
FAO/PL:1968/M/9/1
WHO/FOOD ADD./69.35
1968 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
Issued jointly by FAO and WHO
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Committee on Pesticide Residues, which met in Geneva, 9-16 December,
1968.
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
WORLD HEALTH ORGANIZATION
Geneva, 1969
OXYTHIOQUINOX
IDENTITY
Chemical names
6-methyl-2-oxo-1,3-dithiolo-[4,5-b]quinoxaline (IUPAC)
Synonyms
Chinomethionat
Oxythiochinox
Morestan(R) (trade name)
Formula
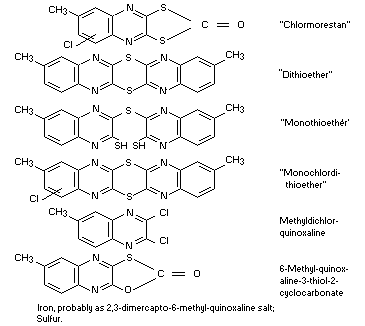
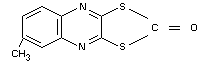 Other information on identity and properties
The technical compound contains at least 80 per cent pure active
ingredient and the following by-products in varying concentrations:
Other information on identity and properties
The technical compound contains at least 80 per cent pure active
ingredient and the following by-products in varying concentrations:
 EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
No data available.
Acute toxicity
Animal Route LD50 (mg/kg References
body-weight)
Mouse (M) i.p. 650 DuBois and Raymund, 1962
Mouse (F) i.p. 700 DuBois and Raymund, 1962
Rat (M) oral > 2500 Bayer, 1959
Rat (F) oral 3000 DuBois, 1961
Rat (M) i.p. 700 DuBois, 1961
Rat (F) i.p. 600 DuBois, 1961
Guinea-pig (M) oral 1500 DuBois and Raymund, 1962
Guinea-pig (F) i.p. 350 DuBois and Raymund, 1962
Chicken oral >500 DuBois, 1962
Cat oral >1000 Bayer, 1959
Short-term studies
Rat. A group of 10 male rats was treated daily with 100 mg/kg(in one
per cent aqueous tragacanth suspension) by stomach tube, for 28 days.
One death occurred after 16 days; autopsy of this animal revealed
kidney damage. Loss of appetite and body-weight were observed in the
other animals after 20 doses. The haematogram was normal (Bayer,
1959).
Four groups of 12 male and 12 female rats were fed 0, 10, 25 or 50 ppm
in the diet for 16 weeks without any deaths. Food intake was
unaffected in both sexes, as was body-weight gain in the females. A
slight transient depression of body-weight gain was observed between
four and 12 weeks, in the males fed 50 ppm. Based on data from five
male and five female animals per group, gross and histopathology were
comparable to the controls. Depression of absolute liver and kidney
weights, apparent in all male test groups was not dose related (Doull
et al., 1963).
Six groups, each comprising five male weanling rats were fed for 90
days, dietary levels of 0, 10, 25, 60, 150 and 500 ppm active
ingredient of oxythioquinox as the 25 per cent wettable powder.
Body-weight gain was depressed at the 500 ppm level, probably due, at
least in part, to diet rejection. Liver to body-weight ratio was
significantly increased at 500 ppm. Investigations of liver microsomal
enzyme activity (EPN detoxification, O-demethylase, and acetoacetic
acid synthesis) showed depressed activity in all systems at 500 ppm,
and acetoacetic acid synthesis was also inhibited at 150 ppm. Analysis
of liver tissue failed to reveal the presence of oxythioquinox using a
method sensitive to the detection of 0.5 ppm (Carlson and DuBois,
1968).
Oral administration of an aqueous emulsion five times weekly for four
months to groups of 10 male rats at dose levels of 0, 10, 25, 50, 100
or 250 mg/kg did not cause any mortality. Haematograms and urinalysis
were normal at all dose levels. However, clinical symptoms (hair loss)
were apparent at 250 mg/kg after seven weeks. Body-weight gain was
reduced in the 100, and 250 mg/kg groups and liver-weight ratios were
increased at these levels. Histological damage to liver cells was
apparent at 250 mg/kg (Kimmerle, 1963).
In the three generation rat study, described under "Special studies.
Reproduction", liver damage was observed in the FO generation in the
animals fed 500 ppm. In this group, gross pathology was normal but
histopathology showed 60 per cent of the animals displaying toxic
injury (periportally arranged swollen liver epithelial cells, with
several pyknotic nuclei) with a further eight per cent showing
questionable injury (Hecht and Grundmann, 1964).
Dog. Groups of two male and two female dogs were fed 0, 10, 25 or 50
ppm in dry diet for 28 months. Body-weight, food intake, general
behaviour and appearance, haematograms, serum glutamic oxaloacetic
transaminase, and serum glutamic-pyruvic transaminase determinations,
organ weights (absolute and organ to body-weight ratios) and gross
pathology of treated animals were all comparable to the controls.
Histological examination revealed inflammatory cell foci in the livers
of all the dogs which received 25 and 50 ppm; but the same effect was
noted in two-thirds of the 10 ppm group and in three of the four
controls (Doull et al., 1966).
Long-term studies
Rat. Technical oxythioquinox (91 per cent pure) was administered to
groups of 50 male and 50 female rats at dietary levels of 10, 25, 60,
125 or 500 ppm, for two years. One hundred male and 100 female rats
served as untreated controls. Clinical symptoms in the form of yellow
staining of the hair of the paws were apparent at 60 ppm, the
frequency increasing with increasing dose level. At 500 ppm, abdominal
and facial fur were also stained yellow. Body-weight gain was
depressed in both sexes, and food intake was depressed in the female
at 500 ppm. Incidence of mortality, haematograms, urinalysis, and
gross pathology were all comparable to the controls. Marked liver
hypertrophy was shown, as evidenced by absolute organ weights, the
increase being significant in all test groups except the females fed
25 ppm. Thyroid weights were also significantly increased in males at
150 and 500 ppm, and in females at 25 and 60 ppm. There was a tendency
towards decreased adrenal weights in males at 150 and 500 ppm, and in
females at 500 ppm. Pituitary enlargement was, however, comparable in
all groups (Lorke and Loser, 1966a).
Histopathologically, vacuolar cytoplasmic swelling was noted in the
liver at all levels, the incidence tending to increase with increasing
dose level. Necrotic lesions were observed only at 500 ppm. Bile duct
hyperplasia occurred in six out of 38 of the rats fed 500 ppm. In the
testes reduced spermatogenesis was significantly more frequent at 150
and 500 ppm. Tumour incidence was unrelated to the dose level
(Grundmann and Hobik, 1966a),
Special studies
Reproduction
Rat. In a three-generation rat study, 91 per cent pure technical
oxythioquinox was incorporated in the diets of groups of eight male
and 16 female rats (except for the F2b breeding animals where 10
males and 20 females were utilized). At levels of 0, 10, 25, 60 and
150 ppm, no effects on adult body-weight, incidence of pregnancy,
litter size, birth and weaning weights, or survival to four weeks were
noted up to and including 60 ppm. At 150 ppm, incidence of pregnancy
was reduced in both first generation litters and litter size was
reduced in one litter in each generation. At 500 ppm, pregnancy was
inhibited. Cross breeding studies showed the inhibition to be due to
male infertility. Treated females mated with untreated males produced
litters. but the litter size was markedly reduced. The 500 ppm group
was discontinued after the first attempted mating. Body-weight gain
was reduced in female rats and doubtfully in male rats at 500 ppm.
Abnormalities observed during the study included uni- and bilateral
anophthalmia, and unspecified abnormalities of the incisor teeth.
These abnormalities were randomly distributed between groups. In the
F1a litters, one hairless offspring occurred in the 60 and 150 ppm
groups.
Organ weights and histopathology on two male and two female animals in
the F3b litter were comparable to the controls (Lorke and Loser,
1966; Grundmann and Hobik, 1966).
Observations in man
Compressions containing dry or moist oxythioquinox were applied to the
forearms of nine human volunteers for varying periods of two, four,
eight or 24 hours. Exposures for 24 hours resulted in reddening of the
skin and occasional swelling and blistering, the incidence and
severity of these injuries being more marked with the moist product
(Bayer, 1959).
Comments
Adequate data are available on acute and short-term studies. However,
a no-effect level has not been demonstrated in long-term studies in
rat. Liver hypertrophy is extensive at 10 ppm, the lowest dose tested.
At present it is not possible to estimate a daily acceptable intake
for man. Until further data are available, foods should not be
permitted to contain any residues of oxythioquinox and its possible
metabolites from treated plants.
Further information is needed concerning the metabolic fate of this
pesticide in various animals including man; particularly further
research is needed to ascertain its effect on spermatogenesis and
whether this phenomenon occurs in primates. Since it has been reported
that there is an injurious effect to skin upon prolonged application,
it will be necessary to do further studies on the cutaneous toxicity
of this compound including studies related to the question of
photo-sensitization.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Pre-harvest treatment
Oxythioquinox is used as fungicide and acaricide on a large number of
crops. As a fungicide, it is used against mildew diseases on pome,
stone and soft fruit, strawberries, grapes and cucurbits and has a
protective and curative action (Grewe et al., 1965; Wäckers et al.,
1965). As an acaricide, it is effective not only against susceptible
mite strains, but also against strains which are resistant to other
acaricides (Unterstenhöfer et al., 1965). A good insecticidal side
effect has been observed against Psylla piri (Unterstenhöfer et al.,
1965).
Established pre-harvest intervals are as follows:
Pre-harvest
Country Crop interval (days)
Denmark Tree fruit 8
Cucumbers 4
Germany Tree fruit and vegetables 14
Cucumbers 4
United Kingdom Tree fruit 21
Gooseberries, black currants 14
Pre-harvest
Country Crop interval (days)
Marrow 7
Cucumbers grown under glass 3
Finland - 14
Holland Tree fruit 28
Cucumbers 3
Israel Apples, citrus fruit, grapes 14
tomatoes, strawberries,
egg-plants, peppers 7
Italy - 5
Yugoslavia Tree fruit 14
Cucumbers 7
Austria Tree fruit 14
Vegetables 4
Poland Tree fruit and vegetables 21
Cucumbers 3
Sweden General 7
Cucumbers 4
Switzerland Tree fruit 21
Vegetables 5
Spain Cucumbers 10
Other crops 15
Post-harvest treatments
No use.
