
Pesticide residues in food 2001
First draft prepared by
A. Bartholomaeus
Chemicals and Non-prescription Medicines Branch
Therapeutic Goods Administration, Canberra ACT, Australia
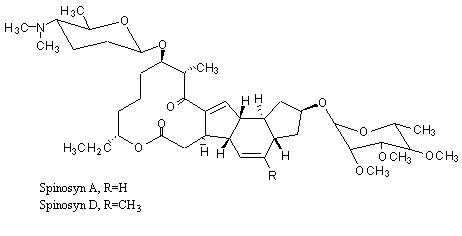
Spinosad is the ISO approved name for a mixture of compounds formed as a fermentation product of the soil organism Saccharopolyspora spinosa. The mixture comprises approximately 10 related chemicals, with proteinaceous, carbohydrate and inorganic salt compounds derived from the fermentation process. Two closely related compounds, spinosyn A and spinosyn D, in a ratio of approximately 6:1 or 7:1, represent about 88% of the composition of spinosad and are responsible for most of its insecticidal activity. Spinosyn A and spinosyn D differ only in respect to substitution of a hydrogen by a methyl group at a position that is not metabolically labile. The remainder of spinosad is made up of a number of closely related spinosyns, which differ in the location of other minor substitutions at various sites around the molecule (Figure 1).

Figure 1. Structure of spinosyn A and D
Spinosad, an insecticide which acts by causing rapid excitation of the insect nervous system, is a new compound and has not previously been evaluated by JMPR.
(a) Absorption, distribution and excretion
Groups of five Fischer 344 rats of each sex were given a single dose of [14C]spinosyn A (purity, > 96%) by gavage as a suspension in aqueous 0.5% methylcellulose, at a dose of 10 or 100 mg/kg bw. Other groups were given 14 daily doses of unlabelled spinosyn A by gavage at 10 mg/kg bw, followed on day 15 by a single dose of [14C]spinosyn A at 10 mg/kg bw. All animals were killed 7 days after the last dose. The plasma concentration of radiolabel was followed for 72 h in two groups of three rats of each sex given a single dose of [14C]spinosyn A by gavage at 10 or 100 mg/kg bw. The time to the maximal plasma concentration (Cmax) was 1 h in both males and females at the lower dose and 6 h in males and 12 h in females at the higher dose. The time to half the Cmax was 6 h in males and 12 h in females at the lower dose and 12 h in males and 24 h in females at the higher dose. The distribution of radiolabel in tissues was therefore assessed in groups of three animals, at 6 and 12 h in males and 2 and 24 h in females at 100 mg/kg bw, and at 1 and 6 h in males and 1 and 12 h in females at 10 mg/kg bw. Radiolabel was determined in the adrenals, blood, bone, brain, heart, duodenum, gastrointestinal tract (including contents), gonads, kidneys, liver, lung, mesenteric lymph nodes, perirenal fat, skeletal muscle, skin, spleen, thymus and thyroid and in the carcass. Urine, faeces and expired CO2 were also analysed for radiolabel. Tissues from animals given repeated doses were sampled 1 h after dosing and again at 6 h in males and 12 h in females. Biliary excretion through bile-duct cannulae was determined in three rats of each sex for 24 h after a single dose of [14C]spinosyn A by gavage at 10 or 100 mg/kg bw. The animals were killed 24 h after dosing, and radiolabel was determined in blood, skin and carcass and in collected urine, faeces and CO2. The study complied with the requirements of GLP and OECD guideline 417.
More than 90% of the radiolabelled dose was recovered in all groups. In animals killed 7 days after dosing, faecal excretion accounted for 85–88% of the administered dose in males and 81–82% in females, mostly within the first 24 h, while 6–10% of the dose was excreted in the urine. The radiolabel in tissues and the carcass accounted for < 3% of the dose. Faecal elimination was biphasic, the half-lives for the initial and terminal phases for males being 9 and 28 h at the lower dose, 14 and 25 h at the higher dose and 9 and 31 h after repeated doses, and those for females being 11 and 35 h at the lower dose, 29 and 42 h at the higher dose and 10 and 44 h after repeated doses.
All tissues sampled at the Cmax and one-half the Cmax contained measurable radiolabel, but there was at least a 10-fold reduction in concentration between the sacrifice at one-half the Cmax and the final sacrifice for all groups. An increase in concentration in some tissues between these sacrifice times indicated that the distribution in tissues was incomplete. At the lower dose, the highest tissue concentrations (0.3–0.6 µg/g, expressed as equivalents) after 168 h were found in the kidneys, liver (males only), lymph nodes and fat; at the higher dose, the highest concentrations (7–13 µg/g in males and 0.8–41 µg/g in females, as equivalents) after 168 h were found in the kidneys, lymph nodes, fat and thyroid. In animals given repeated doses, 0.2–0.3 µg/g remained in fat, kidney and lymph nodes (females only) after 168 h. Approximately equal amounts of radiolabel were recovered in the bile of male and female rats, with 41% (higher dose) and 44% (lower dose) in males and 41% (higher dose) and 38% (lower dose) in females. Male and female rats excreted similar amounts in the faeces (20–23%). The radiolabel recovered in bile, faeces, urine, exhaled CO2, skin and remaining carcass accounted for > 90% of that administered in both sexes at both the lower and higher dose. On the basis of the radiolabel detected in urine and bile, 70–80% of both doses was absorbed in both sexes. Given that no bile would be eliminated in the faeces of these animals, the approximately 20% of the dose recovered from faeces represents unabsorbed spinosyn A. The overall elimination rates were rapid, as 77–91% and 57–83% of the radiolabel in males and females, respectively, was recovered in excreta within 24 h and < 3% of the dose was found in tissues and carcass by 168 h after dosing. A proposed metabolic pathway for spinosyn A and D is provided in Figure 2 (Domoradzki et al., 1995).
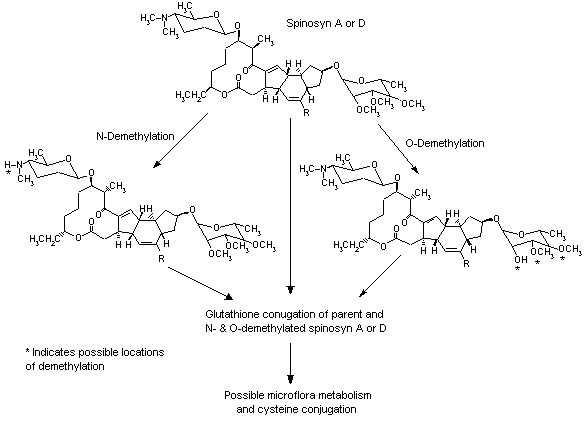
Figure 2. Proposed metabolic pathway for spinosyn A and D
The absorption, distribution and elimination of radiolabel were studied in groups of three female Fischer 344 rats given [14C]spinosyn A (purity, 97%) at 10 mg/kg bw per day as a suspension in aqueous Methocel for 3 or 7 days and killed 1 or 7 days or 1, 7, 14 or 21 days after the last dose. The study was conducted in accordance with the requirements of GLP and OECD guideline 417. The mean total recovery of radiolabel was 87–93%. Most of the recovered radiolabel was excreted in urine (4–6%) and faeces (74–87%) during the first 24 h after administration. The concentrations of residues in tissues declined rapidly on cessation of dosing and were generally below the limit of detection 21 days after the end of the 7-day dosing period. After both the 3-day and the 7-day dosing periods, the total concentration of tissue residues declined to < 0.5% of the administered dose within 24 h. Although the concentration in thyroid 24 h after cessation of dosing for 7 days (2 µg/g of tissue expressed as equivalents) was not as high as in many other tissues, it declined more slowly, remaining at approximately 0.5 µg/g of tissue at 7 days and 0.25 µg/g of tissue at 14 days after cessation of dosing, while the concentrations in other tissues were < 0.1 µg/g of tissue 14 days at this time (Thalaker, 1996).
Groups of five Fischer 344 rats of each sex were given a single dose of [14C]spinosyn D (purity, 95.6%) at 100 mg/kg bw by gavage as an aqueous suspension in 0.5% methylcellulose ether, and the concentration of radiolabel was determined in faeces and urine collected for 7 days, expired CO2 collected for 72 h and in the adrenals, bone, brain, duodenum, fat, gastrointestinal tract (plus contents), gonads, heart, kidneys, liver, lungs, skeletal muscle, spleen, skin, thymus, thyroid, mesenteric lymph nodes, blood and carcass at terminal sacrifice 168 h after dosing. The study was conducted in accordance with the requirements of GLP and OECD Guideline 417.
More than 90% of the administered dose was recovered. Faecal excretion accounted for 84% of the administered dose in males and 92% in females, with 68–73% recovered within the first 24 h. Urinary excretion accounted for 4.9% of the dose in males and 2.8% in females. Spinosyn D was eliminated via the faeces and urine in a biphasic manner, the mean half-lives being 6 h for the initial and 30 h for the terminal phases of faecal excretion and 5 and 33 h for urinary excretion. Less than 0.05% of the radiolabel was recovered as exhaled CO2. At terminal sacrifice, the radiolabel in tissues and carcass accounted for < 1% of the dose in both males and females, and the final cage wash accounted for < 3% of the dose. The highest concentrations of radiolabel were detected in fat, liver, kidneys and mesenteric lymph nodes (Mendrala et al., 1995a).
Three male Fischer 344 rats were given a single dose of [14C]spinosyn D (purity, 95.6%) by gavage in an aqueous suspension in 0.5% methylcellulose ether at approximately 100 mg/kg bw, and bile (from a bile-duct cannula), faeces, urine and CO2 were collected for 24 h. The rats were then killed, and the concentration of radiolabel remaining in the tissues and carcass was determined. The study was conducted in accordance with the requirements of GLP and OECD Guideline 417. No overt signs of toxicity were reported. About 95% of the radiolabel was recovered. The proportion of the administered dose recovered in excreta was 22–55% in faeces, 28–40% in bile, 3% in urine and < 1% in expired CO2 or in the cage wash. At sacrifice, the tissues and carcass contained about 21% of the radiolabelled dose. Given that no bile would be eliminated in the faeces of these animals, the authors argued that the approximately 34% of radiolabel excreted in faeces represented unabsorbed spinosyn D. Oral absorption was estimated to account for > 70% of the administered dose (Mendrala et al., 1995b).
[14C]Spinosyn A in dipropylene glycol was applied at a dose of 50 mg/kg bw (10 mg/cm2) to the shaved skin of Fischer 344 rats under an occlusive dressing for 24 h, and the animals were killed. The study was conducted in accordance with the requirements of GLP and the Pesticide Assessment Guidelines of the USA’s Environmental Protection Agency (Section 85-1). Approximately 94–95% of the administered dose was recovered, but 1% was absorbed, as determined from recovery in urine, faeces, carcass, tissues and expired air. A second group was similarly treated, but, after 24 h of treatment, the administration site was washed and then re-occluded with fresh bandaging for 120 h, at which time the animals were killed. About 2% of the applied dose was absorbed, and 93–94% was recovered (Domoradzki & Shabrang, 1996).
In the study of Domoradzki et al. (1995), described above, conjugation with glutathione, either directly or after O or N demethylation, was identified as the major path of metabolism. Glutathione and cysteine conjugates of spinosyn A and of O-demethylated spinosyn A were tentatively identified as metabolites in faeces, as well as unconjugated O-demethylated spinosyn A. The biliary and urinary metabolites were tentatively identified as glutathione conjugates of spinosyn A and of O-demethylated spinosyn A. The metabolites identified in liver were the glutathione conjugates of spinosyn A and of O-demethylated spinosyn A. Unconjugated O- and N-demethylated spinosyn A were identified in the liver, lung, kidney, thyroid and plasma.
In the study of Mendrala et al. (1995a), described above, about half of the administered dose was rapidly eliminated as unchanged spinosyn D and its cysteine conjugate in the faeces of both male and female rats. About 3% of the dose recovered in faeces was identified as an N-demethylated metabolite of spinosyn D and its glutathione conjugate. A cysteine conjugate of spinosyn D was tentatively identified as the main faecal metabolite, accounting for 9–12% of the radiolabelled dose. The authors proposed that the cysteine conjugate was formed from the metabolism of glutathione conjugates by gut microflora, which is a reasonable hypothesis. The glutathione conjugates of spinosyn D and of N-demethylated spinosyn D were tentatively identified in urine and faecal specimens from both sexes. A number of minor metabolites were isolated from urine and faeces but were not identified as there was insufficient material (< 3% of the dose).
In the study of Mendrala et al. (1995b), described above, metabolites were isolated and identified from pooled bile samples collected at intervals of 2–4 h and 6–8 h. The metabolism of spinosyn D followed essentially the same pathway as that of spinosyn A (Figure 2). Conjugation with glutathione, secretion into the bile and excretion in the faeces were identified as the main routes of metabolism and elimination. Less than 0.03% of the dose was eliminated as unchanged spinosyn D. The main biliary metabolite of spinosyn D was tentatively identified as its glutathione conjugate. Other metabolites identified were the glutathione conjugates of O- and N-demethylated spinosyn D. Several minor metabolites were isolated in the bile.
The acute toxicity of spinosad is summarized in Table 1.
Table 1. Acute toxicity of technical-grade spinosad and 1:1 spinosyn A and D in male and female animals
|
Species |
Strain |
Purity |
% A/% D |
Route |
Vehicle |
LD50/LC50 (mg/kg |
Reference |
|
Spinosad |
|||||||
|
Mouse |
CD-1 |
87.9 |
NR |
Oral |
0.5% aqueous methyl cellulose |
> 5000 |
Gilbert et al. (1994) |
|
Mouse |
CD-1 |
88.0 |
NR |
Oral |
0.5% aqueous methyl cellulosev |
Males, 6100 |
Gilbert & Yano (1996) |
|
Rat |
Fischer 344 |
87.9 |
NR |
Oral |
0.5% aqueous methyl cellulose |
Females, > 5000 |
Gilbertet al. (1994)a |
|
Rat |
Fischer 344 |
88.0 |
NR |
Oral |
0.5% aqueous methyl cellulose |
Males, > 7500 |
Gilbert & Yano (1996) |
|
Rat |
Fischer 344 |
78.2 |
NR |
Oral |
10% aqueous acacia |
> 2000 (no deaths) |
Wright et al. (1992a) |
|
Rat |
Fischer 344 |
88.0 |
|
Inhalationb |
|
> 5.2 |
Wolff et al. (1992) |
|
Rabbit |
New Zealand white |
87.9 |
|
Dermal |
Water |
> 2000 (no deaths) |
Gilbert (1994a) |
|
Rabbit |
New Zealand white |
88.2 |
NR |
Dermal |
Water |
> 5000 (no deaths) |
Gilbert (1994b) |
|
Rabbit |
New Zealand white |
87.9 |
NR |
Dermal |
|
>5000 (no deaths) |
Laska et al. (1992) |
|
Spinosyn A and D |
|||||||
|
Rat |
Fischer 344 |
96.3 |
46.1/50.2 |
Oral |
0.5% aqueous methyl cellulose |
Males, 4400 |
Stebbins & Brooks (1999a) |
|
Rabbits |
New Zealand white |
96.3 |
46.1/50.2 |
Dermal |
0.5% aqueous methyl cellulose |
> 5000 |
Stebbins & Brooks 1999b) |
|
|
All studies complied with the requirements of GLP and the respective OECD or USA Environmental Protection Agency guidelines. |
|
|
NR, not reported |
|
a |
In a previous study (details not provided), there were no deaths at 2000 mg/kg bw. In this study, four of five males died. By combining the results of this study with those of Wright et al. (1992a), Dow calculate an LD50 of 3700 mg/kg bw. |
|
b |
Median equivalent aerodynamic diameter, 2.96 µm, with a geometric standard deviation of 2.67 µm |
(ii) Ocular and dermal irritation and dermal sensitization
In a study conducted in accordance with GLP requirements and a modification of OECD guideline 404 (higher dose and duration of exposure), groups of five New Zealand white rabbits of each sex received an application of spinosad (purity, 88%) at 5000 mg/kg bw on intact skin under a semi-occlusive dressing for 24 h. After removal of the dressing and any residual test compound, the application site was assessed for signs of irritation at 1 h and then daily for 14 days. A gross pathological examination was conducted on all animals. There were no deaths, clinical signs of toxicity or dermal irritation during the study, and all animals gained weight normally. There were no gross pathological lesions attributed to treatment with spinosad (Laska et al., 1992).
In a study conducted in accordance with GLP requirements and OECD guideline 404, spinosad (purity, 87.9%) was applied to the intact skin of New Zealand white rabbits at a dose of 500 mg moistened with water under a semi-occlusive dressing for 4 h. No dermal irritation was seen (Gilbert, 1994b).
No deaths, clinical signs of toxicity or dermal irritation and no gross pathological lesions attributed to treatment were seen in groups of five New Zealand white rabbits of each sex that received an application of spinosad (purity, 88%) at 5000 mg/kg bw on intact skin under a semi-occlusive dressing for 24 h and followed up for 14 days. The study complied with GLP and was conducted in accordance with OECD guideline 404 (Laska et al., 1992).
In groups of five New Zealand white rabbits of each sex that received a dermal application of a mixture of 46.1% spinosyn A and 50.2% spinosyn D at 5000 mg/kg bw on intact skin under a semi-occlusive dressing for 4 h and followed up for 72 h, there were no deaths, clinical signs of toxicity or dermal irritation and no gross pathological lesions attributed to treatment. The study complied with GLP and was conducted in accordance with OECD guideline 404 (Stebbins & Brooks, 1999c).
Administration of 100 mg of spinosad (purity, 87.9%) into one eye of each of six New Zealand white rabbits produced conjunctival redness (average Draize score, 1.7) and chemosis (average score, 1.3) in all treated eyes within 1 h, which resolved in all but one animal (redness with a score of 1) by 24 h and in the remaining animal by 48 h. Spinosad is a slight eye irritant. The study complied with GLP and was conducted in accordance with OECD guideline 405 (Gilbert 1994c).
Administration of 100 mg of a 50:50 mixture of 46.1% spinosyn A and 50.2% spinosyn D to one eye of each of three New Zealand white rabbits produced slight conjunctival redness (average Draize score, 1), slight chemosis (average score, 1) and slight discharge (average score, 1.3) in all treated eyes at 1 h, which resolved in all but one animal (redness and chemosis with scores of 1) by 24 h and in the remaining animal by 48 h. The test substance was a slight eye irritant. The study complied with GLP and was conducted in accordance with OECD guideline 405 (Stebbins & Brooks, 1999d).
No evidence of delayed contact hypersensitivity was seen in Hartley albino guinea-pigs treated with with spinosad (purity, 87.9%) by the Buehler method, with induction and challenge doses of 400 mg. The sensitivity of the method was confirmed in a positive control group. The study complied with GLP and was conducted in accordance with OECD guideline 406 (Gilbert, 1994d).
In a study of maximization with spinosad (purity, 88%) in female Hartley guinea–pigs, 0.1 ml of 0.5% spinosad and 0.1 ml of a 1% emulsion of spinosad in Freund complete adjuvant were injected subcutaneously at two sites on opposite sides of a shaved area of the scapula region on test day 1 for induction. Sodium lauryl sulfate (10% in vaseline) was applied to the test area on day 6, and, on day 7, 0.2 ml of spinosad was applied to the test area and covered with an occlusive dressing for 48 h. After 3 weeks, 0.1 ml of spinosad was applied to a shaved area of the abdominal lateral region and maintained under an occlusive dressing for 24 h. There was no evidence of skin sensitization. A concurrent positive control group treated with dinitrochlorobenzene gave appropriate responses. The study complied with GLP and was conducted in accordance with OECD guideline 406 (Shibata, 1996).
In a study of skin sensitization with the Buehler method in male Hartley guinea-pigs, a 50:50 mixture of 46.1% spinosyn A and 50.2% spinosyn D was applied at a dose of 0.4 g moistened with 0.5% methylcellulose solution three times 1 week apart for induction. The animals were challenged with the same preparation. There was no evidence of skin sensitization. The study complied with GLP and was conducted in accordance with OECD guideline 406 (Stebbins & Brooks, 1999e).
(b) Short-term studies of toxicity
Mice
In a study conducted in accordance with the principles of GLP and OECD guideline 408, groups of 10 CD-1 mice of each sex were given diets containing spinosad (purity, 77.6%) at a concentration of 0, 50, 150, 450 or 1200 ppm for 3 months, equal to 0, 6, 18, 57 and 110 mg/kg bw per day for males and 0, 8, 23, 72 and 140 mg/kg bw per day for females. Toxicity was assessed by determining clinical signs at least daily, body weight at least weekly, haematological end-points (clotting parameters, erythrocyte, total and differential leukocyte and platelet counts, erythrocyte volume fraction, mean haemoglobin concentration, mean corpuscular volume and mean corpuscular haemoglobin concentration) and clinical chemical parameters (alanine and aspartate aminotransferase activity and albumin, globulin, total protein, bilirubin, cholesterol, triglyceride, glucose, creatinine and urea nitrogen concentrations) before sacrifice, gross and histopathological appearance (adrenals, heart, prostate, aorta, Harderian gland, lachrymal gland, bone, kidneys, skin, bone marrow, brain, liver, lungs, spleen, lymph nodes, epididymides, muscle, gonads, eyes, thymus, peripheral nerve, thyroid, gall-bladder, trachea, urinary bladder, uterus, femur and joint, pituitary, tongue, parathyroid, mammary glands and spinal cord) and the weights of the kidneys, liver, heart, spleen, ovaries, testes and brain. Samples of liver, kidney and lung from three animals of each sex per group were examined ultrastructurally.
Three males and two females at 1200 ppm died before day 44 due to hepatic necrosis, and the remaining animals at this dose lost approximately 25% of their initial body weight, were cachetic and had mild to moderate microcytic, hypochromic anaemia and marked neutrophilic leukocytosis. Their neutrophils had cytoplasmic basophilia and nuclear hypersegmentation, indicating degeneration and prolonged circulation. Increased alkaline phosphatase activity (by three times that of controls in males and 2.5 times in females), alanine aminotransferase activity (by 15 times in males and 11 times in females), aspartate aminotransferase activity (by eight times in males and five times in females) and globulin concentration (by 12% in males and 41% in females) were seen, with lower albumin concentrations (by 30% in the two sexes). Males had decreased glucose (by 50%), bilirubin (by 33%), cholesterol (by 25%) and triglyceride concentrations (by 55%), and females had decreased glucose (by 60%) and bilirubin concentrations (by 35%). The groups at 1200 ppm were discontinued on day 44, and all animals were necropsied.
