
FAO/PL:1969/M/17/1
WHO/FOOD ADD./70.38
1969 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
Issued jointly by FAO and WHO
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Group on Pesticide Residues, which met in Rome, 8 - 15 December 1969.
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
WORLD HEALTH ORGANIZATION
Rome, 1970
ETHOXYQUIN
IDENTITY
Chemical name
6-ethoxy-1,2-dihydro-2,2,4-trimethylquinoline or
1,2-dihydro-6-ethoxy-2,2,4-trimethylquinoline.
Synonym
Stop Scald, Santoquin
In some countries formulations containing ethoxyquin may appear under
different trade names.
Structural formula
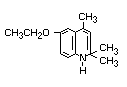 Other relevant chemical properties
The technical material as manufactured in the USA (Santoquin) is
claimed to contain 100 percent active ingredients, but is not 100
percent ethoxyquin. E.g. "Stop Scald" is a 70 percent emulsion of
Santoquin in water with added emulsifiers. It is a dark liquid varying
in color from yellow to black. The color is claimed to be independent
of the antioxidant or biological activity of the chemical.
Boiling Point: 125°C at 1-2 mm Hg.
Specific gravity: 1.028 to 1.032
Solubility: Miscible with animal and vegetable fats and oils.
Insoluble in water.
Viscosity at: 32°F 20,000 centipoises
73 197 "
122 17 "
158 7 "
(Monsanto, 1958).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Studies in chickens resulted in a 99 percent recovery of a single dose
of C14-labelled ethoxyquin within 48 hours. Continuous administration
of 125-137 ppm of ethoxyquin in the diet resulted in accumulation of
approximately 0.1 ppm of ethoxyquin and/or its metabolites in the
liver and fat per week during the first 12 weeks. Accumulation in
muscle and other edible tissue was barely detectable. Withdrawal of
ethoxyquin from the diet resulted in a loss of 79-90 percent of the
tissue residue in a 6 to 18 hour period. The excretion products
comprised 15 percent unchanged ethoxyquin, the remainder probably
being N-glucuronide and N-acetyl derivatives (Monsanto, 1956).
When ethoxiquin was given in the diet of rats for 10 days at a
concentration of 50 ppm, accumulation in the liver (2.1 - 4.8 ppm) and
kidney (2.1 - 2.7 ppm) was observed. Concentrations in fat and
skeletal muscle were less than 1 ppm (Wilson, 1956a; Wilson et al.,
1959).
Rats preconditioned for several weeks on a diet containing 50 ppm of
unlabelled ethoxyquin were given a single oral dose of 1.5 mg of
ethoxyquin C14-labelled in the 2 and 4 positions of the heterocyclic
ring. In two days 30 percent of the radioactivity was excreted in
urine, 34 percent in faeces. In four days and seven days 40-60 percent
and 58 percent was excreted in respectively, and 30-40 percent and 36
percent was excreted in faeces respectively. C14-carbon dioxide in
respired air was detected on the first day only, and comprised 0.7
percent of the administered dose (Wilson, 1956a Wilson et al., 1959).
In rats, repeated administration of ethoxyquin results in residues in
the kidney as well as in the fat and liver. A greater degree of
metabolic breakdown may occur than in chicken, because about 1 percent
of the C14 administered is exhaled as C14-carbon dioxide in rats as
compared with 0.2 percent in chickens (Monsanto, 1956).
Pregnant rats treated as above, and administered the labellet
ethoxyquin nine days prior to parturition indicated that placental
transfer of ethoxyquin occurs, because newborn young contained 0.12 to
0.21 ppm ethoxyquin in their tissues. Milk samples from two female
rats fed a diet of 50 ppm of ethoxyquin for 10 days had residues in
milk of 0.12 ppm and 0.19 ppm (Wilson, 1956a; Wilson et al., 1959).
Metabolic studies in dog indicated that ethoxyquin, per se, is not
excreted in the urine to any appreciable extent, but is excreted as
four unidentified metabolites (probably glucoronates). There was no
evidence of the ethoxy-group being split from the molecule during
metabolism. Results demonstrated that elimination is chiefly by the
kidneys and only to a small degree by way of the faeces (Hanzal,
1955).
TOXICOLOGICAL STUDIES
Special studies on reproduction
Rat
After 40 days on a slightly tocopherol-deficient diet containing 0,
250, 500 or 1000 ppm ethoxyquin, rats were bred to produce three
consecutive litters. The first litter offspring were utilized to
produce a second generation litter. The top dose level was discarded
after production of one litter. No effects on reproduction, as
reflected by fertility, litter size, or survival of offspring were
observed. The animals receiving the experimental diet produced young
and raised them more successfully than the controls. The 500 ppm diet
was more effective than the 250 ppm (Wilson, 1956b; Wilson and DeEds,
1959).
Groups, each of from five to nine rats, one to ten days pregnant, were
fed 0, 125, 375 or 1125 ppm ethoxyquin in the diet. Litter size,
stillbirths, survival to weanling, and weanling weights were all
comparable to the control rats (Derse, 1956).
Groups, each of eight or nine female rats, were placed on diets
containing 0, 125, 375 or 1125 ppm ethoxyquin on the day of mating.
Gestation time was comparable in all groups. However, litter size was
slightly depressed at the 375 ppm level and above, and at 1125 ppm
incidence of stillbirths was increased, and survival to weaning was
decreased (Derse, 1956).
Acute toxicity
LD50
Animal Route mg/kg body-weight References
Mouse i.p. 800-1000 Wilson and DeEds, 1959
Mouse i.v. 178 Wilson and DeEds, 1959
Mouse inhalation 3000 Kel'man, 1965
Rat oral ca.800 Wilson and DeEds, 1959
Rat inhalation 3150 Kel'man, 1965
Chicken oral 8000-10000 Maclay, 1954
Short-term studies
Chicken
Six groups of 70 four-day old cockerels were fed 0, 7.5, 15, 30, 75 or
750 ppm ethoxyquin in the diet for 12 weeks. No adverse effects were
observed on body-weight, food-consumption, mortality, gross-, or
histopathology (Maclay, 1954).
No adverse effects were observed in cockerels fed diets containing
7.5, 15, 30, 75 or 750 ppm ethoxyquin for 12 weeks (Colorado A. and
Experimental Station, ca. 1954).
Nor were any adverse effects observed in chicks fed diets containing
75, 750 or 1500 ppm for eight weeks (Halloran, 1952).
Chicks were fed 0.25 percent of ethoxyquin in their diet for six
weeks. The concentration of ethoxyquin in the livers was significantly
higher when the dietary level of protein was 17 percent than when the
level was 23 percent. (March et al., 1968).
Dog
Mixed groups, each of three dogs, were given oral doses by capsule
five times weekly for one year at dose-levels of 0, 3, 10, 50 or 100
mg/kg body-weight. Feeding the 100 mg/kg group was terminated after
sit weeks due to toxic effects, and the dogs were sacrificed at nine
weeks. One female animal at 3 mg/kg developed histoplasmosis about 40
weeks after initiation of the study, resulting in abnormal findings
when compared to the control dogs. All such finding could be
attributed to the infection. In the remaining animals body-weight was
depressed at 100 mg/kg; bromosulphthalein retention was increased at
10 mg/kg and above, indicating liver dysfunction; abdominal tenderness
was apparent in two of three dogs (male) at 10 mg/kg, and in two of
three dogs (one male and one female) at 50 mg/kg; erythrocyte
sedimentation rate was increased in one of three dogs at 50 mg/kg, and
in all three dogs at 100 mg/kg; haemograms showed reduced haematocrit,
haemoglobin, and erythrocyte counts in two of three dogs at 50 mg/kg,
and in all dogs at 100 mg/kg; urin-analysis indicated dark amber,
green or blackish brown urine at 50 and 100 mg/kg; there was increased
heart, liver, and kidney to body-weight ratios in all dose-levels, but
there does not appear to be any dose relationship in these increases;
histopathology indicated liver-stress, and fatty renal nephrosis at
the 10, 50 and 100 mg/kg dose-levels (Hanzal, 1955).
Rabbit
Undiluted ethoxyquin emulsion (70 percent) was applied to the clipped
intact skin of three rabbits, and removed after 24 hours. Slight
erythema occurred after 24 hours in all animals with barely
perceptible redness remaining after 48 hours in only one animal. The
compound was classed as a mild skin-irritant under the conditions
described (Kelly 1960).
Rat
Three groups of 10 male and 10 female rats which were fed 0, 0.2, and
0.4 percent ethoxyquin in their diet for 200 days displayed an initial
depression in growth rate (statistically significant only at the 0.4
level) in both test groups. The weight loss was not recovered during
the course of the experiment. Haemograms in all groups were
comparable. At autopsy, liver and kidney weights were elevated in both
sexes in both test groups. Histological examination showed
pyelonephritis in the males fed 0.2 and 0.4 percent, and doubtfully at
0.4 percent in the females. Thyroid hyperplasia was noted in males at
0.4 percent (Wilson and DeEds, 1959).
Groups of 10 male rats were fed 0, 62, 125, 250, 500, 1000 or 2000 ppm
ethoxyquin in the diet. Similarly, groups of 10 females were fed 0,
500, 1000 or 2000 ppm, and groups of five females 125 or 250 ppm. Half
the animals in some of the groups of 10 were autopsied after 200 days
on the diet. Body-weight gain was depressed in both sexes at 2000 ppm.
At autopsy, kidney to body-weight ratio was elevated in the males at
250 ppm and above, and in the females at 2000 ppm. Liver to
body-weight ratios were elevated at 1000 and 2000 ppm in males, and at
2000 ppm in females, Wilson and DeEds, 1959). Histopathological
examination showed kidney lesions at 500 ppm and above in the males,
and at 2000 ppm in the females; inclusions were observed in liver cell
cytoplasm in males at 2000 ppm, and in females at 2000 ppm (Cox,
1953).
Long-term studies
Dog
Ethoxyquin was fed to two groups of 14 dogs of mixed sex, at dietary
levels of 0 or 300 ppm for five years. No effects were observed on
haematology, urinalysis, clinical chemistry (aerum glutamic-oxalic
transaminase, blood urea nitrogen and bromosulphthalein retention),
organ weights or organ to body-weight ration, body-weight and
gross- or histopathology (Monsanto, 1966).
Rat
Groups, each of approximately 10 male and 10 female rats, were fed
levels of 0, 62, 125, 250, 500, 1000, 2000 and 4000 ppm of ethoxyquin
in their diets for periods of up to two years. The animals were
sacrificed for autopsy after 200, 400, 600 or 715 days. Mortality
rates at all dose levels were not significantly different from the
controls. Reduced body-weight gain was significant at 2000 ppm after
225 days in the male animals and after 21 days in the females. After
200 days increased liver to body-weight and increased kidney to
body-weight ratios were found at 250 ppm in the males and at 1000 ppm
in the females. Haemoglobin values for both sexes were normal 100 and
300 days after the start of the experiment in those rats fed 2000 and
4000 ppm. Histological changes in the renal cortex were clearly
evident after 200 days in the male rats receiving 2000 and 4000 ppm,
but not in the females. All other organs were normal in both sexes
after 200 days. After 400 days lesions in the kidneys
(pyelonephritis), liver and thyroid were clearly evident in the males
only. Similar lesions were evident for periods up to 717 days in both
sexes, although more marked in the males, and occasional tumours were
evident after 700 days but were unrelated to the dose-level, and were
also evident in the controls. No clearly defined effects were evident
from feeding 62 ppm, but minute lesions were present in the kidneys of
two of the males receiving the 500 ppm diet. It appeared difficult to
distinguish the abnormalities in the group examined after 700 days
with pathological manifestations associated with senility after that
time (Wilson and DeEds, 1959).
OBSERVATION IN MAN
In 20-years experience in the production of ethoxyquin there was no
indication of any cases of skin irritation or sensitivity (Kelly,
1960). However, cases of apparent sensitivity to ethoxyquin have been
reported in fruit handlers. Multiple cases of dermatitis have occurred
among employees handling freshly sprayed apples moist with solutions
of 70 percent formulated ethoxyquin. Patch-testing in volunteers
indicated that these skin reactions were not due to direct irritation,
but were the result of sensitization (Wood, 1965).
COMMENT
Studies in the rat and the dog indicate that toxic effects upon the
liver and kidney occur when dietary levels of 250 ppm and above are
given to the rat and 10 mg/kg or higher daily doses are given to dogs.
Administration of 300 ppm in the diet, however, was well tolerated by
dogs over a five-year period. There was an increase in the number of
stillbirths and a decrease in the litter size and in the incidence of
survival-to-weaning in rats fed 1125 ppm but not in those fed 125 ppm.
Lower doses (ca. 50 ppm) are rapidly excreted in the rat, dog and
chicken. Ethoxyquin is apparently converted to water soluble
metabolites and as long at these conversion mechanisms are functioning
there appears little likelihood of storage of the compound in the body
fat.
The occurrence of dermatitis among fruit packers suggests that
sensitization could be a problem among those who are handling
ethoxyquin.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Dog: 300 ppm in diet, equivalent to 7.5 mg/kg body-weight/day
Rat: 125 ppm in diet, equivalent to 6.25 mg/kg body-weight/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.06 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
Ethoxyquin is used as a pre-harvest treatment for the control of scald
of susceptible apple varieties. It is applied once as a full cover
spray within two days of harvest at a dosage rate of 3 pints of a
commercial formulation containing 70 percent active ingredient per 100
gallons of water (2700 ppm active ingredient).
Post-harvest treatments
For a post-harvest treatment of apples and pears, ethoxyquin is used
as a 15-30 sec. dip or a 15 sec. spray prior to or as the fruit passes
over the grading line. The dosage rate used is 2 to 3 pints of a
commercial formulation containing 70 percent active ingredient per 100
gallons of water (1800-2700 ppm active ingredient). The fruit is
treated no later than one week after harvest.
The above information on use patterns was obtained from product labels
from Canada, United States, United Kingdom and Italy.
Other uses
Ethoxyquin is also used as an antioxidant in spices, fish meal,
poultry feeds, swine feeds and other animal feeds.
Other information pertinent to use pattern
This monograph is confined to information pertaining to only the
pesticidal uses of ethoxyquin on apples and pears.
Ethoxyquin manufactured in the United States is sold in the following
countries: Sweden, Norway, Denmark, United Kingdom, Netherlands,
Belgium-Luxembourg, France, W. Germany, Austria, Switzerland, Finland,
Spain, Italy, Rep. of South Africa, Mexico, Colombia, Venezuela, Peru,
Chile, Brazil, Philippines, Australia, New Zealand, United States and
Canada (Monsanto - Direct communication, 1969).
Information concerning uses in countries other than Canada, United
States, United Kingdom and Italy, such as Israel, Japan, West Germany
and Sweden, where it was understood that ethoxyquin was manufactured,
was not available to the Joint Meeting.
RESIDUES RESULTING FROM SUPERVISED TRIALS
The results reported in Tables 1, 2, 3 for apples and 6 and 7 for
pears are from supervised trials made in various geographical
locations in the United States (Monsanto, 1961). Those in Tables 4 and
5 for apples, are from supervised trials carried out in New Zealand
(Padfield et al., 1963).
