
FAO/PL:1968/M/9/1
WHO/FOOD ADD./69.35
1968 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
Issued jointly by FAO and WHO
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Committee on Pesticide Residues, which met in Geneva, 9-16 December,
1968.
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
WORLD HEALTH ORGANIZATION
Geneva, 1969
CRUFOMATE
IDENTITY
Chemical name
4-tert-butyl-2-chlorophenyl methyl methylphosphoramidate
O-(4-tertbutyl-2-chlorophenyl) methyl methylphosphoramidate
(IUPAC)
Synonyms
Ruelene(R), Dowco(R)132, Montrel(R), Rulex(R)
Structural formula
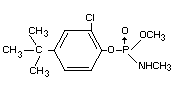 Other information on identity and properties
The recrystallized technical product melts at 52.5-59.2°C. When pure,
crufomate is a white solid melting at 62-62.5°C. The compound is
stable from room temperature to 60°C. It is unstable in strongly acid
and in alkaline media. At room temperature, it is freely soluble in
most of the common organic solvents except for the paraffin solvents
in which its solubility is about one to three per cent by weight. Its
solubility in water is 0.5 per cent by weight.
Crufomate is formulated in several concentrations either for direct
use or for application after suitable dilution (emulsifiable
concentrates, pour-ons, systemic insecticide, anthelmintic, sheep and
goat wormer).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Following an 11 mg/kg intramuscular dosage of 32P labelled crufomated
to a yearling steer, radioactivity at the site of the treatment
dropped from 16 milliroentgens per hour immediately following the
treatment to 1.5 within two days and 0 after 13 days. Residues of
crufomate were practically non-existent after 14 days. Peak levels of
radioactivity occurred in the blood, urine and faeces in two to eight
hours, 12-18 hours, and 18-30 hours respectively. Fat tissues
contained less than 0.2 ppm of radioactive compounds after 14 days.
Red blood cholinesterase activity was depressed no more than 25 per
cent. The end product of the metabolism of crufomate is phosphoric
acid (Plapp. 1960).
A 21-day study of the metabolism of radioactive crufomate using acute
oral dosages of 100 and 200 mg/kg of body-weight in sheep shows
residues in the urine consisting of crufomate and several hydrolysis
products. Over 85 per cent of the administered 32P was recovered in
the excreta. The 32P in the urine, amounting to 75 per cent of the
dose, was primarily in the form of the hydrolysis products of
crufomate. Some of the crufomate was hydrolyzed completely to
inorganic phosphate and retained in the animal tissues and bones.
Crufomate itself was not found in the tissues after seven days,
indicating fairly rapid metabolism. Residues of crufomate and several
hydrolysis products were found in the blood and plasma shortly after
treatment, but within two days the crufomate decreased to a low level
(Bauriedel and Swank, 1962).
Other metabolic studies in sheep (Chamberlain and Gatterdam, 1960),
and in poultry (Buttram and Arthur, 1961) showed rapid breakdown of
crufomate. The likely mammalian metabolites of crufomate do not cause
inhibition of cholinesterase (Smith, 1968).
Acute toxicity (oral)
Animal Sex LD50 (mg/kg References
body-weight)
Rat male 950 McCollister et al., 1968
Rat female 770 McCollister et al., 1968
Rabbit mixed 490 McCollister et al., 1968
Guinea-pig male 1 000 McCollister et al., 1968
Dog male >1 000 McCollister et al., 1968
Dog female >750 McCollister et al., 1968
Sheep. Lambs received a single dose of 100, 200 or 400 mg/kg. Signs
of intoxication appeared at 200 mg/kg or more. Whole blood
cholinesterase activity was depressed to 50 per cent at 100 mg/kg,
and was markedly depressed at higher doses (Galvin et al., 1960).
Symptoms are highly variable and mainly caused by central nervous
system disturbances. At necropsy severe congestion of lungs and
kidneys, enlargement of liver and scattered haemorrhages were found
(Radeleff, 1964).
Short-term studies
Rat. Rats (10 males and 10 females per group) were started at the
age of 45 days on diets containing 10, 30, 100, 300 or 1000 ppm
crufomate. During a 90-day experimental period no evidence of
ill-effect was found when judged by general appearance and behaviour,
growth, mortality or food consumption records, haematological
determinations, final body and organ weight ratios, and gross and
microscopic examination of tissues. Results of measurements of
cholinesterase activity in blood and brain show that at 41 days there
was a peculiarly uniform depression of cholinesterase activity in the
plasma of both male and female rats over the entire range of 10-1000
ppm. The same phenomenon, although not as marked, was seen at 69 days,
indicating that a higher intake of chemical per unit of body-weight
during the early period way have been partially responsible.
Measurement of the erythrocyte-and brain-cholinesterase activities
showed little or no effect at the 30 ppm level of crufomate. In the
groups of rats from each dietary level which were placed on the
control ration for 48 days following the 90-day test period, recovery
of blood and brain cholinesterase was complete (McCollister et al.,
1968).
Dog. Groups comprising one male and one female dog were given diets
containing 0, 15, 40, 125 or 250 ppm crufomate for 75 days. Dogs that
received 250 ppm crufomate in their diets for 75 days showed no
evidence of adverse effect by any of the criteria examined except
cholinesterase activity and liver histopathology. Plasma-,
erythrocyte- and brain-cholinesterase levels were decreased to about
65 per cent of the control values. Slight centrilobular granular
degeneration was observed on microscopic examination of the liver.
Cholinesterase measurements were within the normal range in dogs at
the 125 ppm level. There was no evidence of adverse effect in dogs
that received 15 or 40 ppm dietary crufomate for 75 days, as judged by
general appearance and behaviour, growth, mortality, food consumption,
haematological indices, cholinesterase activities, final body and
organ weights and gross and microscopic appearance of the tissues
(McCollister et al., 1968).
Groups of dogs, each containing four males and four females per group,
were given diets containing 0, 10, 20, 200 or 2000 ppm of crufomate
for up to two years. The dogs on 2000 ppm crufomate were transferred
to the control diet after 30 days and sacrificed on days 94 and 95 of
the study. After one year, one male and one female from each of the
other groups were sacrificed for tissue examination. Cholinesterase
determinations were made at 30, 60 and 90 days after the start of the
study and at six-week intervals up to one year and every three months
thereafter. The animals on the highest level of crufomate manifested
gross signs of intoxication, values for serum alkaline phosphatase and
transaminase were elevated, plasma cholinesterase was reduced to 55
per cent of the pre-test average, and red cell cholinesterase was
depressed to 19 per cent of the pre-test values. In those dogs that
received 200 ppm crufomate in their diet plasma and erythrocyte
cholinesterase activity was depressed slightly. Other biochemical,
haematological findings and gross and microscopic examinations of the
tissues were comparable to the controls. Groups of male and female
dogs that received diets containing 10 or 20 ppm for two years showed
no evidence of adverse effect according to any of the criteria applied
(McCollister et al,, 1968).
Long-term studies
Rat. Groups of rats (25 of each sex) were started on diets
containing 1, 10, 100 or 1000 ppm of crufomate. Fifteen rats of each
sex from each group were designated for a two-year study. The
remaining 10 of each sex were killed at 12 or 18 months. Additional
groups of rats (five of each sex) were given diets containing 20, 40,
60 or 80 ppm of crufomate for 20 months.
Both male and female rats that received 1000 ppm crufomate in their
diets showed retardation of growth during the second year of the
study. Necropsy and microscopic examination of these rats after 24
months revealed atrophy of the muscles of the hindlegs and slight
degeneration of the sciatic nerve. A reduction in testis weight to
about one half, reflecting degeneration and atrophy of seminiferous
tubules, was seen at the 1000 ppm level after 12 and 18 months and
again at 24 months. The only evidence of my effect at dosage levels
below 1000 ppm was depression of cholinesterase. Brain cholinesterase
activity was depressed to 38 to 50 per cent of control for the rats
fed 1000 ppm but was within normal range at the lower dosage levels.
Male rats showed no significant effect on plasma cholinesterase at 100
ppm and below, or in red cells at 40 ppm and below. Corresponding
values for female rats were 40 ppm for plasma and 60 ppm for red cell
cholinesterase (McCollister et al., 1968).
Special studies
(a) Fertility and reproduction
Groups of four male and 12 female rats were maintained on diets
containing 0, 10 and 100 ppm crufomate through a three-generation
study involving two litters per generation and assessing fertility,
reproduction and lactation. In addition, a fourth group received 1 ppm
crufomate for the first generation and 500 ppm for the remaining two
generations of the experiment. In all these groups no evidence was
found of adverse effect on fertility, gestation, viability or
lactation, as well as body-weights at weaning and mating.
Cholinesterase inhibition was not investigated (McCollister at al.,
1968).
Forty bulls, three to seven years old, were given a single oral dose
of crufomate at 50 mg/kg or sprayed to wetness with 0.75 per cent
dispersion of a crufomate wettable powder without affecting the
motility of the spermatozoa after one and four weeks (McGregor 1960).
An acute oral dosage of 100 mg/kg of crufomate had no effect on the
fertility of male rats, rabbits or guinea-pigs (McCollister, 1959).
(b) Neurotoxicity
Intraperitoneal injections of crufomate into 25 mature hens at dosage
rates of 500, 750 and 1000 mg/kg caused mortalities in one out of
seven, one out of four and 10 out of 14, respectively, of the birds
treated. Of the surviving 31 treated hens 26 developed ataxia and
paralysis. Those able to take food and water made uneventful and
complete recovery within 90 days of treatment. Histopathological
examination of nerve tissue revealed no departure from normal (Hymas
and Stevenson, 1961).
(c) Potentiation
Single oral doses of crufomate were administered jointly to rats with
each of 14 commercial cholinesterase inhibiting insecticides (13
organo-phosphorus compounds and one carbamate). There was no effect
other than additive (ratios 0.7 to 2.2) for the LD50 values except
for crufomate-malathion (fivefold increase). Dietary feeding studies
in dogs for 12 weeks with diets containing 100 ppm malathion plus 20
ppm crufomate, or 10 ppm malathion plus 20 ppm crufomate, showed no
significant evidence of potentiation (McCollister et al., 1968).
(d) Studies on the metabolite
Rat. Groups of rats (25 of each sex) were fed 100, 300, 1000, and
3000 ppm 4-tert-butyl-2-chlorophenol (the hydrolytic metabolite of
crufomate) in the diet for two years. Rats fed the dosage levels of
100, 300 or 1000 ppm showed no significant evidence of adverse effects
as judged by general appearance, behaviour, growth, mortality,
incidence of tumorous growths, haematological studies, serum urea
nitrogen and alkaline phosphatase determination. Final average body
and organ weights and gross and microscopic effects after examination
of the tissues were normal. Rats fed dietary levels of 3000 ppm showed
moderate microscopic liver changes and increased senility in the
heart, kidney and liver (McCollister, 1964).
Dog. Groups of eight dogs (four of each sex) were fed diets
containing 20, 200 or 2000 ppm 4-tert-butyl-2-chlorophenol for two
years. The male dogs on 2000 ppm showed atrophy and degenerative
changes in the seminiferous tubules of the testes. No other gross or
microscopic effect was observed at any dose level (Dieterich et al.,
1965).
Observations in man
Groups of three adult males per test were given one, two or four
tablets containing 200 mg of crufomate daily for seven days.
Laboratory tests consisted of complete blood counts, urinalysis, red
blood cell cholinesterase response and serum glutamic pyruvic
transaminase determinations. The subjects were interrogated concerning
side effects. The second and third groups showed a delayed 50-80 per
cent red blood cell cholinesterase depression after completion of the
treatments. No other abnormalities were noted in the laboratory tests
(Campbell, 1962).
Comments
The most sensitive criterion upon which to judge the safety of
crufomate upon ingestion by animals or human subjects is its
cholinesterase-inhibiting properties. The short-term and long-term
studies are adequate. Because the only study available in man was too
limited, it is desirable that further metabolic studies in man should
be carried out to show that 4-tert-butyl-2-chlorophenol is the main
metabolite also in this species.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Rat: 40 ppm in the diet, equivalent to 2 mg/kg per day
Dog: 40 ppm in the diet, equivalent to 1 mg/kg per day
Man: 200 mg/day, equivalent to about 3 mg/kg per day
Estimate of acceptable daily intake for man
0-0.1 mg/kg per day
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Crufomate is used on or in cattle, sheep, and goats to control
internal cattle grubs and external horn flies and cattle lice (Anon.,
1966). Applications of crufomate for cattle grub control have been by
injection, oral administration, and spraying (McGregor et al., 1959),
but the simple pour-on application of a low volume of insecticide on
the backs of cattle has been found best (Rogoff and Kohler, 196O).
Formulations of crufomate may be sprayed or poured on to cattle during
the warmer months of the year. However, in the United States of
America during October through February, treatment is restricted
depending on the locality. The dosage rate ranges from about 15 to 50
mg/kg of body-weight.
As a pour-on applied to the backline of cattle, an emulsifiable
concentrate of crufomate is diluted with water to apply about 39 mg/kg
for grubs, horn flies and lice, and about 52 mg/kg for internal
helminth parasite control.
Another treatment of internal helminth parasites of livestock involves
the oral drench (introduction into rumen directly) of 50 to 75 mg/kg.
The following internal parasites of livestock are examples of genera
for which this treatment has been recommended: Haemonchus and
Osteragia (stomach worms), Tricho-strongylus (hairworms),
Strongyloides (threadworm), Bunostomum (hookworm),
Oesophagostomum (nodular worms), Nematodirus (thin-necked
strongyle), Trichuris (whipworms), Cooperia (Cooper's worms),
Oestrus ovis (head grub) and others.
Retreatment for horn flies and cattle lice should not be done more
often than every 28 days and not within 28 days of slaughter. Animals
under stress from sickness, crowding, excitement or surgery should not
be treated. Lactating dairy cows or dry dairy cows within 28 days of
freshening should not be treated. General recommendations for the
current use of cruformate are given in Anon. (1967, 1968).
Residues resulting from supervised trials
When crufomate is used as directed, its residues decrease to a
negligible level 28 days after external treatment and 14 days after
internal treatment. Residues of crufomate found in supervised trials
are summarized in the following table:
Rate of Post-treatment Residues* Reference
Animal application interval (days) (ppm)
(mg/kg)
Cattle 75 14 No residues Anon. (1962b)
(<0.04) fat,
lean meat
Sheep 89.8 14 No residues Claborn et al.
(<0.02) fat, (1960)
internal organs
Lambs 200 7 No residues Bauriedel and
(<0.05) fat, Swank (1962)
lean meat
Cattle 50 7 0.3-1.3** Mussel and
fat, less in Ludwig (1961)
other tissues
Cattle 100 7 0.6-1.6** Mussel and
fat, less in Ludwig (1961)
other tissues
(continued)
Rate of Post-treatment Residues* Reference
Animal application interval (days) (ppm)
(mg/kg)
Cattle 50 7 0.07-0.2** fat Mussel and
Ludwig (1961)
Cattle 100 7 0.1-2.4** fat Mussel and
Ludwig (1961)
Cattle 52 7 0.05-0.14 fat Dishburger and
14 <0.02-0.05 fat Rice (1968)
21 no residues
(<0.02) fat
Calves 52 and 104 28 0.1-0.22 fat Rice (1964)
Lactating 49-66 1 0.195 milk Leahy and
cow 2 no detectable Brown (1963)
residues
(<0.05) milk
Lactating 37.5 1 0.14-0.3 milk Anon. 1962a
cow 2 0.0 milk
Steers 50 7 0.1 phenol in Kutchinski
fat (limit of (1960)
sensitivity, 0.1)
* When one figure is given, residue is maximum value.
** No residues (<0.1 ppm) after 28 days.
Fate of residues
In animals
Six possible routes for the hydrolysis of crufomate to inorganic
phosphate are shown below (Bauriedel and Swank, 1962).
