
FAO/PL:1968/M/9/1
WHO/FOOD ADD./69.35
1968 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
Issued jointly by FAO and WHO
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Committee on Pesticide Residues, which met in Geneva, 9-16 December,
1968.
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
WORLD HEALTH ORGANIZATION
Geneva, 1969
FENCHLORPHOS
IDENTITY
Chemical names
0,0-dimethyl 0-2,4,5-trichlorophenyl phosphorothioate;
0,0-dimethyl 0-(2,4,5-trichlorophenyl) phosphorothioate
(IUPAC)
Synonyms
Ronnel, Ectoral(R), Etrolene(R), Nankor(R), Trolene(R), Korlan(R)
Formula
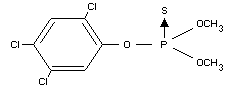 Other information on identity and properties
Technical fenchlorphos is a light tan solid melting at 34-37°C (41°C
when pure). It has a mild mercaptan odour. It is stable at
temperatures up to 60°C and in neutral and slightly acid media. It is
unstable in alkaline and strongly acid media and upon prolonged
exposure in aqueous preparations. In solution, most cations other than
copper have little or no effect. At room temperature, the compound is
soluble in most organic solvents and is practically insoluble in
water.
The technical product is supplied in a variety of concentrations and
formulations (emulsifiable concentrate, wettable powder, mineral
blocks, mineral granules, medicated premix).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
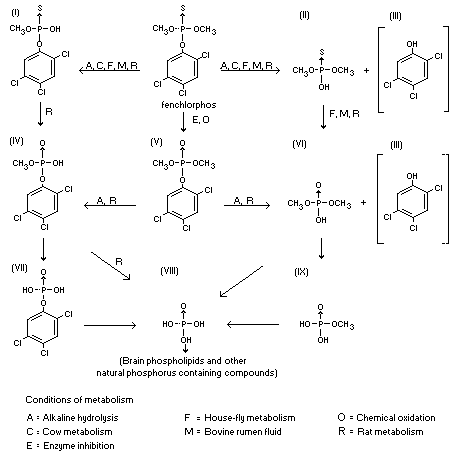
Fenchlorphos and its oxygen analogue are susceptible to hydrolysis in
animals at either the methyl-phosphate or phenylphosphate bond.
Hydrolysis at both sites has been demonstrated with alkali and bovine
rumen juice; and in rat, house-fly, and cow metabolic studies (Plapp
et al., 1958a, 1958b; Menzie, 1966). The following pathways in animals
are suggested from these studies:
Other information on identity and properties
Technical fenchlorphos is a light tan solid melting at 34-37°C (41°C
when pure). It has a mild mercaptan odour. It is stable at
temperatures up to 60°C and in neutral and slightly acid media. It is
unstable in alkaline and strongly acid media and upon prolonged
exposure in aqueous preparations. In solution, most cations other than
copper have little or no effect. At room temperature, the compound is
soluble in most organic solvents and is practically insoluble in
water.
The technical product is supplied in a variety of concentrations and
formulations (emulsifiable concentrate, wettable powder, mineral
blocks, mineral granules, medicated premix).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Fenchlorphos and its oxygen analogue are susceptible to hydrolysis in
animals at either the methyl-phosphate or phenylphosphate bond.
Hydrolysis at both sites has been demonstrated with alkali and bovine
rumen juice; and in rat, house-fly, and cow metabolic studies (Plapp
et al., 1958a, 1958b; Menzie, 1966). The following pathways in animals
are suggested from these studies:
 P32-labelled fenchlorphos was given orally to rats at a dose level of
100 mg/kg body-weight. The highest concentration was reached in the
tissues after 12 hours. The residues persisted for more than seven
days and were found to consist of fenchlorphos and its oxygen
analogue. These compounds were hydrolysed at either the
methyl-phosphate or the phenyl-phosphate linkage and the resulting
phosphoric acids were excreted in the urine. In the rat approximately
60 per cent of the administered dose was excreted within two days. In
a cow treated orally with 100 mg/kg body-weight the metabolism was
identical to that in the rat but excretion in the urine did not occur
as rapidly. After the first day, 20 per cent of the dose had been
excreted and 50 per cent after six days. A milk sample taken eight
hours after treatment contained 30 ppm of fenchlorphos but this level
had decreased to 0.4 ppm after seven days. A calf fed this milk at
various intervals never showed more than 1.5 ppm of fenchlorphos and
metabolites in its blood, nor was there any depression in
cholinesterase level (Plapp and Casida, 1958a).
Sheep given oral doses of 100 mg/kg of P32-labelled fenchlorphos
attained the highest concentrations in the blood after four hours.
Within 21 days 70 per cent of the administered dose had appeared in
the urine and seven per cent in the faeces. The concentration in the
fat fell from about 30 ppm after one week to less than 1 ppm after six
weeks (Millar, 1965).
The oxygen analogue of fenchlorphos is a more potent cholinesterase
inhibitor than fenchlorphos itself, but the principal hydrolytic
mammalian metabolites are poor inhibitors of cholinesterase being
equal to or less active than the parent compound (Leng, 1958a; Smith,
1968).
Acute toxicity (oral)
LD50 (mg/kg
Animal Sex body-weight) References
Mouse Female 2140 McCollister et al.,
1959
Rat Male 1740 "
Rat Female > 2000 "
Rat Male 1250 Gaines, 1960
Rat Female 2630 Gaines, 1960
Guinea-pig Male 3140 McCollister et al.,
1959
Rabbit Male and 640 "
female
Dog Male and > 500 "
female (causes emesis)
Short-term studies
Rat. Groups of 20 rats (10 of each sex) were fed 0.5, 1.5, 5, 15 and
50 mg/kg/day of fenchlorphos in their diets for 105 days. Mortality
records, food consumption, growth and final average body and organ
weights were closely comparable in experimental and control groups.
Histologic examination revealed no evidence of adverse effects
attributable to fenchlorphos, except in the male and female rats at
the 50 mg/kg/day level where there was some evidence in the liver of a
slight granular degeneration or cloudy swelling of the parenchymal
cells of the entire lobule. In the kidneys, there appeared to be some
cloudy swelling and vacuolation of the renal tubular epithelium with
very slight interstitial nephritis. Following 105-day administration
of fenchlorphos in the diet, all rats, including those that had
received the 50 mg/kg/day level, showed essentially complete recovery
of any depressed cholinesterase activity within six to eight weeks
after being placed on the control ration only. Microscopic examination
of the tissues from the animals fed 50 mg/kg/day, sacrificed after 30
and 45 days on the recovery ration, showed no evidence of any residual
pathological effects (McCollister et al., 1959).
Dog. One female dog per dosage level was fed 10, 30, 100 and 300 ppm
fenchlorphos in the diet for 12 months. All dogs were normal in
behaviour, appetite and growth. Haematological findings, final body
and organ weights and terminal blood urea nitrogen values showed no
significant deviations from the control. Microscopic examination of
all major organs revealed no evidence of detrimental effects
attributable to the test compound. However, the general pattern of
cholinesterase measurements indicated no significant changes in plasma
or red blood cell cholinesterase activity in the dogs that received
the equivalent of the 10, 30 or 100 ppm of fenchlorphos in their diet,
although there was significant depression at higher levels
(McCollister et al., 1959).
In another study, groups of dogs (four of each sex) were fed
fenchlorphos in their diet twice daily, six days a week for two years
at dosage levels of 1, 3 or 10 mg/kg/day. No evidence of adverse
effect was seen as judged by body-weight gain, food intake, general
appearances and behaviour, routine clinical laboratory investigations,
organ weight analysis, gross and microscopic examination of the
tissues, and brain or red cell cholinesterase activity. Plasma
cholinesterase activity in the dogs receiving 10 mg/kg/day was
consistently significantly below that of the controls. The depression
at the 3 mg/kg/day dosage was significant on only 50 per cent of the
occasions tested, and only very rarely in the dogs fed 1 mg/kg/day.
The over-all average of plasma cholinesterase activity measurements
for the dogs fed the 3 mg/kg/day dose level was 82 per cent of the
pre-treatment control activity (Noel et al., 1965).
Long-term studies
Rat. Groups of rats each containing 10 or 15 males and 10 or 15
females were fed diets containing 0.5, 1.5, 5, 15 and 50 mg/kg/day of
fenchlorphos for periods up to two years. All groups appeared normal
in general appearance and behaviour. Growth curves, mortality records,
haematological data, food consumption and organ weights showed no
significant differences from the control groups. In the rats fed 50
mg/kg/day histological examination of both sexes revealed central
lobular, granular degeneration and some necrosis of the parenchymal
cells in the liver, and evidence of cloudy swelling of tubular
epithelium in the kidneys together with interstitial nephritis. No
gross or histological abnormalities were observed in the rats fed dose
levels below 50 mg/kg/day. After two years no significant inhibition
of red blood cell or brain cholinesterase was observed in rats of
either sex receiving fenchlorphos at levels of 5 mg/kg/day or lower.
Male rats were more sensitive to plasma cholinesterase inhibition than
wore females, the no-effect levels being 5 mg/kg/day and 0.5 mg/kg/day
respectively (McCollister et al., 1959).
Special studies
(a) Reproduction
Groups of eight male and 16 female rats were maintained on diets
containing 100 or 300 ppm of fenchlorphos through three generations
with two litters per generation. Fertility, reproduction and lactation
were normal as judged by indices of fertility, gestation, viability
and lactation, body-weight records and teratological examination of
foetuses. A similar group of rats maintained at a level of 1000 ppm
through three generations showed no significant effects except for the
viability and lactation indices in the F1b and F2b generations, and
slightly depressed average body-weights. All three dietary
concentrations lowered plasma cholinesterase activity. Red cell
cholinesterase was not depressed at the 100 ppm dietary level;
however, depression was seen at the 300 and 1000 ppm dietary levels
(McCollister et al., 1967).
(b) Potentiation
Single oral dosages of fenchlorphos were administered jointly to rats
with 10 other commercial organo-phosphorus insecticides. No
significant potentiation problem was seen for such combinations under
ordinary conditions of field application and use of fenchlorphos
(McCollister et al., 1959). Dietary combinations of fenchlorphos (100
ppm) with EPN (20 or 70 ppm) or malathion (100 ppm) fed to dogs for 10
weeks produced additive or less than additive red blood cell or plasma
cholinesterase inhibition effects (Keller, 1961).
(c) Neurotoxicity
Nineteen mature hens were given intraperitoneal injections of either
2000 or 3000 mg/kg of fenchlorphos dissolved in diethyl succinate. The
surviving hens (10 of the 16 given 3000 mg/kg) showed no neurotoxic
effects within the 90-day observation period following treatment
(Hymas, 1961).
(d) Studies on the metabolite, 2,4,5-trichlorophenol
Groups of young male and female rats were fed dietary levels of 100,
300, 1000, 3000 and 10 000 ppm of 2,4,5-trichlorophenol for 98 days.
Records were kept concerning appearance, behaviour, mortality, food
consumption, body and organ weights and terminal haematological tests
(urea nitrogen, leucocyte counts, haematocrits and haemoglobin
values). No evidence of adverse effects was noted at the 1000 ppm
levels or less. At 3000 and 10 000 ppm the rats showed diuresis and
slight pathological changes of the kidney and liver, and at the 10 000
ppm level there was a slight decrease in growth (McCollister et al.,
1961).
Observations in man
Twenty human subjects were fed varying amounts of fenchlorphos in 250
mg portions over a period of three to seven days. Ten of these
volunteers received a total of 2500 mg each over three days, for an
average of about 11.9 mg/kg/day. Seven individuals discontinued the
treatment at the end of three days because of side effects which
included sporadic abdominal cramps, anorexia, blurred vision,
diarrhoea, headache, heartburn, malaise, nausea and weakness. The
three remaining individuals, who suffered no symptoms, received a
total of 9500 mg each over seven days, for an average of 19.4
mg/kg/day. Red blood cell cholinesterase depression and marked plasma
cholinesterase depression occurred in the latter three cases after 72
hours, one and two weeks. In another test, 10 persons received a total
of 3000 mg each over four days for an average of 10.7 mg/kg/day with
no symptoms related to the treatment. Another group of nine
individuals under treatment for schistosomiasis received the 10.7
mg/kg/day for 14 days without any effect on red blood cell
cholinesterase. Plasma cholinesterase was reduced in both tests,
markedly in eight of them after 72 hours. No other clinical signs or
symptoms of toxicity due to the treatments were seen in
haematological, liver function and urinalysis tests (Gould, 1961;
Slomka, 1964).
Comments
On the basis of many short- and long-term experiments on animals
including reproduction studies, the tolerability of relatively high
doses is evident without ascertaining any irreversible changes in the
organism. These data confirm experience from medical treatment of
humans. An additive effect with some organo-phosphorus compounds was
reported. Possible effect on aliesterase activity was not followed up.
For the present the most sensitive reported effect from oral doses of
fenchlorphos is the inhibition of cholinesterase activity. The
no-effect level for red blood cell cholinesterase inhibition in dogs
and rats is 3-5 mg/kg/day, for plasma cholinesterase inhibition it
varies from 0.5-5 mg/kg/day. The data from human volunteers are
inadequate.
TOXICOLOGICAL EVALUATION
Levels causing no significant toxicological effect
Rat: 0.5 mg/kg per day;
Dog: 1 mg/kg per day.
Estimate of acceptable daily intake for man
0-0.01 mg/kg body-weight.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Fenchlorphos is used to control ectoparasites, such as horn-flies,
screw-worms, ticks, fleece-worms (fly strike), lice and sheep keds of
livestock (swine, cattle, sheep). Premises such as farm buildings,
food processing plants, restaurants, supermarkets, food-packing
plants, dwellings, yards, parks and outdoor areas are treated with
fenchlorphos for control of flies, mosquitos, roaches, bed-bugs,
silverfish, fleas, ticks, spiders and ants. Applications are made in
the form of sprays, "pour-ons", dips, dusts, aerosols, cattle
backrubbers, oral drenches, smears and baits (Anon., 1967). Sprays are
used at one-half to two per cent fenchlorphos for buildings and
yards, wetting them sufficiently to result in a dose of approximately
216-864 mg/m2 of surface area. Coverage of livestock with
three-quarter per cent spray or less results in doses of less than 25
mg/kg of body-weight. The Dow Chemical Co. is preparing new
recommendations for withdrawal intervals between treatment and
slaughter for cattle, sheep and goats. At present, withdrawal periods
range from two to 12 weeks between last treatment and slaughter. Beef
cattle and dairy heifers are fed insecticide mineral blocks or
granules containing 5.5 per cent fenchlorphos for the control of horn
flies and cattle grubs. Cattle grub control is also obtained by
feeding cattle for seven consecutive days on food treated with 0.6 per
cent fenchlorphos (18 mg/kg/day).
The registered uses for fenchlorphos in the United States of America
are given in Anon. (1966, 1968). The registered plant uses of
fenchlorphos are for most fruit and vegetables in the Netherlands and
for bananas in the United States of America and Canada.
Residues resulting from supervised trials
Residues of fenchlorphos accumulate to a high degree in the fat of
animals and tend to be retained there. Most residue data were
therefore determined in the fat (Plapp and Casida, 1958; Ivey et al.,
1967). It has not been determined whether the oxygen analogue is
similarly stored. Several studies, utilizing cholinesterase inhibition
for analysis, indicated that appreciable residues of the oxygen
analogue did not occur. Verification of this point with modern
instrumentation (glc) would be desirable.
Residues from typical supervised trials are summarized in the
following table:
Dosage No. or duration Days after Residues
Treatment Animal active of last in Reference
ingredient treatments treatment tissue* ppm
Spray Cattle 1%, 1 gal 1 7 3.0-7.5 )
fat )
> Anon., 1959
14 0.66-1.6 )
fat )
Tip-spray Sheep 0.4%, 600 ml 1 7 0.35 fat )
> Miller, 1965
14 0.04 fat )
Back-rubber Cattle 1 or 2% for 28 days 14 0.0 fat, Ivey et al.,
int. 1967
organs
Pour-on Calf 4 oz. 5% 5 times 7 0.23-0.45) Dishburger
formulation 3 week fat ) et al., 1966
each time intervals 14 0.0-0.21 )
fat )
21 0.0-0.04 )
fat
Dip Sheep 0.5% 3 times, 28 5.7 fat )
2 week > Anon., 1959
intervals 43 1.2 fat )
Bedding Pig 5% for 28 0-14 0.0-0.03 )
granules days fat > Teekell
) et al., 1963
(continued)
Dosage No. or duration Days after Residues
Treatment Animal active of last in Reference
ingredient treatments treatment tissue* ppm
Litter Hen 5% granules ) 0.0-0.04 ) Miller, 1962;
) egg yolk )
) for 10-50 )
4% emulsion ) days 0.0 egg ) Smith at al.,
) white ) 1965;
5% dust ) 0.0 ) Dishburger &
liver, ) Rice, 1965
muscle )
Spray Cow 2 qts 0.5% 1 1 0.21 milk)
5 0.05 milk) Claborn et al.,
10 0.00 milk) 1965
Oral Cow 145 mg/kg 1 1 0.47 milk)
3 0.02 milk) Leahy, 1960
Back-rubber Cow 2% in oil 4 times < 0.01 milk Ivey et al., 1967
daily for
14 days
Barn Cow 36 mg/ft2 1 - 0.02 milk Leng, 1958b
* If one figure is listed it is a maximum value.
Fate of residues
In animals
After administration of P32-labelled fenchlorphos to a cow, the
highest levels of radioactivity appeared in the fat, kidneys and
lungs. In another test, 18 mg/kg/day was fed for seven days to cattle
and the concentration in the blood fell to 0.01-0.05-ppm 72 hours
after feeding was stopped.
The major measurable residue occurring in significant quantities is
fenchlorphos. Residues of the oxygen analogue, if present, appear to
be negligible in cows (Plapp and Casida, 1958).
Evidence of residues in food, in commerce or at consumption
Analyses were made in 1968 by the United States Department of
Agriculture of 149 tissue samples from slaughter houses located in
Louisiana, Texas, Oklahoma, Indiana and Nebraska (Stewart,1968). From
0.01-0.10 ppm of fenchlorphos was found in only four of the samples. A
level of 0.25 ppm in tissues was used as the working tolerance
limitation for organo-phosphorus insecticides.
In total diet studies conducted by the United States Food and Drug
Administration between June 1964 and June 1968 on 104 composites in
each of four food classes, only trace amounts (trace = <0.0005 ppm)
were found in about one per cent of the composites (Duggan. 1968).
Methods of residue analysis
(For key to metabolite numerals, see above chart)
Enzymatic inhibition
Although fenchlorphos is a poor inhibitor of cholinesterase in
vitro, it can be converted by treatment with bromine into the oxygen
analogue which is a potent cholinesterase inhibitor. Metabolites I,
III, IV and VII are poor inhibitors of fly head cholinesterase using
the colorimetric method described by Ellman et al. (1961). Presumably,
metabolites II, VI, VIII and IX are also poor inhibitors.