Other uses
Oxythioquinox is used against mildew and mites on ornamentals (Grewe
et al., 1965; Wäckers et al., 1965; Besemer et al., 1963).
Residues resulting from supervised trials
In the following table, residue values are given after application at
the recommended concentrations:
Residue at harvest
Pre-harvest (ppm)
Crop Number of interval
treatments (days) Range Average
Apples 1-4 7-10 n.d.-1.5 0.2
(United
States of 1 7 0.8 0.8
America)
Pears 3 7-8 0.1-0.8 0.4
(United
States of 1 7-8 0.2-2.1 1.2
America
Strawberries 2 7-8 n.d.-2.2 1.0
(United
States of 7 n.d. n.d.
America
Grapes 2 7 n.d.-9.4 6.0
(United
States of 7 12.5 12.5
America)
Cucumbers 2 4 n.d. n.d.
Summer 4 1-7 n.d.-0.9 0.6
squash 4 1-7 n.d.-1.2 0.9
Winter 4 1-8 n.d.-0.1 n.d.
squash 4 1-8 0.5-0.6 0.5
Alfalfa 1 7 0.3-1.1 0.9
(United 7 3.6 3.6
States of 14 0.1-0.3 0.1
America 14 3.0 3.0
Fate of residues
General comments
The half-life of oxythioquinox on apples, pears, and goose berries is
between five and nine days (Grewe et al., 1965). Loss probably is
caused not only by enzymatic processes, but also by physical and
chemical influences in the environment. Wash-off may occur only a
short time after application. After a few days even heavy rainfall
causes no further noticeable loss. Probably oxythioquinox is dissolved
in the wax layers of the plant surfaces (Grewe et al., 1965).
In diluted ammoniacal solution, oxythioquinox is instantly saponified
to form 2,3-dithiol-6-methylquinoxaline with liberation of carbonate
(Grewe et al., 1965). This substance, which is also formed in plants
(Chemagro Internal Report), is probably unstable to light, as shown by
experiments with its analogue 2,3-dithiolquinoxaline, which is formed
under similar conditions from Eradex, a compound related to
oxythioquinox (Tietz et al., 1962).
In soils
The half-life of oxythioquinox in soils is about 60 days
(Farbenfabriken Bayer A.G., private communication).
In plants
When oxythioquinox C14 was applied to growing oranges and apples,
there was a steady decrease in the amount of total radioactivity.
While activity on the surface was due only to oxythioquinox itself
free and bound 2,3-dithio-6-methyl quinoxaline and other, unknown
metabolites were found in the peel, reaching a maximum at 7-14 days
and then decreasing slowly. The unknown metabolites were not
extractable with organic solvents. Reduction followed by treatment
with boron trifluoride-methanol and diazomethane solubilized
approximately 80 per cent of the insoluble material. Perhaps this
consists of conjugated compounds with carboxylic groups or phenolic or
glycosidic hydroxyls. In the pulp only traces of radioactivity were
found (Chemagro Internal Report).
In animals
When rats were fed carbonyl-14C-labelled oxythioquinox. it was found
that most of the radioactivity was exhaled as 14CO2. After
2,3-14C- and 35S-labelled active ingredient was fed to rats, no
14CO2 was found in the air expired. In this particular case, most
of the activity was present in the urine and in the faeces. A small
proportion of the activity was found in the blood plasma in
protein-bound form. The activity is not present as oxythioquinox
itself, but partially as 2,3-dithiol-6-methyl quinoxaline, in traces
as the corresponding dihydroxy compound, and as other still
unidentified metabolites. The quinoxaline ring is apparently not
metabolized in the animal body (Chemagro Internal Report).
In storage and processing
Washing of oranges containing 0.4 ppm oxythioquinox reduced the
residue to the non-detectable level (Chemagro Internal Report).
Peeling reduces residues to values below 1 ppm.
ppm in peel ppm in pulp (seven
(seven days days after
after application)
Crop application)
Oranges 0.2-4.4 n.d.-0.2
Lemons 0.2-1.2 n.d.
Grapefruit n.d.-1.8 n.d.-0.2
Apples 1.0-4.1 0.1-0.7
(Farbenfabriken Bayer A.G., private communication)
After processing oranges containing 0.4 ppm oxythioquinox, residues of
less than 0.1 ppm were detected in frozen and heated juice, chopped
peel and cattle feed, press liquor and molasses. A residue level of
1.7 ppm was found only in cold pressed citrus oil (Chemagro Internal
Report).
Evidence of residues in food in commerce or at consumption
No data available.
Methods of residue analysis
Analytical methods appear to be sufficient for present purposes. A
colorimetric method has been developed by Havens et al., 1964. It is
an adaption of a method to determine residues of "Eradex New" in plant
material (Tietz et al., 1962). The procedure involves a hydrolysis
with concentrated ammonium hydroxide to give
2,3-dithiol-6-methylquinoxaline. Subsequent treatment with ammonical
nickel reagent gives a red-coloured chelate which is measured at
540 mµ. The limit of sensitivity is 0.14 ppm. This method gives poor
results, when large amounts of oil are present in the sample.
For the determination of residues in orange peel. a method has been
described based on a combination of thin-layer chromatography and
cathode ray polarography (Hearth et al., 1966). Sensitivity: 0.5 ppm.
Gas chromatographic determination with an electron capture detector is
used for moist crops, dry hops, and for crops with high oil content.
Sensitivity: 0.1 ppm for most crops (Vogeler et al., 1967; Chemagro
Internal Reports).
National tolerances
No registrations have been agreed in the United States of America. A
petition will be filed for the following tolerances:
Walnuts: 0.1 ppm
Cucumbers, water melons, winter squash: 0.75 ppm
Apples, pears, melons (except water melons), summer squash: 1.5 ppm
Citrus, grapes: 2.5 ppm
Papayas: 5.0 ppm
Strawberries: 6 ppm
In Germany the tolerance for cucumbers is 0.1 ppm. For pome fruit, a
tolerance of 0.3 ppm has been proposed.
In the Netherlands and Switzerland, a tolerance of 0.1 ppm has been
agreed for fruit, vegetables, and cucumbers.
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Appraisal
This compound is widely used as a fungicide and an acaricide in many
countries. The technical product contains at least 80 per cent pure
active ingredient and at least six by-products in varying
concentrations. Further information on the composition of the
technical product is required, together with information on the
quantities being used in each country. The residues consist of the
parent compound, one identified metabolite and other metabolites not
yet identified. Accordingly further information on the chemical nature
of the terminal residues is also required together with the nature of
degradation products in plants and animals.
A variety of analytical methods are available which would be adequate
to detect residues down to 0.1 ppm, including gas-liquid
chromatography, thin-layer chromatography, polarography. However, if
tolerances of 0.1 ppm or below are to be considered, improved methods
of analysis would be required.
Recommendations
Since an acceptable daily intake has not been established, tolerances
cannot be recommended.
Further work or information
Required (before an acceptable daily intake or tolerances can be
established)
1. Information on the nature of terminal residues in plants and
animal products.
2. Data from countries other than the United States of America on
the required rates and frequencies of application, pre-harvest
intervals, and the resultant residues.
3. Further data on residue levels in raw agricultural products
moving in commerce.
4. Data on residue levels in total diet studies.
5. Comparative evaluation of methods of analysis for regulatory
purposes.
6. Experimental studies on the metabolism responsible for liver
hyperplasia in rats.
7. Biochemical studies on excretion and metabolism.
8. Two-year studies on rats at lower dosage.
9. Further information on anti-spermatogenic effects.
Desirable
1. Collaborative studies to establish a referee method.
2. Studies of metabolism in various animals including man.
3. Further studies on the cutaneous toxicity, including studies
related to the question of photosensitization.
REFERENCES
Bayer. Active ingredient Dr Sasse Ss2074. Farbenfabriken Bayer
Basemer, A. F. H. and Immikhuizen, E. (1963) Biologisch Veld-en
Kasonderzoek van Insekticiden, Acariciden en Fungiciden in
Tuinbouwgewassen. P.D.-Jaarboek 1962, Wageningen, No. 138: 131-146
Carlson, B. P. and DuBois, K. P. (1968) Effects of feeding various
dietary levels of Morestan to male rats for 90 days. University of
Chicago. Unpublished report
Doull, J., DiGiacomo, R., Root, M., Vesselinovitch, D. and Meskauskas,
J. (1966) Chronic oral toxicity of Morestan (Bayer 36205) to male and
female dogs. University of Chicago. Unpublished report
Doull, J., Root, M. and Gowan, J. (1963) Subacute oral toxicity of
Morestan (Bayer 36205) to male and female rats. University of Chicago.
Unpublished report
DuBois, K. P. (1961) Intraperitoneal and oral toxicity of Bayer 36205
to rats. University of Chicago. Unpublished report
DuBois, K. P. (1962) The acute oral toxicity of Bayer 36205 to
chickens. University of Chicago. Unpublished report
DuBois, K. P. and Raymund, A. B. (1962) The acute toxicity of Bayer
36205 to mice, guinea pigs and rats. University of Chicago.
Unpublished report
Grewe, F. and Kaspers, H. (1965) Morestan, a new fungicide of the
2,3-disubstituted quinoxaline group for controlling powdery mildews.
Pflanzenschutz-Nachrichten "Bayer", 18: 1-23
Grundmann, E. and Hobik, H. P. (1966a) Bay 36205, two-year feeding
test with rats. Histology. Farbenfabriken Bayer. Unpublished report
Grundmann, E. and Hobik, H. P. (1966b) Bay 36205 Generation test.
Histology. Farbenfabriken Bayer. Unpublished report
Havens, R., Adams, J. M. and Anderson, C. A. (1964) Colorimetric
determination of 6-methyl-2,3-quinoxalinedithiol cyclic carbonate
(Morestan) residues in apples and pears. J. Agr. Food Chem.,
12: 247-248
Hearth, F. E., Ott, D. E. and Gunther, F. A. (1966)
Oscillopolarographic analysis of Morestan residues in Valencia orange
rind following thin layer chromatography. J. Assoc. off. agric.
