
AGP:1970/M/12/1
WHO/FOOD ADD/71.42
1970 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
Issued jointly by FAO and WHO
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Group on Pesticide Residues, which met in Rome, 9-16 November, 1970.
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
WORLD HEALTH ORGANIZATION
Rome, 1971
2,4-D
IDENTITY
Chemical name
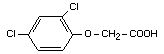
2,4-dichlorophenoxyacetic acid
Synonyms
2,4-D acid
Structural formula
 Other relevant chemical properties
White powder: m.p., 140.5°C; solubility in water at room temperature
is 620 mg/1; soluble in aqueous alkali and in alcohols; insoluble in
petroleum oils. V.p., 0.4 mm Hg at 160°C. Can be used as alkali metal
salt, amine salts and esters.
Purity
Technical, 98 percent pure
EVALUATION FOR ACCEPTABLE DAILY INTAKE
N.B. Except when otherwise specified, toxicity studies on 2,4-D are
assumed to have been performed using the free acid.
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
When administered orally as an amine salt, 2,4-D is rapidly absorbed
in animals, concentrations in plasma reaching a peak after two hours
for chickens, or four to seven hours for rats, calves and pigs.
Similar results are seen after oral administration of alkali metal
salts of 2,4-D. In the case of an ester of 2,4-D, absorption by the
oral route is poor, as evidenced by low plasma and tissue levels
following administration. The intact eater has not been detected in
plasma or urine following treatment to rats, pigs or calves; only the
free acid is found, a fact which indicates that hydrolysis occurs
before absorption. Absorbed 2,4-D is distributed throughout the body;
levels in liver, kidney, spleen and lung reach a maximum after six
hours and after this time levels in these organs often exceed the
level in plasma, particularly in chickens and rats. There appears to
be little variation of distribution between the sexes. In a study with
pigs, high levels of 2,4-D were also found in endocrine glands and in
the excretory organs. The brain level was usually low, relative to the
plasma level. However, 2,4-D passes the placental barrier, as
evidenced by experiments in pigs. The distribution pattern after
administering an ester of 2,4-D is similar to that of the salts,
although tissue levels are lower. A partial binding of 2,4-D to plasma
proteins may occur. The biological half-life in plasma for chickens,
rats, calves and pigs was found to be 8, 3, 8 and 12 hours,
respectively. Elimination of 2,4-D when administered as an ester is
less rapid than that of salts. Excretion is principally via the
kidney, and only low levels have been found in the faeces or bile.
Hens have been reported to excrete 2,4-D in their eggs, the compound
occurring largely in the yolk (Erne, 1966a, 1966b).
Rats were given by stomach-tube doses of 2,4-D labelled with 14-carbon
in the 1 or 2 positions; the compounds had specific activities of 3.03
mCi/mM and 1 mCi/mM, respectively. They were given in doses of 1, 5,
10, 40, 60, 80 or 100 mg per rat. Urine, faeces and expired air were
collected. No radioactivity was detected in the breath at any time
during three days following administration. In the rats given 1 to 10
mg of 2,4-D, 94 to 99 percent was excreted over a 72 hour period,
mostly during the first 24 hours. In animals fed the higher doses of
20 to 100 mg, the percentage excreted bore an inverse linear
relationship to the close administered for both sexes, being 75.5
percent. After 144 hours in the rats given the maximum dose of 100 mg.
This changed pattern of excretion indicated a slower excretion of the
higher doses. In the case of six rats which were given 1 mg of
labelled 2,4-D per rat and a group of seven rats which were given 80
mg, these animals were sacrificed at varying intervals from 1 to 41
hours following administration, and levels of radioactivity in various
organs and tissues were determined. Radioactivity was found in all
tissues examined and reached a maximum six to eight hours after
administration. Excretion was also rapid and after 41 hours in the
animals given 80 mg, only the liver and kidney contained more than 1
ppm (on a dry tissue weight basis) of 2,4-D, the figures for these
organs being 1.5 and 1.8 ppm, respectively. With the rats given 1 mg,
levels below 0.1 ppm were reached in all organs except the stomach
after 12 hours (Khanna and Fang, 1966).
A phenomenon of declining plasma levels following repeated treatment
with 2,4-D has also been observed in chickens and pigs and has been
suggested as being indicative of the animal's developing an adaption
to 2,4-D (Erne, 1966a). See also under 'Fate of residues'.
In a case of fatal poisoning in many 2,4-D was found in blood, urine
and tissues of all organs the brain had the lowest concentration
- less than one fiftieth of that in the blood (Nielzen et al., 1965).
Effects on enzymes and other biochemical parameters
In rats which were given 10 mg/kg body-weight of the sodium salt of
2,4-D by stomach tube for 120 days, the oxygen uptake decreased from
2.14 to 1.88 mm3/mg of dry tissue in the heart, from 2.3 to 2.2 in
the liver and from 5.7 to 2.32 in the kidneys (Stanosz, 1969).
The effect of ingested phenoxy-acid herbicides on muscular function
has been suggested to be related to interference with carbohydrate
metabolism. Transitory diabetiform conditions have been reported in
individuals spraying these compounds. However, hyperglycaemia or
glycosuria could not be reproduced with certainty in rabbits; only one
in five animals responded in this manner following administration of
doses ranging from 125 to 500 mg/kg body-weight from 6 to 50 days.
(Lorenzen and Lyngsoe, 1957; Dalgaard-Mikkelsen and Poulsen, 1962).
Attention has been drawn to the increase in nitrate content which has
been observed in certain plants after spraying with 2,4-D. This
increase has been thought to be the cause of toxic effects which have
been observed in grazing animals. Abortions in cattle have also been
attributed to this phenomenon. Information on this possible indirect
toxic action of 2,4-D has been summarized (Way, 1969). Manifestation
of toxicity appears, however, to be confined to farm animals, and no
reports have been encountered which would indicate that human food
exposed to 2,4-D as a growing crop has become toxic in this manner.
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Mouse
Repeated injections of 2,4-D have not influenced the growth rate of
two transplated sarcomas in mice. Mitotic counts performed at various
intervals in these tissues after a single injection of 200 mg/kg body-
weight of 2,4-D did not differ significantly from a control not
treated with 2,4-D (Bucher, 1946).
Groups of 18 mice of each sex from two hybrid strains were given 2,4-D
from seven days of age for 18 months. The compounds were given daily
by gavage at levels of 0, 46.4 or 100 mg/kg body-weight until weaning,
after which time 2,4-D was incorporated into the diet at the
corresponding levels of 0, 149 or 323 ppm, respectively. There was no
significant increase in tumours between the controls and the groups
given the two levels of 2,4-D (Innes et al., 1969).
Special studies on reproduction
Chicken embryo
When injected into fertile eggs at 5 or 10 mg/egg, 2,4-D did not
produce malformations, although it was stated that certain
phenoxyacetic acids produced feather blanching. The percentage hatch
was 70 and 50 percent for the eggs receiving the two respective levels
of 2,4-D (Dunachie and Fletcher, 1967).
Chicken
Groups of three-day old chicks were given 0 (25 chicks) or 1000 ppm
(29 chicks) of 2,4-D in their drinking water. Weight-gain, age of
sexual maturity and onset of egg production did not differ between the
test and control groups. However, the number, and possibly the weight,
of the eggs was reduced in the group given 2,4-D (Björkland and Erne,
1966).
Mouse
Mice which underwent daily injections of about 90 mg/kg body-weight of
2,4-D became pregnant and bore apparently normal litters at the end of
a normal gestation period (Bucher, 1946).
Rat
Groups, each comprising five newly mated female rats, received 0 or
1000 ppm of 2,4-D in their drinking water during their pregnancy and
for a further ten months. Pregnancy and parturition were normal.
Litter size was not significantly different between the test and
control groups. No malformations were observed in the young nor were
any clinical or morphological abnormalities revealed. After weaning,
the young rats were given 0 or 1000 ppm of 2,4-D in the same manner as
their parents for up to two years. The group receiving 2,4-D had
reduced food intake and depressed growth rate. Clinical-chemical
parameters were normal. Mortality was higher in the group given 2,4-D.
Relative organ weights and gross and histopathological examination
revealed no changes in the test group (Björkland and Erne, 1966).
It has been stated that the long-term feeding to rats of potatoes
which had been treated with 2,4-D affected reproduction. In the F2
generation, and even more in the F3 generation, the ability to
reproduce was reduced in the animals fed these potatoes (An der Lan,
1966).
Pig
A female pig was fed 500 ppm of an amine salt of 2,4-D during her
entire seventh pregnancy and for a further six weeks. Anorexia was
observed. One mummified foetus and 15 underdeveloped live young were
delivered. Ten of these died by the first day. Autopsy revealed a
generalized anaemia. No malformations were observed. The surviving
five young were fed 500 ppm of 2,4-D amine salt; growth was retarded
and locomotor disturbances were observed. Clinical-chemistry results
were the same as described under the chronic feeding study for the pig
(Björkland and Erne, 1966).
Special studies on toxicity of impurities
There is no information that the presence of chlorinated
dibenzodioxins, such as occur in some batches of technical 2,4,5-T,
has been looked for in technical 2,4-D. It is reported that the
condensation of the phenol precursors to form such compounds could not
occur in the manufacture of 2,4-D (Dow, 1970).
TABLE I
Acute Toxicity of 2,4-D
LD50
Animal Route mg/kg References
body-weight
Chicken oral 380-765 (amine salt) Bjorn and Northen, 1948
Chicken (mixed) oral 541 Rows and Hymas, 1954
Mouse oral 375 Hill and Carlisle, 1947
Mouse (m) oral 368 Rowe and Hymas, 1954
Mouse s.c. 280 Bucher, 1946
Rat oral 666 Hill and Carlisle, 1947
Rat (m) oral 375 Rowe and Hymas, 1954
Guinea,pig oral 1000 Hill and Carlisle, 1947
Guinea pig
(mixed) oral 469 Rowe and Hymas, 1954
Rabbit oral 800 Hill and Carlisle, 1947
Dog oral 100 Drill and Hiratzka, 1953
Dog oral 541 Rowe and Hymas, 1954
Monkey oral > 428 Hill and Carlisle, 1947
For man the lethal dose of 2,4-D has been estimated to be greater than
80 mg/kg body-weight (Nielsen et al., 1965).
Symptoms of myotonia in mice were evident after the parenteral
administration of 150-200 mg/kg body-weight (Bucher, 1946).
Initial signs following the administration of a lethal dose of 2,4-D
to dogs were often not present until six hours following oral
administration. The animal then became ataxic with progressive
increase in spasm. Pathological changes were limited to the
gastrointestinal tract, lungs and liver. Death in most cases appeared
to be due to hepatic congestion or to pneumonia, which followed the
development of anorexia, weight loss and myotonia (Drill and Hiratzka,
1953).
No toxic symptoms were observed when monkeys received 214 mg/kg
body-weight orally, but 428 mg/kg caused nausea, vomiting, lethargy,
muscle incoordination and head drop. All species reacted similarly;
death from large doses was thought to be due to ventricular
fibrillation (Hill and Carlisle, 1947).
Short-term studies
Chicken
Groups, each of five chickens, were given an amine salt of 2,4-D three
times a week in oral doses of 0, 0.28, 2.8, 28, or 280 mg/kg
body-weight (acid equivalent) for up to four weeks (a total of 12
doses). Weight gain was reduced only at the 280 mg/kg level (Bjorn and
Northen, 1948).
In the study described under "Reproduction" three day old chicks were
given 0 or 1000 ppm of 2,4-D. In the group given 2,4-D, gross
pathology revealed enlarged kidneys, and histopathology showed changes
in the kidney tissue mostly in the proximal convoluted tubules
(Björkland and Erne, 1966).
Mouse
Injection of 50 to 90 mg/kg body-weight of 2,4-D subcutaneously to
mice for three weeks to three months failed to produce a clear-cut
syndrome of chronic toxicity. Levels of 70 mg/kg retarded growth,
however, probably due to reduced food intake (Bucher, 1946).
Rat
Rats were fed a dietary level of 1000 ppm of 2,4-D for one month
without harmful effects (Hill and Carlisle, 1947).
Groups each comprising five or six young female rats were given 0, 3,
10, 30, 100 or 300 mg/kg body-weight of 2,4-D by stomach tube five
times a week for periods up to four weeks. The animals which received
30 mg/kg or less showed no adverse effects, as judged by gross
appearance, behaviour, mortality, growth, haematological values,
blood-urea nitrogen, organ weights, gross pathology and
histopathology. The rats on 100 mg/kg showed varying degrees of
gastrointestinal irritation, slight cloudy swelling of the liver and
depressed growth rate. The animals given 300 mg/kg failed rapidly and
died principally of severe gastrointestinal irritation (Rowe and
Hymas, 1954).
In a separate experiment, groups each of five young female rats, were
fed dietary levels of 0, 100, 300, 1000, 3000 or 10,000 ppm of 2,4-D
for periods up to 113 days. The animals fed 300 ppm or less showed no
adverse effects as judged by the parameters described in the previous
experiment. Those given 1000 ppm had increased mortality, depressed
growth rate, slightly increased liver weight and slight cloudy
swelling of the liver. The animals fed 3000 or 10,000 ppm were
sacrificed after 12 days because of food refusal and rapid
weight-loss. Autopsy revealed increased liver and kidney weights and
there were slight pathological changes in the organs (Rowe and Hymas,
1954).
Guinea pig
Guinea pigs tolerated 10 oral doses of 100 mg/kg bodyweight of 2,4-D
given over a 12 day period. Inhalation of the sodium salt of 2,4-D as
a dust failed to produce systemic effects (Hill and Carlisle, 1947).
Dog
Groups of from two to four dogs of mixed sexes were given 0, 2, 5, 10
or 20 mg/kg body-weight of 2,4-D orally by capsule on five days a week
for 13 weeks. All the dogs given 10 mg/kg or less survived the 90-day
test period. Of two male dogs given 20 mg/kg, one died after 18 days,
the other after 25 days. A female given 20 mg/kg/day in two divided
doses died after 49 days; another male dog on the same dose regimen
survived the 90-day period. Loss of weight occurred only in the dogs
which ultimately died, and this phenomenon began seven to 12 days
prior to death. Dogs that survived 90 days were free of symptoms: in
the others, ataxia and increased muscle tonus occurred prior to death.
The blood picture was normal, except for a terminal fall in lymphocyte
count prior to death. Weights of thyroid, adrenals, heart, liver and
kidney were not different from the controls in the surviving animals;
in two of the dogs that died, there was a slight increase in heart and
kidney weights. Two dogs, one receiving 2 and the other 20 mg/kg
showed redness in the duodenum. Histopathology revealed no changes in
heart, lungs, thyroid, adrenal or testes. Two dogs receiving an
unspecified level of 2,4-D showed areas of focal necrosis in the
liver, which was considered not to be related to administration of
2,4-D (Drill and Hiratzka, 1953).
Pig
An amine salt of 2,4-D and 2,4-D ester were given to young pigs in
doses of 50, 100 or 300 mg/kg body-weight at varying intervals up to
103 days. Symptoms of intoxication were similar to those reported from
studies with laboratory animals, and autopsy revealed similar
pathological findings. Clinical signs of anorexia and retarded growth
were evident in one pig given 51 doses of 50 mg/kg during 103 days
(Björklund and Erne, 1966).
On the basis of this experiment it has been suggested that the pig may
be more sensitive to 2,4-D than other animals tested, since the
no-effect level appears to be less than 50 mg/kg body-weight/day
(Erne, 1966a).
Five pigs were fed an amine salt of 2,4-D at 0 or 500 ppm in their
diet for up to 12 months. Food consumption and growth rate was reduced
in the controls, and after about one month, three animals developed
locomotor disturbances of increasing severity. The animals were
sacrificed after two to 12 months. Organ weights were normal, and
gross pathological changes were not evident. Clinical-chemical
observations involved lowered haemoglobin and haematocrit values,
elevation of glutamic-oxaloacetic transaminase and reduced albumin and
albumin-globulin ratios in the treated animals (Björklund and Erne,
1966).
Cattle
When steers were given oral doses of 0, 50, 100, 200 or 250 mg/kg
body-weight of an amine salt of 2,4-D for five days a week, poisoning
was evident at 250 mg/kg after 15 treatments and at 100 mg/kg after 86
treatments. A dose of 50 mg/kg given 112 times had no effect on one
steer (Palmer, 1963).
Long-term studies
No complete long-term studies appear to have been conducted.
OBSERVATIONS IN MAN
A man has been reported to have taken 500 mg of 2,4-D daily for three
weeks (approximately 8 mg/kg body-weight) without experiencing any
harmful effects. No further details are given (Assouly, 1951).
A man aged 23 committed suicide by oral intake of the dimethylamine
salt of 2,4-D. Pronounced degenerative changes of the ganglion cells
of the brain were found upon histological examination (Nielsen et al.,
1965).
Subjective clinical symptoms have been reported among workers using
various esters of and the sodium salts of 2.4-D. Individuals
complained of rapid fatigue, headache, liver pains, loss of appetite,
etc. Certain functional shifts were noted in the cardiovascular
system, and sensitivity to taste and smell was lowered (Fetisov,
1966).
In workers employed in factories manufacturing 2,4-dichlorophenol and
other chlorinated phenols, a moderately high incidence of urinary
porphyria, chloracne and hirsutism has been reported. The authors
suggest that a highly chlorinated phenolic ether may be the compound
responsible (Bleiberg at al., 1964).
Clinical symptoms of peripheral neuropathy have been reported in three
individuals who had direct skin contact with an eater of 2,4-D. An
electromyographic examination supported the diagnosis (Goldstein et
al., 1959).
COMMENTS
Information on the absorption and excretion of 2,4-D in several
species indicates that the compound is rapidly excreted unchanged, and
that it is not stored in the tissues in mammals. Reproduction studies
are limited to a single generation but do not indicate that 2,4-D is a
teratogen. There is no information that toxic chlorinate
dibenzodioxins have been looked for in commercial 2,4-D.
Increased nitrate formation has been reported to occur in plants
treated with 2,4-D and is thought to have resulted in toxic effects in
grazing animals. This observation is of concern if 2,4-D is to be used
on edible crops.
Attention was drawn to the absence of any complete long-term studies
in any species. For this reason, no acceptable daily intake for 2,4-D
could be established.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
A pre- or post-emergent selective herbicide in cereals used for
prevention of preharvest fruit drop, the production of harvest fruit
drop, the production of seedless fruit and the regulation of the
growth of plants.
Acid in oil formulations control many broad-leaved annual and
perennial weeds on roadside verges, around farm buildings, etc.
FATE OF RESIDUES
In animals
When a cow was fed 5 ppm of 2,4-D in a 23 kg ration for five days,
none of the herbicide could be detected in the milk or faeces
throughout the study; the analytical limit of detection was 0.1 ppm.
The compound was found to disappear from the rumen, due presumably to
dilution, followed by absorption on the gut wall and by decomposition.
When 2,4-D was added to artificially collected rumen at a level of 2
ppm it was not decomposed (Gutenmann and Lisk, 1963; Gutenmann et al.,
1963).
In pigs which developed symptoms of poisoning following repeated
administration of from 50 to 300 mg/kg body-weight of an amine salt of
2,4-D or of 2,4-D ester, high plasma levels of 2,4-D were found (200
to 400 µg/ml) even 24 hours after the dose was given. Elimination
occurred at an enhanced rate after repeated administration and
eventually decreased to about 10, µg/ml (Björkland and Erne, 1966).
A capsule containing 539.6 µCi of 2-14 C-labelled 2,4-D was
administered orally to a female sheep of weight 26.6 kg. This dose was
equivalent to 4 mg/kg body-weight, which was stated to be the amount
that the animal would receive while grazing in a treated pasture.
Urine and faeces were collected separately, and blood samples were
withdrawn from the jugular vein at intervals after administration of
the dose.
The animal was sacrificed after four days, and tissues were assayed
for carbon-14. Levels of radioactivity in the blood rose during the
first half hour following treatment and reached a peak after one and a
half hours. By 24 hours, the radioactivity level in blood had
decreased to background. About 15 percent of the original dose of
2,4-D was found in the urine after one and a quarter hours, 50 percent
after eight and a half hours and 96 percent after 70 hours. Total
collected faeces contained about 1.4 percent of the dose. Paper
chromatography and electrophoresis from urine extracts revealed only
one spot of the same Rf as 2,4-D, indicating that the compound is
excreted unchanged in the urine. Tissue levels, as determined
following slaughter four days after administration, contained less
than 0.05 ppm radioactive equivalents, with the exception of thyroid
and urinary bladder, which contained 0.56 and 0.5 ppm, respectively.
The nature of these residues in tissue was not determined (Clark et
al., 1964).
In plants
It has been demonstrated by a number of workers that 2,4-D is
translocated in plants (Dhillon and Lucas, 1950; Holley et al., 1950;
Fang et al., 1951), since free phenoxyacetic acids could be isolated
from parts well removed from the site of application. Jaworski and
Butts (1952) demonstrated the formation of a complex of 2,4-D in
plants, and Holley et al. (1950) indicated that plants could detoxify
2,4-D to form hydroxylated products. Weintraub and Norman, (1950) and
McIlrath and Ingle (1953), showed that this destruction was not rapid
in all plants. 14C-labelled material led to the recognition that
some of the applied material was complexed (Jaworski and Butts, 1952).
Existence of oxidative degradation of 2,4-D was shown by Holley et al.
