

WHO FOOD ADDITIVES SERIES: 53
First draft prepared by
J. Wongtavatchai
Faculty of Veterinary Medicine, Chulalongkorn University, Bangkok, Thailand
J.G. McLean
Camberwell, Victoria, Australia
F. Ramos
Laboratório de Bromatologia, Nutrição e Hidrologia, Faculdade de Farmácia, Universidade de Coimbra, Portugal
and
D. Arnold
Berlin, Germany
Chloramphenicol is a broad-spectrum antibiotic with historical veterinary uses in all major food-producing animals and with current uses in humans and companion animals. Chloramphenicol was evaluated previously by the Committee at its twelfth, thirty-second and forty-second meetings (Annex 1, references 17, 80 and 110). A number of other agencies have also reviewed chloramphenicol (e.g. International Agency for Research on Cancer (IARC), 1990; European Committee for Veterinary Medicinal Products, 1994; United States Food and Drug Administration, 1985). Concerns have been expressed about the genotoxicity of chloramphenicol and its metabolites, its embryo- and fetotoxicity, its carcinogenic potential in humans and the lack of a dose-response relationship for aplastic anaemia caused by treatment with chloramphenicol in humans. Deficiencies identified in data on the toxicity of chloramphenicol include information necessary for the assessment of carcinogenicity and effects on reproduction. An acceptable daily intake (ADI) has never been allocated and consequently a maximum residue limit (MRL) has not been assigned. This has resulted in the restriction of the use of chloramphenicol in veterinary medicine to non-food use.
Chloramphenicol was originally isolated from the soil organism Streptomyces venezuelae in 1947, but is now produced synthetically. Three common forms are used for systemic therapy, depending on the route of administration; a free base form of chloramphenicol, chloramphenicol palmitate and chloramphenicol succinate. Other formulations are also available for topical use. Chloramphenicol usually acts as a bacteriostatic, but at higher concentrations or against some very susceptible organisms it can be bactericidal. It is used in the treatment of human infection with Salmonella typhi (typhoid) and other forms of salmonellosis, and other life-threatening infections of the central nervous system and respiratory tract (Parfitt, 1999). In veterinary medicine, chloramphenicol is used for the treatment of a variety of infections in animals, particularly those caused by anaerobic bacteria or those that are resistant to other antimicrobial agents. Chloramphenicol in animals is well absorbed by both oral and parenteral routes (Plumb, 2002).
There is good evidence for a haemotoxic effect of chloramphenicol in humans, with two forms of toxicity being described. The first is a commonly occurring, dose-related reversible bone-marrow depression, which develops during treatment and is reversible following the withdrawal of the drug. The second is a severe aplastic anaemia, which is non-dose-related and often irreversible.
This monograph summarizes the recently published literature and submitted unpublished information on the toxicity of chloramphenicol.
The usual therapeutic range for chloramphenicol in serum in most animal species is 5-15 µg/ml. After dosing, chloramphenicol is widely distributed throughout the body. The volume of distribution of chloramphenicol reported in companion animals is 1.8 l/kg in the dog, 2.4 l/kg in the cat and 1.41 l/kg in the horse. Hepatic metabolism by a glucuronidative mechanism is the principle pathway for which chloramphenicol undergoes biotransformation to an inactive metabolite, chloramphenicol glucuronide. Only about 5-15% of the drug is excreted unchanged in the urine. Dogs excrete only about 6% of unchanged drug into the urine. Cats have a limited ability to form glucuronide conjugates with drugs and therefore excrete chloramphenicol more slowly than other animals, with 25% or more of the administered dose excreted unchanged in the urine. The elimination half-life of chloramphenicol is 1.1-5.0 h in dog, <1 h in foals and ponies, and 4-8 h in cats (Adams, 1995; Plumb, 2002).
The pharmacokinetic properties of orally administered chloramphenicol in broiler chickens indicate that chloramphenicol is rapidly absorbed. At a dose of 30 or 50 mg/kg bw, the drug reached the maximum plasma concentration at 0.72 h or 0.60 h, it was eliminated with a mean half-life (t1/2 beta) of 6.87 or 7.41 h and had a bioavailability of 29% or 38% respectively. A concentration of chloramphenicol of >5 µg/ml was achieved in plasma at 15 min, and persisted up to 2 or 4 h after administration of chloramphenicol at a dose of 30 or 50 mg/kg bw. When chickens received an oral dose of chloramphenicol at 50 mg/kg bw once daily for 4 days, three metabolites, dehydrochloramphenicol; nitrophenylaminopropanedione-chloramphenicol (NPAP-chloramphenicol); and nitrosochloramphenicol were found in kidney, liver and muscle. The study found a slow clearance of residues, particularly of the NPAP and nitrosochloramphenicol residues, which were detected in tissues at 12 days after dosing (Anadon et al., 1994).
Plasma concentrations of chloramphenicol were determined in four calves given four oral doses of chloramphenicol palmitate, each corresponding to a dose of chloramphenicol of 25 mg/kgbw, at 12 h intervals. After the fourth dose, the plasma concentration of chloramphenicol reached a steady state of 5-6 µg/ml. The half-life of elimination was 4.5 h. Dehydrochloramphenicol at a concentration of 3-7 µg/ml was also detected in the plasma. The authors suggested that dehydrochloramphenicol, which is a metabolite produced by intestinal bacteria and suggested to be associated with fatal aplastic anaemia in human, may occur in edible tissues of animals treated with chloramphenicol (Gassner & Wuethrich, 1994).
Several metabolites of chloramphenicol were identified in urine samples obtained from male Wistar rats and from a human volunteer given tritiated chloramphenicol at a dose of 10 mg/kg bw by mouth. In rats, the two most abundant metabolites detected in the first 24 h by high-performance liquid chromatography (HPLC) and gas chromatography-mass spectrometry (GC-MS) were chloramphenicol-base and chloramphenicol-acetylarylamine. The remaining metabolites were unchanged chloramphenicol, chloramphenicol-oxamic acid, chloramphenicol-alcohol, chloramphenicol-glucuronide and chloramphenicol-oxamylethanolamine. Similar end-products were also found in the human volunteer. The amount of chloramphenicol-oxamylethanolamine, which is an end-product of chloramphenicol biotransformation that was previously reported in birds, represented 0.74% and 1.37% of the ingested radioactivity found in the rat and human urine samples. The formation of chloramphenicol-oxamylethanolamine as an end-product of the metabolism of chloramphenicol by the liver was proven by the release of chloramphenicol-oxamylethanolamine after incubation of tritiated chloramphenicol with hepatocyte microsomes from rats treated with phenobarbital (Cravedi et al., 1995).
Chloramphenicol-aldehyde as a metabolic product of chloramphenicol was identified in a study in four children with major infections treated with chloramphenicol at a dose of 50 mg/kg bw per day). The residues in samples of urine collected during the treatment were analysed using HPLC and GC-MS. Results indicated the existence of compounds with characteristics corresponding to the synthesized chloramphenicol-aldehyde derivatives. The author concluded that chloramphenicol-aldehyde, a metabolite that was toxic to bone marrow and previously observed only in rat hepatic tissue, was a new metabolite in humans (Holt, 1995).
A study performed in vitro in human bone-marrow cells from 72 donors showed that chloramphenicol succinate was metabolized to chloramphenicol and other metabolites. In all 72 samples, the HPLC analysis of cell-free supernatant obtained from samples of bone marrow incubated with chloramphenicol succinate for 3 h at 37°C revealed a substance with a retention time corresponding to that of chloramphenicol. Other metabolites, nitrosochloramphenicol and unidentified metabolites, were also presented in some bone marrow samples. The study referred to the ultimate toxic derivatives of chloramphenicol produced in the bone marrow in situ as resulting from the metabolic biotransformation of the prodrug, thus indicating the marrow as both the site of metabolic conversion and the target of injury (Ambekar et al., 2000).
Catalan et al. (1993) reported that chloramphenicol induced sister chromatid exchange in bovine lymphocyte cultures, an effect that is indicative of DNA damage and repair, and also observed a delay in the cell cycle.
The cytotoxicity and genotoxicity of chloramphenicol and six metabolites were investigated in human bone-marrow cells (RiBM cells) in vitro. The six metabolites tested in the study were: nitrosochloramphenicol, chloramphenicol-glucuronide, chloramphenicol base (NAPD), and an alcohol derivative (hydroxy-amphenicol, HAP), dehydrochloramphenicol and nitrophenyl-aminopropanedione-chloramphenicol (NPAP-chloramphenicol). The cytotoxic effect was demonstrated by inhibition of incorporation of tritiated thymidine into DNA. The genotoxic effect was evaluated by the induction of DNA single-strand breaks. Cytotoxic effects were found with three metabolites, nitrosochloramphenicol, dehydrochloramphenicol and NPAP-chloramphenicol, at concentrations ranging from 2 × 105 to 2 × 104 mol/l. Nitrosochloramphenicol appeared to be the most potent cytotoxic compound tested, while chloramphenicol-glucuronide and HAP were not cytotoxic in RiBM cells. A similar cytotoxic response was reported earlier in human peripheral blood lymphocytes, but dehydrochloramphenicol was the most inhibitory compound. Genotoxic potential was observed with nitrosochloramphenicol and dehydrochloramphenicol at a concentration of 1-2 × 104 mol/l, with a dose-response pattern; chloramphenicol and other metabolites were devoid of genotoxic effect at concentrations up to 4 × 103 mol/l. On the basis of the response found in RiBM cells compared with the previous investigation using peripheral blood lymphocytes, the authors concluded that RiBM cells were much less susceptible to the genotoxic effect of chloramphenicol metabolites than were human lymphocytes (Lafarge-Frayssinet et al., 1994; Robbana-Barnat et al., 1997).
An increase in apoptosis in marrow progenitor cells was reported in patients with aplastic anaemia (Philpott et al., 1995). The first evaluation of apoptosis in toxicity caused by chloramphenicol was assessed in vitro in a study using a monkey kidney-derived cell line and human haematopoietic progenitor cells from human neonatal cord blood. At a concentration of 2-5 mmol/l, chloramphenicol caused apoptosis in dividing cells of both systems. In a subsequent study of myelotoxicity in vivo, morphological evidence of apoptosis was seen in erythroid and myeloid precursors in femoral marrow of B6C3F1 mice given chloramphenicol at a dose of 200 mg/kg bw. The authors suggested that effect of chloramphenicol is at the differentiation stage of the committed marrow progenitor cells, rather than the replication stage of the stem cells, and therefore this response appears to be paralleling the reversible bone-marrow depression seen in the treated patient (Holt et al., 1997, 1998).
The observation that chloramphenicol induces apoptosis in haemopoietic stem cells was confirmed by additional studies using models in vitro and in vivo. Phenotypic analyses using flow cytometry (with a fluorescence-activated cell sorter, FACS) have demonstrated the induction of apoptosis in purified human bone-marrow CD34+ cells treated with chloramphenicol (Kong et al., 1999). A link between this cytotoxicity and chloramphenicol-induced apoptosis was confirmed in vivo in BALB/c mice treated orally with a single high dose of chloramphenicol at 4000 mg/kg bw or with thiamphenicol. Apoptosis in femoral mononuclear cells sampled at 36 h after dosing, as indicated by morphological evidence for increased numbers of apoptotic nucleated marrow cells, was only induced by chloramphenicol, while thiamphenicol gave a negative result. The authors suggested that the induction of apoptosis in marrow progenitor cells might account for chloramphenicol-induced toxicity associated with aplastic anaemia in humans (Turton et al., 2002b).
Inhibition of protein synthesis in the mitochondria of bone-marrow cells has been considered as a mechanism by which bone-marrow depression is induced by chloramphenicol. The underlying cytotoxicity may be caused by the similarity between mitochondrial ribosomes and bacterial ribosomes, both of which are 70S. Thus chloramphenicol can also inhibit mitochondrial protein synthesis in mammalian cells, particularly in erythropoietic cells, which appear to be sensitive to the drug (Sande & Mandell, 1993; Kucers et al., 1997). It was reasoned that the inhibition of mitochondrial protein synthesis suppressed the division of mitochondria and resulted in the formation of megamitochondria. Investigation of the toxicity caused by chloramphenicol in mouse hepatic cells in vivo, however, showed that antioxidants prevented the formation of megamitochondria (Matsuhashi et al., 1996). The role of antioxidants in reducing the cytotoxic effects of chloramphenicol was also reported to occur in vitro in a study using a monkey kidney-derived cell line and haematopoietic progenitor cells from human neonatal cord blood. Also, in cells in culture, the cytotoxic effects of chloramphenicol on apoptosis and suppression of progenitor cell growth were not pronounced when cells were co-cultured with antioxidants such as mercaptoethylamine or vitamin C (Holt et al., 1997). Both studies suggested that toxicity caused by chloramphenicol relates intimately to oxidative stress, with a possible link between a metabolic event—the production of free radicals—and bone marrow suppression.
The cytotoxic potential of chloramphenicol with regard to cell membrane function was examined in a study investigating inhibition of protozoan motility. The effect of chloramphenicol on the locomotion of the protozoan Tetrahymena pyriformis, a model widely used for the evaluation of toxicity in excitable tissue, was tested. Chloramphenicol appeared to depress the motility of the test organism more effectively than did chloramphenicol succinate, the hydrophilic form of chloramphenicol. Results suggested that chloramphenicol, with its hydrophobic free form, has the ability to partition into the lipid bilayer of the cell membrane and thus the potential to cause membrane-mediated toxic effects. The authors postulated that such effects might explain the acute toxicity of chloramphenicol in excitable tissues, such as myocardium, and are a possible mechanism for chloramphenicol-induced cardiovascular collapse in neonates, or "grey baby syndrome" (Wu et al., 1996).
In contrast to the membrane-mediated toxic effects observed in Tetrahymena spp., chloramphenicol had no adverse effects on the morphologic characteristics and migration of canine corneal epithelial cells in vitro. A monolayer of cultured cells from the canine corneal epithelium was treated with a number of different antibiotics after a defect had been made on the monolayer. The toxicity of the antibiotics was determined by the morphologic characteristics and the migration of treated cells. Pure antibiotics were used at a concentration similar to that in tears, obtained with topical use of the commercially available antibiotic products in humans. Comparison between control cells and cells treated with antibiotics indicated that chloramphenicol had no cytopathologic effects on the monolayer and cellular morphologic characteristics, and migration of the treated cells was similar to that of control cells (Hendrix et al., 2001).
Two types of chloramphenicol-induced toxicity in humans have been widely discussed. The first is a frequently occurring, dose-related, bone-marrow depression that develops during treatment with chloramphenicol. The condition is seen as a mild anaemia, with decreased haemoglobin concentrations and reticulocytopenia, with the bone marrow showing reduced erythroid precursors, increased myeloid : erythroid cell ratio and vacuolation of erythroid cells. The patient returns to normal after drug withdrawal. Inhibition of protein synthesis in bone-marrow cells has been proposed as the mechanism of these effects (Kucers et al., 1997). The second is a severe, non-dose-related aplastic anaemia, which is irreversible. Aplastic anaemia is evident as severe pancytopenia in peripheral blood, with an acellular or hypocellular bone marrow. This might also result in leukaemia in humans (Dollery, 1999; Turton et al., 2002a).
Severe bone-marrow failure induced by chloramphenicol in man is relatively infrequent. Susceptibility to chloramphenicol-induced aplastic anaemia and leukaemia in man is considered to involve a genetic element. It has been suggested that chloramphenicol-induced aplastic anaemia and leukaemia are related to the DNA damage caused by nitrosochloramphenicol, which is a product of the reduction of the para-nitro group of chloramphenicol. The ability to reduce the para-nitro group to the nitroso derivative is genetically determined, and thus gives rise to an individual metabolic predisposition to such drug-induced conditions. The assumption is supported by investigations in vitro and in vivo demonstrating that the haematological response to chloramphenicol in mice is partly strain-dependent (Festing et al., 2001). However, the exact biochemical mechanism responsible for aplastic anaemia in man has not yet been elucidated.
A battery of toxicological studies was performed in an attempt to develop a rodent model for chloramphenicol-induced aplastic anaemia in humans. However, recent studies have confirmed several previous reports that no suitable or reliable laboratory animal model of aplastic anaemia exists, although administration of chloramphenicol succinate in rodent models induces haematological changes comparable to the chloramphenicol-induced reversible, dose-dependent bone-marrow depression seen in humans (Young & Maciejewski, 1997; Holt et al., 1998; Yallop et al., 1998; Turton et al., 1999).
Haematoxicity induced by chloramphenicol was investigated in a study in CD-1 weanling mice. Animals were given chloramphenicol at a dose of 1400 mg/kg bw by gavage daily for 10 days, and blood samples were taken at 1, 4 and 15 days after the last dose. Haematological data at day 1 after dosing showed a significant reduction in erythrocytes, erythrocyte volume fraction and haemoglobin values, which returned to normal by day 4 or 15. The investigator suggested that the reversible, dose-dependent anaemia seen in man could develop in CD-1 mice given chloramphenicol succinate (Turton et al., 1999).
In a study of the potential of chloramphenicol succinate and thiamphenicol to induce aplastic anaemia, female BALB/c mice were given chloramphenicol succinate at 2000 mg/kg bw per day or thiamphenicol at 850 mg/kg bw per day by gavage for 17 days. On days 1, 13, 22, 41, 98 and 179 after the final dose, blood and marrow samples were collected for haematological examination and assays for haematopoietic stem cells. Chloramphenicol succinate and thiamphenicol were found to have similar effects. Significant reductions in values for peripheral blood parameters (erythrocyte count, erythrocyte volume fraction and haemoglobin concentration) and bone marrow parameters (erythroid colony forming units and granulocyte-macrophage colony forming units) were found in samples at day 1 after dosing. At the later sampling times, values for all the observed parameters gradually returned to normal, and there was no evidence of marrow suppression by the end of the experiment. On the basis of this observation, the authors determined that chloramphenicol succinate and thiamphenicol induced reversible anaemia in BALB/c mice; however, aplastic anaemia does not appear in the BALB/c mouse (Turton et al., 2000).
The induction of haematoxicity by administration of chloramphenicol was attempted in another rodent species, after the induction in mice was unsuccessful. Guinea-pigs were examined for susceptibility to bone-marrow depression induced by chloramphenicol succinate. In a dose range-finding study, chloramphenicol succinate administered at a dose of 825 mg/kg bw for 16 days induced changes comparable to the reversible bone-marrow depression seen in humans, but there was no evidence of late-stage marrow depression, as would be seen in marrow aplasia. The authors concluded that rodents are not susceptible to myelotoxicity induced by chloramphenicol. The guinea-pig, like the mouse and rat, serves as a model for early events, but is not a good model for aplastic anaemia induced by chloramphenicol in man (Turton et al., 2002a).
Despite the well-recognized potential toxicity of chloramphenicol in humans, the drug is considered by most experts to be of low toxicity in adult companion animals when they are appropriately dosed. The development of aplastic anaemia as seen in humans does not appear to be a significant problem in animals. However, a dose-related reversible bone-marrow suppression is seen in all species, primarily after long-term therapy. Early signs of bone-marrow toxicity can include vacuolation of many of the early cells of the myeloid and erythroid series, lymphocytopenia, and neutropenia. Other adverse effects that may be noted in animals treated with chloramphenicol include anorexia, vomiting, diarrhoea and depression. Cats tend to be more sensitive to developing adverse reactions to chloramphenicol than are dogs; cats given chloramphenicol at a dose of 50 mg/kg bw every 12 h for 2-3 weeks do develop in high incidence of adverse effects (Plumb, 2002).
A bone marrow disorder was reported in a study of toxicity in a dog that was dosed orally with chloramphenicol at 300 mg/kg bw per day for 14 days. The results showed a decrease in the number of total erythroid cells, with proportional increase in myeloid cells, yielding a significantly increased myeloid to erythroid cell ratio (Baig et al., 1994).
Breeder turkey hens given drinking-water containing chloramphenicol at a concentration of 500 mg/l for 4 days showed a decrease in egg production. The toxic effects of chloramphenicol were mortality and cessation of egg production, which were more severe when a combination of chloramphenicol and monensin was given (Friedman et al., 1998).
Overall analysis of toxicity with chloramphenicol has suggested that the most serious toxic effect, aplastic anaemia reported in humans, is not seen in animals. However, reversible, dose-related bone-marrow suppression can be observed in all species given chloramphenicol in excessive doses or for prolonged periods. Other signs of toxicity caused by chloramphenicol are evident in animals in susceptible states, e.g. in neonatal animals or pregnant animals in which the hepatic biotransformation of chloramphenicol is impaired, or where the drug causes a decrease in protein synthesis in the fetus. However, owing to the potential toxicity of chloramphenicol in humans, and because of the possibility that metabolites of chloramphenicol might be found in the edible tissues of animals treated with chloramphenicol, the use of chloramphenicol in veterinary practice is not permitted in food-producing animals in many countries.
Toxicity caused by chloramphenicol in humans has been widely discussed because it induces bone-marrow depression. The more common dose-related bone-marrow depression is evident when the daily dose of chloramphenicol is >4 g in humans. Toxicity is reversible if the treatment is discontinued or the dosage is reduced. A more serious and unpredictable reaction is aplastic anaemia, which is not considered to be dose-related. Although the incidence of aplastic anaemia has been shown to correlate with several risk factors, it is estimated to occur with a frequency of 1 in 24 000-40 000 courses of treatment with chloramphenicol. Mortality from aplastic anaemia occurs in >50% of cases (Greenwood, 2000; Maluf et al., 2002).
Rappeport & Bunn (1994) suggested that aplastic anaemia in humans is an idiosyncratic reaction to chloramphenicol, which has an immunological basis and which is related to the nitrobenzene structure. This hypothesis is supported by clinical evidence showing that 40-50% patients with aplastic anaemia have a partial or complete response to a variety of immunosuppressive agents.
Young (2002) reviewed the pathophysiology of aplastic anaemia and reported that most cases can be characterized by a T-cell mediated destruction of bone-marrow haematopoietic cells.
This aberrant immune response may be a reaction to chemicals, drugs or viral infections, but endogenous antigens may also be involved. Many drugs can cause idiosyncratic haematopoietic failure; however, it is rare that patients, some of whom may have only ingested small quantities of the drug, show bone-marrow failure as a complication. Owing to the idiosyncratic nature of the response, it is difficult to study aplastic anaemia, and animal models do not exist (Young & Maciejewski, 1997). The therapeutic use of chloramphenicol has been followed by the development of aplastic anaemia in humans. This was particularly notable in the period following the introduction of chloramphenicol as a therapeutic agent in 1948, and before its association with aplastic anaemia had been recognized.
Other indications of toxicity associated with treatment with chloramphenicol in humans have been recognized. Circulatory collapse ("grey baby syndrome") has occurred in human neonates treated with chloramphenicol. This adverse reaction may be explained by the poor hepatic biotransformation of the drug in neonates as a result of slow glucuronidation of chloramphenicol. Toxic concentrations of chloramphenicol in blood and tissues develop secondary to an inability to conjugate the drug or to excrete the conjugate efficiently. However, the precise reason for the occurrence of cardiovascular collapse in grey baby syndrome is poorly understood. It has been stated that nitro-reduction derivatives of chloramphenicol might play a role in causing hypotension, and the hypothesis was assessed by perfusion of chloramphenicol through the isolated lobules of human placenta. A decrease in blood pressure was found at the time coinciding with a peak in concentration of nitric oxide, which is a product of the nitroreduction of chloramphenicol (Holt & Bajoria, 1999).
The potential for an adverse reaction induced by treatment with chloramphenicol is of critical importance in seriously ill or compromised patients. In patients with pre-existing haematologic abnormalities or hepatic failure, or in neonates, chloramphenicol is only used when no other effective antibiotics are available. Chloramphenicol has not been determined to be safe for use during pregnancy. The drug may decrease protein synthesis in the fetus, particularly in the bone marrow. Chloramphenicol is found in human milk at 50% of serum concentrations in humans and therefore the drug should be given with extreme caution to nursing mothers (Greenwood, 2000; Plumb, 2002).
The most serious adverse effect of treatment with chloramphenicol in humans is its association with acquired aplastic anaemia. Many population-based studies have been carried out to identify etiological factors associated with aplastic anaemia and to determine a link between the use of chloramphenicol and the development of marrow aplasia. Young & Alter (1994) reported that the published estimates of incidence of aplastic anaemia are significantly influenced by the methods used to acquire the data, and the diagnostic exclusion criteria. The incidence estimates reported in some former studies were too high owing to the inclusion of cases improperly classified as aplastic anaemia; the reported incidence of aplastic anaemia declines when rigorous diagnostic criteria have been applied. On the basis of an extensive review, the authors concluded that the incidence rate for aplastic anaemia is 2-6 cases per million population, with most cases of aplastic anaemia being classified as idiopathic (Young & Alter, 1994).
