
FAO Meeting Report No. PL/1965/10/1
WHO/Food Add./27.65
EVALUATION OF THE TOXICITY OF PESTICIDE RESIDUES IN FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Committee on Pesticides in Agriculture and
the WHO Expert Committee on Pesticide Residues, which met in Rome,
15-22 March 19651
Food and Agriculture Organization of the United Nations
World Health Organization
1965
1 Report of the second joint meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Committee on Pesticide
Residues, FAO Meeting Report No. PL/1965/10; WHO/Food Add./26.65
DEMETON
Chemical name
Demeton consists of a mixture of two isomers;
(X) Demeton-O and (II) Demeton-S.
(I) diethyl 2-(ethylthio)ethyl phosphorothionate; O,O-diethyl
O-[2-(ethylthio)ethyl]phosphorothioate.
(II) diethyl S-[2-(ethylthio)ethyl]phosphorothiolate;
O,O-diethyl S[2-(ethylthio)ethyl]-phosphorothioate.
Synonyms
(I) P=S systox; thiono systox; mercaptofos; systox.
(II) P=O systox; isosystox; thiol systox; mercaptofos tiolovy.
Empirical formula
C8H19O3PS2
Structural formula
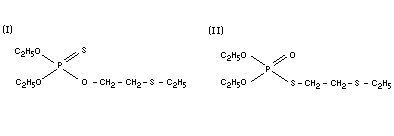 BIOLOGICAL DATA
Biochemical aspects
The thiono and thiol isomers of demeton are metabolized by plants
and animals, degraded to a number of derivatives. In the mouse, 50-70%
of an orally-administered dose is eliminated in the urine within 24
hours. The principal pathway of metabolism for both isomers is the
oxidation of the mercapto sulfur of the ethyl mecapto ethyl moiety to
the sulfoxide and sulfone.
A secondary pathway involves the oxidation of the thiono sulfur
to produce the phosphate and its sulfoxide and sulfone. Finally, both
isomers and their metabolites are degraded by hydrolysis of the
P-O- or P-S- bond to form the alcohol and acid, with the exception of
the sulfone of demeton-S which gives ethyl vinyl sulfone and diethyl
phosphorothioate (Fukuto et al., 1955; March et al., 1955).
The demeton isomers and a number of derivatives are highly active
in vivo and in vitro as cholinesterase inhibitors.
In vitro: molar concentrations necessary to produce 50%
cholinesterase inhibition expressed as I50 are as follows (Barnes &
Denz, 1954; DuBois et al., 1956; Wirth, 1958).
The sulfoxide and the sulfone of Demeton-O are less toxic than
the parent compound (LD50 respectively for the rat, oral, 100 mg/kg
and 90 mg/kg body-weight) (Wirth, 1958). The sulfoxide and the sulfone
of Demeton-S have about the same toxicity as technical demeton, the
oral LD50 for the rat being approximately 2.0 for both derivatives
(DuBois et al., 1956; Wirth, 1958).
Short-term studies
Rat. Fifty ppm of demeton in the diet for 112 days produced the
typical symptoms of depressed cholinesterase activity, especially in
the first week. No histological changes were observed. Three ppm for
77 days caused only a small inhibition of cholinesterase (Schrader,
1957).
Groups of 12 to 18 female rats fed 50 ppm (48% Demeton-S) for 16
weeks showed signs of poisoning and the growth-rate was affected. No
histological changes were observed. Groups receiving 10 and 20 ppm
showed severe inhibition of the brain and blood cholinesterase
activity, while 3 ppm still gave 30% inhibition of blood
cholinesterase activity. One ppm did not cause significant inhibition
(Barnes & Denz, 1954).
The daily administration of 0.75 mg/kg of Demeton-S for 3 days
caused a progressive decrease in the cholinesterase activity of the
brain, serum and submaxillary glands (DuBois et al., 1956).
Groups of 5 rats were given 65 oral doses of 0.4, 0.66 and 1.89
mg/kg body-weight of technical demeton over a period of 90 days. One
animal at the highest dose level died. No other signs of intoxication
were seen (Deichmann & Rakoczy, 1955).
Guinea-pig. With daily doses of 2.5 mg of demeton (30%
Demeton-S per kg body-weight) for 48 days, no toxic changes were
observed (10 animals), but there was an inhibition of blood
cholinesterase activity (Bär, 1954).
Dog. Groups of 2 dogs (one female and one male) were given
technical demeton for 24 weeks. No significant depression of plasma
cholinesterase activity was found with 0.025 mg/kg body-weight per
day. With 0.047 mg/kg body-weight per day significant plasma
inhibition was found after 16 weeks (Frawley & Fuyat, 1957).