(1950) and Weintraub et al. (1952b) Holley et al. 1950) and Holley
(1952) demonstrated the evolution of 14CO2 after treating plants
with 14C-2,4-D, and the formation of a product more water soluble
than 2,4-D acid. Evolution of 14CO2 from plants treated with either
methylene-labelled or carboxyl-labelled 2,4-D has been shown by Holley
(1952) and Weintraub et al. (1952a, 1954). The side chain of
phenoxyacetic acids can be removed by such plants as currants
(Luckwill and Lloyd-Jones, 1960), wild cucumber (Slife et al., 1962)
and tick beans (Canny and Marcus, 1960). Roots have been shown by
Cann and Marcus (1960) to be of greater efficiency in decarboxylation
than shoots. Not all of the carbon liberated from breakdown of the
side chain of phenoxyacetic acids is evolved as 14CO2, but some is
incorporated into plant constituents (Weintraub at al., 1952a;
Luckwill and Lloyd-Jones, 1960). Luckwill and Lloyd-Jones (1960)
studied the degradation of 2,4-D by currants, apples and strawberries.
They showed that carboxyl-labelled phenoxyacetic acid more readily
yielded 14CO2 than did the methylene-labelled acid.
By spectrophotometric analysis, Schieferstein (1957) showed that the
rate of penetration of 2,4-D through ivy cuticle varied with the age,
and hence thickness of the cuticle. Orgell (1957) found cationic and
anionic surfactants hindered the sorption of 2,4-D at low pH; nonionic
surfactants had little effect. At high pH, cationic surfactants caused
sorption, whereas anionic and non-ionic surfactants had little effect.
2,4-D applied to roots may move to tops only in minute quantities, and
only after some days; applied to leaves, it may pass downward into the
stem and roots in quantity within a few hours.
In soil
2,4-D has been shown to be biodegradable in soils by DeRose and Newman
(1948), Brown and Mitchell (1948) and Audus (1950). Audus (1964)
listed numerous micro-organisms which are capable of degrading 2,4-D.
Evidence of residues in food in commerce or at consumption
After application of 2,4-D to bean plants and oats, 25-60% was found
in tissues, but only 0.33-1.66% in oats (Boyle, 1954). Some
unpublished data was presented (BASF, 1970) regarding residues of
2,4-D in cereals (grain and straw) found to occur following
applications of the herbicide either alone or in admixture with other
phenoxyacid herbicides (MCPA, mecoprop). A summary of the data
relating to 2,4-D is given in Table II. Williams (1964) was unable to
detect 2,4-D in a number of total diet samples at a sensitivity of
0.01 ppm. Duggan and Weatherwax (1967) in total diet studies found
herbicide chemicals infrequently, averaging an intake of about 0.01
mg/kg of which one-third was 2,4-D. Very small amounts of 2,4-D were
found in oils and fats (0.001 mg in 1964/5, and sugars and sugar
products (0.004 mg 1964/5, and 0.002 mg in 1965/6).
TABLE II
Residues of 2,4-D in cereals
Crop Rate of Intervals (days) Residues found
Application Applic.-Harvest Grain Straw
Barley 375 g/ha 64 <0.01 0.08
77 <0.01 0.02
TABLE II (cont'd)
Residues of 2,4-D in cereals
Crop Rate of Intervals (days) Residues found
Application Applic.-Harvest Grain Straw
Barley 520 g/ha 64 n.d.1 0.1
77 n.d. n.d
735 g/ha 64 0.015 0.34
77 n.d. n.d
375 g/ha 76 n.d. 0.02
89 n.d. 0.04
Wheat 520 g/ha 76 n.d. n.d
89 n.d. n.d
735 g/ha 76 n.d. 0.03
89 n.d. n.d
375 g/ha 76 0.01 0.02
89 n.d. n.d
Oats 520 g/ha 76 n.d. n.d
89 n.d. n.d
735 g/ha 76 n.d. 0.02
89 n.d. n.d
375 g/ha 76 n.d. 0.04
89 n.d. n.d
Rye 520 g/ha 76 n.d. n.d
89 n.d. n.d
735 g/ha 76 n.d. 0.04
89 n.d. <0.02
1 n.d. = not detected.
METHODS OF RESIDUE ANALYSIS
Residues of 2,4-D can be determined after suitable extraction,
separation and isolation by gas chromatography, ultraviolet
spectrophotometry, fluorimetry, thinlayer and paper chromatography.
The preferred method is to methylate the isolated 2,4-D and determine
by GLC. This method has been used for the following sample substrates:
forage (Hagin and Linscott, 1965; Gutenmann and Lisk, 1963; Yip and
Neys, 1966)
oysters (Duffy and Shelfoon, 1967), water (Devine and Zweig, 1969),
milk (Yip and Neys, 1966; Crosby and Bowers, 1966)
in fruit peel (Meagher, 1966), citrus fruit (Erickson and Hield, 1962)
and pineapple and orange peel (Hendrickson and Meagher, 1969).
Benvenue et al. (1962) determined residues of 2,4-D in dry crops and
walnuts by microcoulometric gas chromatography. Clerk et al. (1967),
extracted animal tissues and, after isolation of the 2,4-D, hydrolysed
it to 2,4-dichlorophenol, purified by steam distillation and then used
GLC. 2,4-D can be determined colorimetrically by the use of
chromotropic acid in sulphuric acid at a wavelength of 565 nm
(Marquardt and Luce, 1951 and 1955; Erickson and Brannaman, 1954). Aly
and Faust (1964) suggested using 6-amino-1-naphthol-3-sulphonic acid
and 6-anilino-1-naphthol-3-sulphonic acid, which are more sensitive
(1´-2´ times) than chromotropic acid. Marquardt and Luce (1961)
cleaved the phenoxy acid with pyridine hydrochloride, releasing the
phenol derivative 2,4-dichlorophenol, added 4-amino antipyrine and
potassium ferricyanide, and determined the complex at 515 nm.
Ultraviolet spectrophotometry at a wavelength of 284 nm was used by
Warshowsky and Schantz (1950), Aly and Faust (1963) and Gordon and
Beroza (1952). Kuznetsov and Gagarina (1962) determined 2,4-D in
plants by paper chromatographic separation, elution from strips and
colorimetric determination with butyl rhodamine at a wavelength of 564
nm. Salo and Makinen (1964) used silica gel thin-layer chromatography
with Blankopher DCB and examination under ultraviolet light at 360 nm
for visualization. Abbott et al. (1964) used paper and thin-layer
chromatography for detection, separation and identification. Guilbault
and Sadar (1969) used a thin-layer chromatographic separation followed
by a fluorimetric determination based on the breakdown of 4-methyl
umbelliferone heptanoate by lipase.
NATIONAL TOLERANCES
Country Crop Tolerance(ppm)
United States of Apples, pears, 5
America lemons, oranges,
(December 1969) grapefruits
Barley, oat, rye, 20
wheat and forage
Netherlands Barley, oat, rye, 0.5
wheat and grain
Vegetables, 0.05
(except potatoes)
fruits of vegetables
and fruit crops
Fed. Rep. of Germany Leafy and other 0.05
sprouting vegetables,
fruiting vegetables,
root vegetables
APPRAISAL
2,4-D is used in cereals as a selective pre- and post-emergence
herbicide for the control of broad-leaved weeds. It in also used to
prevent early fruit drop, as a plant growth regulator and for weed
control on roadside verges, around buildings, etc. Some evidence from
supervised trials regarding the possible occurrence of residues of
2,4-D in cereal grain and straw was provided. Insufficient information
relating to residues resulting from other uses was available. Total
diet studies in the U.S.A. have very occasionally revealed minimal
quantities of 2,4-D in oils and fats or sugar and sugar products. No
evidence regarding the need to establish practical residue limits was
apparent. Gas chromatographic methods are available which should be
adaptable for regulatory purposes where required.
Since no acceptable daily intake had been established, it was decided
not to recommend tolerances. Nevertheless, it was decided to record
that following officially acceptable use in various countries.
residues of 2,4-D can occur in the following crops:
Wheat, barley, oats, rye and grain 0.02 ppm
Wheat, barley, oats, rye and straw 0.5 ppm
FURTHER WORK OR INFORMATION
REQUIRED (before an acceptable daily intake for man can
be established).
1. An adequate long-term oral study in a rodent
species and at least a two-year study in a
nonrodent mammalian species.
2. A three-generation reproduction study in at
least one mammalian species.
3. Information on the nature of the impurities
occurring in commercial preparations of 2,4-D.
DESIRABLE
1. Further information on the metabolism of 2,4-D
in plants, laboratory animals and man.
2. Elucidation of the problem of increased nitrate
formation in plants treated with 2,4-D, to
determine if this phenomenon could create a
toxic hazard to man.
3. Information on the occurrence of 2,4-D residues
in crops other than cereals.
REFERENCES
Abbott, D.C., Egan, H., Hammond, E.W. and Thomson, J. (1964) The
chromatographic detection and determination of organo-chlorine
herbicides in soil and water. Analyst, 89: 480-488
Aly, O.M. and Faust, S.D. (1963) Determination of
2,4-dichloro-phenoxyacetic acid in surface water, J.Amer. Waterworks
Assoc., 55: 639-646
Aly, O.M. and Faust, S.D. (1964) Determination of phenoxyacetic acids
with J and phenyl J acids. Anal.Chem., 36: 2200-2201
An der Lan, H. (1966) The present situation of toxicology in the field
of crop protection. Res. Rev., 15: 31-43
Assouly, M. (1951) Désherbants sélectifs et substances de croissance.
Aperçu technique. Effet pathologique our l'homme an cours de la
fabrication de l'ester de 2,4-d. Arch.Mal.Prof., 12: 26-30
Audus, L.J. (1950) Biological detoxication of
2,4-dichlorophenoxyacetic acid in soils. Nature, 166: 356
Audus, L.J. (1964) The physiology and biochemistry of herbicides.
Academic Press
BASF (1970) Residues of 2,4-D in cereal grain and straw. (unpublished
report). Badische Anilin und Soda Fabriken
Bevenue, A., Zweig, G. and Nash, N.L. (1962) Residue determination of
2,4-dichlorophenoxyacetic acid in dry crops and walnuts. J. Assoc.
Offic. Agric. Chem., 45: 990-993
Björklund, N-E. and Erne, K. (1966) Toxicological studies of
phenoxyacetic herbicides in animals, Acta vet. scand., 7: 364-390
Bjorn, M.K. and Northen, H.T. (1948) Effects of
2,4-dichlorophenoxyacetic acid on chicks. Science, 108: 479-480
Bleiberg, J., Wallen, M., Brodkin, R. and Applebaum, I.L. (1964)
Industrially acquired porphyria. Arch.Dermatol., 89: 793-797
Boyle, F.P. (1954) Physiology and chemistry of
2,4-dichlorophenoxyacetic acid on resistant and non-resistant plants.
Congr. intern. botan., Paris, Rapps et communs., 8: 184-185
Brown, J.W. and Mitchell, J.W. (1948) Inactivation of 2,4
dichlorophenoxyacetic acid in soil as effected by soil moisture,
temperature, the addition of manure and autoclaving. Botan. Gaz.,
109: 314-323
Bucher, N.L.R. (1946) Effects of 2,4-dichlorophenoxyacetic acid on
experimental animals. Proc. Soc. exp. Biol. Med., 63: 204-205
Canny, M.J. and Marcus, K. (1960) Metabolism of
2,4-dichlorophenoxyacetic acid in bean plants. Australian J. Biol.
Sci., 13: 486-491
Clark, D.E., Young, J.E., Younger, R.L., Hunt, L.M. and McLaran, J.K.
(1964) The fate of 2,4-dichlorophenoxyacetic acid in sheep. J. Agr.
Food Chem., 12: 43-45
Clark, D.E., Wright, F.C. and Hunt, L.M. (1967) Determination of 2,4-D
residues in animal tissues. J. Ag. Food Chem. 15: 171-173
Crosby, D.G. and Bowers, J.B. (1966) Determination of 2,4-D residues
in animal products, Bull. Environ. Contam. Toxicol., 1: 104-107
Dalgaard-Mikkelsen, S. and Poulsen, E. (1962) Toxicology of
herbicides. Pharmacol. Rev., 14: 225-250
De Rose, H.R. and Newman, A.S. (1948) The effect of certain plant
growth regulators on soil microorganism and microbial processes, Proc.
Soil Sci. Soc. Amer., 12: 217-221
Devine, J.M. and Zweig, G. (1969) Note on the determination of some
chlorophenoxy herbicides and their esters in water. J. Assoc. Offic.
Anal. Chem., 52: 187-189
Dhillon, A.S. and Lucas, E.H. (1950) Absorption, translocation and
persistence of 2,4-D in some plants. Botan. Gaz., 112: 198-207
Dow (1970) Information provided verbally to WHO
Drill, V.A. and Hiratzka, T. (1953) Toxicity of
2,4-dichlorophenoxyacetic acid and 2,4,5-trichlorophenoxyacetic acid.
Arch. industr. Hyg., 7: 61-67
Duggan, R.E. and Weatherwax, J.R. (1967) Dietary intake of pesticide
chemicals. Science, 157: 1006-1010
Dunachie, J.F. and Fletcher, W.W. (1967) Effect of some herbicides on
the hatching rate of hen's eggs. Nature, 215: 1406-1407
Erickson, L.C. and Brannaman, B.L. (1954) Chromatropic acid method for
determining 2,4-D residues. Hilgardia, 23: 175-184
Erickson, L.C. and Hield, H.Z. (1962) Determination of some
2,4-dichlorophenoxyacetic acid in citrus fruit. J. Agr. Food Chem.,
10: 204-207
Erne, K. (1966(a)) Distribution and elimination of chlorinated
phenoxyacetic acids in animals. Act Vet. Scand. 7: 240-256
Erne K. (1966(b)) Animal metabolism of phenoxyacetic herbicides. Act
Vet. Scand., 7: 264-271
Fang, S.C., Jaworski, G., Logan, A.V., Freed, V.H. and Butts, J.S.
(1951) The absorption of radioactive 2,4-dichlorophenoxyacetic acid
and the translocation of carbon-14 by bean plants. Arch. Biochem.
Biophys., 32: 249-255
Fetisov, M.I. (1966) Problems of occupational hygiene in work with
herbicides of 2,4-D group (in Russian). Gig. sanit., 9: 28-31
Goldstein, N.P., Jones, P.H. and Brown, J.R. Peripheral neuropathy
after exposure to an ester of 2,4-D, J. Amer. med. Ass., 171:
1306-1309
Gordon, N. and Beroza, M. (1952) Spectrophotometric determination of
small quantities of 2,4-dichlorophenoxyacetic acid and
2,4,5-trichlorophenoxyacetic acid. Anal. Chem., 24: 1968-1971
Guilbault, G.G. and Sadar, M.H. (1969) Fluorometric determination of
pesticides. Anal. Chem., 41: 366-368
Gutenmann, W.H., Hardee, D.D., Holland, R.F. and Lisk, D.J. (1963)
Residue studies with 2,4-dichlorophenoxyacetic acid herbicides in the
dairy cow and in a natural and artificial rumen. Dairy Sci., 46:
1287-1288
Gutenmann, W.H. and List, D.J. (1963) Rapid determination of 4(2,4-DB)
and a metabolite, 2,4-D, in treated forage by electron affinity
spectroscopy. J. Agr. Food Chem., 11: 304-306
Hagin, R.D. and Linscott, D.L. (1965) Determination of
4(2,4-dichlorophenoxybutyric acid (2,4-DB) and
2,4-dichlorophenoxyacetic acid (2,4-D) in forage plants. J. Agr. Food
Chem., 13: 123-125
Hendrickson, R. and Meagher, W.R. (1969) Spray residues of 2,4-D and
2,4,5-TP in "pineapple" orange peel. J. Agr. Food Chem., 17: 601-603
Hill, E.V. and Carlisle, H. (1947) Toxicity of
2,4-dichlorophenoxyacetic acid for experimental animals. J. industr.
Hyg. Toxicol., 29: 85-95
Holley, R.W., Boyle, F.P. and Hand, D.B. (1950) Fate of radioactive
2,4-dichlorophenoxyacetic acid in bean plants. Arch. Biochem., 27:
143-151
Holley, R.W. (1952) Studies of the fate of radioactive 2,4-D acid in
bean plants. II. A water soluble transformator product of 2,4-D. Arch.
Biochem. Biophys., 35: 171-175
Innes, J.R.M., Ulland, B.M., Valerii, M.G., Petrucelli, L., Fishbein,
L., Hart, E.R., Pallotta, A.J., Bates, R.R., Falk, H.C., Gart, J.J.,
Klein, M., Mitchell, I. and Peter, J. (1969) Bioassay of pesticides
and industrial chemicals for tumorigenicity in mice: a preliminary
note, J. nat. Cancer Inst., 42: 1101-1114
Jaworski, E.G., and Butts, J.S. (1952) Plant metabolism II. The
metabolism of carbon 14 labeled 2,4-dichlorophenoxyacetic acid in bean
plants. Arch. Biochem. Biophys., 38: 207-218
Khanna, S. and Fang, S.C. (1966) Metabolism of C14-labelled 2,4
dichlorophenoxyacetic acid in rats. J. Agr. Fd. Chem., 14: 500-503
Kuznetsov, V.J. and Gagarina, M.I. (1962) Photometric determination of
2,4-dichlorophenoxyacetic acid (2,4-D) with butyl rhodamine. Zhur.
Anal. Khim., 17: 235-237
Lorenzen, I. and Lyngse, J. (1957) En undersgelse over virkningen af
et kloreret fenoxyacetat pa kulhydratstofskiftet pa kaniner. Nord.
hyg. T., 38: 153-157
Luckwill, L.C. and Lloyd-Jones, C.P. (1960) Metabolism of plant growth
regulators. I. 2,4-Dichlorophenoxyacetic acid (2,4-D) in leaves of red
and black currant. Ann. Appl. Biol., 48: 613-625
Marquardt, R.P. and Luce, E.N. (1951) Determination of small amounts
of 2,4-dichlorophenoxyacetic acid in milk, Anal. Chem., 23:
1484-1486
Marquardt, R.P. and Luce, E.N. (1955) Determination of
2,4-dichlorophenoxyacetic acid (2,4-D) in grain and seed. J. Agr. Food
Chem., 3: 51-53
Marquardt, R.P. and Luce, E.N. (1961) A new procedure for determining
phenoxyacid herbicides in agricultural products. J. Agr. Food Chem.,
9: 266-270
McIlnath, W.J. and Ingle, D.R. (1953) Further evidence of the
persistence of 2,4-D in cotton. Plant Physiol., 28: 693-696
Meaghen, W.R. (1966) Determination of 2,4-dichlorophenoxyacetic acid
and 2(2,4,5-trichlorophenoxy) propionic acid in citrus by electron
capture gas chromatography. J. Agr. Food Chem., 14: 374-377
Nielsen, K., Kaempe, B., and Jensen-Holm, J. (1965) Fatal poisoning in
man by 2,4-dichlorophenoxyacetic acid (2,4-D): determination of the
agents in forensic materials. Acta pharmacol. toxicol., 22: 224-254
Orgell, W.H. (1957) Absorptive properties of plant cuticles. Proc.
Iowa Acad. Sci., 64: 189-198
Palmer, J.S. (1963) Chronic toxicity of 2,4-D alkanolamine salts to
cattle, J. Amer. vet. Med. Ass., 143: 398-399
Rowe, V.K. and Hymas, T.A. (1954) Summary of toxicological information
on 2,4-D and 2,4,5-T type herbicides and an evaluation of the hazards
to livestock associated with their use. Amer. J. vet. Res., 15:
622-629
Salo, T. and Makinen, R.Z. (1964) Thin-layer chromatography of
substances used for treatment of citrus fruit peel: -biphenyl,
o-phenyl phenol and 2,4-D. Z. Lebensm. u. Forsch., 125: 170-171
Schieferstein, R.H. (1957) Development of protective structures of the
plant cuticle. Ph. D. dissertation, Iowa State College, Ames, Iowa,
U.S.A.
Slife, J.W., Key, J.L., Yamaguchi, S. and Crafts, A.D. (1962)
Penetration, translocation and metabolism of 2,4-D and 2,3,5-T in wild
and cultivated cucumber plants. Weeds, 10: 29-35
Stanosz, S. (1969) Effect of sodium 2,4-dichlorophenoxyacetate on
tissue respiration in parenchymatous organs of rats. (in Polish). Med.
Piacy, 20b: 600-605. Chem. Abstr., 73: (1970)
Warshowsky, B. and Schantz, E.J. (1950) Determination of
2,4-dichlorophenoxyacetic acid in soil. Anal. Chem., 22: 460-463
Way, J.M. (1969) Toxicity and hazards to man, domestic animals and
wildlife from some commonly used auxin herbicides. Res. Rev., 26:
37-62
Weintraub, R.L. and Norman, A.G. (1950) Recovery of growth regulator
from plants treated with 2,4-dichlorophenoxyacetic acid. Science,
111: 493-494
Weintraub, R.L., Brown, J.W., Fields, M. and Rohan, J. (1952a)
Metabolism of 2,4-dichlorophenoxyacetic acid. I. C14O2 production
by bean plants treated with labeled 2,4-dichlorophenoxyacetic acids.
Plant Physiol., 27: 292-301
Weintraub, R.L., Yeatman, J.N., Lockhart, J.A., Reinhart, J.H. and
Fields, M. (1952b) Metabolism of 2,4-dichlorophenoxyacetic acid. II.
Metabolism of the side chain by bean plants. Arch. Biochem. Biophys.,
40: 277-285
Weintraub, R.L., Reinhart, J.H., Scherff, R.A. and Schisler, L. (1954)
Metabolism of 2,4-dichlorophenoxyacetic acid. III. Metabolism and
persistence in dormant plant tissue. Plant Physiol., 29: 303-304
Williams, S. (1964) Pesticide residues in total diet samples. J.