There are only a few recently documented cases of aplastic anaemia in patients that were sensitive to chloramphenicol. Possible etiologic factors associated with aplastic anaemia were identified in 151 Turkish patients who met the diagnostic criteria. The findings suggested that these cases of aplastic anaemia were most often idiopathic (99 out of 151 cases). The most common identifiable etiologic factor was the use of drugs (23 out of 151 cases), which were mainly non-steroidal anti-inflammatory agents, while chloramphenicol appeared to be specified in 1 out of 23 cases of drug use associated with aplastic anaemia. Exposure to benzene was the second most common causal agent in the studied cases (19 out of 151 cases) (Alnigenis et al., 2001).
In an investigation of potential risk factors associated with aplastic anaemia in the state of Parana, Brazil, the statistical evaluation of 125 cases of aplastic anaemia showed no positive association between use of chloramphenicol and development of aplastic anaemia. Instead, the causes of aplastic anaemia in Brazil were apparently identified as common factors related to the disease, such as exposure to certain chemicals. The incidence found in this study was similar to that reported in Thailand and Europe (Maluf et al., 2002).
Additional reports evaluating the correlations between the incidence of aplastic anaemia and use of chloramphenicol were documented in cases of aplastic anaemia in Nigeria and in Nepal. In a 5-year prospective study in Nigeria, it was estimated that aplastic anaemia developed in 0.002% of non-obstetric patients treated with chloramphenicol. Of 18 cases of aplastic anaemia diagnosed in Nepal, 16 were identified as being idiopathic and one was found to be associated with toxicity caused by treatment with chloramphenicol. Both studies concluded that chloramphenicol-induced aplastic anaemia is rare (Durosinmi & Ajayi, 1993; Sah et al., 1999).
It has been claimed that the topical ophthalmic use of chloramphenicol causes bone-marrow aplasia, but this issue has not been completely resolved. Recent observations have shown that the use of chloramphenicol as a topical eye medication is unlikely to introduce aplastic anaemia. Two extensive population-based studies in industrialized and developing countries presented no support for the claim that eyedrops containing chloramphenicol increase the risk of aplastic anaemia. The investigators found that there was no history of use of eyedrops containing chloramphenicol in more than 400 cases of aplastic anaemia examined. On the basis of this observation, the authors disagreed with the general recommendation stating that use of eyedrops containing chloramphenicol should be avoided because of an increased risk of aplastic anaemia (Wiholm etal., 1998).
Serum concentrations of chloramphenicol were monitored in a study in 40 patients treated with eyedrops containing chloramphenicol. HPLC with a minimum limit of detection (LOD) of 1 mg/l was used to measure the serum accumulation of chloramphenicol after topical therapy. After a course of treatment in which the mean dose of chloramphenicol received in 1 week of treatment was 8.0 mg, and in 2 weeks was 15.3mg, serum concentrations of chloramphenicol were below the limit of detection. The authors considered that the topical use of chloramphenicol was not a risk factor for induction of dose-related toxicity in bone marrow, and the suspension of use of topical chloramphenicol in ophthalmic practice was questioned (Walker et al., 1998).
Despite the failure of epidemiological studies to find an association between the topical use chloramphenicol and development of aplastic anaemia, the hypothesis of a metabolic predisposition in individuals predisposed to blood dyscrasias cannot be disregarded. Small doses of chloramphenicol similar to those used in topical therapy may cause this idiosyncratic reaction in certain individuals. In 1993, 23 cases of blood dyscrasias in patients treated topically with chloramphenicol for ophthalmic purposes were reported to the national register of drug-induced ocular side-effects in Oregon, USA (Fraunfelder et al., 1993).
In a critical review of the potential risk of developing aplastic anaemia attributable to topical use of chloramphenicol, the authors postulated that the risk posed by topical use of chloramphenicol may be similar to that of orally administered chloramphenicol. This is because topical administration achieves systemic effects by absorption through the conjunctival membrane or through drainage down the lacrimal duct followed by absorption from the gastrointestinal tract. In their view, it is not possible to justify subjecting patients to such potential risk, and therefore ocular chloramphenicol should be used only when there is no alternative (Doona & Walsh, 1995).
Contact sensitivity to chloramphenicol is rare in humans. Two cases of skin ulcer, secondary to contact dermatitis, were reported in a woman aged 48 years and a man aged 46 years. Both patients had applied chloramphenicol in the form of chloromycetin cream to their wounds for about 1 month and developed skin ulcer at the application sites. Hypersensitivity to chloramphenicol was confirmed by patch tests in both patients. The ulcer healed after use of the drug was discontinued (Matsumoto et al., 1998).
The teratogenic risk of chloramphenicol was studied in a population-based dataset of the Hungarian case-control surveillance of congenital abnormalities, 1980-1996. Retrospective investigation of the effects of oral treatment with chloramphenicol during pregnancy was implemented in 38 151 pregnant women who had healthy babies (control group) and 22 865 pregnant women who had congenitally abnormal newborns or fetuses. The case-control pair analysis of pregnant women who were treated in the second month or third month of pregnancy did not reveal any teratogenic potential of chloramphenicol in humans. The authors concluded that treatment with chloramphenicol during early pregnancy presents little, if any, teratogenic risk to the fetus in humans (Czeizel et al., 2000). However, the human embryo implants at day 6-7 of gestation and organogenesis begins at day 21; heightened susceptibility to malformations occurs during this period (Rogers & Kavlock, 2001). Therefore, this study may have overlooked the occurrence of early abnormalities.
Although the use of chloramphenicol in veterinary medicine has been restricted to non-food animals, residues have been found in samples taken from domestically produced animals in national monitoring programmes and foods, and in samples moving in international trade. For example, results of analyses carried out in Germany in the years 2000-2002 in accordance with directive 96/23/EEC showed that a small fraction (<0.2%) of all samples (n> 17 500) taken at farms and slaughter houses contained residues of chloramphenicol. The concentrations that were found in a total of 11 positive samples from fattening cattle, swine and poultry ranged from 0.3 to 3.3 µg/kg with eight values of <1 µg/kg (BVL, 2003). Unfortunately, comparable information was not available from many countries and, the limits of detection or quantification (LOD or LOQ) of the analytical methods used in some countries were—until recently—too high to allow quantification of traces of chloramphenicol.
The Health and Consumer Protection Directorate-General of the European Commission communicated ranges of concentrations of chloramphenicol in some food items. These had been reported by Member States during the years 2000-2003 (European Commission, 2004). The information included concentrations for the following commodities: skimmed milk powder (range, 0.021-1.23 µg/kg), milk products (range, 0.3-1.27 µg/kg), honey (range, 0.3-4.0 µg/kg; one sample at 38.7 µg/kg), washed pollen (0.58 µg/kg), shrimps (range, 0.1-7.7 µg/kg; two samples at 31.89 and 297 µg/kg), crabmeat (range, 0.3-1 µg/kg), crayfish (range, 0.14-6.3 µg/kg), casings (range, 0.5-2.9 µg/kg), rabbit meat (0.3 µg/kg), turkey breasts (0.82 µg/kg), and chicken breasts (range, 0.4-1.2 µg/kg).
The presence of residues of chloramphenicol has caused major food scares in the past 2 to 3 years. Shrimps, prawns, food products from other aquatic animals, honey, royal jelly, meat and offal, casings, rabbit, poultry meat and milk powder are among the commodities in which the drug was found. Many of the shipments of commodities in which residues of chloramphenicol were found originated in south-east Asia.
The Food Standards Agency of the United Kingdom, for example, has published a table with test results (Food Standards Agency, 2002/2003) obtained with samples of honey that were on sale in the United Kingdom. Using a method with a "reporting limit" (RL) of 0.3 µg/kg, the results ranged from "none detected above the RL set" to a maximum of 7.2 µg/kg. Several samples also contained streptomycin, for which the RL was 50 µg/kg. A group of 20 samples of honey that were analysed in the Netherlands (Voedsel en Waren Autoriteit, 2004) gave an average mass concentration of 1.9 µg/kg (range, 0.06-5.9 µg/kg). Positive findings of residues of chloramphenicol in honey from different geographic regions have also been reported in the scientific literature (Verzegnassi et al., 2003). Most of the contaminated honey originated from China. Reybroeck (2003) analysed samples of honey available on the Belgian market; samples were screened using an enzyme-linked immunosorbent assay (ELISA) (LOD, 0.1-0.3 µg/kg, depending on clean-up). Positive samples were subjected to high-performance liquid chromatography-mass spectrometry (HPLC-MS) confirmatory analysis (LOD, 0.1 µg/kg). Of the samples with known origin, only samples of Chinese origin contained residues of chloramphenicol (31 out of 40 samples). It has been hypothesized that contamination of honey with chloramphenicol could be related to treatments against foulbrood disease (Dharmananda, 2003).
While some of the positive findings are most likely to be the result of intentional uses of chloramphenicol rather than of environmental contamination, it has also been argued that very low concentrations of chloramphenicol detected in certain foods of animal origin, e.g. in poultry and in products from aquaculture, could perhaps be derived from environmental sources—either from chloramphenicol produced naturally by microorganisms or from residues resulting from past uses, which still persist in the environment.
Examples of low concentrations from information published by the Food Standards Agency of Ireland (Food Standards Agency of Ireland, 2002/2003) for the year 2002 are summarized in Table 1. The Food Standards Agency of Ireland has placed on the Internet information on alert/non-alert notifications of the Member States of the European Union concerning residues of chloramphenicol in shrimps, prawns, fish, fishery products and several other food commodities. A review of the data shows that at least nine of the Member States had communicated such notifications. In the majority of approximately 110 short summaries on notifications, no quantitative data on residue concentrations were communicated. However, an evaluation of a total of 47 quantitative results (33 for shrimp samples, 4 for prawn samples, 1 for fish and 9 for crabmeat, crayfish and surimi samples) showed the following distribution characteristics of the concentrations found: range, 0.1-34 µg/kg; median: 0.5 µg/kg.
Table 1. Range of concentrations of chloramphenicol found in samples of aquaculture products
|
Lower class limit |
Upper class limit |
Number of results |
|
0.1 |
0.5 |
25 |
|
>0.5 |
1.0 |
7 |
|
>1.0 |
1.5 |
2 |
|
>1.5 |
2.0 |
0 |
|
>2.0 |
2.5 |
2 |
|
>2.5 |
3.0 |
0 |
|
>3.0 |
3.5 |
3 |
|
>3.5 |
4.0 |
1 |
|
>4.0 |
4.5 |
1 |
|
>4.5 |
5.0 |
1 |
|
>5.0 |
34.0 |
5 |
From Food Standards Agency of Ireland (2002/2003).
Although these data are not representative and the samples were not all independent, it cannot be ruled out that there could exist two distributions of concentrations of residues:
The countries involved were not equally represented and the above numbers of samples are too low for any valid comparison between countries of origin. Another set of results was made available for evaluation by the Committee: the average value of a population of 50 samples of shrimp in which the presence of chloramphenicol was confirmed indicated an average value of 0.25 µg/kg, (range, 0.06-0.69 µg/kg), with two outlying values of 3.0 and 3.7 µg/kg (Voedsel en Waren Autoriteit, 2004).
The Centre of Analytical Services and Experimentation of Ho Chi Minh City conducted a validation study of GC and HPLC-MS methods for detection of chloramphenicol; a number of shrimp samples were analysed. During the first three quarters of 2002, a total of 44 samples were found to contain chloramphenicol (range of concentrations, 0.4-1.4 µg/kg) (Ngoc Son, 2002).
The Fourteenth Session of the FAO/WHO Codex Alimentarius Committee for Residues of Veterinary Drugs in Foods (CCRVDF) discussed the possibility that such traces of chloramphenicol found in food-producing animals could originate from environmental contaminations, rather than from intentional use. The report of the session reflects the discussion as follows (Joint FAO/WHO Food Standards Programme, 2003):
114. The request from Indonesia to consider the elaboration of an MRL for chloramphenicol in shrimp was addressed by the Joint Secretariat who discussed the possibility that this compound could find its way into animal tissues via other routes than its use as a veterinary drug. Limited data showed that chloramphenicol may persist in the environment or even be formed by soil microorganisms. Hypothetically, very low levels found in animal products could therefore not be related to the use of chloramphenicol as a veterinary drug. Several delegations stressed that it would be premature to draw any conclusions or to discuss a possible classification as a contaminant and that illegal use of the drug was a primary concern. It was noted that international trade had been disrupted severely during the past year by the rejection of products which had been contaminated at very low levels with chloramphenicol and some other veterinary drugs. The Committee noted the offer of the FAO Secretariat to JECFA to examine the potential persistence of chloramphenicol in the environment or its formation by soil microorganisms on the basis of data to be provided by Indonesia.
An attempt to investigate these possibilities has been made in the present monograph. After discussion of the development and current status of analytical methods, essentially two hypotheses for a possible environmental origin for very low concentrations of residues in foods were tested. In the first hypothetical scenario it is assumed that:
The second hypothetical scenario assumes that food-producing animals may currently still occasionally be exposed to persisting environmental residues of chloramphenicol resulting from historical veterinary uses.
This monograph also assesses the hypothetical dietary intakes resulting from low-level contamination of seafood with residues of chloramphenicol and compares these intakes with the lowest known human therapeutic exposures.
The ideal method for the analysis of residues of chloramphenicol should be sensitive, accurate and precise, and provide unambiguous information on the identity of the analyte. Furthermore, it should be as cost-effective and robust as possible. In practice, it is difficult to develop methods that combine all these characteristics. Therefore, the analytical strategies used for the control of residues of chloramphenicol in animal tissues frequently include the initial application of a screening method, followed by a confirmatory analysis of those samples that gave positive results with the screening method.
Typically, the three basic steps used in the majority of methods of analysis for chloramphenicol are:
This step usually includes homogenization and extraction of the tissue with suitable organic solvents, the separation of liquids and solids and the removal of lipids from the crude extract. Temperature control during storage and extraction of the sample is important in order to avoid metabolism in vitro, particularly in samples of liver and kidney, respectively, owing to the action of metabolizing enzymes (Parker & Shaw, 1988; Sanders et al., 1991). Analysis of liver and kidney requires enzymatic hydrolysis of conjugates of chloramphenicol; this step can apparently be omitted when working with trout tissues (Baradat et al., 1993; Mottier et al., 2003).
Use of the following organic solvents has been described for the extraction of chloramphenicol:
The lipids were removed through solvent partition, using:
—nHexane (Nagata & Saeki, 1992; Kijak, 1994; Epstein, 1994; Chevalier et al., 1995; Gude et al., 1995; Li et al., 2001; Perez et al., 2002; Pfenning et al., 2002a; Turnipseed et al., 2002; Storey et al., 2003; Gantverg et al., 2003; Pfenning et al., 2003);
—A mixture of nhexane and chloroform (1:1) (Sanders et al., 1991);
—nHeptane (Rupp et al., 2003; Stuart et al., 2003).
There are numerous procedures used to purify the primary extract in order to remove substances interfering with the detection and quantification step. Solid-phase extraction is the most widely used technique for purification in the analysis of residues of chloramphenicol in food matrices (Chevalier et al., 1995; Neuhaus et al., 2002; Turnipseed et al., 2002; Storey et al., 2003; Gantverg et al., 2003; Pfenning et al., 2003; Posyniak et al., 2003; Mottier et al., 2003; Impens et al., 2003).
There are, however, other purification techniques, for example, immunoaffinity chromatography (van de Water et al., 1989; Gude et al., 1995), which takes advantage of highly selective hapten-antibody interactions.
In past decades, several analytical methods have been developed and reviewed for the detection and quantification of chloramphenicol in foods and biological fluids.
Gas chromatography: the presence of polar functional groups in the chloramphenicol molecule requires a derivatization step, usually through a sylilation reaction, before gas chromatography analysis. The attachment of particular functional groups onto the chloramphenicol molecule may also lower the LOD of the electron capture detector (ECD) and MS. The silylation reaction is usually catalysed by acids or bases. Frequently used silylation reagents include:
ECD has been widely used for the analysis of chloramphenicol. The GC-ECD method for analysis of chloramphenicol in muscle of prawns, described by Munns et al. (1994) for example, reached a LOD of 1 µg/kg.
When GC is coupled with MS, the most frequently applied ionization techniques are chemical ionization (CI) and electron impact (EI).
Negative chemical ionization (NCI) presents a limited number of fragments; however, the molecular ion is always part of the spectrum. Owing to the presence of two chlorine atoms in the chloramphenicol molecule, GC-MS in NCI mode is one of the most reliable techniques, and it is the most commonly used method for the confirmation of residues of chloramphenicol. The LOD can be <0.1 mg/kg in muscular tissue (van Ginkel et al., 1990; Epstein, 1994; Borner et al., 1995). GC-MS in EI mode is less sensitive; however, it produces spectra of fragments, which are reproducible with different instruments, and can therefore be stored in electronic databases for reference purposes.
HPLC: The development of atmospheric pressure ionization (API) mass detectors, coupled HPLC, has become one of the most reliable and widespread techniques in the analysis of residues of chloramphenicol. This combination of liquid chromatography and mass spectrometry (LC-MS) enables the detection and quantification, without derivatization, of polar non-volatile analytes, such as chloramphenicol.
Neuhaus et al. (2002) have described an LC-MS/MS method with a LOD of 0.08 µg/kg and an LOQ of 0.3 µg/kg in prawns. Mottier et al. (2003) have developed a method for meat (chicken, turkey, pork and beef) and aquatic products (crab, prawn and fish), using liquid chromatography (electrospray ionization) tandem mass spectrometry (LC(ESI)-MS/MS), with isotopic dilution, reaching LOD and LOQ values of 0.003 µg/kg and 0.01 µg/kg, respectively.
Impens et al. (2003) have described an analytical strategy for the screening and confirmation of residues of chloramphenicol in prawn tissue, using ELISA for screening and GC-MS/MS and LC-MS/MS for confirmation; both selective techniques reached LODs of 0.1 µg/kg.
Gantverg et al. (2003) have developed a very sensitive method for the detection and confirmation of chloramphenicol in pork and beef muscle and in urine. After extraction, chloramphenicol was determined through LC-MS/MS in CI mode at negative atmospheric pressure, and by GC-MS in EI mode.
In summary, GC-MS and LC-MS/MS are, nowadays, the most reliable techniques for the determination of residues of chloramphenicol in edible animal tissues.
Selected examples of the development of analytical methods between 1990 and 2003 are listed in Table 2.
The European Union has recently defined minimum required performance limits (MPRLs) for analytical methods used for the determination of substances for which no permitted limit has been established, and in particular for those substances, like chloramphenicol, whose use is not authorized or specifically prohibited by Community legislation. For chloramphenicol, a MRPL of 0.3 µg/kg was established (European Commission, 2003).
Table 2. Overview of the development of analytical methods for residues of chloramphenicol in foods of animal origin
|
Analytical method |
Food matrix |
LOD |
LOQ |
Levels reported (µg/kg) |
Reference |
|
GC(NICI)-MS |
Muscle/egg |
0.1 |
>0.1 |
— |
van Ginkel et al. (1990) |
|
LC/UV |
Calf muscle |
1 |
— |
— |
Sanders et al. (1991) |
|
LC-MS |
Some aquatic species |
0.1 |
0.3 |
— |
Van de Riet et al. (1992) |
|
GC(NICI)-MS |
Bovine muscle |
0.6 |
— |
— |
Epstein (1994) |
|
GC(NICI)-MS |
Cows' milk |
0.5a |
— |
— |
Kijak (1994) |
|
GC-ECD |
Shrimp |
1 |
— |
— |
Munns (1994) |
|
GC(NICI)-HRMS |
Egg |
0.3 |
0.5 |
— |
Borner et al. (1995) |
|
GC-ECD |
Egg |
0.3 |
0.5 |
— |
|
|
HPLC (RP) |
Foie gras |
2.5 |
— |
— |
Chevalier et al. |
|
LC/UV |
Pasteurized milk |
50a |
— |
— |
Perez et al. (2002) |
|
LC-MS/MS ESI(-) |
Shrimp |
<0.5 |
— |
— |
Pfenning et al. (2002b) |
|
LC-MS/MS |
Shrimp |
0.08 |
0.3 |
— |
Neuhaus et al. (2002) |
|
GC-ECD |
Shrimp |
0.05 |
— |
0.65-0.72 |
Ngoc Son (2002) |
|
GC(NCI)-MS |
Shrimp |
0.3 |
— |
— |
|
|
LC-MS/MS APCI(-) |
Shrimp |
0.1 |
— |
— |
|
|
LC-MS/MS APCI |
Equine, porcine and bovine muscle |
0.02 |
— |
— |
Gantverg et al. (2003) |
|
GC-MS/MS |
Shrimp |
0.1 |
— |
— |
Impens et al. (2003) |
|
LC-MS/MS |
Shrimp |
0.1 |
— |
— |
|
|
LC(ESI)-MS/MS |
Chicken meat |
0.003 |
0.01 |
— |
Mottier et al. (2003) |
|
LC-MS/MS (ESI) |
Shrimp, crab |
0.1 |
— |
— |
Storey et al. (2003) |
|
GC-ECD |
Bovine muscle |
— |
0.25 |
— |
United States Department of Agriculture (2003) |
|
GC(NICI)-MS |
Bovine muscle |
— |
0.25 |
— |
APCI, atmospheric pressure chemical ionization; ECD, electron capture detection; ESI, ion electrospray; ESI(-), negative ion electrospray; GC, gas chromatography; HRMS, high-resolution mass spectrometry; LC, liquid chromatography; LOD, limit of detection; LOQ, limit of quantification; MS, mass spectrometry; NICI, negative ion chemical ionization; RP, reverse phase; UV, ultraviolet.
a (µg/l).
Chloramphenicol was first described as a new antibiotic produced by cultures of an actinomycete isolated from soil by Ehrlich et al. (1947). The soil samples from which the strains were isolated were collected from a mulched field near Caracas, Venezuela (strain ATCC 10712) and from a compost soil on the horticultural farm of the Illinois Agricultural Experiment Station at Urbana (strain ATCC 10595), respectively. It was demonstrated by Ehrlich et al. (1948) that this actinomycete was a new species. The dynamics of the synthesis of chloramphenicol were studied under laboratory conditions by Legator & Gottlieb (1953), who showed that the peak concentration of chloramphenicol in the culture medium was reached hours after the growth peak of the microorganisms. The antibiotic was not accumulated intracellularly. Addition of chloramphenicol to the culture medium inhibited the synthesis of the antibiotic.
Chloramphenicol was also isolated from the soil actinomycete Streptosporangium viridogriseum var. kofuense by Tamura et al. (1971) and from the marine snail Lunatia heros (moon snail) by Price et al. (1981).
The biosynthetic route of chloramphenicol starts with the general shikimate pathway for assembling aromatic structures. It then branches at chorismic acid to generate pamino-phenylalanine, which serves as an advanced precursor of the pnitrophenylserinol moiety of chloramphenicol (He et al., 2001; Lewis et al., 2003). 3’-O-Acetyl-chloramphenicol, which is commonly formed from chloramphenicol by many resistant bacteria, has also been isolated from the antibiotic-producing organism. It has been suggested that it is a protected intermediate in chloramphenicol biosynthesis, implicating acetylation as a self-resistance mechanism in the producing organism (Gross et al., 2002). 3’-O-Acetyl-chloramphenicol esterase activity was detected in cell-free extracts of strains of Streptomyces venezuela, other Streptomyces spp. and Streptosporangium viridogriseum var. kofuense, which produced chloramphenicol (Nakano et al., 1977).
Gottlieb & Siminoff (1952) studied the adsorption, stability, and rate of production of chloramphenicol in soil under different laboratory conditions. Chloramphenicol was poorly adsorbed to soil. When the drug was added to sterilized soil at a concentration of 50 mg/kg, approximately 80% could be recovered over the whole observation period of 14 days. When the same experiment was carried out using non-sterile soil, the antibiotic was slowly degraded. When sterilized soil was infested with S. venezuelae and was incubated for long periods, the authors were able to show the presence in soil of chloramphenicol formed by the microorganism following a lag phase of several weeks. The highest concentration measured was 1.12 mg/kg. Table 3 summarizes some of the results obtained in experiments designed to study the interaction of S. venezuelae and chloramphenicol-sensitive Bacillus subtilis. The table gives the results of the control experiments in which S. venezuelae was the only microorganism added.