Analytical methods based on the high sensitivity of the manometric
measurement of cholinesterase inhibition of the oxygen analogue were
used to detect residues of fenchlorphos. Sensitivities ranged from
0.005-0.5 ppm in analyses of milk, fat, blood, egg white and egg yolk.
Beam and Hankinson (1964) have described a colorimetric cholinesterase
inhibition assay for fenchlorphos and other pesticides in milk. A
variety of other esterase methods for fenchlorphos, some utilizing
paper or thin-layer chromatography, have also been reported (McKinley
and Read, 1962; McKinley and Johal, 1963; Coffin and Savary, 1964;
el-Refai and Hopkins, 1965). While some of these methods of analysis
are very sensitive to fenchlorphos and its oxygen analogue, they are
not suitable for routine regulatory purposes.
2,4,5-Trichlorophenol colorimetric detection method
Fenchlorphos can be determined by hydrolysis to the
2,4,5-trichlorophenol moiety, which is in turn analysed by the
4-aminoantipyrine colorimetric method. The method, with various
modifications, has a sensitivity of 0.02-0.1 ppm in pea pods, pea
vines, peas, tomatoes, bananas and in body tissues of sheep and cattle
(Duggan et al., 1967; Claborn and Ivey, 1964).
Tapernoux and Magat (1963) have described a method based on
ultra-violet spectrometry of the phenol for residues of fenchlorphos
in beef, meat and fat. Most colorimetric methods for fenchlorphos do
not distinguish between the phenol (III) and the parent compound and
give, therefore, a maximum limit rather than an actual quantity.
Gas chromatographic methods
A gas chromatographic method of analysis for fenchlorphos appears to
be the method of choice based on accuracy, specificity, sensitivity
and speed. Gas chromatographic methods utilizing a large variety of
detectors (micro-coulometric, electron-capture, thermionic, flame
photometric, microwave-powered emission) for the determination of
fenchlorphos in many foods have been described (Bache and Lisk, 1967;
Burke and Holswade, 1964, 1966; Burke and Giuffrida, 1964; Egan et
al., 1964; Gehrt, 1967; Horiguchi et al., 1964; Moye, 1967; Nelson,
1966; Onley and Bertuzzi, 1966; Stevens, 1967). Sensitivities are in
the order of 0.001-0.1 ppm.
Gas chromatography multi-pesticide residue studies by a number of FDA
laboratories of the United States of America, using an
electron-capture detector (ECGC), validated a sensitivity of about
0.01 ppm for fenchlorphos in apples, oats, cabbage, peppers, grapes,
feed pellets, green peppers, red cabbage, rutabagas, olive oil, and
cheese (Wells, 1967).
Fenchlorphos residues have also been determined by use of ECGC with a
sensitivity of 0.001 ppm in milk and 0.005 ppm in body tissues from
cattle after proper extraction and clean-up methods. Fenchlorphos
recoveries of 87 per cent from milk, 77 per cent from fat and 85-94
per cent from other tissues may be obtained (Claborn and Ivey, 1965).
Hydrolysis of fenchlorphos and its phenol metabolites may be used to
determine the phenol. Wessel (1967, 1968) reported on a collaborative
study of the accuracy of residue determinations for fenchlorphos added
to lettuce and apples using the potassium chloride thermionic
detection (KCITD) and ECGC. Average recoveries were 91-94 per cent
with KCITD and 94-98 per cent by ECGC for residues of fenchlorphos
added at the 0.5 ppm level to apples and the 5.0 ppm level to lettuce.
There is little doubt that several of the methods cited may be useful
for either regulatory or referee purposes (but only for the parent
compound or its phenol hydrolysis product).
The lack of a quantitative residue method to analyse for the oxygen
analogue of fenchlorphos, which has been mentioned in metabolic
studies, has been noted. The determination of this compound by flame
photometric gas chromatography with virtually no clean-up can probably
be accomplished by the general procedure described by Beroza and
Bowman (1968); with other detectors, a clean-up would probably be
necessary. Clean-up of milk and crude crop extracts by sweep
co-distillation prior to determining fenchlorphos by gas
chromatography with thermionic detection has been reported (Storherr
and Watts, 1965; Watts and Storherr, 1967) and is undoubtedly useful
for this purpose.
Thin-layer chromatography
Methods for the separation and detection of fenchlorphos and other
organo-phosphorus insecticide residues on thin layer chromatograms
have been described by Abbott et al. (1967), Bunyan (1964), el-Refai
and Hopkins (1965), Fischer (1968), Kovacs (1964), Ragab (1967, 1967a)
and Rahn and Urban (1964). Such methods can be valuable for
confirmation of identity.
National tolerances
Country Crop Tolerance (ppm)
Canada Bananas (peel) 0.5
Netherlands Fruit, vegetables (except
potatoes), unprocessed
cereals, unsifted flour 0.4
United States of Bananas (peel) 0.5
America
Fenchlorphos is currently registered for use on a "no residue" basis
for animal applications and/or building pest control operations in the
following areas:
United States Japan United Kingdom of
of America Korea Great Britain and
Canada Australia Northern Ireland
Argentina New Zealand Germany
Brazil Italy Switzerland
Uruguay France Union of South Africa
India Finland Algeria
Republic of Sweden Ivory Coast
China (Taiwan) Netherlands Jordan
Malaysia Spain Israel
Viet-Nam Portugal Turkey
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Appraisal
This compound is used in many countries to control ectoparasites of
livestock; it is fed to animals, applied externally, and used on
premises. At present its application on plants is very limited, and
adequate residue data and use patterns were not available for study
for the purpose of recommending tolerances for raw agricultural
plant products.
Residues resulting from animal treatment consist essentially of
fenchlorphos although there may be some oxygen analogue and its
phenolic hydrolysis product present. Good agricultural practice
results in residues for which a 7.5 ppm tolerance of fenchlorphos in
the fat of meat is required. This residue is well within limits set by
the acceptable daily intake (0.01 mg/kg) because fat of meat comprises
a small fraction of the total diet.
Residues in milk diminished to about 0.02 ppm three or four days after
dosing dairy cow at rates of intake higher than normal.
Yolk of eggs from treated hens (dust or emulsifiable concentrate)
contained as much as 0.04 ppm of the insecticide.
Residues of fenchlorphos were not found in appreciable amount in
cattle tissues from slaughter houses and in total diet studies in the
United States of America.
Published gas-liquid chromatography methods of determining residues of
fenchlorphos appear to be adequate for regulatory purposes. One of
these should be selected for regulatory purposes and one should be
evaluated as a referee method.
Residue data are available only from the United States of America and
the United Kingdom. Use pattern and residue data from other countries
have not been submitted.
Recommendations
Temporary tolerances
The following temporary tolerances, to be in effect until 1972, are to
apply to raw agricultural products moving in commerce unless otherwise
indicated. In the case of fruit and vegetables the tolerances should
be applied as soon as practicable after harvest and in any event prior
to actual retail to the public. In the case of commodities entering
international trade, the tolerances should be applied by the importing
country at the point of entry or as soon as practicable thereafter.
Meat (on fat basis, at slaughter) 7.5 ppm
Yolk of eggs 0.05 ppm
Whole milk 0.04 ppm
The above tolerances include the oxygen analogue. In the case of
commodities entering international trade, the tolerances should be
applied by the importing country at the point of entry or as soon as
practicable thereafter.
Further work or information
Required before 30 June 1972:
1. If use of the compound is to be extended to fruit and vegetables,
data from several countries on the required rates and frequencies of
application, pre-harvest intervals, and the resultant residues.
2. Data from several countries other than the United States of America
and the United Kingdom on animal use patterns and resultant residues.
3. Data on residue levels in raw agricultural products moving in
commerce.
4. Data from countries other than the United States of America on
residue levels found in total diet studies.
5. Comparative evaluation of methods of analysis for regulatory
purposes.
Desirable:
1. Collaborative studies to establish a referee method.
2. Estimation of effect on aliesterase activity in animals and man.
3. Determination of no-effect level with respect to cholinesterase
activity in man.
4. Data on the fate of the trichlorophenol metabolite.
5. More adequate human data.
REFERENCES
Abbot, D. C., Burridge, A. S., Thomson, J. and Webb K. S. (1967) A
thin-layer chromatographic screening test for organophosphorus
pesticide residues. Analyst, 92: 170-175
Anon. (1959) Residue data in support of application of KORLAN(R) for
use on livestock. The Dow Chemical Company, Midland, Michigan.
Unpublished report
Anon. (1966) Ronnel, U.S.D.A. Summary of registered agricultural
pesticide chemical uses. Issued October 1, 1966. Washington, D.C.
Anon. (1967) Suggested guide for the use of insecticides to control
insects affecting crops, livestock, households, stored products,
forests and forest products, 1967. Agr. Handbook No. 331, U.S.D.A.,
Supt. of Documents, U.S. Government Printing Office, Washington, D.C.
20402
Anon. (1968) Technical Information Bulletin. 1968 registered livestock
and household uses for Ronnel and registered livestock uses for
"RUELENE". The Dow Chemical Company, Midland, Michigan 48640
Bache, C. A. and Lisk, D. J. (1967) Selective residue determination of
sulfur-, halogen- and phosphorus-containing pesticides by
helium-plasma emission spectrometry. J. Ass. Offic. Anal. Chem., 50:
1246-1250
Beam, J. E. and Hankinson, D. J. (1964) Application of the
acetyl-cholinesterase inhibition method for detecting organophosphate
residues and elated compounds in milk. J. Dairy Sci., 47: 1297-1305
Beroza, M. and Bowman, M. C. (1968) Gas chromatography of pesticide
residues containing phosphorus or sulfur with the flame photometric
detector. Environmental Sci. Technol., 2: 450-457
Brody, S. S. and Chaney, J. E. (1966) Flame photometric detector. The
application of a specific detector for phosphorus and for sulfur
compounds sensitive to subnanogram quantities. J. Gas Chromatogr.,
4: 42-46
Bunyan, P. J. (1964) The detection of organo-phosphorus pesticides on
thin-layer chromatograms. Analyst, 89: 615-618
Burke, J. and Giuffrida, L. (1964) Investigations of electron capture
gas chromatography for analysis of multiple chlorinated pesticide
residues in vegetables. J. Ass. Offic. Agr. Chem., 47: 326-345
Burke, J. and Holswade, W. (1964) Gas chromatography with
microcoulometric detection for pesticide residue analysis. J. Ass.
Ofic. Agr. Chem., 47: 845-859
Burke, J. A. and Holswade, W. (1966) A gas chromatographic column for
pesticide residue analysis: Retention times and response data. J. Ass.
Offic. Anal. Chem., 49: 374-385
Claborn, H. V. and Ivey, M. C. (1964) Colorimetric determination of
nemacide and Ronnel in animal tissues. J. Ass. Offic. Agr. Chem.,
47: 871-875
Claborn, H. V. and Ivey, M. C. (1965) Determination of
0,0-dimethyl 0-2,4,5-trichlorophenyl phosphorothioate in animal
tissues and milk. J. Agr. Food Chem., 13: 353-354
Claborn, H. V., Mann, H. D., Berry, I. L. and Hoffman, R. A. (1965)
Comparisons of residues in milk resulting from two types of spray
applications of DDT, Shell compound 4072 and Ronnel. J. Econ.
Entomol., 58:922-923
Coffin, D. E. and Savary, G. (1964) Procedure for extraction and
clean-up of plant material prior to determination of organophosphate
residues. J. Ass. Offic. Agr. Chem., 47: 875-881
Dishburger, H. J. and Rice, J. R. (1965) Residue analysis for Ronnel
in tissues from chickens confined on litter treated with KORLAN(R).
The Dow Chemical Company, Lake Jackson, Texas. Unpublished report
Dishburger, H. J., Rice, J. R. and Muniza, R. A. (1966) Ronnel
residues in the fat of cattle following multiple pour-on applications
of KORLAN(R). The Dow Chemical Company, Lake Jackson, Texas.
Unpublished report
Duggan, R. E. (1968) U.S. Dept. of Health, Education and Welfare, Food
and Drug Administration, Washington, D.C. Private communication
Duggan, R. E., Barry, H. C., Johnson, L. Y. and Williams, S. (1967)
Pesticide Analytical Manual, vol. II. Methods for individual pesticide
residues (Ronnel). U.S. Dept. of Health, Education and Welfare, Food
and Drug Administration, Washington, D.C. (July, 1967 revision)
Egan, H., Hammond, E. W. and Thomson, J. (1964) The analysis of
organo-phosphorus pesticide residues by gas chromatography. Analyst,
89: 175-178
Ellman, G. L., Courtney, D. K., Andres, Jr, V., Featherstone, R.M.
(1961) A new and rapid colorimetric determination of acetyl-
cholinesterase activity. Biochem. Pharmacol., 7: 88-95
el-Refai, A., and Hopkins, T. L. (1965) Thin-layer chromatography and
cholinesterase detection of several phosphorothiono insecticides and
their oxygen analogs. J. Agr. Food Chem., 13: 477-481
Fischer, R. (1968) Identification and quantitative estimation of
organophosphorus insecticides in biological material. III. Arch.
Toxicol., 23: 129-135
Gaines, T. B. (1960) The acute toxicity of pesticides to rats.
Toxicol. appl. Pharmacol., 2: 88-99
Gehrt, A. J. (1967) Ultraviolet spectrophotometric and gas
chromatographic methods for Ronnel in feeds. J. Ass. Offic. Anal.
Chem., 50: 257-261
Gould, A. H. (1961) Human volunteer tests concerning the oral toxicity
of Ronnel. Arnold H. Gould, M.D., Washington D.C. Unpublished report
Horiguchi, M., Ishida, M. and Higosaki, N. (1964) Determination of
some organophosphorus insecticides by gas-liquid partition
chromatography. Chem. Pharm. Bull. (Tokyo), 12:(11), 1315-1319
Hymas, T. A. (1961) Checking Ronnel for neurotoxic effect in 20-month
old hens. To G. E. Lynn, The Dow Chemical Co. Unpublished report
Ivey, M. C., Eschle, J. L., Claborn, H. V. and Graham, O. H. (1967)
Ronnel residues in the meat and milk of cattle exposed to
Ronnel-impregnated backrubber used for horn-fly control. J. Econ.
Entomol., 60: 712-716
Keller, J. G. (1961) Ten-week dietary feeding. Dogs. (Potentiation
study) Final report revised. Hazleton Laboratories Inc. Unpublished
report
Kovacs, M. F. (1964) Thin-layer chromatography for organo
thiophosphate pesticide residue determination
Leahy, J. S. (1960) Determination of the rate of excretion of
0,0-dimethyl 0-2,4,5-trichlorophenyl phosphorothioate in cows
milk following oral administration of Ronnel. Nutritional Research
Unit, Huntingdon, England. Unpublished report
Leng, M. L. (1958a) Cholinesterase inhibitory power of various Ronnel
metabolites. The Dow Chemical Co. Unpublished report
Leng, M. L. (1958b) Ultramicrodetermination of 0,0-dimethyl
0-2,4,5-trichlorophenyl phosphorothioate and its phosphate analogue
in milk. The Dow Chemical Company, Midland, Michigan. Unpublished
report
McCollister, D. D., Oyen, F. and Rows, V. K. (1959) Toxicological
studies of 0,0-dimethyl-0-(2,4,5-trichlorophenyl) phosphorothioate
(Ronnel) in laboratory animals. J. Agr. Food Chem., 7: 689-693
McCollister, D. D., Lockwood, D. T. and Rowe, V. K. (1961) Toxicologic
information on 2,4,5-trichlorophenol. Toxicol. appl. Pharmacol., 3:
63-70
McCollister, D. D., Copeland, J. R. and Oyen, F. (1967) Results of
fertility and reproduction studies in rats maintained on diets
containing Ronnel. The Dow Chemical Co. Unpublished report
McKinley, W. P. and Johal, P. S. (1963) Esterase inhibition technique
for the detection of organophosphorus pesticides, II. Simplified
version for routine checking. J. Ass Offic. Agr. Chem., 46: 840-842
McKinley, W. P. and Read, S. I. (1962) Esterase inhibition technique
for the detection of organophosphorus pesticides. J. Ass. Offic. Agr.
Chem., 45: 467-473
Menzie, C. (1966) Metabolism of pesticides. Wildlife No. 96, Fish and
Wildlife Service, U.S. Dept. of Interior, pages 167-169
Millar, K. R. (1965) Residues in tissues of sheep following dosing and
tip spraying with 32P-labelled Ronnel. New Zealand J. Agric. Res.,
8: 302-312
Miller, P. W. (1962) Ronnel in eggs from chickens treated with various
applications of KORLAN(R). The Dow Chemical Company, Midland,
Michigan. Unpublished report.
Moye, H. A. (1962) An improved microwave emission gas chromatography
detector for pesticide residue analysis. Anal. Chem., 39: 1441-1445
Nelson, R. C. (1966) Screening procedure for organothiophosphate
pesticide residues on fruits and vegetables by micro-coulometric gas
chromatography. J. Ass. Offic. Anal. Chem. 49: 763-765
Noel, P. R., Mawdesley-Thomas, L. E., Chesterman, H., Jolly, D. and
Street, A. E. (1965) Ronnel. Chronic toxicity study in the dog. Final
report. Huntingdon Research Centre. Unpublished report
Onley, J. H. and Bertuzzi, P. F. (1966) Rapid extraction procedure for
chlorinated pesticide residues in raw animal tissues and fat and meat
products. J. Ass. Offic. Anal. Chem., 49: 370-374
Plapp, F. W. and Casida, J. E. (1958a) Bovine Metabolism of
organophosphorus insecticides, metabolic fate of 0,0-dimethyl
0-(2,4,5-trichlorophenyl) phosphorothioate in rats and a cow.
J. Agr. Food Chem., 6: 662-667
Plapp, F. W. and Casida, J. E. (1958b) Hydrolysis of the
alkyl-phosphate bond in certain dialkyl aryl phosphorothioate
insecticides by rats, cockroaches and alkali, J. Econ. Entomol., 51:
800-803
Ragab, M. T. H. (1967) Direct fluorescent detection of
organothiophosphorus pesticides and some of their sulfur-containing
breakdown products after thin-layer chromatography. J. Ass. Offic.
Anal. Chem., 50: 1088-1098
Ragab, M. T. H. (1967a) 4-(p-nitrobenzyl) pyridine as a spray
reagent for organophosphorus pesticides and some of their breakdown
products on thin-layer chromatograms. Bull. environmtl. Contamination
Toxicol., 2: 279-288
Rahn, H. W. and Urban, G. (1964) Detection of insecticidal phosphoric
acid esters and their hydrolytic cleavage products. Pharmazie, 19:
597-602
Slokma, M. B. (1964) Toxicity of Ronnel with special reference to
humans. Pitman-Moore Research Center, The Dow Chemical Co. Unpublished
report
Smith, C. T., Shaw, F. R., Anderson, D. L., Callahan, R. A. and
Ziener, W. H. (1965) Ronnel residues in eggs of poultry. J. Econ.
