
AGP:1970/M/12/1
WHO/FOOD ADD/71.42
1970 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
Issued jointly by FAO and WHO
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Group on Pesticide Residues, which met in Rome, 9-16 November, 1970.
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
WORLD HEALTH ORGANIZATION
Rome, 1971
DITHIOCARBAMATE FUNGICIDES
(other than mancozeb)
Explanation
The dithiocarbamate fungicides: ferbam, mancozeb, maneb, nabam,
thiram, zineb and ziram were evaluated at the Joint FAO/WHO Meeting in
1967. Although the biochemical data were limited, temporary acceptable
daily intakes (ADI's) were established for all of these compounds, but
it was pointed out that these ADI's are to be applicable to the parent
compounds only (FAO/WHO, 1968). The following monograph addendum
collectively summarizes data that have become available since that
time. It is recognized that, in spite of their chemical similarity,
the biochemical and toxicological properties of these fungicides vary
considerably. However, the availability of new data did not justify
producing a separate monograph for each compound. In the cast of
mancozeb, however, important new information comparing the metabolism
in animals with that in plants had become available, and this compound
is the subject of a separate monograph.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Biotransformation
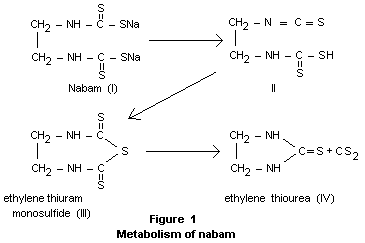
The dithiocarbamate compounds are thought to breakdown to
alkylthioureas, carbon disulfide and hydrogen sulfide. The scheme
shown in Figure 1 has been suggested for the breakdown of nabam (Falk
et al., 1965).
Breakdown of nabam, as well an zineb and maneb, in aqueous systems has
been demonstrated to product ethylene thiourea and ethylene thiuram
monosulfide (Vonk and Kaars Sijpesteijn, 1970).
With maneb, zineb and nabam, ethylene thiourea and ethylenediamine
were found in all cases, often as the end products of breakdown.
Levels of the intermediates, such as ethylene-bis thiuram
monosulfide and ethylene di-isothiocyanate, varied according to which
compound was studied (Engst and Schnaak, 1970).
Further work on the metabolism of various dithiocarbamate fungicides
is reported to be in progress in The Netherlands (Verschuuren, 1970).
Effect on enzymes and other biochemical parameters
The effect on inhalation of maneb and zineb on five isoenzymes of
lactic dehydrogenase in rat testes was studied. With maneb-poisoned
animals, an increase in the isoenzyme related to aerobic metabolism
and a decrease in that related to anaerobic metabolism was observed.
There was no effect on those isoenzymes in the case of animals exposed
to zineb (Izmirova et al., 1969).
 Information on the effect of zineb or maneb on various enzyme systems
in the rat is reported under "Short-term studies" (Bankowska et al.,
1970).
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Mouse
Groups of 18 mice of each sex from two hybrid strains were given
various dithiocarbamate fungicides from seven days of age for 18
months. The compounds were given daily by gavage from day seven of age
until weaning; thereafter, the compounds were added to the diet in
such a concentration that the animals received the corresponding
amount. The compounds and the respective amounts administered were:
ferbam, 10 mg/kg body-weight then 32 ppm in the diet; maneb, 46.4
mg/kg, then 158 ppm; nabam 21.5 mg/kg then 73 ppm; thiram, 10 mg/kg
then 26 ppm and zineb, 464 mg/kg then 1298 ppm. In all cases there was
no significant increase in tumours compared with a control group of
mice. However, when ethylene thiourea (a metabolite of some
dithiocarbamates) was administered at 215 mg/kg body-weight and then
after weaning incorporated into the diet at 646 ppm, the total
incidence of tumours was 14 out of 16 for the males and 18 out of 18
for the females in one strain; and 18 out of 18 and 12 out of 16,
respectively, for the males and females in the other strain (Innes et
al., 1969),
Technical ziram (97.6 percent) was administered orally to mice
(strains C57B1 and A) in doses 0 or 75 mg/kg body-weight twice a week
over a period of 2.5 months. This regimen comprises a total of 20
doses equivalent to 1500 mg/kg body-weight. A positive control group
was exposed in the same way to urethane to enable comparison of
results. The occurrence of adenomas in lungs and livers was studied
after the end of the exposure at intervals of 1.5 months during six
months. Adenomas were ascertained in the lungs only. The occurrence
was: in strain A in the ziram group 51.2 percent, in the urethane
group 100 percent and in the negative control group 42.6 percent. The
differences are not statistically significant. The first adenomas were
apparent after three months. In strain C57 in the ziram group, 7.4
percent adenomas were found, in the urethane group 25.6 percent and in
the negative control group 0 percent. The results are statistically
significant according to the T test (T=2.08; P=0.05) but not according
to the chi squared test (chi2= 2.18; P=0.15). The occurrence of
adenomas was ascertained after six months. On the basis of the results
of the experiments, ziram is considered a weak blastogen (Khicenko and
Chernov, 1968).
Mouse and rat
Zineb, ziram, maneb and urethane have been administered orally once a
week during six weeks to mice of strains A and C57 and to rats (SPF).
The total dose of single substances in the rats (200-250 g in weight)
was 120 mg (ca. 600 mg/kg body-weight/6 weeks), in mice (19-22 g) 60
mg (ca. 3000 mg/kg/6/weeks), with the exception of ziram, where the
total dose represented only half this amount i.e. 30 mg (ca. 1500
mg/kg-6 weeks). Comparison was made between the activity of
glucoso-6-phosphate dehydrogenase in liver and deoxyribonuclease in
serum under development of proliferative changes in the lungs. With
regard to zineb, ziram and urethane, the correlation was ascertained
in rate only between the early blastogenic changes in lungs and the
deoxyribonuclease activity in the serum. In case of the weak
blastogens, zineb and ziram, maximum increase of ferment activity was
apparent in the ninth month and lung changes in the 12th month after
the exposure. In the urethane group, these reactions in rats were
observed earlier (4,5 or 6 months and 9 months, respectively). Neither
the activity of glucoso-6-phosphate dehydrogenase in the liver of mice
and rats nor that of deoxyribonuclease in serum changed, and there was
no correlation with proliferative changes in the lungs (Chepinoga et
al., 1969).
Special studies on mutagenicity
The mutagenic activity of zineb and ziram is considered to be not
significant using the Muller-5 test in Drosophila melanogaster, when
compared with the spontaneous mutation rate of 0.14 percent (Benes and
Sram, 1969). See also "Observations in Man".
Special studies on reproduction
Chick embryo
The toxicity of the dithiocarbamate fungicides, ferbam, nabam, thiram
and ziram, was determined for the chick embryo. It was determined that
in order to avoid mortality from the solvent, propylene glycol, the
injections must be made at a very early stage in the development of
the embryo. The compounds were injected into the air chamber of
fertile eggs prior to incubation. On days four and six, the eggs were
candled to determine in which the embryos had died. The LD50 values
in mg/egg were for: ferbam, 0.0022; nabam, 0.14; thiram, 0.0019 and
ziram, 0.0021. The LD50 of thiram when injected into the yolk sac was
much higher than when injected into the air chamber, namely 0.018
mg/egg. The toxicity of thiram and ziram was reduced by simultaneous
injection of cysteine. It was suggested that there was a reduction of
these dithiocarbamates to less toxic materials (Gebhart and van
Logten, 1968).
Rat
Groups, each comprising 10 male and 10 female rats were given, twice
weekly, oral doses of 0, 700 or 1400 mg/kg body-weight of maneb for
four and a half months. (This dose regimen is equivalent to a daily
intake of 0, 200 or 400 mg/kg body-weight.) No clinical abnormalities
in the parent generation were observed. There were six births in the
group given the lower dose. There was a moderately high incidence of
stillbirths and imperfect skull development, but the actual numbers
compared with the controls is not stated (Kaloyanova et al., 1967).
The effect of the fungicide maneb on the embryonal development and the
generative function of rate was studied. For studying the potential
embryotoxic effect of maneb, mature white rat females with regular
ovulation were used. During the whole pregnancy, these female rats
were administered maneb by gavage every second day (as a suspension in
milk) at a dose level of 50 mg/kg body-weight (0.001 LD50). An
embryotoxic effect of the preparation was ascertained, i.e. abnormal
development of the embryos (dead embryos and resorption), stillborn
young and those incapable of living. In 21 percent of the pregnancies,
the unfavourable effect of maneb on the course of the pregnancy was
apparent when compared with 12 percent in the controls (Marcon, 1969).
Immature female and male rats, of body-weight 80-100 g, were exposed
to maneb for a month, the dose level being 50 mg/kg body-weight. When
mature (after two and one half months from the beginning of the
exposure), the control males were mated with the exposed females and
vice versa. A decline of fertility in both sexes was observed. This
effect, however, was not permanent, the function of the gonads being
restored in three and one half months (Marcon, 1969).
Hamster
Groups of from nine to 14 pregnant female hamsters received an oral
dose of 0, 31, 63, 125 or 500 mg/kg bodyweight of thiram in
dimethylsulfoxide solution on day seven or eight of gestation. No
litters were produced from the group given 500 mg/kg, and the
mortality of the parents was 60 percent. Incidence of deformed young
was significantly greater for the animals given 125 or 250 mg/kg of
thiram compared to the controls given dimethylsulfoxide alone.
Abnormalities included fused ribs, deformed tails and head defects
including all degrees of exencephaly. A similar study using
carboxymethyl cellulose as a solvent at 0, 125, 250, 300 or 500 mg/kg
body-weight of thiram produced a lower incidence of malformation both
in the treated and control animals, although the percentage incidence
of terata was increased in the 250 mg/kg groups and higher compared to
the controls (Robens, 1969).