I50 × 10-6 M in 30 minutes at 37°C
Rat Human Human Rat References
brain serum erythrocyte plasma
cholinesterase cholinesterase cholinesterase cholinesterase
Demeton-S (48%) 4.0 2.4 Mühlmann & Tietz, 1956
Demeton-O 4.26* 1.9* 4.65* DuBois et al., 1956
Sulfoxide 4.75* 3.1* 6.2* "
Sulfone 48.30* 17.2* 51.0* "
Demeton-S 0.078* 0.078* 0.31* "
0.21 Barnes & Denz, 1954
Sulfoxide 3.58* 1.5* 2.56* DuBois et al., 1956
2.2 Barnes & Denz, 1954
Sulfone 2.86* 0.52* 1.69* DuBois et al., 1956
2.0 Barnes & Denz, 1954
* In 40 minutes at 37° celsius
Animal Route Substance LD50 mg/kg References
body-weight
Mouse Intraperitoneal Demeton (Demeton-S) 5.6-7 DuBois et al., 1956
Mühlmann & Tietz, 1956
Oral Demeton (technical) 2.5-14 Barnes & Denz, 1954
(diverse mixtures) Borgmann & Hunold, 1954
Gaines, 1960
Metcalf, 1955
Schrader, 1957
Oral Demeton (Demeton-O) 7.5-30 Metcalf, 1955
Schrader, 1957
Wirth, 1958
Oral Demeton (Demeton-O) 1.5-16.2 Borgmann & Hunold, 1954
DuBois et al., 1956
Metcalf, 1955
Schrader, 1957
Wirth, 1958
Intraperitoneal Demeton 3 DuBois & Coon, 1955
Intraperitoneal Demeton (Demeton-S) 1.5 DuBois et al., 1956
Guinea-pig Intraperitoneal Demeton (Demeton-S) 5.5 DuBois et al., 1956
Acute toxicity
Cattle. Technical demeton (0.1 mg/kg body-weight) fed in
capsules to a cow for 3 consecutive days caused severe symptom of
poisoning. After feeding demeton-treated hay containing an average
concentration of 41 ppm to 3 cows for 56 days, no adverse effects on
weight changes and milk production were observed, but during the last
6 days of feeding them was a decrease in erythrocyte cholinesterase
activity (Dahm & Jacobson, 1956).
Man. Groups of 5 subjects were given demeton for 30 days, in
the following amounts: 4.125 mg, 4.5 mg, 4.875 mg per day. Each period
was followed by a second control period of 30 days. The plasma and
erythrocyte cholinesterase activity remained within 20% of the control
values during the administration of demeton (Moeller & Rider, 1965).
Long-term studies
No data available.
Comments on experimental studies reported
Long-term toxicity tests on rats using demeton have not been
carried out. The toxicity of technical demeton is dependent upon the
proportion of each isomer in the mixture. The toxic effect of demeton
results from the action of the unchanged isomers and, moreover, of
different metabolic products. Demeton-S and its metabolic products are
more toxic than Demeton-O.
EVALUATION
Level causing no significant toxicological effect in animals
Rat. The estimated maximum no-effect level is 1 ppm, equivalent
to 0.05 mg/kg body-weight.
Dog. 0.025 mg/kg body-weight seems to be the no-effect level.
Man. In man a dose equivalent to approximately 0.07 mg/kg
body-weight per day showed no effect.
Estimate of acceptable daily intake for man
0-0.0025 mg/kg body-weight per day.
This value is based on experiments carried out with specimens of
technical demeton and does not therefore take account of the chemical
changes in the crop.
Further work desirable
Chemical composition and toxicity of the residues. Reproduction
studies in the rat.
REFERENCES
Bär, F. (1954) Arzneimittel-Forsch., 4. 668
Barnes, J. M. & Denz, F. A. (1954) Brit. J. Industr. Med., 11, 11
Borgmann, W. & Hanold, G. A. (1954) Report from Max von Pettenkofer
Institute
Dahm, P. A. & Jacobson, N. L. (1956) J. Agric. Food Chem., 4, 150
Deichmann, W. B. & Rakoczy, R. (1955) Arch. industr. Hlth, 11, 324
DuBois, K. P. & Coon, M. J. (1955) Arch. industr. Hyg., 6, 9
DuBois, K. P., Murphy, S. D. & Thursh, D. R. (1956) Arch. industr.
