
AGP:1970/M/12/1
WHO/FOOD ADD/71.42
1970 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
Issued jointly by FAO and WHO
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Group on Pesticide Residues, which met in Rome, 9-16 November, 1970.
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
WORLD HEALTH ORGANIZATION
Rome, 1971
2,4,5-T
IDENTITY
Chemical name
2,4,5-trichlorophenoxyacetic acid
Synonyms
2,4,5-T Acid
Weedone 2,4,5-T
Structural formula
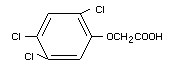 Other relevant chemical properties
The pure acid is a white crystalline solid with a melting point of
158°C and a water solubility of 278 mg/l. It is soluble in acetone,
ethanol and ether (Martin, 1968). The salts with alkali metals and
amines are water soluble but insoluble in petroleum oils; esters are
water insoluble but soluble in oils. The technical acid is stable and
non-corrosive.
Purity
The technical acid has a melting point at approximately 150-151°c. A
typical production lot assayed 95+ percent
2,4,5-trichlorophenoxyacetic acid, 2.9 percent
dichloromethoxyphenoxyacetic acids, 0.6 percent related
trichlorophenoxyacetic acid, 0.5 percent dichlorophenoxyacetic acids,
0.4 percent bis-(2,4,5-trichlorophenoxy)-acetic acid and less than
0.5 ppm 2,3,7,8-tetrachlorodibenzo-p-dioxin (Dow, 1970a). This last
impurity, hereinafter referred to as the "dioxin", is a highly toxic
potent chloracnegen and has been reported to be present in one sample
of commercially produced 2,4,5-T at a level of approximately 27 ppm
(Courtney et al., 1970a; Emerson et al., 1970).
2,4,5-T is formulated for use as a herbicide in water soluble
formulations of various amine salts (e.g. triethylamine) or as
formulations of esters (e.g. iso-octyl) dispersible in oil and/or
water.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
The plasma half-life of an amine salt of 2,4,5-T has been reported to
be approximately three hours in the rat, following the administration
of a single dose of 100 mg/kg body-weight (Erne, 1966a). See also
"Fate of residues, in animals".
Mice were injected with a single dose of 100 mg/kg body-weight of
2,4,5-T in dimethylsulfoxide solution. The animals were sacrificed at
various intervals after injection and analyzed in toto for 2,4,5-T.
The amounts recovered as percentage of the amount injected indicated
decreasing levels at the following time intervals after dosing: at 0
hours, 77.1 percent; at 16 hours, 56.9 percent and at 24 hours, 23.7
percent (Zielinski and Fishbein, 1967).
Effect on enzymes and other biochemical parameters
As is the case with 2,4-D and other auxin herbicides, it appears that
treatment of plants with 2,4,5-T may result in an increased nitrate
formation and produce toxic effects in grazing animals (Way, 1969).
See also the monograph on 2,4-D.
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Mouse
Groups of 18 mice of each sex, from two hybrid strains, were given
2,4,5-T acid from seven days of age for 18 months. The compound was
given by gavage at 0 or 21.5 mg/kg body-weight until weaning, after
which time 2,4,5-T was incorporated into the diet at 0 or 60 ppm.
There was no significant increase in tumour incidence between the
control and treated groups (Innes et al., 1969).
Special studies on teratogenicity
Mouse
Two strains of pregnant mice received one or more of the following
daily dose levels of 2,4,5-T: 0, 21.5, 46.4 or 113 mg/kg body-weight.
The compound was administered subcutaneously in dimethyl sulfoxide
solution or orally in honey on gestation days 6 to 14 or days 9 to 17
or days 6 to 15 inclusive. A significant increase in cleft palate and
cystic kidney was observed in the foetuses from the animals which were
given 46.4 or 113 mg/kg on days 6 to 14 or 9 to 17 of gestation. In
the group given 113 mg/kg orally, there was increased foetal
mortality. The level of "dioxin" impurity in the sample of 2,4,5-T
used was approximately 30 ppm; thus the animals received at 0, 0.6,
1.4 or 3.4 µg/kg of the "dioxin". The authors concluded that the
sample of 2,4,5-T used was teratogenic and foetocidal in the two
strains of mice when administered orally or subcutaneously throughout
the period of organogenesis (Courtney et al., 1970a).
Additional studies have been conducted with three strains of mice
using (a) purified 2,4,5-T estimated to contain less than 0.1 ppm of
the "dioxin", (b) commercially produced 2,4,5-T containing
approximately 0.5 ppm of the "dioxin", (c) 2,4,5-T from a chemical
supply house, (d) the sample of 2,4,5-T used in the previously
described study and (e) pure "dioxin". The compounds were administered
to pregnant mice by subcutaneous injection in dimethylsulfoxide on
gestation days 6 to 15 inclusive (ten doses) unless otherwise noted.
The doses of 2,4,5-T used were 50, 100, 113 or 150 mg/kg body-weight/
day. The purified sample (a) was tested in one strain of mice at a
level of 100 mg/kg/day only, when a significant increase over the
controls of cleft palate and "kidney involvement" (not otherwise
described) was observed. In the same strain of mice, the sample of
commercial 2,4,5-T (b) gave no adverse effects at doses of 50 or 100
mg/kg/day. At 150 mg/kg/day this sample, (b), resulted in a
significant number of cleft palates and increased foetal mortality,
but resulted in no "kidney involvement". In another strain of mice,
the commercial samples of 2,4,5-T (b) and (c) were judged to have
caused an increase in cleft palate at doses of 100 mg and 113
mg/kg/day, respectively. There was no increased "kidney involvement"
or foetal mortality from either sample. In the third strain of mice,
100 mg/kg/day of commercial 2,4,5-T, (b), caused no significant
increase in cleft palate or "kidney involvement", but there was an
increase in foetal mortality. Pure "dioxin" at doses of 1 or 3 ug/kg
body-weight caused "kidney involvement" in all tests, but resulted in
significant increases in cleft palates and foetal mortality only in
some tests, not in others (Courtney at al., 1970b).
Rat
2,4,5-T containing approximately 30 ppm of the "dioxin" impurity was
administered orally to one strain of rats at daily dose levels of 0,
4.6, 10 or 46.4 mg/kg body-weight on gestation days 10 to 15
inclusive. The lowest dose level of 4.6 mg/kg produced a significant
increase in the percentage of abnormal litters or incidence of foetal
mortality, The high dose levels produced a dose related incidence of
increased foetal mortality and abnormal foetuses. Cystic kidney
appeared to be the major manifestation of abnormality (Courtney et
al., 1970a).
Rats were administered various samples of the 2,4,5-T orally as
follows: (a) "pure" at 150 mg/body-weight/day (days 13 to 14 only),
(b) commercial production at 10, 21.5, 46.4 and 80 mg/kg/day, (d) the
sample containing 30 ppm of the "dioxin" at 10 and 21.5 mg/kg/day. No
terata were reported in any test. Increased foetal mortality resulted
from the "pure" (a) sample at the 150 mg/kg/day dose given on days 13
and 14 of gestation, and with the commercial production sample (b) at
a dose of 80 mg/kg/day (LD40 dose for the dams). The sample (d) at
the dose of 10 mg/kg/day gave an increase in "kidney involvement" that
was statistically significant; however, there was no increase noted at
the 21.5 mg/kg/day dose. Pure "dioxin" administered at 0.5 and 2 µg/kg
body-weight resulted in no terata or increase in foetal mortality, but
both doses were judged to have resulted in significant increases in
"kidney involvement" (Courtney at al., 1970b).
Five treatment groups, each consisting of 25 female rats were
administered 1, 3, 6, 12 or 24 mg/kg/body-weight/day of 2,4,5-T
containing <1 ppm of the "dioxin" by gavage on gestation days 6
through 15 inclusive. A single group of 50 females served as controls.
The following studies were made: clinical observations, maternal
body-weights (pre-breeding and day 20), number and position of
foetuses and resorptions, number of corpora lutea, pup weight and
sex, gross external examination of pups and microscopic examination
for intestinal haemorrhage in pups. Representative stained histologic
sections through the head, thorax and abdomen of ten control and ten
foetuses from dams administered the high dose levels were studied for
histopathologic changes. No clinical or gross pathologic signs of
adverse chemical effect were observed in the treated dams. The other
observations did not reveal any teratogenic or embryotoxic effects
(Emerson at al., 1970).
Pure "dioxin" was administered by gavage to rats at dose levels of 0,
0.03, 0.125, 0.5, 2.0 and 8.0 µg/kg body-weight/day to groups of 24
(controls) and 12 (treatment) animals on gestation days 6 to 15
inclusive. The foetuses were taken by caesarean section on day 20 of
gestation.
No differences were observed in the foetuses taken from dams treated
at the dosage of 0.03 µg/kg/day and the controls. At the 0.125
µg/kg/day dosage there was a slight decrease in average weight of
foetuses, three dead foetuses, incidence of intestinal haemorrhage
(18/127) and of subcutaneous oedema (22/80). One foetus at this level
had a rudimentary tail. At the 0.5 ug/kg/day level, the number of
foetuses was reduced and the number of resorptions and foetal deaths
was increased to six. The average weight of the viable foetuses was
slightly decreased. The incidence of intestinal haemorrhage (36/99)
and subcutaneous oedema (31/65) was markedly increased over that seen
in the 0.125 µg/kg/day treatment. At the 2.0 µg/kg/day level, only
seven viable foetuses were obtained. These were from four of the 11
litters examined. Resorptions were numerous, intestinal haemorrhage
was frequent (4/7) and subcutaneous oedema was present in all of the
four foetuses examined. One foetus from this treatment level was found
to have a kinked tail and two of its feet were somewhat misshapen, but
skeletal examination revealed no evidence of bone abnormalities. The
8.0 µg/kg/day dosage level proved to be toxic to the dams. There were
no viable foetuses in the dams examined on day 20 of gestation. All
resorptions occurred early, and no evidence of foetal tissue was
found. Skeletal examinations of foetuses from all dams used in this
experiment revealed delayed ossification of some sternebrae and skull
bones. This manifestation occurred generally throughout the various
groups, including controls (Sparschu et al., 1970).
Rabbit
Groups of 20 pregnant female rabbits each were given oral doses of
2,4,5-T containing <1 ppm of the "dioxin" by capsule from gestation
day 6 to 18 inclusive. The daily doses of 2,4,5-T administered were 0,
10, 20 and 40 mg/kg body-weight. The rabbits ware artificially
inseminated, and caesarean sections were performed on day 29. The
following studies were made: clinical signs, maternal body-weight,
conception rate, gross visceral examination, number of corpora lutea
and implantations, number of kits, resorptions and still-births; kit
weight and sex; gross external examinations; viability following
24-hour incubation; detailed visceral and skeletal examination of kits
from the control and 40 mg/kg groups. There were no dose-related
trends evident from the results of these studies. A high incidence of
neonatal mortality, which was not compound-related, was observed in
the control and treated groups. a 40 mg/kg level dam aborted on day
24; the litter was apparently completely resorbed, and bacterial focal
hepatic necrosis was observed in the dam. This animal had started to
lose weight prior to treatment with 2,4,5-T. It was concluded that
under the conditions of the study, 2,4,5-T was not embryotoxic or
teratogenic in rabbits when administered orally during the period of
organogenesis (Emerson et al., 1970).
Acute toxicity
LD50 mg/kg
Animal Route body-weight References
Mouse oral 389 Rowe and Hymas, 1954
Rat oral 500 Rowe and Hymas, 1954
Guinea pig oral 381 Rowe and Hymas, 1954
Dog oral >100 Drill and Hiratzka, 1953
In a study in pigs, to compare the acute toxicity of 2,4,5-T as
compared to 2,4-D, it is reported that single doses of 100 mg/kg
body-weight caused anorexia, vomiting, diarrhoea and ataxia only in
the two pigs given 2,4,5-T. At autopsy, gastrointestinal irritation
and congestion of the liver and kidney were observed (Björklund and
Erne, 1966).
Short-term studies
Turkey
Groups of male turkeys received 0.25 percent of an ester of 2,4,5-T in
their diet for 11 days. Based upon the amount of food consumed, the
birds received the equivalent of 62 mg/kg body-weight of the free
2,4,5-T acid. There was no appreciable change in the rate of
body-weight gain or in food consumption (Roberts and Rogers, 1957).
Rat
Groups of ten male and ten female rats per group were maintained for
90 days on diets containing 2,4,5-T which had < 1 ppm of the "dioxin"
impurity. The levels in the diet were adjusted so that the animals
received 0, 3, 10, 30 or 100 mg/kg body-weight of 2,4,5-T daily.
Visual observation revealed no changes in appearance or behaviour in
any of the rats, nor were there any deaths. Evidence of
compound-related effects was minimal and was limited to the animals
given 30 and 100 mg/kg. Changes found in both sexes fed 100 mg/kg/day
included depression in body-weight gain, slight decrease in food
intake and elevated serum alkaline phosphatase levels. Gross necropsy
examination revealed an inconsistent slight paleness and accentuated
lobular pattern of the livers of some rats of both sexes at this dose
level with inconsistent minimal amounts of hepatocellular swelling
observed upon histopathologic examination. Male rats at this dose also
had slightly increased serum glutamic-pyruvic transaminase levels and
slight decreases in red cell counts and in haemoglobin. There were
some detectable similar changes at 30 mg/kg/day, but these changes
were considered to be of questionable toxicological significance. At
doses of 3 or 10 mg/kg/day, there were no compound-related changes
observed (Dow, 1970b).
Groups of male and female rats (ten of each sex per group) were
maintained for 90 days on diets containing 0, 100, 300, 1000 and 3000
ppm of a standardized mixture of mono- di-, and tripropylene glycol
butyl ether esters of 2,4,5-T (equivalent to 0, 6.2, 18.6, 62 and 186
mg/kg body-weight of free acid). No evidence of adverse effects was
noted at the 100 or 300 ppm levels, based on gross appearance and
behaviour, mortality, food consumption, haematological indices and
gross and microscopic examination of the tissues. Increase in
spleen-weight in the males fed 100 and 300 ppm and increased
body-weight of the females fed 100 ppm was considered to be unrelated
to the administration of 2,4,5-T ester, and no pathological changes
were observed. At the 1000 ppm level, histopathology revealed slight
cloudy swelling of the parenchymal cells with central lobular necrosis
in the liver in two of ten animals of both sexes examined, as well as
some hypercellularity of the glomerular tuft, with cloudy swelling of
the renal tubular epithelium in the females. There was significant
increase in the average weight of the kidney in male rats at this
level, but there were no differences compared to the control group
with respect to all other above-mentioned parameters. At the 3000 ppm
level, growth retardation was evident and kidney to body-weight ratios
were increased in the males only. The livers of both sexes were large
and light in colour and histopathology revealed a generalized cloudy
swelling of the parenchymal cells and a slight central lobular
necrosis. The kidneys displayed some cloudy swelling of the tubular
epithelium, which was more marked in the females than the males. Some
kidney necrosis was also evident in the females. Serum alkaline
phosphatase was elevated in the males at this level (Dow, 1961).
Dog
2,4,5-T was administered orally in capsules to groups of from two to
four adult mongrel dogs, of mixed sex, for five days a week over a
13-week period. The levels administered were 0, 2, 5, 10 and 20 mg/kg
body-weight/day. All dogs receiving 10 mg/kg or less survived the 90
day test period. The dogs on 20 mg/kg/day died between days 11 and 75.