Special studies on the effects on thyroid function
Rat
Groups, each comprising 10 rats of unspecified sex, received orally 0
or 3 500 mg/kg body-weight of maneb or 2 400 mg/kg of zineb presumably
as a single dose. After a period of 24 hours had elapsed following the
administration of the compounds, the animals were injected
intraperitoneally with 1 mCi of carrier free 131I. The animals that
received zineb accumulated nine times less, and those given maneb 4.5
times less 131I the control group. This goitrogenic effect of these
dithiocarbamates was considered to be related to their metabolites
which are derivatives of thiourea (Ivanova et al., 1967). See also
"Observations in man".
Acute toxicity
LD50 Reference
Compound Animal Route mg/kg
body-weight
Zineb mouse (M) i.p. 2 400 Lessel and
(approx.) Cliffe, 1961
Zineb rat (M) s.c. >5 600 Lessel and
Cliffe, 1961
Zineb guinea-pig (F) oral >4 800 Lessel and
Cliffe, 1961
Short-term studies
Rat
Groups of 10 male and 10 female newly-weaned rats were given 0, 15,
60, 250 or 1000 mg/kg body-weight of zineb by gavage for five days a
week over a four week period. Blood samples were taken during the
final week of feeding. Some of the animals were sacrificed for autopsy
immediately upon termination of the period of administration of zineb;
others were kept for two weeks without receiving zineb prior to
sacrifice. No effects attributable to zineb were detected at levels of
250 mg/kg or lower. At 1000 mg/kg the kidneys were enlarged in the
animals of both sexes, but no histological changes were apparent in
that organ. The thyroids were not enlarged, although histological
examination indicated a slight hyperplasia in the females given 1000
mg/kg. Weight gain in the animals was normal, as was the blood
picture. There were no histological changes in any tissue other than
in the thyroid. One female animal given 1000 mg/kg died of an unknown
cause. Withdrawal of zineb appeared to result in a reversal of the
effect on the kidney and thyroid, as evidenced by examination of the
animals sacrificed two weeks after discontinuing treatment (Lessel and
Cliffe, 1961).
Male rats were maintained for six weeks on diets containing 0, 500 or
5000 ppm of either maneb or zineb. Gross and histopathological changes
in the thyroid gland, reduced assimilation of 124I and slightly
reduced respiratory activity of the liver mitochondria at 5000 ppm
were observed in the test groups. No significant alterations were
observed with respect to the following parameters: mitochondrial
cytochrome oxidase; glucose-6-phosphate dehydrogenase in the
erythrocyte and liver homogenates; contents of cytochromes a3, b and
c; content of flavoproteides in the mitochondria and content of
oxidized and reduced nicotineamidoadenine in liver and kidney
homogenates (Bankowska et al., 1970).
Long-term studies
Rat
Studies are reported to be underway in the Netherlands with rats.
Preliminary results indicated that certain dithiocarbamate fungicides
may affect the reticuloendothelial and haematopoietic systems as
evidenced by the occurrence of slight anaemia, as well as effects on
the thymus, mesenteric lymph nodes and thyroid function. Details are
not yet available (Verschuuren, 1970).
OBSERVATIONS IN MAN
Mutagenic activity of ziram was studied in nine workers (four men and
five women) exposed for three to five years to a concentration of 1.95
- 3.7 mg/m3 air using the test of chromosome aberrations in
peripheral leucocytes. The control group consisted of four humans
(three women and one man). The number of aberrated cells in the group
exposed to ziram was 5.9 percent and in the control group 0.75 percent
(Pilinskaya, 1970).
The damage of thyroid gland function has been observed not only in
animals but in humans, too. Having examined 25 workers coming into
contact with thiram, Ryznkova and Smirnova found a symptomology of
hyperthroidosis in nine workers from this group; surgical treatment
was necessary in three cases (Korablev, 1969).
COMMENTS
Since the last evaluation of the dithiocarbamate fungicides considered
in this monograph, adequate information on the biotransformation in
plants has not been forthcoming. Such information is needed to
determine if the main metabolites are the same in animals as in
plants. The Meeting was informed that a considerable amount of work on
a number of dithiocarbamates is now in progress in The Netherlands.
This work involves short and long term studies and studies on
mechanisms of action and metabolism. Preliminary information from this
work indicates the existence of some effects on the
reticuloendothelial and haematopoietic system, as evidenced by a
slight anaemia as well as changes in the thymus, mesenteric lymph
nodes and thyroid function. Work reported from eastern Europe
indicates that certain dithiocarbamate fungicides affect reproductive
physiology, may have a carcinogenic potential and disturb the thyroid
function.
For these reasons it was considered that the temporary acceptable
daily intakes should remain at the same figures as previously
established (and be applicable to the parent compounds only), pending
consideration of the results of the studies in progress.
TOXICOLOGICAL EVALUATION
Levels causing no toxicological effects
Ferbam
Rat: 250 ppm in the diet, equivalent to 12.5 mg/kg
body-weight/day
Dog: 5 mg/kg body-weight
Maneb
Rat: 250 ppm in the diet, equivalent to 12.5 mg/kg
body-weight/day
Nabam
No long-term studies reported. See FAO/WHO, 1968
Thiram
Rat: 48 ppm in the diet, approximately equivalent to 2.5 mg/kg
body-weight/day
Dog: 5 mg/kg body-weight/day
Zineb
Not found in the long term study previously reported. See
FAO/WHO, 1968
Ziram
Rat: 250 ppm in the diet, equivalent to 12.5 mg/kg
body-weight/day
Dog: 5 mg/kg body-weight/day
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.025 mg/kg body-weight, applicable to the parent compounds only,
and to the sum of all the dithiocarbamate fungicides if more than one
is present.
EVALUATION FOR TOLERANCES
Metabolism
In the study of some ethylene-bis-dithiocarbamates, rapid
decomposition of nabam, maneb and zineb in an aqueous environment,
mainly to ethylene thiourea and ethylene thiram monosulfide, was
confirmed (Vonk and Kaars Sijpesteijn, 1970). But only ethylene
thiourea was taken up readily in the plant, being converted to an
unidentified product to some extent: no ethylene thiram monosulfide
was found in the plant.
METHODS OF RESIDUE ANALYSIS
Currently, the most commonly used method is still the nonspecific one
involving the determination of carbon disulphide evolved on treatment
with acid. Modifications to improve recoveries have been made by
various workers (McLeod and McCully, 1969; Rangaswamy, Poornima and
Majumder, 1970).
FURTHER WORK OR INFORMATION
REQUIRED (by June 1973)
1. Elucidation of the effect on the reticuloendothelial and
haematopoietic systems.
2. Further clarification of the possible carcinogenic effect of
these compounds
3. Elucidation of the effect on reproductive physiology
4. Elucidation of the effect on thyroid function
REQUIRED (before tolerances can be recommended)
Studies on the biotransformation of the compound in plants to
determine the chemical nature of residues, and appropriate
toxicological studies on these residues.
REFERENCES
Bankowska, J., Bojanowska, A., Komorowska-Malewska, W., Krawczynski,
K., Majle, T., Syrowatka, T. and Wiadrowska, B. (1970). The studies on
the influence of zineb and maneb on the functional state of the
thyroid gland and some related enzymatic systems (in Polish).
Roczn.Zak.Hig. (Warsz.), 21 (2): 117-127
Benes., V. and Sram, R. (1969) Mutagenic activity of some pesticides
in Drosophila melanogaster. Industr.Med., 38 (12): 50-52
Chepinoga, O.P., Zastavnyuk, N.P. and Zadorozhnaya, N.A. (1969).
Blastogenic hazard of some derivatives of the dithiocarbamic acid and
approaches to the detection (in Russian). Gigiyena primeneniya,
toksikologiya pesticidov i klinika otravleniy (Kiev), 7: 153-166
Engst, R. and Schnaak, W. (1970) Investigations of the metabolism of
the fungicidal ethylene-bis-dithiocarbamates maneb and zineb. III
The cause of the reaction of degradation. Z. Lebensm.
Untersuch.-Forsch., 143: 99-103
Falk, H.L., Thompson, S.J. and Kotin, P. (1965) Carcinogenic potential
of pesticides. Arch. environm. Hlth., 10: 847-858
FAO/WHO (1968) 1967 Evaluations of some pesticide residues in food
FAO/PL: 1967/M/11/1; WHO/Food Add./68.30
Gebhardt, D.O.E. and van Logten, M.J. (1968). The chick embryo test as
used in the study of the toxicity of certain dithiocarbamates.
Toxicol. appl. pharmacol., 13: 316-324
Innes, J.R.M., Ulland, B.B., Valerio, M.G., Petrucelli, L., Fishbein,
L., Hart, E.R., Pallotta, A.J., Bates, R.R., Falk, H.C., Gart, J.J.,
Klein, M., Mitchell, I. and Peter, J. (1969) Bioassay of pesticides
and industrial chemicals for tumorigenicity in mice: a preliminary
note. J.nat.Cancer Inst., 42: 1101-1114
Ivanova, L., Sheytanov, M. and Mosheva-Ismirova, N. (1967) Changes in
the functional state of the thyroid gland upon acute intoxication with
certain dithiocarbamates - zineb and maneb. C.R.Aced.bulg.Sci., 20
(9): 1011-1013
Izmirova, N., Izmirov, I. and Ivanova, L. (1969) The effect of zineb
and maneb on the isoenzymes of lactic dehydrogenase in the testes of
rats. C.R.Acad. bulg.Sci., 22 (2): 225-227
Kaloyanava, F., Ivanova, L. and Alexiev, B. (1967) The influence of
large doses of maneb on the progeny of albino rats. C.R.Acad.bulg.