Assoc. Offic. Agric. Chem., 47: 815-821
Yip, G. and Meys, R.E. (1966) Analysis of 2,4-D residues in milk and
forage. Weeds, 14: 167-170
Other relevant chemical properties
White powder: m.p., 140.5°C; solubility in water at room temperature
is 620 mg/1; soluble in aqueous alkali and in alcohols; insoluble in
petroleum oils. V.p., 0.4 mm Hg at 160°C. Can be used as alkali metal
salt, amine salts and esters.
Purity
Technical, 98 percent pure
EVALUATION FOR ACCEPTABLE DAILY INTAKE
N.B. Except when otherwise specified, toxicity studies on 2,4-D are
assumed to have been performed using the free acid.
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
When administered orally as an amine salt, 2,4-D is rapidly absorbed
in animals, concentrations in plasma reaching a peak after two hours
for chickens, or four to seven hours for rats, calves and pigs.
Similar results are seen after oral administration of alkali metal
salts of 2,4-D. In the case of an ester of 2,4-D, absorption by the
oral route is poor, as evidenced by low plasma and tissue levels
following administration. The intact eater has not been detected in
plasma or urine following treatment to rats, pigs or calves; only the
free acid is found, a fact which indicates that hydrolysis occurs
before absorption. Absorbed 2,4-D is distributed throughout the body;
levels in liver, kidney, spleen and lung reach a maximum after six
hours and after this time levels in these organs often exceed the
level in plasma, particularly in chickens and rats. There appears to
be little variation of distribution between the sexes. In a study with
pigs, high levels of 2,4-D were also found in endocrine glands and in
the excretory organs. The brain level was usually low, relative to the
plasma level. However, 2,4-D passes the placental barrier, as
evidenced by experiments in pigs. The distribution pattern after
administering an ester of 2,4-D is similar to that of the salts,
although tissue levels are lower. A partial binding of 2,4-D to plasma
proteins may occur. The biological half-life in plasma for chickens,
rats, calves and pigs was found to be 8, 3, 8 and 12 hours,
respectively. Elimination of 2,4-D when administered as an ester is
less rapid than that of salts. Excretion is principally via the
kidney, and only low levels have been found in the faeces or bile.
Hens have been reported to excrete 2,4-D in their eggs, the compound
occurring largely in the yolk (Erne, 1966a, 1966b).
Rats were given by stomach-tube doses of 2,4-D labelled with 14-carbon
in the 1 or 2 positions; the compounds had specific activities of 3.03
mCi/mM and 1 mCi/mM, respectively. They were given in doses of 1, 5,
10, 40, 60, 80 or 100 mg per rat. Urine, faeces and expired air were
collected. No radioactivity was detected in the breath at any time
during three days following administration. In the rats given 1 to 10
mg of 2,4-D, 94 to 99 percent was excreted over a 72 hour period,
mostly during the first 24 hours. In animals fed the higher doses of
20 to 100 mg, the percentage excreted bore an inverse linear
relationship to the close administered for both sexes, being 75.5
percent. After 144 hours in the rats given the maximum dose of 100 mg.
This changed pattern of excretion indicated a slower excretion of the
higher doses. In the case of six rats which were given 1 mg of
labelled 2,4-D per rat and a group of seven rats which were given 80
mg, these animals were sacrificed at varying intervals from 1 to 41
hours following administration, and levels of radioactivity in various
organs and tissues were determined. Radioactivity was found in all
tissues examined and reached a maximum six to eight hours after
administration. Excretion was also rapid and after 41 hours in the
animals given 80 mg, only the liver and kidney contained more than 1
ppm (on a dry tissue weight basis) of 2,4-D, the figures for these
organs being 1.5 and 1.8 ppm, respectively. With the rats given 1 mg,
levels below 0.1 ppm were reached in all organs except the stomach
after 12 hours (Khanna and Fang, 1966).
A phenomenon of declining plasma levels following repeated treatment
with 2,4-D has also been observed in chickens and pigs and has been
suggested as being indicative of the animal's developing an adaption
to 2,4-D (Erne, 1966a). See also under 'Fate of residues'.
In a case of fatal poisoning in many 2,4-D was found in blood, urine
and tissues of all organs the brain had the lowest concentration
- less than one fiftieth of that in the blood (Nielzen et al., 1965).
Effects on enzymes and other biochemical parameters
In rats which were given 10 mg/kg body-weight of the sodium salt of
2,4-D by stomach tube for 120 days, the oxygen uptake decreased from
2.14 to 1.88 mm3/mg of dry tissue in the heart, from 2.3 to 2.2 in
the liver and from 5.7 to 2.32 in the kidneys (Stanosz, 1969).
The effect of ingested phenoxy-acid herbicides on muscular function
has been suggested to be related to interference with carbohydrate
metabolism. Transitory diabetiform conditions have been reported in
individuals spraying these compounds. However, hyperglycaemia or
glycosuria could not be reproduced with certainty in rabbits; only one
in five animals responded in this manner following administration of
doses ranging from 125 to 500 mg/kg body-weight from 6 to 50 days.
(Lorenzen and Lyngsoe, 1957; Dalgaard-Mikkelsen and Poulsen, 1962).
Attention has been drawn to the increase in nitrate content which has
been observed in certain plants after spraying with 2,4-D. This
increase has been thought to be the cause of toxic effects which have
been observed in grazing animals. Abortions in cattle have also been
attributed to this phenomenon. Information on this possible indirect
toxic action of 2,4-D has been summarized (Way, 1969). Manifestation
of toxicity appears, however, to be confined to farm animals, and no
reports have been encountered which would indicate that human food
exposed to 2,4-D as a growing crop has become toxic in this manner.
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Mouse
Repeated injections of 2,4-D have not influenced the growth rate of
two transplated sarcomas in mice. Mitotic counts performed at various
intervals in these tissues after a single injection of 200 mg/kg body-
weight of 2,4-D did not differ significantly from a control not
treated with 2,4-D (Bucher, 1946).
Groups of 18 mice of each sex from two hybrid strains were given 2,4-D
from seven days of age for 18 months. The compounds were given daily
by gavage at levels of 0, 46.4 or 100 mg/kg body-weight until weaning,
after which time 2,4-D was incorporated into the diet at the
corresponding levels of 0, 149 or 323 ppm, respectively. There was no
significant increase in tumours between the controls and the groups
given the two levels of 2,4-D (Innes et al., 1969).
Special studies on reproduction
Chicken embryo
When injected into fertile eggs at 5 or 10 mg/egg, 2,4-D did not
produce malformations, although it was stated that certain
phenoxyacetic acids produced feather blanching. The percentage hatch
was 70 and 50 percent for the eggs receiving the two respective levels
of 2,4-D (Dunachie and Fletcher, 1967).
Chicken
Groups of three-day old chicks were given 0 (25 chicks) or 1000 ppm
(29 chicks) of 2,4-D in their drinking water. Weight-gain, age of
sexual maturity and onset of egg production did not differ between the
test and control groups. However, the number, and possibly the weight,
of the eggs was reduced in the group given 2,4-D (Björkland and Erne,
1966).
Mouse
Mice which underwent daily injections of about 90 mg/kg body-weight of
2,4-D became pregnant and bore apparently normal litters at the end of
a normal gestation period (Bucher, 1946).
Rat
Groups, each comprising five newly mated female rats, received 0 or
1000 ppm of 2,4-D in their drinking water during their pregnancy and
for a further ten months. Pregnancy and parturition were normal.
Litter size was not significantly different between the test and
control groups. No malformations were observed in the young nor were
any clinical or morphological abnormalities revealed. After weaning,
the young rats were given 0 or 1000 ppm of 2,4-D in the same manner as
their parents for up to two years. The group receiving 2,4-D had
reduced food intake and depressed growth rate. Clinical-chemical
parameters were normal. Mortality was higher in the group given 2,4-D.
Relative organ weights and gross and histopathological examination
revealed no changes in the test group (Björkland and Erne, 1966).
It has been stated that the long-term feeding to rats of potatoes
which had been treated with 2,4-D affected reproduction. In the F2
generation, and even more in the F3 generation, the ability to
reproduce was reduced in the animals fed these potatoes (An der Lan,
1966).
Pig
A female pig was fed 500 ppm of an amine salt of 2,4-D during her
entire seventh pregnancy and for a further six weeks. Anorexia was
observed. One mummified foetus and 15 underdeveloped live young were
delivered. Ten of these died by the first day. Autopsy revealed a
generalized anaemia. No malformations were observed. The surviving
five young were fed 500 ppm of 2,4-D amine salt; growth was retarded
and locomotor disturbances were observed. Clinical-chemistry results
were the same as described under the chronic feeding study for the pig
(Björkland and Erne, 1966).
Special studies on toxicity of impurities
There is no information that the presence of chlorinated
dibenzodioxins, such as occur in some batches of technical 2,4,5-T,
has been looked for in technical 2,4-D. It is reported that the
condensation of the phenol precursors to form such compounds could not
occur in the manufacture of 2,4-D (Dow, 1970).
TABLE I
Acute Toxicity of 2,4-D
LD50
Animal Route mg/kg References
body-weight
Chicken oral 380-765 (amine salt) Bjorn and Northen, 1948
Chicken (mixed) oral 541 Rows and Hymas, 1954
Mouse oral 375 Hill and Carlisle, 1947
Mouse (m) oral 368 Rowe and Hymas, 1954
Mouse s.c. 280 Bucher, 1946
Rat oral 666 Hill and Carlisle, 1947
Rat (m) oral 375 Rowe and Hymas, 1954
Guinea,pig oral 1000 Hill and Carlisle, 1947
Guinea pig
(mixed) oral 469 Rowe and Hymas, 1954
Rabbit oral 800 Hill and Carlisle, 1947
Dog oral 100 Drill and Hiratzka, 1953
Dog oral 541 Rowe and Hymas, 1954
Monkey oral > 428 Hill and Carlisle, 1947
For man the lethal dose of 2,4-D has been estimated to be greater than
80 mg/kg body-weight (Nielsen et al., 1965).
Symptoms of myotonia in mice were evident after the parenteral
administration of 150-200 mg/kg body-weight (Bucher, 1946).
Initial signs following the administration of a lethal dose of 2,4-D
to dogs were often not present until six hours following oral
administration. The animal then became ataxic with progressive
increase in spasm. Pathological changes were limited to the
gastrointestinal tract, lungs and liver. Death in most cases appeared
to be due to hepatic congestion or to pneumonia, which followed the
development of anorexia, weight loss and myotonia (Drill and Hiratzka,
1953).
No toxic symptoms were observed when monkeys received 214 mg/kg
body-weight orally, but 428 mg/kg caused nausea, vomiting, lethargy,
muscle incoordination and head drop. All species reacted similarly;
death from large doses was thought to be due to ventricular
fibrillation (Hill and Carlisle, 1947).
Short-term studies
Chicken
Groups, each of five chickens, were given an amine salt of 2,4-D three
times a week in oral doses of 0, 0.28, 2.8, 28, or 280 mg/kg
body-weight (acid equivalent) for up to four weeks (a total of 12
doses). Weight gain was reduced only at the 280 mg/kg level (Bjorn and
Northen, 1948).
In the study described under "Reproduction" three day old chicks were
given 0 or 1000 ppm of 2,4-D. In the group given 2,4-D, gross
pathology revealed enlarged kidneys, and histopathology showed changes
in the kidney tissue mostly in the proximal convoluted tubules
(Björkland and Erne, 1966).
Mouse
Injection of 50 to 90 mg/kg body-weight of 2,4-D subcutaneously to
mice for three weeks to three months failed to produce a clear-cut
syndrome of chronic toxicity. Levels of 70 mg/kg retarded growth,
however, probably due to reduced food intake (Bucher, 1946).
Rat
Rats were fed a dietary level of 1000 ppm of 2,4-D for one month
without harmful effects (Hill and Carlisle, 1947).
Groups each comprising five or six young female rats were given 0, 3,
10, 30, 100 or 300 mg/kg body-weight of 2,4-D by stomach tube five
times a week for periods up to four weeks. The animals which received
30 mg/kg or less showed no adverse effects, as judged by gross
appearance, behaviour, mortality, growth, haematological values,
blood-urea nitrogen, organ weights, gross pathology and
histopathology. The rats on 100 mg/kg showed varying degrees of
gastrointestinal irritation, slight cloudy swelling of the liver and
depressed growth rate. The animals given 300 mg/kg failed rapidly and
died principally of severe gastrointestinal irritation (Rowe and
Hymas, 1954).
In a separate experiment, groups each of five young female rats, were
fed dietary levels of 0, 100, 300, 1000, 3000 or 10,000 ppm of 2,4-D
for periods up to 113 days. The animals fed 300 ppm or less showed no
adverse effects as judged by the parameters described in the previous
experiment. Those given 1000 ppm had increased mortality, depressed
growth rate, slightly increased liver weight and slight cloudy
swelling of the liver. The animals fed 3000 or 10,000 ppm were
sacrificed after 12 days because of food refusal and rapid
weight-loss. Autopsy revealed increased liver and kidney weights and
there were slight pathological changes in the organs (Rowe and Hymas,
1954).
Guinea pig
Guinea pigs tolerated 10 oral doses of 100 mg/kg bodyweight of 2,4-D
given over a 12 day period. Inhalation of the sodium salt of 2,4-D as
a dust failed to produce systemic effects (Hill and Carlisle, 1947).
Dog
Groups of from two to four dogs of mixed sexes were given 0, 2, 5, 10
or 20 mg/kg body-weight of 2,4-D orally by capsule on five days a week
for 13 weeks. All the dogs given 10 mg/kg or less survived the 90-day
test period. Of two male dogs given 20 mg/kg, one died after 18 days,
the other after 25 days. A female given 20 mg/kg/day in two divided
doses died after 49 days; another male dog on the same dose regimen
survived the 90-day period. Loss of weight occurred only in the dogs
which ultimately died, and this phenomenon began seven to 12 days
prior to death. Dogs that survived 90 days were free of symptoms: in
the others, ataxia and increased muscle tonus occurred prior to death.
The blood picture was normal, except for a terminal fall in lymphocyte
count prior to death. Weights of thyroid, adrenals, heart, liver and
kidney were not different from the controls in the surviving animals;
in two of the dogs that died, there was a slight increase in heart and
kidney weights. Two dogs, one receiving 2 and the other 20 mg/kg
showed redness in the duodenum. Histopathology revealed no changes in
heart, lungs, thyroid, adrenal or testes. Two dogs receiving an
unspecified level of 2,4-D showed areas of focal necrosis in the
liver, which was considered not to be related to administration of
2,4-D (Drill and Hiratzka, 1953).
Pig
An amine salt of 2,4-D and 2,4-D ester were given to young pigs in
doses of 50, 100 or 300 mg/kg body-weight at varying intervals up to
103 days. Symptoms of intoxication were similar to those reported from
studies with laboratory animals, and autopsy revealed similar
pathological findings. Clinical signs of anorexia and retarded growth
were evident in one pig given 51 doses of 50 mg/kg during 103 days
(Björklund and Erne, 1966).
On the basis of this experiment it has been suggested that the pig may
be more sensitive to 2,4-D than other animals tested, since the
no-effect level appears to be less than 50 mg/kg body-weight/day
(Erne, 1966a).
Five pigs were fed an amine salt of 2,4-D at 0 or 500 ppm in their
diet for up to 12 months. Food consumption and growth rate was reduced
in the controls, and after about one month, three animals developed
locomotor disturbances of increasing severity. The animals were
sacrificed after two to 12 months. Organ weights were normal, and
gross pathological changes were not evident. Clinical-chemical
observations involved lowered haemoglobin and haematocrit values,
elevation of glutamic-oxaloacetic transaminase and reduced albumin and
albumin-globulin ratios in the treated animals (Björklund and Erne,
1966).
Cattle
When steers were given oral doses of 0, 50, 100, 200 or 250 mg/kg
body-weight of an amine salt of 2,4-D for five days a week, poisoning
was evident at 250 mg/kg after 15 treatments and at 100 mg/kg after 86
treatments. A dose of 50 mg/kg given 112 times had no effect on one
steer (Palmer, 1963).
Long-term studies
No complete long-term studies appear to have been conducted.
OBSERVATIONS IN MAN
A man has been reported to have taken 500 mg of 2,4-D daily for three
weeks (approximately 8 mg/kg body-weight) without experiencing any
harmful effects. No further details are given (Assouly, 1951).
A man aged 23 committed suicide by oral intake of the dimethylamine
salt of 2,4-D. Pronounced degenerative changes of the ganglion cells
of the brain were found upon histological examination (Nielsen et al.,
1965).
Subjective clinical symptoms have been reported among workers using
various esters of and the sodium salts of 2.4-D. Individuals
complained of rapid fatigue, headache, liver pains, loss of appetite,
etc. Certain functional shifts were noted in the cardiovascular
system, and sensitivity to taste and smell was lowered (Fetisov,
1966).
In workers employed in factories manufacturing 2,4-dichlorophenol and
other chlorinated phenols, a moderately high incidence of urinary
porphyria, chloracne and hirsutism has been reported. The authors
suggest that a highly chlorinated phenolic ether may be the compound
responsible (Bleiberg at al., 1964).
Clinical symptoms of peripheral neuropathy have been reported in three
individuals who had direct skin contact with an eater of 2,4-D. An
electromyographic examination supported the diagnosis (Goldstein et
al., 1959).
COMMENTS
Information on the absorption and excretion of 2,4-D in several
species indicates that the compound is rapidly excreted unchanged, and
that it is not stored in the tissues in mammals. Reproduction studies
are limited to a single generation but do not indicate that 2,4-D is a
teratogen. There is no information that toxic chlorinate
dibenzodioxins have been looked for in commercial 2,4-D.
Increased nitrate formation has been reported to occur in plants
treated with 2,4-D and is thought to have resulted in toxic effects in
grazing animals. This observation is of concern if 2,4-D is to be used
on edible crops.
Attention was drawn to the absence of any complete long-term studies
in any species. For this reason, no acceptable daily intake for 2,4-D
could be established.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
A pre- or post-emergent selective herbicide in cereals used for
prevention of preharvest fruit drop, the production of harvest fruit
drop, the production of seedless fruit and the regulation of the
growth of plants.
Acid in oil formulations control many broad-leaved annual and
perennial weeds on roadside verges, around farm buildings, etc.
FATE OF RESIDUES
In animals
When a cow was fed 5 ppm of 2,4-D in a 23 kg ration for five days,
none of the herbicide could be detected in the milk or faeces
throughout the study; the analytical limit of detection was 0.1 ppm.
The compound was found to disappear from the rumen, due presumably to
dilution, followed by absorption on the gut wall and by decomposition.
When 2,4-D was added to artificially collected rumen at a level of 2
ppm it was not decomposed (Gutenmann and Lisk, 1963; Gutenmann et al.,
1963).
In pigs which developed symptoms of poisoning following repeated
administration of from 50 to 300 mg/kg body-weight of an amine salt of
2,4-D or of 2,4-D ester, high plasma levels of 2,4-D were found (200
to 400 µg/ml) even 24 hours after the dose was given. Elimination
occurred at an enhanced rate after repeated administration and
eventually decreased to about 10, µg/ml (Björkland and Erne, 1966).
A capsule containing 539.6 µCi of 2-14 C-labelled 2,4-D was
administered orally to a female sheep of weight 26.6 kg. This dose was
equivalent to 4 mg/kg body-weight, which was stated to be the amount
that the animal would receive while grazing in a treated pasture.
Urine and faeces were collected separately, and blood samples were
withdrawn from the jugular vein at intervals after administration of
the dose.
The animal was sacrificed after four days, and tissues were assayed
for carbon-14. Levels of radioactivity in the blood rose during the
first half hour following treatment and reached a peak after one and a
half hours. By 24 hours, the radioactivity level in blood had
decreased to background. About 15 percent of the original dose of
2,4-D was found in the urine after one and a quarter hours, 50 percent
after eight and a half hours and 96 percent after 70 hours. Total
collected faeces contained about 1.4 percent of the dose. Paper
chromatography and electrophoresis from urine extracts revealed only
one spot of the same Rf as 2,4-D, indicating that the compound is
excreted unchanged in the urine. Tissue levels, as determined
following slaughter four days after administration, contained less
than 0.05 ppm radioactive equivalents, with the exception of thyroid
and urinary bladder, which contained 0.56 and 0.5 ppm, respectively.
The nature of these residues in tissue was not determined (Clark et
al., 1964).
In plants
It has been demonstrated by a number of workers that 2,4-D is
translocated in plants (Dhillon and Lucas, 1950; Holley et al., 1950;
Fang et al., 1951), since free phenoxyacetic acids could be isolated
from parts well removed from the site of application. Jaworski and
Butts (1952) demonstrated the formation of a complex of 2,4-D in
plants, and Holley et al. (1950) indicated that plants could detoxify
2,4-D to form hydroxylated products. Weintraub and Norman, (1950) and
McIlrath and Ingle (1953), showed that this destruction was not rapid
in all plants. 14C-labelled material led to the recognition that
some of the applied material was complexed (Jaworski and Butts, 1952).
Existence of oxidative degradation of 2,4-D was shown by Holley et al.
