
WHO FOOD ADDITIVES SERIES: 52
ALIPHATIC AND AROMATIC ETHERS
First draft prepared by
Professor I.G. Sipes
Department of Pharmacology, College of Medicine, University of Arizona, Tucson, Arizona, USA
1. EVALUATION
1.1 Introduction
The Committee evaluated a group of 29 aliphatic and aromatic flavouring agents (see Table 1) that included eucalyptol (No. 1234) and anisole (No. 1241) by the Procedure for the Safety Evaluation of Flavouring Agents (see Figure 1, Introduction). These agents have not been evaluated previously by the Committee. Benzyl butyl ether (No. 1253) and dibenzyl ether (No. 1256) were evaluated for specifications only at the twenty-fourth meeting (Annex 1, reference 53).
Twenty-three of the 29 flavouring agents (Nos 1231–1239, 1241–1246, 1248–1255) have been reported to occur naturally in foods. They have been detected in fruits, vegetables, alcoholic beverages, cheese, oil and tea (Maarse et al., 1999).
Table 1. Summary of results of the safety evaluations of aliphatic and aromatic ethers used as flavouring agentsa
|
Flavouring agent |
No. |
CAS No. and structure |
Step A3b Does intake exceed the threshold for human intake? |
Step A4 Is the substance or its metabolites endogenous? |
Step A5 Adequate margin of safety for substance or related substance? |
Comments on predicted metabolism |
Conclusion based on current intake |
|
Structural class I |
|
|
|
|
|
|
|
|
Anisole |
1241 |
100-66-3
 |
No
Europe: 0.03
USA: 0.01 |
NR |
NR |
See note 1 |
No safety concern |
|
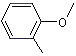
o-Methylanisole |
1242 |
578-58-5
 |
No
Europe: 3
USA: 0.06 |
NR |
NR |
See note 1 |
No safety concern |
|
p-Methylanisole |
1243 |
104-93-8
 |
No
Europe: 0.5
USA: 15 |
NR |
NR |
See note 2 |
No safety concern |
|
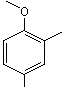
2,4-Dimethylanisole |
1245 |
6738-23-4
 |
No
Europe: ND
USA: 0.2 |
NR |
NR |
See note 3 |
No safety concern |
|
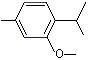
1-Methyl-3-methoxy-4-isopropylbenzene |
1246 |
1076-56-8
 |
No
Europe: 2
USA: 0.1 |
NR |
NR |
See note 3 |
No safety concern |
|
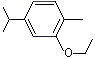
Carvacryl ethyl ether |
1247 |
4732-13-2
 |
No
Europe: 0.1
USA: 0.02 |
NR |
NR |
See note 3 |
No safety concern |
|
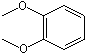
1,2-Dimethoxybenzene |
1248 |
91-16-7
 |
No
Europe: ND
USA: 20 |
NR |
NR |
See note 3 |
No safety concern |
|
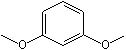
m-Dimethoxybenzene |
1249 |
151-10-0
 |
No
Europe: 5
USA: 2 |
NR |
NR |
See note 3 |
No safety concern |
|
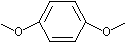
p-Dimethoxybenzene |
1250 |
150-78-7
 |
No
Europe: 18
USA: 7 |
NR |
NR |
See note 3 |
No safety concern |
|
Structural class II |
|
|
|
|
|
|
|
|
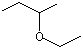
sec-Butyl ethyl ether |
1231 |
2679-87-0
 |
No
Europe: 8
USA: 0.3 |
NR |
NR |
See note 4 |
No safety concern |
|
C1-Ethoxy-3-methyl-2-butene |
1232 |
22094-00-4
 |
No
Europe: 0.9
USA: 2 |
NR |
NR |
See note 4 |
No safety concern |
|
1,4-Cineole |
1233 |
470-67-7
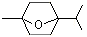 |
No
Europe: 5
USA: 146 |
NR |
NR |
See note 5 |
No safety concern |
|
Eucalyptol |
1234 |
70-82-6
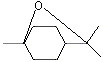 |
Yes
Europe: 1439
USA: 1954 |
No |
Yes. The NOEL for eucalyptol of >32 mg/kg bw per day (Roe et al., 1979) is approximately 1000 times greater than the estimated daily intakes of 24 µg/kg bw in Europe and 33 µg/kg bw in the USA when used as a flavouring agent |
See note 5 |
No safety concern |
|
Nerol oxide |
1235 |
1786-08-9
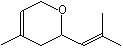 |
No
Europe: 1
USA: 0.7 |
NR |
NR |
See note 5 |
No safety concern |
|
2,2,6-Trimethyl-6-vinyltetrahydropyran |
1236 |
7392-19-0
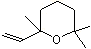 |
No
Europe: 0.01
USA: 8 |
NR |
NR |
See note 5 |
No safety concern |
|
CTetrahydro-4-methyl-2-(2-methylpropen-1-yl) pyran |
1237 |
16409-43-1
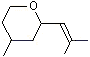 |
No
Europe: 4
USA: 0.2 |
NR |
NR |
See note 5 |
No safety concern |
|
Theaspirane |
1238 |
36431-72-8
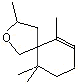 |
No
Europe: 2
USA: 0.1 |
NR |
NR |
See note 5 |
No safety concern |
|
Cycloionone |
1239 |
5552-30-7
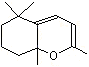 |
No
Europe: ND
USA: 2 |
NR |
NR |
See note 5 |
No safety concern |
|
Benzyl ethyl ether |
1252 |
539-30-0
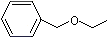 |
No
Europe: 0.003
USA: 2 |
NR |
NR |
See notes 3 and 6 |
No safety concern |
|
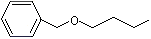
Benzyl butyl ether |
1253 |
588-67-0
 |
No
Europe: ND
USA: 0.02 |
NR |
NR |
See notes 3 and 6 |
No safety concern |
|
CMethyl phenethyl ether |
1254 |
3558-60-9
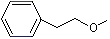 |
No
Europe: 31
USA: 0.01 |
NR |
NR |
See notes 3 and 6 |
No safety concern |
|
Structural class III |
|
|
|
|
|
|
|
|
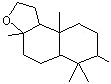
1,5,5,9-Tetramethyl-13- oxatricyclo (8.3.0.0(4,9)) tridecane |
1240 |
3738-00-9
 |
No
Europe: 1
USA: 0.1 |
NR |
NR |
See note 5 |
No safety concern |
|
p-Propylanisole |
1244 |
104-45-0
 |
Yes
Europe: 23
USA: 114 |
No |
Yes. The NOEL of 300 mg/kg bw per day for the related substance p-propenylanisole (trans-anethole) (Vavasour, 1999) Annex 1, reference 138) is >100 000 times greater than the daily intakes of p-propylanisole in Europe (0.4 µg/kg bw) and in the USA (2 µg/kg bw) when used as a flavouring agent |
See note 7 |
No safety concern |
|
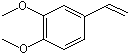
C3,4-Dimethoxy-1-vinylbenzene |
1251 |
6380-23-0
 |
No
Europe: ND
USA: 0.01 |
NR |
NR |
See notes 3 and 6 |
No safety concern |
|
Diphenyl ether |
1255 |
101-84-8
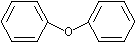 |
No
Europe: 14
USA: 5 |
NR |
NR |
See note 8 |
No safety concern |
|
Dibenzyl ether |
1256 |
103-50-4
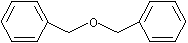 |
Yes
Europe: 0.6
USA: 241 |
No |
Yes. The NOEL of 196 mg/kg bw per day and >620 mg/kg bw per (females) day (males) for dibenzyl ether (Burdock & Ford, 1992) is >10 000 000 and >10 000 times greater than the estimated daily intakes of 0.01 µg/kg bw in Europe and 4 µg/kg bw in the USA, respectively, when used as a flavouring agent |
See notes 3 and 6 |
No safety concern |
|
beta-Naphthyl methyl ether |
1257 |
93-04-9
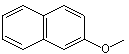 |
No
Europe: ND
USA: 0.01 |
NR |
NR |
See note 9 |
No safety concern |
|
Cbeta-Naphthyl ethyl ether |
1258 |
93-18-5
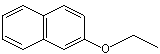 |
No
Europe: ND
USA: 4 |
NR |
NR |
See note 9 |
No safety concern |
|
beta-Naphthyl isobutyl ether |
1259 |
2173-57-1
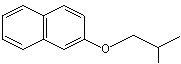 |
No
Europe: 1
USA: 2 |
NR |
NR |
See note 9 |
No safety concern |
|
CAS: Chemical Abstracts Service; ND: no intake data reported; NR: not required for evaluation because consumption of the substance was determined to be of no safety concern at Step A3 of the Procedure. |
|
a |
Step 2: All of the flavouring agents in this group are expected to be metabolized to innocuous products. |
|
b |
The threshold for human intake for structural classes I, II and III are 1800 µg/day, 540 µg/day and 90 µg/day, respectively. All intake values are expressed in mg per day. |
| |
The combined per capita intakes of flavouring agents in structural class I is 29 µg per day in Europe and 44 µg per day in the USA. The combined per capita intake of flavouring agents in structural class II is 1491 µg per day in Europe and 2115 µg per day in the USA. The combined per capita intake of flavouring agents in structural class III is 40 µg per day in Europe and 366 µg per day in the USA. |
|
Notes: |
|
1. |
Metabolized primarily by p-hydroxylation with O-demethylation, and o-hydroxylation is the minor pathway |
|
2. |
Metabolized primarily by m-hydroxylation with O-demethylation |
|
3. |
Metabolized by O-demethylation |
|
4. |
Metabolized by cytochrome P450-catalysed O-dealkylation to the corresponding alcohol and aldehyde, followed by complete oxidation in the fatty acid pathway and tricarboxylic acid cycle |
|
5. |
Oxidized by cytochrome P450 isoenzymes to polar metabolites, followed by conjugation with glucuronic acid and excretion in the urine |
|
6. |
Metabolized by ring hydroxylation |
|
7. |
Metabolized by O-demethylation, alpha and omega-1 oxidation of the side chain and side chain degradation |
|
8. |
Metabolized by ring hydroxylation followed by conjugation with glucuronic acid and excretion |
|
9. |
Excreted as a glucuronic acid conjugate with the methyl ether linkage intact |
1.2 Estimated daily intake
The total annual volume of production of the 29 flavouring agents in this group is approximately 11 000 kg in Europe (International Organization of the Flavour Industry, 1995) and 19 000 kg in the USA (National Academy of Sciences, 1970, 1982 and 1987; Lucas et al., 1999). More than 90% of the total annual volume of production in Europe and >75% in the USA is accounted for by eucalyptol (No. 1234). The estimated daily per capita intake of eucalyptol in Europe and the USA is 1439 µg and 1954 µg, respectively. The daily per capita intakes of the other flavouring agents in the group range from 0.003–241 µg/day (National Academy of Sciences, 1970, 1982 and 1987; International Organization of the Flavour Industry, 1995; Lucas et al., 1999). The daily per capita intake of each agent in Europe and in the USA is reported in Table 1.
1.3 Absorption, distribution, metabolism and elimination
The aliphatic ethers in this group are either open chain (Nos 1231–1232) or cyclic compounds (Nos 1233–1240). The open-chain aliphatic compounds can be expected to undergo O-dealkylation to yield the corresponding aldehyde and alcohol, followed by complete oxidation in the fatty acid pathway and tricarboxylic acid cycle (Krantz & Carr, 1969). The alicyclic ethers can be expected to undergo either ring hydroxylation or side-chain oxidation followed by conjugation with glucuronic acid and excretion in the urine (Madyastha & Chadha, 1986; Asakawa et al., 1988).
Most of the aromatic flavouring agents in this group have single benzene ring structures with an ether group and one or more simple saturated (Nos 1241–1250 and 1252–1254) or unsaturated (No. 1251) side-chains. Some have dual methoxy groups (Nos 1248–1251). Others in this group have two aromatic rings, which are either separate (Nos 1255 and 1256) or fused (Nos 1257–1259). These aromatic ethers are expected to be metabolized by one or more of three pathways (ring hydroxylation, O-dealkylation, or side-chain oxidation), depending on the location of the substituents, and then conjugated with glucuronic acid, sulfate or glycine (Bray et al., 1955; Daly, 1970; Law & Chakrabarti, 1983; Sangster et al., 1983; Takahara et al., 1986).
1.4 Application of the Procedure for the Safety Evaluation of Flavouring Agents
|
Step 1. |
In applying the Procedure for the Safety Evaluation of Flavouring Agents (see Figure 1, Introduction) to the 29 flavouring agents in this group, the Committee assigned nine (Nos 1241–1243 and 1245–1250) to structural class I. Twelve flavouring agents (Nos 1231–1239, and 1252–1254) were assigned to structural class II and the remaining eight (Nos 1240, 1244, 1251, 1255–1259) were assigned to structural class III (Cramer et al., 1978). |
|
Step 2. |
All the flavouring agents in this group are expected to be metabolized to innocuous products. The evaluation of all agents in this group therefore proceeded via the A-side of the decision-tree. |
|
Step A3. |
The estimated daily per capita intakes of all nine of the flavouring agents in structural class I, 11 of the 12 agents in structural class II, and six of the eight agents in structural class III are below the threshold of concern (i.e. 1800 µg for class I, 540 µg for class II and 90 µg for class III). The Committee concluded that these 26 substances would not be expected to be of safety concern when used as flavouring agents at currently estimated levels of intake. Intake of one of the agents in structural class II, eucalyptol (No. 1234), and two agents in structural class III, p-propylanisole (No. 1244) and dibenzyl ether (No. 1256), exceed the thresholds of concern for class II and III. The daily intake of eucalyptol per capita has been reported to be 1439 µg in Europe and 1954 µg in the USA. The daily intake per capita of p-propylanisole is 23 µg in Europe and 114 µg in the USA. The daily intake per capita of dibenzyl ether is 0.6 µg in Europe and 241 µg in the USA. Accordingly, the evaluation of these agents proceeded to step A4. |
|
Step A4. |
None of these three flavouring agents is endogenous in humans. The evaluation of these substances therefore proceeded to step A5. |
|
Step A5. |
The no-observed-effect level (NOEL) of >32 mg/kg bw per day (Roe et al., 1979) for eucalyptol (No. 1234) is approximately 1000 times greater than the estimated intake of eucalyptol from its use as a flavouring agent in Europe (24 µg/kg bw per day) and in the USA (33 µg/kg bw per day)1. The NOEL of 300 mg/kg bw per day for p-propenylanisole, identified by the Committee (Annex 1, reference 138), provides a margin of safety that is approximately 150 000 times greater than the highest estimated intake of p-propylanisole (No. 1244) from its use as a flavouring agent (0.4 µg/kg bw per day in Europe and 2 µg/kg bw per day in the USA). The NOEL of 196 mg/kg bw per day (Burdock & Ford, 1992) for dibenzyl ether (No. 1256) provides a margin of safety that is 50 000 times greater than the highest estimated intake of dibenzyl ether from its use as a flavouring agent (0.01 µg/kg bw per day in Europe and 4 µg/kg bw per day in the USA). The Committee therefore concluded that the safety of these agents raises no concern at their currently estimated levels of use. |
Table 1 summarizes the evaluations of the 29 aliphatic and aromatic ethers (Nos 1231–1259) in this group.
1.5 Consideration of combined intakes from use as flavouring agents
All 29 agents in this group are expected to be metabolized efficiently and the available metabolic pathways would not be saturated. Evaluation of all the data indicated no safety concern associated with combined intake.
1.6 Consideration of secondary components
Two members of this group, 1,4-cineole (No. 1233) and benzyl butyl ether (No. 1253), have a minimum assay value <95%. Information on the safety of the secondary components of these three compounds is summarized in Annex 6 (Summary of the safety evaluation of secondary components of flavouring agents with minimum assay values of less than 95%). 1,8-Cineole (No. 1234), the secondary component in 1,4-cineole, was evaluated at the present meeting, while benzyl alcohol (No. 25), the secondary component in benzyl butyl ether, was evaluated at a previous meeting (Annex 1, reference 122). The secondary components in these two flavouring agents were considered not to present a safety concern.
1.7 Conclusions
The Committee concluded that none of the flavouring agents in this group of aliphatic and aromatic ethers would raise a safety concern at the currently estimated levels of intake. Other data on the toxicity and metabolism of these aromatic and aliphatic ethers were consistent with the results of the safety evaluation.