Sci., 20 (10): 1109-1112
Khicenko, I.I. and Chernov, O.V. (1968) Experimental study of
potential blastogenic effect of ziram (in Russian). Gigiyena
primeneniya, toksikologya pesticidov i klinika otravleniy (Kiev),6:
770-776
Korablev, M.V. (1969) Toxicological characteristics of dithiocarbamate
acid derivative employed in the national economy and medicine
(Literature survey) (in Russian). Farmakol. i Toksicol., 32 (3):
356-362
Lessel, B. and Cliffe, E.E. (1961) The mammalian toxicity of metiram.
Short term (4-week) oral toxicity. Unpublished report, submitted by
Boots Pure Drug Co. Ltd.
McLeod, H.A. and McCully, K.A. (1969) Head space procedure for
screening food samples for dithiocarbamate residues. J.Assn.Off.
Anal.Chem.,52: 1226
Marcon, L.V. (1969) The effect of maneb on the embryonal development
of generative function of rats (in Russian). Farmakol. i Toksikol.,
32 (6): 731-732
Pilinskaya, M.A. (1970) Chromosome aberrations in the persons
contacted with ziram (in Russian). Genetika,6 (7): 157-163
Rangaswamy, J.R., Poornima, P. and Majumder, S.K. (1970) Rapid
colorimetric method for estimation of thiram residues in grain; also
ferbam and ziram. J.Assn.Off.Anal.Chem., 53: 519-522, 1043-1044
Robens, J.F. (1969) Teratologic studies of carbaryl, diazinon, norea,
disulfiram and thiram in small laboratory animals. Toxicol.appl.
Pharmacol., 15: 152-163
Verschuuren, H.G. (1970) Information provided verbally to the 1970
Joint FAO/WHO Meeting on Pesticide Residues on work being conducted on
dithiocarbamate fungicides in The Netherlands
Vonk, J.W. and Kaars Sijpesteijn, A., (1970) Fate in plants of
ethylene-bis-dithiocarbamate fungicides and their decomposition
products. Ann.appl.Biol., 65: 489-496
Information on the effect of zineb or maneb on various enzyme systems
in the rat is reported under "Short-term studies" (Bankowska et al.,
1970).
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Mouse
Groups of 18 mice of each sex from two hybrid strains were given
various dithiocarbamate fungicides from seven days of age for 18
months. The compounds were given daily by gavage from day seven of age
until weaning; thereafter, the compounds were added to the diet in
such a concentration that the animals received the corresponding
amount. The compounds and the respective amounts administered were:
ferbam, 10 mg/kg body-weight then 32 ppm in the diet; maneb, 46.4
mg/kg, then 158 ppm; nabam 21.5 mg/kg then 73 ppm; thiram, 10 mg/kg
then 26 ppm and zineb, 464 mg/kg then 1298 ppm. In all cases there was
no significant increase in tumours compared with a control group of
mice. However, when ethylene thiourea (a metabolite of some
dithiocarbamates) was administered at 215 mg/kg body-weight and then
after weaning incorporated into the diet at 646 ppm, the total
incidence of tumours was 14 out of 16 for the males and 18 out of 18
for the females in one strain; and 18 out of 18 and 12 out of 16,
respectively, for the males and females in the other strain (Innes et
al., 1969),
Technical ziram (97.6 percent) was administered orally to mice
(strains C57B1 and A) in doses 0 or 75 mg/kg body-weight twice a week
over a period of 2.5 months. This regimen comprises a total of 20
doses equivalent to 1500 mg/kg body-weight. A positive control group
was exposed in the same way to urethane to enable comparison of
results. The occurrence of adenomas in lungs and livers was studied
after the end of the exposure at intervals of 1.5 months during six
months. Adenomas were ascertained in the lungs only. The occurrence
was: in strain A in the ziram group 51.2 percent, in the urethane
group 100 percent and in the negative control group 42.6 percent. The
differences are not statistically significant. The first adenomas were
apparent after three months. In strain C57 in the ziram group, 7.4
percent adenomas were found, in the urethane group 25.6 percent and in
the negative control group 0 percent. The results are statistically
significant according to the T test (T=2.08; P=0.05) but not according
to the chi squared test (chi2= 2.18; P=0.15). The occurrence of
adenomas was ascertained after six months. On the basis of the results
of the experiments, ziram is considered a weak blastogen (Khicenko and
Chernov, 1968).
Mouse and rat
Zineb, ziram, maneb and urethane have been administered orally once a
week during six weeks to mice of strains A and C57 and to rats (SPF).
The total dose of single substances in the rats (200-250 g in weight)
was 120 mg (ca. 600 mg/kg body-weight/6 weeks), in mice (19-22 g) 60
mg (ca. 3000 mg/kg/6/weeks), with the exception of ziram, where the
total dose represented only half this amount i.e. 30 mg (ca. 1500
mg/kg-6 weeks). Comparison was made between the activity of
glucoso-6-phosphate dehydrogenase in liver and deoxyribonuclease in
serum under development of proliferative changes in the lungs. With
regard to zineb, ziram and urethane, the correlation was ascertained
in rate only between the early blastogenic changes in lungs and the
deoxyribonuclease activity in the serum. In case of the weak
blastogens, zineb and ziram, maximum increase of ferment activity was
apparent in the ninth month and lung changes in the 12th month after
the exposure. In the urethane group, these reactions in rats were
observed earlier (4,5 or 6 months and 9 months, respectively). Neither
the activity of glucoso-6-phosphate dehydrogenase in the liver of mice
and rats nor that of deoxyribonuclease in serum changed, and there was
no correlation with proliferative changes in the lungs (Chepinoga et
al., 1969).
Special studies on mutagenicity
The mutagenic activity of zineb and ziram is considered to be not
significant using the Muller-5 test in Drosophila melanogaster, when
compared with the spontaneous mutation rate of 0.14 percent (Benes and
Sram, 1969). See also "Observations in Man".
Special studies on reproduction
Chick embryo
The toxicity of the dithiocarbamate fungicides, ferbam, nabam, thiram
and ziram, was determined for the chick embryo. It was determined that
in order to avoid mortality from the solvent, propylene glycol, the
injections must be made at a very early stage in the development of
the embryo. The compounds were injected into the air chamber of
fertile eggs prior to incubation. On days four and six, the eggs were
candled to determine in which the embryos had died. The LD50 values
in mg/egg were for: ferbam, 0.0022; nabam, 0.14; thiram, 0.0019 and
ziram, 0.0021. The LD50 of thiram when injected into the yolk sac was
much higher than when injected into the air chamber, namely 0.018
mg/egg. The toxicity of thiram and ziram was reduced by simultaneous
injection of cysteine. It was suggested that there was a reduction of
these dithiocarbamates to less toxic materials (Gebhart and van
Logten, 1968).
Rat
Groups, each comprising 10 male and 10 female rats were given, twice
weekly, oral doses of 0, 700 or 1400 mg/kg body-weight of maneb for
four and a half months. (This dose regimen is equivalent to a daily
intake of 0, 200 or 400 mg/kg body-weight.) No clinical abnormalities
in the parent generation were observed. There were six births in the
group given the lower dose. There was a moderately high incidence of
stillbirths and imperfect skull development, but the actual numbers
compared with the controls is not stated (Kaloyanova et al., 1967).
The effect of the fungicide maneb on the embryonal development and the
generative function of rate was studied. For studying the potential
embryotoxic effect of maneb, mature white rat females with regular
ovulation were used. During the whole pregnancy, these female rats
were administered maneb by gavage every second day (as a suspension in
milk) at a dose level of 50 mg/kg body-weight (0.001 LD50). An
embryotoxic effect of the preparation was ascertained, i.e. abnormal
development of the embryos (dead embryos and resorption), stillborn
young and those incapable of living. In 21 percent of the pregnancies,
the unfavourable effect of maneb on the course of the pregnancy was
apparent when compared with 12 percent in the controls (Marcon, 1969).
Immature female and male rats, of body-weight 80-100 g, were exposed
to maneb for a month, the dose level being 50 mg/kg body-weight. When
mature (after two and one half months from the beginning of the
exposure), the control males were mated with the exposed females and
vice versa. A decline of fertility in both sexes was observed. This
effect, however, was not permanent, the function of the gonads being
restored in three and one half months (Marcon, 1969).
Hamster
Groups of from nine to 14 pregnant female hamsters received an oral
dose of 0, 31, 63, 125 or 500 mg/kg bodyweight of thiram in
dimethylsulfoxide solution on day seven or eight of gestation. No
litters were produced from the group given 500 mg/kg, and the
mortality of the parents was 60 percent. Incidence of deformed young
was significantly greater for the animals given 125 or 250 mg/kg of
thiram compared to the controls given dimethylsulfoxide alone.
Abnormalities included fused ribs, deformed tails and head defects
including all degrees of exencephaly. A similar study using
carboxymethyl cellulose as a solvent at 0, 125, 250, 300 or 500 mg/kg
body-weight of thiram produced a lower incidence of malformation both
in the treated and control animals, although the percentage incidence
of terata was increased in the 250 mg/kg groups and higher compared to
the controls (Robens, 1969).
Special studies on the effects on thyroid function
Rat
Groups, each comprising 10 rats of unspecified sex, received orally 0
or 3 500 mg/kg body-weight of maneb or 2 400 mg/kg of zineb presumably
as a single dose. After a period of 24 hours had elapsed following the
administration of the compounds, the animals were injected
intraperitoneally with 1 mCi of carrier free 131I. The animals that
received zineb accumulated nine times less, and those given maneb 4.5
times less 131I the control group. This goitrogenic effect of these
dithiocarbamates was considered to be related to their metabolites
which are derivatives of thiourea (Ivanova et al., 1967). See also
"Observations in man".
