
FAO Meeting Report No. PL/1965/10/1
WHO/Food Add./27.65
EVALUATION OF THE TOXICITY OF PESTICIDE RESIDUES IN FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Committee on Pesticides in Agriculture and
the WHO Expert Committee on Pesticide Residues, which met in Rome,
15-22 March 19651
Food and Agriculture Organization of the United Nations
World Health Organization
1965
1 Report of the second joint meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Committee on Pesticide
Residues, FAO Meeting Report No. PL/1965/10; WHO/Food Add./26.65
DEMETON-S-METHYL SULFOXIDE
Chemical name
S dimethyl-[2-(ethylthionyl)ethyl]-phosphorothiolate;
O,O-dimethyl-S [2(ethyl-sulfinyl)ethyl]phosphorothioate.
Synonyms
Isomethylsystox-sulfoxide; metasystox R; oxydemeton-methyl;
meta-isosystox sulfoxide.
Empirical formula
C6H15O4PS2
Structural formula
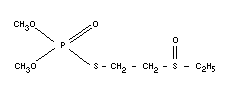 BIOLOGICAL DATA
Biochemical aspects
Demeton-S-methyl sulfoxide is produced in plants from the
metabolism of demeton-S-methyl. The sulfoxide is further broken down
by plants and animals. After injection into mice, 97-98% is rapidly
eliminated (Niessen et al., 1963).
In vitro: Molar concentrations necessary to produce 50%
inhibition of sheep erythrocyte cholinesterase expressed as; I50 in
30 minutes at 37°C are as follows (Heath & Vandekar, 1957):
demeton-S-methyl P=O isomer 6.5 × 10-5
demeton-S-methyl sulfoxide 4.1 × 10-5
demeton-S-methyl sulfone 2.3 × 10-5
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse Oral 30 DuBois & Plzak, 1962
Mouse Intraperitoneal 8-12 DuBois & Plzak, 1962
DuBois & Plzak, 1962
Rat Oral 30-75 Mühlmann & Tietz, 1956
Schrader, 1963
Rat Intraperitoneal 20 DuBois & Plzak, 1962
Rat Intravenous 47 Heath & Vandekar, 1957
Guinea-pig Oral 120 DuBois & Plzak, 1962
Guinea-pig Intraperitoneal 30 DuBois & Plzak, 1962
Rat. In groups of 20 rats, administration of the sulfoxide by
mouth in doses of 5 mg/kg body-weight daily for 3 months caused no
signs of intoxication or pathological changes, and 10 mg/kg
body-weight for 21 days caused an inhibition of cholinesterase
activity after 4.6 days (Wirth, 1958).
Groups of 6 males and 6 female rats received concentrations of 20
ppm or less in the diet for a period of 16 weeks: no significant
influence on growth-rate or food consumption was observed. Ten ppm or
less caused no significant depression of erythrocyte cholinesterase
activity. Gross and microscopic examination of the tissues of rats
revealed no indication of toxic effects except for fatty changes in
the livers of some of the rats fed 10 ppm and 20 ppm (Bär, 1963). 50
ppm for 6 months had no effect on weight gains in a group of 6 rats
and showed no pathological changes attributable to the action of the
compound. The brain and blood cholinesterase activity was strongly
inhibited (Vandekar, 1958). Concentrations of 100 and 200 ppm produced
signs of intoxication in the first 3 weeks of the experiment.
Dog. Diets containing 5, 10 and 20 ppm have been fed to male
and female beagle dogs for periods of 12 weeks. None of these dose
levels produced significant changes in food consumption or body-weight
or gave rise to cholinergic symptoms. Levels of 10 ppm or less did not
cause significant inhibition of serum or erythrocyte cholinesterase
activity (Root et al., 1963).
Long-term studies
No data available.
Comments on experimental studies reported
When compared with highly purified demeton-S-methyl,
demeton-S-methyl sulfoxide is about 30% more toxic than
demeton-S-methyl. The inhibition of cholinesterase activity is
greater. Long-term toxicity tests on rats have not been carried out.
EVALUATION
Level causing no significant toxicological effect
Rat. 10 ppm, equivalent to 1 mg/kg body-weight, causes no
significant inhibition of cholinesterase.
Dog. 10 ppm - equivalent to 0.25 mg/kg body-weight per day did
not show an effect.
Estimate of acceptable daily intake for man
0-0.0025 mg/kg body-weight per day.
Further work desirable
Chemical composition and toxicity of the residues. Observations
on the effect in man. Reproduction studies in the rat.
