
WHO FOOD ADDITIVES SERIES: 49
First draft prepared by Gladwin Roberts1 & Carl Cerniglia2
1Chemical Products Assessment Section, Therapeutic Goods Administration, Department of Health and Aged Care, Canberra, Australia; and
2National Center for Toxicological Research, Food and Drug Administration, Arkansas, USA
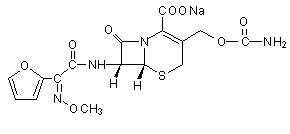
Cefuroxime is a cephalosporin antibacterial agent with activity against a range of gram-positive and gram-negative bacteria. The chemical name of cefuroxime sodium is sodium (Z)-(6R,7R)-3-(carbamoyloxymethyl)-7-[2-(2-furyl)-2-(methoxy-imino)acetamide]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate. Its structure is shown in Figure 1. The sodium salt is used in veterinary medicine for the treatment of mastitis and is available in two formulations, both applied by intramammary infusion: one for the treatment of clinical mastititis in lactating cattle and the second for the treatment of sub-clinical mastitis in dry cows and to prevent new infections during the dry period. Cefuroxime is also used in human medicine, as either the sodium salt or as the 1-acetoxyethyl ester (known as cefuroxime axetil).
Cefuroxime has not previously been evaluated by the Committee.

Figure 1. Structure of cefuroxime sodium
Rats
Groups of three Wistar rats were given [14C]cefuroxime sodium as a single intravenous or intramuscular injection of 25 mg/kg bw or as an intravenous infusion of 50 mg/kg bw over 40 min. The blood concentrations of radiolabel were highest within 30 min of the end of administration and then rapidly declined, with initial half-times of 0.5–1 h. By 24 h, the blood concentrations were < 10% of the respective peak. It was distributed widely, kidney and liver having higher concentrations of radiolabel than blood; particularly low concentrations were detected in the central nervous system, muscle and fat. Dosing of female rats on day 18 of gestation resulted in very low concentrations of radiolabel in fetal tissue and amniotic fluid. Relatively high concentrations were detected in the milk of lactating rats, and the peak represented 18% of blood concentrations after 1 h. About 80% of a parenteral dose was excreted in urine, and the remainder was excreted in bile, predominantly during the first 24 h. A portion of the radiolabel in bile was subject to enterohepatic recirculation. Daily intramuscular injections for up to 14 days did not affect the kinetics in blood or excretion in urine (Nanbo et al., 1979; Okumura et al., 1979).
In anaesthetized rats given a single intravenous injection of 20 mg/kg bw of cefuroxime, the bioavailability in brain was 4.2% that in plasma, thus confirming the low penetration of the blood–brain barrier (Tsai et al., 1999).
The absorption of cefuroxime axetil in the small intestine of anaesthetized rats was investigated in situ by perfusion at a concentration of 12, 59, 120 or 200 µmol/l. Absorption across the intestinal mucosa appeared to occur as a result of an active transport mechanism. Some drug was hydrolysed in the lumen of the intestine, the proportion increasing from 16 to 25% with increasing concentration (Ruiz-Balaguer, et al., 1997).
In seven rats given a single dose of 2 mg of cefuroxime axetil by gavage, the peak plasma concentration of cefuroxime was found at about 30 min. About 25–30% of the dose was bioavailable, and a similar amount was considered to have been immediately excreted in the bile. The relatively low bioavailability appears to be due to hydrolysis in the intestine, which reduces the amount of the ester available for absorption, and hydrolysis in blood, which facilitates rapid excretion of cefuroxime in the urine (Ruiz-Carretero et al., 2000).
Rabbits
Groups of four rabbits were given single intravenous or intramuscular injections or twice daily intramuscular injections of cefuroxime at a dose of 25 mg/kg bw for 15 days. The peak blood concentrations were reached within 15 min after each administration and then rapidly declined. About 75–80% of the drug was recovered in urine within 24 h. No cefuroxime was detected in organs 24 h after the final dose (Okumura et al., 1979).
Dogs
Three male beagle dogs were given a single intravenous injection of 25 mg/kg bw [14C]cefuroxime sodium. The concentration of radiolabel in blood peaked immediately after dosing and then decreased, with an initial half-time of 0.7 h. About 90% of the dose was excreted in urine; recovery of about 8% in faeces suggested limited excretion in bile (Nanbo et al., 1979). In beagle dogs given an intramuscular dose of 25 mg/kg bw cefuroxime, 4% of the dose was detected in bile; the concentrations were maximal 1–2 h after administration and gradually decreased thereafter up to 24 h (Okumura et al., 1979).
Plasma was taken from one male and one female beagle dog given cefuroxime axetil by gavage at a dose of 100, 400 or 1600 mg/kg bw per day. Samples were withdrawn after treatment for 1, 36, 169 and 188 days. Peak concentrations of cefuroxime in plasma were found within 2 h of administration, and the bioavailability was claimed to be proportional to the dose. Unchanged cefuroxime axetil was also detected in the plasma of the female given 400 mg/kg bw per day and in both animals at 1600 mg/kg bw per day, attaining a highest concentration representing about 10% of the peak. De-esterification led to formation of acetaldehyde and acetic acid (Spurling et al., 1986).
Humans
Cefuroxime sodium was poorly absorbed through the gastrointestinal tract. After oral administration of 1000 mg to two volunteers, only 1% of the administered dose was recovered in urine, and the plasma concentrations were stated to be only just measurable. When cefuroxime sodium was injected, at least 95% of the dose was recovered in the urine, mainly in unchanged form (Foord, 1976).
Cefuroxime is widely distributed in the body, entering body fluids and tissues when given at therapeutic concentrations. It crossed the placenta and has been detected in breast milk (Parfitt, 1999). The concentrations of cefuroxime in cerebrospinal fluid were about 10% of those in plasma (Mandell & Petri, 1996). Injection of 750 mg of cefuroxime to women during pregnancy, labour or caesarean section resulted in significant concentrations in the amniotic fluid and umbilical blood vessels and in plasma from the neonates (Craft et al., 1981; Phillipson & Stiernstedt, 1982).
Moderate amounts of cefuroxime axetil were absorbed after five oral doses of 125, 250, 500 × 2 and 1000 mg to 12 male volunteers. The bioavailability was about 36% in fasted individuals and 50% after food intake. A linear relationship was found between the dose in fed subjects and both the area under the plasma concentration–time curve and the peak plasma concentration. The peak plasma concentration was 43% greater in fed subjects than in fasted subjects (Finn et al., 1987). Cefuroxime axetil was hydrolysed in the intestinal mucosa and blood to yield cefuroxime; the resulting concentrations in plasma were variable (Parfitt, 1999). The plasma half-time was 1–2 h, the maximum plasma concentration after oral administration was 6.3 mg/l, and the degree of protein binding was 33–50% (Kalman & Barriere, 1990). Cefuroxime was excreted primarily in urine (Williams & Harding, 1984), and secretion by the renal tubules was responsible for 43–54% of the renal elimination (Foord, 1976). The kinetics was unaffected by repeated oral dosing for 7 days (Sommers et al., 1984).
Rats and dogs
Up to 97% of administered [14C]cefuroxime sodium remained unchanged in the plasma and urine of rats and dogs treated parenterally. However, an unidentified metabolite was detected in the bile of rats, which accounted for about 27% of the material present, the remainder being unchanged parent drug (Nanbo et al., 1979). In another study, in rats given cefuroxime by intramuscular injection, thin-layer chromatography showed that the only compound present in urine, bile and plasma was the parent. The metabolites produced in rats and dogs were not identified (Okumura et al., 1979).
Humans
Cefuroxime axetil mixed with human blood in vitro was rapidly de-esterified to cefuroxime, with a half-time of 3.5 min. Similarly, in 12 male volunteers given a single oral dose of 1.5 g of cefuroxime axetil, hydrolysis of the ester was virtually complete, and cefuroxime was the only compound detected in blood (Harding et al., 1984). In 11 children given a single oral dose of 10, 15 or 20 mg/kg bw cefuroxime axetil, the unchanged ester was found in the urine of only four subjects and accounted for < 0.1% of the administered dose (Powell et al., 1991).
The studies of acute toxicity did not comply with good laboratory practice (GLP). As shown in Table 1, cefuroxime was of low acute toxicity in mice and rats after oral or parenteral administration and was slightly toxic in rabbits when administered parenterally.
Table 1. Results of studies of the acute toxicity of cefuroxime sodium in male and female rodents
|
Species |
Route |
LD50 (mg/kg bw) |
Reference |
|
Mouse |
Oral |
> 10 000 |
Tamura et al. (1979) |
|
Rat |
Oral |
> 10 000 |
Tamura et al. (1979) |
|
Mouse |
Intraperitoneal |
> 10 000 |
Tamura et al. (1979) |
|
Rat |
Intraperitoneal |
~ 10 000 |
Tamura et al. (1979) |
|
Mouse |
Subcutaneous |
> 10 000 |
Capel-Edwards et al. (1979); |
|
Rat |
Subcutaneous |
> 10 000 |
Capel-Edwards et al. (1979); |
|
Mouse |
Intravenous |
10 400 |
Capel-Edwards et al. (1979); |
|
Rat |
Intravenous |
> 8 000 |
Capel-Edwards et al. (1979); |
|
Rabbit |
Intravenous |
> 1 500 |
Tamura et al. (1979) |
|
Mouse |
Intramuscular |
> 2 000 |
Tamura et al. (1979) |
|
Rat |
Intramuscular |
> 2 000 |
Tamura et al. (1979) |
|
Rabbit |
Intramuscular |
> 300 |
Tamura et al. (1979) |
The only adverse effect seen after oral administration was diarrhoea. In animals that died after parenteral injection, swelling of the caecum was the main effect seen at autopsy, and destruction of cortical renal tubules was observed only in rats (Tamura et al., 1979).
Two cats and four dogs were given a single intramuscular injection of 2 g/kg bw cefuroxime sodium as a 40% solution in water. No signs of toxicity were seen, and the only treatment-related effect was discomfort at the injection site, which persisted for a few minutes after injection. Cynomolgus monkeys given 1.5–2.0 g/kg bw intramuscularly showed slight loss of body weight, and some had diarrhoea (Glaxo Laboratories Ltd, 1986; Capel-Edwards et al., 1979).
Rats
A series of studies that did not comply with GLP were conducted in rats given cefuroxime sodium parenterally.
Groups of five male and five female CD rats received cefuroxime sodium in 0.9% saline solution intravenously at a dose of 0, 50, 100, 200 or 400 mg/kg bw per day for 29 (males) or 30 (females) days. Treatment had no effects on body weight, general condition, haematological, blood biochemical or urinary end-points, organ weights or lesions. As only a summary of this study was available, the absence of findings could not be confirmed independently (Glaxo Laboratories Ltd, 1986).
Groups of eight male and eight female Charles River CD rats were given cefuroxime sodium in 0.9% saline solution by subcutaneous injection at a dose of 0, 100, 200, 400 or 800 mg/kg bw per day for 33 (males) or 34 (females) days. There were no toxic signs, no effects on body-weight gain and no changes in urinary parameters. Inflammation and ulceration at the injection site were noted in animals at the highest dose. Haemoglobin concentration and erythrocyte volume fraction were reduced in some animals given 200 mg/kg bw per day or more, although the effect was significant only at the highest dose. The serum potassium concentration was increased in females at 100 and 800 mg/kg bw per day. Lymphatic dilatation and lymphocyte infiltration were seen in the colonic mucosa in some animals at 800 mg/kg bw per day. There were no significant effects at 100 mg/kg bw per day. As only a summary of this study was available, the absence of findings cannot be confirmed independently (Glaxo Laboratories Ltd, 1986).
