
FAO Meeting Report No. PL/1965/10/1
WHO/Food Add./27.65
EVALUATION OF THE TOXICITY OF PESTICIDE RESIDUES IN FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Committee on Pesticides in Agriculture and
the WHO Expert Committee on Pesticide Residues, which met in Rome,
15-22 March 19651
Food and Agriculture Organization of the United Nations
World Health Organization
1965
1 Report of the second joint meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Committee on Pesticide
Residues, FAO Meeting Report No. PL/1965/10; WHO/Food Add./26.65
DIMETHRIN
Chemical name
2,4-dimethylbenzyl ester of chrysanthemumic acid.
Empirical formula
C19H26O2
Structural formula
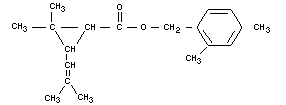 BIOLOGICAL DATA
Biochemical aspects
Preliminary studies in rabbits suggested that dimethrin is
excreted as chrysanthemumic acid and the glucuronide of
2,4-dimethylbenzoic acid (Ambrose, 1964). Later, the two acids were
isolated and positively identified (Masri et al., 1964a). Urine from
rabbits and rats gave positive reduction tests with Benedict's
reagent, which became negative when dimethrin was removed from the
diet (Ambrose, 1964).
Acute toxicity
Large doses produce lassitude, anorexia, weight loss, stupor, and
death. Hyperactivity and convulsions such as occur in poisoning by
pyrethrins are not seen.
Rats tolerated 14 800 mg/kg and guinea-pigs tolerated 9860 mg/kg
given by stomach-tube (Ambrose, 1964). Mice tolerated doses as high as
20 000 mg/kg but some were killed by 40 000 mg/kg (Preri, 1959).
Short-term studies
Rat. Fifteen oral doses at the rate of 9860 mg/kg caused no
abnormality of body-weight or general appearance, or gross
pathological findings (Ambrose, 1964).
However, Cox (1962a) and later Masri et al. (1964b) reported
microscopic changes in many liver cells of all rats fed 15 000 or
30 000 ppm for three months and in a smaller proportion of the cells
of some rats maintained at a dietary level of 6000 ppm. The presence
of changes in the liver of rats fed 3000 ppm was questionable. Cox
considered the changes similar to those produced by DDT. This
possibility was investigated by Kimbrough, Gaines & Ortega (1964) who
found that a dietary level of 20 000 ppm for 100 days did, in fact,
produce an increase of liver weight and liver cell changes
indistinguishable by light microscopy from those produced by DDT and
described earlier by Ortega et al. (1956). Furthermore, the
morphological details as revealed by electron microscopy are entirely
similar to those already described for DDT (Ortega, personal
communication 1964; Ortega, 1962).
The morphological changes produced by high dietary levels of
dimethrin are confined to the liver; other organs studied were: heart,
lung, spleen, kidney, adrenal, bladder, thyroid, pancreas, intestine,
stomach, ovary, and testis (Cox, 1962; Masri et al., 1964a).
Furthermore, the liver cell changes produced by feeding dimethrin at a
concentration of 20 000 ppm for three months were completely reversed
within three months. Shorter recovery periods were not investigated
(Cox, 1962b; Masri et al., 1964a).
Rabbit. Four animals received technical dimethrin by
stomach-tube at a rate of 500 mg/kg/day six days per week for a total
of 80 to 90 doses; three of them showed more than the normal amount of
fibrous tissue around the bile ducts, and one showed a slight
accumulation of fat in the parenchymal cells of the liver. Rabbits
that received both technical dimethrin and piperonyl butoxide each at
a dosage of 125 mg/kg/day six days per week for 89 doses also showed
an accumulation of fat in the parenchymal cells (Kimbrough, 1965).
Dog. Two dogs given technical dimethrin by capsule at the rate
of 125 mg/kg/day for 105 doses showed moderate accumulation of fat in
the parenchymal cells of the liver (Kimbrough, 1965).
