
WHO FOOD ADDITIVES SERIES: 52
ALICYCLIC, ALICYCLIC-FUSED AND AROMATIC-FUSED RING LACTONES
First draft prepared by
Dr A. Mattia
Division of Biotechnology and GRAS Notice Review, Office of Food Additive Safety, Center for Food Safety and Applied Nutrition, Food & Drug Administration, College Park, Maryland, United States
and
Dr A.G. Renwick
Clinical Pharmacology Group, University of Southampton, Southampton, England
1. EVALUATION
1.1 Introduction
The Committee evaluated a group of 16 flavouring agents (see Table 1) by the Procedure for the Safety Evaluation of Flavouring Agents (see Figure 1, Introduction). This group included:
- Four aliphatic lactones (Nos 1157–1160) that form open-chain acyclic hydroxycarboxylic acids upon hydrolysis1;
- Six gamma- or delta-lactones fused to an alicyclic ring (cyclohexyl or decalin ring systems) (Nos 1161–1166); and
- Six gamma- or delta-lactones fused to a benzene ring (Nos 1167–1172).
The Committee had previously evaluated one member of the group, dihydro-coumarin (No. 1171), at its thirty-fifth meeting (Annex 1, reference 88), but no ADI was established. The Committee noted that metabolites of dihydrocoumarin have been identified in rabbit urine (Furuya, 1958). The toxicological data considered were derived from studies of acute toxicity in mice, rats, and guinea pigs, a 14-week study in rats in which the dosage was uncertain owing to loss of the test substance during storage (Hagan et al., 1967), a 90-day study in rats treated with a single dose (Trubek, 1958), and a study in which three dogs were treated with one of two doses for 2 years (without a control group) (Hagan et al., 1967). No adverse effects were reported, but the Committee considered these data to be inadequate. At the thirty-fifth meeting, the Committee stated that the results of a short-term study in a rodent species and metabolic studies to determine the extent of conversion of dihydrocoumarin to coumarin would be needed before dihydrocoumarin could be re-evaluated.
Eight of the 16 flavouring agents in this group (Nos 1157, 1162–1164, 1168–1171) have been reported to occur naturally in foods and have been detected in rose-apple, celery stalks, soya bean, black and green tea, boiled beef and peppermint oil (Maarse et al., 1999).
1.2 Estimated daily intake
The total annual volume of production of the 16 flavouring agents in this group is approximately 12 200 kg in Europe (International Organization of the Flavour Industry, 1995) and 10 000 kg in the USA (National Academy of Sciences, 1982; Lucas et al., 1999). More than 80% of the total annual volume of production in Europe and more than 84% in the USA is accounted for by dihydrocoumarin (No. 1171). The estimated daily per capita intakes in Europe and the USA for dihydrocoumarin are 1.4 and 1.1 mg, respectively (International Organization of the Flavour Industry, 1995; Lucas et al., 1999. The daily per capita intakes of all the other flavouring agents in the group are in the range of 0.07–298 mg (International Organization of the Flavour Industry, 1995; Lucas et al., 1999), most values being at the lower end of this range. The daily per capita intake of each agent in Europe and in the USA is reported in Table 1.
Table 1. Summary of results of safety evaluations of alicyclic, alicyclic-fused and aromatic-fused ring lactones used asflavouring agentsa
|
Flavouring agent |
No. |
CAS number and structure |
Step A3 Does intake exceed the threshold for human intake?b |
Step A4 Is the flavouring agent or are its metabolites endogenous? |
Comments based on predicted metabolism |
Conclusion based on current intake |
|
Structural class I |
|
|
|
|
|
|
|
4-Hydroxy-4-methyl-5-hexenoic acid gamma-lactone |
1157 |
1073-11-6
 |
No
Europe: ND
USA: 3 |
NR |
See note 1 |
No safety concern |
|
(+/-) 3-Methyl-gamma-decalactone |
1158 |
67663-01-8
 |
No
Europe: ND
USA: 5 |
NR |
See note 1 |
No safety concern |
|
4-Hydroxy-4-methyl-7-cis-decenoic acid gamma_lactone |
1159 |
70851-61-5
 |
No
Europe: ND
USA: 13 |
NR |
See note 1 |
No safety concern |
|
Tuberose lactone |
1160 |
153175-57-6
 |
No
Europe: ND
USA: 11 |
NR |
See note 1 |
No safety concern |
|
Structural class III |
|
|
|
|
|
|
|
Dihydromintlactone |
1161 |
92015-65-1
 |
No
Europe: ND
USA: 12 |
NR |
See note 2 |
No safety concern |
|
Mintlactone |
1162 |
13341-72-5
 |
No
Europe: 4
USA: 9 |
NR |
See note 2 |
No safety concern |
|
Dehydromenthofurolactone |
1163 |
75640-26-5
 |
No
Europe: 2
USA: 9 |
NR |
See note 3 |
No safety concern |
|
(+/-)-(2,6,6-Trimethyl-2-hydroxycyclohexylidene) acetic acid gamma-lactone |
1164 |
15356-74-8
 |
No
Europe: ND
USA: 0.9 |
NR |
See note 2 |
No safety concern |
|
Sclareolide |
1165 |
564-20-5
 |
No
Europe: 1
USA: 6 |
NR |
See note 2 |
No safety concern |
|
Octahydrocoumarin |
1166 |
4430-31-3
 |
No
Europe: ND
USA: 0.07 |
NR |
See note 4 |
No safety concern |
|
Structural class III |
|
|
|
|
|
|
|
2-(4-Methyl-2-hydroxyphenyl)propionic acid gamma-lactone |
1167 |
65817-24-5
 |
No
Europe: ND
USA: 2 |
Yes. The NOELs of 5.42 and 6.55 mg/ kg bw per day for males and females, respectively, for the related substance 3-propylidenephthalide (No. 1168) (Posternak et al.,1969) are >100 000 times greater than the estimated intake of 2-(4-methyl-2-hydroxyphenyl) propionic acid gamma-lactone in the USA(0.03 µg/kg bw per day) when used as a flavouring agent |
See note 5 |
No safety concern |
|
3-Propylidenephthalide |
1168 |
17369-59-4
 |
No
Europe: 20
USA: 52 |
Yes. The NOELs of 5.42 and 6.55 mg/kg bw per day for males and females, respectively, (Posternak et al.,1969) are >1000 times greater than the estimated intakes of 3-propylidenephthalide in Europe (0.3 µg/kg bw per day) and in the USA (0.9 µg/kg bw per day) when used as a flavouring agent |
See note 6 |
No safety concern |
|
3-n-Butylphthalide |
1169 |
6066-49-5
 |
No
Europe: 0.6
USA: 0.4 |
Yes. The NOELs of 5.42 and 6.55 mg/kg bw per day for males and females, respectively, for the related substance 3-propylidenephthalide (No. 1168) (Posternak et al.,1969) are >100 000 times greater than the estimated intakes of 3-n-butylphthalide inEurope (0.01 µg/kg bw per day) and in the USA(0.006 µg/kg bw perday) when used asa flavouring agent |
See note 6 |
No safety concern |
|
3-Butylidenephthalide |
1170 |
551-08-6
 |
No
Europe: 10
USA: 7 |
Yes. The NOELs of 5.42 and 6.55 mg/kg bw per day for males and females, respectively for therelated substance 3-propylidenephthalide (No. 1168) (Posternak et al.,1969) are >10 000 times greater than the estimated intakes of 3-butylidenephthalide in Europe (0.2 µg/kg bw per day) and in the USA(0.1 µg/kg bw per day) when used as a flavouring agent |
See note 6 |
No safety concern |
|
Dihydrocoumarin |
1171 |
119-84-6
 |
Yes
Europe: 1415
USA: 1111 |
Yes. Several safety studies are available as discussed in the text |
See note 7 |
No safety concern |
|
6-Methylcoumarin |
1172 |
92-48-8
 |
Yes
Europe: 298
USA: 96 |
Yes. Several safety studies are available as discussed in the text |
See note 8 |
No safety concern |
Notes to Table 1
|
CAS: Chemical Abstracts Service; ND: no data on intake reported; NR: not required for evaluation because consumption of the substance wasdetermined to be of no safety concern at step A3 of the Procedure |
|
a |
Step 2: Ten flavouring agents (Nos 1157–1166) in this group were predicted to be metabolized to innocuous products. The evaluation of theseflavouring agents therefore proceeded via the A-side of the decision tree. The aromatic-fused ring lactones (Nos 1167–1172) are in structuralclass III; limited metabolic data exist for this subgroup of flavouring agents. The evaluation of these six flavouring agents therefore proceeded via the B-side of the decision tree |
|
b |
The thresholds for human intake for structural classes I and III are 1800 and 90 µg/day, respectively. All intake values are expressed in µg/day. The combined intakes of flavouring agents in structural class I is 32 µg/person per day in the USA. The combined intake of flavouring agents instructural class III is 1751 µg/person per day in Europe and 1305 µg/person per day in the USA |
|
Notes: |
|
1. |
Hydrolysed to a hydroxycarboxylic acid, followed by beta-oxidative cleavage to yield metabolites that are completely metabolized in the citric acid cycle |
|
2. |
Hydrolysed to a hydroxycarboxylic acid, followed by excretion; oxidation of ring substituents or the ring itself to yield polar hydroxylatedmetabolites that may be excreted in the urine |
|
3. |
Hydrolysed to a ketoacid, followed by excretion as the glucuronic acid conjugate or oxidation of ring substituents to yield polar hydroxylatedmetabolites that may be excreted in the urine |
|
4. |
Hydrolysed to a hydroxycarboxylic acid, followed by beta-oxidative cleavage to a polar hydroxyacid that may be excreted free or conjugated withglucuronic acid |
|
5. |
Hydrolysed to hydroxycarboxylic acid, followed by glucuronic acid or glycine conjugation |
|
6. |
Hydrolysed to hydroxycarboxylic acid or keto carboxylic acid, followed by excretion as the glucuronic acid or glycine conjugate |
|
7. |
Hydrolysed followed by beta-oxidation to yield o-hydroxybenzoic acid which is excreted primarily in the urine unchanged or as the glycine conjugate |
|
8. |
Oxidized to yield 7-hydroxy-6-methylcoumarin via ring hydroxylation or coumarin-6-carboxylic acid via methyl group oxidation. Excretion ofthese conjugates either in the free form or as glycine conjugates |
1.3 Absorption, distribution, metabolism and elimination
Lactones are formed by acid-catalysed intramolecular cyclization of 4- or 5-carbon hydroxycarboxylic acids to yield five- (gamma-) or six- (delta-) membered lactone rings, respectively. The stability of the lactone ring in an aqueous environment is pH-dependent. In blood, lactones would hydrolyse rapidly to the open-chain hydroxycarboxylic acid (Fishbein & Bessman, 1966; Roth & Giarman, 1966; Guidotti & Ballotti, 1970). On the basis of the results of studies of structurally-related lactones (Billecke et al., 2000), the four aliphatic lactones (Nos 1157–1160) in the group can be expected to hydrolyse to the corresponding hydroxycarboxylic acid, and then undergo beta-oxidative cleavage to yield metabolites that are com-pletely metabolized in the citric acid cycle (Voet & Voet, 1990; Nelson & Cox, 2000).
The metabolic options open to lactones fused to alicyclic rings (Nos 1161–1166) include excretion as the open-chain hydroxycarboxylic acid derivative, hydroxylation of ring alkyl substituents producing polar metabolites that may be excreted (Madyastha & Raj, 1993), or oxidative degradation of the carboxylic acid side-chain to yield polar alicyclic or aromatic carboxylic acids that are excreted unchanged or in conjugated form (Brewster et al., 1977a).
Metabolic pathways available to aromatic fused-ring lactones (Nos 1167–1172) include excretion as the glycine or glutamine conjugates of the open-chain hydroxycarboxylic acid derivative, or oxidation or reduction of the side-chain and subsequent excretion as the glucuronic acid conjugate (Ambrose et al., 1933; James et al., 1972).
1.4 Application of the Procedure for the Safety Evaluation of Flavouring Agents
|
Step 1. |
In applying the Procedure for the Safety Evaluation of Flavouring Agents, the Committee assigned four of the 16 agents (Nos 1157–1160) to structural class I. The remaining 12 agents (Nos 1161–1172) were assigned to structural class III (Cramer et al., 1978). |
|
Step 2. |
Ten flavouring agents (Nos 1157–1166) in this group are expected to be metabolized to innocuous products. The evaluation of these flavour-ing agents therefore proceeded via the A-side of the decision-tree. |
| |
Limited metabolic data exist for the aromatic-fused ring lactones (Nos 1167–1172). The evaluation of these six flavouring agents therefore proceeded via the B-side of the decision tree. |
|
Step A3. |
The estimated daily per capita intakes of all four of the flavouring agents in structural class I (Nos 1157–1160) and six agents (Nos 1161–1166) in structural class III are below the threshold for concern (i.e. 1800 µg/day for class I and 90 µg/day for class III). The Committee con-cluded that these 10 substances would not be expected to be of safety concern at current estimated levels of intake as flavouring agents. |
|
Step B3. |
The estimated daily per capita intakes of four of the flavouring agents in structural class III are below the threshold for concern for their class (i.e. 90 µg/day). Accordingly, the evaluation of these four agents proceeded to step B4. |
| |
The estimated daily per capita intakes of the remaining two substances in structural class III, dihydrocoumarin (No. 1171) and 6-methyl-coumarin (No. 1172), exceed the threshold of concern for their class (i.e. 90 µg/day). The estimated intake of dihydrocoumarin is 1415 mg/ person per day in Europe and 1111 µg/person per day in the USA. The estimated intake of 6-methylcoumarin (No. 1172) is 298 µg/person per day in Europe and 96 µg/person per day in the USA. In accordance with the Procedure, more extensive data are needed to perform a safety evaluation of flavouring agents exceeding the threshold for their structural class at step B3. |
|
Step B4. |
The NOELs of 5.42 and 6.55 mg/kg bw per day (Posternak et al., 1969) for males and females, respectively, for 3-propylidenephthalide (No. 1168) are 1000 times greater than its estimated intake of 0.3 µg/kg bw per day in Europe and 0.9 µg/kg bw per day in the USA. |
| |
The NOELs for 3-propylidenephthalide are appropriate to evaluate 2-(4-methyl-2-hydroxyphenyl)propionic acid gamma-lactone (No. 1167), 3-n-butylphthalide (No. 1169), and 3-butylidenephthalide (No. 1170) because these substances are structurally related and undergo similar pathways of metabolism. The NOELs of 5.42 and 6.55 mg/kg bwper day (Posternak et al., 1969) for males and females, respectively, for 3-propylidenephthalide are 100 000 times greater than the estimated intake from use as a flavouring agent of 2-(4-methyl-2-hydrox-yphenyl)propionic acid gamma-lactone in the USA (0.03 µg/kg bw per day), 100 000 times greater than the estimated intake of 3-n-butylphthalide in Europe (0.01 µg/kg bw per day) and in the USA (0.006 µg/kg bw per day), and 10 000 times greater than the estimated intake of 3-butylidenephthalide in Europe (0.2 µg/kg bw per day) and in the USA (0.1 µg/kg bw per day). The Committee concluded that these substances would not pose a safety concern at currently estimated levels of intake. Table 1 summarizes the evaluations of the members of this group. |
1.5 Consideration of flavouring agents with high intakes evaluated by the B-side of the decision-tree
1.5.1 Dihydrocoumarin (No. 1171)
More extensive data on metabolism and toxicity were considered to complete the safety evaluation of dihydrocoumarin (No. 1171).
Dihydrocoumarin is a delta-lactone fused to a benzene ring and is not a member of the class of aromatic coumarin derivatives. Dihydrocoumarin lacks alpha,beta-unsaturation in the lactone ring, which is a key structural feature in the metabolism of coumarin. Coumarin is principally metabolized in humans and primates by electrophilic substitution (i.e. 7-hydroxylation), and in rats and several other species by epoxidation of the alkene function to form mainly 3-hydroxycoumarin (Egan et al., 1990; Van Iersel et al., 1994). The absence of a double bond in dihydrocoumarin precludes the formation of the reactive epoxide and subsequent metabolites. The metabolic fate of dihydrocoumarin closely resembles that of simple aliphatic delta-lactones. Dihydrocoumarin and aliphatic lactones hydrolyse to the corresponding ring-opened hydroxyacids (Billecke et al., 2000). Hydrolysis of dihydrocoumarin yields a substituted 3-phenylpropanoic acid derivative that is expected to undergo either conjugation or side-chain oxidation to yield the corresponding benzoic acid derivative (Furuya, 1958; Pollitt, 1974).
Mice
In a 13-week study, groups of B6C3F1 mice received dihydrocoumarin in corn oil by gavage at a dose of up to 1600 mg/kg bw, once daily, 5 days a week. No gross or microscopic lesions were observed in either sex at any dose, although body-weight gain was reduced in males and females at the highest dose and females at 800 mg/kg bw showed changes in organ weights. The NOEL for dihydrocoumarin was 400 mg/kg bw per day in B6C3F1 mice (National Toxicology Program, 1993).
In a study of the potential carcinogenicity of dihydrocoumarin, B6C3F1 mice received dihydrocoumarin in corn oil by gavage at a dose of up to 800 mg/kg bw, once daily, 5 days per week for 2 years No significant differences in survival rates, final mean body weights or clinical findings were reported in the treated animals as compared with the controls. The only increase in neoplasia associated with the administration of dihydrocoumarin was in the incidences of hepatocellular adenoma and hepatocellular adenoma and carcinoma (combined), seen at all doses in females only. This effect reflects the high incidence of spontaneous liver tumours in this hybrid mouse and thus the heightened sensitivity to enhancement of liver neoplasia (Maronpot et al., 1987; Haseman et al., 1994, 1998). In view of the nature of the findings, the Committee concluded that observations of hepatic neoplasms in this bioassay in mice are not relevant to the safety of dihydrocoumarin in humans at low levels of intake from use as a flavouring agent (National Toxicology Program, 1993).
In a 13-week study, groups of Fischer 344/N rats received dihydrocoumarin in corn oil by gavage at a dose of up to 1200 mg/kg bw, once daily, 5 days per week. In males only, body weight was decreased at the highest dose. Changes in enzymes and other constituents of blood plasma were reported at doses of >300 mg/kg bw. Increases in liver and kidney weights were observed at the two higher doses. Centrilobular hepatocellular hypertrophy, ranging in severity from minimal to mild, was reported in the livers of animals of each sex at a dose of >300 mg/kg bw. No adverse treatment-related effects were observed in either male or female rats receiving dihydrocoumarin at 75 and 150 mg/kg bw (National Toxicology Program, 1993).
In a study of the potential carcinogenicity of dihydrocoumarin, Fischer 344/N rats received doses of up to 600 mg/kg bw in corn oil by gavage, on 5 days per week for 2 years. A significant dose-related decrease in the survival of male rats, attributed to progressive degenerative nephropathy leading to renal failure, was reported after week 92. Nephropathy was reported in control and treated rats of each sex. Although the incidence of nephropathy was greater in male rats, the findings were significant only in females at the two higher doses. Microscopic examination revealed a statistically significant, dose-related increase in renal tubule hyperplasia in male rats only. A significant increase in the incidence of renal tubule adenomas was observed in males treated with 600 mg dihydrocoumarin/kg bw when compared with the control group, but there was no evidence of malignant renal tubule neoplasms in male rats at any dose. Increases in the incidence of renal tubule hyperplasia or renal tubule adenomas were not observed in female rats. The Committee concluded that the renal hyperplastic and neoplastic effects observed are sex- and species-specific and not dose-related, and that these effects reflect the sensitivity of the male rat kidney to chronic progressive nephropathy and neoplasia. The NOEL was 300 mg/kg bw per day (National Toxicology Program, 1993).