2. RELEVANT BACKGROUND INFORMATION
2.1 Additional considerations on intake
Of the 29 substances in this group, 23 have been reported to occur naturally in foods (Table 2). Aliphatic and aromatic ethers have been detected in a variety of foods including fruits, vegetables, alcoholic beverages, coffee and tea (Maarse et al., 1999). The ether with the highest annual volume of production, eucalyptol (No. 1234), is widely distributed in the plant kingdom (see Table 3). The oils derived from these plants have been reported to contain significant amounts of eucalyptol. In fact, eucalyptol is one of the few substances obtained exclusively by isolation from the essential oil of eucalyptus, which contains up to 85% eucalyptol (Bauer & Garbe, 1985). The percentage of eucalyptol in essential oils is: eucalyptus oil, 47.7–85% (Bauer & Garbe, 1985; Silvestre et al., 1997); rosemary oil, 14–50% (Ravid et al., 1993), cardamon oil, 23.40–51.30% (Bernhard et al., 1971); basil oil, 0.94–12.91% (Marotti et al., 1996); spearmint oil, 0.8–2.6% (Smith et al., 1963; Murray et al., 1972); peppermint oil, 1.40–7.30% (Derbesy et al., 1991); and corn mint oil, trace amounts–2.57% (Gasic et al., 1992). The consumption ratio calculated for eucalyptol is approximately 23, indicating that exposure occurs predominantly from the consumption of traditional foods and essential oils (i.e. consumption ratio, >1) (Stofberg & Kirschman, 1985; Stofberg & Grundschober, 1987; Lucas et al., 1999; Maarse et al., 1999) (see Tables 2 and 3).
Table 2. Annual volumes of production of aliphatic and aromatic ethers used as flavouring agents
|
Flavouring agent (No.) |
Most recent annual volume (kg)a |
Intakeb ("eaters only") |
Annual volume in naturally occurring foods (kg)c |
Consumption ratiod |
|
µg/day |
µg/kg bw per day |
|
sec-Butyl ethyl ether (1231) |
|
Europe |
57 |
8 |
0.1 |
|
|
| |
|
|
|
|
|
|
USA |
2 |
0.3 |
0.005 |
+ |
NA |
|
1-Ethoxy-3-methyl-2-butene (1232) |
|
Europe |
6 |
0.9 |
0.01 |
|
|
|
USAe |
11 |
2 |
0.03 |
+f |
NA |
|
1,4-Cineole (1233) |
|
Europe |
32 |
5 |
0.08 |
|
|
|
USA |
1 107 |
146 |
2 |
17 |
0.02 |
|
Eucalyptol (1234) |
|
Europe |
10 087 |
1439 |
24 |
|
|
|
USA |
14 832 |
1954 |
33 |
337 484 |
23 |
|
Nerol oxide (1235) |
|
Europe |
7 |
1 |
0.02 |
|
|
|
USA |
5 |
0.7 |
0.01 |
+ |
NA |
|
2,2,6-Trimethyl-6-vinyltetrahydropyran (1236) |
|
Europe |
0.1 |
0.01 |
0.0002 |
|
|
|
USAe |
45 |
8 |
0.1 |
18 |
0.4 |
|
Tetrahydro-4-methyl-2-(2-methylpropen-1-yl)pyran (1237) |
|
Europe |
31 |
4 |
0.07 |
|
|
|
USA |
2 |
0.2 |
0.004 |
+ |
NA |
|
Theaspirane (1238) |
|
Europe |
14 |
2 |
0.03 |
|
|
|
USA |
0.9 |
0.1 |
0.002 |
+ |
NA |
|
Cycloionone (1239) |
|
Europe |
ND |
ND |
ND |
|
|
|
USAe |
9 |
2 |
0.03 |
+ |
NA |
|
1,5,5,9-Tetramethyl-13-oxatricyclo(8.3.0.0(4,9))tridecane (1240) |
|
Europe |
10 |
1 |
0.02 |
|
|
|
USA |
0.9 |
0.1 |
0.002 |
- |
NA |
|
Anisole (1241) |
|
Europe |
0.2 |
0.03 |
0.0005 |
|
|
|
USA |
0.05 |
0.01 |
0.0001 |
+ |
NA |
|
o-Methylanisole (1242) |
|
Europe |
20 |
3 |
0.05 |
|
|
|
USA |
0.5 |
0.06 |
0.001 |
+ |
NA |
|
p-Methylanisole (1243) |
|
Europe |
4 |
0.5 |
0.01 |
|
|
|
USA |
113 |
15 |
0.2 |
+ |
NA |
|
p-Propylanisole (1244) |
|
Europe |
161 |
23 |
0.4 |
|
|
|
USA |
862 |
114 |
2 |
+ |
NA |
|
2,4-Dimethylanisole (1245) |
|
Europe |
ND |
ND |
ND |
|
|
|
USAe |
1 |
0.2 |
0.003 |
+ |
NA |
|
1-Methyl-3-methoxy-4-isopropylbenzene (1246) |
|
Europe |
14 |
2 |
0.03 |
|
|
|
USA |
0.5 |
0.1 |
0.001 |
+ |
NA |
|
Carvacryl ethyl ether (1247) |
|
Europe |
0.7 |
0.1 |
0.002 |
|
|
|
USA |
0.2 |
0.02 |
0.0004 |
- |
NA |
|
1,2-Dimethoxybenzene (1248) |
|
Europe |
ND |
ND |
ND |
|
|
|
USAe |
113 |
20 |
0.3 |
+ |
NA |
|
m-Dimethoxybenzene (1249) |
|
Europe |
38 |
5 |
0.09 |
|
|
|
USA |
17 |
2 |
0.04 |
+ |
NA |
|
p-Dimethoxybenzene (1250) |
|
Europe |
124 |
18 |
0.3 |
|
|
|
USA |
54 |
7 |
0.1 |
+ |
NA |
|
3,4-Dimethoxy-1-vinylbenzene (1251) |
|
Europe |
ND |
ND |
ND |
|
|
|
USAg |
0.05 |
0.01 |
0.0001 |
3 347 |
66 940 |
|
Benzyl ethyl ether (1252) |
|
Europe |
0.02 |
0.003 |
0.00005 |
|
|
|
USAg |
14 |
2 |
0.04 |
15 |
1 |
|
Benzyl butyl ether (1253) |
|
Europe |
ND |
ND |
ND |
|
|
|
USAg |
0.1 |
0.02 |
0.0003 |
+ |
NA |
|
Methyl phenethyl ether (1254) |
|
Europe |
216 |
31 |
0.5 |
|
|
|
USA |
0.1 |
0.01 |
0.0002 |
+ |
NA |
|
Diphenyl ether (1255) |
|
Europe |
100 |
14 |
0.2 |
|
|
|
USA |
39 |
5 |
0.1 |
+ |
NA |
|
Dibenzyl ether (1256) |
|
Europe |
4 |
0.6 |
0.01 |
|
|
|
USA |
1 828 |
241 |
4 |
- |
NA |
|
beta-Naphthyl methyl ether (1257) |
|
Europe |
ND |
ND |
ND |
|
|
|
USAg |
0.05 |
0.01 |
0.0001 |
- |
NA |
|
beta-Naphthyl ethyl ether (1258) |
|
Europe |
ND |
ND |
ND |
|
|
|
USA |
29 |
4 |
0.06 |
- |
NA |
|
beta-Naphthyl isobutyl ether (1259) |
|
Europe |
10 |
1 |
0.02 |
|
|
|
USAg |
9 |
2 |
0.03 |
- |
NA |
|
Total |
|
|
|
|
|
|
Europe |
10 929 |
|
|
|
|
|
USA |
19 095 |
|
|
|
|
|
NA, not available; ND, no intake data reported; +, reported to occur naturally in foods (Maarse et al., 1999), but no quantitative data; -, not reported to occur naturally in foods |
|
a |
From International Organization of the Flavour Industry (1995) and Lucas et al. (1999) or National Academy of Sciences (1970, 1982, 1987). |
|
b |
Intake (mg/person per day) calculated as follows: |
| |
[(annual volume, kg) × (1 × 109 µg/kg)]/[population × survey correction factor × 365 days], |
| |
where population (10%, "eaters only") =32 × 106 for Europe and 26 × 106 for the USA; |
| |
where correction factor =0.6 for Europe and USA National Academy of Sciences surveys and 0.8 for the Lucas et al. survey in the USA, representing the assumption that only 60% and 80% of the annual volume of flavouring agent, respectively, was reported in the poundage surveys (International Organization of the Flavour Industry, 1995; Lucas et al., 1999; National Academy of Sciences, 1970, 1982, 1987). |
| |
Intake (µg/kg bw per day) calculated as follows: (µg/person per day)/body weight, where body weight =60 kg. Slight variations may occur from rounding. |
|
c |
Quantitative data for the United States reported by Stofberg & Grundschober (1987). |
|
d |
The consumption ratio was calculated as follows: (annual consumption via food, |
| |
kg)/(most recent reported volume as a flavouring agent, kg) |
|
e |
The volume cited is the anticipated annual volume, which was the maximum amount of flavouring agent estimated to be used annually by the manufacturer at the time the material was proposed for flavour use. Subsequent national surveys (National Academy of Sciences, 1970, 1982, 1987; Lucas et al., 1999) revealed no reported use of the agent as a flavouring agent. |
|
f |
Natural occurrence data were reported in a private communication to FEMA (1983, 1985, 1990). |
|
g |
Annual volume reported in previous USA surveys (NAS, 1970; 1982; 1987). |
Table 3. Consumption of eucalyptol from foods and essential oils in the USA
|
Food |
Annual consumption of this food in the USA (kg/year) |
Concentration of eucalyptol in food (kg/kg food)c |
Annual consumption of eucalyptol via this food in the USA (kg)d |
|
Cinnamon |
1 840 000a |
0.020 |
36 800 |
|
Peppermint oil |
460 000a |
0.085 |
39 100 |
|
Spearmint oil |
230 000a |
0.026 |
5 980 |
|
Cornmint oil |
21 137b |
0.03 |
634 |
|
Eucalyptol oil |
82 100b |
0.8657 |
71 074 |
|
Ginger |
879 969b |
0.112 |
98 557 |
|
Rosemary |
207 745b |
0.410 |
85 175 |
|
Sage oil |
776b |
0.211 |
164 |
|
Total |
|
|
337 484 |
| |
|
|
|
|
Flavouring agent |
Annual consumption via food in the USA (kg) |
Annual consumption as added flavouring agent in the USA (kg)b |
Consumption ratioe |
|
Eucalyptol |
337 484 |
14 832 |
23 |
|
a |
From Stofberg & Grundschober (1987) |
|
b |
From Lucas et al. (1999) |
|
c |
From Maarse et al. (1999) |
|
d |
Annual consumption of this food in USA (kg/year) × concentration of eucalyptol in this food (kg/kg food) =annual consumption of eucalyptol via this food in the USA (kg) |
|
e |
The consumption ratio is calculated as follows: (annual consumption via food, kg)/(most recent reported volume as flavouring agent, kg) |
Quantitative data on natural occurrence and consumption ratios reported for two other flavouring agents (3,4-dimethoxy-1-vinylbenzene (No. 1251); and benzyl ethyl ether (No. 1252)) also indicate that exposure occurs predominantly from consumption of traditional foods (Stofberg & Kirschman, 1985; Stofberg & Grundschober, 1987) (Table 2).
2.2 Biological data
2.2.1 Biochemical data
(a) Absorption, distribution and excretion
The simple aliphatic and aromatic ethers in this group are expected to be rapidly absorbed from the gastrointestinal tract and excreted in the urine.
(i) Aliphatic ethers (Nos 1231–1240)
Data on absorption, metabolism and urinary excretion of the aliphatic acyclic ethers in animals are available for the structurally related substance methyl tert-butyl ether2. In rats, methyl tert-butyl ether is rapidly absorbed, metabolized and excreted after oral administration at a dose of 400 mg/kg bw, and the reported halflife is 30 min in the blood. No tissue-specific affinity was observed (Miller et al., 1997).
In rabbits, peak plasma concentrations of the alicyclic substance, eucalyptol (No. 1234) and its major unconjugated metabolites occurred within 30 min and 1 h, respectively, after oral administration of a dose of 200 mg/kg bw. The parent ether reached a maximum plasma concentration of 840 µg/dl within 30 min, while the plasma concentration of the principal unconjugated metabolite, (+)-2-exo-hydroxy-1,8-cineole, peaked at 2400 µg/dl within 1 h and then decreased slowly between 2 h and 6 h. Peak plasma concentration (1250 µg/dl) of the major conju-gated metabolite, (+)-2-exo-hydroxy-1,8-cineole, occurred 1.5–2 h after dosing (Miyazawa et al., 1989). In mice, blood concentrations of eucalyptol reached a peak 5 min after oral administration of 4, 20, or 40 µl of rosemary oil containing 39% eucalyptol (approximately equivalent to 52, 260 and 520 mg/kg bw of eucalyptol, respectively). At 260 mg/kg bw, blood concentrations remained fairly constant over the following 90 min, while at 520 mg/kg bw, the peak blood concentration dropped to 60% of the maximum value and remained in that range for the following 80 min (Kovar et al., 1987). These results indicate that at doses of up to 200 mg/kg bw, eucalyptol undergoes rapid absorption into the blood, metabolism and conjugation to polar metabolites. At higher doses, however, metabolism appears to be slower, probably due to saturation of the metabolic pathway.
The intrinsic clearance (CL’int =Vmax/Km) of eucalyptol was studied in microsomes from male Hooded Wistar rats and humans. To determine the effect on clearance caused by induction of metabolism, rats were treated once a day for 6 days with either a mixture of terpenes (eucalyptol, 255 mg/kg bw; p-cymene, 4 mg/kg bw; l-limonene, 34 mg/kg bw; and alpha-pinene, 103 mg/kg bw) by gavage or with phenobarbitone at a dose of 80 mg/kg bw. Liver microsomes from the rats treated with the mixture of terpenes or with phenobarbitone, and from control rats, and pooled liver microsomes from seven male patients were incubated with eucalyptol (5–200 µmol/l) for 10 min (except for microsomes from rats treated with phenobarbitone, which were incubated for 5 min). Intrinsic clearance values were as follows: control rats, 27.5 µl/µg of protein per min; terpene-treated rats, 258.2 µl/mg of protein per min; phenobarbitone-treated rats, 1824.7 µl/mg of protein per min; and for human patients, 11.6 µl/mg of protein per min. Rats treated with terpenes or with phenobarbitone metabolize eucalyptol at a higher rate than rats that have not been induced, suggesting that cytochrome P450 (CYP450) isoenzymes have been induced (Pass et al., 2001).
Volunteers exposed for 20 min to air passing over 4 ml of eucalyptol, via a closed breathing circuit, showed biphasic elimination of eucalyptol from the blood. The half-life for distribution was 6.7 min, while the half-life for elimination was 104.6 min (Jager et al., 1996).
(ii) Aromatic ethers (Nos 1241–1259)
In rabbits given anisole (No. 1241) at a dose of 500 mg/kg bw, or diphenyl ether (No. 1255) at a dose of 500 mg/kg bw by stomach tube, 80% of the administered dose was excreted in the urine within 24 h. The unchanged ethers were not detected in the urine and no smell of anisole or diphenyl ether was detected on the breath of the rabbits (Bray et al., 1953).
Most the administered dose of p-methylanisole (No. 1243) given to six rabbits by gavage was excreted in the urine within 24 h (Bray et al., 1955).
Female Wistar albino rats treated by oral intubation and male CD-1 mice treated intraperitoneally received a single dose of [14C]p-propylanisole (No. 1244) (labelled at the methoxy position) of 0.05, 0.5, 5, 50, 500 or 1500 mg/kg bw. At the lowest dose, most of the radiolabel was excreted as 14CO2 in the expired air (81.6% and 74.5% in rats and mice, respectively). Radiolabelled metabolites were also excreted in the urine (8.0% and 15.0% in rats and mice, respectively). At the highest dose, approximately equal amounts of radiolabel were excreted in the urine (37.1% and 38.0% in rats and mice, respectively) and in the expired air (47.2% and 49.9% in rats and mice, respectively) within 72 h, suggesting that the O-demethylation pathway becomes saturated at the higher doses administered. Approximately 1–5% of the radiolabel was excreted in the faeces regardless of the administered dose (Sangster et al., 1983).
In two humans given gelatine capsules containing 100 µg of p-propylanisole labelled with 14C at the methoxy position, approximately 67% of the administered dose was recovered within 48 h, most of which was recovered in the expired air (43% eliminated after 8 h), and the remainder in the urine (23.8, 24.4, and 24.8% eliminated after 8, 24, and 48 h, respectively) (Sangster et al., 1987).
Male Sprague-Dawley rats were given ring-labelled [14C]diphenyl ether (No. 1255) by intraperitoneal injection at a single dose of 5 mg/kg bw or by gavage at a single dose of 10 mg/kg bw. Radiolabel was detected in all organs and tissues within 1 h after intraperitoneal injection, with peak concentrations in the liver, lung, kidney and spleen being 2–10 times higher than those in the muscle, brain, heart, fats and testes. Rats given the radiolabelled diphenyl ether by gavage excreted at least 90% of the radiolabel within 3 days, with approximately 80% and 10% of the administered dose being detected in the urine and faeces, respectively (Law et al., 1983).
Simple-substituted diphenyl ethers exhibit a similar pharmacokinetic profile. More than 74% of an intravenous dose (176 µg/kg bw) of ring-labelled [14C]p-chlorodiphenyl ether administered to male Sprague-Dawley rats was excreted in the urine and faeces within 1 week. More than 90% of the radiolabel excreted in the urine was accounted for by one metabolite. When radiolabelled p-chorodiphenyl ether was incubated with rat liver microsomes, >70% was converted to a single metabolite within 30 min, while 7% was irreversibly bound to microsomal protein (Chui et al., 1987).