Sheep. A concentration of 100 ppm in drinking-water given for 2
days may produce diarrhoea in sheep, but the effect has not been
observed in horses, rats, mice, rabbits, or hens (Barnes, 1963).
Long-term studies
Rat. According to Ambrose, 1964, dietary levels as high as 20
000 ppm for 52 weeks caused no change in growth rate, survival, food
consumption, haemoglobin, red and white cell counts or blood sugar.
Dietary levels of 10 000 and 20 000 ppm produced increased
liver-to-body-weight ratios which, however, returned to normal within
six weeks after feeding was stopped. Liver pathology was specifically
denied (Ambrose, 1964). However, this conclusion is contradicted by
the results of short-term tests.
Comments on the experimental studies reported and evaluation
Rats tolerate a dietary level of 3000 ppm (equivalent to 150
mg/kg/day) without any significant differences in the microscopic
morphology of the liver or in any other parameter between the treated
and control animals. An acceptable daily intake for man cannot be
established until the nature and significance of the liver changes
reported in rats at higher doses have been determined.
Further work required
Studies should be made of the long-term effects of dimethrin on
the liver of at least 1 species and an effort should be made to
evaluate the liver changes in the rat. This study should involve the
compound alone and in combination with major synergists. A method
should be developed to analyse a metabolite of dimethrin in human
urine so that absorption of the compound by men with prolonged and
intensive exposure to it may be measured.
REFERENCES
Ambrose, A. M. (1964) Toxicol. Appl. Pharmacol., 6, 112
Barnes, J. M. (1963) Letter to WHO dated 21 June 1963
Cox, A. J. jr (1962a) Unpublished report May 18
Cox, A. J. jr (1962b) Unpublished report August 13
Kimbrough, R. D., Gaines, T. B. & Ortega, P. (1964) Unpublished report
Kimbrough, R. D. (1965) Unpublished report February 16
Masri, M. S., Jones, F. T., Lundin, R. E., Bailey, G. F. & DeEds, F.
(1964a) Toxicol. Appl. Pharmacol., 6, 711
Masri, M. S., Hendrickson, A. P., Cox, A. J. jr & DeEds, F. (1964b)
Toxicol. Appl. Pharmacol., 6, 716
Ortega, P., Hayes, W. J., jr, Durham, W. F. & Mattson, A. M. (1956)
Publ. Health Monogr. Nr. 43, PHS Pub. No. 484
Ortega, P. (1962) Fed. Proc., 21, 306
Preri, R. J. (1959) Assay Report from Wisconsin Alumni Research
Foundation, W. A. R. F. No. 9110894-9110895
BIOLOGICAL DATA
Biochemical aspects
Preliminary studies in rabbits suggested that dimethrin is
excreted as chrysanthemumic acid and the glucuronide of
2,4-dimethylbenzoic acid (Ambrose, 1964). Later, the two acids were
isolated and positively identified (Masri et al., 1964a). Urine from
rabbits and rats gave positive reduction tests with Benedict's
reagent, which became negative when dimethrin was removed from the
diet (Ambrose, 1964).
Acute toxicity
Large doses produce lassitude, anorexia, weight loss, stupor, and
death. Hyperactivity and convulsions such as occur in poisoning by
pyrethrins are not seen.
Rats tolerated 14 800 mg/kg and guinea-pigs tolerated 9860 mg/kg
given by stomach-tube (Ambrose, 1964). Mice tolerated doses as high as
20 000 mg/kg but some were killed by 40 000 mg/kg (Preri, 1959).
Short-term studies
Rat. Fifteen oral doses at the rate of 9860 mg/kg caused no
abnormality of body-weight or general appearance, or gross
pathological findings (Ambrose, 1964).
