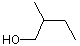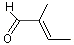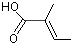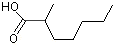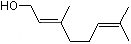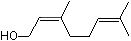WHO FOOD ADDITIVES SERIES: 52
ALIPHATIC BRANCHED-CHAIN SATURATED AND UNSATURATED ALCOHOLS, ALDEHYDES, ACIDS, AND RELATED ESTERS
First draft prepared by
Mrs I.M.E.J. Pronk
Center for Substances and Integrated Risk Assessment, National Institute for Public Health and the Environment, Bilthoven, Netherlands
1. EVALUATION
1.1 Introduction
The Committee evaluated a group of flavouring agents comprising 32 aliphatic branched-chain saturated and unsaturated alcohols, aldehydes, acids, and related esters (see Table 1) by the Procedure for the Safety Evaluation of Flavouring Agents (see Figure 1, Introduction). The Committee had previously evaluated two members of this group; citronellol (No. 1219) and citral (No. 1225) were both evaluated by the Committee at its eleventh meeting (Annex 1, reference 14), when conditional acceptable daily intakes (ADIs)1 of 0–0.25 mg/kg bw and 0–1 mg/kg bw respectively, were allocated. Citronellol and citral were re-evaluated at the twenty-third meeting of the Committee (Annex 1, reference 50) as part of a group of terpenoid flavouring agents, including geranyl acetate, linalool and linalyl acetate. A group ADI of 0–0.5 mg/kg bw, expressed as citral, was established for citral, geranyl acetate, citronellol, linalool, and linalyl acetate on the basis of their clearly-defined metabolism, rapid excretion, and low toxicity in short-term studies. The Committee maintained, however, that a long-term study was required for at least one member of this group. At its forty-ninth meeting (Annex 1, reference 131), the Committee evaluated a group of 26 geranyl, neryl, citronellyl, and rhodinyl esters formed from branched-chain terpenoid alcohols and aliphatic acyclic linear and branched-chain carboxylic acids by the Procedure. Two-year studies of carcinogenicity had been conducted for a mixture of two of these esters, geranyl acetate and citronellyl acetate. The Committee concluded that there were no safety concerns for any of the 26 substances under the low levels of intake arising from their use as flavouring agents and maintained the group ADI for citral, geranyl acetate, citronellol, linalool, and linalyl acetate. Likewise, at its fifty-first meeting (Annex 1, reference 137) when the Committee re-evaluated linalool and linalyl acetate, the group ADI was maintained.
Table 1. Summary of results of safety evaluations of aliphatic branched-chain saturated and unsaturated alcohols, aldehydes, acids, and related esters used as flavouring agentsa
|
Flavouring agent |
No. |
CAS No. and structure |
Step A3b Does intake exceed the threshold for human intake? |
Step A4 Is the flavouring agent or are its metabolites endogenous? |
Step A5 Adequate margin of safety for the flavouring agent or related substance? |
Comments on predicted metabolism |
Conclusion based on current intake |
|
Structural class I |
|
|
|
|
|
|
|
|
(+/-) 2-Methyl-1-butanol |
1199 |
137-32-6
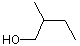 |
No
Europe: ND
USA: 35 |
NR |
NR |
See note 1 |
No safety concern |
|
3-Methyl-2-buten-1-ol |
1200 |
556-82-1
 |
No
Europe: 5.4
USA: 3.8 |
NR |
NR |
See note 1 |
No safety concern |
|
2-Methyl-2-butenal |
1201 |
1115-11-3
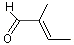 |
No
Europe: 0.7
USA: 0.2 |
NR |
NR |
See note 1 |
No safety concern |
|
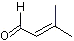
3-Methyl-2-butenal |
1202 |
107-86-8
 |
No
Europe: 3.9
USA: 0.5 |
NR |
NR |
See note 1 |
No safety concern |
|
Ammonium isovalerate (ammonium salt of isovaleric acid) |
1203 |
7563-33-9
 |
No
Europe: 18
USA: 16 |
NR |
NR |
See note 2 |
No safety concern |
|
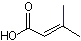
3-Methylcrotonic acid |
1204 |
541-47-9
 |
No
Europe: 121
USA: 0.01 |
NR |
NR |
See note 2 |
No safety concern |
|
trans-2-Methyl-2-butenoic acid |
1205 |
80-59-1
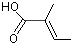 |
No
Europe: 4.9
USA: 1.6 |
NR |
NR |
See note 2 |
No safety concern |
|
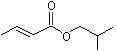
Isobutyl 2-butenoate |
1206 |
589-66-2
 |
No
Europe: 0.5
USA: 45 |
NR |
NR |
See note 3 |
No safety concern |
|
2-Methylallyl butyrate |
1207 |
7149-29-3
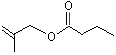 |
No
Europe: ND
USA: 0.2 |
NR |
NR |
See note 3 |
No safety concern |
|
4-Methyl-2-pentenal |
1208 |
5362-56-1
 |
No
Europe: 0.3
USA: 0.2 |
NR |
NR |
See note 1 |
No safety concern |
|
2-Methyl-2-pentenal |
1209 |
623-36-9
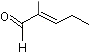 |
No
Europe: 4
USA: 0.2 |
NR |
NR |
See note 1 |
No safety concern |
|
2-Methyl-2-pentenoic acid |
1210 |
3142-72-1
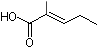 |
No
Europe: 42
USA: 20 |
NR |
NR |
See note 2 |
No safety concern |
|
2,4-Dimethyl-2-pentenoic acid |
1211 |
66634-97-7
 |
No
Europe: 0.1
USA: 0.1 |
NR |
NR |
See note 2 |
No safety concern |
|
2-Methylheptanoic acid |
1212 |
1188-02-9
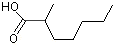 |
No
Europe: 17
USA: 6 |
NR |
NR |
See note 2 |
No safety concern |
|
Isobutyl angelate |
1213 |
7779-81-9
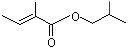 |
No
Europe: 0.1
USA: 0.1 |
NR |
NR |
See note 3 |
No safety concern |
|
2-Butyl-2-butenal |
1214 |
25409-08-9
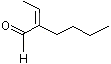 |
No
Europe: ND
USA: 0.01 |
NR |
NR |
See note 4 |
No safety concern |
|
2-Isopropyl-5-methyl-2-hexenal |
1215 |
35158-25-9
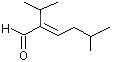 |
No
Europe: 0.3
USA: 0.01 |
NR |
NR |
See note 4 |
No safety concern |
|
2-Ethyl-2-heptenal |
1216 |
10031-88-6
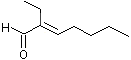 |
No
Europe: 0.01
USA: 0.1 |
NR |
NR |
See note 4 |
No safety concern |
|
2-Methyl-2-octenal |
1217 |
73757-27-4
 |
No
Europe: ND
USA: 7.9 |
NR |
NR |
See note 1 |
No safety concern |
|
4-Ethyloctanoic acid |
1218 |
16493-80-4
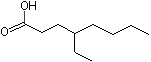 |
No
Europe: ND
USA: 4 |
NR |
NR |
See note 2 |
No safety concern |
|
dl-Citronellol |
1219 |
106-22-9
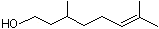 |
No
Europe: 370
USA: 771 |
NR |
NR |
See note 4 |
* |
|
Citronellal |
1220 |
106-23-0
 |
No
Europe: 945
USA: 324 |
NR |
NR |
See note 4 |
No safety concern |
|
3,7-Dimethyl-6-octenoic acid |
1221 |
502-47-6
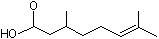 |
No
Europe: 3.1
USA: 0.2 |
NR |
NR |
See note 4 |
No safety concern |
|
Rhodinol |
1222 |
6812-78-8
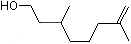 |
No
Europe: 53
USA: 8.4 |
NR |
NR |
See note 4 |
No safety concern |
|
Geraniol |
1223 |
106-24-1
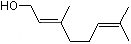 |
No
Europe: 640
USA: 315 |
NR |
NR |
See note 4 |
No safety concern |
|
Nerol |
1224 |
106-25-2
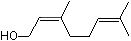 |
No
Europe: 290
USA: 171 |
NR |
NR |
See note 4 |
No safety concern |
|
Citral (Mixture of the trans and cis isomers geranial and neral) |
1225 |
5392-40-5
 |
Yes
Europe: 6849
USA: 6990 |
No |
Yes. The NOEL of 60 mg/kg bw per day (National Toxicology Program, 2003) for citral is >500 times more than the estimated daily intakes of 114 mg/kg bw in Europe and 117 mg/kg bw in the USA when used a flavouring agent |
See note 4 |
* |
|
8-Ocimenyl acetate |
1226 |
197098-61-6
 |
No
Europe: ND
USA: 7.7 |
NR |
NR |
See note 4 |
No safety concern |
|
2,6-Dimethyl-10-methylene-2,6,11-dodecatrienal |
1227 |
60066-88-8
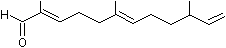 |
No
Europe: 5.1
USA: 0.5 |
NR |
NR |
See note 4 |
No safety concern |
|
3,7,11-Trimethyl-2,6,10-dodecatrienal |
1228 |
19317-11-4
 |
No
Europe: ND
USA: 0.2 |
NR |
NR |
See note 4 |
No safety concern |
|
12-Methyltridecanal |
1229 |
75853-49-5
 |
No
Europe: ND
USA: 0.5 |
NR |
NR |
See note 1 |
No safety concern |
|
Farnesol |
1230 |
4602-84-0
 |
No
Europe: 9
USA: 2.6 |
NR |
NR |
See note 4 |
No safety concern |
|
CAS: Chemical Abstracts Service; ND: no intake data reported; NR: not required for evaluation because consumption of the agent was determined to be of no safety concern at Step A3 of the Procedure. |
|
a |
Step 2: All of the flavouring agents in this group are expected to be metabolized to innocuous products. |
|
b |
The threshold for human intake for structural class I is 1800 µg/day. All intake values are expressed in µg/day. The combined per capita intake of flavouring agents in structural class I is 9382 µg per day in Europe and 8732 µg per day in the USA. |
|
* |
A group ADI of 0–0.5 mg/kg bw, expressed as citral, was established for citral, citronellol, geranyl acetate, linalool, and linalyl acetate by the Committee at its twenty-third meeting (Annex 1, reference 50), which was maintained at the present meeting. Use of citronellol and citral as flavouring agents is subsumed in the group ADI. |
|
Notes: |
|
1. |
Primarily oxidized to corresponding carboxylic acid that may enter the beta-oxidation pathway yielding shorter chain carboxylic acids that are subsequently metabolized to CO2 via the tricarboxylic acid pathway. |
|
2. |
Metabolized primarily via the beta-oxidation pathway yielding shorter chain carboxylic acids that are subsequently metabolized to CO2 via the tricarboxylic acid pathway. |
|
3. |
Hydrolysed to the corresponding alcohol and carboxylic acid, then participates in the pathway cited in notes 1 and 2. |
|
4. |
Oxidized to corresponding carboxylic acid. The acid may undergo partial beta-oxidation, be excreted or undergo omega-oxidation to yield polar polyoxygenated metabolites that are excreted free or conjugated primarily in the urine. If unsaturation is present, the polar polyoxygenated metabolites may also form hydrogenation or hydration metabolites. |
Twenty-four of the 32 flavouring agents (Nos 1199–1202, 1204–1206, 1208–1210, 1212, 1213, 1215, 1217–1221, 1223–1225, 1227, 1228, 1230) have been reported to occur naturally in foods. The substances that occur naturally in the highest abundance are the monoterpene primary alcohols, aldehydes, and carboxylic acids. They have been detected in fruits such as raspberries, strawberries, bananas, kumquats, and myrtle berries, as well as in many alcoholic beverages, including beer, wine, whisky, brandy and rum. They are most abundant, however, in citrus fruits and in many spices and spice oils, including ginger, coriander, cinnamon, mustard, chamomile, sage and thyme (Maarse et al., 1999).
1.2 Estimated daily intake
The total annual volume of production of the 32 flavouring agents in this group is approximately 66 000 kg in Europe (International Organization of the Flavour Industry, 1995) and 66 000 kg in the USA (National Academy of Sciences, 1970, 1975, 1982, 1987, as reported in National Academy of Sciences, 1989; Lucas et al., 1999). More than 95% of the total annual volume of production in Europe and the USA is accounted for by citronellol (No. 1219), citronellal (No. 1220), geraniol (No. 1223), nerol (No. 1224), and citral (No. 1225). Of these, citral accounts for approximately 73% of the total annual volume of production in Europe and 80% in the USA. The estimated daily per capita intakes of citral in Europe and the USA are 6849 mg and 6990 mg, respectively. The daily intakes per capita of all the other flavouring agents in the group are estimated to be in the range of 0.01–945 µg, most values being below 50 µg. The daily per capita intake of each agent in Europe and in the USA is reported in Table 2.
