
FAO Meeting Report No. PL/1965/10/1
WHO/Food Add./27.65
EVALUATION OF THE TOXICITY OF PESTICIDE RESIDUES IN FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Committee on Pesticides in Agriculture and
the WHO Expert Committee on Pesticide Residues, which met in Rome,
15-22 March 19651
Food and Agriculture Organization of the United Nations
World Health Organization
1965
1 Report of the second joint meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Committee on Pesticide
Residues, FAO Meeting Report No. PL/1965/10; WHO/Food Add./26.65
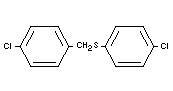
CHLORBENSIDE
Chemical name
4-chlorobenzyl 4-chlorophenyl sulfide.
Synonyms
Chlorparaside; chlorsulfacide.
Empirical formula
C13H10Cl2S
Structural formula
 BIOLOGICAL DATA
Biochemical aspects
After 13 weeks of feeding at a 10-ppm dose level there was no
appreciable chlorbenside in any tissue of the rats except fat and
possibly liver. At 100 ppm there were measurable concentrations in
liver, kidney and fat but not in the blood or brain; whilst at 1000
ppm there were measurable concentrations in all organs, although the
amount in the blood was barely detectable. The greatest amount of
chlorbenside found in any one animal was 184 µg in a whole rat which
had received the highest dose level of 1000 ppm. This was about one
fiftieth of the daily intake. The total body load fell to about one
sixth within 2 weeks of stopping treatment (Boots, 1957).
Rabbits were given chlorbenside orally in a dose of about 300
mg/kg body-weight and the excretion of sulfide (chlorbenside),
sulfoxide and sulfone in urine and faeces was followed. 97.4% of the
dose was recovered unchanged from the faeces, and traces of sulfoxide
were present in the urine (Boots, 1957).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse Oral > 3 000 pure sulfide Boots, 1957
> 3 000 pure sulfoxide
> 3 000 pure sulfone
>10 000 technical sulfide Boots, 1957
>10 000 technical sulfone
Rat Oral >10 000 technical sulfide Boots, 1957
Short-term studies
Mouse. Each of 3 pure samples (sulfide, sulfoxide, sulfone) was
given daily by stomach tube to a group of 10 male mice at a dose of
1000 mg/kg body-weight 6 times a week for 14 days (12 doses total):
enlargement of the liver was observed and a few deaths occurred
(Boots, 1957).
Rat. Daily oral doses of 50 mg/kg body-weight of technical
sulfide or sulfone were given to rats for a period of 3 weeks; no
toxic effects resulted. Daily doses of 250 mg/kg body-weight for the
same period caused enlargement of the liver without any histological
evidence of liver damage. There was no other toxic effect (Boots,
1957).
Several small groups of rats, each consisting of 2 males and 2
females, were given daily oral doses of chlorbenside in aqueous acacia
suspensions for 3 to 4 weeks at dose levels up to 10 000 mg/kg
body-weight. Doses were administered by stomach-tube. Doses of 5000
mg/kg body-weight and over were sub-divided in 2 half-doses daily. The
only systemic effect of the 10 000 mg/kg body-weight daily dose was
the liver enlargement. There were mild cellular changes in the liver,
consisting of vacuolation of the lobular cells. The increase in
liver-weight occurred at all doses, and was graded according to dose.
The reduction of growth occurred only on doses of 5000 mg/kg
body-weight and above, and the vacuolation of liver cells was marked
at the highest dose level of 10 000 mg/kg body-weight daily (Boots,
1957).
Additional experiments in rats showed that the major part of the
liver hypertrophy occurs in the first few weeks of feeding
chlorbenside and continued feeding after this point has little further
effect.
The liver hypertrophy is reversible, and the liver-weight returns
nearly to normal within two weeks of stopping treatment (Boots, 1957).
Breeding rats (4 groups and 2 control groups each of 6 breeding
pairs) were maintained throughout 3 generations on diets containing
100 ppm or 20 ppm of technical sulfide, 20 ppm of pure sulfoxide or 20
ppm of technical sulfone. The treatment had no effect on fertility
through 3 generations (Boots, 1957).
