
Pesticide residues in food - 2002 - Joint FAO/WHO Meeting on Pesticide Residues
First draft prepared by
S. Logan
Chemical Product Assessment Section, Therapeutic Goods Administration
Department of Health and Ageing, Canberra, Australia
The fungicide tolylfluanid (N-dichlorofluoromethylthio-N’,N’-dimethyl-N-para-tolylsulfamide) was last reviewed toxicologically by the Joint Meeting in 1988 (Annex 1, reference 53), which established an ADI of 0–0.1 mg/kg bw. It was considered by the present Meeting within the periodic review programme of the Codex Committee on Pesticide Residues.
Single doses of tolylfluanid labelled with 14C at the fluoridichloromethylsulfenyl group were administered orally to rats. At doses of 0.1–20 mg/kg bw, 50–60% of the administered activity was eliminated in urine and 20–30% in faeces within 48 h. Urinary elimination took place with a half-life of 2–3 h during the first 24 h and then more slowly, with a half-life of about 40 h from the third day. In rats with biliary fistulae given 0.5 mg/kg bw by intraduodenal administration, about 6% of the total radioactivity was eliminated in bile. When tolylfluanid was administered orally at a dose of 5 mg/kg bw, however, 16% of the activity was exhaled within 48 h, and the maximal plasma concentration (about 1.5 ppm) was attained after 2 h. While the plasma half-life was 2–3 h during the first 6–8 h, it slowed to about 40 h after 3 days. Of the administered activity, 6% was retained (excluding gastrointestinal tract) for 8 h after administration, 2% for 48 h, 1% for 6 days and 0.5% for 12 days. The highest concentrations were found in the thyroid gland, with 5 ppm 1 day after administration and 1 ppm after 10 days. Whole-body autoradiography after intravenous injection of 10 mg/kg bw confirmed this observation (Weber et al., 1977).
A dose of 2 or 20 mg/kg bw of 14C-ring-labelled tolylfluanid was given orally to male and female Wistar rats. A further group of rats of each sex was pretreated for 14 days with non-radioactive tolylfluanid at a dose of 2 mg/kg bw before receiving radioactive compound. Tolylfluanid was quickly and almost completely absorbed (> 95%); the peak plasma concentration was reached within 1.5 h. Within 48 h, 75–80% of the radioactivity was excreted in urine (half-life, 4–8 h), 14–25% in faeces and 0.06% in expired air. In animals with biliary fistulae, biliary secretion accounted for about 14% of the total elimination. Total residual radioactivity represented 0.07–0.2% of the total administered dose. Higher concentrations of radioactivity were found in liver and kidney (three to seven times the mean body concentration), and lower concentrations were found in perirenal fat, brain, gonads and thyroid (three to nine times less than the mean body concentration). Renal clearance was 3.1–4 ml/min, which corresponds to normal renal plasma flow in rats. No effect of dose was observed on pharmacokinetics (Abbink & Weber, 1988).
Groups of BOR: WISW (SPF Cpb) Wistar rats were given phenyl ring-labelled tolylfluanid at 2 mg/kg bw or at 1 mg/kg bw in combination with unlabelled tolylfluanid at 99 mg/kg bw by gavage. Another group of rats received unlabelled tolylfluanid at 2 mg/kg bw per day for 14 days and then a dose of 2 mg/kg bw of radiolabelled tolylfluanid by gavage. The plasma concentration peaked after about 1 h. By 24 h, the plasma 14C concentration had decreased by about 10-fold after 100 mg/kg bw and by about 100-fold after 2 mg/kg bw. The distribution volume in steady state was more than 14 times the body volume, indicating that 14C had been distributed rapidly into peripheral compartments. The short mean residence time in the central compartment of 14 h indicated rapid redistribution into plasma before excretion. The radioactivity was rapidly excreted, 87–100% of the administered dose being excreted within 48 h. The main route of excretion was the urine, particularly in females, which excreted < 85% of the administered dose by this route. The tissue concentrations of 14C at 48 h were low after 100 mg/kg bw. Sex differences were seen in some tissues, with higher concentrations in erythrocytes, kidney, brain and skin in females and higher concentrations in thyroid in males (Klein, 1991).
Six Wistar (Bor:WISW SPF Cpb) rats were dosed orally and one was dosed by intravenous injection with ring-labelled tolylfluanid at 4 mg per rat, corresponding to a dose of 20 mg/kg bw. The rat treated intravenously was killed 5 min after dosing, and those treated orally were killed 2, 4, 8, 24, 48 and 72 h after dosing. After oral administration, the radioactivity was absorbed relatively rapidly, as radioactivity was detected in all body tissues except compact bone 2 h after treatment. The kidney, liver and intestinal tract had markedly higher concentrations than other tissues. After intravenous injection, the pattern was quite different, with high concentrations in all tissues, including fat, probably due to the presence of unchanged parent compound, which is relatively lipophilic. After oral administration, the distribution pattern was that of a hydrophilic substance, with no evidence of storage in a particular organ or tissue. This suggests rapid metabolism of the parent compound. The presence of high concentrations in the renal medulla and renal papillary area 2 h after oral dosing indicated rapid, extensive renal excretion. Rapid faecal excretion also appeared to occur. Over the 24 h after dosing, the concentrations in tissues and organs decreased rapidly, while depletion was much slower during the subsequent 48 h. A slightly increased concentration of radioactivity in the infraorbital gland may have indicated some elimination in lachrymal fluid (Weber, 1988).
A lactating goat was given 14C-ring-labelled tolylfluanid at a dose of 10 mg/kg bw per day by gavage for 3 days. Blood was taken 0.25, 0.5, 1, 2, 3, 4, 6, 8 and 24 h after the first dose. Milk was sampled before dosing and 8 h after dosing. Urine and faeces were collected at 24-h intervals. The goat was killed 50 h after the first dose (2 h after the last dose), and the liver, kidney, muscle and fat were removed, weighed and sampled for radioactivity. The plasma concentration peaked at 2.2 µg/ml 50 min after dosing. By 50 h after the first dose, the highest concentrations were seen in kidney (37 mg/kg), followed by liver (20 mg/kg). Low concentrations were found in fat (1.5 mg/kg) and muscle (0.53 mg/kg). The main route of excretion was urine (49% of the administered dose). Faecal excretion accounted for 10% of the administered dose, and only 0.24% was found in milk. The plasma concentration fell in a biphasic manner, with a half-life of 1.6 h during the first 6 h after dosing and a half-life of 9.1 h in the terminal phase. The mean residence time was 7.3 h. Approximately 62% of the administered dose was recovered (Ecker & Weber, 1995).
Groups of five white Leghorn laying hens were dosed orally with 14C-ring-labelled tolylfluanid at a dose of 5 mg/kg bw. Blood samples were taken 0.25, 0.5, 1, 2, 3, 4, 6, 8 and 24 h after dosing. A second group was given 5 mg/kg bw per day for 3 days. Excreta and eggs were collected over each 24-h period. The birds were killed 56 h after the first dose (8 h after the final dose). The peak plasma concentration was 0.52 µg/ml and was seen 3 h after dosing. By 24 h after dosing, the plasma concentration was 0.018 µg/ml. At the end of the study, the highest concentrations were found in kidney (0.47 mg/kg) and liver (0.23 mg/kg); lower concentrations were found in eggs in the ovary (0.048 mg/kg), skin (0.045 mg/kg), breast muscle (0.037 mg/kg), thigh muscle (0.027 mg/kg), eggs collected during the last period before sacrifice (0.024 mg/kg) and fat (0.019 mg/kg). Of the administered dose, 84% was excreted in urine and faeces and < 0.01% in eggs. Total recovery of radioactivity was 84%; as the gastrointestinal tract was not collected, much of the residual radioactivity may have been in the intestinal contents. The plasma concentrations fell in a biphasic manner, with a half-life of 1 h during the first 6 h after dosing and a half-life of 12 h in the terminal phase. The mean residence time was approximately 9 h (Ecker & Weber, 1996).
[fluorodichloromethyl-14C]Tolylfluanid was administered orally or intravenously to rats at a single dose of 5 or 10 mg/kg bw. The main metabolite in the urine of treated rats was thiazolidine-2-thioxo-4-carbonic acid, which accounted for 74% of the radioactivity in urine 8 h after intravenous administration and for 50–63% after oral administration. Tolylfluanid was not detected in urine (Ecker, 1978).
After administration of a single oral dose of 20 mg/kg bw of [14C-benzene ring]tolylfluanid to male Wistar rats, 90% of the radioactivity in urine and 70% of that in faeces was due to one metabolite, 4-dimethylaminosulfonylaminobenzoic acid. A minor metabolite (6% of total radioactivity) was not characterized (Ecker & Brauner, 1987).
The metabolism of radiolabelled tolylfluanid was studied in BOR:WISW (SPF Cpb) Wistar rats given an oral dose of 2 or 100 mg/kg bw. The metabolic products were analysed in urine and faeces by high-performance liquid chromatography, nuclear magnetic resonance and mass spectroscopy with reference standards. Urine analysis showed the presence of two major metabolites, dimethylaminosulfonylaminobenzoic acid and monomethylamino-sulfonylaminobenzoic acid, which made up 68% and 5% of the recovered radioactivity, respectively. Two unknown metabolites constituted 2.3% of the recovered radioactivity. In faeces, the metabolite profile varied with dosing regimen. After a single or repeated doses of 2 mg/kg bw, the metabolites consisted mainly of dimethylaminosulfonotoluidine and dimethylaminosulfonyl-aminobenzoic acid. After the dose of 100 mg/kg bw, tolylfluanid accounted for 56% of the recovered radioactivity, with dimethylaminosulfonotoluidine and dimethylaminosulfonylamino-benzoic acid making up about 20%. Thus, a low dose of tolylfluanid was well absorbed and almost completely metabolized, the metabolites being excreted rapidly mainly in the urine. After a high dose, some of the tolylfluanid remained unabsorbed and was excreted unchanged (Klein, 1991).
A lactating goat was dosed with 14C-ring-labelled tolylfluanid as described above, and muscle, liver, kidney, fat, milk and urine samples were analysed for metabolites. The main metabolites detected in all media were different from those in rats and consisted of N-[4-(dimethyl-aminosulfonylamide)benzoyl]glycine and N-[4-(methylaminosulfonylamido)benzoyl]glycine, the hippuric acid conjugates of N-(4-dimethylaminosulfonylamido)benzoic acid and N-(4-methylaminosulfonylamido)benzoic acid. These four metabolites made up most of the residue in all tissues and organs. Other metabolites identified were 4-(dimethylaminosulfonylamide)-benzylic alcohol, 4-(methylaminosulfonylamide)benzylic alcohol, 4-dimethylaminosulfono-toluidine and 4-methylaminosulfotoluidid. No parent compound was detected in any tissue (Ecker & Weber, 1995).
Laying hens were dosed with 14C-ring-labelled tolylfluanid as described above. Kidney, liver, breast and thigh muscle, skin, subcutaneous fat and eggs were collected and used to identify and quantify metabolites. The main metabolite in muscle (56%), eggs (37%) and liver (11%) was 4-(dimethylaminosulfonylamido)benzoic acid; 4-(monomethylaminosulfonylamido)benzoic acid predomeinated in muscle (13%) and liver (12%) but occurred in small amounts in eggs (3.3%). N-[4-(Dimethylaminosulfonylamido)benzoyl]glycine was found in eggs (6.5%) but in none of the tissues. 4-Dimethylaminosulfotoluidid was the main metabolite in fat (66%) and was found at low concentrations in eggs (3.8%) and muscle (0.7%); 4-monomethylaminosulfotoluidid was not found in fat and occurred at low concentrations in eggs (3.6%) and muscle (0.8%) (Ecker & Weber, 1996).
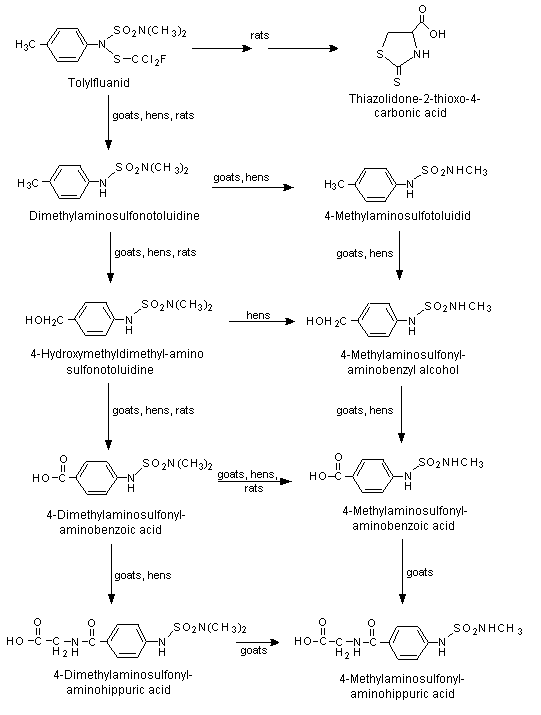
A proposed metabolic pathway for tolylfluanid in rats, goats and hens is shown in Figure 1.

Figure 1. Proposed metabolic pathway for tolylfluanid
(a) Lethal doses
The acute toxicity of tolylfluanid after administration dermally and by inhalation is summarized in Table 1. In rats, the oral LD50 was > 5000 mg/kg bw, the dermal LD50 was > 5000 mg/kg bw, and the inhalation LC50 (4-h exposure) was > 0.16–1 mg/l air (depending on particle size, 2.5–20 µm).
Table 1. Acute toxicity of tolylfluanid
|
Species |
Strain |
Sex |
Route |
Purity (%) |
LD50/LC50 |
Reference |
|
Mouse |
Oral |
NR |
> 1000 |
Kimmerle (1964) |
||
|
Mouse |
CF1 |
Male |
Oral |
99.6 |
> 1000 |
Kimmerle (1968) |
|
Rat |
Male, female |
Oral |
NR |
> 1000 |
Kimmerle (1964) |
|
|
Rat |
Wistar |
Male, female |
Oral |
99.6 |
> 1000 |
Kimmerle (1968) |
|
Rat |
Male |
Oral |
98.7 |
> 2500 |
Kimmerle & Lorke (1967) |
|
|
Rat |
Wistar Bor:WISW (SPF-Cpb) |
Female |
Oral |
91.5 |
> 5000 |
Heimann & Pauluhn (1983) |
|
Rat |
Wistar Hsd Win: Wu |
Male, female |
Oral |
98.9 |
> 5000 |
Bomann (1995a) |
|
Rabbit |
Oral |
NR |
> 1000 |
Kimmerle (1964) |
||
|
Rabbit |
Male, female |
Oral |
NR |
250–500 |
Kimmerle (1968) |
|
|
Guinea-pig |
Oral |
NR |
1000 |
Kimmerle (1964) |
||
|
Guinea-pig |
Purbright |
Female |
Oral |
NR |
250–500 |
Kimmerle (1968) |
|
Cat |
Oral |
NR |
> 1000 |
Kimmerle (1964) |
||
|
Cat |
Male, female |
Oral |
NR |
> 500 |
Kimmerle (1968) |
|
|
Sheep |
Male, female |
Oral |
99.2 |
620–1200 |
Hoffmann (1983) |
|
|
Rat |
Male |
Dermal |
NR |
> 500 |
Kimmerle (1964) |
|
|
Rat |
Wistar Bor:WISW (SPF-Cpb) |
Male, female |
Dermal |
91.5 |
> 5000 |
Heimann & Pauluhn (1983) |
|
Rat |
Wistar Hsd Win: Wu |
Male, female |
Dermal |
98.9 |
> 5000 |
Bomann (1995b) |
|
Rat |
Wistar II |
Male |
Inhalation |
99.6 |
0.26 |
Kimmerle (1968) |
|
Rat |
Male |
Inhalationa |
98.7 |
0.26 |
Kimmerle & Lorke (1967) |
|
|
Rat |
Wistar BOR:WISW (SPF-Cpb) |
Male |
Inhalation |
98.3 |
0.20 |
Märtins (1996a) |
|
Rat |
Wistar BOR:WISW (SPF-Cpb) |
Female |
Inhalation |
98.3 (micronized) |
0.16 |
Märtins (1996a) |
|
Rat |
Wistar Hsd Cpb:WU (SPF) |
Male, female |
Inhalation |
97.2 (technical-grade) |
> 1 |
Pauluhn (1997) |
|
Rat |
Wistar Hsd Cpb:WU (SPF) |
Male, female |
Inhalation |
98.6 (technical-grade) |
0.38 |
Pauluhn (2001) |
|
Rat |
Male |
Intraperitoneal |
NR |
15 |
Kimmerle (1964) |
|
|
Rat |
Wistar II |
Male |
Intraperitoneal |
NR |
20 |
Kimmerle (1968) |
|
Rat |
Wistar II |
Female |
Intraperitoneal |
NR |
26 |
Kimmerle (1968) |
|
Rat |
Wistar Bor:WISW (SPF-Cpb) |
Male |
Intraperitoneal |
91.5 |
15 |
Heimann & Pauluhn (1983) |
|
Rat |
Wistar Bor:WISW (SPF-Cpb) |
Female |
Intraperitoneal |
91.5 |
16 |
Heimann & Pauluhn (1983) |
NR, not reported
a Dynamic spraying with Lutrol/ethanol. Mice, guinea-pigs and rabbits were more sensitive than rats to tolylfluanid, but there was insufficient information to determine an LC50.
After administration by gavage or intraperitoneal injection, rats had disturbed behaviour (unspecified) and decreased mobility at 500 mg/kg bw orally and 1 mg/kg bw intraperitoneally and dyspnoea, with disturbed behaviour persisting for up to 5 days. Rolling, staggering and spastic gait were also seen in rats after intraperitoneal dosing, with a swollen abdomen and post-mortem signs associated with local irritation (Heimann & Pauluhn, 1983). In mice, guinea-pigs, rabbits and cats, tolylfluanid caused general deterioration of condition and (in cats only) vomiting (Kimmerle, 1968). In sheep treated orally, anorexia, weakness of the extremities and loose faeces were seen (Hoffmann, 1983). Rabbits dosed orally with 500 mg/kg bw showed no abnormalities in liver function (bromsulfothalein test and measurements of alanine aminotransferase and serum sorbitol dehydrogenase activity) at 1 or 24 h or 7 days after dosing (Kimmerle, 1964). The clinical signs after exposure by inhalation included extreme breathing difficulties, respiratory sounds and sneezing, serous nasal discharge and cyanosis, as well as morphological changes in the respiratory tract of animals that died, consistent with the severe respiratory irritancy of the substance (Märtins, 1996a; Pauluhn, 1997). The lesser toxicity of the technical-grade material in comparison with the micronized material may have been due to the larger particle size in the technical-grade material (17–20 µm compared with 2–2.5 µm in the micronized material). In a later study with a particle size of 4 µm, tolylfluanid had moderate to high acute toxicity (Pauluhn, 2001).
(b) Dermal and ocular irritation and dermal sensitization
The results of tests for dermal and ocular irritation and dermal sensitization with tolylfluanid are summarized in Table 2.
Table 2. Results of tests for dermal and ocular irritation and dermal sensitization with tolylfluanid
|
Species |
Strain |
Sex |
End-point |
Purity (%) |
Result |
Reference |
|
Rabbit |
Skin irritation (24 h) |
NR |
Not irritating |
Kimmerle (1964) |
||
|
Rabbit |
New Zealand white |
Male, female |
Skin irritation (24 h) |
91.5 |
Not irritating |
Heimann & Pauluhn (1983) |
|
Rabbit |
HC:New Zealand white |
Skin irritation (4 h) |
99.1 |
Not irritating |
Pauluhn (1984) |
|
|
Rabbit |
HC:New Zealand white |
Skin irritation (4 h) |
98.5 |
Severely irritating |
Krötlinger (1994a) |
|
|
Rabbit |
HC:New Zealand white |
Skin irritation (4 h) |
1% aqueous solution |
Not irritating |
Krötlinger (1994a) |
|
|
Rabbit |
Eye irritation |
NR |
Not irritating |
Kimmerle (1964) |
||
|
Rabbit |
New Zealand white |
Male, female |
Eye irritation |
91.5 |
Severely irritating |
Heimann & Pauluhn (1983) |
|
Rabbit |
HC:New Zealand white |
Eye irritation |
99.1 |
Highly irritating |
Pauluhn (1984) |
|
|
Rabbit |
HC:New Zealand white |
Eye irritation |
98.5 |
Moderately irritating |
Krötlinger (1994a) |
|
|
Rabbit |
HC:New Zealand white |
Eye irritation |
1 aqueous solution |
Not irritating |
Krötlinger (1994a) |
|
|
Guinea-pig |
Pirbright white W 58 |
Male |
Skin sensitization |
91.5 |
Sensitizing |
Heimann (1983 ) |
|
Guinea-pig |
DHPW |
Male |
Skin sensitization |
98.7 or 98.5 |
Not sensitizing |
Diesing (1990) |
|
Guinea-pig |
SPF Bor:DHPW |
Male |
Skin sensitization |
98.5 |
Sensitizing |
Diesing (1991) |
|
Mice |
Hsd Win:NMRI |
Female |
Local lymph node assay |
98.9 |
Possibly sensitizing |
Vohr (2001) |
|
Mice |
Hsd Win:NMRI |
Female |
Local lymph node assay |
98.9 |
Sensitizing |
Vohr (2002) |
NR, not reported
In a study of skin sensitization with the Magnusson and Klingman maximization test in guinea-pigs, the concentrations of tolylfluanid (purity, 91.5%) used were 1% for intradermal induction, 0.6% for topical induction and 0.3% for topical challenge. Tolylfluanid sensitized the skin in 10 treated animals and two naive controls (Heimann, 1983). In a Buehler test with a 50% solution for induction and 25% and 50% for challenge, tolylfluanid did not sensitize skin (Diesing, 1990). In an open epicutaneous skin sensitization test, animals were induced with 1%, 3%, 10% or 30% on 5 days/week for 4 weeks. Challenge doses were given 4, 6 and 8 weeks later, at concentrations of 1%, 3%, 10% and 30% tolylfluanid at the first challenge, 0.03%, 0.1%, 0.3% and 1% tolylfluanid suspension at the second challenge at 6 weeks and 0.003%, 0.01%, 0.03% and 0.1% for the final challenge at 8 weeks. The number of animals with erythematous reactions and the intensity of the reaction decreased with decreasing challenge dose over the range 1–0.003%. The threshold dose for initiation of a sensitization reaction appeared to be 0.01%. Tolylfluanid was thus a skin sensitizer in guinea-pigs (Diesing, 1991).
In an assay in female mice, 1% tolylfluanid increased the cell count in local lymph nodes, indicating sensitization. The weight of the local lymph nodes was also increased, perhaps reflecting a response to irritation, and more lymph fluid was present in the tissue. Ear swelling and weight were also increased. Overall, the findings were consistent with a sensitization reaction, but irritation could not be ruled out (Vohr, 2001).
In another assay in female mice, tolylfluanid at 0.2% and 1% in a dimethylacetamide, acetone and ethanol vehicle increased the cell count in local lymph nodes. Ear swelling and ear weight were increased at 1%, indicating a reaction to irritation, but this was not seen at 0.2%. Tolylfluanid was considered to have both sensitizing potential and irritating properties (Vohr, 2002).
Rats
No abnormal clinical signs or deaths were reported in groups of 10 rats given tolylfluanid (purity not specified) by gavage at a dose of 100, 200 or 330 mg/kg bw per day for 5 days or by intraperitoneal injection at 1.5, 5 or 30 mg/kg bw per day for 5 days (Kimmerle, 1964).
Groups of 15 SPF Wistar rats of each sex recieved diets containing tolylfluanid (purity, 99%) at a concentration of 150, 500, 1500 or 4500 ppm, resulting in mean intakes of 13, 46, 130 and 400 mg/kg bw per day for males and 18, 60, 180 and 510 mg/kg bw per day for females A group of 30 rats of each sex served as controls. Animals were examined for clinical signs and deaths daily, and food consumption and body weight were measured weekly. Haematological, clinical chemical and urinary end-points were measured after 1 and 3 months of treatment in five animals of each sex per group. The haematological end-points included haemoglobin, eythrocyte volume fraction, erythrocyte count, total and differential leukocyte counts, mean corpuscular volume, mean corpuscular haemoglobin, reticulocyte count, thrombocyte count and prothrombin time. The clinical chemical end-points included alkaline phosphatase, alanine and aspartate aminotransferase and glutamate dehydrogenase activity and bilirubin, total protein, glucose and cholesterol concentrations. The urine analyses included glucose, haemoglobin, pH, ketones, bile pigments, protein, microscopic sediment, urea and creatinine. At the end of the study, all animals were killed and examined grossly, and the thyroid, thymus, heart, lung, liver, spleen, kidney, suprarenal glands and testes or ovaries were weighed. The heart, lung liver, spleen, kidney, suprarenal glands, pituitary gland, thyroid gland, parathyroid gland, testes, epididymides, accessory reproductive gland, ovaries and uterus from five animals of each sex per group, and the pancreas, oesophagus, stomach, intestine, lymph node, thymus, bladder, brain, eyes, aorta, quadriceps muscle, femur and bone marrow from five animals of each sex in the control group and that at 4500 ppm were examined histopathologically.