In a study in rats fed diets containing 0.76% dihydrocoumarin (equivalent to 580 mg/kg bw), relative liver weights were significantly increased in the treated group compared with the controls, but no microscopic abnormalities were observed (Lake et al., 1994). Dihydrocoumarin did not markedly affect the activities of carnitine acetyltransferase and palmitoyl-coenzyme A (CoA) oxidation, indicating that dihydrocoumarin is unlikely to be a rodent liver peroxisome proliferator (Lock et al., 1989).
As noted by the Committee at its thirty-fifth meeting, no effects were reported in three short-term studies in rats fed dihydrocoumarin in the diet (Trubek, 1957, 1958; Hagan et al., 1967). Although it was not possible to determine a NOEL from these studies, they did provide additional data that support the safe use of dihydrocoumarin as a flavouring agent at its current level of intake.
As also noted at the thirty-fifth meeting, no effects were reported in a long-term study in dogs fed dihydrocoumarin in the diet. The data obtained in this study were limited by the small number of animals tested (Hagan et al., 1967).
Dihydrocoumarin was tested in various assays for genotoxicity in vitro. The results of the assays for reverse mutation and unscheduled DNA synthesis were negative (Prival et al., 1982; Brusick, 1982b; National Toxicology Program, 1983; Curren, 1986). Cytotoxicity was reported in a mouse lymphoma assay in the presence of an endogenous metabolic activation system, however, similar assays for forward mutation without metabolic activation produced negative results (Cifone, 1982b, 1984). Negative results were obtained in six out of seven studies of chromosomal aberration in Chinese hamster ovary cells (Galloway, 1983; National Toxicology Program, 1993). A dose-related increase in sister chromatid exchange was found in the same cell line (National Toxicology Program, 1983), but this isolated positive result was not considered evidence for genotoxicity. A test for micronucleus formation in rats in vivo gave negative results (National Toxicology Program, 1993). The Committee concluded that the data indicated that dihydrocoumarin is not genotoxic.
5.1.2 6-Methylcoumarin (No. 1172)
More extensive data on metabolism and toxicity were considered in order to complete the safety evaluation of 6-methylcoumarin (No. 1172).
It is anticipated that humans will metabolize 6-methylcoumarin via methyl group oxidation to the corresponding benzoic acid derivative, which can also be readily excreted. 6-Methylcoumarin may also undergo ring hydroxylation to form the corresponding 7-hydroxy metabolite, followed by excretion as the glucuronic acid conjugate. At high doses, metabolism via the 3,4-epoxide is at most a minor pathway, even in individuals exhibiting decreased activity of CYP2A6.
When 6-methylcoumarin was administered daily by gavage to male and female B6C3F1 mice for 13 weeks at a dose of up to 800 mg/kg bw, no toxicologically significant changes were reported in clinical, macroscopic or microscopic examinations at any dose. Prostration, bradycardia, bradypnoea, hypoactivity, hypothermia, and loss of the grasping reflex were reported at the highest dose only (National Toxicology Program, 2002).
In a 13-week study, 6-methylcoumarin was given to rats by gavage at a dose of up to 1200 mg/kg bw per day. All rats receiving the highest dose of 1200 mg/kg bw and one male rat receiving 600 mg/kg bw died during week 1 of the study. Decreases in body weight were reported in males and females receiving 600 mg 6-methylcoumarin/kg bw, relative to controls. The clinical effects, including hypoactivity, lachrymation, ataxia, impaired righting reflex and decreased limb tone, were reported in animals of each sex at 600 mg/kg bw. A decrease in serum cholinesterase activity was reported in females receiving 300 mg/kg bw. No other changes were reported in haematological, serum biochemical or urinary parameters at any dose. Necropsy of all animals receiving 1200 mg/kg bw revealed microscopic hepatic lesions that varied in the degree of congestion, degeneration, necrosis and hepatitis. Increased mean absolute and relative liver weights were reported in males and females receiving doses of 300 and 600 mg/kg bw; however, these changes were not accompanied by any substance-related macroscopic observations. No treatment-related effects were reported in animals receiving a dose of 150 mg/kg bw per day (National Toxicology Program, 2002).
In a 14-week study, groups of weanling Osborne-Mendel rats were fed diets containing 6-methylcoumarin at a concentration of 0, 1000 or 10 000 ppm (equivalent to 0, 100 and 1000 mg/kg bw). No significant differences in general health and behaviour, or body weight and food consumption were reported in the treated animals as compared with the controls. Haematological examinations performed at the end of the study did not reveal any treatment-related effects. No effects on organ weights, or macroscopic or microscopic changes in the tissues were reported at any dose.
In a 2-year feeding study, Osborne-Mendel rats were fed diets containing 6-methylcoumarin at concentrations of up to 15 000 ppm (equivalent to 750 mg/kg bw). Depression in growth rates was noted in males receiving doses of 375 or 750 mg/kg bw, but in females only at the higher dose. Hepatic effects in males and females receiving a dose of 750 mg/kg bw included fatty metamorphosis, very slight bile duct proliferation, and focal telangiectasis. No treatment-related effects were observed at doses up to and including 175 mg/kg bw per day in males and 375 mg/kg bw per day in females (Hagan et al., 1967).
The results of a 13-week study in rats fed diets containing 0.82% 6-methylcoumarin (corresponding to 695 mg/kg bw) revealed a slight vacuolation of hepatocytes in three out of eight treated animals; however, no increases in plasma aminotransferase activity and no bile-duct hyperplasia or cholangiofibrosis were reported (Lake et al., 1994). 6-Methylcoumarin treatment increased mixed function oxidase activity (i.e. 7-ethoxycoumarin O-deethylase activity), but it did not markedly affect the activities of carnitine acetyltransferase and palmitoyl-coenzyme A oxidation, indicating that 6-methylcoumarin is unlikely to be a rodent liver peroxisome proliferator (Lock et al., 1989).
No effects were reported in a limited study in dogs given 6-methylcoumarin at a dose of 200 mg/kg bw per day in gelatine capsules for 2 or 4 weeks. In a long-term study, no effects were reported in dogs fed diets containing 6-methylcoumarin at a concentration providing a dose of 50 mg/kg bw per day (Hagan et al., 1967). The data obtained in these studies are limited because only one or two dogs were tested per group (Levenstein, 1954).
6-Methylcoumarin was not genotoxic in a number of assays for reverse mutation with Salmonella typhimurium strains (Brusick, 1982a; Wild et al., 1983; Haworth et al., 1983); marginally positive results were reported in a single assay (Wild et al., 1983). Negative results were reported in a mouse lymphoma assay (Cifone, 1982a; National Toxicology Program, 1993). No increase in the frequency of mutation was observed in a test for sex-linked recessive lethal mutation in Drosophila melanogaster (Wild et al., 1983). A test for micronucleus formation in mice in vivo gave negative results in females and equivocal results in males (Witt et al., 2000); the positive results were not confirmed in a similar study (Wild et al., 1983). The Committee concluded that the data indicated that 6-methylcoumarin is not genotoxic.
In a 13-week study in rats given dihydrocoumarin, a NOEL of 150 mg/kg bw per day was identified (National Toxicology Program, 1993). This NOEL is about 5000 times greater than the estimated per capita intake of dihydrocoumarin in Europe (24 mg/kg bw per day) and in the USA (19 mg/kg bw per day). In rats, the NOEL for dihydrocoumarin in the 2-year study by gavage was 300 mg/kg bw per day (National Toxicology Program, 1993). These NOELs are 10 000 times greater than the estimated intake of dihydrocoumarin in Europe (24 mg/kg bw per day) and in the USA (19 mg/kg bw per day). In a 13-week study in rats, a NOEL of 150 mg/kg bw per day was found (National Toxicology Program, 2002). This NOEL is 30 000 times greater than the estimated intake of 6-methylcoumarin in Europe (5 mg/kg bw per day) and in the USA (2 mg/kg bw per day). Understanding of their metabolism and the available data on toxicity led the Committee to conclude that the safety of dihydrocoumarin and 6-methylcoumarin would not be expected to present a safety concern at current levels of intake (Table 1).
1.6 Consideration of secondary components
Three members of this group of flavouring agents (Nos 1158, 1160, and 1164) have minimum assay values of <95%. Information on the safety of the secondary components of these three compounds is summarized in Annex 6 (Summary of the safety evaluation of secondary components of flavouring agents with minimum assay values of less than 95%). The secondary components of No. 1158 (heptan-1-ol) and No. 1160 (gamma-dodecalactone and 2(3H)-furanone, dihydro-5-(2-octenyl)-(Z )) were evaluated at the forty-ninth meeting. None of the secondary components was considered to present a safety concern at current levels of intake. The secondary components of No. 1164 (2,9-dimethyl 3,8-decanedione and 4-hydroxy-5,6-oxo beta-ionone) have not been previously evaluated. However, compounds that are structurally related to the secondary components of No. 1164 (3,4-hexandione and beta-ionone) were evaluated at the fifty-first meeting, and were considered not to present a safety concern at current intake levels. On this basis, the secondary components of No. 1164 were considered not to present a safety concern at current levels of intake.
1.7 Consideration of combined intakes from use as flavouring agents
All 16 agents in this group are expected to be efficiently metabolized and would not saturate metabolic pathways. Evaluation of all the data indicated no safety concern associated with combined intake.
1.8 Conclusions
The Committee concluded that none of the 16 flavouring agents in this group of alicyclic, alicyclic-fused and aromatic-fused ring lactones would present safety concerns at the current estimated levels of intake. More extensive data on metabolism and toxicity were considered in the evaluations of dihydrocoumarin (No. 1172) and 6-methylcoumarin (No. 1171) in accordance with the application of the Procedure in the case of flavouring agents with high intakes evaluated by the B-side of the decision-tree. Other data on the toxicity and metabolism of these ring lactones were consistent with the results of the safety evaluation.
2. RELEVANT BACKGROUND INFORMATION
2.1 Additional considerations on intake
The concentrations of aliphatic lactones intentionally added to food are similar to those that occur naturally in food. Eight of the lactones in this group have been reported to occur naturally in foods and have been detected in rose-apple, celery stalks, soya bean, black and green tea, boiled beef and peppermint oil (Maarse et al., 1999). The annual volumes of production of members of this group are reported in Table 2. The lactone with the greatest total annual volume of production, dihy-drocoumarin, has been detected in sweet grass oil. Its hydrolysis product, 3-(2-hydroxyphenyl)propanoic acid, has been detected in sherry, and in white and red wine. Lactones have also been identified as components of commonly-used spices. The annual consumption of (+/-)-(2,6,6-trimethyl-2-hydroxycyclohexylidene) acetic acid gamma-lactone (No. 1164) as a component of paprika is 0.39 mg/kg2 (American Spice Trade Association, 2000), that is, 10 times greater than the daily intake of 0.01 µg/kg bw from its use as a flavouring agent in the USA. Quantitative data on natural occurrence reported for one flavouring agent in the group, 3-n-butylphthalide (No. 1169), demonstrate that intake occurs predominantly from the consumption of food (i.e. consumption ratio, >1) (National Academy of Sciences, 1982; Stofberg & Kirschman, 1985; Stofberg & Grundschober, 1987; Maarse et al., 1999).
Table 2. Annual volumes of production of alicyclic, alicyclic-fused and aromatic-fused ring lactones used as flavouring agents in Europe and the USA
|
Agent (No.) |
Most recent annual volume (kg)a |
Intakeb |
Annual volume in naturally occurring foods (kg)c |
Consumption ratiod |
|
µg/day |
µg/kg bw per day |
|
4-Hydroxy-4-methyl-5-hexenoic acid gamma-lactone (1157) |
|
Europe |
ND |
ND |
ND |
|
|
|
USAe |
18 3 |
0.05 |
+NA |
|
|
|
(+/-) 3-Methyl-g-decalactone (1158) |
|
Europe |
ND |
ND |
ND |
|
|
|
USAe |
30 |
5 |
0.09 |
- |
NA |
|
4-Hydroxy-4-methyl-7-cis-decenoic acid gamma-lactone (1159) |
|
Europe |
ND |
ND |
ND |
|
|
|
USAe |
75 |
13 |
0.2 |
- |
NA |
|
Tuberose lactone (1160) |
|
Europe |
ND |
ND |
ND |
|
|
|
USAe |
60 |
11 |
0.2 |
- |
NA |
|
Dihydromintlactone (1161) |
|
Europe |
ND |
ND |
ND |
|
|
|
USAe |
66 |
12 |
0.2 |
- |
NA |
|
Mintlactone (1162) |
|
Europe |
29 |
4 |
0.07 |
|
|
|
USAe |
50 |
9 |
0.1 |
+ |
NA |
|
Dehydromenthofurolactone (1163) |
|
Europe |
12 |
2 |
0.03 |
|
|
|
USAe |
50 |
9 |
0.1 |
+ |
NA |
|
(+/-)-(2,6,6-Trimethyl-2-hydroxycyclohexylidene) acetic acid gamma-lactone (1164) |
|
Europe |
ND |
ND |
ND |
|
|
|
USAe |
5 |
0.9 |
0.01 |
+ |
NA |
|
Sclareolide (1165) |
|
Europe |
9 |
1 |
0.02 |
|
|
|
USAf |
49 |
6 |
0.1 |
- |
NA |
|
Octahydrocoumarin (1166) |
|
Europe |
ND |
ND |
ND |
|
|
|
USA |
0.5 |
0.07 |
0.001 |
- |
NA |
|
2-(4-Methyl-2-hydroxyphenyl) propionic acid gamma-lactone (1167) |
|
Europe |
ND |
ND |
ND |
|
|
|
USAe |
9 |
2 |
0.03 |
- |
NA |
|
3-Propylidenephthalide (1168) |
|
Europe |
139 |
20 |
0.3 |
|
|
|
USA |
395 |
52 |
0.9 |
+ |
NA |
|
3-n-Butylphthalide (1169) |
|
Europe |
4 |
0.6 |
0.01 |
|
|
|
USAg |
2 |
0.4 |
0.006 |
2318 |
1159 |
|
3-Butylidenephthalide (1170) |
|
Europe |
71 |
10 |
0.2 |
|
|
|
USA |
54 |
7 |
0.1 |
+ |
NA |
|
Dihydrocoumarin (1171) |
|
Europe |
9916 |
1415 |
24 |
|
|
|
USA |
8437 |
1111 |
19 |
+ |
NA |
|
6-Methylcoumarin (1172) |
|
Europe |
2091 |
298 |
5 |
|
|
|
USA |
730 |
96 |
2 |
- |
NA |
|
Total |
|
|
|
|
|
|
Europe |
12 271 |
|
|
|
|
|
USA |
10 031 |
|
|
|
|
|
NA, not available; ND, no data reported; +, reported to occur naturally in foods (Maarse et al., 1999), but no quantitative data; -, not reported to occur naturally in foods |
|
a |
From International Organization of the Flavour Industry (1995) and Lucas et al. (1999) or National Academy of Sciences (1982) |
|
b |
Intake (µg/person/day) was calculated as follows: [(annual volume, kg) × (1 × 109 mg/kg)/(population × survey correction factor × 365 days)], where population (10%, "eaters only") =32 × 106 for Europe and 26 × 106 for the USA. The correction factor =0.6 for Europe and USA National Academy of Sciences surveys and 0.8 for the Lucas et al. USA survey representing the assumption that only 60% and 80% of the annual flavour volume, respectively, was reported in the poundage surveys (National Academy of Sciences, 1982; International Organization of the Flavour Industry, 1995; Lucas et al., 1999) |
| |
Intake µg/kg bw day) was calculated as follows: [(µg/person per day)/body weight], where body weight =60 kg. Slight variations may occur from rounding Quantitative data for the United States reported by Stofberg & Grundschober (1987). |
|
d |
The consumption ratio was calculated as follows: (annual consumption in food, kg)/(most recent reported volume as a flavouring agent, kg) |
|
e |
Anticipated annual volume, which was the maximum amount of flavour estimated to be used annually by the manufacturer at the time the material was proposed for flavour use. National surveys (National Academy of Sciences, 1970, 1982 or 1987; Lucas et al., 1999) revealed no reported use of the agent as a flavouring agent at that time |
|
f |
Annual volume reported by industry for the year 2000 (Private communication to the Flavor and Extract Manufacturers Association, 2002) |
|
g |
Annual volume reported in the USA (National Academy of Sciences, 1982) |
2.2 Biological data
2.2.1 Biochemical data
(a) Hydrolysis and the effect of pH
In nature, lactones are formed by acid-catalysed intramolecular cyclization of 4- or 5-carbon hydroxycarboxylic acids to yield 5- (gamma-) or 6- (delta-) membered lactone rings, respectively (see Figure 1). Smaller (3- or 4-membered rings) and larger (>7-membered rings) lactones form in a similar manner, but are relatively unstable compared with gamma- and delta-lactones. In an aqueous environment, a pH-dependent equilibrium is established between the open-chain hydroxycarboxylic acid and the lactone ring. In basic media, such as blood, the formation of the open-chain hydroxycarboxylate anion is favoured, while in acidic media, such as urine, the formation of the lactone ring is favoured. The stability of the lactone ring in an acidic environment is shown by the observation that the urine from 10 normal human adults contains a variety of aliphatic lactones such as gamma-valerolactone, gamma-hexalactone, and delta-hexalactone (Zlatkis & Liebich, 1971).
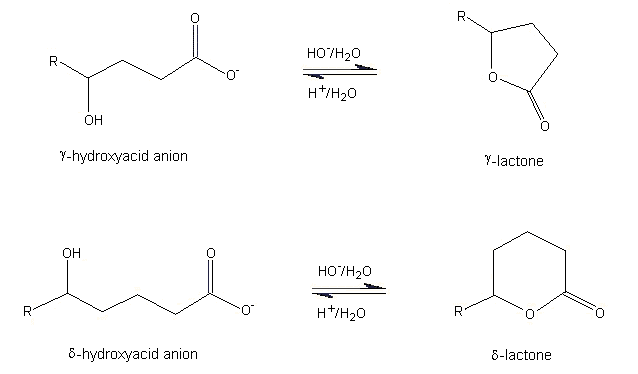
Figure 1. Equilibrium of gamma- and delta-hydroxycarboxylate anion and lactone
Studies of hydrolysis have been performed using structurally-related gamma-lactones formed from linear saturated hydroxycarboxylic acids with varying lengths of carbon chain (C4, C5, C9, C10, C11, C12, and C16). Studies of hydrolysis with gamma-butyrolactone have been conducted in rats, guinea-pigs, rabbits, cats, dogs and humans (Roth & Giarman, 1966). Significant hydrolysis of gamma-butyrolactone has been reported to occur upon incubation with liver or plasma lactonase for 10 min (Fishbein & Bessman, 1966). The hydrolysis product, gamma-hydroxybutyric acid, was detected in the muscles and fat of rats given gamma-butyrolactone intravenously at a dose of 500 mg/kg (Roth & Giarman, 1966) and in the blood and brain of rats given gamma-butyrolactone orally at the same dose (Guidotti & Ballotti, 1970). The half-life for the conversion of the lactone ring to the open-chain hydroxycarboxylate anion in the whole blood of rats is <1 min (Roth & Giarman, 1966). In rats, the hydrolysis of lactones administered intravenously may be catalysed, in part, by a lactonase present primarily in the plasma and liver. A similar gamma-lactonase that catalyses the hydrolysis of 4- to 8-carbon lactones has been detected in human blood (Fishbein & Bessman, 1966). Higher concentrations of the form of lactone found in the brain and muscles of rats after intravenous administration confirm that the lactonase is more active in plasma.