Hlth., 13, 606
Frawley, J. P. & Fuyat, H. N. (1957) J. Agric. Food Chem., 5, 346
Fukuto, T. R., Metcalf, R. L., March, R. B. & Maxon, M. G. (1955)
J. econ. Ent., 48, 347
Gaines, Th. B. (1960) Toxicol. Appl. Pharmacol., 2, 88-99
March, R. B., Metcalf, R. L., Fukuto, T. R. & Maxon, M. G. (1955)
J. econ. Ent., 48, 355
Metcalf, R. L. (1955) Organic Insecticides, Interscience, New York
Moeller, H. C. & Rider, J. A. (1963) Fed. Proc., 22 (171), 187
Mühlmann, R. & Tietz, H. (1956) Höfchen-Briefe, 9, 116
Schrader, G. (1957) Communication to Farbenfabriken Bayer AG,
Leverkusen, Agnew. Chemie, 69, 86
Wirth, W. (1958) Arch. exp. Path. Pharmacol., 234, 352
BIOLOGICAL DATA
Biochemical aspects
The thiono and thiol isomers of demeton are metabolized by plants
and animals, degraded to a number of derivatives. In the mouse, 50-70%
of an orally-administered dose is eliminated in the urine within 24
hours. The principal pathway of metabolism for both isomers is the
oxidation of the mercapto sulfur of the ethyl mecapto ethyl moiety to
the sulfoxide and sulfone.
A secondary pathway involves the oxidation of the thiono sulfur
to produce the phosphate and its sulfoxide and sulfone. Finally, both
isomers and their metabolites are degraded by hydrolysis of the
P-O- or P-S- bond to form the alcohol and acid, with the exception of
the sulfone of demeton-S which gives ethyl vinyl sulfone and diethyl
phosphorothioate (Fukuto et al., 1955; March et al., 1955).
The demeton isomers and a number of derivatives are highly active
in vivo and in vitro as cholinesterase inhibitors.
In vitro: molar concentrations necessary to produce 50%
cholinesterase inhibition expressed as I50 are as follows (Barnes &
Denz, 1954; DuBois et al., 1956; Wirth, 1958).
The sulfoxide and the sulfone of Demeton-O are less toxic than
the parent compound (LD50 respectively for the rat, oral, 100 mg/kg
and 90 mg/kg body-weight) (Wirth, 1958). The sulfoxide and the sulfone
of Demeton-S have about the same toxicity as technical demeton, the
oral LD50 for the rat being approximately 2.0 for both derivatives
(DuBois et al., 1956; Wirth, 1958).
Short-term studies
Rat. Fifty ppm of demeton in the diet for 112 days produced the
typical symptoms of depressed cholinesterase activity, especially in
the first week. No histological changes were observed. Three ppm for
77 days caused only a small inhibition of cholinesterase (Schrader,
1957).
Groups of 12 to 18 female rats fed 50 ppm (48% Demeton-S) for 16
weeks showed signs of poisoning and the growth-rate was affected. No
histological changes were observed. Groups receiving 10 and 20 ppm
showed severe inhibition of the brain and blood cholinesterase
activity, while 3 ppm still gave 30% inhibition of blood
cholinesterase activity. One ppm did not cause significant inhibition
(Barnes & Denz, 1954).
The daily administration of 0.75 mg/kg of Demeton-S for 3 days
caused a progressive decrease in the cholinesterase activity of the
brain, serum and submaxillary glands (DuBois et al., 1956).
Groups of 5 rats were given 65 oral doses of 0.4, 0.66 and 1.89
mg/kg body-weight of technical demeton over a period of 90 days. One
animal at the highest dose level died. No other signs of intoxication
were seen (Deichmann & Rakoczy, 1955).
Guinea-pig. With daily doses of 2.5 mg of demeton (30%
Demeton-S per kg body-weight) for 48 days, no toxic changes were
observed (10 animals), but there was an inhibition of blood
cholinesterase activity (Bär, 1954).
Dog. Groups of 2 dogs (one female and one male) were given
technical demeton for 24 weeks. No significant depression of plasma
cholinesterase activity was found with 0.025 mg/kg body-weight per
day. With 0.047 mg/kg body-weight per day significant plasma
inhibition was found after 16 weeks (Frawley & Fuyat, 1957).