There was no effect on haemoglobin; red cell, white cell or
differential count of dogs that survived or died during the study, nor
were there any changes in the organ weights among the dogs that
survived the study. Histopathology revealed no significant changes in
the heart, lungs, thyroid, ovaries, testes, adrenals, or liver.
Duodenal hyperaemia in one dog at 10 mg/kg and one at 20 mg/kg was
evident as well as early infiltration of the mucosal cells. An
occasional slight increase in the number of casts in kidney sections
was not dose-related and was considered to be of doubtful
significance. (Drill and Hiratzka, 1953).
Long-term studies
No comprehensive long-term studies appear to have been conducted.
OBSERVATIONS IN MAN
The effect of work exposure to 2,4,5-T on the health of employees
engaged in the manufacture of the herbicide has been studied. A total
of 130 employees having a work experience from two months to over
three years and range of exposure of 2 to 8 mg of 2,4,5-T per day were
studied. The workers were given extensive physical examinations,
including a battery of at least 20 laboratory tests. No differences
were found between the groups of men exposed to 2,4,5-T and a control
group of 4600 men. In addition, karyotyping was carried out on 52
exposed men. There was no indication that 2,4,5-T exposure had
affected the structural integrity or rearranged the genetic material
of the lymphocyte chromosomes (Dow, 1970c).
In workers employed in factories manufacturing chlorinated phenols, a
moderately high incidence of urinary porphyria, chloracne and
hirsutism has been reported. The authors suggest that a highly
chlorinated phenolic ether may be the compound responsible (Bleiberg
et al., 1964).
Sporadic outbreaks of severe acne have been encountered in workers in
chemical plants where 2,4,5-trichlorophenol is manufactured or used.
It is stated that one of the agents responsible is
2,3,7,8-tetrachlorodibenzo-p-dioxin (Anon, 1970).
COMMENT
Considerable information on the potential teratogenic effect of
2,4,5-T of varying degrees of purity is available in mice, rats and
rabbits. The evidence so far presented is inconclusive to determine
whether the teratogenic effects shown in mice and to a lesser extent
in rats are due solely to the presence of the impurity
2,3,7,8-tetrachlorodibenzo-p-dioxin (called the "dioxin").
Information from two independent laboratories appear to indicate some
conflicting results with respect to teratogenicity.
Thus, in one study with mice using a "pure" sample of 2,4,5-T (i.e.
one alleged to contain <0.1 ppm of the "dioxin") foetal abnormalities
were produced at the only level tested, which was 100 mg/kg
body-weight. This level would be equivalent to receiving less than
0.01 µg/kg body-weight of the "dioxin". On the other hand, when 0.03
µg/kg of the "dioxin" alone was administered to pregnant rats, there
were no terata produced, although abnormalities were evident at 0.125
µg/kg and higher levels of the "dioxin". In rabbit reproduction
studies, using 2,4,5-T containing <1 ppm of the "dioxin", no terata
were produced at any dose level, although foetal mortality was evident
at 40 mg/kg body-weight.
The need for further studies with respect to teratogenicity in several
species, including, if possible, non-human primates was stressed. It
was pointed out that there is no scientifically verifiable evidence
that 2,4,5-T has caused teratogenic effects in man.
Attention was drawn to the reported occurrence of chloracne and other
unpleasant toxic effects encountered among some workers involved in
the manufacture of 2,4,5-T and/or related compounds.
The toxic effects reported to be due to increased nitrate formation in
plants treated with 2,4,5-T were not considered to present a hazard to
man under conditions of normal use of the compound.
Because of the reported teratogenic effects, which may or may not be
related to impurities in the samples of 2,4,5-T used, and because
there are no comprehensive long-term feeding studies in any species
(or a two-year feeding study in a non-rodent mammalian species), an
acceptable daily intake for man could not be established. It was
recognized that measurable levels of 2,4,5-T or its breakdown products
could appear in food commodities (see following). In such cases, the
establishment of an acceptable daily intake for 2,4,5-T will be
necessary in assessing the problem of pesticide residues in food. It
was stressed that in order to establish such a figure, a level causing
no toxicological effect would have to be determined. It was further
emphasized that indiscriminate use of 2,4,5-T should be discouraged.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Control of certain weeds in cereal crops and lawns, spot control of
nettles in pasture and used selectively in forestry for control of
woody weeds. Used mainly on non-edible crops.
FATE OF RESIDUES
In animals
Erne (1966 a and b) studied the distribution and elimination of
2,4,5-T in farm animals; amine and alkali salts were readily absorbed
and distributed in the body. The highest tissue levels were found in
liver, kidney, spleen and lungs; the levels found in these organs
exceeded levels found in plasma.
St. John et al., (1964) studied the fate of 2,4,5-T in cattle; it was
eliminated an soluble salts in the urine, and no residue was found in
the milk. Urine and milk samples were analysed from cows fed 2,4,5-T
over a period of four days; over 90 percent was recovered from the
urine excreted over a period of six days.
In plant
After foliar application, 2,4,5-T, being a lipoid-soluble compound,
seem to be limited to the phloem in its movement out of leaves, being
accumulated in the more active metabolic region. Residual 2,4,5-T has
been shown to persist on the leaf surface of apricots or at least one
month following foliar application of 14C labelled 2,4,5-T (Maxie et
al., 1956). No evidence was obtained for metabolism of 2,4,5-T in
either leaves or fruits of apricots.
Evidence of residues in food in commerce or at consumption
Some unpublished data were presented (Boehringer Sohn, 1970) regarding
residues of 2,4,5-T in cereals (grain and straw) found to occur
following applications of the herbicide, either alone or in admixture
with other phenoseyacid herbicides (recoprop, MCPA, 2,4-DP). A summary
of the data relating to 2,4,5-T is given in Table I.
TABLE I
Residues of 2,4,5-T in cereals
Interval
Rate of (days) Residues found, ppm
Crop Application Applic.
g/ha Harvest Grain Straw
Barley 120 64 0.04 0.80
77 n.d.1 0.13
89 - 0.06
100 - n.d.
380 64 n.d. 0.30
77 n.d. 0.12
89 - 0.04
100 - n.d.
520 64 0.04 0.40
77 n.d. 0.10
89 - 0.04
100 - n.d.
Wheat 120 76 0.03 0.17
89 0.025 0.09
380 76 0.03 0.15
89 0.02 0.11
520 76 0.03 0.21
89 0.02 0.11
Oats 120 76 n.d. 0.20
89 n.d. n.d.
380 76 n.d. n.d.
89 n.d. n.d.
520 76 n.d. 0.23
89 n.d. n.d.
Rye 120 76 0.02 0.15
89 n.d. 0.10
380 76 0.02 0.15
89 n.d. 0.08
520 76 0.02 1.0
89 n.d. 0.22
1 n.d. = not detected
METHODS OF RESIDUE ANALYSIS
Residues of 2,4,5-T may be determined by suitable combinations of
extraction, separation and end determination by ultraviolet
spectrophotemetry, gas chromatography, paper and thin-layer
chromatography and radiometric methods. Bradley and Thompson (1964)
used GLC of the methyl esters after methylation with diazomethane.
Gordon and Beroza (1952), after extraction and separation by partition
chromatography, determined 2,4,5-T by spectrophotometry at 289 nm;
this method may be used for alfalfa hay extracts. By use of paper and
thin-layer chromatography for detection, separation and
identification, Abbott et al. (1964) determined 2,4,5-T in soil and
water. Hindin et al. (1964) analysed surface and ground water by paper
chromatography and GLC. Edgerton and Lisk (1963) determined 2,4,5-T in
applies by radio-isotopic and GLC methods. Clark (1969) determined
2,4,5-T and its propylene glycol butyl ether esters in animal tissue,
blood and urine. He converted to methyl esters and analysed by
microcoulometric GLC; recoveries at 0.05 - 20 ppm levels of 2,4,5-T
were 89.3 - 93.6% and of the ester 70.5 - 92.5%. Gas chromatography of
the methyl ester of 2,4,5-T should be suitable as a procedure for
regulatory purposes.
NATIONAL TOLERANCES
Country Crop Tolerance
(ppm)
Netherlands Vegetables (except 0.1
potatoes), fruits of
vegetables, fruit crops.
Fed. Repub. Germany Leafy, other sprouting 0.01
vegetables, fruiting
vegetables, root
vegetables
APPRAISAL
2,4,5-T in a selective herbicide, mostly used for the control of woody
weeds in non-edible crops, forestry, etc. There is a limited need in
parts of Europe for its use in cereals, in admixtures with other
phenoxyacid herbicides, for the control of certain weed species.
Evidence regarding the residues that can accrue in grain and straw
following such uses was provided. No evidence regarding the need to
establish practical residue limits was apparent. Gas chromatographic
methods are available which should be adaptable for regulatory
purposes where required.
When used an recommended for the limited use on some cereals, the
following residues of 2,4,5-T can occur:
Wheat, barley, oats, rye grain 0.05 ppm
Wheat, barley, oats, rye straw 1 ppm
FURTHER WORK OR INFORMATION
REQUIRED (before an acceptable daily intake for man can be
established)
1. An adequate long-term oral study in order to establish a no-effect
level using (i) a commercially available material, (ii) the purest
available 2,4,5-T and (iii) 2,3,7,8-tetrachlorodibenzo-p-dioxin.
2. Studies on reproduction and teratogenicity with 2,4,5-T using (i) a
commercially available material, (ii) the purest available 2,4,5-T
and (iii) 2,3,7,8-tetrachlorodibenzo-p-dioxin.
DESIRABLE
Information on the availability of acceptable methods for the
detection and determination of chlorinated dibenzodioxin impurities in
technical 2,4,5-T and its formulations at the 0.01 - 0.05 ppm level.
Current attempts to standardize the specifications of 2,4,5-T products
in regard to their content of chlorinated dibenzodioxin compounds
should be continued and encouraged.
REFERENCES
Abbott, D.C., Egan, H., Hammond, E.W. and Thomson, J. (1964) The
chromatographic detection and determination of organo-chlorine
herbicides in soil and water. Analyst, 89: 480-488
Anon. (1970) Another herbicide on the blacklist. Nature, 226:
309-311
Bjorklund, N-E and Erne, K. (1966) Toxicological studies of
phenoxyacetic herbicides in animals. Acta Vet. Scand., 7: 364-390
Bleiberg, J., Wallen, M., Brodkin, R, and Applebaum, I.L, (1964)
Industrially acquired porphyria. Arch, Dermatol., 89: 793-797
Boehringer Sohn. (1970) Residues of 2,4,5-T in cereals and straw.
Unpublished report
Bradley, J.K. and Thompson, W.K. Determination of 2,4-D and 2,4,5-T in
lawn fertilisers. J. Sci.Fd.Agr., 15: 673-677
Clark, D.E. (1969) Determination of 2,4,5-trichlorophenoxyacetic acid
and its propylene glycol butyl ether esters in animal tissue, blood
and urine. J.Agr.Fd. Chem., 17: 1168-1170
Courtney, K.D., Gaylor, D.W., Hogan, M.D., Falk, H.L., Baton, R.R. and
Mitchell, I. (1970a) Teratogenic evaluation of 2,4,5-T. Science,
168: 864-866
Courtney, K.D., Moore, J.A., Gaylor, D.W., Hogen, M.D. and Falk, H.L.
(1970b) Summary teratogen study. Typescript draft of record of 15
April 1970 hearing on 2,4,5-T before the Sub-committee on Energy,
National Resources and the Environment of the U.S. Senate Committee on
Commerce, 225-232 (does not appear in final printed report)
Dow (1961) Results of 90-day dietary feeding studies on Dowanol 97B
(mono-, di- and tripropylene glycol butyl ether) esters of 2,4,5-T in
rats. Unpublished data, The Dow Chemical Company
Dow (1970a) Assay of a commercial production lot of 2,4,5-T.
Unpublished data, The Dow Chemical Company
Dow (1970b) A 90-day dietary feeding study on 2,4,5-T
trichlorophenoxyacetic acid in rats. Unpublished data, The Dow
Chemical Company
Dow (1970c) A study of the effects of work exposure to 2,4,5-T on
employee health. Unpublished data, The Dow Chemical Company
Drill, V.A. and Hiratzka, T. (1953) Toxicity of
2,4-dichlorophenoxyacetic acid and 2,4,5-trichlorophenoxyacetic acid,
a report on their acute and chronic toxicity in dogs. Arch. Ind. Hyg.
Occup. Med., 7: 61-67
Edgerton, L.J. and Lisk, D.L. (1963) Determination of residues of
2,4,5-trichlorophenoxyacetic acid in apples by radioisotope and gas
chromatographic methods. Proc. Amer. Soc. Hort. Sci., 83: 120-125
Emerson, J.L., Thompson, D.J., Strebing, R.J., Gerbig, C.G. and
Robinson, V.B. (1970) Teratogenic studies on
2,4,5-trichlorophenoxyacetic acid in the rat and rabbit. Paper on rat
data presented at Society of Toxicology, Atlanta, Georgia, March 17.
Submitted to Fd. Cosmet. Toxicol. for publication
Erne, K. (1966a) Distribution and elimination of chlorinated
phenoxyacetic acids in animals. Acta. Vet. Scand., 7: 240-256
Erne, K. (1966b) Animal metabolism of phenoxyacetic herbicides. Acta
Vet. Scand., 7: 264-271
Gordon, N. and Beroza, M. (1952) Spectrophotometric determination of
small quantities of 2,4-dichlorophenoxyacetic acid and
2,4,5-trichlorophenoxyacetic acid. Anal. Chem., 24: 1968-1971
Hindin, E., May, D.S. and Dunstan, G.E. (1964) Collection and analysis
of synthetic organic pesticides from surface and ground water.
Res.Rev. 7: 130-156
Innes, J.R.M., Ulland, B.M., Valerio, M.G., Petrucelli, L., Fishbein,
L., Hart, E.R., Pallotta, A.J., Bates, R.R., Falk, H.C., Gart, J.J.,
Klein, M., Mitchell, I. and Peter, J. (1969) Bioassay of pesticides
and industrial chemicals for tumorigenicity in mice: a preliminary
note. J. nat. Cancer Inst., 42: 1101-1114
Maxie, E.C. and Crane, J.C. (1956) Some metabolic effects of
2,4,5-trichlorophenoxyacetic acid on Tilton apricot fruits. Proc.
Amer. Soc. Hort. Sci., 68: 113-123
Martin, H. (1968) Pesticide manual, British Crop Protection Council,
page 401
Roberts, R.E. and Rogers, B.J. (1957) The effect of 2,4,5-T brush
spray on turkeys. Poultry Science, 36: 703-705
Rowe, V.K. and Hymas, T.A. (1954) Summary of toxicological information
on 2,4-D and 2,4,5-T type herbicides and an evaluation of the hazards
to livestock associated with their use. Am. J. Vet. Res., 15:
622-629
Sparschu, G.L., Dunn, F.L. and Rowe, V.K. (1970) Teratogenic study of
2,3,7,8-tetrachlorodibenzo-p-dioxin in the rat. Paper presented at
Society of Toxicology Atlanta, Georgia, March 17. Mss. submitted to
Fd. Cosmet. Toxicol. for publication
St. John, L.E., Wagner. D.G. and Lisk, D.J. (1964) Fate of atrasine,
Kuron, Silvex and 2,4,5-T in the dairy cow. J.Dairy Sci., 47:
1267-1270
Way, J.M. (1969) Toxicity and hazards to man, domestic animals and
wildlife from some commonly used auxin herbicides. Res. Rev.26:
37-62
Zielinski, W.L. Jr. and Fishbein, L. (1967) Gas chromatographic
measurement of disappearance rates of 2,4,-D and 2,4,5-T acids and
2,4-D esters in mice. J. Agr. Fd. Chem. 15: 841-844
Other relevant chemical properties
The pure acid is a white crystalline solid with a melting point of
158°C and a water solubility of 278 mg/l. It is soluble in acetone,
ethanol and ether (Martin, 1968). The salts with alkali metals and
amines are water soluble but insoluble in petroleum oils; esters are
water insoluble but soluble in oils. The technical acid is stable and
non-corrosive.