Male Sprague-Dawley rats were given 14C-labelled 2,2’,4,4’,5-pentachlorodiphenyl ether (a substance that is structurally related to p-chorodiphenyl ether) at a dose of 10 mg/kg bw by intravenous injection or by gavage. Radiolabel was present in all tissues examined, with peak concentrations detected in fat tissue, followed by the skin, liver and kidneys. Other tissues examined included the brain, muscle, spleen and heart. The amount of radiolabel in fat tissue peaked at day 4 while in all other tissues and in blood a peak was attained within 1 h. Radiolabel rapidly decreased in all tissues and the blood, with the exception of fat tissue, by day 21. In rats treated by gavage, 55% and 1.3% of the radiolabel was excreted in the faeces and the urine, respectively, within 7 days (Komsta et al., 1998).
(b) Biotransformation
Several metabolic options are available to aliphatic and aromatic ethers. One pathway for aliphatic and aromatic ethers is O-dealkylation to form the corresponding aldehydes and alcohols, if a suitable alkyl substituent (methyl or ethyl) is attached to the ether oxygen. The resulting alcohols may be further oxidized and then conjugated and excreted, while the aldehydes (i.e. acetaldehyde and formaldehyde) are oxidized to carboxylic acids that participate in fundamental biochemical pathways, including the fatty acid pathway and tricarboxylic acid cycle. In a second pathway, the aliphatic acyclic or aromatic moiety may undergo CYP450-induced C-oxidation (ring hydroxylation) or side-chain oxidation, followed by conjugation with sulfate or glucuronic acid, and then excretion.
(i) Aliphatic ethers (Nos 1231–1240)
Aliphatic ethers undergo NADPH-dependent, CYP450-catalysed O-dealkylation to the corresponding alcohols and aldehydes (Brady et al., 1990). In male Sprague-Dawley rats treated by intraperitoneal injection, methyl tert-butyl ether (at a dose of 1 or 5 ml/kg bw) was O-demethylated to form tert-butyl alchol and formaldehyde (Brady et al., 1990). The metabolism of methyl tert-butyl ether was inhibited by 35% by monoclonal antibodies to CYP2E1, indicating that the substance is partly metabolized by this isozyme. Pretreatment of rats with methyl tert-butyl ether at a dose of 1 or 5 ml/kg bw caused induction of CYP2B1; however, there was no change in the activity of CYP2E1 (Brady et al., 1990). In other studies of methyl tert-butyl ether administered orally, additional minor oxidation metabolites were identified, including 2-methyl-1,2-propanediol and alpha-hydroxybutyric acid (Bernauer et al., 1998).
Diethyl ether undergoes O-demethylation to ethanol and acetaldehyde, followed by oxidation to acetate, which eventually enters the citric acid cycle (Krantz & Carr, 1969). Therefore, sec-butyl ethyl ether (No. 1231) and 1-ethoxy-3-methyl-2-butene (No. 1232) are likely to undergo O-dealkylation to form the corresponding alcohols and aldehydes, which are expected to subsequently participate in the fatty acid pathway and tricarboxylic acid cycle.
In humans and laboratory animals, alicyclic ethers, such as 1,4-cineole and eucalyptol (1,8-cineole), are oxidized by CYP450 isoenzymes to yield polar hydroxylated metabolites, which are conjugated and excreted or further oxidized and excreted. Cleavage of the ether is a minor metabolic pathway (Hiroi et al., 1995; Miyazawa & Shindo, 2001; Miyazawa et al., 2001a, b).
The metabolism of eucalyptol (No. 1234) has been studied in a number of animal species. This monoterpene cyclic ether principally undergoes ring hydroxylation to form 2- or 3-hydroxy-1,8-cineole, which is subsequently excreted as the glucuronic acid conjugate (Williams, 1959b) (see Figure 1). In male albino rats given eucalyptol at a dose of 800 mg/kg bw by gavage, the major metabolites included 2- and 3-hydroxy-1,8-cineole, and 1,8-dihydroxy-10-carboxy-p-menthane, which was possibly formed by oxidation of the metabolite p-menthane-1,8-diol produced by cleavage of the ether linkage (Madyastha & Chadha, 1986).
Figure 1. Metabolism of eucalyptol in rats and humans
These results are consistent with those of a later study (Pass et al., 2001; also described under 2.1.1(a)). Hooded Wistar rats were treated once a day for 6 days with either a mixture of terpenes (eucalyptol, 255 mg/kg bw; p-cymene, 4 mg/kg bw; l-limonene, 34 mg/kg bw; and alpha-pinene, 103 mg/kg bw) by gavage or with phenobarbitone at a dose of 80 mg/kg bw. Liver microsomes prepared from rats treated with either the mixture of terpenes or with phenobarbitone, and from control rats, and pooled liver microsomes from seven male patients were incubated with eucalyptol (5–200 µmol/l) for 10 min (except for microsomes from rats treated with phenobarbitone, which were incubated for 5 min). The microsomes from rats treated with the mixture of terpenes produced similar amounts of 2- and 3-hydroxy-1,8-cineole and lesser amounts of the 9-hydroxy-1,8-cineole metabolite. Of the six metabolites detected in the microsomes from rats treated with phenobarbitone, 2-hydroxy-1,8-cineole was the major metabolite, followed by 3- and 9-hydroxy-1,8-cineole. The remaining three metabolites included trace amounts of 7-hydroxy-1,8-cineole, 9-cineolic acid and one unknown hydroxycineole metabolite. In microsomes from the control rats (no treatment), 3-hydroxy-1,8-cineole was the primary metabolite, followed by 2- and 9-hydroxycineole. 2-Hydroxy-1,8-cineole was the major metabolite identified in pooled microsomes from human liver, while 9-hydroxy-1,8-cineole was the minor metabolite. The authors concluded that hydroxylation of the alicyclic ring positions rather than oxidation of methyl substituents is the preferred metabolic route in rats and humans (Pass et al., 2001).
The metabolism of eucalyptol and 1,4-cineole (No. 1233) was studied in rat and human liver microsomes and in recombinant CYP450 enzymes expressed in insect cells, in which human CYP450 and NADPH-P450 reductase cDNAs had been introduced. Insect cells that expressed recombinant human CYP450 enzymes oxidized 1,4-cineole to 2-exo-hydroxy-1,8-cineole; recombinant CYP3A4 showed the greatest activity, followed by 2B6 and 2A6 (Miyazawa et al., 2001a). Eucalyptol and 1,4-cineole were oxidized at high rates to 2-exo-hydroxy-1,8-cineole (see Figure 1) and 2-exo-hydroxy-1,4-cineole3, respectively, by the enzymes in the CYP3A family, specifically, CYP3A4 in both rat and human liver microsomes (Miyazawa et al., 2001a, b; Miyazawa & Shindo, 2001). Earlier studies indicate that members of the CYP3A and 2B family of isoenzymes are also induced by eucalyptol. In a study in which hepatic microsomes were prepared from male Sprague-Dawley rats that had been intraperitoneally injected with eucalyptol at a dose of 300 mg/bw kg once a day for five days, and then killed, assays for metabolic activities and expression of individual forms of CYP450 (1A1, 2A2, 2B1, 2C11, 2E11, 3A2 and 4A2) showed that eucalyptol induces CYP450 isoenzymes 2B1 and 3A2 (Hiroi et al., 1995).
In a study to investigate the inhibitory effects of eucalyptol on the activities of CYP1A1, 1A2 and 2B1, female Wistar rats were treated with either beta-napthoflavone at a dose of 80 mg/kg bw per day by intraperitoneal injection for 4 consecutive days, or with drinking-water containing 0.1% (w/v) sodium phenobarbital for 4 days plus a single injection of sodium phenobarbital of 40 mg/kg bw on day 5. In microsomes from rats treated with beta-napthoflavone, eucalyptol caused 50% inhibition of the activity of methoxyresorufin-O-demethylase (a selective marker for CYP1A2) at concentrations of >300 µmol/l (the IC50), but had no inhibitory effect on the activity of ethoxyresorufin-O-deethylase (a selective marker for CYP1A1) at concentrations of <150 µmol/l. In microsomes from rats treated with phenobarbital, eucalyptol inhibited the activity of pentoxyresorufin-O-depenylase (a selective marker for CYP2B1) with an IC50 of 4.7 µmol/l. The authors concluded that eucalyptol is a selective inhibitor of CYP2B1, a very weak inhibitor of CYP1A2, and has no inhibitory effect on CYP1A1 (De-Oliveira et al., 1999).
In rabbits, 1,4-cineole is metabolized by ring- and side-chain hydroxylation. Urinary metabolites collected over 3 days after administration of 10 000 mg of 1,4-cineole included the ring hydroxylation product 3,8-dihydroxy-1,4-cineole, the side-chain hydroxylation product 9-hydroxy-1,4-cineole and its corresponding carboxylic acid, 1,4-cineole-9-carboxylic acid. Other metabolites included 8,9-dihydroxy-1,4-cineole and 1,4-cineole-8-en-9-ol. No evidence of ether cleavage was observed at this dose (Asakawa et al., 1988).
(ii) Aromatic ethers (Nos 1241–1259)
The aromatic ethers in this group are metabolized by ring hydroxylation, cleavage of the methyl ether (O-demethylation), and/or oxidation of the ring substituents.
Several studies have demonstrated that anisole (No. 1241) principally undergoes CYP450-induced ring-hydroxylation preferentially at the para position, with O-demethylation and ortho- hydroxylation as the minor pathways (Daly & Jerina, 1969; Daly, 1970; Takahara et al., 1986; Ohi et al., 1992). After a 15-min incubation in vitro with liver microsomes obtained from rats treated with 3-methylcholanthrene, [2–2H]anisole (25–50 µmol) underwent ortho-and para-hydroxylation to form 2- and 4-hydroxyanisole, respectively (Daly & Jerina, 1969). In a similar study, anisole (50 µmol) was incubated for 15 min with liver homogenates from rats treated with 3-methylcholanthrene. The resulting metabolites included p-hydroxyanisole (4 µmol), o-hydroxyanisole (0.8 mmol) and phenol (0.2 µmol). Thus, in liver microsomes in vitro, the major metabolic pathway for anisole is p-hydroxylation, while O-demethylation and o-hydroxylation are minor pathways (Daly, 1970).
The effect of concentration of oxygen on the metabolism of anisole was investigated in a later study in which microsomes from the liver of rats treated with phenobarbital were incubated with anisole (2 mmol/l) for 1 h at different concentrations of oxygen (24, 34, 54, 74, 113 or 223 µmol/l). Metabolites identified at all concentrations of oxygen were the O-demethylated product, phenol, and the aromatic hydroxylated products, p-hydroxyanisole and o-hydroxyanisole. The formation rates and composition of these metabolites were dependent on concentration of oxygen, as the amount of O-demethylated product decreased at concentrations of oxygen of <60 µmol/l (typical pressure of oxygen in the liver was 35 µmol/l) (Takahara et al., 1986). In a second study using the same protocol, more p-hydroxyanisole than phenol and o-hydroxyanisole was formed at all oxygen concentrations (Ohi et al., 1992).
Experiments with anisole in vivo confirm that ring-hydroxylation predominates over O-demethylation. Analysis of urine collected from rabbits 24 h after treatment by gavage with anisole at a dose of 0.5 g/kg bw revealed that 2% of the excreted anisole was unconjugated, 48% was conjugated with glucuronic acid, and 29% was conjugated with sulfate. The metabolites of anisole are p-methoxyphenol (major) and o-methoxyphenol (minor). No evidence of ether cleavage was detected in either anisole or diphenyl ether (Bray et al., 1953). Incubation of anisole (2 mmol) with rabbit liver microsomes for 1 h resulted in ether cleavage to phenol and formaldehyde at a relative enzymatic dealkylation rate of 6% from p-ethoxyacetanilide to p-acetamidophenol. The results of this study demonstrate that anisole undergoes limited ether cleavage in the rabbit (Axelrod, 1956).
ortho-Substituted anisoles (e.g. o-methylanisole, No. 1242) are metabolized in the same way as anisole, mainly by hydroxylation at the para-position, but with less O-demethylation. As expected, alkyl substituents at the para-position, (e.g. p-methylanisole) block para-hydroxylation, leading to an increase in O-demethylation and hydroxylation at the meta-position. Incubation of rat liver homogenate with o-methylanisole (50 µmol/l) for 15 min yields the ring-hydroxylation products 4-hydroxy-2-methylanisole (major product) and 6-hydroxy-2-methylanisole (minor product), and to a lesser extent, the O-demethylation product, o-cresol (trace amounts). Incubation with p-methylanisole (50 µmol/l) yields the O-demethylation product p-cresol (major product) and to a lesser extent, the ring hydroxylation products, 2-hydroxy-4-methylanisole (minor product) and 3-hydroxy-4-methylanisole (minor product) (Daly, 1970).
In rabbits treated by gavage, p-methylanisole (No. 1243; 700 mg) undergoes mainly methyl group oxidation to yield anisic acid (p-methoxybenzoic acid), which is excreted as the glucuronic acid conjugate in the urine. A smaller amount (27%) of p-methylanisole is demethylated and excreted in the urine as the sulfate or glucuronic acid conjugate of p-cresol. In humans and dogs, anisic acid (p-methoxybenzoic acid) is excreted as conjugates of glucuronic acid and glycine (Bray et al., 1955).
[14C]p-Propylanisole (No. 1244) labelled at the methoxy position is mostly metabolized via O-demethylation, alpha- and omega-1 oxidation of the side-chain, and side-chain degradation (see Figure 2). In a study in groups of female Wistar albino rats given [14C]p-propylanisole by oral intubation and in male CD-1 mice given [14C]p-propylanisole intraperitoneally at a dose of 0.05, 0.5, 5, 50, 500 or 1500 mg/kg bw for both species, at the lowest dose, more radiolabel was excreted as 14CO2 in expired air (81.6% and 74.5 % in rats and mice, respectively) than in urine (8.0% and 15.0% in rats and mice, respectively) within 72 h. As the dose increased, a metabolic shift to alpha and omega-1 hydroxylation occurred, yielding greater amounts of the glucuronic acid urinary conjugates of 1- and 2-hydroxy-p-propyl anisole and the side-chain degradation product, p-methoxybenzoic acid conjugated with glycine (Sangster et al., 1983). Plausible metabolic routes for p-propylanisole (No. 1244) in rats and mice, on the basis of the results of this study and of other available studies in the literature with structurally related substances such as trans-anethole (p-propenylanisole) and estragole, are presented in Figure 2.
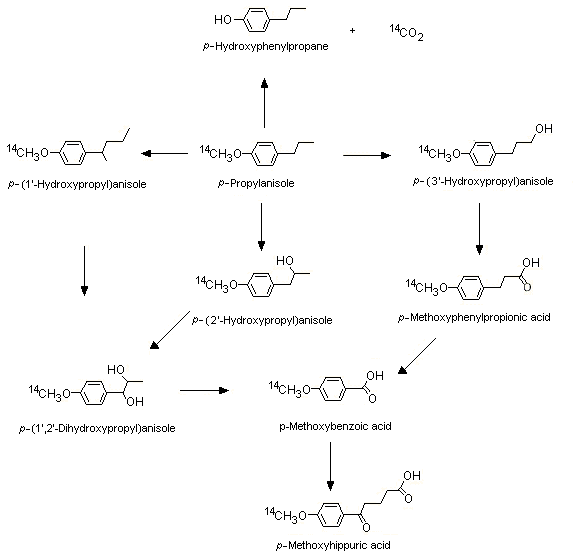
Figure 2. Metabolism of p-propylanisole in rats and mice
At low doses, the O-demethylation pathway for the metabolism of p-propylanisole predominates in humans as well. In a study in humans, two male volunteers were fed a gelatine capsule containing 100 µg of [14C]p-propylanisole (equivalent to a dose of 1.5 µg/kg bw). Most (42.7%) of the radiolabel was excreted as exhaled 14CO2 within 48 h, demonstrating that O-demethylation was the principal metabolic pathway for p-propylanisole. Other identified metabolic products included those derived from side-chain oxidation (e.g. the glucuronic acid conjugates of 1- and 2-hydroxy-p-propylanisoles and 1,2-dihydroxy-p-propylanisoles) and side-chain oxidative degradation (4-methyoxybenzoic acid) (Sangster et al., 1987).
p-Dimethoxybenzene (No. 1250), administered at a dose of 700 mg/kg bw by gavage in rabbits, undergoes extensive O-demethylation to p-methoxyphenol (34%), followed by excretion as a glucuronic acid or sulfate conjugate. Trace amounts of hydroquinone were reported. O-Demethylation of p-dimethoxybenzene was also reported to occur in rabbit liver slices in vitro (Bray et al., 1955).
Ring-labelled [14C]diphenyl ether (No. 1255), administered at a dose of 10 mg/kg bw by gavage in male Sprague-Dawley rats, was extensively metabolized to its mono-, di-, and trihydroxylated derivatives. The metabolites were 2-hydroxy-, 4-hydroxy-, 4,4’-dihydroxy-, 4-methoxy-mono-hydroxy-and 4-methoxy-dihydroxy-diphenyl ether (Law et al., 1983; Law & Chakrabarti, 1983). Incubation of labelled diphenyl ether with rat liver microsomes demonstrated that the hepatic mixed function oxidase system mediates rapid hydroxylation of the diphenyl ether (Vmax =23.3 pmol/mg microsomal protein per min; Kmax =1.33 × 10-4mol) (Law & Chakrabarti, 1983).