Other information on identity and properties
The recrystallized technical product melts at 52.5-59.2°C. When pure,
crufomate is a white solid melting at 62-62.5°C. The compound is
stable from room temperature to 60°C. It is unstable in strongly acid
and in alkaline media. At room temperature, it is freely soluble in
most of the common organic solvents except for the paraffin solvents
in which its solubility is about one to three per cent by weight. Its
solubility in water is 0.5 per cent by weight.
Crufomate is formulated in several concentrations either for direct
use or for application after suitable dilution (emulsifiable
concentrates, pour-ons, systemic insecticide, anthelmintic, sheep and
goat wormer).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Following an 11 mg/kg intramuscular dosage of 32P labelled crufomated
to a yearling steer, radioactivity at the site of the treatment
dropped from 16 milliroentgens per hour immediately following the
treatment to 1.5 within two days and 0 after 13 days. Residues of
crufomate were practically non-existent after 14 days. Peak levels of
radioactivity occurred in the blood, urine and faeces in two to eight
hours, 12-18 hours, and 18-30 hours respectively. Fat tissues
contained less than 0.2 ppm of radioactive compounds after 14 days.
Red blood cholinesterase activity was depressed no more than 25 per
cent. The end product of the metabolism of crufomate is phosphoric
acid (Plapp. 1960).
A 21-day study of the metabolism of radioactive crufomate using acute
oral dosages of 100 and 200 mg/kg of body-weight in sheep shows
residues in the urine consisting of crufomate and several hydrolysis
products. Over 85 per cent of the administered 32P was recovered in
the excreta. The 32P in the urine, amounting to 75 per cent of the
dose, was primarily in the form of the hydrolysis products of
crufomate. Some of the crufomate was hydrolyzed completely to
inorganic phosphate and retained in the animal tissues and bones.
Crufomate itself was not found in the tissues after seven days,
indicating fairly rapid metabolism. Residues of crufomate and several
hydrolysis products were found in the blood and plasma shortly after
treatment, but within two days the crufomate decreased to a low level
(Bauriedel and Swank, 1962).
Other metabolic studies in sheep (Chamberlain and Gatterdam, 1960),
and in poultry (Buttram and Arthur, 1961) showed rapid breakdown of
crufomate. The likely mammalian metabolites of crufomate do not cause
inhibition of cholinesterase (Smith, 1968).
Acute toxicity (oral)
Animal Sex LD50 (mg/kg References
body-weight)
Rat male 950 McCollister et al., 1968
Rat female 770 McCollister et al., 1968
Rabbit mixed 490 McCollister et al., 1968
Guinea-pig male 1 000 McCollister et al., 1968
Dog male >1 000 McCollister et al., 1968
Dog female >750 McCollister et al., 1968
Sheep. Lambs received a single dose of 100, 200 or 400 mg/kg. Signs
of intoxication appeared at 200 mg/kg or more. Whole blood
cholinesterase activity was depressed to 50 per cent at 100 mg/kg,
and was markedly depressed at higher doses (Galvin et al., 1960).
Symptoms are highly variable and mainly caused by central nervous
system disturbances. At necropsy severe congestion of lungs and
kidneys, enlargement of liver and scattered haemorrhages were found
(Radeleff, 1964).
Short-term studies
Rat. Rats (10 males and 10 females per group) were started at the
age of 45 days on diets containing 10, 30, 100, 300 or 1000 ppm
crufomate. During a 90-day experimental period no evidence of
ill-effect was found when judged by general appearance and behaviour,
growth, mortality or food consumption records, haematological
determinations, final body and organ weight ratios, and gross and
microscopic examination of tissues. Results of measurements of
cholinesterase activity in blood and brain show that at 41 days there
was a peculiarly uniform depression of cholinesterase activity in the
plasma of both male and female rats over the entire range of 10-1000
ppm. The same phenomenon, although not as marked, was seen at 69 days,
indicating that a higher intake of chemical per unit of body-weight
during the early period way have been partially responsible.
Measurement of the erythrocyte-and brain-cholinesterase activities
showed little or no effect at the 30 ppm level of crufomate. In the
groups of rats from each dietary level which were placed on the
control ration for 48 days following the 90-day test period, recovery
of blood and brain cholinesterase was complete (McCollister et al.,
1968).
Dog. Groups comprising one male and one female dog were given diets
containing 0, 15, 40, 125 or 250 ppm crufomate for 75 days. Dogs that
received 250 ppm crufomate in their diets for 75 days showed no
evidence of adverse effect by any of the criteria examined except
cholinesterase activity and liver histopathology. Plasma-,
erythrocyte- and brain-cholinesterase levels were decreased to about
65 per cent of the control values. Slight centrilobular granular
degeneration was observed on microscopic examination of the liver.
Cholinesterase measurements were within the normal range in dogs at
the 125 ppm level. There was no evidence of adverse effect in dogs
that received 15 or 40 ppm dietary crufomate for 75 days, as judged by
general appearance and behaviour, growth, mortality, food consumption,
haematological indices, cholinesterase activities, final body and
organ weights and gross and microscopic appearance of the tissues
(McCollister et al., 1968).
Groups of dogs, each containing four males and four females per group,
were given diets containing 0, 10, 20, 200 or 2000 ppm of crufomate
for up to two years. The dogs on 2000 ppm crufomate were transferred
to the control diet after 30 days and sacrificed on days 94 and 95 of
the study. After one year, one male and one female from each of the
other groups were sacrificed for tissue examination. Cholinesterase
determinations were made at 30, 60 and 90 days after the start of the
study and at six-week intervals up to one year and every three months
thereafter. The animals on the highest level of crufomate manifested
gross signs of intoxication, values for serum alkaline phosphatase and
transaminase were elevated, plasma cholinesterase was reduced to 55
per cent of the pre-test average, and red cell cholinesterase was
depressed to 19 per cent of the pre-test values. In those dogs that
received 200 ppm crufomate in their diet plasma and erythrocyte
cholinesterase activity was depressed slightly. Other biochemical,
haematological findings and gross and microscopic examinations of the
tissues were comparable to the controls. Groups of male and female
dogs that received diets containing 10 or 20 ppm for two years showed
no evidence of adverse effect according to any of the criteria applied
(McCollister et al,, 1968).
Long-term studies
Rat. Groups of rats (25 of each sex) were started on diets
containing 1, 10, 100 or 1000 ppm of crufomate. Fifteen rats of each
sex from each group were designated for a two-year study. The
remaining 10 of each sex were killed at 12 or 18 months. Additional
groups of rats (five of each sex) were given diets containing 20, 40,
60 or 80 ppm of crufomate for 20 months.
Both male and female rats that received 1000 ppm crufomate in their
diets showed retardation of growth during the second year of the
study. Necropsy and microscopic examination of these rats after 24
months revealed atrophy of the muscles of the hindlegs and slight
degeneration of the sciatic nerve. A reduction in testis weight to
about one half, reflecting degeneration and atrophy of seminiferous
tubules, was seen at the 1000 ppm level after 12 and 18 months and
again at 24 months. The only evidence of my effect at dosage levels
below 1000 ppm was depression of cholinesterase. Brain cholinesterase
activity was depressed to 38 to 50 per cent of control for the rats
fed 1000 ppm but was within normal range at the lower dosage levels.
Male rats showed no significant effect on plasma cholinesterase at 100
ppm and below, or in red cells at 40 ppm and below. Corresponding
values for female rats were 40 ppm for plasma and 60 ppm for red cell
cholinesterase (McCollister et al., 1968).
Special studies
(a) Fertility and reproduction
Groups of four male and 12 female rats were maintained on diets
containing 0, 10 and 100 ppm crufomate through a three-generation
study involving two litters per generation and assessing fertility,
reproduction and lactation. In addition, a fourth group received 1 ppm
crufomate for the first generation and 500 ppm for the remaining two
generations of the experiment. In all these groups no evidence was
found of adverse effect on fertility, gestation, viability or
lactation, as well as body-weights at weaning and mating.
Cholinesterase inhibition was not investigated (McCollister at al.,
1968).
Forty bulls, three to seven years old, were given a single oral dose
of crufomate at 50 mg/kg or sprayed to wetness with 0.75 per cent
dispersion of a crufomate wettable powder without affecting the
motility of the spermatozoa after one and four weeks (McGregor 1960).
An acute oral dosage of 100 mg/kg of crufomate had no effect on the
fertility of male rats, rabbits or guinea-pigs (McCollister, 1959).
(b) Neurotoxicity
Intraperitoneal injections of crufomate into 25 mature hens at dosage
rates of 500, 750 and 1000 mg/kg caused mortalities in one out of
seven, one out of four and 10 out of 14, respectively, of the birds
treated. Of the surviving 31 treated hens 26 developed ataxia and
paralysis. Those able to take food and water made uneventful and
complete recovery within 90 days of treatment. Histopathological
examination of nerve tissue revealed no departure from normal (Hymas
and Stevenson, 1961).
(c) Potentiation
Single oral doses of crufomate were administered jointly to rats with
each of 14 commercial cholinesterase inhibiting insecticides (13
organo-phosphorus compounds and one carbamate). There was no effect
other than additive (ratios 0.7 to 2.2) for the LD50 values except
for crufomate-malathion (fivefold increase). Dietary feeding studies
in dogs for 12 weeks with diets containing 100 ppm malathion plus 20
ppm crufomate, or 10 ppm malathion plus 20 ppm crufomate, showed no
significant evidence of potentiation (McCollister et al., 1968).
(d) Studies on the metabolite
Rat. Groups of rats (25 of each sex) were fed 100, 300, 1000, and
3000 ppm 4-tert-butyl-2-chlorophenol (the hydrolytic metabolite of
crufomate) in the diet for two years. Rats fed the dosage levels of
100, 300 or 1000 ppm showed no significant evidence of adverse effects
as judged by general appearance, behaviour, growth, mortality,
incidence of tumorous growths, haematological studies, serum urea
nitrogen and alkaline phosphatase determination. Final average body
and organ weights and gross and microscopic effects after examination
of the tissues were normal. Rats fed dietary levels of 3000 ppm showed
moderate microscopic liver changes and increased senility in the
heart, kidney and liver (McCollister, 1964).
Dog. Groups of eight dogs (four of each sex) were fed diets
containing 20, 200 or 2000 ppm 4-tert-butyl-2-chlorophenol for two
years. The male dogs on 2000 ppm showed atrophy and degenerative
changes in the seminiferous tubules of the testes. No other gross or
microscopic effect was observed at any dose level (Dieterich et al.,
1965).
Observations in man
Groups of three adult males per test were given one, two or four
tablets containing 200 mg of crufomate daily for seven days.
Laboratory tests consisted of complete blood counts, urinalysis, red
blood cell cholinesterase response and serum glutamic pyruvic
transaminase determinations. The subjects were interrogated concerning
side effects. The second and third groups showed a delayed 50-80 per
cent red blood cell cholinesterase depression after completion of the
treatments. No other abnormalities were noted in the laboratory tests
(Campbell, 1962).
Comments
The most sensitive criterion upon which to judge the safety of
crufomate upon ingestion by animals or human subjects is its
cholinesterase-inhibiting properties. The short-term and long-term
studies are adequate. Because the only study available in man was too
limited, it is desirable that further metabolic studies in man should
be carried out to show that 4-tert-butyl-2-chlorophenol is the main
metabolite also in this species.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Rat: 40 ppm in the diet, equivalent to 2 mg/kg per day
Dog: 40 ppm in the diet, equivalent to 1 mg/kg per day
Man: 200 mg/day, equivalent to about 3 mg/kg per day
Estimate of acceptable daily intake for man
0-0.1 mg/kg per day
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Crufomate is used on or in cattle, sheep, and goats to control
internal cattle grubs and external horn flies and cattle lice (Anon.,
1966). Applications of crufomate for cattle grub control have been by
injection, oral administration, and spraying (McGregor et al., 1959),
but the simple pour-on application of a low volume of insecticide on
the backs of cattle has been found best (Rogoff and Kohler, 196O).
Formulations of crufomate may be sprayed or poured on to cattle during
the warmer months of the year. However, in the United States of
America during October through February, treatment is restricted
depending on the locality. The dosage rate ranges from about 15 to 50
mg/kg of body-weight.
As a pour-on applied to the backline of cattle, an emulsifiable
concentrate of crufomate is diluted with water to apply about 39 mg/kg
for grubs, horn flies and lice, and about 52 mg/kg for internal
helminth parasite control.
Another treatment of internal helminth parasites of livestock involves
the oral drench (introduction into rumen directly) of 50 to 75 mg/kg.
The following internal parasites of livestock are examples of genera
for which this treatment has been recommended: Haemonchus and
Osteragia (stomach worms), Tricho-strongylus (hairworms),
Strongyloides (threadworm), Bunostomum (hookworm),
Oesophagostomum (nodular worms), Nematodirus (thin-necked
strongyle), Trichuris (whipworms), Cooperia (Cooper's worms),
Oestrus ovis (head grub) and others.
Retreatment for horn flies and cattle lice should not be done more
often than every 28 days and not within 28 days of slaughter. Animals
under stress from sickness, crowding, excitement or surgery should not
be treated. Lactating dairy cows or dry dairy cows within 28 days of
freshening should not be treated. General recommendations for the
current use of cruformate are given in Anon. (1967, 1968).
Residues resulting from supervised trials
When crufomate is used as directed, its residues decrease to a
negligible level 28 days after external treatment and 14 days after
internal treatment. Residues of crufomate found in supervised trials
are summarized in the following table:
Rate of Post-treatment Residues* Reference
Animal application interval (days) (ppm)
(mg/kg)
Cattle 75 14 No residues Anon. (1962b)
(<0.04) fat,
lean meat
Sheep 89.8 14 No residues Claborn et al.
(<0.02) fat, (1960)
internal organs
Lambs 200 7 No residues Bauriedel and
(<0.05) fat, Swank (1962)
lean meat
Cattle 50 7 0.3-1.3** Mussel and
fat, less in Ludwig (1961)
other tissues
Cattle 100 7 0.6-1.6** Mussel and
fat, less in Ludwig (1961)
other tissues
(continued)
Rate of Post-treatment Residues* Reference
Animal application interval (days) (ppm)
(mg/kg)
Cattle 50 7 0.07-0.2** fat Mussel and
Ludwig (1961)
Cattle 100 7 0.1-2.4** fat Mussel and
Ludwig (1961)
Cattle 52 7 0.05-0.14 fat Dishburger and
14 <0.02-0.05 fat Rice (1968)
21 no residues
(<0.02) fat
Calves 52 and 104 28 0.1-0.22 fat Rice (1964)
Lactating 49-66 1 0.195 milk Leahy and
cow 2 no detectable Brown (1963)
residues
(<0.05) milk
Lactating 37.5 1 0.14-0.3 milk Anon. 1962a
cow 2 0.0 milk
Steers 50 7 0.1 phenol in Kutchinski
fat (limit of (1960)
sensitivity, 0.1)
* When one figure is given, residue is maximum value.
** No residues (<0.1 ppm) after 28 days.
Fate of residues
In animals
Six possible routes for the hydrolysis of crufomate to inorganic
phosphate are shown below (Bauriedel and Swank, 1962).
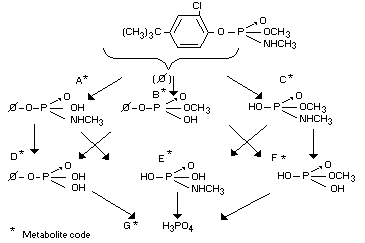 B, C, D, F and G have been identified, definitely or tentatively, as
metabolites in addition to the 4-tert-butyl-2-chlorophenol
hydrolysis product.
Several studies with P32 labelled crufomate administered orally and
by injection to cattle, sheep, and hens have shown that crufomate is
eliminated rapidly as hydrolysis products or hydrolyzed to inorganic
phosphate which may be retained. (Bauriedel and Swank, 1962; Brady and
Arthur, 1962; Buttram and Arthur, 1961; Plapp, 1960; Chamberlain and
Gatterdam, 1960).
