
FAO/PL:1968/M/9/1
WHO/FOOD ADD./69.35
1968 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
Issued jointly by FAO and WHO
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Committee on Pesticide Residues, which met in Geneva, 9-16 December,
1968.
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
WORLD HEALTH ORGANIZATION
Geneva, 1969
DIOXATHION
IDENTITY
Chemical name
2,3-p-dioxanedithiol-S,S-bis-(O,O-diethyl phosphorodithioate)
Synonyms
Delnav (R), AC 528
Formula
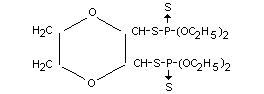 Other information on identity and properties
The technical product contains 70 per cent cis- and trans-
isomers of phosphorodithioate at a 1:2 ratio. The remaining 30 per
cent contains related compounds described by Arthur and Casida (1959)
as:
(a) ca. 10 per cent ethylphosphorothioates and
ethylphosphorodithioates
(b) ca. 1 per cent bis (diethyoxyphosphinothioyl) disulfide
(c) ca. 5 per cent 2-p-dioxenethiol-S-(O,O-diethylphos-phorodithioate)
(diethylphosphorothioic acid and salts
(d) (diethylphosphorodithioic acid and salts
(oxygen analogues of the principal component, and of (c) above
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Rats were treated orally with P32-labelled dioxathion which possessed
a ratio of cis- to trans-isomers approximating that of the
technical product. At levels of 1 and 5 µg/kg over a 10-day period,
metabolites occurred primarily in the urine and to a lesser extent in
the faeces. More than 95 per cent of the radioactivity found in the
urine was in the form of hydrolysis products. The hydrolytic products
excreted in the urine during the first 12 hours after treatment with
15 mg/kg were identified as diethyl phosphoric acid, 0,0-diethyl
phosphorothionic and 0,0-diethyl phosphorodithioic acid. A similar
group of metabolites was encountered after in vitro metabolism
studies were made using rat liver slices. The presence of these
hydrolytic metabolites indicates that enzymatic cleavage of the
phosphorothiolate grouping occurs at the carbon-sulfur as well as at
the phosphorus-sulfur bonds, along with considerable oxidation before
and after hydrolysis. A daily dose of 5 mg/kg of dioxathion given
orally to rats over seven consecutive days resulted in a maximum
accumulation of 0.6 ppm in the fat. After 10 days' withdrawal the
level in the fat fell to 0.1 ppm or lower. At daily doses of 10 mg/kg
a level of 0.6 ppm was reached in the fat after three days, and this
level held constant through continued feeding for 21 days. When rats
were given an oral dose of dioxathion (25 mg/kg body-weight), the only
tissues containing detectable dioxathion were: fat 0.34 ppm cis- and
0.68 ppm trans-isomer; kidney 0.08 ppm cis- and 0.14 trans- and
muscle 0.05 ppm cis- and 0.05 trans-. All other tissues contained
less than 0.01 ppm dioxathion. The cis- and trans-isomers were
stored in tissue to a greater extent than the other technical product
components. In the biological systems studied the disulfide compound
was the least stable of the radioactive components used. The
cis- and trans-isomers were always similar in stability and much
more stable than the dioxene component. The single exception was with
human plasma where the dioxene was the most stable of the technical
dioxathion components studied (Arthur and Casida, 1959)
Dioxathion possesses in vitro anticholinesterase activity; it has
a molar I50 of 7.6 × 10-7 for isolated beef erythrocyte
cholinesterase. Oxidation, chemically, with bromine water, or,
biologically, with fortified rat liver homogenates increased the in
vitro activity approximately 20-fold. The enzyme system responsible
for the activation was found in the supernatent layer of the liver
homogenates following centrifugation (Frawley et al., 1963).
Dioxathion is also an inhibitor of aliesterase and experiments
relating to this property are described under "Special Studies, (c)
Potentiation".
As has been observed in other organo-phosphorus compounds, acute doses
of dioxathion stimulate the pituitary-adrenal system and increase the
production of adaptive liver enzymes. A single dose of 50 mg/kg
administered intraperitoneally, to rats, produced, after 15 hours, a
maximum average cholinesterase depression of 33 per cent of control
which was accompanied by a four-to five-fold increase in hepatic
alkaline phosphatase and tyrosine-alpha-ketoglutarate transaminase.
Daily intraperitoneal injections of 15 mg/kg for 10 days reduced brain
cholinesterase to 20 per cent of control and produced a significant,
but much lower increase in hepatic adaptive enzymes than in the group
receiving the single 50 mg/kg dose (Murphy, 1966).
Acute toxicity
LD50
Animal Route Solvent mg/kg body-weight References
Mouse (M) Oral Maize oil 176 Frawley et
al., 1963
Rat (M) Oral Peanut oil 43 Gaines, 1960
Rat (F) Oral Peanut oil 23 Gaines, 1960
Rat (F) i.p. Ethanol-propylene 30 Frawley et
glycol al., 1963
(20:80)
Dog Oral None 10-40 Frawley et
al., 1963
The cis-isomer has approximately four times the toxicity of the
trans-isomer (subcutaneous rat LD50: cis, 66-86 mg/kg; trans,
230-290 mg/kg). The symptoms following acute exposure to dioxathion
are typical of parasympathetic stimulation and death is usually
preceded by clonic convulsions. The rate of onset of symptoms was
observed to be slower than with some other organo-phosphorus
compounds, presumably due to less rapid conversion to the oxygen
analogue. Maximum inhibition of cholinesterase at all sources of the
enzyme occurred within one hour following an intraperitoneal injection
of 13 mg/kg given to rats, with a recovery time of from two to three
weeks (Frawley et al., 1963).
Short-term studies
Rat
Groups, each of 25 male and 25 female rats, were fed 0, 1, 3, 10, 100
and 500 ppm of dioxathion in their diet. Duration of the test diet was
13 weeks, except that the animals fed 500 ppm were sacrificed after
one week because of marked food refusal and loss of body-weight. In
addition, groups of five male and five female rats were sacrificed at
given intervals prior to termination of the 13-week feeding period,
for determination of erythrocyte, plasma and brain cholinesterase
activity. Of the rats fed 100 ppm, only the females showed minimal
symptoms of parasympathetic stimulation. At all the lower doses no sex
difference due to dioxathion could be observed with respect to any of
the parameters considered. Marked brain, plasma and erythrocyte
cholinesterase depression occurred at the 100 ppm level. At the 10 ppm
level, brain cholinesterase was normal, but plasma and erythrocyte
cholinesterase were significantly depressed. At the 3 ppm and 1 ppm
levels, cholinesterase activity was normal in all tissues examined.
Recovery at all levels was rapid for plasma and slow for brain and
erythrocyte cholinesterase. Gross and histological examinations
revealed no pathological changes in the animals fed 100 ppm or lower
doses.
Dog
Dioxathion was administered to dogs, five days a week for a one- to
two-week period, at dosages from 0.25 mg/kg to 8.0 mg/kg. Three of the
four dogs given 8.0 mg/kg displayed the typical syndrome of
parasympathetic stimulation, which gradually subsided after
withdrawal, and all dogs were free of symptoms 10 days after the last
dose. No such effects were evident in the dogs receiving doses below
8.0 mg/kg. Plasma cholinesterase was inhibited at doses of 0.8 mg/kg
and above and erythrocyte cholinesterase at doses of 2.5 mg/kg and
above. Rapid recovery of plasma cholinesterase occurred, the level
being normal two weeks after withdrawal, however there was only slow
recovery of erythrocyte cholinesterase and it was still not complete
after five weeks. Another group of dogs fed 0.075 mg/kg and lower
doses, five days a week for 90 days showed no inhibition of either
plasma or erythrocyte cholinesterase when periodic tests were made
(Frawley et al., 1963).
Special studies
(a) Reproduction
Rat. A three-generation reproduction study was conducted on groups
of weanling rats fed 3 ppm and 10 ppm for 79 days before mating. No
abnormal pathologic changes were found in any of the parental
generations after 39 weeks. No adverse effect on reproductive
performance, fertility, lactation or litter size was found at either
level. The progeny were viable, normal in size and anatomical
structure. Findings among all test animals, three parental generations
and six litters of progeny, were comparable to control animals for all
parameters (Kennedy et al., 1968).
(b) Neurotoxicity
Using TOCP (triorthocresyl phosphate) as a positive control, oral
doses from 10 to 1000 mg/kg and subcutaneous doses from 25 to 200
mg/kg of dioxathion were administered to a total of 75 mature hens.
The hens treated with TOCP at 500 mg/kg developed typical neurological
symptoms associated with myelin degeneration whereas the hens treated
with dioxathion either died from the acutely toxic dose or recovered
without development of the neurological symptoms (Frawley et al.,
1963).
(c) Potentiation
Four organo-phosphorus insecticides, including dioxathion, and one
carbamate insecticide, carbaryl, were administered both individually
and in combination to rats. Potentiation, based upon percentage
mortality, occurred when malathion and dioxathion were administered
simultaneously, but it was greatly enhanced when dioxathion was
administered four hours before malathion. Dioxathion and carbaryl also
potentiated each other, but only when an interval existed before the
administration of the carbaryl (Hagan et al., 1961).
To evaluate further the potentiation of dioxathion and malathion a
subacute feeding study was made whereby dogs were administered oral
doses of 0.1, 0.2 and 0.4 mg/kg of dioxathion or 0.2, 0.4 and 0.8
mg/kg of malathion daily, for a pre-treatment period of six weeks. The
dogs pre-treated with dioxathion were then given malathion and those
pre-treated with malathion given dioxathion for an additional six
weeks. When administered according to this schedule, dioxathion and
malathion did not show any enhanced inhibition of plasma or
erythrocyte cholinesterase (Zaratzian of al., 1961).
In another experiment, dioxathion was fed to rats along with 14 other
organo-phosphorus compounds and also with the carbamate, carbaryl. No
significant potentiation, on the basis of the LD50 was observed when
the compounds were administered simultaneously. However, when
dioxathion was administered four hours prior to malathion, significant
potentiation occurred (Frawley et al., 1963).
A direct measurement of aliesterase activity was made, using diethyl
succinate and tributyrin as substrates. Rats were fed dietary levels
of 0, 0.2, 1, 5, and 25 ppm of dioxathion for given periods up to 13
weeks. Four groups were sacrificed at separate intervals during the
feeding period, and a fifth group was sacrificed four weeks after
return to a normal diet. The rates of hydrolysis of the substrates
were used as a measure of aliesterase activity in the liver and serum.
Cholinesterase activity was also measured in these tissues. A dietary
level of 1 ppm caused slight inhibition of aliesterase in the liver,
whereas at 5 ppm no cholinesterase inhibition was evident. Similar
difference in activity was found for serum. Maximum inhibition of
aliesterase occurred early in the feeding period and complete recovery
of activity had resulted by four weeks after return to a normal diet.
These results indicate that aliesterase is much more sensitive to
inhibition by dioxathion than is cholinesterase (DuBois et al., 1968).
Observation in man
Five female and five male adult human volunteers received 0.075
mg/kg/day of dioxathion, orally for 28 days. Plasma and erythrocyte
cholinesterase measurements were made at frequent intervals and no
significant change from pre-treatment levels was found. After 28 days,
the treatment was varied and during the following 28 days two subjects
continued to receive 0.075 mg/kg/day, two received 0.150 mg/kg/day and
the remaining six received 0.075 mg/kg/day of dioxathion along with
0.150 mg/kg/day of malathion. Erythrocyte cholinesterase levels were
not affected by any of these doses. Plasma cholinesterase was not
affected by the continuing dose of 0.075 mg/kg/day but 10-20 per cent
inhibition was observed in the two subjects which received the higher
dose of 0.150 mg/kg/day. This depression was thought to be significant
because during a 17-day post-treatment period the activity returned to
pre-treatment level. Subjects receiving the combined
dioxathion-malathion treatment did not appear to display any
cholinesterase depression related to the experimental treatment. All
clinical findings were similar to those observed in a control group of
subjects which received placebos. Blood counts, coagulation times and
prothrombin times were normal in all subjects (Frawley et al., 1963).
Comments
The toxicological studies reported above have been conducted on
technical dioxathion which contains 70 per cent dioxathion.
Short-term studies in rats and dogs including reproduction studies in
rats demonstrated that all test doses failed to produce any
morphologic changes. Conventional two-year chronic toxicity studies
have not been conducted on dioxathion. The toxic action of this
compound is restricted to inhibition of cholinesterase enzymes. The
reported studies prove similar susceptibility in man and dogs. The
significance of aliesterase inhibition with regard to metabolism of
some chemicals is apparent, and the higher sensitivity to aliesterase
inhibition was taken into account when estimating the acceptable daily
intake.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Rat: 3 ppm in the diet, equivalent to 0.15 mg/kg per day
Dog: 0.075 mg/kg,per day
Man: 0.075 mg/kg per day
Estimate of acceptable daily intake for man
0-0.0015 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Pre-harvest treatments
The major uses of dioxathion as an agricultural insecticide are given
in the following table. Dioxathion is formulated as an emulsifiable
concentrate.
Rate of Pre-harvest
Crop Pest application interval
(kg/ha) (days)
Citrus: grapefruit, mites 0.03-0.06 kg/100 1 0
oranges, citrus thrips
limes, lemons,
tangerines,
tangelos
apples,* pears, mites 0.06 kg/100 1 7
quinces coddling moths (max.7.56 kg/ha)
apple maggots
grapes mites, grape 0.95 kg/ha (spray) 14
leafhoppers 1.5 kg/ha (dust)
* Apple pomace from treated fruit should not be fed to dairy or
meat animals.
Dioxathion is applied to beef cattle, sheep, goats and swine in the
form of sprays and dips of 0.15 per cent concentration and by
backrubber. Use is not permitted on dairy animals. It is effective in
controlling ticks, lice, horn flies, screw worms and sheep ked. Repeat
applications can be made after 2-week intervals.
Post-harvest treatments
No use is known for application on stored products.
Other uses
In addition to the major uses listed above, dioxathion is used for
controlling mites on walnuts, on stone fruit prior to fruit formation,
ornamentals and beans grown for shelled dry beans.
Residues resulting from supervised trials
Data in the form of unpublished reports (Hercules, 1958-1961),
retained at FAO headquarters in Rome, indicate residues likely to
occur from dioxathion application to plants and animals. These data
show the rate of decline of residues from various application rates
including that representative of the use pattern (above). Unless
otherwise stated the residues are expressed in terms of technical
dioxathion (which contains 70 per cent cis- and trans-isomers) in
contrast to United States and Canada tolerances which are for the
total of cis- and trans-isomers.
Citrus
Residues in citrus, confined to the peel, are persistent with an
indicated half-life of 75-100 days. No residues exceeding 0.03 ppm
were detected in the pulp. Gunther et al. (1958) studied the deposit
and persistence of dioxathion on field sprayed mature navel oranges
and lemons in California. Maximum residues occurred from 2 to 21 days
after application. Residues measured on the day of spraying were
slightly lower, probably due to loss in handling. Data are summarized
in the following table:
Rate of Pre-harvest Residue range
Crop application interval (at harvest)
(kg/100 l) (days) (ppm)
whole
peel fruit
Navel 0.02 0-21 1.0-3.5 0.2-0.8
oranges 0.04 0-21 3.8-4.8 0.8-1.1
0.09 0-21 6.3-9.7 1.4-2.1
temple 0.06 0-28 5.4-11.7 1.1-2.3
oranges
lemons 0.02 0-28 4.6-9.0 1.4-2.7
0.04 0-28 8.4-14.7 2.5-4.4
0.09 0-28 13.2-23.4 4.0-7.0
* Calculated on basis of average peel weights 22 per cent and
30 per cent for oranges and lemons, respectively.
Grapes
Two spray studies were made in the United States of America (one in
California and one in Delaware) to determine dioxathion residues in
grapes. In the California study Thompson seedless grapes (varying from
fruit ready for harvest to fruit two months prior to harvest) were
treated with one application of dioxathion at 0.95 kg/ha. None of the
resulting residues, even those on samples taken three hours after
application, exceeded 0.4 ppm. Residues from double the application
rate did not exceed 1 ppm. In the Delaware study grapes were treated
with two applications at 0.95 kg/ha. The first application was made
when small fruit was present and the second application was made eight
weeks later, a few weeks prior to harvest. Residues from this study
showed a rapid initial decrease from an average of 13.5 ppm three
hours after the initial spraying to an estimated 2.0 ppm 10 days
later. Thereafter the residues exhibited a high degree of persistence
decreasing to an average of 1 ppm 56 days after the spray application.
A second application on grapes which contained a 1 ppm residue from
the first application resulted in an initial average residue of 5.3
ppm which again rapidly declined in the first nine days to an
estimated 2 ppm. Again this residue showed persistence during the
remaining days until harvest, reaching a 1.8 ppm average at 21 days.
At the recommended pre-harvest period of 14 days, the Delaware study
showed a maximum residue of 2.4 ppm and an average residue of 2.0 ppm
(technical dioxathion).