In humans, paraoxonase (PON1), a serum enzyme belonging to the class of A-carboxyesterases (Aldridge, 1953), is known to rapidly hydrolyse a broad range of aliphatic lactone substrates, including beta-, gamma-, delta-, and omega-lactones, lactones fused to alicyclic rings (such as 2-(2-hydroxycyclopent-4-enyl)ethanoic acid gamma-lactone), and lactones fused to aromatic rings (such as dihydrocoumarin (No. 1171)) (Billecke et al., 2000). The activities of paraoxonase isoenzymes (Q and R) in human blood exhibit a bimodal distribution that is accounted for by a Q/R (glutamine or arginine) polymorphism with Q-type homozygotes showing a lower activity than QR heterozygotes or R homozygotes (Humbert et al., 1993).
Incubation of human R-type PON1 (1 mmol/l) with the aliphatic lactones gamma-butyrolactone, gamma-valerolactone, gamma-decanolactone and undecano-gamma-lactone resulted in hydrolysis rates of 9.1, 7.0, 19.0 and 13.0 µmol/min per ml substrate, respectively (Billecke et al., 2000). The alicyclic fused-ring lactone, 2-(2-hydroxycyclopent-4-enyl)ethanoic acid gamma-lactone, is hydrolysed at a slower rate, <3 µmol/min per ml substrate by the Q and R isoenzymes of PON1 (Billecke et al., 2000).
Relative rates of hydrolysis are more rapid for lactones fused to benzene rings. Incubation of R-type PON1 (1 mmol/l) with the aromatic delta-lactone dihydrocoumarin (No. 1171) resulted in a hydrolysis rate of 17.0 µmol/min per ml of substrate, suggesting that PON1 has a high affinity for aromatic lactones. gamma-Lactones fused to a benzene ring also undergo rapid hydrolysis of the lactone ring. Homogentisic acid lactone (2,5-dihydroxyphenylethanoic acid lactone) and 2-hydroxyphenylethanoic acid gamma-lactone are rapidly hydrolysed (50 and 13.5 µmol/min per ml, respectively) to the corresponding hydroxycarboxylic acids in the presence of R-type PON1 (1 mmol/l) (Billecke et al., 2000). Q and R isoenzymes exhibit similar activities for aromatic lactones; however, introduction of a double bond in the lactone ring retards hydrolysis. Coumarin (2-hydroxyphenyl)propanoic acid delta-lactone) is not hydrolysed by either PON1 isoenzyme (Billecke et al., 2000). On the basis of this observation, it is anticipated that 6-methylcoumarin (No. 1172), which also contains a double bond, will not be hydrolysed in vivo. This conclusion is consistent with the observation that major metabolites of 6-methylcoumarin are not the products of lactone ring hydrolysis (see section 2.2.1(c)). The above data indicate that dihydrocoumarin and other lactones fused to aromatic rings will be readily hydrolysed in vivo to the open-chain hydroxycarboxylic acid form. Therefore, dihydrocoumarin will hydrolyse in vivo to form 3-(o-hydroxyphenyl) propionic acid (o-HPPA).
A 1-h incubation of 1 mmol 4,4-dibutyl-gamma-butyrolactone and omega-6-hexadecenlactone with 50 ml of simulated intestinal fluid resulted in 92% and 96% hydrolysis, respectively, yielding the ring-opened hydroxycarboxylic acids (Morgareidge, 1962). Ninety-two percent of the omega-6-hexadecenlactone was hydrolysed within the first 15 min of incubation (Morgareidge, 1962).
Incubation of 1 mmol of gamma-valerolactone and gamma-undecalactone with 50 ml of simulated intestinal fluid resulted in 32% and 58% hydrolysis within 4 h, respectively (Morgareidge, 1962). Doubling the volume of intestinal fluid resulted in a 50% and 62% hydrolysis of gamma-valerolactone and gamma-undecalactone, respectively, within the same period of time (Morgareidge, 1962). Conversely, hydrolysis (93%) occurred within 1 h of incubation of 200 mg of gamma-valerolactone with 50 ml of rat liver homogenate (Morgareidge, 1963).
Incubation of gamma-nonalactone and gamma-undecalactone with rat liver homogenate in buffer solution at pH 7.5 resulted in 62–94% and 26–40% hydrolysis within 1 h, respectively (Morgareidge, 1963). After 1 h, 81–88% and 45–70% hydrolysis of gamma-nonalactone and gamma-undecalactone, respectively, occurred at pH 8.0 (Morgareidge, 1963).
The pharmacologic activity of many drugs depends on lactone ring hydrolysis. Simvastatin, a widely-used cholesterol-lowering agent, is a delta-lactone prodrug that is ring-opened to yield a beta-hydroxy acid that inhibits the activity of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase used in cholesterol synthesis. Human cholecystectomy patients with T-tube drainage given a single oral dose of 100 mg [14C]simvastatin show the presence of the open-chain hydroxycarboxylic acid in bile in vivo within 12 h of administration (Cheng et al., 1994).
With the exception of 6-methylcoumarin (No. 1172), data on the hydrolysis of a wide variety of lactones in simulated intestinal fluid, rat liver homogenate, and human blood serum support the conclusion that lactones will hydrolyse before absorption or upon entering the systemic circulation.
(b) Absorption, distribution and excretion
At the physiological pH of the intestines, aliphatic lactones (Nos 1157–1160) exist in the ring-opened form (i.e. as hydroxycarboxylic acids). Studies show that hydroxycarboxylic acids are absorbed from the gastrointestinal tract, rapidly metabolized, and excreted from the body.
Most data available on the disposition of lactones formed from simple aliphatic gamma- or delta-hydroxycarboxylic acids are obtained from studies on gamma-butyrolactone (i.e. 4-hydroxybutyric acid gamma-lactone). These data were included in the original review on aliphatic lactones (Annex 1, reference 132). A brief overview is presented below.
gamma-Butyrolactone is absorbed rapidly and completely from the intestinal tract when given orally at a dose of >100 mg/kg to male Sprague-Dawley rats (Guidotti & Ballotti, 1970; Lettieri & Fung, 1978). Once absorbed, gamma-hydroxybutyric acid is completely oxidized in the fatty acid pathway and citric acid cycle (Nelson & Cox, 2000). Respiratory excretion of 14C-labelled CO2 was detected 4 min after males were injected with radiolabelled gamma-hydroxybutyric acid (sodium salt), while peak recovery occurred 15 min after administration. Approximately 60% of the administered dose was recovered as expired 14CO2 within 2.5 h (Roth & Giarman, 1966).
As documented in the discussion on hydrolysis (see section 2.2.1(a)), gamma- or delta-lactones fused to alicyclic rings (e.g. cyclohexyl ring system) (Nos 1161–1166) are absorbed as the open-chain cycloalkyl-substituted hydroxycarboxylic acids. Therefore, substances such as dihydromintlactone (No. 1161) and octahydrocoumarin (No. 1166) exist in vivo as the ring-opened form 2-hydroxycyclohexyacetic acid derivative and 3-(2-hydroxycyclohexyl) propanoic acid, respectively. Information on cyclohexyl- and cyclopentyl-substituted carboxylic acids provides relevant data on the absorption, distribution and excretion of these substances.
Greater than 98% of an oral dose of 100 mg/kg bw of [14C]cyclohexanecarboxylate salt administered to male Wistar albino rats is excreted in the urine within 7 h. Less than 1% is excreted in the faeces or expired air (Brewster et al., 1977a). Greater than 97% of a dose of 200 mg/kg bw of [14C]cyclohexanecarboxylate salt given to male Wistar rats via the duodenal cannula is recovered as five different urinary and biliary metabolites within 7 h. The rate of excretion increased as the dose decreased from 200 to 0.5 mg/kg bw. At the lowest dose (0.5 mg/kg bw), 95% of the administered dose was excreted within the first 90 min (Brewster et al., 1977a).
Other studies have shown dose-dependent clearance of cyclohexanecarboxylic acids from the body. Female Sprague-Dawley rats injected with a single dose of cyclohexanecarboxylic acid at 67, 133, or 311 mg/kg bw showed a decrease in renal and blood clearance rates after 24 h and 48 h, as the dose increased. At the lowest dose (67 mg/kg bw), blood and renal clearance rates were reported to be 27.6 and 3.99 ml/min per kg bw, respectively, and at the highest dose (311 mg/kg bw), they were reported to be 8.50 and 0.76 ml/min per kg bw, respectively. These results suggest that the pathway by which cyclohexanecarboxylic acids are eliminated from the body can be saturated, but only at relatively high doses (Liu & Pollack, 1993).
Dihydrocoumarin (No. 1171) and four other aromatic lactones in the group (Nos 1167–1170) are either gamma- or delta-lactones fused to a benzene ring. Having no double bond in the lactone ring, these substances exist in vivo as the ring-opened form (i.e. 3-(2-hydroxyphenyl)propanoic acid and 2-hydroxyphenylacetic acid derivatives). In the case of dihydrocoumarin, data indicate that the open-chain form is excreted mainly unchanged as the glycine conjugate in the urine. In rabbits, a "considerable amount" of dihydrocoumarin administered orally was detected in the urine as the glycine conjugate of the ring-opened form, o-hydroxyphenylpropionic acid (Furuya, 1958; Morgareidge, 1962, 1963).
The only lactone in the group that is not readily hydrolysed either before absorption or in the systemic circulation is 6-methylcoumarin (No. 1172). The absorption, distribution, and excretion of 6-methylcoumarin can be predicted on the basis of analogy with the structurally related substance coumarin. Coumarin is rapidly absorbed from the intestinal tract after oral administration in rats and humans. Five minutes after a single oral dose of 3-[14C]coumarin was administered to rats, 14C was detected in the serum, liver, and kidney. A peak concentration was reached after 45–60 min. Within 48 h, 10% and 70% of the dose was eliminated in the faeces and urine of rats, respectively (Feuer et al., 1966). Male Wistar rats given benzene 14C-labelled (U)coumarin by gavage as a single dose of either 10, 100, or 150 mg/kg bw in arachis oil were reported to excrete 33.4%, 31.2% or 28.6% of the administered dose in the urine within 24 h (Huwer et al., 1991).
In humans, coumarin was detected in the blood minutes after ingestion of an oral dose of 0.857 mg/kg bw. A peak plasma concentration was reached within 10–20 min. Greater than 80% of the administered dose was excreted in the urine within 24 h (Ritschel et al., 1977). On the basis of these data, it is anticipated that 6-methylcoumarin will be rapidly absorbed and excreted primarily in the urine of mammals.
All 16 lactones in the group of aliphatic, alicyclic-fused and aromatic-fused ring lactones used as flavouring agents are expected to be readily absorbed from the gastrointestinal tract, metabolized and excreted, predominantly in the urine.
(c) Metabolism
(i) Aliphatic lactones (Nos 115–1160)
Four of the 16 substances in this group (4-hydroxy-4-methyl-5-hexenoic acid gamma-lactone, No. 1157; (+/-)3-methyl-g-decalactone, No. 1158; 4-hydroxy-4-methyl-7-cis-decenoic acid gamma-lactone, No. 1159; tuberose lactone, No. 1160) are formed via the intramolecular cyclization of a gamma-membered aliphatic hydroxycarboxylic acid. The metabolic fate of these aliphatic lactones can be predicted on the basis of analogy with the known biotransformations of structurally-related lactones.
In general, the open-chain hydroxycarboxylic acid formed from gamma- or delta-lactones is converted via acetyl coenzyme A (CoA) to the corresponding hydroxythioester. The hydroxythioester then undergoes beta-oxidization and cleavage to yield an acetyl CoA fragment and a new a-hydroxythioester, which undergoes a-decarboxylation to yield a linear carboxylic acid and eventually, CO2 and H2O (Nelson & Cox, 2000).
In vivo, gamma-butyrolactone is rapidly metabolized and eliminated primarily as respiratory CO2 and urinary metabolites (National Toxicology Program, 1992). Intermediates formed during the beta-oxidation of gamma-hydroxycarboxylic acids have been detected in humans (Walkenstein et al., 1964; Lee, 1977). These intermediates include (S)-3,4-dihydroxybutyric acid, glycolic acid, and 3-oxobutyric acid, which have been detected in the urine after administration of an oral dose of 1000 mg of gamma-hydroxybutanoic acid lactone (Lee, 1977).
If the lactone is formed from a hydroxycarboxylic acid-containing unsaturation process, beta-oxidation and cleavage of acetyl CoA units will continue along the carbon chain until the position of unsaturation is reached. If the unsaturation begins at an odd-numbered carbon, acetyl CoA fragmentation will eventually yield a 3-enoyl CoA, which is converted to trans-Delta2-enoyl CoA before entering the fatty acid pathway. If unsaturation begins at an even-numbered carbon, acetyl CoA frag-mentation will yield a Delta 2-enoyl CoA product, which acts as a substrate for further fatty acid oxidation (Nelson & Cox, 2000).
The chain length and the position and size of the alkyl substituents influence the principal metabolic pathways utilized for detoxication of branched-chain hydroxycarboxylic acids. Short-chain (<C6) branched aliphatic hydroxycarboxylic acids may be excreted unchanged as the glucuronic acid conjugate, or undergo alpha- or beta-oxidation followed by cleavage and complete metabolism to CO2 via the fatty acid pathway and the tricarboxylic acid cycle (Nelson & Cox, 2000). Alternatively, as chain length, substitution and lipophilicity increase, the hydroxycarboxylic acid may undergo a combination of omega-, omega-1 and beta-oxidation to yield polar hydroxycarboxylic acid, ketoacid, and hydroxydiacid metabolites which may be excreted as the glucuronic acid or sulfate conjugates in the urine and, to a lesser extent, in the faeces (Diliberto et al., 1990). Long-chain, methyl-substituted carboxylic acids are, to some extent, omega-oxidized in animals to form diacids, which can be detected in the urine (Williams, 1959).
Acids with a methyl substituent located at a 2- or 4-position, as in 4-hydroxy-4-methyl-7-cis-decenoic acid gamma-lactone (No. 1159), are extensively metabolized to CO2 in the fatty acid pathway via beta-oxidation and cleavage of the longer branched chain. If the methyl group is located at the 3-position, as in (+/-) 3-methyl-gamma-decalactone (No. 1158), beta-oxidation occurs in the shorter branched chain. Larger alkyl substituents (>C2) located at the alpha- or beta-position inhibit metabolism to CO2 (Deuel Jr., 1957; Albro, 1975; Deisinger et al., 1994) in which case there is either direct conjugation of the acid with glucuronic acid, or Omega-oxidation leading to diacid metabolites which may be conjugated and excreted.
(ii) Lactones fused to alicyclic ring systems (Nos 1161–1166)
The formation of the ring-opened hydroxycarboxylic acid fused to a cycloalkyl ring provides for a polar substrate capable of excretion, unchanged or conjugated, in the urine. In the acidic pH of the urine, the open-chain hydroxycarboxylic acid derivative is present as the ring-closed lactone. The presence of mintlactone (No. 1162) in the urine of rats given pulegone is evidence that the lactone may be excreted unchanged (Madyastha & Raj, 1993).
If alkyl substituents are present on the alicyclic ring (Nos 1161–1165), two principal metabolic options exist. Lactone ring hydrolysis may be followed by direct excretion in the urine. Alternatively, oxidation of ring substituents or the ring itself yields polar polyhydroxylated carboxylic acid metabolites that may be excreted primarily in the urine.
Hydrolysis of the lactone ring in dehydromenthofurolactone (No. 1163) yields the enolic form of a ketocarboxylic acid, 2-(2-keto-4-methylcyclohexyl)propionic acid (also known as 9-carboxypulegone) (see Figure 2). The ketocarboxylic acid is excreted unchanged as the glucuronic acid conjugate or undergoes side-chain oxidation to yield additional hydroxylated metabolites that are also detected in the urine. Ring- and side-chain hydroxylated metabolites of both dehydromenthofurolactone and its ring-opened form (9-carboxypulegone) have been detected in the urine of rats given either pulegone or menthofuran (Madyastha & Raj, 1993).
Figure 2. Metabolism of dehydromenthofurolactone (No. 1163)
In addition to being excreted unchanged or undergoing hydroxylation of ring substituents, the open-chain form of the lactone may undergo oxidative degradation of the side-chain. If the lactone is a delta-lactone fused to an alicyclic ring (No. 1166, octahydrocoumarin), it may be hydrolysed to a carboxylic acid containing an odd number of carbons (one or three) in the side-chain. beta-Oxidation and cleavage of the octahydrocoumarin side-chain yields 3-hydroxycyclohexanecarboxylic acid, which may subsequently be excreted unchanged in the urine, or aromatized to a benzoic acid derivative and excreted mainly as the hippuric acid. If the side-chain acid contains an even number of carbons, such as dihydromintlactone (No. 1161), the resulting hydroxycarboxylic acid may be excreted unchanged or in conjugated form and the ring may be cleaved to yield polar metabolites that may be excreted in the urine.
In perfused rat liver, the related substance, cyclohexanecarboxylic acid, which contains an odd number of carbons in the side-chain, is primarily metabolized to hippuric acid. Small amounts of the glucuronic acid conjugate of the parent acid, hexahydrohippuric acid, 3,4,5,6-tetrahydrohippuric acid, unchanged cyclohexanecarboxylic acid, and benzoic acid also were detected (Brewster et al., 1977b). Benzoyl glucuronide was found in the bile of rats as an in vivo metabolite of cyclohexanecarboxylic acid (Brewster et al., 1977a).
On the basis of these data, it is concluded that the metabolic options available to lactones fused to alicyclic rings include excretion as the open-chain hydroxycarboxylic acid derivative, hydroxylation of ring alkyl substituents leading to polar metabolites that may be excreted, or oxidative degradation of the carboxylic acid side-chain yielding polar alicyclic or aromatic carboxylic acids that are excreted unchanged or in conjugated form in the urine.
(iii) Lactones fused to aromatic ring systems (Nos 1167–1172)
gamma-Lactones (Nos 1167–1170)
The four gamma-lactones (Nos 1167–1170) fused to a benzene ring are hydrolysed in vivo to the corresponding 2-(2-hydroxyphenyl)propionic acid (No. 1167) or 2-(2-hydroxyalkyl)benzoic acid (Nos 1168–1170) derivatives. The phenylpropionic acid derivative is excreted as the glycine or glutamine conjugates while the 2-(2-hydrox-yalkyl)benzoic acid (phthalides) derivatives may be excreted directly, or the side-chain oxygenated functional group (alcohol or enolic alcohol) may be oxidized (alcohol) or reduced (keto formed from enol). The reduced form is subsequently conjugated and excreted.