Purity
The technical acid has a melting point at approximately 150-151°c. A
typical production lot assayed 95+ percent
2,4,5-trichlorophenoxyacetic acid, 2.9 percent
dichloromethoxyphenoxyacetic acids, 0.6 percent related
trichlorophenoxyacetic acid, 0.5 percent dichlorophenoxyacetic acids,
0.4 percent bis-(2,4,5-trichlorophenoxy)-acetic acid and less than
0.5 ppm 2,3,7,8-tetrachlorodibenzo-p-dioxin (Dow, 1970a). This last
impurity, hereinafter referred to as the "dioxin", is a highly toxic
potent chloracnegen and has been reported to be present in one sample
of commercially produced 2,4,5-T at a level of approximately 27 ppm
(Courtney et al., 1970a; Emerson et al., 1970).
2,4,5-T is formulated for use as a herbicide in water soluble
formulations of various amine salts (e.g. triethylamine) or as
formulations of esters (e.g. iso-octyl) dispersible in oil and/or
water.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
The plasma half-life of an amine salt of 2,4,5-T has been reported to
be approximately three hours in the rat, following the administration
of a single dose of 100 mg/kg body-weight (Erne, 1966a). See also
"Fate of residues, in animals".
Mice were injected with a single dose of 100 mg/kg body-weight of
2,4,5-T in dimethylsulfoxide solution. The animals were sacrificed at
various intervals after injection and analyzed in toto for 2,4,5-T.
The amounts recovered as percentage of the amount injected indicated
decreasing levels at the following time intervals after dosing: at 0
hours, 77.1 percent; at 16 hours, 56.9 percent and at 24 hours, 23.7
percent (Zielinski and Fishbein, 1967).
Effect on enzymes and other biochemical parameters
As is the case with 2,4-D and other auxin herbicides, it appears that
treatment of plants with 2,4,5-T may result in an increased nitrate
formation and produce toxic effects in grazing animals (Way, 1969).
See also the monograph on 2,4-D.
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Mouse
Groups of 18 mice of each sex, from two hybrid strains, were given
2,4,5-T acid from seven days of age for 18 months. The compound was
given by gavage at 0 or 21.5 mg/kg body-weight until weaning, after
which time 2,4,5-T was incorporated into the diet at 0 or 60 ppm.
There was no significant increase in tumour incidence between the
control and treated groups (Innes et al., 1969).
Special studies on teratogenicity
Mouse
Two strains of pregnant mice received one or more of the following
daily dose levels of 2,4,5-T: 0, 21.5, 46.4 or 113 mg/kg body-weight.
The compound was administered subcutaneously in dimethyl sulfoxide
solution or orally in honey on gestation days 6 to 14 or days 9 to 17
or days 6 to 15 inclusive. A significant increase in cleft palate and
cystic kidney was observed in the foetuses from the animals which were
given 46.4 or 113 mg/kg on days 6 to 14 or 9 to 17 of gestation. In
the group given 113 mg/kg orally, there was increased foetal
mortality. The level of "dioxin" impurity in the sample of 2,4,5-T
used was approximately 30 ppm; thus the animals received at 0, 0.6,
1.4 or 3.4 µg/kg of the "dioxin". The authors concluded that the
sample of 2,4,5-T used was teratogenic and foetocidal in the two
strains of mice when administered orally or subcutaneously throughout
the period of organogenesis (Courtney et al., 1970a).
Additional studies have been conducted with three strains of mice
using (a) purified 2,4,5-T estimated to contain less than 0.1 ppm of
the "dioxin", (b) commercially produced 2,4,5-T containing
approximately 0.5 ppm of the "dioxin", (c) 2,4,5-T from a chemical
supply house, (d) the sample of 2,4,5-T used in the previously
described study and (e) pure "dioxin". The compounds were administered
to pregnant mice by subcutaneous injection in dimethylsulfoxide on
gestation days 6 to 15 inclusive (ten doses) unless otherwise noted.
The doses of 2,4,5-T used were 50, 100, 113 or 150 mg/kg body-weight/
day. The purified sample (a) was tested in one strain of mice at a
level of 100 mg/kg/day only, when a significant increase over the
controls of cleft palate and "kidney involvement" (not otherwise
described) was observed. In the same strain of mice, the sample of
commercial 2,4,5-T (b) gave no adverse effects at doses of 50 or 100
mg/kg/day. At 150 mg/kg/day this sample, (b), resulted in a
significant number of cleft palates and increased foetal mortality,
but resulted in no "kidney involvement". In another strain of mice,
the commercial samples of 2,4,5-T (b) and (c) were judged to have
caused an increase in cleft palate at doses of 100 mg and 113
mg/kg/day, respectively. There was no increased "kidney involvement"
or foetal mortality from either sample. In the third strain of mice,
100 mg/kg/day of commercial 2,4,5-T, (b), caused no significant
increase in cleft palate or "kidney involvement", but there was an
increase in foetal mortality. Pure "dioxin" at doses of 1 or 3 ug/kg
body-weight caused "kidney involvement" in all tests, but resulted in
significant increases in cleft palates and foetal mortality only in
some tests, not in others (Courtney at al., 1970b).
Rat
2,4,5-T containing approximately 30 ppm of the "dioxin" impurity was
administered orally to one strain of rats at daily dose levels of 0,
4.6, 10 or 46.4 mg/kg body-weight on gestation days 10 to 15
inclusive. The lowest dose level of 4.6 mg/kg produced a significant
increase in the percentage of abnormal litters or incidence of foetal
mortality, The high dose levels produced a dose related incidence of
increased foetal mortality and abnormal foetuses. Cystic kidney
appeared to be the major manifestation of abnormality (Courtney et
al., 1970a).
Rats were administered various samples of the 2,4,5-T orally as
follows: (a) "pure" at 150 mg/body-weight/day (days 13 to 14 only),
(b) commercial production at 10, 21.5, 46.4 and 80 mg/kg/day, (d) the
sample containing 30 ppm of the "dioxin" at 10 and 21.5 mg/kg/day. No
terata were reported in any test. Increased foetal mortality resulted
from the "pure" (a) sample at the 150 mg/kg/day dose given on days 13
and 14 of gestation, and with the commercial production sample (b) at
a dose of 80 mg/kg/day (LD40 dose for the dams). The sample (d) at
the dose of 10 mg/kg/day gave an increase in "kidney involvement" that
was statistically significant; however, there was no increase noted at
the 21.5 mg/kg/day dose. Pure "dioxin" administered at 0.5 and 2 µg/kg
body-weight resulted in no terata or increase in foetal mortality, but
both doses were judged to have resulted in significant increases in
"kidney involvement" (Courtney at al., 1970b).
Five treatment groups, each consisting of 25 female rats were
administered 1, 3, 6, 12 or 24 mg/kg/body-weight/day of 2,4,5-T
containing <1 ppm of the "dioxin" by gavage on gestation days 6
through 15 inclusive. A single group of 50 females served as controls.
The following studies were made: clinical observations, maternal
body-weights (pre-breeding and day 20), number and position of
foetuses and resorptions, number of corpora lutea, pup weight and
sex, gross external examination of pups and microscopic examination
for intestinal haemorrhage in pups. Representative stained histologic
sections through the head, thorax and abdomen of ten control and ten
foetuses from dams administered the high dose levels were studied for
histopathologic changes. No clinical or gross pathologic signs of
adverse chemical effect were observed in the treated dams. The other
observations did not reveal any teratogenic or embryotoxic effects
(Emerson at al., 1970).
Pure "dioxin" was administered by gavage to rats at dose levels of 0,
0.03, 0.125, 0.5, 2.0 and 8.0 µg/kg body-weight/day to groups of 24
(controls) and 12 (treatment) animals on gestation days 6 to 15
inclusive. The foetuses were taken by caesarean section on day 20 of
gestation.
No differences were observed in the foetuses taken from dams treated
at the dosage of 0.03 µg/kg/day and the controls. At the 0.125
µg/kg/day dosage there was a slight decrease in average weight of
foetuses, three dead foetuses, incidence of intestinal haemorrhage
(18/127) and of subcutaneous oedema (22/80). One foetus at this level
had a rudimentary tail. At the 0.5 ug/kg/day level, the number of
foetuses was reduced and the number of resorptions and foetal deaths
was increased to six. The average weight of the viable foetuses was
slightly decreased. The incidence of intestinal haemorrhage (36/99)
and subcutaneous oedema (31/65) was markedly increased over that seen
in the 0.125 µg/kg/day treatment. At the 2.0 µg/kg/day level, only
seven viable foetuses were obtained. These were from four of the 11
litters examined. Resorptions were numerous, intestinal haemorrhage
was frequent (4/7) and subcutaneous oedema was present in all of the
four foetuses examined. One foetus from this treatment level was found
to have a kinked tail and two of its feet were somewhat misshapen, but
skeletal examination revealed no evidence of bone abnormalities. The
8.0 µg/kg/day dosage level proved to be toxic to the dams. There were
no viable foetuses in the dams examined on day 20 of gestation. All
resorptions occurred early, and no evidence of foetal tissue was
found. Skeletal examinations of foetuses from all dams used in this
experiment revealed delayed ossification of some sternebrae and skull
bones. This manifestation occurred generally throughout the various
groups, including controls (Sparschu et al., 1970).
Rabbit
Groups of 20 pregnant female rabbits each were given oral doses of
2,4,5-T containing <1 ppm of the "dioxin" by capsule from gestation
day 6 to 18 inclusive. The daily doses of 2,4,5-T administered were 0,
10, 20 and 40 mg/kg body-weight. The rabbits ware artificially
inseminated, and caesarean sections were performed on day 29. The
following studies were made: clinical signs, maternal body-weight,
conception rate, gross visceral examination, number of corpora lutea
and implantations, number of kits, resorptions and still-births; kit
weight and sex; gross external examinations; viability following
24-hour incubation; detailed visceral and skeletal examination of kits
from the control and 40 mg/kg groups. There were no dose-related
trends evident from the results of these studies. A high incidence of
neonatal mortality, which was not compound-related, was observed in
the control and treated groups. a 40 mg/kg level dam aborted on day
24; the litter was apparently completely resorbed, and bacterial focal
hepatic necrosis was observed in the dam. This animal had started to
lose weight prior to treatment with 2,4,5-T. It was concluded that
under the conditions of the study, 2,4,5-T was not embryotoxic or
teratogenic in rabbits when administered orally during the period of
organogenesis (Emerson et al., 1970).
Acute toxicity
LD50 mg/kg
Animal Route body-weight References
Mouse oral 389 Rowe and Hymas, 1954
Rat oral 500 Rowe and Hymas, 1954
Guinea pig oral 381 Rowe and Hymas, 1954
Dog oral >100 Drill and Hiratzka, 1953
In a study in pigs, to compare the acute toxicity of 2,4,5-T as
compared to 2,4-D, it is reported that single doses of 100 mg/kg
body-weight caused anorexia, vomiting, diarrhoea and ataxia only in
the two pigs given 2,4,5-T. At autopsy, gastrointestinal irritation
and congestion of the liver and kidney were observed (Björklund and
Erne, 1966).
Short-term studies
Turkey
Groups of male turkeys received 0.25 percent of an ester of 2,4,5-T in
their diet for 11 days. Based upon the amount of food consumed, the
birds received the equivalent of 62 mg/kg body-weight of the free
2,4,5-T acid. There was no appreciable change in the rate of
body-weight gain or in food consumption (Roberts and Rogers, 1957).
Rat
Groups of ten male and ten female rats per group were maintained for
90 days on diets containing 2,4,5-T which had < 1 ppm of the "dioxin"
impurity. The levels in the diet were adjusted so that the animals
received 0, 3, 10, 30 or 100 mg/kg body-weight of 2,4,5-T daily.
Visual observation revealed no changes in appearance or behaviour in
any of the rats, nor were there any deaths. Evidence of
compound-related effects was minimal and was limited to the animals
given 30 and 100 mg/kg. Changes found in both sexes fed 100 mg/kg/day
included depression in body-weight gain, slight decrease in food
intake and elevated serum alkaline phosphatase levels. Gross necropsy
examination revealed an inconsistent slight paleness and accentuated
lobular pattern of the livers of some rats of both sexes at this dose
level with inconsistent minimal amounts of hepatocellular swelling
observed upon histopathologic examination. Male rats at this dose also
had slightly increased serum glutamic-pyruvic transaminase levels and
slight decreases in red cell counts and in haemoglobin. There were
some detectable similar changes at 30 mg/kg/day, but these changes
were considered to be of questionable toxicological significance. At
doses of 3 or 10 mg/kg/day, there were no compound-related changes
observed (Dow, 1970b).
Groups of male and female rats (ten of each sex per group) were
maintained for 90 days on diets containing 0, 100, 300, 1000 and 3000
ppm of a standardized mixture of mono- di-, and tripropylene glycol
butyl ether esters of 2,4,5-T (equivalent to 0, 6.2, 18.6, 62 and 186
mg/kg body-weight of free acid). No evidence of adverse effects was
noted at the 100 or 300 ppm levels, based on gross appearance and
behaviour, mortality, food consumption, haematological indices and
gross and microscopic examination of the tissues. Increase in
spleen-weight in the males fed 100 and 300 ppm and increased
body-weight of the females fed 100 ppm was considered to be unrelated
to the administration of 2,4,5-T ester, and no pathological changes
were observed. At the 1000 ppm level, histopathology revealed slight
cloudy swelling of the parenchymal cells with central lobular necrosis
in the liver in two of ten animals of both sexes examined, as well as
some hypercellularity of the glomerular tuft, with cloudy swelling of
the renal tubular epithelium in the females. There was significant
increase in the average weight of the kidney in male rats at this
level, but there were no differences compared to the control group
with respect to all other above-mentioned parameters. At the 3000 ppm
level, growth retardation was evident and kidney to body-weight ratios
were increased in the males only. The livers of both sexes were large
and light in colour and histopathology revealed a generalized cloudy
swelling of the parenchymal cells and a slight central lobular
necrosis. The kidneys displayed some cloudy swelling of the tubular
epithelium, which was more marked in the females than the males. Some
kidney necrosis was also evident in the females. Serum alkaline
phosphatase was elevated in the males at this level (Dow, 1961).
Dog
2,4,5-T was administered orally in capsules to groups of from two to
four adult mongrel dogs, of mixed sex, for five days a week over a
13-week period. The levels administered were 0, 2, 5, 10 and 20 mg/kg
body-weight/day. All dogs receiving 10 mg/kg or less survived the 90
day test period. The dogs on 20 mg/kg/day died between days 11 and 75.