Pome fruits
Tests were conducted in Indiana, New Mexico and North Carolina on
apples using five to eight cover sprays at various dosages. In apples,
as with citrus, residues remained in the peel. Samples taken one week
after the last application, where the maximum recommended use
programme was followed (seven cover sprays using 0.06 kg/100 1) had
the following residues.
Location Range Average
(ppm) (ppm)
Indiana 4.3-4.9 4.6
New Mexico 5.7-8.2 6.5
North Carolina 5.6-6.6 6.0
The samples taken above were picked one to five weeks prior to normal
harvest time. Dioxathion residues are quite persistent, growth
dilution being the primary cause of residue decline. The average
half-life indicated in the three studies above is about 11 weeks.
Livestock
Studies conducted by the USDA and summarized below furnish information
on the site of residue deposition, magnitude of residue, build-up and
decline pattern, and metabolic fate of dioxathion in livestock.
Chamberlain et al. (1960) treated steers with 32P-labelled technical
dioxathion using a 16.2 mg/kg dermal application and 4.56 mg/kg oral
administration. Plapp et al. (1960) gave steers an 8.8 mg/kg dermal
application of 32P-labelled technical dioxathion. Studies on the
deposition of residue in tissues were conducted by Jackson et al.
(1962) using dermal applications of 0.15 per cent and 0.25 per cent
spray.
In all studies the largest residue was found on the hair of the animal
and to a lesser but still appreciable degree on the hide. The only
significant accumulation of absorbed residue in animal tissue occurred
in the fat with only traces (0.1 ppm or less) present in the liver,
kidney and muscle. The residue in the fat reached a peak two to seven
days after spraying and then declined to 0.1 ppm or less two to three
weeks after spraying with the recommended 0.15 per cent spray. No
accumulative residue build-up was noted in the fat of cattle after six
applications with the 0.15 per cent spray following the recommended
two-week interval between applications.
The following table indicates the level of residue found in the fat
from the three studies.
Dermal application Days after Residues in fat
rate application ppm
0.15 per cent spray 2 0.37-0.95 (0.73 av.)
0.25 per cent spray 2 0.91-1.15 (1.05 av.)
8.8 mg/kg 7 0.2
16.2 mg/kg 7 1.5
The radio-labelled studies showed that the bulk of absorbed dioxathion
was rapidly metabolized and that 10-20 per cent of that dermally
applied was excreted in the urine in one week principally as diethyl
phosphoric, diethyl thiophosphoric and diethyl dithiophosphoric acids.
A higher percentage of the dose was eliminated in the urine and faeces
of orally treated animals. The maximum radioactivity in the blood
occurred at three hours in the dermally treated steer with a maximum
blood cholinesterase inhibition of 32 per cent, whereas the orally
treated steer exhibited a maximum cholinesterase depression of 83 per
cent and a maximum absorption of radioactivity in the blood after 12
hours.
The residue pattern in sheep, hogs and shorn goats is the same as for
cattle with the following decreasing order of residues in the fat:
cattle > shorn goats > sheep > hogs. Residues in the fat of hogs
did not exceed 0.1 ppm even with exaggerated treatment. The maximum
decrease in blood cholinesterase activity in sheep and goats (60-80
per cent of normal) occurred two days after one dermal application
but returned to normal in about one week. The depression in shorn
goats was the most severe (20-40 per cent of normal, two days after
application) and returned to about normal two weeks after the
application.
Three dairy cows were fed dioxathion daily for 28 days at a rate of
0.33 to 0.4 mg/kg body-weight. This is the maximum amount which would
occur in a diet of 50 per cent citrus pulp which had a residue of 23
ppm. Residues of 0.06-0.12 ppm (0.08 ppm average) were found in the
fat. It can therefore be estimated that the total residue in the fat
of cattle from a combination of dermal treatment and ingestion of
treated citrus pulp would not be likely to exceed 1.0 ppm based on the
cis- and trans-isomers.
No residues were found in the meat of cattle in any of the studies.
Determinations for dioxathion in the milk of the dairy cows fed
0.33-0.4 mg/kg body-weight daily for 28 days were negative with an
analytical sensitivity of 0.01-0.02 ppm.
In plants
Casida and Ahmed (1959) studied the behaviour of 32P-labelled
technical dioxathion components on lima bean, cabbage, cotton and
tomato plants. They showed that the cis- and trans-isomers were
the least susceptible components to volatization from plant surfaces.
Hydrolysis of dioxathion components on plant surfaces did not occur
readily. Hydrolysis that did occur was primarily with absorbed
materials and in this case the cis- and trans-isomers hydrolyzed
more slowly than the other components. The rapid hydrolysis of
absorbed dioxathion components was further demonstrated with bean
seedlings which readily absorbed radioactive components through the
roots from water containing 40 ppm of each of the dioxathion
components. After 1.5 days absorption time through the roots, the
cis- and trans-isomers in the bean foliage were about 70 per cent
hydrolyzed.
The formation of more polar derivatives and more potent in vitro
cholinesterase inhibitors does occur on exposure to sunlight or after
application to plants. However, Casida and Ahmed (1959) found that the
amounts of unhydrolyzed but more polar materials in aged plant
residues were but a small fraction (10 per cent on cabbages, 1 per
cent on beans) of the unchanged cis- and trans-isomers. The more
polar non-hydrolyzed derivatives from the dioxene component formed
more readily and disappeared more rapidly than the cis- and
trans-isomer derivatives.
Casida and Ahmed (1959) also found that the cis- to trans-isomer
ratio (about 1:2) held nearly constant after application of technical
dioxathion to plants. No conversion of one geometrical isomer to the
other occurred nor did the cis- and trans-isomers on plants from
any dioxene derivative.
The confinement of dioxathion residue in citrus to the peel is
associated with its solubility in the waxes and oils in that portion
of the fruit. Any conversion of the cis- and trans-isomers to a
metabolite must occur rather slowly since dioxathion disappears from
the peel at a slow rate. No appreciable cholinesterase inhibiting
material was detected in lemon pulp (edible portion) 71 days after an
excessive 0.18 kg/100 1 spray treatment in a field trial.
In animals
The general metabolic pattern described for rats in the section
entitled "Evaluation for Acceptable Daily Intake" appears to be
similar for livestock. The only significant residue stored in cattle
occurred in the fat with 75 per cent of the residue as the cis- and
trans-isomer and 21 per cent as the dioxene fraction. The metabolic
products in rat and cattle urine and faeces were similar following
oral administration.
Evidence of residues in food in commerce or at consumption
Total diet and food survey data for a limited number of
organo-phosphorus pesticide residues collected by the United States
Food and Drug Administration were obtained using a GLC method which
includes a Florisil column cleanup as described by Mills et al.
(1963). Pardue and Watts (1968) found that a standard of dioxathion
added to a Florisil column was not recovered in either the 6 per
cent, 15 per cent or 50 per cent ethyl ether in petroleum ether
eluate. Consequently, no applicable surveillance data are currently
available for dioxathion. (See discussion below under "Methods of
residue analysis".)
In storage and processing
Apples and pears are usually pared and cored before dehydration. Since
dioxathion residues in these fruits are confined to the peel, no
residue would be expected in the dried fruit.
The solubility of dioxathion is such that no appreciable residue would
be expected in fruit juice; however, small residues may result from
physical carry-over.
In the preparation of cattle feed from citrus, a 62 per cent residue
loss was demonstrated in the drying operation. This plant scale test
involved adding 4 ppm dioxathion to the press cake just prior to
drying with combustion gas at 200-300°F. An average residue of 22.6
ppm was found in the finished product. A small scale laboratory test
with lemon peel demonstrated an over-all residue loss of 42 per cent
as a result of liming, pressing and mild drying at 122°F.
In a controlled experiment with dioxathion added to wet apple pomace,
a 60 per cent residue loss occurred during drying in a commercial
processing unit. No milk-residue studies have been carried out with a
feeding level as high as that expected from the use of dried pomace
from treated apples; therefore such feed should not be used for dairy
or meat animals.
Methods of residue analysis
The analytical method described here is specific for the major
components, the cis- and trans-isomers of
2,3-p-dioxanedithiol-S,S-bis-(O,O-diethyl phosphorodithioate). These
are the most persistent and abundant constituents and their
determination is the most effective measurement of the toxic hazard of
the residue. Unhydrolyzed metabolic conversion products or other
components of the technical product which are excluded by the
analytical procedure used have been absent or found only in small
amounts in the vegetable and animal systems investigated and at a much
lower level than the unchanged cis- and trans-isomers of
dioxathion and completely hydrolyzed components.
The method described by Dunn (1958) extracts the residue with hexane
or isopropyl alcohol. Cleanup is achieved by use of an acid alumina
column. Waxes are readily eluted with hexane and dioxathion is eluted
with benzene. Final cleanup is done by partition chromatography on
Celite 545 with a solvent pair such as acetonitrile and hexane.
Dioxathion is determined by cleaving the molecule with mercuric
chloride to yield 2,3-dichloro-p-dioxane as one of the reaction
products. Hydrolysis of this product yields ethylene glycol and
glyoxal, the latter being determined colorimetrically as glyoxal
2,4-dinitrophenylosazone. The intensity of the colour formed in basic
dimethyl formamide solution is measured at absorbance peak 614 mµ and
the quantity estimated by comparison with a standard absorbance curve
prepared from technical or pure dioxathion.
The cleavage reaction is peculiar to thio acetals and thus is specific
for the pesticide dioxathion as no other pesticide residues known are
glyoxal precursors and the cleanup procedures exclude possible
precursors in the sample material.
The method is sensitive to 5 µg. The following table indicates the
result of method validation studies:
Fortification Recovery Blank
Sample level, ppm (per cent.) values
Citrus peel 1-10 96-103 0.1
Citrus pulp 0-03 100 0.008
Apples 1-30 95-101 0.1
Grapes 0.1-20 100 0.07 or less
Animal fat 1.0 84-90 0.2 or less
A multi-residue method for a large number of organo-phosphorus
pesticides and alteration products is being developed by the United
States Food and Drug Administration. It is anticipated that this
procedure using a charcoal column cleanup prior to determination by
GLC equipped with a KCl thermionic detector will soon be available for
the practical accumulation of total diet and surveillance data on a
large number of organo-phosphorus compounds. Watts et al. (1968a and
b) described a charcoal column cleanup procedure and determined the
recovery of about 60 organo-phosphorus pesticides and alteration
products through this cleanup. In addition they investigated the
applicability of three widely used GLC columns for determination of
this large group of pesticides. The columns were packed with 80-100
mesh, Gas Chrom Q coated with (a) 10 per cent DC 200, (b) 2 per cent
diethylene glycol succinate (2 per cent DEGS) and (c) a 1:1 mixed
column with 10 per cent DC 200 and 15 per cent QF1.
They found that 0.1 ppm dioxathion added to an ethyl acetate extract
of kale was 95 per cent recovered in the charcoal column cleanup. All
three GLC columns were adequate for thermionic detection. The 2 per
cent DEGS column gave the best sensitivity with 1.5 ng dioxathion
needed for a 30-50 per cent scale deflection.
National tolerances
Expressed as the cis- and trans-isomers of
2,3-p-dioxane-dithiol-S,S-bis-(O,O-diethyl phosphorodithioate).
Country Crop Tolerance
(ppm)
Canada apples, pears, quinces 4.9
citrus: grapefruit, oranges
lemons, limes, tangerines
tangelos 2.5
grapes 2.0
animals: beef cattle, sheep,
goats, swine 1.0
Netherlands leaf and sprout vegetables,
fruit vegetables, pulses,
fruit including grapes 0.4
United States apples, pears, quinces 4.9
of America
citrus: grapefruits, oranges
lemons, limes, tangerines
tangelos 2.8
grapes 2.1
(continued)
Country Crop Tolerance
(ppm)
animals: beef cattle, sheep,
coats, swine 1.0 (in fat)
West leaf and sprout vegetables,
Germany fruit vegetables, pulses,
fruit including grapes 0.4
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Appraisal
Dioxathion is a narrow spectrum insecticide and acaricide and its use
in agriculture is limited to a small number of food commodities. If
its use were to extend, a reappraisal of the recommendations would be
necessary. Therefore, the recommendations for limits should be on a
"temporary" basis.
Although figures are available for residues at stated times after
application, no data are available for losses during subsequent
storage and processing, except for the dehydration of citrus peel in
which case there is about a 60 per cent loss during drying.
No data are available on the nature of the residues derived from the
impurities in the technical product. No results are available from
total diet studies or from surveillance of food in commerce.
There are colorimetric analytical methods which are suitable for
regulatory purposes and for development as referee methods.
Recommendations
Temporary tolerances
The following temporary tolerances, to be in effect until 1972, are to
apply to raw agricultural products moving in commerce unless otherwise
indicated. In the case of fruit and vegetables the tolerances should
be applied as soon as practicable after harvest and in any event prior
to actual retail to the public. In the case of commodities entering
international trade, the tolerances should be applied by the importing
country at the point of entry or as soon as practicable thereafter.
Citrus 3.0
Pome fruits 5.0
Grapes 2.0
Meat 1.0 (not to include poultry)
These tolerances apply to the total of cis- and trans-isomers of
the principal active ingredient.
Further work or information
Required before 30 June 1972
1. Determination and identification of impurities.
2. Data on the disappearance of residues during storage and
processing, including residues from impurities in the technical
product.
3. Data on residue levels in raw agricultural products moving in
commerce.
4. Data on residue levels in total diet studies.
Desirable
1. Estimation of the effect on aliesterase activity in dogs.
2. Long-term studies in rats.
REFERENCES
Arthur, B. W. and Casida, J. E., (1959) Biological activity and
metabolism of Hercules AC-528 components in rats and cockroaches.
J. Econ. Entomol., 52: 20-27
Casida, J. E. and Ahmed, M. K. (1959) Mechanism of residue loss of
Hercules 528 components on plant foliage. J. Econ. Entomol., 52:
111-115
Chamberlain, W. F., Gatterdam, P. E. and Hopkins, D. E. (1960)
Metabolism of P32-Delnav in cattle. J. Econ. Entomol., 53: 672-675
DuBois, K. P., Kinoshita, F. K. and Frawley, J. P. (1968) Quantitative
measurement of aliesterase inhibition by EPN
(0-ethyl-0-p-nitrophenyl phenylphosphonothioate) and Delnav.
Toxicol. appl. Pharmacol. (In press)
Dunn, C. L. (1958) Determination of 2,3-p-dioxanedithiol S,S-bis (0,0
-diethyl phosphorodithioate). J. Agr. Food Chem., 6: 203-209
Frawley, J. P., Weir, R., Tusing, T., DuBois, K. P. and Calandra, J.
C. (1963) Toxicologic investigations on Delnav(R) Toxicol. appl.
Pharmacol., 5: 605-624
Gaines, T. B. (1960) The acute toxicity of pesticides to rats.
Toxicol. appl. Pharmacol., 2: 88-99
Gunther, F. A., Jeppson, L. R., Barkley, J. H., Elliott, L. M. and
Blinn, R. C. (1958) Persistence of residues of 2,3-p-dioxanedithiol
S,S-bis(0,0-diethyl phosphorodithioate) as an acaricide on and in
mature lemons and oranges. J. Agr. and Food Chem., 6: 210-211
Hagan, E. C., Jenner, R. M. and Fitzhugh, O. G. (1961) Acute oral
toxicity and potentiation studies with anticholinesterase compounds.
Fed. Proc., 20: 432
Hercules. (1958-1961) Hercules Powder Co. Inc. Unpublished reports on
Delnav residues
Jackson, J. B., Radeleff, R. D., Roberts, R. H. et al. (1962) Acute
toxicity of Delnav and its residues in tissues of livestock. J. Econ.
Entomol., 55: 669-702
Kennedy, G., Frawley, J. P. and Calandra, J. C. (1968) Multigeneration
reproduction study in rats fed Delnav, Herban and Toxaphene. Toxicol.
appl. Pharmacol. (In press)
Mills, P. A., Onley, J. H. and Gaither, R. A. (1963) Rapid method for
chlorinated pesticide residues in nonfatty foods. J. Assoc. Offic.
Agric. Chem., 46: 186-191
Murphy, S. D. (1966) Response of adaptive liver enzymes to acute
poisoning by organophosphate insecticides. Toxicol. appl. Pharmacol.,
8: 266-276
Pardue, J. R. and Watts, R. R. (1968) U.S. Food and Drug
Administration. Private communication
Plapp, F. W., Bigley, W. S. and Darrow, D. I. (1960) Studies on the
metabolism and residues of P32-labelled Delnav in Hereford steer.
J. Econ. Entomol., 53: 60-64
Watts, R. R., Storherr, R. W., Pardue, J. R. et al. (1968a) A widely
applicable charcoal column cleanup method for organophosphorus
pesticide residues in crop extracts. Presented at the 1968 annual
meeting of the Association of Official Analytical Chemists,
Washington, D.C., Oct. 14, 1968
Watts, R. R. and Storherr, R. W. (1968b) Gas chromatographic
determination of organophosphorus pesticide residues. General aspects
of potassium thermionic detection, retention times and response data
for three columns. Presented at the 1968 annual meeting of the
Association of Official Analytical Chemists, Washington, D.C., Oct.