Groups of 18 male and 18 female SD-JCL rats were given cefuroxime in distilled water by subcutaneous injection at a dose of 200, 500 or 1500 mg/kg bw per day on 6 days per week for 35 days. A similar group of controls was given saline. An additional group of six males and six females given the highest dose was retained for a 5-week recovery period after the end of treatment. No deaths or signs of overt toxicity were noted, and there were no effects on body-weight gain, water intake or urinary end-points. A significant fall in erythrocyte count was seen in males at the highest dose and in females at all doses. Significant decreases in haemoglobin concentration and erythrocyte volume fraction were noted in animals of each sex given the highest dose. An increase in leukocyte numbers occurred in males a 500 or 1500 mg/kg bw per day.
Increased serum alanine aminotransferase activity was seen in animals of each sex at 1500 mg/kg bw per day and increased alkaline phosphatase activity in males at 500 and 1500 mg/kg bw per day. Bilirubin concentrations were decreased in all groups of treated males and in females at 500 and 1500 mg/kg bw per day. A significant increase in blood glucose concentration was seen in males at the two higher doses, while significant decreases were seen in all groups of treated females. The albumin concentration was decreased and the potassium concentrations were increased in animals at 1500 mg/kg bw per day. At this dose, males showed decreased liver weights. The weight of the kidney was increased in males, and caecal swelling was seen in animals of each sex at the two higher doses of cefuroxime. All the changes were fully or partially reversed on cessation of dosing. No compound- related effects were found on histological examination. A NOEL was not identified (Ito et al., 1979a).
Groups of 18 male and 18 female SD-JCL rats were given cefuroxime in distilled water by intraperitoneal injection at a dose of 100, 300 or 1000 mg/kg bw per day on 6 days per week for 35 days. A similar group of controls was given saline. An additional group of six males and six females at the highest dose was allowed a 5-week recovery period after the end of treatment. No deaths or signs of overt toxicity were noted, and there were no effects on body-weight gain or urinary end-points. Females at 1000 mg/kg bw per day had slightly increased water intake. Erythrocyte counts were significantly decreased in males at the highest dose, and increased values were found in females at 300 and 1000 mg/kg bw per day. The haemoglobin concentration and erythrocyte volume fraction were not affected. Blood glucose concentrations were decreased in males and blood urea nitrogen concentrations were decreased in females at 300 and 1000 mg/kg bw per day but with no dose–response relationship. The potassium concentration was decreased in females at 300 and 1000 mg/kg bw per day and increased in males at 100 and 1000 mg/kg bw per day. Caecal swelling was noted in animals at 300 and 1000 mg/kg bw per day. There were no compound-related effects on organ weights or on histological appearance. All changes were reversed on cessation of dosing. There were no significant effects at 100 mg/kg bw per day (Ito et al., 1979a).
Groups of seven male and seven female CD rats were given cefuroxime sodium in 0.9% saline by subcutaneous injection at a dose of 0, 100, 300 or 900 mg/kg bw per day for 91 days. The groups given the control and highest dose contained an additional seven rats of each sex which were allowed a 21-day recovery period at the end of dosing. There were no effects on general condition, blood biochemistry or body weight. The groups given 300 and 900 mg/kg bw per day showed reduced erythrocyte count, haemoglobin concentration and erythrocyte volume fraction. Reticulocyte counts were slightly increased in male rats that had reduced erythrocyte counts. Total leukocyte counts were increased in females at the two higher doses, but the differential counts were unaffected. Prothrombin times were slightly increased in animals of each sex given the highest dose and in males at 300 mg/kg bw per day. Urine volume, urinary electrolyte excretion and water intake were increased and specific gravity decreased in animals at 900 mg/kg bw per day. The spleen weight was increased in females at 900 mg/kg bw per day, but the only pathological findings were inflammation, haemorrhage and haemosiderin deposition at the injection sites in rats at 300 and 900 mg/kg bw per day. The changes were reversed at the end of the recovery period. There were no significant effects at 100 mg/kg bw per day. As only a summary of this study was available, the findings cannot be confirmed independently (Glaxo Laboratories Ltd, 1986).
Groups of 10 male and 10 female CD rats were given cefuroxime sodium in 0.9% saline by subcutaneous injection at a dose of 0, 50, 150 or 450 mg/kg bw per day for 26 weeks. Body-weight gain and general condition were unaffected, except for localized swelling at the injection site. There were slight reductions in erythrocyte volume fraction, haemoglobin concentration and erythrocyte counts, and increases in reticulocyte counts in animals at 450 mg/kg bw per day. The serum protein concentration was slightly decreased and sodium and potassium excretion was increased in animals at 450 mg/kg bw per day. The only pathological changes were those associated with physical damage and inflammatory reactions at the injection site. There were no significant effects at 150 mg/kg bw per day. As only a summary of this study was available, the findings cannot be confirmed independently (Glaxo Laboratories Ltd, 1986).
Groups of 26 male and 26 female SD-JCL rats were given cefuroxime in distilled water by subcutaneous injection at a dose of 100, 250 or 750 mg/kg bw per day on 6 days per week for up to 26 weeks. Similar groups of controls were given saline. Animals were killed after 3 months (eight rats of each sex) and 6 months (10 rats of each sex) of treatment, and after a 3-month recovery period (eight rats of each sex). There were no overt signs of toxicity and no significant effects on urinary end-points. Body-weight gain was slightly depressed in males at 750 mg/kg bw per day despite a small increase in food intake. Erythrocyte count, haemoglobin concentration and erythrocyte volume fraction were lower in females at all doses. Significant decreases were seen in serum aspartate aminotransferase activity in males at all doses and in females at 750 mg/kg bw per day and in bilirubin concentration in males at 250 mg/kg bw per day and in males and females at 750 mg/kg bw per day. Slight decreases in cholesterol, total protein and albumin concentration were seen in rats at the highest dose. Kidney weights were increased in the groups given 250 or 750 mg/kg bw per day, but the effect was not associated with pathological alterations. Caecal swelling was seen in animals of each sex at 250 and 750 mg/kg bw per day after 3 and 6 months of treatment. None of these changes was seen at the end of the recovery period. A NOEL was not identified (Ito et al., 1979b).
Groups of 26 male and 26 female SD-JCL rats were given cefuroxime in distilled water by intraperitoneal injection at a dose of 50, 125 or 375 mg/kg bw per day on 6 days per week for 26 weeks. A similar group of controls was given saline. Animals were killed after 3 months (eight rats of each sex) and 6 months (10 rats of each sex) of treatment and after a 3-month recovery period (eight rats of each sex). There were no overt signs of toxicity and no deaths that could be attributed to treatment. Body-weight gain was unaffected; however, feed conversion was reduced at 3 months in animals at 125 or 375 mg/kg bw per day, gradually returning to control levels thereafter. There were no effects on urinary parameters. Leukocyte counts were decreased in females at 125 and 375 mg/kg bw per day; at the highest dose, this effect was associated with an increase in the ratio of neutrophils to lymphocytes. A decreased total protein concentration in males at the highest dose was the only consistent biochemical alteration. Caecal swelling was seen in animals of each sex at 375 mg/kg bw per day at 3 months only, but no histopathological findings were attributable to administration of the compound. The leukocyte counts remained low during the recovery period, whereas all other changes were reversed to control levels. No effects were seen at 50 mg/kg bw per day (Ito et al., 1979b).
Dogs
A series of studies that did not conform to GLP were conducted, in which dogs were treated with cefuroxime sodium parenterally and with cefuroxime axetil by oral administration.
Groups of two male and two female beagle dogs were given cefuroxime sodium in 0.9% saline by intramuscular injection at a dose of 0, 60, 180 or 540 mg/kg bw per day for 10 or 11 days. All the dogs gained weight and remained in good health. Haematological, blood biochemical and urinary end-points were unremarkable. The weights of the liver and kidney relative to body weight were increased at 540 mg/kg bw per day, but the lesions were limited to irritation at the injection site. As only a summary of this study was available, the findings cannot be confirmed independently (Glaxo Laboratories Ltd, 1986).
Groups of three male and three female beagle dogs were given cefuroxime sodium in saline by subcutaneous injection at a dose of 0, 50 or 500 mg/kg bw per day on 6 days per week for 5 weeks. One animal of each sex per group was allowed a 4-week recovery period at the end of treatment. No overt toxicity was seen, but body-weight loss was seen in male dogs at 500 mg/kg bw per day. Haematological and urinary analyses revealed no significant findings. The bilirubin concentration was decreased in both groups of treated males, but the difference was seen before the beginning of dosing. Organ weights and gross appearance were unaffected. At the end of the recovery period, the end-points in control and treated animals were similar. No effects were seen at 50 mg/kg bw per day (Ito et al., 1979c).
Groups of three male and three female beagle dogs were given cefuroxime sodium in saline by intravenous injection at a dose of 0, 25 or 250 mg/kg bw per day on 6 days per week for 5 weeks. One animal of each sex per group was allowed a 4-week recovery period at the end of treatment. Body-weight loss was observed in males at the higher dose. There were no signs of overt toxicity and no effects on haematological, blood biochemical or urinary end-points, organ weights or histopathological appearance. At the end of the recovery period, all parameters were similar in control and treated animals. No effects were seen at 25 mg/kg bw per day (Ito et al., 1979c).
Cefuroxime axetil was administered as an aqueous suspension to groups of four male and four female beagle dogs twice daily by gavage for 27 weeks. The equivalent doses of cefuroxime were 0, 100, 400 and 1600 mg/kg bw per day. Additional groups of two males and two females given 0 or 400 mg/kg bw per day were allowed a 3-week recovery period. Two dogs were killed during the study because of intercurrent disease (polyarteritis). The only signs seen in the remaining animals were occasional vomiting and salivation at the highest dose. Body-weight gain was slightly retarded in the group at this dose. Ophthalmic, electrocardiographic and urinary parameters were unaltered. At the highest dose, erythrocyte count, haemoglobin concentration and erythrocyte volume fraction were lower and the reticulocyte number was higher than in controls. Prolonged prothrombin and activated partial thromboplastin times were observed in males at 1600 mg/kg bw per day, and the concentration of coagulation factor VII was reduced in this group. Plasma total protein, albumin and cholesterol concentrations were reduced and that of triglycerides increased at 1600 mg/kg bw per day. The weight of the kidneys of males at this dose was increased. No differences were seen between groups after the recovery period. None of the macroscopic or microscopic findings was attributable to treatment. The NOEL was 400 mg/kg bw per day (Spurling et al., 1986).
Groups of three male and three female beagle dogs were given cefuroxime sodium by subcutaneous or intramuscular injection at a dose of 0, 50, 150 or 450 mg/kg bw per day for 6 months. The route of administration was varied during the study to minimize the irritating effects of the injections. The compound was initially given in saline, but distilled water was used as the vehicle from day 25. Controls and dogs at 50 mg/kg bw per day were given intramuscular injections into the hind legs, while animals at the higher doses received subcutaneous injections.
After 12 weeks of treatment, one animal at the highest dose developed a Heinz body haemolytic anaemia and was killed after a further 2 weeks. Otherwise, the condition and body-weight gain of dogs was generally good throughout the study. Discomfort arose at the site of injection, particularly after the subcutaneous doses. The mean corpuscular haemoglobin concentrations were slightly reduced in dogs at 150 and 450 mg/kg bw per day. Serum iron values were consistently reduced (significant after 8 weeks), and serum iron binding capacity was increased in animals at the highest dose throughout the study. Analyses of blood biochemistry, urine and organ weights showed no compound-related effects. The only pathological changes were inflammatory reactions at the injection sites in some treated dogs and changes to the bone marrow and reticuloendothelial system consistent with severe haemolytic anaemia in the animal killed at week 14. No effects were seen at 50 mg/kg bw per day. As only a summary of this study was available, the findings could not be confirmed independently (Glaxo Laboratories Ltd, 1986).
