
WHO FOOD ADDITIVES SERIES: 52
HYDROXYPROPENYLBENZENES (addendum)
First draft prepared by
Professor I.G. Sipes
Department of Pharmacology, College of Medicine, University of Arizona, Tucson, Arizona, USA
1. EVALUATION
1.1 Introduction
The Committee evaluated a group of flavouring agents that included nine hydroxy- or alkoxy-substituted propenylbenzenes (see Table 1), commonly recognized as isoeugenol derivatives, by the Procedure for the Safety Evaluation of Flavouring Agents (see Figure 1, Introduction). These agents have not been evaluated previously by the Committee.
Three of the nine flavouring agents (Nos 1260, 1265 and 1266) have been reported to occur naturally in foods. They have been detected in blueberries, mushrooms, ginger, raw fatty fish, and pork (Maarse et al. 1999).
Table 1. Summary of results of the safety evaluations of hydroxypropenylbenzenes used as flavouring agentsa
|
Flavouring agent |
No. |
CAS no. and structure |
Step A3 Does intake exceed the threshold for human intake?b |
Step A4 Is the flavouring agent or are its metabolites endogenous? |
Step A5 Adequate margin of safety for the flavouring agent or related substance? |
Comments |
Conclusion based on current intake |
|
Structural class I |
|
|
|
|
|
|
|
|
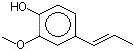
Isoeugenol |
1260 |
97-54-1
 |
No
Europe: 117
USA: 43 |
NR |
NR |
See note 1 |
No safety concern |
|
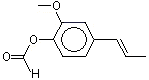
Isoeugenyl formate |
1261 |
7774-96-1
 |
No
Europe: ND
USA: 0.2 |
NR |
NR |
See note 2 |
No safety concern |
|
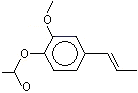
Isoeugenyl acetate |
1262 |
93-29-8
 |
No
Europe: 0.7
USA: 11 |
NR |
NR |
See note 3 |
No safety concern |
|
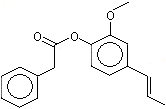
Isoeugenyl phenylacetate |
1263 |
120-24-1
 |
No
Europe: ND
USA: 0.3 |
NR |
NR |
See note 4 |
No safety concern |
|
Propenylguaethol |
1264 |
94-86-0
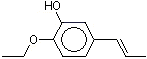 |
No
Europe: 44
USA: 354 |
NR |
NR |
See note 1 |
No safety concern |
|
4-Propenyl-2,6-dimethoxyphenol |
1265 |
20675-95-0
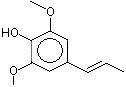 |
No
Europe: ND
USA: 2 |
NR |
NR |
See note 1 |
No safety concern |
|
Structural Class III |
|
|
|
|
|
|
|
|
Isoeugenyl methyl ether |
1266 |
93-16-3
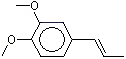 |
Yes
Europe: 128
USA: 129 |
No |
Yes; The NOEL of 6 mg/kg bw per day for isoeugenol methyl ether (Osborne, 1981) is >1000 times greater than the daily intakes of 2 mg/kg bw per day in Europe and the USA as a flavouring agent |
See note 5 |
No safety concern |
|
Isoeugenyl ethyl ether |
1267 |
7784-67-0
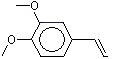 |
No
Europe: ND
USA: 0.009 |
NR |
NR |
See note 5 |
No safety concern |
|
Isoeugenyl benzyl ether |
1268 |
120-11-6
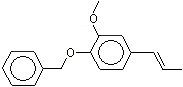 |
No
|
NR |
NR |
See note 5 |
No safety concern |
|
CAS, Chemical Abstracts Service; ND, no intake data reported; NR, not required for evaluation because consumption of the substance was determined to be of no safety concern at Step A3 of the procedure |
|
a |
Step 2: All of the agents in this group are expected to be metabolized to innocuous products |
|
b |
The threshold for human intake for structural classes I and III are 1800 µg/day and 90 µg/day, respectively. All intake values are expressed in µg per day. The combined per capita intake of flavouring agents in structural class I is 162 µg per day in Europe and 411 µg per day in the USA. The combined per capita intake of flavouring agents in structural class III is 129 µg per day in Europe and 130 µg per day in the USA |
|
Notes: |
|
1. |
Detoxicated primarily by conjugation of the phenolic OH group with sulfate or glucuronic acid and excreted mainly in the urine |
|
2. |
Hydrolysed to isoeugenol and formic acid, which is oxidized to CO2 and H2O |
|
3. |
Hydrolysed to isoeugenol and acetic acid, which is absorbed from the gastrointestinal tract and acts as a precursor for synthesis ofbiomolecules |
|
4. |
Hydrolysed to isoeugenol and phenylacetic acid, which is endogenous in humans and excreted as the glutamine conjugate |
|
5. |
Detoxicated primarily by O-demethylation at the (m) or (p)-methoxy substituent to yield the corresponding phenol followed by excretion in the urine as the sulfate or glucuronic acid conjugate |
1.2 Estimated daily intake
The total annual volume of production of the nine flavouring agents in this group is approximately 2000 kg in Europe (International Organization of the Flavour Industry, 1995) and 4100 kg in the USA (National Academy of Sciences, 1970, 1982; Lucas et al., 1999). More than 80% of the total annual volume of production in Europe is accounted for by isoeugenol (No. 1260) and isoeugenyl methyl ether (No. 1266), and >65% of the total annual volume of production in the USA is accounted for by propenylguaethol (No. 1264). The estimated daily per capita intake of isoeugenol is approximately 120 µg in Europe and 40 µg in the USA. The estimated daily per capita intake of isoeugenyl methyl ether is approximately 130 µg in Europe and 130 µg in the USA. The estimated daily per capita intake of propenylguaethol is approximately 40 µg in Europe and 350 µg per day in the USA. The daily per capita intakes of the other flavouring agents in the group range from 0.009–11 µg (National Academy of Sciences, 1970, 1982; International Organization of the Flavour Industry, 1995; Lucas et al., 1999), with most being <1 µg. The daily per capita intake of each agent in Europe and in the USA is reported in Table 1.
1.3 Absorption, distribution, metabolism and elimination
Six (Nos 1260–1265) of the nine flavouring agents contain a free phenolic OH group or are simple phenolic esters. The remaining three agents (Nos 1266–1268) are propenyl benzene derivatives that contain a methoxy, ethoxy, or benzoxy substituents on the para- (p) position and a methoxy substituent on the meta (m) position.
Isoeugenol derivatives containing a phenolic OH group (Nos 1260, 1264 and 1265) are rapidly absorbed from the gastrointestinal tract and are metabolized principally in the liver via conjugation of the phenolic hydroxy group with sulfate or glucuronic acid. The conjugates are subsequently excreted, primarily in the urine (Badger et al., 2002; Fuciarelli, 2001).
Esters of isoeugenol (Nos 1261–1263) are hydrolysed in vivo by carboxylesterases (Heymann, 1980). Upon hydrolysis the product, isoeugenol, is conjugated and excreted while the component carboxylic acids are metabolized in well-recognized biochemical pathways (Williams, 1959).
The alkoxypropenylbenzene derivatives (Nos 1266–1268) in this group primarily undergo O-demethylation of either the (m) or (p)-methoxy substituent to yield the corresponding isoeugenol derivative that is then excreted as the sulfate or glucuronic acid conjugate (Solheim & Scheline, 1976; Annex 1, reference 137 ).
1.4 Application of the procedure for the safety evaluation of flavouring agents
|
Step 1. |
In applying the Procedure for the Safety Evaluation of Flavouring Agents to the above-mentioned flavouring agents, the Committee assigned six of the nine flavouring agents (Nos 1260–1265) to structural class I. The remaining three flavouring agents (Nos 1266–1268) were assigned to structural class III (Cramer et al., 1978). |
|
Step 2. |
All the flavouring agents in this group are expected to be metabolized to innocuous products. The evaluation of all agents in this group therefore proceeded via the A-side of the decision-tree. |
|
Step A3. |
The estimated daily per capita intakes of all six of the flavouring agents in structural class I and two of the three agents in structural class III are below the threshold of concern (i.e. 1800 µg for class I and 90 µg for class III). The Committee concluded that the safety of these eight flavouring agents raises no concern at their currently estimated levels of intake. One of the agents in structural class III, isoeugenyl methyl ether (No. 1266), exceeds the threshold of concern. The daily per capita intake of isoeugenyl methyl ether is 128 µg in Europe and 129 µg in the USA. Accordingly, the evaluation of isoeugenyl methyl ether proceeded to step A4. |
|
Step A4. |
Isoeugenyl methyl ether is not endogenous in humans. The evaluation therefore proceeded to step A5. |
|
Step A5. |
A NOEL of 100 mg/kg bw per day for isoeugenyl methylether (No. 1266) was identified from a 28-day study in rats fed diets containing isoeugenyl methylether (Osborne, 1981). In another study of longer duration (13 weeks), no adverse effects were observed in rats given diets containing isoeugenyl methyl ether at a dose of 6 mg/kg bw per day. The NOEL of 6 mg/kg bw per day was >1000 times greater than the estimated intake of isoeugenyl methyl ether from its use as a flavouring agent in Europe and in the USA (2 µg/kg bw per day in each case). On the basis of these data, the Committee concluded that isoeugenyl methyl ether is not expected to be of safety concern at currently estimated levels of use. |
Table 1 summarizes the evaluations of nine hydroxypropenylbenzenes (Nos 1260–1268).