2-(4-Methyl-2-hydroxyphenyl)propionic acid gamma-lactone (No. 1167) is a phenylacetic acid with an additional methyl group at position 2. As such, it is structurally related to phenylacetic acid. In two men, an average of 91% and 7% of an oral dose of [carboxy-14C]-phenylacetic acid of 1.0 mg/kg bw is excreted within 24 h as glutamine and taurine3 conjugates, respectively. Unlike in most other animals, only a trace amount of the glycine conjugate has been detected in humans (James et al., 1972). The distribution and type of conjugation in humans is relatively unaffected by continued ingestion of phenylacetic acid. After being fed the acid at increasing daily doses (1000–10 000 mg) over 97 days, humans consistently excreted >90% of the administered dose as the phenylacetylglutamine conjugate at 24-h intervals (Ambrose et al., 1933). Similarly, Old and New World monkeys conjugate phenylacetic acid with glutamine and to a lesser extent, taurine. However, significant quantities of phenylacetic acid (1–44%) are excreted unconjugated. In carnivores (e.g. dog, cat, ferret), glycine conjugation predominates with no detectible amountsof glutamine conjugation. Likewise in rodents and lagomorphs (rabbits), phenylacetic acid is excreted primarily as the glycine conjugate. Unconjugated phenylacetic acid and minute amounts of taurine conjugates are also excreted. In rats, >94% of a dose of phenylyacetic acid of 80 mg/kg bw given by intraperitoneal injection is excreted as the glycine conjugate within 24 h (James et al., 1972).
Phthalides (3-propylidenephthalide, No. 1168; 3-n-butylphthalide, No. 1169; 3-butylidenephthalide, No. 1170) are lactones formed from intramolecular cyclization of o-2-hydroxyethylbenzoic acid derivatives. Hydrolysis of alpha-alkyl-substituted phthalide (No. 1169) would yield the corresponding secondary alcohol attached to a benzoic acid skeleton. Hydrolysis of alpha-alkenyl-substituted phthalides (Nos 1168 and 1170) would yield the enol form of the corresponding ketone attached to a benzoic acid skeleton. The benzoic acid moiety may conjugate with glycine and be excreted mainly as the hippurate, while the ketone function produced by hydrolysis of propylidene- and butylidene-phthalide may be reduced to the corresponding alcohol, and excreted as the glucuronic acid conjugate (Nelson & Cox, 2000).
In animals, mitochondrial acid CoA ligases activate benzoates to form an intermediate CoA thioester, which reacts with the amino acids glycine and glutamine.
The resulting glycine conjugate of benzoic acid is efficiently removed from circulation during a single pass through the kidney (Killenberg & Webster, 1980). The ketocarboxylic acids produced by hydrolysis of two phthalides (Nos 1168 and 1170) may be reduced to the corresponding alcohol by aromatic ketone reductase (Felsted & Bachur, 1980). Reduction of other aromatic ketones (e.g. acetophenone) occurs stereoselectively (Culp & McMahon, 1968). However, it is difficult to predict the stereospecificity of the reaction since aromatic ketone reductase consists of multiple enzymes with different absolute stereospecific or stereocatalytic properties. It is anticipated that humans will hydrolyse phthalides to their corresponding ketocarboxylic acid (Nos 1168 and 1170) or hydroxycarboxylic acid (No. 1169), which may form the corresponding hippurate and subsequently be excreted in the urine or undergo ketone reduction to yield a secondary alcohol that may also be excreted as the corresponding glucuronic acid conjugate.
On the basis of these data, it was concluded that the metabolic options available to lactones fused to aromatic rings include excretion as the glycine or glutamine conjugates of the open-chain hydroxycarboxylic acid derivative, or oxidation or reduction of the side-chain and subsequent excretion as the glucuronic acid conjugate.
Dihydrocoumarin (No. 1171)
In animals, dihydrocoumarin is hydrolysed to the corresponding ring-opened hydroxycarboxylic acid, o-HPPA (see Figure 3). In rabbits given a single dose of dihydrocoumarin of 200 mg/kg bw by gavage, the principal urinary metabolites identified by qualitative thin-layer chromatography included the ring-opened form o-HPPA and the glycine conjugate of o-HPPA. Minor urinary metabolites included o-coumaric acid, o-coumarylglycine, coumarin, 7-hydroxycoumarin and 3-hydroxycoumarin (Furuya, 1958). Dihydrocoumarin also undergoes ring opening to yield o-HPPA when incubated with rat caecal extract (Scheline, 1968).
Figure 3. Metabolism of dihydrocoumarin (No. 1171)
After hydrolysis, o-HPPA may also undergo beta-oxidation and cleavage to yield o-hydroxybenzoic acid (salicylic acid), which is excreted primarily in the urine unchanged or as the glycine conjugate (Morgareidge, 1962, 1963) (see Figure 3). In humans, the structurally-related acids, 3-phenylpropionic acid (Pollitt, 1974) and 3-phenylpropenoic acid (i.e. cinnamic acid) (Williams, 1959) undergo efficient beta-oxidation and cleavage to yield benzoic acid, which is excreted predominantly in the urine as hippuric acid. In rats and mice, cinnamic acid is metabolized mainly to hippuric acid (Nutley et al., 1994); in rats, however, o-methoxycinnamic acid is metabolized mainly to the beta-hydroxy derivative that is excreted as the glucuronic acid conjugate (Samuelson et al., 1986).
It is anticipated that humans will hydrolyse dihydrocoumarin to form o-HPPA, which is either excreted unchanged, conjugated with glycine, or beta-oxidized and cleaved to yield o-hydroxybenzoic acid (i.e. salicylic acid) (see Figure 3).
6-Methylcoumarin (No. 1172)
Unlike aliphatic lactones, which are readily hydrolysed in vivo to the corresponding hydroxy acids (Morgareidge, 1962, 1963), fused ring aromatic lactones like 6-methylcoumarin (No. 1172) and coumarin contain a relatively stable lactone ring. Increased stability may result from extended conjugation of the aromatic ring with the alpha, beta-unsaturated lactone ring present in coumarin derivatives. Therefore, it is expected that oxidation competes favourably with lactone ring hydrolysis in vivo.
6-Methylcoumarin is a methyl-substituted coumarin ring system, which is expected to be metabolized via oxidation of the methyl group to yield the corresponding aromatic carboxylic acid, or via ring oxidation. In the latter case, oxidation of the benzene ring (i.e. 7-hydroxylation) or the lactone ring alkene (i.e. 3,4-epoxidation) is possible. Compared with oxidation pathways for lactone rings, 6-methylcoumarin is expected to exhibit an increased relative rate of benzene ring hydroxylation at the C7 position owing to the presence of a methyl substituent (Lake et al., 1994).
The demethylated analogue, coumarin, is rapidly absorbed and metabolized in humans (Cohen, 1979). 7-Hydroxycoumarin is the principal metabolite in humans and other primates (Shilling et al., 1969; Waller & Chasseaud, 1981; Egan et al., 1990; Cholerton et al., 1992; Rautio et al., 1992) formed by first-pass metabolism in the liver (Van Iersel et al., 1994). 7-Hydroxycoumarin is then rapidly conjugated with glucuronic acid (Ritschel et al., 1977) and excreted in the urine. 7-Hydroxylation of coumarin is catalysed by cytochrome P450 isoenzymes 2A6 (CYP2A6) and 2B6 (CYP2B6). Gene subfamilies CYP2A and CYP2B, which are expressed to form these enzymes, have been isolated from human hepatic mRNA derived from chromosome 19 (Miles et al., 1990; Forrester et al., 1992). Volunteers excreted varying amounts of 7-hydroxycoumarin (Cholerton et al., 1992; Rautio et al., 1992; Van Iersel et al., 1994) suggesting that some interindividual variation may exist in the gene expression of CYP2A6 (Miles et al., 1990; Yamano et al., 1990; Pearce et al., 1992; Yamazaki et al., 1992).
In the alternate 3-hydroxylation pathway, which predominates in rats, coumarin is oxidized via a 3,4-epoxide intermediate to yield mainly o-HPAA. This pathway may compete favourably in humans at high concentrations of substrate (Fentem & Frey, 1992; Van Iersel et al., 1994), presumably when CYP2A6 approaches saturation or when gene expression limits the production of CYP2A6. o-HPAA accounted for only 1–6% of a 200 mg dose of coumarin given to humans (Shilling et al., 1969). In rats, coumarin is predominantly oxidized to o-HPAA, presumably via a 3,4-epoxide, some of which reacts with glutathione at the alpha-position (Huwer et al., 1991). Additionally, the epoxide may bind covalently to cytoplasmic proteins (Lake et al., 1989; Peters et al., 1991), form a 3,4-diol, or rearrange to yield 3-hydroxycoumarin (Lewis et al., 1994).
2.2.2 Toxicological studies
Typically, the toxicological studies described in the monograph are organized according to duration (i.e. short-term, long-term, and carcinogenicity), flavouring agent and species. However, in the interest of preserving the integrity of the studies performed by the National Toxicology Program (National Toxicology Program) on dihydrocoumarin (No. 1171) and 6-methylcoumarin (No. 1172), the short-term studies and studies of carcinogenicity will be discussed in the section on long-term studies (see 2.2.2 (c)) in the sequence in which they were conducted.
(a) Acute toxicity
Oral LD50 values have been reported for 9 of the 16 substances in this group and are summarized in Table 3. In mice, oral LD50 values ranged from 1010 to 3050 mg/kg bw (Levenstein, 1953, 1954; Pellmont, 1970). In rats, oral LD50 values ranged from 465 to >5000 mg/kg bw (Jenner et al., 1964; Posternak, 1965; Moreno, 1972, 1973, 1975, 1976, 1980; Feuer, 1974; BASF, 1976; Lewis & Palanker, 1979; Buch, 1981; Collier, 1982; Reagan & Becci, 1984; Serota, 1984). In guinea-pigs, an oral LD50 for dihydrocoumarin of 1760 mg/kg bw has been reported (Jenner et al., 1964).
Table 3. Studies of the acute oral toxicity of alicyclic, alicyclic-fused and aromatic-fused ring lactones
|
No. |
Flavouring agent |
Species |
Sex |
LD50
(mg/kg bw) |
Reference |
|
1162 |
Mintlactone |
Rat |
M, F |
530a |
Collier (1982) |
|
1163 |
Dehydromenthofurolactone |
Rat |
M, F |
2253 |
Reagan & Becci (1984) |
|
1165 |
Sclareolide |
Rat |
M, F |
>5000 |
Lewis & Palanker (1979) |
|
1166 |
Octahydrocoumarin |
Rat |
M, F |
3302 |
Buch (1981) |
|
1166 |
Octahydrocoumarin |
Rat |
NR |
3900 |
Moreno (1978) |
|
1166 |
Octahydrocoumarin |
Rat |
M, F |
3840 |
BASF (1976) |
|
1168 |
3-Propylidenephthalide |
Rat |
NR |
1650 |
Moreno (1975) |
|
1169 |
3-n-Butylphthalide |
Mouse |
NR |
1850 |
Pellmont (1970) |
|
1169 |
3-n-Butylphthalide |
Rat |
NR |
2450 |
Moreno (1976) |
|
1170 |
3-Butylidenephthalide |
Rat |
NR |
1850 |
Moreno (1980) |
|
1170 |
3-Butylidenephthalide |
Rat |
NR |
2200 |
Posternak (1965) |
|
1171 |
Dihydrocoumarin |
Mouse |
M, F |
1010 |
Levenstein (1953) |
|
1171 |
Dihydrocoumarin |
Rat |
M, F |
1460 |
Jenner et al. (1964) |
|
1171 |
Dihydrocoumarin |
Rat |
M |
1650 |
Moreno (1972) |
|
1171 |
Dihydrocoumarin |
Guinea-pig |
M, F |
1760 |
Jenner et al. (1964) |
|
1172 |
6-Methylcoumarin |
Mouse |
NR |
3050 |
Levenstein (1954) |
|
1172 |
6-Methylcoumarin |
Rat |
NR |
465 |
Feuer (1974) |
|
1172 |
6-Methylcoumarin |
Rat |
NR |
1680 |
Moreno (1973) |
|
1172 |
6-Methylcoumarin |
Rat |
M, F |
844 |
Serota (1984) |
M, male; F, female; NR, not reported
a Dose converted to mg/kg using specific gravity of 1.06
These results demonstrate that the acute toxicity of alicyclic, alicyclic-fused and aromatic-fused ring lactones is low.
(b) Short-term studies of toxicity
(i) Aliphatic lactones (Nos 1157–1160)
Numerous short-term studies of toxicity have been conducted on aliphatic lactones. At its forty-ninth meeting, the Committee evaluated a group of aliphatic lactones, which included 19 lactones formed from simple aliphatic linear saturated hydroxycarboxylic acids (Annex 1, reference 132). Upon review of the available data, the Committee noted that class I lactones would have a low order of toxicity when administered orally, due to their simple chemical structures and efficient modes of metabolism. On this basis, the Committee concluded that the group of aliphatic lactones presented no safety concern at current levels of intake when used as flavouring agents.
The four aliphatic gamma-lactones in this group are new flavouring agents and were not included in the earlier review. These flavouring agents (4-hydroxy-4-methyl-5-hexenoic acid gamma-lactone, No. 1157; (+/-)3-methyl-gamma-decalactone, No. 1158; 4-hydroxy-4-methyl-7-cis-decenoic acid gamma-lactone, No. 1159; tuberose lactone, No. 1160) are all formed from simple aliphatic linear saturated hydroxycarboxylic acids and have all been assigned to class I (Cramer et al., 1978). Therefore, the toxicological profiles of these lactones can be predicted on the basis of analogy with the known toxicological profiles of the structurally-related lactones previously evaluated by the Committee (Annex 1, reference 132).
(ii) Lactones fused to alicyclic ring systems (Nos 1161–1166)
Dihydromintlactone (No 1161)
Groups of five male and five female Sprague-Dawley rats were given diets containing dihydromintlactone (No. 1161) at concentrations calculated to provide an average daily intake of 0, 5.5, 15.5, or 27.8 mg/kg bw, for 28 days (Cormack et al., 2000). The animals were examined daily for overt signs of toxicity, general health, overt changes in water consumption and behavioural changes. Individual body weights, and food, and water consumption were recorded weekly. Evaluations of haematology and blood chemistry parameters were performed for all animals at the end of the study. All animals were subjected to gross necropsy examination, and the major organs and tissues (e.g. brain, heart, kidneys, liver, and lungs) of animals receiving the highest dose (27.8 mg/kg bw per day) and of control animals were subjected to complete histopathological evaluation. There were no deaths, clinical signs of toxicity, or changes in functional parameters (i.e. behavioural, functional performance, and sensory reactivity) reported in any of the animals during the study. A statistically significant decrease (p <0.01) in body-weight gain and a slight (13%) decrease in food consumption were reported in week 2 in males receiving a dose of 27.8 mg/kg bw per day, but not thereafter. No changes in body-weight gain or food consumption were observed in any other groups. No significant differences were observed in haematological or blood chemistry parameters between animals in the control group and animals treated with dihydromintlactone. At necropsy, there were no significant differences in absolute and relative organ weights between groups treated with dihydromintlactone and the controls. Gross and histopathological examinations did not reveal any abnormalities related to the administration of dihydromintlactone. Although the decreased body weights and food consumption at week 2 in males receiving the highest dose were statistically significant, the authors concluded that this isolated finding was of minimal toxicological significance owing to the recovery observed during weeks 3 and 4 of the study. Nevertheless, the NOEL for dihydromintlactone was reported by the study authors as 15.5 mg/kg bw per day for males and >27.8 mg/kg bw per day for females (Cormack et al., 2000).
Dehydromenthofurolactone (No. 1163)
In a 21-day range-finding study, groups of five male and five female Sprague-Dawley rats were given daily doses of dehydromenthofurolactone of 0 or 100 mg/kg bw in the diet. The animals were observed daily for mortality and overt physical and behavioural abnormalities. Individual body weights and food consumption were recorded before initiation of the study and every 3 days until study termination, when all animals were killed and subjected to a complete gross necropsy. A complete set of tissues and organs was excised from each animal, and liver and kidney tissues were examined microscopically. No deaths or outward clinical signs of toxicity were reported in any of the animals during the study. A significant decrease in body-weight gain in the treated animals was reported on days 1–7 of the study, but not thereafter. Significant but sporadic decreases in food consumption were reported in males on days 4–7, and in females on days 1–4, 7–10, and 13–15 of the study. Females treated with dehydromenthofurolactone were reported to have significantly reduced absolute body weights when compared with controls on days 7–22 of the study. No significant difference in absolute body weights was reported between males treated with dehydromenthofurolactone and controls. The significantly increased absolute and relative liver weights reported in the treated males were not accompanied by any microscopic abnormalities. No significant differences in absolute and relative organ weights were observed in females treated with dehydromenthofurolactone when compared with controls. No gross or microscopic pathological effects related to the administration of dehydromenthofurolactone were reported. The author concluded that 100 mg/kg bw per day was an appropriate high dose for a subsequent 90-day study in rats (Becci, 1986).
Groups of 20 male and 20 female Sprague-Dawley rats were fed diets containing dehydromenthofurolactone at concentrations calculated to provide an average daily intake of 0, 1, 10, or 100 mg/kg bw for 13 weeks. The animals were observed daily for mortality, pharmacotoxic signs and overt behavioural changes. Individual body weights and food consumption were recorded before initiation of the study and weekly thereafter. Clinical chemistry tests and gross necropsies were conducted on randomly selected animals (five of each sex per group) after 4 weeks of treatment with dehydromenthofurolactone and on all surviving animals after 13 weeks. At necropsy, the absolute and relative organ weights of all animals were determined. Tissues from controls and from rats receiving the highest dose (100 mg/kg bw per day) were fixed and stained for microscopic examination. On the basis of the initial findings, microscopic examinations of potential target organs (i.e. oesophagus and stomach) were extended to include animals receiving the low dose (1 mg/kg bw per day) and the intermediate dose (10 mg/kg bw per day).
No mortality attributable to dehyromenthofurolactone was reported. There was no significant difference in body weight reported between animals receiving the two lower doses and control animals throughout the duration of the study. Conversely, body weights and food consumption of animals receiving the highest dose animals were significantly lower (p >0.05) than those of the control animals throughout the study. Since no difference in food conversion (body-weight gain per gram of food consumed) was reported at any dose, the decreased food consumption and body-weight gains of the animals receiving the highest dose were attributed to the decreased palatability of the diet at this dose. Oesophageal and gastric changes in animals given the highest dose may also have had an effect on food consumption.
The incidence of slight to mild hyperkeratosis and epithelial thickening of the oesophageal mucosa was significantly increased (p <0.05) in the groups receiving the two higher doses when compared with controls. Rats given the highest dose exhibited a significant increase in the incidence of hyperkeratosis of the squamous forestomach at termination. However, the authors did not consider these observations to be of any toxicological significance since they were not associated with any other dose-related biological alterations (i.e. basal cell proliferation). Instead, the authors attributed these changes to the irritation of the mucosa, resulting from the continuous ingestion of high doses of dehydromenthofurolactone. No other lesions were reported.
Increased relative liver weights were reported in both sexes at 10 and 100 mg/ kg bw per day, but not at 1.0 mg/kg bw per day. However, since absolute liver weights were unchanged, the increased relative organ weights were attributed to the lower body weights of rats. Increased concentrations of total protein and albumin in the serum were noted only in males at the highest dose. This was not considered to be biologically important because the increase did not affect the albumin:globulin ratio at any dose and it was not accompanied by any changes in clinical, haematological, or urine analysis parameters. On the basis of the findings of the study, the author reported the NOEL for dehydromenthofurolactone to be 1.0 mg/kg bw per day (Voss, 1985).