Acute toxicity
LD50 Reference
Compound Animal Route mg/kg
body-weight
Zineb mouse (M) i.p. 2 400 Lessel and
(approx.) Cliffe, 1961
Zineb rat (M) s.c. >5 600 Lessel and
Cliffe, 1961
Zineb guinea-pig (F) oral >4 800 Lessel and
Cliffe, 1961
Short-term studies
Rat
Groups of 10 male and 10 female newly-weaned rats were given 0, 15,
60, 250 or 1000 mg/kg body-weight of zineb by gavage for five days a
week over a four week period. Blood samples were taken during the
final week of feeding. Some of the animals were sacrificed for autopsy
immediately upon termination of the period of administration of zineb;
others were kept for two weeks without receiving zineb prior to
sacrifice. No effects attributable to zineb were detected at levels of
250 mg/kg or lower. At 1000 mg/kg the kidneys were enlarged in the
animals of both sexes, but no histological changes were apparent in
that organ. The thyroids were not enlarged, although histological
examination indicated a slight hyperplasia in the females given 1000
mg/kg. Weight gain in the animals was normal, as was the blood
picture. There were no histological changes in any tissue other than
in the thyroid. One female animal given 1000 mg/kg died of an unknown
cause. Withdrawal of zineb appeared to result in a reversal of the
effect on the kidney and thyroid, as evidenced by examination of the
animals sacrificed two weeks after discontinuing treatment (Lessel and
Cliffe, 1961).
Male rats were maintained for six weeks on diets containing 0, 500 or
5000 ppm of either maneb or zineb. Gross and histopathological changes
in the thyroid gland, reduced assimilation of 124I and slightly
reduced respiratory activity of the liver mitochondria at 5000 ppm
were observed in the test groups. No significant alterations were
observed with respect to the following parameters: mitochondrial
cytochrome oxidase; glucose-6-phosphate dehydrogenase in the
erythrocyte and liver homogenates; contents of cytochromes a3, b and
c; content of flavoproteides in the mitochondria and content of
oxidized and reduced nicotineamidoadenine in liver and kidney
homogenates (Bankowska et al., 1970).
Long-term studies
Rat
Studies are reported to be underway in the Netherlands with rats.
Preliminary results indicated that certain dithiocarbamate fungicides
may affect the reticuloendothelial and haematopoietic systems as
evidenced by the occurrence of slight anaemia, as well as effects on
the thymus, mesenteric lymph nodes and thyroid function. Details are
not yet available (Verschuuren, 1970).
OBSERVATIONS IN MAN
Mutagenic activity of ziram was studied in nine workers (four men and
five women) exposed for three to five years to a concentration of 1.95
- 3.7 mg/m3 air using the test of chromosome aberrations in
peripheral leucocytes. The control group consisted of four humans
(three women and one man). The number of aberrated cells in the group
exposed to ziram was 5.9 percent and in the control group 0.75 percent
(Pilinskaya, 1970).
The damage of thyroid gland function has been observed not only in
animals but in humans, too. Having examined 25 workers coming into
contact with thiram, Ryznkova and Smirnova found a symptomology of
hyperthroidosis in nine workers from this group; surgical treatment
was necessary in three cases (Korablev, 1969).
COMMENTS
Since the last evaluation of the dithiocarbamate fungicides considered
in this monograph, adequate information on the biotransformation in
plants has not been forthcoming. Such information is needed to
determine if the main metabolites are the same in animals as in
plants. The Meeting was informed that a considerable amount of work on
a number of dithiocarbamates is now in progress in The Netherlands.
This work involves short and long term studies and studies on
mechanisms of action and metabolism. Preliminary information from this
work indicates the existence of some effects on the
reticuloendothelial and haematopoietic system, as evidenced by a
slight anaemia as well as changes in the thymus, mesenteric lymph
nodes and thyroid function. Work reported from eastern Europe
indicates that certain dithiocarbamate fungicides affect reproductive
physiology, may have a carcinogenic potential and disturb the thyroid
function.
For these reasons it was considered that the temporary acceptable
daily intakes should remain at the same figures as previously
established (and be applicable to the parent compounds only), pending
consideration of the results of the studies in progress.
TOXICOLOGICAL EVALUATION
Levels causing no toxicological effects
Ferbam
Rat: 250 ppm in the diet, equivalent to 12.5 mg/kg
body-weight/day
Dog: 5 mg/kg body-weight
Maneb
Rat: 250 ppm in the diet, equivalent to 12.5 mg/kg
body-weight/day
Nabam
No long-term studies reported. See FAO/WHO, 1968
Thiram
Rat: 48 ppm in the diet, approximately equivalent to 2.5 mg/kg
body-weight/day
Dog: 5 mg/kg body-weight/day
Zineb
Not found in the long term study previously reported. See
FAO/WHO, 1968
Ziram
Rat: 250 ppm in the diet, equivalent to 12.5 mg/kg
body-weight/day
Dog: 5 mg/kg body-weight/day
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.025 mg/kg body-weight, applicable to the parent compounds only,
and to the sum of all the dithiocarbamate fungicides if more than one
is present.
EVALUATION FOR TOLERANCES
Metabolism
In the study of some ethylene-bis-dithiocarbamates, rapid
decomposition of nabam, maneb and zineb in an aqueous environment,
mainly to ethylene thiourea and ethylene thiram monosulfide, was
confirmed (Vonk and Kaars Sijpesteijn, 1970). But only ethylene
thiourea was taken up readily in the plant, being converted to an
unidentified product to some extent: no ethylene thiram monosulfide
was found in the plant.
METHODS OF RESIDUE ANALYSIS
Currently, the most commonly used method is still the nonspecific one
involving the determination of carbon disulphide evolved on treatment
with acid. Modifications to improve recoveries have been made by
various workers (McLeod and McCully, 1969; Rangaswamy, Poornima and
Majumder, 1970).
FURTHER WORK OR INFORMATION
REQUIRED (by June 1973)
1. Elucidation of the effect on the reticuloendothelial and
haematopoietic systems.
2. Further clarification of the possible carcinogenic effect of
these compounds
3. Elucidation of the effect on reproductive physiology
4. Elucidation of the effect on thyroid function
REQUIRED (before tolerances can be recommended)
Studies on the biotransformation of the compound in plants to
determine the chemical nature of residues, and appropriate
toxicological studies on these residues.
REFERENCES
Bankowska, J., Bojanowska, A., Komorowska-Malewska, W., Krawczynski,
K., Majle, T., Syrowatka, T. and Wiadrowska, B. (1970). The studies on
the influence of zineb and maneb on the functional state of the
thyroid gland and some related enzymatic systems (in Polish).
Roczn.Zak.Hig. (Warsz.), 21 (2): 117-127
Benes., V. and Sram, R. (1969) Mutagenic activity of some pesticides
in Drosophila melanogaster. Industr.Med., 38 (12): 50-52
Chepinoga, O.P., Zastavnyuk, N.P. and Zadorozhnaya, N.A. (1969).
Blastogenic hazard of some derivatives of the dithiocarbamic acid and
approaches to the detection (in Russian). Gigiyena primeneniya,
toksikologiya pesticidov i klinika otravleniy (Kiev), 7: 153-166
Engst, R. and Schnaak, W. (1970) Investigations of the metabolism of
the fungicidal ethylene-bis-dithiocarbamates maneb and zineb. III
The cause of the reaction of degradation. Z. Lebensm.
Untersuch.-Forsch., 143: 99-103
Falk, H.L., Thompson, S.J. and Kotin, P. (1965) Carcinogenic potential
of pesticides. Arch. environm. Hlth., 10: 847-858
FAO/WHO (1968) 1967 Evaluations of some pesticide residues in food
FAO/PL: 1967/M/11/1; WHO/Food Add./68.30
Gebhardt, D.O.E. and van Logten, M.J. (1968). The chick embryo test as
used in the study of the toxicity of certain dithiocarbamates.
Toxicol. appl. pharmacol., 13: 316-324
Innes, J.R.M., Ulland, B.B., Valerio, M.G., Petrucelli, L., Fishbein,
L., Hart, E.R., Pallotta, A.J., Bates, R.R., Falk, H.C., Gart, J.J.,
Klein, M., Mitchell, I. and Peter, J. (1969) Bioassay of pesticides
and industrial chemicals for tumorigenicity in mice: a preliminary
note. J.nat.Cancer Inst., 42: 1101-1114
Ivanova, L., Sheytanov, M. and Mosheva-Ismirova, N. (1967) Changes in
the functional state of the thyroid gland upon acute intoxication with
certain dithiocarbamates - zineb and maneb. C.R.Aced.bulg.Sci., 20
(9): 1011-1013
Izmirova, N., Izmirov, I. and Ivanova, L. (1969) The effect of zineb
and maneb on the isoenzymes of lactic dehydrogenase in the testes of
rats. C.R.Acad. bulg.Sci., 22 (2): 225-227
Kaloyanava, F., Ivanova, L. and Alexiev, B. (1967) The influence of
large doses of maneb on the progeny of albino rats. C.R.Acad.bulg.
Sci., 20 (10): 1109-1112
Khicenko, I.I. and Chernov, O.V. (1968) Experimental study of
potential blastogenic effect of ziram (in Russian). Gigiyena
primeneniya, toksikologya pesticidov i klinika otravleniy (Kiev),6:
770-776
Korablev, M.V. (1969) Toxicological characteristics of dithiocarbamate
acid derivative employed in the national economy and medicine
(Literature survey) (in Russian). Farmakol. i Toksicol., 32 (3):
356-362
Lessel, B. and Cliffe, E.E. (1961) The mammalian toxicity of metiram.
Short term (4-week) oral toxicity. Unpublished report, submitted by
Boots Pure Drug Co. Ltd.
