
FAO Meeting Report No. PL/1965/10/1
WHO/Food Add./27.65
EVALUATION OF THE TOXICITY OF PESTICIDE RESIDUES IN FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Committee on Pesticides in Agriculture and
the WHO Expert Committee on Pesticide Residues, which met in Rome,
15-22 March 19651
Food and Agriculture Organization of the United Nations
World Health Organization
1965
1 Report of the second joint meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Committee on Pesticide
Residues, FAO Meeting Report No. PL/1965/10; WHO/Food Add./26.65
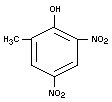
DNOC
Chemical name
2-methyl-4,6-dinitrophenol; 2,4-dinitro-6-methylphenol;
2,4-dinitro-o-cresol; 3,5-dinitro-o-cresol.
Synonyms
DNC
Empirical formula
C7H6N2O5
Structural formula
 BIOLOGICAL DATA
Biochemical aspects
Like other dinitrophenols, DNOC increases the oxidative
metabolism and heat production by direct cellular reaction. DNOC
affects enzyme systems by inhibiting the formation of adenosine
triphosphate and by blocking oxidative phosphorylation.
In the blood, DNOC combines with the plasm proteins to form
compounds that persist for variable times depending on the species
(King & Harvey, 1953). In mammals and plants DNOC is reduced to a
number of amino compounds (Wessels, 1960).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Oral 26-30 Lehman, 1951
Negherbon, 1959
Rat Intraperitoneal 28.5 Lawford et al., 1954
Mouse Intraperitoneal 24-26 Lawford et al., 1954
Guinea-pig Intraperitoneal 22.5 Lawford et al., 1954
Rabbit Intraperitoneal 23.5 Lawford et al., 1954
Goat Oral 100 Negherbon, 1959
Short-term studies
Rat. Groups of 5-10 male rats were given diets containing 7.8,
15.6, 31.2, 62.5, 125, 250, 500 and 1000 ppm DNOC for 105 days.
Dosages from 7.8 to 31.2 ppm did not affect growth or food
consumption. Growth and food consumption were slightly increased in
the 62.5 and 125 ppm groups. Dosages of 125 and 250 ppm killed 60% of
the rats and growth was inhibited in the survivors on 250 ppm. The
animals with 500 and 1000 ppm failed to grow and died in 2 or 3 days.
No histopathological changes were noted in any organ (Ambrose, 1942).
Groups of 20 male rats were given diets containing 20, 50, 100,
200, 500 and 1000 ppm DNOC for 6 months. Growth was normal in those on
20, 50 and 100 ppm; with 200 ppm, growth was decreased but not
significantly; with 500 ppm there was poor weight gain and with 1000
ppm half the rats died by the tenth day. The reminder were killed for
histopathological examination. Slight cloudy swelling of the liver at
1000 ppm was found (Spencer et al., 1948).
Duckling. A group of nine ducklings given 0.25% DNOC showed
bilateral cataracts within 24 hours. This dosage was lethal to all
ducklings within 2 days (Spencer et al., 1948).
Man. Two volunteers received 75 mg/day orally for 7 days. In
one subject, lassitude, headache and malaise occurred on the seventh
day. In the second no symptoms appeared (Negherbon, 1959).
Five male volunteers were given daily doses of 75 mg of pure
DNOC orally for 5 days. This dose ranged from 0.92 to 1.27 mg/kg
body-weight per day. The volunteers on the highest dose developed
symptoms of lassitude, headache and general malaise on the fifth day.
Doses of the order of 1 mg/kg body-weight resulted in concentrations
of 15-20 µg of DNOC per g of blood after 3-5 days. Symptoms occur when
the concentration in the blood is of the order of 20 µg per g of blood
(Harvey et al., 1951).
Bilateral cataracts have occurred from the use of DNOC in the
treatment of obesity (Bidstrup & Payne, 1951).
Comments on the experimental studies reported
Two relatively short studies in rats have indicated that a
dosage of 100 ppm of DNOC in the diet is a "no-effect" level. However,
human studies have shown that DNOC is more toxic to man than was
indicated by these rat studies. Studies with ducks and reports of
human use in obesity show that DNOC may produce cataracts.
EVALUATION
Estimate of acceptable daily intake for man
In view of the fact that the maximum no-effect level in the rat
was established on a six-month experiment only and because of the high
toxicity of DNOC to man no maximum acceptable daily intake for man can
be established.
Further work required
Chemical composition and toxicity of the plant residues.
REFERENCES
Ambrose, A. M. (1942) J. Pharmacol. exp. Ther., 76, 245
Bidstrup, P. L. & Payne, D. J. H. (1951) Brit. med. J., 2, 16
Harvey, D. G., Bidstrup, P. L. & Bonnell, J. A. L. (1951) Brit. med.