Summary of residue data
The residues of ethoxyquin on apples and pears when applied as a dip
or spray on a whole-fruit basis did not exceed 1.2 ppm from U.S.A.
data. However, from New Zealand, levels up to 2.8 ppm on apples have
been reported and one variety, Dougherty, which appears to have the
ability to retain fairly high levels of residue, was reported to have
levels up to 4.4 ppm. Results obtained in New Zealand also indicate
that use of a warm dip may result in residues of ethoxyquin on apples
as high as 6 ppm. Experimental results suggest that the uptake of
ethoxyquin is likely to increase as apple fruit matures and cooled
fruit appears to show lower residues than fruit treated at normal air
temperature. The results of trials carried out in the United States
indicate that residues found as a result of spraying fruit on the tree
are generally less than when apples or pears are dipped post-harvest.
FATE OF RESIDUES
Ethoxyquin, instead of existing as a single molecule, changes
spontaneously into a complex resulting from oxidation. These complex
structures are still effective biologically. No major degradation
change occurs in the ethoxyquin molecule. The structure of the
oxidation product has not been definitely determined but the
structures of the first reaction product(s) which fit the observations
most closely are suggested to be:
Other relevant chemical properties
The technical material as manufactured in the USA (Santoquin) is
claimed to contain 100 percent active ingredients, but is not 100
percent ethoxyquin. E.g. "Stop Scald" is a 70 percent emulsion of
Santoquin in water with added emulsifiers. It is a dark liquid varying
in color from yellow to black. The color is claimed to be independent
of the antioxidant or biological activity of the chemical.
Boiling Point: 125°C at 1-2 mm Hg.
Specific gravity: 1.028 to 1.032
Solubility: Miscible with animal and vegetable fats and oils.
Insoluble in water.
Viscosity at: 32°F 20,000 centipoises
73 197 "
122 17 "
158 7 "
(Monsanto, 1958).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Studies in chickens resulted in a 99 percent recovery of a single dose
of C14-labelled ethoxyquin within 48 hours. Continuous administration
of 125-137 ppm of ethoxyquin in the diet resulted in accumulation of
approximately 0.1 ppm of ethoxyquin and/or its metabolites in the
liver and fat per week during the first 12 weeks. Accumulation in
muscle and other edible tissue was barely detectable. Withdrawal of
ethoxyquin from the diet resulted in a loss of 79-90 percent of the
tissue residue in a 6 to 18 hour period. The excretion products
comprised 15 percent unchanged ethoxyquin, the remainder probably
being N-glucuronide and N-acetyl derivatives (Monsanto, 1956).
When ethoxiquin was given in the diet of rats for 10 days at a
concentration of 50 ppm, accumulation in the liver (2.1 - 4.8 ppm) and
kidney (2.1 - 2.7 ppm) was observed. Concentrations in fat and
skeletal muscle were less than 1 ppm (Wilson, 1956a; Wilson et al.,
1959).
Rats preconditioned for several weeks on a diet containing 50 ppm of
unlabelled ethoxyquin were given a single oral dose of 1.5 mg of
ethoxyquin C14-labelled in the 2 and 4 positions of the heterocyclic
ring. In two days 30 percent of the radioactivity was excreted in
urine, 34 percent in faeces. In four days and seven days 40-60 percent
and 58 percent was excreted in respectively, and 30-40 percent and 36
percent was excreted in faeces respectively. C14-carbon dioxide in
respired air was detected on the first day only, and comprised 0.7
percent of the administered dose (Wilson, 1956a Wilson et al., 1959).
In rats, repeated administration of ethoxyquin results in residues in
the kidney as well as in the fat and liver. A greater degree of
metabolic breakdown may occur than in chicken, because about 1 percent
of the C14 administered is exhaled as C14-carbon dioxide in rats as
compared with 0.2 percent in chickens (Monsanto, 1956).
Pregnant rats treated as above, and administered the labellet
ethoxyquin nine days prior to parturition indicated that placental
transfer of ethoxyquin occurs, because newborn young contained 0.12 to
0.21 ppm ethoxyquin in their tissues. Milk samples from two female
rats fed a diet of 50 ppm of ethoxyquin for 10 days had residues in
milk of 0.12 ppm and 0.19 ppm (Wilson, 1956a; Wilson et al., 1959).
Metabolic studies in dog indicated that ethoxyquin, per se, is not
excreted in the urine to any appreciable extent, but is excreted as
four unidentified metabolites (probably glucoronates). There was no
evidence of the ethoxy-group being split from the molecule during
metabolism. Results demonstrated that elimination is chiefly by the
kidneys and only to a small degree by way of the faeces (Hanzal,
1955).
TOXICOLOGICAL STUDIES
Special studies on reproduction
Rat
After 40 days on a slightly tocopherol-deficient diet containing 0,
250, 500 or 1000 ppm ethoxyquin, rats were bred to produce three
consecutive litters. The first litter offspring were utilized to
produce a second generation litter. The top dose level was discarded
after production of one litter. No effects on reproduction, as
reflected by fertility, litter size, or survival of offspring were
observed. The animals receiving the experimental diet produced young
and raised them more successfully than the controls. The 500 ppm diet
was more effective than the 250 ppm (Wilson, 1956b; Wilson and DeEds,
1959).
Groups, each of from five to nine rats, one to ten days pregnant, were
fed 0, 125, 375 or 1125 ppm ethoxyquin in the diet. Litter size,
stillbirths, survival to weanling, and weanling weights were all
comparable to the control rats (Derse, 1956).
Groups, each of eight or nine female rats, were placed on diets
containing 0, 125, 375 or 1125 ppm ethoxyquin on the day of mating.
Gestation time was comparable in all groups. However, litter size was
slightly depressed at the 375 ppm level and above, and at 1125 ppm
incidence of stillbirths was increased, and survival to weaning was
decreased (Derse, 1956).
Acute toxicity
LD50
Animal Route mg/kg body-weight References
Mouse i.p. 800-1000 Wilson and DeEds, 1959
Mouse i.v. 178 Wilson and DeEds, 1959
Mouse inhalation 3000 Kel'man, 1965
Rat oral ca.800 Wilson and DeEds, 1959
Rat inhalation 3150 Kel'man, 1965
Chicken oral 8000-10000 Maclay, 1954
Short-term studies
Chicken
Six groups of 70 four-day old cockerels were fed 0, 7.5, 15, 30, 75 or
750 ppm ethoxyquin in the diet for 12 weeks. No adverse effects were
observed on body-weight, food-consumption, mortality, gross-, or
histopathology (Maclay, 1954).
No adverse effects were observed in cockerels fed diets containing
7.5, 15, 30, 75 or 750 ppm ethoxyquin for 12 weeks (Colorado A. and
Experimental Station, ca. 1954).
Nor were any adverse effects observed in chicks fed diets containing
75, 750 or 1500 ppm for eight weeks (Halloran, 1952).
Chicks were fed 0.25 percent of ethoxyquin in their diet for six
weeks. The concentration of ethoxyquin in the livers was significantly
higher when the dietary level of protein was 17 percent than when the
level was 23 percent. (March et al., 1968).
Dog
Mixed groups, each of three dogs, were given oral doses by capsule
five times weekly for one year at dose-levels of 0, 3, 10, 50 or 100
mg/kg body-weight. Feeding the 100 mg/kg group was terminated after
sit weeks due to toxic effects, and the dogs were sacrificed at nine
weeks. One female animal at 3 mg/kg developed histoplasmosis about 40
weeks after initiation of the study, resulting in abnormal findings
when compared to the control dogs. All such finding could be
attributed to the infection. In the remaining animals body-weight was
depressed at 100 mg/kg; bromosulphthalein retention was increased at
10 mg/kg and above, indicating liver dysfunction; abdominal tenderness
was apparent in two of three dogs (male) at 10 mg/kg, and in two of
three dogs (one male and one female) at 50 mg/kg; erythrocyte
sedimentation rate was increased in one of three dogs at 50 mg/kg, and
in all three dogs at 100 mg/kg; haemograms showed reduced haematocrit,
haemoglobin, and erythrocyte counts in two of three dogs at 50 mg/kg,
and in all dogs at 100 mg/kg; urin-analysis indicated dark amber,
green or blackish brown urine at 50 and 100 mg/kg; there was increased
heart, liver, and kidney to body-weight ratios in all dose-levels, but
there does not appear to be any dose relationship in these increases;
histopathology indicated liver-stress, and fatty renal nephrosis at
the 10, 50 and 100 mg/kg dose-levels (Hanzal, 1955).
Rabbit
Undiluted ethoxyquin emulsion (70 percent) was applied to the clipped
intact skin of three rabbits, and removed after 24 hours. Slight
erythema occurred after 24 hours in all animals with barely
perceptible redness remaining after 48 hours in only one animal. The
compound was classed as a mild skin-irritant under the conditions
described (Kelly 1960).
Rat
Three groups of 10 male and 10 female rats which were fed 0, 0.2, and
0.4 percent ethoxyquin in their diet for 200 days displayed an initial
depression in growth rate (statistically significant only at the 0.4
level) in both test groups. The weight loss was not recovered during
the course of the experiment. Haemograms in all groups were
comparable. At autopsy, liver and kidney weights were elevated in both
sexes in both test groups. Histological examination showed
pyelonephritis in the males fed 0.2 and 0.4 percent, and doubtfully at
0.4 percent in the females. Thyroid hyperplasia was noted in males at
0.4 percent (Wilson and DeEds, 1959).
Groups of 10 male rats were fed 0, 62, 125, 250, 500, 1000 or 2000 ppm
ethoxyquin in the diet. Similarly, groups of 10 females were fed 0,
500, 1000 or 2000 ppm, and groups of five females 125 or 250 ppm. Half
the animals in some of the groups of 10 were autopsied after 200 days
on the diet. Body-weight gain was depressed in both sexes at 2000 ppm.
At autopsy, kidney to body-weight ratio was elevated in the males at
250 ppm and above, and in the females at 2000 ppm. Liver to
body-weight ratios were elevated at 1000 and 2000 ppm in males, and at
2000 ppm in females, Wilson and DeEds, 1959). Histopathological
examination showed kidney lesions at 500 ppm and above in the males,
and at 2000 ppm in the females; inclusions were observed in liver cell
cytoplasm in males at 2000 ppm, and in females at 2000 ppm (Cox,
1953).
Long-term studies
Dog
Ethoxyquin was fed to two groups of 14 dogs of mixed sex, at dietary
levels of 0 or 300 ppm for five years. No effects were observed on
haematology, urinalysis, clinical chemistry (aerum glutamic-oxalic
transaminase, blood urea nitrogen and bromosulphthalein retention),
organ weights or organ to body-weight ration, body-weight and
gross- or histopathology (Monsanto, 1966).
Rat
Groups, each of approximately 10 male and 10 female rats, were fed
levels of 0, 62, 125, 250, 500, 1000, 2000 and 4000 ppm of ethoxyquin
in their diets for periods of up to two years. The animals were
sacrificed for autopsy after 200, 400, 600 or 715 days. Mortality
rates at all dose levels were not significantly different from the
controls. Reduced body-weight gain was significant at 2000 ppm after
225 days in the male animals and after 21 days in the females. After
200 days increased liver to body-weight and increased kidney to
body-weight ratios were found at 250 ppm in the males and at 1000 ppm
in the females. Haemoglobin values for both sexes were normal 100 and
300 days after the start of the experiment in those rats fed 2000 and
4000 ppm. Histological changes in the renal cortex were clearly
evident after 200 days in the male rats receiving 2000 and 4000 ppm,
but not in the females. All other organs were normal in both sexes
after 200 days. After 400 days lesions in the kidneys
(pyelonephritis), liver and thyroid were clearly evident in the males
only. Similar lesions were evident for periods up to 717 days in both
sexes, although more marked in the males, and occasional tumours were
evident after 700 days but were unrelated to the dose-level, and were
also evident in the controls. No clearly defined effects were evident
from feeding 62 ppm, but minute lesions were present in the kidneys of
two of the males receiving the 500 ppm diet. It appeared difficult to
distinguish the abnormalities in the group examined after 700 days
with pathological manifestations associated with senility after that
time (Wilson and DeEds, 1959).
OBSERVATION IN MAN
In 20-years experience in the production of ethoxyquin there was no
indication of any cases of skin irritation or sensitivity (Kelly,
1960). However, cases of apparent sensitivity to ethoxyquin have been
reported in fruit handlers. Multiple cases of dermatitis have occurred
among employees handling freshly sprayed apples moist with solutions
of 70 percent formulated ethoxyquin. Patch-testing in volunteers
indicated that these skin reactions were not due to direct irritation,
but were the result of sensitization (Wood, 1965).
COMMENT
Studies in the rat and the dog indicate that toxic effects upon the
liver and kidney occur when dietary levels of 250 ppm and above are
given to the rat and 10 mg/kg or higher daily doses are given to dogs.
Administration of 300 ppm in the diet, however, was well tolerated by
dogs over a five-year period. There was an increase in the number of
stillbirths and a decrease in the litter size and in the incidence of
survival-to-weaning in rats fed 1125 ppm but not in those fed 125 ppm.
Lower doses (ca. 50 ppm) are rapidly excreted in the rat, dog and
chicken. Ethoxyquin is apparently converted to water soluble
metabolites and as long at these conversion mechanisms are functioning
there appears little likelihood of storage of the compound in the body
fat.
The occurrence of dermatitis among fruit packers suggests that
sensitization could be a problem among those who are handling
ethoxyquin.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Dog: 300 ppm in diet, equivalent to 7.5 mg/kg body-weight/day
Rat: 125 ppm in diet, equivalent to 6.25 mg/kg body-weight/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.06 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
Ethoxyquin is used as a pre-harvest treatment for the control of scald
of susceptible apple varieties. It is applied once as a full cover
spray within two days of harvest at a dosage rate of 3 pints of a
commercial formulation containing 70 percent active ingredient per 100
gallons of water (2700 ppm active ingredient).
Post-harvest treatments
For a post-harvest treatment of apples and pears, ethoxyquin is used
as a 15-30 sec. dip or a 15 sec. spray prior to or as the fruit passes
over the grading line. The dosage rate used is 2 to 3 pints of a
commercial formulation containing 70 percent active ingredient per 100
gallons of water (1800-2700 ppm active ingredient). The fruit is
treated no later than one week after harvest.
The above information on use patterns was obtained from product labels
from Canada, United States, United Kingdom and Italy.
Other uses
Ethoxyquin is also used as an antioxidant in spices, fish meal,
poultry feeds, swine feeds and other animal feeds.
Other information pertinent to use pattern
This monograph is confined to information pertaining to only the
pesticidal uses of ethoxyquin on apples and pears.
Ethoxyquin manufactured in the United States is sold in the following
countries: Sweden, Norway, Denmark, United Kingdom, Netherlands,
Belgium-Luxembourg, France, W. Germany, Austria, Switzerland, Finland,
Spain, Italy, Rep. of South Africa, Mexico, Colombia, Venezuela, Peru,
Chile, Brazil, Philippines, Australia, New Zealand, United States and
Canada (Monsanto - Direct communication, 1969).
Information concerning uses in countries other than Canada, United
States, United Kingdom and Italy, such as Israel, Japan, West Germany
and Sweden, where it was understood that ethoxyquin was manufactured,
was not available to the Joint Meeting.
RESIDUES RESULTING FROM SUPERVISED TRIALS
The results reported in Tables 1, 2, 3 for apples and 6 and 7 for
pears are from supervised trials made in various geographical
locations in the United States (Monsanto, 1961). Those in Tables 4 and
5 for apples, are from supervised trials carried out in New Zealand
(Padfield et al., 1963).
