
FAO Meeting Report No. PL/1965/10/1
WHO/Food Add./27.65
EVALUATION OF THE TOXICITY OF PESTICIDE RESIDUES IN FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Committee on Pesticides in Agriculture and
the WHO Expert Committee on Pesticide Residues, which met in Rome,
15-22 March 19651
Food and Agriculture Organization of the United Nations
World Health Organization
1965
1 Report of the second joint meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Committee on Pesticide
Residues, FAO Meeting Report No. PL/1965/10; WHO/Food Add./26.65
PHENYLMERCURIC ACETATE
Chemical name
Phenylmercuric acetate
Synonym
PMA
Empirical formula
C8H8O2Hg
Structural formula
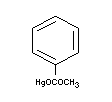 BIOLOGICAL DATA
Biochemical aspects
In rats, phenylmercuric acetate is readily absorbed from the
gastro-intestinal tract (Fitzhugh et al., 1950; Princkett et al.,
1950). The major route of elimination of mercury after oral,
intramuscular or intravenous administration of PMA is by way of the
bile and excretion into the alimentary tract (Berlin & Ullberg, 1963;
Princkett et al., 1950).
PMA given orally and intramuscularly to 87 chicks,
intramuscularly to 12 rats, and intravenously to 4 dogs, was absorbed
apparently unchanged. After about 96 hours no PMA could be detected in
the tissues, but inorganic mercury accumulated in the liver and kidney
(Miller et al., 1960). These findings were confirmed by studies with
radioactive PMA (Berlin & Ullberg, 1963). Excretion by the kidneys was
in the form of inorganic mercury and not as PMA (Berlin, 1963; Miller
et al., 1960). One explanation of this may be that PMA is
protein-bound in the blood.
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Intraperitoneal 10 Swensson, 1952
(approx. lethal dose)
Mouse Oral 70 Goldberg et al., 1950
Chick Oral 60 Miller et al., 1960
(approx. lethal dose)
Short-term studies
Rat. Rats were given intraperitoneal injections of PMA at
dosages of 1-2.5 mg/kg body-weight every other day for 4 weeks. The
animals showed gradually increasing apathy, loss of weight, and
finally neurological symptoms (ataxia and paresis), especially at the
higher dosages. Histopathological examination revealed damage to the
granular layer and the Purkinje cells of the cerebellum, and to the
spinal cord (Swensson, 1952).
Rabbit. A rabbit was fed with a diet containing PMA for 130
days, the total amount of mercury consumed during the experimental
period being 770 mg. The animal showed marked growth depression and
died after 130 days. Chemical analysis revealed large amounts of
mercury in the organs - 29 mg/kg organ-weight in the kidney, 0.52
mg/kg in the liver and 5.18 mg/kg in the gastro-intestinal tract
- whereas a control rabbit showed only 0.06 mg/kg in the kidney and
traces in the liver. Another rabbit fed a diet containing PMA for 100
days received a total amount of 6.9 mg of mercury. There was no
abnormality in appearance or growth. The contents of mercury in the
organs were 0.455 mg/kg in the kidney and 0.042 mg/kg in the liver
(Kluge et al., 1938).
Guinea-pig. A guinea-pig was fed a diet containing PMA for 670
days and consumed a total of 20.4 mg during the whole experimental
period. No ill-effects were observed in general appearance or growth.
The mercury content of the kidney was 4.76 mg/kg organ-weight, whereas
that of a control animal was 0.3 mg/kg (Kluge et al., 1938).
Long-term studies
Rat. Groups, each of 10-12 male and 10-12 female rats, were fed
diets containing 0.1, 0.5, 2.5, 10, 40 and 160 ppm of PMA for 2 years.
The growth was significantly retarded at 40 ppm and upward, and also
retarded in males at 10 ppm. The average survival period was reduced
at 160 ppm, while other dosage levels did not affect the mortality
rates. Gross pathological examination revealed enlargement and
granularity of the kidney, and moderate paleness of the viscera
suggestive of anaemia at 0.5 ppm and upward. Microscopic studies
demonstrated severe damage of the tubules of the kidney at 10 ppm in
females at one year, and there was detectable kidney damage at 0.5 ppm
in both sexes at 2 years. In males, marked changes in the renal
tubules were observed at 160 ppm at one year, and moderate to slight
at 40 ppm in both sexes. There were also some changes in the bone
marrow and caeca at high dosage levels. Accumulation of mercury
occurred in the organs and the storage of mercury in the kidney and
liver in the group at 0.1 ppm PMA was higher than that in the control
group (Fitzhugh et al., 1950).
