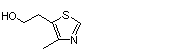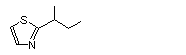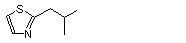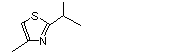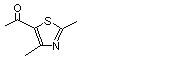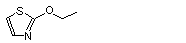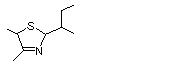WHO FOOD ADDITIVES SERIES: 50
SULFUR-CONTAINING HETEROCYCLIC COMPOUNDS
First draft prepared by
Dr P.J. Abbott
Australia New Zealand Food Authority, Canberra, ACT, Australia
and Professor A.G. Renwick
Clinical Pharmacology Group, University of Southampton, Southampton, England
1. EVALUATION
The Committee evaluated a group of 30 flavouring agents comprising sulfur-containing heterocyclic compounds (see Table 1) using the Procedure for the Safety Evaluation of Flavouring Agents (See Figure 1). The group is composed of both five- and six-member S-containing aromatic and non-aromatic heterocyclic compounds, including thiazole itself and derivatives of thiazole, dithiazine, thiazoline and thiophene. Thiazole is a five-membered aromatic heterocyclic compound containing sulfur and nitrogen atoms in the 1- and 3-ring positions, respectively (Nos 1030–1044 and 1054–1057).
Table 1. Summary of the results of safety evaluations of sulfur-containing heterocyclic compoundsa
|
Flavouring agent |
No. |
CAS No. and structure |
Step 2
Predicted to be metabolized to innocuous products? |
Steps A3 / B3b
Does intake exceed the threshold for structural class? |
Step A4
Is the flavouring agent or are its metabolites endogenous? |
Step A5/B4
Adequate margin of safety for the flavouring agent or related substance? |
Conclusion based on current intake |
|
Structural class II |
|
|
|
|
|
|
|
|
Thiamine hydrochloride |
1030 |
67-03-8
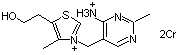 |
Yesc
A side |
No
Europe: 2900
USA: 1200 |
No |
A5 Yes.
The NOEL of 36 mg/kg bw per day is > 500 times the estimated intake of thiamine hydrochloride when used as a flavouring agent. |
No safety concern |
|
4-Methyl-5-thiazoleethanol |
1031 |
137-00-8
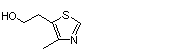 |
Yesd
A side |
No
Europe: 170
USA: 380 |
N/R |
N/R |
No safety concern |
|
Thiazole |
1032 |
288-47-1
 |
No
B side |
No
Europe: 0.01
USA: 0.07 |
N/R |
B5 Yes
The NOEL of 0.92 mg/kg bw per day for the related flavouring agent No. 1039 is > 900 000 times ithe estimated intake of thiazole when used as a flavouring agent. |
No safety concern |
|
2-(1-Methylpropyl)-thiazole |
1033 |
18277-27-5
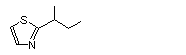 |
No
B side |
No
Europe: 0.03
USA: 0.01 |
N/R |
B5 Yes
The NOEL of 0.92 mg/kg bw per day for the related flavouring agent No. 1039 is > 1 million times the estimated intake of 2-(1-methylpropyl)-thiazole when used as a flavouring agent. |
No safety concern |
|
2-Isobutylthiazole |
1034 |
18640-74-9
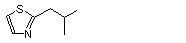 |
No
B side |
No
Europe: 3
USA: 0.4 |
N/R |
B5 Yes
The NOEL of 0.92 mg/kg bw per day for the related flavouring agent No. 1039 is 10 000 times the estimated intake of 2-isobutylthiazole when used as a flavouring agent. |
No safety concern |
|
4,5-Dimethylthiazole |
1035 |
3581-91-7
 |
No
B side |
No
Europe: 0.2
USA: 0.4 |
N/R |
B5 Yes
The NOEL of 0.92 mg/kg bw per day for the related flavouring agent No. 1039 is > 200 000 times the estimated intake of 4,5-dimethylthiazole when used as a flavouring agent. |
No safety concern |
|
2,4,5-Trimethylthiazole |
1036 |
13623-11-5
 |
No
B side |
No
Europe: 1
USA: 0.3 |
N/R |
B5 Yes
The NOEL of 0.92 mg/kg bw per day for the related flavouring agent No. 1039 is > 90 000 times the estimated intake of 2,4,5-trimethylthiazole when used as a flavouring agent. |
No safety concern |
|
2-Isopropyl-4-methylthiazole |
1037 |
15679-13-7
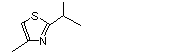 |
No
B side |
No
Europe: 22
USA: 10 |
N/R |
B5 Yes
The NOEL of 0.92 mg/kg bw per day for the related flavouring agent No. 1039 is > 2000 times the estimated intake of 2-isopropyl-4-methylthiazole when used as a flavouring agent. |
No safety concern |
|
4-Methyl-5-vinylthiazole |
1038 |
1759-28-0
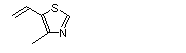 |
No
B side |
No
Europe: 2
USA: 0.2 |
N/R |
B5 Yes
The NOEL of 0.92 mg/kg bw per day for the related flavouring agent No. 1039 is > 20 000 times the estimated intake of 4-methyl-5-vinylthiazole when used as a flavouring agent. |
No safety concern |
|
2,4-Dimethyl-5-vinylthiazole |
1039 |
65505-18-2
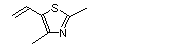 |
No
B side |
No
Europe: ND
USA: 0.007 |
N/R |
B5 Yes
The NOEL of 0.92 mg/kg bw per day is > 9 million times the estimated intake of 2,4-dimethyl-5-vinylthiazole when used as a flavouring agent. |
No safety concern |
|
2-Acetylthiazole |
1041 |
24295-03-2
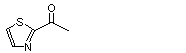 |
No
B side |
No
Europe: 11
USA: 10 |
N/R |
B5 Yes
The NOEL of 50 mg/kg bw per day is > 200 000 times the estimated intake of 2-acetylthiazole when used as a flavouring agent. |
No safety concern |
|
2-Propionylthiazole |
1042 |
43039-98-1
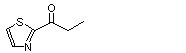 |
No
B side |
No
Europe: ND
USA: 0.2 |
N/R |
B5 Yes
The NOEL of 50 mg/kg bw per day for the related flavouring agent No. 1041 is > 10 million times the estimated intake of 2-propionylthiazole when used as a flavouring agent. |
No safety concern |
|
4-Methylthiazole |
1043 |
693-95-8
 |
No
B side |
No
Europe: 0.1
USA: 0.05 |
N/R |
B5 Yes
The NOEL of 0.92 mg/kg bw per day for the related flavouring agent No. 1039 is > 400 000 times the estimated intake of 4-methylthiazole when used as a flavouring agent. |
No safety concern |
|
2-Ethyl-4-methylthiazole |
1044 |
15679-12-6
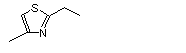 |
No
B side |
No
Europe: 4
USA: 1 |
N/R |
B5 Yes
The NOEL of 0.92 mg/kg bw per day for the related flavouring agent No. 1039 is > 10 000 times the estimated intake of 2-ethyl-4-methylthiazole when used as a flavouring agent. |
No safety concern |
|
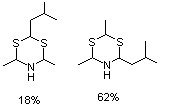
2-Isobutyl-4,6-dimethyldihydro-1,3,5-dithiazine and 4-isobutyl-2,6-dimethyldihydro-1,3,5-dithiazine (mixture) |
1046 |
101517-87-7
101517-86-6
 |
No
B side |
No
Europe: 0.1
USA: 0.05 |
N/R |
B5 Yes
The NOEL of 11 mg/kg bw per day is > 5 million times the estimated intake of a mixture of 2-isobutyl-4,6-dimethyl- and 4-isobutyl-2,6-dimethyldihydro-1,3,5-dithiazine when used as a flavouring agent. |
No safety concern |
|
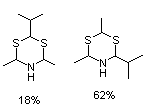
2-Isopropyl-4,6-dimethyl 2,6-dimethyldihydro-1,3,5-dithiazine and 4-isopropyl-2,6-dimethyldihydro-1,3,5-dithiazine (mixture) |
1047 |
104691-41-0
104691-40-9
 |
No
B side |
No
Europe: ND
USA: 0.07 |
N/R |
B5 Yes
The NOEL of 11 mg/kg bw per day is > 10 million times the estimated intake of a mixture of 2-isopropyl-4,6-dimethyl and 4-isopropyl-2,6-dimethyldihydro-1,3,5-dithiazine when used as a flavouring agent. |
No safety concern |
|
2,4,6-Triisobutyl-5,6-dihydro-4H-1,3,5-dithiazine |
1048 |
74595-94-1
 |
No
B side |
No
Europe: ND
USA: 2.6 |
N/R |
B5 Yes
The NOEL of 11 mg/kg bw per day for the related flavouring agent No. 1046 is > 200 000 times the estimated intake of 2,4,6-triisobutyl-5,6-dihydro-4H-1,3,5-dithiazine when used as a flavouring agent. |
No safety concern |
|
2,4,6-Trimethyldihydro-4H-1,3,5-dithiazine |
1049 |
638-17-5
 |
No
B side |
No
Europe: ND
USA: 3.3 |
N/R |
B5 Yes
The NOEL of 11 mg/kg bw per day for the related flavouring agent No. 1046 is > 5 million times the estimated intake of 2,4,6-trimethyldihydro-4H-1,3,5-dithiazine when used as a flavouring agent. |
No safety concern |
|
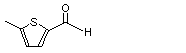
5-Methyl-2-thiophene-carboxyaldehyde |
1050 |
13679-70-4
 |
No
B side |
No
Europe: 1
USA: 0.01 |
N/R |
B5 Yes
The NOEL of 290 mg/kg bw per day for the related flavouring agent No. 1053 is > 20 million times the estimated intake of 5-methyl-2-thiophene-carboxyaldehyde when used as a flavouring agent. |
No safety concern |
|
3-Acetyl-2,5-dimethylthiophene |
1051 |
2530-10-1
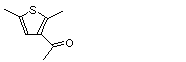 |
No
B side |
No
Europe: 22
USA: 0.2 |
N/R |
B5 Yes
The NOEL of 290 mg/kg bw per day for the related flavouring agent No. 1053 is > 700 000 times the estimated intake of 3-acetyl-2,5-dimethylthiophene when used as a flavouring agent. |