Summary of residue data
The residues of ethoxyquin on apples and pears when applied as a dip
or spray on a whole-fruit basis did not exceed 1.2 ppm from U.S.A.
data. However, from New Zealand, levels up to 2.8 ppm on apples have
been reported and one variety, Dougherty, which appears to have the
ability to retain fairly high levels of residue, was reported to have
levels up to 4.4 ppm. Results obtained in New Zealand also indicate
that use of a warm dip may result in residues of ethoxyquin on apples
as high as 6 ppm. Experimental results suggest that the uptake of
ethoxyquin is likely to increase as apple fruit matures and cooled
fruit appears to show lower residues than fruit treated at normal air
temperature. The results of trials carried out in the United States
indicate that residues found as a result of spraying fruit on the tree
are generally less than when apples or pears are dipped post-harvest.
FATE OF RESIDUES
Ethoxyquin, instead of existing as a single molecule, changes
spontaneously into a complex resulting from oxidation. These complex
structures are still effective biologically. No major degradation
change occurs in the ethoxyquin molecule. The structure of the
oxidation product has not been definitely determined but the
structures of the first reaction product(s) which fit the observations
most closely are suggested to be:
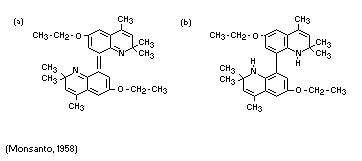 TABLE 1
Pre-harvest treatments - applied as a fruit and foliage spray just prior to picking of apples.
Interval between
Treatment Rate Apple Treatment and Residue (ppm)
(ppm ethoxyquin) Variety Sampling (days) Fresh Apples Stored Apples
900 Paragon 13 - 14 0 - 0.2
Winesap
1800 " 13 - 14 0.03 - 0.35
1500 McIntosh 23 - 24 0 - 0.01
44 0 - 0.11
3000 McIntosh 23 0 0 - 0.16
2000 Wealthy 53 0.01 - 0.10
0 Greening 53 0.00 - 0.11
2000 " 22 0.00 - 0.13
53 0.01 - 0.11
2000 McIntosh 20 0.00 - 0.01
51 0.00
TABLE 2
Post-harvest treatments - using a 15 second dip or applied as a spray.
Interval between
Treatment Rate Apple Treatment and Residue (ppm)
(ppm ethoxyquin) Variety Sampling (days) Fresh Apples Stored Apples
1000 Red
Delicious 2 0.07, 0.42
31 0.13, 0.07
2000 " 3 0.20, 0.34
32 0.14, 1.17
3000 " 3 0.48, 0.34
32 0.53, 1.00
1000 Jonathon 4 0.03, 0.18
32 0.11
2000 " 4 0.62, 0.43
" 33 0.04, 0.04
3000 " 4 0.44, 0.81
" 33 0.09 0.04
2000 Wealthy 23 0.00 - 0.62
" 55 0.00 - 0.20
3000 " 23 0.01 - 0.81
" 55 0.04 - 0.14
0 Greening 50 0.00 - 0.11
2000 " 20 0.03 - 0.13
" 50 0.00
2000 McIntosh 19 0.00
49 0.00
TABLE 3
Post-harvest treatments - using a 15 second dip.
Treatment Rate Apple Interval Residues (ppm)
(ppm ethoxyquin) Variety (days)* Peel Pulp Whole Apple
0 Jonathon 1 0.2 - 0.3 0.03 - 0.04
1000 " 1 3.6 - 4.1 0.47 - 0.54
2000 " 1 4.5 - 4.7 0.61 - 0.64
3000 " 1 5.4 - 5.7 0.67 - 0.70
4000 " 1 8.0 - 7.5 0.94 - 1.00
0 McIntosh 7 0.07, 0.11 0.08, 0.11 0.08, 0.11
3600 " 7 1.45, 1.45 0.14, 0.14 0.29, 0.30
0 Cortland 7 0.07, 0.16 0.07, 0.09 0.08, 0.08
2700 " 7 1.75, 1.80 0.07, 0.11 0.30, 0.32
* Interval between treatment and sampling
TABLE 4
Residues on apples dipped or sprayed with 1800 ppm ethoxyquin.
The samples of fruit for analysis were withdrawn from the
line during normal operation and residue determinations were
made as soon as possible after treatment.
Residue (ppm)
Apple First Pick Second Pick
Variety Treatment March-April March-May
Delicious Cool dip 1.4 1.2
Warm dip 1.5 1.9
Spray 1.0 0.8
Granny Smith Cool dip 1.1 2.8
Warm dip 1.6 4.8
Spray 1.5 0.7
Rome Beauty Cool dip 0.7 1.4
Warm dip 2.4 1.8
Spray 0.9 1.3
Sturmer Cool dip 0.5 1.6
Warm dip 1.8 2.7
Spray 1.8 2.3
Dougherty Cool dip 2.6 4.4
Warm dip 1.9 6.0
Spray 2.2 3.0
TABLE 5
Ethoxyquin residues on apples after treatment in commercial spay tunnels.
Residue analyses were made as soon as possible after treatment.
Treatment Rate Apple Fruit
(ppm ethoxyquin) Variety Temperature Residue (ppm)
1800 Sturmer Air temp. 1.6
Cooled 38°F 0.7
1800 Granny Smith Air temp. 0.7 - 1.7
Cooled 33°F 0.5 - 1.0
2700 Air temp. 2.2
Cooled 33°f 1.5
TABLE 6
Pre-harvest treatments - applied an a fruit and foliage spray just
prior to picking of pears.
Interval between
Treatment Rate Pear Treatment and Residues (ppm)
(ppm ethoxyquin) Variety Sampling (days) Fresh Fruit Stored Fruit
1000 Anjou 23 - 25 0.01
70 - 71 0.00
2000 " 23 - 25 0.01 - 0.04
70 - 71 0.00
TABLE 7
Post-harvest treatments using a 15 second dip.
Residues (ppm)
Treatment Rate Pear Interval Whole Stored
(ppm ethoxyquin) Variety (days)* Pulp Peel Fruit Fruit
900 Anjou 53 0.00
1800 " 53 0.00
1000 " 53 0.03
1500 " 53 0.03
2000 " 53 0.01
2700 " 7 0.04 - 0.05 4.7 - 4.9 0.66
3600 " 7 0.04 - 0.09 7.6 - 8.3 1.09
5400 " 7 0.04 - 0.08 7.9 - 9.2 1.07
* Interval between treatment and sampling.
Evidence of residues in food in commerce or at consumption
No information available.
METHODS OF RESIDUE ANALYSIS
Methods using ultraviolet spectrophotometry of spectrofluorometry have
been proposed for the determination of residues of ethoxyquin in food,
feed and animal tissue. The spectrophotometric methods of Choy et al.
(1963) and Alicino et al. (1963) both include clean-up stages to
separate ethoxyquin from other anti-oxidants such as butylated
hydroxyanisole and butylated hydroxytoluene before estimation of
absorbance at 296 nm. A spectrofluorometric procedure for ethoxyquin
in feeding stuffs (Gordon et al., 1964) was adapted for use on samples
of eggs, chicken muscle and liver (Van Deren and Jaworski, 1966). It
is relatively non-specific for ethoxyquin and residues of other
pesticides or of other naturally occurring compounds may interfere.
Collaborative study of this method (Van Deren and Jaworski, 1967) led
to its adoption as an official, first action procedure (Anon, 1968).
The ethoxyquin is extracted with iso-octane, cleaned-up by an
acid-alkaline extraction procedure and determined by examining the
fluorescence of an iso-octane solution. The suitability of this method
for application to fruit for regulatory purposes needs further
evaluation.
NATIONAL TOLERANCES
Tolerances for Fruit
Canada - 3 ppm on apples
U.K. - 3 ppm on apples
U.S.A. - 3 ppm on apples and pears
(for ethoxyquin and other naturally occurring
fluorescent materials).
New Zealand - 3 ppm on apples
Tolerances derived from use as additives in animal feeds
Canada - 3 ppm in livers of poultry
0.5 ppm in meat, poultry meat and eggs
U.S.A. - 3 ppm in poultry livers and fat
0.5 ppm in eggs and meat
APPRAISAL
In a review of the literature very few articles were found on
ethoxyquin other than those on its efficacy in controlling scald. The
abstracting journals reviewed were, Review of Applied Mycology,
Biological Abstracts, Chemical Abstracts and Pesticide Documentation
Bulletin.
Data available are based on ethoxyquin as manufactured in the U.S.A.
and used in several other countries. Ethoxyquin is also manufactured
in Israel, Japan, West Germany and Sweden. The amount and nature of
minor constituents and amount of ethoxyquin monomer in products other
than U.S.A. manufacture in unknown. It is used for the control of
scald in apples (and to a lesser extent pears), as both pre-harvest
treatments and post-harvest dips and sprays.
The residues of ethoxyquin on apples and pears when applied as a dip
or spray on a whole-fruit basis did not exceed 1.2 ppm from U.S.A.
data. However, from New Zealand, levels up to 2.8 ppm on apples have
been reported and one variety, Dougherty, which appears to have the
ability to retain fairly high residue, was reported to have levels up
to 4.4 ppm. Results obtained in New Zealand also indicate that use of
a warm dip may result in residues of ethoxyquin on apples as high as 6
ppm. Experimental results suggest that the uptake of ethoxyquin is
likely to increase as apple fruit matures. Cooled fruit appears to
have lower residues than fruit treated at normal air temperature. The
results of trials carried out in the U.S.A. indicate that residues
found as a result of spraying fruit on the tree are generally less
than when apples or pears are dipped post-harvest.
Ethoxyquin, instead of existing as a single molecule, changes
spontaneously into a complex resulting from oxidation. These complex
structures are still effective biologically. No major degradation
change occurs in the ethoxyquin molecule. The structure of the
oxidation product has not been definitely determined, but the
structures of the first reaction product(s) which fit the observations
most closely are given in the monograph.
The fluorometric method is relatively non-specific for ethoxyquin on
apples and pears. Many naturally occurring fluorescent materials may
interfere. It is also possible that residues of other pesticides used
on apples may fluoresce or be converted into fluorescent compounds.
More suitable spectrofluometric methods available for animal products
should be adopted and evaluated.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES OR PRACTICAL
RESIDUE LIMITS
TOLERANCES
Apples and pears 3.0 ppm
DESIRABLE
1. Additional reproduction studies to investigate the effect on
survival rate of offspring of rats.
2. A method of analysis for apples and pears suitable for regulatory
purposes.
3. Composition and purity of this compound as manufactured in several
countries and the amount of ethoxyquinmonomer in the products from
various manufacturers.
REFERENCES
Alicino, N.J., Klein, H.C., Quattrone, J.J. and Choy, T.K. (1963)
Determination of butylated hydroxyanisole, butylated, hydroxytoluene,
and ethoxyquin in hydrocarbon-soluble samples. J. Agr. Fd Chem., 11:
496-8
Anon (1968) Changes in Official Method of Analysis. J. Assoc. Offic.
Anal. Chem., 51:453-4
Choy, T., Alicino, N.J., Klein, H.C. and Quattrone, J.J. (1963)
Determination of ethoxyquin by ultraviolet spectrophotometry. J. Agri.
Fd Chem., 11:340-2
Choy, T., Alicino, N.J., Klein, H.C. and Quattrone, J.J. (1963)
Determination of ethoxyquin by ultraviolet spectrophotometry. J.Agri.
Fd Chem., 11:340-2
Colorado, A. and M. Experimental Station. (1954) The chronic toxicity
of the antioxidant ca. 6-ethoxy, 2,2,4-trimethyl, 1,2-dihydroquinoline
(Santoquin, Monsanto). Unpublished, undated, report of studies
conducted during 1952-53. Submitted by Monsanto Chemical Company.
Cox, A.J. (1953) 6-ethoxy, 2,2,4-trimethyl-1,2-dihydroquinoline
(EMHQ). Histological report on sections of rat tissues stained with
haematoxylin-eosin. Unpublished report submitted by Monsanto Chemical
Company.
Derse, P. (1956) Assay report. Unpublished report of Wisconsin Alumni
Research Foundation submitted by Monsanto Chemical Company
Gordon, R.S. Conkin, R.A. and Machlin, L.J. (1964) Determination of
ethoxyquin in feeds. J. Assoc. Offic. Agric. Chem., 47:512-6
Halloran, H.R. (1952) Chick toxicity tests on Santoflex AW.
Unpublished report from the Poultry Products of Central California,
Petaluma, to California Department of Agriculture submitted by
Monsanto Chemical Company.
Hansal, R.F. (1955) Final report and addendum. Chronic oral
administration-dogs-metabolic studies. Unpublished report of Hazleton
Laboratories submitted by Monsanto Chemical Company
Kelly, R.G. (1960) Supplementary toxicity information on Santoquin
(6-ethoxy-2,2,4-trimethyl-1,2-dihydroquinoline). Unpublished report
prepared and submitted by Monsanto Chemical Company
Kel'man, G.Y. (1965) Comparative toxicity of Santoflex A
(6-ethoxy-2,2,4-trimethyl-1,2-dihydroquinoline) and acetoneanyl
(1,2-dihydro-2,2,4-trimethylquinoline). Keuchuk i Rezina 24:40-41
[(Chem. Abstr. 63:1136g (1965)]
Mackay, V.D. (1954) Toxicity data in support of the use of 0.015% of
6-ethoxy-2,2,4-trimethyl-1,2-dihydroquinoline on alfalfa meal for
carotene preservation. Unpublished report of Western Utilization
Research Branch, U.S.D.A., submitted by Monsanto Chemical Company
March, B.E., Biely, J. and Coates, V. (1968) The influence of diet on
toxicity of the anti-oxidant
1,2-dihydro-6-ethoxy-2,2,4-trimethylquinoline. Can. J. Physiol.
Pharmacol. 46:139-43. [Chem. Abstr. 68:102955m (1968)]
Monsanto (1956) Santoquin for use in Poultry Feeds, Unpublished report
prepared and submitted by Monsanto Chemical Company
Monsanto. (1956) Petition proposing the issuance of a regulation
permitting the use of Santoquin as an antioxidant for addition to
poultry feeds. Unpublished report. October
Monsanto. (1961) Information to support label registration request for
"Stop-Scald" (ethoxyquin) on apples and pears to control common scald
and petition for tolerance for ethoxyquin on pears. Unpublished
report.
Monsanto. (1966) Five year Santoquin feeding study in dogs.
Unpublished collection of reports prepared and submitted by Monsanto
Chemical Company
Monsanto. (1969) Direct communication
Padfield, C.A.S., Atkinson, J.D., Clark, P.J. (1963) Control of apple
scald by ethoxyquin. N.Z.J. Agr. Res. 6:245-52
Van Deren, J.M. and Jaworski, E.G. (1966) Ethoxyquin (Santonin (R) in
eggs, chicken muscle and chicken liver. J. Assoc. Offic. Anal. Chem.,
49:712-4
Van Deren, J.M. and Jaworski, E.G. (1967) Collaborative study of the
determination of ethoxyquin in chick tissue and eggs. J. Assoc. Offic.
