
FAO Meeting Report No. PL/1965/10/1
WHO/Food Add./27.65
EVALUATION OF THE TOXICITY OF PESTICIDE RESIDUES IN FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Committee on Pesticides in Agriculture and
the WHO Expert Committee on Pesticide Residues, which met in Rome,
15-22 March 19651
Food and Agriculture Organization of the United Nations
World Health Organization
1965
1 Report of the second joint meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Committee on Pesticide
Residues, FAO Meeting Report No. PL/1965/10; WHO/Food Add./26.65
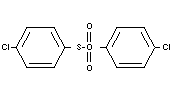
CHLORFENSON
Chemical name
p-chlorophenyl-p-chlorobenzene sulfonate;
4-chlorophenyl-4-chlorobenzene-sulfonate.
Synonyms
Ovotram; Ovex; Mitran; Orthotran; Ovotex; Difenson; CPCBS.
Empirical formula
C12H8O3Cl2S
Structural formula
 BIOLOGICAL DATA
Biochemical aspects
Absorption from the alimentary tract of dogs and rats after
single oral doses varying from 15 to 500 mg/kg body-weight was
variable. Intravenous doses of 15 mg/kg body-weight in dogs
disappeared from the plasma within 22 hours and less than 1% of the
total dose was recovered in the urine. There is evidence that
accumulation occurs in depot fat of dogs after the administration of
50 mg/kg body-weight daily for 6 months. Only traces could be found in
the fat depots and tissues or organs in dogs given doses of 15 and 5
mg/kg body-weight per day for the same period (Dow, 1962).
Female rats tended to accumulate chlorfenson in depot fat more
than males after dose levels of 300 and 3000 ppm in the diet for 370
days. Small quantities were also found in the liver and muscles. At
the level of 100 ppm no traces could be found in tissues or organs of
rats (Dow, 1962).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Oral 2 000 Dow, 1951
Dow, 1962
Short-term studies
Rat. Female rats (5 per group) were fed 300, 1000, 3000 and
10 000 ppm in the diet for 130 days. Those at 300 ppm showed no
evidence of adverse effects as judged by general appearance and
behaviour, mortality rate, growth, organ weights, and microscopic
examination of the tissues. The animals receiving 1000 and 3000 ppm
showed an increase in liver and kidney weights, and slight
histological degenerative changes in these organs. Those on the
highest dose level, 10 000 ppm, showed more pronounced effects (Dow,
1962).
Dog. Sixteen male and female dogs were divided into 4 groups
and were given 5, 15 and 50 mg/kg body-weight per day for 6 months. At
the lowest dose level, 5 mg, no effect was reported. At doses of 15
and 50 mg/kg body-weight per day only a questionable increase in the
average weights of the liver were found. No histological changes were
noted in this organ (Dow, 1962).
Long-term studies
Rat. Groups of 80 rats (40 female and 40 male were maintained
for one year on diets containing 100, 300 and 3000 ppm of chlorfenson.
Growth retardation, increases in average liver and kidney weight and
histological changes in these organs were found in the rats receiving
300 and 3000 ppm. At 100 ppm only a slight increase in liver weight
and histological changes in liver and kidneys were found (Dow, 1951).
The slight changes in the liver were characterized by the presence of
congestion and cloudy swelling, mainly in the portal areas which had
the appearance of beginning portal cirrhosis without fibrosis. The
kidneys showed some evidence of glomerular fibrosis with degeneration,
vacuole formation, cloudy swelling of the tubular epithelium, and the
presence of casts, oedema and congestion. Half the rats from this
experiment were placed on the normal diet for 30 days. Gross
examination at autopsy showed no abnormalities in the animals that had
previously received 100 and 300 ppm of chlorfenson in the diet.
Microscopic examination of the tissues of the rats that had received
100 ppm showed slight pathological changes in the liver and kidney.
The liver showed beginning portal cirrhosis and a few large fat
vacuoles and areas of necrosis. The kidney showed glomerular and
tubular fibrosis (Dow, 1962).
