D., Krska, R., Grasserbauer, M. & Broekaert, J.A.C. (1998) Determination of thrichothecene mycotoxins in wheat by use of supercritical fluid extraction and HPLC-DAD or GC-ECD. J. Chromatogr. A, 795, 297–304.
Junta Nacional de Granos (1984) Resolución No. 26120/84. [Sampling grains methodology], Buenos Aires (in Spanish).
Kamimura, H. (1989) Removal of mycotoxins during food processing. In: Natori, S., Hashimoto, K. & Ueno, Y., eds, Mycotoxins and Phycotoxins ’88, Amsterdam: Elsevier Science Publisher, pp.169–176.
Kamimura, H., Nishijima, M., Yasuda, K., Saito, K., Ibe, A., Nagayama, T., Ushiyama, H. & Naoi, Y. (1981) Simultaneous detection of several Fusarium mycotoxins in cereals, grains and foodstuffs. J. Assoc. Off. Anal. Chem., 64, 1067–1073.
Kanai, K. & Kondo, E. (1984) Decreased resistance to myobacterial infection in mice fed a trichothecene compound (T-2 toxin). Jpn. J. Med. Sci. Biol., 37, 97–104.
Karlovski, P. (1999) Biological detoxification of fungal toxins and its use in plant breeding, feed and food production. Nat. Toxins, 7, 1–23.
Kawamura, O., Satoshi, N., Sonomi, S., Ohtani, K., Yoshitugu, S., Tanaka, T. & Ueno, Y. (1990) Survey of T-2 toxin in cereals by an indirect enzyme-linked immunosorbent assay. Food Agric. Immunol., 2, 173–180.
Kempf, H.J. & Wolf, G. (1989) Erwina herbicola as a biocontrol agent of Fusarium culmorum and Puccinia recondita f. sp. Tritici on wheat. Phytopathology, 79, 990–994.
Kiessling, K., Pettersson, H., Sandholm, K. & Olsen, M. (1984) Metabolism of aflatoxin, ochratoxin, zearalenone, and three trichothecenes by intact rumen fluid, rumen protozoa, and rumen bacteria. Applied Environ. Microbiol., 47, 1070–1073.
Kotal, F., Holadova, K., Hajslova, J., Poustak, J. & Radova, Z. (1999) Determination of trichothecenes in cereals. J. Chromatogr. A, 830, 219–225.
Krivobok, S., Olivier, P., Marzin, D.R., Seiglemurandi, F. & Steinman, R. (1987) Study of the genotoxic potential of 17 mycotoxins with the SOS chromotest. Mutagenesis, 2, 433–439.
Krska, R. (1998) Performance of modern sample preparation techniques in the analysis of Fusarium mycotoxins in cereals. J. Chromatogr. A, 815, 49–57.
Krska, R., Schuhmacher, R., Weingärtner, J. & Grasserbauer, M. (1997) Modern sample preparation techniques for the analysis of Fusarium mycotoxins in cereals. Cereal Res. Communications, 25, 327–329.
Kubena, L.F., Harvey, R.B., Huff, W.E., Corrier, D.E., Phillips, T.D. & Rottinghaus, G.E. (1990) Efficacy of a hydrated sodium calcium aluminosilicate to reduce the toxicity of aflatoxin and T-2 toxin. Poult. Sci., 69, 1078–1086.
Kuczuk, M.H., Benson, P.M., Heath, H. & Hayes, A.W. (1978) Evaluation of the mutagenic potential of mycotoxins using Salmonella typhimurium and Saccharomyces cerevisiae. Mutat. Res., 53, 11–20.
Kumagai, S. & Shimizu, T. (1988) Effects of Fusarenon-X and T-2 toxin on intestinal absorption of monosaccharide in rats. Arch. Toxicol., 61, 489–495.
Lafarge-Frayssinet, C., Lespinats, G., Lafont, P., Loisillier, F., Mousset, S., Rosenstein, Y. & Frayssinet, C. (1979) Immunosuppressive effects of Fusarium cells to mitogens. Proc. Soc. Exp. Biol. Med., 160, 302–311.
Lafarge-Frayssinet, C., DeCloitre, F., Mousset, S., Martin, M. & Frayssinet, C. (1981) Induction of DNA single strand breaks by T-2 toxin, a trichothecene metabolites of Fusarium, effect on lymphoid organs and liver. Mutat. Res., 88, 115–124.
Lafarge-Frayssinet, C., Chakor, K., Lafont, P. & Frayssinet C. (1990) Transplacental transfer of T2-toxin, pathological effect. J. Exp. Pathol. Toxicol. Oncol., 10, 64–68.
Langseth, W. (2000) [A survey on the occurrence of mycotoxins in cereals on the Norwegian market.] Report from National Veterinary Institute, Norway (in Norwegian).
Langseth, W. & Rundberget, T. (1998) Instrumental methods for determination of nonmacrocyclic trichothecenes in cereals, foodstuff and cultures. J. Chromatogr. A, 815, 103–121.
Langseth, W. & Rundberget, T. (2000) The occurrence of HT-2 toxin and other trichothecenes in Norwegian cereals. Mycopathologia, 147, 157–165.
Langseth, W., Elen, O. & Rundberget, T. (2000) Occurrence of mycotoxins in Norwegian cereals 1988–1999. National Veterinary Institute, Department of Chemistry, Norway. Unpublished data submitted by Wenche Langseth.
Lansden, J.A., Cole, R.J., Dorner, J.W., Cox, R.H., Cutler, H.G. & Clark, J.D. (1978) A new trichothecene mycotoxin isolated from Fusarium tricinctum. J. Agric. Food Chem., 26, 246–249.
Lauren, D.R. & Agnew, M.P. (1991) Multitoxin screening method for fusarium mycotoxins in grains. J. Agric. Food Chem., 39, 502–507.
Lautraite, S., Parent-Massin, D., Rio, B. & Hoellinger, H. (1995) Comparison of toxicity induced by T-2 toxin on human and rat granulo-monocytic progenitors with an in vitro model. Hum. Exp. Toxicol., 14, 672–678.
Lautraite, S., Parent-Massin, D., Rio, B. & Hoellinger, H. (1996) Comparison of toxicity induced by HT-2 toxin on human and rat granulo-monocytic progenitors with an in vitro model. Hum. Exp. Toxicol., 15, 208–213.
Lawrence, J.F. & Scott, P.M. (1993) Determination of mycotoxins and phycotoxins. In: Barceló, D., ed., Environmental Analysis: Techniques, Applications and Quality Assurance, Amsterdam: Elsevier, pp. 273–309.
Leonov, A.N. (1977) Current view of the chemical nature of factors responsible for alimentary toxic aleukia. In: Rodricks, J.V., Hesseltine, C.W. & Mehlman, M.A., eds, Mycotoxins in Human and Animal Health, Park Forest South, Illinois: Pathotox Publishers, pp. 323–328.
Lew, H., Adler., A., Thimm, N., Krska,K., Wiedner G. & Schuh, M. (2000a) Occurrence of toxigenic fungi and related mycotoxins in plants, food and feed in Austria. COST 835. Unpublished. Submitted by A. Logrieco.
Lew, H., Adler, A., Thimm, N., Krska,K., Wiedner G. & Schuh, M. (2000b) Fusarium species and Fusarium toxins in Austrian maize. Bodenkultur (in press).
Li, G., Shinozuka, J., Uetsuka, K., Nakayama, H. & Doi, K. (1997) T-2 toxin-induced apoptosis in intestinal crypt epithelial cells of mice. Exp. Toxicol. Pathol., 49, 447–450.
Liao, L.L., Grollman, A.P. & Horwitz, S.B. (1976) Mechanism of action of the 12,13-epoxytrichothecene anguidine: An inhibitor of protein synthesis. Biochim. Biophys. Acta, 454, 273–284.
Ligler, F.S., Bredehorst, R., Talebian, A., Shriver, L.C., Hammer, C.F., Sheridan, J.P., Vogel, C.W. & Gaber, B.P. (1987) A homogeneous immunoassay for the mycotoxin T-2 utilizing liposomes, monoclonal antibodies, and complement. Anal. Biochem., 163, 369–375.
Lindenfelser, L.A., Lillehoj, E.B. & Burmeister, H.R. (1974) Aflatoxin and trichothecene toxins, skin tumor induction and synergistic acute toxicity in white mice. J. Natl Cancer Inst., 52, 113–116.
Linnainmaa, K., Sorsa, M. & Ilus, T. (1979) Epoxytrichothecene mycotoxins as c-mitotic agents in Allium. Hereditas, 90, 151–156.
Liu, W., Langseth, W., Skinnes, H., Elen, O.N. & Sundheim, L. (1997) Comparison of visual head blight ratings, seed infection levels, and deoxynivalenol production for assessment of resistance in cereals inoculated with fusarium culmorum. Eur. J. Plant Pathol., 103, 589–595.
Lorenzana, R.M., Beasley, V.R., Buck, W.B., Ghent, A.W., Lundren, G.R. & Poppenga, R.H. (1985) Experimental T-2 toxicosis in swine. I Changes in cardiac output, aortic mean pressure, catecholamines, 6- keto PGFa, thromboxane B2, and acid base parameters. Fundam. Appl. Toxicol., 5, 879–892.
Luo, X. (1988) Outbreaks of moldy cereals poisoning in China. In: Issues in Food Safety, Washington DC: Toxicology Forum, Inc., pp. 56–63.
Luo, Y., Yoshizawa, T. & Katayama, T. (1990) Comparative study on the natural occurrence of fusarium mycotoxins (trichothecenes and zearalenone) in corn and wheat from high- and low-risk areas for human esophageal cancer in china. Appl. Environ. Microbiol., 56, 3723–3726.
Lutsky, I. & Mor, N. (1981) Experimental alimentary toxic aleukia in cats. Lab. Anim. Sci., 31, 43–47.
Lutsky, I., Mor, N., Yagen, B. & Joffe, A.Z. (1978) The role of T-2 toxin in experimental alimentary aleukia, a toxicity study in cats. Toxicol. Appl. Pharmacol., 43, 111–124.
MacDonald, E.J., Covan, K.R. & Smith, T.K. (1988) Effect of acute oral doses of T-2 toxin on tissue concentrations of biogenic amines in the rat. J. Anim. Sci., 66, 434–441.
Malone, B.R., Humphrey, C.W., Romer, T.R. & Richard, J.L. (1998) One-step solid phase extraction clean-up and fluorometric analysis of deoxynivalenol in grains. J. AOAC Int., 81, 448–452.
Mann, D.D., Buening, G.M., Hook, B. & Osweiler, G.D. (1983) Effects of T-2 mycotoxin on bovine serum proteins. Am. J. Vet. Res., 44, 1757–1759.
Mann, D.D., Buening, G.M., Osweiler, G.D. & Hook, B. (1984) Effect of subclinical levels of T-2 toxin on the bovine cellular immune system. Can. J. Comp. Med., 48, 308–312.
Mao, W., Lumsden, R.D., Lewis, J.A. & Hebbar, P.K. (1998) Seed treatment using pre-infiltration and biocontrol agents to reduce damping-off of corn caused by species of Pythium and Fusarium. Plant Dis., 82, 294–299.
Marasas, W.F.O., Bamburg, J.R., Smalley, E.B., Strong, F.M., Ragland, W.L. & Degurse, P.E. (1969) Toxic effects on trout, rats and mice of T-2 toxin produced by the fungus Fusarium tricinctum. Toxicol. Appl. Pharmacol., 15, 471–482.