Monkeys
In a study that did not comply with GLP, groups of two male and two female cynomolgus monkeys were given cefuroxime sodium in 0.9% saline by intramuscular injection at a dose of 0, 150 or 450 mg/kg bw per day for 28 days. No overt signs of toxicity were seen, although some animals in each treated group produced softer faeces than normal for a few days at the beginning of treatment. Animals at 450 mg/kg bw per day showed transient reductions in erythrocyte count and haemoglobin concentration (significant at day 7), with moderate leukocytosis (neutrophilia and eosinophilia) in males only. These changes had largely reversed by the end of the study. Blood biochemistry and urinary parameters showed no alterations, and there were no compound-related effects on organ weights. Histological examination revealed only inflammatory reactions at injection sites. As only a summary of this study was available, the findings cannot be confirmed independently. A NOEL was not identified (Glaxo Laboratories Ltd, 1986).
A battery of tests that complied with appropriate standards was conducted to address the genotoxicity of cefuroxime sodium. The results are summarized in Table 2.
Table 2. Results of tests for genotoxicity with cefuroxime sodium
|
End-point |
Test object |
Concentration |
Result |
Reference |
|
Reverse mutationa |
S. typhimurium TA1535, |
0.0013–0.8 µg/ml |
Negative |
Ballantyne (1996) |
|
TA98 ± S9 |
0.0013–1.0 µg/ml |
Negative |
||
|
TA100 ± S9 |
0.031–0.5 µg/ml |
Negative |
||
|
Reverse mutationa |
E. coli strains WP2 pKM101 ± S9 |
0.0013–0.8 µg/ml |
Negative |
Ballantyne (1996) |
|
WP2 uvrA pKM101 ± S9 |
0.00026–0.16 µg/ml |
Negative |
||
|
Reverse mutationb |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 ± S9 |
0.05–2.0 µg/ml |
Negative |
Tweats (1977) |
|
Gene conversionc |
Saccharomyces cerevisiae JD1± S9 |
100–5000 µg/ml |
Negative |
Tweats (1977) |
|
Forward mutationd |
Thymidine kinase (tk) locus in mouse lymphoma |
140–4500 µg/ml |
Negative |
Fellows (1995) |
|
Chromosomal damage in vitroe |
Human peripheral blood lymphocytes in culture |
750–1500 µg/ml, 20 h exposure –S9 |
Positive |
Marshall, A. (1996) |
|
2200–4500 µg/ml, 3 h exposure + S9 |
Negative |
|||
|
480 µg/ml, 44 h exposure –S9 |
Positive |
|||
|
4500 µg/ml, 3 h exposure ±S9 |
Negative |
|||
|
Chromosomal damage in vivof |
Micronucleus formation in bone marrow from CR/H female mice |
Two intraperitoneal doses of 100, 1000 or 10 000 mg/kg bw, 24 h apart |
Negative |
Tweats et al. (1980) |
|
S9, 9000 × g supernatant of rat liver used for metabolic activation |
|
|
a |
Positive controls were 2-nitrofluorene for TA98; sodium azide for TA100 and TA1535; ICR-191 for TA1537 and N-methyl-N’´-nitro-N-nitrosoguanidine (MNNG) for E. coli in the absence of S9; 2-aminoanthracene for all S. typhimurium and E. coli strains in the presence of S9. |
|
b |
Positive controls were 2-acetamidofluorene for TA98, methyl methanesulfonate for TA100, MNNG for TA1535, 9-aminoacridine for TA1537 and hycanthone methanesulfonate for TA1538 in the presence and absence of S9. |
|
c |
Positive controls were hycanthone methanesulfonate in the presence and absence of S9. |
|
d |
Positive controls were 4-nitroquinoline 1-oxide in the absence of S9 and benzo[a]pyrene in the presence of S9. |
|
e |
Positive controls were 4-nitroquinoline 1-oxide in the absence of S9 and cyclophosphamide in the presence of S9. |
|
f |
Positive control was adryamicin by intraperitoneal injection. |
As expected, cefuroxime was toxic to the bacterial strains used in the mutagenicity assays. Consequently, only relatively low concentrations of cefuroxime sodium could be tested in these studies. The selected concentrations were based on the results of tests for cytotoxicity. Negative results were obtained, except in the test for induction of chromosomal aberrations in cultured human peripheral blood lymphocytes obtained from one woman and one man. In this test, a positive result was seen in the absence of the metabolizing system. Prolonged exposure, for 20–44 h, was required to obtain a positive effect, and no chromosomal damage was observed after exposure for 3 h. The aberrations were predominantly chromatid deletions, with very few chromosome rearrangements. Although the aberration frequencies were markedly higher than in controls, the magnitude of the response was less than proportional to the concentration of cefuroxime. This may have been due to inhibition of mitosis, which increased from 14% to 54% as the concentration was increased. Nevertheless, these positive results obtained in vitro were not confirmed in a test for micronucleus formation in vivo in mice receiving cefuroxime sodium at intraperitoneal doses of up to 10 000 mg/kg bw.
Similar clastogenic effects were seen in cultured human lymphocytes treated with two other cephalosporins, cephalonium (Marshall, R., 1995) and cephalexin (Riley, 1996). Another cephalosporin, ceftiofur sodium, did not induce reverse mutation in bacteria, mutations in mammalian cells in vitro or micronuclei in vivo. However, chromosomal damage was seen in Chinese hamster ovary cells after exposure in vitro for 44 h, but not for shorter times (Aaron et al., 1995a). These data on ceftiofur were reviewed by the Committee at its forth-fifth meeting and were considered to be of no biological significance (Annex I, reference 120). Further investigations suggested that the clastogenic effect of ceftiofur is associated with inhibition of cell division (Aaron et al., 1995b).
A series of studies that did not conform to GLP were conducted with parenteral administration of cefuroxime sodium.
Mice
Groups of 12 male and 30 female CRH mice were given cefuroxime sodium in distilled water by subcutaneous injection at a dose of 800, 1600 or 3200 mg/kg bw per day. Controls were given 0.9% saline. After 60 days, each male was caged with two or three female mice at the same dose When pregnancy was detected, the female mice were re-housed. When most of the female mice were pregnant, the males were killed. The females were divided into two approximately equal groups per dose: one group was killed on day 18 of gestation, and the females in the other group were allowed to deliver and rear their litters. The latter group received their final dose on day 18 of gestation. Three weeks after the birth of the F1 pups, the F0 dams and all but one male and one female from each litter were killed. When the F1 pups were 4 weeks old, one male from one litter was selected and re-housed with one female from another litter to give rise to the F2 generation. The F1 dams and the F2 pups were killed on postnatal day 21.
The only treatment-related sign during the study was subcutaneous swelling at the site of injection. There were no treatment-related effects on body-weight gain, conception rate, numbers of implantation sites or resorptions, numbers of live fetuses, litter weights or the proportions of male mice in either generation. There were no fetal abnormalities, and the body-weight gain and physical development of the pups was unaffected. No reproductive effects were observed at any dose (Griffiths, 1975a).
Rats
Groups of 20 male and 20 female Sprague-Dawley rats were given cefuroxime sodium in physiological saline by subcutaneous injection at a dose of 0, 200, 400 or 800 mg/kg bw per day. Males were treated from 60 days before and during mating, while females were treated from 14 days before mating until day 7 of gestation. The dams were killed on day 20 of gestation. Pain during drug administration was noted in animals given the highest dose. Males at doses of 400 mg/kg bw per day and above showed slight increases in food intake, and water intake was increased in all treated groups. Body-weight gain was suppressed in treated females during the latter part of gestation. There were no differences in conception rates or in the numbers of corpora lutea, implantations, resorptions or live fetuses. Fetal body weights and the numbers of malformed fetuses were not influenced by treatment. Neither fertility nor reproduction was affected at any dose (Otaka et al., 1979).
Mice
Groups of 10–17 pregnant female CRH mice were given subcutaneous injections of cefuroxime sodium in 0.9% saline solution at a dose of 0, 800, 1600, 3200 or 6400 mg/kg bw per day on days 6–15 of gestation and killed on day 18 of gestation. The animals remained in good condition, and there were no effects on body-weight gain or the numbers of implantation sites, resorptions or live fetuses. Litter weights and the sex ratio of fetuses were unaffected, and no fetal abnormalities were noted. Fetal development was not affected at any dose (Troughton & Bartholomew, 1975a).
Rats
Groups of 30 pregnant Sprgaue-Dawley rats were given cefuroxime sodium in physiological saline by subcutaneous injection at a dose of 0, 200, 400, 800 or 1600 mg/kg bw per day on days 7–17 of gestation. Twenty females in each group were killed on day 20 of gestation, and the remaining 10 were allowed to deliver and rear their pups for 21 days. Dams in the latter groups were killed on postnatal day 21, and the F1 offspring matured up to 10 weeks of age. One male and two female F1 rats per dam in each treatment group were mated; one of the two females was killed on day 20 of gestation, and the other was allowed to deliver and rear the F2 pups for 3 weeks. Pain, bleeding and haematomas at the injection site were common at the highest dose. Dams in all groups showed slight depression in body-weight gain and increased water intake and caecal weight. In the phase at which teratogenesis was examined, lower body weights were seen in F1 fetuses at 800 and 1600 mg/kg bw per day, but this effect was not seen in full-term pups. No effects were found on the numbers of implantations, resorptions or live fetuses, sex ratios, the incidences of fetal abnormalities or postnatal development or reproductive capacity in either generation. There were no effects on fetuses at 400 mg/kg bw per day (Otaka et al., 1979).
Groups of 30 pregnant Sprgaue-Dawley rats were given cefuroxime sodium in physiological saline by intravenous injection at a dose of 0, 200, 400 or 800 mg/kg bw per day on days 7–17 of gestation. Twenty females in each group were killed on day 20 of gestation, and the remaining 10 were allowed to deliver and rear their pups for 21 days. Dams in the latter groups were killed on postnatal day 21, and the F1 offspring matured up to 10 weeks of age. One male and two female F1 rats per dam in each treatment group were mated; one of the two females was killed on day 20 of gestation, and the other was allowed to deliver and rear the F2 pups for 3 weeks. Twitching after injection was noted at doses of 400 mg/kg bw per day and above. Slight depression in body-weight gain was seen in dams at the highest dose and increased water intake and caecal weight in all treated groups. No effects were found on the numbers of implantations, resorptions or live fetuses, sex ratios, the incidences of fetal abnormalities or postnatal development or reproductive capacity in either generation. There were no effects on fetuses at any dose (Otaka et al., 1979).
Rabbits
Groups of 5–11 pregnant Dutch rabbits were given cefuroxime sodium in 0.9% saline by intramuscular injection at a dose of 0, 50, 100, 200 or 400 mg/kg bw per day on days 6–18 of gestation and were killed on day 29 of gestation. Four does given 400 mg/kg bw per day, one given 200 mg/kg bw per day and one given 100 mg/kg bw per day died during the study. The rabbits developed diarrhoea before death, probably due to disturbances of the gut flora. Surviving does showed no effects on body-weight gain, and there were no effects on the numbers of implantation sites, resorptions or live fetuses. The incidence of fetal abnormalities was similar in all groups. Fetal development was not affected at any dose (Troughton & Bartholomew, 1975b).
Groups of 10 pregnant New Zealand white rabbits were given cefuroxime sodium in physiological saline by intravenous or subcutaneous injection at a dose of 0, 25, 50, 100 or 150 mg/kg bw per day on days 6–18 of gestation and were killed on day 28 of gestation. No deaths occurred during the study, and the body weights were similar to those of controls. Caecal weight was increased in does at the highest dose. No differences were seen in the numbers of implants, resorptions or live fetuses, in the sex ratio, in fetal weights or in the incidences of fetal abnormalities in any group. Fetal development was not affected at any dose (Furuhashi et al., 1979).
