
Pesticide residues in food - 2002 - Joint FAO/WHO Meeting on Pesticide Residues
First draft prepared by
I. Dewhurst
Pesticides Safety Directorate, Department for Environment, Food and Rural Affairs,
Kings Pool, York, England
Flutolanil (alpha,alpha,alpha-tri-fluoro-3’-isopropoxy-ortho-toluanilide) is a systemic benzanilide fungicide. It specifically inhibits the succinate dehydrogenase complex (EC 1.3.99.1, complex II) of Basidiomycetes but not those of fungi of other classes. Succinate dehydrogenase is an iron–sulfur protein that is an integral part of the inner mitochondrial membrane and a key element in the electron transport chain of mammals. Flutolanil has not been evaluated previously by JMPR.
The available studies on the toxicity of flutolanil were performed between 1977 and 1990. Of the 26 studies evaluated, 13 included statements of compliance with good laboratory practice (GLP), and most of the other studies had been checked by quality assurance departments and were conducted in laboratories that subsequently were certified for GLP. The GLP status or indications of quality assurance procedures are described in the summary of each study. Many of the studies were performed to Japanese guidelines and often including investigations over and above those required in the comparable OECD guideline. Unless stated otherwise, the standard of reporting was adequate.
The absorption and metabolism of orally administered flutolanil were investigated in two studies in rats, in which the concentrations of residues in tissues were also determined. No studies of toxicokinetics have been performed in other species.
In a study that complied with GLP, performed in 1992, [aniline ring-U-14C]flutolanil (specific activity; 20 mCi/mmol; radiochemical purity, > 99%) suspended in a vehicle consisting of 1% Tween 80 and 0.5% carboxymethyl cellulose was given orally to groups of three male and three female CD (Sprague-Dawley-derived) rats. Unlabelled flutolanil (purity, 99.9% or 97.6%) was also used in preparation of the solutions. The animals received either a single dose of 20 mg/kg bw [14C]flutolanil; consecutive doses of 20 mg/kg bw per day of unlabelled flutolanil for 14 days followed by single dose of [14C]flutolanil on day 15; or a single dose of 1000 mg/kg [14C]flutolanil. After administration, urine was collected three times on day 1, then daily; and faeces and cage wash were collected daily. After sample collection on day 7, the animals were killed, and blood and 12 tissue samples were obtained. The radioactivity in the samples was determined by liquid scintillation counting after appropriate processing. Volatile 14C production was not determined as an earlier study showed that < 0.1% of the dose had been exhaled.
The total recovery was acceptable at the lower dose but was < 90% at the higher dose (Table 1). Most of the radioactivity was excreted within 24 h, approximately half the urinary excretion occurring within 12 h, indicating relatively rapid absorption. The extent of absorption, as determined by urinary excretion, varied with dose and repeated dosing (Table 1), indicating saturation of absorption by large doses. There was evidence of induction of flutolanil metabolism by repeated dosing. Similar results were found in the two sexes.
Table 1. Radioactivity recovered in urine and faeces from rats given [14C]flutolanil (mean ± standard deviation)
|
Dose (mg/kg) and dose regimen |
Sex |
Per cent of administered radioactivity |
|||||
|
24 h |
168 h |
||||||
|
Urine |
Faeces |
Totala |
Urine |
Faeces |
Totala |
||
|
20 × 1 |
Male |
40 ± 12 |
34 ± 20 |
76 |
45 ± 15 |
42 ± 29 |
90 |
|
Female |
37 ± 8 |
37 ± 12 |
79 |
41 ± 10 |
41 ± 13 |
88 |
|
|
20 × (14 + 1) |
Male |
66 ± 14 |
24 ± 7 |
92 |
70 ± 16 |
29 ± 9 |
103 |
|
Female |
65 ± 20 |
28 ± 6 |
98 |
71 ± 22 |
32 ± 8 |
109 |
|
|
1000 × 1 |
Male |
6 ± 3 |
76 ± 11 |
83 |
7 ± 4 |
78 ± 13 |
86 |
|
Female |
9 ± 3 |
64 ± 11 |
75 |
10 ± 4 |
67 ± 12 |
79 |
|
From Eschbach (1992)
a Including cage wash
The concentration and distribution of radioactivity in blood and tissues at 7 days was minimal, representing < 0.2% of that administered to any animal, indicating no significant potential for bioaccumulation. The tissue concentrations varied considerably between animals in the same group. The only tissue in which consistent concentrations were found was the liver, in which the mean was about 10 times those in blood. The amount of radioactivity in the livers was higher in females in all groups than in males (2.4 ± 1.1 versus 0.68 ± 0.38 µg/g at 1000 mg/kg bw; 0.23 ± 0.04 versus 0.12 ± 0.01 µg/g after repeated doses) (Eschbach, 1992).
In a study that did not comply with GLP, reported in 1982, the absorption, distribution and excretion of flutolanil were investigated in groups of four male CRJ:CD(SD) rats (three for routine observations and one for autoradiography). The animals received [aniline-U-14C]flutolanil (purity, > 99%; specific activity, 34 µCi/mg) by gavage in olive oil at a dose of 20 or 100 mg/kg bw. Radioactivity in blood, urine, faeces and tissues was determined by liquid scintillation counting, after appropriate extraction and processing, or by autoradiography. Blood samples were taken from the tail vein at various times between 15 min and 48 h after dosing. Autoradiography and examinations for tissue distributions were conducted on rats killed after 0.5, 2, 6 and72 h. Exhaled 14C was not measured, as a previous study had indicated that it accounted for < 0.1% of the administered dose.
The findings were similar but not identical to those shown in Table 1, the differences being attributed to use of an oil-based rather than an aqueous vehicle. About 80% of the administered dose was excreted within the first 24 h, and total excretion at 7 days was > 96%. Urine was the predominant route of excretion, accounting for 65–70% at both doses, as seen after repeated dosing (Table 1). The concentration in whole blood peaked at 2 h, with values of 4.2 ± 2 µg/ml at 20 mg/kg bw and 12 ± 3 µg/ml at 100 mg/kg bw, falling to < 1 µg/ml at 24 h, expressed as equivalents. After a decline, a second peak in the blood concentration of radioactivity was seen, at 6 h, indicating some enterohepatic circulation. The peak concentrations in the tissues of animals given 20 mg/kg bw were seen at 2 h in liver (15 ± 3 µg/g) and kidney (10 ± 3 µg/g). The results of autoradiography were consistent with the measured absorption rates and distribution in tissues (Aizawa, 1982).
In a study in which flutolanil was given in the diet to Sprague-Dawley rats for 4 weeks, analyses of brain, liver, blood, kidney and fat showed low concentrations in animals receiving > 2000 ppm (Table 2). The highest concentrations of residues were found in adipose tissue and liver. There was minimal retention of flutolanil. The lowest dose of 400 ppm was equal to 36 mg/kg bw per day in males and 41 mg/kg bw per day in females. Comparison of the results at 400 ppm with those at 20 mg/kg bw in the study of Eschbach (1992), described above, indicated that flutolanil that has no significant propensity to accumulate. The increase in residue concentration with increasing dose indicated that the saturation of absorption seen after administration by gavage does not occue after dietary intake (Tsuchiya & Sugimoto, 1977).
Table 2. Concentrations (ppm) of residues of flutolanil in tissues from rats receiving flutolanil for 4 weeks (mean)
|
Tissue |
Dose (ppm) |
|||||||||
|
0 |
400 |
2000 |
10 000 |
50 000 |
||||||
|
Male |
Female |
Male |
Female |
Male |
Female |
Male |
Female |
Male |
Female |
|
|
Blood |
< 0.11 |
< 0.06 |
< 0.11 |
< 0.06 |
< 0.11 |
< 0.06 |
< 0.11 |
0.10 |
< 0.11 |
0.11 |
|
Kidney |
< 0.06 |
< 0.09 |
< 0.06 |
< 0.09 |
< 0.07 |
< 0.09 |
< 0.07 |
< 0.09 |
0.64 |
0.38 |
|
Liver |
< 0.06 |
< 0.09 |
< 0.06 |
< 0.09 |
0.46 |
0.25 |
0.94 |
1.0 |
3.9 |
4.2 |
|
Brain |
< 0.08 |
< 0.15 |
< 0.08 |
< 0.15 |
0.11 |
< 0.15 |
0.12 |
< 0.15 |
0.31 |
0.25 |
|
Adipose |
< 0.11 |
< 0.50 |
< 0.11 |
< 0.50 |
0.49 |
2.4 |
2.6 |
1.1 |
20 |
9.5 |
From Tsuchiya & Sugimoto (1977)
An analysis of tissue samples from the study in rats given repeated doses showed that flutolanil was present only in fat and at low concentrations: 0.2 ppm at 2000 ppm and 0.6 ppm at 10 000 ppm, which are similar to those found after a single dose. As the analytical method was only for the parent compound, any metabolites that might have contributed to the residues after a single dose would not have been detected. However, as the metabolites formed from flutolanil are relatively polar and are excreted primarily in urine (see below), the findings from the study with repeated doses indicate that flutolanil has little if any potential to bioaccumulate (Katoh et al., 1982).
Samples of urine and faeces from the study by Eschbach (1992) were analysed for metabolites. Composite samples of urine and faeces were made for each group and time up to 72 h. The urine samples were diluted and extracted three times with ether, the aqueous phases being subjected to acid and alkaline treatment plus acid and enzyme (glucuronidase and sulfatase) hydrolysis. Lyophilized faecal samples were extracted into methanol and then with cold and boiling water; the water-soluble fractions were hydrolysed chemically (with HCl) or enzymically (with glucuronidase and sulfatase) and re-extracted. The presence of metabolites was investigated by comparison with standards by thin-layer chromatography, autoradiography and liquid scintillation counting.
The recoveries were generally low (< 50%), but there were no additional spots on the chromatography plates, indicating poor efficiency rather than missed metabolites. The results presented in Table 3 should thus be taken as qualitative rather than quantitative. The main metabolite in urine was alpha,alpha,alpha-tri-fluoro-3’-hydroxy-ortho-toluanilide (M4; desisopropyl flutolanil), present mainly as a conjugate that was released by 0.1 N HCl but not by glucuronidase or sulfatase. The concentrations of M4 were significantly higher in animals dosed repeatedly at 20 mg/kg bw per day than in animals dosed once. Trace amounts of alpha,alpha,alpha-tri-fluoro-5’-hydroxy-3’-methoxy-ortho-toluanilide were seen in some samples. All other metabolites represented < 0.3% of the administered dose. Parent compound was not detected in urine. In faeces, most of the radioactivity was extracted into the methanol phase, primarily as the parent compound, with low concentrations of M4. There was no evidence of cleavage at the amido bridge. Comparison of the results by sex and dose showed that females conjugated flutolanil more extensively than males and that repeated dosing increased the extent of metabolism to M4 and conjugates (Eschbach, 1992).
