
FAO/PL:1968/M/9/1
WHO/FOOD ADD./69.35
1968 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
Issued jointly by FAO and WHO
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Committee on Pesticide Residues, which met in Geneva, 9-16 December,
1968.
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
WORLD HEALTH ORGANIZATION
Geneva, 1969
OXYDEMETON-METHYL
IDENTITY
Chemical name
O,O-dimethyl S-2-(ethylsulfinyl)ethyl phosphorothioate
S-[2-(ethylsulphinyl)ethyl] OO-dimethyl phosphorothioate
(IUPAC)
Synonyms
demeton-S-methyl sulfoxide (English version)
demeton-O-methyl sulfoxide (German version)
Metasystox R(R)
Formula
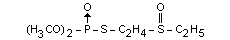 Other information on identity and properties
Technical oxydemeton-ethyl contains 85 per cent to 90 per cent of
the above phosphorothioate. Unless otherwise stated, all biological
and residue investigations have been conducted on this technical
material. The remaining 10-15 per cent were not identified.
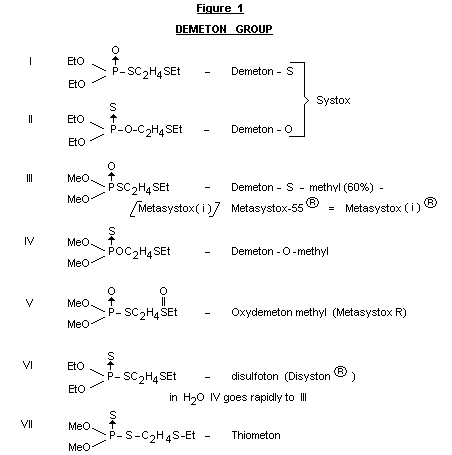
The various compounds in the so-called "demeton" group are shown in
Figure 1. The meeting noted that a range of these compounds are, or
have been, on the market. These have been available in various
formulations, and under various names, as tabulated in Figure 1. In
water, compound IV isomerizes to compound III. Compounds III and IV
would be expected to yield compound V as a common intermediate or
terminal residue. Thiometon (compound VII) would also be expected to
yield compound III and then V. Studies of the fate of the compound
appear to be limited to one involving the use of
radioisotopically-labelled material. The fate of V is also implied by
other studies with the O-ethyl analogue.
Other information on identity and properties
Technical oxydemeton-ethyl contains 85 per cent to 90 per cent of
the above phosphorothioate. Unless otherwise stated, all biological
and residue investigations have been conducted on this technical
material. The remaining 10-15 per cent were not identified.
The various compounds in the so-called "demeton" group are shown in
Figure 1. The meeting noted that a range of these compounds are, or
have been, on the market. These have been available in various
formulations, and under various names, as tabulated in Figure 1. In
water, compound IV isomerizes to compound III. Compounds III and IV
would be expected to yield compound V as a common intermediate or
terminal residue. Thiometon (compound VII) would also be expected to
yield compound III and then V. Studies of the fate of the compound
appear to be limited to one involving the use of
radioisotopically-labelled material. The fate of V is also implied by
other studies with the O-ethyl analogue.
 RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Pre-harvest treatments
Oxydemeton-ethyl has been registered on a "no-residue basis" in the
United States of America for a limited number of crops; however, these
registrations are under review. It has been proposed for use in the
United States as an emulsifiable spray on a variety of crops as
indicated in Table I to control aphids, mites, thrips, and
leafhoppers.
TABLE I
Rate of Pre-harvest
Crop application Number of interval
(kg/ha) treatments (days)
Apples, pears, plums 0.03 kg/100 l 1-3 7-30
(applied as full
coverage spray)
Blackberries, 0.03-0.04 kg/100 l 1 14
raspberries (not to exceed
2.29 kg/ha)
Strawberries 0.48-0.95 2 3
Citrus 0.03-0.04 kg/100 l 2 3
(applied as full
coverage spray)
Muskmelon, 0.48-0.63 1-3 14
cantaloupe, pumpkin,
winter squash,
head lettuce,
cotton
Watermelon 0.48-0.63 2 7
Summer squash 0.48-0.63 1 1
Cucumbers, peppers 0.48-0.63 2 0
Eggplant, cabbage 0.48-0.63 1-3 7
potatoes, turnips,
corn
TABLE I (continued)
Rate of Pre-harvest
Crop application Number of interval
(kg/ha) treatments (days)
Leaf lettuce, turnip 0.48-0.63 2 28-30
tops, sugar beets
Walnuts 0.48 1
Oxydemeton-methyl is used in at least 14 European countries but
specific conditions of use were not available to the meeting.
Generally, a pre-harvest interval of 20-30 days is practised.
Switzerland and Finland specify a 6-week pre-harvest interval;
Yugoslavia, 8 weeks; and Portugal and Austria, 5 weeks. The United
Kingdom recommends a 2-week pre-harvest interval for cereal grains.
Post-harvest treatments
No post-harvest treatment is known.
Other uses
Oxydemeton-methyl is used for the control of a number of insects on
ornamentals and for a variety of field crops grown only for seed
purposes.
Residues resulting from supervised trials
Data have been accumulated from field trials to show residues likely
to occur from the recommended use of oxydemeton-methyl as summarized
in Table I. These unpublished data, submitted by Chemagro Corporation
to the United States Food and Drug Administration in the form of
pesticide petitions, are summarized in Table II. The major portion of
the data was obtained by a total organic phosphorous method of
analysis with sensitivities of 0.1-0.3 ppm (see Methods of
analysis). A gas chromatographic method was used for the data on
lettuce, sugar beets and walnuts.
Residues on apples appear to be the most persistent with an estimated
half-life of greater than 30 days. Generally, where high initial
residues were found either because of the type of crop or from an
exaggerated dosage, the dissipation rate was rapid for the first
several days and then became more gradual as the residue dropped below
1.0 ppm. In the majority of crops the estimated half-life was 7 days
or less.
The use of oxydemeton-methyl on plants which would be used for animal
feed prompted a milk and meat residue study. 32P-labelled
oxydemeton-methyl was orally administered to two cows for 14 days at a
dosage level equivalent to 5 ppm in fresh forage. By a method
sensitive to about 0.005 ppm for both milk and tissue samples, no
residues were detected in the milk samples collected daily nor in the
liver, kidney, muscle, fat and various other organ samples examined at
0 and 7 days after termination of the feeding.
Feeding of the closely related Di-Syston to dairy cows at levels of 5
and 12 ppm showed no residues in milk or meat by a method sensitive to
0.005 ppm in milk and 0.02 ppm in meat.