Anal. Chem., 50:844-7
Wilson, R.H. (1956a) Distribution and excretion of oral Santoquin
C14, Unpublished reports of Western Utilization Research Branch,
U.S.D.A. submitted by Monsanto Chemical Company
Wilson, R.H. (1956b) Final report - reproduction in rats receiving
Santoquin. Unpublished report of Western Regional Utilization Research
Branch, U.S.D.A. submitted by Monsanto Chemical Company
Wilson, R.H. and DeEds, F. (1959) Toxicity studies on the antioxidant
6-ethoxy-1,2-dihydro-2,2,4-trimethylquinoline. J. Agr. Food Chem.
7:203-6
Wilson, R.H., Thomas, J.O., Thompson, C.R., Lanner, H.F. and Kohler,
G.O. (1959) Absorption, metabolism and excretion of the antioxidant,
6-ethoxy-1,2-dihydro-2,2,4-trimethylquinoline. J. Agr. Food Chem.,
7:206-9
Wood, W.S. (1965) Untitled report of dermatitis cases in fruit
handlers. Unpublished report from the Medical Dental Building,
Vancouver, B.C. submitted by Monsanto Chemical Company
TABLE 1
Pre-harvest treatments - applied as a fruit and foliage spray just prior to picking of apples.
Interval between
Treatment Rate Apple Treatment and Residue (ppm)
(ppm ethoxyquin) Variety Sampling (days) Fresh Apples Stored Apples
900 Paragon 13 - 14 0 - 0.2
Winesap
1800 " 13 - 14 0.03 - 0.35
1500 McIntosh 23 - 24 0 - 0.01
44 0 - 0.11
3000 McIntosh 23 0 0 - 0.16
2000 Wealthy 53 0.01 - 0.10
0 Greening 53 0.00 - 0.11
2000 " 22 0.00 - 0.13
53 0.01 - 0.11
2000 McIntosh 20 0.00 - 0.01
51 0.00
TABLE 2
Post-harvest treatments - using a 15 second dip or applied as a spray.
Interval between
Treatment Rate Apple Treatment and Residue (ppm)
(ppm ethoxyquin) Variety Sampling (days) Fresh Apples Stored Apples
1000 Red
Delicious 2 0.07, 0.42
31 0.13, 0.07
2000 " 3 0.20, 0.34
32 0.14, 1.17
3000 " 3 0.48, 0.34
32 0.53, 1.00
1000 Jonathon 4 0.03, 0.18
32 0.11
2000 " 4 0.62, 0.43
" 33 0.04, 0.04
3000 " 4 0.44, 0.81
" 33 0.09 0.04
2000 Wealthy 23 0.00 - 0.62
" 55 0.00 - 0.20
3000 " 23 0.01 - 0.81
" 55 0.04 - 0.14
0 Greening 50 0.00 - 0.11
2000 " 20 0.03 - 0.13
" 50 0.00
2000 McIntosh 19 0.00
49 0.00
TABLE 3
Post-harvest treatments - using a 15 second dip.
Treatment Rate Apple Interval Residues (ppm)
(ppm ethoxyquin) Variety (days)* Peel Pulp Whole Apple
0 Jonathon 1 0.2 - 0.3 0.03 - 0.04
1000 " 1 3.6 - 4.1 0.47 - 0.54
2000 " 1 4.5 - 4.7 0.61 - 0.64
3000 " 1 5.4 - 5.7 0.67 - 0.70
4000 " 1 8.0 - 7.5 0.94 - 1.00
0 McIntosh 7 0.07, 0.11 0.08, 0.11 0.08, 0.11
3600 " 7 1.45, 1.45 0.14, 0.14 0.29, 0.30
0 Cortland 7 0.07, 0.16 0.07, 0.09 0.08, 0.08
2700 " 7 1.75, 1.80 0.07, 0.11 0.30, 0.32
* Interval between treatment and sampling
TABLE 4
Residues on apples dipped or sprayed with 1800 ppm ethoxyquin.
The samples of fruit for analysis were withdrawn from the
line during normal operation and residue determinations were
made as soon as possible after treatment.
Residue (ppm)
Apple First Pick Second Pick
Variety Treatment March-April March-May
Delicious Cool dip 1.4 1.2
Warm dip 1.5 1.9
Spray 1.0 0.8
Granny Smith Cool dip 1.1 2.8
Warm dip 1.6 4.8
Spray 1.5 0.7
Rome Beauty Cool dip 0.7 1.4
Warm dip 2.4 1.8
Spray 0.9 1.3
Sturmer Cool dip 0.5 1.6
Warm dip 1.8 2.7
Spray 1.8 2.3
Dougherty Cool dip 2.6 4.4
Warm dip 1.9 6.0
Spray 2.2 3.0
TABLE 5
Ethoxyquin residues on apples after treatment in commercial spay tunnels.
Residue analyses were made as soon as possible after treatment.
Treatment Rate Apple Fruit
(ppm ethoxyquin) Variety Temperature Residue (ppm)
1800 Sturmer Air temp. 1.6
Cooled 38°F 0.7
1800 Granny Smith Air temp. 0.7 - 1.7
Cooled 33°F 0.5 - 1.0
2700 Air temp. 2.2
Cooled 33°f 1.5
TABLE 6
Pre-harvest treatments - applied an a fruit and foliage spray just
prior to picking of pears.
Interval between
Treatment Rate Pear Treatment and Residues (ppm)
(ppm ethoxyquin) Variety Sampling (days) Fresh Fruit Stored Fruit
1000 Anjou 23 - 25 0.01
70 - 71 0.00
2000 " 23 - 25 0.01 - 0.04
70 - 71 0.00
TABLE 7
Post-harvest treatments using a 15 second dip.
Residues (ppm)
Treatment Rate Pear Interval Whole Stored
(ppm ethoxyquin) Variety (days)* Pulp Peel Fruit Fruit
900 Anjou 53 0.00
1800 " 53 0.00
1000 " 53 0.03
1500 " 53 0.03
2000 " 53 0.01
2700 " 7 0.04 - 0.05 4.7 - 4.9 0.66
3600 " 7 0.04 - 0.09 7.6 - 8.3 1.09
5400 " 7 0.04 - 0.08 7.9 - 9.2 1.07
* Interval between treatment and sampling.
Evidence of residues in food in commerce or at consumption
No information available.
METHODS OF RESIDUE ANALYSIS
Methods using ultraviolet spectrophotometry of spectrofluorometry have
been proposed for the determination of residues of ethoxyquin in food,
feed and animal tissue. The spectrophotometric methods of Choy et al.
(1963) and Alicino et al. (1963) both include clean-up stages to
separate ethoxyquin from other anti-oxidants such as butylated
hydroxyanisole and butylated hydroxytoluene before estimation of
absorbance at 296 nm. A spectrofluorometric procedure for ethoxyquin
in feeding stuffs (Gordon et al., 1964) was adapted for use on samples
of eggs, chicken muscle and liver (Van Deren and Jaworski, 1966). It
is relatively non-specific for ethoxyquin and residues of other
pesticides or of other naturally occurring compounds may interfere.
Collaborative study of this method (Van Deren and Jaworski, 1967) led
to its adoption as an official, first action procedure (Anon, 1968).
The ethoxyquin is extracted with iso-octane, cleaned-up by an
acid-alkaline extraction procedure and determined by examining the
fluorescence of an iso-octane solution. The suitability of this method
for application to fruit for regulatory purposes needs further
evaluation.
NATIONAL TOLERANCES
Tolerances for Fruit
Canada - 3 ppm on apples
U.K. - 3 ppm on apples
U.S.A. - 3 ppm on apples and pears
(for ethoxyquin and other naturally occurring
fluorescent materials).
New Zealand - 3 ppm on apples
Tolerances derived from use as additives in animal feeds
Canada - 3 ppm in livers of poultry
0.5 ppm in meat, poultry meat and eggs
U.S.A. - 3 ppm in poultry livers and fat
0.5 ppm in eggs and meat
APPRAISAL
In a review of the literature very few articles were found on
ethoxyquin other than those on its efficacy in controlling scald. The
abstracting journals reviewed were, Review of Applied Mycology,
Biological Abstracts, Chemical Abstracts and Pesticide Documentation
Bulletin.
Data available are based on ethoxyquin as manufactured in the U.S.A.
and used in several other countries. Ethoxyquin is also manufactured
in Israel, Japan, West Germany and Sweden. The amount and nature of
minor constituents and amount of ethoxyquin monomer in products other
than U.S.A. manufacture in unknown. It is used for the control of
scald in apples (and to a lesser extent pears), as both pre-harvest
treatments and post-harvest dips and sprays.
The residues of ethoxyquin on apples and pears when applied as a dip
or spray on a whole-fruit basis did not exceed 1.2 ppm from U.S.A.
data. However, from New Zealand, levels up to 2.8 ppm on apples have
been reported and one variety, Dougherty, which appears to have the
ability to retain fairly high residue, was reported to have levels up
to 4.4 ppm. Results obtained in New Zealand also indicate that use of
a warm dip may result in residues of ethoxyquin on apples as high as 6
ppm. Experimental results suggest that the uptake of ethoxyquin is
likely to increase as apple fruit matures. Cooled fruit appears to
have lower residues than fruit treated at normal air temperature. The
results of trials carried out in the U.S.A. indicate that residues
found as a result of spraying fruit on the tree are generally less
than when apples or pears are dipped post-harvest.
Ethoxyquin, instead of existing as a single molecule, changes
spontaneously into a complex resulting from oxidation. These complex
structures are still effective biologically. No major degradation
change occurs in the ethoxyquin molecule. The structure of the
oxidation product has not been definitely determined, but the
structures of the first reaction product(s) which fit the observations
most closely are given in the monograph.
The fluorometric method is relatively non-specific for ethoxyquin on
apples and pears. Many naturally occurring fluorescent materials may
interfere. It is also possible that residues of other pesticides used
on apples may fluoresce or be converted into fluorescent compounds.
More suitable spectrofluometric methods available for animal products
should be adopted and evaluated.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES OR PRACTICAL
RESIDUE LIMITS
TOLERANCES
Apples and pears 3.0 ppm
DESIRABLE
1. Additional reproduction studies to investigate the effect on
survival rate of offspring of rats.
2. A method of analysis for apples and pears suitable for regulatory
purposes.
3. Composition and purity of this compound as manufactured in several
countries and the amount of ethoxyquinmonomer in the products from
various manufacturers.
REFERENCES
Alicino, N.J., Klein, H.C., Quattrone, J.J. and Choy, T.K. (1963)
Determination of butylated hydroxyanisole, butylated, hydroxytoluene,
and ethoxyquin in hydrocarbon-soluble samples. J. Agr. Fd Chem., 11:
496-8
Anon (1968) Changes in Official Method of Analysis. J. Assoc. Offic.
Anal. Chem., 51:453-4
Choy, T., Alicino, N.J., Klein, H.C. and Quattrone, J.J. (1963)
Determination of ethoxyquin by ultraviolet spectrophotometry. J. Agri.
Fd Chem., 11:340-2
Choy, T., Alicino, N.J., Klein, H.C. and Quattrone, J.J. (1963)
Determination of ethoxyquin by ultraviolet spectrophotometry. J.Agri.
Fd Chem., 11:340-2
Colorado, A. and M. Experimental Station. (1954) The chronic toxicity
of the antioxidant ca. 6-ethoxy, 2,2,4-trimethyl, 1,2-dihydroquinoline
(Santoquin, Monsanto). Unpublished, undated, report of studies
conducted during 1952-53. Submitted by Monsanto Chemical Company.
Cox, A.J. (1953) 6-ethoxy, 2,2,4-trimethyl-1,2-dihydroquinoline
(EMHQ). Histological report on sections of rat tissues stained with
haematoxylin-eosin. Unpublished report submitted by Monsanto Chemical
Company.
Derse, P. (1956) Assay report. Unpublished report of Wisconsin Alumni
Research Foundation submitted by Monsanto Chemical Company
Gordon, R.S. Conkin, R.A. and Machlin, L.J. (1964) Determination of
ethoxyquin in feeds. J. Assoc. Offic. Agric. Chem., 47:512-6
Halloran, H.R. (1952) Chick toxicity tests on Santoflex AW.
Unpublished report from the Poultry Products of Central California,
Petaluma, to California Department of Agriculture submitted by
Monsanto Chemical Company.
Hansal, R.F. (1955) Final report and addendum. Chronic oral
administration-dogs-metabolic studies. Unpublished report of Hazleton
Laboratories submitted by Monsanto Chemical Company
Kelly, R.G. (1960) Supplementary toxicity information on Santoquin
(6-ethoxy-2,2,4-trimethyl-1,2-dihydroquinoline). Unpublished report
prepared and submitted by Monsanto Chemical Company
Kel'man, G.Y. (1965) Comparative toxicity of Santoflex A
(6-ethoxy-2,2,4-trimethyl-1,2-dihydroquinoline) and acetoneanyl
(1,2-dihydro-2,2,4-trimethylquinoline). Keuchuk i Rezina 24:40-41
[(Chem. Abstr. 63:1136g (1965)]
Mackay, V.D. (1954) Toxicity data in support of the use of 0.015% of
6-ethoxy-2,2,4-trimethyl-1,2-dihydroquinoline on alfalfa meal for
carotene preservation. Unpublished report of Western Utilization
Research Branch, U.S.D.A., submitted by Monsanto Chemical Company
March, B.E., Biely, J. and Coates, V. (1968) The influence of diet on
toxicity of the anti-oxidant
1,2-dihydro-6-ethoxy-2,2,4-trimethylquinoline. Can. J. Physiol.
Pharmacol. 46:139-43. [Chem. Abstr. 68:102955m (1968)]
Monsanto (1956) Santoquin for use in Poultry Feeds, Unpublished report
prepared and submitted by Monsanto Chemical Company
Monsanto. (1956) Petition proposing the issuance of a regulation
permitting the use of Santoquin as an antioxidant for addition to
poultry feeds. Unpublished report. October
Monsanto. (1961) Information to support label registration request for
"Stop-Scald" (ethoxyquin) on apples and pears to control common scald
and petition for tolerance for ethoxyquin on pears. Unpublished
report.
Monsanto. (1966) Five year Santoquin feeding study in dogs.
Unpublished collection of reports prepared and submitted by Monsanto
Chemical Company
Monsanto. (1969) Direct communication
Padfield, C.A.S., Atkinson, J.D., Clark, P.J. (1963) Control of apple
scald by ethoxyquin. N.Z.J. Agr. Res. 6:245-52
Van Deren, J.M. and Jaworski, E.G. (1966) Ethoxyquin (Santonin (R) in
eggs, chicken muscle and chicken liver. J. Assoc. Offic. Anal. Chem.,
49:712-4
Van Deren, J.M. and Jaworski, E.G. (1967) Collaborative study of the
determination of ethoxyquin in chick tissue and eggs. J. Assoc. Offic.
Anal. Chem., 50:844-7
Wilson, R.H. (1956a) Distribution and excretion of oral Santoquin
C14, Unpublished reports of Western Utilization Research Branch,
U.S.D.A. submitted by Monsanto Chemical Company
Wilson, R.H. (1956b) Final report - reproduction in rats receiving
Santoquin. Unpublished report of Western Regional Utilization Research
Branch, U.S.D.A. submitted by Monsanto Chemical Company
Wilson, R.H. and DeEds, F. (1959) Toxicity studies on the antioxidant
6-ethoxy-1,2-dihydro-2,2,4-trimethylquinoline. J. Agr. Food Chem.