Table 3. Pattern of extraction and metabolites detected in urine and faeces of rats given flutolanil (mean per cent of administered dose at 72 h)
|
Extraction medium |
Metabolite |
Dose |
|||||
|
1 × 20 mg/kg bw |
14+1 × 20 mg/kg bw per day |
1 × 1000 mg/kg bw |
|||||
|
Male |
Female |
Male |
Female |
Male |
Female |
||
|
Urine (total) |
|
45 |
40 |
70 |
70 |
7 |
10 |
|
Ether |
Total |
3.2 |
2.3 |
3.8 |
8.0 |
0.8 |
1.4 |
|
|
M4 |
0.8 |
0.1 |
0.6 |
1.0 |
0.1 |
0.1 |
|
|
M7 |
– |
– |
< 0.1 |
1.0 |
– |
0.1 |
|
Glucuronides |
Total |
3.9 |
8.8 |
1.7 |
3.0 |
0.4 |
1.5 |
|
|
M4 |
0.3 |
0.4 |
– |
– |
– |
– |
|
Sulfates |
Total |
3.2 |
4.5 |
6.1 |
12 |
1.0 |
2.2 |
|
|
M4 |
0.3 |
0.3 |
1.8 |
3.9 |
0.5 |
0.8 |
|
HCl |
Total |
18 |
21 |
15 |
24 |
3.2 |
2.9 |
|
|
M4 |
4.2 |
1.0 |
9.9 |
12 |
1.0 |
0.8 |
|
|
M7 |
0.1 |
– |
< 0.1 |
1.3 |
0.6 |
0.1 |
|
Faeces (total) |
42 |
40 |
28 |
31 |
78 |
66 |
|
|
Methanol |
Total |
34 |
37 |
27 |
23 |
52 |
36 |
|
|
M4 |
1.4 |
1.1 |
0.9 |
1.8 |
0.5 |
0.5 |
|
|
Flutolanil |
20 |
27 |
17 |
9.0 |
32 |
25 |
|
Glucuronides |
Total |
0.3 |
0.3 |
0.3 |
0.2 |
0.6 |
0.2 |
|
|
Flutolanil |
– |
0.1 |
0.1 |
– |
– |
– |
|
Sulfates |
Total |
0.4 |
0.2 |
0.3 |
0.3 |
0.5 |
0.1 |
|
|
Flutolanil |
– |
0.1 |
0.1 |
0.1 |
0.4 |
– |
|
HCl |
Total |
0.9 |
0.6 |
0.6 |
1.0 |
0.5 |
0.8 |
|
|
Flutolanil |
0.2 |
0.1 |
0.1 |
0.1 |
0.2 |
0.6 |
From Eschbach (1992); M4, alpha,alpha,alpha-tri-fluoro-3’-hydroxy-ortho-toluanilide; M7, alpha,alpha,alpha-tri-fluoro-4’-hydroxy-3’-methoxy-ortho-toluanilide
Urine and faeces from the study by Aizawa (1982) in which CRJ:CD(SD) rats were given flutolanil at 20 or 100 mg/kg bw by gavage were processed by acid and base treatments, solvent extraction, incubation with glucuronidase or sulfatase and boiling acid reflux and were then analysed for metabolites by thin-layer chromatography by comparison with 10 postulated metabolites. The results of this study were somewhat different from those reported by Eschbach (1992), significantly higher levels of conjugates being present (Table 4). The differences were attributed to use of an oil-based rather than an aqueous vehicle. The main metabolite was M4 in free and conjugated forms. As seen by Eschbach (1992), there was no evidence of cleavage at the amido bridge. Trace concentrations (< 3%) of a number of metabolites and their conjugates were also detected in urine and faeces (Table 4) (Aizawa, 1982).
Table 4. Pattern of extraction and metabolites detected in urine and faeces after 72 h from male rats given flutolanil at 20 mg/kg bw
|
Extraction medium |
Metabolite |
Mean per cent of administered dose |
|
Faeces (total) |
|
26 |
|
Methanol |
Total |
18 |
|
|
M4 |
5.2 |
|
Flutolanil |
1.2 |
|
|
|
M8 |
2.4 |
|
|
M2 |
2.1 |
|
Water-soluble |
|
|
|
Glucuronides |
Total |
2 |
|
Sulfates |
Total |
2 |
|
HCl |
Total |
3 |
|
Urine (total) |
|
69 |
|
Ether |
Total |
5.7 |
|
Flutolanil |
2.3 |
|
|
Water-soluble |
|
|
|
Glucuronides |
Total |
16 |
|
|
M4 |
12 |
|
Sulfates |
Total |
42 |
|
|
M4 |
37 |
|
HCl |
Total |
53 |
|
|
M4 |
47 |
From Aizawa (1982); M4, alpha,alpha,alpha-tri-fluoro-3’-hydroxy-ortho-toluanilide; M2, M8, metabolites 2 and 8, not further identified
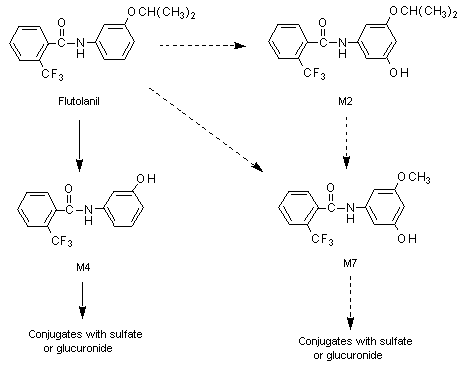
The proposed metabolic pathway for flutolanil in rats is shown in Figure 1.

Figure 1. Proposed metabolic pathway of flutolanil in rats
Solid lines, major pathways; dotted lines, minor routes
M2, 5’hydroxyflutolanil; M4, alpha,alpha,alpha-tri-fluoro-3’-hydroxy-ortho-toluanilide;
M7, alpha,alpha,alpha-tri-fluoro-5’-hydroxy-3’-methoxy-ortho-toluanilide
The dermal absorption of a commercial formulation of flutolanil through human epidermis in vitro was investigated in a study that complied with GLP, conducted in 1999. The formulation was prepared with [14C]flutolanil to give an activity of 6.3 MBq (specific activity, 141 dpm/µg). Human epidermal membranes were prepared from whole skin obtained from the abdomen post mortem. Undiluted formulation was applied at 10 µl/cm2 and left unoccluded for 24 h. Receptor fluid (50% aqueous ethanol at 32 °C) was sampled routinely and analysed by liquid scintillation counting. At the end of the test, the chamber and epidermis were washed and the radioactivity determined. A conservative assessment, including residue in the epidermal sample and that penetrating the skin, gave an overall dermal penetration rate of < 0.5% within 24 h, indicating that flutolanil is poorly absorbed through human epidermis when applied as a concentrated formulation. No data were presented for dilutions that are actually used (Ward, 1999).
(a) General toxicity
The acute toxicity of flutolanil is summarized in Table 5. The vehicle used in all except the studies by inhalation was physiological saline and 1% Tween® 80. The only signs of toxicity were transient lethargy and reduced body-weight gain.
Table 5. Acute toxicity of flutolanil
|
Species |
Strain |
Sex |
Route |
LD50 (mg/kg bw)/ |
Purity (%) |
Reference |
|
Rat |
Fischer 344 |
M & F |
Oral |
> 10 000 |
98.2 |
Kosaka & Saito (1982a) |
|
Mouse |
ICR |
M & F |
Oral |
>10 000 |
98.2 |
Kosaka & Saito (1982b) |
|
Rat |
Fischer 344 |
M & F |
Dermal |
> 5000 |
98.2 |
Kosaka & Saito (1982a) |
|
Rat |
Fischer 344 |
M & F |
Inhalation |
> 6 |
98.7 |
Tsuda et al. (1984) |
|
Rat |
Fischer 344 |
M & F |
Intraperitoneal |
> 10 000 |
98.2 |
Kosaka & Saito (1982a) |
|
Mouse |
ICR |
M & F |
Intraperitoneal |
> 10 000 |
98.2 |
Kosaka & Saito (1982b) |
|
Rat |
Fischer 344 |
M & F |
Subcutaneous |
> 10 000 |
98.2 |
Kosaka & Saito (1982a) |
|
Mouse |
ICR |
M & F |
Subcutaneous |
> 10 000 |
98.2 |
Kosaka & Saito (1982b) |
(b) Ocular irritation
In a study that complied with GLP and did not deviate significantly from OECD guideline 405 (1987), reported in 1986, the acute ocular irritancy of flutolanil (purity, 97.5%) was investigated in six male New Zealand white rabbits, which received a dose of 0.1 g instilled into the conjunctival sac. There was no evidence of an initial pain response. Conjunctival redness (score = 1) was seen in all animals at 1 h, and the redness was still present at 48 h in four animals. All eyes were normal by 72 h (Cummins, 1986a).
(c) Dermal irritation
In study that complied with GLP and was performed according to OECD guideline 404 (1981) with no significant deviations, reported in 1986, the acute dermal irritancy of flutolanil (purity, 97.5%) was investigated in six male New Zealand white rabbits, which received an application of 0.5 g (moistened with 0.2 ml water) on a shorn area of 6 cm2 for 4 h under a semi-occlusive dressing. There were no deaths or clinical signs of systemic toxicity and no erythema or oedema (scores = 0 ) at any time up to 72 h, when the study was terminated (Cummins, 1986b).
(d) Dermal sensitization
In a study that complied with GLP and did not deviate significantly from the requirements of OECD guideline 406 (1992), reported in 1986, the skin sensitizing potential of futolanil (purity, 97.5% ) was investigated in groups of 20 female Dunkin-Hartley guinea-pigs in a Magnusson and Kligman maximization protocol. The intradermal induction concentration was 2% w/v in acetone/paraffin oil or 1% w/v in acetone/adjuvant. A 60% solution in acetone was used for the topical induction and challenge applications, with application of 10% sodium lauryl sulfate 24 h before induction. The concentrations were chosen on the basis of irritancy and administration difficulties found in a screening test. Erythema was seen after intradermal and topical induction. Two treated animals died between topical induction and challenge, of unknown causes. The challenge elicited no response in the test group (Cummins, 1986c).
(a) Oral administration
Mice
In a range-finding study that complied with GLP, reported in 1987, which was not designed to comply with the relevant OECD guideline (408), groups of 12 CD-1 (ICR) BR mice of each sex received flutolanil (purity, 97.6%) in the diet at a concentration of 0, 500, 5000 or 50 000 ppm for 90 days. Body weight, food consumption and clinical signs were monitored routinely; no clinical chemistry, urine analysis or haematology was performed. All animals were necropsied grossly, and the adrenals, liver, bone marrow and spleen from controls and animals at the highest concentration, and livers from animals at the two lower concentrations were examined histologically. The findings were assessed statistically with the Dunnett test or Student t test.
The homogeneity, stability and achieved concentrations in the diet were confirmed analytically to be within ± 10% of nominal. The mean intakes were 0, 70, 680 and 7500 mg/kg bw per day for males and 0, 80, 880 and 8800 mg/kg bw per day for females. There were no clinical signs of toxicity. Food consumption, water consumption and gross and microscopic findings were typical for the mouse strain and showed no evidence of treatment-related effects. One female at the lowest concentration died in week 9, but this was considered not to be treatment related as there were no deaths at higher concentrations. Body-weight gain and food conversion efficiency were reduced at 50 000 ppm in both sexes and in females at 5000 ppm, although with no clear dose–response relationship. The absolute and relative weights of the liver were increased in animals at the highest concentration (Table 6). The findings at 5000 ppm were considered to be minimal, with no clear dose–response relationship and thus not adverse. No NOAEL could be identified, owing to the limited investigations (Aughton, 1987).
Table 6. Changes in body weight and absolute and relative weights of the liver in mice given flutolanil in the diet for 13 weeks (mean ± standard deviation)
|
Dietary concentration (ppm) |
Body weight (g) |
Liver weight |
||||
|
Males |
Females |
Absolute (g) |
Relative (%) |
|||
|
Males |
Females |
Males |
Females |
|||
|
0 |
41 ± 3 |
33 ± 3 |
2.1 ± 0.3 |
1.8 ± 0.3 |
5.3 ± 0.4 |
5.5 ± 0.7 |
|
500 |
40 ± 4 |
33 ± 4 |
2.0 ± 0.3 |
1.7 ± 0.3 |
5.1 ± 0.5 |
5.4 ± 0.5 |
|
5000 |
41 ± 3 |
30 ± 2* |
2.2 ± 0.3 |
1.6 ± 0.3 |
5.5 ± 0.5 |
5.6 ± 0.8 |
|
50 000 |
38 ± 3* |
31 ± 2 |
2.6 ± 0.3** |
2.2 ± 0.4** |
7.0 ± 0.8** |
7.2 ± 0.8** |
From Aughton (1987)
* p < 0.05; ** p < 0.01; t test
Rats
In a study checked by quality assurance, reported in 1986, which was performed in accordance with OECD guideline 408 (1981) with no significant deviations, groups of 10 Sprague-Dawley CD rats of each sex received diets containing flutolanil (purity, 97.5%) at a concentration of 0, 500, 4000 or 20 000 ppm for 13 weeks. Body weight, food consumption and extensive clinical signs were monitored routinely. Samples were taken from fasted animals for clinical chemistry and haematology (no clotting parameters measured) before sacrifice; no samples were taken for urine analysis. Ophthalmoscopy was performed before treatment and at sacrifice. Extensive gross and microscopic examinations were performed on control and animals at the highest concentration; in animals at the two lower concentrations, microscopic examination was limited to lung, liver and kidneys. Findings were assessed statistically.
