Pesticide residues in food - 2002 - Joint FAO/WHO Meeting on Pesticide Residues
First draft prepared by
C. Vleminckx
Scientific Institute of Public Health, Division Toxicology,
Brussels, Belgium.
Metalaxyl is a 1:1 mixture of (R)-2-[(2,6-dimethylphenyl)methoxyacetylamino]propionic acid methyl ester (R-enantiomer) and (S)-2-[(2,6-dimethylphenyl)methoxyacetylamino]propionic acid methyl ester (S-enantiomer). Technical-grade metalaxyl-M consists of a minimum of 97% of the R-enantiomer and 3% of the S-enantiomer. The two compounds are fungicides used in agriculture, horticulture and forestry, which act by inhibiting mycelial growth and spore formation. Metalaxyl-M has not been evaluated previously; however, the toxicity of metalaxyl was evaluated by the 1982 Joint Meeting (Annex 1, reference 38), which established an ADI of 0–0.03 mg/kg bw on the basis of a NOAEL of 2.5 mg/kg bw per day in a 2-year study in rats.
All the studies with metalaxyl-M were conducted according to current guidelines of the OECD, Commission of the European Communities and the FIFRA of the USA and also in accordance with the principles of good laboratory practice. Several of the studies performed with metalaxyl were finalized before the OECD guidelines and regulations for good laboratory practice were enacted. Nevertheless, all the relevant studies were subjected to quality assurance and, with few exceptions, their protocols complied with today’s guideline requirements.
Absorption, distribution and excretion are generally passive processes that are similar for enantiomers, but enzymatic metabolism and protein binding to plasma or tissue proteins can show a high degree of stereoselectivity. Hence, enantiomers can be metabolized at different rates and even along different routes, although this is less common (Caldwell, 1995). A detailed comparative investigation of the metabolism of both metalaxyl-M and metalaxyl is therefore indicated. All the studies of metabolism were conducted with metalaxyl or metalaxyl-M uniformly labelled with 14C on the phenyl ring.
Rats
The metabolic fate of [14C]metalaxyl (radiochemical purity, > 99%) was followed in four male and four female RAI rats given a single oral dose of 0.5 or 25 mg/kg bw. The animals were kept in individual metabolism cages, and urine, faeces and expired CO2 were collected separately for analysis at 24-h intervals. When the rats were killed 144 h after dosing, the liver, fat, kidney, muscle, blood, heart, brain, lungs, spleen, ovary, testis and remaining carcass were examined for residual radioactivity.
In both sexes, irrespective of dose, more than 60% of the administered radioactivity was excreted within 24 h, and the compound was almost completely eliminated within 144 h (Table 1). While renal elimination was the preferred route in female rats, males excreted greater amounts in faeces. Males and females at 0.5 mg/kg bw excreted 37% and 55% of the administered dose in urine and 66% and 45% in faeces, respectively; while males and females at 25 mg/kg bw excreted 38% and 63% in urine and 63% and 35% in faeces, respectively. Less than 0.02% of the dose appeared in expired air. In animals at 0.5 mg/kg bw, residues were found at levels above the limit of quantitative determination only in liver, blood and carcass, still accounting for < 0.005 ppm of metalaxyl equivalents (Table 2). Animals at 25 mg/kg bw showed higher concentrations, with 0.1–0.23 ppm in liver, carcass, fat and blood and < 0.1 ppm of metalaxyl equivalents in all other tissues. The concentration of residual radioactivity in tissues was generally higher in females than in males. Two-dimensional thin-layer chromatography (TLC) of the urine in various solvent systems demonstrated the presence of four to six major metabolite fractions and about 10 minor ones, most of the metabolites being relatively polar. The pattern of metabolites was not significantly influenced by dose or by the sex of the animals. No unchanged metalaxyl was detected in urine. The results of this study show that orally administered metalaxyl is readily absorbed from the gut into the general circulation and rapidly excreted in rats. The preferred route of excretion is via the urine for females and the faeces for males. Because of the rapid elimination of the compound, the residual radioactivity in tissues was generally low (Hamboeck, 1977, 1981a).
Table 1. Kinetics of excretion of [14C]metalaxyl (% of administered dose) by rats treated by gavage
|
Route of excretion |
Dose (mg/kg bw) |
|||
|
0.49 |
0.54 |
24 |
27 |
|
|
Urine |
||||
|
0–24 h |
27 |
38 |
28 |
46 |
|
24–48 h |
7.5 |
11 |
6.7 |
9.9 |
|
48–72 h |
1.9 |
3.9 |
1.7 |
4.4 |
|
72–144 h |
0.85 |
2.0 |
0.82 |
2.5 |
|
Total |
37 |
55 |
38 |
63 |
|
Faeces |
||||
|
0–24 h |
38 |
26 |
34 |
18 |
|
24–48 h |
20 |
14 |
24 |
13 |
|
48–72 h |
4,0 |
2.7 |
3.3 |
3.3 |
|
72–144 h |
3.5 |
1.9 |
1.4 |
1.3 |
|
Total |
66 |
45 |
63 |
35 |
|
Total excretion |
100 |
100 |
100 |
98 |
|
Tissue residues |
0.08 |
0.12 |
0.12 |
0.20 |
|
Cage wash |
0.67 |
0.36 |
0.33 |
1.7 |
From Hamboeck (1977)
Table 2. Residual radioactivity (ppm of metalaxyl equivalents) in rat tissues 6 days after a single oral doses by gavage
|
Tissue |
Dose (mg/kg bw) |
|||
|
0.49 |
0.54 |
24 |
27 |
|
|
Carcass |
LOQ |
0.003 |
0.093 |
0.17 |
|
Liver |
0.002 |
0.004 |
0.15 |
0.22 |
|
Fat |
< LOQ > LOD |
< LOQ > LOD |
0.056 |
0.19 |
|
Kidney |
< LOQ > LOD |
LOQ |
0.032 |
0.063 |
|
Muscle |
LOQ |
< LOQ > LOD |
0.009 |
0.016 |
|
Blood |
< LOQ > LOD |
0.002 |
0.068 |
0.12 |
|
Brain |
< LOQ > LOD |
< LOQ > LOD |
0.009 |
0.019 |
|
Lungs |
< LOQ > LOD |
LOQ |
0.032 |
0.074 |
|
Testis |
< LOQ > LOD |
– |
0.005 |
– |
|
Ovary |
– |
< LOQ > LOD |
– |
0.046 |
From Hamboeck (1977); LOQ, limit of quantification; LOD, limit of detection
The absorption, distribution and excretion of [14C]metalaxyl (radiochemical purity, > 98%) were studied in groups of five male and five female Sprague-Dawley rats given metalaxyl at a single oral dose of 2 or 80 mg/kg bw or 2 mg/kg bw by intravenous injection. The animals were kept in individual metabolism cages, and urine, faeces and expired CO2 were collected separately for analysis at 24-h intervals for 3 days after dosing. Biliary excretion was investigated, and enterohepatic circulation was monitored for 24 h by injecting 0.4 ml of bile collected for 6 h from male rats given metalaxyl at 80 mg/kg bw intravenously. Blood samples were taken from the caudal vein (after oral administration) or jugular vein (after intravenous injection) at various times, and portions of the samples were radioassayed. To prevent enterohepatic circulation during determination of the rate of disappearance of radioactivity from blood in animals given 2 mg/kg bw intravenously, their bile ducts were cannulated under anaesthesia, and the concentration of metalaxyl in whole blood and plasma was measured. The distribution of radioactivity in plasma, blood, brain, thyroid, lung, heart, thymus, liver, kidney, adrenal, spleen, pancreas, duodenum, testis, uterus, ovary, abdominal fat and hypogastrium, abdominal and dorsal skin, femoral muscle and bone marrow was measured 1, 24 and 72 h after administration.
After gavage, the compound was taken up readily into the general circulation. At 2 mg/kg bw, the maximum concentration (Cmax) of radioactivity in blood was reached after 20 min in males (0.48 µg/ml) and 40 min in females (0.93 µg/ml) (Table 3). The decline in radioactivity showed a biphasic relationship, with half-times of 1.1 and 72 h in males and 2 and 22 h in females for the first and second phase, respectively. In the group given [14C]metalaxyl at 80 mg/kg bw, the concentration in blood reached a maximum more slowly than with 2 mg/kg bw, by 40 min after administration in males and by 100 min in females, the Cmax in females (38 µg/ml) again being higher than that in males (19 µg/ml). The half-times were 1.5 h and 125 h in males and 3 h and 96 h in females for the first and second phases, respectively. The decreasing blood concentrations after 6 h suggested enterohepatic circulation of metalaxyl or its metabolites.
Table 3. Concentrations of radioactivity in blood (µg equivalents/ml) after a single oral or intravenous dose of [14C]metalaxyl
|
Time |
Route |
Dose (mg/kg bw) |
|||
|
Oral |
2 |
80 |
|||
|
Males |
Females |
Males |
Females |
||
|
20 min |
0.48 |
0.87 |
19 |
23 |
|
|
40 min |
0.37 |
0.93 |
19 |
32 |
|
|
60 min |
0.25 |
0.85 |
16 |
34 |
|
|
80 min |
0.19 |
0.75 |
12 |
38 |
|
|
100 min |
0.13 |
0.64 |
9.9 |
38 |
|
|
2 h |
0.10 |
0.62 |
8.0 |
38 |
|
|
3 h |
0.08 |
0.34 |
5.6 |
32 |
|
|
4 h |
0.07 |
0.26 |
4.2 |
28 |
|
|
5 h |
0.06 |
– |
3.6 |
21 |
|
|
6 h |
0.09 |
0.22 |
4.5 |
16 |
|
|
8 h |
0.09 |
0.18 |
2.4 |
8.4 |
|
|
10 h |
0.09 |
0.15 |
2.6 |
5.6 |
|
|
12 h |
0.10 |
0.13 |
2.7 |
4.1 |
|
|
24 h |
0.09 |
0.09 |
2.3 |
3.1 |
|
|
Intravenous |
2 mg/kg bw (bile-duct cannulated) |
||||
|
Males |
Females |
||||
|
30 s |
2.6 |
2.6 |
|||
|
1 min |
2.6 |
2.7 |
|||
|
2 min |
2.2 |
2.6 |
|||
|
3 min |
1.9 |
2.3 |
|||
|
4 min |
1.8 |
2.3 |
|||
|
5 min |
1.7 |
2.2 |
|||
|
7.5 min |
1.5 |
2.1 |
|||
|
10 min |
1.3 |
1.8 |
|||
|
15 min |
1.2 |
1.7 |
|||
|
20 min |
1.0 |
1.5 |
|||
|
25 min |
0.85 |
1.4 |
|||
|
30 min |
0.78 |
1.4 |
|||
From Uesugi (1988)
The rate of disappearance of radioactivity from blood of animals treated intravenously with metalaxyl at 2 mg/kg bw fitted a two-compartment model. The half-time in whole blood was 0.42 h in males and 0.64 h in females, and the half-time in plasma was 0.41 h in males and 0.56 h in females.
The distribution of radioactivity in tissues after oral administration of metalaxyl is shown in Table 4. The concentration of radioactivity in all organs except brain and in tissues of all treated animals reached a maximum 1 h after administration and was higher than that in plasma. At 2 mg/kg bw, high concentrations of radioactivity were observed after 1 h in liver, kidney and duodenum in males and in thyroid, liver, kidney, duodenum and abdominal fat in females. The concentrations in these organs and in plasma in females was higher than that in males. Thereafter, the concentrations in most organs declined gradually, and by 72 h after administration the concentrations in liver and kidney had decreased to one-sixth to one-tenth of the values at 1 h. At 80 mg/kg bw, high concentrations of radioactivity were observed in thyroid, liver, kidney, duodenum and abdominal fat in males and in thyroid, liver, kidney, adrenal, spleen, duodenum and fat in females. After 1 h, the concentrations in most organs declined gradually, and the values at 72 h were one-half to one-tenth of that at 1 h.
Table 4. Distribution of radioactivity (µg equivalent/g) in rat tissues after a single oral administration of [14C]metalaxyl at 2 / 80 mg/kg bw
|
Tissue |
Time after exposure (h) |
|||||
|
1 |
24 |
72 |
||||
|
Males |
Females |
Males |
Females |
Males |
Females |
|
|
Plasma |
0.08 / 22 |
0.40 / 245 |
0.05 / 5.0 |
0.03 / 2.4 |
0.01 / 0.32 |
0.01 / 0.56 |
|
Blood |
0.09 / 33 |
0.36 / 37 |
0.05 / 3.2 |
0.08 / 8.0 |
0.05 / 1.6 |
0.08 / 1.6 |
|
Brain |
0.06 / 12 |
0.31 / 28 |
0.06 / 3.3 |
0.03 / 2.2 |
0.03 / 1.5 |
0.04 / 2.0 |
|
Thyroid |
0.17 / 79 |
1.1 / 52 |
0.33 / 59 |
0.03 / 5.8 |
0.23 / 15 |
0.25 / 27 |
|
Liver |
0.48 / 49 |
1.4 / 56 |
0.28 / 11 |
0.22 / 8.8 |
0.07 / 5.6 |
0.10 / 4.0 |
|
Kidney |
0.45 / 72 |
1.5 / 58 |
0.27 / 26 |
0.25 / 14 |
0.08 / 5.9 |
0.10 / 7.0 |
|
Adrenal |
0.11 / 35 |
0.74 / 59 |
0.26 / 11 |
0.06 / 4.3 |
0.15 / 6.6 |
0.20 / 12 |
|
Spleen |
0.21 / 29 |
0.43 / 65 |
0.16 /15 |
0.05 / 5.8 |
0.08 / 5.7 |
0.13 / 7.3 |
|
Duodenum |
0.73 / 60 |
1.3 / 81 |
0.21 / 24 |
0.30 / 16 |
0.10 / 2.6 |
0.14 / 7.3 |
|
Abdomen fat |
0.07 / 36 |
1.7 / 84 |
0.11 / 9.1 |
0.03 / 5.3 |
0.03 / 2.3 |
0.07 / 3.6 |
From Uesugi (1988)
The amounts of radioactivity excreted in urine and faeces and in expired air are shown in Table 5. Urinary and faecal excretion was rapid, both males and females excreting 67–84% of the administered dose within 24 h and 92–100% within 72 h. The amounts excreted in expired air by rats at 2 mg/kg bw was below the level of detection at all times, while those at 80 mg/kg bw excreted 0.001–0.006% of the administered dose.
Table 5. Cumulative excretion of radioactivity (% of dose) in rat urine, faeces and expired air after a single oral administration of [14C]metalaxyl
|
Dose (mg/kg bw) |
Time (h) |
Males |
Females |
||||||
|
Urine |
Faeces |
Total |
Expired air |
Urine |
Faeces |
Total |
Expired air |
||
|
2 |
0–24 |
31 |
43 |
74 |
ND |
49 |
22 |
71 |
ND |
|
48 |
37 |
53 |
90 |
ND |
57 |
31 |
88 |
ND |
|
|
72 |
39 |
56 |
95 |
ND |
60 |
33 |
92 |
ND |
|
|
80 |
0–24 |
46 |
39 |
84 |
0.006 |
54 |
13 |
66 |
0.001 |
|
48 |
50 |
48 |
98 |
0.005 |
65 |
27 |
92 |
ND |
|
|
72 |
51 |
50 |
100 |
0.001 |
67 |
30 |
97 |
0.003 |
|
ND, not detected
Biliary excretion of radioactivity is shown in Table 6. When cannulated rats were given [14C]metalaxyl at 2 mg/kg bw orally, males excreted 31% in bile within 1 h, 49% within 2 h and 71% within 24 h, while females excreted 11% within 1 h, 33% within 2 h and 66% within 24 h. A clear difference between males and females was seen in the amount excreted in bile after the high dose of metalaxyl, males excreting 15% within 1 h, 29% within 2 h and 69% within 24 h and females excreting 1.2% within 1 h, 4.4% within 2 h and 54% within 24 h. After intravenous injection of [14C]metalaxyl, male rats excreted 30% in bile within 10 min, 67% within 30 min and 91% within 5 h, and females excreted 9.1% within 10 min, 36% within 30 min and 91% within 5 h, indicating differences in the transport and metabolism of metalaxyl by the liver and bile duct.
Table 6. Cumulative biliary excretion of radioactivity (% of administered dose) after a single oral dose of [14C]metalaxyl to bile-cannulated rats
|
Time |
Route |
Dose (mg/kg bw) |
|||
|
Oral |
2 |
80 |
|||
|
Males |
Females |
Males |
Females |
||
|
0–1 h |
31 |
11 |
15 |
1.2 |
|
|
2 h |
49 |
33 |
29 |
4.4 |
|
|
3 h |
58 |
49 |
37 |
9.9 |
|
|
4 h |
63 |
56 |
40 |
17 |
|
|
5 h |
65 |
59 |
43 |
23 |
|
|
6 h |
66 |
60 |
46 |
28 |
|
|
8 h |
68 |
62 |
51 |
36 |
|
|
10 h |
69 |
64 |
55 |
40 |
|
|
12 h |
70 |
64 |
58 |
43 |
|
|
24 h |
71 |
66 |
69 |
54 |
|
|
Urine, 24 h |
24 |
29 |
22 |
14 |
|
|
Intravenous |
2 |
||||
|
Males |
Females |
||||
|
0–10 min |
30 |
9.1 |
|||
|
20 min |
55 |
24 |
|||
|
30 min |
67 |
36 |
|||
|
40 min |
74 |
44 |
|||
|
50 min |
78 |
51 |
|||
|
1 h |
81 |
57 |
|||
|
2 h |
88 |
77 |
|||
|
3 h |
90 |
85 |
|||
|
4 h |
90 |
89 |
|||
|
5 h |
91 |
91 |
|||
|
Urine, 5 h |
7.7 |
3.7 |
|||
From Uesugi (1988)
In rats that received bile from male rats given [14C]metalaxyl intravenously at 80 mg/kg bw, males again excreted 0.9% of the administered dose in bile within 1 h, 8.9% within 10 h and 46% within 24 h, and females excreted 0.8% within 1 h, 11% within 10 h and 19% within 24 h. Males excreted 9.1% in urine within 24 h and females excreted 6.3%. These results strongly support the existence of enterohepatic circulation of metalaxyl or its metabolites.
The results of this study show that orally administered metalaxyl is rapidly absorbed in rats through the digestive tract. The twofold higher Cmax in females than in males may have been due to different excretory rates into bile, and the subsequent biphasic pattern of disappearance showed enterohepatic circulation. Metalaxyl and its metabolites were excreted rapidly in urine and faeces, the differences between the sexes being due to the differences in biliary excretion. The higher excretion rate in urine by females suggests qualitative and quantitative differences in the metabolites in bile, a difference in the reabsorption rate from the digestive tract and a different flow to enterohepatic circulation. The rates of excretion in bile were high in both sexes. No difference was found in the total amounts excreted by males and females over 24 h, but males showed a higher excretion rate at earlier times, as confirmed in experiments with intravenous administration. The average bioavailability of metalaxyl was thus about 90%. Metalaxyl translocated readily to all tissues except brain, with maximal amounts 1 h after oral administration, decreasing to relatively low concentrations in all tissues by 72 h (Uesugi, 1988).
The absorption, distribution and excretion of [14C]metalaxyl (radiochemical purity, 97.2–97.3%) were studied after intravenous and oral administrations to groups of five male and five female rats (Taconic Farms, Germantown, New York, USA). Group 1 received a single intravenous injection of 1.1 mg/kg bw, groups 2 and 4 received single oral doses of 1.1 and 200 mg/kg bw, respectively, and Group 3 received 14 daily doses of 1.1 mg/kg bw unradiolabelled compound (purity, 96.5%) orally, followed by a single oral dose of [14C]metalaxyl. For each group, one additional male and one female were designated as controls and received the vehicle only (isotonic saline for intravenous administration, ethanol and PEG-200 for oral administration). Faeces and urine were collected at various times for 7 days. Rats were killed at the completion of the study, and specified tissues collected. The study was performed in compliance with the principles of good laboratory practice (GLP) with quality assurance (QA) certification, and the protocol was in accordance with guideline 85-1 of the FIFRA Subdivision F and mostly in compliance with OECD TG 417 (1984) and TM B36 from Annex V of Directive 87/302 of the European Commission.
The total average recoveries were 102.8% for group 1, 99.7% for group 2, 103.0% for group 3 and 101.6% for group 4 (Table 7). Excretion of radioactivity was rapid and complete in all groups (Table 8), the amount eliminated in excreta ranging from 95.5% to 109.4%. More than 89% was eliminated within the first 48 h after dosing. The pattern of elimination was different in males and females, elimination via faeces predominating in males (60%) and elimination in urine predominating in females (70%). The similar patterns of excretion after oral and intravenous administration indicate that the compound was well absorbed. The high recovery of radioactivity in the faeces of intravenously dosed rats (59% in males, 36% n females) suggests the involvement of biliary secretion, which was more extensive in males.
Table 7. Recovery of radioactivity (per cent of total dose) in rats after oral or intravenous administration of [14C]metalaxyl
|
Route of excretion |
Dose (mg/kg bw) |
|||||||
|
1.1 (intravenous) |
1.1 (oral) |
1.4 (oral with pretreatment) |
200 (oral) |
|||||
|
Males |
Females |
Males |
Females |
Males |
Females |
Males |
Females |
|
|
Urine |
44 |
66 |
35 |
67 |
32 |
74 |
47 |
70 |
|
Faeces |
59 |
36 |
62 |
35 |
64 |
35 |
54 |
31 |
|
Tissues |
0.37 |
0.42 |
0.31 |
0.56 |
0.29 |
0.44 |
0.30 |
0.31 |
|
Erythrocytes |
0.012 |
0.013 |
0.009 |
0.009 |
0.008 |
0.007 |
0.007 |
0.009 |
|
Plasma |
0.003 |
0.004 |
0.002 |
0.004 |
0.001 |
0.002 |
0.001 |
0.001 |
|
Total |
103.9 |
101.8 |
97.4 |
102.0 |
95.9 |
110.0 |
101.2 |
102.1 |
From Jameson (1990)
Table 8. Elimination of [14C]metalaxyl (per cent of administered dose) by rats after oral or intravenous treatment
|
Route of excretion |
Dose (mg/kg bw) |
|||||||
|
1.1 (intravenous) |
1.1 (oral) |
1.4 (oral with pretreatment) |
200 (oral) |
|||||
|
Males |
Females |
Males |
Females |
Males |
Females |
Males |
Females |
|
|
Urine |
||||||||
|
12 h |
20 |
35 |
15 |
32 |
14 |
38 |
31 |
40 |
|
24 h |
31 |
50 |
26 |
51 |
25 |
59 |
38 |
54 |
|
48 h |
39 |
59 |
32 |
60 |
30 |
68 |
44 |
64 |
|
72 h |
41 |
62 |
34 |
62 |
31 |
71 |
45 |
67 |
|
168 h |
44 |
65 |
35 |
66 |
32 |
74 |
46 |
69 |
|
Faeces |
||||||||
|
12 h |
NS |
NS |
NS |
NS |
NS |
NS |
NS |
NS |
|
24 h |
40 |
25 |
44 |
24 |
47 |
24 |
39 |
17 |
|
48 h |
54 |
33 |
57 |
31 |
60 |
33 |
50 |
28 |
|
72 h |
57 |
35 |
60 |
33 |
62 |
34 |
53 |
30 |
|
168 h |
59 |
36 |
62 |
35 |
64 |
35 |
54 |
31 |
From Jameson (1990); NS, no sample collected
At the low dose, the concentrations of [14C]metalaxyl equivalents were low in all tissues (Table 9). After 7 days, the highest concentrations of residue (average for males and females) were found in intestine (0.03 ppm) and liver (0.007 ppm). The tissue levels were not affected by the route of administration. At the high dose, residues were measurable in all tissues, the highest concentrations at 7 days being observed in the intestine (3.1 ppm) and liver (0.81 ppm). No significant difference between the sexes was seen at either dose. In all groups, < 1% of the dose was recovered in tissues after 7 days. Low concentrations were found in blood fractions; the highest average values at the high dose (males and females) were 0.62 ppm for erythrocytes and 0.057 ppm for plasma.
Table 9. Residual radioactivity (ppm metalaxyl equivalents) in rats after oral or intravenous administration of [14C]metalaxyl
|
Tissue |
Dose (mg/kg bw) |
|||||||
|
1.1 (intravenous) |
1.1 (oral) |
1.4 (oral with pretreatment) |
200 (oral) |
|||||
|
Males |
Females |
Males |
Females |
Males |
Females |
Males |
Females |
|
|
Bone |
0.00029 |
0.00041 |
0.00027 |
0.00038 |
0.00048 |
0.00035 |
0.045 |
0.094 |
|
Brain |
< LOQ |
< LOQ |
0.0013 |
< LOQ |
0.0002 |
0.00028 |
0.055 |
0.10 |
|
Fat |
0.00036 |
< LOQ |
< LOQ |
0.0021 |
< LOQ |
< LOQ |
0.38 |
0.67 |
|
Gonads |
< LOQ |
0.0011 |
< LOQ |
0.0012 |
< LOQ |
0.00099 |
0.028 |
0.25 |
|
Kidneys |
0.0016 |
0.0028 |
0.0012 |
0.0023 |
0.0017 |
0.0021 |
0.16 |
0.28 |
|
Liver |
0.0054 |
0.010 |
0.0037 |
0.0090 |
0.004 |
0.0082 |
0.64 |
0.98 |
|
Lungs |
0.0024 |
0.0068 |
0.00044 |
0.0056 |
< LOQ |
0.0037 |
0.12 |
0.20 |
|
Muscle |
< LOQ |
< LOQ |
< LOQ |
0.00034 |
< LOQ |
< LOQ |
0.066 |
0.11 |
|
Spleen |
< LOQ |
0.0029 |
< LOQ |
0.0012 |
0.0012 |
0.0020 |
0.086 |
0.17 |
|
Stomach |
0.0008 |
0.00032 |
< LOQ |
< LOQ |
0.0013 |
0.045 |
0.071 |
0.15 |
|
Intestine |
0.021 |
0.030 |
0.029 |
0.045 |
0.018 |
0.0026 |
3.5 |
2.7 |
|
Residual carcass |
0.0013 |
0.0018 |
0.00028 |
0.0030 |
0.0023 |
0.0026 |
0.18 |
1.1 |
|
Erythrocytes |
0.004 |
0.006 |
0.003 |
0.004 |
0.003 |
0.004 |
0.51 |
0.72 |
|
Plasma |
0.0007 |
0.001 |
0.0005 |
0.001 |
0.0003 |
0.0005 |
0.050 |
0.063 |
From Jameson (1990); LOQ, limit of quantification
This study confirmed the previous finding that metalaxyl is rapidly and well absorbed and eliminated, with differences in excretion between males and females. Pre-treatment with 14 daily doses of 1 mg/kg bw of unlabelled metalaxyl or intravenous administration did not affect the rate or route of excretion. Reflecting the rapid elimination of the compound, the residual radioactivity in tissues was generally low; even after an oral dose of 200 mg/kg bw, the concentrations was < 1 ppm in all tissues (Jameson, 1990).