Analysis of urine collected 24 h after adminsistration of diphenyl ether (No. 1255) at a dose of 500 mg/kg bw by gavage in rabbits revealed that 15% of the administered dose was excreted as unconjugated phenolic metabolites of diphenyl ether; 63% was conjugated with glucuronic acid, and 12% was conjugated with sulfate. The principal initial metabolite of diphenyl ether was p-hydroxyphenyl phenyl ether; a minor metabolite, presumed to be di-(p-hydroxyphenyl) ether, was also reported. The hydroxylated products of diphenyl ether were unconjugated (15%), conjugated with glucuronic acid (63%) or conjugated with sulfate (12%). No evidence of ether cleavage was reported (Bray et al., 1953).
The metabolites of diphenyl ether, 2-hydroxy-, 4-hydroxy-, 4,4’-dihydroxy-, 4-methoxy-mono-hydroxy- and 4-methoxy-dihydroxy-diphenyl ether, but not the parent ether, were found in the urine of guinea-pigs treated with diphenyl ether at a dose of 28 mg/kg bw by intraperitoneal injection. The authors also noted the presence of free and conjugated diphenyl ether metabolites in the urine of guinea-pigs (Poon et al., 1986).
beta-Naphthyl methyl ether (No. 1257) was excreted as a glucuronic acid conjugate in which the methyl ether linkage was identified intact. The exact position of the glucuronic acid residue on the naphthyl moiety was not identified (Williams, 1959a).
In conclusion, the straight-chain aliphatic ethers, sec-butyl ethyl ether (No. 1231) and 1-ethoxy-3-methyl-2-butene (No. 1232) may undergo O-dealkylation in vivo to yield the corresponding alcohol and aldehyde that subsequently undergo complete oxidation in the fatty acid pathway and tricarboxylic acid cycle. Alicyclic ethers principally undergo ring-hydroxylation by CYP450, conjugation with glucuronic acid and then excretion in the urine. The aromatic ethers may undergo ring-hydroxylation, O-demethylation or side-chain oxidation, depending upon the position of the substituents, followed by conjugation with glucuronic acid, sulfate or glycine. The data demonstrate that the substances in this group are rapidly absorbed, distributed, metabolized and excreted.
2.2.2 Toxicological studies
(a) Acute toxicity
Oral LD50 values have been reported for 17 of the 29 substances in this group and are summarized in Table 4. In rats, LD50 values range from 1680 mg/kg bw for eucalyptol (No. 1234) to >5000 mg/kg bw for nerol oxide (No. 1235), diphenyl ether (No. 1255), beta-naphthyl methyl ether (No. 1257), and beta-naphthyl isobutyl ether (No. 1259), demonstrating that the acute oral toxicity of these aliphatic and aromatic ethers is extremely low (Brownlee, 1940; Pecchiani & Saffiotti, 1957; Jenner et al., 1964; Taylor et al., 1964; Bär & Greipentog, 1967; Hart & Wong, 1971; Weir & Wong, 1971a, b; Moreno, 1973, 1977, 1978, 1980, 1981; Levenstein, 1974; Wohl, 1974; Clark et al., 1979; Sauer-Freeman, 1980; Birch, 1992; Gillman, 1997).
Table 4. Studies of the acute oral toxicity of aliphatic and aromatic ethers
|
No. |
Flavouring agent |
Species |
Sex |
LD50 (mg/kg bw) |
Reference |
|
1232 |
1-Ethoxy-3-methyl-2-butene |
Mouse |
M |
24 h: 1000–8000 |
Bahler & Bonetti (1973) |
| |
|
|
|
14 days: 1000–4000 |
|
|
1233 |
1,4-Cineole |
Rat |
NR |
3100 |
Moreno (1981) |
|
1234 |
Eucalyptol |
Rat |
M, F |
2480 |
Bär & Greipentrog (1967) |
|
1234 |
Eucalyptol |
Rat |
M, F |
2480 |
Jenner et al. (1964) |
|
1234 |
Eucalyptol |
Rat |
M, F |
1680 |
Brownlee (1940) |
|
1235 |
Nerol oxide |
Rat |
NR |
>5000 |
Moreno (1980) |
|
1236 |
2,2,6-Trimethyl-6-vinyltetrahydropyran |
Rat |
M, F |
2700 |
Sauer-Freeman (1980) |
| |
|
|
M |
2800 |
|
| |
|
|
F |
2750 |
|
|
1236 |
2,2,6-Trimethyl-6-vinyltetrahydropyran |
Mouse |
M, F |
4000–8000 |
Roure (1979) |
|
1241 |
Anisole |
Rat |
M, F |
3700 |
Taylor et al. (1964) |
|
1241 |
Anisole |
Rat |
M, F |
3700 |
Bär & Greipentrog (1967) |
|
1241 |
Anisole |
Rat |
M, F |
3700 |
Jenner et al. (1964) |
|
1243 |
p-Methylanisole |
Rat |
M, F |
1920 |
Hart & Wong (1971) |
|
1244 |
p-Propylanisole |
Rat |
M, F |
4400 |
Taylor et al. (1964) |
|
1244 |
p-Propylanisole |
Rat |
M, F |
4400 |
Jenner et al. (1964) |
|
1244 |
p-Propylanisole |
Mouse |
NR |
7300 |
Jenner et al. (1964) |
|
1244 |
p-Propylanisole |
Rat |
M, F |
4400 |
Bär & Greipentrog (1967) |
|
1245 |
2,4-Dimethylanisole |
Rat |
M, F |
>2000 |
Gilman (1997) |
|
1249 |
m-Dimethoxybenzene |
Rat |
M, F |
2500 |
Moreno (1978) |
|
1249 |
m-Dimethoxybenzene |
Rat |
M, F |
2560 |
Bär & Greipentrog (1967) |
|
1250 |
p-Dimethoxybenzene |
Rat |
M, F |
3600 |
Moreno (1973) |
|
1254 |
Methyl phenethyl ether |
Rat |
M, F |
4100 |
Moreno (1977) |
|
1255 |
Diphenyl ether |
Rat |
M, F |
3370 |
Weir & Wong (1971a) |
|
1255 |
Diphenyl ether |
Rat |
F |
3990a, 5660b |
Pecchiai & Saffiotti (1957) |
|
1255 |
Diphenyl ether |
Rat |
F |
4100c |
Clark et al. (1979) |
|
1255 |
Diphenyl ether |
Rat |
M, F |
2450 |
Birch (1992) |
|
1256 |
Dibenzyl ether |
Rat |
M, F |
2500 |
Wohl (1974) |
|
1257 |
beta-Naphthyl methyl ether |
Rat |
M, F |
>5000 |
Levenstein (1974) |
|
1257 |
beta -Naphthyl methyl ether |
Mouse |
M, F |
825 |
Schafer & Bowles (1985) |
|
1258 |
beta Naphthyl ethyl ether |
Rat |
M, F |
3110 |
Weir & Wong (1971b) |
|
1258 |
beta -Naphthyl ethyl ether |
Mouse |
M, F |
1213 |
Schafer & Bowles (1985) |
|
1259 |
beta -Naphthyl isobutyl ether |
Rat |
M, F |
5930 |
Jenner et al. (1964) |
|
1259 |
beta -Naphthyl isobutyl ether |
Rat |
M, F |
>5000 |
Moreno (1978) |
|
1259 |
beta -Naphthyl isobutyl ether |
Rat |
M, F |
5930 |
Bär & Greipentrog (1967) |
|
M, male; F, female, NR, not reported |
|
a |
LD50 for diphenyl ether only |
|
b |
LD50 for a mixture containing 73.6% diphenyl ether and 26.4% diphenyl |
|
c |
LD50 for a mixture containing 72% diphenyl ether and 26% diphenyl |
In mice, LD50 values range from 825 mg/kg for beta-naphthyl methyl ether up to 8000 mg/kg bw for 1-ethoxy-3-methyl-2-butene (No. 1232), 2,2,6-trimethyl-6-vinyltetrahydropyran (No. 1236), and beta-naphthyl ethyl ether (No. 1258), confirming the low toxicity of aliphatic and aromatic ethers (Jenner et al., 1964; Bahler & Bonetti, 1973; Roure Inc., 1979; Schafer & Bowles, 1985).
(b) Short-term studies of toxicity
Short-term studies of toxicity and long-term studies of toxicity and carcinogenicity have been reported for 12 agents in the group. The results of studies with eucalyptol (No. 1234), tetrahydro-4-methyl-2-(2-methylpropen-1-yl)pyran (No. 1237), cycloionone (No. 1239), p-methylanisole (No. 1243), p-propylanisole (No. 1244), carvacryl ethyl ether (No. 1247), 1,2-dimethoxybenzene (No. 1248), m-dimethoxybenzene (No. 1249), diphenyl ether (No. 1255), dibenzyl ether (No. 1256), beta-naphthyl ethyl ether (No. 1258) are described below and summarized in Table 5.
Table 5. Results of short-term studies of toxicity and long-term studies of toxicity and carcinogenicity with aliphatic and aromatic ethers
|
No. |
Flavouring agent |
Species; sex |
No. of test groupsa/ no. per groupb |
Route |
Duration (days) |
NOEL (mg/kg bw per day) |
Reference |
|
Short-term studies of toxicity |
|
1234 |
Eucalyptol |
Rat; M, F |
4/12 |
Gavage |
28 |
300 (M)
1200c (F) |
National Toxicology Program (1987a) |
|
1234 |
Eucalyptol |
Rat; M, F |
4/12 |
Dietd |
28 |
NE (M)
1500c (F) |
National Toxicology Program (1987a) |
|
1234 |
Eucalyptol |
Mice; M, F |
4/12 |
Gavage |
28 |
1200c |
National Toxicology Program (1987b) |
|
1234 |
Eucalyptol |
Mice; M, F |
4/12 |
Dietd |
28 |
562.5 (M)
1125 (F) |
National Toxicology Program (1987b) |
|
1237 |
Tetrahydro-4-methyl-2-(2-methylpropen-1-yl)pyran |
Rat; M, F |
1/20–32 |
Diet |
90 |
2.514c (M)
2.805c (F) |
Posternak et al. (1969) |
|
1239 |
Cycloionone |
Rat; M, F |
1/10 |
Diet |
14 |
36.6c (M)
33.7c (F) |
Wnorowski (1997) |
|
1239 |
Cycloionone |
Rat; M, F |
4/10 |
Gavage |
28 |
120 |
Wnorowski (1998) |
|
1243 |
p-Methylanisole |
Rat; M, F |
3/20 |
Gavage |
28 |
40 |
Brunsborg et al. (1994) |
|
1244 |
p-Propylanisole |
Rat; M |
1/20 |
Gavage |
32 |
NE |
Hagan et al. (1967) |
|
1244 |
p-Propylanisole |
Rat; M, F |
3/20 |
Diet |
133(19 weeks) |
<100 |
Hagan et al. (1967) |
|
1247 |
Carvacryl ethyl ether |
Rat; M, F |
1/10 |
Diet |
14 |
<22 (M)
22c (F) |
Gill & Van Miller (1987) |
|
1248 |
1,2-Dimethoxybenzene |
Rat; M, F |
1/10 |
Diet |
14 |
10c |
Trimmer et al. (1992) |
|
1249 |
m-Dimethoxybenzene |
Rat; M, F |
1/30 |
Diet |
90 |
9.6c (M)
11.2c (F) |
Oser et al. (1965) |
|
1249 |
m-Dimethoxybenzene |
Rat; M, F |
NR |
Gavage |
84 |
10c |
Bär & Greipentrog (1967) |
|
1250 |
p-Dimethoxybenzene |
Rat; M, F |
1/5 or 10 |
Diet |
28 or 56 |
1000c |
Altmann et al. (1985) |
|
1255 |
Diphenyl ether |
Rat; M, F |
3/40 |
Diet |
91 |
250c |
Johnson et al. (1992) |
|
1256 |
Dibenzyl ether |
Rat; M, F |
1/30 |
Diet |
90 |
3.33c |
Oser (1964) |
|
1256 |
Dibenzyl ether |
Rat; M, F |
3/20–32 |
Diet |
91 |
620c (M)
196 (F) |
Burdock & Ford (1992) |
|
1258 |
beta-Naphthyl ethyl ether |
Rat; M, F |
1/30 |
Diet |
90 |
5.1c (M)
5.7c (F) |
Oser et al. (1965) |
|
1258 |
beta-Naphthyl ethyl ether |
Rat; NR |
NR |
Gavage |
84 |
5.0c |
Bär & Greipentrog (1967) |
|
Long-term studies of toxicity and carcinogenicity |
|
1234 |
Eucalyptol |
Mice; M |
2/52 |
Gavagee |
560
(80 weeks) |
32c |
Roe et al. (1979) |
|
1249 |
m-Dimethoxybenzene |
Rat; M, F |
2/20 or 40 |
Diet |
730
(2 years) |
250c |
Bär & Greipentrog (1967) |
|
1255 |
Diphenyl ether |
Rat; M |
2/8 |
Diet |
390 (13 months) |
530c |
Pecchiai & Saffiotti (1957) |
|
M, male; F, female; NR, not reported; NE, not established |
|
a |
Total number of test groups does not include control animals |
|
b |
Total number per test group includes both male and female animals Study performed with either a single dose or multiple doses that had produced no effect. The value is therefore not a true NOEL, but is the highest dose tested that produced no adverse effects. The actual NOEL may be higher |
|
d |
Eucalyptol microencapsulated in the feed |
|
e |
Eucalyptol in a mixture also containing chloroform and peppermint oil |
(i) Eucalyptol (No. 1234)
Rats
Groups of six male and six female Fischer 344 rats were given eucalyptol either by gavage or in the diet, in encapsulated form, for 28 days. Encapsulated eucalyptol was administered at a concentration of 3750, 7500, 15 000, or 30 000 ppm, equivalent to approximately 187.5, 375, 750, or 1500 mg/kg bw per day, respectively (Food & Drug Administration, 1993). In rats treated by gavage, eucalyptol was administered at a dose of 150, 300, 600, or 1200 mg/kg bw per day. Control groups received the vehicle or diet alone, or no treatment by gavage. Body-weight gain was decreased among male rats fed eucalyptol at the three highest doses in the diet (375, 750, and 1500 mg/kg bw per day), but these decreases were not statistically significant compared with values for controls. A statistically significant decrease in body-weight gain was reported in males given eucalyptol by gavage at the two highest doses (600 and 1200 mg/kg bw per day). No changes in body-weight gain were reported in treated females compared with controls. No statistically significant differences in food or water consumption were detected among males and females treated with eucalyptol either by gavage or in the diet, compared with controls. No changes in absolute or relative weights of the liver, right kidney, heart, brain, thymus, lungs, or right testicle (males) were reported in any treated animals compared with the untreated or vehicle controls.
Histopathological examination revealed evidence of centrilobular cytoplasmic vacuolization and centrilobular fatty tissue changes at all doses in males receiving diets containing eucalyptol, but these effects were not dose-related. These types of cytoplasmic alterations are typical of adaptive metabolic changes in the liver resulting from continuous exposure to high doses of test substances; these changes are reversible in nature. Other changes observed mainly at the two higher doses in males included cytoplasmic alterations of the kidney tubular epithelium and parotid salivary gland. However, there was no evidence of these types of alterations or any other alterations in females at any dose.
In rats treated by gavage, there was similar evidence of histopathology of the liver in males at 600 and 1200 mg/kg bw per day (3/6 and 6/6 animals, respectively). As for animals given diets containing eucalyptol, there was no evidence of histopathology in female rats at any dose. The authors concluded that female rats appeared to be more resistant to the effects of the test substance than males (National Toxicology Program, 1987a).
Mice
In another study conducted by the National Toxicology Program, groups of six male and six female B6C3F1 hybrid mice were given eucalyptol either in encapsulated form in the diet or by gavage, for 28 days. Encapsulated eucalyptol was provided at a concentration of 3750, 7500, 15 000, or 30 000 ppm in the diet (equivalent to approximately 562.5, 1125, 2250 or 4500 mg/kg bw per day, respectively (Food & Drug Administration, 1993). In mice treated by gavage, eucalyptol was given at a dose of 150, 300, 600, or 1200 mg/kg bw per day. Control groups received the vehicle or diet alone, or no treatment by gavage. There were no statistically significant dose-related differences in body weight, absolute organ weight, and food or water consumption between any of the treated animals and the controls. The brain weight : body weight ratios of female mice at the highest dose (4500 mg/kg bw per day) of encapsulated eucalyptol was significantly higher than those of controls and of all other treated groups. The liver weight:body weight ratios of male mice at the three higher doses (1125, 2250 and 4500 mg/kg bw per day) of encapsulated eucalyptol were significantly higher than those of controls and of animals at the lowest dose (562.5 mg/kg bw per day).