Sclareolide (No. 1165)
Groups of five male and five female Sprague-Dawley rats were fed diets containing sclareolide at a dose of 0 or 10 mg/kg bw per day for 14 days. Based on food consumption values, the average daily intakes of sclareolide for weeks 1 and 2 were reported to be 8.19 and 7.52 mg/kg bw for males and 7.95 and 7.92 mg/kg bw for females, respectively. Concurrent controls were maintained. Mortality, food consumption, body weight, detailed clinical signs, absolute and relative organ weights, gross pathology and histopathology were recorded for all rats. There were no deaths or outward clinical signs of toxicity reported in any of the animals. Comparisons of food intake and body weight revealed no significant differences between treated animals and controls. Compared with controls, mean body weights were significantly increased and mean relative kidney weights were significantly decreased in treated females at week 2. The authors did not consider these observations to be of any toxicological significance because they were not accompanied by any evidence of histopathology. No treatment-related effects were reported in the treated rats on gross pathological and histological examinations of the liver and kidneys (Terrill, 1990).
(iii) Lactones used to aromatic ring systems (Nos 1167–1172)
3-Propylidenephthalide (No. 1168)
Groups of 14 male and 14 female Charles River CD rats were fed diets containing 3-propylidenephthalide for 13 weeks. The animals received diets containing 3-propylidenephthalide at a concentration of 47, 78, or 94 ppm from 0–4, 5–10, or 11–13 weeks, respectively. The average daily intake of 3-propylidenephthalide over the 13-week period was calculated to be approximately 5.42 mg/kg bw for males and 6.55 mg/kg bw for females. Concurrent controls were maintained. Individual body weights, food consumption and efficiency of food use were measured weekly. Haematological and clinical chemistry examinations were performed on 50% of the animals at week 7 and on all of the animals at the end of the study. At necropsy, gross and histopathological examinations were performed on all animals.
No differences in body weight, food consumption and efficiency of food use were reported in treated animals when compared with the controls. Concentration of haemoglobin, erythrocyte count, erythrocyte volume fraction and total and differential leukocyte counts for controls and treated animals were similar through-out the study. The relative weights of the liver and kidneys of the treated animals did not differ significantly from those of the controls. Gross necropsy and histopathological examination revealed no dose-related lesions in any treated animals (Posternak et al., 1969).
Dihydrocoumarin (No. 1171)
A 13-week study in rats was designed to investigate the hepatotoxic potential of dihydrocoumarin (Lake et al., 1994). Groups of six to eight male Sprague-Dawley rats were fed diets containing 0.76% dihydrocoumarin, calculated to provide an average daily intake of 580 mg/kg bw. Concurrent controls were maintained. Although relative liver weights were significantly increased in the treated group compared with the controls, histopathological examination of animals treated with dihydrocoumarin revealed no abnormalities. Dihydrocoumarin did not markedly affect the activities of carnitine acetyltransferase or palmitoyl-CoA oxidation (Lake et al., 1994), indicating that dihydrocoumarin is unlikely to be a rodent liver peroxisome proliferator (Lock et al., 1989).
Groups of 10 male and 10 female weanling Osborne-Mendel rats were fed diets containing dihydrocoumarin at a concentration of 0, 1000 or 10 000 ppm for 14 weeks (Hagan et al., 1967). These concentrations were calculated to provide an average daily intake of dihydrocoumarin of 0, 100 or 1000 mg/kg bw, respectively (Food & Drug Administration, 1993). However, a limitation of this study is the inability to determine the actual exposure levels owing to loss of the test substance in the diet during storage. No difference in general health and behaviour was reported in the treated animals when compared with the controls. Weekly measurements of body weight and food consumption revealed no significant difference between treated and control groups of rats. Haematological examinations performed at study termination showed no treatment-related effects in any of the animals. Additionally, no effects on organ weights, or macroscopic or microscopic changes in the tissues were reported upon necropsy (Hagan et al., 1967).
A blend of four compounds, which included dihydrocoumarin at a concentration of 15 ppm, was fed ad libitum to a group of 12 male and 12 female rats for 12 weeks. This daily ration was adjusted to provide a dose of dihydrocoumarin of approximately 5.58 mg/kg bw per day. Concurrent controls were maintained. Measurements of body weight, food consumption, and efficiency of food use were performed weekly. After the 12-week period, the urine of rats (three of each sex per group) was examined for the presence of sugar and albumin, and blood concentrations of haemoglobin were determined. The rats were subjected to gross necropsy and complete histopathological examinations of the liver and kidneys. There were no deaths reported in any of the treated animals during the study. The treated animals appeared normal in general health and behaviour throughout the study. The efficiency of food utilization was significantly depressed in the treated males (p <0.05) and females (p <0.01) when compared with the controls. The growth, final mean body weights, and absolute and relative liver and kidney weights of the treated animals were not significantly different from those of the controls. No other treatment-related effects were reported in any rats upon clinical (i.e. haematology and urine analysis) and gross necropsy examinations (Trubek, 1957).
In a follow-up study, groups of 10 male and 10 female rats were fed diets containing dihydrocoumarin at a concentration providing approximately 0 or 110 mg/kg bw per day for 12 weeks. Measurements of body weight, food consumption, and efficiency of food use were performed weekly. After the 12-week period, urine from three rats of each sex per group was examined for the presence of sugar and albumin, and blood concentration of haemoglobin was determined. The animals were subjected to gross necropsy and complete histopathological examinations of the liver and kidneys. No adverse effects were reported in the treated rats with respect to general health and behaviour, growth, body weight, food consumption and efficiency of food use. At necropsy, gross examination did not reveal any significant differences in absolute and relative liver and kidney weights between treated and control animals. No treatment-related effects on clinical parameters (i.e. haematology and urine analysis) were reported. On the basis of these results,the author concluded that dihydrocoumarin was not the component responsible for the depression in efficiency of food use observed in the previous study (Trubek, 1957, 1958).
6-Methylcoumarin (No. 1172)
Mice
Groups of 10 male and 10 female B6C3F1 mice were given 6-methylcoumarin by gavage for 13 weeks at a dose of 0, 50, 100, 200, 400, or 800 mg/kg bw per day. The mice were observed twice daily for signs of toxicity, moribundity, and mortality. General health and body-weight measurements were recorded weekly. At study termination, clinical tests were conducted on all surviving mice. All mice were subjected to gross necropsy and histopathological examinations. Three mice at 800 mg/kg bw and one mouse at 400 mg/kg bw died during the course of the study. The final mean body and organ weights of all treated mice were similar to those of the controls. No toxicologically significant changes were reported in the clinical, macroscopic and microscopic examinations of male or female mice at any dose. Prostration, bradycardia, bradypnoea, hypoactivity, hypothermia, and the loss of the grasping reflex were reported at 800 mg/kg bw per day. No such effects were reported in any other group (National Toxicology Program, 2002).
Rats
Groups of 10 male and 10 female rats were given 6-methylcoumarin by gavage at a dose of 0, 75, 150, 300, 600, or 1200 mg/kg bw per day for 13 weeks. The rats were observed twice daily for signs of toxicity, morbundity, and mortality. Individual body weights were recorded weekly. At study termination, clinical tests were conducted on all surviving rats. All rats were subjected to gross necropsy and histopathological examination. All rats at 1200 mg/kg bw per day and one male at 600 mg/kg bw per day died during week 1 of the study. A statistically significant decrease (p <0.05) in mean body-weight gain was reported in males and females at 600 mg/kg bw per day when compared with controls at week 13 of the study, whereas only a slight decrease was reported in males at 300 mg/kg bw per day. Clinical effects, including hypoactivity, lacrimation, ataxia, impaired righting reflex and decreased limb tone, were reported in both sexes at 600 and 1200 mg/kg bw per day. No changes related to treatment with 6-methylcoumarin were reported in haematological, serum biochemical, or urine analysis parameters for rats at any dose. Although a significant decrease in serum cholinesterase activity was reported in females at 300 and 600 mg/kg bw per day, possible clinical effects of the decrease (i.e. ataxia) were observed only at 600 and 1200 mg/kg bw per day. Necropsy of all animals in the group receiving the highest dose revealed microscopic lesions that varied in the degree of congestion, degeneration, necrosis and hepatitis. Increased mean absolute and relative liver weights were reported in males and females at 300 and 600 mg/kg bw per day; however, these changes were not accompanied by any treatment-related macroscopic observations. No treatment-related effects were reported at 150 mg/kg bw per day (National Toxicology Program, 2002).
Groups of 10 male and 10 female weanling Osborne-Mendel rats were fed diets containing 6-methylcoumarin at a concentration of 0, 1000 or 10 000 ppm for 14 weeks (Hagan et al., 1967). These dietary concentrations were calculated to provide average daily intakes of 0, 100 and 1000 mg/kg bw, respectively (Food & Drug Administration, 1993). No significant differences in general health and behaviour were reported in the treated animals when compared with the controls. Weekly measurements of body weight and food consumption revealed no significant difference between treated and control groups. Haematological examinations performed at study termination showed no treatment-related effects in any of the animals. No effects on organ weights, or macroscopic or microscopic changes in the tissues were reported at any dose (Hagan et al., 1967).
The mechanism of hepatotoxicity of coumarin and related coumarin derivatives, including 6-methylcoumarin, has been studied in rats. For 13 weeks, groups of male Sprague-Dawley rats were fed diets containing 0, 0.5 or 0.75% coumarin (corresponding to an average daily intake of 0, 395 or 535 mg/kg bw, respectively) or 0.82% 6-methylcoumarin4 (corresponding to 695 mg/kg bw). At the end of the 13-week study period, the rats were killed and livers were excised for biochemical and morphological examination. Although relative liver weights and activities of gamma-glutamyl transferase were increased for all treated groups, only animals treated with coumarin exhibited significant dose-related increases in the activities of the plasma aminotransferases alanine transferase (ALT) and aspartate transferase (AST) when compared with the controls. Histopathological examination of animals treated with coumarin revealed vacuolation of centrilobular hepatocytes, bile duct hyperplasia, and cholangiofibrosis, particularly in rats given the diet containing 0.75% coumarin. No increase in plasma activities of aminotransferase and no bile duct hyperplasia or cholangiofibrosis were observed in animals maintained on the diet containing 6-methylcoumarin; however, a slight vacuolation of hepatocytes was observed in three out of eight treated animals. Unlike coumarin, treatment with 6-methylcoumarin increased the activity of mixed function oxidase (i.e. 7-ethoxycoumarin O-deethylase activity). 6-Methylcoumarin did not markedly affect the activities of carnitine acetyltransferase and palmitoyl-CoA oxidation, indicating that 6-methylcoumarin is unlikely to be a rodent liver peroxisome proliferator (Lake et al., 1994).
Dogs
In a study with limitations, groups of two dogs received 6-methylcoumarin in gelatin capsules at a dose of 200 mg/kg bw per day for 2 or 4 weeks. There were no outward clinical signs of toxicity reported in any of the animals during the study. At necropsy, macroscopic and microscopic examination of major organs, including the liver, revealed no morphological or histological changes attributable to treatment with 6-methylcoumarin (Levenstein, 1954).
(c) Long-term studies of toxicity and carcinogenicity
Dihydrocoumarin (No. 1171)
Mice
Groups of 10 male and 10 female B6C3F1 mice received dihydrocoumarin (3,4-dihydrocoumarin) in corn oil by gavage at a dose of 0, 100, 200, 400, 800, or 1600 mg/kg bw, once daily, 5 days per week for 13 weeks. The animals were observed twice daily for mortality. General health, body weight and clinical observations were recorded weekly. In addition, the mice were palpated once weekly to detect masses. At the end of the study, necropsies were performed on all animals. Major organs including the brain, lungs, heart, liver and kidneys were weighed. Clinical pathology was conducted on all surviving mice at necropsy. Complete histopathologic examinations were performed on all control animals, animals receiving dihydrocoumarin at 1600 mg/kg bw per day and all animals that died before the end of the study. Tissue sections from select organs, including the liver and kidneys, were removed, fixed and stained for microscopic examination. Eight males and five females receiving dihydrocoumarin at 1600 mg/kg bw per day died within the first 5 weeks of the study. The deaths of two males in the control group and in the group receiving a dose of 100 mg/kg bw per day were attributed to dosing accidents. The body-weight gain of males and females at all doses was similar to that of the controls, with the exception of the two surviving males at 1600 mg/kg bw per day that exhibited a significant decrease (p <0.05) in body-weight gain. Significant increases in absolute (p <0.05) and relative (p <0.01) liver weights and rel-ative (p <0.05) kidney weights were also reported in these two surviving males. The absolute (p <0.01) and relative (p <0.01) liver weights of the five surviving females at 1600 mg/kg bw per day and the absolute (p <0.01) liver weights of the females at 800 mg/kg bw per day were significantly greater than those of the controls. No gross or microscopic lesions were observed in either sex at any dose. The NOEL for dihydrocoumarin was 800 and 400 mg/kg bw per day in male and female B6C3F1 mice respectively (National Toxicology Program, 1993).
In a study of carcinogenicity with dihydrocoumarin, groups of 70 male and 70 female B6C3F1 mice received dihydrocoumarin in corn oil by gavage at a dose of 0, 200, 400 or 800 mg/kg bw per day, 5 days per week for 2 years (National Toxicology Program, 1993). The animals were observed twice daily for mortality and morbidity. Body weights and clinical findings were recorded weekly for the first 13 weeks, and monthly thereafter. At the end of the study, necropsy was performed on all animals. No significant difference in survival rates, final mean body weights or clinical findings was reported in the treated animals when compared with the controls. There were also no biologically significant effects on haematological or clinical chemistry parameters reported in any of the groups at an interim evaluation at 15 months.
The incidences of nephropathy, and renal tubule hyperplasia, adenomas and carcinomas in males at all doses were not significantly different from those in controls (see Table 4). The combined incidence of renal tubule adenoma or carcinoma in males (0/50 controls; 1/51 at 200 mg/kg bw; 2/51 at 400 mg/kg bw; 2/49 at 800 mg/kg bw) was not considered to be treatment-related because of low incidence and the lack of a dose–response relationship. No neoplasms of the kidney were reported in either treated or control female mice.
Table 4. Incidences of renal neoplasms in male mice treated with dihydrocoumarin by gavage for 2 years
|
Renal neoplasm |
Dose (mg/kg bw) |
|
0 (control) |
200 |
400 |
800 |
|
Nephropathy |
45/50 (90%) |
46/51 (90%) |
45/51 (88%) |
43/49 (88%) |
|
Renal tubule hyperplasiaa |
0/50 (0%) |
1/51 (2%) |
3/51 (6%) |
1/49 (2%) |
|
Renal tubule adenomaa |
0/50 (0%) |
0/51 (0%) |
2/51 (4%) |
1/49 (2%) |
|
Renal tubule carcinomaa |
0/50 (0%) |
1/51 (2%) |
0/51 (0%) |
1/49 (2%) |
|
Renal tubule adenoma or carcinomaa |
0/50 (0%) |
1/51 (2%) |
2/51 (4%) |
2/49 (4%) |
|
From National Toxicology Program (1993) |
|
a |
Combined examination of single and step sections of formalin-fixed kidney tissues |
Neoplastic and non-neoplastic lesions associated with treatment with dihydrocoumarin developed principally in the liver in mice (see Table 5). There was an increase in the incidence of hepatocellular adenomas and combined hepatocellular adenomas and carcinomas in treated female mice. compared with that of the control group; however, the incidence of these neoplasms was not dose-related. The number of treated females with multiple hepatocellular adenomas was increased. The increased incidence of neoplasms in females was not considered to be clear evidence of a carcinogenic response. In general, the incidence of hepatocellular neoplasms in treated and control males was greater than in treated and control females, indicating the increased susceptibility of the male mouse liver to neoplastic changes. However, the incidences of hepatocellular adenomas in treated male mice were not significantly different from the control group. The number of males with multiple hepatocellular adenomas was moderately increased in the groups given 400 and 800 mg/kg bw. In addition, no increased incidence of malignant liver neoplasms was observed in treated males at any dose.
Table 5. Incidences of hepatocellular neoplasms in mice treated with dihydrocoumarin by gavage for 2 years
|
Hepatic neoplasm |
Dose (mg/kg bw) |
|
0 (control) |
200 |
400 |
800 |
|
Male |
|
|
|
|
|
Hepatocellular adenoma |
29/50 (58%) |
23/51 (45%) |
36/51 (71%) |
31/50 (62%) |
|
Hepatocellular carcinoma |
11/50 (22%) |
11/51 (22%) |
11/51 (22%) |
6/50 (12%) |
|
Hepatoblastoma |
0/50 (0%) |
0/51 (0%) |
0/51 (0%) |
2/50 (4%) |
|
Combined ratesa |
36/50 (72%) |
30/51 (59%) |
40/51 (78%) |
34/50 (68%) |
|
Female |
|
|
|
|
|
Hepatocellular adenoma |
10/51 (20%) |
20/50 (40%) |
22/50 (44%) |
20/52 (38%) |
|
Hepatocellular carcinoma |
3/51 (6%) |
2/50 (4%) |
4/50 (8%) |
6/52 (12%) |
|
Combined ratesb |
13/51 (25%) |
21/50 (42%) |
25/50 (50%) |
24/52 (46%) |
|
From National Toxicology Program (1993) |
|
a |
Historical incidence for 2-year studies of administration in corn oil by gavage with vehicle control groups (mean ±standard deviation, 370/901 (41.1% ±15.5%); range, 14–72%. Includes adenoma, carcinoma, or hepatoblastoma (combined) |
|
b |
Historical incidence: 129/898 (14.4% ±8.1%); range, 2–34%. Includes adenoma or carcinoma (combined) |
The incidence of alveolar/bronchiolar adenoma in male mice was increased at 200 and 400 mg/kg bw per day. This was not considered to be related to treatment as the increase was slight and there was no dose-response relationship.
The National Toxicology Program report concluded: "There was no evidence of carcinogenic activity of 3,4-dihydrocoumarin in male B6C3F1 mice receiving 200, 400, or 800 mg/kg. There was some evidence of carcinogenic activity in female B6C3F1 mice based on increased incidences of hepatocellular adenoma, and hepatocellular adenoma and carcinoma (combined)."
The primary neoplastic effects observed in mice treated by gavage in the 2-year study by National Toxicology Program were associated with the liver. The high incidence of hepatocellular adenomas and carcinomas in both control and treated groups of males and females demonstrate the sensitivity of the B6C3F1 mouse liver to neoplastic changes. The high incidences of hepatocellular adenoma in treated and control males were not significantly different. The incidence of adenomas in male controls was higher than in any group of treated females, demonstrating the heightened sensitivity of the male mouse liver. Although the incidence of hepatocellular adenomas and combined hepatocellular adenomas and carcinomas in all groups of treated females was greater than in the control group, the response was not related to dose. The incidence of hepatocellular neoplasms in treated males was not significantly different from that in the controls, but the incidence of hepatocellular neoplasms was higher in both these groups than than in the groups of treated and control females. The profile of neoplastic responses is consistent with the historically high background incidence of hepatocellular neoplasms in male and female B6C3F1 mice (Maronpot et al., 1987).
A recent analysis of the historical spontaneous incidence of liver neoplasms in control male and female B6C3F1 mice has revealed background incidences of liver adenoma/carcinomas of 42.2% for males and 23.6% for females (Haseman et al., 1998). Increasing rates of incidence of liver tumours are strongly correlated with increasing mean body weights in control and test mice, achieved by changing the National Toxicology Program protocol to use individual rather than group housing (Haseman et al., 1994). Treated males and females housed individually attained mean body weights of >50 and >49 g, respectively, from weeks 53 to 103 of the study. Analysis of the effect of body weight on the incidence of liver neoplasms for control mice in the National Toxicology Program database revealed that in males with mean body weights of >49 g the incidence of liver tumours was 54% among controls for feeding studies and 62% among controls for inhalation studies. The incidence of liver tumours was 35% among females with mean body weights of >49 g in the control groups for both feeding studies and inhalation studies. In addition, other factors such as administration of corn oil as the gavage vehicle and the presence of Heliobacter hepaticus in males contribute to the high background incidence of this type of neoplasm in both sexes of mice (Haseman et al., 1998).