I50 × 10-6 M in 30 minutes at 37°C
Rat Human Human Rat References
brain serum erythrocyte plasma
cholinesterase cholinesterase cholinesterase cholinesterase
Demeton-S (48%) 4.0 2.4 Mühlmann & Tietz, 1956
Demeton-O 4.26* 1.9* 4.65* DuBois et al., 1956
Sulfoxide 4.75* 3.1* 6.2* "
Sulfone 48.30* 17.2* 51.0* "
Demeton-S 0.078* 0.078* 0.31* "
0.21 Barnes & Denz, 1954
Sulfoxide 3.58* 1.5* 2.56* DuBois et al., 1956
2.2 Barnes & Denz, 1954
Sulfone 2.86* 0.52* 1.69* DuBois et al., 1956
2.0 Barnes & Denz, 1954
* In 40 minutes at 37° celsius
Animal Route Substance LD50 mg/kg References
body-weight
Mouse Intraperitoneal Demeton (Demeton-S) 5.6-7 DuBois et al., 1956
Mühlmann & Tietz, 1956
Oral Demeton (technical) 2.5-14 Barnes & Denz, 1954
(diverse mixtures) Borgmann & Hunold, 1954
Gaines, 1960
Metcalf, 1955
Schrader, 1957
Oral Demeton (Demeton-O) 7.5-30 Metcalf, 1955
Schrader, 1957
Wirth, 1958
Oral Demeton (Demeton-O) 1.5-16.2 Borgmann & Hunold, 1954
DuBois et al., 1956
Metcalf, 1955
Schrader, 1957
Wirth, 1958
Intraperitoneal Demeton 3 DuBois & Coon, 1955
Intraperitoneal Demeton (Demeton-S) 1.5 DuBois et al., 1956
Guinea-pig Intraperitoneal Demeton (Demeton-S) 5.5 DuBois et al., 1956
Acute toxicity
Cattle. Technical demeton (0.1 mg/kg body-weight) fed in
capsules to a cow for 3 consecutive days caused severe symptom of
poisoning. After feeding demeton-treated hay containing an average
concentration of 41 ppm to 3 cows for 56 days, no adverse effects on
weight changes and milk production were observed, but during the last
6 days of feeding them was a decrease in erythrocyte cholinesterase
activity (Dahm & Jacobson, 1956).
Man. Groups of 5 subjects were given demeton for 30 days, in
the following amounts: 4.125 mg, 4.5 mg, 4.875 mg per day. Each period
was followed by a second control period of 30 days. The plasma and
erythrocyte cholinesterase activity remained within 20% of the control
values during the administration of demeton (Moeller & Rider, 1965).
Long-term studies
No data available.
Comments on experimental studies reported
Long-term toxicity tests on rats using demeton have not been
carried out. The toxicity of technical demeton is dependent upon the
proportion of each isomer in the mixture. The toxic effect of demeton
results from the action of the unchanged isomers and, moreover, of
different metabolic products. Demeton-S and its metabolic products are
more toxic than Demeton-O.
EVALUATION
Level causing no significant toxicological effect in animals
Rat. The estimated maximum no-effect level is 1 ppm, equivalent
to 0.05 mg/kg body-weight.
Dog. 0.025 mg/kg body-weight seems to be the no-effect level.
Man. In man a dose equivalent to approximately 0.07 mg/kg
body-weight per day showed no effect.
Estimate of acceptable daily intake for man
0-0.0025 mg/kg body-weight per day.
This value is based on experiments carried out with specimens of
technical demeton and does not therefore take account of the chemical
changes in the crop.
Further work desirable
Chemical composition and toxicity of the residues. Reproduction
studies in the rat.
REFERENCES
Bär, F. (1954) Arzneimittel-Forsch., 4. 668
Barnes, J. M. & Denz, F. A. (1954) Brit. J. Industr. Med., 11, 11
Borgmann, W. & Hanold, G. A. (1954) Report from Max von Pettenkofer
Institute
Dahm, P. A. & Jacobson, N. L. (1956) J. Agric. Food Chem., 4, 150
Deichmann, W. B. & Rakoczy, R. (1955) Arch. industr. Hlth, 11, 324
DuBois, K. P. & Coon, M. J. (1955) Arch. industr. Hyg., 6, 9
DuBois, K. P., Murphy, S. D. & Thursh, D. R. (1956) Arch. industr.
