
FAO/PL:1969/M/17/1
WHO/FOOD ADD./70.38
1969 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
Issued jointly by FAO and WHO
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Group on Pesticide Residues, which met in Rome, 8 - 15 December 1969.
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
WORLD HEALTH ORGANIZATION
Rome, 1970
FOLPET
IDENTITY
Chemical name
N-(trichloromethylthio)phthalimide
Synonyms
Phaltan(R)
Structural formula
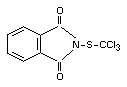 Other relevant chemical properties
The pure material is a white, crystalline solid, m.p. 117°C. It is
practically insoluble in water at room temperature and is only
slightly soluble in organic solvents. The technical product is 90%
pure. The impurities in the technical product consist of up to 4.5
percent phthalimide, up to 2.5 percent water, up to 2.5 percent
calcium carbonate, and less than 1 percent unidentified products. It
is formulated as 50 percent wettable powder (Ortho Phaltan 50 W); a 75
percent wettable powder is also formulated. Folpet is stable when dry
but hydrolyses slowly in water at room temperature, rapidly at high
temperatures or under alkaline conditions.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
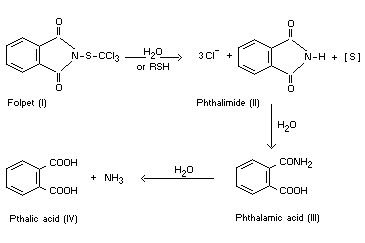
The hydrolysis products of folpet (I) are phthalimide (II), chloride
ions, and various inorganic forms of sulphur. Phthalimide is further
hydrolysed to phthalic acid (IV) and ammonia. No organochlorine or
organosulphur products have been detected. In the presence of
sulfhydryl compounds folpet is degraded extremely rapidly giving the
same products as from hydrolysis. In blood, the sulphenimide bond of
folpet is rapidly cleaved with the formation of phthalimide, the half
life being about one minute. The hydrolysis therefore appears to
proceed as follows (Dye, 1969):
Other relevant chemical properties
The pure material is a white, crystalline solid, m.p. 117°C. It is
practically insoluble in water at room temperature and is only
slightly soluble in organic solvents. The technical product is 90%
pure. The impurities in the technical product consist of up to 4.5
percent phthalimide, up to 2.5 percent water, up to 2.5 percent
calcium carbonate, and less than 1 percent unidentified products. It
is formulated as 50 percent wettable powder (Ortho Phaltan 50 W); a 75
percent wettable powder is also formulated. Folpet is stable when dry
but hydrolyses slowly in water at room temperature, rapidly at high
temperatures or under alkaline conditions.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
The hydrolysis products of folpet (I) are phthalimide (II), chloride
ions, and various inorganic forms of sulphur. Phthalimide is further
hydrolysed to phthalic acid (IV) and ammonia. No organochlorine or
organosulphur products have been detected. In the presence of
sulfhydryl compounds folpet is degraded extremely rapidly giving the
same products as from hydrolysis. In blood, the sulphenimide bond of
folpet is rapidly cleaved with the formation of phthalimide, the half
life being about one minute. The hydrolysis therefore appears to
proceed as follows (Dye, 1969):
 Because the group attached to the imide nitrogen is the same in folpet
as it is in captan, it is reasonable to assume that in the presence of
sulfhydryl groups (e.g. as in cysteine) the trichloromethylthio group
will break down to give chloride ions by a series of reactions similar
to those described in the monograph on captan (Owens, 1969).
Because the trichloromethylthio moiety is the same in both captan and
folpet, the only difference in the two compounds being that the ring
portion of folpet is aromatic, it has been assumed that all metabolic
data for captan relative to the trichloromethylthio portion of the
molecule will also be applicable to folpet (Dye, 1969) (see the
monograph on captan).
Special studies on reproduction
Rat
Four groups, each of 8 male and 16 female rats, were fed technical
folpet (94.4 percent purity) at dietary levels of 0, 100, 500 and 1000
ppm (active ingredient) in a three-generation study. Parental animals
were on the test diet for 79 days before mating and continuously
thereafter. There were no adverse effects on body-weight gain or
terminal organ-weights of parental animals, or on reproductive
performance, fertility, lactation, litter-size or incidence of
stillbirths in any test group. Pup survival at 5 and 21 days, and
weanling weights in all test groups were comparable with the controls.
Histopathology performed on parental animals of each generation and on
F3b weanlings in the 0 and 1000 ppm groups revealed no changes which
could be correlated with the ingestion of folpet (Kennedy et al.,
1967a).
Special studies on teratogenicity
Chicken-egg
Folpet was injected in dimethylsulphoxide solution into the yolk or
air cell of fresh fertile chicken eggs at levels which varied from 3
to 20 mg/kg egg-weight. The eggs were incubated and non-viable embryos
and hatched chicks were examined for gross abnormalities. In a total
of 830 eggs injected with folpet the incidence of malformations was
8.19 percent. The metabolites of folpet, phthalimide (305 eggs) and
phthalic acid (290 eggs) were also injected under similar conditions
using dimethylsulphoxide as a solvent for phthalimide and ethanol for
phthalic acid. A control group comprising over 1500 eggs was also
injected with dimethylsulphoxide alone and another group of several
thousand eggs with ethanol alone. The incidence of malformation was
3.93 percent for phthalimide, 3.10 percent for phthalic acid and less
than 2.0 percent for the controls. Micromelia amelia and phocomelia
accounted for most of the deformities (Varrett et al., 1969).
Hamster
Groups of 10 pregnant Syrian Golden hamsters were fed technical folpet
at dietary levels approximately equivalent to 0, 125, 250, 500 or 1000
(eight animals only) mg/kg body-weight per day from gestation days 4
to 15 inclusive. On day 15 of gestation all animals were sacrificed
and foetal examination was carried out. Maternal body-weight gains
were decreased over the feeding period in the 250 and 500 mg/kg groups
while at the highest level there was a weight loss recorded. There was
an increase in the number of resorption sites at the two highest
dose-levels compared with the control group. Growth appeared to be
retarded in the foetuses from all test groups. The mean number of
foetuses per litter was reduced at the two highest dose-levels. There
were no gross or skeletal abnormalities attributable to folpet at any
of the levels used (Arnold et al., 1968).
A companion study, in which folpet was given by intubation, was
carried out. Hamsters were given a single dose of 0 (11 pregnant
females), 125 (9 pregnant females), 250 (5) or 500 (8) mg/kg
body-weight, half of each group being intubated on day 7 and the
remainder on day 8. A positive control group was given thalidomide,
1000 mg/kg body-weight, on day 7. In this experiment the number of
foetal resorption sites in all groups was higher than normal. The high
incidence was in the 125 mg/kg group (5.8 per litter) and the low
incidence was in the 500 mg/kg group (2.5 per litter). The number of
young per litter was lower in all test groups than in the control.
There were no gross physical or skeletal anomalies in the test groups
which could be associated with folpet treatment. No abnormalities were
found in the group treated with thalidomide (Arnold et al., 1968).
Monkey
Groups of from four to six pregnant monkeys (Rhesus and stumptailed
macque) were given oral doses of 10, 25 and 75 mg/kg body-weight of
folpet daily on days 21 through 34 of gestation. Thalidomide was given
as a positive control to groups of 9 or 11 monkeys at levels 5 and 10
mg/kg body-weight respectively. All foetuses from animals given folpet
were grossly normal except for one Rhesus foetus each from the 25 and
75 mg/kg dose-levels which had 13 pairs of ribs. No abortions occurred
at the 25 and 75 mg/kg levels of folpet but one abortion occurred at
the 10 mg/kg level in day 54 of gestation. Abortions and foetal
deformities occurred in the groups given thalidomide (Vondruska,
1969).
Rabbit
Groups of pregnant New Zealand albino rabbits were given folpet at
dose-levels of 0, 18.75 (5 rabbits), 37.5 (5) or 75 (7) mg/kg
body-weight on gestation days 6 to 18 inclusive. The dose was
administered by gelatin capsule. Positive control animals were given
thalidomide at various dose levels. On day 29 each doe was sacrificed
and the young removed by Caesarean section. Folpet produced signs of
toxicity in the dose. At the two higher dose levels there was a loss
in body-weight over the period of treatment (days 6 to 18). Also at
these dose-levels the incidence of foetal resorption was higher than
in the negative control group. There appeared to be a compound-related
effect on mortality. Examination of 80 embryos from folpet-treated
rabbits revealed no gross abnormalities; internal structural formation
was normal and well-defined skeletal development was observed.
Thalidomide treatment in the positive controls resulted in malformed
young (Kennedy et al., 1967c; Kennedy et al., 1968).
Groups of pregnant New Zealand white rabbits were given folpet at dose
levels of 75 or 150 mg/kg body-weight. Thalidomide was also given to a
positive control group at the same dose-levels. Thalidomide but not
folpet produced a teratological response (McLaughlin et al., 1969).
Rat
Pregnant female rats of the Charles River strain were given 0, 100 (10
animals) or 500 (5 animals) mg/kg body-weight of technical folpet by
oral intubation on days 6 to 15 for the lower dose and days 8 to 10
for the higher dose-level. Trypan Blue was given by subcutaneous
injection, 50 mg/kg body-weight, on days 8 to 10 to a fourth group
serving as a positive control. All rats were sacrificed on the
twentieth day of gestation. Examination of a total of 169 foetuses
revealed no significant increase in the incidence of abnormalities in
the groups given folpet. Internal structural formation was normal, the
young were present in normal numbers, and were well-formed, Trypan
Blue treatments produced malformed young as expected (Kennedy et al.,
1967b).
A group of 10 pregnant female rats (Charles River and Sprague Dawley
derived strain) was given oral doses of 100 mg/kg body-weight/day of
folpet from day 6 to day 15 of gestation and another group of four
pregnant rats was given oral doses of 500 mg/kg body-weight/day from
day 8 to day 10. Examination of 120 foetuses in the 100 mg/kg group,
49 foetuses in the 500 mg/kg group and 200 foetuses from an untreated
control group gave no evidence of abnormalities related to the
administration of folpet (Kennedy et al., 1968).
Studies on the metabolite phthalimide
Rabbit (teratogenic study)
Groups of 10 female Dutch Belted Rabbits were given 0 or 75 mg/kg
body-weight of phthalimide (the hydrolytic metabolite of folpet), via
gelatin capsule, on day 6 through 16. A treated control group received
75 mg/kg of thalidomide over the same period. On day 28 the rabbits
were killed, the young removed by Caesarean section, and examined for
abnormalities. No adverse effects of phthalimide were noted on the
parental females or on the 24-hour survival rate of the young. No
abnormalities, external, internal or skeletal, were seen in the test
group but were present in the treated controls. Resorption of foetuses
occurred in three of seven does in the thalidomide group and in one of
ten in the phthalimide group. This animal lost 220 grams in weight
between day 11 and 16. Three pups were aborted on day 25, and three
resorption sites were present which accounted for all the implantation
sites
Acute toxicity
LD50mg/kg
Animal Route body-weight References
Rat oral >10,000 Elsea, 1956
Rabbit percutaneous >22,600 Kay and Calandra, 1960
Short-term studies
Dog
Four groups of dogs (three males and three females per group) were
given folpet at dose-levels of 0, 250, 1000 and 1500 mg/kg
body-weight. The compound was given orally by capsule 5 days a week
for 17 months. At 12 months 2-3 dogs (male and female) from each group
were sacrificed for pathologic study. The remainder were sacrificed
for pathological study after 17 months. Total mean weight-gain over
the duration of the test was depressed in both sexes at the highest
dose-level. None of the animals died during the test. Haematologic
studies, urinalyses, liver function tests (bromosulphthalein
retention), serum alkaline phosphatase and blood urea nitrogen
determinations all showed no unusual findings. There were no gross or
microscopic pathologic changes which could be correlated with the test
material (Key and Calandra, 1961a).
Rat
Groups each comprising 10 male and 10 female rats were fed dietary
levels of 0, 0.1, 0.32 and 1 percent of folpet for 12 weeks. Growth
was normal except in the male rats fed the 1 percent level where there
was a significant decrease. There were no gross abnormalities.
Histopathological examination of liver, kidneys, adrenals, intestines,
lungs and gonads of two male and two female animals of each group
revealed no abnormalities (Weir, 1956).
Long-term studies
Rat
Folpet was fed to four groups of rats at dietary levels of 0, 0.1,
0.32 and 1.0 percent for 17 months. Each test group consisted of 30
males and 30 females, with 60 rats of each sex in the control group.
After 12 months on the test, 5 males and 5 females of each test group
(10 of each in the controls), were killed for pathologic study.
Body-weight data showed a slight adverse effect on growth of rats fed
1 percent folpet in their diet. An increase in spleen to body-weight
ratio in this group, observed at 12 months was not present in the rats
examined after 17 months on the diet. There were no effects on
mortality, tumour incidence, haematologic studies, urinalysis, gross
or histopathology that were attributable to the feeding of folpet (Kay
and Calandra, 1961b).
COMMENT
In the two species used in acute toxicity studies, an LD50 value was
not obtained. The 17-month study on dogs and the 17-month rat study
indicate a high tolerance, by these species, to chronic exposure to
this material. These studies should, however, have been of at least
two-years duration to determine if there may be a potential for
carcinogenicity. Reproduction and teratogenicity studies on folpet,
have been carried out on rats, hamsters, and two strains of rabbits.
No evidence of teratogenic effects has been reported. As there are
indications of toxic effects on the mothers in the teratogenicity
studies with hamsters and rabbits, it is recommended that further
studies be done on these two species. Because of the uncertainty
regarding embryotoxicity, and since the long-term study in rats was
only 17 months' duration, a temporary adi is recommended.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Rat: 3,200 ppm in diet, equivalent to 160 mg/kg body-weight/day
Dog: 1,000 mg/kg body-weight/day
Estimate of temporary acceptable daily intake for man
0-0.16 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Folpet is a protective fungicide used mainly for foliage application
at about 0.1 percent active ingredient.