The clinical signs in animals at 50 and 150 ppm were similar to those seen in controls, but at higher concentrations rough or oily coats, thinness, rapid respiration, hypoactivity, hypothermia, ventral and perineal soiling, alopecia and swollen tail were observed commonly. Transient but statistically significant reductions in body weight and body-weight gain occurred in males at 450 ppm, the terminal body weight being 92% that of controls. Also at this dietary concentration, statistically significant decreases were seen in haemoglobin (–22%), packed cell volume (–11%), albumin concentration (–10%) and lymphocyte count (–33%) and increases in alkaline phosphatase activity (+36%) in males; a statistically significant increase in neutrophil count (+72%) in females; and a statistically significant increase in aspartate aminotransferase activity (twice control value in both sexes) and statistically significant decreases in mean corpuscular volume (–8% in males, –10% in females) and mean corpuscular haemoglobin (–10% in males, –11% in females). Significantly increased absolute and relative weights of the liver were seen in both sexes (0.04% in controls, 0.05% at 450 ppm) and relative weight of the spleen in females at 450 ppm (23 mg/10 g in controls, 37 mg/10 g at 450 ppm). Treatment-related gross alterations were seen only in animals at 1200 ppm and consisted of altered appearance of the liver (eight males, nine females, described only as ‘whole tissue alteration’) and kidneys (six males, three females, ‘whole tissue alteration’), adhesions (two males, five females) and lesions of the liver (nine mice of each sex), enlarged spleen (four males, eight females) and enlarged lymph nodes (10 mice of each sex). The term ‘whole tissue alteration’ was used to group a number of gross pathological observations affecting an entire tissue, including alterations in colour, texture and appearance.
Gross, microscopic and ultrastructural examination revealed extensive effects in a wide range of tissues, most notably vacuolation. Histological alterations observed only at 1200 ppm consisted of: acute necrotizing and chronic inflammation of the renal capsule, acute capsular inflammation and severe multifocal necrosis of the liver, granular vascular leukocytosis and vacuolation of cardiac myocytes, intramural histiocytes and macrophages, acute diffuse interstitial inflammation of the lung, acute diffuse inflammation of the spleen, acute inflammation and severe necrosis of the lymph node, atrophy of the thymus, lymphoid vacuolation of the ileum and vacuolation of the pituitary. The incidences of other histopathological lesions occurring at concentrations other than that terminated prematurely are summarized in Table 2. Electron microscopy of tissues from three animals of each sex per group revealed an increased intensity (but not incidence) of cytoplasmic lamellar inclusion bodies, from minimal in controls, to moderate in the liver and lungs and moderate–severe in the kidneys of mice at 450 ppm.
Table 2. Histological alterations in mice given spinosad in the diet for 90 days (males/females)
|
Lesion and intensity |
Concentration in diet (ppm) |
||||
|
|
0 |
50 |
150 |
450 |
1200 |
|
Kidney |
|||||
|
Multifocal cortical tubular cyst: Slight to moderate |
0/0 |
0/0 |
0/0 |
0/1 |
4/0 |
|
Cortical multifocal tubular regeneration: Slight to moderate |
0/0 |
0/0 |
0/0 |
7/1 |
2/0 |
|
Cortical tubular vacuolation: Minimal to marked |
0/0 |
0/0 |
1/0 |
10/2 |
10/10 |
|
Liver |
|||||
|
Centrilobular cytomegaly: Slight |
0/0 |
0/0 |
1/0 |
6/0 |
2/0 |
|
Focal/multifocal granuloma: Minimal to slight |
0/0 |
0/0 |
0/0 |
0/4 |
0/0 |
|
Acute multifocal inflammation: Slight to moderate |
0/0 |
0/0 |
0/0 |
0/1 |
9/10 |
|
Centrilobular hepatocellular vacuolation: Minimal to slight |
0/0 |
0/0 |
0/0 |
8/6 |
0/0 |
|
Diffuse hepatocellular vacuolation: Minimal to slight |
0/0 |
0/0 |
0/1 |
0/3 |
0/0 |
|
Moderate to marked |
0/0 |
0/0 |
0/0 |
0/0 |
10/10 |
|
Vacuolation, Kupffer cell: Minimal. |
0/0 |
0/0 |
0/0 |
0/4 |
0/0 |
|
Lung |
|||||
|
Multifocal alveolar macrophages: Minimal to moderate |
0/0 |
0/0 |
0/0 |
10/8 |
10/9 |
|
Spleen |
|||||
|
Lymphoid vacuolar change: Minimal to slight |
0/0 |
0/0 |
4/0 |
8/7 |
4/4 |
|
Lymphoid vacuolar change: Moderate |
0/0 |
0/0 |
0/0 |
1/0 |
6/5 |
|
Haematopoiesis: Moderate |
0/0 |
0/0 |
0/0 |
2/0 |
9/8 |
|
Multifocal lymphocytic necrosis: Slight |
0/0 |
0/0 |
0/0 |
10/7 |
6/5 |
|
Lymph node |
|||||
|
Lymphoid vacuolar change: Slight to moderate |
0/0 |
0/0 |
0/2 |
7/7 |
5/6 |
|
Histiocytosis: Slight to moderate |
0/0 |
0/0 |
1/0 |
7/6 |
8/6 |
|
Multifocal lymphocytic necrosis: Slight to moderate |
0/0 |
0/0 |
0/2 |
7/7 |
3/5 |
|
Thymus |
|||||
|
Lymphoid vacuolar change: Slight to moderate |
0/0 |
0/0 |
0/0 |
3/5 |
0/0 |
|
Multifocal lymphocytic necrosis: Slight to moderate |
0/0 |
0/0 |
0/0 |
3/5 |
0/0 |
|
Pancreas |
|||||
|
Diffuse acinar atrophy: Slight |
0/0 |
0/0 |
0/0 |
2/0 |
5/0 |
|
Diffuse acinar vacuolation: Slight to moderate |
0/0 |
0/0 |
0/0 |
10/7 |
9/5 |
|
Tongue |
|||||
|
Chronic multifocal inflammation: Slight |
0/0 |
0/0 |
0/0 |
2/2 |
3/5 |
|
Multifocal regeneration, muscular layer: Minimal to slight |
0/0 |
0/0 |
0/0 |
2/4 |
1/1 |
|
Multifocal vacuolation: Slight |
0/0 |
0/0 |
0/0 |
1/2 |
6/6 |
|
Stomach |
|||||
|
Multifocal glandular dilation: Slight to minimal |
2/1 |
3/1 |
5/3 |
3/4 |
0/0 |
|
Multifocal glandular dilation: Moderate to marked |
0/0 |
0/0 |
0/0 |
7/5 |
10/10 |
|
Histiocytosis: Minimal to moderate |
0/0 |
0/0 |
0/0 |
4/2 |
7/6 |
|
Mucosal hyaline droplets: Slight to moderate |
0/0 |
0/0 |
0/0 |
5/4 |
4/1 |
|
Acute diffuse mucosal inflammation: Minimal to moderate |
0/0 |
0/0 |
0/0 |
4/4 |
7/6 |
|
Chronic diffuse mucosal inflammation: Slight |
0/0 |
0/0 |
0/0 |
5/3 |
1/2 |
|
Multifocal mucosal mineralisation:Minimal to slight |
0/0 |
0/0 |
4/3 |
6/8 |
6/8 |
|
Multifocal mucosal necrosis: Minimal to slight |
0/0 |
0/0 |
1/0 |
4/6 |
3/4 |
|
Ovary |
|||||
|
Vacuolation: Moderate to marked |
0 |
0 |
1 |
9 |
9 |
|
Oviduct |
|||||
|
Mucosal vacuolation: Slight to marked |
0 |
0 |
0 |
7 |
8 |
|
Uterus |
|||||
|
Submucosal histiocytosis: Minimal to moderate |
0 |
0 |
0 |
5 |
7 |
|
Mucosal vacuolation: Slight to marked |
0 |
0 |
0 |
9 |
8 |
|
Cervix |
|||||
|
Vacuolation: Slight to marked |
0 |
0 |
0 |
4 |
5 |
|
Vagina |
|||||
|
Vacuolation: Slight to marked |
0 |
0 |
0 |
3 |
7 |
|
Epididymis |
|||||
|
Mucosal vacuolation: Slight to moderate |
0 |
0 |
0 |
1 |
10 |
|
Skeletal muscle |
|||||
|
Multifocal degeneration: Slight |
0/0 |
0/0 |
0/0 |
3/1 |
5/4 |
|
Multifocal regeneration: Slight |
0/0 |
0/0 |
0/0 |
3/3 |
5/3 |
|
Bone marrow |
|||||
|
Granulocytic hypercellularity |
0/0 |
0/0 |
0/0 |
0/0 |
10/10 |
|
Multifocal necrosis: Minimal to slight |
0/0 |
0/0 |
0/0 |
0/1 |
6/5 |
|
Adrenal |
|||||
|
Chronic capsular inflammation: Moderate to marked |
0/0 |
0/0 |
0/0 |
0/0 |
2/2 |
|
Vacuolation, zona reticularis: Slight |
0/0 |
0/0 |
0/0 |
2/0 |
8/0 |
From Grothe et al. (1992a)
Lymphoid-cell vacuolar changes (vacuolation), necrosis and/or histiocytosis were reported in the spleen and lymph nodes from mice at 150 ppm, in the thymus at 450 ppm and in ileal Peyer patches at 1200 ppm; the changes in the spleen and lymph nodes were associated with enlargement at 1200 ppm. In the kidneys, cortical tubule cytoplasmic vacuoles seen at 150 ppm were associated with clusters of regenerating cortical cells at doses > 450 ppm, reflecting vacuolar degeneration. Acute or chronic renal capsule inflammation was seen at 1200 ppm. The hepatic effects consisted of hepatocellular vacuolation in females at doses > 150 ppm and in males at 1200 ppm, centrilobular cytomegaly in males at doses > 150 ppm, inflammatory changes in females at > 450 ppm and in males at 1200 ppm, granulomatous changes in females at 450 ppm and severe multifocal necrosis (foci of coagulation necrosis and fibrosis surrounded by thick bands of nuclear debris and necrotic inflammatory cells) at 1200 ppm. The inflammatory and necrotic zones were surrounded by areas of degenerative, enlarged, finely vacuolated hepatocytes. In areas that were not necrotic there were multifocal areas of acute inflammation.
Other findings attributed to treatment included increased incidences over those in control animals of lesions affecting the heart (vacuolation and granulocytic vascular leukocytosis), lung (inflammatory changes including necrosis), spleen (inflammatory changes), lymph nodes (inflammation and severe necrosis), bone marrow (granulocytic hypercellularity), pituitary gland (vacuolation), adrenal gland (inflammation) and cervix (histiocytosis) at 1200 ppm; lesions in the spleen (haematopoiesis and necrosis), lung (intra-alveolar macrophages), thymus (necrosis), pancreas (acinar atrophy, vacuolation), tongue (inflammatory changes, regeneration of muscle layer, vacuolation), stomach (moderate-to-marked glandular dilatation, histiocytosis, mucosal hyaline droplets and inflammatory changes), oviduct (mucosal inflammation), uterus (histiocytosis and inflammatory changes), cervix (vacuolation), vagina and epididymides (vacuolation), skeletal muscle (degenerative changes), bone marrow (necrosis) and adrenal gland (vacuolation) at doses > 450 ppm; lesions in lymph nodes (lymphocyte necrosis) and stomach (mucosal mineralization and necrosis) at doses > 150 ppm; and lesions in the ovary (vacuolation) at doses > 50 ppm. The histopathological finding of vacuolation corresponded to ultrastructural evidence of cytoplasmic lamellar inclusion bodies.
The vacuolar changes observed were considered by the authors to be consistent with phospholipidosis, a condition resulting from accumulation of polar lipids in lysosomes. The NOAEL was 50 ppm, equal to 6 mg/kg bw per day, on the basis of multiple histological alterations at 150 ppm (Grothe et al., 1992a).
Rats
In a study conducted in accordance with GLP requirements and OECD guideline 407, groups of five male Fischer 344 rats were given diets containing spinosad (purity, 88%; 76.1% spinosyn A, 11.9% spinosyn D), spinosyn A alone (purity, 96.2%) or spinosyn D alone (purity, 93.0%) at a dietary concentration of 0 (10 animals), 1000 or 3000 ppm for 28 days, equal to 86 and 220 mg/kg bw per day of spinosad, 86 and 220 mg/kg bw per day of spinosyn A and 86 and 250 mg/kg bw per day of spinosyn D. The animals were evaluated for clinical chemical parameters (albumin, alanine aminotransferase, aspartate aminotransferase, 5’-nucleotidase, Ca, Cl, Na, K, creatinine, globulin, sorbitol dehydrogenase, total protein, blood urea nitrogen), haematological end-points (erythrocyte, differential and total leukocyte and platelets counts, erythrocyte volume fraction, haemoglobin, mean corpuscular haemoglobin, erythrocyte sedimentation rate, mean corpuscular volume, mean corpuscular haemoglobin concentration), urinary parameters (appearance, specific gravity, glucose, ketones, bacteria, occult blood, pH, protein, urobilirubin, bilirubin, epithelial cells, urate crystals, erythrocytes, leukocytes), organ weights (adrenals, brain, gonads, heart, kidneys, liver, lungs, pituitary, pancreas, prostate, spleen, thyroid, thymus, uterus) and histological appearance of the adrenals, heart, prostate, aorta, ileum, rectum, blood smear, jejunum, salivary gland, bone, kidneys, skin, bone marrow, lachrymal gland, spinal cord, caecum, lung, spleen, colon, duodenum, mammary gland, stomach, epididymides, testes, eyes, skeletal muscle, thymus, peripheral nerve, thyroid, gall-bladder, oesophagus, trachea, Harderian glands, urinary bladder, pancreas, femur and joint, pituitary, tongue, parathyroid, larynx, nasal tissues and any gross lesions.
There were no deaths and no treatment-related clinical signs or ophthalmic effects. The body weight and body-weight gain of rats at 3000 ppm of each compound were lower than those of controls, attaining statistical significance for spinosad (19% and 34% lower, respectively) and spinosyn A (17% and 32%) but not spinosyn D (5.5% and 10%). Reduced weight gains were associated with lower food consumption (32% and 29% lower than controls for spinosad and spinosyn A, respectively), with a reduction also seen for groups at 1000 ppm of spinosad (10%). The significant haematological changes (p 0.05) that were seen included reduced erythrocyte count (approximately 25% below control value), haemoglobin (–50%), erythrocyte volume fraction (–50%), mean corpuscular volume (–30%) and mean corpuscular haemoglobin (–30%) and increased platelet count (approximately twice control value) in groups at 3000 ppm of spinosad or spinosyn A.
Morphological examinations revealed increased incidences of erythrocyte polychromasia and hypochromasia and misshapen, enlarged platelets in rats at 3000 ppm of spinosad and spinosyn A. Significant clinical chemical changes (p 0.05) occurred at 3000 ppm and consisted of decreased total protein (–11%) and globulin (–14%) concentrations after intake of spinosad; decreased albumin concentration (–5%), decreased alkaline phosphatase activity (–30%) and increased cholesterol concentration (1.7–2 times control value) after intake of spinosad or spinosyn A; increased aspartate aminotransferase activity (2–2.5 times control) after intake of spinosad and spinosyn D (and a non-significant increase of 80% with spinosyn A); and increased K (11%) after intake of spinosyn A. Urinary parameters were unaffected.
The weight of the kidney relative to that of brain was significantly decreased by 10% in rats given spinosad at 3000 ppm, and the spleen weight was significantly increased by 20% with spinosyn A. The absolute spleen weights were also significantly increased in groups given spinosyn A or spinosyn D at 3000 ppm. Gross pathological examination revealed oedema of the glandular gastric mucosa and haemolysed blood in the lumen of the stomach in all rats given spinosad or spinosyn A at 3000 ppm. The histopathological changes (Table 3) consisted of increased incidences and/or severity of vacuolar changes in the epithelial cells of the thyroid and tubular epithelial cells in the kidneys (all compounds > 1000 ppm) and in the lymph nodes and thymus (spinosad and spinosyn D), epididymides (spinosad and spinosyn A) and jejunum and seminal vesicles (spinosad) at 3000 ppm. Other effects seen at 3000 ppm were epithelial cell aggregation in skeletal muscle (spinosyn A and spinosyn D), increased extramedullary haematopoiesis in spleen and bone marrow, increased mitotic figures in the glandular mucosa of the stomach (associated with regenerative changes) and alveolar histiocytosis (spinosad and spinosyn A). All compounds at the dietary concentration of 3000 ppm reduced the occurrence of protein droplets in the renal tubule epithelium and degeneration or regeneration in the glandular stomach. A NOAEL could not be identified as histological alterations were seen at all dietary concentrations (McGuirk et al., 1994).
Table 3. Pathological findings attributable to treatment in rats given spinosad, spinosyn A or spinosyn D in the diet for 28 days
|
Lesion |
Concentration in diet (ppm) |
||||||
|
Control |
Spinosad |
Spinosyn A |
Spinosyn D |
||||
|
0 |
1000 |
3000 |
1000 |
3000 |
1000 |
3000 |
|
|
No. of animals |
10 |
5 |
5 |
5 |
5 |
5 |
5 |
|
Stomach |
|||||||
|
Mucosal glandular oedema |
0 |
0 |
5 |
0 |
5 |
0 |
0 |
|
Haemolysed blood |
0 |
0 |
5 |
0 |
5 |
0 |
0 |
|
Degeneration or regeneration of glandular mucosa: |
0 |
0 |
5 |
0 |
5 |
0 |
4 |
|
Slight to moderate |
|
|
|
|
|
|
|
|
Bone marrow |
|||||||
|
Haematopoiesis |
0 |
0 |
5 |
0 |
5 |
0 |
0 |
|
Epididymides |
|||||||
|
Vacuolation of epithelial cells |
0 |
0 |
4 |
0 |
4 |
0 |
0 |
|
Jejunum |
|||||||
|
Vacuolation |
0 |
0 |
2 |
0 |
1 |
0 |
1 |
|
Kidney |
|||||||
|
Vacuolation |
2 |
5 |
5 |
5 |
5 |
5 |
5 |
|
Decreased protein droplets, tubules |
0 |
0 |
4 |
0 |
5 |
0 |
3 |
|
Lung |
|||||||
|
Alveolar histiocytosis |
0 |
0 |
5 |
0 |
5 |
0 |
1 |
|
Mesenteric lymph nodes |
|||||||
|
Vacuolation |
2 |
1 |
5 |
3 |
5 |
1 |
3 |
|
Skeletal muscle |
|||||||
|
Aggregation of epithelial cells |
0 |
0 |
1 |
0 |
3 |
0 |
3 |
|
Spleen |
|||||||
|
Extramedullary haematopoiesis |
0 |
0 |
4 |
0 |
4 |
0 |
0 |
|
Seminal vesicles |
|||||||
|
Vacuolation, epithelial cells |
0 |
0 |
2 |
0 |
0 |
0 |
0 |
|
Thymus |
|||||||
|
Vacuolation, macrophages |
0 |
0 |
2 |
0 |
0 |
0 |
2 |
|
Thyroid |
|||||||
|
Vacuolation of epithelial cells: Slight to moderate |
0 |
5 |
5 |
5 |
5 |
4 |
4 |
From McGuirk et al. (1994)
In a study conducted in accordance with GLP, groups of 10 male Fischer 344 rats were given diets containing spinosad (purity, 88%; 76.1% spinosyn A, 11.9% spinosyn D) at a concentration of 0, 250, 1000 or 1500 ppm for 4 weeks. Groups of 10 rats were killed at 2 and 4 weeks, and, to assess the reversibility of any effects, a further four groups of animals at 0, 1000 and 1500 ppm were killed after an additional 2, 4, 8 or 22 weeks on a normal diet. The actual intake of spinosad was calculated to be 21, 82 and 120 mg/kg bw per day at the respective dietary concentrations. The experimental parameters determined were clinical signs, body weights, food consumption, serum phospholipid and cholesterol concentrations, gross lesions, kidney and thyroid weights and histological appearance of the epididymides, jejunum, kidneys, liver, lung, mediastinal and mesenteric lymph nodes, seminal vesicles, spleen, thymus and thyroid glands. Only animals at 0 and 1000 ppm that were allowed to recover were examined histologically, as the authors argued that the extent of vacuolation observed at 1000 ppm was sufficient to demonstrate the rate and extent of recovery.
The body-weight gain of animals at 1000 and 1500 ppm was 9–10% lower than that of controls, and their feed consumption was approximately 6% lower. A slight but significant increase in serum cholesterol concentration was observed in males at 1500 ppm at week 2; as this change was not found at week 4, it was discounted as toxicologically irrelevant. The weights of the kidney and thyroid relative to body weight were significantly increased in rats at 1500 ppm, by approximately 6 and 24%, respectively. Slight to very slight vacuolation of the thyroid (follicular epithelial cells) and kidney (tubule epithelial cells) was seen at 1000 and 1500 ppm and of the thymus (macrophages) and spleen (macrophages) at 1500 ppm after 2 weeks of treatment. At completion of the 4-week treatment, vacuolation was confined predominantly to the kidney and thyroid in rats at 1000 and 1500 ppm and to the thymus in those at 1500 ppm, isolated individuals at 1500 ppm showing vacuolation also in the spleen, mesenteric lymph node and liver. During the recovery phase, the vacuolation of the kidney resolved rapidly, with no effects observed after 2 weeks on a normal diet. The thyroid vacuolation resolved more slowly and was still observed in all but one or two animals at 1000 ppm after 2, 4 and 8 weeks on a normal diet; however, this lesion was not detectable 22 weeks after cessation of treatment. The NOAEL was 250 ppm, equal to 21 mg/kg bw per day, on the basis of vacuolation in the kidney and thyroid at 1000 ppm (Yano & Liberacki, 1999a).
In a study conducted in accordance with GLP requirements and OECD guideline 408, groups of 10 male and 10 female Fischer 344 rats were given diets containing spinosad (purity, 77.6%; ratio of spinsoynA:spinosyn D, approximately 5:1) at a concentration of 0, 500, 1000, 2000 or 4000 ppm for 3 months, equal to 0, 34, 69, 130 and 270 mg/kg bw per day for males and 0, 39, 78, 150 and 310 mg/kg bw per day for females. The experimental parameters determined were clinical signs, body weight, food consumption, ophthalmic end-points, haematological parameters (clotting parameters, reticulocyte, erythrocyte, total and differential leukocyte and platelet counts, erythrocyte volume fraction, haemoglobin, mean corpuscular haemoglobin, mean corpuscular volume, mean corpuscular haemoglobin concentration), clinical chemical end-points (albumin, globulin, total protein, bilirubin, cholesterol, triglyceride, glucose, electrolyte, creatinine and urea nitrogen concentrations and the activities of alanine and aspartate aminotransferases, creatine phosphokinase and gamma-glutamyl transpeptidase) and urinary parameters (appearance, specific gravity, glucose, ketones, bacteria, occult blood, pH, protein, bilirubin and leukocytes), gross and microscopic appearance (adrenal, heart, prostate, aorta, small and large intestine, salivary gland, Harderian gland, sternum, bone, kidney, skin, bone marrow, brain, liver, lung, spleen, lymph node, stomach, epididymus, muscle, gonad, eye, thymus, peripheral nerve, thyroid, oesophagus, trachea, urinary bladder, pancreas, uterus, prostate, femur and joint, pituitary, tongue and parathyroid) and organ weights.
