
FAO Meeting Report No. PL/1965/10/1
WHO/Food Add./27.65
EVALUATION OF THE TOXICITY OF PESTICIDE RESIDUES IN FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Committee on Pesticides in Agriculture and
the WHO Expert Committee on Pesticide Residues, which met in Rome,
15-22 March 19651
Food and Agriculture Organization of the United Nations
World Health Organization
1965
1 Report of the second joint meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Committee on Pesticide
Residues, FAO Meeting Report No. PL/1965/10; WHO/Food Add./26.65
METHYL PARATHION
Chemical name
Dimethyl-4-nitrophenyl phosphorothionate;
O,O-dimethyl-O-(-4-nitrophenyl) phosphorothioate.
Synonyms
Methaphos; Wolfatox; Dimethylparathion; Metacide.
Empirical formula
C8H10O5NSP
Structural formula
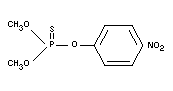 BIOLOGICAL DATA
Biochemical aspects
Methyl parathion has biological properties similar to those of
parathion. Methyl parathion is oxidized to methyl paraoxon, mainly in
the liver, and this has a weaker inhibitory effect on cholinesterase
than paraoxon (Davison, 1955a; Hazleton, 1955). The molar I50 of
methyl paraoxon (rat brain 30 min. at 37°) was 4.0 × 10-8 (Davison,
1955b).
In guinea-pigs given orally 32P-labelled methyl parathion, the
compound began to enter the organs at once and the maximum tissue
levels were reached in 1-2 hours. A high concentration of the compound
was found in the liver (Gar et al., 1958).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Oral 9.0-42.0* Deichmann et al., 1952
Gaines, 1960
Metcalf, 1955
(continued)
Animal Route LD50 mg/kg References
body-weight
Rat Oral 9.7-14.8 Deichmann et al., 1952
(pure product) Gaines, 1960
Metcalf, 1955
Rat Intraperitoneal 3.5 DuBois & Coon, 1952
Mouse Oral 32.1 Ikeda, 1962
* Dependent on the sex of the animal, the vehicle used and the purity
of the sample.
Short-term studies
Dog. Groups of 2 dogs, one male and one female, were maintained
on diets containing 5, 20 and 50 ppm of methyl parathion for 12 weeks.
The two highest dosage levels produced significant depression in
erythrocyte cholinesterase activity. Plasm cholinesterase activity was
significantly depressed at 50 ppm but only a doubtful change was seen
at 20 ppm. The 5 ppm level produced no significant inhibition of
cholinesterase activity (Williams et al., 1959).
Man. A group of 5 subjects was given 3 mg of methyl parathion
orally per day for 28 days, 3.5 mg per day for 28 days, and 4.0 mg per
day for 43 days. No depression of erythrocyte or plasm cholinesterase
activity occurred and no side-effects were seen (Moeller & Rider,
1961).
A group of 5 subjects was given daily 4.5 mg during 30 days
followed by 5 mg for 29 days. Another group received 5.5 mg for 20
days followed by 6 mg for 29 days and the last group received 6.5 mg
for 35 days, followed by 7 mg for 24 days. The maximum depression of
whole-blood cholinesterase activity was 15% (Moeller & Rider, 1962).
Groups of 5 subjects were given methyl parathion for 30 days in
the following amounts: 7 mg per day, 7.5 mg per day, 8 and 9 mg per
day. The plasma and erythrocyte cholinesterase activities remained
within 20% of the control values (Moeller & Rider, 1963).
Long-term studies
No information available.
Comments on experimental studies reported
The biochemical and toxicological studies on this compound are
not as extensive as in the case of parathion but it would seem to have
been adequately investigated in man from the point of view of its
effect on cholinesterase activity.
EVALUATION
Level causing no significant toxicological effect
The highest dietary dose having no effect on cholinesterase
activity was 7-9 mg per day in man, equivalent to approximately 0.1
mg/kg body-weight per day.
Estimate of acceptable daily intake for man
0-0.01 mg/kg body-weight per day.
Further work desirable
Reproduction studies in the rat.