There was no effect on haemoglobin; red cell, white cell or
differential count of dogs that survived or died during the study, nor
were there any changes in the organ weights among the dogs that
survived the study. Histopathology revealed no significant changes in
the heart, lungs, thyroid, ovaries, testes, adrenals, or liver.
Duodenal hyperaemia in one dog at 10 mg/kg and one at 20 mg/kg was
evident as well as early infiltration of the mucosal cells. An
occasional slight increase in the number of casts in kidney sections
was not dose-related and was considered to be of doubtful
significance. (Drill and Hiratzka, 1953).
Long-term studies
No comprehensive long-term studies appear to have been conducted.
OBSERVATIONS IN MAN
The effect of work exposure to 2,4,5-T on the health of employees
engaged in the manufacture of the herbicide has been studied. A total
of 130 employees having a work experience from two months to over
three years and range of exposure of 2 to 8 mg of 2,4,5-T per day were
studied. The workers were given extensive physical examinations,
including a battery of at least 20 laboratory tests. No differences
were found between the groups of men exposed to 2,4,5-T and a control
group of 4600 men. In addition, karyotyping was carried out on 52
exposed men. There was no indication that 2,4,5-T exposure had
affected the structural integrity or rearranged the genetic material
of the lymphocyte chromosomes (Dow, 1970c).
In workers employed in factories manufacturing chlorinated phenols, a
moderately high incidence of urinary porphyria, chloracne and
hirsutism has been reported. The authors suggest that a highly
chlorinated phenolic ether may be the compound responsible (Bleiberg
et al., 1964).
Sporadic outbreaks of severe acne have been encountered in workers in
chemical plants where 2,4,5-trichlorophenol is manufactured or used.
It is stated that one of the agents responsible is
2,3,7,8-tetrachlorodibenzo-p-dioxin (Anon, 1970).
COMMENT
Considerable information on the potential teratogenic effect of
2,4,5-T of varying degrees of purity is available in mice, rats and
rabbits. The evidence so far presented is inconclusive to determine
whether the teratogenic effects shown in mice and to a lesser extent
in rats are due solely to the presence of the impurity
2,3,7,8-tetrachlorodibenzo-p-dioxin (called the "dioxin").
Information from two independent laboratories appear to indicate some
conflicting results with respect to teratogenicity.
Thus, in one study with mice using a "pure" sample of 2,4,5-T (i.e.
one alleged to contain <0.1 ppm of the "dioxin") foetal abnormalities
were produced at the only level tested, which was 100 mg/kg
body-weight. This level would be equivalent to receiving less than
0.01 µg/kg body-weight of the "dioxin". On the other hand, when 0.03
µg/kg of the "dioxin" alone was administered to pregnant rats, there
were no terata produced, although abnormalities were evident at 0.125
µg/kg and higher levels of the "dioxin". In rabbit reproduction
studies, using 2,4,5-T containing <1 ppm of the "dioxin", no terata
were produced at any dose level, although foetal mortality was evident
at 40 mg/kg body-weight.
The need for further studies with respect to teratogenicity in several
species, including, if possible, non-human primates was stressed. It
was pointed out that there is no scientifically verifiable evidence
that 2,4,5-T has caused teratogenic effects in man.
Attention was drawn to the reported occurrence of chloracne and other
unpleasant toxic effects encountered among some workers involved in
the manufacture of 2,4,5-T and/or related compounds.
The toxic effects reported to be due to increased nitrate formation in
plants treated with 2,4,5-T were not considered to present a hazard to
man under conditions of normal use of the compound.
Because of the reported teratogenic effects, which may or may not be
related to impurities in the samples of 2,4,5-T used, and because
there are no comprehensive long-term feeding studies in any species
(or a two-year feeding study in a non-rodent mammalian species), an
acceptable daily intake for man could not be established. It was
recognized that measurable levels of 2,4,5-T or its breakdown products
could appear in food commodities (see following). In such cases, the
establishment of an acceptable daily intake for 2,4,5-T will be
necessary in assessing the problem of pesticide residues in food. It
was stressed that in order to establish such a figure, a level causing
no toxicological effect would have to be determined. It was further
emphasized that indiscriminate use of 2,4,5-T should be discouraged.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Control of certain weeds in cereal crops and lawns, spot control of
nettles in pasture and used selectively in forestry for control of
woody weeds. Used mainly on non-edible crops.
FATE OF RESIDUES
In animals
Erne (1966 a and b) studied the distribution and elimination of
2,4,5-T in farm animals; amine and alkali salts were readily absorbed
and distributed in the body. The highest tissue levels were found in
liver, kidney, spleen and lungs; the levels found in these organs
exceeded levels found in plasma.
St. John et al., (1964) studied the fate of 2,4,5-T in cattle; it was
eliminated an soluble salts in the urine, and no residue was found in
the milk. Urine and milk samples were analysed from cows fed 2,4,5-T
over a period of four days; over 90 percent was recovered from the
urine excreted over a period of six days.
In plant
After foliar application, 2,4,5-T, being a lipoid-soluble compound,
seem to be limited to the phloem in its movement out of leaves, being
accumulated in the more active metabolic region. Residual 2,4,5-T has
been shown to persist on the leaf surface of apricots or at least one
month following foliar application of 14C labelled 2,4,5-T (Maxie et
al., 1956). No evidence was obtained for metabolism of 2,4,5-T in
either leaves or fruits of apricots.
Evidence of residues in food in commerce or at consumption
Some unpublished data were presented (Boehringer Sohn, 1970) regarding
residues of 2,4,5-T in cereals (grain and straw) found to occur
following applications of the herbicide, either alone or in admixture
with other phenoseyacid herbicides (recoprop, MCPA, 2,4-DP). A summary
of the data relating to 2,4,5-T is given in Table I.
TABLE I
Residues of 2,4,5-T in cereals
Interval
Rate of (days) Residues found, ppm
Crop Application Applic.
g/ha Harvest Grain Straw
Barley 120 64 0.04 0.80
77 n.d.1 0.13
89 - 0.06
100 - n.d.
380 64 n.d. 0.30
77 n.d. 0.12
89 - 0.04
100 - n.d.
520 64 0.04 0.40
77 n.d. 0.10
89 - 0.04
100 - n.d.
Wheat 120 76 0.03 0.17
89 0.025 0.09
380 76 0.03 0.15
89 0.02 0.11
520 76 0.03 0.21
89 0.02 0.11
Oats 120 76 n.d. 0.20
89 n.d. n.d.
380 76 n.d. n.d.
89 n.d. n.d.
520 76 n.d. 0.23
89 n.d. n.d.
Rye 120 76 0.02 0.15
89 n.d. 0.10
380 76 0.02 0.15
89 n.d. 0.08
520 76 0.02 1.0
89 n.d. 0.22
1 n.d. = not detected
METHODS OF RESIDUE ANALYSIS
Residues of 2,4,5-T may be determined by suitable combinations of
extraction, separation and end determination by ultraviolet
spectrophotemetry, gas chromatography, paper and thin-layer
chromatography and radiometric methods. Bradley and Thompson (1964)
used GLC of the methyl esters after methylation with diazomethane.
Gordon and Beroza (1952), after extraction and separation by partition
chromatography, determined 2,4,5-T by spectrophotometry at 289 nm;
this method may be used for alfalfa hay extracts. By use of paper and
thin-layer chromatography for detection, separation and
identification, Abbott et al. (1964) determined 2,4,5-T in soil and
water. Hindin et al. (1964) analysed surface and ground water by paper
chromatography and GLC. Edgerton and Lisk (1963) determined 2,4,5-T in
applies by radio-isotopic and GLC methods. Clark (1969) determined
2,4,5-T and its propylene glycol butyl ether esters in animal tissue,
blood and urine. He converted to methyl esters and analysed by
microcoulometric GLC; recoveries at 0.05 - 20 ppm levels of 2,4,5-T
were 89.3 - 93.6% and of the ester 70.5 - 92.5%. Gas chromatography of
the methyl ester of 2,4,5-T should be suitable as a procedure for
regulatory purposes.
NATIONAL TOLERANCES
Country Crop Tolerance
(ppm)
Netherlands Vegetables (except 0.1
potatoes), fruits of
vegetables, fruit crops.
Fed. Repub. Germany Leafy, other sprouting 0.01
vegetables, fruiting
vegetables, root
vegetables
APPRAISAL
2,4,5-T in a selective herbicide, mostly used for the control of woody
weeds in non-edible crops, forestry, etc. There is a limited need in
parts of Europe for its use in cereals, in admixtures with other
phenoxyacid herbicides, for the control of certain weed species.
Evidence regarding the residues that can accrue in grain and straw
following such uses was provided. No evidence regarding the need to
establish practical residue limits was apparent. Gas chromatographic
methods are available which should be adaptable for regulatory
purposes where required.
When used an recommended for the limited use on some cereals, the
following residues of 2,4,5-T can occur:
Wheat, barley, oats, rye grain 0.05 ppm
Wheat, barley, oats, rye straw 1 ppm
FURTHER WORK OR INFORMATION
REQUIRED (before an acceptable daily intake for man can be
established)
1. An adequate long-term oral study in order to establish a no-effect
level using (i) a commercially available material, (ii) the purest
available 2,4,5-T and (iii) 2,3,7,8-tetrachlorodibenzo-p-dioxin.
2. Studies on reproduction and teratogenicity with 2,4,5-T using (i) a
commercially available material, (ii) the purest available 2,4,5-T
and (iii) 2,3,7,8-tetrachlorodibenzo-p-dioxin.
DESIRABLE
Information on the availability of acceptable methods for the
detection and determination of chlorinated dibenzodioxin impurities in
technical 2,4,5-T and its formulations at the 0.01 - 0.05 ppm level.
Current attempts to standardize the specifications of 2,4,5-T products
in regard to their content of chlorinated dibenzodioxin compounds
should be continued and encouraged.
REFERENCES
Abbott, D.C., Egan, H., Hammond, E.W. and Thomson, J. (1964) The
chromatographic detection and determination of organo-chlorine
herbicides in soil and water. Analyst, 89: 480-488
Anon. (1970) Another herbicide on the blacklist. Nature, 226:
309-311
Bjorklund, N-E and Erne, K. (1966) Toxicological studies of
phenoxyacetic herbicides in animals. Acta Vet. Scand., 7: 364-390
Bleiberg, J., Wallen, M., Brodkin, R, and Applebaum, I.L, (1964)
Industrially acquired porphyria. Arch, Dermatol., 89: 793-797
Boehringer Sohn. (1970) Residues of 2,4,5-T in cereals and straw.
Unpublished report
Bradley, J.K. and Thompson, W.K. Determination of 2,4-D and 2,4,5-T in
lawn fertilisers. J. Sci.Fd.Agr., 15: 673-677
Clark, D.E. (1969) Determination of 2,4,5-trichlorophenoxyacetic acid
and its propylene glycol butyl ether esters in animal tissue, blood
and urine. J.Agr.Fd. Chem., 17: 1168-1170
Courtney, K.D., Gaylor, D.W., Hogan, M.D., Falk, H.L., Baton, R.R. and
Mitchell, I. (1970a) Teratogenic evaluation of 2,4,5-T. Science,
168: 864-866
Courtney, K.D., Moore, J.A., Gaylor, D.W., Hogen, M.D. and Falk, H.L.
(1970b) Summary teratogen study. Typescript draft of record of 15
April 1970 hearing on 2,4,5-T before the Sub-committee on Energy,
National Resources and the Environment of the U.S. Senate Committee on
Commerce, 225-232 (does not appear in final printed report)
Dow (1961) Results of 90-day dietary feeding studies on Dowanol 97B
(mono-, di- and tripropylene glycol butyl ether) esters of 2,4,5-T in
rats. Unpublished data, The Dow Chemical Company
Dow (1970a) Assay of a commercial production lot of 2,4,5-T.
Unpublished data, The Dow Chemical Company
Dow (1970b) A 90-day dietary feeding study on 2,4,5-T
trichlorophenoxyacetic acid in rats. Unpublished data, The Dow
Chemical Company
Dow (1970c) A study of the effects of work exposure to 2,4,5-T on
employee health. Unpublished data, The Dow Chemical Company
Drill, V.A. and Hiratzka, T. (1953) Toxicity of
2,4-dichlorophenoxyacetic acid and 2,4,5-trichlorophenoxyacetic acid,
a report on their acute and chronic toxicity in dogs. Arch. Ind. Hyg.
Occup. Med., 7: 61-67
Edgerton, L.J. and Lisk, D.L. (1963) Determination of residues of
2,4,5-trichlorophenoxyacetic acid in apples by radioisotope and gas
chromatographic methods. Proc. Amer. Soc. Hort. Sci., 83: 120-125
Emerson, J.L., Thompson, D.J., Strebing, R.J., Gerbig, C.G. and
Robinson, V.B. (1970) Teratogenic studies on
2,4,5-trichlorophenoxyacetic acid in the rat and rabbit. Paper on rat
data presented at Society of Toxicology, Atlanta, Georgia, March 17.
Submitted to Fd. Cosmet. Toxicol. for publication
Erne, K. (1966a) Distribution and elimination of chlorinated
phenoxyacetic acids in animals. Acta. Vet. Scand., 7: 240-256
Erne, K. (1966b) Animal metabolism of phenoxyacetic herbicides. Acta
Vet. Scand., 7: 264-271
Gordon, N. and Beroza, M. (1952) Spectrophotometric determination of
small quantities of 2,4-dichlorophenoxyacetic acid and
2,4,5-trichlorophenoxyacetic acid. Anal. Chem., 24: 1968-1971
Hindin, E., May, D.S. and Dunstan, G.E. (1964) Collection and analysis
of synthetic organic pesticides from surface and ground water.
Res.Rev. 7: 130-156
Innes, J.R.M., Ulland, B.M., Valerio, M.G., Petrucelli, L., Fishbein,
L., Hart, E.R., Pallotta, A.J., Bates, R.R., Falk, H.C., Gart, J.J.,
Klein, M., Mitchell, I. and Peter, J. (1969) Bioassay of pesticides
and industrial chemicals for tumorigenicity in mice: a preliminary
note. J. nat. Cancer Inst., 42: 1101-1114
Maxie, E.C. and Crane, J.C. (1956) Some metabolic effects of
2,4,5-trichlorophenoxyacetic acid on Tilton apricot fruits. Proc.
Amer. Soc. Hort. Sci., 68: 113-123
Martin, H. (1968) Pesticide manual, British Crop Protection Council,
page 401
Roberts, R.E. and Rogers, B.J. (1957) The effect of 2,4,5-T brush
spray on turkeys. Poultry Science, 36: 703-705
Rowe, V.K. and Hymas, T.A. (1954) Summary of toxicological information
on 2,4-D and 2,4,5-T type herbicides and an evaluation of the hazards
to livestock associated with their use. Am. J. Vet. Res., 15:
622-629
Sparschu, G.L., Dunn, F.L. and Rowe, V.K. (1970) Teratogenic study of
2,3,7,8-tetrachlorodibenzo-p-dioxin in the rat. Paper presented at
Society of Toxicology Atlanta, Georgia, March 17. Mss. submitted to
Fd. Cosmet. Toxicol. for publication
St. John, L.E., Wagner. D.G. and Lisk, D.J. (1964) Fate of atrasine,
Kuron, Silvex and 2,4,5-T in the dairy cow. J.Dairy Sci., 47:
1267-1270
Way, J.M. (1969) Toxicity and hazards to man, domestic animals and
wildlife from some commonly used auxin herbicides. Res. Rev.26:
37-62
Zielinski, W.L. Jr. and Fishbein, L. (1967) Gas chromatographic
measurement of disappearance rates of 2,4,-D and 2,4,5-T acids and
2,4-D esters in mice. J. Agr. Fd. Chem. 15: 841-844
 Other relevant chemical properties
The pure acid is a white crystalline solid with a melting point of
158°C and a water solubility of 278 mg/l. It is soluble in acetone,
ethanol and ether (Martin, 1968). The salts with alkali metals and
amines are water soluble but insoluble in petroleum oils; esters are
water insoluble but soluble in oils. The technical acid is stable and
non-corrosive.