There was no significant dose-related histopathology reported in any of the treated mice. A minimal, but dose-related, hypertrophy of the centrilobular hepatocytes was reported in males receiving encapsulated eucalyptol at the three higher doses (control, 0/6; 562.5 mg/kg bw per day, 0/6; 1125 mg/kg bw per day, 1/6; 2250 mg/kg bw per day, 5/6; 4500 mg/kg bw per day, 6/6) and in females at the two higher doses (control, 0/6; 562.5 mg/kg bw per day, 1/6; 1125 mg/kg bw per day, 0/6; 2250 mg/kg bw per day, 4/6; 4500 mg/kg bw per day, 6/6), which the authors attributed to continuous exposure of the tissues to eucalyptol over a 24 h period, as compared with the mice treated by gavage, that were exposed to bolus doses. Oesophageal and stomach lesions were reported in mice treated by gavage; these lesions were attributed by the authors to possible gouging by the gavage needles. The authors concluded that, compared with Fischer 344 rats, B6C3F1 hybrid mice are less susceptible to the effects of eucalyptol, regardless of whether it is administered by gavage or encapsulated and mixed in the diet (National Toxicology Program, 1987b).
(ii) Tetrahydro-4-methyl-2-(2-methylpropen-1-yl)pyran (No. 1237)
In a 90-day study, groups of 10–16 male and 10–16 female Charles River rats were given basal diets containing tetrahydro-4-methyl-2-(methylpropen-1-yl)pyran at a concentration calculated to provide average daily intakes of 2.514 and 2.805 mg/kg bw per day for male and female rats, respectively. Animals were housed in pairs of the same sex and given food and water ad libitum. After 90 days of treatment, all the animals were killed, subjected to detailed necropsy, and liver and kidneys were excised for weighing. A wide range of tissues and organs from each animal were preserved and histopathological examinations were performed on major organs and tissues (i.e. kidney, liver, heart, and lung). Weekly measurements of body weight and food intake showed no significant differences between treated and control animals. Measurement of haematological (haemoglobin, erythrocyte volume fraction, erythrocyte count, and total and differential leukocyte counts) and clinical chemistry (blood urea) parameters at weeks 7 and 13 revealed no significant difference between treated and control rats. The authors concluded that tetrahydro-4-methyl-2-(methylpropen-1-yl)pyran had no effects in rats at the concentrations given (Posternak et al., 1969).
(iii) Cycloionone (No. 1239)
Groups of five male and five female Sprague-Dawley rats were given diets containing cycloionone at a concentration of 0 or 420 ppm for 14 days. On the basis of weekly measurement of body weight and food consumption, the average daily intake was calculated to be 36.6 and 33.7 mg/kg bw for males and females, respectively. Animals were observed daily for gross signs of toxicity. Body weight and food consumption were measured on days 8 and 15. On day 15, gross necropsies were performed on all animals and the kidneys and liver of each animal were removed, weighed, and prepared for histological evaluation. All animals in the study survived and appeared healthy. Measurement of body-weight gain, food consumption, and absolute and relative liver and kidney weights revealed no significant differences between test and control animals. Gross necropsy and histopathological examination of kidney and liver tissues revealed no evidence of lesions related to administration of the test material (Wnorowski, 1997).
In a follow-up 28-day study, five male and five female Sprague-Dawley rats were given cycloionone at a concentration of 0, 30, 120, 400 or 1000 mg/kg bw per day by gavage. Parameters evaluated included body weight, food consumption, mortality, standard clinical chemistry and haematology analyses, and gross necropsy. Histopathological examinations of a variety of organs were performed on the controls and the animals at the highest dose (1000 mg/kg bw per day). Further histopathological examinations of animals at lower doses were conducted in order to identify a NOEL.
The authors reported no gross signs of toxicity, abnormal behaviour, or mortality. Food consumption and measurements of haematological parameters were not significantly different between treated and control animals. A significant decrease in body weight was reported for the female rats at the highest dose during the last week of the study, but over the entire treatment period rats in all groups gained weight. No significant differences in body-weight gain were reported between the treated and control groups during the course of the study. At necropsy, the liver of one female at the highest dose appeared haemorrhagic (one lobe). Compared with controls, relative liver weights were significantly increased in male and female rats at 400 and 100 mg/kg bw per day, as well as the relative kidney weights of male rats at the highest dose. Statistically significant differences in clinical chemistry parameters were reported between treated and control rats. The serum concentrations of total protein were significantly increased in males at the two higher doses (400 and 1000 mg/kg bw per day). Concentrations of globulin were also significantly increased in males and females at the highest dose (1000 mg/kg bw per day; p <0.01). Concentrations of globulin were also significantly increased in females at 120 mg/kg bw per day (p <0.05), but were not increased in females at 400 mg/kg bw per day compared with controls; consequently, the increase was not considered by the study authors to be related to treatment. Gamma-glutamyl transpeptidase activities were significantly increased in female rats at the highest dose (1000 mg/kg bw per day; p <0.01). There was a significant decrease in the activities of alkaline phosphatase reported in females at the highest dose (p <0.05) and in males at the two higher doses (400 and 1000 mg/kg bw per day; p <0.05 and p <0.01, respectively). Concentrations of glucose were significantly (p <0.01) decreased and concentrations of albumin were significantly (p <0.05) increased in the males at the highest dose. Concentrations of urea nitrogen were significantly decreased in females at the highest dose (p <0.01).
Histopathological findings included thyroid follicular-cell hyperplasia in male and female rats at 400 and 1000 mg/kg bw per day, testes seminiferous tubule degeneration in males at 1000 mg/kg bw per day and in one male at 400 mg/kg bw per day, kidney hyaline droplets in tubular epithelial cells in male and female rats at 400 and 1000 mg/kg bw per day, and subtle liver cytoplasmic vacuolization and cytomegaly in males at 400 and 1000 mg/kg bw per day and in females at 1000 mg/kg bw per day. The authors reported that the NOEL for cycloionone was 120 mg/kg bw per day for male and female rats treated by gavage for 28 days (Wnorowski, 1998).
(iv) p-Methylanisole (No. 1243)
In a 28-day study, groups of 10 male and 10 female Wistar rats were given p-methylanisole at a dose of 0, 40, 120, or 240 mg/kg bw per day by gavage in soybean oil. The rats were examined twice daily, and body weight and food and water intakes were measured weekly during the study period. Blood samples were obtained from all of the rats after week 3 for evaluation of haematological and clinical chemistry parameters. The rats were killed after 28 days and subjected to a full gross necropsy. Histopathological examinations of the whole organs and tissues of animals receiving the highest dose (240 mg/kg bw per day) and control animals were performed. There were no deaths during the study period, nor were any clinical signs of illness reported. No significant differences in food and water consumption, body-weight gain, or relative organ weights were observed between treated and control groups. Statistically significant decreases in serum creatinine and urea levels were reported in both male and female rats at the intermediate (120 mg/kg bw per day) and highest doses. It was also reported that the male rats in these two groups experienced alterations in water balance, as suggested by a decreased erythrocyte volume fraction relative to that in controls. The authors considered this result to be ambiguous, since concentrations of urinary creatinine were not abnormal. Additionally, no lesions of the kidneys were noted microscopically and no significant differences in absolute or relative kidney weights were reported between treated rats and controls. Concentrations of haemoglobin were decreased at the intermediate and highest doses; however, according to the authors, this result was not biologically significant. At necropsy, histological examinations revealed no dose-related lesions of any organs. The NOEL for p-methylanisole administered by gavage was 40 mg/kg bw per day in male and female rats (Brunsborg et al., 1994).
(v) p-Propylanisole (No. 1244)
For 32 days, groups of 20 male Osborne-Mendel weanling rats were given corn oil containing p-propylanisole at a dose of 0 or 2000 mg/kg bw per day, which was gradually raised to 5000 mg/kg bw per day. Sixteen animals survived long enough to receive the maximum dose of 5000 mg/kg bw per day, while a total of seven animals survived until the end of the study (day 32). Gross pathology of treated rats revealed flaky white material and minute ulcers on the mucosa of the forestomach, while histopathological examination revealed a moderately severe hyperkeratosis of the stratified squamous epithelium in the forestomach. Both of these effects were most likely attributable to the administration of the test material by gavage. Moderate osteoporosis was also reported in treated rats (Hagan et al., 1967).
In a follow-up study, groups of 10 male and 10 female weanling Osborne-Mendel rats were fed p-propylanisole at a concentration of 0, 1000, 2500 or 10 000 ppm in the diet for 19 weeks (Hagan et al., 1967), calculated to provide average daily intakes of 0, 100, 250 or 1000 mg/kg bw, respectively (Food & Drug Administration, 1993). Measurement of the concentration of p-propylanisole in the diet revealed a 16% loss over a 1-week period. Weekly measurements of body weight and food consumption revealed no significant differences between treated and control groups. Haematological examinations performed at termination of the study showed no treatment-related effects in any of the treated animals. No effects on organ weights were reported and gross and histopathological examination of the rats failed to reveal any lesions that could be associated with administration of p-propylanisole. Slight to very slight osteoporosis was observed in rats at all three doses of p-propylanisole (100 mg/kg bw per day, 4/10; 250 mg/kg bw per day, 6/10; 1000 mg/kg bw per day, 6/10), as well as in control animals (1/10); however, the statistical significance of these observations was not reported. No other effects were reported and additional detail on this study is not available. However, in two long-term studies with the related flavouring agent p-propenylanisole4 (trans-anethole) in rats (Vavasour, 1999), previously reviewed by the Committee (Annex 1, reference 138), no evidence of osteoporosis was reported at any dose.
(vi) Carvacryl ethyl ether (No. 1247)
Groups of five female and five male Sprague-Dawley Fischer 344 rats were given diets containing carvacryl ethyl ether at a dose of 0 or 22 mg/kg bw per day for 14 days. Animals were observed for mortality and clinical signs of toxicity at 1, 4, and 24 h after administration, and daily thereafter for 14 days. Body weights were measured before the initiation of the study and on days 6 and 14. Food consumption was measured on days 7 and 14. All rats were killed after 14 days and subjected to a complete necropsy, including histological examination of the livers and kidneys. There was no mortality or clinical signs of toxicity reported throughout the course of the study. Measurement of body weights 1 day before treatment and on days 6 and 14 of the study revealed a significant decrease in body-weight gain in males after 6 and 14 days of treatment, compared with that in the control group. Food consumption in males was reduced during the first 7 days of the study, possibly due to reduced palatability of the diet. No significant difference in absolute body weight was reported between treated and control rats. A statistically significant (p <0.01) decrease in absolute liver and kidney weights and liver weight relative to body weight were reported for males. No biologically significant indications of toxicity were observed for carvacryl ethyl ether on the basis of physical signs of toxicity, gross necropsy, or histopathological examination of the liver and kidneys of rats. The decreases in liver and kidney weights in treated male rats were attributed to abnormally high liver and kidney weights of animals in the control groups (Gill & Van Miller, 1987).
(vii) 1,2-Dimethoxybenzene (No. 1248)
In a 14-day study of toxicity, groups of five male and five female Charles River rats were fed diets containing 1,2-dimethoxybenzene at a dose of 0 or 10 mg/kg bw per day. Observations included body weight, food consumption, and organ weights. Necropsies and histopathological examinations of the kidneys and livers were performed on all animals. All animals survived to study termination and no treatment-related effects on body weight, food consumption, and organ weights were reported. Dermal abnormalities (i.e. scabs and sores) observed in both controls and rats treated with 1,2-dimethoxybenzene were not considered to be treatment-related. Discoloration of the lungs noted in two rats per group was attributed to the method of asphyxiation used in the study (Trimmer et al., 1992).
(viii) m-Dimethoxybenzene (No. 1249)
Groups of 15 male and 15 female Wistar-derived FDRL rats received diets containing m-dimethoxybenzene at concentrations providing estimated daily intakes of 0 or 10 mg/kg bw for 90 days. The actual intakes of m-dimethoxybenzene in male and female rats were reported to be 9.6 and 11.2 mg/kg bw per day, respectively. Food and water consumption were similar in the treated and control groups. Evaluations of haematological and blood chemical parameters made for eight rats of each sex at week 6, and for all rats at week 12, revealed no significant differences between treated and control groups. At necropsy, measurement of liver and kidney weights revealed no significant differences between the treated and control groups. Histological and gross pathological examinations revealed no significant tissue alterations related to treatment with m-dimethoxybenzene (Oser et al., 1965).
In a 12-week study, an unspecified number of male and female rats was given m-dimethoxybenzene at a dose of 10 mg/kg bw per day by gavage; no adverse effects were reported in any animals (Bär & Griepentrog, 1967).
(ix) p-Dimethoxybenzene (No. 1250)
The potential effects of p-dimethoxybenzene on the forestomach were examined in groups of 5–10 male and 5–10 female Wistar rats maintained on diets containing 0 or 2% p-dimethoxybenzene for 4 or 8 weeks. The concentrations of p-dimethoxybenzene used were calculated to provide an average daily intake of 0 or 1000 mg/kg bw (Food & Drug Administration, 1993). At necropsy, no macroscopically visible lesions of the forestomach were reported in treated rats, nor were there any treatment-related effects in the glandular stomach and oesophagus (Altmann et al., 1985).
(x) Diphenyl ether (No. 1255)
Groups of 20 male and 20 female Sprague-Dawley rats were given diets containing diphenyl ether at a concentration of 0, 200, 1000, or 5000 ppm for 13 weeks. These concentrations were calculated to provide average daily intakes of diphenyl ether of 0, 10, 50, or 250 mg/kg bw, respectively (Food & Drug Administration, 1993). The animals were observed daily and body weight and food consumption were measured weekly. Necropsies were performed on 10 males and 10 females per group at 13 weeks and on the remaining animals at 4 weeks after cessation of treatment. Mean body weight, body-weight gain and food consumption were significantly decreased in all rats at the highest dose (250 mg/kg bw per day) and in females at 50 mg/kg bw per day. The authors attributed these affects to the reduced palatability of the diet. Evaluations of haematological and serum chemical parameters and urine analysis of treated rats showed that values were within the normal range. Gross necropsy and histological examinations of a wide range of organs and tissues did not reveal any pathological changes that could be associated with treatment with diphenyl ether. The authors concluded that diets containing diphenyl ether did not induce systemic toxicity at any concentration tested. The NOEL was >250 mg/kg bw per day in rats (Johnson et al., 1992).
(xi) Dibenzyl ether (No. 1256)
Groups 15 male and 15 female Wistar-derived FDRL rats were given dibenzyl ether in the feed at a concentration calculated to provide a daily intake of 3.33 mg/kg bw for a period of 90 days. The rats were observed daily for mortality, physical appearance, and behaviour. Weekly measurements of body weight and food intake were made, and the efficiency of food use was calculated after 12 weeks. After week 6, eight rats of each sex per group were randomly selected for evaluation of haematological and clinical chemistry parameters (i.e. concentration of haemoglobin, erythrocyte volume fraction, total and differential leukocytes, glucose and urea nitrogen). After 12 weeks, these parameters were evaluated in all animals, and complete urine analysis was carried out on pooled samples from three animals of the same sex. There were no signs of toxicity in any of the rats throughout the study. No significant differences were reported between treated and control rats with regard to parameters of growth, food intake, efficiency of food use, haematology, clinical chemistry, urine analysis, and absolute and relative organ weights. Furthermore, gross and histopathological examinations of major organs and tissues (e.g. liver, kidney, spleen, stomach, adrenals, heart, lungs, and brain) failed to show any lesions that could be related to treatment with dibenzyl ether (Oser, 1964).
Groups of male and female Crl : CD(SD)BR albino rats5 were given diets containing dibenzyl ether at concentrations calculated to provide average daily intakes of 0, 62, 196 or 620 mg/kg bw for 91 consecutive days. Dietary mixtures were analysed and adjusted weekly. Concentration analyses revealed that dietary mixtures provided the test substance to within 8% of the target value. Daily observations of clinical signs and mortality, and weekly measurements of body weight, food intake and efficiency of food use showed no significant differences between test and control groups. Evaluation of haematological and clinical chemistry parameters, and urine analysis performed on all test animals and on half the control animals at weeks 6 and 12 showed significant decreases (p <0.05) in erythrocyte count, haemoglobin, and erythrocyte volume fraction in females at the intermediate (196 mg/kg bw per day) and highest dose (620 mg/kg bw per day) at week 6. By week 12, statistically significant decreases in erythrocyte count and erythrocyte volume fraction were reported in females at the intermediate dose, but not at the highest dose. Treated male rats showed no significant changes in any haematological parameters evaluated during the study. On the basis of these observations, and in view of the fact that the erythrocyte count and haematological values were within normal limits for this strain of rat at the testing laboratory, the authors concluded that the haematological changes observed in female rats were transient in nature and were of no toxicological significance.
At necropsy, there was a statistically significant increase in absolute and relative liver weight (p <0.05) in females at the highest dose, possibly related to induction of the metabolism of dibenzyl ether. Gross and histopathological examinations of rats failed to reveal any evidence of toxicity that could be attributed to treatment with dibenzyl ether (Burdock & Ford, 1992). The NOELs for dibenzyl ether of 196 mg/kg bw per day in females and >620 mg/kg bw per day in males are >10 000 000 and >10 000 times greater than the daily per capita intakes of 0.01 µg/kg bw per day (International Organization of the Flavour Industry, 1995) and 4 µg/kg bw per day (Lucas et al., 1999) from the use of dibenzyl ether as a flavouring agent in Europe and the USA, respectively (see Table 2).