Pre-harvest treatments
Rates of application and intervals between treatment and harvest are:
Citrus fruits - 0.12 percent active ingredients applied
at 2/3 petal fall, 2 weeks after petal
fall, on full flush growth in August and
September. Use up to harvest.
Other fruits - 0.09-0.12 percent active ingredient,
applied as needed at 7-14 day intervals
up to harvest.
Avocados - 0.18 percent active ingredient, applied
as needed at 2 to 3 week intervals up to
harvest.
Small fruits and berries - 1.1 to 2.2 kg a.i./ha, applied as needed
at a 7 - 10 day intervals up to harvest.
Cranberries - 5.0 kg a.i./ha, applied as needed at
10-14 day intervals up to 30 days of
harvest.
Vegetables - 1.1 to 4.5 kg a.i./ha, applied as needed
at 7 to 10 day intervals up to harvest.
Celery - Same schedule up to 7 days of harvest.
Post-harvest treatments
No post-harvest treatments are recommended.
Other uses
Ornamentals - 0.12 percent a.i., applied as needed at 7-10 day
intervals.
RESIDUES RESULTING FROM SUPERVISED TRIALS
The residue data are from treatments made under commercial conditions
in the U.S.A. (Table I). It is found that the residue patterns of
folpet follow those of captan. Rain causes a loss of both compounds.
The development of the wax as the fruit ripens tends to retain the
residue. Generally the initial level of folpet is reduced by one half
within a week or two. The residue levels of the fruit pulp and fruit
juice are found much lower than those of the parent whole fruits.
TABLE I
Residues from field trials (Dye, 1969)
Rate of Pre-harvest Folpet
application Number of interval residue
Crop (kg a.i. per ha) treatments (days) (ppm)
Apples (0.1% to run-off) 1 0-3 2.2-10.5
Blueberries 2.8 (0.1%) 1-6 0-2 7.0-23.4
Cherries (0.1-0.2%) 3-5 0-1 3.7-8.9
Fresh currants 0.25-0.37 1-5 0-1 8.0-33.0
Grapes 2.7-3.4 (0.1%) 1-4 0-1 7.6-25.0
Grapefruits (0.1-0.2%) 1 0-1 2.4-5.9
Oranges (0.1-0.2%) 1 0-1 2.9-8.1
Raspberries 1.1-2.8 (0.1%) 1-3 0-1 4.6-13.9
Strawberries 0.3-2.8 1-8 0-1 1.7-5.7
Cantaloup,
whole 2.3 1 0-1 0.6-1.1
Cucumber 2.3 1 0-1 0.8-1.9
Onion, bulb (0.1%) 7-8 0-7 0.4-1.2
Tomatoes 1.0-2.3 1 0-1 1.7-4.1
Watermelons,
rind (0.2%) 14 0.0-0.3
pulp (0.2%) 14 0.0-0.1
FATE OF RESIDUES
General comments
The remarks under 'BIOCHEMICAL ASPECTS' earlier in this monograph,
particularly those relating to the hydrolysis of folpet, pertain to
the behaviour of residues in crops or foods both before and after
consumption.
In animals
In animals folpet is expected to be subject to the similar degradative
reactions as in plants but no actual data have been available for
evaluation.
In plants
No published data are available from actual studies of the residues in
plants. It is highly probable however that degradation products of
hydrolysis as outlined above could be found; but this requires further
confirmatory studies.
In soil
The instability of the compound would not permit build-up in soils.
However, no experimental data were available for confirmation.
In storage and processing
No general quantitative estimates about the effect of washing on the
folpet residues can be made. It is, however, believed that a
relatively high portion of residues could be removed. This is
supported by a single figure on oranges on which washing reduced a
residue of 8.1 ppm down to 1.1 ppm (Dye, 1969).
Peeling of fruits is also expected to reduce folpet residues even more
than washing but lack of data does not allow definite conclusions to
be drawn.
In refrigerated storage as well as in frozen products folpet residues
are expected to be very stable. Any special treatment, e.g. blanching,
before such storage however may reduce the folpet residues. Data are
not available for final evaluation. The only figures available
concerned frozen cherries which contained a folpet residue about 10
percent of the residue level of unprocessed cherries (Dye, 1969).
Juice extracted from folpet treated grapes have contained small
amounts of folpet. The residues in juice in most instances were less
than 1 ppm although there were few figures up to 5 ppm (Dye, 1969).
The possible occurrence of folpet in grape juice is of practical
importance because folpet can cause slight suppression of fermentation
and produce a poor flavour in wines (Chalkov and Vanev, 1968).
The canning process is expected to be very destructive to folpet
residues. The only data available are for canned cherries which were
found to contain a residue less than the detection limit of the
analytical method although the unprocessed cherries had residues up to
5.6 ppm (Dye, 1969).
METHODS OF RESIDUE ANALYSIS
A method of residue analysis (Anon., 1960) is based on extraction with
benzene, clean-up with activated carbon, and reaction with resorcinol
to give a yellow product which is measured in a colorimeter. This
method, however, cannot distinguish between folpet and captan, but
gives the sum of these two compounds. Folpet does not migrate into
plant tissues; surface stripping is considered adequate for removing
residues.
A method is developed to distinguish between folpet and captan (Anon.,
1961). The sensitivity of the method is not satisfactory.
Polarographic methods have been set up for determining derivatives of
folpet and captan (Anon., 1962a; 1962b; Nangniot, 1966).
Gas chromatographic methods developed for captan (Kilgore et al.,
1967); Bevenue and Ogata, 1968; Pomerantz and Ross, 1968) and
detecting residues an low as 0.01 ppm can be used for folpet as well.
Folpet has been determined with sufficient accuracy at a residue level
of 2 ppm in red and white wines by an electron capture gas
chromatographic method (Matta, 1968) using pentane for extraction and
Florisil for cleanup.
For regulatory purposes the GLC method has to be further developed.
NATIONAL TOLERANCES
Tolerance
Country (ppm) Crop
Canada 30 Celery
Canada 25 Apples, avocados, blackberries,
blueberries, boysenberries,
cantaloups, cherries, citrus
fruits, cranberries, crabapples,
cucumber, currants, dewberries,
garlic, gooseberries, grapes,
honeydew melons, huckleberries,
leeks, lettuce, loganberries,
muskmelons, onions, pumpkins,
raspberries, shallots,
strawberries, summer squash,
tomatoes, watermelons, winter
squash
(continued)
Tolerance
Country (ppm) Crop
Germany (Fed.Rep.) 15 Fruits, grapes, hops
Hungary 10 Fruits, grapes
Netherlands 20 All crops
United States of
America 50 Celery, cherries, leeks,
lettuce, onions (green),
shallots
United States of 25 Apples, avocados, blackberries,
America blueberries, boysenberries,
crabapples, cranberries,
currants, dewberries,
gooseberries, grapes,
huckleberries, loganberries,
raspberries, strawberries,
tomatoes
United States of 15 Cucumbers, garlic, melons,
America onions (dry bulb), pumpkins,
summer squash, winter squash
United States of 15 Citrus fruits (interim
America tolerance)
APPRAISAL
Folpet is used to control fungus diseases on tree fruits, citrus
fruits, avocadoes, small fruits and berries, vegetables and
ornamentals. Concentration of the foliage sprays is recommended to
about 0.1 percent active ingredient. Folpet in nonphytotoxic, though
some injuries are reported on pears and apples. It in stable when dry,
but hydrolyses slowly in water at room temperature, rapidly at higher
temperatures or under alkaline conditions. National tolerances
established for the residues of folpet in various commodities vary
from 10 to 50 ppm. Folpet is formulated as 50 percent and 75 percent
wettable powder.
The residue data available to the Meeting were from treatments made
under commercial conditions in the U.S.A. A large variety of crops
were covered. It is found that the residue patterns of folpet follow
those of captan. The wash-off effect of rain may be pronounced.
Generally the initial level of folpet is reduced by one half within a
week or two. The residue levels of the fruit pulp and fruit juice are
found much lower than those of the entire parent fruits. The effect of
processing on the residues may be pronounced.
The main degradation mechanisms of folpet in plants are postulated to
be the same as those of captan resulting from the reaction with
sulfhydryl compounds, mainly phthalimide, to phthalic acid, free
chlorine ion, and inorganic sulphur compounds. The degradation
products of folpet in plants are assumed to be the same as in animals.
The documentation of folpet refers to a colorimetric analysis of
residues of folpet. The method uses a colour reaction with resorcinol
and is not specific for folpet. (It reacts with captan, too). For
differentiating folpet and captan, UV spectrometry can be applied.
Good agricultural practice was considered to produce residue levels
determined one day after application as indicated under the following
headings:
RECOMMENDATION FOR TOLERANCES, TEMPORARY TOLERANCES OR
PRACTICAL RESIDUE LIMITS
TEMPORARY TOLERANCES (effective to 1973)
Apples 10 ppm
Blueberries 25 ppm
Cantaloups, whole fruit 2 ppm
Cherries 15 ppm
Citrus fruits 10 ppm
Cucumbers 2 ppm
Currants, fresh 30 ppm
Grapes 25 ppm
Onions 2 ppm
Raspberries 15 ppm
Strawberries 5 ppm
Tomatoes 5 ppm
Water melons 2 ppm
Insufficient information was available to enable evaluation of
residues or tolerances to be suggested for blackberries, cranberries
and celery.
FURTHER WORK OR INFORMATION
REQUIRED (before 30 June 1973)
1. Long-term studies of sufficient duration to test for possible
carcinogenic effects.
2. Additional studies on the effects of the compound on reproductive
physiology.
3. Further studies on metabolism, especially on the
trichloromethylthio-moiety.
4. Further data on the nature of terminal residues in plants as well
as on the magnitude of the degradation product: magnitude in
relation to the parent compound or toxicological importance.
5. Further data on the degradation mechanisms of folpet.
6. Data on the necessary rates and frequencies of application,
pre-harvest intervals and the resultant residues, from countries
other than the U.S.A.
7. Data on residue levels in raw agricultural products moving in
commerce.
8. Qualitative and quantitative data on fate of residues in washing,
blanching, storing and thermal processing of the treated crops.
DESIRABLE
1. Information on the fate of the compound in soil.
2. Evaluation of the analytical methods by collaborative studies
taking into account the possible presence of structurally related
compounds, e.g. captan and captafol.
REFERENCES
Anon. (1960) The analysis of residues of captan and folpet. Residue
method RM-1. Chevron Chemical Co. Unpub. Rept.
Anon. (1961) The determination of and differentiation between
residues of Phaltan and captan. Residue method RM-1A. Chevron
Chemical Co. Unpub. Rept.
Anon. (1962a) Centre de recherches de phytopharmacie à Gembloux
(Belgique) Résultats inédits
Anon. (1962b) Institut agronomique de l'etat (Gembloux-Belgique).
Résultats inédits
Arnold, D., Kodras, R. and Fancher, O.E. (1968) Teratogenicity study
on Phaltan Golden Syrian hamsters Unpub. Rept. of Industrial
Bio-Test Laboratories, submitted by Chevron Chemical Co.
Bevenue, A., and Ogata, J.N. (1968) The examination of mixtures of
captan and Phaltan by gas chromatography. J. Chromatog. 36:529-31
Chalkov, I. and Vanev, S. (1968) Determination of the effect of some
new fungicides, used to control gray rot in grapes under field
conditions, on the enzymic activity of yeasts. Lozarstvo Vinar
(Sofia) 17:33-40 (Chem. Abstr. 69:34 908 s, 1968)
Dye, D.F. (1969) Folpet. Unpub. Summary Rept. submitted to FAO and
WHO by Chevron Chemical Co.
Elsea, J.R. (1956) Phthalimide captan analog. Acute oral
administration. Unpub. Rept. of Hazleton Laboratories submitted by
Chevron Chemical Co.
Kay, J.H. and Calandra, J.C. (1960) Acute percutaneous and eye
irritation studies on Phaltan. Unpub. Rept. of Industrial Bio-Test
Laboratories submitted by Chevron Chemical Co.
Kay, J.H. and Calandra, J.C. (1961a) Chronic oral toxicity of
Phaltan. Pure bread beagle dogs, (with addendum report. Pathological
findings). Unpub. Rept. of Industrial Bio-Test Laboratories
submitted by Chevron Chemical Co.
Kay, J.H. and Calandra, J.C. (1961b) Chronic oral toxicity of
Phaltan. Albino rats. Unpub. Rept. of Industrial Bio-Test
Laboratories submitted by Chevron Chemical Co.
Kennedy, G., Fancher, O.E. and Calandra J.C. (1967a) Three generation
reproduction study in albino rats-Phaltan. Final Rept. Unpub.
Rept. of Industrial Bio-Test Laboratories submitted by Chevron
Chemical Co.
Kennedy, G., Fancher, O.E. and Calandra, J.C. (1967b) Rat
teratogenicity study. Captan, Difolatan and Phaltan. Unpub. Rept.
of Industrial Bio-Test Laboratories submitted by Chevron Chemical Co.
Kennedy, G., Jackson, G., Fancher, O.E. and Calandra, J.C. (1967c)
Rabbit teratogenicity study. Captan and Phaltan. Unpub. Rept. of
Industrial Bio-Test Laboratories submitted by Chevron Chemical Co.
Kennedy, G., Fancher, O.E. and Calandra, J.C. (1968) An investigation
of the teratogenic potential of captan, folpet and Difolatan. Toxicol.
appl. Pharmacol. 13:42-430
Kilgore, W.W., Winterlin, W. and White, R. (1967) Gas chromatographic
determinations of captan residues. J. Agr. Food Chem. 15:1035-37
Matta, M. (1968) Gas chromatographic determination of chlorinated
pesticide residues in wine. Vini Ital. 10:171-4 (Chem. Abstr.