Soil dissipation studies were conducted in various types of soil in
different geographical areas. When rota-tilled into the soil to give
an initial concentration of 2 ppm in the soil, no residues were found
during a period of 30-355 days after the application. Spraying of
0.31-0.94 kg/ha in 1-3 applications resulted in residue values of 0.1
ppm 27 days after the last application. (Not clear in petition whether
soil or foliage application.) Foliar application to plants apparently
does not result in accumulation of residues in the soil.
TABLE II
Pre-harvest Residue
Crop Initial interval (at harvest)
residue (ppm) (days) (ppm)
Raspberries 0.8-3.9 14 0.1-0.2
Blackberries - 14 0.1-0.6
Strawberries 0.4-3.2 3 0.2-1.7
Apples 0.8-1.5 7 0.5-1.4
Pears - 30 0.1 or less
Plums - 21 0.8
Oranges - 7 0.2-1.1 (peel)
<0.1-0.4 (whole fruit)
Lemons - 7 0.1-3.4 (peel)
<0.1-1.0 (whole fruit)
<0.1-0.2 (pulp)
Grapefruit - 7 0.1-0.3 (peel)
Cantaloups and
pumpkins - 14 <0.1
Winter squash - 14 <0.1-0.4
Summer squash - 1 <0.1-0.8
Watermelons - 7 <0.1
Cucumbers 0.2-0.7 0 0.2-0.7
Peppers 0.1-0.6 0 0.1-0.6
Eggplant 0.1-0.2 7 <0.1-1.0
Corn (maize) - 7 0.1-0.5 (kernels)
<0.1-0.3 (husks or cobs)
Corn fodder - 7 0.4-2.2
Cabbage 1.2 at 3 days 7 0.2-0.9
Head lettuce 10.7 14 <0.05-1.9
Leaf lettuce 0.8-24.3 at 3 days 28 <0.05-1.9
Turnip tops 17.1 28 0.2-1.1
Sugar beet tops - 30 <0.05-0.2
Turnips 0.3 7 <0.1-0.2
Sugar beets - 30 <0.05
Potatoes - 7 <0.1
Walnuts - 30 0.1 or less
Cottonseed 0.1 (3 days) 14 <0.1
Fate of residues
In plants
Mühlmann and Tietz (1956) reported on studies of the residues
resulting from the treatment of Vicia faba, sugar beets, cucumbers,
potatoes, and cabbages with Methylsosystox and its sulfoxide
(oxydemeton) and sulfone. It was shown that the major residue
component which formed very rapidly was the sulfoxide. This converted
very slowly to the sulfone which did not concentrate very greatly. The
authors concluded that the speed of oxidation and decomposition of the
active substances did not appear to go at a fixed rate but depended in
part on weather conditions, especially temperature, and light
conditions.
The fate of Methylisosystox compounds appears to follow the same route
as the ethyl analogues; however, there are no quantitative data on
comparative rates.
In storage and processing
Storage of treated samples of alfalfa, apples, cabbage, green oat
forage and raspberries at 0° to -10°F for 0-33 days did not result in
appreciable loss of oxydemeton-methyl residues. (Method sensitivity
about 0.1-0.3 ppm.)
Prune trees treated with 0.48 kg/ha in one application had a gross
residue of 0.13 ppm on the fresh fruit 40 days after treatment. The
residue 10 days following sun drying was 0.19 ppm. No appreciable
accumulation of residue is expected in dried fruit (method sensitivity
about 0.1 ppm).
Valencia orange trees were treated with two applications at 0.06
kg/100 1.The fruit was harvested three days after the second
application and processed immediately. No residue was detectable in
the fresh fruit pulp. A residue of 0.8 ppm found on the orange peel
was reduced to 0.6 ppm after washing. None of the processed products
(e.g. juice, oil, water-phase emulsion, cattle feed and molasses)
contained any detectable residues (method sensitivity about 0.1 ppm).
Radiometric studies in sugar beet products using 32P-labelled
oxydemeton-ethyl and the corresponding sulfone showed that 85
per cent of the radioactivity was present in the form of degradation
products in the juice. Liming and oxalation of the juice completed the
decomposition of the remaining sulfoxide and sulfone to products which
were not chloroform extractable. Radioactivity in the pulp, 8 per
cent from the sulfoxide treatment and 5 per cent from the sulfone
treatment, was in the form of decomposition products which remained in
the aqueous phase after chloroform extraction.
Evidence of residues in food in commerce or at consumption
No data available.
Methods of residue analysis
At the present time no adequate method of analysis which could be used
to enforce tolerances on samples of unknown spray history is available
for oxydemeton-methyl.
The major portion of the residue data presented in this monograph was
obtained by a method which measures total organic phosphorus. This
method provides satisfactory data on the level of residues from
supervised trials and measures the parent compound as well as the
sulfone analogue. However, it is nonspecific and not satisfactory for
enforcement of tolerances on foods in commerce. The method consists of
solvent extraction, cleanup with an activated carbon column, digestion
by mineral acids and spectrophotometric determination based on the
phospho-molybdenum blue reaction. Modified cleanup procedures are
available for oily samples, milk and meat. The method has a
sensitivity of 0.1-0.3 ppm. Recoveries ranged from 50-142 per cent
but were generally in the range of 70-100 per cent at fortification
levels of 0.1-2.3 ppm. Blanks gave values of <0.1-0.4 ppm. Similar
results were obtained in fortification studies with the sulfone
analogue.
A gas chromatographic procedure has been developed which consists of
solvent extractions, oxidation by potassium permanganate to convert
the sulfoxide to the sulfone, and gas-liquid chromatography
determination of the total sulfone using a phosphorus-sensitive
thermionic emission detector. Among the 30 crops tested recoveries
ranged from 75-117 per cent for the parent compound and 74-109 per
cent for the sulfone at fortification levels of 0.1 and 0.4 ppm.
Blank values of <0.01-<0.05 ppm were obtained. Adequate data are not
available to evaluate the performance of this method with walnuts,
milk and meat samples.
Since some of the commonly used organo-phosphorus chemicals yield
interfering peaks in gas-liquid chromatography, this method when used
as proposed with only one column is inadequate for specific
identification of residues of oxydemeton. If the above procedure can
be supplemented by an identification technique such as that offered by
a second column or an isolation step such as thin-layer
chromatography, a suitable enforcement method might be realized which
would lend assurance of positive residue identification and eliminate
possible interference from other organo-phosphorus pesticides and
degradation products.