The absorption, distribution, metabolism and excretion of metalaxyl-M and metalaxyl were compared at two doses of [phenyl-U-14C]-labelled test substance (radiochemical purity, 98.5% and 97.3%, respectively) in groups of four male and four female Tif:RAIf (SPF) rats. Groups received metalaxyl or metalaxyl-M at a single oral dose of 1 or 100 mg/kg bw. Urine and faeces were collected at 24-h intervals up to 168 h after treatment, and urine was collected after 8 h (12 h from the group given the higher dose of metalaxyl-M). Blood samples were taken 0.25, 0.5, 1, 2, 4, 8, 24 and 48 h after dosing from all animals and from three additional rats of each sex per dose in the group given metalaxyl-M at the higher dose. Seven days after dosing, bone, brain, fat, gonads, heart, kidneys, liver, lungs, plasma, skeletal muscle, spleen, uterus, whole blood and residual carcass were taken for analysis. The study was performed in compliance with the principles of GLP (with QA certification).
The concentrations of radioactivity from both compounds reached a maximum in blood within 0.5–1 h after administration, irrespective of the dose, except that the maximum in females at the higher dose group of metalaxyl occurred at 4 h (Table 10). The short half-times of 9–14 h, indicating rapid depletion from blood, were also independent of test substance, dose or sex of the animals. The areas under the blood concentration–time curve (AUC) of metalaxyl-M and metalaxyl were similar for the lower dose but increased proportionally at the higher dose, except in females at the higher dose of metalaxyl, for which the AUC was 179-fold higher than at the lower dose. Generally, the bioavailability of both compounds was higher in females than in males.
Table 10. Blood kinetics of metalaxyl-M and metalaxyl after oral administration to rats
|
Kinetics |
[phenyl-U-14C]Metalaxyl-M |
[phenyl-U-14C]Metalaxyl |
||||||
|
1 mg/kg bw |
100 mg/kg bw |
1 mg/kg bw |
100 mg/kg bw |
|||||
|
Male |
Female |
Male |
Female |
Male |
Female |
Male |
Female |
|
|
Cmax (µg parent equivalent) |
0.07 |
0.21 |
26 |
17 |
0.08 |
0.23 |
18 |
28 |
|
Tmax (h) |
0.5 |
0.5 |
0.5 |
1.0 |
0.5 |
1.0 |
0.5 |
4.0 |
|
Half-time (h) |
14 |
12 |
11 |
10 |
12 |
9.4 |
11 |
8.5 |
|
AUC0–48 h (µg × h g–1) |
0.9 |
1.4 |
120 |
130 |
0.9 |
1.5 |
83 |
270 |
From Müller (1997)
Actual doses: metalaxyl-M, 1 and 110 mg/kg bw for males and females; metalaxyl, 1.2 and 100 mg/kg bw for males and 1.1 and 120 mg/kg bw for females
The urinary excretion and tissue residues indicated the extent of absorption was similar for metalaxyl-M (37–62%) and metalaxyl (48–61%) (Table 11). As shown previously, most of an oral dose of metalaxyl is eliminated with the bile. As the absorption process is generally not influenced by chirality, it can be assumed that both test compounds were completely absorbed. Distribution occurred rapidly: 7 days after the low dose of either substance, the concentrations of residues in all tissues were very low, not exceeding 0.010 ppm of parent equivalents (Table 12). The pattern of distribution was similar at the higher dose but approximately 100-fold greater. Depletion of the racemate metalaxyl from adipose tissue was markedly slower than that of the R-enantiomer metalaxyl-M, owing to a slightly greater tendency of the racemate to form lipophilic metabolites than the R-enantiomer and subsequent deposition in adipose tissue. However, this metabolic difference applied only to 0.01% and 0.03% of the dose of metalaxyl-M and metalaxyl, respectively.
Table 11. Absorption (per cent of dose) of metalaxyl-M and metalaxyl after oral administration to rats
|
Tissue |
[phenyl-U-14C]Metalaxyl-M |
[phenyl-U-14C]Metalaxyl |
||||||
|
1 mg/kg bw |
100 mg/kg bw |
1 mg/kg bw |
100 mg/kg bw |
|||||
|
Male |
Female |
Male |
Female |
Male |
Female |
Male |
Female |
|
|
Urine |
50 |
62 |
37 |
46 |
47 |
20 |
49 |
59 |
|
Tissues |
0.23 |
0.27 |
0.17 |
0.24 |
0.16 |
0.55 |
0.17 |
0.43 |
|
Apparent absorption |
50 |
62 |
37 |
47 |
48 |
61 |
49 |
60 |
From Müller (1997)
Table 12. Tissue residues (ppm of parent equivalents) 7 days after oral administration to rats
|
Tissue |
[phenyl-U-14C]Metalaxyl-M |
[phenyl-U-14C]Metalaxyl |
||||||
|
1 mg/kg bw |
100 mg/kg bw |
1 mg/kg bw |
100 mg/kg bw |
|||||
|
Male |
Female |
Male |
Female |
Male |
Female |
Male |
Female |
|
|
Bone |
< LOD |
< LOD |
0.020 |
0.027 |
< LOD |
< LOD |
0.021 |
0.037 |
|
Brain |
< LOQ |
0.001 |
0.030 |
0.046 |
0.001 |
0.001 |
0.040 |
0.069 |
|
Fat |
< LOQ |
< LOQ |
0.032 |
0.043 |
0.001 |
0.002 |
0.246 |
0.29 |
|
Heart |
0.001 |
0.001 |
0.047 |
0.064 |
= LOQ |
0.001 |
0.062 |
0.090 |
|
Kidneys |
0.001 |
0.002 |
0.100 |
0.17 |
0.001 |
0.002 |
0.097 |
0.20 |
|
Liver |
0.005 |
0.009 |
0.456 |
0.56 |
0.004 |
0.009 |
0.307 |
0.74 |
|
Lungs |
0.001 |
0.010 |
0.089 |
0.15 |
0.001 |
0.009 |
0.082 |
0.14 |
|
Muscle |
< LOQ |
= LOQ |
0.028 |
0.039 |
< LOQ |
= LOQ |
0.044 |
0.047 |
|
Ovary |
– |
< LOD |
– |
0.043 |
– |
= LOD |
– |
0.083 |
|
Plasma |
< LOQ |
< LOQ |
0.009 |
0.017 |
< LOD |
< LOQ |
0.008 |
0.022 |
|
Spleen |
0.001 |
0.003 |
0.073 |
0.12 |
0.001 |
0.002 |
0.067 |
0.13 |
|
Testes |
= LOD |
– |
0.016 |
– |
< LOQ |
– |
0.016 |
– |
|
Uterus |
– |
= LOD |
– |
0.031 |
– |
< LOD |
– |
0.04 |
|
Carcass |
0.002 |
0.002 |
0.132 |
0.23 |
0.001 |
0.006 |
0.14 |
0.47 |
|
Total residues (% of dose) |
0.23 |
0.27 |
0.17 |
0.24 |
0.16 |
0.55 |
0.17 |
0.43 |
From Müller (1997); LOQ, limit of quantification: LOD, limit of detection
LOD, limit of detection; LOQ, limit of quantification
The excretion pattern was essentially the same for metalaxyl-M and metalaxyl (Table 13). In all groups, females showed slightly greater renal elimination than males. With both compounds, the administered dose was rapidly and almost completely eliminated, independently of dose or the sex of the animals. The blood kinetics, absorption, distribution and rate and route of excretion were not influenced by chirality (Müller, 1997).
Table 13. Excretion of metalaxyl-M and metalaxyl (per cent of dose) after oral administration
|
Tissue |
[phenyl-U-14C]Metalaxyl-M |
[phenyl-U-14C]Metalaxyl |
||||||
|
1 mg/kg bw |
100 mg/kg bw |
1 mg/kg bw |
100 mg/kg bw |
|||||
|
Male |
Female |
Male |
Female |
Male |
Female |
Male |
Female |
|
|
Urine |
||||||||
|
0–24 h |
40 |
49 |
31 |
36 |
40 |
49 |
44 |
49 |
|
24–48 h |
7.7 |
10 |
5.0 |
7.5 |
5.3 |
7.5 |
4.1 |
7.5 |
|
48–72 h |
1.1 |
1.7 |
0.8 |
2.0 |
0.9 |
1.3 |
0.6 |
1.3 |
|
72–168 h |
0.8 |
1.2 |
0.8 |
1.5 |
1.0 |
1.6 |
0.4 |
1.6 |
|
Subtotal |
50 |
62 |
37 |
46 |
47 |
60 |
49 |
59 |
|
Faeces |
||||||||
|
0–24 h |
36 |
27 |
49 |
38 |
40 |
24 |
45 |
27 |
|
24–48 h |
10 |
8.1 |
8.5 |
9.3 |
8.9 |
6.4 |
5.3 |
7.8 |
|
48–72 h |
1.4 |
0.7 |
1.1 |
1.5 |
1.0 |
1.0 |
0.9 |
1.0 |
|
72–168 h |
0.9 |
0.5 |
0.8 |
0.8 |
0.6 |
1.1 |
0.4 |
1.0 |
|
Subtotal |
48 |
37 |
59 |
50 |
50 |
33 |
52 |
36 |
|
Cage wash |
0.67 |
1.0 |
0.64 |
1.5 |
0.88 |
3.0 |
0.20 |
1.5 |
|
Total excretion |
99 |
100 |
97 |
98 |
98 |
96 |
100 |
97 |
From Müller (1997)
Rats
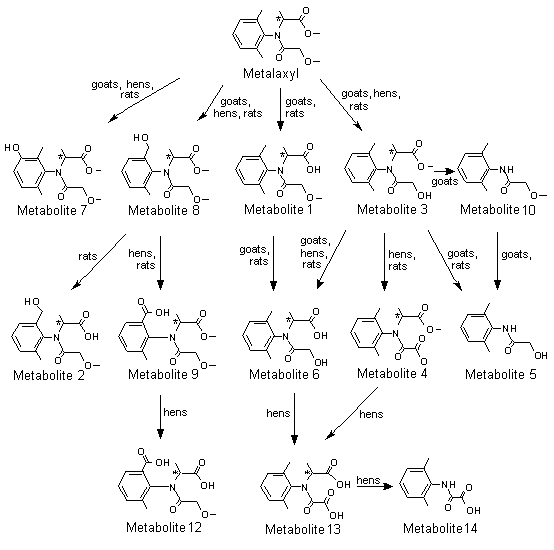
A generalized metabolic pathway for metalaxyl and metalaxyl-M in rats is shown in Figure 1.

Figure 1. Proposed metabolic pathway for metalaxyl and metalaxyl-M in rats, goats and hens
Metabolite 1, N-(2,6-dimethylphenyl)-N-(methoxyacetyl)alanine; metabolite 2,
N-[(2-hydroxymethyl)-6-methylphenyl]-N-(methoxyacetyl)alanine; metabolite 3,
N-(2,6-dimethyphenyl)-N-(hydroxyacetyl)alanine methyl ester; metabolite 4,
N-(carboxycarbonyl)-N-(2,6-dimethylphenyl)alanine methyl ester; metabolite 5,
N-hydroxyacetyl-2,6-dimethylaniline; metabolite 6, N-(2,6-dimethylphenyl)-N-(hydroxyacetyl)alanine;
metabolite 7, N-(2,6-dimethyl-5-hydroxyphenyl)-N-(methoxyacetyl)alanine methyl ester;
metabolite 8, N-(2-hydroxymethyl-6-methylphenyl)-N-(methoxyacetyl)alanine methyl ester;
metabolite 9, N-(2-carboxy-6-methylphenyl)-N-(methoxyacetyl)alanine methyl ester; metabolite 10,
N-(2,6-dimethylphenyl)methoxy-acetamide; metabolite 11, N-[(2,6-dimethylphenyl)alanine; metabolite 12,
N-(2-carboxy-6-methylphenyl)-N-methoxyacetyl)alanine; metabolite 13,
N-(carboxycarbonyl)-N-(2,6-dimethylphenyl)alanine; metabolite 14, [(2,6-dimethylphenyl)amino]oxoacetic acid
Conjugates not shown
* Chiral centre
The degradation of [14C]metalaxyl (purity, > 99%) was investigated in a preliminary study in 16 female Tif:RAI (SPF) rats after a single oral dose of 28 mg/kg bw. Urine and faeces were collected for 48 h. Within that time, 64% of the radioactivity was excreted in urine and 33% in faeces. The identified metabolites accounted for about 30% of the radioactivity in urine, equivalent to 19% of the dose. The following urinary metabolites were identified chromato-graphically and spectroscopically: N-(2-hydroxymethyl-6-methylphenyl)-N-(methoxyacetyl)-alanine methyl ester (14% of the dose, metabolite 8 ‘B’ isomer), N-(2,6-dimethylphenyl)hydroxy-acetamide or N-hydroxyacetyl-2,6-dimethylaniline (3%, metabolite 5), N-(2,6-dimethylphenyl)-N-(methoxyacetyl)alanine (2%, metabolite 1) and N-(2,6-dimethylphenyl)methoxyacetamide (only in free form, 0.3%, metabolite 10).
The structures identified so far show that the metabolism of metalaxyl proceeds primarily via oxidative and hydrolytic processes: (i) methyl ester hydrolysis, (ii) N-dealkylation, (iii) methyl ether cleavage and (iv) benzylic methyl oxidation. Most of the metabolites formed were subsequently conjugated by glucuronic acid and excreted via the kidney. They were therefore found in urine in both free and conjugated forms. Products formed by ring hydroxylation in the aniline moiety, as reported for lidocaine, mepivacaine and bupivacaine, which contain the same aniline moiety, were not found in this study (Hamboeck, 1978).
In a follow-up to the previous studies, the metabolic fate of [14C]metalaxyl (radiochemical purity, > 98%) was investigated in 24 female Tif:RAI (SPF) rats after administration of a single oral dose of 28 mg/kg bw. Urine and faeces were collected for 48 h. Within this time, 58% of the radioactivity was excreted in urine and 32% in faeces. The metabolites present in urine and faeces that were identified chromatographically and/or spectroscopically were N-(2,6-dimethylphenyl)-N-(hydroxyacetyl)alanine (39% of the dose, metabolite 6), N-(2-hydroxymethyl-6-methylphenyl)-N-(methoxyacetyl)alanine methyl ester (14%, metabolite 8 ‘A’ isomer), N-(2-6-dimethylphenyl)-N-(methoxyacetyl)alanine (4.1%, metabolite 1), N-hydroxyacetyl-2,6-dimethylaniline (2.9%, metabolite 5), N-(2-carboxy-6-methylphenyl)-N-(methoxyacetyl)alanine methyl ester (1.2%, metabolite 9), N-(2,6-dimethyphenyl)-N-(hydroxyacetyl)alanine methyl ester (0.9%, metabolite 3), 4-(2,6-dimethylphenyl)-3-methylmorpholine-2,5-dione (0.6%, isomeric lactone form of metabolite 6), metalaxyl (0.4%) and N-(2,6-dimethyl-5-hydroxyphenyl)-N-(methoxyacetyl)alanine methyl ester (0.3%, metabolite 7). The urinary metabolites were partially conjugated with glucuronic acid.
At least four independent pathways of biotransformation degrade metalaxyl in rats: (i) hydrolytic cleavage of the carboxyl methyl ester group, (ii) hydrolytic (or oxidative) cleavage of the methyl ether moiety, (iii) oxidation of the toluene methyl side-chain to the benzylic alcohol derivative and (iv) oxidation of the phenyl moiety to form phenols. Secondary biotransformation pathways are N-dealkylation at the 2-aniline propionic acid bond, oxidation of benzylic alcohol to the benzoic acid derivative and conjugation of metabolites with glucuronic acid.
Metalaxyl was effectively metabolized by rats, preferably by hydrolytic and oxidative reactions. The products formed were readily excreted in urine and faeces, and also as conjugates with glucuronic acid in urine. Urine and faeces generally contained the same metabolite structures, indicating their common origin from the general circulation (Hamboeck, 1981b).
The distribution, excretion and metabolism of [14C]metalaxyl (radiochemical purity, 96.9%) were studied after intravenous and oral administrations to groups of five male and five female Sprague-Dawley rats. Groups 1 and 2 received a single dose of 1.1 mg/kg bw intravenously or orally. Group 3 received metalaxyl (purity, 96.5%) in 14 daily oral doses of 1.4 mg/kg bw and on day 15 received [14C]metalaxyl at a single oral dose of 1.1 mg/kg bw. Group 4 received [14C]metalaxyl at a single oral dose of 200 mg/kg bw. Urine and faeces were collected for 7 days, and the radioactive residues were quantified. Rats were killed 168 h after dosing, and tissues were taken for determination of residues. Urine was characterized by TLC and sequential enzymatic hydrolysis. Faeces were extracted with methanol:water (80:20, v:v) to solubilize a minimum of 91% of the radioactive residue. The study was performed in accordance with the FIFRA guidelines (40 CFR part 158.135) for general metabolism studies and in compliance with the principles of GLP (with QA certification).
[14C]Metalaxyl was almost completely eliminated (93–98%) in the urine and faeces of rats within 72 h after intravenous or oral administration. Excretion was rapid and complete. Excretion occurred primarily via the kidneys in females (70%) and in faeces in males (60%). The similarity of the elimination profiles in rats treated intravenously and orally indicated that metalaxyl was well absorbed. Biliary excretion was suggested by the high faecal recoveries in male (59%) and female (36%) rats after intravenous administration. The concentrations in tissues were low in all groups at the lower dose, the highest values being found in intestine (0.045 ppm) and liver (0.008 ppm). A 200-fold increase was found with the higher dose, the highest values again being found in intestine (3.5 ppm) and liver (0.98 ppm). These findings would result from localized dynamic enterohepatic circulation, with metalaxyl conjugates and intestinal beta-glucuronidase moving through a conjugation, deconjugation, reabsorption and reconjugation cycle to eliminate the xenobiotic.
In urine, the patterns of metabolites were qualitatively similar, regardless of sex and dose. Metabolism was extensive, with a possible 33 metabolites found. Ten metabolites were identified by co-chromatography with standards. Nine metabolites (including metalaxyl) were purified and identified from mass and nuclear magnetic resonance spectra. Metabolites 6, 1, 9 and 8 ‘B’ isomer were the major metabolites in rat urine (Table 14), while metabolites 5 and 4 (N-(carboxycarbonyl)-N-(2,6-dimethylphenyl)alanine methyl ester) were present in moderate amounts. Metabolite 8 ‘A’ isomer, N-(2,6-dimethylphenyl)alanine (metabolite 11) and metabolite 7 were minor metabolites. Metalaxyl co-chromatographed with metabolite 3 as minor components, and a new metabolite, N-[(2-hydroxymethyl)-6-methylphenyl]-N-(methoxyacetyl)alanine (metabolite 2), was identified. Free metabolites represented 7.9–30% of the dose, accounting for 20–51% of the total 14C-labelled residue in urine. Conjugates represented 50–97% of urinary radioactivity. After enzyme hydrolysis, most of the metabolites could be partitioned into ethyl acetate and co-chromatographed with free metabolites and standards. Urinary conjugation included glucuronide, sulfate and possibly peptide adducts.
Table 14. Major metabolites of metalaxyl in rats
|
Chemical name |
Metabolite no. |
Abundance (% of dose) |
|||
|
Urine |
Faeces |
||||
|
Male |
Female |
Male |
Female |
||
|
N-(2,6-Dimethylphenyl)-N-(methoxyacetyl)alanine methyl ester |
Metalaxyl |
||||
|
0.1 |
0.2–1.8 |
0.4–0.8 |
0.2–0.4 |
||
|
N-(2,6-Dimethylphenyl)-N-(hydroxyacetyl)alanine methyl ester |
3 |
||||
|
N-(2-Hydroxymethyl-6-methylphenyl)-N-(methoxyacetyl)alanine methyl ester |
8 |
0.1–0.6 |
0.9–5.7 |
1.0–4.9 |
1.1–2.9 |
|
N-(2,6-Dimethyl-5-hydroxyphenyl)-N-(methoxyacetyl)alanine methyl ester |
7 |
< 0.1 |
0.1 |
0.1–0.2 |
< 0.1 |
|
N-Hydroxyacetyl-2,6-dimethylaniline |
5 |
1.0–1.8 |
0.7–1.3 |
0.1–0.7 |
0.1 |
|
N-(2,6-Dimethylphenyl)-N-(methoxyacetyl)alanine |
1 |
0.1 |
0.6–4.9 |
1.7–2.2 |
0.3–0.4 |
|
N-(2,6-Dimethylphenyl)-N-(hydroxyacetyl)alanine |
6 |
3.2–6.1 |
10–20 |
7.1–10.4 |
|
|
9.0–11 |
|||||
|
N-(2-Carboxy-6-methylphenyl)-N-(methoxyacetyl)alanine methyl ester |
9 |
1.2–2.1 |
1.5–2.6 |
0.7–1.2 |
|
|
N-(Carboxycarbonyl)-N-(2,6-dimethylphenyl)alanine methyl ester |
4 |
0.2–0.4 |
0.5–1.2 |
0.2 |
0.2 |
|
N-[(2-Hydroxymethyl)-6-methylphenyl]-N-(methoxyacetyl)alanine |
2 |
0.1–0.4 |
0.6–2.3 |
0.1–0.3 |
0.1–0.4 |
From Itterly (1990)
In faeces, metabolite 6 was the main metabolite in females. Metabolites 6 and 9 co-chromatographed as a major zone for males. Metabolite 9 was present in moderate amounts in females, and metabolites 1 and 8 ‘B’ isomer were found in both sexes. Metalaxyl and metabolite 3 co-chromatographed as minor components. Metabolites 3 ‘A’ isomer and 5, 4, 11, 7 and 2 were all minor faecal metabolites. Unconjugated metabolites identified in faecal extracts represented 11–19% of the dose and accounted for 46–76% of the total 14C-labelled residue. Metabolite conjugation accounted for 29–51% of the faecal radioactivity, which was hydrolysed predominantly to aglycones with beta-glucuronidase (18–42%). Sulfate conjugates accounted for 3.8–12% of the radioactivity.
The extensive biotransformation of metalaxyl in rats is accounted for by demethylation, N-dealkylation and hydroxylation, followed by glucuronide and sulfate conjugation. Metalaxyl was extensively metabolized in all groups, little or no unchanged metalaxyl being eliminated in urine or faeces. TLC showed that the biotransformation products were similar in all groups, and approximately the same relative proportions were observed in urine and faeces. A sizeable proportion of metabolites was conjugated in both urine and faeces, especially at the lower dose. The metabolic route appeared to involve three major and one minor pathways (see Figure 1). Demethylation of the ether gave the alcohol, metabolite 3, with stepwise demethylation of the ester forming the alcohol acid, metabolite 6, which was the major metabolite in urine and faeces. Further oxidation of the alcohol, metabolite 3, formed the acid, metabolite 4. N-Dealkylation of metabolite 3 gave metabolite 5, the hydroxyacetamide. Oxidation of the aromatic methyl of metalaxyl formed the benzylic alcohol isomers, A and B, of metabolite 8. The methyl ester of isomer A was demethylated, forming an acid, metabolite 2, the benzylic alcohol of metabolite 1. The B isomer was oxidized to the benzoic acid, metabolite 9. Demethylation of the ester of metalaxyl formed the acid ether, metabolite 1, which was a major urinary metabolite in females at the higher dose and a major faecal metabolite in males. In the minor pathway, metalaxyl underwent hydroxylation at the meta position on the phenyl ring, forming a mixture of isomers of metabolite 7. All the metabolites that were isolated undergo phase II conjugation reactions and are present as glucuronide and sulfate conjugates (Itterly, 1990).
The pattern of metabolites of the R-enantiomer, metalaxyl-M, and the racemate, metalaxyl, in rats was also investigated in the study of Müller (1997), the protocol of which is described above. Faeces were extracted with acetonitrile and acetonitrile:water, such that 91–95% of the radioactivity present was extracted, and the composite urines from each group and the faecal extracts were analysed quantitatively by two-dimentional TLC.
Apart from stereochemistry, the metabolic patterns of metalaxyl-M and metalaxl were similar (Tables 15 and 16). Both were extensively metabolized, yielding 17 and 13 metabolite fractions in urine and faeces, respectively, independently of dose and the sex of the animals. The dose- and sex-related differences in the concentrations of metabolites metalaxyl found by Itterly (1990) were also seen for metalaxyl-M. Only the concentration of metabolite fraction U4 in male rats at the higher dose depended markedly on stereochemistry. Therefore, the metabolic pathways deduced for metalaxyl are also valid for metalaxyl-M (see Figure 1).