Rabbit. Daily oral doses of 250 mg/kg body-weight of technical
sulfide or sulfone given to rabbits for a period of 4 weeks had no
toxic effects (Boots, 1957).
Long-term studies
Rat. Groups of 10 albino rats, 5 of each sex, were maintained
on diets containing 20, 100 and 1000 ppm of technical sulfide, and the
same 3 concentrations of technical sulfone. Three similar groups were
maintained on the basic diet as controls. They were placed on the
experimental diets when the rats were 5-6 weeks of age. With the
exception of 3 animals of each sex which were killed after 6 months or
after 1 year for pathological examination, the remaining 4 animals in
each group were maintained on their respective diets for 2 years, and
the survivors were killed at the end of this time. The main organs
were taken for histological examination. No deaths occurred which
could be attributed to the effects of treatment, and there was no
effect on the length of life-span, weight gain and blood picture. The
livers were enlarged in both sexes fed 1000 ppm of either compound and
in females fed 100 ppm. There was no significant enlargement in males
fed 100 ppm or in either sex fed 20 ppm. There was no microscopic
abnormality of the liver. The kidneys of males fed 1000 ppm of either
compound showed a slight increase in weight. There was no effect on
the kidneys of males on the two lower doses, nor in any of the
females. There were no other gross or microscopic pathological changes
that could be attributed to treatment (Boots, 1957).
Dog. Eight mongrel females approximately one to one-and-a-half
years old were maintained for one year on daily oral doses of 60 mg of
technical sulfide, pure sulfoxide or technical sulfone. This is
equivalent to approximately 5 mg/kg body-weight daily. There were no
toxic effects on growth, appetite, general health, blood picture,
liver function or kidney function and no gross or microscopic
pathological changes were found (Boots, 1957).
Comments on experimental studies reported
Long-term studies of two years' duration in rats and one year in
dogs were carried out. Other studies in mice, rats and rabbits of
shorter duration were reported. Tissue residue and excretion studies
showed that the pesticide is not cumulative.
EVALUATION
Level causing no significant toxicological effect in the rat
Twenty ppm in the diet of the rat is equivalent to 1 mg/kg
body-weight; in the dog 5 mg/kg body-weight.
Estimate of acceptable daily intakes for man
0-0.01 mg/kg body-weight.
Further work desirable
Additional long-term studies in the rat and the dog.
REFERENCE
Boots Pure Drug Co. Ltd., Nottingham, England (1957) Unpublished
Report
BIOLOGICAL DATA
Biochemical aspects
After 13 weeks of feeding at a 10-ppm dose level there was no
appreciable chlorbenside in any tissue of the rats except fat and
possibly liver. At 100 ppm there were measurable concentrations in
liver, kidney and fat but not in the blood or brain; whilst at 1000
ppm there were measurable concentrations in all organs, although the
amount in the blood was barely detectable. The greatest amount of
chlorbenside found in any one animal was 184 µg in a whole rat which
had received the highest dose level of 1000 ppm. This was about one
fiftieth of the daily intake. The total body load fell to about one
sixth within 2 weeks of stopping treatment (Boots, 1957).
Rabbits were given chlorbenside orally in a dose of about 300
mg/kg body-weight and the excretion of sulfide (chlorbenside),
sulfoxide and sulfone in urine and faeces was followed. 97.4% of the
dose was recovered unchanged from the faeces, and traces of sulfoxide
were present in the urine (Boots, 1957).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse Oral > 3 000 pure sulfide Boots, 1957
> 3 000 pure sulfoxide
> 3 000 pure sulfone
>10 000 technical sulfide Boots, 1957
>10 000 technical sulfone
Rat Oral >10 000 technical sulfide Boots, 1957
Short-term studies
Mouse. Each of 3 pure samples (sulfide, sulfoxide, sulfone) was
given daily by stomach tube to a group of 10 male mice at a dose of
1000 mg/kg body-weight 6 times a week for 14 days (12 doses total):
enlargement of the liver was observed and a few deaths occurred
(Boots, 1957).