National tolerances
Country Crop Tolerance (ppm)
Germany Leafy and other sprouting 0.4
vegetables, fruit vegetables,
legumes, fruit, grapes and
hops
Netherlands No restrictions 0.4
Switzerland Kernel fruit and grapes 0.5
Tolerances proposed by manufacturer
Australia 0.5
Austria 0.4
United States Corn (fodder and forage) 2.5
of America strawberries and leaf lettuce 1.75
apples, blackberries,
raspberries,
plums, oranges, lemons,
National tolerances (cont'd)
Country Crop Tolerance (ppm)
United States grapefruit, cabbage,
of America turnip tops 1.5
summer squash, cucumbers, head
lettuce, eggplant 1.0
peppers 0.75
corn (kernel and cob) 0.5
pears, melons, watermelons,
pumpkins, winter squash,
walnuts, sugar beet
tops, turnips 0.3
potatoes, sugar beets,
cottonseed 0.1
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Appraisal
This compound is a general systemic insecticide and acaricide and is
used on plants in many countries. The meeting noted that a range of
closely related compounds are, or have been, on the market.
There appears to be no information on the fate of residue during
storage and processing. This is important because existing data
suggest that residues tend to be persistent.
In the gas chromatographic method of analysis the sulfoxide is
oxidized to the sulfone, so that the application of this method
provides no information on the relative proportions of the sulfone and
sulfoxide in the original residue. At the present time no suitable
methods are available for regulatory or referee purposes.
Recommendations
Withdrawal (see above) of the earlier-established acceptable daily
intake and lack of quantitative and qualitative information on the
terminal residues preclude any recommendations for tolerances.
Further work or information
Required (before acceptable daily intake or tolerances can be
established)
1. Further information on the nature and persistence of residues,
especially as a result of comparative studies with the O-ethyl
analogues.
2. Specification of the compound (or compounds) in actual
agricultural use.
3. Studies to compare the metabolite fate in plants, animals and
man.
4. Adequate long-term studies in two species.
Desirable
1. Further data on residue levels in raw agricultural products
moving in commerce.
2. Investigation of cholinesterase inhibition in man.
REFERENCE
Mühlmann, R. and Tietz, H. (1956) The chemical behaviour of
Methyli-sosystox in the living plant and the problem of residues.
Höfchen Briefe 2/1956. Farbenfabriken Bayer, Leverkusen
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Pre-harvest treatments
Oxydemeton-ethyl has been registered on a "no-residue basis" in the
United States of America for a limited number of crops; however, these
registrations are under review. It has been proposed for use in the
United States as an emulsifiable spray on a variety of crops as
indicated in Table I to control aphids, mites, thrips, and
leafhoppers.
TABLE I
Rate of Pre-harvest
Crop application Number of interval
(kg/ha) treatments (days)
Apples, pears, plums 0.03 kg/100 l 1-3 7-30
(applied as full
coverage spray)
Blackberries, 0.03-0.04 kg/100 l 1 14
raspberries (not to exceed
2.29 kg/ha)
Strawberries 0.48-0.95 2 3
Citrus 0.03-0.04 kg/100 l 2 3
(applied as full
coverage spray)
Muskmelon, 0.48-0.63 1-3 14
cantaloupe, pumpkin,
winter squash,
head lettuce,
cotton
Watermelon 0.48-0.63 2 7
Summer squash 0.48-0.63 1 1
Cucumbers, peppers 0.48-0.63 2 0
Eggplant, cabbage 0.48-0.63 1-3 7
potatoes, turnips,
corn
TABLE I (continued)
Rate of Pre-harvest
Crop application Number of interval
(kg/ha) treatments (days)
Leaf lettuce, turnip 0.48-0.63 2 28-30
tops, sugar beets
Walnuts 0.48 1
Oxydemeton-methyl is used in at least 14 European countries but
specific conditions of use were not available to the meeting.
Generally, a pre-harvest interval of 20-30 days is practised.
Switzerland and Finland specify a 6-week pre-harvest interval;
Yugoslavia, 8 weeks; and Portugal and Austria, 5 weeks. The United
Kingdom recommends a 2-week pre-harvest interval for cereal grains.
Post-harvest treatments
No post-harvest treatment is known.
Other uses
Oxydemeton-methyl is used for the control of a number of insects on
ornamentals and for a variety of field crops grown only for seed
purposes.
Residues resulting from supervised trials
Data have been accumulated from field trials to show residues likely
to occur from the recommended use of oxydemeton-methyl as summarized
in Table I. These unpublished data, submitted by Chemagro Corporation
to the United States Food and Drug Administration in the form of
pesticide petitions, are summarized in Table II. The major portion of
the data was obtained by a total organic phosphorous method of
analysis with sensitivities of 0.1-0.3 ppm (see Methods of
analysis). A gas chromatographic method was used for the data on
lettuce, sugar beets and walnuts.
Residues on apples appear to be the most persistent with an estimated
half-life of greater than 30 days. Generally, where high initial
residues were found either because of the type of crop or from an
exaggerated dosage, the dissipation rate was rapid for the first
several days and then became more gradual as the residue dropped below
1.0 ppm. In the majority of crops the estimated half-life was 7 days
or less.
The use of oxydemeton-methyl on plants which would be used for animal
feed prompted a milk and meat residue study. 32P-labelled
oxydemeton-methyl was orally administered to two cows for 14 days at a
dosage level equivalent to 5 ppm in fresh forage. By a method
sensitive to about 0.005 ppm for both milk and tissue samples, no
residues were detected in the milk samples collected daily nor in the
liver, kidney, muscle, fat and various other organ samples examined at
0 and 7 days after termination of the feeding.
Feeding of the closely related Di-Syston to dairy cows at levels of 5
and 12 ppm showed no residues in milk or meat by a method sensitive to
0.005 ppm in milk and 0.02 ppm in meat.
Soil dissipation studies were conducted in various types of soil in
different geographical areas. When rota-tilled into the soil to give
an initial concentration of 2 ppm in the soil, no residues were found
during a period of 30-355 days after the application. Spraying of
0.31-0.94 kg/ha in 1-3 applications resulted in residue values of 0.1
ppm 27 days after the last application. (Not clear in petition whether
soil or foliage application.) Foliar application to plants apparently
does not result in accumulation of residues in the soil.