REFERENCES
Davison, A. N. (1955a) Biochem., J., 61, 203
Davison, A. N. (1955b) Biochem. J., 60, 399
Deichmann, W. B., Pugliese, W. & Cassidy, J. (1952) A.M.A. Arch.
industr. Hyg., 5, 523
DuBois, K. P. & Coon, J. M. (1952) A.M.A. Arch. industr. Hyg., 6,
9
Gaines, T. B. (1960) Toxicol. appl. Pharmacol., 2, 88
Gar, K. Sazonova, N. A., Fadeer, Y. N. Vladimirova, I. L. & Golubeva,
N. A. (1958) Org. Insektofungitsidy i Gerbitsidy, 93 (From Chem.
Abstr., 54, 15688)
Hazleton, L. W. (1955) Agr. Food Chem., 3, 312
Ikeda, Y. (1962) Report to the Japan Academy of Sciences
Metcalf, R. L. (1955) Organic insecticides, Interscience, New York
Moeller, H. C. & Rider, J. A. (1961) Fed. Proc., 20, 434
Moeller, H. C. & Rider, J. A. (1962) Fed. Proc., 21, 451
Moeller, H. C. & Rider, J. A. (1963) Fed. Proc., 22, 189
Williams, M. W., Fuyat, H. N. & Fitzhugh, O. G. (1959) Toxicol.
appl. Pharmacol., 1, 1
BIOLOGICAL DATA
Biochemical aspects
Methyl parathion has biological properties similar to those of
parathion. Methyl parathion is oxidized to methyl paraoxon, mainly in
the liver, and this has a weaker inhibitory effect on cholinesterase
than paraoxon (Davison, 1955a; Hazleton, 1955). The molar I50 of
methyl paraoxon (rat brain 30 min. at 37°) was 4.0 × 10-8 (Davison,
1955b).
In guinea-pigs given orally 32P-labelled methyl parathion, the
compound began to enter the organs at once and the maximum tissue
levels were reached in 1-2 hours. A high concentration of the compound
was found in the liver (Gar et al., 1958).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Oral 9.0-42.0* Deichmann et al., 1952
Gaines, 1960
Metcalf, 1955
(continued)
Animal Route LD50 mg/kg References
body-weight
Rat Oral 9.7-14.8 Deichmann et al., 1952
(pure product) Gaines, 1960
Metcalf, 1955
Rat Intraperitoneal 3.5 DuBois & Coon, 1952
Mouse Oral 32.1 Ikeda, 1962
* Dependent on the sex of the animal, the vehicle used and the purity
of the sample.
Short-term studies
Dog. Groups of 2 dogs, one male and one female, were maintained
on diets containing 5, 20 and 50 ppm of methyl parathion for 12 weeks.
The two highest dosage levels produced significant depression in
erythrocyte cholinesterase activity. Plasm cholinesterase activity was
significantly depressed at 50 ppm but only a doubtful change was seen
at 20 ppm. The 5 ppm level produced no significant inhibition of
cholinesterase activity (Williams et al., 1959).
Man. A group of 5 subjects was given 3 mg of methyl parathion
orally per day for 28 days, 3.5 mg per day for 28 days, and 4.0 mg per
day for 43 days. No depression of erythrocyte or plasm cholinesterase
activity occurred and no side-effects were seen (Moeller & Rider,
1961).
A group of 5 subjects was given daily 4.5 mg during 30 days
followed by 5 mg for 29 days. Another group received 5.5 mg for 20
days followed by 6 mg for 29 days and the last group received 6.5 mg
for 35 days, followed by 7 mg for 24 days. The maximum depression of
whole-blood cholinesterase activity was 15% (Moeller & Rider, 1962).
Groups of 5 subjects were given methyl parathion for 30 days in
the following amounts: 7 mg per day, 7.5 mg per day, 8 and 9 mg per
day. The plasma and erythrocyte cholinesterase activities remained
within 20% of the control values (Moeller & Rider, 1963).
Long-term studies
No information available.
Comments on experimental studies reported
The biochemical and toxicological studies on this compound are
not as extensive as in the case of parathion but it would seem to have
been adequately investigated in man from the point of view of its
effect on cholinesterase activity.
EVALUATION
Level causing no significant toxicological effect
The highest dietary dose having no effect on cholinesterase
activity was 7-9 mg per day in man, equivalent to approximately 0.1
mg/kg body-weight per day.
Estimate of acceptable daily intake for man
0-0.01 mg/kg body-weight per day.
Further work desirable
Reproduction studies in the rat.
REFERENCES
Davison, A. N. (1955a) Biochem., J., 61, 203
Davison, A. N. (1955b) Biochem. J., 60, 399
Deichmann, W. B., Pugliese, W. & Cassidy, J. (1952) A.M.A. Arch.
industr. Hyg., 5, 523
DuBois, K. P. & Coon, J. M. (1952) A.M.A. Arch. industr. Hyg., 6,
9
Gaines, T. B. (1960) Toxicol. appl. Pharmacol., 2, 88
Gar, K. Sazonova, N. A., Fadeer, Y. N. Vladimirova, I. L. & Golubeva,
N. A. (1958) Org. Insektofungitsidy i Gerbitsidy, 93 (From Chem.
Abstr., 54, 15688)
Hazleton, L. W. (1955) Agr. Food Chem., 3, 312
Ikeda, Y. (1962) Report to the Japan Academy of Sciences
Metcalf, R. L. (1955) Organic insecticides, Interscience, New York
Moeller, H. C. & Rider, J. A. (1961) Fed. Proc., 20, 434
Moeller, H. C. & Rider, J. A. (1962) Fed. Proc., 21, 451
Moeller, H. C. & Rider, J. A. (1963) Fed. Proc., 22, 189
Williams, M. W., Fuyat, H. N. & Fitzhugh, O. G. (1959) Toxicol.