J., 2, 13
King, E. & Harvey, D. G. (1953) Biochem. J., 53, 185 and 196
Lawford, D. J., King, E. & Harvey, D. S. (1954) J. Pharm.
Pharmacol., 6, 619-624
Lehman, A. J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Negherbon, W. O. (1959) Handbook of Toxicology, Philadelphia &
London, Saunders, vol. III
Spencer, H. C., Rowe, V. K., Adams, E. M. & Irish, D. D. (1948)
J. industr. Hyg., 30, 10
Wessels, J. S. C. (1960) Bioph. Biochem. Acta, 38, 195
BIOLOGICAL DATA
Biochemical aspects
Like other dinitrophenols, DNOC increases the oxidative
metabolism and heat production by direct cellular reaction. DNOC
affects enzyme systems by inhibiting the formation of adenosine
triphosphate and by blocking oxidative phosphorylation.
In the blood, DNOC combines with the plasm proteins to form
compounds that persist for variable times depending on the species
(King & Harvey, 1953). In mammals and plants DNOC is reduced to a
number of amino compounds (Wessels, 1960).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Oral 26-30 Lehman, 1951
Negherbon, 1959
Rat Intraperitoneal 28.5 Lawford et al., 1954
Mouse Intraperitoneal 24-26 Lawford et al., 1954
Guinea-pig Intraperitoneal 22.5 Lawford et al., 1954
Rabbit Intraperitoneal 23.5 Lawford et al., 1954
Goat Oral 100 Negherbon, 1959
Short-term studies
Rat. Groups of 5-10 male rats were given diets containing 7.8,
15.6, 31.2, 62.5, 125, 250, 500 and 1000 ppm DNOC for 105 days.
Dosages from 7.8 to 31.2 ppm did not affect growth or food
consumption. Growth and food consumption were slightly increased in
the 62.5 and 125 ppm groups. Dosages of 125 and 250 ppm killed 60% of
the rats and growth was inhibited in the survivors on 250 ppm. The
animals with 500 and 1000 ppm failed to grow and died in 2 or 3 days.
No histopathological changes were noted in any organ (Ambrose, 1942).
Groups of 20 male rats were given diets containing 20, 50, 100,
200, 500 and 1000 ppm DNOC for 6 months. Growth was normal in those on
20, 50 and 100 ppm; with 200 ppm, growth was decreased but not
significantly; with 500 ppm there was poor weight gain and with 1000
ppm half the rats died by the tenth day. The reminder were killed for
histopathological examination. Slight cloudy swelling of the liver at
1000 ppm was found (Spencer et al., 1948).
Duckling. A group of nine ducklings given 0.25% DNOC showed
bilateral cataracts within 24 hours. This dosage was lethal to all
ducklings within 2 days (Spencer et al., 1948).
Man. Two volunteers received 75 mg/day orally for 7 days. In
one subject, lassitude, headache and malaise occurred on the seventh
day. In the second no symptoms appeared (Negherbon, 1959).
Five male volunteers were given daily doses of 75 mg of pure
DNOC orally for 5 days. This dose ranged from 0.92 to 1.27 mg/kg
body-weight per day. The volunteers on the highest dose developed
symptoms of lassitude, headache and general malaise on the fifth day.
Doses of the order of 1 mg/kg body-weight resulted in concentrations
of 15-20 µg of DNOC per g of blood after 3-5 days. Symptoms occur when
the concentration in the blood is of the order of 20 µg per g of blood
(Harvey et al., 1951).
Bilateral cataracts have occurred from the use of DNOC in the
treatment of obesity (Bidstrup & Payne, 1951).
Comments on the experimental studies reported
Two relatively short studies in rats have indicated that a
dosage of 100 ppm of DNOC in the diet is a "no-effect" level. However,
human studies have shown that DNOC is more toxic to man than was
indicated by these rat studies. Studies with ducks and reports of
human use in obesity show that DNOC may produce cataracts.
EVALUATION
Estimate of acceptable daily intake for man
In view of the fact that the maximum no-effect level in the rat
was established on a six-month experiment only and because of the high
toxicity of DNOC to man no maximum acceptable daily intake for man can
be established.
Further work required
Chemical composition and toxicity of the plant residues.
REFERENCES
Ambrose, A. M. (1942) J. Pharmacol. exp. Ther., 76, 245
Bidstrup, P. L. & Payne, D. J. H. (1951) Brit. med. J., 2, 16
Harvey, D. G., Bidstrup, P. L. & Bonnell, J. A. L. (1951) Brit. med.
J., 2, 13
King, E. & Harvey, D. G. (1953) Biochem. J., 53, 185 and 196
Lawford, D. J., King, E. & Harvey, D. S. (1954) J. Pharm.