Comments on experimental studies reported
It is clear from the biochemical studies that PMA may give rise
to mercury accumulation in the tissues and the long-term study in the
rat failed to demonstrate a no-effect level.
EVALUATION
Level causing no significant toxicological effect in rat
A no-effect level has not been demonstrated.
Estimate of acceptable daily intake for man
The level of 0.1 ppm, equivalent to 0.005 mg/kg body-weight per
day, produced a slight effect in the rat. Even if this figure were to
be adopted as a maximum no-effect level and the customary safety
factor applied this would give an acceptable daily intake for man of
0.00005 mg/kg body-weight. This is tantamount to zero. It is
undesirable that for the general population there should be any
increase in the natural intake of mercury. The same considerations
apply to other phenylmercury salts.
REFERENCES
Berlin, M. (1963) Arch. environm. Hlth, 6, 626
Berlin, M. & Ullberg, S. (1963) Arch. environm. Hlth, 6, 602
Fitzhugh, O. G. Nelson, A. A. Laug, E. P. & Kunze, F. M. (1950)
A.M.A. Arch. industr. Hyg., 2, 433
Goldberg, A. A., Shapero, M. & Wilder, E. (1950) J. Pharm. (Lond.),
2, 20
Kluge, H., Tschubel, H. & Zitek, A. (1938) Z. Untersuch.
Lebensmitt., 76, 322
Miller, V. L., Klavano, P. A. & Csonka, E. (1960) Toxicol. appl.
Pharmacol., 2, 344
Princkett, C. S., Laug, E. P. & Kunze, F. M. (1950) Proc. Soc. exp.
Biol. (N.Y.), 73, 585
Swensson, A. (1952) Acta med. scand., 143, 365
BIOLOGICAL DATA
Biochemical aspects
In rats, phenylmercuric acetate is readily absorbed from the
gastro-intestinal tract (Fitzhugh et al., 1950; Princkett et al.,
1950). The major route of elimination of mercury after oral,
intramuscular or intravenous administration of PMA is by way of the
bile and excretion into the alimentary tract (Berlin & Ullberg, 1963;
Princkett et al., 1950).
PMA given orally and intramuscularly to 87 chicks,
intramuscularly to 12 rats, and intravenously to 4 dogs, was absorbed
apparently unchanged. After about 96 hours no PMA could be detected in
the tissues, but inorganic mercury accumulated in the liver and kidney
(Miller et al., 1960). These findings were confirmed by studies with
radioactive PMA (Berlin & Ullberg, 1963). Excretion by the kidneys was
in the form of inorganic mercury and not as PMA (Berlin, 1963; Miller
et al., 1960). One explanation of this may be that PMA is
protein-bound in the blood.
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Intraperitoneal 10 Swensson, 1952
(approx. lethal dose)
Mouse Oral 70 Goldberg et al., 1950
Chick Oral 60 Miller et al., 1960
(approx. lethal dose)
Short-term studies
Rat. Rats were given intraperitoneal injections of PMA at
dosages of 1-2.5 mg/kg body-weight every other day for 4 weeks. The
animals showed gradually increasing apathy, loss of weight, and
finally neurological symptoms (ataxia and paresis), especially at the
higher dosages. Histopathological examination revealed damage to the
granular layer and the Purkinje cells of the cerebellum, and to the
spinal cord (Swensson, 1952).
Rabbit. A rabbit was fed with a diet containing PMA for 130
days, the total amount of mercury consumed during the experimental
period being 770 mg. The animal showed marked growth depression and
died after 130 days. Chemical analysis revealed large amounts of
mercury in the organs - 29 mg/kg organ-weight in the kidney, 0.52
mg/kg in the liver and 5.18 mg/kg in the gastro-intestinal tract
- whereas a control rabbit showed only 0.06 mg/kg in the kidney and
traces in the liver. Another rabbit fed a diet containing PMA for 100
days received a total amount of 6.9 mg of mercury. There was no
abnormality in appearance or growth. The contents of mercury in the
organs were 0.455 mg/kg in the kidney and 0.042 mg/kg in the liver
(Kluge et al., 1938).
Guinea-pig. A guinea-pig was fed a diet containing PMA for 670
days and consumed a total of 20.4 mg during the whole experimental
period. No ill-effects were observed in general appearance or growth.
The mercury content of the kidney was 4.76 mg/kg organ-weight, whereas
that of a control animal was 0.3 mg/kg (Kluge et al., 1938).
Long-term studies
Rat. Groups, each of 10-12 male and 10-12 female rats, were fed
diets containing 0.1, 0.5, 2.5, 10, 40 and 160 ppm of PMA for 2 years.