TABLE II
Pre-harvest Residue
Crop Initial interval (at harvest)
residue (ppm) (days) (ppm)
Raspberries 0.8-3.9 14 0.1-0.2
Blackberries - 14 0.1-0.6
Strawberries 0.4-3.2 3 0.2-1.7
Apples 0.8-1.5 7 0.5-1.4
Pears - 30 0.1 or less
Plums - 21 0.8
Oranges - 7 0.2-1.1 (peel)
<0.1-0.4 (whole fruit)
Lemons - 7 0.1-3.4 (peel)
<0.1-1.0 (whole fruit)
<0.1-0.2 (pulp)
Grapefruit - 7 0.1-0.3 (peel)
Cantaloups and
pumpkins - 14 <0.1
Winter squash - 14 <0.1-0.4
Summer squash - 1 <0.1-0.8
Watermelons - 7 <0.1
Cucumbers 0.2-0.7 0 0.2-0.7
Peppers 0.1-0.6 0 0.1-0.6
Eggplant 0.1-0.2 7 <0.1-1.0
Corn (maize) - 7 0.1-0.5 (kernels)
<0.1-0.3 (husks or cobs)
Corn fodder - 7 0.4-2.2
Cabbage 1.2 at 3 days 7 0.2-0.9
Head lettuce 10.7 14 <0.05-1.9
Leaf lettuce 0.8-24.3 at 3 days 28 <0.05-1.9
Turnip tops 17.1 28 0.2-1.1
Sugar beet tops - 30 <0.05-0.2
Turnips 0.3 7 <0.1-0.2
Sugar beets - 30 <0.05
Potatoes - 7 <0.1
Walnuts - 30 0.1 or less
Cottonseed 0.1 (3 days) 14 <0.1
Fate of residues
In plants
Mühlmann and Tietz (1956) reported on studies of the residues
resulting from the treatment of Vicia faba, sugar beets, cucumbers,
potatoes, and cabbages with Methylsosystox and its sulfoxide
(oxydemeton) and sulfone. It was shown that the major residue
component which formed very rapidly was the sulfoxide. This converted
very slowly to the sulfone which did not concentrate very greatly. The
authors concluded that the speed of oxidation and decomposition of the
active substances did not appear to go at a fixed rate but depended in
part on weather conditions, especially temperature, and light
conditions.
The fate of Methylisosystox compounds appears to follow the same route
as the ethyl analogues; however, there are no quantitative data on
comparative rates.
In storage and processing
Storage of treated samples of alfalfa, apples, cabbage, green oat
forage and raspberries at 0° to -10°F for 0-33 days did not result in
appreciable loss of oxydemeton-methyl residues. (Method sensitivity
about 0.1-0.3 ppm.)
Prune trees treated with 0.48 kg/ha in one application had a gross
residue of 0.13 ppm on the fresh fruit 40 days after treatment. The
residue 10 days following sun drying was 0.19 ppm. No appreciable
accumulation of residue is expected in dried fruit (method sensitivity
about 0.1 ppm).
Valencia orange trees were treated with two applications at 0.06
kg/100 1.The fruit was harvested three days after the second
application and processed immediately. No residue was detectable in
the fresh fruit pulp. A residue of 0.8 ppm found on the orange peel
was reduced to 0.6 ppm after washing. None of the processed products
(e.g. juice, oil, water-phase emulsion, cattle feed and molasses)
contained any detectable residues (method sensitivity about 0.1 ppm).
Radiometric studies in sugar beet products using 32P-labelled
oxydemeton-ethyl and the corresponding sulfone showed that 85
per cent of the radioactivity was present in the form of degradation
products in the juice. Liming and oxalation of the juice completed the
decomposition of the remaining sulfoxide and sulfone to products which
were not chloroform extractable. Radioactivity in the pulp, 8 per
cent from the sulfoxide treatment and 5 per cent from the sulfone
treatment, was in the form of decomposition products which remained in
the aqueous phase after chloroform extraction.
Evidence of residues in food in commerce or at consumption
No data available.
Methods of residue analysis
At the present time no adequate method of analysis which could be used
to enforce tolerances on samples of unknown spray history is available
for oxydemeton-methyl.
The major portion of the residue data presented in this monograph was
obtained by a method which measures total organic phosphorus. This
method provides satisfactory data on the level of residues from
supervised trials and measures the parent compound as well as the
sulfone analogue. However, it is nonspecific and not satisfactory for
enforcement of tolerances on foods in commerce. The method consists of
solvent extraction, cleanup with an activated carbon column, digestion
by mineral acids and spectrophotometric determination based on the
phospho-molybdenum blue reaction. Modified cleanup procedures are
available for oily samples, milk and meat. The method has a
sensitivity of 0.1-0.3 ppm. Recoveries ranged from 50-142 per cent
but were generally in the range of 70-100 per cent at fortification
levels of 0.1-2.3 ppm. Blanks gave values of <0.1-0.4 ppm. Similar
results were obtained in fortification studies with the sulfone
analogue.
A gas chromatographic procedure has been developed which consists of
solvent extractions, oxidation by potassium permanganate to convert
the sulfoxide to the sulfone, and gas-liquid chromatography
determination of the total sulfone using a phosphorus-sensitive
thermionic emission detector. Among the 30 crops tested recoveries
ranged from 75-117 per cent for the parent compound and 74-109 per
cent for the sulfone at fortification levels of 0.1 and 0.4 ppm.
Blank values of <0.01-<0.05 ppm were obtained. Adequate data are not
available to evaluate the performance of this method with walnuts,
milk and meat samples.
Since some of the commonly used organo-phosphorus chemicals yield
interfering peaks in gas-liquid chromatography, this method when used
as proposed with only one column is inadequate for specific
identification of residues of oxydemeton. If the above procedure can
be supplemented by an identification technique such as that offered by
a second column or an isolation step such as thin-layer
chromatography, a suitable enforcement method might be realized which
would lend assurance of positive residue identification and eliminate
possible interference from other organo-phosphorus pesticides and
degradation products.
National tolerances
Country Crop Tolerance (ppm)
Germany Leafy and other sprouting 0.4
vegetables, fruit vegetables,
legumes, fruit, grapes and
hops
Netherlands No restrictions 0.4
Switzerland Kernel fruit and grapes 0.5
Tolerances proposed by manufacturer
Australia 0.5
Austria 0.4
United States Corn (fodder and forage) 2.5
of America strawberries and leaf lettuce 1.75
apples, blackberries,
raspberries,
plums, oranges, lemons,
National tolerances (cont'd)
Country Crop Tolerance (ppm)
United States grapefruit, cabbage,
of America turnip tops 1.5
summer squash, cucumbers, head
lettuce, eggplant 1.0
peppers 0.75
corn (kernel and cob) 0.5
pears, melons, watermelons,
pumpkins, winter squash,
walnuts, sugar beet
tops, turnips 0.3
potatoes, sugar beets,
cottonseed 0.1
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Appraisal
This compound is a general systemic insecticide and acaricide and is
used on plants in many countries. The meeting noted that a range of
closely related compounds are, or have been, on the market.
There appears to be no information on the fate of residue during
storage and processing. This is important because existing data
suggest that residues tend to be persistent.
In the gas chromatographic method of analysis the sulfoxide is
oxidized to the sulfone, so that the application of this method
provides no information on the relative proportions of the sulfone and
sulfoxide in the original residue. At the present time no suitable
methods are available for regulatory or referee purposes.
Recommendations
Withdrawal (see above) of the earlier-established acceptable daily
intake and lack of quantitative and qualitative information on the
terminal residues preclude any recommendations for tolerances.