Owing to the deaths of five males and five females at 4000 ppm, this group was discontinued on day 44, and all animals were necropsied. The cause of death was substantial, treatment-related weight loss, the weights of males being 41% those of controls and those of females 56% of controls by week 6. The clinical signs seen in most or all animals at this dietary concentration were laboured breathing, thinness, piloerection and ‘distension of the penis’, with chromorhinorrhoea and hypothermia in one female and two males. Significantly lower body weights were reported in animals at 2000 ppm, from week 11 in males (12% below control) and females (13%) from week 2. At 4000 ppm, the haematological changes seen consisted of regenerative changes in erythrocytes (anisocytosis, polychromasia and erythroblastosis). At 2000 ppm, significant reductions were seen in the erythrocyte count (–11%, males only), haemoglobin (–40% in males, –6% in females), packed cell volume (–33%, males only), mean corpuscular volume (–35% in males, –10% in females) and mean corpuscular haemoglobin (–33% in males, –4% in females). At 4000 ppm, reticulocyte counts were increased (by 10% in males, 46% in females) and leukocyte counts were increased non-significantly in males (+26%) and significantly in females (+34%). Prothrombin time was slightly but significantly decreased in males at dietary concentrations > 500 ppm (15.6 s in controls, 14.3 s at 500 ppm, 14.3 s at 1000 ppm, 13.6 s at 2000 ppm). Examination of the data for individual animals revealed consistent values in each group, indicating that the effect was not due to outliers in either the control or treated groups. The changes in mean values for clinical chemical parameters in animals at 4000 ppm were increased serum inorganic phosphorus (+19% in males, +60% in females), blood urea nitrogen (+70% in males, +80% in females) and cholesterol concentration (+60%, males only), alanine aminotransferase activity (1.5 and 4 times control value in males and females, respectively), aspartate aminotransferase activity (3 and 4 times control), alkaline phosphatase activity (2 and 3 times control), gamma-glutamyl transferase activity (2 and 3 times control) and creatine phosphokinase activity (1.2 and 3 times control) and reduced triglyceride concentration (–35%, males only). Significant differences from control values at other dietary concentrations were increased inorganic phosphorus (+10% in males, +35% in females) and aspartate aminotransferase activity (3 and 3 times control) at 2000 ppm, increased cholesterol concentrations at > 1000 ppm (+15% in males, +26% in females at 1000 ppm; +37% in males, +31% in females at 2000 ppm), increased alanine aminotransferase activity in males (+34% at 1000 ppm, +50% at 2000 ppm) and increased blood urea nitrogen in females (+67% at 1000 ppm, +61% at 2000 ppm), and increased activities of alkaline phosphatase (+67%), alanine aminotransferase (+20%) and g-glutamyl transpeptidase (+27%) in females at 2000 ppm. Urine analysis revealed a slight but significant decrease in mean pH in females at doses > 1000 ppm (8.2 in controls, 8.0 at 500 ppm, 6.6 at 1000 ppm, 5.4 at 2000 ppm) and in males at 2000 ppm (8.2, 8,2, 7.8, 6.4, respectively).
Significant changes in the absolute and relative (to body weight) weights of organs were reported as follows: increased adrenal and thyroid weights and decreased uterine weights at 2000 ppm, increased liver weights in females at > 500 ppm and in males at > 1000 ppm, increased heart and spleen weights in females at > 1000 ppm and in both sexes at 2000 ppm, and increased kidney weights at > 1000 ppm (Table 4). The Meeting considered that the increased absolute and relative thyroid weights at 1000 ppm, although not statistically significant, were both treatment-related and toxicologically significant, as a clear dose–response relationship was observed, the thyroid is a known target organ of spinosad, and histological effects were observed in this organ at the same dose. Although the weights of a number of other organs relative to body weight were increased at 2000 ppm, the Meeting considered the effects to be secondary to lower terminal body weights reported at that concentration.
Table 4. Changes in absolute (g) and relative (mg/100 g) organ weights in rats given diets containing spinosad for 90 days
|
Dietary concentration (ppm) |
Body weight (g) |
Kidney |
Liver |
Heart |
||||||||||
|
|
|
|
Absolute |
Relative |
Absolute |
Relative |
Absolute |
Relative |
||||||
|
|
M |
F |
M |
F |
M |
F |
M |
F |
M |
F |
M |
F |
M |
F |
|
0 |
330 |
200 |
1.9 |
1.2 |
560 |
610 |
8.5 |
4.7 |
2.6 |
2.4 |
880 |
590 |
260 |
300 |
|
500 |
360 |
200 |
2.1 |
1.3* |
580 |
630 |
9.5 |
5.1* |
2.6 |
2.5* |
940 |
650* |
260 |
320 |
|
1000 |
340 |
200 |
2.1* |
1.4* |
610* |
710* |
9.7* |
5.5* |
2.8* |
2.8* |
930 |
670* |
270 |
340* |
|
2000 |
290* |
170* |
2.1 |
1.4* |
710* |
830 |
9.1 |
6.0* |
3.1* |
3.5* |
1000* |
700* |
350* |
400* |
|
Dietary concentration (ppm) |
Spleen |
Uterus |
Thyroid and parathyroids |
Adrenals |
||||||||||
|
|
Absolute |
Relative |
Absolute |
Relative |
Absolute |
Relative |
Absolute |
Relative |
||||||
|
|
M |
F |
M |
F |
|
|
M |
F |
M |
F |
M |
F |
M |
F |
|
0 |
640 |
440 |
190 |
220 |
670 |
340 |
20 |
14 |
5.8 |
7.0 |
48 |
57 |
14 |
29 |
|
500 |
670 |
450 |
180 |
220 |
510 |
250 |
19 |
15 |
5.3 |
7.4 |
50 |
60 |
14 |
29 |
|
1000 |
680 |
560* |
200 |
280* |
520 |
270 |
25 |
17 |
7.1 |
8.7 |
53 |
62 |
15 |
32 |
|
2000 |
1200* |
840* |
400* |
480* |
460* |
270 |
42* |
26* |
14* |
15* |
58* |
69* |
20* |
40* |
From Grothe et al. (1992b)
* p < 0.05
Gross, histopathological and ultrastructural examination revealed extensive effects in a wide range of tissues, most notably vacuolation. Findings observed only or predominantly in nine or 10 animals at 4000 ppm were distension of the caecum (in nine males and 10 females), small testes (in four males), ‘distension of the penis’ (seven animals), minimal to moderate chronic multifocal inflammatory necrosis of the liver (four males, three females), multifocal hepatocellular vacuolation of the liver (10 of each sex), acute multifocal inflammation of the lung (eight males, seven females), lymphoid vacuolar changes in the spleen (10 males, nine females), necrosis of the lymph node (three males, one female), atrophy of the thymus (six of each sex), lymphoid vacuolar changes in the thymus (five of each sex), diffuse acinar vacuolation of the pancreas (10 males, nine females), atrophy of the pancreas (two males, three females), diffuse mucosal fibrosis (seven males, two females), glandular mineralization of the stomach (nine males, seven females), glandular epithelial vacuolation of the stomach (nine males, seven females), mucosal vacuolation of the oviduct (nine animals), mucosal vacuolation of the vagina (three animals), moderate to marked hypospermatogenesis (10 animals), mucosal vacuolation of the epididymides (10 animals) and bone-marrow hypocellularity (10 males, eight females). The incidences of gross pathological and histopathological findings at other dietary concentrations are summarized in Table 5.
Table 5. Gross and histopathological lesions in groups of 9–10 rats given spinosad in the diet for 90 days (males/females)
|
Tissue and lesion |
Dietary concentration (ppm) |
||||
|
0 |
500 |
1000 |
2000 |
4000 |
|
|
Gross examination |
|||||
|
Kidney |
|||||
|
Whole tissue alteration |
0/0 |
0/0 |
0/0 |
2/4 |
1/0 |
|
Liver |
|||||
|
Hepato-diaphragmatic nodule. |
0/0 |
0/0 |
0/0 |
1/2 |
0/0 |
|
Whole tissue alteration |
0/0 |
0/0 |
0/0 |
0/9 |
10/9 |
|
Lung |
|||||
|
Whole brown, firm, mottled |
0/0 |
0/0 |
0/0 |
0/9 |
10/9 |
|
Lymph node |
|||||
|
Enlarged |
0/0 |
0/0 |
0/7 |
10/10 |
0/0 |
|
Spleen |
|||||
|
Enlarged |
0/0 |
0/0 |
0/0 |
10/10 |
0/0 |
|
Stomach |
|||||
|
Lesion |
0/0 |
0/0 |
0/0 |
10/10 |
0/0 |
|
Testes |
|||||
|
Enlarged |
0 |
0 |
0 |
8 |
0 |
|
Thyroid |
|||||
|
Whole tissue alteration |
0/0 |
0/0 |
0/0 |
10/10 |
0/0 |
|
Histopathological examination |
|||||
|
Kidney |
|||||
|
Cortical tubule necrosis: Minimal to slight |
0/0 |
0/0 |
0/0 |
0/2 |
6/2 |
|
Multifocal cortical tubular vacuolation: Slight to moderate |
0/0 |
0/0 |
0/0 |
10/10 |
10/10 |
|
Liver |
|||||
|
Multifocal granuloma: Minimal to moderate |
0/0 |
0/0 |
0/9 |
8/10 |
10/10 |
|
Multifocal Kupffer cell vacuolation: Minimal to moderate |
0/0 |
0/0 |
0/9 |
8/10 |
10/10 |
|
Heart |
|||||
|
Progressive cardiomyopathy: Slight |
0/0 |
0/0 |
1/1 |
1/3 |
0/0 |
|
Multifocal vacuolation: Minimal to slight |
0/0 |
0/0 |
0/1 |
0/1 |
2/1 |
|
Lung |
|||||
|
Alveolar macrophages: Minimal to slight |
0/1 |
0/0 |
0/0 |
3/10 |
10/10 |
|
Spleen |
|||||
|
Haematopoiesis: Slight to moderate |
0/0 |
0/0 |
0/0 |
10/9 |
0/0 |
|
Histiocytosis: Minimal to marked |
2/1 |
3/0 |
9/8 |
10/10 |
0/0 |
|
Lymph node |
|||||
|
Lymphoid cell vacuolar change: Slight to moderate |
0/0 |
0/0 |
0/0 |
0/3 |
9/9 |
|
Histiocytosis: minimal to marked |
2/5 |
7/6 |
9/9 |
10/10 |
9/9 |
|
Thymus |
|||||
|
Histiocytosis: Slight to moderate |
0/0 |
0/0 |
0/0 |
3/1 |
4/2 |
|
Pancreas |
|||||
|
Diffuse acinar atrophy: Slight |
0/0 |
0/0 |
0/1 |
0/1 |
2/3 |
|
Stomach |
|||||
|
Glandular dilatation: Minimal to slight |
3/2 |
4/2 |
5/5 |
8/4 |
9/7 |
|
Focal hyperkeratosis |
0/0 |
0/0 |
0/0 |
7/7 |
0/0 |
|
Ileum |
|||||
|
Histiocytosis of Peyer patches: Moderate |
0/0 |
0/0 |
0/0 |
1/1 |
1/0 |
|
Uterus |
|||||
|
Mucosal vacuolation: Minimal to moderate |
0 |
0 |
2 |
9 |
10 |
|
Adrenal |
|||||
|
Cortical vacuolation: Slight |
9/0 |
9/0 |
7/1 |
3/4 |
10/8 |
|
Thyroid |
|||||
|
Acute multifocal inflammation: Minimal to slight |
0/0 |
0/0 |
0/0 |
5/2 |
0/0 |
|
Diffuse follicular epithelial vacuolation: Minimal to marked |
0/0 |
6/0 |
10/9 |
10/10 |
10/10 |
|
Skeletal muscle |
|||||
|
Multifocal degeneration: Minimal to moderate |
0/0 |
0/0 |
1/7 |
10/10 |
9/10 |
|
Multifocal regeneration: Minimal to moderate |
0/0 |
1/0 |
0/5 |
10/10 |
5/9 |
From Grothe et al. (1992b)
The term ‘whole tissue alteration’ was used by the pathologist to group a variety of macroscopic findings affecting an entire tissue, such as discolouration, with or without mottling, and varying degrees of alteration to the texture of the tissue.
Histopathological changes attributed to treatment were lymphoid-cell vacuolar changes and/or histiocytosis in the spleen, thymus, lymph nodes and ileal Peyer patches at dietary concentrations > 1000 ppm; splenic enlargement was seen at 2000 ppm. The lymphoid vacuolar changes were characterized by the presence of large vacuoles in the cytoplasm of lymphocytes or lymphoblasts, while histiocytosis was characterized by collections of histiocyte–macrophage-type cells with faintly vacuolar cytoplasm in the sinusoids of lymphoid tissues. In kidneys, cytoplasmic vacuoles were seen in the cortical tubules at concentrations > 2000 ppm, accompanied by necrotic changes; together, these lesions were reported to be consistent with vacuolar degeneration. Hepatocellular vacuolation and granulomatous inflammatory changes (the inflammatory response was considered secondary to vacuolar changes in Kupffer cells) were reported in females at concentrations > 1000 ppm and in males at 2000 ppm. The changes in animals at 4000 ppm were more pronounced and associated with necrosis. Other findings attributed to treatment included an increase in the severity of vacuolation of cardiac myocytes and cardiomyopathy at > 1000 ppm, myopathy of skeletal muscle (characterized by multifocal fibrils undergoing degeneration or regeneration) in females at > 1000 ppm and in males at 2000 ppm, adrenocortical vacuolation in females at > 1000 ppm, intra-alveolar macrophages in females at > 2000 ppm and in males at 4000 ppm, vacuolation of pancreatic acinar cells at 4000 ppm, and focal hyperkeratosis in the stomach associated with linear papillary or nodular proliferation of the gastric ridge at 2000 ppm. Other findings in the stomach were increased incidences of vacuolation in the epithelium of the gastric mucosa, mucosal fibrosis and glandular mineralization, and an increased severity of gastric glandular dilatation at 4000 ppm. The increased incidence of excessive fluid in the caecum and caecomegaly reported at dietary concentrations > 500 ppm was considered secondary to a detrimental effect on gut flora that caused osmotic disturbances; however, no histopathological findings were reported in the caecum. An increased incidence of vacuolar changes was also reported in the uterus at dietary concentrations > 1000 ppm, in the oviduct and vagina at 4000 ppm and in the thyroid follicular epithelia at concentrations > 500 ppm. Gross examination of the thyroids showed them to be enlarged and yellow. Hypospermatogenesis (characterized by the presence of immature forms in the tubules due to maturation arrest) and epididymal vacuolation were reported at 4000 ppm. The histopathological finding of vacuolation corresponded to ultrastructural evidence of cytoplasmic lamellar inclusion bodies (Table 6). Although some pathological findings seen at concentrations 2000 ppm, were not reported at 4000 ppm, the reduced duration of intake at the latter concentration or autolysis may have been responsible.
Table 6. Numbers of animals with cytoplasmic lamellar inclusion bodies in groups of three male and three female rats given spinosad in the diet for 90 days
|
Tissue |
Degree |
Control |
2000 ppm |
4000 ppm |
|
Liver |
Minimal |
4 |
0 |
0 |
|
Slight |
0 |
6 |
6 |
|
|
Kidney |
Minimal |
4 |
0 |
0 |
|
Moderate to marked |
0 |
6 |
6 |
|
|
Lung |
Minimal |
4 |
0 |
NE |
|
Slight to marked |
0 |
6 |
|
|
|
Spleen |
Minimal |
6 |
0 |
NE |
|
Slight to moderate |
0 |
6 |
|
|
|
Thyroid |
Minimal |
2 |
0 |
NE |
|
Severe |
0 |
6 |
|
From Grothe et al. (1992b). NE, not examined
A NOAEL could not be identified as vacuolatory changes, liver weight changes and decreased prothrombin times were seen at dietary concentrations > 500 ppm. The study authors suggested that the changes found may have been the result of prolonged inhibition of phospholipid catabolism and possibly protein synthesis. They also argued that, given the severity and type of lesions occurring at 500 ppm, the affects were probably reversible (Grothe et al., 1992b).
In a study conducted in accordance with GLP requirements and OECD guideline 408, groups of 10 male and 10 female Fischer 344 rats were given diets containing spinosad (purity, 96.3%; ratio of spinosyn A to spinosyn D, 1:1) at a concentration of 0, 120, 600 or 1000 ppm for 13 weeks, corresponding to actual achieved intakes of 0, 7.7, 39 and 65 mg/kg bw per day for males and 0, 9.2, 47 and 80 mg/kg bw per day for females. The following parameters were measured: clinical signs at least daily, ophthalmic end-points before the study and at necropsy, body weights and food consumption at least weekly, haematological parameters (erythrocyte, total and differential leukocyte and platelet counts, erythrocyte volume fraction, haemoglobin), clinical chemical end-points (albumin, globulin, total protein, bilirubin, cholesterol, triglyceride, glucose, electrolyte, creatinine and urea nitrogen concentrations and alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase and creatine phosphokinase activities), urine analysis (appearance, specific gravity, glucose, ketones, occult blood, pH, protein, bilirubin), organ weights (brain, liver, kidneys, heart, adrenals, gonads, spleen, thyroid, parathyroid) and gross and histopathology of the adrenals, heart, prostate, aorta, small and large intestine, salivary gland, Harderian gland, sternum, lachrymal gland, bone, kidneys, skin, bone marrow, brain, liver, lungs, spleen, lymph nodes, stomach, epididymides, muscle, gonads, eyes, thymus, peripheral nerve, thyroid, oesophagus, trachea, urinary bladder, pancreas, uterus, femur and joint, pituitary, tongue, oral and nasal tissues, parathyroid, vagina, cervix and mammary gland.
A slight, dose-related, statistically significant reduction in platelet count was seen in both sexes at 600 and 1000 ppm (males, 630, 610, 580, 560 × 103/mm3; females, 700, 650, 600, 590 × 103/mm3). This effect was discounted by the study authors on the basis that the values for males were within the range of other controls in the laboratory (550–680) and those for females were only slightly outside this range (630–900), despite the attainment of statistical significance and the clear trend in both sexes. Other 90-day studies with this strain of rat have shown slight to marked increases in platelet counts at dietary concentrations at or above the highest used in this study, which is consistent with the authors’ conclusion. At 1000 ppm, slight, statistically significant increases in total protein (< 8%) and albumin (< 6%) concentrations were seen in both sexes, and alanine and aspartate aminotransferase activities were significantly increased in males (by 50–60%) and non-significantly in females (by 50%). In both sexes at 1000 ppm, the absolute and relative thyroid weights were significantly increased (+20% above control value). In females at 600 and 1000 ppm, significant increases were found in the weights of the liver (+8%, +19%), spleen (+10%, +43%), heart (+6%, +11%) and kidney (+8%, +12%), and females at 1000 ppm also had signicantly increased adrenal weights (+16%). Animals of each sex at 600 and 1000 ppm had watery caecal contents.
Treatment-related histological effects were confined to animals at the two higher dietary concentrations and involved the thyroid, spleen, lymph nodes, liver (females), kidneys (males) and stomach (females) (Table 7). The changes in organ weights in females were considered by the authors to be unrelated to treatment on the basis that the values were within the range for relevant controls in other studies in the laboratory. However, the effects are consistent with those seen in other 90-day studies with spinosad in this strain of rat, they correlated in most cases with histological findings in the same organs, were statistically significant and were not due to outliers in the data for individual animals. The Meeting therefore concluded that they were treatment-related. The epithelial cells lining the thyroid follicles were particularly sensitive, with vacuolation seen in all animals at 600 and 1000 ppm. In the spleen, lymph nodes, liver and kidneys, aggregation of reticuloendothelial cells was observed. In the stomach and liver of females at concentrations > 600 ppm, individual cell necrosis was also seen. The NOAEL was 120 ppm, equal to 7.7 mg/kg bw per day (Yano & Liberacki, 1999b).
Table 7. Incidences of histopathological lesions in rats given diets containing spinosad for 90 days
|
Lesion |
Dietary concentration (ppm) |
|||||||
|
Males |
Females |
|||||||
|
0 |
120 |
600 |
1000 |
0 |
120 |
600 |
1000 |
|
|
Thyroid |
||||||||
|
Follicular epithelial cell vacuolation: Very slight |
0/10 |
0/10 |
9/10 |
2/10 |
0/10 |
0/10 |
8/10 |
1/10 |
|
Follicular epithelial cell vacuolation: Slight |
0 |
0 |
1/10 |
8/10 |
0 |
0 |
2/19 |
9/10 |
|
Spleen |
||||||||
|
Reticuloendothelial cell aggregation: Very slight |
3/10 |
4/10 |
5/10 |
2/10 |
0/10 |
1/10 |
6/10 |
3/10 |
|
Reticuloendothelial cell aggregation: Slight |
0 |
0 |
0 |
4/10 |
0 |
0 |
0 |
7/10 |
|
Lymph nodes |
||||||||
|
Aggregates of reticuloendothelial cells: Very slight to slight |
|
|
|
|
|
|
|
|
|
Mediastinal |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
2/10 |
8/10 |
|
Mesenteric |
2/10 |
2/10 |
3/10 |
10/10 |
0/10 |
0/10 |
8/10 |
10/10 |
|
Submandibular |
0/10 |
– |
– |
1/8 |
0/7 |
0/8 |
2/9 |
6/8 |
|
Liver |
|
|
|
|
|
|
|
|
|
Mononuclear-cell aggregation: Very slight |
1/10 |
0/10 |
0/10 |
5/10 |
3/10 |
2/10 |
0/10 |
0/10 |
|
Reticuloendothelial cell aggregation: Very slight to slight |
0/10 |
0/10 |
0/10 |
1/10 |
2/10 |
1/10 |
9/10 |
9/10 |
|
Individual cell necrosis: Very slight |
2/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
8/10 |
0 |
|
Kidneys |
||||||||
|
Mononuclear cell aggregation: Very slight |
0/10 |
0/10 |
0/10 |
3/10 |
1/10 |
– |
– |
2/10 |
|
Stomach |
||||||||
|
Necrosis, individual cells, glandular mucosa |
– |
– |
– |
– |
0/10 |
0/10 |
0/10 |
6/10 |
From Yano & Liberacki (1999b); –, no animals examined
In a study conducted in accordance with GLP requirements and OECD guideline 408, groups of 10 Fischer 344 rats of each sex were given diets containing spinosad (purity, 88%) at a nominal concentration of 0, 30, 60, 120 or 600 ppm for 13 weeks. Two recovery groups of 10 rats of each sex at 0 or 600 ppm were returned to a normal diet for 4 weeks before sacrifice. The approximate actual achieved doses of spinosad were 0, 2.2, 4.3, 8.6 and 43 mg/kg bw per day for males and 0, 2.6, 5.2, 10 and 52 mg/kg bw per day for females. The experimental parameters determined included clinical signs, body weight, food consumption, ophthalmic parameters, haematological end-points (erythrocyte, total and differential leukocyte and platelet counts, erythrocyte volume fraction, haemoglobin, erythrocyte sedimentation rate), clinical chemical parameters (albumin, globulin, total protein, bilirubin, cholesterol, triglyceride, glucose, thyroxine, electrolyte, creatinine and urea nitrogen concentrations and alanine and aspartate aminotransferase and creatine phosphokinase activities), urine analysis (appearance, specific gravity, glucose, ketones, bacteria, occult blood, pH, protein, bilirubin), gross and histopathological appearance of animals at 0 and 600 ppm (adrenals, heart, prostate, aorta, small and large intestine, salivary gland, bone, kidneys, skin, bone marrow, spinal cord, brain, liver, lungs, spleen, mammary gland, stomach, epididymides, muscle, gonads, eyes, thymus, eyes, peripheral nerve, thyroid, oesophagus, trachea, Harderian glands, urinary bladder, pancreas, uterus, femur and joint, pituitary, vagina, tongue, parathyroid and cervix), histopathological appearance of organs of animals at other doses (lungs, liver, kidneys, thyroid, spleen, thymus and lymph nodes) and the weights of the adrenals, brain, gonads, heart, kidneys, liver, spleen and thyroid. No haematological or urological parameters were assessed at the end of the recovery period, but creatinine was measured in males and total protein, albumin, globulin and cholesterol in females, and tissues from the thyroid gland and adjacent tissues (oesophagus, larynx, parathyroid gland and trachea) of animals that had been allowed to recover were subjected to histopathological examinations.