(1950) and Weintraub et al. (1952b) Holley et al. 1950) and Holley
(1952) demonstrated the evolution of 14CO2 after treating plants
with 14C-2,4-D, and the formation of a product more water soluble
than 2,4-D acid. Evolution of 14CO2 from plants treated with either
methylene-labelled or carboxyl-labelled 2,4-D has been shown by Holley
(1952) and Weintraub et al. (1952a, 1954). The side chain of
phenoxyacetic acids can be removed by such plants as currants
(Luckwill and Lloyd-Jones, 1960), wild cucumber (Slife et al., 1962)
and tick beans (Canny and Marcus, 1960). Roots have been shown by
Cann and Marcus (1960) to be of greater efficiency in decarboxylation
than shoots. Not all of the carbon liberated from breakdown of the
side chain of phenoxyacetic acids is evolved as 14CO2, but some is
incorporated into plant constituents (Weintraub at al., 1952a;
Luckwill and Lloyd-Jones, 1960). Luckwill and Lloyd-Jones (1960)
studied the degradation of 2,4-D by currants, apples and strawberries.
They showed that carboxyl-labelled phenoxyacetic acid more readily
yielded 14CO2 than did the methylene-labelled acid.
By spectrophotometric analysis, Schieferstein (1957) showed that the
rate of penetration of 2,4-D through ivy cuticle varied with the age,
and hence thickness of the cuticle. Orgell (1957) found cationic and
anionic surfactants hindered the sorption of 2,4-D at low pH; nonionic
surfactants had little effect. At high pH, cationic surfactants caused
sorption, whereas anionic and non-ionic surfactants had little effect.
2,4-D applied to roots may move to tops only in minute quantities, and
only after some days; applied to leaves, it may pass downward into the
stem and roots in quantity within a few hours.
In soil
2,4-D has been shown to be biodegradable in soils by DeRose and Newman
(1948), Brown and Mitchell (1948) and Audus (1950). Audus (1964)
listed numerous micro-organisms which are capable of degrading 2,4-D.
Evidence of residues in food in commerce or at consumption
After application of 2,4-D to bean plants and oats, 25-60% was found
in tissues, but only 0.33-1.66% in oats (Boyle, 1954). Some
unpublished data was presented (BASF, 1970) regarding residues of
2,4-D in cereals (grain and straw) found to occur following
applications of the herbicide either alone or in admixture with other
phenoxyacid herbicides (MCPA, mecoprop). A summary of the data
relating to 2,4-D is given in Table II. Williams (1964) was unable to
detect 2,4-D in a number of total diet samples at a sensitivity of
0.01 ppm. Duggan and Weatherwax (1967) in total diet studies found
herbicide chemicals infrequently, averaging an intake of about 0.01
mg/kg of which one-third was 2,4-D. Very small amounts of 2,4-D were
found in oils and fats (0.001 mg in 1964/5, and sugars and sugar
products (0.004 mg 1964/5, and 0.002 mg in 1965/6).
TABLE II
Residues of 2,4-D in cereals
Crop Rate of Intervals (days) Residues found
Application Applic.-Harvest Grain Straw
Barley 375 g/ha 64 <0.01 0.08
77 <0.01 0.02
TABLE II (cont'd)
Residues of 2,4-D in cereals
Crop Rate of Intervals (days) Residues found
Application Applic.-Harvest Grain Straw
Barley 520 g/ha 64 n.d.1 0.1
77 n.d. n.d
735 g/ha 64 0.015 0.34
77 n.d. n.d
375 g/ha 76 n.d. 0.02
89 n.d. 0.04
Wheat 520 g/ha 76 n.d. n.d
89 n.d. n.d
735 g/ha 76 n.d. 0.03
89 n.d. n.d
375 g/ha 76 0.01 0.02
89 n.d. n.d
Oats 520 g/ha 76 n.d. n.d
89 n.d. n.d
735 g/ha 76 n.d. 0.02
89 n.d. n.d
375 g/ha 76 n.d. 0.04
89 n.d. n.d
Rye 520 g/ha 76 n.d. n.d
89 n.d. n.d
735 g/ha 76 n.d. 0.04
89 n.d. <0.02
1 n.d. = not detected.
METHODS OF RESIDUE ANALYSIS
Residues of 2,4-D can be determined after suitable extraction,
separation and isolation by gas chromatography, ultraviolet
spectrophotometry, fluorimetry, thinlayer and paper chromatography.
The preferred method is to methylate the isolated 2,4-D and determine
by GLC. This method has been used for the following sample substrates:
forage (Hagin and Linscott, 1965; Gutenmann and Lisk, 1963; Yip and
Neys, 1966)
oysters (Duffy and Shelfoon, 1967), water (Devine and Zweig, 1969),
milk (Yip and Neys, 1966; Crosby and Bowers, 1966)
in fruit peel (Meagher, 1966), citrus fruit (Erickson and Hield, 1962)
and pineapple and orange peel (Hendrickson and Meagher, 1969).
Benvenue et al. (1962) determined residues of 2,4-D in dry crops and
walnuts by microcoulometric gas chromatography. Clerk et al. (1967),
extracted animal tissues and, after isolation of the 2,4-D, hydrolysed
it to 2,4-dichlorophenol, purified by steam distillation and then used
GLC. 2,4-D can be determined colorimetrically by the use of
chromotropic acid in sulphuric acid at a wavelength of 565 nm
(Marquardt and Luce, 1951 and 1955; Erickson and Brannaman, 1954). Aly
and Faust (1964) suggested using 6-amino-1-naphthol-3-sulphonic acid
and 6-anilino-1-naphthol-3-sulphonic acid, which are more sensitive
(1´-2´ times) than chromotropic acid. Marquardt and Luce (1961)
cleaved the phenoxy acid with pyridine hydrochloride, releasing the
phenol derivative 2,4-dichlorophenol, added 4-amino antipyrine and
potassium ferricyanide, and determined the complex at 515 nm.
Ultraviolet spectrophotometry at a wavelength of 284 nm was used by
Warshowsky and Schantz (1950), Aly and Faust (1963) and Gordon and
Beroza (1952). Kuznetsov and Gagarina (1962) determined 2,4-D in
plants by paper chromatographic separation, elution from strips and
colorimetric determination with butyl rhodamine at a wavelength of 564
nm. Salo and Makinen (1964) used silica gel thin-layer chromatography
with Blankopher DCB and examination under ultraviolet light at 360 nm
for visualization. Abbott et al. (1964) used paper and thin-layer
chromatography for detection, separation and identification. Guilbault
and Sadar (1969) used a thin-layer chromatographic separation followed
by a fluorimetric determination based on the breakdown of 4-methyl
umbelliferone heptanoate by lipase.
NATIONAL TOLERANCES
Country Crop Tolerance(ppm)
United States of Apples, pears, 5
America lemons, oranges,
(December 1969) grapefruits
Barley, oat, rye, 20
wheat and forage
Netherlands Barley, oat, rye, 0.5
wheat and grain
Vegetables, 0.05
(except potatoes)
fruits of vegetables
and fruit crops
Fed. Rep. of Germany Leafy and other 0.05
sprouting vegetables,
fruiting vegetables,
root vegetables
APPRAISAL
2,4-D is used in cereals as a selective pre- and post-emergence
herbicide for the control of broad-leaved weeds. It in also used to
prevent early fruit drop, as a plant growth regulator and for weed
control on roadside verges, around buildings, etc. Some evidence from
supervised trials regarding the possible occurrence of residues of
2,4-D in cereal grain and straw was provided. Insufficient information
relating to residues resulting from other uses was available. Total
diet studies in the U.S.A. have very occasionally revealed minimal
quantities of 2,4-D in oils and fats or sugar and sugar products. No
evidence regarding the need to establish practical residue limits was
apparent. Gas chromatographic methods are available which should be
adaptable for regulatory purposes where required.
Since no acceptable daily intake had been established, it was decided
not to recommend tolerances. Nevertheless, it was decided to record
that following officially acceptable use in various countries.
residues of 2,4-D can occur in the following crops:
Wheat, barley, oats, rye and grain 0.02 ppm
Wheat, barley, oats, rye and straw 0.5 ppm
FURTHER WORK OR INFORMATION
REQUIRED (before an acceptable daily intake for man can
be established).
1. An adequate long-term oral study in a rodent
species and at least a two-year study in a
nonrodent mammalian species.
2. A three-generation reproduction study in at
least one mammalian species.
3. Information on the nature of the impurities
occurring in commercial preparations of 2,4-D.
DESIRABLE
1. Further information on the metabolism of 2,4-D
in plants, laboratory animals and man.
2. Elucidation of the problem of increased nitrate
formation in plants treated with 2,4-D, to
determine if this phenomenon could create a
toxic hazard to man.
3. Information on the occurrence of 2,4-D residues
in crops other than cereals.
REFERENCES
Abbott, D.C., Egan, H., Hammond, E.W. and Thomson, J. (1964) The
chromatographic detection and determination of organo-chlorine
herbicides in soil and water. Analyst, 89: 480-488
Aly, O.M. and Faust, S.D. (1963) Determination of
2,4-dichloro-phenoxyacetic acid in surface water, J.Amer. Waterworks
Assoc., 55: 639-646
Aly, O.M. and Faust, S.D. (1964) Determination of phenoxyacetic acids
with J and phenyl J acids. Anal.Chem., 36: 2200-2201
An der Lan, H. (1966) The present situation of toxicology in the field
of crop protection. Res. Rev., 15: 31-43
Assouly, M. (1951) Désherbants sélectifs et substances de croissance.
Aperçu technique. Effet pathologique our l'homme an cours de la
fabrication de l'ester de 2,4-d. Arch.Mal.Prof., 12: 26-30
Audus, L.J. (1950) Biological detoxication of
2,4-dichlorophenoxyacetic acid in soils. Nature, 166: 356
Audus, L.J. (1964) The physiology and biochemistry of herbicides.
Academic Press
BASF (1970) Residues of 2,4-D in cereal grain and straw. (unpublished
report). Badische Anilin und Soda Fabriken
Bevenue, A., Zweig, G. and Nash, N.L. (1962) Residue determination of
2,4-dichlorophenoxyacetic acid in dry crops and walnuts. J. Assoc.
Offic. Agric. Chem., 45: 990-993
Björklund, N-E. and Erne, K. (1966) Toxicological studies of
phenoxyacetic herbicides in animals, Acta vet. scand., 7: 364-390
Bjorn, M.K. and Northen, H.T. (1948) Effects of
2,4-dichlorophenoxyacetic acid on chicks. Science, 108: 479-480
Bleiberg, J., Wallen, M., Brodkin, R. and Applebaum, I.L. (1964)
Industrially acquired porphyria. Arch.Dermatol., 89: 793-797
Boyle, F.P. (1954) Physiology and chemistry of
2,4-dichlorophenoxyacetic acid on resistant and non-resistant plants.
Congr. intern. botan., Paris, Rapps et communs., 8: 184-185
Brown, J.W. and Mitchell, J.W. (1948) Inactivation of 2,4
dichlorophenoxyacetic acid in soil as effected by soil moisture,
temperature, the addition of manure and autoclaving. Botan. Gaz.,
109: 314-323
Bucher, N.L.R. (1946) Effects of 2,4-dichlorophenoxyacetic acid on
experimental animals. Proc. Soc. exp. Biol. Med., 63: 204-205
Canny, M.J. and Marcus, K. (1960) Metabolism of
2,4-dichlorophenoxyacetic acid in bean plants. Australian J. Biol.
Sci., 13: 486-491
Clark, D.E., Young, J.E., Younger, R.L., Hunt, L.M. and McLaran, J.K.
(1964) The fate of 2,4-dichlorophenoxyacetic acid in sheep. J. Agr.
Food Chem., 12: 43-45
Clark, D.E., Wright, F.C. and Hunt, L.M. (1967) Determination of 2,4-D
residues in animal tissues. J. Ag. Food Chem. 15: 171-173
Crosby, D.G. and Bowers, J.B. (1966) Determination of 2,4-D residues
in animal products, Bull. Environ. Contam. Toxicol., 1: 104-107
Dalgaard-Mikkelsen, S. and Poulsen, E. (1962) Toxicology of
herbicides. Pharmacol. Rev., 14: 225-250
De Rose, H.R. and Newman, A.S. (1948) The effect of certain plant
growth regulators on soil microorganism and microbial processes, Proc.
Soil Sci. Soc. Amer., 12: 217-221
Devine, J.M. and Zweig, G. (1969) Note on the determination of some
chlorophenoxy herbicides and their esters in water. J. Assoc. Offic.
Anal. Chem., 52: 187-189
Dhillon, A.S. and Lucas, E.H. (1950) Absorption, translocation and
persistence of 2,4-D in some plants. Botan. Gaz., 112: 198-207
Dow (1970) Information provided verbally to WHO
Drill, V.A. and Hiratzka, T. (1953) Toxicity of
2,4-dichlorophenoxyacetic acid and 2,4,5-trichlorophenoxyacetic acid.
Arch. industr. Hyg., 7: 61-67
Duggan, R.E. and Weatherwax, J.R. (1967) Dietary intake of pesticide
chemicals. Science, 157: 1006-1010
Dunachie, J.F. and Fletcher, W.W. (1967) Effect of some herbicides on
the hatching rate of hen's eggs. Nature, 215: 1406-1407
Erickson, L.C. and Brannaman, B.L. (1954) Chromatropic acid method for
determining 2,4-D residues. Hilgardia, 23: 175-184
Erickson, L.C. and Hield, H.Z. (1962) Determination of some
2,4-dichlorophenoxyacetic acid in citrus fruit. J. Agr. Food Chem.,
10: 204-207
Erne, K. (1966(a)) Distribution and elimination of chlorinated
phenoxyacetic acids in animals. Act Vet. Scand. 7: 240-256
Erne K. (1966(b)) Animal metabolism of phenoxyacetic herbicides. Act
Vet. Scand., 7: 264-271
Fang, S.C., Jaworski, G., Logan, A.V., Freed, V.H. and Butts, J.S.
(1951) The absorption of radioactive 2,4-dichlorophenoxyacetic acid
and the translocation of carbon-14 by bean plants. Arch. Biochem.
Biophys., 32: 249-255
Fetisov, M.I. (1966) Problems of occupational hygiene in work with
herbicides of 2,4-D group (in Russian). Gig. sanit., 9: 28-31
Goldstein, N.P., Jones, P.H. and Brown, J.R. Peripheral neuropathy
after exposure to an ester of 2,4-D, J. Amer. med. Ass., 171:
1306-1309
Gordon, N. and Beroza, M. (1952) Spectrophotometric determination of
small quantities of 2,4-dichlorophenoxyacetic acid and
2,4,5-trichlorophenoxyacetic acid. Anal. Chem., 24: 1968-1971
Guilbault, G.G. and Sadar, M.H. (1969) Fluorometric determination of
pesticides. Anal. Chem., 41: 366-368
Gutenmann, W.H., Hardee, D.D., Holland, R.F. and Lisk, D.J. (1963)
Residue studies with 2,4-dichlorophenoxyacetic acid herbicides in the
dairy cow and in a natural and artificial rumen. Dairy Sci., 46:
1287-1288
Gutenmann, W.H. and List, D.J. (1963) Rapid determination of 4(2,4-DB)
and a metabolite, 2,4-D, in treated forage by electron affinity
spectroscopy. J. Agr. Food Chem., 11: 304-306
Hagin, R.D. and Linscott, D.L. (1965) Determination of
4(2,4-dichlorophenoxybutyric acid (2,4-DB) and
2,4-dichlorophenoxyacetic acid (2,4-D) in forage plants. J. Agr. Food
Chem., 13: 123-125
Hendrickson, R. and Meagher, W.R. (1969) Spray residues of 2,4-D and
2,4,5-TP in "pineapple" orange peel. J. Agr. Food Chem., 17: 601-603
Hill, E.V. and Carlisle, H. (1947) Toxicity of
2,4-dichlorophenoxyacetic acid for experimental animals. J. industr.
Hyg. Toxicol., 29: 85-95
Holley, R.W., Boyle, F.P. and Hand, D.B. (1950) Fate of radioactive
2,4-dichlorophenoxyacetic acid in bean plants. Arch. Biochem., 27:
143-151
Holley, R.W. (1952) Studies of the fate of radioactive 2,4-D acid in
bean plants. II. A water soluble transformator product of 2,4-D. Arch.
Biochem. Biophys., 35: 171-175
Innes, J.R.M., Ulland, B.M., Valerii, M.G., Petrucelli, L., Fishbein,
L., Hart, E.R., Pallotta, A.J., Bates, R.R., Falk, H.C., Gart, J.J.,
Klein, M., Mitchell, I. and Peter, J. (1969) Bioassay of pesticides
and industrial chemicals for tumorigenicity in mice: a preliminary
note, J. nat. Cancer Inst., 42: 1101-1114
Jaworski, E.G., and Butts, J.S. (1952) Plant metabolism II. The
metabolism of carbon 14 labeled 2,4-dichlorophenoxyacetic acid in bean
plants. Arch. Biochem. Biophys., 38: 207-218
Khanna, S. and Fang, S.C. (1966) Metabolism of C14-labelled 2,4
dichlorophenoxyacetic acid in rats. J. Agr. Fd. Chem., 14: 500-503
Kuznetsov, V.J. and Gagarina, M.I. (1962) Photometric determination of
2,4-dichlorophenoxyacetic acid (2,4-D) with butyl rhodamine. Zhur.
Anal. Khim., 17: 235-237
Lorenzen, I. and Lyngse, J. (1957) En undersgelse over virkningen af
et kloreret fenoxyacetat pa kulhydratstofskiftet pa kaniner. Nord.
hyg. T., 38: 153-157
Luckwill, L.C. and Lloyd-Jones, C.P. (1960) Metabolism of plant growth
regulators. I. 2,4-Dichlorophenoxyacetic acid (2,4-D) in leaves of red
and black currant. Ann. Appl. Biol., 48: 613-625
Marquardt, R.P. and Luce, E.N. (1951) Determination of small amounts
of 2,4-dichlorophenoxyacetic acid in milk, Anal. Chem., 23:
1484-1486
Marquardt, R.P. and Luce, E.N. (1955) Determination of
2,4-dichlorophenoxyacetic acid (2,4-D) in grain and seed. J. Agr. Food
Chem., 3: 51-53
Marquardt, R.P. and Luce, E.N. (1961) A new procedure for determining
phenoxyacid herbicides in agricultural products. J. Agr. Food Chem.,
9: 266-270
McIlnath, W.J. and Ingle, D.R. (1953) Further evidence of the
persistence of 2,4-D in cotton. Plant Physiol., 28: 693-696
Meaghen, W.R. (1966) Determination of 2,4-dichlorophenoxyacetic acid
and 2(2,4,5-trichlorophenoxy) propionic acid in citrus by electron
capture gas chromatography. J. Agr. Food Chem., 14: 374-377
Nielsen, K., Kaempe, B., and Jensen-Holm, J. (1965) Fatal poisoning in
man by 2,4-dichlorophenoxyacetic acid (2,4-D): determination of the
agents in forensic materials. Acta pharmacol. toxicol., 22: 224-254
Orgell, W.H. (1957) Absorptive properties of plant cuticles. Proc.
Iowa Acad. Sci., 64: 189-198
Palmer, J.S. (1963) Chronic toxicity of 2,4-D alkanolamine salts to
cattle, J. Amer. vet. Med. Ass., 143: 398-399
Rowe, V.K. and Hymas, T.A. (1954) Summary of toxicological information
on 2,4-D and 2,4,5-T type herbicides and an evaluation of the hazards
to livestock associated with their use. Amer. J. vet. Res., 15:
622-629
Salo, T. and Makinen, R.Z. (1964) Thin-layer chromatography of
substances used for treatment of citrus fruit peel: -biphenyl,
o-phenyl phenol and 2,4-D. Z. Lebensm. u. Forsch., 125: 170-171
Schieferstein, R.H. (1957) Development of protective structures of the
plant cuticle. Ph. D. dissertation, Iowa State College, Ames, Iowa,
U.S.A.
Slife, J.W., Key, J.L., Yamaguchi, S. and Crafts, A.D. (1962)
Penetration, translocation and metabolism of 2,4-D and 2,3,5-T in wild
and cultivated cucumber plants. Weeds, 10: 29-35
Stanosz, S. (1969) Effect of sodium 2,4-dichlorophenoxyacetate on
tissue respiration in parenchymatous organs of rats. (in Polish). Med.
Piacy, 20b: 600-605. Chem. Abstr., 73: (1970)
Warshowsky, B. and Schantz, E.J. (1950) Determination of
2,4-dichlorophenoxyacetic acid in soil. Anal. Chem., 22: 460-463
Way, J.M. (1969) Toxicity and hazards to man, domestic animals and
wildlife from some commonly used auxin herbicides. Res. Rev., 26:
37-62
Weintraub, R.L. and Norman, A.G. (1950) Recovery of growth regulator
from plants treated with 2,4-dichlorophenoxyacetic acid. Science,
111: 493-494
Weintraub, R.L., Brown, J.W., Fields, M. and Rohan, J. (1952a)
Metabolism of 2,4-dichlorophenoxyacetic acid. I. C14O2 production
by bean plants treated with labeled 2,4-dichlorophenoxyacetic acids.
Plant Physiol., 27: 292-301
Weintraub, R.L., Yeatman, J.N., Lockhart, J.A., Reinhart, J.H. and
Fields, M. (1952b) Metabolism of 2,4-dichlorophenoxyacetic acid. II.
Metabolism of the side chain by bean plants. Arch. Biochem. Biophys.,
40: 277-285
Weintraub, R.L., Reinhart, J.H., Scherff, R.A. and Schisler, L. (1954)
Metabolism of 2,4-dichlorophenoxyacetic acid. III. Metabolism and
persistence in dormant plant tissue. Plant Physiol., 29: 303-304
Williams, S. (1964) Pesticide residues in total diet samples. J.