14, 1968
Zaratzian, V. L., Arnault, L. T., Michel, T. C. and Fitzhugh, O. G.
(1961) Effects of organic phosphates Delnav and Malathion in the dog.
Fed. Proc., 20: 432
Other information on identity and properties
The technical product contains 70 per cent cis- and trans-
isomers of phosphorodithioate at a 1:2 ratio. The remaining 30 per
cent contains related compounds described by Arthur and Casida (1959)
as:
(a) ca. 10 per cent ethylphosphorothioates and
ethylphosphorodithioates
(b) ca. 1 per cent bis (diethyoxyphosphinothioyl) disulfide
(c) ca. 5 per cent 2-p-dioxenethiol-S-(O,O-diethylphos-phorodithioate)
(diethylphosphorothioic acid and salts
(d) (diethylphosphorodithioic acid and salts
(oxygen analogues of the principal component, and of (c) above
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Rats were treated orally with P32-labelled dioxathion which possessed
a ratio of cis- to trans-isomers approximating that of the
technical product. At levels of 1 and 5 µg/kg over a 10-day period,
metabolites occurred primarily in the urine and to a lesser extent in
the faeces. More than 95 per cent of the radioactivity found in the
urine was in the form of hydrolysis products. The hydrolytic products
excreted in the urine during the first 12 hours after treatment with
15 mg/kg were identified as diethyl phosphoric acid, 0,0-diethyl
phosphorothionic and 0,0-diethyl phosphorodithioic acid. A similar
group of metabolites was encountered after in vitro metabolism
studies were made using rat liver slices. The presence of these
hydrolytic metabolites indicates that enzymatic cleavage of the
phosphorothiolate grouping occurs at the carbon-sulfur as well as at
the phosphorus-sulfur bonds, along with considerable oxidation before
and after hydrolysis. A daily dose of 5 mg/kg of dioxathion given
orally to rats over seven consecutive days resulted in a maximum
accumulation of 0.6 ppm in the fat. After 10 days' withdrawal the
level in the fat fell to 0.1 ppm or lower. At daily doses of 10 mg/kg
a level of 0.6 ppm was reached in the fat after three days, and this
level held constant through continued feeding for 21 days. When rats
were given an oral dose of dioxathion (25 mg/kg body-weight), the only
tissues containing detectable dioxathion were: fat 0.34 ppm cis- and
0.68 ppm trans-isomer; kidney 0.08 ppm cis- and 0.14 trans- and
muscle 0.05 ppm cis- and 0.05 trans-. All other tissues contained
less than 0.01 ppm dioxathion. The cis- and trans-isomers were
stored in tissue to a greater extent than the other technical product
components. In the biological systems studied the disulfide compound
was the least stable of the radioactive components used. The
cis- and trans-isomers were always similar in stability and much
more stable than the dioxene component. The single exception was with
human plasma where the dioxene was the most stable of the technical
dioxathion components studied (Arthur and Casida, 1959)
Dioxathion possesses in vitro anticholinesterase activity; it has
a molar I50 of 7.6 × 10-7 for isolated beef erythrocyte
cholinesterase. Oxidation, chemically, with bromine water, or,
biologically, with fortified rat liver homogenates increased the in
vitro activity approximately 20-fold. The enzyme system responsible
for the activation was found in the supernatent layer of the liver
homogenates following centrifugation (Frawley et al., 1963).
Dioxathion is also an inhibitor of aliesterase and experiments
relating to this property are described under "Special Studies, (c)
Potentiation".
As has been observed in other organo-phosphorus compounds, acute doses
of dioxathion stimulate the pituitary-adrenal system and increase the
production of adaptive liver enzymes. A single dose of 50 mg/kg
administered intraperitoneally, to rats, produced, after 15 hours, a
maximum average cholinesterase depression of 33 per cent of control
which was accompanied by a four-to five-fold increase in hepatic
alkaline phosphatase and tyrosine-alpha-ketoglutarate transaminase.
Daily intraperitoneal injections of 15 mg/kg for 10 days reduced brain
cholinesterase to 20 per cent of control and produced a significant,
but much lower increase in hepatic adaptive enzymes than in the group
receiving the single 50 mg/kg dose (Murphy, 1966).
Acute toxicity
LD50
Animal Route Solvent mg/kg body-weight References
Mouse (M) Oral Maize oil 176 Frawley et
al., 1963
Rat (M) Oral Peanut oil 43 Gaines, 1960
Rat (F) Oral Peanut oil 23 Gaines, 1960
Rat (F) i.p. Ethanol-propylene 30 Frawley et
glycol al., 1963
(20:80)
Dog Oral None 10-40 Frawley et
al., 1963
The cis-isomer has approximately four times the toxicity of the
trans-isomer (subcutaneous rat LD50: cis, 66-86 mg/kg; trans,
230-290 mg/kg). The symptoms following acute exposure to dioxathion
are typical of parasympathetic stimulation and death is usually
preceded by clonic convulsions. The rate of onset of symptoms was
observed to be slower than with some other organo-phosphorus
compounds, presumably due to less rapid conversion to the oxygen
analogue. Maximum inhibition of cholinesterase at all sources of the
enzyme occurred within one hour following an intraperitoneal injection
of 13 mg/kg given to rats, with a recovery time of from two to three
weeks (Frawley et al., 1963).
Short-term studies
Rat
Groups, each of 25 male and 25 female rats, were fed 0, 1, 3, 10, 100
and 500 ppm of dioxathion in their diet. Duration of the test diet was
13 weeks, except that the animals fed 500 ppm were sacrificed after
one week because of marked food refusal and loss of body-weight. In
addition, groups of five male and five female rats were sacrificed at
given intervals prior to termination of the 13-week feeding period,
for determination of erythrocyte, plasma and brain cholinesterase
activity. Of the rats fed 100 ppm, only the females showed minimal
symptoms of parasympathetic stimulation. At all the lower doses no sex
difference due to dioxathion could be observed with respect to any of
the parameters considered. Marked brain, plasma and erythrocyte
cholinesterase depression occurred at the 100 ppm level. At the 10 ppm
level, brain cholinesterase was normal, but plasma and erythrocyte
cholinesterase were significantly depressed. At the 3 ppm and 1 ppm
levels, cholinesterase activity was normal in all tissues examined.
Recovery at all levels was rapid for plasma and slow for brain and
erythrocyte cholinesterase. Gross and histological examinations
revealed no pathological changes in the animals fed 100 ppm or lower
doses.
Dog
Dioxathion was administered to dogs, five days a week for a one- to
two-week period, at dosages from 0.25 mg/kg to 8.0 mg/kg. Three of the
four dogs given 8.0 mg/kg displayed the typical syndrome of
parasympathetic stimulation, which gradually subsided after
withdrawal, and all dogs were free of symptoms 10 days after the last
dose. No such effects were evident in the dogs receiving doses below
8.0 mg/kg. Plasma cholinesterase was inhibited at doses of 0.8 mg/kg
and above and erythrocyte cholinesterase at doses of 2.5 mg/kg and
above. Rapid recovery of plasma cholinesterase occurred, the level
being normal two weeks after withdrawal, however there was only slow
recovery of erythrocyte cholinesterase and it was still not complete
after five weeks. Another group of dogs fed 0.075 mg/kg and lower
doses, five days a week for 90 days showed no inhibition of either
plasma or erythrocyte cholinesterase when periodic tests were made
(Frawley et al., 1963).
Special studies
(a) Reproduction
Rat. A three-generation reproduction study was conducted on groups
of weanling rats fed 3 ppm and 10 ppm for 79 days before mating. No
abnormal pathologic changes were found in any of the parental
generations after 39 weeks. No adverse effect on reproductive
performance, fertility, lactation or litter size was found at either
level. The progeny were viable, normal in size and anatomical
structure. Findings among all test animals, three parental generations
and six litters of progeny, were comparable to control animals for all
parameters (Kennedy et al., 1968).
(b) Neurotoxicity
Using TOCP (triorthocresyl phosphate) as a positive control, oral
doses from 10 to 1000 mg/kg and subcutaneous doses from 25 to 200
mg/kg of dioxathion were administered to a total of 75 mature hens.
The hens treated with TOCP at 500 mg/kg developed typical neurological
symptoms associated with myelin degeneration whereas the hens treated
with dioxathion either died from the acutely toxic dose or recovered
without development of the neurological symptoms (Frawley et al.,
1963).
(c) Potentiation
Four organo-phosphorus insecticides, including dioxathion, and one
carbamate insecticide, carbaryl, were administered both individually
and in combination to rats. Potentiation, based upon percentage
mortality, occurred when malathion and dioxathion were administered
simultaneously, but it was greatly enhanced when dioxathion was
administered four hours before malathion. Dioxathion and carbaryl also
potentiated each other, but only when an interval existed before the
administration of the carbaryl (Hagan et al., 1961).
To evaluate further the potentiation of dioxathion and malathion a
subacute feeding study was made whereby dogs were administered oral
doses of 0.1, 0.2 and 0.4 mg/kg of dioxathion or 0.2, 0.4 and 0.8
mg/kg of malathion daily, for a pre-treatment period of six weeks. The
dogs pre-treated with dioxathion were then given malathion and those
pre-treated with malathion given dioxathion for an additional six
weeks. When administered according to this schedule, dioxathion and
malathion did not show any enhanced inhibition of plasma or
erythrocyte cholinesterase (Zaratzian of al., 1961).
In another experiment, dioxathion was fed to rats along with 14 other
organo-phosphorus compounds and also with the carbamate, carbaryl. No
significant potentiation, on the basis of the LD50 was observed when
the compounds were administered simultaneously. However, when
dioxathion was administered four hours prior to malathion, significant
potentiation occurred (Frawley et al., 1963).
A direct measurement of aliesterase activity was made, using diethyl
succinate and tributyrin as substrates. Rats were fed dietary levels
of 0, 0.2, 1, 5, and 25 ppm of dioxathion for given periods up to 13
weeks. Four groups were sacrificed at separate intervals during the
feeding period, and a fifth group was sacrificed four weeks after
return to a normal diet. The rates of hydrolysis of the substrates
were used as a measure of aliesterase activity in the liver and serum.
Cholinesterase activity was also measured in these tissues. A dietary
level of 1 ppm caused slight inhibition of aliesterase in the liver,
whereas at 5 ppm no cholinesterase inhibition was evident. Similar
difference in activity was found for serum. Maximum inhibition of
aliesterase occurred early in the feeding period and complete recovery
of activity had resulted by four weeks after return to a normal diet.
These results indicate that aliesterase is much more sensitive to
inhibition by dioxathion than is cholinesterase (DuBois et al., 1968).
Observation in man
Five female and five male adult human volunteers received 0.075
mg/kg/day of dioxathion, orally for 28 days. Plasma and erythrocyte
cholinesterase measurements were made at frequent intervals and no
significant change from pre-treatment levels was found. After 28 days,
the treatment was varied and during the following 28 days two subjects
continued to receive 0.075 mg/kg/day, two received 0.150 mg/kg/day and
the remaining six received 0.075 mg/kg/day of dioxathion along with
0.150 mg/kg/day of malathion. Erythrocyte cholinesterase levels were
not affected by any of these doses. Plasma cholinesterase was not
affected by the continuing dose of 0.075 mg/kg/day but 10-20 per cent
inhibition was observed in the two subjects which received the higher
dose of 0.150 mg/kg/day. This depression was thought to be significant
because during a 17-day post-treatment period the activity returned to
pre-treatment level. Subjects receiving the combined
dioxathion-malathion treatment did not appear to display any
cholinesterase depression related to the experimental treatment. All
clinical findings were similar to those observed in a control group of
subjects which received placebos. Blood counts, coagulation times and
prothrombin times were normal in all subjects (Frawley et al., 1963).
Comments
The toxicological studies reported above have been conducted on
technical dioxathion which contains 70 per cent dioxathion.
Short-term studies in rats and dogs including reproduction studies in
rats demonstrated that all test doses failed to produce any
morphologic changes. Conventional two-year chronic toxicity studies
have not been conducted on dioxathion. The toxic action of this
compound is restricted to inhibition of cholinesterase enzymes. The
reported studies prove similar susceptibility in man and dogs. The
significance of aliesterase inhibition with regard to metabolism of
some chemicals is apparent, and the higher sensitivity to aliesterase
inhibition was taken into account when estimating the acceptable daily
intake.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Rat: 3 ppm in the diet, equivalent to 0.15 mg/kg per day
Dog: 0.075 mg/kg,per day
Man: 0.075 mg/kg per day
Estimate of acceptable daily intake for man
0-0.0015 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Pre-harvest treatments
The major uses of dioxathion as an agricultural insecticide are given
in the following table. Dioxathion is formulated as an emulsifiable
concentrate.
Rate of Pre-harvest
Crop Pest application interval
(kg/ha) (days)
Citrus: grapefruit, mites 0.03-0.06 kg/100 1 0
oranges, citrus thrips
limes, lemons,
tangerines,
tangelos
apples,* pears, mites 0.06 kg/100 1 7
quinces coddling moths (max.7.56 kg/ha)
apple maggots
grapes mites, grape 0.95 kg/ha (spray) 14
leafhoppers 1.5 kg/ha (dust)
* Apple pomace from treated fruit should not be fed to dairy or
meat animals.
Dioxathion is applied to beef cattle, sheep, goats and swine in the
form of sprays and dips of 0.15 per cent concentration and by
backrubber. Use is not permitted on dairy animals. It is effective in
controlling ticks, lice, horn flies, screw worms and sheep ked. Repeat
applications can be made after 2-week intervals.
Post-harvest treatments
No use is known for application on stored products.
Other uses
In addition to the major uses listed above, dioxathion is used for
controlling mites on walnuts, on stone fruit prior to fruit formation,
ornamentals and beans grown for shelled dry beans.
Residues resulting from supervised trials
Data in the form of unpublished reports (Hercules, 1958-1961),
retained at FAO headquarters in Rome, indicate residues likely to
occur from dioxathion application to plants and animals. These data
show the rate of decline of residues from various application rates
including that representative of the use pattern (above). Unless
otherwise stated the residues are expressed in terms of technical
dioxathion (which contains 70 per cent cis- and trans-isomers) in
contrast to United States and Canada tolerances which are for the
total of cis- and trans-isomers.
Citrus
Residues in citrus, confined to the peel, are persistent with an
indicated half-life of 75-100 days. No residues exceeding 0.03 ppm
were detected in the pulp. Gunther et al. (1958) studied the deposit
and persistence of dioxathion on field sprayed mature navel oranges
and lemons in California. Maximum residues occurred from 2 to 21 days
after application. Residues measured on the day of spraying were
slightly lower, probably due to loss in handling. Data are summarized
in the following table:
Rate of Pre-harvest Residue range
Crop application interval (at harvest)
(kg/100 l) (days) (ppm)
whole
peel fruit
Navel 0.02 0-21 1.0-3.5 0.2-0.8
oranges 0.04 0-21 3.8-4.8 0.8-1.1
0.09 0-21 6.3-9.7 1.4-2.1
temple 0.06 0-28 5.4-11.7 1.1-2.3
oranges
lemons 0.02 0-28 4.6-9.0 1.4-2.7
0.04 0-28 8.4-14.7 2.5-4.4
0.09 0-28 13.2-23.4 4.0-7.0
* Calculated on basis of average peel weights 22 per cent and
30 per cent for oranges and lemons, respectively.
Grapes
Two spray studies were made in the United States of America (one in
California and one in Delaware) to determine dioxathion residues in
grapes. In the California study Thompson seedless grapes (varying from
fruit ready for harvest to fruit two months prior to harvest) were
treated with one application of dioxathion at 0.95 kg/ha. None of the
resulting residues, even those on samples taken three hours after
application, exceeded 0.4 ppm. Residues from double the application
rate did not exceed 1 ppm. In the Delaware study grapes were treated
with two applications at 0.95 kg/ha. The first application was made
when small fruit was present and the second application was made eight
weeks later, a few weeks prior to harvest. Residues from this study
showed a rapid initial decrease from an average of 13.5 ppm three
hours after the initial spraying to an estimated 2.0 ppm 10 days
later. Thereafter the residues exhibited a high degree of persistence
decreasing to an average of 1 ppm 56 days after the spray application.
A second application on grapes which contained a 1 ppm residue from
the first application resulted in an initial average residue of 5.3
ppm which again rapidly declined in the first nine days to an
estimated 2 ppm. Again this residue showed persistence during the
remaining days until harvest, reaching a 1.8 ppm average at 21 days.
At the recommended pre-harvest period of 14 days, the Delaware study
showed a maximum residue of 2.4 ppm and an average residue of 2.0 ppm
(technical dioxathion).
Pome fruits
Tests were conducted in Indiana, New Mexico and North Carolina on
apples using five to eight cover sprays at various dosages. In apples,
as with citrus, residues remained in the peel. Samples taken one week
after the last application, where the maximum recommended use
programme was followed (seven cover sprays using 0.06 kg/100 1) had
the following residues.