The two products that may be expected as residues are cruformate and
its phenolic hydrolysis product. The other hydrolysis products
(metabolites A, B, C, D, E, F) appear to be too polar to be stored
appreciably; the studies with P32 labelled crufomate verify the rapid
elimination of these products.
Evidence of residues in food in commerce or at consumption
No residues of crufomate were found in 149 tissue samples taken in
1968 from slaughter-houses in five states (Indiana, Louisiana,
Nebraska, Oklahoma, and Texas) in the United States of America
(Stewart, 1968).
Methods of residue analysis
Because crufomate is used almost exclusively for the control of
arthropods and worms on or in animals, residue analyses for this
chemical and its metabolites have been conducted primarily on meat,
milk, blood, and animal tissues.
For the residue analyses at the present time, the method of choice
based on sensitivity, specificity, and speed of analysis would appear
to be gas chromatography with a thermionic or flame photometric
detector for sensing phosphorus compounds, after removal of fat (if
necessary) by a hexane-acetonitrile or similar solvent partition.
Burke (1965) and Bowman and Beroza (1965) determined the compound
itself by gas chromatography. Bowman and Beroza (1967) later
determined it in milk and silage by temperature-programmed gas
chromatography with the flame photometric detector as part of a
multicomponent analysis. No clean-up other than a hexane-acetonitrile
partition was needed to remove the fat from the milk extract. The
fraction partitioning into the upper fat-containing phase is readily
calculated from the p-value, which is 0.031 in this system (Bowman
and Beroza, 1965). Recoveries at levels of fortification of 0.05 and
0.2 ppm were generally better than 90 per cent with a sensitivity of
about 0.01 ppm. In an unpublished communication Dishburger and Rice
(1968) determined crufomate in animal fat tissue. They used liquid
chromatography on silicic acid for a clean-up prior to analysis of the
eluate by gas chromatography with thermionic detection. Sensitivity
was 0.02 ppm and recoveries averaged 92 per cent at levels of 0.02 to
0.06 ppm. The use of electron capture, microcoulometric, and
electrolytic-conductivity detectors for the gas chromatographic
analysis of crufomate appears feasible but has not been evaluated.
Early methods used in the analysis of crufomate residues were
manometric housefly-head cholinesterase inhibition procedures
developed by The Dow Chemical Co. They required modifications
depending on the substrate being analysed. A procedure for extracting
crufomate from milk developed by Timmerman et al. (1961) was about 90
per cent efficient. Leahy and Taylor (1963) determined crufomate in
milk by paper chromatography of the milk extract, acid digestion of
the portion of the paper chromatogram containing the pesticide, and
determination of the phosphorus content colorimetrically.
For confirmation of identity of residues, Elgar (1967) suggested
independent parameters that might be used. When enough material can be
isolated, spectroscopy (infra-red, ultra-violet, mass) is excellent
for such confirmations. Bowman and Beroza (1967), reported gas
chromatographic retention times on three liquid phases for crufomate.
They also gave partitioning data (p-values) in six binary solvent
systems, which may be used for identification or for establishing the
quantitative validity of gas chromatographic peaks. Rf values in
paper (Leahy and Taylor, 1963; McKinley and Read, 1962) and thin-layer
chromatography (El-Refai and Hopkins, 1965; Ragab, 1967) can be
useful. The combined use of these and other methods (chemical
derivatives, bio-assay) will make identifications more certain.
The 4-tert-butyl-2-chlorophenol metabolite of crufomate was
determined spectrophotometrically in beef fat and meat with a
sensitivity of 0.1 ppm and an average recovery of 80 per cent after a
clean-up by liquid chromatography and steam distillation, coupling
with diazotized p-nitroaniline, and chromatography of the resulting
dye (Anon., 1960). The presence of a chlorine atom may allow
quantification by electron-capture gas chromatography.
National tolerances
Registration in the United States on a non-residue basis (Anon., 1965)
is to be superseded by the establishment of negligible or finite
residue tolerances. In other countries such as Great Britain, Ireland,
European Economic Community countries, all Latin American countries,
Australia, New Zealand, and Canada, products containing crufomate are
registered on a non-residue basis when used for external parasite
control. Crufomate is registered for internal parasite control of
livestock in Great Britain, Ireland, Spain, Australia, New Zealand,
Brazil, Uruguay, Argentina, Colombia, Ecuador, Venezuela, Paraguay,
Central American countries, Mexico, Canada and the United States of
America.
The Dow Chemical Company is presently reviewing their residue and
toxicological data on crufomate and may soon recommend shortening or
elimination of withdrawal time specifications.
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Appraisal
Crufomate is used internally and is applied externally to livestock as
an insecticide; it is also used as an anthelmintic. In its principal
uses, it is administered long before slaughter so there will be no
residues from such applications in meat. Another use, of secondary
importance, is accompanied by a recommended minimum of 14 days between
treatment and slaughter.
Lactating cattle experimentally treated by pour-on oral drench yielded
milk that did not contain residues in excess of 0.05 ppm after two
days.
A monitoring survey, conducted in 1968 on cattle tissue from five
states of the United States of America, detected no residues in any of
the 149 samples examined.
Several gas chromatographic methods for the compound, which appear to
be suitable for regulatory purposes or for evaluation as a referee
procedure, have been reported.
Recommendations
The following temporary tolerances (to be in effect until 1972) are to
apply to raw agricultural products moving in commerce unless otherwise
indicated. In the case of commodities entering international trade,
the tolerances should be applied by the importing country at the point
of entry or as soon as practicable thereafter.
Temporary tolerances
Meat (on fat basis) (at slaughter) 1 ppm
Whole milk 0.05 ppm
Further work or information
Required before 30 June 1972
1. Data from countries other than the United States of America and the
United Kingdom on the use pattern and resultant residues.
2. Further data on the use pattern and resultant residues in milk from
treated animals, using gas-liquid chromatographic methods.
3. Data on residues in the non-fatty portion of meat and meat
products.
4. Comparative evaluation of gas-liquid chromatographic methods for
regulatory purposes.
Desirable
1. Collaborative studies to establish a referee method.
2. More extensive studies on cholinesterase effects in man.
3. Studies on the metabolism in man to show that
4-tert-butyl-2-chlorophenol is the main metabolite in that species.
REFERENCES
Anon. (1960) Determination of 4-tert-butyl-2-chlorophenol in the
meat and the fat of cattle. Residue determination method ACR 60.8. The
Dow Chemical Co. Midland, Michigan
Anon. (1962a) Residues of Ruelene (R) in milk from a single oral
drench application of Ruelene wormer sheep and goat drench. The Dow
Chemical Co., Midland, Michigan. Unpublished report
Anon. (1962b) Residues of Ruelene in fat and lean meat from cattle
treated with Ruelene in the feed and by oral drench. The Dow Chemical
Co., Midland, Michigan. Unpublished report
Anon. (1965) U.S.D.A. Summary of registered agricultural pesticide
chemical uses (4-tert-butyl-2-chlorophenyl methyl
methylphosphoramidate). p. 767, Issued October 1, 1965, Pesticides
Regulation Division, Agricultural Research Service, U.S. Department of
Agriculture, Washington, D.C.
Anon. (1966) Ruelene (R) systemic insecticide, The Dow Chemical Co.,
Midland, Michigan
Anon. (1967) Suggested guide for the use of insecticides to control
insects affecting crops, livestock, households, stored products,
forests, and forest products, 1967. Agriculture Handbook No. 331,
U.S.D.A. Supt. of Documents, U.S. Government Printing Office,
Washington, D.C.
Anon. (1968) Technical information bulletin. 1968 registered livestock
and household uses for Ronnel and registered livestock uses for
"Ruelene". The Dow Chemical Co., Midland, Michigan
Bauriedel, W. R. and Swank, M. G. (1960) Residue and metabolism of
radioactive Ruelene (R), 4-tertiary-butyl-2-chlorophenyl methyl
methylphosphoramidate, administered as a single oral dose to cattle.
Dow Chemical Co. Unpublished report
Bauriedel, W. R. and Simmons, B. L. (1961) Residue and metabolism of
radioactive Ruelene (R), 4-tertiary-butyl-2-chlorophenyl methyl
methylphosphoramidate, administered to a steer by intrarumenal
injection. Dow Chemical Co. Unpublished report
Bauriedel, W. R. and Swank, M. G. (1962) Residue and metabolism of
radioactive 4-tert-butyl-2-chlorophenyl methyl
methylphosphoramidate, administered as a single oral dose to sheep. J.
Agr. Food. Chem., 10: 150-154
Bowman, M. C. and Beroza, M. (1965) Extraction p-values of
pesticides and related compounds in six solvent systems. J. Ass.
Offic. Agr. Chem., 48: 943-952
Bowman, M. C. and Beroza, M. (1967) Temperature programmed gas
chromatography of twenty phosphorus-containing insecticides on four
different columns and its application to the analysis of milk and corn
silage. J. Ass. Offic. Anal. Chem., 50: 1228-1236
Brady, U. E., jr and Arthur, B. W. (1962) Absorption and metabolism of
Ruelene(R) by Arthropods. J. Econ. Entom., 55: 833-836
Burch, G. R. (1963) Report on Ruelene(R) study in dogs. Pitman-Moore
Division, The Dow Chemical Co., Indianapolis, Indiana. Unpublished
report
Burke, J. A. (1965) Gas chromatography of pesticide residue analysis:
Some practical aspects. J. Ass. Offic. Agr. Chem., 48: 1037-1058
Buttram, J. R. and Arthur, B. W. (1961) Magnitude and nature of
residues in tissues and eggs of poultry receiving Ruelene (R) in the
feed. J. Econ. Entom., 54: 456-460
Campbell, P. J. (1960) Study of Ruelene. Stough-Wisdom Research Inc.
Unpublished report
Chamberlain, W. F. and Gatterdam, P. E. (1960) Third report on studies
with P32 labeled Ruelene. Livestock Insects Investigations,
Entomology Research Division, U.S.D.A., Kerrville, Texas. Unpublished
report
Claborn, H. V., Mann, H. D. and Ivey, M. C. (1960) Second report on
studies with P32 labelled Ruelene, part II, studies on residues in
tissues. Pesticide Chemicals Research Branch, Entomology Research
Division, U.S.D.A., Kerrville, Texas. Unpublished report
Dieterich, W. H., Paynter, O. E. and Weir, R. J. (1965) The chronic
toxicity of Ruelene and a Ruelene derivative in beagle dogs. Hazleton
Laboratories. Paper presented to the Society of Toxicology,
Williamsburg, Virginia
Dishburger, H. J. and Rice, J. R. (1968) Residues in omental fat of
cattle following pour-on application of Ruelene (R) 8R and 25E
insecticide formulations. The Dow Chemical Co., Lake Jackson, Texas.
Unpublished report
Elgar, K. E. (1967) Confirmation of identity, in report by Egan, H.,
pesticide residues. J. Ass. Offic. Anal. Chem., 50: 1069
El-Refai, A. and Hopkins, T. L. (1965) Thin-layer chromatography and
cholinesterase detection of several phosphorothiono insecticides and
their oxygen analogs. J. Agr. Food Chem., 13: 477-481
Galvin, T. J., Bell, R. R. and Turk, R. D. (1960) Anthelmintics for
ruminants. II. Anthelmintic activity and toxicity of Ruelene in sheep.
Amer. J. vet. Res., 21: 1058-1061
Hymas, T. A. and Stevenson, G. T. (1961) Attempt to produce
neurotoxicity by the injection of Ruelene or Ronnel into mature
Leghorn hens. Dow Chemical Co. Unpublished report
Jackson, J. B., Drummond, R. O., Buck, W. B. and Hunt, L. M. (1960)
Toxicity of organic phosphorus insecticides to horses. J. Econ.
Entomol., 53: 602-604
Kutschinski, A. H. (1960) Determination of
4-tert-butyl-2-chlorophenol residues in the meat and fat of cattle
sprayed with 4-tert-butyl-2-chlorophenyl methyl
methylphosphoramidate (Ruelene(R)). The Dow Chemical Co., Midland,
Michigan. Unpublished report
Leahy, J. S. and Brown, F. G. (1963) Residues of
4-tert-butyl-2-chlorophenyl methyl methylphosphoramidate in the milk
of dairy cows following dermal application. The Veterinary Record,
75: (39) 1000-1001
Leahy, J. S. and Taylor, T. (1963) The determination of residues of
"Ruelene" in milk. Analyst, 88: 882-885
McCollister, D. D. (1959) Fertility studies with male laboratory
animals given single doses of Ruelene. Dow Chemical Co. Unpublished
report
McCollister, D. D. (1964) Results of two-year dietary feeding studies
of 4-tert-butyl-2-chlorophenol in rats. Dow Chemical Co. Unpublished
report
McCollister, D. D., Olson, K. J., Rowe, V. K., Paynter, O. E., Weir,
R. J. and Dieterich, W. H. (1968) Toxicology of
4-tert-butyl-2-chlorophenyl methyl methylphosphoramidate (Ruelene)
in laboratory animals. Food and Cosmetic Toxicology, 6: 185-198
McGregor, W. S., Ludwig, P. D. and Wade, L. L. (1959) Progress report
on Ruelene for cattle grub control. Down to Earth, Fall, 1959, pp. 1-2
McGregor, W. S. (1960) The effects of Ruelene (R) treatments on the
fertility of range bulls. Dow Chemical Co. Unpublished report
McKinley, W. P. and Read, S. I. (1962) Esterase inhibition technique
for the detection of organophosphorus pesticides. J. Ass. Offic. Agr.
Chem., 45: 467-473
Mussell, D. R. and Ludwig, P. D. (1961) Residues of Ruelene(R) in
animal tissues when applied as a spray to cattle. The Dow Chemical
Co., Midland, Michigan. Unpublished report
Plapp, F. W. (1960) Studies on the metabolism of P32 Ruelene (R) in a
Hereford steer. Agricultural Research Service, U.S.D.A., Corvallis,
Oregon. Unpublished report
Radeleff, R. D. (1964) Organophosphorus compounds. Ruelene (R),
O-4-tert-butyl-2-chlorophenyl methyl methylphosphoramidate.
Veterinary Toxicology, Lea and Febger, Philadelphia, Pa., pp. 209-210
Ragab, M. T. H. (1967) Direct fluorescent detection of
organothiophosphorus pesticides and some of the sulfur-containing
breakdown products after thin-layer chromatography. J. Ass. Offic.
Anal. Chem., 50: 1088-1098
Rice, J. R. (1964) Residue determination of Ruelene in bovine tissue
and Ruelene TF-88 pour-on application. The Dow Chemical Co., Lake
Jackson, Texas. Unpublished report
Rogoff, W. M. and Kohler, P. H. (1960) Effectiveness of Ruelene (R)
applied as localized "pour-on" and as spray for cattle grub control.
J. Econ. Entomol., 53: 814-817
Sherman, M. and Ross, E. (1961) Acute and subacute toxicity of
insecticides for chicks. Toxicol. appl. Pharmacol., 3: 521-533
Skerman, K. D. (1962) Mintrel(R). A new anthelmintic for sheep.
Aust. vet. J., pp. 324-334
Smith, G. N. (1968) Cholinesterase inhibition of crufomate (Ruelene
(R) insecticide) and metabolites. Dow Chemical Co. Unpublished report
Stewart, J. H. (1968) U.S. Department of Agriculture, Washington, D.C.
Private communication
Timmerman, J. A., jr, Dorough, H. W., Buttram, J. R. and Arthur, B. W.
(1961) In vitro stability and recovery of insecticides from milk.
J. Econ. Entomol. 54: 441-444
Weidenbach, C. P., Radeleff, R. D. and Buck, W. B. (1962)
Toxicological studies of Ruelene(R) 4-tert-butyl-2-chlorophenyl
methyl methylphosphoramidate. J. Amer. vet. med. Ass., 140: 460-463
B, C, D, F and G have been identified, definitely or tentatively, as
metabolites in addition to the 4-tert-butyl-2-chlorophenol
hydrolysis product.