Further work or information
Required (before acceptable daily intake or tolerances can be
established)
1. Further information on the nature and persistence of residues,
especially as a result of comparative studies with the O-ethyl
analogues.
2. Specification of the compound (or compounds) in actual
agricultural use.
3. Studies to compare the metabolite fate in plants, animals and
man.
4. Adequate long-term studies in two species.
Desirable
1. Further data on residue levels in raw agricultural products
moving in commerce.
2. Investigation of cholinesterase inhibition in man.
REFERENCE
Mühlmann, R. and Tietz, H. (1956) The chemical behaviour of
Methyli-sosystox in the living plant and the problem of residues.
Höfchen Briefe 2/1956. Farbenfabriken Bayer, Leverkusen
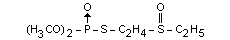 Other information on identity and properties
Technical oxydemeton-ethyl contains 85 per cent to 90 per cent of
the above phosphorothioate. Unless otherwise stated, all biological
and residue investigations have been conducted on this technical
material. The remaining 10-15 per cent were not identified.
The various compounds in the so-called "demeton" group are shown in
Figure 1. The meeting noted that a range of these compounds are, or
have been, on the market. These have been available in various
formulations, and under various names, as tabulated in Figure 1. In
water, compound IV isomerizes to compound III. Compounds III and IV
would be expected to yield compound V as a common intermediate or
terminal residue. Thiometon (compound VII) would also be expected to
yield compound III and then V. Studies of the fate of the compound
appear to be limited to one involving the use of
radioisotopically-labelled material. The fate of V is also implied by
other studies with the O-ethyl analogue.
Other information on identity and properties
Technical oxydemeton-ethyl contains 85 per cent to 90 per cent of
the above phosphorothioate. Unless otherwise stated, all biological
and residue investigations have been conducted on this technical
material. The remaining 10-15 per cent were not identified.
The various compounds in the so-called "demeton" group are shown in
Figure 1. The meeting noted that a range of these compounds are, or
have been, on the market. These have been available in various
formulations, and under various names, as tabulated in Figure 1. In
water, compound IV isomerizes to compound III. Compounds III and IV
would be expected to yield compound V as a common intermediate or
terminal residue. Thiometon (compound VII) would also be expected to
yield compound III and then V. Studies of the fate of the compound
appear to be limited to one involving the use of
radioisotopically-labelled material. The fate of V is also implied by
other studies with the O-ethyl analogue.
 RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Pre-harvest treatments
Oxydemeton-ethyl has been registered on a "no-residue basis" in the
United States of America for a limited number of crops; however, these
registrations are under review. It has been proposed for use in the
United States as an emulsifiable spray on a variety of crops as
indicated in Table I to control aphids, mites, thrips, and
leafhoppers.
TABLE I
Rate of Pre-harvest
Crop application Number of interval
(kg/ha) treatments (days)
Apples, pears, plums 0.03 kg/100 l 1-3 7-30
(applied as full
coverage spray)
Blackberries, 0.03-0.04 kg/100 l 1 14
raspberries (not to exceed
2.29 kg/ha)
Strawberries 0.48-0.95 2 3
Citrus 0.03-0.04 kg/100 l 2 3
(applied as full
coverage spray)
Muskmelon, 0.48-0.63 1-3 14
cantaloupe, pumpkin,
winter squash,
head lettuce,
cotton
Watermelon 0.48-0.63 2 7
Summer squash 0.48-0.63 1 1
Cucumbers, peppers 0.48-0.63 2 0
Eggplant, cabbage 0.48-0.63 1-3 7
potatoes, turnips,
corn
TABLE I (continued)
Rate of Pre-harvest
Crop application Number of interval
(kg/ha) treatments (days)
Leaf lettuce, turnip 0.48-0.63 2 28-30
tops, sugar beets
Walnuts 0.48 1
Oxydemeton-methyl is used in at least 14 European countries but
specific conditions of use were not available to the meeting.
Generally, a pre-harvest interval of 20-30 days is practised.
Switzerland and Finland specify a 6-week pre-harvest interval;
Yugoslavia, 8 weeks; and Portugal and Austria, 5 weeks. The United
Kingdom recommends a 2-week pre-harvest interval for cereal grains.
Post-harvest treatments
No post-harvest treatment is known.
Other uses
Oxydemeton-methyl is used for the control of a number of insects on
ornamentals and for a variety of field crops grown only for seed
purposes.
Residues resulting from supervised trials
Data have been accumulated from field trials to show residues likely
to occur from the recommended use of oxydemeton-methyl as summarized
in Table I. These unpublished data, submitted by Chemagro Corporation
to the United States Food and Drug Administration in the form of
pesticide petitions, are summarized in Table II. The major portion of
the data was obtained by a total organic phosphorous method of
analysis with sensitivities of 0.1-0.3 ppm (see Methods of
analysis). A gas chromatographic method was used for the data on
lettuce, sugar beets and walnuts.
Residues on apples appear to be the most persistent with an estimated
half-life of greater than 30 days. Generally, where high initial
residues were found either because of the type of crop or from an
exaggerated dosage, the dissipation rate was rapid for the first
several days and then became more gradual as the residue dropped below
1.0 ppm. In the majority of crops the estimated half-life was 7 days
or less.
The use of oxydemeton-methyl on plants which would be used for animal
feed prompted a milk and meat residue study. 32P-labelled
oxydemeton-methyl was orally administered to two cows for 14 days at a
dosage level equivalent to 5 ppm in fresh forage. By a method
sensitive to about 0.005 ppm for both milk and tissue samples, no
residues were detected in the milk samples collected daily nor in the
liver, kidney, muscle, fat and various other organ samples examined at
0 and 7 days after termination of the feeding.
Feeding of the closely related Di-Syston to dairy cows at levels of 5
and 12 ppm showed no residues in milk or meat by a method sensitive to
0.005 ppm in milk and 0.02 ppm in meat.
Soil dissipation studies were conducted in various types of soil in
different geographical areas. When rota-tilled into the soil to give
an initial concentration of 2 ppm in the soil, no residues were found
during a period of 30-355 days after the application. Spraying of
0.31-0.94 kg/ha in 1-3 applications resulted in residue values of 0.1
ppm 27 days after the last application. (Not clear in petition whether
soil or foliage application.) Foliar application to plants apparently
does not result in accumulation of residues in the soil.