Hlth., 13, 606
Frawley, J. P. & Fuyat, H. N. (1957) J. Agric. Food Chem., 5, 346
Fukuto, T. R., Metcalf, R. L., March, R. B. & Maxon, M. G. (1955)
J. econ. Ent., 48, 347
Gaines, Th. B. (1960) Toxicol. Appl. Pharmacol., 2, 88-99
March, R. B., Metcalf, R. L., Fukuto, T. R. & Maxon, M. G. (1955)
J. econ. Ent., 48, 355
Metcalf, R. L. (1955) Organic Insecticides, Interscience, New York
Moeller, H. C. & Rider, J. A. (1963) Fed. Proc., 22 (171), 187
Mühlmann, R. & Tietz, H. (1956) Höfchen-Briefe, 9, 116
Schrader, G. (1957) Communication to Farbenfabriken Bayer AG,
Leverkusen, Agnew. Chemie, 69, 86
Wirth, W. (1958) Arch. exp. Path. Pharmacol., 234, 352
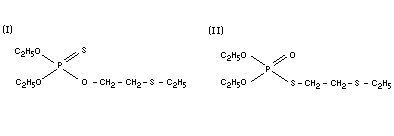 BIOLOGICAL DATA
Biochemical aspects
The thiono and thiol isomers of demeton are metabolized by plants
and animals, degraded to a number of derivatives. In the mouse, 50-70%
of an orally-administered dose is eliminated in the urine within 24
hours. The principal pathway of metabolism for both isomers is the
oxidation of the mercapto sulfur of the ethyl mecapto ethyl moiety to
the sulfoxide and sulfone.
A secondary pathway involves the oxidation of the thiono sulfur
to produce the phosphate and its sulfoxide and sulfone. Finally, both
isomers and their metabolites are degraded by hydrolysis of the
P-O- or P-S- bond to form the alcohol and acid, with the exception of
the sulfone of demeton-S which gives ethyl vinyl sulfone and diethyl
phosphorothioate (Fukuto et al., 1955; March et al., 1955).
The demeton isomers and a number of derivatives are highly active
in vivo and in vitro as cholinesterase inhibitors.
In vitro: molar concentrations necessary to produce 50%
cholinesterase inhibition expressed as I50 are as follows (Barnes &
Denz, 1954; DuBois et al., 1956; Wirth, 1958).
The sulfoxide and the sulfone of Demeton-O are less toxic than
the parent compound (LD50 respectively for the rat, oral, 100 mg/kg
and 90 mg/kg body-weight) (Wirth, 1958). The sulfoxide and the sulfone
of Demeton-S have about the same toxicity as technical demeton, the
oral LD50 for the rat being approximately 2.0 for both derivatives
(DuBois et al., 1956; Wirth, 1958).
Short-term studies
Rat. Fifty ppm of demeton in the diet for 112 days produced the
typical symptoms of depressed cholinesterase activity, especially in
the first week. No histological changes were observed. Three ppm for
77 days caused only a small inhibition of cholinesterase (Schrader,
1957).
Groups of 12 to 18 female rats fed 50 ppm (48% Demeton-S) for 16
weeks showed signs of poisoning and the growth-rate was affected. No
histological changes were observed. Groups receiving 10 and 20 ppm
showed severe inhibition of the brain and blood cholinesterase
activity, while 3 ppm still gave 30% inhibition of blood
cholinesterase activity. One ppm did not cause significant inhibition
(Barnes & Denz, 1954).
The daily administration of 0.75 mg/kg of Demeton-S for 3 days
caused a progressive decrease in the cholinesterase activity of the
brain, serum and submaxillary glands (DuBois et al., 1956).
Groups of 5 rats were given 65 oral doses of 0.4, 0.66 and 1.89
mg/kg body-weight of technical demeton over a period of 90 days. One
animal at the highest dose level died. No other signs of intoxication
were seen (Deichmann & Rakoczy, 1955).
Guinea-pig. With daily doses of 2.5 mg of demeton (30%
Demeton-S per kg body-weight) for 48 days, no toxic changes were
observed (10 animals), but there was an inhibition of blood
cholinesterase activity (Bär, 1954).
Dog. Groups of 2 dogs (one female and one male) were given
technical demeton for 24 weeks. No significant depression of plasma
cholinesterase activity was found with 0.025 mg/kg body-weight per
day. With 0.047 mg/kg body-weight per day significant plasma
inhibition was found after 16 weeks (Frawley & Fuyat, 1957).