Table 2. Annual volumes of production of aliphatic branched-chain saturated and unsaturated alcohols, aldehydes, acids, and related esters used as flavouring agents in Europe and the USA
|
Flavouring agent (No.) |
Most recent annual volume (kg)a |
Intakeb (‘eaters only’) |
Annual volume in naturally occurring foods (kg)c |
Consumption ratiod |
|
µg/day |
µg/kg bw per day |
|
(+/-) 2-Methyl-1-butanol (1199) |
|
Europe |
NR |
NA |
NA |
|
|
|
USAe |
200 |
35 |
0.6 |
+ |
NA |
|
3-Methyl-2-buten-1-ol (1200) |
|
Europe |
38 |
5.4 |
0.1 |
|
|
|
USA |
29 |
3.8 |
0.1 |
6 156 |
212 |
|
2-Methyl-2-butenal (1201) |
|
Europe |
5 |
0.7 |
0.01 |
|
|
|
USA |
1.4 |
0.2 |
0.003 |
3 871 |
2 765 |
|
3-Methyl-2-butenal (1202) |
|
Europe |
27 |
3.9 |
0.1 |
|
|
|
USAf |
2.7 |
0.5 |
0.01 |
+ |
NA |
|
Ammonium isovalerate (1203) |
|
Europe |
127 |
18 |
0.3 |
|
|
|
USA |
118 |
16 |
0.3 |
- |
NA |
|
3-Methylcrotonic acid (1204) |
|
Europe |
850 |
121 |
2 |
|
|
|
USA |
0.05 |
0.01 |
0.0001 |
+ |
NA |
|
trans-2-Methyl-2-butenoic acid (1205) |
|
Europe |
34 |
4.9 |
0.1 |
|
|
|
USA |
12 |
1.6 |
0.03 |
77 |
6 |
|
Isobutyl 2-butenoate (1206) |
|
Europe |
3.8 |
0.5 |
0.01 |
|
|
|
USA |
340 |
45 |
0.7 |
+ |
NA |
|
2-Methylallyl butyrate (1207) |
|
Europe |
NR |
NA |
NA |
|
|
|
USAf |
1.4 |
0.2 |
0.004 |
- |
NA |
|
4-Methyl-2-pentenal (1208) |
|
Europe |
2 |
0.3 |
0.005 |
|
|
|
USA |
1.8 |
0.2 |
0.004 |
+ |
NA |
|
2-Methyl-2-pentenal (1209) |
|
Europe |
28 |
4 |
0.1 |
|
|
|
USA |
1.8 |
0.2 |
0.004 |
855 |
475 |
|
2-Methyl-2-pentenoic acid (1210) |
|
Europe |
297 |
42 |
0.7 |
|
|
|
USA |
154 |
20 |
0.3 |
+ |
NA |
|
2,4-Dimethyl-2-pentenoic acid (1211) |
|
Europe |
1 |
0.1 |
0.002 |
|
|
|
USA |
0.9 |
0.1 |
0.002 |
- |
NA |
|
2-Methylheptanoic acid (1212) |
|
Europe |
118 |
17 |
0.3 |
|
|
|
USA |
45 |
6 |
0.1 |
+ |
NA |
|
Isobutyl angelate (1213) |
|
Europe |
1 |
0.1 |
0.002 |
|
|
|
USAf |
0.5 |
0.1 |
0.001 |
+ |
NA |
|
2-Butyl-2-butenal (1214) |
|
Europe |
NR |
NA |
NA |
|
|
|
USAf |
0.05 |
0.01 |
0.0001 |
- |
NA |
|
2-Isopropyl-5-methyl-2-hexenal (1215) |
|
Europe |
2 |
0.3 |
0.005 |
|
|
|
USA |
0.05 |
0.01 |
0.0001 |
+ |
NA |
|
2-Ethyl-2-heptenal (1216) |
|
Europe |
0.1 |
0.01 |
0.0002 |
|
|
|
USAf |
0.5 |
0.1 |
0.001 |
- |
NA |
|
2-Methyl-2-octenal (1217) |
|
Europe |
NR |
NA |
NA |
|
|
|
USAe |
45 |
7.9 |
0.1 |
+ |
NA |
|
4-Ethyloctanoic acid (1218) |
|
Europe |
NR |
NA |
NA |
|
|
|
USAe |
23 |
4 |
0.1 |
+ |
NA |
|
dl-Citronellol (1219) |
|
Europe |
2 591 |
370 |
6.2 |
|
|
|
USA |
5 851 |
771 |
13 |
1578 |
0.3 |
|
Citronellal (1220) |
|
Europe |
6625 |
945 |
16 |
|
|
|
USA |
2458 |
324 |
5.4 |
1088 |
0.4 |
|
3,7-Dimethyl-6-octenoic acid (1221) |
|
Europe |
22 |
3.1 |
0.1 |
|
|
|
USA |
1.8 |
0.2 |
0.004 |
+ |
NA |
|
Rhodinol (1222) |
|
Europe |
373 |
53 |
0.9 |
|
|
|
USA |
64 |
8.4 |
0.1 |
- |
NA |
|
Geraniol (1223) |
|
Europe |
4488 |
640 |
11 |
|
|
|
USA |
2390 |
315 |
5.2 |
20856 |
9 |
|
Nerol (1224) |
|
Europe |
2032 |
290 |
4.8 |
|
|
|
USA |
1302 |
171 |
2.9 |
603 |
0.5 |
|
Citral (1225) |
|
Europe |
47997 |
6849 |
114 |
|
|
|
USA |
53070 |
6990 |
117 |
38073 |
0.7 |
|
8-Ocimenyl acetate (1226) |
|
Europe |
NR |
NA |
NA |
|
|
|
USAe |
44 |
7.7 |
0.1 |
- |
NA |
|
2,6-Dimethyl-10-methylene-2,6,11-dodecatrienal (1227) |
|
Europe |
36 |
5.1 |
0.1 |
|
|
|
USA |
3.6 |
0.5 |
0.01 |
+ |
NA |
|
3,7,11-Trimethyl-2,6,10-dodecatrienal (1228) |
|
Europe |
NR |
NA |
NA |
|
|
|
USAe |
1.3 |
0.2 |
0.004 |
+ |
NA |
|
12-Methyltridecanal (1229) |
|
Europe |
NR |
NA |
NA |
|
|
|
USAe |
3 |
0.5 |
0.01 |
- |
NA |
|
Farnesol (1230) |
|
|
|
|
|
|
Europe |
63 |
9 |
0.1 |
|
|
|
USA |
20 |
2.6 |
0.04 |
243 |
12 |
|
Total |
|
|
|
|
|
|
Europe |
65761 |
|
|
|
|
|
USA |
66186 |
|
|
|
|
|
NA, not applicable; NR, not reported; +, reported to occur naturally in foods (Maarse et al., 1999), but quantitative data were not available; -, not reported to occur naturally in foods |
|
a |
From International Organization of the Flavour Industry (1995) and Lucas et al. (1999) or National Academy of Sciences (1989) |
|
b |
Intake expressed as µg/person per day was calculated as follows: [(annual volume, kg) × (1 × 109 µg/kg)/(population × survey correction factor × 365 days)], where population (10%, "eaters only") =32 × 106 for Europe and 26 × 106 for the USA. The correction factor =0.6 for Europe and USA National Academy of Sciences surveys and 0.8 for the USA, representing the assumption that only 60% and 80% of the annual volume of the flavour, respectively, was reported in the poundage surveys (Lucas et al., 1999; International Organization of the Flavour Industry, 1995; National Academy of Sciences, 1989). Intake expressed as µg/kg bw per day calculated as follows: [(µg/person per day)/body weight], where body weight =60 kg. Slight variations may occur from rounding. Quantitative data for the USA reported by Stofberg & Grundschober (1987) |
|
d |
Calculated as follows: (annual consumption in food, kg)/(most recent reported volume as a flavouring agent, kg) |
|
e |
The volume cited is the current anticipated annual volume, which was the maximum amount of flavouring agent estimated to be used annually by the manufacturer at the time the material was proposed for flavour use. National surveys (National Academy of Sciences 1970, 1975, 1982, or 1987 as reported in National Academy of Sciences (1989); Lucas et al., 1999) revealed no reported use of the substance as a flavouring agent at that time. |
|
f |
Annual volume reported in previous USA surveys (National Academy of Sciences 1970, 1975, 1982, or 1987, as reported in National Academy of Sciences (1989)) |
1.3 Absorption, distribution, metabolism, and elimination
The four esters in this group can be expected to be hydrolysed by esterases to the corresponding alcohols and carboxylic acids (Heymann, 1980). Once formed, the latter substances, together with the other alcohols, acids and aldehydes in this group, are readily absorbed from the gastrointestinal tract. On the basis of the results of a study with citral, after absorption the substances can be expected to be distributed rapidly throughout the body, metabolized, and excreted as polar metabolites in the urine, faeces, and expired air. There is no evidence for accumulation in the body (Diliberto et al., 1988).
The substances in this group share common metabolic pathways. Shorter branched-chain aliphatic alcohols, aldehydes, and acids undergo beta-oxidative cleavage to yield intermediates of the amino acid and/or fatty acid metabolic pathways. These intermediates are completely metabolized to CO2 via the tricarboxylic acid cycle. As chain length and substitution increase, the alcohols and aldehydes undergo a combination of omega-, omega-1 and beta-oxidation, and selective dehydrogenation and hydration to yield polar acidic metabolites. Therefore, all of the flavouring agents in this group will eventually be either completely oxidized or oxidized to polar metabolites that are excreted primarily in the urine (Fischer & Bielig, 1940; Williams, 1959; Chadha & Madyastha, 1984; Voet & Voet, 1990; Diliberto et al., 1990).
1.4 Application of the Procedure for the Safety Evaluation of Flavouring Agents
|
Step A1. |
In applying the Procedure for the Safety Evaluation of Flavouring Agents (see Figure 1, Introduction), the Committee assigned all of the flavouring agents in this group to structural class I (Cramer et al., 1978). |
|
Step A2. |
All the flavouring agents in this group are expected to be metabolized to innocuous products. The evaluation of all agents in this group therefore proceeded via the A-side of the decision-tree. |
|
Step A3. |
The estimated daily per capita intakes of 31 of the 32 flavouring agents are below the threshold of concern for structural class I (1800 mg). The Committee concluded that the safety of these 31 flavouring agents raises no concern at their currently estimated levels of intake as flavouring agents. One of the agents, citral (No. 1225), exceeds the threshold of concern for class I. The daily per capita intake of citral is 6849 mg in Europe and 6990 mg in the USA. Accordingly, the evaluation of citral proceeded to step A4. |
|
Step A4. |
Citral is not endogenous in humans. The evaluation of citral therefore proceeded to step A5. |
|
Step A5. |
The no-observed-effect level (NOEL) of 60 mg/kg bw per day for citral (No. 1225) from a 2-year study of carcinogenicity (National Toxicology Program, 2003) is approximately 500 times greater than the estimated intake of citral from its use as a flavouring agent in Europe (114 mg/kg bw per day) and in the USA (117 mg/kg bw per day). The Committee therefore concluded that citral would not pose a safety concern at the currently estimated level of intake. |
Table 1 summarizes the evaluations of the 32 aliphatic branched-chain, saturated and unsaturated alcohols, aldehydes, acids, and related esters (Nos 1199–1230) in this group.
1.5 Consideration of combined intakes from use as flavouring agents
In the unlikely event that all 32 of these flavouring agents were to be consumed concurrently on a daily basis, the estimated combined per capita intake would exceed the human intake threshold for structural class I (1800 µg per day). However, the agents in this group are expected to be metabolized efficiently and the available metabolic pathways would not be saturated. Evaluation of all the data indicated no safety concern associated with combined intake.
1.6 Consideration of secondary components
Seven members of this group of flavouring agents (Nos 1209, 1211, 1219–1223) have minimum assay values of <95%. Information on the safety of the secondary components of these seven compounds is summarized in Annex 6 (Summary of the safety evaluation of secondary components of flavouring agents with minimum assay values of <95%). The secondary components of No. 1209 (propionaldehyde and propionic acid) were evaluated at the forty-ninth meeting (Annex 1, reference 131) and were considered not to present a safety concern at current levels of intake. The secondary component of No. 1211 (4-methyl-2-methylenevaleric acid) has not been evaluated previously; however, a structurally related substance (isovaleric acid) was evaluated at the forty-ninth meeting and considered not to present a safety concern at current levels of intake. Two of the secondary components of No. 1219 (geraniol and citronellal) were evaluated at the present meeting, while the remaining secondary component (citronellyl acetate) was evaluated at the fifty-ninth meeting; none of the secondary components was considered to present a safety concern at current intake levels. One of the secondary components of No. 1220 (eucalyptol) was evaluated at the present meeting, while three other secondary components (linalool, isopulegol, and citronellyl acetate) were evaluated by the Committee at its fifty-first, fifty-fifth and fifty-ninth meetings. None of the secondary components of No. 1220 was considered to present a safety concern on the basis of current intake levels. One of the secondary components of No. 1221 (citronellal) was evaluated at the present meeting, and considered not to present a safety concern at current levels of intake. Some of the secondary components of Nos 1221–1223 (citronellyl, neryl, and geranyl acetate esters) are expected to be hydrolysed to the corresponding terpene alcohols (citronellol, nerol, and geraniol), which were evaluated at the present meeting, and acetic acid, which was evaluated at the forty-ninth meeting. On this basis, none of the secondary components of Nos 1221–1223 was considered to present a safety concern on the basis of current levels of intake.
1.7 Conclusions
The Committee maintained the previously established group ADI of 0–0.5 mg/kg bw, expressed as citral, for citral, citronellol, geranyl acetate, linalool, and linalyl acetate. The Committee noted that the estimated combined intake for citronellol (Table 1), citral (Table 1), geranyl acetate (Annex 1, reference 131), linalool (Annex 1, reference 137), and linalyl acetate (Annex 1, reference 137) is approximately 0.20 mg/kg bw per day in Europe and 0.15 mg/kg bw per day in the USA, and therefore does not exceed the group ADI. It was also noted that even the total combined daily intake of all 32 flavouring agents under evaluation (approximately 0.15 mg/kg bw in Europe and the USA) is less than the group ADI. The Committee concluded that the safety of the flavouring agents in this group of aliphatic branched-chain saturated and unsaturated alcohols, aldehydes, acids, and related esters would not raise concern at the currently estimated levels of intake. The Committee noted that all of the available data on toxicity and metabolism of the flavouring agents in the group were consistent with the results of the safety evaluation.
2. RELEVANT BACKGROUND INFORMATION
2.1 Explanation
The relevant background information summarizes the key scientific data applicable to the safety evaluation of 32 aliphatic branched-chain saturated and unsaturated alcohols, aldehydes, acids, and related esters used as flavouring agents (see Table 1). All members of this group are aliphatic, acyclic, branched-chain substances containing a primary oxygenated functional group and one or more alkyl substituents (for the esters after hydrolysis only). The carbon chain may be saturated or unsaturated. Members of the group that exhibit the highest annual volumes of use as flavouring agents include a series of naturally occurring 10-carbon terpene primary alcohols and aldehydes (citral, geraniol, nerol, citronellol, and citronellal).