7:203-6
Wilson, R.H., Thomas, J.O., Thompson, C.R., Lanner, H.F. and Kohler,
G.O. (1959) Absorption, metabolism and excretion of the antioxidant,
6-ethoxy-1,2-dihydro-2,2,4-trimethylquinoline. J. Agr. Food Chem.,
7:206-9
Wood, W.S. (1965) Untitled report of dermatitis cases in fruit
handlers. Unpublished report from the Medical Dental Building,
Vancouver, B.C. submitted by Monsanto Chemical Company
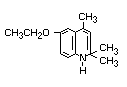 Other relevant chemical properties
The technical material as manufactured in the USA (Santoquin) is
claimed to contain 100 percent active ingredients, but is not 100
percent ethoxyquin. E.g. "Stop Scald" is a 70 percent emulsion of
Santoquin in water with added emulsifiers. It is a dark liquid varying
in color from yellow to black. The color is claimed to be independent
of the antioxidant or biological activity of the chemical.
Boiling Point: 125°C at 1-2 mm Hg.
Specific gravity: 1.028 to 1.032
Solubility: Miscible with animal and vegetable fats and oils.
Insoluble in water.
Viscosity at: 32°F 20,000 centipoises
73 197 "
122 17 "
158 7 "
(Monsanto, 1958).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Studies in chickens resulted in a 99 percent recovery of a single dose
of C14-labelled ethoxyquin within 48 hours. Continuous administration
of 125-137 ppm of ethoxyquin in the diet resulted in accumulation of
approximately 0.1 ppm of ethoxyquin and/or its metabolites in the
liver and fat per week during the first 12 weeks. Accumulation in
muscle and other edible tissue was barely detectable. Withdrawal of
ethoxyquin from the diet resulted in a loss of 79-90 percent of the
tissue residue in a 6 to 18 hour period. The excretion products
comprised 15 percent unchanged ethoxyquin, the remainder probably
being N-glucuronide and N-acetyl derivatives (Monsanto, 1956).
When ethoxiquin was given in the diet of rats for 10 days at a
concentration of 50 ppm, accumulation in the liver (2.1 - 4.8 ppm) and
kidney (2.1 - 2.7 ppm) was observed. Concentrations in fat and
skeletal muscle were less than 1 ppm (Wilson, 1956a; Wilson et al.,
1959).
Rats preconditioned for several weeks on a diet containing 50 ppm of
unlabelled ethoxyquin were given a single oral dose of 1.5 mg of
ethoxyquin C14-labelled in the 2 and 4 positions of the heterocyclic
ring. In two days 30 percent of the radioactivity was excreted in
urine, 34 percent in faeces. In four days and seven days 40-60 percent
and 58 percent was excreted in respectively, and 30-40 percent and 36
percent was excreted in faeces respectively. C14-carbon dioxide in
respired air was detected on the first day only, and comprised 0.7
percent of the administered dose (Wilson, 1956a Wilson et al., 1959).
In rats, repeated administration of ethoxyquin results in residues in
the kidney as well as in the fat and liver. A greater degree of
metabolic breakdown may occur than in chicken, because about 1 percent
of the C14 administered is exhaled as C14-carbon dioxide in rats as
compared with 0.2 percent in chickens (Monsanto, 1956).
Pregnant rats treated as above, and administered the labellet
ethoxyquin nine days prior to parturition indicated that placental
transfer of ethoxyquin occurs, because newborn young contained 0.12 to
0.21 ppm ethoxyquin in their tissues. Milk samples from two female
rats fed a diet of 50 ppm of ethoxyquin for 10 days had residues in
milk of 0.12 ppm and 0.19 ppm (Wilson, 1956a; Wilson et al., 1959).
Metabolic studies in dog indicated that ethoxyquin, per se, is not
excreted in the urine to any appreciable extent, but is excreted as
four unidentified metabolites (probably glucoronates). There was no
evidence of the ethoxy-group being split from the molecule during
metabolism. Results demonstrated that elimination is chiefly by the
kidneys and only to a small degree by way of the faeces (Hanzal,
1955).
TOXICOLOGICAL STUDIES
Special studies on reproduction
Rat
After 40 days on a slightly tocopherol-deficient diet containing 0,
250, 500 or 1000 ppm ethoxyquin, rats were bred to produce three
consecutive litters. The first litter offspring were utilized to
produce a second generation litter. The top dose level was discarded
after production of one litter. No effects on reproduction, as
reflected by fertility, litter size, or survival of offspring were
observed. The animals receiving the experimental diet produced young
and raised them more successfully than the controls. The 500 ppm diet
was more effective than the 250 ppm (Wilson, 1956b; Wilson and DeEds,
1959).
Groups, each of from five to nine rats, one to ten days pregnant, were
fed 0, 125, 375 or 1125 ppm ethoxyquin in the diet. Litter size,
stillbirths, survival to weanling, and weanling weights were all
comparable to the control rats (Derse, 1956).
Groups, each of eight or nine female rats, were placed on diets
containing 0, 125, 375 or 1125 ppm ethoxyquin on the day of mating.
Gestation time was comparable in all groups. However, litter size was
slightly depressed at the 375 ppm level and above, and at 1125 ppm
incidence of stillbirths was increased, and survival to weaning was
decreased (Derse, 1956).
Acute toxicity
LD50
Animal Route mg/kg body-weight References
Mouse i.p. 800-1000 Wilson and DeEds, 1959
Mouse i.v. 178 Wilson and DeEds, 1959
Mouse inhalation 3000 Kel'man, 1965
Rat oral ca.800 Wilson and DeEds, 1959
Rat inhalation 3150 Kel'man, 1965
Chicken oral 8000-10000 Maclay, 1954
Short-term studies
Chicken
Six groups of 70 four-day old cockerels were fed 0, 7.5, 15, 30, 75 or
750 ppm ethoxyquin in the diet for 12 weeks. No adverse effects were
observed on body-weight, food-consumption, mortality, gross-, or
histopathology (Maclay, 1954).
No adverse effects were observed in cockerels fed diets containing
7.5, 15, 30, 75 or 750 ppm ethoxyquin for 12 weeks (Colorado A. and
Experimental Station, ca. 1954).
Nor were any adverse effects observed in chicks fed diets containing
75, 750 or 1500 ppm for eight weeks (Halloran, 1952).
Chicks were fed 0.25 percent of ethoxyquin in their diet for six
weeks. The concentration of ethoxyquin in the livers was significantly
higher when the dietary level of protein was 17 percent than when the
level was 23 percent. (March et al., 1968).
Dog
Mixed groups, each of three dogs, were given oral doses by capsule
five times weekly for one year at dose-levels of 0, 3, 10, 50 or 100
mg/kg body-weight. Feeding the 100 mg/kg group was terminated after
sit weeks due to toxic effects, and the dogs were sacrificed at nine
weeks. One female animal at 3 mg/kg developed histoplasmosis about 40
weeks after initiation of the study, resulting in abnormal findings
when compared to the control dogs. All such finding could be
attributed to the infection. In the remaining animals body-weight was
depressed at 100 mg/kg; bromosulphthalein retention was increased at
10 mg/kg and above, indicating liver dysfunction; abdominal tenderness
was apparent in two of three dogs (male) at 10 mg/kg, and in two of
three dogs (one male and one female) at 50 mg/kg; erythrocyte
sedimentation rate was increased in one of three dogs at 50 mg/kg, and
in all three dogs at 100 mg/kg; haemograms showed reduced haematocrit,
haemoglobin, and erythrocyte counts in two of three dogs at 50 mg/kg,
and in all dogs at 100 mg/kg; urin-analysis indicated dark amber,
green or blackish brown urine at 50 and 100 mg/kg; there was increased
heart, liver, and kidney to body-weight ratios in all dose-levels, but
there does not appear to be any dose relationship in these increases;
histopathology indicated liver-stress, and fatty renal nephrosis at
the 10, 50 and 100 mg/kg dose-levels (Hanzal, 1955).
Rabbit
Undiluted ethoxyquin emulsion (70 percent) was applied to the clipped
intact skin of three rabbits, and removed after 24 hours. Slight
erythema occurred after 24 hours in all animals with barely
perceptible redness remaining after 48 hours in only one animal. The
compound was classed as a mild skin-irritant under the conditions
described (Kelly 1960).
Rat
Three groups of 10 male and 10 female rats which were fed 0, 0.2, and
0.4 percent ethoxyquin in their diet for 200 days displayed an initial
depression in growth rate (statistically significant only at the 0.4
level) in both test groups. The weight loss was not recovered during
the course of the experiment. Haemograms in all groups were
comparable. At autopsy, liver and kidney weights were elevated in both
sexes in both test groups. Histological examination showed
pyelonephritis in the males fed 0.2 and 0.4 percent, and doubtfully at
0.4 percent in the females. Thyroid hyperplasia was noted in males at
0.4 percent (Wilson and DeEds, 1959).
Groups of 10 male rats were fed 0, 62, 125, 250, 500, 1000 or 2000 ppm
ethoxyquin in the diet. Similarly, groups of 10 females were fed 0,
500, 1000 or 2000 ppm, and groups of five females 125 or 250 ppm. Half
the animals in some of the groups of 10 were autopsied after 200 days
on the diet. Body-weight gain was depressed in both sexes at 2000 ppm.
At autopsy, kidney to body-weight ratio was elevated in the males at
250 ppm and above, and in the females at 2000 ppm. Liver to
body-weight ratios were elevated at 1000 and 2000 ppm in males, and at
2000 ppm in females, Wilson and DeEds, 1959). Histopathological
examination showed kidney lesions at 500 ppm and above in the males,
and at 2000 ppm in the females; inclusions were observed in liver cell
cytoplasm in males at 2000 ppm, and in females at 2000 ppm (Cox,
1953).
Long-term studies
Dog
Ethoxyquin was fed to two groups of 14 dogs of mixed sex, at dietary
levels of 0 or 300 ppm for five years. No effects were observed on
haematology, urinalysis, clinical chemistry (aerum glutamic-oxalic
transaminase, blood urea nitrogen and bromosulphthalein retention),
organ weights or organ to body-weight ration, body-weight and
gross- or histopathology (Monsanto, 1966).
Rat
Groups, each of approximately 10 male and 10 female rats, were fed
levels of 0, 62, 125, 250, 500, 1000, 2000 and 4000 ppm of ethoxyquin
in their diets for periods of up to two years. The animals were
sacrificed for autopsy after 200, 400, 600 or 715 days. Mortality
rates at all dose levels were not significantly different from the
controls. Reduced body-weight gain was significant at 2000 ppm after
225 days in the male animals and after 21 days in the females. After
200 days increased liver to body-weight and increased kidney to
body-weight ratios were found at 250 ppm in the males and at 1000 ppm
in the females. Haemoglobin values for both sexes were normal 100 and
300 days after the start of the experiment in those rats fed 2000 and
4000 ppm. Histological changes in the renal cortex were clearly
evident after 200 days in the male rats receiving 2000 and 4000 ppm,
but not in the females. All other organs were normal in both sexes
after 200 days. After 400 days lesions in the kidneys
(pyelonephritis), liver and thyroid were clearly evident in the males
only. Similar lesions were evident for periods up to 717 days in both
sexes, although more marked in the males, and occasional tumours were
evident after 700 days but were unrelated to the dose-level, and were
also evident in the controls. No clearly defined effects were evident
from feeding 62 ppm, but minute lesions were present in the kidneys of
two of the males receiving the 500 ppm diet. It appeared difficult to
distinguish the abnormalities in the group examined after 700 days
with pathological manifestations associated with senility after that
time (Wilson and DeEds, 1959).
OBSERVATION IN MAN
In 20-years experience in the production of ethoxyquin there was no
indication of any cases of skin irritation or sensitivity (Kelly,
1960). However, cases of apparent sensitivity to ethoxyquin have been
reported in fruit handlers. Multiple cases of dermatitis have occurred
among employees handling freshly sprayed apples moist with solutions
of 70 percent formulated ethoxyquin. Patch-testing in volunteers
indicated that these skin reactions were not due to direct irritation,
but were the result of sensitization (Wood, 1965).
COMMENT
Studies in the rat and the dog indicate that toxic effects upon the
liver and kidney occur when dietary levels of 250 ppm and above are
given to the rat and 10 mg/kg or higher daily doses are given to dogs.
Administration of 300 ppm in the diet, however, was well tolerated by
dogs over a five-year period. There was an increase in the number of
stillbirths and a decrease in the litter size and in the incidence of
survival-to-weaning in rats fed 1125 ppm but not in those fed 125 ppm.
Lower doses (ca. 50 ppm) are rapidly excreted in the rat, dog and
chicken. Ethoxyquin is apparently converted to water soluble
metabolites and as long at these conversion mechanisms are functioning
there appears little likelihood of storage of the compound in the body
fat.
The occurrence of dermatitis among fruit packers suggests that
sensitization could be a problem among those who are handling
ethoxyquin.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Dog: 300 ppm in diet, equivalent to 7.5 mg/kg body-weight/day
Rat: 125 ppm in diet, equivalent to 6.25 mg/kg body-weight/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.06 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
Ethoxyquin is used as a pre-harvest treatment for the control of scald
of susceptible apple varieties. It is applied once as a full cover
spray within two days of harvest at a dosage rate of 3 pints of a
commercial formulation containing 70 percent active ingredient per 100
gallons of water (2700 ppm active ingredient).
Post-harvest treatments
For a post-harvest treatment of apples and pears, ethoxyquin is used
as a 15-30 sec. dip or a 15 sec. spray prior to or as the fruit passes
over the grading line. The dosage rate used is 2 to 3 pints of a
commercial formulation containing 70 percent active ingredient per 100
gallons of water (1800-2700 ppm active ingredient). The fruit is
treated no later than one week after harvest.
The above information on use patterns was obtained from product labels
from Canada, United States, United Kingdom and Italy.
Other uses
Ethoxyquin is also used as an antioxidant in spices, fish meal,
poultry feeds, swine feeds and other animal feeds.
Other information pertinent to use pattern
This monograph is confined to information pertaining to only the
pesticidal uses of ethoxyquin on apples and pears.
Ethoxyquin manufactured in the United States is sold in the following
countries: Sweden, Norway, Denmark, United Kingdom, Netherlands,
Belgium-Luxembourg, France, W. Germany, Austria, Switzerland, Finland,
Spain, Italy, Rep. of South Africa, Mexico, Colombia, Venezuela, Peru,
Chile, Brazil, Philippines, Australia, New Zealand, United States and
Canada (Monsanto - Direct communication, 1969).
Information concerning uses in countries other than Canada, United
States, United Kingdom and Italy, such as Israel, Japan, West Germany
and Sweden, where it was understood that ethoxyquin was manufactured,
was not available to the Joint Meeting.
RESIDUES RESULTING FROM SUPERVISED TRIALS
The results reported in Tables 1, 2, 3 for apples and 6 and 7 for
pears are from supervised trials made in various geographical
locations in the United States (Monsanto, 1961). Those in Tables 4 and
5 for apples, are from supervised trials carried out in New Zealand
(Padfield et al., 1963).
Summary of residue data
The residues of ethoxyquin on apples and pears when applied as a dip
or spray on a whole-fruit basis did not exceed 1.2 ppm from U.S.A.
data. However, from New Zealand, levels up to 2.8 ppm on apples have
been reported and one variety, Dougherty, which appears to have the
ability to retain fairly high levels of residue, was reported to have
levels up to 4.4 ppm. Results obtained in New Zealand also indicate
that use of a warm dip may result in residues of ethoxyquin on apples
as high as 6 ppm. Experimental results suggest that the uptake of
ethoxyquin is likely to increase as apple fruit matures and cooled
fruit appears to show lower residues than fruit treated at normal air
temperature. The results of trials carried out in the United States
indicate that residues found as a result of spraying fruit on the tree
are generally less than when apples or pears are dipped post-harvest.