Rat. Daily oral doses of 50 mg/kg body-weight of technical
sulfide or sulfone were given to rats for a period of 3 weeks; no
toxic effects resulted. Daily doses of 250 mg/kg body-weight for the
same period caused enlargement of the liver without any histological
evidence of liver damage. There was no other toxic effect (Boots,
1957).
Several small groups of rats, each consisting of 2 males and 2
females, were given daily oral doses of chlorbenside in aqueous acacia
suspensions for 3 to 4 weeks at dose levels up to 10 000 mg/kg
body-weight. Doses were administered by stomach-tube. Doses of 5000
mg/kg body-weight and over were sub-divided in 2 half-doses daily. The
only systemic effect of the 10 000 mg/kg body-weight daily dose was
the liver enlargement. There were mild cellular changes in the liver,
consisting of vacuolation of the lobular cells. The increase in
liver-weight occurred at all doses, and was graded according to dose.
The reduction of growth occurred only on doses of 5000 mg/kg
body-weight and above, and the vacuolation of liver cells was marked
at the highest dose level of 10 000 mg/kg body-weight daily (Boots,
1957).
Additional experiments in rats showed that the major part of the
liver hypertrophy occurs in the first few weeks of feeding
chlorbenside and continued feeding after this point has little further
effect.
The liver hypertrophy is reversible, and the liver-weight returns
nearly to normal within two weeks of stopping treatment (Boots, 1957).
Breeding rats (4 groups and 2 control groups each of 6 breeding
pairs) were maintained throughout 3 generations on diets containing
100 ppm or 20 ppm of technical sulfide, 20 ppm of pure sulfoxide or 20
ppm of technical sulfone. The treatment had no effect on fertility
through 3 generations (Boots, 1957).
Rabbit. Daily oral doses of 250 mg/kg body-weight of technical
sulfide or sulfone given to rabbits for a period of 4 weeks had no
toxic effects (Boots, 1957).
Long-term studies
Rat. Groups of 10 albino rats, 5 of each sex, were maintained
on diets containing 20, 100 and 1000 ppm of technical sulfide, and the
same 3 concentrations of technical sulfone. Three similar groups were
maintained on the basic diet as controls. They were placed on the
experimental diets when the rats were 5-6 weeks of age. With the
exception of 3 animals of each sex which were killed after 6 months or
after 1 year for pathological examination, the remaining 4 animals in
each group were maintained on their respective diets for 2 years, and
the survivors were killed at the end of this time. The main organs
were taken for histological examination. No deaths occurred which
could be attributed to the effects of treatment, and there was no
effect on the length of life-span, weight gain and blood picture. The
livers were enlarged in both sexes fed 1000 ppm of either compound and
in females fed 100 ppm. There was no significant enlargement in males
fed 100 ppm or in either sex fed 20 ppm. There was no microscopic
abnormality of the liver. The kidneys of males fed 1000 ppm of either
compound showed a slight increase in weight. There was no effect on
the kidneys of males on the two lower doses, nor in any of the
females. There were no other gross or microscopic pathological changes
that could be attributed to treatment (Boots, 1957).
Dog. Eight mongrel females approximately one to one-and-a-half
years old were maintained for one year on daily oral doses of 60 mg of
technical sulfide, pure sulfoxide or technical sulfone. This is
equivalent to approximately 5 mg/kg body-weight daily. There were no
toxic effects on growth, appetite, general health, blood picture,
liver function or kidney function and no gross or microscopic
pathological changes were found (Boots, 1957).
Comments on experimental studies reported
Long-term studies of two years' duration in rats and one year in
dogs were carried out. Other studies in mice, rats and rabbits of
shorter duration were reported. Tissue residue and excretion studies
showed that the pesticide is not cumulative.