REFERENCES
Bär, F. (1963) Personal communication
DuBois, K. & Plzak, G. J. (1962) Toxicol. Appl. Pharmacol., 4, 621
Heath, D. F. & Vandekar, M. (1957) Biochem. J., 67, 187
Mühlmann, R. & Tietz, H. (1956) Höfchen-Briefe, 9, 116
Niessen, H., Tietz, H., Hecht, J. & Kimmerli, G. (1963)
Arch. Toxikol., 20. 44
Root, M., Gowan, J. & Doull, J. (1963) Confidential report.
Schrader, G. (1963) Die Entwicklung neuer insectizider
Phosphorsäure-Ester, Verlag Chemie GMBH, Weinheim
Vandekar, M. (1958) Brit. J. industr. Med., 15, 158
Wirth, W. (1958) Arch. exp. Path. Phamacol., 234, 352
BIOLOGICAL DATA
Biochemical aspects
Demeton-S-methyl sulfoxide is produced in plants from the
metabolism of demeton-S-methyl. The sulfoxide is further broken down
by plants and animals. After injection into mice, 97-98% is rapidly
eliminated (Niessen et al., 1963).
In vitro: Molar concentrations necessary to produce 50%
inhibition of sheep erythrocyte cholinesterase expressed as; I50 in
30 minutes at 37°C are as follows (Heath & Vandekar, 1957):
demeton-S-methyl P=O isomer 6.5 × 10-5
demeton-S-methyl sulfoxide 4.1 × 10-5
demeton-S-methyl sulfone 2.3 × 10-5
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse Oral 30 DuBois & Plzak, 1962
Mouse Intraperitoneal 8-12 DuBois & Plzak, 1962
DuBois & Plzak, 1962
Rat Oral 30-75 Mühlmann & Tietz, 1956
Schrader, 1963
Rat Intraperitoneal 20 DuBois & Plzak, 1962
Rat Intravenous 47 Heath & Vandekar, 1957
Guinea-pig Oral 120 DuBois & Plzak, 1962
Guinea-pig Intraperitoneal 30 DuBois & Plzak, 1962
Rat. In groups of 20 rats, administration of the sulfoxide by
mouth in doses of 5 mg/kg body-weight daily for 3 months caused no
signs of intoxication or pathological changes, and 10 mg/kg
body-weight for 21 days caused an inhibition of cholinesterase
activity after 4.6 days (Wirth, 1958).
Groups of 6 males and 6 female rats received concentrations of 20
ppm or less in the diet for a period of 16 weeks: no significant
influence on growth-rate or food consumption was observed. Ten ppm or
less caused no significant depression of erythrocyte cholinesterase
activity. Gross and microscopic examination of the tissues of rats
revealed no indication of toxic effects except for fatty changes in
the livers of some of the rats fed 10 ppm and 20 ppm (Bär, 1963). 50
ppm for 6 months had no effect on weight gains in a group of 6 rats
and showed no pathological changes attributable to the action of the
compound. The brain and blood cholinesterase activity was strongly
inhibited (Vandekar, 1958). Concentrations of 100 and 200 ppm produced
signs of intoxication in the first 3 weeks of the experiment.
Dog. Diets containing 5, 10 and 20 ppm have been fed to male
and female beagle dogs for periods of 12 weeks. None of these dose
levels produced significant changes in food consumption or body-weight
or gave rise to cholinergic symptoms. Levels of 10 ppm or less did not
cause significant inhibition of serum or erythrocyte cholinesterase
activity (Root et al., 1963).
Long-term studies
No data available.
Comments on experimental studies reported
When compared with highly purified demeton-S-methyl,
demeton-S-methyl sulfoxide is about 30% more toxic than
demeton-S-methyl. The inhibition of cholinesterase activity is
greater. Long-term toxicity tests on rats have not been carried out.
EVALUATION
Level causing no significant toxicological effect
Rat. 10 ppm, equivalent to 1 mg/kg body-weight, causes no
significant inhibition of cholinesterase.
Dog. 10 ppm - equivalent to 0.25 mg/kg body-weight per day did
not show an effect.
Estimate of acceptable daily intake for man
0-0.0025 mg/kg body-weight per day.
Further work desirable
Chemical composition and toxicity of the residues. Observations
on the effect in man. Reproduction studies in the rat.
REFERENCES
Bär, F. (1963) Personal communication
DuBois, K. & Plzak, G. J. (1962) Toxicol. Appl. Pharmacol., 4, 621
Heath, D. F. & Vandekar, M. (1957) Biochem. J., 67, 187
Mühlmann, R. & Tietz, H. (1956) Höfchen-Briefe, 9, 116
Niessen, H., Tietz, H., Hecht, J. & Kimmerli, G. (1963)
Arch. Toxikol., 20. 44
Root, M., Gowan, J. & Doull, J. (1963) Confidential report.