Marrs, T.C., Eddington, A.G. & Price, P.N. (1985) Experimental T-2 toxicosis in guinea pigs. Hum. Toxicol., 4,114–115.
Martin, L.J., Morse, J.D. & Anthony, A. (1986) Quantitative cytophotometric analysis of brain neuronal RNA and protein changes in acute T-2 mycotoxin poisoned rats. Toxicon, 24, 933–941.
Märtlbauer, E., Dietrich, R. & Terplan, G. (1991) [Experience with immunoassays for the determination of mycotoxins in food.] Arch. Lebensmittelhyg., 42, 3–6 (in German).
Masuko, H., Ueno, Y., Otokawa, M. & Yaginuma, K. (1977) The enhancing effect of T-2 toxin on delayed hyper-sensitivity in mice. Jpn. J. Med. Sci. Biol., 30, 159–164.
Maycock, R. & Utley, D. (1985) J. Chromatogr., 347, 429–433.
Mezes M., Barta M. & Nagy G. (1999) Comparative investigation on the effect of T-2 mycotoxin on lipid peroxidation and antioxidant status in different poultry species. Res. Vet. Sci., 66, 19–23.
Miller, J.D. (1994) Epidemiology of Fusarium ear diseases of cereals. In: Miller, J.D. & Trenholm, H.L., eds, Mycotoxins in Grain—Compounds Other than Aflatoxin, St Paul, Minnesota: Eagan Press, pp. 19–36.
Mills, C.E.N., Alcock, S.M., Lee, H.A. & Morgan, M.R.A. (1990) An enzyme-linked immunosorbent assay for deoxynivalenol in wheat, utilizing novel hapten derivatization procedures. Food Agric. Immunol., 2, 109–118.
Mirocha, C.J. & Abbas, H.K. (1989) Chemistry, occurrence and toxicology of the haemorrhagic mycotoxin (wortmannin) produced by Fusarium. In, Natori, S., Hashimoto, K. & Ueno, Y., eds, Mycotoxins and Phycotoxins, Amsterdam: Elsevier Science Publishers.
Mirocha, C.J. & Pathre, S. (1973) Identification of the toxic principle in a sample of poefusarin. Appl. Microbiol., 26, 719–724.
Mirocha, C.J., Kolaczkowski, E., Xie, W., Yu, H. & Jelen. H.-H. (1998) Analysis of deoxynivalenol and its derivatives (batch and single kernel) using gas chromatography / mass spectrometry. J. Agric. Food Chem., 46, 1414–1418.
Möller, T.E. & Gustavsson, H.F. (1992) Determination of type A and B trichothecenes in cereals by gas chromatography with electron capture detection. J. AOAC Int., 75, 1049–1053.
Mühlemann, M., Lüthy, J. & Hübner. (1997a) Mycotoxin contamination of food in Ecuador. A: Aflatoxins. Mitt. Geb. Lebensm. Hyg., 88, 474–496.
Mühlemann, M., Lüthy, J. & Hübner. (1997b) Mycotoxin contamination of food in Ecuador. B: Ochratoxin A, Deoxynivalenol, T-2 toxin and fumonisin. Mitt. Geb- Lebensm. Hyg., 88, 593–612.
Mulders, J. & Van Impelen-Peek Hilde, A.M. (1986) Gas chromatographic determination of deoxynivalenol in cereals. Z. Lebensm.Untersuchforsch., 183, 406–409.
Müller, H. & Schwadorf, K. (1993) Natural occurrence of Fusarium toxins in wheat grown in a southwestern area of Germany. Mycopathologia, 121, 115–121.
Müller, H.M., Reimann, J., Schumacher, U. & Schwadorf, K. (1997a) Fusarium toxins in wheat harvested during six years in an area of southwest Germany. Nat. Toxins, 5, 24–30.
Müller, H., Reinmann, J., Schumacher, U. & Schwadorf, K. (1997b) Natural occurrence of Fusarium toxins in barley harvested during five years in an area of southwest Germany. Mycopathologia, 137, 185–192.
Müller, H.M., Reimann, J., Schumacher, U., & Schwadorf, K. (1998) Natural occurrence of Fusarium toxins in oats harvested during five years in an area of southwest Germany. Food Addit. Contam., 15, 801–806.
Munsch, N. & Mueller, W.E.G. (1980) Effect of T-2 toxin on DNA polymerases and terminal deoxynucleotidyl transferase of Molt-4 and Nu-8 cell lines. Immunopharmacology, 2, 313–318.
Nakamura, Y., Takeda, S., Ogasawara, K., Karashimada, T. & Ando K. (1951) [A study on toxicosis caused by Akakabi poisoned wheat flour.] Hokkaido Doritsu Eiseikenkyujoho, 2, 35–46 (in Japanese).
Norkost (1997) GEMS/Food spreadsheet.
Norppa, H., Penttila, M., Sorsa, M., Hintikka, E.L. & Ilus, T. (1980) Mycotoxin T-2 of Fusarium tricinctum and chromosome changes in Chinese hamster bone marrow. Hereditas, 93, 329–332.
Ogasawara, K. (1965) [Akakabi toxicosis.] J. Food Hyg. Soc. Jpn, 6, 81–82 (in Japanese).
Ohta, M., Isii, K. & Ueno, Y. (1977) Metabolism of trichothecene mycotoxins. Part 1. Microsomal deacetylation of T-2 toxin in animal tissues. J. Biochem. (Tokyo), 82, 1591–1598.
Ohtsubo, K. & Saito, M. (1977) Chronic effects of trichothecene toxins. In: Rodricks, J.V., Hesseltine, C.W. & Mehlman, M.A., eds, Mycotoxins in Human and Animal Health, Park Forest South, Illinois: Pathotox Publishers, pp. 255–262.
Olavarria, J. (1992) [Mycotoxins quantification in grain and subproducts. Problems associated with sampling and procedure for application limits.] IPA La Platina, 67, 18–25.
Oldham, J.W., Allred, L.E., Milo, G.E., Kindig, O. & Capen, C.C. (1980) The toxicological evaluation of the mycotoxins T-2 and T-2 tetraol using normal human fibroblasts in vitro. Toxicol. Appl. Pharmacol., 52, 159–168.
Onji, Y., Aoki, Y., Tani, N., Umebayashi, K., Kitada, Y. & Dohi, Y. (1998) Direct analysis of several Fusarium mycotoxins in cereals by capillary gas chromatography–mass spectrometry. J. Chromatogr. A, 815, 59–65.
Otokawa, M., Shibahara, Y. & Egashira, Y. (1979) The inhibitory effect of T-2 toxin on tolerance induction of delayed-type hypersensitivity in mice. Jpn. J. Med. Sci. Biol., 32, 37–46.
Pace, J.D. (1986) Metabolism and clearance of T-2 mycotoxin in perfused rat livers. Fundam. Appl. Toxicol., 7, 424–433.
Pace, J.G., Watts, M.R., Burrows, E.P., Dinterman, R.E., Matson, C., Hauer, E.C. & Wannemacher, R.W., Jr (1985) Fate and distribution of 3H labeled T-2 mycotoxin in guinea pigs. Toxicol. Appl. Pharmacol., 80, 377–385.
Pang, V.F., Lorenzana, R.M., Beasley, V.R., Buck, W.B. & Haschek, W.M. (1987a) Experimental T-2 toxicosis in swine. III. Morphologic changes following intravascular administration of T-2 toxin. Fundam. Appl. Toxicol., 8, 298–309.
Pang, V.F., Lambert, R.J., Felsburg, P.J., Beasley, V.R., Buck, W.B. & Haschek, W.M. (1987b) Experimental T-2 toxicosis in swine following inhalation exposure, effects on pulmonary and systemic immunity and morphologic changes. Toxicol. Pathol., 15, 308–319.
Pang, V.F., Lambert, R.J., Felsburg, P.J., Beasley, V.R., Buck, W.B. & Haschek, W.M. (1988) Experimental T-2 toxicosis in swine following inhalation exposure, clinical signs and effects on hematology, serum biochemistry and immune response. Fundam. Appl. Toxicol., 11, 100–109.
Parent-Massin, D. & Thouvenot, D. (1995) In vitro toxicity of trichothecenes on rat haematopoietic progenitors. Food Addit. Contam., 12, 41–49.
Parent-Massin, D., Fuselier, R. & Thouvenot, D., (1994) In vitro toxicity of trichothecenes on human haematopoietic progenitors. Food Addit. Contam., 11, 441–447.
Park, J.J. & Chu, F.S. (1996) Assessment of immunochemical methods for the analysis of trichothecene mycotoxins in naturally occurring moldy corn. J. AOAC Int., 79, 465–471.
Park, J.C., Zong, M.S. & Chang, I.-M. (1991) Survey of the presence of the fusarium mycotoxins nivalenol, deoxynivalenol and T-2 toxin in Korean cereals of the 1989 harvest. Food Addit. Contam., 8, 447–451.
Park, J.J., Smalley, E.B. & Chu, F. S. (1996) Natural occurrence of Fusarium mycotoxins in field samples from the 1992 Wisconsin corn crop. Appl. Environ. Microbiol., 62, 1642–1648.
Parry, D.W., Jenkinson, P. & McLeod, L. (1995) Fusarium ear blight (scab) in small grain cereals: A review. Plant Pathol., 44, 207–238.
Patel, S., Hazel, C.M., Winterton, A.G. & Mortby, E. (1996) Survey of ethnic foods for mycotoxins. Food Addit. Contam., 13, 833–841.
Patey, A.L. & Gilbert, J. (1989) Fate of Fusarium mycotoxins in cereals during food processing and methods for their detoxification. In: Chenkowski, J., ed., Fusarium: Mycotoxins, Taxonomy and Pathogenicity, Amsterdam: Elsevier, pp. 399–420.
Patterson, D.S.P., Matthews, J.G., Shreeve, B.J., Roberts, B.A., McDonald, S.M. & Hayes, A.W. (1979) The failure of trichothecene mycotoxins and whole cultures of Fusarium tricinctum to cause experimental haemorrhagic syndromes in calves and pigs. Vet. Rec., 105, 252–253.
Paucod, J.C., Krivobok, S. & Vidal, D. (1990) Immunotoxicity testing of mycotoxins T-2 and patulin on BALB/c mice. Acta Microbiol. Hung., 37, 331–339.
Pawlosky, R.J. & Mirocha, C.J. (1984) Structure of a metabolic derivative of T-2 toxin (TC-6) based on mass spectrometry. J. Agric. Food Chem., 32, 1420–1423.
Pettersson, H. (1992) Trichothecene analysis in Swedish cereals 1987–1990. In: Proceedings of the 3rd European Seminar on Fusarium-Mycotoxins, Taxonomy, Pathogenicity and Host Resistance, 22–24 September 1992, Radzikow: Plant Breeding and Acclimatization Institute, pp. 37–41.
Pettersson, H. (2000) Department of Animal Nutrition and Management at the Agricultural University, Sweden. Unpublished data submitted to WHO and FAO, GEMS/FOOD file.
Pfeiffer, R.L., Swanson, S.P. & Buck, W.B. (1988) Metabolism of T-2 toxin in rats, effects of dose, route, and time. J. Agric. Food Chem., 36, 1227–1232.
Pier, A.C., Cysewski, S.J., Richard, J.L., Baetz, A.L. & Mitchell, L. (1976) Experimental mycotoxicoses in calves with aflatoxin, ochratoxin, rubratoxin, and T-2 toxin. In, Proceedings of the 80th Annual Meeting of the US Animal Health Association, Miami Beach, Florida, 7–12 November 1976, Richmond, Virginia: US Animal Health Association, pp. 130–148.