McLeod, H.A. and McCully, K.A. (1969) Head space procedure for
screening food samples for dithiocarbamate residues. J.Assn.Off.
Anal.Chem.,52: 1226
Marcon, L.V. (1969) The effect of maneb on the embryonal development
of generative function of rats (in Russian). Farmakol. i Toksikol.,
32 (6): 731-732
Pilinskaya, M.A. (1970) Chromosome aberrations in the persons
contacted with ziram (in Russian). Genetika,6 (7): 157-163
Rangaswamy, J.R., Poornima, P. and Majumder, S.K. (1970) Rapid
colorimetric method for estimation of thiram residues in grain; also
ferbam and ziram. J.Assn.Off.Anal.Chem., 53: 519-522, 1043-1044
Robens, J.F. (1969) Teratologic studies of carbaryl, diazinon, norea,
disulfiram and thiram in small laboratory animals. Toxicol.appl.
Pharmacol., 15: 152-163
Verschuuren, H.G. (1970) Information provided verbally to the 1970
Joint FAO/WHO Meeting on Pesticide Residues on work being conducted on
dithiocarbamate fungicides in The Netherlands
Vonk, J.W. and Kaars Sijpesteijn, A., (1970) Fate in plants of
ethylene-bis-dithiocarbamate fungicides and their decomposition
products. Ann.appl.Biol., 65: 489-496
 Information on the effect of zineb or maneb on various enzyme systems
in the rat is reported under "Short-term studies" (Bankowska et al.,
1970).
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Mouse
Groups of 18 mice of each sex from two hybrid strains were given
various dithiocarbamate fungicides from seven days of age for 18
months. The compounds were given daily by gavage from day seven of age
until weaning; thereafter, the compounds were added to the diet in
such a concentration that the animals received the corresponding
amount. The compounds and the respective amounts administered were:
ferbam, 10 mg/kg body-weight then 32 ppm in the diet; maneb, 46.4
mg/kg, then 158 ppm; nabam 21.5 mg/kg then 73 ppm; thiram, 10 mg/kg
then 26 ppm and zineb, 464 mg/kg then 1298 ppm. In all cases there was
no significant increase in tumours compared with a control group of
mice. However, when ethylene thiourea (a metabolite of some
dithiocarbamates) was administered at 215 mg/kg body-weight and then
after weaning incorporated into the diet at 646 ppm, the total
incidence of tumours was 14 out of 16 for the males and 18 out of 18
for the females in one strain; and 18 out of 18 and 12 out of 16,
respectively, for the males and females in the other strain (Innes et
al., 1969),
Technical ziram (97.6 percent) was administered orally to mice
(strains C57B1 and A) in doses 0 or 75 mg/kg body-weight twice a week
over a period of 2.5 months. This regimen comprises a total of 20
doses equivalent to 1500 mg/kg body-weight. A positive control group
was exposed in the same way to urethane to enable comparison of
results. The occurrence of adenomas in lungs and livers was studied
after the end of the exposure at intervals of 1.5 months during six
months. Adenomas were ascertained in the lungs only. The occurrence
was: in strain A in the ziram group 51.2 percent, in the urethane
group 100 percent and in the negative control group 42.6 percent. The
differences are not statistically significant. The first adenomas were
apparent after three months. In strain C57 in the ziram group, 7.4
percent adenomas were found, in the urethane group 25.6 percent and in
the negative control group 0 percent. The results are statistically
significant according to the T test (T=2.08; P=0.05) but not according
to the chi squared test (chi2= 2.18; P=0.15). The occurrence of
adenomas was ascertained after six months. On the basis of the results
of the experiments, ziram is considered a weak blastogen (Khicenko and
Chernov, 1968).
Mouse and rat
Zineb, ziram, maneb and urethane have been administered orally once a
week during six weeks to mice of strains A and C57 and to rats (SPF).
The total dose of single substances in the rats (200-250 g in weight)
was 120 mg (ca. 600 mg/kg body-weight/6 weeks), in mice (19-22 g) 60
mg (ca. 3000 mg/kg/6/weeks), with the exception of ziram, where the
total dose represented only half this amount i.e. 30 mg (ca. 1500
mg/kg-6 weeks). Comparison was made between the activity of
glucoso-6-phosphate dehydrogenase in liver and deoxyribonuclease in
serum under development of proliferative changes in the lungs. With
regard to zineb, ziram and urethane, the correlation was ascertained
in rate only between the early blastogenic changes in lungs and the
deoxyribonuclease activity in the serum. In case of the weak
blastogens, zineb and ziram, maximum increase of ferment activity was
apparent in the ninth month and lung changes in the 12th month after
the exposure. In the urethane group, these reactions in rats were
observed earlier (4,5 or 6 months and 9 months, respectively). Neither
the activity of glucoso-6-phosphate dehydrogenase in the liver of mice
and rats nor that of deoxyribonuclease in serum changed, and there was
no correlation with proliferative changes in the lungs (Chepinoga et
al., 1969).
Special studies on mutagenicity
The mutagenic activity of zineb and ziram is considered to be not
significant using the Muller-5 test in Drosophila melanogaster, when
compared with the spontaneous mutation rate of 0.14 percent (Benes and
Sram, 1969). See also "Observations in Man".
Special studies on reproduction
Chick embryo
The toxicity of the dithiocarbamate fungicides, ferbam, nabam, thiram
and ziram, was determined for the chick embryo. It was determined that
in order to avoid mortality from the solvent, propylene glycol, the
injections must be made at a very early stage in the development of
the embryo. The compounds were injected into the air chamber of
fertile eggs prior to incubation. On days four and six, the eggs were
candled to determine in which the embryos had died. The LD50 values
in mg/egg were for: ferbam, 0.0022; nabam, 0.14; thiram, 0.0019 and
ziram, 0.0021. The LD50 of thiram when injected into the yolk sac was
much higher than when injected into the air chamber, namely 0.018
mg/egg. The toxicity of thiram and ziram was reduced by simultaneous
injection of cysteine. It was suggested that there was a reduction of
these dithiocarbamates to less toxic materials (Gebhart and van
Logten, 1968).
Rat
Groups, each comprising 10 male and 10 female rats were given, twice
weekly, oral doses of 0, 700 or 1400 mg/kg body-weight of maneb for
four and a half months. (This dose regimen is equivalent to a daily
intake of 0, 200 or 400 mg/kg body-weight.) No clinical abnormalities
in the parent generation were observed. There were six births in the
group given the lower dose. There was a moderately high incidence of
stillbirths and imperfect skull development, but the actual numbers
compared with the controls is not stated (Kaloyanova et al., 1967).
The effect of the fungicide maneb on the embryonal development and the
generative function of rate was studied. For studying the potential
embryotoxic effect of maneb, mature white rat females with regular
ovulation were used. During the whole pregnancy, these female rats
were administered maneb by gavage every second day (as a suspension in
milk) at a dose level of 50 mg/kg body-weight (0.001 LD50). An
embryotoxic effect of the preparation was ascertained, i.e. abnormal
development of the embryos (dead embryos and resorption), stillborn
young and those incapable of living. In 21 percent of the pregnancies,
the unfavourable effect of maneb on the course of the pregnancy was
apparent when compared with 12 percent in the controls (Marcon, 1969).
Immature female and male rats, of body-weight 80-100 g, were exposed
to maneb for a month, the dose level being 50 mg/kg body-weight. When
mature (after two and one half months from the beginning of the
exposure), the control males were mated with the exposed females and
vice versa. A decline of fertility in both sexes was observed. This
effect, however, was not permanent, the function of the gonads being
restored in three and one half months (Marcon, 1969).
Hamster
Groups of from nine to 14 pregnant female hamsters received an oral
dose of 0, 31, 63, 125 or 500 mg/kg bodyweight of thiram in
dimethylsulfoxide solution on day seven or eight of gestation. No
litters were produced from the group given 500 mg/kg, and the
mortality of the parents was 60 percent. Incidence of deformed young
was significantly greater for the animals given 125 or 250 mg/kg of
thiram compared to the controls given dimethylsulfoxide alone.
Abnormalities included fused ribs, deformed tails and head defects
including all degrees of exencephaly. A similar study using
carboxymethyl cellulose as a solvent at 0, 125, 250, 300 or 500 mg/kg
body-weight of thiram produced a lower incidence of malformation both
in the treated and control animals, although the percentage incidence
of terata was increased in the 250 mg/kg groups and higher compared to
the controls (Robens, 1969).
Special studies on the effects on thyroid function
Rat
Groups, each comprising 10 rats of unspecified sex, received orally 0
or 3 500 mg/kg body-weight of maneb or 2 400 mg/kg of zineb presumably
as a single dose. After a period of 24 hours had elapsed following the
administration of the compounds, the animals were injected
intraperitoneally with 1 mCi of carrier free 131I. The animals that
received zineb accumulated nine times less, and those given maneb 4.5
times less 131I the control group. This goitrogenic effect of these
dithiocarbamates was considered to be related to their metabolites
which are derivatives of thiourea (Ivanova et al., 1967). See also
"Observations in man".