Entomol., 58: 1160-1161
Smith, G. N. (1968) Cholinesterase inhibition of fenchlorphos and
metabolites. The Dow Chemical Co. Unpublished report
Stevens, R. K. (1967) A rapid specific method for the gas
chromatographic determination of organophosphate pesticides in cold
pressed citrus oil. J. Ass. Offic. Anal. Chem., 50: 1236-1242
Stewart, J. H. (1968) U.S. Department of Agriculture, Washington, D.C.
Private communication
Storherr, R. W. and Watts, R. R. (1965) A sweep codistillation cleanup
method for organophosphate pesticides. J. Ass. Offic. Agr. Chem.,
48: 11-54-58
Tapernoux, A. and Magat, A. (1963) Analysis of beef meat and fat for
residues of Ronnel, an organophosphate used in the treatment of bovine
hypodermosis. Ann. Fals. Expert. Chim., 56: (658) 266-270
Teekell, R. A., Rice, J. R. and McGregor, W. S. (1963) Ronnel residues
in pig fat after being on litter treated with KORLAN(R) insecticide
granules. The Dow Chemical Company, Lake Jackson, Texas. Unpublished
report.
Watts, R. R. and Storherr, R. W. (1967) Sweep codistillation clean-up
for milk for determination of organophosphate and chlorinated
hydrocarbon pesticides. J. Ass. Offic. Anal. Chem., 50: 581-585
Wells, C. E. (1967) Validation study of a method for pesticide
residues in foods and animal feeds. J. Ass. Offic. Anal. Chem., 50:
1205-1215
Wessel, J. R. (1967) Collaborative study of a method for multiple
organophosphorus pesticides in non-fatty foods. J. Ass. Offic. Anal.
Chem., 50: 430-439
Wessel, J. R. (1968) Collaborative study of three gas chromatographic
dual detection systems for analysis of multiple chlorinated and
organophosphorus pesticides. J. Ass. Offic. Anal. Chem., 51: 666-675
P32-labelled fenchlorphos was given orally to rats at a dose level of
100 mg/kg body-weight. The highest concentration was reached in the
tissues after 12 hours. The residues persisted for more than seven
days and were found to consist of fenchlorphos and its oxygen
analogue. These compounds were hydrolysed at either the
methyl-phosphate or the phenyl-phosphate linkage and the resulting
phosphoric acids were excreted in the urine. In the rat approximately
60 per cent of the administered dose was excreted within two days. In
a cow treated orally with 100 mg/kg body-weight the metabolism was
identical to that in the rat but excretion in the urine did not occur
as rapidly. After the first day, 20 per cent of the dose had been
excreted and 50 per cent after six days. A milk sample taken eight
hours after treatment contained 30 ppm of fenchlorphos but this level
had decreased to 0.4 ppm after seven days. A calf fed this milk at
various intervals never showed more than 1.5 ppm of fenchlorphos and
metabolites in its blood, nor was there any depression in
cholinesterase level (Plapp and Casida, 1958a).
Sheep given oral doses of 100 mg/kg of P32-labelled fenchlorphos
attained the highest concentrations in the blood after four hours.
Within 21 days 70 per cent of the administered dose had appeared in
the urine and seven per cent in the faeces. The concentration in the
fat fell from about 30 ppm after one week to less than 1 ppm after six
weeks (Millar, 1965).
The oxygen analogue of fenchlorphos is a more potent cholinesterase
inhibitor than fenchlorphos itself, but the principal hydrolytic
mammalian metabolites are poor inhibitors of cholinesterase being
equal to or less active than the parent compound (Leng, 1958a; Smith,
1968).
Acute toxicity (oral)
LD50 (mg/kg
Animal Sex body-weight) References
Mouse Female 2140 McCollister et al.,
1959
Rat Male 1740 "
Rat Female > 2000 "
Rat Male 1250 Gaines, 1960
Rat Female 2630 Gaines, 1960
Guinea-pig Male 3140 McCollister et al.,
1959
Rabbit Male and 640 "
female
Dog Male and > 500 "
female (causes emesis)
Short-term studies
Rat. Groups of 20 rats (10 of each sex) were fed 0.5, 1.5, 5, 15 and
50 mg/kg/day of fenchlorphos in their diets for 105 days. Mortality
records, food consumption, growth and final average body and organ
weights were closely comparable in experimental and control groups.
Histologic examination revealed no evidence of adverse effects
attributable to fenchlorphos, except in the male and female rats at
the 50 mg/kg/day level where there was some evidence in the liver of a
slight granular degeneration or cloudy swelling of the parenchymal
cells of the entire lobule. In the kidneys, there appeared to be some
cloudy swelling and vacuolation of the renal tubular epithelium with
very slight interstitial nephritis. Following 105-day administration
of fenchlorphos in the diet, all rats, including those that had
received the 50 mg/kg/day level, showed essentially complete recovery
of any depressed cholinesterase activity within six to eight weeks
after being placed on the control ration only. Microscopic examination
of the tissues from the animals fed 50 mg/kg/day, sacrificed after 30
and 45 days on the recovery ration, showed no evidence of any residual
pathological effects (McCollister et al., 1959).
Dog. One female dog per dosage level was fed 10, 30, 100 and 300 ppm
fenchlorphos in the diet for 12 months. All dogs were normal in
behaviour, appetite and growth. Haematological findings, final body
and organ weights and terminal blood urea nitrogen values showed no
significant deviations from the control. Microscopic examination of
all major organs revealed no evidence of detrimental effects
attributable to the test compound. However, the general pattern of
cholinesterase measurements indicated no significant changes in plasma
or red blood cell cholinesterase activity in the dogs that received
the equivalent of the 10, 30 or 100 ppm of fenchlorphos in their diet,
although there was significant depression at higher levels
(McCollister et al., 1959).
In another study, groups of dogs (four of each sex) were fed
fenchlorphos in their diet twice daily, six days a week for two years
at dosage levels of 1, 3 or 10 mg/kg/day. No evidence of adverse
effect was seen as judged by body-weight gain, food intake, general
appearances and behaviour, routine clinical laboratory investigations,
organ weight analysis, gross and microscopic examination of the
tissues, and brain or red cell cholinesterase activity. Plasma
cholinesterase activity in the dogs receiving 10 mg/kg/day was
consistently significantly below that of the controls. The depression
at the 3 mg/kg/day dosage was significant on only 50 per cent of the
occasions tested, and only very rarely in the dogs fed 1 mg/kg/day.
The over-all average of plasma cholinesterase activity measurements
for the dogs fed the 3 mg/kg/day dose level was 82 per cent of the
pre-treatment control activity (Noel et al., 1965).
Long-term studies
Rat. Groups of rats each containing 10 or 15 males and 10 or 15
females were fed diets containing 0.5, 1.5, 5, 15 and 50 mg/kg/day of
fenchlorphos for periods up to two years. All groups appeared normal
in general appearance and behaviour. Growth curves, mortality records,
haematological data, food consumption and organ weights showed no
significant differences from the control groups. In the rats fed 50
mg/kg/day histological examination of both sexes revealed central
lobular, granular degeneration and some necrosis of the parenchymal
cells in the liver, and evidence of cloudy swelling of tubular
epithelium in the kidneys together with interstitial nephritis. No
gross or histological abnormalities were observed in the rats fed dose
levels below 50 mg/kg/day. After two years no significant inhibition
of red blood cell or brain cholinesterase was observed in rats of
either sex receiving fenchlorphos at levels of 5 mg/kg/day or lower.
Male rats were more sensitive to plasma cholinesterase inhibition than
wore females, the no-effect levels being 5 mg/kg/day and 0.5 mg/kg/day
respectively (McCollister et al., 1959).
Special studies
(a) Reproduction
Groups of eight male and 16 female rats were maintained on diets
containing 100 or 300 ppm of fenchlorphos through three generations
with two litters per generation. Fertility, reproduction and lactation
were normal as judged by indices of fertility, gestation, viability
and lactation, body-weight records and teratological examination of
foetuses. A similar group of rats maintained at a level of 1000 ppm
through three generations showed no significant effects except for the
viability and lactation indices in the F1b and F2b generations, and
slightly depressed average body-weights. All three dietary
concentrations lowered plasma cholinesterase activity. Red cell
cholinesterase was not depressed at the 100 ppm dietary level;
however, depression was seen at the 300 and 1000 ppm dietary levels
(McCollister et al., 1967).
(b) Potentiation
Single oral dosages of fenchlorphos were administered jointly to rats
with 10 other commercial organo-phosphorus insecticides. No
significant potentiation problem was seen for such combinations under
ordinary conditions of field application and use of fenchlorphos
(McCollister et al., 1959). Dietary combinations of fenchlorphos (100
ppm) with EPN (20 or 70 ppm) or malathion (100 ppm) fed to dogs for 10
weeks produced additive or less than additive red blood cell or plasma
cholinesterase inhibition effects (Keller, 1961).
(c) Neurotoxicity
Nineteen mature hens were given intraperitoneal injections of either
2000 or 3000 mg/kg of fenchlorphos dissolved in diethyl succinate. The
surviving hens (10 of the 16 given 3000 mg/kg) showed no neurotoxic
effects within the 90-day observation period following treatment
(Hymas, 1961).
(d) Studies on the metabolite, 2,4,5-trichlorophenol
Groups of young male and female rats were fed dietary levels of 100,
300, 1000, 3000 and 10 000 ppm of 2,4,5-trichlorophenol for 98 days.
Records were kept concerning appearance, behaviour, mortality, food
consumption, body and organ weights and terminal haematological tests
(urea nitrogen, leucocyte counts, haematocrits and haemoglobin
values). No evidence of adverse effects was noted at the 1000 ppm
levels or less. At 3000 and 10 000 ppm the rats showed diuresis and
slight pathological changes of the kidney and liver, and at the 10 000
ppm level there was a slight decrease in growth (McCollister et al.,
1961).
Observations in man
Twenty human subjects were fed varying amounts of fenchlorphos in 250
mg portions over a period of three to seven days. Ten of these
volunteers received a total of 2500 mg each over three days, for an
average of about 11.9 mg/kg/day. Seven individuals discontinued the
treatment at the end of three days because of side effects which
included sporadic abdominal cramps, anorexia, blurred vision,
diarrhoea, headache, heartburn, malaise, nausea and weakness. The
three remaining individuals, who suffered no symptoms, received a
total of 9500 mg each over seven days, for an average of 19.4
mg/kg/day. Red blood cell cholinesterase depression and marked plasma
cholinesterase depression occurred in the latter three cases after 72
hours, one and two weeks. In another test, 10 persons received a total
of 3000 mg each over four days for an average of 10.7 mg/kg/day with
no symptoms related to the treatment. Another group of nine
individuals under treatment for schistosomiasis received the 10.7
mg/kg/day for 14 days without any effect on red blood cell
cholinesterase. Plasma cholinesterase was reduced in both tests,
markedly in eight of them after 72 hours. No other clinical signs or
symptoms of toxicity due to the treatments were seen in
haematological, liver function and urinalysis tests (Gould, 1961;
Slomka, 1964).
Comments
On the basis of many short- and long-term experiments on animals
including reproduction studies, the tolerability of relatively high
doses is evident without ascertaining any irreversible changes in the
organism. These data confirm experience from medical treatment of
humans. An additive effect with some organo-phosphorus compounds was
reported. Possible effect on aliesterase activity was not followed up.
For the present the most sensitive reported effect from oral doses of
fenchlorphos is the inhibition of cholinesterase activity. The
no-effect level for red blood cell cholinesterase inhibition in dogs
and rats is 3-5 mg/kg/day, for plasma cholinesterase inhibition it
varies from 0.5-5 mg/kg/day. The data from human volunteers are
inadequate.
TOXICOLOGICAL EVALUATION
Levels causing no significant toxicological effect
Rat: 0.5 mg/kg per day;
Dog: 1 mg/kg per day.
Estimate of acceptable daily intake for man
0-0.01 mg/kg body-weight.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Fenchlorphos is used to control ectoparasites, such as horn-flies,
screw-worms, ticks, fleece-worms (fly strike), lice and sheep keds of
livestock (swine, cattle, sheep). Premises such as farm buildings,
food processing plants, restaurants, supermarkets, food-packing
plants, dwellings, yards, parks and outdoor areas are treated with
fenchlorphos for control of flies, mosquitos, roaches, bed-bugs,
silverfish, fleas, ticks, spiders and ants. Applications are made in
the form of sprays, "pour-ons", dips, dusts, aerosols, cattle
backrubbers, oral drenches, smears and baits (Anon., 1967). Sprays are
used at one-half to two per cent fenchlorphos for buildings and
yards, wetting them sufficiently to result in a dose of approximately
216-864 mg/m2 of surface area. Coverage of livestock with
three-quarter per cent spray or less results in doses of less than 25
mg/kg of body-weight. The Dow Chemical Co. is preparing new
recommendations for withdrawal intervals between treatment and
slaughter for cattle, sheep and goats. At present, withdrawal periods
range from two to 12 weeks between last treatment and slaughter. Beef
cattle and dairy heifers are fed insecticide mineral blocks or
granules containing 5.5 per cent fenchlorphos for the control of horn
flies and cattle grubs. Cattle grub control is also obtained by
feeding cattle for seven consecutive days on food treated with 0.6 per
cent fenchlorphos (18 mg/kg/day).
The registered uses for fenchlorphos in the United States of America
are given in Anon. (1966, 1968). The registered plant uses of
fenchlorphos are for most fruit and vegetables in the Netherlands and
for bananas in the United States of America and Canada.
Residues resulting from supervised trials
Residues of fenchlorphos accumulate to a high degree in the fat of
animals and tend to be retained there. Most residue data were
therefore determined in the fat (Plapp and Casida, 1958; Ivey et al.,
1967). It has not been determined whether the oxygen analogue is
similarly stored. Several studies, utilizing cholinesterase inhibition
for analysis, indicated that appreciable residues of the oxygen
analogue did not occur. Verification of this point with modern
instrumentation (glc) would be desirable.
Residues from typical supervised trials are summarized in the
following table:
Dosage No. or duration Days after Residues
Treatment Animal active of last in Reference
ingredient treatments treatment tissue* ppm
Spray Cattle 1%, 1 gal 1 7 3.0-7.5 )
fat )
> Anon., 1959
14 0.66-1.6 )
fat )
Tip-spray Sheep 0.4%, 600 ml 1 7 0.35 fat )
> Miller, 1965
14 0.04 fat )
Back-rubber Cattle 1 or 2% for 28 days 14 0.0 fat, Ivey et al.,
int. 1967
organs
Pour-on Calf 4 oz. 5% 5 times 7 0.23-0.45) Dishburger
formulation 3 week fat ) et al., 1966
each time intervals 14 0.0-0.21 )
fat )
21 0.0-0.04 )
fat
Dip Sheep 0.5% 3 times, 28 5.7 fat )
2 week > Anon., 1959
intervals 43 1.2 fat )
Bedding Pig 5% for 28 0-14 0.0-0.03 )
granules days fat > Teekell
) et al., 1963
(continued)
Dosage No. or duration Days after Residues
Treatment Animal active of last in Reference
ingredient treatments treatment tissue* ppm
Litter Hen 5% granules ) 0.0-0.04 ) Miller, 1962;
) egg yolk )
) for 10-50 )
4% emulsion ) days 0.0 egg ) Smith at al.,
) white ) 1965;
5% dust ) 0.0 ) Dishburger &
liver, ) Rice, 1965
muscle )
Spray Cow 2 qts 0.5% 1 1 0.21 milk)
5 0.05 milk) Claborn et al.,
10 0.00 milk) 1965
Oral Cow 145 mg/kg 1 1 0.47 milk)
3 0.02 milk) Leahy, 1960
Back-rubber Cow 2% in oil 4 times < 0.01 milk Ivey et al., 1967
daily for
14 days
Barn Cow 36 mg/ft2 1 - 0.02 milk Leng, 1958b
* If one figure is listed it is a maximum value.
Fate of residues
In animals
After administration of P32-labelled fenchlorphos to a cow, the
highest levels of radioactivity appeared in the fat, kidneys and
lungs. In another test, 18 mg/kg/day was fed for seven days to cattle
and the concentration in the blood fell to 0.01-0.05-ppm 72 hours
after feeding was stopped.
The major measurable residue occurring in significant quantities is
fenchlorphos. Residues of the oxygen analogue, if present, appear to
be negligible in cows (Plapp and Casida, 1958).
Evidence of residues in food, in commerce or at consumption
Analyses were made in 1968 by the United States Department of
Agriculture of 149 tissue samples from slaughter houses located in
Louisiana, Texas, Oklahoma, Indiana and Nebraska (Stewart,1968). From
0.01-0.10 ppm of fenchlorphos was found in only four of the samples. A
level of 0.25 ppm in tissues was used as the working tolerance
limitation for organo-phosphorus insecticides.
In total diet studies conducted by the United States Food and Drug
Administration between June 1964 and June 1968 on 104 composites in
each of four food classes, only trace amounts (trace = <0.0005 ppm)
were found in about one per cent of the composites (Duggan. 1968).
Methods of residue analysis
(For key to metabolite numerals, see above chart)
Enzymatic inhibition
Although fenchlorphos is a poor inhibitor of cholinesterase in
vitro, it can be converted by treatment with bromine into the oxygen
analogue which is a potent cholinesterase inhibitor. Metabolites I,
III, IV and VII are poor inhibitors of fly head cholinesterase using
the colorimetric method described by Ellman et al. (1961). Presumably,
metabolites II, VI, VIII and IX are also poor inhibitors.
Analytical methods based on the high sensitivity of the manometric
measurement of cholinesterase inhibition of the oxygen analogue were
used to detect residues of fenchlorphos. Sensitivities ranged from
0.005-0.5 ppm in analyses of milk, fat, blood, egg white and egg yolk.
Beam and Hankinson (1964) have described a colorimetric cholinesterase
inhibition assay for fenchlorphos and other pesticides in milk. A
variety of other esterase methods for fenchlorphos, some utilizing
paper or thin-layer chromatography, have also been reported (McKinley
and Read, 1962; McKinley and Johal, 1963; Coffin and Savary, 1964;
el-Refai and Hopkins, 1965). While some of these methods of analysis
are very sensitive to fenchlorphos and its oxygen analogue, they are
not suitable for routine regulatory purposes.
2,4,5-Trichlorophenol colorimetric detection method
Fenchlorphos can be determined by hydrolysis to the
2,4,5-trichlorophenol moiety, which is in turn analysed by the
4-aminoantipyrine colorimetric method. The method, with various
modifications, has a sensitivity of 0.02-0.1 ppm in pea pods, pea
vines, peas, tomatoes, bananas and in body tissues of sheep and cattle
(Duggan et al., 1967; Claborn and Ivey, 1964).