2.2 Additional considerations on intake
Volumes of production and intake values for each flavouring agent are reported in Table 2.
Twenty-four of the 32 flavouring agents in the group have been reported to occur naturally in traditional foods (Maarse et al., 1999; Table 2). Quantitative data on natural occurrence data have been reported for 10 flavouring agents in the group (Stofberg & Grundschober, 1987). The consumption of 3-methyl-2-buten-1-ol (No. 1200), 2-methyl-2-butenal (No. 1201), trans-2-methyl-2-butenoic acid (No. 1205), 2-methyl-2-pentenal (No. 1209), geraniol (No. 1223), and farnesol (No. 1230) is derived predominantly from their presence in traditional foods (i.e. they have a consumption ratio >1; Table 2). The remaining four substances, dl-citronellol (No. 1219), citronellal (No. 1220), nerol (No. 1224), and citral (No. 1225) are not consumed primarily from traditional foods (consumption ratio, <1; Table 2).
2.3 Biological data
2.3.1 Biochemical data
(a) Hydrolysis, absorption, distribution, and excretion
The four esters in this group of flavouring agents (Nos 1206, 1207, 1213, and 1226) are expected to be hydrolysed to the corresponding alcohols and carboxylic acids. Hydrolysis of aliphatic esters is catalysed by classes of enzymes recognized as carboxylesterases or esterases, the most important of which are the beta-esterases. In mammals, these enzymes occur in most tissues throughout the body, but they predominate in the hepatocytes (Heymann, 1980). The substrate specificity of beta-carboxylesterases has been correlated with the structure of the alcohol and carboxylic acid moieties (Heyman, 1980).
No hydrolysis data have been provided for the four esters of the present group. However, there are data on hydrolysis in vitro for some related short-chain aliphatic saturated and unsaturated esters indicating that these can be hydrolysed in vivo in the gut to yield the corresponding alcohols and carboxylic acids of the esters prior to absorption (Longland et al., 1977; Grundschober, 1977). Allyl hexanoate, isoamyl butyrate, isoamyl isovalerate, and isoamyl hexanoate were shown to be hydrolysed in artificial gastric juice with half-lives of 1120, 660, 295, and 146 min, respectively, while hydrolysis in artificial pancreatic juice was faster (half-lives of 2, 11, 10, and 38 min, respectively). Preparations of rat liver homogenate and small intestinal mucosa were found to be much more efficient in hydrolysing esters than the artificial gastrointestinal juices: the half-lives for isoamyl butyrate and allyl hexanoate were 0.07–0.5 and 0.1–4 s, respectively (Longland et al., 1977). Grundschober (1977) found that citronellyl acetate (15 µl/l) and allyl hexanoate (60 µl/l) were completely hydrolysed within 2 h by simulated intestinal fluid containing pancreatin at pH7.5.
Data on absorption, distribution, and excretion are available for 2-methyl-1-butanol (No. 1199) and citral (No. 1225).
When rats were given 2-methyl-1-butanol (No. 1199) by intraperitoneal injection in four equal doses of 250 mg/kg bw at 15-min intervals, maximum blood concentration was 550 mg/l. Blood concentrations decreased over the next 9 h. Of the total dose of 1000 mg/kg bw, only 5.6% was excreted in air and 2% in the urine. The remainder was metabolized, first to the corresponding aldehyde and then to the acid (Haggard et al., 1945).
Male Wistar rats and male LACA mice were given a single dose of 14C-labelled citral (No. 1225) at a dose of 5, 770, or 960 mg/kg bw for rats, and 100 mg/kg bw for mice, by gavage. Citral underwent rapid absorption from the gastrointestinal tract and distribution throughout the body, independent of the dose administered. In both species, the radiolabel was excreted rapidly, with most being excreted within 24 h, predominantly in the urine, but also in exhaled air (as 14CO2) and faeces. Excretion was essentially complete by 96 h in rats and by 120 h in mice (Phillips et al., 1976).
Male Fischer F344 rats were given citral labelled with 14C at the C1 and C2 positions in a single oral dose of 5, 50, or 500 mg/kg bw or an intravenous dose of 5 mg/kg bw. After 72 h, the animals were sacrificed and tissues and excreta analysed for radioactivity. Most radiolabel was excreted in the urine, faeces, and expired air as 14CO2 or [14C]citral within 24 h, regardless of the dose or route of administration. At the lowest oral dose, 83% of the radiolabel was recovered within 72 h (51% in urine, 12% in faeces, 17% as expired 14CO2, <1% as expired [14C]citral, and 3% in total tissues). Production of 14CO2 essentially ceased 12 h after treatment, and the amount of 14C found in any tissue was very small (<2%). This excretion profile did not change much with increasing oral dose, although both in this study and that of Phillips et al. (1976) oxidation to CO2 was somewhat greater at the lowest dose.
After intravenous administration, citral was rapidly eliminated from the blood: <25% of the administered dose remained in the blood 2 min after administration. Within 5 min, no unmetabolized citral could be detected in the blood. Elimination of radioactivity from the blood followed three phases: a rapid first phase with an elimination half-life of 11 min, a slower intermediate phase with a half-life of 43 min, and a terminal phase with a half-life of 27 h. Within 72 h after treatment, 79% of the dose was recovered in urine (58%), faeces (7%), expired 14CO2 (8%), expired 14C-citral (<1%), and tissues (6%). Elimination was essentially complete within 24 h. In bile duct-cannulated rats it was shown that approximately 27% of an intravenous dose of 5 mg/kg bw was eliminated via the bile within 4 h of dosing. No unmetabolized citral was detected in the bile. The somewhat greater faecal excretion (4–9%) of citral by the oral route versus the intravenous route suggests that the oral dose was not completely absorbed (Diliberto et al., 1988).
The same authors conducted a study in which multiple doses were administered to ascertain whether citral could induce its own metabolism and thereby affect disposition and excretion. Male rats were treated orally with unlabelled citral at a dose of 5 mg/kg bw per day for 10 days, followed by treatment with [14C]citral in a single oral dose of 5 mg/kg bw for the study of disposition or a single intravenous dose of 5 mg/kg bw for the biliary excretion study. Repeated exposure increased biliary excretion to approximately 36%, but did not affect the disposition pattern of citral in rats (Diliberto et al., 1988).
From these studies it can concluded that citral is rapidly absorbed, metabolized and excreted in the urine, faeces, and expired air. There is evidence of enterohepatic circulation of citral metabolites. Tissue distribution is widespread, but there is no evidence of bioaccumulation.
(b) Metabolism
Upon hydrolysis, the four esters in this group produce short-chain acids (C2–C5; two unsaturated, of which one branched, and two saturated, unbranched) and alcohols (C4–C10; all branched, two saturated and two unsaturated). The group further consists of branched-chain saturated and unsaturated primary alcohols (C5–C15), aldehydes (C5–C15), and acids (C5–C10). The Committee previously reviewed data on the metabolism of linear and branched-chain, saturated and unsaturated aliphatic acyclic alcohols, aldehydes, acids, and related esters (Annex 1, references 132, 138). General aspects of their metabolism have been described (Annex 1, reference 131, 137). Additional relevant data are available on some terpenoid substances of the present group of flavouring agents, mainly geraniol (No. 1223) and citral (No. 1225).
Male IISc rats were given [1-3H]geraniol in daily doses of 800 mg/kg bw by gavage for 20 consecutive days. Five urinary metabolites were identified via two primary pathways. In one pathway, the alcohol is oxidized to yield geranic acid (3,7-dimethyl-2,6-octadienoic acid) which is subsequently hydrated to yield 3, 7-dimethyl-3-hydroxy-6-octenoic acid (3-hydroxy citronellic acid). In a second pathway, the alcohol undergoes selective omega-oxidation of the C8-methyl to yield 8-hydroxygeraniol and 8-carboxygeraniol, the latter of which undergoes further oxidation to the principal urinary metabolite 2,6-dimethyl-2,6-octadienedioic acid (Hildebrandt acid) (Chadha & Madyastha, 1984) (see Figure 1). It was demonstrated that administration of geraniol at a dose of 600 mg/kg bw by gavage for 1, 3 or 6 days induced expression of rat liver microsomal cytochrome P450 and geraniol hydroxylation, but not the activities of rat liver microsomal cytochrome b5, NADPH-cytochrome c reductase, and NADH-cytochrome c reductase, nor the activities of these enzymes in rat lung microsomes (Chadha & Madyastha, 1984). Rabbits are also capable of omega-oxidation of geraniol, as both the Hildebrandt acid and its dihydro form (2,6-dimethyl-2-octendioic acid; reduced or dihydro-Hildebrandt acid) were isolated from the urine of treated animals (Fischer & Bielig, 1940; Asano & Yamakawa, 1950). In both rabbits and rats, the omega-hydroxylation is mediated by the cytochrome P450 system and requires NADPH and oxygen (Licht & Coscia, 1978; Chadha & Madyastha, 1982). It has been demonstrated that not only rat liver microsomes are capable of omega-hydroxylating geraniol, but also rat lung and kidney microsomes (Chadha & Madyastha, 1982).
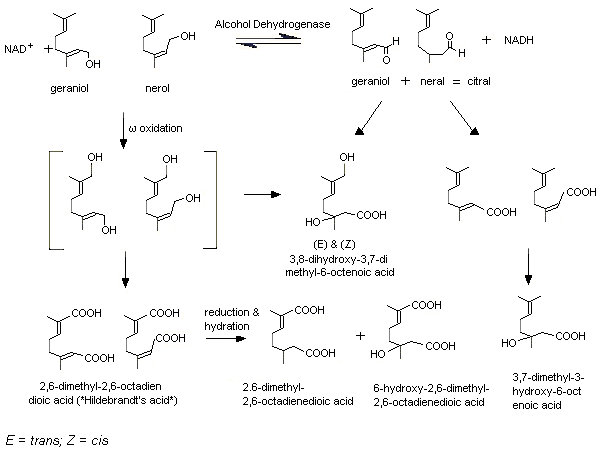
Figure 1. Metabolism of geraniol, nerol, and citral
In rats, citral, a mixture of the corresponding aldehyde of geraniol (geranial) and the aldehyde cis-isomer (neral), is metabolized via similar alcohol and omega-oxidation pathways (Diliberto et al., 1990). In male Fisher 344 rats given [1,2-14C]citral at a dose of 5 or 500 mg/kg bw by gavage, citral was rapidly metabolized and excreted as metabolites. The major metabolite identified in the bile was the glucuronide of geranic acid. In the urine of these rats, several carboxylic acids were identified (e.g. geranic acid, Hildebrandt acid, and dihydro-Hildebrandt acid), resulting from oxidation of the aldehyde function, from omega-oxidation and further reduction and hydration of the unsaturation at C2 (Diliberto et al., 1990) (Figure 1). Hepatic reduction of the aldehyde may precede oxidation pathways, as experiments in vitro revealed that citral is not oxidized by rat hepatic aldehyde dehydrogenase (ALDH) to the corresponding acids. In fact, citral was found to be a potent inhibitor of ALDH-mediated oxidation of acetaldehyde, and was reduced to the corresponding alcohols by rat hepatic alcohol dehydrogenase (ADH). These alcohols could then possibly undergo cytochrome P450-mediated omega-hydroxylation, with the resulting diols being substrates for oxidation (Boyer & Petersen, 1990). Treatment of rats with citral also induced hepatic cytochrome P450 and glucuronyl transferase (Parke & Rahman, 1969).
A similar metabolic fate as that of geraniol and citral was found for nerol (No. 1224), citronellol (No. 1219), citronellal (No. 1220), and citronellic acid (No. 1221). In rabbits given citronellol by gavage, dihydro-Hildebrandt acid and an alcohol precursor (8-hydroxy-3,7-dimethyl-6-octenoic acid) have been reported as urinary metabolites (Fischer & Bielig, 1940). Rat lung microsomes have been shown capable of omega-hydroxylation of citronellol and nerol (Chadha & Madyastha, 1982); a similar reaction has been reported for nerol with rabbit liver microsomes (Licht & Coscia, 1978). In rabbits, citronellic acid was metabolized to dihydro-Hildebrandt acid (Asano & Yamakawa, 1950).
In rabbits, citronellal is metabolized to dihydro-Hildebrandt acid after subcutaneous injection (Asano & Yamakawa, 1950) and oral administration (Ishida et al., 1989). This indicates omega-oxidation. Three other metabolites were found in the urine of rabbits after oral administration: trans- and cis-menthane-3,8-diol and isopregol. These metabolites were the result of cyclization of citronellal, and accounted for <10% of the administered dose. The formation of trans- and cis-menthane-3,8-diol has been confirmed in vitro, after 3 h of incubation of citronellal with fresh gastric fluid isolated from male rabbits (Ishida et al., 1989).
2.3.2 Toxicological studies
(a) Acute toxicity
Oral LD50 values have been reported for 14 of the 32 flavouring agents in this group; two of these substances have been tested in both mice and rats, one only in mice, and the other 11 only in rats (see Table 3). In mice, oral LD50 values ranged from 1150 to 8764 mg/kg bw (Hoffmann-LaRoche, 1967a, b; Schafer & Bowles, 1985). In rats, oral LD50 values ranged from to 810 to >17 000 mg/kg bw, with all but one being >2000 mg/kg bw (Smyth et al., 1954; Yamakawa, 1962; Jenner et al., 1964; Moreno, 1972, 1973, 1974, 1977, 1978, 1980; Sterner & Stiglic, 1976; BASF, 1978; 1981; Rowe & McCollister, 1982). These LD50 values indicate that the acute oral toxicity of aliphatic branched chain saturated and unsaturated alcohols, aldehydes, acids and related esters is low.