The homogeneity, stability and achieved concentrations in the diet were determined analytically and considered to be generally acceptable. The achieved intakes were corrected for the low recoveries in about 90% of the samples containing the lowest concentration. The only death was that of a control male. The clinical signs were similar in all groups. Body-weight gain was reduced slightly (by 5%) but not statistically significantly in animals at 20 000 ppm. The food consumption of treated males was generally increased, but there was no consistent dose–response relationship. Small but statistically significant reductions in mean corpuscular haemoglobin, mean cell volume and platelet count in some groups showed no dose–response relationship and were considered not to be adverse effects of treatment. The only consistent changes in clinical chemistry were small but statistically significant reductions in total bilirubin and increases in albumin in animals at the high and intermediate concentrations (Table 7). Serum enzyme markers of hepatotoxicity were unaltered by treatment. Increased liver and thyroid weights were seen at the two higher concentrations. The incidences of gross and microscopic lesions were similar in all groups and consistent with those seen in rats of this age.
Table 7. Findings in rats given flutolanil in the diet for 13 weeks (mean ± standard deviation)
|
Parameter |
Dietary concentration (ppm) |
|||||||
|
0 |
500 |
4000 |
20 000 |
|||||
|
Males |
Females |
Males |
Females |
Males |
Females |
Males |
Females |
|
|
Intake (mg/kg bw per day) |
0 |
0 |
34 |
40 |
230 |
340 |
1500 |
1700 |
|
Mean cell volume (µm3) |
51 ± 2 |
51 ± 2 |
49 ± 3 |
50 ± 1 |
50 ± 2 |
49 ± 3* |
50 ± 2 |
51 ± 2 |
|
Mean corpuscular haemoglobin (fg) |
22 ± 1 |
23 ± 1 |
21 ± 1* |
22 ± 1 |
21 ± 1 |
22 ± 1** |
22 ± 1 |
22 ± 1 |
|
Platelet count (108/ml) |
12 ± 2 |
11 ± 1 |
10 ± 2* |
11 ± 1 |
11 ± 1 |
11 ± 2 |
11 ± 1 |
12 ± 1 |
|
Albumin concentration (g/dl) |
3.5 ± 0.2 |
3.6 ± 0.1 |
3.6 ± 0.1 |
3.8 ± 0.2 |
3.6 ± 0.1 |
3.8 ± 0.1* |
3.7 ± 0.2* |
3.9 ± 0.2* |
|
Bilirubin concentration (µg/dl) |
28 ± 7 |
34 ± 0.1 |
28 ± 12 |
32 ± 4 |
28 ± 4 |
27 ± 5 |
24 ± 7 |
26 ± 10* |
|
|
|
|
|
|
|
|
|
|
|
Body weight (g) |
490 ± 51 |
270 ± 26 |
500 ± 43 |
270 ± 27 |
510 ± 35 |
270 ± 18 |
470 ± 26 |
260 ± 12 |
|
Liver weight (absolute; g) |
13 ± 2 |
6.8 ± 0.4 |
14 ± 2 |
6.9 ± 0.5 |
14 ± 2 |
7.5 ± 0.4** |
14 ± 1 |
8.1 ± 0.3** |
|
Liver weight (relative; %) |
2.9 ± 0.2 |
2.8 ± 0.2 |
3.0 ± 0.3 |
2.7 ± 0.2 |
3.0 ± 0.3 |
3.0 ± 0.2* |
3.3 ± 0.1** |
3.4 ± 0.1** |
|
Thyroid weight (relative; ± 105) |
5.7 ± 0.9 |
6.6 ± 1.3 |
5.7 ± 0.8 |
5.8 ± 1.2 |
7.0 ± 1.1* |
6.6 ± 1.4 |
6.9 ± 0.8* |
7.5 ± 1.5 |
|
Thyroid weight (absolute; range; mg) |
22–31 |
12–20 |
22–34 |
11–21 |
26–40 |
11–22 |
27–34 |
14–25 |
From Atkinson & Daly (1986a)
* p < 0.05; ** p < 0.01; analysis of variance
The pattern of findings at 4000 ppm indicates an adaptive response to administration of a high concentration of xenobiotic. In the absence of histopathological lesions, the 10% increase in liver weight was considered not to be adverse in itself. The NOAEL was 4000 ppm, equal to 230 mg/kg bw per day, on the basis of the pattern of changes seen at 20 000 ppm (Atkinson & Daly, 1986a).
Dogs
In a study accredited by quality assurance and performed according to OECD guideline 409 (1981) with no significant deviations, reported in 1986, groups of four beagle dogs of each sex received flutolanil (purity, 97.5%) in gelatin capsules at a dose of 0, 80, 400 or 2000 mg/kg bw per day for 95–98 days. No vehicle was used, and control groups received an empty capsule. Clinical signs, deaths and food and water consumption were assessed routinely. Ophthalmic examinations were performed before treatment and just before sacrifice. Samples for haematology and clinical chemistry were taken before treatment and at weeks 6 and 13. At sacrifice, all animals underwent extensive gross and histopathological examination. An appropriate range of statistical tests was used.
The content of the capsules does not appear to have been confirmed, although the stability of flutolanil was demonstrated. There were no treatment-related deaths or clinical signs. The findings on ophthalmoscopy were similar in treated and control animals. Treated females gained 1.3–1.5 kg (20–25%) more weight than controls (0.9 kg; 15%), even though their food consumption was slightly reduced (< 5%); in the absence of a dose–response relationship, this finding was considered not to be adverse. Water consumption varied between groups but with no dose–response relationship. Reduced erythrocyte count, erythrocyte volume fraction and haemoglobin concentration relative to those of controls and animals at the other two doses indicated an effect in dogs at the highest dose. The only clinical chemical finding of note was raised alkaline phosphatase activity in males at the lowest and highest doses and females at the highest dose (Table 8). The absence of an effect in males at the intermediate dose and the high value before treatment in males at the lowest dose indicate that the finding at the lowest dose was not treatment-related; interpretation of the results for females is confounded by the relatively low value in controls.
Table 8. Findings in dogs given flutolanil in gelatin capsules for 13 weeks (mean ± standard deviation)
|
Parameter |
Time |
Dose (mg/kg bw per day) |
|||||||
|
0 |
80 |
400 |
2000 |
||||||
|
Males |
Females |
Males |
Females |
Males |
Females |
Males |
Females |
||
|
Erythrocyte count (109/ml) |
Before test |
6.5 ± 0.6 |
6.7 ± 0.6 |
6.0 ± 0.3 |
5.9 ± 0.5 |
6.2 ± 0.3 |
6.5 ± 0.5 |
6.2 ± 0.1 |
6.3 ± 0.5 |
|
Week 6 |
6.7 ± 0.4 |
7.3 ± 0.3 |
6.2 ± 0.5 |
6.4 ± 0.3 |
6.8 ± 0.5 |
6.8 ± 0.7 |
5.9 ± 0.4* |
6.4 ± 0.4 |
|
|
Week 13 |
6.6 ± 0.5 |
7.0 ± 0.4 |
6.7 ± 0.2 |
6.5 ± 0.1 |
6.8 ± 0.3 |
6.8 ± 0.6 |
5.8 ± 0.6 |
6.4 ± 0.6 |
|
|
Erythrocyte volume fraction (%) |
Before test |
45 ± 4 |
45 ± 4 |
42 ± 3 |
42 ± 3 |
43 ± 3 |
46 ± 4 |
43 ± 1 |
45 ± 3 |
|
Week 13 |
46 ± 3 |
49 ± 2 |
47 ± 1 |
46 ± 1 |
48 ± 2 |
48 ± 4 |
42 ± 5 |
46 ± 3 |
|
|
Haemoglobin concentration (g/dl) |
Before test |
16 ± 1.3 |
16 ± 1.5 |
15 ± 1.2 |
15 ± 0.8 |
16 ± 1.0 |
17 ± 1.3 |
16 ± 0.5 |
16 ± 1.0 |
|
Week 13 |
18 ± 1.3 |
19 ± 0.6 |
18 ± 0.6 |
18 ± 0.8 |
19 ± 0.7 |
19 ± 1.4 |
17 ± 2.0 |
18 ± 1.4 |
|
|
Alkaline phosphatase activity (IU/l) |
Before test |
72 ± 17 |
68 ± 19 |
100 ± 10 |
85 ± 35 |
75 ± 10 |
100 ± 16 |
79 ± 26 |
110 ± 12 |
|
Week 13 |
52 ± 8 |
60 ± 17 |
81 ± 17* |
70 ± 25 |
58 ± 10 |
93 ± 17 |
120 ± 16** |
110 ± 15* |
|
|
Body weight (kg) |
|
9.5 ± 1.4 |
7.4 ± 0.7 |
9.1 ± 0.9 |
8.1 ± 0.6 |
9.9 ± 0.7 |
8.0 ± 0.8 |
9.6 ± 0.7 |
8.0 ± 1.1 |
|
Liver weight (absolute) (g) |
|
300 ± 24 |
240 ± 46 |
280 ± 29 |
270 ± 31 |
300 ± 10 |
290 ± 19 |
380 ± 45** |
320 ± 24* |
|
Liver weight (relative) (%) |
|
3.1 ± 0.4 |
3.3 ± 0.5 |
3.0 ± 0.4 |
3.3 ± 0.5 |
3.1 ± 0.3 |
3.7 ± 0.3 |
3.9 ± 0.2** |
4.0 ± 0.5 |
|
Thyroid weight (absolute) (g) |
|
0.74 ± 0.12 |
0.57 ± 0.7 |
0.70 ± 0.21 |
0.58 ± 0.24 |
0.73 ± 0.15 |
0.69 ± 0.15 |
0.92 ± 0.24 |
0.70 ± 0.18 |
|
Thyroid weight (relative) (× 105) |
|
7.7 ± 0.3 |
7.7 ± 0.3 |
7.8 ± 2.6 |
7.2 ± 0.4 |
7.4 ± 1.8 |
8.6 ± 1.7 |
9.5 ± 2.0 |
8.8 ± 2.6 |
From Atkinson & Daly (1986b)
* p < 0.05; ** p < 0.01; analysis of variance
The absolute and relative weights of the liver were increased significantly in animals of each sex at 2000 mg/kg bw per day. The weight of the thyroid was increased by > 20% in animals at 2000 mg/kg bw per day and in females at 400 mg/kg bw per day, although the increase was not statistically significant. The only histopathological finding associated with treatment was an increase in the ‘severity’ of pallor and hepatocyte swelling (due to presumed glycogen deposition) in animals of each sex at 400 and 2000 mg/kg bw per day; the finding at 400 mg/kg bw per day was minimal but was considered notable by the examining pathologist.
The NOAEL was 80 mg/kg bw per day on the basis of altered liver tissues at 400 mg/kg bw per day (Atkinson & Daly, 1986b).
In a study verified by quality assurance and conducted in 1982, groups of six beagle dogs of each sex, approximately 6 months of age at commencement of the study, received nine batches of flutolanil (purity, 97.5–99.4%) in gelatin capsules for 24 months at a dose of 0, 50, 250 or 1250 mg/kg bw per day given 30 min after the daily feed of 350 g. In addition to routine observations for this type of study, body temperature and pulse were measured weekly 6 h after dosing), electrocardiography and ophthalmoscopy were performed before treatment and at termination, sperm parameters were measured at termination and the estrus cycle regularly. Urine, faeces and blood samples were obtained before treatment and at 1.5, 3, 6, 12, 18 and 24 months. Haematological examinations included measurements of prothrombin time. Excretion of bromsulfothalein (liver function test) and phenolsulfonthalein (kidney function test) was investigated at 24 months. Extensive gross and histopathological examinations were performed on all animals. The results were analysed by analysis of variance or Student t test as appropriate.