No safety concern |
|
4--Methyl-5-thiazoleethanol acetate |
1054 |
656-53-1
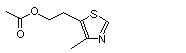 |
Yese
A side |
No
Europe: 10
USA: 3 |
N/R |
N/R |
No safety concern |
|
Structural class III |
|
|
|
|
|
|
|
|
Benzothiazole |
1040 |
95-16-9
 |
No
B side |
No
Europe: 1
USA: 0.2 |
N/R |
B5 Yes
The NOEL of 5.1 mg/kg bw per day is > 20 000 times the estimated intake of benzothiazole when used as a flavouring agent. |
No safety concern |
|
4,5-Dimethyl-2-isobutyl-3-thiazoline |
1045 |
65894-83-9
 |
No
B side |
No
Europe: 0.01
USA: 4 |
N/R |
B5 Yes
The NOEL of 1.2 mg/kg bw per day for the related flavouring agent No. 1059 is > 10 000 times the estimated intake of 4,5-dimethyl-2-isobutyl-3-thiazoline when used as a flavouring agent. |
No safety concern |
|
2-Thienyl mercaptan |
1052 |
7774-74-5
 |
No
B side |
No
Europe: 0.01
USA: 0.03 |
N/R |
B5 Yes
The NOEL of 290 mg/kg bw per day for the related flavouring agent No. 1053 is > 700 million times the estimated intake of 2-thienyl mercaptan when used as a flavouring agent. |
No safety concern |
|
2-Thienyl disulfide |
1053 |
6911-51-9
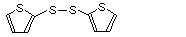 |
No
B side |
No
Europe: ND
USA: 0.07 |
N/R |
B5 Yes
The NOEL of 290 mg/kg bw per day is > 200 million times the estimated intake of 2-thienyl disulfide when used as a flavouring agent. |
No safety concern |
|
2,4-Dimethyl-5-acetylthiazole |
1055 |
38205-60-6
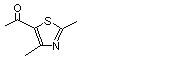 |
No
B side |
No
Europe: 0.01
USA: 2 |
N/R |
B5 Yes
The NOEL of 24 mg/kg bw per day is > 10 million times the estimated intake of 2,4-dimethyl-5-acetylthiazole when used as a flavouring agent. |
No safety concern |
|
2-Ethoxythiazole |
1056 |
15679-19-3
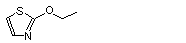 |
No
B side |
No
Europe: 0.01
USA: 0.12 |
N/R |
B5 Yes
The NOEL of 50 mg/kg bw per day for the related flavouring agent No. 1041 is > 20 million times the estimated intake of 2-ethoxythiazole when used as a flavouring agent. |
No safety concern |
|
2-Methyl-5-methoxythiazole |
1057 |
38205-64-0
 |
No
B side |
No
Europe: ND
USA: 0.01 |
N/R |
B5 Yes
The NOEL of 8.6 mg/kg bw per day is > 40 million times the estimated intake of 2-methyl-5-methoxythiazole when used as a flavouring agent. |
No safety concern |
|
4,5-Dimethyl-2-ethyl-3-thiazoline |
1058 |
76788-46-0
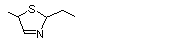 |
No
B side |
No
Europe: ND
USA: 0.01 |
N/R |
B5 Yes
The NOEL of 1.2 mg/kg bw per day for the related flavouring agent No. 1059 is > 6 million times the estimated intake of 4,5-dimethyl-2-ethyl-3-thiazoline when used as a flavouring agent. |
No safety concern |
|
2-(2-Butyl)-4,5-dimethyl-3-thiazoline |
1059 |
65894-82-8
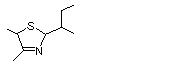 |
No
B side |
No
Europe: ND
USA: 5 |
N/R |
B5 Yes
The NOEL of 1.2 mg/kg bw per day is > 10 000 times the estimated intake of 2-(2-butyl)-4,5-dimethyl-3-thiazoline when used as a flavouring agent. |
No safety concern |
|
CAS: Chemical Abstracts Service; ND: no intake data reported; N/R: not required for evaluation. |
|
a |
Step 1. Twenty-one flavouring agents are in structural class II and nine flavouring agents are in structural class III. |
|
b |
The thresholds for human intake for structural classes II and III are 540 µg/day and 90 µg/day, respectively. All intake values are expressed in µg/day. The combined per capita intake of flavouring agents in structural class II in 3100 µg/day in Europe and 1600 µg/day in the USA. The combined per capita intake of flavouring agents in structural class III is 1 µg/day in Europe and 11 µg/day in the USA. |
|
c |
Enzymatically cleaved to yield 4-methyl-5-thiazoleethanol (No. 1031) and 2-methyl-4-amino-5-hydroxymethylpyrimidine. The thiazole and pyrimidine fragments are further oxidized to yield 4-methylthiazole-4-acetic acid and the 5-pyrimidine carboxylic acid derivative, respectively, which, together with thiamine, are excreted in the urine. Can also be converted to 2-methyl-4-amino-5-formylaminopyrimidine and thiamine acetic acid. |
|
d |
Oxidized to yield 4-methylthiazole-4-acetic acid and excreted in the urine (See note c) |
|
e |
Hydrolyzed to yield 4-methylthiazole-4-acetic acid and excreted in urine (See note c) |
Dithiazine is a six-membered non-aromatic heterocyclic compound containing two sulfur atoms and one nitrogen atom in the 1-, 3- and 5-positions, respectively (Nos 1046–1049). Thiazoline (partially reduced thiazole) is a five-membered non-aromatic heterocyclic compound containing sulfur and nitrogen atoms in the 1- and 3-positions, respectively (Nos 1045, 1058 and 1059). Thiophene is a five-membered aromatic heterocyclic compound containing a sulfur atom in the 1-position (Nos 1050–1053). Except for thiazole (No. 1032), all the flavouring agents in this group are ring-substituted with one or more of the following functional groups: alkyl, alkenyl, aryl, alcohol, keto, thiol and disulfide.
The Committee has not previously evaluated any of the members of this group.
Eighteen flavouring agents in the group have been detected as natural components of food, and quantitative data have been reported on the natural occurrence of eight agents (Nos 1032, 1034, 1036, 1040, 1041, 1043 and 1050). The foods in which one or more of these flavouring agents can be found include lean meat, beans, nuts, whole-grain cereals, fish, coffee, milk, beer, peanuts, popcorn, pork liver, shrimp, tomato, potato, grapes and apples (Maarse et al., 1999).
1.2 Estimated daily intake
The total annual volume of production of the 30 sulfur-containing heterocyclic compounds in this group was reported to be 22 000 kg in Europe (International Organization of the Flavor Industry, 1995) and 12 000 kg in the USA (Lucas et al., 1999). These values are equivalent to total daily per capita intakes of 3100 µg in Europe and 1600 µg in the USA.
In both Europe and the USA, thiamine hydrochloride (vitamin B1; No. 1030) and its principal metabolite, 4-methyl-5-thiazoleethanol (No. 1031), accounted for approximately 98% of the total per capita dietary intake of the flavouring agents in this group. In Europe, thiamine hydrochloride and 4-methyl-5-thiazoleethanol accounted for approximately 92% (2900 µg/day) and 6% (170 µg/day), respectively, of the total per capita intake. In the USA, thiamine hydrochloride and 4-methyl-5-thiazoleethanol accounted for approximately 75% (1200 µg/day) and 23% (380 µg/day), respectively, of the total per capita intake of the flavouring agents in this group.
The estimated intakes of the remaining flavouring agents are between 0.01 and 22 µg/day in both Europe and the USA, with most intakes below 4 µg/day.
1.3 Absorption, distribution, metabolism and elimination
Thiazole and its derivatives are metabolized primarily by side-chain oxidation or oxidation of the ring sulfur or nitrogen atoms (Rance, 1989); however, other routes of metabolism, involving ring cleavage, are possible. Derivatives of dithiazine, which are cyclic sulfides, are expected to be metabolized primarily by S-oxidation to yield the corresponding sulfoxides and sulfones. Thiazoline is predicted to be similarly metabolized. Thiamine hydrochloride (No. 1030) at an intake of less than 5 mg/day is readily absorbed in the small intestine by an active transport system, followed by conversion to the coenzyme, thiamine pyrophosphate (Hegsted, et al., 1976; Wilson, 1982; Tietz, 1986). Thiamine is metabolized primarily to a pyrimidine and a thiazole (4-methyl-5-thiazoleethanol, No. 1031) fragment. These fragments are further oxidized and excreted in the urine.
Thiophene derivatives are metabolized primarily by S-oxidation, followed by conjugation with glutathione; however, other routes of metabolism, involving ring cleavage, are also possible. The resulting mercapturic acid derivative is eliminated in the urine (Dansette et al., 1992; Valadon et al., 1996). The aromatic-fused thiazole, 2-acetylthiazole (benzothiazole; No. 1041), which lacks ring substituents, is metabolized by thiazole ring cleavage, yielding a series of free and conjugated ortho-aminophenyl sulfone and sulfoxide metabolites (Wilson et al., 1991).