Purity
The technical acid has a melting point at approximately 150-151°c. A
typical production lot assayed 95+ percent
2,4,5-trichlorophenoxyacetic acid, 2.9 percent
dichloromethoxyphenoxyacetic acids, 0.6 percent related
trichlorophenoxyacetic acid, 0.5 percent dichlorophenoxyacetic acids,
0.4 percent bis-(2,4,5-trichlorophenoxy)-acetic acid and less than
0.5 ppm 2,3,7,8-tetrachlorodibenzo-p-dioxin (Dow, 1970a). This last
impurity, hereinafter referred to as the "dioxin", is a highly toxic
potent chloracnegen and has been reported to be present in one sample
of commercially produced 2,4,5-T at a level of approximately 27 ppm
(Courtney et al., 1970a; Emerson et al., 1970).
2,4,5-T is formulated for use as a herbicide in water soluble
formulations of various amine salts (e.g. triethylamine) or as
formulations of esters (e.g. iso-octyl) dispersible in oil and/or
water.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
The plasma half-life of an amine salt of 2,4,5-T has been reported to
be approximately three hours in the rat, following the administration
of a single dose of 100 mg/kg body-weight (Erne, 1966a). See also
"Fate of residues, in animals".
Mice were injected with a single dose of 100 mg/kg body-weight of
2,4,5-T in dimethylsulfoxide solution. The animals were sacrificed at
various intervals after injection and analyzed in toto for 2,4,5-T.
The amounts recovered as percentage of the amount injected indicated
decreasing levels at the following time intervals after dosing: at 0
hours, 77.1 percent; at 16 hours, 56.9 percent and at 24 hours, 23.7
percent (Zielinski and Fishbein, 1967).
Effect on enzymes and other biochemical parameters
As is the case with 2,4-D and other auxin herbicides, it appears that
treatment of plants with 2,4,5-T may result in an increased nitrate
formation and produce toxic effects in grazing animals (Way, 1969).
See also the monograph on 2,4-D.
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Mouse
Groups of 18 mice of each sex, from two hybrid strains, were given
2,4,5-T acid from seven days of age for 18 months. The compound was
given by gavage at 0 or 21.5 mg/kg body-weight until weaning, after
which time 2,4,5-T was incorporated into the diet at 0 or 60 ppm.
There was no significant increase in tumour incidence between the
control and treated groups (Innes et al., 1969).
Special studies on teratogenicity
Mouse
Two strains of pregnant mice received one or more of the following
daily dose levels of 2,4,5-T: 0, 21.5, 46.4 or 113 mg/kg body-weight.
The compound was administered subcutaneously in dimethyl sulfoxide
solution or orally in honey on gestation days 6 to 14 or days 9 to 17
or days 6 to 15 inclusive. A significant increase in cleft palate and
cystic kidney was observed in the foetuses from the animals which were
given 46.4 or 113 mg/kg on days 6 to 14 or 9 to 17 of gestation. In
the group given 113 mg/kg orally, there was increased foetal
mortality. The level of "dioxin" impurity in the sample of 2,4,5-T
used was approximately 30 ppm; thus the animals received at 0, 0.6,
1.4 or 3.4 µg/kg of the "dioxin". The authors concluded that the
sample of 2,4,5-T used was teratogenic and foetocidal in the two
strains of mice when administered orally or subcutaneously throughout
the period of organogenesis (Courtney et al., 1970a).
Additional studies have been conducted with three strains of mice
using (a) purified 2,4,5-T estimated to contain less than 0.1 ppm of
the "dioxin", (b) commercially produced 2,4,5-T containing
approximately 0.5 ppm of the "dioxin", (c) 2,4,5-T from a chemical
supply house, (d) the sample of 2,4,5-T used in the previously
described study and (e) pure "dioxin". The compounds were administered
to pregnant mice by subcutaneous injection in dimethylsulfoxide on
gestation days 6 to 15 inclusive (ten doses) unless otherwise noted.
The doses of 2,4,5-T used were 50, 100, 113 or 150 mg/kg body-weight/
day. The purified sample (a) was tested in one strain of mice at a
level of 100 mg/kg/day only, when a significant increase over the
controls of cleft palate and "kidney involvement" (not otherwise
described) was observed. In the same strain of mice, the sample of
commercial 2,4,5-T (b) gave no adverse effects at doses of 50 or 100
mg/kg/day. At 150 mg/kg/day this sample, (b), resulted in a
significant number of cleft palates and increased foetal mortality,
but resulted in no "kidney involvement". In another strain of mice,
the commercial samples of 2,4,5-T (b) and (c) were judged to have
caused an increase in cleft palate at doses of 100 mg and 113
mg/kg/day, respectively. There was no increased "kidney involvement"
or foetal mortality from either sample. In the third strain of mice,
100 mg/kg/day of commercial 2,4,5-T, (b), caused no significant
increase in cleft palate or "kidney involvement", but there was an
increase in foetal mortality. Pure "dioxin" at doses of 1 or 3 ug/kg
body-weight caused "kidney involvement" in all tests, but resulted in
significant increases in cleft palates and foetal mortality only in
some tests, not in others (Courtney at al., 1970b).
Rat
2,4,5-T containing approximately 30 ppm of the "dioxin" impurity was
administered orally to one strain of rats at daily dose levels of 0,
4.6, 10 or 46.4 mg/kg body-weight on gestation days 10 to 15
inclusive. The lowest dose level of 4.6 mg/kg produced a significant
increase in the percentage of abnormal litters or incidence of foetal
mortality, The high dose levels produced a dose related incidence of
increased foetal mortality and abnormal foetuses. Cystic kidney
appeared to be the major manifestation of abnormality (Courtney et
al., 1970a).
Rats were administered various samples of the 2,4,5-T orally as
follows: (a) "pure" at 150 mg/body-weight/day (days 13 to 14 only),
(b) commercial production at 10, 21.5, 46.4 and 80 mg/kg/day, (d) the
sample containing 30 ppm of the "dioxin" at 10 and 21.5 mg/kg/day. No
terata were reported in any test. Increased foetal mortality resulted
from the "pure" (a) sample at the 150 mg/kg/day dose given on days 13
and 14 of gestation, and with the commercial production sample (b) at
a dose of 80 mg/kg/day (LD40 dose for the dams). The sample (d) at
the dose of 10 mg/kg/day gave an increase in "kidney involvement" that
was statistically significant; however, there was no increase noted at
the 21.5 mg/kg/day dose. Pure "dioxin" administered at 0.5 and 2 µg/kg
body-weight resulted in no terata or increase in foetal mortality, but
both doses were judged to have resulted in significant increases in
"kidney involvement" (Courtney at al., 1970b).
Five treatment groups, each consisting of 25 female rats were
administered 1, 3, 6, 12 or 24 mg/kg/body-weight/day of 2,4,5-T
containing <1 ppm of the "dioxin" by gavage on gestation days 6
through 15 inclusive. A single group of 50 females served as controls.
The following studies were made: clinical observations, maternal
body-weights (pre-breeding and day 20), number and position of
foetuses and resorptions, number of corpora lutea, pup weight and
sex, gross external examination of pups and microscopic examination
for intestinal haemorrhage in pups. Representative stained histologic
sections through the head, thorax and abdomen of ten control and ten
foetuses from dams administered the high dose levels were studied for
histopathologic changes. No clinical or gross pathologic signs of
adverse chemical effect were observed in the treated dams. The other
observations did not reveal any teratogenic or embryotoxic effects
(Emerson at al., 1970).
Pure "dioxin" was administered by gavage to rats at dose levels of 0,
0.03, 0.125, 0.5, 2.0 and 8.0 µg/kg body-weight/day to groups of 24
(controls) and 12 (treatment) animals on gestation days 6 to 15
inclusive. The foetuses were taken by caesarean section on day 20 of
gestation.
No differences were observed in the foetuses taken from dams treated
at the dosage of 0.03 µg/kg/day and the controls. At the 0.125
µg/kg/day dosage there was a slight decrease in average weight of
foetuses, three dead foetuses, incidence of intestinal haemorrhage
(18/127) and of subcutaneous oedema (22/80). One foetus at this level
had a rudimentary tail. At the 0.5 ug/kg/day level, the number of
foetuses was reduced and the number of resorptions and foetal deaths
was increased to six. The average weight of the viable foetuses was
slightly decreased. The incidence of intestinal haemorrhage (36/99)
and subcutaneous oedema (31/65) was markedly increased over that seen
in the 0.125 µg/kg/day treatment. At the 2.0 µg/kg/day level, only
seven viable foetuses were obtained. These were from four of the 11
litters examined. Resorptions were numerous, intestinal haemorrhage
was frequent (4/7) and subcutaneous oedema was present in all of the
four foetuses examined. One foetus from this treatment level was found
to have a kinked tail and two of its feet were somewhat misshapen, but
skeletal examination revealed no evidence of bone abnormalities. The
8.0 µg/kg/day dosage level proved to be toxic to the dams. There were
no viable foetuses in the dams examined on day 20 of gestation. All
resorptions occurred early, and no evidence of foetal tissue was
found. Skeletal examinations of foetuses from all dams used in this
experiment revealed delayed ossification of some sternebrae and skull
bones. This manifestation occurred generally throughout the various
groups, including controls (Sparschu et al., 1970).
Rabbit
Groups of 20 pregnant female rabbits each were given oral doses of
2,4,5-T containing <1 ppm of the "dioxin" by capsule from gestation
day 6 to 18 inclusive. The daily doses of 2,4,5-T administered were 0,
10, 20 and 40 mg/kg body-weight. The rabbits ware artificially
inseminated, and caesarean sections were performed on day 29. The
following studies were made: clinical signs, maternal body-weight,
conception rate, gross visceral examination, number of corpora lutea
and implantations, number of kits, resorptions and still-births; kit
weight and sex; gross external examinations; viability following
24-hour incubation; detailed visceral and skeletal examination of kits
from the control and 40 mg/kg groups. There were no dose-related
trends evident from the results of these studies. A high incidence of
neonatal mortality, which was not compound-related, was observed in
the control and treated groups. a 40 mg/kg level dam aborted on day
24; the litter was apparently completely resorbed, and bacterial focal
hepatic necrosis was observed in the dam. This animal had started to
lose weight prior to treatment with 2,4,5-T. It was concluded that
under the conditions of the study, 2,4,5-T was not embryotoxic or
teratogenic in rabbits when administered orally during the period of
organogenesis (Emerson et al., 1970).
Acute toxicity
LD50 mg/kg
Animal Route body-weight References
Mouse oral 389 Rowe and Hymas, 1954
Rat oral 500 Rowe and Hymas, 1954
Guinea pig oral 381 Rowe and Hymas, 1954
Dog oral >100 Drill and Hiratzka, 1953
In a study in pigs, to compare the acute toxicity of 2,4,5-T as
compared to 2,4-D, it is reported that single doses of 100 mg/kg
body-weight caused anorexia, vomiting, diarrhoea and ataxia only in
the two pigs given 2,4,5-T. At autopsy, gastrointestinal irritation
and congestion of the liver and kidney were observed (Björklund and
Erne, 1966).
Short-term studies
Turkey
Groups of male turkeys received 0.25 percent of an ester of 2,4,5-T in
their diet for 11 days. Based upon the amount of food consumed, the
birds received the equivalent of 62 mg/kg body-weight of the free
2,4,5-T acid. There was no appreciable change in the rate of
body-weight gain or in food consumption (Roberts and Rogers, 1957).
Rat
Groups of ten male and ten female rats per group were maintained for
90 days on diets containing 2,4,5-T which had < 1 ppm of the "dioxin"
impurity. The levels in the diet were adjusted so that the animals
received 0, 3, 10, 30 or 100 mg/kg body-weight of 2,4,5-T daily.
Visual observation revealed no changes in appearance or behaviour in
any of the rats, nor were there any deaths. Evidence of
compound-related effects was minimal and was limited to the animals
given 30 and 100 mg/kg. Changes found in both sexes fed 100 mg/kg/day
included depression in body-weight gain, slight decrease in food
intake and elevated serum alkaline phosphatase levels. Gross necropsy
examination revealed an inconsistent slight paleness and accentuated
lobular pattern of the livers of some rats of both sexes at this dose
level with inconsistent minimal amounts of hepatocellular swelling
observed upon histopathologic examination. Male rats at this dose also
had slightly increased serum glutamic-pyruvic transaminase levels and
slight decreases in red cell counts and in haemoglobin. There were
some detectable similar changes at 30 mg/kg/day, but these changes
were considered to be of questionable toxicological significance. At
doses of 3 or 10 mg/kg/day, there were no compound-related changes
observed (Dow, 1970b).
Groups of male and female rats (ten of each sex per group) were
maintained for 90 days on diets containing 0, 100, 300, 1000 and 3000
ppm of a standardized mixture of mono- di-, and tripropylene glycol
butyl ether esters of 2,4,5-T (equivalent to 0, 6.2, 18.6, 62 and 186
mg/kg body-weight of free acid). No evidence of adverse effects was
noted at the 100 or 300 ppm levels, based on gross appearance and
behaviour, mortality, food consumption, haematological indices and
gross and microscopic examination of the tissues. Increase in
spleen-weight in the males fed 100 and 300 ppm and increased
body-weight of the females fed 100 ppm was considered to be unrelated
to the administration of 2,4,5-T ester, and no pathological changes
were observed. At the 1000 ppm level, histopathology revealed slight
cloudy swelling of the parenchymal cells with central lobular necrosis
in the liver in two of ten animals of both sexes examined, as well as
some hypercellularity of the glomerular tuft, with cloudy swelling of
the renal tubular epithelium in the females. There was significant
increase in the average weight of the kidney in male rats at this
level, but there were no differences compared to the control group
with respect to all other above-mentioned parameters. At the 3000 ppm
level, growth retardation was evident and kidney to body-weight ratios
were increased in the males only. The livers of both sexes were large
and light in colour and histopathology revealed a generalized cloudy
swelling of the parenchymal cells and a slight central lobular
necrosis. The kidneys displayed some cloudy swelling of the tubular
epithelium, which was more marked in the females than the males. Some
kidney necrosis was also evident in the females. Serum alkaline
phosphatase was elevated in the males at this level (Dow, 1961).
Dog
2,4,5-T was administered orally in capsules to groups of from two to
four adult mongrel dogs, of mixed sex, for five days a week over a
13-week period. The levels administered were 0, 2, 5, 10 and 20 mg/kg
body-weight/day. All dogs receiving 10 mg/kg or less survived the 90
day test period. The dogs on 20 mg/kg/day died between days 11 and 75.
There was no effect on haemoglobin; red cell, white cell or
differential count of dogs that survived or died during the study, nor
were there any changes in the organ weights among the dogs that
survived the study. Histopathology revealed no significant changes in
the heart, lungs, thyroid, ovaries, testes, adrenals, or liver.
