
FAO Meeting Report No. PL/1965/10/1
WHO/Food Add./27.65
EVALUATION OF THE TOXICITY OF PESTICIDE RESIDUES IN FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Committee on Pesticides in Agriculture and
the WHO Expert Committee on Pesticide Residues, which met in Rome,
15-22 March 19651
Food and Agriculture Organization of the United Nations
World Health Organization
1965
1 Report of the second joint meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Committee on Pesticide
Residues, FAO Meeting Report No. PL/1965/10; WHO/Food Add./26.65
CHLORTHION
Chemical name
O,O-dimethyl-O-(3-chloro-4-nitrophenyl)-phosphorothioate;
O,O-dimethyl-O-3-chloro-4-nitrophenyl phosphorothionate.
Empirical formula
C8H9O5NSCIP
Structural formula
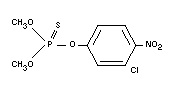 BIOLOGICAL DATA
Biochemical aspects
Chlorthion is activated to a potent cholinesterase inhibitor,
chloroxon, in animals and plants. It is an active inhibitor of rat
brain cholinesterase (DuBois et al., 1953). The molar I50 of
chloroxon for human plasma cholinesterase (30 min. at 37°) in vitro
is 4 × 10-7 (Fallscheer & Cook, 1956). Its in vivo mammalian
toxicity is high in comparison with its low in vitro activity; this
is ascribed to slow absorption from the peritoneal cavity, the
gastro-intestinal tract and its slow passage into the central and
peripheral nervous systems. However, when administered
intraperitoneally in large doses (1000 mg/kg body-weight) it can gain
access to and inhibit the cholinesterase activity of the brain. As it
is the 3-chloro-derivative of "methyl parathion" it will probably be
metabolized in a similar manner to the latter compound by oxidative
replacement of the sulfur by oxygen to produce the active form of the
insecticide. Such a metabolite does not appear to have been described
and there are no reports in the literature to indicate whether the
chloronitrophenyl residue is excreted as 3-chloro-4-nitrophenol, as
would be expected by analogy with parathion. Few hydrolytic products
have been found in rat urine after the administration of this compound
(Plapp & Casida, 1958). There are no details of its metabolism in
plants.
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Oral 625-1 500 DuBois et al., 1953
Klimmer & Pfaff, 1955
Rat Intraperitoneal 750 DuBois et al., 1953
Mouse Oral 1 250 Klimmer & Pfaff, 1955
Guinea-pig Intraperitoneal 525 Klimmer & Pfaff, 1955
Differences in acute oral toxicity may be due to the purity of
the material, in particular, the presence of more toxic oxidation
products and of dimethyl-2-chloro-4-nitrophenyl thionophosphate. The
vehicle used is also important. Combination of chlorthion with
malathion and other phosphorothionates is said to result in a
potentiation of its oral toxicity to mice (Rosenberg & Coon, 1958).
Short-term studies
Rat. Intraperitoneal injections of undiluted chlorthion to
groups of 5 rats produced no mortality at a dose level of 50 mg/kg
body-weight daily for 60 days; 100 mg/kg body-weight daily for 60 days
resulted in 40% mortality. With 200 mg/kg body-weight daily all
animals died within 5-10 days. The symptoms prior to death were
similar to those observed for more toxic organo-phosphorus
insecticides. Serum cholinesterase activity was decreased more than
that of brain or submaxillary gland, probably owing to slow absorption
into the tissues. At the lowest dose level (50 mg/kg body-weight
daily) the serum cholinesterase activity fell to below 50% of normal
after the first dose (DuBois et al., 1953).
Rats (4 groups of 13 male and 4 groups of 13 female) fed for 120
days on diets containing 10, 20 and 50 ppm of chlorthion showed no
significant change in growth rate, food consumption, or general
appearance and no symptoms of cholinergic stimulation. Pathological
examination showed no difference in the gross or microscopic features
of the tissues compared with control animals. The animals fed at the
50 ppm level showed slight reduction in brain and submaxillary gland
cholinesterase activity levels and a marked decrease in serum
cholinesterase activity amounting to 76% inhibition. Reduction in
serum cholinesterase activity was also noted at the 20 ppm level (61%
inhibition) and at 10 ppm (27% inhibition). Reversal of the inhibition
occurred within 2 weeks following removal of chlorthion from the diet
(DuBois et al., 1956).