Table 3. Studies of acute oral toxicity with aliphatic branched-chain saturated and unsaturated alcohols, aldehydes, acids, andrelated esters
|
No. |
Flavouring agent |
Species |
Sex |
LD50 (mg/kg bw) |
Reference |
|
1199 |
(+/-) 2-Methyl-1-butanol |
Rat |
NR |
4 010 |
Rowe & McCollister (1982) |
|
1200 |
3-Methyl-2-buten-1-ol |
Rat |
NR |
810 |
Moreno (1977) |
|
1205 |
trans-2-Methyl-2-butenoic acid |
Mouse |
NR |
1 150 |
Schafer & Bowles (1985) |
|
1209 |
2-Methyl-2-pentenal |
Rat |
M |
4 290 |
Smyth et al. (1954) |
|
1210 |
2-Methyl-2-pentenoic acid |
Rat |
M |
<5 000 |
Moreno (1980) |
|
1215 |
2-Isopropyl-5-methyl-2-hexenal |
Rat |
NR |
>5 000 |
Moreno (1973) |
|
1219 |
dl-Citronellol |
Rat |
NR |
3 450 |
Moreno (1973) |
|
1220 |
Citronellal |
Rat |
NR |
>5 000 |
Moreno (1973) |
|
1221 |
3,7-Dimethyl-6-octenoic acid |
Rat |
NR |
2 610 |
Moreno (1978) |
|
1222 |
Rhodinol |
Rat |
NR |
>5 000 |
Moreno (1973) |
|
1223 |
Geraniola |
Rat |
M, F |
3 600 |
Jenner et al. (1964) |
|
1223 |
Geraniol |
Rat |
NR |
4 800 |
Yamawaki (1962) |
|
1224 |
Nerol |
Rat |
M |
4 500 |
Moreno (1972) |
|
1225 |
Citral |
Mouse |
M, F |
3 297 |
Hoffmann-LaRoche (1967a) |
|
1225 |
Citral (synthetic) |
Mouse |
M |
2 007 |
Hoffmann-LaRoche (1967b) |
|
1225 |
Citral (refined) |
Mouse |
M, F |
2 464 |
Hoffmann-LaRoche (1967b) |
|
1225 |
Citral |
Rat |
M, F |
4 960 |
Jenner et al. (1964) |
|
1225 |
Citral |
Rat |
NR |
6 800 |
BASF (1978) |
|
1230 |
Farnesol |
Mouse |
M, F |
8 764 |
Hoffmann-LaRoche (1967a) |
|
1230 |
Farnesol |
Rat |
M, F |
>5 000 |
BASF (1981) |
|
1230 |
Farnesol |
Rat |
NR |
>5 000 |
Moreno (1974) |
|
1230 |
Farnesol |
Rat |
M, F |
>20 ml/kg (17 742b) |
Sterner & Stiglic (1976) |
|
M, male; F, female; NR, not reported |
|
a |
Geraniol extra (a mixture of 3,7-dimethyl-2,6-octadienol and 3,7-dimethyl-1,6-octadienol) |
|
b |
Calculated using a density of 0.8871 (Merck, 1997) |
(b) Short-term studies of toxicity
Short-term studies of toxicity were available for four of the 32 substances in this group (Oser 1958 a, b; Hagan et al., 1967; Posternak, 1968; Dieter et al., 1993; National Toxicology Program, 2003). The results of these studies are summarized in Table 4 and described below.
Table 4. Results of short-term studies of toxicity and long-term studies of toxicity and carcinogenicity with aliphatic branched-chain unsaturated alcohols, aldehydes, acids, and related esters
|
No. |
Flavouring agent |
Species; sex |
No. of test groupsa/ no. per groupb |
Route |
Duration |
NOEL (mg/kg bw) per day |
Reference |
|
Short-term studies of toxicity |
|
1211 |
2,4-Dimethyl-2-pentenoic acid |
Rat; M, F |
1/28 |
Dietc |
13 weeks |
1.36 (M)d
1.55 (F)d |
Posternak (1968) |
|
1219 |
dl-Citronellole |
Rat; M, F |
1/20 |
Diet |
12 weeks |
51 (M)d,f
56 (F)d,f |
Oser (1958b) |
|
1223 |
Geraniolg |
Rat; M, F |
1/10 |
Diet |
16 weeks |
500d |
Hagan et al. (1967) |
|
1223 |
Geraniolg |
Rat; M, F |
1/10 |
Diet |
27–28 weeks |
50d |
Hagan et al. (1967) |
|
1225 |
Citral |
Mouse; M, F |
3/10 |
Gavageh |
12 days |
<534 |
Dieter et al. (1993) |
|
1225 |
Citral |
Mouse; M, F |
5/10 |
Dieti |
14 days |
4275 |
Dieter et al. (1993) |
|
1225 |
Citral |
Mouse; M, F |
4/20 |
Dieti |
14 weeks |
<745 (M)
<790 (F) |
National Toxicology Program (2003) |
|
1225 |
Citral |
Rat; M, F |
3/10 |
Gavageh |
12 days |
1140 (M)
2280 (F)d |
Dieter et al. (1993) |
|
1225 |
Citral |
Rat; M, F |
5/10 |
Dieti |
14 days |
570 |
Dieter et al. (1993) |
|
1225 |
Citralj |
Rat; M, F |
1/21 |
Diet |
12 weeks |
52 (M)d,k
60 (F)d,k |
Oser (1958a) |
|
1225 |
Citral |
Rat; M, F |
3/20 |
Diet |
13 weeks |
500d,l |
Hagan et al. (1967) |
|
1225 |
Citral |
Rat; M, F |
4/20 |
Dieti |
14 weeks |
<345 (M)
675 (F) |
National Toxicology Program (2003) |
|
Long-term studies of toxicity and carcinogenicity |
|
1225 |
Citral |
Mouse; M, F |
3/100 |
Dieti |
104–105 weeks |
120 (M)
60 (F) |
National Toxicology Program (2003) |
|
1225 |
Citral |
Rat; M, F |
3/100 |
Dieti |
104–105 weeks |
100 |
National Toxicology Program (2003) |
| |
Geranyl acetate/Citronellyl acetatem |
Mouse; M, F |
2/100 |
Gavageh |
103 weeks |
—n |
National Toxicology Program (1987) |
| |
Geranyl acetate/Citronellyl acetatem |
Rat; M, F |
2/100 |
Gavageh |
103 weeks |
1000 (710/290) |
National Toxicology Program (1987) |
|
M, male; F, female; NR, not reported |
|
a |
Does not include control animals |
|
b |
Includes both male and female animals |
|
c |
Administered in an emulsion with gum arabic |
|
d |
As neither a single dose nor multiple doses had any adverse effects, this dose is not a true NOEL but the highest dose tested that had no adverse effects. The actual NOEL may be higher. |
|
e |
Administered as a mixture of citronellol and linalool (1 : 1, w/w) |
|
f |
Dose given for citronellol |
|
g |
Administered as geraniol extra (mixture of 3,7-dimethyl-2,6-octadienol and 3,7-dimethyl-1,6-octadienol) |
|
h |
Administered in corn oil |
|
i |
Administered microencapsulated in the diet |
|
j |
Administered as a mixture of citral and citral diethyl acetal (1 : 1, w/w) |
|
k |
Dose given for citral |
|
l |
Measurement of the concentration of citral in the diet revealed a loss of 58% during 1 week due to the volatility of the test substance |
|
m |
Structurally related terpenoid esters administered as a mixture: geranyl acetate, 71%; citronellyl acetate, 29% |
|
n |
There was no NOEL owing to dosing errors and low survival associated with infections |
(i) Citral (No. 1225)
Mice
Groups of five male and five female B6C3F1 mice (aged 28 days) were given citral in corn oil by gavage for 12 days or fed microencapsulated citral in the diet for 14 days. The microencapsulated citral was administered at concentrations of 0, 0.63, 1.25, 2.5, 5, or 10% (w/w), to achieve average daily intakes of 0, 534, 1068, 2137, 4275, or 8550 mg/kg bw, respectively. The microcapsules were composed of a sugar and starch mixture; the chemical load of citral in the microcapsules being 37.8%, with a geranial : neral ratio of approximately 2 : 1. In the study in mice treated by gavage, the doses used were equivalent to the three lower doses achieved in the study in mice given citral in the diet, i.e. 534, 1068, and 2137 mg/kg bw. Animals were weighed at the beginning of the study and on days 4, 7, 11, and 14. Food consumption and clinical signs of toxicity were recorded on the same days, starting on day 4. At the end of the period of treatment, necropsy was performed on all. Organ weights were obtained for the right kidney, liver, and spleen, and histological examinations were performed on lung, liver, kidney, spleen, nasal cavity, stomach, brain, heart, as well as on all gross lesions.
In the study of mice treated by gavage, all mice at 2137 mg/kg bw per day and two males at 1068 mg/kg bw per day died before completion of the study. Final body weights and weights of kidney and spleen were not affected, but there was a dose-related increase in liver weights in both females (approximately 20–35%) and males (approximately 10–30%). Cytoplasmic fatty vacuolization of hepatocytes was reported in females in the groups receiving the intermediate and highest doses and in males receiving the highest dose. Necrosis, ulceration, and acute inflammation of the forestomach were observed in mice receiving the highest dose, and inflammation and/or hyperplasia of the forestomach were observed in approximately half the animals receiving the intermediate dose. According to the authors, this direct irritating effect of citral is consistent with reports for other chemicals that cause irritation of the skin and mucous membranes. The NOEL was <534 mg/kg bw per day on the basis of increased liver weights.
No mortality occurred during the study in mice given diets containing citral. In male mice, a transient decrease in food consumption was noted in the group receiving the diet containing 10% citral at day 4, and at day 7; by days 11 and 14, however, food consumption was above that of the control animals, as it was for males receiving the diets containing 2.5 and 5% citral. Higher food intakes were also noted in females at the three highest doses as the study progressed. Significant reductions in body-weight gain in all animals at the highest dose were observed at day 4, and although male mice recovered, the final body weights of animals of both sexes at the highest dose were significantly lower (approximately 10%) than those of the controls. The authors associated the failure of female mice to recover with food scattering or inefficient food use. Upon histopathological examination, no treatment-related lesions were reported at any dose. The NOEL for citral was 4275 mg/kg bw per day, on the basis of decreased body weights (Dieter et al., 1993).
In a 14-week study of toxicity, groups of 10 male and 10 female B6C3F1 mice (aged 6 weeks) were given diets containing microencapsulated citral (purity, 97.5%) at a concentration of 3900, 7800, 15 600, or 31 300 mg/kg of diet. Additional groups received untreated diet (untreated controls) or diet containing placebo microcapsules (vehicle controls). The concentrations used provided average daily intakes of 745, 1840, 3915, and 8110 mg/kg bw for males and 790, 1820, 3870, and 7550 mg/kg bw for females. The microcapsules were composed of a mixture of sugar and starch, and contained 31.3% citral, with a geranial : neral ratio of approximately 2 : 1. Animals were observed twice daily for general health and behaviour, and clinical findings were recorded weekly. Body weight was measured at the start of the study and then weekly until termination of the study. Food consumption was recorded twice weekly for females and weekly for males. At termination, all animals were necropsied and weights of heart, right kidney, liver, lung, right testis, and thymus were recorded. A complete histopathological examination was performed on animals in the group receiving the highest dose (31 300 mg/kg bw), and on the animals in the untreated and vehicle control groups. In addition, histopathological examination of the forestomach and ovaries of animals at lower doses was conducted in order to identify a NOEL. Blood for haematological analysis was taken from all mice surviving to the end of the study. Clinical chemistry was not performed. The study complied with good laboratory practice (GLP) guidelines.
In the second week of the study, four male mice in the group receiving the highest dose were killed in a moribund condition. There was a significant and dose-related decrease in final mean body weights and body-weight gains at all doses (final body weights deceased by 15–48% in males and by 12–45% in females). At 15 600 and 31 300 mg/kg bw, mice were generally thin and lethargic; a few males at 7800 mg/kg bw were also thin. Compared with controls, food consumption of animals at all doses increased during treatment, probably due to scattering of feed because of poor palatability. Lymphocyte and leukocyte counts were reduced in males at all doses, and females at the two highest doses. Organ weight changes at all doses reflected the differences in body weights between groups. Significant increases in the incidences of mild forestomach hyperkeratosis and epithelial hyperplasia were observed in females at 15 600 mg/kg bw (5 out of 10) but not at 31 300 mg/kg bw (0 out of 10). These lesions were also observed in one male at 31 300 mg/kg bw (1 out of 10). Incidences of ovarian atrophy were significantly increased at 15 600 mg/kg bw (moderate atrophy) and at 31 300 mg/kg bw (marked atrophy). The diagnosis of atrophy was based on an absence or reduction in the number of corpora lutea with no effect on primary, secondary, or antral follicles. However, upon review by the National Toxicology Program Pathology Working Group, the lesions were characterized as hypoplasia and were considered to be a secondary effect resulting from the poor condition of the treated female mice. The NOEL for citral was <745 mg/kg bw per day in male mice on the basis of decreased body weights and body-weight gains, and reductions in lymphocyte and leukocyte counts. In female mice, the NOEL was <790 mg/kg bw per day on the basis of decreased body weights and body-weight gains (National Toxicology Program, 2003).
Rats
Groups of five male and five female Fischer 344/N rats (aged 28 days) were given citral in corn oil by gavage for 12 days or fed diets containing microencapsulated citral for 14 days. Diets contained microencapsulated citral at a concentration of 0, 0.63, 1.25, 2.5, 5, or 10% (w/w), calculated to achieve average daily intakes of 0, 142, 285, 570, 1140, or 2280 mg/kg bw, respectively. The microcapsules were composed of a mixture of sugar and starch; the chemical load of citral in the microcapsules was 37.8%, with a geranial : neral ratio of approximately 2 : 1. In the study of rats treated by gavage, the doses administered were equivalent to the three highest doses achieved in the feeding study, i.e. 570, 1140, and 2280 mg/kg bw. Animals were weighed at the beginning of the study and on days 4, 7, 11, and 14. Food consumption and clinical signs of toxicity were recorded on the same days, starting on day 4. At the end of the period of treatment, necroscopy was performed on all animals. Organ weights were obtained for the right kidney, liver, and spleen, and histological examinations were performed on lung, liver, kidney, spleen, nasal cavity, stomach, as well as on all gross lesions.