1.5 Consideration of combined intakes from use as flavouring agents
All agents in this group are expected to be metabolized efficiently and the available metabolic pathways would not be saturated. Evaluation of all the data indicated no safety concerns associated with combined intake.
1.6 Conclusions
The Committee concluded that the flavouring agents in this group of hydroxypropenylbenzenes would not be of safety concern at the currently estimated levels of intake. Other data on the toxicity and metabolism of these hydroxypropenylbenzenes were consistent with the results of the safety evaluation.
2. RELEVANT BACKGROUND INFORMATION
2.1 Additional considerations on intake
Quantitative natural occurrence data and consumption ratios reported for isoeugenol (No. 1260), propenylguaethol (No. 1264), and isoeugenyl methyl ether (No. 1266) indicate that exposure occurs predominantly from consumption of traditional foods (i.e. consumption ratio, >1) (Stofberg & Kirschman, 1985; Stofberg & Grundschober, 1987). Volumes of production and intake values for each flavouring agent in this group are shown in Table 2.
Table 2. Annual volumes of production of hydroxypropenylbenzenes used as flavouring agents
|
Agent (No.) |
Most recent annual volume (kg)a |
Intakeb |
Annual volume in naturally occurring foods (kg)c |
Consumption ratiod |
|
µg/day |
µg/kg bw per day |
|
Isoeugenol (1260) |
|
|
|
|
|
|
Europe |
817 |
117 |
2 |
|
|
|
USA |
327 |
43 |
0.7 |
2162 |
7 |
|
Isoeugenyl formate (1261) |
|
|
|
|
|
|
Europe |
ND |
ND |
ND |
|
|
|
USAe |
1 |
0.2 |
0.003 |
— |
NA |
|
Isoeugenyl acetate (1262) |
|
|
|
|
|
|
Europe |
5 |
0.7 |
0.01 |
|
|
|
USA |
86 |
11 |
0.2 |
— |
NA |
|
Isoeugenyl phenylacetate (1263) |
|
|
|
|
|
|
Europe |
ND |
ND |
ND |
|
|
|
USA |
2 |
0.3 |
0.004 |
— |
NA |
|
Propenylguaethol (1264) |
|
|
|
|
|
|
Europe |
311 |
44 |
0.7 |
|
|
|
USA |
2685 |
354 |
6 |
— |
NA |
|
4-Propenyl-2,6-dimethoxyphenol (1265) |
|
|
|
|
|
|
Europe |
ND |
ND |
ND |
ND |
|
|
USAf |
11 |
2 |
0.03 |
108 |
10 |
|
Isoeugenyl methyl ether (1266) |
|
|
|
|
|
|
Europe |
894 |
128 |
2 |
|
|
|
USA |
980 |
129 |
2 |
110 |
0.1 |
|
Isoeugenyl ethyl ether (1267) |
|
|
|
|
|
|
Europe |
ND |
ND |
ND |
|
|
|
USAe |
0.05 |
0.009 |
0.0001 |
— |
NA |
|
Isoeugenyl benzyl ether (1268) |
|
|
|
|
|
|
Europe |
8 |
1 |
0.02 |
|
|
|
USA |
11 |
1 |
0.02 |
— |
NA |
|
TOTAL |
|
|
|
|
|
|
Europe |
2035 |
|
|
|
|
|
USA |
|
|
|
|
4103 |
NA, not available; ND, no intake data reported; +, reported to occur naturally in foods (Maarse et al., 1999), but no quantitative data; -, not reported to occur naturally in foods
a From International Organization of the Flavour Industry (1995) and Lucas et al. (1999) or National Academy of Sciences (1970, 1982)
b Intake (mg/person per day) calculated as follows: [(annual volume, kg) × (1 × 109 mg/kg)]/[population × survey correction factor × 365 days], where population (10%, "eaters only") = 32 × 106 for Europe and 26 × 106 for the USA; where correction factor = 0.6 for Europe and USA National Academy of Sciences surveys and 0.8 for the Lucas et al. survey in the USA representing the assumption that only 60% and 80% of the annual volume of flavouring agent, respectively, was reported in the poundage surveys (International Organization of the Flavour Industry, 1995; Lucas et al., 1999; National Academy of Sciences, 1970, 1982). Intake (µg/kg bw per day) calculated as follows: [(µg/person per day)/body weight], where body weight =60 kg. Slight variations may occur from rounding
c Quantitative data for the United States reported by Stofberg & Grundschober (1987)
d The consumption ratio is calculated as follows: (annual consumption via food, kg)/(most recent reported volume as a flavouring substance, kg)
e Annual volume reported in previous surveys in the USA (National Academy of Sciences, 1970; 1982)
f The volume cited is the anticipated annual volume of production, which was the maximum amount of flavouring agent estimated to be used annually by the manufacturer at the time the material was proposed for flavour use. Subsequent national surveys (National Academy of Sciences, 1970, 1982, 1987; Lucas et al., 1999), if applicable, revealed no reported use of the agent as a flavouring agent
2.2 Biological data
2.2.1 Biochemical data
(a) Hydrolysis
In general, aromatic esters are hydrolysed in vivo through the catalytic activity of carboxylesterases (Heymann, 1980; Anders, 1989), the most important of which are the A-esterases. Carboxylesterases are found in the endoplasmic reticulum of most mammalian tissues; however they are most abundant in hepatocytes (Anders, 1989; Graffner-Nordberg et al., 1998; Hosokawa et al., 2001).
In a study of the hydrolysis of the structurally related ester, phenyl acetate1, using pig liver carboxylesterase, the Km (substrate concentration at which half the true maximum velocity of an enzyme-catalysed reaction is achieved) and Vmax (maximum velocity of an enzyme-catalysed reaction) values for phenyl acetate were reported to be 0.43 mmol/l and 438 mmol/min per mg protein, respectively, at a substrate (phenyl acetate) concentration of 0.2–3 mmol/l (Jung & Heymann, 1979). A second phenolic ester, o-tolyl acetate (o-methylphenyl acetate) was 60% hydrolysed in vitro after incubation with pancreatin for 2 h at 37 °C (Grundschober, 1977). Phenyl 2-hydroxybenzoate (phenyl salicylate) is hydrolysed to phenol and 2-hydroxybenzoic acid in humans, as shown in a study in which one man was given one capsule containing 90 mg of phenyl salicylate per hour for 8 h. Urine was collected for 72 h after the first dose, in 8 h collection periods. Analysis of total urinary phenol showed a peak concentration of 472 ppm during the second collection period. The concentration of free urinary phenol peaked at 25 ppm during the same period. Approximately 60 h after the first dose, concentrations of both total and free urinary phenol returned to baseline levels (7 and 1 ppm, respectively) (Fishbeck et al., 1975).
Recent studies have revealed that isoeugenyl acetate (No 1262) undergoes extensive hydrolysis when incubated with rat hepatocytes or with microsomes prepared from rat liver. For example, incubation of isoeugenyl acetate (500 µmol/l) with hepatocytes (2 million cells) resulted in the complete hydrolysis of the ester to isoeugenol within 15–20 min. Hydrolytic activity was greatly enriched in the endoplasmic reticulum (i.e. microsomal fraction) of the liver. Rat blood also hydrolysed isoeugenyl acetate at a rate of 1600 nmol/ml per min (personal communication from Professor G. Sipes, University of Arizona, Tucson, Arizona, USA to the Flavor and Extract Manufacturers Association (FEMA), Washington, DC, USA; submitted to WHO by FEMA).
The aromatic phenols and carboxylic acids resulting from hydrolysis are readily absorbed and metabolized in well-recognized biochemical pathways.
(b) Absorption, distribution and excretion
In humans, rats, and mice, orally administered hydroxypropenylbenzene derivatives are rapidly absorbed from the gastrointestinal tract and predominantly metabolized in the liver via phase II conjugation of the phenolic hydroxy (OH) group to form sulfate and glucuronic acid conjugates. These conjugates are eliminated primarily in the urine.
For the flavouring agents that do not contain a free phenolic OH group (Nos 1266–1268), the predominant metabolic pathways include O-demethylation to yield an isoeugenol derivative, and omega-oxidation of the terminal methyl group to yield a benzoic acid derivative. In O-demethylation, either (m)- or (p)-methoxy-substituted isoeugenol is converted to the corresponding isoeugenol derivative containing a free phenolic OH group. The phenol is then excreted in the urine as the sulfate or glucuronic acid conjugate. Carbon dioxide produced by O-demethylation is eliminated in the expired air (Solheim & Scheline, 1976). At low doses, this is the predominant detoxication pathway in animals (Sangster et al., 1987). As doses increase, omega-oxidation, and to a lesser extent, epoxidation of the propenyl side-chain compete favourably with O-demethylation. Other minor metabolites of these three substituted isoeugenol derivatives can be formed.