Tapernoux and Magat (1963) have described a method based on
ultra-violet spectrometry of the phenol for residues of fenchlorphos
in beef, meat and fat. Most colorimetric methods for fenchlorphos do
not distinguish between the phenol (III) and the parent compound and
give, therefore, a maximum limit rather than an actual quantity.
Gas chromatographic methods
A gas chromatographic method of analysis for fenchlorphos appears to
be the method of choice based on accuracy, specificity, sensitivity
and speed. Gas chromatographic methods utilizing a large variety of
detectors (micro-coulometric, electron-capture, thermionic, flame
photometric, microwave-powered emission) for the determination of
fenchlorphos in many foods have been described (Bache and Lisk, 1967;
Burke and Holswade, 1964, 1966; Burke and Giuffrida, 1964; Egan et
al., 1964; Gehrt, 1967; Horiguchi et al., 1964; Moye, 1967; Nelson,
1966; Onley and Bertuzzi, 1966; Stevens, 1967). Sensitivities are in
the order of 0.001-0.1 ppm.
Gas chromatography multi-pesticide residue studies by a number of FDA
laboratories of the United States of America, using an
electron-capture detector (ECGC), validated a sensitivity of about
0.01 ppm for fenchlorphos in apples, oats, cabbage, peppers, grapes,
feed pellets, green peppers, red cabbage, rutabagas, olive oil, and
cheese (Wells, 1967).
Fenchlorphos residues have also been determined by use of ECGC with a
sensitivity of 0.001 ppm in milk and 0.005 ppm in body tissues from
cattle after proper extraction and clean-up methods. Fenchlorphos
recoveries of 87 per cent from milk, 77 per cent from fat and 85-94
per cent from other tissues may be obtained (Claborn and Ivey, 1965).
Hydrolysis of fenchlorphos and its phenol metabolites may be used to
determine the phenol. Wessel (1967, 1968) reported on a collaborative
study of the accuracy of residue determinations for fenchlorphos added
to lettuce and apples using the potassium chloride thermionic
detection (KCITD) and ECGC. Average recoveries were 91-94 per cent
with KCITD and 94-98 per cent by ECGC for residues of fenchlorphos
added at the 0.5 ppm level to apples and the 5.0 ppm level to lettuce.
There is little doubt that several of the methods cited may be useful
for either regulatory or referee purposes (but only for the parent
compound or its phenol hydrolysis product).
The lack of a quantitative residue method to analyse for the oxygen
analogue of fenchlorphos, which has been mentioned in metabolic
studies, has been noted. The determination of this compound by flame
photometric gas chromatography with virtually no clean-up can probably
be accomplished by the general procedure described by Beroza and
Bowman (1968); with other detectors, a clean-up would probably be
necessary. Clean-up of milk and crude crop extracts by sweep
co-distillation prior to determining fenchlorphos by gas
chromatography with thermionic detection has been reported (Storherr
and Watts, 1965; Watts and Storherr, 1967) and is undoubtedly useful
for this purpose.
Thin-layer chromatography
Methods for the separation and detection of fenchlorphos and other
organo-phosphorus insecticide residues on thin layer chromatograms
have been described by Abbott et al. (1967), Bunyan (1964), el-Refai
and Hopkins (1965), Fischer (1968), Kovacs (1964), Ragab (1967, 1967a)
and Rahn and Urban (1964). Such methods can be valuable for
confirmation of identity.
National tolerances
Country Crop Tolerance (ppm)
Canada Bananas (peel) 0.5
Netherlands Fruit, vegetables (except
potatoes), unprocessed
cereals, unsifted flour 0.4
United States of Bananas (peel) 0.5
America
Fenchlorphos is currently registered for use on a "no residue" basis
for animal applications and/or building pest control operations in the
following areas:
United States Japan United Kingdom of
of America Korea Great Britain and
Canada Australia Northern Ireland
Argentina New Zealand Germany
Brazil Italy Switzerland
Uruguay France Union of South Africa
India Finland Algeria
Republic of Sweden Ivory Coast
China (Taiwan) Netherlands Jordan
Malaysia Spain Israel
Viet-Nam Portugal Turkey
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Appraisal
This compound is used in many countries to control ectoparasites of
livestock; it is fed to animals, applied externally, and used on
premises. At present its application on plants is very limited, and
adequate residue data and use patterns were not available for study
for the purpose of recommending tolerances for raw agricultural
plant products.
Residues resulting from animal treatment consist essentially of
fenchlorphos although there may be some oxygen analogue and its
phenolic hydrolysis product present. Good agricultural practice
results in residues for which a 7.5 ppm tolerance of fenchlorphos in
the fat of meat is required. This residue is well within limits set by
the acceptable daily intake (0.01 mg/kg) because fat of meat comprises
a small fraction of the total diet.
Residues in milk diminished to about 0.02 ppm three or four days after
dosing dairy cow at rates of intake higher than normal.
Yolk of eggs from treated hens (dust or emulsifiable concentrate)
contained as much as 0.04 ppm of the insecticide.
Residues of fenchlorphos were not found in appreciable amount in
cattle tissues from slaughter houses and in total diet studies in the
United States of America.
Published gas-liquid chromatography methods of determining residues of
fenchlorphos appear to be adequate for regulatory purposes. One of
these should be selected for regulatory purposes and one should be
evaluated as a referee method.
Residue data are available only from the United States of America and
the United Kingdom. Use pattern and residue data from other countries
have not been submitted.
Recommendations
Temporary tolerances
The following temporary tolerances, to be in effect until 1972, are to
apply to raw agricultural products moving in commerce unless otherwise
indicated. In the case of fruit and vegetables the tolerances should
be applied as soon as practicable after harvest and in any event prior
to actual retail to the public. In the case of commodities entering
international trade, the tolerances should be applied by the importing
country at the point of entry or as soon as practicable thereafter.
Meat (on fat basis, at slaughter) 7.5 ppm
Yolk of eggs 0.05 ppm
Whole milk 0.04 ppm
The above tolerances include the oxygen analogue. In the case of
commodities entering international trade, the tolerances should be
applied by the importing country at the point of entry or as soon as
practicable thereafter.
Further work or information
Required before 30 June 1972:
1. If use of the compound is to be extended to fruit and vegetables,
data from several countries on the required rates and frequencies of
application, pre-harvest intervals, and the resultant residues.
2. Data from several countries other than the United States of America
and the United Kingdom on animal use patterns and resultant residues.
3. Data on residue levels in raw agricultural products moving in
commerce.
4. Data from countries other than the United States of America on
residue levels found in total diet studies.
5. Comparative evaluation of methods of analysis for regulatory
purposes.
Desirable:
1. Collaborative studies to establish a referee method.
2. Estimation of effect on aliesterase activity in animals and man.
3. Determination of no-effect level with respect to cholinesterase
activity in man.
4. Data on the fate of the trichlorophenol metabolite.
5. More adequate human data.
REFERENCES
Abbot, D. C., Burridge, A. S., Thomson, J. and Webb K. S. (1967) A
thin-layer chromatographic screening test for organophosphorus
pesticide residues. Analyst, 92: 170-175
Anon. (1959) Residue data in support of application of KORLAN(R) for
use on livestock. The Dow Chemical Company, Midland, Michigan.
Unpublished report
Anon. (1966) Ronnel, U.S.D.A. Summary of registered agricultural
pesticide chemical uses. Issued October 1, 1966. Washington, D.C.
Anon. (1967) Suggested guide for the use of insecticides to control
insects affecting crops, livestock, households, stored products,
forests and forest products, 1967. Agr. Handbook No. 331, U.S.D.A.,
Supt. of Documents, U.S. Government Printing Office, Washington, D.C.
20402
Anon. (1968) Technical Information Bulletin. 1968 registered livestock
and household uses for Ronnel and registered livestock uses for
"RUELENE". The Dow Chemical Company, Midland, Michigan 48640
Bache, C. A. and Lisk, D. J. (1967) Selective residue determination of
sulfur-, halogen- and phosphorus-containing pesticides by
helium-plasma emission spectrometry. J. Ass. Offic. Anal. Chem., 50:
1246-1250
Beam, J. E. and Hankinson, D. J. (1964) Application of the
acetyl-cholinesterase inhibition method for detecting organophosphate
residues and elated compounds in milk. J. Dairy Sci., 47: 1297-1305
Beroza, M. and Bowman, M. C. (1968) Gas chromatography of pesticide
residues containing phosphorus or sulfur with the flame photometric
detector. Environmental Sci. Technol., 2: 450-457
Brody, S. S. and Chaney, J. E. (1966) Flame photometric detector. The
application of a specific detector for phosphorus and for sulfur
compounds sensitive to subnanogram quantities. J. Gas Chromatogr.,
4: 42-46
Bunyan, P. J. (1964) The detection of organo-phosphorus pesticides on
thin-layer chromatograms. Analyst, 89: 615-618
Burke, J. and Giuffrida, L. (1964) Investigations of electron capture
gas chromatography for analysis of multiple chlorinated pesticide
residues in vegetables. J. Ass. Offic. Agr. Chem., 47: 326-345
Burke, J. and Holswade, W. (1964) Gas chromatography with
microcoulometric detection for pesticide residue analysis. J. Ass.
Ofic. Agr. Chem., 47: 845-859
Burke, J. A. and Holswade, W. (1966) A gas chromatographic column for
pesticide residue analysis: Retention times and response data. J. Ass.
Offic. Anal. Chem., 49: 374-385
Claborn, H. V. and Ivey, M. C. (1964) Colorimetric determination of
nemacide and Ronnel in animal tissues. J. Ass. Offic. Agr. Chem.,
47: 871-875
Claborn, H. V. and Ivey, M. C. (1965) Determination of
0,0-dimethyl 0-2,4,5-trichlorophenyl phosphorothioate in animal
tissues and milk. J. Agr. Food Chem., 13: 353-354
Claborn, H. V., Mann, H. D., Berry, I. L. and Hoffman, R. A. (1965)
Comparisons of residues in milk resulting from two types of spray
applications of DDT, Shell compound 4072 and Ronnel. J. Econ.
Entomol., 58:922-923
Coffin, D. E. and Savary, G. (1964) Procedure for extraction and
clean-up of plant material prior to determination of organophosphate
residues. J. Ass. Offic. Agr. Chem., 47: 875-881
Dishburger, H. J. and Rice, J. R. (1965) Residue analysis for Ronnel
in tissues from chickens confined on litter treated with KORLAN(R).
The Dow Chemical Company, Lake Jackson, Texas. Unpublished report
Dishburger, H. J., Rice, J. R. and Muniza, R. A. (1966) Ronnel
residues in the fat of cattle following multiple pour-on applications
of KORLAN(R). The Dow Chemical Company, Lake Jackson, Texas.
Unpublished report
Duggan, R. E. (1968) U.S. Dept. of Health, Education and Welfare, Food
and Drug Administration, Washington, D.C. Private communication
Duggan, R. E., Barry, H. C., Johnson, L. Y. and Williams, S. (1967)
Pesticide Analytical Manual, vol. II. Methods for individual pesticide
residues (Ronnel). U.S. Dept. of Health, Education and Welfare, Food
and Drug Administration, Washington, D.C. (July, 1967 revision)
Egan, H., Hammond, E. W. and Thomson, J. (1964) The analysis of
organo-phosphorus pesticide residues by gas chromatography. Analyst,
89: 175-178
Ellman, G. L., Courtney, D. K., Andres, Jr, V., Featherstone, R.M.
(1961) A new and rapid colorimetric determination of acetyl-
cholinesterase activity. Biochem. Pharmacol., 7: 88-95
el-Refai, A., and Hopkins, T. L. (1965) Thin-layer chromatography and
cholinesterase detection of several phosphorothiono insecticides and
their oxygen analogs. J. Agr. Food Chem., 13: 477-481
Fischer, R. (1968) Identification and quantitative estimation of
organophosphorus insecticides in biological material. III. Arch.
Toxicol., 23: 129-135
Gaines, T. B. (1960) The acute toxicity of pesticides to rats.
Toxicol. appl. Pharmacol., 2: 88-99
Gehrt, A. J. (1967) Ultraviolet spectrophotometric and gas
chromatographic methods for Ronnel in feeds. J. Ass. Offic. Anal.
Chem., 50: 257-261
Gould, A. H. (1961) Human volunteer tests concerning the oral toxicity
of Ronnel. Arnold H. Gould, M.D., Washington D.C. Unpublished report
Horiguchi, M., Ishida, M. and Higosaki, N. (1964) Determination of
some organophosphorus insecticides by gas-liquid partition
chromatography. Chem. Pharm. Bull. (Tokyo), 12:(11), 1315-1319
Hymas, T. A. (1961) Checking Ronnel for neurotoxic effect in 20-month
old hens. To G. E. Lynn, The Dow Chemical Co. Unpublished report
Ivey, M. C., Eschle, J. L., Claborn, H. V. and Graham, O. H. (1967)
Ronnel residues in the meat and milk of cattle exposed to
Ronnel-impregnated backrubber used for horn-fly control. J. Econ.
Entomol., 60: 712-716
Keller, J. G. (1961) Ten-week dietary feeding. Dogs. (Potentiation
study) Final report revised. Hazleton Laboratories Inc. Unpublished
report
Kovacs, M. F. (1964) Thin-layer chromatography for organo
thiophosphate pesticide residue determination
Leahy, J. S. (1960) Determination of the rate of excretion of
0,0-dimethyl 0-2,4,5-trichlorophenyl phosphorothioate in cows
milk following oral administration of Ronnel. Nutritional Research
Unit, Huntingdon, England. Unpublished report
Leng, M. L. (1958a) Cholinesterase inhibitory power of various Ronnel
metabolites. The Dow Chemical Co. Unpublished report
Leng, M. L. (1958b) Ultramicrodetermination of 0,0-dimethyl
0-2,4,5-trichlorophenyl phosphorothioate and its phosphate analogue
in milk. The Dow Chemical Company, Midland, Michigan. Unpublished
report
McCollister, D. D., Oyen, F. and Rows, V. K. (1959) Toxicological
studies of 0,0-dimethyl-0-(2,4,5-trichlorophenyl) phosphorothioate
(Ronnel) in laboratory animals. J. Agr. Food Chem., 7: 689-693
McCollister, D. D., Lockwood, D. T. and Rowe, V. K. (1961) Toxicologic
information on 2,4,5-trichlorophenol. Toxicol. appl. Pharmacol., 3:
63-70
McCollister, D. D., Copeland, J. R. and Oyen, F. (1967) Results of
fertility and reproduction studies in rats maintained on diets
containing Ronnel. The Dow Chemical Co. Unpublished report
McKinley, W. P. and Johal, P. S. (1963) Esterase inhibition technique
for the detection of organophosphorus pesticides, II. Simplified
version for routine checking. J. Ass Offic. Agr. Chem., 46: 840-842
McKinley, W. P. and Read, S. I. (1962) Esterase inhibition technique
for the detection of organophosphorus pesticides. J. Ass. Offic. Agr.
Chem., 45: 467-473
Menzie, C. (1966) Metabolism of pesticides. Wildlife No. 96, Fish and
Wildlife Service, U.S. Dept. of Interior, pages 167-169
Millar, K. R. (1965) Residues in tissues of sheep following dosing and
tip spraying with 32P-labelled Ronnel. New Zealand J. Agric. Res.,
8: 302-312
Miller, P. W. (1962) Ronnel in eggs from chickens treated with various
applications of KORLAN(R). The Dow Chemical Company, Midland,
Michigan. Unpublished report.
Moye, H. A. (1962) An improved microwave emission gas chromatography
detector for pesticide residue analysis. Anal. Chem., 39: 1441-1445
Nelson, R. C. (1966) Screening procedure for organothiophosphate
pesticide residues on fruits and vegetables by micro-coulometric gas
chromatography. J. Ass. Offic. Anal. Chem. 49: 763-765
Noel, P. R., Mawdesley-Thomas, L. E., Chesterman, H., Jolly, D. and
Street, A. E. (1965) Ronnel. Chronic toxicity study in the dog. Final
report. Huntingdon Research Centre. Unpublished report
Onley, J. H. and Bertuzzi, P. F. (1966) Rapid extraction procedure for
chlorinated pesticide residues in raw animal tissues and fat and meat
products. J. Ass. Offic. Anal. Chem., 49: 370-374
Plapp, F. W. and Casida, J. E. (1958a) Bovine Metabolism of
organophosphorus insecticides, metabolic fate of 0,0-dimethyl
0-(2,4,5-trichlorophenyl) phosphorothioate in rats and a cow.
J. Agr. Food Chem., 6: 662-667
Plapp, F. W. and Casida, J. E. (1958b) Hydrolysis of the
alkyl-phosphate bond in certain dialkyl aryl phosphorothioate
insecticides by rats, cockroaches and alkali, J. Econ. Entomol., 51:
800-803
Ragab, M. T. H. (1967) Direct fluorescent detection of
organothiophosphorus pesticides and some of their sulfur-containing
breakdown products after thin-layer chromatography. J. Ass. Offic.
Anal. Chem., 50: 1088-1098
Ragab, M. T. H. (1967a) 4-(p-nitrobenzyl) pyridine as a spray
reagent for organophosphorus pesticides and some of their breakdown
products on thin-layer chromatograms. Bull. environmtl. Contamination
Toxicol., 2: 279-288
Rahn, H. W. and Urban, G. (1964) Detection of insecticidal phosphoric
acid esters and their hydrolytic cleavage products. Pharmazie, 19:
597-602
Slokma, M. B. (1964) Toxicity of Ronnel with special reference to
humans. Pitman-Moore Research Center, The Dow Chemical Co. Unpublished
report
Smith, C. T., Shaw, F. R., Anderson, D. L., Callahan, R. A. and
Ziener, W. H. (1965) Ronnel residues in eggs of poultry. J. Econ.
Entomol., 58: 1160-1161
Smith, G. N. (1968) Cholinesterase inhibition of fenchlorphos and
metabolites. The Dow Chemical Co. Unpublished report
Stevens, R. K. (1967) A rapid specific method for the gas
chromatographic determination of organophosphate pesticides in cold
pressed citrus oil. J. Ass. Offic. Anal. Chem., 50: 1236-1242
Stewart, J. H. (1968) U.S. Department of Agriculture, Washington, D.C.
Private communication
Storherr, R. W. and Watts, R. R. (1965) A sweep codistillation cleanup
method for organophosphate pesticides. J. Ass. Offic. Agr. Chem.,
48: 11-54-58
Tapernoux, A. and Magat, A. (1963) Analysis of beef meat and fat for
residues of Ronnel, an organophosphate used in the treatment of bovine
hypodermosis. Ann. Fals. Expert. Chim., 56: (658) 266-270
Teekell, R. A., Rice, J. R. and McGregor, W. S. (1963) Ronnel residues
in pig fat after being on litter treated with KORLAN(R) insecticide
granules. The Dow Chemical Company, Lake Jackson, Texas. Unpublished
report.