The flutolanil content of the capsules does not appear to have been confirmed analytically. There were no deaths or clinical signs of systemic toxicity. The occurrence of emesis, soft faeces and salivation increased in the groups at the two higher doses after 15 months of treatment. The food consumption and body weight of animals at the highest dose were decreased after about 18 months. The reduction in body weight of these animals during the final 6 months was about 0.8 kg (7%), whereas all other groups showed increases of 0.1–0.2 kg (1–2%) during the same period. Water consumption, body temperature (~ 38.5 şC), pulse rate, sperm quality and quantity, urine, haematological and clinical chemical end-points (including alkaline phosphatase) and bromsulfothalein and phenolsulfonthalein clearance were similar in all groups. The electrocardiogram was stated to be unaffected by treatment (limited traces presented). Estrus cycling was somewhat irregular in some bitches at 50 and 250 mg/kg bw per day, but this was considered to be unrelated to treatment as it was not affected at the highest dose. The absolute weights of organs were similar in the treated and control groups. Some organ weights were increased relative to body weight in animals at the highest dose, the increase for the heart reaching statistical significance (by about 10%; p < 0.01) in both sexes. The relative liver weight was increased by <10%.
The only notable finding at gross examination was hyperaemia of the gastrointestinal tract in animals at the highest dose. Histological examination showed no clear differences in the pattern of findings in treated and control animals. The gastrointestinal effects may have been a local effect due to administration of the capsules, but it is unusual for such effects to become more prevalent towards the end of a study. The authors did not propose a mechanism for the gastrointestinal lesions but noted that they were consistent with irritation. The absence of effects on the liver, as had been seen in the shorter study in dogs, might be due to the fact that the systemic dose of flutolanil was reduced due to vomiting during the latter part of the study.
The NOAEL was 50 mg/kg bw per day, on the basis of gastrointestinal effects at 250 mg/kg bw per day (Sato 1982).
(b) Dermal exposure
Rats
In a study that complied with GLP and was performed according to OECD 410 (1981) with no significant deviations, reported in 1990, groups of five male and five female Sprague-Dawley CD rats received an application of flutolanil (purity, 97.6%) moistened with distilled water on shaved dorsal skin (5 cm × 5 cm) at a dose of 0 or 1000 mg/kg bw per day on 5 days per week for 3 weeks. The area was covered with a semi-occlusive bandage for 6 h. Clinical signs, body weight and food consumption were measured routinely, and samples for clinical chemistry, haematology (no clotting parameters measured) and urine analysis were taken before sacrifice. Ophthalmoscopy was performed before treatment and at sacrifice. Gross and histopathological examinations were limited to skin, liver, kidneys and gross lesions. Various statistical analyses were performed.
There were no clinical signs of toxicity. One treated male was found dead on day 19, the only finding being an enlarged liver and congestion, reported as typical of findings in animals that are not exsanguinated at sacrifice. There were no treatment-related effects on body weight, food consumption, clinical chemistry, haematology or ophthalmoscopy. An apparent decrease in adrenal weights in treated males was attributed to values for controls that were outside the range in other controls in the same laboratory; the weights of the adrenals in females were unaffected by treatment. The histopathological findings were similar in treated and control groups. There was no evidence of local skin lesions. No NOAEL could be identified as the possibility that the death was treatment-related could not be ruled out (Auletta, 1990).
Mice
In a study that complied with GLP, reported in 1990, groups of 52 CD-1 mice of each sex received diets containing flutolanil (purity, 97.6%) at a concentration of 0, 300, 1500, 7000 or 30 000 ppm for 79 weeks. The animals were examined routinely for clinical signs, deaths, body-weight gain and food consumption. Samples for blood smears were taken from the tail vein of controls and animals at the highest concentration in weeks 50 and 75; haematological examinations (excluding clotting times) were performed on samples taken from the retro-orbital sinus of 10 mice of each sex per group in week 77. All animals underwent a detailed gross necropsy. Extensive microscopic examinations were performed on animals that died during the study, all controls and animals at the highest concentration. Anomalies, liver, kidney and lungs from mice at the lowest and intermediate concentrations were also examined microscopically. The results were analysed by a range of statistical tests.
The homogeneity, stability and achieved concentration in the diet were confirmed analytically. Initial poor homogeneity at 300 ppm was remedied by a change to the mixing procedure in week 9. The mean achieved concentrations were within 5% of nominal, giving intakes of 0, 32, 160, 740 and 3300 mg/kg bw per day for males and 0, 34, 170, 840 and 3700 mg/kg bw per day for females. Survival was not adversely affected by flutolanil, treated groups tending to survive longer than controls (Table 9). Appearance and behaviour were similar in all groups. Body-weight gain was reduced for males receiving 30 000 ppm (by 17%) and females receiving concentrations > 7000 ppm (by 13%) during the first 6 months of the study. Food consumption was only slightly reduced (by 5%) in treated groups, indicating that the impaired weight gain was not due entirely to reduced food consumption.
Table 9. Findings in CD-1 mice given diets containing flutolanil for up to 79 weeks (mean ± standard deviation)
|
Parameter |
Dietary concentration (ppm) |
|||||||||
|
0 |
300 |
1500 |
7000 |
30 000 |
||||||
|
Male |
Female |
Male |
Female |
Male |
Female |
Male |
Female |
Male |
Female |
|
|
Survival (%) |
62 |
60 |
63 |
81 |
63 |
85 |
77 |
85 |
69 |
77 |
|
Body-weight gain, week 24 (g) |
24 ± 5 |
16 ± 5 |
24 ± 6 |
16 ± 6 |
23 ± 6 |
16 ± 6 |
23 ± 6 |
14 ± 4* |
20 ± 4* |
14 ± 4** |
|
Terminal body weight (g) |
50 ± 7 |
44 ± 8 |
49 ± 6 |
45 ± 7 |
48 ± 6 |
43 ± 7 |
52 ± 7 |
41 ± 6 |
47 ± 6 |
39 ± 7* |
|
Liver weight (absolute, g) |
3.2 ± 1.2 |
2.0 ± 0.4 |
3.3 ± 1.3 |
2.0 ± 0.5 |
3.0 ± 0.7 |
1.9 ± 0.4 |
3.3 ± 1.1 |
2.1 ± 0.4 |
3.5 ± 1.1 |
2.4 ± 1.3 |
|
Liver weight (relative, %) |
6.5 ± 2.5 |
4.7 ± 0.9 |
6.9 ± 2.9 |
4.6 ± 1.1 |
6.3 ± 1.7 |
4.4 ± 0.7 |
6.3 ± 1.9 |
5.1 ± 0.8 |
7.3 ± 2.1 |
6.3 ± 2.9** |
|
|
|
|
|
|
|
|
|
|
|
|
|
Hepatocellular carcinoma |
0–17%b |
0–2%b |
|
|
|
|
|
|
|
|
|
3 |
0 |
9 |
0 |
3 |
1 |
3 |
0 |
5 |
0 |
|
|
Hepatocellular adenoma |
4–19%b |
0–2%b |
|
|
|
|
|
|
|
|
|
3 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
7 |
1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Peri-acinar fatty vacuolation |
4 |
1 |
6 |
2 |
9 |
0 |
14*a |
0 |
9 |
2 |
|
Pan-acinar fatty vacuolation |
0 |
0 |
1 |
1 |
0 |
2 |
0 |
0 |
2 |
5*a |
|
Centri-acinar fatty vacuolation |
0 |
2 |
0 |
0 |
0 |
0 |
0 |
2 |
0 |
7 |
From Martin (1990)
* p < 0.05 ** p < 0.01, t test
*a p < 0.05, Fisher exact test
b Other controls in the same laboratory
Blood smears taken at week 75 showed that animals at 30 000 ppm flutolanil had a slightly (15%) increased ratio of lymphocytes to neutrophils; however, as the ratios in treated animals were typical of those in younger mice, this finding was considered not to be adverse. Variations in erythrocyte parameters were seen in males receiving concentrations > 7000 ppm, but as the changes were small, within the range of control values and did not show a dose–response relationship; they were considered not to be an adverse effect of treatment. The absolute and relative weights of the liver were increased in animals at 30 000 ppm, most markedly in females; a reduced absolute heart weight and an increased relative weight of the brain at this concentration were a reflection of the reduced body weight.
Gross examination revealed no other signs of a treatment-related effect. The non-neoplastic findings were typical of those in aged mice, the general pattern indicating that treated animals had fewer degenerative change than controls. Treatment-related findings were confined primarily to the liver, with increased pan-acinar and centri-acinar fatty vacuolation in females at the highest concentration. The increased peri-acinar fatty vacuolation in males at > 1500 ppm was potentially related to treatment, as the liver is a target organ; however, there was no clear evidence of a dose–response relationship over a 20-fold dose range and no increase in severity over the typical background findings. The peri-acinar vacuolation in males was considered not to be of a severity consistent with impaired liver function. Increased absolute (15%) and relative (24%) spleen weights in females at the highest concentration had no pathological correlates and were not statistically significant. The only tumours for which the incidences were increased were hepatocellular adenoma and carcinoma in males. The increases were not statistically significant (Fisher exact test), individually or combined (Table 9), and, for animals at the highest concentration, were within the range for male CD-1 mice in the testing facility and in generic data from the supplier.
The Meeting concluded that flutolanil was not carcinogenic in this study. The incidences of hepatocellular tumours in males at the highest concentration were within control ranges and were not statistically significantly different from those in controls. Furthermore, the intake at the highest concentration was more than three times the recommended limit dose for such studies. The only organ that showed any consistent pattern of non-neoplastic effects was the liver in animals at 30 000 ppm, with increased weights in both sexes and fatty vacuolation in females. The reduced body-weight gain during the first 6 months of the study was not related to reduced food consumption and was therefore considered to be treatment-related, being consistent both with findings in other studies and the mechanism of action of flutolanil as a disrupter of mitochondrial electron transport.
The NOAEL was 1500 ppm, equal to 170 mg/kg bw per day, on the basis of a statistically significant decrease in body-weight gain in females at 7000 ppm. The NOAEL for carcinogenicity was 30 000 ppm, equal to 3300 mg/kg bw per day, the highest dose tested (Martin, 1990).
Rats
Groups of 50 male and 50 female Crj:CD rats received diets containing flutolanil (six lots; purity, 97.5–99.2%) at a concentration of 0, 40, 200, 2000 or 10 000 ppm for 2 years, in a study audited for quality assurance and performed in 1980–82. Six animals of each sex per group were killed at interim sacrifice at 3 months and 10 of each sex per group at 12 months. Body weight, clinical signs and food consumption were recorded routinely; but no ophthalmic examinations were performed. Urine analysis and clinical chemical and haematological examinations (excluding clotting parameters) were performed at 6 and 18 months on 10 animals of each sex per group and at interim and terminal sacrifices. At sacrifice, a gross examination was performed,11 organs were weighed, and 29 tissues (including eyes) and any abnormalities found were examined microscopically with a range of stains. Animals found dead or killed in extremis were examined as far as possible. The concentrations of flutolanil residues in liver, kidney and fat were determined in 10 animals in each group at terminal sacrifice. The study was performed in accordance with OECD guideline 453 (1981), although the examination of eyes was limited (no ophthalmoscopy, use of 10% formalin rather than a more specific preservative).
The stability and achieved concentrations in the diet were confirmed analytically. The mean intakes of flutolanil were 0, 1.8, 9, 87 and 460 mg/kg bw per day for males and 0, 2.1, 10, 100 and 540 mg/kg bw per day for females. The survival rate was similar in control and treated groups and was considered acceptable, being in excess of 50% in all groups until week 93 and > 43% in groups at the highest concentration at termination. No treatment-related clinical signs were found. The body-weight gain of males at the highest concentration was reduced during the first 3 months of the study (p < 0.05; t test) but not subsequently (Table 10). The body weights of males at the intermediate concentration and all treated females were frequently higher than those of controls, possibly due to with increased food consumption at the beginning of the study. As there was no dose–response relationship over a 250-fold range, it is unlikely that flutolanil exerted a pharmacological effect on dietary control mechanisms.