Male Fischer 344 rats given [14C]isoeugenol (No. 1260) at a single oral dose of 156 mg/kg bw excreted >85% of the administered dose as the glucuronic acid and sulfate conjugate in the urine within 72 h. No parent compound was detected in the blood after oral administration. Similarly, male rats given [14C]isoeugenol at a single intravenous dose of 15.6 mg/kg bw excreted 82% of the administered dose as the glucuronic acid and sulfate conjugates in the urine within 72 h. At 1 h and 48 h after administration, 1% and 12% of the radiolabel, respectively, was recovered in the blood. After administration by either route, approximately 10% of the administered dose was excreted in the faeces and <0.1% was recovered in expired air. Less than 0.25% of the radiolabel remained in selected tissues (Badger et al., 2002).
The results of toxicokinetic studies conducted with isoeugenol administered by gavage or intravenously to Fischer 344/N rats and B6C3F1 mice indicate that isoeugenol undergoes extensive first-pass metabolism (Fuciarelli, 2001). Isoeugenol was detected in the plasma of rats and mice 2 min after the administration by gavage of single doses of 17 and 35 mg/kg bw to rats and mice. The time at which peak plasma concentrations (Tmax) were attained was shown to be short, with values ranging from between 2 and 20 min in rats, and between 5 and 20 min in mice. Collectively, these data indicate that isoeugenol is rapidly absorbed from the gastrointestinal tract. However, the results indicate that isoeugenol has a low bioavailability2 (approximately 14% for rats at 17 mg/kg bw and approximately 35% for mice at 35 mg/kg bw). On the basis of low bioavailability, the authors concluded that isoeugenol undergoes first-pass metabolism before systemic distribution. Short terminal elimination rate constants (t1/b) for both species also indicate that isoeugenol is rapidly eliminated from the systemic circulation. The high total clearance (Ctot) values reported for rats and mice further support the conclusion that isoeugenol is rapidly and extensively eliminated from systemic circulation after administration by gavage (Fuciarelli, 2001).
In groups of male Wistar rats given a single dose of 200 mg/kg bw of isoeugenol methyl ether (No. 1266) by oral or intraperitoneal administration, 79% and 90% of the administered dose was eliminated in the urine within 24 h (Solheim & Scheline, 1976). Subsequent analysis of the urine failed to detect any parent compound. Studies with p-methoxypropenylbenzene (trans-anethole)3 in mice and rats (Annex 1, reference 137 ) support the conclusion that hydroxy- and methoxypropenylbenzene derivatives are rapidly absorbed, metabolized, and eliminated. Furthermore, the rapid absorption and elimination of alkoxypropenylbenzene derivatives was supported by data on the metabolism of p-methoxypropenylbenzene in humans.
In two men given a single dose of 1 mg of trans-[methoxy-14C]anethole, 81% of the radiolabel was either excreted in the urine or exhaled as 14CO2 within 8 h. After 48 h, the total recovery of radiolabel was 88% of the administered dose (Sangster et al., 1987). In a study in five persons (body weights, 62–77 kg) given 1, 50, or 250 mg of trans-[methoxy-14C]anethole orally (approximately equivalent to 0.015, 0.87, or 4 mg/kg bw, respectively) in three separate doses, most of the radiolabel was eliminated in the expired air and urine within the first 8 h. The dose administered was shown to have no effect on the rate or route of excretion (Caldwell & Sutton, 1988). In a third study in five volunteers given a single dose of trans-anethole of 500 mg (equivalent to approximately 12 mg/kg bw), >57% of the administered dose was excreted in the urine within 24 h (Le Bourhis, 1973).
(c) Metabolism
After absorption, orally administered hydroxy- and alkoxypropenylbenzene derivatives are completely metabolized in humans, rats and mice. Pharmacokinetic and metabolic information on isoeugenol (No. 1260), isoeugenol methyl ether (No. 1266), and related alkoxypropenylbenzene derivatives (e.g. trans-anethole) indicate that hydroxypropenylbenzenes primarily undergo conjugation of the phenolic OH group with sulfate or glucuronic acid, followed by excretion mainly in the urine. Dealkylation of ring alkoxy substituents and oxidation of the propenyl side-chain are minor metabolic pathways for hydroxypropenylbenzene derivatives.
In the absence of a free phenolic hydroxy group (Nos 1266–1268), alkoxypropenylbenzenes undergo O-dealkylation and side-chain oxidation (omega-oxidation and epoxidation). At low concentrations, O-dealkylation is the predominant pathway, yielding the corresponding phenol derivative (Newberne et al., 1999; Annex 1, reference 137). As the dose increases, omega-oxidation of the propenyl side-chain and epoxidation of the double bond compete with O-dealkylation. In omega-oxidation, the propenyl side-chain undergoes terminal methyl oxidation to yield a cinnamyl alcohol derivative that is successively oxidized to the corresponding cinnamic acid derivative. The acid may be conjugated with glycine and excreted, or it may undergo beta-oxidation, cleavage, and conjugation to the corresponding hippuric acid derivative. In epoxidation, the propenyl double bond forms a labile epoxide that is detoxicated via the action of epoxide hydrolyase to form the corresponding diol. The epoxide also may be detoxicated via glutathione transferase to form the corresponding glutathione conjugate (Annex 1, reference 137 ).
Isoeugenol (No. 1260), which contains a free phenolic hydroxy group, is also readily conjugated with glucuronic acid and sulfate, and subsequently excreted (Williams, 1959; Badger et al., 2002). given to In male Fischer 344 rats given [14C]isoeugenol in a single oral dose of 156 mg/kg bw or a single intravenous dose of 15.6 mg, >80% of the administered dose was excreted as the glucuronic acid and sulfate conjugate in the urine within 72 h. With both routes of administration, approximately 10% of the administered dose was excreted in the faeces and <0.1% was recovered in expired air (Badger et al., 2002).
Data on isoeugenol are consistent with metabolic data on the structurally related substance, eugenol (3-methoxy-4-hydroxyallylbenzene). The metabolic fate of eugenol has been studied in female Wistar rats and male CD-1 mice given ring-labelled [14C]eugenol in a single dose of 0.5, 5, 50 or 1000 mg/kg bw by gavage. More than 50% of urinary radiolabel was accounted for by glucuronic acid or sulfate conjugates of eugenol, with sulfate conjugates being predominant at low doses, and glucuronic acid conjugates assuming increasing importance with increasing dose. Other minor routes of metabolism included O-demethylation to yield 3,4-dihydroxyallylbenzene, reduction of the allylic double bond to yield 4-hydroxy-3-methoxypropylbenzene, and a combination of O-demethylation and epoxidation of the side-chain to yield the corresponding 2’,3’-diol. The allylic reduction is mediated by the microbial flora of the gastrointestinal tract of the rat (Sutton et al., 1985).
A study in eight humans showed that >55% of a single oral dose of eugenol4 of 150 mg (approximately 2.5 mg/kg bw), was excreted as the glucuronic acid and sulfate conjugates in the urine. Other minor conjugated urinary metabolites include cis- and trans-isoeugenol formed by double bond isomerization, a thiol-substituted methoxyhydroxybenzene derivative (approximately 11%), and diol and hydroxy-acid metabolites (13%) presumably formed via hydrolysis and subsequent oxidation of an epoxide intermediate (Fischer et al., 1990). Eugenol epoxide was either rapidly converted to the corresponding diol by cytosolic epoxide hydrolase, or conjugated with glutathione and excreted.
In male rats given a single oral dose of 200 mg of eugenol in 1 ml of olive oil, the specific activity of UDP-glucuronyl transferase in the liver increased gradually during 12 h and reached its maximum (0.277 µmol/min per mg of protein, corresponding to 165% of the control value of 0.169 µmol/min per mg of protein) 48 h after administration. The specific activity declined to 142% of the control value (0.240 µmol/min per mg of protein) after 72 h, providing additional evidence for glucuronic acid conjugation of isoeugenol. There was also an increase in the excretion of glucuronide ethers in urine (35 mg/12 h per rat) collected during the 0–12 or 12–24 h after administration of eugenol; the excretion of glucuronide ethers decreased rapidly to normal levels (5 mg/12 h per rat) after 24 h (Yuasa, 1974).
The three flavouring agents in this group (isoeugenyl formate, (No. 1261); isoeugenyl acetate (No. 1262); isoeugenyl phenylacetate (No. 1263)) that contain an ester substituent in the para-position are expected to be hydrolysed to isoeugenol and the corresponding carboxylic acid (i.e. formic acid, acetic acid, and phenylacetic acid, respectively). In humans and other primates, formic acid is oxidized to carbon dioxide and water primarily in the liver (Tephly, 1991). Acetic acid is readily absorbed from the gastrointestinal tract where it acts as a precursor for the synthesis of biomolecules, such as phospholipids, steroids, and fatty acids (Nelson & Cox, 2000). Phenylacetic acid is endogenous in humans, as it occurs as one of the end-products in the metabolism of L-phenylalanine. It is excreted primarily as the glutamine conjugate (James et al., 1972; Silverman et al., 1986).