1.4 Application of the Procedure for the Safety Evaluation of Flavouring Agents
The stepwise evaluation of the 30 sulfur-containing heterocyclic compounds is described in detail below and summarized in Table 1.
|
Step 1. |
All the flavouring agents in this group are heterocyclic compounds, and five of these (Nos 1040, 1050, 1051, 1052 and 1053) are also aromatic 11 . One of the aromatic heterocyclic flavouring agents, benzothiazole (No. 1040) is unsubstituted and was therefore placed in structural class III (Cramer et al., 1978). Of the other four substituted compounds, two (Nos 1050 and 1051) are common constituents of food and were therefore classified in structural class II, while the other two (Nos 1052 and 1053) are not common constituents of food and were therefore placed in structural class III. |
| |
Of the 25 non-aromatic heterocyclic flavouring agents, 19 (Nos 1030–1039, 1041–1044, 1046–1049 and 1054) are or are structurally closely related to common constituents of food and were therefore placed in structural class II. The remaining six flavouring agents (Nos 1045 and 1055–1059) were classified in structural class III. |
|
Step 2. |
The data on most individual members of the group were, in most cases, insufficient to allow conclusions about their probable metabolic fate. The metabolism of thiamine hydrochloride (No. 1030) and its metabolite, 4-methyl-5-thiazoleethanol (No. 1031), is well characterized, and both are metabolized to innocuous products. 4-Methyl-5-thiazoleethanol acetate (No. 1054) is expected to be readily hydrolysed to 4-methyl-5-thiazoleethanol. The evaluation of all three flavouring agents therefore proceeded via the A (left-hand) side of the scheme. For all other flavouring agents in the group, the evaluation proceeded via the B (right-hand) side of the scheme. |
|
Step A3. |
With respect to those flavouring agents evaluated via the A side of the scheme, the estimated daily per capita intakes of 4-methyl-5-thiazoleethanol (No. 1031) and 4-methyl-5-thiazoleethanol acetate (No. 1054) are below the threshold for human intake for structural class II (540 µg), and therefore these flavouring agents are not expected to raise any safety concern. The daily per capita intake of thiamine hydrochloride (No. 1030) is 2900 µg in Europe and 1200 µg in the USA. The intake of this flavouring agent therefore exceeds the threshold for human intake of compounds in structural class II, and its evaluation proceeded to step A4. |
|
Step A4. |
Thiamine hydrochloride (No. 1030) is not endogenous; therefore, its evaluation proceeded to step A5. |
|
Step A5. |
The NOEL of 36 mg/kg bw per day for thiamine hydrochloride (No. 1030) in a 90-day dietary study in rats is more than 500 times the estimated intake of this substance from its use as a flavouring agent in Europe (48 µg/kg bw per day) and more than 1000 times the estimated intake in the USA (22 µg/kg bw per day). Thiamine hydrochloride would therefore not be expected to be a safety concern. |
|
Step B3 |
With regard to those flavouring agents evaluated via the B side of the scheme, the estimated daily per capita intakes of all 18 flavouring agents in structural class II (Nos 1032–1039, 1041–1044 and 1046–1051) are below the threshold for human intake for structural class II (540 µg per person per day). Similarly, the estimated daily per capita intakes of all nine flavouring agents in structural class III (Nos 1040, 1045, 1052, 1053 and 1055–1059) are below the threshold for human intake for compounds in this class (90 µg per person per day). The evaluation of all of these flavouring agents therefore proceeded to step B4. |
|
Step B4 |
The NOEL for 2,4-dimethyl-5-vinylthiazole (No. 1039) in a 90-day dietary study in rats was 0.92 mg/kg bw per day, and this NOEL is appropriate for the structurally related flavouring agents, thiazole (No. 1032), 2-(1-methylpropyl)thiazole (No. 1033), 2-isobutylthiazole (No. 1034), 4,5-dimethylthiazole (No. 1035), 2,4,5-trimethylthiazole (No. 1036), 2-isopropyl-4-methylthiazole (No. 1037), 4-methyl-5-vinylthiazole (No. 1038), 4-methylthiazole (No. 1043) and 2-ethyl-4-methylthiazole (No. 1044). |
The NOEL for 2-acetylthiazole (No. 1041) in a 90-day dietary study in rats was 50 mg/kg bw per day, and this NOEL is appropriate for the structurally related flavouring agent, 2-propionylthiazole (No. 1042).
The NOEL for 2-(2-butyl)-4,5-dimethyl-3-thiazoline (No. 1059) in a 90-day dietary study in rats was 1.2 mg/kg bw per day, and this NOEL is appropriate for the structurally related flavouring agents, 4,5-dimethyl-2-isobutyl-3-thiazoline (No. 1045) and 4,5-dimethyl-2-ethyl-3-thiazoline (No. 1058).
The NOEL for a mixture of 2-isobutyl-4,6-dimethyl and 4-isobutyl-2,6-dimethyldihydro-1,3,5-dithiazine (No. 1046) and of a mixture of 2-isopropyl-4,6-dimethyl and 4-isopropyl-2,6-dimethyldihydro-1,3,5-dithiazine (No. 1047) in a 14-day dietary study in rats was 11 mg/kg bw per day, and this NOEL is appropriate for the structurally related agents, 2,4,6-triisobutyl-5,6-dihydro-4H-1,3,5-dithiazine (No. 1048) and 2,4,6-trimethyldihydro-4H-1,3,5-dithiazine (No. 1049).
The NOEL for 2-thienyl disulfide (No. 1053) in a 90-day dietary study in rats was 290 mg/kg bw per day, and this NOEL is appropriate for the structurally related agents 5-methyl-2-thiophene carboxyaldehyde (No. 1050), 3-acetyl-2,5-dimethylthiophene (No. 1051) and 2-thienyl mercaptan (No. 1052).
The NOEL for benzothiazole (No. 1040) in a 90-day dietary study in rats was 5.1 mg/kg bw per day.
The NOEL for 2,4-dimethyl-5-acetylthiazole (No. 1055) in a dietary study in rats was 24 mg/kg bw per day.
The NOEL for 2-methyl-5-methoxythiazole (No. 1057) in a 90-day dietary study in rats was 8.6 mg/kg bw per day.
For all of the above-mentioned flavouring agents (Nos 1032–1053, 1055–1059), therefore, a NOEL exists either for the substance itself, which provides an adequate margin of safety under the intended conditions of use, or for a structurally related substance, which is high enough to accommodate any perceived difference in toxicity between the substance itself and the related substance.
The NOEL used to evaluate each flavouring agent and the margins of safety they provide on the basis of current levels of intake are summarized in Table 2. On the basis of these data, these flavouring agents would not be expected to be a safety concern.
Table 2. Margins of safety for the flavouring agents proceeding via the B side of the Scheme
|
Flavouring agent |
No. |
Related flavouring agent(s) |
No. |
NOEL (mg/kg bw per day) |
Reference |
Highest estimated intake (mg/kg bw |
Margin of safety per day) |
|
Structural class II |
|
|
|
|
|
|
|
|
Thiazole |
1032 |
2,4-Dimethyl-5-vinylthiazole |
1039 |
0.92a |
Posternak et al. (1969) |
0.001 |
> 900 000 |
|
2-(1-Methylpropyl)-thiazole |
1033 |
2,4-Dimethyl-5-vinylthiazole |
1039 |
0.92a |
Posternak et al. (1969) |
0.0005 |
> 1 million |
|
2-Isobutylthiazole |
1034 |
2,4-Dimethyl-5-vinylthiazole |
1039 |
0.92a |
Posternak et al. (1969) |
0.05 |
> 10 000 |
|
4,5-Dimethylthiazole |
1035 |
2,4-Dimethyl-5-vinylthiazole |
1039 |
0.92a |
Posternak et al. (1969) |
0.004 |
> 200 000 |
|
2,4,5-Trimethylthiazole |
1036 |
2,4-Dimethyl-5-vinylthiazole |
1039 |
0.92a |
Posternak et al. (1969) |
0.01 |
> 90 000 |
|
2-Isopropyl-4-methylthiazole |
1037 |
2,4-Dimethyl-5-vinylthiazole |
1039 |
0.92a |
Posternak et al. (1969) |
0.4 |
> 2000 |
|
4-Methyl-5-vinylthiazole |
1038 |
2,4-dimethyl-5-vinylthiazole |
1039 |
0.92a |
Posternak et al. (1969) |
0.04 |
> 20 000 |
|
2,4-Dimethyl-5-vinylthiazole |
1039 |
NA |
NA |
0.92a |
Posternak et al. (1969) |
0.0001 |
> 9 million |
|
2-Acetylthiazole |
1041 |
NA |
NA |
50a |
Wheldon et al. (1970) |
0.2 |
> 200 000 |
|
2-Propionylthiazole |
1042 |
2-Acetylthiazole |
1041 |
50a |
Wheldon et al. (1970) |
0.003 |
> 10 million |
|
4-Methylthiazole |
1043 |
2,4-Dimethyl-5-vinylthiazole |
1039 |
0.92a |
Posternak et al. (1969) |
0.002 |
> 400 000 |
|
2-Ethyl-4-methylthiazole |
1044 |
2,4-Dimethyl-5-vinylthiazole |
1039 |
0.92a |
Posternak et al. (1969) |
0.06 |
> 10 000 |
|
2-Isobutyl-4,6- dimethyldihdro-1,3,5-dithiazine and 4-isobutyl-2,6-di-methyldihydro-1,3,5-dithiazine (mixture) |
1046 |
NA |
NA |
11b |
Rush (1989a,b) |
0.002 |
> 5 million |
|
2-Isopropyl-4,6- dimethyldihydro-1,3,5-dithiazine and 4-isopropyl-2,6-dimethyldihydro-1,3,5-dithiazine (mixture) |
1047 |
NA |
NA |
11b |
Rush (1989a,b) |
0.001 |
> 10 million |
|
2,4,6-Triisobutyl-5,6-dihydro-4H-1,3,5-dithiazine |
1048 |
2-Isobutyl-4,6-dimethyl and 4- isobutyl-2,6-dimethyldihydro-1,3,5-dithiazine (mixture) |
1046 |
11b |
Rush (1989a,b) |
0.04 |
> 200 000 |
|
2,4,6-Trimethyldihydro-4H-1,3,5-dithiazine |
1049 |
2-Isobutyl-4,6-dimethyl and 4- isobutyl-2,6-dimethyldihydro-1,3,5-dithiazine |
1046 |
11b |
Rush (1989a,b) |
0.002 |
> 5 million |
|
5-Methyl-2-thiophene-carboxyaldehyde |
1050 |
2-Thienyl disulfide |
1053 |
290a |
Morgareidge & Oser (1970) |
0.01 |
> 20 million |
|
3-Acetyl-2,5-dimethylthiophene |
1051 |
2-Thienyl disulfide |
1053 |
290a |
Morgareidge & Oser (1970) |
0.4 |
> 700 000 |
|
Structural class III |
|
|
|
|
|
|
|
|
Benzothiazole |
1040 |
NA |
NA |
5.1a |
Margareidge (1971) |
0.02 |
> 20 000 |
|
4,5-Dimethyl-2-isobutyl-3-thiazoline |
1045 |
2-(2-Butyl)-4,5-dimethyl-3-thiazoline |
1059 |
1.2a |
Babish (1978) |
0.07 |
> 10 000 |
|
2-Thienyl mercaptan |
1052 |
2-Thienyl disulfide |
1053 |
290a |
Morgareidge & Oser (1970) |
0.0004 |
> 700 million |
|
2-Thienyl disulfide |
1053 |
NA |
NA |
290a |
Morgareidge (1971) |
0.001 |
> 200 million |
|
2,4-Dimethyl-5-acetylthiazole |
1055 |
NA |
NA |
24a |
Shellenberger (1971) |
0.03 |
> 10 million |
|
2-Ethoxythiazole |
1056 |
2-Acetylthiazole |
1041 |
50a |
Wheldon et al. (1970) |
0.002 |
> 20 million |
|
2-Methyl-5-methoxythiazole |
1057 |
NA |
NA |
8.6a |
Posternak et al. (1975) |
0.0002 |
> 40 million |
|
4,5-Dimethyl-2-ethyl-3-thiazoline |
1058 |
2-(2-Butyl)-4,5-dimethyl-3-thiazoline |
1059 |
1.2a |
Babish (1978) |
0.0002 |
> 6 million |
|
2-(2-Butyl)-4,5-dimethyl-3-thiazoline |
1059 |
NA |
NA |
1.2a |
Babish (1978) |
0.09 |
> 10 000 |
NA, not applicable
a 90-day study in rats
b 14-day study in rats
1.5 Consideration of combined intake from use as flavouring agents
Although the 30 flavouring agents in this group are unlikely to produce common metabolites, consideration of their combined intake is appropriate since all are likely to be conjugated with glutathione before excretion. In the unlikely event that all 21 flavouring agents in structural class II were to be consumed simultaneously on a daily basis, the estimated combined per capita intakes (3100 µg/day in Europe and 1600 µg/day in the USA) would exceed the threshold of human intake for their structural class (540 µg per person). Since, at these levels of intake, the conjugation capacity of the glutathione pool is unlikely to be depleted, this level of intake is not anticipated to be a safety concern. In the unlikely event that the nine flavouring agents in structural class III were to be consumed simultaneously on a daily basis, the estimated combined per capita intakes (1 µg/day in Europe and 11 µg/day in the USA) would not exceed the threshold for human intake for their structural class (90 µg per person).