Table 15. Quantitative pattern of metabolites of metalaxyl-M and metalaxyl in urine of rats (per cent of dose)
|
Metabolite fraction |
[phenyl-U-14C]Metalaxyl-M |
[phenyl-U-14C]Metalaxyl |
||||||
|
1 mg/kg bw |
100 mg/kg bw |
1 mg/kg bw |
100 mg/kg bw |
|||||
|
Male |
Female |
Male |
Female |
Male |
Female |
Male |
Female |
|
|
U1 |
5.2 |
3.6 |
7.2 |
3.4 |
5.7 |
3.4 |
4.9 |
4.3 |
|
U2 |
– |
1.5 |
0.4 |
1.6 |
– |
1.3 |
0.2 |
0.8 |
|
U3 |
21 |
27 |
12 |
16 |
18 |
23 |
10 |
20 |
|
U4 |
2.4 |
1.0 |
2.6 |
1.2 |
2.8 |
1.0 |
13 |
2.5 |
|
U5 |
0.4 |
0.3 |
0.4 |
0.6 |
0.2 |
0.4 |
0.5 |
0.8 |
|
U6 |
0.3 |
– |
0.1 |
0.2 |
– |
– |
– |
0.1 |
|
U7 |
0.5 |
– |
0.3 |
0.1 |
– |
– |
0.2 |
0.1 |
|
U8 |
0.7 |
0.7 |
0.9 |
0.7 |
0.4 |
0.6 |
0.6 |
0.7 |
|
U9 |
– |
– |
0.2 |
– |
– |
– |
0.1 |
– |
|
U10 |
4.9 |
8.1 |
3.4 |
6.3 |
6.2 |
9.0 |
6.6 |
9.8 |
|
U11 |
– |
2.0 |
3.6 |
1.2 |
0.9 |
2.7 |
4.0 |
2.2 |
|
U12 |
2.7 |
1.6 |
2.2 |
1.6 |
2.2 |
1.1 |
1.4 |
1.2 |
|
U13 |
1.4 |
0.4 |
– |
1.6 |
– |
1.1 |
0.2 |
2.9 |
|
U14 |
1.5 |
1.1 |
1.1 |
0.8 |
0.9 |
1.0 |
0.7 |
0.7 |
|
U15 |
0.8 |
3.1 |
1.2 |
3.9 |
1.1 |
2.6 |
1.3 |
4.1 |
|
U16 |
0.9 |
0.5 |
0.5 |
1.5 |
0.4 |
1.2 |
0.6 |
1.7 |
|
U17 |
0.3 |
– |
– |
1.0 |
0.3 |
1.0 |
– |
1.3 |
|
Unidentified radioactivity |
6.5 |
9.7 |
2.8 |
3.0 |
6.8 |
9.2 |
3.7 |
4.0 |
|
Sum |
49 |
61 |
36 |
45 |
46 |
59 |
48 |
58 |
From Müller (1997)
U, urinary metabolite
Table 16. Quantitative pattern of metabolites of metalaxyl-M and metalaxyl in faeces of rats (per cent of dose)
|
Metabolite fraction |
[phenyl-U-14C]Metalaxyl-M |
[phenyl-U-14C]Metalaxyl |
||||||
|
1 mg/kg bw |
100 mg/kg bw |
1 mg/kg bw |
100 mg/kg bw |
|||||
|
Male |
Female |
Male |
Female |
Male |
Female |
Male |
Female |
|
|
Unextracted |
3.0 |
2.1 |
3.9 |
2.6 |
4.9 |
2.2 |
4.5 |
2.1 |
|
F1 |
– |
– |
2.1 |
1.6 |
0.8 |
0.3 |
2.7 |
1.0 |
|
F2 |
– |
0.8 |
5.9 |
6.3 |
0.8 |
1.3 |
3.0 |
2.1 |
|
F3 |
– |
– |
0.4 |
0.2 |
0.4 |
– |
0.6 |
0.3 |
|
F4 |
7.3 |
2.9 |
5.0 |
2.8 |
13 |
1.9 |
9.7 |
3.6 |
|
F5 |
0.6 |
0.4 |
0.4 |
0.4 |
0.3 |
0.3 |
0.5 |
0.3 |
|
F6 |
14 |
15 |
12 |
16 |
12 |
13 |
11 |
12 |
|
F7 |
3.7 |
0.6 |
2.7 |
1.1 |
1.8 |
0.4 |
1.8 |
0.9 |
|
F8 |
– |
0.3 |
3.0 |
0.9 |
1.5 |
0.2 |
2.8 |
0.7 |
|
F9 |
3.5 |
0.6 |
0.2 |
0.7 |
– |
0.5 |
0.1 |
0.7 |
|
F10 |
0.8 |
0.3 |
0.6 |
0.3 |
0.6 |
0.3 |
0.9 |
0.5 |
|
F11 |
2.1 |
2.3 |
5.8 |
4.5 |
3.1 |
2.7 |
5.9 |
3.2 |
|
F12 |
2.3 |
1.3 |
4.6 |
2.9 |
1.3 |
0.9 |
3.3 |
1.5 |
|
F13 |
2.2 |
1.7 |
5.0 |
4.5 |
0.9 |
1.5 |
2.1 |
2.5 |
|
Unidentified radioactivity |
8.6 |
8.1 |
6.3 |
3.9 |
7.8 |
5.9 |
2.9 |
4.0 |
|
Sum |
49 |
61 |
36 |
45 |
46 |
59 |
48 |
58 |
From Müller (1997)
F, faecal metabolite
The study of Itterly (1990) showed that metalaxyl undergoes extensive phase II reactions, namely conjugation with sulfuric acid and glucuronic acid. Sulfonation and glucuronidation are competing pathways for hydroxylated metalaxyl metabolites, and sulfonation can be superseded by glucuronidation at increasing concentrations of substrate, resulting in a quantitative shift of metabolite distribution. These reactions are catalysed by sulfontransferases and UDP-glucuronyltransferases, which are known to discriminate between enantiomers. In the case of metabolite U4, the preferred substrate for a sex- and dose-dependent shift of conjugation appeared to be the S-enantiomer, as the concentration of the R-enantiomer was not affected by the dose. The pattern of metabolites in urine and faeces thus indicated that, aside from stereochemistry, the metabolic pathways of metalaxyl-M (the R-enantiomer) and metalaxyl (the racemate) are similar (Müller, 1997).
Goats
One lactating goat was given 7 ppm of [14C]metalaxyl in gelatine capsules orally for 10 consecutive days, and urine, faeces, milk, volatiles and CO2 were collected daily. Blood was collected every other day and on the day of sacrifice, day 10. At sacrifice, 24 h after the last dose, brain, omental fat, skeletal fat, tenderloin muscle, leg muscle, heart, kidney, liver and intestinal contents were sampled. The radioactivity in urine was characterized by partitioning, TLC and electrophoresis.
At sacrifice, 94% of the administered radioactivity was found in urine and 12% in faeces; the rumen and intestinal contents contained only 0.77% of the total. Less than 0.01% of the administered dose was excreted in milk, which contained concentrations of 0.003–0.008 ppm, and little was found in the blood (0.06%) or tissues (0.87%). The residual radioactivity was highest in liver (0.057 ppm), skeletal fat (0.023 ppm) and kidneys (0.019 ppm). The total recovery of radioactivity was 107.22%. The goat metabolized metalaxyl to polar, organic acidic products. Enzyme analysis indicated that the metabolites were natural conjugates rather than simple metabolites. Two-dimensional TLC comparisons of the metabolites in goat and rat urine showed that the metabolites were the same, goat urine containing more polar metabolites than rat urine.
Thus, [14C]metalaxyl fed to one goat was rapidly absorbed, metabolized and excreted. Radioactivity did not accumulate in tissues and was not secreted in milk. The results of TLC suggested that metalaxyl is metabolized by the same pathways in goats and rats (Marco, 1978).
In a study conducted according to Guideline 171-4 of FIFRA Subdivision O and in compliance with the principles of GLP (with QA certification), two lactating goats were given [14C]metalaxyl (radiochemical purity, > 97%) in gelatine capsules at a dose of 150 mg/kg bw per day for 4 days. The dose was equivalent to a dietary concentration of 77 ppm. During the dosing period, urine and faeces were collected daily and milk twice a day. The goats were killed 6 and 7 h after the last dose, and blood and samples of leg muscle, omental fat, perirenal fat, kidney, tenderloin, gall-bladder, liver, heart and rumen were collected. Urine, tissue and milk extracts were treated with glucuronidase and profiled on TLC.
During the observation period, a total of 76% of the administered radioactivity was eliminated, with 67% in urine, 9.3% in faeces and 0.1% in milk (Table 17). The concentrations of residues in milk were highest on day 4, amounting to 0.12 and 0.415 ppm metalaxyl equivalents for the two goats. At sacrifice, 3.8% of the administered radioactivity was found in the intestinal tract. The tissues contained 1% of the total dose, individual residual concentrations ranging from 0.065 ppm metalaxyl equivalents in tenderloin to 2.3 ppm in kidney (Table 18).
Table 17. Mean excretion of [14C]metalaxyl in two goats given a concentration of 77 ppm
|
Day |
Urine |
Faeces |
Milk |
|
|
% total dose |
ppm metalaxyl equivalents |
|||
|
1 |
18 |
2.6 |
0.02 |
0.058 |
|
2 |
18 |
2.8 |
0.02 |
0.064 |
|
3 |
17 |
3.2 |
0.03 |
0.088 |
|
4 |
14 |
0.64 |
0.03 |
0.27 |
|
Total |
66 |
9.3 |
0.10 |
|
From Emrani (1990, 1991)
Table 18. Mean residues of [14C]metalaxyl equivalents in two goats 6–7 h after administration of a dose equivalent to 77 ppm
|
Tissue |
Per cent |
ppm |
|
Tenderloin |
0.25 |
0.094 |
|
Leg muscle |
0.28 |
0.11 |
|
Liver |
0.20 |
1.6 |
|
Kidney |
0.03 |
1.7 |
|
Omental fat |
0.03 |
0.12 |
|
Heart |
< 0.01 |
0.17 |
|
Blood |
0.16 |
0.34 |
|
Rumen and intestinal contents |
3.8 |
|
|
Total recovery |
81 |
From Emrani (1990, 1991)
Co-chromatography allowed identification of 28–73% of the radiactive residues present in tissues, 90% of those in milk and 78% of the radioactive materials in urine. The main metabolites of metalaxyl in urine were metabolites 6 (43%), 3 (3.9%), 1 (1.6%), 7 (< 1.7%) and both isomers of metabolite 8 (8.7% A; 19% B) (see Figure 1). In tissues, the main metabolites identified were metabolite 6 and both isomers of metabolite 8. The main metabolite in milk was metabolite 3, mostly conjugated to fatty acids (66%).
Thus, in goats, metalaxyl was hydrolysed to the ester alcohol and the acid alcohol, which may be N-dealkylated. Alternatively, oxidation can lead to either benzylic alcohol or phenolic derivatives. Most of the urinary metabolites were present as glucuronic acid conjugates. The main metabolites in milk appeared to be lipophilic conjugates of the acid alcohol or the ester alcohol (Emrani, 1990, 1991).
Chickens
Five laying hens were given a diet containing [14C]metalaxyl (radiochemical purity, > 97%) at a concentration of 100 ppm, equivalent to 10 mg/kg bw per day, for 4 days. Eggs and excreta were collected daily. The birds were killed 6 h after the last dose, and selected tissues were taken to determine the amount and nature of the metabolites. The study was conducted according to Guideline 171-4 of the FIFRA Subdivision O and in compliance with the principles of GLP (with QA certification). Metalaxyl was almost completely eliminated in the excreta (91%) (Table 19). Only marginal amounts of radioactivity were detected in eggs (0.04% in whites and < 0.04% in yolks) and 0.9% in edible tissues. The concentrations of residue levels were about 0.5 ppm in most organs, only gizzard, liver and kidney containing larger amounts (Table 20). In eggs, the concentrations were 0.13–0.18 ppm in whites and 0.014–0.21 ppm in yolks. The excreta contained mostly (28%) metabolite 6, 2-[(1-carboxyethyl) (methoxyacetyl)amino]-3-methyl benzoic acid (metabolite 12), metabolites 3 and 8 and unchanged metalaxyl (see Figure 1). Minor amounts of metabolite 9, [(2,6-dimethylphenyl)amino]-oxoacetic acid (metabolite 14), N-(carboxycarbonyl)-N-(2,6-dimethylphenyl) alanine (metabolite 13) and metabolite 4 were also found. Additional, unidentified metabolites were detected. Chromatographic examination showed that most of the residual radioactivity in tissues was in the form of the A and B isomers of N-[2-(hydroxymethyl)-6-methylphenyl]-N-(hydroxyacetyl)alanine (metabolites P1 and P2) and metabolites 6 and 8. The main residues in egg yolk were metabolites 6, P1 and P2. The whites contained mainly metabolites P1, 8, P2 and P3 (the structure of which is only partially known) and unchanged metalaxyl. Most of the metabolites were glucuronic, sulfuric or fatty acid conjugates.
Table 19. Excretion of [14C]metalaxyl by hens given a concentration of 100 ppm
|
Day |
Excreta |
Egg white |
Egg yolk |
||
|
% total dose |
ppm metalaxyl equivalents |
% total dose |
ppm metalaxyl equivalents |
||
|
1 |
23 |
0.01 |
0.13 |
< 0.01 |
0.014 |
|
2 |
22 |
0.01 |
0.17 |
< 0.01 |
0.066 |
|
3 |
27 |
0.01 |
0.16 |
0.01 |
0.14 |
|
4 |
18 |
0.01 |
0.18 |
0.01 |
0.21 |
|
Total |
92 |
||||
From Kennedy (1990, 1991)
Table 20. Mean residues of [14C]metalaxyl equivalents in hens 6 h after administration of a diet containing 100 ppm
|
Tissue |
% total dose |
ppm metalaxyl equivalents |
|
Skin and attached fat |
0.05 |
0.32 |
|
Peritoneal fat |
0.02 |
0.25 |
|
Breast muscle |
0.25 |
0.55 |
|
Thigh muscle |
0.31 |
0.67 |
|
Liver |
0.14 |
1.4 |
|
Kidney |
0.04 |
1.5 |
|
Gizzard |
0.08 |
1.4 |
|
Total recovery |
92 |
From Kennedy (1990, 1991)
The results show that the metabolism of metalaxyl in laying hens initially involves oxidation and demethylation of the parent compound. Sequential demethylation of the ether and the ester groups gives first the alcohol, metabolite 3, and then the hydroxy acid, metabolite 6. The ester alcohol, metabolite 3, undergoes conjugation with both fatty acids and glucuronic acid, the latter known as metabolite P3a. Oxidation of the benzylic carbon of metalaxyl produces the benzylic alcohol, metabolite 8, which undergoes sequential demethylation of the ether moiety to give metabolite P0 (N-[(2-hydroxymethyl)-6-methylphenyl]-N-(methoxyacetyl)alanine), the free acid of metabolite 8, and the ester moieties to give metabolites P1 and P2. These metabolites can undergo conjugation with fatty acids. The benzylic alcohol of metabolite 8 also forms the sulfuric acid conjugate P4 and to a minor extent the benzoic acid, metabolite 9.
Comparison of the metabolites found in hen excreta with those found in goat and rat urine indicates that the major metabolic pathways in hens are substantially the same as those in goats and rats (Figure 1). The differences between species are due primarily to the faster metabolic rate and greater tendency for oxidative transformation in hens (Kennedy, 1990, 1991).
All the data suggest similar pathways in all three species (Emrani, 1990, 1991).
The dermal absorption of [phenyl-U-14C]-metalaxyl-M (purity, 97.3%), formulated as an emulsifiable concentrate containing active substance at 480 g/l, was tested in groups of 12 male Tif:RAIf rats at a dose of 0.094 mg/cm2 (2% dilution) or 4.7 mg/cm2 (undiluted formulation) in a volume of 100 µl. The substance was applied for 8 h inside a 10-cm2 ring, which was glued to the clipped skin of the animals and covered with non-occlusive tape. Subgroups of four animals per dose were killed at 8 (directly after washing of the skin), 24 and 48 h, and urine and faeces were collected. The washing solution, including cotton swabs, ring and covering tape, and the cage washing solution were also collected. Whole blood, plasma, treated and untreated skin and carcasses were retained after sacrifice. Blood samples were taken 0.5, 1, 2, 4, 6, 8, 12, 24 and 48 h after application. The study was performed in compliance with the principles of GLP (with QA certification).
The lower dose of metalaxyl-M was rapidly absorbed; a first maximum in blood (0.06 ppm) was reached 1 h after application (Table 21), and a second maximum (0.045 ppm) was reached after 12 h. The concentration of radioactivity in blood decreased considerably between 12 and 48 h after treatment. After administration of the higher dose, the first maximum in blood (0.44 ppm) was reached 8 h after application. The wash-off effect—an increase in blood concentration after removal of the compound from the skin—was more pronounced than at the lower dose, and the second maximum (1.5 ppm) was reached at 24 h. The concentration of radioactivity in blood decreased between 24 and 48 h.
Table 21. Kinetics of radioactivity (ppm of equivalents) from [phenyl-U-14C]metalaxyl-M in four rats 48 h after receiving a dermal application
|
Time (h) |
Dose (mg/cm2) |
|
|
0.094 |
4.7 |
|
|
0.5 |
0.030 |
< LOQ |
|
1 |
0.057 |
< LOQ |
|
2 |
0.035 |
0.41 |
|
4 |
0.024 |
< LOQ |
|
6 |
0.029 |
0.41 |
|
8 |
0.036 |
0.44 |
|
12 |
0.044 |
0.94 |
|
24 |
0.026 |
1.5 |
|
48 |
0.0099 |
0.42 |
From Mewes (1998a); LOQ, limit of quantification
Within 8 h, 26% of the lower dose and 3% of the higher dose had been absorbed systemically (Table 22); 35% of the lower and 16% of the higher dose were absorbed within 48 h. At both doses, systemic absorption increased even after the substance had been removed from skin, but the absorbed metalaxyl-M was rapidly excreted in the urine and faeces. Some substance remained on the skin after washing at 8 h, but the amount decreased rapidly up to 48 h, indicating that metalaxyl-M in the epidermis and dermis during exposure was systemically absorbed after washing. The actual amounts absorbed suggest that the absorption process was saturated at the higher dose. The rate of penetration was only six times higher at the higher dose (18 µg cm2/h) than at the lower dose (3 µg cm2/h) although the dose increased by a factor of 50. The absorbed radioactivity was excreted in similar amounts in urine and feces. The terminal concentrations in blood and plasma after the lower dose were similar 8 and 24 h after application but had decreased considerably by 48 h (Table 23). With the higher dose, the blood and plasma concentrations increased between 8 and 24 h but decreased thereafter.
Table 22. Recovery (% applied dose) of radioactivity after dermal exposure of rats to [phenyl-U-14C]metalaxyl-M
|
Site |
Dose (mg/cm2) |
|||||
|
0.094 |
4.7 |
|||||
|
8 h |
24 h |
48 h |
8 h |
24 h |
48 h |
|
|
Urine |
2.3 |
12 |
14 |
0.33 |
2.8 |
6.4 |
|
Faeces |
0.07 |
9.1 |
16 |
< 0.01 |
2.3 |
6.4 |
|
Cage wash |
0.32 |
0.69 |
0.47 |
0.04 |
0.18 |
0.66 |
|
Control skin and blood |
0.04 |
0.04 |
0.01 |
< 0.01 |
0.01 |
0.07 |
|
Residual carcass |
23 |
14 |
4.7 |
2.6 |
3.9 |
3.0 |
|
Systemic absorption |
26 |
35 |
35 |
3.0 |
9.3 |
16 |
|
Treated skin |
20 |
8.5 |
5.5 |
15 |
10 |
11 |
|
Skin wash |
60 |
56 |
54 |
82 |
81 |
71 |
|
Recovery |
100 |
100 |
95 |
100 |
100 |
98 |
|
Absorbed dosea |
46 |
441 |
40 |
18 |
19 |
27 |
From Mewes (1998a)
a Systemic absorption plus treated skin
Table 23. Terminal concentrations in blood and plasma (ppm equivalents) of radioactivity after dermal exposure of rats to [phenyl-U-14C]metalaxyl-M
|
Medium |
Dose (mg/cm2) |
|||||
|
0.094 |
4.7 |
|||||
|
8 h |
24 h |
48 h |
8 h |
24 h |
48 h |
|
|
Blood |
0.031 |
0.030 |
0.012 |
0.30 |
0.42 |
0.32 |
|
Plasma |
0.042 |
0.033 |
0.0075 |
0.39 |
0.48 |
0.26 |
From Mewes (1998a)
The Meeting considered that at least some of the substantial amount of metalaxyl-M remaining on treated skin after washing was available for systemic absorption. On the basis of the average values for the sum of systemic absorption and skin deposition at 8, 24 and 48 h, the absorption was 44% of the lower dose and 22% of the higher dose (Mewes, 1998a).
Penetration of [phenyl-U-14C]metalaxyl-M (purity, 97.3%), formulated as an emulsifiable concentrate containing 480 g/l of active substance, through rat and human epidermis was compared in vitro. Epidermal membranes were set up in flow-through diffusion cells, and the perfusates were collected at defined intervals. Metalaxyl-M was applied at 0.083, 0.76 or 40 mg/cm2 to rat epidermis and 0.083, 0.77 or 40 mg/cm2 to human epidermis for 48 h. The two lower doses reflected the concentrations used for foliar (0.2% dilution) and soil application (2% dilution), respectively, and the highest dose represented undiluted formulation. The study was performed in compliance with the principles of GLP (with QA certification).
As seen in Table 24, metalaxyl-M at concentrations used in the field and as undiluted formulation penetrated human skin more slowly and to a significantly smaller extent than through rat skin. The ratio for rat:human was 6:1 at the lowest dose and 3:1 at the highest dose. The 50:50 mixture of ethanol:water used as receptor fluid in the study may have resulted in an artificially high value for absorption (Mewes, 1998b).
Table 24. Penetration of metalaxyl-M through rat and human epidermis in vitro
|
Rat |
Human |
Rat |
Human |
Rat |
Human |
|
|
Penetration |
||||||
|
12 h |
58 |
17 |
56 |
24 |
2.6 |
0.3 |
|
24 h |
62 |
24 |
61 |
34 |
6.0 |
1.3 |
|
48 h |
66 |
35 |
64 |
50 |
13 |
3.3 |
|
Lag time (h) |
0.7 |
1.7 |
0.5 |
1.4 |
2.0 |
7.0 |
|
Flux constant (µg× cm2/h; absorption rate) |
12 |
2.0 |
130 |
36 |
110 |
35 |
|
Ratio rat:human |
6:1 |
4:1 |
3:1 |
|||
|
Permeability coefficient (cm/h) |
11 × 10–3 |
1.9 × 10–3 |
13 × 10–3 |
3.6 × 10–3 |
0.21 × 10–3 |
0.07 × 10–3 |
From Mewes (1998b)
The results of this study were used to compare dermal absorption between species semi-quantitatively. Thus, human dermal absorption in vivo was estimated as the systemic absorption in rats in vivo divided by the factors for species differences determined in vitro. A figure of 10% for dermal absorption was considered an appropriate compromise, in view of the uncertainties in the individual studies. This figure was based on a dermal absorption of 40% for rats in vivo and a fourfold correction for human skin in vitro.
(a) Lethal doses
The acute toxicity of metalaxyl-M after administration by the oral, dermal and inhalation routes is summarized in Table 25.
Table 25. Studies of the acute toxicity of metalaxyl-M
|
Species |
Strain |
Route |
Vehicle |
LD50 (mg/kg bw; 95% CI or range) LC50 (mg/l air) |
Reference |
|
Mouse |
Tif MAG (SPF) |
Oral solution |
Aqueous 0.5% CMC |
Males, > 1000 |
Winkler (1996a) |
|
Rat |
Sprague-Dawley-derived Tif RAI (SPF) |
Oral |
Aqueous 0.5% CMC solution |
670 (440–1000) |
Schoch (1994a) |
|
Rat |
Wistar |
Inhalation, 4 h, whole-body |
Aerosol |
> 2.3 (highest attainable concentration) |
Arts (1995) |
|
Rat |
Sprague-Dawley-derived Tif RAI (SPF) |
Dermal |
> 2000 |
Schoch (1994b) |
All studies were performed in male and female animals according to good laboratory practice, with quality assurance certfication
CMC, carboxymethylcellulose
Groups of five male and five female fasted mice were given metalaxyl-M (purity, 97.1%) orally at a dose of 500 or 1000 mg/kg bw for males and 200, 500 or 1000 mg/kg bw for females, and were observed for 14 days before sacrifice. The experimental protocol was not fully in compliance with OECD TG 401 (1987) or TM B1 from Annex V of Directive 92/69/EEC, as only two doses were given to males. Two of five males at 1000 mg/kg bw and one of five females at doses >500 mg/kg bw died, and and three of five females were found dead and two were killed for humane reasons. At 1000 mg/kg bw, male and female animals showed the following treatment-related symptoms: ventral or lateral recumbency, severe dyspnoea, reduced locomotor activity, convulsions, tremor and tonic spasms. Ataxia, hunched posture and piloerection were found in surviving males only, all of which recovered fully by day 7. At 500 mg/kg bw, ventral or lateral recumbency were seen in two males and two females, and dyspnoea, reduced locomotor activity, hunched posture and piloerection were seen in all animals, whereas tonic spasms, convulsions and ataxia were seen in individual animals. All males had recovered by day 4 and all surviving females by day 2. At 200 mg/kg bw, all females presented with hunched posture and piloerection but fully recovered within 1 day. At autopsy, no deviations from normal morphology were found. The body weights of surviving animals were not affected by the treatment (Winkler, 1996c).
