
FAO Meeting Report No. PL/1965/10/1
WHO/Food Add./27.65
EVALUATION OF THE TOXICITY OF PESTICIDE RESIDUES IN FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Committee on Pesticides in Agriculture and
the WHO Expert Committee on Pesticide Residues, which met in Rome,
15-22 March 19651
Food and Agriculture Organization of the United Nations
World Health Organization
1965
1 Report of the second joint meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Committee on Pesticide
Residues, FAO Meeting Report No. PL/1965/10; WHO/Food Add./26.65
CHLORPROPHAM
Chemical names
Isopropyl N-(3-chlorophenyl)carbmate; isopropyl
chlorocarbanilate
Synonyms
CIPC
Empirical formula
C10H12O2N Cl
Structural formula
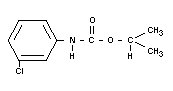 BIOLOGICAL DATA
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Oral 5000-8000 van Esch & van Genderen, 1956;
Westrick at al., 1953
Rat Intraperitoneal 700 van Esch & van Genderen, 1956
Rabbit Oral approx. 5000 Westrick et al., 1953
Short-term studies
Mouse. Groups each of 30 mice, 15 females and 15 males, were
given the following oral doses of chlorpropham : One group, a single
dose of 750 mg/kg body-weight; a second group, once a week an oral
dose of 750 mg/kg body-weight; and a third group, 1000 ppm in the diet
for 6 months. Chlorpropham and also ethylurethane were used as
initiators and croton oil was painted on the skin as a promoter.
Although in some of the original experiments skin papillomas developed
following applications of croton oil in mice fed clorpropham, this
result could not be confirmed in a number or subsequent experiments
carried on in the same institution. No lung tumours were found in
these experiments. Positive controls given ethylurethane in the place
of chlorpropham developed both skin papillomas and lung adenomas (van
Esch et al., 1958; van Esch, 1965).
Rat. Groups of 10 rats were fed diets containing 310, 1250,
5000 and 20 000 ppm of chlorpropham for 90 days. All groups showed an
increase in weight and food-intake as compared with the controls. The
mean liver weights of the animals receiving 1250, 5000 and 20 000 ppm
were significantly higher than those of the controls (no significant
changes in kidney weight). No microscopic changes in the liver and the
kidney after 90 days were found (Westrick et al., 1953).
Dog. Groups of 4 dogs (2 male and 2 female) were given 200,
2000 and 20 000 ppm of chlorpropham in their diet for one year. All
animals showed initial weight loss, but only the animals receiving 20
000 ppm failed to surpass the average starting weight for the group
during the experimental period; no animals died and there was no
apparent morbidity. Lower haemoglobin and haematocrit values were
found in the first six-month feeding period on 20 000 ppm but not at
the end of the 12-month feeding period. The weights of the liver and
spleen relative to body-weight increased at the 20 000 ppm level
(Larson et al., 1960).
Pig. Pigs, 4 per group, received 3300 ppm of chlorpropham in
their diet for 19 weeks. There was no effect on weight gain, and at
autopsy no pathological abnormalities were observed. The liver, kidney
and spleen were histologically normal. No changes in the blood
picture, except a slight decrease in haemoglobin content in the
nineteenth week, were present (van Esch & van Genderen, 1956).
Long-term studies
Rat. Groups each of 50 rats, 25 males and 25 females, were
administered 200, 2000 and 20 000 ppm chlorpropham in their diet for 2
years. No abnormalities were observed at concentrations of 200 and
2000 ppm. At 20 000 ppm growth was depressed, the mortality rate of
the males was increased, and the haematocrit and haemoglobin values
were lowered; increases in liver and spleen weights relative to
body-weight were observed. Tumour incidence in the treated animals was
not greater than in the control animals (Larson et al., 1960).
Comments on experimental work reported
The suspicion that chlorpropham could be a co-carcinogen for mice
could not be confirmed. In other species experiments purposely
designed did not indicate any tumorigenic effect.
EVALUATION
Not enough toxicological data on animals are available at present
to set no-effect levels.
Further work required
Biochemical studies; long-term feeding studies in other species
than the rat.