The Committee concluded that observations of hepatic neoplasms in mice in the National Toxicology Program bioassay are not relevant to the safety of dihydrocoumarin in humans at low levels of intake from use as a flavouring agent. This conclusion is based on the high incidence of spontaneous hepatocellular neoplasms (adenomas and carcinomas) in B6C3F1 mice, which is related to body-weight changes, use of corn oil as the gavage vehicle, and the presence of Heliobacter hepaticus, the absence of consistent data on the dose-response relationship, the lack of hepatocellular neoplastic effects in the parallel study in rats (discussed below), and the relatively high doses administered that are 1000–10 000 times greater than the intake of 24 µg/kg bw per day in Europe and 19 µg/kg bw per day in the USA from use of dihydrocoumarin as a flavouring agent (see Table 1). On the basis of what is known about the metabolism of structurally-related substances, humans may metabolize low intakes of dihydrocoumarin via a different principal pathway (i.e. beta-oxidation and cleavage) than that (i.e. beta-hydroxylation) used by rodents at high intakes (Samuelson et al., 1986).
Rats
Groups of 10 male and 10 female Fischer 344/N rats received dihydrocoumarin in corn oil by gavage at a dose of 0, 75, 150, 300, 600 or 1200 mg/kg bw, once daily, 5 days per week for 13 weeks. The animals were observed twice daily for mortality. General health, body weight and observations for clinical signs of toxicity were recorded weekly. In addition, the rats were palpated for masses once per week. At the end of the study, necropsies were performed on all animals, and major organs, including the brain, lungs, heart, liver and kidneys, were examined and weighed. Clinical tests for pathology were conducted on all surviving rats at necropsy. Complete histopathologic examinations were performed on all control animals, on animals receiving the highest dose (1200 mg/kg bw per day) and on all animals that died before the end of the study. Tissue sections from selected organs, including the liver and kidneys, were removed, fixed and stained for micro-scopic examination. Two males and five females at 1200 mg/kg bw per day died during the course of the study. The death of one control male was attributed to a dosing accident. The final mean body weight and body-weight gain of males in the group receiving 1200 mg/kg bw per day were significantly (p <0.01) lower than those of the controls. The mean body weights of all other treated males and females were similar to or slightly greater than those of the controls. The significant differences in haematology (i.e. platelet and erythrocyte counts, haemoglobin concentrations) reported between the treated and control animals were not considered to be clinically important because they were consistent with the anticoagulant effects of dihydrocoumarin. The biological significance of other reported changes in enzymes and other constitutents in blood plasma at doses >300 mg/kg bw could not be determined. The absolute and relative liver and kidney weights of males and females at doses of 600 and 1200 mg/kg bw were significantly (p <0.01) greater than those of the controls. Centrilobular hepatocellular hypertrophy, ranging in severity from minimal to mild, was reported in the liver of both sexes at 300, 600 and 1200 mg/kg bw. No adverse treatment-related effects were observed in either males or females at doses of 75 and 150 mg/kg bw. On the basis of these results, the NOEL for dihydrocoumarin was 150 mg/kg bw per day in Fischer 344/N rats (National Toxicology Program, 1993).
In a study of carcinogenicity conducted by the National Toxicology Program, groups of 60 male and 60 female Fischer 344/N rats received dihydrocoumarin in corn oil by gavage 5 days per week for 2 years, providing a dose of 0, 150, 300, or 600 mg/kg bw. The animals were observed twice daily for mortality and morbidity. Body weights and clinical findings were recorded weekly for the first 13 weeks, and monthly thereafter. Evaluations of haematology and clinical chemistry parameters were performed at a 15-month interim evaluation. At necropsy, all tissues and organs were examined for gross lesions and all major tissues were fixed and stained for microscopic evaluation. A significant dose-related decrease in the survival of males was reported (27/60 at 0 mg/kg; 12/60 at 150 mg/kg; 8/60 at 300 mg/kg; 2/60 at 600 mg/kg) with the majority of deaths occurring after week 92. The decreased survival was attributed to progressive degenerative nephropathy leading to renal failure. Typically, the severity of nephropathy increased with dose. After week 6, the mean body-weight gain of the males at the highest dose was 5–10% lower than that of the control animals (National Toxicology Program, 1993).
In a related stop-exposure evaluation, groups of male Fischer 344/N rats were given dihydrocoumarin in corn oil by gavage at a dose of 600 mg/kg bw per day for 9 or 15 months, followed by corn oil only until the end of the study. After 9 and 15 months, half the animals were necropsied, while the remainder received corn oil until death or the end of the study. The severity of nephropathy in the stop-exposure group was significantly greater than that of the animals sacrificed at 9 or 15 months. This indicates that this form of nephropathy is a progressive degenerative disease associated with the ageing process in rats. Any chemically-related nephropathy observed is probably irreversible, especially in Fischer 344/N males.
No significant change in survival rates, mean body weights or clinical findings related to treatment with dihydrocoumarin was reported in treated females at any dose.
Haematology and clinical chemistry performed at the 15-month interim evaluation revealed statistically significant increased serum activities of alanine aminotransferase (ALT), alkaline phosphatase (ALP), sorbitol dehydrogenase, or gamma-glutamyl transferase (GGT) in males at 300 and 600 mg/kg bw per day. Increased activities of ALP and GGT were reported for female rats at 600 mg/kg bw per day. Although significant changes in the activity of hepatic enzymes are normally associated with altered liver function, the increases in ALT, ALP, and GGT activites were not accompanied by histopathological evidence of hepatocellular degeneration or necrosis.
Nephropathy was reported in both sexes of control and treated rats (see Table 6). Although the incidence of nephropathy was greater in males than in females, there was no significant difference between treated and control males. The greater susceptibility of the male rat to chronic nephropathy made it vulnerable to chemical toxicity as the study progressed. Microscopic examination of combined single and step sections of the kidneys of male rats revealed a statistically significant, dose-related increase in renal tubule hyperplasia (control, 0/50; 150 mg/kg, 5/48; 300 mg/kg, 6/47; 600 mg/kg, 8/50). Only in males treated with 600 mg/kg bw was there a significant (p <0.05) difference in the incidence of renal tubule adenomas when compared with the control group. Although the total incidence of renal tubule adenomas (10/145) in all groups of treated males was significantly greater than the incidence of neoplasms in historical controls (8/1019), the results were not considered to be clear evidence of carcinogenicity because there was no evidence of malignant renal tubule neoplasms in males at any dose. Although renal transitional cell carcinomas were reported in two males that died early in the study (i.e. at days 444 and 528) at 600 mg/kg bw, no evidence of transitional cell carcinomas was found in the majority (86%) of males surviving longer than 528 days. In addition, step-sections showed no evidence for a treatment-related response of the renal transitional cells in the kidneys of males. No significant difference in the incidence of renal tubule hyperplasia or renal tubule adenoma was observed between control and treated groups of females.
Table 6. Incidences of renal neoplasms in rats treated with dihydrocoumarin by gavage for 2 years
| |
Incidence/no. of animals in group (%) |
| |
0 mg/kg
(control) |
150
mg/kg bw |
300
mg/kg bw |
600
mg/kg bw |
|
Male |
|
|
|
|
|
Nephropathy |
50/50 (100%) |
47/48 (98%) |
47/47 (100%) |
47/50 (94%) |
|
Renal tubule hyperplasiac |
0/50 (0%) |
5/48 (10%)a |
6/47 (13%)a |
8/50 (16%)b |
|
Renal tubule adenomac |
1/50 (2%) |
1/48 (2%) |
3/47 (6%) |
6/50 (12%)a |
|
Female |
|
|
|
|
|
Nephropathy |
20/50 (40%) |
20/49 (41%) |
37/49 (76%)b |
31/49 (63%)b |
|
Renal tubule hyperplasiaa |
0/50 (0%) |
0/49 (0%) |
0/49 (0%) |
2/49 (4%) |
|
Renal tubule adenomaa |
1/50 (2%) |
1/49 (2%) |
1/49 (2%) |
0/49 (0%) |
|
From National Toxicology Program (1993) |
|
a |
Significantly (p <0.05) different from control group by the logistics regression test (2-year study) |
|
b |
Significantly (p <0.01) different from control group |
|
c |
Combined single and step sections |
Unlike the treated male rats in the 2-year bioassay by the National Toxicology Program, the treated female rats that exhibited increased nephropathy at 300 and 600 mg/kg bw did not exhibit an increased incidence of renal tubule hyperplasia or renal tubule neoplasms. The incidences of renal tubule hyperplasia and neoplasms in male mice were not dose-related or statistically significant when compared with those of the controls. In addition, no renal histopathology was observed in studies in rats fed with dihydrocoumarin at doses of up to 500 mg/kg bw per day for 14 weeks, nor in a study in dogs fed with dihydrocoumarin at doses of up to 150 mg /kg bw per day for 2 years (Trubek, 1957, 1958; Hagan et al., 1967). Therefore, the renal hyperplastic and neoplastic effects are sex- and species-specific and not dose-related.
The National Toxicology Program report concluded that: "Under the conditions of these 2-year gavage studies, there was some evidence of carcinogenic activity of 3,4-dihydrocoumarin in male F344/N rats based on increased incidences of renal tubule adenomas and focal hyperplasia. The transitional cell carcinomas in two 600 mg/kg males may also have been chemical related. There was no evidence of carcinogenic activity of 3,4-dihydrocoumarin in female F344/N rats receiving 150, 300 or 600 mg/kg."
The male rat kidney has been shown to be a unique target organ for the carcinogenic effects of a variety of chemical substances (Burdock et al., 1990; EPA, 1991). Analysis by researchers at the National Toxicology Program (Haseman et al., 1998) has shown that the survival rates of Fischer 344 male rats used as controls have decreased significantly over the last decade, from 66% to <50%. One of the major causes of death is severe nephropathy, which has been increasing in incidence in more recent control groups (Haseman et al., 1994). On the basis of this evidence, it can be concluded that the renal effects of dihydrocoumarin in the male rat are, indeed, species- and sex-specific phenomena, and in all probability, reflect the sensitivity of the male rat kidney to chronic progressive nephropathy, focal hyperplasia, and specific tumourigenic responses. It is unclear whether the incidence of transitional cell carcinomas reported in two male rats early in the study is related to treatment with dihydrocoumarin at a dose of 600 mg/kg bw per day. It was reported that older males showed no evidence of renal transitional cell changes at any dose.
The interaction of test substances with spontaneous, age-related renal disease in laboratory rats has recently been reviewed (Hard, 1998). On the basis of a comprehensive review of renal tumours of all types reported in bioassays performed by the National Toxicology Program, it seems that the interaction of chemical agents and spontaneous chronic progressive nephropathy occurs at two levels; first, to exacerbate the rate of chronic progressive nephropathy, and second, to stimulate tubule hyperplasia into foci of atypical hyperplasia eventually leading to adenomas. The induction of tumours via this pathway normally produces a minimal response in male rats, leading to a low incidence of tumours of relatively small size and low grade. Such is the case with dihydrocoumarin.
A significant (p <0.01) increase in the incidence of forestomach ulcers in male rats was reported at the end of the study, but not at the 15-month interim evaluation. Although an increased incidence of ulcers, squamous hyperplasia, and chronic inflammation occurred in males at all doses, the effects were not dose-related. No significant forestomach effects were reported in female rats at any dose. Two neoplasms (a squamous cell papilla and carcinoma) reported in female rats at 600 mg/kg bw could not be attributed to treatment because of the low incidence of the neoplasms and of focal hyperplasia (National Toxicology Program, 1993).
Dogs
Mongrel and beagle dogs were given capsules containing dihydrocoumarin at a dose of 50 mg/kg bw (one male and one female) or 150 mg/kg bw (one female), daily, six times a week, for 2 years with no treatment-related effects at either dose (Hagan et al., 1967). The animals were weighed weekly. Haematological examinations were performed three times before the start of treatment and at 2 weeks, 1 month, 3 months, 6 months and 1 year after treatment and then at yearly intervals until termination. At necropsy, the major organs were weighed and a complete histological examination was performed. All tissues were examined macroscopically and microscopically for treatment-related changes. No difference was reported in body weight and growth of the treated animals when compared with the controls. No treatment-related effects were reported in the macroscopic or microscopic tissue examinations or haematological parameters at either dose. This study is of limited value, however, owing to the lack of a control group, the small number of test animals, and the composition of the test groups (Hagan et al., 1967).
6-Methylcoumarin (No. 1172) Rats
In a long-term study, groups of 10 male and 10 female Osborne-Mendel rats were fed diets containing 6-methylcoumarin at a concentration of 500, 1000, 3500, 7500, or 15 000 ppm for 2 years (Hagan et al., 1967). Daily intake of 6-methylcoumarin was calculated to be approximately 25, 50, 175, 375, or 750 mg/kg bw, respectively (Food & Drug Administration, 1993). Body weights, food intake, and general condition were recorded weekly. Haematological examinations, undertaken at 3, 6, 12 and 22 months, included erythrocyte counts, haemoglobin counts and erythrocyte volume fractions. At termination, the tissues of all rats were examined for macroscopic and microscopic changes. Depression in growth rates was noted in males at 375 mg/kg bw per day. Depression of growth rate was severe in males and moderate in females at a dose of 750 mg/kg bw. Hepatic effects in males and females at this dose included fatty metamorphosis, very slight bile duct proliferation, and focal telangiectasis. No treatment-related effects were observed at intakes up to and including 175 mg/kg bw per day in males and 375 mg/kg bw per day in females (Hagan et al., 1967).
Dogs
Dogs (one male, one female) were given capsules containing 6-methylcoumarin at a dose of 50 mg/kg bw per day, 6 days per week for 2 years (Hagan et al., 1967). The animals were weighed weekly. Haematological examinations were performed before the start of treatment, at 2 weeks, 1, 3 and 6 months and 1 year after treatment and then at yearly intervals until termination. At necropsy, the major organs were weighed and a complete histological examination was per-formed. All tissues were examined macroscopically and microscopically for treatment-related changes. No differences were reported in the body weight and growth of the treated animals when compared with the controls. No treatment-related effects were reported in the macroscopic or microscopic tissue examinations or in the evaluation of haematological parameters. Unfortunately, the small number of test animals in this study limits its value (Hagan et al., 1967).
Table 7 summarizes the results of short-term and long-term studies of toxicity and carcinogenicity.
Table 7. Results of short-term and long-term studies of toxicity and carcinogenicity with alicyclic, alicyclic-fused and aromatic-fused ring lactones
|
No. |
Flavouring agent |
Species; sex |
No. of test groupsa/ no. per groupb |
Route of administration |
Duration
(days) |
NOEL
(mg/kg bw per day) |
Reference |
|
Short-term studies of toxicity |
|
1161 |
Dihydromintlactone |
Rat; M, F |
3/10 |
Diet |
28 |
15.5 (M)c
>27.8 (F)c |
Cormack et al. (2000) |
|
1163 |
Dehydromenthofurolactone |
Rat; M, F |
1/10 |
Diet |
21 |
>100c |
Becci (1986) |
|
1163 |
Dehydromenthofurolactone |
Rat; M, F |
3/40 |
Diet |
90 |
1.0 |
Voss (1985) |
|
1165 |
Sclareolide |
Rat; M, F |
1/10 |
Diet |
14 |
7.86 (M)d
7.94 (F)e |
Terrill (1990) |
|
1168 |
3-Propylidenephthalide |
Rat; M, F |
1/28 |
Diet |
90 |
>5.42 (M)c
>6.55 (F)c |
Posternak et al. (1969) |
|
1171 |
Dihydrocoumarin |
Rat; M |
1/8 |
Diet |
90 |
<580 |
Lake et al. (1994) |
|
1171 |
Dihydrocoumarin |
Rat; M, F |
2/20 |
Diet |
98 |
>1000c |
Hagan et al. (1967) |
|
1171 |
Dihydrocoumarin |
Rat; M, F |
1/24 |
Diet |
84 |
>5.58c |
Trubek (1957) |
|
1171 |
Dihydrocoumarin |
Rat; M, F |
1/20 |
Diet |
84 |
>110c |
Trubek (1958) |
|
1172 |
6-Methylcoumarin |
Mouse; M, F |
5/20 |
Gavage |
90 |
200 |
National Toxicology Program (2002) |
|
1172 |
6-Methylcoumarin |
Rat; M, F |
5/20 |
Gavage |
90 |
150 |
National Toxicology Program (2002) |
|
1172 |
6-Methylcoumarin |
Rat; M, F |
2/20 |
Diet |
98 |
>1000c |
Hagan et al. (1967) |
|
1172 |
6-Methylcoumarin |
Rat; M |
1/8 |
Diet |
90 |
>695c |
Lake et al. (1994) |
|
1172 |
6-Methylcoumarin |
Dog |
1/2 |
Diet |
28 |
>200c |
Levenstein (1954) |
|
Long-term studies of toxicity and carcinogenicity |
|
1171 |
Dihydrocoumarin |
Mouse; M, F |
5/20 |
Gavage |
90 |
800 (M)
400 (F) |
National Toxicology Program (1993) |
|
1171 |
Dihydrocoumarin |
Mouse; M, F |
3/140 |
Gavage |
721 |
800 (M)
<200 (F) |
National Toxicology Program (1993) |
|
1171 |
Dihydrocoumarin |
Rat; M, F |
5/20 |
Gavage |
90 |
150 |
National Toxicology Program (1993) |
|
1171 |
Dihydrocoumarin |
Rat; M, F |
3/120 |
Gavage |
721 |
300 (M)
600 (F) |
National Toxicology Program (1993) |
|
1171 |
Dihydrocoumarin |
Dog; M, F |
1/2
1/1 |
Diet |
730 |
>150c |
Hagan et al. (1967) |
|
1172 |
6-Methylcoumarin |
Rat; M, F |
5/20 |
Diet |
730 |
175 (M)
375 (F) |
Hagan et al. (1967) |
|
1172 |
6-Methylcoumarin |
Dog; M, F |
1/2 |
Diet |
730 |
>50c |
Hagan et al. (1967) |
|
M, Male; F, Female; NR, Not reported |
|
a |
Total number of test groups does not include control animals |
|
b |
Total number per test group includes both male and female animals |
|
c |
Study performed with either a single dose or multiple doses that produced no adverse effect. The value is therefore not a true NOEL, but is the highest dose tested that produced no adverse effects. The actual NOAEL may be higher |
|
d |
Average intake calculated based on food consumption for weeks 1 (8.19 mg/kg bw) and 2 (7.52 mg/kg bw) |
|
e |
Average intake calculated based on food consumption for weeks 1 (7.95 mg/kg bw) and 2 (7.92 mg/kg bw) |
(d) Genotoxicity
(i) In vitro
Testing for genotoxicity in vitro has been performed with five representative members (Nos 1164, 1166, 1168, 1171 and 1172) of the group of alicyclic, alicyclic-fused and aromatic-fused ring lactones used as flavouring agents (see Table 8).