EVALUATION
Level causing no significant toxicological effect in the rat
Twenty ppm in the diet of the rat is equivalent to 1 mg/kg
body-weight; in the dog 5 mg/kg body-weight.
Estimate of acceptable daily intakes for man
0-0.01 mg/kg body-weight.
Further work desirable
Additional long-term studies in the rat and the dog.
REFERENCE
Boots Pure Drug Co. Ltd., Nottingham, England (1957) Unpublished
Report
 BIOLOGICAL DATA
Biochemical aspects
After 13 weeks of feeding at a 10-ppm dose level there was no
appreciable chlorbenside in any tissue of the rats except fat and
possibly liver. At 100 ppm there were measurable concentrations in
liver, kidney and fat but not in the blood or brain; whilst at 1000
ppm there were measurable concentrations in all organs, although the
amount in the blood was barely detectable. The greatest amount of
chlorbenside found in any one animal was 184 µg in a whole rat which
had received the highest dose level of 1000 ppm. This was about one
fiftieth of the daily intake. The total body load fell to about one
sixth within 2 weeks of stopping treatment (Boots, 1957).
Rabbits were given chlorbenside orally in a dose of about 300
mg/kg body-weight and the excretion of sulfide (chlorbenside),
sulfoxide and sulfone in urine and faeces was followed. 97.4% of the
dose was recovered unchanged from the faeces, and traces of sulfoxide
were present in the urine (Boots, 1957).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse Oral > 3 000 pure sulfide Boots, 1957
> 3 000 pure sulfoxide
> 3 000 pure sulfone
>10 000 technical sulfide Boots, 1957
>10 000 technical sulfone
Rat Oral >10 000 technical sulfide Boots, 1957
Short-term studies
Mouse. Each of 3 pure samples (sulfide, sulfoxide, sulfone) was
given daily by stomach tube to a group of 10 male mice at a dose of
1000 mg/kg body-weight 6 times a week for 14 days (12 doses total):
enlargement of the liver was observed and a few deaths occurred
(Boots, 1957).
Rat. Daily oral doses of 50 mg/kg body-weight of technical
sulfide or sulfone were given to rats for a period of 3 weeks; no
toxic effects resulted. Daily doses of 250 mg/kg body-weight for the
same period caused enlargement of the liver without any histological
evidence of liver damage. There was no other toxic effect (Boots,
1957).
Several small groups of rats, each consisting of 2 males and 2
females, were given daily oral doses of chlorbenside in aqueous acacia
suspensions for 3 to 4 weeks at dose levels up to 10 000 mg/kg
body-weight. Doses were administered by stomach-tube. Doses of 5000
mg/kg body-weight and over were sub-divided in 2 half-doses daily. The
only systemic effect of the 10 000 mg/kg body-weight daily dose was
the liver enlargement. There were mild cellular changes in the liver,
consisting of vacuolation of the lobular cells. The increase in
liver-weight occurred at all doses, and was graded according to dose.
The reduction of growth occurred only on doses of 5000 mg/kg
body-weight and above, and the vacuolation of liver cells was marked
at the highest dose level of 10 000 mg/kg body-weight daily (Boots,
1957).
Additional experiments in rats showed that the major part of the
liver hypertrophy occurs in the first few weeks of feeding
chlorbenside and continued feeding after this point has little further
effect.
The liver hypertrophy is reversible, and the liver-weight returns
nearly to normal within two weeks of stopping treatment (Boots, 1957).
Breeding rats (4 groups and 2 control groups each of 6 breeding
pairs) were maintained throughout 3 generations on diets containing
100 ppm or 20 ppm of technical sulfide, 20 ppm of pure sulfoxide or 20
ppm of technical sulfone. The treatment had no effect on fertility
through 3 generations (Boots, 1957).
Rabbit. Daily oral doses of 250 mg/kg body-weight of technical
sulfide or sulfone given to rabbits for a period of 4 weeks had no
toxic effects (Boots, 1957).