Acute toxicity
LD50 Reference
Compound Animal Route mg/kg
body-weight
Zineb mouse (M) i.p. 2 400 Lessel and
(approx.) Cliffe, 1961
Zineb rat (M) s.c. >5 600 Lessel and
Cliffe, 1961
Zineb guinea-pig (F) oral >4 800 Lessel and
Cliffe, 1961
Short-term studies
Rat
Groups of 10 male and 10 female newly-weaned rats were given 0, 15,
60, 250 or 1000 mg/kg body-weight of zineb by gavage for five days a
week over a four week period. Blood samples were taken during the
final week of feeding. Some of the animals were sacrificed for autopsy
immediately upon termination of the period of administration of zineb;
others were kept for two weeks without receiving zineb prior to
sacrifice. No effects attributable to zineb were detected at levels of
250 mg/kg or lower. At 1000 mg/kg the kidneys were enlarged in the
animals of both sexes, but no histological changes were apparent in
that organ. The thyroids were not enlarged, although histological
examination indicated a slight hyperplasia in the females given 1000
mg/kg. Weight gain in the animals was normal, as was the blood
picture. There were no histological changes in any tissue other than
in the thyroid. One female animal given 1000 mg/kg died of an unknown
cause. Withdrawal of zineb appeared to result in a reversal of the
effect on the kidney and thyroid, as evidenced by examination of the
animals sacrificed two weeks after discontinuing treatment (Lessel and
Cliffe, 1961).
Male rats were maintained for six weeks on diets containing 0, 500 or
5000 ppm of either maneb or zineb. Gross and histopathological changes
in the thyroid gland, reduced assimilation of 124I and slightly
reduced respiratory activity of the liver mitochondria at 5000 ppm
were observed in the test groups. No significant alterations were
observed with respect to the following parameters: mitochondrial
cytochrome oxidase; glucose-6-phosphate dehydrogenase in the
erythrocyte and liver homogenates; contents of cytochromes a3, b and
c; content of flavoproteides in the mitochondria and content of
oxidized and reduced nicotineamidoadenine in liver and kidney
homogenates (Bankowska et al., 1970).
Long-term studies
Rat
Studies are reported to be underway in the Netherlands with rats.
Preliminary results indicated that certain dithiocarbamate fungicides
may affect the reticuloendothelial and haematopoietic systems as
evidenced by the occurrence of slight anaemia, as well as effects on
the thymus, mesenteric lymph nodes and thyroid function. Details are
not yet available (Verschuuren, 1970).
OBSERVATIONS IN MAN
Mutagenic activity of ziram was studied in nine workers (four men and
five women) exposed for three to five years to a concentration of 1.95
- 3.7 mg/m3 air using the test of chromosome aberrations in
peripheral leucocytes. The control group consisted of four humans
(three women and one man). The number of aberrated cells in the group
exposed to ziram was 5.9 percent and in the control group 0.75 percent
(Pilinskaya, 1970).
The damage of thyroid gland function has been observed not only in
animals but in humans, too. Having examined 25 workers coming into
contact with thiram, Ryznkova and Smirnova found a symptomology of
hyperthroidosis in nine workers from this group; surgical treatment
was necessary in three cases (Korablev, 1969).
COMMENTS
Since the last evaluation of the dithiocarbamate fungicides considered
in this monograph, adequate information on the biotransformation in
plants has not been forthcoming. Such information is needed to
determine if the main metabolites are the same in animals as in
plants. The Meeting was informed that a considerable amount of work on
a number of dithiocarbamates is now in progress in The Netherlands.
This work involves short and long term studies and studies on
mechanisms of action and metabolism. Preliminary information from this
work indicates the existence of some effects on the
reticuloendothelial and haematopoietic system, as evidenced by a
slight anaemia as well as changes in the thymus, mesenteric lymph
nodes and thyroid function. Work reported from eastern Europe
indicates that certain dithiocarbamate fungicides affect reproductive
physiology, may have a carcinogenic potential and disturb the thyroid
function.
For these reasons it was considered that the temporary acceptable
daily intakes should remain at the same figures as previously
established (and be applicable to the parent compounds only), pending
consideration of the results of the studies in progress.
TOXICOLOGICAL EVALUATION
Levels causing no toxicological effects
Ferbam
Rat: 250 ppm in the diet, equivalent to 12.5 mg/kg
body-weight/day
Dog: 5 mg/kg body-weight
Maneb
Rat: 250 ppm in the diet, equivalent to 12.5 mg/kg
body-weight/day
Nabam
No long-term studies reported. See FAO/WHO, 1968
Thiram
Rat: 48 ppm in the diet, approximately equivalent to 2.5 mg/kg
body-weight/day
Dog: 5 mg/kg body-weight/day
Zineb
Not found in the long term study previously reported. See
FAO/WHO, 1968
Ziram
Rat: 250 ppm in the diet, equivalent to 12.5 mg/kg
body-weight/day
Dog: 5 mg/kg body-weight/day
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.025 mg/kg body-weight, applicable to the parent compounds only,
and to the sum of all the dithiocarbamate fungicides if more than one
is present.
EVALUATION FOR TOLERANCES
Metabolism
In the study of some ethylene-bis-dithiocarbamates, rapid
decomposition of nabam, maneb and zineb in an aqueous environment,
mainly to ethylene thiourea and ethylene thiram monosulfide, was
confirmed (Vonk and Kaars Sijpesteijn, 1970). But only ethylene
thiourea was taken up readily in the plant, being converted to an
unidentified product to some extent: no ethylene thiram monosulfide
was found in the plant.
METHODS OF RESIDUE ANALYSIS
Currently, the most commonly used method is still the nonspecific one
involving the determination of carbon disulphide evolved on treatment
with acid. Modifications to improve recoveries have been made by
various workers (McLeod and McCully, 1969; Rangaswamy, Poornima and
Majumder, 1970).
FURTHER WORK OR INFORMATION
REQUIRED (by June 1973)
1. Elucidation of the effect on the reticuloendothelial and
haematopoietic systems.
2. Further clarification of the possible carcinogenic effect of
these compounds
3. Elucidation of the effect on reproductive physiology
4. Elucidation of the effect on thyroid function
REQUIRED (before tolerances can be recommended)
Studies on the biotransformation of the compound in plants to
determine the chemical nature of residues, and appropriate
toxicological studies on these residues.
REFERENCES
Bankowska, J., Bojanowska, A., Komorowska-Malewska, W., Krawczynski,
K., Majle, T., Syrowatka, T. and Wiadrowska, B. (1970). The studies on
the influence of zineb and maneb on the functional state of the
thyroid gland and some related enzymatic systems (in Polish).
Roczn.Zak.Hig. (Warsz.), 21 (2): 117-127
Benes., V. and Sram, R. (1969) Mutagenic activity of some pesticides
in Drosophila melanogaster. Industr.Med., 38 (12): 50-52
Chepinoga, O.P., Zastavnyuk, N.P. and Zadorozhnaya, N.A. (1969).
Blastogenic hazard of some derivatives of the dithiocarbamic acid and
approaches to the detection (in Russian). Gigiyena primeneniya,
toksikologiya pesticidov i klinika otravleniy (Kiev), 7: 153-166
Engst, R. and Schnaak, W. (1970) Investigations of the metabolism of
the fungicidal ethylene-bis-dithiocarbamates maneb and zineb. III
The cause of the reaction of degradation. Z. Lebensm.
Untersuch.-Forsch., 143: 99-103
Falk, H.L., Thompson, S.J. and Kotin, P. (1965) Carcinogenic potential
of pesticides. Arch. environm. Hlth., 10: 847-858
FAO/WHO (1968) 1967 Evaluations of some pesticide residues in food
FAO/PL: 1967/M/11/1; WHO/Food Add./68.30
Gebhardt, D.O.E. and van Logten, M.J. (1968). The chick embryo test as
used in the study of the toxicity of certain dithiocarbamates.
Toxicol. appl. pharmacol., 13: 316-324
Innes, J.R.M., Ulland, B.B., Valerio, M.G., Petrucelli, L., Fishbein,
L., Hart, E.R., Pallotta, A.J., Bates, R.R., Falk, H.C., Gart, J.J.,
Klein, M., Mitchell, I. and Peter, J. (1969) Bioassay of pesticides
and industrial chemicals for tumorigenicity in mice: a preliminary
note. J.nat.Cancer Inst., 42: 1101-1114
Ivanova, L., Sheytanov, M. and Mosheva-Ismirova, N. (1967) Changes in
the functional state of the thyroid gland upon acute intoxication with
certain dithiocarbamates - zineb and maneb. C.R.Aced.bulg.Sci., 20
(9): 1011-1013
Izmirova, N., Izmirov, I. and Ivanova, L. (1969) The effect of zineb
and maneb on the isoenzymes of lactic dehydrogenase in the testes of
rats. C.R.Acad. bulg.Sci., 22 (2): 225-227
Kaloyanava, F., Ivanova, L. and Alexiev, B. (1967) The influence of
large doses of maneb on the progeny of albino rats. C.R.Acad.bulg.
Sci., 20 (10): 1109-1112
Khicenko, I.I. and Chernov, O.V. (1968) Experimental study of
potential blastogenic effect of ziram (in Russian). Gigiyena
primeneniya, toksikologya pesticidov i klinika otravleniy (Kiev),6:
770-776
Korablev, M.V. (1969) Toxicological characteristics of dithiocarbamate
acid derivative employed in the national economy and medicine
(Literature survey) (in Russian). Farmakol. i Toksicol., 32 (3):
356-362
Lessel, B. and Cliffe, E.E. (1961) The mammalian toxicity of metiram.
Short term (4-week) oral toxicity. Unpublished report, submitted by
Boots Pure Drug Co. Ltd.
McLeod, H.A. and McCully, K.A. (1969) Head space procedure for
screening food samples for dithiocarbamate residues. J.Assn.Off.
Anal.Chem.,52: 1226
Marcon, L.V. (1969) The effect of maneb on the embryonal development
of generative function of rats (in Russian). Farmakol. i Toksikol.,
32 (6): 731-732
Pilinskaya, M.A. (1970) Chromosome aberrations in the persons
contacted with ziram (in Russian). Genetika,6 (7): 157-163
Rangaswamy, J.R., Poornima, P. and Majumder, S.K. (1970) Rapid
colorimetric method for estimation of thiram residues in grain; also
ferbam and ziram. J.Assn.Off.Anal.Chem., 53: 519-522, 1043-1044
Robens, J.F. (1969) Teratologic studies of carbaryl, diazinon, norea,
disulfiram and thiram in small laboratory animals. Toxicol.appl.