69:66469 r, 1968)
McLaughlin, J., Jr., Reynaldo, E.F., Lamar, J.K. and Marliac, J.P.
(1969) Teratology studies in rabbits with captan, folpet and
Thalidomide. Toxicol. appl. Pharmacol. 14:641
Nangniot, P. (1966) L'application des méthodes électrochimiques à
l'étude des résidus de pesticides. Meded Rijksfac. Landbouwwetensch.
31:447-73
Owens, R. G. (1969) Metabolism of fungicides and related compounds.
Ann. N.Y. Acad. Sci. 100:144-52
Palazzolo, R., Fancher, O.E. and Calandra, J.C. (1966) Rabbit
reproduction study. Phthalimide. Unpub. Rept. of Industrial Bio-Test
Laboratories submitted by Chevron Chemical Co.
Pomerantz, I.H. and Rose, R. (1968) Captan and structurally related
compounds: thin-layer and gas-liquid chromatography. J. Assoc.
Offic. Anal. Chem. 51:1058-62
Varrett, M.J., Mutchler, M.K., Scott, W.F., Reynaldo, E.F. and
McLaughlin, J. (1969) Teratogenic effects of captan and related
compounds in the developing chick embryo. Ann. N.Y. Acad. Sci.
160:334-43
Vondruska, J.F. (1969) Teratologic investigation of Phaltan in
Macaca mulatta (Rhesus monkey) and Macaca arctoides
(stumptailed macque).
Unpub. Rept. of Industrial Bio-Test Laboratories submitted by
Chevron Chemical Co.
Weir, R.J. (1956) N-trichloromethylthiophthalimide, recrystallized
phthalimide, (phthalimide analog of captan). Subacute
feeding-rats. Supplement to report dated 23 July, 1956. Unpub.
Rept. submitted by Hazleton Laboratories to California Spray -
Chemical Co.
Because the group attached to the imide nitrogen is the same in folpet
as it is in captan, it is reasonable to assume that in the presence of
sulfhydryl groups (e.g. as in cysteine) the trichloromethylthio group
will break down to give chloride ions by a series of reactions similar
to those described in the monograph on captan (Owens, 1969).
Because the trichloromethylthio moiety is the same in both captan and
folpet, the only difference in the two compounds being that the ring
portion of folpet is aromatic, it has been assumed that all metabolic
data for captan relative to the trichloromethylthio portion of the
molecule will also be applicable to folpet (Dye, 1969) (see the
monograph on captan).
Special studies on reproduction
Rat
Four groups, each of 8 male and 16 female rats, were fed technical
folpet (94.4 percent purity) at dietary levels of 0, 100, 500 and 1000
ppm (active ingredient) in a three-generation study. Parental animals
were on the test diet for 79 days before mating and continuously
thereafter. There were no adverse effects on body-weight gain or
terminal organ-weights of parental animals, or on reproductive
performance, fertility, lactation, litter-size or incidence of
stillbirths in any test group. Pup survival at 5 and 21 days, and
weanling weights in all test groups were comparable with the controls.
Histopathology performed on parental animals of each generation and on
F3b weanlings in the 0 and 1000 ppm groups revealed no changes which
could be correlated with the ingestion of folpet (Kennedy et al.,
1967a).
Special studies on teratogenicity
Chicken-egg
Folpet was injected in dimethylsulphoxide solution into the yolk or
air cell of fresh fertile chicken eggs at levels which varied from 3
to 20 mg/kg egg-weight. The eggs were incubated and non-viable embryos
and hatched chicks were examined for gross abnormalities. In a total
of 830 eggs injected with folpet the incidence of malformations was
8.19 percent. The metabolites of folpet, phthalimide (305 eggs) and
phthalic acid (290 eggs) were also injected under similar conditions
using dimethylsulphoxide as a solvent for phthalimide and ethanol for
phthalic acid. A control group comprising over 1500 eggs was also
injected with dimethylsulphoxide alone and another group of several
thousand eggs with ethanol alone. The incidence of malformation was
3.93 percent for phthalimide, 3.10 percent for phthalic acid and less
than 2.0 percent for the controls. Micromelia amelia and phocomelia
accounted for most of the deformities (Varrett et al., 1969).
Hamster
Groups of 10 pregnant Syrian Golden hamsters were fed technical folpet
at dietary levels approximately equivalent to 0, 125, 250, 500 or 1000
(eight animals only) mg/kg body-weight per day from gestation days 4
to 15 inclusive. On day 15 of gestation all animals were sacrificed
and foetal examination was carried out. Maternal body-weight gains
were decreased over the feeding period in the 250 and 500 mg/kg groups
while at the highest level there was a weight loss recorded. There was
an increase in the number of resorption sites at the two highest
dose-levels compared with the control group. Growth appeared to be
retarded in the foetuses from all test groups. The mean number of
foetuses per litter was reduced at the two highest dose-levels. There
were no gross or skeletal abnormalities attributable to folpet at any
of the levels used (Arnold et al., 1968).
A companion study, in which folpet was given by intubation, was
carried out. Hamsters were given a single dose of 0 (11 pregnant
females), 125 (9 pregnant females), 250 (5) or 500 (8) mg/kg
body-weight, half of each group being intubated on day 7 and the
remainder on day 8. A positive control group was given thalidomide,
1000 mg/kg body-weight, on day 7. In this experiment the number of
foetal resorption sites in all groups was higher than normal. The high
incidence was in the 125 mg/kg group (5.8 per litter) and the low
incidence was in the 500 mg/kg group (2.5 per litter). The number of
young per litter was lower in all test groups than in the control.
There were no gross physical or skeletal anomalies in the test groups
which could be associated with folpet treatment. No abnormalities were
found in the group treated with thalidomide (Arnold et al., 1968).
Monkey
Groups of from four to six pregnant monkeys (Rhesus and stumptailed
macque) were given oral doses of 10, 25 and 75 mg/kg body-weight of
folpet daily on days 21 through 34 of gestation. Thalidomide was given
as a positive control to groups of 9 or 11 monkeys at levels 5 and 10
mg/kg body-weight respectively. All foetuses from animals given folpet
were grossly normal except for one Rhesus foetus each from the 25 and
75 mg/kg dose-levels which had 13 pairs of ribs. No abortions occurred
at the 25 and 75 mg/kg levels of folpet but one abortion occurred at
the 10 mg/kg level in day 54 of gestation. Abortions and foetal
deformities occurred in the groups given thalidomide (Vondruska,
1969).
Rabbit
Groups of pregnant New Zealand albino rabbits were given folpet at
dose-levels of 0, 18.75 (5 rabbits), 37.5 (5) or 75 (7) mg/kg
body-weight on gestation days 6 to 18 inclusive. The dose was
administered by gelatin capsule. Positive control animals were given
thalidomide at various dose levels. On day 29 each doe was sacrificed
and the young removed by Caesarean section. Folpet produced signs of
toxicity in the dose. At the two higher dose levels there was a loss
in body-weight over the period of treatment (days 6 to 18). Also at
these dose-levels the incidence of foetal resorption was higher than
in the negative control group. There appeared to be a compound-related
effect on mortality. Examination of 80 embryos from folpet-treated
rabbits revealed no gross abnormalities; internal structural formation
was normal and well-defined skeletal development was observed.
Thalidomide treatment in the positive controls resulted in malformed
young (Kennedy et al., 1967c; Kennedy et al., 1968).
Groups of pregnant New Zealand white rabbits were given folpet at dose
levels of 75 or 150 mg/kg body-weight. Thalidomide was also given to a
positive control group at the same dose-levels. Thalidomide but not
folpet produced a teratological response (McLaughlin et al., 1969).
Rat
Pregnant female rats of the Charles River strain were given 0, 100 (10
animals) or 500 (5 animals) mg/kg body-weight of technical folpet by
oral intubation on days 6 to 15 for the lower dose and days 8 to 10
for the higher dose-level. Trypan Blue was given by subcutaneous
injection, 50 mg/kg body-weight, on days 8 to 10 to a fourth group
serving as a positive control. All rats were sacrificed on the
twentieth day of gestation. Examination of a total of 169 foetuses
revealed no significant increase in the incidence of abnormalities in
the groups given folpet. Internal structural formation was normal, the
young were present in normal numbers, and were well-formed, Trypan
Blue treatments produced malformed young as expected (Kennedy et al.,
1967b).
A group of 10 pregnant female rats (Charles River and Sprague Dawley
derived strain) was given oral doses of 100 mg/kg body-weight/day of
folpet from day 6 to day 15 of gestation and another group of four
pregnant rats was given oral doses of 500 mg/kg body-weight/day from
day 8 to day 10. Examination of 120 foetuses in the 100 mg/kg group,
49 foetuses in the 500 mg/kg group and 200 foetuses from an untreated
control group gave no evidence of abnormalities related to the
administration of folpet (Kennedy et al., 1968).
Studies on the metabolite phthalimide
Rabbit (teratogenic study)
Groups of 10 female Dutch Belted Rabbits were given 0 or 75 mg/kg
body-weight of phthalimide (the hydrolytic metabolite of folpet), via
gelatin capsule, on day 6 through 16. A treated control group received
75 mg/kg of thalidomide over the same period. On day 28 the rabbits
were killed, the young removed by Caesarean section, and examined for
abnormalities. No adverse effects of phthalimide were noted on the
parental females or on the 24-hour survival rate of the young. No
abnormalities, external, internal or skeletal, were seen in the test
group but were present in the treated controls. Resorption of foetuses
occurred in three of seven does in the thalidomide group and in one of
ten in the phthalimide group. This animal lost 220 grams in weight
between day 11 and 16. Three pups were aborted on day 25, and three
resorption sites were present which accounted for all the implantation
sites
Acute toxicity
LD50mg/kg
Animal Route body-weight References
Rat oral >10,000 Elsea, 1956
Rabbit percutaneous >22,600 Kay and Calandra, 1960
Short-term studies
Dog
Four groups of dogs (three males and three females per group) were
given folpet at dose-levels of 0, 250, 1000 and 1500 mg/kg
body-weight. The compound was given orally by capsule 5 days a week
for 17 months. At 12 months 2-3 dogs (male and female) from each group
were sacrificed for pathologic study. The remainder were sacrificed
for pathological study after 17 months. Total mean weight-gain over
the duration of the test was depressed in both sexes at the highest
dose-level. None of the animals died during the test. Haematologic
studies, urinalyses, liver function tests (bromosulphthalein
retention), serum alkaline phosphatase and blood urea nitrogen
determinations all showed no unusual findings. There were no gross or
microscopic pathologic changes which could be correlated with the test
material (Key and Calandra, 1961a).
Rat
Groups each comprising 10 male and 10 female rats were fed dietary
levels of 0, 0.1, 0.32 and 1 percent of folpet for 12 weeks. Growth
was normal except in the male rats fed the 1 percent level where there
was a significant decrease. There were no gross abnormalities.
Histopathological examination of liver, kidneys, adrenals, intestines,
lungs and gonads of two male and two female animals of each group
revealed no abnormalities (Weir, 1956).
Long-term studies
Rat
Folpet was fed to four groups of rats at dietary levels of 0, 0.1,
0.32 and 1.0 percent for 17 months. Each test group consisted of 30
males and 30 females, with 60 rats of each sex in the control group.
After 12 months on the test, 5 males and 5 females of each test group
(10 of each in the controls), were killed for pathologic study.
Body-weight data showed a slight adverse effect on growth of rats fed
1 percent folpet in their diet. An increase in spleen to body-weight
ratio in this group, observed at 12 months was not present in the rats
examined after 17 months on the diet. There were no effects on
mortality, tumour incidence, haematologic studies, urinalysis, gross
or histopathology that were attributable to the feeding of folpet (Kay
and Calandra, 1961b).
COMMENT
In the two species used in acute toxicity studies, an LD50 value was
not obtained. The 17-month study on dogs and the 17-month rat study
indicate a high tolerance, by these species, to chronic exposure to
this material. These studies should, however, have been of at least
two-years duration to determine if there may be a potential for
carcinogenicity. Reproduction and teratogenicity studies on folpet,
have been carried out on rats, hamsters, and two strains of rabbits.
No evidence of teratogenic effects has been reported. As there are
indications of toxic effects on the mothers in the teratogenicity
studies with hamsters and rabbits, it is recommended that further
studies be done on these two species. Because of the uncertainty
regarding embryotoxicity, and since the long-term study in rats was
only 17 months' duration, a temporary adi is recommended.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Rat: 3,200 ppm in diet, equivalent to 160 mg/kg body-weight/day
Dog: 1,000 mg/kg body-weight/day
Estimate of temporary acceptable daily intake for man
0-0.16 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Folpet is a protective fungicide used mainly for foliage application
at about 0.1 percent active ingredient.
Pre-harvest treatments
Rates of application and intervals between treatment and harvest are:
Citrus fruits - 0.12 percent active ingredients applied
at 2/3 petal fall, 2 weeks after petal
fall, on full flush growth in August and
September. Use up to harvest.
Other fruits - 0.09-0.12 percent active ingredient,
applied as needed at 7-14 day intervals
up to harvest.
Avocados - 0.18 percent active ingredient, applied
as needed at 2 to 3 week intervals up to
harvest.
Small fruits and berries - 1.1 to 2.2 kg a.i./ha, applied as needed
at a 7 - 10 day intervals up to harvest.
Cranberries - 5.0 kg a.i./ha, applied as needed at
10-14 day intervals up to 30 days of
harvest.
Vegetables - 1.1 to 4.5 kg a.i./ha, applied as needed
at 7 to 10 day intervals up to harvest.
Celery - Same schedule up to 7 days of harvest.
Post-harvest treatments
No post-harvest treatments are recommended.
Other uses
Ornamentals - 0.12 percent a.i., applied as needed at 7-10 day
intervals.
RESIDUES RESULTING FROM SUPERVISED TRIALS
The residue data are from treatments made under commercial conditions
in the U.S.A. (Table I). It is found that the residue patterns of
folpet follow those of captan. Rain causes a loss of both compounds.