Several studies with P32 labelled crufomate administered orally and
by injection to cattle, sheep, and hens have shown that crufomate is
eliminated rapidly as hydrolysis products or hydrolyzed to inorganic
phosphate which may be retained. (Bauriedel and Swank, 1962; Brady and
Arthur, 1962; Buttram and Arthur, 1961; Plapp, 1960; Chamberlain and
Gatterdam, 1960).
The two products that may be expected as residues are cruformate and
its phenolic hydrolysis product. The other hydrolysis products
(metabolites A, B, C, D, E, F) appear to be too polar to be stored
appreciably; the studies with P32 labelled crufomate verify the rapid
elimination of these products.
Evidence of residues in food in commerce or at consumption
No residues of crufomate were found in 149 tissue samples taken in
1968 from slaughter-houses in five states (Indiana, Louisiana,
Nebraska, Oklahoma, and Texas) in the United States of America
(Stewart, 1968).
Methods of residue analysis
Because crufomate is used almost exclusively for the control of
arthropods and worms on or in animals, residue analyses for this
chemical and its metabolites have been conducted primarily on meat,
milk, blood, and animal tissues.
For the residue analyses at the present time, the method of choice
based on sensitivity, specificity, and speed of analysis would appear
to be gas chromatography with a thermionic or flame photometric
detector for sensing phosphorus compounds, after removal of fat (if
necessary) by a hexane-acetonitrile or similar solvent partition.
Burke (1965) and Bowman and Beroza (1965) determined the compound
itself by gas chromatography. Bowman and Beroza (1967) later
determined it in milk and silage by temperature-programmed gas
chromatography with the flame photometric detector as part of a
multicomponent analysis. No clean-up other than a hexane-acetonitrile
partition was needed to remove the fat from the milk extract. The
fraction partitioning into the upper fat-containing phase is readily
calculated from the p-value, which is 0.031 in this system (Bowman
and Beroza, 1965). Recoveries at levels of fortification of 0.05 and
0.2 ppm were generally better than 90 per cent with a sensitivity of
about 0.01 ppm. In an unpublished communication Dishburger and Rice
(1968) determined crufomate in animal fat tissue. They used liquid
chromatography on silicic acid for a clean-up prior to analysis of the
eluate by gas chromatography with thermionic detection. Sensitivity
was 0.02 ppm and recoveries averaged 92 per cent at levels of 0.02 to
0.06 ppm. The use of electron capture, microcoulometric, and
electrolytic-conductivity detectors for the gas chromatographic
analysis of crufomate appears feasible but has not been evaluated.
Early methods used in the analysis of crufomate residues were
manometric housefly-head cholinesterase inhibition procedures
developed by The Dow Chemical Co. They required modifications
depending on the substrate being analysed. A procedure for extracting
crufomate from milk developed by Timmerman et al. (1961) was about 90
per cent efficient. Leahy and Taylor (1963) determined crufomate in
milk by paper chromatography of the milk extract, acid digestion of
the portion of the paper chromatogram containing the pesticide, and
determination of the phosphorus content colorimetrically.
For confirmation of identity of residues, Elgar (1967) suggested
independent parameters that might be used. When enough material can be
isolated, spectroscopy (infra-red, ultra-violet, mass) is excellent
for such confirmations. Bowman and Beroza (1967), reported gas
chromatographic retention times on three liquid phases for crufomate.
They also gave partitioning data (p-values) in six binary solvent
systems, which may be used for identification or for establishing the
quantitative validity of gas chromatographic peaks. Rf values in
paper (Leahy and Taylor, 1963; McKinley and Read, 1962) and thin-layer
chromatography (El-Refai and Hopkins, 1965; Ragab, 1967) can be
useful. The combined use of these and other methods (chemical
derivatives, bio-assay) will make identifications more certain.
The 4-tert-butyl-2-chlorophenol metabolite of crufomate was
determined spectrophotometrically in beef fat and meat with a
sensitivity of 0.1 ppm and an average recovery of 80 per cent after a
clean-up by liquid chromatography and steam distillation, coupling
with diazotized p-nitroaniline, and chromatography of the resulting
dye (Anon., 1960). The presence of a chlorine atom may allow
quantification by electron-capture gas chromatography.
National tolerances
Registration in the United States on a non-residue basis (Anon., 1965)
is to be superseded by the establishment of negligible or finite
residue tolerances. In other countries such as Great Britain, Ireland,
European Economic Community countries, all Latin American countries,
Australia, New Zealand, and Canada, products containing crufomate are
registered on a non-residue basis when used for external parasite
control. Crufomate is registered for internal parasite control of
livestock in Great Britain, Ireland, Spain, Australia, New Zealand,
Brazil, Uruguay, Argentina, Colombia, Ecuador, Venezuela, Paraguay,
Central American countries, Mexico, Canada and the United States of
America.
The Dow Chemical Company is presently reviewing their residue and
toxicological data on crufomate and may soon recommend shortening or
elimination of withdrawal time specifications.
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Appraisal
Crufomate is used internally and is applied externally to livestock as
an insecticide; it is also used as an anthelmintic. In its principal
uses, it is administered long before slaughter so there will be no
residues from such applications in meat. Another use, of secondary
importance, is accompanied by a recommended minimum of 14 days between
treatment and slaughter.
Lactating cattle experimentally treated by pour-on oral drench yielded
milk that did not contain residues in excess of 0.05 ppm after two
days.
A monitoring survey, conducted in 1968 on cattle tissue from five
states of the United States of America, detected no residues in any of
the 149 samples examined.
Several gas chromatographic methods for the compound, which appear to
be suitable for regulatory purposes or for evaluation as a referee
procedure, have been reported.
Recommendations
The following temporary tolerances (to be in effect until 1972) are to
apply to raw agricultural products moving in commerce unless otherwise
indicated. In the case of commodities entering international trade,
the tolerances should be applied by the importing country at the point
of entry or as soon as practicable thereafter.
Temporary tolerances
Meat (on fat basis) (at slaughter) 1 ppm
Whole milk 0.05 ppm
Further work or information
Required before 30 June 1972
1. Data from countries other than the United States of America and the
United Kingdom on the use pattern and resultant residues.
2. Further data on the use pattern and resultant residues in milk from
treated animals, using gas-liquid chromatographic methods.
3. Data on residues in the non-fatty portion of meat and meat
products.
4. Comparative evaluation of gas-liquid chromatographic methods for
regulatory purposes.
Desirable
1. Collaborative studies to establish a referee method.
2. More extensive studies on cholinesterase effects in man.
3. Studies on the metabolism in man to show that
4-tert-butyl-2-chlorophenol is the main metabolite in that species.
REFERENCES
Anon. (1960) Determination of 4-tert-butyl-2-chlorophenol in the
meat and the fat of cattle. Residue determination method ACR 60.8. The
Dow Chemical Co. Midland, Michigan
Anon. (1962a) Residues of Ruelene (R) in milk from a single oral
drench application of Ruelene wormer sheep and goat drench. The Dow
Chemical Co., Midland, Michigan. Unpublished report
Anon. (1962b) Residues of Ruelene in fat and lean meat from cattle
treated with Ruelene in the feed and by oral drench. The Dow Chemical
Co., Midland, Michigan. Unpublished report
Anon. (1965) U.S.D.A. Summary of registered agricultural pesticide
chemical uses (4-tert-butyl-2-chlorophenyl methyl
methylphosphoramidate). p. 767, Issued October 1, 1965, Pesticides
Regulation Division, Agricultural Research Service, U.S. Department of
Agriculture, Washington, D.C.
Anon. (1966) Ruelene (R) systemic insecticide, The Dow Chemical Co.,
Midland, Michigan
Anon. (1967) Suggested guide for the use of insecticides to control
insects affecting crops, livestock, households, stored products,
forests, and forest products, 1967. Agriculture Handbook No. 331,
U.S.D.A. Supt. of Documents, U.S. Government Printing Office,
Washington, D.C.
Anon. (1968) Technical information bulletin. 1968 registered livestock
and household uses for Ronnel and registered livestock uses for
"Ruelene". The Dow Chemical Co., Midland, Michigan
Bauriedel, W. R. and Swank, M. G. (1960) Residue and metabolism of
radioactive Ruelene (R), 4-tertiary-butyl-2-chlorophenyl methyl
methylphosphoramidate, administered as a single oral dose to cattle.
Dow Chemical Co. Unpublished report
Bauriedel, W. R. and Simmons, B. L. (1961) Residue and metabolism of
radioactive Ruelene (R), 4-tertiary-butyl-2-chlorophenyl methyl
methylphosphoramidate, administered to a steer by intrarumenal
injection. Dow Chemical Co. Unpublished report
Bauriedel, W. R. and Swank, M. G. (1962) Residue and metabolism of
radioactive 4-tert-butyl-2-chlorophenyl methyl
methylphosphoramidate, administered as a single oral dose to sheep. J.
Agr. Food. Chem., 10: 150-154
Bowman, M. C. and Beroza, M. (1965) Extraction p-values of
pesticides and related compounds in six solvent systems. J. Ass.
Offic. Agr. Chem., 48: 943-952
Bowman, M. C. and Beroza, M. (1967) Temperature programmed gas
chromatography of twenty phosphorus-containing insecticides on four
different columns and its application to the analysis of milk and corn
silage. J. Ass. Offic. Anal. Chem., 50: 1228-1236
Brady, U. E., jr and Arthur, B. W. (1962) Absorption and metabolism of
Ruelene(R) by Arthropods. J. Econ. Entom., 55: 833-836
Burch, G. R. (1963) Report on Ruelene(R) study in dogs. Pitman-Moore
Division, The Dow Chemical Co., Indianapolis, Indiana. Unpublished
report
Burke, J. A. (1965) Gas chromatography of pesticide residue analysis:
Some practical aspects. J. Ass. Offic. Agr. Chem., 48: 1037-1058
Buttram, J. R. and Arthur, B. W. (1961) Magnitude and nature of
residues in tissues and eggs of poultry receiving Ruelene (R) in the
feed. J. Econ. Entom., 54: 456-460
Campbell, P. J. (1960) Study of Ruelene. Stough-Wisdom Research Inc.
Unpublished report
Chamberlain, W. F. and Gatterdam, P. E. (1960) Third report on studies
with P32 labeled Ruelene. Livestock Insects Investigations,
Entomology Research Division, U.S.D.A., Kerrville, Texas. Unpublished
report
Claborn, H. V., Mann, H. D. and Ivey, M. C. (1960) Second report on
studies with P32 labelled Ruelene, part II, studies on residues in
tissues. Pesticide Chemicals Research Branch, Entomology Research
Division, U.S.D.A., Kerrville, Texas. Unpublished report
Dieterich, W. H., Paynter, O. E. and Weir, R. J. (1965) The chronic
toxicity of Ruelene and a Ruelene derivative in beagle dogs. Hazleton
Laboratories. Paper presented to the Society of Toxicology,
Williamsburg, Virginia
Dishburger, H. J. and Rice, J. R. (1968) Residues in omental fat of
cattle following pour-on application of Ruelene (R) 8R and 25E
insecticide formulations. The Dow Chemical Co., Lake Jackson, Texas.
Unpublished report
Elgar, K. E. (1967) Confirmation of identity, in report by Egan, H.,
pesticide residues. J. Ass. Offic. Anal. Chem., 50: 1069
El-Refai, A. and Hopkins, T. L. (1965) Thin-layer chromatography and
cholinesterase detection of several phosphorothiono insecticides and
their oxygen analogs. J. Agr. Food Chem., 13: 477-481
Galvin, T. J., Bell, R. R. and Turk, R. D. (1960) Anthelmintics for
ruminants. II. Anthelmintic activity and toxicity of Ruelene in sheep.
Amer. J. vet. Res., 21: 1058-1061
Hymas, T. A. and Stevenson, G. T. (1961) Attempt to produce
neurotoxicity by the injection of Ruelene or Ronnel into mature
Leghorn hens. Dow Chemical Co. Unpublished report
Jackson, J. B., Drummond, R. O., Buck, W. B. and Hunt, L. M. (1960)
Toxicity of organic phosphorus insecticides to horses. J. Econ.
Entomol., 53: 602-604
Kutschinski, A. H. (1960) Determination of
4-tert-butyl-2-chlorophenol residues in the meat and fat of cattle
sprayed with 4-tert-butyl-2-chlorophenyl methyl
methylphosphoramidate (Ruelene(R)). The Dow Chemical Co., Midland,
Michigan. Unpublished report
Leahy, J. S. and Brown, F. G. (1963) Residues of
4-tert-butyl-2-chlorophenyl methyl methylphosphoramidate in the milk
of dairy cows following dermal application. The Veterinary Record,
75: (39) 1000-1001
Leahy, J. S. and Taylor, T. (1963) The determination of residues of
"Ruelene" in milk. Analyst, 88: 882-885
McCollister, D. D. (1959) Fertility studies with male laboratory
animals given single doses of Ruelene. Dow Chemical Co. Unpublished
report
McCollister, D. D. (1964) Results of two-year dietary feeding studies
of 4-tert-butyl-2-chlorophenol in rats. Dow Chemical Co. Unpublished
report
McCollister, D. D., Olson, K. J., Rowe, V. K., Paynter, O. E., Weir,
R. J. and Dieterich, W. H. (1968) Toxicology of
4-tert-butyl-2-chlorophenyl methyl methylphosphoramidate (Ruelene)
in laboratory animals. Food and Cosmetic Toxicology, 6: 185-198
McGregor, W. S., Ludwig, P. D. and Wade, L. L. (1959) Progress report
on Ruelene for cattle grub control. Down to Earth, Fall, 1959, pp. 1-2
McGregor, W. S. (1960) The effects of Ruelene (R) treatments on the
fertility of range bulls. Dow Chemical Co. Unpublished report
McKinley, W. P. and Read, S. I. (1962) Esterase inhibition technique
for the detection of organophosphorus pesticides. J. Ass. Offic. Agr.
Chem., 45: 467-473
Mussell, D. R. and Ludwig, P. D. (1961) Residues of Ruelene(R) in
animal tissues when applied as a spray to cattle. The Dow Chemical
Co., Midland, Michigan. Unpublished report
Plapp, F. W. (1960) Studies on the metabolism of P32 Ruelene (R) in a
Hereford steer. Agricultural Research Service, U.S.D.A., Corvallis,
Oregon. Unpublished report
Radeleff, R. D. (1964) Organophosphorus compounds. Ruelene (R),
O-4-tert-butyl-2-chlorophenyl methyl methylphosphoramidate.
Veterinary Toxicology, Lea and Febger, Philadelphia, Pa., pp. 209-210
Ragab, M. T. H. (1967) Direct fluorescent detection of
organothiophosphorus pesticides and some of the sulfur-containing
breakdown products after thin-layer chromatography. J. Ass. Offic.
Anal. Chem., 50: 1088-1098
Rice, J. R. (1964) Residue determination of Ruelene in bovine tissue
and Ruelene TF-88 pour-on application. The Dow Chemical Co., Lake
Jackson, Texas. Unpublished report
Rogoff, W. M. and Kohler, P. H. (1960) Effectiveness of Ruelene (R)
applied as localized "pour-on" and as spray for cattle grub control.
J. Econ. Entomol., 53: 814-817
Sherman, M. and Ross, E. (1961) Acute and subacute toxicity of
insecticides for chicks. Toxicol. appl. Pharmacol., 3: 521-533
Skerman, K. D. (1962) Mintrel(R). A new anthelmintic for sheep.
Aust. vet. J., pp. 324-334
Smith, G. N. (1968) Cholinesterase inhibition of crufomate (Ruelene
(R) insecticide) and metabolites. Dow Chemical Co. Unpublished report
Stewart, J. H. (1968) U.S. Department of Agriculture, Washington, D.C.