TABLE II
Pre-harvest Residue
Crop Initial interval (at harvest)
residue (ppm) (days) (ppm)
Raspberries 0.8-3.9 14 0.1-0.2
Blackberries - 14 0.1-0.6
Strawberries 0.4-3.2 3 0.2-1.7
Apples 0.8-1.5 7 0.5-1.4
Pears - 30 0.1 or less
Plums - 21 0.8
Oranges - 7 0.2-1.1 (peel)
<0.1-0.4 (whole fruit)
Lemons - 7 0.1-3.4 (peel)
<0.1-1.0 (whole fruit)
<0.1-0.2 (pulp)
Grapefruit - 7 0.1-0.3 (peel)
Cantaloups and
pumpkins - 14 <0.1
Winter squash - 14 <0.1-0.4
Summer squash - 1 <0.1-0.8
Watermelons - 7 <0.1
Cucumbers 0.2-0.7 0 0.2-0.7
Peppers 0.1-0.6 0 0.1-0.6
Eggplant 0.1-0.2 7 <0.1-1.0
Corn (maize) - 7 0.1-0.5 (kernels)
<0.1-0.3 (husks or cobs)
Corn fodder - 7 0.4-2.2
Cabbage 1.2 at 3 days 7 0.2-0.9
Head lettuce 10.7 14 <0.05-1.9
Leaf lettuce 0.8-24.3 at 3 days 28 <0.05-1.9
Turnip tops 17.1 28 0.2-1.1
Sugar beet tops - 30 <0.05-0.2
Turnips 0.3 7 <0.1-0.2
Sugar beets - 30 <0.05
Potatoes - 7 <0.1
Walnuts - 30 0.1 or less
Cottonseed 0.1 (3 days) 14 <0.1
Fate of residues
In plants
Mühlmann and Tietz (1956) reported on studies of the residues
resulting from the treatment of Vicia faba, sugar beets, cucumbers,
potatoes, and cabbages with Methylsosystox and its sulfoxide
(oxydemeton) and sulfone. It was shown that the major residue
component which formed very rapidly was the sulfoxide. This converted
very slowly to the sulfone which did not concentrate very greatly. The
authors concluded that the speed of oxidation and decomposition of the
active substances did not appear to go at a fixed rate but depended in
part on weather conditions, especially temperature, and light
conditions.
The fate of Methylisosystox compounds appears to follow the same route
as the ethyl analogues; however, there are no quantitative data on
comparative rates.
In storage and processing
Storage of treated samples of alfalfa, apples, cabbage, green oat
forage and raspberries at 0° to -10°F for 0-33 days did not result in
appreciable loss of oxydemeton-methyl residues. (Method sensitivity
about 0.1-0.3 ppm.)
Prune trees treated with 0.48 kg/ha in one application had a gross
residue of 0.13 ppm on the fresh fruit 40 days after treatment. The
residue 10 days following sun drying was 0.19 ppm. No appreciable
accumulation of residue is expected in dried fruit (method sensitivity
about 0.1 ppm).
Valencia orange trees were treated with two applications at 0.06
kg/100 1.The fruit was harvested three days after the second
application and processed immediately. No residue was detectable in
the fresh fruit pulp. A residue of 0.8 ppm found on the orange peel
was reduced to 0.6 ppm after washing. None of the processed products
(e.g. juice, oil, water-phase emulsion, cattle feed and molasses)
contained any detectable residues (method sensitivity about 0.1 ppm).
Radiometric studies in sugar beet products using 32P-labelled
oxydemeton-ethyl and the corresponding sulfone showed that 85
per cent of the radioactivity was present in the form of degradation
products in the juice. Liming and oxalation of the juice completed the
decomposition of the remaining sulfoxide and sulfone to products which
were not chloroform extractable. Radioactivity in the pulp, 8 per
cent from the sulfoxide treatment and 5 per cent from the sulfone
treatment, was in the form of decomposition products which remained in
the aqueous phase after chloroform extraction.
Evidence of residues in food in commerce or at consumption
No data available.
Methods of residue analysis
At the present time no adequate method of analysis which could be used
to enforce tolerances on samples of unknown spray history is available
for oxydemeton-methyl.
The major portion of the residue data presented in this monograph was
obtained by a method which measures total organic phosphorus. This
method provides satisfactory data on the level of residues from
supervised trials and measures the parent compound as well as the
sulfone analogue. However, it is nonspecific and not satisfactory for
enforcement of tolerances on foods in commerce. The method consists of
solvent extraction, cleanup with an activated carbon column, digestion
by mineral acids and spectrophotometric determination based on the
phospho-molybdenum blue reaction. Modified cleanup procedures are
available for oily samples, milk and meat. The method has a
sensitivity of 0.1-0.3 ppm. Recoveries ranged from 50-142 per cent
but were generally in the range of 70-100 per cent at fortification
levels of 0.1-2.3 ppm. Blanks gave values of <0.1-0.4 ppm. Similar
results were obtained in fortification studies with the sulfone
analogue.
A gas chromatographic procedure has been developed which consists of
solvent extractions, oxidation by potassium permanganate to convert
the sulfoxide to the sulfone, and gas-liquid chromatography
determination of the total sulfone using a phosphorus-sensitive
thermionic emission detector. Among the 30 crops tested recoveries
ranged from 75-117 per cent for the parent compound and 74-109 per
cent for the sulfone at fortification levels of 0.1 and 0.4 ppm.
Blank values of <0.01-<0.05 ppm were obtained. Adequate data are not
available to evaluate the performance of this method with walnuts,
milk and meat samples.
Since some of the commonly used organo-phosphorus chemicals yield
interfering peaks in gas-liquid chromatography, this method when used
as proposed with only one column is inadequate for specific
identification of residues of oxydemeton. If the above procedure can
be supplemented by an identification technique such as that offered by
a second column or an isolation step such as thin-layer
chromatography, a suitable enforcement method might be realized which
would lend assurance of positive residue identification and eliminate
possible interference from other organo-phosphorus pesticides and
degradation products.
National tolerances
Country Crop Tolerance (ppm)
Germany Leafy and other sprouting 0.4
vegetables, fruit vegetables,
legumes, fruit, grapes and
hops
Netherlands No restrictions 0.4
Switzerland Kernel fruit and grapes 0.5
Tolerances proposed by manufacturer
Australia 0.5
Austria 0.4
United States Corn (fodder and forage) 2.5
of America strawberries and leaf lettuce 1.75
apples, blackberries,
raspberries,
plums, oranges, lemons,
National tolerances (cont'd)
Country Crop Tolerance (ppm)
United States grapefruit, cabbage,
of America turnip tops 1.5
summer squash, cucumbers, head
lettuce, eggplant 1.0
peppers 0.75
corn (kernel and cob) 0.5
pears, melons, watermelons,
pumpkins, winter squash,
walnuts, sugar beet
tops, turnips 0.3
potatoes, sugar beets,
cottonseed 0.1
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Appraisal
This compound is a general systemic insecticide and acaricide and is
used on plants in many countries. The meeting noted that a range of
closely related compounds are, or have been, on the market.