No deaths and no treatment-related abnormal clinical signs were observed during treatment. The groups did not differ in body weight. Body-weight gain was decreased by < 10% and food consumption by 13% in females at 4500 ppm, but these effects were considered not to be of biological significance. There were no changes in haematological, clinical chemical or urine end-points. No abnormalities were seen on gross examination post mortem. The relative liver weight was increased in males at concentrations >1500 ppm and in females at 4500 ppm. The relative kidney weight was increased in females at concentrations > 1500 ppm and the relative weight of the suprarenal gland was increased in females at concentrations > 500 ppm. No treatment-related changes were seen on histopathological examination. As the changes in organ weights were not accompanied by clinical chemical or histopathological changes, they were considered to be of no biological significance. The NOAEL was 4500 ppm, equal to 400 mg/kg bw per day (JMPR, 1988; amended by reference to Bomhard & Schilde, 1976).
Groups of 10 male and 10 female Hsd/Win:WU Wistar rats were given diets containing tolylfluanid (purity, 97.5%) at a concentration of 0, 300, 1650 or 9000 ppm for 13 weeks, equal to 0, 20, 110 and 640 mg/kg bw per day for males and 0, 23, 130 and 740 mg/kg bw per day for females. Two groups, at 0 and 9000 ppm, were held for recovery for a further 4 weeks without treatment before being killed. The diet was analysed for the content and stability of tolylfluanid. Animals were examined for clinical signs and deaths daily, and food consumption and body weight were measured weekly. An ophthalmological examination was done before treatment and in controls and rats at the highest concentration at the end of the study. Haematology, clinical chemistry and urine analysis were performed after 1 and 3 months of treatment and at the end of the recovery period. Erythrocyte and leukocyte parameters, platelets, Heinz bodies and clotting times were assessed. The clinical chemical examinations included determination of alanine and aspartate aminotransferase, alkaline phosphatase and glutamate dehydrogenase activity, total bilirubin, cholesterol, Ca, Cl, K, Na, P, creatinine, glucose, triglyceride, blood urea nitrogen, albumin, total protein, thyroxine-binding capacity, thyroid-stimulating hormone (TSH), tri-iodothyronine and thyroxine. At the end of the study, all rats were examined grossly, and the adrenals, heart, brain, gonads, kidneys, liver, lungs, spleen and thyroid were weighed and examined comprehensively by histology As no abnormalities were found at the end of the treatment phase, organs were not examined histologically at the end of the recovery phase.
Clinical signs were unaffected by treatment. The weight gain of animals at 9000 ppm was slightly reduced, resulting in terminal weights that were 94% and 96% those of controls for males and females, respectively. Although the reduction in weight gain was small and was not reflected in absolute daily feed intake, slightly decreased food use efficiency was observed in animals at this dose: 71 g/kg bw per day for males, with 67 g/kg bw per day for controls, and 82 g/kg bw per day for females, with 75 g/kg bw per day for controls. Water intake was increased in males at 9000 ppm (100 g/kg bw per day, with 93 g/kg bw per day for controls) and in females at 1650 and 9000 ppm (120 and 130 g/kg bw per day, with 105 g/kg bw per day for controls). No treatment-related ophthalmological effects were observed at sacrifice in animals at the highest dietary concentration.
With the exception of small, isolated deviations from control, with no dose–response relationship, there were no changes in haematological parameters in treated animals. The clinical chemical effects included slight alterations in liver enzymes and more significant alterations in thyroid parameters in both sexes at 9000 ppm and in males at 1650 ppm. These effects resolved during the 4-week recovery period, with the exception of thyroxine-binding capacity, which remained elevated in males at 9000 ppm (0.93; control, 0.87). These effects were similar to those seen with the related compound, dichlofluanid. Intermittent increases in cholesterol concentrations and decreased creatinine concentrations were also seen, which may have been associated with changes in liver function. The changes in clinical chemical end-points are summarized in Table 3. No treatment-related alterations were seen on gross or microscopic examination. Other than a slight increase in the relative liver weight in males (5%) at 9000 ppm, which was not observed in animals allowed to recover, there were no treatment-related effects on organ weights. The NOAEL was 300 ppm, equal to 20 mg/kg bw per day, on the basis of altered thyroid parameters in males at the next highest dose (Dreist, 1995).
Table 3. Changes in clinical chemical end-points in rats given diets containing tolylfluanid
|
End-point |
Week |
Dietary concentration (ppm) |
|||||||||||
|
Males |
Females |
||||||||||||
|
0 |
300 |
1650 |
9000 |
0 |
300 |
1650 |
9000 |
||||||
|
Aspartate aminotransferase activity (U/l) |
4 |
39 |
37 |
37 |
35* |
44 |
40 |
48 |
40 |
||||
|
|
13 |
35 |
31 |
34 |
34 |
40 |
37 |
37 |
31 |
||||
|
Alanine aminotransferase activity (U/l) |
4 |
42 |
41 |
37** |
28** |
35 |
36 |
35 |
21** |
||||
|
|
13 |
32 |
29 |
28 |
25% |
36 |
32 |
31 |
20** |
||||
|
Alkaline phosphatase activity (U/l) |
4 |
540 |
460* |
430* |
440* |
310 |
320 |
300 |
270* |
||||
|
|
13 |
250 |
210* |
200** |
200** |
160 |
170 |
150 |
130* |
||||
|
Glutamate dehydrogenase activity (U/l) |
4 |
2.4 |
1.9 |
2.3 |
5.0* |
2.0 |
2.2 |
2.7 |
2.2 |
||||
|
|
13 |
7.8 |
3.2 |
6.1 |
9.7 |
13 |
4.5 |
9.5 |
3.5 |
||||
|
Cholesterol (mmol/l) |
4 |
1.9 |
2.0 |
2.1 |
2.2* |
2.0 |
2.0 |
2.2 |
2.4* |
||||
|
|
13 |
2.4 |
2.3 |
2.3 |
2.1 |
2.2 |
1.9 |
2.1 |
2.3 |
||||
|
Creatinine (µmol/l) |
4 |
41 |
42 |
40 |
37** |
46 |
44 |
45 |
44 |
||||
|
|
13 |
45 |
49 |
46 |
42 |
47 |
44* |
46* |
44* |
||||
|
Tri-iodothyronine (nmol/l) |
4 |
1.4 |
1.2* |
1.2* |
1.2** |
1.3 |
1.3 |
1.3 |
1.3 |
||||
|
|
13 |
1.3 |
1.4 |
1.6* |
1.5** |
1.3 |
1.4 |
1.3 |
1.3 |
||||
|
Thyroxine (nmol/l) |
4 |
64 |
59 |
58 |
54* |
52 |
55 |
46 |
39** |
||||
|
|
13 |
49 |
53 |
55* |
50 |
41 |
48 |
43 |
37 |
||||
|
Thyroxine-binding capacity |
4 |
0.84 |
0.85 |
0.86* |
0.90* |
0.75 |
0.77 |
0.79 |
0.84** |
||||
|
|
13 |
0.75 |
0.76 |
0.79 |
0.84** |
0.85 |
0.88 |
0.89** |
0.90** |
||||
|
Thyroid-stimulating hormone (µg/l) |
4 |
8.4 |
7.7 |
9.8 |
12* |
7.5 |
7.7 |
8.3 |
10** |
||||
|
|
13 |
3.7 |
3.1 |
4.9 |
8.3* |
2.2 |
1.8 |
2.9 |
3.2** |
||||
From Dreist (1995)
* p < 0.05; ** p < 0.01
Groups of 10 BOR:WISW (SPF-Cpb) rats of each sex were exposed to a micronized powder form of tolylfluanid (purity, 98.8%) by nose-only inhalation for 6 h/day on 5 consecutive days at a nominal concentration of 0, 0.0015, 0.015 or 0.15 mg/l (actual concentrations, 0, 0.0016, 0.014 and 0.12 mg/l). The mean particle size was 1.6–2.6 µm. The rats were observed twice daily (once daily on weekends) for abnormal clinical signs, including changes in the skin and fur, eyes, mucous membranes, respiratory, circulatory and autonomic and central nervous systems, somatomotor activity and behaviour. Particular attention was paid to tremors, convulsions, salivation, diarrhoea, lethargy, somnolence or coma. The time of death was recorded as accurately as possible. A battery of functional observational (FOB) examinations was administered after the fifth dose and on subsequent days if any decrease in reflexes was noted. The reflexes tested included visual placing response, grip strength, abdominal muscle tone, corneal and pupillary reflexes, pinnal reflex, righting reflex, tail-pinch response and startle reflex after either sound or touch. Body weight was measured before treatment and on days 4, 7 and 14. The rectal temperature was measured 15–30 min after exposure on days 0, 4 and 7 and in the morning after exposure. Haematological and clinical chemical end-points were measured on day 7 in five animals of each sex per group. The haematological parameters examined included leukocyte, erythrocyte and reticulocyte counts, erythrocyte volume fraction, haemoglobin, mean corpuscular volume, mean corpuscular haemoglobin concentration, mean corpuscular haemoglobin, Heinz bodies, platelet count and clotting time. The clinical chemical parameters measured included alanine and aspartate aminotransferase, alkaline phosphatase, glutamate dehydrogenase and lactate dehydrogenase activity and bilirubin, triglycerides and cholesterol. Five animals of each sex per group were killed on day 7 and the remaining rats on day 14 on. All rats were examined grossly post mortem, and the adrenals, brain, heart, kidney, liver, lungs, spleen and thymus were weighed and the organ:body weight and organ:brain weight ratios were calculated. No histopathological examination was done.
Six males and nine females at 0.12 mg/l died before the third exposure. The survivors were not exposed again, but were allowed to recover. No deaths occurred at lower doses. Abnormal clinical signs were also seen at 0.12 mg/l, including bradypnoea, extreme breathing difficulty, respiratory sounds, cyanosis, sneezing, serous or bloody nasal discharge, salivation, decreased motility, ungroomed coat, piloerection, prostration, tremor and limpness. All survivors recovered within 8 days, and no abnormal clinical signs were seen at lower doses. There were no treatment-related effects in the FOB tests. Weight loss was seen at 0.12 mg/l, but there was no effect on body-weight gain at lower doses. Hypothermia was seen at 0.12 mg/l immediately after exposure, the mean body temperature being decreased by 7.2 °C in males and 8 °C in females. There were no treatment-related changes in haematological or clinical chemical parameters.
The lungs of rats found dead were dark red, spongy and puffy, and white foamy material was found in the trachea. The livers were pale with distinct lobulation, and red areas were found in the thymus. Gross examination at sacrifice revealed dark-red discolouration of the lungs of rats at 0.12 mg/l. Increased lung weight was seen at this dose, consistent with the other signs of lung irritation. Decreased thymus weight was also seen at 0.12 mg/l, which the sponsor considered was related to the stress due to respiratory irritation. Decreased liver weight was seen at the interim sacrifice in males at all doses and in females at 0.014 mg/l. All changes in organ weights were reversed by the time of the terminal sacrifice. The changes in absolute and relative organ weights are presented in Table 4. Results for animals at 0.12 mg/l are not included as few animals at this dose survived to the interim sacrifice. No NOAEC could be identified, as changes in liver weight were seen in males at 0.0016 mg/l (Märtins, 1996b).
Table 4. Changes in organ weights at interim sacrifice in rats exposed to tolylfluanid by inhalation
|
Organ weight |
Concentration (mg/l) |
|||||
|
Males |
Females |
|||||
|
0 |
0.0016 |
0.014 |
0 |
0.0016 |
0.014 |
|
|
Absolute thymus weight (mg) |
410 |
370 |
410 |
310 |
360 |
290 |
|
Thymus weight relative to body weight (mg/100 g) |
180 |
160 |
190 |
160 |
190 |
160 |
|
Absolute lung weight (mg) |
1 200 |
1100 |
1200 |
1000 |
1100 |
1100 |
|
Lung weight relative to body weight (mg/100 g) |
500 |
490 |
540 |
540 |
560 |
590 |
|
Absolute liver weight (mg) |
10 000 |
9200* |
9000* |
7100 |
6900 |
6300* |
|
Liver weight relative to body weight (mg/100 g) |
4 500 |
4100* |
4100* |
3700 |
3600 |
3400 |
From Dreist (1995)
* p < 0.05; ** p < 0.01
Groups of 10 BOR:WISW (SPF-Cpb) rats of each sex were exposed to a micronized powder form of tolylfluanid (purity, 98.8%) by nose-only inhalation for 6 h/day on 5 consecutive days at a nominal concentration of 0, 0.0015, 0.015 or 0.15 mg/l (actual concentrations, 0, 0.0016, 0.014 and 0.12 mg/l). The mean particle size was 1.6–2.6 µm. The rats were observed twice daily (once daily on weekends) for abnormal clinical signs, including changes in the skin and fur, eyes, mucous membranes, respiratory, circulatory and autonomic and central nervous systems, somatomotor activity and behaviour. Particular attention was paid to tremors, convulsions, salivation, diarrhoea, lethargy, somnolence or coma. The time of death was recorded as accurately as possible. A battery of functional observational (FOB) examinations was administered after the fifth dose and on subsequent days if any decrease in reflexes was noted. The reflexes tested included visual placing response, grip strength, abdominal muscle tone, corneal and pupillary reflexes, pinnal reflex, righting reflex, tail-pinch response and startle reflex after either sound or touch. Body weight was measured before treatment and on days 4, 7 and 14. The rectal temperature was measured 15–30 min after exposure on days 0, 4 and 7 and in the morning after exposure. Haematological and clinical chemical end-points were measured on day 7 in five animals of each sex per group. The haematological parameters examined included leukocyte, erythrocyte and reticulocyte counts, erythrocyte volume fraction, haemoglobin, mean corpuscular volume, mean corpuscular haemoglobin concentration, mean corpuscular haemoglobin, Heinz bodies, platelet count and clotting time. The clinical chemical parameters measured included alanine and aspartate aminotransferase, alkaline phosphatase, glutamate dehydrogenase and lactate dehydrogenase activity and bilirubin, triglycerides and cholesterol. Five animals of each sex per group were killed on day 7 and the remaining rats on day 14 on. All rats were examined grossly post mortem, and the adrenals, brain, heart, kidney, liver, lungs, spleen and thymus were weighed and the organ:body weight and organ:brain weight ratios were calculated. No histopathological examination was done.
Six males and nine females at 0.12 mg/l died before the third exposure. The survivors were not exposed again, but were allowed to recover. No deaths occurred at lower doses. Abnormal clinical signs were also seen at 0.12 mg/l, including bradypnoea, extreme breathing difficulty, respiratory sounds, cyanosis, sneezing, serous or bloody nasal discharge, salivation, decreased motility, ungroomed coat, piloerection, prostration, tremor and limpness. All survivors recovered within 8 days, and no abnormal clinical signs were seen at lower doses. There were no treatment-related effects in the FOB tests. Weight loss was seen at 0.12 mg/l, but there was no effect on body-weight gain at lower doses. Hypothermia was seen at 0.12 mg/l immediately after exposure, the mean body temperature being decreased by 7.2 °C in males and 8 °C in females. There were no treatment-related changes in haematological or clinical chemical parameters.
The lungs of rats found dead were dark red, spongy and puffy, and white foamy material was found in the trachea. The livers were pale with distinct lobulation, and red areas were found in the thymus. Gross examination at sacrifice revealed dark-red discolouration of the lungs of rats at 0.12 mg/l. Increased lung weight was seen at this dose, consistent with the other signs of lung irritation. Decreased thymus weight was also seen at 0.12 mg/l, which the sponsor considered was related to the stress due to respiratory irritation. Decreased liver weight was seen at the interim sacrifice in males at all doses and in females at 0.014 mg/l. All changes in organ weights were reversed by the time of the terminal sacrifice. The changes in absolute and relative organ weights are presented in Table 4. Results for animals at 0.12 mg/l are not included as few animals at this dose survived to the interim sacrifice. No NOAEC could be identified, as changes in liver weight were seen in males at 0.0016 mg/l (Märtins, 1996b).
Table 4. Changes in organ weights at interim sacrifice in rats exposed to tolylfluanid by inhalation
|
Organ weight |
Concentration (mg/l) |
|||||
|
Males |
Females |
|||||
|
0 |
0.0016 |
0.014 |
0 |
0.0016 |
0.014 |
|
|
Absolute thymus weight (mg) |
410 |
370 |
410 |
310 |
360 |
290 |
|
Thymus weight relative to body weight (mg/100 g) |
180 |
160 |
190 |
160 |
190 |
160 |
|
Absolute lung weight (mg) |
1 200 |
1100 |
1200 |
1000 |
1100 |
1100 |
|
Lung weight relative to body weight (mg/100 g) |
500 |
490 |
540 |
540 |
560 |
590 |
|
Absolute liver weight (mg) |
10 000 |
9200* |
9000* |
7100 |
6900 |
6300* |
|
Liver weight relative to body weight (mg/100 g) |
4 500 |
4100* |
4100* |
3700 |
3600 |
3400 |
From Märtins (1996b)
* p < 0.05
Groups of 10 BOR:WISW (SPF-Cpb) rats of each sex were exposed to a micronized powder form of tolylfluanid (purity 98.3%) by nose-only inhalation for 6 h/day on 5 days per week for 4 weeks at a nominal concentration of 0, 0.0002, 0.0015, 0.01 or 0.05 mg/l (actual concentrations, 0, 0.0002, 0.0015, 0.0098 and 0.05 mg/l). The mean particle size was 1.9–2.6 µm. Exposure to 0.05 mg/l resulted in deaths and severe clinical signs, and all surviving rats at this dose were killed on day 14. An additional five animals of each sex per group were exposed to 0, 0.01 or 0.05 mg/l, and those at 0.01 mg/l were allowed to recover for up to 5 weeks. The animals were observed twice daily (once daily on weekends) for abnormal clinical signs, including changes in skin and fur, eyes, mucous membranes, respiratory, circulatory and autonomic and central nervous systems, somatomotor activity and behaviour. Particular attention was paid to tremors, convulsions, salivation, diarrhoea, lethargy, somnolence or coma. The time of death was recorded as accurately as possible. FOB tests were administered after the fifth dose and on subsequent days if any decrease in reflexes was noted. The reflexes tested included visual placing response, grip strength, abdominal muscle tone, corneal and pupillary reflexes, pinnal reflex, righting reflex, tail-pinch response and startle reflex after either sound or touch. Body weight was measured weekly. Rectal temperature was measured 15–30 min after exposure on days 0, 7, 21 and 56. An ophthalmological examination was done on five animals of each sex per group before treatment and on day 20. Haematological and clinical chemical end-points were measured at the end of the study and the end of the recovery phase. The haematological parameters examined included leukocyte, erythrocyte and reticulocyte counts, erythrocyte volume fraction, haemoglobin, mean corpuscular volume, mean corpuscular haemoglobin concentration, mean corpuscular haemoglobin, Heinz bodies, platelet count and clotting time. The clinical chemical parameters included aspartate and alanine aminotransferase, alkaline phosphatase, glutamate dehydrogenase, lactate dehydrogenase, gamma-glutamyl transferase and creatine kinase activity and glucose, urea, bilirubin, creatinine, total protein, albumin, triglycerides, cholesterol, Na, K, Mg, Ca, P and Cl. The liver was tested for the concentrations of triglycerides and glycogen and the activity of cytochrome P450, aminopyrine-N-demethylase, para-nitrosoanisole-O-demethylase, 7-ethoxy-coumarin deethylase, 7-ethoxy-resorufin deethylase, aldrin epoxidase, epoxide hydrolase, glutathione-S-transferase and UDP-glucuronyl transferase. Urine was analysed at the end of exposure from 10 animals of each sex per group for sediment composition, pH, volume, protein, glucose, blood, bilirubin, urobilinogen, ketone bodies and osmolality. All rats were examined grossly post mortem, and the adrenals, brain, heart, kidney, liver, lungs, ovaries, spleen, testes, thyroid and thymus were weighed and organ:body weight and organ:brain weight ratios were calculated. A complete histopathological examination was done. The medial thyroid was left attached to the larynx or trachea to obviate artefacts of preparation.
Five males and seven females at 0.05 mg/l died between day 7 (males) or 6 (females) and day 13, and all rats at this dose, including those allowed to recover, were killed on day 14. Abnormal clinical signs were seen in all rats at 0.0098 and 0.05 mg/l, including bradypnoea, laboured breathing, abnormal respiratory sounds, serous nasal discharge and red incrustations on the nose. At 0.05 mg/l, the signs also included extreme breathing difficulty, cyanosis, red incrustation on the eyes, decreased motility, sluggishness, prostration, ungroomed coat and piloerection. The signs increased in severity during the exposure period. No abnormal clinical signs were seen in rats at 0.0098 mg/l during recovery. There was no clear sex difference in clinical signs. No effects were seen on reflexes, grip strength or foot splay. Body weight was statistically significantly lower at 0.05 mg/l in both males (decreased by 9–15%) and females (3–7%). The author reported that no effects were seen on body weight in any other group; however, the body-weight gain of females at 0.0098 mg/l was decreased by 38% in comparison with controls during the study, and that of males at this dose was decreased by 26% during the first 12 days of the study. These effects were considered to be treatment-related. Body-weight gain returned to normal during the recovery period. Rectal temperature was decreased in animals at 0.05 mg/l after exposure but was not affected in any other groups. Ophthalmoscopic examination showed no treatment-related abnormalities, although corneal dystrophy was seen in all groups.
Haematological examination showed a statistically significant decrease in mean corpuscular volume in males at 0.0015 mg/l; however, this was considered incidental as the value was within the range of that of other controls in the same laboratory, and no other changes were seen. The reticulocyte count was increased in both sexes at 0.05 mg/l (by 11%, 10%, 7%, 13% and 36% in males and 12%, 12%, 14%, 12% and 33% in females at 0, 0, 0.0002, 0.0015, 0.0098 and 0.05 mg/l, respectively). The values at 0.05 mg/l were not affected by any individual outlyiers, and the author indicated that they were within the range of other controls. The range quoted was 38 ± 17 for males and 28 ± 11 for females, which would exclude the values seen at other doses in females. The Meeting therefore considered that the increases at 0.05 mg/l were treatment-related. No haematological effects were seen at other doses.
Clinical chemistry showed elevated alkaline phosphatase activity at 0.0098 and 0.05 mg/l. The changes were relatively small, with no clear dose–response relationship, and were considered not to be treatment-related. Marginal changes in sodium and alpha1-globulin concentrations, were found but were within the range of other controls and were considered not to be treatment-related. The UDP-glucuronyltransferase activity in the liver was statistically significantly decreased in females at 0.05 mg/l and statistically nonsignificantly decreased in females at 0.0098 mg/l and males at 0.05 mg/l. This finding was treatment-related but of questionable toxicological relevance, particularly as the values were within the range of those of other controls in the same laboratory.
Gross examination post mortem revealed changes in the lung at 0.05 mg/l, including discolouration and deflated pulmonary segments. No macroscopic changes were seen at other doses. The absolute and relative weights of the lung were increased in rats at 0.05 mg/l. The thyroids in this group also appeared to be markedly heavier, although no gross or microscopic changes were reported. The increased thyroid weight was seen in all rats in the group and may have been due to a weighing error. The changes in organ weights are summarized in Table 5.
Table 5. Changes in organ weights in rats exposed to tolyfluanid by inhalation
|
Organ weight |
Concentration (mg/l) |
|||||||||
|
Males |
Females |
|||||||||
|
0 |
0.0002 |
0.0015 |
0.0098 |
0.05 |
0 |
0.0002 |
0.0015 |
0.0098 |
0.05 |
|
|
Absolute lung weight (mg) |
1240 |
1180 |
1190 |
1170 |
1460 |
1050 |
1040 |
1040 |
1030 |
1330 |
|
Relative lung weight (mg/100 g bw) |
470 |
450 |
470 |
460 |
770 |
560 |
540 |
560 |
560 |
800 |
|
Absolute thyroid weight (mg) |
6 |
5 |
6 |
7 |
260 |
6 |
6 |
5 |
5 |
230 |
|
Relative thyroid weight (mg/100 g bw) |
2 |
2 |
2 |
3 |
130 |
3 |
3 |
3 |
3 |
140 |
From Märtins (1997)
Microscopic examination showed squamous metaplasia and hyperkeratosis of the anterior nasal cavities, squamous metaplasia of the larynx, epithelial desquamation and round-cell infiltration of the trachea, increased mucus secretion in the bronchi and lung fibrosis at 0.05 mg/l. At 0.0098 mg/l, hyperkeratosis of the anterior region of the nasal cavities was seen, which was reversed at the end of the treatment period. No other treatment-related changes were seen. In detailed bone-marrow smears, an increased relative count of segmented neutrophils was seen at 0.05 mg/l. This was considered treatment-related. The NOAEC was 0.0015 mg/l (Märtins, 1997).