Pineiro, M., Scott, P.M. & Kanhere, S.R. (1996) Mycotoxin producing potential of Fusarium graminearum isolates from Uruguayan barley. Mycopathologia, 132, 167–172.
Plattner, R.D., Beremand, M.N. & Powell, R.G. (1989) Analysis of trichothecene mycotoxins by mass spectrometry and tandem mass spectrometry. Tetrahedron, 45, 2251–2262.
Prado, G., Oliveira, M., Ferreira, S., Corrêa, T. & Affonso, B. (1997) [Natural occurrence of deoxynivalenol and T-2 toxin in post harvest corn.] Cienc. Tecnol. Aliment., 17, 259–262 (in Portuguese).
Quiroga, N., Resnik, S.L., Pacin, A., Martínez, E., Pagano, A., Riccobene, I. & Neira, S. (1995) Natural occurrence of trichothecenes and zearalenone in Argentinean wheat. Food Control, 6, 201–204.
Rafai, P. & Tuboly, S. (1982) Effect of T-2 toxin on adrenocortical function and immune response in growing pigs. Zbl. Vet. Med., B29, 558–565.
Rafai, P., Bata, A., Vanyi, A., Papp, Z., Brydl, E., Jakab, L., Tuboly, S. & Tury, E. (1995a) Effect of various levels of T-2 toxin on the clinical status, performance and metabolism of growing pigs. Vet. Rec., 136, 485–489.
Rafai, P., Tuboly, S., Bata, A., Tilly, P., Vanyi, A., Papp, Z., Jakab, L. & Tury, E. (1995b) Effect of various levels of T-2 toxin in the immune system of growing pigs. Vet. Rec., 136, 511–514.
Rajakylä, E., Laasasenaho, K. & Sakkers, P.J.D. (1987) Determination of mycotoxins in grain by high-performance liquid chromatography and thermospray liquid chromatography–mass spectrometry. J. Chromatogr., 384, 391–402.
Rava, E. (1996) Mycotoxins in maize products of the 1994–1995 marketing season. Mycotoxin Res., 12 , 25–30.
Rava, E., Viljoen, J.H., Kailmeyer, H. & de Jager, A. (1996) Fungi and mycotoxins in South African maize of the 1993 crop. Mycotoxin Res., 12, 15–24.
Razzazi-Fazeli, E., Böhm, J. & Luf, W. (1999) Determination of nivalenol and deoxynivalenol in wheat using liquid chromatography-–mass spectrometry with negative ion atmospheric pressure chemical ionisation. J. Chromatogr. A, 854, 45–55-
Reutter, M. (1999) Zearalenone and deoxynivalenol in cereals and feed stuffs of Schleswig-Holstein: Investigation of the harvest 1998. In: Proceedings of the 21st Mycotoxin Workshop, 7–9 June 1999, Jena, Germany, Jena: Gesellschaft für Mykotoxinforschung e.V., Bundesinstitut für gesundheitlichen Verbraucherschutz und Veterinärmedizin.
Ridascreen DON (2000) [Enzyme immunoassay for the quantitative determination of deoxynivalenol] (Art.No. 2901), R-Biopharm GmbH, Darmstadt, Germany (in German).
Rio, B., Lautraite, S. & Parent-Massin, D. (1997) In vitro toxicity of trichothecenes on human erythroblastic progenitors. Hum. Exp. Toxicol. 16, 673–679.
Robison, T.S., Mirocha, C.J., Kurtz, H.J., Behrens, J.C., Weaver, G.A. & Chi, M.S. (1979a) Distribution of tritium labeled T-2 toxin in swine. J. Agric. Food Chem., 27, 1411–1413.
Robison, T.S., Mirocha, C.J., Kurtz, H.J., Behrens, J.C., Chi, M.S., Weaver, G.A. & Nystrom, S.D. (1979b) Transmission of T-2 toxin into bovine and porcine milk. J. Dairy Sci., 62, 637–641.
Romer, T.R. (1986) Use of small charcoal/alumina cleanup columns in determination of trichothecene mycotoxins in foods and feeds. J. Assoc. Off. Anal. Chem., 69, 699–703.
Rosen, R.T. & Rosen, J.D. (1983) Quanitification and confirmation of four fusarium mycotoxins in corn by gas chromatography–mass spectrometry–selected ion monitoring. J. Chromatogr., 283, 223–230.
Rosenstein, Y. & Lafarge-Frayssinet, C. (1983) Inhibitory effect of Fusarium T-2 toxin on lymphoid DNA and protein synthesis. Toxicol. Appl. Pharmacol., 70, 283–288.
Rosenstein, Y., Lafarge-Frayssinet, C., Lespinats, G., Loisillier, F., Lafont, P. & Frayssinet, C. (1979) Immunosuppressive activity of Fusarium toxins. Effects on antibody synthesis and skin grafts of crude extracts of T-2 toxin and diacetoxyscirpenol. Immunology, 36, 111–118.
Rousseaux, C.G. & Schiefer, H.B. (1987) Maternal toxicity, embryolethality and abnormal fetal development in CD-1 mice following one oral dose of T-2 toxin. J. Appl. Toxicol., 7, 281–288.
Rousseaux, C.G., Schiefer, H.B. & Hancock, D.S. (1986) Reproductive and teratological effects of continuous low-level dietary T-2 toxin in female CD-1 mice for two generations. J. Appl. Toxicol., 6, 179–184.
Rukmini, C., Prasad, J.S. & Rao, K. (1980) Effect of feeding T-2 toxin to rats and monkeys. Food Cosmet. Toxicol., 18, 267–270.
Ryu, J.C., Yang, J.S., Song, Y.S., Kwon, O.S., Park, J. & Chang, I.M. (1996) Survey of natural occurrence of trichothecene mycotoxins and zearalenone in Korean cereals harvested in 1992 using gas chromatography/mass spectrometry. Food Addit. Contam., 13, 333–341.
Saito, M. & Ohtsubo, K. (1974) Trichothecene toxins of Fusarium species. In: Purchase, I.F.H., ed., Mycotoxins, Amsterdam: Elsevier Science Publishers, pp. 263–281.
Sano, A., Satoshi, M., Masao, S. & Shoji, T. (1987) High-performance liquid chromatographic method for determining trichothecene mycotoxins by post-column fluorescence derivatization. J. Chromatogr., 410, 427–436.
Sarkisov, A.C., Kvaschina, E.S., Korneev, N.E., Koroleva, V.P., Gerasimova, P.N. & Akilova, N.S. (1944) [Harmfulness of cereals over-wintered in the field.] Veterinariya, 11–12, 39–41 (in Russian).
Sato, N., Ueno, Y. & Enomoto, M. (1975) Toxicological approaches to the toxic metabolites of Fusaria. Part 8. Acute and subacute toxicities of T-2 toxin in cats. Jpn. J. Pharmacol., 25, 263–270.
Saubois, A., Nepote, M.C. & Basilico, J.C. (1992) Incidence of Fusarium toxins in corn and milling byproducts Arch. Latinoam. Nutr., 42, 168–172.
Schappert, K.T. & Khachatourians, G.C. (1986) Effects of T-2 toxin on induction of petite mutants and mitochondrial function in Saccharomyces cerevisiae. Curr. Genet., 10, 671–679.
Schiefer, H.B., Rousseaux, C.G., Hancock, D.S. & Blakley, B.R. (1987) Effects of low-level long-term oral exposure to T-2 toxin in CD-1 mice. Food Chem. Toxicol., 25, 593–601.
Schollenberger, M., Lauber, U., Terry Jara, H., Suchy, S., Drochner, W. & Müller, H.-M. (1998) Determination of eight trichothecenes by gas chromatography–mass spectrometry after sample clean-up by two-stage solid phase extraction. J. Chromatogr. A, 815, 123–132.
Schollenberger, M., Suchy, S., Jara, H.T., Drochner, W. & Muller, H.M. (1999) A survey of Fusarium toxins in cereal-based foods marketed in an area of southwest Germany. Mycopathologia, 147, 49–57.
Schollenberger, M., Jara, H.T., Suchy, S., Drochner, W. & Muller, H.M. (2000a) Fusarium toxins in wheat flour collected in an area of southwest Germany. Institute of Animal Nutrition, Hohenheim, Stuttgart, Germany. Unpublished data submitted to WHO and FAO by U. Lauber.
Schollenberger, M., Suchy, S., Jara, H.T., Rüfle, M., Drochner, W. & Muller, H.M. (2000b) Further survey of Fusarium toxins in cereal-based foods marketed in an area of southwest Germany. Institute of Animal Nutrition, Hohenheim, Stuttgart, Germany. Unpublished data submitted to WHO and FAO by U. Lauber.
Schollenberger, M., Jara, H.T., Suchy, S., Drochner, W. & Muller, H.M. (2000c)Occurrence of Fusarium toxins in wheat for food use harvested 1998 in southwest Germany. Institute of Animal Nutrition, Hohenheim, Stuttgart, Germany. Unpublished data submitted to WHO and FAO by U. Lauber.
Schuhmacher, R., Apfalter, S., Krska, R. & Grasserbauer, M. (1996) Quality assurance for the analysis of the mycotoxins deoxynivalenol in cereals. In: Proceedings der österreich Lebensmittelchemikertage 1996.
Schwadorf, K. & Müller, H.M. (1991) Determination of trichothecenes in cereals by gas chromatography with ion trap detection. J. Chromatogr., 32, 137–142.
Scott, P. (1991) Possibilities of reduction or elimination of mycotoxins present in cereal grain. In: Chenkowski, J., ed., Cereal Grain: Mycotoxins, Fungi and Quality in Drying and Storage, Amsterdam: Elsevier, pp. 529–572.
Scott, P.M. (1997) Multi-year monitoring of Canadian grains and grain-based foods for trichothecenes and zearalenone. Food Addit. Contam., 14, 333–339.
Scott, P.M. & Trucksess, M.W. (1997) Application of immunoaffinity columns to mycotoxin analysis. J. AOAC Int., 80, 941–949.
Scott, P.M., Lawrence, J.W. & van Walbeek, W. (1970) Detection of mycotoxins by thin-layer chromatography: Application to screening of fungal extracts. Appl. Microbiol., 20, 839–842.
Scott, P.M., Kanhere, S.R. & Tarter, E.J. (1986) Determination of nivalenol and deoxynivalenol in cereals by electron-capture gas chromatography. J. Assoc. Off. Anal. Chem., 69, 889–893.
Scott, P.M., Lombaert, G.A., Pellaers, P., Bacler, S., Kanhere, S.R., Sun, W.F., Lau, P.-Y. & Weber, D. (1989) Application of capillary gas chromatography to a survey of wheat for five trichothecenes. Food Addit. Contam., 6, 489–500.
Scott, P.M., Kanhere, S.R. & Weber D. (1993) Analysis of Canadian and imported beers for Fusarium mycotoxins by gas chromatography–mass spectrometry. Food Addit. Contam., 10, 381–389.
Scudamore, K.A., Nawaz, S. & Hetmanski, M.T. (1998) Mycotoxins in ingredients of animal feeding stuffs: II. Determination of mycotoxins in maize and maize products. Food Addit. Contam., 15, 30–55.
Seidel, V., Lang, B., Fraissler, S., Lang, C., Schiller, K., Filek, G. & Lindner W. (1993) Analysis of trace levels of trichothecene mycotoxins in Austrian cereals by gas chromatography with electron capture detection. Chromatographia, 37, 191–200.