(xii) beta-Naphthyl ethyl ether (No. 1258)
For 90 days, groups of 15 male and 15 female Wistar-derived FDRL rats received beta-naphthyl ethyl ether in the diet at estimated daily intake levels of 0 or 5 mg/kg bw. The actual dietary intakes of beta-naphthyl ethyl ether in male and female rats were reported to be 5.1 and 5.7 mg/kg bw per day, respectively. Weekly measurements of food and water consumption showed no significant difference between treated and control groups of rats. Results of haematological and blood chemical determinations made on eight rats of each sex at week 6, and in all rats at week 12, were reported to be within the range of normal values. At necropsy, liver and kidney weights were recorded and revealed no significant differences between test and control groups. Histopathological examinations were performed on the following organs from half of the animals in each group: liver, kidneys, stomach, small and large intestines, spleen, pancreas, heart, lungs, bone marrow, muscle, brain, spinal cord, bladder, adrenals, thyroid, pituitary, gonads, salivary glands, and lymph nodes. Histological and gross pathological examinations revealed no significant tissue alterations in the rats related to administration of beta-naphthyl ethyl ether (Oser et al., 1965). The NOEL’s of 5.1 and 5.7 mg/kg bw per day for male and female rats, respectively, are >100 000 times the daily per capita intake of 0.06 µg/kg bw per day (Lucas et al., 1999) from the use of beta-naphthyl ethyl ether as a flavouring agent in the USA.
In a 12-week study, beta-naphthyl ethyl ether was given by gavage to an unspecified number of rats at a dose of 5.0 mg/kg bw per day. No effects were reported that could be attributed to beta-naphthyl ethyl ether treatment of the rats (Bär & Griepentrog, 1967).
(c) Long-term studies of toxicity and carcinogenicity
(i) Eucalyptol (No. 1234)
In a study of the possible carcinogenic effects of toothpaste constituents, including chloroform, eucalyptol, and peppermint oil, eucalyptol was administered at at dose of 8 or 32 mg/kg bw per day by gavage, 6 days per week for 80 weeks to groups of 52 male specific pathogen-free CFLP mice. Control groups of 52 mice were either untreated or received a toothpaste base without chloroform, peppermint, or eucalyptol (vehicle control). Animals were housed four per cage and given food and water ad libitum. Mice were weighed weekly for the first 6 months and then every 2 weeks during the last 6 months of the study. Food consumption was recorded on a cage-by-cage basis. Animals were observed twice daily and those found dead or in a moribund condition during the study were subjected to gross examination. At week 80, animals were killed and organ weights for the adrenals, kidneys, liver, lungs, and spleen were recorded. All macroscopically identified tumours were examined histopathologically, together with tissues from the liver, kidneys, lungs, and brain.
No treatment-related changes were reported for the following parameters: food consumption, body weight, organ weights, and clinical signs of toxicity. Necropsy and organ weight measurements showed no treatment-related differences between test and control groups. Histopathological examination revealed no significant differences between test, control, or vehicle control groups in the incidence or severity of tumours of the liver, lung, kidney, or malignant lymphoma (Roe et al., 1979).
The NOEL for eucalyptol was >32 mg/kg bw per day, which is approximately 1000 times the daily per capita intakes of 24 µg/kg bw per day (International Organization of the Flavour Industry, 1995) and 33 µg/kg bw per day (Lucas et al., 1999) from the use of eucalyptol as a flavouring agent in Europe and the USA, respectively.
(ii) m-Dimethoxybenzene (No. 1249)
Groups of rats (male : female ratio unspecified) received diets containing 0.1% (20 rats) or 0.5% (40 rats) m-dimethoxybenzene, (equivalent to dietary intakes of 50 mg/kg bw and 250 mg/kg bw, respectively (Food and Drug Administration, 1993)) for up to 2 years. Examination of appearance, behaviour, body weight and histolopathology yielded no significant differences between treated and control groups. Spontaneous diseases present in some of the animals were equally frequent among the test and control animals (Bär & Griepentrog, 1967).
(iii) Diphenyl ether (No. 1255)
In a 13-month study, groups of eight male albino rats were given diets containing either diphenyl ether only at an estimated daily intake of 530 mg/kg bw (0.5 cm3/kg bw) or as part of a mixture of diphenyl (26.4%) and diphenyl ether (73.6%) at estimated daily intakes of 137 and 396 mg/kg bw (0.5 cm3/kg bw), respectively. Histopathological examinations were performed on the liver, kidney, spleen, heart, lung, thyroid and parathyroid glands, adrenal glands, pancreas, testicles, stomach and intestines. No tumours were observed in the rats treated with either diphenyl ether alone or as part of the mixture. No control group was used in this study (Pecchiani & Saffiotti, 1957).
(d) Genotoxicity
Testing for genotoxicity has been performed with 12 representative substances in this group. Except for isolated positive results at cytotoxic concentrations (Galloway et al., 1987; Heck et al., 1989), assays for genotoxicity in vitro gave negative results. Negative results were also obtained in the assay for micronucleus formation in mice in vivo. The results of these tests are summarized in Table 6 and described below.
Table 6. Results of studies of genotoxicity with aliphatic and aromatic ethers
|
No. |
Flavouring agent |
End-point |
Test system |
Dose or concentration |
Result |
Reference |
|
In vitro |
|
1234 |
Eucalyptol |
Reverse mutation |
S. typhimurium TA102, TA100, TA98, TA97 |
250–2500 µg/plate |
Negativea |
Gomes-Carneiro et al. (1998) |
|
1234 |
Eucalyptol |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537 |
3.3–3333 µg/plate |
Negativea,b |
Haworth et al. (1983) |
|
1234 |
Eucalyptol |
Sister chromatid exchange |
Chinese hamster ovary cells |
50–500 µg/mlc |
Positivedd |
Galloway et al. (1987) |
|
600–800 µg/ml |
Negativee |
|
1234 |
Eucalyptol |
Sister chromatid exchange |
Chinese hamster ovary CHO K-1 cells |
10, 33.3 and 100 µmol/l
(1.5, 5.1 and 15.4 µg/ml)f |
Negatived |
Sasaki et al. (1989) |
|
1234 |
Eucalyptol |
Chromosomal aberrations |
Chinese hamster ovary cells |
479–663 µg/ml |
Negatived |
Galloway et al. (1987) |
|
630–810 µg/ml |
Negativee |
|
1234 |
Eucalyptol |
DNA repair |
Bacillus subtilis H17 (rec+) and M45 (rec-) |
18 µg/disk |
Negativeg |
Oda et al. (1979) |
|
1234 |
Eucalyptol |
DNA repair |
Bacillus subtilis H17 (rec+) and M45 (rec-) |
<20 µl/disk (20 000 µg/disk)h |
Negativeg |
Yoo (1986) |
|
1241 |
Anisole |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537 |
3 µmol/plate (324 µg/plate)i |
Negativea |
Florin et al. (1980) |
|
1241 |
Anisole |
Sister chromatid exchange |
Human lymphocytes |
0–2.0 mmol/l (0–216 µg/ml)i |
Negative |
Jansson et al. (1988) |
|
1243 |
p-Methylanisole |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537 |
3 µmol/plate (367 µg/plate)j |
Negativea |
Florin et al. (1980) |
|
1243 |
p-Methylanisole |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
50 000 µg/plate |
Negativea |
Heck et al. (1989) |
|
1243 |
p-Methylanisole |
Unscheduled DNA synthesis |
Rat hepatocytes |
188 µg/ml |
Positive |
Heck et al. (1989) |
|
1244 |
p-Propylanisole |
Reverse mutation |
S. typhimurium TA98, TA100, (1983) TA1535, TA1537, TA1538 |
<750 µg/plate |
Negativea |
Wild et al. |
|
1244 |
p-Propylanisole |
Unscheduled DNA synthesis |
Rat hepatocytes |
>5 × 10 -3mol/l (751 µg/ml)k and above |
Negative |
Howes et al. (1990) |
|
1248 |
1,2-Dimethoxybenzene |
Reverse mutation |
S. typhimurium TA100 |
0.1, 1, 10, 100 and 1000 µg/plate |
Negative |
Rapson et al. (1980) |
|
1249 |
m-Dimethoxybenzene |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
<3.6 mg/plate |
Negativea |
Wild et al. (1983) |
|
1250 |
p-Dimethoxybenzene |
Reverse mutation |
S. typhimurium TA98, TA100, (1983) TA1535, TA1537 |
10–900 µg/plate |
Negativea,b |
Haworth et al. |
|
1255 |
Diphenyl ether |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537 |
3 µmol/plate (511 µg/plate)l |
Negativea |
Florin et al. (1980) |
|
1255 |
Diphenyl ether |
Reverse mutation |
S. typhimurium TA98, TA100, TA1532, TA1535, TA1537, TA1538, TA2636 |
0.1–500 µg/plate |
Negativea |
Pagano et al. (1983) |
|
1255 |
Diphenyl ether |
Reverse mutation |
S. typhimurium TA100, TA97, TA98, TA102 |
<Cytotoxic concentrations (>10 -2mmol/l or 17 µg/ml)l |
Negativea |
Pagano et al. (1988) |
|
1255 |
Diphenyl ether |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537 |
3.3–333.3 µg/plate |
Negativea,b |
Haworth et al. (1983) |
|
1255 |
Diphenyl ether |
Reverse mutation |
S. typhimurium TA100, TA1538, TA98, TA1537, TA1535 |
1–10 000 µg/plate |
Negativea |
Clark et al. (1979) |
|
1255 |
Diphenyl ether |
Reverse mutation |
S. typhimurium TA1535, TA100, TA1538, TA98, TA1537, TA1978 |
5 and 10 µl/plate (5000 and 11 000 µg/plate)m |
Negaitvea |
Westinghouse (1984) |
|
1255 |
Diphenyl ether |
Mutations |
Saccharomyces cerevisiae D7 |
Up to 1 mmol/l (170 µg/ml)l |
Negativea |
Pagano et al. (1983) |
|
1255 |
Diphenyl ether |
Chromosomal aberrations |
Chinese hamster ovary cells |
5–5000 µg/ml |
Negativea |
SanSebastian (1989) |
|
1255 |
Diphenyl ether |
Unscheduled DNA synthesis |
Rat hepatocytes |
0.5–100 µg/ml |
Negative |
Mirsalis and Bakke (1987) |
|
1255 |
Diphenyl ether |
Unscheduled DNA synthesis |
Rat hepatocytes |
0.1–1000 µg/mln |
Negative |
Farr (1987a) |
|
1256 |
Dibenzyl ether |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
<3.6 mg/plate |
Negativea |
Wild et al. (1983) |
|
1257 |
beta-Naphthyl methyl ether |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537 |
3 µmol/plate (475 µg/plate)o |
Negativea |
Florin et al. (1980) |
|
1258 |
beta-Naphthyl ethyl ether |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
<3.6 mg/plate |
Negativea |
Wild et al. (1983) |
|
1258 |
beta-Naphthyl ethyl ether |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537 |
3 µmol/plate (517 µg/plate)p |
Negativea |
Florin et al. (1980) |
|
1259 |
beta-Naphthyl isobutyl ether |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
<1 mg/plate |
Negativea |
Wild et al. (1983) |
|
In vivo |
|
1244 |
p-Propylanisole |
Micronucleus formation |
Mice |
750, 1125, 1500 mg/kg |
Negativeq |
Wild et al. (1983) |
|
1244 |
p-Propylanisole |
Sex-linked recessive lethal mutation |
Drosophila melanogaster |
5 mmol/l (751 µg/ml)k |
Negativer |
Wild et al. (1983) |
|
1249 |
m-Dimethoxybenzene |
Micronucleus formation |
Mice |
558, 966, or 1382 mg/kg bw |
Negativeq |
Wild et al. (1983) |
|
1249 |
m-Dimethoxybenzene |
Sex-linked recessive lethal mutation |
Drosophila melanogaster |
25 mmol/l (3454 µg/ml)s |
Negative |
Wild et al. (1983) |
|
1256 |
Dibenzyl ether |
Micronucleus formation |
Mice |
400, 700 or 1000 mg/kg bwt |
Negativeq |
Wild et al. (1983) |
|
1256 |
Dibenzyl ether |
Sex-linked recessive lethal mutation |
Drosophila melanogaster |
10 mmol/l (1983 µg/ml)u |
Negative |
Wild et al. (1983) |
|
1258 |
beta-Naphthyl ethyl ether |
Micronucleus formation |
Mice |
344, 603, 861 mg/kg bw |
Negativeq |
Wild et al. (1983) |
|
1258 |
beta-Naphthyl ethyl ether |
Sex-linked recessive lethal mutation |
Drosophila melanogaster |
25 mmol/l (4,306 µg/ml)p |
Negative |
Wild et al. (1983) |
|
1259 |
beta-Naphthyl isobutyl ether |
Micronucleus formation |
Mice |
800, 1,400, or 2000 mg/kg bw |
Negativeq |
Wild et al. (1983) |
|
1259 |
beta-Naphthyl isobutyl ether |
Sex-linked recessive lethal mutation |
Drosophila melanogaster |
25 mmol/l |
Negativev |
Wild et al. (1983) |
Notes to Table 6
|
a |
With or without metabolic activation |
|
b |
Pre-incubation method |
|
c |
Lowest dose to give a significant increase in sister chromatid exchange: Trial I—500 µg/ml, Trial II—200 µg/ml |
|
d |
Without metabolic activation |
|
e |
With metabolic activation |
|
f |
Calculated using relative molecular mass of eucalyptol =154.25 |
|
g |
Foreign language article, data available from English abstract and/or tables |
|
h |
Calculated using density of eucalyptol =0.921–0.924 (Food Chemical Codex, 1996) |
|
i |
Calculated using relative molecular mass of anisole =108.14 |
|
j |
Calculated using relative molecular mass of p-methylanisole =122.17 |
|
k |
Calculated using relative molecular mass of p-propylanisole =150.22 |
|
l |
Calculated using relative molecular mass of diphenyl ether =170.21 |
|
m |
Calculated using density of diphenyl ether =1.07 (Arctander, 1969) |
|
n |
These values are for a mixture containing 73.5% diphenyl ether and 26.5% biphenyl |
|
o |
Calculated using relative molecular mass of _-naphthyl methyl ether =158.2 |
|
p |
Calculated using relative molecular mass of _-naphthyl ethyl ether =172.23 |
|
q |
Administered via intraperitoneal injection |
|
r |
In one of the four tests using I-propylanisole, high frequencies of sex-linked recessive lethal mutation were observed in two broods, which were significantly (p <0.01) above the control value. However, the authors noted that four doubles (two lethal mutations from one male) were observed in the test, and due to the lack of effects seen in the other three tests, the doubles were considered pre-existing and of spontaneous origin (Wild et al., 1983). |
|
s |
Calculated using relative molecular mass of m-dimethoxybenzene =138.17 |
|
t |
Administered twice within a 24-h period |
|
u |
Calculated using relative molecular mass of dibenzyl ether=198.27 |
|
v |
A slight increase "with a borderline significance of p =0.05" in frequencies of sex-linked recessive lethal mutations was reported in the second brood of three, which was considered of questionable relevance and not a positive result (Wild et al., 1983). The "borderline" significance reported (p =0.05) appears to be due to the abnormally low frequency of sex-linked recessive lethal mutations in the corresponding control brood (control brood II: 34/17734 or 0.19%) when compared to the control groups of the other two broods (control brood I: 42/18188 or 0.23%and control brood III: 50/16980 or 0.29%) |
In vitro
Negative results were reported in the standard Ames assay when various strains of Salmonella typhimurium (TA97, TA98, TA100, TA102, TA1532, TA1535, TA1537, TA1538, TA1978 and TA2636) were incubated with eucalyptol (No. 1234), anisole (No. 1241), p-methylanisole (No. 1243), p-propylanisole (No. 1244), 1,2-dimethoxybenzene (No. 1248), m-dimethoxybenzene (No. 1249), p-dimethoxy-benzene (No. 1250), diphenyl ether (No. 1255), dibenzyl ether (No. 1256), beta-naphthyl methyl ether (No. 1257), beta-naphthyl ethyl ether (No. 1258), or beta-naphthyl isobutyl ether (No. 1259) at concentrations of up to 50 000 µg/plate, with and without metabolic activation (Clark et al., 1979; Florin et al., 1980; Rapson et al., 1980; Haworth et al., 1983; Pagano et al., 1983, 1988; Wild et al., 1983; Westinghouse Electric Corporation, 1984; Heck et al., 1989; Gomes-Carneiro et al., 1998).
Eucalyptol (No. 1234) was tested in assays for sister chromatid exchange in Chinese hamster ovary cells in vitro (Galloway et al., 1987; Sasaki et al., 1989). A statistically significant increase (p <0.05) in the incidence of sister chromatid exchanges in the absence of metabolic activation was reported at high concentrations (200–500 µg/ml) that induced cell cycle delay (Galloway et al., 1987). This finding was, however, not confirmed in a subsequent study that also used eucalyptol at concentrations that extended into the toxic range (Sasaki et al., 1989), nor was any increased incidence of sister chromatid exchange found in the presence of metabolic activation (Galloway et al., 1987). In an assay for sister chromatid exchange in human lymphocytes in vitro, anisole (No. 1241) did not induce sister chromatid exchange at concentrations of up to 2 mmol/l (216 µg/ml)6 (Jansson et al., 1988).