Groups of 20 rats (10 females and 10 males) were given a diet
containing 0.63, 1.25, 2.5, 5, 15 and 50 mg/kg body-weight per day for
two years. Increases in the average weights of liver and kidney and
histopathological changes in these organs were noted at doses of 2.5
mg/kg and above. Microscopically there were fatty changes in the liver
and chronic glomerulonephritis in the kidneys.
In the groups of rats that had ingested doses of 1.25 and 0.63
mg/kg per day, no significant effects were found as judged by general
appearance and behaviour, growth, mortality rate, food consumption,
average organ weights, and gross and microscopic examination of the
tissues (Dow, 1962; Weil & McCollister, 1963).
Comments on the experimental studies reports
Adequate experimental data on the short-term and long-term
toxicity in the rat and short-term toxicity in the dog have been
presented. Almost all the information obtainable on long-term toxicity
has been derived from one reliably conducted study with adequate
numbers of animals. Biochemical data on the accumulation and excretion
of chlorfenson have also been presented, but no information is
available on its metabolism.
EVALUATION
Level causing no significant toxicological effect in the rat
The maximum no-effect level in rats is 25 ppm equivalent to 1.25
mg/kg body-weight per day.
Estimate of acceptable daily intake for man
0-0.01 mg/kg body-weight.
Further work desirable
Metabolic studies in animals. Short-and long-term studies in
other species than the rat.
REFERENCES
Dow Chemical Company (1951) Midland Michigan, Technical Bulletin
Dow Chemical Company (1962) Midland Michigan, Unpublished data
Weil, C. S. & McCollister, D. D. (1963) Agric. Food Chem., 11, 486
BIOLOGICAL DATA
Biochemical aspects
Absorption from the alimentary tract of dogs and rats after
single oral doses varying from 15 to 500 mg/kg body-weight was
variable. Intravenous doses of 15 mg/kg body-weight in dogs
disappeared from the plasma within 22 hours and less than 1% of the
total dose was recovered in the urine. There is evidence that
accumulation occurs in depot fat of dogs after the administration of
50 mg/kg body-weight daily for 6 months. Only traces could be found in
the fat depots and tissues or organs in dogs given doses of 15 and 5
mg/kg body-weight per day for the same period (Dow, 1962).
Female rats tended to accumulate chlorfenson in depot fat more
than males after dose levels of 300 and 3000 ppm in the diet for 370
days. Small quantities were also found in the liver and muscles. At
the level of 100 ppm no traces could be found in tissues or organs of
rats (Dow, 1962).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Oral 2 000 Dow, 1951
Dow, 1962
Short-term studies
Rat. Female rats (5 per group) were fed 300, 1000, 3000 and
10 000 ppm in the diet for 130 days. Those at 300 ppm showed no
evidence of adverse effects as judged by general appearance and
behaviour, mortality rate, growth, organ weights, and microscopic
examination of the tissues. The animals receiving 1000 and 3000 ppm
showed an increase in liver and kidney weights, and slight
histological degenerative changes in these organs. Those on the
highest dose level, 10 000 ppm, showed more pronounced effects (Dow,
1962).
Dog. Sixteen male and female dogs were divided into 4 groups
and were given 5, 15 and 50 mg/kg body-weight per day for 6 months. At
the lowest dose level, 5 mg, no effect was reported. At doses of 15
and 50 mg/kg body-weight per day only a questionable increase in the
average weights of the liver were found. No histological changes were
noted in this organ (Dow, 1962).
Long-term studies
Rat. Groups of 80 rats (40 female and 40 male were maintained
for one year on diets containing 100, 300 and 3000 ppm of chlorfenson.
Growth retardation, increases in average liver and kidney weight and
histological changes in these organs were found in the rats receiving
300 and 3000 ppm. At 100 ppm only a slight increase in liver weight
and histological changes in liver and kidneys were found (Dow, 1951).