Shepherd, M.J. & Gilbert, J. (1988) Long-term storage stability of deoxynivalenol standard reference solutions. J. Agric. Food Chem., 36, 305–308.
Shinozuka, J., Li, G., Uetsuka, K., Nakayama, H. & Doi, K. (1997a) Process of the development of T-2 toxin-induced apoptosis in the lymphoid organs of mice. Exp. Anim., 46, 117–126.
Shinozuka, J., Li, G., Kiatipattanasakul, W., Uetsuka, K., Nakayama, H. & Doi, K. (1997b) T-2 toxin-induced apoptosis in lymphoid organs of mice. Exp. Toxicol. Pathol., 49, 387–392.
Shinozuka, J., Suzuki, M., Noguchi, N., Sugimoto, T., Uetsuka, K., Nakayama, H. & Doi, K. (1998) T-2 toxin-induced apoptosis in hematopoietic tissues of mice. Toxicol. Pathol., 26, 674–681.
Shinozuka, J., Tsutsui, S., Ishigami, N., Ueno-Yamanouchi, A., Nakayam, N. & Doi, K. (1999) Development of apoptosis and changes in apoptosis-related genes expression in the mouse thymus following T-2 toxin-inoculation. J. Toxicol. Pathol., 12, 77–81.
Sinha, R.C., Savard, M.E. & Laum, R. (1995) Production of monoclonal antibodies for the specific detection of deoxynivalenol and 15-acetyldeoxynivalenol by ELISA. J. Agric. Food Chem., 43, 1740–1744.
Sinovec, S. & Jovanovic, M. (1993) Development of pathomorphologic alterations in the liver, kidney and heart of rats treated with T-2 toxin. Acta Vet. (Beograd), 43, 1555–1564.
Sintov, A., Bialer, M. & Yagen, B. (1986) Pharmacokinetics of T-2 toxin and its metabolite HT-2 toxin, after intravenous administration in dogs. Drug Metab. Disposition, 14, 250–254.
Sintov, A., Bialer, M. & Yagen, B. (1987) Pharmacokinetics of T-2 tetraol, a urinary metabolite of the trichothecene mycotoxin, T-2 toxin, in dogs. Xenobiotica, 17, 941–950.
Sintov, A., Bialer, M. & Yagen, B. (1988) Pharmacokinetics and protein binding of trichothecene mycotoxins, T-2 toxin and HT-2 toxin, in dogs. Toxicon, 26, 153–160.
Siren, A.L. & Feuerstein, G. (1986) Effect of T-2 toxin on regional blood flow and vascular resistance in the conscious rat. Toxic. Appl. Pharmacol., 38, 438–444.
Sirkka, U., Nieminen, S.A. & Ylitalo, P. (1992) Acute neurobehavioral toxicity of trichothescene T-2 toxin in the rat, Pharmacol. Toxicol., 70, 111–114.
Soares, L.M. & Furlani, R. (1996) Survey of mycotoxins in wheat and wheat products sold in health food stores of the city of Campinas, State of Sao Paulo. Rev. Microbiol., 27, 41–45.
Sorsa, M., Linssainmaa, K., Paittela, M. & Ilus, T. (1980) Evaluation of the mutagenicity of epoxytrichothecene mycotoxins in Drosophila melanogaster. Hereditas, 92, 163–165.
Stafford, M.E. & McLaughlin, C.S. (1973) Trichodermin, a possible inhibitor of the termination process of protein synthesis. J. Cell. Physiol., 82, 121–128.
Stanford, G.K., Hood, R.D. & Hayes, A.W. (1975) Effect of prenatal administration of T-2 toxin to mice. Res. Commun. Chem. Pathol. Pharmacol., 10, 743–746.
Stenwig, H., Langseth, W. & Sandli, D. (1992) The occurrence of thrichothecenes and moulds in barley and oats sampled in Norway. In: Proceedings of the 3rd European Seminar on Fusarium-Mycotoxins, Taxonomy, Pathogenicity and Host Resistance, 22–24 September 1992, Radzikow: Plant Breeding and Acclimatization Institute, pp. 42–50.
Steyn, P.S., Thiel, P.G. & Trinder, D.W. (1991) In: Smith, J.E. & Henderson, R.S., eds, Mycotoxins and Animal Foods, Boca Raton, Florida: CRC Press Inc., pp. 165–221.
Stoloff, L. (1997) Mycotoxicology Newsletter VIII, No. 2.
Stratton, G.W., Robinson, A.R., Smith, H.C., Kittilsen, L. & Barbour, M.. (1993) Levels of five mycotoxins in grains harvested in Atlantic Canada as measured by high performance liquid chromatography. Arch. Environ. Contam. Toxicol., 24, 399–409.
Sugamata, M., Hattori, T., Ihara, T., Okumura, H., Yoshino, N. & Ueno, Y. (1998) Fine structural changes and apoptotic cell death by T-2 mycotoxin. J. Toxicol. Sci., 23 (Suppl. 2), 148–154.
Sugita-Konishi, Y., Hara, K.Y., Kasuga, F. & Kumagai, S. (1998) The effects of trichothecenes on host defense against infectious diseases. Maikotokishin (Tokyo), 47, 19–23.
Swanson, S.P., Dahlem, A.M., Rood, H.D., Côte, L.-M., Buck, W.B. & Yoshizawa, T. (1986) Gas chromatographic analysis of milk for deoxynivalenol and its metabolite dom-1. J. Assoc. Off. Anal. Chem., 69, 41–43.
Swanson, S.P., Nicoletti, J., Rood, H.D., Jr, Buck, W.B., Cote, L.M. & Yoshizawa, T. (1987) Metabolism of three trichothecene mycotoxins, T-2 toxin, diacetoxyscirpenol and deoxynivalenol, by bovine rumen microorganisms. J. Agric. Food Chem., 32, 1181–1183.
Sydenham, E.W. & Thiel, P.G. (1987) The simultaneous determination of diacetoxyscirpenol and T-2 toxin in fungal cultures and grain samples by capillary gas chromatography. Food Addit. Contam., 4, 277–284.
Sylvia, V.L., Phillips, T.D., Clemint, B.A., Green, J.L., Kubena, L.F. & Heidelbaugh, N.D. (1986) Effect of vesicular arbuscular mycorrhizal fungi and phosphorus on the survival and growth of flowering dogwood (cornus-Florida) J. Chromatogr., 362, 79–85.
Tacke, B.K. & Casper, H.H. (1996) Determination of deoxynivalenol in wheat, barley and malt by column cleanup and gas chromatography with electron capture detection. J. AOAC Int., 79, 472–475.
Tai, J.H. & Pestka, J.J. (1988) Impaired murine resistance to salmonella typhimurium following oral exposure to the trichothecene T-2 toxin. Food Chem. Toxicol., 26, 691–698.
Takahashi , H., Osada, K., Yazaki, H. & Kimura, S. (1992) [Detection of mutagenic activity of mycotoxins by Salmonella typhimurium/microsome assay and ultra-weak chemiluminescence.] Nippon Eiyo Shokuryo Gakkaishi, 45, 169–173 (in Japanese).
Tanaka, T., Hasegawa, A., Matsuki, Y., Ishii, K. & Ueno Y. (1985) Improved methodology for the simultaneous detection of the trichothecene mycotoxins deoxynivalenol and nivalenol in cereals Food Addit. Contam., 2, 125–137.
Tanaka, T., Akihiko, H., Matsuki, Y., Lee, U.S. & Ueno, Y. (1986) A limited survey of fusarium mycotoxins nivalenol, deoxynivalenol and zearalenone in 1984 UK harvested wheat and barley. Food Addit. Contam., 3, 247–252.
Tanaka, T., Yamamoto, S., Akihiko, H., Aoki, N., Besling, J.R., Yoshitsugu, S. & Ueno, Y. (1990) A survey of the natural occurrence of fusarium mycotoxins, deoxynivalenol, nivalenol and zearalenone, in cereals harvested in the Netherlands. Mycopathologia, 110, 19–22.
Thompson, W.L. & Wannemacher, R.W., Jr (1986) Structure–function relationships of 12,13-epoxytrichothecene mycotoxins in cell culture, comparison to whole animal lethality. Toxicon, 24, 985–994.
Thompson, W.L. & Wannemacher, R.W., Jr (1990) In vivo effects of T-2 mycotoxin on synthesis of proteins and DNA in rat tissues, Toxicol. Appl. Pharmacol., 105, 483–491.
Thurman, J.D., Creasia, D.A., Quance, J.L. & Johnson, A.J. (1986) Adrenal cortical necrosis caused by T-2 mycotoxicosis in female, but not male, mice. Am. J. Vet. Res., 47, 1122–1124.
Thust, R., Kneist, S. & Huehne, V. (1983) [Genotoxicity of Fusarium mycotoxins (nivalenol, T-2 toxin and zearalenone) in Chinese hamster V79-E cells in vitro.] Arch. Geschwulstforsch., 53, 9–15 (in German).
Thuvander, A., Wikman, C. & Gadhasson, I. (1999) In vitro exposure of human lymphocytes to trichothecenes, individual variation in sensitivity and effects of combined exposure on lymphocyte function. Food Chem. Toxicol., 37, 639–648.
Thuvander, A., Möller, T., Enghardt Barbieri, H., Janson, A., Salomonsson, A.C. & Olsen, M. (2000) Dietary intake of some important mycotoxins by the Swedish population. Food Addit. Contam. (in press).
Tochinai, Y. (1933) [On scab of cereals and grains. I and II.] Bochugai Zassi, 20, 106–114, 175–188 (in Japanese).
Tomar, R.S., Blakley, B.R. & Coteau, W.E. (1988) In vitro effects of T-2 toxin on the mitogen responsiveness and antibody-producing ability of human lymphocytes. Toxicol. Lett., 40, 109–117.
Trenholm, H.L., Warner, R.M. & Prelusky, D.B. (1985) Assessment of extraction procedures in the analysis of naturally contaminated grain products deoxynivalenol (vomitoxin). J. Assoc. Off. Anal. Chem., 68, 645–649.
Trucksess, M.W. (1995) Mycotoxins. J. AOAC Int., 78, 135–141.
Trucksess, M.W., Nesheim, S. & Eppley, R.M. (1984) Thin layer chromatographic determination of deoxynivalenol in wheat and corn. J. Assoc. Off. Anal. Chem., 67, 40–43.
Trucksess, M.W., Flood, M.T., Mossoba, M.M. & Page, S.W. (1987) High-performance thin-layer chromatographic determination of deoxynivalenol, fusarenon-X, and nivalenol in barley, corn, and wheat. J. Agric. Food Chem., 35, 445–448.
Trusal, L.R. (1985) Morphological changes in CHO and Vero cells treated with T-2 mycotoxin. Correlation with inhibition of protein synthesis. Cell Biochem. Funct., 3, 205–216.
Trusal, L.R. & O’Brien, J.C. (1986) Ultrastructural effects of T-2 mycotoxins on rat hepatocytes in vitro. Toxicon, 24, 481–488.
Tsunoda, H., Tsuruta, O., Matsunami, S. & Ishii, S. (1957) [Studies on the microorganisms which deteriorate the stored cereals and grains. XIV.] Shokuryokenkyujo Hokoku, 12, 27–33 (in Japanese).
Ueno, Y. (1984) Toxicological features of T-2 toxin and related trichothecenes. Fundam. Appl. Toxicol., 4, S124–S132.