1.6 Conclusions
On the basis of the predicted metabolism and the NOELs for some members of the group, the Committee concluded that the 30 sulfur-containing heterocyclic compounds in this group would not raise safety concerns at current levels of intake.
The Committee noted that, for the three flavouring agents that were predicted to be metabolized to innocuous products, the available data on toxicity were consistent with the results of the safety evaluation.
2. RELEVANT BACKGROUND INFORMATION
2.1 Explanation
This monograph summarizes the key data relevant to the evaluation of the 30 flavouring substances in this group. The group included both five- and six-member sulfur-containing aromatic and non-aromatic heterocycles, namely, thiazole and derivatives of thiazole, dithiazine, thiazoline and thiophene. Thiazole derivatives are five-membered aromatic heterocycles containing sulfur and nitrogen atoms in the 1- and 3-ring positions, respectively (Nos 1030–1044 and 1054–1057). Thiazoline derivatives (containing partially reduced thiazole) are five-membered non-aromatic heterocycles containing sulfur and nitrogen atoms in the 1- and 3-ring positions, respectively (Nos 1045, 1058 and 1059). Dithiazine derivatives are six-membered non-aromatic heterocycles containing two sulfur atoms and one nitrogen (Nos 1046–1049). Thiophene derivatives are five-membered aromatic heterocycles containing a sulfur atom in the 1-ring position (Nos 1050–1053). Except for thiazole (No. 1032), all the substances in this group are ring-substituted with one or more of the following functional groups: alkyl, alkenyl, aryl, alcohol, keto, thiol and disulfide.
2.2 Additional considerations on intake
The total annual volume of production of the 30 sulfur-containing heterocyclic compounds in this group was reported to be 22 000 kg in Europe (International Organization of the Flavor Industry, 1995) and 12 000 kg in the USA (Lucas et al., 1999). In both Europe and the USA, 98% of the total volume of use was accounted for by thiamine hydrochloride (vitamin B1) and its metabolite, 4-methyl-5-thiazole ethanol (see Table 3).
Table 3. Annual volumes of production of sulfur-containing heterocyclic compounds used as flavouring agents in Europe and the USA
|
Substance (No.) |
Most recent annual production volume (kg)a |
Intakeb |
Consumption ratioc |
|
|
µg/day |
µg/kg bw
per day |
|
|
Thiamine hydrochloride (1030) |
|
Europe |
20 000 |
2900 |
48 |
NA |
|
USA |
9 300 |
1200 |
20 |
NA |
|
4-Methyl-5-thiazoleethanol (1031) |
|
Europe |
1 200 |
170 |
3 |
NA |
|
USA |
2 800 |
380 |
6 |
NA |
|
Thiazole (1032) |
|
Europe |
0.1 |
0.01 |
0.0002 |
180 |
|
USA |
0.5 |
0.07 |
0.001 |
35 |
|
2-(1-Methylpropyl)thiazole (1033) |
|
Europe |
0.2 |
0.03 |
0.0005 |
NA |
|
USA |
0.1 |
0.01 |
0.0002 |
NA |
|
2-Isobutylthiazole (1034) |
|
Europe |
19 |
3 |
0.05 |
37 |
|
USA |
2.7 |
0.4 |
0.006 |
261 |
|
4,5-Dimethylthiazole (1035) |
|
Europe |
1.5 |
0.2 |
0.004 |
1 |
|
USA |
2.7 |
0.4 |
0.006 |
0.4 |
|
2,4,5-Trimethylthiazole (1036) |
|
Europe |
5 |
1 |
0.01 |
214 |
|
USA |
2.3 |
0.3 |
0.005 |
465 |
|
2-Isopropyl-4-methylthiazole (1037) |
|
Europe |
160 |
22 |
0.4 |
NA |
|
USA |
73 |
10 |
0.2 |
NA |
|
4-Methyl-5-vinylthiazole (1038) |
|
Europe |
17 |
2 |
0.04 |
NA |
|
USA |
1.4 |
0.2 |
0.003 |
NA |
|
2,4-Dimethyl-5-vinylthiazole (1039) |
|
Europe |
N/D |
N/D |
N/D |
NA |
|
USA |
0.05 |
0.007 |
0.0001 |
NA |
|
Benzothiazole (1040) |
|
Europe |
10 |
1 |
0.02 |
0.4 |
|
USA |
1.4 |
0.2 |
0.003 |
3 |
|
2-Acetylthiazole (1041) |
|
Europe |
80 |
11 |
0.2 |
0.2 |
|
USA |
73 |
10 |
0.2 |
0.2 |
|
2-Propionylthiazole (1042) |
|
Europe |
N/D |
N/D |
N/D |
NA |
|
USA |
1.5 |
0.2 |
0.003 |
NA |
|
4-Methylthiazole (1043) |
|
Europe |
0.8 |
0.1 |
0.002 |
1 200 |
|
USA |
0.4 |
0.05 |
0.001 |
2 400 |
|
2-Ethyl-4-methylthiazole (1044) |
|
Europe |
26 |
4 |
0.06 |
NA |
|
USA |
5.4 |
1 |
0.01 |
NA |
|
4,5-Dimethyl-2-isobutyl-3-thiazoline (1045) |
|
Europe |
0.1 |
0.01 |
0.0002 |
NA |
|
USA |
30 |
4 |
0.07 |
NA |
|
Mixture of 2-isobutyl-4,6-dimethyl- and 4-isobutyl-2,6-dimethyldihydro-1,3,5-dithiazine (1046) |
|
Europe |
1 |
0.1 |
0.002 |
NA |
|
USA |
0.4 |
0.05 |
0.001 |
NA |
|
Mixture of 2-isobutyl-4,6-dimethyl and 4-isobutyl-2,6-dimethyldihydro-1,3,5-dithiazine (1047) |
|
Europe |
N/D |
N/D |
N/D |
NA |
|
USA |
0.5 |
0.07 |
0.001 |
NA |
|
2,4,6-Triisobutyl-5,6-dihydro-4H-1,3,5-dithiazine (1048) |
|
Europe |
N/D |
N/D |
N/D |
NA |
|
USA |
20 |
2.6 |
0.04 |
NA |
|
2,4,6-Trimethyldihydro-4H-1,3,5-dithiazine (1049) |
|
Europe |
N/D |
N/D |
N/D |
NA |
|
USA |
25 |
3.3 |
0.05 |
NA |
|
5-Methyl-2-thiophenecarboxyaldehyde (1050) |
|
Europe |
6 |
1 |
0.01 |
76 |
|
USA |
0.05 |
0.01 |
0.0002 |
9 100 |
|
3-Acetyl-2,5-dimethylthiophene (1051) |
|
Europe |
150 |
22 |
0.4 |
NA |
|
USA |
1.8 |
0.2 |
0.004 |
NA |
|
2-Thienyl mercaptan (1052) |
|
Europe |
0.1 |
0.01 |
0.0002 |
NA |
|
USA |
0.2 |
0.03 |
0.0004 |
NA |
|
2-Thienylsulfide (1053) |
|
Europe |
N/D |
N/D |
N/D |
NA |
|
USA |
0.5 |
000 |
0.001 |
NA |
|
4-Methyl-5-thiazoleethanol acetate (1054) |
|
Europe |
71 |
10 |
0.2 |
NA |
|
USA |
25 |
3 |
0.03 |
NA |
|
2,4-Dimethyl-5-acetylthiazole (1055) |
|
Europe |
0.1 |
0.01 |
0.0001 |
NA |
|
USA |
13 |
2 |
0.03 |
NA |
|
2-Ethoxythiazole (1056) |
|
Europe |
0.1 |
0.01 |
0.0002 |
NA |
|
USA |
0.9 |
0.12 |
0.002 |
NA |
|
2-Methyl-5-methoxythiazole (1057) |
|
Europe |
N/D |
N/D |
N/D |
NA |
|
USA |
0.1 |
0.01 |
0.0002 |
NA |
|
4,5-Dimethyl-2-ethyl-3-thiazoline (1058) |
|
Europe |
N/D |
N/D |
N/D |
NA |
|
USA |
0.1 |
0.01 |
0.0002 |
NA |
|
2-(2-Butyl)-4,5-dimethyl-3-thiazoline (1059) |
|
Europe |
N/D |
N/D |
N/D |
NA |
|
USA |
40 |
5 |
0.09 |
NA |
|
Total annual volume |
|
Europe |
22 000 |
|
|
|
|
USA |
12 000 |
|
|
|
|
NA, not available; N/D, no intake data reported |
|
a |
From International Organization of the Flavor Industry (1995) and Lucas et al. (1999) |
|
b |
Intake (µg/person per day) calculated as follows: [(annual volume, kg) × (1 × 109 µg/kg)/(population × survey correction factor × 365 days)], where population (10%, ‘eaters only’) = 32 × 106 for Europe and 26 × 106 for the USA. The correction factor = 0.6 for Europe and 0.8 for the USA, representing the assumption that only 60% and 80% of the annual production volume of the flavour, resepctively, was reported in the poundage surveys. Intake (µg/kg bw per day) calculated as follows: [(µg/person per day)/body weight], where body weight = 60 kg. Slight variations may occur from rounding. |
|
c |
Calculated as follows: (annual consumption in food, kg)/(most recently reported volume as a flavouring agent, kg) |
Eighteen of the 30 flavouring agents in this group have been reported to occur naturally in foods (Maarse et al., 1999). Quantitative data on natural occurrence and consumption ratios have been reported for eight substances in the group (Nos 1032, 1034–1036, 1040, 1041,1043 and 1050), which indicate that the consumptionof six substances (Nos 1032, 1034, 1036, 1040, 1043 and 1050) derives predominantly from traditional foods (i.e., consumption ratio > 1) (Stofberg & Kirschman, 1985; Stofberg & Grundschober, 1987) (see Table 1). Thiamine hydrochloride (No. 1030) is vitamin B1 and is found in most plants and in animal tissues, the main sources being lean meat, beans, nuts, whole-grain cereals and fish (Lehninger, 1987). Other thiazole and thiophene derivatives have been detected in foods including coffee, milk, beer, peanuts, popcorn, beef, pork liver, fish, shrimp, tomatoes, potatoes, grapes and apples (Maarse et al., 1999).