Dog. Chlorthion fed to 4 dogs (2 male, 2 female) at dose levels
of 0.5, 2, 5 and 15 ppm in the diet produced no depression of plasma
and erythrocyte cholinesterase levels at 0.5, 2 and 5 ppm and gave
only a questionable depression at 15 ppm over a period of 12 weeks
(Williams et al., 1959).
Long-term studies
No data available.
Comments on the experimental studies reported
Although reliable acute toxicity data are available, no long-term
toxicity tests on animals have been reported. No information is
available about toxicity in man.
EVALUATION
Level causing no significant toxic effects in the rat and dog
The data so far reported are not sufficient for an estimate to be
made of an acceptable daily intake for man, especially as long-term
studies are indicated because of the chemical nature of this compound.
Further work required
Chemical composition and toxicity of the residues. Observations
on the effect in man. Long-term studies in rats including reproduction
studies.
REFERENCES
DuBois, K. P., Doull, J., Deroin, J. & Cummings, O. K. (1953)
Arch. industr. Hyg., 8, 350-8
DuBois, K. P., Doull, J. & Rehfuss, P. A. (1956) Report from
Department of Pharmacology, University of Chicago
Fallscheer, H. O. & Cook, J. W. (1956) J. Assoc. official Agr.
Chem., 39, 691
Klimmer, O. R. & Pfaff, W. (1955) Arzneimitt-Forsch, 5
Plapp, P. W. & Casida, J. E. (1958) J. econ. Ent., 51, 800
Rosenberg, P. & Coon, J. M. (1958) Proc. Soc. exp. Biol. (N.Y.),
97, 836-9
Williams, M. W., Fuyat, H. N. & Fitzhugh, O. G. (1959) Toxicol.
Appl. Pharmacol., 1, 1-7
BIOLOGICAL DATA
Biochemical aspects
Chlorthion is activated to a potent cholinesterase inhibitor,
chloroxon, in animals and plants. It is an active inhibitor of rat
brain cholinesterase (DuBois et al., 1953). The molar I50 of
chloroxon for human plasma cholinesterase (30 min. at 37°) in vitro
is 4 × 10-7 (Fallscheer & Cook, 1956). Its in vivo mammalian
toxicity is high in comparison with its low in vitro activity; this
is ascribed to slow absorption from the peritoneal cavity, the
gastro-intestinal tract and its slow passage into the central and
peripheral nervous systems. However, when administered
intraperitoneally in large doses (1000 mg/kg body-weight) it can gain
access to and inhibit the cholinesterase activity of the brain. As it
is the 3-chloro-derivative of "methyl parathion" it will probably be
metabolized in a similar manner to the latter compound by oxidative
replacement of the sulfur by oxygen to produce the active form of the
insecticide. Such a metabolite does not appear to have been described
and there are no reports in the literature to indicate whether the
chloronitrophenyl residue is excreted as 3-chloro-4-nitrophenol, as
would be expected by analogy with parathion. Few hydrolytic products
have been found in rat urine after the administration of this compound
(Plapp & Casida, 1958). There are no details of its metabolism in
plants.
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Oral 625-1 500 DuBois et al., 1953
Klimmer & Pfaff, 1955
Rat Intraperitoneal 750 DuBois et al., 1953
Mouse Oral 1 250 Klimmer & Pfaff, 1955
Guinea-pig Intraperitoneal 525 Klimmer & Pfaff, 1955
Differences in acute oral toxicity may be due to the purity of
the material, in particular, the presence of more toxic oxidation
products and of dimethyl-2-chloro-4-nitrophenyl thionophosphate. The
vehicle used is also important. Combination of chlorthion with
malathion and other phosphorothionates is said to result in a
potentiation of its oral toxicity to mice (Rosenberg & Coon, 1958).
Short-term studies
Rat. Intraperitoneal injections of undiluted chlorthion to
groups of 5 rats produced no mortality at a dose level of 50 mg/kg
body-weight daily for 60 days; 100 mg/kg body-weight daily for 60 days
resulted in 40% mortality. With 200 mg/kg body-weight daily all
animals died within 5-10 days. The symptoms prior to death were
similar to those observed for more toxic organo-phosphorus
insecticides. Serum cholinesterase activity was decreased more than
that of brain or submaxillary gland, probably owing to slow absorption
into the tissues. At the lowest dose level (50 mg/kg body-weight
daily) the serum cholinesterase activity fell to below 50% of normal
after the first dose (DuBois et al., 1953).