FATE OF RESIDUES
Ethoxyquin, instead of existing as a single molecule, changes
spontaneously into a complex resulting from oxidation. These complex
structures are still effective biologically. No major degradation
change occurs in the ethoxyquin molecule. The structure of the
oxidation product has not been definitely determined but the
structures of the first reaction product(s) which fit the observations
most closely are suggested to be:
Other relevant chemical properties
The technical material as manufactured in the USA (Santoquin) is
claimed to contain 100 percent active ingredients, but is not 100
percent ethoxyquin. E.g. "Stop Scald" is a 70 percent emulsion of
Santoquin in water with added emulsifiers. It is a dark liquid varying
in color from yellow to black. The color is claimed to be independent
of the antioxidant or biological activity of the chemical.
Boiling Point: 125°C at 1-2 mm Hg.
Specific gravity: 1.028 to 1.032
Solubility: Miscible with animal and vegetable fats and oils.
Insoluble in water.
Viscosity at: 32°F 20,000 centipoises
73 197 "
122 17 "
158 7 "
(Monsanto, 1958).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Studies in chickens resulted in a 99 percent recovery of a single dose
of C14-labelled ethoxyquin within 48 hours. Continuous administration
of 125-137 ppm of ethoxyquin in the diet resulted in accumulation of
approximately 0.1 ppm of ethoxyquin and/or its metabolites in the
liver and fat per week during the first 12 weeks. Accumulation in
muscle and other edible tissue was barely detectable. Withdrawal of
ethoxyquin from the diet resulted in a loss of 79-90 percent of the
tissue residue in a 6 to 18 hour period. The excretion products
comprised 15 percent unchanged ethoxyquin, the remainder probably
being N-glucuronide and N-acetyl derivatives (Monsanto, 1956).
When ethoxiquin was given in the diet of rats for 10 days at a
concentration of 50 ppm, accumulation in the liver (2.1 - 4.8 ppm) and
kidney (2.1 - 2.7 ppm) was observed. Concentrations in fat and
skeletal muscle were less than 1 ppm (Wilson, 1956a; Wilson et al.,
1959).
Rats preconditioned for several weeks on a diet containing 50 ppm of
unlabelled ethoxyquin were given a single oral dose of 1.5 mg of
ethoxyquin C14-labelled in the 2 and 4 positions of the heterocyclic
ring. In two days 30 percent of the radioactivity was excreted in
urine, 34 percent in faeces. In four days and seven days 40-60 percent
and 58 percent was excreted in respectively, and 30-40 percent and 36
percent was excreted in faeces respectively. C14-carbon dioxide in
respired air was detected on the first day only, and comprised 0.7
percent of the administered dose (Wilson, 1956a Wilson et al., 1959).
In rats, repeated administration of ethoxyquin results in residues in
the kidney as well as in the fat and liver. A greater degree of
metabolic breakdown may occur than in chicken, because about 1 percent
of the C14 administered is exhaled as C14-carbon dioxide in rats as
compared with 0.2 percent in chickens (Monsanto, 1956).
Pregnant rats treated as above, and administered the labellet
ethoxyquin nine days prior to parturition indicated that placental
transfer of ethoxyquin occurs, because newborn young contained 0.12 to
0.21 ppm ethoxyquin in their tissues. Milk samples from two female
rats fed a diet of 50 ppm of ethoxyquin for 10 days had residues in
milk of 0.12 ppm and 0.19 ppm (Wilson, 1956a; Wilson et al., 1959).
Metabolic studies in dog indicated that ethoxyquin, per se, is not
excreted in the urine to any appreciable extent, but is excreted as
four unidentified metabolites (probably glucoronates). There was no
evidence of the ethoxy-group being split from the molecule during
metabolism. Results demonstrated that elimination is chiefly by the
kidneys and only to a small degree by way of the faeces (Hanzal,
1955).
TOXICOLOGICAL STUDIES
Special studies on reproduction
Rat
After 40 days on a slightly tocopherol-deficient diet containing 0,
250, 500 or 1000 ppm ethoxyquin, rats were bred to produce three
consecutive litters. The first litter offspring were utilized to
produce a second generation litter. The top dose level was discarded
after production of one litter. No effects on reproduction, as
reflected by fertility, litter size, or survival of offspring were
observed. The animals receiving the experimental diet produced young
and raised them more successfully than the controls. The 500 ppm diet
was more effective than the 250 ppm (Wilson, 1956b; Wilson and DeEds,
1959).
Groups, each of from five to nine rats, one to ten days pregnant, were
fed 0, 125, 375 or 1125 ppm ethoxyquin in the diet. Litter size,
stillbirths, survival to weanling, and weanling weights were all
comparable to the control rats (Derse, 1956).
Groups, each of eight or nine female rats, were placed on diets
containing 0, 125, 375 or 1125 ppm ethoxyquin on the day of mating.
Gestation time was comparable in all groups. However, litter size was
slightly depressed at the 375 ppm level and above, and at 1125 ppm
incidence of stillbirths was increased, and survival to weaning was
decreased (Derse, 1956).
Acute toxicity
LD50
Animal Route mg/kg body-weight References
Mouse i.p. 800-1000 Wilson and DeEds, 1959
Mouse i.v. 178 Wilson and DeEds, 1959
Mouse inhalation 3000 Kel'man, 1965
Rat oral ca.800 Wilson and DeEds, 1959
Rat inhalation 3150 Kel'man, 1965
Chicken oral 8000-10000 Maclay, 1954
Short-term studies
Chicken
Six groups of 70 four-day old cockerels were fed 0, 7.5, 15, 30, 75 or
750 ppm ethoxyquin in the diet for 12 weeks. No adverse effects were
observed on body-weight, food-consumption, mortality, gross-, or
histopathology (Maclay, 1954).
No adverse effects were observed in cockerels fed diets containing
7.5, 15, 30, 75 or 750 ppm ethoxyquin for 12 weeks (Colorado A. and
Experimental Station, ca. 1954).
Nor were any adverse effects observed in chicks fed diets containing
75, 750 or 1500 ppm for eight weeks (Halloran, 1952).
Chicks were fed 0.25 percent of ethoxyquin in their diet for six
weeks. The concentration of ethoxyquin in the livers was significantly
higher when the dietary level of protein was 17 percent than when the
level was 23 percent. (March et al., 1968).
Dog
Mixed groups, each of three dogs, were given oral doses by capsule
five times weekly for one year at dose-levels of 0, 3, 10, 50 or 100
mg/kg body-weight. Feeding the 100 mg/kg group was terminated after
sit weeks due to toxic effects, and the dogs were sacrificed at nine
weeks. One female animal at 3 mg/kg developed histoplasmosis about 40
weeks after initiation of the study, resulting in abnormal findings
when compared to the control dogs. All such finding could be
attributed to the infection. In the remaining animals body-weight was
depressed at 100 mg/kg; bromosulphthalein retention was increased at
10 mg/kg and above, indicating liver dysfunction; abdominal tenderness
was apparent in two of three dogs (male) at 10 mg/kg, and in two of
three dogs (one male and one female) at 50 mg/kg; erythrocyte
sedimentation rate was increased in one of three dogs at 50 mg/kg, and
in all three dogs at 100 mg/kg; haemograms showed reduced haematocrit,
haemoglobin, and erythrocyte counts in two of three dogs at 50 mg/kg,
and in all dogs at 100 mg/kg; urin-analysis indicated dark amber,
green or blackish brown urine at 50 and 100 mg/kg; there was increased
heart, liver, and kidney to body-weight ratios in all dose-levels, but
there does not appear to be any dose relationship in these increases;
histopathology indicated liver-stress, and fatty renal nephrosis at
the 10, 50 and 100 mg/kg dose-levels (Hanzal, 1955).
Rabbit
Undiluted ethoxyquin emulsion (70 percent) was applied to the clipped
intact skin of three rabbits, and removed after 24 hours. Slight
erythema occurred after 24 hours in all animals with barely
perceptible redness remaining after 48 hours in only one animal. The
compound was classed as a mild skin-irritant under the conditions
described (Kelly 1960).
Rat
Three groups of 10 male and 10 female rats which were fed 0, 0.2, and
0.4 percent ethoxyquin in their diet for 200 days displayed an initial
depression in growth rate (statistically significant only at the 0.4
level) in both test groups. The weight loss was not recovered during
the course of the experiment. Haemograms in all groups were
comparable. At autopsy, liver and kidney weights were elevated in both
sexes in both test groups. Histological examination showed
pyelonephritis in the males fed 0.2 and 0.4 percent, and doubtfully at
0.4 percent in the females. Thyroid hyperplasia was noted in males at
0.4 percent (Wilson and DeEds, 1959).
Groups of 10 male rats were fed 0, 62, 125, 250, 500, 1000 or 2000 ppm
ethoxyquin in the diet. Similarly, groups of 10 females were fed 0,
500, 1000 or 2000 ppm, and groups of five females 125 or 250 ppm. Half
the animals in some of the groups of 10 were autopsied after 200 days
on the diet. Body-weight gain was depressed in both sexes at 2000 ppm.
At autopsy, kidney to body-weight ratio was elevated in the males at
250 ppm and above, and in the females at 2000 ppm. Liver to
body-weight ratios were elevated at 1000 and 2000 ppm in males, and at
2000 ppm in females, Wilson and DeEds, 1959). Histopathological
examination showed kidney lesions at 500 ppm and above in the males,
and at 2000 ppm in the females; inclusions were observed in liver cell
cytoplasm in males at 2000 ppm, and in females at 2000 ppm (Cox,
1953).
Long-term studies
Dog
Ethoxyquin was fed to two groups of 14 dogs of mixed sex, at dietary
levels of 0 or 300 ppm for five years. No effects were observed on
haematology, urinalysis, clinical chemistry (aerum glutamic-oxalic
transaminase, blood urea nitrogen and bromosulphthalein retention),
organ weights or organ to body-weight ration, body-weight and
gross- or histopathology (Monsanto, 1966).
Rat
Groups, each of approximately 10 male and 10 female rats, were fed
levels of 0, 62, 125, 250, 500, 1000, 2000 and 4000 ppm of ethoxyquin
in their diets for periods of up to two years. The animals were
sacrificed for autopsy after 200, 400, 600 or 715 days. Mortality
rates at all dose levels were not significantly different from the
controls. Reduced body-weight gain was significant at 2000 ppm after
225 days in the male animals and after 21 days in the females. After
200 days increased liver to body-weight and increased kidney to
body-weight ratios were found at 250 ppm in the males and at 1000 ppm
in the females. Haemoglobin values for both sexes were normal 100 and
300 days after the start of the experiment in those rats fed 2000 and
4000 ppm. Histological changes in the renal cortex were clearly
evident after 200 days in the male rats receiving 2000 and 4000 ppm,
but not in the females. All other organs were normal in both sexes
after 200 days. After 400 days lesions in the kidneys
(pyelonephritis), liver and thyroid were clearly evident in the males
only. Similar lesions were evident for periods up to 717 days in both
sexes, although more marked in the males, and occasional tumours were
evident after 700 days but were unrelated to the dose-level, and were
also evident in the controls. No clearly defined effects were evident
from feeding 62 ppm, but minute lesions were present in the kidneys of
two of the males receiving the 500 ppm diet. It appeared difficult to
distinguish the abnormalities in the group examined after 700 days
with pathological manifestations associated with senility after that
time (Wilson and DeEds, 1959).
OBSERVATION IN MAN
In 20-years experience in the production of ethoxyquin there was no
indication of any cases of skin irritation or sensitivity (Kelly,
1960). However, cases of apparent sensitivity to ethoxyquin have been
reported in fruit handlers. Multiple cases of dermatitis have occurred
among employees handling freshly sprayed apples moist with solutions
of 70 percent formulated ethoxyquin. Patch-testing in volunteers
indicated that these skin reactions were not due to direct irritation,
but were the result of sensitization (Wood, 1965).
COMMENT
Studies in the rat and the dog indicate that toxic effects upon the
liver and kidney occur when dietary levels of 250 ppm and above are
given to the rat and 10 mg/kg or higher daily doses are given to dogs.
Administration of 300 ppm in the diet, however, was well tolerated by
dogs over a five-year period. There was an increase in the number of
stillbirths and a decrease in the litter size and in the incidence of
survival-to-weaning in rats fed 1125 ppm but not in those fed 125 ppm.
Lower doses (ca. 50 ppm) are rapidly excreted in the rat, dog and
chicken. Ethoxyquin is apparently converted to water soluble
metabolites and as long at these conversion mechanisms are functioning
there appears little likelihood of storage of the compound in the body
fat.
The occurrence of dermatitis among fruit packers suggests that
sensitization could be a problem among those who are handling
ethoxyquin.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Dog: 300 ppm in diet, equivalent to 7.5 mg/kg body-weight/day
Rat: 125 ppm in diet, equivalent to 6.25 mg/kg body-weight/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.06 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
Ethoxyquin is used as a pre-harvest treatment for the control of scald
of susceptible apple varieties. It is applied once as a full cover
spray within two days of harvest at a dosage rate of 3 pints of a
commercial formulation containing 70 percent active ingredient per 100
gallons of water (2700 ppm active ingredient).
Post-harvest treatments
For a post-harvest treatment of apples and pears, ethoxyquin is used
as a 15-30 sec. dip or a 15 sec. spray prior to or as the fruit passes
over the grading line. The dosage rate used is 2 to 3 pints of a
commercial formulation containing 70 percent active ingredient per 100
gallons of water (1800-2700 ppm active ingredient). The fruit is
treated no later than one week after harvest.
The above information on use patterns was obtained from product labels
from Canada, United States, United Kingdom and Italy.
Other uses
Ethoxyquin is also used as an antioxidant in spices, fish meal,
poultry feeds, swine feeds and other animal feeds.
Other information pertinent to use pattern
This monograph is confined to information pertaining to only the
pesticidal uses of ethoxyquin on apples and pears.
Ethoxyquin manufactured in the United States is sold in the following
countries: Sweden, Norway, Denmark, United Kingdom, Netherlands,
Belgium-Luxembourg, France, W. Germany, Austria, Switzerland, Finland,
Spain, Italy, Rep. of South Africa, Mexico, Colombia, Venezuela, Peru,
Chile, Brazil, Philippines, Australia, New Zealand, United States and
Canada (Monsanto - Direct communication, 1969).
Information concerning uses in countries other than Canada, United
States, United Kingdom and Italy, such as Israel, Japan, West Germany
and Sweden, where it was understood that ethoxyquin was manufactured,
was not available to the Joint Meeting.
RESIDUES RESULTING FROM SUPERVISED TRIALS
The results reported in Tables 1, 2, 3 for apples and 6 and 7 for
pears are from supervised trials made in various geographical
locations in the United States (Monsanto, 1961). Those in Tables 4 and
5 for apples, are from supervised trials carried out in New Zealand
(Padfield et al., 1963).