The absolute and relative weights of the heart and liver of males at 600 ppm were slightly increased, by 10 and 6%, respectively, and all but the increased absolute liver weight were statistically significant. The absolute weights of the heart and spleen were significantly increased in females by 9% each. In the absence of histological findings in these organs, the authors considered them to be unrelated to treatment. The altered liver, heart and spleen weights were, however, consistent with effects seen in these organs in other studies, and, while the magnitude of the changes and the lack of clinical or histological correlates suggests they are likely to be of minimal toxicological significance, a relationship with treatment cannot be discounted. Organ weights were unaffected after the recovery period. Histopathological findings were restricted to slight vacuolation and enlargement of the epithelial cells lining the thyroid follicles of males at 600 ppm and decreased staining intensity seen occasionally in thyroid follicular cell colloid. The severity, but not the incidence, of epithelial cell vacuolation was partially reversible during the 4-week recovery period. Serum thyroxine concentrations were not significantly affected by treatment, although the value for males at 600 ppm was 10% lower than that of controls (4.1 µg/dL in controls and 4.1, 4.2, 4.3 and 3.6 µg/dL at 30, 60, 120 and 600 ppm, respectively), and an examination of data for individual animals did not reveal the presence of outliers to which the apparent decline might be attributed. Multiple renal adenomas and a focus of hyperplasia of the tubule epithelium were noted in one male rat at 600 ppm, and, although these types of lesions are unusual in rats of this strain and age, similar renal tumours have been reported in a female control in the same laboratory. As similar lesions were not observed in other 90-day studies with spinosad in this strain of rat at higher dietary concentrations, the observation was considered by the Meeting to be unrelated to treatment. The NOAEL was 120 ppm, equal to 8.6 mg/kg bw per day (Yano & Bond, 1994).
In a study conducted in accordance with GLP requirements and OECD guideline 412, groups of 10 male and 10 female Fischer 344 rats were exposed (nose only) to an atmosphere containing spinosad (purity, 88%; 76.1% spinosyn A and 11.9% spinosyn D) at a concentration of 0, 0.3, 1.4 or 9.5 mg/m3 for 6 h/day, 5 days per week, for 14 days. Half the animals of each group were killed at the end of the exposure period, and the remainder were maintained without treatment for a further 15 days to evaluate recovery. The mass median aerodynamic diameter of the particles was 1–1.6 µm (geometric standard deviation, 2.4–4.2). The effects of spinosad were assessed by evaluating clinical signs at least daily, body weight and food consumption weekly, haematological end-points (erythrocyte, total and differential leukocyte and platelet counts, erythrocyte volume fraction, haemoglobin, cell morphology, clotting time), clinical chemical parameters (alkaline phosphatase, alanine and aspartate aminotransferase and creatine phosphokinase activities, urea nitrogen, creatinine, albumin, globulin, glucose, bilirubin, cholesterol, triglyceride and electrolyte concentrations) and gross appearance. Urinary parameters (appearance, specific gravity, pH, bilirubin, glucose, ketones, proteins, blood, urobilinogen) and the weights and histopathological appearance of organs from controls and those at the high concentration (adrenals, heart, prostate, aorta, small and large intestine, salivary gland, lachrymal gland, Harderian gland, bone, kidneys, skin, bone marrow, brain, liver, lungs, spleen, lymph nodes, stomach, epididymides, muscle, gonads, eyes and optic nerve, thymus, peripheral nerve, thyroid, oesophagus, trachea, urinary bladder, pancreas, prostate, cervix, vagina, spinal cord, mammary glands, oral and nasal tissues, uterus, femur and joint, pituitary, tongue and parathyroid) were measured only for the main group at the end of exposure. No effects were observed in any group, either at the end of treatment or after the recovery period. In the absence of any effects, the NOAEL was 9.5 mg/m3, the highest concentration tested (Yano & McGuirk, 1999).
Rabbits
In a study conducted in accordance with GLP requirements and FIFRA guideline 82-2, spinosad (purity, 88%) was evaluated for its potential to induce dermal irritation and systemic toxicity in New Zealand white rabbits after repeated dermal exposure. Groups of four male and six female rabbits received spinosad at a dose of 0 or 1000 mg/kg bw on the intact skin under a semi-occlusive dressing for 6 h/day for 21 days. The animals were examined for clinical signs, body weight, food consumption, ophthalmic end-points, clinical chemical end-points (alanine and aspartate aminotransferase activities and albumin, 5’-nucleotidase, Ca, Cl, Na, K, P, creatinine, globulin, sorbitol dehydrogenase, total protein, blood urea nitrogen concentrations), haematological parameters (erythrocyte, total and differential leukocyte and platelet counts, erythrocyte volume fraction, haemoglobin, mean corpuscular haemoglobin, eyrthrocyte sedimentation rate, mean corpuscular volume, mean corpuscular haemoglobin concentration), urinary end-points (appearance, specific gravity, glucose, ketones, bacteria, occult blood, pH, protein, urobilirubin, bilirubin, epithelial cells, urate crystals, erythrocytes, leukocytes), organ weights (adrenals, gonads, heart, kidneys, liver, lungs, pituitary, pancreas, prostate, thyroid, thymus, uterus) and histological appearance of the adrenals, heart, prostate, aorta, ileum, rectum, blood smear, jejunum, salivary gland, bone, kidneys, skin, bone marrow, spinal cord, liver, caecum, lungs, colon, lymph nodes, duodenum, mammary gland, stomach, skeletal muscle, testes, eyes, thymus, peripheral nerve, thyroid, oesophagus, trachea, ovaries, urinary bladder, pancreas, uterus, femur and joint, pituitary, tongue and parathyroid. There were no effects on any parameter examined. The NOAEL was 1000 mg/kg bw per day (Wright et al., 1992b).
In a study complying with GLP standards and OECD guideline 410, spinosad (purity, 88%; 76.1% spinosyn A and 11.9% spinosyn D) was applied to the intact skin of groups of five New Zealand white rabbits of each sex at a dose of 0, 100, 500 or 1000 mg/kg bw for 6 h/day under a semi-occlusive dressing 15 times over 21 days. The doses were selected on the basis of the results of a preliminary study in which no clinical signs of toxicity or of dermal irritation were seen in male rabbits exposed dermally to spinosad at 500 or 1000 mg/kg bw for 6 h/day for 4 consecutive days. The experimental parameters determined were clinical signs, food and water consumption, ophthalmic parameters, irritation (weekly and at necropsy), clinical chemical end-points (alkaline phosphatase and alanine and aspartate aminotransferase activities, albumin, bilirubin, 5’-nucleotidase, Ca, Cl, creatinine, globulin, glucose, K, sorbitol dehydrogenase, protein, Na and urea nitrogen concentrations) and haematological parameters (erythrocyte, total and differential leukocyte and platelet counts, erythrocyte volume fraction, haemoglobin). Histopathological examinations were conducted on untreated and treated skin from all animals and on the liver, kidney and stomach from rabbits at 0 or 1000 mg/kg bw per day. Liver, kidney and testes were weighed. Treatment had no effect on any parameter tested. The NOAEL was 1000 mg/kg bw per day (Vedula & Yano, 1994).
Dogs
In a study complying with GLP standards, pairs of one male and one female beagles were given diets containing spinosad (purity, 88%) at a concentration of 0, 200, 2000 or 4000 ppm for 4 weeks, equal to 6.5, 62 and 120 mg/kg bw per day for males and 6.8, 54 and 92 mg/kg bw per day for females. The following experimental parameters were determined: clinical signs, body weight, food consumption, ophthalmic parameters, haematological end-points (erythrocyte, leukocyte and platelet counts, erythrocyte volume fraction, haemoglobin, mean corpuscular haemoglobin, mean corpuscular volume, mean corpuscular haemoglobin concentration, eyrthrocyte sedimentation rate), clinical chemical end-points (albumin, globulin, total protein, bilirubin, cholesterol, triglyceride, glucose, electrolyte, creatinine and urea nitrogen concentrations and the activities of alanine and aspartate aminotransferase, creatine phosphokinase and gamma-glutamyl transpeptidase), urinary end-points (appearance, specific gravity, glucose, ketones, occult blood, pH, protein, bilirubin, leukocytes), organ weights (adrenals, brain, gonads, heart, kidneys, liver, lungs, pituitary, pancreas, prostate, spleen, thyroid with parathyroids) and histological appearance of the adrenals, heart, prostate, aorta, small and large intestine, salivary gland, bone, kidneys, skin, bone marrow, lachrymal gland, spinal cord, brain, liver, lungs, spleen, lymph nodes, sternum, mammary gland, stomach, epididymides, muscle, gonads, thymus, eye with optic nerve, peripheral nerve, thyroid, gall-bladder, oesophagus, trachea, urinary bladder, pancreas, uterus, femur and joint, pituitary, tongue, parathyroid, penis, nasopharynx, buccal cavity, diaphragm and oviducts. Histopathological examinations were conducted on tissues from control animals and those at 4000 ppm and on the following tissues from animals at 200 and 2000 ppm: brain (pons), pituitary, thyroids, parathyroids, adrenals, faucial tonsils, spleen, lymph nodes (cervical), liver, pancreas, intestinal tract and all gross lesions. The results could not be analysed statistically, as the groups were too small.
Both animals at 4000 ppm were killed in extremis on day 23 owing to body-weight losses of 0.5 kg in the male and 1 kg in the female, accompanied by reduced food consumption and, in the male, hindlimb weakness. The clinical signs before sacrifice were loose stools with blood and/or mucus and vomiting. Loose or watery stools were also reported for the male at 2000 ppm, and the female at this concentration lost 0.6 kg, which was associated with a 40–60% reduction in food consumption. Leukocyte counts were increased by approximately 50% over initial values in both animals at 4000 ppm, but, at 2000 ppm, the leukocyte and platelet counts were decreased in the female by 50% and 70%, respectively. Increased activities of alkaline phosphatase, aspartate aminotransferase (by 2–4 times the control value) and alanine aminotransferase (3–4 times control) were seen in animals at dietary concentrations > 2000 ppm, and the concentration of inorganic phosphorus was decreased (by > 60%) in the female at 2000 ppm and in the male at 4000 ppm (by 30%). A 30–40% decrease in the albumin:globulin ratio was seen secondary to increased globulin concentrations in females at dietary concentrations > 2000 ppm and in the male at 4000 ppm. Increased triglyceride concentrations was seen in males > 2000 ppm and in the female at 4000 ppm, increased total cholesterol at 4000 ppm and increased potassium in males > 2000 ppm and in the female at 4000 ppm. Urine analysis revealed occult blood in the male and a reduced pH in the female at 4000 ppm.
As this was a dose range-finding study, the organs of the animals at 4000 ppm which died prematurely were not weighed. In males, thyroid weights were increased by 30% at 200 ppm and by 100% at 2000 ppm and pancreas weights by 50% at 2000 ppm. Liver weights were increased by 25 and 40% and kidney weights by 30% and 40% in the male and female at 2000 ppm, respectively. The weight of the spleen of the female at 2000 ppm was increased threefold. Although the relative adrenal weights were increased in females at 200 and 2000 ppm by 40% and 85%, the weights in males at these concentrations were decreased by 25% and 42%. Gross examination revealed pale livers and red spots in the colon or ileo-caecal region of the intestine in males at concentrations > 2000 ppm and in the female at 4000 ppm, and white foamy fluid in the stomach of the female at 4000 ppm.
In both the male and the female at 4000 ppm, histopathological examination revealed cytoplasmic vacuolation or vacuolated cell aggregation in the brain, spinal cord, pituitary, thymus, thyroid, parathyroid, adrenal, faucial tonsil, spleen, bone, bone marrow, lymph nodes, salivary glands, liver, pancreas, gastrointestinal tract, nasal cavity and larynx. Other changes at 4000 ppm were foamy-cell aggregation in the lung, arteritis in the brain, heart, gall-bladder, nasal cavity, lung, kidney, and epididymides of the male dog and lung and urinary bladder of the female, inflammatory cell infiltration in the liver, microgranulomas in the spleen, mucosal atrophy in the stomach, focal haemorrhage in the intestinal mucosa of the colon or caecum, bone-marrow necrosis, lymph node adenitis, acinar-cell atrophy of the pancreas, rhinitis, tracheitis in the male and thymic atrophy in the female. At 2000 ppm, cytoplasmic vacuolation or vacuolated (foamy) cell aggregation was reported in the liver, spleen, brain, thyroid, faucial tonsil, lymph nodes, pancreas, ileum, caecum, colon, rectum and lung; inflammatory cell infiltration in the liver in both sexes; microgranulomas in the spleen, focal haemorrhage in the caecum and haematoma and endocarditis in the heart of the male and congestion and microgranulomas in the spleen and acinar atrophy of the pancreas in the female. At 200 ppm, microgranulomas of the liver, focal haemorrhage in the caecum and arteritis in the lung of the male, and microgranuloma in the spleen and arteritis in the lung of the female were seen. Although the authors of the study considered that the effects observed at 200 ppm were not treatment-related, the Meeting considered that no NOAEL could be identified, as the histological lesions and effects on organ weights seen at 200 ppm were consistent with those seen at higher concentrations and as only one animal of each sex received each concentration (Enomoto, 1992).
In a study conducted in accordance with GLP requirements and OECD guideline 409, groups of four beagles of each sex were given diets containing spinosad (purity, 88%) at a nominal concentration of 0, 150, 300 or 900 (females)/1350 (males) ppm for 13 weeks, equal to 0, 4.9, 9.7 and 33 mg/kg bw per day for males and 0, 5.4, 10 and 30 mg/kg bw per day for females. The experimental parameters determined were: clinical signs, body weight, food consumption, ophthalmic parameters, haematological end-points (reticulocyte, erythrocyte, total and differential leukocyte and platelet counts, erythrocyte volume fraction, haemoglobin, mean corpuscular haemoglobin, mean corpuscular volume, mean corpuscular haemoglobin concentration, eyrthrocyte sedimentation rate), clinical chemical parameters (albumin, globulin, total protein, bilirubin, cholesterol, triglyceride, glucose, electrolyte, creatinine and urea nitrogen concentrations and alanine aminotransferase, aspartate aminotransferase, creatine phosphokinase and gamma-glutamyl transpeptidase activities) and urinary end-points (appearance, specific gravity, glucose, ketones, bacteria, occult blood, pH, protein, bilirubin, leukocytes), gross and histological appearance (adrenals, heart, aorta, prostate, small and large intestine, salivary gland, sternum, bone, kidneys, skin, bone marrow, liver, lungs, spleen, lymph nodes, stomach, epididymides, muscle, gonads, eyes, thymus, peripheral nerve, thyroid, parathyroid, gall-bladder, oesophagus, trachea, urinary bladder, pancreas, uterus, femur and joint, pituitary, tongue and parathyroid) and the weights of the adrenals, brain, gonads, heart, kidneys, liver, pituitary, pancreas, spleen and thyroids with parathyroids.
The highest concentration was reduced to 900 ppm for males from day 38 as one male in this group had to be killed in extremis at week 5 after showing unsteadiness, decreased activity and body-weight loss Two males (including the one that died) at 900/1350 ppm had periocular sebum and watery black stools. Loose stools were common in females at 900 ppm. At week 13, the mean body weights of males and females at the highest concentration were 82 and 88% of control values, respectively, and their mean food consumption was 84 and 89% of control values. In males at 900/1350 ppm, significant (p 0.05) reductions in erythrocyte volume fraction (by 27%), haemoglobin (27%), erythrocyte count (21–24%) and reticulocyte count (85%, week 13 only) were observed at weeks 7 and 13. Although the leukocyte counts were approximately 60 and 80% of control values at weeks 7 and 13 and the reticulocyte count was 38% of the control values at week 7, the differences did not attain statistical significance. In females, significantly lower values were obtained for mean erythrocyte volume fraction (11% lower than control), haemoglobin (14%) and mean corpuscular haemoglobin (5%) at week 13 and for leukocyte count (38%), lymphocyte count (43%) and platelet count (38%) at week 7. Although not statistically significant, the leukocyte and lymphocyte counts at week 13 for animals at 300 and 900 ppm were 66 and 76% and 62 and 63% of control values, respectively. These haematological findings, in conjunction with bone-marrow necrosis and hypocellularity seen histologically are consistent with a diagnosis of the early stages of aplastic anaemia. In males at 900/1350 ppm, significantly lower serum albumin concentration (22% below control) and albumin:globulin ratio (45%), and increased serum globulin (140% of control value), total cholesterol (130%) and triglyceride (133%) concentrations (week 13 only) were observed in weeks 7 and 13. In females, significantly increased aspartate aminotransferase (335%) and alanine aminotransferase activities (726%), total cholesterol (149%), triglyceride (176%) and serum globulin concentrations (131%) and decreased phosphorus concentration (18%) and albumin:globulin ratio (38%) were observed in week 7, and increased aspartate aminotransferase activity (506%) and decreased serum albumin concentration (22%) and albumin:globulin ratio (37%) at week 13. Although there was also a 10-fold increase in the mean alanine aminotransferase activity over the control value in females at 900 ppm at week 13, this was attributable to an extreme value in an individual animal. Females at 900 ppm had a significant reduction in urinary pH at week 13. The aspartate aminotransferase activity in males at 900/1350 ppm was elevated non-significantly in weeks 7 and 13 (260 and 191% of control), as were the values for alanine aminotransferase activity (221 and 149%).
Table 8 shows the alterations in organ weights. The relative (to body weight) weight of the spleen (+72%) was increased in females and that of the thyroid (+80%) in males, and the weights of the liver (+60% in males, +36% in females) and kidney (+45% in males, +26% in females) were increased significantly at the highest dietary concentration. The weight of the pancreas was increased and that of the ovary decreased in females at > 150 ppm; however, there was considerable variation in individual values within each group, which greatly outweighed the variation in the mean values between groups. Furthermore, a dose–response relationship was not seen at 150 or 300 ppm, and no histological alterations were observed in either organ at 150 ppm. The Meeting therefore considered that the apparent effect on the pancreas and ovaries at 150 and 300 ppm was not treatment-related. Gross examination of animals at 900/1350 ppm revealed increased incidences of whitish (granular) areas of the gastric mucosa, distension of the stomach with food and pale livers in both sexes, emaciation, thymic atrophy, pancreatic oedema, lymph node enlargement, brown–yellow spots on the lungs, red spots on the gastric mucosa and thyroid and periocular sebum in males, and hepatic and splenic enlargement and hepatic pallor in females.
Table 8. Changes in weights of organs relative to body weight in dogs given spinosad in the diet for 90 days
|
Dietary concentration (ppm) |
Body weight |
Kidney |
Liver |
Spleen |
Thyroid |
Ovaries |
Pancreas |
||||||
|
|
M |
F |
M |
F |
M |
F |
M |
F |
M |
F |
|
M |
F |
|
0 |
10 |
9.3 |
0.40 |
0.39 |
2.5 |
2.6 |
– |
0.22 |
0.0070 |
0.0077 |
0.020 |
0.14 |
0.17 |
|
150 |
10 |
8.7 |
0.40 |
0.42 |
2.5 |
2.6 |
– |
0.24 |
0.0084 |
0.012* |
0.015 |
0.20 |
0.22 |
|
300 |
10 |
8.9 |
0.43 |
0.41 |
2.6 |
2.7 |
– |
0.24 |
0.0086 |
0.0091 |
0.016 |
0.18 |
0.20 |
|
900 |
8.2 |
8.2 |
0.58 |
0.49 |
4.0 |
3.5 |
– |
0.38 |
0.013* |
0.010* |
0.012 |
0.25* |
0.27 |
From Harada (1994); –, no effect
* p 0.05
Histopathological examination (Table 9) revealed increased incidences of vacuolation of cells of the spleen, lymph node, faucial tonsil, ileum, caecum, colon, rectum and pancreas, spermatid giant cells, foamy cell aggregation in the lung, and atrophy of the gastric mucosa in animals at dietary concentrations > 300 ppm. At the highest concentration, there were increased incidences of focal necrosis and cellular depletion in sternal and femoral bone marrow, increased haematopoiesis in the femoral bone marrow and spleen in females, cellular vacuolation in the liver, testis, pituitary, thyroid and parathyroid, brain and spinal cord, atrophy of the splenic white pulp, acinar cells of the salivary gland and thymus, Kupffer cell proliferation, and arteritis in the cerebrum, lung, epididymis, testes, spinal cord and optic nerve. The NOAEL was 150 ppm, equal to 4.9 mg/kg bw per day, on the basis of multiple histological alterations at higher concentrations (Harada, 1994).