(c) Peri- and postnatal toxicity
Mice
Groups of 12–15 pregnant CRH strain mice were given cefuroxime sodium in 0.9% saline by subcutaneous injection at a dose of 0, 800, 1600 or 3200 mg/kg bw per day from day 16 of gestation to 23 days after parturition. The dams and pups were killed on postnatal day 23. The severity of local tissue damage at the injection site was dose-dependent. There were no effects on maternal body weight, length of gestation or sex ratio. The litter sizes were reduced at 1600 and 3200 mg/kg bw per day but with no dose–response relationship. Postnatal development of pups and the proportion of pups successfully weaned were unaffected. There were no effects on reproductive capacity at 800 mg/kg bw per day (Griffiths, 1975b).
Rats
Groups of 20 pregnant Sprague-Dawley rats were given cefuroxime sodium in physiological saline by subcutaneous injection at a dose of 0, 200, 400 or 800 mg/kg bw per day from day 17 of gestation to day 20 after parturition. The dams were killed on postnatal day 21. One male and two female F1 offspring per dam in each group were mated at 10 weeks of age; one of the two females was killed on day 20 of gestation, and the other was allowed to deliver and rear the F2 pups for 3 weeks. Body-weight gain and food and water intakes were increased in F0 dams in all groups during lactation. No differences in gestation or parturition or in the numbers of implantations or live pups, sex ratios or body weights of offspring were found in any generation. Caecal weights at the time of weaning were increased in male and female F1 pups of dams at 400 and 800 mg/kg bw per day. Pre- and postnatal development, fertility and reproductive capacity were unaffected at any dose (Otaka et al., 1979).
Rabbits
Groups of 10–16 pregnant Dutch rabbits were given cefuroxime sodium in 0.9% saline by intramuscular injection at a dose of 0, 50, 100 or 200 mg/kg bw per day from day 19 of gestation to 6 weeks after parturition. Surviving does and offspring were killed on postnatal days 42–44; however, several deaths occurred before parturition: one at the lowest dose, two at the intermediate dose and six at the highest dose. The deaths were attributed to perturbation of the maternal gut flora and secondary toxaemic effects on the liver. Another three does given 200 mg/kg bw per day either aborted or lost their entire litters soon after parturition. There were no effects on maternal body weight, length of gestation, sex ratio or litter size. The weights of pups of does at the highest dose were consistently low at parturition and throughout lactation, but their postnatal development and survival were unaffected. There were no effects on offspring of does at 100 mg/kg bw per day (Griffiths, 1975c).
Studies on nephrotoxic potential that did not comply with GLP were carried out in rats, rabbits and dogs.
Rats
Groups of 10 male and 10 female Sprague-Dawley JCL rats were given cefuroxime sodium in distilled water as a single injection, either subcutaneously at 0, 1000 or 3000 mg/kg bw or as intravenously at 0, 500 or 1500 mg/kg bw. It was not clear when the tests were conducted or when the animals were killed, but they were probably killed 24 h after treatment. The specific gravity and sodium and potassium content of urine were increased in females at 3000 mg/kg bw, and the urinary sodium concentration was decreased in males at this dose. Decreased blood concentrations of creatinine were seen in females, of potassium in males and of uric acid in males and females, all at 3000 mg/kg bw. The blood concentrations of magnesium in females at 1500 mg/kg bw and of urea nitrogen in both sexes at 500 and 1500 mg/kg bw were increased. Renal function tests and histopathological examination of a range of tissues revealed no treatment-related changes (Ito et al., 1979d).
Groups of 10 male and 10 female Sprague-Dawley JCL rats were given cefuroxime sodium in distilled water by subcutaneous injection at a dose of 0, 800 or 1600 mg/kg bw per day or by intravenous injection at 0, 400 or 800 mg/kg bw per day for 1 week. The results of urine analysis were unremarkable. At 1600 mg/kg bw per day given subcutaneously, the blood concentrations of glucose, protein and albumin were decreased in males, those of creatinine and uric acid were decreased in females, and that of sodium was increased in females. The blood urea nitrogen concentration was increased at both intravenous doses, and the blood concentrations of uric acid and sodium were increased at 800 mg/kg bw per day. Renal function tests and histopathological examination of a range of tissues revealed no treatment-related changes (Ito et al., 1979d).
Rabbits
Groups of five male and five female New Zealand white rabbits were given cefuroxime sodium in distilled water as a single intramuscular injection of 0, 100 or 300 mg/kg bw. It was not clear when the tests were conducted or when the animals were killed, but they were probably killed 24 h after treatment. The urinary chloride concentration was increased in females at both doses. Tests for blood biochemistry and renal function and histopathological examination of a range of tissues revealed no treatment-related changes (Ito et al., 1979d).
Groups of five male and five female New Zealand white rabbits were given cefuroxime sodium in distilled water by intramuscular injection at a dose of 0 or 100 mg/kg bw per day for 1 or 4 weeks. After 4 weeks of dosing, increased urine volume associated with decreased sodium, potassium and chloride concentrations were observed in treated females. Increased creatinine and phenolsulfonphthalein clearance were seen in females treated for 1 week but not in those treated for 4 weeks. Blood biochemical and histopathological examination of a range of tissues revealed no treatment-related changes (Ito et al., 1979d).
Dogs
Groups of three male and three female beagle dogs were given cefuroxime sodium in distilled water as a single intravenous injection of 0 or 1000 mg/kg bw. Urinary and blood analyses gave unremarkable results. Phenolsulfonphthalein clearance was increased in treated females. Histopathological examination of a range of tissues revealed no treatment-related changes (Ito et al., 1979d).
Groups of three male and three female beagle dogs were given cefuroxime sodium in distilled water by intravenous injection at a dose of 0, 250 or 500 mg/kg bw per day for 1 week. No treatment-related changes were found in the results of urinary analysis or blood biochemistry, tests for renal function or histopathological examination of a range of tissues (Ito et al., 1979d).
Dogs
In a study that did not comply with GLP, two male beagle dogs were given cefuroxime sodium in saline by subcutaneous injection at a dose of 0, 100 or 300 mg/kg bw per day for 10 days. One dog was given a single daily dose, while the other was given three divided doses, 8 h apart. There were no effects on body weight or general condition. Blood samples were taken 1, 4 and 8 h after injection on the first and eighth days and 24 h after the initial dose on the first, second and tenth days. The activities of alkaline phosphatase, alanine and aspartate aminotransferases, sorbitol dehydrogenase and glutamate dehydrogenase were not affected at any time. No histopathological changes were observed in the liver. As only a summary of this study was available, the findings cannot be confirmed independently (Glaxo Laboratories Ltd, 1986).
Several studies were available on the effects of orally administered cefuroxime on the faecal bacterial flora of humans. These studies were carried out according to appropriate standards for study protocol and conduct.
Six healthy male volunteers aged 22–40 (weighing 70–86 kg) were given 10 oral doses of 60 mg of cefuroxime axetil, 8 h apart, for a total dose of about 8.5 mg/kg bw. Bowel function was recorded, and faecal samples were collected for microbio-logical analysis 2 days before and during treatment. Three of the six volunteers had diarrhoea during the dosing period which lasted for 2 days. Another had mild abdominal discomfort for 1 day. A decline in the number of Escherichia coli and coliforms (> 2 log) was seen in the faeces of the three men who developed diarrhoea. Similarly, there was a > 2 log-fold decrease in the number of Streptococcus faecalis in two men with diarrhoea. S. faecalis could not be detected in the third. The count of Candida spp. increased from 103 to 109 in two men with diarrhoea and to 105 in the other. It had been shown previously that oral doses of cefuroxime and other cephalosporins can increase the proportion of yeast (Candida albicans) in the gastrointestinal flora (Thomakos et al., 1998). The number of Bacteroides spp. declined from 1010 to 103 and those of Peptostreptococcus and Peptococcus spp. decreased from 1010 to < 103 in two of the men with diarrhoea and to 5 × 106 in the third. After cessation of dosing, the microbial counts returned to pre-treatment levels. There were no effects on Clostridium spp. (Wise et al., 1984).
Oral administration of cefuroxime axetil at a dose of 250 mg twice daily for 4.5 days to 10 healthy volunteers (five men, five women; mean age, 35 years) caused gastrointestinal disturbances. One of the subjects developed a feeling of nausea, and five reported a bloated feeling; seven reported soft faeces, and two detected a change in odour. Diarrhoea developed in four volunteers, and six reported an increased number of defaecations during a day. One woman developed vaginitis. Bowel function returned to normal within 5 days of completing treatment. Decreased total numbers of anaerobes and total aerobes were found in several individuals. The number of Enterobacteriaceae was reduced or even nil in six persons. Those of Streptococcus and Candida spp. were increased. Pseudomonas aeruginosa appeared in the faeces of four persons during the treatment period. Clostridium difficile and C. perfringens were not detected in any faecal samples, and no clostridial toxins were found. In three persons in whom Enterobacteriaceae were eliminated, recolonization with the sensitive species E. coli, Citrobacter freundii or Klebsiella ozaenae occurred. The populations of faecal flora returned to pre-treatment proportions about 7 days after cessation of treatment (Leigh et al., 1990).
Treatment of eight patients for acute exacerbation of bronchitis with cefuroxime axetil at a dose of 250 mg (~ 4.1 mg/kg bw) twice a day for 10 days resulted in reduced numbers of Staphylococci, Enterobacteriaceae and clostridial populations. These returned to pre-treatment levels 14 days after cessation of treatment. C. difficile appeared in three patients (Novelli et al., 1995). In another study, in which 15 recipients of liver transplants were given 6.3 g of cefuroxime (100 mg/kg bw), 0.6 g of tobramycin and 0.5 g of nystatin three times daily for 19–21 days to suppress the gut flora, only a few samples contained C. difficile. The diarrhoea induced by the treatment was self-limiting (Hove et al., 1996).
Minimum inhibitory concentrations (MICs) have been reported for a range of bacterial species.
Cefuroxime was included for comparison in a multicentre trial of agents against more than 42 000 gram-positive and gram-negative organisms. This survey from 79 medical centres and community hospitals in the USA made it possible to establish MIC50 and MIC90 values for Proteus mirabilis, P. vulgaris, Escherichia coli and Enterococcus faecalis (Murray et al., 1994). These data and data for the same organisms reported by Marshall, A. (1995) are shown in Table 3. The results are based on an inoculum density of 107 CFU/ml. The two reports show closely similar values.
Table 3. Minimum inhibitory concentrations (MICs) of clinical isolates in two studies
|
Organism |
Murray et al. (1994) |
Marshall, A. (1995) |
||
|
|
MIC50 |
MIC90 |
MIC50 |
MIC90 |
|
Escherichia coli |
4 |
8 (10 942) |
4 |
4 (10) |
|
Proteus mirabilis |
2 |
4 (3 822) |
1 |
2 (7) |
|
Proteus vulgaris |
> 64 |
> 64 (341) |
256 |
256 (3) |
|
Enterococcus faecalis |
32 |
32 (2 624) |
8 |
256 (10) |
Numbers in parentheses are numbers of isolates studied.
MIC values for cefuroxime (sodium) against 100 bacterial strains, comprising 10 isolates of 10 genera derived from human gut isolates, were determined by an agar dilution method (Table 4). The bacteria tested are usually considered the most relevant and representative of human gut flora. Of the 10 groups tested, five genera (Enterococcus spp., Lactobacillus spp., Bifidobacterium spp., Peptostreptococcus spp. and Clostridium spp.) showed a strong inoculum effect (more than two antibiotic dilutions) between the undiluted culture and a 10–2 dilution, and two genera (Bacteroides spp. and Bifidobacterium spp.) showed an inoculum effect between the 10–2 and 10–4 dilutions. The information available indicates that the lowest relevant MIC50, at the highest inoculum level of 109 CFU/ml, was 8 µg/ml for Bifidobacterium spp. (Marshall, R., 1995).