The slight changes in the liver were characterized by the presence of
congestion and cloudy swelling, mainly in the portal areas which had
the appearance of beginning portal cirrhosis without fibrosis. The
kidneys showed some evidence of glomerular fibrosis with degeneration,
vacuole formation, cloudy swelling of the tubular epithelium, and the
presence of casts, oedema and congestion. Half the rats from this
experiment were placed on the normal diet for 30 days. Gross
examination at autopsy showed no abnormalities in the animals that had
previously received 100 and 300 ppm of chlorfenson in the diet.
Microscopic examination of the tissues of the rats that had received
100 ppm showed slight pathological changes in the liver and kidney.
The liver showed beginning portal cirrhosis and a few large fat
vacuoles and areas of necrosis. The kidney showed glomerular and
tubular fibrosis (Dow, 1962).
Groups of 20 rats (10 females and 10 males) were given a diet
containing 0.63, 1.25, 2.5, 5, 15 and 50 mg/kg body-weight per day for
two years. Increases in the average weights of liver and kidney and
histopathological changes in these organs were noted at doses of 2.5
mg/kg and above. Microscopically there were fatty changes in the liver
and chronic glomerulonephritis in the kidneys.
In the groups of rats that had ingested doses of 1.25 and 0.63
mg/kg per day, no significant effects were found as judged by general
appearance and behaviour, growth, mortality rate, food consumption,
average organ weights, and gross and microscopic examination of the
tissues (Dow, 1962; Weil & McCollister, 1963).
Comments on the experimental studies reports
Adequate experimental data on the short-term and long-term
toxicity in the rat and short-term toxicity in the dog have been
presented. Almost all the information obtainable on long-term toxicity
has been derived from one reliably conducted study with adequate
numbers of animals. Biochemical data on the accumulation and excretion
of chlorfenson have also been presented, but no information is
available on its metabolism.
EVALUATION
Level causing no significant toxicological effect in the rat
The maximum no-effect level in rats is 25 ppm equivalent to 1.25
mg/kg body-weight per day.
Estimate of acceptable daily intake for man
0-0.01 mg/kg body-weight.
Further work desirable
Metabolic studies in animals. Short-and long-term studies in
other species than the rat.
REFERENCES
Dow Chemical Company (1951) Midland Michigan, Technical Bulletin
Dow Chemical Company (1962) Midland Michigan, Unpublished data
Weil, C. S. & McCollister, D. D. (1963) Agric. Food Chem., 11, 486
 BIOLOGICAL DATA
Biochemical aspects
Absorption from the alimentary tract of dogs and rats after
single oral doses varying from 15 to 500 mg/kg body-weight was
variable. Intravenous doses of 15 mg/kg body-weight in dogs
disappeared from the plasma within 22 hours and less than 1% of the
total dose was recovered in the urine. There is evidence that
accumulation occurs in depot fat of dogs after the administration of
50 mg/kg body-weight daily for 6 months. Only traces could be found in
the fat depots and tissues or organs in dogs given doses of 15 and 5
mg/kg body-weight per day for the same period (Dow, 1962).
Female rats tended to accumulate chlorfenson in depot fat more
than males after dose levels of 300 and 3000 ppm in the diet for 370
days. Small quantities were also found in the liver and muscles. At
the level of 100 ppm no traces could be found in tissues or organs of
rats (Dow, 1962).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Oral 2 000 Dow, 1951
Dow, 1962
Short-term studies
Rat. Female rats (5 per group) were fed 300, 1000, 3000 and
10 000 ppm in the diet for 130 days. Those at 300 ppm showed no
evidence of adverse effects as judged by general appearance and
behaviour, mortality rate, growth, organ weights, and microscopic
examination of the tissues. The animals receiving 1000 and 3000 ppm
showed an increase in liver and kidney weights, and slight
histological degenerative changes in these organs. Those on the
highest dose level, 10 000 ppm, showed more pronounced effects (Dow,
1962).
