
FAO Meeting Report No. PL/1965/10/1
WHO/Food Add./27.65
EVALUATION OF THE TOXICITY OF PESTICIDE RESIDUES IN FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Committee on Pesticides in Agriculture and
the WHO Expert Committee on Pesticide Residues, which met in Rome,
15-22 March 19651
Food and Agriculture Organization of the United Nations
World Health Organization
1965
1 Report of the second joint meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Committee on Pesticide
Residues, FAO Meeting Report No. PL/1965/10; WHO/Food Add./26.65
ALLETHRIN
Chemical name
d1-2-allyl-4-hydroxy-3-methyl-2-cyclopenten-1-one esters of cis
and trans d1-chrysanthemum monocarboxylic acid.
Synonyms
allyl homologue of cinerin I (see monograph on pyrethrins)
Empirical formula
C19H26O3 (Mol. wt. 302.4)
Structural formula
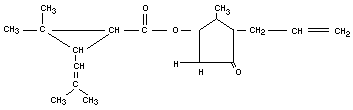 Relevant physical and chemical properties
Allethrin is a synthetic pyrethroid and has chemical properties
similar to those of pyrethrins, however it is more stable on exposure
to heat and sunlight. Detoxification occurs by double bond
hydrogenation of acid or allyl side chains, and it hydrolyses to
chrysanthemic acid and 2-allyl-3-methyl-2, 4-cyclopentadienone.
Allylrethrolone and chrysanthemic acid exist as optical isomers.
Chrysanthemic acid also exists as geometric isomers. There are eight
optical and geometric isomers of allethrin, all possibly present in
the technical material. Insecticidal activity depends on the
proportions of the various isomers present (Negherbon, 1959).
Uses
Allethrin, alone or in combination with synergists, is used in
many instances where pyrethrins are used for the same purpose. If
price is reduced in the future, use may be expanded. Principal
formulations are technical allethrin (usually 95%); 1% dust; 0.1% to
0.6% aerosol formulations and in distillate formulations with or
without synergists and other ingredients. When used alone it is exempt
from tolerances for residues (in Canada and U.S.A.) except for
post-harvest application to grain, with no limitations on use. Residue
tolerances have been established when used in conjunction with
synergists. It is particularly useful as dusts on a wide range of
horticultural crops when other pesticides cannot be used close to date
of harvest. It is recommended in synergized formulations as aerosols
or dips for post-harvest use on fruits and berries, in storage and in
processing plants. Agricultural premises, including milk rooms, are
treated with allethrin aerosols or surface sprays where residues of
other pesticides must be avoided. Direct application is approved for
beef and dairy cattle, sheep, goats, poultry and swine. Post-harvest
use for stored grain (surface treatment) has also been approved in
some countries.
Residues
Methods described for pyrethrins residue analysis are applicable
for allethrin, except that the standard is made from allethrin. A
colorimetric method for allethrin residues in milk and meat has been
described (McClelland and Moore, 1958). The method is reported
accurate to 0.1 ppm or 10 mmg/100g sample. Allethrin was not found in
the milk of dairy cows, which had been sprayed daily for three weeks,
or in the meat tissue of a female goat that had been sprayed daily for
five weeks, all with a large overdose of spray. No information is
available on the chemical nature of terminal residues in treated
crops.
Effect on treated crop
There is no information available on the effect of allethrin on
treated crops.
BIOLOGICAL DATA
Acute toxicity
Animal Route LD50 mg/kg Reference
body-weight
Rat, male Oral 920 Carpenter et al., 1950
Rat, female Oral 900 "
Mouse, male Oral 480 "
20% in Deo-base
Mouse, male, Oral 1580 "
5% in Deo-base
Rabbit, male Oral 4290 "
Short-term studies
Rat. Rats showed a slight decrease in growth-rate when fed
commercial allethrin at a dietary level of 5000 ppm and a more nearly
normal growth-rate when fed the same concentration of purified
allethrin. A dietary level of 2500 ppm apparently produced no clinical
effect. No examination of the liver was reported (Ambrose & Robbins,
1951).
In another study rats that were fed allethrin for 16 weeks showed
no gross effect at 5000 ppm but did show tremor and convulsions at 10
000 ppm (Lehman, 1952).