Watts, R. R. and Storherr, R. W. (1967) Sweep codistillation clean-up
for milk for determination of organophosphate and chlorinated
hydrocarbon pesticides. J. Ass. Offic. Anal. Chem., 50: 581-585
Wells, C. E. (1967) Validation study of a method for pesticide
residues in foods and animal feeds. J. Ass. Offic. Anal. Chem., 50:
1205-1215
Wessel, J. R. (1967) Collaborative study of a method for multiple
organophosphorus pesticides in non-fatty foods. J. Ass. Offic. Anal.
Chem., 50: 430-439
Wessel, J. R. (1968) Collaborative study of three gas chromatographic
dual detection systems for analysis of multiple chlorinated and
organophosphorus pesticides. J. Ass. Offic. Anal. Chem., 51: 666-675
 Other information on identity and properties
Technical fenchlorphos is a light tan solid melting at 34-37°C (41°C
when pure). It has a mild mercaptan odour. It is stable at
temperatures up to 60°C and in neutral and slightly acid media. It is
unstable in alkaline and strongly acid media and upon prolonged
exposure in aqueous preparations. In solution, most cations other than
copper have little or no effect. At room temperature, the compound is
soluble in most organic solvents and is practically insoluble in
water.
The technical product is supplied in a variety of concentrations and
formulations (emulsifiable concentrate, wettable powder, mineral
blocks, mineral granules, medicated premix).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Fenchlorphos and its oxygen analogue are susceptible to hydrolysis in
animals at either the methyl-phosphate or phenylphosphate bond.
Hydrolysis at both sites has been demonstrated with alkali and bovine
rumen juice; and in rat, house-fly, and cow metabolic studies (Plapp
et al., 1958a, 1958b; Menzie, 1966). The following pathways in animals
are suggested from these studies:
Other information on identity and properties
Technical fenchlorphos is a light tan solid melting at 34-37°C (41°C
when pure). It has a mild mercaptan odour. It is stable at
temperatures up to 60°C and in neutral and slightly acid media. It is
unstable in alkaline and strongly acid media and upon prolonged
exposure in aqueous preparations. In solution, most cations other than
copper have little or no effect. At room temperature, the compound is
soluble in most organic solvents and is practically insoluble in
water.
The technical product is supplied in a variety of concentrations and
formulations (emulsifiable concentrate, wettable powder, mineral
blocks, mineral granules, medicated premix).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Fenchlorphos and its oxygen analogue are susceptible to hydrolysis in
animals at either the methyl-phosphate or phenylphosphate bond.
Hydrolysis at both sites has been demonstrated with alkali and bovine
rumen juice; and in rat, house-fly, and cow metabolic studies (Plapp
et al., 1958a, 1958b; Menzie, 1966). The following pathways in animals
are suggested from these studies:
 P32-labelled fenchlorphos was given orally to rats at a dose level of
100 mg/kg body-weight. The highest concentration was reached in the
tissues after 12 hours. The residues persisted for more than seven
days and were found to consist of fenchlorphos and its oxygen
analogue. These compounds were hydrolysed at either the
methyl-phosphate or the phenyl-phosphate linkage and the resulting
phosphoric acids were excreted in the urine. In the rat approximately
60 per cent of the administered dose was excreted within two days. In
a cow treated orally with 100 mg/kg body-weight the metabolism was
identical to that in the rat but excretion in the urine did not occur
as rapidly. After the first day, 20 per cent of the dose had been
excreted and 50 per cent after six days. A milk sample taken eight
hours after treatment contained 30 ppm of fenchlorphos but this level
had decreased to 0.4 ppm after seven days. A calf fed this milk at
various intervals never showed more than 1.5 ppm of fenchlorphos and
metabolites in its blood, nor was there any depression in
cholinesterase level (Plapp and Casida, 1958a).
Sheep given oral doses of 100 mg/kg of P32-labelled fenchlorphos
attained the highest concentrations in the blood after four hours.
Within 21 days 70 per cent of the administered dose had appeared in
the urine and seven per cent in the faeces. The concentration in the
fat fell from about 30 ppm after one week to less than 1 ppm after six
weeks (Millar, 1965).
The oxygen analogue of fenchlorphos is a more potent cholinesterase
inhibitor than fenchlorphos itself, but the principal hydrolytic
mammalian metabolites are poor inhibitors of cholinesterase being
equal to or less active than the parent compound (Leng, 1958a; Smith,
1968).
Acute toxicity (oral)
LD50 (mg/kg
Animal Sex body-weight) References
Mouse Female 2140 McCollister et al.,
1959
Rat Male 1740 "
Rat Female > 2000 "
Rat Male 1250 Gaines, 1960
Rat Female 2630 Gaines, 1960
Guinea-pig Male 3140 McCollister et al.,
1959
Rabbit Male and 640 "
female
Dog Male and > 500 "
female (causes emesis)
Short-term studies
Rat. Groups of 20 rats (10 of each sex) were fed 0.5, 1.5, 5, 15 and
50 mg/kg/day of fenchlorphos in their diets for 105 days. Mortality
records, food consumption, growth and final average body and organ
weights were closely comparable in experimental and control groups.
Histologic examination revealed no evidence of adverse effects
attributable to fenchlorphos, except in the male and female rats at
the 50 mg/kg/day level where there was some evidence in the liver of a
slight granular degeneration or cloudy swelling of the parenchymal
cells of the entire lobule. In the kidneys, there appeared to be some
cloudy swelling and vacuolation of the renal tubular epithelium with
very slight interstitial nephritis. Following 105-day administration
of fenchlorphos in the diet, all rats, including those that had
received the 50 mg/kg/day level, showed essentially complete recovery
of any depressed cholinesterase activity within six to eight weeks
after being placed on the control ration only. Microscopic examination
of the tissues from the animals fed 50 mg/kg/day, sacrificed after 30
and 45 days on the recovery ration, showed no evidence of any residual
pathological effects (McCollister et al., 1959).
Dog. One female dog per dosage level was fed 10, 30, 100 and 300 ppm
fenchlorphos in the diet for 12 months. All dogs were normal in
behaviour, appetite and growth. Haematological findings, final body
and organ weights and terminal blood urea nitrogen values showed no
significant deviations from the control. Microscopic examination of
all major organs revealed no evidence of detrimental effects
attributable to the test compound. However, the general pattern of
cholinesterase measurements indicated no significant changes in plasma
or red blood cell cholinesterase activity in the dogs that received
the equivalent of the 10, 30 or 100 ppm of fenchlorphos in their diet,
although there was significant depression at higher levels
(McCollister et al., 1959).
In another study, groups of dogs (four of each sex) were fed
fenchlorphos in their diet twice daily, six days a week for two years
at dosage levels of 1, 3 or 10 mg/kg/day. No evidence of adverse
effect was seen as judged by body-weight gain, food intake, general
appearances and behaviour, routine clinical laboratory investigations,
organ weight analysis, gross and microscopic examination of the
tissues, and brain or red cell cholinesterase activity. Plasma
cholinesterase activity in the dogs receiving 10 mg/kg/day was
consistently significantly below that of the controls. The depression
at the 3 mg/kg/day dosage was significant on only 50 per cent of the
occasions tested, and only very rarely in the dogs fed 1 mg/kg/day.
The over-all average of plasma cholinesterase activity measurements
for the dogs fed the 3 mg/kg/day dose level was 82 per cent of the
pre-treatment control activity (Noel et al., 1965).
Long-term studies
Rat. Groups of rats each containing 10 or 15 males and 10 or 15
females were fed diets containing 0.5, 1.5, 5, 15 and 50 mg/kg/day of
fenchlorphos for periods up to two years. All groups appeared normal
in general appearance and behaviour. Growth curves, mortality records,
haematological data, food consumption and organ weights showed no
significant differences from the control groups. In the rats fed 50
mg/kg/day histological examination of both sexes revealed central
lobular, granular degeneration and some necrosis of the parenchymal
cells in the liver, and evidence of cloudy swelling of tubular
epithelium in the kidneys together with interstitial nephritis. No
gross or histological abnormalities were observed in the rats fed dose
levels below 50 mg/kg/day. After two years no significant inhibition
of red blood cell or brain cholinesterase was observed in rats of
either sex receiving fenchlorphos at levels of 5 mg/kg/day or lower.
Male rats were more sensitive to plasma cholinesterase inhibition than
wore females, the no-effect levels being 5 mg/kg/day and 0.5 mg/kg/day
respectively (McCollister et al., 1959).
Special studies
(a) Reproduction
Groups of eight male and 16 female rats were maintained on diets
containing 100 or 300 ppm of fenchlorphos through three generations
with two litters per generation. Fertility, reproduction and lactation
were normal as judged by indices of fertility, gestation, viability
and lactation, body-weight records and teratological examination of
foetuses. A similar group of rats maintained at a level of 1000 ppm
through three generations showed no significant effects except for the
viability and lactation indices in the F1b and F2b generations, and
slightly depressed average body-weights. All three dietary
concentrations lowered plasma cholinesterase activity. Red cell
cholinesterase was not depressed at the 100 ppm dietary level;
however, depression was seen at the 300 and 1000 ppm dietary levels
(McCollister et al., 1967).
(b) Potentiation
Single oral dosages of fenchlorphos were administered jointly to rats
with 10 other commercial organo-phosphorus insecticides. No
significant potentiation problem was seen for such combinations under
ordinary conditions of field application and use of fenchlorphos
(McCollister et al., 1959). Dietary combinations of fenchlorphos (100
ppm) with EPN (20 or 70 ppm) or malathion (100 ppm) fed to dogs for 10
weeks produced additive or less than additive red blood cell or plasma
cholinesterase inhibition effects (Keller, 1961).
(c) Neurotoxicity
Nineteen mature hens were given intraperitoneal injections of either
2000 or 3000 mg/kg of fenchlorphos dissolved in diethyl succinate. The
surviving hens (10 of the 16 given 3000 mg/kg) showed no neurotoxic
effects within the 90-day observation period following treatment
(Hymas, 1961).
(d) Studies on the metabolite, 2,4,5-trichlorophenol
Groups of young male and female rats were fed dietary levels of 100,
300, 1000, 3000 and 10 000 ppm of 2,4,5-trichlorophenol for 98 days.
Records were kept concerning appearance, behaviour, mortality, food
consumption, body and organ weights and terminal haematological tests
(urea nitrogen, leucocyte counts, haematocrits and haemoglobin
values). No evidence of adverse effects was noted at the 1000 ppm
levels or less. At 3000 and 10 000 ppm the rats showed diuresis and
slight pathological changes of the kidney and liver, and at the 10 000
ppm level there was a slight decrease in growth (McCollister et al.,
1961).
Observations in man
Twenty human subjects were fed varying amounts of fenchlorphos in 250
mg portions over a period of three to seven days. Ten of these
volunteers received a total of 2500 mg each over three days, for an
average of about 11.9 mg/kg/day. Seven individuals discontinued the
treatment at the end of three days because of side effects which
included sporadic abdominal cramps, anorexia, blurred vision,
diarrhoea, headache, heartburn, malaise, nausea and weakness. The
three remaining individuals, who suffered no symptoms, received a
total of 9500 mg each over seven days, for an average of 19.4
mg/kg/day. Red blood cell cholinesterase depression and marked plasma
cholinesterase depression occurred in the latter three cases after 72
hours, one and two weeks. In another test, 10 persons received a total
of 3000 mg each over four days for an average of 10.7 mg/kg/day with
no symptoms related to the treatment. Another group of nine
individuals under treatment for schistosomiasis received the 10.7
mg/kg/day for 14 days without any effect on red blood cell
cholinesterase. Plasma cholinesterase was reduced in both tests,
markedly in eight of them after 72 hours. No other clinical signs or
symptoms of toxicity due to the treatments were seen in
haematological, liver function and urinalysis tests (Gould, 1961;
Slomka, 1964).
Comments
On the basis of many short- and long-term experiments on animals
including reproduction studies, the tolerability of relatively high
doses is evident without ascertaining any irreversible changes in the
organism. These data confirm experience from medical treatment of
humans. An additive effect with some organo-phosphorus compounds was
reported. Possible effect on aliesterase activity was not followed up.
For the present the most sensitive reported effect from oral doses of
fenchlorphos is the inhibition of cholinesterase activity. The
no-effect level for red blood cell cholinesterase inhibition in dogs
and rats is 3-5 mg/kg/day, for plasma cholinesterase inhibition it
varies from 0.5-5 mg/kg/day. The data from human volunteers are
inadequate.
TOXICOLOGICAL EVALUATION
Levels causing no significant toxicological effect
Rat: 0.5 mg/kg per day;
Dog: 1 mg/kg per day.
Estimate of acceptable daily intake for man
0-0.01 mg/kg body-weight.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Fenchlorphos is used to control ectoparasites, such as horn-flies,
screw-worms, ticks, fleece-worms (fly strike), lice and sheep keds of
livestock (swine, cattle, sheep). Premises such as farm buildings,
food processing plants, restaurants, supermarkets, food-packing
plants, dwellings, yards, parks and outdoor areas are treated with
fenchlorphos for control of flies, mosquitos, roaches, bed-bugs,
silverfish, fleas, ticks, spiders and ants. Applications are made in
the form of sprays, "pour-ons", dips, dusts, aerosols, cattle
backrubbers, oral drenches, smears and baits (Anon., 1967). Sprays are
used at one-half to two per cent fenchlorphos for buildings and
yards, wetting them sufficiently to result in a dose of approximately
216-864 mg/m2 of surface area. Coverage of livestock with
three-quarter per cent spray or less results in doses of less than 25
mg/kg of body-weight. The Dow Chemical Co. is preparing new
recommendations for withdrawal intervals between treatment and
slaughter for cattle, sheep and goats. At present, withdrawal periods
range from two to 12 weeks between last treatment and slaughter. Beef
cattle and dairy heifers are fed insecticide mineral blocks or
granules containing 5.5 per cent fenchlorphos for the control of horn
flies and cattle grubs. Cattle grub control is also obtained by
feeding cattle for seven consecutive days on food treated with 0.6 per
cent fenchlorphos (18 mg/kg/day).
The registered uses for fenchlorphos in the United States of America
are given in Anon. (1966, 1968). The registered plant uses of
fenchlorphos are for most fruit and vegetables in the Netherlands and
for bananas in the United States of America and Canada.
Residues resulting from supervised trials
Residues of fenchlorphos accumulate to a high degree in the fat of
animals and tend to be retained there. Most residue data were
therefore determined in the fat (Plapp and Casida, 1958; Ivey et al.,
1967). It has not been determined whether the oxygen analogue is
similarly stored. Several studies, utilizing cholinesterase inhibition
for analysis, indicated that appreciable residues of the oxygen
analogue did not occur. Verification of this point with modern
instrumentation (glc) would be desirable.
Residues from typical supervised trials are summarized in the
following table:
Dosage No. or duration Days after Residues
Treatment Animal active of last in Reference
ingredient treatments treatment tissue* ppm
Spray Cattle 1%, 1 gal 1 7 3.0-7.5 )
fat )
> Anon., 1959
14 0.66-1.6 )
fat )
Tip-spray Sheep 0.4%, 600 ml 1 7 0.35 fat )
> Miller, 1965
14 0.04 fat )
Back-rubber Cattle 1 or 2% for 28 days 14 0.0 fat, Ivey et al.,
int. 1967
organs
Pour-on Calf 4 oz. 5% 5 times 7 0.23-0.45) Dishburger
formulation 3 week fat ) et al., 1966
each time intervals 14 0.0-0.21 )
fat )
21 0.0-0.04 )
fat
Dip Sheep 0.5% 3 times, 28 5.7 fat )
2 week > Anon., 1959
intervals 43 1.2 fat )
Bedding Pig 5% for 28 0-14 0.0-0.03 )
granules days fat > Teekell
) et al., 1963
(continued)
Dosage No. or duration Days after Residues
Treatment Animal active of last in Reference
ingredient treatments treatment tissue* ppm
Litter Hen 5% granules ) 0.0-0.04 ) Miller, 1962;
) egg yolk )
) for 10-50 )
4% emulsion ) days 0.0 egg ) Smith at al.,
) white ) 1965;
5% dust ) 0.0 ) Dishburger &
liver, ) Rice, 1965
muscle )
Spray Cow 2 qts 0.5% 1 1 0.21 milk)
5 0.05 milk) Claborn et al.,
10 0.00 milk) 1965
Oral Cow 145 mg/kg 1 1 0.47 milk)
3 0.02 milk) Leahy, 1960
Back-rubber Cow 2% in oil 4 times < 0.01 milk Ivey et al., 1967
daily for
14 days
Barn Cow 36 mg/ft2 1 - 0.02 milk Leng, 1958b
* If one figure is listed it is a maximum value.
Fate of residues
In animals
After administration of P32-labelled fenchlorphos to a cow, the
highest levels of radioactivity appeared in the fat, kidneys and
lungs. In another test, 18 mg/kg/day was fed for seven days to cattle
and the concentration in the blood fell to 0.01-0.05-ppm 72 hours
after feeding was stopped.
The major measurable residue occurring in significant quantities is
fenchlorphos. Residues of the oxygen analogue, if present, appear to
be negligible in cows (Plapp and Casida, 1958).
Evidence of residues in food, in commerce or at consumption
Analyses were made in 1968 by the United States Department of
Agriculture of 149 tissue samples from slaughter houses located in
Louisiana, Texas, Oklahoma, Indiana and Nebraska (Stewart,1968). From
0.01-0.10 ppm of fenchlorphos was found in only four of the samples. A
level of 0.25 ppm in tissues was used as the working tolerance
limitation for organo-phosphorus insecticides.
In total diet studies conducted by the United States Food and Drug
Administration between June 1964 and June 1968 on 104 composites in
each of four food classes, only trace amounts (trace = <0.0005 ppm)
were found in about one per cent of the composites (Duggan. 1968).
Methods of residue analysis
(For key to metabolite numerals, see above chart)
Enzymatic inhibition
Although fenchlorphos is a poor inhibitor of cholinesterase in
vitro, it can be converted by treatment with bromine into the oxygen
analogue which is a potent cholinesterase inhibitor. Metabolites I,
III, IV and VII are poor inhibitors of fly head cholinesterase using
the colorimetric method described by Ellman et al. (1961). Presumably,
metabolites II, VI, VIII and IX are also poor inhibitors.
Analytical methods based on the high sensitivity of the manometric
measurement of cholinesterase inhibition of the oxygen analogue were
used to detect residues of fenchlorphos. Sensitivities ranged from
0.005-0.5 ppm in analyses of milk, fat, blood, egg white and egg yolk.
Beam and Hankinson (1964) have described a colorimetric cholinesterase
inhibition assay for fenchlorphos and other pesticides in milk. A
variety of other esterase methods for fenchlorphos, some utilizing
paper or thin-layer chromatography, have also been reported (McKinley
and Read, 1962; McKinley and Johal, 1963; Coffin and Savary, 1964;
el-Refai and Hopkins, 1965). While some of these methods of analysis
are very sensitive to fenchlorphos and its oxygen analogue, they are
not suitable for routine regulatory purposes.