appl. Pharmacol., 1, 1
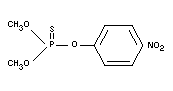 BIOLOGICAL DATA
Biochemical aspects
Methyl parathion has biological properties similar to those of
parathion. Methyl parathion is oxidized to methyl paraoxon, mainly in
the liver, and this has a weaker inhibitory effect on cholinesterase
than paraoxon (Davison, 1955a; Hazleton, 1955). The molar I50 of
methyl paraoxon (rat brain 30 min. at 37°) was 4.0 × 10-8 (Davison,
1955b).
In guinea-pigs given orally 32P-labelled methyl parathion, the
compound began to enter the organs at once and the maximum tissue
levels were reached in 1-2 hours. A high concentration of the compound
was found in the liver (Gar et al., 1958).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Oral 9.0-42.0* Deichmann et al., 1952
Gaines, 1960
Metcalf, 1955
(continued)
Animal Route LD50 mg/kg References
body-weight
Rat Oral 9.7-14.8 Deichmann et al., 1952
(pure product) Gaines, 1960
Metcalf, 1955
Rat Intraperitoneal 3.5 DuBois & Coon, 1952
Mouse Oral 32.1 Ikeda, 1962
* Dependent on the sex of the animal, the vehicle used and the purity
of the sample.
Short-term studies
Dog. Groups of 2 dogs, one male and one female, were maintained
on diets containing 5, 20 and 50 ppm of methyl parathion for 12 weeks.
The two highest dosage levels produced significant depression in
erythrocyte cholinesterase activity. Plasm cholinesterase activity was
significantly depressed at 50 ppm but only a doubtful change was seen
at 20 ppm. The 5 ppm level produced no significant inhibition of
cholinesterase activity (Williams et al., 1959).
Man. A group of 5 subjects was given 3 mg of methyl parathion
orally per day for 28 days, 3.5 mg per day for 28 days, and 4.0 mg per
day for 43 days. No depression of erythrocyte or plasm cholinesterase
activity occurred and no side-effects were seen (Moeller & Rider,
1961).
A group of 5 subjects was given daily 4.5 mg during 30 days
followed by 5 mg for 29 days. Another group received 5.5 mg for 20
days followed by 6 mg for 29 days and the last group received 6.5 mg
for 35 days, followed by 7 mg for 24 days. The maximum depression of
whole-blood cholinesterase activity was 15% (Moeller & Rider, 1962).
Groups of 5 subjects were given methyl parathion for 30 days in
the following amounts: 7 mg per day, 7.5 mg per day, 8 and 9 mg per
day. The plasma and erythrocyte cholinesterase activities remained
within 20% of the control values (Moeller & Rider, 1963).
Long-term studies
No information available.
Comments on experimental studies reported
The biochemical and toxicological studies on this compound are
not as extensive as in the case of parathion but it would seem to have
been adequately investigated in man from the point of view of its
effect on cholinesterase activity.
EVALUATION
Level causing no significant toxicological effect
The highest dietary dose having no effect on cholinesterase
activity was 7-9 mg per day in man, equivalent to approximately 0.1
mg/kg body-weight per day.
Estimate of acceptable daily intake for man
0-0.01 mg/kg body-weight per day.
Further work desirable
Reproduction studies in the rat.
REFERENCES
Davison, A. N. (1955a) Biochem., J., 61, 203
Davison, A. N. (1955b) Biochem. J., 60, 399
Deichmann, W. B., Pugliese, W. & Cassidy, J. (1952) A.M.A. Arch.
industr. Hyg., 5, 523
DuBois, K. P. & Coon, J. M. (1952) A.M.A. Arch. industr. Hyg., 6,
9
Gaines, T. B. (1960) Toxicol. appl. Pharmacol., 2, 88
Gar, K. Sazonova, N. A., Fadeer, Y. N. Vladimirova, I. L. & Golubeva,
N. A. (1958) Org. Insektofungitsidy i Gerbitsidy, 93 (From Chem.
Abstr., 54, 15688)
Hazleton, L. W. (1955) Agr. Food Chem., 3, 312
Ikeda, Y. (1962) Report to the Japan Academy of Sciences
Metcalf, R. L. (1955) Organic insecticides, Interscience, New York
Moeller, H. C. & Rider, J. A. (1961) Fed. Proc., 20, 434
Moeller, H. C. & Rider, J. A. (1962) Fed. Proc., 21, 451
Moeller, H. C. & Rider, J. A. (1963) Fed. Proc., 22, 189
Williams, M. W., Fuyat, H. N. & Fitzhugh, O. G. (1959) Toxicol.
appl. Pharmacol., 1, 1
BIOLOGICAL DATA
Biochemical aspects
Methyl parathion has biological properties similar to those of
parathion. Methyl parathion is oxidized to methyl paraoxon, mainly in
the liver, and this has a weaker inhibitory effect on cholinesterase
than paraoxon (Davison, 1955a; Hazleton, 1955). The molar I50 of
methyl paraoxon (rat brain 30 min. at 37°) was 4.0 × 10-8 (Davison,
1955b).