The daily per capita intake of this group of flavouring substances is 3100 µg/day in Europe (52 µg/kg bw per day) and 1600 µg/day in the USA (27 µg/kg bw per day). Thiamine hydrochloride accounted for approximately 92% of the total per capita intake in Europe, while in the USA, thiamine hydrochloride accounted for approximately 75% of the total per capita intake. The high per capita intake of thiamine hydrochloride is likely to be the result of its use to fortify food products rather than of its use as a flavour.
2.3 Biological data
2.3.1 Biochemical data
(a) Absorption and excretion
Little specific information was available on the absorption and transformation of individual members of this group of flavouring substances, apart from thiamine hydrochloride (No. 1030). The absorption and excretion of thiamine hydrochloride is well understood (Informatics, Inc., 1974; Tietz, 1986; Lehninger, 1987). It is readily absorbed in the small intestine via an active transport process at an intake of < 5 mg/day (Tietz, 1986). Passive diffusion across the small intestine becomes significant at intakes > 5 mg/day (Wilson, 1982). In the jejunal mucosa, thiamine is phosphorylated to yield thiamine pyrophosphate. Both free and phosphorylated thiamine occur in the plasma. Thiamine pyrophosphate functions as a Mg(II)-coordinated coenzyme involved in several biochemical processes in which transfer of an aldehyde group occurs. It also has functions in energy production and the biosynthesis of lipids and acetylcholine (Lehninger, 1987).
A thiazole derivative of possible clinical importance, 1-(2,6-difluorophenyl)-1H,3H-thiazolo[3,4-a]benzimidazole, was rapidly absorbed when administered orally at a dose of 50 mg/kg bw to male CD2F1 mice, and was detected in the plasma up to 30 min after dosing; a single elimination phase with a half-life of 6 min was noted. Of the administered dose, only 0.4% was recovered in the urine as the intact compound. The major and minor urinary metabolites were the thiazole ring sulfoxide and sulfone, respectively (El Dareer et al., 1993).
Four compounds (Nos 1050–1053) in the group are derivatives of thiophene. In a study in male Sprague-Dawley rats, thiophene tritiated at the C-1 and C-4 positions was injected intraperitoneally at a dose of 200 mg/kg bw. About 31% of the radioactivity was excreted between 0 and 15 h, while 4% was excreted between 15 and 50 h. More than 94% of the urinary radiolabel was accounted for by a single metabolite, the 2-mercapturic acid derivative of 1,4-dihydrothiophene-S-oxide (Dansette et al., 1992).
Excretion of a related thiophene derivative, 3-benzothiophene, was examined in male Sprague-Dawley rats. After an intraperitoneal injection of 30 mg/kg bw of [14C-keto]3-benzoylthiophene, 20% of the radiolabel was recovered in the urine within 24 h. About 15% of the urinary radiolabel could be accounted for by a mixture of two diastereoisomers of the mercapturic acid conjugate of 4,5-dihydro-3-benzolythiophene (Valadon et al., 1996).
(b) Biotransformation
(i) Metabolism of thiazole and its derivatives (Nos 1030–1044 and 1054–1059)
Thiazole is a five-membered heterocycle containing sulfur and nitrogen atoms in the 1- and 3-ring positions. Derivatives of thiazole in this group of flavouring substances are substituted in various combinations at the 2-, 4- or 5-position.
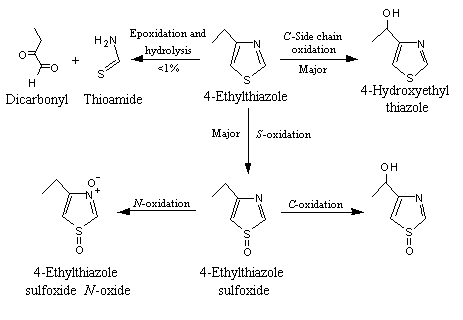
Two primary metabolic routes are potentially available to unsubstituted thiazole (No. 1032). The first is oxidation of the sulfur to form the corresponding sulfoxide and sulfone, and this route is the most favoured. The second, electrophilic attack at ring carbons (usually C-5), is unlikely because thiazoles do not readily undergo electrophilic substitution reactions (Rance, 1989). Addition of alkyl and acyl substituents to the thiazole ring increases the potential metabolic routes available. Alkyl- and acyl-substituted thiazole derivatives (Nos 1033–1039, 1041–1044 and 1054–1059) are primarily metabolized via side-chain oxidation and ring S- and N-oxidation. The major metabolites are readily excreted in the urine either free or as glutathione conjugates (see Figure 1). A minor metabolic pathway (< 0.5%) is ring cleavage followed by C-oxidation to yield alpha-diketone and thioamide fragments.
Figure 1. Metabolism of alkylthiazoles
(ii) Metabolism of thiamine hydrochloride (No. 1030) and a metabolite (4-methyl-5-thiazole ethanol) (No. 1031)
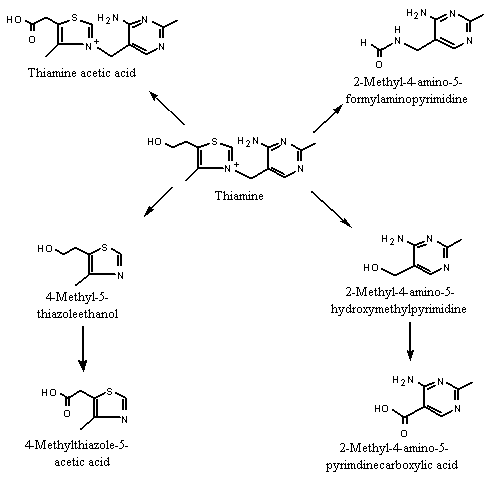
Thiamine is metabolized via two major pathways. In one, thiamine is cleaved to yield 4-methyl-5-thiazoleethanol (No. 1031) and 2-methyl-4-amino-5-hydroxymethyl-pyrimidine. The thiazole and pyrimidine fragments are further oxidized to yield 4-methylthiazole-5-acetic acid and the 5-pyrimidine carboxylic acid derivative, respectively, which, together with thiamine, are excreted in the urine (Tietz, 1986). In other pathways, thiamine is converted to 2-methyl-4-amino-5-formylamino-pyrimidine and thiamine acetic acid (Hanley & Fenwick, 1989) (see Figure 2).
Figure 2. Metabolism of thiamine and 4-methyl-5-thiazoleethanol
(iii) Metabolism of benzothiazole (No. 1040)
The fused-ring thiazole derivative, benzothiazole, is metabolized in guinea-pigs primarily by thiazole ring cleavage. A dose of 30 mg/kg bw given by intraperitoneal injection was metabolized to the free and conjugated forms of ortho-aminophenyl methyl sulfide, ortho-aminophenyl methyl sulfoxide and ortho-aminophenyl methyl sulfone. Small amounts of the N-hydroxy derivative of the sulfoxide and sulfone were also detected (Wilson et al., 1991).
(iv) Metabolism of related (non-flavouring) thiazole derivatives
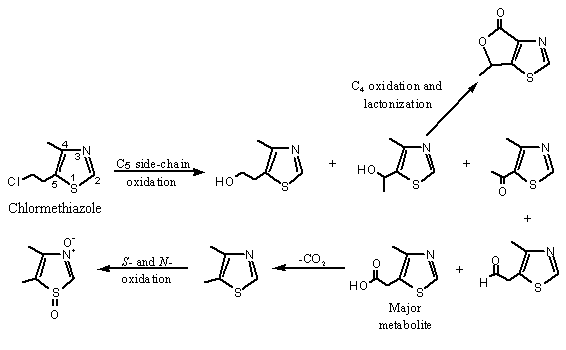
The therapeutic agent chlormethiazole is an alkyl- and chloroalkyl-substituted thiazole derivative [5-(2-chloroethyl)-4-methylythiazole] that is metabolized by oxidation of the alkyl and chloroalkyl substituents and by S- and N-oxidation. In volunteers treated orally, chlormethiazole was metabolized via side-chain oxidation of the ethyl C-1 position. The major urinary metabolite is 5-(acetic acid)-4-methylthiazole (see Figure 3). Methyl oxidation at C-4 yields 5-(hydroxyethyl)-4-thiazolecarboxylic acid lactone. The ring sulfoxide, the ring N-oxide and the combined ring S- and N-oxides of the side-chain were also found in urine. This was the first reported instance of sulfoxidation of a thiazole sulfur and oxidation of two different heteroatoms in the same heterocyclic aromatic ring in vivo (Offen et al., 1985).