Table 3. Production of chloramphenicol in sterilized soil infested with S. venezuelae
|
Time (days) |
Concentration of chloramphenicol |
Number of cells |
PH |
Concentration of chloramphenicol |
Number of cells (105/g) |
PH |
Concentration of chloramphenicol |
Number of cells |
PH |
|
Experiment 1 |
Experiment 2 |
Experiment 3 |
|||||||
|
0 |
— |
2.00 |
5.90 |
— |
0.39 |
5.90 |
0.0 |
38.7 |
5.45 |
|
7 |
0.00 |
23.30 |
5.57 |
0.00 |
35.50 |
5.05 |
0.0 |
12.0 |
5.05 |
|
20 |
0.00 |
28.50 |
5.10 |
1.12 |
48.20 |
5.60 |
0.0 |
37.0 |
5.63 |
|
36 |
0.58 |
18.20 |
5.45 |
— |
— |
— |
— |
— |
— |
|
65 |
— |
— |
— |
0.56 |
62.50 |
5.63 |
0.5 |
30.0 |
5.45 |
|
93 |
0.50 |
168.00 |
5.75 |
— |
— |
— |
— |
— |
— |
|
100 |
— |
— |
— |
0.82 |
945.00 |
5.85 |
0.79 |
1230.0 |
5.78 |
When organic substrates were added to the soil before sterilization, the production of chloramphenicol increased after the addition of S. venezuelae. Under the most favourable conditions of growth (in the presence of tryptone), chloramphenicol accumulated in the soil at a concentration of 25.0-27.8 mg/kg during 18-31 days of incubation. Addition of 1% of more "natural" substrates like alfalfa, corn stover and soybean straw also increased the production of chloramphenicol. However, only in the presence of alfalfa were significant quantities (concentration, 1.4mg/kg) observed.
These results should not be interpreted to mean that S. venezuelae produces chloramphenicol in soil in appreciable amounts under natural conditions. Natural soil is not usually a good substrate for production of antibiotic (Gottlieb, 1976). Ehrlich's group has investigated soils from 91 cultivated and grassland sites in nine states of the USA and from 13 other countries and found that soil samples were either infested with Streptomyces venezuelae or were not infested. No chloramphenicol was identified in extracts from either of these soils. Initially, the LOD of chloramphenicol was 0.3 mg/kg using a test based on the antimicrobial activity of chloramphenicol. In other experiments with LOD of 0.05 mg/kg in a chemical assay selective for the nitro group in the chloramphenicol molecule, these negative results were confirmed. If the soils were sterilized before seeding, chloramphenicol was found and identified in the infested soils (Ehrlich et al., 1952a, 1952b).
In their soil studies, Ehrlich et al. also investigated the recovery of smaller amounts of chloramphenicol added to non-sterilized soil. When chloramphenicol was incubated in sterilized soil at a concentration of 4.6 mg/kg for 92 days at 23-27 °C, recoveries were approximately 40% throughout the observation period. When the same incubation was performed with non-sterile samples of soil, the concentrations of chloramphenicol declined as a function of the incubation time as shown in Table 4. A graph of the same data (see Figure 1) suggests a biphasic curve for the degradation of chloramphenicol in soil. However, the second phase could be the result of the closeness to the LOQ of the observed concentrations. For the values ranging sufficiently above the LOQ (days 0-17 of the experiment), a half-life in the order of 3-4 days was estimated.
Whether antibiotics are produced in soil in appreciable amounts by indigenous soil organisms has remained a scientific dispute for several decades. Only recently, it has been demonstrated that an antibiotic can be synthesized in detectable amounts in soil. Using biosensor methods with very low LODs, Hansen et al. (2001) have demonstrated the presence of oxytetracycline produced by Streptomyces rimosus in untreated soil. However, similar studies with chloramphenicol were not found.
Table 4. Recovery of chloramphenicol from non-sterile soil after the addition of chloramphenicol at 4.6 mg/kg
|
Time (days) |
Recovery of chloramphenicol from soil (mg/kg) |
||
|
1/24 (1 h) |
1.900 |
1.700 |
2.200 |
|
1 |
1.300 |
1.300 |
1.200 |
|
2 |
0.850 |
1.000 |
0.890 |
|
3 |
0.770 |
0.740 |
0.820 |
|
4 |
0.550 |
0.240 |
0.470 |
|
5 |
0.600 |
0.690 |
0.600 |
|
7 |
0.250 |
0.290 |
0.440 |
|
11 |
0.063 |
0.069 |
0.270 |
|
17 |
0.130 |
0.130 |
0.150 |
|
29 |
0.041 |
0.093 |
0.041 |
|
45 |
<0.027 |
0.036 |
0.033 |
|
92 |
<0.027 |
<0.027 |
<0.027 |
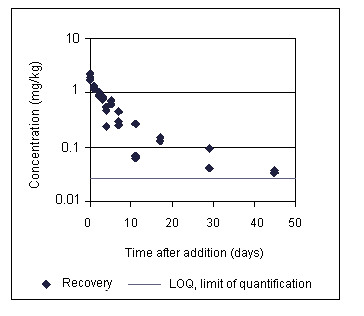
Figure 1. Stability of chloramphenicol in non-sterile soil
The Food and Drug Administration of the USA has published an environmental assessment of chloramphenicol in the context of a proposal made in March 1985 to withdraw approval of new animal drug applications (NADAs) using chloramphenicoloral solution (Food and Drug Administration, 1985). Further information was obtained from the Hazardous Substances Databank, a database of the National Library of Medicine's TOXNET system (Hazardous Substances Databank, 2003). The information available from these sources (most of the original literature cited was not available for this review) is summarized as follows. The solubility of chloramphenicol in water at 25°C is 2.5 g/l over a wide range of pH. Chloramphenicol is not adsorbed to clay or soil to any significant degree and therefore has very high mobility in soil. Adsorption to sediment and bioconcentration in aquatic organisms should not be important processes. Chloramphenicol is degraded by biological, chemical, and photolytic means and undergoes oxidation, reduction and condensation reactions upon exposure to light in aqueous solution. Photochemical decomposition of chloramphenicol in vitro by ultraviolet-A (UV-A) light leads to the formation of pnitrobenzaldehyde (pNB), pnitrobenzoic acid (pNBA) and pnitrosobenzoic acid (pNOBA); the latter comprises up to 45% by molarity of the starting amount of chloramphenicol (de Vries et al., 1994).
The half-life of chloramphenicol in soil at 25°C is 4.5 days; in pond water the half-life is 10.3 days at 25 °C and pH 8, and 20.8 days at 37°C and pH 6.
The log Kow for chloramphenicol is 1.14. With regard to sorption coefficients to soil solids (Kd,solid), the range of values for chloramphenicol is in the same group as, for example, olaquindox, sulfamethazine, sulfathiazole, and metronidazole, which also appear to have little sorption affinity to soil particles, as is evidenced by their low values of Kd,solid (0.2-2 l/kg) (Tolls, 2001; Rabolle & Spliid, 2000).
Lai et al. (1995) estimated Kd,solid values of 0.4 l/kg for a freshwater sediment (salinity, 0 g/kg ; pH 7.7; sulfate, 4.8 mmol/l) from an eel pond in Taiwan, China, and of 0.2 l/kg for a marine sediment (salinity, 33 g/kg; pH 8.2; sulfate, 25.6 mmol/l) from a shrimp farm in Taiwan, China. The authors used top sediment (0-5cm). The typical concentration of chloramphenicol was 60-70 mg/l throughout all experiments. However, possible effects of the concentration of chloramphenicol were also studied using concentrations of 100, 200, and 400 mg/l. The average rates of chloramphenicol transformation were much higher under anaerobic conditions than under aerobic conditions. Some selected results are summarized in Table 5. Chloramphenicol also degraded very slowly in sterilized slurries.
Table 5. Rate of degradation (mg/l per day) of chloramphenicol in slurries of aquaculture pond soils
|
Condition of incubation |
Sediment |
Concentration of chloramphenicol (mg/l) |
||
|
100 |
200 |
400 |
||
|
Aerobic |
Freshwater eel pond |
6.4 |
9.2 |
9.7 |
|
Marine shrimp pond |
1.6 |
1.8 |
1.6 |
|
|
Anaerobic |
Freshwater eel pond |
20.7 |
21.1 |
20.4 |
|
Marine shrimp pond |
20.3 |
21.3 |
15.6 |
|
Although the physicochemical mechanism of sorption interactions is not known, it is possible that chloramphenicol might form charge-transfer complexes with soil constituents (Haderlein & Schwarzenbach, 1993).
Lai et al. (1997) added sodium chloride to brackish water top sediment (0-5cm) slurries (10% slurry; salinity, 24 g/kg; pH 7.9) of a shrimp pond to obtain salinities of 30 and 36 g/kg and conducted sorption and transformation experiments at final concentrations of chloramphenicol of 60-70 mg/l and temperatures of 20-25 °C. The experiments were carried out under either anaerobic or aerobic conditions. While sorption to soil of chloramphenicol was not influenced by changes in salinities and by aerobic or anaerobic conditions, the compound was much more stable under aerobic conditions. Increasing salinities also slowed down the degradation process under aerobic conditions but not under anaerobic conditions. Selected results from this study are summarized in Table 6.
Table 6. Influence of salinity and oxygen on sorption and stability of chloramphenicol in slurries of pond soils (brackish water)
|
Salinity |
% Chlorphenicol adsorbed within 1 h |
-ka |
t1/2 |
-ka |
t1/2 |
|
|
Aerobic |
Anaerobic |
Aerobic |
Anaerobic |
|||
|
24 |
22 |
25 |
0.067 |
10.0 |
0.573 |
1.2 |
|
30 |
18 |
24 |
0.031 |
23.0 |
0.642 |
1.1 |
|
36 |
17 |
18 |
0.012 |
57.8 |
0.578 |
1.2 |
From Lai et al. (1997).
a k is the first order rate constant of the degradation of chloramphenicol. Its dimension is 1/day.
Chien et al. (1999) studied the degradation of chloramphenicol in aquaculture pond sediment. Freshwater (salinity, 0 g/kg) eel pond sediment slurries (10% w/v) were treated with sodium chloride to obtain salinities of 12, 24 and 36 g/kg. There were no significant differences in sorption rate either between aerobic and anaerobic conditions or among various salinities. Degradation of chloramphenicol fitted well to an exponential curve. The degradation rates under anaerobic conditions were significantly greater than those under aerobic conditions. As salinity increased, the degradation rates decreased under both aerobic and anaerobic conditions in this experiment. Selected results are summarized in Table 7.
Table 7. Influence of salinity and oxygen on sorption and stability of chloramphenicol in slurries of pond soils (eel pond)
|
Salinity |
% Chloramphenicol adsorbed within 1 h |
-ka |
t1/2 |
-ka |
t1/2 |
|
|
Aerobic |
Anaerobic |
Aerobic |
Anaerobic |
|||
|
0 |
28 |
23 |
0.297 |
2.4 |
1.915 |
0.4 |
|
12 |
25 |
21 |
0.155 |
4.5 |
0.957 |
0.7 |
|
24 |
27 |
25 |
0.080 |
8.9 |
0.495 |
1.4 |
|
36 |
28 |
23 |
0.039 |
18.4 |
0.286 |
2.4 |
From Chien et al. (1999).
a k is the first order rate constant of the degradation of chloramphenicol. Its dimension is 1/day.
These studies demonstrate that chloramphenicol can be quite stable under suitable aerobic and ionic conditions and at normal pH.
Hirsch et al. (1999) have estimated that 20.1 million daily doses of chloramphenicol were prescribed in 1995 in Germany for human medical use (indications and doses not given). They estimate that 5-10% of the doses were excreted unchanged and 70-90% were excreted as the glucuronide. When they analysed 10 samples of sewage treatment plant effluents with a LOQ of 0.02 µg/l, they found one sample containing 0.56 µg/l of chloramphenicol. Of 52 samples of surface water, four contained chloramphenicol at concentrations greater than the LOQ, with a maximum of 0.06 µg/l in one sample. As a general rule, concentrations of antibiotic residues in sewage treatment plant effluents were approximately one order of magnitude higher than the concentrations found in surface water. In 59 samples of ground water, no chloramphenicol residues at concentrations greater than the LOQ were found. Chloramphenicol residues were not found in samples taken during a study conducted in the USA (Kolpin et al., 2002). It was also not found in samples of groundwater, surface water and drinking-water analysed in a recent study conducted in the Netherlands (Stolker et al., 2003) and using sensitive analytical methods with a LOQ for chloramphenicol of 0.005 µg/l (Versteegh et al., 2003).
Hamscher et al. (2003) studied sedimentation dust collected between 1981 and 2000 in a pig finishing unit (350-420 animals). Each year, 10-15 samples were collected over periods of 14-30 days. One randomly selected sample was analysed for each year. Chloramphenicol was detected in three out of 20 samples (representing the years of sampling 1989, 1991, and 1992) at concentrations of 1.96, 0.07, and 5.49 mg/kg, respectively. The samples had been stored for more than 10 years before analysis.
The mere isolation of chloramphenicol-resistant microorganisms from the environment, including soil, can probably not be used as an argument for the presence of the drug. The phenomenon of resistance is too complex and its occurrence does not need to be related to any history of the use of chloramphenicol itself. For example, Kardavy et al. (2000) isolated chloramphenicol-resistant species of Providencia rettgeri from the gut of larvae of the oil fly inhabiting the 40 000-year-old asphalt seeps of Rancho La Brea in California. They found a correlation between antibiotic resistance and organic solvent tolerance, which could be explained by the presence of an active efflux pump maintained by the constant selective pressure of the solvent-rich environment. These efflux pumps expel a broad range of comparatively hydrophobic antibiotics (chloramphenicol, erythromycin, nitrofurantoin, novobiocin, rifampin, spectinomycin, and vancomycin), most of which contain aromatic ring systems. Providencia spp. are also known as agents of nosocomial infections. Efflux pumps play also an important role in resistant strains of other bacterial species (Malléa et al., 2003).
Resistance to chloramphenicol in Salmonella enterica serovar typhimurium isolated from cattle in the USA has drastically increased over the years (Davis et al., 1999) owing to sharply increased occurrence of isolates displaying DT104-linked resistance. Although the use of chloramphenicol is prohibited in the USA, it was never authorized for use in food-producing animals and monitoring results do not suggest widespread illegal use. A gene conferring cross-resistance to florfenicol and chloramphenicol has been isolated from Salmonella enterica serovar typhimurium (S. typhimurium) DT104 (Bolton et al., 1999). A conjugative plasmid, pOLA52, conferring resistance to the antibiotic growth promoter olaquindox has been isolated from Escherichia coli from swine manure. It also confers resistance to ampicillin and chloramphenicol and has a high frequency of transfer between strains of E. coli (Sørensen et al., 2003).
Petersen et al. (2002) have studied the development of antibiotic resistance in integrated fish farms in Asia. The farms used antimicrobial agents and animal manure was shed directly into fish ponds as fertilizer in these farms. Three of the farms were using chloramphenicol in ducks and pigs. The impact of the use of antibiotics on the development of antimicrobial resistance among the indicator microorganisms was greatest at the beginning of a fish production cycle. However, the most significant increase in resistance to chloramphenicol occurred on a farm where this antibiotic was not used (amoxicillin, enrofloxacin, norfloxacin, tylosin were used on this farm).
For such reasons, the many reports dealing with resistance phenomena discovered in the environment have not been used here as an argument for the presence of chloramphenicol in the environment.
Table 8 provides an example and short summary of the relationship between age, live weight, feed intake and daily live-weight gain in pigs raised in the western hemisphere (Peer et al., 2001).
Table 8. Typical feed intakes and live-weight gain in pigs raised in the western hemisphere
|
Production class |
Live weight (kg) |
Average daily gain (kg) |
Dry matter intake |
|
|
(kg/animal per day) |
(kg of dry matter/kg average daily gain) |
|||
|
Starter |
10-20 |
0.450 |
0.80-0.84 |
1.78-1.87 |
|
Grower |
20-50 |
0.700 |
1.61-1.91 |
2.04-2.73 |
|
Finisher |
50-100 |
0.820 |
2.63-3.29 |
2.94-4.01 |
From Peer et al. (2001).
A review of recent research papers indicates that the situation in south-east Asia could differ significantly from that observed in, for example, certain agricultural areas of the USA. Figure 2 summarizes the results of this review. More details are given in the Appendix I at the end of this document. From these details it can be seen that the typical daily dry matter intake of the animals is in the same order in the different hemispheres. However, the growth rates of the pigs used in the studies in south-east Asia were comparatively lower.
The data for the pigs in Iowa in Figure 2 were retrieved from the Internet (The pig site, 2003). The data for pigs in south-east Asia were taken from the studies summarized in the tables given in Appendix I at the end of this monograph. These data were collected from published results of research projects carried out in southeast Asia. They may give an incomplete picture and real conditions may vary largely from country to country, and between regions and provinces of a given country, and may also be subject to rapid changes. However, a complete review of animal feeding practices is outside the scope of this monograph. The information provided in the Appendix I is limited to the minimum necessary to derive suitable relationships between live weight, live-weight gain and feed intake since this information is needed to estimate soil ingestion as function of growth and intake of dry matter of the animals, and to relate hypothetical environmental doses of chloramphenicol to estimated soil ingestion.
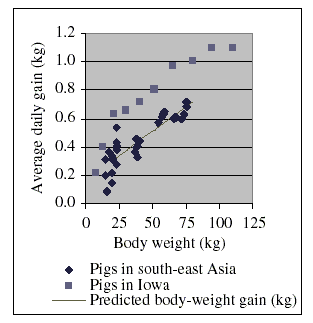
Figure 2. Comparison of growth rates of pigs raised in different regions of the world
Body weight is the mean of the initial and the final body weight
of a feeding period. Average daily gain is the average over the whole feeding period.
As a result of the data discussed in this section, one can assume for simple model calculations that pigs generally eat approximately 35 g of dry matter per kg bw per day. Using the data in Figure 2, the average daily live-weight gain is estimated as a function of body weight (within the limits of 20 g and 75 kg body weight) according to the formula:
Average daily live-weight gain (g) = 0.164 g + 0.00693 (g/kg) × Body weight (kg)
It is not within the scope of this monograph to describe the range of different production systems. An example of data that have been gathered in the western hemisphere can be found in tabular form in a publication of the National Academy of Sciences of the USA (National Academy of Sciences, 1994). However, under the hypothesis that soil ingestion could be the cause of chloramphenicol residues in tissues and edible products, extensive systems—as they still exist, for example, in south-east Asia, with free-ranging scavenging chickens receiving supplementary feeds—are of particular interest. Local breeds may play an important role, at least regionally (Nguyen Dang Vang & Le Viet Ly, 2000). Some of these breeds have small bodies and growth rates and feed conversion may vary largely (Tran Thi Mai Phuong et al., 2003). A limited amount of data from south-east Asia were available from published results of research projects. These results, which are not necessarily representative, are summarized in more detail in Appendix I. Selected data are presented in Figure 3. The study of Duong Duy Dong (2003) provided useful data for model calculations.
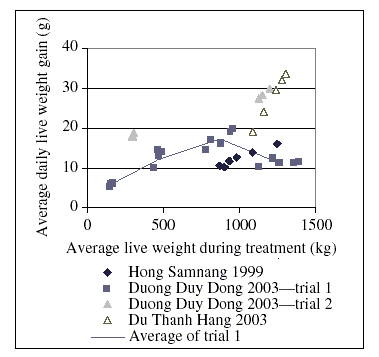
Figure 3. Growth rate of chickens under different feeding conditions
As a result of the evaluation of the data discussed in this section, the daily live-weight gain of chickens with a body weight of between 150 and 1000 g was calculated according to the formula:
Average daily live-weight gain (g) = 3.599 g + 0.01623 (g/kg) × Body weight (kg)
The intake of dry matter is approximately 3 g per g of average daily live-weight gain.
Chloramphenicol is not highly systemically bioavailable after per-oral administration to ruminating cattle. The drug is largely degraded by microflora in the rumen. Corresponding calculations have not, therefore, been performed for cattle.
Soil ingestion varies seasonally and according to farm management. Using the titanium content of faeces as a stable indicator of soil ingestion, Thornton & Abrahams (1983) found that grazing cattle involuntarily ingest from 1% to nearly 18% of their dry matter intake as soil; sheep may ingest up to 30%. Abrahams et al. (2003) studied rates of soil ingestion by sheep grazing on metal-enriched flood-plain soils and found very high rates of soil intake during the winter/spring season with maximum rates during March, when soil ingestion exceeded 30% of the dry matter intake at two of the 11 sites investigated. No detailed quantitative data on soil ingestion of chickens and pigs were available for evaluation, although the phenomenon of soil ingestion in (food) animals is well recognized and frequently described in the literature.
Fries et al. (1982) investigated soil ingestion by dairy cattle using quantitative analysis of titanium in faecal samples and in soils to which the animals had access. Selected results are summarized in Table 9.
Table 9. Soil ingestion by dairy cattle
|
Description of the group of animals |
Range of mean soil ingestion (% of dry matter intake) |
|||
|
Lower bound |
Upper bound |
|||
|
Mean |
Standard error |
Mean |
Standard error |
|
|
Lactating cows |
||||
|
Confined to concrete |
0.14 |
0.02 |
0.53 |
0.05 |
|
Housed in freestall barns with soil bedding |
0.35 |
0.06 |
0.64 |
0.18 |
|
Access to unpaved lots with no vegetation |
0.60 |
0.07 |
0.96 |
0.22 |
|
Yearling heifers and dry cows |
||||
|
Confined to concrete |
0.52 |
0.11 |
0.81 |
0.19 |
|
Access to unpaved lots with no vegetation |
0.25 |
0.04 |
2.41 |
0.26 |
|
Access to unpaved lots with sparse vegetation |
1.56 |
0.21 |
3.77 |
1.50 |
|
On pasture but receiving supplemental feed |
1.38 |
0.33 |
2.43 |
0.50 |
From Fries et al. (1982).
Using the information collected in section 6, hypothetical intakes of chloramphenicol were calculated as a function of intake of dry matter and corresponding soil intake of the animals. It was assumed that soil represented 2% of dry matter intake. The concentration of chloramphenicol in soil was set at one of the following concentrations:
The following steps were performed in the calculations1:
The hypothetical cumulative intake of chloramphenicol by a single animal whose live weight increases from 20 kg to 75 kg during 119 days (the average time required to achieve this body-weight gain) varies from <183 µg to 91.3 mg under these conditions. The results are summarized in Table 10. The cumulative intake overestimates the amount of chloramphenicol in the body of the animal. This value is much smaller since—even if the drug were 100% bioavailable—every daily dose is partly eliminated before the next dose is ingested.
Table 10. Hypothetical cumulative intake through ingestion of soil containing chloramphenicol, in pigs
|
Average daily gain |
Dry matter intake |
Soil intake |
Day |
Body weight |
Concentration of chloramphenicol in soil (µg/g)( |
Cumulative intake of chloramphenicol (µg) |
|
0.164 + 0.00693 × |
35 |
2% of dry matter intake |
1 |
20 |
<0.05 |
<0.7 |
|
119 |
75 |
<0.05 |
<183 |
In order to correctly estimate the amount of chloramphenicol in the body, bioavailability and elimination rate must be known. Unfortunately, the pharmacokinetic behaviour of low doses of chloramphenicol in pigs is not known. The elimination half-life for high doses is not well understood because quantitative determinations of chloramphenicol in plasma have usually not been extended to sufficiently long time periods after the administration of the dose. Boertz (1984) and Boertz et al. (1985) have carried out a residue study using 24 pigs each with a body weight of approximately 100 kg. The animals were given a single subcutaneous injection of chloramphenicol of 30 mg/kg bw. Two animals were slaughtered at each of 12 time-points between 4 h and 30 days after dosing. The kinetics in plasma and in all tissues examined suggest that there are at least two elimination phases, the first one characterized by a half-life of 6-10 h and a second one with a half-life of up to 100 h. There was excellent agreement between the results obtained with radioimmunoassay and with GC-ECD. However, the number of data points covering the terminal phase was too small to estimate the parameters of this phase with sufficient accuracy. A study using the same dose was carried out later in the same laboratory with the aim of producing reference material (Balizs & Arnold, 1989). Blood samples from five pigs were taken to predict the appropriate time of slaughter in order to obtain a muscle sample with a concentration of chloramphenicol of approximately 10 µg/kg. The seven time-points used covered the period between 1 h and 107 h. The following half lives were found under these conditions in the five animals: 10.6, 7.4, 6.5, 10.9 and 14.2 h, respectively. The data obtained in these two studies are summarized in Figure 4.