Pharmacol., 6, 619-624
Lehman, A. J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Negherbon, W. O. (1959) Handbook of Toxicology, Philadelphia &
London, Saunders, vol. III
Spencer, H. C., Rowe, V. K., Adams, E. M. & Irish, D. D. (1948)
J. industr. Hyg., 30, 10
Wessels, J. S. C. (1960) Bioph. Biochem. Acta, 38, 195
 BIOLOGICAL DATA
Biochemical aspects
Like other dinitrophenols, DNOC increases the oxidative
metabolism and heat production by direct cellular reaction. DNOC
affects enzyme systems by inhibiting the formation of adenosine
triphosphate and by blocking oxidative phosphorylation.
In the blood, DNOC combines with the plasm proteins to form
compounds that persist for variable times depending on the species
(King & Harvey, 1953). In mammals and plants DNOC is reduced to a
number of amino compounds (Wessels, 1960).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Oral 26-30 Lehman, 1951
Negherbon, 1959
Rat Intraperitoneal 28.5 Lawford et al., 1954
Mouse Intraperitoneal 24-26 Lawford et al., 1954
Guinea-pig Intraperitoneal 22.5 Lawford et al., 1954
Rabbit Intraperitoneal 23.5 Lawford et al., 1954
Goat Oral 100 Negherbon, 1959
Short-term studies
Rat. Groups of 5-10 male rats were given diets containing 7.8,
15.6, 31.2, 62.5, 125, 250, 500 and 1000 ppm DNOC for 105 days.
Dosages from 7.8 to 31.2 ppm did not affect growth or food
consumption. Growth and food consumption were slightly increased in
the 62.5 and 125 ppm groups. Dosages of 125 and 250 ppm killed 60% of
the rats and growth was inhibited in the survivors on 250 ppm. The
animals with 500 and 1000 ppm failed to grow and died in 2 or 3 days.
No histopathological changes were noted in any organ (Ambrose, 1942).
Groups of 20 male rats were given diets containing 20, 50, 100,
200, 500 and 1000 ppm DNOC for 6 months. Growth was normal in those on
20, 50 and 100 ppm; with 200 ppm, growth was decreased but not
significantly; with 500 ppm there was poor weight gain and with 1000
ppm half the rats died by the tenth day. The reminder were killed for
histopathological examination. Slight cloudy swelling of the liver at
1000 ppm was found (Spencer et al., 1948).
Duckling. A group of nine ducklings given 0.25% DNOC showed
bilateral cataracts within 24 hours. This dosage was lethal to all
ducklings within 2 days (Spencer et al., 1948).
Man. Two volunteers received 75 mg/day orally for 7 days. In
one subject, lassitude, headache and malaise occurred on the seventh
day. In the second no symptoms appeared (Negherbon, 1959).
Five male volunteers were given daily doses of 75 mg of pure
DNOC orally for 5 days. This dose ranged from 0.92 to 1.27 mg/kg
body-weight per day. The volunteers on the highest dose developed
symptoms of lassitude, headache and general malaise on the fifth day.
Doses of the order of 1 mg/kg body-weight resulted in concentrations
of 15-20 µg of DNOC per g of blood after 3-5 days. Symptoms occur when
the concentration in the blood is of the order of 20 µg per g of blood
(Harvey et al., 1951).
Bilateral cataracts have occurred from the use of DNOC in the
treatment of obesity (Bidstrup & Payne, 1951).
Comments on the experimental studies reported
Two relatively short studies in rats have indicated that a
dosage of 100 ppm of DNOC in the diet is a "no-effect" level. However,
human studies have shown that DNOC is more toxic to man than was
indicated by these rat studies. Studies with ducks and reports of
human use in obesity show that DNOC may produce cataracts.
EVALUATION
Estimate of acceptable daily intake for man
In view of the fact that the maximum no-effect level in the rat
was established on a six-month experiment only and because of the high
toxicity of DNOC to man no maximum acceptable daily intake for man can
be established.
Further work required
Chemical composition and toxicity of the plant residues.
REFERENCES
Ambrose, A. M. (1942) J. Pharmacol. exp. Ther., 76, 245
Bidstrup, P. L. & Payne, D. J. H. (1951) Brit. med. J., 2, 16
Harvey, D. G., Bidstrup, P. L. & Bonnell, J. A. L. (1951) Brit. med.
J., 2, 13
King, E. & Harvey, D. G. (1953) Biochem. J., 53, 185 and 196
Lawford, D. J., King, E. & Harvey, D. S. (1954) J. Pharm.