Location Range Average
(ppm) (ppm)
Indiana 4.3-4.9 4.6
New Mexico 5.7-8.2 6.5
North Carolina 5.6-6.6 6.0
The samples taken above were picked one to five weeks prior to normal
harvest time. Dioxathion residues are quite persistent, growth
dilution being the primary cause of residue decline. The average
half-life indicated in the three studies above is about 11 weeks.
Livestock
Studies conducted by the USDA and summarized below furnish information
on the site of residue deposition, magnitude of residue, build-up and
decline pattern, and metabolic fate of dioxathion in livestock.
Chamberlain et al. (1960) treated steers with 32P-labelled technical
dioxathion using a 16.2 mg/kg dermal application and 4.56 mg/kg oral
administration. Plapp et al. (1960) gave steers an 8.8 mg/kg dermal
application of 32P-labelled technical dioxathion. Studies on the
deposition of residue in tissues were conducted by Jackson et al.
(1962) using dermal applications of 0.15 per cent and 0.25 per cent
spray.
In all studies the largest residue was found on the hair of the animal
and to a lesser but still appreciable degree on the hide. The only
significant accumulation of absorbed residue in animal tissue occurred
in the fat with only traces (0.1 ppm or less) present in the liver,
kidney and muscle. The residue in the fat reached a peak two to seven
days after spraying and then declined to 0.1 ppm or less two to three
weeks after spraying with the recommended 0.15 per cent spray. No
accumulative residue build-up was noted in the fat of cattle after six
applications with the 0.15 per cent spray following the recommended
two-week interval between applications.
The following table indicates the level of residue found in the fat
from the three studies.
Dermal application Days after Residues in fat
rate application ppm
0.15 per cent spray 2 0.37-0.95 (0.73 av.)
0.25 per cent spray 2 0.91-1.15 (1.05 av.)
8.8 mg/kg 7 0.2
16.2 mg/kg 7 1.5
The radio-labelled studies showed that the bulk of absorbed dioxathion
was rapidly metabolized and that 10-20 per cent of that dermally
applied was excreted in the urine in one week principally as diethyl
phosphoric, diethyl thiophosphoric and diethyl dithiophosphoric acids.
A higher percentage of the dose was eliminated in the urine and faeces
of orally treated animals. The maximum radioactivity in the blood
occurred at three hours in the dermally treated steer with a maximum
blood cholinesterase inhibition of 32 per cent, whereas the orally
treated steer exhibited a maximum cholinesterase depression of 83 per
cent and a maximum absorption of radioactivity in the blood after 12
hours.
The residue pattern in sheep, hogs and shorn goats is the same as for
cattle with the following decreasing order of residues in the fat:
cattle > shorn goats > sheep > hogs. Residues in the fat of hogs
did not exceed 0.1 ppm even with exaggerated treatment. The maximum
decrease in blood cholinesterase activity in sheep and goats (60-80
per cent of normal) occurred two days after one dermal application
but returned to normal in about one week. The depression in shorn
goats was the most severe (20-40 per cent of normal, two days after
application) and returned to about normal two weeks after the
application.
Three dairy cows were fed dioxathion daily for 28 days at a rate of
0.33 to 0.4 mg/kg body-weight. This is the maximum amount which would
occur in a diet of 50 per cent citrus pulp which had a residue of 23
ppm. Residues of 0.06-0.12 ppm (0.08 ppm average) were found in the
fat. It can therefore be estimated that the total residue in the fat
of cattle from a combination of dermal treatment and ingestion of
treated citrus pulp would not be likely to exceed 1.0 ppm based on the
cis- and trans-isomers.
No residues were found in the meat of cattle in any of the studies.
Determinations for dioxathion in the milk of the dairy cows fed
0.33-0.4 mg/kg body-weight daily for 28 days were negative with an
analytical sensitivity of 0.01-0.02 ppm.
In plants
Casida and Ahmed (1959) studied the behaviour of 32P-labelled
technical dioxathion components on lima bean, cabbage, cotton and
tomato plants. They showed that the cis- and trans-isomers were
the least susceptible components to volatization from plant surfaces.
Hydrolysis of dioxathion components on plant surfaces did not occur
readily. Hydrolysis that did occur was primarily with absorbed
materials and in this case the cis- and trans-isomers hydrolyzed
more slowly than the other components. The rapid hydrolysis of
absorbed dioxathion components was further demonstrated with bean
seedlings which readily absorbed radioactive components through the
roots from water containing 40 ppm of each of the dioxathion
components. After 1.5 days absorption time through the roots, the
cis- and trans-isomers in the bean foliage were about 70 per cent
hydrolyzed.
The formation of more polar derivatives and more potent in vitro
cholinesterase inhibitors does occur on exposure to sunlight or after
application to plants. However, Casida and Ahmed (1959) found that the
amounts of unhydrolyzed but more polar materials in aged plant
residues were but a small fraction (10 per cent on cabbages, 1 per
cent on beans) of the unchanged cis- and trans-isomers. The more
polar non-hydrolyzed derivatives from the dioxene component formed
more readily and disappeared more rapidly than the cis- and
trans-isomer derivatives.
Casida and Ahmed (1959) also found that the cis- to trans-isomer
ratio (about 1:2) held nearly constant after application of technical
dioxathion to plants. No conversion of one geometrical isomer to the
other occurred nor did the cis- and trans-isomers on plants from
any dioxene derivative.
The confinement of dioxathion residue in citrus to the peel is
associated with its solubility in the waxes and oils in that portion
of the fruit. Any conversion of the cis- and trans-isomers to a
metabolite must occur rather slowly since dioxathion disappears from
the peel at a slow rate. No appreciable cholinesterase inhibiting
material was detected in lemon pulp (edible portion) 71 days after an
excessive 0.18 kg/100 1 spray treatment in a field trial.
In animals
The general metabolic pattern described for rats in the section
entitled "Evaluation for Acceptable Daily Intake" appears to be
similar for livestock. The only significant residue stored in cattle
occurred in the fat with 75 per cent of the residue as the cis- and
trans-isomer and 21 per cent as the dioxene fraction. The metabolic
products in rat and cattle urine and faeces were similar following
oral administration.
Evidence of residues in food in commerce or at consumption
Total diet and food survey data for a limited number of
organo-phosphorus pesticide residues collected by the United States
Food and Drug Administration were obtained using a GLC method which
includes a Florisil column cleanup as described by Mills et al.
(1963). Pardue and Watts (1968) found that a standard of dioxathion
added to a Florisil column was not recovered in either the 6 per
cent, 15 per cent or 50 per cent ethyl ether in petroleum ether
eluate. Consequently, no applicable surveillance data are currently
available for dioxathion. (See discussion below under "Methods of
residue analysis".)
In storage and processing
Apples and pears are usually pared and cored before dehydration. Since
dioxathion residues in these fruits are confined to the peel, no
residue would be expected in the dried fruit.
The solubility of dioxathion is such that no appreciable residue would
be expected in fruit juice; however, small residues may result from
physical carry-over.
In the preparation of cattle feed from citrus, a 62 per cent residue
loss was demonstrated in the drying operation. This plant scale test
involved adding 4 ppm dioxathion to the press cake just prior to
drying with combustion gas at 200-300°F. An average residue of 22.6
ppm was found in the finished product. A small scale laboratory test
with lemon peel demonstrated an over-all residue loss of 42 per cent
as a result of liming, pressing and mild drying at 122°F.
In a controlled experiment with dioxathion added to wet apple pomace,
a 60 per cent residue loss occurred during drying in a commercial
processing unit. No milk-residue studies have been carried out with a
feeding level as high as that expected from the use of dried pomace
from treated apples; therefore such feed should not be used for dairy
or meat animals.
Methods of residue analysis
The analytical method described here is specific for the major
components, the cis- and trans-isomers of
2,3-p-dioxanedithiol-S,S-bis-(O,O-diethyl phosphorodithioate). These
are the most persistent and abundant constituents and their
determination is the most effective measurement of the toxic hazard of
the residue. Unhydrolyzed metabolic conversion products or other
components of the technical product which are excluded by the
analytical procedure used have been absent or found only in small
amounts in the vegetable and animal systems investigated and at a much
lower level than the unchanged cis- and trans-isomers of
dioxathion and completely hydrolyzed components.
The method described by Dunn (1958) extracts the residue with hexane
or isopropyl alcohol. Cleanup is achieved by use of an acid alumina
column. Waxes are readily eluted with hexane and dioxathion is eluted
with benzene. Final cleanup is done by partition chromatography on
Celite 545 with a solvent pair such as acetonitrile and hexane.
Dioxathion is determined by cleaving the molecule with mercuric
chloride to yield 2,3-dichloro-p-dioxane as one of the reaction
products. Hydrolysis of this product yields ethylene glycol and
glyoxal, the latter being determined colorimetrically as glyoxal
2,4-dinitrophenylosazone. The intensity of the colour formed in basic
dimethyl formamide solution is measured at absorbance peak 614 mµ and
the quantity estimated by comparison with a standard absorbance curve
prepared from technical or pure dioxathion.
The cleavage reaction is peculiar to thio acetals and thus is specific
for the pesticide dioxathion as no other pesticide residues known are
glyoxal precursors and the cleanup procedures exclude possible
precursors in the sample material.
The method is sensitive to 5 µg. The following table indicates the
result of method validation studies:
Fortification Recovery Blank
Sample level, ppm (per cent.) values
Citrus peel 1-10 96-103 0.1
Citrus pulp 0-03 100 0.008
Apples 1-30 95-101 0.1
Grapes 0.1-20 100 0.07 or less
Animal fat 1.0 84-90 0.2 or less
A multi-residue method for a large number of organo-phosphorus
pesticides and alteration products is being developed by the United
States Food and Drug Administration. It is anticipated that this
procedure using a charcoal column cleanup prior to determination by
GLC equipped with a KCl thermionic detector will soon be available for
the practical accumulation of total diet and surveillance data on a
large number of organo-phosphorus compounds. Watts et al. (1968a and
b) described a charcoal column cleanup procedure and determined the
recovery of about 60 organo-phosphorus pesticides and alteration
products through this cleanup. In addition they investigated the
applicability of three widely used GLC columns for determination of
this large group of pesticides. The columns were packed with 80-100
mesh, Gas Chrom Q coated with (a) 10 per cent DC 200, (b) 2 per cent
diethylene glycol succinate (2 per cent DEGS) and (c) a 1:1 mixed
column with 10 per cent DC 200 and 15 per cent QF1.
They found that 0.1 ppm dioxathion added to an ethyl acetate extract
of kale was 95 per cent recovered in the charcoal column cleanup. All
three GLC columns were adequate for thermionic detection. The 2 per
cent DEGS column gave the best sensitivity with 1.5 ng dioxathion
needed for a 30-50 per cent scale deflection.
National tolerances
Expressed as the cis- and trans-isomers of
2,3-p-dioxane-dithiol-S,S-bis-(O,O-diethyl phosphorodithioate).
Country Crop Tolerance
(ppm)
Canada apples, pears, quinces 4.9
citrus: grapefruit, oranges
lemons, limes, tangerines
tangelos 2.5
grapes 2.0
animals: beef cattle, sheep,
goats, swine 1.0
Netherlands leaf and sprout vegetables,
fruit vegetables, pulses,
fruit including grapes 0.4
United States apples, pears, quinces 4.9
of America
citrus: grapefruits, oranges
lemons, limes, tangerines
tangelos 2.8
grapes 2.1
(continued)
Country Crop Tolerance
(ppm)
animals: beef cattle, sheep,
coats, swine 1.0 (in fat)
West leaf and sprout vegetables,
Germany fruit vegetables, pulses,
fruit including grapes 0.4
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Appraisal
Dioxathion is a narrow spectrum insecticide and acaricide and its use
in agriculture is limited to a small number of food commodities. If
its use were to extend, a reappraisal of the recommendations would be
necessary. Therefore, the recommendations for limits should be on a
"temporary" basis.
Although figures are available for residues at stated times after
application, no data are available for losses during subsequent
storage and processing, except for the dehydration of citrus peel in
which case there is about a 60 per cent loss during drying.
No data are available on the nature of the residues derived from the
impurities in the technical product. No results are available from
total diet studies or from surveillance of food in commerce.
There are colorimetric analytical methods which are suitable for
regulatory purposes and for development as referee methods.
Recommendations
Temporary tolerances
The following temporary tolerances, to be in effect until 1972, are to
apply to raw agricultural products moving in commerce unless otherwise
indicated. In the case of fruit and vegetables the tolerances should
be applied as soon as practicable after harvest and in any event prior
to actual retail to the public. In the case of commodities entering
international trade, the tolerances should be applied by the importing
country at the point of entry or as soon as practicable thereafter.
Citrus 3.0
Pome fruits 5.0
Grapes 2.0
Meat 1.0 (not to include poultry)
These tolerances apply to the total of cis- and trans-isomers of
the principal active ingredient.
Further work or information
Required before 30 June 1972
1. Determination and identification of impurities.
2. Data on the disappearance of residues during storage and
processing, including residues from impurities in the technical
product.
3. Data on residue levels in raw agricultural products moving in
commerce.
4. Data on residue levels in total diet studies.
Desirable
1. Estimation of the effect on aliesterase activity in dogs.
2. Long-term studies in rats.
REFERENCES
Arthur, B. W. and Casida, J. E., (1959) Biological activity and
metabolism of Hercules AC-528 components in rats and cockroaches.
J. Econ. Entomol., 52: 20-27
Casida, J. E. and Ahmed, M. K. (1959) Mechanism of residue loss of
Hercules 528 components on plant foliage. J. Econ. Entomol., 52:
111-115
Chamberlain, W. F., Gatterdam, P. E. and Hopkins, D. E. (1960)
Metabolism of P32-Delnav in cattle. J. Econ. Entomol., 53: 672-675
DuBois, K. P., Kinoshita, F. K. and Frawley, J. P. (1968) Quantitative
measurement of aliesterase inhibition by EPN
(0-ethyl-0-p-nitrophenyl phenylphosphonothioate) and Delnav.
Toxicol. appl. Pharmacol. (In press)
Dunn, C. L. (1958) Determination of 2,3-p-dioxanedithiol S,S-bis (0,0
-diethyl phosphorodithioate). J. Agr. Food Chem., 6: 203-209
Frawley, J. P., Weir, R., Tusing, T., DuBois, K. P. and Calandra, J.
C. (1963) Toxicologic investigations on Delnav(R) Toxicol. appl.
Pharmacol., 5: 605-624
Gaines, T. B. (1960) The acute toxicity of pesticides to rats.
Toxicol. appl. Pharmacol., 2: 88-99
Gunther, F. A., Jeppson, L. R., Barkley, J. H., Elliott, L. M. and
Blinn, R. C. (1958) Persistence of residues of 2,3-p-dioxanedithiol
S,S-bis(0,0-diethyl phosphorodithioate) as an acaricide on and in
mature lemons and oranges. J. Agr. and Food Chem., 6: 210-211
Hagan, E. C., Jenner, R. M. and Fitzhugh, O. G. (1961) Acute oral
toxicity and potentiation studies with anticholinesterase compounds.
Fed. Proc., 20: 432
Hercules. (1958-1961) Hercules Powder Co. Inc. Unpublished reports on
Delnav residues
Jackson, J. B., Radeleff, R. D., Roberts, R. H. et al. (1962) Acute
toxicity of Delnav and its residues in tissues of livestock. J. Econ.
Entomol., 55: 669-702
Kennedy, G., Frawley, J. P. and Calandra, J. C. (1968) Multigeneration
reproduction study in rats fed Delnav, Herban and Toxaphene. Toxicol.
appl. Pharmacol. (In press)
Mills, P. A., Onley, J. H. and Gaither, R. A. (1963) Rapid method for
chlorinated pesticide residues in nonfatty foods. J. Assoc. Offic.
Agric. Chem., 46: 186-191
Murphy, S. D. (1966) Response of adaptive liver enzymes to acute
poisoning by organophosphate insecticides. Toxicol. appl. Pharmacol.,
8: 266-276
Pardue, J. R. and Watts, R. R. (1968) U.S. Food and Drug
Administration. Private communication
Plapp, F. W., Bigley, W. S. and Darrow, D. I. (1960) Studies on the
metabolism and residues of P32-labelled Delnav in Hereford steer.
J. Econ. Entomol., 53: 60-64
Watts, R. R., Storherr, R. W., Pardue, J. R. et al. (1968a) A widely
applicable charcoal column cleanup method for organophosphorus
pesticide residues in crop extracts. Presented at the 1968 annual
meeting of the Association of Official Analytical Chemists,
Washington, D.C., Oct. 14, 1968
Watts, R. R. and Storherr, R. W. (1968b) Gas chromatographic
determination of organophosphorus pesticide residues. General aspects
of potassium thermionic detection, retention times and response data
for three columns. Presented at the 1968 annual meeting of the
Association of Official Analytical Chemists, Washington, D.C., Oct.