Metalaxyl-M (purity, 97.9%) was administered orally to groups of five male and five female fasted rats at a dose of 500, 1000 or 2000 mg/kg bw for males and 200 or 500 mg/kg bw for females, and the animals were observed for 14 days before sacrifice. The experimental protocol was not fully in compliance with OECD TG 401 (1987) or TM B1 from Annex V of Directive 92/69/EEC, as only two doses were given to females. Four of five females at 500 mg/kg bw, three of five males at 1000 mg/kg bw and all males at 2000 mg/kg bw died on the day of administration after observation of non-specific symptoms such as piloerection, abnormal body position, dyspnoea and reduced locomotor activity. Convulsions and/or tonic spasms occurred in two males and four females given 500 mg/kg bw and all males at 1000 and 2000 mg/kg bw. Ataxia was seen in two females at 200 mg/kg bw and tremor in one male at 500 mg/kg bw. Four females at 500 mg/kg showed increased irritability. Males at 2000 mg/kg bw showed vocalization, respiratory sounds and cyanosis. The surviving animals recovered within 3–6 days. At autopsy, one male at 2000 mg/kg bw (which died shortly after treatment) had a spotted thymus. No deviations from normal morphology were found in the other animals (Schoch, 1994a).
Five male and five female rats were exposed to metalaxyl-M (purity, 97.1%) for 4 h in a nose-only system to a mean aerial concentration of 2.3 mg/l, the highest attainable concentration, measured in the breathing zone. The test atmosphere was generated by nebulizing the material into small droplets with a compressed air-driven machine. The mass median aerodynamic diameter of the particles was 2.1 µm with a geometric standard deviation of 1.4 µm, which ensured exposure of the bronchioles and alveoli of the animals. The animals were observed for 14 days before sacrifice. The experimental protocol was in compliance with OECD TG 403 (1981) and TM B2 from Annex V of Directive 92/69/EEC. No deaths occurred. Slight shallow breathing was observed in all rats during the first 3 h of exposure, and clear restlessness was seen from the second hour of exposure and onwards. Two female rats also showed slightly decreased breathing frequency during the last hour of exposure. Shortly after exposure, four females had a hunched appearance, and one female had slightly decreased breathing frequency and incoordination. The only abnormalities seen during the 14-day observation period were fatty, yellow, discoloured fur and a small alopecic area in one female. No abnormalities in body-weight gain were observed. At autopsy, no abnormalities were found (Arts, 1995).
Five male and five female rats received dermal applications of metalaxyl-M (purity, 97.3%) at a dose of 2000 mg/kg bw under a semi-occlusive dressing. The dressing was removed after 24 h, and the animals were observed for 14 days before sacrifice. The experimental protocol was in compliance with OECD TG 402 (1987) and TM B3 from Annex V of Directive 92/69/EEC. No deaths occurred, and no clinical symptoms or signs of local irritation were found. At autopsy, no deviations from normal morphology were noted (Schoch, 1994b).
Thus, in rats, the oral LD50 was 380 mg/kg bw for females and 950 mg/kg bw for males; the dermal LD50 was > 2000 mg/kg bw, and the LC50 (4-h exposure) was > 2.3 mg/l of air. In addition to nonspecific clinical signs, reduced locomotor activity, ataxia, convulsions, tremor and spasms were seen after treatment with metalaxyl-M; however, these effects occurred only at or near lethal doses and were considered not to be indicative of neurotoxic potential (see below). Surviving animals recovered within 3–6 days.
The acute toxicity of metalaxyl is summarized in Table 26. Metalaxyl had qualitatively and quantitatively similar effects to the R-enantiomer, metalaxyl-M. All of the studies except that reported by Hartmann (1992) were considered only as providing additional information in view of the limited data presented. In the study of Naidu & Radhakrishnamurty (1978), rats developed symptoms of central nervous system poisoning including tremors, twiches, tonic extension, loss of rightening reflex, ataxia and hypnosis within 5–10 min of intraperitoneal injection of metalaxyl. Death occurred within 10–15 min at 300–400 mg/kg bw and within 1–2 h at lower doses.
Table 26. Studies of the acute toxicity of metalaxyl
|
Species |
Strain |
Route and vehicle |
Dose (mg/kg bw) |
Purity (%) |
LD50 (mg/kg bw; |
Reference |
|
Mouse |
Tif MAG (SPF) |
Oral, CMC |
320, 460, 600, 1000, 2200 |
99.4 |
790 (630–990) |
Sachsse & Bathe (1976) |
|
Rat |
Tif RAIf (SPF), fasted |
Oral, CMC |
220, 460, 780, 1000, 1300, 2200 |
99.4 |
670 (520–870) |
Sachsse & Bathe (1976) |
|
Rabbit |
Himalayan |
Oral, CMC |
220, 460, 1000, 3600 |
99.4 |
700 (500–960) |
Sachsse & Ullmann (1976) |
|
Rat |
Tif RAIf (SPF) |
Inhalation, 4 h, nose only, aerosol |
3600 mg/m3 air (maximum attainable concentration |
96.1 |
> 3.6 |
Hartmann (1992) |
|
Rat |
Tif RAIf (SPF) |
Dermal |
2200, 3200 |
99.4 |
> 3200 |
Sachsse & Bathe (1976) |
|
Rabbit |
Himalayan |
Dermal |
1000, 6000 |
99.4 |
> 6000 |
Sachsse & Ullmann (1978) |
|
Rat |
Wistar-CTF |
Intraperitoneal, DMSO |
Males, 250, 275, 300, 400 |
95.8 |
Males, 300 |
Naidu & Radhakrishnamurty (1988) |
|
Females, 200, 250, 270, 300 |
||||||
|
Rat |
99.4 |
310 |
Sachsse & Bathe (1976) |
CMC, carboxymethylcellulose
(b) Dermal and ocular irritation and dermal sensitization
Dermal irritation: The dermal irritation potential of metalaxyl-M (purity, 97.3%) was tested in three male New Zealand white rabbits to which 0.5 ml of test material was applied under an occlusive dressing for 4 h. The skin reactions were evaluated 1, 24, 48 and 72 h after removal of the patch. Slight erythema (score 1 in two rabbits) was observed only 1 h after removal of the bandages, but no skin reactions were seen subsequently in any animals, and the study was terminated. Metalaxyl-M was not irritating to the skin. The study was performed according to GLP, and the protocol was in compliance with TM B4 from Annex V of Directive 92/69/EEC (Marty, 1994a).
Ocular irritation: The ocular irritation potential of metalaxyl-M (purity, 97.3%) was tested in one male and two female New Zealand white rabbits which received 0.1 ml of the substance in the conjunctival sac of the left eye; the other eye was left untreated to serve as a control. The eyes were examined for irritation with a slip-lamp, and any ocular reactions were recorded 1, 24, 48, 72 h and 7, 10, 14, 17 and 21 days after instillation. Clear signs of ocular irritation were seen in all animals, comprising corneal opacity grades 1–2, grade 1 iridal lesions and conjunctival redness and chemosis (grades 2 and 1, respectively). Vascularization of the cornea was observed in two animals on days 7 and 10, but all symptoms were reversed within 14 days. In the third animal, vascularization was observed on day 14, and corneal opacity was still present at the end of the observation period. As the changes were not fully reversible in all animals, the substance is considered a severe ocular irritant. The study was performed according to GLP, and the protocol was in compliance with TM B5 from Annex V of Directive 92/69/EEC (Marty, 1994b).
Dermal sensitization: The skin sensitization potential of metalaxyl-M (purity, 97.3%) was assessed in 10 male and 10 female Pirbright white guinea-pigs in the Magnusson-Kligman maximization test. The animals received one intradermal injection of 0.1 ml of a 5% solution of metalaxyl-M in peanut oil with Freund adjuvant, followed after 1 week by one epidermal application of 0.4 g of undiluted metalaxyl-M. The animals were challenged on day 21 with a 30% emulsion of metalaxyl-M in vaseline. After the dressing had been removed, on day 10, irritation of the application site was observed in all treated animals. One animal showed grade 1 skin reactions 24 and 48 h after the challenge application, but all the others remained unaffected. The test substance was considered not to be sensitizing. The study was performed according to GLP, and the protocol was in compliance with TM B6 from Annex V of Directive 92/69/EEC (Marty, 1994c).
The potential of metalaxyl-M (purity, 96.6%) to induce delayed contact hypersensitivity was evaluated in a Buehler test. On the basis of the results of a preliminary test, 10 albino Crl:(HA)BR guinea-pigs were given undiluted material for both the induction phase and the challenge. In the induction phase, each animal received metalaxyl-M once a week for 3 weeks, at a dose of 0.4 ml on an adhesive patch, which was semi-occluded and left in place for 6 h. Two weeks after application of the third induction dose, a challenge dose of 0.4 ml was administered in the same manner. No dermal reactions were observed in the animals in either the induction or the challenge phase of the study. The study was performed according to GLP and to Guideline 81-6 of the Environmental Protection Agency (USA) (EPA). The protocol was not fully in compliance with TM B6 from Annex V of Directive 92/69/EE, as 10 animals were used instead of 20 (Glaza, 1995).
Studies on dermal and ocular irritation and dermal sensitization with metalaxyl are summarized in Table 27. The studies of Sachsse & Ullmann (1976b,c) were considered only to provide additional information, since limited data were presented. In the study of Sachsse & Ullmann (1976c), approximately 100 mg of metalaxyl were placed in the conjunctival sacs of three male and three female rabbits. The eyes of three of the six rabbits were rinsed 30 s after instillation of the test material. No irritation was found in any of the rinsed eyes, but the unwashed eyes showed irritation of the conjunctiva and cornea for 3 days. They were clear by day 4. While the degree of corneal involvement appeared to be minimal, it could not be evaluated subjectively as only total scores according to the Draize (1977) scale were presented. As rinsing the eyes prevented irritation, the irritation in the unwashed eyes may have been related to the physical nature of the particles rather than to the chemical itself.
Table 27. Irritation and sensitization potential of metalaxyl
|
Species |
Conditions |
Purity (%) |
Results |
Reference |
|
Dermal irritation |
||||
|
Rabbit (3 male, 3 female) |
24 h exposure, occlusive |
99.4 |
Not irritating |
Sachsse & Ullmann (1976b) |
|
Ocular irritation |
||||
|
Rabbit (6) |
Eyes of 3 rabbits rinsed and eyes of 3 not rinsed after treatment |
99.4 |
Minimally irritating |
Sachsse & Ullmann (1976c) |
|
Dermal sensitization |
||||
|
Guinea-pig |
Optimization test, 0.1% suspension (induction and challenge) |
99.4 |
Not sensitizing |
Sachsse & Ullmann (1976d) |
|
Guinea-pig |
Modified Buehler test, 25% (induction and challenge) |
96.1 |
Not sensitizing |
Arcelin (1991) |
In the study of Sachsse & Ullmann (1976d), groups of 10 male and 10 female guinea-pigs were given a series of intracutaneous injections of metalaxyl as a 0.1% suspension in polyethylene glycol and saline, according to the optimization test of Mauer et al. (1975). Dinitrochlorobenzene served as the positive control. Seven of 20 treated animals and one of 20 vehicle control animals showed positive reactions after challenge, while all 20 animals in the positive control group gave positive reactions. The authors considered that metalaxyl did not have skin sensitizing potential in guinea-pigs. In the metalaxyl-treated group, the average increase in skin reactions after challenge over that seen after induction was only marginal, by a factor of 1.2. In contrast, in the positive control group, the average increase in skin reactions at challenge was 18-fold. The reactions in the vehicle control group during induction were more marked than in the metalaxyl-treated group at induction and challenge by factors of 1.7 and 1.3, respectively. Use of an irritating vehicle and irritating concentrations of metalaxyl at challenge made it difficult to differentiate sensitization reactions from irritation. The results were below the threshold for significance set by the laboratory (p = 0.02). The Meeting considered that the results were equivocal but do not represent a positive effect.
Metalaxyl was not irritating to the skin of rabbits and did not sensitize guinea-pigs skin. It was slightly irritating to the eyes of rabbits, in contrast to metalaxyl-M, which was a severe ocular irritant.
Several short-term studies of toxicity have been conducted with metalaxyl-M, by oral administration in rats and dogs and by dermal application in rats. In earlier studies, racemic metalaxyl was tested by oral administration in rats and dogs, dermal application in rabbits and inhalation in rats.
Rats
A study was conducted to detect possible differences in the toxicological properties of metalaxyl-M and the racemate metalaxyl. The study was in compliance with the principles of GLP (with QA certification), and the protocol was fully in compliance with OECD TG 407 (1981) and TM B7 from Annex V of Directive 92/69/EEC. Metalaxyl-M (purity, 96.1%) and metalaxyl (purity, 97.3%) suspended in water containing 0.5% carboxymethylcellulose (CMC) and 0.1% Tween 80 were administered to groups of five male and five female Sprague-Dawley-derived rats (Tif:RAIf (SPF) hybrids of RII/1 RII/2) at a dose of 0, 10, 50, 150 or 300 mg/kg bw per day, 7 days/week for 4 weeks by gavage. The study was terminated after 28 days of treatment, and all animals were examined clinically and histopathologically.
No deaths occurred. Animals given doses > 150 mg/kg bw per day showed transient hypoactivity after the first treatment with metalaxyl-M, and two females at this dose were prostrate. No further clinical signs were observed. Body-weight gain and food consumption were similar in treated and untreated rats. The mean water consumption of males at the highest dose was reduced by 10% throughout treatment with metalaxyl-M, whereas treatment with metalaxyl increased the mean water consumption of animals at this dose by 10% in males and 22% in females.
The haematological examinations revealed no changes. A minimally lower plasma sodium concentration, a minimally higher plasma chloride concentration and a tendency to decreased urea concentration were recorded among males at the highest dose of both metalaxyl-M and metalaxyl. In addition, males treated with metalaxyl-M at this dose had a minimally lower plasma bilirubin concentration, and females showed increased plasma albumin and globulin concentrations and reduced plasma bilirubin. The differences from untreated controls were statistically significant for animals receiving metalaxyl-M at 300 mg/kg bw per day and for those given metalaxyl at 150 and 300 mg/kg bw per day.
No treatment-related gross changes in organs were seen at necropsy. The absolute and relative weights of the liver were increased by 9–12% in males and females at the highest dose of metalaxyl and females at the highest dose of metalaxyl-M.
Histopathological examination revealed minimal-to-moderate hypertrophy of centrilobular hepatocytes in females given metalaxyl-M at 150 mg/kg bw per day and in both sexes at 300 mg/kg bw per day. Minimal hepatocellular hypertrophy was also seen in the females given metalaxyl at 300 mg/kg bw per day. A minimally increased incidence of extramedullary haematopoiesis in the spleen was found in females given the two higher doses of metalaxyl.
In summary, treatment of rats with metalaxyl and metalaxyl-M resulted in similar effects. The main target organ of both substances was the liver, which reacted to treatment with hypertrophy. Metalaxyl-M had no unexpected toxicological properties that would differentiate the R-enantiomer from the racemic form of metalaxyl qualitatively. Quantitative differences in the effects of the two substances were generally minimal and remained within the range of biological variation. It is therefore justified to conclude that the two compounds are toxicologically equivalent. The NOAEL for both substances was 300 mg/kg bw per day, the highest dose tested, as the effects on the liver observed at doses > 150 mg/kg bw per day were considered not to be adverse (Gerspach, 1994).
Metalaxyl (purity, 94.6%) was administered by gastric intubation to groups of 10 male and 10 female Tif:RAIf (SPF) rats at an initial daily dose of 0, 10, 30 or 100 mg/kg bw. As the treatment provoked no overt toxic reaction, the doses were raised to 0, 30, 100 and 300 mg/kg bw per day from day 15 to 21 and finally to 0, 60, 200 and 600 mg/kg bw per day from day 22 to 28. The study was performed before GLP guidelines and EEC or OECD test guidelines were enacted. The study was not considered in the final evaluation, as the doses were increased during treatment, no neurological examination beyond normal clinical inspections were conducted and no histopathological examination was done.
No deaths occurred. After the dose had been increased to 600 mg/kg bw per day, i.e. to near the acute oral LD50 for rats, tremors were observed. On subsequent days, the animals adapted to the treatment, and no clinical symptoms were seen on days 26, 27 and 28.
No treatment-related effects were noted on body-weight gain or food consumption. Ophthalmic examination, haematology, blood biochemistry and urine analysis revealed no treatment-related changes. The mean absolute and relative weights of the liver were increased in all treated groups, with a positive trend from control to highest dose. This effect was more pronounced in females than in males. In males at the highest dose, the absolute weight of the testes was significantly increased. The relative weight of the adrenals was significantly increased in females at the highest dose. The only gross pathological change observed was atrophy of the left testis in one male at the highest dose. This study indicated that the liver responded to treatment with hypertrophy, as shown by the dose-related increases in absolute and relative liver weights (Sachsse, 1979).
In a study conducted in compliance with the principles of GLP (with QA certification), groups of 10 male and 10 female Sprague-Dawley-derived rats (Tif:RAIf (SPF), hybrids of RII/1 × RII/2) were given diets containing technical-grade metalaxyl-M (purity, 97.1%) at a concentration of 25, 50, 250, 625 or 1250 ppm for 3 months. A group of 10 rats of each sex receiving the vehicle (acetone) served as controls. An additional group of 10 rats of each sex from the control and highest-dose groups were kept for a 4-week recovery period before sacrifice. The doses were selected to allow direct comparison with the previous short-term study conducted with metalaxyl. The doses were equal to mean intakes of 1.7, 3.5, 17, 45 and 91 mg/kg bw per day for males and 1.9, 3.7, 18, 49 and 95 mg/kg bw per day for females. Overt signs of toxicity were recorded daily, and body weight, food consumption and water consumption were recorded weekly throughout the study. Ophthalmoscopy was performed on animals from the control and highest-dose groups before treatment, towards the end (day 87) of treatment and towards the end of the recovery period (day 115). Haematological, blood chemical and urine analyses were carried out on all surviving treated animals at the end of treatment and at the end of the recovery period. All animals killed after treatment underwent a detailed necropsy and comprehensive microscopic evaluation of tissues; the livers of animals allowed to recover for 4 weeks were examined. The protocol complied generally with OECD TG 408 (1981) and TM B26 from Annex V of Directive 87/302/EEC except that the maximum tolerated dose was not reached.
No treatment-related differences were found between treated animals and controls during treatment, with no deaths or clinical signs and similar body-weight gain and food and water consumption in treated and control animals. No ophthalmological changes were noted, and haematology, clinical chemistry and urine analysis revealed no changes that could be attributed to treatment.
At termination, no treatment-related gross changes were found in organs. The absolute and relative weights of the organs were unaffected by treatment, although a slight (maximum, 7%) increase in mean liver:body weight ratio was seen in females at concentrations > 625 ppm. Histopathological examination revealed a dose-related occurrence of hepatocellular inclusion bodies (consisting of ring- or whorl-shaped eosinophilic particles located within the cytoplasm of perilobular hepatocytes) in males at 625 and 1250 ppm. The occurrence of these inclusions was sometimes associated with enlargement of perilobular hepatocytes. Females at these concentrations showed increased incidences of minimal hepatocellular hypertrophy, located centrilobularly. Both findings were completely reversed within the 4-week recovery period.
Treatment with metalaxyl-M at these concentrations was thus well tolerated. The NOAEL was 1250 ppm, equal to 91 mg/kg bw per day, the highest dose tested, as the effects on the liver observed at 625 ppm were considered not to be adverse (Gerspach, 1995).
Groups of 20 male and 20 female Sprague-Dawley rats received diets containing metalaxyl (purity, 99%) at a concentration of 0, 50, 250 or 1250 ppm for 3 months. Five males and five females from the control and highest-dose groups were kept for a 4-week recovery period before they were killed. The doses were equal to mean intakes of 3.2, 16 and 79 mg/kg bw per day for males and 3.5, 18 and 86 mg/kg bw per day for females. Overt signs of toxicity were recorded daily, and body weight and food consumption were recorded weekly throughout the study. Ophthalmoscopy was performed on all animals before treatment and on 10 males and females from the control and highest-dose groups during weeks 5, 9 and 13. Animals allowed to recover were examined during week 17. Haematological, blood chemical and urine analyses were carried out on all surviving treated animals at weeks 5, 9 and 13 and at week 17 for rats allowed to recover. Animals that died or were killed during the the study and all animals that survived until scheduled sacrifice were autopsied. A comprehensive microscopic evaluation of tissues from all treated animals was carried out, and the livers and ovaries from the five female rats killed after the 4-week recovery period were examined. The study was performed before GLP guidelines and EEC or OECD test guidelines were enacted; however, the protocol complied with the major requirements of OECD TG 408 (1981).
No deaths and no overt signs of toxicity were reported. Ophthalmic examination revealed no treatment-related changes. Males at the highest dose had minimally decreased body-weight gain (terminal weight, 97% of control) and food consumption (week 13, 94% of control). Haematology, blood biochemistry and urine analysis revealed no treatment-related changes. At autopsy, no macroscopic changes were seen, and there were no relevant changes in organ weights. Histopathological examination revealed minimal hypertrophy in a few hepatocytes in the females at the two higher concentrations, with incidences of 0/20, 0/20, 2/20 and 7/20. Large ovarian cysts were reported in 5/20 females at 1250 ppm, but these were regarded as of doubtful significance. In the group allowed to recover, no changes were noted in the liver, and the ovaries were similar to those of controls.
The maximum tolerated dose was not reached in this study. As the liver-cell hypertrophy at 1250 ppm was considered not to be adverse, this concentration, 79 mg/kg bw per day, was the NOAEL (Drake, 1977).
In a study performed in compliance with the principles of GLP (with QA certification), groups of 20 male and 20 female Sprague-Dawley-derived rats (Tif:RAIf (SPF)) received diets containing metalaxyl (purity, 93.5%) at a concentration of 0, 10, 50, 250 or 1250 ppm for 3 months. The doses were equal to mean intakes of 0.66, 3.5, 15 and 72 mg/kg bw per day for males and 0.67, 3.6, 16 and 74 mg/kg bw per day for females. Overt signs of toxicity were recorded daily, and body weight and food consumption were recorded weekly throughout the study. Ocular examinations and an auditory test were performed before and after treatment. Animals allowed to recover were examined during week 17. Haematological, blood chemical and urine analyses were carried out on 10 rats of each sex per group in weeks 4, 8 and 12. At the end of treatment, all animals were subjected to a detailed necropsy and a comprehensive microscopic evaluation of tissues. The study was performed before EEC or OECD test guidelines were enacted; however, the protocol complied with the major requirements of OECD TG 408 (1981).
No deaths or clinical signs were seen. Body-weight gain and food consumption remained unaffected by treatment. The laboratory investigations revealed a slight decrease in leukocyte count for males at the highest concentration at week 12. At necropsy, slightly increased adrenal weights were seen in males at concentrations > 50 ppm (160%, 180% and 170% of control values). Histopathological examination of the organs and tissues revealed no changes related to administration of metalaxyl.
The maximum tolerated dose was not reached in this study. The concentration of 10 ppm, equal to 0.66 mg/kg bw per day, represents a conservative NOAEL on the basis of the changes in adrenal weights in males. However, the effect was not observed in females and was not confirmed by blood biochemistry or histopathology. Moreover, this effect has not been observed in other studies. This study was not taken into account in the final evaluation (Gfeller, 1980).
In a study of the toxicological effects of repeated inhalation of the pyrolysis products of cigarette tobacco treated with metalaxyl, groups of 10 male and 10 female Fischer 344 rats were exposed for 5 days per week, for 13 weeks, to the smoke from 18 cigarettes that had been spiked with 0, 130, 3900 or 13 000 ppm of metalaxyl (technical-grade, purity not specified). In addition, five groups of two rats of each sex were exposed once to the smoke of each concentration of spiked tobacco and to ambient air, before initiation of the 90-day study, for analyses of plasma nicotine. The criteria used to evaluate treatment-related effects included death, moribundity, appearance, behaviour, body weight, clinical pathology, absolute and relative organ weights, gross pathology and histopathology. The study was conducted in compliance with the principles of GLP (with QA).
No distinct clinical changes were noted in any of the treated animals during the study. No treatment-related effects or trends were observed in body-weight gain, food consumption, haematological or clinical chemical parameters or gross or histological appearance. This study was not used in the final evaluation (Coate, 1982).
In a study conducted in compliance with the principles of GLP (with QA certification), metalaxyl-M (purity, 97.1%) suspended in 1% (w/v) carboxymethylcellulose in 0.1% (w/v) aqueous polysorbate 80 was applied to the clipped skin of groups of five Sprague-Dawley-derived (Tif:RAIf (SPF) hybrids of RII/1 × RII/2) rats of each sex at a dose of 0, 50, 250 or 1000 mg/kg bw per day under an occlusive dressing for 6 h/day, 5 days/week, for 4 weeks. No application was made on the weekend days of weeks 1–3. Clinical signs, body weight, food consumption and deaths were monitored throughout the study. Haematological and blood chemical analyses were performed at the end of treatment. At sacrifice, the animals were examined macroscopically and organ weights were recorded. Organs and tissues were collected, prepared for histopathological evaluation and examined microscopically. The protocol of the study complied with the major requirements of OECD TG 410 (1981) and TM B9 from Annex V of Directive 92/69/EEC.