Table 4. Minimum inhibitory concentrations (MICs) of cefuroxime against predominant intestinal microflora at three inoculum levels
|
Genus |
MIC50 (µg/ml) |
||
|
Nominal 109 CFU |
Nominal 107 CFU |
Nominal 105 CFU |
|
|
Escherichia coli |
8 |
4 |
2 |
|
Proteus spp. |
8 |
2 |
1 |
|
Enterococcus spp. |
256 |
8 |
4 |
|
Lactobacillus spp. |
128 |
4 |
1 |
|
Fusobacterium spp. |
128 |
0.5 |
0.062 |
|
Bacteroides spp. |
> 256 |
256 |
8 |
|
Bifidobacterium spp. |
8 |
1 |
0.25 |
|
Peptostreptococcus spp. |
128 |
4 |
1 |
|
Clostridium spp. |
256 |
32 |
2 |
|
Eubacterium spp. |
32 |
32 |
16 |
In a limited study of the susceptibility of 124 bacteria of aerobic origin isolated from patients with spontaneous bacterial peritonitis, the MIC50 and MIC90 values for E. coli were both 4 µg/ml, and the MIC50 for Enterococcus spp. was > 16 µg/ml (Sader et al.,1995).
In a study specifically designed to determine the effects of inoculum size on MIC values, a clear inoculum effect was seen in several bacteria. Many cephalosporin antibiotics have an inoculum effect, which may be related to drug deactivation or metabolism (Goldstein et al., 1991). The significance of this observation is twofold. First, at high inocula, the activity of cefuroxime is greatly reduced; therefore, use of MICs for concentrations of organisms lower than those found in vivo in the gastrointestinal tract will result in overestimates of the potential of cefuroxime to affect the microflora adversely. Secondly, the data show potential beta-lactamase production, which in vivo would lead to inactivation of cefuroxime. This conclusion is supported by the work of Barry et al. (1977).
The effects of co-culture on the MIC and minimum bactericidal concentration of cefuroxime against 10 combinations of bacteria from the human gastrointestinal microflora were determined by agar dilution and by broth microdilution. The results (Table 5) showed that the activity of cefuroxime was significantly reduced, by a factor of up to 20, by co-culture as compared with monoculture. The relatively sensitive Fusobacterium mortiferum strain became less susceptible when tested in co-culture with a more resistant, beta-lactamase-producing Proteus vulgaris strain. This was not observed in all cases. The results also showed that the gut microrganisms can protect themselves against the antimicrobial effects of cefuroxime, thus affording protection of the overall gut ecosystem (Pridmore, 1996). Indeed, significant beta-lactamase activity has been detected in human faeces (Nord et al., 1989). Particular organisms are known to produce the enzyme, and some, like Bacteroides fragilis, have strains that produce high concentrations (Cornick et al., 1990). In a study of the beta-lactamase activity in bacteria used to determine the MICs of cefuroxime, 21 of the 100 strains representative of the human gut flora had intrinsic beta-lactamase activity, and a further eight isolates produced beta-lactamase in the presence of cefuroxime (Marshall, A., 1996).
Table 5. Effect of co-culture of intestinal microflora on the microbiological activity of cefuroxime
|
Organism |
Individual broth |
Individual |
MBC in co-culture |
|
Bifidobacterium adolescentis |
0.25 |
0.25 |
0.125 |
|
Bacteroides distasonis |
> 256 |
> 256 |
> 256 |
|
Proteus vulgaris |
> 256 |
> 256 |
> 256 |
|
Fusobacterium mortiferum |
0.25 |
0.25 |
4 |
|
Enterococcus faecalis |
> 256 |
> 256 |
> 256 |
|
Bifidobacterium spp. |
4 |
256 |
> 256 |
|
Enterococcus faecalis |
> 256 |
> 256 |
> 256 |
|
Bifidobacterium spp. |
16 |
32 |
64 |
|
Proteus mirabilis |
16 |
32 |
16 |
|
Clostridium perfringens |
4 |
64 |
16 |
|
Bacteroides distasonis |
32 |
32 |
2 |
|
Peptostreptococcus spp. |
1 |
2 |
2 |
|
Proteus vulgaris |
> 256 |
> 256 |
> 256 |
|
Bifidobacterium spp. |
1 |
2 |
2 |
|
Bacteroides uniformis |
> 256 |
> 256 |
> 256 |
|
Peptostreptococcus magnus |
1 |
1 |
2 |
|
Proteus mirabilis |
32 |
128 |
128 |
|
Fusobacterium spp. |
0.016 |
0.016 |
0.031 |
|
Peptostreptococcus asaccharolyticus |
0.25 |
0.25 |
0.25 |
|
Fusobacterium spp. |
0.016 |
0.016 |
0.031 |
MBC, minimum bactericidal concentration
Cefuroxime is unaffected by many of the common beta-lactamases and is effective against bacterial strains that are resistant to other beta-lactam antibiotics. Cefuroxime is stable to many bacterial beta-lactamases, especially plasmid-mediated enzymes that are commonly found in Enterobacteriaceae (Medical Economics Co., 2002).
A model was used to assess the effects of cefuroxime on bacteria representative of the human gut flora in vitro under conditions that mimicked those in the human gut. Two concentrations were used: one was based on the maximum concentration of cefuroxime residues expected to be found in the human gut, and the second was comparable to the MICs for cefuroxime against the respective human gut isolates. The effects of cefuroxime were investigated against five groups of bacteria (Clostridium sporogenes, Cl. difficile, Peptostreptococcus magnus, P. anaerobius, and Bacteroides spp.) found in the human gut. The drug was added to sterile anaerobic cooked-meat medium supplemented with pepsin and incubated anaerobically at pH 2 and 37 oC for 1 h. After adjustment to pH 7 and the addition of physiological levels of bile salts and pancreatin, the tester strain was added, and the culture was incubated anaerobically for 18 h. The results are given in Table 6.
Table 6. Effects of cefuroxime on human intestinal microflora in vitro
|
Bacterial strain |
Cefuroxime concentration |
Inoculum density |
Total viable count (cfu/ml) |
Cefuroxime MIC (mg/ml) |
|
|
On inoculation |
After 18 h incubation |
||||
|
Clostridium sporogenes |
0 |
|
5.0 × 106 |
9.8 × 108 |
|
|
2 |
8.0 × 108 |
4.8 × 106 |
8.1 × 107 |
2 |
|
|
4 |
|
5.1 × 106 |
6.3 × 106 |
|
|
|
Clostridium difficile |
0 |
|
1.1 × 106 |
6.4 × 108 |
|
|
2 |
1.6 × 108 |
1.6 × 106 |
1.0 × 107 |
256 |
|
|
4 |
|
1.3 × 106 |
6.3 × 106 |
|
|
|
Peptostreptococcus magnus |
0 |
3.2 × 108 |
3.9 × 106 |
3.7 × 106 |
0.5 |
|
2 |
|
3.7 × 108 |
6.9 × 107 |
|
|
|
Peptostreptococcus anaerobius |
0 |
4.8 × 108 |
6.0 × 106 |
2.5 × 108 |
1 |
|
2 |
|
5.1 × 107 |
5.6 × 107 |
|
|
|
Bacteroides thetaiotaomicron |
0 |
|
1.2 × 106 |
7.6 × 108 |
|
|
2 |
1.0 × 108 |
6.0 × 105 |
9.6 × 107 |
256 |
|
|
256 |
|
6.5 × 105 |
2.8 × 107 |
|
|
|
Bacteroides spp. |
0 |
|
7.2 × 105 |
8.0 × 108 |
|
|
2 |
1.5 × 108 |
6.0 × 105 |
4.5 × 108 |
2 |
|
|
256 |
|
7.2 × 105 |
< 2.5 × 105 |
|
|
|
Lactobacillus spp.a |
0 |
|
2.2 × 106 |
6.3 × 107 |
|
|
2 |
3.5 × 108 |
1.7 × 106 |
1.0 × 107 |
4 |
|
|
128 |
|
2.1 × 106 |
< 2.5 × 105 |
|
|
|
Lactobacillus spp. |
0 |
|
3.4 × 106 |
2.6 × 108 |
|
|
2 |
4.0 × 108 |
2.7 × 106 |
2.1 × 108 |
256 |
|
|
128 |
|
2.2 × 106 |
8.3 × 107 |
|
|
|
Escherichia coli a |
0 |
|
9.2 × 106 |
1.1 × 109 |
|
|
2 |
9.3 × 108 |
9.8 × 106 |
2.9 × 108 |
2 |
|
|
8 |
|
8.7 × 107 |
8.7 × 108 |
|
|
|
Escherichia coli |
0 |
|
1.0 × 107 |
1.2 × 109 |
|
|
2 |
1.1 × 109 |
7.7 × 106 |
3.9 × 108 |
4 |
|
|
8 |
|
1.0 × 107 |
1.9 × 108 |
|
|
a
Duplicate names indicate different strains/isolates.Eight of the 10 strains tested showed no pronounced change or increase in viable count. The results obtained for 10 bacterial strains in this investigation suggest that cefuroxime had no detectable effects at the concentrations used, which were equal to or greater than those likely to be achieved in the human gut in vivo. Reduced numbers of one strain of Bacteroides spp. and one strain of Lactobacillus spp. were found, but in both cases only after exposure to very high concentrations (> 128 µg/ml) chosen as the geometric mean MIC against these organisms (Pridmore, 1995).
Published reviews of adverse effects in patients treated with cefuroxime sodium and cefuroxime axetil at therapeutic doses indicate that these drugs are generally well tolerated by adults and children. Gastrointestinal disturbances such as stomatitis, nausea and diarrhoea, particularly after oral dosing, occur frequently and are due to the effects of the drug on the colonic microflora. Administration by intramuscular injection commonly causes irritation, thrombophlebitis and pain at the injection site. Hypersensitivity reactions are relatively uncommon, and central nervous system toxicity is rare. Increased liver enzyme activity and blood urea nitrogen concentration and anaemia and other haematological abnormalities (i.e. eosinophilia, leukopenia, neutropenia and positive Coombs test [antibodies to red blood cells}) have been observed occasionally. These changes have been mild, asymptomatic and transient and are regarded as clinically inconsequential (Smith & Le Frock, 1983; Perry & Brogden, 1996). Pseudomembranous colitis is infrequent and occurs at a low incidence (Parfitt, 1999).
High doses of cefuroxime were associated with the development of reversible encephalopathy characterized by dulled sensibility or stupor, myoclonic jerks and asterixis (negative myoclonus) in four patients. Three of the patients were over 60 years of age and had renal failure, and the condition arose after two to three intravenous injections of 750 mg. The other patient had been given 125 mg of cefuroxime axetil orally three times a day for 2 years and had then received a dose of 6000 mg for 4 days. In each case, the condition resolved on cessation of treatment (Herishanu et al., 1998).
A study was conducted in 106 women who received cefuroxime during the first trimester of pregnancy. The women were enrolled prospectively and paired for age, smoking habits and alcohol consumption. The daily dose of cefuroxime was 500–1000 mg, and the duration of treatment was 5–10 days. The risk for spontaneous abortion, fetal distress or malformations in neonates was not increased. The rate of induced abortions was significantly higher in the treated group, but the number of live births, the birth weights and postnatal motor development were similar in the two groups (Berkovitch et al., 2000).