I50 × 10-6 M in 30 minutes at 37°C
Rat Human Human Rat References
brain serum erythrocyte plasma
cholinesterase cholinesterase cholinesterase cholinesterase
Demeton-S (48%) 4.0 2.4 Mühlmann & Tietz, 1956
Demeton-O 4.26* 1.9* 4.65* DuBois et al., 1956
Sulfoxide 4.75* 3.1* 6.2* "
Sulfone 48.30* 17.2* 51.0* "
Demeton-S 0.078* 0.078* 0.31* "
0.21 Barnes & Denz, 1954
Sulfoxide 3.58* 1.5* 2.56* DuBois et al., 1956
2.2 Barnes & Denz, 1954
Sulfone 2.86* 0.52* 1.69* DuBois et al., 1956
2.0 Barnes & Denz, 1954
* In 40 minutes at 37° celsius
Animal Route Substance LD50 mg/kg References
body-weight
Mouse Intraperitoneal Demeton (Demeton-S) 5.6-7 DuBois et al., 1956
Mühlmann & Tietz, 1956
Oral Demeton (technical) 2.5-14 Barnes & Denz, 1954
(diverse mixtures) Borgmann & Hunold, 1954
Gaines, 1960
Metcalf, 1955
Schrader, 1957
Oral Demeton (Demeton-O) 7.5-30 Metcalf, 1955
Schrader, 1957
Wirth, 1958
Oral Demeton (Demeton-O) 1.5-16.2 Borgmann & Hunold, 1954
DuBois et al., 1956
Metcalf, 1955
Schrader, 1957
Wirth, 1958
Intraperitoneal Demeton 3 DuBois & Coon, 1955
Intraperitoneal Demeton (Demeton-S) 1.5 DuBois et al., 1956
Guinea-pig Intraperitoneal Demeton (Demeton-S) 5.5 DuBois et al., 1956
Acute toxicity
Cattle. Technical demeton (0.1 mg/kg body-weight) fed in
capsules to a cow for 3 consecutive days caused severe symptom of
poisoning. After feeding demeton-treated hay containing an average
concentration of 41 ppm to 3 cows for 56 days, no adverse effects on
weight changes and milk production were observed, but during the last
6 days of feeding them was a decrease in erythrocyte cholinesterase
activity (Dahm & Jacobson, 1956).
Man. Groups of 5 subjects were given demeton for 30 days, in
the following amounts: 4.125 mg, 4.5 mg, 4.875 mg per day. Each period
was followed by a second control period of 30 days. The plasma and
erythrocyte cholinesterase activity remained within 20% of the control
values during the administration of demeton (Moeller & Rider, 1965).
Long-term studies
No data available.
Comments on experimental studies reported
Long-term toxicity tests on rats using demeton have not been
carried out. The toxicity of technical demeton is dependent upon the
proportion of each isomer in the mixture. The toxic effect of demeton
results from the action of the unchanged isomers and, moreover, of
different metabolic products. Demeton-S and its metabolic products are
more toxic than Demeton-O.
EVALUATION
Level causing no significant toxicological effect in animals
Rat. The estimated maximum no-effect level is 1 ppm, equivalent
to 0.05 mg/kg body-weight.
Dog. 0.025 mg/kg body-weight seems to be the no-effect level.
Man. In man a dose equivalent to approximately 0.07 mg/kg
body-weight per day showed no effect.
Estimate of acceptable daily intake for man
0-0.0025 mg/kg body-weight per day.
This value is based on experiments carried out with specimens of
technical demeton and does not therefore take account of the chemical
changes in the crop.
Further work desirable
Chemical composition and toxicity of the residues. Reproduction
studies in the rat.
REFERENCES
Bär, F. (1954) Arzneimittel-Forsch., 4. 668
Barnes, J. M. & Denz, F. A. (1954) Brit. J. Industr. Med., 11, 11
Borgmann, W. & Hanold, G. A. (1954) Report from Max von Pettenkofer
Institute
Dahm, P. A. & Jacobson, N. L. (1956) J. Agric. Food Chem., 4, 150
Deichmann, W. B. & Rakoczy, R. (1955) Arch. industr. Hlth, 11, 324
DuBois, K. P. & Coon, M. J. (1955) Arch. industr. Hyg., 6, 9
DuBois, K. P., Murphy, S. D. & Thursh, D. R. (1956) Arch. industr.