Treatment with metalaxyl-M produced no clinical signs or behavioural changes and no signs of local irritation. Although the food intake of treated and control animals was similar, males at the highest dose gained less weight than controls. At the end of treatment, the mean body weight of males at the highest dose was 6% lower and the mean body-weight gain 21% lower than that of controls. Haematological and blood chemical analyses gave no indication of a treatment-related effect. Males at the highest dose had a 16% decreased mean spleen weight, and the liver:body weight ratios were increased by 8% in males and 16% in females. Macroscopic and histopathological examination showed no treatment-related findings. Dermal treatment with metalaxyl-M was thus well tolerated, with no irritating effect. The NOAEL for systemic effects was 1000 mg/kg bw per day, the highest dose tested, as the modifications in liver and spleen weight were not confirmed at necropsy or by histopathological findings (Gerspach, 1998).
Rabbits
In a study conducted in compliance with the principles of GLP (with QA certification), powdered metalaxyl (purity, 92%) was applied to the clipped skin of groups of 10 male and 10 female New Zealand white rabbits at a dose of 0, 10, 100 or 1000 mg/kg bw per day under a semi-occlusive dressing for 6 h/day, 5 days/week, for 3 weeks. The skin of half of the animals was abraded once a week in order to enhance dermal absorption. Clinical signs, deaths and signs of dermal irritation were recorded daily throughout the study. Body weight and food consumption were monitored at the beginning of treatment and then twice a week until sacrifice or death. Haematological and blood chemical analyses were performed before and at the end of treatment. After death or at sacrifice, the animals were examined macroscopically, and organ weights were recorded. Organs and tissues were collected, prepared for histopathological evaluation and examined microscopically. The study was performed before EEC or OECD test guidelines were enacted; however, the protocol of the study complied with the major requirements of OECD TG 410 (1981).
The application was tolerated at all doses with no signs of systemic toxicity. Body weight and food consumption were similar in all groups. One male at 100 mg/kg bw per day was found dead on day 20, but the cause of death was not reported. Abnormal skin reactions were limited to lesions from the tape used to secure the dressing, and pimple-like eruptions were seen in all groups at a frequency that was not dose-related; they were considered not to be related to treatment. Haematological and clinical chemical analyses and post-mortem examinations for organ weight, gross and histopathological lesions in brain, pituitary, heart, thyroid, adrenals, genital organs, liver, kidney and skin revealed no treatment-related changes. The experimental procedure induced multifocal dermatitis in treated and untreated areas of the skin in both treated and control rabbits. Dermal treatment with metalaxyl was thus well tolerated. The NOAEL was 1000 mg/kg bw per day, the highest dose tested (Calkins, 1980).
Dogs
The toxicity of metalaxyl-M in dogs was investigated in a 13-week study of dietary administration. Metalaxyl was previously tested in a 6-month study with administration in the diet and in a 2-year study with administration in capsules.
In a study conducted in compliance with the principles of GLP (with QA certification), groups of four male and four female beagle dogs were given diets containing technical-grade metalaxyl-M (purity, 97.1%) in acetone at a concentration of 0, 50, 125, 250 or 1250 ppm for 13 weeks. The doses were selected to allow direct comparison with the previous short-term study conducted with metalaxyl and were equal to a mean daily intake of 1.6, 4.1, 7.3 and 39 mg/kg bw per day for males and 1.6, 4.3, 7.9 and 40 mg/kg bw per day for females. Overt signs of toxicity, body weight and food consumption were recorded daily throughout the study. Ophthalmoscopy was performed before and at the end of treatment. Haematological, blood chemical and urine analyses were conducted before treatment and at weeks 7 and 13. At 13 weeks, all animals were killed and necropsied, and a comprehensive histological examination was carried out. The protocol was generally in compliance with OECD TG 409 (1981) and TM B27 from Annex V of Directive 87/302/EEC, except that the maximum tolerated dose was not reached.
Treatment caused no deaths or any clinical signs of toxicological significance, and the ophthalmic examination revealed no changes. All dogs ate similar quantities of food, and the body-weight development was similar in treated and control groups. No treatment-related changes in haematological parameters were observed. Increased alkaline phosphatase activity was seen in males and females at the highest concentration after 7 weeks (190% increase over controls for males and 220% increase for females) and 13 weeks (210% for males and 260% for females) of treatment. Treatment had no effect on the urine parameters investigated. At sacrifice, the mean absolute (25% in males, 28% in females) and relative (25% in males, 33% in females) weights of the liver were increased in animals at the highest concentration. Macro- and histopathological examination revealed no changes of toxicological significance. Metalaxyl-M was thus well tolerated at concentrations < 1250 ppm. The liver was the target organ, as indicated by increases in relative and absolute weights and in alkaline phosphatase activity. The NOAEL was 250 ppm, equal to 7.3 mg/kg bw per day (Altmann, 1995).
In a study conducted in compliance with the principles of GLP (with QA certification), groups of six male and six female beagle dogs were given diets containing technical-grade metalaxyl (purity, 92%) in acetone at a concentration of 0, 50, 250 or 1000 ppm for 6 months. Two additional dogs of each sex from the control group and that at 1000 ppm were kept for a 4-week recovery period before they were killed. The doses were equal to mean daily intakes of 1.6, 7.8 and 31 mg/kg bw per day for males and 1.7, 7.4 and 32 mg/kg bw per day for females. Overt signs of toxicity, body weight and food consumption were recorded daily throughout the study. Ophthalmoscopy was performed before and at the end of treatment. Haematological and blood chemical analyses were conducted before treatment and at 30-day intervals until termination of dosing. Urine was analysed before treatment and at 60-day intervals until termination of dosing. Blood samples were collected from dogs allowed to recover 4 weeks after termination of dosing, and urine was collected before necropsy. At 6 months, all animals were killed and subjected to a detailed necropsy and a comprehensive histological examination. The protocol generally complied with OECD TG 409 (1981) and TM B27 from Annex V of Directive 87/302/EEC, although the duration of treatment exceeded the basic guideline requirements. Nevertheless, it did not exceed 10% of the normal life span of the dogs, and the investigation can be considered a short-term study.
No deaths occurred and no treatment-related changes were found in clinical signs, body-weight gain, food consumption or ophthalmic parameters. In comparison with the control animals, the erythrocyte count, erythrocyte volume fraction and haemoglobin concentration were significantly lower in males at the highest concentration from day 60 of treatment onwards, and these haematological changes were still present in males allowed to recover, though they were not statistically significant. Blood biochemical analyses revealed a slight increase in plasma alkaline phosphatase activity in animals of each sex at the highest concentration from day 30 onwards, which became statistically significant during the second half of the treatment period. After 180 days of treatment, the activity was 170% of the control value in males and 190% in females. In the group allowed to recover, the alkaline phosphatase activity was only minimally above the control values (120% of control value in males and 110% in females). Urinary parameters were all within normal limits, and no trends by dietary concentration were detected.
Although not statistically significant, there appeared to be a dose-related increase in absolute liver weight, with increases of 8% at 50 ppm, 14% at 250 ppm and 16% at 1000 ppm, and in the liver:body weights ratios in males and especially in females, with increases of 2% at 50 ppm, 13% at 250 ppm and 20% at 1000 ppm. These changes were reversible in males and less pronounced in females when allowed to recover. The difference in liver:brain weight ratio attained statistical significance (120% of control values) in females at 1000 ppm after 180 days of treatment; the value was 110% of control in the group allowed to recover. Gross and histopathological examination showed no treatment-related changes.
The NOAEL was 250 ppm, equal to 7.8 mg/kg bw per day, on the basis of increases in alkaline phosphatase activity and liver weight at 1000 ppm (Beck & deWard, 1981).
Metalaxyl (purity, 92.7–94.1%) was administered in gelatine capsules to groups of six male and six female beagle dogs at a dose of 0, 0.8, 8 or 80 mg/kg bw per day once a day, 7 days a week, for 2 years. All surviving animals were killed at termination of the study at week 103. The study complied with the principles of GLP (with QA certification), and the protocol was generally in compliance with OECD TG 409 (1981) although it was begun in 1980, before adoption of OECD TG.
Animals at the highest dose frequently showed spasms and/or salivation, especially during the first 52 weeks of treatment. The symptoms usually occurred 10–30 min after dosing and disappeared within 0.5–2 min of their onset. Two males and two females died during weeks 20–52. There were no treatment-related effects on body weight, although male dogs at 0.8 mg/kg bw per day weighed slightly more than controls during the study. There were no treatment-related effects on food consumption, water consumption or ophthalmic end-points.
A slight decrease or a decreasing trend in the specific gravity of the urine was observed in males at 80 mg/kg bw per day from 13 weeks of treatment. Mild anaemia was observed in animals at the highest dose. The reductions (10–20%) in erythrocyte count, haemoglobin concentration and erythrocyte volume fraction became evident only at 26 weeks and were slightly more pronounced in males than in females. The values for all three haematological end-points in males were within the range of other controls in the laboratory in weeks 26 and 52. For females, the values for all three end-points in week 26 and for erythrocyte volume fraction in week 52 were within the range of other controls; the other two parameters were marginally lower than those of other controls in week 52.
Animals at 80 mg/kg bw per day had significantly increased serum activities of alkaline phosphatase and alanine aminotransferase as compared with controls (Table 28). The values for alkaline phosphatase during the study were 1.5–2.5 times higher than those determined before initiation of treatment and 2–5.6 times those in the corresponding control groups. The activity in males at 8 mg/kg bw per day and in controls decreased gradually during the study from that determined before initiation of treatment, and only a slight (maximum, twofold) but statistically significant increase in alkaline phosphatase activity was found. The increase in alanine aminotransferase activity in males at the intermediate dose was slight (maximum, 50%) and often not dose-related. Animals at the highest dose also had elevated albumin, total protein and calcium concentrations. Males showed decreased aspartate aminotransferase activity and globulin and creatinine concentrations.
Table 28. Activity of alkaline phosphatase and alanine aminotransferase in dogs given metalaxyl in gelatine capsules for 2 years (U/l; group mean ± standard deviation)
|
Time after treatment (weeks) |
Dose (mg/kg bw per day) |
|||||||
|
0 |
0.8 |
8 |
80 |
|||||
|
Males |
Females |
Males |
Females |
Males |
Females |
Males |
Females |
|
|
Alakaline phosphatase |
||||||||
|
Before |
227 ± 26 |
217 ± 69 |
200 ± 27 |
211 ± 38 |
224 ± 33 |
225 ± 49 |
196 ± 19* |
208 ± 24 |
|
4 |
218 ± 35 |
190 ± 39 |
190 ± 33 |
182 ± 45 |
216 ± 33 |
218 ± 55 |
296 ± 100 |
408 ± 204* |
|
13 |
148 ± 29 |
148 ± 38 |
149 ± 51 |
140 ± 45 |
203 ± 42* |
157 ± 31 |
395 ± 215* |
390 ± 185* |
|
26 |
107 ± 24 |
132 ± 20 |
122 ± 47 |
109 ± 38 |
169 ± 53* |
132 ± 39 |
443 ± 166* |
292 ± 77** |
|
52 |
88 ± 27 |
112 ± 41 |
125 ± 74 |
94 ± 35 |
176 ± 68* |
118 ± 43 |
367 ± 161* |
421 ± 339 |
|
78 |
78 ± 25 |
110 ± 46 |
108 ± 49 |
93 ± 67 |
160 ± 68* |
93 ± 34 |
451 ± 191* |
442 ± 457 |
|
103 |
75 ± 23 |
110 ± 40 |
137 ± 83 |
76 ± 28 |
186 ± 82* |
94 ± 26 |
423 ± 139 * |
460 ± 389 |
|
Alanine aminotransferase |
||||||||
|
Before |
22 ± 3 |
25 ± 4 |
25 ± 6 |
30 ± 25 |
27 ± 8 |
23 ± 6 |
33 ± 17 |
31 ± 6 |
|
4 |
20 ± 3 |
27 ± 5 |
24 ± 6 |
27 ± 5 |
27 ± 5* |
29 ± 7 |
25 ± 5 |
27 ± 3 |
|
13 |
22 ± 4 |
25 ± 3 |
25 ± 4 |
25 ± 6 |
29 ± 6* |
26 ± 3 |
28 ± 6 |
33 ± 9 |
|
26 |
21 ± 4 |
23 ± 3 |
25 ± 5 |
24 ± 5 |
28 ± 12 |
25 ± 3 |
39 ± 10* |
49 ± 19* |
|
52 |
22 ± 6 |
24 ± 3 |
29 ± 9 |
25 ± 5 |
28 ± 11 |
26 ± 5 |
65 ± 24* |
56 ± 32 |
|
78 |
22 ± 5 |
25 ± 4 |
28 ± 13 |
25 ± 3 |
30 ± 9 |
27 ± 5 |
66 ± 17* |
96 ± 31* |
|
103 |
22 ± 6 |
27 ± 5 |
27 ± 8 |
23 ± 6 |
33 ± 9* |
27 ± 6 |
86 ± 31* |
86 ± 30* |
From Harada (1984)
Six animals examined per data point, except males at 80 mg/kg bw per day, only five of which were examined after 26 weeks of treatment and only four thereafter, and females at this dose, of which only four were examined from week 52 onwards
* Significantly different from controls at p < 0.05; ** significantly different from controls at p < 0.001
At necropsy, animals at the highest dose frequently showed enlargement of the liver and increased liver weights, with increases in absolute weight of 58% in males and 16% in females and an increase in relative weight of 16% in both sexes. Males also had significantly increased kidney weights (by 34%) in comparison with controls. Histopathology revealed no specific changes attributable to treatment. The incidences of hepatic lesions such as focal inflammation, fibrosis and brown pigment deposition tended to be higher in animals at 80 mg/kg bw per day than in controls; however, the lesions could not definitively be related to treatment as they were relatively mild.
The changes in alkaline phosphatase and alanine aminotransferase activity observed in animals at the highest dose indicated hepatic effects of treatment and were considered to correspond to the increased liver weights. However, even though the difference in alkaline phosphatase activity between males at 8 mg/kg bw per day and concurrent controls was statistically significant, it was slight, and, in the absence of other biologically significant changes in clinical chemistry and histopathology, was considered not to be adverse
The liver was the target organ of metalaxyl, as indicated by the changes in blood chemistry and increased liver weight. Although a decrease in urine specific gravity and increased kidney weights were observed in males, there were no histopathological abnormalities in the kidney, and it is not clear whether this effect was related to treatment. The haematological end-points indicating anaemia occurred only after long-term treatment and were not relevant to acute exposure. The NOAEL was 8 mg/kg bw per day (Harada, 1984).
Mice
Groups of 60 male and 60 female ICI-derived Swiss mice were given diets containing metalaxyl (purity, 93–94.6%) at a concentration of 0, 50, 250 or 1250 ppm for 104 weeks, equal to mean daily intakes of 4, 19 and 100 mg/kg bw per day for males and 4.6, 23 and 120 mg/kg bw per day for females. These values were re-calculated from individual values for food consumption and body weight, taking into account the content of the test compound in the food batches used. In earlier evaluations of this study, standard conversion factors were used to calculate the intake in mg/kg bw per day. The study was performed in compliance with the principles of GLP (with QA certification), and the design complied with the requirements of OECD TG 451 (1981), although the treatment period was longer than that usually recommended for mice (18 months).
The animals showed no clinical signs of toxicity, and the mortality rate was similar in all groups. At 78 weeks, the survival rates were 53%, 55%, 57% and 50% for males and 67%, 53%, 53% and 52% for females in the control group and at the low, intermdiate and high dietary concentrations, respectively. At 104 weeks, the mortality rates were increased, with survival rates of 8%, 10%, 10% and 17% for males and 13%, 13%, 8% and 10% for females, respectively. During weeks 11–30 of treatment, males at 1250 ppm gained less weight (12 g) than control males (17 g), corresponding to a reduction of 31% and leading to a difference of 9% at week 30. The animals recovered slowly thereafter, but the cumulative body-weight gain was still reduced at week 30 (by 12%) and week 56 (by 10%) for males at 1250 ppm. A slight, 8% reduction in body-weight gain in comparison with controls was observed in weeks 0–10 among females at 1250 ppm. The terminal body weights of treated and untreated groups were similar. The food consumption and water intake of treated and control animals were essentially identical throughout the treatment period. Food conversion efficiency was reduced in males at 1250 ppm during weeks 11–30.
Macroscopic and histopathological examination of a wide range of tissues, including blood and bone marrow smears, revealed no significant treatment-related changes. The histopathological results for the main target organ, the liver, are outlined in Table 29.
Table 29. Incidences of histopathological liver changes in mice given diets containing metalaxyl for 2 years
|
Change and distribution in time |
Dietary concentration (ppm) |
|||||||
|
0 |
50 |
250 |
1250 |
|||||
|
Males |
Females |
Males |
Females |
Males |
Females |
Males |
Females |
|
|
Fatty vacuolation (total incidence) |
||||||||
|
Dead at 0–52 weeks |
1/10 |
3/7 |
1/8 |
1/10 |
0/9 |
0/5 |
2/7 |
10/14 |
|
Dead at 53–104 weeks |
20/46 |
25/44 |
19/46 |
14/42 |
20/45 |
16/49 |
18/43 |
26/40 |
|
Killed at 104 weeks |
2/4 |
6/8 |
0/6 |
2/8 |
1/6 |
0/5 |
7/10 |
4/5 |
|
Total |
23/60 |
34/59 |
20/60 |
17/60 |
21/60 |
16/59 |
27/60 |
40/59 |
|
Adenomas |
||||||||
|
Dead at 0–52 weeks |
1/10 |
0/7 |
0/8 |
0/10 |
0/9 |
0/5 |
1/7 |
0/14 |
|
Dead at 53–104 weeks |
10/46 |
4/44 |
19/46 |
1/42 |
13/45 |
5/49 |
13/43 |
1/40 |
|
Killed at 104 weeks |
2/4 |
0/8 |
2/6 |
2/8 |
1/6 |
1/5 |
6/10 |
1/5 |
|
Total |
13/60 |
4/59 |
21/60 |
3/60 |
14/60 |
6/59 |
20/60 |
2/59 |
|
Carcinomas |
||||||||
|
Dead at 0–52 weeks |
0/10 |
0/7 |
0/8 |
0/10 |
0/9 |
0/5 |
0/7 |
0/14 |
|
Dead at 53–104 weeks |
1/46 |
1/44 |
1/46 |
0/42 |
2/45 |
1/49 |
3/43 |
1/40 |
|
Killed at 104 weeks |
0/4 |
0/8 |
0/6 |
0/8 |
1/6 |
0/5 |
0/10 |
0/5 |
|
Total |
1/60 |
1/59 |
1/60 |
0/60 |
3/60 |
1/59 |
3/60 |
1/59 |
|
Adenomas plus carcinomas |
||||||||
|
Dead at 0–52 weeks |
1/10 |
0/7 |
0/8 |
0/10 |
0/9 |
0/5 |
1/7 |
0/14 |
|
Dead at 53–104 weeks |
11/46 |
5/44 |
20/46 |
1/42 |
15/45 |
6/49 |
16/43 |
2/40 |
|
Killed at 104 weeks |
2/4 |
0/8 |
2/6 |
2/8 |
2/6 |
1/5 |
6/10 |
1/5 |
|
Total |
14/60 |
5/59 |
22/60 |
3/60 |
17/60 |
7/59 |
23/60 |
3/59 |
From McSheehy et al. (1980a,b)
There was no evidence of a carcinogenic response to treatment. A re-evaluation of the slides of the liver did not alter this conclusion, and the liver tumours in male mice were considered not to be treatment-related. This conclusion is supported by the following findings: (i) The liver tumours were age-related, occurring predominantly after 18 months of the study. (ii) The survival of the animals was not affected by administration of metalaxyl. (iii) Neither general health nor body weight or food consumption was compromised by treatment. (iv) The presence of Tyzzeria did not obscure the presence of liver tumours, and the non-neoplastic lesions in the liver (hepatocytic fatty vacuolation and bile-duct proliferation) were no different in control and treated groups and would therefore not have affected the distribution of liver tumours. (v) The liver tumour incidence was not statistically significantly different (chi2, 2 × 4) between control and treated groups (p = 0.12). (vi) The liver tumour incidence did not show a dose-related trend (Mantel trend test; p = 0.15). (vii) The liver tumour incidence in the control group (23%) was within the range of that in six other control groups in the same laboratory (12–37%).
Treatment with metalaxyl did not increase the incidence of benign or malignant tumours in mice of either sex. The NOAEL was 250 ppm, equal to 19 mg/kg bw per day, on the basis of slight body-weight loss in male mice at 1250 ppm (McSheehy et al., 1980a,b).
Rats
Groups of 80 male and 80 female CD Sprague-Dawley-derived rats were fed diets containing technical-grade metalaxyl (purity, 93–96.4%) at a concentration of 0, 50, 250 or 1250 ppm for 2 years, equal to mean daily intakes of 1.7, 8.7 and 43 mg/kg bw per day for males and 2, 10 and 55 mg/kg bw per day for females. The doses were selected on the basis of a 3-month study of toxicity, which had shown decreased body-weight gain and effects on the liver at 1250 ppm. Ten animals of each sex per dose were killed after 55 weeks of treatment, while the remaining animals were maintained on the treated diet until terminal sacrifice after 105 weeks. The study was performed in compliance with the principles of GLP (with QA certification), and the design complied generally with the requirements of OECD TG 453 (1981), although the highest concentration was high enough for a carcinogenicity study but not for a toxicity study, gamma-glutamyl transpeptidase activity was not measured and the ovaries were not weighed.
No deaths and no clinical signs of toxicity resulted from treatment. While no differences between treated and control animals were seen in food consumption or absolute body weight, the body-weight gain of females at 1250 ppm was reduced transiently in weeks 26–52 (by 10% in comparison with controls). Haematological, clinical chemical and urine parameters remained within normal limits, and no remarkable intergroup differences were found.
At interim sacrifice, the liver:body weight ratio was significantly increased (120% of control) in females at 1250 ppm. At terminal sacrifice, increased absolute and relative liver weights were found in animals of each sex at this concentration. The increase in relative liver weight in males at 250 ppm was due at least partly to a decreased carcass weight in this group. The increased relative weights of the liver observed in females at 1250 ppm in week 55 and in males at 250 and 1250 ppm in weeks 105 were not accompanied by underlying hepatic damage, indicating that the changes in weight indicated a mild change in the liver that was not adverse.
An increased severity of eosinophilic vacuolation was seen in males at the two higher concentrations that were killed at week 55. This finding was considered to represent a transitory response that was not adverse because it was not observed in animals dying during the study or at terminal sacrifice. Centriacinar, periacinar and panacinar hepatocytic vacuolation in the liver of various degrees of severity was observed, indicating fatty changes. The centriacinar and panacinar vacuolation was not related to treatment. In males, no significant, dose-dependent increase in the incidence of hepatocytic vacuolation was observed in any treated group. In animals that died up to week 52, no relationship with treatment was found for hepatocyte vacuolation in the few animals affected. Increased incidences of periacinar hepatocyte vacuolation were observed in all treated females at 55 and 105 weeks, but the distribution among groups was uneven and there was no clear relationship with dose. The increase in severity was not dose-related at any time, except in females at 1250 ppm at terminal sacrifice, in which the severity was increased in comparison with concurrent controls and with females at this concentration at earlier times. Therefore, only a usually slight-to-moderate increase in the incidence of periacinar hepatocytic vacuolation, which was considered to be a fatty change and occurred spontaneously in control animals, was observed in metalaxyl-treated females, with an increase in severity only for females at the highest concentration at week 105. Except in the these animals, there was a slight decrease in the overall average severity of hepatic vacuolation between sacrifice at week 55 and at week 105. A second histopathological evaluation (Faccini, 1985) confirmed the overall result of marginal changes in the liver. It also showed an increased incidence of centrilobular hepatocytomegaly in male and female rats at 1250 ppm and possibly a marginally higher incidence in males at 250 ppm. A higher incidence of foci of cellular alteration was seen in males at 250 ppm, with a possible trend towards the same effect in females at this concentration.
A statistically significant increase (p < 0.05) was found in the incidence of C-cell (parafollicular cell) adenomas in females at 250 ppm (Table 30). However, no tumours were detected in animals that died before 78 weeks of treatment, no dose–response relationship was established, and the incidence was within that of controls from 15 contemporary studies in the same laboratory (total adenomas, 0–15%; adenomas in females killed at termination of study: 0–30%). Therefore, the distribution was considered to be fortuitous and not related to treatment with metalaxyl.
Table 30. Incidences of C-cell adenomas of the thyroid in rats given diets containing metalaxyl for 2 years
|
Time of death |
Dietary concentration (ppm) |
|||||||
|
0 |
50 |
250 |
1250 |
|||||
|
Males |
Females |
Males |
Females |
Males |
Females |
Males |
Females |
|
|
Dead at 0–52 weeks |
0/6 |
0/0 |
0/3 |
0/2 |
0/2 |
0/4 |
0/6 |
0/4 |
|
Killed after 55 weeks |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
|
Dead at 53–105 weeks |
2/44 |
0/38 |
1/36 |
4/42 |
3/46 |
5/37 |
3/36 |
0/42 |
|
Killed after 105 weeks (%) |
2/20 (10) |
2/32 (3.6) |
0/31 (0) |
3/26 (12) |
1/22 (4.5) |
5/28 (18) |
1/28 (3.6) |
5/24 (21) |
|
Total (%) |
4/80 (5) |
2/80 (2.5) |
1/80 (1.3) |
7/80 (8.8) |
4/80 (5.0) |
10/79 (13)* |
4/80 (5.0) |
5/80 (6.3) |
From Ashby & Whitney (1980a,b)
* Statistically significant at p < 0.005
Thus, metalaxyl administered to rats at concentrations < 1250 ppm in the diet for 2 years was well tolerated. The finding of hepatocytic vacuolation is an age-related finding and was considered not to be adverse. The hepatocellular changes were not accompanied by degenerative, irreversible or cytotoxic lesions such as necroses, which can be observed in the cases of severe fatty change of the liver, and no accompanying inflammatory changes were found. Blood chemistry showed no altered liver parameters pointing to hepatocellular damage. In general, most evidence suggests that moderate hepatic fatty change alone does not impair hepatic function (Haschek & Rousseaux, 1991). Centrilobular hepatocytomegaly was considered to be the main effect, occurring in rats of each sex at the highest concentration. This change might have been a consequence of a mild enzyme-inducing effect of the compound (Uesugi, 1988). The treatment had no effect on the incidence or distribution of neoplastic lesions. A re-evaluation of C-cell (parafollicular cell) adenomas in the thyroid gland did not alter this position. The NOAEL was 1250 ppm, equal to 43 mg/kg bw per day, the highest concentration tested, as the mild liver changes seen at this concentration were considered not to be adverse (Ashby & Whitney, 1980a,b).