Chemists, 49: 774-778
Hecht, G. and Grundmann, E. (1964) Report on testing of Morestan for
toxic effects on the liver. Farbenfabriken Bayer. Unpublished report
Kimmerle, G. (1963) Four months' feeding test on rats with the active
ingredient Ss2074. Farbenfabriken Bayer. Unpublished report
Lorke, D. and Loser, E. (1966a) Bay 36205 studies of chronic toxicity
to rats. Farbenfabriken Bayer. Unpublished report
Lorke, D. and Loser, E. (1966b) Bay 36205 studies of chronic toxicity
to rats. Farbenfabriken Bayer. Unpublished report
Tietz, M. et al. (1962) Method of determining residues of the
acaricide Eradex New in plant material. Pflanzenschutz-Nachrichten
"Bayer", 15: 166-171
Vogeler, K. and Niessen, H. (1967) Gas chromatographic determination
of Morestan residues in plants. Pflanzenschutz-Nachrichten "Bayer",
20: 550-556
Wäckers, R. and van den Berge, C. (1965) Experiences with Morestan in
Dutch fruit farming and market gardening. Pflanzenschutz-Nachrichten
"Bayer", 18: 34-44
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
No data available.
Acute toxicity
Animal Route LD50 (mg/kg References
body-weight)
Mouse (M) i.p. 650 DuBois and Raymund, 1962
Mouse (F) i.p. 700 DuBois and Raymund, 1962
Rat (M) oral > 2500 Bayer, 1959
Rat (F) oral 3000 DuBois, 1961
Rat (M) i.p. 700 DuBois, 1961
Rat (F) i.p. 600 DuBois, 1961
Guinea-pig (M) oral 1500 DuBois and Raymund, 1962
Guinea-pig (F) i.p. 350 DuBois and Raymund, 1962
Chicken oral >500 DuBois, 1962
Cat oral >1000 Bayer, 1959
Short-term studies
Rat. A group of 10 male rats was treated daily with 100 mg/kg(in one
per cent aqueous tragacanth suspension) by stomach tube, for 28 days.
One death occurred after 16 days; autopsy of this animal revealed
kidney damage. Loss of appetite and body-weight were observed in the
other animals after 20 doses. The haematogram was normal (Bayer,
1959).
Four groups of 12 male and 12 female rats were fed 0, 10, 25 or 50 ppm
in the diet for 16 weeks without any deaths. Food intake was
unaffected in both sexes, as was body-weight gain in the females. A
slight transient depression of body-weight gain was observed between
four and 12 weeks, in the males fed 50 ppm. Based on data from five
male and five female animals per group, gross and histopathology were
comparable to the controls. Depression of absolute liver and kidney
weights, apparent in all male test groups was not dose related (Doull
et al., 1963).
Six groups, each comprising five male weanling rats were fed for 90
days, dietary levels of 0, 10, 25, 60, 150 and 500 ppm active
ingredient of oxythioquinox as the 25 per cent wettable powder.
Body-weight gain was depressed at the 500 ppm level, probably due, at
least in part, to diet rejection. Liver to body-weight ratio was
significantly increased at 500 ppm. Investigations of liver microsomal
enzyme activity (EPN detoxification, O-demethylase, and acetoacetic
acid synthesis) showed depressed activity in all systems at 500 ppm,
and acetoacetic acid synthesis was also inhibited at 150 ppm. Analysis
of liver tissue failed to reveal the presence of oxythioquinox using a
method sensitive to the detection of 0.5 ppm (Carlson and DuBois,
1968).
Oral administration of an aqueous emulsion five times weekly for four
months to groups of 10 male rats at dose levels of 0, 10, 25, 50, 100
or 250 mg/kg did not cause any mortality. Haematograms and urinalysis
were normal at all dose levels. However, clinical symptoms (hair loss)
were apparent at 250 mg/kg after seven weeks. Body-weight gain was
reduced in the 100, and 250 mg/kg groups and liver-weight ratios were
increased at these levels. Histological damage to liver cells was
apparent at 250 mg/kg (Kimmerle, 1963).
In the three generation rat study, described under "Special studies.
Reproduction", liver damage was observed in the FO generation in the
animals fed 500 ppm. In this group, gross pathology was normal but
histopathology showed 60 per cent of the animals displaying toxic
injury (periportally arranged swollen liver epithelial cells, with
several pyknotic nuclei) with a further eight per cent showing
questionable injury (Hecht and Grundmann, 1964).
Dog. Groups of two male and two female dogs were fed 0, 10, 25 or 50
ppm in dry diet for 28 months. Body-weight, food intake, general
behaviour and appearance, haematograms, serum glutamic oxaloacetic
transaminase, and serum glutamic-pyruvic transaminase determinations,
organ weights (absolute and organ to body-weight ratios) and gross
pathology of treated animals were all comparable to the controls.
Histological examination revealed inflammatory cell foci in the livers
of all the dogs which received 25 and 50 ppm; but the same effect was
noted in two-thirds of the 10 ppm group and in three of the four
controls (Doull et al., 1966).
Long-term studies
Rat. Technical oxythioquinox (91 per cent pure) was administered to
groups of 50 male and 50 female rats at dietary levels of 10, 25, 60,
125 or 500 ppm, for two years. One hundred male and 100 female rats
served as untreated controls. Clinical symptoms in the form of yellow
staining of the hair of the paws were apparent at 60 ppm, the
frequency increasing with increasing dose level. At 500 ppm, abdominal
and facial fur were also stained yellow. Body-weight gain was
depressed in both sexes, and food intake was depressed in the female
at 500 ppm. Incidence of mortality, haematograms, urinalysis, and
gross pathology were all comparable to the controls. Marked liver
hypertrophy was shown, as evidenced by absolute organ weights, the
increase being significant in all test groups except the females fed
25 ppm. Thyroid weights were also significantly increased in males at
150 and 500 ppm, and in females at 25 and 60 ppm. There was a tendency
towards decreased adrenal weights in males at 150 and 500 ppm, and in
females at 500 ppm. Pituitary enlargement was, however, comparable in
all groups (Lorke and Loser, 1966a).
Histopathologically, vacuolar cytoplasmic swelling was noted in the
liver at all levels, the incidence tending to increase with increasing
dose level. Necrotic lesions were observed only at 500 ppm. Bile duct
hyperplasia occurred in six out of 38 of the rats fed 500 ppm. In the
testes reduced spermatogenesis was significantly more frequent at 150
and 500 ppm. Tumour incidence was unrelated to the dose level
(Grundmann and Hobik, 1966a),
Special studies
Reproduction
Rat. In a three-generation rat study, 91 per cent pure technical
oxythioquinox was incorporated in the diets of groups of eight male
and 16 female rats (except for the F2b breeding animals where 10
males and 20 females were utilized). At levels of 0, 10, 25, 60 and
150 ppm, no effects on adult body-weight, incidence of pregnancy,
litter size, birth and weaning weights, or survival to four weeks were
noted up to and including 60 ppm. At 150 ppm, incidence of pregnancy
was reduced in both first generation litters and litter size was
reduced in one litter in each generation. At 500 ppm, pregnancy was
inhibited. Cross breeding studies showed the inhibition to be due to
male infertility. Treated females mated with untreated males produced
litters. but the litter size was markedly reduced. The 500 ppm group
was discontinued after the first attempted mating. Body-weight gain
was reduced in female rats and doubtfully in male rats at 500 ppm.
Abnormalities observed during the study included uni- and bilateral
anophthalmia, and unspecified abnormalities of the incisor teeth.
These abnormalities were randomly distributed between groups. In the
F1a litters, one hairless offspring occurred in the 60 and 150 ppm
groups.
Organ weights and histopathology on two male and two female animals in
the F3b litter were comparable to the controls (Lorke and Loser,
1966; Grundmann and Hobik, 1966).
Observations in man
Compressions containing dry or moist oxythioquinox were applied to the
forearms of nine human volunteers for varying periods of two, four,
eight or 24 hours. Exposures for 24 hours resulted in reddening of the
skin and occasional swelling and blistering, the incidence and
severity of these injuries being more marked with the moist product
(Bayer, 1959).
Comments
Adequate data are available on acute and short-term studies. However,
a no-effect level has not been demonstrated in long-term studies in
rat. Liver hypertrophy is extensive at 10 ppm, the lowest dose tested.
At present it is not possible to estimate a daily acceptable intake
for man. Until further data are available, foods should not be
permitted to contain any residues of oxythioquinox and its possible
metabolites from treated plants.
Further information is needed concerning the metabolic fate of this
pesticide in various animals including man; particularly further
research is needed to ascertain its effect on spermatogenesis and
whether this phenomenon occurs in primates. Since it has been reported
that there is an injurious effect to skin upon prolonged application,
it will be necessary to do further studies on the cutaneous toxicity
of this compound including studies related to the question of
photo-sensitization.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Pre-harvest treatment
Oxythioquinox is used as fungicide and acaricide on a large number of
crops. As a fungicide, it is used against mildew diseases on pome,
stone and soft fruit, strawberries, grapes and cucurbits and has a
protective and curative action (Grewe et al., 1965; Wäckers et al.,
1965). As an acaricide, it is effective not only against susceptible
mite strains, but also against strains which are resistant to other
acaricides (Unterstenhöfer et al., 1965). A good insecticidal side
effect has been observed against Psylla piri (Unterstenhöfer et al.,
1965).
Established pre-harvest intervals are as follows:
Pre-harvest
Country Crop interval (days)
Denmark Tree fruit 8
Cucumbers 4
Germany Tree fruit and vegetables 14
Cucumbers 4
United Kingdom Tree fruit 21
Gooseberries, black currants 14
Pre-harvest
Country Crop interval (days)
Marrow 7
Cucumbers grown under glass 3
Finland - 14
Holland Tree fruit 28
Cucumbers 3
Israel Apples, citrus fruit, grapes 14
tomatoes, strawberries,
egg-plants, peppers 7
Italy - 5
Yugoslavia Tree fruit 14
Cucumbers 7
Austria Tree fruit 14
Vegetables 4
Poland Tree fruit and vegetables 21
Cucumbers 3
Sweden General 7
Cucumbers 4
Switzerland Tree fruit 21
Vegetables 5
Spain Cucumbers 10
Other crops 15
Post-harvest treatments
No use.
Other uses
Oxythioquinox is used against mildew and mites on ornamentals (Grewe
et al., 1965; Wäckers et al., 1965; Besemer et al., 1963).