Groups of 10 Wistar rats (Hsd Cpb: WU (SPF)) of each sex were exposed to tolylfluanid (purity, 98.9%) by directed flow at an intended concentration of 0, 0.001, 0.004 or 0.015 mg/l for 6 h/day, 5 days per week for 4 weeks. The tolylfluanid was not micronized, as it was considered that this process might increase its toxicity by causing degradation to sulfenic acid derivatives, which are known pneumotoxicants. The humidity of the air used during exposure was increased to 60% to decrease potentiation of irritation by the dry dust formulation. The aerosol was generated by blowing dry air over the test material; metered volumes of air were then removed and replaced with moistened air to increase the humidity to 60%. The temperature and humidity in the test chamber were measured and recorded. The achieved tolylfluanid concentrations were determined by gravimetric analysis of the filters in the chamber, and samples for particle-size distribution were taken in the vicinity of the breathing zone. The achieved concentrations were 0, 0.0012, 0.004 and 0.011 mg/l, with mass median aerodynamic diameters of 0, 3.5, 3.7 and 4.0 µm, respectively. The percentages of particles with a diameter < 3 µm were 0, 44%, 42% and 37%, respectively.
Body weight was measured twice a week before treatment. Rats were examined for appearance and behaviour twice daily during exposure and daily when not exposed. Respiratory function was assessed in four spontaneously breathing, conscious rats of each sex per group. The rats were allowed sufficient time to acclimatize to the measuring device before assessment of respiratory rate, tidal volume, respiratory minute volume, peak inspiratory and expiratory times and the duration of apnoeic periods. Colonic temperature was recorded within 30 min of the end of exposure. Clinical chemical end-points were measured after 4 weeks of exposure. The haematological parameters examined included erythrocyte, thrombocyte and reticulocyte counts, total and differential leucokyte counts, erythrocyte volume fraction, haemoglobin, mean corpuscular volume, mean corpuscular haemoglobin concentration, mean corpuscular haemoglobin, clotting times and Heinz bodies. The clinical chemical parameters included aspartate and alanine aminotransferase, glutamate dehydrogenase, lactate dehydrogenase, alkaline phosphatase and gamma-glutamyl transferase activity and albumin, glucose, urea, bilirubin, creatinine, total protein, triglycerides, cholesterol, Na, K, Ca, Mg, P and Cl. The liver was assessed for N-demethylase, O-demethylase and cytochrome P450 activity and triglyceride concentration. An ophthalmological examination was done on five animals of each sex per group before exposure, and on five of each sex in the control and high-dose groups at the end of exposure; other groups were examined only if abnormalities were detected. At the end of the study, all rats were examined grossly post mortem, and the adrenals, brain, heart, kidney, liver, lungs, ovaries, spleen, testes, thymus and uterus were weighed. The oesophagus, eyes, eyelids, extraorbital lachrymal glands, Harderian glands, head, larynx, liver, lungs, lymph nodes, pharynx, trachea and any organs or tissues with macroscopic abnormalities from all rats were examined histologically.
No deaths occurred during the study. Clinical signs were seen in all rats at 0.011 mg/l and included tachypnoea and irregular breathing patterns. In males, these effects were seen only on days 10 and 11, while in females they were seen from day 10 to day 29. When lung function was assessed on days 14–18, a slight increase in tidal volume was found in all treated rats, with no treatment-related difference in minute volume or respiratory rate. No effects on reflexes were detected. Treated rats had a slightly higher colonic temperature than controls. Body-weight gain was lower in all groups of treated rats throughout the study, with weight gains of 50, 27, 34 and 25 g in males and 27, 16, 15 and 14 g in females at 0, 0.0012, 0.004 and 0.011 mg/l, respectively. No treatment-related changes were found on haematological examination. Decreased cholesterol concentrations were seen in treated males, which were not dose-related, and in females at 0.004 and 0.011 mg/l, with concentrations of 1.6, 1.3, 1.2 and 1.3 mmol/l in males and 1.5, 1.4, 1.2 and 1.2 mmol/l in females. There were no effects on liver enzymes, but the liver triglyceride concentration was decreased in females at 0.011 mg/l. No treatment-related changes were seen on ophthalmological examination or post mortem.
The weight of the lungs was slightly increased in all groups of treated males and in females at 0.011 mg/l. The thymus weight was decreased in rats at 0.004 and 0.011 mg/l, the effect being more noticeable in males than females. The changes in organ weights are summarized in Table 6. Histopathological examination showed an increased incidence of mucus and cells in the lumen of the larynx and focal inflammation and epithelial squamous metaplasia of the larynx in all treated groups. In the lungs, peribronchial infiltration was seen in all treated groups; peribronchial fibrosis seen in females at 0.004 and 0.011 mg/l and in males at 0.011 mg/l. Epithelial changes were seen in all groups of treated males and in females at 0.004 and 0.011 mg/l (Table 7). No other treatment-related histopathological changes were reported. No NOAEC could be identified; the LOAEC was 0.0012 mg/l (Pauluhn, 2002).
Table 6. Changes in organ weights in rats exposed to tolylfluanid by inhalation
|
Organ weight |
Concentration (mg/l) |
|||||||
|
Males |
Females |
|||||||
|
0 |
0.0012 |
0.004 |
0.011 |
0 |
0.0012 |
0.004 |
0.011 |
|
|
Absolute lung weight (mg) |
1180 |
1230 |
1200 |
1200 |
990 |
960 |
940 |
1040 |
|
Lung weight relative to body weight (mg/100 g) |
410 |
490** |
440* |
460** |
190 |
500 |
510 |
550** |
|
Absolute thymus weight (mg) |
340 |
340 |
290 |
280 |
310 |
280 |
270 |
270 |
|
Thymus weight relative to body weight (mg/100 g) |
120 |
130 |
110 |
100 |
150 |
150 |
150 |
140 |
From Pauluhn (2002)
Table 7. Histopathological changes in rats exposed to tolylfluanid by inhalation
|
Histopathological change |
Concentration (mg/l) |
|||||||
|
Males |
Females |
|||||||
|
0 |
0.0012 |
0.004 |
0.011 |
0 |
0.0012 |
0.004 |
0.011 |
|
|
Larynx: mucus and cells in lumen |
0 |
1 |
3 |
4 |
0 |
1 |
2 |
1 |
|
Larynx: focal inflammation |
8 |
10 |
10 |
10 |
6 |
10 |
10 |
10 |
|
Larynx: epithelial squamous metaplasia |
0 |
10 |
10 |
10 |
0 |
10 |
10 |
10 |
|
Lung: peribronchial infiltration |
0 |
4 |
5 |
10 |
0 |
3 |
7 |
10 |
|
Lung: peribronchial fibrosis |
0 |
2 |
1 |
10 |
0 |
0 |
3 |
5 |
|
Lung: epithelial changes |
0 |
1 |
3 |
10 |
0 |
0 |
4 |
8 |
From Pauluhn (2002)
Rabbits
Groups of five Mount Vizcacha rabbits of each sex received tolylfluanid (purity, 98.9%) dermally at a dose of 500 mg/kg bw per day for 14 days. The material was spread on plastic sheets which were applied to clipped skin and secured with leather corsets for 24 h. The rabbits were housed individually with free access to food and water. Blood and urine were collected for haematology, urine analysis and tests for liver and kidney function before treatment, at the end of treatment and at the end of a 14-day observation phase,. The haematological parameters examined were haemoglobin, erythrocyte, leukocyte and thrombocyte counts, erythrocyte volume fraction and mean corpuscular volume. The liver function tests included determination of serum transaminases, sorbitol dehydrogenase, bilirubin and albumin. Urine was analysed for glucose, albumin, blood and bile pigments; the serum urea concentration was also measured. No differences were found between control and treated rabbits with regard to clinical signs, body weight, haematology or liver or renal function (JMPR, 1988; amended by reference to Kimmerle & Solmecke, 1971)
Groups of five HC New Zealand white rabbits of each sex received dermal applications of suspensions of tolylfluanid (purity, 98.6%) in 2% v/v Cremophor EL in normal saline at a dose of 0 (control), 1, 30 or 300 mg/kg bw per day for 3 weeks; satellite groups received 0 and 300 mg/kg bw per day for a study of the reversibility of effects over 14 days. Males received 17 applications of the test substance and females 18 applications. The rabbits were housed individually under controlled conditions, with free access to food and water. The back and flanks of each rabbit were clipped 1 day before dosing and twice weekly for the remainder of the study. Tolylfluanid suspensions (2 ml/kg bw) were placed on small pads consisting of four layers of gauze, which were applied to the clipped application sites and held in position for 6 h with adhesive dressings. The rabbits were restrained during treatment. At the end of each 6-h application, the dressings were removed and the test area was washed with soap and water. Skin irritation on the test sites was assessed on a Draize (1977) scale 24 h after the beginning of each dosing period, i.e. immediately before application of the next dose. In order to quantify any swelling at the application site, skin thickness was assessed before treatment and on days 5, 8, 12, 19 and 22 of the study, and the mean of two readings with a skinfold calliper was recorded. Animals were observed at least once a day for clinical and other signs. Body weight and food consumption were determined weekly. Haematological and blood chemical end-points were measured 6 days before treatment and at necropsy or the end of the recovery phase. The haematological tests included erythrocyte, leukocyte, differential leukocyte, reticulocyte and platelet counts, erythrocyte volume fraction, haemoglobin, calculated mean corpuscular volume, mean corpuscular haemoglobin, mean corpuscular haemoglobin concentration and the presence of Heinz bodies. The blood chemical tests comprised estimates of inorganic P, Na, K, Ca and Cl, urea, glucose, creatinine, cholesterol, total bilirubin, total protein and albumin concentrations, and aspartate and alanine aminotransferase, alkaline phosphatase and glutamate dehydrogenase activity. Liver samples were taken at necropsy and assessed for N- and O-demethylase, cytochrome P450 and triglycerides. At necropsy, any gross pathological changes were noted, and the brain, thyroid, heart, lungs, liver, kidneys, adrenals, spleen, testes and ovaries were weighed. A wide range of organs from animals in the control group and at 300 mg/kg bw per day was examined histopathologically, while examinations of animals at 1 and 30 mg/kg bw per day were restricted to the skin and any organs with macroscopic changes.
No treatment-related deaths, clinical signs or changes in food consumption or body weight occurred. Haematological parameters were not affected. Statistically significant reductions in lymphocyte counts seen in females at 30 and 300 mg/kg bw per day were considered to be of no biological significance, as the values were similar to those of other controls. Blood and liver chemistry were likewise unaffected by treatment. At necropsy, no gross pathological findings, organ weight changes or histopathological changes (except in the skin) were found that were related to treatment, as the responses were isolated, restricted to one sex and showed no dose–response relationship. The only tolylfluanid-induced effects were on the skin at the application sites. No erythematous responses were seen in control groups; skin reddening was observed during treatment in 8/10 animals at 1 mg/kg bw per day and in all animals at 30 and 300 mg/kg bw per day. The mean erythema scores for the three groups showed a positive dose–response relationship for severity of effect and for the onset of erythema. Individual animals showed a range of erythema, with scores ranging from ‘very slight’ through ‘moderate’ to ‘distinct’. The mean scores over the study were 0.32, 0.66 and 1.2 for the groups at 1, 30 and 300 mg/kg bw per day, respectively. In addition, scaly skin changes within the application area were apparent in both males and females in all treated groups, with incidences of 0/19, 3/10, 9/10 and 20/20 at 0, 1, 30 and 300 mg/kg bw per day, respectively. Other dermal changes (e.g. incrustations, open wounds) were seen only in animals at 300 mg/kg bw per day. Skin-fold thickness was enhanced in males at the highest dose and in females at all doses. Histopathological dermal changes (inflammation, infiltrates, acanthosis and hyperkeratosis) were found in all treated groups. These effects were largely reversible during the 2-week recovery phase, hyperkeratosis being the only dermal finding at termination of the test.
Thus, dermal application of tolylfluanid at doses up to 300 mg/kg bw per day for 21 days had no systemic toxic effect. All the doses caused erythema, skin lesions, increased skin thickness and histopathological changes that appeared to be reversible, as judged by the responses in the group at 300 mg/kg bw per day that was allowed to recover. A NOAEL could not be identified, as skin reactions were seen at the lowest dose tested (Kolb et al., 1995).
Dogs
Groups of four male and four female beagles were given diets containing tolylfluanid (purity, 99.7%) at a concentration of 0, 330, 1000 or 3000 ppm for 92 days, equivalent to intakes of 0, 11, 31 and 90 mg/kg bw per day for males and 0, 11, 32 and 98 mg/kg bw per day for females on the basis of final body weight. The animals were housed individually under controlled conditions throughout the study and given 250 g of dry food mashed with water daily. They were inspected daily for abnormal clinical signs and food consumption; body weight was recorded weekly. An ophthalmoscopic examination, and the pupillary reflex, patellar reflex, flexor reflex and extensor thrust were tested before treatment and in weeks 7 and 13. Haematological, clinical chemical and urine parameters were measured before treatment and in weeks 7 and 13. The erythrocyte volume fraction, haemoglobin and erythrocyte, total and differential leukocyte and reticulocyte counts were assessed, and alanine and aspartate aminotransferase and alkaline phosphatase activity and glucose, urea, creatinine, bilirubin and total protein concentrations were determined. The volume and specific gravity of urine were measured, and the urine was tested for albumin, sugar, blood, pH and bilirubin. The sediment was examined microscopically. All tissues were examined grossly and histopathologically post mortem, and the heart, lung, liver, kidney, spleen, pancreas, testes, ovaries, thyroid, adrenal, thymus, prostate, brain and pituitary were weighed.
Animals at 3000 ppm had an ungroomed appearance, a bristly coat and decreased activity from week 5 of the study. By the end of the study, these animals showed slight emaciation. Food intake was decreased in 6/8 dogs at 3000 ppm, by 7% in males and 10% in females. Body-weight gain was decreased at 3000 ppm, by 57% in males and 63% in females. No abnormalities were found in reflexes or on ophthalmological examination. The haematological examination showed no abnormalities. Alanine and aspartate aminotransferase activity was decreased in males at 3000 ppm in weeks 7 and 13, but the toxicological significance of this effect was unclear. Alkaline phosphatase activity was increased in males at 1000 and 3000 ppm and in females at 3000 ppm in weeks 7 and 13. The increases in males at 1000 ppm were slight and were considered not to be treatment-related. No abnormalities were found in urine analysis.
No treatment-related pathological abnormalities were found grossly post mortem. While the weights of a range of organs were increased at 3000 ppm, including the liver, kidney, spleen, thyroid and adrenal gland, these were generally not dose-related. Histopathological examination showed an increased intensity of periodic acid–Schiff staining in hepatocytes from animals at 3000 ppm. This, with the increased liver weight at this dose and the increased alkaline phosphatase activity, indicates some effects on the liver at this dose. The NOAEL was 1000 ppm, equivalent to 31 mg/kg bw per day (JMPR, 1988; amended by reference to Hoffmann & Mirea, 1974).
Groups of four male and four female beagles were given gelatin capsules containing tolylfluanid (purity, 99.2%) as a 90% premix at a dose of 0, 2.5, 12 or 62 mg/kg bw per day from week 1 to 33 and 0, 2.5, 12 or 120 mg/kg bw per day from week 34 to week 52. Up to week 16, 400 g of food were given daily to each dog; subsequently, 450 g per animal per day were supplied. Behaviour and appearance were checked several times daily; food consumption was noted daily, and water consumption was estimated. Body weight was measured weekly. The pupil reaction, corneal reflex, patellar tendon reflex, stretch reflex and righting reflex were assessed before treatment and in weeks 3, 6, 13, 26, 39 and 52; body temperature and pulse rate were also measured at these times. In dogs at 0 and 62/120 mg/kg bw per day, these parameters were also examined in weeks 36 and 45. An ophthalmoscopic examination was done on all dogs before treatment and in weeks 13, 26, 39 and 52. Blood was taken before treatment and in weeks 3, 6, 13, 26, 36 (control and highest dose), 39, 45 (control and highest dose) and 52. The haematological parameters examined were erythrocyte volume fraction, haemoglobin, erythrocyte, total and differential leukocyte, thrombocyte and reticulocyte counts, mean corpuscular volume, mean corpuscular haemoglobin, mean corpuscular haemoglobin concentration, thromboplastin time and blood sedimentation rate. The clinical chemical parameters examined were blood glucose, urea, creatinine, total protein, aspartate and alanine aminotransferase, alkaline phosphatase and glutamate dehydrogenase activity, bilirubin, cholesterol, serum protein, Na, K, Ca and Cl. In week 40, kidney function was tested with phenolsulfonphthalein Urine was collected for 6 h at the same times as blood, and the urine volume and specific gravity were measured. The protein, glucose, blood, bilirubin, ketones and pH of the urine were measured, and the sediment was examined microscopically. All dogs were killed at the end of the study. A gross examination was done post mortem, and the brain, heart, lungs, liver, spleen, kidneys, thyroid, adrenals, testicles, prostate, ovaries and pancreas were weighed. A liver sample was taken and cytochrome P450 and N-demethylase activities were determined. Bone-marrow smears were prepared and examined. A full range of tissues was fixed for histopathological examination.
There were no deaths and no treatment-related effects on appearance or behaviour. The body-weight gain of male controls (+4.4 kg in 12 months) was above the average for other controls in the laboratory (+2.8 kg), but it was significantly reduced at 62 mg/kg bw per day (+1.9 kg in 12 months). Females at this dose also had reduced body-weight gain (+1.7 kg in 12 months). Animals at 62 mg/kg bw per day vomited frequently after administration of the compound. They also had significantly increased serum alanine aminotransferase and glutamate dehydrogenase activities and a retarding of the physiological age-related decrease in alkaline phosphatase activity. No liver alterations were found, however, histologically.
An effect on renal function was seen at the highest dose. Urea and creatinine concentrations were significantly increased, and glycosuria and proteinuria were found in two animals. Slight alterations in cortical tubules (dilatation, epithelial flattening, focal hypertrophy and/or desquamation of the epithelia) were found in all animals at the highest dose. Slightly reduced serum K concentrations were found at the highest dose as compared with control values, possibly as a result of the frequent vomiting. No treatment-related effects were seen on haematological or ophthalmological parameters. The NOAEL was 12 mg/kg bw per day (JMPR, 1988; amended by reference to von Keutz & Nash, 1986).
Tolylfluanid (purity, 96.7–97.2%) was given to groups of four beagles of each sex orally in gelatine capsules at a dose of 0, 5, 20 or 80 mg/kg bw per day for 52 weeks. The animals were housed individually under controlled conditions with free access to food. Initially, each animal was given 350 g of food per day; this was increased in week 27 to 400 g. The animals were observed twice daily for abnormal clinical signs, including changes in appearance and behaviour. Body weight was measured weekly, and food consumption was determined daily. A neurological examination, including assessment of the pupillary reaction, corneal reflex, patellar reflex and stretch and righting reflexes, was administered in weeks 7, 13, 26, 39 and 52, and body temperature was measured. Blood pressure was measured, and an electrocardiogram was performed on each dog once before treatment and in weeks 7, 13, 26, 39 and 52, both before and 2 h after dosing. An ophthalmological examination was done before treatment and in weeks 8, 13, 26, 39 and 52. Blood samples were taken twice before treatment and in weeks 2, 7, 13, 26, 39 and 50. Total and differential leukocyte, erythrocyte, thrombocyte and reticulocyte counts, blood sedimentation rate, haemoglobin, methaemoglobin, erythrocyte volume fraction, mean corpuscular haemoglobin, mean corpuscular volume, mean corpuscular haemoglobin concentration, partial thromboplastin time and prothrombin time were measured. Aspartate and alanine aminotransferase, glutamate dehydrogenase, alkaline phosphatase, gamma-glutamyl transferase, lactate dehydrogenase and creatine kinase activity, protein, albumin, urea, creatinine, bilirubin, cholesterol, glucose, Na, K, Ca, Cl, Mg, P, triglycerides, tri-iodothyronine, thyroxine and thyroxine-binding capacity were determined. Urine was collected before treatment and in weeks 2, 7, 13, 26, 39 and 50. The volume, density, pH, sodium, creatinine, urea, bilirubin, K, blood, protein, glucose, calcium, urobilinogen, ketone bodies, sediment and P concentrations were determined. At the end of the study, all dogs were killed and examined grossly. Liver samples were taken and frozen, and N-demethylase, O-demethylase and cytochrome P450 activities and triglyceride concentration were determined. The brain, heart, liver, lungs, spleen, thymus, adrenals, kidneys, pancreas, thyroid, pituitary, testes, prostate, uterus and ovaries were weighed. A full histopathological examination was done. The fluoride concentration in the bones and teeth was determined.
No deaths occured during the study. Diarrhoea was seen in all groups, with occasional vomiting; however, these were not treatment-related. There were no treatment-related effects on food consumption, body weight or haematological or clinical chemical parameters. No effect was seen on thyroid hormone concentrations. In females at 80 mg/kg bw per day, N-demethylase activity in the liver was slightly elevated, but this effect was not statistically significant and was the result of an abnormally high value in one individual; it was considered not to be treatment-related. No treatment-related effects were found in urine or on physical examination. No treatment-related effects were seen on organ weights or on macroscopic appearance. Histopathological examination showed an increased incidence of vacuolation in the liver in treated females (in 2, 1, 0 and 2 males and 0, 2, 2 and 2 females at 0, 5, 20 or 80 mg/kg bw per day); however, there was no dose–response relationship and this is a common finding in dogs. It was considered not to be treatment-related. A slight increase in lipofuscin deposits in the kidney was seen in males at 20 and 80 mg/kg bw per day, but this also was considered not to be treatment-related. The fluoride concentration in bones was elevated in males at 80 mg/kg bw per day and in females at doses > 20 mg/kg bw per day. The fluoride concentration in teeth was elevated in males at 80 mg/kg bw per day and in females at doses > 5 mg/kg bw per day, although this effect was not dose-related. As increased fluoride concentrations in bones and teeth were seen in females at 5 mg/kg bw per day, a NOAEL could not be identified (Wetzig & Schilde, 1997, 1998).
Mice
Groups of 50 NMRI mice of each sex were given diets containing technical-grade tolylfluanid (purity, 99.1%) in a 90% pre-mix at a concentration of 0, 200, 1000 or 5000 ppm for 104 weeks, equal to an average of 33, 160 and 770 mg/kg bw per day for males and 46, 200 and 950 mg/kg bw per day for females. The animals were observed twice a day for deaths or adverse physical or behavioural signs. Body weights and food consumption were recorded weekly until week 8, then fortnightly for the rest of the study. Haematological (haemoglobin, erythrocyte volume fraction, erythrocyte and total and differential leukocyte counts) and clinical chemical end-points (alkaline phosphatase, glutamate pyruvate transaminase and urea) were evaluated before the start of the study and after 12 and 24 months. All animals, including those which died or were killed before the end of the study, were dissected, and the brain, heart, lung, liver, spleen, kidneys, adrenals, thyroids and testes or ovaries were weighed. Tissues were prepared for histopathological examination. For technical reasons, the treated groups were started 5 months after the control group. This discrepancy in time was considered by the author to be the explanation for the greater body-weight gain and food consumption (about 30% higher) of control animals and for the differences between control and treated groups in haematological and clinical end-points. No dose-related difference in any of these parameters was found in treated animals.
The mortality rate in all female groups was higher than that in males, but no dose-related effect was evident. At necropsy, hepatocellular adenomas and pulmonary adenomas were found in all control and treated groups. The incidence of liver tumours was increased in males, but no treatment-related effect was evident (10/51, 6/50, 13/50 and 6/49 at 0, 200, 1000 and 5000 ppm, respectively). The incidence of lung adenomas was 17/51, 17/50, 20/50 and 20/49 in males and 9/49, 6/50, 14/50 and 7/51 in females at 0, 200, 1000 and 5000 ppm, respectively. The incidences, latency, variety and organ distribution of these tumours did not indicate a dose-related effect. This study is unsuitable for identifying a NOAEL, as the control group did not provide adequate comparison for a range of findings (JMPR, 1988; amended with reference to Mohr, 1982).