Assoc. Offic. Agric. Chem., 47: 815-821
Yip, G. and Meys, R.E. (1966) Analysis of 2,4-D residues in milk and
forage. Weeds, 14: 167-170
 Other relevant chemical properties
White powder: m.p., 140.5°C; solubility in water at room temperature
is 620 mg/1; soluble in aqueous alkali and in alcohols; insoluble in
petroleum oils. V.p., 0.4 mm Hg at 160°C. Can be used as alkali metal
salt, amine salts and esters.
Purity
Technical, 98 percent pure
EVALUATION FOR ACCEPTABLE DAILY INTAKE
N.B. Except when otherwise specified, toxicity studies on 2,4-D are
assumed to have been performed using the free acid.
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
When administered orally as an amine salt, 2,4-D is rapidly absorbed
in animals, concentrations in plasma reaching a peak after two hours
for chickens, or four to seven hours for rats, calves and pigs.
Similar results are seen after oral administration of alkali metal
salts of 2,4-D. In the case of an ester of 2,4-D, absorption by the
oral route is poor, as evidenced by low plasma and tissue levels
following administration. The intact eater has not been detected in
plasma or urine following treatment to rats, pigs or calves; only the
free acid is found, a fact which indicates that hydrolysis occurs
before absorption. Absorbed 2,4-D is distributed throughout the body;
levels in liver, kidney, spleen and lung reach a maximum after six
hours and after this time levels in these organs often exceed the
level in plasma, particularly in chickens and rats. There appears to
be little variation of distribution between the sexes. In a study with
pigs, high levels of 2,4-D were also found in endocrine glands and in
the excretory organs. The brain level was usually low, relative to the
plasma level. However, 2,4-D passes the placental barrier, as
evidenced by experiments in pigs. The distribution pattern after
administering an ester of 2,4-D is similar to that of the salts,
although tissue levels are lower. A partial binding of 2,4-D to plasma
proteins may occur. The biological half-life in plasma for chickens,
rats, calves and pigs was found to be 8, 3, 8 and 12 hours,
respectively. Elimination of 2,4-D when administered as an ester is
less rapid than that of salts. Excretion is principally via the
kidney, and only low levels have been found in the faeces or bile.
Hens have been reported to excrete 2,4-D in their eggs, the compound
occurring largely in the yolk (Erne, 1966a, 1966b).
Rats were given by stomach-tube doses of 2,4-D labelled with 14-carbon
in the 1 or 2 positions; the compounds had specific activities of 3.03
mCi/mM and 1 mCi/mM, respectively. They were given in doses of 1, 5,
10, 40, 60, 80 or 100 mg per rat. Urine, faeces and expired air were
collected. No radioactivity was detected in the breath at any time
during three days following administration. In the rats given 1 to 10
mg of 2,4-D, 94 to 99 percent was excreted over a 72 hour period,
mostly during the first 24 hours. In animals fed the higher doses of
20 to 100 mg, the percentage excreted bore an inverse linear
relationship to the close administered for both sexes, being 75.5
percent. After 144 hours in the rats given the maximum dose of 100 mg.
This changed pattern of excretion indicated a slower excretion of the
higher doses. In the case of six rats which were given 1 mg of
labelled 2,4-D per rat and a group of seven rats which were given 80
mg, these animals were sacrificed at varying intervals from 1 to 41
hours following administration, and levels of radioactivity in various
organs and tissues were determined. Radioactivity was found in all
tissues examined and reached a maximum six to eight hours after
administration. Excretion was also rapid and after 41 hours in the
animals given 80 mg, only the liver and kidney contained more than 1
ppm (on a dry tissue weight basis) of 2,4-D, the figures for these
organs being 1.5 and 1.8 ppm, respectively. With the rats given 1 mg,
levels below 0.1 ppm were reached in all organs except the stomach
after 12 hours (Khanna and Fang, 1966).
A phenomenon of declining plasma levels following repeated treatment
with 2,4-D has also been observed in chickens and pigs and has been
suggested as being indicative of the animal's developing an adaption
to 2,4-D (Erne, 1966a). See also under 'Fate of residues'.
In a case of fatal poisoning in many 2,4-D was found in blood, urine
and tissues of all organs the brain had the lowest concentration
- less than one fiftieth of that in the blood (Nielzen et al., 1965).
Effects on enzymes and other biochemical parameters
In rats which were given 10 mg/kg body-weight of the sodium salt of
2,4-D by stomach tube for 120 days, the oxygen uptake decreased from
2.14 to 1.88 mm3/mg of dry tissue in the heart, from 2.3 to 2.2 in
the liver and from 5.7 to 2.32 in the kidneys (Stanosz, 1969).
The effect of ingested phenoxy-acid herbicides on muscular function
has been suggested to be related to interference with carbohydrate
metabolism. Transitory diabetiform conditions have been reported in
individuals spraying these compounds. However, hyperglycaemia or
glycosuria could not be reproduced with certainty in rabbits; only one
in five animals responded in this manner following administration of
doses ranging from 125 to 500 mg/kg body-weight from 6 to 50 days.
(Lorenzen and Lyngsoe, 1957; Dalgaard-Mikkelsen and Poulsen, 1962).
Attention has been drawn to the increase in nitrate content which has
been observed in certain plants after spraying with 2,4-D. This
increase has been thought to be the cause of toxic effects which have
been observed in grazing animals. Abortions in cattle have also been
attributed to this phenomenon. Information on this possible indirect
toxic action of 2,4-D has been summarized (Way, 1969). Manifestation
of toxicity appears, however, to be confined to farm animals, and no
reports have been encountered which would indicate that human food
exposed to 2,4-D as a growing crop has become toxic in this manner.
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Mouse
Repeated injections of 2,4-D have not influenced the growth rate of
two transplated sarcomas in mice. Mitotic counts performed at various
intervals in these tissues after a single injection of 200 mg/kg body-
weight of 2,4-D did not differ significantly from a control not
treated with 2,4-D (Bucher, 1946).
Groups of 18 mice of each sex from two hybrid strains were given 2,4-D
from seven days of age for 18 months. The compounds were given daily
by gavage at levels of 0, 46.4 or 100 mg/kg body-weight until weaning,
after which time 2,4-D was incorporated into the diet at the
corresponding levels of 0, 149 or 323 ppm, respectively. There was no
significant increase in tumours between the controls and the groups
given the two levels of 2,4-D (Innes et al., 1969).
Special studies on reproduction
Chicken embryo
When injected into fertile eggs at 5 or 10 mg/egg, 2,4-D did not
produce malformations, although it was stated that certain
phenoxyacetic acids produced feather blanching. The percentage hatch
was 70 and 50 percent for the eggs receiving the two respective levels
of 2,4-D (Dunachie and Fletcher, 1967).
Chicken
Groups of three-day old chicks were given 0 (25 chicks) or 1000 ppm
(29 chicks) of 2,4-D in their drinking water. Weight-gain, age of
sexual maturity and onset of egg production did not differ between the
test and control groups. However, the number, and possibly the weight,
of the eggs was reduced in the group given 2,4-D (Björkland and Erne,
1966).
Mouse
Mice which underwent daily injections of about 90 mg/kg body-weight of
2,4-D became pregnant and bore apparently normal litters at the end of
a normal gestation period (Bucher, 1946).
Rat
Groups, each comprising five newly mated female rats, received 0 or
1000 ppm of 2,4-D in their drinking water during their pregnancy and
for a further ten months. Pregnancy and parturition were normal.
Litter size was not significantly different between the test and
control groups. No malformations were observed in the young nor were
any clinical or morphological abnormalities revealed. After weaning,
the young rats were given 0 or 1000 ppm of 2,4-D in the same manner as
their parents for up to two years. The group receiving 2,4-D had
reduced food intake and depressed growth rate. Clinical-chemical
parameters were normal. Mortality was higher in the group given 2,4-D.
Relative organ weights and gross and histopathological examination
revealed no changes in the test group (Björkland and Erne, 1966).
It has been stated that the long-term feeding to rats of potatoes
which had been treated with 2,4-D affected reproduction. In the F2
generation, and even more in the F3 generation, the ability to
reproduce was reduced in the animals fed these potatoes (An der Lan,
1966).
Pig
A female pig was fed 500 ppm of an amine salt of 2,4-D during her
entire seventh pregnancy and for a further six weeks. Anorexia was
observed. One mummified foetus and 15 underdeveloped live young were
delivered. Ten of these died by the first day. Autopsy revealed a
generalized anaemia. No malformations were observed. The surviving
five young were fed 500 ppm of 2,4-D amine salt; growth was retarded
and locomotor disturbances were observed. Clinical-chemistry results
were the same as described under the chronic feeding study for the pig
(Björkland and Erne, 1966).
Special studies on toxicity of impurities
There is no information that the presence of chlorinated
dibenzodioxins, such as occur in some batches of technical 2,4,5-T,
has been looked for in technical 2,4-D. It is reported that the
condensation of the phenol precursors to form such compounds could not
occur in the manufacture of 2,4-D (Dow, 1970).
TABLE I
Acute Toxicity of 2,4-D
LD50
Animal Route mg/kg References
body-weight
Chicken oral 380-765 (amine salt) Bjorn and Northen, 1948
Chicken (mixed) oral 541 Rows and Hymas, 1954
Mouse oral 375 Hill and Carlisle, 1947
Mouse (m) oral 368 Rowe and Hymas, 1954
Mouse s.c. 280 Bucher, 1946
Rat oral 666 Hill and Carlisle, 1947
Rat (m) oral 375 Rowe and Hymas, 1954
Guinea,pig oral 1000 Hill and Carlisle, 1947
Guinea pig
(mixed) oral 469 Rowe and Hymas, 1954
Rabbit oral 800 Hill and Carlisle, 1947
Dog oral 100 Drill and Hiratzka, 1953
Dog oral 541 Rowe and Hymas, 1954
Monkey oral > 428 Hill and Carlisle, 1947
For man the lethal dose of 2,4-D has been estimated to be greater than
80 mg/kg body-weight (Nielsen et al., 1965).
Symptoms of myotonia in mice were evident after the parenteral
administration of 150-200 mg/kg body-weight (Bucher, 1946).
Initial signs following the administration of a lethal dose of 2,4-D
to dogs were often not present until six hours following oral
administration. The animal then became ataxic with progressive
increase in spasm. Pathological changes were limited to the
gastrointestinal tract, lungs and liver. Death in most cases appeared
to be due to hepatic congestion or to pneumonia, which followed the
development of anorexia, weight loss and myotonia (Drill and Hiratzka,
1953).
No toxic symptoms were observed when monkeys received 214 mg/kg
body-weight orally, but 428 mg/kg caused nausea, vomiting, lethargy,
muscle incoordination and head drop. All species reacted similarly;
death from large doses was thought to be due to ventricular
fibrillation (Hill and Carlisle, 1947).
Short-term studies
Chicken
Groups, each of five chickens, were given an amine salt of 2,4-D three
times a week in oral doses of 0, 0.28, 2.8, 28, or 280 mg/kg
body-weight (acid equivalent) for up to four weeks (a total of 12
doses). Weight gain was reduced only at the 280 mg/kg level (Bjorn and
Northen, 1948).
In the study described under "Reproduction" three day old chicks were
given 0 or 1000 ppm of 2,4-D. In the group given 2,4-D, gross
pathology revealed enlarged kidneys, and histopathology showed changes
in the kidney tissue mostly in the proximal convoluted tubules
(Björkland and Erne, 1966).
Mouse
Injection of 50 to 90 mg/kg body-weight of 2,4-D subcutaneously to
mice for three weeks to three months failed to produce a clear-cut
syndrome of chronic toxicity. Levels of 70 mg/kg retarded growth,
however, probably due to reduced food intake (Bucher, 1946).
Rat
Rats were fed a dietary level of 1000 ppm of 2,4-D for one month
without harmful effects (Hill and Carlisle, 1947).
Groups each comprising five or six young female rats were given 0, 3,
10, 30, 100 or 300 mg/kg body-weight of 2,4-D by stomach tube five
times a week for periods up to four weeks. The animals which received
30 mg/kg or less showed no adverse effects, as judged by gross
appearance, behaviour, mortality, growth, haematological values,
blood-urea nitrogen, organ weights, gross pathology and
histopathology. The rats on 100 mg/kg showed varying degrees of
gastrointestinal irritation, slight cloudy swelling of the liver and
depressed growth rate. The animals given 300 mg/kg failed rapidly and
died principally of severe gastrointestinal irritation (Rowe and
Hymas, 1954).
In a separate experiment, groups each of five young female rats, were
fed dietary levels of 0, 100, 300, 1000, 3000 or 10,000 ppm of 2,4-D
for periods up to 113 days. The animals fed 300 ppm or less showed no
adverse effects as judged by the parameters described in the previous
experiment. Those given 1000 ppm had increased mortality, depressed
growth rate, slightly increased liver weight and slight cloudy
swelling of the liver. The animals fed 3000 or 10,000 ppm were
sacrificed after 12 days because of food refusal and rapid
weight-loss. Autopsy revealed increased liver and kidney weights and
there were slight pathological changes in the organs (Rowe and Hymas,
1954).
Guinea pig
Guinea pigs tolerated 10 oral doses of 100 mg/kg bodyweight of 2,4-D
given over a 12 day period. Inhalation of the sodium salt of 2,4-D as
a dust failed to produce systemic effects (Hill and Carlisle, 1947).
Dog
Groups of from two to four dogs of mixed sexes were given 0, 2, 5, 10
or 20 mg/kg body-weight of 2,4-D orally by capsule on five days a week
for 13 weeks. All the dogs given 10 mg/kg or less survived the 90-day
test period. Of two male dogs given 20 mg/kg, one died after 18 days,
the other after 25 days. A female given 20 mg/kg/day in two divided
doses died after 49 days; another male dog on the same dose regimen
survived the 90-day period. Loss of weight occurred only in the dogs
which ultimately died, and this phenomenon began seven to 12 days
prior to death. Dogs that survived 90 days were free of symptoms: in
the others, ataxia and increased muscle tonus occurred prior to death.
The blood picture was normal, except for a terminal fall in lymphocyte
count prior to death. Weights of thyroid, adrenals, heart, liver and
kidney were not different from the controls in the surviving animals;
in two of the dogs that died, there was a slight increase in heart and
kidney weights. Two dogs, one receiving 2 and the other 20 mg/kg
showed redness in the duodenum. Histopathology revealed no changes in
heart, lungs, thyroid, adrenal or testes. Two dogs receiving an
unspecified level of 2,4-D showed areas of focal necrosis in the
liver, which was considered not to be related to administration of
2,4-D (Drill and Hiratzka, 1953).
Pig
An amine salt of 2,4-D and 2,4-D ester were given to young pigs in
doses of 50, 100 or 300 mg/kg body-weight at varying intervals up to
103 days. Symptoms of intoxication were similar to those reported from
studies with laboratory animals, and autopsy revealed similar
pathological findings. Clinical signs of anorexia and retarded growth
were evident in one pig given 51 doses of 50 mg/kg during 103 days
(Björklund and Erne, 1966).
On the basis of this experiment it has been suggested that the pig may
be more sensitive to 2,4-D than other animals tested, since the
no-effect level appears to be less than 50 mg/kg body-weight/day
(Erne, 1966a).
Five pigs were fed an amine salt of 2,4-D at 0 or 500 ppm in their
diet for up to 12 months. Food consumption and growth rate was reduced
in the controls, and after about one month, three animals developed
locomotor disturbances of increasing severity. The animals were
sacrificed after two to 12 months. Organ weights were normal, and
gross pathological changes were not evident. Clinical-chemical
observations involved lowered haemoglobin and haematocrit values,
elevation of glutamic-oxaloacetic transaminase and reduced albumin and
albumin-globulin ratios in the treated animals (Björklund and Erne,
1966).
Cattle
When steers were given oral doses of 0, 50, 100, 200 or 250 mg/kg
body-weight of an amine salt of 2,4-D for five days a week, poisoning
was evident at 250 mg/kg after 15 treatments and at 100 mg/kg after 86
treatments. A dose of 50 mg/kg given 112 times had no effect on one
steer (Palmer, 1963).
Long-term studies
No complete long-term studies appear to have been conducted.
OBSERVATIONS IN MAN
A man has been reported to have taken 500 mg of 2,4-D daily for three
weeks (approximately 8 mg/kg body-weight) without experiencing any
harmful effects. No further details are given (Assouly, 1951).
A man aged 23 committed suicide by oral intake of the dimethylamine
salt of 2,4-D. Pronounced degenerative changes of the ganglion cells
of the brain were found upon histological examination (Nielsen et al.,
1965).
Subjective clinical symptoms have been reported among workers using
various esters of and the sodium salts of 2.4-D. Individuals
complained of rapid fatigue, headache, liver pains, loss of appetite,
etc. Certain functional shifts were noted in the cardiovascular
system, and sensitivity to taste and smell was lowered (Fetisov,
1966).
In workers employed in factories manufacturing 2,4-dichlorophenol and
other chlorinated phenols, a moderately high incidence of urinary
porphyria, chloracne and hirsutism has been reported. The authors
suggest that a highly chlorinated phenolic ether may be the compound
responsible (Bleiberg at al., 1964).
Clinical symptoms of peripheral neuropathy have been reported in three
individuals who had direct skin contact with an eater of 2,4-D. An
electromyographic examination supported the diagnosis (Goldstein et
al., 1959).
COMMENTS
Information on the absorption and excretion of 2,4-D in several
species indicates that the compound is rapidly excreted unchanged, and
that it is not stored in the tissues in mammals. Reproduction studies
are limited to a single generation but do not indicate that 2,4-D is a
teratogen. There is no information that toxic chlorinate
dibenzodioxins have been looked for in commercial 2,4-D.
Increased nitrate formation has been reported to occur in plants
treated with 2,4-D and is thought to have resulted in toxic effects in
grazing animals. This observation is of concern if 2,4-D is to be used
on edible crops.
Attention was drawn to the absence of any complete long-term studies
in any species. For this reason, no acceptable daily intake for 2,4-D
could be established.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
A pre- or post-emergent selective herbicide in cereals used for
prevention of preharvest fruit drop, the production of harvest fruit
drop, the production of seedless fruit and the regulation of the
growth of plants.
Acid in oil formulations control many broad-leaved annual and
perennial weeds on roadside verges, around farm buildings, etc.
FATE OF RESIDUES
In animals
When a cow was fed 5 ppm of 2,4-D in a 23 kg ration for five days,
none of the herbicide could be detected in the milk or faeces
throughout the study; the analytical limit of detection was 0.1 ppm.
The compound was found to disappear from the rumen, due presumably to
dilution, followed by absorption on the gut wall and by decomposition.
When 2,4-D was added to artificially collected rumen at a level of 2
ppm it was not decomposed (Gutenmann and Lisk, 1963; Gutenmann et al.,
1963).
In pigs which developed symptoms of poisoning following repeated
administration of from 50 to 300 mg/kg body-weight of an amine salt of
2,4-D or of 2,4-D ester, high plasma levels of 2,4-D were found (200
to 400 µg/ml) even 24 hours after the dose was given. Elimination
occurred at an enhanced rate after repeated administration and
eventually decreased to about 10, µg/ml (Björkland and Erne, 1966).
A capsule containing 539.6 µCi of 2-14 C-labelled 2,4-D was
administered orally to a female sheep of weight 26.6 kg. This dose was
equivalent to 4 mg/kg body-weight, which was stated to be the amount
that the animal would receive while grazing in a treated pasture.
Urine and faeces were collected separately, and blood samples were
withdrawn from the jugular vein at intervals after administration of
the dose.
The animal was sacrificed after four days, and tissues were assayed
for carbon-14. Levels of radioactivity in the blood rose during the
first half hour following treatment and reached a peak after one and a
half hours. By 24 hours, the radioactivity level in blood had
decreased to background. About 15 percent of the original dose of
2,4-D was found in the urine after one and a quarter hours, 50 percent
after eight and a half hours and 96 percent after 70 hours. Total
collected faeces contained about 1.4 percent of the dose. Paper
chromatography and electrophoresis from urine extracts revealed only
one spot of the same Rf as 2,4-D, indicating that the compound is
excreted unchanged in the urine. Tissue levels, as determined
following slaughter four days after administration, contained less
than 0.05 ppm radioactive equivalents, with the exception of thyroid
and urinary bladder, which contained 0.56 and 0.5 ppm, respectively.
The nature of these residues in tissue was not determined (Clark et
al., 1964).
In plants
It has been demonstrated by a number of workers that 2,4-D is
translocated in plants (Dhillon and Lucas, 1950; Holley et al., 1950;
Fang et al., 1951), since free phenoxyacetic acids could be isolated
from parts well removed from the site of application. Jaworski and
Butts (1952) demonstrated the formation of a complex of 2,4-D in
plants, and Holley et al. (1950) indicated that plants could detoxify
2,4-D to form hydroxylated products. Weintraub and Norman, (1950) and
McIlrath and Ingle (1953), showed that this destruction was not rapid
in all plants. 14C-labelled material led to the recognition that
some of the applied material was complexed (Jaworski and Butts, 1952).
Existence of oxidative degradation of 2,4-D was shown by Holley et al.
