
FAO Meeting Report No. PL/1965/10/1
WHO/Food Add./27.65
EVALUATION OF THE TOXICITY OF PESTICIDE RESIDUES IN FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Committee on Pesticides in Agriculture and
the WHO Expert Committee on Pesticide Residues, which met in Rome,
15-22 March 19651
Food and Agriculture Organization of the United Nations
World Health Organization
1965
1 Report of the second joint meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Committee on Pesticide
Residues, FAO Meeting Report No. PL/1965/10; WHO/Food Add./26.65
CAPTAN
Chemical names
N-(trichloromethylthio)cyclohex-4-ene-1,2 dicarboxyimide;
N-trichloromethylthio-4-cyclohexene-1,2-dicarboximide;
N-(trichloromethylthio)-3a,4,7,7a-tetra-hydrophthalimide.
Empirical formula
C9H8O2NCl3S
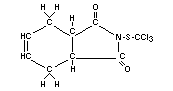
Structural formula
 BIOLOGICAL DATA
Biochemical aspects
In yeast, it seems that the active principle of captan is the
thiophosgene formed from the trichloromethylthio group. Thiophosgene
will probably combine with active groups as - OH, -SH2 and NH2
within the cell (Lukens & Sisler, 1958). In a fungus, captan gave a
marked depression of the activity of the enzyme glutamine
dehydrogenase and a stimulation of diphosphopyridine nucleotide
oxydase (Byrde et al., 1956).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Oral 9 000-15 000 Link et al., 1956
(given as Spector, 1956
wettable powder)
Rat Intraperitoneal 50-100 Link et al., 1956
Short-term studies
Dog. Groups of 4 dogs, 2 males and 2 females, were fed 400,
4000 and 12 000 ppm of technical captan for 66 weeks. The animals on
the highest level, 12 000 ppm, showed slightly enlarged kidneys and
livers. No histological changes occurred at any dose level (Fitzhugh,
1963).
Pig. Seven pigs fed 480 ppm captan in their diet for 14 weeks
showed good weight gains. Haematological examination did not show any
abnormalities. Gross and histopathological study of the liver and
kidney revealed no abnormalities. No residual captan (i.e., <0.1 ppm)
was found in the tissues (Link et al., 1956).
Pigs fed 4000 ppm of captan in the diet for 25 weeks showed no
symptoms, but no further observations were made (Johnson, 1954).
Chick. Chicks received 320 ppm captan in their food for 74
days. No abnormalities were found (Link et al., 1956).
Long-term studies
Rat. Groups each of 20 rats, 10 males and 10 females, were fed
1000 and 5000 ppm of captan in their diet for 2 years. Another group
of 20 rats received 10 000 ppm of captan partly technical and partly
recrystallized for one year. In the group receiving 1000 ppm in the
diet a reduction in weight occurred for the last 16 weeks of the
experiment. Female rats receiving 5000 ppm and both sexes on diets
containing 10 000 ppm of captan showed growth depression and higher
mortality. At autopsy atrophied testes were found in the animals on
the highest dose level. Organ weights, blood picture, tumour frequency
and histological studies were not significantly different from those
in controls (Weir, 1956).
In another experiment 30 rats of each sex were fed 1000 ppm of
technical captan for 17 months and compared to two untreated control
groups. Body-weight gains, food consumption, survival rate and tumour
incidence were comparable in treated and untreated animals (Industrial
Bio-Test Laboratories, Inc., 1961).
Comments on the experimental studies reports
Long-term experiments in rats and short-term experiments in rats,
dogs and pigs have been carried out. There is no information about the
metabolism of the compound.
Level causing no significant effect in animals
Rat: 1000 ppm equivalent with 50 mg/kg body-weight per day
Dog: 4000 ppm " " 100 mg/kg " " " "
Pig: 480 ppm " " 19 mg/kg " " " "
Estimate of acceptable daily intake for man
0-0.1 mg/kg body-weight per day.
Further work desirable
Determination and evaluation of toxicity of the residues
occurring in the plant. Studies on the metabolism in animals.
Extension of the long-term studies in rats and long-term studies in
other species at dosages designed to find maximum no-effect levels.