Rats (4 groups of 13 male and 4 groups of 13 female) fed for 120
days on diets containing 10, 20 and 50 ppm of chlorthion showed no
significant change in growth rate, food consumption, or general
appearance and no symptoms of cholinergic stimulation. Pathological
examination showed no difference in the gross or microscopic features
of the tissues compared with control animals. The animals fed at the
50 ppm level showed slight reduction in brain and submaxillary gland
cholinesterase activity levels and a marked decrease in serum
cholinesterase activity amounting to 76% inhibition. Reduction in
serum cholinesterase activity was also noted at the 20 ppm level (61%
inhibition) and at 10 ppm (27% inhibition). Reversal of the inhibition
occurred within 2 weeks following removal of chlorthion from the diet
(DuBois et al., 1956).
Dog. Chlorthion fed to 4 dogs (2 male, 2 female) at dose levels
of 0.5, 2, 5 and 15 ppm in the diet produced no depression of plasma
and erythrocyte cholinesterase levels at 0.5, 2 and 5 ppm and gave
only a questionable depression at 15 ppm over a period of 12 weeks
(Williams et al., 1959).
Long-term studies
No data available.
Comments on the experimental studies reported
Although reliable acute toxicity data are available, no long-term
toxicity tests on animals have been reported. No information is
available about toxicity in man.
EVALUATION
Level causing no significant toxic effects in the rat and dog
The data so far reported are not sufficient for an estimate to be
made of an acceptable daily intake for man, especially as long-term
studies are indicated because of the chemical nature of this compound.
Further work required
Chemical composition and toxicity of the residues. Observations
on the effect in man. Long-term studies in rats including reproduction
studies.
REFERENCES
DuBois, K. P., Doull, J., Deroin, J. & Cummings, O. K. (1953)
Arch. industr. Hyg., 8, 350-8
DuBois, K. P., Doull, J. & Rehfuss, P. A. (1956) Report from
Department of Pharmacology, University of Chicago
Fallscheer, H. O. & Cook, J. W. (1956) J. Assoc. official Agr.
Chem., 39, 691
Klimmer, O. R. & Pfaff, W. (1955) Arzneimitt-Forsch, 5
Plapp, P. W. & Casida, J. E. (1958) J. econ. Ent., 51, 800
Rosenberg, P. & Coon, J. M. (1958) Proc. Soc. exp. Biol. (N.Y.),
97, 836-9
Williams, M. W., Fuyat, H. N. & Fitzhugh, O. G. (1959) Toxicol.
Appl. Pharmacol., 1, 1-7
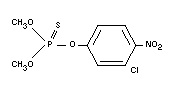 BIOLOGICAL DATA
Biochemical aspects
Chlorthion is activated to a potent cholinesterase inhibitor,
chloroxon, in animals and plants. It is an active inhibitor of rat
brain cholinesterase (DuBois et al., 1953). The molar I50 of
chloroxon for human plasma cholinesterase (30 min. at 37°) in vitro
is 4 × 10-7 (Fallscheer & Cook, 1956). Its in vivo mammalian
toxicity is high in comparison with its low in vitro activity; this
is ascribed to slow absorption from the peritoneal cavity, the
gastro-intestinal tract and its slow passage into the central and
peripheral nervous systems. However, when administered
intraperitoneally in large doses (1000 mg/kg body-weight) it can gain
access to and inhibit the cholinesterase activity of the brain. As it
is the 3-chloro-derivative of "methyl parathion" it will probably be
metabolized in a similar manner to the latter compound by oxidative
replacement of the sulfur by oxygen to produce the active form of the
insecticide. Such a metabolite does not appear to have been described
and there are no reports in the literature to indicate whether the
chloronitrophenyl residue is excreted as 3-chloro-4-nitrophenol, as
would be expected by analogy with parathion. Few hydrolytic products
have been found in rat urine after the administration of this compound
(Plapp & Casida, 1958). There are no details of its metabolism in
plants.
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Oral 625-1 500 DuBois et al., 1953
Klimmer & Pfaff, 1955
Rat Intraperitoneal 750 DuBois et al., 1953
Mouse Oral 1 250 Klimmer & Pfaff, 1955
Guinea-pig Intraperitoneal 525 Klimmer & Pfaff, 1955
Differences in acute oral toxicity may be due to the purity of
the material, in particular, the presence of more toxic oxidation
products and of dimethyl-2-chloro-4-nitrophenyl thionophosphate. The
vehicle used is also important. Combination of chlorthion with
malathion and other phosphorothionates is said to result in a
potentiation of its oral toxicity to mice (Rosenberg & Coon, 1958).