However, Cox (1962a) and later Masri et al. (1964b) reported
microscopic changes in many liver cells of all rats fed 15 000 or
30 000 ppm for three months and in a smaller proportion of the cells
of some rats maintained at a dietary level of 6000 ppm. The presence
of changes in the liver of rats fed 3000 ppm was questionable. Cox
considered the changes similar to those produced by DDT. This
possibility was investigated by Kimbrough, Gaines & Ortega (1964) who
found that a dietary level of 20 000 ppm for 100 days did, in fact,
produce an increase of liver weight and liver cell changes
indistinguishable by light microscopy from those produced by DDT and
described earlier by Ortega et al. (1956). Furthermore, the
morphological details as revealed by electron microscopy are entirely
similar to those already described for DDT (Ortega, personal
communication 1964; Ortega, 1962).
The morphological changes produced by high dietary levels of
dimethrin are confined to the liver; other organs studied were: heart,
lung, spleen, kidney, adrenal, bladder, thyroid, pancreas, intestine,
stomach, ovary, and testis (Cox, 1962; Masri et al., 1964a).
Furthermore, the liver cell changes produced by feeding dimethrin at a
concentration of 20 000 ppm for three months were completely reversed
within three months. Shorter recovery periods were not investigated
(Cox, 1962b; Masri et al., 1964a).
Rabbit. Four animals received technical dimethrin by
stomach-tube at a rate of 500 mg/kg/day six days per week for a total
of 80 to 90 doses; three of them showed more than the normal amount of
fibrous tissue around the bile ducts, and one showed a slight
accumulation of fat in the parenchymal cells of the liver. Rabbits
that received both technical dimethrin and piperonyl butoxide each at
a dosage of 125 mg/kg/day six days per week for 89 doses also showed
an accumulation of fat in the parenchymal cells (Kimbrough, 1965).
Dog. Two dogs given technical dimethrin by capsule at the rate
of 125 mg/kg/day for 105 doses showed moderate accumulation of fat in
the parenchymal cells of the liver (Kimbrough, 1965).
Sheep. A concentration of 100 ppm in drinking-water given for 2
days may produce diarrhoea in sheep, but the effect has not been
observed in horses, rats, mice, rabbits, or hens (Barnes, 1963).
Long-term studies
Rat. According to Ambrose, 1964, dietary levels as high as 20
000 ppm for 52 weeks caused no change in growth rate, survival, food
consumption, haemoglobin, red and white cell counts or blood sugar.
Dietary levels of 10 000 and 20 000 ppm produced increased
liver-to-body-weight ratios which, however, returned to normal within
six weeks after feeding was stopped. Liver pathology was specifically
denied (Ambrose, 1964). However, this conclusion is contradicted by
the results of short-term tests.
Comments on the experimental studies reported and evaluation
Rats tolerate a dietary level of 3000 ppm (equivalent to 150
mg/kg/day) without any significant differences in the microscopic
morphology of the liver or in any other parameter between the treated
and control animals. An acceptable daily intake for man cannot be
established until the nature and significance of the liver changes
reported in rats at higher doses have been determined.
Further work required
Studies should be made of the long-term effects of dimethrin on
the liver of at least 1 species and an effort should be made to
evaluate the liver changes in the rat. This study should involve the
compound alone and in combination with major synergists. A method
should be developed to analyse a metabolite of dimethrin in human
urine so that absorption of the compound by men with prolonged and
intensive exposure to it may be measured.
REFERENCES
Ambrose, A. M. (1964) Toxicol. Appl. Pharmacol., 6, 112
Barnes, J. M. (1963) Letter to WHO dated 21 June 1963
Cox, A. J. jr (1962a) Unpublished report May 18
Cox, A. J. jr (1962b) Unpublished report August 13
Kimbrough, R. D., Gaines, T. B. & Ortega, P. (1964) Unpublished report
Kimbrough, R. D. (1965) Unpublished report February 16
Masri, M. S., Jones, F. T., Lundin, R. E., Bailey, G. F. & DeEds, F.