Residues resulting from supervised trials
In the following table, residue values are given after application at
the recommended concentrations:
Residue at harvest
Pre-harvest (ppm)
Crop Number of interval
treatments (days) Range Average
Apples 1-4 7-10 n.d.-1.5 0.2
(United
States of 1 7 0.8 0.8
America)
Pears 3 7-8 0.1-0.8 0.4
(United
States of 1 7-8 0.2-2.1 1.2
America
Strawberries 2 7-8 n.d.-2.2 1.0
(United
States of 7 n.d. n.d.
America
Grapes 2 7 n.d.-9.4 6.0
(United
States of 7 12.5 12.5
America)
Cucumbers 2 4 n.d. n.d.
Summer 4 1-7 n.d.-0.9 0.6
squash 4 1-7 n.d.-1.2 0.9
Winter 4 1-8 n.d.-0.1 n.d.
squash 4 1-8 0.5-0.6 0.5
Alfalfa 1 7 0.3-1.1 0.9
(United 7 3.6 3.6
States of 14 0.1-0.3 0.1
America 14 3.0 3.0
Fate of residues
General comments
The half-life of oxythioquinox on apples, pears, and goose berries is
between five and nine days (Grewe et al., 1965). Loss probably is
caused not only by enzymatic processes, but also by physical and
chemical influences in the environment. Wash-off may occur only a
short time after application. After a few days even heavy rainfall
causes no further noticeable loss. Probably oxythioquinox is dissolved
in the wax layers of the plant surfaces (Grewe et al., 1965).
In diluted ammoniacal solution, oxythioquinox is instantly saponified
to form 2,3-dithiol-6-methylquinoxaline with liberation of carbonate
(Grewe et al., 1965). This substance, which is also formed in plants
(Chemagro Internal Report), is probably unstable to light, as shown by
experiments with its analogue 2,3-dithiolquinoxaline, which is formed
under similar conditions from Eradex, a compound related to
oxythioquinox (Tietz et al., 1962).
In soils
The half-life of oxythioquinox in soils is about 60 days
(Farbenfabriken Bayer A.G., private communication).
In plants
When oxythioquinox C14 was applied to growing oranges and apples,
there was a steady decrease in the amount of total radioactivity.
While activity on the surface was due only to oxythioquinox itself
free and bound 2,3-dithio-6-methyl quinoxaline and other, unknown
metabolites were found in the peel, reaching a maximum at 7-14 days
and then decreasing slowly. The unknown metabolites were not
extractable with organic solvents. Reduction followed by treatment
with boron trifluoride-methanol and diazomethane solubilized
approximately 80 per cent of the insoluble material. Perhaps this
consists of conjugated compounds with carboxylic groups or phenolic or
glycosidic hydroxyls. In the pulp only traces of radioactivity were
found (Chemagro Internal Report).
In animals
When rats were fed carbonyl-14C-labelled oxythioquinox. it was found
that most of the radioactivity was exhaled as 14CO2. After
2,3-14C- and 35S-labelled active ingredient was fed to rats, no
14CO2 was found in the air expired. In this particular case, most
of the activity was present in the urine and in the faeces. A small
proportion of the activity was found in the blood plasma in
protein-bound form. The activity is not present as oxythioquinox
itself, but partially as 2,3-dithiol-6-methyl quinoxaline, in traces
as the corresponding dihydroxy compound, and as other still
unidentified metabolites. The quinoxaline ring is apparently not
metabolized in the animal body (Chemagro Internal Report).
In storage and processing
Washing of oranges containing 0.4 ppm oxythioquinox reduced the
residue to the non-detectable level (Chemagro Internal Report).
Peeling reduces residues to values below 1 ppm.
ppm in peel ppm in pulp (seven
(seven days days after
after application)
Crop application)
Oranges 0.2-4.4 n.d.-0.2
Lemons 0.2-1.2 n.d.
Grapefruit n.d.-1.8 n.d.-0.2
Apples 1.0-4.1 0.1-0.7
(Farbenfabriken Bayer A.G., private communication)
After processing oranges containing 0.4 ppm oxythioquinox, residues of
less than 0.1 ppm were detected in frozen and heated juice, chopped
peel and cattle feed, press liquor and molasses. A residue level of
1.7 ppm was found only in cold pressed citrus oil (Chemagro Internal
Report).
Evidence of residues in food in commerce or at consumption
No data available.
Methods of residue analysis
Analytical methods appear to be sufficient for present purposes. A
colorimetric method has been developed by Havens et al., 1964. It is
an adaption of a method to determine residues of "Eradex New" in plant
material (Tietz et al., 1962). The procedure involves a hydrolysis
with concentrated ammonium hydroxide to give
2,3-dithiol-6-methylquinoxaline. Subsequent treatment with ammonical
nickel reagent gives a red-coloured chelate which is measured at
540 mµ. The limit of sensitivity is 0.14 ppm. This method gives poor
results, when large amounts of oil are present in the sample.
For the determination of residues in orange peel. a method has been
described based on a combination of thin-layer chromatography and
cathode ray polarography (Hearth et al., 1966). Sensitivity: 0.5 ppm.
Gas chromatographic determination with an electron capture detector is
used for moist crops, dry hops, and for crops with high oil content.
Sensitivity: 0.1 ppm for most crops (Vogeler et al., 1967; Chemagro
Internal Reports).
National tolerances
No registrations have been agreed in the United States of America. A
petition will be filed for the following tolerances:
Walnuts: 0.1 ppm
Cucumbers, water melons, winter squash: 0.75 ppm
Apples, pears, melons (except water melons), summer squash: 1.5 ppm
Citrus, grapes: 2.5 ppm
Papayas: 5.0 ppm
Strawberries: 6 ppm
In Germany the tolerance for cucumbers is 0.1 ppm. For pome fruit, a
tolerance of 0.3 ppm has been proposed.
In the Netherlands and Switzerland, a tolerance of 0.1 ppm has been
agreed for fruit, vegetables, and cucumbers.
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Appraisal
This compound is widely used as a fungicide and an acaricide in many
countries. The technical product contains at least 80 per cent pure
active ingredient and at least six by-products in varying
concentrations. Further information on the composition of the
technical product is required, together with information on the
quantities being used in each country. The residues consist of the
parent compound, one identified metabolite and other metabolites not
yet identified. Accordingly further information on the chemical nature
of the terminal residues is also required together with the nature of
degradation products in plants and animals.
A variety of analytical methods are available which would be adequate
to detect residues down to 0.1 ppm, including gas-liquid
chromatography, thin-layer chromatography, polarography. However, if
tolerances of 0.1 ppm or below are to be considered, improved methods
of analysis would be required.
Recommendations
Since an acceptable daily intake has not been established, tolerances
cannot be recommended.
Further work or information
Required (before an acceptable daily intake or tolerances can be
established)
1. Information on the nature of terminal residues in plants and
animal products.
2. Data from countries other than the United States of America on
the required rates and frequencies of application, pre-harvest
intervals, and the resultant residues.
3. Further data on residue levels in raw agricultural products
moving in commerce.
4. Data on residue levels in total diet studies.
5. Comparative evaluation of methods of analysis for regulatory
purposes.
6. Experimental studies on the metabolism responsible for liver
hyperplasia in rats.
7. Biochemical studies on excretion and metabolism.
8. Two-year studies on rats at lower dosage.
9. Further information on anti-spermatogenic effects.
Desirable
1. Collaborative studies to establish a referee method.
2. Studies of metabolism in various animals including man.
3. Further studies on the cutaneous toxicity, including studies
related to the question of photosensitization.
REFERENCES
Bayer. Active ingredient Dr Sasse Ss2074. Farbenfabriken Bayer
Basemer, A. F. H. and Immikhuizen, E. (1963) Biologisch Veld-en
Kasonderzoek van Insekticiden, Acariciden en Fungiciden in
Tuinbouwgewassen. P.D.-Jaarboek 1962, Wageningen, No. 138: 131-146
Carlson, B. P. and DuBois, K. P. (1968) Effects of feeding various
dietary levels of Morestan to male rats for 90 days. University of
Chicago. Unpublished report
Doull, J., DiGiacomo, R., Root, M., Vesselinovitch, D. and Meskauskas,
J. (1966) Chronic oral toxicity of Morestan (Bayer 36205) to male and
female dogs. University of Chicago. Unpublished report
Doull, J., Root, M. and Gowan, J. (1963) Subacute oral toxicity of
Morestan (Bayer 36205) to male and female rats. University of Chicago.
Unpublished report
DuBois, K. P. (1961) Intraperitoneal and oral toxicity of Bayer 36205
to rats. University of Chicago. Unpublished report
DuBois, K. P. (1962) The acute oral toxicity of Bayer 36205 to
chickens. University of Chicago. Unpublished report
DuBois, K. P. and Raymund, A. B. (1962) The acute toxicity of Bayer
36205 to mice, guinea pigs and rats. University of Chicago.
Unpublished report
Grewe, F. and Kaspers, H. (1965) Morestan, a new fungicide of the
2,3-disubstituted quinoxaline group for controlling powdery mildews.
Pflanzenschutz-Nachrichten "Bayer", 18: 1-23
Grundmann, E. and Hobik, H. P. (1966a) Bay 36205, two-year feeding
test with rats. Histology. Farbenfabriken Bayer. Unpublished report
Grundmann, E. and Hobik, H. P. (1966b) Bay 36205 Generation test.
Histology. Farbenfabriken Bayer. Unpublished report
Havens, R., Adams, J. M. and Anderson, C. A. (1964) Colorimetric
determination of 6-methyl-2,3-quinoxalinedithiol cyclic carbonate
(Morestan) residues in apples and pears. J. Agr. Food Chem.,
12: 247-248
Hearth, F. E., Ott, D. E. and Gunther, F. A. (1966)
Oscillopolarographic analysis of Morestan residues in Valencia orange
rind following thin layer chromatography. J. Assoc. off. agric.