Pharmacol., 15: 152-163
Verschuuren, H.G. (1970) Information provided verbally to the 1970
Joint FAO/WHO Meeting on Pesticide Residues on work being conducted on
dithiocarbamate fungicides in The Netherlands
Vonk, J.W. and Kaars Sijpesteijn, A., (1970) Fate in plants of
ethylene-bis-dithiocarbamate fungicides and their decomposition
products. Ann.appl.Biol., 65: 489-496
Information on the effect of zineb or maneb on various enzyme systems
in the rat is reported under "Short-term studies" (Bankowska et al.,
1970).
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Mouse
Groups of 18 mice of each sex from two hybrid strains were given
various dithiocarbamate fungicides from seven days of age for 18
months. The compounds were given daily by gavage from day seven of age
until weaning; thereafter, the compounds were added to the diet in
such a concentration that the animals received the corresponding
amount. The compounds and the respective amounts administered were:
ferbam, 10 mg/kg body-weight then 32 ppm in the diet; maneb, 46.4
mg/kg, then 158 ppm; nabam 21.5 mg/kg then 73 ppm; thiram, 10 mg/kg
then 26 ppm and zineb, 464 mg/kg then 1298 ppm. In all cases there was
no significant increase in tumours compared with a control group of
mice. However, when ethylene thiourea (a metabolite of some
dithiocarbamates) was administered at 215 mg/kg body-weight and then
after weaning incorporated into the diet at 646 ppm, the total
incidence of tumours was 14 out of 16 for the males and 18 out of 18
for the females in one strain; and 18 out of 18 and 12 out of 16,
respectively, for the males and females in the other strain (Innes et
al., 1969),
Technical ziram (97.6 percent) was administered orally to mice
(strains C57B1 and A) in doses 0 or 75 mg/kg body-weight twice a week
over a period of 2.5 months. This regimen comprises a total of 20
doses equivalent to 1500 mg/kg body-weight. A positive control group
was exposed in the same way to urethane to enable comparison of
results. The occurrence of adenomas in lungs and livers was studied
after the end of the exposure at intervals of 1.5 months during six
months. Adenomas were ascertained in the lungs only. The occurrence
was: in strain A in the ziram group 51.2 percent, in the urethane
group 100 percent and in the negative control group 42.6 percent. The
differences are not statistically significant. The first adenomas were
apparent after three months. In strain C57 in the ziram group, 7.4
percent adenomas were found, in the urethane group 25.6 percent and in
the negative control group 0 percent. The results are statistically
significant according to the T test (T=2.08; P=0.05) but not according
to the chi squared test (chi2= 2.18; P=0.15). The occurrence of
adenomas was ascertained after six months. On the basis of the results
of the experiments, ziram is considered a weak blastogen (Khicenko and
Chernov, 1968).
Mouse and rat
Zineb, ziram, maneb and urethane have been administered orally once a
week during six weeks to mice of strains A and C57 and to rats (SPF).
The total dose of single substances in the rats (200-250 g in weight)
was 120 mg (ca. 600 mg/kg body-weight/6 weeks), in mice (19-22 g) 60
mg (ca. 3000 mg/kg/6/weeks), with the exception of ziram, where the
total dose represented only half this amount i.e. 30 mg (ca. 1500
mg/kg-6 weeks). Comparison was made between the activity of
glucoso-6-phosphate dehydrogenase in liver and deoxyribonuclease in
serum under development of proliferative changes in the lungs. With
regard to zineb, ziram and urethane, the correlation was ascertained
in rate only between the early blastogenic changes in lungs and the
deoxyribonuclease activity in the serum. In case of the weak
blastogens, zineb and ziram, maximum increase of ferment activity was
apparent in the ninth month and lung changes in the 12th month after
the exposure. In the urethane group, these reactions in rats were
observed earlier (4,5 or 6 months and 9 months, respectively). Neither
the activity of glucoso-6-phosphate dehydrogenase in the liver of mice
and rats nor that of deoxyribonuclease in serum changed, and there was
no correlation with proliferative changes in the lungs (Chepinoga et
al., 1969).
Special studies on mutagenicity
The mutagenic activity of zineb and ziram is considered to be not
significant using the Muller-5 test in Drosophila melanogaster, when
compared with the spontaneous mutation rate of 0.14 percent (Benes and
Sram, 1969). See also "Observations in Man".
Special studies on reproduction
Chick embryo
The toxicity of the dithiocarbamate fungicides, ferbam, nabam, thiram
and ziram, was determined for the chick embryo. It was determined that
in order to avoid mortality from the solvent, propylene glycol, the
injections must be made at a very early stage in the development of
the embryo. The compounds were injected into the air chamber of
fertile eggs prior to incubation. On days four and six, the eggs were
candled to determine in which the embryos had died. The LD50 values
in mg/egg were for: ferbam, 0.0022; nabam, 0.14; thiram, 0.0019 and
ziram, 0.0021. The LD50 of thiram when injected into the yolk sac was
much higher than when injected into the air chamber, namely 0.018
mg/egg. The toxicity of thiram and ziram was reduced by simultaneous
injection of cysteine. It was suggested that there was a reduction of
these dithiocarbamates to less toxic materials (Gebhart and van
Logten, 1968).
Rat
Groups, each comprising 10 male and 10 female rats were given, twice
weekly, oral doses of 0, 700 or 1400 mg/kg body-weight of maneb for
four and a half months. (This dose regimen is equivalent to a daily
intake of 0, 200 or 400 mg/kg body-weight.) No clinical abnormalities
in the parent generation were observed. There were six births in the
group given the lower dose. There was a moderately high incidence of
stillbirths and imperfect skull development, but the actual numbers
compared with the controls is not stated (Kaloyanova et al., 1967).
The effect of the fungicide maneb on the embryonal development and the
generative function of rate was studied. For studying the potential
embryotoxic effect of maneb, mature white rat females with regular
ovulation were used. During the whole pregnancy, these female rats
were administered maneb by gavage every second day (as a suspension in
milk) at a dose level of 50 mg/kg body-weight (0.001 LD50). An
embryotoxic effect of the preparation was ascertained, i.e. abnormal
development of the embryos (dead embryos and resorption), stillborn
young and those incapable of living. In 21 percent of the pregnancies,
the unfavourable effect of maneb on the course of the pregnancy was
apparent when compared with 12 percent in the controls (Marcon, 1969).
Immature female and male rats, of body-weight 80-100 g, were exposed
to maneb for a month, the dose level being 50 mg/kg body-weight. When
mature (after two and one half months from the beginning of the
exposure), the control males were mated with the exposed females and
vice versa. A decline of fertility in both sexes was observed. This
effect, however, was not permanent, the function of the gonads being
restored in three and one half months (Marcon, 1969).
Hamster
Groups of from nine to 14 pregnant female hamsters received an oral
dose of 0, 31, 63, 125 or 500 mg/kg bodyweight of thiram in
dimethylsulfoxide solution on day seven or eight of gestation. No
litters were produced from the group given 500 mg/kg, and the
mortality of the parents was 60 percent. Incidence of deformed young
was significantly greater for the animals given 125 or 250 mg/kg of
thiram compared to the controls given dimethylsulfoxide alone.
Abnormalities included fused ribs, deformed tails and head defects
including all degrees of exencephaly. A similar study using
carboxymethyl cellulose as a solvent at 0, 125, 250, 300 or 500 mg/kg
body-weight of thiram produced a lower incidence of malformation both
in the treated and control animals, although the percentage incidence
of terata was increased in the 250 mg/kg groups and higher compared to
the controls (Robens, 1969).
Special studies on the effects on thyroid function
Rat
Groups, each comprising 10 rats of unspecified sex, received orally 0
or 3 500 mg/kg body-weight of maneb or 2 400 mg/kg of zineb presumably
as a single dose. After a period of 24 hours had elapsed following the
administration of the compounds, the animals were injected
intraperitoneally with 1 mCi of carrier free 131I. The animals that
received zineb accumulated nine times less, and those given maneb 4.5
times less 131I the control group. This goitrogenic effect of these
dithiocarbamates was considered to be related to their metabolites
which are derivatives of thiourea (Ivanova et al., 1967). See also
"Observations in man".
Acute toxicity
LD50 Reference
Compound Animal Route mg/kg
body-weight
Zineb mouse (M) i.p. 2 400 Lessel and
(approx.) Cliffe, 1961
Zineb rat (M) s.c. >5 600 Lessel and
Cliffe, 1961
Zineb guinea-pig (F) oral >4 800 Lessel and
Cliffe, 1961
Short-term studies
Rat
Groups of 10 male and 10 female newly-weaned rats were given 0, 15,
60, 250 or 1000 mg/kg body-weight of zineb by gavage for five days a
week over a four week period. Blood samples were taken during the
final week of feeding. Some of the animals were sacrificed for autopsy
immediately upon termination of the period of administration of zineb;
others were kept for two weeks without receiving zineb prior to
sacrifice. No effects attributable to zineb were detected at levels of
250 mg/kg or lower. At 1000 mg/kg the kidneys were enlarged in the
animals of both sexes, but no histological changes were apparent in
that organ. The thyroids were not enlarged, although histological
examination indicated a slight hyperplasia in the females given 1000
mg/kg. Weight gain in the animals was normal, as was the blood
picture. There were no histological changes in any tissue other than
in the thyroid. One female animal given 1000 mg/kg died of an unknown
cause. Withdrawal of zineb appeared to result in a reversal of the
effect on the kidney and thyroid, as evidenced by examination of the
animals sacrificed two weeks after discontinuing treatment (Lessel and
Cliffe, 1961).