The growth was significantly retarded at 40 ppm and upward, and also
retarded in males at 10 ppm. The average survival period was reduced
at 160 ppm, while other dosage levels did not affect the mortality
rates. Gross pathological examination revealed enlargement and
granularity of the kidney, and moderate paleness of the viscera
suggestive of anaemia at 0.5 ppm and upward. Microscopic studies
demonstrated severe damage of the tubules of the kidney at 10 ppm in
females at one year, and there was detectable kidney damage at 0.5 ppm
in both sexes at 2 years. In males, marked changes in the renal
tubules were observed at 160 ppm at one year, and moderate to slight
at 40 ppm in both sexes. There were also some changes in the bone
marrow and caeca at high dosage levels. Accumulation of mercury
occurred in the organs and the storage of mercury in the kidney and
liver in the group at 0.1 ppm PMA was higher than that in the control
group (Fitzhugh et al., 1950).
Comments on experimental studies reported
It is clear from the biochemical studies that PMA may give rise
to mercury accumulation in the tissues and the long-term study in the
rat failed to demonstrate a no-effect level.
EVALUATION
Level causing no significant toxicological effect in rat
A no-effect level has not been demonstrated.
Estimate of acceptable daily intake for man
The level of 0.1 ppm, equivalent to 0.005 mg/kg body-weight per
day, produced a slight effect in the rat. Even if this figure were to
be adopted as a maximum no-effect level and the customary safety
factor applied this would give an acceptable daily intake for man of
0.00005 mg/kg body-weight. This is tantamount to zero. It is
undesirable that for the general population there should be any
increase in the natural intake of mercury. The same considerations
apply to other phenylmercury salts.
REFERENCES
Berlin, M. (1963) Arch. environm. Hlth, 6, 626
Berlin, M. & Ullberg, S. (1963) Arch. environm. Hlth, 6, 602
Fitzhugh, O. G. Nelson, A. A. Laug, E. P. & Kunze, F. M. (1950)
A.M.A. Arch. industr. Hyg., 2, 433
Goldberg, A. A., Shapero, M. & Wilder, E. (1950) J. Pharm. (Lond.),
2, 20
Kluge, H., Tschubel, H. & Zitek, A. (1938) Z. Untersuch.
Lebensmitt., 76, 322
Miller, V. L., Klavano, P. A. & Csonka, E. (1960) Toxicol. appl.
Pharmacol., 2, 344
Princkett, C. S., Laug, E. P. & Kunze, F. M. (1950) Proc. Soc. exp.
Biol. (N.Y.), 73, 585
Swensson, A. (1952) Acta med. scand., 143, 365
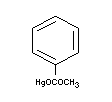 BIOLOGICAL DATA
Biochemical aspects
In rats, phenylmercuric acetate is readily absorbed from the
gastro-intestinal tract (Fitzhugh et al., 1950; Princkett et al.,
1950). The major route of elimination of mercury after oral,
intramuscular or intravenous administration of PMA is by way of the
bile and excretion into the alimentary tract (Berlin & Ullberg, 1963;
Princkett et al., 1950).
PMA given orally and intramuscularly to 87 chicks,
intramuscularly to 12 rats, and intravenously to 4 dogs, was absorbed
apparently unchanged. After about 96 hours no PMA could be detected in
the tissues, but inorganic mercury accumulated in the liver and kidney
(Miller et al., 1960). These findings were confirmed by studies with
radioactive PMA (Berlin & Ullberg, 1963). Excretion by the kidneys was
in the form of inorganic mercury and not as PMA (Berlin, 1963; Miller
et al., 1960). One explanation of this may be that PMA is
protein-bound in the blood.
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Intraperitoneal 10 Swensson, 1952
(approx. lethal dose)
Mouse Oral 70 Goldberg et al., 1950
Chick Oral 60 Miller et al., 1960
(approx. lethal dose)
Short-term studies
Rat. Rats were given intraperitoneal injections of PMA at
dosages of 1-2.5 mg/kg body-weight every other day for 4 weeks. The
animals showed gradually increasing apathy, loss of weight, and
finally neurological symptoms (ataxia and paresis), especially at the
higher dosages. Histopathological examination revealed damage to the
granular layer and the Purkinje cells of the cerebellum, and to the
spinal cord (Swensson, 1952).