Table 8. Results of studies of genotoxicity with alicyclic, alicyclic-fused and aromatic-fused lactones
|
No. |
Flavouring agent |
End-point |
Test object |
Dose or concentration |
Results |
Reference |
|
In vitro |
|
|
|
|
|
|
|
1164 |
(+/-)-(2,6,6-Trimethyl-2-hydroxycyclohexylidene) acetic acid gamma-lactone |
Reverse mutation |
S. typhimurium TA98, TA100, and TA1537 |
100 µg/plate |
Negativea |
Kinae et al. (1981) |
|
1164 |
(+/-)-(2,6,6-Trimethyl-2-hydroxycyclohexylidene) acetic acid gamma-lactone |
DNA repair |
B. subtilis H-17 (rec+) and M-45 (rec-) |
10 mg/disk
(10 000 µg/disk) |
Negative |
Kinae et al. (1981) |
|
1166 |
Octahydrocoumarin |
Reverse mutation |
S. typhimurium TA98 and TA100 |
<5000 µg/plate |
Negativea,b |
Watanabe and Tukada (1989) |
|
1168 |
3-Propylidenephthalide |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA97, and TA1537 |
3.3–400 µg/plate |
Positivea,b,f |
Zeiger et al. (1988) |
|
1171 |
Dihydrocoumarin |
Reverse mutation |
S. typhimurium TA1535, TA1537, TA1538, TA98, and TA100 |
<75 µl/plate(88 950 µg/plate)c |
Negativea |
Brusick (1982b) |
|
1171 |
Dihydrocoumarin |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535 and TA1537 |
10–6666 µg/plate |
Negativea |
National Toxicology Program (1993) |
|
1171 |
Dihydrocoumarin |
Reverse mutation |
S. typhimurium and TA98, TA100, TA1535, TA1537 |
<10 mg/plate
(<10 000 µg/plate) |
Negativea,d |
Prival et al. (1982) |
|
1171 |
Dihydrocoumarin |
Forward mutation |
Mouse lymphoma L5178Y TK +/-cells |
200–500 nl/ml (237–593 µg/ml)c |
Weakly positivee |
Cifone (1982b) |
|
1171 |
Dihydrocoumarin |
Forward mutation |
Mouse lymphoma L5178Y TK +/-cells |
400–800 nl/ml (474–949 µg/ml)c |
Negativegf |
Cifone (1982b) |
|
1171 |
Dihydrocoumarin |
Forward mutation |
Mouse lymphoma L5178Y TK +/-cells |
<2500 nl/ml (2965 µg/ml)c |
Positivee |
Cifone (1984) |
|
1171 |
Dihydrocoumarin |
Forward mutation |
Mouse lymphoma L5178Y TK +/-cells |
<2500 nl/ml (2965 µg/ml)c |
Negativeg |
Cifone (1984) |
|
1171 |
Dihydrocoumarin |
Unscheduled DNA synthesis |
Rat hepatocytes |
0.03–4.0 µl/ml
(35.6–4744 µg/ml)c |
Negative |
Curren (1986) |
|
1171 |
Dihydrocoumarin |
Chromosomal aberrations |
Chinese hamster ovary cells |
0.01–1.0 µl/ml
(11.9–1186 µg/ml)c |
Negativee |
Galloway (1983) |
|
1171 |
Dihydrocoumarin |
Chromosomal aberrations |
Chinese hamster ovary cells |
33.3–333 nl/ml
(39.5–395 µg/ml)c |
Negativee |
Galloway (1983) |
|
1171 |
Dihydrocoumarin |
Chromosomal aberrations |
Chinese hamster ovary cells |
500, 1000 and 1600 µg/ml |
Negativee |
National Toxicology Program (1993) |
|
1171 |
Dihydrocoumarin |
Chromosomal aberrations |
Chinese hamster ovary cells |
100, 160 and 500 µg/ml |
Negative |
National Toxicology Program (1993) |
|
1171 |
Dihydrocoumarin |
Sister chromatid exchanges |
Chinese hamster ovary cells |
50–300 µg/ml |
Positive |
National Toxicology Program (1993) |
|
1171 |
Dihydrocoumarin |
Sister chromatid exchanges |
Chinese hamster ovary cells |
50–1000 µg/ml |
Negativee |
National Toxicology Program (1993) |
|
1171 |
Dihydrocoumarin |
Sister chromatid exchanges |
Chinese hamster ovary cells |
1600 and 2000 µg/ml |
Positivee |
National Toxicology Program (1993) |
|
1172 |
6-Methylcoumarin |
Reverse mutation |
S. typhimurium TA100 |
<3.6 mg/plate
(<3600 µg/plate) |
Marginally positivee |
Wild et al. (1983) |
|
1172 |
6-Methylcoumarin |
Reverse mutation |
S. typhimurium TA100 |
<3.6 mg/plate (<3600 µg/plate) |
Negative |
Wild et al. (1983) |
|
1172 |
6-Methylcoumarin |
Reverse mutation |
S. typhimurium TA98, TA1535, TA1537, and TA1538 |
<3.6 mg/plate (<3600 µg/plate) |
Negativea |
Wild et al. (1983) |
|
1172 |
6-Methylcoumarin |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, and TA1537 |
33–3333 µg/plate |
Negativea,b |
Haworth et al.(1983) |
|
1172 |
6-Methylcoumarin |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537 and TA1538 |
1–5000 µg/plate |
Negativea |
Brusick (1982a) |
|
1172 |
6-Methylcoumarin |
Forward mutation |
Mouse lymphoma L5178Y Tk +/-cells |
6.25–100 µg/ml |
Negativee |
Cifone (1982) |
|
1172 |
6-Methylcoumarin |
Forward mutation |
Mouse lymphoma L5178Y Tk +/-cells |
15.6–250 µg/ml |
Negative |
Cifone (1982) |
|
In vivo |
|
|
|
|
|
|
|
1171 |
Dihydrocoumarin |
Micronucleus formation |
Mouse peripheral blood cells |
400, 800 and 1600 mg/kg bw |
Negative |
National Toxicology Program (1993) |
|
1172 |
6-Methylcoumarin |
Sex-linked recessive lethal mutation |
Drosophila melanogaster |
10 mmol/l
(1602 µg/ml)g |
Negative |
Wild et al. (1983) |
|
1172 |
6-Methylcoumarin |
Micronucleus formation |
Mouse peripheral blood cells |
200 and 400 mg/kg |
Equivocal (M)h
Negative (F) |
Witt et al. (2000) |
|
1172 |
6-Methylcoumarin |
Micronucleus formation |
Mouse bone-marrow cells |
160, 240, and 320 mg/kg |
Negativei |
Wild et al. (1983) |
|
a |
With and without metabolic activation |
|
b |
Pre-incubation method |
|
c |
Calculated based on specifi c gravity of dihydrocoumarin =1.186 -1.192 (Food and Chemical Codex, 1996) |
|
d |
Plate incorporation method |
|
e |
With metabolic activation |
|
f |
A two-fold increase in revertants was reported at one concentration only |
|
gf |
Without metabolic activation |
|
g |
Calculated based on the relative molecular weight of 6-methylcoumarin =160.17 |
|
h |
Although the statistical analysis yielded a positive trend test (p =0.006), and the frequency of micronucleus formation was signifi cantly elevatedabove the control value (p =0.0072), the result was concluded to be equivocal in male mice due to the very small increase in the frequency ofmicronucleus normochromatic erythrocytes (NCE) observed (<0.5 per 1000 NCE) |
|
i |
Administered by intraperitoneal injection |
Negative results were reported in the Ames assay when Salmonella typhimurium strains (TA97, TA98, TA100, TA1535, TA1537 and TA1538) were incu-bated with 100 µg of (+/-)-(2,6,6-trimethyl-2-hydroxycyclohexylidene) acetic acid gamma-lactone (No. 1164) per plate (Kinae et al., 1981), up to 5000 µg of octahydrocoumarin (No. 1166) per plate (Watanabe & Tukada, 1989), or up to 75 µl (88 950 µg)5 of dihydrocoumarin (No. 1171) per plate (Brusick, 1982b; Prival et al., 1982; National Toxicology Program, 1993), with and without metabolic activation.
In a similar assay for reverse mutation, concentrations of up to 400 µg of 3-propylidenephthalide (No. 1168) per plate yielded a mutagenic response in the presence of metabolic activation in S. typhimurium strains TA97, TA98, TA100, TA1535 and TA1537 (Zeiger et al., 1988). As the purity of the 3-propylidenephthalide sample was unknown, the authors could not conclusively determine whether the mutagenic response was caused by the test material or by possible contaminants present in the sample. These results in vitro have not been confirmed by a standard in vivo assay.
At concentrations of up to 3.6 mg of 6-methylcoumarin (No. 1172) per plate, a slight but significant increase in the number of revertants of one strain of S. typhimurium, TA100, was reported, but only in the presence of metabolic activation (Wild et al., 1983). Negative results were reported in four strains (TA98, TA1535, TA1537, and TA1538) with or without metabolic activation (Wild et al., 1983). In further assays for reverse mutation, 6-methylcoumarin yielded negative results in S. typhimurium strains TA98, TA100, TA1535, TA1537 and TA1538 at concentrations of up to 5000 µg/plate, with or without metabolic activation (Brusick, 1982a; Haworth et al., 1983). On this basis, the marginally positive result in one strain is considered to be an isolated incident, and cannot be used to conclusively characterize the mutagenic potential of 6-methylcoumarin.
Negative results were reported in an assay for DNA repair in which (+/-)-(2,6,6-trimethyl-2-hydroxycyclohexylidene) acetic acid gamma-lactone (No. 1164) was incubated with Bacillus subtillis strains (H17 and M45) at concentrations of up to 10 mg/disk (10 000 µg/disk) (Kinae et al., 1981).
Concentrations of dihydrocoumarin of up to 2500 nl/ml (2965 µg/ml) were reported to be mutagenic in mouse lymphoma L5178Y Tk +/- cells only in the presence of metabolic activation (Cifone, 1982b, 1984). The authors did not consider the positive results to be a conclusive determination of mutagenicity because increases in mutant frequency were only detected at cytotoxic concentrations in the presence of metabolic activation (Cifone, 1982b, 1984). It has since been proven that non-physiological culture conditions, such as low pH and high osmolality, may produce positive results in similar assays in the absence of genotoxic materials (Brusick, 1986). The effect of low pH has been observed mainly in the presence of metabolic activation and is believed to be an effect of the acidic environment created by the S9 constituents produced at low pH (Cifone, 1985; Brusick, 1987). In similar assays, negative results were reported for dihydrocoumarin in assays for forward mutation in mouse lymphoma L5178Y Tk+/- cells at concentrations of up to 2500 nl/ml (2965 µg/ml) in the absence of metabolic activation (Cifone, 1982b, 1984). 6-Methylcoumarin was not mutagenic in mouse lymphoma L5178Y Tk +/- cells at concentrations of up to 250 µg/ml, with or without metabolic activation (Cifone, 1982a).
Dihydrocoumarin did not induce unscheduled DNA synthesis in rat hepatocytes at concentrations of up to 4.0 µl/ml (4744 µg/ml) (Curren, 1986).
Dihydrocoumarin did not induce chromosomal aberrations in Chinese hamster ovary (CHO) cells, at doses of up to 1600 µg/ml with metabolic activation and up to 500 µg/ml without metabolic activation (Galloway, 1983; National Toxicology Program, 1993). Dihydrocoumarin induced a dose-related increase in sister chro-matid exchange in CHO cells at a concentration of up to 300 µg/ml, in the absence of metabolic activation (National Toxicology Program, 1993). In the presence of metabolic activation, a significant increase in sister chromatid exchange was observed in CHO cells only at the two highest dihydrocoumarin doses tested (1600 and 2000 µg/ml). However, cytotoxicity was clearly evident at a dose of 2000 µg/ml (National Toxicology Program, 1993). The isolated positive results from assays for cytogenetic indicator sister chromatid exchange in CHO cells are clearly out-weighed by the overwhelming negative evidence from the studies of chromosomal aberration in the same cell type.
(ii) In vivo
The genotoxic potential of 6-methylcoumarin was studied in a Basc test for induction of sex-linked recessive lethal mutations in adult Drosophila melanogaster (Wild et al., 1983). The observed frequency of mutation was not increased when a 10 mmol/l (1602 µg/ml)6 solution of 6-methylcoumarin was fed to the flies for 3 days.
No significant increase in the frequency of micronucleated erythrocytes was reported in peripheral blood samples obtained from male and female B6C3F1 mice after 13 weeks of treatment with dihydrocoumarin at doses of up to 1600 mg/kg bw per day (National Toxicology Program, 1993). A test for micronucleus forma-tion in peripheral blood from B6C3F1 mice given 6-methylcoumarin at a dose of 200 or 400 mg/kg bw per day was reported to produce negative results in females and equivocal results in males, owing to the very small increase in the frequency of micronucleus normochromatic erythrocytes (NCE) observed (<0.5 increase per 1000 NCE) (Witt et al., 2000). In a similar study, groups of NMRI mice given 6-methylcoumarin intraperitoneally at a dose of 160, 240 or 320 mg/kg bw showed no increase in micronucleated erythrocytes in samples of bone marrow, 30 h after treatment (Wild et al., 1983).
Discussion of genotoxicity data
Alicyclic, alicyclic-fused and aromatic-fused ring lactones used as flavouring agents are not mutagenic in vitro in the Ames or DNA repair assays. In the assay in mouse lymphoma cells, positive results obtained only in the presence of metabolic activation from S9 could be explained as a well-known artefact of the presence of S9. The negative results obtained at the same concentrations in the absence of metabolic activation support this possibility. The predominance of negative results for dihydrocoumarin in CHO cells in vitro and in assays in vivo suggests a lack of genotoxicity. Taking into account the above results and the fact that these substances are rapidly metabolized in vivo to compounds of lower toxicological potential, it is concluded that the alicyclic, alicyclic-fused and aromatic-fused ring lactones used as flavouring agents exhibit low genotoxic potentials.
3. References
Albro, P.W. (1975) The metabolism of 2-ethylhexanol in rats. Xenobiotica, 5(10), 625–636.
Aldridge, W.N. (1953) Serum Esterases 1. Two types of esterase (a and b) hydrolysing p-nitrophenyl acetate, propionate, and butyrate, and a method for their determination. Biochem J., 53110–117.
Ambrose, A.M., Power, F.W. & Sherwin, C.P. (1933) Further studies on the detoxication of phenylacetic acid. Journal of Biological Chemistry, 101, 669–675.
American Spice Trade Association (2000) Spice Statistics 2000: The American Spice Trade Association Report.
BASF (1976) Acute toxicity studies on octahydrocoumarin. Unpublished report to the Research Institute of Fragrance Materials, Englewood Cliffs, New Jersey, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Becci, P.J. (1986) 21-Day dietary dose-range study in rats with SRA 84–11 (dehydromenthofurolactone). Unpublished report. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Billecke, S., Draganov, D., Counsell, R., Stetson, P., Watson, C., Hsu, C. & La Du, B.N. (2000) Human serum paraoxonase (PON1) isozymes Q and R hydrolyze lactones and cyclic carbonate esters. Drug Metabol. Dispos., 28, 1335–1342.
Brewster, D., Jones, R.S. & Parke, D.V. (1977a) The metabolism of cyclohexanecarboxylate in the rat. Biochem. J., 164, 595–600.
Brewster, D., Jones, R.S. & Parke, D.V. (1977b) The metabolism of cyclohexanecarboxylic acid in the isolated perfused rat liver. Xenobiotica, 7, 601–609.
Brusick, D.J. (1982a) Mutagenicity evaluation of 6-methylcoumarin in the Ames Salmonella/microsome plate test. LBI Project No. 20988. Unpublished report. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Brusick, D.J. (1982b) Mutagenicity evaluation of dihydrocoumarin in the Ames Salmonella/microsome plate test. LIB Project No. 20988. Unpublished report. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Brusick, D.J. (1986) Genotoxic effects in cultured mammalian cells produced by low pH treatment conditions and increased ion concentrations. Environ. Mutagen., 8, 879–886.
Brusick, D.J. (1987) Implications of treatment-condition-induced genotoxicity for chemical screening and data interpretation. Mutat. Res., 189, 1–6.
Buch, S.A. (1981) Acute oral toxicity of cyclohexyl lactone in the rat. Unpublished report. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Burdock, G.A., Wagner, B.M., Smith, R.L., Munro, I.C. & Newberne, P.M. (1990) 15. GRAS Substances. Food Technol., 44, 78–86.
Cheng, H., Schwartz, M.S., Vickers, S., Gilbert, J.D., Amin, R.D., Depuy, B., Liu, L., Rogers, J.D., Pond, S.M., Duncan, C.A., Olah, T.V. & Bayne, W.F. (1994) Metabolic disposition of simvastatin in patients with T-tube drainage. Drug Metabol. Dispos., 22, 139–142.
Cholerton, S., Idle, M.E., Vas, A., Gonzalez, F.J. & Idle, J.R. (1992) Comparison of a novel thin-layer chromatographic-fluorescence detection method with a spectrofluorimetric method for the determination of 7-hydroxycoumarin in human urine. J. Chromatography A, 575, 325–330.
Cifone, M.A. (1982a) Mutagenicity evaluation of 6-methylcoumarin in the mouse lymphoma forward mutation assay. LIB Project No. 20989. Unpublished report. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Cifone, M.A. (1982b) Mutagenicity evaluation of dihydrcoumarin in the mouse lymphoma forward mutation assay. LIB Project No. 20989. Unpublished report. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Cifone, M.A. (1984) Mutagenicity evaluation of dihydrocoumarin in the mouse lymphoma forward mutation assay. LIB Project No. 20989. Unpublished report. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Cifone, M.A. (1985) Relationship between increases in the mutant frequency in L5178Y TK+/- mouse lymphoma cells at low pH and metabolic activation. Environ. Mutagen., 7 (Suppl 3), 27.
Cohen, A.J. (1979) Critical review of the toxicology of coumarin with special reference to interspecies differences in metabolism and hepatotoxic response and their significance to man. Food Cosmet. Toxicol., 17, 277–289.
Collier, T.A. (1982) Determination of the acute oral median lethal dose (LD50) of menthofurolactone in the rat. Unpublished report. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Cormack, H., Mullee, D. & Brooks, P.N. (2000) Twenty-eight day repeated dose oral (dietary) toxicity study in the rat. Unpublished report. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Cramer, G.M., Ford, R.A. & Hall, R.L. (1978) Estimation of toxic hazard—A decision tree approach. Food Cosmet. Toxicol., 16, 255–276.
Culp, H.W. & McMahon, R.E. (1968) Reductase for aromatic aldehydes and ketones. J. Biol. Chem., 243, 848–852.
Curren, R.D. (1986) Unscheduled DNA synthesis in rat primary hepatocytes of dihydrocoumarin. Study Number T4429.380. Unpublished report. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Deisinger, P.J., Boatman, R.J. & Guest, D. (1994) Metabolism of 2-ethylhexanol administered orally and dermally to the female Fischer 344 rat. Xenobiotica, 24, 429–440.
Deuel, Jr., H.J. (1957) The oxidation and metabolism of triglycerides, fatty acids, and glycerol in the animal body. In: The Lipids: Their Chemistry and Biochemistry, New York: Interscience Publishers, pp. 71–99 & 291–300.
Diliberto, J.J., Srinivas, P., Overstreet, D., Usha, G., Burka, L.T. & Birnbaum, L.S. (1990) Metabolism of citral, an alpha,beta-unsaturated aldehyde, in male F344 rats. Drug Metabol. Dispos., 18, 866–875.
Egan, D., O’Kennedy, R., Moran, E., Cox, D., Prossier, E. & Thorne, R.D. (1990) The pharmacology, metabolism, analysis and applications of coumarin and coumarin-related compounds. Drug Metabol. Rev., 22, 503–529.