In guinea-pigs given orally 32P-labelled methyl parathion, the
compound began to enter the organs at once and the maximum tissue
levels were reached in 1-2 hours. A high concentration of the compound
was found in the liver (Gar et al., 1958).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Oral 9.0-42.0* Deichmann et al., 1952
Gaines, 1960
Metcalf, 1955
(continued)
Animal Route LD50 mg/kg References
body-weight
Rat Oral 9.7-14.8 Deichmann et al., 1952
(pure product) Gaines, 1960
Metcalf, 1955
Rat Intraperitoneal 3.5 DuBois & Coon, 1952
Mouse Oral 32.1 Ikeda, 1962
* Dependent on the sex of the animal, the vehicle used and the purity
of the sample.
Short-term studies
Dog. Groups of 2 dogs, one male and one female, were maintained
on diets containing 5, 20 and 50 ppm of methyl parathion for 12 weeks.
The two highest dosage levels produced significant depression in
erythrocyte cholinesterase activity. Plasm cholinesterase activity was
significantly depressed at 50 ppm but only a doubtful change was seen
at 20 ppm. The 5 ppm level produced no significant inhibition of
cholinesterase activity (Williams et al., 1959).
Man. A group of 5 subjects was given 3 mg of methyl parathion
orally per day for 28 days, 3.5 mg per day for 28 days, and 4.0 mg per
day for 43 days. No depression of erythrocyte or plasm cholinesterase
activity occurred and no side-effects were seen (Moeller & Rider,
1961).
A group of 5 subjects was given daily 4.5 mg during 30 days
followed by 5 mg for 29 days. Another group received 5.5 mg for 20
days followed by 6 mg for 29 days and the last group received 6.5 mg
for 35 days, followed by 7 mg for 24 days. The maximum depression of
whole-blood cholinesterase activity was 15% (Moeller & Rider, 1962).
Groups of 5 subjects were given methyl parathion for 30 days in
the following amounts: 7 mg per day, 7.5 mg per day, 8 and 9 mg per
day. The plasma and erythrocyte cholinesterase activities remained
within 20% of the control values (Moeller & Rider, 1963).
Long-term studies
No information available.
Comments on experimental studies reported
The biochemical and toxicological studies on this compound are
not as extensive as in the case of parathion but it would seem to have
been adequately investigated in man from the point of view of its
effect on cholinesterase activity.
EVALUATION
Level causing no significant toxicological effect
The highest dietary dose having no effect on cholinesterase
activity was 7-9 mg per day in man, equivalent to approximately 0.1
mg/kg body-weight per day.
Estimate of acceptable daily intake for man
0-0.01 mg/kg body-weight per day.
Further work desirable
Reproduction studies in the rat.
REFERENCES
Davison, A. N. (1955a) Biochem., J., 61, 203
Davison, A. N. (1955b) Biochem. J., 60, 399
Deichmann, W. B., Pugliese, W. & Cassidy, J. (1952) A.M.A. Arch.
industr. Hyg., 5, 523
DuBois, K. P. & Coon, J. M. (1952) A.M.A. Arch. industr. Hyg., 6,
9
Gaines, T. B. (1960) Toxicol. appl. Pharmacol., 2, 88
Gar, K. Sazonova, N. A., Fadeer, Y. N. Vladimirova, I. L. & Golubeva,
N. A. (1958) Org. Insektofungitsidy i Gerbitsidy, 93 (From Chem.
Abstr., 54, 15688)
Hazleton, L. W. (1955) Agr. Food Chem., 3, 312
Ikeda, Y. (1962) Report to the Japan Academy of Sciences
Metcalf, R. L. (1955) Organic insecticides, Interscience, New York
Moeller, H. C. & Rider, J. A. (1961) Fed. Proc., 20, 434
Moeller, H. C. & Rider, J. A. (1962) Fed. Proc., 21, 451
Moeller, H. C. & Rider, J. A. (1963) Fed. Proc., 22, 189
Williams, M. W., Fuyat, H. N. & Fitzhugh, O. G. (1959) Toxicol.
appl. Pharmacol., 1, 1