Table 9. Numbers of dogs with histopathological lesionsin dogs after receiveing diets containing spinosad for 90 days (males/females)
|
Tissue and lesion |
Dietary concentration (ppm) |
|||
|
0 |
150 |
300 |
900/1350 |
|
|
Thymus |
||||
|
Atrophy |
0/0 |
0/0 |
0/1 |
2/0 |
|
Bone marrow |
||||
|
Sternal: Focal necrosis/cellular depletion |
0/0 |
0/0 |
0/0 |
1/2 |
|
Femoral: Focal necrosis/cellular depletion |
0/0 |
0/0 |
0/0 |
1/3 |
|
Femoral: Increased haematopoiesis |
0/0 |
0/0 |
0/0 |
0/1 |
|
Spleen |
||||
|
Vacuolated cell aggregation in white pulp |
0/0 |
0/0 |
1/1 |
4/4 |
|
Atrophic white pulp |
0/0 |
0/0 |
0/0 |
4/1 |
|
Lymph nodes |
||||
|
Cervical: Vacuolated cell aggregation in lymph follicles |
0/0 |
0/0 |
0/2 |
4/4 |
|
Mesenteric: Vacuolated cell aggregation in lymph follicles |
0/0 |
0/0 |
1/2 |
4/4 |
|
Faucial tonsil: Vacuolated cell aggregation in lymph follicles |
0/0 |
0/0 |
2/34/4 |
|
|
Lung |
||||
|
Foamy cell aggregation |
0/0 |
0/0 |
0/1 |
4/4 |
|
Stomach |
||||
|
Atrophic mucosa |
0/0 |
0/0 |
0/2 |
4/4 |
|
Ileum |
||||
|
Vacuolated cell aggregation in lymph follicles |
0/0 |
0/0 |
1/3 |
4/4 |
|
Caecum |
||||
|
Vacuolated cell aggregation in lymph follicles |
0/0 |
0/0 |
0/2 |
3/4 |
|
Colon |
||||
|
Vacuolated cell aggregation in lymph follicles |
0/0 |
|
2/1 |
4/4 |
|
Rectum |
||||
|
Vacuolated cell aggregation in lymph follicles |
0/0 |
0/0 |
2/0 |
3/4 |
|
Liver |
||||
|
Vacuolated hepatocytes |
0/0 |
0/0 |
0/0 |
3/1 |
|
Kupffer cell proliferation |
0/0 |
0/0 |
0/0 |
3/3 |
|
Pancreas |
||||
|
Vacuolated acinar cells |
0/0 |
0/0 |
2/0 |
4/3 |
|
Testes |
||||
|
Giant spermatid cells |
0 |
0 |
1 |
2 |
|
Arteritis |
0 |
0 |
0 |
2 |
|
Vacuolated seminiferous epithelial cells |
0 |
0 |
0 |
3 |
|
Decreased spermatogenesis |
0 |
0 |
0 |
1 |
|
Epididymis |
||||
|
Arteritis |
0 |
0 |
0 |
2 |
|
Thyroid |
||||
|
Vacuolated C-cells |
0/0 |
0/0 |
0/0 |
1/3 |
|
Parathyroid |
||||
|
Vacuolated glandular cells |
0/0 |
0/0 |
0/0 |
4/4 |
|
Brain |
||||
|
Cerebrum: Arteritis in meninx |
0/0 |
0/0 |
0/0 |
1/0 |
|
Cerebellum: Vacuolated nerve cells |
0/0 |
0/0 |
0/0 |
0/1 |
|
Pons: Vacuolated nerve cells |
0/0 |
0/0 |
0/0 |
3/1 |
|
Spinal cord |
||||
|
Cervical: Vacuolated nerve cells |
0/0 |
0/0 |
0/0 |
4/0 |
|
Thoracic: Vacuolated nerve cells |
0/0 |
0/0 |
0/0 |
2/2 |
|
Thoracic: Arteritis in nerve root/meninx |
0/0 |
0/0 |
0/0 |
2/0 |
|
Lumbar: Vacuolated nerve cells |
0/0 |
0/0 |
0/0 |
3/3 |
|
Eye |
||||
|
Arteritis in optic nerve |
0/0 |
0/0 |
0/0 |
2/0 |
From Harada (1994)
In a study conducted in accordance with GLP requirements and OECD guideline 452, groups of four beagle dogs of each sex were given diets containing spinosad (purity, 87.2%) for 12 months. The dietary concentration was 0, 50, 100 or 300 ppm in weeks 1–13, at which time the amount of feed was decreased from 300 g/dog per day to 250 g/dog per day in order to ‘prevent the dogs from becoming obese’; subsequently, the concentration of spinosad in the diet was increased to 0, 60, 120 and 360 ppm from weeks 14–52 to compensate for the reduced food intake. The experimental parameters determined were deaths, clinical signs, body weight, food consumption, ophthalmic end-points, neurological assessment (‘functional observational battery’) at week 49 or 50, haematological end-points (erythrocyte, total and differential leukocyte and platelet counts, erythrocyte volume fraction, haemoglobin, mean corpuscular haemoglobin, mean corpuscular volume, mean corpuscular haemoglobin concentration, eyrthrocyte sedimentation rate), clinical chemical parameters (albumin, globulin, total protein, bilirubin, cholesterol, triglyceride, glucose, electrolyte, creatinine and urea nitrogen concentrations and the activities of alanine and aspartate aminotransferase, creatine phosphokinase and g-glutamyl transpeptidase), urinary parameters (appearance, specific gravity, glucose, ketones, bacteria, occult blood, pH, protein, bilirubin, leukocytes), organ weights (adrenals, brain, gonads, heart, kidneys, liver, lungs, pituitary, pancreas, prostate, spleen, thyroid, thymus, uterus) and gross and histopathological appearance (adrenals, heart, prostate, aorta, small and large intestine, salivary gland, bone, kidneys, skin, bone marrow, brain, liver, lungs, spleen, lymph nodes, stomach, epididymides, muscle, gonads, eyes, thymus, peripheral nerve, thyroid, gall-bladder, oesophagus, trachea, urinary bladder, pancreas, uterus, femur and joint, pituitary, tongue and parathyroid). The approximate, average achieved doses were 1.4, 2.7 and 8.5 mg/kg bw per day for males and 1.3, 2.7 and 8.2 mg/kg bw per day for females.
Effects were seen at 300/360 ppm only. Slight but significantly increased aspartate aminotransferase activity (control, 34 U/l; 300/360 ppm, 50 U/l) and triglyceride concentration (41 and 54 mg/dl) were observed in males in week 26 only. As the values for these two parameters were higher than the control values for each dog, the finding is unlikely to be incidental. Alanine aminotransferase activity was increased nonsignificantly in males at week 26 (44 U/l for controls, 113 U/l at 300/360 ppm) and week 52 (40 and 83 U/l), but the increase was confined to three of the four animals, the fourth animal having normal values at both week 26 and 52. The relative thyroid weight was slightly increased in males (12%) and significantly in females (55%). Vacuolated cell aggregates were seen in the spleen, mesenteric and cervical (females only) lymph nodes and faucial tonsil in both sexes and in the ileum, caecum, colon and rectum in males. Two males also showed vacuolation of glandular cells of the parathyroid gland. The NOAEL was 100 ppm, equal to 2.7 mg/kg bw per day, on the basis of the occurrence of tissue vacuolation and alterations in clinical chemistry at 300/360 ppm (Harada, 1995).
(c) Long-term studies of toxicity and carcinogenicity
Mice
In a study conducted in accordance with GLP requirements and OECD guideline 451, groups of 70 CD-1 mice of each sex received diets containing spinosad (purity, 88%; 76.1% spinosyn A, 11.9% spinosyn D) at a concentration of 0, 25, 80 or 360 ppm for up to 18 months, equal to 0, 3.4, 11 and 51 mg/kg bw per day for males and 0, 4.3, 14 and 67 mg/kg bw per day for females. Ten animals of each sex per group were killed at 3 and 12 months. Females at 360 ppm were killed on day 455 because they had markedly reduced body-weight gain, and excessive deaths occurred. The tissues from this group were not examined, as the authors argued that the maximum tolerated dose had been exceeded and the results for these animals would be of questionable value. Toxicity was assessed by determining clinical signs at least daily, body weight and food consumption at regular intervals, ophthalmic end-points before treatment and at sacrifice, haematological end-points (erythrocyte, total and differential leukocyte and platelet counts, erythrocyte volume fraction, haemoglobin) and clinical chemical parameters (albumin, globulin, total protein, bilirubin, cholesterol, triglyceride, glucose, electrolyte, creatinine and urea nitrogen concentrations and alanine and aspartate aminotransferase and alkaline phosphatase activities) before each kill, gross appearance at each kill, histological changes in the adrenals, heart, prostate, aorta, small and large intestine, salivary gland, Harderian gland, sternum, lachrymal gland, bone, kidneys, skin, bone marrow, brain, liver, lungs, spleen, lymph nodes, stomach, epididymides, muscle, gonads, eyes, thymus, peripheral nerve, thyroid, gall-bladder, oesophagus, trachea, urinary bladder, pancreas, uterus, pituitary, tongue, parathyroid, oral and nasal tissues, vagina and cervix from controls and groups at the highest concentration at each interim sacrifice (subsets of other groups examined), and at 18 months in controls, females at 80 ppm and males at 360 ppm. The brain, heart, liver, kidneys, testes and spleen were weighed.
Treatment-related effects were found only at 360 ppm. The mortality rates were substantially increased by the end of the study, being 24% for male and 18% for female controls, 35% for males and 26% for females at 25 ppm, 29% for males and 13% for females at 80 ppm and 44% for males and 60% for females at 360 ppm, exceeding the acceptable limits for a maximum tolerated dose. Body-weight gain was 10–15% less than that of controls, resulting in significantly lower terminal weights, and food intake was slightly reduced in females. Clinical signs of toxicity (dermatitis of the ear, lachrymation, thin appearance, perineal soiling and roughened coat) were observed.
Slight, transient anaemia consisting of significantly decreased erythrocyte volume fraction (10–12% below control value) and haemoglobin (by 8–16%) was seen in both sexes at 3 months and in males and to a lesser extent in females at 12 months, but was not apparent in males at 18 months (females not examined). A slight but significant decrease in albumin concentration (10% below control) and total protein concentration (6–11%) was observed in both sexes at 12 months and to a similar extent in males at 18 months. At 12 months, the phosphate (27%) and sodium (8%) concentrations in females were significantly elevated. An increase in the incidence and severity of hypochromasia and a slightly but significantly decreased calcium concentration (5%) were seen in males at 18 months. Liver weights were increased in both sexes from 3 months, the relative weights (to body weight) being 20 and 27% greater than those of controls for males and females at 12 months, respectively, and the spleen weights were increased in both sexes at 3 months (76% greater than controls for males, 47% for females) and 12 months (110% for males, 30% for females), the relative weights generally being significantly different from those of controla. Gross examination revealed increased incidences of decreased fat and thickening of the glandular portion of the stomach in females at 12 months. At terminal sacrifice, increased incidences of chronic active inflammation of the pinna and a decreased incidence of distension of the ovarian bursa were found in females and an increased incidence of perineal soiling, decreased fat and thickening of the gastric glandular mucosa in both sexes.
Histopathological examination revealed vacuolation in a number of tissues at all scheduled sacrifices (3, 12 and 18 months); the tissues affected were the cervix, epididymides, mesenteric lymph nodes, ovaries, pancreas, parathyroid, uterus and vagina at 3 months; epididymides, mesenteric lymph nodes, ovaries, pancreas and parathyroid at 12 months; and epididymides, pancreas and parathyroid at 18 months. Other effects were degeneration or regeneration of the kidneys and increased extramedullary haematopoiesis in the spleen at 3 months; aggregates of alveolar macrophages in the lungs, histiocytosis in the mesenteric lymph nodes, myopathy of skeletal muscle and tongue and inflammation, hyperplasia or hyperkeratosis of the gastric mucosa at 3, 12 and 18 months; and aggregates of reticuloendothelial cells in the liver at 18 months. The key findings are summarized in Table 10. There were no tumours that could be attributed to treatment. The NOAEL was 80 ppm, equal to 11 mg/kg bw per day, on the basis of multiple histological alterations and clinical chemical changes and effects on organ weights at 360 ppm (Bond et al., 1995a).
Table 10. Gross and histopathological findings in mice given spinosad in the diet for 18 months
|
Time and finding |
Dietary concentration (ppm) |
|||||||
|
0 |
25 |
80 |
360 |
|||||
|
M |
F |
M |
F |
M |
F |
M |
F |
|
|
12 months (10 animals) |
||||||||
|
Epididymides |
||||||||
|
Epithelial cell vacuolation: Very slight |
10 |
|
9 |
|
9 |
|
0 |
|
|
Epithelial cell vacuolation: Slight |
0 |
|
0 |
|
0 |
|
10 |
|
|
Lungs |
||||||||
|
Aggregates of alveolar macrophages |
3 |
2 |
4 |
1 |
3 |
1 |
9 |
9 |
|
Lymph nodes (mesenteric) |
||||||||
|
Sinus histiocytosis |
1 |
1 |
1 |
0 |
0 |
2 |
8 |
5 |
|
Vacuolation, macrophage(s), slight |
0 |
0 |
0 |
0 |
0 |
0 |
3 |
3 |
|
Ovaries |
||||||||
|
Vacuolation: Very slight |
|
5 |
|
6 |
|
5 |
|
2 |
|
Vacuolation: Slight |
|
1 |
|
0 |
|
0 |
|
8 |
|
Pancreas |
||||||||
|
Vacuolation: Very slight |
7 |
7 |
9 |
9 |
8 |
7 |
1 |
1 |
|
Vacuolation: Slight |
1 |
2 |
0 |
0 |
0 |
0 |
7 |
8 |
|
Parathyroid |
||||||||
|
Vacuolation: Slight |
0 |
0 |
0 |
0 |
0 |
0 |
9 |
7 |
|
Skeletal muscle |
||||||||
|
Myopathy |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
3 |
|
Stomach |
||||||||
|
Hyperplasia, glandular mucosa |
6 |
4 |
5 |
3 |
3 |
4 |
10 |
10 |
|
Chronic inflammation, glandular mucosa |
2 |
3 |
1 |
4 |
1 |
2 |
10 |
10 |
|
Tongue |
||||||||
|
Myopathy: Slight |
0 |
0 |
0 |
0 |
0 |
0 |
3 |
6 |
|
18 months (50 animals) |
||||||||
|
Chronic active inflammation of pinna |
0 |
1 |
5 |
0 |
1 |
1 |
4 |
13 |
|
Perineal soiling |
1 |
1 |
4 |
1 |
4 |
1 |
8 |
7 |
|
Decreased amount of fat |
11 |
6 |
8 |
11 |
6 |
5 |
24 |
21 |
|
Distended ovarian bursa |
|
21 |
|
17 |
|
27 |
|
8 |
|
Diffuse thickening of glandular mucosa of the stomach |
5 |
2 |
1 |
4 |
3 |
0 |
35 |
32 |
|
Epididymides |
||||||||
|
Epithelial cell vacuolation: Very slight |
45 |
|
44 |
|
43 |
|
2 |
|
|
Epithelial cell vacuolation: Slight |
1 |
|
0 |
|
0 |
|
48* |
|
|
Liver |
||||||||
|
Aggregates of reticuloendothelial cells |
41 |
43 |
35 |
39 |
36 |
45 |
26 |
|
|
Lungs |
||||||||
|
Aggregates of alveolar macrophages |
6 |
10 |
6 |
3 |
11 |
8 |
40* |
|
|
Lymph node |
||||||||
|
Sinus histiocytosis |
1 |
1 |
0 |
3 |
3 |
1 |
13* |
|
|
Pancreas |
||||||||
|
Vacuolation of acini: Very slight |
22 |
16 |
15 |
17 |
22 |
17 |
22 |
|
|
Vacuolation of acini: Slight |
8 |
5 |
5 |
7 |
5 |
9 |
23* |
|
|
Parathyroid |
||||||||
|
Vacuolation: Slight |
3 |
0 |
2 |
1 |
5 |
0 |
40* |
|
|
Skeletal muscle |
||||||||
|
Myopathy |
0 |
0 |
0 |
0 |
0 |
0 |
5* |
|
|
Stomach |
||||||||
|
Hyperkeratosis, nonglandular mucosa |
2 |
6 |
2 |
2 |
0 |
0* |
20* |
|
|
Hyperplasia, glandular mucosa |
26 |
28 |
26 |
25 |
26 |
30 |
40* |
|
|
Hyperplasia, nonglandular mucos: Slight |
1 |
1 |
1 |
1 |
0 |
0 |
12* |
|
|
Chronic inflammation, glandular mucosa |
12 |
18 |
17 |
18 |
12 |
15 |
43* |
|
|
Tongue |
||||||||
|
Myopathy: Slight |
0 |
0 |
0 |
0 |
0 |
0 |
24* |
|
From Bond et al. (1995a)*Significantly different from control
Because of the early deaths of females at 360 ppm in the previous study, an 18-month study was conducted in which groups of 60 CD-1 mice of each sex received diets containing spinosad (purity, 88%; 76.1% spinosyn A, 11.9% spinosyn D) at a concentration of 0, 8 or 240 ppm for 18 months, equal to 0, 1.1 and 33 mg/kg bw per day for males and 0, 1.3 and 42 mg/kg bw per day for females. Ten animals of each sex per group were killed at 12 months for interim investigations. Toxicity was assessed by determining clinical signs at least daily, body weight and food consumption at regular intervals, ophthalmic end-points before treatment and at the kills, haematological parameters (erythrocyte, total and differential leukocyte and platelet counts, erythrocyte volume fraction, haemoglobin) and clinical chemical parameters (albumin, globulin, total protein, bilirubin, cholesterol, triglyceride, glucose, electrolyte, creatinine and urea nitrogen concentrations and alanine and aspartate aminotransferase and alkaline phosphatase activities) before each kill, gross examination at each kill and histopathological examination of females at 0 and 240 ppm only (adrenals, heart, prostate, aorta, small and large intestine, salivary gland, Harderian gland, sternum, lachrymal gland, bone, kidneys, skin, bone marrow, brain, liver, lungs, spleen, lymph nodes, stomach, epididymides, muscle, gonads, eyes, thymus, peripheral nerve, thyroid, gall-bladder, oesophagus, trachea, urinary bladder, pancreas, uterus, pituitary, tongue, parathyroid, oral and nasal tissues, vagina and cervix) and the weights of the brain, heart, liver, kidneys, testes and spleen. The study was conducted in accordance with GLP requirements and OECD guideline 451.
Mortality rates were unaffected. Body-weight gain was lower in both sexes at 240 ppm early in the study but was similar to control values thereafter, reflecting acclimatization to the study diet. A statistically significant increase observed in leukocyte count in females at 8 and 240 ppm (approximately twice control values) at 12 months was probably due to a low control value, as the control value at 18 months was similar those of animals at 8 and 240 ppm at both the 12-month interim kill and terminal sacrifice at 18 months. The weight of the liver relative to body weight was significantly increased (by 8–10%) in both sexes at 18 months. Gross examination revealed thickening of the glandular portion of the stomach in both sexes at 240 ppm.
Histopathological examination of females at 0 and 240 ppm showed that the alterations at 240 ppm were similar to that observed at 360 ppm in the previous study, consisting primarily of sinus histiocytosis of the mesenteric lymph nodes, vacuolation of pancreatic acini, vacuolation of the parathyroid, aggregates of alveolar macrophages and alveolar cell hyperplasia in the lungs, skeletal muscle and tongue myopathy and inflammation and hyperplasia or hyperkeratosis of the gastric mucosa (Table 11).
Table 11. Histopathological findings at 18 months in control female mice and females given a diet containing spinosad at 240 ppm (n=50) for 18 months
|
Lesion |
Control |
240 ppm |
|
Lung |
||
|
Aggregates of alveolar macrophages |
8 |
39* |
|
Lung |
||
|
Alveolar cell hyperplasia |
2 |
6 |
|
Lymph node |
||
|
Sinus histiocytosis |
2 |
20* |
|
Pancreas |
||
|
Vacuolation of acini |
35 |
41 |
|
Parathyroid |
||
|
Vacuolation, slight |
2 |
25* |
|
Skeletal muscle |
||
|
Myopathy |
0 |
22* |
|
Stomach |
||
|
Hyperkeratosis, nonglandular mucosa |
7 |
19* |
|
Hyperplasia, glandular mucosa |
18 |
48* |
|
Hyperplasia, nonglandular mucosa, |
3 |
22* |
|
Chronic inflammation, glandular mucosa |
14 |
39* |
|
Tongue |
||
|
Myopathy, slight |
0 |
17* |
From Bond et al. (1996)* Significantly different from control
Both the nature and incidence of tumours at 240 ppm were similar to those in controls. As limited investigations were carried out on in males and in females at 8 ppm, a NOAEL could not be identified in this study. The results support, however, the NOAEL of 80 ppm (equal to 11 mg/kg bw per day) in the previous study (Bond et al., 1996).
Rats
Groups of 65 Fischer 344 rats of each sex received diets containing spinosad (purity, 88%; 76.1% spinosyn A and 11.9% spinosyn D) at a concentration of 0, 50, 200, 500 or 1000 ppm, equal to 0, 2.4, 9.5, 24 and 49 mg/kg bw per day for males and 0, 3, 12, 30 and 63 mg/kg bw per day for females, for 24 months. Fifty animals of each sex per group were scheduled to receive spinosad for 2 years, 10 of each sex per group were used to assess toxicity and five of each sex per group were used to assess neurotoxicity (see below). Clinical signs of toxicity, body weight and food consumption were assessed at regular intervals during the study, and ophthalmic examinations were performed before treatment and at sacrifice. Haematological parameters (erythrocyte, total and differential leukocyte and platelet counts, erythrocyte volume fraction, haemoglobin), clinical chemical end-points (albumin, globulin, total protein, bilirubin, cholesterol, triglyceride, glucose, electrolyte, creatinine and urea nitrogen concentrations and alkaline phosphatase, alanine and aspartate aminotransferase and creatine phosphokinase activities) and urological parameters (appearance, specific gravity, glucose, ketones, urobilinogen, occult blood, pH, protein, bilirubin, leukocytes) were assessed at 6 and 12 months in 10 animals of each sex per satellite group and at 18 and 24 months in 10 and 20 surviving rats, respectively. Gross examinations were conducted on 10 animals of each sex per satellite group after 12 months, on all decedents and at final sacrifice. Histological examination of all gross lesions, the larynx, nasal and oral tissues, cervix, adrenals, heart, prostate, aorta, small and large intestine, salivary gland, Harderian gland, lachrymal gland, bone, kidneys, skin, bone marrow, brain, liver, lungs, spleen, lymph nodes, stomach, epididymides, muscle, gonads, eyes, thymus, peripheral nerve, thyroid, oesophagus, trachea, urinary bladder, pancreas, uterus, mammary gland, pituitary, tongue and parathyroid was conducted, and the weights of the adrenals, brain, gonads, heart, kidneys, liver, lungs, pituitary, pancreas, prostate, spleen, thyroid, thymus and uterus were determined in 10 rats of each sex per group after 12 months, on all decedents and on surviving control and treated animals. At terminal sacrifice, a limited subset of tissues from animals at 50 and 200 ppm were examined histologically; these comprised the liver, kidney, lungs, thyroid with parathyroids, tissues in which the incidence of alterations at 500 or 1000 ppm suggested a possible treatment-related lesion and tissues with gross lesions. The study complied with the requirements of GLP and OECD guideline 453.