The development of the wax as the fruit ripens tends to retain the
residue. Generally the initial level of folpet is reduced by one half
within a week or two. The residue levels of the fruit pulp and fruit
juice are found much lower than those of the parent whole fruits.
TABLE I
Residues from field trials (Dye, 1969)
Rate of Pre-harvest Folpet
application Number of interval residue
Crop (kg a.i. per ha) treatments (days) (ppm)
Apples (0.1% to run-off) 1 0-3 2.2-10.5
Blueberries 2.8 (0.1%) 1-6 0-2 7.0-23.4
Cherries (0.1-0.2%) 3-5 0-1 3.7-8.9
Fresh currants 0.25-0.37 1-5 0-1 8.0-33.0
Grapes 2.7-3.4 (0.1%) 1-4 0-1 7.6-25.0
Grapefruits (0.1-0.2%) 1 0-1 2.4-5.9
Oranges (0.1-0.2%) 1 0-1 2.9-8.1
Raspberries 1.1-2.8 (0.1%) 1-3 0-1 4.6-13.9
Strawberries 0.3-2.8 1-8 0-1 1.7-5.7
Cantaloup,
whole 2.3 1 0-1 0.6-1.1
Cucumber 2.3 1 0-1 0.8-1.9
Onion, bulb (0.1%) 7-8 0-7 0.4-1.2
Tomatoes 1.0-2.3 1 0-1 1.7-4.1
Watermelons,
rind (0.2%) 14 0.0-0.3
pulp (0.2%) 14 0.0-0.1
FATE OF RESIDUES
General comments
The remarks under 'BIOCHEMICAL ASPECTS' earlier in this monograph,
particularly those relating to the hydrolysis of folpet, pertain to
the behaviour of residues in crops or foods both before and after
consumption.
In animals
In animals folpet is expected to be subject to the similar degradative
reactions as in plants but no actual data have been available for
evaluation.
In plants
No published data are available from actual studies of the residues in
plants. It is highly probable however that degradation products of
hydrolysis as outlined above could be found; but this requires further
confirmatory studies.
In soil
The instability of the compound would not permit build-up in soils.
However, no experimental data were available for confirmation.
In storage and processing
No general quantitative estimates about the effect of washing on the
folpet residues can be made. It is, however, believed that a
relatively high portion of residues could be removed. This is
supported by a single figure on oranges on which washing reduced a
residue of 8.1 ppm down to 1.1 ppm (Dye, 1969).
Peeling of fruits is also expected to reduce folpet residues even more
than washing but lack of data does not allow definite conclusions to
be drawn.
In refrigerated storage as well as in frozen products folpet residues
are expected to be very stable. Any special treatment, e.g. blanching,
before such storage however may reduce the folpet residues. Data are
not available for final evaluation. The only figures available
concerned frozen cherries which contained a folpet residue about 10
percent of the residue level of unprocessed cherries (Dye, 1969).
Juice extracted from folpet treated grapes have contained small
amounts of folpet. The residues in juice in most instances were less
than 1 ppm although there were few figures up to 5 ppm (Dye, 1969).
The possible occurrence of folpet in grape juice is of practical
importance because folpet can cause slight suppression of fermentation
and produce a poor flavour in wines (Chalkov and Vanev, 1968).
The canning process is expected to be very destructive to folpet
residues. The only data available are for canned cherries which were
found to contain a residue less than the detection limit of the
analytical method although the unprocessed cherries had residues up to
5.6 ppm (Dye, 1969).
METHODS OF RESIDUE ANALYSIS
A method of residue analysis (Anon., 1960) is based on extraction with
benzene, clean-up with activated carbon, and reaction with resorcinol
to give a yellow product which is measured in a colorimeter. This
method, however, cannot distinguish between folpet and captan, but
gives the sum of these two compounds. Folpet does not migrate into
plant tissues; surface stripping is considered adequate for removing
residues.
A method is developed to distinguish between folpet and captan (Anon.,
1961). The sensitivity of the method is not satisfactory.
Polarographic methods have been set up for determining derivatives of
folpet and captan (Anon., 1962a; 1962b; Nangniot, 1966).
Gas chromatographic methods developed for captan (Kilgore et al.,
1967); Bevenue and Ogata, 1968; Pomerantz and Ross, 1968) and
detecting residues an low as 0.01 ppm can be used for folpet as well.
Folpet has been determined with sufficient accuracy at a residue level
of 2 ppm in red and white wines by an electron capture gas
chromatographic method (Matta, 1968) using pentane for extraction and
Florisil for cleanup.
For regulatory purposes the GLC method has to be further developed.
NATIONAL TOLERANCES
Tolerance
Country (ppm) Crop
Canada 30 Celery
Canada 25 Apples, avocados, blackberries,
blueberries, boysenberries,
cantaloups, cherries, citrus
fruits, cranberries, crabapples,
cucumber, currants, dewberries,
garlic, gooseberries, grapes,
honeydew melons, huckleberries,
leeks, lettuce, loganberries,
muskmelons, onions, pumpkins,
raspberries, shallots,
strawberries, summer squash,
tomatoes, watermelons, winter
squash
(continued)
Tolerance
Country (ppm) Crop
Germany (Fed.Rep.) 15 Fruits, grapes, hops
Hungary 10 Fruits, grapes
Netherlands 20 All crops
United States of
America 50 Celery, cherries, leeks,
lettuce, onions (green),
shallots
United States of 25 Apples, avocados, blackberries,
America blueberries, boysenberries,
crabapples, cranberries,
currants, dewberries,
gooseberries, grapes,
huckleberries, loganberries,
raspberries, strawberries,
tomatoes
United States of 15 Cucumbers, garlic, melons,
America onions (dry bulb), pumpkins,
summer squash, winter squash
United States of 15 Citrus fruits (interim
America tolerance)
APPRAISAL
Folpet is used to control fungus diseases on tree fruits, citrus
fruits, avocadoes, small fruits and berries, vegetables and
ornamentals. Concentration of the foliage sprays is recommended to
about 0.1 percent active ingredient. Folpet in nonphytotoxic, though
some injuries are reported on pears and apples. It in stable when dry,
but hydrolyses slowly in water at room temperature, rapidly at higher
temperatures or under alkaline conditions. National tolerances
established for the residues of folpet in various commodities vary
from 10 to 50 ppm. Folpet is formulated as 50 percent and 75 percent
wettable powder.
The residue data available to the Meeting were from treatments made
under commercial conditions in the U.S.A. A large variety of crops
were covered. It is found that the residue patterns of folpet follow
those of captan. The wash-off effect of rain may be pronounced.
Generally the initial level of folpet is reduced by one half within a
week or two. The residue levels of the fruit pulp and fruit juice are
found much lower than those of the entire parent fruits. The effect of
processing on the residues may be pronounced.
The main degradation mechanisms of folpet in plants are postulated to
be the same as those of captan resulting from the reaction with
sulfhydryl compounds, mainly phthalimide, to phthalic acid, free
chlorine ion, and inorganic sulphur compounds. The degradation
products of folpet in plants are assumed to be the same as in animals.
The documentation of folpet refers to a colorimetric analysis of
residues of folpet. The method uses a colour reaction with resorcinol
and is not specific for folpet. (It reacts with captan, too). For
differentiating folpet and captan, UV spectrometry can be applied.
Good agricultural practice was considered to produce residue levels
determined one day after application as indicated under the following
headings:
RECOMMENDATION FOR TOLERANCES, TEMPORARY TOLERANCES OR
PRACTICAL RESIDUE LIMITS
TEMPORARY TOLERANCES (effective to 1973)
Apples 10 ppm
Blueberries 25 ppm
Cantaloups, whole fruit 2 ppm
Cherries 15 ppm
Citrus fruits 10 ppm
Cucumbers 2 ppm
Currants, fresh 30 ppm
Grapes 25 ppm
Onions 2 ppm
Raspberries 15 ppm
Strawberries 5 ppm
Tomatoes 5 ppm
Water melons 2 ppm
Insufficient information was available to enable evaluation of
residues or tolerances to be suggested for blackberries, cranberries
and celery.
FURTHER WORK OR INFORMATION
REQUIRED (before 30 June 1973)
1. Long-term studies of sufficient duration to test for possible
carcinogenic effects.
2. Additional studies on the effects of the compound on reproductive
physiology.
3. Further studies on metabolism, especially on the
trichloromethylthio-moiety.
4. Further data on the nature of terminal residues in plants as well
as on the magnitude of the degradation product: magnitude in
relation to the parent compound or toxicological importance.
5. Further data on the degradation mechanisms of folpet.
6. Data on the necessary rates and frequencies of application,
pre-harvest intervals and the resultant residues, from countries
other than the U.S.A.
7. Data on residue levels in raw agricultural products moving in
commerce.
8. Qualitative and quantitative data on fate of residues in washing,
blanching, storing and thermal processing of the treated crops.
DESIRABLE
1. Information on the fate of the compound in soil.
2. Evaluation of the analytical methods by collaborative studies
taking into account the possible presence of structurally related
compounds, e.g. captan and captafol.
REFERENCES
Anon. (1960) The analysis of residues of captan and folpet. Residue
method RM-1. Chevron Chemical Co. Unpub. Rept.
Anon. (1961) The determination of and differentiation between
residues of Phaltan and captan. Residue method RM-1A. Chevron
Chemical Co. Unpub. Rept.
Anon. (1962a) Centre de recherches de phytopharmacie à Gembloux
(Belgique) Résultats inédits
Anon. (1962b) Institut agronomique de l'etat (Gembloux-Belgique).
Résultats inédits
Arnold, D., Kodras, R. and Fancher, O.E. (1968) Teratogenicity study
on Phaltan Golden Syrian hamsters Unpub. Rept. of Industrial
Bio-Test Laboratories, submitted by Chevron Chemical Co.
Bevenue, A., and Ogata, J.N. (1968) The examination of mixtures of
captan and Phaltan by gas chromatography. J. Chromatog. 36:529-31
Chalkov, I. and Vanev, S. (1968) Determination of the effect of some
new fungicides, used to control gray rot in grapes under field
conditions, on the enzymic activity of yeasts. Lozarstvo Vinar
(Sofia) 17:33-40 (Chem. Abstr. 69:34 908 s, 1968)
Dye, D.F. (1969) Folpet. Unpub. Summary Rept. submitted to FAO and
WHO by Chevron Chemical Co.
Elsea, J.R. (1956) Phthalimide captan analog. Acute oral
administration. Unpub. Rept. of Hazleton Laboratories submitted by
Chevron Chemical Co.
Kay, J.H. and Calandra, J.C. (1960) Acute percutaneous and eye
irritation studies on Phaltan. Unpub. Rept. of Industrial Bio-Test
Laboratories submitted by Chevron Chemical Co.
Kay, J.H. and Calandra, J.C. (1961a) Chronic oral toxicity of
Phaltan. Pure bread beagle dogs, (with addendum report. Pathological
findings). Unpub. Rept. of Industrial Bio-Test Laboratories
submitted by Chevron Chemical Co.
Kay, J.H. and Calandra, J.C. (1961b) Chronic oral toxicity of
Phaltan. Albino rats. Unpub. Rept. of Industrial Bio-Test
Laboratories submitted by Chevron Chemical Co.
Kennedy, G., Fancher, O.E. and Calandra J.C. (1967a) Three generation
reproduction study in albino rats-Phaltan. Final Rept. Unpub.
Rept. of Industrial Bio-Test Laboratories submitted by Chevron
Chemical Co.
Kennedy, G., Fancher, O.E. and Calandra, J.C. (1967b) Rat
teratogenicity study. Captan, Difolatan and Phaltan. Unpub. Rept.
of Industrial Bio-Test Laboratories submitted by Chevron Chemical Co.
Kennedy, G., Jackson, G., Fancher, O.E. and Calandra, J.C. (1967c)
Rabbit teratogenicity study. Captan and Phaltan. Unpub. Rept. of
Industrial Bio-Test Laboratories submitted by Chevron Chemical Co.
Kennedy, G., Fancher, O.E. and Calandra, J.C. (1968) An investigation
of the teratogenic potential of captan, folpet and Difolatan. Toxicol.
appl. Pharmacol. 13:42-430
Kilgore, W.W., Winterlin, W. and White, R. (1967) Gas chromatographic
determinations of captan residues. J. Agr. Food Chem. 15:1035-37
Matta, M. (1968) Gas chromatographic determination of chlorinated
pesticide residues in wine. Vini Ital. 10:171-4 (Chem. Abstr.
69:66469 r, 1968)
McLaughlin, J., Jr., Reynaldo, E.F., Lamar, J.K. and Marliac, J.P.
(1969) Teratology studies in rabbits with captan, folpet and
Thalidomide. Toxicol. appl. Pharmacol. 14:641
Nangniot, P. (1966) L'application des méthodes électrochimiques à
l'étude des résidus de pesticides. Meded Rijksfac. Landbouwwetensch.
31:447-73
Owens, R. G. (1969) Metabolism of fungicides and related compounds.
Ann. N.Y. Acad. Sci. 100:144-52
Palazzolo, R., Fancher, O.E. and Calandra, J.C. (1966) Rabbit
reproduction study. Phthalimide. Unpub. Rept. of Industrial Bio-Test
Laboratories submitted by Chevron Chemical Co.
Pomerantz, I.H. and Rose, R. (1968) Captan and structurally related
compounds: thin-layer and gas-liquid chromatography. J. Assoc.
Offic. Anal. Chem. 51:1058-62
Varrett, M.J., Mutchler, M.K., Scott, W.F., Reynaldo, E.F. and
McLaughlin, J. (1969) Teratogenic effects of captan and related
compounds in the developing chick embryo. Ann. N.Y. Acad. Sci.
160:334-43
Vondruska, J.F. (1969) Teratologic investigation of Phaltan in
Macaca mulatta (Rhesus monkey) and Macaca arctoides
(stumptailed macque).