The case records of 78 women aged 19–38 years who had been treated with cefuroxime during pregnancy were analysed retrospectively. Treatment had been given during the first trimester to 13 women, during the second trimester to 19 women and during the third trimester to 46 women. The physical and mental development of the children born to these mothers was evaluated for 18 months after birth. No abnormalities were detected (Manka et al., 2000).
The Committee considered the results of studies on pharmacokinetics and metabolism, acute and short-term toxicity, genotoxicity, fertility, reproductive and developmental toxicity, toxicity to the kidney and liver, microbiological safety and studies in humans. Many of the older studies were available as summary reports only and thus could not be assessed, nor was it possible to determine whether the studies had been carried out according to appropriate standards for study protocol and conduct. The newer studies on genotoxicity and microbiological activity were carried out according to appropriate standards.
Toxicological data
Cefuroxime, when administered as cefuroxime sodium, was poorly absorbed from the gastrointestinal tract of humans after oral administration. When injected into rats and humans, cefuroxime was widely distributed throughout the body. Metabolism was negligible in rats, dogs and humans; however, the Committee noted that significant transformation occurred after intramammary treatment of cows with cefuroxime sodium. Elimination occurred rapidly, largely in the urine, in rats, rabbits, dogs and humans and also in food-producing animals. A small proportion was excreted in the bile of rats and dogs. More absorption occurred after oral administration of cefuroxime axetil in rats, dogs and humans. The ester was rapidly hydrolysed in the intestinal mucosa and blood to yield cefuroxime, acetaldehyde and acetic acid. Thus, the spectrum of toxicity of cefuroxime axetil in vivo would be the same as that of cefuroxime.
In a study summarized in the residue monograph (FAO, 2002), which was conducted according to good laboratory practice (GLP), eight lactating dairy cows (weighing 402–599 kg) were given [14C]cefuroxime sodium by intramammary infusion after each of three successive milkings at a nominal dose of 250 mg per quarter per treatment, for a total dose of 3000 mg. Approximately 95% of the dose administered to two of the cows held in metabolic cages was accounted for, with 78% in milk, 10% in urine, 4.6% in faeces and 2.4% in the cage wash. The concentration of cefuroxime equivalent (0.10 mg/kg) in blood peaked 24 h after treatment and declined steadily to 0.01 mg/kg at 196 h. The concentrations were higher in the plasma fraction (0.13 mg/kg at 24 h) than in serum. The elimination appeared to follow a biphasic model, suggesting distribution in a body compartment other than blood or plasma. Elimination was primarily in milk (75–82%) and urine (6–14%). Parent compound accounted for about 20% of the residue in urine, while the remaining residues were in three unidentified polar fractions separated by liquid chromatography. Parent compound also accounted for about 20% of the total residues in milk during and immediately after treatment, declining to about 6% several days after treatment and later to < 2%. The Committee considered that factors other than metabolism, such as compound instability, might have accounted for much of this depletion from milk; however, no data were available to confirm this hypothesis. At slaughter 7 days after the third dosing, no antimicrobiologically active residues were extracted from the kidney, the edible tissue with the highest concentration of radiolabelled residues. About 60% of the radiolabel was extractable from kidney, but very little co-eluted in chromatographic analysis with the parent compound.
Cefuroxime sodium was of low acute toxicity when given orally, and it was not lethal at single oral doses of up to 10 000 mg/kg bw in mice and rats. The main adverse effect seen was diarrhoea. Parenteral administration by the subcutaneous, intramuscular, intraperitoneal or intravenous route of single doses of up to 2000 mg/kg bw was also well tolerated in mice, rats, cats, dogs and cynomolgus monkeys. Rabbits were the most sensitive to the acute toxicity of cefuroxime.
In short-term studies of toxicity, rats were given parenteral doses of cefuroxime sodium for periods of 29–91 days (50–1500 mg/kg bw per day) and 26 weeks (50–750 mg/kg bw per day). Reductions in erythrocyte count, haemoglobin concentration and erythrocyte volume fraction were observed consistently at doses of 100–1500 mg/kg bw per day, and decreased serum protein concentrations were detected in some studies in animals given doses of 375–1500 mg/kg bw per day. The only pathological change, apart from inflammation and haemorrhage at the injection site, was swelling of the caecum at doses of 250–1500 mg/kg bw per day. No effects were seen at 50 mg/kg bw per day for 26 weeks.
Dogs were given cefuroxime sodium parenterally for periods of 10–35 days (50–540 mg/kg bw per day) and 26 weeks (50–450 mg/kg bw per day). Body-weight loss was observed at 250 and 500 mg/kg bw per day for 35 days, but body weight was not affected at any dose during the 26-week study. Haemoglobin concentrations were reduced at 150 and 450 mg/kg bw per day for 26 weeks, and lower serum iron concentrations were found at 450 mg/kg bw per day. One animal given 450 mg/kg bw per day developed severe haemolytic anaemia after 12 weeks. The pathological changes in other dogs were restricted to inflammatory reactions at the injection site. No effects were found at a dose of 50 mg/kg bw per day for 26 weeks.
Dogs were treated orally with aqueous solutions of cefuroxime axetil, providing a dose of 0, 100, 400 or 1600 mg/kg bw per day as cefuroxime, for 27 weeks. Treatment-related effects were seen only at 1600 mg/kg bw per day. Body-weight gain was retarded. Erythrocyte count, haemoglobin concentration and erythrocyte volume fraction were reduced, and the reticulocyte count was increased. Clotting times were prolonged, the concentrations of coagulation factor VII and plasma total protein, albumin and cholesterol were reduced, and the concentration of triglycerides was increased. The weight of the kidneys was increased in males, but there was no histological change attributable to cefuroxime. The NOEL was 400 mg/kg bw per day.
Cynomolgus monkeys were given cefuroxime sodium by intramuscular injection at a dose of 0, 150 or 450 mg/kg bw per day for 28 days. Soft faeces were reported in some animals in each treatment group, but no further details were available. At the highest dose, the erythrocyte count and haemoglobin concentration were reduced during the initial stages of the study. Inflammation at the injection site was the sole pathological finding.
Assays covering an adequate range of genotoxic end-points were conducted with cefuroxime sodium. The results were negative, with the exception of an assay for chromosomal aberration in vitro in the absence of metabolic activation, but effects were seen only after prolonged exposure. The Committee concluded that this result, when taken in conjunction with the negative results in a test for micronucleus formation in the bone marrow of mice treated intraperitoneally, was not of biological significance. It concluded that cefuroxime does not pose a genotoxic hazard.
No long-term studies of toxicity were carried out with cefuroxime. The drug has no significant genotoxic activity and is not chemically related to known carcinogens. Furthermore, cefuroxime was poorly absorbed from the gastrointestinal tract. No neoplastic or preneoplastic lesions were observed in 26-week studies in rats and dogs given repeated parenteral doses. Under the circumstances, the Committee concluded that studies of carcinogenicity were unnecessary.
Multigeneration studies of reproductive toxicity and peri- and postnatal toxicity were carried out in mice (800–3200 mg/kg bw per day) and rats (200–800 mg/kg bw per day) treated subcutaneously. Fertility and reproduction in parental animals and postnatal development of the offspring were unaffected at any dose. Developmental toxicity was investigated in mice (800–6400 mg/kg bw per day) and rats (200–1600 mg/kg bw per day) treated subcutaneously. There was no effect on fetal development.
Rabbits received cefuroxime at intramuscular doses of 50–400 mg/kg bw per day during gestation and/or until 6 weeks after parturition. A dose-related increase in the mortality rate of dams was observed at all doses, with diarrhoea occurring before death. This effect was probably due to disturbances of the gut flora, a recognized phenomenon in rabbits given antimicrobial agents. There were no effects on fetal development or postnatal growth and survival at any dose.
Special studies designed to identify potential renal toxicity were undertaken in rats (given single doses of up to 3000 mg/kg bw and up to 1800 mg/kg bw per day for 1 week), rabbits (given single doses of up to 300 mg/kg bw and 100 mg/kg bw per day for 4 weeks) and dogs (given single doses of 1000 mg/kg bw and up to 500 mg/kg bw per day for 1 week). There was no evidence of renal toxicity.
In a special study to investigate hepatic toxicity in dogs, the concentrations of a range of serum liver enzymes were not affected by doses of cefuroxime of 100 and 300 mg/kg bw per day for 10 days.
Humans treated with cefuroxime sodium or cefuroxime axetil have generally shown few toxic side-effects. Gastrointestinal disturbances are frequent, particularly after oral dosing, and irritation and pain commonly occur at sites of injection. Hypersensitivity reactions are relatively uncommon, and central nervous system toxicity is rare. Increased serum concentrations of liver enzymes and blood urea nitrogen and anaemia and other haematological abnormalities are observed occasionally. These changes are mild and are generally regarded as inconsequential. In elderly patients with renal failure, which impedes the elimination of cefuroxime, higher systemic concentrations have been associated with reversible encephalopathy. Data from use during pregnancy, although limited, did not show any adverse effects on the developing fetus or the neonate.
The most relevant study for determining a toxicological NOEL is the 27-week study in dogs given cefuroxime axetil orally. Owing to the rapid conversion of the axetil ester to cefuroxime, the effects observed are likely to be due to the latter. Studies in which cefuroxime sodium was given by parenteral injection are less relevant for assessing the acceptable intake of cefuroxime in food. Hence, the NOEL for toxicity was 400 mg/kg bw per day. A safety factor of 100 was considered appropriate in view of the existing database for a compound with a long history of use. Therefore, an ADI of 0–4 mg/kg bw could be established on the basis of the toxicological data.
Microbiological data
Oral administration of cefuroxime axetil at therapeutic doses of 2.5–8 mg/kg bw per day to human patients or volunteers resulted in gastrointestinal disturbances. The signs and symptoms included soft faeces, nausea, a bloated feeling and diarrhoea. Further investigation showed that these overt signs were associated with decreased numbers of bacteria or, in extreme cases, elimination of certain colonic flora. In a few subjects, there was evidence of overgrowth of pathogenic bacteria or yeasts, suggesting disruption of the protective barrier effect in the colon. The populations of colonic flora returned to normal within 7–14 days after cessation of treatment.
Cefuroxime has been tested for its inhibitory activity against microorganisms representative of the human colonic microflora. The most sensitive species were Bifidobacterium spp., with an MIC50 value of 8 mg/ml at an inoculum density of 109 CFU/ml. It has been shown that the activity of cefuroxime is reduced when large inocula are used or when two or more organisms are combined in co-culture, possibly due to the production of beta-lactamase. In an in-vitro model of the gut that mimics the conditions found in the human colon, cefuroxime at very high concentrations inhibited the growth of two of 10 co-culture combinations of bacteria from the human gastrointestinal tract. These results indicate that the intestinal microflora can protect themselves from the antimicrobial effects of cefuroxime, thus affording protection to the overall gut ecosystem.