Rabbit. A rabbit was fed with a diet containing PMA for 130
days, the total amount of mercury consumed during the experimental
period being 770 mg. The animal showed marked growth depression and
died after 130 days. Chemical analysis revealed large amounts of
mercury in the organs - 29 mg/kg organ-weight in the kidney, 0.52
mg/kg in the liver and 5.18 mg/kg in the gastro-intestinal tract
- whereas a control rabbit showed only 0.06 mg/kg in the kidney and
traces in the liver. Another rabbit fed a diet containing PMA for 100
days received a total amount of 6.9 mg of mercury. There was no
abnormality in appearance or growth. The contents of mercury in the
organs were 0.455 mg/kg in the kidney and 0.042 mg/kg in the liver
(Kluge et al., 1938).
Guinea-pig. A guinea-pig was fed a diet containing PMA for 670
days and consumed a total of 20.4 mg during the whole experimental
period. No ill-effects were observed in general appearance or growth.
The mercury content of the kidney was 4.76 mg/kg organ-weight, whereas
that of a control animal was 0.3 mg/kg (Kluge et al., 1938).
Long-term studies
Rat. Groups, each of 10-12 male and 10-12 female rats, were fed
diets containing 0.1, 0.5, 2.5, 10, 40 and 160 ppm of PMA for 2 years.
The growth was significantly retarded at 40 ppm and upward, and also
retarded in males at 10 ppm. The average survival period was reduced
at 160 ppm, while other dosage levels did not affect the mortality
rates. Gross pathological examination revealed enlargement and
granularity of the kidney, and moderate paleness of the viscera
suggestive of anaemia at 0.5 ppm and upward. Microscopic studies
demonstrated severe damage of the tubules of the kidney at 10 ppm in
females at one year, and there was detectable kidney damage at 0.5 ppm
in both sexes at 2 years. In males, marked changes in the renal
tubules were observed at 160 ppm at one year, and moderate to slight
at 40 ppm in both sexes. There were also some changes in the bone
marrow and caeca at high dosage levels. Accumulation of mercury
occurred in the organs and the storage of mercury in the kidney and
liver in the group at 0.1 ppm PMA was higher than that in the control
group (Fitzhugh et al., 1950).
Comments on experimental studies reported
It is clear from the biochemical studies that PMA may give rise
to mercury accumulation in the tissues and the long-term study in the
rat failed to demonstrate a no-effect level.
EVALUATION
Level causing no significant toxicological effect in rat
A no-effect level has not been demonstrated.
Estimate of acceptable daily intake for man
The level of 0.1 ppm, equivalent to 0.005 mg/kg body-weight per
day, produced a slight effect in the rat. Even if this figure were to
be adopted as a maximum no-effect level and the customary safety
factor applied this would give an acceptable daily intake for man of
0.00005 mg/kg body-weight. This is tantamount to zero. It is
undesirable that for the general population there should be any
increase in the natural intake of mercury. The same considerations
apply to other phenylmercury salts.
REFERENCES
Berlin, M. (1963) Arch. environm. Hlth, 6, 626
Berlin, M. & Ullberg, S. (1963) Arch. environm. Hlth, 6, 602
Fitzhugh, O. G. Nelson, A. A. Laug, E. P. & Kunze, F. M. (1950)
A.M.A. Arch. industr. Hyg., 2, 433
Goldberg, A. A., Shapero, M. & Wilder, E. (1950) J. Pharm. (Lond.),
2, 20
Kluge, H., Tschubel, H. & Zitek, A. (1938) Z. Untersuch.
Lebensmitt., 76, 322
Miller, V. L., Klavano, P. A. & Csonka, E. (1960) Toxicol. appl.
Pharmacol., 2, 344
Princkett, C. S., Laug, E. P. & Kunze, F. M. (1950) Proc. Soc. exp.
Biol. (N.Y.), 73, 585
Swensson, A. (1952) Acta med. scand., 143, 365
BIOLOGICAL DATA
Biochemical aspects
In rats, phenylmercuric acetate is readily absorbed from the
gastro-intestinal tract (Fitzhugh et al., 1950; Princkett et al.,
1950). The major route of elimination of mercury after oral,
intramuscular or intravenous administration of PMA is by way of the
bile and excretion into the alimentary tract (Berlin & Ullberg, 1963;
Princkett et al., 1950).
PMA given orally and intramuscularly to 87 chicks,
intramuscularly to 12 rats, and intravenously to 4 dogs, was absorbed
apparently unchanged. After about 96 hours no PMA could be detected in
the tissues, but inorganic mercury accumulated in the liver and kidney
(Miller et al., 1960). These findings were confirmed by studies with
radioactive PMA (Berlin & Ullberg, 1963). Excretion by the kidneys was
in the form of inorganic mercury and not as PMA (Berlin, 1963; Miller
et al., 1960). One explanation of this may be that PMA is
protein-bound in the blood.