There appears to be no information on the fate of residue during
storage and processing. This is important because existing data
suggest that residues tend to be persistent.
In the gas chromatographic method of analysis the sulfoxide is
oxidized to the sulfone, so that the application of this method
provides no information on the relative proportions of the sulfone and
sulfoxide in the original residue. At the present time no suitable
methods are available for regulatory or referee purposes.
Recommendations
Withdrawal (see above) of the earlier-established acceptable daily
intake and lack of quantitative and qualitative information on the
terminal residues preclude any recommendations for tolerances.
Further work or information
Required (before acceptable daily intake or tolerances can be
established)
1. Further information on the nature and persistence of residues,
especially as a result of comparative studies with the O-ethyl
analogues.
2. Specification of the compound (or compounds) in actual
agricultural use.
3. Studies to compare the metabolite fate in plants, animals and
man.
4. Adequate long-term studies in two species.
Desirable
1. Further data on residue levels in raw agricultural products
moving in commerce.
2. Investigation of cholinesterase inhibition in man.
REFERENCE
Mühlmann, R. and Tietz, H. (1956) The chemical behaviour of
Methyli-sosystox in the living plant and the problem of residues.
Höfchen Briefe 2/1956. Farbenfabriken Bayer, Leverkusen
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Pre-harvest treatments
Oxydemeton-ethyl has been registered on a "no-residue basis" in the
United States of America for a limited number of crops; however, these
registrations are under review. It has been proposed for use in the
United States as an emulsifiable spray on a variety of crops as
indicated in Table I to control aphids, mites, thrips, and
leafhoppers.
TABLE I
Rate of Pre-harvest
Crop application Number of interval
(kg/ha) treatments (days)
Apples, pears, plums 0.03 kg/100 l 1-3 7-30
(applied as full
coverage spray)
Blackberries, 0.03-0.04 kg/100 l 1 14
raspberries (not to exceed
2.29 kg/ha)
Strawberries 0.48-0.95 2 3
Citrus 0.03-0.04 kg/100 l 2 3
(applied as full
coverage spray)
Muskmelon, 0.48-0.63 1-3 14
cantaloupe, pumpkin,
winter squash,
head lettuce,
cotton
Watermelon 0.48-0.63 2 7
Summer squash 0.48-0.63 1 1
Cucumbers, peppers 0.48-0.63 2 0
Eggplant, cabbage 0.48-0.63 1-3 7
potatoes, turnips,
corn
TABLE I (continued)
Rate of Pre-harvest
Crop application Number of interval
(kg/ha) treatments (days)
Leaf lettuce, turnip 0.48-0.63 2 28-30
tops, sugar beets
Walnuts 0.48 1
Oxydemeton-methyl is used in at least 14 European countries but
specific conditions of use were not available to the meeting.
Generally, a pre-harvest interval of 20-30 days is practised.
Switzerland and Finland specify a 6-week pre-harvest interval;
Yugoslavia, 8 weeks; and Portugal and Austria, 5 weeks. The United
Kingdom recommends a 2-week pre-harvest interval for cereal grains.
Post-harvest treatments
No post-harvest treatment is known.
Other uses
Oxydemeton-methyl is used for the control of a number of insects on
ornamentals and for a variety of field crops grown only for seed
purposes.
Residues resulting from supervised trials
Data have been accumulated from field trials to show residues likely
to occur from the recommended use of oxydemeton-methyl as summarized
in Table I. These unpublished data, submitted by Chemagro Corporation
to the United States Food and Drug Administration in the form of
pesticide petitions, are summarized in Table II. The major portion of
the data was obtained by a total organic phosphorous method of
analysis with sensitivities of 0.1-0.3 ppm (see Methods of
analysis). A gas chromatographic method was used for the data on
lettuce, sugar beets and walnuts.
Residues on apples appear to be the most persistent with an estimated
half-life of greater than 30 days. Generally, where high initial
residues were found either because of the type of crop or from an
exaggerated dosage, the dissipation rate was rapid for the first
several days and then became more gradual as the residue dropped below
1.0 ppm. In the majority of crops the estimated half-life was 7 days
or less.
The use of oxydemeton-methyl on plants which would be used for animal
feed prompted a milk and meat residue study. 32P-labelled
oxydemeton-methyl was orally administered to two cows for 14 days at a
dosage level equivalent to 5 ppm in fresh forage. By a method
sensitive to about 0.005 ppm for both milk and tissue samples, no
residues were detected in the milk samples collected daily nor in the
liver, kidney, muscle, fat and various other organ samples examined at
0 and 7 days after termination of the feeding.
Feeding of the closely related Di-Syston to dairy cows at levels of 5
and 12 ppm showed no residues in milk or meat by a method sensitive to
0.005 ppm in milk and 0.02 ppm in meat.
Soil dissipation studies were conducted in various types of soil in
different geographical areas. When rota-tilled into the soil to give
an initial concentration of 2 ppm in the soil, no residues were found
during a period of 30-355 days after the application. Spraying of
0.31-0.94 kg/ha in 1-3 applications resulted in residue values of 0.1
ppm 27 days after the last application. (Not clear in petition whether
soil or foliage application.) Foliar application to plants apparently
does not result in accumulation of residues in the soil.
TABLE II
Pre-harvest Residue
Crop Initial interval (at harvest)
residue (ppm) (days) (ppm)
Raspberries 0.8-3.9 14 0.1-0.2
Blackberries - 14 0.1-0.6
Strawberries 0.4-3.2 3 0.2-1.7
Apples 0.8-1.5 7 0.5-1.4
Pears - 30 0.1 or less
Plums - 21 0.8
Oranges - 7 0.2-1.1 (peel)
<0.1-0.4 (whole fruit)
Lemons - 7 0.1-3.4 (peel)
<0.1-1.0 (whole fruit)
<0.1-0.2 (pulp)
Grapefruit - 7 0.1-0.3 (peel)
Cantaloups and
pumpkins - 14 <0.1
Winter squash - 14 <0.1-0.4
Summer squash - 1 <0.1-0.8
Watermelons - 7 <0.1
Cucumbers 0.2-0.7 0 0.2-0.7
Peppers 0.1-0.6 0 0.1-0.6
Eggplant 0.1-0.2 7 <0.1-1.0
Corn (maize) - 7 0.1-0.5 (kernels)
<0.1-0.3 (husks or cobs)
Corn fodder - 7 0.4-2.2
Cabbage 1.2 at 3 days 7 0.2-0.9
Head lettuce 10.7 14 <0.05-1.9
Leaf lettuce 0.8-24.3 at 3 days 28 <0.05-1.9
Turnip tops 17.1 28 0.2-1.1
Sugar beet tops - 30 <0.05-0.2
Turnips 0.3 7 <0.1-0.2
Sugar beets - 30 <0.05
Potatoes - 7 <0.1
Walnuts - 30 0.1 or less
Cottonseed 0.1 (3 days) 14 <0.1
Fate of residues
In plants
Mühlmann and Tietz (1956) reported on studies of the residues
resulting from the treatment of Vicia faba, sugar beets, cucumbers,
potatoes, and cabbages with Methylsosystox and its sulfoxide
(oxydemeton) and sulfone. It was shown that the major residue
component which formed very rapidly was the sulfoxide. This converted
very slowly to the sulfone which did not concentrate very greatly. The
authors concluded that the speed of oxidation and decomposition of the
active substances did not appear to go at a fixed rate but depended in
part on weather conditions, especially temperature, and light
conditions.