Unpub. Rept. of Industrial Bio-Test Laboratories submitted by
Chevron Chemical Co.
Weir, R.J. (1956) N-trichloromethylthiophthalimide, recrystallized
phthalimide, (phthalimide analog of captan). Subacute
feeding-rats. Supplement to report dated 23 July, 1956. Unpub.
Rept. submitted by Hazleton Laboratories to California Spray -
Chemical Co.
 Other relevant chemical properties
The pure material is a white, crystalline solid, m.p. 117°C. It is
practically insoluble in water at room temperature and is only
slightly soluble in organic solvents. The technical product is 90%
pure. The impurities in the technical product consist of up to 4.5
percent phthalimide, up to 2.5 percent water, up to 2.5 percent
calcium carbonate, and less than 1 percent unidentified products. It
is formulated as 50 percent wettable powder (Ortho Phaltan 50 W); a 75
percent wettable powder is also formulated. Folpet is stable when dry
but hydrolyses slowly in water at room temperature, rapidly at high
temperatures or under alkaline conditions.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
The hydrolysis products of folpet (I) are phthalimide (II), chloride
ions, and various inorganic forms of sulphur. Phthalimide is further
hydrolysed to phthalic acid (IV) and ammonia. No organochlorine or
organosulphur products have been detected. In the presence of
sulfhydryl compounds folpet is degraded extremely rapidly giving the
same products as from hydrolysis. In blood, the sulphenimide bond of
folpet is rapidly cleaved with the formation of phthalimide, the half
life being about one minute. The hydrolysis therefore appears to
proceed as follows (Dye, 1969):
Other relevant chemical properties
The pure material is a white, crystalline solid, m.p. 117°C. It is
practically insoluble in water at room temperature and is only
slightly soluble in organic solvents. The technical product is 90%
pure. The impurities in the technical product consist of up to 4.5
percent phthalimide, up to 2.5 percent water, up to 2.5 percent
calcium carbonate, and less than 1 percent unidentified products. It
is formulated as 50 percent wettable powder (Ortho Phaltan 50 W); a 75
percent wettable powder is also formulated. Folpet is stable when dry
but hydrolyses slowly in water at room temperature, rapidly at high
temperatures or under alkaline conditions.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
The hydrolysis products of folpet (I) are phthalimide (II), chloride
ions, and various inorganic forms of sulphur. Phthalimide is further
hydrolysed to phthalic acid (IV) and ammonia. No organochlorine or
organosulphur products have been detected. In the presence of
sulfhydryl compounds folpet is degraded extremely rapidly giving the
same products as from hydrolysis. In blood, the sulphenimide bond of
folpet is rapidly cleaved with the formation of phthalimide, the half
life being about one minute. The hydrolysis therefore appears to
proceed as follows (Dye, 1969):
 Because the group attached to the imide nitrogen is the same in folpet
as it is in captan, it is reasonable to assume that in the presence of
sulfhydryl groups (e.g. as in cysteine) the trichloromethylthio group
will break down to give chloride ions by a series of reactions similar
to those described in the monograph on captan (Owens, 1969).
Because the trichloromethylthio moiety is the same in both captan and
folpet, the only difference in the two compounds being that the ring
portion of folpet is aromatic, it has been assumed that all metabolic
data for captan relative to the trichloromethylthio portion of the
molecule will also be applicable to folpet (Dye, 1969) (see the
monograph on captan).
Special studies on reproduction
Rat
Four groups, each of 8 male and 16 female rats, were fed technical
folpet (94.4 percent purity) at dietary levels of 0, 100, 500 and 1000
ppm (active ingredient) in a three-generation study. Parental animals
were on the test diet for 79 days before mating and continuously
thereafter. There were no adverse effects on body-weight gain or
terminal organ-weights of parental animals, or on reproductive
performance, fertility, lactation, litter-size or incidence of
stillbirths in any test group. Pup survival at 5 and 21 days, and
weanling weights in all test groups were comparable with the controls.
Histopathology performed on parental animals of each generation and on
F3b weanlings in the 0 and 1000 ppm groups revealed no changes which
could be correlated with the ingestion of folpet (Kennedy et al.,
1967a).
Special studies on teratogenicity
Chicken-egg
Folpet was injected in dimethylsulphoxide solution into the yolk or
air cell of fresh fertile chicken eggs at levels which varied from 3
to 20 mg/kg egg-weight. The eggs were incubated and non-viable embryos
and hatched chicks were examined for gross abnormalities. In a total
of 830 eggs injected with folpet the incidence of malformations was
8.19 percent. The metabolites of folpet, phthalimide (305 eggs) and
phthalic acid (290 eggs) were also injected under similar conditions
using dimethylsulphoxide as a solvent for phthalimide and ethanol for
phthalic acid. A control group comprising over 1500 eggs was also
injected with dimethylsulphoxide alone and another group of several
thousand eggs with ethanol alone. The incidence of malformation was
3.93 percent for phthalimide, 3.10 percent for phthalic acid and less
than 2.0 percent for the controls. Micromelia amelia and phocomelia
accounted for most of the deformities (Varrett et al., 1969).
Hamster
Groups of 10 pregnant Syrian Golden hamsters were fed technical folpet
at dietary levels approximately equivalent to 0, 125, 250, 500 or 1000
(eight animals only) mg/kg body-weight per day from gestation days 4
to 15 inclusive. On day 15 of gestation all animals were sacrificed
and foetal examination was carried out. Maternal body-weight gains
were decreased over the feeding period in the 250 and 500 mg/kg groups
while at the highest level there was a weight loss recorded. There was
an increase in the number of resorption sites at the two highest
dose-levels compared with the control group. Growth appeared to be
retarded in the foetuses from all test groups. The mean number of
foetuses per litter was reduced at the two highest dose-levels. There
were no gross or skeletal abnormalities attributable to folpet at any
of the levels used (Arnold et al., 1968).
A companion study, in which folpet was given by intubation, was
carried out. Hamsters were given a single dose of 0 (11 pregnant
females), 125 (9 pregnant females), 250 (5) or 500 (8) mg/kg
body-weight, half of each group being intubated on day 7 and the
remainder on day 8. A positive control group was given thalidomide,
1000 mg/kg body-weight, on day 7. In this experiment the number of
foetal resorption sites in all groups was higher than normal. The high
incidence was in the 125 mg/kg group (5.8 per litter) and the low
incidence was in the 500 mg/kg group (2.5 per litter). The number of
young per litter was lower in all test groups than in the control.
There were no gross physical or skeletal anomalies in the test groups
which could be associated with folpet treatment. No abnormalities were
found in the group treated with thalidomide (Arnold et al., 1968).
Monkey
Groups of from four to six pregnant monkeys (Rhesus and stumptailed
macque) were given oral doses of 10, 25 and 75 mg/kg body-weight of
folpet daily on days 21 through 34 of gestation. Thalidomide was given
as a positive control to groups of 9 or 11 monkeys at levels 5 and 10
mg/kg body-weight respectively. All foetuses from animals given folpet
were grossly normal except for one Rhesus foetus each from the 25 and
75 mg/kg dose-levels which had 13 pairs of ribs. No abortions occurred
at the 25 and 75 mg/kg levels of folpet but one abortion occurred at
the 10 mg/kg level in day 54 of gestation. Abortions and foetal
deformities occurred in the groups given thalidomide (Vondruska,
1969).
Rabbit
Groups of pregnant New Zealand albino rabbits were given folpet at
dose-levels of 0, 18.75 (5 rabbits), 37.5 (5) or 75 (7) mg/kg
body-weight on gestation days 6 to 18 inclusive. The dose was
administered by gelatin capsule. Positive control animals were given
thalidomide at various dose levels. On day 29 each doe was sacrificed
and the young removed by Caesarean section. Folpet produced signs of
toxicity in the dose. At the two higher dose levels there was a loss
in body-weight over the period of treatment (days 6 to 18). Also at
these dose-levels the incidence of foetal resorption was higher than
in the negative control group. There appeared to be a compound-related
effect on mortality. Examination of 80 embryos from folpet-treated
rabbits revealed no gross abnormalities; internal structural formation
was normal and well-defined skeletal development was observed.
Thalidomide treatment in the positive controls resulted in malformed
young (Kennedy et al., 1967c; Kennedy et al., 1968).
Groups of pregnant New Zealand white rabbits were given folpet at dose
levels of 75 or 150 mg/kg body-weight. Thalidomide was also given to a
positive control group at the same dose-levels. Thalidomide but not
folpet produced a teratological response (McLaughlin et al., 1969).
Rat
Pregnant female rats of the Charles River strain were given 0, 100 (10
animals) or 500 (5 animals) mg/kg body-weight of technical folpet by
oral intubation on days 6 to 15 for the lower dose and days 8 to 10
for the higher dose-level. Trypan Blue was given by subcutaneous
injection, 50 mg/kg body-weight, on days 8 to 10 to a fourth group
serving as a positive control. All rats were sacrificed on the
twentieth day of gestation. Examination of a total of 169 foetuses
revealed no significant increase in the incidence of abnormalities in
the groups given folpet. Internal structural formation was normal, the
young were present in normal numbers, and were well-formed, Trypan
Blue treatments produced malformed young as expected (Kennedy et al.,
1967b).
A group of 10 pregnant female rats (Charles River and Sprague Dawley
derived strain) was given oral doses of 100 mg/kg body-weight/day of
folpet from day 6 to day 15 of gestation and another group of four
pregnant rats was given oral doses of 500 mg/kg body-weight/day from
day 8 to day 10. Examination of 120 foetuses in the 100 mg/kg group,
49 foetuses in the 500 mg/kg group and 200 foetuses from an untreated
control group gave no evidence of abnormalities related to the
administration of folpet (Kennedy et al., 1968).
Studies on the metabolite phthalimide
Rabbit (teratogenic study)
Groups of 10 female Dutch Belted Rabbits were given 0 or 75 mg/kg
body-weight of phthalimide (the hydrolytic metabolite of folpet), via
gelatin capsule, on day 6 through 16. A treated control group received
75 mg/kg of thalidomide over the same period. On day 28 the rabbits
were killed, the young removed by Caesarean section, and examined for
abnormalities. No adverse effects of phthalimide were noted on the
parental females or on the 24-hour survival rate of the young. No
abnormalities, external, internal or skeletal, were seen in the test
group but were present in the treated controls. Resorption of foetuses
occurred in three of seven does in the thalidomide group and in one of
ten in the phthalimide group. This animal lost 220 grams in weight
between day 11 and 16. Three pups were aborted on day 25, and three
resorption sites were present which accounted for all the implantation
sites
Acute toxicity
LD50mg/kg
Animal Route body-weight References
Rat oral >10,000 Elsea, 1956
Rabbit percutaneous >22,600 Kay and Calandra, 1960
Short-term studies
Dog
Four groups of dogs (three males and three females per group) were
given folpet at dose-levels of 0, 250, 1000 and 1500 mg/kg
body-weight. The compound was given orally by capsule 5 days a week
for 17 months. At 12 months 2-3 dogs (male and female) from each group
were sacrificed for pathologic study. The remainder were sacrificed
for pathological study after 17 months. Total mean weight-gain over
the duration of the test was depressed in both sexes at the highest
dose-level. None of the animals died during the test. Haematologic
studies, urinalyses, liver function tests (bromosulphthalein
retention), serum alkaline phosphatase and blood urea nitrogen
determinations all showed no unusual findings. There were no gross or
microscopic pathologic changes which could be correlated with the test
material (Key and Calandra, 1961a).
Rat
Groups each comprising 10 male and 10 female rats were fed dietary
levels of 0, 0.1, 0.32 and 1 percent of folpet for 12 weeks. Growth
was normal except in the male rats fed the 1 percent level where there
was a significant decrease. There were no gross abnormalities.
Histopathological examination of liver, kidneys, adrenals, intestines,
lungs and gonads of two male and two female animals of each group
revealed no abnormalities (Weir, 1956).
Long-term studies
Rat
Folpet was fed to four groups of rats at dietary levels of 0, 0.1,
0.32 and 1.0 percent for 17 months. Each test group consisted of 30
males and 30 females, with 60 rats of each sex in the control group.
After 12 months on the test, 5 males and 5 females of each test group
(10 of each in the controls), were killed for pathologic study.
Body-weight data showed a slight adverse effect on growth of rats fed
1 percent folpet in their diet. An increase in spleen to body-weight
ratio in this group, observed at 12 months was not present in the rats
examined after 17 months on the diet. There were no effects on
mortality, tumour incidence, haematologic studies, urinalysis, gross
or histopathology that were attributable to the feeding of folpet (Kay
and Calandra, 1961b).
COMMENT
In the two species used in acute toxicity studies, an LD50 value was
not obtained. The 17-month study on dogs and the 17-month rat study
indicate a high tolerance, by these species, to chronic exposure to
this material. These studies should, however, have been of at least
two-years duration to determine if there may be a potential for
carcinogenicity. Reproduction and teratogenicity studies on folpet,
have been carried out on rats, hamsters, and two strains of rabbits.
No evidence of teratogenic effects has been reported. As there are
indications of toxic effects on the mothers in the teratogenicity
studies with hamsters and rabbits, it is recommended that further
studies be done on these two species. Because of the uncertainty
regarding embryotoxicity, and since the long-term study in rats was
only 17 months' duration, a temporary adi is recommended.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Rat: 3,200 ppm in diet, equivalent to 160 mg/kg body-weight/day
Dog: 1,000 mg/kg body-weight/day
Estimate of temporary acceptable daily intake for man
0-0.16 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Folpet is a protective fungicide used mainly for foliage application
at about 0.1 percent active ingredient.
Pre-harvest treatments
Rates of application and intervals between treatment and harvest are:
Citrus fruits - 0.12 percent active ingredients applied
at 2/3 petal fall, 2 weeks after petal
fall, on full flush growth in August and
September. Use up to harvest.