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Intraperitoneal 10 Swensson, 1952
(approx. lethal dose)
Mouse Oral 70 Goldberg et al., 1950
Chick Oral 60 Miller et al., 1960
(approx. lethal dose)
Short-term studies
Rat. Rats were given intraperitoneal injections of PMA at
dosages of 1-2.5 mg/kg body-weight every other day for 4 weeks. The
animals showed gradually increasing apathy, loss of weight, and
finally neurological symptoms (ataxia and paresis), especially at the
higher dosages. Histopathological examination revealed damage to the
granular layer and the Purkinje cells of the cerebellum, and to the
spinal cord (Swensson, 1952).
Rabbit. A rabbit was fed with a diet containing PMA for 130
days, the total amount of mercury consumed during the experimental
period being 770 mg. The animal showed marked growth depression and
died after 130 days. Chemical analysis revealed large amounts of
mercury in the organs - 29 mg/kg organ-weight in the kidney, 0.52
mg/kg in the liver and 5.18 mg/kg in the gastro-intestinal tract
- whereas a control rabbit showed only 0.06 mg/kg in the kidney and
traces in the liver. Another rabbit fed a diet containing PMA for 100
days received a total amount of 6.9 mg of mercury. There was no
abnormality in appearance or growth. The contents of mercury in the
organs were 0.455 mg/kg in the kidney and 0.042 mg/kg in the liver
(Kluge et al., 1938).
Guinea-pig. A guinea-pig was fed a diet containing PMA for 670
days and consumed a total of 20.4 mg during the whole experimental
period. No ill-effects were observed in general appearance or growth.
The mercury content of the kidney was 4.76 mg/kg organ-weight, whereas
that of a control animal was 0.3 mg/kg (Kluge et al., 1938).
Long-term studies
Rat. Groups, each of 10-12 male and 10-12 female rats, were fed
diets containing 0.1, 0.5, 2.5, 10, 40 and 160 ppm of PMA for 2 years.
The growth was significantly retarded at 40 ppm and upward, and also
retarded in males at 10 ppm. The average survival period was reduced
at 160 ppm, while other dosage levels did not affect the mortality
rates. Gross pathological examination revealed enlargement and
granularity of the kidney, and moderate paleness of the viscera
suggestive of anaemia at 0.5 ppm and upward. Microscopic studies
demonstrated severe damage of the tubules of the kidney at 10 ppm in
females at one year, and there was detectable kidney damage at 0.5 ppm
in both sexes at 2 years. In males, marked changes in the renal
tubules were observed at 160 ppm at one year, and moderate to slight
at 40 ppm in both sexes. There were also some changes in the bone
marrow and caeca at high dosage levels. Accumulation of mercury
occurred in the organs and the storage of mercury in the kidney and
liver in the group at 0.1 ppm PMA was higher than that in the control
group (Fitzhugh et al., 1950).
Comments on experimental studies reported
It is clear from the biochemical studies that PMA may give rise
to mercury accumulation in the tissues and the long-term study in the
rat failed to demonstrate a no-effect level.
EVALUATION
Level causing no significant toxicological effect in rat
A no-effect level has not been demonstrated.
Estimate of acceptable daily intake for man
The level of 0.1 ppm, equivalent to 0.005 mg/kg body-weight per
day, produced a slight effect in the rat. Even if this figure were to
be adopted as a maximum no-effect level and the customary safety
factor applied this would give an acceptable daily intake for man of
0.00005 mg/kg body-weight. This is tantamount to zero. It is
undesirable that for the general population there should be any
increase in the natural intake of mercury. The same considerations
apply to other phenylmercury salts.
REFERENCES
Berlin, M. (1963) Arch. environm. Hlth, 6, 626
Berlin, M. & Ullberg, S. (1963) Arch. environm. Hlth, 6, 602
Fitzhugh, O. G. Nelson, A. A. Laug, E. P. & Kunze, F. M. (1950)
A.M.A. Arch. industr. Hyg., 2, 433
Goldberg, A. A., Shapero, M. & Wilder, E. (1950) J. Pharm. (Lond.),
2, 20
Kluge, H., Tschubel, H. & Zitek, A. (1938) Z. Untersuch.
Lebensmitt., 76, 322
Miller, V. L., Klavano, P. A. & Csonka, E. (1960) Toxicol. appl.
Pharmacol., 2, 344
Princkett, C. S., Laug, E. P. & Kunze, F. M. (1950) Proc. Soc. exp.
Biol. (N.Y.), 73, 585
Swensson, A. (1952) Acta med. scand., 143, 365