In summary, the major metabolic pathway for isoeugenol, isoeugenyl esters, and other derivatives (Nos 1260–1265) containing a free phenolic OH group involves rapid conjugation with sulfate or glucuronic acid, followed by excretion in the urine. Minor metabolic pathways involve the O-dealkylation of ring alkoxy substituents, or oxidation of the propenyl side-chain via omega-oxidation or epoxidation.
Alkoxypropenylbenzene derivatives (Nos 1266–1268) are detoxicated primarily by O-dealkylation at the meta- or para-positions to yield the corresponding phenol, followed by excretion in the urine as the sulfate or glucuronic acid conjugate. omega-Oxidation is a minor metabolic pathway for alkoxypropenylbenzene derivatives. For groups of five male albino Wistar rats given isoeugenyl methyl ether (No. 1266) at a single oral dose of 200 or 400 mg/kg bw, the principal bile metabolites included those formed by 3- and 4-O-demethylation, and omega-oxidation (3,4-dimethoxycinnamic acid and glycine conjugate). More than 77% of urinary metabolites are formed by a combination of O-demethylation and omega-oxidation, yielding mainly 4-hydroxy-3-methoxycinnamic acid, the glycine conjugates of 3,4-dimethoxybenzoic acid, and 3,4-dimethoxycinnamic acid. Metabolites resulting from the epoxidation pathway account for <1% of the urinary metabolites. The unchanged parent compound was reported not to occur in the urine of rats (Solheim & Scheline, 1976).
Data on the metabolism of the structurally related alkoxypropenylbenzene derivative, trans-anethole, in humans are consistent with data for isoeugenol methyl ether in animals. In five persons given a single dose of 500 mg (approximately 12 mg/kg bw), trans-anethole was metabolized primarily by side-chain oxidation to yield p-methoxybenzoic acid (52%) and p-hydroxybenzoic acid (5%), then excreted in the urine (LeBourhis, 1973). In 2 men, a single oral dose of 1 mg of trans-[methoxy-14C]anethole was metabolized primarily by O-demethylation and beta-oxidation of the side-chain. During the first 8 h, approximately 20% of the administered dose was metabolized by O-demethylation and excreted as 14CO2 in exhaled air. Within 24 h, approximately 60% of the administered dose was metabolized by the beta-oxidation pathway and excreted in the urine as p-methoxybenzoic acid (3.5%) and its glycine conjugate, p-methoxyhippuric acid (56%). Approximately 3% of the administered dose was metabolized via epoxidation to yield a mixture of diastereomeric diols of 1-(4’-methoxyphenyl)propane-1,2-diol (Sangster et al., 1987).
Data acquired from studies in rodents provide additional evidence that the metabolic fate of alkoxypropenylbenzene derivatives is species-, sex-, dose-, and time-dependent (Annex 1, reference 137). In the rat, trans-anethole administered at a low dose is detoxicated by O-demethylation to yield CO2 in expired air and conjugated phenolic derivatives, which are subsequently excreted in the urine. Increasing doses of trans-anethole lead to saturation of the O-demethylation pathway, resulting in use of alternative metabolic pathways, such as omega-oxidation and epoxidation. In mice, increasing doses of trans-anethole result in induction of an omega-oxidation pathway that competes favourably with O-demethylation for detoxication of trans-anethole, with only extremely small amounts eliminated via epoxidation. Overall, the data suggest that at low levels of exposure, alkoxypropenylbenzene derivatives are efficiently detoxicated in rodents and humans by O-dealkyation and omega-oxidation pathways.
2.2.2 Toxicological studies
(a) Acute toxicity
Oral LD50 values have been reported for six of the nine substances in this group and are summarized in Table 3. In rats, LD50 values range from 286 mg/kg bw for isoeugenol (No. 1260) to 4900 mg/kg bw for isoeugenyl benzyl ether (No. 1268) (Jenner et al., 1964; Bär & Greipentrog, 1967; Keating, 1972; Levenstein & Wolven, 1972; Moreno, 1973; Piccirillo & Hartman, 1982; Piccirillo, 1984a, b). An oral LD50 of 1410 mg/kg bw for isoeugenol has also been reported in guinea-pigs (Jenner et al., 1964). These results show that the acute oral toxicity of hydroxypropenylbenzenes is low.
Table 3. Studies of acute oral toxicity of hydroxypropenylbenzenes
|
No. |
Flavouring agent |
Species |
Sex |
LD50 (mg/kg bw) |
Reference |
|
1162 |
Mintlactone |
Rat |
M, F |
530a |
Collier (1982) |
|
1163 |
Dehydromenthofurolactone |
Rat |
M, F |
2253 |
Reagan & Becci (1984) |
|
1165 |
Sclareolide |
Rat |
M, F |
>5000 |
Lewis & Palanker (1979) |
|
1166 |
Octahydrocoumarin |
Rat |
M, F |
3302 |
Buch (1981) |
|
1166 |
Octahydrocoumarin |
Rat |
NR |
3900 |
Moreno (1978) |
|
1166 |
Octahydrocoumarin |
Rat |
M, F |
3840 |
BASF (1976) |
|
1168 |
3-Propylidenephthalide |
Rat |
NR |
1650 |
Moreno (1975) |
|
1169 |
3-n-Butylphthalide |
Mouse |
NR |
1850 |
Pellmont (1970) |
|
1169 |
3-n-Butylphthalide |
Rat |
NR |
2450 |
Moreno (1976) |
|
1170 |
3-Butylidenephthalide |
Rat |
NR |
1850 |
Moreno (1980) |
|
1170 |
3-Butylidenephthalide |
Rat |
NR |
2200 |
Posternak (1965) |
|
1171 |
Dihydrocoumarin |
Mouse |
M, F |
1010 |
Levenstein (1953) |
|
1171 |
Dihydrocoumarin |
Rat |
M, F |
1460 |
Jenner et al. (1964) |
|
1171 |
Dihydrocoumarin |
Rat |
M |
1650 |
Moreno (1972) |
|
1171 |
Dihydrocoumarin |
Guinea-pig |
M, F |
1760 |
Jenner et al. (1964) |
|
1172 |
6-Methylcoumarin |
Mouse |
NR |
3050 |
Levenstein (1954) |
|
1172 |
6-Methylcoumarin |
Rat |
NR |
465 |
Feuer (1974) |
|
1172 |
6-Methylcoumarin |
Rat |
NR |
1680 |
Moreno (1973) |
|
1172 |
6-Methylcoumarin |
Rat |
M, F |
844 |
Serota (1984) |
M, male; F, female; NR, not reported
a Dose converted to mg/kg using specific gravity of 1.06
(b) Short-term studies of toxicity
In addition to short-term studies with isoeugenol (No. 1260), propenylguaethol (No. 1264), isoeugenyl methyl ether (No. 1266), and isoeugenyl benzyl ether (No. 1268), the substantial volume of data on short-term toxicity, long-term toxicity and carcinogenicity that is available for the structurally related substance, trans-anethole (Annex 1, reference 137 ), is not included in this review. The results of short-term studies of toxicity are summarized in Table 4.
Table 4. Results of short-term studies of toxicity with hydroxypropenylbenzenes
|
No. |
Flavouring agent |
Species; sex |
No. of test groupsa/no. per groupb |
Route |
Duration (days) |
NOEL (mg/kg bw per day) |
Reference |
|
1260 |
Isoeugenol |
Mouse; M, F |
5/20 |
Gavage |
98 |
300c |
National Toxicology Program (2002) |
|
1260 |
Isoeugenol |
Rat; M, F |
5/20 |
Gavage |
98 |
F: 37.5c
M: <37.5c |
National Toxicology Program (2002) |
|
1260 |
Isoeugenol |
Rat; M, F |
1/10 |
Gavage |
112 |
1000d |
Hagan et al. (1967) |
|
1264 |
Propenylguaethol |
Rat; M, F |
3/20 |
Gavage |
29 |
250 |
Terrill (1991) |
|
1266 |
Isoeugenyl methyl ether |
Rat; M, F |
3/32 |
Diet |
28 |
100 |
Purchase et al. (1992) |
|
1266 |
Isoeugenyl methyl ether |
Rat; M, F |
1/48 |
Diet |
90 |
6d |
Osborne (1981) |
|
1268 |
Isoeugenyl benzyl ether |
Rat; M, F |
3/20 |
Gavage |
28 |
60 |
Boe et al. (1989) |
|
M, male; F, female |
|
a |
Total number of test groups does not include control animals |
|
b |
Total number per test group includes both male and female animals |
|
c |
NOEL based on limited information obtained from the National Toxicology Program Preliminary Report (2002) |
|
d |
Study performed with either a single dose or multiple doses that produced no effect. The value is therefore not a true NOEL, but is the highest dose tested that produced no adverse effects. The actual NOEL may be higher |
(i) Isoeugenol (No. 1260)
Mice
In a preliminary report on a 14-week study performed by the National Toxicology Program, groups of 10 male and 10 female B6C3F1 mice were given isoeugenol at a dose of 0, 37.5, 75, 150, 300, or 600 mg/kg bw per day in corn oil by gavage. General health, body weight and clinical observations were recorded weekly. At the end of the study, necropsies were performed on all animals and selected organs were weighed. A complete histopathological evaluation was conducted. No clinical signs of toxicity were reported in any of the treated animals throughout the study, and all animals survived the duration of the study. At termination, a 14% decrease in mean body weight was reported in males given isoeugenol at a dose of 600 mg/kg bw per day, as compared with the controls. In the absence of a dose-related response, the small magnitude of change observed in the absolute and relative liver weights of the treated animals compared with controls led the authors to conclude that these changes were not treatment related. Minimal to moderate degeneration of the olfactory epithelium degeneration was observed in all males and females at 600 mg/kg bw per day. This was accompanied by olfactory nerve fiber degradation in five males and eight females at this dose. The finding of hyperplasia of the forestomach squamous epithelium at all doses was considered ambiguous owing to the low incidence, low severity (minimal to mild), and the absence of a dose-related response (National Toxicology Program, 2002).