REFERENCES
van Esch, G. J. (1965) Personal communication concerning data from the
National Institute of Public Health, Utrecht, The Netherlands
van Esch, G. J. & van Genderen. H. (1956) Preliminary report of the
National Institute of Public Health
van Esch, G. J., van Genderen, H. & Vink, H. H. (1958) Brit. J.
Cancer, 3, 355
Larson, F. S., Crawford, E. M., Blackwell Smith, R., Hennigar, G. R.,
Haag, H. B. & Finnegan, J. K. (1960) Toxicol. Appl. Pharmacol., 2,
659
Westrick, M. L., Gross, P. & Schrenk, H. H. (1953) Report from Ind.
Hyg. Foundation America to Pittsburgh Plate Glass Company,
June/September and September/December
BIOLOGICAL DATA
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Oral 5000-8000 van Esch & van Genderen, 1956;
Westrick at al., 1953
Rat Intraperitoneal 700 van Esch & van Genderen, 1956
Rabbit Oral approx. 5000 Westrick et al., 1953
Short-term studies
Mouse. Groups each of 30 mice, 15 females and 15 males, were
given the following oral doses of chlorpropham : One group, a single
dose of 750 mg/kg body-weight; a second group, once a week an oral
dose of 750 mg/kg body-weight; and a third group, 1000 ppm in the diet
for 6 months. Chlorpropham and also ethylurethane were used as
initiators and croton oil was painted on the skin as a promoter.
Although in some of the original experiments skin papillomas developed
following applications of croton oil in mice fed clorpropham, this
result could not be confirmed in a number or subsequent experiments
carried on in the same institution. No lung tumours were found in
these experiments. Positive controls given ethylurethane in the place
of chlorpropham developed both skin papillomas and lung adenomas (van
Esch et al., 1958; van Esch, 1965).
Rat. Groups of 10 rats were fed diets containing 310, 1250,
5000 and 20 000 ppm of chlorpropham for 90 days. All groups showed an
increase in weight and food-intake as compared with the controls. The
mean liver weights of the animals receiving 1250, 5000 and 20 000 ppm
were significantly higher than those of the controls (no significant
changes in kidney weight). No microscopic changes in the liver and the
kidney after 90 days were found (Westrick et al., 1953).
Dog. Groups of 4 dogs (2 male and 2 female) were given 200,
2000 and 20 000 ppm of chlorpropham in their diet for one year. All
animals showed initial weight loss, but only the animals receiving 20
000 ppm failed to surpass the average starting weight for the group
during the experimental period; no animals died and there was no
apparent morbidity. Lower haemoglobin and haematocrit values were
found in the first six-month feeding period on 20 000 ppm but not at
the end of the 12-month feeding period. The weights of the liver and
spleen relative to body-weight increased at the 20 000 ppm level
(Larson et al., 1960).
Pig. Pigs, 4 per group, received 3300 ppm of chlorpropham in
their diet for 19 weeks. There was no effect on weight gain, and at
autopsy no pathological abnormalities were observed. The liver, kidney
and spleen were histologically normal. No changes in the blood
picture, except a slight decrease in haemoglobin content in the
nineteenth week, were present (van Esch & van Genderen, 1956).
Long-term studies
Rat. Groups each of 50 rats, 25 males and 25 females, were
administered 200, 2000 and 20 000 ppm chlorpropham in their diet for 2
years. No abnormalities were observed at concentrations of 200 and
2000 ppm. At 20 000 ppm growth was depressed, the mortality rate of
the males was increased, and the haematocrit and haemoglobin values
were lowered; increases in liver and spleen weights relative to
body-weight were observed. Tumour incidence in the treated animals was
not greater than in the control animals (Larson et al., 1960).
Comments on experimental work reported
The suspicion that chlorpropham could be a co-carcinogen for mice
could not be confirmed. In other species experiments purposely
designed did not indicate any tumorigenic effect.
EVALUATION
Not enough toxicological data on animals are available at present
to set no-effect levels.
Further work required
Biochemical studies; long-term feeding studies in other species
than the rat.
REFERENCES
van Esch, G. J. (1965) Personal communication concerning data from the
National Institute of Public Health, Utrecht, The Netherlands
van Esch, G. J. & van Genderen. H. (1956) Preliminary report of the
National Institute of Public Health
van Esch, G. J., van Genderen, H. & Vink, H. H. (1958) Brit. J.