Schrader, G. (1963) Die Entwicklung neuer insectizider
Phosphorsäure-Ester, Verlag Chemie GMBH, Weinheim
Vandekar, M. (1958) Brit. J. industr. Med., 15, 158
Wirth, W. (1958) Arch. exp. Path. Phamacol., 234, 352
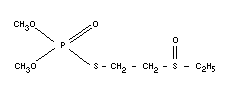 BIOLOGICAL DATA
Biochemical aspects
Demeton-S-methyl sulfoxide is produced in plants from the
metabolism of demeton-S-methyl. The sulfoxide is further broken down
by plants and animals. After injection into mice, 97-98% is rapidly
eliminated (Niessen et al., 1963).
In vitro: Molar concentrations necessary to produce 50%
inhibition of sheep erythrocyte cholinesterase expressed as; I50 in
30 minutes at 37°C are as follows (Heath & Vandekar, 1957):
demeton-S-methyl P=O isomer 6.5 × 10-5
demeton-S-methyl sulfoxide 4.1 × 10-5
demeton-S-methyl sulfone 2.3 × 10-5
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse Oral 30 DuBois & Plzak, 1962
Mouse Intraperitoneal 8-12 DuBois & Plzak, 1962
DuBois & Plzak, 1962
Rat Oral 30-75 Mühlmann & Tietz, 1956
Schrader, 1963
Rat Intraperitoneal 20 DuBois & Plzak, 1962
Rat Intravenous 47 Heath & Vandekar, 1957
Guinea-pig Oral 120 DuBois & Plzak, 1962
Guinea-pig Intraperitoneal 30 DuBois & Plzak, 1962
Rat. In groups of 20 rats, administration of the sulfoxide by
mouth in doses of 5 mg/kg body-weight daily for 3 months caused no
signs of intoxication or pathological changes, and 10 mg/kg
body-weight for 21 days caused an inhibition of cholinesterase
activity after 4.6 days (Wirth, 1958).
Groups of 6 males and 6 female rats received concentrations of 20
ppm or less in the diet for a period of 16 weeks: no significant
influence on growth-rate or food consumption was observed. Ten ppm or
less caused no significant depression of erythrocyte cholinesterase
activity. Gross and microscopic examination of the tissues of rats
revealed no indication of toxic effects except for fatty changes in
the livers of some of the rats fed 10 ppm and 20 ppm (Bär, 1963). 50
ppm for 6 months had no effect on weight gains in a group of 6 rats
and showed no pathological changes attributable to the action of the
compound. The brain and blood cholinesterase activity was strongly
inhibited (Vandekar, 1958). Concentrations of 100 and 200 ppm produced
signs of intoxication in the first 3 weeks of the experiment.
Dog. Diets containing 5, 10 and 20 ppm have been fed to male
and female beagle dogs for periods of 12 weeks. None of these dose
levels produced significant changes in food consumption or body-weight
or gave rise to cholinergic symptoms. Levels of 10 ppm or less did not
cause significant inhibition of serum or erythrocyte cholinesterase
activity (Root et al., 1963).
Long-term studies
No data available.
Comments on experimental studies reported
When compared with highly purified demeton-S-methyl,
demeton-S-methyl sulfoxide is about 30% more toxic than
demeton-S-methyl. The inhibition of cholinesterase activity is
greater. Long-term toxicity tests on rats have not been carried out.
EVALUATION
Level causing no significant toxicological effect
Rat. 10 ppm, equivalent to 1 mg/kg body-weight, causes no
significant inhibition of cholinesterase.
Dog. 10 ppm - equivalent to 0.25 mg/kg body-weight per day did
not show an effect.
Estimate of acceptable daily intake for man
0-0.0025 mg/kg body-weight per day.
Further work desirable
Chemical composition and toxicity of the residues. Observations
on the effect in man. Reproduction studies in the rat.
REFERENCES
Bär, F. (1963) Personal communication
DuBois, K. & Plzak, G. J. (1962) Toxicol. Appl. Pharmacol., 4, 621
Heath, D. F. & Vandekar, M. (1957) Biochem. J., 67, 187
Mühlmann, R. & Tietz, H. (1956) Höfchen-Briefe, 9, 116
Niessen, H., Tietz, H., Hecht, J. & Kimmerli, G. (1963)
Arch. Toxikol., 20. 44
Root, M., Gowan, J. & Doull, J. (1963) Confidential report.