The mutagenic or genotoxic potential of technical-grade metalaxyl and metalaxyl-M was investigated in a battery of tests in vitro and in vivo (Tables 31 and 32).
Table 31. Results of the studies of genotoxicity conducted with metalaxyl
|
End-point |
Test object |
Concentration |
Purity (%) |
Results |
GLP or QA |
Reference |
|
In vitro |
||||||
|
Reverse mutation |
S. typhimurium TA98, TA100, TA102, TA1535, TA1537 |
20, 78, 313, 1250, 5000 µg/plate in acetone |
95.7 |
Negativea,b |
GLP, QA |
Deparade & Arni (1985) |
|
Mitotic gene conversion, mitotic crossing over and reverse mutation |
Saccharomyces cerevisiae D7 |
400, 2000, 10 000 µg/ml in DMSO |
94.1 |
Negativea |
Compliance statement |
Arni & Müller (1982) |
|
Forward mutation |
Mouse lymphoma L5178Y cells, Tk locus |
+ S9: 0.062, 0.12, 0.25, 0.5 mg/ml |
94.1 |
Negativea |
Compliance statement |
Strasser & Müller (1982) |
|
Chromosomal aberration |
Chinese hamster ovary cells |
150, 300, 900, 1200 µg/ml in DMSO |
NR |
– S9: positive at 1200 µg/ml (cytotoxicity) |
GLP, QA |
Ivett (1986) |
|
Chromosomal aberration |
Human peripheral blood lymphocytes |
10, 30, 100, 300, 1000 µg/ml in DMSO |
99 |
– S9: dose-dependent increase |
Hrelia et al. (1996) |
|
|
Unscheduled DNA synthesis |
Human fibroblasts |
4, 20, 100, 500 µg/ml in DMSO, no S9 |
94.1 |
Negative |
Compliance statement |
Puri & Müller (1982) |
|
Unscheduled DNA synthesis |
Primary rat hepatocytes |
16, 80, 400, 2000 µg/ml in DMSO |
94.1 |
Negative |
GLP, QA |
Puri & Müller (1985) |
|
Cell transformation |
BALB/c 3T3 cells |
50, 250, 500 µg/ml in DMSO |
99.9 |
– S9: positive at 500 µg/ml (cytotoxic) |
Perocco et al. (1995) |
|
|
In vivo |
||||||
|
Micronucleus formation |
Tif:MAGf (SPF) mice |
Single oral doses of 78, 160, 310 mg/kg bw in arachis oil; sampling 16, 14, 48 h later |
96.1 |
Negative |
GLP, QA |
Hertner & Arni (1992) |
|
Micronucleus formation |
CD-1 mice, 5 males per group, bone-marrow cells |
Single intraperitoneal doses of 75, 150, 300 mg/kg bw in corn oil; sampling at 24 h |
99 |
Negative |
Hrelia et al. (1996) |
|
|
Nucleus anomalies |
Chinese hamsters (5 males, 5 females per group); bone-marrow cells |
Oral, 600, 1200, 2400 mg/kg bw in CMC; 2 days; sampling 24 h after second treatment |
98 |
Negative |
Compliance statement |
Langauer & Müller (1979) |
|
Dominant lethal mutation |
NMRI mice (20 males per group) |
Single oral doses of 65, 200 mg/kg bw in CMC |
99.4 |
Negative |
Compliance statement |
Fritz (1978a) |
S9, exogenous metabolic activation system from 9000 × g fraction of rat liver induced with Aroclor; DMSO, dimethyl sulfoxide;
CMC, carboxymethylcellulose; NR, not reported. Positive control substances were used in all assays and gave the expected results.
a With and without metabolic activation
b Experimental protocol mostly in compliance with OECD TG 471 (1983)
Table 32. Results of the studies of genotoxicity conducted with metalaxyl-M
|
End-point |
Test object |
Concentration |
Purity (%) |
Results |
GLP or QA |
Reference |
|
In vitro |
||||||
|
Reverse mutation |
S. typhimurium TA98, TA100, TA102, TA1535, TA1537; E. coli WP2 uvrA |
310, 620, 1200, 2500, 5000 µg/plate in DMSO |
97.3 |
Negativea,b |
GLP, QA |
Hertner (1994a) |
|
Chromosomal aberration |
Chinese hamster ovary cells |
– S9: 18-h treatment: 130, 250, 510, 1000 µg/ml 42-h treatment: 250, 510, 1000 µg/ml |
97.3 |
Negativea |
GLP, QA |
Hertner (1994b) |
|
Unscheduled DNA synthesis |
Primary rat hepatocytes |
1) 4.9, 20, 78, 160, 310, 620 µg/ml |
97.1 |
Negative |
GLP, QA |
Ogorek (2000) |
|
In vivo |
||||||
|
Micronucleus formation |
Ico:CD1(CRL) mice |
Single oral |
97.1 |
Negativee |
GLP, QA |
Deparade (1999) |
S9, exogenous metabolic activation system from 9000 × g fraction of rat liver induced with Aroclor; DMSO, dimethyl sulfoxide;
CMC, carboxymethylcellulose
Positive control substances were used in all assays and gave the expected results.
a With and without metabolic activation
b Experimental protocol mostly in compliance with OECD TG 471 (1983)
Metalaxyl at a concentration of 10 000 µg/ml in the study by Arni & Müller (1982) reduced the colony count in comparison with controls. In a second experiment, a growth-inhibiting effect was observed at 8000 µg/ml with and without metabolic activation. The study was performed before GLP guidelines were enacted and was therefore not performed with formal quality assurance. The experimental protocol generally complied with OECD TG 480 and 481 (1986) and was considered acceptable.
In the study of Strasser & Müller (1982) on forward mutation, the first assay, in the presence of metabolic activation, gave an equivocal response; comparison of the mutant frequency in treated cultures with the control revealed a factor of 2.6 at the highest concentration, which was barely higher than the threshold value of 2.5. No concentration-related response was demonstrable. A repeat assay failed to confirm the observation. The experimental protocol was generally in compliance with OECD TG 476 (1984), and the study was considered acceptable.
In the study of Ivett (1986) on chromosomal aberration, complete cellular toxicity was found at 1.6 mg/ml and 5 mg/ml of metalaxyl both with and without metabolic activation. Monolayer confluency was reduced at 1200 µg/ml in assays for aberrations (by 30% in the absence of metabolic activation, 20% in the presence). In the test without activation, fewer mitotic cells were observable at the highest dose tested. A significant increase in the percentage of chromosomally aberrant cells was observed only in the absence of activation at 1200 µg/ml, with an increased frequency of endoreduplicated cells. There were no significant increases or dose-related trends in chromosomally aberrant cells at the other doses. The experimental protocol was not fully in compliance with OECD 473 (1983), as the experiment was not duplicated and the purity of the compound was not stated. The study was therefore not used in the evaluation.
In the study of Hrelia et al. (1996) on chromosomal aberration, peripheral blood lymphocytes were obtained from two healthy donors, and an exogenous metabolic activation system was obtained from the livers of rats induced with phenobarbital and beta-naphthoflavone. The frequency of aberrations reached statistical significance at doses of metalaxyl of 300 and 1000 µg/ml in the absence of metabolic activation; the maximum frequency observed at the highest dose was 3.4-fold greater than in the corresponding control. No cytotoxicity was observed, as indicated by the comparable mitotic indexes in treated and control cultures. Chromatid breaks were the main type of aberration observed. This study was considered to provide only additional information.
In the study of Puri & Müller (1982) on unscheduled DNA synthesis, the results of the toxicity test indicated that the highest concentration that would allow at least 25% viability of treated cells was 500 µg/ml. The study was performed before GLP guidelines were enacted and was therefore not performed with formal quality assurance. The experimental protocol was not fully in compliance with OECD 482 (1986), as the experiment was not repeated and no assay was performed with metabolic activation. The study was considered to provide only additional information.
In a second study on unscheduled DNA synthesis (Puri & Müller, 1985), seven concentrations between 250 and 8000 µg/ml were tested. No viable cells were found at concentrations > 4000 µg/ml. The experimental protocol was not fully in compliance with OECD 482 (1986), as the experiment was not repeated. The study was considered to provide additional information.
In the investigation of Perocco et al. (1995) for cell transformation, an effect was detectable only in cultures at level II and was probably due to induction of additional cell proliferation, allowing transformation amplification in these experimental conditions. The cell clonal efficiency was reduced by metalaxyl at the highest dose tested in the absence of metabolic activation; the relative clonal efficiency at that dose was 46%, whereas in the presence of bioactivation it was 78%. The assay for cell transformation in vitro is considered to reflect part of the carcinogenesis process in vivo. However, the end-points of none of the tests in vitro has an established mechanistic link with cancer. It is assumed that some of these tests can detecting tumour promoters. The results of this assay were not confirmed in a second experiment, and this study was not used in the evaluation.
In the study of Hertner & Arni (1992) for micronucleus formation in mice treated in vivo, the highest dose used was the maximum tolerated dose, as demonstrated in a preliminary test for tolerability. Doses > 450 mg/kg bw caused death. At 310 mg/kg bw, reduced locomotor activity, convulsions, hunched posture and piloerection were observed, mainly within the first few hours. No effect was observed on the ratio of polychromatic to normochromatic cells. The experimental protocol generally complied with OECD TG 474 (1983), and the study was considered acceptable.
In the study of micronucleus formation by Hrelia et al. (1996), there was no direct evidence of cytotoxicity in bone marrow, as shown by the comparable ratio of polychromatic to normochromatic cells in control and treated animals. Studies of distribution show that metalaxyl is well distributed in the body and that its bioavailability in blood is good. It is therefore unlikely that the negative results obtained in vivo were due to poor absorption or distribution in the target organ, the bone marrow. This study was considered to provide only additional information.
In the study of Langauer & Müller (1979) on nucleus anomalies in bone-marrow cells, various types of anomalies were recorded, including single Howell–Jolly bodies, fragments of nuclei in erythrocytes, micronuclei in erythroblasts, micronuclei in leukopoietic cells and polyploid cells. The study was performed before GLP guidelines were enacted and was therefore not performed with formal quality assurance. The experimental protocol was not fully in compliance with OECD 474 (1983), as various types of anomalies were considered beside micronuclei in erythrocytes, and too few cells were counted. The slides for three animals of each sex per dose were examined instead of five. The test was conducted according to a scientific protocol that was valid at that time and was considered acceptable.
In the test for dominant lethal mutation reported by Fritz (1978a), the doses were selected on the basis of acute toxicity, the highest dose corresponding to about one-third and the lowest dose to about one-ninth of the LD50. No signs of intolerance were observed in the mice immediately after treatment; however, one male in each treated group died during mating periods VI and III, respectively. The mating ratio and the numbers of implantations, early and late resorptions and live fetuses were similar in treated and untreated groups. The positive control, thiotepa, gave the expected results. The study was performed before GLP guidelines were enacted and was therefore not performed with formal quality assurance. The experimental protocol was not fully in compliance with OECD 478 (1984), as only two doses were tested and no concurrent positive control was used, although positive control data were available from another test conducted in the same laboratory. The study was considered to be acceptable.
In the tests conducted with metalaxyl-M, Hertner (1994a) reported that flow cytometry revealed weak cell cycle arrest after exposure to metalaxyl-M in the absence of metabolic activation. The highest concentration tested suppressed mitotic activity. The experimental protocol generally complied with OECD TG 473 (1983), and the study was considered acceptable.
In the study of unscheduled DNA synthesis reported by Ogorek (2000), cytotoxicity was evaluated within the original DNA repair assay with 11 concentrations of metalaxyl-M, increasing from 4.9 µg/ml to 5000 µg/ml. At the three highest concentrations (1250, 2500 and 5000 µg/ml), no viable cells were found. The eight lower concentrations decreased the viability in a dose-dependent manner by 5–50%. The experimental protocol complied with OECD TG 482 (1986).
In the study of micronucleus formation in vivo, Deparade (1999) reported that nearly all males and females at 500 mg/kg bw performed creeping movements and became recumbent shortly after administration. Most of the animals recovered some hours later. Three males at 800 mg/kg bw (24-h sampling time) were killed when moribund and were replaced by reserve animals. Males at 400 mg/kg bw also performed creeping movements occasionally. No symptoms of toxicity were noted in females at 125 and 250 mg/kg bw or in males at 200 mg/kg bw. A decrease in the ratio of polychromatic to normochromatic cells was observed in males at 800 mg/kg bw (by 27%) and in females at 500 mg/kg bw (by 12%) that were killed after 24 h. In animals killed after 48 h, the ratio of polychromatic to normochromatic cells also decreased in treated animals, by 20% in males and 7.5% in females. The experimental protocol complied with OECD TG 474 (1997).
The results of the tests for genotoxicity with both metalaxyl and metalaxyl-M were therefore negative, with the exception of an assay with metalaxyl for chromosomal aberration in vitro, in which an increased frequency was observed in the absence of metabolic activation at cytotoxic doses.
Rats
In a study conducted in compliance with the principles of GLP (with QA certification), groups of male and female Charles River COBS CD rats received diets containing metalaxyl (purity, 93.5%) at a concentration of 0, 50, 250 or 1250 ppm for three generations, resulting in intakes of 4.1 (3.2–4.7), 21 (17–23) and 96 (84–100) mg/kg bw per day for males and 4.6 (3.6–5.1), 24 (20–27) and 150 (100–240) mg/kg bw per day for females. The values were calculated from data on food consumption and body weight for the F0, F1 and F2b generations before mating and corrected by the analytical food content. The litter sizes were not standardized. All sacrificed animals were subjected to macroscopic examination. The test was conducted according to Guideline 83-4 of FIFRA Subdivision F. The experimental protocol was generally in compliance with TM B35 from Annex V of Directive 87/302/EEC or OECD TG 416 (1983).
In the first generation (F0), 25 males and females in each group were mated. After weaning of the first litter (F1a), all the animals were re-mated, and the resulting litters (F1b) were reared to day 21 post partum, when 25 males and females were selected from each group to form the basis for the F2 generation. A third gestation of 15 F0 animals per dose was terminated after 20 days. The skeletons and viscera of the fetuses were examined for malformations. The F1b animals were mated when they were at least 90 days of age. The resulting litters (F2a) were killed after weaning at 21 days of age, and the parental animals were mated a second time. Ten dams per group were killed on day 20 of gestation, and the fetuses were examined for malformations, while 14 dams were allowed to rear their litters. At weaning, 12 male and female pups from each group were kept on their diet for 90 days, and then their organs were weighed. The third generation was produced from another 12 males and 24 females from the F2b litters. These animals were also re-mated after production of the F3a generation. After weaning of the F3b pups, the study was terminated. The organs from 10 pups of each sex from the control group and at 1250 ppm were weighed and examined histologically.
No treatment-related effects were seen in the parental animals, with the possible exception of a slight retardation in body-weight development in males of the F1b generation at the highest concentration during the first 10 weeks after selection. However, the finding was not reproduced in the F0 or F2 generation and was therefore considered to be of equivocal toxicological significance. The food consumption of females of the F0 and F1b generations at 50 and 250 ppm was lower than that of the other groups, but this effect was considered not to be toxicologically significant. Parameters of reproduction and fertility and of fetal and neonatal development remained unaffected in all groups and generations. At sacrifice, the mean liver weights of F2b females at 1250 ppm were slightly increased (111% of control values). This effect was considered not to be adverse, and reproductive performance and offspring development were unaffected, even at the highest concentration of 1250 ppm. The NOAEL for parental, offspring and reproduction toxicity was 1250 ppm, equal to 96 mg/kg bw per day, the highest dose tested (Cozens, 1980).
Rats
Three studies of developmental toxicity were conducted in rats, one with metalaxyl-M and two with metalaxyl.
In a study conducted in compliance with the principles of GLP (with QA certification), groups of 24 mated Sprague-Dawley-derived Tif:RAIf (SPF) hybrids of RII/1 × RII/2 rats were given metalaxyl-M (purity, 97.1%) by gavage in an aqueous solution of CMC (0.5% w/w) at a dose of 0, 10, 50 or 250 mg/kg bw per day on days 6–15 of gestation. The dams were killed on day 21 after conception, and the fetuses were removed surgically. The study was conducted according to OECD TG 414 (1981) and TM B31 from Annex V of Directive 87/302/EEC.
No clinical signs considered to be treatment-related were seen. One dam at the highest dose was killed when moribund on day 12 of gestation, the condition probably being due to an intubation error. In animals at the highest dose, body-weight development was significantly reduced during treatment, body-weight gain on days 6–11 being 73% of that in controls and that on days 6–16 being 88% of control. The body-weight gain was comparable to that of untreated controls on days 16–21. A slight, transient reduction in food intake was noted during the treatment period in dams receiving 50 and 250 mg/kg bw. No treatment-related macroscopic findings were seen at necropsy of dams.
Treatment had no effect on the mean numbers of corpora lutea, implantation sites, early or late resorptions or post-implantation loss. There were no dead or aborted fetuses. Treatment also had no effect on the number of live fetuses, fetal sex ratio or fetal body weight. No treatment-related external, skeletal or visceral malformations, anomalies or variations were observed among the fetuses.
Metalaxyl-M thus had no embryotoxic or teratogenic potential in rats. The NOAEL for maternal toxicity was 50 mg/kg bw per day, on the basis of a reduction in body-weight gain and feed consumption at a higher dose. The slight, transient reduction in food consumption in animals at 50 mg/kg bw per day was considered not to be adverse as, in that group, no significant effect was noted on body-weight development or any other sign of maternal toxicity. The NOAEL for developmental toxicity was 250 mg/kg bw per day, the highest dose tested (Khalil, 1995).
Metalaxyl (purity, 99.4%) in 2% aqueous CMC was administered by gavage to groups of 25 successfully mated Sprague-Dawley-derived Tif:RAI (SPF) rats on days 6–15 of gestation at a dose of 0, 20, 60 or 120 mg/kg bw per day. The dams were killed on day 21 after conception. The study was performed before GLP guidelines and EEC or OECD test guidelines were enacted. The major deviations from protocol OECD TG 414 (1981) concerned visceral abnormalities, which were observed in only 1/3 fetuses, and the results for body-weight gain and food consumption, which were not outlined in detail. The study was considered to provide only additional information.
The dams at the highest dose showed mild apathy after administration of the substance from day 4 of treatment. In comparison with untreated controls, food consumption was slightly reduced in all treated groups during the first 5 days. A diagram (no numerical data were reported) indicated that the marginal decrease in food consumption of dams at 20 mg/kg bw per day was only transient at day 6. Body-weight gain was slightly depressed at the two higher doses.
The mean numbers of implantations sites and early or late resorptions were similar in all groups. The progeny were largely unaffected by the treatment. No malformations were found in treated groups. In the original report, a marginally increased incidence of slightly retarded ossification of the fifth sternebrae in fetuses at the highest dose was reported to be related to slight maternal toxicity. As the increase was only slight (57% of treated fetuses in comparison with 44% of controls) and the sum of incidences of delayed ossification was not increased at 120 mg/kg bw, a relation to treatment was considered to be doubtful (94% of controls, 79% at the lowest dose, 74% at the intermediate dose and 85% at 120 mg/kg bw). As no effect of treatment was seen on fetal body weight, it is unlikely that growth was retarded at the highest dose.
Metalaxyl had no embryotoxic or teratogenic potential in rats under the experimental conditions of this study. The NOAEL for maternal toxicity was 20 mg/kg bw per day, on the basis of a reduction in body-weight gain at higher doses. The NOAEL for developmental toxicity was 120 mg/kg bw per day, the highest dose tested (Fritz, 1978b).
In a study performed according to Guideline 83-3 of FIFRA Subdivision F and in compliance with the principles of GLP (with QA certification), groups of 27 or 38 (highest dose) mated Charles River COBS CD rats received matalaxyl (purity, 96.8%) in 1% aqueous CMC by gavage at a dose of 0, 50, 250 or (initially) 575 mg/kg bw per day on days 6–15 of gestation. Owing to maternal deaths, the highest dose was reduced to 500 and then to 400 mg/kg bw per day after 2 days of treatment. The surviving dams were killed on day 20 of gestation, and the fetuses were removed surgically for examination. The experimental protocol complied with TM B31 from Annex V of Directive 87/302/EEC.
Overt maternal toxicity was still seen at the two higher doses, evidenced most notably by death, transient convulsions within minutes of dosing, loss of activity, reduced or absent righting reflex and inadequate grooming. Animals at these doses also showed significantly depressed body-weight gain during treatment (90% and 84% of control values at 250 and 400 mg/kg bw, respectively). A slight reduction in mean body-weight gain was also seen in animals at the highest dose throughout gestation (days 0–20: 90% of control value). Food consumption remained comparable in all groups.
No meaningful intergroup differences were seen in fetal parameters; however, some skeletal variations (presacral vertebrae, fewer than normal ribs, reduced ossification of skull, unossified pubic bones) were reported at 400 mg/kg bw per day. The incidences of most were within the range of other controls in the same laboratory, with the exception of unossified pubic bones, which occurred in 14% of the litters and in only 0–6% of other controls.
Metalaxyl had no embryotoxic or teratogenic potential under the experimental conditions of this study. The NOAEL for maternal toxicity was 50 mg/kg bw per day, on the basis of a reduction in body-weight gain at higher doses and clinical signs (including convulsions) at 250 mg/kg bw per day. The NOAEL for developmental toxicity was 400 mg/kg bw per day, the highest dose tested, as the incidence of only one variation was outside the range of other controls in the same laboratory and may have been secondary to maternal toxicity (Leng & Schardein, 1985).
Rabbits
Metalaxyl (purity not specified) suspended in 2% aqueous CMC was administered by gavage to groups of 20 mated Chinchilla SPF rabbits at a dose of 0, 5, 10 or 20 mg/kg bw per day on days 6–18 of gestation. The study was performed before GLP guidelines and EEC or OECD test guidelines were enacted. Major deviations from protocol OECD TG 414 (1981) were found with regard to the descriptions of body-weight gain and food consumption, which were not given in detail. The study was considered to provide only additional information.
Does at the two higher doses reacted to treatment with a dose-related reduction in food consumption and a slight reduction in body-weight gain. Parameters of reproduction were comparable in all groups, and fetal development was not affected. The few instances of malformations observed in the group at 10 mg/kg bw per day (one agenesis of the left kidney and ureter, one hypoplasia of kidneys) and that at 20 mg/kg bw per day (one agenesis of the right kidney and ureter) were considered to be spontaneous and unrelated to treatment. Renal maldevelopment was the most frequent type of malformation found in untreated controls in other studies with the breed of rabbits used in this study.
Metalaxyl did not adversely affect embryonic or fetal development in rabbits under the experimental conditions of this study. No teratogenic potential was found. The NOAEL for maternal toxicity was 5 mg/kg bw per day, on the basis of reductions in body-weight gain and food consumption at higher doses. The NOAEL for developmental toxicity was 20 mg/kg bw per day, the highest dose tested (Fritz & Becker, 1978).
In a study conducted according to Guideline 83-3 of FIFRA Subdivision F and in compliance with the principles of GLP (with QA certification), groups of 18 inseminated Dutch belted rabbits were given metalaxyl (purity, 96.8%) suspended in 1% aqueous CMC by gavage at a dose of 0, 30, 150 or 300 mg/kg bw per day on days 7–19 of gestation. The dams were killed on day 28 of gestation, and the fetuses were removed surgically for examination. The experimental protocol complied with TM B31 from Annex V of Directive 87/302/EEC.
Reduced faecal output was observed for a few rabbits at the highest dose. Animals at this dose lost weight during treatment (average, 71 g), and the overall body-weight gain was 21% lower than the control value throughout the study. Food consumption was similarly reduced in the same group (77% of control value on days 7–20). In the absence of any effect on body weight or food consumption, the slightly decreased body-weight gain (24% lower than control value on days 0–28) in animals at the intermediate dose was considered to be unrelated to treatment. Furthermore, the differences in body-weight gain were more marked before and after the treatment period than during treatment. The variation in body-weight gain might have been due to the slightly reduced initial body-weight and thus to generally reduced body-weight gain in this group.
No treatment-related differences were seen in parameters of reproduction, fetal body weight or the incidences of fetal malformations or variations in treated and control groups.
Metalaxyl had no embryotoxic or teratogenic potential in rabbits under the experimental conditions of this study. The NOAEL for maternal toxicity was 150 mg/kg bw per day, on the basis of reductions in body-weight gain and food consumption at the next highest dose. The NOAEL for developmental toxicity was 300 mg/kg bw per day, the highest dose tested (Laughlin & Schardein, 1984).
The effects of metalaxyl on drug metabolizing enzymes were examined during a study of the kinetics and metabolism of this substance in rats. Five male rats were given metalaxyl orally at a dose of 40 mg/kg bw per day for 7 days or 80 mg/kg bw per day for 3 or 7 days. Controls received 0.6% methylcellulose solution for 3 or 7 days. Phenobarbital was used as a positive control and was administered intraperitoneally at a dose of 80 mg/kg bw per day for 3 days. The animals were fasted for 20 h after the final administration and killed. The livers were homogenized, and the supernatant and microsomal fractions were prepared according to standard methods in order to determine the concentrations and activity of microsomal enzymes.