Figure 4. Plasma kinetics of parent chloramphenicol in pigs given a single subcutaneous dose at 30 mg/kg bw
The following assumptions were made and corresponding calculations were performed in order to obtain a crude estimate of the hypothetical amounts in the bodies of the animals corresponding to the cumulative intake shown in Table 10:
The results are summarized in Table 11.
Table 11. Estimated amounts of chloramphenicol in the body resulting from soil ingestion, in pigsa
|
Concentration of chloramphenicol in soil (µg/g) |
<0.05 |
1 |
25 |
|
|
Cumulative intake of chloramphenicol (µg/animal) |
<183 |
3651 |
91 267 |
|
|
Daily dose (µg/kg bw) |
0.035 |
0.7 |
17.5 |
|
|
Half-life I (h) |
Half-life II (h) |
Estimated amount of chloramphenicol in the bodies of the pigs after 119 days (µg) |
||
|
2 |
— |
<0.0006-<2.62 |
0.013-52.5 |
0.32-1312 |
|
4 |
— |
<0.042-<2.66 |
0.84-53.3 |
21-1333 |
|
6 |
— |
<0.17-<2.80 |
3.5-55.9 |
87-1399 |
|
8 |
— |
<0.37-<2.99 |
7.4-59.9 |
186-1498 |
|
10 |
— |
<0.61-<3.23 |
12.1-64.6 |
303-1615 |
|
10 |
100 |
<1.26-<3.88 |
25.2-77.7 |
630-1942 |
|
100 |
— |
<12.8-<16.3 |
274-326 |
6838-8150 |
|
a |
Results are given as the range between the minimum amounts (calculated for the time-point immediately before the last dose) and the maximum amounts (calculated for the time-point immediately after the last dose). |
The apparent volume of distribution for chloramphenicol in pigs is given as 1.4 l/kg (Kroker, 1994). The studies of Boertz et al. (1984) have shown that concentrations of chloramphenicol in muscle were directly proportional to the concentrations found in plasma. Assuming 100% bioavailability of the ingested chloramphenicol, a crude estimate of maximum residue concentrations expected in pig muscle would result in the values presented in Table 12.
Table 12. Estimated muscle concentrations of chloramphenicol derived from soil ingestion, in pigsa
|
Concentration of chloramphenicol in soil (µg/g) |
<0.05 |
1 |
25 |
|
|
Cumulative intake of chloramphenicol (µg/animal) |
<183 |
3651 |
91 267 |
|
|
Half-life I (h) |
Half-life II (h) |
Estimated concentration of chloramphenicol in muscle (µg/kg) |
||
|
2 |
— |
<0.000-<0.025 |
0.000-0.5 |
0.003-12.5 |
|
4 |
— |
<0.000-<0.025 |
0.008-0.51 |
0.2-12.7 |
|
6 |
— |
<0.002-<0.027 |
0.033-0.53 |
0.83-13.3 |
|
8 |
— |
<0.004-<0.029 |
0.070-0.57 |
1.77-14.3 |
|
10 |
— |
<0.006-<0.031 |
0.115-0.62 |
2.89-15.4 |
|
10 |
100 |
<0.012-<0.037 |
0.24-0.74 |
6-18.5 |
|
100 |
— |
<0.12-<0.16 |
2.6-3.1 |
60.8-77.6 |
From Boertz et al. (1984).
* Results are given as the range between the minimum amounts (calculated for the time-point immediately before the last dose) and the maximum amounts (calculated for the time-point immediately after the last dose).
For a given dose and all other conditions remaining constant the values of the minima and maxima and the differences between the extreme values depend only on the half-lives of elimination. This is illustrated in Figure 5 for constant multiple doses of 17.5 µg/kg bw per day and hypothetical elimination half-lives of 4, 10 and 100 h, respectively.
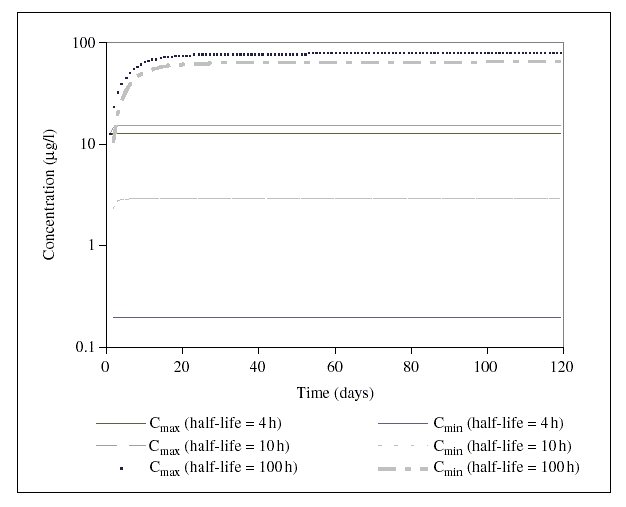
Figure 5. Effect of elimination half-life on steady-state concentrations
The uncertainties of the calculations are:
The calculations performed for chickens are similar to those described in section 6.1 for pigs. Therefore the description of the individual steps of the calculations already explained is not repeated here. The formulae describing live-weight gain and dry matter intake were developed in section 4.2. The basic assumptions and the results are summarized in Table 12. Only the maximum amounts and concentrations calculated for the time-point immediately after the last dose are given in Table 13.
Table 13. Hypothetical muscle concentrations of chloramphenicol derived from ingestion of soil, in chickens
|
Average daily gain (g) |
Dry matter intake |
Soil intake |
Concentration of chloramphenicol in soil (µg/g) |
Body weight (kg) |
Cumulative intake of chloramphenicol (µg) |
Amount in body (µg) |
Concentration in muscle (µg/kg) |
||||
|
Day 1 |
Day 75 |
Day 1 |
Day 75 |
Day 1 |
Day 75 |
Day 1 |
Day 75 |
||||
|
3.599 + 0.01623 × bw |
3 × average daily gain |
2% of dry matter intake |
<0.05 |
150 |
1002 |
<0.018 |
<2.6 |
— |
<0.02 |
— |
<0.02 |
|
0.02 |
|||||||||||
|
1 |
150 |
1002 |
0.362 |
52 |
— |
0.45 |
— |
0.30 |
|||
|
25 |
150 |
1002 |
9.050 |
1308 |
— |
11.30 |
— |
8.10 |
|||
Using a half-life of 7 h and a bioavailability of 35%.
A half-life of 7 h and a bioavailability of 35% were used for the above calculations on the basis of data published by Anadon et al. (1994). These authors have studied bioavailability, pharmacokinetics and residues of chloramphenicol in chickens. The pharmacokinetic properties (on the basis of a two-compartment open model) of chloramphenicol were determined in broiler chickens after intravenous and after oral administration. After oral administration at a dose of 30 or 50 mg/kg bw, chloramphenicol was absorbed rapidly (time to maximal concentration, 0.72 or 0.60 h, respectively) and eliminated with a mean half-life (t1/2 beta) of 6.87 or 7.41 h, respectively. The bioavailability was 29% at a concentration of chloramphenicol of 30 mg/kg bw and 38% at 50 mg/kg bw.
The uncertainties of the calculations are similar to those described for the model in pigs:
This section discusses the hypothetical possibility that residues resulting from past treatments of (humans and) animals could persist in the environment. Chloramphenicol was widely used in food producing animals in nearly all regions of the world until, within about the past 10 years, many countries and regions, including the European Union, imposed a complete ban on all uses in such animals in order to protect the health of consumers. A wide range of formulations, dosages and routes of administration, which cannot be reviewed here, were in use in terrestrial animals. Table 14 provides some additional examples of historical uses in aquaculture in Asia (Arthur et al., 2000). In Asia, chloramphenicol was widely used as a veterinarian antibiotic in aquaculture, according to a contemporary view (Somjetlertcharoen, 2002).
Table 14. Some historical uses of chloramphenicol in aquaculture in Asia
|
Country |
Type of culture or product |
Examples of uses for disease control |
Reference |
|
Bangladesh |
Coastal shrimp culture |
Occasional use, in a small percentage of—mainly semi-intensive—farms; no further details given |
Phillips (2000) |
|
China |
Valuable broodstock, larval shrimp |
Bath treatment, 1-1.8 mg/kg |
Jiang (2000) |
|
Other species of high value, such as eel and soft-shelled turtle |
Injection at 20 mg/kg bw; oral treatment at 25-100 mg/kg bw |
||
|
India |
Extensive and semi-intensive carp farms and semi-intensive shrimp farms |
No details given |
Pathak et al. (2000) |
|
Indonesia |
Shrimp culture |
Immersion therapy at 5 mg/kg |
Supriyadi & Rukyani (2000) |
|
Malaysia and Singapore |
Aquaculture; no further details given |
"It is usually administered through feed at 3-5g/kg." |
Shariff et al. (2000) |
|
Philippines |
Penaeus monodon hatcheries |
Every other day from Z1 to harvest, long bath, at 1 mg/kg; or 3 days, long bath, at 2-4 mg/kg |
Cruz-Lacierda et al. (2000) |
|
Penaeus monodon grow-out ponds |
Medicated feed, 2-2.5g/kg of feed; five times daily for 3 days |
||
|
Spotted scat (Scatophagus argus) |
Bath for 10 h, 50 mg/kg or oral administration, 500-750 mg/kg of feed given at 3-10% body weight for 5-7 days, or by injection at 15 mg/kg of fish |
||
|
Sri Lanka |
Larvae and post-larvae in shrimp hatcheries |
10-15 mg/kg; no further details given |
Wijegoonawardena & Siriwardena (2000) |
|
Eel |
Daily for 21 days by oral administration at 100 mg/kg bw |
Liao et al. (2000) |
|
|
Taiwan, China |
Shrimp |
For bath, at 1-10 mg/kg |
From Arthur et al. (2000).
Environmental residues of chloramphenicol in farm animals are most likely to originate from excreta of treated animals and a significant risk of secondary contamination could be assumed for farming systems in which manure is used as fertilizer.
Integrated farming of fish or shrimp and livestock, for example, combines aquaculture with production of pigs, ducks, chicken, and/or other livestock animals. Farming of such animals produces manure that may be used as soil fertilizer; however, it is also possible to make use of the nutrients contained in this manure in fish or shrimp ponds. These nutrients are taken up by bacteria and microalgae, which themselves are food for filtrating organisms, mostly zooplankton. Some of these organisms are then consumed by fish or shrimp.
According to the literature (Vincke, 1991), the number of pigs required per 10 000 m2 of pond area varies from 40 to 300. Typically, about 100 weaned piglets (aged 2 months; average body weight, 12-15 kg) may be used per 10 000 m2 (ponds are frequently much smaller than 10 000 m2). The pigs reach a body weight of 70-85 kg after 6-7 months. The pigsty may be constructed near the fish pond in traditional systems. Manure is then washed down to the pond.
If production of duck and fish is combined, ponds provide living and foraging space for both. A variety of strains of duck are used, each having a different fattening period. About 750-4000 ducklings may be stocked per 10 000 m2, these animals reaching slaughter weight within 7-9 weeks. Both ducks and chickens (broilers or layers, 1000-6000 animals per 10 000 m2) are traditionally reared in pens beside or over the ponds.
If, in such systems, the terrestrial animals were to be treated with chloramphenicol, drug residues contained in their excreta would constantly be added to and diluted by the pond water, and would disappear according to the rate of degradation discussed in previous sections (half-life, 10-20 days in pond water; see section 5.4), and also according to the extent to which the water of the pond is exchanged.
However, it is also possible that fresh manure may be either directly applied to soil or that it remains in the housing or in a specific storage area before further use. In cases where manure is stored or processed, potential residues of chloramphenicol would be subject to degradation processes; however, published information about the kinetics of degradation of chloramphenicol under various conditions of storage and processing of animal excreta is extremely limited (see section 8.5). De Liguoro et al. (2003) demonstrated that processes that occur between the production of faeces and the application of manure to the soil are very effective in reducing the load of tylosin and oxytetracycline in the environment. Tylosin degraded rapidly in manure of treated Simmental calves, and was no longer detected 45 days after the last treatment. However, the half-life of oxytetracycline in manure was 30 days, and after 5 months maturation, oxytetracycline at a concentration of 820 µg/kg of manure was still detected.
In outside storage areas in particular, chloramphenicol would also be washed out and diluted by rainfall and possibly end up in basins retaining urine and manure fluids, where the conditions of stability of this compound are unknown and might be different from those prevailing in solid manure.
There are many ways of processing manure before its use as fertilizer. In some regions, farmyard manure is the traditional manure. It is a decomposed mixture of cattle dung and urine, together with straw and litter used as bedding material and feed not consumed by the animals. The waste material is collected regularly and placed in trenches finally plastered over with cow dung or earth slurry. It becomes ready for application as fertilizer after 3-4 months (Indiaagronet, 2003).
Frequently, the processing of livestock manure by anaerobic digestion in "bio-digesters" is a key component in integrated aquaculture systems. The main products from the biodigester are biogas and effluent, which is a potential fertilizer because the anaerobic digestion process results in conversion of organic nitrogen of the manure to ionized ammonia (NH4+) (San Thy & Preston, 2003). Effluent may be superior to raw manure in supporting a higher yield of biomass in agricultural crops (Le Ha Chau, 1998a, 1998b). Fish may also grow faster when ponds are fertilized with biodigester effluent instead of unprocessed manure (Pich Sophin & Preston, 2001). Chloramphenicol is not likely to be stable under the described conditions of processing of manure.
The literature offers a wide range of numbers characterizing the quantity of manure produced by different species of animal under different conditions of husbandry, according to the way of processing the manure (e.g. capacity, initial loading, daily volumes added, water addition and retention times in biodigesters), and the amounts added to soil and/or ponds either in terms of dry matter or nitrogen per 10 000 m2. The amount of manure or biodigester solids or effluents added also largely depends on the aquatic species and its density in the pond. It may be added directly or suspended in special manure bags. Fertilization is a basic part of pond preparation since plankton has to be available in adequate quantities before stocking with fish or shrimp.
The values given for production of excreta by terrestrial farm animals in the western hemisphere in Table 15 have been transformed into metric units from the non-metric units used in the original literature (Barker et al., 2003). Other sources give different values that are up to 25% higher or lower. Unfortunately, the term "manure" is rarely defined.
Table 15. Production of faeces and urine by farm animals
|
Species or production class |
Live weight (kg) |
Faeces and urine productiona |
Total solids (tonnes/year per tonne of liveweight) |
||
|
(kg/head per day) |
(tonnes/ head per year) |
(tonnes/year per tonne of liveweight |
|||
|
Dairy cows |
635.00 |
55.50 |
20.20 |
31.9 |
4.43 |
|
Beef cattle |
362.90 |
22.00 |
7.53 |
20.8 |
3.05 |
|
Pigs |
61.20 |
5.03 |
1.72 |
28.1 |
2.90 |
|
Laying hens |
1.81 |
0.12 |
0.04 |
23.5 |
5.85 |
|
Broiler chicken |
0.91 |
0.07 |
0.02 |
24.0 |
6.14 |
|
Duck |
1.36 |
0.15 |
0.05 |
33.3 |
9.00 |
From Barker et al. (2003).
a As voided.
Manure output by domestic animals is affected by many factors, which could differ widely from region to region in the world. In the tropics, a common method of comparing farm livestock is the tropical livestock unit (TLU) (LEAD Virtual Center, 2004). The TLU is based on the cow with a body weight of 250 kg and other animals are compared with it using (weight)3/4 for scaling. The TLUs for selected tropical livestock are given in Table 16.
Table 16. TLU equivalents of some farm animals
|
Animal |
TLU |
|
Cow |
1.00 |
|
Bull |
1.20 |
|
Heifer |
0.70 |
|
Calf |
0.50 |
|
Asian Buffalo |
1.20 |
|
Goat |
0.15 |
|
Sheep |
0.15 |
|
Sow |
0.20 |
|
100 Chickens |
0.60 |
TLU, tropical livestock unit.
The annual production of manure by one TLU (250 kg live weight) in an extensive system is roughly estimated to be about 1000 kg of dry matter. This estimate, which is not greatly different from the values given in Table 15, is based on the assumptions of a daily feed intake varying from 2 to 2.5% of body weight and a digestibility of feed varying from 40-60% (LEAD Virtual Center, 2004b).
In order to avoid over-sophisticated calculations performed using a purely hypothetical background, the following simplifying assumptions may be made. When pigs or cattle are treated with chloramphenicol they receive a dose of 50 mg/kg bw (per-oral, intramuscular or subcutaneous administration in pigs, and intramuscular or subcutaneous administration in cattle, except non-ruminating calves in which the per-oral route is useful) daily over 7 days. Chicken may be treated orally with drinking-water containing chloramphenicol (250 mg/l) over 7 days. Water intake (Guyer, 1996) by chickens is approximately 0.3 l/day per animal.
The resulting total doses would be approximately 87.5 g for a cow with a body weight of 250 kg, 26.25 g for a pig with a body weight of 75 kg, and 0.525 g per chicken. Assuming that a maximum of 50% of the dose is excreted as parent drug or hydrolysable conjugate of the parent drug in the combined mixed excreta within the treatment period plus an additional 3 days, these amounts would be contained in approximately 152 kg of beef cattle excreta, 62 kg of pig excreta and 0.7-1.5 kg of chicken excreta.
The expected concentrations of chloramphenicol equivalents, therefore, would range in the order of 0.29 g equivalents (chloramphenicol plus conjugates) per kg of bovine excreta, 0.21 g per kg of pig excreta and 0.18-0.38 g per kg of chicken excreta. Therefore one could expect to find chloramphenicol equivalents at a concentration of roughly 0.3g per kg of combined liquid and solid excreta from some major farm animals.
The degradation of chloramphenicol in manure has not been studied systematically. Since the molecule has a number of functional groups (e.g. the aromatic nitro group, the dichloro-acetyl side-chain) that could be attacked by known biotic processes, the behaviour of the compound cannot be predicted. As with other aromatic nitro compounds, the nitro group is expected to be particularly sensitive under anaerobic conditions (Hallas & Alexander, 1983). Chloramphenicol—like several other antibiotics (Johnson, 1994; Sanz et al., 1996; Lallai et al., 2002)— is, on the other hand, itself a powerful inhibitor of many anaerobic degradation processes. There are a few examples in the literature of situations in which the concentrations and persistence of other antibiotics and feed additives have been determined in various kinds of manure.
Runsey et al. (1977) conducted experiments in which chlortetracycline was used in a combination of feed additive for feedlot beef cattle. Fresh manure, stored manure, runoff water, manure weathered on pasture, and soil from pasture fertilized with manure were analysed for the additives. Seventeen percent of the chlorotetracycline fed to cattle appeared in fresh manure and 11% appeared in manure stored for 12 weeks. Aga et al. (2003) used an ELISA method to screen manure samples collected from hog lagoons and cattle feedlots for the presence of tetracycline residues. The concentrations measured varied from below the LOD (0.5 ng/kg) to 200 mg/kg. The degradation of tetracyclines in soil-applied manure was also followed using ELISA.; detectable concentrations were found for up to 28 days. Analysis of selected manure extracts by liquid chromatography with mass spectrometry (LC-MS) showed lower concentrations of total tetracyclines compared with the values obtained by ELISA, indicating the presence of other structurally related compounds or transformation products of tetracyclines. De Liguoro et al. (2003) followed the levels of oxytetracycline and tylosin over time in faeces, bedding and manure, and then in the soil of a manured field and surrounding drainage courses, after oral treatment of calves. Fifty Simmental calves were treated for 5 days with oxytetracycline at 60 mg/kg bw per day. After 15 days, the animals were treated for 5 days with tylosin at 20 mg/kg bw per day. Tylosin degraded rapidly and was no longer detected in manure 45 days after cessation of treatment, and no trace of the compound was detected in soil or surrounding water (LOD, 10 µg/l). The half-life of oxytetracycline in manure was 30 days, and the compound was still detectable in this matrix (concentration, 820 µg/kg) after 5 months maturation. Hamscher et al. (2002) investigated the distribution and persistence of tetracyclines and tylosin in a field fertilized twice with liquid manure. On the first fertilization, the manure contained tetracycline at 4.0 mg/kg and chlortetracycline at 0.1 mg/kg. Similar concentrations were applied again 1 year later. Soil sampling was performed 1 and 7 months after the first application and 1 month after the second application. At the third sampling time, the highest average concentrations of tetracycline of 86.2 (soil sublayer, 0-10 cm), 198.7 (10-20 cm), and 171.7 µg/kg (20-30 cm) and chlortetracycline at 4.6-7.3 µg/kg (all three sublayers) were found, indicating that tetracylines persisted and accumulated in the soil. Oxytetracycline and tylosin could not be detected in any sample analysed.
Campagnolo et al. (2002) analysed samples from swine waste storage lagoons and surface water and groundwater obtained from sites proximal to large-scale swine and poultry operations for multiple classes of antimicrobial compounds, and found multiple antimicrobial residues (commonly at concentrations of >100 µg/l) in storage lagoons. Schlusener et al. (2003) developed sensitive methods for the analysis of macrolides and ionophores and tiamulin in manure. The maximum concentrations found in manure samples were tiamulin at 43 µg/kg and salinomycin at 11 µg/kg. Ingerslev & Halling-Sorensen (2001) studied the biodegradability of olaquindox, metronidazole, and tylosin, in soil-manure slurries with 50 g of soil per litre. None of these substances persisted in the biodegradation experiments. Degradation half-lives for the primary degradation were: tylosin, 3.3-8.1 days; olaquindox, 5.8-8.8 days; and metronidazole, 13.1-26.9 days. Loke et al. (2000) studied the stability of tylosin A in manure under methanogenic conditions. Tylosin A is the major component (usually about 90% and not less than 80%) of tylosin. The half-life was less than two days. The authors could not determine whether the decrease in the concentration of tylosin A under aerobic and anaerobic conditions is caused by sorption, or by abiotic or biotic chemical degradation.
Haller et al. (2002) analysed six grab samples taken in Switzerland from manure pits on farms where medicinal feed had been applied and found total sulfonamide concentrations of up to 20 mg/kg of liquid manure.
The feed additive roxarsone is excreted unchanged by poultry. In experiments conducted by Garbarino et al. (2003) it was also found to be stable in fresh dried litter. However, when water was added to litter at about 50% w/w and the mixture was allowed to compost at 40°C, roxarsone disappeared and arsenate was formed within about 30 days. Increasing the amount of water and the incubation temperature increased the rate of degradation. The degradation process was most likely to be biotic.
According to the calculations performed in section 7, a concentration of 0.3g/kg of manure as estimated in section 8.4 would be high enough to cause significant residues in tissues of, for example, chickens scavenging on unprocessed manure within approximately ten half-life periods of the chloramphenicol residues. In practice, however, the excreta of treated animals would probably be diluted with excreta from untreated animals.
Assuming half-lives of <1 day to several days (depending on conditions such as temperature, water content, content of bedding material and particles), manure should no longer play a role as a potential source of contamination several weeks after discontinuation of the use of chloramphenicol in terrestrial animals. Since chloramphenicol is relatively unstable under anaerobic conditions, it would most likely not survive the process of farmyard manure production described above and the biodigester process. However, the compound could probably survive for several weeks or longer if administered to soil or grassland shortly after excretion by treated animals. Quantifiable amounts of residues could be taken up by free-ranging animals for a certain period of time. As mentioned in section 5.5, chloramphenicol could persist for many years in dry dusts formed during the mixing of chloramphenicol with dry feeds. The high concentrations of chloramphenicol present in small quantities of dust could occasionally cause residues in some animals, even a long time after the last use of chloramphenicol on a farm.
If unprocessed manure were to be directly added to aquatic systems, two different "worst-case" scenarios could be discussed:
Hypothetical "worst case" scenario 1: the daily excreta of 10 pigs with a body weight of 75 kg are washed directly into a pond with an area of 1000 m2 and a depth of 1 m. After treatment of all animals as assumed in section 5.4, 0.5 × 26.25 × 10 = 0.13 kg of chloramphenicol equivalents (parent drug plus conjugates) would be added by this means to the pond during the days following treatment, resulting in a maximum concentration of <0.13 kg/1000 m3 or <130 µg/l. The half-life would be <10-20 days. Water exchange rates need also be considered in this scenario. There are no data enabling an estimate to be made of the quantities that could be taken up, e.g. through the gills, by aquatic species under these conditions. However, one cannot exclude the formation of traces of detectable residues in tissues. The problem would no longer exist several months after discontinuation of treatment of terrestrial animals.