Eucalyptol (No. 1234) did not induce chromosomal aberrations in Chinese hamster ovary cells at concentrations ranging from 479 to 663 µg/ml without metabolic activation, and from 630 to 810 µg/ml with metabolic activation (Galloway et al., 1987). Diphenyl ether (No. 1255), at concentrations of 5 to 5000 µg/ml, did not induce chromosomal aberrations in Chinese hamster ovary cells with or without metabolic activation (SanSebastian, 1989).
In an abstract for a preliminary screening study that was not published, p-methylanisole (No. 1243) was tested in an assay for unscheduled DNA synthesis in vitro using hepatocytes isolated from adult male Fischer or Sprague-Dawley rats. Positive responses were reported for p-methylanisole, but only at cytotoxic concentrations (188 µg/ml; relative survival, 60–78%). At lower non-cytotoxic concentrations (5–100 µg/ml), there was no evidence of unscheduled DNA synthesis (Heck et al., 1989). Furthermore, incubation of the related substance p-propylanisole (No. 1244) with rat hepatocytes showed no evidence of unscheduled DNA synthesis (Howes et al., 1990). Diphenyl ether gave negative results in two separate assays for unscheduled DNA synthesis in rat hepatocytes in vitro at concentrations ranging from 0.5 to 100 µg/ml (Mirsalis & Bakke, 1987) and from 0.1 to 1000 µg/ml7 (Farr, 1987a).
In an assay for DNA repair in Bacillus subtilis H17 and M45 (rec assay), eucalyptol gave negative results at concentrations ranging from 18 to 20 000 mg/disc8 (Oda et al., 1979; Yoo, 1986).
In vivo
In an assay for micronucleus formation in bone marrow cells, male and female NMRI mice received single injections of p-propylanisole at a dose of 750, 1125, or 1500 mg/kg bw in olive oil. The mice were killed 30 h after injection. Results were expressed as mean number of micronucleated polychromatic erythrocytes per 1000 polychromated erythrocytes. There was no evidence of an increase in the incidence of micronucleated polychromatic erythrocytes at any of the concentrations of p-propylanisole tested when compared with the values for controls(Wild et al., 1983).
Assays for micronucleus formation were also performed with four other aromatic ethers. There was no evidence of an increase in the frequency of micronucleated polychromatic erythrocytes reported when male and female NMRI mice were given intraperitoneal injections of m-dimethoxybenzene (No. 1249) at up to 1382 mg/kg bw, dibenzyl ether (No. 1256) at up to 1000 mg/kg bw, beta-naphthyl ethyl ether (No. 1258) at up to 861 mg/kg bw, or beta-naphthyl isobutyl ether (No. 1259) at up to 2000 mg/kg bw (Wild et al., 1983).
Assays for sex-linked recessive lethal mutations in Drosophila melanogaster were performed using 5 mmol/l of p-propylanisole (No. 1244), 25 mmol/l of m-dimethoxybenzene (No. 1249), 10 mmol/l of dibenzyl ether (No. 1256), 25 mmol/l of beta-naphthyl ethyl ether (No. 1258), or 25 mmol/l of beta-naphthyl isobutyl ether (No. 1259) (Wild et al., 1983). None of these substances was reported to give positive results in this assay (Wild et al., 1983).
In one of four assays with p-propylanisole, the frequency of sex-linked recessive lethal mutations was significantly increased (p <0.01), a result that was not confirmed when the assay was repeated three times at the same test concentration (5 mmol/l). For beta-naphthyl isobutyl ether, a slight increase in sex-linked recessive lethal mutations "with a borderline significance of p =0.05" was reported only in the second of three broods analysed, which the authors concluded to be of questionable relevance. The "borderline" significance was due to the abnormally low frequency of sex-linked recessive lethal mutations in the corresponding control brood for the second brood (0.19%) compared with the values for controls (0.23 and 0.29%) for the other two broods.
Conclusion
The Committee concluded that there was no confirmed evidence of genotoxicity for any of the aliphatic or aromatic ethers used as flavouring agents.
(e) Reproductive toxicity
(i) Eucalyptol (No. 1234)
Sprague-Dawley rats were given olive oil containing Rowachol® (a mixture containing: alpha/beta-pinene, 17%; l-menthol, 32%; menthone, 6%; borneol, 5%; d-camphene, 5%; cineole (eucalyptol), 2%; and rheochrysin, 0.1%) at an oral dose of 0.16, 0.80, or 1.60 ml/kg (approximately equivalent to dietary intakes of eucalyptol of 0, 3, 15 and 30 mg/kg bw per day, respectively) once daily for 6 days, from days 9–14 of gestation. A control group of rats received olive oil at an oral dose of 0.80 ml/kg bw. Necropsies were performed on day 20 of gestation. No statistically significant differences in maternal body-weight gain, number of implantation sites, placental weight, intrauterine mortality or fetal weight were reported between the groups treated with Rowachol® and the control group. At the highest dose (1.60 ml/kg per day), there were significant reductions in maternal, placental, fetal and newborn body weight compared with controls. Although newborn body weights were significantly decreased at the highest dose, development recovered after 1 week. Additionally, fetuses of animals receiving the highest dose did not show any retarded ossification. There were no gross, visceral or skeletal anomalies at the highest dose, nor was there any significant difference in the incidence of fetal malformations reported between rats treated with Rowachol® and controls. No teratogenic effect was observed for Rowachol® at any dose (Hasegawa & Toda, 1978).
(ii) Diphenyl ether (No. 1255)
Groups of five mated female Sprague-Dawley rats received a mixture (Therminol® VP-1) containing 73.5% diphenyl ether and 26.5% biphenyl at a single daily dose of 0, 100, 200, 400, 800, and 1500 mg/kg bw per day (approximately equivalent to dietary intakes of diphenyl ether of 0, 73.5, 147, 294, 588, and 1102 mg/kg bw per day, respectively) on days 6–15 of gestation. The animals were observed twice daily for clinical signs of toxicity and mortality, and food consumption was measured on days 0–20 of gestation. Body weights and clinical signs of toxicity were recorded on days 0, 6, 10, 12, 15 and 20 of gestation. The animals were sacrificed on day 20 of gestation and subjected to gross necropsy and examinations of corpora lutea and the uterus. Fetuses were weighed, sexed and examined for external malformations. Deaths were reported in 4/5 rats (80%) at 400 mg/kg bw per day during days 8–15 of gestation, and in 1/5 (20%) rats at 1500 mg/kg bw per day on day 15 of gestation. With the exception of the group receiving 400 mg/kg bw per day, in which the rate of pregnancy was 80%, the rate of pregnancy was reported to be 100% in all treatment groups. Rats receiving Therminol® at a dose of 400, 800, or 1500 mg/kg bw per day had staining of the fur in the ano-genital area, as well as signs of excessive salivation. Maternal body-weight gain was affected in a dose-related manner in the groups receiving Therminol® at a dose of 100, 200, or 800 mg/kg bw per day, and weight loss was reported in rats at 1500 mg/kg bw per day. Maternal food consumption was decreased at all doses when compared with that of the controls during days 6–15 of the treatment period; however, during the post-treatment period (days 15–20 of gestation), food consumption and weight gain at all doses were reported to be significantly greater than those of controls. There were no significant differences in the number of corpora lutea, uterine implantation sites, or preimplantation loss indices reported between rats treated with Therminol® and rats in the control groups. The mean number of viable fetuses and resorptions per dam did not differ significantly at 100, 200, or 400 mg/kg bw per day or in controls. Significantly increased frequencies of uterine resorptions and significantly decreased numbers of viable fetuses per litter were reported at 800 mg/kg bw per day. Fetal weights at 1500 mg/kg bw per day were significantly lower than those of controls. No treatment-related effects were noted upon gross examinations of the dams. An external malformation (unilateral microphthalmia) was noted in one of the fetuses of the group receiving a dose of 800 mg/kg bw per day; however, no fetal malformations were reported in the other treated groups (Farr, 1987b).
3. REFERENCES
Altmann, H.J., Grunow, W., Wester, P.W. & Mohr, U. (1985) Induction of forestomach lesions by butylhydroxyanisole and structurally related substances. Arch. Toxicol. 8, 114–116.
Arctander, S. (1969) Perfume and Flavor Chemicals. Vol. 1. No. 1081, Rutgers University, Montclair, New Jersey, USA.
Asakawa, Y., Toyota, M. & Ishida, T. (1988) Biotansformation of 1,4-cineole, a monoterpene ether. Xenobiotica 18, 1129–1134.
Axelrod, J. (1956) The enzymatic cleavage of aromatic ethers. Biochem. J. 63, 634–639.
Bahler, B. & Bonetti, E.P. (1973) Determination of the oral and intraperitoneal LD50 of 1-ethoxy-2-methyl-2-butene in the mouse. Private communication to the Flavor and Extract Manufacturers Association. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Bär, V.F. & Greipentrog, F. (1967) Die Situation in der gesundheitlichen Beurteilung der Aromatisierungsmittel fur Lebensmittel. (Where we stand concerning the evaluation of flavoring substances from the viewpoint of health). Medizin Ernahr. 8, 244–251.
Bauer, K. & Garbe, D. (1985) Eucalyptus Oils. In: Common Fragrance and Flavor Materials: Preparation, Properties and Uses. Deerfield Beach, Florida, pp. 124–125.
Bernauer U., Amberg, A.; Scheutzow, D. & Dekant, W. (1998) Biotransformation of 12C- and 2–13C-Labeled methyl tert-Butyl ether, ethyl tert-Butyl ether and tert Butyl alcohol in rats: identification of metabolites in urine by 13C nuclear magnetic resonance and gas chromatography/mass spectrometry. Chem. Res. Toxicol. 11, 651–658.
Bernhard, R.A., Wijesekera, R.O.B. & Chichester, C.O. (1971) Terpenoids of cardomon oil and their comparative distribution among varieties. Phytochemistry 10, 177–184.
Birch, M. (1992) Toxicological investigations with diphenyl oxide in rats and rabbits. Submission to EPA. Unpublished report to the Flavor and Extract Manufacturers Association. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Brady, J.F., Xiao, F., Ning, S.M. & Yang, C.S. (1990) Metabolism of methyl t-butyl ether by hepatic microsomes. Arch. Toxicol. 64, 157–160.
Bray, H.G., James, S.P., Thorpe, W.V. & Wasdell, M.R. (1953) Metabolism of ethers in rabbit. I. Anisole and diphenyl ethers. Biochem. J. 54, 547–550.
Bray, H.G., Craddock, V.M. & Thorpe, W.V. (1955) Metabolism of ethers in rabbit. II. Nuclear-substituted anisoles. Biochem. J. 60, 225–232.
Brownlee, G. (1940) A pharmacological examination of cineole and phellandrene. Q.J. Pharm. Pharmac. 13, 130–137.
Brunsborg, B., Meyer, O., Wurtzen, G. & Olsen, P. (1994) Four-week toxicity study of 4-methoxyltoulene in rats. Toxicol. Lett. 73, 209–212.
Burdock, G.A. & Ford, R.A. (1992) Safety evaluation of dibenzyl ether. Fd. Cosmet. Toxicol. 30, 559–566.
Chui, Y.C., Addison, R.F. & Law, F.C.P. (1987) Studies of the pharmacokinetics and metabolism of 4-chlorodiphenyl ether in rats. Drug Metab. Dispos. 15, 44–50.
Clark, C.R., Marshall, T.C., Merickel, B.S., Sanchez, A., Brownstein, D.G. & Hobbs, C.H. (1979) Toxicological assessment of heat transfer fluids proposed for use in solar energy applications. Toxicol.Appl. Pharm. 51, 529–535.
Cramer, G.M., Ford, R.A. & Hall, R.L. (1978) Estimation of toxic hazard—a decision tree approach. Food. Cosmet. Toxicol. 16, 255–276.
Daly, J. (1970) Metabolism of acetanilide and anisoles with rat liver microsomes. Biochem. Pharmacol. 19, 2979–2993.
Daly, J. & Jerina, D. (1969) Migration of deuterium during aryl hydroxylation. Arch. Biochem. Biophys. 134, 266–268.
Derbesy, M., Uzio, R., Boyer, D. & Cozon, V. (1991) Application de chromatographie phase chirale a l’analyse des huiles essentielles de mentha piperita. Ann. Fals. Exp. Chim. 84, 205–216.
De-Oliveira, A.C.A.X., Fidalgo, A.A. & Paumgartten, F.J.R. (1999) In vitro inhibition of liver monooxygenases by beta-ionone, 1,8-cineole, (-)-menthol and terpineol. Toxic. 135, 33–41.
Farr, C.H. (1987a) The evaluation of the potential of Therminol VP-1 to induce unscheduled DNA synthesis in primary rat hepatocytes. Submission to EPA. Unpublished report to the Flavor and Extract Manufacturers Association. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Farr, C.H. (1987b) Range-finding teratology study in rats with Therminol VP-1. Submission to EPA. Unpublished report to the Flavor and Extract Manufacturers Association. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Flavor and Extract Manufacturers Association (1983, 1985, 1990) Natural occurrence in food. Private communication to the Flavor and Extract Manufacturers Association. Submitted to WHO by the Flavor and Extract Manufacturers Association, Washington DC. USA.
Florin, I., Rutberg, L., Curvall, M. & Enzell, C.R. (1980) Screening of tobacco smoke constituents for mutagenicity testing using Ames test. Toxicology. 18, 219–232.
Food and Drug Administration (1993) Priority-based assessment of food additives (PAFA) database, Washington DC: Center for food safety and applied nutrition, p. 58.
Food Chemical Codex (1996) Flavor Chemicals: Eucalyptol, 4th Ed., pp. 496–497.
Galloway, S.M., Armstrong, M.J., Reuben, C., Colman, S., Brown, B., Cannon, C., Bloom, A.D., Nakamura, F., Ahmed, M., Duk, S., Rimpo, J., Margolin, B.H., Resnick, M.A., Anderson, B. & Zeiger, E. (1987) Chromosome aberrations and sister chromatid exchanges in Chinese hamster ovary cells: evaluation of 108 chemicals. Environ. Mol. Mutagen. 10, 1–175.
Gasic, O., Mimica-Dukie, N. & Adamovic, D. (1992) Variability of content and composition of essential oils of different Mentha arvenesis L. var. piperascens cultivars. J. Essent. Oil Res. 4, 49–56.
Gill, M.W. & Van Miller, J.P. (1987) Fourteen-day dietary minimum toxicity screen (MTS) in albino rats. Unpublished report to the Research Institute of Fragrance Materials, Englewood Cliffs, New Jersey, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Gillman, M.R. (1997) Acute oral toxicity study, limit test. Unpublished report to the Flavor and Extract Manufactures Association. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Gomes-Carneiro, M., Felzenszwalb, I. & Paumgartten, F.J.R. (1998) Mutagenicity testing of (±)-camphor, 1,8-cineole, citral, citronella, (-)-menthol and terpineol with the Salmonella/microsome assay. Mutat. Res. 416, 129–136.
Hagan, E.C., Hansen, W.H., Fitzhugh, O.G., Jenner, P.M., Jones, W.I., Taylor, J.M., Long, E.L., Nelson, A.A. & Brouwer, J.B. (1967) Food flavourings and compounds of related structure. II. Subacute and chronic toxicity. Food Cosmet. Toxicol. 5, 141–157.
Hart, E.R. & Wong, L.C.K. (1971) Acute oral toxicity studies in rats, acute dermal toxicity and primary skin irritation studies in rabbits of fragrance materials. Unpublished report to the Research Institute of Fragrance Materials, Englewood Cliffs, New Jersey, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Hasegawa, M. & Toda, T. (1978) Teratological studies on Rowachol, remedy for cholelithiasis. Effect of Rowachol administered to pregnant rats during organogenesis on pre- and post-natal development of their offspring. Oyo Yakuri. 15, 1109–1119.
Haworth, S., Lawlor, T., Mortelmans, K., Speck, W. & Zeigler, E. (1983) Salmonella mutagenicity test results for 250 chemicals. Environ. Mutagen. 5(Suppl. 1), 3–142.
Heck, J.D., Vollmuth, T.A., Cifone, M.A., Jagannath, D.R., Myhr, B. & Curren, R.D. (1989) An evaluation of food flavoring ingredients in a genetic toxicity screening battery. The Toxicologist 9, 257.
Hiroi, T., Miyazaki, Y., Kobayashi, Y., Imaoka, S. & Funae, Y. (1995) Induction of hepatic P450’s in rat by essential wood and leaf oils. Xenobiotica. 25, 457–467.
Howes, A.J., Chan, V.S.W. & Caldwell, J. (1990) Structure-specificity of the genotoxicity of some naturally occurring alkenylbenzenes determined by the unscheduled DNA synthesis in rat hepatocytes. Food. Chem. Toxicol. 28, 537–542.
International Organization of the Flavor Industry (1995). European inquiry on volume use. Unpublished report. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Jäger, W., Nasĕl, B., Nasĕl, C., Binder, R., Stimpfl, T., Vycudilik, W. & Buchbauer, G. (1996) Pharmacokinetic studies of the fragrance compound 1,8-cineol in humans during inhalation. Chem. Senses 21, 477–480.