14, 1968
Zaratzian, V. L., Arnault, L. T., Michel, T. C. and Fitzhugh, O. G.
(1961) Effects of organic phosphates Delnav and Malathion in the dog.
Fed. Proc., 20: 432
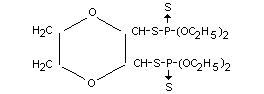 Other information on identity and properties
The technical product contains 70 per cent cis- and trans-
isomers of phosphorodithioate at a 1:2 ratio. The remaining 30 per
cent contains related compounds described by Arthur and Casida (1959)
as:
(a) ca. 10 per cent ethylphosphorothioates and
ethylphosphorodithioates
(b) ca. 1 per cent bis (diethyoxyphosphinothioyl) disulfide
(c) ca. 5 per cent 2-p-dioxenethiol-S-(O,O-diethylphos-phorodithioate)
(diethylphosphorothioic acid and salts
(d) (diethylphosphorodithioic acid and salts
(oxygen analogues of the principal component, and of (c) above
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Rats were treated orally with P32-labelled dioxathion which possessed
a ratio of cis- to trans-isomers approximating that of the
technical product. At levels of 1 and 5 µg/kg over a 10-day period,
metabolites occurred primarily in the urine and to a lesser extent in
the faeces. More than 95 per cent of the radioactivity found in the
urine was in the form of hydrolysis products. The hydrolytic products
excreted in the urine during the first 12 hours after treatment with
15 mg/kg were identified as diethyl phosphoric acid, 0,0-diethyl
phosphorothionic and 0,0-diethyl phosphorodithioic acid. A similar
group of metabolites was encountered after in vitro metabolism
studies were made using rat liver slices. The presence of these
hydrolytic metabolites indicates that enzymatic cleavage of the
phosphorothiolate grouping occurs at the carbon-sulfur as well as at
the phosphorus-sulfur bonds, along with considerable oxidation before
and after hydrolysis. A daily dose of 5 mg/kg of dioxathion given
orally to rats over seven consecutive days resulted in a maximum
accumulation of 0.6 ppm in the fat. After 10 days' withdrawal the
level in the fat fell to 0.1 ppm or lower. At daily doses of 10 mg/kg
a level of 0.6 ppm was reached in the fat after three days, and this
level held constant through continued feeding for 21 days. When rats
were given an oral dose of dioxathion (25 mg/kg body-weight), the only
tissues containing detectable dioxathion were: fat 0.34 ppm cis- and
0.68 ppm trans-isomer; kidney 0.08 ppm cis- and 0.14 trans- and
muscle 0.05 ppm cis- and 0.05 trans-. All other tissues contained
less than 0.01 ppm dioxathion. The cis- and trans-isomers were
stored in tissue to a greater extent than the other technical product
components. In the biological systems studied the disulfide compound
was the least stable of the radioactive components used. The
cis- and trans-isomers were always similar in stability and much
more stable than the dioxene component. The single exception was with
human plasma where the dioxene was the most stable of the technical
dioxathion components studied (Arthur and Casida, 1959)
Dioxathion possesses in vitro anticholinesterase activity; it has
a molar I50 of 7.6 × 10-7 for isolated beef erythrocyte
cholinesterase. Oxidation, chemically, with bromine water, or,
biologically, with fortified rat liver homogenates increased the in
vitro activity approximately 20-fold. The enzyme system responsible
for the activation was found in the supernatent layer of the liver
homogenates following centrifugation (Frawley et al., 1963).
Dioxathion is also an inhibitor of aliesterase and experiments
relating to this property are described under "Special Studies, (c)
Potentiation".
As has been observed in other organo-phosphorus compounds, acute doses
of dioxathion stimulate the pituitary-adrenal system and increase the
production of adaptive liver enzymes. A single dose of 50 mg/kg
administered intraperitoneally, to rats, produced, after 15 hours, a
maximum average cholinesterase depression of 33 per cent of control
which was accompanied by a four-to five-fold increase in hepatic
alkaline phosphatase and tyrosine-alpha-ketoglutarate transaminase.
Daily intraperitoneal injections of 15 mg/kg for 10 days reduced brain
cholinesterase to 20 per cent of control and produced a significant,
but much lower increase in hepatic adaptive enzymes than in the group
receiving the single 50 mg/kg dose (Murphy, 1966).
Acute toxicity
LD50
Animal Route Solvent mg/kg body-weight References
Mouse (M) Oral Maize oil 176 Frawley et
al., 1963
Rat (M) Oral Peanut oil 43 Gaines, 1960
Rat (F) Oral Peanut oil 23 Gaines, 1960
Rat (F) i.p. Ethanol-propylene 30 Frawley et
glycol al., 1963
(20:80)
Dog Oral None 10-40 Frawley et
al., 1963
The cis-isomer has approximately four times the toxicity of the
trans-isomer (subcutaneous rat LD50: cis, 66-86 mg/kg; trans,
230-290 mg/kg). The symptoms following acute exposure to dioxathion
are typical of parasympathetic stimulation and death is usually
preceded by clonic convulsions. The rate of onset of symptoms was
observed to be slower than with some other organo-phosphorus
compounds, presumably due to less rapid conversion to the oxygen
analogue. Maximum inhibition of cholinesterase at all sources of the
enzyme occurred within one hour following an intraperitoneal injection
of 13 mg/kg given to rats, with a recovery time of from two to three
weeks (Frawley et al., 1963).
Short-term studies
Rat
Groups, each of 25 male and 25 female rats, were fed 0, 1, 3, 10, 100
and 500 ppm of dioxathion in their diet. Duration of the test diet was
13 weeks, except that the animals fed 500 ppm were sacrificed after
one week because of marked food refusal and loss of body-weight. In
addition, groups of five male and five female rats were sacrificed at
given intervals prior to termination of the 13-week feeding period,
for determination of erythrocyte, plasma and brain cholinesterase
activity. Of the rats fed 100 ppm, only the females showed minimal
symptoms of parasympathetic stimulation. At all the lower doses no sex
difference due to dioxathion could be observed with respect to any of
the parameters considered. Marked brain, plasma and erythrocyte
cholinesterase depression occurred at the 100 ppm level. At the 10 ppm
level, brain cholinesterase was normal, but plasma and erythrocyte
cholinesterase were significantly depressed. At the 3 ppm and 1 ppm
levels, cholinesterase activity was normal in all tissues examined.
Recovery at all levels was rapid for plasma and slow for brain and
erythrocyte cholinesterase. Gross and histological examinations
revealed no pathological changes in the animals fed 100 ppm or lower
doses.
Dog
Dioxathion was administered to dogs, five days a week for a one- to
two-week period, at dosages from 0.25 mg/kg to 8.0 mg/kg. Three of the
four dogs given 8.0 mg/kg displayed the typical syndrome of
parasympathetic stimulation, which gradually subsided after
withdrawal, and all dogs were free of symptoms 10 days after the last
dose. No such effects were evident in the dogs receiving doses below
8.0 mg/kg. Plasma cholinesterase was inhibited at doses of 0.8 mg/kg
and above and erythrocyte cholinesterase at doses of 2.5 mg/kg and
above. Rapid recovery of plasma cholinesterase occurred, the level
being normal two weeks after withdrawal, however there was only slow
recovery of erythrocyte cholinesterase and it was still not complete
after five weeks. Another group of dogs fed 0.075 mg/kg and lower
doses, five days a week for 90 days showed no inhibition of either
plasma or erythrocyte cholinesterase when periodic tests were made
(Frawley et al., 1963).
Special studies
(a) Reproduction
Rat. A three-generation reproduction study was conducted on groups
of weanling rats fed 3 ppm and 10 ppm for 79 days before mating. No
abnormal pathologic changes were found in any of the parental
generations after 39 weeks. No adverse effect on reproductive
performance, fertility, lactation or litter size was found at either
level. The progeny were viable, normal in size and anatomical
structure. Findings among all test animals, three parental generations
and six litters of progeny, were comparable to control animals for all
parameters (Kennedy et al., 1968).
(b) Neurotoxicity
Using TOCP (triorthocresyl phosphate) as a positive control, oral
doses from 10 to 1000 mg/kg and subcutaneous doses from 25 to 200
mg/kg of dioxathion were administered to a total of 75 mature hens.
The hens treated with TOCP at 500 mg/kg developed typical neurological
symptoms associated with myelin degeneration whereas the hens treated
with dioxathion either died from the acutely toxic dose or recovered
without development of the neurological symptoms (Frawley et al.,
1963).
(c) Potentiation
Four organo-phosphorus insecticides, including dioxathion, and one
carbamate insecticide, carbaryl, were administered both individually
and in combination to rats. Potentiation, based upon percentage
mortality, occurred when malathion and dioxathion were administered
simultaneously, but it was greatly enhanced when dioxathion was
administered four hours before malathion. Dioxathion and carbaryl also
potentiated each other, but only when an interval existed before the
administration of the carbaryl (Hagan et al., 1961).
To evaluate further the potentiation of dioxathion and malathion a
subacute feeding study was made whereby dogs were administered oral
doses of 0.1, 0.2 and 0.4 mg/kg of dioxathion or 0.2, 0.4 and 0.8
mg/kg of malathion daily, for a pre-treatment period of six weeks. The
dogs pre-treated with dioxathion were then given malathion and those
pre-treated with malathion given dioxathion for an additional six
weeks. When administered according to this schedule, dioxathion and
malathion did not show any enhanced inhibition of plasma or
erythrocyte cholinesterase (Zaratzian of al., 1961).
In another experiment, dioxathion was fed to rats along with 14 other
organo-phosphorus compounds and also with the carbamate, carbaryl. No
significant potentiation, on the basis of the LD50 was observed when
the compounds were administered simultaneously. However, when
dioxathion was administered four hours prior to malathion, significant
potentiation occurred (Frawley et al., 1963).
A direct measurement of aliesterase activity was made, using diethyl
succinate and tributyrin as substrates. Rats were fed dietary levels
of 0, 0.2, 1, 5, and 25 ppm of dioxathion for given periods up to 13
weeks. Four groups were sacrificed at separate intervals during the
feeding period, and a fifth group was sacrificed four weeks after
return to a normal diet. The rates of hydrolysis of the substrates
were used as a measure of aliesterase activity in the liver and serum.
Cholinesterase activity was also measured in these tissues. A dietary
level of 1 ppm caused slight inhibition of aliesterase in the liver,
whereas at 5 ppm no cholinesterase inhibition was evident. Similar
difference in activity was found for serum. Maximum inhibition of
aliesterase occurred early in the feeding period and complete recovery
of activity had resulted by four weeks after return to a normal diet.
These results indicate that aliesterase is much more sensitive to
inhibition by dioxathion than is cholinesterase (DuBois et al., 1968).
Observation in man
Five female and five male adult human volunteers received 0.075
mg/kg/day of dioxathion, orally for 28 days. Plasma and erythrocyte
cholinesterase measurements were made at frequent intervals and no
significant change from pre-treatment levels was found. After 28 days,
the treatment was varied and during the following 28 days two subjects
continued to receive 0.075 mg/kg/day, two received 0.150 mg/kg/day and
the remaining six received 0.075 mg/kg/day of dioxathion along with
0.150 mg/kg/day of malathion. Erythrocyte cholinesterase levels were
not affected by any of these doses. Plasma cholinesterase was not
affected by the continuing dose of 0.075 mg/kg/day but 10-20 per cent
inhibition was observed in the two subjects which received the higher
dose of 0.150 mg/kg/day. This depression was thought to be significant
because during a 17-day post-treatment period the activity returned to
pre-treatment level. Subjects receiving the combined
dioxathion-malathion treatment did not appear to display any
cholinesterase depression related to the experimental treatment. All
clinical findings were similar to those observed in a control group of
subjects which received placebos. Blood counts, coagulation times and
prothrombin times were normal in all subjects (Frawley et al., 1963).
Comments
The toxicological studies reported above have been conducted on
technical dioxathion which contains 70 per cent dioxathion.
Short-term studies in rats and dogs including reproduction studies in
rats demonstrated that all test doses failed to produce any
morphologic changes. Conventional two-year chronic toxicity studies
have not been conducted on dioxathion. The toxic action of this
compound is restricted to inhibition of cholinesterase enzymes. The
reported studies prove similar susceptibility in man and dogs. The
significance of aliesterase inhibition with regard to metabolism of
some chemicals is apparent, and the higher sensitivity to aliesterase
inhibition was taken into account when estimating the acceptable daily
intake.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Rat: 3 ppm in the diet, equivalent to 0.15 mg/kg per day
Dog: 0.075 mg/kg,per day
Man: 0.075 mg/kg per day
Estimate of acceptable daily intake for man
0-0.0015 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Pre-harvest treatments
The major uses of dioxathion as an agricultural insecticide are given
in the following table. Dioxathion is formulated as an emulsifiable
concentrate.
Rate of Pre-harvest
Crop Pest application interval
(kg/ha) (days)
Citrus: grapefruit, mites 0.03-0.06 kg/100 1 0
oranges, citrus thrips
limes, lemons,
tangerines,
tangelos
apples,* pears, mites 0.06 kg/100 1 7
quinces coddling moths (max.7.56 kg/ha)
apple maggots
grapes mites, grape 0.95 kg/ha (spray) 14
leafhoppers 1.5 kg/ha (dust)
* Apple pomace from treated fruit should not be fed to dairy or
meat animals.
Dioxathion is applied to beef cattle, sheep, goats and swine in the
form of sprays and dips of 0.15 per cent concentration and by
backrubber. Use is not permitted on dairy animals. It is effective in
controlling ticks, lice, horn flies, screw worms and sheep ked. Repeat
applications can be made after 2-week intervals.
Post-harvest treatments
No use is known for application on stored products.
Other uses
In addition to the major uses listed above, dioxathion is used for
controlling mites on walnuts, on stone fruit prior to fruit formation,
ornamentals and beans grown for shelled dry beans.
Residues resulting from supervised trials
Data in the form of unpublished reports (Hercules, 1958-1961),
retained at FAO headquarters in Rome, indicate residues likely to
occur from dioxathion application to plants and animals. These data
show the rate of decline of residues from various application rates
including that representative of the use pattern (above). Unless
otherwise stated the residues are expressed in terms of technical
dioxathion (which contains 70 per cent cis- and trans-isomers) in
contrast to United States and Canada tolerances which are for the
total of cis- and trans-isomers.
Citrus
Residues in citrus, confined to the peel, are persistent with an
indicated half-life of 75-100 days. No residues exceeding 0.03 ppm
were detected in the pulp. Gunther et al. (1958) studied the deposit
and persistence of dioxathion on field sprayed mature navel oranges
and lemons in California. Maximum residues occurred from 2 to 21 days
after application. Residues measured on the day of spraying were
slightly lower, probably due to loss in handling. Data are summarized
in the following table:
Rate of Pre-harvest Residue range
Crop application interval (at harvest)
(kg/100 l) (days) (ppm)
whole
peel fruit
Navel 0.02 0-21 1.0-3.5 0.2-0.8
oranges 0.04 0-21 3.8-4.8 0.8-1.1
0.09 0-21 6.3-9.7 1.4-2.1
temple 0.06 0-28 5.4-11.7 1.1-2.3
oranges
lemons 0.02 0-28 4.6-9.0 1.4-2.7
0.04 0-28 8.4-14.7 2.5-4.4
0.09 0-28 13.2-23.4 4.0-7.0
* Calculated on basis of average peel weights 22 per cent and
30 per cent for oranges and lemons, respectively.
Grapes
Two spray studies were made in the United States of America (one in
California and one in Delaware) to determine dioxathion residues in
grapes. In the California study Thompson seedless grapes (varying from
fruit ready for harvest to fruit two months prior to harvest) were
treated with one application of dioxathion at 0.95 kg/ha. None of the
resulting residues, even those on samples taken three hours after
application, exceeded 0.4 ppm. Residues from double the application
rate did not exceed 1 ppm. In the Delaware study grapes were treated
with two applications at 0.95 kg/ha. The first application was made
when small fruit was present and the second application was made eight
weeks later, a few weeks prior to harvest. Residues from this study
showed a rapid initial decrease from an average of 13.5 ppm three
hours after the initial spraying to an estimated 2.0 ppm 10 days
later. Thereafter the residues exhibited a high degree of persistence
decreasing to an average of 1 ppm 56 days after the spray application.
A second application on grapes which contained a 1 ppm residue from
the first application resulted in an initial average residue of 5.3
ppm which again rapidly declined in the first nine days to an
estimated 2 ppm. Again this residue showed persistence during the
remaining days until harvest, reaching a 1.8 ppm average at 21 days.
At the recommended pre-harvest period of 14 days, the Delaware study
showed a maximum residue of 2.4 ppm and an average residue of 2.0 ppm
(technical dioxathion).
Pome fruits
Tests were conducted in Indiana, New Mexico and North Carolina on
apples using five to eight cover sprays at various dosages. In apples,
as with citrus, residues remained in the peel. Samples taken one week
after the last application, where the maximum recommended use
programme was followed (seven cover sprays using 0.06 kg/100 1) had
the following residues.