It was shown that rats (and dogs) withstand repeated inhalation
of allethrin aerosols at many times the concentration in air that
would possibly be reached under practical conditions. The method of
dosage did not lend itself to measurement on the basis of milligram
per kilogram of body-weight (Carpenter et al., 1950).
Dog. Four male and four female dogs fed allethrin at a rate of
50 mg/kg/day for two years showed no gross or microscopical effects.
At higher dosage levels in other groups, there were convulsions,
progressively shortened survival, and non-specific pathological
changes (Lehman, 1965).
Man. There are apparently no reports of allergy to allethrin
comparable to those associated with pyrethrum and pyrethrins. However,
since the types of exposure have not been the same the possibility of
allergy to allethrin cannot be excluded.
Comments on the experimental studies reported and evaluation
Rats apparently tolerate prolonged feeding at a rate of 125
mg/kg/day but the exact duration of the test was not stated.
A dosage of 50 mg/kg/day for 2 years produced no detectable
effect in dogs.
Because no study of the liver seems to have been made in rats and
because no study has been made in any species for more than half its
life-span, it is not possible to estimate an acceptable daily intake
for man.
Further work required
Long-term studies in at least two species are required in which
particular attention is paid to possible effects on the liver. The
metabolism of the substance should be explored.
REFERENCES
Ambrose, A. M. & Robbins, D. J. (1951) Fed. Proc., 10, 276
Carpenter, C. P., Weil, C. S., Pozzani, U. C. & Smyth, J. F., jr
(1950) Arch. industr. Hyg., 2, 420
Lehman, A. J. (1952) Quart. Bull. Assoc. Food and Drug Officials
U.S., 16, 47
Lehman, A. J. (1965) Summaries of pesticide toxicity (In press)
McClellan, D. B. & Moore, J. B. (1958) J. Agr. Food Chem., 6, 463
Negherbon, W. (1959) Handbook of Toxicology, vol. 3, Saunders,
Philadelphia
Relevant physical and chemical properties
Allethrin is a synthetic pyrethroid and has chemical properties
similar to those of pyrethrins, however it is more stable on exposure
to heat and sunlight. Detoxification occurs by double bond
hydrogenation of acid or allyl side chains, and it hydrolyses to
chrysanthemic acid and 2-allyl-3-methyl-2, 4-cyclopentadienone.
Allylrethrolone and chrysanthemic acid exist as optical isomers.
Chrysanthemic acid also exists as geometric isomers. There are eight
optical and geometric isomers of allethrin, all possibly present in
the technical material. Insecticidal activity depends on the
proportions of the various isomers present (Negherbon, 1959).
Uses
Allethrin, alone or in combination with synergists, is used in
many instances where pyrethrins are used for the same purpose. If
price is reduced in the future, use may be expanded. Principal
formulations are technical allethrin (usually 95%); 1% dust; 0.1% to
0.6% aerosol formulations and in distillate formulations with or
without synergists and other ingredients. When used alone it is exempt
from tolerances for residues (in Canada and U.S.A.) except for
post-harvest application to grain, with no limitations on use. Residue
tolerances have been established when used in conjunction with
synergists. It is particularly useful as dusts on a wide range of
horticultural crops when other pesticides cannot be used close to date
of harvest. It is recommended in synergized formulations as aerosols
or dips for post-harvest use on fruits and berries, in storage and in
processing plants. Agricultural premises, including milk rooms, are
treated with allethrin aerosols or surface sprays where residues of
other pesticides must be avoided. Direct application is approved for
beef and dairy cattle, sheep, goats, poultry and swine. Post-harvest
use for stored grain (surface treatment) has also been approved in
some countries.
Residues
Methods described for pyrethrins residue analysis are applicable
for allethrin, except that the standard is made from allethrin. A
colorimetric method for allethrin residues in milk and meat has been
described (McClelland and Moore, 1958). The method is reported
accurate to 0.1 ppm or 10 mmg/100g sample. Allethrin was not found in
the milk of dairy cows, which had been sprayed daily for three weeks,
or in the meat tissue of a female goat that had been sprayed daily for
five weeks, all with a large overdose of spray. No information is
available on the chemical nature of terminal residues in treated
crops.
Effect on treated crop
There is no information available on the effect of allethrin on
treated crops.