Hypothetical "worstcase"scenario 2: unprocessed undiluted manure of treated animals is used in pond preparation. In practice the amounts would vary widely depending on, for example, the nitrogen requirements of the particular system. Assuming, for example, that in the preparation phase of a pond with an area of 10 000 m2 a maximum primary dose of 3000 kg of unprocessed manure contaminated with chloramphenicol is applied when the water depth is 10 cm, followed by two maximum secondary doses of 400 kg each applied when the water depth is 30 cm and 100 cm, respectively (e.g. Pathak et al., 2000), the maximum total dose of chloramphenicol would be <0.3 × 3800, that is, <1140 g, depending on the degree of decomposition of active chloramphenicol residues. This amount would probably be quickly dissolved in the water and result in an initial concentration of <1.14 kg/10 000 m3 or <114 µg/l, which would decrease owing to degradation and water exchange. In this example, the true concentrations of chloramphenicol could be much less than 114 µg/l since the excreta would have been collected over a certain period of time, which would favour decomposition of chloramphenicol. Again, one could not exclude the possibility that the remaining chloramphenicol could lead to concentrations of residues in aquatic species at greater than the LOQ. However, such problems would rapidly disappear after discontinuation of the therapeutic use of chloramphenicol in terrestrial animals.
Another source of environmental contamination is the direct therapeutic use of chloramphenicol in aquatic species. In particular, its use in medicated feed had the potential to leave residues in the pond environment. Addition of medicated feed containing chloramphenicol at 2.5 g/kg of feed, five times per day for 3 days could mean, in practice, the introduction of up to 30 kg of feed/day per 10 000 m2 (e.g. Cruz-Lacierda et al., 2000) depending on the density of the aquatic species. With this amount of feed, 22.5 g of chloramphenicol would be introduced into a pond with an area of 1000 m2, for example; at least 15% of this dose would not be consumed (Primavera, 1994) by the aquatic species. Depending on the stability of the feed particles in water (Obaldo, 2001), and of the coating in particular, chloramphenicol could leach out at a rate of <0.5-5% per h. Assuming more stable particles and good quality coating, all the chloramphenicol contained in the feed could also fall down to the mud on the pond bottom and persist there with half-lives of up to several weeks, depending on conditions, such as oxygen content and salinity.
In summary, all the hypothetical considerations performed in this section suggest that after the implementation of a complete ban on the use of chloramphenicol in farm animals, the problems discussed in this section would vanish within several months.
The problems of potential significant carry-over in, for example, feed mills are not discussed in this document.
Assuming occasional contamination of fish or shellfish containing chloramphenicol at a concentration of 0.5 µg/kg, the additional chloramphenicol burden for average and preferential eaters of fish and shellfish could be estimated using data on consumption habits. Such data are not available at the international level. However, some data have been published for certain regions and for certain populations with very high consumption of fish and shellfish.
A survey of 212 people living in Singapore was conducted by Burger et al. (2002) to examine the relative importance of fish, shellfish, and other meat in the diet. From the authors' discussion of their findings in the context of international surveys on fish consumption, it appears that people in the Far East eat significantly more fish than do most people whose families have lived for generations in industrialized societies. In the study by Burger et al., it was found that people ate, on average, fish for about ten meals per week, chicken for eight meals per week, and shrimp and pork for about six meals each per week. While only 8% of people never ate fish, 18% ate fish at all 21 meals per week, and >20% ate shellfish for all 21 meals. Therefore, it seems to be appropriate to base an estimate of high seafood intake by preferential eaters on data obtained in surveys of people of Asian origin.
Sechena et al. (2003) described and quantified rates of seafood consumption, and acquisition and preparation habits of 202 first- and second-generation Asian-American and Pacific Islanders from 10 ethnic groups (Cambodian, Chinese, Phillippine, Hmong, Japanese, Korean, Lao, Mien, Samoan, and Vietnamese) in King County, Washington in 1997. Participants in the study were all consumers of seafood; only one person did not eat shellfish, which was otherwise the predominant seafood consumed by these people (45.9% of all seafood consumed). Rates of fish consumption were skewed considerably for all fish groups, indicating that a few respondents had a larger consumption rate than other respondents. The 90th percentile of all consumption rates for "all seafood" consumption was 3.928 g/kg bw per day.
Using this conservative estimate of seafood intake and multiplying it by the median concentration (0.5 µg/kg) of chloramphenicol found in the published alerts from the Food Standards Agency of Ireland, mentioned above, would result in an estimated daily intake of chloramphenicol of approximately 2 × 109 g/kg bw, or 0.12 µg for a person with a body weight of 60 kg. This estimate of intake could be slightly low since other products of animal origin could also occasionally contain traces of chloramphenicol.
Systemic drug absorption after ocular administration is well known and has been reported in hundreds of research papers for a large number of substances by numerous research groups. For a better understanding of these variable findings, it is important to consider the anatomy and physiology of the eye.
After topical ocular administration of drugs, the highly vascularized conjunctival and nasal mucosa are the major sites of systemic absorption. Drug absorption by this route bypasses the first-pass effects in the gastrointestinal tract and in the liver. However, local ophthalmic bioavailability can be very low, since the tear fluid is rapidly drained from the lower conjunctival sac through the puncta and the lachrymal duct into the lachrymal sac, from where it passes through the nasolachrymal duct into the inferior nasal meatus. From here, the fluid moves to the nasopharynx where it is swallowed into the gastrointestinal tract. It has been reported that drainage of the administered dose via the nasolacrimal system into the nasopharynx and the gastrointestinal tract takes place when the volume of fluid in the eye exceeds the normal lacrimal volume of 7-10 µl. Thus, the portion of the dose that is not eliminated by spillage is drained quickly and the time that the dose is in contact with the surfaces of the cornea and sclera is reduced to approximately 2 min (Saettone, 2002). According to Saettone (2003), the rate of loss of drug from the eye can be such that only 1-5% or less of the drug applied topically as a solution reaches the inner eye. For example, using timolol as a probe, Alvan et al. (1980) found that 12-88% of the dose administered was lost when 16 volunteers received two drops of 0.5% timolol ophthalmic solution in each eye, twice daily for 2 weeks. These anatomical and physiological properties of the eye explain the short pre-corneal residence time and the poor bioavailability of many eyedrop solutions that do not contain a viscosity agent in their formulation to prolong residence time (Sirbat et al., 2000).
In summary, there are three possible systemic absorption sites for drugs administered topically to the eye—conjunctival, inferior nasal, and gastrointestinal mucosa—and total bioavailability, therefore, should be assessed by estimating drug concentration in plasma or urine.
Part of the current knowledge of systemic bioavailability of drugs administered topically to the eye has accumulated from observations of systemic adverse effects after the use of topical ophthalmic preparations (Gerber et al., 1990; Flach, 1994; Jones et al., 1996; Shiuey & Eisenberg, 1996; Diamond, 1997). Urtti & Salminen (1993) emphasize the need to take into account the problem of systemic drug absorption in designing ocular drug and dosage forms to minimize systemic absorption and increase the oculospecificity of drugs, for example, reducing volume and increasing viscosity of eyedrops, controlling drug release from depot preparations, prodrug-derivatization, and addition of vasoconstrictive agents.
In many other studies in humans and animals, the systemic availability of ophthalmic drugs has been convincingly demonstrated by pharmacokinetic research. A few examples of hundreds of papers may be given here. Anderson (1980) determined that systemic absorption of epinephrine and dipivefrin hydrochloride was 55-65% of the ocularly applied dose. Chiang et al. (1983) gave delta 9-tetrahydrocannabinol to rabbits by ophthalmic administration, measured plasma concentrations, and compared with intravenous data to establish bioavailability. Kumar et al. (1985) studied the systemic absorption, plasma concentrations (maximum plasma concentrations are achieved within 10-20 min after topical instillation) and cardiovascular effects of ophthalmic solutions of phenylephrine hydrochloride. Salminen (1990) reviewed literature on human plasma concentrations after instillation of ocular timolol, levobunolol, atropine, cyclopentolate, scopolamine, phenylephrine, betamethasone and technetium-99. Kaila et al. (1999) determined the mean bioavailability of ocularly administered atropine to be 63.5% (n = 6; range, 19-95%). Sasaki et al. (2000) using tilisolol as a model substance and carboxymethylcellulose sodium salt as viscous polymer developed an in vivo pharmacokinetic model that accounts for corneal diffusion in albino rabbits and predicts the concentration of beta-blockers in the anterior segments, and characterizes the systemic absorption of instilled drug with ophthalmic viscous vehicle. Vainio-Jylha et al. (2001) studied the concentration of betaxolol in plasma after its topical ocular use. The drug showed a biphasic concentration-time curve in plasma, the first peak occurring already after several minutes, and concentrations were detectable even at 12 h after dosing. Korte et al. (2002) estimated the systemic bioavailability of timolol in eyedrops containing 0.5% timolol, and compared the cardiopulmonary effects of intravenous and ophthalmic timolol. The peak concentration of ophthalmic timolol in plasma was measured in most subjects within 15 min after drug administration. The systemic bioavailability was 78.0% (n = 8). Ophthalmic timolol resembled intravenous timolol in terms of systemic bioavailability, plasma kinetics, and cardiopulmonary effects. A recent article reviews the principles of systemic absorption of insulin applied topically to the eye. The physiological and pharmaceutical considerations for formulation development and the strategy of improving the systemic absorption and bioavailability of insulin are also discussed (Lee et al., 2002).
The above examples were given since with chloramphenicol alone, a substance that typically easily passes through all biological membranes and barriers, the systemic bioavailability after topical application is a controversial subject of discussion. However, this discussion has taken place in the absence of adequate studies using sensitive analytical methods.
In an early study by Trope et al. (1979), five children aged <9 years received eyedrops containing chloramphenicol, which were administered every 2 h to each eye for 5-7 days. Systemic absorption was not detected with the available assay methods. Walker et al. (1998) used HPLC with a limit of detection of 1 mg/l to investigate whether serum accumulation of chloramphenicol occurred after topical therapy in 40 patients. The mean dose of chloramphenicol received from eyedrops after 1 week of treatment was 8.0 mg, and after 2 weeks, 15.3 mg. As the authors had expected, chloramphenicol failed to accumulate to detectable levels. Contrary to these findings, chloramphenicol applied topically to the eye in ointment produced bacteriostatic concentrations of chloramphenicol in the aqueous humor, which lasted for several hours (George & Hanna, 1977). Hanna et al. (1978) reported that repeated drops of solutions containing 0.5% chloramphenicol for several hours were required to produce a concentration of chloramphenicol of 1 µg/ml in the aqueous humor. Yamada & Hiraki (1995) studied the ocular pharmacokinetics in rabbits of a combination formulation containing 0.25% chloramphenicol (CP) under various experimental conditions. When they sealed the cornea with cyanoacrylate glue to block transcorneal absorption, the absorbed fraction of chloramphenicol was <10% of that in the control, indicating that most of the chloramphenicol in the aqueous humor was derived from the transcorneal route. Ismail & Morton (1995) evaluated the bioavailability of three commercial chloramphenicol ophthalmic products supplemented with [14C]chloramphenicol. Samples of 50 µl were applied to the corneas of isolated bovine eyes. Their results indicate a greater bioavailability of chloramphenicol in ophthalmic ointments than in a liquid preparation, which gave extremely low levels of chloramphenicol in the aqueous humour and corneal.
Typical concentrations of chloramphenicol in eyedrops and ointments are 5 µg/µl (0.5%) and 10 µg/µl (1%), respectively. The recommended frequency of application varies. A dosage regimen that has been recommended on the basis of clinical trial suggests four daily treatments. Under these conditions, acute bacterial conjunctivitis was 88% healed within 9 days of treatment (Laerum et al., 1994).
The locally absorbed fraction of the drug dose enters the anterior chamber by penetration through the three layers and two membranes of the cornea. When a drop (50-75 µl) is applied to the eye it becomes diluted owing to reflex tearing, and the volume that is in excess of the normal lacrimal volume is drained from the eye. The average maximum capacity of the human conjunctival sac is 25-30 µl (Peterson, lecture notes). In consequence, it can be estimated that 66-75% of the administered dose is lost immediately, and that a maximum of 0.33 × 5 × 50 = 82.5 µg per application would be available for possible absorption through the cornea. Ointments have prolonged contact with the cornea, which improves absorption. Chloramphenicol apparently exhibits good penetration if the residence time is sufficiently long. Beasley et al. (1975) gave topical applications of an ophthalmic solution containing 0.5% chloramphenicol to patients at various times before cataract surgery. Aqueous humor obtained at the time of surgery contained intact chloramphenicol at a concentration of 3.5-6.7 µg/ml 1-2 h after topical administration. Aqueous humor is the watery fluid produced by the ciliary body that fills the space between the lens and cornea of the eye, which serves as a nutrient delivery system for the avascular cells in the cornea and eventually drains into a vein or lymphatic vessel. The normal human aqueous humor production during daytime is 2.75 ± 0.65 µl/min (mean ± standard deviation (SD)), while at night production is approximately half that amount (Larsson, 1998). The volume of the anterior chamber is dependent on age. In normal healthy subjects in two age groups, the volume of the anterior chamber was 247 ± 39 µl in the younger group (aged 20-30 years, n = 51) and 160 ± 39 µl in the older group (aged ≥60 years, n= 53) (Toris et al., 1999).
Using these values, the fraction of the bioavailable dose that is absorbed through the cornea per application of one drop (0.6-1.6 µg or 2.4-6.4 µg per day) during a treatment period can be calculated. This value probably represents a low estimate of systemic absorption, as it does not include absorption occurring at other possible sites described above. Nevertheless, this low estimate of systemic absorption of chloramphenicol from eyedrops is up to 50 times higher than the intake estimate calculated in section 7 and is of about the same order of magnitude as the intake corresponding to the highest contaminated seafood sample found in 2002.
No toxicological data on chloramphenicol were submitted to the Committee at its present meeting. The current evaluation was made on the basis of an extensive review of the scientific literature, particularly that published since the forty-second meeting, and with a focus on the toxicological data in humans.
Reports on uptake and metabolism in humans and animals showed that chloramphenicol is rapidly absorbed when administered orally and that it is extensively metabolized. A study with human bone marrow in vitro also showed evidence of metabolism in this tissue.
A number of studies with chloramphenicol and several metabolites of chloramphenicol have shown that they are cytotoxic to bone marrow in vitro.
In order to assess the genotoxicity of chloramphenicol, the Committee reassessed the results of tests reported at its thirty-second and forty-second meetings, and also considered new studies available in the published literature. Chloramphenicol was shown to cause DNA damage in a human fibroblast cell line and in primary cultures of rat hepatocytes, but not in human bone-marrow cells in vitro. The results of tests for reverse mutation in bacteria were mostly negative. In mammalian cells in vitro, chloramphenicol consistently gave positive results in tests for chromosomal aberrations, but results of tests for gene mutations and for sister chromatid exchange were inconsistent. Overall, these results indicated that chloramphenicol was genotoxic in vitro.
In tests for genotoxicity in vivo, chloramphenicol caused chromosomal aberrations in the bone marrow of mice, but gave negative results in tests for micronucleus formation in the bone marrow of mice and rats. It is not clear why contrasting results were obtained in these two assays, but the Committee considered that it was prudent to regard chloramphenicol as a mutagen in somatic cells in vivo.
According to data on heritable mutation reviewed by the Committee at previous meetings, chloramphenicol gave negative results in tests for dominant lethal mutation in mice and in Drosophila melanogaster.
At its present meeting, the Committee reviewed studies on genotoxicity with chloramphenicol and its metabolites in human bone marrow cells or peripheral blood lymphocytes in vitro. Only nitrosochloramphenicol and dehydrochloramphenicol induced DNA strand breaks, while chloramphenicol and other metabolites were without effects. This confirmed the results of previous studies that showed that some of the metabolites of chloramphenicol are genotoxic.
No adequate studies were available to evaluate the carcinogenicity of chloramphenicol in experimental animals. Chloramphenicol has been classified as "probably carcinogenic in humans" by IARC (IARC, 1990).
A number of toxicological studies in rodents were conducted in an effort to develop a model for chloramphenicol-induced aplastic anaemia in humans. While bone-marrow depression was confirmed in these studies, it did not progress to the characteristic aplastic anaemia of humans and was therefore not considered to be a suitable model for the disease in humans. The reversible bone-marrow depression that is seen in animals and humans receiving chloramphenicol can be attributed to its cytotoxicity.
A number of reports of epidemiological studies on the oral and injectable use of chloramphenicol in humans were reviewed; they confirmed that chloramphenicol was toxic to the bone marrow. In many cases, the toxic effects could be reversed by reducing or discontinuing treatment with chloramphenicol. There are, however, cases of aplastic anaemia that appear to be unrelated to dose and that are associated with a high mortality rate. In humans, the aplastic anaemia that is attributable to treatment with chloramphenicol is often fatal and is an idiosyncratic reaction that may have an immunological component. There is also evidence that some of the survivors of aplastic anaemia induced by chloramphenicol subsequently develop leukaemia.
The ophthalmic use of chloramphenicol represents the lowest therapeutic dose of the compound. Epidemiological data relating to the ophthalmic use of chloramphenicol in humans suggest that this form of administration is unlikely to be associated with aplastic anaemia. While any occurrence of aplastic anaemia associated with this form of administration is extremely rare, it is not possible to quantify the absolute risk of the ophthalmic use of chloramphenicol in humans because of the low background occurrence of idiopathic aplastic anaemia.
No adequate studies were available to fully assess potential reproductive toxicity with chloramphenicol. However, chloramphenicol has been shown to be embryotoxic and fetotoxic in a number of laboratory animal species.
In a case-control study in humans, the authors concluded that oral treatment with chloramphenicol in the second and third months of pregnancy presents little, if any, teratogenic risk. However, it is difficult to determine if any effects might occur during the first month of pregnancy.
Residue data
Most countries in the world do not permit the use of chloramphenicol in food-producing animals, in order to protect the health of consumers. Despite such restrictions, chloramphenicol has been detected in food samples collected in national monitoring programmes during the past 2 years and these residues have caused safety concerns. Shrimps, prawns, food products from aquatic animals, honey, royal jelly, meat and offal, sausage casings, rabbit and poultry meat and milk powder were among the commodities in which the drug was detected.
Chloramphenicol—an environmental contaminant?
While the results of monitoring clearly indicate intentional uses of chloramphenicol in some cases, it has also been argued that very low levels of chloramphenicol, such as those found in poultry and in products from aquaculture, could result from environmental contamination. The possibility that chloramphenicol could persist in the environment, or even be formed by soil microorganisms was discussed by the Fourteenth Session of CCRVDF, held in 2003 (Joint FAO/WHO Food Standards Programme, 2003).
Chloramphenicol was first described as an antibiotic produced by cultures of an actinomycete isolated from soil by Ehrlich et al. (1947). The soil samples were collected from a mulched field near Caracas, Venezuela, and from a compost soil on the horticultural farm of the Illinois Agricultural Experiment Station at Urbana. In studies conducted in 1952, the adsorption, stability, and rate of production of chloramphenicol in soil under different laboratory conditions were determined. When sterilized soil was inoculated with Streptomyces venezuelae and was incubated for long periods, the presence in soil of chloramphenicol formed by the microorganism after a lag phase of several weeks was demonstrated. The highest concentration measured was 1.12 mg/kg. However, when workers of the same group and in the same year analysed samples of normal soils collected from 91 cultivated and grassland sites from nine states of the USA and from 13 other countries, no chloramphenicol was identified in extracts from these soils. In this initial study (1952), the limit of detection of chloramphenicol was 0.3 mg/kg (turbidimetric assay using Shigella sonnel). In other experiments with an improved LOD of 0.05 mg/kg, and including the 91 samples of the previous study, these results were confirmed. Chloramphenicol was found and identified in soils only when organic material was added before sterilization and seeding with Streptomyces venezuela.
Whether antibiotics are produced in soil in detectable amounts by indigenous soil organisms has remained a subject of scientific dispute for several decades. It was only recently demonstrated that an antibiotic could be synthesized in detectable amounts in soil. Using biosensor methods with very low LODs, Streptomyces rimosus was found to produce oxytetracycline in untreated soil. However, similar studies have not been carried out for chloramphenicol.
With this background information and on the basis of an extensive search of the current literature, the Committee at its present meeting examined the following two hypothetical scenarios to explain potential environmental contamination of foods of animal origin with chloramphenicol:
Scenario 1
Assuming that:
The Committee conducted a number of simple model calculations to estimate hypothetical intakes of chloramphenicol as a function of soil intake of animals. On the basis of data in the literature, it was assumed that 2% of the dry matter intake of pigs and chickens was soil. The concentration of chloramphenicol in soil was set at one of the following values:
The following steps were performed in the calculations:
The Committee concluded that concentrations of chloramphenicol in soil, as they were found under laboratory conditions in the presence of organic material, would suffice to explain occasional traces of chloramphenicol in tissues and products of free-ranging and/or scavenging livestock animals. With the LOD achieved in the 1950s, however, it was not possible to demonstrate the production of detectable amounts of chloramphenicol in soil. No further empirical data have been obtained since 1952. The possibility that chloramphenicol, produced naturally by soil microorganisms, could lead to the residues found in food-producing animals cannot be ruled out, but remains an unexplored hypothesis that is currently not supported by experimental data.
Scenario 2
Assuming that:
The Committee reviewed published literature in order to investigate the conditions of integrated farming as a potential cause of chloramphenicol residues in food of animal origin. Chloramphenicol was in use in farm animals as a veterinary drug before authorizations were withdrawn in many countries and regions. Before these restrictions, significant amounts of manure, probably containing intact chloramphenicol, had been used as fertilizers. Concentrations of chloramphenicol in fresh manure and certain patterns of its use in integrated farming of aquatic species could, in principle, also explain low concentrations of residue in certain farm animals, such as scavenging chickens, free-ranging pigs and in aquatic animals. However, when reviewing the available information on the half-life of chloramphenicol under different environmental conditions, no evidence was found to show that chloramphenicol could persist in the environment for periods longer than several months, except in dry dusts. Therefore, if there was any risk of food contamination resulting from historical use in the farm environment these problems should disappear within several months of cessation of the use of chloramphenicol. Similar considerations apply to the persistence of chloramphenicol in aquaculture after past uses of the drug as medicated feed that had been directly applied in ponds.
Analytical methods
In the past decade, several methods have been developed for the screening, quantification and confirmation of chloramphenicol in foods.
Screening for chloramphenicol could be performed with validated ELISA kits. The majority of these kits had a LOD of <1 µg/kg. However, confirmatory methods needed to be used in order to avoid false-positive results. For confirmatory purposes, highly sensitive methods based on GC-MS, either electron impact or negative chemical ionization mode, have been used. More recently, LC-MS/MS methods allow the determination and identification of chloramphenicol in food commodities such as honey, meat (chicken, turkey, pork and beef), fish and shellfish, at concentrations of <1 µg/kg. The LOD and LOQ are as low as 0.05 µg/kg and 0.1 µg/kg, respectively.
Conclusions
The Committee concluded that:
Valid analytical methods are available to monitor low concentrations of chloramphenicol in foods; however, confirmatory methods require sophisticated and expensive equipment.
Since the advent of sensitive routine analytical methods for the quantitative determination of residues of chloramphenicol in foods of animal origin at concentrations far less than 1 µg/kg, illegal use of this drug is no longer attractive. Although only limited kinetic residue data exist that describe the terminal elimination from "deep compartments" (Arnold & Somogyi, 1986), these data suggest that the withholding times necessary to allow residues to deplete below 1 µg/kg with reasonable statistical certainty are already in the order of 15 to much longer than 30 days, depending on species, product, dose and route of administration. Therefore, the relatively frequent observation of residues far less than 1 µg/kg in monitoring programmes has stimulated a discussion on whether traces of chloramphenicol in foods could occasionally originate from environmental contamination.
The possible production of chloramphenicol in natural soils was investigated 50 years ago at the time of the discovery of chloramphenicol as a product of a soil microorganism. With the methodologies of that era it was not possible to convincingly demonstrate the production of appreciable amounts of chloramphenicol in soil. The question has never again been examined using adequate analytical methods. However, reliable laboratory experiments conducted by the group that discovered chloramphenicol have shown that in the presence of organic material as it may well occur on farmland chloramphenicol could be formed in concentrations up to 1 mg/kg. However no further empirical data have been generated since that time. These concentrations in soil which were found under laboratory conditions would suffice to explain occasional traces of chloramphenicol in tissues and products of free ranging and/or scavenging livestock animals.
Chloramphenicol was in common use as a veterinary drug before authorizations were withdrawn in many countries and regions. Before such restrictions, enormous amounts of manure likely to contain active chloramphenicol were used as fertilizers. Concentrations of chloramphenicol in fresh manure and certain patterns of its use could in principle explain low residue concentrations in certain farm animals, e.g. in scavenging chickens, free-ranging pigs and in aquatic animals. However, no convincing evidence has been found to show that chloramphenicol could persist in the environment for long times—except in dry dusts.