Dog. Sixteen male and female dogs were divided into 4 groups
and were given 5, 15 and 50 mg/kg body-weight per day for 6 months. At
the lowest dose level, 5 mg, no effect was reported. At doses of 15
and 50 mg/kg body-weight per day only a questionable increase in the
average weights of the liver were found. No histological changes were
noted in this organ (Dow, 1962).
Long-term studies
Rat. Groups of 80 rats (40 female and 40 male were maintained
for one year on diets containing 100, 300 and 3000 ppm of chlorfenson.
Growth retardation, increases in average liver and kidney weight and
histological changes in these organs were found in the rats receiving
300 and 3000 ppm. At 100 ppm only a slight increase in liver weight
and histological changes in liver and kidneys were found (Dow, 1951).
The slight changes in the liver were characterized by the presence of
congestion and cloudy swelling, mainly in the portal areas which had
the appearance of beginning portal cirrhosis without fibrosis. The
kidneys showed some evidence of glomerular fibrosis with degeneration,
vacuole formation, cloudy swelling of the tubular epithelium, and the
presence of casts, oedema and congestion. Half the rats from this
experiment were placed on the normal diet for 30 days. Gross
examination at autopsy showed no abnormalities in the animals that had
previously received 100 and 300 ppm of chlorfenson in the diet.
Microscopic examination of the tissues of the rats that had received
100 ppm showed slight pathological changes in the liver and kidney.
The liver showed beginning portal cirrhosis and a few large fat
vacuoles and areas of necrosis. The kidney showed glomerular and
tubular fibrosis (Dow, 1962).
Groups of 20 rats (10 females and 10 males) were given a diet
containing 0.63, 1.25, 2.5, 5, 15 and 50 mg/kg body-weight per day for
two years. Increases in the average weights of liver and kidney and
histopathological changes in these organs were noted at doses of 2.5
mg/kg and above. Microscopically there were fatty changes in the liver
and chronic glomerulonephritis in the kidneys.
In the groups of rats that had ingested doses of 1.25 and 0.63
mg/kg per day, no significant effects were found as judged by general
appearance and behaviour, growth, mortality rate, food consumption,
average organ weights, and gross and microscopic examination of the
tissues (Dow, 1962; Weil & McCollister, 1963).
Comments on the experimental studies reports
Adequate experimental data on the short-term and long-term
toxicity in the rat and short-term toxicity in the dog have been
presented. Almost all the information obtainable on long-term toxicity
has been derived from one reliably conducted study with adequate
numbers of animals. Biochemical data on the accumulation and excretion
of chlorfenson have also been presented, but no information is
available on its metabolism.
EVALUATION
Level causing no significant toxicological effect in the rat
The maximum no-effect level in rats is 25 ppm equivalent to 1.25
mg/kg body-weight per day.
Estimate of acceptable daily intake for man
0-0.01 mg/kg body-weight.
Further work desirable
Metabolic studies in animals. Short-and long-term studies in
other species than the rat.
REFERENCES
Dow Chemical Company (1951) Midland Michigan, Technical Bulletin
Dow Chemical Company (1962) Midland Michigan, Unpublished data
Weil, C. S. & McCollister, D. D. (1963) Agric. Food Chem., 11, 486
BIOLOGICAL DATA
Biochemical aspects
Absorption from the alimentary tract of dogs and rats after
single oral doses varying from 15 to 500 mg/kg body-weight was
variable. Intravenous doses of 15 mg/kg body-weight in dogs
disappeared from the plasma within 22 hours and less than 1% of the
total dose was recovered in the urine. There is evidence that
accumulation occurs in depot fat of dogs after the administration of
50 mg/kg body-weight daily for 6 months. Only traces could be found in
the fat depots and tissues or organs in dogs given doses of 15 and 5
mg/kg body-weight per day for the same period (Dow, 1962).