Private communication
Timmerman, J. A., jr, Dorough, H. W., Buttram, J. R. and Arthur, B. W.
(1961) In vitro stability and recovery of insecticides from milk.
J. Econ. Entomol. 54: 441-444
Weidenbach, C. P., Radeleff, R. D. and Buck, W. B. (1962)
Toxicological studies of Ruelene(R) 4-tert-butyl-2-chlorophenyl
methyl methylphosphoramidate. J. Amer. vet. med. Ass., 140: 460-463
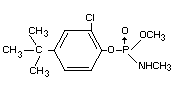 Other information on identity and properties
The recrystallized technical product melts at 52.5-59.2°C. When pure,
crufomate is a white solid melting at 62-62.5°C. The compound is
stable from room temperature to 60°C. It is unstable in strongly acid
and in alkaline media. At room temperature, it is freely soluble in
most of the common organic solvents except for the paraffin solvents
in which its solubility is about one to three per cent by weight. Its
solubility in water is 0.5 per cent by weight.
Crufomate is formulated in several concentrations either for direct
use or for application after suitable dilution (emulsifiable
concentrates, pour-ons, systemic insecticide, anthelmintic, sheep and
goat wormer).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Following an 11 mg/kg intramuscular dosage of 32P labelled crufomated
to a yearling steer, radioactivity at the site of the treatment
dropped from 16 milliroentgens per hour immediately following the
treatment to 1.5 within two days and 0 after 13 days. Residues of
crufomate were practically non-existent after 14 days. Peak levels of
radioactivity occurred in the blood, urine and faeces in two to eight
hours, 12-18 hours, and 18-30 hours respectively. Fat tissues
contained less than 0.2 ppm of radioactive compounds after 14 days.
Red blood cholinesterase activity was depressed no more than 25 per
cent. The end product of the metabolism of crufomate is phosphoric
acid (Plapp. 1960).
A 21-day study of the metabolism of radioactive crufomate using acute
oral dosages of 100 and 200 mg/kg of body-weight in sheep shows
residues in the urine consisting of crufomate and several hydrolysis
products. Over 85 per cent of the administered 32P was recovered in
the excreta. The 32P in the urine, amounting to 75 per cent of the
dose, was primarily in the form of the hydrolysis products of
crufomate. Some of the crufomate was hydrolyzed completely to
inorganic phosphate and retained in the animal tissues and bones.
Crufomate itself was not found in the tissues after seven days,
indicating fairly rapid metabolism. Residues of crufomate and several
hydrolysis products were found in the blood and plasma shortly after
treatment, but within two days the crufomate decreased to a low level
(Bauriedel and Swank, 1962).
Other metabolic studies in sheep (Chamberlain and Gatterdam, 1960),
and in poultry (Buttram and Arthur, 1961) showed rapid breakdown of
crufomate. The likely mammalian metabolites of crufomate do not cause
inhibition of cholinesterase (Smith, 1968).
Acute toxicity (oral)
Animal Sex LD50 (mg/kg References
body-weight)
Rat male 950 McCollister et al., 1968
Rat female 770 McCollister et al., 1968
Rabbit mixed 490 McCollister et al., 1968
Guinea-pig male 1 000 McCollister et al., 1968
Dog male >1 000 McCollister et al., 1968
Dog female >750 McCollister et al., 1968
Sheep. Lambs received a single dose of 100, 200 or 400 mg/kg. Signs
of intoxication appeared at 200 mg/kg or more. Whole blood
cholinesterase activity was depressed to 50 per cent at 100 mg/kg,
and was markedly depressed at higher doses (Galvin et al., 1960).
Symptoms are highly variable and mainly caused by central nervous
system disturbances. At necropsy severe congestion of lungs and
kidneys, enlargement of liver and scattered haemorrhages were found
(Radeleff, 1964).
Short-term studies
Rat. Rats (10 males and 10 females per group) were started at the
age of 45 days on diets containing 10, 30, 100, 300 or 1000 ppm
crufomate. During a 90-day experimental period no evidence of
ill-effect was found when judged by general appearance and behaviour,
growth, mortality or food consumption records, haematological
determinations, final body and organ weight ratios, and gross and
microscopic examination of tissues. Results of measurements of
cholinesterase activity in blood and brain show that at 41 days there
was a peculiarly uniform depression of cholinesterase activity in the
plasma of both male and female rats over the entire range of 10-1000
ppm. The same phenomenon, although not as marked, was seen at 69 days,
indicating that a higher intake of chemical per unit of body-weight
during the early period way have been partially responsible.
Measurement of the erythrocyte-and brain-cholinesterase activities
showed little or no effect at the 30 ppm level of crufomate. In the
groups of rats from each dietary level which were placed on the
control ration for 48 days following the 90-day test period, recovery
of blood and brain cholinesterase was complete (McCollister et al.,
1968).
Dog. Groups comprising one male and one female dog were given diets
containing 0, 15, 40, 125 or 250 ppm crufomate for 75 days. Dogs that
received 250 ppm crufomate in their diets for 75 days showed no
evidence of adverse effect by any of the criteria examined except
cholinesterase activity and liver histopathology. Plasma-,
erythrocyte- and brain-cholinesterase levels were decreased to about
65 per cent of the control values. Slight centrilobular granular
degeneration was observed on microscopic examination of the liver.
Cholinesterase measurements were within the normal range in dogs at
the 125 ppm level. There was no evidence of adverse effect in dogs
that received 15 or 40 ppm dietary crufomate for 75 days, as judged by
general appearance and behaviour, growth, mortality, food consumption,
haematological indices, cholinesterase activities, final body and
organ weights and gross and microscopic appearance of the tissues
(McCollister et al., 1968).
Groups of dogs, each containing four males and four females per group,
were given diets containing 0, 10, 20, 200 or 2000 ppm of crufomate
for up to two years. The dogs on 2000 ppm crufomate were transferred
to the control diet after 30 days and sacrificed on days 94 and 95 of
the study. After one year, one male and one female from each of the
other groups were sacrificed for tissue examination. Cholinesterase
determinations were made at 30, 60 and 90 days after the start of the
study and at six-week intervals up to one year and every three months
thereafter. The animals on the highest level of crufomate manifested
gross signs of intoxication, values for serum alkaline phosphatase and
transaminase were elevated, plasma cholinesterase was reduced to 55
per cent of the pre-test average, and red cell cholinesterase was
depressed to 19 per cent of the pre-test values. In those dogs that
received 200 ppm crufomate in their diet plasma and erythrocyte
cholinesterase activity was depressed slightly. Other biochemical,
haematological findings and gross and microscopic examinations of the
tissues were comparable to the controls. Groups of male and female
dogs that received diets containing 10 or 20 ppm for two years showed
no evidence of adverse effect according to any of the criteria applied
(McCollister et al,, 1968).
Long-term studies
Rat. Groups of rats (25 of each sex) were started on diets
containing 1, 10, 100 or 1000 ppm of crufomate. Fifteen rats of each
sex from each group were designated for a two-year study. The
remaining 10 of each sex were killed at 12 or 18 months. Additional
groups of rats (five of each sex) were given diets containing 20, 40,
60 or 80 ppm of crufomate for 20 months.
Both male and female rats that received 1000 ppm crufomate in their
diets showed retardation of growth during the second year of the
study. Necropsy and microscopic examination of these rats after 24
months revealed atrophy of the muscles of the hindlegs and slight
degeneration of the sciatic nerve. A reduction in testis weight to
about one half, reflecting degeneration and atrophy of seminiferous
tubules, was seen at the 1000 ppm level after 12 and 18 months and
again at 24 months. The only evidence of my effect at dosage levels
below 1000 ppm was depression of cholinesterase. Brain cholinesterase
activity was depressed to 38 to 50 per cent of control for the rats
fed 1000 ppm but was within normal range at the lower dosage levels.
Male rats showed no significant effect on plasma cholinesterase at 100
ppm and below, or in red cells at 40 ppm and below. Corresponding
values for female rats were 40 ppm for plasma and 60 ppm for red cell
cholinesterase (McCollister et al., 1968).
Special studies
(a) Fertility and reproduction
Groups of four male and 12 female rats were maintained on diets
containing 0, 10 and 100 ppm crufomate through a three-generation
study involving two litters per generation and assessing fertility,
reproduction and lactation. In addition, a fourth group received 1 ppm
crufomate for the first generation and 500 ppm for the remaining two
generations of the experiment. In all these groups no evidence was
found of adverse effect on fertility, gestation, viability or
lactation, as well as body-weights at weaning and mating.
Cholinesterase inhibition was not investigated (McCollister at al.,
1968).
Forty bulls, three to seven years old, were given a single oral dose
of crufomate at 50 mg/kg or sprayed to wetness with 0.75 per cent
dispersion of a crufomate wettable powder without affecting the
motility of the spermatozoa after one and four weeks (McGregor 1960).
An acute oral dosage of 100 mg/kg of crufomate had no effect on the
fertility of male rats, rabbits or guinea-pigs (McCollister, 1959).
(b) Neurotoxicity
Intraperitoneal injections of crufomate into 25 mature hens at dosage
rates of 500, 750 and 1000 mg/kg caused mortalities in one out of
seven, one out of four and 10 out of 14, respectively, of the birds
treated. Of the surviving 31 treated hens 26 developed ataxia and
paralysis. Those able to take food and water made uneventful and
complete recovery within 90 days of treatment. Histopathological
examination of nerve tissue revealed no departure from normal (Hymas
and Stevenson, 1961).
(c) Potentiation
Single oral doses of crufomate were administered jointly to rats with
each of 14 commercial cholinesterase inhibiting insecticides (13
organo-phosphorus compounds and one carbamate). There was no effect
other than additive (ratios 0.7 to 2.2) for the LD50 values except
for crufomate-malathion (fivefold increase). Dietary feeding studies
in dogs for 12 weeks with diets containing 100 ppm malathion plus 20
ppm crufomate, or 10 ppm malathion plus 20 ppm crufomate, showed no
significant evidence of potentiation (McCollister et al., 1968).
(d) Studies on the metabolite
Rat. Groups of rats (25 of each sex) were fed 100, 300, 1000, and
3000 ppm 4-tert-butyl-2-chlorophenol (the hydrolytic metabolite of
crufomate) in the diet for two years. Rats fed the dosage levels of
100, 300 or 1000 ppm showed no significant evidence of adverse effects
as judged by general appearance, behaviour, growth, mortality,
incidence of tumorous growths, haematological studies, serum urea
nitrogen and alkaline phosphatase determination. Final average body
and organ weights and gross and microscopic effects after examination
of the tissues were normal. Rats fed dietary levels of 3000 ppm showed
moderate microscopic liver changes and increased senility in the
heart, kidney and liver (McCollister, 1964).
Dog. Groups of eight dogs (four of each sex) were fed diets
containing 20, 200 or 2000 ppm 4-tert-butyl-2-chlorophenol for two
years. The male dogs on 2000 ppm showed atrophy and degenerative
changes in the seminiferous tubules of the testes. No other gross or
microscopic effect was observed at any dose level (Dieterich et al.,
1965).
Observations in man
Groups of three adult males per test were given one, two or four
tablets containing 200 mg of crufomate daily for seven days.
Laboratory tests consisted of complete blood counts, urinalysis, red
blood cell cholinesterase response and serum glutamic pyruvic
transaminase determinations. The subjects were interrogated concerning
side effects. The second and third groups showed a delayed 50-80 per
cent red blood cell cholinesterase depression after completion of the
treatments. No other abnormalities were noted in the laboratory tests
(Campbell, 1962).
Comments
The most sensitive criterion upon which to judge the safety of
crufomate upon ingestion by animals or human subjects is its
cholinesterase-inhibiting properties. The short-term and long-term
studies are adequate. Because the only study available in man was too
limited, it is desirable that further metabolic studies in man should
be carried out to show that 4-tert-butyl-2-chlorophenol is the main
metabolite also in this species.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Rat: 40 ppm in the diet, equivalent to 2 mg/kg per day
Dog: 40 ppm in the diet, equivalent to 1 mg/kg per day
Man: 200 mg/day, equivalent to about 3 mg/kg per day
Estimate of acceptable daily intake for man
0-0.1 mg/kg per day
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Crufomate is used on or in cattle, sheep, and goats to control
internal cattle grubs and external horn flies and cattle lice (Anon.,
1966). Applications of crufomate for cattle grub control have been by
injection, oral administration, and spraying (McGregor et al., 1959),
but the simple pour-on application of a low volume of insecticide on
the backs of cattle has been found best (Rogoff and Kohler, 196O).
Formulations of crufomate may be sprayed or poured on to cattle during
the warmer months of the year. However, in the United States of
America during October through February, treatment is restricted
depending on the locality. The dosage rate ranges from about 15 to 50
mg/kg of body-weight.
As a pour-on applied to the backline of cattle, an emulsifiable
concentrate of crufomate is diluted with water to apply about 39 mg/kg
for grubs, horn flies and lice, and about 52 mg/kg for internal
helminth parasite control.
Another treatment of internal helminth parasites of livestock involves
the oral drench (introduction into rumen directly) of 50 to 75 mg/kg.
The following internal parasites of livestock are examples of genera
for which this treatment has been recommended: Haemonchus and
Osteragia (stomach worms), Tricho-strongylus (hairworms),
Strongyloides (threadworm), Bunostomum (hookworm),
Oesophagostomum (nodular worms), Nematodirus (thin-necked
strongyle), Trichuris (whipworms), Cooperia (Cooper's worms),
Oestrus ovis (head grub) and others.
Retreatment for horn flies and cattle lice should not be done more
often than every 28 days and not within 28 days of slaughter. Animals
under stress from sickness, crowding, excitement or surgery should not
be treated. Lactating dairy cows or dry dairy cows within 28 days of
freshening should not be treated. General recommendations for the
current use of cruformate are given in Anon. (1967, 1968).
Residues resulting from supervised trials
When crufomate is used as directed, its residues decrease to a
negligible level 28 days after external treatment and 14 days after
internal treatment. Residues of crufomate found in supervised trials
are summarized in the following table:
Rate of Post-treatment Residues* Reference
Animal application interval (days) (ppm)
(mg/kg)
Cattle 75 14 No residues Anon. (1962b)
(<0.04) fat,
lean meat
Sheep 89.8 14 No residues Claborn et al.
(<0.02) fat, (1960)
internal organs
Lambs 200 7 No residues Bauriedel and
(<0.05) fat, Swank (1962)
lean meat
Cattle 50 7 0.3-1.3** Mussel and
fat, less in Ludwig (1961)
other tissues
Cattle 100 7 0.6-1.6** Mussel and
fat, less in Ludwig (1961)
other tissues
(continued)
Rate of Post-treatment Residues* Reference
Animal application interval (days) (ppm)
(mg/kg)
Cattle 50 7 0.07-0.2** fat Mussel and
Ludwig (1961)
Cattle 100 7 0.1-2.4** fat Mussel and
Ludwig (1961)
Cattle 52 7 0.05-0.14 fat Dishburger and
14 <0.02-0.05 fat Rice (1968)
21 no residues
(<0.02) fat
Calves 52 and 104 28 0.1-0.22 fat Rice (1964)
Lactating 49-66 1 0.195 milk Leahy and
cow 2 no detectable Brown (1963)
residues
(<0.05) milk
Lactating 37.5 1 0.14-0.3 milk Anon. 1962a
cow 2 0.0 milk
Steers 50 7 0.1 phenol in Kutchinski
fat (limit of (1960)
sensitivity, 0.1)
* When one figure is given, residue is maximum value.
** No residues (<0.1 ppm) after 28 days.
Fate of residues
In animals
Six possible routes for the hydrolysis of crufomate to inorganic
phosphate are shown below (Bauriedel and Swank, 1962).