Figure 3. Metabolism of chlormethiazole
Chlormethiazole has also been reported to undergo nucleophilic attack at C2 (Grupe & Spiteller, 1982).
Oral administration of the thiazole derivative, 4-tert-butyl-2-methylthiazole, to mice resulted in limited ring C-oxidation. Of the administered dose, 0.25% was excreted as ring fragmentation products in the urine within 24 h. These products included an alpha-dicarbonyl metabolite (3,3-dimethyl-2-oxobutanal) and a thioamide metabolite (thioacetamide). On the basis of the structure of the dicarbonyl fragment, it was postulated that the 4,5-double bond of the thiazole ring undergoes epoxidation, then hydrolysis to yield 4,5-diol. The diols then undergo hydrolytic cleavage to yield the corresponding carbonyl derivatives and thioacetamide (Mizutani et al., 1994).
The thioamide metabolite is postulated to be responsible for the nephrotoxicity of 4-tert-butyl-2-methylthiazole (Mizutani et al., 1993).
(v) Metabolism of thiazoline derivatives (Nos 1045, 1058 and 1059)
Thiazoline (containing a partially reduced thiazole ring) is a five-membered, non-aromatic hererocycle containing sulfur and nitrogen atoms in the 1- and 3-ring positions. Derivatives of thiazoline in this group of flavouring substances are substituted in the 2-, 4- and 5-positions. Substances in this group are expected to be metabolized primarily by oxidation of the ring sulfur.
(vi) Metabolism of dithiazine derivatives (Nos 1046–1049)
Dithiazine is a six-membered, non-aromatic heterocycle containing two sulfur atoms (at the 1- and 3-positions) and one nitrogen (at the 5-position). The derivatives of diathiazine in this group of flavouring substances are substituted at the 2-, 4- and 6-positions.
No specific information was available on the metabolism of the dithiazine derivatives, but it is expected to be similar to that of thiazole derivatives. Because of the presence of alkyl substituents, metabolism is expected to be primarily via side-chain oxidation and ring S- and N-oxidation.
(vii) Metabolism of thiophene derivatives (Nos 1050–1053)
Thiophene is a five-membered aromatic heterocycle containing a sulfur atom in the 1-position. Derivatives of thiophene in this group of flavouring substances are substituted in the 1-, 2- and 4- positions.
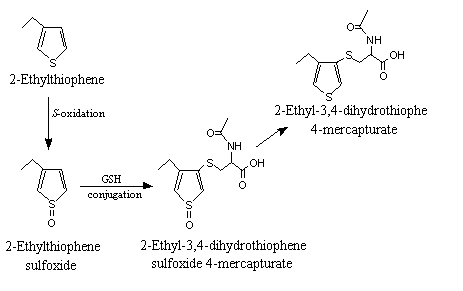
Thiophene and its derivatives follow a similar metabolic pathway to that of thiazole and its derivatives, namely, S-oxidation and side-chain C-oxidation. In a study in Sprague-Dawley rats given an intraperitoneal injection of radiolabelled thiophene at a dose of 200 mg/kg bw, 94% of the radiolabel in the urine was accounted for by the 2-mercapturic acid derivative of 1,4-dihydrothiophene-S-oxide. The authors concluded that it was formed by S-oxidation, yielding the thiophene sulfoxide, which underwent further conjugation with glutathione at position 2 (Dansette et al., 1992).
Ring-substituted thiophenes also undergo S-oxidation and glutathione conjugation (see Figure 4). In a study in which radiolabelled 3-benzoylthiophene was injected intraperitoneally into Sprague-Dawley rats, 15% of the urinary radiolabel was accounted for by a mixture of two diastereomers of the mercapturic acid conjugate of 4,5-dihydro-3-benzoylthiophene. The N-acetylcysteine residue (mercapturate) is bonded to the 4-position of the thiophene ring. Experiments with rat liver microsomes confirmed that thiophene sulfoxide is a reactive intermediate in the conversion of 3-benzothiophene to the dihydromercapturic acid metabolite (Valadon et al., 1996).
Figure 4. Metabolism of alkylthiophenes
2.3.2 Toxicological studies
(a) Acute toxicity
Oral LD50 values have been reported for 18 of the 30 substances in this group. With the exception of thiamine hydrochloride, the values in rats ranged from 460 to 1400 mg/kg bw (Sprince et al., 1974; Posternak et al., 1975; Babish, 1977; Mondino, 1981; Moreno, 1981; Piccirillo, 1982a,b; Reddy & Mayhew, 1986). The LD50 of thiamine hydrochloride in rats was 3700 mg/kg bw. The LD50 values in mice were in the range 400–4800 mg/kg bw (Calvery & Nelson, 1944; Oser, 1970; Shellenberger, 1970; Babish, 1978; Moran & Easterday, 1980).
(b) Short-term studies of toxicity
The results of short-term studies of toxicity with substances in this group are summarized in Table 4.
Table 4. Results of short-term studies of the toxicity of sulfur-containing heterocyclic compounds administered in the diet
|
No. |
Substance |
Species, sex |
No. test groupsa/no. per groupb |
Duration
(days) |
NOEL
(mg/kg bw
per day) |
Reference |
|
1030 |
Thiamine hydrochloride |
Rat; M/F |
1/30 |
90 |
33–40 |
Oser (1964) |
|
1031 |
4-Methyl-5-thiazole ethanol |
Rat; M/F |
1/30 |
90 |
11-14 |
Oser (1964) |
|
1039 |
2,4-Dimethyl-5-vinylthiazole |
Rat; M/F |
1/32 |
90 |
0.92 (M) |
Posternak et al. (1969) |
|
1.0 (F) |
|
1040 |
Benzothiazole |
Rat; M/F |
1/30 |
90 |
5.1 |
Morgareidge (1971) |
|
1041 |
2-Acetylthiazole |
Rat; M/F |
1/30 |
90 |
50 |
Wheldon et al. (1970) |
|
Mixture of 2-isobutyl-4,6-dimethyl and 4-isobutyl-2,6-dimethyldihydro-1,3,5-dithaizole |
Rat; M/F |
1/5 |
14 |
11 |
Rush (1989a,b) |
|
Mixture of 2-isopropyl-4,6-dimethyl and 4-isopropyl-2,6-dimethyldihydro-1,3,5-dithaizole |
Rat, M/F |
1/5 |
14 |
11 |
Rush (1989a,b) |
|
1053 |
2-Thienyldisulfide |
Rat; M/F |
1/30 |
90 |
9300 |
Morgareidge & Oser (1970) |
|
1055 |
2,4-Dimethyl-5-acetylthiazole |
Rat; M/F |
1/46 |
90 |
25 (M) |
Shellenberger (1971) |
|
24 (F) |
|
1057 |
2-Methyl-5-methoxythiazole |
Rat; M/F |
1/32 |
90 |
8.8 (M) |
Posternak et al. (1975) |
|
90 |
8.6 (F) |
|
1059 |
2-(2-Butyl)-4,5-dimethyl-3-thiazoline |
Rat; M/F |
1/30 |
90 |
1.2 (M) |
Babish (1978) |
|
1.3 (F) |
a Does not include control animals
b Includes both male and female animals
(i) Thiamine hydrochloride (No. 1030) and 4-methyl-5-thiazoleethanol (No. 1031)
In a 90-day feeding study, groups of 15 FDRL strain rats of each sex were maintained on a diet which provided a daily intake of 5 g/kg bw of an imitation meat flavour containing thiamine hydrochloride (No. 1030) at a concentration of 13 mg/kg. Analysis of the meat flavour revealed that 40–50% of the thiamine hydrochloride had degraded to 4-methyl-5-thiazoleethanol (No. 1031). Thus, the daily intake of thiamine hydrochloride was 33–44 mg/kg bw, and the daily intake of 4-methyl-5-thiazoleethanol was 11–14 mg/kg bw. The animals were observed daily for behaviour, appearance and survival. Body weights and food intake were reported weekly. At 6 and 12 weeks, the following clinical parameters were measured: blood haemoglobin and haematocrit, total and differential leukocyte counts, blood glucose and urea nitrogen and urinary pH, albumin and glucose. All animals were necropsied at termination of the study. A range of tissues was examined microscopically.
Daily observations revealed no treatment-related effects, and weekly measure-ment of body weights and food consumption revealed no significant differences between test and control animals. Haematological examinations, blood chemical determinations and urine analysis performed during weeks 6 and 12 showed normal values. The gross appearance was unremarkable, and liver and kidney weights were normal. Histopathological examination did not reveal any evidence of treatment-related effects. The increase in tubular dilatation observed in the kidneys of 2/15 males and 4/15 females was considered to be the result of a high intake of protein and salt and not treatment-related. The NOEL was 36 mg/kg bw per day (median value) for thiamine hydrochloride and 12 mg/kg bw per day (median value) for 4-methyl-5-thiazoleethanol (Oser, 1964).
(ii) 2,4-Dimethyl-5-vinylthiazole (No. 1039)
In a 90-day study, groups of 16 Charles River CD rats of each sex were maintained on diets containing 2,4-dimethyl-5-vinylthiazole at concentrations adjusted to provide an intake of approximately 0.92 mg/kg bw per day for males and 1.0 mg/kg bw per day for females. Clinical observations were recorded daily, and food consumption and body weights were determined weekly. During weeks 7 and 13 of the study, haematological and clinical chemical (blood urea) determinations were conducted on 50% of the animals. At the end of the study, all animals were necropsied, and the liver and kidneys were weighed. A wide range of tissues and organs from each animal was preserved, and major organs and tissues were examined histologically.
There were no clinical signs of toxicity, and body weights and food consumption were normal. There were no treatment-related changes in clinical chemical parameters. Organ weights were normal, and histopathological examination did not reveal any treatment-related changes. The NOEL was 0.92 mg/kg bw per day (Posternak et al., 1969).
(iii) Benzothiazole (No. 1040)
In a 90-day study, groups of 15 FDRL rats of each sex were maintained on a diet which provided a daily intake of benzothiazole (No. 1040) at 5.1 mg/kg bw. All animals were observed daily for behaviour, appearance and survival; body weights and food intake were recorded weekly. At weeks 6 and 12, the following clinical parameters were measured: blood haemoglobin and erythrocyte volume fraction, total and differential leukocyte counts, blood glucose and urea nitrogen concentrations and urinary pH, albumin and glucose. All animals were necropsied at the termination of the study, and a range of tissues was examined microscopically.