Long-term studies
Rat. Groups of 10 albino rats, 5 of each sex, were maintained
on diets containing 20, 100 and 1000 ppm of technical sulfide, and the
same 3 concentrations of technical sulfone. Three similar groups were
maintained on the basic diet as controls. They were placed on the
experimental diets when the rats were 5-6 weeks of age. With the
exception of 3 animals of each sex which were killed after 6 months or
after 1 year for pathological examination, the remaining 4 animals in
each group were maintained on their respective diets for 2 years, and
the survivors were killed at the end of this time. The main organs
were taken for histological examination. No deaths occurred which
could be attributed to the effects of treatment, and there was no
effect on the length of life-span, weight gain and blood picture. The
livers were enlarged in both sexes fed 1000 ppm of either compound and
in females fed 100 ppm. There was no significant enlargement in males
fed 100 ppm or in either sex fed 20 ppm. There was no microscopic
abnormality of the liver. The kidneys of males fed 1000 ppm of either
compound showed a slight increase in weight. There was no effect on
the kidneys of males on the two lower doses, nor in any of the
females. There were no other gross or microscopic pathological changes
that could be attributed to treatment (Boots, 1957).
Dog. Eight mongrel females approximately one to one-and-a-half
years old were maintained for one year on daily oral doses of 60 mg of
technical sulfide, pure sulfoxide or technical sulfone. This is
equivalent to approximately 5 mg/kg body-weight daily. There were no
toxic effects on growth, appetite, general health, blood picture,
liver function or kidney function and no gross or microscopic
pathological changes were found (Boots, 1957).
Comments on experimental studies reported
Long-term studies of two years' duration in rats and one year in
dogs were carried out. Other studies in mice, rats and rabbits of
shorter duration were reported. Tissue residue and excretion studies
showed that the pesticide is not cumulative.
EVALUATION
Level causing no significant toxicological effect in the rat
Twenty ppm in the diet of the rat is equivalent to 1 mg/kg
body-weight; in the dog 5 mg/kg body-weight.
Estimate of acceptable daily intakes for man
0-0.01 mg/kg body-weight.
Further work desirable
Additional long-term studies in the rat and the dog.
REFERENCE
Boots Pure Drug Co. Ltd., Nottingham, England (1957) Unpublished
Report
BIOLOGICAL DATA
Biochemical aspects
After 13 weeks of feeding at a 10-ppm dose level there was no
appreciable chlorbenside in any tissue of the rats except fat and
possibly liver. At 100 ppm there were measurable concentrations in
liver, kidney and fat but not in the blood or brain; whilst at 1000
ppm there were measurable concentrations in all organs, although the
amount in the blood was barely detectable. The greatest amount of
chlorbenside found in any one animal was 184 µg in a whole rat which
had received the highest dose level of 1000 ppm. This was about one
fiftieth of the daily intake. The total body load fell to about one
sixth within 2 weeks of stopping treatment (Boots, 1957).
Rabbits were given chlorbenside orally in a dose of about 300
mg/kg body-weight and the excretion of sulfide (chlorbenside),
sulfoxide and sulfone in urine and faeces was followed. 97.4% of the
dose was recovered unchanged from the faeces, and traces of sulfoxide
were present in the urine (Boots, 1957).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse Oral > 3 000 pure sulfide Boots, 1957
> 3 000 pure sulfoxide
> 3 000 pure sulfone
>10 000 technical sulfide Boots, 1957
>10 000 technical sulfone
Rat Oral >10 000 technical sulfide Boots, 1957
Short-term studies
Mouse. Each of 3 pure samples (sulfide, sulfoxide, sulfone) was
given daily by stomach tube to a group of 10 male mice at a dose of
1000 mg/kg body-weight 6 times a week for 14 days (12 doses total):
enlargement of the liver was observed and a few deaths occurred
(Boots, 1957).
Rat. Daily oral doses of 50 mg/kg body-weight of technical
sulfide or sulfone were given to rats for a period of 3 weeks; no
toxic effects resulted. Daily doses of 250 mg/kg body-weight for the
same period caused enlargement of the liver without any histological
evidence of liver damage. There was no other toxic effect (Boots,
1957).