Table 10. Findings in Crj:CD rats given diets containing flutolanil for up to 2 years (mean ± standard deviation)
|
Parameter |
Dietary concentration (ppm) |
|||||||||
|
0 |
40 |
200 |
2000 |
10 000 |
||||||
|
Males |
Females |
Males |
Females |
Males |
Females |
Males |
Females |
Males |
Females |
|
|
Body weight, week 10 (g) |
480 ± 53 |
290 ± 23 |
480 ± 47 |
300 ± 30 |
490 ± 47 |
300 ± 22 |
470 ± 47 |
300 ± 23 |
450 ± 48* |
300 ± 31 |
|
Body weight, week 71 (g) |
790 ± 110 |
520 ± 79 |
820 ± 110 |
570 ± 110 |
810 ± 110 |
570 ± 120 |
840 + 120 |
550 ± 81 |
810 ± 100 |
540 ± 76 |
|
Food intake, week 10 (g/day) |
25 ± 2.2 |
19 ± 2.1 |
28 ± 2.6** |
23 ± 2.2** |
29 ± 2.4** |
21 ± 2.3** |
29 ± 2.5** |
22 ± 2.4** |
29 ± 2.5** |
23 ± 3.2** |
|
Erythrocyte volume fraction, 18 months (%) |
24 ± 1.0 |
22 ± 0.9 |
22 ± 3.4 |
20 ± 3.4 |
22 ± 2.4 |
21 ± 1.4 |
23 ± 4.0 |
20 ± 2.3* |
22 ± 1.4 |
20 ± 1.3** |
|
Haemoglobin concentration, 18 months (g/dl) |
7.7 ± 0.2 |
7.7 ± 0.4 |
7.2 ± 1.5 |
7.2 ± 1.3 |
7.2 ± 0.9 |
7.4 ± 0.5 |
7.7 ± 1.5 |
6.8 ± 0.8** |
7.7 ± 0.3 |
7.0 ± 0.5** |
|
Mean corpuscular haemoglobin, 18 months (pg) |
18 ± 0.7 |
19 ± 0.7 |
18 ± 2.5 |
19 ± 1.7 |
17 ± 1.8 |
19 ± 0.8 |
19 ± 2.3 |
19 ± 0.8* |
18 ± 0.6* |
18 ± 0.7* |
|
Total cholesterol, 24 months (mg/dl) |
160 ± 66 |
130 ± 92 |
150 ± 62 |
130 ± 92 |
150 ± 69 |
110 ± 30 |
130 ± 57 |
120 ± 29 |
160 ± 57 |
96 ± 32* |
|
Calcium, 24 months (mg/dl) |
9.9 ± 0.5 |
10 ± 1.7 |
10 ± 0.5 |
10 ± 1.2 |
10 ± 0.5 |
11 ± 1.5 |
10 ± 0.5 |
11 ± 1.4 |
10 ± 1.1* |
10 ± 1.1 |
|
Phosphate, 24 months (mg/dl) |
5.2 ± 0.6 |
3.8 ± 1.4 |
5.5 ± 0.7 |
3.8 ± 0.7 |
5.2 ± 1.0 |
3.6 ± 0.6 |
5.6 ± 0.8 |
3.8 ± 0.9 |
6.5 ± 2.4* |
3.5 ± 0.6 |
|
Aspartate aminotransferase activity, 24 months (mIU/ml) |
110 ± 32 |
140 ± 54 |
110 ± 38 |
180 ± 116 |
130 ± 44 |
130 ± 30 |
120 ± 40 |
140 ± 39 |
93 ± 30* |
120 ± 30 |
|
Blood urea nitrogen, 24 months (mg/dl) |
17 ± 4 |
18 ± 5 |
20 ± 10 |
20 ± 11 |
18 ± 5 |
17 ± 4 |
18 ± 7 |
14 ±4* |
33 ± 34 |
16 ± 4 |
From Katoh et al. (1982)
* p < 0.05; ** p < 0.01; t test
Altered erythrocyte parameters (reduced haemoglobin concentration, erythrocyte volume fraction and mean corpuscular haemoglobin) were seen in females receiving 2000 or 10 000 ppm at 18 months only; the erythrocyte count was unaffected. Interpretation of these findings was difficult, as there was no clear dose–response relationship and the values for haemoglobin concentration and erythrocyte volume fraction in all groups at 18 months were notably higher than those at 12 or 24 months. The authors considered the pattern of effects at in females 10 000 ppm to be treatment-related and adverse. Other variations in haematological parameters were not consistent with dose or duration and were within normal physiological ranges. They were thus considered not to be adverse effects of treatment.
Variations in a number of clinical chemical parameters were seen during the study, but most showed no consistency over time or dose, and a number, such as decreased bilirubin in older animals, were considered not to be adverse. Some of the alterations in animals at the highest concentration indicated a link with treatment: increased blood urea nitrogen, calcium and phosphate in males, decreased aspartate aminotransferase activity in males and females throughout the study and reduced cholesterol in females at 24 months. All the values except those for cholesterol were reported to be within the normal range. The results of urine analysis were generally similar in treated and control groups, with no indication of treatment-related nephropathy. Variations in the results of clinical chemistry and urine analysis within groups and over time (especially at 18 months) were greater than those usually reported (e.g. for sodium, potassium and chloride), possibly masking any small changes.
At the 3-month interim sacrifice, a number of organs from flutolanil-treated animals showed statistically significant (p < 0.05) changes in weight relative to those of controls, including reduced absolute weights of the thymus and pituitary in all treated males, increased weights of the liver in animals of each sex at the highest concentration and increased kidney weights in males at this concentration. The changes in the thymus and pituitary did not show a clear dose–response relationship and diminished when corrected for body weight. Females at the highest concentration had increased relative pituitary weights (Table 11). At the 12-month interim sacrifice, the only notable effect on organ weights was an increase in liver weight. At terminal sacrifice, increased liver and kidney weights were seen at the highest concentration. Wide variations within groups (standard deviations as large as the mean) hindered evaluation of changes in the weights of many other organs.
Table 11. Weights of organs in Crj:CD rats given diets containing flutolanil for up to 2 years (mean ± standard deviation)
|
Organ |
Time (months) |
Relative (mg/100 g) or absolute (g) weight |
Dietary concentration (ppm) |
|||||||||
|
0 |
40 |
200 |
2000 |
10 000 |
||||||||
|
Males |
Females |
Males |
Females |
Males |
Females |
Males |
Females |
Males |
Females |
|||
|
Thyroid |
3 |
Relative |
5 ± 1.3 |
6 ± 1.1 |
4 ± 0.3 |
6 ± 1.0 |
4 ± 0.4 |
6 ± 1.7 |
5 ± 0.5 |
6 ± 1.2 |
5 ± 1.0 |
7 ± 1.4 |
|
Pituitary |
3 |
Relative |
2.7 ± 0.2 |
4.6 ± 0.7 |
2.3 ± 0.3* |
4.9 ± 1.0 |
2.4 ± 0.2 |
6.3 ± 3.7 |
2.5 ± 0.3 |
4.9 ± 0.9 |
2.5 ± 0.1* |
5.7 ± 1.1 |
|
Liver |
3 |
Relative |
18 ± 3 |
10 ± 0.5 |
17 ± 2 |
9 ± 1 |
16 ± 3 |
10 ± 1 |
16 ± 1 |
10 ± 1 |
18 ± 1 |
12 ± 1* |
|
Liver |
24 |
Absolute |
23 ± 4 |
16 ± 4 |
22 ± 3 |
17 ± 4 |
22 ± 4 |
18 ± 4 |
22 ± 3 |
17 ± 5 |
24 ± 3 |
19 ± 5* |
|
Liver |
24 |
Relative |
2.8 ± 0.4 |
2.8 ± 0.5 |
2.6 ± 0.4 |
3.0 ± 1.0 |
2.7 ± 0.4 |
2.8 ± 0.9 |
2.6 ± 0.3 |
2.8 ± 0.5 |
3.1 ± 0.4** |
3.0 ± 0.6 |
|
Kidney |
3 |
Absolute |
3.6 ± 0.5 |
2.0 ± 0.2 |
3.5 ± 0.3 |
2.1 ± 0.2 |
3.3 ± 0.4 |
2.1 ± 0.1 |
3.2 ± 0.2 |
2.2 ± 0.4 |
3.4 ± 0.2 |
2.2 ± 0.1 |
|
Kidney |
24 |
Absolute |
5.5 ± 1.0 |
2.9 ± 0.7 |
5.4 ± 1.1 |
2.9 ± 0.7 |
5.1 ± 1.1 |
2.9 ± 0.7 |
5.1 ± 0.7 |
3.1 ± 0.8 |
6.4 ± 2.0 |
3.0 ± 0.4 |
|
Kidney |
24 |
Relative |
0.7 ± 0.1 |
0.6 ± 0.2 |
0.6 ± 0.1 |
0.6 ± 0.3 |
0.6 ± 0.1 |
0.5 ± 0.1 |
0.6 ± 0.1 |
0.5 ± 0.1 |
0.9 ± 0.4* |
0.5 ± 0.1 |
From Katoh et al. (1982)
* p < 0.05; ** p < 0.01; t test
Gross pathological investigations did not show any treatment-related effects. A minimal increase in ‘red change’ of the lung in males at the highest concentration was not observed in females and was not correlated with any microscopic findings.
Microscopic examinations allowed identification of a range of findings typical of aged rats. A number of the lesions (Table 12) occurred at higher incidence in some treated groups than in controls; however, many showed no dose–response relationship and/or no pattern consistent with other findings. Lesions associated with administration of flutolanil included vacuolar degeneration of the liver, decreased cellular elements of the spleen (also described as atrophy in the report) in males at concentrations > 2000 ppm, reticulocyte proliferation in the spleen and bone marrow (‘dyshaematopoiesis’) in females at the highest concentration. The patterns of nephrosis and prostate duct dilatation changed during the study, with apparent increases in treated groups at 12 months but with no dose–response relationship over a 250-fold range. These findings were not reproduced at termination. There were no adverse effects of treatment on the thyroid.
Table 12. Non-neoplastic lesions in rats given diets containing flutolanil for up to 2 years
|
Lesion |
Dietary concentration (ppm) |
|||||||||
|
0 |
40 |
200 |
2000 |
10 000 |
||||||
|
Males |
Females |
Males |
Females |
Males |
Females |
Males |
Females |
Males |
Females |
|
|
Terminal sacrifice |
||||||||||
|
Lung, atelectasia |
8/21 |
19/26 |
23/26* |
20/26 |
15/19 |
16/20 |
15/19 |
19/22 |
19/22* |
19/24 |
|
Spleen, decreased cellular elements |
1/21 |
4/26 |
4/26 |
5/26 |
3/19 |
1/20 |
6/19* |
3/22 |
6/22* |
6/24 |
|
Spleen, reticulocyte proliferation |
0/21 |
0/26 |
0/26 |
2/26 |
0/19 |
2/20 |
0/19 |
4/22 |
1/22 |
6/24* |
|
Prostate duct, dilatation (> 3), 12 months |
1/10 |
|
5/10 |
|
5/10 |
|
5/10 |
|
6/10* |
|
|
Prostate duct, dilatation (> 3), 24 months |
11/21 |
|
8/26 |
|
4/19* |
|
4/19* |
|
2/22* |
|
|
Kidney, nephrosis (> 2), 12 months |
6/10 |
1/10 |
4/10 |
3/10 |
7/10 |
4/10 |
4/10 |
4/10 |
5/10 |
5/10 |
|
Kidney, nephrosis (> 2), 24 months |
16/21 |
6/26 |
21/26 |
5/26 |
11/19 |
2/20 |
12/19 |
5/22 |
18/22 |
4/24 |
|
Liver, vacuolar degeneration (> 2), 12 months |
0/10 |
2/10 |
1/10 |
3/10 |
0/10 |
2/10 |
1/10 |
2/10 |
5/10 |
|
|
Liver, vacuolar degeneration (> 2), 24 months |
2/21 |
9/26 |
4/26 |
11/26 |
2/19 |
10/20 |
4/19 |
8/22 |
1/22 |
16/24* |
|
Liver, granulation |
0/21 |
6/26 |
1/26 |
3/26 |
0/19 |
2/20 |
0/19 |
3/22 |
1/22 |
10/24 |
|
Bone marrow, dyshaematopoiesis |
7/21 |
7/26 |
8/26 |
10/26 |
8/19 |
8/20 |
7/19 |
11/22 |
8/22 |
13/24* |
|
Died before study termination |
||||||||||
|
Liver, vacuolar degeneration (> 2) |
7/28 |
11/24 |
6/25 |
12/24 |
4/30 |
15/30 |
8/30 |
16/28 |
11/29 |
18/26 |
|
Spleen, reticulocyte proliferation |
0/28 |
2/24 |
0/25 |
7/24 |
0/30 |
5/30 |
0/30 |
3/28 |
1/29 |
1/26 |
|
Spleen, decreased cellular elements |
6/28 |
2/24 |
6/25 |
5/24 |
7/30 |
6/30 |
2/30 |
8/28 |
6/29 |
12/26* |
|
Prostate duct dilatation (> 3) |
7/28 |
|
12/25 |
|
18/30 |
|
11/30 |
|
13/29 |
|
From Katoh et al. (1982)
The overall incidences of tumours were similar in treated and control groups (Table 13), and typical of CD rats. A number of tumours were present at greater incidences in treated groups than in concurrent controls. In general, these were sporadic, showed no dose–response relationship and were not statistically significant, and the values were within the range of generic data for the strain of rat [data for the test facility were reported to be no longer available]. The pattern of pituitary tumours varied considerably between groups, with an increased incidence of chromophobe adenomas but a decrease in chromophobe adenocarcinomas in treated females. The overall incidences of pituitary tumours were considered to indicate that flutolanil is not tumorigenic in the pituitary gland.