Location Range Average
(ppm) (ppm)
Indiana 4.3-4.9 4.6
New Mexico 5.7-8.2 6.5
North Carolina 5.6-6.6 6.0
The samples taken above were picked one to five weeks prior to normal
harvest time. Dioxathion residues are quite persistent, growth
dilution being the primary cause of residue decline. The average
half-life indicated in the three studies above is about 11 weeks.
Livestock
Studies conducted by the USDA and summarized below furnish information
on the site of residue deposition, magnitude of residue, build-up and
decline pattern, and metabolic fate of dioxathion in livestock.
Chamberlain et al. (1960) treated steers with 32P-labelled technical
dioxathion using a 16.2 mg/kg dermal application and 4.56 mg/kg oral
administration. Plapp et al. (1960) gave steers an 8.8 mg/kg dermal
application of 32P-labelled technical dioxathion. Studies on the
deposition of residue in tissues were conducted by Jackson et al.
(1962) using dermal applications of 0.15 per cent and 0.25 per cent
spray.
In all studies the largest residue was found on the hair of the animal
and to a lesser but still appreciable degree on the hide. The only
significant accumulation of absorbed residue in animal tissue occurred
in the fat with only traces (0.1 ppm or less) present in the liver,
kidney and muscle. The residue in the fat reached a peak two to seven
days after spraying and then declined to 0.1 ppm or less two to three
weeks after spraying with the recommended 0.15 per cent spray. No
accumulative residue build-up was noted in the fat of cattle after six
applications with the 0.15 per cent spray following the recommended
two-week interval between applications.
The following table indicates the level of residue found in the fat
from the three studies.
Dermal application Days after Residues in fat
rate application ppm
0.15 per cent spray 2 0.37-0.95 (0.73 av.)
0.25 per cent spray 2 0.91-1.15 (1.05 av.)
8.8 mg/kg 7 0.2
16.2 mg/kg 7 1.5
The radio-labelled studies showed that the bulk of absorbed dioxathion
was rapidly metabolized and that 10-20 per cent of that dermally
applied was excreted in the urine in one week principally as diethyl
phosphoric, diethyl thiophosphoric and diethyl dithiophosphoric acids.
A higher percentage of the dose was eliminated in the urine and faeces
of orally treated animals. The maximum radioactivity in the blood
occurred at three hours in the dermally treated steer with a maximum
blood cholinesterase inhibition of 32 per cent, whereas the orally
treated steer exhibited a maximum cholinesterase depression of 83 per
cent and a maximum absorption of radioactivity in the blood after 12
hours.
The residue pattern in sheep, hogs and shorn goats is the same as for
cattle with the following decreasing order of residues in the fat:
cattle > shorn goats > sheep > hogs. Residues in the fat of hogs
did not exceed 0.1 ppm even with exaggerated treatment. The maximum
decrease in blood cholinesterase activity in sheep and goats (60-80
per cent of normal) occurred two days after one dermal application
but returned to normal in about one week. The depression in shorn
goats was the most severe (20-40 per cent of normal, two days after
application) and returned to about normal two weeks after the
application.
Three dairy cows were fed dioxathion daily for 28 days at a rate of
0.33 to 0.4 mg/kg body-weight. This is the maximum amount which would
occur in a diet of 50 per cent citrus pulp which had a residue of 23
ppm. Residues of 0.06-0.12 ppm (0.08 ppm average) were found in the
fat. It can therefore be estimated that the total residue in the fat
of cattle from a combination of dermal treatment and ingestion of
treated citrus pulp would not be likely to exceed 1.0 ppm based on the
cis- and trans-isomers.
No residues were found in the meat of cattle in any of the studies.
Determinations for dioxathion in the milk of the dairy cows fed
0.33-0.4 mg/kg body-weight daily for 28 days were negative with an
analytical sensitivity of 0.01-0.02 ppm.
In plants
Casida and Ahmed (1959) studied the behaviour of 32P-labelled
technical dioxathion components on lima bean, cabbage, cotton and
tomato plants. They showed that the cis- and trans-isomers were
the least susceptible components to volatization from plant surfaces.
Hydrolysis of dioxathion components on plant surfaces did not occur
readily. Hydrolysis that did occur was primarily with absorbed
materials and in this case the cis- and trans-isomers hydrolyzed
more slowly than the other components. The rapid hydrolysis of
absorbed dioxathion components was further demonstrated with bean
seedlings which readily absorbed radioactive components through the
roots from water containing 40 ppm of each of the dioxathion
components. After 1.5 days absorption time through the roots, the
cis- and trans-isomers in the bean foliage were about 70 per cent
hydrolyzed.
The formation of more polar derivatives and more potent in vitro
cholinesterase inhibitors does occur on exposure to sunlight or after
application to plants. However, Casida and Ahmed (1959) found that the
amounts of unhydrolyzed but more polar materials in aged plant
residues were but a small fraction (10 per cent on cabbages, 1 per
cent on beans) of the unchanged cis- and trans-isomers. The more
polar non-hydrolyzed derivatives from the dioxene component formed
more readily and disappeared more rapidly than the cis- and
trans-isomer derivatives.
Casida and Ahmed (1959) also found that the cis- to trans-isomer
ratio (about 1:2) held nearly constant after application of technical
dioxathion to plants. No conversion of one geometrical isomer to the
other occurred nor did the cis- and trans-isomers on plants from
any dioxene derivative.
The confinement of dioxathion residue in citrus to the peel is
associated with its solubility in the waxes and oils in that portion
of the fruit. Any conversion of the cis- and trans-isomers to a
metabolite must occur rather slowly since dioxathion disappears from
the peel at a slow rate. No appreciable cholinesterase inhibiting
material was detected in lemon pulp (edible portion) 71 days after an
excessive 0.18 kg/100 1 spray treatment in a field trial.
In animals
The general metabolic pattern described for rats in the section
entitled "Evaluation for Acceptable Daily Intake" appears to be
similar for livestock. The only significant residue stored in cattle
occurred in the fat with 75 per cent of the residue as the cis- and
trans-isomer and 21 per cent as the dioxene fraction. The metabolic
products in rat and cattle urine and faeces were similar following
oral administration.
Evidence of residues in food in commerce or at consumption
Total diet and food survey data for a limited number of
organo-phosphorus pesticide residues collected by the United States
Food and Drug Administration were obtained using a GLC method which
includes a Florisil column cleanup as described by Mills et al.
(1963). Pardue and Watts (1968) found that a standard of dioxathion
added to a Florisil column was not recovered in either the 6 per
cent, 15 per cent or 50 per cent ethyl ether in petroleum ether
eluate. Consequently, no applicable surveillance data are currently
available for dioxathion. (See discussion below under "Methods of
residue analysis".)
In storage and processing
Apples and pears are usually pared and cored before dehydration. Since
dioxathion residues in these fruits are confined to the peel, no
residue would be expected in the dried fruit.
The solubility of dioxathion is such that no appreciable residue would
be expected in fruit juice; however, small residues may result from
physical carry-over.
In the preparation of cattle feed from citrus, a 62 per cent residue
loss was demonstrated in the drying operation. This plant scale test
involved adding 4 ppm dioxathion to the press cake just prior to
drying with combustion gas at 200-300°F. An average residue of 22.6
ppm was found in the finished product. A small scale laboratory test
with lemon peel demonstrated an over-all residue loss of 42 per cent
as a result of liming, pressing and mild drying at 122°F.
In a controlled experiment with dioxathion added to wet apple pomace,
a 60 per cent residue loss occurred during drying in a commercial
processing unit. No milk-residue studies have been carried out with a
feeding level as high as that expected from the use of dried pomace
from treated apples; therefore such feed should not be used for dairy
or meat animals.
Methods of residue analysis
The analytical method described here is specific for the major
components, the cis- and trans-isomers of
2,3-p-dioxanedithiol-S,S-bis-(O,O-diethyl phosphorodithioate). These
are the most persistent and abundant constituents and their
determination is the most effective measurement of the toxic hazard of
the residue. Unhydrolyzed metabolic conversion products or other
components of the technical product which are excluded by the
analytical procedure used have been absent or found only in small
amounts in the vegetable and animal systems investigated and at a much
lower level than the unchanged cis- and trans-isomers of
dioxathion and completely hydrolyzed components.
The method described by Dunn (1958) extracts the residue with hexane
or isopropyl alcohol. Cleanup is achieved by use of an acid alumina
column. Waxes are readily eluted with hexane and dioxathion is eluted
with benzene. Final cleanup is done by partition chromatography on
Celite 545 with a solvent pair such as acetonitrile and hexane.
Dioxathion is determined by cleaving the molecule with mercuric
chloride to yield 2,3-dichloro-p-dioxane as one of the reaction
products. Hydrolysis of this product yields ethylene glycol and
glyoxal, the latter being determined colorimetrically as glyoxal
2,4-dinitrophenylosazone. The intensity of the colour formed in basic
dimethyl formamide solution is measured at absorbance peak 614 mµ and
the quantity estimated by comparison with a standard absorbance curve
prepared from technical or pure dioxathion.
The cleavage reaction is peculiar to thio acetals and thus is specific
for the pesticide dioxathion as no other pesticide residues known are
glyoxal precursors and the cleanup procedures exclude possible
precursors in the sample material.
The method is sensitive to 5 µg. The following table indicates the
result of method validation studies:
Fortification Recovery Blank
Sample level, ppm (per cent.) values
Citrus peel 1-10 96-103 0.1
Citrus pulp 0-03 100 0.008
Apples 1-30 95-101 0.1
Grapes 0.1-20 100 0.07 or less
Animal fat 1.0 84-90 0.2 or less
A multi-residue method for a large number of organo-phosphorus
pesticides and alteration products is being developed by the United
States Food and Drug Administration. It is anticipated that this
procedure using a charcoal column cleanup prior to determination by
GLC equipped with a KCl thermionic detector will soon be available for
the practical accumulation of total diet and surveillance data on a
large number of organo-phosphorus compounds. Watts et al. (1968a and
b) described a charcoal column cleanup procedure and determined the
recovery of about 60 organo-phosphorus pesticides and alteration
products through this cleanup. In addition they investigated the
applicability of three widely used GLC columns for determination of
this large group of pesticides. The columns were packed with 80-100
mesh, Gas Chrom Q coated with (a) 10 per cent DC 200, (b) 2 per cent
diethylene glycol succinate (2 per cent DEGS) and (c) a 1:1 mixed
column with 10 per cent DC 200 and 15 per cent QF1.
They found that 0.1 ppm dioxathion added to an ethyl acetate extract
of kale was 95 per cent recovered in the charcoal column cleanup. All
three GLC columns were adequate for thermionic detection. The 2 per
cent DEGS column gave the best sensitivity with 1.5 ng dioxathion
needed for a 30-50 per cent scale deflection.
National tolerances
Expressed as the cis- and trans-isomers of
2,3-p-dioxane-dithiol-S,S-bis-(O,O-diethyl phosphorodithioate).
Country Crop Tolerance
(ppm)
Canada apples, pears, quinces 4.9
citrus: grapefruit, oranges
lemons, limes, tangerines
tangelos 2.5
grapes 2.0
animals: beef cattle, sheep,
goats, swine 1.0
Netherlands leaf and sprout vegetables,
fruit vegetables, pulses,
fruit including grapes 0.4
United States apples, pears, quinces 4.9
of America
citrus: grapefruits, oranges
lemons, limes, tangerines
tangelos 2.8
grapes 2.1
(continued)
Country Crop Tolerance
(ppm)
animals: beef cattle, sheep,
coats, swine 1.0 (in fat)
West leaf and sprout vegetables,
Germany fruit vegetables, pulses,
fruit including grapes 0.4
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Appraisal
Dioxathion is a narrow spectrum insecticide and acaricide and its use
in agriculture is limited to a small number of food commodities. If
its use were to extend, a reappraisal of the recommendations would be
necessary. Therefore, the recommendations for limits should be on a
"temporary" basis.
Although figures are available for residues at stated times after
application, no data are available for losses during subsequent
storage and processing, except for the dehydration of citrus peel in
which case there is about a 60 per cent loss during drying.
No data are available on the nature of the residues derived from the
impurities in the technical product. No results are available from
total diet studies or from surveillance of food in commerce.
There are colorimetric analytical methods which are suitable for
regulatory purposes and for development as referee methods.
Recommendations
Temporary tolerances
The following temporary tolerances, to be in effect until 1972, are to
apply to raw agricultural products moving in commerce unless otherwise
indicated. In the case of fruit and vegetables the tolerances should
be applied as soon as practicable after harvest and in any event prior
to actual retail to the public. In the case of commodities entering
international trade, the tolerances should be applied by the importing
country at the point of entry or as soon as practicable thereafter.
Citrus 3.0
Pome fruits 5.0
Grapes 2.0
Meat 1.0 (not to include poultry)
These tolerances apply to the total of cis- and trans-isomers of
the principal active ingredient.
Further work or information
Required before 30 June 1972
1. Determination and identification of impurities.
2. Data on the disappearance of residues during storage and
processing, including residues from impurities in the technical
product.
3. Data on residue levels in raw agricultural products moving in
commerce.
4. Data on residue levels in total diet studies.
Desirable
1. Estimation of the effect on aliesterase activity in dogs.
2. Long-term studies in rats.
REFERENCES
Arthur, B. W. and Casida, J. E., (1959) Biological activity and
metabolism of Hercules AC-528 components in rats and cockroaches.
J. Econ. Entomol., 52: 20-27
Casida, J. E. and Ahmed, M. K. (1959) Mechanism of residue loss of
Hercules 528 components on plant foliage. J. Econ. Entomol., 52:
111-115
Chamberlain, W. F., Gatterdam, P. E. and Hopkins, D. E. (1960)
Metabolism of P32-Delnav in cattle. J. Econ. Entomol., 53: 672-675
DuBois, K. P., Kinoshita, F. K. and Frawley, J. P. (1968) Quantitative
measurement of aliesterase inhibition by EPN
(0-ethyl-0-p-nitrophenyl phenylphosphonothioate) and Delnav.
Toxicol. appl. Pharmacol. (In press)
Dunn, C. L. (1958) Determination of 2,3-p-dioxanedithiol S,S-bis (0,0
-diethyl phosphorodithioate). J. Agr. Food Chem., 6: 203-209
Frawley, J. P., Weir, R., Tusing, T., DuBois, K. P. and Calandra, J.
C. (1963) Toxicologic investigations on Delnav(R) Toxicol. appl.
Pharmacol., 5: 605-624
Gaines, T. B. (1960) The acute toxicity of pesticides to rats.
Toxicol. appl. Pharmacol., 2: 88-99
Gunther, F. A., Jeppson, L. R., Barkley, J. H., Elliott, L. M. and
Blinn, R. C. (1958) Persistence of residues of 2,3-p-dioxanedithiol
S,S-bis(0,0-diethyl phosphorodithioate) as an acaricide on and in
mature lemons and oranges. J. Agr. and Food Chem., 6: 210-211
Hagan, E. C., Jenner, R. M. and Fitzhugh, O. G. (1961) Acute oral
toxicity and potentiation studies with anticholinesterase compounds.
Fed. Proc., 20: 432
Hercules. (1958-1961) Hercules Powder Co. Inc. Unpublished reports on
Delnav residues
Jackson, J. B., Radeleff, R. D., Roberts, R. H. et al. (1962) Acute
toxicity of Delnav and its residues in tissues of livestock. J. Econ.
Entomol., 55: 669-702
Kennedy, G., Frawley, J. P. and Calandra, J. C. (1968) Multigeneration
reproduction study in rats fed Delnav, Herban and Toxaphene. Toxicol.
appl. Pharmacol. (In press)
Mills, P. A., Onley, J. H. and Gaither, R. A. (1963) Rapid method for
chlorinated pesticide residues in nonfatty foods. J. Assoc. Offic.
Agric. Chem., 46: 186-191
Murphy, S. D. (1966) Response of adaptive liver enzymes to acute
poisoning by organophosphate insecticides. Toxicol. appl. Pharmacol.,
8: 266-276
Pardue, J. R. and Watts, R. R. (1968) U.S. Food and Drug
Administration. Private communication
Plapp, F. W., Bigley, W. S. and Darrow, D. I. (1960) Studies on the
metabolism and residues of P32-labelled Delnav in Hereford steer.
J. Econ. Entomol., 53: 60-64
Watts, R. R., Storherr, R. W., Pardue, J. R. et al. (1968a) A widely
applicable charcoal column cleanup method for organophosphorus
pesticide residues in crop extracts. Presented at the 1968 annual
meeting of the Association of Official Analytical Chemists,
Washington, D.C., Oct. 14, 1968
Watts, R. R. and Storherr, R. W. (1968b) Gas chromatographic
determination of organophosphorus pesticide residues. General aspects
of potassium thermionic detection, retention times and response data
for three columns. Presented at the 1968 annual meeting of the
Association of Official Analytical Chemists, Washington, D.C., Oct.