Other fruits - 0.09-0.12 percent active ingredient,
applied as needed at 7-14 day intervals
up to harvest.
Avocados - 0.18 percent active ingredient, applied
as needed at 2 to 3 week intervals up to
harvest.
Small fruits and berries - 1.1 to 2.2 kg a.i./ha, applied as needed
at a 7 - 10 day intervals up to harvest.
Cranberries - 5.0 kg a.i./ha, applied as needed at
10-14 day intervals up to 30 days of
harvest.
Vegetables - 1.1 to 4.5 kg a.i./ha, applied as needed
at 7 to 10 day intervals up to harvest.
Celery - Same schedule up to 7 days of harvest.
Post-harvest treatments
No post-harvest treatments are recommended.
Other uses
Ornamentals - 0.12 percent a.i., applied as needed at 7-10 day
intervals.
RESIDUES RESULTING FROM SUPERVISED TRIALS
The residue data are from treatments made under commercial conditions
in the U.S.A. (Table I). It is found that the residue patterns of
folpet follow those of captan. Rain causes a loss of both compounds.
The development of the wax as the fruit ripens tends to retain the
residue. Generally the initial level of folpet is reduced by one half
within a week or two. The residue levels of the fruit pulp and fruit
juice are found much lower than those of the parent whole fruits.
TABLE I
Residues from field trials (Dye, 1969)
Rate of Pre-harvest Folpet
application Number of interval residue
Crop (kg a.i. per ha) treatments (days) (ppm)
Apples (0.1% to run-off) 1 0-3 2.2-10.5
Blueberries 2.8 (0.1%) 1-6 0-2 7.0-23.4
Cherries (0.1-0.2%) 3-5 0-1 3.7-8.9
Fresh currants 0.25-0.37 1-5 0-1 8.0-33.0
Grapes 2.7-3.4 (0.1%) 1-4 0-1 7.6-25.0
Grapefruits (0.1-0.2%) 1 0-1 2.4-5.9
Oranges (0.1-0.2%) 1 0-1 2.9-8.1
Raspberries 1.1-2.8 (0.1%) 1-3 0-1 4.6-13.9
Strawberries 0.3-2.8 1-8 0-1 1.7-5.7
Cantaloup,
whole 2.3 1 0-1 0.6-1.1
Cucumber 2.3 1 0-1 0.8-1.9
Onion, bulb (0.1%) 7-8 0-7 0.4-1.2
Tomatoes 1.0-2.3 1 0-1 1.7-4.1
Watermelons,
rind (0.2%) 14 0.0-0.3
pulp (0.2%) 14 0.0-0.1
FATE OF RESIDUES
General comments
The remarks under 'BIOCHEMICAL ASPECTS' earlier in this monograph,
particularly those relating to the hydrolysis of folpet, pertain to
the behaviour of residues in crops or foods both before and after
consumption.
In animals
In animals folpet is expected to be subject to the similar degradative
reactions as in plants but no actual data have been available for
evaluation.
In plants
No published data are available from actual studies of the residues in
plants. It is highly probable however that degradation products of
hydrolysis as outlined above could be found; but this requires further
confirmatory studies.
In soil
The instability of the compound would not permit build-up in soils.
However, no experimental data were available for confirmation.
In storage and processing
No general quantitative estimates about the effect of washing on the
folpet residues can be made. It is, however, believed that a
relatively high portion of residues could be removed. This is
supported by a single figure on oranges on which washing reduced a
residue of 8.1 ppm down to 1.1 ppm (Dye, 1969).
Peeling of fruits is also expected to reduce folpet residues even more
than washing but lack of data does not allow definite conclusions to
be drawn.
In refrigerated storage as well as in frozen products folpet residues
are expected to be very stable. Any special treatment, e.g. blanching,
before such storage however may reduce the folpet residues. Data are
not available for final evaluation. The only figures available
concerned frozen cherries which contained a folpet residue about 10
percent of the residue level of unprocessed cherries (Dye, 1969).
Juice extracted from folpet treated grapes have contained small
amounts of folpet. The residues in juice in most instances were less
than 1 ppm although there were few figures up to 5 ppm (Dye, 1969).
The possible occurrence of folpet in grape juice is of practical
importance because folpet can cause slight suppression of fermentation
and produce a poor flavour in wines (Chalkov and Vanev, 1968).
The canning process is expected to be very destructive to folpet
residues. The only data available are for canned cherries which were
found to contain a residue less than the detection limit of the
analytical method although the unprocessed cherries had residues up to
5.6 ppm (Dye, 1969).
METHODS OF RESIDUE ANALYSIS
A method of residue analysis (Anon., 1960) is based on extraction with
benzene, clean-up with activated carbon, and reaction with resorcinol
to give a yellow product which is measured in a colorimeter. This
method, however, cannot distinguish between folpet and captan, but
gives the sum of these two compounds. Folpet does not migrate into
plant tissues; surface stripping is considered adequate for removing
residues.
A method is developed to distinguish between folpet and captan (Anon.,
1961). The sensitivity of the method is not satisfactory.
Polarographic methods have been set up for determining derivatives of
folpet and captan (Anon., 1962a; 1962b; Nangniot, 1966).
Gas chromatographic methods developed for captan (Kilgore et al.,
1967); Bevenue and Ogata, 1968; Pomerantz and Ross, 1968) and
detecting residues an low as 0.01 ppm can be used for folpet as well.
Folpet has been determined with sufficient accuracy at a residue level
of 2 ppm in red and white wines by an electron capture gas
chromatographic method (Matta, 1968) using pentane for extraction and
Florisil for cleanup.
For regulatory purposes the GLC method has to be further developed.
NATIONAL TOLERANCES
Tolerance
Country (ppm) Crop
Canada 30 Celery
Canada 25 Apples, avocados, blackberries,
blueberries, boysenberries,
cantaloups, cherries, citrus
fruits, cranberries, crabapples,
cucumber, currants, dewberries,
garlic, gooseberries, grapes,
honeydew melons, huckleberries,
leeks, lettuce, loganberries,
muskmelons, onions, pumpkins,
raspberries, shallots,
strawberries, summer squash,
tomatoes, watermelons, winter
squash
(continued)
Tolerance
Country (ppm) Crop
Germany (Fed.Rep.) 15 Fruits, grapes, hops
Hungary 10 Fruits, grapes
Netherlands 20 All crops
United States of
America 50 Celery, cherries, leeks,
lettuce, onions (green),
shallots
United States of 25 Apples, avocados, blackberries,
America blueberries, boysenberries,
crabapples, cranberries,
currants, dewberries,
gooseberries, grapes,
huckleberries, loganberries,
raspberries, strawberries,
tomatoes
United States of 15 Cucumbers, garlic, melons,
America onions (dry bulb), pumpkins,
summer squash, winter squash
United States of 15 Citrus fruits (interim
America tolerance)
APPRAISAL
Folpet is used to control fungus diseases on tree fruits, citrus
fruits, avocadoes, small fruits and berries, vegetables and
ornamentals. Concentration of the foliage sprays is recommended to
about 0.1 percent active ingredient. Folpet in nonphytotoxic, though
some injuries are reported on pears and apples. It in stable when dry,
but hydrolyses slowly in water at room temperature, rapidly at higher
temperatures or under alkaline conditions. National tolerances
established for the residues of folpet in various commodities vary
from 10 to 50 ppm. Folpet is formulated as 50 percent and 75 percent
wettable powder.
The residue data available to the Meeting were from treatments made
under commercial conditions in the U.S.A. A large variety of crops
were covered. It is found that the residue patterns of folpet follow
those of captan. The wash-off effect of rain may be pronounced.
Generally the initial level of folpet is reduced by one half within a
week or two. The residue levels of the fruit pulp and fruit juice are
found much lower than those of the entire parent fruits. The effect of
processing on the residues may be pronounced.
The main degradation mechanisms of folpet in plants are postulated to
be the same as those of captan resulting from the reaction with
sulfhydryl compounds, mainly phthalimide, to phthalic acid, free
chlorine ion, and inorganic sulphur compounds. The degradation
products of folpet in plants are assumed to be the same as in animals.
The documentation of folpet refers to a colorimetric analysis of
residues of folpet. The method uses a colour reaction with resorcinol
and is not specific for folpet. (It reacts with captan, too). For
differentiating folpet and captan, UV spectrometry can be applied.
Good agricultural practice was considered to produce residue levels
determined one day after application as indicated under the following
headings:
RECOMMENDATION FOR TOLERANCES, TEMPORARY TOLERANCES OR
PRACTICAL RESIDUE LIMITS
TEMPORARY TOLERANCES (effective to 1973)
Apples 10 ppm
Blueberries 25 ppm
Cantaloups, whole fruit 2 ppm
Cherries 15 ppm
Citrus fruits 10 ppm
Cucumbers 2 ppm
Currants, fresh 30 ppm
Grapes 25 ppm
Onions 2 ppm
Raspberries 15 ppm
Strawberries 5 ppm
Tomatoes 5 ppm
Water melons 2 ppm
Insufficient information was available to enable evaluation of
residues or tolerances to be suggested for blackberries, cranberries
and celery.
FURTHER WORK OR INFORMATION
REQUIRED (before 30 June 1973)
1. Long-term studies of sufficient duration to test for possible
carcinogenic effects.
2. Additional studies on the effects of the compound on reproductive
physiology.
3. Further studies on metabolism, especially on the
trichloromethylthio-moiety.
4. Further data on the nature of terminal residues in plants as well
as on the magnitude of the degradation product: magnitude in
relation to the parent compound or toxicological importance.
5. Further data on the degradation mechanisms of folpet.
6. Data on the necessary rates and frequencies of application,
pre-harvest intervals and the resultant residues, from countries
other than the U.S.A.
7. Data on residue levels in raw agricultural products moving in
commerce.
8. Qualitative and quantitative data on fate of residues in washing,
blanching, storing and thermal processing of the treated crops.
DESIRABLE
1. Information on the fate of the compound in soil.
2. Evaluation of the analytical methods by collaborative studies
taking into account the possible presence of structurally related
compounds, e.g. captan and captafol.
REFERENCES
Anon. (1960) The analysis of residues of captan and folpet. Residue
method RM-1. Chevron Chemical Co. Unpub. Rept.
Anon. (1961) The determination of and differentiation between
residues of Phaltan and captan. Residue method RM-1A. Chevron
Chemical Co. Unpub. Rept.
Anon. (1962a) Centre de recherches de phytopharmacie à Gembloux
(Belgique) Résultats inédits
Anon. (1962b) Institut agronomique de l'etat (Gembloux-Belgique).
Résultats inédits
Arnold, D., Kodras, R. and Fancher, O.E. (1968) Teratogenicity study
on Phaltan Golden Syrian hamsters Unpub. Rept. of Industrial
Bio-Test Laboratories, submitted by Chevron Chemical Co.
Bevenue, A., and Ogata, J.N. (1968) The examination of mixtures of
captan and Phaltan by gas chromatography. J. Chromatog. 36:529-31
Chalkov, I. and Vanev, S. (1968) Determination of the effect of some
new fungicides, used to control gray rot in grapes under field
conditions, on the enzymic activity of yeasts. Lozarstvo Vinar
(Sofia) 17:33-40 (Chem. Abstr. 69:34 908 s, 1968)
Dye, D.F. (1969) Folpet. Unpub. Summary Rept. submitted to FAO and
WHO by Chevron Chemical Co.
Elsea, J.R. (1956) Phthalimide captan analog. Acute oral
administration. Unpub. Rept. of Hazleton Laboratories submitted by
Chevron Chemical Co.
Kay, J.H. and Calandra, J.C. (1960) Acute percutaneous and eye
irritation studies on Phaltan. Unpub. Rept. of Industrial Bio-Test
Laboratories submitted by Chevron Chemical Co.
Kay, J.H. and Calandra, J.C. (1961a) Chronic oral toxicity of
Phaltan. Pure bread beagle dogs, (with addendum report. Pathological
findings). Unpub. Rept. of Industrial Bio-Test Laboratories
submitted by Chevron Chemical Co.
Kay, J.H. and Calandra, J.C. (1961b) Chronic oral toxicity of
Phaltan. Albino rats. Unpub. Rept. of Industrial Bio-Test
Laboratories submitted by Chevron Chemical Co.
Kennedy, G., Fancher, O.E. and Calandra J.C. (1967a) Three generation
reproduction study in albino rats-Phaltan. Final Rept. Unpub.
Rept. of Industrial Bio-Test Laboratories submitted by Chevron
Chemical Co.
Kennedy, G., Fancher, O.E. and Calandra, J.C. (1967b) Rat
teratogenicity study. Captan, Difolatan and Phaltan. Unpub. Rept.
of Industrial Bio-Test Laboratories submitted by Chevron Chemical Co.
Kennedy, G., Jackson, G., Fancher, O.E. and Calandra, J.C. (1967c)
Rabbit teratogenicity study. Captan and Phaltan. Unpub. Rept. of
Industrial Bio-Test Laboratories submitted by Chevron Chemical Co.
Kennedy, G., Fancher, O.E. and Calandra, J.C. (1968) An investigation
of the teratogenic potential of captan, folpet and Difolatan. Toxicol.
appl. Pharmacol. 13:42-430
Kilgore, W.W., Winterlin, W. and White, R. (1967) Gas chromatographic
determinations of captan residues. J. Agr. Food Chem. 15:1035-37
Matta, M. (1968) Gas chromatographic determination of chlorinated
pesticide residues in wine. Vini Ital. 10:171-4 (Chem. Abstr.
69:66469 r, 1968)
McLaughlin, J., Jr., Reynaldo, E.F., Lamar, J.K. and Marliac, J.P.
(1969) Teratology studies in rabbits with captan, folpet and
Thalidomide. Toxicol. appl. Pharmacol. 14:641
Nangniot, P. (1966) L'application des méthodes électrochimiques à
l'étude des résidus de pesticides. Meded Rijksfac. Landbouwwetensch.