Several small groups of rats, each consisting of 2 males and 2
females, were given daily oral doses of chlorbenside in aqueous acacia
suspensions for 3 to 4 weeks at dose levels up to 10 000 mg/kg
body-weight. Doses were administered by stomach-tube. Doses of 5000
mg/kg body-weight and over were sub-divided in 2 half-doses daily. The
only systemic effect of the 10 000 mg/kg body-weight daily dose was
the liver enlargement. There were mild cellular changes in the liver,
consisting of vacuolation of the lobular cells. The increase in
liver-weight occurred at all doses, and was graded according to dose.
The reduction of growth occurred only on doses of 5000 mg/kg
body-weight and above, and the vacuolation of liver cells was marked
at the highest dose level of 10 000 mg/kg body-weight daily (Boots,
1957).
Additional experiments in rats showed that the major part of the
liver hypertrophy occurs in the first few weeks of feeding
chlorbenside and continued feeding after this point has little further
effect.
The liver hypertrophy is reversible, and the liver-weight returns
nearly to normal within two weeks of stopping treatment (Boots, 1957).
Breeding rats (4 groups and 2 control groups each of 6 breeding
pairs) were maintained throughout 3 generations on diets containing
100 ppm or 20 ppm of technical sulfide, 20 ppm of pure sulfoxide or 20
ppm of technical sulfone. The treatment had no effect on fertility
through 3 generations (Boots, 1957).
Rabbit. Daily oral doses of 250 mg/kg body-weight of technical
sulfide or sulfone given to rabbits for a period of 4 weeks had no
toxic effects (Boots, 1957).
Long-term studies
Rat. Groups of 10 albino rats, 5 of each sex, were maintained
on diets containing 20, 100 and 1000 ppm of technical sulfide, and the
same 3 concentrations of technical sulfone. Three similar groups were
maintained on the basic diet as controls. They were placed on the
experimental diets when the rats were 5-6 weeks of age. With the
exception of 3 animals of each sex which were killed after 6 months or
after 1 year for pathological examination, the remaining 4 animals in
each group were maintained on their respective diets for 2 years, and
the survivors were killed at the end of this time. The main organs
were taken for histological examination. No deaths occurred which
could be attributed to the effects of treatment, and there was no
effect on the length of life-span, weight gain and blood picture. The
livers were enlarged in both sexes fed 1000 ppm of either compound and
in females fed 100 ppm. There was no significant enlargement in males
fed 100 ppm or in either sex fed 20 ppm. There was no microscopic
abnormality of the liver. The kidneys of males fed 1000 ppm of either
compound showed a slight increase in weight. There was no effect on
the kidneys of males on the two lower doses, nor in any of the
females. There were no other gross or microscopic pathological changes
that could be attributed to treatment (Boots, 1957).
Dog. Eight mongrel females approximately one to one-and-a-half
years old were maintained for one year on daily oral doses of 60 mg of
technical sulfide, pure sulfoxide or technical sulfone. This is
equivalent to approximately 5 mg/kg body-weight daily. There were no
toxic effects on growth, appetite, general health, blood picture,
liver function or kidney function and no gross or microscopic
pathological changes were found (Boots, 1957).
Comments on experimental studies reported
Long-term studies of two years' duration in rats and one year in
dogs were carried out. Other studies in mice, rats and rabbits of
shorter duration were reported. Tissue residue and excretion studies
showed that the pesticide is not cumulative.
EVALUATION
Level causing no significant toxicological effect in the rat
Twenty ppm in the diet of the rat is equivalent to 1 mg/kg
body-weight; in the dog 5 mg/kg body-weight.
Estimate of acceptable daily intakes for man
0-0.01 mg/kg body-weight.
Further work desirable
Additional long-term studies in the rat and the dog.
REFERENCE
Boots Pure Drug Co. Ltd., Nottingham, England (1957) Unpublished
Report