In the study in rats treated by gavage, all rats survived the duration of the study, and neither final body weights nor organ weights were affected by treatment. Upon histopathological examination, only mild hyperplasia of the squamous epithelium of the forestomach in two males receiving the highest dose was observed. The NOEL for citral was 1140 mg/kg bw per day in male rats on the basis of forestom-ach lesions. In female rats, the NOEL for citral was 2280 mg/kg bw per day, the highest dose tested.
No mortality occurred during the study in rats given diets containing citral. Reduced food consumption was observed at day 4 in both sexes given diets containing 5% and 10% citral. This reduction persisted at day 7 in animals given 10% citral, but was absent or only minimal thereafter. Significant reductions in body-weight gain in males and females given the two highest doses were observed from day 4, and although both male and female rats recovered, the final body weights of both sexes at these doses were significantly lower than those of controls (in males, approximately 19–35%, in females, approximately 12–32%). In addition, at the highest dose the absolute weights of the liver, kidney and spleen were decreased in males and females. The only histopathological finding in rats was minimal to mild hyperplasia and/or squamous metaplasia of the respiratory epithelium of the anterior portion of the nasal passages of rats at 5% and 10% citral. There was no inflammatory response associated with these changes, and there was no evidence of atypia or keratinization of cells. The authors noted that these morphological changes were similar to those produced by other, previously tested irritant chemicals. The NOEL for citral was 570 mg/kg bw per day on the basis of nasal lesions and decreased body weights (Dieter et al., 1993).
Groups of 10 male and 11 female weanling rats (aged 27–29 days old) were given diets containing equal parts (by weight) of citral and citral diethyl acetal at an intended dose of 0, or 100 mg/kg bw per day (50 mg of each substance) for 12 weeks. The actual average intake was 104 mg/kg bw per day (52 mg of each substance) for males and 119 mg flavour/kg bw per day (60 mg of each substance) for females.
Treatment did not influence physical appearance and behaviour. Weekly measurements of body weight, food consumption, and efficiency of food use revealed slight (not statistically significant) decreases in these parameters in treated males but not in females. Limited urine analysis and haematology performed for three rats of each sex per group revealed no differences in concentrations of urinary sugar and albumin and in blood haemoglobin. At necropsy, gross examination of all animals was performed and weights of liver and kidney were recorded. No differences were found between control and treated animals. Histopathology was not performed, although tissues were preserved at necropsy. The NOEL for citral was 52 mg/kg bw per day for male rats and 60 mg/kg bw per day for female rats, the highest dose tested (Oser, 1958a).
Groups of 10 male and 10 female weanling Osborne-Mendel rats were given diets containing citral at a concentration of 0, 1000, 2500, or 10 000 mg/kg of diet, calculated (Food & Drug Administration, 1993) to provide average daily intakes of 0, 50, 125, and 500 mg/kg bw, respectively, for 13 weeks. However, the actual intakes of citral were lower; measurements of the concentration of citral in the diet revealed a 58% loss during one week, due to the volatility of the test substance. Body weight, food intake and general condition were recorded weekly. Haematological examinations carried out at termination of the study included measurements of leukocyte and erythrocyte counts, haemoglobin and erythrocyte volume fraction. At necropsy, all animals were examined macroscopically and liver, kidneys, heart, spleen, and testes were weighed. For three to four rats of each sex per group for the group receiving the highest dose and the controls, these organs, together with abdominal and thoracic viscera and bone, bone marrow, and muscle from one hind leg, were preserved and subjected to histopathological examination.
No treatment-related effects on growth, haematology and organ weights were observed, and there were no macroscopic or microscopic changes in the tissues. The NOEL for citral was 500 mg/kg bw per day, the highest dose tested (Hagan et al., 1967).
In a 14-week study of toxicity, groups of 10 male and 10 female Fischer 344/N rats (aged 6 weeks) were given diets containing microencapsulated citral (purity, 97.5%) at a concentration of 3900, 7800, 15 600, or 31 300 mg/kg of diet. Additional groups received untreated diet (untreated controls) or diet containing placebo microcapsules (vehicle controls). Diets containing citral at the three lower concentrations provided average daily intakes of 345, 820, and 1785 mg/kg bw for males and 335, 675, and 1330 mg/kg bw for females. All rats in the group receiving the highest were killed in a moribund condition in the second week of the study and the daily average intake was not calculated for these animals. The microcapsules were composed of a mixture of sugar and starch, and contained 31.3% citral, with a geranial : neral ratio of approximately 2 : 1. Animals were observed twice daily for general health and behaviour, and clinical findings were recorded weekly. Weight measurements were taken initially and then weekly until termination of the study. Food consumption was recorded twice weekly. Blood was taken for haematology and clinical chemistry analysis at days 4 and 22 from rats in the clinical pathology part of the study, and at week 14 from all surviving animals involved in the core part of the study. At termination, all rats in the core study were necropsied and weights of heart, right kidney, liver, lung, right testis, and thymus were recorded. A full histopathological examination was conducted on animals in the groups of untreated and vehicle controls, and on animals in the groups receiving 15 600 or 31 300 mg/kg. In addition, histopathological examination was carried out on the bone marrow, forestomach, and kidneys (male rats only) of animals in groups at lower doses, in order to identify a NOEL. The study complied with GLP guidelines.
Clinical signs of toxicity observed at 31 300 mg/kg included listlessness, hunched posture, absent or slow paw reflex, and dull eyes. Compared with control groups, there was a dose-related decrease in final mean body weights and body-weight gains for all surviving males (by 5–27%) and females (by 4–12%). Food consumption at the two highest doses (15 600 and 31 300 mg/kg) was significantly reduced during the first week of the study, possibly due to poor palatability. As the study progressed, food consumption increased in all groups, including controls. Several transient effects on haematological and clinical chemistry parameters were observed, mainly at the highest dose, but sometimes at all doses (in a dose-related manner). Most changes were consistent with physiological responses related to decreased food and possibly water consumption. Minor changes in organ weights appeared to be related to changes in body weight. At necropsy, no treatment-induced gross lesions were observed; however, microscopic evaluation revealed increased incidences of forestomach hyperplasia and hyperkeratosis at 31 300 mg/kg, reaching levels of statistical significance in females. Significant, but slight bone marrow atrophy was seen at the two higher doses (15 600 and 31 300 mg/kg), which was accompanied at the highest dose accompanied by bone marrow haemorrhage. Three out of 10 males at the lowest dose (3900 mg/kg) and all males at 7800 and 15 600 mg/kg exhibited minimal to mild nephropathy and granular casts of the renal tubules. Increased incidences of nephropathy were not associated with an increase in protein droplets. The authors concluded that the observed renal lesions were unlikely to be mediated by alpha2-microglobulin. In addition to these effects, atrophy of the thymus was observed in males and females at 31 300 mg/kg; males in this group also had aspermia in the testes. The NOEL for citral was <345 mg/kg bw per day in male rats, on the basis of nephropathy. In female rats, the NOEL was 675 mg/kg bw per day, on the basis of decreased body weights and body-weight gains, and bone marrow atrophy (National Toxicology Program, 2003).
(ii) 2,4-Dimethyl-2-pentenoic acid (No. 1211)
In a 13-week study of toxicity, groups of 14 male and 14 female Sprague Dawley CD rats were given diets containing 2,4-dimethyl-2-pentenoic acid at a concentration of 11, 19, and 22 mg/kg of diet at weeks 0–4, 5–10, and 11–13, respectively. The substance was added to the diet in an emulsion with gum arabic. The average daily intake of 2,4-dimethyl-2-pentenoic acid during the experiment was 1.36 mg/kg bw per day for males and 1.55 mg/kg bw per day for females. Control animals received a basal diet containing the gum arabic emulsion without the test material.
Daily observation for clinical signs revealed no treatment-related effects. Weekly measurement of food consumption, body weight, and efficiency of food use showed a statistically significant decrease of 10% in efficiency of food use in treated males compared with that in the control group. However, body weights of these males were only slightly lower than those of controls, while food consumption was slightly increased (both not statistically significantly). Haematological examinations and clinical chemistry (blood urea) determinations conducted on seven animals of each sex per group during week 7 and on all animals during week 13 of the study revealed increases in blood concentration of urea in treated males and females in week 7 (statistically significantly only in females) and in treated males in week 13. Leukocyte counts were significantly increased in treated females at both time-points. The toxicological significance of these changes is unclear. At the end of the period of treatment, all animals were killed, detailed necropsies were performed, and liver and kidney weights were measured. There was no difference in organ weight between treated and control groups for either sex. Gross and histopathological examination revealed no evidence of treatment-related alterations. The NOEL for 2,4-dimethyl-2-pentenoic acid was 1.36 mg/kg bw per day in male rats and 1.55 mg/kg bw per day in female rats, the highest dose tested (Posternak, 1968).
(iii) dl-Citronellol (No. 1219)
Groups of 10 male and 10 female weanling rats (aged 27–29 days) were given diets containing equal parts (by weight) of citronellol and linalool at concentrations intended to provide a dose of 0, or 100 mg/kg bw per day (50 mg of each substance) for 12 weeks. The actual average intake was 102 mg/kg bw per day (51 mg of each substance) for males and 112 mg/kg bw per day (56 mg of each substance) for females.
Treatment did not influence physical appearance and behaviour. Weekly measurements revealed slight, but significant reductions in body-weight gain in males after 4 weeks; although these animals recovered, the final body weight and the net body-weight gain were lower than those of controls (approximately 10%). Food consumption and food use efficiency were also slightly decreased (approximately 5%) in treated males. These parameters were not affected in treated females. The authors attributed the effects in males to reduced palatability of the diet, and considered these effects to be biologically insignificant. Limited urine analysis and haematology in three rats of each sex per group revealed no differences in concentrations of urinary sugar and albumin and in blood haemoglobin. At necropsy, gross examination of all animals was performed and weights of liver and kidney were recorded. No differences were found between treated animals and controls. Histopathology was not performed, although tissues were preserved at necropsy. The NOEL for citronellol was 51 mg/kg bw per day in male rats and 56 mg/kg bw per day in female rats, the highest dose tested (Oser, 1958b).
(iv) Geraniol (No. 1223)
Groups of five male and five female weanling Osborne-Mendel rats were given diet containing geraniol extra, a mixture of 3,7-dimethyl-2,6-octadienol and 3,7-dimethyl-1,6-octadienol, at a concentration of 10 000 mg/kg of diet, calculated (Food & Drug Administration, 1993) to provide an average daily intake of 500 mg/kg bw, for 16 weeks. In a second study, the same strain and number of animals were given diet containing geraniol extra at a concentration of 1000 mg/kg of diet, calculated (Food & Drug Administration, 1993) to provide an average daily intake of 50 mg/kg bw, for 27–28 weeks. A control group of 10 male and 10 female rats was used in each study. Body weight, food intake and general condition were recorded weekly. Haematological examinations made at the termination of the studies included measurements of leukocyte and erythrocyte counts, haemoglobin and erythrocyte volume fraction. At necropsy, all animals were examined macroscopically and liver, kidneys, heart, spleen, and testes were weighed. For three to four rats of each sex per group, these organs along with abdominal and thoracic viscera and bone, bone marrow, and muscle from one hind leg were preserved and subjected to histopathological examination.
In both studies, no treatment-related effects on growth, haematological parameters or organ weights, or on macroscopic or microscopic changes in the tissues were observed. The NOELs for geraniol were 500 and 50 mg/kg bw per day in the first and second study, respectively, the highest doses tested (Hagan et al., 1967).
(c) Long-term studies of toxicity and carcinogenicity
Studies of carcinogenicity were only available for one of the substances under evaluation: citral (National Toxicology Program, 2003). The results of these studies are summarized in Table 4 and described below. However, studies of carcinogenicity in mice and rats have also been performed with a mixture of geranyl acetate (71%) and citronellyl acetate (29%) (National Toxicology Program, 1987), two acetate esters that are expected to be hydrolysed rapidly to geraniol and citronellol, respectively. These studies were considered by the Committee at its forty-ninth meeting (Annex 1, reference 131, 132) in the safety evaluation of a group of 26 esters derived from branched-chain terpenoid alcohols and aliphatic acyclic linear and branched-chain carboxylic acids. The Committee decided that a study of carcinogenicity, in which groups of 50 male and 50 female B6C3F1 mice received this mixture of geranyl acetate (71%) and citronellyl acetate (29%) in corn oil at a dose of 0, 500, or 1000 mg/kg bw per day, 5 days per week for 103 weeks by gavage, was inadequate, due to dosing errors and the low survival associated with infections. In groups of 50 male and 50 female Fischer 344/N rats given this mixture at a dose of 0, 1000 or 2000 mg/kg bw per day, 5 days per week for 103 weeks by gavage, the NOEL for the mixture was 1000 mg/kg bw per day, corresponding to an estimated dose of 710 mg/kg bw per day of geranyl acetate and 290 mg/kg bw per day of citronellyl acetate.
(i) Citral (No. 1225)
Mice
In a study of carcinogenicity, conducted in compliance with GLP, groups of 50 male and 50 female B6C3F1 mice (aged 6 weeks) were fed diets containing microencapsulated citral (purity, 94%) at a concentrations of 0 (untreated control), 0 (vehicle control receiving diet with placebo microcapsules), 500, 1000, or 2000 mg/kg of diet for 104–105 weeks. These concentrations provided average daily intakes of approximately 0, 0, 60, 120, and 260 mg/kg bw per day, respectively. The microcapsules, 50–100 µm in diameter were composed of a mixture of sugar and starch and contained 32.3% citral, with a geranial:neral ratio of approximately 2 : 1. The diets (NTP-2000) used in the study contained less protein and more fibre and fat than diets (NIH-07) previously used in 2-year studies conducted by the National Toxicology Program.
Clinical findings and body weights were monitored on days 0 (body weights only), 8, and 36 and then every 4 weeks until the end of the study. Seven-day food consumption was measured every 4 weeks during the study. Gross and histopathologic examinations were performed on all animals at termination of the study.