Environmental Protection Agency (EPA) (1991) Alpha 2m-globulin association with chemically induced renal toxicity and neoplasia in the male rat. Publication No. EPA/ 625/3–91019F.
Food and Chemicals Codex (1996) Food and Chemicals Codex, 4th Ed. Washington DC, USA: National Academy Press.
Food & Drug Administration (1993) Priority-based assessment of food additives (PAFA) database center for food safety and applied nutrition.
Felsted, R.L. & Bachur, N.R. (1980) Ketone reductases. In: Jackoby, W., Enzymatic Basis of Detoxification, Vol. I, New York: Academic Press, pp. 281–293.
Fentem, J.H. & Frey, J.R. (1992) Metabolism of coumarin by rat, gerbil and human liver microsomes. Xenobiotica, 22, 357–367.
Feuer, G., Goldberg, L. & Gibson, K.I. (1966) Liver response tests. VII. Coumarin metabolism in relation to the inhibition of rat-liver glucose 6-phosphate. Food Chem. Toxicol., 4, 157–167.
Feuer, G. (1974) The metabolism and biological actions of coumarins. Vol. 10. In: West, G.B., ed, Progress in Medicinal Chemistry, Amsterdam: Elsevier Science Publishers, pp. 85–158.
Fishbein, W.N. & Bessman, S.P. (1966) Purification and properties of an enzyme in human blood and rat liver microsomes catalyzing the formation and hydrolysis of gamma-lactones. I. Tissue localization, stoichiometry, specificity distinction from esterase. J. Biol. Chem., 241, 4835–4841.
Forrester, L.M., Henderson, C.J., Glancey, M.J., Back, D.J., Park, B.K., Ball, S.E., Kitteringham, N.R., McLaren, A.W., Miles, J.S., Skett, P. & Wolf, C.R. (1992) Relative expression of cytochrome P-450 isoenzymes in human liver and association with the metabolism of drugs and xenobiotics. Biochem. J., 281, 359–368.
Furuya, T. (1958) Studies on the metabolism of the naturally occurring coumarins. V. Urinary metabolites of coumarin and dihydrocoumarin. Chem. Pharmaceut. Bulletin, 6, 701–706.
Galloway, S.M. (1983) Mutagenicity evaluation of dihydrocoumarin in an in vitro cytogenetic assay measuring chromosome aberration frequencies in Chinese hamster ovary (CHO) cells. LIB Project No. 20990. Unpublished report. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Guidotti, A. & Ballotti, P.L. (1970) Relationship between pharmacological effects and blood and brain levels of gamma-butyrolactone and gamma-hydroxybutyrate. Biochem. Pharmacol., 19, 883–895.
Hagan, E.C., Hansen, W.H., Fitzhugh, O.G., Jenner, P.M., Jones, W.I., Taylor, J.M., Long, E.L., Nelson, A.M. & Brouwer, J.B. (1967) Food flavorings and compounds of related structure. II. Subacute and chronic toxicity. Food Cosmet. Toxicol, 5, 141–157.
Hard, G.C. (1998) Mechanisms of chemically induced renal carcinogenesis in the laboratory rodent. Toxicol. Pathol., 26, 104–112.
Haseman, J.K., Eustis, S.L. & Ward, J.M. (1994) Contributing causes of death in rats and the utilization of this information in the statistical evaluation of tumor data. In: Mohr, V., Capan, C. & Dungworth, D., eds, Pathology of Aging Animals, Vol. 1. Washington DC: ILSI Press, pp. 629–638.
Haseman, J.K., Hailey, J.R. & Morris, R.W. (1998) Spontaneous neoplasm incidences in Fischer 344 rats and B6C3F1 mice in two-year carcinogenicity studies: A National Toxicology Program update. Toxicol. Pathol., 26, 428–441.
Haworth, S., Lawlor, T., Mortelmans, K., Speck, W. & Zeiger, E. (1983) Salmonella mutagenicity test results for 250 chemicals. Environ. Mutagen., 5(Suppl.1), 3–142.
Humbert, R., Adler, D.A., Disteche, C.M., Hassett, C., Omleoinski, C.J. & Purlong, C.E. (1993) The molecular basis of the human serum paraoxonase activity polymorphism. Nat. Genet., 3, 73–76.
Huwer, T., Altmann, H.-J., Grunow, W., Lenhardt, S., Przybylski, M. & Eisenbrand, G. (1991) Coumarin mercapturic acid isolated from rat urine indicates metabolic formation of coumarin 3,4-epoxide. Chem. Res. Toxicol., 4, 586–590.
International Organization of the Flavor Industry (1995). European inquiry on volume use. Unpublished report. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
James, M.O., Smith, R.L., Williams, R.T. & Reidenberg, M. (1972) The conjugation of phenylacetic acid in man, sub-human primates, and some non-primate species. Proc. R. Soc. Lond. B, 182, 25–35.
Jenner, P.M., Hagan, E.C., Taylor, J.M., Cook, E.L. & Fitzhugh, O.G. (1964) Food flavorings and compounds of related structure I. Acute oral toxicity. Food Cosmet. Toxicol., 2, 327–343.
Killenberg, P.G. & Webster, L.T. (1980) Conjugation by peptide bond formation. In: Jakoby, W., ed, Enzymatic Basis of Detoxification, Vol. II. New York: Academic Press, pp. 141–164.
Kinae, N., Hashizume, T., Makita, T., Tomita, I., Kimura, I. & Kanamori, H. (1981) Studies on the toxicity of pulp and paper mill effluents—II. Mutagenicity of the extracts of the liver from spotted sea trout (Nibea mitsukurii). Water Res., 15, 25–30.
Lake, B.G., Gray, T.J.B., Evans, J.G., Lewis, D.F.V., Beamand, J.A. & Hue, K.L. (1989) Studies on the mechanism of coumarin-induced toxicity in rat hepatocytes: comparison with dihydrocoumarin and other coumarin metabolites. Toxicol. Appl. Pharmacol., 97, 311–323.
Lake, B.G., Evans, J.G., Lewis, D.F.V. & Price, R.J. (1994) Comparison of the hepatic effects of coumarin, 3,4-dimethylcoumarin, dihydrocoumarin and 6-methylcoumarin in the rat. Food Chem. Toxicol., 32, 743–751.
Lee, C.R. (1977) Evidence for the beta-oxidation of orally administered 4-hydroxybutyrate in humans. Biochem. Med., 17, 284–291.
Lettieri, J. & Fung, H.-L. (1978) Improved pharmacological activity via pro-drug modification: comparative pharmacokinetics of sodium gamma-hydroxybutyrate and gamma-butyrolactone. Res. Commun. Chem. Pathol. Pharmacol., 22, 107–118.
Levenstein, I. (1953) Acute toxicity of dihydrocoumain in Swiss albino mice. Unpublished report. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Levenstein, I. (1954) Toxicity of 6-methylcoumarin. Unpublished report. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Lewis, C.A. & Palanker, A.L. (1979) Acute toxicity studies in rats and rabbits. Unpublished report to the Research Institute of Fragrance Materials, Englewood Cliffs, New Jersey, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Lewis, D.F.V., Lake B.G., Ioannides, C. & Parke, D.V. (1994) Inhibition of rat hepatic aryl hydrocarbon hydroxylase activity by a series of 7-hydroxy coumarins: QSAR studies. Xenobiotica, 24, 829–838.
Liu, M.J. & Pollack, G.M. (1993) Pharmacokinetics and pharmacodynamics of valproate analogs in rats. II. Pharmacokinetics of octanoic acid, cyclohexanecarboxylic acid, and 1-methyl-1-cyclohexanecarboxylic acid. Biopharmaceutics and Drug Disposition, 14, 325–339.
Lock, E.A., Mitchell, A.M. & Elcombe, C.R. (1989) Biochemical mechanisms on induction of hepatic peroxisomes proliferation. Ann. Rev. Pharmacol. Toxicol., 29, 145–163.
Lucas, C.D., Putnam, J.M. & Hallagan, J.B. (1999) Flavor and Extract Manufacturers Association of the United States 1995 Poundage and Technical Effects Update Survey, Washington DC: Flavor and Extract Manufacturers Association of the United States.
Maarse, H., Visscher, C.A., Willemsens, L.C. & Boelens, M.H. (1999). Volatile Components In Food—Qualitative And Quantitative Data. Zeist: Centraal Instituut Voor Voedingson-derzioek TNO.
Madyastha, K.M. & Raj, C.P. (1993) Studies on the metabolism of a monoterpene ketone, R-(+)-pulegone—a hepatotoxin in the rat: Isolation and characterization of new metabolites. Xenobiotica, 23, 509–518.
Maronpot, R.R., Haseman, J.K., Boorman, G.A., Eustis, S.E., Rao, G.N. & Huff, J.E. (1987) Liver lesions in B6C3F1 mice: The National Toxicology Program, experience and position. Arch. Toxicol., 10(Suppl.), 10–26.
Miles, J.S., McClaren, A.W., Forrester, L.M., Glancey, M.J., Lang, M.A. & Wolf, C.R. (1990) Identification of the human liver cytochrome P-450 responsible for coumarin 7-hydroxylase activity. Biochem. J., 267, 365–371.
Moreno, O.M. (1972) Acute toxicity studies in mice, rats and rabbits. Unpublished report to the Research Institute of Fragrance Materials, Englewood Cliffs, New Jersey, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Moreno, O.M. (1973) Acute toxicity studies on rats and rabbits. Unpublished report to the Research Institute of Fragrance Materials, Englewood Cliffs, New Jersey, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Moreno, O.M. (1975) Acute toxicity studies on rats, rabbits and guinea pigs. Unpublished report to the Research Institute of Fragrance Materials, Englewood Cliffs, New Jersey, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Moreno, O.M. (1976) Acute toxicity studies in rats, mice, rabbits and guinea pigs. Unpublished report to the Research Institute of Fragrance Materials, Englewood Cliffs, New Jersey, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Moreno, O.M. (1978) Acute toxicity studies in rats, mice, rabbits, and guinea pigs. Unpublished report to the Research Institute of Fragrance Materials, Englewood Cliffs, New Jersey, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA
Moreno, O.M. (1980) Acute toxicity studies. Unpublished report to the Research Institute of Fragrance Materials, Englewood Cliffs, New Jersey, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Morgareidge, K. (1962) In vitro digestion of four lactones. Unpublished report. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Morgareidge, K. (1963) In vitro digestion of three lactones. Unpublished report. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
National Academy of Sciences (1970, 1982, 1987) Evaluating the Safety of Food Chemicals. Washington DC, USA.
National Toxicology Program (1992) Toxicology and carcinogenesis studies of gamma-butyrolactone (CAS 96–48–0) in F344/N rats and B6C3F1 mice (gavage studies) (Report No. NTP TR 406).
National Toxicology Program (1993) Toxicology and carcinogenesis studies of 3,4-dihydrocoumarin (CAS 119–84–6) in F344/N rats and B6C3F1 mice (gavage studies) (Report No. NTP TR 423).
National Toxicology Program (2002) Data Tables: Toxicology and carcinogenesis studies of methyl coumarin (CAS 92–48–8) in F344/N rats and B6C3F1 mice (gavage).
Nelson, D.L. & Cox, M.M. (2000) Lehninger Principles of Biochemistry. New York: Worth Publishers, Inc.
Nutley, B.P., Farmer, P. & Caldwell, J. (1994) Metabolism of trans-cinnamic acid in the rat and the mouse and its variation with dose. Food Chem. Toxicol., 32, 877–886.
Pearce, R., Greenway, D. & Parkinson, A. (1992) Species differences and interindividual variation in the level of cytochrome P-450 2A enzymes: effects of coumarin, dicumarol and testosterone oxidation. Arch. Biochem. Biophys., 298, 211–225.
Pellmont, B. (1970) Acute toxicity of 3-n-butylphthalide. Unpublished report. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Peters, M.M., Walters, D.G., Van Ommens, B., Van Bladerens, P.J. & Lake, B.G. (1991) Effect of inducers of cytochrome P-450 on the metabolism fo [3–14C]coumarin by rat hepatic microsomes. Xenobiotica, 21, 499–514.
Pollitt, R.J. (1974) Phenylpropionic acid in the urine of patients with phenylketouria and normals. Clin. Chim. Acta, 55, 317–322.
Posternak, J.M. (1965) Acute toxicity of the flavoring substance TT 135 (3-butylidenephthalide). Unpublished report. Submitted to WHO by the Flavor and Extract Manufacturers Associaton of the United States, Washington DC, USA.
Posternak, J.M., Linder, A. & Vodoz, C.A. (1969) Summaries of toxicological data. Toxicological tests on flavoring matters. Food Cosmet. Toxicol., 7, 405–407.
Prival, M.J., Sheldon, Jr., A.T. & Popkin, D. (1982) Evaluation, using Salmonella typhimurium, of the mutagenicity of seven chemicals found in cosmetics. Food Chem. Toxicol., 20, 427–432.
Rautio, A., Kraul, H., Kojo, A., Salmela, E. & Pelkonen, O. (1992) Interindividual variability of coumarin 7-hydroxylation in healthy volunteers. Pharmacogenet., 2, 227–233.
Reagan, E.L. & Becci, P.J. (1984) Acute oral LD50 study of dehydromenthofurolactone in Sprague-Dawley rats. Unpublished report. Submitted to WHO by the Flavor and Extract Manufacturers Association, Washington DC, USA.
Ritschel, W.A., Brady, M.E., Tan, H.S.I., Hoffmann, K.A., Yiu, I.M. & Grummich, K.W. (1977) Pharmacokinetics of coumarin and its 7-hydroxy-metabolites upon intravenous and peroral administration of coumarin in man. Euro. J. Clin. Pharmacol., 12, 457–461.
Roth, R.H. & Giarman, N.J. (1966) gamma-Butyrolactone and gamma-hydroxybutyric acid. I. Distribution and metabolism. Biochem. Pharmacol., 15, 1333–1348.
Samuelson, O.B., Brenna, J., Solheim, E. & Scheline, R.R. (1986) Metabolism of the cinnamon substituent o-methoxyciannamaldehyde in the rat. Xenobiotica, 16, 845–852.
Scheline, R.R. (1968) Studies on the role of the intestinal microflora in the metabolism of coumarin in rats. Acta Pharmacol. Toxicol., 26, 325–331.
Serota, D.G. (1984) 14-Day single dose subacute toxicity study of 6-methylcoumarin. Unpublished report to the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Shilling, W.H., Crampton, R.F. & Longland, R.C. (1969) Metabolism of coumarin in man. Nature, 221, 664–665.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of flavoring materials: A mechanism for setting priorities for safety evaluation. Food Chem. Toxicol., 23, 857–860.
Stofberg, J. and Grundschober, F. (1987) Consumption ratio and food predominance of flavoring materials. Perfumer Flavorist, 12, 27.
Terrill, J.B. (1990) 14-Day oral toxicity study with sclareolide in rats. Unpublished report. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Trubek (1957) Toxicological screening of components of food flavors. Class XI. Miscellaneous components. Unpublished report to the Research Institute of Fragrance Materials, Englewood Cliffs, New Jersey, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Trubek (1958) Toxicological screening of components of food flavors. Class XI-B benzodihydropyrone. Unpublished report to the Research Institute of Fragrance Materials, Englewood Cliffs, New Jersey, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Van Iersel, M.L.P.S., Henderson, C.J., Walters, D.G., Price, R.J., Wolf, C.R. & Lake, B.G. (1994) Metabolism of [3-14C] coumarin by human liver microsomes. Xenobiotica, 24, 795–803.
Voss, K.A. (1985) 90-Day dietary toxicity study of SRA 84-11 in Sprague-Dawley rats (dehydromenthofurolactone). Unpublished report. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Voet, D. and Voet, J.G. (1990). Biochemistry, New York: John Wiley and Sons.
Walkenstein, S.S., Wiser, R., Gudmundsen, C. and Kimmel, H. (1964) Metabolism of gamma-hydorxybutyric acid. Biochim. Biophys. Acta, 86, 640–642.
Waller, A.R. & Chasseaud, L.F. (1981) The metabolic fate of [14C]coumarin in baboons. Food Cosmet. Toxicol., 19, 1–6.
Watanabe, S. & Tukada, S. (1989) Mutagenicity tests with cyclohexyl lactone in Salmonella typhimurium. Unpublished report. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Wild, D., King, M.T., Gocke, E. & Eckhardt, K. (1983) Study of artificial flavouring substances for mutagenicity in the Salmonella/microsome, basc and micronucleus tests. Food Chem. Toxicol., 21, 707–719.
Williams, R.T. (1959) Cyclohexanecarboxylic acid and its derivatives. In: Detoxication Mechanisms: The metabolism and detoxication of drugs, toxic substances and other organic compounds, London: Chapman & Hall Ltd. pp.119–126.
Witt, K.L., Knapton, A., Wehr, C.M., Hook, G.J., Mirsalis, J., Shelby, M.D. & MacGregor, J.T. (2000) Micronucleated erythrocyte frequency in peripheral blood of B6C3F1 mice from short-term, prechronic, and chronic studies of the NTP carcinogenesis bioassay program. Environ. Mol. Mutagen., 36, 163–194.
Yamano, S., Tatsuno, J. & Gonzalez, F.J. (1990) The CYP2A3 gene product catalyses coumarin 7-hydroxylation in human liver microsomes. Biochem., 29, 1322–1329.
Yamazaki, H.I.I., Yun, C.H., Guengerich, F.P. & Shimada, T. (1992) Cytochrome P-4502E1 and 2A6 enzymes as major catalysts for metabolic activation of N-nitrosodialkyamines and tobacco-related nitrosamines in human liver microsomes. Carcinogenesis, 13, 1789–1794.
Zeiger, E., Anderson, B., Haworth, S., Lawlor, T. & Mortelmans, K. (1988) Salmonella muta-genicity tests: IV. Results from the testing of 300 chemicals. Environ. Mol. Mutagen., 11(Suppl. 12), 1–158.
Zlatkis, A. & Liebich, H.M. (1971) Profile of volatile metabolites in human urine. Clin. Chem., 17, 592–594.
ENDNOTES:
- A group of 35 aliphatic lactones of similar structure was evaluated by the Committee at its forty-ninth meeting (Annex 1, reference 132).
- Total consumption of paprika in the USA in 2000 was 52 771 000 lbs. The average concentration of (+/-)-(2,6,6-trimethyl-2-hydroxycyclohexylidene) acetic acid gamma-lactone (No. 1164) found in paprika is 7.5 ppm. Consumption of (+/-)-(2,6,6-trimethyl-2-hydroxycyclohexylidene) acetic acid gamma-lactone via paprika =52 771 000 × 7.5 ppm) (American Spice Trade Association, 2000).
 Taurine
Taurine- Diets containing 0.82% 6-methylcoumarin and 0.75% coumarin contain equimolar amounts of the two substances.
- Calculated based on specific gravity of dihydrocoumarin =1.186 -1.192 (Food and Chemicals Codex, 1996).
- Calculated based on the relative molecular mass of 6-methylcoumarin =160.17.



















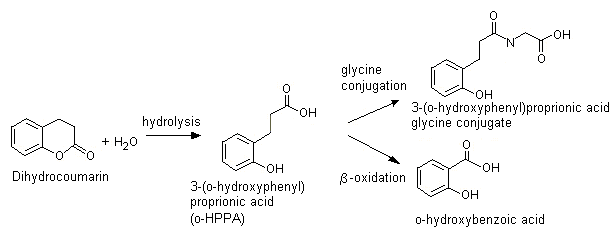
 Taurine
Taurine