2,4,5-Trichlorophenol colorimetric detection method
Fenchlorphos can be determined by hydrolysis to the
2,4,5-trichlorophenol moiety, which is in turn analysed by the
4-aminoantipyrine colorimetric method. The method, with various
modifications, has a sensitivity of 0.02-0.1 ppm in pea pods, pea
vines, peas, tomatoes, bananas and in body tissues of sheep and cattle
(Duggan et al., 1967; Claborn and Ivey, 1964).
Tapernoux and Magat (1963) have described a method based on
ultra-violet spectrometry of the phenol for residues of fenchlorphos
in beef, meat and fat. Most colorimetric methods for fenchlorphos do
not distinguish between the phenol (III) and the parent compound and
give, therefore, a maximum limit rather than an actual quantity.
Gas chromatographic methods
A gas chromatographic method of analysis for fenchlorphos appears to
be the method of choice based on accuracy, specificity, sensitivity
and speed. Gas chromatographic methods utilizing a large variety of
detectors (micro-coulometric, electron-capture, thermionic, flame
photometric, microwave-powered emission) for the determination of
fenchlorphos in many foods have been described (Bache and Lisk, 1967;
Burke and Holswade, 1964, 1966; Burke and Giuffrida, 1964; Egan et
al., 1964; Gehrt, 1967; Horiguchi et al., 1964; Moye, 1967; Nelson,
1966; Onley and Bertuzzi, 1966; Stevens, 1967). Sensitivities are in
the order of 0.001-0.1 ppm.
Gas chromatography multi-pesticide residue studies by a number of FDA
laboratories of the United States of America, using an
electron-capture detector (ECGC), validated a sensitivity of about
0.01 ppm for fenchlorphos in apples, oats, cabbage, peppers, grapes,
feed pellets, green peppers, red cabbage, rutabagas, olive oil, and
cheese (Wells, 1967).
Fenchlorphos residues have also been determined by use of ECGC with a
sensitivity of 0.001 ppm in milk and 0.005 ppm in body tissues from
cattle after proper extraction and clean-up methods. Fenchlorphos
recoveries of 87 per cent from milk, 77 per cent from fat and 85-94
per cent from other tissues may be obtained (Claborn and Ivey, 1965).
Hydrolysis of fenchlorphos and its phenol metabolites may be used to
determine the phenol. Wessel (1967, 1968) reported on a collaborative
study of the accuracy of residue determinations for fenchlorphos added
to lettuce and apples using the potassium chloride thermionic
detection (KCITD) and ECGC. Average recoveries were 91-94 per cent
with KCITD and 94-98 per cent by ECGC for residues of fenchlorphos
added at the 0.5 ppm level to apples and the 5.0 ppm level to lettuce.
There is little doubt that several of the methods cited may be useful
for either regulatory or referee purposes (but only for the parent
compound or its phenol hydrolysis product).
The lack of a quantitative residue method to analyse for the oxygen
analogue of fenchlorphos, which has been mentioned in metabolic
studies, has been noted. The determination of this compound by flame
photometric gas chromatography with virtually no clean-up can probably
be accomplished by the general procedure described by Beroza and
Bowman (1968); with other detectors, a clean-up would probably be
necessary. Clean-up of milk and crude crop extracts by sweep
co-distillation prior to determining fenchlorphos by gas
chromatography with thermionic detection has been reported (Storherr
and Watts, 1965; Watts and Storherr, 1967) and is undoubtedly useful
for this purpose.
Thin-layer chromatography
Methods for the separation and detection of fenchlorphos and other
organo-phosphorus insecticide residues on thin layer chromatograms
have been described by Abbott et al. (1967), Bunyan (1964), el-Refai
and Hopkins (1965), Fischer (1968), Kovacs (1964), Ragab (1967, 1967a)
and Rahn and Urban (1964). Such methods can be valuable for
confirmation of identity.
National tolerances
Country Crop Tolerance (ppm)
Canada Bananas (peel) 0.5
Netherlands Fruit, vegetables (except
potatoes), unprocessed
cereals, unsifted flour 0.4
United States of Bananas (peel) 0.5
America
Fenchlorphos is currently registered for use on a "no residue" basis
for animal applications and/or building pest control operations in the
following areas:
United States Japan United Kingdom of
of America Korea Great Britain and
Canada Australia Northern Ireland
Argentina New Zealand Germany
Brazil Italy Switzerland
Uruguay France Union of South Africa
India Finland Algeria
Republic of Sweden Ivory Coast
China (Taiwan) Netherlands Jordan
Malaysia Spain Israel
Viet-Nam Portugal Turkey
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Appraisal
This compound is used in many countries to control ectoparasites of
livestock; it is fed to animals, applied externally, and used on
premises. At present its application on plants is very limited, and
adequate residue data and use patterns were not available for study
for the purpose of recommending tolerances for raw agricultural
plant products.
Residues resulting from animal treatment consist essentially of
fenchlorphos although there may be some oxygen analogue and its
phenolic hydrolysis product present. Good agricultural practice
results in residues for which a 7.5 ppm tolerance of fenchlorphos in
the fat of meat is required. This residue is well within limits set by
the acceptable daily intake (0.01 mg/kg) because fat of meat comprises
a small fraction of the total diet.
Residues in milk diminished to about 0.02 ppm three or four days after
dosing dairy cow at rates of intake higher than normal.
Yolk of eggs from treated hens (dust or emulsifiable concentrate)
contained as much as 0.04 ppm of the insecticide.
Residues of fenchlorphos were not found in appreciable amount in
cattle tissues from slaughter houses and in total diet studies in the
United States of America.
Published gas-liquid chromatography methods of determining residues of
fenchlorphos appear to be adequate for regulatory purposes. One of
these should be selected for regulatory purposes and one should be
evaluated as a referee method.
Residue data are available only from the United States of America and
the United Kingdom. Use pattern and residue data from other countries
have not been submitted.
Recommendations
Temporary tolerances
The following temporary tolerances, to be in effect until 1972, are to
apply to raw agricultural products moving in commerce unless otherwise
indicated. In the case of fruit and vegetables the tolerances should
be applied as soon as practicable after harvest and in any event prior
to actual retail to the public. In the case of commodities entering
international trade, the tolerances should be applied by the importing
country at the point of entry or as soon as practicable thereafter.
Meat (on fat basis, at slaughter) 7.5 ppm
Yolk of eggs 0.05 ppm
Whole milk 0.04 ppm
The above tolerances include the oxygen analogue. In the case of
commodities entering international trade, the tolerances should be
applied by the importing country at the point of entry or as soon as
practicable thereafter.
Further work or information
Required before 30 June 1972:
1. If use of the compound is to be extended to fruit and vegetables,
data from several countries on the required rates and frequencies of
application, pre-harvest intervals, and the resultant residues.
2. Data from several countries other than the United States of America
and the United Kingdom on animal use patterns and resultant residues.
3. Data on residue levels in raw agricultural products moving in
commerce.
4. Data from countries other than the United States of America on
residue levels found in total diet studies.
5. Comparative evaluation of methods of analysis for regulatory
purposes.
Desirable:
1. Collaborative studies to establish a referee method.
2. Estimation of effect on aliesterase activity in animals and man.
3. Determination of no-effect level with respect to cholinesterase
activity in man.
4. Data on the fate of the trichlorophenol metabolite.
5. More adequate human data.
REFERENCES
Abbot, D. C., Burridge, A. S., Thomson, J. and Webb K. S. (1967) A
thin-layer chromatographic screening test for organophosphorus
pesticide residues. Analyst, 92: 170-175
Anon. (1959) Residue data in support of application of KORLAN(R) for
use on livestock. The Dow Chemical Company, Midland, Michigan.
Unpublished report
Anon. (1966) Ronnel, U.S.D.A. Summary of registered agricultural
pesticide chemical uses. Issued October 1, 1966. Washington, D.C.
Anon. (1967) Suggested guide for the use of insecticides to control
insects affecting crops, livestock, households, stored products,
forests and forest products, 1967. Agr. Handbook No. 331, U.S.D.A.,
Supt. of Documents, U.S. Government Printing Office, Washington, D.C.
20402
Anon. (1968) Technical Information Bulletin. 1968 registered livestock
and household uses for Ronnel and registered livestock uses for
"RUELENE". The Dow Chemical Company, Midland, Michigan 48640
Bache, C. A. and Lisk, D. J. (1967) Selective residue determination of
sulfur-, halogen- and phosphorus-containing pesticides by
helium-plasma emission spectrometry. J. Ass. Offic. Anal. Chem., 50:
1246-1250
Beam, J. E. and Hankinson, D. J. (1964) Application of the
acetyl-cholinesterase inhibition method for detecting organophosphate
residues and elated compounds in milk. J. Dairy Sci., 47: 1297-1305
Beroza, M. and Bowman, M. C. (1968) Gas chromatography of pesticide
residues containing phosphorus or sulfur with the flame photometric
detector. Environmental Sci. Technol., 2: 450-457
Brody, S. S. and Chaney, J. E. (1966) Flame photometric detector. The
application of a specific detector for phosphorus and for sulfur
compounds sensitive to subnanogram quantities. J. Gas Chromatogr.,
4: 42-46
Bunyan, P. J. (1964) The detection of organo-phosphorus pesticides on
thin-layer chromatograms. Analyst, 89: 615-618
Burke, J. and Giuffrida, L. (1964) Investigations of electron capture
gas chromatography for analysis of multiple chlorinated pesticide
residues in vegetables. J. Ass. Offic. Agr. Chem., 47: 326-345
Burke, J. and Holswade, W. (1964) Gas chromatography with
microcoulometric detection for pesticide residue analysis. J. Ass.
Ofic. Agr. Chem., 47: 845-859
Burke, J. A. and Holswade, W. (1966) A gas chromatographic column for
pesticide residue analysis: Retention times and response data. J. Ass.
Offic. Anal. Chem., 49: 374-385
Claborn, H. V. and Ivey, M. C. (1964) Colorimetric determination of
nemacide and Ronnel in animal tissues. J. Ass. Offic. Agr. Chem.,
47: 871-875
Claborn, H. V. and Ivey, M. C. (1965) Determination of
0,0-dimethyl 0-2,4,5-trichlorophenyl phosphorothioate in animal
tissues and milk. J. Agr. Food Chem., 13: 353-354
Claborn, H. V., Mann, H. D., Berry, I. L. and Hoffman, R. A. (1965)
Comparisons of residues in milk resulting from two types of spray
applications of DDT, Shell compound 4072 and Ronnel. J. Econ.
Entomol., 58:922-923
Coffin, D. E. and Savary, G. (1964) Procedure for extraction and
clean-up of plant material prior to determination of organophosphate
residues. J. Ass. Offic. Agr. Chem., 47: 875-881
Dishburger, H. J. and Rice, J. R. (1965) Residue analysis for Ronnel
in tissues from chickens confined on litter treated with KORLAN(R).
The Dow Chemical Company, Lake Jackson, Texas. Unpublished report
Dishburger, H. J., Rice, J. R. and Muniza, R. A. (1966) Ronnel
residues in the fat of cattle following multiple pour-on applications
of KORLAN(R). The Dow Chemical Company, Lake Jackson, Texas.
Unpublished report
Duggan, R. E. (1968) U.S. Dept. of Health, Education and Welfare, Food
and Drug Administration, Washington, D.C. Private communication
Duggan, R. E., Barry, H. C., Johnson, L. Y. and Williams, S. (1967)
Pesticide Analytical Manual, vol. II. Methods for individual pesticide
residues (Ronnel). U.S. Dept. of Health, Education and Welfare, Food
and Drug Administration, Washington, D.C. (July, 1967 revision)
Egan, H., Hammond, E. W. and Thomson, J. (1964) The analysis of
organo-phosphorus pesticide residues by gas chromatography. Analyst,
89: 175-178
Ellman, G. L., Courtney, D. K., Andres, Jr, V., Featherstone, R.M.
(1961) A new and rapid colorimetric determination of acetyl-
cholinesterase activity. Biochem. Pharmacol., 7: 88-95
el-Refai, A., and Hopkins, T. L. (1965) Thin-layer chromatography and
cholinesterase detection of several phosphorothiono insecticides and
their oxygen analogs. J. Agr. Food Chem., 13: 477-481
Fischer, R. (1968) Identification and quantitative estimation of
organophosphorus insecticides in biological material. III. Arch.
Toxicol., 23: 129-135
Gaines, T. B. (1960) The acute toxicity of pesticides to rats.
Toxicol. appl. Pharmacol., 2: 88-99
Gehrt, A. J. (1967) Ultraviolet spectrophotometric and gas
chromatographic methods for Ronnel in feeds. J. Ass. Offic. Anal.
Chem., 50: 257-261
Gould, A. H. (1961) Human volunteer tests concerning the oral toxicity
of Ronnel. Arnold H. Gould, M.D., Washington D.C. Unpublished report
Horiguchi, M., Ishida, M. and Higosaki, N. (1964) Determination of
some organophosphorus insecticides by gas-liquid partition
chromatography. Chem. Pharm. Bull. (Tokyo), 12:(11), 1315-1319
Hymas, T. A. (1961) Checking Ronnel for neurotoxic effect in 20-month
old hens. To G. E. Lynn, The Dow Chemical Co. Unpublished report
Ivey, M. C., Eschle, J. L., Claborn, H. V. and Graham, O. H. (1967)
Ronnel residues in the meat and milk of cattle exposed to
Ronnel-impregnated backrubber used for horn-fly control. J. Econ.
Entomol., 60: 712-716
Keller, J. G. (1961) Ten-week dietary feeding. Dogs. (Potentiation
study) Final report revised. Hazleton Laboratories Inc. Unpublished
report
Kovacs, M. F. (1964) Thin-layer chromatography for organo
thiophosphate pesticide residue determination
Leahy, J. S. (1960) Determination of the rate of excretion of
0,0-dimethyl 0-2,4,5-trichlorophenyl phosphorothioate in cows
milk following oral administration of Ronnel. Nutritional Research
Unit, Huntingdon, England. Unpublished report
Leng, M. L. (1958a) Cholinesterase inhibitory power of various Ronnel
metabolites. The Dow Chemical Co. Unpublished report
Leng, M. L. (1958b) Ultramicrodetermination of 0,0-dimethyl
0-2,4,5-trichlorophenyl phosphorothioate and its phosphate analogue
in milk. The Dow Chemical Company, Midland, Michigan. Unpublished
report
McCollister, D. D., Oyen, F. and Rows, V. K. (1959) Toxicological
studies of 0,0-dimethyl-0-(2,4,5-trichlorophenyl) phosphorothioate
(Ronnel) in laboratory animals. J. Agr. Food Chem., 7: 689-693
McCollister, D. D., Lockwood, D. T. and Rowe, V. K. (1961) Toxicologic
information on 2,4,5-trichlorophenol. Toxicol. appl. Pharmacol., 3:
63-70
McCollister, D. D., Copeland, J. R. and Oyen, F. (1967) Results of
fertility and reproduction studies in rats maintained on diets
containing Ronnel. The Dow Chemical Co. Unpublished report
McKinley, W. P. and Johal, P. S. (1963) Esterase inhibition technique
for the detection of organophosphorus pesticides, II. Simplified
version for routine checking. J. Ass Offic. Agr. Chem., 46: 840-842
McKinley, W. P. and Read, S. I. (1962) Esterase inhibition technique
for the detection of organophosphorus pesticides. J. Ass. Offic. Agr.
Chem., 45: 467-473
Menzie, C. (1966) Metabolism of pesticides. Wildlife No. 96, Fish and
Wildlife Service, U.S. Dept. of Interior, pages 167-169
Millar, K. R. (1965) Residues in tissues of sheep following dosing and
tip spraying with 32P-labelled Ronnel. New Zealand J. Agric. Res.,
8: 302-312
Miller, P. W. (1962) Ronnel in eggs from chickens treated with various
applications of KORLAN(R). The Dow Chemical Company, Midland,
Michigan. Unpublished report.
Moye, H. A. (1962) An improved microwave emission gas chromatography
detector for pesticide residue analysis. Anal. Chem., 39: 1441-1445
Nelson, R. C. (1966) Screening procedure for organothiophosphate
pesticide residues on fruits and vegetables by micro-coulometric gas
chromatography. J. Ass. Offic. Anal. Chem. 49: 763-765
Noel, P. R., Mawdesley-Thomas, L. E., Chesterman, H., Jolly, D. and
Street, A. E. (1965) Ronnel. Chronic toxicity study in the dog. Final
report. Huntingdon Research Centre. Unpublished report
Onley, J. H. and Bertuzzi, P. F. (1966) Rapid extraction procedure for
chlorinated pesticide residues in raw animal tissues and fat and meat
products. J. Ass. Offic. Anal. Chem., 49: 370-374
Plapp, F. W. and Casida, J. E. (1958a) Bovine Metabolism of
organophosphorus insecticides, metabolic fate of 0,0-dimethyl
0-(2,4,5-trichlorophenyl) phosphorothioate in rats and a cow.
J. Agr. Food Chem., 6: 662-667
Plapp, F. W. and Casida, J. E. (1958b) Hydrolysis of the
alkyl-phosphate bond in certain dialkyl aryl phosphorothioate
insecticides by rats, cockroaches and alkali, J. Econ. Entomol., 51:
800-803
Ragab, M. T. H. (1967) Direct fluorescent detection of
organothiophosphorus pesticides and some of their sulfur-containing
breakdown products after thin-layer chromatography. J. Ass. Offic.
Anal. Chem., 50: 1088-1098
Ragab, M. T. H. (1967a) 4-(p-nitrobenzyl) pyridine as a spray
reagent for organophosphorus pesticides and some of their breakdown
products on thin-layer chromatograms. Bull. environmtl. Contamination
Toxicol., 2: 279-288
Rahn, H. W. and Urban, G. (1964) Detection of insecticidal phosphoric
acid esters and their hydrolytic cleavage products. Pharmazie, 19:
597-602
Slokma, M. B. (1964) Toxicity of Ronnel with special reference to
humans. Pitman-Moore Research Center, The Dow Chemical Co. Unpublished
report
Smith, C. T., Shaw, F. R., Anderson, D. L., Callahan, R. A. and
Ziener, W. H. (1965) Ronnel residues in eggs of poultry. J. Econ.
Entomol., 58: 1160-1161
Smith, G. N. (1968) Cholinesterase inhibition of fenchlorphos and
metabolites. The Dow Chemical Co. Unpublished report
Stevens, R. K. (1967) A rapid specific method for the gas
chromatographic determination of organophosphate pesticides in cold
pressed citrus oil. J. Ass. Offic. Anal. Chem., 50: 1236-1242
Stewart, J. H. (1968) U.S. Department of Agriculture, Washington, D.C.