Chemists, 49: 774-778
Hecht, G. and Grundmann, E. (1964) Report on testing of Morestan for
toxic effects on the liver. Farbenfabriken Bayer. Unpublished report
Kimmerle, G. (1963) Four months' feeding test on rats with the active
ingredient Ss2074. Farbenfabriken Bayer. Unpublished report
Lorke, D. and Loser, E. (1966a) Bay 36205 studies of chronic toxicity
to rats. Farbenfabriken Bayer. Unpublished report
Lorke, D. and Loser, E. (1966b) Bay 36205 studies of chronic toxicity
to rats. Farbenfabriken Bayer. Unpublished report
Tietz, M. et al. (1962) Method of determining residues of the
acaricide Eradex New in plant material. Pflanzenschutz-Nachrichten
"Bayer", 15: 166-171
Vogeler, K. and Niessen, H. (1967) Gas chromatographic determination
of Morestan residues in plants. Pflanzenschutz-Nachrichten "Bayer",
20: 550-556
Wäckers, R. and van den Berge, C. (1965) Experiences with Morestan in
Dutch fruit farming and market gardening. Pflanzenschutz-Nachrichten
"Bayer", 18: 34-44
 Other information on identity and properties
The technical compound contains at least 80 per cent pure active
ingredient and the following by-products in varying concentrations:
Other information on identity and properties
The technical compound contains at least 80 per cent pure active
ingredient and the following by-products in varying concentrations:
 EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
No data available.
Acute toxicity
Animal Route LD50 (mg/kg References
body-weight)
Mouse (M) i.p. 650 DuBois and Raymund, 1962
Mouse (F) i.p. 700 DuBois and Raymund, 1962
Rat (M) oral > 2500 Bayer, 1959
Rat (F) oral 3000 DuBois, 1961
Rat (M) i.p. 700 DuBois, 1961
Rat (F) i.p. 600 DuBois, 1961
Guinea-pig (M) oral 1500 DuBois and Raymund, 1962
Guinea-pig (F) i.p. 350 DuBois and Raymund, 1962
Chicken oral >500 DuBois, 1962
Cat oral >1000 Bayer, 1959
Short-term studies
Rat. A group of 10 male rats was treated daily with 100 mg/kg(in one
per cent aqueous tragacanth suspension) by stomach tube, for 28 days.
One death occurred after 16 days; autopsy of this animal revealed
kidney damage. Loss of appetite and body-weight were observed in the
other animals after 20 doses. The haematogram was normal (Bayer,
1959).
Four groups of 12 male and 12 female rats were fed 0, 10, 25 or 50 ppm
in the diet for 16 weeks without any deaths. Food intake was
unaffected in both sexes, as was body-weight gain in the females. A
slight transient depression of body-weight gain was observed between
four and 12 weeks, in the males fed 50 ppm. Based on data from five
male and five female animals per group, gross and histopathology were
comparable to the controls. Depression of absolute liver and kidney
weights, apparent in all male test groups was not dose related (Doull
et al., 1963).
Six groups, each comprising five male weanling rats were fed for 90
days, dietary levels of 0, 10, 25, 60, 150 and 500 ppm active
ingredient of oxythioquinox as the 25 per cent wettable powder.
Body-weight gain was depressed at the 500 ppm level, probably due, at
least in part, to diet rejection. Liver to body-weight ratio was
significantly increased at 500 ppm. Investigations of liver microsomal
enzyme activity (EPN detoxification, O-demethylase, and acetoacetic
acid synthesis) showed depressed activity in all systems at 500 ppm,
and acetoacetic acid synthesis was also inhibited at 150 ppm. Analysis
of liver tissue failed to reveal the presence of oxythioquinox using a
method sensitive to the detection of 0.5 ppm (Carlson and DuBois,
1968).
Oral administration of an aqueous emulsion five times weekly for four
months to groups of 10 male rats at dose levels of 0, 10, 25, 50, 100
or 250 mg/kg did not cause any mortality. Haematograms and urinalysis
were normal at all dose levels. However, clinical symptoms (hair loss)
were apparent at 250 mg/kg after seven weeks. Body-weight gain was
reduced in the 100, and 250 mg/kg groups and liver-weight ratios were
increased at these levels. Histological damage to liver cells was
apparent at 250 mg/kg (Kimmerle, 1963).
In the three generation rat study, described under "Special studies.
Reproduction", liver damage was observed in the FO generation in the
animals fed 500 ppm. In this group, gross pathology was normal but
histopathology showed 60 per cent of the animals displaying toxic
injury (periportally arranged swollen liver epithelial cells, with
several pyknotic nuclei) with a further eight per cent showing
questionable injury (Hecht and Grundmann, 1964).
Dog. Groups of two male and two female dogs were fed 0, 10, 25 or 50
ppm in dry diet for 28 months. Body-weight, food intake, general
behaviour and appearance, haematograms, serum glutamic oxaloacetic
transaminase, and serum glutamic-pyruvic transaminase determinations,
organ weights (absolute and organ to body-weight ratios) and gross
pathology of treated animals were all comparable to the controls.
Histological examination revealed inflammatory cell foci in the livers
of all the dogs which received 25 and 50 ppm; but the same effect was
noted in two-thirds of the 10 ppm group and in three of the four
controls (Doull et al., 1966).
Long-term studies
Rat. Technical oxythioquinox (91 per cent pure) was administered to
groups of 50 male and 50 female rats at dietary levels of 10, 25, 60,
125 or 500 ppm, for two years. One hundred male and 100 female rats
served as untreated controls. Clinical symptoms in the form of yellow
staining of the hair of the paws were apparent at 60 ppm, the
frequency increasing with increasing dose level. At 500 ppm, abdominal
and facial fur were also stained yellow. Body-weight gain was
depressed in both sexes, and food intake was depressed in the female
at 500 ppm. Incidence of mortality, haematograms, urinalysis, and
gross pathology were all comparable to the controls. Marked liver
hypertrophy was shown, as evidenced by absolute organ weights, the
increase being significant in all test groups except the females fed
25 ppm. Thyroid weights were also significantly increased in males at
150 and 500 ppm, and in females at 25 and 60 ppm. There was a tendency
towards decreased adrenal weights in males at 150 and 500 ppm, and in
females at 500 ppm. Pituitary enlargement was, however, comparable in
all groups (Lorke and Loser, 1966a).
Histopathologically, vacuolar cytoplasmic swelling was noted in the
liver at all levels, the incidence tending to increase with increasing
dose level. Necrotic lesions were observed only at 500 ppm. Bile duct
hyperplasia occurred in six out of 38 of the rats fed 500 ppm. In the
testes reduced spermatogenesis was significantly more frequent at 150
and 500 ppm. Tumour incidence was unrelated to the dose level
(Grundmann and Hobik, 1966a),
Special studies
Reproduction
Rat. In a three-generation rat study, 91 per cent pure technical
oxythioquinox was incorporated in the diets of groups of eight male
and 16 female rats (except for the F2b breeding animals where 10
males and 20 females were utilized). At levels of 0, 10, 25, 60 and
150 ppm, no effects on adult body-weight, incidence of pregnancy,
litter size, birth and weaning weights, or survival to four weeks were
noted up to and including 60 ppm. At 150 ppm, incidence of pregnancy
was reduced in both first generation litters and litter size was
reduced in one litter in each generation. At 500 ppm, pregnancy was
inhibited. Cross breeding studies showed the inhibition to be due to
male infertility. Treated females mated with untreated males produced
litters. but the litter size was markedly reduced. The 500 ppm group
was discontinued after the first attempted mating. Body-weight gain
was reduced in female rats and doubtfully in male rats at 500 ppm.
Abnormalities observed during the study included uni- and bilateral
anophthalmia, and unspecified abnormalities of the incisor teeth.
These abnormalities were randomly distributed between groups. In the
F1a litters, one hairless offspring occurred in the 60 and 150 ppm
groups.
Organ weights and histopathology on two male and two female animals in
the F3b litter were comparable to the controls (Lorke and Loser,
1966; Grundmann and Hobik, 1966).
Observations in man
Compressions containing dry or moist oxythioquinox were applied to the
forearms of nine human volunteers for varying periods of two, four,
eight or 24 hours. Exposures for 24 hours resulted in reddening of the
skin and occasional swelling and blistering, the incidence and
severity of these injuries being more marked with the moist product
(Bayer, 1959).
Comments
Adequate data are available on acute and short-term studies. However,
a no-effect level has not been demonstrated in long-term studies in
rat. Liver hypertrophy is extensive at 10 ppm, the lowest dose tested.
At present it is not possible to estimate a daily acceptable intake
for man. Until further data are available, foods should not be
permitted to contain any residues of oxythioquinox and its possible
metabolites from treated plants.
Further information is needed concerning the metabolic fate of this
pesticide in various animals including man; particularly further
research is needed to ascertain its effect on spermatogenesis and
whether this phenomenon occurs in primates. Since it has been reported
that there is an injurious effect to skin upon prolonged application,
it will be necessary to do further studies on the cutaneous toxicity
of this compound including studies related to the question of
photo-sensitization.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Pre-harvest treatment
Oxythioquinox is used as fungicide and acaricide on a large number of
crops. As a fungicide, it is used against mildew diseases on pome,
stone and soft fruit, strawberries, grapes and cucurbits and has a
protective and curative action (Grewe et al., 1965; Wäckers et al.,
1965). As an acaricide, it is effective not only against susceptible
mite strains, but also against strains which are resistant to other
acaricides (Unterstenhöfer et al., 1965). A good insecticidal side
effect has been observed against Psylla piri (Unterstenhöfer et al.,
1965).
Established pre-harvest intervals are as follows:
Pre-harvest
Country Crop interval (days)
Denmark Tree fruit 8
Cucumbers 4
Germany Tree fruit and vegetables 14
Cucumbers 4
United Kingdom Tree fruit 21
Gooseberries, black currants 14
Pre-harvest
Country Crop interval (days)
Marrow 7
Cucumbers grown under glass 3
Finland - 14
Holland Tree fruit 28
Cucumbers 3
Israel Apples, citrus fruit, grapes 14
tomatoes, strawberries,
egg-plants, peppers 7
Italy - 5
Yugoslavia Tree fruit 14
Cucumbers 7
Austria Tree fruit 14
Vegetables 4
Poland Tree fruit and vegetables 21
Cucumbers 3
Sweden General 7
Cucumbers 4
Switzerland Tree fruit 21
Vegetables 5
Spain Cucumbers 10
Other crops 15
Post-harvest treatments
No use.
Other uses
Oxythioquinox is used against mildew and mites on ornamentals (Grewe
et al., 1965; Wäckers et al., 1965; Besemer et al., 1963).
Residues resulting from supervised trials
In the following table, residue values are given after application at
the recommended concentrations:
Residue at harvest
Pre-harvest (ppm)
Crop Number of interval
treatments (days) Range Average
Apples 1-4 7-10 n.d.-1.5 0.2
(United
States of 1 7 0.8 0.8
America)
Pears 3 7-8 0.1-0.8 0.4
(United
States of 1 7-8 0.2-2.1 1.2
America
Strawberries 2 7-8 n.d.-2.2 1.0
(United
States of 7 n.d. n.d.