Table 13. Tumour incidences in Crj:CD rats given diets containing flutolanil, with rates for generic CD rat controls (%)
|
Lesion |
Dietary concentration (ppm) |
Incidence (%) in other controls in the same laboratory |
||||||||||
|
0 |
40 |
200 |
2000 |
10 000 |
||||||||
|
Males |
Females |
Males |
Females |
Males |
Females |
Males |
Females |
Males |
Females |
Males |
Females |
|
|
Adrenals, phaeochromocytoma |
0 |
1 |
3 |
0 |
4 |
2 |
2 |
2 |
4 |
0 |
1.4–23 |
NR |
|
Liver, cholangioma |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
1 |
1 |
0–1.4 |
0–1.4 |
|
Lungs, adenoma |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
1 |
NR |
1.3–4 |
|
Pancreas, islet-cell adenoma |
6 |
0 |
2 |
3 |
5 |
1 |
3 |
1 |
2 |
2 |
NR |
1.4–14 |
|
Pituitary |
|
|
|
|
|
|
|
|
|
|
|
|
|
Chromophobe |
7 |
10 |
3 |
18 |
11 |
17 |
11 |
15 |
8 |
16 |
|
|
|
Chromophobe |
12 |
24 |
7 |
15 |
12 |
16 |
10 |
13* |
9 |
11* |
NR |
NR |
|
Chromophobe and |
0 |
1 |
0 |
0 |
0 |
3 |
0 |
1 |
3 |
0 |
|
|
|
Thyroid, follicular adenoma |
2 |
0 |
0 |
0 |
0 |
1 |
2 |
0 |
0 |
2 |
NR |
1–14 |
|
Urinary bladder, papilloma |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
1 |
2 |
0–3 |
0–1.6 |
|
Uterus, adenocarcinoma |
|
0 |
|
1 |
|
0 |
|
0 |
|
0 |
NR |
1–1.4 |
|
Blood, lymphocytic leukaemia |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0–1.4 |
NR |
|
Total tumour-bearing animals |
31 |
47 |
21 |
47 |
36 |
48 |
31 |
45 |
31 |
45 |
NR |
NR |
From Katoh et al. (1982)
NR, not relevant
* p < 0.05; Fisher exact
Two rare tumours were seen at increased incidences in the groups at the two higher concentrations: liver cholangioma and urinary bladder papilloma. Although the incidences of these two tumour types were not statistically significant, none occurred in the groups at 0, 40 and 200 ppm. Furthermore, the liver is a target organ for flutolanil. The overall incidence of cholangioma was 3/200 (1.5%) (1/50 in males and 1/50 in females at 10 000 ppm and 1/50 in females at 2000 ppm group) in the two groups combined, which is not significantly greater than the generic control incidence of < 1.4%. The incidence of urinary bladder papillomas (1/50 in females at 2000 ppm, 1/50 in males at 10 000 ppm, 2/50 in females at 10 000 ppm) may have been marginally greater than that in female generic controls. As the incidence range in male generic controls was 0–3%, the single papilloma seen in a male at the highest concentration may have been spontaneous. The combined incidence for the two sexes at the two higher concentrations was 2%, which is little different from the maximum incidence in female generic controls of 1.6%. Combining incidence data for the groups at the two higher concentrations may not be inappropriate in view of equivocal evidence for saturation of absorption of flutolanil at high doses. There was no evidence of a hyperplastic response in the bladder of flutolanil-treated animals.
Thus, the main non-neoplastic effects were on initial body-weight gain, the liver, the spleen and erythrocytes. Although many of the effects showed no or minimal dose–response relationships, the overall pattern of findings indicated that 2000 ppm (equal to 87 mg/kg bw per day) was an effect level. Flutolanil did not increase the overall tumour incidence, although a low incidence of rare urinary bladder papillomas and cholangiomas in the groups at the two higher concentrations was noted. The NOAEL was 200 ppm, equal to 9 mg/kg bw per day, on the basis of a pattern of effects at 2000 ppm, including reduced erythrocyte parameters, decreased spleen cellularity and carcinogenicity (Katoh et al., 1982).
Data on the genotoxicity of flutolanil are summarized in Table 14. Flutolanil has been tested for genotoxicity in a range of assays in vitro and in an assay for micronuclei in bone marrow of mice treated in vivo. Negative results were seen in assays for bacterial reverse mutation, bacterial DNA repair, mammalian gene mutation, unscheduled DNA synthesis in rat hepatocytes, clastogenicity in human lymphocytes and chromosomal effects (micronucleus induction) in vivo. A weak positive result was reported in an assay for chromosomal aberration in Chinese hamster lung cells in the presence of metabolic activation. This finding was observed at a moderately cytotoxic concentration and was not reproduced in a study with human lymphocytes in which higher concentrations were used. In two of the studies (for reverse mutation in vitro and micronucleus formation in vivo), flutolanil of exceptionally high purity (> 99%) was used, whereas the minimum purity of the technical-grade material is 96.8%. To address this issue, the mutagenicity of four impurities present in the technical-grade material was tested. They were found to give negative results in assays for reverse mutation1 . The overall weight of evidence indicates that flutolanil (technical) is not genotoxic.
Table 14. Results of studies for genotoxicity with flutolanil
|
End-point |
Test object |
Dose |
Purity (%) |
GLP or QA |
Result |
Reference |
|
In vitro |
|
|
|
|
|
|
|
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538; E. coli WP2 uvrA |
10–25 000 µg/plate |
99.4 |
No |
Negative + S9 |
Moriya & Ohta (1981) |
|
DNA repair |
B. subtilis H17, M45 |
20–10000 µg/disk |
99.4 |
No |
Negative |
Moriya & Ohta (1981) |
|
Forward mutation |
L5178Y mouse lymphoma cells |
6–100 µg/ml |
97.6 |
GLP |
Negative + S9 |
Heidemann (1989) |
|
Unscheduled DNA synthesis |
Wistar rat hepatocytes (primary culture) |
2.7–80 µg/ml |
97.6 |
GLP |
Negative |
Fautz (1989) |
|
Chromosomal aberrations |
Human peripheral lymphocytes |
125–1000 µg/ml |
97.6 |
GLP |
Negative +S9 |
Jenkinson (1990) |
|
Chromosomal aberrations |
Chinese hamster lung (Don) cells |
0, 12, 24, 48 µg/ml |
97.5 |
QA |
Positive +S9 |
Tokiwa (1986) |
|
In vivo |
|
|
|
|
|
|
|
Micronucleus induction |
BDF-1 mice, bone marrow |
0, 6400, 8000, |
99.8 |
QA |
Negative |
Sasaki (1983) |
GLP, good laboratory practice; QA, quality assurance
Rats
A two-generation (one litter per generation) study in Crl:CD(SD)BR rats was reported in 1992, which contained statements of compliance with GLP and which was performed according to OECD guideline 416 (1983) with no significant deviations. Groups of 30 rats of each sex received diets containing flutolanil (purity, 97.6% or 98.3%) at a concentration of 0, 200, 2000 or 20 000 ppm before mating until the end of gestation (males) or the end of lactation (females). The F0 animals were 42 days of age at initiation of treatment and 112 days old at mating. Groups of 30 F1 animals of each sex were treated for 98 days before mating one to one. Examinations for survival, clinical signs, body weight and food consumption were performed routinely. Reproductive performance and outcome were determined appropriately. The litter sizes were reduced to four of each sex on day 4, when numbers permitted. All adult animals and on all pups found dead or culled at day 4 were examined grossly post mortem. Liver, spleen, reproductive tissues and gross lesions from controls and adult animals at the high concentration were examined histopathologically.
The homogeneity of the test diets was acceptable. The concentrations of flutolanil in occasional batches of diet differed from the nominal values by > 15%, although the mean concentrations in the diet were within 7% of nominal. The intakes of flutolanil during the various phases of the study are shown in Table 15. There was no evidence of treatment-related mortality or morbidity in adult animals. The body weights were similar in all groups, with the exception of a transient reduction in F0 males at the two higher concentrations in week 3, which was attributed to defective water provision and was not repeated in the F1 generation. The food consumption patterns were similar for treated and control groups. Dose-related increases in relative and absolute liver weights were seen in animals of each sex, achieving statistical significance at 20 000 ppm (Table 16). Histopathological examination showed similar patterns of lesions in controls and treated animals, with no evidence of increased hepatocellular hypertrophy or necrosis. The only finding of note was an increase in the incidence of atrophy of the testicular germinal epithelium in F1 males at 20 000 ppm (6/30, with 1/30 in controls), but this was considered not to be adverse as it was not seen in the F0 generation, was unilateral, was not associated with poor reproductive performance and was not statistically significant (p = 0.052; Fisher exact test, one-sided). The increased liver weights were greater in F0 than in F1 animals, indicating no specific sensitivity associated with intake during development or early life. The 10% increase in liver weights was considered to be an adaptive response to the high concentrations of ingested flutolanil (20 000 ppm corresponding 1600 mg/kg bw per day) and not adverse in the absence of histopathological changes. The NOAEL for general toxicity was 20 000 ppm.
Table 15. Intakes (mg/kg bw per day) of flutolanil by rats during various stages of a two-generation study of reproductive toxicity (means)
|
Dietary concentration (ppm) |
F0 generation |
F1 generation |
||||
|
Before mating |
Gestation (females) |
Before mating |
Gestation (females) |
|||
|
Males |
Females |
Males |
Females |
|||
|
200 |
16 |
19 |
18 |
16 |
20 |
16 |
|
2000 |
160 |
190 |
180 |
160 |
190 |
160 |
|
20 000 |
1600 |
1900 |
1900 |
1600 |
2000 |
1600 |
From Schroeder (1991)
Table 16. Absolute and relative weights of the liver (mean + standard deviation) in rats given diets containing flutolanil over two generations
|
Dietary concentration (ppm) |
F0 males |
F0 females |
F1 males |
F1 females |
||||
|
g |
g/100 g |
g |
g/100 g |
g |
g/100 g |
g |
g/100 g |
|
|
0 |
18 + 2.5 |
3.6 ± 0.3 |
9.6 ± 1.2 |
3.2 ± 0.3 |
20 ± 3.4 |
3.4 ± 0.4 |
10 ± 1.8 |
3.2 ± 0.6 |
|
200 |
19 ± 2.6 |
3.6 ± 0.3 |
9.6 ± 1.2 |
3.3 ± 0.3 |
20 ± 3.1 |
3.5 ± 0.4 |
9.8 ± 1.2 |
3.1 ± 0.3 |
|
2000 |
19 ± 2.4 |
3.7 ± 0.3 |
9.8 ± 1.2 |
3.3 ± 0.3 |
20 ± 2.8 |
3.4 ± 0.4 |
10 ± 1.6 |
3.3 ± 0.4 |
|
20000 |
20 ± 2.1 |
3.9 ± 0.2** |
11 ± 1.4** |
3.7 ± 0.3** |
20 ± 2.9 |
3.5 ± 0.3 |
11 ± 1.5 |
3.5 ± 0.3** |
From Schroeder (1991)
** p < 0.01; Dunnett test
Mating performance and pregnancy and fertility rates were comparable in control and treated groups. In the F0 generation, the pregnancy rates were > 90% in all groups; however, in the F1 generation, the pregnancy rate was about 80% for both the controls and animals at the intermediate concentration. Male fertility rates in the F0 (93–97%) and F1 (78–96%, with the lowest rates in control animals) matings were unaffected by flutolanil. The length of gestation (22 days), litter size (12–13), sex ratio, pup survival and pup weights were similar in all groups in both generations. An apparent increase in the number of dead pups seen in all treated F0 groups (2–3%) appeared to be related to a low incidence in controls (0.5%), as all groups had mean litter sizes of > 12 live pups; the effect was not repeated in the F1 generation. Overall, there were no treatment-related effects on reproduction at 20 000 ppm.