Jansson, T., Curvall, M., Hedin, A. & Enzell, C.R. (1988) In vitro studies of the biological effects of cigarette smoke condensate. III. Induction of SCE by some phenolic and related constituents derived from cigarette smoke—A study of structure-activity relationships. Mutat. Res. 206, 17–24.
Jenner, P.M., Hagan, E.C., Taylor, J.M., Cook, E.L. & Fitzhugh, O.G. (1964) Food flavorings and compounds of related structure. I. Acute oral toxicity. Food. Cosmet. Toxicol. 2, 327–343.
Johnson, W.D., Ehrlich, J.P., Hatoum, N.S. & Yermakoff, J.K. (1992) Thirteen week oral toxicity study of diphenyl ether in rats. The Toxicologist 12, 117.
Komsta, E., Chu, I., Villeneuve, D.C., Benoit, F.M. & Murdoch, D. (1988) Tissue distribution metabolism and excretion of 2,2’,4,4’,5-pentachlorodiphenyl ether in the rat. Arch. Toxicol. 62, 258–262.
Kovar, K.A., Gropper, B., Friess, D. & Ammon, H.P.T. (1987) Blood levels of 1,8-cineole and locomotor activity of mice after inhalation and oral administration of rosemary oil. Planta Medica 53, 315–318.
Krantz Jr., J.C. & Carr, C.J. (1969) The Pharmocologic Principles of Medical Practice, 7th Ed., Baltimore: Williams and Wilkins, pp. 97, 112.
Law, F.C.P. & Chakrabarti, S. (1983) Irreversible binding of 14C-diphenyl ether-derived radioactivity to liver microsomes in vitro and tissue proteins in vivo. Drug Chem. Toxicol. 6, 285–294.
Law, F.C.P., Song, Y.Y. & Chakrabarti, S. (1983) Disposition and metabolism of diphenyl ether in rats. Xenobiotica. 13, 627–633.
Levenstein, I. (1974) Acute toxicity study in rats and rabbits. Unpublished report to the Research Institute of Fragrance Materials, Englewood Cliffs, New Jersey, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Lucas, C.D., Putnam, J.M. & Hallagan, J.B. (1999) Flavor and Extract Manufacturers Association of the United States 1995 Poundage and Technical Effects Update Survey, Washington DC: Flavor and Extract Manufacturers Association of the United States.
Maarse, H., Visscher, C.A., Willemsens, L.C. & Boelens, M.H. (1999). Volatile Components In Food—Qualitative And Quantitative Data. Zeist: Centraal Instituut Voor Voedingsonderzioek TNO.
Madyastha, K.M. & Chadha, A. (1986). Metabolism of 1,8-cineole in rat: Its effects on liver and lung microsomal cytochrome P-450 systems. Bull. Environ. Contam. Toxicol. 37, 759–766.
Marotti, M., Piccaglia, R. & Giovanelli, E. (1996) Differences in essential oil composition of basil (Ocimum basilicum L.) Italian cultivars related to morphological characteristics. J. Agric. Food Chem. 44, 3926–3929.
Miller, M.J., Ferdinandi, E.S., Klan, M., Andrews, L.S., Douglas, J.F. & Kneiss, J.J. (1997) Pharmacokinetics and disposition of methyl tert-butyl ether in Fischer-344 rats. J. Appl. Toxicol., 17(Suppl. 1), S3–S12.
Mirsalis, J.C. & Bakke, J.P. (1987) Evaluation of the potential of diphenyl oxide to induce unscheduled DNA synthesis in primary rat hepatocyte cultures. Submission to EPA. Unpublished report to the Flavor and Extract Manufacturers Association. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Miyazawa, M., Kameoka, H., Morinaga, K., Negoro, K. & Mura, N. (1989) Hydroxycineole: four new metabolites of 1,8-cineole in rabbits. J. Agric. Food Chem. 37, 222–226.
Miyazawa, M. & Shindo, M. (2001) Biotransformation of 1,8-cineole by human liver microsomes. Nat. Prod. Lett. 15, 49–53.
Miyazawa, M., Shindo, M. & Shimada, T. (2001a) Roles of cytochrome P450 3A enzymes in the 2-hydroxylation of 1,4-cineole, a monoterpene cyclic ether, by rat and human liver microsomes. Xenobiotica. 31, 713–723.
Miyazawa, M., Shindo, M. & Shimada, T. (2001b). Oxidation of 1,8-cineole, the monoterpene cyclic ether originated from Eucalyptus polybractea, by cytochrome P4503A enzymes in rat and human liver microsomes. Drug Metab. Dispos. 29(2), 200–205.
Moreno, O.M. (1973) Acute toxicity studies on rats and rabbits. Unpublished report to the Research Institute of Fragrance Materials, Englewood Cliffs, New Jersey, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Moreno, O.M. (1977) Acute toxicity studies in rats, rabbits and guinea pigs. Unpublished report to the Research Institute of Fragrance Materials, Englewood Cliffs, New Jersey, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Moreno, O.M. (1978) Acute toxicity studies in rats, mice, rabbits and guinea pigs. Unpublished report to the Research Institute of Fragrance Materials, Englewood Cliffs, New Jersey, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Moreno, O.M. (1980) Acute toxicity studies. Unpublished report to the Research Institute of Fragrance Materials, Englewood Cliffs, New Jersey, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Moreno, O.M. (1981) Acute toxicity studies. Unpublished report to the Research Institute of Fragrance Materials, Englewood Cliffs, New Jersey, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Murray, M.J., Faas, W. & Marble, P. (1972) Effects of plant maturity on oil composition of several spearmint species grown in Indiana and Michigan. Crop Science 12, 723–728.
National Academy of Sciences (1970, 1982, 1987) Evaluating the Safety of food chemicals. Washington DC, USA.
National Toxicology Program (1987a) Twenty-eight day gavage and encapsulated feed study on 1,8-cineole in Fischer 344 rats. NTP Chem. No. 15-NTP Expt. Nos. 5014-02 and 5014-06; NCTR Expt. Nos.: 380 and 439.
National Toxicology Program (1987b) Twenty-eight day gavage and encapsulated feed study on 1,8-cineole in B6C3F1 hybrid mice. NTP Chem. No. 15-NTP Expt. Nos. 5014-03 and 5014-07; NCTR Expt. Nos.: 389 and 440.
Oda, Y., Hamono, Y., Inoue, K., Yamamoto, H, Niihara, T. & Kunita, N. (1979) Mutagenicity of food flavours in bacteria. Shokuhin Eisei Hen 9, 177–181.
Ohi, H., Takahara, E., Ohta, S. & Hirobe, M. (1992) Effects of oxygen concentration on the metabolism of anisole homolougues by rat liver microsomes. Xenobiotica 22, 1329–1337.
Oser, B. (1964) Feeding study with dibenzyl ether in rats. Private communication to the Flavor and Extract Manufacturers Association. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Oser, B.L., Carson, S. & Oser, M. (1965) Toxicological tests on flavouring matters. Food. Cosmet. Toxicol. 3, 563–569.
Pagano, G., Esposito, A., Giordano, G.G., Vamvakinos, E., Quinto, I., Bronzetti, G., Bauer, C., Corsi, C., Nieri, R. & Ciajolo, A. (1983) Genotoxicity and teratogenicity of diphenyl and diphenyl ether: A study of sea urchins, yeast and Salmonella typhimurium. Terat. Carcin. Mutagen. 3, 377–393.
Pagano, G., Cipollaro, M., Corsale, G., Morte, R.D., Esposito, A., Giordano, G.G., Micallo, G., Quinto, I. & Staiano, N. (1988) Comparitive toxicity of diphenyl, diphenyl ether, and some of their hydroxy derivatives. Médecine Biologie Environnement 16, 291–297.
Pass, G.J., McClean, S., Stupans, I. & Davies, N. (2001) Microsomal metabolism of the terpene 1,8-cineole in the common brushtail possum (Trichosurus vulpecula), koala (Phascolarctos cinereus), rat and human. Xenobiotica 31, 205–221.
Pecchiani, L. & Saffiotti, U. (1957) Study of toxicity of diphenyl, oxydiphenyl, and their mixtures (dowtherm). Medna Lav. 48, 247–254.
Poon, G., Chui, Y.C. & Law, F.C.P. (1986) Biotransformation of diphenyl ether by trout and guinea-pigs after intraperitoneal administration. 16, 795–800, 01, 01A.
Posternak, J.M., Linder, A. & Vodoz, C.A. (1969) Summary of toxicological data. Food Chem. Toxicol. 7, 405–407.
Rapson, W.H., Nazar, M.A. & Butsky, V.V. (1980) Mutagenicity produced by aqueous chlorination of organic compounds. Bull. Environ. Contam. Toxicol. 24, 590–596.
Ravid, U., Putievsky, E. & Katzir, I. (1993) Determination of the enantiomeric composition of (1R) (+)- and (1S) (-)-camphor in essential oils of some lamiaceae and compositae herbs. Flavour and Fragrance Journal 8, 225–228.
Roe, F.J., Palmer, A.K., Worden, A.N. & Van Abbé, N.J. (1979) Safety evaluation of tooth-paste containing chloroform. I. Long-term studies in mice. J. Environ. Path. Toxicol., 2, 799–819.
Roure Inc. (1979) Orientierende prüfung auf acute intraperitoneale und orale toxizität an der SPF-albino maus. Unpublished report to the Flavor and Extract Manufacturers Association. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Sangster, S.A., Caldwell, J., Hutt, A.J. & Smith, R.L. (1983) The metabolism of p-propylanisole in the rat and mouse and its variation with dose. Food Chem. Toxicol. 21, 263–271.
Sangster, S.A., Caldwell, J., Hutt, A.J. & Smith, R. (1987) The metabolic disposition of [methoxy-14C]-labelled trans-anethole, estragole and p-propylanisole in human volunteers. Xenobiotica 17, 1223–1232.
SanSebastian, J. (1989) In Vitro chromosome aberration analysis in Chinese hamster ovary (CHO) cells. Submission to EPA. Unpublished report to the Flavor and Extract Manufacturers Association. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Sasaki, Y.F., Imanishi, H., Ohta, T. & Shirasu, Y. (1989) Modifying effects of components of plant essence on the induction of sister-chromatid exchanges in cultured Chinese hamster ovary cells. Mutat. Res., 226, 103–110.
Sauer-Freeman, C. (1980) Acute oral median lethality toxicity study in rats (LD50). Unpub-lished report to the Flavor and Extract Manufacturers Association. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Schafer Jr., E.W. & Bowles Jr., W.A. (1985) Acute oral toxicity and repellency of 933 chemicals to house and deer mice. Arch. Environ. Contamin. Toxicol. 14, 111–129.
Scientific Committee on Food (2002) Opinion of the Scientific Committee on Food on eucalyptol, expressed on 17 April 2002, SCF/CS/FLAV/FLAVOUR/20 ADD2 Final, 23 April 2002.
Silvestre, A.J.D., Cavaleiro, J.A.S., Delmond, B., Filliatre, C. & Bourgeois, G. (1997) Analysis of the variation of the essential oil composition of Eucalyptus globulus Labill from Protugal using multivariate statistical analysis. Industrial Crops and Products 6, 27–33.
Smith, D.M., Skakum, W. & Levi, L. (1963) Determination of botanical and geographical origin of spearmint oils by gas chromatographic and ultraviolet analysis. J. Agric. Food Chem. 11, 268–276.
Stofberg, J. & Kirschman, J. C. (1985) The consumption ratio of flavoring materials: A mechanism for setting priorities for safety evaluation. Food. Chem. Toxicol. 23, 857–860.
Stofberg, J. and Grundschober, F. (1987) Consumption ratio and food predominance of flavoring materials. Perfumer Flavorist 12, 27.
Takahara, E., Ohta, S. & Hirobe, M. (1986) Effect of oxygen concentration on the metabolic pathway of anisole in rat liver. Biochem. Pharmacol. 35, 544–546.
Taylor, J.M., Jenner, P.M. & Jones, W.I. (1964) A comparison of toxicity of some allyl, propenyl, and propyl compounds in the rat. Toxicol. Appl. Pharmacol. 6, 378–87.
Trimmer, G.W., Phillips, R.D. & Damico, J.S. (1992) 14-Day subchronic oral toxicity study in the rat. East Millstone, N.J. Unpublished report to the Flavor and Extract Manufacturers Association. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Vavasour, E.J. (1999) trans-Anethole (addendum). In: Safety evaluation of certain food additives. WHO Food Additive Series, No. 42, Geneva.
Weir, R.J. & Wong, L.C.K. (1971a) Acute toxicity studies. Unpublished report to the Research Institute of Fragrance Materials, Englewood Cliffs, New Jersey, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Weir, R.J. & Wong, L.C.K (1971b) Acute oral toxicity studies—rats; Acute dermal toxicity studies—rabbits; Primary skin irritation—rabbits. Unpublished report to the Flavor and Extract Manufacturers Association. Submitted to WHO by the Flavor and Extract Manufacturers Association, Washington DC, USA.
Westinghouse Electric Corporation (1984) Submission to EPA. Unpublished report to the Flavor and Extract Manufacturers Association. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Wild, D., King, M.T., Gocke, E. & Eckhardt, K. (1983) Study of artificial flavouring substances for mutagenicity in the Salmonella/microsome, basc, and micronucleus tests. Food. Chem. Toxicol. 21, 707–719.
Williams, R.T. (1959a) The metabolism of aromatic alcohols, ethers, aldehydes, ketones and quinines. In: Detoxication Mechanisms—The metabolism and detoxication of drugs, toxic substances and other organic compounds, 2nd Ed., Chapter 10, London: Chapman and Hall Ltd., pp. 318–347.
Williams, R.T. (1959b) The metabolism of terpenes and camphors. In: Detoxication Mechanisms—The metabolism and detoxication of drugs, toxic substances and other organic compounds, 2nd Ed, Chapter 17, London: Chapman and Hall Ltd, pp. 519–543.
Wnorowski, G. (1997) 14-day dietary toxicity study:rats. Private communication to the Flavor and Extract Manufactures Association. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Wnorowski, G. (1998) Twenty-eight day repeat dose oral toxicity study of cycloionone in the rat. Private communication to the Flavor and Extract Manufacturers Association. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Wohl, A. (1974) Acute oral and dermal toxicity studies. Unpublished report to the Research Institute of Fragrance Materials, Englewood Cliffs, New Jersey, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Yoo, Y.S. (1986) Mutagenic and antimutagenic activities of flavouring agents used in foodstuffs. Journal of the Osaka City Medical Center 34, 267–288.
ENDNOTES:
- The Committee was informed that certain products, such as lozenges/hard candies, may contain high concentrations of eucalyptol and noted that theoretical estimates of the daily per capita intake of eucalyptol could be as great as 16 mg (Scientific Committee on Food, 2002). Data on the frequency of use of such products were not available to the Committee, thus it was not possible to estimate long-term intake. The Committee noted, however, that even at an intake of 16 mg/person per day, a margin of safety of approximately 120 existed between this intake and the NOEL of >32 mg/kg bw per day identified in a long-term study in rats.
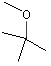 Methyl tert-butyl ether.
Methyl tert-butyl ether.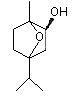 2-Exo-Hydroxy-1,4-cineole.
2-Exo-Hydroxy-1,4-cineole. 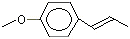 p-Propenylanisole.
p-Propenylanisole. - Control group, 24 males and 24 females; 62 mg/kg bw per day, 16 males and 16 females; 196 mg/kg bw per day, 12 males and 12 females; and 620 mg/kg bw per day, 10 males and 10 females.
- Calculated based on relative molecular mass of anisole =108.14.
- CThe concentrations tested (0.1, 0.5, 1, 5, 10, 50, 100, 250, 500 and 1000 µg/ml) represent a mixture of diphenyl ether (73.5%) and biphenyl (26.5%).
- CCalculated based on density of eucalyptol =0.921–0.924 (Food Chemical Codex, 1996).











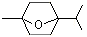


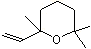



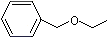










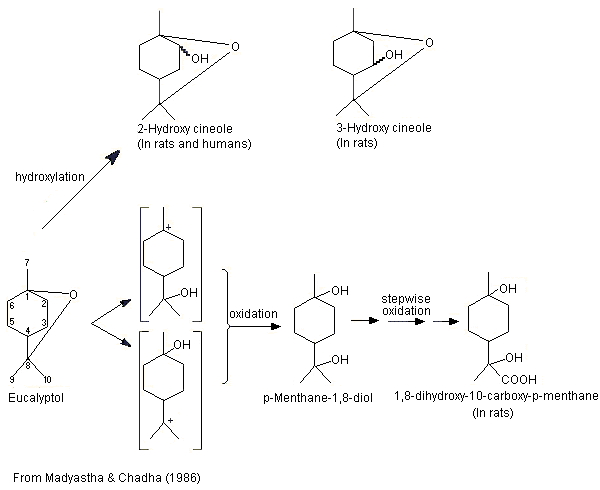

 Methyl tert-butyl ether.
Methyl tert-butyl ether. 2-Exo-Hydroxy-1,4-cineole.
2-Exo-Hydroxy-1,4-cineole.  p-Propenylanisole.
p-Propenylanisole.