Groups of 50 SPF-bred B6C3F1 mice of each sex were given diets containing tolylfluanid (purity, 97.2–98.3%) at a concentration of 0, 60, 300, 1500 or 7500 ppm for 105 weeks. An additional 10 mice of each sex per group were fed diets containing tolylfluanid at these concentrations for 53 weeks. The concentrations were equal to doses of 0, 15, 76, 380 and 2300 mg/kg bw per day for males and 0, 24, 120, 610 and 3000 mg/kg bw per day for females. The doses were chosen on the basis of those used in the previous study (Mohr, 1982). The diet was checked for homogeneity immediately after preparation, and the stability and content of the test substance was verified during the study. The mice were inspected twice daily (once on weekends) for deaths and abnormal clinical signs and examined weekly for changes in body surface, orifices, posture, general behaviour, respiration and excretions. Body weight was measured weekly throughout the study, and food and water consumption were measured weekly for 13 weeks then monthly for the rest of the study for 20 mice per group. Blood samples were taken in weeks 52/53, 79 and 104/105 from 10 mice of each sex per group. The total and differential leukocyte and erythrocyte counts, erythrocyte morphology, haemoglobin, erythrocyte volume fraction, mean corpuscular haemoglobin, mean corpuscular haemoglobin concentration, mean corpuscular volume, platelet count and thromboplastin time were determined. Alkaline phosphatase and aspartate and alanine aminotransferase activity and albumin, bilirubin, cholesterol, total protein, urea, creatinine and glucose concentrations were determined. Fluoride concentrations in teeth and bones were determined in weeks 54 and 106 on 10 animals of each sex per group. All mice were examined macroscopically after death or sacrifice, with complete histopathological examination of tissues that were viable for examination. The adrenals, brain, kidneys, liver, lung, spleen and testes from all animals were weighed at scheduled sacrifice.
All males and 59/60 females at 7500 ppm had white discolouration of the teeth, which was not seen at lower concentrations. The incidences of piloerection and hair loss were slightly increased at 7500 ppm. There were no treatment-related effects on mortality rates. In females, there was no treatment-related effect on body weight, but males at 1500 ppm gained less weight than controls from week 44 and the body weights of males at 7500 ppm were lower than those of controls from week 2. The maximum difference in body weight was 10% at 1500 ppm and 20% at 7500 ppm. No differences in food consumption were seen in females or in males at concentrations up to 1500 ppm. At 7500 ppm, males had increased food consumption, with a 14% greater intake than controls on a per animal basis and a 29% greater intake than controls per kilogram body weight. Water consumption was increased in both sexes at 7500 ppm, by 12% in males and 13% in females on a per animal basis (24% and 12% in males and females per kilogram body weight).
Slight changes in haemoglobin and erythrocyte volume fraction were seen in both sexes at 7500 ppm, but these were not consistent and considered to be of no biological significance. In males, the thrombocyte count was slightly increased at 7500 ppm at both observation times, and prothrombin time was slightly increased in males at 104 weeks. The effects were within the ranges for other controls and were considered to be of no toxicological significance. Clinical chemistry showed increased alkaline phosphatase activity at 1500 and 7500 ppm, and the protein, cholesterol and glucose concentrations were decreased in males at 7500 ppm in week 105. These findings were considered to be treatment-related. The fluoride concentration in bones and teeth was increased in males at 300, 1500 and 7500, while the fluoride concentration in teeth was increased in females at 1500 and 7500 ppm and in bones at 300, 1500 and 7500 ppm (Table 8).
Table 8. Fluoride concentrations in bones and teeth of mice given diets containing tolylfluanid
|
Fluoride (mg/g ash) |
Week |
Dietary concentration (ppm) |
|||||||||
|
Males |
Females |
||||||||||
|
0 |
60 |
300 |
1500 |
7500 |
0 |
60 |
300 |
1500 |
7500 |
||
|
Teeth |
53 |
0.26 |
0.31 |
0.52* |
1.2* |
3.3* |
0.25 |
0.29 |
0.52 |
1.1* |
3.6* |
|
Teeth |
105 |
0.40 |
0.51 |
1.0* |
1.9* |
5.2* |
0.45 |
0.50 |
0.70 |
1.7* |
6.2* |
|
Bone |
53 |
1.1 |
1.2 |
1.8* |
4.0* |
9.9* |
0.93 |
1.1 |
1.7* |
3.6* |
9.4* |
|
Bone |
105 |
1.4 |
1.7 |
2.5 |
5.5* |
11* |
1.2 |
1.5* |
2.4* |
5.6* |
13* |
From Leser & Ruhl-Fehlert (1996)
* p < 0.05
The liver weights were increased in females at 7500 ppm and the kidney weight in males at concentrations > 1500 ppm. Macroscopic examination showed tooth discolouration in all mice at 7500 ppm at the interim kill and in virtually all mice at 7500 ppm at the terminal kill, but was not seen at lower doses. At the terminal kill, other signs consistent with fluoride deposition were discolouration of the skull cap (31 males and 39 females at 7500 ppm) and discolouration and thickening of the bone (1 male at 7500 ppm, and 0, 0, 3, 2 and 10 females at 0, 60, 300, 1500 and 7500 ppm, respectively). Histopathological abnormalities seen in the liver at the interim kill were centrilobular hypertrophy of hepatocytes, basophilic intranuclear inclusion bodies and lymphohistiocytic infiltration. In the kidney, the severity of vacuolization of the tubular epithelium was increased at 1500 and 7500 ppm in males. Subperiostial hyperostosis was seen in the skull cap of females at 1500 and 7500 ppm, and endochondral hyperostosis was seen in the sternum of females at 300, 1500 and 7500 ppm. The incidences of these findings are summarized in Table 9.
Table 9. Histopathological changes after 12 months in mice given diets containing tolylfluanid
|
Histopathological change |
Dietary concentration (ppm) |
|||||||||
|
Males |
Females |
|||||||||
|
0 |
60 |
300 |
1500 |
7500 |
0 |
60 |
300 |
1500 |
7500 |
|
|
Liver: centrilobular hypertrophy |
3 |
0 |
0 |
7 |
10 |
0 |
0 |
0 |
0 |
2 |
|
Liver: basophilic intranuclear inclusion bodies |
0 |
0 |
0 |
0 |
3 |
0 |
0 |
0 |
0 |
0 |
|
Liver: lymphohistiocytic infiltration |
1 |
1 |
1 |
1 |
0 |
1 |
0 |
1 |
4 |
4 |
|
Kidney: vacuolization of tubular epithelium |
9 |
10 |
9 |
10 |
10 |
0 |
0 |
0 |
0 |
0 |
|
Skull cap: subperiostial hyperostosis |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
4 |
|
Sternum: enchondral hyperostosis |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
5 |
10 |
From Leser & Ruhl-Fehlert (1996)
At the terminal kill, histopathological changes were found in the musculoskeletal system of animals at 1500 and 7500 ppm. Focal mineralization, which may indicate an effect on calcium homeostasis, was seen at decreased incidence in a number of tissues, while an increase in kidney papillary mineralization was seen at 7500 ppm. Liver changes were seen at 1500 and 7500 ppm, and the severity of vacuolation in the proximal tubules was increased in males at these concentrations. An increased incidence in lenticular degeneration was seen in males at 7500 ppm. These findings are summarized in Table 10.
Table 10. Histopathological changes after 24 months in mice given diets containing tolylfluanid
|
Histopathological change |
Dietary concentration (ppm) |
|||||||||
|
Males |
Females |
|||||||||
|
0 |
60 |
300 |
1500 |
7500 |
0 |
60 |
300 |
1500 |
7500 |
|
|
Nasal cavity: hyperostosis |
0 |
0 |
0 |
0 |
9 |
0 |
2 |
1 |
6 |
43 |
|
Skull cap: hyperostosis |
0 |
1 |
1 |
5 |
17 |
2 |
4 |
6 |
23 |
44 |
|
Spinal cord: hyperostosis |
0 |
0 |
0 |
0 |
0 |
4 |
1 |
3 |
8 |
44 |
|
Femur: focal spongiosus |
2 |
4 |
1 |
3 |
4 |
5 |
5 |
8 |
12 |
37 |
|
Sternum: hyperostosis |
3 |
1 |
1 |
1 |
2 |
4 |
5 |
9 |
8 |
48 |
|
Cerebrum: mineralization |
39 |
43 |
42 |
36 |
22 |
34 |
26 |
20 |
19 |
5 |
|
Brainstem: mineralization |
4 |
7 |
6 |
1 |
0 |
1 |
1 |
1 |
0 |
0 |
|
Kidney: cortical mineralization |
38 |
33 |
28 |
18 |
16 |
0 |
0 |
1 |
2 |
0 |
|
Kidney: papillary mineralization |
2 |
1 |
3 |
11 |
34 |
6 |
2 |
7 |
5 |
17 |
|
Liver: centrilobular hypertrophy |
2 |
2 |
0 |
26 |
46 |
0 |
0 |
0 |
0 |
0 |
|
Liver: peripheral hypertrophy |
0 |
0 |
0 |
0 |
0 |
4 |
1 |
1 |
14 |
19 |
|
Liver: nuclear inclusion bodies |
1 |
0 |
0 |
10 |
36 |
0 |
0 |
0 |
0 |
0 |
|
Liver: unicellular necrosis |
0 |
1 |
0 |
2 |
25 |
0 |
0 |
0 |
0 |
0 |
|
Liver: biliary cystic change |
0 |
0 |
1 |
0 |
1 |
1 |
0 |
2 |
1 |
9 |
|
Eyes: lenticular degeneration |
5 |
7 |
5 |
9 |
31 |
3 |
6 |
8 |
2 |
3 |
From Leser & Ruhl-Fehlert (1996)
There was no treatment-related increase in the incidence of tumours. On the basis of fluoride deposition in the bones and teeth and histopathological changes in the bone in females at 300 ppm, the NOAEL was 60 ppm, equal to 15 mg/kg bw per day (Leser & Ruhl-Fehlert, 1996).
Rats
Groups of 50 BOR:WISW (SPF Cpb) rats of each sex (100 of each sex for controls) were fed diets containing tolylfluanid (purity, 98.8%) in 90% pre-mix at a concentration of 0, 300, 1500 or 7500 ppm for 2 years, equivalent to 0, 20, 80 and 430 mg/kg bw per day for males and 0, 20, 110 and 580 mg/kg bw per day for females. Additional rats were maintained for examinations and interim sacrifice at 6 and 12 months. The animals were inspected twice daily (once on weekends) for abnormal clinical signs and deaths. Body weight was measured weekly until week 29, then every 2 weeks for the rest of the study. Food consumption was measured weekly. Clinical examinations were made on five animals of each sex per group at 3, 6 and 12 months and 10 of each sex per group at 24 months, and urine was collected over 16 h. Haematological evaluation included erythrocyte, total and differential leukocyte, thrombocyte and reticulocyte counts and measurement of haemoglobin, erythrocyte volume fraction, mean corpuscular haemoglobin, mean corpuscular volume and thromboplastin time. Plasma alkaline phosphatase, aspartate and alanine aminotransferase and glutamate dehydrogenase activities and creatinine, urea, glucose, cholesterol, bilirubin and total protein concentrations were measured. In urine, glucose, blood, protein, pH, ketones, bilirubin, urobilinogen and protein concentrations were determined, and the sediment was examined microscopically. All animals that died during the study or which were killed when moribund were examined macroscopically, and those organs considered evaluable were fixed and examined. Five rats of each sex per group were killed at 6 and 12 months, and all remaining rats were killed, dissected and examined macroscopically at the end of the study. The thyroids, thymus (6 and 12 months only), heart, lung, liver, spleen, kidneys, adrenals, testes and ovaries were weighed. A full range of tissues was examined histopathologically.
The animals at the highest concentration had reduced food consumption (by 10%) and reduced (by about 10%) body-weight gain; appearance, behaviour and mortality were not affected by treatment. Clinical laboratory tests showed random alteration of some blood parameters (mean corpuscular volume, mean corpuscular haemoglobin, reticulocyte, polymorphonuclear cell and lymphocyte counts). There was no dose-related change in liver enzymes and no gross or histopathological evidence of liver damage. Urine analysis did not indicate any dose-related renal alteration. The values for other clinical chemical parameters were within normal ranges. On autopsy, bone alterations (diffuse hyperostosis of the rib bones, hardened cranial bones, focal hyperostosis of the skull caps) were found in both sexes and faster growth of the incisors of the upper jaw in males only at 1500 and 7500 ppm. These effects were considered to be due to increased fluorine intake from the active ingredient.
Tumours of the endocrine and reproductive systems accounted for about 80% of those seen in the study. Only malignant uterine tumours appeared at higher incidence in all treated groups as compared with controls, with incidences of 3/50, 8/50, 12/50 and 13/50 at 0, 300, 1500 and 7500 ppm, respectively. As an unusually low incidence of these fairly common tumours was found in control animals, a second, concurrent control group of 50 rats was examined, in which the incidence of malignant uterine tumours was similar (9/50) to that of the treated group. The test therefore did not indicate that tolylfluanid is carcinogenic. The NOAEL was 300 ppm, equivalent to 20 mg/kg bw per day (JMPR, 1988; amended by reference to Krötlinger & Löser, 1982).
Groups of 50 BOR:WISW (SPF Cpb) Wistar rats of each sex were given diets containing tolylfluanid (purity, 97.2–98.3%) at a concentration of 0, 60, 300, 1500 or 7500 ppm for 105 weeks, and groups of 10 animals of each sex were treated for 52 weeks. These concentrations resulted in doses of 0, 3.6, 18, 90 and 500 mg/kg bw per day for males and 0, 4.2, 21, 100 and 580 mg/kg bw per day for females. The animals underwent a detailed examination each week, including palpation of body surfaces and examination of orifices, posture, respiration and excretions. Body weight was measured weekly, and food and water consumption was measured weekly for the first 13 weeks then every 4 weeks until the end of the study. An ophthalmological examination was conducted before treatment and at weeks 52 and 104. Blood was collected for haematology and clinical chemistry in weeks 27/28, 53/54, 79 and 105 from 10 animals of each sex per group. Total and differential leukocyte, erythrocyte and platelet counts, erythrocyte morphology, haemoglobin, erythrocyte volume fraction, mean corpuscular haemoglobin, mean corpuscular haemoglobin concentration, mean corpuscular volume and thromboplastin time (week 105 only) were measured. Alkaline phosphatase and aspartate and alanine aminotransferase activity and albumin, bilirubin, cholesterol, creatinine, total protein, triglycerides, urea, Cl, Ca, K, Na, inorganic P and glucose were measured. Urine was collected over 16 h at the same intervals, and the concentrations of blood, bilirubin, glucose, ketone bodies, pH, protein, sediment, urobilinogen, Cl, creatinine, K, Na, protein and urea and the volume and osmolality were measured. At weeks 52 and 105, the fluoride concentrations in the bones and teeth of 10 rats of each sex per group were measured. A full macroscopic examination was performed on all rats that died during the study, and suitable tissues were preserved for histopathological examination. At the interim and final sacrifices, full macroscopic and histopathological examinations were done, and the adrenals, brain, heart, liver, lungs, kidneys, spleen and testes were weighed.
The incisors of rats at 7500 ppm required cutting more frequently than those of other animals, perhaps due to increased fluoride deposition, resulting in stronger teeth that were more resistant to abrasion. There were no treatment-related changes in the incidence of palpable masses, and no treatment-related effects on mortality rates. No abnormalities were found on ophthalmological examination. The body weight of rats at 7500 ppm was statistically significantly lower than that of controls from week 1 until the end of the study, the final weight being 9% lower for males and 12% lower for females. The body-weight gain of animals at 7500 ppm was decreased throughout the study, and their food and water consumption was slightly increased on the basis of grams per kilogram body weight but not on the basis of grams per animal per day.
No treatment-related haematological changes were observed during the study. In week 105, the urea and creatinine concentrations in males at 7500 ppm were increased, but this finding was due to high concentrations in one rat and is considered not to be treatment-related. Serum alkaline phosphatase and alanine aminotransferase activities were decreased in rats at 1500 and 7500 ppm, but the toxicological relevance of this finding is unclear. These findings are presented in Table 11.
Table 11. Changes in serum enzyme activity in rats given diets containing tolylfluanid
|
Clinical chemical parameter (U/l) |
Week |
Dietary concentration (ppm) |
|||||||||
|
Males |
Females |
||||||||||
|
0 |
60 |
300 |
1500 |
7500 |
0 |
60 |
300 |
1500 |
7500 |
||
|
Alkaline phosphatase |
27 |
210 |
210 |
200 |
200 |
170* |
140 |
140 |
140 |
130 |
110 |
|
53 |
170 |
170 |
170 |
160 |
140** |
100 |
120 |
110 |
100 |
87 |
|
|
79 |
170 |
160 |
170 |
140* |
120* |
110 |
95 |
120 |
98 |
81 |
|
|
105 |
140 |
150 |
140 |
130 |
110 |
100 |
110 |
110 |
97 |
74** |
|
|
Alanine aminotransferase |
27 |
39 |
42 |
38 |
34 |
28* |
45 |
41 |
37 |
41 |
28** |
|
53 |
41 |
42 |
39 |
37 |
30* |
45 |
47 |
42 |
34** |
35** |
|
|
79 |
40 |
42 |
45 |
31 |
30 |
42 |
45 |
52 |
42 |
39 |
|
|
105 |
31 |
34 |
32 |
28 |
23 |
40 |
41 |
44 |
36 |
36 |
|
From Leser et al. (1996, 1997)
* p < 0.05; ** p < 0.01
Urine analysis showed a tendency towards decreased osmolality and increased urine volume and K and Cl excretion at 7500 ppm. Urine osmolality was statistically significantly decreased and urine volume statistically significantly increased in all treated females at week 53; however, this was due to an abnormally low urine volume in control females and was considered not to be treatment-related. These findings are presented in Table 12.
Table 12. Findings of urine analysis in rats given diets containing tolylfluanid
|
Urine parameter |
Week |
Dietary concentration (ppm) |
|||||||||
|
Males |
Females |
||||||||||
|
0 |
60 |
300 |
1500 |
7500 |
0 |
60 |
300 |
1500 |
7500 |
||
|
Osmolality (mosmol/kg) |
27 |
1450 |
1210 |
1100 |
1040 |
800 |
950 |
880 |
880 |
770 |
740 |
|
53 |
1540 |
1430 |
1460 |
1290 |
1220 |
1920 |
1100* |
990** |
1190* |
750** |
|
|
79 |
1440 |
1560 |
1570 |
1160 |
920 |
1120 |
1080 |
1320 |
1100 |
890 |
|
|
105 |
1250 |
1320 |
1320 |
1420 |
1120 |
830 |
820 |
920 |
880 |
770 |
|
|
Urine volume (ml) |
27 |
6 |
8 |
7 |
10 |
11 |
5 |
7 |
7 |
9 |
11 |
|
53 |
5 |
6 |
5 |
6 |
7 |
2 |
5* |
7** |
6* |
9** |
|
|
79 |
6 |
5 |
4 |
7 |
10 |
5 |
6 |
6 |
6 |
8 |
|
|
105 |
6 |
5 |
6 |
5 |
7 |
7 |
7 |
8 |
7 |
9 |
|
|
Potassium (mol/l) |
27 |
1.2 |
1.2 |
1.1 |
1.3 |
1.3 |
0.57 |
0.73 |
0.84* |
0.82* |
1.0** |
|
53 |
1.0 |
1.0 |
0.97 |
1.1 |
1.3 |
0.49 |
0.72* |
0.72* |
0.79* |
0.88* |
|
|
79 |
0.96 |
0.94 |
0.97 |
1.2 |
1.4 |
0.93 |
0.99 |
0.79 |
0.96 |
1.2 |
|
|
105 |
1.1 |
0.95 |
1.4 |
1.0 |
1.1 |
0.86 |
0.88 |
1.1 |
1.0 |
0.95 |
|
|
Chloride (mol/l) |
27 |
0.38 |
0.35 |
0.28 |
0.41 |
0.60 |
0.17 |
0.17 |
0.21 |
0.26 |
0.45** |
|
53 |
0.26 |
0.21 |
0.21 |
0.31 |
0.55 |
0.08 |
0.10 |
0.13 |
0.14 |
0.23** |
|
|
79 |
0.20 |
0.22 |
0.25 |
0.30 |
0.52* |
0.30 |
0.30 |
0.17* |
0.32 |
0.56* |
|
|
105 |
0.25 |
0.21 |
0.26 |
0.29 |
0.47 |
0.30 |
0.30 |
0.35 |
0.38 |
0.41 |
|
From Leser et al. (1996, 1997)
* p < 0.05; ** p < 0.01
The fluoride content of bones and teeth was increased in animals at dietary concentrations > 1500 ppm during the study; the concentrations in teeth were increased at 300 ppm in week 53 but not in week 105. The findings are presented in Table 13.
Table 13. Fluoride content of bones and teeth of rats given diets containing tolylfluanid
|
Fluoride (mg/g ash) |
Week |
Dietary concentration (ppm) |
|||||||||
|
Males |
Females |
||||||||||
|
0 |
60 |
300 |
1500 |
7500 |
0 |
60 |
300 |
1500 |
7500 |
||
|
Teeth |
53 |
0.12 |
0.17 |
0.34* |
0.75* |
2.7* |
0.15 |
0.19 |
0.39* |
0.95* |
3.6* |
|
105 |
0.31 |
0.25 |
0.45 |
1.1* |
3.5* |
0.33 |
0.28 |
0.43 |
1.1* |
3.8* |
|
|
Bone |
53 |
0.41 |
0.53 |
0.89 |
2.4* |
4.7* |
0.65 |
0.76 |
1.2 |
3.2* |
7.7* |
|
105 |
0.38 |
0.83 |
1.3 |
3.3* |
9.7* |
0.81 |
0.94 |
1.4 |
3.5* |
11* |
|
From Leser et al. (1996, 1997)
* p < 0.05; ** p < 0.01
Relevant macroscopic findings in rats that died during the study and in rats killed at the interim and terminal sacrifices were whitish discolouration of the skull at 7500 ppm and discolouration of the teeth in females at 7500 ppm and in males at concentrations > 300 ppm. These findings were considered to be related to fluoride deposition. At the interim sacrifice, the histopathological changes included focal hyperostosis in the skull with altered bone matrix at 7500 ppm, follicular mineralization of the thyroid in females at 7500 ppm, Leydig-cell hyperplasia in males at 7500 ppm and hepatocyte hypertrophy in females at 7500 ppm. These findings are presented in Table 14. At the termination, histopathological changes were seen in the liver, bones, kidneys, thyroid glands and lungs, mainly from animals at 7500 ppm. The increased incidence of Leydig-cell hyperplasia seen at the interim sacrifice was not confirmed at the final sacrifice. The findings are presented in Table 15.
Table 14. Histopathological changes seen at interim (12 month) sacrifice in groups of 10 rats of each sex given diets containing tolylfluanid
|
Histopathological change |
Dietary concentration (ppm) |
|||||||||
|
Males |
Females |
|||||||||
|
0 |
60 |
300 |
1500 |
7500 |
0 |
60 |
300 |
1500 |
7500 |
|
|
Skull: focal hyperostosis |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
2 |
4 |
|
Skull: altered bone matrix |
0 |
0 |
0 |
0 |
3 |
0 |
0 |
0 |
0 |
2 |
|
Thyroid: follicular mineralization |
7 |
4 |
4 |
2 |
4 |
2 |
2 |
2 |
3 |
6 |
|
Testes: Leydig-cell hyperplasia |
0 |
0 |
1 |
1 |
4 |
|||||
|
Liver: Hepatocyte hypertrophy |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
1 |
1 |
3 |
From Leser et al. (1996, 1997)
Table 15. Histopathological changes seen at terminal (2 years) sacrifice in rats given diets containing tolylfluanid
|
Histopathological change |
Dietary concentration (ppm) |
|||||||||
|
Males |
Females |
|||||||||
|
0 |
60 |
300 |
1500 |
7500 |
0 |
60 |
300 |
1500 |
7500 |
|
|
Liver: portal cytoplasm change |
0 |
0 |
0 |
0 |
19 |
0 |
0 |
0 |
0 |
2 |
|
Liver: focal cytoplasm change |
0 |
0 |
0 |
0 |
6 |
0 |
0 |
0 |
0 |
0 |
|
Liver: hepatocellular alteration |
0 |
0 |
0 |
0 |
0 |
1 |
2 |
0 |
1 |
35 |
|
Liver: hepatocellular vacuolation |
7 |
7 |
7 |
7 |
6 |
7 |
9 |
5 |
5 |
38 |
|
Liver: focal fatty change |
3 |
0 |
2 |
2 |
0 |
2 |
1 |
1 |
2 |
8 |
|
Sternum: osteopetrosis |
0 |
0 |
0 |
0 |
41 |
0 |
0 |
0 |
0 |
29 |
|
Skull: focal hyperostosis |
0 |
2 |
0 |
1 |
31 |
0 |
0 |
1 |
0 |
25 |
|
Kidney: tubular pigment deposition |
0 |
0 |
0 |
1 |
1 |
4 |
2 |
0 |
3 |
36 |
|
Kidney: papillary mineralization |
4 |
1 |
1 |
5 |
22 |
27 |
29 |
19 |
25 |
28 |
|
Testes: Leydig-cell hyperplasia |
25 |
26 |
25 |
18 |
18 |
|||||
|
Thyroid: follicular-cell hyperplasia |
2 |
1 |
0 |
0 |
7 |
1 |
0 |
1 |
0 |
7 |
|
Thyroid: follicular-cell adenoma |
0 |
1 |
1 |
1 |
5 |
0 |
0 |
0 |
0 |
5 |
|
Thyroid: follicular-cell carcinoma |
0 |
0 |
0 |
0 |
1 |
0 |
1 |
0 |
0 |
0 |
|
Lung: alveolar histiocytosis |
19 |
17 |
19 |
24 |
27 |
13 |
9 |
12 |
11 |
24 |
From Leser et al. (1996, 1997)
The only treatment-related neoplasms were thyroid follicular-cell adenomas, which were associated with thyroid follicular-cell hyperplasia, perhaps associated with the increased concentrations of TSH and alterations in the thyroid feedback mechanism. The liver changes seen were consistent with the decreased enzyme activity in animals at 7500 ppm and were considered not to be adverse. The histopathological effects in the kidney in rats at 7500 ppm correlated with the increased urine volume and decreased osmolality, suggesting progressive renal damage. On the basis of increased fluoride deposition in teeth at 300 ppm, the NOAEL was 60 ppm, equal to 3.6 mg/kg bw per day (Leser et al., 1996, 1997).