Rats
In a preliminary report on a 14-week study performed by the National Toxicology Program, groups of 10 male and 10 female Fischer 344 rats were given isoeugenol at a dose of 0, 37.5, 75, 150, 300, or 600 mg/kg bw per day in corn oil by gavage. Body weights and clinical observations were recorded weekly. At termination, evaluations of haematology and clinical chemistry were conducted. All animals were necropsied, and selected organs were weighed. Survival, general health and behaviour of the treated animals were similar to those of the controls throughout the study. A decrease of 10–15% in mean body weights was reported in males at 600 mg/kg bw compared with controls. In a special study, groups of 10 male and 10 female rats were given isoeugenol at same doses listed above by gavage for 4 weeks and 2 days. The rats were killed on day 31. Serum gastin concentrations, stomach pH and hepatic CYP1A1 and CYP2B analysis were performed and results were comparable to those for control groups. Microscopic examination of the stomachs revealed no treatment related changes. A significant (p <0.01) increase in absolute and relative liver weights was reported in females at 600 mg/kg bw per day. No treatment-related neoplastic lesions were reported. Non-neoplastic lesions were limited to centrilobular hyperplasia of the liver in seven females at 300 kg bw per day and in nine females at 600 mg/kg bw per day. Minimal to mild olfactory epithelial degeneration, as classified by the authors, was found in all treated animals of both sexes, with the exception of females at 37.5 mg/kg bw per day (National Toxicology Program, 2002).
Groups of five male and five female weanling Osborne-Mendel rats were given diets containing isoeugenol at a concentration of 10 000 ppm for 16 weeks. The corresponding average daily intake was calculated to be 1000 mg/kg bw per day (Food & Drug Administration, 1993). No significant differences in general health and behaviour were reported in the treated animals compared with the controls. Weekly measurements of body weight and food consumption revealed no differences between treated and control groups. Haematological examinations performed at termination showed no treatment-related effects in any of the treated animals. No effects on organ weights were observed nor were there any macroscopic or microscopic changes in the tissues due to isoeugenol (Hagan et al., 1967).
(ii) Propenylguaethol (No. 1264)
Groups of 10 male and 10 female Sprague-Dawley rats were given propenylguaethol at a dose of 0, 250, 1250, or 2500 mg/kg bw per day twice daily for 29 days by gavage in a 1% solution of methyl cellulose (Terrill, 1991). Animals were monitored twice daily for mortality and moribundity, and body weights, food consumption and detailed clinical observations were recorded weekly. Blood samples were obtained for evaluation of clinical chemistry and haematological parameters (1) at initiation of the study, from 10 animals randomly selected from a pool of available animals that had been fasted overnight; and (2) before termination of the study, from animals were injected with ketamine. At necropsy, major organs (i.e. brain, liver, heart, kidneys, thymus, testes with epididymides, thyroids with parathyroids, adrenals, ovaries, and pituitary) were removed, weighed, and tissues were preserved in 10% formalin. All tissues from the control group and the group receiving the highest dose, and tissues from the heart, liver, kidneys, and gross lesions from groups receiving the lowest and intermediate doses were preserved for examination by microscopy.
All animals survived to study termination. No differences in body weight or food consumption were reported in any of the treated animals when compared with the controls. Haematological examinations revealed a significant increase in platelet count in females at the intermediate (1250 mg/kg bw per day) and highest dose (2500 mg/kg bw per day). Total serum protein and gamma-glutamyl transferase activity were increased in both sexes at the highest dose and in females at the intermediate dose. Increased concentrations of serum albumin were also reported in both sexes at the intermediate and the highest dose. Slight but statistically significant decreases in blood urea nitrogen concentrations were observed in females at the highest dose. A significant (p <0.05) increase in relative liver and kidney weights were reported at the intermediate and the highest dose compared with the control animals. Significant increases (p <0.05) in absolute liver weight occurred in male and female rats at the highest dose. Only males at the highest dose exhibited a significant increase (p <0.05) in absolute kidney weights. The increases in organ weights were not accompanied by any treatment-related histopathological changes. No treatment-related effects were reported in the animals receiving propenylguaethol at the lowest dose (250 mg/kg bw per day) (Terrill, 1991).
(iii) Isoeugenyl methyl ether (No. 1266)
Groups of 16 male and 16 female Sprague-Dawley rats were given diets containing isoeugenol methyl ether at concentrations providing daily intakes of 0, 30, 100 or 300 mg/kg bw for 28 days (Purchase et al., 1992). No differences in general health and behaviour were reported in the treated animals. Weekly food intake and measurements of body-weight gain in the treated groups were similar those of the controls throughout the study. The urine, haematological and serum analyses conducted at the conclusion of the study did not reveal any treatment-related effects. A significant (p <0.001) increase in relative liver weights was reported in male and female animals at 300 mg/kg bw per day, whereas significantly increased (p <0.01) absolute liver weights were reported only in males at the highest dose. At 300 mg/kg bw per day, alanine aminotransferase activity was significantly elevated in females. In addition, multifocal parenchymal necrosis was observed in the liver of one female at 300 mg/kg bw per day. The authors did not attribute the increased relative liver weights to hepatotoxicity in view of the lack of accompanying treatment-related histopathological findings in males and females at 300 mg/kg bw per day. No treatment-related effects were reported in the animals given isoeugenol methyl ether at a dose of 100 mg/kg bw (Purchase et al., 1992).
Groups of 24 male and 24 female Crl : CD(SD)BR albino rats were fed diets containing isoeugenyl methyl ether at a dose of 6 mg/kg per day for 13 weeks. All animals were observed twice daily throughout the study for mortality and signs of toxicity. Evaluations of haematological and blood chemistry parameters and urine analyses were performed on randomly selected rats (12 of each sex per dose) at weeks 6 and 12. No toxicologically significant variations in any of the parameters examined were reported between treated animals and controls. Weekly measurements of body weights and food consumption revealed no significant differences between test animals and controls. At necropsy, gross and histopathological examinations revealed no lesions that could be associated with administration of the isoeugenyl methyl ether. Statistically significant variations in organ weights of treated animals compared with the controls were limited to reduced absolute thyroid weights in males and females, and reduced relative weights of both lobes in females and the right lobe in males; however, these differences were not associated with any morphological changes and consequently, were considered by the authors not to be toxicologically significant (Osborne, 1981).
(iv) Isoeugenyl benzyl ether (No. 1268)
Groups of 10 male and 10 female Wistar rats were given isoeugenyl benzyl ether at a dose of 0, 60, 120, or 240 mg/kg bw per day for 28 days by gavage in soybean oil. The rats were observed twice per day, and body weight, and food and water intake were recorded weekly. Haematological and clinical chemistry analyses were performed on eight rats of each sex per group on days 21 or 22 of treatment. At necropsy, histopathological examination of tissues revealed no dose-related changes. A significant decrease in body weight and in blood urea was reported in females at 120 mg/kg bw per day and in both sexes at 240 mg/kg bw per day. Furthermore, plasma glucose concentrations were significantly reduced in both sexes at 120 and 240 mg/kg bw per day. At the highest dose (240 mg/kg bw per day), this was accompanied by significantly increased relative liver weights in males and females, and significantly increased absolute liver weights in females. No treatment-related effects were observed at 60 mg/kg bw per day (Boe et al., 1989).
(c) Genotoxicity
Four representative members (Nos 1260, 1263, 1264, and 1268) of the group of hydroxypropenylbenzenes have been tested for genotoxicity in vitro (see Table 5).