Hlth., 13, 606
Frawley, J. P. & Fuyat, H. N. (1957) J. Agric. Food Chem., 5, 346
Fukuto, T. R., Metcalf, R. L., March, R. B. & Maxon, M. G. (1955)
J. econ. Ent., 48, 347
Gaines, Th. B. (1960) Toxicol. Appl. Pharmacol., 2, 88-99
March, R. B., Metcalf, R. L., Fukuto, T. R. & Maxon, M. G. (1955)
J. econ. Ent., 48, 355
Metcalf, R. L. (1955) Organic Insecticides, Interscience, New York
Moeller, H. C. & Rider, J. A. (1963) Fed. Proc., 22 (171), 187
Mühlmann, R. & Tietz, H. (1956) Höfchen-Briefe, 9, 116
Schrader, G. (1957) Communication to Farbenfabriken Bayer AG,
Leverkusen, Agnew. Chemie, 69, 86
Wirth, W. (1958) Arch. exp. Path. Pharmacol., 234, 352
BIOLOGICAL DATA
Biochemical aspects
The thiono and thiol isomers of demeton are metabolized by plants
and animals, degraded to a number of derivatives. In the mouse, 50-70%
of an orally-administered dose is eliminated in the urine within 24
hours. The principal pathway of metabolism for both isomers is the
oxidation of the mercapto sulfur of the ethyl mecapto ethyl moiety to
the sulfoxide and sulfone.
A secondary pathway involves the oxidation of the thiono sulfur
to produce the phosphate and its sulfoxide and sulfone. Finally, both
isomers and their metabolites are degraded by hydrolysis of the
P-O- or P-S- bond to form the alcohol and acid, with the exception of
the sulfone of demeton-S which gives ethyl vinyl sulfone and diethyl
phosphorothioate (Fukuto et al., 1955; March et al., 1955).
The demeton isomers and a number of derivatives are highly active
in vivo and in vitro as cholinesterase inhibitors.
In vitro: molar concentrations necessary to produce 50%
cholinesterase inhibition expressed as I50 are as follows (Barnes &
Denz, 1954; DuBois et al., 1956; Wirth, 1958).
The sulfoxide and the sulfone of Demeton-O are less toxic than
the parent compound (LD50 respectively for the rat, oral, 100 mg/kg
and 90 mg/kg body-weight) (Wirth, 1958). The sulfoxide and the sulfone
of Demeton-S have about the same toxicity as technical demeton, the
oral LD50 for the rat being approximately 2.0 for both derivatives
(DuBois et al., 1956; Wirth, 1958).
Short-term studies
Rat. Fifty ppm of demeton in the diet for 112 days produced the
typical symptoms of depressed cholinesterase activity, especially in
the first week. No histological changes were observed. Three ppm for
77 days caused only a small inhibition of cholinesterase (Schrader,
1957).
Groups of 12 to 18 female rats fed 50 ppm (48% Demeton-S) for 16
weeks showed signs of poisoning and the growth-rate was affected. No
histological changes were observed. Groups receiving 10 and 20 ppm
showed severe inhibition of the brain and blood cholinesterase
activity, while 3 ppm still gave 30% inhibition of blood
cholinesterase activity. One ppm did not cause significant inhibition
(Barnes & Denz, 1954).
The daily administration of 0.75 mg/kg of Demeton-S for 3 days
caused a progressive decrease in the cholinesterase activity of the
brain, serum and submaxillary glands (DuBois et al., 1956).
Groups of 5 rats were given 65 oral doses of 0.4, 0.66 and 1.89
mg/kg body-weight of technical demeton over a period of 90 days. One
animal at the highest dose level died. No other signs of intoxication
were seen (Deichmann & Rakoczy, 1955).
Guinea-pig. With daily doses of 2.5 mg of demeton (30%
Demeton-S per kg body-weight) for 48 days, no toxic changes were
observed (10 animals), but there was an inhibition of blood
cholinesterase activity (Bär, 1954).
Dog. Groups of 2 dogs (one female and one male) were given
technical demeton for 24 weeks. No significant depression of plasma
cholinesterase activity was found with 0.025 mg/kg body-weight per
day. With 0.047 mg/kg body-weight per day significant plasma
inhibition was found after 16 weeks (Frawley & Fuyat, 1957).