The fate of Methylisosystox compounds appears to follow the same route
as the ethyl analogues; however, there are no quantitative data on
comparative rates.
In storage and processing
Storage of treated samples of alfalfa, apples, cabbage, green oat
forage and raspberries at 0° to -10°F for 0-33 days did not result in
appreciable loss of oxydemeton-methyl residues. (Method sensitivity
about 0.1-0.3 ppm.)
Prune trees treated with 0.48 kg/ha in one application had a gross
residue of 0.13 ppm on the fresh fruit 40 days after treatment. The
residue 10 days following sun drying was 0.19 ppm. No appreciable
accumulation of residue is expected in dried fruit (method sensitivity
about 0.1 ppm).
Valencia orange trees were treated with two applications at 0.06
kg/100 1.The fruit was harvested three days after the second
application and processed immediately. No residue was detectable in
the fresh fruit pulp. A residue of 0.8 ppm found on the orange peel
was reduced to 0.6 ppm after washing. None of the processed products
(e.g. juice, oil, water-phase emulsion, cattle feed and molasses)
contained any detectable residues (method sensitivity about 0.1 ppm).
Radiometric studies in sugar beet products using 32P-labelled
oxydemeton-ethyl and the corresponding sulfone showed that 85
per cent of the radioactivity was present in the form of degradation
products in the juice. Liming and oxalation of the juice completed the
decomposition of the remaining sulfoxide and sulfone to products which
were not chloroform extractable. Radioactivity in the pulp, 8 per
cent from the sulfoxide treatment and 5 per cent from the sulfone
treatment, was in the form of decomposition products which remained in
the aqueous phase after chloroform extraction.
Evidence of residues in food in commerce or at consumption
No data available.
Methods of residue analysis
At the present time no adequate method of analysis which could be used
to enforce tolerances on samples of unknown spray history is available
for oxydemeton-methyl.
The major portion of the residue data presented in this monograph was
obtained by a method which measures total organic phosphorus. This
method provides satisfactory data on the level of residues from
supervised trials and measures the parent compound as well as the
sulfone analogue. However, it is nonspecific and not satisfactory for
enforcement of tolerances on foods in commerce. The method consists of
solvent extraction, cleanup with an activated carbon column, digestion
by mineral acids and spectrophotometric determination based on the
phospho-molybdenum blue reaction. Modified cleanup procedures are
available for oily samples, milk and meat. The method has a
sensitivity of 0.1-0.3 ppm. Recoveries ranged from 50-142 per cent
but were generally in the range of 70-100 per cent at fortification
levels of 0.1-2.3 ppm. Blanks gave values of <0.1-0.4 ppm. Similar
results were obtained in fortification studies with the sulfone
analogue.
A gas chromatographic procedure has been developed which consists of
solvent extractions, oxidation by potassium permanganate to convert
the sulfoxide to the sulfone, and gas-liquid chromatography
determination of the total sulfone using a phosphorus-sensitive
thermionic emission detector. Among the 30 crops tested recoveries
ranged from 75-117 per cent for the parent compound and 74-109 per
cent for the sulfone at fortification levels of 0.1 and 0.4 ppm.
Blank values of <0.01-<0.05 ppm were obtained. Adequate data are not
available to evaluate the performance of this method with walnuts,
milk and meat samples.
Since some of the commonly used organo-phosphorus chemicals yield
interfering peaks in gas-liquid chromatography, this method when used
as proposed with only one column is inadequate for specific
identification of residues of oxydemeton. If the above procedure can
be supplemented by an identification technique such as that offered by
a second column or an isolation step such as thin-layer
chromatography, a suitable enforcement method might be realized which
would lend assurance of positive residue identification and eliminate
possible interference from other organo-phosphorus pesticides and
degradation products.
National tolerances
Country Crop Tolerance (ppm)
Germany Leafy and other sprouting 0.4
vegetables, fruit vegetables,
legumes, fruit, grapes and
hops
Netherlands No restrictions 0.4
Switzerland Kernel fruit and grapes 0.5
Tolerances proposed by manufacturer
Australia 0.5
Austria 0.4
United States Corn (fodder and forage) 2.5
of America strawberries and leaf lettuce 1.75
apples, blackberries,
raspberries,
plums, oranges, lemons,
National tolerances (cont'd)
Country Crop Tolerance (ppm)
United States grapefruit, cabbage,
of America turnip tops 1.5
summer squash, cucumbers, head
lettuce, eggplant 1.0
peppers 0.75
corn (kernel and cob) 0.5
pears, melons, watermelons,
pumpkins, winter squash,
walnuts, sugar beet
tops, turnips 0.3
potatoes, sugar beets,
cottonseed 0.1
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Appraisal
This compound is a general systemic insecticide and acaricide and is
used on plants in many countries. The meeting noted that a range of
closely related compounds are, or have been, on the market.
There appears to be no information on the fate of residue during
storage and processing. This is important because existing data
suggest that residues tend to be persistent.
In the gas chromatographic method of analysis the sulfoxide is
oxidized to the sulfone, so that the application of this method
provides no information on the relative proportions of the sulfone and
sulfoxide in the original residue. At the present time no suitable
methods are available for regulatory or referee purposes.
Recommendations
Withdrawal (see above) of the earlier-established acceptable daily
intake and lack of quantitative and qualitative information on the
terminal residues preclude any recommendations for tolerances.
Further work or information
Required (before acceptable daily intake or tolerances can be
established)
1. Further information on the nature and persistence of residues,
especially as a result of comparative studies with the O-ethyl
analogues.
2. Specification of the compound (or compounds) in actual
agricultural use.
3. Studies to compare the metabolite fate in plants, animals and
man.
4. Adequate long-term studies in two species.
Desirable
1. Further data on residue levels in raw agricultural products
moving in commerce.
2. Investigation of cholinesterase inhibition in man.
REFERENCE
Mühlmann, R. and Tietz, H. (1956) The chemical behaviour of
Methyli-sosystox in the living plant and the problem of residues.
Höfchen Briefe 2/1956. Farbenfabriken Bayer, Leverkusen