Cancer, 3, 355
Larson, F. S., Crawford, E. M., Blackwell Smith, R., Hennigar, G. R.,
Haag, H. B. & Finnegan, J. K. (1960) Toxicol. Appl. Pharmacol., 2,
659
Westrick, M. L., Gross, P. & Schrenk, H. H. (1953) Report from Ind.
Hyg. Foundation America to Pittsburgh Plate Glass Company,
June/September and September/December
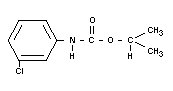 BIOLOGICAL DATA
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Oral 5000-8000 van Esch & van Genderen, 1956;
Westrick at al., 1953
Rat Intraperitoneal 700 van Esch & van Genderen, 1956
Rabbit Oral approx. 5000 Westrick et al., 1953
Short-term studies
Mouse. Groups each of 30 mice, 15 females and 15 males, were
given the following oral doses of chlorpropham : One group, a single
dose of 750 mg/kg body-weight; a second group, once a week an oral
dose of 750 mg/kg body-weight; and a third group, 1000 ppm in the diet
for 6 months. Chlorpropham and also ethylurethane were used as
initiators and croton oil was painted on the skin as a promoter.
Although in some of the original experiments skin papillomas developed
following applications of croton oil in mice fed clorpropham, this
result could not be confirmed in a number or subsequent experiments
carried on in the same institution. No lung tumours were found in
these experiments. Positive controls given ethylurethane in the place
of chlorpropham developed both skin papillomas and lung adenomas (van
Esch et al., 1958; van Esch, 1965).
Rat. Groups of 10 rats were fed diets containing 310, 1250,
5000 and 20 000 ppm of chlorpropham for 90 days. All groups showed an
increase in weight and food-intake as compared with the controls. The
mean liver weights of the animals receiving 1250, 5000 and 20 000 ppm
were significantly higher than those of the controls (no significant
changes in kidney weight). No microscopic changes in the liver and the
kidney after 90 days were found (Westrick et al., 1953).
Dog. Groups of 4 dogs (2 male and 2 female) were given 200,
2000 and 20 000 ppm of chlorpropham in their diet for one year. All
animals showed initial weight loss, but only the animals receiving 20
000 ppm failed to surpass the average starting weight for the group
during the experimental period; no animals died and there was no
apparent morbidity. Lower haemoglobin and haematocrit values were
found in the first six-month feeding period on 20 000 ppm but not at
the end of the 12-month feeding period. The weights of the liver and
spleen relative to body-weight increased at the 20 000 ppm level
(Larson et al., 1960).
Pig. Pigs, 4 per group, received 3300 ppm of chlorpropham in
their diet for 19 weeks. There was no effect on weight gain, and at
autopsy no pathological abnormalities were observed. The liver, kidney
and spleen were histologically normal. No changes in the blood
picture, except a slight decrease in haemoglobin content in the
nineteenth week, were present (van Esch & van Genderen, 1956).
Long-term studies
Rat. Groups each of 50 rats, 25 males and 25 females, were
administered 200, 2000 and 20 000 ppm chlorpropham in their diet for 2
years. No abnormalities were observed at concentrations of 200 and
2000 ppm. At 20 000 ppm growth was depressed, the mortality rate of
the males was increased, and the haematocrit and haemoglobin values
were lowered; increases in liver and spleen weights relative to
body-weight were observed. Tumour incidence in the treated animals was
not greater than in the control animals (Larson et al., 1960).
Comments on experimental work reported
The suspicion that chlorpropham could be a co-carcinogen for mice
could not be confirmed. In other species experiments purposely
designed did not indicate any tumorigenic effect.
EVALUATION
Not enough toxicological data on animals are available at present
to set no-effect levels.
Further work required
Biochemical studies; long-term feeding studies in other species
than the rat.
REFERENCES
van Esch, G. J. (1965) Personal communication concerning data from the
National Institute of Public Health, Utrecht, The Netherlands
van Esch, G. J. & van Genderen. H. (1956) Preliminary report of the
National Institute of Public Health
van Esch, G. J., van Genderen, H. & Vink, H. H. (1958) Brit. J.
Cancer, 3, 355
Larson, F. S., Crawford, E. M., Blackwell Smith, R., Hennigar, G. R.,
Haag, H. B. & Finnegan, J. K. (1960) Toxicol. Appl. Pharmacol., 2,
659
Westrick, M. L., Gross, P. & Schrenk, H. H. (1953) Report from Ind.