I50 × 10-6 M in 30 minutes at 37°C
Rat Human Human Rat References
brain serum erythrocyte plasma
cholinesterase cholinesterase cholinesterase cholinesterase
Demeton-S (48%) 4.0 2.4 Mühlmann & Tietz, 1956
Demeton-O 4.26* 1.9* 4.65* DuBois et al., 1956
Sulfoxide 4.75* 3.1* 6.2* "
Sulfone 48.30* 17.2* 51.0* "
Demeton-S 0.078* 0.078* 0.31* "
0.21 Barnes & Denz, 1954
Sulfoxide 3.58* 1.5* 2.56* DuBois et al., 1956
2.2 Barnes & Denz, 1954
Sulfone 2.86* 0.52* 1.69* DuBois et al., 1956
2.0 Barnes & Denz, 1954
* In 40 minutes at 37° celsius
Animal Route Substance LD50 mg/kg References
body-weight
Mouse Intraperitoneal Demeton (Demeton-S) 5.6-7 DuBois et al., 1956
Mühlmann & Tietz, 1956
Oral Demeton (technical) 2.5-14 Barnes & Denz, 1954
(diverse mixtures) Borgmann & Hunold, 1954
Gaines, 1960
Metcalf, 1955
Schrader, 1957
Oral Demeton (Demeton-O) 7.5-30 Metcalf, 1955
Schrader, 1957
Wirth, 1958
Oral Demeton (Demeton-O) 1.5-16.2 Borgmann & Hunold, 1954
DuBois et al., 1956
Metcalf, 1955
Schrader, 1957
Wirth, 1958
Intraperitoneal Demeton 3 DuBois & Coon, 1955
Intraperitoneal Demeton (Demeton-S) 1.5 DuBois et al., 1956
Guinea-pig Intraperitoneal Demeton (Demeton-S) 5.5 DuBois et al., 1956
Acute toxicity
Cattle. Technical demeton (0.1 mg/kg body-weight) fed in
capsules to a cow for 3 consecutive days caused severe symptom of
poisoning. After feeding demeton-treated hay containing an average
concentration of 41 ppm to 3 cows for 56 days, no adverse effects on
weight changes and milk production were observed, but during the last
6 days of feeding them was a decrease in erythrocyte cholinesterase
activity (Dahm & Jacobson, 1956).
Man. Groups of 5 subjects were given demeton for 30 days, in
the following amounts: 4.125 mg, 4.5 mg, 4.875 mg per day. Each period
was followed by a second control period of 30 days. The plasma and
erythrocyte cholinesterase activity remained within 20% of the control
values during the administration of demeton (Moeller & Rider, 1965).
Long-term studies
No data available.
Comments on experimental studies reported
Long-term toxicity tests on rats using demeton have not been
carried out. The toxicity of technical demeton is dependent upon the
proportion of each isomer in the mixture. The toxic effect of demeton
results from the action of the unchanged isomers and, moreover, of
different metabolic products. Demeton-S and its metabolic products are
more toxic than Demeton-O.
EVALUATION
Level causing no significant toxicological effect in animals
Rat. The estimated maximum no-effect level is 1 ppm, equivalent
to 0.05 mg/kg body-weight.
Dog. 0.025 mg/kg body-weight seems to be the no-effect level.
Man. In man a dose equivalent to approximately 0.07 mg/kg
body-weight per day showed no effect.
Estimate of acceptable daily intake for man
0-0.0025 mg/kg body-weight per day.
This value is based on experiments carried out with specimens of
technical demeton and does not therefore take account of the chemical
changes in the crop.
Further work desirable
Chemical composition and toxicity of the residues. Reproduction
studies in the rat.
REFERENCES
Bär, F. (1954) Arzneimittel-Forsch., 4. 668
Barnes, J. M. & Denz, F. A. (1954) Brit. J. Industr. Med., 11, 11
Borgmann, W. & Hanold, G. A. (1954) Report from Max von Pettenkofer
Institute
Dahm, P. A. & Jacobson, N. L. (1956) J. Agric. Food Chem., 4, 150
Deichmann, W. B. & Rakoczy, R. (1955) Arch. industr. Hlth, 11, 324
DuBois, K. P. & Coon, M. J. (1955) Arch. industr. Hyg., 6, 9
DuBois, K. P., Murphy, S. D. & Thursh, D. R. (1956) Arch. industr.
Hlth., 13, 606
Frawley, J. P. & Fuyat, H. N. (1957) J. Agric. Food Chem., 5, 346
Fukuto, T. R., Metcalf, R. L., March, R. B. & Maxon, M. G. (1955)
J. econ. Ent., 48, 347
Gaines, Th. B. (1960) Toxicol. Appl. Pharmacol., 2, 88-99
March, R. B., Metcalf, R. L., Fukuto, T. R. & Maxon, M. G. (1955)
J. econ. Ent., 48, 355
Metcalf, R. L. (1955) Organic Insecticides, Interscience, New York
Moeller, H. C. & Rider, J. A. (1963) Fed. Proc., 22 (171), 187
Mühlmann, R. & Tietz, H. (1956) Höfchen-Briefe, 9, 116
Schrader, G. (1957) Communication to Farbenfabriken Bayer AG,
Leverkusen, Agnew. Chemie, 69, 86
Wirth, W. (1958) Arch. exp. Path. Pharmacol., 234, 352