Short-term studies
Rat. Intraperitoneal injections of undiluted chlorthion to
groups of 5 rats produced no mortality at a dose level of 50 mg/kg
body-weight daily for 60 days; 100 mg/kg body-weight daily for 60 days
resulted in 40% mortality. With 200 mg/kg body-weight daily all
animals died within 5-10 days. The symptoms prior to death were
similar to those observed for more toxic organo-phosphorus
insecticides. Serum cholinesterase activity was decreased more than
that of brain or submaxillary gland, probably owing to slow absorption
into the tissues. At the lowest dose level (50 mg/kg body-weight
daily) the serum cholinesterase activity fell to below 50% of normal
after the first dose (DuBois et al., 1953).
Rats (4 groups of 13 male and 4 groups of 13 female) fed for 120
days on diets containing 10, 20 and 50 ppm of chlorthion showed no
significant change in growth rate, food consumption, or general
appearance and no symptoms of cholinergic stimulation. Pathological
examination showed no difference in the gross or microscopic features
of the tissues compared with control animals. The animals fed at the
50 ppm level showed slight reduction in brain and submaxillary gland
cholinesterase activity levels and a marked decrease in serum
cholinesterase activity amounting to 76% inhibition. Reduction in
serum cholinesterase activity was also noted at the 20 ppm level (61%
inhibition) and at 10 ppm (27% inhibition). Reversal of the inhibition
occurred within 2 weeks following removal of chlorthion from the diet
(DuBois et al., 1956).
Dog. Chlorthion fed to 4 dogs (2 male, 2 female) at dose levels
of 0.5, 2, 5 and 15 ppm in the diet produced no depression of plasma
and erythrocyte cholinesterase levels at 0.5, 2 and 5 ppm and gave
only a questionable depression at 15 ppm over a period of 12 weeks
(Williams et al., 1959).
Long-term studies
No data available.
Comments on the experimental studies reported
Although reliable acute toxicity data are available, no long-term
toxicity tests on animals have been reported. No information is
available about toxicity in man.
EVALUATION
Level causing no significant toxic effects in the rat and dog
The data so far reported are not sufficient for an estimate to be
made of an acceptable daily intake for man, especially as long-term
studies are indicated because of the chemical nature of this compound.
Further work required
Chemical composition and toxicity of the residues. Observations
on the effect in man. Long-term studies in rats including reproduction
studies.
REFERENCES
DuBois, K. P., Doull, J., Deroin, J. & Cummings, O. K. (1953)
Arch. industr. Hyg., 8, 350-8
DuBois, K. P., Doull, J. & Rehfuss, P. A. (1956) Report from
Department of Pharmacology, University of Chicago
Fallscheer, H. O. & Cook, J. W. (1956) J. Assoc. official Agr.
Chem., 39, 691
Klimmer, O. R. & Pfaff, W. (1955) Arzneimitt-Forsch, 5
Plapp, P. W. & Casida, J. E. (1958) J. econ. Ent., 51, 800
Rosenberg, P. & Coon, J. M. (1958) Proc. Soc. exp. Biol. (N.Y.),
97, 836-9
Williams, M. W., Fuyat, H. N. & Fitzhugh, O. G. (1959) Toxicol.
Appl. Pharmacol., 1, 1-7
BIOLOGICAL DATA
Biochemical aspects
Chlorthion is activated to a potent cholinesterase inhibitor,
chloroxon, in animals and plants. It is an active inhibitor of rat
brain cholinesterase (DuBois et al., 1953). The molar I50 of
chloroxon for human plasma cholinesterase (30 min. at 37°) in vitro
is 4 × 10-7 (Fallscheer & Cook, 1956). Its in vivo mammalian
toxicity is high in comparison with its low in vitro activity; this
is ascribed to slow absorption from the peritoneal cavity, the
gastro-intestinal tract and its slow passage into the central and
peripheral nervous systems. However, when administered
intraperitoneally in large doses (1000 mg/kg body-weight) it can gain
access to and inhibit the cholinesterase activity of the brain. As it
is the 3-chloro-derivative of "methyl parathion" it will probably be
metabolized in a similar manner to the latter compound by oxidative
replacement of the sulfur by oxygen to produce the active form of the
insecticide. Such a metabolite does not appear to have been described
and there are no reports in the literature to indicate whether the
chloronitrophenyl residue is excreted as 3-chloro-4-nitrophenol, as
would be expected by analogy with parathion. Few hydrolytic products
have been found in rat urine after the administration of this compound
(Plapp & Casida, 1958). There are no details of its metabolism in
plants.