(1964a) Toxicol. Appl. Pharmacol., 6, 711
Masri, M. S., Hendrickson, A. P., Cox, A. J. jr & DeEds, F. (1964b)
Toxicol. Appl. Pharmacol., 6, 716
Ortega, P., Hayes, W. J., jr, Durham, W. F. & Mattson, A. M. (1956)
Publ. Health Monogr. Nr. 43, PHS Pub. No. 484
Ortega, P. (1962) Fed. Proc., 21, 306
Preri, R. J. (1959) Assay Report from Wisconsin Alumni Research
Foundation, W. A. R. F. No. 9110894-9110895
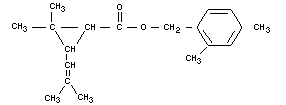 BIOLOGICAL DATA
Biochemical aspects
Preliminary studies in rabbits suggested that dimethrin is
excreted as chrysanthemumic acid and the glucuronide of
2,4-dimethylbenzoic acid (Ambrose, 1964). Later, the two acids were
isolated and positively identified (Masri et al., 1964a). Urine from
rabbits and rats gave positive reduction tests with Benedict's
reagent, which became negative when dimethrin was removed from the
diet (Ambrose, 1964).
Acute toxicity
Large doses produce lassitude, anorexia, weight loss, stupor, and
death. Hyperactivity and convulsions such as occur in poisoning by
pyrethrins are not seen.
Rats tolerated 14 800 mg/kg and guinea-pigs tolerated 9860 mg/kg
given by stomach-tube (Ambrose, 1964). Mice tolerated doses as high as
20 000 mg/kg but some were killed by 40 000 mg/kg (Preri, 1959).
Short-term studies
Rat. Fifteen oral doses at the rate of 9860 mg/kg caused no
abnormality of body-weight or general appearance, or gross
pathological findings (Ambrose, 1964).
However, Cox (1962a) and later Masri et al. (1964b) reported
microscopic changes in many liver cells of all rats fed 15 000 or
30 000 ppm for three months and in a smaller proportion of the cells
of some rats maintained at a dietary level of 6000 ppm. The presence
of changes in the liver of rats fed 3000 ppm was questionable. Cox
considered the changes similar to those produced by DDT. This
possibility was investigated by Kimbrough, Gaines & Ortega (1964) who
found that a dietary level of 20 000 ppm for 100 days did, in fact,
produce an increase of liver weight and liver cell changes
indistinguishable by light microscopy from those produced by DDT and
described earlier by Ortega et al. (1956). Furthermore, the
morphological details as revealed by electron microscopy are entirely
similar to those already described for DDT (Ortega, personal
communication 1964; Ortega, 1962).
The morphological changes produced by high dietary levels of
dimethrin are confined to the liver; other organs studied were: heart,
lung, spleen, kidney, adrenal, bladder, thyroid, pancreas, intestine,
stomach, ovary, and testis (Cox, 1962; Masri et al., 1964a).
Furthermore, the liver cell changes produced by feeding dimethrin at a
concentration of 20 000 ppm for three months were completely reversed
within three months. Shorter recovery periods were not investigated
(Cox, 1962b; Masri et al., 1964a).
Rabbit. Four animals received technical dimethrin by
stomach-tube at a rate of 500 mg/kg/day six days per week for a total
of 80 to 90 doses; three of them showed more than the normal amount of
fibrous tissue around the bile ducts, and one showed a slight
accumulation of fat in the parenchymal cells of the liver. Rabbits
that received both technical dimethrin and piperonyl butoxide each at
a dosage of 125 mg/kg/day six days per week for 89 doses also showed
an accumulation of fat in the parenchymal cells (Kimbrough, 1965).
Dog. Two dogs given technical dimethrin by capsule at the rate
of 125 mg/kg/day for 105 doses showed moderate accumulation of fat in
the parenchymal cells of the liver (Kimbrough, 1965).
Sheep. A concentration of 100 ppm in drinking-water given for 2
days may produce diarrhoea in sheep, but the effect has not been
observed in horses, rats, mice, rabbits, or hens (Barnes, 1963).
Long-term studies
Rat. According to Ambrose, 1964, dietary levels as high as 20
000 ppm for 52 weeks caused no change in growth rate, survival, food
consumption, haemoglobin, red and white cell counts or blood sugar.