Ueno, Y. & Kubota, K. (1976) DNA-attacking ability of carcinogenic mycotoxins in recombination-deficient mutant cells of Bacillus subtilis. Cancer Res., 36, 445–451.
Ueno, Y., Ishii, K., Sato, N., Shimada, N., Tsunoda, H., Sawano, M. & Enomoto, M. (1973a) Screening of trichothecene producing fungi and the comparative toxicity of isolated mycotoxins. Jpn. J. Pharmacol., 23 (Suppl.), 133.
Ueno, Y., Sato, N., Ishii, K., Sakai, K., Tsunoda, H. & Enomoto, M. (1973b) Biological and chemical detection of trichothene mycotoxins of Fusarium species. Appl. Microbiol., 25, 699–704.
Usleber, E., Renz, V., Märtlbauer, E. & Terplan, G. (1992) Studies on the application of enzyme immunoassays for the fusarium mycotoxins deoxynivalenol, 3-acetyldeoxynivalenol, and zearalenone. J. Vet. Med.B., 39, 617–627.
Vega, H.M., Saelzer, F.R., Herlitz, B.E., Rios, G.G., Bastias, T.C., Olavarria, M.J., Madariaga, B.R. & Rebufel, A.P. (1998) [Mycotoxins from Fusarium spp. In corn grown in Chile. 1995–1996 harvest.] Alimentos, 23, 43–58 (in Spanish).
Velazco, V., Faifer, G.C. & Godoy, H.M. (1996) Differential effects of T-2 toxin on bone marrow and spleen erythropoiesis in mice. Food Chem. Toxicol., 34, 371–375.
Veldman, A., Borggreve, G.G., Mulers, E.J. & Van de Lagemaat, D. (1992) Occurrence of the mycotoxins ochratoxin A, zearalenone and deoxynivalenol in feed components. Food Addit. Contam., 9, 647–655.
Venturini, M.C., Quiroga, M.A., Risso, C., Di Lorenzo, Y., Omata, L. & Godoy, H. (1996) Mycotoxin T-2 and aflatoxin B1 as immunosuppressors in mice chronically Infected with Toxoplasma gondii. J. Comp. Pathol., 115, 229–237.
Veratox test, Neogene Corporation, 1998, USA/Canada
Vesonder R.F., Ciegler, A. & Jensen, A.H. (1973) Isolation of the emetic principle from Fusarium infected corn. Appl. Environ. Microbiol., 26, 1008.
Visconti, A. & Mirocha, C.J. (1985) Identification of various T-2 toxin metabolites in chicken excreta and tissues. Appl. Environ. Microbiol., 49, 1246–1250.
Vrabcheva, T., Gessler, R., Usleber, E. & Martlbauer, E. (1996) First survey on the natural occurrence of Fusarium mycotoxins in Bulgarian wheat. Mycopathologia, 136, 47–52.
Wang, J., Fitzpatrick, D.W. & Wilson, J.R. (1993a) Effect of dietary T-2 toxin on biogenic monoamines in discrete areas of the rat brain. Food Chem. Toxicol., 31, 191–197.
Wang, Z.G., Feng, J.N. & Tong, Z.J. (1993b) Human toxicosis caused by moldy rice contaminated with fusarium and T-2toxin. Biomed. Environ. Sci., 6, 65–70.
Wang, J., Fitzpatrick, D.W. & Wilson, J.R. (1998a) Effects of the trichothecene mycotoxin T-2 toxin on neurotransmitters andmetabolites in discrete areas of the rat brain. Food Chem. Toxicol., 36, 947–953.
Wang, J., Fitzpatrick, D.W. & Wilson, J.R. (1998b) Effects of T-2 toxin on blood–brain barrier permeability monamine oxidase activity and protein synthesis in rats. Food Chem. Toxicol., 36, 955–961.
Weaver, G.A., Kurtz, H.J., Bates, F.Y., Chi, M.S., Mirocha, C.J., Behrens, J.C. & Robison, T.S. (1978) Acute and chronic toxicity of T-2 mycotoxin in swine. Vet. Rec., 103, 531–535.
Wehner, F.C., Marasas, W.F.O. & ThieL, P.G. (1978) Lack of mutagenicity to Salmonella typhimurium of some Fusarium mycotoxins. Appl. Environ. Microbiol., 35, 659–662.
Weingärtner, J., Krska, R., Praznik, W., Grasserbauer, M. & Lew, H. (1997) Use of Mycosep multifunctional clean-up columns for the determination of trichothecenes in wheat by electron-capture gas chromatography. Fresenius J. Anal. Chem., 357, 1206–1210.
WHO (1985) Guidelines for the Study of Dietary Intakes of Chemical Contaminants. WHO Offset Publication No. 87. Geneva.
WHO (1990) Selected Mycotoxins: Ochratoxins, Trichothecenes, Ergot (Environmental Health Criteria 105), Geneva.
WHO (1998) GEMS/Food Regional Diets. Regional Per Capita Consumption of Raw and Semi-processed Agricultural Commodities. WHO/FSF/FOS/98.3. Geneva.
WHO (2000) Report on the Joint FAO/WHO Workshop on Methodology for Exposure Assessment of Contaminants and Toxins, 7–8 June 2000. Geneva.
Wilson, D.J. & Gentry, P.A. (1985) T-2 toxin can cause vasoconstriction in an in vitro bovine ear perfusion system. Toxicol. Appl. Pharmacol., 79, 159–165.
Wyatt, R.D., Harris, J.R., Hamilton, P.B. & Burmeister, H.R. (1972) Possible outbreaks of fusariotoxicosis in avians. Avian Dis., 16, 1123–1130.
Wyatt, R.D., Hamilton, P.B. & Burmeister, H.R. (1973) Effects of T-2 toxin in broiler chickens. Poult. Sci., 52, 1853–1859.
Wyatt, R.D., Doerr, J.A., Hamilton, P.B. & Burmeister, H.R. (1975) Egg production shell thickness and other physiological parameters of laying hens affected by T-2 toxin. Appl. Microbiol., 29, 641–645.
Xu, Y.C., Zhang, G.S. & Chu, F.S. (1988) Enzyme-linked immunosorbent assay for deoxynivalenol in corn and wheat. J. Assoc. Off. Anal. Chem., 71, 946–949.
Yang, C.H. & Luo, X.Y. (2000) Method for determination of T-2 toxin in wheat with indirect enzyme-linked immunosorbent assay (ELISA), via e-mail
Yang, S. & Xia, Q.J. (1988a) Papilloma of forestomach induced by Fusarium T-2 toxin in mice. Chin. J. Oncol., 10, 339–341.
Yang, S. & Xia, Q.J. (1988b) Studies on the promoting and initiating effects of Fusarium T-2 toxin. Chin. J. Oncol., 17, 107–110.
Yang, C.H., Luo, X.Y., Ji, R. & Liu, C. (1992) A survey of natural occurrence of T-2 toxin in wheat. Acta Microbiol. Sin., 32, 450–455.
Yarom, R., More, R., Sherman, Y. & Yagen, B. (1983) T-2 toxin-induced pathology in the heart of rats. Br. J. Exp. Pathol., 64, 570–577.
Yarom, R., More, R., Eldor, A. & Yagen, B. (1984a) The effect of T-2 toxin on human platelets. Toxicol. Appl. Pharmacol., 73, 210–217.
Yarom, R., Sherman, Y., More, R., Ginsburg, I., Borinski, R. & Yagen, B. (1984b) T-2 toxin effect on bacterial infection and leukocyte functions. Toxicol. Appl. Pharmacol., 75, 60–68.
Yarom, R., Sherman, Y., Bergmann, F., Sintor, A. & Berman, L.D. (1987) T-2 toxin effect on rat aorta: Cellular changes in vivo and growth of smooth muscle cells in vitro. Exp. Mol. Pathol., 47, 143–153.
Yoshino, N., Takizawa, M., Tashiro, F., Honda, M. & Ueno, Y. (1997) T-2 toxin induced apoptosis in human peripheral blood lymphocytes in vitro. Res. Commun. Biochem. Cell Mol. Biol., 1, 218–227.
Yoshizawa, T., Swanson, S.P. & Mirocha, C.J. (1980) T-2 metabolites in the excreta of broiler chickens administered 3H-labeled T-2 toxin. Appl. Environ. Microbiol., 39, 1172–1177.
Yoshizawa, T., Mirocha, C.J., Behrens, J.C. & Swanson, S.P. (1981) Metabolic fate of T-2 toxin in a lactating cow. Food Cosmet. Toxicol., 19, 31–39.
Yoshizawa, T., Sakamoto, T., Ayano, Y. & Mirocha, C.J. (1982a) [Chemical structures of new metabolites of T-2 toxin.] Proc. Jpn. Assoc. Mycotoxiol., 15, 13–15 (in Japanese).
Yoshizawa, T., Sakamoto, T., Ayano, Y. & Mirocha, C.J. (1982b) 3'-Hydroxy-T-2 and 3'-hydroxy HT-2 toxins, new metabolites of T-2 toxin, a trichothecene mycotoxin, in animals. Agric. Biol. Chem., 46, 2613–2615.
Yoshizawa, T., Hiroaki, T. & Ohi, T. (1983) Structure of a novel metabolite from deoxynivalenol, a trichothecene mycotoxin, in animals. Agric. Biol. Chem., 47, 2133–2135.
Yoshizawa, T., Sakamoto, T. & Okamoto, K. (1984) In vitro formation of 3'-hydroxy T-2 and 3'-hydroxy HT-2 toxins form T-2 toxin by liver homogenates from mice and monkeys. Appl. Environ. Microbiol., 47, 130–134.
Yoshizawa, T., Okamoto, K., Sakamoto, T. & Kuwamura, K. (1985a) In vivo metabolism of T-2 toxin, a trichothecene mycotoxin. On the formation of deepoxidation products. Proc. Jpn. Assoc. Mycotoxicol., 21, 9–12.
Yoshizawa, T., Sakamoto, T. & Kuwamuar, K. (1985b) Structures of deepoxytrichthecene metabolites from 3'-hydroxy HT-2 and T-2 tetraol in rats. Appl. Environ. Microbiol., 50, 676–679.
Young, C.J. & Games, D.E. (1993) Analysis of fusarium mycotoxins by supercritical fluid chromatography with ultraviolet or mass spectrometric detection. J. Chromatogr. A, 653, 374–379.
Zabe, N., Duncan, K., Kruger, S., Cahill, L., McAlice, B. & Kohn, B. (2000) Mycotoxin detection in foods using immunoaffinity columns coupled with liquid chromatography In: Proceedings of the X International IUPAC Symposium of Mycotoxins and Phytotoxins, 21–25 May 2000, São Paulo, Brazil.
Zamir, N., Zamir, D., Eiden, L.E., Palkovits, M., Brownstein, M.J., Eshay, R.L., Weber, E., Froden, A.I. & Feuerstein, G. (1985) Methionine and leucine enkephalin in rat neurohypophysis, different response to osmotic stimuli and T-2 toxin. Science, 228, 606–608.
Zhu, G.F., Cheng, S.J. & Li, M.H. (1987) The genotoxic effects of T-2 toxin, a trichothecene produced by Fusarium fungi. Acta Biol. Exp. Sin., 20, 129–134.