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Oral 625-1 500 DuBois et al., 1953
Klimmer & Pfaff, 1955
Rat Intraperitoneal 750 DuBois et al., 1953
Mouse Oral 1 250 Klimmer & Pfaff, 1955
Guinea-pig Intraperitoneal 525 Klimmer & Pfaff, 1955
Differences in acute oral toxicity may be due to the purity of
the material, in particular, the presence of more toxic oxidation
products and of dimethyl-2-chloro-4-nitrophenyl thionophosphate. The
vehicle used is also important. Combination of chlorthion with
malathion and other phosphorothionates is said to result in a
potentiation of its oral toxicity to mice (Rosenberg & Coon, 1958).
Short-term studies
Rat. Intraperitoneal injections of undiluted chlorthion to
groups of 5 rats produced no mortality at a dose level of 50 mg/kg
body-weight daily for 60 days; 100 mg/kg body-weight daily for 60 days
resulted in 40% mortality. With 200 mg/kg body-weight daily all
animals died within 5-10 days. The symptoms prior to death were
similar to those observed for more toxic organo-phosphorus
insecticides. Serum cholinesterase activity was decreased more than
that of brain or submaxillary gland, probably owing to slow absorption
into the tissues. At the lowest dose level (50 mg/kg body-weight
daily) the serum cholinesterase activity fell to below 50% of normal
after the first dose (DuBois et al., 1953).
Rats (4 groups of 13 male and 4 groups of 13 female) fed for 120
days on diets containing 10, 20 and 50 ppm of chlorthion showed no
significant change in growth rate, food consumption, or general
appearance and no symptoms of cholinergic stimulation. Pathological
examination showed no difference in the gross or microscopic features
of the tissues compared with control animals. The animals fed at the
50 ppm level showed slight reduction in brain and submaxillary gland
cholinesterase activity levels and a marked decrease in serum
cholinesterase activity amounting to 76% inhibition. Reduction in
serum cholinesterase activity was also noted at the 20 ppm level (61%
inhibition) and at 10 ppm (27% inhibition). Reversal of the inhibition
occurred within 2 weeks following removal of chlorthion from the diet
(DuBois et al., 1956).
Dog. Chlorthion fed to 4 dogs (2 male, 2 female) at dose levels
of 0.5, 2, 5 and 15 ppm in the diet produced no depression of plasma
and erythrocyte cholinesterase levels at 0.5, 2 and 5 ppm and gave
only a questionable depression at 15 ppm over a period of 12 weeks
(Williams et al., 1959).
Long-term studies
No data available.
Comments on the experimental studies reported
Although reliable acute toxicity data are available, no long-term
toxicity tests on animals have been reported. No information is
available about toxicity in man.
EVALUATION
Level causing no significant toxic effects in the rat and dog
The data so far reported are not sufficient for an estimate to be
made of an acceptable daily intake for man, especially as long-term
studies are indicated because of the chemical nature of this compound.
Further work required
Chemical composition and toxicity of the residues. Observations
on the effect in man. Long-term studies in rats including reproduction
studies.
REFERENCES
DuBois, K. P., Doull, J., Deroin, J. & Cummings, O. K. (1953)
Arch. industr. Hyg., 8, 350-8
DuBois, K. P., Doull, J. & Rehfuss, P. A. (1956) Report from
Department of Pharmacology, University of Chicago
Fallscheer, H. O. & Cook, J. W. (1956) J. Assoc. official Agr.
Chem., 39, 691
Klimmer, O. R. & Pfaff, W. (1955) Arzneimitt-Forsch, 5
Plapp, P. W. & Casida, J. E. (1958) J. econ. Ent., 51, 800
Rosenberg, P. & Coon, J. M. (1958) Proc. Soc. exp. Biol. (N.Y.),
97, 836-9
Williams, M. W., Fuyat, H. N. & Fitzhugh, O. G. (1959) Toxicol.
Appl. Pharmacol., 1, 1-7