Owing to high mortality rates among controls and rats at 1000 ppm (28% male controls, 30% female controls, 88% males and 60% females at 1000 ppm) in weeks 102 and 62, reduced body weight gains (by > 10–15%) at this concentration and clinical signs of overt toxicity (perineal soiling, rapid respiration, thin appearance, decreased body fat reserves), these groups were discontinued on day 714 for males and 611 for females. The mean terminal body weight was approximately 82% those of controls before termination, but food consumption was unaffected. Although a number of statistically significant changes were seen in haematological and clinical chemical parameters at the interim kills, most were minimal and did not persist to final sacrifice and were therefore considered to be transient or incidental. Potentially treatment-related alterations were seen in alkaline phosphatase and aspartate aminotransferase activities and blood urea nitrogen concentration, although in each case the increase was modest and, with the exception of aspartate aminotransferase activity, was not correlated with histological alterations at sacrifice. Alkaline phosphatase activity was significantly increased in males at 500 and 1000 ppm at 18 months (by 24% and 43%, respectively) and in females at 1000 ppm at 6 and 12 months (by 28% and 32%, respectively) and nonsignificantly at 18 months (26%). Aspartate aminotransferase activity was significantly increased in males at 1000 ppm at 12 (35%) and 18 months (42%) and in females at 18 months (54%) and correlated with, but was not necessarily caused by, lesions observed in the heart at this concentration. The blood urea nitrogen concentration was significantly increased in females at 1000 ppm at 6, 12 and 18 months (by 16%, 56% and 38%) and in males at 6 and 18 months (by 12% and 24%). The globulin concentration was slightly but significantly increased in males at 500 ppm at 18 and 24 months (by 11% and 14%), in females at 500 ppm from 6 months onwards (by 9%, 17%, 11% and 11%), and in both sexes at 1000 ppm at 12 and 18 months (by 9% and 14% in males, 13% and 8% in females). The values were, however, generally within the range seen in other controls in the laboratory. Statistically significant, treatment-related changes in organ weights were seen at 12 months, consisting of increased absolute and relative weights of the heart (dose-related from 500 ppm in females), kidney, liver (absolute weights significant in females only), spleen and thyroid at 1000 ppm. An increase in absolute and relative ovarian weights at concentrations > 500 ppm was dose-related, and adrenal weights were increased at 1000 ppm in females at 12 months. At 24 months, the absolute and relative thyroid weights were increased in both sexes, and the heart and kidney weights were increased in females at 500 ppm. Multifocal pale areas on the lungs were observed in 1/10 males and 8/10 females at 1000 ppm at 12 months.
The histological lesions seen (Table 12) were vacuolation in the kidneys and thyroid, inflammation in the liver, thyroid and prostate, extramedullary haematopoiesis of the spleen and degeneration or regeneration of the glandular mucosa of the stomach. The incidence and/or severity of aggregation of reticuloendothelial cells was increased in the larynx, liver, lymph nodes and spleen. At 500 ppm, there were increased incidences of vacuolation of epithelial cells in the thyroid in both sexes. An increase in the intensity, from very slight to slight or moderate, of reticuloendothelial cell aggregation was observed in the livers of females at 1000 ppm at 12 months, in the mesenteric lymph nodes of both sexes at 12 and 24 months and in the spleen of females at 12 months.
Table 12. Principal histopathological findings in rats given diets containing spinosad for 2 years
|
Lesion |
Dietary concentration (ppm) |
|||||||||
|
0 |
50 |
200 |
500 |
1000 |
||||||
|
M |
F |
M |
F |
M |
F |
M |
F |
M |
F |
|
|
12 months (n = 10) |
||||||||||
|
Kidney |
||||||||||
|
Vacuolation of tubules: Slight |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
9 |
|
Larynx |
||||||||||
|
Aggregation of reticuloendothelial cells: Slight to very slight |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
2 |
7 |
|
Liver |
||||||||||
|
Focal or multifocal subacute to chronic inflammation:Very slight |
1 |
1 |
1 |
1 |
0 |
1 |
2 |
3 |
6 |
10 |
|
Prostate |
||||||||||
|
Acute inflammation |
4 |
|
2 |
|
3 |
|
6 |
|
8 |
|
|
Spleen |
||||||||||
|
Increased extramedullary haematopoiesis |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
10 |
|
Stomach |
||||||||||
|
Degeneration or regeneration of glandular mucosa: Very slight |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
9 |
|
Thyroid |
||||||||||
|
Vacuolation of epithelial cells |
0 |
0 |
0 |
0 |
0 |
0 |
10 |
10 |
10 |
10 |
|
Subacute to chronic inflammation |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
3 |
10 |
9 |
|
24 months (n = 50) |
||||||||||
|
Bone marrow |
||||||||||
|
Myeloid hyperplasia |
1 |
1 |
1 |
2 |
1 |
1 |
1 |
7 |
– |
|
|
Larynx |
||||||||||
|
Inflammation: Subacute to chronic |
6 |
7 |
2 |
13 |
1 |
9 |
5 |
19 |
– |
|
|
Liver |
||||||||||
|
Dilatation of sinusoids; focal: Very slight |
1 |
1 |
2 |
2 |
0 |
6 |
0 |
8 |
– |
|
|
Lung |
||||||||||
|
Focal or multifocal subacute to chronic inflammation |
14 |
18 |
17 |
16 |
13 |
13 |
16 |
37 |
– |
|
|
Thyroid gland |
||||||||||
|
Necrosis |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
16 |
– |
|
|
Vacuolation of epithelial cells |
0 |
6 |
0 |
6 |
7 |
34 |
48 |
42 |
– |
|
|
Inflammation: Subacute to chronic |
0 |
1 |
0 |
0 |
0 |
0 |
3 |
32 |
– |
|
From Bond et al. (1995b)
The gross findings at termination of groups at 1000 ppm were limited to decreased body fat, increased incidences of perineal soiling, serosanguinous fluid in the thoracic cavity and pale areas on the lungs of a substantial proportion of the animals. A small proportion also had mottled left atria and 21/50 males and 11/50 females had a thrombus in the left atrium. The size of the thyroid gland was increased in four of seven animals. At scheduled sacrifice of the remaining groups, increased incidences and/or severity were seen of myeloid hyperplasia, inflammation of the larynx, lungs and thyroid, necrosis of the thyroid in females at 500 ppm, slight dilatation of hepatic sinusoids in females and vacuolation of thyroidal epithelial cells in both sexes at concentrations > 200 ppm. There were also increased incidences of altered eosinophilic hepatocellular cells (in 0, 4, 7 and 2 males and 0, 6, 9 and 2 females at 0, 50, 200, 500 and 1000 ppm, respectively) and mineralization of pulmonary blood vessels (in 0, 11, 13, 0 and 0 males and 1, 12, 9, 0 and 9 females at 0, 50, 200, 500 and 1000 ppm, respectively); however, the incidences were not dose-related, and the finding is likely to be incidental. The nature, incidence and time to onset of tumours was similar in all groups. The NOAEL was 50 ppm, equal to 2.4 mg/kg bw per day, on the basis of histopathological effects at higher concentrations (Bond et al., 1995b).
The results of studies of the genotoxicity of spinosad are summarized in Table 13.
Table 13. Results of studies of the genotoxicity of spinosad
|
End-point |
Test object |
Concentration |
Purity |
Results |
Reference |
|
In vitro |
|||||
|
Reverse mutation |
S. typhimurium TA1535, TA1537, TA98, TA100; E. coli WP2uvrA– |
310–5000 µg/plate in DMSO |
88.0% |
Negative ± S9 |
Garriott et al. (1992a)a |
|
Reverse mutation |
S. typhimurium TA1535, TA98, TA100; E. coli WP2uvrA– |
100–5000 µg/plate in DMSO |
88.0% |
Negative ± S9 |
Lawlor (1996a)b |
|
|
S. typhimurium TA1537 |
50–2500 µg/plate –S9 |
|
Negative |
|
|
Forward mutation |
L5178Y Tk+/– mouse lymphoma cells |
1–35 µg/ml –S9 |
88.0% |
Negative ± S9 |
Garriot et al. (1992b)c |
|
Chromosomal aberration |
Chinese hamster ovary cells |
20–35 µg/ml –S9 |
88.0% |
Negative ± S9 |
Garriott et al. (1992c)d |
|
Unscheduled DNA synthesis |
|
0.5–1000 and 0.01–50 µg/ml, in DMSO |
88.0% |
Negative ± S9 |
Garriott et al. (1992d)e |
|
In vivo |
|||||
|
Micronucleus formation |
Male and female ICR mice |
0, 500, 1000, 2000 mg/kg bw per day × 2 days, orally in 10% acacia in water |
88.0% |
Negative |
Garriott et al. (1992e)f |
|
|
S9, 9000 x g supernatant of Aroclor 1254-induced rat liver; DMSO, dimethyl sulfoxide |
|
|
GLP and QA statements and positive controls included |
|
a |
Test in triplicate. In a preliminary test, concentrations of 1500 and 5000 µg/plate were cytotoxic to TA1537 only. Spinosad promoted the growth of auxotrophs due to trace amounts of histidine and other amino acids as impurities. Absence of revertants was confirmed by replicate plate assay. |
|
b |
In a preliminary test, cytotoxicity was seen in TA100 at 3330 and 5000 µg/plate, TA98 at > 2500 µg/plate, TA1535 at 5000 µg/plate, TA1537 > 1250 µg/plate. Trace amino acid residues were removed before testing. |
|
c |
Concentration-dependent cytotoxicity seen ± S9, obviating interpretation of result –S9 at > 25 µg/ml |
|
d |
Conducted in duplicate; cytotoxicity demonstrated in a preliminary study at > 100 µg/ml |
|
e |
Conducted in duplicate; cytotoxicity observed at concentrations > 10 µg/ml |
|
f |
Five animal of each sex per dose |
In a study conducted to GLP standards and OECD guideline 416, groups of 30 Sprague-Dawley rats of each sex were fed diets containing spinosad (purity, 88%; 76.1% spinosyn A and 11.9% spinosyn D) at concentrations regularly adjusted to deliver an actual dose of 0, 3, 10 or 100 mg/kg bw per day for two generations. After 10 weeks, F0 rats were mated to produce F1a litters, of which 30 rats of each sex per dose were randomly selected to be F1 parental animals. These were mated about 12 weeks after weaning of the last F1a litter to produce F2 litters. One week after weaning of F1a litters, F0 parents were again mated to produce F1b litters. All litters were culled to four pups of each sex when possible on day 4 post partum. Clinical signs of toxicity, body weight and food consumption were assessed at regular intervals throughout the study. The litter parameters assessed included litter size at birth, numbers of live and dead pups on days 0, 1, 4, 7, 14 and 21, and sex and weight on days 1, 4 (before and after culling), 7, 14 and 21. Gross examinations were conducted on all F0 and F1 adults after the last litters were weaned. The liver, kidneys, heart, spleen and thyroid were weighed. Potential target organs and reproductive tissues from all controls and animals at the highest dose were examined histologically. Examination of tissues from rats at the lowest and intermediate doses was restricted to the cervix, coagulating glands, epididymides, heart, kidneys, liver, mesenteric lymph node, ovaries, oviduct, parathyroid, pituitary, prostate, seminal vesicles, spleen, stomach, testes, thyroid, urinary bladder, uterus and vagina. At necropsy, the plasma concentrations of thyroid hormones were determined in 10 F1 animals of each sex. At the time of weaning, 10 pups of each sex per dose from the F1a, F1b and F2 litters were randomly selected for gross examination; the tissues were not examined histologically.
Treatment-related effects on parental animals were observed only at 100 mg/kg bw per day. The clinical signs consisted of increased perineal soiling and vaginal bleeding during lactation of F1a and F2 pups, and F1 females also had an increased incidence of dystocia. The body-weight gain of F0 males was significantly reduced (6%), the body weight of F0 dams during gestation was significantly reduced, and the body-weight gains between parturition and the end of lactation of F1a and F1b pups were approximately 16% lower than those of controls. At 100 mg/kg bw per day, the number of pups born alive was significantly reduced by 20–35% in all litters of both parental generations (F1a, F1b and F2 litters), and the pup body weights were 8–12% lower than in controls on days 14 and 21 of lactation (significant only on day 21). Significantly increased relative weights of the heart, kidney, liver, spleen and thyroid were seen in F0 parents (in males and females: 22%/19%, 13%/19%, 19%/14%, 19%/42%, 128%/26% above control values, respectively) and F1 parents (in males and females: 22%/14%, 15%/13%, 17%/16%, 42%/16%, 71%/30% above control values, respectively) at 100 mg/kg bw per day. Macroscopically, an increased incidence of pale foci was seen in the lungs of F0 adults, two F0 animals had resorbed or dead fetuses, and the incidence of watery caecal contents was increased in F1 adults. Histologically, an increase in severity but not incidence was seen for the following findings: degeneration with or without inflammation in the heart, multifocal tubule degeneration or regeneration in the kidneys, sinus histiocytosis of the mesenteric lymph node, and thyroid epithelial-cell vacuolation. Increases in severity and/or incidence was seen for the following effects: inflammation or aggregation of alveolar macrophages in the lungs, sinus histiocytosis in the spleen, inflammation of the prostate, inflammation and necrosis of the thyroid and dilatation of the stomach with or without cellular debris.
The clinical observations in F1a and F2 pups were attributable to maternal toxicity and included stomachs void of milk and being cold to touch at doses > 10 mg/kg bw per day in F1a litters and at 100 mg/kg bw per day in F2 litters, thinness or decreased activity in F1a pups at 100 mg/kg bw per day, and an increased incidence of cannibalization of F2 pups at 100 mg/kg bw per day. The effects in pups of the F1a generation at 10 mg/kg bw per day were confined almost entirely to one litter, in which all pups were affected and all died by postnatal day 2. The dam produced a normal litter of healthy pups after the F1b mating. Of the remaining 395 F1a pups at this dose, only one had a stomach devoid of milk and two were cold to touch. No other litter of F1b or F2 pups was affected at this dose. Given the nonspecific nature of these effects and the occurrence only in pups of one dam, the finding is probably incidental. The effects in pups at 100 mg/kg bw per day were likely to be secondary to maternal toxicity rather than a direct effect on the pups. The gross and histopathological findings are summarized in Table 14.
Table 14. Gross and histopathological findings in a two-generation study of reproductive toxicity in rats
|
Lesion |
Dose (mg/kg bw per day ) |
|||||||
|
0 |
3 |
10 |
100 |
|||||
|
M |
F |
M |
F |
M |
F |
M |
F |
|
|
Gross findings (n = ~ 30) |
||||||||
|
F0: Pale lungs, generalized multifocal |
0 |
2 |
0 |
1 |
0 |
1 |
6 |
8 |
|
F1: Watery contents of caecum |
2 |
5 |
0 |
1 |
5 |
6 |
20 |
17 |
|
F0 histopathological findings (n=30) |
|
|
|
|
|
|
|
|
|
Lung |
||||||||
|
Subacute or chronic inflammation, multifocal alveoli or septa: Very slight |
4 |
62 |
3 |
0 |
4 |
7 |
10 |
|
|
Subacute or chronic inflammation, multifocal alveoli or septa: Slight |
0 |
0 |
0 |
0 |
0 |
0 |
3 |
2 |
|
Multifocal aggregates of alveolar macrophages: Very slight to slight |
10 |
6 |
4 |
4 |
8 |
6 |
12 |
9 |
|
Multifocal aggregates of alveolar macrophages: Moderate |
0 |
0 |
0 |
0 |
2 |
1 |
12 |
10 |
|
Prostate |
||||||||
|
Chronic active, multifocal inflammation: Very slight |
1 |
|
2 |
|
1 |
|
7 |
|
|
Chronic active, multifocal inflammation: Slight |
0 |
|
0 |
|
0 |
|
4 |
|
|
Spleen |
||||||||
|
Sinus histiocytosis: Very slight |
0 |
0 |
0 |
0 |
0 |
0 |
9 |
20 |
|
Sinus histiocytosis: Slight |
0 |
0 |
0 |
0 |
0 |
0 |
21 |
6 |
|
Thyroid |
||||||||
|
Chronic active multifocal inflammation of interstitium: Very slight |
0 |
0 |
0 |
1 |
0 |
0 |
11 |
7 |
|
Chronic active multifocal inflammation of interstitium: Slight to mmoderate |
0 |
0 |
0 |
0 |
0 |
0 |
16 |
2 |
|
Focal necrosis: Very slight |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
|
Multifocal necrosis: Very slight to slight |
0 |
0 |
0 |
0 |
0 |
0 |
10 |
4 |
|
F1 histopathological findings |
||||||||
|
Kidneys |
||||||||
|
Multifocal mineralization of tubules: Very slight |
5 |
20 |
6 |
19 |
5 |
17 |
10 |
15 |
|
Multifocal mineralization of tubules: Severe |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
|
Lung |
||||||||
|
Subacute or chronic inflammation, multifocal alveoli or septa: Very slight |
4 |
5 |
2 |
1 |
3 |
3 |
13 |
16 |
|
Subacute or chronic inflammation, multifocal alveoli or septa: Slight |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
1 |
|
Multifocal aggregates of alveolar macrophages: Very slight |
4 |
5 |
3 |
5 |
5 |
2 |
12 |
11 |
|
Multifocal aggregates of alveolar macrophages: Slight to moderate |
1 |
0 |
0 |
0 |
2 |
0 |
8 |
7 |
|
Prostate |
||||||||
|
Chronic active, multifocal inflammation: Very slight |
3 |
|
2 |
|
3 |
|
7 |
|
|
Chronic active, multifocal inflammation: Moderate |
0 |
|
0 |
|
0 |
|
3 |
|
|
Spleen |
||||||||
|
Sinus histiocytosis: Very slight |
0 |
0 |
0 |
0 |
0 |
0 |
13 |
17 |
|
Sinus histiocytosis: Slight to moderate |
0 |
0 |
0 |
0 |
0 |
0 |
14 |
5 |
|
Stomach |
||||||||
|
Dilatation of glandular mucosa: Very slight |
0 |
1 |
0 |
0 |
0 |
1 |
1 |
11 |
|
Thyroid |
||||||||
|
Chronic active focal inflammation of interstitium: Very slight |
1 |
0 |
0 |
0 |
0 |
1 |
0 |
5 |
|
Chronic active multifocal inflammation of interstitium: Very slight to slight |
0 |
0 |
0 |
0 |
0 |
0 |
16 |
6 |
|
Multifocal necrosis: Very slight |
0 |
0 |
0 |
0 |
0 |
0 |
6 |
2 |
From Breslin et al. (1994)
Despite the histological alterations in the thyroids of rats at the highest dose, the total thyroxine concentration in the serum was unaffected. No effects were observed in F0 or F1 adults at 3 or 10 mg/kg bw per day. The NOAEL for parental toxicity was 10 mg/kg bw per day on the basis of clinical signs of toxicity, reduced body-weight gain, effects on organ weights and multiple histological alterations at 100 mg/kg bw per day. The NOAEL for reproductive effects was 10 mg/kg bw per day on the basis of decreased litter size at 100 mg/kg bw per day. The NOAEL for developmental toxicity was 10 mg/kg bw per day on the basis of a reduction in the number of pups per litter and clinical signs (cold to touch and stomachs devoid of milk) at 100 mg/kg bw per day, these effects probably reflecting maternal toxicity (Breslin et al., 1994).
Rats
In a study conducted to GLP standards and OECD guideline 414, groups of 30 pregnant Sprague-Dawley rats were given spinosad (purity, 88%; 76.1% spinosyn A and 11.9% spinosyn D) as a suspension in an aqueous solution of 500 ppm methyl cellulose ether by gavage on days 6–15 of gestation (day 0 designated first day of gestation) at a dose of 0, 10, 50 or 200 mg/kg bw per day. Deaths, clinical signs of toxicity, body weight and food consumption were assessed at regular intervals throughout the study. All surviving rats were killed on day 21 of gestation and examined grossly. The parameters reported were the number and position of fetuses in utero, the numbers of live and dead fetuses, the number and position of resorptions, the number of corpora lutea, fetal sex ratio and body weight and any gross external alterations. Visceral examinations were conducted on one-half of the fetuses in each litter, and the other half were subjected to skeletal examinations. The liver, kidney, spleen, thyroid gland, trachea, ovaries, oviducts, uterus, cervix and vagina were taken from two control rats and five at 200 mg/kg bw per day and processed for histological evaluation, and the liver, kidney, spleen, heart and gravid uterus were weighed.
Mean maternal body-weight gain was significantly decreased (10%) in dams at 200 mg/kg bw per day during treatment but was similar to that of controls over the entire gestation period owing to a compensatory weight gain on cessation of treatment. Water consumption was increased in dams at 200 mg/kg bw per day from day 16 of gestation. The number of fetuses per litter and the number of viable litters were unaffected. Visceral and skeletal examinations of fetuses revealed a slight, equivocal increase in the fetal and litter incidences of delayed ossification of sternebrae at doses > 50 mg/kg bw per day (with incidences of 7%, 6.8%, 10% and 11% in fetuses and 33%, 39%, 45% and 56% in litters at 0, 10, 50 and 200 mg/kg bw per day, respectively). Given the flat dose–response relationship for this observation and the high background incidence, this apparent affect was considered incidental to treatment.