Results of surveys for T-2 toxin showing concentrations and distribution of
contamination in food commodities
|
Country/ Region |
Commodity |
Year/ Season |
No. of samples |
LOQ |
n < LOQ |
Mean/Max |
|
South |
Yellow maize |
1993 |
236 |
250a |
236 |
|
|
South Africa |
White maize |
1994–95 |
143 |
250a |
143 |
|
|
Yellow maize |
|
148 |
250a |
148 |
|
|
|
Maize products |
|
158 |
250a |
158 |
|
|
|
Americas |
||||||
|
Argentina |
Maize |
1987–89 |
100 |
100a |
85 |
163/2400 |
|
Argentina |
Wheat |
1986 |
261 |
500a |
241 |
13/NA |
|
Brazil |
Wheat |
1990 |
20 |
100a |
18 |
60/800 |
|
Brazil |
Wheat and wheat products |
1991 |
38 |
100a |
38 |
|
|
Brazil, Paraná |
Maize |
1994–95 |
80 |
50a |
79 |
1.3/104 |
|
Brazil, Goiás |
Maize |
|
8 |
50a |
8 |
|
|
Canada |
Durum wheat |
1987 |
29 |
400 |
29 |
|
|
Soft winter wheat |
1988 |
17 |
400 |
17 |
|
|
|
Maize |
1989 |
16 |
400 |
16 |
|
|
|
Western hard wheat |
1991 |
108 |
400 |
108 |
|
|
|
Canada |
Wheat |
1990 |
29 |
150a |
29 |
|
|
Barley |
1990 |
23 |
150a |
23 |
|
|
|
Chile |
Maize |
1995–96 |
68 |
10a |
68 |
|
|
Ecuador |
Rice |
1992–94 |
99 |
50 |
93 |
27/NA |
|
Beans |
|
76 |
50 |
65 |
30/NA |
|
|
Maize |
|
90 |
50 |
78 |
30.7/NA |
|
|
Europe |
||||||
|
Austria |
Maize |
1996 |
46 |
100 |
45 |
5.6/260 |
|
Maize |
1997 |
58 |
100 |
58 |
|
|
|
Maize |
1998 |
48 |
100 |
46 |
6/150 |
|
|
Bulgaria |
Wheat |
1995 |
140 |
40a |
139 |
0.39/55 |
|
Finland |
Oats |
1987–88 |
21 |
15a |
19 |
5.6/73 |
|
Barley |
|
30 |
15a |
30 |
|
|
|
Wheat |
|
40 |
15a |
40 |
|
|
|
Rye |
|
31 |
15a |
30 |
0.55/17 |
|
|
Finland |
Oats |
1998 |
10 |
20 |
9 |
2.3/23 |
|
Germany |
Wheat |
1987 |
84 |
1–3a |
62 |
21/249 |
|
Germany |
Wheat |
1989 |
78 |
1–5a |
73 |
0.66/12 |
|
Wheat |
1990 |
80 |
1–5a |
71 |
5.7/136 |
|
|
Wheat |
1991 |
80 |
1–5a |
77 |
0.4/16 |
|
|
Wheat |
1992 |
78 |
1-5a |
78 |
|
|
|
Wheat |
1993 |
45 |
1-5a |
27 |
8/94 |
|
|
Germany |
Barley |
1987 |
44 |
1–5a |
40 |
0.5/9 |
|
Barley |
1989 |
40 |
1–5a |
39 |
0.4/16 |
|
|
Barley |
1990 |
47 |
1–5a |
46 |
0.1/6 |
|
|
Barley |
1991 |
51 |
1–5a |
40 |
6.3/110 |
|
|
Barley |
1992 |
58 |
1–5a |
41 |
21/300 |
|
|
Germany |
Oats |
1987 |
56 |
1–5a |
30 |
10/57 |
|
Oats |
1989 |
56 |
1–5a |
39 |
8.2/86 |
|
|
Oats |
1990 |
54 |
1–5a |
21 |
12/26 |
|
|
Oats |
1991 |
51 |
1–5a |
37 |
15/220 |
|
|
Oats |
1992 |
55 |
1–5a |
38 |
76/1700 |
|
|
Germany |
Wheat |
1998 |
56 |
6 |
56 |
|
|
Germany |
White wheat flour (ash content, 400 and550 mg/100 g) |
1999 |
28 |
6 |
28 |
|
|
|
White wheat flour (ash content, 1100 mg/100 g) |
1999 |
13 |
6 |
13 |
|
|
|
White wheat flour (ash content, 1600–1700 mg/100 g) |
1999 |
19 |
6 |
18 |
0.21/4 |
|
Germany |
Bread |
1999 |
107 |
6 |
105 |
0.07/4 |
|
Noodles |
1999 |
39 |
6 |
36 |
0.8/12 |
|
|
Germany |
Bread and related products |
Jan–July 1998 |
96 |
6 |
95 |
0.04/4 |
|
Noodles |
|
29 |
6 |
29 |
|
|
|
Germany |
Breakfast cereals |
Jan–July 1998 |
32 |
6 |
30 |
0.19/7 |
|
Infant foods |
|
25 |
6 |
25 |
|
|
|
Rice |
|
26 |
6 |
25 |
0.73/19 |
|
|
Cereal foods |
|
29 |
6 |
23 |
3.8/39 |
|
|
Norway |
Oats, home grown |
1996 |
14 |
30a |
9 |
41/320 |
|
1997 |
14 |
30a |
6 |
47/195 |
||
|
1998 |
22 |
30a |
18 |
7/42 |
||
|
1999 |
20 |
30a |
12 |
26/91 |
||
|
Wheat, home grown |
1996 |
34 |
30a |
34 |
|
|
|
1997 |
10 |
30a |
10 |
|
||
|
1998 |
24 |
30a |
24 |
|
||
|
Wheat, home grown |
1996 |
28 |
20a |
28 |
|
|
|
1997 |
25 |
20a |
25 |
|
||
|
1998 |
35 |
20a |
35 |
|
||
|
Sweden |
Wheat |
1990 |
88 |
50 |
88 |
|
|
Oats |
1990 |
71 |
50 |
44 |
43/430 |
|
|
Barley |
1990 |
39 |
50 |
39 |
|
|
|
Rye |
1990 |
5 |
50 |
5 |
|
|
|
Wheat |
1991 |
92 |
50 |
92 |
|
|
|
Oats |
1991 |
38 |
50 |
33 |
10/90 |
|
|
Wheat |
1992 |
13 |
50 |
13 |
|
|
|
Oats |
1992 |
2 |
50 |
2 |
|
|
|
Wheat |
1993 |
2 |
50 |
2 |
|
|
|
Oats |
1993 |
10 |
50 |
10 |
|
|
|
Oats |
1994 |
34 |
30 |
25 |
34/532 |
|
|
Oats |
1996 |
80 |
30 |
71 |
18/271 |
|
|
Oats |
1997 |
84 |
20 |
65 |
8/94 |
|
|
Oats |
1998 |
33 |
20 |
23 |
10/58 |
|
|
Barley |
1998 |
10 |
20 |
10 |
|
|
|
Sweden |
Wheat |
10/1996– |
59 |
10 |
59 |
|
|
Oats |
06/1998 |
23 |
10 |
23 |
|
|
|
Rye |
|
28 |
10 |
28 |
|
|
|
Wheat |
1999 |
75 |
25 |
75 |
|
|
|
Oats |
1999 |
10 |
25 |
10 |
|
|
|
Rye |
1999 |
19 |
25 |
19 |
|
|
|
United |
Wheat |
1999 |
53 |
20a |
53 |
|
|
Kingdom |
|
|
|
|
|
|
|
Asia |
||||||
|
Korea, Republic of |
Maize, barley, rice, millet |
1989 |
28 |
15a |
28 |
|
|
Korea, Republic of |
Barley |
July 1992 |
30 |
5a |
30 |
|
|
Maize |
March 1992 |
15 |
5a |
15 |
|
|
|
India |
Maize |
1994–97 |
197 |
100a |
188 |
NA/40 |
|
China, Linxian |
Wheat |
1995 |
25 |
100a |
25 |
|
|
China, Linqu County |
Raw maize |
1996 |
12 |
500a |
12 |
|
|
Maize meal |
1996 |
13 |
500a |
13 |
|
|
|
Cooked pancake |
1996 |
14 |
500a |
14 |
|
|
|
China |
Wheat grain |
Summer 1990 |
330 |
1 |
66 |
53/120 |
|
China |
Wheat grain |
Summer 1992 |
147 |
1 |
34 |
NR/820 |
|
China |
Wheat grain |
Summer 1994 |
157 |
1 |
62 |
14/100 |
|
Country/ Region |
Commodity |
Year/ Season |
90th %ile (µg/kg) |
n > 100(µg/kg |
References |
Sampling procedure |
|
South |
Yellow maize |
1993 |
|
|
P,S, Rava et al. (1996); A, Sydenham & Thiel (1987) |
Samples collected at harvest from silos in main production zones |
|
South Africa |
White maize |
1994–95 |
|
|
P,S, Rava (1996); A, Sydenham & Thiel (1987) |
Samples collected from mills throughout country |
|
Yellow maize |
|
|
|
|||
|
Maize products |
|
|
|
|||
|
Americas |
||||||
|
Argentina |
Maize |
1987–89 |
|
|
P, Saubois et al. (1992); S, Junta Nacional de Granos (1984), COPANT (1998), Jewers (1987); A, Kamimura et al. (1981) |
Samples,10 kg; analytical sample, 50 g |
|
Argentina |
Wheat |
1986 |
|
|
P, Quiroga et al. (1995); S, Apro et al. (1987); A, Trucksess et al. (1984) |
See Trichothecenes Appendix 6 |
|
Brazil |
Wheat |
1990 |
360 |
2 |
P,S, Furlong et al. (1995); A, Furlong & Valente Soares (1995) sample, 1 kg |
Samples from experimental plots in wheat-growing areas of São Paulo; 3–10 kg; laboratory |
|
Brazil |
Wheat and wheat products |
1991 |
|
|
P,S, Soares & Furlani (1996); A, Furlong & Valente Soares (1995) |
1-kg samples purchased fromhealth-food shops |
|
Brazil, Paraná |
Maize |
1994–95 |
|
1 |
P, Prado et al. (1997); S, Fonseca (1991); A, ELISA |
See Trichothecenes Appendix 6 |
|
Brazil, Goiás |
Maize |
|
|
|
|
|
|
Canada |
Durum wheat |
1987 |
|
|
P,S, Scott (1997); A, Scott et al. (1989) |
2–5-kg samples collected inmediately after harvest or at mills |
|
Soft winter wheat |
1988 |
|
|
|||
|
Maize |
1989 |
|
|
|||
|
Western hard wheat |
1991 |
|
|
|||
|
Canada |
Wheat |
1990 |
|
|
P,S,A, Stratton et al. (1993) |
3-kg samples collected at harvest and ground in Romer mill; subsamples, 250 g |
|
Barley |
1990 |
|
|
|||
|
Chile |
Maize |
1995–96 |
|
|
P,A, Vega et al. (1998); S, Olavarría (1992) |
See Trichothecenes Appendix 6 |
|
Ecuador |
Rice |
1992–94 |
|
|
P, Mühlemann et al. (1997a,b); S, Mühlemann et al. (1997a); A, Veratox™ |
Mixed samples of1.2–3 kg collected at random from various climatic regions taking into account variety and type of storage |
|
Beans |
|
|
|
|||
|
Maize |
|
|
|
|||
|
Europe |
||||||
|
Austria |
Maize |
1996 |
|
1 |
Lew et al. (2000a); A, Lew et al. (2000b) |
Composite samples of about 60 kg collected, homogeneized, and reduced to 6-kg; 1 kg ground in home mill |
|
Maize |
1997 |
|
|
|||
|
Maize |
1998 |
|
2 |
|||
|
Bulgaria |
Wheat |
1995 |
|
|
P,S,A, Vrabcheva et al. (1996) |
|
|
Finland |
Oats |
1987–88 |
|
|
P,S,A, Hietaniemi & Kumpulainen (1991) |
2–3-kg samples collected from Finnish State granaries and private farmers |
|
Barley |
|
|
|
|||