Other information on identity and properties
The recrystallized technical product melts at 52.5-59.2°C. When pure,
crufomate is a white solid melting at 62-62.5°C. The compound is
stable from room temperature to 60°C. It is unstable in strongly acid
and in alkaline media. At room temperature, it is freely soluble in
most of the common organic solvents except for the paraffin solvents
in which its solubility is about one to three per cent by weight. Its
solubility in water is 0.5 per cent by weight.
Crufomate is formulated in several concentrations either for direct
use or for application after suitable dilution (emulsifiable
concentrates, pour-ons, systemic insecticide, anthelmintic, sheep and
goat wormer).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Following an 11 mg/kg intramuscular dosage of 32P labelled crufomated
to a yearling steer, radioactivity at the site of the treatment
dropped from 16 milliroentgens per hour immediately following the
treatment to 1.5 within two days and 0 after 13 days. Residues of
crufomate were practically non-existent after 14 days. Peak levels of
radioactivity occurred in the blood, urine and faeces in two to eight
hours, 12-18 hours, and 18-30 hours respectively. Fat tissues
contained less than 0.2 ppm of radioactive compounds after 14 days.
Red blood cholinesterase activity was depressed no more than 25 per
cent. The end product of the metabolism of crufomate is phosphoric
acid (Plapp. 1960).
A 21-day study of the metabolism of radioactive crufomate using acute
oral dosages of 100 and 200 mg/kg of body-weight in sheep shows
residues in the urine consisting of crufomate and several hydrolysis
products. Over 85 per cent of the administered 32P was recovered in
the excreta. The 32P in the urine, amounting to 75 per cent of the
dose, was primarily in the form of the hydrolysis products of
crufomate. Some of the crufomate was hydrolyzed completely to
inorganic phosphate and retained in the animal tissues and bones.
Crufomate itself was not found in the tissues after seven days,
indicating fairly rapid metabolism. Residues of crufomate and several
hydrolysis products were found in the blood and plasma shortly after
treatment, but within two days the crufomate decreased to a low level
(Bauriedel and Swank, 1962).
Other metabolic studies in sheep (Chamberlain and Gatterdam, 1960),
and in poultry (Buttram and Arthur, 1961) showed rapid breakdown of
crufomate. The likely mammalian metabolites of crufomate do not cause
inhibition of cholinesterase (Smith, 1968).
Acute toxicity (oral)
Animal Sex LD50 (mg/kg References
body-weight)
Rat male 950 McCollister et al., 1968
Rat female 770 McCollister et al., 1968
Rabbit mixed 490 McCollister et al., 1968
Guinea-pig male 1 000 McCollister et al., 1968
Dog male >1 000 McCollister et al., 1968
Dog female >750 McCollister et al., 1968
Sheep. Lambs received a single dose of 100, 200 or 400 mg/kg. Signs
of intoxication appeared at 200 mg/kg or more. Whole blood
cholinesterase activity was depressed to 50 per cent at 100 mg/kg,
and was markedly depressed at higher doses (Galvin et al., 1960).
Symptoms are highly variable and mainly caused by central nervous
system disturbances. At necropsy severe congestion of lungs and
kidneys, enlargement of liver and scattered haemorrhages were found
(Radeleff, 1964).
Short-term studies
Rat. Rats (10 males and 10 females per group) were started at the
age of 45 days on diets containing 10, 30, 100, 300 or 1000 ppm
crufomate. During a 90-day experimental period no evidence of
ill-effect was found when judged by general appearance and behaviour,
growth, mortality or food consumption records, haematological
determinations, final body and organ weight ratios, and gross and
microscopic examination of tissues. Results of measurements of
cholinesterase activity in blood and brain show that at 41 days there
was a peculiarly uniform depression of cholinesterase activity in the
plasma of both male and female rats over the entire range of 10-1000
ppm. The same phenomenon, although not as marked, was seen at 69 days,
indicating that a higher intake of chemical per unit of body-weight
during the early period way have been partially responsible.
Measurement of the erythrocyte-and brain-cholinesterase activities
showed little or no effect at the 30 ppm level of crufomate. In the
groups of rats from each dietary level which were placed on the
control ration for 48 days following the 90-day test period, recovery
of blood and brain cholinesterase was complete (McCollister et al.,
1968).
Dog. Groups comprising one male and one female dog were given diets
containing 0, 15, 40, 125 or 250 ppm crufomate for 75 days. Dogs that
received 250 ppm crufomate in their diets for 75 days showed no
evidence of adverse effect by any of the criteria examined except
cholinesterase activity and liver histopathology. Plasma-,
erythrocyte- and brain-cholinesterase levels were decreased to about
65 per cent of the control values. Slight centrilobular granular
degeneration was observed on microscopic examination of the liver.
Cholinesterase measurements were within the normal range in dogs at
the 125 ppm level. There was no evidence of adverse effect in dogs
that received 15 or 40 ppm dietary crufomate for 75 days, as judged by
general appearance and behaviour, growth, mortality, food consumption,
haematological indices, cholinesterase activities, final body and
organ weights and gross and microscopic appearance of the tissues
(McCollister et al., 1968).
Groups of dogs, each containing four males and four females per group,
were given diets containing 0, 10, 20, 200 or 2000 ppm of crufomate
for up to two years. The dogs on 2000 ppm crufomate were transferred
to the control diet after 30 days and sacrificed on days 94 and 95 of
the study. After one year, one male and one female from each of the
other groups were sacrificed for tissue examination. Cholinesterase
determinations were made at 30, 60 and 90 days after the start of the
study and at six-week intervals up to one year and every three months
thereafter. The animals on the highest level of crufomate manifested
gross signs of intoxication, values for serum alkaline phosphatase and
transaminase were elevated, plasma cholinesterase was reduced to 55
per cent of the pre-test average, and red cell cholinesterase was
depressed to 19 per cent of the pre-test values. In those dogs that
received 200 ppm crufomate in their diet plasma and erythrocyte
cholinesterase activity was depressed slightly. Other biochemical,
haematological findings and gross and microscopic examinations of the
tissues were comparable to the controls. Groups of male and female
dogs that received diets containing 10 or 20 ppm for two years showed
no evidence of adverse effect according to any of the criteria applied
(McCollister et al,, 1968).
Long-term studies
Rat. Groups of rats (25 of each sex) were started on diets
containing 1, 10, 100 or 1000 ppm of crufomate. Fifteen rats of each
sex from each group were designated for a two-year study. The
remaining 10 of each sex were killed at 12 or 18 months. Additional
groups of rats (five of each sex) were given diets containing 20, 40,
60 or 80 ppm of crufomate for 20 months.
Both male and female rats that received 1000 ppm crufomate in their
diets showed retardation of growth during the second year of the
study. Necropsy and microscopic examination of these rats after 24
months revealed atrophy of the muscles of the hindlegs and slight
degeneration of the sciatic nerve. A reduction in testis weight to
about one half, reflecting degeneration and atrophy of seminiferous
tubules, was seen at the 1000 ppm level after 12 and 18 months and
again at 24 months. The only evidence of my effect at dosage levels
below 1000 ppm was depression of cholinesterase. Brain cholinesterase
activity was depressed to 38 to 50 per cent of control for the rats
fed 1000 ppm but was within normal range at the lower dosage levels.
Male rats showed no significant effect on plasma cholinesterase at 100
ppm and below, or in red cells at 40 ppm and below. Corresponding
values for female rats were 40 ppm for plasma and 60 ppm for red cell
cholinesterase (McCollister et al., 1968).
Special studies
(a) Fertility and reproduction
Groups of four male and 12 female rats were maintained on diets
containing 0, 10 and 100 ppm crufomate through a three-generation
study involving two litters per generation and assessing fertility,
reproduction and lactation. In addition, a fourth group received 1 ppm
crufomate for the first generation and 500 ppm for the remaining two
generations of the experiment. In all these groups no evidence was
found of adverse effect on fertility, gestation, viability or
lactation, as well as body-weights at weaning and mating.
Cholinesterase inhibition was not investigated (McCollister at al.,
1968).
Forty bulls, three to seven years old, were given a single oral dose
of crufomate at 50 mg/kg or sprayed to wetness with 0.75 per cent
dispersion of a crufomate wettable powder without affecting the
motility of the spermatozoa after one and four weeks (McGregor 1960).
An acute oral dosage of 100 mg/kg of crufomate had no effect on the
fertility of male rats, rabbits or guinea-pigs (McCollister, 1959).
(b) Neurotoxicity
Intraperitoneal injections of crufomate into 25 mature hens at dosage
rates of 500, 750 and 1000 mg/kg caused mortalities in one out of
seven, one out of four and 10 out of 14, respectively, of the birds
treated. Of the surviving 31 treated hens 26 developed ataxia and
paralysis. Those able to take food and water made uneventful and
complete recovery within 90 days of treatment. Histopathological
examination of nerve tissue revealed no departure from normal (Hymas
and Stevenson, 1961).
(c) Potentiation
Single oral doses of crufomate were administered jointly to rats with
each of 14 commercial cholinesterase inhibiting insecticides (13
organo-phosphorus compounds and one carbamate). There was no effect
other than additive (ratios 0.7 to 2.2) for the LD50 values except
for crufomate-malathion (fivefold increase). Dietary feeding studies
in dogs for 12 weeks with diets containing 100 ppm malathion plus 20
ppm crufomate, or 10 ppm malathion plus 20 ppm crufomate, showed no
significant evidence of potentiation (McCollister et al., 1968).
(d) Studies on the metabolite
Rat. Groups of rats (25 of each sex) were fed 100, 300, 1000, and
3000 ppm 4-tert-butyl-2-chlorophenol (the hydrolytic metabolite of
crufomate) in the diet for two years. Rats fed the dosage levels of
100, 300 or 1000 ppm showed no significant evidence of adverse effects
as judged by general appearance, behaviour, growth, mortality,
incidence of tumorous growths, haematological studies, serum urea
nitrogen and alkaline phosphatase determination. Final average body
and organ weights and gross and microscopic effects after examination
of the tissues were normal. Rats fed dietary levels of 3000 ppm showed
moderate microscopic liver changes and increased senility in the
heart, kidney and liver (McCollister, 1964).
Dog. Groups of eight dogs (four of each sex) were fed diets
containing 20, 200 or 2000 ppm 4-tert-butyl-2-chlorophenol for two
years. The male dogs on 2000 ppm showed atrophy and degenerative
changes in the seminiferous tubules of the testes. No other gross or
microscopic effect was observed at any dose level (Dieterich et al.,
1965).
Observations in man
Groups of three adult males per test were given one, two or four
tablets containing 200 mg of crufomate daily for seven days.
Laboratory tests consisted of complete blood counts, urinalysis, red
blood cell cholinesterase response and serum glutamic pyruvic
transaminase determinations. The subjects were interrogated concerning
side effects. The second and third groups showed a delayed 50-80 per
cent red blood cell cholinesterase depression after completion of the
treatments. No other abnormalities were noted in the laboratory tests
(Campbell, 1962).
Comments
The most sensitive criterion upon which to judge the safety of
crufomate upon ingestion by animals or human subjects is its
cholinesterase-inhibiting properties. The short-term and long-term
studies are adequate. Because the only study available in man was too
limited, it is desirable that further metabolic studies in man should
be carried out to show that 4-tert-butyl-2-chlorophenol is the main
metabolite also in this species.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Rat: 40 ppm in the diet, equivalent to 2 mg/kg per day
Dog: 40 ppm in the diet, equivalent to 1 mg/kg per day
Man: 200 mg/day, equivalent to about 3 mg/kg per day
Estimate of acceptable daily intake for man
0-0.1 mg/kg per day
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Crufomate is used on or in cattle, sheep, and goats to control
internal cattle grubs and external horn flies and cattle lice (Anon.,
1966). Applications of crufomate for cattle grub control have been by
injection, oral administration, and spraying (McGregor et al., 1959),
but the simple pour-on application of a low volume of insecticide on
the backs of cattle has been found best (Rogoff and Kohler, 196O).
Formulations of crufomate may be sprayed or poured on to cattle during
the warmer months of the year. However, in the United States of
America during October through February, treatment is restricted
depending on the locality. The dosage rate ranges from about 15 to 50
mg/kg of body-weight.
As a pour-on applied to the backline of cattle, an emulsifiable
concentrate of crufomate is diluted with water to apply about 39 mg/kg
for grubs, horn flies and lice, and about 52 mg/kg for internal
helminth parasite control.
Another treatment of internal helminth parasites of livestock involves
the oral drench (introduction into rumen directly) of 50 to 75 mg/kg.
The following internal parasites of livestock are examples of genera
for which this treatment has been recommended: Haemonchus and
Osteragia (stomach worms), Tricho-strongylus (hairworms),
Strongyloides (threadworm), Bunostomum (hookworm),
Oesophagostomum (nodular worms), Nematodirus (thin-necked
strongyle), Trichuris (whipworms), Cooperia (Cooper's worms),
Oestrus ovis (head grub) and others.
Retreatment for horn flies and cattle lice should not be done more
often than every 28 days and not within 28 days of slaughter. Animals
under stress from sickness, crowding, excitement or surgery should not
be treated. Lactating dairy cows or dry dairy cows within 28 days of
freshening should not be treated. General recommendations for the
current use of cruformate are given in Anon. (1967, 1968).
Residues resulting from supervised trials
When crufomate is used as directed, its residues decrease to a
negligible level 28 days after external treatment and 14 days after
internal treatment. Residues of crufomate found in supervised trials
are summarized in the following table:
Rate of Post-treatment Residues* Reference
Animal application interval (days) (ppm)
(mg/kg)
Cattle 75 14 No residues Anon. (1962b)
(<0.04) fat,
lean meat
Sheep 89.8 14 No residues Claborn et al.
(<0.02) fat, (1960)
internal organs
Lambs 200 7 No residues Bauriedel and
(<0.05) fat, Swank (1962)
lean meat
Cattle 50 7 0.3-1.3** Mussel and
fat, less in Ludwig (1961)
other tissues
Cattle 100 7 0.6-1.6** Mussel and
fat, less in Ludwig (1961)
other tissues
(continued)
Rate of Post-treatment Residues* Reference
Animal application interval (days) (ppm)
(mg/kg)
Cattle 50 7 0.07-0.2** fat Mussel and
Ludwig (1961)
Cattle 100 7 0.1-2.4** fat Mussel and
Ludwig (1961)
Cattle 52 7 0.05-0.14 fat Dishburger and
14 <0.02-0.05 fat Rice (1968)
21 no residues
(<0.02) fat
Calves 52 and 104 28 0.1-0.22 fat Rice (1964)
Lactating 49-66 1 0.195 milk Leahy and
cow 2 no detectable Brown (1963)
residues
(<0.05) milk
Lactating 37.5 1 0.14-0.3 milk Anon. 1962a
cow 2 0.0 milk
Steers 50 7 0.1 phenol in Kutchinski
fat (limit of (1960)
sensitivity, 0.1)
* When one figure is given, residue is maximum value.
** No residues (<0.1 ppm) after 28 days.
Fate of residues
In animals
Six possible routes for the hydrolysis of crufomate to inorganic
phosphate are shown below (Bauriedel and Swank, 1962).
 B, C, D, F and G have been identified, definitely or tentatively, as
metabolites in addition to the 4-tert-butyl-2-chlorophenol
hydrolysis product.
Several studies with P32 labelled crufomate administered orally and
by injection to cattle, sheep, and hens have shown that crufomate is
eliminated rapidly as hydrolysis products or hydrolyzed to inorganic
phosphate which may be retained. (Bauriedel and Swank, 1962; Brady and
Arthur, 1962; Buttram and Arthur, 1961; Plapp, 1960; Chamberlain and
Gatterdam, 1960).
The two products that may be expected as residues are cruformate and
its phenolic hydrolysis product. The other hydrolysis products
(metabolites A, B, C, D, E, F) appear to be too polar to be stored
appreciably; the studies with P32 labelled crufomate verify the rapid
elimination of these products.
Evidence of residues in food in commerce or at consumption
No residues of crufomate were found in 149 tissue samples taken in
1968 from slaughter-houses in five states (Indiana, Louisiana,
Nebraska, Oklahoma, and Texas) in the United States of America
(Stewart, 1968).