Schrader, G. (1963) Die Entwicklung neuer insectizider
Phosphorsäure-Ester, Verlag Chemie GMBH, Weinheim
Vandekar, M. (1958) Brit. J. industr. Med., 15, 158
Wirth, W. (1958) Arch. exp. Path. Phamacol., 234, 352
BIOLOGICAL DATA
Biochemical aspects
Demeton-S-methyl sulfoxide is produced in plants from the
metabolism of demeton-S-methyl. The sulfoxide is further broken down
by plants and animals. After injection into mice, 97-98% is rapidly
eliminated (Niessen et al., 1963).
In vitro: Molar concentrations necessary to produce 50%
inhibition of sheep erythrocyte cholinesterase expressed as; I50 in
30 minutes at 37°C are as follows (Heath & Vandekar, 1957):
demeton-S-methyl P=O isomer 6.5 × 10-5
demeton-S-methyl sulfoxide 4.1 × 10-5
demeton-S-methyl sulfone 2.3 × 10-5
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse Oral 30 DuBois & Plzak, 1962
Mouse Intraperitoneal 8-12 DuBois & Plzak, 1962
DuBois & Plzak, 1962
Rat Oral 30-75 Mühlmann & Tietz, 1956
Schrader, 1963
Rat Intraperitoneal 20 DuBois & Plzak, 1962
Rat Intravenous 47 Heath & Vandekar, 1957
Guinea-pig Oral 120 DuBois & Plzak, 1962
Guinea-pig Intraperitoneal 30 DuBois & Plzak, 1962
Rat. In groups of 20 rats, administration of the sulfoxide by
mouth in doses of 5 mg/kg body-weight daily for 3 months caused no
signs of intoxication or pathological changes, and 10 mg/kg
body-weight for 21 days caused an inhibition of cholinesterase
activity after 4.6 days (Wirth, 1958).
Groups of 6 males and 6 female rats received concentrations of 20
ppm or less in the diet for a period of 16 weeks: no significant
influence on growth-rate or food consumption was observed. Ten ppm or
less caused no significant depression of erythrocyte cholinesterase
activity. Gross and microscopic examination of the tissues of rats
revealed no indication of toxic effects except for fatty changes in
the livers of some of the rats fed 10 ppm and 20 ppm (Bär, 1963). 50
ppm for 6 months had no effect on weight gains in a group of 6 rats
and showed no pathological changes attributable to the action of the
compound. The brain and blood cholinesterase activity was strongly
inhibited (Vandekar, 1958). Concentrations of 100 and 200 ppm produced
signs of intoxication in the first 3 weeks of the experiment.
Dog. Diets containing 5, 10 and 20 ppm have been fed to male
and female beagle dogs for periods of 12 weeks. None of these dose
levels produced significant changes in food consumption or body-weight
or gave rise to cholinergic symptoms. Levels of 10 ppm or less did not
cause significant inhibition of serum or erythrocyte cholinesterase
activity (Root et al., 1963).
Long-term studies
No data available.
Comments on experimental studies reported
When compared with highly purified demeton-S-methyl,
demeton-S-methyl sulfoxide is about 30% more toxic than
demeton-S-methyl. The inhibition of cholinesterase activity is
greater. Long-term toxicity tests on rats have not been carried out.
EVALUATION
Level causing no significant toxicological effect
Rat. 10 ppm, equivalent to 1 mg/kg body-weight, causes no
significant inhibition of cholinesterase.
Dog. 10 ppm - equivalent to 0.25 mg/kg body-weight per day did
not show an effect.
Estimate of acceptable daily intake for man
0-0.0025 mg/kg body-weight per day.
Further work desirable
Chemical composition and toxicity of the residues. Observations
on the effect in man. Reproduction studies in the rat.
REFERENCES
Bär, F. (1963) Personal communication
DuBois, K. & Plzak, G. J. (1962) Toxicol. Appl. Pharmacol., 4, 621
Heath, D. F. & Vandekar, M. (1957) Biochem. J., 67, 187
Mühlmann, R. & Tietz, H. (1956) Höfchen-Briefe, 9, 116
Niessen, H., Tietz, H., Hecht, J. & Kimmerli, G. (1963)
Arch. Toxikol., 20. 44
Root, M., Gowan, J. & Doull, J. (1963) Confidential report.
Schrader, G. (1963) Die Entwicklung neuer insectizider
Phosphorsäure-Ester, Verlag Chemie GMBH, Weinheim
Vandekar, M. (1958) Brit. J. industr. Med., 15, 158
Wirth, W. (1958) Arch. exp. Path. Phamacol., 234, 352