Table 5. Results of studies of genotoxicity with hydroxypropenylbenzenes
|
No. |
Flavouring agent |
End-point |
Test system |
Concentration or dose |
Results |
Reference |
|
In vitro |
|
|
|
|
|
|
|
1260 |
Isoeugenol |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
2, 20, and 200 µg/plate |
Negativea |
Hsia et al. (1979) |
|
1260 |
Isoeugenol |
Reverse mutation |
S. typhimurium TA98 and TA100 |
0.05–100 µl/plate
(54.1–108 200 µg/plate)b |
Negativea |
Rockwell & Raw (1979) |
|
1260 |
Isoeugenol |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537 |
3 µmol/plate
(493 mg/plate)c |
Negatived |
Florin et al. (1980) |
|
1260 |
Isoeugenol |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
0.8 mg/plate
(800 µg/plate) |
Negative |
Douglas et al. (1980) |
|
1260 |
Isoeugenol |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
60, 120, and 300 µg/plate |
Negatived |
Sekizawa & Shibamoto (1982) |
|
1260 |
Isoeugenol |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
1.0 µl/plate
(1082 mg/plate)b |
Negatived |
DeGraff (1983) |
|
1260 |
Isoeugenol |
Reverse mutation |
S. typhimurium TA97, TA98, TA100, TA1535, TA1537 |
<800 µg/plate
|
Negatived |
Mortelmans et al. (1986) |
|
1260 |
Isoeugenol |
Reverse mutation |
S. typhimurium TA97 and TA102 |
<0.5 mg/plate
(500 µg/plate) |
Negatived |
Fujita & Sasaki (1987) |
|
1260 |
Isoeugenol |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
1000 µg/plate |
Negatived |
Heck et al. (1989) |
|
1260 |
Isoeugenol |
Point mutation |
E. coli WP2uvrA |
60, 120, and 300 µg/plate |
Negatived |
Sekizawa & Shibamoto (1982) |
|
1260 |
Isoeugenol |
DNA repair |
B. subtillis H 17 (rec+) and M 45 (rec-) |
22.0 µg/disk |
Negative |
Oda et al. (1979) |
|
1260 |
Isoeugenol |
DNA repair |
B. subtillis H 17 (rec+) and M 45 (rec-) |
0.8 mg/disk
(800 µg/disk) |
Negativee |
Sekizawa & Shibamoto (1982) |
|
1260 |
Isoeugenol |
Sister chromatid exchange |
Chinese hamster ovary cells |
10, 33.3, and100 µmol/l (1.64,5.47, and 16.42 µg/ml)c |
Negativee |
Sasaki et al. (1989) |
|
1260 |
Isoeugenol |
Sister chromatid exchange |
Human lymphocytes |
0.5 mmol/l
(82 µg/ml)c |
Positive |
Jansson et al. (1986) |
|
1260 |
Isoeugenol |
Unscheduled DNA synthesis |
Mouse hepatocytes |
<1000 µmol/l
(164.2 µg/ml)c |
Negative |
Burkey et al. (2000) |
|
1260 |
Isoeugenol |
Unscheduled DNA synthesis |
Rat hepatocytes |
<1000 µmol/l
(164.2 µg/ml)c |
Negative |
Burkey et al. (2000) |
|
1263 |
Isoeugenyl phenylacetate |
Reverse mutation |
S. typhimurium TA1535, TA1537, TA1538, TA98 and TA100 |
<3.6 mg/plate
(3600 µg/plate) |
Negatived |
Wild et al. (1983) |
|
1263 |
Isoeugenyl phenylacetate |
Unscheduled DNA synthesis |
Rat hepatocytes |
<30 µg/ml |
Negative |
San & Reece (2003) |
|
1264 |
Propenylguaethol |
Reverse mutation |
S. typhimurium TA1535, TA1537, TA1538, TA98 and TA100 |
<3.6 mg/plate
(3600 µg/plate) |
Negatived |
Wild et al. (1983) |
|
1264 |
Propenylguaethol |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
<1000 µg/plate
|
Negatived |
Jagannath (1982) |
|
1264 |
Propenylguaethol |
Forward mutation |
Mouse lymphoma L5178Y TK +/-cells |
1.875 to 100 µg/ml |
Positivea,f |
Cifone (1983) |
|
1264 |
Propenylguaethol |
Forward mutation |
Mouse lymphoma L5178Y TK +/-cells |
7.81 to 125 µg/ml |
Negativee,f |
Cifone (1983) |
|
1264 |
Propenylguaethol |
Unscheduled DNA Synthesis |
Rat primary hepatocytes |
1.01–50.4 µg/ml |
Negative |
Cifone (1988) |
|
1268 |
Isoeugenyl benzyl ether |
Reverse mutation |
S. typhimurium TA1535, TA1537, TA1538, TA98 and TA100 |
<3.6 mg/plate
(3600 µg/plate) |
Negatived |
Wild et al. (1983) |
|
1268 |
Isoeugenyl benzyl ether |
Unscheduled DNA synthesis |
Rat hepatocytes |
<20 µg/ml |
Negative |
San & Reece (2003) |
|
In vivo |
|
|
|
|
|
|
|
1263 |
Isoeugenyl phenylacetate |
Sex linked recessive lethal chromosomes |
D. melanogaster |
25 mmol/l
(7059 µg/ml)g |
Negativeh |
Wild et al. (1983) |
|
1263 |
Isoeugenyl phenylacetate |
Micronucleus induction |
Mouse |
564 987, and 1410 mg/kg |
Negativei,j |
Wild et al. (1983) |
|
1264 |
Propenylguaethol |
Sex linked recessive lethal chromosomes |
D. melanogaster |
10 mmol/l
(1782 µg/ml)k |
Negativeh |
Wild et al. (1983) |
|
1264 |
Propenylguaethol |
Micronucleus induction |
Mouse |
649, 1298, and 1947 mg/kg bw |
Negativej |
Wild et al. (1983) |
|
1268 |
Isoeugenyl benzyl ether |
Sex linked recessive lethal chromosomes |
D. melanogaster |
2.5 mmol/l
(636 µg/ml)l |
Negativeh |
Wild et al. (1983) |
|
1268 |
Isoeugenyl benzyl ether |
Micronucleus induction |
Mouse |
508, 1016, and 1524 mg/kg bw |
Negativei,j |
Wild et al. (1983) |
|
a |
With metabolic activation |
|
b |
Calculated based on density =1.079 to 1.085 g/ml (Food Chemicals Codex, 1996) |
|
c |
Calculated based on relative molecular mass =164.2 |
|
d |
With and without metabolic activation |
|
e |
Without metabolic activation |
|
f |
With concomitant cytotoxicity |
|
g |
Calculated based on the relative molecular mass of ioseugenyl phenylacetate =282.34 |
|
h |
Administered orally |
|
i |
Administered twice within 24 h |
|
j |
Administered intraperitoneally |
|
k |
Calculated based on relative molecular mass of propenylguaethol =178.23 |
|
l |
Calculated based on relative molecular mass of isoeugenyl benzyl ether =254.33 |
In vitro
Negative results were reported in the standard Ames assay when various strains of Salmonella typhimurium (TA97, TA98, TA100, TA102, TA1535, TA1537, TA1538) were incubated with isoeugenol (No. 1260) (Hsia et al., 1979; Rockwell & Raw, 1979; Douglas et al., 1980; Florin et al., 1980; Sekizawa & Shibamoto, 1982; DeGraff, 1983; Mortelmans et al., 1986; Fujita & Sasaki, 1987; Heck et al., 1989), isoeugenyl phenylacetate (No. 1263) (Wild et al., 1983), propenylguaethol (No. 1264) (Jagannath, 1982; Wild et al., 1983), or isoeugenol benzyl ether (No. 1268) (Wild et al., 1983) at concentrations of up to 100 µl/plate (108 200 µg/plate)5, with and without metabolic activation.
Isoeugenol did not exhibit mutagenic potential at a concentration of up to 300 µg/plate, with and without metabolic activation, in an assay for point mutations in Escherichia coli (Sekizawa & Shibamoto, 1982). In an assay for DNA repair in Bacillus subtilis, isoeugenol displayed no evidence of genotoxic potential at a concentration of 22 µg/disk (Oda et al., 1979). In a subsequent study, no significant genotoxic effects were observed with isoeugenol at a higher concentration (800 µg/disk), with dimethylsulfoxide as solvent. Inconclusive results were obtained when ethanol was used as the solvent, due to varying growth rates in the tester strains (Sekizawa & Shibamoto, 1982). Therefore, isoeugenol is negative in the B. subtilis rec assay.
Isoeugenol gave negative results in an assay for chromatid breaks and sister chromatid exchanges in Chinese hamster ovary cells (Sasaki et al., 1989), but caused an increase in sister chromatid exchanges in human lymphocytes, albeit at a high concentration (0.5 mmol/l (82 µg/ml))6 (Jansson et al., 1986). Such effects may be produced by test substances at concentrations that produce high levels of cytotoxicity, involving lysosomal breakdown and release of DNAase, rather than by the direct action of the test substance on DNA (Zajac-Kaye & Ts’o, 1984; Bradley et al., 1987).
Uniformly negative results were reported for isoeugenol (Burkey et al., 2000) and propenylguaethol (Cifone, 1988) at concentrations of up to 1000 µmol/l (164.2 µg/ml)6 in assays for unscheduled DNA synthesis conducted in mouse and rat hepatocytes. In studies in primary cultures of rat hepatocytes, isoeugenol phenylacetate (1–30 µg/ml) and isoeugenyl benzyl ether (1.25–20 µg/ml) did not induce unscheduled DNA synthesis (San & Reece, 2003).
Propenylguaethol induced an increase in mutations when incubated with L5178Y Tk +/- mouse lymphoma cells (the mouse lymphoma assay), but only in the presence of metabolic activation (Cifone, 1983). The results for simple aliphatic and aromatic substances in the presence of metabolic activation do not agree with the results of other standardized assays for genotoxicity. This test, which has poor selectivity for genotoxicity, is sensitive to culture conditions of low pH and high osmolality (Caldwell, 1993). Substances (aromatic ethers, phenols, aldehydes, carboxylic acids, lactones) that have a potentially acidifying or ionizing influence on the culture medium have been shown to produce false positive results in this and other assays (Heck et al., 1989).