A decision-tree for evaluating the potential effect of veterinary drug residues on human intestinal microflora was developed by the Committee at its fifty-second meeting (Annex 1, reference 140) and is reproduced in Figure 1. At its present meeting, the Committee used the decision-tree to answer the following questions in its assessment of cefuroxime:
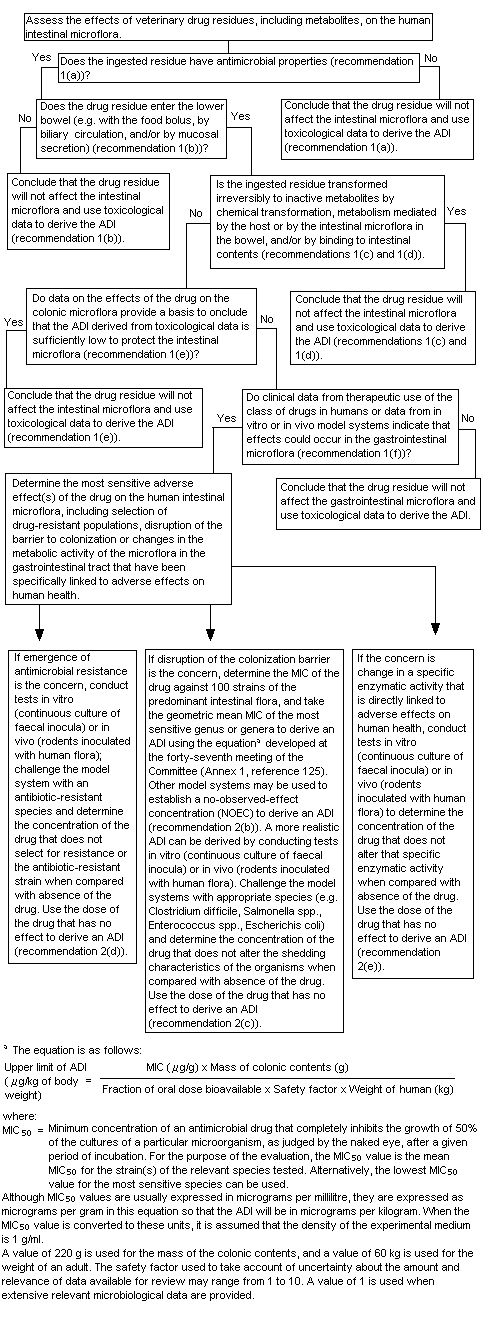
Figure 1. Decision-tree for determining the potential adverse effects of residues of veterinary antimicrobial drugs on the human intestinal microflora
1. Does the ingested residue have antimicrobial properties?
Yes. The parent compound is present as a residue and has been shown to have antimicrobial activity. Significant transformation occurred after intramammary treatment of cows with [14C]cefuroxime sodium, but the degradation products were not identified and their antimicrobial activity is unknown.
2. Does the drug residue enter the lower bowel by any route?
Yes. Cefuroxime sodium is poorly absorbed in mammals, including humans. As approximately 1% of an oral dose of cefuroxime was absorbed systemically in humans, most of an oral dose enters the intestines.
3. Is the ingested residue transformed irreversibly to inactive metabolites by chemical transformation, metabolism mediated by the host or intestinal microflora in the bowel and/or by binding to intestinal contents?
No specific information was available on the metabolism of cefuroxime by intestinal microflora. Cefuroxime undergoes negligible metabolism in rats, dogs and humans; however, intestinal microflora have the potential to deactivate cefuroxime, as they have beta-lactamase enzymes. As there was no direct evidence that cefuroxime is metabolized to inactive metabolites by the intestinal microflora, it was assumed that microbiological activity is retained in the human gastrointestinal tract.
4. Do data on the effects of the drug on the colonic microflora provide a basis to conclude that the ADI derived from toxicological data is sufficiently low to protect the intestinal microflora?
No. A number of studies, including those to establish MIC50 values, experiments with co-cultures, investigations of beta-lactamase activity and pH effects and studies with volunteers, have indicated that cefuroxime may have adverse effects on the intestinal microflora.
5. Do clinical data from the therapeutic use of the class of drugs in humans or data from in-vitro or in-vivo model systems indicate that effects could occur in the gastrointestinal flora?
Yes. Gastrointestinal effects are the most commonly reported adverse reactions to therapeutic use of cefuroxime in humans; they include diarrhoea, nausea, vomiting and abdominal pain.
6. Determine the most sensitive adverse effect of the drug on the human intestinal microflora.
The available data indicate that disruption of the colonization barrier is the main concern with cefuroxime, rather than emergence of resistance. In clinical practice, cefuroxime is used to treat patients with acute sinusitis, otitis media, chronic bronchitis and skin and urinary-tract infections. Cefuroxime is active against many typical aerobic gram-positive and gram-negative bacteria associated with these infections. There is always concern about decreased susceptibility to antimicrobial agents with increasing resistance; however, cefuroxime is not indicated for use against the predominant anaerobic bacteria commonly associated with the human gastro-intestinal tract, and the antimicrobial resistance of such organisms has not been reported.
7. If disruption of the colonization barrier is the concern, determine the MIC of the drug against 100 strains of predominant intestinal flora and take the geometric mean MIC of the most sensitive genus or genera to derive an ADI using the formula for estimating an ADI. Other model systems may be used to establish an NOEC to derive an ADI.
An evaluation of the MIC50 values for relevant gastrointestinal microflora provides a figure of 8 µg/ml for Bifidobacterium spp. This value can be used to calculate a microbiological ADI, as follows:

where:
MIC50 = minimum inhibitory concentration for 50% of strains of the most sensitive relevant organism. The MIC50 for the most sensitive relevant genus of the gut flora was 8 µg/ml (8 µg/g) for Bifidobacterium spp.
MCC = mass of colonic contents; a value of 220 g, determined by the Committee at its forty-seventh meeting (Annex 1, reference 125), was used in the calculation.
FA = available fraction of the dose: the microbiologically active residue is cefuroxime. As only about 1% of an oral dose of cefuroxime was systemically absorbed in humans, the fraction of the dose available to the gastrointestinal microflora is 100%. Thus, the value in the equation is unity (1).
SF = safety factor: the magnitude of the safety factor depends on the quality and quantity of the microbiological data available. A value of 1 is appropriate when extensive relevant microbiological data are available, as is the case in the current assessment. Thus, the safety factor should be unity (1).
BW = body weight: a value of 60 kg has been adopted for an adult.
Hence,
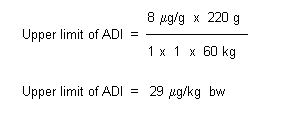
The Committee noted that there is significant transformation of cefuroxime in cattle, which does not occur in laboratory animals or humans. The toxicological activity of the majority of the residue has not been characterized. Therefore, the Committee established a temporary ADI of 0–30 mg/kg bw on the basis of the MIC50 for Bifidobacterium spp., pending the results of studies to (1) identify the residues in milk and clarify whether the residues other than parent compound are due primarily to metabolism or to non-metabolic decomposition of parent cefuroxime; and (2) characterize the toxicological significance of non-parent residues in milk. This information should be provided for evaluation in 2004. The ADI is based on a microbiological end-point and is significantly lower than it would be if it were based on a toxicological end-point. An extra safety factor was not applied to account for the fact that the ADI is temporary, because the concern is the possible toxicity of the transformation products rather then their antimicrobial activity.
Aaron, C.S., Yu, R.L., Harbach, P.R., Mazurek, J.M., Swenson, D.H., Kirkland, D., Marshall, R. & McEnaney, S. (1995a) Comparative mutagenicity testing of ceftiofur sodium: I. Positive results in in vitro cytogenetics. Mutat. Res., 345, 27–35.
Aaron, C.S., Yu, R.L., Bacon, J.A., Kirkland, D., McEnaney, S. & Marshall, R. (1995b) Comparative mutagenicity testing of ceftiofur sodium: II. Cytogenetic damage induced in vitro by ceftiofur is reversible and is due to cell cycle delay. Mutat. Res., 345, 37–47.
Ballantyne, M. (1996) Cefuroxime sodium: Reverse mutation in histidine-requiring strains of Salmonella typhimurium and tryptophan-requiring strains of Escherichia coli using the Microtitre® fluctuation technique. Unpublished report No. 808/60-1052 from Corning Hazleton, Harrogate, United Kingdom. Submitted to WHO by Schering-Plough Animal Health, Middlesex, United Kingdom.
Barry, A.L., Thornsberry, C., Jones, R.N., Fuchs, P.C., Gavan, T.L. & Gerlach, E.H. (1977) Cefuroxime, an in vitro comparison with six other cephalosporins. Proc. R. Soc. Med., 70 (Suppl. 9), 63–71.
Berkovitch, M., Segal-Socher, I., Greenberg, R., Bulkowshtein, M., Arnon, J., Merlob, P. & Or-Noy, A. (2000) First trimester exposure to cefuroxime: A prospective cohort study. Br. J. Clin. Pharmacol., 50, 161–165.
Capel-Edwards, K., Atkinson, R.M. & Pratt, D.A.H. (1979) Toxicological studies on cefuroxime sodium. Toxicology, 13, 1–5.
Cornick, N.A., Cuchural, G., Snydman, D.R., Jacobus, N.V., Iannini, P., Hill, G., Cleary, T., O’Keefe, J.P., Pierson, C. & Finegold, S.M. (1990) The antimicrobial susceptibility patterns of the Bacteroides fragilis group in the United States, 1987. J. Antimicrob. Chemother., 25, 1011–1019.
Craft, I., Mullinger, B.M. & Kennedy, M.R.K. (1981) Placental transfer of cefuroxime. Br. J. Obstet. Gynaecol., 88, 141–145.
FAO (2002) FAO Food and Nutrition Paper, No. 41/14, in preparation.
Fellows, M. (1995) Cefuroxime: Mutation at the thymidine kinase (tk) locus of mouse lymphoma L5178Y cells using the Microtitre® fluctuation technique. Unpublished report No. 808/79-1052 from Corning Hazleton, Harrogate, United Kingdom. Submitted to WHO by Schering-Plough Animal Health, Middlesex, United Kingdom.
Finn, A., Straughn, A., Meyer, M. & Chubb, J. (1987) Effect of dose and food on the bioavailability of cefuroxime axetil. Biopharm. Drug Disposition, 8, 519–526.
Foord, R.D. (1976) Cefuroxime: Human pharmacokinetics. Antimicrob. Agents Chemother., 9, 741–747.
Furuhashi, T., Nomura, A., Ikeya, E. & Nakazawa, M. (1979) Teratological study on cefuroxime in rabbits. Chemotherapy (Tokyo), 27 (Suppl. 6), 273–279.
Glaxo Laboratories Ltd (1986) Zinacef. Product licence No. 0004/0263. Unpublished report No. TOXDIG/44 from Glaxo Laboratories Ltd. Submitted to WHO by Schering-Plough Animal Health, Middlesex, United Kingdom.
Goldstein, E.J.C., Citron, D.M. & Cherubin, C.E. (1991) Comparison of the inoculum effect of cefoxitin and other cephalosporins and of beta-lactamase inhibitors and their penicillin-derived components on the Bacteroides fragilis group. Antimicrob. Agents Chemother., 35, 1868–1874.
Griffiths, H.M. (1975a) Effect of repeated subcutaneous administration of cephalosporin 640/359 on fertility in CRH mice. Unpublished report No. 75TA18, from Glaxo Research Ltd, United Kingdom. Submitted to WHO by Schering-Plough Animal Health, Middlesex, United Kingdom.
Griffiths, H.M. (1975b) Effect of repeated subcutaneous administration of cephalosporin 640/359 on lactation in CRH mice. Unpublished report No. 75TA15 from Glaxo Research Ltd, United Kingdom. Submitted to WHO by Schering-Plough Animal Health, Middlesex, United Kingdom.
Griffiths, H.M. (1975c) Effect of repeated intramuscular administration of cephalosporin 640/359 on lactation in Dutch rabbits. Unpublished report No. 75TA20 from Glaxo Research Ltd, United Kingdom. Submitted to WHO by Schering-Plough Animal Health, Middlesex, United Kingdom.
Harding, S.M., Williams, P.E.O. & Ayrton, J. (1984) Pharmacology of cefuroxime as the 1-acetoxyethyl ester in volunteers. Antimicrob. Agents Chemother., 25, 78–82.
Herishanu, Y.O., Zlotnik, M., Mostoslavsky, M., Podgaietski, M., Frisher, S. & Wirguin, I. (1998) Cefuroxime-induced encephalopathy. Neurology, 50, 1873–1875.