Microphthalmia was observed in one fetus at 50 mg/kg bw per day (0.4%) and two at 200 mg/kg bw per day (0.8%), in different litters. The incidence was not statistically significant and was small in terms of the absolute numbers of fetuses affected. Microphthalmia is a relatively rare malformation: the published incidence in other animals from the same source as that used in this study (Charles River Laboratories, USA) during the period in which the study was performed showed only five cases of microphthalmia in 64 789 fetuses, giving an incidence of 0.008% and a maximum incidence of 0.41% (MARTA, 1996). As unilateral developmental effects are also unusual, the apparent increase in the incidence of microphthalmia might be considered incidental. Observations that indicate that unilateral developmental ophthalmic effects do exist and can be triggered by xenobiotic agents include induction of unilateral microphthalmia and anophthalmia in rats with trypan blue (Land et al., 1976) and the existence of a strain of rats with hereditary unilateral microphthalmia (Tokunaga et al., 1987). Conversely, developmental effects such as microphthalmia tend to occur in clusters, and an earlier database of controls (Hood, 1997) showed a maximal incidence of microphthalmia of 1.7%. A clustering of microphthalmia was reported at the Huntingdon Research Centre in the United Kingdom (Palmer, 1977), where only 1:8637 CFY rat fetuses showed microphthalmia between 1973 and 1976, but six to eight fetuses among 600 did so in a subsequent 2-month period. Data supplied by the company indicate that incidences of microphthalmia similar to that observed in this study had occurred in the same laboratory randomly in control and test groups over a number of years. Similarly, data from Huntingdon Life Sciences (1988–93) revealed scattered incidences of microphthalmia or anophthalmia commensurate with those observed in this study. This information provides considerable support for the proposition that the observed incidence of microphthalmia was an incidental cluster effect, independent of treatment. This conclusion is further supported by the absence of microphthalmia in the study of reproductive toxicity and the absence of other developmental effects that would usually be observed concurrently with a teratogenic effect. Consequently, the observation of microphthalmia in this study was considered to be a cluster effect incidental to treatment.
Other embryo and fetal parameters evaluated and maternal organ weights were not affected by treatment. The NOAEL for maternal toxicity was 50 mg/kg bw per day on the basis of reduced body-weight gain at 200 mg/kg bw per day. The NOAEL for developmental toxicity was 200 mg/kg bw per day, the highest dose tested, in the absence of effects at this dose (Liberacki et al., 1993).
Rabbits
In a study conducted to GLP standards and in accordance with OECD guideline 414, groups of 20 pregnant New Zealand white rabbits were given spinosad (purity, 88%; 76.1% spinosyn A and 11.9% spinosyn D) as a suspension in 500 ppm aqueous methyl cellulose ether by gavage at a dose of 0, 2.5, 10 or 50 mg/kg bw per day on days 7–19 of gestation (day of breeding designated day 0 of gestation). Deaths, clinical signs of toxicity, body weight and food consumption were assessed at regular intervals throughout the study. Gross examinations were conducted on all animals. All does surviving to day 28 of gestation were killed and their fetuses removed surgically. The observations reported were the number and position of fetuses in utero, the numbers of live and dead fetuses, the number and position of resorptions, the number of corpora lutea, fetal sex ratio and body weight and any gross external alterations. In addition, the weights of the liver (with gall-bladder), kidney and gravid uterus were recorded.
Faecal output was reduced at 50 mg/kg bw per day. These does also lost weight from the beginning of treatment (–4 g), and their body-weight gain at the end of treatment was 30% lower than that of controls. Two does at the highest dose aborted on days 22 and 27 of gestation and were killed. There were no treatment-related effects on any of the embryo or fetal parameters evaluated. Gross examination of one doe that aborted revealed serosanguinous ascites, atelectasia (left apical lobe), multifocal pale areas in the wall of the gall-bladder, mucoid tracheal exudate, a dark focus in the cortex of one kidney, decreased ingesta in the digestive tract and a haemorrhage in the vaginal wall; the other rabbit that aborted appeared normal. The NOAEL for maternal toxicity was 10 mg/kg bw per day on the basis of reduced body-weight gain and abortion in does at 50 mg/kg bw per day. The NOAEL for embryonal and fetal toxicity was 50 mg/kg bw per day, the highest dose tested, in the absence of effects at this dose (Vedula et al., 1994).
In a study conducted in accordance with GLP requirements and FIFRA guideline 81-8, groups of 10 male and 10 female Fischer 344 rats received spinosad (purity, 88.0%; 76.1% spinosyn A and 11.9% spinosyn D) in 500 ppm aqueous methylcellulose at a single dose of 0, 200, 630 or 2000 mg/kg bw (active ingredient, doses adjusted for purity) by gavage. A ‘functional observational battery’ and tests for motor activity, forelimb and hindlimb grip strength and landing foot splay were used to assess neurobehavioural changes in 10 rats of each sex per group before treatment, after 5–6 h and on days 8 and 15 of the study. Body weights were also determined. Gross and neurohistopathological examinations were performed at necropsy on five animals of each sex in the control group and that at the highest dose, on the olfactory lobe, cerebellum, thalamus and hypothalamus, midbrain, pons, cerebellum, medulla oblongata, trigeminal ganglion, pituitary gland, eyes with optic nerves, spinal cord, peripheral nerves, dorsal root ganglia, nasal tissues with olfactory epithelium and skeletal muscles. No deaths occurred during the study. The neurobehavioural and motor activity assays revealed no treatment-related effects. There were no gross pathological or neurohistopathological findings attributable to treatment. The NOAEL was 2000 mg/kg bw per day (Albee et al., 1994).
In an evaluation of neurotoxicity conducted concurrently with a 13-week study of toxicity, groups of 10 Fischer 344 rats of each sex were fed diets containing spinosad (purity, 88.0%; 76.1% spinosyn A and 11.9% spinosyn D) at a concentration of 0, 30, 60, 120 or 600 ppm, 7 days a week for 13 weeks, equal to 0, 2.2, 4.3, 8.6 and 43 mg/kg bw per day for males and 0, 2.6, 5.2, 10 and 52 mg/kg bw per day for females. The study was conducted in accordance with GLP requirements and FIFRA guideline 82-7. A ‘functional observational battery’ and tests for grip performance, hindlimb landing, foot splay and motor activity were administered to 10 animals of each sex per group before treatment and monthly during dosing. At 13 weeks, the central and peripheral nervous systems of five animals of each sex per group were evaluated. No treatment-related effects were observed at any dose. The NOAEL was 600 ppm, equivalent to 43 mg/kg bw per day (Wilmer et al., 1993).
In the long-term study of toxicity and carcinogenicity of Bond et al. (1995b) in Fischer rats, described above, satellite groups of 10 rats of each sex per group were evaluated for neurotoxic effects of treatment. At 12 months, a ‘functional observational battery’ consisting of hand holding and open field observations, tests for forelimb and hindlimb grip and landing foot splay and an automated test of motor activity were conducted before treatment and then at 3, 6, 9 and 12 months. At 12 months, the central and peripheral nervous systems of five animals of each sex in the control group and at the highest dose were evaluated. The only potentially treatment-related effects were an increased incidence of very slight focal hyperplasia of the anterior pituitary (pars distalis) in males (in 0/5 controls and 2/5 at 1000 ppm) and very slight multifocal degeneration of individual fibres of the trigeminal nerve in both sexes (in 1/5 male controls, 3/5 males at 1000 ppm, 0/5 female controls, 3/5 females at 1000 ppm). Given the low intensity of these findings, the absence of any observed functional deficit in these or the animals in the main study, and the absence of similar alterations in other neural tissue, these observations were considered to be incidental to treatment. The NOAEL for neurotoxicity was 1000 ppm, equal to 49 mg/kg bw per day, the highest dose tested, in the absence of treatment-related effects (Spencer & Yano, 1995).
In the 12-month study of toxicity of Harada (1995) in beagle dogs, described above, all animals in each group were evaluated for any neurotoxic effects of treatment after acclimatization. The animals were assessed for general sensory function, rectal temperature, response to auditory and somatosensory stimulation, visual function (including pupillary light reflexes, diameter and equality of pupils), visual, tactile and proprioceptive placing reactions, extensor postural thrust reaction and the presence of spontaneous and positional nystagmus, tonic deviations of the limbs or eyes (strabismus) and impaired righting reactions. In addition, the cranial nerves (except the olfactory and accessory nerves) and reflexes were assessed. No neurological abnormalities were found that could be attributed to treatment. The NOAEL was 300 ppm, equal to 8.2 mg/kg bw per day (Holliday, 1994).
(ii) Mechanisms of lysosomal vacuolation
There is evidence for a common mechanism for the reversible lysosomal vacuolation observed in a range of tissues in mice, rats and dogs. Ultrastructurally, the vacuolated lysosomes have the appearance of lysosomal lamellar bodies. Such histological and ultrastructural changes may arise through processes that prevent degradation of the cell constituents normally processed in lysosomes, by enzyme inhibition or interference in the intra-lysosomal environment required for optimal enzyme activity, or by an excess of substrate (Lüllmann-Rauch, 1979; Reasor, 1989). In humans, a range of relatively rare genetic enzyme deficiencies are responsible for this type of lysosomal dysfunction. Mucopolysaccharidoses (e.g. Hunter syndrome, Hurley syndrome) and sphingolipidoses (e.g. Gaucher disease, Niemann-Pick disease), for example, result in accumulation of metabolites in lysosomes due to an excess of substrate resulting from a deficiency in the extra-lysosomal enzymes that normally act on the respective substance (Robbins & Cotran, 1979). Thus, inhibition of an unidentified enzyme responsible for degradation of a normal metabolic by-product, with consequent accumulation of that by-product in lysosomes, is a possible explanation for the observed vacuolation.
The sponsor presented an alternative mechanism for the widespread vacuolation. They noted that the cytoplasm of the affected cells contained clear vacuoles consisting of variable numbers of secondary lysosomes which contained concentric membrane lamellae. The light microscopic and ultrastructural appearance of these cytoplasmic lamellar bodies was consistent with lesions induced by amphiphilic cationic compounds (Schneider, 1992). The general chemical structure of spinosad—a basic amine group attached over a short side-chain to a hydrophobic moiety—is also consistent with the general structure of amphiphilic cationic compounds. The mechanism of the intracellular accumulation of lamellar bodies induced by amphiphilic cationic compounds has been reported (Lüllmann-Rauch et al., 1978; Reasor, 1989): The non-protonated compound enters cells by diffusion; once within the cell cytoplasm, the compound can diffuse into lysosomes and become protonated in the acidic environment. The protonated compound then forms a complex with phospholipids (polar lipids) within the lysosome by hydrophobic and electrostatic forces. This lipid complex becomes positively charged and is protected from normal enzymatic attack by phospholipases. Lipid complexes accumulate within the cell due to the diffusion gradient between the plasma and the cytosol and lysosomes, and by the reduced ability to degrade these complexes enzymatically. This accumulation can be reversed by withdrawal of the compound, thus reducing the diffusion gradient and allowing dissociation of the complex between the compound and phospholipids within the cell.
The generalized, reversible vacuolation and lysosomal lamellar bodies seen after relatively high doses of spinosad are consistent with the hypothesis suggested by the company. The effect might also be consistent with local enzyme inhibition, either within the lysosomes themselves or more generally in the affected tissues. Given the wide variety of cationic amphiphilic compounds which cause effects similar to those seen with spinosad and the clear association between kinetics and tissue sensitivity, the Meeting considered the hypothesis proposed by Dow AgroSciences to be the more likely explanation.
Spinosyn B and spinosyn K are metabolites of spinosyn A in plants and rats. Spinosyn B is N-demethylated spinosyn A, and spinosyn K is one of the possible O-demethylated derivatives of spinosyn A.
In male and female CD-1 mice given spinosyn B (purity, 93.5%) orally in aqueous methyl cellulose, the LD50 was 3200 mg/kg bw. All mice at 5000 mg/kg bw and none at 2000 mg/kg bw died (Gilbert & Stebbins, 1996). In male and female CD-1 mice given spinosyn K (purity, 99%) orally in aqueous methyl cellulose, the LD50 was > 5000 mg/kg bw (Gilbert, 1996).
Neither spinosyn B (purity, 93.5%) not spinosyn K (purity, 98.7%) induced reverse mutation in Salmonella typhimurium TA1535, TA1537, TA98 or TA100 or Escherichia coli WP2uvrA– at doses of 0.10–3300 µg/plate in dimethyl sulfoxide for spinosyn B and 0.33–5000 µg/plate for spinosyn K, in the presence or absence of an exogenous metabolic activation system from rat liver (S9). The tests were conducted in triplicate, and positive controls were included. In preliminary experiments with spinosyn B, cytotoxicity was seen in E. coli WP2uvrA and S. typhimurium TA100 at > 330 µg/plate with S9 and at > 67 µg/plate without S9. In preliminary experiments with spinosyn K, cytotoxicity was seen in TA100 at > 330 µg/plate with S9 and at > 100 µg/plate without S9, and in E. coli WP2uvrA at > 3300 µg/plate with S9 and at > 100 µg/plate without S9 (Lawlor, 1996b,c).
The pharmacokinetics and metabolism of the two principal constituents of spinosad, spinosyn A and D, are very similar. Oral administration of spinosyn A or D to rats resulted in rapid but incomplete absorption of > 70% of the dose. Peak blood concentrations of radiolabel were achieved 1 h after administration of 10 mg/kg bw and 2–6 h after administration of 100 mg/kg bw. This delay in achieving peak blood concentrations is likely to reflect saturation of absorption at higher doses. Elimination occurs primarily in the faeces (70–90%) via the bile, and < 10% was recovered from urine. Most of the administered radiolabel was recovered within 24 h. The half-times for spinosyn A and D radiolabel were 25–42 h and 29–33 h, respectively. A large proportion of the material excreted in the faeces had been absorbed and eliminated in the bile, primarily as glutathione conjugates of N- and O-demethylated spinosyns A and D. Excretion as exhaled 14CO2 was negligible. The highest concentrations of tissue residues were identified in fat, liver, kidneys, and lymph nodes. Although the concentrations in the thyroid were not high in comparison with many other tissues shortly after administration of spinosyn A or D, the rate of decline was slow and ultimately resulted in higher concentrations in the thyroid than in other tissues, where the decline was more rapid. Absorbed spinosyn A and D were extensively biotransformed, with glutathione conjugates of N- or O-demethylated spinosyn A or D as the predominant metabolites.
Technical-grade spinosad had little acute toxicity after oral or dermal administration or inhalation; the LD50 values after oral administration were consistently > 2000 mg/kg bw and generally > 5000 mg/kg bw in rats and mice. In one study, however, four of five male rats died after administration of 5000 mg/kg bw by gavage. The LD50 in rabbits treated dermally was > 5000 mg/kg bw, and the LC50 after inhalation in rats was > 5.2 mg/l.
Spinosad was not irritating to the skin of rabbits and not sensitizing to the skin of guinea-pigs. It caused slight eye irritation in rabbits, which resolved within 48 h.
An extensive range of effects was observed in both short- and long-term studies with repeated doses, and the effects were similar in mice, rats, and dogs. In short-term studies in mice, rats, and dogs, tissue vacuolation was a consistent observation at the LOAEL. In mice, the overall NOAEL in the 90-day study was 6 mg/kg bw per day, and increased liver weight was also observed at the LOAEL. In rats, the overall NOAEL was 8.6 mg/kg bw per day in three 90-day studies and 21 mg/kg bw per day in two 28-day studies, with increased liver weights again observed at the LOAEL in the 90-day studies. In dogs, the LOAEL in a 28-day study was the lowest dose tested, 6.5 mg/kg bw per day; the NOAEL in a 90-day study was 4.9 mg/kg bw per day; and the NOAEL in a 12-month study was 2.7 mg/kg bw per day. Increased thyroid weights were observed in the 28- and 90-day studies in dogs at doses at and above the LOAEL, in addition to tissue vacuolation. In dogs, however, the lymphatic system was more sensitive to vacuolation than the thyroid, the lymphatic lesions occurring at the LOAEL in both the 90-day and 12-month studies.
In long-term studies in mice and rats, tissue vacuolation and other histological alterations were again observed at doses at and above the LOAEL. In mice, the lungs, lymph nodes, stomach, and tongue were the main organs affected at doses above the NOAEL of 11 mg/kg bw per day. The main histological findings were chronic inflammation, hyperplasia, and hyperkeratosis of the stomach, vacuolation of the parathyroid, pancreas, ovaries, and epididymal epithelial cells, and myopathy of the tongue. In rats, the NOAEL in the 2-year study was 2.4 mg/kg bw per day. The primary organ affected at the LOAEL of 9.5 mg/kg bw per day was the thyroid; the lungs, liver, larynx, and bone marrow were affected at higher doses. Vacuolation was limited to the epithelial cells of the thyroid gland, and inflammation was observed in the thyroid, lung, and larynx. Bone-marrow hyperplasia and slight dilatation of liver sinusoids were also observed.
Strong similarities in other toxic effects were found between species and in the short- and the long-term studies. At the higher doses used, spinosad was toxic in multiple organs of mice, rats and dogs, resulting in increased serum activity of liver, muscle and cardiac enzymes (alanine aminotransferase, alkaline phosphatase, aspartate aminotransferase and creatinine phosphokinase), microcytic hypochromic anaemia and increased spleen, thyroid and liver weights. The histological alterations in a wide range of organs were similar in all species tested, the predominant lesions being cellular vacuolation, inflammatory changes (including necrosis), histiocytosis, regenerative and degenerative changes, increased haematopoiesis and skeletal myopathy. In the long-term study in rats, the thyroid was the most sensitive organ overall, effects occurring at lower doses than in other organs and resolving more slowly after withdrawal of treatment.
Vacuolation in the thyroid, the most sensitive toxicological end-point overall, was seen in both short- and long-term studies in rats and was reversible in two studies: a 28-day study in males fed diets containing concentrations equal to doses 120 mg/kg bw per day and a 13-week study in rats of each sex fed diets containing concentrations 40–50 mg/kg bw per day.
Selected tissues from rats and mice in the short-term studies of toxicity were examined by electron microscopy, and the vacuolation was found to be associated with cytoplasmic lamellar inclusion bodies, reflecting a lysosomal storage disorder. While such disorders may arise through a variety of mechanisms which prevent degradation of cell constituents that are usually processed in the lysosomes, spinosad probably acts mainly through a physicochemical mechanism associated with its cationic amphiphilic structure (having both lipophilic and hydrophilic properties in one molecule).
A comparison of spinosad, spinosyn A and spinosyn D in a 28-day study in rats treated in the diet revealed notable differences in the toxicological profiles of spinosyn A and D. The toxicological effects of spinosyn A were closely similar to those of spinosad, but spinosyn D failed to produce most of the haematological and clinical chemical alterations seen with spinosad or spinosyn A. Consequently, minor variations in the relative proportions of spinosyn A and D in the technical-grade active ingredient are unlikely to alter its toxicological profile significantly.
In long-term studies in mice and rats treated in the diet at doses up to 51 and 49 mg/kg bw per day, respectively, there was no evidence that spinosad is carcinogenic.
Spinosad gave negative results in an adequate range of assays for genotoxicity in vivo and in vitro. The Meeting concluded that spinosad is not genotoxic.
Given the absence of both genotoxicity in appropriate short-term tests and carcinogenicity in long-term studies in rats and mice, the Meeting concluded that spinosad is unlikely to pose a carcinogenic risk to humans.
The reproductive toxicity of spinosad was investigated in a two-generation study in rats. The reproductive effects, a reduced number of pups per litter and clinical alterations in F1a and F2 pups, reported only at a dietary concentration adjusted to deliver a constant dose of 100 mg/kg bw per day, the highest dose tested, were attributed to nonspecific parental toxicity rather than to a specific toxic effect on the reproductive system. A reduction in the number of pups per litter was observed in each of three generations of pups at 100 mg/kg bw per day. As a similar finding was not observed at 200 mg/kg bw per day in the study of developmental toxicity in rats, the reduction in pup number per litter may reflect preimplantation losses. The NOAEL for reproductive toxicity was 10 mg/kg bw per day.
In a study of developmental toxicity in rats, dams were given doses up to 200 mg/kg bw per day. Slightly reduced maternal body-weight gain was observed at the highest dose. Unilateral microphthalmia was found at external examination in two fetuses in separate litters at 200 mg/kg bw per day and in one at 50 mg/kg bw per day. Although this is a rare spontaneous malformation in rats, it was discounted as a cluster effect incidental to treatment, for two reasons. First, a similar incidence of this malformation occurred randomly in control and other groups in studies conducted in the same laboratory with the same strain of rat over a number of years; secondly, it occurred in the absence of the other developmental effects which normally accompany a treatment-related increase in the incidence of malformation. The absence of ocular malformations in the study of reproductive toxicity at doses up to 100 mg/kg bw per day provides further support for this conclusion. On this basis, the NOAEL for maternal toxicity in rats was 50 mg/kg bw per day, and that for developmental toxicity was 200 mg/kg bw per day, the highest dose tested. In a study of developmental toxicity in rabbits, the does were given spinosad on days 7–19 of gestation at doses 50 mg/kg bw per day, with no evidence of embryo or fetal effects, despite maternal toxicity, consisting of weight loss, abortion, and clinical signs at the highest dose. The NOAEL for maternal toxicity was 10 mg/kg bw per day, and that for embryo and fetal toxicity was 50 mg/kg bw per day, the highest dose tested.
Neurotoxicity was investigated in rats by giving them a single dose of 2000 mg/kg bw, doses 43 mg/kg bw per day for 3 months, or doses 49 mg/kg bw per day for 12 months. Comprehensive behavioural and histopathological investigations revealed no evidence of neurotoxicity.
The Meeting concluded that the existing database was adequate to characterize the potential hazards of spinosad to fetuses, infants and children.
The most sensitive overall toxicological end-point was thyroid vacuolation in rats treated in the diet in the 2-year study of toxicity and carcinogenicity. The Meeting established an ADI of 0–0.02 mg/kg bw on the basis of the NOAEL of 2.4 mg/kg bw per day in this study and a 100-fold safety factor.
Spinosad has little acute toxicity. In studies with repeated doses, no acute toxicological alerts were observed that might indicate the need for establishing an acute reference dose.