|
Wheat |
|
|
|
|||
|
Rye |
|
|
|
|||
|
Finland |
Oats |
1998 |
23 |
0 |
P,S,A, Eskola et al. (2000a,b) |
See Trichothecenes Appendix 6 |
|
Germany |
Wheat |
1987 |
|
|
P,S, Müller & Schwadorf (1993); A, Schwadorf & Müller (1991) |
Samples collected randomly from farms 1–4 weeks after harvest by Governmental advisory board |
|
Germany |
Wheat |
1989 |
|
|
P,S, Müller & Schwadorf (1993); A, Schwadorf & Müller (1991) |
Samples of 700 g to 1 kg collected randomly from farms 1–4 weeks after harvest by Governmental advisory board |
|
Wheat |
1990 |
|
|
|||
|
Wheat |
1991 |
|
|
|||
|
Wheat |
1992 |
|
|
|||
|
Wheat |
1993 |
|
|
|||
|
Germany |
Barley |
1987 |
|
|
P,S, Muller et al. (1997b); A, Schwadorf & Müller (1991) |
Samples of 700 g to 1 kg collected randomly from farms1–4 weeks after harvest by Governmental advisory board |
|
Barley |
1989 |
|
|
|||
|
Barley |
1990 |
|
|
|||
|
Barley |
1991 |
|
|
|||
|
Barley |
1992 |
|
|
|||
|
Germany |
Oats |
1987 |
|
|
P,S, Müller et al. (1998); A, Schwadorf & Müller (1991) |
Samples of 700 g to 1 kg collected randomly from farms1–4 weeks after harvest by Governmental advisory board |
|
Oats |
1989 |
|
|
|||
|
Oats |
1990 |
|
|
|||
|
Oats |
1991 |
|
|
|||
|
Oats |
1992 |
|
|
|||
|
Germany |
Wheat |
1998 |
|
|
P,S, Schollenberger et al. (2000c); A, Schollenberger et al. (1998) |
Samples collected at random from storage; subsamples of100 g milled (1 mm) and 10 g taken for analysis |
|
Germany |
White wheat flour (ash content, 400 and550 mg/100 g) |
1999 |
|
|
P,S, Schollenberger et al. (2000a); A, Schollenberger et al. (1998) |
Samples of 5 kg collected at random from shops, mixed, and 10 g taken for analysis |
|
White wheat flour (ash content, 1100 mg/100 g) |
1999 |
|
|
|
|
|
|
White wheat flour (ash content, 1600–1700 mg/100 g) |
1999 |
|
|
|
|
|
|
Germany |
Bread |
1999 |
|
|
P,S, Schollenberger et al. (2000b); A, Schollenberger et al. (1998) |
Samples > 100 g dried at 40 şC, ground in home mill (1.5 mm), and 10 g taken for analysis |
|
Noodles |
1999 |
|
|
|||
|
Germany |
Bread and related products |
Jan–July 1998 |
|
|
P,S, Schollenberger et al. (1999); A, Schollenberger et al. (1998) |
Samples dried at 40 şC, ground in home mill (1.5 mm), and 25 g taken for analysis |
|
Noodles |
|
|
|
|||
|
Germany |
Breakfast cereals |
Jan–July 1998 |
|
|
P,S, Schollenberger et al. (1999); A, Schollenberger et al. (1998) |
Samples ground in home mill (1.5 mm), and 25 g taken for analysis |
|
Infant foods |
|
|
|
|||
|
Rice |
|
|
|
|||
|
Cereal foods |
|
|
|
|||
|
Norway |
Oats, home grown |
1996 |
95 |
2 |
S, Langseth (2000); P and A, Langseth (2000); Langseth & Rundberget (2000) |
See Trichothecenes Appendix 6 |
|
1997 |
113 |
3 |
||||
|
1998 |
38 |
0 |
||||
|
1999 |
79 |
0 |
||||
|
Wheat, home grown |
1996 |
|
|
|||
|
1997 |
|
|
||||
|
1998 |
|
|
||||
|
Wheat, home grown |
1996 |
|
|
|||
|
1997 |
|
|
||||
|
1998 |
|
|
||||
|
Sweden |
Wheat |
1990 |
|
|
P,S, Pettersson (2000); A, Pettersson (1992) |
Samples of about 1 kg collected from trials and plots and dried and milled; subsamples of 20 g analysed |
|
Oats |
1990 |
118 |
17 |
|||
|
Barley |
1990 |
|
|
|||
|
Rye |
1990 |
|
|
|||
|
Wheat |
1991 |
|
|
|||
|
Oats |
1991 |
|
0 |
|||
|
Wheat |
1992 |
|
|
|||
|
Oats |
1992 |
|
|
|||
|
Wheat |
1993 |
|
|
|||
|
Oats |
1993 |
|
|
|||
|
Oats |
1994 |
82 |
3 |
|||
|
Oats |
1996 |
39 |
6 |
|||
|
Oats |
1997 |
26.5 |
0 |
|||
|
Oats |
1998 |
43.2 |
0 |
|||
|
Barley |
1998 |
|
|
|||
|
Sweden |
Wheat |
10/1996– |
|
|
P,S, Thuvander et al. (2000); A, Möller & Gustavsson (1992) |
Composite samples of about 1 kg collected at the inflow of cereals to mill, during storage in mill, or in production; analytical sample, 50 g |
|
Oats |
06/1998 |
|
|
|||
|
Rye |
|
|
|
|||
|
Wheat |
1999 |
|
|
|||
|
Oats |
1999 |
|
|
|||
|
Rye |
1999 |
|
|
|||
|
United |
Wheat |
1999 |
|
|
P,S,A, Home Grown |
|
|
Kingdom |
|
|
|
|
Cereals Authority (2000) |
|
|
Asia |
||||||
|
Korea, Republic of |
Maize, barley, rice, millet |
1989 |
|
|
P,S,A, Park et al. (1991) |
Samples collected from farms in four provinces |
|
Korea, Republic of |
Barley |
July 1992 |
|
|
P,S, Ryu et al. (1996); A, Tanaka et al. (1985) |
Samples collected from six provinces |
|
Maize |
March 1992 |
|
|
|||
|
India |
Maize |
1994–97 |
|
|
P,S, Janardhana et al. (1999); A, ELISA |
Samples from 14 districts of Karnataka representing different cultivars collected directly from farmers, production plots, and regulating markets |
|
China, Linxian |
Wheat |
1995 |
|
|
P,S, Gao & Yoshizawa (1997); A, Luo et al. (1990) |
|
|
China, Linqu County |
Raw maize |
1996 |
|
|
P,S,A, Groves et al. (1999) |
3 households in seven villages selected at random from among those known to prepare sour pancakes; in each household, 5 specimens collected to represent successive stages of processing |
|
Maize meal |
1996 |
|
|
|||
|
Cooked pancake |
1996 |
|
|
|||
|
China |
Wheat grain |
Summer 1990 |
130 |
|
P,A, Yang et al. (1992); S, GB5009 |
National standard methods for food chemistry; 15 subsamples of100 g |
|
China |
Wheat grain |
Summer 1992 |
NR |
|
P, Chen et al. (1995); A, Yang et al. (1992); S, GB5009 |
National standard methods for food chemistry;15 subsamples of100 g |
|
China |
Wheat grain |
Summer 1994 |
47 |
|
P, He et al. (1998); A, Yang et al. (1992); S, GB5009 |
National standard methods for food chemistry;15 subsamples of 100 g |
NR, not reported
a
Limit of detectionResults of surveys for HT-2 toxin showing concentrations and distribution of
contamination in food commodities
|
Country/ Region |
Commodity |
Year/ Season |
No. of samples |
LOQ |
n < LOQ |
Mean/Max |
|
America |
||||||
|
Brazil |
Wheat |
1990 |
20 |
500a |
20 |
|
|
Brazil |
Wheat and wheat products |
1991 |
38 |
500a |
38 |
|
|
Canada |
Soft winter wheat |
1988 |
17 |
100 |
16 |
5.9/100 |
|
Maize |
1989 |
16 |
100 |
15 |
14/230 |
|
|
Western hard wheat |
1991 |
108 |
100 |
106 |
5/310 |
|
|
Chile |
Maize |
1995–96 |
68 |
10a |
68 |
|
|
Europe |
||||||
|
Austria |
Maize |
1996 |
46 |
50 |
45 |
2.4/110 |
|
Maize |
1997 |
58 |
50 |
58 |
|
|
|
Maize |
1998 |
48 |
50 |
46 |
4/120 |
|
|
Finland |
Oats |
1987–88 |
21 |
15a |
19 |
3.7/44 |
|
Barley |
|
30 |
15a |
30 |
|
|
|
Wheat |
|
40 |
15a |
40 |
|
|
|
Rye |
|
31 |
15a |
30 |
0.03/23 |
|
|
Finland |
Barley |
1998 |
15 |
20 |
13 |
4.1/41 |
|
Oats |
1998 |
10 |
20 |
7 |
21/95 |
|
|
Germany |
Wheat |
1987 |
84 |
1–3a |
78 |
0.7/20 |
|
Germany |
Wheat |
1989 |
78 |
1–5a |
72 |
1.4/22 |
|
Wheat |
1990 |
80 |
1–5a |
79 |
0.2/17 |
|
|
Wheat |
1991 |
80 |
1–5a |
80 |
|
|
|
Wheat |
1992 |
78 |
1–5a |
73 |
3/150 |
|
|
Wheat |
1993 |
45 |
1–5a |
45 |
|
|
|
Germany |
Oats |
1987 |
56 |
1–5a |
40 |
59/2000 |
|
Oats |
1989 |
56 |
1–5a |
52 |
21/520 |
|
|
Oats |
1990 |
54 |
1–5a |
54 |
|
|
|
Oats |
1991 |
51 |
1–5a |
51 |
|
|
|
Oats |
1992 |
55 |
1–5a |
55 |
|
|
|
Germany |
Barley |
1987 |
44 |
1–5a |
42 |
1.0/32 |
|
Barley |
1989 |
40 |
1–5a |
37 |
1.1/18 |
|
|
Barley |
1990 |
47 |
1–5a |
45 |
0.4/10 |
|
|
Barley |
1991 |
51 |
1–5a |
51 |
|
|
|
Barley |
1992 |
58 |
1–5a |
53 |
13/290 |
|
|
Germany |
Wheat |
1998 |
56 |
18 |
44 |
3.7/51 |
|
Germany |
White wheat flour (ash content, 400 and 550 mg/100 g) |
1999 |
28 |
18 |
28 |
|
|
|
|
|
|
|
||
|
Germany |
White wheat flour (ash content, 1000 mg/ 100 g) |
1999 |
13 |
18 |
12 |
0.9/12 |
|
White wheat flour (ash content, 1600–1700 mg/100 g) |
1999 |
19 |
18 |
16 |
1.9/12 |
|
|
Germany |
Bread |
1999 |
107 |
18 |
105 |
0.22/12 |
|
Noodles |
1999 |
39 |
18 |
37 |
0.6/12 |
|
|
Germany |
Bread and related products |
Jan–July 1998 |
96 |
18 |
83 |
2.2/32 |
|
Noodles |