Private communication
Storherr, R. W. and Watts, R. R. (1965) A sweep codistillation cleanup
method for organophosphate pesticides. J. Ass. Offic. Agr. Chem.,
48: 11-54-58
Tapernoux, A. and Magat, A. (1963) Analysis of beef meat and fat for
residues of Ronnel, an organophosphate used in the treatment of bovine
hypodermosis. Ann. Fals. Expert. Chim., 56: (658) 266-270
Teekell, R. A., Rice, J. R. and McGregor, W. S. (1963) Ronnel residues
in pig fat after being on litter treated with KORLAN(R) insecticide
granules. The Dow Chemical Company, Lake Jackson, Texas. Unpublished
report.
Watts, R. R. and Storherr, R. W. (1967) Sweep codistillation clean-up
for milk for determination of organophosphate and chlorinated
hydrocarbon pesticides. J. Ass. Offic. Anal. Chem., 50: 581-585
Wells, C. E. (1967) Validation study of a method for pesticide
residues in foods and animal feeds. J. Ass. Offic. Anal. Chem., 50:
1205-1215
Wessel, J. R. (1967) Collaborative study of a method for multiple
organophosphorus pesticides in non-fatty foods. J. Ass. Offic. Anal.
Chem., 50: 430-439
Wessel, J. R. (1968) Collaborative study of three gas chromatographic
dual detection systems for analysis of multiple chlorinated and
organophosphorus pesticides. J. Ass. Offic. Anal. Chem., 51: 666-675
P32-labelled fenchlorphos was given orally to rats at a dose level of
100 mg/kg body-weight. The highest concentration was reached in the
tissues after 12 hours. The residues persisted for more than seven
days and were found to consist of fenchlorphos and its oxygen
analogue. These compounds were hydrolysed at either the
methyl-phosphate or the phenyl-phosphate linkage and the resulting
phosphoric acids were excreted in the urine. In the rat approximately
60 per cent of the administered dose was excreted within two days. In
a cow treated orally with 100 mg/kg body-weight the metabolism was
identical to that in the rat but excretion in the urine did not occur
as rapidly. After the first day, 20 per cent of the dose had been
excreted and 50 per cent after six days. A milk sample taken eight
hours after treatment contained 30 ppm of fenchlorphos but this level
had decreased to 0.4 ppm after seven days. A calf fed this milk at
various intervals never showed more than 1.5 ppm of fenchlorphos and
metabolites in its blood, nor was there any depression in
cholinesterase level (Plapp and Casida, 1958a).
Sheep given oral doses of 100 mg/kg of P32-labelled fenchlorphos
attained the highest concentrations in the blood after four hours.
Within 21 days 70 per cent of the administered dose had appeared in
the urine and seven per cent in the faeces. The concentration in the
fat fell from about 30 ppm after one week to less than 1 ppm after six
weeks (Millar, 1965).
The oxygen analogue of fenchlorphos is a more potent cholinesterase
inhibitor than fenchlorphos itself, but the principal hydrolytic
mammalian metabolites are poor inhibitors of cholinesterase being
equal to or less active than the parent compound (Leng, 1958a; Smith,
1968).
Acute toxicity (oral)
LD50 (mg/kg
Animal Sex body-weight) References
Mouse Female 2140 McCollister et al.,
1959
Rat Male 1740 "
Rat Female > 2000 "
Rat Male 1250 Gaines, 1960
Rat Female 2630 Gaines, 1960
Guinea-pig Male 3140 McCollister et al.,
1959
Rabbit Male and 640 "
female
Dog Male and > 500 "
female (causes emesis)
Short-term studies
Rat. Groups of 20 rats (10 of each sex) were fed 0.5, 1.5, 5, 15 and
50 mg/kg/day of fenchlorphos in their diets for 105 days. Mortality
records, food consumption, growth and final average body and organ
weights were closely comparable in experimental and control groups.
Histologic examination revealed no evidence of adverse effects
attributable to fenchlorphos, except in the male and female rats at
the 50 mg/kg/day level where there was some evidence in the liver of a
slight granular degeneration or cloudy swelling of the parenchymal
cells of the entire lobule. In the kidneys, there appeared to be some
cloudy swelling and vacuolation of the renal tubular epithelium with
very slight interstitial nephritis. Following 105-day administration
of fenchlorphos in the diet, all rats, including those that had
received the 50 mg/kg/day level, showed essentially complete recovery
of any depressed cholinesterase activity within six to eight weeks
after being placed on the control ration only. Microscopic examination
of the tissues from the animals fed 50 mg/kg/day, sacrificed after 30
and 45 days on the recovery ration, showed no evidence of any residual
pathological effects (McCollister et al., 1959).
Dog. One female dog per dosage level was fed 10, 30, 100 and 300 ppm
fenchlorphos in the diet for 12 months. All dogs were normal in
behaviour, appetite and growth. Haematological findings, final body
and organ weights and terminal blood urea nitrogen values showed no
significant deviations from the control. Microscopic examination of
all major organs revealed no evidence of detrimental effects
attributable to the test compound. However, the general pattern of
cholinesterase measurements indicated no significant changes in plasma
or red blood cell cholinesterase activity in the dogs that received
the equivalent of the 10, 30 or 100 ppm of fenchlorphos in their diet,
although there was significant depression at higher levels
(McCollister et al., 1959).
In another study, groups of dogs (four of each sex) were fed
fenchlorphos in their diet twice daily, six days a week for two years
at dosage levels of 1, 3 or 10 mg/kg/day. No evidence of adverse
effect was seen as judged by body-weight gain, food intake, general
appearances and behaviour, routine clinical laboratory investigations,
organ weight analysis, gross and microscopic examination of the
tissues, and brain or red cell cholinesterase activity. Plasma
cholinesterase activity in the dogs receiving 10 mg/kg/day was
consistently significantly below that of the controls. The depression
at the 3 mg/kg/day dosage was significant on only 50 per cent of the
occasions tested, and only very rarely in the dogs fed 1 mg/kg/day.
The over-all average of plasma cholinesterase activity measurements
for the dogs fed the 3 mg/kg/day dose level was 82 per cent of the
pre-treatment control activity (Noel et al., 1965).
Long-term studies
Rat. Groups of rats each containing 10 or 15 males and 10 or 15
females were fed diets containing 0.5, 1.5, 5, 15 and 50 mg/kg/day of
fenchlorphos for periods up to two years. All groups appeared normal
in general appearance and behaviour. Growth curves, mortality records,
haematological data, food consumption and organ weights showed no
significant differences from the control groups. In the rats fed 50
mg/kg/day histological examination of both sexes revealed central
lobular, granular degeneration and some necrosis of the parenchymal
cells in the liver, and evidence of cloudy swelling of tubular
epithelium in the kidneys together with interstitial nephritis. No
gross or histological abnormalities were observed in the rats fed dose
levels below 50 mg/kg/day. After two years no significant inhibition
of red blood cell or brain cholinesterase was observed in rats of
either sex receiving fenchlorphos at levels of 5 mg/kg/day or lower.
Male rats were more sensitive to plasma cholinesterase inhibition than
wore females, the no-effect levels being 5 mg/kg/day and 0.5 mg/kg/day
respectively (McCollister et al., 1959).
Special studies
(a) Reproduction
Groups of eight male and 16 female rats were maintained on diets
containing 100 or 300 ppm of fenchlorphos through three generations
with two litters per generation. Fertility, reproduction and lactation
were normal as judged by indices of fertility, gestation, viability
and lactation, body-weight records and teratological examination of
foetuses. A similar group of rats maintained at a level of 1000 ppm
through three generations showed no significant effects except for the
viability and lactation indices in the F1b and F2b generations, and
slightly depressed average body-weights. All three dietary
concentrations lowered plasma cholinesterase activity. Red cell
cholinesterase was not depressed at the 100 ppm dietary level;
however, depression was seen at the 300 and 1000 ppm dietary levels
(McCollister et al., 1967).
(b) Potentiation
Single oral dosages of fenchlorphos were administered jointly to rats
with 10 other commercial organo-phosphorus insecticides. No
significant potentiation problem was seen for such combinations under
ordinary conditions of field application and use of fenchlorphos
(McCollister et al., 1959). Dietary combinations of fenchlorphos (100
ppm) with EPN (20 or 70 ppm) or malathion (100 ppm) fed to dogs for 10
weeks produced additive or less than additive red blood cell or plasma
cholinesterase inhibition effects (Keller, 1961).
(c) Neurotoxicity
Nineteen mature hens were given intraperitoneal injections of either
2000 or 3000 mg/kg of fenchlorphos dissolved in diethyl succinate. The
surviving hens (10 of the 16 given 3000 mg/kg) showed no neurotoxic
effects within the 90-day observation period following treatment
(Hymas, 1961).
(d) Studies on the metabolite, 2,4,5-trichlorophenol
Groups of young male and female rats were fed dietary levels of 100,
300, 1000, 3000 and 10 000 ppm of 2,4,5-trichlorophenol for 98 days.
Records were kept concerning appearance, behaviour, mortality, food
consumption, body and organ weights and terminal haematological tests
(urea nitrogen, leucocyte counts, haematocrits and haemoglobin
values). No evidence of adverse effects was noted at the 1000 ppm
levels or less. At 3000 and 10 000 ppm the rats showed diuresis and
slight pathological changes of the kidney and liver, and at the 10 000
ppm level there was a slight decrease in growth (McCollister et al.,
1961).
Observations in man
Twenty human subjects were fed varying amounts of fenchlorphos in 250
mg portions over a period of three to seven days. Ten of these
volunteers received a total of 2500 mg each over three days, for an
average of about 11.9 mg/kg/day. Seven individuals discontinued the
treatment at the end of three days because of side effects which
included sporadic abdominal cramps, anorexia, blurred vision,
diarrhoea, headache, heartburn, malaise, nausea and weakness. The
three remaining individuals, who suffered no symptoms, received a
total of 9500 mg each over seven days, for an average of 19.4
mg/kg/day. Red blood cell cholinesterase depression and marked plasma
cholinesterase depression occurred in the latter three cases after 72
hours, one and two weeks. In another test, 10 persons received a total
of 3000 mg each over four days for an average of 10.7 mg/kg/day with
no symptoms related to the treatment. Another group of nine
individuals under treatment for schistosomiasis received the 10.7
mg/kg/day for 14 days without any effect on red blood cell
cholinesterase. Plasma cholinesterase was reduced in both tests,
markedly in eight of them after 72 hours. No other clinical signs or
symptoms of toxicity due to the treatments were seen in
haematological, liver function and urinalysis tests (Gould, 1961;
Slomka, 1964).
Comments
On the basis of many short- and long-term experiments on animals
including reproduction studies, the tolerability of relatively high
doses is evident without ascertaining any irreversible changes in the
organism. These data confirm experience from medical treatment of
humans. An additive effect with some organo-phosphorus compounds was
reported. Possible effect on aliesterase activity was not followed up.
For the present the most sensitive reported effect from oral doses of
fenchlorphos is the inhibition of cholinesterase activity. The
no-effect level for red blood cell cholinesterase inhibition in dogs
and rats is 3-5 mg/kg/day, for plasma cholinesterase inhibition it
varies from 0.5-5 mg/kg/day. The data from human volunteers are
inadequate.
TOXICOLOGICAL EVALUATION
Levels causing no significant toxicological effect
Rat: 0.5 mg/kg per day;
Dog: 1 mg/kg per day.
Estimate of acceptable daily intake for man
0-0.01 mg/kg body-weight.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Fenchlorphos is used to control ectoparasites, such as horn-flies,
screw-worms, ticks, fleece-worms (fly strike), lice and sheep keds of
livestock (swine, cattle, sheep). Premises such as farm buildings,
food processing plants, restaurants, supermarkets, food-packing
plants, dwellings, yards, parks and outdoor areas are treated with
fenchlorphos for control of flies, mosquitos, roaches, bed-bugs,
silverfish, fleas, ticks, spiders and ants. Applications are made in
the form of sprays, "pour-ons", dips, dusts, aerosols, cattle
backrubbers, oral drenches, smears and baits (Anon., 1967). Sprays are
used at one-half to two per cent fenchlorphos for buildings and
yards, wetting them sufficiently to result in a dose of approximately
216-864 mg/m2 of surface area. Coverage of livestock with
three-quarter per cent spray or less results in doses of less than 25
mg/kg of body-weight. The Dow Chemical Co. is preparing new
recommendations for withdrawal intervals between treatment and
slaughter for cattle, sheep and goats. At present, withdrawal periods
range from two to 12 weeks between last treatment and slaughter. Beef
cattle and dairy heifers are fed insecticide mineral blocks or
granules containing 5.5 per cent fenchlorphos for the control of horn
flies and cattle grubs. Cattle grub control is also obtained by
feeding cattle for seven consecutive days on food treated with 0.6 per
cent fenchlorphos (18 mg/kg/day).
The registered uses for fenchlorphos in the United States of America
are given in Anon. (1966, 1968). The registered plant uses of
fenchlorphos are for most fruit and vegetables in the Netherlands and
for bananas in the United States of America and Canada.
Residues resulting from supervised trials
Residues of fenchlorphos accumulate to a high degree in the fat of
animals and tend to be retained there. Most residue data were
therefore determined in the fat (Plapp and Casida, 1958; Ivey et al.,
1967). It has not been determined whether the oxygen analogue is
similarly stored. Several studies, utilizing cholinesterase inhibition
for analysis, indicated that appreciable residues of the oxygen
analogue did not occur. Verification of this point with modern
instrumentation (glc) would be desirable.
Residues from typical supervised trials are summarized in the
following table:
Dosage No. or duration Days after Residues
Treatment Animal active of last in Reference
ingredient treatments treatment tissue* ppm
Spray Cattle 1%, 1 gal 1 7 3.0-7.5 )
fat )
> Anon., 1959
14 0.66-1.6 )
fat )
Tip-spray Sheep 0.4%, 600 ml 1 7 0.35 fat )
> Miller, 1965
14 0.04 fat )
Back-rubber Cattle 1 or 2% for 28 days 14 0.0 fat, Ivey et al.,
int. 1967
organs
Pour-on Calf 4 oz. 5% 5 times 7 0.23-0.45) Dishburger
formulation 3 week fat ) et al., 1966
each time intervals 14 0.0-0.21 )
fat )
21 0.0-0.04 )
fat
Dip Sheep 0.5% 3 times, 28 5.7 fat )
2 week > Anon., 1959
intervals 43 1.2 fat )
Bedding Pig 5% for 28 0-14 0.0-0.03 )
granules days fat > Teekell
) et al., 1963
(continued)
Dosage No. or duration Days after Residues
Treatment Animal active of last in Reference
ingredient treatments treatment tissue* ppm
Litter Hen 5% granules ) 0.0-0.04 ) Miller, 1962;
) egg yolk )
) for 10-50 )
4% emulsion ) days 0.0 egg ) Smith at al.,
) white ) 1965;
5% dust ) 0.0 ) Dishburger &
liver, ) Rice, 1965
muscle )
Spray Cow 2 qts 0.5% 1 1 0.21 milk)
5 0.05 milk) Claborn et al.,
10 0.00 milk) 1965
Oral Cow 145 mg/kg 1 1 0.47 milk)
3 0.02 milk) Leahy, 1960
Back-rubber Cow 2% in oil 4 times < 0.01 milk Ivey et al., 1967
daily for
14 days
Barn Cow 36 mg/ft2 1 - 0.02 milk Leng, 1958b
* If one figure is listed it is a maximum value.
Fate of residues
In animals
After administration of P32-labelled fenchlorphos to a cow, the
highest levels of radioactivity appeared in the fat, kidneys and
lungs. In another test, 18 mg/kg/day was fed for seven days to cattle
and the concentration in the blood fell to 0.01-0.05-ppm 72 hours
after feeding was stopped.
The major measurable residue occurring in significant quantities is
fenchlorphos. Residues of the oxygen analogue, if present, appear to
be negligible in cows (Plapp and Casida, 1958).
Evidence of residues in food, in commerce or at consumption
Analyses were made in 1968 by the United States Department of
Agriculture of 149 tissue samples from slaughter houses located in
Louisiana, Texas, Oklahoma, Indiana and Nebraska (Stewart,1968). From
0.01-0.10 ppm of fenchlorphos was found in only four of the samples. A
level of 0.25 ppm in tissues was used as the working tolerance
limitation for organo-phosphorus insecticides.
In total diet studies conducted by the United States Food and Drug
Administration between June 1964 and June 1968 on 104 composites in
each of four food classes, only trace amounts (trace = <0.0005 ppm)
were found in about one per cent of the composites (Duggan. 1968).
Methods of residue analysis
(For key to metabolite numerals, see above chart)
Enzymatic inhibition
Although fenchlorphos is a poor inhibitor of cholinesterase in
vitro, it can be converted by treatment with bromine into the oxygen
analogue which is a potent cholinesterase inhibitor. Metabolites I,
III, IV and VII are poor inhibitors of fly head cholinesterase using
the colorimetric method described by Ellman et al. (1961). Presumably,
metabolites II, VI, VIII and IX are also poor inhibitors.
Analytical methods based on the high sensitivity of the manometric
measurement of cholinesterase inhibition of the oxygen analogue were
used to detect residues of fenchlorphos. Sensitivities ranged from
0.005-0.5 ppm in analyses of milk, fat, blood, egg white and egg yolk.
Beam and Hankinson (1964) have described a colorimetric cholinesterase
inhibition assay for fenchlorphos and other pesticides in milk. A
variety of other esterase methods for fenchlorphos, some utilizing
paper or thin-layer chromatography, have also been reported (McKinley
and Read, 1962; McKinley and Johal, 1963; Coffin and Savary, 1964;
el-Refai and Hopkins, 1965). While some of these methods of analysis
are very sensitive to fenchlorphos and its oxygen analogue, they are
not suitable for routine regulatory purposes.
2,4,5-Trichlorophenol colorimetric detection method
Fenchlorphos can be determined by hydrolysis to the
2,4,5-trichlorophenol moiety, which is in turn analysed by the
4-aminoantipyrine colorimetric method. The method, with various
modifications, has a sensitivity of 0.02-0.1 ppm in pea pods, pea
vines, peas, tomatoes, bananas and in body tissues of sheep and cattle
(Duggan et al., 1967; Claborn and Ivey, 1964).
Tapernoux and Magat (1963) have described a method based on
ultra-violet spectrometry of the phenol for residues of fenchlorphos
in beef, meat and fat. Most colorimetric methods for fenchlorphos do
not distinguish between the phenol (III) and the parent compound and
give, therefore, a maximum limit rather than an actual quantity.
Gas chromatographic methods
A gas chromatographic method of analysis for fenchlorphos appears to
be the method of choice based on accuracy, specificity, sensitivity
and speed. Gas chromatographic methods utilizing a large variety of
detectors (micro-coulometric, electron-capture, thermionic, flame
photometric, microwave-powered emission) for the determination of
fenchlorphos in many foods have been described (Bache and Lisk, 1967;
Burke and Holswade, 1964, 1966; Burke and Giuffrida, 1964; Egan et
al., 1964; Gehrt, 1967; Horiguchi et al., 1964; Moye, 1967; Nelson,
1966; Onley and Bertuzzi, 1966; Stevens, 1967). Sensitivities are in
the order of 0.001-0.1 ppm.