Daily observations reveaed no treatment-related effects, and weekly measure-ments of body weights and food consumption revealed no significant differences between test and control animals. Haematological, blood chemical and urine analyses showed normal values. Gross appearance was unremarkable, and the liver and kidney weights were normal. Histopathological examination showed no evidence of treatment-related effects. The NOEL was 5.1 mg/kg bw per day (Morgareidge, 1971).
(iv) 2-Acetylthiazole (No. 1041)
In a 28-day study, groups of five weanling Cfy Wistar male rats were maintained on diets containing 0, 5000 or 10 000 ppm of 2-acetylthiazole, calculated to provide an average daily intake of 0, 250 or 500 mg/kg bw per day, for 4 weeks. The high dose was reduced to 2500 ppm (125 mg/kg bw per day) on days 4–10. Decreased body weights were found at both the low dose (87%) and the high dose (78%), and slightly increased food consumption and reduced efficiency of food use were reported. At autopsy, no treatment-related changes were observed (Wheldon et al., 1970).
In a 90-day study, groups of 15 male Cfy Wistar rats were maintained on diets containing 0, 100, 1000 or 10 000 ppm of 2-acetylthiazole, equivalent to 0, 5, 50 and 500 mg/kg bw per day, for 13 weeks. The highest dose was maintained for 6 weeks and then raised to 1000 mg/kg bw per day for 7 weeks. Clinical signs were monitored daily, and body weights and food consumption were monitored weekly. Haematological parameters were measured weekly. At the end of the study, all animals were necropsied; the weights of the adrenals, heart, kidneys, liver, lungs, spleen, testes, and thyroid were recorded; and a range of tissues was taken for histopathology.
Reduced body weight and food intake were seen at doses of 50 mg/kg bw per day and above, and decreased food conversion efficiency at the highest dose. Haematological parameters were similar in treated and untreated animals. At necropsy, the relative weights of the liver, adrenals and thyroid were increased in animals at the highest dose. Gross examination showed occasional pallor and mottling of kidneys in some rats, but these findings were not considered significant. Histopathological examination of 13 tissues from five animals at the highest dose revealed minimal fatty changes in the liver. Histopathological examination of the livers of five animals at the low and intermediate doses showed no evidence of treatment-related changes. The NOEL was 50 mg/kg bw per day (Wheldon et al., 1970).
(v) 2- and 4-Isobutyl (No. 1046) and 2- and 4-isopropyl (No. 1047) derivatives of 2,6-dimethyldihydro-1,3,5-dithiazine
In a 14-day study, groups of five male and five female Sprague-Dawley rats were maintained on a diet containing either 2- and 4-isobutyl or 2- and 4-isopropyl derivatives of 2,6-dimethyldihydro-1,3,5-dithiazine. The diets were calculated to provide an average daily intake of 12 mg/kg bw of the isobutyl or isopropyl derivative for males and 11 mg/kg bw of isobutyl or isopropyl derivative for females. During the study, the rats were observed daily for clinical signs of toxicity, and body weight and food consumption were measured weekly. All animals were subjected to a complete gross necroscopy at termination (day 15). No deaths or treatment-related toxic effects were observed. Body-weight gain and food consumption were normal. Histopathological evaluation of the liver and kidneys did not reveal any treatment-related effects. The NOEL for both substances was 11 mg/kg per day (Rush, 1989a,b).
(vi) 5-Methyl-2-thiophenecarboxyaldehyde (No. 1050) and 3-acetyl-2,5- dimethylthiophene (No. 1051)
In a 14-day study, groups of five Fischer 344 rats of each sex were maintained on a diet containing either 5-methyl-2-thiophenecarboxyaldehyde (No. 1050) or 3-acetyl-2,5-dimethylthiophene (No. 1051) at concentrations calculated to provide an estimated average daily intake of 10 mg/kg bw for 14 days. Daily clinical examinations and weekly measurements of body weight and food consumption revealed decreased food consumption (7%, p < 0.01) by males on both substances during the first week. No significant decrease in food consumption was observed among females or males during the second week of the study. On the basis that similar changes were seen with other substances in this study and that the changes in body weight were not accompanied by other signs of toxicity, this was considered not to be a treatment-related effect. At day 14, decreased body-weight gain was observed in males and females given 5-methyl-2-thiophenecarboxyaldehyde (11% and 20%, respectively) and 3-acetyl-2,5-dimethylthiophene (17% and 11%, respectively). At necropsy, decreased absolute (11–14%) and relative liver weights (7–10%, p < 0.05) and decreased absolute kidney weights (8–11%, p < 0.05) were reported for treated males. No difference in absolute or relative liver or kidney weights was observed between control and test females. Macroscopic and histopathological examination did not reveal any gross or microscopic lesions that could be attributed to treatment. A NOEL could not be identified in this study (Gill & Van Miller, 1987).
(vii) 2-Thienyl disulfide (No. 1053)
In a 90-day study, groups of 15 FDRL rats of each sex were maintained on a diet containing 2-thienyl disulfide (No. 1053) which provided a daily intake of 0.29 mg/kg bw. All animals were observed daily for behaviour, appearance and survival; body weights and food intake were recorded weekly. At weeks 6 and 12, the following clinical parameters were measured: blood haemoglobin and erythrocyte volume fraction, total and differential leukocyte counts, blood glucose and urea nitrogen concentrations and urine pH, albumin and glucose. All animals were necropsied at termination of the study, and a range of tissues was examined microscopically.
Daily observations did not reveal any treatment-related effects, and weekly measurement of body weights and food consumption showed no significant differences between test and control animals. Haematological, blood chemical and urine analyses gave normal values. Gross appearance was unremarkable, and the liver and kidney weights were normal. Histopathological examination did not reveal any evidence of treatment-related effects. The NOEL was 0.29 mg/kg bw per day (Morgareidge & Oser, 1970).
(viii) 2,4-Dimethyl-5-acetylthiazole (No. 1055)
In a 90-day study, groups of 23 Sprague-Dawley rats of each sex were maintained on a diet containing 2,4-dimethyl-5-acetylthiazole at a concentration calculated to result in a daily intake of 24 mg/kg bw. Animals were examined daily for clinical appearance and survival and weekly for body weight, food consumption and food use efficiency. Haematological, blood chemical and urine analyses were performed on eight animals of each sex at weeks 6 and 13. At necropsy, all animals were examined grossly, and the heart, kidneys, liver, spleen, testes and ovaries were weighed. Histopathology was carried out on 25 tissues from eight males and eight females and on the liver and kidneys of seven additional males and females.
There were no deaths or clinical signs of toxicity. Body weights and food consumption were normal, and there were no treatment-related changes in clinical parameters. At necropsy, there was an apparent increase in absolute liver and kidney weights in both males and females, which was considered to be due to unusually low average organ weights in the control group. Histopathology did not reveal any treatment-related changes in the liver or kidney or in any other organs. The NOEL was 24 mg/kg bw per day (Shellenberger, 1971).
(ix) 2-Methyl-5-methoxythiazole (No. 1057)
In a 90-day study, groups of 16 Charles River CD rats of each sex were maintained on a diet containing 2-methyl-5-methoxythiazole (No. 1057) at a concentration adjusted to provide an intake of approximately 8.8 mg/kg bw per day for males and 8.6 mg/kg bw per day for females. Clinical observations were recorded daily, and food consumption and body weights were determined weekly. During weeks 7 and 13 of the study, haematological and clinical chemical (blood urea) determinations were conducted on 50% of the animals. At the end of the study, all animals were necropsied, and the liver and kidneys were weighed. A wide range of tissues and organs from each animal was preserved, and major organs and tissues were examined histologically.
There were no clinical signs of toxicity, and body weights and food consumption were normal. No treatment-related changes were found in clinical chemical parameters. The organ weights were normal, and histopathological examination did not reveal any treatment-related changes. The NOEL was 8.6 mg/kg bw per day (Posternak et al., 1975).
(x) 2-(2-Butyl)-4,5-dimethyl-3-thiazoline (No. 1059)
In a 90-day study, groups of 15 FDRL rats of each sex were maintained on a diet containing 2-(2-butyl)-4,5-dimethyl-3-thiazoline (No. 1059), which provided a daily intake of 1.2 mg/kg bw for males and 1.3 mg/kg bw for females. All animals were observed daily for behaviour, appearance and survival; body weights and food intake were reported weekly. At 6 and 12 weeks, the following clinical parameters were measured: blood haemoglobin and erythrocyte volume fraction, total and differential leukocyte counts, blood glucose and urea nitrogen concentrations and urinary pH, albumin and glucose. All animals were necropsied at termination of the study.
Daily observations did not reveal any treatment-related effects, and weekly measurement of body weights and food consumption revealed no significant differences between test and control animals. Haematological, blood chemical and urine analyses revealed normal values. The weights of the liver and kidney were normal. The NOEL was 1.2 mg/kg bw per day (Babish, 1978).
(c) Genotoxicity
Four substances, thiazole (No. 1032), 4,5-dimethylthiazole (No. 1035), 4-methyl-thiazole (No. 1043) and 5-methyl-2-thiophenecarboxyaldehyde (No. 1050), in this group of flavouring substances were testedfor their ability to induce reverse mutation in Salmonella typhimurium strains TA98 and TA100 at doses of 4–100 µmol/plate. The purity of the chemicals was not stated. Positive results were obtained with thiazole in strain TA100 only at a minimum concentration of 4 µmol/plate; however, the mutagenicity that was observed in the absence of an exogenous metabolic activation system was less marked in the presence of such a system, indicating that thaizole does not undergo metabolic activation. The other substances gave uniformly negative results in both strains (Lee et al., 1994). No other tests have been reported.
(d) Other relevant studies
4-Methylthiazole (No. 1043)
In a study to determine the effect of glutathione on the metabolism of thiazoles and, in particular, 4-methylthiazole (No. 1043), groups of mice were given a single oral dose of 495 mg/kg bw alone or 24 h after pretreatment with DL-buthionine sulfoximine, a glutathione depletion agent. In those animals in which glutathione was depleted, treatment with 4-methylthiazole resulted in an increase in relative kidney weight and a significant increase in serum urea nitrogen. In animals treated with 4-methylthiazole alone at doses up to 1.0 mmol/kg bw, there was no effect on kidney weight or serum urea nitrogen concentration (Mizutani et al., 1992).