The mutagenic and genotoxic potential of tolylfluanid was investigated in a battery of tests in vitro and in vivo (Table 16). The results were largely negative. Although some positive findings were reported, they were observed only at cytotoxic doses or contradicted other findings.
Table 16. Results of tests for genotoxicity with tolylfluanid
|
End-point |
Test object |
Concentration |
Purity (%) |
Results |
Reference |
|
In vitro |
|||||
|
Reverse mutationa,b |
S. typhimurium TA98, TA100, TA1535, TA1537 |
0–2500 µg/plate initially, |
100 |
Weakly positive in TA 100 onlyd |
Herbold (1979) |
|
Reverse mutationa,e |
S. typhimurium TA98, TA100, TA1535, TA1537 |
0–5000 µg/plate |
98.5 |
Negative |
Herbold (1994a) |
|
Reverse mutationf,g |
S. cerevisiae S138 and S211alpha |
0–12.5 µg/ml |
99.1 |
Negative |
Hoorn (1984) |
|
Gene mutationh |
Chinese hamster V79 cell line |
4–40 ng/ml –S9, |
98.5 |
Negative |
Heidemann & Miltenburger (1987) |
|
Gene mutationh |
Chinese hamster ovary cells (CHO-K1-BH4) |
0.5–6.0 µg/ml –S9, |
98.5 |
Negative |
den Boer (1987) |
|
Forward mutationh,j |
Mouse lymphoma cells (L5178Y) |
25–300 ng/ml –S9, |
99.1 |
Positivek |
Hoorn (1985) |
|
Chromosomal aberrationh |
Chinese hamster V79 cells |
Cytotoxicity: 0–40 µg/ml |
97.1–98.9 |
Weakly positive in presence of S9 at 30 h incubationl |
Herbold (1996) |
|
Chromosomal aberrationh,m |
Human lymphocytes |
0–10 µg/ml |
99.2 |
Positiven |
Herbold (1984a, 2000a) |
|
Uncheduled DNA synthesisp |
Rat hepatocytes |
Cytotoxicity: 0.98–500 µg/ml, 0.78–200 µg/ml |
98.7 |
Negative |
Brendler-Schwaab (1995) |
|
In vivo |
|||||
|
Micronucleus formationo |
NMRI mouse bone marrow |
2 × 250, 2 × 500 mg/kg bw orally, 24 h apart |
93.4 (90% premix) |
Negative |
Herbold (1980) |
|
Germ-cell mutationp |
NMRI mouse |
0, 500, 1650, 5000 mg/kg bwq |
97.9 |
Negative |
Völkner (1988a) |
|
Micronucleus formationr |
Chinese hamster bone marrow |
4000 mg/kg bw orally, assessed after 6, 24, 48 h |
99.7 |
Negatives |
Herbold (1983, 2000b) |
|
Chromosomal aberration |
Chinese hamster bone marrow |
4000 mg/kg bw orally, assessed after 12 h |
97.9 |
Negative |
Völkner (1990) |
|
Mutationo |
Chinese hamster spermatogonia |
2 × 250, 2 × 500 mg/kg |
93.1 |
Negative |
Herbold (1984b) |
|
Dominant lethal mutationu |
Bor: NMRI mice |
4000 or 8000 mg/kg bwv |
98.8 |
Negative |
Herbold (1986) |
|
Sister chromatid exchangew |
NMRI mice |
0, 500, 1670, 5000 mg/kg bwx |
97.9 |
Negative |
Völkner (1988b) |
|
Recessive gene mutationp |
C57B1/6J × T mice |
0, 1750, 3500, 7000 mg/kg bw orally on day 10 of gestationy |
98.4 |
Negative |
Herbold (1988) |
|
DNA adduct formationp |
Wistar rat lung, liver, thyroid |
0, 1500, 7500 ppm in diet for 21 daysz |
100.5 |
negative |
Calcagnotto et al. (1997) |
|
S9, 9000 × g supernatant of polychlorinated biphenyl-induced rodent liver; QA, quality assurance; GLP, good laboratory practice |
|
|
a |
Positive controls included, S9 fraction of Aroclor-induced rat liver |
|
b |
Cytotoxicity seen at 500 and 2500 µg/plate and from 200 µg/plate in repeat test |
|
c |
Repeat test with TA100 only |
|
d |
TA100 showed an increased mutant frequency to 1.5 times negative control values at 100 µg/plate. In a repeat test, the mutation frequency was increased at 100 and 200 µg/plate by 1.5 times negative control value. |
|
e |
Cytotoxicity seen at ž 10 µg/plate , with limited use of doses up to 80 µg/plate; precipitation seen at 5000 µg/plate |
|
f |
Positive controls included; S9 fraction of Aroclor-induced rat liver, QA statement provided |
|
g |
Extreme cytotoxicity seen from 20 µg/ml |
|
h |
Positive controls included; S9 fraction of Aroclor 1254-induced rat liver, GLP and QA statements included |
|
i |
Cell survival, 24–28% at 40 ng/ml –S9, 32–33% at 3000 ng/ml +S9 |
|
j. |
Total lethality seen at > 300 ng/ml without activation and 7500 ng/ml with activation |
|
k |
Mutations were dose-related but were seen only in the presence of cytotoxicity. |
|
l |
No biologically relevant decrease in mitotic index, but survival index with activation was 46% of control value in this test group. |
|
m |
Cytotoxicity seen from 1 µg/ml |
|
n |
Positive results only in cytotoxic range; however, as findings other than chromosomal gaps were included, the relationship to toxicity is not entirely clear. |
|
o |
Positive control included, study conducted prior to GLP requirement |
|
p |
Positive control included; test performed to GLP and QA |
|
q |
Signs of toxicity, including convulsions and dyspnoea, seen at all doses; no deaths |
|
r |
Positive control included, assessment consistent with GLP according to amendment published in 2000 |
|
s |
Statistically significant increases in aberration frequencies were considered due to abnormally low control values. Values were well below those in positive controls. |
|
t |
Precipitate seen at ž 125 µg/ml; unacceptable toxicity in genotoxicity test at 18 and 20 µg/ml . Positive control was active. |
|
u |
Study conducted prior to GLP requirements; QA statement included |
|
v |
5/50 rats at 4000 mg/kg bw and 12/50 rats at 8000 mg/kg bw died in first few days after dosing, and an additional 1/50 at |
|
4000 mg/kg bw and 2/50 and 8000 mg/kg bw died later in the study. No effects on fertility or pre- or post-implantation losses |
|
|
w |
Positive control included and active; GLP statement included |
|
x |
Deaths in all treated groups, with 2, 7 and 6 mice dying at 500, 1670 and 5000 mg/kg bw, respectively. Treatment-related clinical signs seen in all treated groups |
|
y |
Deaths and toxic signs in all treated groups; signs included apathy, decreased motility, decreased reflexes, weight loss, staggering or high-stepping gait, breathing difficulty and decreased food and water consumption. Survivor recovered fully by 10 days after treatment. No effects on fetal survival or litter size |
|
z |
Depressed food consumption and body-weight gain after treatment |
Rats
Groups of 10 male and 20 female Elberfeld Long Evans FB30 rats were fed diets containing technical-grade tolylfluanid (purity, 98.8%) in a 90% pre-mix at a concentration of 0, 300, 1500 or 7500 ppm, equivalent to 0, 15, 75 and 380 mg/kg bw per day. After 70 days of treatment, pairs of F0 females were mated with one male each. After delivery (F1a), a 4-week lactation period and a 2-week waiting period, F0 rats were mated again. After delivery (F1b), lactation was allowed for 4 weeks, and then the F0 animals were killed. Twenty F1b females and 10 males at each dietary concentration were then mated to obtain the F2a and F2b generations. The study was ended when the F2b generation reached 4 weeks of age. The rats were weighed weekly, and pups were weighed at birth, 5 and 7 days after birth and weekly thereafter. Pups were observed immediately after birth and during lactation.
The F0 generation showed no abnormal clinical signs and no behavioural changes. Body weight was reduced in a dose-related manner for males at 1500 and 7500 ppm and for females at 7500 ppm. Neither the fertility indices not litter size in either mating was affected by treatment. The survival rate to day 5 of pups of the F1b generation at 7500 ppm was slightly reduced. The lactation index (per cent pups surviving for 4 weeks) in the F1a generation at 7500 ppm was reduced, but that of the F1b generation at 7500 ppm was higher than that of controls. The body weight at birth and body-weight gain of pups of the F1a and F1b generations at 7500 ppm were significantly reduced. None of the pups showed malformations.
The F1b generation also showed no abnormal clinical signs and no behavioural changes. The body weight of those at 7500 ppm was reduced. Neither fertility indices nor litter size in either mating was affected by treatment. The survival rate to day 5 was decreased in the F2b generation at 7500 ppm. The lactation index was reduced in both generations at 7500 ppm and in the F2b generation at 1500 ppm; however, the absolute number of pups at 1500 ppm that survived for 4 weeks was the same as in the control group, the lower lactation index being due to a higher number of pups at day 5. The body weight of pups at birth and body-weight gain were reduced in both generations at 7500 ppm. None of the pups showed malformations.
The NOAEL was 300 ppm, equivalent to 15 mg/kg bw per day (JMPR, 1988; amended by reference to Löser, 1980).
Reproductive parameters were assessed in two generations of SPF Wistar Bor:WISW (SPF Cpb) rats, each of the groups of 25 parents group delivering two litters. The rats were housed individually, except during mating, under controlled conditions. About 100 days before the first mating (1:1), the F0 parents were given diets containing tolylfluanid (purity, 89.1–91.5%) at a concentration of 0, 300, 1200 or 4800 ppm, which were continued throughout the rest of the study. Food consumption was recorded twice weekly until mating. Animals were weighed weekly; after confirmation of insemination, the dams were weighed on days 1, 6, 15 and 20. The number of pups in each litter was reduced to eight on day 5 after birth. In the first generation, the F1a pups were weaned at 4 weeks of age and then killed. The F1b pups were weaned at 4 weeks of age, and 25 of each sex per group were selected as the next parental generation, to be mated at about 100 days of age. The F2a and F2b pups were raised to weaning (4 weeks) and then killed. The parent groups (F0 and F1b) were killed after weaning of their second litters.
Reproductive indices, assessed at appropriate times during the study, included the insemination index (number of inseminated females × 100 divided by number of mated females), the fertility index (number of pregnant females × 100 divided by number of inseminated females), the gestation index (number of females with surviving litters × 100 divided by number of pregnant females), the viability index (number of pups still alive after 5 days × 100 divided by number of live pups at birth) and the lactation index (number of pups still alive after 4 weeks × 100 divided by number of surviving pups after 5 days). In addition to the data required to calculate the above indices, the sex of the pups and their mean weights (on a litter basis) were recorded. A note was also made of any pups with external malformations. Litter weights were measured weekly during lactation.
After weaning of their second litters the F0 and F1b parents were killed and examined macroscopically, and the brain, pituitary, adrenals, liver, kidneys, testes, epididymides, seminal vesicles, prostate, ovaries, uterus, vagina and any other tissue showing macroscopic lesions were fixed for histological examination. Histological examinations were restricted to animals in the control group and at 4800 ppm, with the exception of tissues from animals at 300 and 1200 ppm that died during the study and tissues that had macroscopic changes at sacrifice. Food, water and housing conditions were controlled and monitored. Analyses of the feed for tolylfluanid showed that the mixes had satisfactory homogeneity; however, the stability of the compound in the mix containing the lowest concentration was poor after about 5 days. Nevertheless, in six spot checks during the study, the concentrations were within a satisfactory range of the theoretical concentrations.
The male and female F0 and F1b parental animals showed no treatment-related effects on mortality rate or behaviour; however, marked tooth growth was seen at 4800 ppm, with 11/25 F0 males and 25/25 F0 females affected. This effect was much less marked in the F1b parents (males, 0/25; females, 6/25). Although the food intake of the F0 and F1b parents was not appreciably affected by treatment, body weight and body-weight gain were reduced at 1200 and 4800 ppm. Some decreases were also seen at 300 ppm, but these were intermittent and were considered not to be an adverse effect. Analysis of food intakes and body weights enabled calculation of the mean intakes of tolylfluanid by the parental animals: 300 ppm, 23 mg/kg bw per day; 1200 ppm, 97 mg/kg bw per day; and 4800 ppm, 420 mg/kg bw per day.
At sacrifice, the absolute testicular weights and absolute ovarian weights of animals were significantly reduced in animals at dietary concentrations > 1200 ppm and at 4800 ppm, respectively. However, the changes were largely a reflection of the decreased body weights of the animals, since the testis:body weight ratios showed no deviation from control values; although the ratio for ovarian weight still indicated a small but statistically significant effect at 4800 ppm, in the absence of histological changes, its biological significance is doubtful. At sacrifice, pathological examination showed only one feature that was obviously related to treatment, which was hardening of the cranium in 19/25 male and 3/25 female F0 rats at 4800 ppm. When the lesions were examined histologically, they were compatible with hyperostosis. Similar findings had been reported in other studies with tolylfluanid. As the relevant tissues from groups other than F0 animals at 4800 ppm were not available for examination, a NOAEL for this effect could not be identified.
Tolylfluanid appeared to have no biologically significant effect on gestational parameters. Thus, for the four litters, F1a, F1b, F2a and F2b, produced by the parent groups, no treatment-related effects were found on the insemination index, the fertility index, the gestation index, the mean gestation period, the sex ratio of the pups born or the number of stillbirths. The statistically significant reductions in the number of pups born and the mean litter size in the F2b generation at 4800 ppm are unlikely to be of biological significance, as the effects were small and were not seen in the other three litters. Effects that might have been related to treatment were small reductions in pup weights at birth at 4800 ppm and reductions in the weight gain of pups to weaning at 4800 ppm for the F1b, F2a and F2b litters and at 1200 and 4800 ppm for the F1a litter. The 5-day survival (viability index) was not affected to a biologically significant degree by treatment at any dose. The 4-week survival (lactation index) was statistically significantly decreased in F1a pups at all dietary concentrations, while no significant effects were seen for the F1b pups. Within the F1a group, only a poor dose–response relationship was seen for the reduction in lactation index: a marked effect was seen at 4800 ppm (60% decrease) as opposed to 300 and 1200 ppm (30% decrease). The effect was therefore considered not to be treatment-related. The lactation index was decreased in both the F2a and F2b litters at 4800 ppm.
Thus, at doses that produced clear signs of toxicity in the parents (reductions in body weight, hyperostosis and tooth elongation), tolylfluanid had no adverse effects on parental reproductive parameters. Decreased postnatal pup growth (NOAEL, 300 ppm) and survival to weaning (NOAEL, 1200 ppm) were noted. The NOAEL for parental toxicity was 300 ppm, equal to 23 mg/kg bw per day (Holzum & Kaliner, 1989).
In a supplemental two-generation study, with two litters per generation, conducted in a manner directly comparable to the previous study, groups of 30 rats of each sex received diets containing tolylfluanid at 0 or 180 ppm. Minor changes to the protocol included measurement of the viability index as the number of live pups at 4 (rather than 5) days as a percentage of live pups at birth, as the litters were reduced to a maximum of eight pups on day 4 rather than day 5 after birth; and measurement of the lactation index as the number of live pups after 3 (rather than 4) weeks as a percentage of the number of live pups after 4 days (after reductions in litter sizes). In this study, greater care was taken in assessing the tolylfluanid concentrations in the diet, and the analytical results attest to better, more frequent preparation and improved storage conditions, which allowed the concentration to be maintained within acceptable limits. An additional feature was examination of the cranial domes of all rats, whereas, in the previous study, the crania of only animals at 4800 ppm were assessed; hyperostosis was present in both males and females in this group.
In the F0 and F1b parent groups, ingestion at 180 ppm did not affect mortality rates, behaviour, appearance or body weights of male or female rats. Food consumption was consistently lower in the treated groups than in the controls (range, 7–30%), which was attributed to the more frequent (thrice a week) food changes for the treated group in order to maintain the tolylfluanid concentration within acceptable limits. While there is little doubt that the effect might have been due to tolylfluanid, its biological significance is doubtful given the absence of effects on body weight and other parameters. The calculated intakes of tolylfluanid during the study were 16 mg/kg bw per day for F0 males, 17 mg/kg bw per day for F0 females, 20 mg/kg bw per day for F1b males and 22 mg/kg bw per day for F1b females. The overall intake over the two generations and sexes was thus 19 mg/kg bw per day.
At necropsy, no gross pathological changes attributable to treatment were noted. The only histopathological effect that could be attributed to treatment was skull thickening in 29/30 males and 27/30 females of the F0 generation at 180 ppm. This effect was not observed in F1b parents at sacrifice. In a later, amended report, the finding of skull thickening in the F0 generation at 180 ppm was revoked after quantitative evaluation of the skull thicknesses, rather than the qualitative evaluations that formed the basis for the first report. The weights of the liver, spleen, kidney, testes and ovaries were unaffected by treatment.
Treatment of the F0 and F1b parents had no effect on reproductive parameters in the F1a, F1b, F2a and F2b offspring, comprising insemination index, fertilization index, gestation index, gestation period, number of mated females, number of pups born, liveborn index, number of stillbirths, sex ratio, litter size, pup weight, pup body-weight gain to postnatal day 21, viability index to day 4, lactation index, pups with malformations and pups with gross pathological changes at sacrifice. Occasional differences between treated and control groups that reached statistical significance were regarded as being of no biological relevance, as they were minor, isolated in one of the four groups of offspring or within the values for other controls in the same laboratory. The only parameter that was affected was the lactation index, which appeared to be unaffected by treatment in the F1a, F2a and F2b litters but was reduced in the F1b litter (69%, with 91% for controls. The survival rate among other controls at 28 days (rather than 21 days) was > 80% (mean, 94%), although a lactation index of 64% was found in 1/13 tests. In view of the absence of other signs of toxicity, the result for the F1b lactation index might be regarded as an incidental aberration. Overall, tolylfluanid when fed to rats at 180 ppm in the diet appeared to have no adverse effect (Holzum, 1991a, 1995).
A two-generation study was conducted in which Wistar (Bor:WISW) rats received diets containing tolylfluanid (purity, 95.7–97.9%) at a concentration of 0, 100, 700 or 4900 ppm. Each of the parental generations (F0 and F1b) had two litters (F1a, F1b and F2a, F2b). The F0 parents were placed on the tolylfluanid-containing diet 70 days before mating, and the F1b parents were kept on the diets and mated at a minimum of 14 weeks of age. The rats were housed individually (except during mating) under controlled conditions. Initially, 30 rats of each sex per group were used for each of the parental groups, except that only 20 male and 28 female F1b parents at 4900 ppm were available as second-generation parents owing to high pup mortality. If required, all litters were reduced to eight pups on day 4 post partum. The remaining pups were retained to weaning, before being killed. Parental animals were killed for examination after weaning of their second litters.
Analyses of the diets for tolylfluanid content, stability and homogeneity were satisfactory. The diets containing 700 and 4900 ppm were presented weekly, while new diet was offered four times weekly to animals given 100 ppm, because of the instability of tolylfluanid. After verification of insemination, new food was offered on days 0, 7, 14 and 20 of gestation and then on days 0, 4, 7, 14 and 21 post partum. Food consumption was calculated at each change of diet, and the animals (and pups) were weighed at the same time. At times other than gestation and weaning, females were weighed weekly. Animals were observed for clinical and other changes at least twice daily thought the test.
The estrus frequency of 15 parental females per group was assessed during the last 2 weeks of the premating period. After mating (1:1, four night sessions per week for 3 weeks), vaginal smears were taken and the presence of a vaginal plug or the presence of sperm was taken as an indication of insemination (day 0 of gestation). After parturition, live and dead pups were counted, and their sex, litter size, weight and any abnormalities were recorded. Pups were also weighed up to weaning (days 4, 7, 14 and 21 post partum). Insemination, fertility, gestation and viability indices (on days 0, 4 and 21 post partum) were calculated.
After weaning, the pups of the F1a, F2a and F2b generations were examined for malformations and gross pathological signs. Skeletal development was assessed in pups that died on days 1–4 post partum and those culled on day 4 to reduce litter size. After weaning, the F0 and F1b parents were killed and examined macroscopically. The liver, spleen, kidneys, testes and ovaries were weighed and then prepared for histological evaluation, with the thymus, pituitary, adrenals, vagina, uterus, mammary glands, skin, epididymides, coagulation glands, seminal vesicles, prostate, ears, gastrointestinal tract, brain, skull cap, teeth, thyroid and any other tissues with macroscopic changes. Initially, all tissues of animals at 0 and 4900 ppm were assessed, with any tissues that had macroscopic lesions.
There was no treatment-related deaths or effects on behaviour in F0 and F1b parents. ‘Bloody snout’ was a common finding in animals at 700 and 4900 ppm. Increased incisor tooth growth was apparent in F0 females at 4900 ppm and in both sexes of the F1b generation at 4900 ppm. There were no biologically significant changes in food consumption. Body-weight gain was decreased in F0 females at 700 ppm (by 11–14%) and 4900 ppm (by 17–22%). The calculated mean intakes of tolylfluanid by the F0 parents were 0, 7.9, 58 and 450 mg/kg bw per day for males and 0, 9.5, 75 and 570 mg/kg bw per day for females, and those of the F1 parents were 0, 9.1, 70 and 480 mg/kg bw per day for males and 0, 10, 78 and 620 mg/kg bw per day for females.
At termination, no changes in absolute organ weights were apparent in F0 and F1b males. Significant reductions in liver (F0) and spleen (F0 and F1b) weights were found in female parental rats at 4900 ppm; however, the changes were small, and the relative spleen weight was not significantly decreased. In F0 females, the liver:body weight ratio was depressed, and in both generations the kidney:body weight ratio showed an increase. While these effects might be considered to be due to tolylfluanid on the basis of a (shallow) dose–response trend, the absence of adverse histopathological changes as a correlate casts doubt on their toxicological significance. Macroscopic and microscopic evaluation of the F0 and F1b parents revealed no treatment-related changes, apart from discolouration of the teeth and skull cap in some rats at 4900 ppm. In view of the possibility of fluoride-induced hyperostosis, seen in other studies, skull thicknesses were measured. No changes that could be related to treatment were seen.
Tolylfluanid did not affect the reproductive capacity of the F0 and F1b parents, as indicated by the absence of treatment-related effects on estrus frequency, insemination index, fertility index, gestation index, gestation period, number of females mated, litter size, the sex ratio of the pups and their viability index on day 0. No tolylfluanid-induced malformations were present at birth, and no biologically significant depressions in pup weights were recorded. The statistically significant depressions in the weights of F2a pups at 700 and 4900 ppm were small and within the range of values for other controls. Tolylfluanid appeared to have adverse effects on the postnatal development of the pups, seen as reduced weight gain through to weaning at 4900 ppm and reduced viability indices on days 4 and 21 (Table 17)
Table 17. Viability indices in offspring of rats given diets containing tolylfluanid
|
Generation |
Dietary concentration (ppm) |
|||||||
|
Day 4 |
Day 21 |
|||||||
|
0 |
100 |
700 |
4900 |
0 |
100 |
700 |
4900 |
|
|
F1a |
99 |
96 |
98 |
99 |
85 |
95 |
90 |
82 |
|
F1b |
99 |
98 |
97 |
92** |
73 |
75 |
46*** |
34*** |
|
F2a |
95 |
95 |
93 |
88** |
53 |
90 |
51 |
44 |
|
F2b |
96 |
96 |
98 |
94 |
80 |
87 |
59*** |
55*** |
From Pickel & Rinke (1995)
** p < 0.01; *** p < 0.001
Many pups in all groups (including controls) were described as having a thin, cold appearance with a bluish colour and laboured breathing at various times between birth and weaning. On the basis of the numbers of pups involved and the periods over which these effects were seen in individual animals, the severity was greater in pups at the two higher dietary concentrations than in those at the lowest concentration and control animals. The reduction in the viability index on day 21 also reflected this situation, particularly for F1b and F2b pups. Macroscopic examination of the pups after weaning and skeletal examination of 4-day-old pups (and pups that died before postnatal day 4) revealed no treatment-related changes. The incidences of pups that were thin, cold, bluish and had laboured breathing are summarized in Table 18.