Survival in the treated groups of male and female mice was similar to that in the vehicle control group. No clinical signs of toxicity attributable to citral exposure were observed. Mean body weights were generally (statistical significance not specified) lower than mean body weights of the vehicle controls throughout the study in both sexes at the highest dose (males, 4–17%; females, 2–22%). At lower doses, mean body weights were only slightly lower (males, <10%; females, up to 12% at the intermediate dose and <10% at the lowest dose). There was no difference in food consumption between treated and control groups. The incidence of malignant lymphoma was increased in treated female mice (positive trend, with borderline statistical significance at the high dose, p =0.011): untreated control, 4/50 (8%): vehicle control, 3/49 (6%); 500 mg/kg, 5/50 (10%); 1000 mg/kg, 9/50 (18%); 2000 mg/kg, 12/50 (24%). This condition predominantly affected the spleen, mesentric lymph nodes, thymus and to a lesser extent the ovaries. There was no difference in the incidence of malignant lymphoma between treated and vehicle control male mice. A significant positive trend was identified in the incidence of hepatocellular adenoma in treated females. However, pairwise comparison with vehicle controls was not statistically significant, nor were significant trends for hepatocellular carcinoma or for hepatocellular adenoma and carcinoma (combined) noted. Therefore, the positive trend in incidence of hepatocellular adenomas in female mice is presumed to be unrelated to treatment. Inflammation and ulceration of the oral mucosa was seen in all mice, including vehicle controls, and this was associated with penetration of hair shafts into the mucosa and/or tooth sockets. Although the incidences of inflammation in males at the highest dose and inflammation and ulceration in all treated females were significantly increased as compared with controls, it is mentioned by the authors that this is probably not a direct toxic effect attributable to citral. In female mice, increases in the incidences of minimal nephropathy at all doses and in renal tubule mineralization at the lowest and intermediate doses were observed; the toxicological significance of these effects is unclear. Furthermore, similar effects were not observed in the 14-week study in mice. On the basis of decreased body weights, the NOEL for citral was 60 mg/kg bw per day in female mice and 120 mg/kg bw per day in male mice (National Toxicology Program, 2003).
In the National Toxicology Program report, the association between malignant lymphoma in female mice and treatment with citral is questioned. Arguments that support such an association are: (1) a positive trend; (2) statistical significance at the highest dose; (3) incidences that exceed those for historical controls in all but one study using the NTP-2000 diet (range, 6–18% in nine studies, 32% in one study); (4) statistical significance at the two higher doses when compared to the combined data for untreated controls and vehicle controls; and (5) the detection of lymphomas in multiple tissues. Arguments that weaken the association are also mentioned in the National Toxicology Program report: (a) the incidence of malignant lymphoma at the highest dose was within the ranges for historical controls for female mice given the NTP-2000 and NIH-07 diets (range, 6–32% and 6–30%, respectively); and (2) the incidence of malignant lymphoma in the vehicle controls was at the lower end of the ranges for historical controls. Overall, the National Toxicology Program concluded that "under the conditions of this 2-year feed study there was no evidence of carcinogenic activity of citral in male B6C3F1 mice exposed to 500, 1000, or 2000 ppm. There was equivocal evidence of carcinogenic activity in female B6C3F1 mice based on increased incidences of malignant lymphoma" (National Toxicology Program, 2003).
Rats
In a study of carcinogenicity, conducted in compliance with GLP, groups of 50 male and 50 female Fischer 344/N rats (aged 6 weeks) were fed diets containing microencapsulated citral (purity, 94%) at a concentration of 0 (untreated control), 0 (vehicle control receiving feed with placebo microcapsules), 1000, 2000, or 4000 mg/kg of diet for 104–105 weeks. These concentrations provided average daily intakes of 0, 0, 50, 100, and 210 mg/kg bw per day, respectively. Food analysis for concentrations of citral are as specified in the 2-year study in mice (see above). Clinical findings and body weights were monitored on days 0 (body weights only), 8, and 33 and then every 4 weeks until the end of the study. Seven-day food consumption was measured every 4 weeks during the study. Gross and histopathologic examinations were performed on all animals at termination of the study.
Survival of males in all treated groups was greater than that in the vehicle control group. Survival of treated females was similar to that of vehicle controls. No clinical signs of toxicity were observed. Mean body weights of females and males at the highest dose were slightly lower than those of the vehicle controls after week 25 (6–14% and 4–8%, respectively; statistical significance not specified). There was no difference in food consumption between treated and control groups. No neoplastic or non-neoplastic lesions were observed that could be associated with the administration of citral in the diet. There were, however, significant decreases in the incidences of clitoral gland hyperplasia, adenoma, and adenomas or carcinomas (combined) in female rats at 4000 mg/kg. There also was a dose-related decrease in the incidence of mammary gland fibroadenomas in female rats (statistically significantly at 4000 mg/kg). A dose-dependent increase in the incidence of kidney mineralization was observed in males; however, this was also observed in 84% of the males in the vehicle control group, and consequently, it was considered that citral only exacerbated these otherwise spontaneously occurring lesions. Furthermore, similar effects were not observed in the 14-week study in rats. Overall, the National Toxicology Program concluded that "under the conditions of this 2-year feed study there was no evidence of carcinogenic activity of citral in male or female F344/N rats exposed to 1000, 2000, or 4000 ppm". The NOEL for citral was 100 mg/kg bw per day on the basis of decreased body weights (in particular in females) (National Toxicology Program, 2003).
(d) Genotoxicity
Testing for genotoxicity has been carried out with six of the 32 flavouring agents in this group (Nos 1209, 1219, 1220, 1223, 1225, and 1230). The results of these tests are summarized in Table 5 and described below.
Table 5. Results of studies of genotoxicity with aliphatic branched-chain saturated and unsaturated alcohols, aldehydes, acids, and related esters
|
No. |
Flavouring agent |
End-point |
Test object |
Dose or Concentration |
Result |
Reference |
|
In vitro |
|
1209 |
2-Methyl-2-pentenal |
Reverse mutation |
S. typhimurium TA98, TA100 |
0.03–3 µmol/plate
(2.94–294 µg/plate)a |
Negativeb |
Florin et al. (1980) |
|
1219 |
dl-Citronellol |
Reverse mutation |
S. typhimurium TA98 and TA100 |
0.05–100 µl/plate
(0.04–85 µg/plate)c |
Negatived |
Rockwell & Raw (1979) |
|
1219 |
dl-Citronellol |
Rec assay |
B. subtilis M45 and H17 |
17 µg/disk |
Negative |
Oda et al. (1979)e |
|
1220 |
Citronellal |
Reverse mutation |
S. typhimurium TA98, TA100, TA97a, TA102 |
1–300 µg/plate |
Negativeb |
Gomes-Carneiro et al.(1998) |
|
1220 |
Citronellal |
Reverse mutation |
S. typhimurium TA98 and TA 100 |
0.05–500 µg/plate |
Negativeb |
Kasamaki et al. (1982) |
|
1220 |
Citronellal |
Sister chromatid exchange |
Chinese hamster ovary cells |
3.3–100 µmol/l
(0.51–15.4 µg/ml)f |
Negativeg |
Sasaki et al. (1989) |
|
1220 |
Citronellal |
Chromosomal aberration |
Chinese hamster B241 cells |
50 nmol/l
(0.008 µg/ml)f |
Weakly positiveb |
Kasamaki et al.(1982) |
|
1220 |
Citronellal |
Rec assay |
B. subtilis M45 and H17 |
17 µg/disk |
Negative |
Oda et al. (1979)e |
|
1223 |
Geraniol |
Reverse mutation |
S. typhimurium TA 100 |
0.01–1.0 µl
(8.89–889 mg/tube)h |
Negativeb |
Eder et al. (1980) |
|
1223 |
Geraniol |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537 |
3 µmol/plate
(463 µg/plate)f |
Negativeb |
Florin et al. (1980) |
|
1223 |
Geraniol |
Reverse mutation |
S. typhimurium TA92, TA94, TA98, TA100, TA1535, TA1537 |
<500 µg/plate |
Negatived |
Ishidate et al.(1984) |
|
1223 |
Geraniol |
Sister chromatid exchange |
Chinese hamster ovary cells |
33.3–333 µmol/l
(5.14–51.4 µg/ml)f |
Negativeg |
Sasaki et al. (1989) |
|
1223 |
Geraniol |
Chromosomal aberration |
Chinese hamster fibroblast cells |
<125 µg/ml |
Negativeg,i |
Ishidate et al.(1984) |
|
1223 |
Geraniol |
Rec assay |
B. subtilis M45 and H17 |
16 µg/disk |
Negative |
Oda et al. (1979)e |
|
1225 |
Citral |
Reverse mutation |
S. typhimurium TA98, TA100, TA97a, TA102 |
5–700 µg/plate |
Negativeb |
Gomes-Carneiroet al. (1998) |
|
1225 |
Citral |
Reverse mutation |
S. typhimurium TA92, TA94, TA98, TA100, TA1535, TA1537 |
<100 µg/plate |
Negatived |
Ishidate et al.(1984) |
|
1225 |
Citral |
Reverse mutation |
S. typhimurium TA100 |
NR |
Negativeb |
Lutz et al. (1982) |
|
1225 |
Citral |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537 |
1–160 µg/plate |
Negativeb |
Zeiger et al. (1987); National Toxicology Program (2003) |
|
1225 |
Citral |
Mutation |
E. coli WP2uvrA (trp -) |
13–100 µg/plate |
Negative |
Yoo (1986)e |
|
1225 |
Citral |
Sister chromatid exchange |
Chinese hamster ovary cells |
0.289–40.2 µg/ml |
Positiveb |
National Toxicology Program (2003) |
|
1225 |
Citral |
Chromosomal aberration |
Chinese hamster ovary cells |
12.5–60.6 µg/ml |
Negativeb |
National Toxicology Program (2003) |
|
1225 |
Citral |
Chromosomal aberration |
Chinese hamster fibroblast cells |
<30 µg/ml |
Negativeg |
Ishidate et al. (1984) |
|
1225 |
Citral |
Rec assay |
B. subtilis M45 and H17 |
17 µg/disk |
Negative |
Oda et al. (1979)e |
|
1225 |
Citral |
Rec assay |
B. subtilis M45 and H17 |
0.16, 0.32, 0.63 µl/disk
(142, 284, 560 µg/disk)j |
Negative |
Kuroda et al.(1984)e |
|
1.25, 2.5 µl/disk
(1110, 2220 µg/disk) |
Positive |
|
1225 |
Citral |
Rec assay |
B. subtilis M45 and H17 |
<2.5 µl/disk
(<2220 µg/disk) |
Positive |
Yoo (1986)e |
|
1225 |
Citral |
DNA damage |
Mouse fibroblast cells (NTCT 929) |
10–30 µg/ml |
Positive |
Duerksen-Hugheset al. (1999) |
|
1230 |
Farnesol |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537 |
8–5000 µg/plate |
Negativeb |
Creutziger (1989) |
|
1230 |
Farnesol |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537 |
3 µmol/plate
(667 µg/plate)k,l |
Negativeb |
Florin et al. (1980) |
|
In vivo |
|
1225 |
Citral |
Micronucleus formation |
Mouse bone marrow erythrocytes |
250, 500, or 750 mg/kg bwm |
Negative |
National Toxicology Program (2003) |
|
1225 |
Citral |
Micronucleus formation |
Mouse peripheral blood erythrocytes |
745, 1840, 3915, or 8110 mg/kg bw per dayn (males) |
Negative |
National Toxicology Program (2003) |
|
790, 1820, 3870, or 7550 mg/kg bw per dayn (females) |
Negative |
|
a |
Calculated using a relative molecular mass of 98.15 |
|
b |
With and without metabolic activation |
|
c |
Calculated using a density of 0.851 (Merck, 1997) |
|
d |
With metabolic activation |
|
e |
Japanese article, English summary and tables |
|
f |
Calculated using a relative molecular mass of 154.25 |
|
g |
Without metabolic activation |
|
h |
Calculated using a density of 0.8894 (Merck, 1997) |
|
i |
Polyploidy (8%) was observed at the highest dose tested |
|
j |
Calculated using a density of 0.888 (Merck, 1997) |
|
k |
Calculated using a relative molecular mass of 222.37 |
|
l |
Substance precipitated on the plate |
|
m |
Three intraperitoneal injections given at 24-h intervals; male mice only |
|
n |
Microencapsulated citral was administered in the diet for 14 weeks |
In vitro
No evidence of mutagenicity was reported in standard or modified (preincubation method) Ames assays when 2-methyl-2-pentenal (No. 1209; up to 294 µg/plate), dl-citronellol (No.1219; up to 85 µg/plate), citronellal (No.1220; up to 500 µg/plate), geraniol (No.1223; up to 889 µg/plate), citral (No.1225; up to 700 µl/plate), and farnesol (No.1230; up to 5000 µl/plate) were incubated with Salmonella typhimurium strains TA92, TA94, TA97a, TA98, TA100, TA102, TA1535, and/or TA1537 with and without metabolic activation (Rockwell & Raw, 1979; Eder et al., 1980; Florin et al., 1980; Kasamaki et al., 1982; Lutz et al., 1982; Ishidate et al., 1984; Zeiger et al., 1987; Creutziger, 1989; Gomes-Carneiro et al., 1998; National Toxicology Program, 2003). Negative results were reported in a mutation test in which 100 µg/plate of citral was incubated with Escherichia coli WP2 uvrA (Yoo, 1986).
Citronellal (No. 1220) and geraniol (No. 1223) did not induce sister chromatid exchanges in Chinese hamster ovary cells in the absence of metabolic activation at concentrations up to 100 µmol/l (15.4 µg/ml) for citronellal and 333 µmol/l (51.4 µg/ml) for geraniol (Sasaki et al., 1989). In a non-standard assay designed to maximize the frequency of chromosomal aberrations in a Chinese hamster B241 cell line, citronellal at concentrations of 0.008 µg/ml gave weakly positive results with and without metabolic activation (Kasamaki et al., 1982). No evidence of an increase in chromosomal aberrations was reported when geraniol (No. 1223) at concentrations of up to 125 µg/ml was incubated with Chinese hamster fibroblast cells in the absence of metabolic activation (Ishidate et al., 1984), although there was an 8% increase in polyploidy.