Male rats were maintained for six weeks on diets containing 0, 500 or
5000 ppm of either maneb or zineb. Gross and histopathological changes
in the thyroid gland, reduced assimilation of 124I and slightly
reduced respiratory activity of the liver mitochondria at 5000 ppm
were observed in the test groups. No significant alterations were
observed with respect to the following parameters: mitochondrial
cytochrome oxidase; glucose-6-phosphate dehydrogenase in the
erythrocyte and liver homogenates; contents of cytochromes a3, b and
c; content of flavoproteides in the mitochondria and content of
oxidized and reduced nicotineamidoadenine in liver and kidney
homogenates (Bankowska et al., 1970).
Long-term studies
Rat
Studies are reported to be underway in the Netherlands with rats.
Preliminary results indicated that certain dithiocarbamate fungicides
may affect the reticuloendothelial and haematopoietic systems as
evidenced by the occurrence of slight anaemia, as well as effects on
the thymus, mesenteric lymph nodes and thyroid function. Details are
not yet available (Verschuuren, 1970).
OBSERVATIONS IN MAN
Mutagenic activity of ziram was studied in nine workers (four men and
five women) exposed for three to five years to a concentration of 1.95
- 3.7 mg/m3 air using the test of chromosome aberrations in
peripheral leucocytes. The control group consisted of four humans
(three women and one man). The number of aberrated cells in the group
exposed to ziram was 5.9 percent and in the control group 0.75 percent
(Pilinskaya, 1970).
The damage of thyroid gland function has been observed not only in
animals but in humans, too. Having examined 25 workers coming into
contact with thiram, Ryznkova and Smirnova found a symptomology of
hyperthroidosis in nine workers from this group; surgical treatment
was necessary in three cases (Korablev, 1969).
COMMENTS
Since the last evaluation of the dithiocarbamate fungicides considered
in this monograph, adequate information on the biotransformation in
plants has not been forthcoming. Such information is needed to
determine if the main metabolites are the same in animals as in
plants. The Meeting was informed that a considerable amount of work on
a number of dithiocarbamates is now in progress in The Netherlands.
This work involves short and long term studies and studies on
mechanisms of action and metabolism. Preliminary information from this
work indicates the existence of some effects on the
reticuloendothelial and haematopoietic system, as evidenced by a
slight anaemia as well as changes in the thymus, mesenteric lymph
nodes and thyroid function. Work reported from eastern Europe
indicates that certain dithiocarbamate fungicides affect reproductive
physiology, may have a carcinogenic potential and disturb the thyroid
function.
For these reasons it was considered that the temporary acceptable
daily intakes should remain at the same figures as previously
established (and be applicable to the parent compounds only), pending
consideration of the results of the studies in progress.
TOXICOLOGICAL EVALUATION
Levels causing no toxicological effects
Ferbam
Rat: 250 ppm in the diet, equivalent to 12.5 mg/kg
body-weight/day
Dog: 5 mg/kg body-weight
Maneb
Rat: 250 ppm in the diet, equivalent to 12.5 mg/kg
body-weight/day
Nabam
No long-term studies reported. See FAO/WHO, 1968
Thiram
Rat: 48 ppm in the diet, approximately equivalent to 2.5 mg/kg
body-weight/day
Dog: 5 mg/kg body-weight/day
Zineb
Not found in the long term study previously reported. See
FAO/WHO, 1968
Ziram
Rat: 250 ppm in the diet, equivalent to 12.5 mg/kg
body-weight/day
Dog: 5 mg/kg body-weight/day
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.025 mg/kg body-weight, applicable to the parent compounds only,
and to the sum of all the dithiocarbamate fungicides if more than one
is present.
EVALUATION FOR TOLERANCES
Metabolism
In the study of some ethylene-bis-dithiocarbamates, rapid
decomposition of nabam, maneb and zineb in an aqueous environment,
mainly to ethylene thiourea and ethylene thiram monosulfide, was
confirmed (Vonk and Kaars Sijpesteijn, 1970). But only ethylene
thiourea was taken up readily in the plant, being converted to an
unidentified product to some extent: no ethylene thiram monosulfide
was found in the plant.
METHODS OF RESIDUE ANALYSIS
Currently, the most commonly used method is still the nonspecific one
involving the determination of carbon disulphide evolved on treatment
with acid. Modifications to improve recoveries have been made by
various workers (McLeod and McCully, 1969; Rangaswamy, Poornima and
Majumder, 1970).
FURTHER WORK OR INFORMATION
REQUIRED (by June 1973)
1. Elucidation of the effect on the reticuloendothelial and
haematopoietic systems.
2. Further clarification of the possible carcinogenic effect of
these compounds
3. Elucidation of the effect on reproductive physiology
4. Elucidation of the effect on thyroid function
REQUIRED (before tolerances can be recommended)
Studies on the biotransformation of the compound in plants to
determine the chemical nature of residues, and appropriate
toxicological studies on these residues.
REFERENCES
Bankowska, J., Bojanowska, A., Komorowska-Malewska, W., Krawczynski,
K., Majle, T., Syrowatka, T. and Wiadrowska, B. (1970). The studies on
the influence of zineb and maneb on the functional state of the
thyroid gland and some related enzymatic systems (in Polish).
Roczn.Zak.Hig. (Warsz.), 21 (2): 117-127
Benes., V. and Sram, R. (1969) Mutagenic activity of some pesticides
in Drosophila melanogaster. Industr.Med., 38 (12): 50-52
Chepinoga, O.P., Zastavnyuk, N.P. and Zadorozhnaya, N.A. (1969).
Blastogenic hazard of some derivatives of the dithiocarbamic acid and
approaches to the detection (in Russian). Gigiyena primeneniya,
toksikologiya pesticidov i klinika otravleniy (Kiev), 7: 153-166
Engst, R. and Schnaak, W. (1970) Investigations of the metabolism of
the fungicidal ethylene-bis-dithiocarbamates maneb and zineb. III
The cause of the reaction of degradation. Z. Lebensm.
Untersuch.-Forsch., 143: 99-103
Falk, H.L., Thompson, S.J. and Kotin, P. (1965) Carcinogenic potential
of pesticides. Arch. environm. Hlth., 10: 847-858
FAO/WHO (1968) 1967 Evaluations of some pesticide residues in food
FAO/PL: 1967/M/11/1; WHO/Food Add./68.30
Gebhardt, D.O.E. and van Logten, M.J. (1968). The chick embryo test as
used in the study of the toxicity of certain dithiocarbamates.
Toxicol. appl. pharmacol., 13: 316-324
Innes, J.R.M., Ulland, B.B., Valerio, M.G., Petrucelli, L., Fishbein,
L., Hart, E.R., Pallotta, A.J., Bates, R.R., Falk, H.C., Gart, J.J.,
Klein, M., Mitchell, I. and Peter, J. (1969) Bioassay of pesticides
and industrial chemicals for tumorigenicity in mice: a preliminary
note. J.nat.Cancer Inst., 42: 1101-1114
Ivanova, L., Sheytanov, M. and Mosheva-Ismirova, N. (1967) Changes in
the functional state of the thyroid gland upon acute intoxication with
certain dithiocarbamates - zineb and maneb. C.R.Aced.bulg.Sci., 20
(9): 1011-1013
Izmirova, N., Izmirov, I. and Ivanova, L. (1969) The effect of zineb
and maneb on the isoenzymes of lactic dehydrogenase in the testes of
rats. C.R.Acad. bulg.Sci., 22 (2): 225-227
Kaloyanava, F., Ivanova, L. and Alexiev, B. (1967) The influence of
large doses of maneb on the progeny of albino rats. C.R.Acad.bulg.
Sci., 20 (10): 1109-1112
Khicenko, I.I. and Chernov, O.V. (1968) Experimental study of
potential blastogenic effect of ziram (in Russian). Gigiyena
primeneniya, toksikologya pesticidov i klinika otravleniy (Kiev),6:
770-776
Korablev, M.V. (1969) Toxicological characteristics of dithiocarbamate
acid derivative employed in the national economy and medicine
(Literature survey) (in Russian). Farmakol. i Toksicol., 32 (3):
356-362
Lessel, B. and Cliffe, E.E. (1961) The mammalian toxicity of metiram.
Short term (4-week) oral toxicity. Unpublished report, submitted by
Boots Pure Drug Co. Ltd.
McLeod, H.A. and McCully, K.A. (1969) Head space procedure for
screening food samples for dithiocarbamate residues. J.Assn.Off.
Anal.Chem.,52: 1226
Marcon, L.V. (1969) The effect of maneb on the embryonal development
of generative function of rats (in Russian). Farmakol. i Toksikol.,
32 (6): 731-732
Pilinskaya, M.A. (1970) Chromosome aberrations in the persons
contacted with ziram (in Russian). Genetika,6 (7): 157-163
Rangaswamy, J.R., Poornima, P. and Majumder, S.K. (1970) Rapid
colorimetric method for estimation of thiram residues in grain; also
ferbam and ziram. J.Assn.Off.Anal.Chem., 53: 519-522, 1043-1044
Robens, J.F. (1969) Teratologic studies of carbaryl, diazinon, norea,
disulfiram and thiram in small laboratory animals. Toxicol.appl.
Pharmacol., 15: 152-163
Verschuuren, H.G. (1970) Information provided verbally to the 1970
Joint FAO/WHO Meeting on Pesticide Residues on work being conducted on
dithiocarbamate fungicides in The Netherlands
Vonk, J.W. and Kaars Sijpesteijn, A., (1970) Fate in plants of
ethylene-bis-dithiocarbamate fungicides and their decomposition
products. Ann.appl.Biol., 65: 489-496