Hyg. Foundation America to Pittsburgh Plate Glass Company,
June/September and September/December
BIOLOGICAL DATA
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Oral 5000-8000 van Esch & van Genderen, 1956;
Westrick at al., 1953
Rat Intraperitoneal 700 van Esch & van Genderen, 1956
Rabbit Oral approx. 5000 Westrick et al., 1953
Short-term studies
Mouse. Groups each of 30 mice, 15 females and 15 males, were
given the following oral doses of chlorpropham : One group, a single
dose of 750 mg/kg body-weight; a second group, once a week an oral
dose of 750 mg/kg body-weight; and a third group, 1000 ppm in the diet
for 6 months. Chlorpropham and also ethylurethane were used as
initiators and croton oil was painted on the skin as a promoter.
Although in some of the original experiments skin papillomas developed
following applications of croton oil in mice fed clorpropham, this
result could not be confirmed in a number or subsequent experiments
carried on in the same institution. No lung tumours were found in
these experiments. Positive controls given ethylurethane in the place
of chlorpropham developed both skin papillomas and lung adenomas (van
Esch et al., 1958; van Esch, 1965).
Rat. Groups of 10 rats were fed diets containing 310, 1250,
5000 and 20 000 ppm of chlorpropham for 90 days. All groups showed an
increase in weight and food-intake as compared with the controls. The
mean liver weights of the animals receiving 1250, 5000 and 20 000 ppm
were significantly higher than those of the controls (no significant
changes in kidney weight). No microscopic changes in the liver and the
kidney after 90 days were found (Westrick et al., 1953).
Dog. Groups of 4 dogs (2 male and 2 female) were given 200,
2000 and 20 000 ppm of chlorpropham in their diet for one year. All
animals showed initial weight loss, but only the animals receiving 20
000 ppm failed to surpass the average starting weight for the group
during the experimental period; no animals died and there was no
apparent morbidity. Lower haemoglobin and haematocrit values were
found in the first six-month feeding period on 20 000 ppm but not at
the end of the 12-month feeding period. The weights of the liver and
spleen relative to body-weight increased at the 20 000 ppm level
(Larson et al., 1960).
Pig. Pigs, 4 per group, received 3300 ppm of chlorpropham in
their diet for 19 weeks. There was no effect on weight gain, and at
autopsy no pathological abnormalities were observed. The liver, kidney
and spleen were histologically normal. No changes in the blood
picture, except a slight decrease in haemoglobin content in the
nineteenth week, were present (van Esch & van Genderen, 1956).
Long-term studies
Rat. Groups each of 50 rats, 25 males and 25 females, were
administered 200, 2000 and 20 000 ppm chlorpropham in their diet for 2
years. No abnormalities were observed at concentrations of 200 and
2000 ppm. At 20 000 ppm growth was depressed, the mortality rate of
the males was increased, and the haematocrit and haemoglobin values
were lowered; increases in liver and spleen weights relative to
body-weight were observed. Tumour incidence in the treated animals was
not greater than in the control animals (Larson et al., 1960).
Comments on experimental work reported
The suspicion that chlorpropham could be a co-carcinogen for mice
could not be confirmed. In other species experiments purposely
designed did not indicate any tumorigenic effect.
EVALUATION
Not enough toxicological data on animals are available at present
to set no-effect levels.
Further work required
Biochemical studies; long-term feeding studies in other species
than the rat.
REFERENCES
van Esch, G. J. (1965) Personal communication concerning data from the
National Institute of Public Health, Utrecht, The Netherlands
van Esch, G. J. & van Genderen. H. (1956) Preliminary report of the
National Institute of Public Health
van Esch, G. J., van Genderen, H. & Vink, H. H. (1958) Brit. J.
Cancer, 3, 355
Larson, F. S., Crawford, E. M., Blackwell Smith, R., Hennigar, G. R.,
Haag, H. B. & Finnegan, J. K. (1960) Toxicol. Appl. Pharmacol., 2,
659
Westrick, M. L., Gross, P. & Schrenk, H. H. (1953) Report from Ind.
Hyg. Foundation America to Pittsburgh Plate Glass Company,
June/September and September/December