Dietary levels of 10 000 and 20 000 ppm produced increased
liver-to-body-weight ratios which, however, returned to normal within
six weeks after feeding was stopped. Liver pathology was specifically
denied (Ambrose, 1964). However, this conclusion is contradicted by
the results of short-term tests.
Comments on the experimental studies reported and evaluation
Rats tolerate a dietary level of 3000 ppm (equivalent to 150
mg/kg/day) without any significant differences in the microscopic
morphology of the liver or in any other parameter between the treated
and control animals. An acceptable daily intake for man cannot be
established until the nature and significance of the liver changes
reported in rats at higher doses have been determined.
Further work required
Studies should be made of the long-term effects of dimethrin on
the liver of at least 1 species and an effort should be made to
evaluate the liver changes in the rat. This study should involve the
compound alone and in combination with major synergists. A method
should be developed to analyse a metabolite of dimethrin in human
urine so that absorption of the compound by men with prolonged and
intensive exposure to it may be measured.
REFERENCES
Ambrose, A. M. (1964) Toxicol. Appl. Pharmacol., 6, 112
Barnes, J. M. (1963) Letter to WHO dated 21 June 1963
Cox, A. J. jr (1962a) Unpublished report May 18
Cox, A. J. jr (1962b) Unpublished report August 13
Kimbrough, R. D., Gaines, T. B. & Ortega, P. (1964) Unpublished report
Kimbrough, R. D. (1965) Unpublished report February 16
Masri, M. S., Jones, F. T., Lundin, R. E., Bailey, G. F. & DeEds, F.
(1964a) Toxicol. Appl. Pharmacol., 6, 711
Masri, M. S., Hendrickson, A. P., Cox, A. J. jr & DeEds, F. (1964b)
Toxicol. Appl. Pharmacol., 6, 716
Ortega, P., Hayes, W. J., jr, Durham, W. F. & Mattson, A. M. (1956)
Publ. Health Monogr. Nr. 43, PHS Pub. No. 484
Ortega, P. (1962) Fed. Proc., 21, 306
Preri, R. J. (1959) Assay Report from Wisconsin Alumni Research
Foundation, W. A. R. F. No. 9110894-9110895
BIOLOGICAL DATA
Biochemical aspects
Preliminary studies in rabbits suggested that dimethrin is
excreted as chrysanthemumic acid and the glucuronide of
2,4-dimethylbenzoic acid (Ambrose, 1964). Later, the two acids were
isolated and positively identified (Masri et al., 1964a). Urine from
rabbits and rats gave positive reduction tests with Benedict's
reagent, which became negative when dimethrin was removed from the
diet (Ambrose, 1964).
Acute toxicity
Large doses produce lassitude, anorexia, weight loss, stupor, and
death. Hyperactivity and convulsions such as occur in poisoning by
pyrethrins are not seen.
Rats tolerated 14 800 mg/kg and guinea-pigs tolerated 9860 mg/kg
given by stomach-tube (Ambrose, 1964). Mice tolerated doses as high as
20 000 mg/kg but some were killed by 40 000 mg/kg (Preri, 1959).
Short-term studies
Rat. Fifteen oral doses at the rate of 9860 mg/kg caused no
abnormality of body-weight or general appearance, or gross
pathological findings (Ambrose, 1964).
However, Cox (1962a) and later Masri et al. (1964b) reported
microscopic changes in many liver cells of all rats fed 15 000 or
30 000 ppm for three months and in a smaller proportion of the cells
of some rats maintained at a dietary level of 6000 ppm. The presence
of changes in the liver of rats fed 3000 ppm was questionable. Cox
considered the changes similar to those produced by DDT. This
possibility was investigated by Kimbrough, Gaines & Ortega (1964) who
found that a dietary level of 20 000 ppm for 100 days did, in fact,
produce an increase of liver weight and liver cell changes
indistinguishable by light microscopy from those produced by DDT and
described earlier by Ortega et al. (1956). Furthermore, the
morphological details as revealed by electron microscopy are entirely
similar to those already described for DDT (Ortega, personal
communication 1964; Ortega, 1962).