31:447-73
Owens, R. G. (1969) Metabolism of fungicides and related compounds.
Ann. N.Y. Acad. Sci. 100:144-52
Palazzolo, R., Fancher, O.E. and Calandra, J.C. (1966) Rabbit
reproduction study. Phthalimide. Unpub. Rept. of Industrial Bio-Test
Laboratories submitted by Chevron Chemical Co.
Pomerantz, I.H. and Rose, R. (1968) Captan and structurally related
compounds: thin-layer and gas-liquid chromatography. J. Assoc.
Offic. Anal. Chem. 51:1058-62
Varrett, M.J., Mutchler, M.K., Scott, W.F., Reynaldo, E.F. and
McLaughlin, J. (1969) Teratogenic effects of captan and related
compounds in the developing chick embryo. Ann. N.Y. Acad. Sci.
160:334-43
Vondruska, J.F. (1969) Teratologic investigation of Phaltan in
Macaca mulatta (Rhesus monkey) and Macaca arctoides
(stumptailed macque).
Unpub. Rept. of Industrial Bio-Test Laboratories submitted by
Chevron Chemical Co.
Weir, R.J. (1956) N-trichloromethylthiophthalimide, recrystallized
phthalimide, (phthalimide analog of captan). Subacute
feeding-rats. Supplement to report dated 23 July, 1956. Unpub.
Rept. submitted by Hazleton Laboratories to California Spray -
Chemical Co.
Because the group attached to the imide nitrogen is the same in folpet
as it is in captan, it is reasonable to assume that in the presence of
sulfhydryl groups (e.g. as in cysteine) the trichloromethylthio group
will break down to give chloride ions by a series of reactions similar
to those described in the monograph on captan (Owens, 1969).
Because the trichloromethylthio moiety is the same in both captan and
folpet, the only difference in the two compounds being that the ring
portion of folpet is aromatic, it has been assumed that all metabolic
data for captan relative to the trichloromethylthio portion of the
molecule will also be applicable to folpet (Dye, 1969) (see the
monograph on captan).
Special studies on reproduction
Rat
Four groups, each of 8 male and 16 female rats, were fed technical
folpet (94.4 percent purity) at dietary levels of 0, 100, 500 and 1000
ppm (active ingredient) in a three-generation study. Parental animals
were on the test diet for 79 days before mating and continuously
thereafter. There were no adverse effects on body-weight gain or
terminal organ-weights of parental animals, or on reproductive
performance, fertility, lactation, litter-size or incidence of
stillbirths in any test group. Pup survival at 5 and 21 days, and
weanling weights in all test groups were comparable with the controls.
Histopathology performed on parental animals of each generation and on
F3b weanlings in the 0 and 1000 ppm groups revealed no changes which
could be correlated with the ingestion of folpet (Kennedy et al.,
1967a).
Special studies on teratogenicity
Chicken-egg
Folpet was injected in dimethylsulphoxide solution into the yolk or
air cell of fresh fertile chicken eggs at levels which varied from 3
to 20 mg/kg egg-weight. The eggs were incubated and non-viable embryos
and hatched chicks were examined for gross abnormalities. In a total
of 830 eggs injected with folpet the incidence of malformations was
8.19 percent. The metabolites of folpet, phthalimide (305 eggs) and
phthalic acid (290 eggs) were also injected under similar conditions
using dimethylsulphoxide as a solvent for phthalimide and ethanol for
phthalic acid. A control group comprising over 1500 eggs was also
injected with dimethylsulphoxide alone and another group of several
thousand eggs with ethanol alone. The incidence of malformation was
3.93 percent for phthalimide, 3.10 percent for phthalic acid and less
than 2.0 percent for the controls. Micromelia amelia and phocomelia
accounted for most of the deformities (Varrett et al., 1969).
Hamster
Groups of 10 pregnant Syrian Golden hamsters were fed technical folpet
at dietary levels approximately equivalent to 0, 125, 250, 500 or 1000
(eight animals only) mg/kg body-weight per day from gestation days 4
to 15 inclusive. On day 15 of gestation all animals were sacrificed
and foetal examination was carried out. Maternal body-weight gains
were decreased over the feeding period in the 250 and 500 mg/kg groups
while at the highest level there was a weight loss recorded. There was
an increase in the number of resorption sites at the two highest
dose-levels compared with the control group. Growth appeared to be
retarded in the foetuses from all test groups. The mean number of
foetuses per litter was reduced at the two highest dose-levels. There
were no gross or skeletal abnormalities attributable to folpet at any
of the levels used (Arnold et al., 1968).
A companion study, in which folpet was given by intubation, was
carried out. Hamsters were given a single dose of 0 (11 pregnant
females), 125 (9 pregnant females), 250 (5) or 500 (8) mg/kg
body-weight, half of each group being intubated on day 7 and the
remainder on day 8. A positive control group was given thalidomide,
1000 mg/kg body-weight, on day 7. In this experiment the number of
foetal resorption sites in all groups was higher than normal. The high
incidence was in the 125 mg/kg group (5.8 per litter) and the low
incidence was in the 500 mg/kg group (2.5 per litter). The number of
young per litter was lower in all test groups than in the control.
There were no gross physical or skeletal anomalies in the test groups
which could be associated with folpet treatment. No abnormalities were
found in the group treated with thalidomide (Arnold et al., 1968).
Monkey
Groups of from four to six pregnant monkeys (Rhesus and stumptailed
macque) were given oral doses of 10, 25 and 75 mg/kg body-weight of
folpet daily on days 21 through 34 of gestation. Thalidomide was given
as a positive control to groups of 9 or 11 monkeys at levels 5 and 10
mg/kg body-weight respectively. All foetuses from animals given folpet
were grossly normal except for one Rhesus foetus each from the 25 and
75 mg/kg dose-levels which had 13 pairs of ribs. No abortions occurred
at the 25 and 75 mg/kg levels of folpet but one abortion occurred at
the 10 mg/kg level in day 54 of gestation. Abortions and foetal
deformities occurred in the groups given thalidomide (Vondruska,
1969).
Rabbit
Groups of pregnant New Zealand albino rabbits were given folpet at
dose-levels of 0, 18.75 (5 rabbits), 37.5 (5) or 75 (7) mg/kg
body-weight on gestation days 6 to 18 inclusive. The dose was
administered by gelatin capsule. Positive control animals were given
thalidomide at various dose levels. On day 29 each doe was sacrificed
and the young removed by Caesarean section. Folpet produced signs of
toxicity in the dose. At the two higher dose levels there was a loss
in body-weight over the period of treatment (days 6 to 18). Also at
these dose-levels the incidence of foetal resorption was higher than
in the negative control group. There appeared to be a compound-related
effect on mortality. Examination of 80 embryos from folpet-treated
rabbits revealed no gross abnormalities; internal structural formation
was normal and well-defined skeletal development was observed.
Thalidomide treatment in the positive controls resulted in malformed
young (Kennedy et al., 1967c; Kennedy et al., 1968).
Groups of pregnant New Zealand white rabbits were given folpet at dose
levels of 75 or 150 mg/kg body-weight. Thalidomide was also given to a
positive control group at the same dose-levels. Thalidomide but not
folpet produced a teratological response (McLaughlin et al., 1969).
Rat
Pregnant female rats of the Charles River strain were given 0, 100 (10
animals) or 500 (5 animals) mg/kg body-weight of technical folpet by
oral intubation on days 6 to 15 for the lower dose and days 8 to 10
for the higher dose-level. Trypan Blue was given by subcutaneous
injection, 50 mg/kg body-weight, on days 8 to 10 to a fourth group
serving as a positive control. All rats were sacrificed on the
twentieth day of gestation. Examination of a total of 169 foetuses
revealed no significant increase in the incidence of abnormalities in
the groups given folpet. Internal structural formation was normal, the
young were present in normal numbers, and were well-formed, Trypan
Blue treatments produced malformed young as expected (Kennedy et al.,
1967b).
A group of 10 pregnant female rats (Charles River and Sprague Dawley
derived strain) was given oral doses of 100 mg/kg body-weight/day of
folpet from day 6 to day 15 of gestation and another group of four
pregnant rats was given oral doses of 500 mg/kg body-weight/day from
day 8 to day 10. Examination of 120 foetuses in the 100 mg/kg group,
49 foetuses in the 500 mg/kg group and 200 foetuses from an untreated
control group gave no evidence of abnormalities related to the
administration of folpet (Kennedy et al., 1968).
Studies on the metabolite phthalimide
Rabbit (teratogenic study)
Groups of 10 female Dutch Belted Rabbits were given 0 or 75 mg/kg
body-weight of phthalimide (the hydrolytic metabolite of folpet), via
gelatin capsule, on day 6 through 16. A treated control group received
75 mg/kg of thalidomide over the same period. On day 28 the rabbits
were killed, the young removed by Caesarean section, and examined for
abnormalities. No adverse effects of phthalimide were noted on the
parental females or on the 24-hour survival rate of the young. No
abnormalities, external, internal or skeletal, were seen in the test
group but were present in the treated controls. Resorption of foetuses
occurred in three of seven does in the thalidomide group and in one of
ten in the phthalimide group. This animal lost 220 grams in weight
between day 11 and 16. Three pups were aborted on day 25, and three
resorption sites were present which accounted for all the implantation
sites
Acute toxicity
LD50mg/kg
Animal Route body-weight References
Rat oral >10,000 Elsea, 1956
Rabbit percutaneous >22,600 Kay and Calandra, 1960
Short-term studies
Dog
Four groups of dogs (three males and three females per group) were
given folpet at dose-levels of 0, 250, 1000 and 1500 mg/kg
body-weight. The compound was given orally by capsule 5 days a week
for 17 months. At 12 months 2-3 dogs (male and female) from each group
were sacrificed for pathologic study. The remainder were sacrificed
for pathological study after 17 months. Total mean weight-gain over
the duration of the test was depressed in both sexes at the highest
dose-level. None of the animals died during the test. Haematologic
studies, urinalyses, liver function tests (bromosulphthalein
retention), serum alkaline phosphatase and blood urea nitrogen
determinations all showed no unusual findings. There were no gross or
microscopic pathologic changes which could be correlated with the test
material (Key and Calandra, 1961a).
Rat
Groups each comprising 10 male and 10 female rats were fed dietary
levels of 0, 0.1, 0.32 and 1 percent of folpet for 12 weeks. Growth
was normal except in the male rats fed the 1 percent level where there
was a significant decrease. There were no gross abnormalities.
Histopathological examination of liver, kidneys, adrenals, intestines,
lungs and gonads of two male and two female animals of each group
revealed no abnormalities (Weir, 1956).
Long-term studies
Rat
Folpet was fed to four groups of rats at dietary levels of 0, 0.1,
0.32 and 1.0 percent for 17 months. Each test group consisted of 30
males and 30 females, with 60 rats of each sex in the control group.
After 12 months on the test, 5 males and 5 females of each test group
(10 of each in the controls), were killed for pathologic study.
Body-weight data showed a slight adverse effect on growth of rats fed
1 percent folpet in their diet. An increase in spleen to body-weight
ratio in this group, observed at 12 months was not present in the rats
examined after 17 months on the diet. There were no effects on
mortality, tumour incidence, haematologic studies, urinalysis, gross
or histopathology that were attributable to the feeding of folpet (Kay
and Calandra, 1961b).
COMMENT
In the two species used in acute toxicity studies, an LD50 value was
not obtained. The 17-month study on dogs and the 17-month rat study
indicate a high tolerance, by these species, to chronic exposure to
this material. These studies should, however, have been of at least
two-years duration to determine if there may be a potential for
carcinogenicity. Reproduction and teratogenicity studies on folpet,
have been carried out on rats, hamsters, and two strains of rabbits.
No evidence of teratogenic effects has been reported. As there are
indications of toxic effects on the mothers in the teratogenicity
studies with hamsters and rabbits, it is recommended that further
studies be done on these two species. Because of the uncertainty
regarding embryotoxicity, and since the long-term study in rats was
only 17 months' duration, a temporary adi is recommended.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Rat: 3,200 ppm in diet, equivalent to 160 mg/kg body-weight/day
Dog: 1,000 mg/kg body-weight/day
Estimate of temporary acceptable daily intake for man
0-0.16 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Folpet is a protective fungicide used mainly for foliage application
at about 0.1 percent active ingredient.
Pre-harvest treatments
Rates of application and intervals between treatment and harvest are:
Citrus fruits - 0.12 percent active ingredients applied
at 2/3 petal fall, 2 weeks after petal
fall, on full flush growth in August and
September. Use up to harvest.
Other fruits - 0.09-0.12 percent active ingredient,
applied as needed at 7-14 day intervals
up to harvest.
Avocados - 0.18 percent active ingredient, applied
as needed at 2 to 3 week intervals up to
harvest.
Small fruits and berries - 1.1 to 2.2 kg a.i./ha, applied as needed
at a 7 - 10 day intervals up to harvest.
Cranberries - 5.0 kg a.i./ha, applied as needed at
10-14 day intervals up to 30 days of
harvest.
Vegetables - 1.1 to 4.5 kg a.i./ha, applied as needed
at 7 to 10 day intervals up to harvest.
Celery - Same schedule up to 7 days of harvest.
Post-harvest treatments
No post-harvest treatments are recommended.
Other uses
Ornamentals - 0.12 percent a.i., applied as needed at 7-10 day
intervals.
RESIDUES RESULTING FROM SUPERVISED TRIALS
The residue data are from treatments made under commercial conditions
in the U.S.A. (Table I). It is found that the residue patterns of
folpet follow those of captan. Rain causes a loss of both compounds.
The development of the wax as the fruit ripens tends to retain the
residue. Generally the initial level of folpet is reduced by one half
within a week or two. The residue levels of the fruit pulp and fruit
juice are found much lower than those of the parent whole fruits.