(1950) and Weintraub et al. (1952b) Holley et al. 1950) and Holley
(1952) demonstrated the evolution of 14CO2 after treating plants
with 14C-2,4-D, and the formation of a product more water soluble
than 2,4-D acid. Evolution of 14CO2 from plants treated with either
methylene-labelled or carboxyl-labelled 2,4-D has been shown by Holley
(1952) and Weintraub et al. (1952a, 1954). The side chain of
phenoxyacetic acids can be removed by such plants as currants
(Luckwill and Lloyd-Jones, 1960), wild cucumber (Slife et al., 1962)
and tick beans (Canny and Marcus, 1960). Roots have been shown by
Cann and Marcus (1960) to be of greater efficiency in decarboxylation
than shoots. Not all of the carbon liberated from breakdown of the
side chain of phenoxyacetic acids is evolved as 14CO2, but some is
incorporated into plant constituents (Weintraub at al., 1952a;
Luckwill and Lloyd-Jones, 1960). Luckwill and Lloyd-Jones (1960)
studied the degradation of 2,4-D by currants, apples and strawberries.
They showed that carboxyl-labelled phenoxyacetic acid more readily
yielded 14CO2 than did the methylene-labelled acid.
By spectrophotometric analysis, Schieferstein (1957) showed that the
rate of penetration of 2,4-D through ivy cuticle varied with the age,
and hence thickness of the cuticle. Orgell (1957) found cationic and
anionic surfactants hindered the sorption of 2,4-D at low pH; nonionic
surfactants had little effect. At high pH, cationic surfactants caused
sorption, whereas anionic and non-ionic surfactants had little effect.
2,4-D applied to roots may move to tops only in minute quantities, and
only after some days; applied to leaves, it may pass downward into the
stem and roots in quantity within a few hours.
In soil
2,4-D has been shown to be biodegradable in soils by DeRose and Newman
(1948), Brown and Mitchell (1948) and Audus (1950). Audus (1964)
listed numerous micro-organisms which are capable of degrading 2,4-D.
Evidence of residues in food in commerce or at consumption
After application of 2,4-D to bean plants and oats, 25-60% was found
in tissues, but only 0.33-1.66% in oats (Boyle, 1954). Some
unpublished data was presented (BASF, 1970) regarding residues of
2,4-D in cereals (grain and straw) found to occur following
applications of the herbicide either alone or in admixture with other
phenoxyacid herbicides (MCPA, mecoprop). A summary of the data
relating to 2,4-D is given in Table II. Williams (1964) was unable to
detect 2,4-D in a number of total diet samples at a sensitivity of
0.01 ppm. Duggan and Weatherwax (1967) in total diet studies found
herbicide chemicals infrequently, averaging an intake of about 0.01
mg/kg of which one-third was 2,4-D. Very small amounts of 2,4-D were
found in oils and fats (0.001 mg in 1964/5, and sugars and sugar
products (0.004 mg 1964/5, and 0.002 mg in 1965/6).
TABLE II
Residues of 2,4-D in cereals
Crop Rate of Intervals (days) Residues found
Application Applic.-Harvest Grain Straw
Barley 375 g/ha 64 <0.01 0.08
77 <0.01 0.02
TABLE II (cont'd)
Residues of 2,4-D in cereals
Crop Rate of Intervals (days) Residues found
Application Applic.-Harvest Grain Straw
Barley 520 g/ha 64 n.d.1 0.1
77 n.d. n.d
735 g/ha 64 0.015 0.34
77 n.d. n.d
375 g/ha 76 n.d. 0.02
89 n.d. 0.04
Wheat 520 g/ha 76 n.d. n.d
89 n.d. n.d
735 g/ha 76 n.d. 0.03
89 n.d. n.d
375 g/ha 76 0.01 0.02
89 n.d. n.d
Oats 520 g/ha 76 n.d. n.d
89 n.d. n.d
735 g/ha 76 n.d. 0.02
89 n.d. n.d
375 g/ha 76 n.d. 0.04
89 n.d. n.d
Rye 520 g/ha 76 n.d. n.d
89 n.d. n.d
735 g/ha 76 n.d. 0.04
89 n.d. <0.02
1 n.d. = not detected.
METHODS OF RESIDUE ANALYSIS
Residues of 2,4-D can be determined after suitable extraction,
separation and isolation by gas chromatography, ultraviolet
spectrophotometry, fluorimetry, thinlayer and paper chromatography.
The preferred method is to methylate the isolated 2,4-D and determine
by GLC. This method has been used for the following sample substrates:
forage (Hagin and Linscott, 1965; Gutenmann and Lisk, 1963; Yip and
Neys, 1966)
oysters (Duffy and Shelfoon, 1967), water (Devine and Zweig, 1969),
milk (Yip and Neys, 1966; Crosby and Bowers, 1966)
in fruit peel (Meagher, 1966), citrus fruit (Erickson and Hield, 1962)
and pineapple and orange peel (Hendrickson and Meagher, 1969).
Benvenue et al. (1962) determined residues of 2,4-D in dry crops and
walnuts by microcoulometric gas chromatography. Clerk et al. (1967),
extracted animal tissues and, after isolation of the 2,4-D, hydrolysed
it to 2,4-dichlorophenol, purified by steam distillation and then used
GLC. 2,4-D can be determined colorimetrically by the use of
chromotropic acid in sulphuric acid at a wavelength of 565 nm
(Marquardt and Luce, 1951 and 1955; Erickson and Brannaman, 1954). Aly
and Faust (1964) suggested using 6-amino-1-naphthol-3-sulphonic acid
and 6-anilino-1-naphthol-3-sulphonic acid, which are more sensitive
(1´-2´ times) than chromotropic acid. Marquardt and Luce (1961)
cleaved the phenoxy acid with pyridine hydrochloride, releasing the
phenol derivative 2,4-dichlorophenol, added 4-amino antipyrine and
potassium ferricyanide, and determined the complex at 515 nm.
Ultraviolet spectrophotometry at a wavelength of 284 nm was used by
Warshowsky and Schantz (1950), Aly and Faust (1963) and Gordon and
Beroza (1952). Kuznetsov and Gagarina (1962) determined 2,4-D in
plants by paper chromatographic separation, elution from strips and
colorimetric determination with butyl rhodamine at a wavelength of 564
nm. Salo and Makinen (1964) used silica gel thin-layer chromatography
with Blankopher DCB and examination under ultraviolet light at 360 nm
for visualization. Abbott et al. (1964) used paper and thin-layer
chromatography for detection, separation and identification. Guilbault
and Sadar (1969) used a thin-layer chromatographic separation followed
by a fluorimetric determination based on the breakdown of 4-methyl
umbelliferone heptanoate by lipase.
NATIONAL TOLERANCES
Country Crop Tolerance(ppm)
United States of Apples, pears, 5
America lemons, oranges,
(December 1969) grapefruits
Barley, oat, rye, 20
wheat and forage
Netherlands Barley, oat, rye, 0.5
wheat and grain
Vegetables, 0.05
(except potatoes)
fruits of vegetables
and fruit crops
Fed. Rep. of Germany Leafy and other 0.05
sprouting vegetables,
fruiting vegetables,
root vegetables
APPRAISAL
2,4-D is used in cereals as a selective pre- and post-emergence
herbicide for the control of broad-leaved weeds. It in also used to
prevent early fruit drop, as a plant growth regulator and for weed
control on roadside verges, around buildings, etc. Some evidence from
supervised trials regarding the possible occurrence of residues of
2,4-D in cereal grain and straw was provided. Insufficient information
relating to residues resulting from other uses was available. Total
diet studies in the U.S.A. have very occasionally revealed minimal
quantities of 2,4-D in oils and fats or sugar and sugar products. No
evidence regarding the need to establish practical residue limits was
apparent. Gas chromatographic methods are available which should be
adaptable for regulatory purposes where required.
Since no acceptable daily intake had been established, it was decided
not to recommend tolerances. Nevertheless, it was decided to record
that following officially acceptable use in various countries.
residues of 2,4-D can occur in the following crops:
Wheat, barley, oats, rye and grain 0.02 ppm
Wheat, barley, oats, rye and straw 0.5 ppm
FURTHER WORK OR INFORMATION
REQUIRED (before an acceptable daily intake for man can
be established).
1. An adequate long-term oral study in a rodent
species and at least a two-year study in a
nonrodent mammalian species.
2. A three-generation reproduction study in at
least one mammalian species.
3. Information on the nature of the impurities
occurring in commercial preparations of 2,4-D.
DESIRABLE
1. Further information on the metabolism of 2,4-D
in plants, laboratory animals and man.
2. Elucidation of the problem of increased nitrate
formation in plants treated with 2,4-D, to
determine if this phenomenon could create a
toxic hazard to man.
3. Information on the occurrence of 2,4-D residues
in crops other than cereals.
REFERENCES
Abbott, D.C., Egan, H., Hammond, E.W. and Thomson, J. (1964) The
chromatographic detection and determination of organo-chlorine
herbicides in soil and water. Analyst, 89: 480-488
Aly, O.M. and Faust, S.D. (1963) Determination of
2,4-dichloro-phenoxyacetic acid in surface water, J.Amer. Waterworks
Assoc., 55: 639-646
Aly, O.M. and Faust, S.D. (1964) Determination of phenoxyacetic acids
with J and phenyl J acids. Anal.Chem., 36: 2200-2201
An der Lan, H. (1966) The present situation of toxicology in the field
of crop protection. Res. Rev., 15: 31-43
Assouly, M. (1951) Désherbants sélectifs et substances de croissance.
Aperçu technique. Effet pathologique our l'homme an cours de la
fabrication de l'ester de 2,4-d. Arch.Mal.Prof., 12: 26-30
Audus, L.J. (1950) Biological detoxication of
2,4-dichlorophenoxyacetic acid in soils. Nature, 166: 356
Audus, L.J. (1964) The physiology and biochemistry of herbicides.
Academic Press
BASF (1970) Residues of 2,4-D in cereal grain and straw. (unpublished
report). Badische Anilin und Soda Fabriken
Bevenue, A., Zweig, G. and Nash, N.L. (1962) Residue determination of
2,4-dichlorophenoxyacetic acid in dry crops and walnuts. J. Assoc.
Offic. Agric. Chem., 45: 990-993
Björklund, N-E. and Erne, K. (1966) Toxicological studies of
phenoxyacetic herbicides in animals, Acta vet. scand., 7: 364-390
Bjorn, M.K. and Northen, H.T. (1948) Effects of
2,4-dichlorophenoxyacetic acid on chicks. Science, 108: 479-480
Bleiberg, J., Wallen, M., Brodkin, R. and Applebaum, I.L. (1964)
Industrially acquired porphyria. Arch.Dermatol., 89: 793-797
Boyle, F.P. (1954) Physiology and chemistry of
2,4-dichlorophenoxyacetic acid on resistant and non-resistant plants.
Congr. intern. botan., Paris, Rapps et communs., 8: 184-185
Brown, J.W. and Mitchell, J.W. (1948) Inactivation of 2,4
dichlorophenoxyacetic acid in soil as effected by soil moisture,
temperature, the addition of manure and autoclaving. Botan. Gaz.,
109: 314-323
Bucher, N.L.R. (1946) Effects of 2,4-dichlorophenoxyacetic acid on
experimental animals. Proc. Soc. exp. Biol. Med., 63: 204-205
Canny, M.J. and Marcus, K. (1960) Metabolism of
2,4-dichlorophenoxyacetic acid in bean plants. Australian J. Biol.
Sci., 13: 486-491
Clark, D.E., Young, J.E., Younger, R.L., Hunt, L.M. and McLaran, J.K.
(1964) The fate of 2,4-dichlorophenoxyacetic acid in sheep. J. Agr.
Food Chem., 12: 43-45
Clark, D.E., Wright, F.C. and Hunt, L.M. (1967) Determination of 2,4-D
residues in animal tissues. J. Ag. Food Chem. 15: 171-173
Crosby, D.G. and Bowers, J.B. (1966) Determination of 2,4-D residues
in animal products, Bull. Environ. Contam. Toxicol., 1: 104-107
Dalgaard-Mikkelsen, S. and Poulsen, E. (1962) Toxicology of
herbicides. Pharmacol. Rev., 14: 225-250
De Rose, H.R. and Newman, A.S. (1948) The effect of certain plant
growth regulators on soil microorganism and microbial processes, Proc.
Soil Sci. Soc. Amer., 12: 217-221
Devine, J.M. and Zweig, G. (1969) Note on the determination of some
chlorophenoxy herbicides and their esters in water. J. Assoc. Offic.
Anal. Chem., 52: 187-189
Dhillon, A.S. and Lucas, E.H. (1950) Absorption, translocation and
persistence of 2,4-D in some plants. Botan. Gaz., 112: 198-207
Dow (1970) Information provided verbally to WHO
Drill, V.A. and Hiratzka, T. (1953) Toxicity of
2,4-dichlorophenoxyacetic acid and 2,4,5-trichlorophenoxyacetic acid.
Arch. industr. Hyg., 7: 61-67
Duggan, R.E. and Weatherwax, J.R. (1967) Dietary intake of pesticide
chemicals. Science, 157: 1006-1010
Dunachie, J.F. and Fletcher, W.W. (1967) Effect of some herbicides on
the hatching rate of hen's eggs. Nature, 215: 1406-1407
Erickson, L.C. and Brannaman, B.L. (1954) Chromatropic acid method for
determining 2,4-D residues. Hilgardia, 23: 175-184
Erickson, L.C. and Hield, H.Z. (1962) Determination of some
2,4-dichlorophenoxyacetic acid in citrus fruit. J. Agr. Food Chem.,
10: 204-207
Erne, K. (1966(a)) Distribution and elimination of chlorinated
phenoxyacetic acids in animals. Act Vet. Scand. 7: 240-256
Erne K. (1966(b)) Animal metabolism of phenoxyacetic herbicides. Act
Vet. Scand., 7: 264-271
Fang, S.C., Jaworski, G., Logan, A.V., Freed, V.H. and Butts, J.S.
(1951) The absorption of radioactive 2,4-dichlorophenoxyacetic acid
and the translocation of carbon-14 by bean plants. Arch. Biochem.
Biophys., 32: 249-255
Fetisov, M.I. (1966) Problems of occupational hygiene in work with
herbicides of 2,4-D group (in Russian). Gig. sanit., 9: 28-31
Goldstein, N.P., Jones, P.H. and Brown, J.R. Peripheral neuropathy
after exposure to an ester of 2,4-D, J. Amer. med. Ass., 171:
1306-1309
Gordon, N. and Beroza, M. (1952) Spectrophotometric determination of
small quantities of 2,4-dichlorophenoxyacetic acid and
2,4,5-trichlorophenoxyacetic acid. Anal. Chem., 24: 1968-1971
Guilbault, G.G. and Sadar, M.H. (1969) Fluorometric determination of
pesticides. Anal. Chem., 41: 366-368
Gutenmann, W.H., Hardee, D.D., Holland, R.F. and Lisk, D.J. (1963)
Residue studies with 2,4-dichlorophenoxyacetic acid herbicides in the
dairy cow and in a natural and artificial rumen. Dairy Sci., 46:
1287-1288
Gutenmann, W.H. and List, D.J. (1963) Rapid determination of 4(2,4-DB)
and a metabolite, 2,4-D, in treated forage by electron affinity
spectroscopy. J. Agr. Food Chem., 11: 304-306
Hagin, R.D. and Linscott, D.L. (1965) Determination of
4(2,4-dichlorophenoxybutyric acid (2,4-DB) and
2,4-dichlorophenoxyacetic acid (2,4-D) in forage plants. J. Agr. Food
Chem., 13: 123-125
Hendrickson, R. and Meagher, W.R. (1969) Spray residues of 2,4-D and
2,4,5-TP in "pineapple" orange peel. J. Agr. Food Chem., 17: 601-603
Hill, E.V. and Carlisle, H. (1947) Toxicity of
2,4-dichlorophenoxyacetic acid for experimental animals. J. industr.
Hyg. Toxicol., 29: 85-95
Holley, R.W., Boyle, F.P. and Hand, D.B. (1950) Fate of radioactive
2,4-dichlorophenoxyacetic acid in bean plants. Arch. Biochem., 27:
143-151
Holley, R.W. (1952) Studies of the fate of radioactive 2,4-D acid in
bean plants. II. A water soluble transformator product of 2,4-D. Arch.
Biochem. Biophys., 35: 171-175
Innes, J.R.M., Ulland, B.M., Valerii, M.G., Petrucelli, L., Fishbein,
L., Hart, E.R., Pallotta, A.J., Bates, R.R., Falk, H.C., Gart, J.J.,
Klein, M., Mitchell, I. and Peter, J. (1969) Bioassay of pesticides
and industrial chemicals for tumorigenicity in mice: a preliminary
note, J. nat. Cancer Inst., 42: 1101-1114
Jaworski, E.G., and Butts, J.S. (1952) Plant metabolism II. The
metabolism of carbon 14 labeled 2,4-dichlorophenoxyacetic acid in bean
plants. Arch. Biochem. Biophys., 38: 207-218
Khanna, S. and Fang, S.C. (1966) Metabolism of C14-labelled 2,4
dichlorophenoxyacetic acid in rats. J. Agr. Fd. Chem., 14: 500-503
Kuznetsov, V.J. and Gagarina, M.I. (1962) Photometric determination of
2,4-dichlorophenoxyacetic acid (2,4-D) with butyl rhodamine. Zhur.
Anal. Khim., 17: 235-237
Lorenzen, I. and Lyngse, J. (1957) En undersgelse over virkningen af
et kloreret fenoxyacetat pa kulhydratstofskiftet pa kaniner. Nord.
hyg. T., 38: 153-157
Luckwill, L.C. and Lloyd-Jones, C.P. (1960) Metabolism of plant growth
regulators. I. 2,4-Dichlorophenoxyacetic acid (2,4-D) in leaves of red
and black currant. Ann. Appl. Biol., 48: 613-625
Marquardt, R.P. and Luce, E.N. (1951) Determination of small amounts
of 2,4-dichlorophenoxyacetic acid in milk, Anal. Chem., 23:
1484-1486
Marquardt, R.P. and Luce, E.N. (1955) Determination of
2,4-dichlorophenoxyacetic acid (2,4-D) in grain and seed. J. Agr. Food
Chem., 3: 51-53
Marquardt, R.P. and Luce, E.N. (1961) A new procedure for determining
phenoxyacid herbicides in agricultural products. J. Agr. Food Chem.,
9: 266-270
McIlnath, W.J. and Ingle, D.R. (1953) Further evidence of the
persistence of 2,4-D in cotton. Plant Physiol., 28: 693-696
Meaghen, W.R. (1966) Determination of 2,4-dichlorophenoxyacetic acid
and 2(2,4,5-trichlorophenoxy) propionic acid in citrus by electron
capture gas chromatography. J. Agr. Food Chem., 14: 374-377
Nielsen, K., Kaempe, B., and Jensen-Holm, J. (1965) Fatal poisoning in
man by 2,4-dichlorophenoxyacetic acid (2,4-D): determination of the
agents in forensic materials. Acta pharmacol. toxicol., 22: 224-254
Orgell, W.H. (1957) Absorptive properties of plant cuticles. Proc.
Iowa Acad. Sci., 64: 189-198
Palmer, J.S. (1963) Chronic toxicity of 2,4-D alkanolamine salts to
cattle, J. Amer. vet. Med. Ass., 143: 398-399
Rowe, V.K. and Hymas, T.A. (1954) Summary of toxicological information
on 2,4-D and 2,4,5-T type herbicides and an evaluation of the hazards
to livestock associated with their use. Amer. J. vet. Res., 15:
622-629
Salo, T. and Makinen, R.Z. (1964) Thin-layer chromatography of
substances used for treatment of citrus fruit peel: -biphenyl,
o-phenyl phenol and 2,4-D. Z. Lebensm. u. Forsch., 125: 170-171
Schieferstein, R.H. (1957) Development of protective structures of the
plant cuticle. Ph. D. dissertation, Iowa State College, Ames, Iowa,
U.S.A.
Slife, J.W., Key, J.L., Yamaguchi, S. and Crafts, A.D. (1962)
Penetration, translocation and metabolism of 2,4-D and 2,3,5-T in wild
and cultivated cucumber plants. Weeds, 10: 29-35
Stanosz, S. (1969) Effect of sodium 2,4-dichlorophenoxyacetate on
tissue respiration in parenchymatous organs of rats. (in Polish). Med.
Piacy, 20b: 600-605. Chem. Abstr., 73: (1970)
Warshowsky, B. and Schantz, E.J. (1950) Determination of
2,4-dichlorophenoxyacetic acid in soil. Anal. Chem., 22: 460-463
Way, J.M. (1969) Toxicity and hazards to man, domestic animals and
wildlife from some commonly used auxin herbicides. Res. Rev., 26:
37-62
Weintraub, R.L. and Norman, A.G. (1950) Recovery of growth regulator
from plants treated with 2,4-dichlorophenoxyacetic acid. Science,
111: 493-494
Weintraub, R.L., Brown, J.W., Fields, M. and Rohan, J. (1952a)
Metabolism of 2,4-dichlorophenoxyacetic acid. I. C14O2 production
by bean plants treated with labeled 2,4-dichlorophenoxyacetic acids.
Plant Physiol., 27: 292-301
Weintraub, R.L., Yeatman, J.N., Lockhart, J.A., Reinhart, J.H. and
Fields, M. (1952b) Metabolism of 2,4-dichlorophenoxyacetic acid. II.
Metabolism of the side chain by bean plants. Arch. Biochem. Biophys.,
40: 277-285
Weintraub, R.L., Reinhart, J.H., Scherff, R.A. and Schisler, L. (1954)
Metabolism of 2,4-dichlorophenoxyacetic acid. III. Metabolism and
persistence in dormant plant tissue. Plant Physiol., 29: 303-304
Williams, S. (1964) Pesticide residues in total diet samples. J.