Female rats tended to accumulate chlorfenson in depot fat more
than males after dose levels of 300 and 3000 ppm in the diet for 370
days. Small quantities were also found in the liver and muscles. At
the level of 100 ppm no traces could be found in tissues or organs of
rats (Dow, 1962).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Oral 2 000 Dow, 1951
Dow, 1962
Short-term studies
Rat. Female rats (5 per group) were fed 300, 1000, 3000 and
10 000 ppm in the diet for 130 days. Those at 300 ppm showed no
evidence of adverse effects as judged by general appearance and
behaviour, mortality rate, growth, organ weights, and microscopic
examination of the tissues. The animals receiving 1000 and 3000 ppm
showed an increase in liver and kidney weights, and slight
histological degenerative changes in these organs. Those on the
highest dose level, 10 000 ppm, showed more pronounced effects (Dow,
1962).
Dog. Sixteen male and female dogs were divided into 4 groups
and were given 5, 15 and 50 mg/kg body-weight per day for 6 months. At
the lowest dose level, 5 mg, no effect was reported. At doses of 15
and 50 mg/kg body-weight per day only a questionable increase in the
average weights of the liver were found. No histological changes were
noted in this organ (Dow, 1962).
Long-term studies
Rat. Groups of 80 rats (40 female and 40 male were maintained
for one year on diets containing 100, 300 and 3000 ppm of chlorfenson.
Growth retardation, increases in average liver and kidney weight and
histological changes in these organs were found in the rats receiving
300 and 3000 ppm. At 100 ppm only a slight increase in liver weight
and histological changes in liver and kidneys were found (Dow, 1951).
The slight changes in the liver were characterized by the presence of
congestion and cloudy swelling, mainly in the portal areas which had
the appearance of beginning portal cirrhosis without fibrosis. The
kidneys showed some evidence of glomerular fibrosis with degeneration,
vacuole formation, cloudy swelling of the tubular epithelium, and the
presence of casts, oedema and congestion. Half the rats from this
experiment were placed on the normal diet for 30 days. Gross
examination at autopsy showed no abnormalities in the animals that had
previously received 100 and 300 ppm of chlorfenson in the diet.
Microscopic examination of the tissues of the rats that had received
100 ppm showed slight pathological changes in the liver and kidney.
The liver showed beginning portal cirrhosis and a few large fat
vacuoles and areas of necrosis. The kidney showed glomerular and
tubular fibrosis (Dow, 1962).
Groups of 20 rats (10 females and 10 males) were given a diet
containing 0.63, 1.25, 2.5, 5, 15 and 50 mg/kg body-weight per day for
two years. Increases in the average weights of liver and kidney and
histopathological changes in these organs were noted at doses of 2.5
mg/kg and above. Microscopically there were fatty changes in the liver
and chronic glomerulonephritis in the kidneys.
In the groups of rats that had ingested doses of 1.25 and 0.63
mg/kg per day, no significant effects were found as judged by general
appearance and behaviour, growth, mortality rate, food consumption,
average organ weights, and gross and microscopic examination of the
tissues (Dow, 1962; Weil & McCollister, 1963).
Comments on the experimental studies reports
Adequate experimental data on the short-term and long-term
toxicity in the rat and short-term toxicity in the dog have been
presented. Almost all the information obtainable on long-term toxicity
has been derived from one reliably conducted study with adequate
numbers of animals. Biochemical data on the accumulation and excretion
of chlorfenson have also been presented, but no information is
available on its metabolism.
EVALUATION
Level causing no significant toxicological effect in the rat
The maximum no-effect level in rats is 25 ppm equivalent to 1.25
mg/kg body-weight per day.
Estimate of acceptable daily intake for man
0-0.01 mg/kg body-weight.
Further work desirable
Metabolic studies in animals. Short-and long-term studies in
other species than the rat.
REFERENCES
Dow Chemical Company (1951) Midland Michigan, Technical Bulletin
Dow Chemical Company (1962) Midland Michigan, Unpublished data
Weil, C. S. & McCollister, D. D. (1963) Agric. Food Chem., 11, 486