Table 18. Abnormal clinical signs in pups of rats given diets containing tolylfluanid
|
Clinical sign |
Generation |
Dietary concentration (ppm) |
|||||||
|
Males |
Females |
||||||||
|
0 |
100 |
700 |
4900 |
0 |
100 |
700 |
4900 |
||
|
Laboured breathing |
F1a |
6 |
10 |
22 |
26 |
5 |
7 |
19 |
34 |
|
F1b |
6 |
21 |
34 |
28 |
9 |
10 |
27 |
25 |
|
|
F2a |
7 |
7 |
57 |
40 |
2 |
6 |
46 |
40 |
|
|
F2b |
11 |
8 |
30 |
26 |
10 |
1 |
28 |
15 |
|
|
Cold |
F1a |
12 |
8 |
11 |
30 |
17 |
11 |
11 |
18 |
|
F1b |
15 |
28 |
49 |
47 |
10 |
19 |
25 |
30 |
|
|
F2a |
47 |
8 |
43 |
32 |
38 |
4 |
47 |
36 |
|
|
F2b |
16 |
17 |
39 |
40 |
11 |
10 |
37 |
18 |
|
|
Thin |
F1a |
15 |
7 |
3 |
18 |
14 |
4 |
2 |
19 |
|
F1b |
23 |
21 |
36 |
45 |
15 |
15 |
24 |
27 |
|
|
F2a |
55 |
11 |
44 |
38 |
38 |
7 |
46 |
50 |
|
|
F2b |
25 |
15 |
30 |
35 |
7 |
6 |
31 |
20 |
|
|
Bluish |
F1a |
14 |
7 |
19 |
32 |
7 |
7 |
15 |
33 |
|
F1b |
17 |
23 |
34 |
39 |
18 |
17 |
27 |
25 |
|
|
F2a |
34 |
7 |
36 |
32 |
21 |
5 |
34 |
20 |
|
|
F2b |
29 |
15 |
33 |
30 |
15 |
9 |
33 |
19 |
|
From Pickel & Rinke (1995)
The NOAEL was 100 ppm, equal to 7.9 mg/kg bw per day, on the basis of decreased body-weight gain in females, slight clinical signs in parental animals and a decreased viability index in pups (Pickel & Rinke, 1995).
Rats
Groups of 22–24 fertilized female Long-Evans FB 30 rats, 75–95 days old, were given tolylfluanid (purity, 99.9%) at a dose of 0, 100, 300 or 1000 mg/kg bw per day orally on days 6–15 of gestation. The animals were observed routinely for physical appearance, behaviour and body-weight gain. On day 20 of gestation, fetuses were removed surgically, weighed, sexed, inspected for external abnormalities and examined for visceral and bone malformations.
No alteration in physical appearance or behaviour was observed in any group. Two dams at 300 mg/kg bw per day died of effects unrelated to treatment. Body-weight gains were reduced in a dose-dependent manner at 300 and 1000 mg/kg bw per day; however, the dams compensated after the end of treatment. The average fetal weight was reduced by treatment of dams at 300 or 1000 mg/kg bw per day. The resorption ratio at 1000 mg/kg bw per day was slightly, but not statistically significantly, higher than in the control group because of complete loss of embryos by one female. There was no evidence of teratogenicity at any dose. The NOAEL for maternal, fetal and embryotoxic effects was 100 mg/kg bw per day (JMPR, 1988; amended by reference to Machemer, 1976).
Sprague-Dawley (Crl: CD (SD) rats over 12 weeks of age were mated, and groups of 30 inseminated females were given tolylfluanid (purity, 97.5%) prepared as a suspension in 0.5% Alkamuls EL-719 in distilled water at a dose of 0, 100, 300 or 1000 mg/kg bw per day by gavage on days 6–15 of gestation. Analyses confirmed the stability and homogeneity of the formulation and the accuracy of the tolylfluanid concentrations. Food, water and housing conditions were controlled and monitored. The dams were observed daily. Food consumption was assessed on days 1, 6, 7, 12, 16 and 20 of gestation, while body weights were recorded on days 0, 6–16 and 20. On day 20, the dams were killed, and a number of reproductive parameters were assessed, comprising corpora lutea, resorptions, implantations, litter sizes, pre- and post-implantation losses, fertility and gestation indices. In addition, dams were examined macroscopically. The fetuses were checked for viability, sexed, weighed and examined externally for developmental abnormalities. Approximately half were examined for visceral abnormalities and half for skeletal abnormalities. After the visceral examination, the fetuses were fixed, and sections of the head were examined by the Wilson procedure.
No deaths or behavioural changes were seen in the dams during the study. The only clinical sign observed was hair loss in 1, 1, 5 and 8 rats at 0, 100, 300 and 1000 mg/kg bw per day. This was a common finding in developmental studies conducted in the laboratory and may not have been related to treatment. Body weight was reduced in a dose-dependent fashion in all treated groups at the beginning of treatment but was reversed within 2–3 days in the groups at 100 and 300 mg/kg bw per day and within 5 days at 1000 mg/kg bw per day. The body weight of animals at 1000 mg/kg bw per day was significantly lower than that of controls on days 8–20 of gestation. The weight gains in all treated groups during treatment (days 6–16) were significantly lower than that of controls (58, 52, 48 and 34 g at 0, 100, 300 and 1000 mg/kg bw per day, respectively), while the overall weight gains (days 0–20) were significantly lower only at 1000 mg/kg bw per day (144, 136, 136 and 122 mg/kg bw per day at 0, 100, 300 and 1000 mg/kg bw per day, respectively). Food intake was significantly reduced in all three treated groups; the effect quickly reversed at 100 and 300 mg/kg bw per day, but lower food consumption was apparent at day 16 at 1000 mg/kg bw per day. At necropsy, no treatment-related changes were found.
No biologically or statistically significant effects were found on gestation or fertility indices, litter size, post-implantation losses or resorptions. Additionally, fetal viability, fetal and placental weights and the sex ratio of the fetuses were not affected by treatment of the dams. No statistically or biologically significant increases in external, visceral or skeletal variations or malformations were associated with treatment.
A NOAEL for maternal effects could not be identified, given the decreased body-weight gain and food consumption at 100 mg/kg bw per day. No fetal or embryotoxic effects were seen at any dose (Clemens et al., 1995).
Rabbits
Groups of 15 mated female CHBB:HM rabbits were given tolylfluanid (purity, 98.4%) at a dose of 0, 10, 25 or 70 mg/kg bw per day by gavage on days 6–18 of gestation. The compound was made up as a suspension in 0.5% Cremophor EL in water, and doses were delivered in a volume of 5 ml/kg bw. Analyses indicated that the compound was stable in the vehicle over the period during which each new formulation was used (6 days), and two tests indicated satisfactory homogeneity within the suspensions. One analysis of the formulation supplying 10 mg/kg bw per day before treatment indicated a lower than acceptable concentration, but a sample taken after dosage showed a higher (acceptable) content. A similar situation occurred in one set of analyses of the formulation supplying 25 mg/kg bw per day. These deviations (not confirmed on subsequent analysis) cannot be considered to invalidate the study. Food, water and housing conditions were controlled and monitored throughout the study. Animals were observed twice daily (once on weekends) during the study, and food and water consumption was assessed by visual inspection. Body weights were measured on days 0, 6–18 and 29 of gestation. On day 29, the animals were killed and examined grossly. Tissues with macroscopic lesions were fixed and set aside for later histological examination. The dams were examined for the number of corpora lutea, uterine and placental weights and numbers of implantation sites and resorptions. Litter size and fetal sex, viability and weight were determined, and the fetuses were examined for external visceral and skeletal variations and malformations by standard techniques.
No treatment-related deaths or behavioural or abnormal clinical signs were seen in the does. Gross visual inspection showed no differences in food or water intakes among the four groups. Rabbits at 70 mg/kg bw per day lost weight during treatment, while little weight loss was seen in the control group. No differences in weight gain were found among groups during days 0–29 of gestation. Macroscopic and microscopic pathological changes were randomly distributed across all groups, and no signs of dose-related effects were found.
One animal at 70 mg/kg bw per day aborted on day 28 of gestation. The gestation rates were slightly lower at this dose (100%, 92%, 100% and 87% at 0, 10, 25 and 70 mg/kg bw per day, respectively) but were reported to be within the range of other controls in the same laboratory. Although the placental weights did not differ between treated and control animals, 2/15 animals at 70 mg/kg bw per day had macroscopic lesions (necrotic changes and pale, grainy segments). No lesions were found in the placentae of controls or does at 10 mg/kg bw per day, and a minor, pea-sized indurated section in the maternal zone of the placenta was the only change at 25 mg/kg bw per day.
While the litter sizes, fetal weights and sex ratios did not appear to be affected by treatment, more does at 70 mg/kg bw per day had total fetal losses than those at lower doses or controls. While no treatment-related effects were seen on early resorptions (10, 17, 4 and 14 does at 0, 10, 25 and 70 mg/kg bw per day), late resorptions (including one litter of aborted fetuses) were more frequent at 70 mg/kg bw per day (7, 9, 6 and 23 does at 0, 10, 25 and 70 mg/kg bw per day). The numbers of fetuses with malformations were 2, 0, 1, 6 at the four doses, respectively. At 70 mg/kg bw per day, two of the six malformations consisted of a folded retina with a small orbital cavity, which was reported likely to be the result of retarded development. Arthrogryposis of the front limbs (in 1, 0, 1 and 3 kits at 0, 10, 25 and 70 mg/kg bw per day) is a common defect in Himalayan rabbits, and the incidence of 4.2% at 70 mg/kg bw per day was within the range of that for other controls (1.2–4.7%). The incidence of incomplete ossification in the medial phalanx of the forepaw was significantly decreased at 70 mg/kg bw per day (7.7% compared with 47% in controls). No other skeletal variations were seen.
The NOEL was 25 mg/kg bw per day for maternal, embryo and fetotoxicity, on the basis of body-weight reductions, placental lesions, abortion and increased resorptions (Holzum, 1991b, 1994).
A study to assess in more detail the toxicity of tolylfluanid at 70 mg/kg bw per day in pregnant does was conducted under the same conditions as those described above, in which groups of five inseminated female CHBB:HM rabbits were given tolylfluanid (purity, 97.9%) at a dose of 0 or 70 mg/kg bw per day on days 6–18 of gestation. The animals were killed on day 19. The does were observed twice daily for deaths, appearance, behaviour and clinical signs, except on weekends and public holidays when they were observed once daily. Food consumption was determined quantitatively for days 0–6, 6–10, 10–14 and 14–19. Water consumption was determined daily by visual inspection. The does were weighed on day 0 and daily on days 6–19. Blood was drawn from ear veins on day 6 of gestation (before the first dose of tolylfluanid) and on days 7, 14 and 19 and assayed for aspartate and alanine aminotransferase, alkaline phosphatase, glutamate dehydrogenase, lactate dehydrogenase and creatine kinase activity and glucose, cholesterol, triglycerides, creatinine, urea, bilirubin, albumin and total protein concentrations. At the end of the study (day 19 of gestation), the animals were killed and examined grossly; the uteri were examined for implantation sites and the livers were dissected free, weighed and then fixed for histochemical assessments.
No deaths occurred during the study, and no abnormal behaviour or clinical signs were seen. Food consumption was reduced significantly in the treated group when compared with controls over days 6–10 (by 39%) and 10–14 (by 50%) of gestation and statistically non-significantly during days 14–19 of gestation (by 30%). Food consumption over the test period (days 0–19) was lower in treated than in control animals, by 20%. These findings indicate a biologically significant effect of tolylfluanid. Body weight was reduced during treatment in both control and treated groups, the mean loss in the treated animals (–120 g) being greater than that in controls (–40 g).
Normal variations in clinical chemical values coupled with the small group numbers might cloud judgements regarding possible compound-induced changes. While no differences between control and treated groups were seen on days 6 and 7 (i.e. before and after the first dose of tolylfluanid), glutamate dehydrogenase activity and triglyceride concentrations were raised in the treated group on days 14 and 19. Slight (not statistically significant) increases in alanine aminotransferase activity were also seen on these days. A transient increase in creatinine concentrations on day 14 was considered not to be related to treatment. These changes are summarized in Table 19. No differences in liver weights were apparent between the two groups, irrespective of whether absolute weights or organ:body weight ratios were used.
Table 19. Changes in clinical chemistry in inseminated rabbits treated with tolylfluanid
|
Parameter |
Day |
Dose (mg/kg bw per day) |
|
|
0 |
70 |
||
|
Aspartate aminotransferase (U/l) |
6 |
6.1 |
5.6 |
|
7 |
5.3 |
6.4 |
|
|
14 |
6.7 |
8.5 |
|
|
19 |
6.9 |
11 |
|
|
Glutamate dehydrogenase (U/l) |
6 |
1.5 |
1.2 |
|
7 |
1.0 |
1.0 |
|
|
14 |
1.3 |
2.5* |
|
|
19 |
1.6 |
3.3 |
|
|
Triglyceride (mmol/l) |
6 |
0.46 |
0.37 |
|
7 |
0.51 |
0.35* |
|
|
14 |
0.63 |
1.2 |
|
|
19 |
0.96 |
1.8* |
|
From Holzum (1991c)
* p < 0.05
Gross examination showed intestines filled with a watery food mush and gases in 3/5 treated animals and in 1/5 controls. The hepatic changes were restricted to marked lobulation in 2/5 treated animals. Histological examination indicated that these findings were associated with diffuse coarse-droplet steatosis; however, fatty hepatic changes were also present in the control animals. The overall histopathological changes found in this study, expressed as the numbers of animals involved in the control and treated groups, were: diffuse coarse-droplet steatosis, 2/5:3/5; periportal fine droplet steatosis, 1/5:0/5; periportal infiltration, 1/5:2/5; focal Kupffer cell proliferation, 1/5:2/5; and single-cell necrosis, 0/5:1/5. Although the numbers of animals affected did not differ greatly between groups, the intensity of the changes was greater in the treated than in the control group. Thus, tolylfluanid at 70 mg/kg bw per day caused hepatotoxic changes (increased glutamate dehydrogenase activity and triglyceride concentration and gross and microscopic hepatic lesions) in gravid rabbits. Reduced food consumption and body weight were also found (Holzum, 1991c).
In a study of acute neurotoxicity, groups of 12 SPF Wistar (Hsd/Win:WV) rats of each sex were given tolylfluanid (purity, 98.5%) in an aqueous 2% v/v Cremophor EL solution by gavage at a dose of 0, 500, 1000 or 2000 mg/kg bw. Further groups of females received a dose of 0, 50 or 150 mg/kg bw. Analyses confirmed the stability, homogeneity and accuracy of the tolylfluanid dose. The rats were housed individually under controlled conditions with free access to food and water, except for an overnight fast before dosing. The animals were tested in a FOB (Moser, 1989) and for locomotor activity 7 days before dosing, on the day of dosing and on days 7 and 14 after dosing. General clinical and other behavioural changes that fell outside the specific neurotoxicity end-points were noted. Between tests, animals were observed at least twice daily (once on weekends). On test days, the body temperatures and body weights of the animals were recorded. The FOB consisted of a list of observations to be scored under defined conditions: when the animals were in their cages (including posture, piloerection, gait abnormalities, involuntary motor movements, vocalization and other abnormalities); during handling (including ease of removal from the cage, reaction to handling, muscle tone, palpebral closure, pupil size and response, lachrymation and salivation); in an open field (including piloerection, respiratory abnormalities, posture, involuntary muscle movements, stereotypy, bizarre behaviour, gait abnormalities, vocalization, arousal, rearing, urination and defaecation and any other abnormalities); and when a variety of reflexes were initiated (including approach, touch and auditory responses, tail-pinch response, righting reflex, grip strength and foot splay). In order to circumvent biases, all scoring was performed by persons who were unaware of whether the animal had been treated. The FOB tests were initiated about 4.5 h after dosing, as previous tests had indicate that maximal effects were present at this time. Immediately after the FOB had been completed, motor activity and locomotor activity were assessed over 70 min in a figure-of-eight maze in which beam interruptions were automatically recorded as a measure of activity. Motor activity was assessed as the total number of beam interruptions occurring during a test session. Locomotor activity was assessed by eliminating consecutive counts for a given beam, i.e. only one interruption of a given beam was scored until the animal relocated within the maze and interrupted another beam. In addition to total motor activity and locomotor activity counts over the test period, counts for the seven consecutive 10-min intervals within the test period were noted in order to evaluate habituation, i.e. the decrement in activity during the test session.
At the end of the observation period after dosing, all animals were killed and assessed macroscopically. Six rats of each sex per group were selected for histological examination, anaesthetized and perfused for fixation of tissues in situ. The tissues of interest (brain, spinal cord, eyes, optic nerves, spinal nerve roots and ganglia, Gasserian ganglion, gastrocnemius muscle, sciatic, tibial and sural nerves and liver) were dissected free after fixation and prepared for histological examination. Tissues of rats at 0 and 2000 mg/kg bw were examined histologically. When no lesions were found at the highest dose, animals at lower doses were not examined histologically.
No deaths, treatment-related clinical signs or behavioural changes were recorded during the study. Body weights and body-weight gains were not affected by treatment. Males showed no treatment-related changes in the FOB tests on day 0, 7 or 14. Isolated differences (e.g. more rats standing rather than sitting normally in the open field on day 0 at 2000 mg/kg bw; barely perceptible gait incoordination in one rat on day 0 at 1000 mg/kg bw; partially formed faeces in one rat on day 0 at 2000 mg/kg bw) were dismissed on the basis of the absence of dose–response relationships and the fact that the effects were isolated and minor. Females showed a number of more definite, statistically significant responses, which were considered to be treatment-related. These included piloerection in the home cage, during handling and in the open field at 2000 mg/kg bw; decreased activity in the home cage at 2000 mg/kg bw; gait abnormalities in the open field at 2000 mg/kg bw; decreased rearing in the open field at 2000 mg/kg bw; and dose-related decreases in body temperature at 500, 1000 and 2000 mg/kg bw. FOB tests 7 and 14 days after treatment showed no differences between control and treated females.
Unpublished information provided by the laboratory suggested that variations of 15–20% in group rearing activity are not biologically significant. In female rats, the reductions in rearing from control values were –46%, –27%, –25%, –26% and –6% at 2000, 1000, 500, 150 and 50 mg/kg bw. Of these, only the results at 2000 mg/kg bw are statistically significant (p < 0.05). On the basis of the assumption that variations > 20% are biologically significant, however, the responses at 150, 500 and 1000 mg/kg bw were considered to be ‘slight’ but biologically significant. The authors therefore considered that the no-effect level in the FOB tests was 50 mg/kg bw for female rats.
Grip strength and foot splay were unaffected by treatment in both male and female rats. With regard to motor and locomotor activity, data from the pre-treatment trial (and other work in the laboratory) suggested that variations > 20% were biologically significant. Comparisons of control values for motor and locomotor activity showed lower values on the day of treatment than before (–7 days) and after treatment (+7 and +14 days). This is compatible with the animals being in a fasted state when treatment was initiated. In male animals, no treatment-related effects on motor or locomotor activity were seen over the total 70-min trial or in any of the seven 10-min intervals during the test. In contrast, the motor activity of females over the 70-min trial was reduced in a biologically but not statistically significant manner, the reductions with respect to controls being –45%, –43%, –42%, –25% and 0 at 2000, 1000, 500, 150 and 50 mg/kg bw. The results for locomotor activity were more clear-cut, with mean reductions of –58%, –46%, –33%, –33% and –7% at 2000, 1000, 500, 150 and 50 mg/kg bw, respectively. The results for both motor and locomotor activity showed major reductions during the first two 10-min intervals. The reductions at 2000, 1000 and 500 mg/kg bw were statistically significant. No statistically or biologically significant differences in motor activity, locomotor activity or habituation were seen in control or treated groups before treatment (–7 days) or after treatment (+7 and +14 days).
Gross examination of the animals on days 14–15 after dosing revealed no treatment-related lesions. One animal at 1000 mg/kg bw had a dark-red patch in one lung, which was probably due to intrapulmonary injection of some tolylfluanid formulation. No changes were observed in brain weights. Histopathological examination revealed isolated changes that are common in rats. The similar distribution of these findings in controls and rats given 2000 mg/kg bw tolylfluanid indicated the absence of a compound-induced effect.
This study provided evidence of a sex-related difference in sensitivity to tolylfluanid, with males unaffected at the highest dose and females showing slight, nonspecific effects at 50 mg/kg bw. The findings in females were reversible and were general toxic signs rather than specific neurotoxic effects. The NOAEL for general toxicity was 50 mg/kg bw, and that for neurotoxicity was 2000 mg/kg bw (Dreist & Popp, 1994).
Groups of 12 SPF Wistar (HSD/Win:WU) rats of each sex were given diets containing tolylfluanid (purity, 97.5%) at a concentration of 0, 300, 1650 or 9000 ppm for 13 weeks. The diets were prepared weekly. Chemical analyses showed that the stability of tolylfluanid, its homogeneity and its concentrations in the diets were within acceptable limits. The rats were housed individually under controlled conditions, with free access to food and water. They were observed twice daily during the study, and detailed examinations were performed weekly. Food and water consumption and body weights were recorded weekly. FOB tests and assessments of motor and locomotor activity (as described above) were performed in the week before the rats were given the tolylfluanid-containing diet and then during weeks 4, 8 and 13. Body temperatures were recorded at these times. An ophthalmological examination was performed on all animals before treatment and during week 13. At the end of the study, six rats of each sex per group were set aside for perfusion for fixation in situ preparatory to histopathological examination of a wide range of neural entities (as described for the previous study). All animals were examined grossly for pathological signs.
No treatment-related deaths, clinical signs or gross behavioural changes occurred during the study. Body weights and body-weight gains were depressed in both males and females at 9000 ppm, with an incidental decrease in females at 1650 ppm. Food and water intakes (grams per kilogram body weight) were increased in both males and females at 9000 ppm. The calculated mean intakes of tolylfluanid at 0, 300, 1650 and 9000 ppm were 0, 20, 110 and 620 mg/kg bw per day for males and 0, 25, 130 and 770 mg/kg bw per day for females.
No effects were found on the parameters scored in the FOB tests or on fore- or hind-limb grip strength, foot splay or body temperature in male or female rats. An isolated statistically significant finding of decreased hind-limb grip strength in week 8 only in males at 9000 ppm was considered to be related to the decreased body-weight gain, and an isolated statistically significant finding of a profile of home cage posture different from that of controls in week 8 only in females at 1650 ppm was considered incidental to treatment.
No treatment-related differences between control and treated animals were found in motor or locomotor activity. Ophthalmological examination revealed a number of common lesions in both control and treated animals, which were considered not to be treatment-related. No gross pathological changes were seen at necropsy, and no treatment-related changes in brain weights were found. As histopathological examination of animals at 0 and 9000 ppm showed no treatment-related findings, rats at 300 and 1650 ppm were not examined.
Tolylfluanid therefore had no neurotoxic activity at the doses tested, although it did have some toxicity, as judged by reductions in body weight and body-weight gain and alterations in food consumption (Dreist & Popp, 1995).
In female Wistar rats dosed by gavage with triadimefon (purity, 89.5%), tolylfluanid (purity unspecified) or both substances in combination, the LD50 of triadimefon was 1000 mg/kg bw and that of tolylfluanid was > 5000 mg/kg bw. Tolylfluanid at 2500 or 5000 mg/kg bw in combination with triadimefon at 1000 mg/kg bw resulted in the deaths of 3/15 and 5/15 rats, respectively. There was therefore no evidence of potentiation of toxicity (JMPR, 1988; amended by reference to Flucke & Kimmerle, 1978).