REFERENCES
Byrde, R. J. W. et al. (1956) Nature, 178, 638
Fitzhugh, O. G. (1963) Personal communication
Industrial Bio-Test Laboratories, Inc. (1961) Unpublished report
submitted to California Chemical Company
Johnson, D. F. (1954) Southwestern Veterinarian, 8 (1), 30
Link, R. P., Smith, J. C. & Morrill, C. C. (1956) J. Amer. vet. med.
Ass., 128, 614
Lukens, R. J. & Sisler, H. D. (1958) Phytopathology, 48, 235
Spector, W. S., ed. (1956) Handbook of Toxicology, Philadelphia &
London, Saunders, vol. I
Weir, R. J. (1956) Unpublished report of Hazleton Laboratories
BIOLOGICAL DATA
Biochemical aspects
In yeast, it seems that the active principle of captan is the
thiophosgene formed from the trichloromethylthio group. Thiophosgene
will probably combine with active groups as - OH, -SH2 and NH2
within the cell (Lukens & Sisler, 1958). In a fungus, captan gave a
marked depression of the activity of the enzyme glutamine
dehydrogenase and a stimulation of diphosphopyridine nucleotide
oxydase (Byrde et al., 1956).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Oral 9 000-15 000 Link et al., 1956
(given as Spector, 1956
wettable powder)
Rat Intraperitoneal 50-100 Link et al., 1956
Short-term studies
Dog. Groups of 4 dogs, 2 males and 2 females, were fed 400,
4000 and 12 000 ppm of technical captan for 66 weeks. The animals on
the highest level, 12 000 ppm, showed slightly enlarged kidneys and
livers. No histological changes occurred at any dose level (Fitzhugh,
1963).
Pig. Seven pigs fed 480 ppm captan in their diet for 14 weeks
showed good weight gains. Haematological examination did not show any
abnormalities. Gross and histopathological study of the liver and
kidney revealed no abnormalities. No residual captan (i.e., <0.1 ppm)
was found in the tissues (Link et al., 1956).
Pigs fed 4000 ppm of captan in the diet for 25 weeks showed no
symptoms, but no further observations were made (Johnson, 1954).
Chick. Chicks received 320 ppm captan in their food for 74
days. No abnormalities were found (Link et al., 1956).
Long-term studies
Rat. Groups each of 20 rats, 10 males and 10 females, were fed
1000 and 5000 ppm of captan in their diet for 2 years. Another group
of 20 rats received 10 000 ppm of captan partly technical and partly
recrystallized for one year. In the group receiving 1000 ppm in the
diet a reduction in weight occurred for the last 16 weeks of the
experiment. Female rats receiving 5000 ppm and both sexes on diets
containing 10 000 ppm of captan showed growth depression and higher
mortality. At autopsy atrophied testes were found in the animals on
the highest dose level. Organ weights, blood picture, tumour frequency
and histological studies were not significantly different from those
in controls (Weir, 1956).
In another experiment 30 rats of each sex were fed 1000 ppm of
technical captan for 17 months and compared to two untreated control
groups. Body-weight gains, food consumption, survival rate and tumour
incidence were comparable in treated and untreated animals (Industrial
Bio-Test Laboratories, Inc., 1961).
Comments on the experimental studies reports
Long-term experiments in rats and short-term experiments in rats,
dogs and pigs have been carried out. There is no information about the
metabolism of the compound.
Level causing no significant effect in animals
Rat: 1000 ppm equivalent with 50 mg/kg body-weight per day
Dog: 4000 ppm " " 100 mg/kg " " " "
Pig: 480 ppm " " 19 mg/kg " " " "
Estimate of acceptable daily intake for man
0-0.1 mg/kg body-weight per day.
Further work desirable
Determination and evaluation of toxicity of the residues
occurring in the plant. Studies on the metabolism in animals.
Extension of the long-term studies in rats and long-term studies in
other species at dosages designed to find maximum no-effect levels.
REFERENCES
Byrde, R. J. W. et al. (1956) Nature, 178, 638
Fitzhugh, O. G. (1963) Personal communication
Industrial Bio-Test Laboratories, Inc. (1961) Unpublished report
submitted to California Chemical Company
Johnson, D. F. (1954) Southwestern Veterinarian, 8 (1), 30
Link, R. P., Smith, J. C. & Morrill, C. C. (1956) J. Amer. vet. med.