BIOLOGICAL DATA
Acute toxicity
Animal Route LD50 mg/kg Reference
body-weight
Rat, male Oral 920 Carpenter et al., 1950
Rat, female Oral 900 "
Mouse, male Oral 480 "
20% in Deo-base
Mouse, male, Oral 1580 "
5% in Deo-base
Rabbit, male Oral 4290 "
Short-term studies
Rat. Rats showed a slight decrease in growth-rate when fed
commercial allethrin at a dietary level of 5000 ppm and a more nearly
normal growth-rate when fed the same concentration of purified
allethrin. A dietary level of 2500 ppm apparently produced no clinical
effect. No examination of the liver was reported (Ambrose & Robbins,
1951).
In another study rats that were fed allethrin for 16 weeks showed
no gross effect at 5000 ppm but did show tremor and convulsions at 10
000 ppm (Lehman, 1952).
It was shown that rats (and dogs) withstand repeated inhalation
of allethrin aerosols at many times the concentration in air that
would possibly be reached under practical conditions. The method of
dosage did not lend itself to measurement on the basis of milligram
per kilogram of body-weight (Carpenter et al., 1950).
Dog. Four male and four female dogs fed allethrin at a rate of
50 mg/kg/day for two years showed no gross or microscopical effects.
At higher dosage levels in other groups, there were convulsions,
progressively shortened survival, and non-specific pathological
changes (Lehman, 1965).
Man. There are apparently no reports of allergy to allethrin
comparable to those associated with pyrethrum and pyrethrins. However,
since the types of exposure have not been the same the possibility of
allergy to allethrin cannot be excluded.
Comments on the experimental studies reported and evaluation
Rats apparently tolerate prolonged feeding at a rate of 125
mg/kg/day but the exact duration of the test was not stated.
A dosage of 50 mg/kg/day for 2 years produced no detectable
effect in dogs.
Because no study of the liver seems to have been made in rats and
because no study has been made in any species for more than half its
life-span, it is not possible to estimate an acceptable daily intake
for man.
Further work required
Long-term studies in at least two species are required in which
particular attention is paid to possible effects on the liver. The
metabolism of the substance should be explored.
REFERENCES
Ambrose, A. M. & Robbins, D. J. (1951) Fed. Proc., 10, 276
Carpenter, C. P., Weil, C. S., Pozzani, U. C. & Smyth, J. F., jr
(1950) Arch. industr. Hyg., 2, 420
Lehman, A. J. (1952) Quart. Bull. Assoc. Food and Drug Officials
U.S., 16, 47
Lehman, A. J. (1965) Summaries of pesticide toxicity (In press)
McClellan, D. B. & Moore, J. B. (1958) J. Agr. Food Chem., 6, 463
Negherbon, W. (1959) Handbook of Toxicology, vol. 3, Saunders,
Philadelphia
 Relevant physical and chemical properties
Allethrin is a synthetic pyrethroid and has chemical properties
similar to those of pyrethrins, however it is more stable on exposure
to heat and sunlight. Detoxification occurs by double bond
hydrogenation of acid or allyl side chains, and it hydrolyses to
chrysanthemic acid and 2-allyl-3-methyl-2, 4-cyclopentadienone.
Allylrethrolone and chrysanthemic acid exist as optical isomers.
Chrysanthemic acid also exists as geometric isomers. There are eight
optical and geometric isomers of allethrin, all possibly present in
the technical material. Insecticidal activity depends on the
proportions of the various isomers present (Negherbon, 1959).
Uses
Allethrin, alone or in combination with synergists, is used in
many instances where pyrethrins are used for the same purpose. If
price is reduced in the future, use may be expanded. Principal
formulations are technical allethrin (usually 95%); 1% dust; 0.1% to
0.6% aerosol formulations and in distillate formulations with or
without synergists and other ingredients. When used alone it is exempt
from tolerances for residues (in Canada and U.S.A.) except for
post-harvest application to grain, with no limitations on use. Residue
tolerances have been established when used in conjunction with
synergists. It is particularly useful as dusts on a wide range of
horticultural crops when other pesticides cannot be used close to date
of harvest. It is recommended in synergized formulations as aerosols
or dips for post-harvest use on fruits and berries, in storage and in
processing plants. Agricultural premises, including milk rooms, are
treated with allethrin aerosols or surface sprays where residues of
other pesticides must be avoided. Direct application is approved for
beef and dairy cattle, sheep, goats, poultry and swine. Post-harvest
use for stored grain (surface treatment) has also been approved in
some countries.