America
Grapes 2 7 n.d.-9.4 6.0
(United
States of 7 12.5 12.5
America)
Cucumbers 2 4 n.d. n.d.
Summer 4 1-7 n.d.-0.9 0.6
squash 4 1-7 n.d.-1.2 0.9
Winter 4 1-8 n.d.-0.1 n.d.
squash 4 1-8 0.5-0.6 0.5
Alfalfa 1 7 0.3-1.1 0.9
(United 7 3.6 3.6
States of 14 0.1-0.3 0.1
America 14 3.0 3.0
Fate of residues
General comments
The half-life of oxythioquinox on apples, pears, and goose berries is
between five and nine days (Grewe et al., 1965). Loss probably is
caused not only by enzymatic processes, but also by physical and
chemical influences in the environment. Wash-off may occur only a
short time after application. After a few days even heavy rainfall
causes no further noticeable loss. Probably oxythioquinox is dissolved
in the wax layers of the plant surfaces (Grewe et al., 1965).
In diluted ammoniacal solution, oxythioquinox is instantly saponified
to form 2,3-dithiol-6-methylquinoxaline with liberation of carbonate
(Grewe et al., 1965). This substance, which is also formed in plants
(Chemagro Internal Report), is probably unstable to light, as shown by
experiments with its analogue 2,3-dithiolquinoxaline, which is formed
under similar conditions from Eradex, a compound related to
oxythioquinox (Tietz et al., 1962).
In soils
The half-life of oxythioquinox in soils is about 60 days
(Farbenfabriken Bayer A.G., private communication).
In plants
When oxythioquinox C14 was applied to growing oranges and apples,
there was a steady decrease in the amount of total radioactivity.
While activity on the surface was due only to oxythioquinox itself
free and bound 2,3-dithio-6-methyl quinoxaline and other, unknown
metabolites were found in the peel, reaching a maximum at 7-14 days
and then decreasing slowly. The unknown metabolites were not
extractable with organic solvents. Reduction followed by treatment
with boron trifluoride-methanol and diazomethane solubilized
approximately 80 per cent of the insoluble material. Perhaps this
consists of conjugated compounds with carboxylic groups or phenolic or
glycosidic hydroxyls. In the pulp only traces of radioactivity were
found (Chemagro Internal Report).
In animals
When rats were fed carbonyl-14C-labelled oxythioquinox. it was found
that most of the radioactivity was exhaled as 14CO2. After
2,3-14C- and 35S-labelled active ingredient was fed to rats, no
14CO2 was found in the air expired. In this particular case, most
of the activity was present in the urine and in the faeces. A small
proportion of the activity was found in the blood plasma in
protein-bound form. The activity is not present as oxythioquinox
itself, but partially as 2,3-dithiol-6-methyl quinoxaline, in traces
as the corresponding dihydroxy compound, and as other still
unidentified metabolites. The quinoxaline ring is apparently not
metabolized in the animal body (Chemagro Internal Report).
In storage and processing
Washing of oranges containing 0.4 ppm oxythioquinox reduced the
residue to the non-detectable level (Chemagro Internal Report).
Peeling reduces residues to values below 1 ppm.
ppm in peel ppm in pulp (seven
(seven days days after
after application)
Crop application)
Oranges 0.2-4.4 n.d.-0.2
Lemons 0.2-1.2 n.d.
Grapefruit n.d.-1.8 n.d.-0.2
Apples 1.0-4.1 0.1-0.7
(Farbenfabriken Bayer A.G., private communication)
After processing oranges containing 0.4 ppm oxythioquinox, residues of
less than 0.1 ppm were detected in frozen and heated juice, chopped
peel and cattle feed, press liquor and molasses. A residue level of
1.7 ppm was found only in cold pressed citrus oil (Chemagro Internal
Report).
Evidence of residues in food in commerce or at consumption
No data available.
Methods of residue analysis
Analytical methods appear to be sufficient for present purposes. A
colorimetric method has been developed by Havens et al., 1964. It is
an adaption of a method to determine residues of "Eradex New" in plant
material (Tietz et al., 1962). The procedure involves a hydrolysis
with concentrated ammonium hydroxide to give
2,3-dithiol-6-methylquinoxaline. Subsequent treatment with ammonical
nickel reagent gives a red-coloured chelate which is measured at
540 mµ. The limit of sensitivity is 0.14 ppm. This method gives poor
results, when large amounts of oil are present in the sample.
For the determination of residues in orange peel. a method has been
described based on a combination of thin-layer chromatography and
cathode ray polarography (Hearth et al., 1966). Sensitivity: 0.5 ppm.
Gas chromatographic determination with an electron capture detector is
used for moist crops, dry hops, and for crops with high oil content.
Sensitivity: 0.1 ppm for most crops (Vogeler et al., 1967; Chemagro
Internal Reports).
National tolerances
No registrations have been agreed in the United States of America. A
petition will be filed for the following tolerances:
Walnuts: 0.1 ppm
Cucumbers, water melons, winter squash: 0.75 ppm
Apples, pears, melons (except water melons), summer squash: 1.5 ppm
Citrus, grapes: 2.5 ppm
Papayas: 5.0 ppm
Strawberries: 6 ppm
In Germany the tolerance for cucumbers is 0.1 ppm. For pome fruit, a
tolerance of 0.3 ppm has been proposed.
In the Netherlands and Switzerland, a tolerance of 0.1 ppm has been
agreed for fruit, vegetables, and cucumbers.
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Appraisal
This compound is widely used as a fungicide and an acaricide in many
countries. The technical product contains at least 80 per cent pure
active ingredient and at least six by-products in varying
concentrations. Further information on the composition of the
technical product is required, together with information on the
quantities being used in each country. The residues consist of the
parent compound, one identified metabolite and other metabolites not
yet identified. Accordingly further information on the chemical nature
of the terminal residues is also required together with the nature of
degradation products in plants and animals.
A variety of analytical methods are available which would be adequate
to detect residues down to 0.1 ppm, including gas-liquid
chromatography, thin-layer chromatography, polarography. However, if
tolerances of 0.1 ppm or below are to be considered, improved methods
of analysis would be required.
Recommendations
Since an acceptable daily intake has not been established, tolerances
cannot be recommended.
Further work or information
Required (before an acceptable daily intake or tolerances can be
established)
1. Information on the nature of terminal residues in plants and
animal products.
2. Data from countries other than the United States of America on
the required rates and frequencies of application, pre-harvest
intervals, and the resultant residues.
3. Further data on residue levels in raw agricultural products
moving in commerce.
4. Data on residue levels in total diet studies.
5. Comparative evaluation of methods of analysis for regulatory
purposes.
6. Experimental studies on the metabolism responsible for liver
hyperplasia in rats.
7. Biochemical studies on excretion and metabolism.
8. Two-year studies on rats at lower dosage.
9. Further information on anti-spermatogenic effects.
Desirable
1. Collaborative studies to establish a referee method.
2. Studies of metabolism in various animals including man.
3. Further studies on the cutaneous toxicity, including studies
related to the question of photosensitization.
REFERENCES
Bayer. Active ingredient Dr Sasse Ss2074. Farbenfabriken Bayer
Basemer, A. F. H. and Immikhuizen, E. (1963) Biologisch Veld-en
Kasonderzoek van Insekticiden, Acariciden en Fungiciden in
Tuinbouwgewassen. P.D.-Jaarboek 1962, Wageningen, No. 138: 131-146
Carlson, B. P. and DuBois, K. P. (1968) Effects of feeding various
dietary levels of Morestan to male rats for 90 days. University of
Chicago. Unpublished report
Doull, J., DiGiacomo, R., Root, M., Vesselinovitch, D. and Meskauskas,
J. (1966) Chronic oral toxicity of Morestan (Bayer 36205) to male and
female dogs. University of Chicago. Unpublished report
Doull, J., Root, M. and Gowan, J. (1963) Subacute oral toxicity of
Morestan (Bayer 36205) to male and female rats. University of Chicago.
Unpublished report
DuBois, K. P. (1961) Intraperitoneal and oral toxicity of Bayer 36205
to rats. University of Chicago. Unpublished report
DuBois, K. P. (1962) The acute oral toxicity of Bayer 36205 to
chickens. University of Chicago. Unpublished report
DuBois, K. P. and Raymund, A. B. (1962) The acute toxicity of Bayer
36205 to mice, guinea pigs and rats. University of Chicago.
Unpublished report
Grewe, F. and Kaspers, H. (1965) Morestan, a new fungicide of the
2,3-disubstituted quinoxaline group for controlling powdery mildews.
Pflanzenschutz-Nachrichten "Bayer", 18: 1-23
Grundmann, E. and Hobik, H. P. (1966a) Bay 36205, two-year feeding
test with rats. Histology. Farbenfabriken Bayer. Unpublished report
Grundmann, E. and Hobik, H. P. (1966b) Bay 36205 Generation test.
Histology. Farbenfabriken Bayer. Unpublished report
Havens, R., Adams, J. M. and Anderson, C. A. (1964) Colorimetric
determination of 6-methyl-2,3-quinoxalinedithiol cyclic carbonate
(Morestan) residues in apples and pears. J. Agr. Food Chem.,
12: 247-248
Hearth, F. E., Ott, D. E. and Gunther, F. A. (1966)
Oscillopolarographic analysis of Morestan residues in Valencia orange
rind following thin layer chromatography. J. Assoc. off. agric.
Chemists, 49: 774-778
Hecht, G. and Grundmann, E. (1964) Report on testing of Morestan for
toxic effects on the liver. Farbenfabriken Bayer. Unpublished report
Kimmerle, G. (1963) Four months' feeding test on rats with the active
ingredient Ss2074. Farbenfabriken Bayer. Unpublished report
Lorke, D. and Loser, E. (1966a) Bay 36205 studies of chronic toxicity
to rats. Farbenfabriken Bayer. Unpublished report
Lorke, D. and Loser, E. (1966b) Bay 36205 studies of chronic toxicity
to rats. Farbenfabriken Bayer. Unpublished report
Tietz, M. et al. (1962) Method of determining residues of the
acaricide Eradex New in plant material. Pflanzenschutz-Nachrichten
"Bayer", 15: 166-171
Vogeler, K. and Niessen, H. (1967) Gas chromatographic determination
of Morestan residues in plants. Pflanzenschutz-Nachrichten "Bayer",
20: 550-556
Wäckers, R. and van den Berge, C. (1965) Experiences with Morestan in
Dutch fruit farming and market gardening. Pflanzenschutz-Nachrichten
"Bayer", 18: 34-44
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
No data available.