Summary of residue data
The residues of ethoxyquin on apples and pears when applied as a dip
or spray on a whole-fruit basis did not exceed 1.2 ppm from U.S.A.
data. However, from New Zealand, levels up to 2.8 ppm on apples have
been reported and one variety, Dougherty, which appears to have the
ability to retain fairly high levels of residue, was reported to have
levels up to 4.4 ppm. Results obtained in New Zealand also indicate
that use of a warm dip may result in residues of ethoxyquin on apples
as high as 6 ppm. Experimental results suggest that the uptake of
ethoxyquin is likely to increase as apple fruit matures and cooled
fruit appears to show lower residues than fruit treated at normal air
temperature. The results of trials carried out in the United States
indicate that residues found as a result of spraying fruit on the tree
are generally less than when apples or pears are dipped post-harvest.
FATE OF RESIDUES
Ethoxyquin, instead of existing as a single molecule, changes
spontaneously into a complex resulting from oxidation. These complex
structures are still effective biologically. No major degradation
change occurs in the ethoxyquin molecule. The structure of the
oxidation product has not been definitely determined but the
structures of the first reaction product(s) which fit the observations
most closely are suggested to be:
 TABLE 1
Pre-harvest treatments - applied as a fruit and foliage spray just prior to picking of apples.
Interval between
Treatment Rate Apple Treatment and Residue (ppm)
(ppm ethoxyquin) Variety Sampling (days) Fresh Apples Stored Apples
900 Paragon 13 - 14 0 - 0.2
Winesap
1800 " 13 - 14 0.03 - 0.35
1500 McIntosh 23 - 24 0 - 0.01
44 0 - 0.11
3000 McIntosh 23 0 0 - 0.16
2000 Wealthy 53 0.01 - 0.10
0 Greening 53 0.00 - 0.11
2000 " 22 0.00 - 0.13
53 0.01 - 0.11
2000 McIntosh 20 0.00 - 0.01
51 0.00
TABLE 2
Post-harvest treatments - using a 15 second dip or applied as a spray.
Interval between
Treatment Rate Apple Treatment and Residue (ppm)
(ppm ethoxyquin) Variety Sampling (days) Fresh Apples Stored Apples
1000 Red
Delicious 2 0.07, 0.42
31 0.13, 0.07
2000 " 3 0.20, 0.34
32 0.14, 1.17
3000 " 3 0.48, 0.34
32 0.53, 1.00
1000 Jonathon 4 0.03, 0.18
32 0.11
2000 " 4 0.62, 0.43
" 33 0.04, 0.04
3000 " 4 0.44, 0.81
" 33 0.09 0.04
2000 Wealthy 23 0.00 - 0.62
" 55 0.00 - 0.20
3000 " 23 0.01 - 0.81
" 55 0.04 - 0.14
0 Greening 50 0.00 - 0.11
2000 " 20 0.03 - 0.13
" 50 0.00
2000 McIntosh 19 0.00
49 0.00
TABLE 3
Post-harvest treatments - using a 15 second dip.
Treatment Rate Apple Interval Residues (ppm)
(ppm ethoxyquin) Variety (days)* Peel Pulp Whole Apple
0 Jonathon 1 0.2 - 0.3 0.03 - 0.04
1000 " 1 3.6 - 4.1 0.47 - 0.54
2000 " 1 4.5 - 4.7 0.61 - 0.64
3000 " 1 5.4 - 5.7 0.67 - 0.70
4000 " 1 8.0 - 7.5 0.94 - 1.00
0 McIntosh 7 0.07, 0.11 0.08, 0.11 0.08, 0.11
3600 " 7 1.45, 1.45 0.14, 0.14 0.29, 0.30
0 Cortland 7 0.07, 0.16 0.07, 0.09 0.08, 0.08
2700 " 7 1.75, 1.80 0.07, 0.11 0.30, 0.32
* Interval between treatment and sampling
TABLE 4
Residues on apples dipped or sprayed with 1800 ppm ethoxyquin.
The samples of fruit for analysis were withdrawn from the
line during normal operation and residue determinations were
made as soon as possible after treatment.
Residue (ppm)
Apple First Pick Second Pick
Variety Treatment March-April March-May
Delicious Cool dip 1.4 1.2
Warm dip 1.5 1.9
Spray 1.0 0.8
Granny Smith Cool dip 1.1 2.8
Warm dip 1.6 4.8
Spray 1.5 0.7
Rome Beauty Cool dip 0.7 1.4
Warm dip 2.4 1.8
Spray 0.9 1.3
Sturmer Cool dip 0.5 1.6
Warm dip 1.8 2.7
Spray 1.8 2.3
Dougherty Cool dip 2.6 4.4
Warm dip 1.9 6.0
Spray 2.2 3.0
TABLE 5
Ethoxyquin residues on apples after treatment in commercial spay tunnels.
Residue analyses were made as soon as possible after treatment.
Treatment Rate Apple Fruit
(ppm ethoxyquin) Variety Temperature Residue (ppm)
1800 Sturmer Air temp. 1.6
Cooled 38°F 0.7
1800 Granny Smith Air temp. 0.7 - 1.7
Cooled 33°F 0.5 - 1.0
2700 Air temp. 2.2
Cooled 33°f 1.5
TABLE 6
Pre-harvest treatments - applied an a fruit and foliage spray just
prior to picking of pears.
Interval between
Treatment Rate Pear Treatment and Residues (ppm)
(ppm ethoxyquin) Variety Sampling (days) Fresh Fruit Stored Fruit
1000 Anjou 23 - 25 0.01
70 - 71 0.00
2000 " 23 - 25 0.01 - 0.04
70 - 71 0.00
TABLE 7
Post-harvest treatments using a 15 second dip.
Residues (ppm)
Treatment Rate Pear Interval Whole Stored
(ppm ethoxyquin) Variety (days)* Pulp Peel Fruit Fruit
900 Anjou 53 0.00
1800 " 53 0.00
1000 " 53 0.03
1500 " 53 0.03
2000 " 53 0.01
2700 " 7 0.04 - 0.05 4.7 - 4.9 0.66
3600 " 7 0.04 - 0.09 7.6 - 8.3 1.09
5400 " 7 0.04 - 0.08 7.9 - 9.2 1.07
* Interval between treatment and sampling.
Evidence of residues in food in commerce or at consumption
No information available.
METHODS OF RESIDUE ANALYSIS
Methods using ultraviolet spectrophotometry of spectrofluorometry have
been proposed for the determination of residues of ethoxyquin in food,
feed and animal tissue. The spectrophotometric methods of Choy et al.
(1963) and Alicino et al. (1963) both include clean-up stages to
separate ethoxyquin from other anti-oxidants such as butylated
hydroxyanisole and butylated hydroxytoluene before estimation of
absorbance at 296 nm. A spectrofluorometric procedure for ethoxyquin
in feeding stuffs (Gordon et al., 1964) was adapted for use on samples
of eggs, chicken muscle and liver (Van Deren and Jaworski, 1966). It
is relatively non-specific for ethoxyquin and residues of other
pesticides or of other naturally occurring compounds may interfere.
Collaborative study of this method (Van Deren and Jaworski, 1967) led
to its adoption as an official, first action procedure (Anon, 1968).
The ethoxyquin is extracted with iso-octane, cleaned-up by an
acid-alkaline extraction procedure and determined by examining the
fluorescence of an iso-octane solution. The suitability of this method
for application to fruit for regulatory purposes needs further
evaluation.
NATIONAL TOLERANCES
Tolerances for Fruit
Canada - 3 ppm on apples
U.K. - 3 ppm on apples
U.S.A. - 3 ppm on apples and pears
(for ethoxyquin and other naturally occurring
fluorescent materials).
New Zealand - 3 ppm on apples
Tolerances derived from use as additives in animal feeds
Canada - 3 ppm in livers of poultry
0.5 ppm in meat, poultry meat and eggs
U.S.A. - 3 ppm in poultry livers and fat
0.5 ppm in eggs and meat
APPRAISAL
In a review of the literature very few articles were found on
ethoxyquin other than those on its efficacy in controlling scald. The
abstracting journals reviewed were, Review of Applied Mycology,
Biological Abstracts, Chemical Abstracts and Pesticide Documentation
Bulletin.
Data available are based on ethoxyquin as manufactured in the U.S.A.
and used in several other countries. Ethoxyquin is also manufactured
in Israel, Japan, West Germany and Sweden. The amount and nature of
minor constituents and amount of ethoxyquin monomer in products other
than U.S.A. manufacture in unknown. It is used for the control of
scald in apples (and to a lesser extent pears), as both pre-harvest
treatments and post-harvest dips and sprays.
The residues of ethoxyquin on apples and pears when applied as a dip
or spray on a whole-fruit basis did not exceed 1.2 ppm from U.S.A.
data. However, from New Zealand, levels up to 2.8 ppm on apples have
been reported and one variety, Dougherty, which appears to have the
ability to retain fairly high residue, was reported to have levels up
to 4.4 ppm. Results obtained in New Zealand also indicate that use of
a warm dip may result in residues of ethoxyquin on apples as high as 6
ppm. Experimental results suggest that the uptake of ethoxyquin is
likely to increase as apple fruit matures. Cooled fruit appears to
have lower residues than fruit treated at normal air temperature. The
results of trials carried out in the U.S.A. indicate that residues
found as a result of spraying fruit on the tree are generally less
than when apples or pears are dipped post-harvest.
Ethoxyquin, instead of existing as a single molecule, changes
spontaneously into a complex resulting from oxidation. These complex
structures are still effective biologically. No major degradation
change occurs in the ethoxyquin molecule. The structure of the
oxidation product has not been definitely determined, but the
structures of the first reaction product(s) which fit the observations
most closely are given in the monograph.
The fluorometric method is relatively non-specific for ethoxyquin on
apples and pears. Many naturally occurring fluorescent materials may
interfere. It is also possible that residues of other pesticides used
on apples may fluoresce or be converted into fluorescent compounds.
More suitable spectrofluometric methods available for animal products
should be adopted and evaluated.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES OR PRACTICAL
RESIDUE LIMITS
TOLERANCES
Apples and pears 3.0 ppm
DESIRABLE
1. Additional reproduction studies to investigate the effect on
survival rate of offspring of rats.
2. A method of analysis for apples and pears suitable for regulatory
purposes.
3. Composition and purity of this compound as manufactured in several
countries and the amount of ethoxyquinmonomer in the products from
various manufacturers.
REFERENCES
Alicino, N.J., Klein, H.C., Quattrone, J.J. and Choy, T.K. (1963)
Determination of butylated hydroxyanisole, butylated, hydroxytoluene,
and ethoxyquin in hydrocarbon-soluble samples. J. Agr. Fd Chem., 11:
496-8
Anon (1968) Changes in Official Method of Analysis. J. Assoc. Offic.
Anal. Chem., 51:453-4
Choy, T., Alicino, N.J., Klein, H.C. and Quattrone, J.J. (1963)
Determination of ethoxyquin by ultraviolet spectrophotometry. J. Agri.
Fd Chem., 11:340-2
Choy, T., Alicino, N.J., Klein, H.C. and Quattrone, J.J. (1963)
Determination of ethoxyquin by ultraviolet spectrophotometry. J.Agri.
Fd Chem., 11:340-2
Colorado, A. and M. Experimental Station. (1954) The chronic toxicity
of the antioxidant ca. 6-ethoxy, 2,2,4-trimethyl, 1,2-dihydroquinoline
(Santoquin, Monsanto). Unpublished, undated, report of studies
conducted during 1952-53. Submitted by Monsanto Chemical Company.
Cox, A.J. (1953) 6-ethoxy, 2,2,4-trimethyl-1,2-dihydroquinoline
(EMHQ). Histological report on sections of rat tissues stained with
haematoxylin-eosin. Unpublished report submitted by Monsanto Chemical
Company.
Derse, P. (1956) Assay report. Unpublished report of Wisconsin Alumni
Research Foundation submitted by Monsanto Chemical Company
Gordon, R.S. Conkin, R.A. and Machlin, L.J. (1964) Determination of
ethoxyquin in feeds. J. Assoc. Offic. Agric. Chem., 47:512-6
Halloran, H.R. (1952) Chick toxicity tests on Santoflex AW.
Unpublished report from the Poultry Products of Central California,
Petaluma, to California Department of Agriculture submitted by
Monsanto Chemical Company.
Hansal, R.F. (1955) Final report and addendum. Chronic oral
administration-dogs-metabolic studies. Unpublished report of Hazleton
Laboratories submitted by Monsanto Chemical Company
Kelly, R.G. (1960) Supplementary toxicity information on Santoquin
(6-ethoxy-2,2,4-trimethyl-1,2-dihydroquinoline). Unpublished report
prepared and submitted by Monsanto Chemical Company
Kel'man, G.Y. (1965) Comparative toxicity of Santoflex A
(6-ethoxy-2,2,4-trimethyl-1,2-dihydroquinoline) and acetoneanyl
(1,2-dihydro-2,2,4-trimethylquinoline). Keuchuk i Rezina 24:40-41
[(Chem. Abstr. 63:1136g (1965)]
Mackay, V.D. (1954) Toxicity data in support of the use of 0.015% of
6-ethoxy-2,2,4-trimethyl-1,2-dihydroquinoline on alfalfa meal for
carotene preservation. Unpublished report of Western Utilization
Research Branch, U.S.D.A., submitted by Monsanto Chemical Company
March, B.E., Biely, J. and Coates, V. (1968) The influence of diet on
toxicity of the anti-oxidant
1,2-dihydro-6-ethoxy-2,2,4-trimethylquinoline. Can. J. Physiol.
Pharmacol. 46:139-43. [Chem. Abstr. 68:102955m (1968)]
Monsanto (1956) Santoquin for use in Poultry Feeds, Unpublished report
prepared and submitted by Monsanto Chemical Company
Monsanto. (1956) Petition proposing the issuance of a regulation
permitting the use of Santoquin as an antioxidant for addition to
poultry feeds. Unpublished report. October
Monsanto. (1961) Information to support label registration request for
"Stop-Scald" (ethoxyquin) on apples and pears to control common scald
and petition for tolerance for ethoxyquin on pears. Unpublished
report.
Monsanto. (1966) Five year Santoquin feeding study in dogs.
Unpublished collection of reports prepared and submitted by Monsanto
Chemical Company
Monsanto. (1969) Direct communication
Padfield, C.A.S., Atkinson, J.D., Clark, P.J. (1963) Control of apple
scald by ethoxyquin. N.Z.J. Agr. Res. 6:245-52
Van Deren, J.M. and Jaworski, E.G. (1966) Ethoxyquin (Santonin (R) in
eggs, chicken muscle and chicken liver. J. Assoc. Offic. Anal. Chem.,
49:712-4
Van Deren, J.M. and Jaworski, E.G. (1967) Collaborative study of the
determination of ethoxyquin in chick tissue and eggs. J. Assoc. Offic.
Anal. Chem., 50:844-7
Wilson, R.H. (1956a) Distribution and excretion of oral Santoquin
C14, Unpublished reports of Western Utilization Research Branch,
U.S.D.A. submitted by Monsanto Chemical Company
Wilson, R.H. (1956b) Final report - reproduction in rats receiving
Santoquin. Unpublished report of Western Regional Utilization Research
Branch, U.S.D.A. submitted by Monsanto Chemical Company
Wilson, R.H. and DeEds, F. (1959) Toxicity studies on the antioxidant
6-ethoxy-1,2-dihydro-2,2,4-trimethylquinoline. J. Agr. Food Chem.