Residues
Methods described for pyrethrins residue analysis are applicable
for allethrin, except that the standard is made from allethrin. A
colorimetric method for allethrin residues in milk and meat has been
described (McClelland and Moore, 1958). The method is reported
accurate to 0.1 ppm or 10 mmg/100g sample. Allethrin was not found in
the milk of dairy cows, which had been sprayed daily for three weeks,
or in the meat tissue of a female goat that had been sprayed daily for
five weeks, all with a large overdose of spray. No information is
available on the chemical nature of terminal residues in treated
crops.
Effect on treated crop
There is no information available on the effect of allethrin on
treated crops.
BIOLOGICAL DATA
Acute toxicity
Animal Route LD50 mg/kg Reference
body-weight
Rat, male Oral 920 Carpenter et al., 1950
Rat, female Oral 900 "
Mouse, male Oral 480 "
20% in Deo-base
Mouse, male, Oral 1580 "
5% in Deo-base
Rabbit, male Oral 4290 "
Short-term studies
Rat. Rats showed a slight decrease in growth-rate when fed
commercial allethrin at a dietary level of 5000 ppm and a more nearly
normal growth-rate when fed the same concentration of purified
allethrin. A dietary level of 2500 ppm apparently produced no clinical
effect. No examination of the liver was reported (Ambrose & Robbins,
1951).
In another study rats that were fed allethrin for 16 weeks showed
no gross effect at 5000 ppm but did show tremor and convulsions at 10
000 ppm (Lehman, 1952).
It was shown that rats (and dogs) withstand repeated inhalation
of allethrin aerosols at many times the concentration in air that
would possibly be reached under practical conditions. The method of
dosage did not lend itself to measurement on the basis of milligram
per kilogram of body-weight (Carpenter et al., 1950).
Dog. Four male and four female dogs fed allethrin at a rate of
50 mg/kg/day for two years showed no gross or microscopical effects.
At higher dosage levels in other groups, there were convulsions,
progressively shortened survival, and non-specific pathological
changes (Lehman, 1965).
Man. There are apparently no reports of allergy to allethrin
comparable to those associated with pyrethrum and pyrethrins. However,
since the types of exposure have not been the same the possibility of
allergy to allethrin cannot be excluded.
Comments on the experimental studies reported and evaluation
Rats apparently tolerate prolonged feeding at a rate of 125
mg/kg/day but the exact duration of the test was not stated.
A dosage of 50 mg/kg/day for 2 years produced no detectable
effect in dogs.
Because no study of the liver seems to have been made in rats and
because no study has been made in any species for more than half its
life-span, it is not possible to estimate an acceptable daily intake
for man.
Further work required
Long-term studies in at least two species are required in which
particular attention is paid to possible effects on the liver. The
metabolism of the substance should be explored.
REFERENCES
Ambrose, A. M. & Robbins, D. J. (1951) Fed. Proc., 10, 276
Carpenter, C. P., Weil, C. S., Pozzani, U. C. & Smyth, J. F., jr
(1950) Arch. industr. Hyg., 2, 420
Lehman, A. J. (1952) Quart. Bull. Assoc. Food and Drug Officials
U.S., 16, 47
Lehman, A. J. (1965) Summaries of pesticide toxicity (In press)
McClellan, D. B. & Moore, J. B. (1958) J. Agr. Food Chem., 6, 463
Negherbon, W. (1959) Handbook of Toxicology, vol. 3, Saunders,
Philadelphia
Relevant physical and chemical properties
Allethrin is a synthetic pyrethroid and has chemical properties
similar to those of pyrethrins, however it is more stable on exposure
to heat and sunlight. Detoxification occurs by double bond
hydrogenation of acid or allyl side chains, and it hydrolyses to
chrysanthemic acid and 2-allyl-3-methyl-2, 4-cyclopentadienone.
Allylrethrolone and chrysanthemic acid exist as optical isomers.
Chrysanthemic acid also exists as geometric isomers. There are eight
optical and geometric isomers of allethrin, all possibly present in
the technical material. Insecticidal activity depends on the
proportions of the various isomers present (Negherbon, 1959).