Gas chromatography multi-pesticide residue studies by a number of FDA
laboratories of the United States of America, using an
electron-capture detector (ECGC), validated a sensitivity of about
0.01 ppm for fenchlorphos in apples, oats, cabbage, peppers, grapes,
feed pellets, green peppers, red cabbage, rutabagas, olive oil, and
cheese (Wells, 1967).
Fenchlorphos residues have also been determined by use of ECGC with a
sensitivity of 0.001 ppm in milk and 0.005 ppm in body tissues from
cattle after proper extraction and clean-up methods. Fenchlorphos
recoveries of 87 per cent from milk, 77 per cent from fat and 85-94
per cent from other tissues may be obtained (Claborn and Ivey, 1965).
Hydrolysis of fenchlorphos and its phenol metabolites may be used to
determine the phenol. Wessel (1967, 1968) reported on a collaborative
study of the accuracy of residue determinations for fenchlorphos added
to lettuce and apples using the potassium chloride thermionic
detection (KCITD) and ECGC. Average recoveries were 91-94 per cent
with KCITD and 94-98 per cent by ECGC for residues of fenchlorphos
added at the 0.5 ppm level to apples and the 5.0 ppm level to lettuce.
There is little doubt that several of the methods cited may be useful
for either regulatory or referee purposes (but only for the parent
compound or its phenol hydrolysis product).
The lack of a quantitative residue method to analyse for the oxygen
analogue of fenchlorphos, which has been mentioned in metabolic
studies, has been noted. The determination of this compound by flame
photometric gas chromatography with virtually no clean-up can probably
be accomplished by the general procedure described by Beroza and
Bowman (1968); with other detectors, a clean-up would probably be
necessary. Clean-up of milk and crude crop extracts by sweep
co-distillation prior to determining fenchlorphos by gas
chromatography with thermionic detection has been reported (Storherr
and Watts, 1965; Watts and Storherr, 1967) and is undoubtedly useful
for this purpose.
Thin-layer chromatography
Methods for the separation and detection of fenchlorphos and other
organo-phosphorus insecticide residues on thin layer chromatograms
have been described by Abbott et al. (1967), Bunyan (1964), el-Refai
and Hopkins (1965), Fischer (1968), Kovacs (1964), Ragab (1967, 1967a)
and Rahn and Urban (1964). Such methods can be valuable for
confirmation of identity.
National tolerances
Country Crop Tolerance (ppm)
Canada Bananas (peel) 0.5
Netherlands Fruit, vegetables (except
potatoes), unprocessed
cereals, unsifted flour 0.4
United States of Bananas (peel) 0.5
America
Fenchlorphos is currently registered for use on a "no residue" basis
for animal applications and/or building pest control operations in the
following areas:
United States Japan United Kingdom of
of America Korea Great Britain and
Canada Australia Northern Ireland
Argentina New Zealand Germany
Brazil Italy Switzerland
Uruguay France Union of South Africa
India Finland Algeria
Republic of Sweden Ivory Coast
China (Taiwan) Netherlands Jordan
Malaysia Spain Israel
Viet-Nam Portugal Turkey
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Appraisal
This compound is used in many countries to control ectoparasites of
livestock; it is fed to animals, applied externally, and used on
premises. At present its application on plants is very limited, and
adequate residue data and use patterns were not available for study
for the purpose of recommending tolerances for raw agricultural
plant products.
Residues resulting from animal treatment consist essentially of
fenchlorphos although there may be some oxygen analogue and its
phenolic hydrolysis product present. Good agricultural practice
results in residues for which a 7.5 ppm tolerance of fenchlorphos in
the fat of meat is required. This residue is well within limits set by
the acceptable daily intake (0.01 mg/kg) because fat of meat comprises
a small fraction of the total diet.
Residues in milk diminished to about 0.02 ppm three or four days after
dosing dairy cow at rates of intake higher than normal.
Yolk of eggs from treated hens (dust or emulsifiable concentrate)
contained as much as 0.04 ppm of the insecticide.
Residues of fenchlorphos were not found in appreciable amount in
cattle tissues from slaughter houses and in total diet studies in the
United States of America.
Published gas-liquid chromatography methods of determining residues of
fenchlorphos appear to be adequate for regulatory purposes. One of
these should be selected for regulatory purposes and one should be
evaluated as a referee method.
Residue data are available only from the United States of America and
the United Kingdom. Use pattern and residue data from other countries
have not been submitted.
Recommendations
Temporary tolerances
The following temporary tolerances, to be in effect until 1972, are to
apply to raw agricultural products moving in commerce unless otherwise
indicated. In the case of fruit and vegetables the tolerances should
be applied as soon as practicable after harvest and in any event prior
to actual retail to the public. In the case of commodities entering
international trade, the tolerances should be applied by the importing
country at the point of entry or as soon as practicable thereafter.
Meat (on fat basis, at slaughter) 7.5 ppm
Yolk of eggs 0.05 ppm
Whole milk 0.04 ppm
The above tolerances include the oxygen analogue. In the case of
commodities entering international trade, the tolerances should be
applied by the importing country at the point of entry or as soon as
practicable thereafter.
Further work or information
Required before 30 June 1972:
1. If use of the compound is to be extended to fruit and vegetables,
data from several countries on the required rates and frequencies of
application, pre-harvest intervals, and the resultant residues.
2. Data from several countries other than the United States of America
and the United Kingdom on animal use patterns and resultant residues.
3. Data on residue levels in raw agricultural products moving in
commerce.
4. Data from countries other than the United States of America on
residue levels found in total diet studies.
5. Comparative evaluation of methods of analysis for regulatory
purposes.
Desirable:
1. Collaborative studies to establish a referee method.
2. Estimation of effect on aliesterase activity in animals and man.
3. Determination of no-effect level with respect to cholinesterase
activity in man.
4. Data on the fate of the trichlorophenol metabolite.
5. More adequate human data.
REFERENCES
Abbot, D. C., Burridge, A. S., Thomson, J. and Webb K. S. (1967) A
thin-layer chromatographic screening test for organophosphorus
pesticide residues. Analyst, 92: 170-175
Anon. (1959) Residue data in support of application of KORLAN(R) for
use on livestock. The Dow Chemical Company, Midland, Michigan.
Unpublished report
Anon. (1966) Ronnel, U.S.D.A. Summary of registered agricultural
pesticide chemical uses. Issued October 1, 1966. Washington, D.C.
Anon. (1967) Suggested guide for the use of insecticides to control
insects affecting crops, livestock, households, stored products,
forests and forest products, 1967. Agr. Handbook No. 331, U.S.D.A.,
Supt. of Documents, U.S. Government Printing Office, Washington, D.C.
20402
Anon. (1968) Technical Information Bulletin. 1968 registered livestock
and household uses for Ronnel and registered livestock uses for
"RUELENE". The Dow Chemical Company, Midland, Michigan 48640
Bache, C. A. and Lisk, D. J. (1967) Selective residue determination of
sulfur-, halogen- and phosphorus-containing pesticides by
helium-plasma emission spectrometry. J. Ass. Offic. Anal. Chem., 50:
1246-1250
Beam, J. E. and Hankinson, D. J. (1964) Application of the
acetyl-cholinesterase inhibition method for detecting organophosphate
residues and elated compounds in milk. J. Dairy Sci., 47: 1297-1305
Beroza, M. and Bowman, M. C. (1968) Gas chromatography of pesticide
residues containing phosphorus or sulfur with the flame photometric
detector. Environmental Sci. Technol., 2: 450-457
Brody, S. S. and Chaney, J. E. (1966) Flame photometric detector. The
application of a specific detector for phosphorus and for sulfur
compounds sensitive to subnanogram quantities. J. Gas Chromatogr.,
4: 42-46
Bunyan, P. J. (1964) The detection of organo-phosphorus pesticides on
thin-layer chromatograms. Analyst, 89: 615-618
Burke, J. and Giuffrida, L. (1964) Investigations of electron capture
gas chromatography for analysis of multiple chlorinated pesticide
residues in vegetables. J. Ass. Offic. Agr. Chem., 47: 326-345
Burke, J. and Holswade, W. (1964) Gas chromatography with
microcoulometric detection for pesticide residue analysis. J. Ass.
Ofic. Agr. Chem., 47: 845-859
Burke, J. A. and Holswade, W. (1966) A gas chromatographic column for
pesticide residue analysis: Retention times and response data. J. Ass.
Offic. Anal. Chem., 49: 374-385
Claborn, H. V. and Ivey, M. C. (1964) Colorimetric determination of
nemacide and Ronnel in animal tissues. J. Ass. Offic. Agr. Chem.,
47: 871-875
Claborn, H. V. and Ivey, M. C. (1965) Determination of
0,0-dimethyl 0-2,4,5-trichlorophenyl phosphorothioate in animal
tissues and milk. J. Agr. Food Chem., 13: 353-354
Claborn, H. V., Mann, H. D., Berry, I. L. and Hoffman, R. A. (1965)
Comparisons of residues in milk resulting from two types of spray
applications of DDT, Shell compound 4072 and Ronnel. J. Econ.
Entomol., 58:922-923
Coffin, D. E. and Savary, G. (1964) Procedure for extraction and
clean-up of plant material prior to determination of organophosphate
residues. J. Ass. Offic. Agr. Chem., 47: 875-881
Dishburger, H. J. and Rice, J. R. (1965) Residue analysis for Ronnel
in tissues from chickens confined on litter treated with KORLAN(R).
The Dow Chemical Company, Lake Jackson, Texas. Unpublished report
Dishburger, H. J., Rice, J. R. and Muniza, R. A. (1966) Ronnel
residues in the fat of cattle following multiple pour-on applications
of KORLAN(R). The Dow Chemical Company, Lake Jackson, Texas.
Unpublished report
Duggan, R. E. (1968) U.S. Dept. of Health, Education and Welfare, Food
and Drug Administration, Washington, D.C. Private communication
Duggan, R. E., Barry, H. C., Johnson, L. Y. and Williams, S. (1967)
Pesticide Analytical Manual, vol. II. Methods for individual pesticide
residues (Ronnel). U.S. Dept. of Health, Education and Welfare, Food
and Drug Administration, Washington, D.C. (July, 1967 revision)
Egan, H., Hammond, E. W. and Thomson, J. (1964) The analysis of
organo-phosphorus pesticide residues by gas chromatography. Analyst,
89: 175-178
Ellman, G. L., Courtney, D. K., Andres, Jr, V., Featherstone, R.M.
(1961) A new and rapid colorimetric determination of acetyl-
cholinesterase activity. Biochem. Pharmacol., 7: 88-95
el-Refai, A., and Hopkins, T. L. (1965) Thin-layer chromatography and
cholinesterase detection of several phosphorothiono insecticides and
their oxygen analogs. J. Agr. Food Chem., 13: 477-481
Fischer, R. (1968) Identification and quantitative estimation of
organophosphorus insecticides in biological material. III. Arch.
Toxicol., 23: 129-135
Gaines, T. B. (1960) The acute toxicity of pesticides to rats.
Toxicol. appl. Pharmacol., 2: 88-99
Gehrt, A. J. (1967) Ultraviolet spectrophotometric and gas
chromatographic methods for Ronnel in feeds. J. Ass. Offic. Anal.
Chem., 50: 257-261
Gould, A. H. (1961) Human volunteer tests concerning the oral toxicity
of Ronnel. Arnold H. Gould, M.D., Washington D.C. Unpublished report
Horiguchi, M., Ishida, M. and Higosaki, N. (1964) Determination of
some organophosphorus insecticides by gas-liquid partition
chromatography. Chem. Pharm. Bull. (Tokyo), 12:(11), 1315-1319
Hymas, T. A. (1961) Checking Ronnel for neurotoxic effect in 20-month
old hens. To G. E. Lynn, The Dow Chemical Co. Unpublished report
Ivey, M. C., Eschle, J. L., Claborn, H. V. and Graham, O. H. (1967)
Ronnel residues in the meat and milk of cattle exposed to
Ronnel-impregnated backrubber used for horn-fly control. J. Econ.
Entomol., 60: 712-716
Keller, J. G. (1961) Ten-week dietary feeding. Dogs. (Potentiation
study) Final report revised. Hazleton Laboratories Inc. Unpublished
report
Kovacs, M. F. (1964) Thin-layer chromatography for organo
thiophosphate pesticide residue determination
Leahy, J. S. (1960) Determination of the rate of excretion of
0,0-dimethyl 0-2,4,5-trichlorophenyl phosphorothioate in cows
milk following oral administration of Ronnel. Nutritional Research
Unit, Huntingdon, England. Unpublished report
Leng, M. L. (1958a) Cholinesterase inhibitory power of various Ronnel
metabolites. The Dow Chemical Co. Unpublished report
Leng, M. L. (1958b) Ultramicrodetermination of 0,0-dimethyl
0-2,4,5-trichlorophenyl phosphorothioate and its phosphate analogue
in milk. The Dow Chemical Company, Midland, Michigan. Unpublished
report
McCollister, D. D., Oyen, F. and Rows, V. K. (1959) Toxicological
studies of 0,0-dimethyl-0-(2,4,5-trichlorophenyl) phosphorothioate
(Ronnel) in laboratory animals. J. Agr. Food Chem., 7: 689-693
McCollister, D. D., Lockwood, D. T. and Rowe, V. K. (1961) Toxicologic
information on 2,4,5-trichlorophenol. Toxicol. appl. Pharmacol., 3:
63-70
McCollister, D. D., Copeland, J. R. and Oyen, F. (1967) Results of
fertility and reproduction studies in rats maintained on diets
containing Ronnel. The Dow Chemical Co. Unpublished report
McKinley, W. P. and Johal, P. S. (1963) Esterase inhibition technique
for the detection of organophosphorus pesticides, II. Simplified
version for routine checking. J. Ass Offic. Agr. Chem., 46: 840-842
McKinley, W. P. and Read, S. I. (1962) Esterase inhibition technique
for the detection of organophosphorus pesticides. J. Ass. Offic. Agr.
Chem., 45: 467-473
Menzie, C. (1966) Metabolism of pesticides. Wildlife No. 96, Fish and
Wildlife Service, U.S. Dept. of Interior, pages 167-169
Millar, K. R. (1965) Residues in tissues of sheep following dosing and
tip spraying with 32P-labelled Ronnel. New Zealand J. Agric. Res.,
8: 302-312
Miller, P. W. (1962) Ronnel in eggs from chickens treated with various
applications of KORLAN(R). The Dow Chemical Company, Midland,
Michigan. Unpublished report.
Moye, H. A. (1962) An improved microwave emission gas chromatography
detector for pesticide residue analysis. Anal. Chem., 39: 1441-1445
Nelson, R. C. (1966) Screening procedure for organothiophosphate
pesticide residues on fruits and vegetables by micro-coulometric gas
chromatography. J. Ass. Offic. Anal. Chem. 49: 763-765
Noel, P. R., Mawdesley-Thomas, L. E., Chesterman, H., Jolly, D. and
Street, A. E. (1965) Ronnel. Chronic toxicity study in the dog. Final
report. Huntingdon Research Centre. Unpublished report
Onley, J. H. and Bertuzzi, P. F. (1966) Rapid extraction procedure for
chlorinated pesticide residues in raw animal tissues and fat and meat
products. J. Ass. Offic. Anal. Chem., 49: 370-374
Plapp, F. W. and Casida, J. E. (1958a) Bovine Metabolism of
organophosphorus insecticides, metabolic fate of 0,0-dimethyl
0-(2,4,5-trichlorophenyl) phosphorothioate in rats and a cow.
J. Agr. Food Chem., 6: 662-667
Plapp, F. W. and Casida, J. E. (1958b) Hydrolysis of the
alkyl-phosphate bond in certain dialkyl aryl phosphorothioate
insecticides by rats, cockroaches and alkali, J. Econ. Entomol., 51:
800-803
Ragab, M. T. H. (1967) Direct fluorescent detection of
organothiophosphorus pesticides and some of their sulfur-containing
breakdown products after thin-layer chromatography. J. Ass. Offic.
Anal. Chem., 50: 1088-1098
Ragab, M. T. H. (1967a) 4-(p-nitrobenzyl) pyridine as a spray
reagent for organophosphorus pesticides and some of their breakdown
products on thin-layer chromatograms. Bull. environmtl. Contamination
Toxicol., 2: 279-288
Rahn, H. W. and Urban, G. (1964) Detection of insecticidal phosphoric
acid esters and their hydrolytic cleavage products. Pharmazie, 19:
597-602
Slokma, M. B. (1964) Toxicity of Ronnel with special reference to
humans. Pitman-Moore Research Center, The Dow Chemical Co. Unpublished
report
Smith, C. T., Shaw, F. R., Anderson, D. L., Callahan, R. A. and
Ziener, W. H. (1965) Ronnel residues in eggs of poultry. J. Econ.
Entomol., 58: 1160-1161
Smith, G. N. (1968) Cholinesterase inhibition of fenchlorphos and
metabolites. The Dow Chemical Co. Unpublished report
Stevens, R. K. (1967) A rapid specific method for the gas
chromatographic determination of organophosphate pesticides in cold
pressed citrus oil. J. Ass. Offic. Anal. Chem., 50: 1236-1242
Stewart, J. H. (1968) U.S. Department of Agriculture, Washington, D.C.
Private communication
Storherr, R. W. and Watts, R. R. (1965) A sweep codistillation cleanup
method for organophosphate pesticides. J. Ass. Offic. Agr. Chem.,
48: 11-54-58
Tapernoux, A. and Magat, A. (1963) Analysis of beef meat and fat for
residues of Ronnel, an organophosphate used in the treatment of bovine
hypodermosis. Ann. Fals. Expert. Chim., 56: (658) 266-270
Teekell, R. A., Rice, J. R. and McGregor, W. S. (1963) Ronnel residues
in pig fat after being on litter treated with KORLAN(R) insecticide
granules. The Dow Chemical Company, Lake Jackson, Texas. Unpublished
report.
Watts, R. R. and Storherr, R. W. (1967) Sweep codistillation clean-up
for milk for determination of organophosphate and chlorinated
hydrocarbon pesticides. J. Ass. Offic. Anal. Chem., 50: 581-585
Wells, C. E. (1967) Validation study of a method for pesticide
residues in foods and animal feeds. J. Ass. Offic. Anal. Chem., 50:
1205-1215
Wessel, J. R. (1967) Collaborative study of a method for multiple
organophosphorus pesticides in non-fatty foods. J. Ass. Offic. Anal.
Chem., 50: 430-439
Wessel, J. R. (1968) Collaborative study of three gas chromatographic
dual detection systems for analysis of multiple chlorinated and
organophosphorus pesticides. J. Ass. Offic. Anal. Chem., 51: 666-675