Duodenal hyperaemia in one dog at 10 mg/kg and one at 20 mg/kg was
evident as well as early infiltration of the mucosal cells. An
occasional slight increase in the number of casts in kidney sections
was not dose-related and was considered to be of doubtful
significance. (Drill and Hiratzka, 1953).
Long-term studies
No comprehensive long-term studies appear to have been conducted.
OBSERVATIONS IN MAN
The effect of work exposure to 2,4,5-T on the health of employees
engaged in the manufacture of the herbicide has been studied. A total
of 130 employees having a work experience from two months to over
three years and range of exposure of 2 to 8 mg of 2,4,5-T per day were
studied. The workers were given extensive physical examinations,
including a battery of at least 20 laboratory tests. No differences
were found between the groups of men exposed to 2,4,5-T and a control
group of 4600 men. In addition, karyotyping was carried out on 52
exposed men. There was no indication that 2,4,5-T exposure had
affected the structural integrity or rearranged the genetic material
of the lymphocyte chromosomes (Dow, 1970c).
In workers employed in factories manufacturing chlorinated phenols, a
moderately high incidence of urinary porphyria, chloracne and
hirsutism has been reported. The authors suggest that a highly
chlorinated phenolic ether may be the compound responsible (Bleiberg
et al., 1964).
Sporadic outbreaks of severe acne have been encountered in workers in
chemical plants where 2,4,5-trichlorophenol is manufactured or used.
It is stated that one of the agents responsible is
2,3,7,8-tetrachlorodibenzo-p-dioxin (Anon, 1970).
COMMENT
Considerable information on the potential teratogenic effect of
2,4,5-T of varying degrees of purity is available in mice, rats and
rabbits. The evidence so far presented is inconclusive to determine
whether the teratogenic effects shown in mice and to a lesser extent
in rats are due solely to the presence of the impurity
2,3,7,8-tetrachlorodibenzo-p-dioxin (called the "dioxin").
Information from two independent laboratories appear to indicate some
conflicting results with respect to teratogenicity.
Thus, in one study with mice using a "pure" sample of 2,4,5-T (i.e.
one alleged to contain <0.1 ppm of the "dioxin") foetal abnormalities
were produced at the only level tested, which was 100 mg/kg
body-weight. This level would be equivalent to receiving less than
0.01 µg/kg body-weight of the "dioxin". On the other hand, when 0.03
µg/kg of the "dioxin" alone was administered to pregnant rats, there
were no terata produced, although abnormalities were evident at 0.125
µg/kg and higher levels of the "dioxin". In rabbit reproduction
studies, using 2,4,5-T containing <1 ppm of the "dioxin", no terata
were produced at any dose level, although foetal mortality was evident
at 40 mg/kg body-weight.
The need for further studies with respect to teratogenicity in several
species, including, if possible, non-human primates was stressed. It
was pointed out that there is no scientifically verifiable evidence
that 2,4,5-T has caused teratogenic effects in man.
Attention was drawn to the reported occurrence of chloracne and other
unpleasant toxic effects encountered among some workers involved in
the manufacture of 2,4,5-T and/or related compounds.
The toxic effects reported to be due to increased nitrate formation in
plants treated with 2,4,5-T were not considered to present a hazard to
man under conditions of normal use of the compound.
Because of the reported teratogenic effects, which may or may not be
related to impurities in the samples of 2,4,5-T used, and because
there are no comprehensive long-term feeding studies in any species
(or a two-year feeding study in a non-rodent mammalian species), an
acceptable daily intake for man could not be established. It was
recognized that measurable levels of 2,4,5-T or its breakdown products
could appear in food commodities (see following). In such cases, the
establishment of an acceptable daily intake for 2,4,5-T will be
necessary in assessing the problem of pesticide residues in food. It
was stressed that in order to establish such a figure, a level causing
no toxicological effect would have to be determined. It was further
emphasized that indiscriminate use of 2,4,5-T should be discouraged.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Control of certain weeds in cereal crops and lawns, spot control of
nettles in pasture and used selectively in forestry for control of
woody weeds. Used mainly on non-edible crops.
FATE OF RESIDUES
In animals
Erne (1966 a and b) studied the distribution and elimination of
2,4,5-T in farm animals; amine and alkali salts were readily absorbed
and distributed in the body. The highest tissue levels were found in
liver, kidney, spleen and lungs; the levels found in these organs
exceeded levels found in plasma.
St. John et al., (1964) studied the fate of 2,4,5-T in cattle; it was
eliminated an soluble salts in the urine, and no residue was found in
the milk. Urine and milk samples were analysed from cows fed 2,4,5-T
over a period of four days; over 90 percent was recovered from the
urine excreted over a period of six days.
In plant
After foliar application, 2,4,5-T, being a lipoid-soluble compound,
seem to be limited to the phloem in its movement out of leaves, being
accumulated in the more active metabolic region. Residual 2,4,5-T has
been shown to persist on the leaf surface of apricots or at least one
month following foliar application of 14C labelled 2,4,5-T (Maxie et
al., 1956). No evidence was obtained for metabolism of 2,4,5-T in
either leaves or fruits of apricots.
Evidence of residues in food in commerce or at consumption
Some unpublished data were presented (Boehringer Sohn, 1970) regarding
residues of 2,4,5-T in cereals (grain and straw) found to occur
following applications of the herbicide, either alone or in admixture
with other phenoseyacid herbicides (recoprop, MCPA, 2,4-DP). A summary
of the data relating to 2,4,5-T is given in Table I.
TABLE I
Residues of 2,4,5-T in cereals
Interval
Rate of (days) Residues found, ppm
Crop Application Applic.
g/ha Harvest Grain Straw
Barley 120 64 0.04 0.80
77 n.d.1 0.13
89 - 0.06
100 - n.d.
380 64 n.d. 0.30
77 n.d. 0.12
89 - 0.04
100 - n.d.
520 64 0.04 0.40
77 n.d. 0.10
89 - 0.04
100 - n.d.
Wheat 120 76 0.03 0.17
89 0.025 0.09
380 76 0.03 0.15
89 0.02 0.11
520 76 0.03 0.21
89 0.02 0.11
Oats 120 76 n.d. 0.20
89 n.d. n.d.
380 76 n.d. n.d.
89 n.d. n.d.
520 76 n.d. 0.23
89 n.d. n.d.
Rye 120 76 0.02 0.15
89 n.d. 0.10
380 76 0.02 0.15
89 n.d. 0.08
520 76 0.02 1.0
89 n.d. 0.22
1 n.d. = not detected
METHODS OF RESIDUE ANALYSIS
Residues of 2,4,5-T may be determined by suitable combinations of
extraction, separation and end determination by ultraviolet
spectrophotemetry, gas chromatography, paper and thin-layer
chromatography and radiometric methods. Bradley and Thompson (1964)
used GLC of the methyl esters after methylation with diazomethane.
Gordon and Beroza (1952), after extraction and separation by partition
chromatography, determined 2,4,5-T by spectrophotometry at 289 nm;
this method may be used for alfalfa hay extracts. By use of paper and
thin-layer chromatography for detection, separation and
identification, Abbott et al. (1964) determined 2,4,5-T in soil and
water. Hindin et al. (1964) analysed surface and ground water by paper
chromatography and GLC. Edgerton and Lisk (1963) determined 2,4,5-T in
applies by radio-isotopic and GLC methods. Clark (1969) determined
2,4,5-T and its propylene glycol butyl ether esters in animal tissue,
blood and urine. He converted to methyl esters and analysed by
microcoulometric GLC; recoveries at 0.05 - 20 ppm levels of 2,4,5-T
were 89.3 - 93.6% and of the ester 70.5 - 92.5%. Gas chromatography of
the methyl ester of 2,4,5-T should be suitable as a procedure for
regulatory purposes.
NATIONAL TOLERANCES
Country Crop Tolerance
(ppm)
Netherlands Vegetables (except 0.1
potatoes), fruits of
vegetables, fruit crops.
Fed. Repub. Germany Leafy, other sprouting 0.01
vegetables, fruiting
vegetables, root
vegetables
APPRAISAL
2,4,5-T in a selective herbicide, mostly used for the control of woody
weeds in non-edible crops, forestry, etc. There is a limited need in
parts of Europe for its use in cereals, in admixtures with other
phenoxyacid herbicides, for the control of certain weed species.
Evidence regarding the residues that can accrue in grain and straw
following such uses was provided. No evidence regarding the need to
establish practical residue limits was apparent. Gas chromatographic
methods are available which should be adaptable for regulatory
purposes where required.
When used an recommended for the limited use on some cereals, the
following residues of 2,4,5-T can occur:
Wheat, barley, oats, rye grain 0.05 ppm
Wheat, barley, oats, rye straw 1 ppm
FURTHER WORK OR INFORMATION
REQUIRED (before an acceptable daily intake for man can be
established)
1. An adequate long-term oral study in order to establish a no-effect
level using (i) a commercially available material, (ii) the purest
available 2,4,5-T and (iii) 2,3,7,8-tetrachlorodibenzo-p-dioxin.
2. Studies on reproduction and teratogenicity with 2,4,5-T using (i) a
commercially available material, (ii) the purest available 2,4,5-T
and (iii) 2,3,7,8-tetrachlorodibenzo-p-dioxin.
DESIRABLE
Information on the availability of acceptable methods for the
detection and determination of chlorinated dibenzodioxin impurities in
technical 2,4,5-T and its formulations at the 0.01 - 0.05 ppm level.
Current attempts to standardize the specifications of 2,4,5-T products
in regard to their content of chlorinated dibenzodioxin compounds
should be continued and encouraged.
REFERENCES
Abbott, D.C., Egan, H., Hammond, E.W. and Thomson, J. (1964) The
chromatographic detection and determination of organo-chlorine
herbicides in soil and water. Analyst, 89: 480-488
Anon. (1970) Another herbicide on the blacklist. Nature, 226:
309-311
Bjorklund, N-E and Erne, K. (1966) Toxicological studies of
phenoxyacetic herbicides in animals. Acta Vet. Scand., 7: 364-390
Bleiberg, J., Wallen, M., Brodkin, R, and Applebaum, I.L, (1964)
Industrially acquired porphyria. Arch, Dermatol., 89: 793-797
Boehringer Sohn. (1970) Residues of 2,4,5-T in cereals and straw.
Unpublished report
Bradley, J.K. and Thompson, W.K. Determination of 2,4-D and 2,4,5-T in
lawn fertilisers. J. Sci.Fd.Agr., 15: 673-677
Clark, D.E. (1969) Determination of 2,4,5-trichlorophenoxyacetic acid
and its propylene glycol butyl ether esters in animal tissue, blood
and urine. J.Agr.Fd. Chem., 17: 1168-1170
Courtney, K.D., Gaylor, D.W., Hogan, M.D., Falk, H.L., Baton, R.R. and
Mitchell, I. (1970a) Teratogenic evaluation of 2,4,5-T. Science,
168: 864-866
Courtney, K.D., Moore, J.A., Gaylor, D.W., Hogen, M.D. and Falk, H.L.
(1970b) Summary teratogen study. Typescript draft of record of 15
April 1970 hearing on 2,4,5-T before the Sub-committee on Energy,
National Resources and the Environment of the U.S. Senate Committee on
Commerce, 225-232 (does not appear in final printed report)
Dow (1961) Results of 90-day dietary feeding studies on Dowanol 97B
(mono-, di- and tripropylene glycol butyl ether) esters of 2,4,5-T in
rats. Unpublished data, The Dow Chemical Company
Dow (1970a) Assay of a commercial production lot of 2,4,5-T.
Unpublished data, The Dow Chemical Company
Dow (1970b) A 90-day dietary feeding study on 2,4,5-T
trichlorophenoxyacetic acid in rats. Unpublished data, The Dow
Chemical Company
Dow (1970c) A study of the effects of work exposure to 2,4,5-T on
employee health. Unpublished data, The Dow Chemical Company
Drill, V.A. and Hiratzka, T. (1953) Toxicity of
2,4-dichlorophenoxyacetic acid and 2,4,5-trichlorophenoxyacetic acid,
a report on their acute and chronic toxicity in dogs. Arch. Ind. Hyg.
Occup. Med., 7: 61-67
Edgerton, L.J. and Lisk, D.L. (1963) Determination of residues of
2,4,5-trichlorophenoxyacetic acid in apples by radioisotope and gas
chromatographic methods. Proc. Amer. Soc. Hort. Sci., 83: 120-125
Emerson, J.L., Thompson, D.J., Strebing, R.J., Gerbig, C.G. and
Robinson, V.B. (1970) Teratogenic studies on
2,4,5-trichlorophenoxyacetic acid in the rat and rabbit. Paper on rat
data presented at Society of Toxicology, Atlanta, Georgia, March 17.
Submitted to Fd. Cosmet. Toxicol. for publication
Erne, K. (1966a) Distribution and elimination of chlorinated
phenoxyacetic acids in animals. Acta. Vet. Scand., 7: 240-256
Erne, K. (1966b) Animal metabolism of phenoxyacetic herbicides. Acta
Vet. Scand., 7: 264-271
Gordon, N. and Beroza, M. (1952) Spectrophotometric determination of
small quantities of 2,4-dichlorophenoxyacetic acid and
2,4,5-trichlorophenoxyacetic acid. Anal. Chem., 24: 1968-1971
Hindin, E., May, D.S. and Dunstan, G.E. (1964) Collection and analysis
of synthetic organic pesticides from surface and ground water.
Res.Rev. 7: 130-156
Innes, J.R.M., Ulland, B.M., Valerio, M.G., Petrucelli, L., Fishbein,
L., Hart, E.R., Pallotta, A.J., Bates, R.R., Falk, H.C., Gart, J.J.,
Klein, M., Mitchell, I. and Peter, J. (1969) Bioassay of pesticides
and industrial chemicals for tumorigenicity in mice: a preliminary
note. J. nat. Cancer Inst., 42: 1101-1114
Maxie, E.C. and Crane, J.C. (1956) Some metabolic effects of
2,4,5-trichlorophenoxyacetic acid on Tilton apricot fruits. Proc.
Amer. Soc. Hort. Sci., 68: 113-123
Martin, H. (1968) Pesticide manual, British Crop Protection Council,
page 401
Roberts, R.E. and Rogers, B.J. (1957) The effect of 2,4,5-T brush
spray on turkeys. Poultry Science, 36: 703-705
Rowe, V.K. and Hymas, T.A. (1954) Summary of toxicological information
on 2,4-D and 2,4,5-T type herbicides and an evaluation of the hazards
to livestock associated with their use. Am. J. Vet. Res., 15:
622-629
Sparschu, G.L., Dunn, F.L. and Rowe, V.K. (1970) Teratogenic study of
2,3,7,8-tetrachlorodibenzo-p-dioxin in the rat. Paper presented at
Society of Toxicology Atlanta, Georgia, March 17. Mss. submitted to
Fd. Cosmet. Toxicol. for publication
St. John, L.E., Wagner. D.G. and Lisk, D.J. (1964) Fate of atrasine,
Kuron, Silvex and 2,4,5-T in the dairy cow. J.Dairy Sci., 47:
1267-1270
Way, J.M. (1969) Toxicity and hazards to man, domestic animals and
wildlife from some commonly used auxin herbicides. Res. Rev.26:
37-62
Zielinski, W.L. Jr. and Fishbein, L. (1967) Gas chromatographic
measurement of disappearance rates of 2,4,-D and 2,4,5-T acids and
2,4-D esters in mice. J. Agr. Fd. Chem. 15: 841-844
Other relevant chemical properties
The pure acid is a white crystalline solid with a melting point of
158°C and a water solubility of 278 mg/l. It is soluble in acetone,
ethanol and ether (Martin, 1968). The salts with alkali metals and
amines are water soluble but insoluble in petroleum oils; esters are
water insoluble but soluble in oils. The technical acid is stable and
non-corrosive.