Assoc. Offic. Agric. Chem., 47: 815-821
Yip, G. and Meys, R.E. (1966) Analysis of 2,4-D residues in milk and
forage. Weeds, 14: 167-170
Other relevant chemical properties
White powder: m.p., 140.5°C; solubility in water at room temperature
is 620 mg/1; soluble in aqueous alkali and in alcohols; insoluble in
petroleum oils. V.p., 0.4 mm Hg at 160°C. Can be used as alkali metal
salt, amine salts and esters.
Purity
Technical, 98 percent pure
EVALUATION FOR ACCEPTABLE DAILY INTAKE
N.B. Except when otherwise specified, toxicity studies on 2,4-D are
assumed to have been performed using the free acid.
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
When administered orally as an amine salt, 2,4-D is rapidly absorbed
in animals, concentrations in plasma reaching a peak after two hours
for chickens, or four to seven hours for rats, calves and pigs.
Similar results are seen after oral administration of alkali metal
salts of 2,4-D. In the case of an ester of 2,4-D, absorption by the
oral route is poor, as evidenced by low plasma and tissue levels
following administration. The intact eater has not been detected in
plasma or urine following treatment to rats, pigs or calves; only the
free acid is found, a fact which indicates that hydrolysis occurs
before absorption. Absorbed 2,4-D is distributed throughout the body;
levels in liver, kidney, spleen and lung reach a maximum after six
hours and after this time levels in these organs often exceed the
level in plasma, particularly in chickens and rats. There appears to
be little variation of distribution between the sexes. In a study with
pigs, high levels of 2,4-D were also found in endocrine glands and in
the excretory organs. The brain level was usually low, relative to the
plasma level. However, 2,4-D passes the placental barrier, as
evidenced by experiments in pigs. The distribution pattern after
administering an ester of 2,4-D is similar to that of the salts,
although tissue levels are lower. A partial binding of 2,4-D to plasma
proteins may occur. The biological half-life in plasma for chickens,
rats, calves and pigs was found to be 8, 3, 8 and 12 hours,
respectively. Elimination of 2,4-D when administered as an ester is
less rapid than that of salts. Excretion is principally via the
kidney, and only low levels have been found in the faeces or bile.
Hens have been reported to excrete 2,4-D in their eggs, the compound
occurring largely in the yolk (Erne, 1966a, 1966b).
Rats were given by stomach-tube doses of 2,4-D labelled with 14-carbon
in the 1 or 2 positions; the compounds had specific activities of 3.03
mCi/mM and 1 mCi/mM, respectively. They were given in doses of 1, 5,
10, 40, 60, 80 or 100 mg per rat. Urine, faeces and expired air were
collected. No radioactivity was detected in the breath at any time
during three days following administration. In the rats given 1 to 10
mg of 2,4-D, 94 to 99 percent was excreted over a 72 hour period,
mostly during the first 24 hours. In animals fed the higher doses of
20 to 100 mg, the percentage excreted bore an inverse linear
relationship to the close administered for both sexes, being 75.5
percent. After 144 hours in the rats given the maximum dose of 100 mg.
This changed pattern of excretion indicated a slower excretion of the
higher doses. In the case of six rats which were given 1 mg of
labelled 2,4-D per rat and a group of seven rats which were given 80
mg, these animals were sacrificed at varying intervals from 1 to 41
hours following administration, and levels of radioactivity in various
organs and tissues were determined. Radioactivity was found in all
tissues examined and reached a maximum six to eight hours after
administration. Excretion was also rapid and after 41 hours in the
animals given 80 mg, only the liver and kidney contained more than 1
ppm (on a dry tissue weight basis) of 2,4-D, the figures for these
organs being 1.5 and 1.8 ppm, respectively. With the rats given 1 mg,
levels below 0.1 ppm were reached in all organs except the stomach
after 12 hours (Khanna and Fang, 1966).
A phenomenon of declining plasma levels following repeated treatment
with 2,4-D has also been observed in chickens and pigs and has been
suggested as being indicative of the animal's developing an adaption
to 2,4-D (Erne, 1966a). See also under 'Fate of residues'.
In a case of fatal poisoning in many 2,4-D was found in blood, urine
and tissues of all organs the brain had the lowest concentration
- less than one fiftieth of that in the blood (Nielzen et al., 1965).
Effects on enzymes and other biochemical parameters
In rats which were given 10 mg/kg body-weight of the sodium salt of
2,4-D by stomach tube for 120 days, the oxygen uptake decreased from
2.14 to 1.88 mm3/mg of dry tissue in the heart, from 2.3 to 2.2 in
the liver and from 5.7 to 2.32 in the kidneys (Stanosz, 1969).
The effect of ingested phenoxy-acid herbicides on muscular function
has been suggested to be related to interference with carbohydrate
metabolism. Transitory diabetiform conditions have been reported in
individuals spraying these compounds. However, hyperglycaemia or
glycosuria could not be reproduced with certainty in rabbits; only one
in five animals responded in this manner following administration of
doses ranging from 125 to 500 mg/kg body-weight from 6 to 50 days.
(Lorenzen and Lyngsoe, 1957; Dalgaard-Mikkelsen and Poulsen, 1962).
Attention has been drawn to the increase in nitrate content which has
been observed in certain plants after spraying with 2,4-D. This
increase has been thought to be the cause of toxic effects which have
been observed in grazing animals. Abortions in cattle have also been
attributed to this phenomenon. Information on this possible indirect
toxic action of 2,4-D has been summarized (Way, 1969). Manifestation
of toxicity appears, however, to be confined to farm animals, and no
reports have been encountered which would indicate that human food
exposed to 2,4-D as a growing crop has become toxic in this manner.
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Mouse
Repeated injections of 2,4-D have not influenced the growth rate of
two transplated sarcomas in mice. Mitotic counts performed at various
intervals in these tissues after a single injection of 200 mg/kg body-
weight of 2,4-D did not differ significantly from a control not
treated with 2,4-D (Bucher, 1946).
Groups of 18 mice of each sex from two hybrid strains were given 2,4-D
from seven days of age for 18 months. The compounds were given daily
by gavage at levels of 0, 46.4 or 100 mg/kg body-weight until weaning,
after which time 2,4-D was incorporated into the diet at the
corresponding levels of 0, 149 or 323 ppm, respectively. There was no
significant increase in tumours between the controls and the groups
given the two levels of 2,4-D (Innes et al., 1969).
Special studies on reproduction
Chicken embryo
When injected into fertile eggs at 5 or 10 mg/egg, 2,4-D did not
produce malformations, although it was stated that certain
phenoxyacetic acids produced feather blanching. The percentage hatch
was 70 and 50 percent for the eggs receiving the two respective levels
of 2,4-D (Dunachie and Fletcher, 1967).
Chicken
Groups of three-day old chicks were given 0 (25 chicks) or 1000 ppm
(29 chicks) of 2,4-D in their drinking water. Weight-gain, age of
sexual maturity and onset of egg production did not differ between the
test and control groups. However, the number, and possibly the weight,
of the eggs was reduced in the group given 2,4-D (Björkland and Erne,
1966).
Mouse
Mice which underwent daily injections of about 90 mg/kg body-weight of
2,4-D became pregnant and bore apparently normal litters at the end of
a normal gestation period (Bucher, 1946).
Rat
Groups, each comprising five newly mated female rats, received 0 or
1000 ppm of 2,4-D in their drinking water during their pregnancy and
for a further ten months. Pregnancy and parturition were normal.
Litter size was not significantly different between the test and
control groups. No malformations were observed in the young nor were
any clinical or morphological abnormalities revealed. After weaning,
the young rats were given 0 or 1000 ppm of 2,4-D in the same manner as
their parents for up to two years. The group receiving 2,4-D had
reduced food intake and depressed growth rate. Clinical-chemical
parameters were normal. Mortality was higher in the group given 2,4-D.
Relative organ weights and gross and histopathological examination
revealed no changes in the test group (Björkland and Erne, 1966).
It has been stated that the long-term feeding to rats of potatoes
which had been treated with 2,4-D affected reproduction. In the F2
generation, and even more in the F3 generation, the ability to
reproduce was reduced in the animals fed these potatoes (An der Lan,
1966).
Pig
A female pig was fed 500 ppm of an amine salt of 2,4-D during her
entire seventh pregnancy and for a further six weeks. Anorexia was
observed. One mummified foetus and 15 underdeveloped live young were
delivered. Ten of these died by the first day. Autopsy revealed a
generalized anaemia. No malformations were observed. The surviving
five young were fed 500 ppm of 2,4-D amine salt; growth was retarded
and locomotor disturbances were observed. Clinical-chemistry results
were the same as described under the chronic feeding study for the pig
(Björkland and Erne, 1966).
Special studies on toxicity of impurities
There is no information that the presence of chlorinated
dibenzodioxins, such as occur in some batches of technical 2,4,5-T,
has been looked for in technical 2,4-D. It is reported that the
condensation of the phenol precursors to form such compounds could not
occur in the manufacture of 2,4-D (Dow, 1970).
TABLE I
Acute Toxicity of 2,4-D
LD50
Animal Route mg/kg References
body-weight
Chicken oral 380-765 (amine salt) Bjorn and Northen, 1948
Chicken (mixed) oral 541 Rows and Hymas, 1954
Mouse oral 375 Hill and Carlisle, 1947
Mouse (m) oral 368 Rowe and Hymas, 1954
Mouse s.c. 280 Bucher, 1946
Rat oral 666 Hill and Carlisle, 1947
Rat (m) oral 375 Rowe and Hymas, 1954
Guinea,pig oral 1000 Hill and Carlisle, 1947
Guinea pig
(mixed) oral 469 Rowe and Hymas, 1954
Rabbit oral 800 Hill and Carlisle, 1947
Dog oral 100 Drill and Hiratzka, 1953
Dog oral 541 Rowe and Hymas, 1954
Monkey oral > 428 Hill and Carlisle, 1947
For man the lethal dose of 2,4-D has been estimated to be greater than
80 mg/kg body-weight (Nielsen et al., 1965).
Symptoms of myotonia in mice were evident after the parenteral
administration of 150-200 mg/kg body-weight (Bucher, 1946).
Initial signs following the administration of a lethal dose of 2,4-D
to dogs were often not present until six hours following oral
administration. The animal then became ataxic with progressive
increase in spasm. Pathological changes were limited to the
gastrointestinal tract, lungs and liver. Death in most cases appeared
to be due to hepatic congestion or to pneumonia, which followed the
development of anorexia, weight loss and myotonia (Drill and Hiratzka,
1953).
No toxic symptoms were observed when monkeys received 214 mg/kg
body-weight orally, but 428 mg/kg caused nausea, vomiting, lethargy,
muscle incoordination and head drop. All species reacted similarly;
death from large doses was thought to be due to ventricular
fibrillation (Hill and Carlisle, 1947).
Short-term studies
Chicken
Groups, each of five chickens, were given an amine salt of 2,4-D three
times a week in oral doses of 0, 0.28, 2.8, 28, or 280 mg/kg
body-weight (acid equivalent) for up to four weeks (a total of 12
doses). Weight gain was reduced only at the 280 mg/kg level (Bjorn and
Northen, 1948).
In the study described under "Reproduction" three day old chicks were
given 0 or 1000 ppm of 2,4-D. In the group given 2,4-D, gross
pathology revealed enlarged kidneys, and histopathology showed changes
in the kidney tissue mostly in the proximal convoluted tubules
(Björkland and Erne, 1966).
Mouse
Injection of 50 to 90 mg/kg body-weight of 2,4-D subcutaneously to
mice for three weeks to three months failed to produce a clear-cut
syndrome of chronic toxicity. Levels of 70 mg/kg retarded growth,
however, probably due to reduced food intake (Bucher, 1946).
Rat
Rats were fed a dietary level of 1000 ppm of 2,4-D for one month
without harmful effects (Hill and Carlisle, 1947).
Groups each comprising five or six young female rats were given 0, 3,
10, 30, 100 or 300 mg/kg body-weight of 2,4-D by stomach tube five
times a week for periods up to four weeks. The animals which received
30 mg/kg or less showed no adverse effects, as judged by gross
appearance, behaviour, mortality, growth, haematological values,
blood-urea nitrogen, organ weights, gross pathology and
histopathology. The rats on 100 mg/kg showed varying degrees of
gastrointestinal irritation, slight cloudy swelling of the liver and
depressed growth rate. The animals given 300 mg/kg failed rapidly and
died principally of severe gastrointestinal irritation (Rowe and
Hymas, 1954).
In a separate experiment, groups each of five young female rats, were
fed dietary levels of 0, 100, 300, 1000, 3000 or 10,000 ppm of 2,4-D
for periods up to 113 days. The animals fed 300 ppm or less showed no
adverse effects as judged by the parameters described in the previous
experiment. Those given 1000 ppm had increased mortality, depressed
growth rate, slightly increased liver weight and slight cloudy
swelling of the liver. The animals fed 3000 or 10,000 ppm were
sacrificed after 12 days because of food refusal and rapid
weight-loss. Autopsy revealed increased liver and kidney weights and
there were slight pathological changes in the organs (Rowe and Hymas,
1954).
Guinea pig
Guinea pigs tolerated 10 oral doses of 100 mg/kg bodyweight of 2,4-D
given over a 12 day period. Inhalation of the sodium salt of 2,4-D as
a dust failed to produce systemic effects (Hill and Carlisle, 1947).
Dog
Groups of from two to four dogs of mixed sexes were given 0, 2, 5, 10
or 20 mg/kg body-weight of 2,4-D orally by capsule on five days a week
for 13 weeks. All the dogs given 10 mg/kg or less survived the 90-day
test period. Of two male dogs given 20 mg/kg, one died after 18 days,
the other after 25 days. A female given 20 mg/kg/day in two divided
doses died after 49 days; another male dog on the same dose regimen
survived the 90-day period. Loss of weight occurred only in the dogs
which ultimately died, and this phenomenon began seven to 12 days
prior to death. Dogs that survived 90 days were free of symptoms: in
the others, ataxia and increased muscle tonus occurred prior to death.
The blood picture was normal, except for a terminal fall in lymphocyte
count prior to death. Weights of thyroid, adrenals, heart, liver and
kidney were not different from the controls in the surviving animals;
in two of the dogs that died, there was a slight increase in heart and
kidney weights. Two dogs, one receiving 2 and the other 20 mg/kg
showed redness in the duodenum. Histopathology revealed no changes in
heart, lungs, thyroid, adrenal or testes. Two dogs receiving an
unspecified level of 2,4-D showed areas of focal necrosis in the
liver, which was considered not to be related to administration of
2,4-D (Drill and Hiratzka, 1953).
Pig
An amine salt of 2,4-D and 2,4-D ester were given to young pigs in
doses of 50, 100 or 300 mg/kg body-weight at varying intervals up to
103 days. Symptoms of intoxication were similar to those reported from
studies with laboratory animals, and autopsy revealed similar
pathological findings. Clinical signs of anorexia and retarded growth
were evident in one pig given 51 doses of 50 mg/kg during 103 days
(Björklund and Erne, 1966).
On the basis of this experiment it has been suggested that the pig may
be more sensitive to 2,4-D than other animals tested, since the
no-effect level appears to be less than 50 mg/kg body-weight/day
(Erne, 1966a).
Five pigs were fed an amine salt of 2,4-D at 0 or 500 ppm in their
diet for up to 12 months. Food consumption and growth rate was reduced
in the controls, and after about one month, three animals developed
locomotor disturbances of increasing severity. The animals were
sacrificed after two to 12 months. Organ weights were normal, and
gross pathological changes were not evident. Clinical-chemical
observations involved lowered haemoglobin and haematocrit values,
elevation of glutamic-oxaloacetic transaminase and reduced albumin and
albumin-globulin ratios in the treated animals (Björklund and Erne,
1966).
Cattle
When steers were given oral doses of 0, 50, 100, 200 or 250 mg/kg
body-weight of an amine salt of 2,4-D for five days a week, poisoning
was evident at 250 mg/kg after 15 treatments and at 100 mg/kg after 86
treatments. A dose of 50 mg/kg given 112 times had no effect on one
steer (Palmer, 1963).
Long-term studies
No complete long-term studies appear to have been conducted.
OBSERVATIONS IN MAN
A man has been reported to have taken 500 mg of 2,4-D daily for three
weeks (approximately 8 mg/kg body-weight) without experiencing any
harmful effects. No further details are given (Assouly, 1951).
A man aged 23 committed suicide by oral intake of the dimethylamine
salt of 2,4-D. Pronounced degenerative changes of the ganglion cells
of the brain were found upon histological examination (Nielsen et al.,
1965).
Subjective clinical symptoms have been reported among workers using
various esters of and the sodium salts of 2.4-D. Individuals
complained of rapid fatigue, headache, liver pains, loss of appetite,
etc. Certain functional shifts were noted in the cardiovascular
system, and sensitivity to taste and smell was lowered (Fetisov,
1966).
In workers employed in factories manufacturing 2,4-dichlorophenol and
other chlorinated phenols, a moderately high incidence of urinary
porphyria, chloracne and hirsutism has been reported. The authors
suggest that a highly chlorinated phenolic ether may be the compound
responsible (Bleiberg at al., 1964).
Clinical symptoms of peripheral neuropathy have been reported in three
individuals who had direct skin contact with an eater of 2,4-D. An
electromyographic examination supported the diagnosis (Goldstein et
al., 1959).
COMMENTS
Information on the absorption and excretion of 2,4-D in several
species indicates that the compound is rapidly excreted unchanged, and
that it is not stored in the tissues in mammals. Reproduction studies
are limited to a single generation but do not indicate that 2,4-D is a
teratogen. There is no information that toxic chlorinate
dibenzodioxins have been looked for in commercial 2,4-D.
Increased nitrate formation has been reported to occur in plants
treated with 2,4-D and is thought to have resulted in toxic effects in
grazing animals. This observation is of concern if 2,4-D is to be used
on edible crops.
Attention was drawn to the absence of any complete long-term studies
in any species. For this reason, no acceptable daily intake for 2,4-D
could be established.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
A pre- or post-emergent selective herbicide in cereals used for
prevention of preharvest fruit drop, the production of harvest fruit
drop, the production of seedless fruit and the regulation of the
growth of plants.
Acid in oil formulations control many broad-leaved annual and
perennial weeds on roadside verges, around farm buildings, etc.
FATE OF RESIDUES
In animals
When a cow was fed 5 ppm of 2,4-D in a 23 kg ration for five days,
none of the herbicide could be detected in the milk or faeces
throughout the study; the analytical limit of detection was 0.1 ppm.
The compound was found to disappear from the rumen, due presumably to
dilution, followed by absorption on the gut wall and by decomposition.
When 2,4-D was added to artificially collected rumen at a level of 2
ppm it was not decomposed (Gutenmann and Lisk, 1963; Gutenmann et al.,
1963).
In pigs which developed symptoms of poisoning following repeated
administration of from 50 to 300 mg/kg body-weight of an amine salt of
2,4-D or of 2,4-D ester, high plasma levels of 2,4-D were found (200
to 400 µg/ml) even 24 hours after the dose was given. Elimination
occurred at an enhanced rate after repeated administration and
eventually decreased to about 10, µg/ml (Björkland and Erne, 1966).
A capsule containing 539.6 µCi of 2-14 C-labelled 2,4-D was
administered orally to a female sheep of weight 26.6 kg. This dose was
equivalent to 4 mg/kg body-weight, which was stated to be the amount
that the animal would receive while grazing in a treated pasture.
Urine and faeces were collected separately, and blood samples were
withdrawn from the jugular vein at intervals after administration of
the dose.
The animal was sacrificed after four days, and tissues were assayed
for carbon-14. Levels of radioactivity in the blood rose during the
first half hour following treatment and reached a peak after one and a
half hours. By 24 hours, the radioactivity level in blood had
decreased to background. About 15 percent of the original dose of
2,4-D was found in the urine after one and a quarter hours, 50 percent
after eight and a half hours and 96 percent after 70 hours. Total
collected faeces contained about 1.4 percent of the dose. Paper
chromatography and electrophoresis from urine extracts revealed only
one spot of the same Rf as 2,4-D, indicating that the compound is
excreted unchanged in the urine. Tissue levels, as determined
following slaughter four days after administration, contained less
than 0.05 ppm radioactive equivalents, with the exception of thyroid
and urinary bladder, which contained 0.56 and 0.5 ppm, respectively.
The nature of these residues in tissue was not determined (Clark et
al., 1964).
In plants
It has been demonstrated by a number of workers that 2,4-D is
translocated in plants (Dhillon and Lucas, 1950; Holley et al., 1950;
Fang et al., 1951), since free phenoxyacetic acids could be isolated
from parts well removed from the site of application. Jaworski and
Butts (1952) demonstrated the formation of a complex of 2,4-D in
plants, and Holley et al. (1950) indicated that plants could detoxify
2,4-D to form hydroxylated products. Weintraub and Norman, (1950) and
McIlrath and Ingle (1953), showed that this destruction was not rapid
in all plants. 14C-labelled material led to the recognition that
some of the applied material was complexed (Jaworski and Butts, 1952).
Existence of oxidative degradation of 2,4-D was shown by Holley et al.