Methods of residue analysis
Because crufomate is used almost exclusively for the control of
arthropods and worms on or in animals, residue analyses for this
chemical and its metabolites have been conducted primarily on meat,
milk, blood, and animal tissues.
For the residue analyses at the present time, the method of choice
based on sensitivity, specificity, and speed of analysis would appear
to be gas chromatography with a thermionic or flame photometric
detector for sensing phosphorus compounds, after removal of fat (if
necessary) by a hexane-acetonitrile or similar solvent partition.
Burke (1965) and Bowman and Beroza (1965) determined the compound
itself by gas chromatography. Bowman and Beroza (1967) later
determined it in milk and silage by temperature-programmed gas
chromatography with the flame photometric detector as part of a
multicomponent analysis. No clean-up other than a hexane-acetonitrile
partition was needed to remove the fat from the milk extract. The
fraction partitioning into the upper fat-containing phase is readily
calculated from the p-value, which is 0.031 in this system (Bowman
and Beroza, 1965). Recoveries at levels of fortification of 0.05 and
0.2 ppm were generally better than 90 per cent with a sensitivity of
about 0.01 ppm. In an unpublished communication Dishburger and Rice
(1968) determined crufomate in animal fat tissue. They used liquid
chromatography on silicic acid for a clean-up prior to analysis of the
eluate by gas chromatography with thermionic detection. Sensitivity
was 0.02 ppm and recoveries averaged 92 per cent at levels of 0.02 to
0.06 ppm. The use of electron capture, microcoulometric, and
electrolytic-conductivity detectors for the gas chromatographic
analysis of crufomate appears feasible but has not been evaluated.
Early methods used in the analysis of crufomate residues were
manometric housefly-head cholinesterase inhibition procedures
developed by The Dow Chemical Co. They required modifications
depending on the substrate being analysed. A procedure for extracting
crufomate from milk developed by Timmerman et al. (1961) was about 90
per cent efficient. Leahy and Taylor (1963) determined crufomate in
milk by paper chromatography of the milk extract, acid digestion of
the portion of the paper chromatogram containing the pesticide, and
determination of the phosphorus content colorimetrically.
For confirmation of identity of residues, Elgar (1967) suggested
independent parameters that might be used. When enough material can be
isolated, spectroscopy (infra-red, ultra-violet, mass) is excellent
for such confirmations. Bowman and Beroza (1967), reported gas
chromatographic retention times on three liquid phases for crufomate.
They also gave partitioning data (p-values) in six binary solvent
systems, which may be used for identification or for establishing the
quantitative validity of gas chromatographic peaks. Rf values in
paper (Leahy and Taylor, 1963; McKinley and Read, 1962) and thin-layer
chromatography (El-Refai and Hopkins, 1965; Ragab, 1967) can be
useful. The combined use of these and other methods (chemical
derivatives, bio-assay) will make identifications more certain.
The 4-tert-butyl-2-chlorophenol metabolite of crufomate was
determined spectrophotometrically in beef fat and meat with a
sensitivity of 0.1 ppm and an average recovery of 80 per cent after a
clean-up by liquid chromatography and steam distillation, coupling
with diazotized p-nitroaniline, and chromatography of the resulting
dye (Anon., 1960). The presence of a chlorine atom may allow
quantification by electron-capture gas chromatography.
National tolerances
Registration in the United States on a non-residue basis (Anon., 1965)
is to be superseded by the establishment of negligible or finite
residue tolerances. In other countries such as Great Britain, Ireland,
European Economic Community countries, all Latin American countries,
Australia, New Zealand, and Canada, products containing crufomate are
registered on a non-residue basis when used for external parasite
control. Crufomate is registered for internal parasite control of
livestock in Great Britain, Ireland, Spain, Australia, New Zealand,
Brazil, Uruguay, Argentina, Colombia, Ecuador, Venezuela, Paraguay,
Central American countries, Mexico, Canada and the United States of
America.
The Dow Chemical Company is presently reviewing their residue and
toxicological data on crufomate and may soon recommend shortening or
elimination of withdrawal time specifications.
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Appraisal
Crufomate is used internally and is applied externally to livestock as
an insecticide; it is also used as an anthelmintic. In its principal
uses, it is administered long before slaughter so there will be no
residues from such applications in meat. Another use, of secondary
importance, is accompanied by a recommended minimum of 14 days between
treatment and slaughter.
Lactating cattle experimentally treated by pour-on oral drench yielded
milk that did not contain residues in excess of 0.05 ppm after two
days.
A monitoring survey, conducted in 1968 on cattle tissue from five
states of the United States of America, detected no residues in any of
the 149 samples examined.
Several gas chromatographic methods for the compound, which appear to
be suitable for regulatory purposes or for evaluation as a referee
procedure, have been reported.
Recommendations
The following temporary tolerances (to be in effect until 1972) are to
apply to raw agricultural products moving in commerce unless otherwise
indicated. In the case of commodities entering international trade,
the tolerances should be applied by the importing country at the point
of entry or as soon as practicable thereafter.
Temporary tolerances
Meat (on fat basis) (at slaughter) 1 ppm
Whole milk 0.05 ppm
Further work or information
Required before 30 June 1972
1. Data from countries other than the United States of America and the
United Kingdom on the use pattern and resultant residues.
2. Further data on the use pattern and resultant residues in milk from
treated animals, using gas-liquid chromatographic methods.
3. Data on residues in the non-fatty portion of meat and meat
products.
4. Comparative evaluation of gas-liquid chromatographic methods for
regulatory purposes.
Desirable
1. Collaborative studies to establish a referee method.
2. More extensive studies on cholinesterase effects in man.
3. Studies on the metabolism in man to show that
4-tert-butyl-2-chlorophenol is the main metabolite in that species.
REFERENCES
Anon. (1960) Determination of 4-tert-butyl-2-chlorophenol in the
meat and the fat of cattle. Residue determination method ACR 60.8. The
Dow Chemical Co. Midland, Michigan
Anon. (1962a) Residues of Ruelene (R) in milk from a single oral
drench application of Ruelene wormer sheep and goat drench. The Dow
Chemical Co., Midland, Michigan. Unpublished report
Anon. (1962b) Residues of Ruelene in fat and lean meat from cattle
treated with Ruelene in the feed and by oral drench. The Dow Chemical
Co., Midland, Michigan. Unpublished report
Anon. (1965) U.S.D.A. Summary of registered agricultural pesticide
chemical uses (4-tert-butyl-2-chlorophenyl methyl
methylphosphoramidate). p. 767, Issued October 1, 1965, Pesticides
Regulation Division, Agricultural Research Service, U.S. Department of
Agriculture, Washington, D.C.
Anon. (1966) Ruelene (R) systemic insecticide, The Dow Chemical Co.,
Midland, Michigan
Anon. (1967) Suggested guide for the use of insecticides to control
insects affecting crops, livestock, households, stored products,
forests, and forest products, 1967. Agriculture Handbook No. 331,
U.S.D.A. Supt. of Documents, U.S. Government Printing Office,
Washington, D.C.
Anon. (1968) Technical information bulletin. 1968 registered livestock
and household uses for Ronnel and registered livestock uses for
"Ruelene". The Dow Chemical Co., Midland, Michigan
Bauriedel, W. R. and Swank, M. G. (1960) Residue and metabolism of
radioactive Ruelene (R), 4-tertiary-butyl-2-chlorophenyl methyl
methylphosphoramidate, administered as a single oral dose to cattle.
Dow Chemical Co. Unpublished report
Bauriedel, W. R. and Simmons, B. L. (1961) Residue and metabolism of
radioactive Ruelene (R), 4-tertiary-butyl-2-chlorophenyl methyl
methylphosphoramidate, administered to a steer by intrarumenal
injection. Dow Chemical Co. Unpublished report
Bauriedel, W. R. and Swank, M. G. (1962) Residue and metabolism of
radioactive 4-tert-butyl-2-chlorophenyl methyl
methylphosphoramidate, administered as a single oral dose to sheep. J.
Agr. Food. Chem., 10: 150-154
Bowman, M. C. and Beroza, M. (1965) Extraction p-values of
pesticides and related compounds in six solvent systems. J. Ass.
Offic. Agr. Chem., 48: 943-952
Bowman, M. C. and Beroza, M. (1967) Temperature programmed gas
chromatography of twenty phosphorus-containing insecticides on four
different columns and its application to the analysis of milk and corn
silage. J. Ass. Offic. Anal. Chem., 50: 1228-1236
Brady, U. E., jr and Arthur, B. W. (1962) Absorption and metabolism of
Ruelene(R) by Arthropods. J. Econ. Entom., 55: 833-836
Burch, G. R. (1963) Report on Ruelene(R) study in dogs. Pitman-Moore
Division, The Dow Chemical Co., Indianapolis, Indiana. Unpublished
report
Burke, J. A. (1965) Gas chromatography of pesticide residue analysis:
Some practical aspects. J. Ass. Offic. Agr. Chem., 48: 1037-1058
Buttram, J. R. and Arthur, B. W. (1961) Magnitude and nature of
residues in tissues and eggs of poultry receiving Ruelene (R) in the
feed. J. Econ. Entom., 54: 456-460
Campbell, P. J. (1960) Study of Ruelene. Stough-Wisdom Research Inc.
Unpublished report
Chamberlain, W. F. and Gatterdam, P. E. (1960) Third report on studies
with P32 labeled Ruelene. Livestock Insects Investigations,
Entomology Research Division, U.S.D.A., Kerrville, Texas. Unpublished
report
Claborn, H. V., Mann, H. D. and Ivey, M. C. (1960) Second report on
studies with P32 labelled Ruelene, part II, studies on residues in
tissues. Pesticide Chemicals Research Branch, Entomology Research
Division, U.S.D.A., Kerrville, Texas. Unpublished report
Dieterich, W. H., Paynter, O. E. and Weir, R. J. (1965) The chronic
toxicity of Ruelene and a Ruelene derivative in beagle dogs. Hazleton
Laboratories. Paper presented to the Society of Toxicology,
Williamsburg, Virginia
Dishburger, H. J. and Rice, J. R. (1968) Residues in omental fat of
cattle following pour-on application of Ruelene (R) 8R and 25E
insecticide formulations. The Dow Chemical Co., Lake Jackson, Texas.
Unpublished report
Elgar, K. E. (1967) Confirmation of identity, in report by Egan, H.,
pesticide residues. J. Ass. Offic. Anal. Chem., 50: 1069
El-Refai, A. and Hopkins, T. L. (1965) Thin-layer chromatography and
cholinesterase detection of several phosphorothiono insecticides and
their oxygen analogs. J. Agr. Food Chem., 13: 477-481
Galvin, T. J., Bell, R. R. and Turk, R. D. (1960) Anthelmintics for
ruminants. II. Anthelmintic activity and toxicity of Ruelene in sheep.
Amer. J. vet. Res., 21: 1058-1061
Hymas, T. A. and Stevenson, G. T. (1961) Attempt to produce
neurotoxicity by the injection of Ruelene or Ronnel into mature
Leghorn hens. Dow Chemical Co. Unpublished report
Jackson, J. B., Drummond, R. O., Buck, W. B. and Hunt, L. M. (1960)
Toxicity of organic phosphorus insecticides to horses. J. Econ.
Entomol., 53: 602-604
Kutschinski, A. H. (1960) Determination of
4-tert-butyl-2-chlorophenol residues in the meat and fat of cattle
sprayed with 4-tert-butyl-2-chlorophenyl methyl
methylphosphoramidate (Ruelene(R)). The Dow Chemical Co., Midland,
Michigan. Unpublished report
Leahy, J. S. and Brown, F. G. (1963) Residues of
4-tert-butyl-2-chlorophenyl methyl methylphosphoramidate in the milk
of dairy cows following dermal application. The Veterinary Record,
75: (39) 1000-1001
Leahy, J. S. and Taylor, T. (1963) The determination of residues of
"Ruelene" in milk. Analyst, 88: 882-885
McCollister, D. D. (1959) Fertility studies with male laboratory
animals given single doses of Ruelene. Dow Chemical Co. Unpublished
report
McCollister, D. D. (1964) Results of two-year dietary feeding studies
of 4-tert-butyl-2-chlorophenol in rats. Dow Chemical Co. Unpublished
report
McCollister, D. D., Olson, K. J., Rowe, V. K., Paynter, O. E., Weir,
R. J. and Dieterich, W. H. (1968) Toxicology of
4-tert-butyl-2-chlorophenyl methyl methylphosphoramidate (Ruelene)
in laboratory animals. Food and Cosmetic Toxicology, 6: 185-198
McGregor, W. S., Ludwig, P. D. and Wade, L. L. (1959) Progress report
on Ruelene for cattle grub control. Down to Earth, Fall, 1959, pp. 1-2
McGregor, W. S. (1960) The effects of Ruelene (R) treatments on the
fertility of range bulls. Dow Chemical Co. Unpublished report
McKinley, W. P. and Read, S. I. (1962) Esterase inhibition technique
for the detection of organophosphorus pesticides. J. Ass. Offic. Agr.
Chem., 45: 467-473
Mussell, D. R. and Ludwig, P. D. (1961) Residues of Ruelene(R) in
animal tissues when applied as a spray to cattle. The Dow Chemical
Co., Midland, Michigan. Unpublished report
Plapp, F. W. (1960) Studies on the metabolism of P32 Ruelene (R) in a
Hereford steer. Agricultural Research Service, U.S.D.A., Corvallis,
Oregon. Unpublished report
Radeleff, R. D. (1964) Organophosphorus compounds. Ruelene (R),
O-4-tert-butyl-2-chlorophenyl methyl methylphosphoramidate.
Veterinary Toxicology, Lea and Febger, Philadelphia, Pa., pp. 209-210
Ragab, M. T. H. (1967) Direct fluorescent detection of
organothiophosphorus pesticides and some of the sulfur-containing
breakdown products after thin-layer chromatography. J. Ass. Offic.
Anal. Chem., 50: 1088-1098
Rice, J. R. (1964) Residue determination of Ruelene in bovine tissue
and Ruelene TF-88 pour-on application. The Dow Chemical Co., Lake
Jackson, Texas. Unpublished report
Rogoff, W. M. and Kohler, P. H. (1960) Effectiveness of Ruelene (R)
applied as localized "pour-on" and as spray for cattle grub control.
J. Econ. Entomol., 53: 814-817
Sherman, M. and Ross, E. (1961) Acute and subacute toxicity of
insecticides for chicks. Toxicol. appl. Pharmacol., 3: 521-533
Skerman, K. D. (1962) Mintrel(R). A new anthelmintic for sheep.
Aust. vet. J., pp. 324-334
Smith, G. N. (1968) Cholinesterase inhibition of crufomate (Ruelene
(R) insecticide) and metabolites. Dow Chemical Co. Unpublished report
Stewart, J. H. (1968) U.S. Department of Agriculture, Washington, D.C.
Private communication
Timmerman, J. A., jr, Dorough, H. W., Buttram, J. R. and Arthur, B. W.
(1961) In vitro stability and recovery of insecticides from milk.
J. Econ. Entomol. 54: 441-444
Weidenbach, C. P., Radeleff, R. D. and Buck, W. B. (1962)
Toxicological studies of Ruelene(R) 4-tert-butyl-2-chlorophenyl
methyl methylphosphoramidate. J. Amer. vet. med. Ass., 140: 460-463
B, C, D, F and G have been identified, definitely or tentatively, as
metabolites in addition to the 4-tert-butyl-2-chlorophenol
hydrolysis product.
Several studies with P32 labelled crufomate administered orally and
by injection to cattle, sheep, and hens have shown that crufomate is
eliminated rapidly as hydrolysis products or hydrolyzed to inorganic
phosphate which may be retained. (Bauriedel and Swank, 1962; Brady and
Arthur, 1962; Buttram and Arthur, 1961; Plapp, 1960; Chamberlain and
Gatterdam, 1960).