In vivo
Data from studies conducted in vivo support the conclusion that hydroxypropenylbenzene and alkoxypropenylbenzene derivatives do not have any significant genotoxic potential. Isoeugenyl phenylacetate, propenylguaethol, and isoeugenyl benzyl ether did not induce sex-linked recessive lethal mutations in male Drosophila melanogaster when administered orally at a dose of 25, 10, or 2.5 mmol/l, respectively (Wild et al., 1983). Furthermore, there was no evidence of an increase in the incidence of polychromatic mononucleated erythrocytes in standardized assays for micronucleus formation in cells from mice given isoeugenyl phenylacetate, propenylguaethol or isoeugenyl benzyl ether at doses of up to 1410, 1947, and 1524 mg/kg bw, respectively, by injection (Wild et al., 1983).
(d) Reproductive toxicity
(i) Propenylguaethol (No. 1264)
Four groups of ten virgin Crl : CD rats were given propenylguaethol at an oral dose of 0, 250, 1250, or 2500 mg/kg bw by gavage in a 1% solution of methylcellulose once daily, beginning 7 days before cohabitation, throughout cohabitation (maximum of 7 days), gestation, delivery, and until 4 days after parturition. Maternal indices monitored included daily clinical observation, measurement of body weights, food consumption, duration of gestation and fertility parameters (mating and fertility index, gestation index, number of offspring per litter). Offspring indices monitored included daily observation for clinical signs of toxicity, examination for gross external malformations, and measurements of mortality (number of still-borns), viability (pups dying on postnatal days 1–4), body weight and body-weight gain.
Maternal deaths occurred at 1250 mg/kg bw per day (1 out of 10) and 2500 mg/kg bw per day (2 out of 10). Additional clinical observations in the rats that died included decreased motor activity, impaired or loss of righting reflex, a red substance in the vaginal area, loss of righting reflex, chromodacryorrhoea, dystocia, and laboured breathing. During the premating period, a significant incidence of abnormal coloured urine was observed at 1250 and 2500 mg/kg bw per day. Urine-stained fur was observed at significant levels of incidence at the lowest (250 mg/kg bw per day) and highest (2500 mg/kg bw per day) doses during the premating and gestation periods. Excess salivation was observed in a significant number of rats at all doses during the premating and gestation periods, as well as during lactation in rats receiving the highest dose. Reduced body-weight gain and food consumption values were reported at 2500 mg/kg bw per day during gestation and increased body weight values were reported during lactation compared with the control group.
The lack of biologically important or statistically significant differences among the treated groups with regard to size of live litters, pup sex ratios, body weights, viability and morphology indicates that propenylguaethol does not affect the viability and growth of offspring at the same high doses that produce adverse maternal effects. On the basis of these results, the authors did not consider that propenylguaethol adversely affected reproduction or development in rats. The NOEL for propenylguaethol for maternal reproductive effects was <250 mg/kg bw per day, and the NOEL for developmental effects was reported as >2500 mg/kg bw per day (Hoberman, 1990).
3. REFERENCES
Anders, M.W. (1989) Biotransformation and bioactivation of xenobiotics by the kidney. In: Hutson, D.H., Caldwell, J. & Paulson. G.D., eds, Intermediary Xenobiotic Metabolism in Animals, New York: Taylor and Francis, pp. 81–97.
Badger, D.A., Smith, R.L., Bao, J., Kuester, R.K. & Sipes, I.G. (2002) Disposition and metabolism of isoeugenol in the male Fischer-344 rat. Food Chem. Toxicol., 40, 1757–1765.
Bär, V.F. & Greipentrog F. (1967) Die situation in der gesundheitlichen Beurteilung der Aromatisierungsmittel fur Lebensmittel. [Where we stand concerning the evaluation of flavoring substances from the viewpoint of health]. Medizin Ernahr. 8, 244–251 (In German).
Boe, M., Wurtzen G. & Olsen P. (1989) Short term toxicity study in rats dosed with isoeugenol benzyl ether. Drug Chem. Toxicol. 12(2), 165–171.
Bradley, M.O., Taylor, J.M., Armstrong, M.J. & Galloway, S.M. (1987) Relationship among cytotoxicity, lysosomal breakdown, chromosome aberrations and DNA double-strand breaks. Mutat. Res., 189, 69–79.
Burkey, J.L., Sauer, J.M., McQueen, C.A. & Sipes I.G. (2000) Cytotoxicity and genotoxicity of methyleugenol and related congeners—a mechanism of activation for methyleugenol. Mutat. Res., 452, 25–33.
Caldwell, J. & Sutton, J.D. (1988) Influence of dose size on the disposition of trans-[methoxy-14C]anethole in human volunteers. Food Chem. Toxicol. 26, 87–91.
Caldwell, J. (1993) Perspective on the usefulness of the mouse lymphoma assay as an indicator of a genotoxic carcinogen: Ten compounds which are positive in the mouse lymphoma assay. Teratogen. Carcin. Mut., 13, 185–190.
Cifone, M.A. (1983) Mutagenicity evaluation of vanitrope in the mouse lymphoma forward mutation assay. Private communication to the Flavor and Extract Manufacturers Association. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington, DC, USA.
Cifone, M.A. (1988) Mutagenicity test on vanitrope in the rat primary hepatocyte unscheduled DNA synthesis assay. Private communication to the Flavor and Extract Manufacturers Association. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington, DC, USA.
Cramer, G.M., Ford, R.A. & Hall, R.L. (1978) Estimation of toxic hazard—a decision tree approach. Food. Cosmet. Toxicol. 16, 255–276.
DeGraff, W.G. (1983) Mutagenicity evaluation of isoeugenol in the Ames Salmonella/microsome plate test. Private communication to the Flavor and Extract Manufacturers Association. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington, DC, USA.
Douglas, G.R., Nestmann, E.R., Betts, J.L., Mueller, J.C., Lee, E.G.-H., Stich, H.F., San, R.H.C., Brouzes, R.J.P., Chmelauskas, A., Paavila, H.D. & Walden, C.C. (1980) Mutagenic activity in pulp mill effluents. In: Jolley, R.L., Brungs, W.A., Cumming, R.B. & Jacobs, V.A., eds., Water Chlorination: Environmental Impact & Health Effects, Ann Arbor Science Publishers Inc, pp. 865–880.
Fishbeck, W.A., Langer, R.R. & Kociba, R.J. (1975) Elevated urinary phenol levels not related to benzene exposure. Amer. Ind. Hygiene Assoc. J., (AIHAJ), 36, 820–824.
Fischer, I.U., Unruh, G.E. & Dengler, H.J. (1990) The metabolism of eugenol in man. Xenobiotica 20, 209–222.
Florin, I., Rutberg, L., Curvall, M. & Enzell, C.R. (1980) Screening of tobacco smoke constituents for mutagenicity testing using Ames test. Toxicology, 18, 219–232.
Food and Drug Administration (1993) Priority-based assessment of food additives (PAFA) database. Center for Food Safety and Applied Nutrition, p. 58.
Food Chemicals Codex (1996) Flavor Chemicals: Isoeugenol, 4th Ed. pp. 516–517.
Fuciarelli, A.F. (2001) Single-administration toxicokinetic study (intravenous and gavage routes) of isoeugenol in Fischer 344/N rats and B6C3F1 mice (NTP Chem Task Number: CHEM04834). Unpublished report.
Fujita, H. & Sasaki, M. (1987) Mutagenicity test of food additives with Salmonella typhimurium TA97 and TA102. Annual Report of the Tokyo Metropolitan Research Laboratory of Public Health 38, 423–430.
Grundschober, F. (1977) Toxicological assessment of flavouring esters. Toxicology, 8, 387–390.
Hagan, E.C., Hansen, W.H., Fitzhugh, O.G., Jenner, P.M., Jones, W.I., Taylor, J.M., Long, E.L., Nelson, A.A. & Brouwer, J.B. (1967) Food flavourings and compounds of related structure. II. Subacute and chronic toxicity. Food. Cosmet. Toxicol. 5, 141–157.
Heck, J.D., Vollmuth, T.A., Cifone, M.A., Jagannath, D.R., Myhr, B. & Curren, R.D. (1989) An evaluation of food flavoring ingredients in a genetic toxicity screening battery. The Toxicologist, 9, 257.
Heymann, E. (1980) Carboxylesterases and amidases. In: Jakoby, W.B., ed., Enzymatic Basis of Detoxication, 2nd Ed., Vol. II, New York: Academic Press Inc., pp. 291–323.
Hoberman, A.M. (1990) Reproductive and developmental toxicity screening test of B134 (vanitrope) administered orally via gavage to Crl:CD (SD) BR female rats. Private communication to the Flavor and Extract Manufacturers Association. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington, DC, USA.