Ass., 128, 614
Lukens, R. J. & Sisler, H. D. (1958) Phytopathology, 48, 235
Spector, W. S., ed. (1956) Handbook of Toxicology, Philadelphia &
London, Saunders, vol. I
Weir, R. J. (1956) Unpublished report of Hazleton Laboratories
 BIOLOGICAL DATA
Biochemical aspects
In yeast, it seems that the active principle of captan is the
thiophosgene formed from the trichloromethylthio group. Thiophosgene
will probably combine with active groups as - OH, -SH2 and NH2
within the cell (Lukens & Sisler, 1958). In a fungus, captan gave a
marked depression of the activity of the enzyme glutamine
dehydrogenase and a stimulation of diphosphopyridine nucleotide
oxydase (Byrde et al., 1956).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Oral 9 000-15 000 Link et al., 1956
(given as Spector, 1956
wettable powder)
Rat Intraperitoneal 50-100 Link et al., 1956
Short-term studies
Dog. Groups of 4 dogs, 2 males and 2 females, were fed 400,
4000 and 12 000 ppm of technical captan for 66 weeks. The animals on
the highest level, 12 000 ppm, showed slightly enlarged kidneys and
livers. No histological changes occurred at any dose level (Fitzhugh,
1963).
Pig. Seven pigs fed 480 ppm captan in their diet for 14 weeks
showed good weight gains. Haematological examination did not show any
abnormalities. Gross and histopathological study of the liver and
kidney revealed no abnormalities. No residual captan (i.e., <0.1 ppm)
was found in the tissues (Link et al., 1956).
Pigs fed 4000 ppm of captan in the diet for 25 weeks showed no
symptoms, but no further observations were made (Johnson, 1954).
Chick. Chicks received 320 ppm captan in their food for 74
days. No abnormalities were found (Link et al., 1956).
Long-term studies
Rat. Groups each of 20 rats, 10 males and 10 females, were fed
1000 and 5000 ppm of captan in their diet for 2 years. Another group
of 20 rats received 10 000 ppm of captan partly technical and partly
recrystallized for one year. In the group receiving 1000 ppm in the
diet a reduction in weight occurred for the last 16 weeks of the
experiment. Female rats receiving 5000 ppm and both sexes on diets
containing 10 000 ppm of captan showed growth depression and higher
mortality. At autopsy atrophied testes were found in the animals on
the highest dose level. Organ weights, blood picture, tumour frequency
and histological studies were not significantly different from those
in controls (Weir, 1956).
In another experiment 30 rats of each sex were fed 1000 ppm of
technical captan for 17 months and compared to two untreated control
groups. Body-weight gains, food consumption, survival rate and tumour
incidence were comparable in treated and untreated animals (Industrial
Bio-Test Laboratories, Inc., 1961).
Comments on the experimental studies reports
Long-term experiments in rats and short-term experiments in rats,
dogs and pigs have been carried out. There is no information about the
metabolism of the compound.
Level causing no significant effect in animals
Rat: 1000 ppm equivalent with 50 mg/kg body-weight per day
Dog: 4000 ppm " " 100 mg/kg " " " "
Pig: 480 ppm " " 19 mg/kg " " " "
Estimate of acceptable daily intake for man
0-0.1 mg/kg body-weight per day.
Further work desirable
Determination and evaluation of toxicity of the residues
occurring in the plant. Studies on the metabolism in animals.
Extension of the long-term studies in rats and long-term studies in
other species at dosages designed to find maximum no-effect levels.
REFERENCES
Byrde, R. J. W. et al. (1956) Nature, 178, 638
Fitzhugh, O. G. (1963) Personal communication
Industrial Bio-Test Laboratories, Inc. (1961) Unpublished report
submitted to California Chemical Company
Johnson, D. F. (1954) Southwestern Veterinarian, 8 (1), 30
Link, R. P., Smith, J. C. & Morrill, C. C. (1956) J. Amer. vet. med.