The two products that may be expected as residues are cruformate and
its phenolic hydrolysis product. The other hydrolysis products
(metabolites A, B, C, D, E, F) appear to be too polar to be stored
appreciably; the studies with P32 labelled crufomate verify the rapid
elimination of these products.
Evidence of residues in food in commerce or at consumption
No residues of crufomate were found in 149 tissue samples taken in
1968 from slaughter-houses in five states (Indiana, Louisiana,
Nebraska, Oklahoma, and Texas) in the United States of America
(Stewart, 1968).
Methods of residue analysis
Because crufomate is used almost exclusively for the control of
arthropods and worms on or in animals, residue analyses for this
chemical and its metabolites have been conducted primarily on meat,
milk, blood, and animal tissues.
For the residue analyses at the present time, the method of choice
based on sensitivity, specificity, and speed of analysis would appear
to be gas chromatography with a thermionic or flame photometric
detector for sensing phosphorus compounds, after removal of fat (if
necessary) by a hexane-acetonitrile or similar solvent partition.
Burke (1965) and Bowman and Beroza (1965) determined the compound
itself by gas chromatography. Bowman and Beroza (1967) later
determined it in milk and silage by temperature-programmed gas
chromatography with the flame photometric detector as part of a
multicomponent analysis. No clean-up other than a hexane-acetonitrile
partition was needed to remove the fat from the milk extract. The
fraction partitioning into the upper fat-containing phase is readily
calculated from the p-value, which is 0.031 in this system (Bowman
and Beroza, 1965). Recoveries at levels of fortification of 0.05 and
0.2 ppm were generally better than 90 per cent with a sensitivity of
about 0.01 ppm. In an unpublished communication Dishburger and Rice
(1968) determined crufomate in animal fat tissue. They used liquid
chromatography on silicic acid for a clean-up prior to analysis of the
eluate by gas chromatography with thermionic detection. Sensitivity
was 0.02 ppm and recoveries averaged 92 per cent at levels of 0.02 to
0.06 ppm. The use of electron capture, microcoulometric, and
electrolytic-conductivity detectors for the gas chromatographic
analysis of crufomate appears feasible but has not been evaluated.
Early methods used in the analysis of crufomate residues were
manometric housefly-head cholinesterase inhibition procedures
developed by The Dow Chemical Co. They required modifications
depending on the substrate being analysed. A procedure for extracting
crufomate from milk developed by Timmerman et al. (1961) was about 90
per cent efficient. Leahy and Taylor (1963) determined crufomate in
milk by paper chromatography of the milk extract, acid digestion of
the portion of the paper chromatogram containing the pesticide, and
determination of the phosphorus content colorimetrically.
For confirmation of identity of residues, Elgar (1967) suggested
independent parameters that might be used. When enough material can be
isolated, spectroscopy (infra-red, ultra-violet, mass) is excellent
for such confirmations. Bowman and Beroza (1967), reported gas
chromatographic retention times on three liquid phases for crufomate.
They also gave partitioning data (p-values) in six binary solvent
systems, which may be used for identification or for establishing the
quantitative validity of gas chromatographic peaks. Rf values in
paper (Leahy and Taylor, 1963; McKinley and Read, 1962) and thin-layer
chromatography (El-Refai and Hopkins, 1965; Ragab, 1967) can be
useful. The combined use of these and other methods (chemical
derivatives, bio-assay) will make identifications more certain.
The 4-tert-butyl-2-chlorophenol metabolite of crufomate was
determined spectrophotometrically in beef fat and meat with a
sensitivity of 0.1 ppm and an average recovery of 80 per cent after a
clean-up by liquid chromatography and steam distillation, coupling
with diazotized p-nitroaniline, and chromatography of the resulting
dye (Anon., 1960). The presence of a chlorine atom may allow
quantification by electron-capture gas chromatography.
National tolerances
Registration in the United States on a non-residue basis (Anon., 1965)
is to be superseded by the establishment of negligible or finite
residue tolerances. In other countries such as Great Britain, Ireland,
European Economic Community countries, all Latin American countries,
Australia, New Zealand, and Canada, products containing crufomate are
registered on a non-residue basis when used for external parasite
control. Crufomate is registered for internal parasite control of
livestock in Great Britain, Ireland, Spain, Australia, New Zealand,
Brazil, Uruguay, Argentina, Colombia, Ecuador, Venezuela, Paraguay,
Central American countries, Mexico, Canada and the United States of
America.
The Dow Chemical Company is presently reviewing their residue and
toxicological data on crufomate and may soon recommend shortening or
elimination of withdrawal time specifications.
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Appraisal
Crufomate is used internally and is applied externally to livestock as
an insecticide; it is also used as an anthelmintic. In its principal
uses, it is administered long before slaughter so there will be no
residues from such applications in meat. Another use, of secondary
importance, is accompanied by a recommended minimum of 14 days between
treatment and slaughter.
Lactating cattle experimentally treated by pour-on oral drench yielded
milk that did not contain residues in excess of 0.05 ppm after two
days.
A monitoring survey, conducted in 1968 on cattle tissue from five
states of the United States of America, detected no residues in any of
the 149 samples examined.
Several gas chromatographic methods for the compound, which appear to
be suitable for regulatory purposes or for evaluation as a referee
procedure, have been reported.
Recommendations
The following temporary tolerances (to be in effect until 1972) are to
apply to raw agricultural products moving in commerce unless otherwise
indicated. In the case of commodities entering international trade,
the tolerances should be applied by the importing country at the point
of entry or as soon as practicable thereafter.
Temporary tolerances
Meat (on fat basis) (at slaughter) 1 ppm
Whole milk 0.05 ppm
Further work or information
Required before 30 June 1972
1. Data from countries other than the United States of America and the
United Kingdom on the use pattern and resultant residues.
2. Further data on the use pattern and resultant residues in milk from
treated animals, using gas-liquid chromatographic methods.
3. Data on residues in the non-fatty portion of meat and meat
products.
4. Comparative evaluation of gas-liquid chromatographic methods for
regulatory purposes.
Desirable
1. Collaborative studies to establish a referee method.
2. More extensive studies on cholinesterase effects in man.
3. Studies on the metabolism in man to show that
4-tert-butyl-2-chlorophenol is the main metabolite in that species.
REFERENCES
Anon. (1960) Determination of 4-tert-butyl-2-chlorophenol in the
meat and the fat of cattle. Residue determination method ACR 60.8. The
Dow Chemical Co. Midland, Michigan
Anon. (1962a) Residues of Ruelene (R) in milk from a single oral
drench application of Ruelene wormer sheep and goat drench. The Dow
Chemical Co., Midland, Michigan. Unpublished report
Anon. (1962b) Residues of Ruelene in fat and lean meat from cattle
treated with Ruelene in the feed and by oral drench. The Dow Chemical
Co., Midland, Michigan. Unpublished report
Anon. (1965) U.S.D.A. Summary of registered agricultural pesticide
chemical uses (4-tert-butyl-2-chlorophenyl methyl
methylphosphoramidate). p. 767, Issued October 1, 1965, Pesticides
Regulation Division, Agricultural Research Service, U.S. Department of
Agriculture, Washington, D.C.
Anon. (1966) Ruelene (R) systemic insecticide, The Dow Chemical Co.,
Midland, Michigan
Anon. (1967) Suggested guide for the use of insecticides to control
insects affecting crops, livestock, households, stored products,
forests, and forest products, 1967. Agriculture Handbook No. 331,
U.S.D.A. Supt. of Documents, U.S. Government Printing Office,
Washington, D.C.
Anon. (1968) Technical information bulletin. 1968 registered livestock
and household uses for Ronnel and registered livestock uses for
"Ruelene". The Dow Chemical Co., Midland, Michigan
Bauriedel, W. R. and Swank, M. G. (1960) Residue and metabolism of
radioactive Ruelene (R), 4-tertiary-butyl-2-chlorophenyl methyl
methylphosphoramidate, administered as a single oral dose to cattle.
Dow Chemical Co. Unpublished report
Bauriedel, W. R. and Simmons, B. L. (1961) Residue and metabolism of
radioactive Ruelene (R), 4-tertiary-butyl-2-chlorophenyl methyl
methylphosphoramidate, administered to a steer by intrarumenal
injection. Dow Chemical Co. Unpublished report
Bauriedel, W. R. and Swank, M. G. (1962) Residue and metabolism of
radioactive 4-tert-butyl-2-chlorophenyl methyl
methylphosphoramidate, administered as a single oral dose to sheep. J.
Agr. Food. Chem., 10: 150-154
Bowman, M. C. and Beroza, M. (1965) Extraction p-values of
pesticides and related compounds in six solvent systems. J. Ass.
Offic. Agr. Chem., 48: 943-952
Bowman, M. C. and Beroza, M. (1967) Temperature programmed gas
chromatography of twenty phosphorus-containing insecticides on four
different columns and its application to the analysis of milk and corn
silage. J. Ass. Offic. Anal. Chem., 50: 1228-1236
Brady, U. E., jr and Arthur, B. W. (1962) Absorption and metabolism of
Ruelene(R) by Arthropods. J. Econ. Entom., 55: 833-836
Burch, G. R. (1963) Report on Ruelene(R) study in dogs. Pitman-Moore
Division, The Dow Chemical Co., Indianapolis, Indiana. Unpublished
report
Burke, J. A. (1965) Gas chromatography of pesticide residue analysis:
Some practical aspects. J. Ass. Offic. Agr. Chem., 48: 1037-1058
Buttram, J. R. and Arthur, B. W. (1961) Magnitude and nature of
residues in tissues and eggs of poultry receiving Ruelene (R) in the
feed. J. Econ. Entom., 54: 456-460
Campbell, P. J. (1960) Study of Ruelene. Stough-Wisdom Research Inc.
Unpublished report
Chamberlain, W. F. and Gatterdam, P. E. (1960) Third report on studies
with P32 labeled Ruelene. Livestock Insects Investigations,
Entomology Research Division, U.S.D.A., Kerrville, Texas. Unpublished
report
Claborn, H. V., Mann, H. D. and Ivey, M. C. (1960) Second report on
studies with P32 labelled Ruelene, part II, studies on residues in
tissues. Pesticide Chemicals Research Branch, Entomology Research
Division, U.S.D.A., Kerrville, Texas. Unpublished report
Dieterich, W. H., Paynter, O. E. and Weir, R. J. (1965) The chronic
toxicity of Ruelene and a Ruelene derivative in beagle dogs. Hazleton
Laboratories. Paper presented to the Society of Toxicology,
Williamsburg, Virginia
Dishburger, H. J. and Rice, J. R. (1968) Residues in omental fat of
cattle following pour-on application of Ruelene (R) 8R and 25E
insecticide formulations. The Dow Chemical Co., Lake Jackson, Texas.
Unpublished report
Elgar, K. E. (1967) Confirmation of identity, in report by Egan, H.,
pesticide residues. J. Ass. Offic. Anal. Chem., 50: 1069
El-Refai, A. and Hopkins, T. L. (1965) Thin-layer chromatography and
cholinesterase detection of several phosphorothiono insecticides and
their oxygen analogs. J. Agr. Food Chem., 13: 477-481
Galvin, T. J., Bell, R. R. and Turk, R. D. (1960) Anthelmintics for
ruminants. II. Anthelmintic activity and toxicity of Ruelene in sheep.
Amer. J. vet. Res., 21: 1058-1061
Hymas, T. A. and Stevenson, G. T. (1961) Attempt to produce
neurotoxicity by the injection of Ruelene or Ronnel into mature
Leghorn hens. Dow Chemical Co. Unpublished report
Jackson, J. B., Drummond, R. O., Buck, W. B. and Hunt, L. M. (1960)
Toxicity of organic phosphorus insecticides to horses. J. Econ.
Entomol., 53: 602-604
Kutschinski, A. H. (1960) Determination of
4-tert-butyl-2-chlorophenol residues in the meat and fat of cattle
sprayed with 4-tert-butyl-2-chlorophenyl methyl
methylphosphoramidate (Ruelene(R)). The Dow Chemical Co., Midland,
Michigan. Unpublished report
Leahy, J. S. and Brown, F. G. (1963) Residues of
4-tert-butyl-2-chlorophenyl methyl methylphosphoramidate in the milk
of dairy cows following dermal application. The Veterinary Record,
75: (39) 1000-1001
Leahy, J. S. and Taylor, T. (1963) The determination of residues of
"Ruelene" in milk. Analyst, 88: 882-885
McCollister, D. D. (1959) Fertility studies with male laboratory
animals given single doses of Ruelene. Dow Chemical Co. Unpublished
report
McCollister, D. D. (1964) Results of two-year dietary feeding studies
of 4-tert-butyl-2-chlorophenol in rats. Dow Chemical Co. Unpublished
report
McCollister, D. D., Olson, K. J., Rowe, V. K., Paynter, O. E., Weir,
R. J. and Dieterich, W. H. (1968) Toxicology of
4-tert-butyl-2-chlorophenyl methyl methylphosphoramidate (Ruelene)
in laboratory animals. Food and Cosmetic Toxicology, 6: 185-198
McGregor, W. S., Ludwig, P. D. and Wade, L. L. (1959) Progress report
on Ruelene for cattle grub control. Down to Earth, Fall, 1959, pp. 1-2
McGregor, W. S. (1960) The effects of Ruelene (R) treatments on the
fertility of range bulls. Dow Chemical Co. Unpublished report
McKinley, W. P. and Read, S. I. (1962) Esterase inhibition technique
for the detection of organophosphorus pesticides. J. Ass. Offic. Agr.
Chem., 45: 467-473
Mussell, D. R. and Ludwig, P. D. (1961) Residues of Ruelene(R) in
animal tissues when applied as a spray to cattle. The Dow Chemical
Co., Midland, Michigan. Unpublished report
Plapp, F. W. (1960) Studies on the metabolism of P32 Ruelene (R) in a
Hereford steer. Agricultural Research Service, U.S.D.A., Corvallis,
Oregon. Unpublished report
Radeleff, R. D. (1964) Organophosphorus compounds. Ruelene (R),
O-4-tert-butyl-2-chlorophenyl methyl methylphosphoramidate.
Veterinary Toxicology, Lea and Febger, Philadelphia, Pa., pp. 209-210
Ragab, M. T. H. (1967) Direct fluorescent detection of
organothiophosphorus pesticides and some of the sulfur-containing
breakdown products after thin-layer chromatography. J. Ass. Offic.
Anal. Chem., 50: 1088-1098
Rice, J. R. (1964) Residue determination of Ruelene in bovine tissue
and Ruelene TF-88 pour-on application. The Dow Chemical Co., Lake
Jackson, Texas. Unpublished report
Rogoff, W. M. and Kohler, P. H. (1960) Effectiveness of Ruelene (R)
applied as localized "pour-on" and as spray for cattle grub control.
J. Econ. Entomol., 53: 814-817
Sherman, M. and Ross, E. (1961) Acute and subacute toxicity of
insecticides for chicks. Toxicol. appl. Pharmacol., 3: 521-533
Skerman, K. D. (1962) Mintrel(R). A new anthelmintic for sheep.
Aust. vet. J., pp. 324-334
Smith, G. N. (1968) Cholinesterase inhibition of crufomate (Ruelene
(R) insecticide) and metabolites. Dow Chemical Co. Unpublished report
Stewart, J. H. (1968) U.S. Department of Agriculture, Washington, D.C.
Private communication
Timmerman, J. A., jr, Dorough, H. W., Buttram, J. R. and Arthur, B. W.
(1961) In vitro stability and recovery of insecticides from milk.
J. Econ. Entomol. 54: 441-444
Weidenbach, C. P., Radeleff, R. D. and Buck, W. B. (1962)
Toxicological studies of Ruelene(R) 4-tert-butyl-2-chlorophenyl
methyl methylphosphoramidate. J. Amer. vet. med. Ass., 140: 460-463