7:203-6
Wilson, R.H., Thomas, J.O., Thompson, C.R., Lanner, H.F. and Kohler,
G.O. (1959) Absorption, metabolism and excretion of the antioxidant,
6-ethoxy-1,2-dihydro-2,2,4-trimethylquinoline. J. Agr. Food Chem.,
7:206-9
Wood, W.S. (1965) Untitled report of dermatitis cases in fruit
handlers. Unpublished report from the Medical Dental Building,
Vancouver, B.C. submitted by Monsanto Chemical Company
TABLE 1
Pre-harvest treatments - applied as a fruit and foliage spray just prior to picking of apples.
Interval between
Treatment Rate Apple Treatment and Residue (ppm)
(ppm ethoxyquin) Variety Sampling (days) Fresh Apples Stored Apples
900 Paragon 13 - 14 0 - 0.2
Winesap
1800 " 13 - 14 0.03 - 0.35
1500 McIntosh 23 - 24 0 - 0.01
44 0 - 0.11
3000 McIntosh 23 0 0 - 0.16
2000 Wealthy 53 0.01 - 0.10
0 Greening 53 0.00 - 0.11
2000 " 22 0.00 - 0.13
53 0.01 - 0.11
2000 McIntosh 20 0.00 - 0.01
51 0.00
TABLE 2
Post-harvest treatments - using a 15 second dip or applied as a spray.
Interval between
Treatment Rate Apple Treatment and Residue (ppm)
(ppm ethoxyquin) Variety Sampling (days) Fresh Apples Stored Apples
1000 Red
Delicious 2 0.07, 0.42
31 0.13, 0.07
2000 " 3 0.20, 0.34
32 0.14, 1.17
3000 " 3 0.48, 0.34
32 0.53, 1.00
1000 Jonathon 4 0.03, 0.18
32 0.11
2000 " 4 0.62, 0.43
" 33 0.04, 0.04
3000 " 4 0.44, 0.81
" 33 0.09 0.04
2000 Wealthy 23 0.00 - 0.62
" 55 0.00 - 0.20
3000 " 23 0.01 - 0.81
" 55 0.04 - 0.14
0 Greening 50 0.00 - 0.11
2000 " 20 0.03 - 0.13
" 50 0.00
2000 McIntosh 19 0.00
49 0.00
TABLE 3
Post-harvest treatments - using a 15 second dip.
Treatment Rate Apple Interval Residues (ppm)
(ppm ethoxyquin) Variety (days)* Peel Pulp Whole Apple
0 Jonathon 1 0.2 - 0.3 0.03 - 0.04
1000 " 1 3.6 - 4.1 0.47 - 0.54
2000 " 1 4.5 - 4.7 0.61 - 0.64
3000 " 1 5.4 - 5.7 0.67 - 0.70
4000 " 1 8.0 - 7.5 0.94 - 1.00
0 McIntosh 7 0.07, 0.11 0.08, 0.11 0.08, 0.11
3600 " 7 1.45, 1.45 0.14, 0.14 0.29, 0.30
0 Cortland 7 0.07, 0.16 0.07, 0.09 0.08, 0.08
2700 " 7 1.75, 1.80 0.07, 0.11 0.30, 0.32
* Interval between treatment and sampling
TABLE 4
Residues on apples dipped or sprayed with 1800 ppm ethoxyquin.
The samples of fruit for analysis were withdrawn from the
line during normal operation and residue determinations were
made as soon as possible after treatment.
Residue (ppm)
Apple First Pick Second Pick
Variety Treatment March-April March-May
Delicious Cool dip 1.4 1.2
Warm dip 1.5 1.9
Spray 1.0 0.8
Granny Smith Cool dip 1.1 2.8
Warm dip 1.6 4.8
Spray 1.5 0.7
Rome Beauty Cool dip 0.7 1.4
Warm dip 2.4 1.8
Spray 0.9 1.3
Sturmer Cool dip 0.5 1.6
Warm dip 1.8 2.7
Spray 1.8 2.3
Dougherty Cool dip 2.6 4.4
Warm dip 1.9 6.0
Spray 2.2 3.0
TABLE 5
Ethoxyquin residues on apples after treatment in commercial spay tunnels.
Residue analyses were made as soon as possible after treatment.
Treatment Rate Apple Fruit
(ppm ethoxyquin) Variety Temperature Residue (ppm)
1800 Sturmer Air temp. 1.6
Cooled 38°F 0.7
1800 Granny Smith Air temp. 0.7 - 1.7
Cooled 33°F 0.5 - 1.0
2700 Air temp. 2.2
Cooled 33°f 1.5
TABLE 6
Pre-harvest treatments - applied an a fruit and foliage spray just
prior to picking of pears.
Interval between
Treatment Rate Pear Treatment and Residues (ppm)
(ppm ethoxyquin) Variety Sampling (days) Fresh Fruit Stored Fruit
1000 Anjou 23 - 25 0.01
70 - 71 0.00
2000 " 23 - 25 0.01 - 0.04
70 - 71 0.00
TABLE 7
Post-harvest treatments using a 15 second dip.
Residues (ppm)
Treatment Rate Pear Interval Whole Stored
(ppm ethoxyquin) Variety (days)* Pulp Peel Fruit Fruit
900 Anjou 53 0.00
1800 " 53 0.00
1000 " 53 0.03
1500 " 53 0.03
2000 " 53 0.01
2700 " 7 0.04 - 0.05 4.7 - 4.9 0.66
3600 " 7 0.04 - 0.09 7.6 - 8.3 1.09
5400 " 7 0.04 - 0.08 7.9 - 9.2 1.07
* Interval between treatment and sampling.
Evidence of residues in food in commerce or at consumption
No information available.
METHODS OF RESIDUE ANALYSIS
Methods using ultraviolet spectrophotometry of spectrofluorometry have
been proposed for the determination of residues of ethoxyquin in food,
feed and animal tissue. The spectrophotometric methods of Choy et al.
(1963) and Alicino et al. (1963) both include clean-up stages to
separate ethoxyquin from other anti-oxidants such as butylated
hydroxyanisole and butylated hydroxytoluene before estimation of
absorbance at 296 nm. A spectrofluorometric procedure for ethoxyquin
in feeding stuffs (Gordon et al., 1964) was adapted for use on samples
of eggs, chicken muscle and liver (Van Deren and Jaworski, 1966). It
is relatively non-specific for ethoxyquin and residues of other
pesticides or of other naturally occurring compounds may interfere.
Collaborative study of this method (Van Deren and Jaworski, 1967) led
to its adoption as an official, first action procedure (Anon, 1968).
The ethoxyquin is extracted with iso-octane, cleaned-up by an
acid-alkaline extraction procedure and determined by examining the
fluorescence of an iso-octane solution. The suitability of this method
for application to fruit for regulatory purposes needs further
evaluation.
NATIONAL TOLERANCES
Tolerances for Fruit
Canada - 3 ppm on apples
U.K. - 3 ppm on apples
U.S.A. - 3 ppm on apples and pears
(for ethoxyquin and other naturally occurring
fluorescent materials).
New Zealand - 3 ppm on apples
Tolerances derived from use as additives in animal feeds
Canada - 3 ppm in livers of poultry
0.5 ppm in meat, poultry meat and eggs
U.S.A. - 3 ppm in poultry livers and fat
0.5 ppm in eggs and meat
APPRAISAL
In a review of the literature very few articles were found on
ethoxyquin other than those on its efficacy in controlling scald. The
abstracting journals reviewed were, Review of Applied Mycology,
Biological Abstracts, Chemical Abstracts and Pesticide Documentation
Bulletin.
Data available are based on ethoxyquin as manufactured in the U.S.A.
and used in several other countries. Ethoxyquin is also manufactured
in Israel, Japan, West Germany and Sweden. The amount and nature of
minor constituents and amount of ethoxyquin monomer in products other
than U.S.A. manufacture in unknown. It is used for the control of
scald in apples (and to a lesser extent pears), as both pre-harvest
treatments and post-harvest dips and sprays.
The residues of ethoxyquin on apples and pears when applied as a dip
or spray on a whole-fruit basis did not exceed 1.2 ppm from U.S.A.
data. However, from New Zealand, levels up to 2.8 ppm on apples have
been reported and one variety, Dougherty, which appears to have the
ability to retain fairly high residue, was reported to have levels up
to 4.4 ppm. Results obtained in New Zealand also indicate that use of
a warm dip may result in residues of ethoxyquin on apples as high as 6
ppm. Experimental results suggest that the uptake of ethoxyquin is
likely to increase as apple fruit matures. Cooled fruit appears to
have lower residues than fruit treated at normal air temperature. The
results of trials carried out in the U.S.A. indicate that residues
found as a result of spraying fruit on the tree are generally less
than when apples or pears are dipped post-harvest.
Ethoxyquin, instead of existing as a single molecule, changes
spontaneously into a complex resulting from oxidation. These complex
structures are still effective biologically. No major degradation
change occurs in the ethoxyquin molecule. The structure of the
oxidation product has not been definitely determined, but the
structures of the first reaction product(s) which fit the observations
most closely are given in the monograph.
The fluorometric method is relatively non-specific for ethoxyquin on
apples and pears. Many naturally occurring fluorescent materials may
interfere. It is also possible that residues of other pesticides used
on apples may fluoresce or be converted into fluorescent compounds.
More suitable spectrofluometric methods available for animal products
should be adopted and evaluated.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES OR PRACTICAL
RESIDUE LIMITS
TOLERANCES
Apples and pears 3.0 ppm
DESIRABLE
1. Additional reproduction studies to investigate the effect on
survival rate of offspring of rats.
2. A method of analysis for apples and pears suitable for regulatory
purposes.
3. Composition and purity of this compound as manufactured in several
countries and the amount of ethoxyquinmonomer in the products from
various manufacturers.
REFERENCES
Alicino, N.J., Klein, H.C., Quattrone, J.J. and Choy, T.K. (1963)
Determination of butylated hydroxyanisole, butylated, hydroxytoluene,
and ethoxyquin in hydrocarbon-soluble samples. J. Agr. Fd Chem., 11:
496-8
Anon (1968) Changes in Official Method of Analysis. J. Assoc. Offic.
Anal. Chem., 51:453-4
Choy, T., Alicino, N.J., Klein, H.C. and Quattrone, J.J. (1963)
Determination of ethoxyquin by ultraviolet spectrophotometry. J. Agri.
Fd Chem., 11:340-2
Choy, T., Alicino, N.J., Klein, H.C. and Quattrone, J.J. (1963)
Determination of ethoxyquin by ultraviolet spectrophotometry. J.Agri.
Fd Chem., 11:340-2
Colorado, A. and M. Experimental Station. (1954) The chronic toxicity
of the antioxidant ca. 6-ethoxy, 2,2,4-trimethyl, 1,2-dihydroquinoline
(Santoquin, Monsanto). Unpublished, undated, report of studies
conducted during 1952-53. Submitted by Monsanto Chemical Company.
Cox, A.J. (1953) 6-ethoxy, 2,2,4-trimethyl-1,2-dihydroquinoline
(EMHQ). Histological report on sections of rat tissues stained with
haematoxylin-eosin. Unpublished report submitted by Monsanto Chemical
Company.
Derse, P. (1956) Assay report. Unpublished report of Wisconsin Alumni
Research Foundation submitted by Monsanto Chemical Company
Gordon, R.S. Conkin, R.A. and Machlin, L.J. (1964) Determination of
ethoxyquin in feeds. J. Assoc. Offic. Agric. Chem., 47:512-6
Halloran, H.R. (1952) Chick toxicity tests on Santoflex AW.
Unpublished report from the Poultry Products of Central California,
Petaluma, to California Department of Agriculture submitted by
Monsanto Chemical Company.
Hansal, R.F. (1955) Final report and addendum. Chronic oral
administration-dogs-metabolic studies. Unpublished report of Hazleton
Laboratories submitted by Monsanto Chemical Company
Kelly, R.G. (1960) Supplementary toxicity information on Santoquin
(6-ethoxy-2,2,4-trimethyl-1,2-dihydroquinoline). Unpublished report
prepared and submitted by Monsanto Chemical Company
Kel'man, G.Y. (1965) Comparative toxicity of Santoflex A
(6-ethoxy-2,2,4-trimethyl-1,2-dihydroquinoline) and acetoneanyl
(1,2-dihydro-2,2,4-trimethylquinoline). Keuchuk i Rezina 24:40-41
[(Chem. Abstr. 63:1136g (1965)]
Mackay, V.D. (1954) Toxicity data in support of the use of 0.015% of
6-ethoxy-2,2,4-trimethyl-1,2-dihydroquinoline on alfalfa meal for
carotene preservation. Unpublished report of Western Utilization
Research Branch, U.S.D.A., submitted by Monsanto Chemical Company
March, B.E., Biely, J. and Coates, V. (1968) The influence of diet on
toxicity of the anti-oxidant
1,2-dihydro-6-ethoxy-2,2,4-trimethylquinoline. Can. J. Physiol.
Pharmacol. 46:139-43. [Chem. Abstr. 68:102955m (1968)]
Monsanto (1956) Santoquin for use in Poultry Feeds, Unpublished report
prepared and submitted by Monsanto Chemical Company
Monsanto. (1956) Petition proposing the issuance of a regulation
permitting the use of Santoquin as an antioxidant for addition to
poultry feeds. Unpublished report. October
Monsanto. (1961) Information to support label registration request for
"Stop-Scald" (ethoxyquin) on apples and pears to control common scald
and petition for tolerance for ethoxyquin on pears. Unpublished
report.
Monsanto. (1966) Five year Santoquin feeding study in dogs.
Unpublished collection of reports prepared and submitted by Monsanto
Chemical Company
Monsanto. (1969) Direct communication
Padfield, C.A.S., Atkinson, J.D., Clark, P.J. (1963) Control of apple
scald by ethoxyquin. N.Z.J. Agr. Res. 6:245-52
Van Deren, J.M. and Jaworski, E.G. (1966) Ethoxyquin (Santonin (R) in
eggs, chicken muscle and chicken liver. J. Assoc. Offic. Anal. Chem.,
49:712-4
Van Deren, J.M. and Jaworski, E.G. (1967) Collaborative study of the
determination of ethoxyquin in chick tissue and eggs. J. Assoc. Offic.
Anal. Chem., 50:844-7
Wilson, R.H. (1956a) Distribution and excretion of oral Santoquin
C14, Unpublished reports of Western Utilization Research Branch,
U.S.D.A. submitted by Monsanto Chemical Company
Wilson, R.H. (1956b) Final report - reproduction in rats receiving
Santoquin. Unpublished report of Western Regional Utilization Research
Branch, U.S.D.A. submitted by Monsanto Chemical Company
Wilson, R.H. and DeEds, F. (1959) Toxicity studies on the antioxidant
6-ethoxy-1,2-dihydro-2,2,4-trimethylquinoline. J. Agr. Food Chem.
7:203-6
Wilson, R.H., Thomas, J.O., Thompson, C.R., Lanner, H.F. and Kohler,
G.O. (1959) Absorption, metabolism and excretion of the antioxidant,
6-ethoxy-1,2-dihydro-2,2,4-trimethylquinoline. J. Agr. Food Chem.,
7:206-9
Wood, W.S. (1965) Untitled report of dermatitis cases in fruit
handlers. Unpublished report from the Medical Dental Building,
Vancouver, B.C. submitted by Monsanto Chemical Company