Levels relevant to risk assessment
|
Species |
Study |
Effect |
NOAEL |
LOAEL |
|
Mouse |
18-month study of toxicity and carcinogenicitya |
Toxicity |
80 ppm, equal to 11 mg/kg bw per day |
360 ppm equal to 51 mg/kg bw per day |
|
Carcinogenicity |
360 ppm equal to 51mg/kg bw per dayb |
– |
||
|
Rat |
2-year study of toxicity and carcinogenicitya |
Toxicity |
50 ppm, equal to 2.4 mg/kg bw per day |
200 ppm equal to 9.5 mg/kg bw per day |
|
Carcinogenicity |
200 ppm equal to 9.5 mg/kg bw per dayb |
– |
||
|
12-month study of neurotoxicitya |
Neurotoxicity |
1000 ppm, equal to 49 mg/kg bw per dayb |
|
|
|
Two-generation study of reproductive toxicitya |
Parental and offspring toxicity |
10 mg/kg bw per day |
100 mg/kg bw per day |
|
|
Developmental toxicityc |
Maternal toxicity |
50 mg/kg bw per day |
200 mg/kg bw per day |
|
|
Embryo- and feto-toxicity |
200 mg/kg bw per dayb |
– |
||
|
Rabbit |
Developmental toxicityc |
Maternal toxicity |
10 mg/kg bw per day |
50 mg/kg bw per day |
|
Embryo- and feto-toxicity |
50 mg/kg bw per dayb |
– |
||
|
Dog |
12-month study of toxicitya |
Toxicity |
100/120 ppm, equal to 2.7 mg/kg bw per day |
300 ppm, equal to 8.2 mg/kg bw per day |
a
Dietb
Highest dose testedc
GavageEstimate of acceptable daily intake for humans
0–0.02 mg/kg bw
Estimate of acute reference dose
Unnecessary
Studies that would provide information useful for continued evaluation of the compound
Observations in humans
Further investigation of the mechanism of tissue vacuolation
Relevant end-points for setting guidance values for dietary and non-dietary exposure
|
Absorption, distribution, excretion, and metabolism in mammals |
|
|
Spinosyn A |
|
|
Rate and extent of oral absorption |
> 70% |
|
Dermal absorption |
< 1% after 24 h in rats |
|
Distribution |
Rapid; highest concentrations of residues in kidney, lymph nodes, fat, and thyroid at 168 h |
|
Rate and extent of excretion |
Rapid, largely complete within 24 h; faeces, > 80%; urine, 6–10% |
|
Potential for accumulation |
Limited, but decline in thyroid tissue is slow and prolonged |
|
Metabolism in mammals |
Large proportion eliminated unchanged in faeces. Biliary and urinary metabolites primarily glutathione conjugates of spinosyn A and N- and O-demethylated spinosyn A |
|
Spinosyn D |
|
|
Rate and extent of oral absorption |
> 70% |
|
Distribution |
Rapid; highest concentrations of residues in kidney, lymph nodes, fat, and thyroid at 168 h |
|
Rate and extent of excretion |
Rapid, largely complete within 24 h; faeces, > 80%; urine, 6–10% |
|
Potential for accumulation |
Limited, but decline in thyroid tissue is slow and prolonged |
|
Metabolism in mammals |
Large proportion eliminated unchanged in the faeces. Biliary and urinary metabolites primarily glutathione conjugates of spinosyn D and N- and O-demethylated spinosyn D |
|
Toxicologically significant compounds |
Spinosyns A, D |
|
Acute toxicity |
|
|
Spinosad |
|
|
LD50, oral |
Spinosad: > 5000 mg/kg bw, mice and female rats > 2000-< 5000 mg/kg bw, male rats |
|
|
Spinosyn A:D (46:50): males, 4400 mg/kg bw; females, > 5000 mg/kg bw |
|
LD50, dermal |
Spinosad: > 5000 mg/kg bw, rabbit |
|
|
Spinosyn A:D (46:50): males and females, > 5000 mg/kg bw |
|
LC50, inhalation |
> 5.2 mg/l, rats |
|
Dermal irritation |
Not irritating, rabbits |
|
Ocular irritation |
Slight, rabbits |
|
Dermal sensitization |
Not sensitizing, guinea-pigs |
|
Short-term toxicity |
|
|
Target/critical effect |
Many tissues, vacuolation; thyroid, increased weight; liver, increased aspartate aminotransferase activity |
|
Lowest relevant oral NOAEL |
2.7 mg/kg bw per day |
|
Lowest relevant dermal NOAEL |
> 1000 mg/kg bw per day (rabbits, 21 days) |
|
Lowest relevant inhalational NOAEL |
> 9.5 mg/m3 (rats, 14 days) |
|
Long-term toxicity and carcinogenicity |
|
|
Target/critical effect |
Mice, rats, and dogs; many tissues, vacuolation and associated alterations in clinical chemical parameters; anaemia |
|
Lowest relevant NOAEL |
2-year study, rats, 2.4 mg/kg bw per day |
|
Carcinogenicity |
Not carcinogenic, mice, rats |
|
Genotoxicity |
Not genotoxic |
|
Reproductive toxicity |
|
|
Reproductive target/critical effect |
Reduced number of pups per litter, clinical signs in rat pups secondary to maternal toxicity |
|
Lowest relevant reproductive NOAEL |
10 mg/kg bw per day in a two-generation study in rats |
|
Developmental target/critical effect |
No developmental effects in rats or rabbits |
|
Lowest relevant developmental NOAEL |
50 mg/kg bw per day, rabbits |
|
Neurotoxicity/Delayed neurotoxicity |
No evidence of neurotoxicity in a 12-month study in rats at doses up to 49 mg/kg bw per day |
|
Medical data |
No data |
|
Summary |
Value |
Study |
Safety factor |
|
ADI |
0–0.02 mg/kg bw |
2 years, rats |
100 |
|
Acute RfD |
Unnecessary |
|
|
Albee, R.R., Berdasco, N.M. & Yano, B.L. (1994) XDE-105: Acute neurotoxicity study in Fischer 344 rats. Unpublished reports Nos DR-0323-1194-009, DR-0323-1194-009R, DR-0323-1194-009A, DR-0323-1194-009B, DR-0323-1194-009C, DR-0323-1194-009D. Submitted to WHO by Dow AgroSciences, Letcombe, United Kingdom.
Bond, D.M., Stebbins, K.E. & McGuirk, R.J. (1995a) XDE-105: 18-month dietary oncogenicity study in CD-1 mice. Unpublished report No. DR-0323-1194-006(1). Submitted to WHO by Dow AgroSciences, Letcombe, United Kingdom.
Bond, D.M., Yano, B.L., Stebbins, K.E. & McGuirk, R.J. (1995b) DE-105: Two-year chronic toxicity, chronic neurotoxicity and oncogenicity study in Fischer 344 rats. Unpublished reports Nos DR-0323-1194-005 and DR-0323-1194-005I. Submitted to WHO by Dow AgroSciences, Letcombe, United Kingdom
Bond, D.M., Stebbins, K.E. & McGuirk, R.J. (1996) XDE-105: 18-month dietary oncogenicity study in CD-1 mice. Unpublished report No. DR-0323-1194-006(A). Submitted to WHO by Dow AgroSciences, Letcombe, United Kingdom.
Breslin, W.J., Quast, J.F. & Vedula, U. (1994) XDE-105: Two-generation dietary reproduction study in Sprague-Dawley rats. Unpublished reports Nos DR-0323-1194-008A0, DR-0323-1194-008W2, DR-0323-1194-008LO, DR-0323-1194-008P2, DR-0323-1194-008W1, DR-0323-1194-008L2, DR-0323-1194-008L1, DR-0323-1194-008W3 and DR-0323-1194-008. Submitted to WHO by Dow AgroSciences, Letcombe, United Kingdom.
Domoradzki, J.Y. (1995) XDE-105: Comparison of the Metabolism and Tissue Distribution of 14C-Labelled XDE-105(Factor A) and 14C-Labelled XDE-105 (Factor D) in Fischer 344 Rats. Unpublished report No T2.2-187-19 USA. Submitted to WHO by Dow AgroSciences, Letcombe, United Kingdom
Domoradzki, J.Y. & Shabrang, S.N. (1996) Spinosyn A: Probe study dermal absorption of 14C-labelled spinosyn A in Fischer rats. Unpublished report No. DR-0323-1194-029. Submitted to WHO by Dow AgroSciences, Letcombe, United Kingdom.
Domoradzki, J.Y., Stewart, H.S., Gilbert, J.R. & Markham, D.A. (1995) XDE-105 (factor A): Metabolism and distribution of 14C labelled XDE-105 (factor A) in Fischer 344 rats. Unpublished report No. DECO-HET-DR-0323-1194-012. Submitted to WHO by Dow AgroSciences, Letcombe, United Kingdom.
Enomoto, A. (1992) XDE-105: 4-week dose range finding study in dogs. Unpublished report No. IET 91-0126. from Institute of Environmental Toxicology, Kodaira, Tokyo, Japan. Submitted to WHO by Dow AgroSciences, Letcombe, United Kingdom.
Garriott, M.L., Rexroat, M.A. & Grothe, D.W. (1992a) The effect of XDE-105 on the induction of reverse mutations in Salmonella typhimurium and Escherichia coli using the Ames test. Unpublished reports Nos 910820AMS3162, 910917AMS3162, 910924AMS3162. Submitted to WHO by Dow AgroSciences, Letcombe, United Kingdom
Garriott, M.L., Michaelis, K.C. & Grothe, D.W. (1992b) The effect of XDE-105 on the induction of forward mutation at the thymidine kinase locus of L5178Y mouse lymphoma cells. Unpublished report No. 910910MLA3162. Submitted to WHO by Dow AgroSciences, Letcombe, United Kingdom.
Garriott, M.L., Kindig, D.E.F. & Grothe, D.W. (1992c) The effect of XDE-105 on the in vitro induction of chromosome aberrations in Chinese hamster ovary cells. Unpublished reports Nos 910918CAB3162, 911009CAB3162 from Toxicology Research Laboratories, Lilly Research Laboratories. Greenfield, Indianapolis, USA. Submitted to WHO by Dow AgroSciences, Letcombe, United Kingdom.
Garriott, M.L., Yount, D.J. & Grothe, D.W. (1992d) The effect of XDE-105 on the induction of unscheduled DNA synthesis in primary cultures of adult rat hepatocytes. Unpublished reports Nos 910806UDS3162, 910827UDS3162. Submitted to WHO by Dow AgroSciences, Letcombe, United Kingdom.
Garriott, M.L., Brunny, J.D., Kindig, D.E.F. & Grothe, D.W. (1992e) The effect of XDE-105 on the in vivo induction of micronuclei in bone marrow of ICR mice. Unpublished reports Nos 910916MNT3162, 911007MNT3162.
Gilbert, K.S (1994a) XDE-105: Acute dermal toxicity study in New Zealand white rabbits. Unpublished report No. DR-0323-1194-017D. Submitted to WHO by Dow AgroSciences, Letcombe, United Kingdom.
Gilbert, K.S (1994b) XDE-105: Acute dermal toxicity study in New Zealand white rabbits. Unpublished report No. DR-0323-1194-017D1. Submitted to WHO by Dow AgroSciences, Letcombe, United Kingdom.
Gilbert K.S. (1994c) XDE-105: Primary eye irritation study in New Zealand white rabbits. Unpublished report No. DR-0323-1194-017C. Submitted to WHO by Dow AgroSciences, Letcombe, United Kingdom.
Gilbert, K.S. (1994d) XDE-105: Dermal sensitization potential in the Hartley albino guinea pig. Unpublished report No. DR-0323-1194-017E. Submitted to WHO by Dow AgroSciences, Letcombe, United Kingdom
Gilbert, K.S. (1996) Spinosyn K. Acute oral toxicity study in CD-1 mice. Dow Chemical Co., Midland, Michigan, USA, 2 April 1996, DR-0351-5524-001A.
Gilbert, K.S. & Stebbins, K.E. (1996) Spinosyn B: 1996 acute oral toxicity study in CD-1. Dow Chemical Co., Midland, Michigan, USA Unpublished report No. DR-0350-1119-001A. 19 February 1996.
Gilbert, K.S. & Yano, B.L. (1996) DE-105: Acute oral toxicity study in Fischer 344 rats and CD-1 mice. Dow Chemical Co., Midland, Michigan, USA, 6 March 1996, DR-0323-1194-031.
Gilbert, K.S., Johnson, K.A. & Stebbins, K.E. (1994) XDE-105: Acute oral toxicity study in Fischer 344 rats and CD-1 mice. Unpublished reports Nos DR-0323-1194-017A, DR-0323-1194-017R, DR-0323-1194-017M. Submitted to WHO by Dow AgroSciences, Letcombe, United Kingdom
Grothe, D.W., Boss, S.M. & Gries, C.L (1992a) A subchronic toxicity study in CD-1 mice administered XDE-105 in the diet for 3 months. Unpublished report No. M01290 from Toxicology Research Laboratories, Lilly Research Laboratories, USA. Submitted to WHO by Dow AgroSciences, Letcombe, United Kingdom.
Grothe, D.W., Boss, S.M. & Gries, C.L. (1992b) A subchronic toxicity study in Fischer 344 rats administered XDE-105 in the diet for 3 months. Unpublished report No. R20690. Submitted to WHO by Dow AgroSciences, Letcombe, United Kingdom
Harada, T., XDE-105: 13-Week Oral Subchronic Toxicity Study in Dogs, Dow Chemical Japan Ltd., Shinagawa-ku, Tokyo 140, Japan, Unpublished report No IET 91-0079, 01 September 1994
Harada, T. (1995) DE-105: 12-month oral chronic toxicity study in dogs. Unpublished report No. DR-0323-1194-021 (IET 91-0080), from Institute of Environmental Toxicology, Tokyo, Japan. Submitted to WHO by Dow AgroSciences, Letcombe, United Kingdom.
Holliday, T.A. (1994) XDE-105: 12 month oral chronic toxicity study in dogs. Unpublished report No. DECO-HET-DR-1194-021 (IET 91-0080), from Institute of Environmental Toxicology, Tokyo, Japan. Submitted to WHO by Dow AgroSciences, Letcombe, United Kingdom.
Hood, R.D. (1997) In: Handbook of Developmental Toxicology, New York: CRC Press, Appendix B, Historical control data.
Land, P.W., Polley, E.H. & Kernis, M.M. (1976) Patterns of retinal projections to the lateral geniculate nucleus and superior colliculus of rats with induced unilateral congenital eye defects. Brain Res., 103, 394–399.
Laska D.A., Rock G.L. & Grothe D.W. (1992) A 2-week acute dermal irritation and toxicity study in New Zealand white rabbits following a single topical application and 24 hour exposure of XDE-105. Toxicology Research Laboratories, Greenfield, Indianapolis, USA, 23 July 1992.. Unpublished report, Laboratory Project ID.: B06891.
Lawlor, T.E. (1996a) Mutagenicity test on XDE-105 in the Salmonella Escherichia coli /mammalian microsome reverse mutation assay preincubation method. with a confirmatory assay. Unpublished report No. 17341-0-422RT,DR-0323-1194-032 from Corning Hazleton Inc., Vienna, Virginia, USA-
Lawlor, T.E. (1996b) Mutagenicity test on XDE-105 factor B in the Salmonella–Escherichia coli /mammalian microsome reverse mutation assay preincubation method with a confirmatory assay. Unpublished report No. 16974-0-422R from Corning Hazleton Inc., Vienna, Virginia, USA. Submitted to WHO by Dow AgroSciences, Letcombe, United Kingdom.
Lawlor, T.E. (1996c) Mutagenicity test on XDE-105 factor K in the Salmonella–Escherichia coli /mammalian microsome reverse mutation assay preincubation method with a confirmatory assay. Unpublished report No. 17637-0-422R from Corning Hazleton Inc., Vienna, Virginia, USA. Submitted to WHO by Dow AgroSciences, Letcombe, United Kingdom.
Liberacki, A.B., Breslin W.J. & Yano, B.L. (1993) XDE-105: Oral gavage teratology study in Sprague-Dawley rats. Unpublished report No. DR-0323-1194-003. Submitted to WHO by Dow AgroSciences, Letcombe, United Kingdom.
Lüllmamn-Rauch, R. (1979) Drug induced lysosomal storage disorders. In: Dingle, J.T., Jacques, P.J. & Shaw, I.H., eds, Lysosomes in Applied Biology and Therapeutics, Amsterdam: Elsevier North Holland, pp. 49–130.
Lüllmann, H., Lüllmamn-Rauch, R. & Wasserman, O. (1979) Lipidosis induced by lysosomal storage disorders. Biochem. Pharmacol., 27, 1103–1108.
MARTA (1996) Historical control data (1992–1994) for developmental and reproductive toxicity studies using the Crl:CD(SD)BR rat. Published and distributed by Charles River Laboratories.
McGuirk, R.J., Yano, B.L., Freshour, N.L. & Piasecki, D.A. (1994) XDE-105, factor A and factor D: 28-day dietary toxicity study in Fischer 344 rats Unpublished report No. DR-0323-1194-013. Submitted to WHO by Dow AgroSciences, Letcombe, United Kingdom
Mendrala, A.L., Gilbert, J.R., Stewart, H.S. & Domoradzki, J.Y. (1995a) XDE-105 (factor D): Metabolism and tissue distribution of 14C-labelled XDE-105 (factor D) in Fischer 344 rats. Unpublished report No. DECO-HET-DR-0335-0070-00. Submitted to WHO by Dow AgroSciences, Letcombe, United Kingdom.
Mendrala, A.L., Gilbert, J.R., Stewart, H.S. & Domoradzki, J.Y. (1995b) Bile elimination of XDE-105 (factor D) in Fischer 344 rats. Unpublished report No. DECO-HET-DR-0335-0070-002. Submitted to WHO by Dow AgroSciences, Letcombe, United Kingdom.
Palmer, A.K. (1977) Incidence of sporadic malformations, anomalies and variations in random bred laboratory animals. In: Methods in Prenatal Toxicology: Evaluation of Embryonic Effects in Experimental Animals, Teratology Workshop. April 1977, Berlin, pp. 52–71.
Reasor, M. (1989) A review of the biology and toxicological implications of lysosomal lamellar bodies by drugs. Toxicol. App. Pharmacol., 97, 47–56.
Robbins, S.L. & Cotran, R.S. (1979) In: Pathologic Basis of Disease, London: W.B Saunders Co., pp. 243–256.
Schneider, P. (1992) Drug induced lysosomal disorders in laboratory animals: New substances acting on lysosomes. Arch. Toxicol., 66, 23–33.
Shibata, R. (1996) A skin sensitization study of DE-105 in guinea pigs (maximisation test), Bozo Research Centre Inc., Tokyo, Japan. Unpublished report No. B-3106, 19 April 1996.
Spencer, P.J. & Yano, B.L. (1995) XDE-105: Chronic neurotoxicity study in Fischer 344 rats. Unpublished report No. DECO-HET-DR-0323-1194-005N. Submitted to WHO by Dow AgroSciences, Letcombe, United Kingdom.
Stebbins, K.E. & Brooks, K.J. (1999a) Spinosad (spinosyn A & D, 50:50 mixture): Acute oral toxicity study in Fischer 344 rats. Unpublished report No. DR-0360-3530-002, 07 January 1999, Dow AgroSciences, Indianapolis, USA.
Stebbins, K.E. & Brooks, K.J. (1999b) Spinosad (spinosyn A & D, 50:50 mixture): Acute dermal toxicity study in New Zealand white rabbits. Unpublished report No. DR-0360-3530-005, 07 January 1999. Dow AgroSciences, Indianapolis, USA.
Stebbins, K.E. & Brooks, K.J, (1999c) Spinosad (spinosyn A & D, 50:50 mixture): Acute dermal irritation study in New Zealand white rabbits. Unpublished report No. DR-0360-3530-003, 07 January 1999. Dow AgroSciences, Indianapolis, USA.
Stebbins, K.E. & Brooks, K.J. (1999d) Spinosad (spinosyn A & D, 50:50 mixture): Acute eye irritation study in New Zealand white rabbits. Unpublished report dated 07 January 1999. Dow AgroSciences, Indianapolis, USA.
Stebbins, K.E. & Brooks, K.J. (1999e) Spinosad (spinosyn A & D, 50:50 mixture): Dermal sensitisation potential study in Hartley albino guinea pigs. Unpublished report No. DR-360-3530-006, 07 January 1999. Dow AgroSciences, Indianapolis, USA.
Thalaker, F.W. (1996) Bioaccumulation of 14C-spinosyn A in female Fischer 344 rats following repeated oral administration of 14C-spinosyn A. Unpublished report No. DR-0323-1194-043. Submitted to WHO by Dow AgroSciences, Letcombe, United Kingdom
Tokunaga, A., Sugita, S. & Otani, K. (1987) Uncrossed retino-geniculate and retino-tectal projections in the hereditary unilaterally microphthalmic rats. Neurosci. Res., 4, 195–210.
Vedula, U. & Yano, B.L. (1994) XDE-105: Probe and 21-day repeated dose dermal toxicity study in New Zealand white rabbits. Unpublished report No. DR-0323-1194-018. Submitted to WHO by Dow AgroSciences, Letcombe, United Kingdom.
Vedula, U., Yano, B.L. & Breslin, W.J. (1994) XDE-105: Oral gavage teratology study in New Zealand white rabbits. Unpublished report No. DR-0323-1194-011. Submitted to WHO by Dow AgroSciences, Letcombe, United Kingdom.
Wilmer, J.W., Spencer, P.J., Yano, B.L. & Bond, D.M. (1993) XDE-105: 13-week dietary toxicity, 4-week recovery, and 13-week neurotoxicity studies in Fischer 344 rats (neurotoxicity portion). Unpublished report No. DR-0323-1194-001B. Submitted to WHO by Dow AgroSciences, Letcombe, United Kingdom.
Wolff, R.K., Allen, D.L., Williams, G.D. & Grothe, D.W. (1992) The acute inhalation toxicity in the Fischer 344 rat of technical XDE-105. Unpublished report No. R33491.
Wright, F.L., Keaton, M.J. & Grothe, D.W. (1992a) The acute toxicity of XDE-105 administered orally to Fischer 344 rats. Unpublished report No. R42590 from Toxicology Research Laboratories, Lilly Research Laboratories, USA. Submitted to WHO by Dow AgroSciences, Letcombe, United Kingdom.
Wright, F.L., Laska, D.A., Poulsen, R.G. & Grothe, D.W. (1992b) A 21-day subchronic dermal toxicity study of XDE-105 in New Zealand white rabbits. Unpublished report No. B08491 from Lilly Research Laboratories, Greenfield, Indianapolis, USA. Submitted to WHO by Dow AgroSciences, Letcombe, United Kingdom.
Yano, B.L. & Bond, D.M. (1994) XDE-105: 13-week dietary toxicity and 4-week recovery studies in Fischer 344 rats. Unpublished report No. DR-0323-1194-001A. Submitted to WHO by Dow AgroSciences, Letcombe, United Kingdom.
Yano, B.L. & Liberacki, A.B. (1999a) Spinosad: 4-week dietary toxicity and recovery study in Fischer 344 rats. Unpublished reports Nos 981125S, DR-0323-1194-107. Submitted to WHO by Dow AgroSciences, Letcombe, United Kingdom
Yano, B.L. & Liberacki, A.B. (1999b) Spinosad (50% spinosyn A and 50% spinosyn D): 13-week dietary toxicity study in Fischer rats. Unpublished report No. DR-0360-3530-001 from School of Veterinary Medicine, University of California, Davis, California, USA. Submitted to WHO by Dow AgroSciences, Letcombe, United Kingdom.
Yano, B.L. & McGuirk, R.J. (1999) Spinosad technical (DE-105): 14-day nose only aerosol inhalation toxicity and 2-week recovery studies in Fischer 344 rats. Unpublished report No. 991021. Submitted to WHO by Dow AgroSciences, Letcombe, United Kingdom.
See Also:
Toxicological Abbreviations
Spinosad (ICSC)