The effects of metalaxyl and phenobarbital on drug metabolizing enzymes are shown in Table 33. In rats given 40 mg/kg bw per day for 7 days, the activities of cytochrome P450 (CYP), aminopyrine N-demethylase, para-nitroanisole O-demethylase, para-nitrophenol UDP-glucuronyl transferase and dinitrochlorobenzene glutathione transferase were significantly increased compared with controls. Administration of 80 mg/kg bw per day for 3 or 7 days significantly enhanced the activities of all these enzymes and also of NADPH cytochrome C reductase. Administration of phenobarbital for 3 days significantly enhanced the activities of all enzymes examined when compared with controls. Thus, administration of metalaxyl at 40 or 80 mg/kg bw per day orally for 3–7 days significantly increased the concentration and/or the activity of several hepatic enzymes. The content of cytochrome b5 remained unaffected. In comparison with the positive control group treated with phenobarbital, the enzyme-inducing effect of metalaxyl was relatively mild, accounting for about 50% for most of the enzymes (Uesugi, 1988).
Table 33. Effects of metalaxyl and phenobarbital on drug metabolizing enzymes in male rats
|
Parameter |
Control |
Metalaxyl (mg/kg bw per day orally) |
Phenobarbital (intraperitoneally) |
|||
|
3 days |
7 days |
40 for 7 days |
80 for 3 days |
80 for 7 days |
||
|
Body weight (g) |
160 |
200 |
190 |
160 |
180 |
160 |
|
Liver weight (g/100 g bw) |
3.5 |
3.4 |
3.4 |
3.5 |
3.5 |
4.8 |
|
Cytochrome P450 (nmol/mg protein) |
0.54 |
0.55 |
0.65* |
0.77* |
0.73* |
1.6** |
|
Cytochrome b5 (nmol/mg protein) |
0.13 |
0.13 |
0.15 |
0.18 |
0.12 |
0.35* |
|
NADPH-cytochrome C reductase (µmol/min per mg protein) |
0.030 |
0.031 |
0.030 |
0.039* |
0.038* |
0.070** |
|
Aminopyrine N-demethylase (nmol/min per mg protein) |
10 |
10 |
11* |
11* |
11* |
14** |
|
para-Nitroanisole O-demethylase (nmol/min per mg protein) |
0.23 |
0.24 |
0.29 |
0.38** |
0.42** |
0.70** |
|
para-Nitrophenol UDP-glucuronyl transferase (nmol/min per mg protein) |
25 |
25 |
32* |
44** |
36** |
49** |
|
Dinitrochlorobenzene glutathione transferase (µmol/min per mg protein) |
1.1 |
1.1 |
1.3* |
1.3* |
1.4** |
2.2** |
From Uesugi (1988)
* p < 0.05; ** p < 0.01
The ability of metalaxyl to affect specific biomarkers of non-genotoxic co-carcinogenesis was investigated in male and female Swiss Albino CD-1 mice given metalaxyl (purity, 99.5%) dissolved in corn oil as a single intraperitoneal dose of 200 or 400 mg/kg bw or repeated doses of 200 mg/kg bw per day for 3 days. Controls received the vehicle only. Liver, kidney and lung were removed rapidly and processed separately, and a 9000 × g supernatant and microsomal fractions were prepared according to standard methods. The concentration or activity of microsomal enzymes was then determined. The study was considered to provide only additional information.
No significant changes in the absolute or relative weights of the liver, kidney or lung were observed after treatment. Although a single dose did not significantly affect the monooxygenases, a clear example of selective CYP3A induction was recorded in various tissues after repeated treatment. About a threefold increase in CYP3A isoenzymes, as indicated by N-demethylation of aminopyrine, was observed in the liver in both sexes, and about a fivefold increase in the activity of this oxidase was found in kidney. No significant change in the selected biomarkers was observed in lung. A weak but significant reduction in CYP2B1 isoform activity was recorded in the liver of male animals. Liver and kidney CYP3A overexpression was corroborated by western immunoblotting with rabbit polyclonal antibodies to CYP3A1/2. Northern blotting with a CYP3A cDNA biotinylated probe showed that the expression of this isoenzyme in liver is regulated at the mRNA level (Paolini et al., 1996).
The effect of metalaxyl on cardiac activity was investigated in male rats given an intraperitoneal injection of 200, 250 or 300 mg/kg bw. Metalaxyl decreased the heart rate at or near lethal doses (Naidu & Radhakrishnamurty, 1988, 1989).
The metabolism of metalaxyl in plants (Figure 2) and animals is essentially comparable. Studies of acute toxicity, short-term studies of toxicity and studies of genotoxicity have been conducted with the major plant metabolites, which are also the main metabolites found in soil. The metabolites tested were racemates. In potato, metalaxyl was oxidized to benzylic alcohol and benzylic acid, which underwent hydrolysis to the acid metabolite 12 (N-(2-carboxy-6-methylphenyl)-N-methoxyacetyl)alanine). This metabolite was not identified in rats. Metabolite 1(metalaxyl acid) is a primary metabolite of metalaxyl in rats, and metabolite 6 is the major urinary and faecal metabolite of metalaxyl in rats.
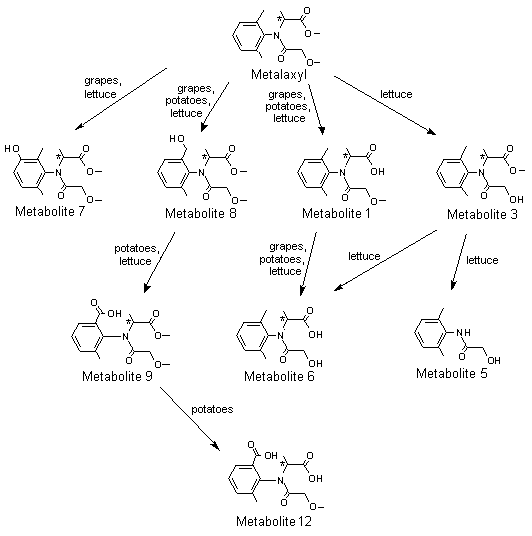
Figure 2. Proposed metabolic pathway of metalaxyl and metalaxyl-M in grapes, potato and lettuce
Metabolite 1, N-(2,6-dimethylphenyl)-N-(methoxyacetyl)alanine; metabolite 3,
N-(2,6-dimethyphenyl)-N-(hydroxyacetyl)alanine methyl ester; metabolite 5,
N-hydroxyacetyl-2,6-dimethylaniline; metabolite 6, N-(2,6-dimethylphenyl)-N-(hydroxyacetyl)alanine;
metabolite 7, N-(2,6-dimethyl-5-hydroxyphenyl)-N-(methoxyacetyl)alanine methyl ester;
metabolite 8, N-(2-hydroxymethyl-6-methylphenyl)-N-(methoxy-acetyl)alanine methyl ester;
metabolite 9, N-(2-carboxy-6-methylphenyl)-N-(methoxyacetyl)alanine methyl ester;
metabolite 12, N-(2-carboxy-6-methylphenyl)-N-methoxyacetyl)alanine
Conjugates not shown
* Chiral centre
Three plant metabolites were tested for acute toxicity; the results are outlined in Table 34. In the study by Winkler (1996b), groups of five male and five female rats were given metabolite 1 (purity, 100%) at a dose of 2000 mg/kg bw (limit test), and the animals were observed for 14 days before sacrifice. The experimental protocol complied with OECD TG 401 (1987). There were no deaths, no remarkable clinical signs and no effect on body weight. At necropsy, no deviations from normal morphology were found in any animal.
In the study of Hartmann (1994), groups of five male and five female rats were given metabolite 12 (purity, 99%) at a dose of 2000 mg/kg bw (limit test), and the animals were observed for 14 days before they were killed. The experimental protocol complied with OECD TG 401 (1987). There were no deaths and no effect on body weight. Piloerection, hunched posture and dyspnoea were seen, but the animals recovered within 3 days. At necropsy, no deviations from normal morphology were found in any animal.
Table 34. Studies of the acute toxicity of metabolites of metalaxyl in male and female Sprague-Dawley-derived Tif:RAI f (SPF) rats
|
Metabolite |
Vehicle |
LD50 (mg/kg bw) |
Reference |
|
Oral administration |
|||
|
1 |
Distilled water |
> 2000 |
Winkler (1996b) |
|
6 |
0.5% CMC in 0.1% aqueous polysorbate 80 |
> 3000 |
Sarasin & Gfeller (1986) |
|
12 |
0.5% CMC in 0.1% aqueous polysorbate 80 |
> 2000 |
Hartmann (1994) |
|
Dermal application |
|||
|
1 |
Distilled water |
> 2000 |
Winkler (1996c) |
|
12 |
Distilled water |
> 2000e |
Winkler (1996d) |
All the studies were conducted according to good laboratory practice with quality assurance.
Metabolite 1: N-(2,6-dimethylphenyl)-N-(methoxyacetyl)alanine; metabolite 6: N-(2,6-dimethylphenyl)-N-(hydroxyacetyl)alanine; metabolite 12: N-(2-carboxy-6-methylphenyl)-N-methoxyacetyl)alanine
CMC, carboxymethylcellulose
In the study of Sarasin & Gfeller (1986), groups of five male and five female rats were given metabolite 6 (purity, 95%) at a dose of 300, 1000 or 3000 mg/kg bw and were observed for 14 days before being killed. The experimental protocol was not fully in compliance with OECD TG 401, as body weights were not measured and necropsy was not performed. The study was nevertheless found to be acceptable. There were no deaths. Dyspnoea, exophthalmia, ruffled fur and curved body weight position were seen, and animals at the highest dose showed sedation 3–5 h after the administration. The animals recovered within 10–12 days.
In the study of Winkler (1996c), groups of five male and five female rats were given metabolite 1 (purity, 100%) at a dose of 2000 mg/kg bw (limit test) and were observed for 14 days before sacrifice. The experimental protocol was in compliance with OECD TG 402 (1987). There were no deaths, no treatment-related clinical signs and no effect on body weight. There were no remarkable findings at the site of skin application. At necropsy, no deviations from normal morphology were found in any animal.
In the study of Winkler (1996d), groups of five male and five female rats were given metabolite 12 (purity, 99%) at a dose of 2000 mg/kg bw (limit test) and were observed for 14 days before sacrifice. The experimental protocol was in compliance with OECD TG 402 (1987). There were no deaths and no treatment-related clinical signs. Slight local erythema at the application site was recorded in one of five males from day 5 through day 11 and in two of five females on days 5 and 6 after treatment. Body-weight loss was recorded in one female during the second week after treatment. At necropsy, no deviations from normal morphology were found in any animal.
Thus, these metabolites had little acute toxicity after oral or dermal administration in rats. No deaths occurred after oral administration at doses that significantly exceeded the LD50 of the parent compound, metalaxyl.
In a study conducted in compliance with the principles of GLP (with QA certification), groups of five male and five female Sprague-Dawley-derived Tif:RAIf (SPF) rats, hybrids of RII/1 × RII/2, were given metabolite 12 (purity, 99%) suspended in water containing 0.5% CMC and 0.1% Tween 80 by gavage at a dose of 0, 10, 50, 200 or 1000 mg/kg bw per day for 28 days. An additional group of five rats of each sex from the control and the highest-dose groups were kept for a 4-week recovery period before sacrifice. Overt signs of toxicity were recorded daily, and body weight, food consumption and water consumption were recorded weekly throughout the study. Detailed clinical observations were performed before treatment and once weekly thereafter. A battery of functional observational tests (FOB) and motor activity tests were conducted at week 4 and 8 (recovery groups only). Haematological, blood chemical and urine analyses were carried out on all surviving animals at the end of treatment and on animals kept for evaluation of reversibility. After they were killed, the animals were examined macroscopically, and organ weights were recorded. Organs and tissues were collected and prepared for histopathological evaluation. The protocol complied with OECD TG 407 (1995) and TM B7 from Annex V of Directive 92/69/EEC.
Daily and weekly clinical observations showed no relevant changes related to treatment. There were no deaths attributable to treatment. Body-weight gain and food and water consumption were similar in treated and control groups. Haematological examination revealed no changes that could be attributed to treatment. Slightly increased plasma glucose and potassium concentrations were recorded in males at the highest dose after 4 weeks of treatment, but these findings were completely reversed after the recovery period. Urine analysis showed a reduced pH value for animals at the highest dose, but after the recovery period no differences were noted between treated rats and controls. No treatment-related changes were found in the FOB or motor activity tests.
At the end of treatment, the mean and relative weights of the heart were slightly increased (6% and 11%, respectively) in males at the highest dose. This finding was reversed within the 4-week recovery period. Macroscopic and microscopic examination revealed no treatment-related changes.
Treatment with metabolite 12 was well tolerated up to the limit dose of 1000 mg/kg bw per day. Only minor and completely reversible changes were observed at the highest dose, which were considered not to be adverse in the absence of any histopathological findings. The NOAEL was 1000 mg/kg bw per day (Gerspach, 1997).
In a study conducted in compliance with the principles of GLP (with QA certification), groups of five male and five female Sprague-Dawley-derived Tif:RAIf (SPF) rats, hybrids of RII/1 × RII/2, were given metabolite 1 (purity, 100%) suspended in water containing 0.5% CMC and 0.1% Tween 80 by gavage at a doses of 0, 10, 50, 200 or 1000 mg/kg bw per day for 28 days. An additional group of five rats of each sex from the control and the highest-dose groups were kept for a 4-week recovery period before sacrifice. Overt signs of toxicity were recorded daily, and body weight, food consumption and water consumption were recorded weekly throughout the study. Detailed clinical observations were performed before treatment and once weekly thereafter. FOB and motor activity tests were conducted at weeks 4 and 8 (recovery groups only). Haematology, blood chemistry and urine analysis were carried out on all surviving animals at the end of the treatment period and at the end of the recovery period on animals kept for evaluation of reversibility. At termination, animals were examined macroscopically, and organ weights were recorded. Organs and tissues were collected and prepared for histopathological evaluation. The protocol complied with OECD TG 407 (1995) and TM B7 from Annex V of Directive 92/69/EEC.
Daily and weekly clinical observations revealed no change of toxicological relevance. No deaths occurred. Body-weight gain and food and water consumptions were similar in treated and control groups. Haematological, blood chemical and urine analyses revealed no changes that could be attributed to treatment. No treatment-related changes were found in the FOB and motor activity tests.
At the end of treatment, the relative weight of the liver in males at the highest dose was minimally increased (by 7%). A tendency to increased liver weights was also noted in females at 50 and 1000 mg/kg bw per day (by 7% and 6%, respectively). These changes were reversed within the recovery period. No treatment-related changes were observed at necropsy. Microscopic examination showed increased incidences of minimal hypertrophy of liver hepatocytes in males at 200 and 1000 mg/kg bw per day and in females at 50, 200 and 1000 mg/kg bw per day. This treatment-related effect disappeared during the recovery period.
Treatment with metabolite 1 was well tolerated, with no signs of overt toxicity. There was no indication that metabolite 1 has neurotoxic potential. The changes in the liver indicate a weak hepatotrophic effect of metabolite 1 in rats. However, in view of the minimal degree of these effects and their complete reversibility, no toxicological importance was attributed to these findings. The NOAEL was 1000 mg/kg bw per day (Frankhauser, 1997).
The genotoxic potential of metabolites 1 and 12 was assessed in several tests in vitro (see Table 35). Both metabolites gave negative results in the presence and absence of metabolic activation.
Table 35. Results of studies of genotoxicity performed with metabolites of metalaxyl
|
End-point |
Test object |
Concentration |
Purity (%) |
Results |
Reference |
|
Metabolite 12 |
|||||
|
Reverse mutation |
S. typhimurium TA98, TA100, TA102, TA1535, TA1537; E. coli WP2 uvrA |
310–5000 µg/plate in DMSO |
99 |
Negativea,b |
Ogorek (1997) |
|
Forward mutation |
Chinese hamster V79 cells hprt locus |
(1) + S9: 74–2000 µg/ml |
99 |
Negativea,c |
Deparade (1998) |
|
(2) + S9: 56–1500 µg/ml |
|||||
|
(3) + S9: 400–1350 µg/ml |
|||||
|
Solvent, DMSO |
|||||
|
Forward mutation |
Mouse lymphoma L5178Y cells, Tk locus |
± S9: 500–3000 µg/ml in DMSO |
99 |
Negativea,d |
Clay (2001) |
|
Chromosomal aberration |
Chinese hamster V79 cells |
750, 1500, 3000 µg/ml in DMSO |
99% |
Negativeae |
Czich (2001) |
|
+ S9: (1) 4-h exposure, harvesting 14 h later |
|||||
|
(2) 18-h exposure, direct harvesting |
|||||
|
(3) 28-h exposure, direct harvesting |
|||||
|
– S9: (1) 4-h exposure, harvesting 14 h later |
|||||
|
(2) 4-h exposure, harvesting 24 h later |
|||||
|
Metabolite 1 |
|||||
|
Reverse mutation |
S. typhimurium TA98, TA100, TA102, TA1535, TA1537; E. coli WP2 uvrA |
310–5000 µg/plate in DMSO |
100 |
Negativea,b |
Deparade (1997) |
|
Forward mutation |
Chinese hamster V79 cells, Hprt locus |
First experiment: |
100 |
Negativea, e |
Ogorek (1998) |
|
Second experiment: |
|
All the studies were conducted in accordance with good laboratory practice and quality assurance. |
|
|
S9, exogenous metabolic activation system from 9000 × g fraction of liver from rats induced with Aroclor; DMSO, dimethyl sulfoxide |
|
|
Positive control substances were used in all assays and gave the expected results. |
|
|
Metabolite 1: N-(2,6-dimethylphenyl)-N-(methoxyacetyl)alanine; metabolite 12: N-(2-carboxy-6-methylphenyl)-N-methoxyacetyl)alanine |
|
|
a |
With and without metabolic activation |
|
b |
Experimental protocol in compliance with OECD TG 471 (1997). The original experiment with and without metabolic activation and the confirmatory experiment without activation were performed as standard plate incorporation assays. The confirmatory experiment with metabolic activation was carried out as preincubation assay. |
|
c |
Experimental protocol in compliance with OECD TG 476 (1997) |
|
d |
Experimental protocol in compliance with OECD TG 476 (1997). Metabolite 12 was tested up to a maximum concentration of 3000 µg/ml, approximately equivalent to 10 mmol/l, the limit concentration for this assay. No significant toxicity was observed in cultures treated with this dose, in the presence or absence of S9 . |
|
e |
Experimental protocol in compliance with OECD TG 476 (1997) |
Routine medical examinations, including anamnesis, physical examination covering blood pressure and comprehensive blood and urine analysis, of employees who had handled metalaxyl-M since 1999 in laboratories, production and formulation plants or field trial projects have revealed no adverse effects. Apart from local dermal effects, no compound-related adverse effects on human health were reported in workers involved in the production of metalaxyl between 1986 and 1994.
No cases of poisoning have been reported in the open literature involving workers in the production and formulation or field use of metalaxyl or metalaxyl-M. Apart from its potential for ocular irritation, metalaxyl-M has little acute toxicity. In studies in experimental animals, the symptoms of acute intoxication were nonspecific and transient.
Investigations with the R-enantiomer were confined to studies of its absorption, distribution, metabolism and excretion, acute and short-term toxicity, mutagenicity and developmental toxicity, and were designed to establish whether there are qualitative or quantitative differences in the toxicological properties of metalaxyl-M and metalaxyl. As described below, none of the studies revealed any unexpected effects of metalaxyl-M, and the quantitative dose–effect relationships found with the racemate and the R-enantiomer were similar. The Meeting therefore concluded that the database on metalaxyl could be used for the toxicological evaluation of metalaxyl-M. Since the previous evaluation of metalaxyl by the Joint Meeting, several new studies have been conducted with the racemate. The present Meeting reviewed the available studies, consisting of the original studies and new studies on absorption, distribution, metabolism and excretion, a 2-year study in dogs and studies of developmental toxicity in rats and rabbits.
All the studies with metalaxyl-M and all the pivotal studies with metalaxyl were certified as being compliant with good laboratory practice.
Studies of the biokinetics and metabolism of both metalaxyl and metalaxyl-M have been performed. The absorption, distribution and excretion of the two compounds were similar, and both were rapidly absorbed and eliminated after oral administration. In rats, maximum blood concentrations were detected 0.5–1 h after administration. The decline in radioactivity was biphasic, with half-lives ranging from 1 to 3 h and 22 to 125 h (depending on the dose) for the first and second phases, respectively. Rats eliminated 90–100% of the total administered dose of either substance within 72 h, with the majority eliminated within 24 h. The rate of urinary excretion of radioactivity was higher in females than in males, whereas the faecal elimination rate was higher in males than in females. The similarity of the excretion pattern of radioactivity after oral and intravenous administration of metalaxyl indicates that the compound was probably well absorbed. Elimination of metalaxyl in the bile was substantial in a study with bile duct-cannulated rats, accounting for 55–70% of the total administered radioactivity (average bioavailability, approximately 90% after oral administration). The concentrations of residues of both compounds in organs and tissues were generally low, reflecting the rapid elimination.
Metalaxyl and metalaxyl-M were both extensively metabolized, showing a similar pattern of metabolites, irrespective of sex and administered dose. The profile of metabolites of metalaxyl was quantitatively similar in all three species studied (rats, goats and hens). Metabolism involved hydrolysis of side-chains and oxidation of the phenyl ring. Most of the phase I metabolites were excreted as conjugates with glucuronic acid and sulfate. Treatment with metalaxyl resulted in modest induction of hepatic and renal cytochrome P450 and some other drug metabolizing enzymes.
The LD50 values in rats treated orally with metalaxyl or metalaxyl-M were 670 and 380–950 mg/kg bw, respectively. The LD50 value in rats after dermal application of either substance was > 2000 mg/kg bw. The LC50 values in rats treated by inhalation for 4 h were > 3.6 mg/l and > 2.3 mg/l, the highest achievable concentrations, for metalaxyl and metalaxyl-M, respectively. Neither substance was irritating to the skin of rabbits, nor did they sensitize the skin of guinea-pigs. Metalaxyl-M was considered to be a severe irritant to the eyes of rabbits, whereas metalaxyl was only slightly irritating. Metalaxyl has been classified by WHO as ‘slightly hazardous’ (WHO, 2000); metalaxyl-M has not been classified.
Metalaxyl-M and metalaxyl showed similar toxicological properties. A comparative 28-day study in rats given metalaxyl-M and metalaxyl by gavage confirmed the toxicological equivalence of the R-enantiomer and the racemate, as the nature of the effects as well as the dose–effect relationships were similar. In studies in mice, rats and dogs treated orally, both substances had low toxicity, and treatment was well tolerated, even at relatively high doses.
The available data indicated that the major target organ is the liver and that the dog is the most sensitive species. Increased absolute and relative liver weights were observed in rats and dogs. Both substances caused hepatocellular enlargement in rats, while dogs showed changes in blood biochemical parameters indicative of hepatocellular damage (increased serum activity of alkaline phosphatase). Mild effects observed in the liver of rodents were considered not to be adverse.
After treatment with metalaxyl for 6 months, dogs showed slightly reduced erythrocyte parameters (red blood cell count, erythrocyte volume fraction and haemoglobin concentration), while no significant haematological effects were detected with metalaxyl-M in a 3-month study.
In 90-day studies in rats given metalaxyl-M or metalaxyl, the NOAEL was 1200 ppm, the highest dose tested, equal to 91 or 79 mg/kg bw per day, respectively. In dogs, the NOAELs were 250 ppm, equal to 7.3 mg/kg bw per day, in a 13- week study with metalaxyl-M and 250 ppm, equal to 7.4 mg/kg bw per day, in a 6-month study with metalaxyl, on the basis of increased alkaline phosphatase activity and liver weights at 1200 ppm of metalaxyl-M and 1000 ppm of metalaxyl.
Treatment of dogs for 2 years at the high dose of 80 mg/kg bw per day resulted in transient clinical signs and the deaths of two of six males and two of six females. The surviving animals showed mild anaemia starting after about 52 weeks of treatment (considered not to be relevant for acute intake) and elevated serum activities of alkaline phosphatase and alanine aminotransferase. In addition, increased liver (both sexes) and kidney (males) weights were noted. The NOAEL was 8 mg/kg bw per day on the basis of effects observed at 80 mg/kg bw per day.
Long-term studies of toxicity and carcinogenicity were conducted with metalaxyl in mice and rats. Male mice showed reduced body-weight gain at a dietary concentration of 1200 ppm (equal to 100 mg/kg bw per day), so that the NOAEL was 250 ppm, equal to 19 mg/kg bw per day. There was no evidence of a carcinogenic response to treatment. In rats, increased absolute and relative liver weights were recorded at a dietary concentration of 1200 ppm (equal to 43 mg/kg bw per day) in animals of each sex and a slight increase in relative liver weight in males at 250 ppm (equal to 8.7 mg/kg bw per day). In the group at the highest dose, histopathological examination revealed centrilobular hepatocyte enlargement and slightly increased incidences of fatty infiltration of liver cells in females. As the findings in the liver were mild and considered unlikely to be adverse, the NOAEL was 43 mg/kg bw per day. There was no evidence of a carcinogenic response to treatment. Since technical-grade metalaxyl contains approximately 50% of the R-enantiomer, the results also apply to metalaxyl-M. The Meeting concluded that metalaxyl and metalaxyl-M are not carcinogenic in rodents.
A comprehensive range of studies of genotoxicity with both metalaxyl and metalaxyl-M gave negative results. The Meeting concluded that neither metalaxyl nor metalaxyl-M is likely to be genotoxic.
In the absence of genotoxic and carcinogenic potential, the Meeting concluded that neither metalaxyl nor metalaxyl-M is likely to pose a carcinogenic risk to humans.