The morphological changes produced by high dietary levels of
dimethrin are confined to the liver; other organs studied were: heart,
lung, spleen, kidney, adrenal, bladder, thyroid, pancreas, intestine,
stomach, ovary, and testis (Cox, 1962; Masri et al., 1964a).
Furthermore, the liver cell changes produced by feeding dimethrin at a
concentration of 20 000 ppm for three months were completely reversed
within three months. Shorter recovery periods were not investigated
(Cox, 1962b; Masri et al., 1964a).
Rabbit. Four animals received technical dimethrin by
stomach-tube at a rate of 500 mg/kg/day six days per week for a total
of 80 to 90 doses; three of them showed more than the normal amount of
fibrous tissue around the bile ducts, and one showed a slight
accumulation of fat in the parenchymal cells of the liver. Rabbits
that received both technical dimethrin and piperonyl butoxide each at
a dosage of 125 mg/kg/day six days per week for 89 doses also showed
an accumulation of fat in the parenchymal cells (Kimbrough, 1965).
Dog. Two dogs given technical dimethrin by capsule at the rate
of 125 mg/kg/day for 105 doses showed moderate accumulation of fat in
the parenchymal cells of the liver (Kimbrough, 1965).
Sheep. A concentration of 100 ppm in drinking-water given for 2
days may produce diarrhoea in sheep, but the effect has not been
observed in horses, rats, mice, rabbits, or hens (Barnes, 1963).
Long-term studies
Rat. According to Ambrose, 1964, dietary levels as high as 20
000 ppm for 52 weeks caused no change in growth rate, survival, food
consumption, haemoglobin, red and white cell counts or blood sugar.
Dietary levels of 10 000 and 20 000 ppm produced increased
liver-to-body-weight ratios which, however, returned to normal within
six weeks after feeding was stopped. Liver pathology was specifically
denied (Ambrose, 1964). However, this conclusion is contradicted by
the results of short-term tests.
Comments on the experimental studies reported and evaluation
Rats tolerate a dietary level of 3000 ppm (equivalent to 150
mg/kg/day) without any significant differences in the microscopic
morphology of the liver or in any other parameter between the treated
and control animals. An acceptable daily intake for man cannot be
established until the nature and significance of the liver changes
reported in rats at higher doses have been determined.
Further work required
Studies should be made of the long-term effects of dimethrin on
the liver of at least 1 species and an effort should be made to
evaluate the liver changes in the rat. This study should involve the
compound alone and in combination with major synergists. A method
should be developed to analyse a metabolite of dimethrin in human
urine so that absorption of the compound by men with prolonged and
intensive exposure to it may be measured.
REFERENCES
Ambrose, A. M. (1964) Toxicol. Appl. Pharmacol., 6, 112
Barnes, J. M. (1963) Letter to WHO dated 21 June 1963
Cox, A. J. jr (1962a) Unpublished report May 18
Cox, A. J. jr (1962b) Unpublished report August 13
Kimbrough, R. D., Gaines, T. B. & Ortega, P. (1964) Unpublished report
Kimbrough, R. D. (1965) Unpublished report February 16
Masri, M. S., Jones, F. T., Lundin, R. E., Bailey, G. F. & DeEds, F.
(1964a) Toxicol. Appl. Pharmacol., 6, 711
Masri, M. S., Hendrickson, A. P., Cox, A. J. jr & DeEds, F. (1964b)
Toxicol. Appl. Pharmacol., 6, 716
Ortega, P., Hayes, W. J., jr, Durham, W. F. & Mattson, A. M. (1956)
Publ. Health Monogr. Nr. 43, PHS Pub. No. 484
Ortega, P. (1962) Fed. Proc., 21, 306
Preri, R. J. (1959) Assay Report from Wisconsin Alumni Research
Foundation, W. A. R. F. No. 9110894-9110895