Assays for sister chromatid exchanges with citral (No. 1225) were performed in Chinese hamster ovary cells. In the absence of metabolic activation, an increase in sister chromatid exchanges of at least 20% that of control cultures was reported at concentrations of 0.289–2.89 µg/ml in the first trial and 7.5–10 µg/ml in the second trial. Toxicity was observed at 8.86 and 20 µg/ml in the first and second trial, respectively. With metabolic activation, an increase of sister chromatid exchanges of at least 20% that of control cultures was reported with citral at 8.68 µg/ml in the first trial and 15.1–40.2 µg/ml in the second trial. Toxicity was reported at 28.9 µg/ml in the first trial; no toxicity was observed in the second trial. Owing to cell cycle delay induced by citral, at the higher concentrations (10 µg/ml without and 20.1–40.2 µg/ml with metabolic activation) extended culture periods were necessary to allow accumulation of sufficient second-division metaphase cells for analysis (National Toxicology Program, 2003). In contrast to these findings, there was no evidence for an increase in chromosomal aberrations with higher concentrations of citral (12.5–25.3 µg/ml without and 30.3–60.6 µg/ml with metabolic activation) (National Toxicology Program, 2003) or, in another chromosomal aberration assay in Chinese hamster fibroblast cells, at concentrations of citral of up to 30 µg/ml, without metabolic activation (Ishidate et al., 1984).
Rec assays for DNA repair in Bacillus subtilis strains M45 and H17 have been performed with dl-citronellol (No. 1219), citronellal (No. 1220), geraniol (No. 1223), and citral (No. 1225). In one study, each of the four agents gave negative results at concentrations of 16 or 17 µg/disc (Oda et al., 1979). Citral gave positive results in two other rec assays (Kuroda et al., 1984; Yoo, 1986) but only at high concen-trations (1110 and 2220 µg/disc). Rec assays performed at lower concentrations of citral (up to 560 µg/disc) were negative (Kuroda et al., 1984).
In a recently developed assay for DNA damage measuring induction of p53 tumour suppressor protein in mouse fibroblasts (NTCT 929 cell line) in vitro, citral gave positive results at concentrations of 10–30 µg/ml after 17 h of incubation. In this assay, increased expression of p53 is considered to indicate the induction of DNA damage (Duerksen-Hughes et al., 1999).
In vivo
Groups of four or five male B63CF1 mice received citral (No. 1225) at a dose of 250, 500, 750, or 1000 mg/kg bw per day by intraperitoneal injection at 24-h intervals for a period of 3 days. A group of animals given corn oil only and another group given cyclophosphamide were used as vehicle and positive controls, respectively. The highest dose of citral was lethal and only the three lower doses were used to evaluate the results of the assay. Twenty-four hours after the third injection, the animals were sacrificed and blood smears were taken from bone marrow cells collected from the femur. Scoring of 2000 polychromatic erythrocytes for formation of micronuclei revealed no increase in micronucleated polychromatic erythrocytes at any dose. The ratio of polychromatic erythrocytes:normochromatic erythrocytes was not determined (National Toxicology Program, 2003).
In addition to the assay for micronuclei formation in bone marrow, an assay for micronuclei formation in mouse peripheral blood erythrocytes was performed. Peripheral blood samples were obtained within 24 h of the final treatment in a 14-week study of toxicity in which female and male B63CF1 mice were given diet containing microencapsulated citral at a dose of up to 7550 and 8110 mg/kg bw per day, respectively. Blood smears were made, fixed and stained, and 1000 normochromatic erythrocytes per animal were scored for the frequency of micronuclei. In addition, the percentage of polychromatic erythrocytes among the total population of erythrocytes was scored. Results for all doses in both males and females showed no increase in micronucleated normochromatic erythrocytes or in the percentage of polychromatic erythrocytes (National Toxicology Program, 2003).
Conclusion
Several aliphatic branched-chain unsaturated alcohols and aldehydes have been tested in the Ames assay and found to be not mutagenic in vitro. In addition to showing a lack of mutagenic potential in the Ames assay, citral gave negative results in assays for mutagenicity in E. coli WP2 uvrA. There was some evidence of DNA damage caused by citral from two rec assays with Bacillus subtilis, but only at very high concentrations. Rec assays performed with lower concentrations of test substance, however, gave negative results for citral as well as for dl-citronellol, citronellal, and geraniol.
Citronellal showed weak evidence of clastogenicity in a non-standard assay for chromosomal aberrations, but gave negative results in assays for sister chromatid exchanges. Geraniol neither induced sister chromatid exchanges nor chromosomal aberrations. Citral showed evidence of activity in assays for sister chromatid exchanges, but increased incubation times were required because of delayed cell cycling. Citral did not induce chromosomal aberrations in vitro nor did it show signs of genotoxicity in assays for micronucleus formation in bone marrow and peripheral erythrocytes in vivo. Citral induced DNA damage in mouse fibroblasts in vitro, as shown by increased expression of P53. This result, however, contrasts with the results of existing assays for genotoxicity with citral, which are largely negative.
On the basis of the results of available studies of genotoxicity, the Committee concluded that members of this group of aliphatic branched-chain saturated and unsaturated alcohols, aldehydes, acids, and related esters are not genotoxic.
(e) Reproductive toxicity
(i) Citral (No. 1225)
Groups of 30 female Sprague Dawley/Charles River rats were given citral orally at a dose of 0 (corn oil control), 50, 160, or 500 mg/kg bw per day from 2 weeks before mating until day 20 of gestation. At that time half the females in each group underwent caesarian section, while the other half delivered naturally and continued to receive treatment until day 21 of lactation.
Maternal toxicity observed at a dose of 160 and 500 mg/kg bw per day included mortality, clinical symptoms of toxicity (urine-stained abdominal fur, decreased motor activity, and excess salivation), as well as significant decreases in body-weight gain during days 0–6 of gestation, and significant increases in food consumption during lactation (days 1–4 post-partum). The only indication of maternal toxicity at a dose of 50 mg/kg bw per day was excess salivation. No significant differences were observed in estrus cycling, duration of cohabitation, rate of pregnancy, or duration of gestation. Slight (not statistically significant) decreases in fetal body weight were reported for fetuses delivered by Caesarean delivery at the intermediate and highest doses. Significantly decreased pup body weights for pups that were delivered naturally were reported at the highest dose. Treatment did not affect the numbers of corpora lutea and implantations, live litter sizes, resorptions, sex ratio, or pup viability up to day 21 of lactation. No gross external alterations were reported in the offspring. The NOEL for citral was 50 mg/kg bw per day for maternal toxicity and 160 mg/kg bw per day for developmental toxicity (Hoberman et al., 1989).
In a screening test for reproductive and developmental toxicity, four groups of 10 virgin Crl:CD BR VAF Plus rats were treated orally by gavage with citral diethyl acetal at a dose of 0, 125, 250, or 500 mg/kg bw per day from 7 days before cohabitation, throughout cohabitation (maximum of 7 days), gestation, delivery, and for 4 day after parturition. Maternal indices monitored included clinical signs of toxicity, mortality, body weights and food consumption, fertility parameters (mating and fertility index, gestation index, numbers of implantations sites and pups per litter), duration of gestation, maternal behaviour, and gross lesions at necropsy. Offspring indices included clinical signs of toxicity, viability, sex ratio, body weights, and examination for gross external malformations.
In the dams, the only effects observed for citral diethyl acetal were clinical signs (not specified), and (not statistically significantly) decreased body weights and body-weight gains at the intermediate and highest doses. At the highest dose, pup body weights were also decreased (not statistically significantly). On this basis, the NOEL for citral was 125 mg/kg bw per day for maternal toxicity and 250 mg/kg bw per day for developmental toxicity (Vollmuth et al., 1990).
In a study of developmental toxicity, groups of 20 pregnant Wistar rats were given citral at a dose of 0, 60, 125, 250, 500, or 1000 mg/kg bw (purity, 95%) in corn oil by gavage, daily during days 6–15 of gestation. On day 21 of gestation the dams were killed and the fetuses removed.
Maternal body-weight gain was significantly reduced at the two higher doses (500 and 1000 mg/kg bw), while the decrease in body-weight gain observed at the two intermediate doses (125 and 250 mg/kg bw) could primarily be attributed to reduced weight of the gravid uterus. At the lowest dose, a transient decrease in body-weight gain was noted on days 6–15 of gestation. At doses of >125 mg/kg bw and higher, there was a dose-dependent decrease in the ratio of pregnant rats (i.e. with implantation sites) to sperm-positive treated rats, which suggested impaired implantation or induction of very early postimplantation losses. Citral also significantly reduced the number of implantations per dam at 125 and 1000 mg/kg bw. An increase in resorptions was observed at 60, 125, and (not statistically significantly) at 250 mg/kg bw, but not at higher doses. Due to the increase in resorptions and the reduced number of implantations per dam, the number of live fetuses was reduced at doses of 60 (not statistically significant), 125, 250 and 1000 mg/kg bw, but not at 500 mg/kg per day. At doses of >125 mg/kg bw, ossification was delayed and fetal weights decreased. An increase in the proportion of fetuses exhibiting haematoma was reported at doses of >250 mg/kg. No treatment-related effects on the incidence of gross structural abnormalities or visceral malformations were seen; however, the frequency of fetuses with one or more minor skeletal anomalies was increased at 125, 250, and 1000 mg/kg bw. Effects on fetal organ weights were limited to increases in absolute and relative spleen weights at >250 mg/kg bw. The NOEL for citral was <60 mg/kg bw per day for maternal toxicity, given that effects on body-weight gain and prenatal losses were observed at all doses tested. The NOEL for developmental toxicity was 60 mg/kg bw per day on the basis of fetal growth retardation and increased incidences of skeletal anomalies. Citral is not teratogenic; the increases in frequencies of fetuses with delayed ossification and skeletal anomalies were not dose-related and occurred at doses that were maternally toxic (Nogueira et al., 1995).
In study of developmental toxicity, groups of 25 pregnant Sprague-Dawley (COBS) CD BR rats were exposed by inhalation to citral (purity, 90%) at a concentration of 0, 10 or 34 ppm (as vapour), or 68 ppm (as an aerosol/vapour mixture)for 6 h per day on days 6 to 15 of gestation. Dams were killed on day 20 of gestation and the fetuses were removed and examined for gross, visceral and skeletal malformations.
Maternal toxicity was only observed at the highest dose, and included mortality, decreased body weight, and clinical signs of toxicity (including ocular opacity, difficulty in breathing, nasal discharge, and salivation). After cessation of treatment, most clinical signs disappeared, and although body weights increased, the net body-weight gain was significantly decreased. The number of corpora lutea, implantations, resorptions, fetal viability, litter size, and sex ratio were not significantly affected by treatment with citral. At the maternally toxic dose, some slight fetotoxic effects, apparent as slightly decreased fetal weights and slightly increased incidences of hypoplastic bones (both not statistically significant), were observed. There were no other treatment-related visceral or skeletal malformations (Gaworski et al., 1992).
(f) Other effects
In short-term studies of toxicity in which citral was administered orally by gavage in corn oil for 3–10 days to male rats, citral induced peroxisome proliferation, hepatomegaly and microsomal induction at extremely high doses (1500 mg/kg bw per day for 5 days or 2400 mg/kg bw per day for 3 or 10 days) (Jackson et al., 1987; Roffey et al., 1990). In the short-term studies under evaluation, increased liver weights were reported in mice but not in rats treated by gavage in 12-day studies by Dieter et al. (1993). In mice, no associated histopathologic hepatic changes were observed. The liver (weight and histopathology) was not affected in studies in which diets containing citral were given to mice and rats for up to 14 weeks. Hence, it is unlikely that citral induced peroxisome proliferation in these studies.
According to the National Toxicology Program (2003), citral has been shown to induce benign and atypical prostatic hyperplasia in a number of studies in rats treated dermally. The mechanism by which citral induces this prostatic hyperplasia is unknown, but it has been suggested that interaction of citral with serum testosterone and/or estrogen may play a role. The strain genotype and endocrine background play a role in the development of the disease, as Wistar and Sprague-Dawley rats, but Fischer 344 or ACI/Ztm rats, were affected after treatment with citral. Consistent with this, the two-year study of carcinogenicity with Fischer 344/N rats treated orally did not reveal any effect on male accessory glands, including all lobes of the prostate (National Toxicology Program, 2003).
Geldof et al. (1992) showed that application of citral directly to the vagina of ovariectomized female Copenhagen rats significantly increased proliferation of the vaginal epithelium. The effect, however, was smaller than that induced by estradiol-17beta. In addition, citral inhibited binding of estradiol-17beta to estrogen receptors, but did not inhibit binding of testeosterone to androgen receptors in vitro. These results suggest that citral has a weak estrogenic activity.
3. REFERENCES
Asano, M. & Yamakawa, T. (1950) The fate of branched chain fatty acids in animal body. I. A contribution to the problem of "Hildebrandt acid". J. Biochem. 37, 321–327.
BASF (1978) Bericht über die Gewerbetoxikologische Vorprüfung (Citral). Unpublished report. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
BASF (1981) Gewerbetoxikologische Grundprüfung (Farnesol). Unpublished report. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Boyer, C.S. & Petersen, D.R. (1990) The metabolism of 3,7-dimethyl-2,6-octadienal (citral) in rat hepatic mitochondrial and cytosolic fractions. Drug Metab. Dispos., 18, 81–86.
Chadha, A. & Madyastha, K.M. (1982) omega-Hydroxylation of acyclic monoterpene alcohols by rat lung microsomes. Biochem. Biophys. Res. Commun. 108, 1271–1277.
Chadha, A. & Madyastha, K.M. (1984) Metabolism of geraniol and linalool in the rat and effects on liver and lung microsomal enzymes. Xenobiotica, 14, 365–374.