Pharmacol., 6, 619-624
Lehman, A. J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Negherbon, W. O. (1959) Handbook of Toxicology, Philadelphia &
London, Saunders, vol. III
Spencer, H. C., Rowe, V. K., Adams, E. M. & Irish, D. D. (1948)
J. industr. Hyg., 30, 10
Wessels, J. S. C. (1960) Bioph. Biochem. Acta, 38, 195
BIOLOGICAL DATA
Biochemical aspects
Like other dinitrophenols, DNOC increases the oxidative
metabolism and heat production by direct cellular reaction. DNOC
affects enzyme systems by inhibiting the formation of adenosine
triphosphate and by blocking oxidative phosphorylation.
In the blood, DNOC combines with the plasm proteins to form
compounds that persist for variable times depending on the species
(King & Harvey, 1953). In mammals and plants DNOC is reduced to a
number of amino compounds (Wessels, 1960).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Oral 26-30 Lehman, 1951
Negherbon, 1959
Rat Intraperitoneal 28.5 Lawford et al., 1954
Mouse Intraperitoneal 24-26 Lawford et al., 1954
Guinea-pig Intraperitoneal 22.5 Lawford et al., 1954
Rabbit Intraperitoneal 23.5 Lawford et al., 1954
Goat Oral 100 Negherbon, 1959
Short-term studies
Rat. Groups of 5-10 male rats were given diets containing 7.8,
15.6, 31.2, 62.5, 125, 250, 500 and 1000 ppm DNOC for 105 days.
Dosages from 7.8 to 31.2 ppm did not affect growth or food
consumption. Growth and food consumption were slightly increased in
the 62.5 and 125 ppm groups. Dosages of 125 and 250 ppm killed 60% of
the rats and growth was inhibited in the survivors on 250 ppm. The
animals with 500 and 1000 ppm failed to grow and died in 2 or 3 days.
No histopathological changes were noted in any organ (Ambrose, 1942).
Groups of 20 male rats were given diets containing 20, 50, 100,
200, 500 and 1000 ppm DNOC for 6 months. Growth was normal in those on
20, 50 and 100 ppm; with 200 ppm, growth was decreased but not
significantly; with 500 ppm there was poor weight gain and with 1000
ppm half the rats died by the tenth day. The reminder were killed for
histopathological examination. Slight cloudy swelling of the liver at
1000 ppm was found (Spencer et al., 1948).
Duckling. A group of nine ducklings given 0.25% DNOC showed
bilateral cataracts within 24 hours. This dosage was lethal to all
ducklings within 2 days (Spencer et al., 1948).
Man. Two volunteers received 75 mg/day orally for 7 days. In
one subject, lassitude, headache and malaise occurred on the seventh
day. In the second no symptoms appeared (Negherbon, 1959).
Five male volunteers were given daily doses of 75 mg of pure
DNOC orally for 5 days. This dose ranged from 0.92 to 1.27 mg/kg
body-weight per day. The volunteers on the highest dose developed
symptoms of lassitude, headache and general malaise on the fifth day.
Doses of the order of 1 mg/kg body-weight resulted in concentrations
of 15-20 µg of DNOC per g of blood after 3-5 days. Symptoms occur when
the concentration in the blood is of the order of 20 µg per g of blood
(Harvey et al., 1951).
Bilateral cataracts have occurred from the use of DNOC in the
treatment of obesity (Bidstrup & Payne, 1951).
Comments on the experimental studies reported
Two relatively short studies in rats have indicated that a
dosage of 100 ppm of DNOC in the diet is a "no-effect" level. However,
human studies have shown that DNOC is more toxic to man than was
indicated by these rat studies. Studies with ducks and reports of
human use in obesity show that DNOC may produce cataracts.
EVALUATION
Estimate of acceptable daily intake for man
In view of the fact that the maximum no-effect level in the rat
was established on a six-month experiment only and because of the high
toxicity of DNOC to man no maximum acceptable daily intake for man can
be established.
Further work required
Chemical composition and toxicity of the plant residues.
REFERENCES
Ambrose, A. M. (1942) J. Pharmacol. exp. Ther., 76, 245
Bidstrup, P. L. & Payne, D. J. H. (1951) Brit. med. J., 2, 16
Harvey, D. G., Bidstrup, P. L. & Bonnell, J. A. L. (1951) Brit. med.
J., 2, 13
King, E. & Harvey, D. G. (1953) Biochem. J., 53, 185 and 196
Lawford, D. J., King, E. & Harvey, D. S. (1954) J. Pharm.
Pharmacol., 6, 619-624
Lehman, A. J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Negherbon, W. O. (1959) Handbook of Toxicology, Philadelphia &
London, Saunders, vol. III
Spencer, H. C., Rowe, V. K., Adams, E. M. & Irish, D. D. (1948)
J. industr. Hyg., 30, 10
Wessels, J. S. C. (1960) Bioph. Biochem. Acta, 38, 195