The NOAEL for general and reproductive toxicity was 20 000 ppm, equal to 1600 mg/kg bw per day, the highest dose tested (Schroeder, 1991).
Rats
In a study that complied with GLP, conducted in 1987 and performed according to OECD guideline 414 (1981), with no significant deviations, groups of 22 mated female CD rats received flutolanil (purity, 97.5%) in 0.5% aqueous methylcellulose by gavage at a dose of 0, 40, 200 or 1000 mg/kg bw per day on days 6–15 of gestation (day 0 = day of mating). The doses were based on the results of a preliminary study (not submitted). The dams were killed on day 20 of gestation, and the reproductive tract was examined for corpora lutea, implantation and resorption sites and live and dead fetuses. The fetuses were weighed, sexed and examined for external abnormalities; then, 50% were dissected and stained (Alizarin red) for skeletal examination and the remainder were examined by Wilson sectioning.
There were no deaths, clinical signs or changes in body-weight gain or food or water consumption indicative of treatment-related maternal toxicity. The numbers of corpora lutea, implantations, resorptions, viable fetuses and fetal weights were similar in all groups and within the ranges of other controls in the laboratory. The fetal incidences of hepatic haemorrhage, displaced testes (slight), abdominal haemorrhage, subcutaneous haemorrhage of the lower jaw and scapula, incomplete ossification of sternebrae and thoracic centra and metacarpal ossification were higher in treated groups than in the concurrent controls. However, these findings were considered not to be treatment-related as the incidences were within the range of other control values and no dose–response relationship was seen over a 25-fold range (Table 17). One fetus of a dam at the highest dose had microphthalmia (left), anophthalmia (right) and severe internal hydrocephaly, which had not been seen in previous studies, together with a number of other abnormalities. Eight other pups from this litter showed no notable abnormalities. This single finding was considered not to represent evidence of developmental toxicity.
Table 17. Findings in fetuses of CD rats treated with flutolanil (per cent), with values for other controls in the same laboratory (mean per cent and range)
|
Abnormality |
Dose (mg/kg bw per day) |
||||
|
0 |
40 |
200 |
1000 |
Other controlsa |
|
|
Hepatic haemorrhage |
1.9 |
5.9 |
8.7 |
7.2 |
10 (0–28) |
|
Displaced testes (slight) |
5.7 |
9.3 |
9.5 |
12 |
3.3 (0–24) |
|
Subcutaneous haemorrhage, lower jaw |
2.5 |
3.9 |
4.7 |
3.3 |
0.5 (0–6.0) |
|
Subcutaneous haemorrhage, scapula |
20 |
24 |
22 |
24 |
29 (6–91) |
|
Abdominal haemorrhage |
0 |
0.7 |
0.7 |
2.0 |
1.8 (0–8.0) |
|
Incomplete ossification of thoracic vertebral centra |
22 |
37* |
26 |
33* |
26 (9–58) |
|
Incomplete ossification of two sternebrae |
66 |
71 |
74* |
69 |
67 (43–85) |
|
Metacarpal ossification (grade 4) |
10 |
19 |
20 |
23* |
(6–71) |
From Lambert (1987)
a > 14 000 fetuses in > 115 studies
* p < 0.05; Mann Whitney U (one-tailed)
The NOAEL for both developmental and maternal toxicity was 1000 mg/kg bw per day (Lambert, 1987).
Rabbits
In a study that complied with GLP, conducted in 1987, which was performed according to OECD guideline 414 (1981), with no significant deviations, groups of 16 mated female New Zealand white rabbits received flutolanil (purity, 97.5%) in 2% aqueous gum arabic by gavage at a dose of 0, 40, 200 or 1000 mg/kg bw per day on days 6–18 of gestation (day 0 = day of mating). The doses were selected on the basis of a preliminary study (not submitted). The dams were killed on day 28 of gestation, and the reproductive tract was examined for corpora lutea, implantation and resorption sites and live and dead fetuses. The fetuses were weighed, sexed, examined for external abnormalities, dissected for observation of visceral abnormalities (Stuckhardt & Poppe, 1984), then stained with Alizarin red for skeletal examination. The dams underwent a gross necropsy, and the liver, kidney, spleen and adrenal were weighed.
One animal at the lowest dose died due to a dosing error. There were no clinical signs or changes in body-weight gain or food consumption indicative of treatment-related maternal toxicity. One control, three at the intermediate dose and three at the high dose had no implants. The numbers of corpora lutea, implantations, resorptions and viable fetuses and fetal weights were similar in all groups and within the range of values for other controls. An apparently dose-related increase in the ratio of female pups was considered not to indicate selective male toxicity, as the numbers of resorptions and deaths were low and there was no overall effect on litter size (Table 18). The overall incidences of abnormalities were low, with only two fetuses (at each of the two higher doses) showing any external, visceral or skeletal abnormalities. The extent of ossification was similar in all groups. Most of the data on fetuses were presented on a litter basis, and individual findings were presented only for gross abnormalities. Given the low incidence of findings, however, this is considered not to be a significant issue. The maternal organ weights and gross findings were similar in all groups. Flutolanil showed no evidence of maternal or fetotoxicity at the highest dose tested. The overall NOAEL was 1000 mg/kg bw per day (Tauchi, 1987).
Table 18. Findings in rabbit fetuses after exposure of dams to flutolanil during gestation
|
Finding |
Dose (mg/kg bw per day) |
|||
|
0 |
40 |
200 |
1000 |
|
|
Male:female fetuses |
60:44 |
54:45 |
51:50 |
44:51 |
|
Resorptions or deaths (per dam) |
1 (0.1) |
0 (0) |
4 (0.3) |
7 (0.5) |
|
Litter size (mean ± SD) |
7.4 ±1.7 |
6.6 ± 1.7 |
7.8 ± 2.6 |
7.3 ± 1.9 |
From Tauchi (1987)
Flutolanil has been produced for almost 20 years. No reports of adverse effects were identified during routine monitoring of production plant workers (Kose & Nakayama, 2002; Mitsumoto & Fuji, 2002).
The available studies on the toxicity of flutolanil were performed between 1977 and 1990. Although a number of the studies were performed before adoption of good laboratory practice, the overall quality of the database and standard of reporting were considered to be adequate.
Two studies of absorption and metabolism in rats were evaluated, in which different vehicles were used. [aniline ring-U-14C]Flutolanil was rapidly absorbed, peak concentrations of radioactivity being achieved in blood and tissue 2 h after dosing. The highest concentrations of radioactivity at 2 h were found in liver and kidney, which were 3.5- and 2.5-fold higher than those in whole blood, respectively. The extent of absorption of an oral dose, as estimated from urinary excretion, varied with dose and with whether single or repeated doses were given. The greatest absorption of an oral dose of 20 mg/kg bw was about 70%. The absorption of a dose of 100 mg/kg bw per day was similar, but that of 1000 mg/kg bw per day fell to about 10%, indicating that there is a plateau for the achieved systemic dose after administration by gavage. Less saturation of absorption was seen after dietary administration, and the concentration of tissue residues and the frequency of liver enlargement generally showed dose-response relationships up to very high doses. Excretion was rapid (> 80% within 24 h), the proportion in urine and faeces varying between studies, with increased urinary excretion after repeated dosing. The primary urinary metabolite, representing up to 57% of the administered dose, was desisopropyl flutolanil, either free or as the glucuronide or, predominantly, the sulfate conjugate. There was evidence of induction of phase-I metabolism and/or conjugation of flutolanil after repeated administration. There was no evidence of cleavage at the amido bridge. Measurement of tissue residues after administration for 28 days or 2 years showed that flutolanil did not bioaccumulate in rats.
Flutolanil has very low acute toxicity after oral (LD50, > 10 000 mg/kg bw), dermal, inhalation, intraperitoneal or subcutaneous administration. No evidence of specific acute toxicity was seen. Flutolanil was neither irritating nor sensitizing to skin but was a slightly irritating to the eye. WHO (2000) has classified flutolanil as ‘unlikely to present an acute hazard in normal use’.
In studies with repeated doses in mice, rats and dogs for up to 2 years, the pattern of effects was comparable, comprising liver enlargement, depression of body-weight gain and mild haematological disturbances, with some evidence of increased thyroid weight seen in shorter studies in rats and dogs. In a 2-year study of toxicity and carcinogenicity in rats, an increased frequency of vacuolar degeneration of the liver was observed at 10 000 ppm (equal to 460 mg/kg bw per day), and splenic effects (decreased cellular elements) were observed at concentrations of 2000 ppm (equal to 87 mg/kg bw per day) and above. All the findings seen in a 90-day study of toxicity in dogs were not reproduced in a 2-year study of toxicity in dogs, perhaps because the vomiting seen at higher doses (250 mg/kg bw per day and above) towards the end of the 2-year study reduced the absorbed dose and might have reversed any effects. A range of other effects was found, with no consistent pattern among studies or species, no clear dose–response relationship and no evidence of an association with treatment. In all three species, flutolanil could be administered at doses in excess of the accepted limit value without any clear evidence of severe toxicity. The NOAELs for non-neoplastic effects were 1500 ppm (equal to 170 mg/kg bw per day) in mice, 200 ppm (equal to 9 mg/kg bw per day) in rats and 50 mg/kg bw per day in dogs.
Flutolanil at a dietary concentration of 30 000 ppm, equivalent to 3300 mg/kg bw per day, increased the incidences of hepatocellular adenomas and carcinomas in mice and produced a 10–20% increase in liver weight. The increases in tumour incidences were not statistically significant, and the values were within the range seen in other controls. The Meeting concluded that the liver tumours were of no significance for human risk assessment.
In rats, flutolanil did not increase the overall tumour incidence or the incidences of hepatocellular or thyroid tumours. Nevertheless, the low, statistically nonsignificant increases in the incidences of uncommon cholangiomas of the liver and papillomas of the urinary bladder at dietary concentrations of 2000 ppm and above were of potential concern, because none were seen at 0, 40 and 200 ppm. The cholangiomas were also of interest in view of the fact that the liver is a target organ for the effects of flutolanil; however, the incidence of cholangiomas (1/50 in males and females at 10 000 ppm and in females at 2000 ppm) resulted in an overall incidence of 3/200 (1.5%) in the two groups combined, which was not significantly greater than the incidence in other controls of up to 1.4%. The incidence of papillomas of the urinary bladder (1/50 in females at 2000 ppm and in males at 10 000 ppm, 2/50 in females at 10 000 ppm) was marginally greater than that in other control groups of females (0–1.6%) and was within the range of other groups of male controls (0–3%). There was no evidence of a hyperplastic response in the bladder of animals given flutolanil. As papillomas of the urinary bladder and cholangiomas of the liver can occur spontaneously, and a clear NOAEL for these tumours was identified at 200 ppm (equal to 9 mg/kg bw per day), the Meeting concluded that the low incidences of these rare tumours were not of significance for the overall risk assessment.
A weak positive result was reported for chromosomal aberration in Chinese hamster lung cells at a moderately cytotoxic concentration of flutolanil in the presence of metabolic activation. Negative results were seen in five other adequate assays in vitro and in an assay for chromosomal effects (micronucleus induction) in vivo. Studies of bacterial gene mutation with four impurities present in the technical-grade material showed that the impurities did not induce reverse mutation. The Meeting concluded that the overall weight of evidence indicates that flutolanil (technical grade) is not genotoxic.