A review indicated that there are two basic mechanisms for the production of thyroid tumours: a direct carcinogenic effect on the thyroid gland and hormone imbalance. Substances that inhibit thyroid hormones can interfere with their production, resulting in a sustained increase in the synthesis and secretion of TSH. This can produce follicular-cell hypertrophy, hyperplasia and, ultimately, neoplasia due to the sustained stimulation. In rodents, increased TSH concentrations (as seen after low dietary iodine or in the presence of a TSH-secreting pituitary tumour) can lead to thyroid tumours. The effects can be reversed by iodine supplementation, thyroid hormone replacement or hypophysectomy. The thyroid has autoregulation, in which receptor sensitivity to TSH is moderated by iodolipids: when iodolipid concentrations are low, the cells are more responsive to TSH. Rats do not have a thyroid-binding globulin protein in their serum. The half-life of thyroxine in rats is about 12 h, whereas it is 5–9 days in humans. Thus, rats have higher basal activity in the thyroid gland and greater sensitivity to changes in hormone concentrations. The incidence of spontaneous thyroid tumours in Fischer 344 rats is 2%, while that in humans is about 0.004%. Thyroid hormone synthesis can be disrupted by sulfonamides, although the effects on thyroid hormone function in persons receiving therapeutic doses of sulfonamides were limited, with slight decreases in thyroid hormone concentrations but no effects on TSH. In rodents, chemicals that induce hepatic microsomal enzymes increase the hepatic disposition of thyroid hormones, resulting in a feedback loop and increased thyroid hormone secretion. In rats given phenobarbital, thyroid tumours can be inhibited by treating them with thyroxine. In humans, long-term treatment with enzyme inducers such as anti-epileptic medication resulted in decreased thyroxine concentrations but normal tri-iodothyronine and TSH concentrations and no increase in tumour incidence. Rodents, and especially rats, therefore appear to be a very conservative model for the induction of thyroid tumours (McClain, 1992).
Groups of 10 Bor:WIW (SPF-Cpb) rats of each sex were given diets containing tolylfluanid (purity, 91.0%) at a concentration of 0, 300, 1500 or 7500 ppm for 4 weeks, equal to 0, 21, 120 and 680 mg/kg bw per day for males and 0, 22, 120 and 750 mg/kg bw per day for females. The rats were housed individually under controlled conditions with free access to food and water. They were inspected twice daily (daily on weekends) for deaths and abnormal clinical signs. Food and water consumption and body weight were measured weekly. Before treatment and on days 7 and 27 for males and 8 and 28 for females, blood samples were taken, and cholesterol, triglyceride, tri-iodothyronine, total thyroxine and TSH concentrations were determined. At the end of the study, all the rats were killed, and a detailed gross examination was done post mortem. The brain, heart, testes, kidneys, adrenals, ovaries and thyroid were weighed, and a full range of tissues was prepared for histopathological examination; however, only the thyroid glands were examined.
During the study, tolylfluanid was determined to be suitably stable in the diet and the diet was homogeneous. No deaths occurred. The only abnormal clinical signs seen were distended abdomens in four males and two females at 7500 ppm. The authors reported that treatment-related effects were found on body weight in rats at 7500 ppm. Overall, there was a slight decrease in body-weight gain in animals at 1500 ppm, particularly in males, the weight gains being 70, 69, 57 and 36 g for males and 19, 18, 16 and 14 g for females at 0, 300, 1500 and 7500 ppm, respectively. Food consumption was increased in rats at 7500 ppm, with slight effects at 1500 ppm in males. Water intake was increased in animals at 7500 ppm.
No treatment-related effects were reported on cholesterol or triglyceride concentrations. Tri-iodothyronine concentrations were decreased in males at 7500 ppm on day 7 and increased in females at 7500 ppm on day 28. Thyroxine concentrations were decreased in animals of each sex at 7500 ppm on each of the two days on which it was measured. In animals at 1500 ppm at the second time, the thyroxine concentration was decreased significantly in males and non-significantly in females. These changes were considered to be of no toxicological significance, as there were no changes in TSH or tri-iodothyronine concentrations at this dose. The TSH concentration was increased in both sexes at 7500 ppm on day 27 or 28. The thyroid hormone changes are summarized in Table 20.
Table 20. Concentrations of thyroid hormones in rats after treatment with tolylfluanid
|
Thyroid hormone |
Day |
Dietary concentration (ppm) |
|||||||
|
Males |
Females |
||||||||
|
0 |
300 |
1500 |
7500 |
0 |
300 |
1500 |
7500 |
||
|
Tri-iodothyronine (nmol/l) |
7/8 |
1.2 |
1.2 |
1.3 |
0.98** |
1.3 |
1.4 |
1.4 |
1.0 |
|
27/28 |
1.4 |
1.4 |
1.3 |
1.3 |
1.2 |
1.4 |
1.4 |
1.8** |
|
|
Thyroxine (nmol/l) |
7/8 |
83 |
87 |
81 |
54** |
80 |
80 |
78 |
52** |
|
27/28 |
89 |
81 |
70** |
50** |
92 |
96 |
84 |
72** |
|
|
Thyroid-stimulating hormone (pg/ml) |
7/8 |
920 |
740 |
920 |
800 |
310 |
540 |
640 |
760 |
|
27/28 |
880 |
830 |
860 |
1500** |
320 |
590 |
615 |
1400** |
|
From Bomhard (1988)
** p < 0.01
Examination post mortem revealed no notable gross pathological findings. The relative kidney weight was increased in both sexes at 7500 ppm and the relative testis weight in males. These increases were considered to be of no toxicological significance but to be related to the decreased body weight seen at this dose. No abnormalities were noted on histopathological examination of the thyroid. The NOAEL for effects on the thyroid was 7500 ppm, equal to 680 mg/kg bw per day (Bomhard, 1988).
The acute LD50 of thiazolidinthioncarbonacid, the main urinary metabolite of tolylfluanid, when suspended in Lutrol and given by oral gavage to male rats was > 1000 mg/kg bw, as no deaths occurred. Abnormal clinical signs (unspecified) were seen in three of three rats at 1000 mg/kg bw, but none were seen at 500 mg/kg bw (Thyssen, 1978).
The acute toxicity of dimethylaminosulf-para-toluidid, a metabolite of tolylfluanid (purity, 99.7%), was assessed in rats and rabbits (Table 21). After oral dosing, abnormal clinical signs (apathy, prostration, sedation, dyspnoea and increased urination) were apparent within 5–45 min and lasted for up to 9 days, with males affected at doses > 1000 mg/kg bw and females at doses > 500 mg/kg bw. Pale, patchy livers were noted in animals that died after oral dosing, but no lesions were found in animals that survived to the end of the 14-day observation period. After dermal dosing, disturbed behaviour (unspecified) and dyspnoea persisting for 3–9 days were seen. No lesions were found at necropsy. After inhalation, no signs of irritation were seen in the eyes or nose. In the ocular irritancy test in rabbits, slight conjunctival redness was seen for 24 h after exposure, which may have been a mechanical effect (Flucke & Thyssen, 1978).
Table 21. Acute toxicity of dimethylaminosulf-para-toluidid
|
Species |
Strain |
Sex |
Route |
Vehicle |
Purity (%) |
LD50 |
LC50 |
|
Rat |
Wistar |
Male |
Oral |
Cremophor EL |
99.7 |
6100 |
|
|
Rat |
Wistar |
Female |
Oral |
Cremophor EL |
99.7 |
1600 |
|
|
Rat |
Wistar |
Male, female |
Dermal |
Cremophor EL |
99.7 |
> 5000 |
|
|
Rat |
Wistar |
Male, female |
Inhalation |
Cremophor EL |
99.7 |
> 0.16 (1 h) |
|
|
> 0.16 (4 h) |
|||||||
|
Rabbit |
New Zealand white |
Male, female |
Dermal |
Not irritating |
|||
|
Rabbit |
New Zealand white |
Male, female |
Ocular |
Slightly irritating |
From Flucke & Thyssen (1978)
Groups of five Wistar rats (Hsd Win:WU) of each sex were dosed orally by gavage with 4-hydroxymethyl dimethylaminosulf-para-toluidid (purity, 97%) in Cremophor EL at 1000 or 5000 mg/kg bw. The animals were observed several times on the day of treatment and at least once a day for 14 days. Body weights were recorded on days 1, 4, 8 and 15. At the end of the test, the rats were killed and examined post mortem. No deaths were seen. At 5000 mg/kg bw, all five males and one female had clinical signs (staggering gait and, in males, dyspnoea, decreased motility and reactivity, ptosis) within 10–15 min of dosing, the effects persisting for up to 2.25 h in males and about 45 min in the affected female. Body-weight gain was not affected by treatment, and no pathological changes were noted at necropsy. The LD50 was > 5000 mg/kg bw (Krötlinger, 1994b).
WAK 6550 (a tolylfluanid metabolite; purity, 98.6%) was tested under the same conditions as in the previous report (Krotlinger, 1994b) but only at 5000 mg/kg bw. No deaths, abnormal clinical signs, changes in body-weight gain or pathological changes were reported. The oral LD50 was > 5000 mg/kg bw (Krotlinger, 1995a).
WAK 6676 (a tolylfluanid metabolite; purity, 98.4%) was tested under the same conditions as in the previous report (Krotlinger, 1994b). No deaths were seen. At 5000 mg/kg bw, decreased motility and reactivity were seen within 15 min of dosing, which lasted for about 4 h. Body-weight gain was unaffected, and no abnormalities were seen at necropsy. The acute oral LD50 was > 5000 mg/kg bw (Krötlinger, 1995b).
WAK 6698 (a tolylfluanid metabolite; purity, 99.6%) was tested under the same conditions as in the previous report (Krotlinger, 1994b) at doses of 100, 1000 and 5000 mg/kg bw. No deaths were recorded. At 1000 mg/kg bw, two of five males and three of five females showed a staggering gait that lasted for 2 h after dosing. At 5000 mg/kg bw, all rats had a staggering gait, decreased mobility and dyspnoea, lasting for 4 h after treatment. Body-weight gain was not affected, and there were no abnormalities post mortem. The acute oral LD50 was > 5000 mg/kg bw (Krötlinger, 1995c).
Studies of the genotoxicity of metabolites of tolylfluanid are summarized in Table 22.
Table 22. Results of studies of genotoxicity with tolylfluanid metabolites
|
End-point |
Test object |
Test substance |
Concentration |
Purity (%) |
Results |
Reference |
|
Reverse mutationa |
S. typhimurium TA98, TA100, TA1535, TA1537 |
4-Hydroxy methyl dimethylaminosulf-para-toluidid |
0–5000 µg/plate |
97.0 |
Negative |
Herbold (1994b) |
|
Reverse mutationa |
S. typhimurium TA98, TA100, TA1535, TA1537 |
WAK 6550 |
0–5000 µg/plate |
98.6 |
Negative |
Herbold (1995a) |
|
Reverse mutationa |
S. typhimurium TA98, TA100, TA1535, TA1537 |
WAK 6676 |
0–5000 µg/plate |
98.4 |
Negative |
Herbold (1995b) |
|
Reverse mutationa |
S. typhimurium TA98, TA100, TA1535, TA1537 |
WAK 6698 |
0–5000 µg/plateb |
99.6 |
Negative |
Herbold (1995c) |
|
Forward mutationa |
Mouse lymphoma L5178Y Tk± |
WAK 6698 |
0–1000 µg/platec |
99.6 |
Negative |
Cifone (1995) |
|
Chromosomal aberrationa |
Chinese hamster V79 cells |
Dimethylaminosulf-para-toluidid d |
800 µg/ml – S9 |
99 |
Positive |
Herbold (2002) |
a Positive controls included; 9000 × g fraction of Aroclor-induced rat liver (S9)
b Bacteriotoxic effects from 158 µg/plate
c Cytotoxic effects from 500 µg/plate
The anti-thyroid activity of a tolylfluanid metabolite, thionamide 2-thiazolidinethione-4-carboxylic acid, was investigated in vitro in a study that was not conducted according to GLP. Many thionamides are known goitrogens and are able to interact with thyroid peroxidase and type I iodothyronine deiodinase. Therefore, the interaction of thionamide 2-thiazolidinethione-4-carboxylic acid and tolylfluanid was investigated with enzymes derived from pig thyroid and rat liver homogenate. Neither substance inhibited thyroid peroxidase-catalysed oxidation of the model substrate guaiacol. Thionamide 2-thiazolidinethione-4-carboxylic acid suppressed thyroid peroxidase-catalysed iodine formation as effectively as did propylthiouracil (the positive control). It also suppressed non-enzymatic iodination of L-tyrosine. It was effectively metabolized by thyroid peroxidase, and conversion products were detected after cooling; however, it did not cause the irreversible inhibition of thyroid peroxidase seen with other thionamides. Thionamide 2-thiazolidinethione-4-carboxylic acid was a weak inhibitor of iodothyronine deiodinase, suggesting that it does not interfere with the formation of tri-iodothyronine from thyroxine. It was considered to act similarly to a number of antithyroid drugs and to be the metabolite most likely to be responsible for the thyroid lesions and TSH concentrations seen after high doses of tolylfluanid (Freyberger, 1995).
In a study conducted before GLP, six male volunteers received a cotton-wool pad containing dry tolylfluanid on the skin of the forearm for 24 h. No signs of irritation were observed (Kimmerle, 1964). In a similar study, no irritation was seen on the skin of 10 male volunteers after a 24-h exposure (Kimmerle, 1968).
Workers in a manufacturing plant where tolylfluanid was produced and formulated were given annual medical examinations which included a medical history, clinical tests, a test for respiratory mobility of the thorax, functional test of the spine, measurements of height, weight, blood sedimentation rate, blood count, urinary status, gamma-glutamyl transferase and serum alanine aminotransferase activity, fluorine excretion in urine, X-ray of the thorax and a lung function test. In the initial report (Faul, 1982), on 60 workers examined over a number of years, one worker had developed a possible skin allergy to tolylfluanid, but no other abnormalities were seen. In a second report (Faul, 1989), two cases of allergic skin disease were associated with exposure to tolylfluanid. Both cases resolved when the workers were not handling tolylfluanid. In the first case, prurient, pustular lesions on both forearms were seen after packing of tolylfluanid formulations; in the second case, exanthema of the face and throat was seen after the worker had spent time in the packing hall. No other abnormalities were found that could be related to exposure to tolylfluanid.
Reports from a number of companies in which tolylfluanid was used at concentrations of 0.8–0.9% as a wood preservative, with application by dipping or brushing, indicated no adverse effects in workers applying the product, in customers using the product at home or in individuals in contact with the treated wood (Imsgard, 1993; Olloz, 1993; Schneeberger, 1993). A company in which a tolylfluanid product was formulated and used in exterior paints and wood preservatives reported no cases of skin or other irritation (Roos, 1993).
Consumption of tolylfluanid at 0.08 mg/kg bw per day would result in a fluoride intake of 0.0003 mg by a child aged 4–6 years weighing 18.5 kg and 0.001 mg by a 70-kg adult. These intakes would be was well below daily exposure to naturally occurring fluoride in food and water, which is 0.5 mg. Treatment of children with 1 mg of fluoride daily did not produce dental fluorosis (Heimann, 2000).
[14C]Tolylfluanid was rapidly and extensively absorbed after oral administration to rats, with peak plasma concentrations of radiolabel 1 h after dosing, followed by rapid metabolism and almost complete excretion, mainly in the urine and to a lesser extent in the bile, within 48 h. High tissue concentrations were seen soon after dosing in the kidney and liver, with lower concentrations in perirenal fat, brain, gonads and thyroid. By 48 h, all tissue concentrations were low.
Metabolism involves cleavage of the fluorodichloromethylthio group from tolylfluanid to form N,N-dimethyl-N’-para-tolysulfamide (dimethylaminosulfotoluidine, DMST). The fluorodi-chloromethylsulfenyl side-chain undergoes further metabolism to form thiazolidine-2-thioxo-4-carboxylic acid, which is the main metabolite in the urine of rats and is of toxicological significance because of its potential anti-thyroid effects. Dimethyltolylsulfamide is also further metabolized, producing a range of metabolites that are not of toxicological significance. The release of the fluoride ion and its distribution in the body have not been clearly characterized.
Tolylfluanid is of low toxicity in mice (LD50, > 1000 mg/kg bw) and rats (LD50, > 5000 mg/kg bw) after oral administration and is of low toxicity in rats (LD50, > 5000 mg/kg bw) after dermal application. It was highly toxic after inhalation for 4 h through the nose only (LC50, 0.16 to > 1 mg/l, depending on particle size and micronization). Common signs observed after single doses were sedation, decreased motility, disturbed behaviour and dyspnoea. After intraperitoneal injection, signs consistent with local irritation were seen. After exposure by inhalation, the signs included extreme difficulties in breathing, sneezing, serous nasal discharge and cyanosis, with histopathological findings consistent with severe respiratory irritation. Tolylfluanid was a severe skin irritant and moderately to severely irritating to the eye. It was a skin sensitizer in a Magnusson and Kligman maximization test, in an open epicutaneous test and in a local lymph node assay in mice, but was not a skin sensitizer in a Buehler test. Overall, tolylfluanid is considered to be a skin sensitizer. WHO has concluded that tolylfluanid is ‘unlikely to present an acute hazard in normal use’ (WHO, 2000).
Decreased body-weight gain was seen in mice and rats given tolylfluanid in the diet at concentrations of 1500 ppm and above in long-term studies, with variable effects on food consumption. Water intake was increased in mice and rats at 7500 ppm. Liver toxicity was seen in mice, rats and dogs at dietary concentrations of 1500 ppm and above, the signs including altered liver enzyme activity, increased liver weights and histopathological changes. Signs of renal toxicity were seen in mice, rats and dogs at 1500 ppm and above, which included decreased urine osmolality and increased urine volume at 7500 ppm, increased kidney weight at 1500 ppm and above and histopathological changes at 7500 ppm.
In all species tested, the concentrations of fluoride in the bone and teeth were increased in a dose-related manner. At high doses, this increase was associated with discolouration, particularly of the skull cap and incisors, in both sexes but starting at lower doses in male rats. In long-term studies, rats at 7500 ppm, equal to 500 mg/kg bw per day, required treatment for overgrown incisors more frequently than controls, presumably because fluoride deposition in the incisors had increased their strength and thus decreased the wear on these teeth. Hyperostosis of the skull and sternum was seen at high doses in mice and rats of either sex, and histopathological changes were seen in the bones of female mice at 300 ppm (equal to 120 mg/kg bw per day) and female rats at 1500 ppm (equal to 100 mg/kg bw per day). In both sexes, increased fluoride deposition was seen at 300 ppm, equal to 76 mg/kg bw per day, in mice and 18 mg/kg bw per day in rats. The NOAEL for fluoride deposition was 60 ppm, equal to 15 mg/kg bw per day, in mice, and 60 ppm, equal to 3.6 mg/kg bw per day, in rats. In dogs, the fluoride concentration in bone was increased in males at doses of 80 mg/kg bw per day and in females at 20 mg/kg bw per day and above, while the fluoride concentration in teeth was increased in males at 80 mg/kg bw per day and in females at all doses including the lowest one tested, 5 mg/kg bw per day, although not in a dose-related manner. The increase in fluoride deposition raises concern because mottling of dental enamel (or dental fluorosis) occurs in humans after exposure to high concentrations of fluoride, particularly where water has a high concentration of fluoride or has been inappropriately supplemented. While this is mainly a cosmetic defect, it is generally recognized as adverse.
Alterations in thyroid hormone levels were observed in a number of studies in rats. These included decreased concentrations of triiodothyronine and thyroxine at 1650 and 9000 ppm in the diet (equal to 110 and 640 mg/kg bw per day, respectively) and increased concentrations of thyroid-stimulating hormone at 9000 ppm in a 13-week study. In a 2-year study, increased incidences of thyroid follicular-cell hyperplasia and adenomas were seen at 7500 ppm in the diet (equal to 500 mg/kg bw per day). As rats do not have thyroid-binding globulin in their serum, they are more sensitive to certain types of thyroid toxicants than are humans. The half-life of thyroxine is about 12 h in rats and 5–9 days in humans. In rats, chemicals that induce hepatic microsomal enzymes increase the hepatic clearance of thyroid hormones, resulting in a compensatory increase in thyroid hormone secretion. This effect is not seen in humans treated with the same substances. It was not clear if this mechanism was involved in the effects on the thyroid seen after dosing with tolylfluanid. One of the metabolites of tolylfluanid, thiazolidine-2-thione-4-carboxylic acid, reversibly inhibits thyroid peroxidase and might have contributed to the effects on the rat thyroid.
No treatment-related tumours were seen in long-term studies in mice, rats or dogs, other than a slight increase in the incidence of thyroid follicular-cell adenomas in rats. This finding was considered unlikely to be of concern at doses that do not perturb thyroid homeostasis in humans.
Tests for genotoxicity in vitro gave negative results in the absence of cytotoxicity. The results of all tests in vivo were negative. The results of tests for the genotoxicity on the metabolites of tolylfluanid were also negative. The Meeting concluded that tolylfluanid is unlikely to be genotoxic.
On the basis of the results of the tests for genotoxicity and carcinogenicity in animals, the Meeting concluded that tolylfluanid is unlikely to pose a carcinogenic risk to humans.
Studies of reproductive toxicity in rats showed effects on reproductive performance, pup survival and pup weight only at doses that were maternally toxic, including 7500 ppm in the diet in a two-generation study in which decreased body-weight gain was seen in females at 7500 ppm and in males at 1500 ppm. In a second two-generation study, decreased pup birth weight and weight gain to weaning and a decreased lactation index were seen at 4800 ppm in the diet, while decreased body-weight gains were seen in the parental animals at 1200 and 4800 ppm. Adverse clinical signs (bloody snouts) and decreased pup viability were seen at 700 ppm (equal to 58 mg/kg bw per day) in a third two-generation study of reproductive toxicity. The contribution of fluoride in milk to these effects was not clearly established, as the concentration was not measured. The NOAEL in this study was 100 ppm, equal to 7.9 mg/kg bw per day.
In a study of developmental toxicity in rats, decreased body-weight gain was observed in dams at 300 and 1000 mg/kg bw per day. Reduced fetal body weight was also seen at these doses, and an increased resorption rate was seen at 1000 mg/kg bw per day. The NOAEL was 100 mg/kg bw per day. In a second study in rats, decreased body-weight gain was seen among dams in all groups (100, 300 and 1000 mg/kg bw per day), but there were no effects on fetuses at any dose. In a study of developmental toxicity in rabbits, decreased maternal body-weight gain and late resorptions were seen at 70 mg/kg bw per day. There were no treatment-related abnormalities, and the Meeting concluded that tolylfluanid is not teratogenic.
In studies of neurotoxicity in rats given single or repeated doses, there was no evidence of neurotoxic effects at any dose. In females, slight decreases in reactivity and motor activity were attributed to the general toxic effects of tolylfluanid, with a NOAEL after acute administration of 50 mg/kg bw.
The Meeting concluded that the existing database on tolylfluanid was adequate to characterize the potential hazards of tolylfluanid to fetuses, infants and children.
Routine medical surveillance of individuals working in tolylfluanid manufacture and formulation plants and of workers using tolylfluanid revealed a low incidence of skin sensitization, but no other adverse effects attributable to tolylfluanid.
The Meeting established an ADI of 0–0.08 mg/kg bw on the basis of the NOAEL of 60 ppm, equal to 3.6 mg/kg bw per day, in the 2-year study in rats, in which increased fluoride deposition was seen at higher doses, and a safety factor of 50. This safety factor was used because of the limited differences noted between species in the deposition of fluoride in bones and teeth after administration of tolylfluanid. The NOAEL in the 2-year study in rats treated in the diet was used in preference to the LOAEL of 5 mg/kg bw per day in the 1-year study in dogs given tolylfluanid by capsule, as increased fluoride concentrations were seen only in the teeth and only in females at the low dose in the study in dogs, without a clear dose–response relationship.