Acute toxicity
Animal Route LD50 (mg/kg References
body-weight)
Mouse (M) i.p. 650 DuBois and Raymund, 1962
Mouse (F) i.p. 700 DuBois and Raymund, 1962
Rat (M) oral > 2500 Bayer, 1959
Rat (F) oral 3000 DuBois, 1961
Rat (M) i.p. 700 DuBois, 1961
Rat (F) i.p. 600 DuBois, 1961
Guinea-pig (M) oral 1500 DuBois and Raymund, 1962
Guinea-pig (F) i.p. 350 DuBois and Raymund, 1962
Chicken oral >500 DuBois, 1962
Cat oral >1000 Bayer, 1959
Short-term studies
Rat. A group of 10 male rats was treated daily with 100 mg/kg(in one
per cent aqueous tragacanth suspension) by stomach tube, for 28 days.
One death occurred after 16 days; autopsy of this animal revealed
kidney damage. Loss of appetite and body-weight were observed in the
other animals after 20 doses. The haematogram was normal (Bayer,
1959).
Four groups of 12 male and 12 female rats were fed 0, 10, 25 or 50 ppm
in the diet for 16 weeks without any deaths. Food intake was
unaffected in both sexes, as was body-weight gain in the females. A
slight transient depression of body-weight gain was observed between
four and 12 weeks, in the males fed 50 ppm. Based on data from five
male and five female animals per group, gross and histopathology were
comparable to the controls. Depression of absolute liver and kidney
weights, apparent in all male test groups was not dose related (Doull
et al., 1963).
Six groups, each comprising five male weanling rats were fed for 90
days, dietary levels of 0, 10, 25, 60, 150 and 500 ppm active
ingredient of oxythioquinox as the 25 per cent wettable powder.
Body-weight gain was depressed at the 500 ppm level, probably due, at
least in part, to diet rejection. Liver to body-weight ratio was
significantly increased at 500 ppm. Investigations of liver microsomal
enzyme activity (EPN detoxification, O-demethylase, and acetoacetic
acid synthesis) showed depressed activity in all systems at 500 ppm,
and acetoacetic acid synthesis was also inhibited at 150 ppm. Analysis
of liver tissue failed to reveal the presence of oxythioquinox using a
method sensitive to the detection of 0.5 ppm (Carlson and DuBois,
1968).
Oral administration of an aqueous emulsion five times weekly for four
months to groups of 10 male rats at dose levels of 0, 10, 25, 50, 100
or 250 mg/kg did not cause any mortality. Haematograms and urinalysis
were normal at all dose levels. However, clinical symptoms (hair loss)
were apparent at 250 mg/kg after seven weeks. Body-weight gain was
reduced in the 100, and 250 mg/kg groups and liver-weight ratios were
increased at these levels. Histological damage to liver cells was
apparent at 250 mg/kg (Kimmerle, 1963).
In the three generation rat study, described under "Special studies.
Reproduction", liver damage was observed in the FO generation in the
animals fed 500 ppm. In this group, gross pathology was normal but
histopathology showed 60 per cent of the animals displaying toxic
injury (periportally arranged swollen liver epithelial cells, with
several pyknotic nuclei) with a further eight per cent showing
questionable injury (Hecht and Grundmann, 1964).
Dog. Groups of two male and two female dogs were fed 0, 10, 25 or 50
ppm in dry diet for 28 months. Body-weight, food intake, general
behaviour and appearance, haematograms, serum glutamic oxaloacetic
transaminase, and serum glutamic-pyruvic transaminase determinations,
organ weights (absolute and organ to body-weight ratios) and gross
pathology of treated animals were all comparable to the controls.
Histological examination revealed inflammatory cell foci in the livers
of all the dogs which received 25 and 50 ppm; but the same effect was
noted in two-thirds of the 10 ppm group and in three of the four
controls (Doull et al., 1966).
Long-term studies
Rat. Technical oxythioquinox (91 per cent pure) was administered to
groups of 50 male and 50 female rats at dietary levels of 10, 25, 60,
125 or 500 ppm, for two years. One hundred male and 100 female rats
served as untreated controls. Clinical symptoms in the form of yellow
staining of the hair of the paws were apparent at 60 ppm, the
frequency increasing with increasing dose level. At 500 ppm, abdominal
and facial fur were also stained yellow. Body-weight gain was
depressed in both sexes, and food intake was depressed in the female
at 500 ppm. Incidence of mortality, haematograms, urinalysis, and
gross pathology were all comparable to the controls. Marked liver
hypertrophy was shown, as evidenced by absolute organ weights, the
increase being significant in all test groups except the females fed
25 ppm. Thyroid weights were also significantly increased in males at
150 and 500 ppm, and in females at 25 and 60 ppm. There was a tendency
towards decreased adrenal weights in males at 150 and 500 ppm, and in
females at 500 ppm. Pituitary enlargement was, however, comparable in
all groups (Lorke and Loser, 1966a).
Histopathologically, vacuolar cytoplasmic swelling was noted in the
liver at all levels, the incidence tending to increase with increasing
dose level. Necrotic lesions were observed only at 500 ppm. Bile duct
hyperplasia occurred in six out of 38 of the rats fed 500 ppm. In the
testes reduced spermatogenesis was significantly more frequent at 150
and 500 ppm. Tumour incidence was unrelated to the dose level
(Grundmann and Hobik, 1966a),
Special studies
Reproduction
Rat. In a three-generation rat study, 91 per cent pure technical
oxythioquinox was incorporated in the diets of groups of eight male
and 16 female rats (except for the F2b breeding animals where 10
males and 20 females were utilized). At levels of 0, 10, 25, 60 and
150 ppm, no effects on adult body-weight, incidence of pregnancy,
litter size, birth and weaning weights, or survival to four weeks were
noted up to and including 60 ppm. At 150 ppm, incidence of pregnancy
was reduced in both first generation litters and litter size was
reduced in one litter in each generation. At 500 ppm, pregnancy was
inhibited. Cross breeding studies showed the inhibition to be due to
male infertility. Treated females mated with untreated males produced
litters. but the litter size was markedly reduced. The 500 ppm group
was discontinued after the first attempted mating. Body-weight gain
was reduced in female rats and doubtfully in male rats at 500 ppm.
Abnormalities observed during the study included uni- and bilateral
anophthalmia, and unspecified abnormalities of the incisor teeth.
These abnormalities were randomly distributed between groups. In the
F1a litters, one hairless offspring occurred in the 60 and 150 ppm
groups.
Organ weights and histopathology on two male and two female animals in
the F3b litter were comparable to the controls (Lorke and Loser,
1966; Grundmann and Hobik, 1966).
Observations in man
Compressions containing dry or moist oxythioquinox were applied to the
forearms of nine human volunteers for varying periods of two, four,
eight or 24 hours. Exposures for 24 hours resulted in reddening of the
skin and occasional swelling and blistering, the incidence and
severity of these injuries being more marked with the moist product
(Bayer, 1959).
Comments
Adequate data are available on acute and short-term studies. However,
a no-effect level has not been demonstrated in long-term studies in
rat. Liver hypertrophy is extensive at 10 ppm, the lowest dose tested.
At present it is not possible to estimate a daily acceptable intake
for man. Until further data are available, foods should not be
permitted to contain any residues of oxythioquinox and its possible
metabolites from treated plants.
Further information is needed concerning the metabolic fate of this
pesticide in various animals including man; particularly further
research is needed to ascertain its effect on spermatogenesis and
whether this phenomenon occurs in primates. Since it has been reported
that there is an injurious effect to skin upon prolonged application,
it will be necessary to do further studies on the cutaneous toxicity
of this compound including studies related to the question of
photo-sensitization.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Pre-harvest treatment
Oxythioquinox is used as fungicide and acaricide on a large number of
crops. As a fungicide, it is used against mildew diseases on pome,
stone and soft fruit, strawberries, grapes and cucurbits and has a
protective and curative action (Grewe et al., 1965; Wäckers et al.,
1965). As an acaricide, it is effective not only against susceptible
mite strains, but also against strains which are resistant to other
acaricides (Unterstenhöfer et al., 1965). A good insecticidal side
effect has been observed against Psylla piri (Unterstenhöfer et al.,
1965).
Established pre-harvest intervals are as follows:
Pre-harvest
Country Crop interval (days)
Denmark Tree fruit 8
Cucumbers 4
Germany Tree fruit and vegetables 14
Cucumbers 4
United Kingdom Tree fruit 21
Gooseberries, black currants 14
Pre-harvest
Country Crop interval (days)
Marrow 7
Cucumbers grown under glass 3
Finland - 14
Holland Tree fruit 28
Cucumbers 3
Israel Apples, citrus fruit, grapes 14
tomatoes, strawberries,
egg-plants, peppers 7
Italy - 5
Yugoslavia Tree fruit 14
Cucumbers 7
Austria Tree fruit 14
Vegetables 4
Poland Tree fruit and vegetables 21
Cucumbers 3
Sweden General 7
Cucumbers 4
Switzerland Tree fruit 21
Vegetables 5
Spain Cucumbers 10
Other crops 15
Post-harvest treatments
No use.
Other uses
Oxythioquinox is used against mildew and mites on ornamentals (Grewe
et al., 1965; Wäckers et al., 1965; Besemer et al., 1963).
Residues resulting from supervised trials
In the following table, residue values are given after application at
the recommended concentrations:
Residue at harvest
Pre-harvest (ppm)
Crop Number of interval
treatments (days) Range Average
Apples 1-4 7-10 n.d.-1.5 0.2
(United
States of 1 7 0.8 0.8
America)
Pears 3 7-8 0.1-0.8 0.4
(United
States of 1 7-8 0.2-2.1 1.2
America
Strawberries 2 7-8 n.d.-2.2 1.0
(United
States of 7 n.d. n.d.
America
Grapes 2 7 n.d.-9.4 6.0
(United
States of 7 12.5 12.5
America)
Cucumbers 2 4 n.d. n.d.