In a three-generation study of reproductive toxicity in rats with metalaxyl, the NOAEL for parental and pup toxicity and for reproductive performance was 1200 ppm, equal to 96 mg/kg bw per day, the highest dose tested. Four studies of developmental toxicity were performed with metalaxyl, two in rats and two in rabbits. They gave no indication of teratogenic or embryotoxic potential, even when the material was administered at a dose close to that which caused maternal lethality. In rats, the NOAELs were 50 mg/kg per day and 400 mg/kg per day (the highest dose tested) for maternal and developmental toxicity, respectively. In rabbits, the NOAELs were 150 mg/kg per day and 300 mg/kg per day (the highest dose tested) for maternal and developmental toxicity, respectively. In view of the similarity of the effects of the two substances and the lack of developmental or reproductive toxicity with metalaxyl, investigation of metalaxyl-M was confined to a study of developmental toxicity in rats. In this study, treatment of pregnant rats at maternally toxic doses had no adverse effect on the pups. The NOAELs were 50 mg/kg bw per day for maternal toxicity and 250 mg/kg bw per day (highest dose tested) for developmental toxicity.
The metabolism of metalaxyl and metalaxyl-M is similar in animals and plants. The acute toxicity of the three major plant metabolites of metalaxyl, N-(2-6-dimethylphenyl)-N-(methoxyacetyl)alanine (M1), N-(2,6-dimethylphenyl)-N-(hydroxyacetyl)alanine (M6) and N-(2-carboxy-6-methylphenyl)-N-methoxyacetyl)alanine (M12), was studied after oral administration. These metabolites showed little toxicity, with LD50 values > 2000 mg/kg bw and NOAELs in 28-day studies > 1000 mg/kg bw per day, the highest dose tested. They also had no mutagenic potential in vitro. On the basis of these results and on the fact that all major plant metabolites except M12 also occur in rats and have thus been investigated in toxicological studies, the Meeting concluded that the plant metabolites are of no toxicological concern for humans.
No cases of adverse effects were reported in personnel involved in the production and formulation of metalaxyl or metalaxyl-M or in the field use of these products.
The Meeting concluded that there were sufficient toxicological data to assess both metalaxyl and metalaxyl-M. It also concluded that the existing database was adequate to characterize the potential hazard of metalaxyl and metalaxyl-M to fetuses, infants and children.
The Meeting established a group ADI of 0–0.08 mg/kg bw for metalaxyl and metalaxyl-M (alone or in combination), on the basis of the NOAEL of 8 mg/kg bw per day in the 2-year study in dogs with metalaxyl and a safety factor of 100.
The Meeting concluded that it was not necessary to establish an acute RfD because metalaxyl and metalaxyl-M have little acute toxicity and, in studies with repeated doses, no toxicological alerts for acute effects were observed that might indicate the need to establish one.
|
Levels relevant to risk assessment |
||||
|
Species |
Study |
Effect |
NOAEL |
LOAEL |
|
Mouse |
2-year study of toxicity and carcinogenicitya,e |
Toxicity |
250 ppm, equal to 19 mg/kg bw per day |
1250 ppm, equal to 100 mg/kg bw per day |
|
Carcinogenicity |
1250 ppm, equal to 100 mg/kg bw per dayd |
– |
||
|
Rat |
2-year study of toxicity and carcinogenicitya,e |
Toxicity |
1250 ppm, equal to 43 mg/ kg bw per dayd |
– |
|
Carcinogenicity |
1250 ppm, equal to 43 mg/kg bw per dayd |
– |
||
|
Three-generation study of reproductive toxicitya,e |
Parental, pup and reproductive toxicity |
1250 ppm, equal to 96 mg/kg bw per dayd |
– |
|
|
Developmental toxicityb,f |
Maternal toxicity |
50 mg/kg bw per day |
250 mg/kg bw per day |
|
|
Embryo- and fetotoxicity |
250 mg/kg bw per dayd |
– |
||
|
Rabbit |
Developmental toxicityb,e |
Maternal toxicity |
150 mg/kg bw per day |
300 mg/kg bw per day |
|
Embryo- and fetotoxicity |
300 mg/kg bw per dayd |
|||
|
Dog |
2-year study of toxicityc,e |
Toxicity |
8 mg/kg bw per day |
80 mg/kg bw per day |
a Dietary administration
b Gavage
c Gelatine capsule
d Highest dose tested
e Study performed with metalaxyl
f Study performed with metalaxyl-M
Estimate of acceptable daily intake for humans
0–0.08 mg/kg bw (group ADI for metalaxyl and metalaxyl-M, alone or in combination)
Estimate of acute reference dose
Unnecessary
Studies that would provide information useful for continued evaluation of the compound
Further observations in humans
List of end-points relevant for setting guidance values for dietary and non-dietary exposure
|
Absorption, distribution, excretion and metabolism in mammals |
|
|
Rate and extent of oral absorption |
Rapid and extensive |
|
Dermal absorption |
10% for spraying dilution and for concentrated EC formulation (in vivo and in vitro data) |
|
Distribution |
Uniformly distributed |
|
Potential for accumulation |
None |
|
Rate and extent of excretion |
Rapid and extensive |
|
Metabolism in animals |
Extensively metabolized |
|
Toxicologically significant compounds |
Parent compounds |
|
Acute toxicity |
|
|
Rat, LD50, oral |
380 mg/kg bw (metalaxyl-M) |
|
670 mg/kg bw (metalaxyl) |
|
|
Rat, LD50 , dermal |
> 2000 mg/kg bw (metalaxyl-M) |
|
> 3200 mg/kg bw (metalaxyl) |
|
|
Rat, LC50, inhalation |
> 2.3 mg/L (4 h, nose-only, aerosol) (metalaxyl-M) |
|
> 3.6 mg/L (4 h) (metalaxyl) |
|
|
Skin irritation |
Not irritating (4 h, rabbit) (metalaxyl-M) |
|
Not irritating (24 h, rabbit) (metalaxyl) |
|
|
Eye irritation |
Severely irritating (rabbit) (metalaxyl-M) |
|
Slightly irritating (rabbit) (metalaxyl) |
|
|
Skin sensitization |
Not sensitizing (Magnusson & Kligman or Buehler) (metalaxyl-M) |
|
Not sensitizing (Mauer or Buehler) (metalaxyl) |
|
|
Short-term studies of toxicity |
|
|
Target/critical effect |
Liver |
|
Lowest relevant oral NOAEL |
8 mg/kg bw per day (dog; 13 weeks with metalaxyl-M and 6 months and 2 years with metalaxyl) |
|
Lowest relevant dermal NOAEL |
1000 mg/kg bw per day (highest dose tested, 4 weeks with metalaxyl-M in rats and 3 weeks with metalaxyl in rabbits) |
|
Lowest relevant inhalation NOAEL |
No relevant data |
|
Genotoxicity |
Not genotoxic |
|
Long-term studies of toxicity and carcinogenicity |
|
|
Target/critical effect |
Liver |
|
Lowest relevant NOAEL |
250 ppm, equal to 19 mg/kg bw per day (2-year study with metalaxyl in mice) |
|
Carcinogenicity |
Not carcinogenic |
|
Reproductive toxicity |
|
|
Reproductive toxicity target/critical effect |
No reproductive effects observed |
|
Lowest relevant NOAEL for reproductive toxicity |
Parents, pups and reproduction: 1250 ppm (equal to 96 mg/kg bw per day, highest dose tested; 3-generation study with metalaxyl in rats) |
|
Developmental toxicity target/critical effect |
No developmental effects at maternally toxic doses |
|
Lowest relevant NOAEL for developmental toxicity |
Maternal: 50 mg/kg bw per day |
|
Embryo- and fetotoxicity: 250 mg/kg bw per day (highest dose tested, metalaxyl-M in rats) |
|
|
Neurotoxicity |
No concerns arising from available information |
|
Other toxicological studies |
Metalaxyl is a mild inducer of xenobiotic metabolizing enzymes in liver and kidney |
|
Toxicity of metabolites |
No toxicological concern |
|
Medical data |
No adverse effects on health of manufacturing personnel |
|
Summary |
Value |
Study |
Safety factor |
|
ADI |
0–0.08 mg/kg bw |
2 years in dogs |
100 |
|
Acute RfD |
Unnecessary |
Altmann, B. (1995) 3-months subchronic dietary toxicity study in beagle dogs. Unpublished report No. 943128, dated 7 August 1995, from Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Arcelin, G. (1991) Contact hypersensitivity to CGA 48988 tech. In albino guinea pigs—Modified Buehler method. Unpublished report dated 10 December 1991 from RCC Research & Consulting Company AG, Itingen, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Arni, P. & Müller, D. (1982) Saccharomyces cerevisiae D7/mammalian-microsome mutagenicity test in vitro with CGA 48988 (test for mutagenic properties in yeast cells). Unpublished report No. 811561, dated 18 January 1982 from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Arts, J.H.E. (1995) Acute (4 hour) inhalation toxicity study with CGA 329351 in rats. Unpublished report No. V94.692, dated 3 January 1995 from TNO Nutrition and Food Research Institute, Zeist, Netherlands. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Ashby, R. & Whitney, J.C. (1980) CGA 48988—Toxicity and oncogenicity in dietary administration to rats for two years. Unpublished report No. 80/CIA009/315, dated 23 September 1980, from Life Science Research Ltd, Eye, Suffolk, England. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Ashby R. & Whitney J.C. (1980a) Evaluation of C-cell (parafollicular cell) adenomas of the thyroid occurring in the study with CGA 48988: Toxicity and oncogenicity in dietary administration to rats for two years. Addendum to unpublished report No. 80/CIA009/315, 23.09.1980 from Life Science Research Ltd, Eye, Suffolk, England. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Beck, L.S. & deWard, J. (1981) Six month chronic oral toxicity study with CGA 48988 technical in beagle dogs. Unpublished report No. 1545, dated 20 January 1981, from Elars Bioresearch Laboratories, USA. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Caldwell, J. (1995) Stereochemical determinants of the nature and consequences of drug metabolism. J. Chromatogr. A, 694, 39–48.
Calkins, J.E. (1980) A 21-day subacute dermal toxicity study in albino rabbits with CGA 48988 technical. Unpublished report No. 410-0226, dated 14 November 1980 from Ciba-Geigy Corp., Greensboro, USA. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Clay, P. (2001) CGA 108906 (metabolite of CGA 329351)—L5178Y TK+/– mouse lymphoma mutation assay. Unpublished report No. VV0261/20003082, dated 29 March 2001, from Central Toxicology Laboratory, Cheshire, England. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Coate, W.B. (1982) Subchronic inhalation study in rats CGA 48988 treated cigarettes. Unpublished report No. 483-202, dated 18 January 1982 from Hazleton Laboratories, Madison, Wisconsin, USA. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Cozens, D. (1980) Effect of CGA 48988 on reproductive function of multiple generations in the rat. Unpublished report No. CBG 181/80254, dated 13 August 1980 from Huntingdon Research Centre Ltd, Huntingdon, England. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Czich, A. (2001) In vitro chromosome aberration test in Chinese hamster V79 cells with CGA 108906 tech. (metabolite of CGA 329351). Unpublished report No. 684301/20003080, dated 29 March 2001, from RCC Cytotest Cell Research GmbH, Rossdorf, Germany. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Deparade E. (1997) Salmonella and Escherichia/mammalian-microsome mutagenicity test. Unpublished report No. 963102, dated 10 January 1997, from Novartis Crop Protection AG, Basel, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Deparade, E. (1998) Gene mutation test with Chinese hamster cells V79. Unpublished report No. 983071, dated 3 December 1998 from Novartis Crop Protection AG, Basel, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Deparade, E. (1999) CGA329351 tech.—Micronucleus test, mouse. Unpublished report No. 993096, dated 27 October 1999 from Novartis Crop Protection AG, Basel, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Deparade, E. & Arni, P. (1985) Salmonella/mammalian-microsome mutagenicity test with CGA 48988 techn. Unpublished report No. 851007, dated 18 November 1985 from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Draize, J.H. (1977) Dermal and eye toxicity tests. In: Principles and Procedures for Evaluating the Toxicity of Household Substances, Washington DC: National Academy of Sciences, pp. 48–49.
Drake, J.C. (1977) 3 months dietary study in rats with compound CGA 48988. Unpublished report No. 8/77/SL, dated 25 February 1977, from Geigy Pharmaceuticals, Stamford Lodge, England. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Emrani, J. (1990) Metabolism of [phi-14C]-metalaxyl in goats. Unpublished report No. ABR-90078, dated 10 October 1990, from Ciba-Geigy Corp., Greensboro, USA. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Emrani, E.J. (1991) Supplemental report on the metabolism of [phi-14C]-metalaxyl in goats. Unpublished report No. ABR-91075, dated 14 November 1991, from Ciba-Geigy Corp., Greensboro, USA. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Faccini, J.M. (1985) Evaluation of the livers of the 2-year carcinogenicity study on rats with CGA 48988. Addendum to unpublished report No. 80/CIA009/315, dated 24 May 1985 from Robens Institute of Industrial Health and Safety, Surrey University, Guildford, Surrey, England. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Fankhauser, H. (1997) 28-days subacute, oral toxicity study in rats (gavage). Unpublished report No. 963103, dated 21 May 1997, from Novartis Crop Protection AG, Stein, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Fritz, H. (1978a) Dominant lethal study CGA 48988 technical mouse (test for cytotoxic or mutagenic effects on male germinal cells). Unpublished report No. 327761, dated 23 February 1978, from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Fritz, H. (1978b) Reproduction study CGA 48988 tech. rat seg. II (test for teratogenic or embryotoxic effects). Unpublished report No. 227716, dated 7 February 1978, from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Fritz, H. & Becker, H. (1978) Reproduction study—rabbit—CGA 48988 tech. seg. II (test for teratogenic or embryotoxic effects). Unpublished report No. 784868, dated 6 December 1978, from Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Gerspach, R. (1994) 28 days subacute, oral toxicity study in rats (gavage). Comparison of toxicity profiles of CGA 329351 tech. and CGA 48988 tech. Unpublished report No. 933180, dated 26 August 1994, from Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Gerspach, R. (1995) 3-month oral toxicity study in rats (administration in food). Unpublished report No. 943127, dated 9 August 1995, from Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Gerspach, R. (1997) 28-days subacute, oral toxicity study in rats (gavage). Unpublished report No. 963128, dated 6 June 1997, from Novartis Crop Protection AG, Stein, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Gerspach, R. (1998) 4-week repeated dose dermal toxicity study in rats. Unpublished report No. 973094, dated 1 September 1998, from Novartis Crop Protection AG, Stein, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Gfeller, W. (1980) 3 month toxicity study on rats with technical CGA 48988. Unpublished report No. 791811, dated 29 December 1980, from Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Glaza, S.M. (1995) Dermal sensitization study of CGA 329351 technical in guinea pigs—closed patch technique. Unpublished report No. CHW 50400231, dated 18 July 1995 from Hazleton Laboratories, Madison, Wisconsin, USA. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Hamboeck, H. (1977) Distribution, degradation and excretion of CGA 48988 in the rat. Unpublished report No. 18/77, dated 6 April 1977, from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Hamboeck, H. (1978) Metabolism of CGA 48988 in the rat. Unpublished report No. 26/78, dated 12 May 1978, from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Hamboeck, H. (1981a) Sex dependency of CGA 48988 metabolism in rats. Unpublished report No. 33/81, dated 23 November 1981 from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Hamboeck, H. (1981b) Metabolic pathways of CGA 48988 in the rat. Unpublished report No. 31/81, dated 7 October 1981 from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Harada, T. (1984) CG 117 (CGA 48988)—24-month oral chronic toxicity study in dogs. Unpublished report dated 6 October 1984, from Institute of Environmental Toxicology, Japan. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Hartmann, H.R. (1992) Unpublished report from Ciba-Geigy Ltd, Basel, Switzerland. Submitted by Ciba-Geigy to Portugal (Rapporteur Member State) in context of Directive 91/414/EEC and reported in the draft monograph report prepared by Portugal in 2000.
Hartmann, H.R. (1994) Acute oral toxicity in the rat. Unpublished report No. 943085, dated 25 August 1994, from Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Haschek, W.M. & Rousseaux, C.G. (1991) Handbook of Toxicologic Pathology, San Diego, California: Academic Press, pp. 279–294.
Hertner, T. (1994a) Salmonella and Escherichia/mammalian-microsome mutagenicity test. Unpublished report No. 943012, dated 17 August 1994, from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Hertner, T. (1994b) Cytogenetic test on Chinese hamster cells in vitro. Unpublished report No. 943013, dated 29 June 1994, from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Hertner, T. & Arni, P. (1992) Micronucleus test in mouse. Unpublished report No. 923096, dated 17 December 1992, from Ciba-Geigy Basel, Genetische Toxikilogie, Basel, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Hrelia, P., Maffei, F., Fimognari, C., Vigagni, F. & Cantelli-Forti, G. (1996) Cytogenetic effects of metalaxyl on human and animal chromosomes. Mutat. Res., 369, 81–86.
Itterly, W. (1990) Characterization and identification of phenyl-[14C]-metalaxyl metabolites in rats. Unpublished report No. ABR-90079, dated 19 October 1990, from Ciba-Geigy Corp., Greensboro, USA. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Ivett, J.L. (1986) Clastogenic evaluation of metalaxyl technical CGA 48988 tech. in an in vitro cytogenetic assay measuring chromosomal aberration frequencies in Chinese hamster ovary (CHO) cells. Unpublished report No. 20990, dated 18 March 1986, from Hazleton Laboratories, Madison, Wisconsin, USA. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Jameson, C.E. (1990) Rat metabolism studies: Tissues and excreta. Unpublished report No. ABC 37613, dated 20 August 1990, from Analytical Bio-Chemistry Lab. Inc., Columbia, USA. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Kennedy, E. (1990) Metabolism of [phi-14C]-metalaxyl in hens. Unpublished report No. ABR-90077, dated 12 October 1990, from Ciba-Geigy Corp., Greensboro, USA. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Kennedy, E. (1991) Supplemental report on the metabolism of [phi-14C]-metalaxyl in hens. Unpublished report No. ABR-91077, dated 14 November 1991, from Ciba-Geigy Corp., Greensboro, USA. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Khalil, S. (1995) Rat oral teratogenicity. Unpublished report No. 943129, dated 3 May 1995, from Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Langauer, M. & Müller, D. (1979) Nucleus anomaly test in somatic interphase nuclei CGA 48988 Chinese hamster (test for mutagenic effects on bone marrow cells). Unpublished report No. 783007, dated 18 June 1979, from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Laughlin, K.A. & Schardein, J.L. (1984) CGA 48988 techn—Teratology study in rabbits. Unpublished report No. 382-098, dated 4 December 1984, from International Research and Development Corp., Mattawan, USA. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Leng, J.M. & Schardein, J.L. (1985) CGA 48988 techn—Teratology study in rats. Unpublished report No. 382-100, dated 3 January 1985, from International Research and Development Corp., Mattawan, USA. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Marco, J.J. (1978) Balance and metabolism of [phi-14C]-CGA 48988 in a lactating goat. Unpublished report No. ABR-78046, dated 24 May 1978, from Ciba-Geigy Corp., Greensboro, USA. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Marty, J.H. (1994a) Acute dermal irritation/corrosion study in the rabbit. Unpublished report No. 943028, dated 17 May 1994, from Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Marty, J.H. (1994b) Acute eye irritation/corrosion study in the rabbit. Unpublished report No. 943029, dated 1 June 1994 from Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Marty, J. (1994c) Skin sensitization test in the guinea pig—maximisation test. Unpublished report No. 943030, dated 16 August 1994, from Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Mauer, T., Thomann, P., Weirich, E.G. & Hess, R. (1975) The pptimization test in the guinea pig. A method for the predictive evaluation of the contact allergenicity of chemicals. Agents Actions, 5, 174–179.
McSheehy, T.W., Macrae, S.M. & Whitney, J.C. (1980a) Oncogenicity in dietary administration to mice for two years with CGA 48988 technical. Unpublished report 80/CIA008/442, dated 31 October 1980, from Ciba-Geigy Corp., Greensboro, USA. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
McSheehy, T.W., Macrae, S.M. & Whitney, J.C. (1980b) Evaluation of male hepatocellular tumors in the study with CGA 48988: Oncogenicity in dietary administration to mice for two years. Addendum to unpublished report 80/CIA008/442, dated 31 October 1980, from Life Science Research Ltd, Eye, Suffolk, England. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Mewes, K.E. (1998a) Dermal absorption of [phenyl-U-14C] CGA 329351 formulated as Ridomil Gold 480 EC (A-9408 B) in the rat. Unpublished report No. 034AM04, dated 14 May 1998, from Novartis Crop Protection AG, Basel, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Mewes, K.E. (1998b) The in vitro percutaneous absorption of [phenyl-U-14C] CGA 329351 formulated as Ridomil R Gold 480 EC (A-9408 B) through rat and human epidermis. Unpublished report No. 034AM05, dated 28 May 1998, from Novartis Crop Protection AG, Basel, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Müller, T. (1997) Comparison of the absorption, distribution, metabolism and excretion of [phenyl-U-14C] CGA 329351 and [phenyl-U-14C] CGA 48988 in the rat. Unpublished report No. 0.4AM04, dated 11 December 1997, from Novartis Crop Protection AG, Basel, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Naidu, K.A. & Radhakrishnamurty, R. (1988) Metalaxyl-induced bradycardia in rats: mediated by alpha-adrenoreceptors. J. Toxicol. Environ. Health, 23, 495–498.
Naidu, K.A. & Radhakrishnamurty, R. (1989) Effect of metalaxyl on heart rate in rats: Role of alpha1-adrenoreceptors. J. Toxicol. Environ. Health, 26, 203–207.
Ogorek, B. (1997) Salmonella and Escherichia/mammalian-microsome mutagenicity test. Unpublished report No. 963127, dated 23 May 1997, from Novartis Crop Protection AG, Basel, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Ogorek, B. (1998) Gene mutation test with Chinese hamster cells V79. Unpublished report No. 983070, dated 19 November 1998, from Novartis Crop Protection AG, Basel, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Ogorek, B. (2000) CGA 329351 tech.—Autoradiographic DNA repair test on rat hepatocytes (OECD conform) in vitro. Unpublished report No. 993097, dated 10 May 2000 from Novartis Crop Protection AG, Basel, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Paolini, M, Mesirca, R., Pozzetti, L., Sapone, A. & Cantelli-Forti, G. (1996) Biomarkers of effect in evaluating metalaxyl cocarcinogenesis—Selective induction of murine CYP 3A isoform. Mutat. Res., 361, 157–164.
Perocco, P., Colacci, A., Bonora, B. & Grilli, S. (1995) In vitro transforming effect of the fungicides metalaxyl and zineb. Teratog. Carcinog. Mutag., 15, 73–80.
Puri, E. & Müller, D. (1982) Autoradiographic DNA repair test on human fibroblasts CGA 48988 (in vitro test for DNA-damaging properties). Unpublished report No. 811660, dated 18 November 1982, from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Puri, E. & Müller, D. (1985) Autoradiographic DNA repair test on rat hepatocytes CGA 48988 techn. Unpublished report No. 851004, dated 25 November 1985, from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Sachsse, K. (1979) 28-days toxicity study on rats with CGA 48988 technical. Unpublished report No. 790325, dated 20 April 1979, from Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Sachsse, K. & Bathe, ? (1976) Unpublished report from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Ciba-Geigy for JMPR 1982.
Sachsse, K. & Ullman, L. (1976a) Unpublished report from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Ciba-Geigy for JMPR 1982.
Sachsse, K. & Ullman, L. (1976b) Unpublished report from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Ciba-Geigy for JMPR 1982.
Sachsse, K. & Ullman, L. (1976c) Eye irritation in the rabbit of technical CGA 48988. Unpublished report dated 13 May 1976 from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Sachsse, K. & Ullman, L. (1976d) Skin sensitizing (contact allergenic) effect in guinea pigs of technical CGA 48988. Unpublished report dated 13 September 1976 from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Sachsse, K. & Ullmann, L. (1978) Unpublished report from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Ciba-Geigy for JMPR 1982.
Sarasin, G. & Gfeller, W. (1986) Approximate acute oral LD50 in the rat (CGA 107955). Unpublished report No. 821243, dated 9 January 1986, from Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Schoch, M. (1994a) Acute oral toxicity in the rat. Unpublished report No. 933179, dated 6 April 1994, from Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Schoch, M. (1994b) Acute dermal toxicity in the rat. Unpublished report No. 943027, dated 5 May 1994, from Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Strasser, F. & Müller, D. (1982) L5178Y/TK+/– mouse lymphoma mutagenicity test CGA 48988 (in vitro test for mutagenic properties of chemical substances in mammalian cells). Unpublished report No. 811258, dated 1 February 1982, from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Uesugi, T. (1988) Studies on the absorption, distribution and excretion of [14C]-metalaxyl in rats. Unpublished report No. 88021, dated 22 August 1988, from New Drug Development Research Center Inc. Iwamizawa, Japan. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
WHO (2000) The WHO recommended classification of pesticides by hazard and guidelines to classification 2000–2202 (WHO/PCS/01.5). Available from the International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland.
Winkler G. (1996a) Acute oral toxicity in the mouse. Unpublished report No. 963013, dated 19 March 1996, from Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Winkler, G. (1996b) Acute oral toxicity in the rat (limit test). Unpublished report No. 963100, dated 1 October 1996, from Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Winkler, G. (1996c) Acute dermal toxicity in the rat (limit test). Unpublished report No. 963101, dated 1 October 1996, from Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
Winkler, G. (1996d) Acute dermal toxicity in the rat (limit test). Unpublished report No. 963126, dated 16 December 1996, from Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by Syngenta Crop Protection AG, Basel, Switzerland
See Also:
Toxicological Abbreviations