Purity
The technical acid has a melting point at approximately 150-151°c. A
typical production lot assayed 95+ percent
2,4,5-trichlorophenoxyacetic acid, 2.9 percent
dichloromethoxyphenoxyacetic acids, 0.6 percent related
trichlorophenoxyacetic acid, 0.5 percent dichlorophenoxyacetic acids,
0.4 percent bis-(2,4,5-trichlorophenoxy)-acetic acid and less than
0.5 ppm 2,3,7,8-tetrachlorodibenzo-p-dioxin (Dow, 1970a). This last
impurity, hereinafter referred to as the "dioxin", is a highly toxic
potent chloracnegen and has been reported to be present in one sample
of commercially produced 2,4,5-T at a level of approximately 27 ppm
(Courtney et al., 1970a; Emerson et al., 1970).
2,4,5-T is formulated for use as a herbicide in water soluble
formulations of various amine salts (e.g. triethylamine) or as
formulations of esters (e.g. iso-octyl) dispersible in oil and/or
water.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
The plasma half-life of an amine salt of 2,4,5-T has been reported to
be approximately three hours in the rat, following the administration
of a single dose of 100 mg/kg body-weight (Erne, 1966a). See also
"Fate of residues, in animals".
Mice were injected with a single dose of 100 mg/kg body-weight of
2,4,5-T in dimethylsulfoxide solution. The animals were sacrificed at
various intervals after injection and analyzed in toto for 2,4,5-T.
The amounts recovered as percentage of the amount injected indicated
decreasing levels at the following time intervals after dosing: at 0
hours, 77.1 percent; at 16 hours, 56.9 percent and at 24 hours, 23.7
percent (Zielinski and Fishbein, 1967).
Effect on enzymes and other biochemical parameters
As is the case with 2,4-D and other auxin herbicides, it appears that
treatment of plants with 2,4,5-T may result in an increased nitrate
formation and produce toxic effects in grazing animals (Way, 1969).
See also the monograph on 2,4-D.
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Mouse
Groups of 18 mice of each sex, from two hybrid strains, were given
2,4,5-T acid from seven days of age for 18 months. The compound was
given by gavage at 0 or 21.5 mg/kg body-weight until weaning, after
which time 2,4,5-T was incorporated into the diet at 0 or 60 ppm.
There was no significant increase in tumour incidence between the
control and treated groups (Innes et al., 1969).
Special studies on teratogenicity
Mouse
Two strains of pregnant mice received one or more of the following
daily dose levels of 2,4,5-T: 0, 21.5, 46.4 or 113 mg/kg body-weight.
The compound was administered subcutaneously in dimethyl sulfoxide
solution or orally in honey on gestation days 6 to 14 or days 9 to 17
or days 6 to 15 inclusive. A significant increase in cleft palate and
cystic kidney was observed in the foetuses from the animals which were
given 46.4 or 113 mg/kg on days 6 to 14 or 9 to 17 of gestation. In
the group given 113 mg/kg orally, there was increased foetal
mortality. The level of "dioxin" impurity in the sample of 2,4,5-T
used was approximately 30 ppm; thus the animals received at 0, 0.6,
1.4 or 3.4 µg/kg of the "dioxin". The authors concluded that the
sample of 2,4,5-T used was teratogenic and foetocidal in the two
strains of mice when administered orally or subcutaneously throughout
the period of organogenesis (Courtney et al., 1970a).
Additional studies have been conducted with three strains of mice
using (a) purified 2,4,5-T estimated to contain less than 0.1 ppm of
the "dioxin", (b) commercially produced 2,4,5-T containing
approximately 0.5 ppm of the "dioxin", (c) 2,4,5-T from a chemical
supply house, (d) the sample of 2,4,5-T used in the previously
described study and (e) pure "dioxin". The compounds were administered
to pregnant mice by subcutaneous injection in dimethylsulfoxide on
gestation days 6 to 15 inclusive (ten doses) unless otherwise noted.
The doses of 2,4,5-T used were 50, 100, 113 or 150 mg/kg body-weight/
day. The purified sample (a) was tested in one strain of mice at a
level of 100 mg/kg/day only, when a significant increase over the
controls of cleft palate and "kidney involvement" (not otherwise
described) was observed. In the same strain of mice, the sample of
commercial 2,4,5-T (b) gave no adverse effects at doses of 50 or 100
mg/kg/day. At 150 mg/kg/day this sample, (b), resulted in a
significant number of cleft palates and increased foetal mortality,
but resulted in no "kidney involvement". In another strain of mice,
the commercial samples of 2,4,5-T (b) and (c) were judged to have
caused an increase in cleft palate at doses of 100 mg and 113
mg/kg/day, respectively. There was no increased "kidney involvement"
or foetal mortality from either sample. In the third strain of mice,
100 mg/kg/day of commercial 2,4,5-T, (b), caused no significant
increase in cleft palate or "kidney involvement", but there was an
increase in foetal mortality. Pure "dioxin" at doses of 1 or 3 ug/kg
body-weight caused "kidney involvement" in all tests, but resulted in
significant increases in cleft palates and foetal mortality only in
some tests, not in others (Courtney at al., 1970b).
Rat
2,4,5-T containing approximately 30 ppm of the "dioxin" impurity was
administered orally to one strain of rats at daily dose levels of 0,
4.6, 10 or 46.4 mg/kg body-weight on gestation days 10 to 15
inclusive. The lowest dose level of 4.6 mg/kg produced a significant
increase in the percentage of abnormal litters or incidence of foetal
mortality, The high dose levels produced a dose related incidence of
increased foetal mortality and abnormal foetuses. Cystic kidney
appeared to be the major manifestation of abnormality (Courtney et
al., 1970a).
Rats were administered various samples of the 2,4,5-T orally as
follows: (a) "pure" at 150 mg/body-weight/day (days 13 to 14 only),
(b) commercial production at 10, 21.5, 46.4 and 80 mg/kg/day, (d) the
sample containing 30 ppm of the "dioxin" at 10 and 21.5 mg/kg/day. No
terata were reported in any test. Increased foetal mortality resulted
from the "pure" (a) sample at the 150 mg/kg/day dose given on days 13
and 14 of gestation, and with the commercial production sample (b) at
a dose of 80 mg/kg/day (LD40 dose for the dams). The sample (d) at
the dose of 10 mg/kg/day gave an increase in "kidney involvement" that
was statistically significant; however, there was no increase noted at
the 21.5 mg/kg/day dose. Pure "dioxin" administered at 0.5 and 2 µg/kg
body-weight resulted in no terata or increase in foetal mortality, but
both doses were judged to have resulted in significant increases in
"kidney involvement" (Courtney at al., 1970b).
Five treatment groups, each consisting of 25 female rats were
administered 1, 3, 6, 12 or 24 mg/kg/body-weight/day of 2,4,5-T
containing <1 ppm of the "dioxin" by gavage on gestation days 6
through 15 inclusive. A single group of 50 females served as controls.
The following studies were made: clinical observations, maternal
body-weights (pre-breeding and day 20), number and position of
foetuses and resorptions, number of corpora lutea, pup weight and
sex, gross external examination of pups and microscopic examination
for intestinal haemorrhage in pups. Representative stained histologic
sections through the head, thorax and abdomen of ten control and ten
foetuses from dams administered the high dose levels were studied for
histopathologic changes. No clinical or gross pathologic signs of
adverse chemical effect were observed in the treated dams. The other
observations did not reveal any teratogenic or embryotoxic effects
(Emerson at al., 1970).
Pure "dioxin" was administered by gavage to rats at dose levels of 0,
0.03, 0.125, 0.5, 2.0 and 8.0 µg/kg body-weight/day to groups of 24
(controls) and 12 (treatment) animals on gestation days 6 to 15
inclusive. The foetuses were taken by caesarean section on day 20 of
gestation.
No differences were observed in the foetuses taken from dams treated
at the dosage of 0.03 µg/kg/day and the controls. At the 0.125
µg/kg/day dosage there was a slight decrease in average weight of
foetuses, three dead foetuses, incidence of intestinal haemorrhage
(18/127) and of subcutaneous oedema (22/80). One foetus at this level
had a rudimentary tail. At the 0.5 ug/kg/day level, the number of
foetuses was reduced and the number of resorptions and foetal deaths
was increased to six. The average weight of the viable foetuses was
slightly decreased. The incidence of intestinal haemorrhage (36/99)
and subcutaneous oedema (31/65) was markedly increased over that seen
in the 0.125 µg/kg/day treatment. At the 2.0 µg/kg/day level, only
seven viable foetuses were obtained. These were from four of the 11
litters examined. Resorptions were numerous, intestinal haemorrhage
was frequent (4/7) and subcutaneous oedema was present in all of the
four foetuses examined. One foetus from this treatment level was found
to have a kinked tail and two of its feet were somewhat misshapen, but
skeletal examination revealed no evidence of bone abnormalities. The
8.0 µg/kg/day dosage level proved to be toxic to the dams. There were
no viable foetuses in the dams examined on day 20 of gestation. All
resorptions occurred early, and no evidence of foetal tissue was
found. Skeletal examinations of foetuses from all dams used in this
experiment revealed delayed ossification of some sternebrae and skull
bones. This manifestation occurred generally throughout the various
groups, including controls (Sparschu et al., 1970).
Rabbit
Groups of 20 pregnant female rabbits each were given oral doses of
2,4,5-T containing <1 ppm of the "dioxin" by capsule from gestation
day 6 to 18 inclusive. The daily doses of 2,4,5-T administered were 0,
10, 20 and 40 mg/kg body-weight. The rabbits ware artificially
inseminated, and caesarean sections were performed on day 29. The
following studies were made: clinical signs, maternal body-weight,
conception rate, gross visceral examination, number of corpora lutea
and implantations, number of kits, resorptions and still-births; kit
weight and sex; gross external examinations; viability following
24-hour incubation; detailed visceral and skeletal examination of kits
from the control and 40 mg/kg groups. There were no dose-related
trends evident from the results of these studies. A high incidence of
neonatal mortality, which was not compound-related, was observed in
the control and treated groups. a 40 mg/kg level dam aborted on day
24; the litter was apparently completely resorbed, and bacterial focal
hepatic necrosis was observed in the dam. This animal had started to
lose weight prior to treatment with 2,4,5-T. It was concluded that
under the conditions of the study, 2,4,5-T was not embryotoxic or
teratogenic in rabbits when administered orally during the period of
organogenesis (Emerson et al., 1970).
Acute toxicity
LD50 mg/kg
Animal Route body-weight References
Mouse oral 389 Rowe and Hymas, 1954
Rat oral 500 Rowe and Hymas, 1954
Guinea pig oral 381 Rowe and Hymas, 1954
Dog oral >100 Drill and Hiratzka, 1953
In a study in pigs, to compare the acute toxicity of 2,4,5-T as
compared to 2,4-D, it is reported that single doses of 100 mg/kg
body-weight caused anorexia, vomiting, diarrhoea and ataxia only in
the two pigs given 2,4,5-T. At autopsy, gastrointestinal irritation
and congestion of the liver and kidney were observed (Björklund and
Erne, 1966).
Short-term studies
Turkey
Groups of male turkeys received 0.25 percent of an ester of 2,4,5-T in
their diet for 11 days. Based upon the amount of food consumed, the
birds received the equivalent of 62 mg/kg body-weight of the free
2,4,5-T acid. There was no appreciable change in the rate of
body-weight gain or in food consumption (Roberts and Rogers, 1957).
Rat
Groups of ten male and ten female rats per group were maintained for
90 days on diets containing 2,4,5-T which had < 1 ppm of the "dioxin"
impurity. The levels in the diet were adjusted so that the animals
received 0, 3, 10, 30 or 100 mg/kg body-weight of 2,4,5-T daily.
Visual observation revealed no changes in appearance or behaviour in
any of the rats, nor were there any deaths. Evidence of
compound-related effects was minimal and was limited to the animals
given 30 and 100 mg/kg. Changes found in both sexes fed 100 mg/kg/day
included depression in body-weight gain, slight decrease in food
intake and elevated serum alkaline phosphatase levels. Gross necropsy
examination revealed an inconsistent slight paleness and accentuated
lobular pattern of the livers of some rats of both sexes at this dose
level with inconsistent minimal amounts of hepatocellular swelling
observed upon histopathologic examination. Male rats at this dose also
had slightly increased serum glutamic-pyruvic transaminase levels and
slight decreases in red cell counts and in haemoglobin. There were
some detectable similar changes at 30 mg/kg/day, but these changes
were considered to be of questionable toxicological significance. At
doses of 3 or 10 mg/kg/day, there were no compound-related changes
observed (Dow, 1970b).
Groups of male and female rats (ten of each sex per group) were
maintained for 90 days on diets containing 0, 100, 300, 1000 and 3000
ppm of a standardized mixture of mono- di-, and tripropylene glycol
butyl ether esters of 2,4,5-T (equivalent to 0, 6.2, 18.6, 62 and 186
mg/kg body-weight of free acid). No evidence of adverse effects was
noted at the 100 or 300 ppm levels, based on gross appearance and
behaviour, mortality, food consumption, haematological indices and
gross and microscopic examination of the tissues. Increase in
spleen-weight in the males fed 100 and 300 ppm and increased
body-weight of the females fed 100 ppm was considered to be unrelated
to the administration of 2,4,5-T ester, and no pathological changes
were observed. At the 1000 ppm level, histopathology revealed slight
cloudy swelling of the parenchymal cells with central lobular necrosis
in the liver in two of ten animals of both sexes examined, as well as
some hypercellularity of the glomerular tuft, with cloudy swelling of
the renal tubular epithelium in the females. There was significant
increase in the average weight of the kidney in male rats at this
level, but there were no differences compared to the control group
with respect to all other above-mentioned parameters. At the 3000 ppm
level, growth retardation was evident and kidney to body-weight ratios
were increased in the males only. The livers of both sexes were large
and light in colour and histopathology revealed a generalized cloudy
swelling of the parenchymal cells and a slight central lobular
necrosis. The kidneys displayed some cloudy swelling of the tubular
epithelium, which was more marked in the females than the males. Some
kidney necrosis was also evident in the females. Serum alkaline
phosphatase was elevated in the males at this level (Dow, 1961).
Dog
2,4,5-T was administered orally in capsules to groups of from two to
four adult mongrel dogs, of mixed sex, for five days a week over a
13-week period. The levels administered were 0, 2, 5, 10 and 20 mg/kg
body-weight/day. All dogs receiving 10 mg/kg or less survived the 90
day test period. The dogs on 20 mg/kg/day died between days 11 and 75.
There was no effect on haemoglobin; red cell, white cell or
differential count of dogs that survived or died during the study, nor
were there any changes in the organ weights among the dogs that
survived the study. Histopathology revealed no significant changes in
the heart, lungs, thyroid, ovaries, testes, adrenals, or liver.