Summer 4 1-7 n.d.-0.9 0.6
squash 4 1-7 n.d.-1.2 0.9
Winter 4 1-8 n.d.-0.1 n.d.
squash 4 1-8 0.5-0.6 0.5
Alfalfa 1 7 0.3-1.1 0.9
(United 7 3.6 3.6
States of 14 0.1-0.3 0.1
America 14 3.0 3.0
Fate of residues
General comments
The half-life of oxythioquinox on apples, pears, and goose berries is
between five and nine days (Grewe et al., 1965). Loss probably is
caused not only by enzymatic processes, but also by physical and
chemical influences in the environment. Wash-off may occur only a
short time after application. After a few days even heavy rainfall
causes no further noticeable loss. Probably oxythioquinox is dissolved
in the wax layers of the plant surfaces (Grewe et al., 1965).
In diluted ammoniacal solution, oxythioquinox is instantly saponified
to form 2,3-dithiol-6-methylquinoxaline with liberation of carbonate
(Grewe et al., 1965). This substance, which is also formed in plants
(Chemagro Internal Report), is probably unstable to light, as shown by
experiments with its analogue 2,3-dithiolquinoxaline, which is formed
under similar conditions from Eradex, a compound related to
oxythioquinox (Tietz et al., 1962).
In soils
The half-life of oxythioquinox in soils is about 60 days
(Farbenfabriken Bayer A.G., private communication).
In plants
When oxythioquinox C14 was applied to growing oranges and apples,
there was a steady decrease in the amount of total radioactivity.
While activity on the surface was due only to oxythioquinox itself
free and bound 2,3-dithio-6-methyl quinoxaline and other, unknown
metabolites were found in the peel, reaching a maximum at 7-14 days
and then decreasing slowly. The unknown metabolites were not
extractable with organic solvents. Reduction followed by treatment
with boron trifluoride-methanol and diazomethane solubilized
approximately 80 per cent of the insoluble material. Perhaps this
consists of conjugated compounds with carboxylic groups or phenolic or
glycosidic hydroxyls. In the pulp only traces of radioactivity were
found (Chemagro Internal Report).
In animals
When rats were fed carbonyl-14C-labelled oxythioquinox. it was found
that most of the radioactivity was exhaled as 14CO2. After
2,3-14C- and 35S-labelled active ingredient was fed to rats, no
14CO2 was found in the air expired. In this particular case, most
of the activity was present in the urine and in the faeces. A small
proportion of the activity was found in the blood plasma in
protein-bound form. The activity is not present as oxythioquinox
itself, but partially as 2,3-dithiol-6-methyl quinoxaline, in traces
as the corresponding dihydroxy compound, and as other still
unidentified metabolites. The quinoxaline ring is apparently not
metabolized in the animal body (Chemagro Internal Report).
In storage and processing
Washing of oranges containing 0.4 ppm oxythioquinox reduced the
residue to the non-detectable level (Chemagro Internal Report).
Peeling reduces residues to values below 1 ppm.
ppm in peel ppm in pulp (seven
(seven days days after
after application)
Crop application)
Oranges 0.2-4.4 n.d.-0.2
Lemons 0.2-1.2 n.d.
Grapefruit n.d.-1.8 n.d.-0.2
Apples 1.0-4.1 0.1-0.7
(Farbenfabriken Bayer A.G., private communication)
After processing oranges containing 0.4 ppm oxythioquinox, residues of
less than 0.1 ppm were detected in frozen and heated juice, chopped
peel and cattle feed, press liquor and molasses. A residue level of
1.7 ppm was found only in cold pressed citrus oil (Chemagro Internal
Report).
Evidence of residues in food in commerce or at consumption
No data available.
Methods of residue analysis
Analytical methods appear to be sufficient for present purposes. A
colorimetric method has been developed by Havens et al., 1964. It is
an adaption of a method to determine residues of "Eradex New" in plant
material (Tietz et al., 1962). The procedure involves a hydrolysis
with concentrated ammonium hydroxide to give
2,3-dithiol-6-methylquinoxaline. Subsequent treatment with ammonical
nickel reagent gives a red-coloured chelate which is measured at
540 mµ. The limit of sensitivity is 0.14 ppm. This method gives poor
results, when large amounts of oil are present in the sample.
For the determination of residues in orange peel. a method has been
described based on a combination of thin-layer chromatography and
cathode ray polarography (Hearth et al., 1966). Sensitivity: 0.5 ppm.
Gas chromatographic determination with an electron capture detector is
used for moist crops, dry hops, and for crops with high oil content.
Sensitivity: 0.1 ppm for most crops (Vogeler et al., 1967; Chemagro
Internal Reports).
National tolerances
No registrations have been agreed in the United States of America. A
petition will be filed for the following tolerances:
Walnuts: 0.1 ppm
Cucumbers, water melons, winter squash: 0.75 ppm
Apples, pears, melons (except water melons), summer squash: 1.5 ppm
Citrus, grapes: 2.5 ppm
Papayas: 5.0 ppm
Strawberries: 6 ppm
In Germany the tolerance for cucumbers is 0.1 ppm. For pome fruit, a
tolerance of 0.3 ppm has been proposed.
In the Netherlands and Switzerland, a tolerance of 0.1 ppm has been
agreed for fruit, vegetables, and cucumbers.
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Appraisal
This compound is widely used as a fungicide and an acaricide in many
countries. The technical product contains at least 80 per cent pure
active ingredient and at least six by-products in varying
concentrations. Further information on the composition of the
technical product is required, together with information on the
quantities being used in each country. The residues consist of the
parent compound, one identified metabolite and other metabolites not
yet identified. Accordingly further information on the chemical nature
of the terminal residues is also required together with the nature of
degradation products in plants and animals.
A variety of analytical methods are available which would be adequate
to detect residues down to 0.1 ppm, including gas-liquid
chromatography, thin-layer chromatography, polarography. However, if
tolerances of 0.1 ppm or below are to be considered, improved methods
of analysis would be required.
Recommendations
Since an acceptable daily intake has not been established, tolerances
cannot be recommended.
Further work or information
Required (before an acceptable daily intake or tolerances can be
established)
1. Information on the nature of terminal residues in plants and
animal products.
2. Data from countries other than the United States of America on
the required rates and frequencies of application, pre-harvest
intervals, and the resultant residues.
3. Further data on residue levels in raw agricultural products
moving in commerce.
4. Data on residue levels in total diet studies.
5. Comparative evaluation of methods of analysis for regulatory
purposes.
6. Experimental studies on the metabolism responsible for liver
hyperplasia in rats.
7. Biochemical studies on excretion and metabolism.
8. Two-year studies on rats at lower dosage.
9. Further information on anti-spermatogenic effects.
Desirable
1. Collaborative studies to establish a referee method.
2. Studies of metabolism in various animals including man.
3. Further studies on the cutaneous toxicity, including studies
related to the question of photosensitization.
REFERENCES
Bayer. Active ingredient Dr Sasse Ss2074. Farbenfabriken Bayer
Basemer, A. F. H. and Immikhuizen, E. (1963) Biologisch Veld-en
Kasonderzoek van Insekticiden, Acariciden en Fungiciden in
Tuinbouwgewassen. P.D.-Jaarboek 1962, Wageningen, No. 138: 131-146
Carlson, B. P. and DuBois, K. P. (1968) Effects of feeding various
dietary levels of Morestan to male rats for 90 days. University of
Chicago. Unpublished report
Doull, J., DiGiacomo, R., Root, M., Vesselinovitch, D. and Meskauskas,
J. (1966) Chronic oral toxicity of Morestan (Bayer 36205) to male and
female dogs. University of Chicago. Unpublished report
Doull, J., Root, M. and Gowan, J. (1963) Subacute oral toxicity of
Morestan (Bayer 36205) to male and female rats. University of Chicago.
Unpublished report
DuBois, K. P. (1961) Intraperitoneal and oral toxicity of Bayer 36205
to rats. University of Chicago. Unpublished report
DuBois, K. P. (1962) The acute oral toxicity of Bayer 36205 to
chickens. University of Chicago. Unpublished report
DuBois, K. P. and Raymund, A. B. (1962) The acute toxicity of Bayer
36205 to mice, guinea pigs and rats. University of Chicago.
Unpublished report
Grewe, F. and Kaspers, H. (1965) Morestan, a new fungicide of the
2,3-disubstituted quinoxaline group for controlling powdery mildews.
Pflanzenschutz-Nachrichten "Bayer", 18: 1-23
Grundmann, E. and Hobik, H. P. (1966a) Bay 36205, two-year feeding
test with rats. Histology. Farbenfabriken Bayer. Unpublished report
Grundmann, E. and Hobik, H. P. (1966b) Bay 36205 Generation test.
Histology. Farbenfabriken Bayer. Unpublished report
Havens, R., Adams, J. M. and Anderson, C. A. (1964) Colorimetric
determination of 6-methyl-2,3-quinoxalinedithiol cyclic carbonate
(Morestan) residues in apples and pears. J. Agr. Food Chem.,
12: 247-248
Hearth, F. E., Ott, D. E. and Gunther, F. A. (1966)
Oscillopolarographic analysis of Morestan residues in Valencia orange
rind following thin layer chromatography. J. Assoc. off. agric.
Chemists, 49: 774-778
Hecht, G. and Grundmann, E. (1964) Report on testing of Morestan for
toxic effects on the liver. Farbenfabriken Bayer. Unpublished report
Kimmerle, G. (1963) Four months' feeding test on rats with the active
ingredient Ss2074. Farbenfabriken Bayer. Unpublished report
Lorke, D. and Loser, E. (1966a) Bay 36205 studies of chronic toxicity
to rats. Farbenfabriken Bayer. Unpublished report
Lorke, D. and Loser, E. (1966b) Bay 36205 studies of chronic toxicity
to rats. Farbenfabriken Bayer. Unpublished report
Tietz, M. et al. (1962) Method of determining residues of the
acaricide Eradex New in plant material. Pflanzenschutz-Nachrichten
"Bayer", 15: 166-171
Vogeler, K. and Niessen, H. (1967) Gas chromatographic determination
of Morestan residues in plants. Pflanzenschutz-Nachrichten "Bayer",
20: 550-556
Wäckers, R. and van den Berge, C. (1965) Experiences with Morestan in
Dutch fruit farming and market gardening. Pflanzenschutz-Nachrichten
"Bayer", 18: 34-44