The occasional daily dietary intake of chloramphenicol by preferential eaters of fish and shellfish contaminated at the levels reported in the year 2002 (median value, 0.5 µg/kg) is more than one order of magnitude lower than the systemically bioavailable fraction of a daily dose of a typical ophthalmic formulation used in human medicine.
As there is evidence that chloramphenicol is a genotoxin in vivo, it is prudent to assume that chloramphenicol could cause some effects, such as cancer, through a genotoxic mechanism for which there is no identifiable threshold dose.
Epidemiological studies in humans show that treatment with chloramphenicol is associated with the induction of aplastic anaemia, which may be fatal. It was not possible to establish any dose-response relationship or threshold dose for the induction of aplastic anaemia.
The Committee noted that aplastic anaemia induced by chloramphenicol is a rare idiosyncratic response in humans, which may have an immunological component. In common with many other idiosyncratic immune system-mediated adverse reactions, no animal model could be developed. As a consequence of these considerations, and because the mechanism of chloramphenicol-induced aplastic anaemia remains unknown, the Committee could not identify any studies in animals or epidemiological studies that would assist the further toxicological evaluation of chloramphenicol.
The Committee concluded that it was not appropriate to establish an ADI for chloramphenicol.
Taking into account the present gaps in scientific knowledge, the Committee could not completely rule out the possibility that occasionally foods are contaminated from environmental sources. The easiest way to rule out this possibility would be a thorough investigation of chloramphenicol concentrations in soil using modern analytical methods.
The Committee was unable to propose options for regulating traces of chloramphenicol in foods. The human exposure which may have resulted from consumption of contaminated seafood in the years 2001-2003 was probably lower than the systemic exposure resulting from the use of ophthalmic formulations. However, the resulting risk could not be estimated.
Abrahams, P.W. & Steigmajer, J. (2003) Soil ingestion by sheep grazing the metal enriched floodplain soils of mid-Wales. Environ. Geochem. Health, 25, 17-24.
Adams, H.R. (1995) Veterinary Pharmacology and Therapeutics, 7th Ed. Ames: Iowa State Press, pp. 820-824.
Aga, D.S., Goldfish, R. & Kulshrestha, P. (2003) Application of ELISA in determining the fate of tetracyclines in land-applied livestock wastes. Analyst, 128, 658-662.
Alnigenis, M.N., Nalcaci, M., Pekcelen, Y., Atamer, T. & Sargin, D. (2001) Possible etiologic factors in 151 Turkish patients with aplastic anemia. Am. J. Hematol., 68, 60-61.
Alvan, G., Calissendorff, B., Seideman, P., Widmark, K. & Widmark, G. (1980) Absorption of ocular timolol. Clin. Pharmacokinet., 5, 95-100.
Ambekar, C.S., Cheung, B., Lee, J., Chan, L.C., Liang, R. & Kumana, C.R. (2000) Metabolism of chloramphenicol succinate in human bone marrow. Eur. J. Clin. Pharmacol., 56, 405-409.
Anadon, A., Bringas, P., Martinez-Larranaga, M.R. & Diaz, M.J. (1994) Bioavailability, pharmacokinetics and residues of chloramphenicol in the chicken. J. Vet. Pharmacol. Ther., 17, 52-58.
Anderson, J.A. (1980) Systemic absorption of topical ocularly applied epinephrine and dipivefrin. Arch. Ophthalmol., 98, 350-353.
Arnold, D. & Somogyi, A. (1986) Chloramphenicol residues in edible tissues of food animals. Proc. Second World Congress on Foodborne Infections and Intoxications, Vol. II, pp. 832-836.
Arthur, J.R., Lavilla-Pitogo, C.R. & Subasinghe, R.P., eds. (2000) Use of Chemicals in Aquaculture in Asia. Proceedings of the Meeting on the Use of Chemicals in Aquaculture in Asia, 20-22 May 1996; Tigbauan, Iloilo, Philippines. Southeast Asian Fisheries Development Center, Aquaculture Department, Tigbauan, Iloilo, Philippines.
Baig, J., Sharma, M.C. & Lal, S.B. (1994) Haemato-biochemical and the bone marrow changes in chloramphenicol-induced toxicosis in dogs. Indian J. Anim. Sci., 64, 712-715.
Balizs, G. & Arnold, D. (1989) Reference standards for residue analysis of chloramphenicol in milk and meat: a critical study. Chromatographia, 27, 489-494.
Baradat, M., Alary, J. & Cravedi, J.P. (1993) Residues of chloramphenicol in rainbow trout. In: Proceedings of the Euroresidue II Conference. Veldhoven, Netherlands, 3-5 May, 1993.
Barker, J.C., Hodges, S.C. & Walls, F.R. (2003) Livestock manure production rates and nutrient content. In: 2003 North Carolina Agricultural Chemicals Manual, Chapter X. The College of Agriculture and Life Sciences, North Carolina State University, Raleigh, North Carolina (http://ipm.ncsu.edu/agchem/chptr10/1011.pdf, accessed 18 May 2004).
Beasley, H., Boltralik, J.J. & Baldwin, H.A (1975) Chloramphenicol in aqueous humour after topical application. Arch. Ophthalmol., 93, 184-185.
Berry, D.J. (1987) Reversed-phase high-performance liquid chromatographic determination of chloramphenicol in small biological samples. J. Chromatogr., 385, 337-341.
Boertz, A.K. (1984) Radioimmunologische und gaschromatographische Untersuchungen zum Rückstandsver-halten von Chloramphenicol beim Schwein. Dissertation (in German), Technische Universität, Berlin, Germany.
Boertz, A.K., Arnold, D. & Somogyi, A. (1985) [Behaviour of chloramphenicol residues following intramuscular administration in swine] (in German) Z. Ernährungswiss. 24, 113-119.
Borner, S., Fry, H., Balizs, G. & Kroker, R. (1995) Confirmation of chloramphenicol residues in egg by gas chromatography/high-resolution mass spectrometry and comparison of quantitation with gas chromatography—electron capture detection. J. AOAC Int., 78, 1153-1160.
Bolton, L.F., Kelley, L.C., Lee, M.D., Fedorka-Cray, P.J. & Maurer, J.J. (1999) Detection of multidrug-resistant Salmonella enterica serotype typhimurium DT104 based on a gene which confers cross-resistance to florfenicol and chloramphenicol. J. Clin. Microbiol., 37, 1348-1351.
Bories, G.F., Peleran, J.C. & Wal, J.M. (1983) Liquid chromatographic determination and mass spectrometric confirmation of chloramphenicol residues in animal tissues. J. AOAC Int., 66, 1521-1526.
Bui Van Chinh, Nguyen Huu Tao & Do Viet Minh (1993) Growing and ensiling soybean forage between rice crops as a protein supplement for pigs in north Vietnam. Livestock Research for Rural Development, 5(1) (http://www.cipav.org.co/lrrd/, accessed 18 May 2004).
Burger, J., Fleischer, J. & Gochfeld, M. (2002) Fish, shellfish, and meat meals of the public in Singapore. Environ. Res., 92, 254-261.
BVL (2003) Communication from the Bundesamt fuer Verbraucherschutz und Lebensmittelsicherheit, Berlin, Germany to the FAO JECFA Secretariat.
Campagnolo, E.R., Johnson, K.R., Karpati, A., Rubin, C.S., Kolpin, D.W., Meyer, M.T., Esteban, J.E., Currier, R.W., Smith, K., Thu, K.M. & McGeehin, M. (2002) Antimicrobial residues in animal waste and water resources proximal to large-scale swine and poultry feeding operations. Sci. Total Environ., 299, 89-95.
Chevalier, M., Pochard, M.F. & Bel, B. (1995) Determination of chloramphenicol residues by reverse phase high-performance liquid chromatography in foies gras. Food Addit. Contam., 12, 101-106.
Catalan, J., Moreno, C. & Arruga, M.V. (1993) Sister-chromatid exchanges induced by chloramphenicol on bovine lymphocytes. Mutat. Res., 319, 11-18.
Chiang, C.W., Barnett, G. & Brine, D. (1983) Systemic absorption of delta 9-tetrahydrocannabinol after ophthalmic administration to the rabbit. J. Pharm. Sci., 72, 136-138.
Chien, Y.H., Lai, H.T. & Liu, S.M. (1999). Modelling the effects of sodium chloride on degradation of chloramphenicol in aquaculture pond sediment. Sci. Total Environ., 239, 81-87.
Costa, M.H., et al. (1993) Evaluation of residues of chloramphenicol in liver, kidney, muscle and blood of rabbits and stability studies during storage and after a cooking procedure. In: Proceedings of the Euroresidue II Conference. Veldhoven, Netherlands, 3-5 May, 1993, pp. 246-250.
Cravedi, J., Perdu-Durand, E., Baradat, M., Alary, J., Debrauwer, L. & Bories, G. (1995) Chloramphenicol oxamylethanolamine as an end product of chloramphenicol metabolism in rat and humans: evidence for the formation of a phospholipid adduct. Chem. Res. Toxicol., 8, 642-648.
Cruz-Lacierda, E.R., de la Peña, L.D. & Lumanlan-Mayo, S.C. (2000) The use of chemicals in aquaculture in the Philippines. In: Arthur, J.R., Lavilla-Pitogo, C.R., and Subasinghe, R.P., eds., Use of Chemicals in Aquaculture in Asia. Proceedings of the Meeting on the Use of Chemicals in Aquaculture in Asia, 20-22 May 1996, Tigbauan, Iloilo, Philippines. Published by: Southeast Asian Fisheries Development Center, Aquaculture Department, Tigbauan, Iloilo, Philippines.
Czeizel, A., Rockenbauer, M., Sorensen, H. & Olsen, J. (2000) A population-based case-control teratologic study of oral chloramphenicol treatment during pregnancy. Eur. J. Epidemiol., 16, 323-327.
Davis, M.A., Hancock, D.D., Besser, T.E., Rice, D.H., Gay, J.M., Gay, C., Gearhart, L. & Di Giacomo, R. (1999) Changes in antimicrobial resistance among Salmonella enterica serovar typhimurium isolates from humans and cattle in the north-western United States, 1982-1997. Emerg. Infect. Dis., 5(6), (http://www.cdc.gov/ncidod/eid/vol5no6/davis.htm, accessed 18 May 2004).
De Liguoro, M., Cibin, V., Capolongo, F., Halling-Sorensen, B. & Montesissa, C. (2003) Use of oxytetracycline and tylosin in intensive calf farming: evaluation of transfer to manure and soil. Chemosphere, 52, 203-212.
de Vries, H., Hemelaar, P.J., Gevers, A.C. & Beyersbergen van Henegouwen, G.M. (1994) Photoreactivity of chloramphenicol in vitro and in vivo. Photochem. Photobiol., 60, 249-252.
Dharmananda, S. (2003) Traces of chloramphenicol in Chinese bee products: origin, development, and resolution. Institute for Traditional Medicine, Portland, Oregon (http://www.itmonline.org/arts/bees.htm, accessed 18 May 2004).
Diamond, J.P. (1997) Systemic adverse effects of topical ophthalmic agents. Implications for older patients. Drugs Aging, 11, 352-360.
Dollery, C. (1999) Therapeutic Drugs. Edinburgh: Churchill Livingstone, pp. C168-C172.
Doona, M. & Walsh, J.B. (1995) Use of chloramphenicol as topical eye medication: time to cry halt? BMJ, 310, 1217-1218.
Durosinmi, M. & Ajayi, A. (1993) A prospective study of chloramphenicol induced aplastic anaemia in Nigerians. Trop. Geogr. Med., 45, 159-161.
Du Thanh Hang (2003) Effect of level of fresh duckweed (Lemna minor) in diets of chickens raised in confinement. In: Preston, R. & Ogle, B., eds, Proceedings of Final National Seminar-Workshop on Sustainable Livestock Production on Local Feed Resources. HUAF-SAREC, Hue City, 25-28 March, 2003 (http://www.mekarn.org/sarec03/ hanghue.htm, accessed 18 May 2004).
Duong Duy Dong (2003) Use of rubber seed meal in diets for colored feather chickens. In: Preston, R. & Ogle, B., eds, Proceedings of Final National Seminar-Workshop on Sustainable Livestock Production on Local Feed Resources. HUAF-SAREC, Hue City, 25-28 March, 2003 (http://www.mekarn.org/sarec03/donguaf.htm, accessed 18 May 2004).
Ehrlich, J., Bartz, Q.R., Smith, R.M., Joslyn, D.A. & Burkholder, P.R. (1947) Chloromycetin, a new antibiotic from a soil actinomycete. Science, 106, 417.
Ehrlich, J., Gottlieb, D., Burkholder, P.R., Anderson, L.E. & Pridham, D.G. (1948) Streptomyces venezuelae, n. sp., the source of chloromycetin. J. Bacteriol., 56, 456-467.
Ehrlich, J., Anderson, L.E., Coffey, G.L. & Gottlieb, D. (1952a) Streptomyces venezuelae: soil studies. Antibiot. Chemother., 2, 595-596.
Ehrlich, J., Anderson, L.E., Coffey, G.L. & Gottlieb, D. (1952b) Streptomyces venezuelae: further soil studies. Antibiot. Chemother., 3, 1141-1148.
European Committee for Veterinary Medicinal Products (1994) Chloramphenicol summary report. European Agency for the Evaluation of Medicinal Products (http://www.emea.eu.int/pdfs/vet/mrls/chloramphenicol.pdf, accessed 18 May 2004).
Epstein, R. (1994) International validation study for the determination of chloramphenicol in bovine muscle. J. AOAC Int., 77, 570-576.
European Commission (2003) Commission Decision 2003/181/CE, 13 March 2003. Official Journal of the European Communities., L71, pp. 17-18.
European Commission (2004) Communication from the Health and Consumer Protection Directorate-General of the European Commission to the FAO JECFA Secretariat.
Farooq, M., Mian, M.A., Durrani, F.R. & Syed, M. (2002) Feed consumption and efficiency of feed utilization by egg type layers for egg production. Livestock Research for Rural Development, 14(1) (http://www.cipav.org.co/lrrd/, accessed 18 May 2004).
Food and Drug Administration (1985) Chloramphenicol oral solution; opportunity for hearing. Federal Register, 50, 27059-2706 (also http://www.fda.gov/cvm/efoi/ea/Agency_ Initiated_Agency/Chloramphenicol_EA.pdf, accessed 18 May 2004).
Festing, M.F., Diamanti, P. & Turton, J.A. (2001) Strain differences in haematological response to chloramphenicol succinate in mice: implications for toxicological research. Food Chem. Toxicol., 39, 375-383.
Flach, A.J. (1994) Systemic toxicity associated with topical ophthalmic medications. J. Fla. Med. Assoc., 81, 256-260.
Food Standards Agency (2002/2003) Results of FSA testing programme for chloramphenicol in honey. Tuesday, 19 February 2002 (http://www.foodstandards.gov.uk/multimedia/ webpage/resultshoneytest, accessed 18 May 2004).
Food Standards Agency of Ireland (2002/2003) Archive Alert Notifications (http://www.fsai.ie/alerts/archive/index.asp, accessed 18 May 2004).
Fraunfelder, F.T., Morgan, R.L. & Yunis, A.A. (1993) Blood dyscrasias and topical ophthalmic chloramphenicol. Am. J. Ophthamol., 115, 812-813.
Friedman, Y., Weismann, Y., Avidar, Y. & Bogin, E. (1998) The toxic effects of monensin and chloramphenicol on laying turkey breeder hens. Avian Pathol., 27, 205-208.
Fries, G.F., Marrow, G.S. & Snow, P.A. (1982) Soil ingestion by dairy cattle. J. Dairy Sci., 65, 611-618.
Gantverg, A., Shishani, I. & Hoffman, M. (2003) Determination of chloramphenicol in animal tissues and urine liquid chromatography-tandem mass spectrometry versus gas chromatography-mass spectrometry. Anal. Chim. Acta, 483, 125-135.
Garbarino, J.R., Bednar, A.J., Rutherford, D.W., Beyer, R.S. & Wershaw, R.L. (2003) Environmental fate of roxarsone in poultry litter. I. Degradation of roxarsone during composting. Environ. Sci. Technol., 37, 1509-1514.
Gassner, B. & Wuethrich, A. (1994) Pharmacokinetic and toxicology aspects of the medication of beef-type calves with an oral formulation of chloramphenicol palmitate. J. Vet. Pharmacol. Ther., 17, 279-283.
Gerber, S.L., Cantor, L.B. & Brater, D.C. (1990) Systemic drug interactions with topical glaucoma medications. Surv. Ophthalmol., 35, 205-218.
George, F.J. & Hanna, C. (1977) Ocular penetration of chloramphenicol. Effects of route of administration. Arch. Ophthalmol., 95, 879-882.
Gottlieb, D. & Siminoff, P. (1952) The production and role of antibiotics in soil II. Chloromycetin. Phytopathology, 42, 91-97.
Gottlieb, D. (1976) The production and role of antibiotics in soil. J. Antibiot. (Tokyo), 29, 987-1000.
Greenwood, D. (2000) Antimicrobial Chemotherapy, 4th Ed., New York: Oxford University Press.
Gross, F., Lewis, E.A., Piraee, M., van Pee, K.H., Vining, L.C. & White, R.L. (2002) Isolation of 3’-O-acetylchloramphenicol: a possible intermediate in chloramphenicol biosynthesis. Bioorg. Med. Chem. Lett., 12, 283-286.
Gude, T., Preiss, A. & Rubach, K. (1995) Determination of chloramphenicol in muscle, liver, kidney and urine of pigs by means of immunoaffinity chromatography and gas chromatography with electron capture detection. J. Chromatogr. B, 673, 197-204.
Guyer, P.Q. (1996) Livestock water quality (Report No. G79-467-A). University of Nebraska, Institute of Agriculture and Natural Resources (http://www.ianr.unl.edu/pubs/beef/ g467.htm#as, accessed 18 May 2004).
Haderlein, S.B. & Schwarzenbach, R.P. (1993) Environ. Sci. Technol., 27, 316-326.
Haller, M.Y., Muller, S.R., McArdell, C.S., Alder, A.C. & Suter, M.J. (2002) Quantification of veterinary antibiotics (sulfonamides and trimethoprim) in animal manure by liquid chromatography-mass spectrometry. J. Chromatogr. A, 952, 111-120.
Hamscher, G., Sczesny, S., Hoper, H. & Nau, H. (2002) Determination of persistent tetracycline residues in soil fertilized with liquid manure by high-performance liquid chromatography with electrospray ionization tandem mass spectrometry. Anal. Chem., 74, 1509-1518.
Hamscher, G., Pawelzick, H.T., Sczesny, S., Nau, H. & Hartung, J. (2003) Antibiotics in dust originating from a pig-fattening farm: a new source of health hazard for farmers? Environ. Health. Perspect., 111, 1590-1594 (also available at http://ehpnet1.niehs.nih.gov/ members/2003/6288/6288.pdf, accessed 18 May 2004).
Hallas, L.E. & Alexander, M. (1983) Microbial transformation of nitroaromatic compounds in sewage effluent. Appl. Environ. Microbiol., 45, 1234-1241.
Hanna, C., Massey, J.Y., Hendrickson, R.O., Williamson, J., Jones, E.M. & Wilson, P. (1978) Ocular penetration of topical chloramphenicol in humans. Arch. Ophthalmol., 96, 1258-1261.
Hansen, L.H., Ferrari, B., Sorensen, A.H., Veal, D. & Sorensen, S.J. (2001) Detection of oxytetracycline production by Streptomyces rimosus in soil microcosms by combining whole-cell biosensors and flow cytometry. Appl. Environ. Microbiol., 67, 239-244.
He, J., Magarvey, N., Piraee, M. & Vining, L.C. (2001) The gene cluster for chloramphenicol biosynthesis in Streptomyces venezuelae ISP5230 includes novel shikimate pathway homologues and a monomodular nonribosomal peptide synthetase gene. Microbiology, 147, 2817-2829.
Hendrix, D.V.H., Ward, D.A & Barnhill, M.A. (2001) Effects of antibiotics on morphologic characteristics and migration of canine corneal epithelial cells in tissue culture. Am. J. Vet. Res., 62, 1664-1669.
Hirsch, R., Ternes, T., Haberer, K. & Kratz, K.L. (1999) Occurrence of antibiotics in the aquatic environment. Sci. Total Environ., 225, 109-118.
Holt, D.E. (1995) The identification and characterisation of chloramphenicol-aldehyde, a new human metabolite of chloramphenicol. Eur. J. DrugMetab. Pharmacokinet., 20, 35-42.
Holt, D.E. & Bajoria, R. (1999) The role of nitro-reduction and nitric oxide in the toxicity of chloramphenicol. Hum. Exp. Toxicol. 18, 111-118.
Holt, D.E., Ryder, T.A., Fairbairn, A., Hurley, R. & Harvey, D. (1997) The myelotoxicity of chloramphenicol: in vitro and in vivo studies. I. In vitro effects on cells in culture. Hum. Exp. Toxicol., 16, 570-576.
Holt, D.E., Andrews, C.M., Payne, J.P., Williams, T.C. & Turton, J.A. (1998) The myelotoxicity of chloramphenicol: in vitro and in vivo studies. II. In vivo myelotoxicity in the B6C3F1 mouse. Hum. Exp. Toxicol., 17, 8-17.
Hong Samnang (1999) Duckweed versus ground soya beans as supplement for scavenging native chickens in an integrated farming system. Livestock Research for Rural Development 11(1) (http://www.cipav.org.co/lrrd/, accessed 18 May 2004).
Hazardous Substances Databank (2003) Data generated from the Hazardous Substances Databank, a database of the National Library of Medicine's TOXNET system on October 31 2003 (http://toxnet.nlm.nih.gov, accessed 18 May 2004).
IARC (1990) IARC Monographs on the evaluation of carcinogenic risk of chemicals to humans, Vol. 50, Chloramphenicol. Lyon: IARCPress, pp. 169-193.
Impens, S., Reybroeck, W., Vercammen, J., Courtheyn, D., Ooghe, S., De Wasch, K., Smedts, W. & De Brabander, H. (2003) Screening and confirmation of chloramphenicol in shrimp tissue using ELISA in combination with GC-MS2 and LC-MS2. Anal. Chim. Acta, 483, 153-163.
Indiaagronet (2003) Farm yard manure (definition) (http://www.indiaagronet.com/ indiaagronet/Manuers_fertilizers/contents/farm_yard_manure.htm, accessed 18 May 2004).
Ingerslev, F. & Halling-Sorensen, B. (2001) Biodegradability of metronidazole, olaquindox, and tylosin and formation of tylosin degradation products in aerobic soil-manure slurries. Ecotoxicol. Environ. Saf., 48, 311-320.
Ismail, S. & Morton, D. (1985) Comparative bioavailability of chloramphenicol ophthalmic product. Honours Project Publications, University of Zimbabwe, Department of Pharmacy (http://uzweb.uz.ac.zw/medicine/pharmacy/pubs/1985.html, accessed 18 May 2004).
Jiang, Y.L. (2000) The use of chemicals in aquaculture in the People's Republic of China. In: Arthur, J.R., Lavilla-Pitogo, C.R. & Subasinghe, R.P., eds, (2000) Use of Chemicals in Aquaculture in Asia. Proceedings of the Meeting on the Use of Chemicals in Aquaculture in Asia, 20-22 May 1996, Tigbauan, Iloilo, Philippines. Southeast Asian Fisheries Development Center, Aquaculture Department, Tigbauan, Iloilo, Philippines, pp. 141-153.
Johnson, R.I. (1994) Effects of antibacterial agents on transformation of fatty acids in sediment from a marine fish farm site. Sci. Total Environ., 152, 143-152.
Joint FAO/WHO Food Standards Programme (2003). Report of the Fourteenth Session of the Codex Committee on Residues of Veterinary Drugs in Foods (ALINORM 03/31 A) (ftp://ftp.fao.org/docrep/fao/meeting/006/y9015e.pdf, accessed 18 May 2004).
Jones, A.L., Keighley, J.E., Gold, W. & Good, A.M. (1996) Eye drops—the hidden poison. Scott. Med. J., 41, 110-112.
Kadavy, D.R., Hornby, J.M., Haverkost, T. & Nickerson, K.W. (2000) Natural antibiotic resistance of bacteria isolated from larvae of the oil fly, Helaeomyia petrolei. Appl. Environ. Microbiol., 66, 4615-4619.
Kaila, T., Korte, J.M. & Saari, K.M. (1999) Systemic bioavailability of ocularly applied 1% atropine eyedrops. Acta Ophthalmol. Scand., 77, 193-196.