In view of the lack of genotoxicity and the finding of statistically nonsignificant increases in tumour incidences, for which clear NOAELs were identified, the Meeting concluded that flutolanil is unlikely to pose a carcinogenic risk to humans.
Flutolanil showed no specific reproductive effects in a two-generation study of reproductive toxicity in rats. The only sign of general toxicity, increased liver weight, occurred at similar frequency in both generations of parents, indicating that no specific effect was associated with exposure in utero or in early life. A slight but statistically nonsignificant increase in the frequency of atrophy of the testicular germinal epithelium in F1 male offspring of dams at the highest dose was not associated with alterations in reproductive performance. The NOAEL for general and reproductive toxicity was 20 000 ppm, equal to 1600 mg/kg bw per day, the highest dose tested.
The results of studies of developmental toxicity in rats and rabbits indicated no specific fetotoxicity or teratogenicity at the highest dose tested, 1000 mg/kg bw per day. The Meeting concluded that flutolanil is not teratogenic.
The Meeting concluded that the available database was adequate to characterize the potential hazard of flutolanil to fetuses, infants and children.
No adverse findings have been reported in workers in production or formulation plants or in operators applying flutolanil.
The Meeting established an ADI of 0–0.09 mg/kg bw on the basis of the NOAEL of 200 ppm, equal to 9 mg/kg bw per day, for effects on erythrocytes and an increase in the incidence of decreased cellular elements of the spleen in the long-term study of toxicity and carcinogenicity in rats, and a safety factor of 100.
The Meeting concluded that it was unnecessary to establish an acute RfD for flutolanil in view of its low acute lethality, the absence of clinical signs and effects pertinent to administration of single doses, and the absence of developmental effects.
|
Levels relevant to risk assessment |
||||
|
Species |
Study |
Effect |
NOAEL |
LOAEL |
|
Mouse |
18-month study of toxicity and carcinogenicitya |
Toxicity |
1500 ppm, equal to 170 mg/kg bw per day |
7000 ppm, equal to 840 mg/kg bw per day |
|
|
|
Carcinogenicity |
30 000 ppm, equal to 3300 mg/kg bw per dayd |
– |
|
Rat |
2-year study of toxicity and carcinogenicitya |
Toxicity |
200 ppm, equal to 9 mg/kg bw per day |
2000 ppm, equal to 87 mg/kg bw per day |
|
|
|
Carcinogenicity |
200 ppm, equal to 9 mg/kg bw per day |
2000 ppm, equal to 87 mg/kg bw per day |
|
|
Two-generation study of reproductive toxicitya |
Parental and pup toxicity |
20 000 ppm, equal to 1600 mg/kg bw per dayd |
|
|
|
Developmental toxicityb |
Maternal, embryo- and fetotoxicity |
1000 mg/kg bw per dayd |
– |
|
Rabbit |
Developmental toxicityb |
Maternal, embryo- and fetotoxicity |
1000 mg/kg bw per dayd |
– |
|
Dog |
2-year study of toxicityc |
Toxicity |
50 mg/kg bw per day |
250 mg/kg bw per day |
aDietary administration
bGavage
cCapsule
d Highest dose tested
Estimate of acceptable daily intake for humans
0–0.09 mg/kg bw
Estimate of acute reference dose
Unnecessary
Studies that would provide information useful for continued evaluation of the compound
Further observations in humans
List of end-points relevant for setting guidance values for dietary and non-dietary exposure
|
Absorption, distribution, excretion and metabolism in mammals |
|
|
Rate and extent of absorption |
Rapid Tmax (2 h ); ~ 70% absorption at 20 mg/kg bw; evidence of saturation at higher doses by gavage |
|
Distribution |
Extensive; highest concentrations in liver, kidney and adipose tissue. |
|
Potential for accumulation |
None (data from 2-year study in rats) |
|
Rate and extent of excretion |
Relatively rapid (~50% within 12 h) |
|
Metabolism in animals |
De-isopropylation and conjugation; some evidence of auto-induction |
|
Toxicologically significant compounds (animals, plants and environment) |
Flutolanil |
|
|
|
|
Acute toxicity |
|
|
Rat, LD50, oral |
> 10 000 mg/kg bw |
|
Rat, LD50, dermal |
> 5000 mg/kg bw |
|
Rat, LC50, inhalation |
(4 h) > 6 mg/l |
|
Skin irritation |
Not irritating |
|
Eye irritation |
Slightly irritating |
|
Skin sensitization |
Not sensitizing (Magnusson and Kligman) |
|
|
|
|
Short term studies of toxicity |
|
|
Target/critical effect |
Liver, erythrocytes, emesis |
|
Lowest relevant oral NOAEL |
50 mg/kg bw per day (2-year study in dogs) |
|
Genotoxicity |
Not genotoxic |
|
|
|
|
Long-term studies of toxicity and carcinogenicity |
|
|
Target/critical effect |
Liver vacuolation, reduced cellular elements in spleen, erythrocyte parameters |
|
Lowest relevant oral NOAEL |
200 ppm, equal to 9 mg/kg bw per day (2-year study in rats) |
|
Carcinogenicity |
Unlikely to pose a risk to humans |
|
|
|
|
Reproductive toxicity |
|
|
Target/critical effect for reproductive toxicity |
None |
|
Lowest relevant NOAEL for reproductive toxicity |
20 000 ppm, equal to 1600 mg/kg bw per day, in rats, highest dose tested |
|
Target/critical effect for developmental toxicity |
None |
|
Lowest relevant NOAEL for developmental toxicity |
1000 mg/kg bw per day in rats and rabbit,s highest dose tested |
|
Neurotoxicity |
No evidence of neurotoxicity in routine studies |
|
Medical data |
No effects reported in production or formulation plant workers or applicators |
|
Summary |
Value |
Study |
Safety factor |
|
ADI |
0.09 |
2 years, rats |
100 |
|
Acute RfD |
Unnecessary |
|
|
Aizawa, H. (1982) Absorption, distribution, excretion and metabolism of flutolanil in rats. Unpublished report No. T-3021 from Mitsubishi-Kasei Institute of Toxicological and Environmental Sciences, Kanagawa, Japan.
Atkinson, J. & Daly, I. (1986a) A three-month oral toxicity study of flutolanil in rats. Unpublished report No. 85-2926 from Biodynamics, Inc., East Millstone, New Jersey, USA. Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Atkinson, J., & Daly, I. (1986b) A three-month oral toxicity study of flutolanil in dogs. Unpublished report No. 85-2927 from Biodynamics, Inc., East Millstone, New Jersey, USA. Submitted to WHO by Nihon Nohyau Co., Ltd, Tokyo, Japan.
Auletta, C. (1990) A 21-day dermal toxicity study in rats with flutolanil. Unpublished report No. 89-3497 from Biodynamics, Inc., East Millstone, New Jersey, USA. Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Aughton, P. (1987) Flutolanil: Preliminary toxicity study by dietary administration to CD-1(ICR)BR mice for 13 weeks. Unpublished report No. NHH/017/FLU from Life Science Research, Eye, Suffolk, United Kingdom. Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Cummins, H.A. (1986a) Flutolanil: Acute dermal irritation/corrosion test in the rabbit. Unpublished report T-3027 from Life Sciences Research Ltd, Suffolk, United Kingdom. Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Cummins, H.A. (1986b) Flutolanil: Acute eye irritation/corrosion test in the rabbit. Unpublished report T-3026 from Life Sciences Research Ltd, Suffolk, United Kingdom. Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Cummins, H.A. (1986c) Flutolanil: Delayed contact hypersensitivity study in guinea-pigs. Unpublished report T-3028 from Life Sciences Research Ltd, Suffolk, United Kingdom. Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Eschbach, J. (1992) Flutolanil: The metabolism of aniline-ring 14C-flutolanil in rats. Unpublished report No. 90076 from Biodynamics, Inc., East Millstone, New Jersey, USA. Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Fautz, R. (1989) Unscheduled DNA synthesis in primary hepatocytes of male rats in vitro. Unpublished report No. 145708 from CCR Cytotest Cell Research, Robdorf, Germany. Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Heidemann, A. (1989) Cell mutation assay at the thymidine kinase (TK+/–) locus in mouse lymphoma L5178Y cells. Unpublished report No. 145607 from CCR Cytotest Cell Research, Robdorf, Germany. Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Jenkinson, P. (1990) Flutolanil: OECD 473 metaphase analysis in human lymphocytes in vitro. Unpublished report No. G261-199/40 from Safepharm Laboratories Ltd, Derby, United Kingdom. Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Katoh, M., Suzuki, A., Ito, M., Chida, T. & Matsuzawa, I. (1982) Chronic toxicity test of NNF-136 in rats. Unpublished report from Mitsubishi-Kasei Institute of Toxicological and Environmental Sciences Co., Ltd, Kanagawa-ken, Japan. Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Kosaka, T. & Saito, T. (1982a) NNF-136: Acute toxicity study in rats. Unpublished report from Institute of Environmental Toxicology, Tokyo, Japan. Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Kosaka, T. & Saito, T. (1982b) NNF-136: Acute toxicity study in mice. Unpublished report from Institute of Environmental Toxicology, Tokyo, Japan. Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Kose, S. & Nakayama, S. (2002) Statement of medical surveillance, Saga plant of Nihon Nohyaku Company, Japan. Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Lambert, E.P. (1987) Teratology study in the rat. Unpublished report No. 87/NHH023/554 from Life Science Research, Eye, Suffolk, United Kingdom. Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Martin, P. (1990) Flutolanil: Oncogenicity study by dietary administration to CD-1 (ICR)BR mice for 78 weeks. Unpublished report No. 89/NHH018/0043 from Life Science Research, Eye, Suffolk, United Kingdom. Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Mitsumoto, S. & Fuji, T. (2002) Statement of medical surveillance, Ube plant of the Central Glass Company, Japan. Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Moriya, M. & Ohta, T. (1981) NNF-136: Microbial mutagenicity study. Unpublished report from Institute of Environmental Toxicology, Tokyo, Japan. Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Sasaki, Y. (1983) Flutolanil: Micronucleus test. Unpublished report from Institute of Environmental Toxicology, Tokyo, Japan. Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Sato, M.(1982) Chronic toxicity of NNF-136 administered orally to beagle dogs for 104 weeks. Unpublished report from Shin Nippon Biomedical Laboratories, Ltd, Kagoshima, Japan. Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Schroeder, R. (1991) A two-generation reproduction study in rats with flutolanil. Unpublished report No. 89-3417 from Biodynamics, Inc., East Millstone, New Jersey, USA. Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Stuckhardt, J.L. & Poppe, S.M. (1984) Fresh visceral examination of rat and rabbit fetuses used in teratogenicity testing. Teratog. Carcinog. Mtag., 4, 181–188.
Tauchi, K. (1987) Teratogenicity study of flutolanil in the rabbit. Unpublished report from Imamichi Institute of Animal Reproduction, Japan. Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Tokiwa, T. (1986) Chromosomal aberration test of flutolanil with cultured mammalian cells. Unpublished report No. NRILS 85-1567 from NRI Life Sciences, Kanagawa-ken, Japan. Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Tsuchiya, K. & Sugimoto, T., (1977) One month subacute toxicity study on flutolanil in rats. Unpublished report No. 54-039 from Institute of Life Science Research, Nihon Nohyaku Co., Ltd, Kawachinagano, Japan. Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Tsuda, S., Iwasaki, M., Yoshida, M., Ikeda, T., Harada, T., & Ebino, K. (1984) Acute inhalation toxicity of flutolanil (Moncut®) to rats. Unpublished report from Institute of Environmental Toxicology, Tokyo, Japan. Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Ward, RJ. (1999) Flutolanil: in vitro absorption from a 450 g/l SC formulation through human epidermis. Unpublished report No. T-3081 from Zeneca Central Toxicology Laboratory, Cheshire, England. Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
WHO (2000) The WHO recommended classification of pesticides by hazard and guidelines to classification 2000–2202 (WHO/PCS/01.5). Available from the International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland.
ENDNOTES
1 These studies were evaluated, but the compounds are not named as the data were considered to represent commercially sensitive information.
See Also: Toxicological Abbreviations Flutolanil (ICSC)