Ass., 128, 614
Lukens, R. J. & Sisler, H. D. (1958) Phytopathology, 48, 235
Spector, W. S., ed. (1956) Handbook of Toxicology, Philadelphia &
London, Saunders, vol. I
Weir, R. J. (1956) Unpublished report of Hazleton Laboratories
BIOLOGICAL DATA
Biochemical aspects
In yeast, it seems that the active principle of captan is the
thiophosgene formed from the trichloromethylthio group. Thiophosgene
will probably combine with active groups as - OH, -SH2 and NH2
within the cell (Lukens & Sisler, 1958). In a fungus, captan gave a
marked depression of the activity of the enzyme glutamine
dehydrogenase and a stimulation of diphosphopyridine nucleotide
oxydase (Byrde et al., 1956).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Oral 9 000-15 000 Link et al., 1956
(given as Spector, 1956
wettable powder)
Rat Intraperitoneal 50-100 Link et al., 1956
Short-term studies
Dog. Groups of 4 dogs, 2 males and 2 females, were fed 400,
4000 and 12 000 ppm of technical captan for 66 weeks. The animals on
the highest level, 12 000 ppm, showed slightly enlarged kidneys and
livers. No histological changes occurred at any dose level (Fitzhugh,
1963).
Pig. Seven pigs fed 480 ppm captan in their diet for 14 weeks
showed good weight gains. Haematological examination did not show any
abnormalities. Gross and histopathological study of the liver and
kidney revealed no abnormalities. No residual captan (i.e., <0.1 ppm)
was found in the tissues (Link et al., 1956).
Pigs fed 4000 ppm of captan in the diet for 25 weeks showed no
symptoms, but no further observations were made (Johnson, 1954).
Chick. Chicks received 320 ppm captan in their food for 74
days. No abnormalities were found (Link et al., 1956).
Long-term studies
Rat. Groups each of 20 rats, 10 males and 10 females, were fed
1000 and 5000 ppm of captan in their diet for 2 years. Another group
of 20 rats received 10 000 ppm of captan partly technical and partly
recrystallized for one year. In the group receiving 1000 ppm in the
diet a reduction in weight occurred for the last 16 weeks of the
experiment. Female rats receiving 5000 ppm and both sexes on diets
containing 10 000 ppm of captan showed growth depression and higher
mortality. At autopsy atrophied testes were found in the animals on
the highest dose level. Organ weights, blood picture, tumour frequency
and histological studies were not significantly different from those
in controls (Weir, 1956).
In another experiment 30 rats of each sex were fed 1000 ppm of
technical captan for 17 months and compared to two untreated control
groups. Body-weight gains, food consumption, survival rate and tumour
incidence were comparable in treated and untreated animals (Industrial
Bio-Test Laboratories, Inc., 1961).
Comments on the experimental studies reports
Long-term experiments in rats and short-term experiments in rats,
dogs and pigs have been carried out. There is no information about the
metabolism of the compound.
Level causing no significant effect in animals
Rat: 1000 ppm equivalent with 50 mg/kg body-weight per day
Dog: 4000 ppm " " 100 mg/kg " " " "
Pig: 480 ppm " " 19 mg/kg " " " "
Estimate of acceptable daily intake for man
0-0.1 mg/kg body-weight per day.
Further work desirable
Determination and evaluation of toxicity of the residues
occurring in the plant. Studies on the metabolism in animals.
Extension of the long-term studies in rats and long-term studies in
other species at dosages designed to find maximum no-effect levels.
REFERENCES
Byrde, R. J. W. et al. (1956) Nature, 178, 638
Fitzhugh, O. G. (1963) Personal communication
Industrial Bio-Test Laboratories, Inc. (1961) Unpublished report
submitted to California Chemical Company
Johnson, D. F. (1954) Southwestern Veterinarian, 8 (1), 30
Link, R. P., Smith, J. C. & Morrill, C. C. (1956) J. Amer. vet. med.
Ass., 128, 614
Lukens, R. J. & Sisler, H. D. (1958) Phytopathology, 48, 235
Spector, W. S., ed. (1956) Handbook of Toxicology, Philadelphia &
London, Saunders, vol. I
Weir, R. J. (1956) Unpublished report of Hazleton Laboratories