Keukens, H.J., Aerts, M.M.L. & Traag, W.A. (1992) Analytical strategy for the regulatory control of residues of chloramphenicol in meat: preliminary studies in milk. J. AOAC Int., 75, 245-256.
Kijak, P.J. (1994) Confirmation of chloramphenicol residues in bovine milk by gas chromatography/mass spectometry. J. AOAC Int., 77, 34-40.
Khieu Borin, Sim Chou & Preston, T.R. (2000) Fresh water fish silage as protein source for growing-fattening pigs fed sugar palm juice. Livestock Research for Rural Development, 12(1) (http://www.cipav.org.co/lrrd/lrrd12/1/bori121 .htm, accessed 18 May 2004).
Kolpin, D.W., Furlong, E.T., Meyer, M.T., Thurman, E.M., Zaugg, S.D., Barber, L.B. & Buxton, H.T. (2002) Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999-2000: a national reconnaissance. Environ. Sci. Technol., 36, 1202-1211.
Kong, C.T., Holt, D.E., Ma, S.K., Lie, A.K.W. & Chan, L.C. (1999) Effects of antioxidants and a caspase inhibitor on chloramphenicol-induced toxicity of human bone marrow and HL-60 cells. Hum. Exp. Toxicol., 19, 530-510.
Korte, J.M., Kaila, T. & Saari, K.M. (2002) Systemic bioavailability and cardiopulmonary effects of 0.5% timolol eyedrops. Graefes Arch. Clin. Exp. Ophthalmol., 240, 430-435.
Kroker, R. (1994) [Chloramphenicol (CP)]. In: Löscher, W., Ungemach, F.R., and Kroker, R., eds, Grundlagen der Pharmakotherapie bei Haus- und Nutztieren, 2nd Ed. (in German), Berlin and Hamburg: Paul Parey, p. 226.
Kucers, A., Crowe, S.M., Grayson, M.L. & Hoy, J.F. (1997) Chloramphenicol and thiamphenicol. In: The Use of Antibiotics. Oxford: Butterworth-Heinemann, pp. 548-599.
Kumar, V., Schoenwald, R.D., Chien, D.S., Packer, A.J. & Choi, W.W. (1985) Systemic absorption and cardiovascular effects of phenylephrine eyedrops. Am. J. Ophthalmol., 99, 180-184.
Laerum, E., Fiskaadal, H.J., Erdal, J.E. & Loberg, R.M. (1994) [Chloramphenicol eyedrops in acute bacterial conjunctivitis. A comparison of two dosage regimes in general practice] [Abstract only, article in Norwegian] Tidsskr Nor Laegeforen, 114, 671-673.
Lafarge-Frayssinet, C., Robbana-Barnat, S., Frayssinet, C., Toucas, L. & Decloitre, F. (1994) Cytotoxicity and DNA damaging potency chloramphenicol and six metabolites: a new evaluation in human lymphocytes and Raji cells. Mutat. Res., 320, 207-215.
Lai, H.T., Liu, S.H. & Chien, Y.H.J. (1995) Transformation of chloramphenicol and oxytetracycline in aquaculture pond sediments. Environ. Sci. Health, A30, 1897-1923.
Lai, H.T., Chien, Y.H. & Liu, S.M. (1997) Transformation of chloramphenicol and oxytetracycline in brackish water sediment under various salinities and aerobic and anaerobic conditions. J. Fish. Soc. Taiwan, 24, 49-61.
Lallai, A., Mura, G. & Onnis, N. (2002). The effects of certain antibiotics on biogas production in the anaerobic digestion of pig waste slurry. Bioresour. Technol., 82, 205-208.
Larsson, L.I. (1998) Techniques for measuring aqueous humor inflow and outflow. Glaucoma World, 12 (http://www.glaucomaworld.net/english/012/e012a04t.html, accessed 18 May 2004). The original paper cited by Larsson is: Brubaker, R.F. (1991) Flow of aqueous humor in humans. The Friedenwald lecture. Invest. Ophthalmol. Vis. Sci., 32, 3145-3166.
LEAD Virtual Center (2004a) Livestock, Environment and Development (LEAD) Initiative. Livestock and Environment Toolbox (http://lead.virtualcenter.org/en/dec/toolbox/ homepage.htm, accessed 18 May 2004). Note: this toolbox provides generic advice on various aspects of the interactions between livestock and the environment).
LEAD Virtual Center (2004b) Livestock, Environment and Development (LEAD) Initiative. Livestock and Environment Toolbox (http://lead.virtualcenter.org/en/dec/toolbox/ Index.htm, accessed 18 May 2004).
Lee, Y.C., Simamora, P., Pinsuwan, S. & Yalkowsky, S.H. (2002) Review on the systemic delivery of insulin via the ocular route. Int. J. Pharm., 233, 1-18.
Legator, M. & Gottlieb, D. (1953) The dynamics of chloramphenicol synthesis. Antibiot. Chemother., 3, 809-817.
Le Ha Chau (1998a) Biodigester effluent versus manure from pigs or cattle as fertilizer for production of cassava foliage (Manihot esculenta). Livestock Research for Rural Development, 10(3) (http://www.cipav.org.co/lrrd/, accessed 18 May 2004).
Le Ha Chau (1998b) Biodigester effluent versus manure, from pigs or cattle, as fertilizer for duckweed (Lemna spp.). Livestock Research for Rural Development, 10(3) (http://www.cipav.org.co/lrrd/, accessed 18 May 2004).
Le Thi Men, Huynh Huu Chi, Ngo Vi Nghia, Nguyen Thi Kim Khang, Ogle B. & Preston, T.R. (2003) Utilization of catfish oil in diets based on dried cassava root waste for crossbred fattening pigs in the Mekong delta of Vietnam. Livestock Research for Rural Development, 15(4) (http://www.cipav.org.co/lrrd, accessed 18 May 2004).
Lewis, E.A., Adamek, T.L., Vining, L.C. & White, R.L. (2003) Metabolites of a blocked chloramphenicol producer. J. Nat. Prod., 66, 62-66.
Li, T.L., Chung-Wang, Y.J. & Shih, Y.C. (2001) Determination and confirmation of chloramphenicol residues in swine muscle and liver. J. Food Science, 67, 21-28.
Liao, I.C., Guo, J.J. & Su, M.S. (2000) The use of chemicals in aquaculture in Taiwan, Province of China. In: Arthur, J.R., Lavilla-Pitogo, C.R. & Subasinghe, R.P., eds. (2000) Use of Chemicals in Aquaculture in Asia. Proceedings of the Meeting on the Use of Chemicals in Aquaculture in Asia, 20-22 May 1996; Tigbauan, Iloilo, Philippines. Southeast Asian Fisheries Development Centre, Aquaculture Department, Tigbauan, Iloilo, Philippines. pp. 193-206.
Loke, M.L., Ingerslev, F., Halling-Sorensen, B. & Tjornelund, J. (2000) Stability of tylosin A in manure containing test systems determined by high-performance liquid chromatography. Chemosphere, 40, 759-765.
Ly, J. (2002) The effect of methionine on digestion indices and N balance of young Mong Cai pigs fed high levels of ensiled cassava leaves. Livestock Research for Rural Development, 14(6) (http://www.cipav.org.co/lrrd/, accessed 18 May 2004).
Ly, J. & Pok Samkol (2001) The nutritive value of ensiled cassava leaves for young Mong Cai pigs fed high levels of protein. Livestock Research for Rural Development, 13(4) (http://www.cipav.org.co/lrrd/, accessed 18 May 2004).
Ly, J., Chhay Ty, Chiev Phiny & Preston, T.R. (2001) Some aspects of the nutritive value of leaf meals of Trichanthera gigantea and Morus alba for Mong Cai pigs. Livestock Research for Rural Development, 13(3). (http://www.cipav.org.co/lrrd/, accessed 18 May 2004).
Ly, J., Hean Pheap, Keo Saeth & Pok Samkol (2002) The effect of DL-methionine supplementation on digestibility and performance traits of growing pigs fed broken rice and water spinach (Ipomoea aquatica). Livestock Research for Rural Development, 14(5) (http://www.cipav.org.co/lrrd/, accessed 18 May 2004).
Maluf, E. (2002) Aplastic anemia in Brazil: incidence and risk factors. Am. J. Hematol., 71, 268-274.
Malléa, M., Mahamoud, A., Chevalier, J., Alibert-Franco, S., Brouant, P., Barbe, J. & Pagès, J.M. (2003). Alkylaminoquinolines inhibit the bacterial antibiotic efflux pump in multidrug-resistant clinical isolates. Biochem J., 376, 801-805.
Matsuhashi, T., Liu, X., Nishizawa, Y., Usukura, J., Wozniak, M. & Wakabayashi, T. (1996) Mechanism of the formation of megamitochondria in the mouse liver induced by chloramphenicol. Toxicol. Lett., 86, 47-54.
Matsumoto, J., Natsuaki, M., Kitano, Y. & Kitagawa, K. (1998) Two cases of skin ulcer due to chloromycetin (R) cream. Hifu, 40, 576-581.
Mottier, P., Parisod, V., Gremaud, E., Guy, P.A., Stadler, R.H. (2003) Determination of the antibiotic chloramphenicol in meat and seafood products by liquid chromatography—electrospray ionisation tandem mass spectrometry. J. Chromatogr. A., 994, 75-84.
Munns, R.K., Holland, D.C., Roybal, J.E., Storey, J.M., Long, A.R., Stehly, G.R. & Plakas, S.M. (1994) Gas chromatographic determination of chloramphenicol residues in shrimp: Interlaboratory study. J. AOAC Int., 77, 596-601.
Nagata, T. & Saeki, M. (1992) Simultaneous determination of thiamphenicol, florfenicol, and chloramphenicol residues in muscles of animals and cultured fish by liquid chromatography. J. Liq. Chromatogr., 15, 2045-2056.
Nakano, H., Matsuhashi, Y., Takeuchi, T. & Umezawa, H. (1977) Distribution of chloramphenicol acetyltransferase and chloramphenicol-3-acetate esterase among Streptomyces and Corynebacterium. J. Antibiot. (Tokyo), 30, 76-82.
National Academy of Sciences (1994) Subcommittee on Poultry Nutrition, National Research Council, National Academy of Sciences. Nutrient Requirements of Poultry, Ninth Revised Edition, pp. 19-34 (http://books.nap.edu/books/0309048923/html/19.html#pagetop, accessed 18 May 2004).
Neuhaus, B.K., Hurlbut, J.A. & Hammack, W. (2002) LC/MS/MS analysis of chloramphenicol in shrimp. U.S. FDA—Laboratory Information Bulletin No. 4290, 18(9) (http://www.cfsan.fda.gov/~acrobat/lib4290.pdf, accessed 18 May 2004).
Ngoc Son, C.P. (2002) Chloramphenicol analysis in shrimp. CASE study of GC and HPLC-MS validation at the Centre of Analytical Services and Experimentation (CASE) of Ho Chi Minh City. Viet Nam: CASE (http://www.hcmuaf.edu.vn/CdHoiThao/ELISA-7-2002/CLORAMSHRIMP.pdf, accessed 18 May 2004).
Nguyen Thi Thuy & Ly, J. (2002) A short-term study of growth and digestibility indices in Mong Cai pigs fed rubber seed meal. Livestock Research for Rural Development, 14(2) (http://www.cipav.org.co/lrrd, accessed 18 May 2004).
Nguyen Dang Vang & Le Viet Ly (2000) A review on poultry production in Vietnam. The National Institute of Animal Husbandry (NIAH) (http://www.vcn.vnn.vn/sp_pape/ spec_00_9_1.htm, accessed 18 May 2004).
Nguyen Thi Loc & Le Khac Huy (2003) Effects of DL-methionine supplementation levels in ensiled cassava root-based diets on the performance and economic efficiency of F1 (large white × Mong Cai) fattening pigs. In: Preston, R. & Ogle, B., eds, Proceedings of Final National Seminar-Workshop on Sustainable Livestock Production on Local Feed Resources, HUAF-SAREC, Hue City, 25-28 March, 2003 (http://www.mekarn.org/ sarec03/lochue.htm, accessed 18 May 2004).
Nguyen Van Lai (1998) On-farm comparison of Mong Cai and large white pigs fed ensiled cassava root, rice bran and duckweed. Livestock Research for Rural Development, 10(3) (http://www.cipav.org.co/lrrd, accessed 18 May 2004).
Obaldo, L.G. (2001) Manufacturing different feed sizes and growth of three size classes of Pacific white shrimp, Litopenaeus vannamei. World Aquaculture Society, January 2001 (http://www.oceanicinstitute.org/research/abstrfeeds_differentfeedsizes.html, accessed 18 May 2004).
Parfitt, K. (1999) Martindale: The Complete Drug Reference, 32nd Ed., London: Pharmaceutical Press, pp. 182-184.
Pathak, S.C., Ghosh, S.K. & Palanisamy, K. (2000). The Use of chemicals in aquaculture in India. In: Arthur, J.R., Lavilla-Pitogo, C.R. & Subasinghe, R.P., eds. (2000) Use of Chemicals in Aquaculture in Asia. Proceedings of the Meeting on the Use of Chemicals in Aquaculture in Asia, 20-22 May 1996; Tigbauan, Iloilo, Philippines. Southeast Asian Fisheries Development Center, Aquaculture Department, Tigbauan, Iloilo, Philippines, pp. 87-112.
Parker, R. & Shaw, I.C. (1988) Determination of chloramphenicol in tissues—problems with in vitro metabolism. Analyst, 113, 1875-1876.
Peer, D.J., Ball, R. & Young, L. (2001) Practical diets for pigs. Ministry of Agriculture and Food, Government of Ontario (http://www.gov.on.ca/OMAFRA/english/livestock/ swine/facts/01-013.htm#top, accessed 18 May 2004)
Perez, N., Gutierrez, R., Noa, M., Diaz, G., Luna, H., Escobar, I. & Munive, Z. (2002) Liquid chromatographic determination of multiple sulfonamides, nitrofurans, and chloramphenicol residues in pasteurized milk. J. AOAC Int., 85, 20-24.
Petersen, A., Andersen, J.S., Kaewmak, T., Somsiri, T. & Dalsgaard, A. (2002). Impact of integrated fish farming on antimicrobial resistance in a pond environment. Appl. Environ. Microbiol., 68, 6036-6042
Peterson, G. School of Pharmacy, University of Tasmania. CSA 311 Clinical Pharmacokinetics, lecture notes online. (http://www.healthsci.utas.edu.au/pharmacy/kinetics/ index.htm, accessed 18 May 2004).
Pfenning, A.P., Madson, M.R., Roybal, J.E., Turnipseed, S.B., Gonzales, S.A., Hurlbut, J.A. & Salmon, G.D. (1998) Simultaneous determination of chloramphenicol, florfenicol, and thiamphenicol residues in milk by gas chromatography with electron capture detection. J. AOAC Int., 81, 714-720.
Pfenning, A., Turnipseed, S., Roybal, J., Burns, C., Madson, M., Storey, J. & Lee, R. (2002a) Confirmation of multiple phenicol residues in shrimp by electrospray LC-MS. U.S. FDA— Laboratory Information Bulletin No. 4284, 18(5) (http://www.cfsan.fda.gov/~frf/ lib4284.html, accessed 18 May 2004).
Pfenning, A., Turnipseed, S., Roybal, J., Burns, C., Madson, M., Storey, J. & Lee, R. (2002b) Confirmation of chloramphenicol residues in crawfish by electrospray LC/MS. U.S. FDA— Laboratory Information Bulletin No. 4289, 18(8) (http://www.cfsan.fda.gov/~frf/ lib4289.html, accessed 18 May 2004).
Pfenning, A., Turnipseed, S., Roybal, J., Madson, M., Lee, R. & Storey, J. (2003) Confirmation of chloramphenicol residue in crab by electrospray LC/MS. U.S. FDA—Laboratory Information Bulletin No. 4294, 19(1) (http://www.cfsan.fda.gov/~frf/lib4294.html, accessed 18 May 2004).
Phillips, M. (2000) The use of chemicals in carp and shrimp aquaculture in Bangladesh, Cambodia, Lao PDR, Nepal, Pakistan, Sri Lanka and Viet Nam. In: Arthur, J.R., Lavilla-Pitogo, C.R. & Subasinghe, R.P., eds. (2000) Use of Chemicals in Aquaculture in Asia. Proceedings of the Meeting on the Use of Chemicals in Aquaculture in Asia, 20-22 May 1996; Tigbauan, Iloilo, Philippines. Southeast Asian Fisheries Development Center, Aquaculture Department, Tigbauan, Iloilo, Philippines, pp. 75-86.
Philpott, N.J., Scopes, J., Marsh, J.C.W., Gordon-Smith, E.C. & Gibson, F.M. (1995) Increased apoptosis in aplastic anaemia bone marrow progenitor cells: possible pathophysiologic significance. Exp. Hematol., 23, 1642-1648.
Pich Sophin & Preston, T.R. (2001) Effect of processing pig manure in a biodigester as fertilizer input for ponds growing fish in polyculture. Livestock Research for Rural Development, 13(6) (http://www.cipav.org.co/lrrd/, accessed 18 May 2004).
Plumb, D.C. (2002) Veterinary Drug Handbook, 4th Ed., Ames: Iowa State Press, pp. 166-169.
Posyniak, A., Zmudzki, J. & Niedzielska, J. (2003) Evaluation of sample preparation for control of chloramphenicol residues in porcine tissues by enzyme-linked immunosorbent assay and liquid chromatography. Anal. Chim. Acta, 483, 307-311.
Prak Kea, Preston, T.R. & Ly, J. (2003) Feed intake, digestibility and N retention of a diet of water spinach supplemented with palm oil and/or broken rice and dried fish for growing pigs. Livestock Research for Rural Development, 15(8) (http://www.cipav.org.co/lrrd/, accessed 18 May 2004).
Primavera, J.H. (1994) Shrimp farming in the Asia-Pacific: environmental and trade issues and regional cooperation. Nautilus Institute Workshop on Trade and Environment in Asia-Pacific: Prospects for Regional Cooperation 23-25 September 1994, East-West Center, Honolulu (http://www.nautilus.org/papers/enviro/trade/shrimp.html, accessed 18 May 2004).
Price, C.A., Lynch, E.M., Bowie, B.A. & Newman, D.J. (1981). Isolation and identification of chloramphenicol from the moon snail, Lunatia heros. J. Antibiot. (Tokyo), 34, 118-119.
Rabolle, M. & Spliid, N.H. (2000) Sorption and mobility of metronidazole, olaquindox, oxytetracycline and tylosin in soil. Chemosphere, 40, 715-722.
Rappeport, J.M. & Bunn, H.P. (1994) Bone marrow failure: aplastic anaemia and other primary bone marrow disorders. In: Isselbacher, K.J. et al., eds, Harrison's Principles of Internal Medicine. New York: McGraw-Hill, pp. 1754-1756.
Reybroeck, W. (2003) Residues of antibiotics and sulphonamides in honey on the Belgian market. APIACTA, 38, 23-30.
Robbana-Barnat, S., Decloitre, F., Frayssinet, C., Seigneurin, J.M., Toucas, L. & Lafarge-Frayssinet, C. (1997) Use of human lymphoblastoid cells to detect the toxic effect of chloramphenicol and metabolites possibly involved in aplastic anemia in man. Drug Chem. Toxicol., 20, 239-253.
Rogers, J.M. & Kavlock, R.J. (2001) Developmental toxicology. In: Klaassen, C.D. ed., Casarett & Doull's Toxicology: The Basic Science of Poisons, 6th Ed., New York: McGraw-Hill, p. 358.
Rowland, M. & Tozer, T.M. (1995) Clinical Pharmakokinetics, Concepts and Applications, 3rd Ed., Baltimore: Williams & Wilkins.
Runsey, T.S., Miller, R.W. & Dinius, D.A. (1977) Residue content of beef feedlot manure after feeding diethylstilbestrol, chlortetracycline and Ronnel and the use of stirofos to reduce population of fly larvae in feedlot manure. Arch. Environ. Contam. Toxicol., 6, 203-212.
Rupp, H.S., Stuart, J.S. & Hurlbut, J.A. (2003) LC/MS/MS analysis of chloramphenicol in crab meat. U.S. FDA—Laboratory Information Bulletin No. 430, 19(4) (http://www.cfsan.fda.gov/~acrobat/lib4302.pdf, accessed 18 May 2004).
Saettone, M.F. (2002) Progress and problems in ophthalmic drug delivery. Business briefing. Pharmatech., 167-170.
Saettone, M.F. (2003) New trends in ocular drug delivery. Archivos de la Sociedad Espanola de oftalmologia, 2 (dated February 2003, editorial).
Sah, S.P., Raj, G.A., Rijal, S. & Karki, P. (1999) Clinico-haematological and management profile of aplastic anaemia—a first series of 18 cases from Nepal. Singapore Med. J., 40, 451-454.
Salminen, L. (1990) Review: systemic absorption of topically applied ocular drugs in humans. J. Ocul. Pharmacol., 6, 243-249.
Sande, M.A. & Mandell, G.L. (1993) Antimicrobial agents. In: Gilman, A.G., Rall, T.W., Nies, A.S. & Taylor, P., eds, Goodman and Gillman's The Pharmacological Basis Of Therapeutics, 8th Ed., New York: McGraw-Hill Inc., pp. 1125-1134.
Sanders, P., Guillot, P., Dagorn, M. & Delmas, J.M. (1991) Liquid chromatographic determination of chloramphenicol in calf tissues: studies of stability in muscle, kidney and liver. J. AOAC Int., 74, 483-486.
Sanders, P. & Laurentie, M. (2004) Personal communication to the FAO JECFA Secretariat.
Sanz, J.L., Rodriguez, N. & Amils, R. (1996) The action of antibiotics on the anaerobic digestion process. Appl. Microbiol. Biotechnol., 46, 587-592.
Sasaki, H., Yamamura, K., Mukai, T., Nishida, K., Nakamura, J., Nakashima, M. & Ichikawa, M. (2000) Pharmacokinetic prediction of the ocular absorption of an instilled drug with ophthalmic viscous vehicle. Biol. Pharm. Bull., 23, 1352-1356.
Sechena, R., Liao, S., Lorenzana, R., Nakano, C., Polissar, N. & Fenske, R. (2003) Asian American and Pacific Islander seafood consumption—a community-based study in King County, Washington. J. Expo. Anal. Environ. Epidemiol., 13, 256-266.
Schlusener, M.P., Bester, K. & Spiteller, M. (2003) Determination of antibiotics such as macrolides, ionophores and tiamulin in liquid manure by HPLC-MS/MS. Anal. Bioanal. Chem., 375, 942-947.
Shariff, M., Nagaraj, G., Chua, F.H.C. & Wang, Y.G. (2000) The use of chemicals in aquaculture in Malaysia and Singapore. In: Arthur, J.R., Lavilla-Pitogo, C.R. & Subasinghe, R.P., eds. (2000) Use of Chemicals in Aquaculture in Asia. Proceedings of the Meeting on the Use of Chemicals in Aquaculture in Asia, 20-22 May 1996; Tigbauan, Iloilo, Philippines. Southeast Asian Fisheries Development Center, Aquaculture Department, Tigbauan, Iloilo, Philippines, pp. 127-140.
Shiuey, Y. & Eisenberg, M.J. (1996) Cardiovascular effects of commonly used ophthalmic medications. Clin. Cardiol., 19, 5-8.
Sirbat, D., Marchal-Heussler, L., Hoffman, M. & Maincent, P. (2000) Moyens d'amélioration de la biodisponibilité pour la voie topique [Ways to improve ocular bioavailability for topical applications]. J. Fr. Ophtalmol., 23, 505-509.
Sørensen, A.H., Hansen, L.H., Johannesen, E. & Sørensen, S.J. (2003). Conjugative plasmid conferring resistance to olaquindox. Antimicrob. Agents Chemother., 47, 798-799.
Somjetlertcharoen, A. (2002) Chloramphenicol in aquaculture. AAHRI Newsletter, 11(1) (http://www.fisheries.go.th/aahri/Health_new/AAHRI/AAHRI/Topics/Newsletter/art54.htm, accessed 18 May 2004).
Stolker, A.A.M., Niesing, W., Hogendoorn, E.A., Versteegh, J.F.M., Fuchs, R. & Brinkman, U.A.T. (2003) Liquid chromatography with triple-quadrupole or quadrupole-time of flight mass spectrometry for screening and confirmation of residues of pharmaceuticals in water. Anal. Bioanal. Chem., 378, 955-963.