Duodenal hyperaemia in one dog at 10 mg/kg and one at 20 mg/kg was
evident as well as early infiltration of the mucosal cells. An
occasional slight increase in the number of casts in kidney sections
was not dose-related and was considered to be of doubtful
significance. (Drill and Hiratzka, 1953).
Long-term studies
No comprehensive long-term studies appear to have been conducted.
OBSERVATIONS IN MAN
The effect of work exposure to 2,4,5-T on the health of employees
engaged in the manufacture of the herbicide has been studied. A total
of 130 employees having a work experience from two months to over
three years and range of exposure of 2 to 8 mg of 2,4,5-T per day were
studied. The workers were given extensive physical examinations,
including a battery of at least 20 laboratory tests. No differences
were found between the groups of men exposed to 2,4,5-T and a control
group of 4600 men. In addition, karyotyping was carried out on 52
exposed men. There was no indication that 2,4,5-T exposure had
affected the structural integrity or rearranged the genetic material
of the lymphocyte chromosomes (Dow, 1970c).
In workers employed in factories manufacturing chlorinated phenols, a
moderately high incidence of urinary porphyria, chloracne and
hirsutism has been reported. The authors suggest that a highly
chlorinated phenolic ether may be the compound responsible (Bleiberg
et al., 1964).
Sporadic outbreaks of severe acne have been encountered in workers in
chemical plants where 2,4,5-trichlorophenol is manufactured or used.
It is stated that one of the agents responsible is
2,3,7,8-tetrachlorodibenzo-p-dioxin (Anon, 1970).
COMMENT
Considerable information on the potential teratogenic effect of
2,4,5-T of varying degrees of purity is available in mice, rats and
rabbits. The evidence so far presented is inconclusive to determine
whether the teratogenic effects shown in mice and to a lesser extent
in rats are due solely to the presence of the impurity
2,3,7,8-tetrachlorodibenzo-p-dioxin (called the "dioxin").
Information from two independent laboratories appear to indicate some
conflicting results with respect to teratogenicity.
Thus, in one study with mice using a "pure" sample of 2,4,5-T (i.e.
one alleged to contain <0.1 ppm of the "dioxin") foetal abnormalities
were produced at the only level tested, which was 100 mg/kg
body-weight. This level would be equivalent to receiving less than
0.01 µg/kg body-weight of the "dioxin". On the other hand, when 0.03
µg/kg of the "dioxin" alone was administered to pregnant rats, there
were no terata produced, although abnormalities were evident at 0.125
µg/kg and higher levels of the "dioxin". In rabbit reproduction
studies, using 2,4,5-T containing <1 ppm of the "dioxin", no terata
were produced at any dose level, although foetal mortality was evident
at 40 mg/kg body-weight.
The need for further studies with respect to teratogenicity in several
species, including, if possible, non-human primates was stressed. It
was pointed out that there is no scientifically verifiable evidence
that 2,4,5-T has caused teratogenic effects in man.
Attention was drawn to the reported occurrence of chloracne and other
unpleasant toxic effects encountered among some workers involved in
the manufacture of 2,4,5-T and/or related compounds.
The toxic effects reported to be due to increased nitrate formation in
plants treated with 2,4,5-T were not considered to present a hazard to
man under conditions of normal use of the compound.
Because of the reported teratogenic effects, which may or may not be
related to impurities in the samples of 2,4,5-T used, and because
there are no comprehensive long-term feeding studies in any species
(or a two-year feeding study in a non-rodent mammalian species), an
acceptable daily intake for man could not be established. It was
recognized that measurable levels of 2,4,5-T or its breakdown products
could appear in food commodities (see following). In such cases, the
establishment of an acceptable daily intake for 2,4,5-T will be
necessary in assessing the problem of pesticide residues in food. It
was stressed that in order to establish such a figure, a level causing
no toxicological effect would have to be determined. It was further
emphasized that indiscriminate use of 2,4,5-T should be discouraged.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Control of certain weeds in cereal crops and lawns, spot control of
nettles in pasture and used selectively in forestry for control of
woody weeds. Used mainly on non-edible crops.
FATE OF RESIDUES
In animals
Erne (1966 a and b) studied the distribution and elimination of
2,4,5-T in farm animals; amine and alkali salts were readily absorbed
and distributed in the body. The highest tissue levels were found in
liver, kidney, spleen and lungs; the levels found in these organs
exceeded levels found in plasma.
St. John et al., (1964) studied the fate of 2,4,5-T in cattle; it was
eliminated an soluble salts in the urine, and no residue was found in
the milk. Urine and milk samples were analysed from cows fed 2,4,5-T
over a period of four days; over 90 percent was recovered from the
urine excreted over a period of six days.
In plant
After foliar application, 2,4,5-T, being a lipoid-soluble compound,
seem to be limited to the phloem in its movement out of leaves, being
accumulated in the more active metabolic region. Residual 2,4,5-T has
been shown to persist on the leaf surface of apricots or at least one
month following foliar application of 14C labelled 2,4,5-T (Maxie et
al., 1956). No evidence was obtained for metabolism of 2,4,5-T in
either leaves or fruits of apricots.
Evidence of residues in food in commerce or at consumption
Some unpublished data were presented (Boehringer Sohn, 1970) regarding
residues of 2,4,5-T in cereals (grain and straw) found to occur
following applications of the herbicide, either alone or in admixture
with other phenoseyacid herbicides (recoprop, MCPA, 2,4-DP). A summary
of the data relating to 2,4,5-T is given in Table I.
TABLE I
Residues of 2,4,5-T in cereals
Interval
Rate of (days) Residues found, ppm
Crop Application Applic.
g/ha Harvest Grain Straw
Barley 120 64 0.04 0.80
77 n.d.1 0.13
89 - 0.06
100 - n.d.
380 64 n.d. 0.30
77 n.d. 0.12
89 - 0.04
100 - n.d.
520 64 0.04 0.40
77 n.d. 0.10
89 - 0.04
100 - n.d.
Wheat 120 76 0.03 0.17
89 0.025 0.09
380 76 0.03 0.15
89 0.02 0.11
520 76 0.03 0.21
89 0.02 0.11
Oats 120 76 n.d. 0.20
89 n.d. n.d.
380 76 n.d. n.d.
89 n.d. n.d.
520 76 n.d. 0.23
89 n.d. n.d.
Rye 120 76 0.02 0.15
89 n.d. 0.10
380 76 0.02 0.15
89 n.d. 0.08
520 76 0.02 1.0
89 n.d. 0.22
1 n.d. = not detected
METHODS OF RESIDUE ANALYSIS
Residues of 2,4,5-T may be determined by suitable combinations of
extraction, separation and end determination by ultraviolet
spectrophotemetry, gas chromatography, paper and thin-layer
chromatography and radiometric methods. Bradley and Thompson (1964)
used GLC of the methyl esters after methylation with diazomethane.
Gordon and Beroza (1952), after extraction and separation by partition
chromatography, determined 2,4,5-T by spectrophotometry at 289 nm;
this method may be used for alfalfa hay extracts. By use of paper and
thin-layer chromatography for detection, separation and
identification, Abbott et al. (1964) determined 2,4,5-T in soil and
water. Hindin et al. (1964) analysed surface and ground water by paper
chromatography and GLC. Edgerton and Lisk (1963) determined 2,4,5-T in
applies by radio-isotopic and GLC methods. Clark (1969) determined
2,4,5-T and its propylene glycol butyl ether esters in animal tissue,
blood and urine. He converted to methyl esters and analysed by
microcoulometric GLC; recoveries at 0.05 - 20 ppm levels of 2,4,5-T
were 89.3 - 93.6% and of the ester 70.5 - 92.5%. Gas chromatography of
the methyl ester of 2,4,5-T should be suitable as a procedure for
regulatory purposes.
NATIONAL TOLERANCES
Country Crop Tolerance
(ppm)
Netherlands Vegetables (except 0.1
potatoes), fruits of
vegetables, fruit crops.
Fed. Repub. Germany Leafy, other sprouting 0.01
vegetables, fruiting
vegetables, root
vegetables
APPRAISAL
2,4,5-T in a selective herbicide, mostly used for the control of woody
weeds in non-edible crops, forestry, etc. There is a limited need in
parts of Europe for its use in cereals, in admixtures with other
phenoxyacid herbicides, for the control of certain weed species.
Evidence regarding the residues that can accrue in grain and straw
following such uses was provided. No evidence regarding the need to
establish practical residue limits was apparent. Gas chromatographic
methods are available which should be adaptable for regulatory
purposes where required.
When used an recommended for the limited use on some cereals, the
following residues of 2,4,5-T can occur:
Wheat, barley, oats, rye grain 0.05 ppm
Wheat, barley, oats, rye straw 1 ppm
FURTHER WORK OR INFORMATION
REQUIRED (before an acceptable daily intake for man can be
established)
1. An adequate long-term oral study in order to establish a no-effect
level using (i) a commercially available material, (ii) the purest
available 2,4,5-T and (iii) 2,3,7,8-tetrachlorodibenzo-p-dioxin.
2. Studies on reproduction and teratogenicity with 2,4,5-T using (i) a
commercially available material, (ii) the purest available 2,4,5-T
and (iii) 2,3,7,8-tetrachlorodibenzo-p-dioxin.
DESIRABLE
Information on the availability of acceptable methods for the
detection and determination of chlorinated dibenzodioxin impurities in
technical 2,4,5-T and its formulations at the 0.01 - 0.05 ppm level.
Current attempts to standardize the specifications of 2,4,5-T products
in regard to their content of chlorinated dibenzodioxin compounds
should be continued and encouraged.
REFERENCES
Abbott, D.C., Egan, H., Hammond, E.W. and Thomson, J. (1964) The
chromatographic detection and determination of organo-chlorine
herbicides in soil and water. Analyst, 89: 480-488
Anon. (1970) Another herbicide on the blacklist. Nature, 226:
309-311
Bjorklund, N-E and Erne, K. (1966) Toxicological studies of
phenoxyacetic herbicides in animals. Acta Vet. Scand., 7: 364-390
Bleiberg, J., Wallen, M., Brodkin, R, and Applebaum, I.L, (1964)
Industrially acquired porphyria. Arch, Dermatol., 89: 793-797
Boehringer Sohn. (1970) Residues of 2,4,5-T in cereals and straw.
Unpublished report
Bradley, J.K. and Thompson, W.K. Determination of 2,4-D and 2,4,5-T in
lawn fertilisers. J. Sci.Fd.Agr., 15: 673-677
Clark, D.E. (1969) Determination of 2,4,5-trichlorophenoxyacetic acid
and its propylene glycol butyl ether esters in animal tissue, blood
and urine. J.Agr.Fd. Chem., 17: 1168-1170
Courtney, K.D., Gaylor, D.W., Hogan, M.D., Falk, H.L., Baton, R.R. and
Mitchell, I. (1970a) Teratogenic evaluation of 2,4,5-T. Science,
168: 864-866
Courtney, K.D., Moore, J.A., Gaylor, D.W., Hogen, M.D. and Falk, H.L.
(1970b) Summary teratogen study. Typescript draft of record of 15
April 1970 hearing on 2,4,5-T before the Sub-committee on Energy,
National Resources and the Environment of the U.S. Senate Committee on
Commerce, 225-232 (does not appear in final printed report)
Dow (1961) Results of 90-day dietary feeding studies on Dowanol 97B
(mono-, di- and tripropylene glycol butyl ether) esters of 2,4,5-T in
rats. Unpublished data, The Dow Chemical Company
Dow (1970a) Assay of a commercial production lot of 2,4,5-T.
Unpublished data, The Dow Chemical Company
Dow (1970b) A 90-day dietary feeding study on 2,4,5-T
trichlorophenoxyacetic acid in rats. Unpublished data, The Dow
Chemical Company
Dow (1970c) A study of the effects of work exposure to 2,4,5-T on
employee health. Unpublished data, The Dow Chemical Company
Drill, V.A. and Hiratzka, T. (1953) Toxicity of
2,4-dichlorophenoxyacetic acid and 2,4,5-trichlorophenoxyacetic acid,
a report on their acute and chronic toxicity in dogs. Arch. Ind. Hyg.
Occup. Med., 7: 61-67
Edgerton, L.J. and Lisk, D.L. (1963) Determination of residues of
2,4,5-trichlorophenoxyacetic acid in apples by radioisotope and gas
chromatographic methods. Proc. Amer. Soc. Hort. Sci., 83: 120-125
Emerson, J.L., Thompson, D.J., Strebing, R.J., Gerbig, C.G. and
Robinson, V.B. (1970) Teratogenic studies on
2,4,5-trichlorophenoxyacetic acid in the rat and rabbit. Paper on rat
data presented at Society of Toxicology, Atlanta, Georgia, March 17.
Submitted to Fd. Cosmet. Toxicol. for publication
Erne, K. (1966a) Distribution and elimination of chlorinated
phenoxyacetic acids in animals. Acta. Vet. Scand., 7: 240-256
Erne, K. (1966b) Animal metabolism of phenoxyacetic herbicides. Acta
Vet. Scand., 7: 264-271
Gordon, N. and Beroza, M. (1952) Spectrophotometric determination of
small quantities of 2,4-dichlorophenoxyacetic acid and
2,4,5-trichlorophenoxyacetic acid. Anal. Chem., 24: 1968-1971
Hindin, E., May, D.S. and Dunstan, G.E. (1964) Collection and analysis
of synthetic organic pesticides from surface and ground water.
Res.Rev. 7: 130-156
Innes, J.R.M., Ulland, B.M., Valerio, M.G., Petrucelli, L., Fishbein,
L., Hart, E.R., Pallotta, A.J., Bates, R.R., Falk, H.C., Gart, J.J.,
Klein, M., Mitchell, I. and Peter, J. (1969) Bioassay of pesticides
and industrial chemicals for tumorigenicity in mice: a preliminary
note. J. nat. Cancer Inst., 42: 1101-1114
Maxie, E.C. and Crane, J.C. (1956) Some metabolic effects of
2,4,5-trichlorophenoxyacetic acid on Tilton apricot fruits. Proc.
Amer. Soc. Hort. Sci., 68: 113-123
Martin, H. (1968) Pesticide manual, British Crop Protection Council,
page 401
Roberts, R.E. and Rogers, B.J. (1957) The effect of 2,4,5-T brush
spray on turkeys. Poultry Science, 36: 703-705
Rowe, V.K. and Hymas, T.A. (1954) Summary of toxicological information
on 2,4-D and 2,4,5-T type herbicides and an evaluation of the hazards
to livestock associated with their use. Am. J. Vet. Res., 15:
622-629
Sparschu, G.L., Dunn, F.L. and Rowe, V.K. (1970) Teratogenic study of
2,3,7,8-tetrachlorodibenzo-p-dioxin in the rat. Paper presented at
Society of Toxicology Atlanta, Georgia, March 17. Mss. submitted to
Fd. Cosmet. Toxicol. for publication
St. John, L.E., Wagner. D.G. and Lisk, D.J. (1964) Fate of atrasine,
Kuron, Silvex and 2,4,5-T in the dairy cow. J.Dairy Sci., 47:
1267-1270
Way, J.M. (1969) Toxicity and hazards to man, domestic animals and
wildlife from some commonly used auxin herbicides. Res. Rev.26:
37-62
Zielinski, W.L. Jr. and Fishbein, L. (1967) Gas chromatographic
measurement of disappearance rates of 2,4,-D and 2,4,5-T acids and
2,4-D esters in mice. J. Agr. Fd. Chem. 15: 841-844