Hosokawa, M., Watanabe, N., Tsukada, E., Fukumoto, M., Chiba, K., Takeya, M., Imai, T., Sasaki, Y.F. & Sato, T. (2001) Multiplicity of carboxylesterase isozymes in mammals and humans: role in metabolic activation of prodrugs. Yakubutsu Dotai (Xenobiotic Metabolism and Disposition) 16(Suppl.), 92–93.
Hsia, M.T.S., Adamovics, J.A. & Kreamer, B.L. (1979) Microbial mutagenicity studies of insect growth regulators and other potential insecticidal compounds in Salmonella typhimurium. Chemosphere, 8, 521–529.
International Organization of the Flavor Industry (1995). European inquiry on volume use. Unpublished report. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Jagannath, D.R. (1982) Mutagenicity evaluation of vanitrope in the Ames Salmonella/microsome plate test. Private communication to the Flavor and Extract Manufacturers Association. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington, DC, USA.
James, M.O., Smith, R.L., Williams, R.T. & Reidegberg, M. (1972) Conjugation of phenylacetic acid in man, subhuman primates and some nonprimate species. Proc. R. Soc. Lond. B Biol. Sci., 182, 25–35.
Jansson T., Curvall, M., Hedin, A. & Enzell, C.R. (1986) In vitro studies of biological effects of cigarette smoke condensate. II. Induction of sister-chromatid exchanges in human lymphocytes by weakly acidic, semivolatile constituents. Mutat. Res., 169, 129–139.
Jenner, P.M., Hagan, E.C., Taylor, J.M., Cook, E.L. & Fitzhugh, O.G. (1964) Food flavorings and compounds of related structure. I. Acute oral toxicity. Food. Cosmet. Toxicol., 2, 327–343.
Jung, W. & Heymann, E. (1979) Characterization of the isoenzymes of pig liver carboxylesterase. II. Kinetic studies. Eur. J. Biochem., 95, 519–525.
Keating, J.W. (1972) Acute toxicity study in rats and rabbits. Unpublished report to the Research Institute of Fragrance Materials, Englewood Cliffs, New Jersey, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington, DC, USA.
LeBourhis, B. (1973) Biological properties of trans-anethole. An attempt to determine the acceptable daily dose. Parfum Cosmet Savons France, 3, 8–9.
Levenstein, I. & Wolven, A.M. (1972) Acute toxicity study in rats and rabbits. Unpublished report to the Research Institute of Fragrance Materials, Englewood Cliffs, New Jersey, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington, DC, USA.
Lucas, C.D., Putnam, J.M. & Hallagan, J.B. (1999) Flavor and Extract Manufacturers Association of the United States 1995 Poundage and Technical Effects Update Survey, Washington DC: Flavor and Extract Manufacturers Association of the United States.
Maarse, H., Visscher, C.A., Willemsens, L.C. & Boelens, M.H. (1999). Volatile components in food—qualitative and quantitative data. Zeist: Centraal Instituut Voor Voedingsonderzioek TNO.
Moreno, O.M. (1973) Acute toxicity studies on rats and rabbits. Unpublished report to the Research Institute of Fragrance Materials, Englewood Cliffs, New Jersey, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington, DC, USA.
Mortelmans, K., Haworth, S., Lawlor, T., Speck, W., Tainer, B. & Zeiger, E. (1986) Salmonella mutagenicity tests: II. Results from the testing of 270 chemicals. Environ. Mutagen., 8, 1–119.
National Academy of Sciences (1970, 1982) Evaluating the Safety of Food Chemicals. Washington, DC.
National Toxicology Program (2002) National Toxicology Program. 90-Day studies on isoeugenol (CAS 97-54-1) in F344/N rats and B6C3F1 mice (gavage): Preliminary report (Study Number G004164-M). Unpublished report. Nelson, D.L. & Cox M.M. (2000) Lehninger: Principles of Biochemistry. New York: Worth Publishers, Inc.
Newberne, P., Smith, R.L., Doull, J., Goodman, J.I., Munro, I.C., Portoghese, P.S., Wagner, B.M., Weil, C.S., Woods, L.A., Adams, T.B., Lucas, C.D. & Ford, R.A. (1999) The FEMA GRAS assessment of trans-anethole used as a flavor substance. Food Chem. Toxicol. 37, 789–811.
Oda Y., Hamono, Y., Inoue, K., Yamamoto, H, Niihara, T. & Kunita, N. (1979) Mutagenicity of food flavours in bacteria. Shokuhin Eisei Hen, 9, 177–181.
Osborne, B.E. (1981) A 91-day single dose level dietary study of eugenyl methyl ether and isoeugenyl methyl ether in the albino rat. Private communication to the Flavor and Extract Manufacturers Association. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington, DC, USA.
Piccirillo, V.J. (1984a) 14-Day single dose subacute toxicity study in the rat with isoeugenol. Private communication to the Flavor and Extract Manufacturers Association. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington, DC, USA.
Piccirillo, V.J. (1984b) 14-Day single dose subacute toxicity study in the rat with vanitrope. Private communication to the Flavor and Extract Manufacturers Association. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington, DC, USA.
Piccirillo, V.J. & Hartman, W.C. (1982) Acute oral toxicity (LD50) study with 4-methyl-2,6-dimethoxy phenol in rats. Private communication to the Flavor and Extract Manufacturers Association. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington, DC, USA.
Purchase, R., Ford, G.P., Creasy, D.M., Brantom, P.G. & Gangolli, S.D. (1992) A 28-day feeding study with methyl isoeugenol in rats. Food Chem. Toxicol. 30, 475–481.
Rockwell, P. & Raw, I. (1979) A mutagenic screening of various herbs, spices and food additives. Nutr. Cancer, 1, 10–15.
San, R.H.C. & Reece, J.D. (2003) Unscheduled DNA synthesis in mammalian cells in vitro. Unpublished report UDS-01-1 from BioReliance, Rockville, MD, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington, DC, USA.
Sangster, S.A., Caldwell, J., Hutt, A.J. & Smith, R. (1987) The metabolic disposition of [methoxy-14C]-labelled trans-anethole, estragole and p-propylanisole in human volunteers. Xenobiotica 17, 1223–1232.
Sasaki, Y.F., Imanishi, H., Ohta, T. & Shirasu, Y. (1989) Modifying effects of components of plant essence on the induction of sister-chromatid exchanges in cultured Chinese hamster ovary cells. Mut. Res., 226, 103–110.
Sekizawa, J. & Shibamoto, T. (1982) Genotoxicity of safrole-related chemicals in microbial test systems. Mutat. Res., 101, 127–140.
Silverman, L.M., Christenson, R.H. & Grant, G.H. (1986) Amino acids and proteins. In: Tietz, N., ed., The Textbook of Clinical Chemistry, Philadelphia: W.B. Saunders Company.
Solheim, E. & Scheline, R.R. (1976) Metabolism of alkenebenzene derivatives in the rat. II. Eugenol and isoeugenol methyl ethers. Xenobiotica 6, 137–150.
Stofberg, J. & Grundschober, F. (1987) Consumption ratio and food predominance of flavoring materials. Perfumer Flavorist, 12, 27.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of flavoring materials: A mechanism for setting priorities for safety evaluation. Food. Chem. Toxicol., 23, 857–860.
Sutton, D.J., Sangster, S.A. & Caldwell, J. (1985) Dose-dependent variation in the disposition of eugenol in the rat. Biochem. Pharmacol., 34, 465–466.
Tephly, T.R. (1991) The toxicity of methanol. Life Sciences, 48, 1031–1041.
Terrill, J.B. (1991) 28-Day oral toxicity study in rats (vanitrope). Private communication to the Flavor and Extract Manufacturers Association. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington, DC, USA.
Wild, D., King, M.T., Gocke, E. & Eckhardt, K. (1983) Study of artificial flavouring substances for mutagenicity in the Salmonella/microsome, basc, and micronucleus tests. Food Chem. Toxicol., 21, 707–719.
Williams, R.T. (1959) The metabolism of terpenes and camphors. In: Detoxication mechanisms—the metabolism and detoxication of drugs, toxic substances and other organic compounds, 2nd Ed., London: Chapman and Hall Ltd., pp. 519–543.
Yuasa, A. (1974) Exp. studies on glucuronidaton. Japanese Journal of Veterinary Science, 36, 427–432.
Zajac-Kaye, M. & Ts’o, P.O. (1984) DNAase I encapsulation in liposomes can induce neoplastic transformation of Syrian hamster embryo cells in culture. Cell, 39, 427–437.
ENDNOTES:
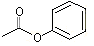 Phenyl acetate
Phenyl acetate - bioavailability is defined as the ratio of area under the plasma concentration-time curve for administration by gavage (AUC(gavage)) divided by the AUC for intravenous administration (AUC(IV)).
 p-Methoxypropenylbenzene (trans-anethole)
p-Methoxypropenylbenzene (trans-anethole)  Eugenol
Eugenol- Calculated based on density of isoeugenol =1.082 g/ml (Sigma-Aldrich)
- Calculated based on the relative molecular mass of isoeugenol =164.2










 Phenyl acetate
Phenyl acetate  p-Methoxypropenylbenzene (trans-anethole)
p-Methoxypropenylbenzene (trans-anethole)  Eugenol
Eugenol