TABLE I
Residues from field trials (Dye, 1969)
Rate of Pre-harvest Folpet
application Number of interval residue
Crop (kg a.i. per ha) treatments (days) (ppm)
Apples (0.1% to run-off) 1 0-3 2.2-10.5
Blueberries 2.8 (0.1%) 1-6 0-2 7.0-23.4
Cherries (0.1-0.2%) 3-5 0-1 3.7-8.9
Fresh currants 0.25-0.37 1-5 0-1 8.0-33.0
Grapes 2.7-3.4 (0.1%) 1-4 0-1 7.6-25.0
Grapefruits (0.1-0.2%) 1 0-1 2.4-5.9
Oranges (0.1-0.2%) 1 0-1 2.9-8.1
Raspberries 1.1-2.8 (0.1%) 1-3 0-1 4.6-13.9
Strawberries 0.3-2.8 1-8 0-1 1.7-5.7
Cantaloup,
whole 2.3 1 0-1 0.6-1.1
Cucumber 2.3 1 0-1 0.8-1.9
Onion, bulb (0.1%) 7-8 0-7 0.4-1.2
Tomatoes 1.0-2.3 1 0-1 1.7-4.1
Watermelons,
rind (0.2%) 14 0.0-0.3
pulp (0.2%) 14 0.0-0.1
FATE OF RESIDUES
General comments
The remarks under 'BIOCHEMICAL ASPECTS' earlier in this monograph,
particularly those relating to the hydrolysis of folpet, pertain to
the behaviour of residues in crops or foods both before and after
consumption.
In animals
In animals folpet is expected to be subject to the similar degradative
reactions as in plants but no actual data have been available for
evaluation.
In plants
No published data are available from actual studies of the residues in
plants. It is highly probable however that degradation products of
hydrolysis as outlined above could be found; but this requires further
confirmatory studies.
In soil
The instability of the compound would not permit build-up in soils.
However, no experimental data were available for confirmation.
In storage and processing
No general quantitative estimates about the effect of washing on the
folpet residues can be made. It is, however, believed that a
relatively high portion of residues could be removed. This is
supported by a single figure on oranges on which washing reduced a
residue of 8.1 ppm down to 1.1 ppm (Dye, 1969).
Peeling of fruits is also expected to reduce folpet residues even more
than washing but lack of data does not allow definite conclusions to
be drawn.
In refrigerated storage as well as in frozen products folpet residues
are expected to be very stable. Any special treatment, e.g. blanching,
before such storage however may reduce the folpet residues. Data are
not available for final evaluation. The only figures available
concerned frozen cherries which contained a folpet residue about 10
percent of the residue level of unprocessed cherries (Dye, 1969).
Juice extracted from folpet treated grapes have contained small
amounts of folpet. The residues in juice in most instances were less
than 1 ppm although there were few figures up to 5 ppm (Dye, 1969).
The possible occurrence of folpet in grape juice is of practical
importance because folpet can cause slight suppression of fermentation
and produce a poor flavour in wines (Chalkov and Vanev, 1968).
The canning process is expected to be very destructive to folpet
residues. The only data available are for canned cherries which were
found to contain a residue less than the detection limit of the
analytical method although the unprocessed cherries had residues up to
5.6 ppm (Dye, 1969).
METHODS OF RESIDUE ANALYSIS
A method of residue analysis (Anon., 1960) is based on extraction with
benzene, clean-up with activated carbon, and reaction with resorcinol
to give a yellow product which is measured in a colorimeter. This
method, however, cannot distinguish between folpet and captan, but
gives the sum of these two compounds. Folpet does not migrate into
plant tissues; surface stripping is considered adequate for removing
residues.
A method is developed to distinguish between folpet and captan (Anon.,
1961). The sensitivity of the method is not satisfactory.
Polarographic methods have been set up for determining derivatives of
folpet and captan (Anon., 1962a; 1962b; Nangniot, 1966).
Gas chromatographic methods developed for captan (Kilgore et al.,
1967); Bevenue and Ogata, 1968; Pomerantz and Ross, 1968) and
detecting residues an low as 0.01 ppm can be used for folpet as well.
Folpet has been determined with sufficient accuracy at a residue level
of 2 ppm in red and white wines by an electron capture gas
chromatographic method (Matta, 1968) using pentane for extraction and
Florisil for cleanup.
For regulatory purposes the GLC method has to be further developed.
NATIONAL TOLERANCES
Tolerance
Country (ppm) Crop
Canada 30 Celery
Canada 25 Apples, avocados, blackberries,
blueberries, boysenberries,
cantaloups, cherries, citrus
fruits, cranberries, crabapples,
cucumber, currants, dewberries,
garlic, gooseberries, grapes,
honeydew melons, huckleberries,
leeks, lettuce, loganberries,
muskmelons, onions, pumpkins,
raspberries, shallots,
strawberries, summer squash,
tomatoes, watermelons, winter
squash
(continued)
Tolerance
Country (ppm) Crop
Germany (Fed.Rep.) 15 Fruits, grapes, hops
Hungary 10 Fruits, grapes
Netherlands 20 All crops
United States of
America 50 Celery, cherries, leeks,
lettuce, onions (green),
shallots
United States of 25 Apples, avocados, blackberries,
America blueberries, boysenberries,
crabapples, cranberries,
currants, dewberries,
gooseberries, grapes,
huckleberries, loganberries,
raspberries, strawberries,
tomatoes
United States of 15 Cucumbers, garlic, melons,
America onions (dry bulb), pumpkins,
summer squash, winter squash
United States of 15 Citrus fruits (interim
America tolerance)
APPRAISAL
Folpet is used to control fungus diseases on tree fruits, citrus
fruits, avocadoes, small fruits and berries, vegetables and
ornamentals. Concentration of the foliage sprays is recommended to
about 0.1 percent active ingredient. Folpet in nonphytotoxic, though
some injuries are reported on pears and apples. It in stable when dry,
but hydrolyses slowly in water at room temperature, rapidly at higher
temperatures or under alkaline conditions. National tolerances
established for the residues of folpet in various commodities vary
from 10 to 50 ppm. Folpet is formulated as 50 percent and 75 percent
wettable powder.
The residue data available to the Meeting were from treatments made
under commercial conditions in the U.S.A. A large variety of crops
were covered. It is found that the residue patterns of folpet follow
those of captan. The wash-off effect of rain may be pronounced.
Generally the initial level of folpet is reduced by one half within a
week or two. The residue levels of the fruit pulp and fruit juice are
found much lower than those of the entire parent fruits. The effect of
processing on the residues may be pronounced.
The main degradation mechanisms of folpet in plants are postulated to
be the same as those of captan resulting from the reaction with
sulfhydryl compounds, mainly phthalimide, to phthalic acid, free
chlorine ion, and inorganic sulphur compounds. The degradation
products of folpet in plants are assumed to be the same as in animals.
The documentation of folpet refers to a colorimetric analysis of
residues of folpet. The method uses a colour reaction with resorcinol
and is not specific for folpet. (It reacts with captan, too). For
differentiating folpet and captan, UV spectrometry can be applied.
Good agricultural practice was considered to produce residue levels
determined one day after application as indicated under the following
headings:
RECOMMENDATION FOR TOLERANCES, TEMPORARY TOLERANCES OR
PRACTICAL RESIDUE LIMITS
TEMPORARY TOLERANCES (effective to 1973)
Apples 10 ppm
Blueberries 25 ppm
Cantaloups, whole fruit 2 ppm
Cherries 15 ppm
Citrus fruits 10 ppm
Cucumbers 2 ppm
Currants, fresh 30 ppm
Grapes 25 ppm
Onions 2 ppm
Raspberries 15 ppm
Strawberries 5 ppm
Tomatoes 5 ppm
Water melons 2 ppm
Insufficient information was available to enable evaluation of
residues or tolerances to be suggested for blackberries, cranberries
and celery.
FURTHER WORK OR INFORMATION
REQUIRED (before 30 June 1973)
1. Long-term studies of sufficient duration to test for possible
carcinogenic effects.
2. Additional studies on the effects of the compound on reproductive
physiology.
3. Further studies on metabolism, especially on the
trichloromethylthio-moiety.
4. Further data on the nature of terminal residues in plants as well
as on the magnitude of the degradation product: magnitude in
relation to the parent compound or toxicological importance.
5. Further data on the degradation mechanisms of folpet.
6. Data on the necessary rates and frequencies of application,
pre-harvest intervals and the resultant residues, from countries
other than the U.S.A.
7. Data on residue levels in raw agricultural products moving in
commerce.
8. Qualitative and quantitative data on fate of residues in washing,
blanching, storing and thermal processing of the treated crops.
DESIRABLE
1. Information on the fate of the compound in soil.
2. Evaluation of the analytical methods by collaborative studies
taking into account the possible presence of structurally related
compounds, e.g. captan and captafol.
REFERENCES
Anon. (1960) The analysis of residues of captan and folpet. Residue
method RM-1. Chevron Chemical Co. Unpub. Rept.
Anon. (1961) The determination of and differentiation between
residues of Phaltan and captan. Residue method RM-1A. Chevron
Chemical Co. Unpub. Rept.
Anon. (1962a) Centre de recherches de phytopharmacie à Gembloux
(Belgique) Résultats inédits
Anon. (1962b) Institut agronomique de l'etat (Gembloux-Belgique).
Résultats inédits
Arnold, D., Kodras, R. and Fancher, O.E. (1968) Teratogenicity study
on Phaltan Golden Syrian hamsters Unpub. Rept. of Industrial
Bio-Test Laboratories, submitted by Chevron Chemical Co.
Bevenue, A., and Ogata, J.N. (1968) The examination of mixtures of
captan and Phaltan by gas chromatography. J. Chromatog. 36:529-31
Chalkov, I. and Vanev, S. (1968) Determination of the effect of some
new fungicides, used to control gray rot in grapes under field
conditions, on the enzymic activity of yeasts. Lozarstvo Vinar
(Sofia) 17:33-40 (Chem. Abstr. 69:34 908 s, 1968)
Dye, D.F. (1969) Folpet. Unpub. Summary Rept. submitted to FAO and
WHO by Chevron Chemical Co.
Elsea, J.R. (1956) Phthalimide captan analog. Acute oral
administration. Unpub. Rept. of Hazleton Laboratories submitted by
Chevron Chemical Co.
Kay, J.H. and Calandra, J.C. (1960) Acute percutaneous and eye
irritation studies on Phaltan. Unpub. Rept. of Industrial Bio-Test
Laboratories submitted by Chevron Chemical Co.
Kay, J.H. and Calandra, J.C. (1961a) Chronic oral toxicity of
Phaltan. Pure bread beagle dogs, (with addendum report. Pathological
findings). Unpub. Rept. of Industrial Bio-Test Laboratories
submitted by Chevron Chemical Co.
Kay, J.H. and Calandra, J.C. (1961b) Chronic oral toxicity of
Phaltan. Albino rats. Unpub. Rept. of Industrial Bio-Test
Laboratories submitted by Chevron Chemical Co.
Kennedy, G., Fancher, O.E. and Calandra J.C. (1967a) Three generation
reproduction study in albino rats-Phaltan. Final Rept. Unpub.
Rept. of Industrial Bio-Test Laboratories submitted by Chevron
Chemical Co.
Kennedy, G., Fancher, O.E. and Calandra, J.C. (1967b) Rat
teratogenicity study. Captan, Difolatan and Phaltan. Unpub. Rept.
of Industrial Bio-Test Laboratories submitted by Chevron Chemical Co.
Kennedy, G., Jackson, G., Fancher, O.E. and Calandra, J.C. (1967c)
Rabbit teratogenicity study. Captan and Phaltan. Unpub. Rept. of
Industrial Bio-Test Laboratories submitted by Chevron Chemical Co.
Kennedy, G., Fancher, O.E. and Calandra, J.C. (1968) An investigation
of the teratogenic potential of captan, folpet and Difolatan. Toxicol.
appl. Pharmacol. 13:42-430
Kilgore, W.W., Winterlin, W. and White, R. (1967) Gas chromatographic
determinations of captan residues. J. Agr. Food Chem. 15:1035-37
Matta, M. (1968) Gas chromatographic determination of chlorinated
pesticide residues in wine. Vini Ital. 10:171-4 (Chem. Abstr.
69:66469 r, 1968)
McLaughlin, J., Jr., Reynaldo, E.F., Lamar, J.K. and Marliac, J.P.
(1969) Teratology studies in rabbits with captan, folpet and
Thalidomide. Toxicol. appl. Pharmacol. 14:641
Nangniot, P. (1966) L'application des méthodes électrochimiques à
l'étude des résidus de pesticides. Meded Rijksfac. Landbouwwetensch.
31:447-73
Owens, R. G. (1969) Metabolism of fungicides and related compounds.
Ann. N.Y. Acad. Sci. 100:144-52
Palazzolo, R., Fancher, O.E. and Calandra, J.C. (1966) Rabbit
reproduction study. Phthalimide. Unpub. Rept. of Industrial Bio-Test
Laboratories submitted by Chevron Chemical Co.
Pomerantz, I.H. and Rose, R. (1968) Captan and structurally related
compounds: thin-layer and gas-liquid chromatography. J. Assoc.
Offic. Anal. Chem. 51:1058-62
Varrett, M.J., Mutchler, M.K., Scott, W.F., Reynaldo, E.F. and
McLaughlin, J. (1969) Teratogenic effects of captan and related
compounds in the developing chick embryo. Ann. N.Y. Acad. Sci.
160:334-43
Vondruska, J.F. (1969) Teratologic investigation of Phaltan in
Macaca mulatta (Rhesus monkey) and Macaca arctoides
(stumptailed macque).
Unpub. Rept. of Industrial Bio-Test Laboratories submitted by
Chevron Chemical Co.
Weir, R.J. (1956) N-trichloromethylthiophthalimide, recrystallized
phthalimide, (phthalimide analog of captan). Subacute
feeding-rats. Supplement to report dated 23 July, 1956. Unpub.
Rept. submitted by Hazleton Laboratories to California Spray -
Chemical Co.