Storey, J., Pfenning, A., Turnipseed, S., Nandrea, G., Lee, R., Burns, C. & Madson, M. (2003) Determination of chloramphenicol residues in shrimp and crab tissues by electrospray triple quadrupole LC/MS/MS. U.S. FDA—Laboratory Information Bulletin No. 4306, 19(6) (http://www.cfsan.fda.gov/~frf/lib4306.html, accessed 18 May 2004).
Stuart, J.S., Rupp, H.S. & Hurlbut, J.A. (2003) LC/MS/MS Analysis of chloramphenicol in crawfish meat. U.S. FDA—Laboratory Information Bulletin No. 4303, 19(4) (http://www.cfsan.fda.gov/~frf/lib4303.html, accessed 18 May 2004).
Supriyadi, H. & Rukyani, A. (2000) The use of chemicals in aquaculture in Indonesia. In: Arthur, J.R., Lavilla-Pitogo, C.R. & Subasinghe, R.P., eds. (2000) Use of Chemicals in Aquaculture in Asia. Proceedings of the Meeting on the Use of Chemicals in Aquaculture in Asia, 20-22 May 1996; Tigbauan, Iloilo, Philippines. Southeast Asian Fisheries Development Center, Aquaculture Department, Tigbauan, Iloilo, Philippines, pp. 113-118.
Tamura, A., Takeda, I., Naruto, S. & Yoshimura, Y. (1971) Chloramphenicol from Streptosporangium viridogriseum var. kofuense. J. Antibiot. (Tokyo), 24, 270.
Tean, B., Sath, K., Samkol, P. & Ly, J. (2002a) Utilization by pigs of diets containing Cambodian rubber seed meal. Livestock Research for Rural Development, 14(1) (http://www.cipav.org.co/lrrd/, accessed 18 May 2004).
Tean, B., Ly, J. & Preston, T.R. (2002b) A study of N utilization in young Mong Cai and Large White pigs fed water spinach and graded levels of rubber seed cake. Livestock Research for Rural Development, 14(3) (http://www.cipav.org.co/lrrd/, accessed 18 May 2004).
The pig site (2003) This website contains extracts of the book, Carr, J., Garth Pig Stockmanship Standards, Iowa State University, 5M Enterprises Limited (http:// www.thepigsite.com/stockstds/Default.asp?display=17, accessed 18 May 2004).
Thornton, I. & Abrahams, P. (1983) Soil ingestion—a major pathway of heavy metals into livestock grazing contaminated land. Sci. Total Environ., 28, 287-294.
Thy, S. & Preston, T.R. (2003) Effluent from biodigesters with different retention times for primary production and feed of Tilapia (Oreochromis niloticus). Livestock Research for RuralDevelopment 15(9) (http://www.cipav.org.co/lrrd/, accessed 18 May 2004).
Tolls, J. (2001) Sorption of veterinary pharmaceuticals in soils: a review. Environ. Sci. Technol., 35, 3397-3406.
Toris, C.B., Yablonski, M.E., Wang, Y.L. & Camras, C.B. (1999) Aqueous humor dynamics in the aging human eye. Am. J. Ophthalmol., 127, 407-412.
Tran Thi Mai Phuong, Nguyen Van Thien & Tran Long (2003) Study on the productivities and meat quality of AC chicken (black-bone chicken) in Vietnam. The National Institute of Animal Husbandry (NIAH) (http://www.vcn .vnn.vn/sp_pape/sp_paper2003/ spaper_5_12_2003_5.htm, accessed 18 May 2004).
Trope, G.E., Lawrence, J.R., Hind, V.M. & Bunney, J. (1979) Systemic absorption of topically applied chloramphenicol eyedrops. Br. J. Ophthalmol., 63, 690-691.
Turnipseed, S., Burns, C., Storey, J., Lee, R. & Pfenning, A. (2002) Confirmation of multiple phenicol residues in honey by electrospray LC/MS. U.S. FDA—Laboratory Information Bulletin No. 4281, 18(5) (http://www.cfsan.fda.gov/~frf/lib4281.html, accessed 18 May 2004).
Turton, J.A., Yallop, D., Andrews, C.M., Fagg, R., York, M. & Williams, T.C. (1999) Haemotoxicity of chloramphenicol succinate in the CD-1 mouse and Wistar Hanover rat. Hum. Exp. Toxicol., 18, 566-576.
Turton, J.A., Andrews, C.M., Havard, A.C. & Williams, T.C. (2002a) Studies on the haemotoxicity of chloramphenicol succinate in the Dunkin Hartley guinea pig. Int. J. Exp. Pathol., 83, 225-238.
Turton, J.A., Havard, A.C., Robinson, S., Holt, D.E., Andrews, C.M., Fagg, R. & Williams, T.C. (2000) An assessment of chloramphenicol and thiamphenicol in the induction of aplastic anaemia in the BALB/c mouse. Food Chem. Toxicol., 38, 925-938.
Turton, J.A., Andrews, C.M., Havard, A.C., Robinson, S., York, M., Williams, T.C. & Gibson, F.M. (2002b) Haemotoxicity of thiamphenicol in the BALB/c mouse and Wistar Hanover rat. Food Chem. Toxicol., 40, 1849-1861.
Urtti, A. & Salminen, L. (1993) Minimizing systemic absorption of topically administered ophthalmic drugs. Surv. Ophthalmol., 37, 435-456.
U.S. Department of Agriculture (2003) Determination and confirmation of chloramphenicol in bovine muscle. SOP No. CLG—CAM.02 dated April 25, 2003, U.S. Department of Agriculture, Food Safety and Inspection Service, Office of Public Health and Science (http://www.fsis.usda.gov/Frame/FrameRedirect.asp?main=/ophs/clg/clg-cam. 02.pdf, accessed 18 May 2004).
Vainio-Jylha, E., Vuori, M.L., Pyykko, K. & Huupponen, R. (2001) Plasma concentration of topically applied betaxolol in elderly glaucoma patients. J. Ocul. Pharmacol. Ther., 17, 207-213.
van Ginkel, L.A., van Rossum, H.J., Zoontjes, P.W., van Blitterswijk, H., Ellen, G., van der Heeft, E., de Jong, A.P.J. M. & Zomer, G. (1990) Development and validation of a gas chromatographic-mass spectrometric procedure for the identification and quantification of residues of chloramphenicol. Anal. Chim. Acta, 237, 61-69.
van der Heeft, E., de Jong, A.P., van Ginkel, L.A., van Rossum, H.J. & Zomer, G. (1991) Conformation and quantification of chloramphenicol in cow's urine, muscle and eggs by electron capture negative ion chemical ionization gas chromatography/mass spectrometry. Biol. Mass Spectrom., 20, 763-770.
Van de Riet, J.M., Potter, R.A., Christie-Fougere, M. & Burns, B.G. (1992) Simultaneous determination of residues of chloramphenicol, thiamphenicol, florfenicol, and florfenicol amine in farmed aquatic species by liquid chromatography/mass spectrometry. J. AOAC Int., 86, 510-514.
van de Water, C., Tebbal, D. & Haagsma, N. (1989) Monoclonal antibody-mediated cleanup procedure for the high-performance liquid chromatographic analysis of chloramphenicol in milk and eggs. J. Chromatogr., 478, 205-215.
Versteegh, J.F.M., Stolker, A.A.M., Niesing, W. & Muller, J.J.A. (2003) Geneesmiddelen in drinkwater en drinkwaterbronnen; Resultaten van het meetprogramma 2002. RIVM rapport 703719004/2003 (http://www.rivm.nl/bibliotheek/rapporten/703719004.html, accessed 18 May 2004).
Verzegnassi, L., Royer, D., Mottier, P. & Stadler, R.H. (2003) Analysis of chloramphenicol in honeys of different geographical origin by liquid chromatography coupled to electrospray ionization tandem mass spectrometry. Food Addit. Contam., 20, 335-342.
Vincke, M.M.J (1991) Integrated farming of fish and livestock: present status and future development. In: T.K. Mukherjee, T.K., editor-in-chief; Phang Siew Moi, Jothi M. Panandam & Yap Siaw Yang, eds, Integrated livestock—fish production systems. Proceedings of the FAO/IPT Workshop on Integrated Livestock-Fish Production Systems, 16-20 December 1991, Institute of Advanced Studies, University of Malaya, Kuala Lumpur, Malaysia. University of Malaya, Kuala Lumpur (http://www.fao.org/docrep/004/ ac155E/AC155E01.htm, accessed 18 May 2004).
Voedsel en Waren Autoriteit (2004) Communication of the Voedsel en Waren Autoriteit of the Netherlands to the FAO JECFA Secretariat.
Walker, S., Diaper, C.J., Bowman, R., Sweeney, G., Seal, D.V. & Kirkness, C.M. (1998). Lack of evidence for systemic toxicity following topical chloramphenicol use. Eye, 12, 875-879.
Wiholm, B.E., Kelly, J.P., Kaufman, D., Issaragrisil, S., Levy, M., Anderson, T. & Shapiro, S. (1998) Relationship of aplastic anaemia to use of chloramphenicol eye drops in two international case-control studies. BMJ, 316, 666-666.
Wijegoonawardena, P.K.M. & Siriwardena, P.P.G.S.N. (2000). The use of chemotherapeutic agents in shrimp hatcheries in Sri Lanka. In: Arthur, J.R., Lavilla-Pitogo, C.R. & Subasinghe, R.P., eds. (2000) Use of Chemicals in Aquaculture in Asia. Proceedings of the Meeting on the Use of Chemicals in Aquaculture in Asia, 20-22 May 1996; Tigbauan, Iloilo, Philippines. Southeast Asian Fisheries Development Center, Aquaculture Department, Tigbauan, Iloilo, Philippines, pp. 185-192.
Wu, C., Clift, P., Fry, C.H. & Henry, J.A. (1996) Membrane action of chloramphenicol measured by protozoan motility inhibition. Arch. Toxicol., 70, 850-853.
Yallop, D., Andrews, W., Holt, D., York, M., Fagg, R., Williams, T. & Turton, J. (1998) Chloramphenicol is haematotoxic at high dose levels in the mouse but not in the rat. Hum. Exp. Toxicol., 17, 57.
Yamada, N. & Hiraki, S. (1995) [Pharmacokinetics of subconjunctivally injected drugs in the anterior segment of rabbit eyes] (In Japanese). Nippon Ganka Gakkai Zasshi, 99, 262-270 (http://www.ncbi. nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=7732915&dopt=Abstract, accessed 18 May 2004).
Young, N.S. & Alter, B.P. (1994) Epidemiology of acquired aplastic anemia. In: Aplastic anemia acquired and inherited. Philadelphia: W.S. Saunders, pp. 24-31.
Young, N.S. & Maciejewski, J. (1997) The pathophysiology of acquired aplastic anemia. N. Engl. J. Med., 336, 1365-1372.
Young, N.S. (2002) Acquired aplastic anemia. Ann. Intern. Med., 136, 534-546.
Summary of data from feeding experiments
Table 1. Feeding experiments with pigs in south-east Asia
|
Breed or production class |
Feeding scheme |
Initial body weight (kg) |
Final body weight(kg) |
Duration of treatment (days) |
Dry matter intake (g/kg LW per day) |
Average daily body-weight gain (kg) |
Reference |
|
|
F1 Large white x Mong Cai |
Rice bran, ensiled cassava roots, maize, fish meal, soya bean meal, supplemented with 0, 0.1, 0.2 or 0.3% DL-methionine (on the basis of dry matter) |
20 |
88.2 |
120 |
<31 |
0.568 |
Nguyen Thi Loc & Le Khac Huy (2003) |
|
|
19.9 |
93.3 |
0.611 |
||||||
|
19.6 |
97.5 |
0.649 |
||||||
|
20 |
95.4 |
0.628 |
||||||
|
Different crossbreeds |
Rice bran, fresh water fish silage (FWFS), sugar palm juice, and water hyacinth (Eichhornia crassipes) |
14-35 |
150 (5 months) |
0.405 |
Khieu Borin et al. (2000) |
|||
|
(0.325-0.476) |
||||||||
|
Crossbred pigs (local x large white) |
Rice bran, maize, fish meal, groundnut cake, soya bean meal plus soya bean foliage silage substituted for 0-38% of total protein in the diet |
0 |
Bui Van Chinh etal. (1993) |
|||||
|
25 |
20.1 |
55.6 |
90 |
– |
0.395 |
|||
|
38 |
20.1 |
56.6 |
90 |
– |
0.406 |
|||
|
0 |
20.2 |
59.4 |
90 |
– |
0.435 |
|||
|
25 |
48.5 |
84.7 |
60 |
– |
0.601 |
|||
|
38 (% of total protein) |
48.5 |
84.5 |
60 |
– |
0.600 |
|||
|
48.7 |
85.3 |
60 |
– |
0.610 |
||||
|
Castrated crossbred male piglets |
Water spinach, broken rice, dried fish plus palm oil |
0 |
10 |
33.6 |
– |
Prak Kea et al. (2003) |
||
|
5 |
9.5 |
31.6 |
– |
|||||
|
10 |
(8-10) |
31.8 |
– |
|||||
|
15 (% of dry matter) |
30.1 |
– |
||||||
|
Castrated male Mong Cai |
Basal diet of cassava bran, wheat bran and fishmeal or basal diet with 30% of protein replaced by leaf meal |
14.5 |
23.3 |
4 x 10 |
35 |
0.22 |
Ly et al. (2001) |
|
|
Castrated Mong Cai male pigs |
Sugar palm syrup and dry fish, substituted by ensiled cassava |
0 |
12.3 |
36.6 |
0.14 |
Ly & Pok Samkol (2001) |
||
|
Large white castrated male and female pigs |
Wheat bran (progressively substituted by 0, 10, 20 and 30% ground whole RSM), dried fish |
13.6 |
26.4 |
42 |
51.0 |
0.315 |
Tean et al. (2002a) |
|
|
10.6 |
27.3 |
61.7 |
0.341 |
|||||
|
10.1 |
24.3 |
61.0 |
0.364 |
|||||
|
8.5 |
20.6 |
64.0 |
0.310 |
|||||
|
Large white castrated male and female pigs (1:1) |
Broken rice, water spinach, supplemented with DL-methionine |
0 |
29.2 |
47.8 |
56 |
32.5 |
0.332 |
Ly et al. (2002) |
|
0.25 |
26.7 |
46.8 |
37.6 |
0.360 |
||||
|
0.50 |
28.0 |
53.8 |
32.3 |
0.442 |
||||
|
0.75 (% of dry matter) |
25.1 |
50.9 |
36.3 |
0.459 |
||||
|
Castrated Mong Cai male pigs |
Sugar palm syrup, dried fresh water fish and ensiled cassava leaves (73.5% of dry matter) supplemented with DL-methionine |
0.5 |
19.2 |
Approximately 30 |
0.143 ± |
Ly (2002) |
||
|
1.0 (% of dry matter) |
22.5 |
0.025 |
||||||
|
Landrace x large white |
Broken rice, rice bran, soybean, extracted, soybean, dehulled, plus |
|||||||
|
1. Cassava root meal, |
57.8 |
92.4 |
26.5-30.7 |
0.685 |
Le Thi Men et al. (2003) |
|||
|
2. Cassava root waste, |
57.3 |
85.5 |
0.598 |
|||||
|
3. Cassava root waste, catfish oil 5% |
57.6 |
94.0 |
0.715 |
|||||
|
4. Cassava root waste, catfish oil 10% |
57.3 |
89.3 |
0.634 |
|||||
|
Mong Cai and Large white female pigs |
Cassava bran, rice bran, dried fresh water fish and whole rubber seed replacing part of the dry matter |
0 |
22.9 |
Mong Cai, 0.273; large white, 0.533; control, 0.377; rubber seed, 0.429 |
Nguyen Thi Thuy & Ly (2002) |
|||
|
Mong Cai piglets and large white pigs |
Rice bran, plus ensiled cassava roots or duck weed (at libitum) |
Ensiled cassava roots |
6.38 |
22.6 |
0.200 |
Nguyen Van Lai (1998) |
||
|
Duck weed |
11.3 |
20.7 |
0.087 |
|||||
|
Castrate male pigs, either Mong Cai or large white |
Fresh, chopped water spinach plus partially defatted rubber seeds (rubber seed cake) replacing part of the daily feed intake .. . |
0 |
30 |
15.2 |
Tean et al. (2002b) |
|||
|
20 |
17.9 |
|||||||
|
30 |
20.5 |
|||||||
|
40 (% replaced) |
23.9 |
RSM, rubber seeed meal; FWFS, fresh water fish silage; LW, live weight.
Table 2. Feeding experiments with chickens in south-east Asia
|
Breed or production class |
Feeding scheme |
Initial age of the animals (days) |
Initial body weight (g) |
Final body weight (g) |
Duration of treatment (days) |
Daily feed intake (g/bird) |
Average daily body-weight gain (g) |
Reference |
|||||
|
Native chickens of both sexes scavenging in two different places, p1 and p2 |
Supplements offered in the evening: 50g broken rice, 50 g duckweed 50 g broken rice, 50 g ground soya beans 50 g of broken rice |
p1 |
p2 |
p1 |
p2 |
70 |
p1a |
p2a |
p1 |
p2 |
Hong Samnang (1999) |
||
|
607 |
542 |
1567 |
1424 |
40.5 |
45.65 |
13.7 |
12.6 |
||||||
|
692 |
523 |
1814 |
1344 |
58.5 |
63.00 |
16.0 |
11.7 |
||||||
|
551 |
499 |
1255 |
241 |
38.7 |
43.2 |
10.1 |
10.6 |
||||||
|
Egg type layers |
Starter ration |
42 |
27.6 |
Farooq et al. (2002) |
|||||||||
|
Grower ration |
84 |
49.3 |
|||||||||||
|
Layer ration |
241 |
131.5 |
|||||||||||
|
Growing and scavenging coloured feather chickens |
Diets containing RSM. The highest two doses of rubber seed (RS) had negative effects on survival rates |
Group (% |
1 |
33.8 |
128 |
10.8** |
Duong Duy Dong (2003) |
||||||
|
(RSM) |
34.0 |
10.6 |
|||||||||||
|
RS0 (0) |
34.4 |
9.7 |
|||||||||||
|
RS10(10) |
34.4 |
11.9 |
|||||||||||
|
RS20 (20) |
33.8 |
11.6 |
|||||||||||
|
RS30 (30) |
34.1 |
9.3 |
|||||||||||
|
RS40 (40) |
|||||||||||||
|
RS50 (50) |
|||||||||||||
|
Tam Hoang chickens |
Diets of RSM |
0 |
|||||||||||
|
5 |
1 |
550 |
1700 |
1-28 |
0-70 |
2.91b |
3.55b |
17.9 |
27.5 |
||||
|
10 (% RSM) |
570 |
1829 |
2.87 |
3.56 |
18.7 |
28.2 |
|||||||
|
Luong Phuong chickens |
Basal diet based on maize and concentrate (30:70 ratio; 16% crude protein in the dry matter) offered ad libitum or restricted to 60, 70, 80 or 90% of the ad libitum intake. On all the diets the birds had free access to fresh duck weed. |
30 |
554 |
1747 |
3.00 |
3.66 |
18.1 |
27.4 |
Du Thanh Hang (2003) |
||||
|
1326C |
30 |
70.1d |
19.2e |
||||||||||
|
1380 |
81.6 |
23.9 |
|||||||||||
|
1395 |
92.9 |
29.56 |
|||||||||||
|
1456 |
99.9 |
31.96 |
|||||||||||
|
1452 |
106.5 |
33.64 |
|||||||||||
RSM, rubber seeed meal.
* Estimated from the data assuming 90% dry matter for broken rice and soya beans and 5% for duckweed.
b Feed conversion rate (kg of feed/kg gain).
c Carcass weight.
d Dry matter intake.
e The authors report the following relationship between dry matter intake (y) and live-weight gain (y): y= 0.415x - 9.75.
Model for the calculation of the concentrations in muscle of chloramphenicol ingested with soil.
|
— |
Chloramphenicol is administered orally (ingestion with soil). The cumulative intake is calculated as follows: |
|
• |
Intake of soil is 2% of the intake of dry matter. |
|
• |
Intake of dry matter is related to body weight (for pigs) or average daily body-weight gain (for chickens). The choice of body weight (pigs) and average daily body-weight gain (chickens) was dictated by the nature of available empirical data. From the available empirical data, linear relationships were derived that were valid for a certain period of growth of the animals. |
|
— |
The site of "measurement" of chloramphenicol within the body is the plasma. |
|
— |
Concentrations of chloramphenicol in plasma and in muscle are assumed to be equal (this is strongly supported by experimental data). |
|
— |
Absorption is the process by which unchanged drug proceeds from the site of administration to the site of "measurement". |
|
— |
The combined processes of distribution and elimination are called disposition, whereby elimination means the loss of the drug from the site of "measurement". |
|
— |
The rate of change of drug in the body is equal to the sum of the rates of absorption and the rate of elimination. |
|
— |
For simplification of the model, the following assumptions were made: |
|
The drug is administered as a single daily dose (the amount contained in the daily dose increases as a function of average daily body weight gain and increased intake of feed). |
|
|
The rates of absorption and distribution are short compared with the rate of elimination. |
|
|
All drug in the body is expressed as equivalents of parent chloramphenicol. |
|
|
— |
Calculations are performed for a range of assumed elimination half-lives (t1/2). |
|
— |
The amount A remaining in the body at time tafter ingestion of the amount A0 is calculated using a mono-exponential term: |
A = A0 × e-kt
|
Only in one example is the sum of two exponential terms used. |
|
|
The relationship between the half-life and the elimination rate constant k is: |
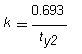
|
— |
The amount remaining in the body just after each of three successive ingestions is calculated according to the following scheme: |
|
Time |
Amount remaining: |
||
|
from 1st ingestion |
from 2nd ingestion |
from 3rd ingestion |
|
|
0 |
A0,1 |
||
|
tau |
A0,1 × e-k tau |
A0,2 |
|
|
2tau |
A0,1 × e-k2 tau |
A0,2 × e-k tau |
A0,3 |
|
— |
The daily ingested amount is not constant, but increases as a function of increasing intake of feed during the growth phase. It is assumed that pigs grow from 20 to 75 kg of body weight within 119 days; and it is assumed that chickens grow from 150 to 1000 g of body weight within 75 days. If the ingested dose is expressed as ingested amount divided by body weight, then it is constant in the model for pigs, since feed intake is related to body weight in that model. However, in the model for chickens, feed intake is related to growth rate. Therefore, the dose decreases steadily. |
|
— |
The amount of the drug remaining in the body at the end of the growth phase is used to calculate the concentration of chloramphenicol in the plasma according to the formula: |
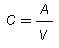
|
where C is the concentration, A is the amount remaining in the body and V is the volume of distribution. Values for V are taken from the literature. |
Alternatives:
The average plasma concentration at steady state after multiple doses can be calculated from (Rowland & Tozer, 1995):
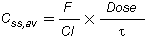
where F is the fraction absorbed (dimensionless), D is the daily dose (µg/kg bw), and Cl is the body clearance 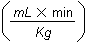
Values for Cl have to be taken from the literature.
Use of this model has been proposed by Sanders & Laurentie (2004).
Like the present monograph, this alternative model assumes:
Table 1. Daily oral dose necessary to maintain an average steady-state concentration of chloramphenicol of 1 µg/kg in muscle
|
Species |
Clearance |
Daily dosea |
Daily intake of feed (g) or water (ml) per kg bw |
Concentration (µg/g or µg/ml) |
||
|
Feed |
Water |
Feed |
Water |
|||
|
Pigs |
4.16 |
6 |
40 |
70 |
0.15 |
0.08 |
|
Poultry |
3.84 |
5.5 |
240 |
150 |
0.02 |
0.04 |
|
6.62 |
9.5 |
0.04 |
0.06 |
|||
a It is assumed that either feed or water is the only source of contamination.
The model gives the following results:
Using the selected model, it can be shown that the conditions described for pigs in Table 1, while producing an average steady-state concentration of chloramphenicol of 1 µg/kg, would at the same time cause a fluctuation at steady state of between approximately 0.06 µg/kg (minimum, shortly before the next dose) and 4.4 µg/kg (maximum, shortly after the last dose).
Clearance, volume of distribution and elimination half-life are linked through the equation:
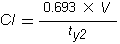
Using, for example in the model for pigs, a clearance value of 4.16 (ml/min × kg) and a volume of distribution of 1400 ml would correspond to a half-life of 233 min or 3.89 h.
FOOTNOTES:
See Also:
Toxicological Abbreviations
Chloramphenicol (WHO Food Additives Series 23)
Chloramphenicol (WHO Food Additives Series 33)
CHLORAMPHENICOL (JECFA Evaluation)
Chloramphenicol (IARC Summary & Evaluation, Volume 50, 1990)