|
29 |
18 |
21 |
3.6/25 |
|
|
Breakfast cereals |
|
32 |
18 |
22 |
4/22 |
|
|
Infant foods |
|
25 |
18 |
24 |
0.5/12 |
|
|
Rice |
|
26 |
18 |
26 |
|
|
|
Cereal foods |
|
29 |
18 |
18 |
8/51 |
|
|
Norway |
Rye, imported |
1996 |
4 |
20a |
4 |
|
|
Wheat, imported |
1996 |
34 |
20a |
33 |
0.6/20 |
|
|
Wheat, imported |
1997 |
10 |
20a |
10 |
|
|
|
Wheat, imported |
1998 |
24 |
20a |
24 |
|
|
|
Oats, home grown |
1996 |
14 |
20a |
0 |
140/400 |
|
|
Oats, home grown |
1997 |
14 |
20a |
0 |
200/710 |
|
|
Oats, home grown |
1998 |
22 |
20a |
6 |
64/520 |
|
|
Oats, home grown |
1999 |
20 |
20a |
3 |
91/240 |
|
|
Wheat, home grown |
1996 |
28 |
20a |
28 |
|
|
|
Wheat, home grown |
1997 |
25 |
20a |
25 |
|
|
|
Wheat, home grown |
1998 |
35 |
20a |
35 |
|
|
|
Sweden |
Wheat |
1990 |
88 |
30 |
88 |
|
|
Oats |
1990 |
71 |
30 |
35 |
70/330 |
|
|
Barley |
1990 |
39 |
30 |
33 |
15/140 |
|
|
Rye |
1990 |
5 |
30 |
5 |
|
|
|
Wheat |
1991 |
92 |
10 |
51 |
12/70 |
|
|
Oats |
1991 |
38 |
10 |
13 |
95/365 |
|
|
Wheat |
1992 |
13 |
10 |
13 |
|
|
|
Oats |
1992 |
2 |
10 |
0 |
40/40 |
|
|
Wheat |
1993 |
2 |
10 |
2 |
|
|
|
Oats |
1993 |
10 |
10 |
10 |
|
|
|
Oats |
1994 |
34 |
20 |
22 |
30/339 |
|
|
Oats |
1996 |
80 |
10 |
48 |
23/303 |
|
|
Oats |
1997 |
84 |
10 |
45 |
21/175 |
|
|
Oats |
1998 |
33 |
10 |
22 |
12/92 |
|
|
Barley |
1998 |
10 |
10 |
10 |
|
|
|
Sweden |
Wheat |
10/1996– |
59 |
10 |
59 |
|
|
Oats |
06/1998 |
23 |
10 |
23 |
|
|
|
Rye |
|
28 |
10 |
28 |
|
|
|
Wheat |
1999 |
75 |
25 |
75 |
|
|
|
Oats |
1999 |
10 |
25 |
10 |
|
|
|
Rye |
1999 |
19 |
25 |
19 |
|
|
|
United Kingdom |
Wheat |
1999 |
53 |
20a |
36 |
20/170 |
|
Asia |
||||||
|
Korea, Republic of |
Barley |
July 1992 |
30 |
5a |
30 |
|
|
Maize |
March 1992 |
15 |
5a |
15 |
|
|
|
China, Linxian |
Wheat |
1995 |
25 |
100a |
25 |
|
|
China, Linqu County |
Raw maize |
1996 |
12 |
500a |
12 |
|
|
Maize meal |
1996 |
13 |
500a |
13 |
|
|
|
Cooked pancake |
1996 |
14 |
500a |
14 |
|
|
|
Country/ Region |
Commodity |
Year/ Season |
90th %ile (µg/kg) |
n > 100(µg/kg |
References |
Sampling procedure |
|
America |
||||||
|
Brazil |
Wheat |
1990 |
|
|
P,S, Furlong et al. (1995); A, Furlong & Valente Soares (1995) |
|
|
Brazil |
Wheat and wheat products |
1991 |
|
|
P,S, Soares & Furlani (1996); A, Furlong & Valente Soares (1995) |
|
|
Canada |
Soft winter wheat |
1988 |
|
|
P,S, Scott (1997); A, Scott et al. (1989) |
2–5-kg samples collected inmediately after harvest or at mills |
|
Maize |
1989 |
|
|
|||
|
Western hard wheat |
1991 |
|
|
|||
|
Chile |
Maize |
1995–96 |
|
|
P,A, Vega et al. (1998); S, Olavarría (1992) |
See Trichothecenes |
|
Europe |
||||||
|
Austria |
Maize |
1996 |
|
1 |
P,S,A, Lew et al. (2000a); A, Lew et al. (2000b) |
Composite samples of about 60 kg collected, homogenized, and reduced to 6-kg samples; ground in home mill (1 kg) |
|
Maize |
1997 |
|
|
|||
|
Maize |
1998 |
|
1 |
|||
|
Finland |
Oats |
1987–88 |
|
|
P,S,A, Hietaniemi & Kumpulainen (1991) |
2–3-kg samples collected from Finnish State granaries and private farmers |
|
Barley |
|
|
|
|||
|
Wheat |
|
|
|
|||
|
Rye |
|
|
|
|||
|
Finland |
Barley |
1998 |
20 |
0 |
P,S,A, Eskola et al. (2000a,b) |
See Trichothecenes |
|
Oats |
1998 |
95 |
0 |
|||
|
Germany |
Wheat |
1987 |
|
|
P,S, Müller & Schwadorf (1993); A, Schwadorf & Müller (1991) |
Samples collected randomly from farms1–4 weeks after harvest by Governmental advisory board |
|
Germany |
Wheat |
1989 |
|
|
Müller et al. (1997a); A, Schwadorf & Müller (1991) |
Samples of 700 g to 1 kg collected randomly from farms1–4 weeks after harvest by Governmental advisory board |
|
Wheat |
1990 |
|
|
|||
|
Wheat |
1991 |
|
|
|||
|
Wheat |
1992 |
|
|
|||
|
Wheat |
1993 |
|
|
|||
|
Germany |
Oats |
1987 |
|
|
P,S, Müller et al. (1998); A, Schwadorf & Müller (1991) |
As above |
|
Oats |
1989 |
|
|
|||
|
Oats |
1990 |
|
|
|||
|
Oats |
1991 |
|
|
|||
|
Oats |
1992 |
|
|
|||
|
Germany |
Barley |
1987 |
|
|
P,S, Muller et al. (1997b); A, Schwadorf & Müller (1991) |
As above |
|
Barley |
1989 |
|
|
|||
|
Barley |
1990 |
|
|
|||
|
Barley |
1991 |
|
|
|||
|
Barley |
1992 |
|
|
|||
|
Germany |
Wheat |
1998 |
12 |
0 |
P,S, Schollenberger et al. (2000c); A, Schollenberger et al. (1998) |
Samples collected at random from storage; subsample, 100 g milled (1 mm) and 10 g taken for analysis |
|
Germany |
White wheat flour (ash content, 400 and 550 mg/100 g) |
1999 |
|
|
P,S, Schollenberger et al. (2000a); A, Schollenberger et al. (1998) |
Samples of 5 kg collected at random from shops, mixed, and 10 g taken for analysis |
|
|
|
|
||||
|
|
|
|
||||
|
|
|
|
||||
|
Germany |
White wheat flour (ash content, 1000 mg/ 100 g) |
1999 |
|
|
P,S, Schollenberger et al. (2000a); A, Schollenberger et al. (1998) |
Samples of 5 kg collected at random from shops, mixed, and 10 g taken for analysis |
|
White wheat flour (ash content, 1600–1700 mg/100 g) |
1999 |
|
|
|||
|
Germany |
Bread |
1999 |
|
|
P,S, Schollenberger et al. (2000b); A, Schollenberger et al. (1998) |
Samples > 100 g dried at 40 şC, ground in home mill (1.5 mm), and 10 g taken for analysis |
|
Noodles |
1999 |
|
|
|||
|
Germany |
Bread and related products |
Jan–July 1998 |
|
|
P,S, Schollenberger et al. (1999); A, Schollenberger et al. (1998) |
Samples dried at 40 şC, ground in home mill (1.5 mm), and 25 g taken for analysis |
|
Noodles |
|
|
|
|||
|
Breakfast cereals |
|
|
|
|||
|
Infant foods |
|
|
|
|||
|
Rice |
|
|
|
|||
|
Cereal foods |
|
|
|
|||
|
Norway |
Rye, imported |
1996 |
|
|
S, Langseth (2000); P,A, Langseth (2000), Langseth & Rundberget (2000) |
See Trichothecenes Appendix 6 |
|
Wheat, imported |
1996 |
|
|
|||
|
Wheat, imported |
1997 |
|
|
|||
|
Wheat, imported |
1998 |
|
|
|||
|
Oats, home grown |
1996 |
295 |
8 |
|||
|
Oats, home grown |
1997 |
546 |
7 |
|||
|
Oats, home grown |
1998 |
93 |
2 |
|||
|
Oats, home grown |
1999 |
190 |
8 |
|||
|
Wheat, home grown |
1996 |
|
|
|||
|
Wheat, home grown |
1997 |
|
|
|||
|
Wheat, home grown |
1998 |
|
|
|||
|
Sweden |
Wheat |
1990 |
|
|
P,S, Pettersson (2000); A, Pettersson (1992) |
Samples of about 1 kg collected from trials and plots, dried, and milled; subsample, 20 g |
|
Oats |
1990 |
230 |
18 |
|||
|
Barley |
1990 |
|
3 |
|||
|
Rye |
1990 |
|
|
|||
|
Wheat |
1991 |
33.5 |
0 |
|||
|
Oats |
1991 |
291 |
13 |
|||
|
Wheat |
1992 |
|
|
|||
|
Oats |
1992 |
|
0 |
|||
|
Wheat |
1993 |
|
|
|||
|
Oats |
1993 |
|
|
|||
|
Oats |
1994 |
91.5 |
3 |
|||
|
Oats |
1996 |
75.7 |
6 |
|||
|
Oats |
1997 |
78 |
4 |
|||
|
Oats |
1998 |
37.2 |
0 |
|||
|
Barley |
1998 |
|
|
|||
|
Sweden |
Wheat |
10/1996– |
|
|
P,S, Thuvander et al. (2000); A, Möller & Gustavsson (1992) |
Composite samples of about 1 kg collected at inflow of cereals to mill, during storage in mill, or during production; nalytical sample, 50 g |
|
Oats |
06/1998 |
|
|
|||
|
Rye |
|
|
|
|||
|
Wheat |
1999 |
|
|
|||
|
Oats |
1999 |
|
|
|||
|
Rye |
1999 |
|
|
|||
|
United Kingdom |
Wheat |
1999 |
70 |
3 |
P,S,A, Home Grown Cereals Authority (2000) |
|
|
Asia |
||||||
|
Korea, Republic of |
Barley |
July 1992 |
|
|
P,S, Ryu et al. (1996); A, Tanaka et al. (1985) |
|
|
Maize |
March 1992 |
|
|
|||
|
China, Linxian |
Wheat |
1995 |
|
|
P,S, Gao & Yoshizawa (1997); A, Luo et al. (1990) |
|
|
China, Linqu County |
Raw maize |
1996 |
|
|
P,S,A, Groves et al. (1999) |
3 households in 7 villages selected at random from among those known to prepare sour pancakes; at each household, 5 specimens collected to represent successive stages of processing |
|
Maize meal |
1996 |
|
|
|||
|
Cooked pancake |
1996 |
|
|
|||
a
Limit of detection
See Also:
Toxicological Abbreviations