(1950) and Weintraub et al. (1952b) Holley et al. 1950) and Holley
(1952) demonstrated the evolution of 14CO2 after treating plants
with 14C-2,4-D, and the formation of a product more water soluble
than 2,4-D acid. Evolution of 14CO2 from plants treated with either
methylene-labelled or carboxyl-labelled 2,4-D has been shown by Holley
(1952) and Weintraub et al. (1952a, 1954). The side chain of
phenoxyacetic acids can be removed by such plants as currants
(Luckwill and Lloyd-Jones, 1960), wild cucumber (Slife et al., 1962)
and tick beans (Canny and Marcus, 1960). Roots have been shown by
Cann and Marcus (1960) to be of greater efficiency in decarboxylation
than shoots. Not all of the carbon liberated from breakdown of the
side chain of phenoxyacetic acids is evolved as 14CO2, but some is
incorporated into plant constituents (Weintraub at al., 1952a;
Luckwill and Lloyd-Jones, 1960). Luckwill and Lloyd-Jones (1960)
studied the degradation of 2,4-D by currants, apples and strawberries.
They showed that carboxyl-labelled phenoxyacetic acid more readily
yielded 14CO2 than did the methylene-labelled acid.
By spectrophotometric analysis, Schieferstein (1957) showed that the
rate of penetration of 2,4-D through ivy cuticle varied with the age,
and hence thickness of the cuticle. Orgell (1957) found cationic and
anionic surfactants hindered the sorption of 2,4-D at low pH; nonionic
surfactants had little effect. At high pH, cationic surfactants caused
sorption, whereas anionic and non-ionic surfactants had little effect.
2,4-D applied to roots may move to tops only in minute quantities, and
only after some days; applied to leaves, it may pass downward into the
stem and roots in quantity within a few hours.
In soil
2,4-D has been shown to be biodegradable in soils by DeRose and Newman
(1948), Brown and Mitchell (1948) and Audus (1950). Audus (1964)
listed numerous micro-organisms which are capable of degrading 2,4-D.
Evidence of residues in food in commerce or at consumption
After application of 2,4-D to bean plants and oats, 25-60% was found
in tissues, but only 0.33-1.66% in oats (Boyle, 1954). Some
unpublished data was presented (BASF, 1970) regarding residues of
2,4-D in cereals (grain and straw) found to occur following
applications of the herbicide either alone or in admixture with other
phenoxyacid herbicides (MCPA, mecoprop). A summary of the data
relating to 2,4-D is given in Table II. Williams (1964) was unable to
detect 2,4-D in a number of total diet samples at a sensitivity of
0.01 ppm. Duggan and Weatherwax (1967) in total diet studies found
herbicide chemicals infrequently, averaging an intake of about 0.01
mg/kg of which one-third was 2,4-D. Very small amounts of 2,4-D were
found in oils and fats (0.001 mg in 1964/5, and sugars and sugar
products (0.004 mg 1964/5, and 0.002 mg in 1965/6).
TABLE II
Residues of 2,4-D in cereals
Crop Rate of Intervals (days) Residues found
Application Applic.-Harvest Grain Straw
Barley 375 g/ha 64 <0.01 0.08
77 <0.01 0.02
TABLE II (cont'd)
Residues of 2,4-D in cereals
Crop Rate of Intervals (days) Residues found
Application Applic.-Harvest Grain Straw
Barley 520 g/ha 64 n.d.1 0.1
77 n.d. n.d
735 g/ha 64 0.015 0.34
77 n.d. n.d
375 g/ha 76 n.d. 0.02
89 n.d. 0.04
Wheat 520 g/ha 76 n.d. n.d
89 n.d. n.d
735 g/ha 76 n.d. 0.03
89 n.d. n.d
375 g/ha 76 0.01 0.02
89 n.d. n.d
Oats 520 g/ha 76 n.d. n.d
89 n.d. n.d
735 g/ha 76 n.d. 0.02
89 n.d. n.d
375 g/ha 76 n.d. 0.04
89 n.d. n.d
Rye 520 g/ha 76 n.d. n.d
89 n.d. n.d
735 g/ha 76 n.d. 0.04
89 n.d. <0.02
1 n.d. = not detected.
METHODS OF RESIDUE ANALYSIS
Residues of 2,4-D can be determined after suitable extraction,
separation and isolation by gas chromatography, ultraviolet
spectrophotometry, fluorimetry, thinlayer and paper chromatography.
The preferred method is to methylate the isolated 2,4-D and determine
by GLC. This method has been used for the following sample substrates:
forage (Hagin and Linscott, 1965; Gutenmann and Lisk, 1963; Yip and
Neys, 1966)
oysters (Duffy and Shelfoon, 1967), water (Devine and Zweig, 1969),
milk (Yip and Neys, 1966; Crosby and Bowers, 1966)
in fruit peel (Meagher, 1966), citrus fruit (Erickson and Hield, 1962)
and pineapple and orange peel (Hendrickson and Meagher, 1969).
Benvenue et al. (1962) determined residues of 2,4-D in dry crops and
walnuts by microcoulometric gas chromatography. Clerk et al. (1967),
extracted animal tissues and, after isolation of the 2,4-D, hydrolysed
it to 2,4-dichlorophenol, purified by steam distillation and then used
GLC. 2,4-D can be determined colorimetrically by the use of
chromotropic acid in sulphuric acid at a wavelength of 565 nm
(Marquardt and Luce, 1951 and 1955; Erickson and Brannaman, 1954). Aly
and Faust (1964) suggested using 6-amino-1-naphthol-3-sulphonic acid
and 6-anilino-1-naphthol-3-sulphonic acid, which are more sensitive
(1´-2´ times) than chromotropic acid. Marquardt and Luce (1961)
cleaved the phenoxy acid with pyridine hydrochloride, releasing the
phenol derivative 2,4-dichlorophenol, added 4-amino antipyrine and
potassium ferricyanide, and determined the complex at 515 nm.
Ultraviolet spectrophotometry at a wavelength of 284 nm was used by
Warshowsky and Schantz (1950), Aly and Faust (1963) and Gordon and
Beroza (1952). Kuznetsov and Gagarina (1962) determined 2,4-D in
plants by paper chromatographic separation, elution from strips and
colorimetric determination with butyl rhodamine at a wavelength of 564
nm. Salo and Makinen (1964) used silica gel thin-layer chromatography
with Blankopher DCB and examination under ultraviolet light at 360 nm
for visualization. Abbott et al. (1964) used paper and thin-layer
chromatography for detection, separation and identification. Guilbault
and Sadar (1969) used a thin-layer chromatographic separation followed
by a fluorimetric determination based on the breakdown of 4-methyl
umbelliferone heptanoate by lipase.
NATIONAL TOLERANCES
Country Crop Tolerance(ppm)
United States of Apples, pears, 5
America lemons, oranges,
(December 1969) grapefruits
Barley, oat, rye, 20
wheat and forage
Netherlands Barley, oat, rye, 0.5
wheat and grain
Vegetables, 0.05
(except potatoes)
fruits of vegetables
and fruit crops
Fed. Rep. of Germany Leafy and other 0.05
sprouting vegetables,
fruiting vegetables,
root vegetables
APPRAISAL
2,4-D is used in cereals as a selective pre- and post-emergence
herbicide for the control of broad-leaved weeds. It in also used to
prevent early fruit drop, as a plant growth regulator and for weed
control on roadside verges, around buildings, etc. Some evidence from
supervised trials regarding the possible occurrence of residues of
2,4-D in cereal grain and straw was provided. Insufficient information
relating to residues resulting from other uses was available. Total
diet studies in the U.S.A. have very occasionally revealed minimal
quantities of 2,4-D in oils and fats or sugar and sugar products. No
evidence regarding the need to establish practical residue limits was
apparent. Gas chromatographic methods are available which should be
adaptable for regulatory purposes where required.
Since no acceptable daily intake had been established, it was decided
not to recommend tolerances. Nevertheless, it was decided to record
that following officially acceptable use in various countries.
residues of 2,4-D can occur in the following crops:
Wheat, barley, oats, rye and grain 0.02 ppm
Wheat, barley, oats, rye and straw 0.5 ppm
FURTHER WORK OR INFORMATION
REQUIRED (before an acceptable daily intake for man can
be established).
1. An adequate long-term oral study in a rodent
species and at least a two-year study in a
nonrodent mammalian species.
2. A three-generation reproduction study in at
least one mammalian species.
3. Information on the nature of the impurities
occurring in commercial preparations of 2,4-D.
DESIRABLE
1. Further information on the metabolism of 2,4-D
in plants, laboratory animals and man.
2. Elucidation of the problem of increased nitrate
formation in plants treated with 2,4-D, to
determine if this phenomenon could create a
toxic hazard to man.
3. Information on the occurrence of 2,4-D residues
in crops other than cereals.
REFERENCES
Abbott, D.C., Egan, H., Hammond, E.W. and Thomson, J. (1964) The
chromatographic detection and determination of organo-chlorine
herbicides in soil and water. Analyst, 89: 480-488
Aly, O.M. and Faust, S.D. (1963) Determination of
2,4-dichloro-phenoxyacetic acid in surface water, J.Amer. Waterworks
Assoc., 55: 639-646
Aly, O.M. and Faust, S.D. (1964) Determination of phenoxyacetic acids
with J and phenyl J acids. Anal.Chem., 36: 2200-2201
An der Lan, H. (1966) The present situation of toxicology in the field
of crop protection. Res. Rev., 15: 31-43
Assouly, M. (1951) Désherbants sélectifs et substances de croissance.
Aperçu technique. Effet pathologique our l'homme an cours de la
fabrication de l'ester de 2,4-d. Arch.Mal.Prof., 12: 26-30
Audus, L.J. (1950) Biological detoxication of
2,4-dichlorophenoxyacetic acid in soils. Nature, 166: 356
Audus, L.J. (1964) The physiology and biochemistry of herbicides.
Academic Press
BASF (1970) Residues of 2,4-D in cereal grain and straw. (unpublished
report). Badische Anilin und Soda Fabriken
Bevenue, A., Zweig, G. and Nash, N.L. (1962) Residue determination of
2,4-dichlorophenoxyacetic acid in dry crops and walnuts. J. Assoc.
Offic. Agric. Chem., 45: 990-993
Björklund, N-E. and Erne, K. (1966) Toxicological studies of
phenoxyacetic herbicides in animals, Acta vet. scand., 7: 364-390
Bjorn, M.K. and Northen, H.T. (1948) Effects of
2,4-dichlorophenoxyacetic acid on chicks. Science, 108: 479-480
Bleiberg, J., Wallen, M., Brodkin, R. and Applebaum, I.L. (1964)
Industrially acquired porphyria. Arch.Dermatol., 89: 793-797
Boyle, F.P. (1954) Physiology and chemistry of
2,4-dichlorophenoxyacetic acid on resistant and non-resistant plants.
Congr. intern. botan., Paris, Rapps et communs., 8: 184-185
Brown, J.W. and Mitchell, J.W. (1948) Inactivation of 2,4
dichlorophenoxyacetic acid in soil as effected by soil moisture,
temperature, the addition of manure and autoclaving. Botan. Gaz.,
109: 314-323
Bucher, N.L.R. (1946) Effects of 2,4-dichlorophenoxyacetic acid on
experimental animals. Proc. Soc. exp. Biol. Med., 63: 204-205
Canny, M.J. and Marcus, K. (1960) Metabolism of
2,4-dichlorophenoxyacetic acid in bean plants. Australian J. Biol.
Sci., 13: 486-491
Clark, D.E., Young, J.E., Younger, R.L., Hunt, L.M. and McLaran, J.K.
(1964) The fate of 2,4-dichlorophenoxyacetic acid in sheep. J. Agr.
Food Chem., 12: 43-45
Clark, D.E., Wright, F.C. and Hunt, L.M. (1967) Determination of 2,4-D
residues in animal tissues. J. Ag. Food Chem. 15: 171-173
Crosby, D.G. and Bowers, J.B. (1966) Determination of 2,4-D residues
in animal products, Bull. Environ. Contam. Toxicol., 1: 104-107
Dalgaard-Mikkelsen, S. and Poulsen, E. (1962) Toxicology of
herbicides. Pharmacol. Rev., 14: 225-250
De Rose, H.R. and Newman, A.S. (1948) The effect of certain plant
growth regulators on soil microorganism and microbial processes, Proc.
Soil Sci. Soc. Amer., 12: 217-221
Devine, J.M. and Zweig, G. (1969) Note on the determination of some
chlorophenoxy herbicides and their esters in water. J. Assoc. Offic.
Anal. Chem., 52: 187-189
Dhillon, A.S. and Lucas, E.H. (1950) Absorption, translocation and
persistence of 2,4-D in some plants. Botan. Gaz., 112: 198-207
Dow (1970) Information provided verbally to WHO
Drill, V.A. and Hiratzka, T. (1953) Toxicity of
2,4-dichlorophenoxyacetic acid and 2,4,5-trichlorophenoxyacetic acid.
Arch. industr. Hyg., 7: 61-67
Duggan, R.E. and Weatherwax, J.R. (1967) Dietary intake of pesticide
chemicals. Science, 157: 1006-1010
Dunachie, J.F. and Fletcher, W.W. (1967) Effect of some herbicides on
the hatching rate of hen's eggs. Nature, 215: 1406-1407
Erickson, L.C. and Brannaman, B.L. (1954) Chromatropic acid method for
determining 2,4-D residues. Hilgardia, 23: 175-184
Erickson, L.C. and Hield, H.Z. (1962) Determination of some
2,4-dichlorophenoxyacetic acid in citrus fruit. J. Agr. Food Chem.,
10: 204-207
Erne, K. (1966(a)) Distribution and elimination of chlorinated
phenoxyacetic acids in animals. Act Vet. Scand. 7: 240-256
Erne K. (1966(b)) Animal metabolism of phenoxyacetic herbicides. Act
Vet. Scand., 7: 264-271
Fang, S.C., Jaworski, G., Logan, A.V., Freed, V.H. and Butts, J.S.
(1951) The absorption of radioactive 2,4-dichlorophenoxyacetic acid
and the translocation of carbon-14 by bean plants. Arch. Biochem.
Biophys., 32: 249-255
Fetisov, M.I. (1966) Problems of occupational hygiene in work with
herbicides of 2,4-D group (in Russian). Gig. sanit., 9: 28-31
Goldstein, N.P., Jones, P.H. and Brown, J.R. Peripheral neuropathy
after exposure to an ester of 2,4-D, J. Amer. med. Ass., 171:
1306-1309
Gordon, N. and Beroza, M. (1952) Spectrophotometric determination of
small quantities of 2,4-dichlorophenoxyacetic acid and
2,4,5-trichlorophenoxyacetic acid. Anal. Chem., 24: 1968-1971
Guilbault, G.G. and Sadar, M.H. (1969) Fluorometric determination of
pesticides. Anal. Chem., 41: 366-368
Gutenmann, W.H., Hardee, D.D., Holland, R.F. and Lisk, D.J. (1963)
Residue studies with 2,4-dichlorophenoxyacetic acid herbicides in the
dairy cow and in a natural and artificial rumen. Dairy Sci., 46:
1287-1288
Gutenmann, W.H. and List, D.J. (1963) Rapid determination of 4(2,4-DB)
and a metabolite, 2,4-D, in treated forage by electron affinity
spectroscopy. J. Agr. Food Chem., 11: 304-306
Hagin, R.D. and Linscott, D.L. (1965) Determination of
4(2,4-dichlorophenoxybutyric acid (2,4-DB) and
2,4-dichlorophenoxyacetic acid (2,4-D) in forage plants. J. Agr. Food
Chem., 13: 123-125
Hendrickson, R. and Meagher, W.R. (1969) Spray residues of 2,4-D and
2,4,5-TP in "pineapple" orange peel. J. Agr. Food Chem., 17: 601-603
Hill, E.V. and Carlisle, H. (1947) Toxicity of
2,4-dichlorophenoxyacetic acid for experimental animals. J. industr.
Hyg. Toxicol., 29: 85-95
Holley, R.W., Boyle, F.P. and Hand, D.B. (1950) Fate of radioactive
2,4-dichlorophenoxyacetic acid in bean plants. Arch. Biochem., 27:
143-151
Holley, R.W. (1952) Studies of the fate of radioactive 2,4-D acid in
bean plants. II. A water soluble transformator product of 2,4-D. Arch.
Biochem. Biophys., 35: 171-175
Innes, J.R.M., Ulland, B.M., Valerii, M.G., Petrucelli, L., Fishbein,
L., Hart, E.R., Pallotta, A.J., Bates, R.R., Falk, H.C., Gart, J.J.,
Klein, M., Mitchell, I. and Peter, J. (1969) Bioassay of pesticides
and industrial chemicals for tumorigenicity in mice: a preliminary
note, J. nat. Cancer Inst., 42: 1101-1114
Jaworski, E.G., and Butts, J.S. (1952) Plant metabolism II. The
metabolism of carbon 14 labeled 2,4-dichlorophenoxyacetic acid in bean
plants. Arch. Biochem. Biophys., 38: 207-218
Khanna, S. and Fang, S.C. (1966) Metabolism of C14-labelled 2,4
dichlorophenoxyacetic acid in rats. J. Agr. Fd. Chem., 14: 500-503
Kuznetsov, V.J. and Gagarina, M.I. (1962) Photometric determination of
2,4-dichlorophenoxyacetic acid (2,4-D) with butyl rhodamine. Zhur.
Anal. Khim., 17: 235-237
Lorenzen, I. and Lyngse, J. (1957) En undersgelse over virkningen af
et kloreret fenoxyacetat pa kulhydratstofskiftet pa kaniner. Nord.
hyg. T., 38: 153-157
Luckwill, L.C. and Lloyd-Jones, C.P. (1960) Metabolism of plant growth
regulators. I. 2,4-Dichlorophenoxyacetic acid (2,4-D) in leaves of red
and black currant. Ann. Appl. Biol., 48: 613-625
Marquardt, R.P. and Luce, E.N. (1951) Determination of small amounts
of 2,4-dichlorophenoxyacetic acid in milk, Anal. Chem., 23:
1484-1486
Marquardt, R.P. and Luce, E.N. (1955) Determination of
2,4-dichlorophenoxyacetic acid (2,4-D) in grain and seed. J. Agr. Food
Chem., 3: 51-53
Marquardt, R.P. and Luce, E.N. (1961) A new procedure for determining
phenoxyacid herbicides in agricultural products. J. Agr. Food Chem.,
9: 266-270
McIlnath, W.J. and Ingle, D.R. (1953) Further evidence of the
persistence of 2,4-D in cotton. Plant Physiol., 28: 693-696
Meaghen, W.R. (1966) Determination of 2,4-dichlorophenoxyacetic acid
and 2(2,4,5-trichlorophenoxy) propionic acid in citrus by electron
capture gas chromatography. J. Agr. Food Chem., 14: 374-377
Nielsen, K., Kaempe, B., and Jensen-Holm, J. (1965) Fatal poisoning in
man by 2,4-dichlorophenoxyacetic acid (2,4-D): determination of the
agents in forensic materials. Acta pharmacol. toxicol., 22: 224-254
Orgell, W.H. (1957) Absorptive properties of plant cuticles. Proc.
Iowa Acad. Sci., 64: 189-198
Palmer, J.S. (1963) Chronic toxicity of 2,4-D alkanolamine salts to
cattle, J. Amer. vet. Med. Ass., 143: 398-399
Rowe, V.K. and Hymas, T.A. (1954) Summary of toxicological information
on 2,4-D and 2,4,5-T type herbicides and an evaluation of the hazards
to livestock associated with their use. Amer. J. vet. Res., 15:
622-629
Salo, T. and Makinen, R.Z. (1964) Thin-layer chromatography of
substances used for treatment of citrus fruit peel: -biphenyl,
o-phenyl phenol and 2,4-D. Z. Lebensm. u. Forsch., 125: 170-171
Schieferstein, R.H. (1957) Development of protective structures of the
plant cuticle. Ph. D. dissertation, Iowa State College, Ames, Iowa,
U.S.A.
Slife, J.W., Key, J.L., Yamaguchi, S. and Crafts, A.D. (1962)
Penetration, translocation and metabolism of 2,4-D and 2,3,5-T in wild
and cultivated cucumber plants. Weeds, 10: 29-35
Stanosz, S. (1969) Effect of sodium 2,4-dichlorophenoxyacetate on
tissue respiration in parenchymatous organs of rats. (in Polish). Med.
Piacy, 20b: 600-605. Chem. Abstr., 73: (1970)
Warshowsky, B. and Schantz, E.J. (1950) Determination of
2,4-dichlorophenoxyacetic acid in soil. Anal. Chem., 22: 460-463
Way, J.M. (1969) Toxicity and hazards to man, domestic animals and
wildlife from some commonly used auxin herbicides. Res. Rev., 26:
37-62
Weintraub, R.L. and Norman, A.G. (1950) Recovery of growth regulator
from plants treated with 2,4-dichlorophenoxyacetic acid. Science,
111: 493-494
Weintraub, R.L., Brown, J.W., Fields, M. and Rohan, J. (1952a)
Metabolism of 2,4-dichlorophenoxyacetic acid. I. C14O2 production
by bean plants treated with labeled 2,4-dichlorophenoxyacetic acids.
Plant Physiol., 27: 292-301
Weintraub, R.L., Yeatman, J.N., Lockhart, J.A., Reinhart, J.H. and
Fields, M. (1952b) Metabolism of 2,4-dichlorophenoxyacetic acid. II.
Metabolism of the side chain by bean plants. Arch. Biochem. Biophys.,
40: 277-285
Weintraub, R.L., Reinhart, J.H., Scherff, R.A. and Schisler, L. (1954)
Metabolism of 2,4-dichlorophenoxyacetic acid. III. Metabolism and
persistence in dormant plant tissue. Plant Physiol., 29: 303-304
Williams, S. (1964) Pesticide residues in total diet samples. J.
Assoc. Offic. Agric. Chem., 47: 815-821
Yip, G. and Meys, R.E. (1966) Analysis of 2,4-D residues in milk and
forage. Weeds, 14: 167-170