14, 1968
Zaratzian, V. L., Arnault, L. T., Michel, T. C. and Fitzhugh, O. G.
(1961) Effects of organic phosphates Delnav and Malathion in the dog.
Fed. Proc., 20: 432
Other information on identity and properties
The technical product contains 70 per cent cis- and trans-
isomers of phosphorodithioate at a 1:2 ratio. The remaining 30 per
cent contains related compounds described by Arthur and Casida (1959)
as:
(a) ca. 10 per cent ethylphosphorothioates and
ethylphosphorodithioates
(b) ca. 1 per cent bis (diethyoxyphosphinothioyl) disulfide
(c) ca. 5 per cent 2-p-dioxenethiol-S-(O,O-diethylphos-phorodithioate)
(diethylphosphorothioic acid and salts
(d) (diethylphosphorodithioic acid and salts
(oxygen analogues of the principal component, and of (c) above
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Rats were treated orally with P32-labelled dioxathion which possessed
a ratio of cis- to trans-isomers approximating that of the
technical product. At levels of 1 and 5 µg/kg over a 10-day period,
metabolites occurred primarily in the urine and to a lesser extent in
the faeces. More than 95 per cent of the radioactivity found in the
urine was in the form of hydrolysis products. The hydrolytic products
excreted in the urine during the first 12 hours after treatment with
15 mg/kg were identified as diethyl phosphoric acid, 0,0-diethyl
phosphorothionic and 0,0-diethyl phosphorodithioic acid. A similar
group of metabolites was encountered after in vitro metabolism
studies were made using rat liver slices. The presence of these
hydrolytic metabolites indicates that enzymatic cleavage of the
phosphorothiolate grouping occurs at the carbon-sulfur as well as at
the phosphorus-sulfur bonds, along with considerable oxidation before
and after hydrolysis. A daily dose of 5 mg/kg of dioxathion given
orally to rats over seven consecutive days resulted in a maximum
accumulation of 0.6 ppm in the fat. After 10 days' withdrawal the
level in the fat fell to 0.1 ppm or lower. At daily doses of 10 mg/kg
a level of 0.6 ppm was reached in the fat after three days, and this
level held constant through continued feeding for 21 days. When rats
were given an oral dose of dioxathion (25 mg/kg body-weight), the only
tissues containing detectable dioxathion were: fat 0.34 ppm cis- and
0.68 ppm trans-isomer; kidney 0.08 ppm cis- and 0.14 trans- and
muscle 0.05 ppm cis- and 0.05 trans-. All other tissues contained
less than 0.01 ppm dioxathion. The cis- and trans-isomers were
stored in tissue to a greater extent than the other technical product
components. In the biological systems studied the disulfide compound
was the least stable of the radioactive components used. The
cis- and trans-isomers were always similar in stability and much
more stable than the dioxene component. The single exception was with
human plasma where the dioxene was the most stable of the technical
dioxathion components studied (Arthur and Casida, 1959)
Dioxathion possesses in vitro anticholinesterase activity; it has
a molar I50 of 7.6 × 10-7 for isolated beef erythrocyte
cholinesterase. Oxidation, chemically, with bromine water, or,
biologically, with fortified rat liver homogenates increased the in
vitro activity approximately 20-fold. The enzyme system responsible
for the activation was found in the supernatent layer of the liver
homogenates following centrifugation (Frawley et al., 1963).
Dioxathion is also an inhibitor of aliesterase and experiments
relating to this property are described under "Special Studies, (c)
Potentiation".
As has been observed in other organo-phosphorus compounds, acute doses
of dioxathion stimulate the pituitary-adrenal system and increase the
production of adaptive liver enzymes. A single dose of 50 mg/kg
administered intraperitoneally, to rats, produced, after 15 hours, a
maximum average cholinesterase depression of 33 per cent of control
which was accompanied by a four-to five-fold increase in hepatic
alkaline phosphatase and tyrosine-alpha-ketoglutarate transaminase.
Daily intraperitoneal injections of 15 mg/kg for 10 days reduced brain
cholinesterase to 20 per cent of control and produced a significant,
but much lower increase in hepatic adaptive enzymes than in the group
receiving the single 50 mg/kg dose (Murphy, 1966).
Acute toxicity
LD50
Animal Route Solvent mg/kg body-weight References
Mouse (M) Oral Maize oil 176 Frawley et
al., 1963
Rat (M) Oral Peanut oil 43 Gaines, 1960
Rat (F) Oral Peanut oil 23 Gaines, 1960
Rat (F) i.p. Ethanol-propylene 30 Frawley et
glycol al., 1963
(20:80)
Dog Oral None 10-40 Frawley et
al., 1963
The cis-isomer has approximately four times the toxicity of the
trans-isomer (subcutaneous rat LD50: cis, 66-86 mg/kg; trans,
230-290 mg/kg). The symptoms following acute exposure to dioxathion
are typical of parasympathetic stimulation and death is usually
preceded by clonic convulsions. The rate of onset of symptoms was
observed to be slower than with some other organo-phosphorus
compounds, presumably due to less rapid conversion to the oxygen
analogue. Maximum inhibition of cholinesterase at all sources of the
enzyme occurred within one hour following an intraperitoneal injection
of 13 mg/kg given to rats, with a recovery time of from two to three
weeks (Frawley et al., 1963).
Short-term studies
Rat
Groups, each of 25 male and 25 female rats, were fed 0, 1, 3, 10, 100
and 500 ppm of dioxathion in their diet. Duration of the test diet was
13 weeks, except that the animals fed 500 ppm were sacrificed after
one week because of marked food refusal and loss of body-weight. In
addition, groups of five male and five female rats were sacrificed at
given intervals prior to termination of the 13-week feeding period,
for determination of erythrocyte, plasma and brain cholinesterase
activity. Of the rats fed 100 ppm, only the females showed minimal
symptoms of parasympathetic stimulation. At all the lower doses no sex
difference due to dioxathion could be observed with respect to any of
the parameters considered. Marked brain, plasma and erythrocyte
cholinesterase depression occurred at the 100 ppm level. At the 10 ppm
level, brain cholinesterase was normal, but plasma and erythrocyte
cholinesterase were significantly depressed. At the 3 ppm and 1 ppm
levels, cholinesterase activity was normal in all tissues examined.
Recovery at all levels was rapid for plasma and slow for brain and
erythrocyte cholinesterase. Gross and histological examinations
revealed no pathological changes in the animals fed 100 ppm or lower
doses.
Dog
Dioxathion was administered to dogs, five days a week for a one- to
two-week period, at dosages from 0.25 mg/kg to 8.0 mg/kg. Three of the
four dogs given 8.0 mg/kg displayed the typical syndrome of
parasympathetic stimulation, which gradually subsided after
withdrawal, and all dogs were free of symptoms 10 days after the last
dose. No such effects were evident in the dogs receiving doses below
8.0 mg/kg. Plasma cholinesterase was inhibited at doses of 0.8 mg/kg
and above and erythrocyte cholinesterase at doses of 2.5 mg/kg and
above. Rapid recovery of plasma cholinesterase occurred, the level
being normal two weeks after withdrawal, however there was only slow
recovery of erythrocyte cholinesterase and it was still not complete
after five weeks. Another group of dogs fed 0.075 mg/kg and lower
doses, five days a week for 90 days showed no inhibition of either
plasma or erythrocyte cholinesterase when periodic tests were made
(Frawley et al., 1963).
Special studies
(a) Reproduction
Rat. A three-generation reproduction study was conducted on groups
of weanling rats fed 3 ppm and 10 ppm for 79 days before mating. No
abnormal pathologic changes were found in any of the parental
generations after 39 weeks. No adverse effect on reproductive
performance, fertility, lactation or litter size was found at either
level. The progeny were viable, normal in size and anatomical
structure. Findings among all test animals, three parental generations
and six litters of progeny, were comparable to control animals for all
parameters (Kennedy et al., 1968).
(b) Neurotoxicity
Using TOCP (triorthocresyl phosphate) as a positive control, oral
doses from 10 to 1000 mg/kg and subcutaneous doses from 25 to 200
mg/kg of dioxathion were administered to a total of 75 mature hens.
The hens treated with TOCP at 500 mg/kg developed typical neurological
symptoms associated with myelin degeneration whereas the hens treated
with dioxathion either died from the acutely toxic dose or recovered
without development of the neurological symptoms (Frawley et al.,
1963).
(c) Potentiation
Four organo-phosphorus insecticides, including dioxathion, and one
carbamate insecticide, carbaryl, were administered both individually
and in combination to rats. Potentiation, based upon percentage
mortality, occurred when malathion and dioxathion were administered
simultaneously, but it was greatly enhanced when dioxathion was
administered four hours before malathion. Dioxathion and carbaryl also
potentiated each other, but only when an interval existed before the
administration of the carbaryl (Hagan et al., 1961).
To evaluate further the potentiation of dioxathion and malathion a
subacute feeding study was made whereby dogs were administered oral
doses of 0.1, 0.2 and 0.4 mg/kg of dioxathion or 0.2, 0.4 and 0.8
mg/kg of malathion daily, for a pre-treatment period of six weeks. The
dogs pre-treated with dioxathion were then given malathion and those
pre-treated with malathion given dioxathion for an additional six
weeks. When administered according to this schedule, dioxathion and
malathion did not show any enhanced inhibition of plasma or
erythrocyte cholinesterase (Zaratzian of al., 1961).
In another experiment, dioxathion was fed to rats along with 14 other
organo-phosphorus compounds and also with the carbamate, carbaryl. No
significant potentiation, on the basis of the LD50 was observed when
the compounds were administered simultaneously. However, when
dioxathion was administered four hours prior to malathion, significant
potentiation occurred (Frawley et al., 1963).
A direct measurement of aliesterase activity was made, using diethyl
succinate and tributyrin as substrates. Rats were fed dietary levels
of 0, 0.2, 1, 5, and 25 ppm of dioxathion for given periods up to 13
weeks. Four groups were sacrificed at separate intervals during the
feeding period, and a fifth group was sacrificed four weeks after
return to a normal diet. The rates of hydrolysis of the substrates
were used as a measure of aliesterase activity in the liver and serum.
Cholinesterase activity was also measured in these tissues. A dietary
level of 1 ppm caused slight inhibition of aliesterase in the liver,
whereas at 5 ppm no cholinesterase inhibition was evident. Similar
difference in activity was found for serum. Maximum inhibition of
aliesterase occurred early in the feeding period and complete recovery
of activity had resulted by four weeks after return to a normal diet.
These results indicate that aliesterase is much more sensitive to
inhibition by dioxathion than is cholinesterase (DuBois et al., 1968).
Observation in man
Five female and five male adult human volunteers received 0.075
mg/kg/day of dioxathion, orally for 28 days. Plasma and erythrocyte
cholinesterase measurements were made at frequent intervals and no
significant change from pre-treatment levels was found. After 28 days,
the treatment was varied and during the following 28 days two subjects
continued to receive 0.075 mg/kg/day, two received 0.150 mg/kg/day and
the remaining six received 0.075 mg/kg/day of dioxathion along with
0.150 mg/kg/day of malathion. Erythrocyte cholinesterase levels were
not affected by any of these doses. Plasma cholinesterase was not
affected by the continuing dose of 0.075 mg/kg/day but 10-20 per cent
inhibition was observed in the two subjects which received the higher
dose of 0.150 mg/kg/day. This depression was thought to be significant
because during a 17-day post-treatment period the activity returned to
pre-treatment level. Subjects receiving the combined
dioxathion-malathion treatment did not appear to display any
cholinesterase depression related to the experimental treatment. All
clinical findings were similar to those observed in a control group of
subjects which received placebos. Blood counts, coagulation times and
prothrombin times were normal in all subjects (Frawley et al., 1963).
Comments
The toxicological studies reported above have been conducted on
technical dioxathion which contains 70 per cent dioxathion.
Short-term studies in rats and dogs including reproduction studies in
rats demonstrated that all test doses failed to produce any
morphologic changes. Conventional two-year chronic toxicity studies
have not been conducted on dioxathion. The toxic action of this
compound is restricted to inhibition of cholinesterase enzymes. The
reported studies prove similar susceptibility in man and dogs. The
significance of aliesterase inhibition with regard to metabolism of
some chemicals is apparent, and the higher sensitivity to aliesterase
inhibition was taken into account when estimating the acceptable daily
intake.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Rat: 3 ppm in the diet, equivalent to 0.15 mg/kg per day
Dog: 0.075 mg/kg,per day
Man: 0.075 mg/kg per day
Estimate of acceptable daily intake for man
0-0.0015 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Pre-harvest treatments
The major uses of dioxathion as an agricultural insecticide are given
in the following table. Dioxathion is formulated as an emulsifiable
concentrate.
Rate of Pre-harvest
Crop Pest application interval
(kg/ha) (days)
Citrus: grapefruit, mites 0.03-0.06 kg/100 1 0
oranges, citrus thrips
limes, lemons,
tangerines,
tangelos
apples,* pears, mites 0.06 kg/100 1 7
quinces coddling moths (max.7.56 kg/ha)
apple maggots
grapes mites, grape 0.95 kg/ha (spray) 14
leafhoppers 1.5 kg/ha (dust)
* Apple pomace from treated fruit should not be fed to dairy or
meat animals.
Dioxathion is applied to beef cattle, sheep, goats and swine in the
form of sprays and dips of 0.15 per cent concentration and by
backrubber. Use is not permitted on dairy animals. It is effective in
controlling ticks, lice, horn flies, screw worms and sheep ked. Repeat
applications can be made after 2-week intervals.
Post-harvest treatments
No use is known for application on stored products.
Other uses
In addition to the major uses listed above, dioxathion is used for
controlling mites on walnuts, on stone fruit prior to fruit formation,
ornamentals and beans grown for shelled dry beans.
Residues resulting from supervised trials
Data in the form of unpublished reports (Hercules, 1958-1961),
retained at FAO headquarters in Rome, indicate residues likely to
occur from dioxathion application to plants and animals. These data
show the rate of decline of residues from various application rates
including that representative of the use pattern (above). Unless
otherwise stated the residues are expressed in terms of technical
dioxathion (which contains 70 per cent cis- and trans-isomers) in
contrast to United States and Canada tolerances which are for the
total of cis- and trans-isomers.
Citrus
Residues in citrus, confined to the peel, are persistent with an
indicated half-life of 75-100 days. No residues exceeding 0.03 ppm
were detected in the pulp. Gunther et al. (1958) studied the deposit
and persistence of dioxathion on field sprayed mature navel oranges
and lemons in California. Maximum residues occurred from 2 to 21 days
after application. Residues measured on the day of spraying were
slightly lower, probably due to loss in handling. Data are summarized
in the following table:
Rate of Pre-harvest Residue range
Crop application interval (at harvest)
(kg/100 l) (days) (ppm)
whole
peel fruit
Navel 0.02 0-21 1.0-3.5 0.2-0.8
oranges 0.04 0-21 3.8-4.8 0.8-1.1
0.09 0-21 6.3-9.7 1.4-2.1
temple 0.06 0-28 5.4-11.7 1.1-2.3
oranges
lemons 0.02 0-28 4.6-9.0 1.4-2.7
0.04 0-28 8.4-14.7 2.5-4.4
0.09 0-28 13.2-23.4 4.0-7.0
* Calculated on basis of average peel weights 22 per cent and
30 per cent for oranges and lemons, respectively.
Grapes
Two spray studies were made in the United States of America (one in
California and one in Delaware) to determine dioxathion residues in
grapes. In the California study Thompson seedless grapes (varying from
fruit ready for harvest to fruit two months prior to harvest) were
treated with one application of dioxathion at 0.95 kg/ha. None of the
resulting residues, even those on samples taken three hours after
application, exceeded 0.4 ppm. Residues from double the application
rate did not exceed 1 ppm. In the Delaware study grapes were treated
with two applications at 0.95 kg/ha. The first application was made
when small fruit was present and the second application was made eight
weeks later, a few weeks prior to harvest. Residues from this study
showed a rapid initial decrease from an average of 13.5 ppm three
hours after the initial spraying to an estimated 2.0 ppm 10 days
later. Thereafter the residues exhibited a high degree of persistence
decreasing to an average of 1 ppm 56 days after the spray application.
A second application on grapes which contained a 1 ppm residue from
the first application resulted in an initial average residue of 5.3
ppm which again rapidly declined in the first nine days to an
estimated 2 ppm. Again this residue showed persistence during the
remaining days until harvest, reaching a 1.8 ppm average at 21 days.
At the recommended pre-harvest period of 14 days, the Delaware study
showed a maximum residue of 2.4 ppm and an average residue of 2.0 ppm
(technical dioxathion).
Pome fruits
Tests were conducted in Indiana, New Mexico and North Carolina on
apples using five to eight cover sprays at various dosages. In apples,
as with citrus, residues remained in the peel. Samples taken one week
after the last application, where the maximum recommended use
programme was followed (seven cover sprays using 0.06 kg/100 1) had
the following residues.