Uses
Allethrin, alone or in combination with synergists, is used in
many instances where pyrethrins are used for the same purpose. If
price is reduced in the future, use may be expanded. Principal
formulations are technical allethrin (usually 95%); 1% dust; 0.1% to
0.6% aerosol formulations and in distillate formulations with or
without synergists and other ingredients. When used alone it is exempt
from tolerances for residues (in Canada and U.S.A.) except for
post-harvest application to grain, with no limitations on use. Residue
tolerances have been established when used in conjunction with
synergists. It is particularly useful as dusts on a wide range of
horticultural crops when other pesticides cannot be used close to date
of harvest. It is recommended in synergized formulations as aerosols
or dips for post-harvest use on fruits and berries, in storage and in
processing plants. Agricultural premises, including milk rooms, are
treated with allethrin aerosols or surface sprays where residues of
other pesticides must be avoided. Direct application is approved for
beef and dairy cattle, sheep, goats, poultry and swine. Post-harvest
use for stored grain (surface treatment) has also been approved in
some countries.
Residues
Methods described for pyrethrins residue analysis are applicable
for allethrin, except that the standard is made from allethrin. A
colorimetric method for allethrin residues in milk and meat has been
described (McClelland and Moore, 1958). The method is reported
accurate to 0.1 ppm or 10 mmg/100g sample. Allethrin was not found in
the milk of dairy cows, which had been sprayed daily for three weeks,
or in the meat tissue of a female goat that had been sprayed daily for
five weeks, all with a large overdose of spray. No information is
available on the chemical nature of terminal residues in treated
crops.
Effect on treated crop
There is no information available on the effect of allethrin on
treated crops.
BIOLOGICAL DATA
Acute toxicity
Animal Route LD50 mg/kg Reference
body-weight
Rat, male Oral 920 Carpenter et al., 1950
Rat, female Oral 900 "
Mouse, male Oral 480 "
20% in Deo-base
Mouse, male, Oral 1580 "
5% in Deo-base
Rabbit, male Oral 4290 "
Short-term studies
Rat. Rats showed a slight decrease in growth-rate when fed
commercial allethrin at a dietary level of 5000 ppm and a more nearly
normal growth-rate when fed the same concentration of purified
allethrin. A dietary level of 2500 ppm apparently produced no clinical
effect. No examination of the liver was reported (Ambrose & Robbins,
1951).
In another study rats that were fed allethrin for 16 weeks showed
no gross effect at 5000 ppm but did show tremor and convulsions at 10
000 ppm (Lehman, 1952).
It was shown that rats (and dogs) withstand repeated inhalation
of allethrin aerosols at many times the concentration in air that
would possibly be reached under practical conditions. The method of
dosage did not lend itself to measurement on the basis of milligram
per kilogram of body-weight (Carpenter et al., 1950).
Dog. Four male and four female dogs fed allethrin at a rate of
50 mg/kg/day for two years showed no gross or microscopical effects.
At higher dosage levels in other groups, there were convulsions,
progressively shortened survival, and non-specific pathological
changes (Lehman, 1965).
Man. There are apparently no reports of allergy to allethrin
comparable to those associated with pyrethrum and pyrethrins. However,
since the types of exposure have not been the same the possibility of
allergy to allethrin cannot be excluded.
Comments on the experimental studies reported and evaluation
Rats apparently tolerate prolonged feeding at a rate of 125
mg/kg/day but the exact duration of the test was not stated.
A dosage of 50 mg/kg/day for 2 years produced no detectable
effect in dogs.
Because no study of the liver seems to have been made in rats and
because no study has been made in any species for more than half its
life-span, it is not possible to estimate an acceptable daily intake
for man.
Further work required
Long-term studies in at least two species are required in which
particular attention is paid to possible effects on the liver. The
metabolism of the substance should be explored.
REFERENCES
Ambrose, A. M. & Robbins, D. J. (1951) Fed. Proc., 10, 276
Carpenter, C. P., Weil, C. S., Pozzani, U. C. & Smyth, J. F., jr
(1950) Arch. industr. Hyg., 2, 420
Lehman, A. J. (1952) Quart. Bull. Assoc. Food and Drug Officials
U.S., 16, 47
Lehman, A. J. (1965) Summaries of pesticide toxicity (In press)
McClellan, D. B. & Moore, J. B. (1958) J. Agr. Food Chem., 6, 463
Negherbon, W. (1959) Handbook of Toxicology, vol. 3, Saunders,
Philadelphia