The Meeting established an acute RfD of 0.5 mg/kg bw on the basis of the NOAEL of 50 mg/kg bw in the study of acute neurotoxicity in rats and a safety factor of 100.
|
Levels relevant to risk assessment |
||||
|
Species |
Study |
Effect |
NOAEL |
LOAEL |
|
Mouse |
2-year study of toxicity and carcinogenicitya |
Toxicity |
60 ppm, equal to |
300 ppm, equal to |
|
Carcinogenicity |
7500 ppm, equal to |
– |
||
|
Rat |
2-year study of toxicity and carcinogenicitya |
Toxicity |
60 ppm, equal to |
300 ppm, equal to |
|
Carcinogenicity |
1500 ppm, equal to |
7500 ppm, equal to |
||
|
Multigeneration study of reproductive toxicitya |
Parental and pup toxicity |
100 ppm, equal to |
700 ppm, equal to |
|
|
Developmental toxicityd |
Maternal toxicity |
– |
100 mg/kg bw per dayc |
|
|
Embryo- and feto-toxicity |
100 mg/kg bw per day |
300 mg/kg bw per day |
||
|
Acute neurotoxicityd |
Decreased motor activity in females |
50 mg/kg bw per day |
150 mg/kg bw per day |
|
|
Rabbit |
Study of developmental toxicityd |
Maternal, embryo and fetotoxicity |
25 mg/kg bw per day |
70 mg/kg bw per day |
|
Dog |
1-year study of toxicitye |
Toxicity |
– |
5 mg/kg bw per dayc |
a Dietary administration
b Highest dose tested
c Lowest dose tested
d Gavage
e Capsule
Estimate of acceptable daily intake for humans
0–0.08 mg/kg bw
Estimate of acute reference dose
0.5 mg/kg bw
Studies that would provide information useful for continued evaluation of the compound
List of end-points relevant for setting guidance values for dietary and non-dietary exposure
|
Absorption, distribution, excretion and metabolism in mammals |
|
|
Rate and extent of oral absorption |
Rapid and extensive |
|
Distribution |
Extensive, highest concentrations in liver and kidney |
|
Potential for accumulation |
Fluoride accumulation in teeth and bones |
|
Rate and extent of excretion |
Complete |
|
Metabolism in animals |
Extensive, with no parent compound in urine or faeces |
|
Toxicologically significant compounds |
Tolylfluanid, thiazolidine-2-thion-4-carbonic acid, dimethylaminosulftoluidine, fluoride |
|
Acute toxicity |
|
|
Rat, LD50, oral |
> 5000 mg/kg bw |
|
Rat, LD50, dermal |
> 5000 mg/kg bw |
|
Rat, LC50, inhalation |
0.16 mg/l (4-h nose-only) |
|
Irritation |
Severe skin irritant and moderate-to-severe eye irritant |
|
Skin sensitization |
Sensitizer (Magnusson & Kligman, Klecak open epicutaneous test, local lymph node assay in mice) |
|
Short-term toxicity |
|
|
Target/critical effect |
Liver, kidney, thyroid |
|
Lowest relevant oral NOAEL |
300 ppm, equal to 20 mg/kg bw per day (13-week, rats) |
|
Lowest relevant dermal NOAEL |
LOAEL, 1 mg/kg bw per day in rabbits, local skin effects; NOAEL, 300 mg/kg bw per day, systemic effects |
|
Lowest relevant inhalation LOAEC |
0.0012 mg/l (4-week, nose-only, rats) |
|
Genotoxicity |
Unlikely to be genotoxic |
|
Long-term toxicity and carcinogenicity |
|
|
Target/critical effect |
Fluoride accumulation in bones and teeth, bone changes |
|
Lowest relevant NOAEL |
60 ppm, equal to 3.6 mg/kg bw per day (2-year, rats, diet) |
|
Carcinogenicity |
Unlikely to pose a carcinogenic risk to humans. |
|
Reproductive toxicity |
|
|
Target/critical effect for reproductive toxicity |
Decreased pup viability at maternally toxic doses |
|
Lowest relevant NOAEL for reproductive toxicity |
100 ppm, equal to 7.9 mg/kg bw per day (2-generation study, diet, rats) |
|
Target/critical effect for developmental toxicity |
Not teratogenic; embryo- and fetotoxic at maternally toxic doses |
|
Lowest relevant NOAEL for developmental toxicity |
25 mg/kg bw per day (rabbits) |
|
Neurotoxicity |
|
|
Acute neurotoxicity |
No specific neurotoxicity seen, general toxicity seen at 150 mg/kg bw; NOAEL, 50 mg/kg bw |
|
Short-term neurotoxicity |
No neurotoxic signs seen |
|
Medical data |
Low incidence of skin sensitization in production workers |
|
Summary |
Value |
Study |
Safety factor |
|
ADI |
0–0.08 |
Rat, 2-year, diet |
50 |
|
Acute RfD |
0.5 |
Rat, acute neurotoxicity |
100 |
Abbink, J. & Weber, H. (1988) Phenyl-UL-14C-tolylfluanid: Investigation of the biokinetic behaviour in the rat. Unpublished report No. PF2989 21 April 1988, amended 10 November 2000, from Bayer AG, Institute of Toxicology, Wuppertal, Germany. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
den Boer, W.C. (1987) Mutagenicity test on KUE 13183b in the CHO/HGPRT forward mutation assay. Unpublished report No. R4204 24 August 1987 from Hazleton Biotechnologies, Netherlands. Submitted to WHO by Bayer AG Bayerwerk.
Bomann, W. (1995a) KUE 13183b (c.n. tolylfluanid). Study for acute oral toxicity in rats. Unpublished report No. 23615 6 January 1995, from Bayer AG, Institute of Toxicology, Wuppertal, Germany. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Bomann W (1995b) KUE 13183B (tolylfluanid). Study for acute dermal toxicity in rats. Unpublished report No. 23616 6 January 1995, from Bayer AG, Institute for Toxicology, Wuppertal, Germany. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Bomhard, E. (1988) KUE 13183b (tolylfluanid, Euparen M active ingredient)—Subacute toxicological study on the question of an effect on the thyroid of rats (four-week feeding test). Unpublished report No. 17183 29 September 1988 from Bayer AG, Toxicology Institute, Wuppertal, Germany. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Bomhard, E. & Schilde, B. (1976) KUE 13183b—Subchronic toxicological study in rats (feeding experiment over 3 months). Unpublished report No. 5929 20 February 1976 from Bayer AG, Toxicology Institute, Wuppertal, Germany. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Brendler-Schwaab, S. (1995) KUE 13183b—Test on unscheduled DNA synthesis in rat liver primary cell cultures in vitro. Unpublished report No. 24436 31 October 1995 from Bayer AG, Institute of Toxicology, Wuppertal, Germany. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Calcagnotto, A.M., Jeffrey, A.M. & Luo, F.-Q. (1997) 32P-Postlabelling assay for detection of adduct formation by tolylfluanid (TF) in rat lung, thyroid and liver DNA. Unpublished report No. R6933 25 August 1997 from American Health Foundation. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Cifone, M.A. (1995) Mutagenicity test on WAK 6698 in the L5178Y TK+/– mouse lymphoma forward mutation assay. Unpublished report 21 December 1995 from Corning Hazleton Inc. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Clemens, G.R., Grosso, D.S. & Hartnagel, R.E. (1995) A developmental study with orally administered KUE 13183b technical in the rat. Unpublished report No. MTD9405 8 June 1995 from Bayer Corp., Drug Safety Evaluation, West Haven, Connecticut, USA. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Diesing, L. (1990) KUE 13183b (tolylfluanid). Study for skin sensitising effect on guinea pigs (Büehler’s patch test). Unpublished report No. 18630 8 January 1990 from Bayer AG, Department of Toxicology, Wuppertal, Germany. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Diesing, L. (1991) KUE 13183b. Study for skin-sensitizing effect on guinea pigs (Klecak open epicutaneous test). Unpublished report No. 19981 11 February 1991 from Bayer AG, Department of Toxicology, Wuppertal, Germay.. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Draize, J.H. (1977) Dermal and eye toxicity tests. In: Principles and Procedures for Evaluating the Toxicity of Household Substances, Washington DC: National Academy of Sciences, pp. 48–49.
Dreist, M. (1995) KUE 13183B (common name tolylfluanid). Subchronic toxicity study in Wistar rats (thirteen week administration in the diet with a four week recovery period). Unpublished report No. 24334, study No. T 3058000, 27 September 1995 from Bayer AG Toxicology, Wuppertal, Germany. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Dreist, M. & Popp, A. (1994) KUE 13183B (common name tolylfluanid). Acute oral neurotoxicity screening studies in rats. Unpublished report No. 23517 5 December 1994 from Bayer AG Toxicology, Wuppertal, Germany. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Dreist, M. & Popp, A. (1995) KUE 13183B (common name tolylfluanid). Subchronic neurotoxicity screening studies in Wistar rats (thirteen-week administration in the diet). Unpublished report No. 24336 29 September 1995 from Bayer AG Toxicology, Wuppertal, Germany. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Ecker, W. (1978) Biotransformation of [14C] tolylfluanid in the rat. Unpublished report No. PF1282 9 November 1978 from Isotope Laboratory Chemistry, Institute for Pharmacokinetics, Wuppertal, Germany. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Ecker, W. & Brauner, A. (1987) Biotransformation of [ring-U-14C] tolylfluanid by the rat following oral administration. Unpublished report No. PF2826 14 July 1987 from Bayer AG Toxicology, Wuppertal, Germany. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Ecker, W. & Weber, H. (1995) [Phenyl-U-14C] tolylfluanid absorption, distribution, excretion and metabolism in a lactating goat. Unpublished report No. PF4106 24 October 1995 from Bayer AG Institute for Metabolism Research and Residue Analysis, Germany. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Ecker, W. & Weber, H. (1996) [Phenyl-U-14C] tolylfluanid absorption, distribution, excretion and metabolism in a laying hens. Unpublished report No. PF4170 15 November 1996 from Bayer AG Institute for Metabolism Research and Residue Analysis, Germany. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Faul, J. (1982) Statement to Pkt IV/1.2.2 of the BBA application form ‘Details of effects on man, internal company experience’. Unpublished report 28 April 1982 from Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Faul, J. (1989) Euparen and Euparen M—In-company occupational medical experience. Unpublished report 4 September 1989 from Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Flucke, W. & Kimmerle, G.. (1978) Triadimefon (MEB 6447) and tolylfluanid (KUE 13183b)—Study for acute combination toxicity. Unpublished report No. 7304 9 February 1978 from Bayer AG Toxicology, Wuppertal, Germany. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Flucke, W. & Thyssen, J. (1978) Dimethylaminosulf-p-toluidid—Study for acute toxicity. Unpublished report No. 7384 March 1978 from Bayer AG Toxicology, Wuppertal, Germany. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Freyberger, A. (1995) Tolylfluanid (KUE 13183b)—In vitro characterisation of the goitrogenic properties of its metabolite 2-thiazolidinethione-4-carboxylic acid (TTCA). Unpublished report No. 24435 31 October 1995 from Bayer AG Toxicology, Wuppertal, Germany. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Heidemann, A. & Miltenburger, H.G. (1987) KUE 13183b, detection of gene mutations in somatic mammalian cells in culture: HGPRT-test with V79 cells. Unpublished report No. R4103 8 May 1987 from Laboratory for Mutagenicity Testing, Technical University, Darmstadt, Germany. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Heimann, K.G. (1983) KUE 13183B. Study for sensitising effects on guinea pigs. Unpublished report No. 11492 24 January 1983 from Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Heimann, K.G. (2000) Assessment of fluoride uptake after prolonged administration of tolylfluanid. Unpublished report 4 April 2000 from Business Group Crop Protection, Development/Registration, Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany
Heimann, K.G. & Pauluhn, J. (1983) KUE 13183B (tolylfluanid, Euparen M active ingredient). Study for acute toxicity. Unpublished report No. 11383 5 January 1983 from Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Herbold, B. (1979) KUE 13183b—Salmonella/microsome test for point mutagenic effects. Unpublished report No. 8265 30 March 1979 from Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Herbold, B. (1980) KUE 13183b (tolylfluanid)—Micronucleus-test on the mouse to evaluate for mutagenic effect. Unpublished report No. 9149 13 May 1980 from Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Herbold, B. (1983) KUE 13183b (c.n. tolylfluanid/Euparen M/Preventol VP OC 3017)—Cytogenetic study of the Chinese hamster’s bone marrow in vivo to evaluate for mutagenic effect. Unpublished report No. 11792 10 May 1983 from Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Herbold, B. (1984a) KUE 13183b (c.n. tolylfluanid)—Cytogenetic study with human lymphocyte cultures in vitro to evaluate for harmful effect on chromosomes. Unpublished report No. 12836 6 August 1984 from Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, FRG
Herbold, B. (1984b) KUE 13183b (c.n tolylfluanid)—Cytogenetic study on the spermatogonia of the Chinese hamster in vivo to evaluate for mutagenic effect. Unpublished report No. 12739 8 June 1984 from Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Herbold, B. (1986) KUE 13183b (c.n tolylfluanid)—Dominant lethal test on the male mouse to evaluate for mutagenic effect. Unpublished report No. 15017 26 August 1986 from Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Herbold, B. (1988) KUE 13183b (c.n tolylfluanid)—Spot test on cross bred C57B1/6J x T stock mouse fetuses to evaluate for induced somatic changes in the genes of the coat pigment cells. Unpublished report No. 16752 31 May 1988 from Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Herbold, B. (1994a) KUE 13183b—Salmonella/microsome test. Unpublished report No. 22843 27 January 1994 from Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Herbold, B. (1994b) WAK 5818—Salmonella/microsome test plate incorporation and preincubation method. Unpublished report No. 23520 6 December 1994 from Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Herbold, B. (1995a) WAK 6550—Salmonella/microsome test plate incorporation and preincubation method. Unpublished report No. 23749 15 February 1995 from Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Herbold, B. (1995b) WAK 667—Salmonella/microsome test plate incorporation and preincubation method. Unpublished report No. 23780 21 February 1995 from Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Herbold, B. (1995c) WAK 6698—Salmonella/microsome test plate incorporation and preincubation method. Unpublished report No. 23821 9 March 1995 from Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, FRG.
Herbold, B. (1996) KUE 13183b—In vitro mammalian chromosome aberration test with Chinese hamster V79 cells. Unpublished report No. 24581 2 January 1996 from Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Herbold, B. (2000a) KUE 13183b—First amendment to final report No. 12836 of 6 August 1984—Cytogenetic investigations in human lymphocyte in vitro to test for clastogenic effects. Unpublished report No. 12836A. 13 October 2000 from Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, FRG
Herbold, B. (2000b) KUE 13183b—First amendment to final report No. 11792 of 10 May 1983—Cytogenetic investigations in the bone marrow of Chinese hamsters in vivo to test for clastogenic effects. Unpublished report No. 11792A 13 October 2000 from Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Herbold, B. (2002) KUE-13183B-DMST. In vitro chromosome aberration test with Chinese hamster V79 cells. Study No. T3070871. Unpublished report No. PH 31854 13 February 2002 from Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Hoffmann, K. (1983) KUE 13183b—Acute toxicity to the sheep after oral administration. Unpublished report No. 11975 2 August 1983 from Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Hoffmann, K. & Mirea, D. (1974) KUE 13183b (tolylfluanid/Euparen M®)—Subchronic toxicity study on dog (thirteen-week feeding experiment). Unpublished report No. 4957 12 September 1974 from Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Holzum, B. (1991a) KUE 13183b (c.n. tolylfluanid)—Two-generation study on rats (supplement to study T1007392). Unpublished report No. 20583 30 August 1991 from Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Holzum, B. (1991b9) KUE 13183b (c.n. tolylfluanid)—Study for embryotoxic effects on rabbits following oral administration. Unpublished report No. 20034 1 March 1991 from Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Holzum B (1991c) KUE 13183b (c.n. tolylfluanid)—Supplementary study for maternal toxicity to gravid rabbits following oral administration. Unpublished report No. 19901 23 January 1991 from Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Holzum, B. (1994) KUE 13183b (c.n. tolylfluanid)—Study for embryotoxic effects on rabbits following oral administration, first amendment to report No. 20034. Unpublished report No. 2034A 15 November 1994 from Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Holzum, B. (1995) KUE 13183b (c.n. tolylfluanid)—Two-generation study on rats (supplement to study T1007392), first amendment to report No. 20583. Unpublished report No. 20583A 10 April 1995 from Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Holzum, B. & Kaliner, G. (1989) KUE 13183b (c.n. tolylfluanid)—2 generation study on rats. Unpublished report No. 17788 3 March 1989 from Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Hoorn, A.J.W. (1984) Mutagenicity evaluation of KUE 13183b (c.n. tolylfluanid) in the reverse mutation induction assay with Saccharomyces cerevisiae strains S138 and S211alpha. Unpublished report No. R3060 29 August 1984 from Litton Bionetics, Netherlands. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Hoorn, A.J.W. (1985) Mutagenicity evaluation of KUE 13183b (c.n. tolylfluanid) in the mouse lymphoma forward mutation assay. Unpublished report No. R3192 February 1985 from Litton Bionetics, Netherlands. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Imsgard, F. (1993) Overview concerning the use of Preventol A4 (tolylfluanid) by GORI. Unpublished report from GORI, DK-6000 Kolding, 2 August 1993. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
von Keutz, E. & Nash, G. (1986) KUE 13183b (tolylfluanid)—Chronic toxicity to dogs after oral administration (twelve-month capsule study). Unpublished report No. 12999 22 July 198 from Institute of Toxicology, Bayer AG.. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Kimmerle, G. (1964) Kühle 13183b (Hat. No. 3363). Unpublished report 12 November 1964 from Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Kimmerle, G. (1968) BAY 49854—Toxicological studies. Unpublished report No. 832 10 June 1968 from Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Kimmerle, G. & Lorke, D. (1967) Toxicological studies on the active ingredient BAY 49854. Unpublished report No. 323 17 May 1967 from Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Kimmerle, G. & Solmecke, B. (1971) Methyl-Euparen, subacute cutaneous application to rabbits. Unpublished report No. 2619 5 March 1971 from Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Klein, O. (1991) [U-phenyl-14C] Tolylfluanid: General rat metabolism study. Unpublished report No. PF3785 7 October 1991, amended 21 September 2000, from Crop Protection Research, Institute for Metabolism Research, Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Kolb, J., Vohr, H.W. & Sander, E. (1995) KUE 13183 b—Subacute dermal toxicity study on rabbits. Unpublished report No. 23712 6 February 1995 from Department of Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Krötlinger, F. (1994a) KUE 13183b (tolylfluanid). Study for skin and eye irritation/corrosion in rabbits. First amendment to report No. 22860 1 February 1994. Unpublished report No. 22860A 5 October 2000 from Fachbereich Toxicologie, Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Krötlinger, F. (1994b) WAK 5818 (tolylfluanid metabolite). Study for acute oral toxicity in rats. Unpublished report No. 23482 15 November 1994 from Bayer AG, Fachbereich Toxicologie, Wuppertal, Germany. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Krötlinger, F. (1995a) WAK 6550 (tolylfluanid metabolite). Study for acute oral toxicity in rats. Unpublished report No. 23613 6 January 1995 from Bayer AG, Fachbereich Toxicologie, Wuppertal, Germany. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Krötlinger, F. (1995b) WAK 6676 (tolylfluanid metabolite). Study for acute oral toxicity in rats. Unpublished report No. 23729 10 February 1995 from Bayer AG, Fachbereich Toxicologie, Wuppertal, Germany. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Krötlinger, F. (1995c) WAK 6698 (tolylfluanid metabolite). Study for acute oral toxicity in rats. Unpublished report No. 23772 21 February 1995 from Bayer AG, Fachbereich Toxicologie, Wuppertal, Germany. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Krötlinger, F. & Löser E (1982) KUE 13183b (tolylfluanid/Euparen M-active ingredient)—Chronic toxicological study in rats (feeding for two years). Unpublished report No. 10978 30 June 1982 from Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Leser, K.H. & Ruhl-Fehlert, C. (1996) KUE 13183b (c.n. tolylfluanid)—Oncogenicity study in B6C3F1 mice (administration in food over 2 years). Unpublished report No. 25548 17 October 1996 from Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Leser, K.H., Rosenbruch, M. & Rinke, M. (1996) KUE 13183b (c.n. tolylfluanid)—Study on chronic toxicity and carcinogenicity in Wistar rats (administration in food over 2 years). Unpublished report No. 25426 13 September 1996 from Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Leser, K.H., Rosenbruch, M. & Rinke, M. (1997) KUE 13183b (c.n. tolylfluanid)—Study on chronic toxicity and carcinogenicity in Wistar rats (administration in food over 2 years). Unpublished report No. 25426A 27 May 1997 from Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Löser, E. (1980) KUE 13183B (Euparen M—active ingredient)—Generation study with rats. Unpublished report No. 9419 29 August 1980 from Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Machemer, L. (1976) KUE 13183B—Studies of embryotoxic and teratogenic effects on rats after oral administration. Unpublished report No. 5888 10 February 1976 from Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Märtins, T. (1996a) KUE 13183B (common name: tolylfluanid). Study on acute inhalation toxicity in rats according to OECD 403. Unpublished report No. 25503 7 October 1996 from Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Märtins, T. (1996b) KUE 13183B. Study on subacute inhalation toxicity in rats (5x6 hours exposure). Unpublished report No. 25437 18 September 1996 from Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Märtins, T. (1997) KUE 13183B. Study on subacute inhalation toxicity in rats (20x6 hours exposure) according to OECD-guideline No. 412. Unpublished report No. 25828 20 January 1997 from Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
McClain, R.M. (1992) Thyroid gland neoplasia: Non-genotoxic mechanisms. Toxicol. Lett., 64/65, 397–408.
Mohr, U. (1982) KUE 13183b—Study for cancerogenic effect on NMRI-mice (feeding study for 104 weeks). Unpublished report No. R2225 22 July 1982 from Department of Experimental Pathology, Medical University, Hanover, Germany. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Moser, V.C. (1989) Screening approaches to neurotoxicity. A functional observational battery. J. Am. Coll. Toxicol., 8, 85–93.
Olloz, F. (1993) Preventol A5/tolylfluanide. Unpublisjed report, 13 June 1993 from Pentol AG, Switzerland. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Pauluhn, J. (1984) KUE 13183B (Euparen M active ingredient). Study for irritant/corrosive effect on skin and eye (rabbit). Unpublished report No. 12362 6 January 1984 from Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Pauluhn, J. (1997) KUE 13183B (common name: tolylfluanid). Study on acute inhalation toxicity in rats according to OECD 403. Unpublished report No. 26653 22 September 1997 from Department of Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Pauluhn, J. (2001) KUE 13183B (common name: tolylfluanid). Study on acute inhalation toxicity in rats according to OECD 403. Unpublished report No. 30639 22 January 2001 from Department of Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Pauluhn, J. (2002) KUE-13183B. Subacute inhalation toxicity on rats (exposure 20 x 6 hour/day for 4 weeks). Study No. T6070315. Unpublished report No. PH 31791 20 February 2002 from Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Pickel, M. & Rinke, M. (1995) KUE 13183B. Two-generation study in rats. Unpublished report No. 23921 10 April 1995 from Department of Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Roos, B. (1993) Tolylfluanid VP OC 3049. Unpublished report 22 August 1993 from Geveko Oy, Finland. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Schneeberger, R. (1993) Unpublished report 20 July 1993 from Blaser & Co. AG, Switzerland. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Thyssen, J. (1978) Determination of acute toxicity (LD50)—KUE 5156 thiazolidinthioncarbonacid, main metabolite in urine of (14C) Euparen (-M). Unpublished report 30 January 1978 from Department of Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Vohr, H.W. (2001) KUE 13183B—Local lymph node assay in mice (LLNA/IMDS). Unpublished report No. 31086 11 June 2001 from Department of Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Vohr, J.W. (2002) KUE 13183B. Local lymph node assay in mice (LLNA/IMDS) (secondary response). Study No. T4071088. Unpublished report No. PJ 31924 8 April 2002 from Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Völkner, W. (1988a) Mouse germ-cell cytogenetic assay with KUE 13183b. Unpublished report No. R4485 1 July 1988 from Cytotest Cell Research GmbH & Co. KG, Germany. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Völkner, W. (1988b) Sister chromatid exchange assay in bone marrow cells of the mouse with KUE 13183b. Unpublished report No. R4422 2 May 1988 from Cytotest Cell Research GmbH & Co. KG, Germany. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Völkner, W. (1990) Chromosome aberration assay in bone marrow cells of Chinese hamster with KUE 13183b. Unpublished report No. R5153 20 September 1990 from Cytotest Cell Research GmbH & Co. KG, Germany. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Weber, H. (1988) [Phenyl-UL-14C] tolylfluanid: Whole-body autoradiographic distribution of the radioactivity in the rat. Unpublished report No. PF2961 7 March 1988, amended 4 November 2000, from Institute for Metabolism Research, Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Weber, H., Patzschke, K. & Wegner, L.A. (1977) Tolylfluanid-14C (Euparen M active substance)—Biokinetic investigations of rats. Unpublished report No. PF1165 29 September 1977 from Isotope Laboratory, Institute for Pharmacokinetics, Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Wetzig, H. & Schilde, B. (1997) KUE 13183b (c.n. tolylfluanid)—Chronic (52 week) oral toxicity study in dogs (study no. T6060604). Unpublished report No. 26664 24 September 1997 from Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
Wetzig, H. & Schilde, B. (1998) KUE 13183b (c.n. tolylfluanid)—Chronic (52 week) oral toxicity study in dogs (study no. T6060604). Unpublished report No. 26664A 17 August 1998 from Bayer AG. Submitted to WHO by Bayer AG, Bayerwerk, Germany.
WHO (2000) The WHO recommended classification of pesticides by hazard and guidelines to classification 2000–2202 (WHO/PCS/01.5). Available from the International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland.
See Also:
Toxicological Abbreviations