Cramer, G.M., Ford, R.A. & Hall, R.L. (1978) Estimation of toxic hazard—a decision tree approach. Food Cosmet. Toxicol. 16, 255–276.
Creutziger, R. (1989) Report on the mutagenicity testing. Salmonella/microsome test (Ames-Test). Test Substance: "Farnesol". Unpublished report No. 5048/00-89 from International Bio Research, Walsrode/Hannover, Germany. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Dieter, M.P., Goehl, T.J., Jameson, C.W., Elwell, M.R., Hildebrandt, P.K. & Yuan, J.H. (1993) Comparison of the toxicity of citral in F344 rats and B6C3F1 mice when administered by microencapsulation in feed or by corn-oil gavage. Food Chem. Toxicol., 31, 463–474.
Diliberto, J.J, Usha, G. & Birnbaum, L.S. (1988) Disposition of citral in male Fischer rats. Drug Metab. Dispos., 16, 721–27.
Diliberto, J.J., Srinivas, P., Overstreet, D., Usha, G., Burka, L.T. & Birnbaum, L.S. (1990) Metabolism of citral, an alpha,beta-unsaturated aldehyde, in male F344 rats. Drug Metab. Dispos., 18, 866–875.
Duerksen-Hughes, P.J., Yang, J. & Ozcan, O. (1999) p53 Induction as a genotoxic test for twenty-five chemicals undergoing in vivo carcinogenicity testing. Environ. Health Per-spect., 107, 805–812.
Eder, E., Neudecker, T., Lutz, D. & Henschler, D. (1980) Mutagenic potential of allyl and allylic compounds, structure-activity relationship as determined by alkylating and direct in vitro mutagenic properties. Biochem. Pharmacol., 29, 993–998.
Food & Drug Administration (1993) Priority-based assessment of food additives (PAFA) database, Centre for Food Safety and Applied Nutrition, Washington DC, USA, 58.
Fischer, F.G. & Bielig, H.-J. (1940) On the hydrogenation of unsaturated substances in the animal body (Biochemical hydrogenations VII). Z. Physiol. Chem., 266, 73–98.
Florin, I., Rutberg, L., Curvall, M. & Enzell, C.R. (1980) Screening of tobacco smoke constituents for mutagenicity using the Ames’ test. Toxicology, 18, 219–232.
Gaworski, C.L., Vollmuth, T.A., York, R.G., Heck, J.D. & Aranyi, C. (1992) Developmental toxicity evaluation of inhaled citral in Sprague-Dawley rats. Food Chem. Toxicol., 30, 269–275.
Geldof, A.A., Engel, C. & Rao, B.R. (1992) Estrogenic action of commonly used fragrant agent citral induces prostatic hyperplasia. Urol. Res., 20, 139–144.
Gomes-Carneiro, M.R., Felzenszwalb, I. & Paumgartten, F.J.R. (1998) Mutagenicity testing of (+)-camphor, 1,8-cineole, citral, citronellal, (-)-menthol and terpineol with the Salmonella / microsome assay. Mutat. Res., 416, 129–136.
Grundschober, F. (1977) Toxicological assessment of flavouring esters. Toxicology, 8, 387–390.
Hagan, E.C., Hansen, W.H., Fitzhugh, O.G., Jenner, P.M., Jones, W.I., Taylor, J.M., Long, E.L., Nelson, A.A. & Brouwer, J.B. (1967) Food flavourings and compounds of related structure. II. Subacute and chronic toxicity. Food Cosmet. Toxicol., 5, 141–157.
Haggard, H.W., Miller, D.P. & Greenberg, L.A. (1945) The amyl alcohols and their ketones: their metabolic fates and comparative toxicities. J. Ind. Hyg. Toxicol., 27, 1–14.
Heymann, E. (1980) Carboxlesterases and amidases. In: Jakoby, W.B., ed, Enzymatic Basis of Detoxication, 2nd Ed., Vol. II, New York: Academic Press, Inc., pp. 291–323.
Hoberman, A.M., Christian, M.S., Bennett, M.B. & Vollmuth, T.A. (1989) Oral general reproduction study of citral in female rats. The Toxicologist, 9, 271.
Hoffmann-LaRoche (1967a) Acute toxicity, eye and skin irritation tests on aromatic compounds. Private communication to Research Institute for Fragrance Materials. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Hoffmann-LaRoche (1967b) Acute toxicity, eye and skin irritation tests on aromatic compounds. Private communication to Research Institute for Fragrance Materials. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
International Organization of the Flavour Industry (1995) European inquiry on volume of use. Unpublished report. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Ishida, T., Toyota, M. & Asakawa, Y. (1989) Terpenoid biotransformation in mammals. V. Metabolism of (+)-citronellal, (±)-7-hydroxycitronellal, citral, (-)-perillaldehyde, (-)-myrtenal, cuminaldehyde, thujone, and (±)-carvone in rabbits. Xenobiotica, 19, 843– 855.
Ishidate, M., Jr, Sofuni, T., Yoshikawa, K., Hayashi, M., Nohmi, T., Sawada, M. & Matsuoka, A. (1984) Primary mutagenicity screening of food additives currently used in Japan. Food Chem. Toxicol., 22, 623–636.
Jackson, G.M., Hall, D.E. & Walker, R. (1987) Comparison of the short-term hepatic effects of orally administered citral in Long Evans hooded and Wistar albino rats. Food Chem. Toxicol., 25, 505–513.
Jenner, P.M., Hagan, E.C., Taylor, J.M., Cook, E.L. & Fitzhugh, O.G. (1964) Food flavourings and compounds of related structure. I. Acute oral toxicity. Food Cosmet. Toxicol., 2, 327–343.
Kasamaki, A., Takahashi, H., Tsumura, N., Niwa, J., Fujita, T. & Urasawa, S. (1982) Genotoxicity of flavoring agents. Mutat. Res., 105, 387–392.
Kuroda, K., Tanaka, S., Yu, Y.S. & Ishibashi, T. (1984) Rec-assay of food additives. Nippon Kosnu Eisei Zasshi, 31, 277–281.
Licht, H.J. & Coscia, C.J. (1978) Cytochrome P-450LM2 mediated hydroxylation of monoterpene alcohols. Biochemistry, 17, 5638–5646.
Longland, R.C., Shilling, W.H. & Gangolli, S.D. (1977) The hydrolysis of flavouring esters by artificial gastrointestinal juices and rat tissue preparations. Toxicology, 8, 197–204.
Lucas, C.D., Putnam, J.M. & Hallagan, J.B. (1999) Flavor and Extract Manufacturers Association of the United States 1995 Poundage and Technical Effects Update Survey, Washington DC: Flavor and Extract Manufacturers Association of the United States.
Lutz, D., Eder, E., Neudecker, T. & Henschler, D. (1982) Structure-mutagenicity relationship in alpha,beta-unsaturated carbonylic compounds and their corresponding allylic alcohols. Mutat. Res. 93, 305–315.
Maarse, H., Visscher, C.A., Willemsens, L.C. & Boelens, M.H. (1999). Volatile Components In Food—Qualitative And Quantitative Data. Zeist: Centraal Instituut Voor Voedingsonderzioek TNO.
Merck (1997) The Merck Index: An encyclopedia of chemicals and drugs. 12th Ed., Version 12.2, on CD-ROM, New Jersey: Merck & Co., Inc. and Chapman & Hall CRCnetBASE Electronic Publishing Division. Moreno O.M. (1972) Acute oral toxicity in rats (Nerol). Unpublished report No. 836-72 from Toxicological Resources, East Millstone, New Jersey, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Moreno O.M. (1973) Acute oral toxicity in rats; dermal toxicity in rabbits (2-Isopropyl-5-methyl-2-hexenal; Citronellol; Citronellal; Rhodinol). Unpublished report Nos. 73–197, 73–88, 73–190, 73–150 from MB Research Laboratories, Inc., Perkasie, Pennsylvania, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Moreno, O.M. (1974) Acute oral toxicity in rats; dermal toxicity in rabbits (Farnesol). Unpublished report No. 74-605 from MB Research Laboratories, Inc., Perkasie, Pennsylvania, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Moreno, O.M. (1977) Acute oral toxicity in rats; dermal toxicity in rabbits (3-Methyl-2-buten-1-ol; trans-2-Methyl-2-butenoic acid). Unpublished report No. 77-1714 from MB Research Laboratories, Inc., Spinnerstown, Pennsylvania, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Moreno, O.M. (1978) Acute oral toxicity in rats; acute dermal toxicity in rabbits (3,7-Dimethyl-6-octenoic acid). Unpublished report No. MB 78-2628 from MB Research Laboratories, Inc., Spinnerstown, Pennsylvania, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Moreno, O.M. (1980) Oral toxicity in rats; dermal toxicity in rabbits (2-Methyl-2-pentenoic acid). Unpublished report No. MB 80-4819 from MB Research Laboratories, Inc., Spinnerstown, Pennsylvania, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
National Academy of Sciences (1989) 1987 Poundage and technical effects update of substances added to food, Committee on Food Additives Survey Data, Food and Nutrition Board, Institute of Medicine, National Academy of Sciences, Washington DC, USA.
National Toxicology Program (1987) Carcinogenesis studies of food grade geranyl acetate (71%) and citronelly acetate (29%) (NTP-TR-252; PB-88–2508). National Technical Information Services, Research Triangle Park, North Carolina, USA. [as evaluated in WHO Food Additives Series 40, 1998 (Annex 1, reference 132)]
National Toxicology Program (2003) Toxicology and carcinogenesis studies of citral (microencapsulated) (CAS No. 5392-40-5) in F344/N rats and B6C3F1 mice (feed studies). (NTP TR 505; NIH Publication No. 01-4439). US Department of Health and Human Services, Public Health Service, National Institutes of Health, USA.
Nogueira, A.C.M.A., Carvalho, R.R., Souza, C.A.M., Chahoud, I. & Paumgartten, F.J.R. (1995) Study on the embryofetotoxicity of citral in the rat. Toxicology, 96, 105–113.
Oda, Y., Hamano, Y., Inoue, K., Yamamoto, H., Niihara, T. & Kunita, N. (1979) Mutagenicity of food flavours in bacteria (1st report). Shokuhin Eisei Hen, 9, 177–181.
Oser, O.M. (1958a) Toxicological screening of components of food flavors. Class X. Citral Compounds. Unpublished report from Food and Drug Research Laboratories, Inc. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Oser, O.M. (1958b) Toxicological Screening of components of food flavors. Class VI. Citronellol and Linalool. Unpublished report from Food and Drug Research Laboratories, Inc. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Parke, D.V. & Rahman, H. (1969) The effects of some terpenoids and other dietary anutrients on hepatic drug-metabolizing enzymes. Biochem. J., 113, 12.
Phillips, J.C., Kingsnorth, J., Gangolli, S.D. & Gaunt, I.F. (1976) Studies on the absorption, distribution and excretion of citral in the rat and mouse. Food Cosmet. Toxicol. 14, 537–540.
Posternak, J.M. (1968) Subacute toxicity (90 days) of chemical 2,4-dimethyl-2-pentenoic acid (TT 118). Unpublished report by Firmenich & Cie. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Rockwell, P. & Raw, I. (1979) A mutagenic screening of various herbs, spices, and food additives. Nutr. Cancer, 1, 10–15.
Roffey, S.J., Walker, R. & Gibson, G.G. (1990) Hepatic peroxisomal and microsomal enzyme induction by citral and linalool in rats. Food Chem. Toxicol. 28, 403–408.
Rowe, V.K. & McCollister, S.B. (1982) Alcohols. In: Clayton, G.D. & Clayton, F.E., eds, Patty’s Industrial Hygiene and Toxicology, 3rd Revised Ed., Vol. 2C, New York: John Wiley & Sons, chapter 35, pp. 4527–4708.
Sasaki, Y.F., Imanishi, H., Ohta, T. & Shirasu, Y. (1989) Modifying effects of components of plant essence on the induction of sister-chromatid exchanges in cultured Chinese hamster ovary cells. Mutat. Res. 226, 103–110.
Schafer, E.W., Jr & Bowles, W.A., Jr (1985) Acute oral toxicity and repellency of 933 chemicals to house and deer mice. Arch. Environ. Contam. Toxicol., 14, 111–129.
Smyth, H.F., Jr, Carpenter, C.P., Weill, C.S. & Pozzani, U.C. (1954) Range-finding toxicity data. List V. A.M.A. Arch. Indust. Hyg. Occup. Med., 10, 61–68.
Sterner, W. & Stiglic, A. (1976) Acute oral toxicity of "Faktor F" in rats. Unpublished report No. 1-4-435/2-76 from International Bio-Research, Inc., Hannover, Germany. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Stofberg, J. & Grundschober, F. (1987) Consumption ratio and food predominance of flavoring materials. Perfumer Flavorist 12, 27.
Voet, D. & Voet, J.G. (1990) Biochemistry. New York: John Wiley & Sons.
Vollmuth, T.A., Bennett, M.B., Hoberman, A.M. & Christian, M.S. (1990) An evaluation of food flavoring ingredients using an in vivo reproductive and developmental toxicity screening test. Teratology, 41, 597.
Williams, R.T. (1959) The metabolism of some aliphatic aldehydes, ketones and acids. In: Detoxication mechanisms. The metabolism and detoxication of drugs, toxic substances and other organic compounds, 2nd Ed., London: Chapman & Hall, Ltd., chapter four, pp. 88–113.
Yamawaki, T. (1962) Pharmacological effects of geraniol. Nippon Yakurigaku Zasshi, 58, 394–400.
Yoo, Y.S. (1986) Mutagenic and antimutagenic activities of flavoring agents used in food-stuffs. J. Osaka City Med. Centre, 34, 267–288.
Zeiger, E., Anderson, B., Haworth, S., Lawlor, T., Mortelmans, K. & Speck, W. (1987) Salmonella mutagenicity tests: III. Results from the testing of 255 chemicals. Environ. Mutag., 9, 1–110.
ENDNOTES:
1 "Conditional ADI", which signifies an ADI with special considerations, is a term no longer used by JECFA.