Hove, H., Tvede, M. & Mortensen, P.B. (1996) Antibiotic-associated diarrhoea, Clostridium difficile, and short-chain fatty acids. Scand. J. Gastroenterol., 31, 688–693.
Ito, R., Kawamura, H., Kajiwara, S., Toida, S., Matsuura, S., Hidano, T., Miyasaka, M., Kimura, H., Takahashi, M. & Tamura, J. (1979a) Study on the safety of cefuroxime (2). Five-week subacute toxicity and five-week recovery in rats. Chemotherapy (Tokyo), 27 (Suppl. 6), 130–151.
Ito, R., Kawamura, H., Kajiwara, S., Toida, S., Matsuura, S., Hidano, T., Miyasaka, M., Kimura, H., Takahashi, M., Saito, N., Seki, Y. & Tamura, J. (1979b) Study on the safety of cefuroxime (4). Six-month chronic toxicity and three-month recovery in rats. Chemotherapy (Tokyo), 27 (Suppl. 6), 171–208.
Ito, R., Kawamura, H., Kajiwara, S., Toida, S., Matsuura, S., Hidano, T., Watanabe, M., Tomizawa, S., Yano, K. & Tamura, J. (1979c) Study on the safety of cefuroxime (3). Five-week subacute toxicity and four-month recovery in beagle dogs. Chemotherapy (Tokyo), 27 (Suppl. 6), 152–170.
Ito, R., Kawamura, H., Kajiwara, S., Toida, S., Matsuura, S., Hidano, T., Miyasaka, M., Kimura, H., Takahashi, M., Saito, N., Seki, Y., Yano, K. & Tamura, J. (1979d) Study on the safety of cefuroxime (5). Nephrotoxicity in rats, rabbits and dogs. Chemotherapy (Tokyo), 27 (Suppl. 6), 209–244.
Kalman, D. & Barriere, S.L. (1990) Review of the pharmacology, pharmacokinetics, and clinical use of cephalosporins. Texas Heart Inst. J., 17, 203–215.
Leigh, D.A., Walsh, B., Leung, A., Tait, S., Peatey, K. & Hancock, P. (1990) The effects of cefuroxime axetil on the faecal flora of healthy volunteers. J. Antimicrob. Chemother., 26, 261–268.
Mandell, G.L. & Petri, W.A. (1996) Antimicrobial agents. Penicillins, cephalosporins and other -lactam antibiotics. In: Hardman, J.G., Limbird, L.E., Molinoff, P.B., Ruddon, R.W. & Gilman, A.G., eds, The Pharmacological Basis of Therapeutics, 9th Ed., London: McGraw-Hill, pp. 1073–1101.
Manka, W., Solowiow, R. & Okrzeja, D. (2000) Assessment of infant development during an 18-month follow-up after treatment of infections in pregnant women with cefuroxime axetil. Drug Saf., 22, 83–88.
Marshall, A. (1995) Determination of minimum inhibitory concentration (MIC) of cefuroxime against bacterial gut isolates at three inoculum levels. Unpublished report No. DWS/020/95 from Don Whitley Scientific, West Yorkshire, United Kingdom. Submitted to WHO by Schering-Plough Animal Health, Middlesex, United Kingdom.
Marshall, A. (1996) Detection of beta-lactamase activity in human gut bacteria used for cefuroxime MIC determination. Unpublished report No. DWS/007/96 from Don Whitley Scientific, West Yorkshire, United Kingdom. Submitted to WHO by Schering-Plough Animal Health, Middlesex, United Kingdom.
Marshall, R. (1995) Cephalonium: Induction of chromosome aberrations in cultured human peripheral blood lymphocytes. Unpublished report No. 808/50-1052 from Corning Hazleton, Harrogate, United Kingdom. Submitted to WHO by Schering-Plough Animal Health, Middlesex, United Kingdom.
Marshall, R. (1996) Cefuroxime sodium: Induction of chromosome aberrations in cultured human peripheral blood lymphocytes. Unpublished report No. 808/59-1052 from Corning Hazleton, Harrogate, United Kingdom. Submitted to WHO by Schering-Plough Animal Health, Middlesex, United Kingdom.
Medical Economics Co. (2002) Physicians‘ Desk Reference, www.pdref.com.
Murray, P.R., Cantrell, H.F. & Lankford, R.B. (1994) Multicenter evaluation of the in vitro activity of piperacillin–tazobactam compared with eleven selected -lactam antibiotics and ciprofloxacin against more than 42,000 aerobic gram-positive and gram-negative bacteria. Diagn. Microbiol. Infect. Dis., 19, 111–120.
Nanbo, T., Takaichi, M., Mitsugi, K., Esumi, Y., Okumura, K., Tsuji, H. & Fukuda, I. (1979) Pharmacokinetics and metabolic study of cefuroxime in rats and dogs. Chemotherapy (Tokyo), 27 (Suppl. 6), 91–103.
Nord, C.E., Bergan,T. & Thorsteinsson, S.B. (1989) Impact of ticarcillin/clavulanate on the intestinal microflora. J. Antimicrob. Chemother., 24 (Suppl. B), 221–226.
Novelli, A., Mazzei, T., Fallani, S., Dei, R., Cassetta, M.I. & Conti, S. (1995) Betalactam therapy and intestinal flora. J. Chemother., 7 (Suppl. 1), 25–31.
Okumura, K., Tsuji, H., Fukuda, I., Takeda, K., Kobayashi, J. & Kato, H. (1979) The absorption, biodistribution, metabolism and excretion of cefuroxime. Chemotherapy (Tokyo), 27 (Suppl. 6), 104–110.
Otaka, T., Kawasaki, H., Furuhashi, T., Sudoh, S., Nomura, A., Shimizu, Y. & Nakazawa, M. (1979) Reproduction study on cefuroxime in rats. Chemotherapy (Tokyo), 27 (Suppl. 6), 245–272.
Parfitt, K., ed. (1999) Martindale. The Complete Drug Reference, 32nd Ed., London: Pharmaceutical Press.
Perry, C.M. & Brogden, R.N. (1996) Cefuroxime axetil. A review of its antibacterial activity, pharmacokinetic properties and therapeutic efficacy. Drugs, 52,125–158.
Phillipson, A. & Stiernstedt, G. (1982) Pharmacokinetics of cefuroxime in pregnancy. Am. J. Obstet. Gynecol., 142, 823–828.
Powell, D.A., James, N.C., Ossi, M.J., Nahata, M.C. & Donn, K.H. (1991) Pharmacokinetics of cefuroxime axetil suspension in infants and children. Antimicrob. Agents Chemother., 35, 2042–2045.
Pridmore, A. (1995) Studies on the survival of selected human gut flora following incubation with cefuroxime in a simple in vitro gut model. Unpublished report No. DWS/026/95 from Don Whitley Scientific, West Yorkshire, United Kingdom. Submitted to WHO by Schering-Plough Animal Health, Middlesex, United Kingdom.
Pridmore A (1996) Determination of the minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MBC) of cefuroxime against selected co-culture combinations of bacterial gut isolates. Unpublished report No. DWS/008/96 from Don Whitley Scientific, West Yorkshire, United Kingdom. Submitted to WHO by Schering-Plough Animal Health, Middlesex, United Kingdom.
Riley, S. (1996) Cephalexin: Induction of chromosome aberrations in cultured human peripheral blood lymphocytes. Unpublished report No. 808/57-1052 from Corning Hazleton, Harrogate, United Kingdom. Submitted to WHO by Schering-Plough Animal Health, Middlesex, United Kingdom.
Ruiz-Balaguer, N., Nacher, A., Casabo, V.G. & Merino, M. (1997) Non-linear intestinal absorption kinetics of cefuroxime axetil in rats. Antimicrob. Agents Chemother., 41, 445–448.
Ruiz-Carretero, P., Nacher, A., Merino-Sanjuan, M. & Casabo, V.G. (2000) Pharmacokinetics and absolute bioavailability of oral cefuroxime axetil in the rat. Int. J. Pharm., 202, 89–96.
Sader, H.S., Runyon, B.A., Erwin, M.E. & Jones, R.N. (1995) Antimicrobial activity of 11 newer and investigational drugs tested against aerobic isolates from spontaneous bacterial peritonitis. Diagn. Microbiol. Infect. Dis., 21, 105–110.
Smith, B.R. & LeFrock, J.L. (1983) Cefuroxime: Antimicrobial activity, pharmacology, and clinical efficacy. Ther. Drug Monit., 5, 149–160.
Sommers, D.K., Van Wyk, M., Williams, P.E.O. & Harding, S.M. (1984) Pharmacokinetics and tolerance of cefuroxime axetil in volunteers during repeat dosing. Antimicrob. Agents Chemother., 25, 344–347.
Spurling, N.W., Harcourt, R.A. & Hyde, J.J. (1986) An evaluation of the safety of cefuroxime axetil during six months oral administration to beagle dogs. J. Toxicol. Sci., 11, 237–277.
Tamura, J., Sato, N., Moriwaki, T., Nagasawa, R., Aoishi, M., Okamura, C., Sutoh, M., Tatami, A. & Ezaki, H. (1979) Study of the safety of cefuroxime (1). Acute toxicity in mice, rats and rabbits. Chemotherapy (Tokyo), 27 (Suppl. 6), 124–129.
Thomakos, N., Maraki, S., Liakakos, T., Macheras, A., Kanavaki, S., Marinis, E., Sehas, M., Margioris, A.N. & Samonis, G. (1998) Effect of cefamandole, cefuroxime and cefoxitin on yeast fecal flora of surgical patients. Chemotherapy, 44, 324–327.
Troughton, H.J. & Bartholomew, J.E. (1975a) Teratogenicity of cephalosporin 640/359: Subcutaneous administration to female CRH mice on days 6 to 15 of pregnancy. Unpublished report from Glaxo Research, United Kingdom. Submitted to WHO by Schering-Plough Animal Health, Middlesex, United Kingdom.
Troughton, H.J. & Bartholomew, J.E. (1975b) Teratogenicity of cephalosporin 640/359: Subcutaneous administration to female Dutch rabbits on days 6 to 18 of pregnancy. Unpublished report from Glaxo Research, United Kingdom. Submitted to WHO by Schering-Plough Animal Health, Middlesex, United Kingdom.
Tsai, T.H., Cheng, F.C., Chen, K.C., Chen, Y.F. & Chen, C.F. (1999) Simultaneous measurement of cefuroxime in rat blood and brain by microdialysis and microbore liquid chromatography. Applications to pharmacokinetics. J. Chromatogr. B Biomed. Sci. Appl., 735, 25–31.
Tweats, D.J. (1977) Mutagenicity screen. Cefuroxime sodium salt II/6 (TH4/01) batch number BRC 1212 (milled). Unpublished report No. 77ZIN23 from Glaxo Research Ltd, United Kingdom. Submitted to WHO by Schering-Plough Animal Health, Middlesex, United Kingdom.
Tweats, D.J., Halliday, I.J. & Tiley, M.J. (1980) Mutagenicity screen. Micronucleus test: Cefuroxime sodium II/6 milled. Unpublished report No. 80TA4 from Glaxo Research Ltd, United Kingdom. Submitted to WHO by Schering-Plough Animal Health, Middlesex, United Kingdom.
Williams, P.E.O. & Harding, S.M. (1984) The absolute bioavailability of oral cefuroxime axetil in male and female volunteers after fasting and after food. J. Antimicrob. Chemother., 13, 191–196.
Wise, R., Bennett, S.A. & Dent, J. (1984) The pharmacokinetics of orally absorbed cefuroxime compared with amoxycillin/clavulanic acid. J. Antimicrob. Chemother., 13, 603–610.
See Also:
Toxicological Abbreviations
Cefuroxime (WHO Food Additives Series 53)
CEFUROXIME (JECFA Evaluation)