3. REFERENCES
Babish, J.G. (1977) Acute oral toxicity of 2-isopropyl-4-methylthiazole in rats. Unpublished report from Food and Drug Research Laboratories, Inc., Maspeth, New York, USA. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Babish, J.G. (1978) 90-day feeding study of 2-(2-butyl)-4,5-dimethyl-3-thiazoline in rats. Unpublished report from Food and Drug Research Laboratories, Inc., Maspeth, New York, USA. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Calvery, H.O. & Nelson, A.A. (1944) Acute toxicity study for 4-methyl-5-thiazoleethanol in mice. Unpublished report. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Cramer, G.M. & Ford, R.A. (1978) Estimation of toxic hazard—A decision tree approach. Food Cosmet. Toxicol., 16, 255–276.
Dansette, P.M., Thang, D.C., El Amri, H. & Mansuy, D. (1992) Evidence for thiophene S-oxide as a primary reactive metabolite of thiophene in vivo: Formation of a dihydrothiophene sulphoxide mercapturic acid. Biochem. Biophys. Res. Communications, 186, 1624–1630.
Decouvelaere, B., Gaillard, C., Kettler, J.P., Martin, S., Gires, P. & Gaillot, J. (1986) Biotransformation of 48740 RP. Unpublished report presented at the 10th European Drug Metabolism Workshop, Guildford, Surrey, England.
El Dareer, S.M., Tillery, K.F., Rose, L.M., Posey, C.F., Struck, R.F., Stiller, S.W. & Hill, D.L (1993) Metabolism and disposition of a thiazolobenzimidazole active against human immunodeficiency virus-1. Drug Metab. Disposition, 21, 231–235.
Gill, M.W. & Van Miller, J.P. (1987) Fourteen-day dietary minimum toxicity (MTS) in albino rats. Unpublished report from Bushy Run Research Centre, Export, Pennsylvania, USA. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Grupe, A. & Spiteller, G. (1982) Unexpected metabolites produced from clomethiazole. J. Chromatogr., 230, 335–344.
Hanley, A.B. & Fenwick, G.R. (1989) Naturally occuring sulphur compounds. In: Damani, L.A., ed., Sulphur Containing Drugs and Related Organic Compounds, Chichester: Ellis Horwood, Vol. 1A, pp. 41–44.
Hegsted, D.M., Chichester, C.O. & Darby, W.J., eds (1976) Present Knowledge in Nutrition, 4th Ed., Washington DC, The Nutrition Foundation Inc.
Informatics, Inc. (1974) Scientific Literature Reviews on Generally Recognized as Safe (GRAS) Ingredients—Thiamine, Washington DC: US Food & Drug Administration.
International Organization of the Flavor Industry (1995) European usage data. Unpublished report. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Lee, H., Bian, S.S. & Chen, Y.L. (1994) Genotoxicity of 1,3-dithiane and 1,4-dithiane in the CHO/SCE assay and in the Salmonella/microsomal test. Mutat. Res., 321, 213–218.
Lehninger, A.L. (1987) Principles of Biochemistry, Worth Publishing Inc., pp. 252–254, 768.
Lucas, C.D., Putnam, J.M. & Hallagan, J.B. (1999) Flavor and Extract Manufacturers’ Association of the United States 1995 Poundage and Technical Effects Update Survey. Washington DC: Flavor and Extract Manufacturers’ Association of the United States.
Maarse, H., Visscher, C.A., Willemsens, L.C. & Boelens, M.H. (1999) Volatile Components in Food—Qualitative and Quantitative Data, Zeist, Centraal Instituut Voor Voedingsonderzioek TNO.
Mizutani, T., Yoshida, K. & Ito, K. (1992) Nephrotoxicity of thiazoles structurally related to thiabendazole in mice depleted of glutathione by treatment with bithionine sulfoximine. Res. Communications Chem. Pathol. Pharmacol., 75, 29–38.
Mizutani, T., Yoshida, K. & Kawazoe, S. (1993) Possible role of thioformamide as a proximate toxicant in the nephrotoxicity of thiabendazole and related thiazoles in the glutathione-depleted mice: Structure–toxicity and metabolism studies. Chem. Res. Toxicol., 6, 174–179.
Mizutani, T., Yoshida, K. & Kawazoe, S. (1994) Formation of toxic metabolites from thiabendazole and other thiazoles in mice. Drug Metab. Disposition, 22, 750–755.
Mondino, A. (1981) Acute toxicity study on methyl-4-thiazol. Unpublished report from Instituto di Ricerche Biomedicine, Rome. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Moran, E.J. & Easterday, O.D. (1980). Acute oral toxicity on selected flavor chemicals. Food Chem. Toxicol., 3, 249–258.
Moreno, O.M. (1981). Single dose oral toxicity/LD50 in rats. Unpublished report by MB Research Laboratories Inc., Spinerstown, Pennsylvania, USA. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Morgareidge, K. (1971) 90-day feeding study with benzothiazole in rats. Unpublished report from Food and Drug Research Laboratories, Inc., Maspeth, New York, USA. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Morgareidge, K. & Oser, B.L. (1970) 90-day feeding studies in rats with 2-thienyldisulfide. Unpublished report from Food and Drug Research Laboratories, Inc., Maspeth, New York, USA. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Offen, C.P., Frearson, M.J., Wilson, K. & Burnett, D. (1985) 4,5-Dimethylthiazole-N-oxide-S-oxide: A metabolite of chloromethiazole in man. Xenobiotica, 15, 503–511.
Oser, B.L. (1964) Subacute (90-day) feeding study with imitation meat flavor IT-2341 in rats. Unpublished report from Food and Drug Research Laboratories, Inc., Maspeth, New York, USA. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Oser, B.L. (1970) The acute oral toxicity of ten compounds. Unpublished report from the Food and Drug Research Laboratories, Inc., Maspeth, New York, USA. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Piccirillo, V.J. (1982a) Final report on acute oral toxicity (LD50) study in the rat with 4,5-dimethylthiazole. Unpublished report from Borriston Laboratories, Inc., Temple Hills, Maryland, USA. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Piccirillo, V.J. (1982b) Acute oral toxicity (LD50) study in the rat with 2-ethoxythiazole. Unpublished report from Borriston Laboratories, Inc., Temple Hills, Maryland, USA. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Posternak, J.M., Linder, A. & Vpodos, C.A. (1969) Summaries of toxicological data: Toxicological tests on flavouring matters. Food Cosmet. Toxicol., 7, 405–407.
Posternak, J.M., Dufour, J.J., Rogg, C. & Vodoz, C.A. (1975) Toxicology tests on flavouring matters. II. Pyrazines and other compounds. Food Cosmet. Toxicol., 13, 487–490.
Rance, D.J. (1989) Sulphur heterocycles. In: Damani, L.A., ed., Sulfur-containing Drugs and Related Organic Compounds. Chemistry, Biochemistry and Toxicology, Chichester, Ellis Horwood, Vol. 1, pp. 217–259.
Reddy, G. & Mayhew, D.A. (1986) Acute oral toxicity (LD50) study in rats with benzothiazole. J. Am. Coll. Toxicol., 4, 666.
Rush, R. (1989a) 14-day dietary toxicity study in rats with 2(4)-isobutyl-4(2),6-dimethyldihydro-1,3,5-dithiazine. Unpublished report from Springborn Laboratories, Inc., Spencerville, Ohio, USA. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Rush, R. (1989b) 14-day dietary toxicity study in rats with 2(4)-isopropyl-4(2),6-dimethyldihydro-1,3,5-dithiazine. Unpublished report from Springborn Laboratories, Inc., Spencerville, Ohio, USA. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Sprince, H., Parker, C.M., Smith, G.G. & Gonzales, L.J. (1974) Protection against acetaldehyde toxicity in the rat by L-cysteine, thiamin and 1,2-methylthiazolidine-4-carboxylic acid. Agents Actions, 4, 125–130.
Shellenberger, T.E. (1970) Acute toxicological evaluations of chemicals with mice, Unpublished report from the Gulf South Research Institute, Iszeria, Louisiana, USA. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Shellenberger, T.E. (1971) Subacute toxicity evaluation of 2,4-dimethyl-5-acetylthiazole with rats. Unpublished report from the Gulf South Research Institute, Iszeria, Louisiana, USA. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
Stofberg, J. & Grundschober, F. (1987) Consumption ratio and food predominance of flavoring materials. Perfumer Flavorist, 12, 27.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of flavouring materials: A mechanism for setting priorities for safety evaluations. Food Chem. Toxicol., 23, 857–860.
Tietz, N.W., ed. (1986) B-vitamins. In: Textbook of Clinical Chemistry, New York, W.B. Saunders Co., pp. 940–945.
Valadon, P., Dansette, P.M., Girault, J-P., Amar, C. & Mansuy, D. (1996) Thiophene sulfoxides as reactive metabolites: Formation upon microsomal oxidation of a 3-aroylthiophenes and fate in the presence of nucleophiles in vitro and in vivo. Chem. Res. Toxicol., 9, 1403–1413.
Wilson, J.D. (1982) Disorder of vitamins—Deficiency, excess and errors of metabolism. In: Harrison’s Principles of Internal Medicine, 10th ed., New York, McGraw-Hill, pp. 461–470.
Wilson, K., Chissick, A.M., Fowler, A.M., Frearson, F.J., Gittins, M. & Swinbourne, F.J. (1991) Metabolism of benzothiazole. 1. Identification of ring-cleavage products. Xenobiotica, 21, 1179–1183.
Wheldon, G.H., Amyes, S.J., Street, A.E., Hague, P.H. & Mawdesley-Thomas, L.E. (1970) Toxicity of Wa4295, Sa 927, SH3048, and Wa 3328 in dietary administration to rats over a period of 13 weeks. Unpublished report from Huntingdon Research Centre, United Kingdom. Submitted to WHO by Flavor and Extract Manufacturers’ Association of the United States.
ENDNOTES
1. According to the decision-tree process, an ‘aromatic’ compound has at least one benzene, furan, thiophene, pyridine or pyrole ring, however substituted and regardless of whether it is fused to another ring.