Location Range Average
(ppm) (ppm)
Indiana 4.3-4.9 4.6
New Mexico 5.7-8.2 6.5
North Carolina 5.6-6.6 6.0
The samples taken above were picked one to five weeks prior to normal
harvest time. Dioxathion residues are quite persistent, growth
dilution being the primary cause of residue decline. The average
half-life indicated in the three studies above is about 11 weeks.
Livestock
Studies conducted by the USDA and summarized below furnish information
on the site of residue deposition, magnitude of residue, build-up and
decline pattern, and metabolic fate of dioxathion in livestock.
Chamberlain et al. (1960) treated steers with 32P-labelled technical
dioxathion using a 16.2 mg/kg dermal application and 4.56 mg/kg oral
administration. Plapp et al. (1960) gave steers an 8.8 mg/kg dermal
application of 32P-labelled technical dioxathion. Studies on the
deposition of residue in tissues were conducted by Jackson et al.
(1962) using dermal applications of 0.15 per cent and 0.25 per cent
spray.
In all studies the largest residue was found on the hair of the animal
and to a lesser but still appreciable degree on the hide. The only
significant accumulation of absorbed residue in animal tissue occurred
in the fat with only traces (0.1 ppm or less) present in the liver,
kidney and muscle. The residue in the fat reached a peak two to seven
days after spraying and then declined to 0.1 ppm or less two to three
weeks after spraying with the recommended 0.15 per cent spray. No
accumulative residue build-up was noted in the fat of cattle after six
applications with the 0.15 per cent spray following the recommended
two-week interval between applications.
The following table indicates the level of residue found in the fat
from the three studies.
Dermal application Days after Residues in fat
rate application ppm
0.15 per cent spray 2 0.37-0.95 (0.73 av.)
0.25 per cent spray 2 0.91-1.15 (1.05 av.)
8.8 mg/kg 7 0.2
16.2 mg/kg 7 1.5
The radio-labelled studies showed that the bulk of absorbed dioxathion
was rapidly metabolized and that 10-20 per cent of that dermally
applied was excreted in the urine in one week principally as diethyl
phosphoric, diethyl thiophosphoric and diethyl dithiophosphoric acids.
A higher percentage of the dose was eliminated in the urine and faeces
of orally treated animals. The maximum radioactivity in the blood
occurred at three hours in the dermally treated steer with a maximum
blood cholinesterase inhibition of 32 per cent, whereas the orally
treated steer exhibited a maximum cholinesterase depression of 83 per
cent and a maximum absorption of radioactivity in the blood after 12
hours.
The residue pattern in sheep, hogs and shorn goats is the same as for
cattle with the following decreasing order of residues in the fat:
cattle > shorn goats > sheep > hogs. Residues in the fat of hogs
did not exceed 0.1 ppm even with exaggerated treatment. The maximum
decrease in blood cholinesterase activity in sheep and goats (60-80
per cent of normal) occurred two days after one dermal application
but returned to normal in about one week. The depression in shorn
goats was the most severe (20-40 per cent of normal, two days after
application) and returned to about normal two weeks after the
application.
Three dairy cows were fed dioxathion daily for 28 days at a rate of
0.33 to 0.4 mg/kg body-weight. This is the maximum amount which would
occur in a diet of 50 per cent citrus pulp which had a residue of 23
ppm. Residues of 0.06-0.12 ppm (0.08 ppm average) were found in the
fat. It can therefore be estimated that the total residue in the fat
of cattle from a combination of dermal treatment and ingestion of
treated citrus pulp would not be likely to exceed 1.0 ppm based on the
cis- and trans-isomers.
No residues were found in the meat of cattle in any of the studies.
Determinations for dioxathion in the milk of the dairy cows fed
0.33-0.4 mg/kg body-weight daily for 28 days were negative with an
analytical sensitivity of 0.01-0.02 ppm.
In plants
Casida and Ahmed (1959) studied the behaviour of 32P-labelled
technical dioxathion components on lima bean, cabbage, cotton and
tomato plants. They showed that the cis- and trans-isomers were
the least susceptible components to volatization from plant surfaces.
Hydrolysis of dioxathion components on plant surfaces did not occur
readily. Hydrolysis that did occur was primarily with absorbed
materials and in this case the cis- and trans-isomers hydrolyzed
more slowly than the other components. The rapid hydrolysis of
absorbed dioxathion components was further demonstrated with bean
seedlings which readily absorbed radioactive components through the
roots from water containing 40 ppm of each of the dioxathion
components. After 1.5 days absorption time through the roots, the
cis- and trans-isomers in the bean foliage were about 70 per cent
hydrolyzed.
The formation of more polar derivatives and more potent in vitro
cholinesterase inhibitors does occur on exposure to sunlight or after
application to plants. However, Casida and Ahmed (1959) found that the
amounts of unhydrolyzed but more polar materials in aged plant
residues were but a small fraction (10 per cent on cabbages, 1 per
cent on beans) of the unchanged cis- and trans-isomers. The more
polar non-hydrolyzed derivatives from the dioxene component formed
more readily and disappeared more rapidly than the cis- and
trans-isomer derivatives.
Casida and Ahmed (1959) also found that the cis- to trans-isomer
ratio (about 1:2) held nearly constant after application of technical
dioxathion to plants. No conversion of one geometrical isomer to the
other occurred nor did the cis- and trans-isomers on plants from
any dioxene derivative.
The confinement of dioxathion residue in citrus to the peel is
associated with its solubility in the waxes and oils in that portion
of the fruit. Any conversion of the cis- and trans-isomers to a
metabolite must occur rather slowly since dioxathion disappears from
the peel at a slow rate. No appreciable cholinesterase inhibiting
material was detected in lemon pulp (edible portion) 71 days after an
excessive 0.18 kg/100 1 spray treatment in a field trial.
In animals
The general metabolic pattern described for rats in the section
entitled "Evaluation for Acceptable Daily Intake" appears to be
similar for livestock. The only significant residue stored in cattle
occurred in the fat with 75 per cent of the residue as the cis- and
trans-isomer and 21 per cent as the dioxene fraction. The metabolic
products in rat and cattle urine and faeces were similar following
oral administration.
Evidence of residues in food in commerce or at consumption
Total diet and food survey data for a limited number of
organo-phosphorus pesticide residues collected by the United States
Food and Drug Administration were obtained using a GLC method which
includes a Florisil column cleanup as described by Mills et al.
(1963). Pardue and Watts (1968) found that a standard of dioxathion
added to a Florisil column was not recovered in either the 6 per
cent, 15 per cent or 50 per cent ethyl ether in petroleum ether
eluate. Consequently, no applicable surveillance data are currently
available for dioxathion. (See discussion below under "Methods of
residue analysis".)
In storage and processing
Apples and pears are usually pared and cored before dehydration. Since
dioxathion residues in these fruits are confined to the peel, no
residue would be expected in the dried fruit.
The solubility of dioxathion is such that no appreciable residue would
be expected in fruit juice; however, small residues may result from
physical carry-over.
In the preparation of cattle feed from citrus, a 62 per cent residue
loss was demonstrated in the drying operation. This plant scale test
involved adding 4 ppm dioxathion to the press cake just prior to
drying with combustion gas at 200-300°F. An average residue of 22.6
ppm was found in the finished product. A small scale laboratory test
with lemon peel demonstrated an over-all residue loss of 42 per cent
as a result of liming, pressing and mild drying at 122°F.
In a controlled experiment with dioxathion added to wet apple pomace,
a 60 per cent residue loss occurred during drying in a commercial
processing unit. No milk-residue studies have been carried out with a
feeding level as high as that expected from the use of dried pomace
from treated apples; therefore such feed should not be used for dairy
or meat animals.
Methods of residue analysis
The analytical method described here is specific for the major
components, the cis- and trans-isomers of
2,3-p-dioxanedithiol-S,S-bis-(O,O-diethyl phosphorodithioate). These
are the most persistent and abundant constituents and their
determination is the most effective measurement of the toxic hazard of
the residue. Unhydrolyzed metabolic conversion products or other
components of the technical product which are excluded by the
analytical procedure used have been absent or found only in small
amounts in the vegetable and animal systems investigated and at a much
lower level than the unchanged cis- and trans-isomers of
dioxathion and completely hydrolyzed components.
The method described by Dunn (1958) extracts the residue with hexane
or isopropyl alcohol. Cleanup is achieved by use of an acid alumina
column. Waxes are readily eluted with hexane and dioxathion is eluted
with benzene. Final cleanup is done by partition chromatography on
Celite 545 with a solvent pair such as acetonitrile and hexane.
Dioxathion is determined by cleaving the molecule with mercuric
chloride to yield 2,3-dichloro-p-dioxane as one of the reaction
products. Hydrolysis of this product yields ethylene glycol and
glyoxal, the latter being determined colorimetrically as glyoxal
2,4-dinitrophenylosazone. The intensity of the colour formed in basic
dimethyl formamide solution is measured at absorbance peak 614 mµ and
the quantity estimated by comparison with a standard absorbance curve
prepared from technical or pure dioxathion.
The cleavage reaction is peculiar to thio acetals and thus is specific
for the pesticide dioxathion as no other pesticide residues known are
glyoxal precursors and the cleanup procedures exclude possible
precursors in the sample material.
The method is sensitive to 5 µg. The following table indicates the
result of method validation studies:
Fortification Recovery Blank
Sample level, ppm (per cent.) values
Citrus peel 1-10 96-103 0.1
Citrus pulp 0-03 100 0.008
Apples 1-30 95-101 0.1
Grapes 0.1-20 100 0.07 or less
Animal fat 1.0 84-90 0.2 or less
A multi-residue method for a large number of organo-phosphorus
pesticides and alteration products is being developed by the United
States Food and Drug Administration. It is anticipated that this
procedure using a charcoal column cleanup prior to determination by
GLC equipped with a KCl thermionic detector will soon be available for
the practical accumulation of total diet and surveillance data on a
large number of organo-phosphorus compounds. Watts et al. (1968a and
b) described a charcoal column cleanup procedure and determined the
recovery of about 60 organo-phosphorus pesticides and alteration
products through this cleanup. In addition they investigated the
applicability of three widely used GLC columns for determination of
this large group of pesticides. The columns were packed with 80-100
mesh, Gas Chrom Q coated with (a) 10 per cent DC 200, (b) 2 per cent
diethylene glycol succinate (2 per cent DEGS) and (c) a 1:1 mixed
column with 10 per cent DC 200 and 15 per cent QF1.
They found that 0.1 ppm dioxathion added to an ethyl acetate extract
of kale was 95 per cent recovered in the charcoal column cleanup. All
three GLC columns were adequate for thermionic detection. The 2 per
cent DEGS column gave the best sensitivity with 1.5 ng dioxathion
needed for a 30-50 per cent scale deflection.
National tolerances
Expressed as the cis- and trans-isomers of
2,3-p-dioxane-dithiol-S,S-bis-(O,O-diethyl phosphorodithioate).
Country Crop Tolerance
(ppm)
Canada apples, pears, quinces 4.9
citrus: grapefruit, oranges
lemons, limes, tangerines
tangelos 2.5
grapes 2.0
animals: beef cattle, sheep,
goats, swine 1.0
Netherlands leaf and sprout vegetables,
fruit vegetables, pulses,
fruit including grapes 0.4
United States apples, pears, quinces 4.9
of America
citrus: grapefruits, oranges
lemons, limes, tangerines
tangelos 2.8
grapes 2.1
(continued)
Country Crop Tolerance
(ppm)
animals: beef cattle, sheep,
coats, swine 1.0 (in fat)
West leaf and sprout vegetables,
Germany fruit vegetables, pulses,
fruit including grapes 0.4
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Appraisal
Dioxathion is a narrow spectrum insecticide and acaricide and its use
in agriculture is limited to a small number of food commodities. If
its use were to extend, a reappraisal of the recommendations would be
necessary. Therefore, the recommendations for limits should be on a
"temporary" basis.
Although figures are available for residues at stated times after
application, no data are available for losses during subsequent
storage and processing, except for the dehydration of citrus peel in
which case there is about a 60 per cent loss during drying.
No data are available on the nature of the residues derived from the
impurities in the technical product. No results are available from
total diet studies or from surveillance of food in commerce.
There are colorimetric analytical methods which are suitable for
regulatory purposes and for development as referee methods.
Recommendations
Temporary tolerances
The following temporary tolerances, to be in effect until 1972, are to
apply to raw agricultural products moving in commerce unless otherwise
indicated. In the case of fruit and vegetables the tolerances should
be applied as soon as practicable after harvest and in any event prior
to actual retail to the public. In the case of commodities entering
international trade, the tolerances should be applied by the importing
country at the point of entry or as soon as practicable thereafter.
Citrus 3.0
Pome fruits 5.0
Grapes 2.0
Meat 1.0 (not to include poultry)
These tolerances apply to the total of cis- and trans-isomers of
the principal active ingredient.
Further work or information
Required before 30 June 1972
1. Determination and identification of impurities.
2. Data on the disappearance of residues during storage and
processing, including residues from impurities in the technical
product.
3. Data on residue levels in raw agricultural products moving in
commerce.
4. Data on residue levels in total diet studies.
Desirable
1. Estimation of the effect on aliesterase activity in dogs.
2. Long-term studies in rats.
REFERENCES
Arthur, B. W. and Casida, J. E., (1959) Biological activity and
metabolism of Hercules AC-528 components in rats and cockroaches.
J. Econ. Entomol., 52: 20-27
Casida, J. E. and Ahmed, M. K. (1959) Mechanism of residue loss of
Hercules 528 components on plant foliage. J. Econ. Entomol., 52:
111-115
Chamberlain, W. F., Gatterdam, P. E. and Hopkins, D. E. (1960)
Metabolism of P32-Delnav in cattle. J. Econ. Entomol., 53: 672-675
DuBois, K. P., Kinoshita, F. K. and Frawley, J. P. (1968) Quantitative
measurement of aliesterase inhibition by EPN
(0-ethyl-0-p-nitrophenyl phenylphosphonothioate) and Delnav.
Toxicol. appl. Pharmacol. (In press)
Dunn, C. L. (1958) Determination of 2,3-p-dioxanedithiol S,S-bis (0,0
-diethyl phosphorodithioate). J. Agr. Food Chem., 6: 203-209
Frawley, J. P., Weir, R., Tusing, T., DuBois, K. P. and Calandra, J.
C. (1963) Toxicologic investigations on Delnav(R) Toxicol. appl.
Pharmacol., 5: 605-624
Gaines, T. B. (1960) The acute toxicity of pesticides to rats.
Toxicol. appl. Pharmacol., 2: 88-99
Gunther, F. A., Jeppson, L. R., Barkley, J. H., Elliott, L. M. and
Blinn, R. C. (1958) Persistence of residues of 2,3-p-dioxanedithiol
S,S-bis(0,0-diethyl phosphorodithioate) as an acaricide on and in
mature lemons and oranges. J. Agr. and Food Chem., 6: 210-211
Hagan, E. C., Jenner, R. M. and Fitzhugh, O. G. (1961) Acute oral
toxicity and potentiation studies with anticholinesterase compounds.
Fed. Proc., 20: 432
Hercules. (1958-1961) Hercules Powder Co. Inc. Unpublished reports on
Delnav residues
Jackson, J. B., Radeleff, R. D., Roberts, R. H. et al. (1962) Acute
toxicity of Delnav and its residues in tissues of livestock. J. Econ.
Entomol., 55: 669-702
Kennedy, G., Frawley, J. P. and Calandra, J. C. (1968) Multigeneration
reproduction study in rats fed Delnav, Herban and Toxaphene. Toxicol.
appl. Pharmacol. (In press)
Mills, P. A., Onley, J. H. and Gaither, R. A. (1963) Rapid method for
chlorinated pesticide residues in nonfatty foods. J. Assoc. Offic.
Agric. Chem., 46: 186-191
Murphy, S. D. (1966) Response of adaptive liver enzymes to acute
poisoning by organophosphate insecticides. Toxicol. appl. Pharmacol.,
8: 266-276
Pardue, J. R. and Watts, R. R. (1968) U.S. Food and Drug
Administration. Private communication
Plapp, F. W., Bigley, W. S. and Darrow, D. I. (1960) Studies on the
metabolism and residues of P32-labelled Delnav in Hereford steer.
J. Econ. Entomol., 53: 60-64
Watts, R. R., Storherr, R. W., Pardue, J. R. et al. (1968a) A widely
applicable charcoal column cleanup method for organophosphorus
pesticide residues in crop extracts. Presented at the 1968 annual
meeting of the Association of Official Analytical Chemists,
Washington, D.C., Oct. 14, 1968
Watts, R. R. and Storherr, R. W. (1968b) Gas chromatographic
determination of organophosphorus pesticide residues. General aspects
of potassium thermionic detection, retention times and response data
for three columns. Presented at the 1968 annual meeting of the
Association of Official Analytical Chemists, Washington, D.C., Oct.
14, 1968
Zaratzian, V. L., Arnault, L. T., Michel, T. C. and Fitzhugh, O. G.
(1961) Effects of organic phosphates Delnav and Malathion in the dog.
Fed. Proc., 20: 432
